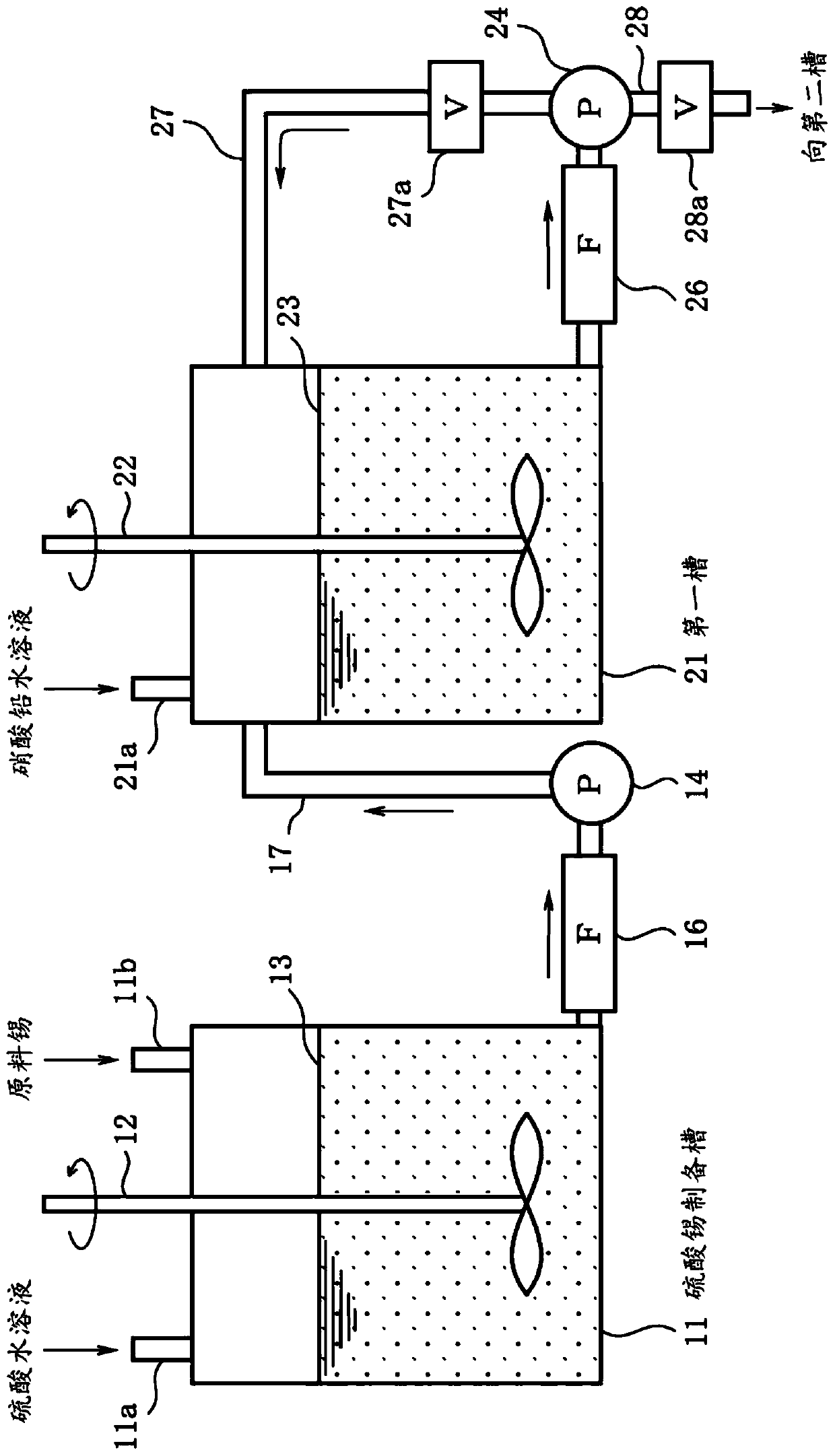
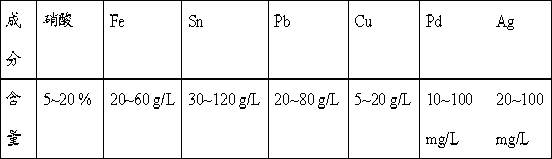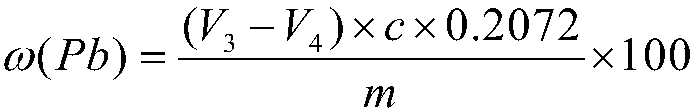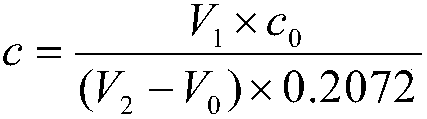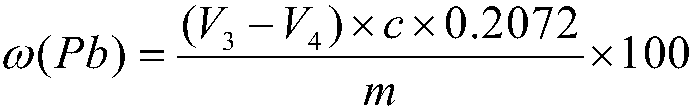Patents
Literature
66 results about "Lead(II) sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
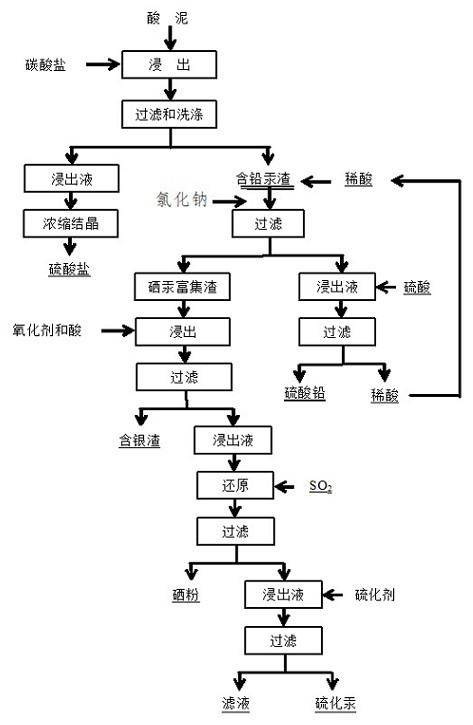
Lead(II) sulfate (PbSO₄) is a white solid, which appears white in microcrystalline form. It is also known as fast white, milk white, sulfuric acid lead salt or anglesite. It is often seen in the plates/electrodes of car batteries, as it is formed when the battery is discharged (when the battery is recharged, then the lead sulfate is transformed back to metallic lead and sulfuric acid on the negative terminal or lead dioxide and sulfuric acid on the positive terminal). Lead sulfate is poorly soluble in water.
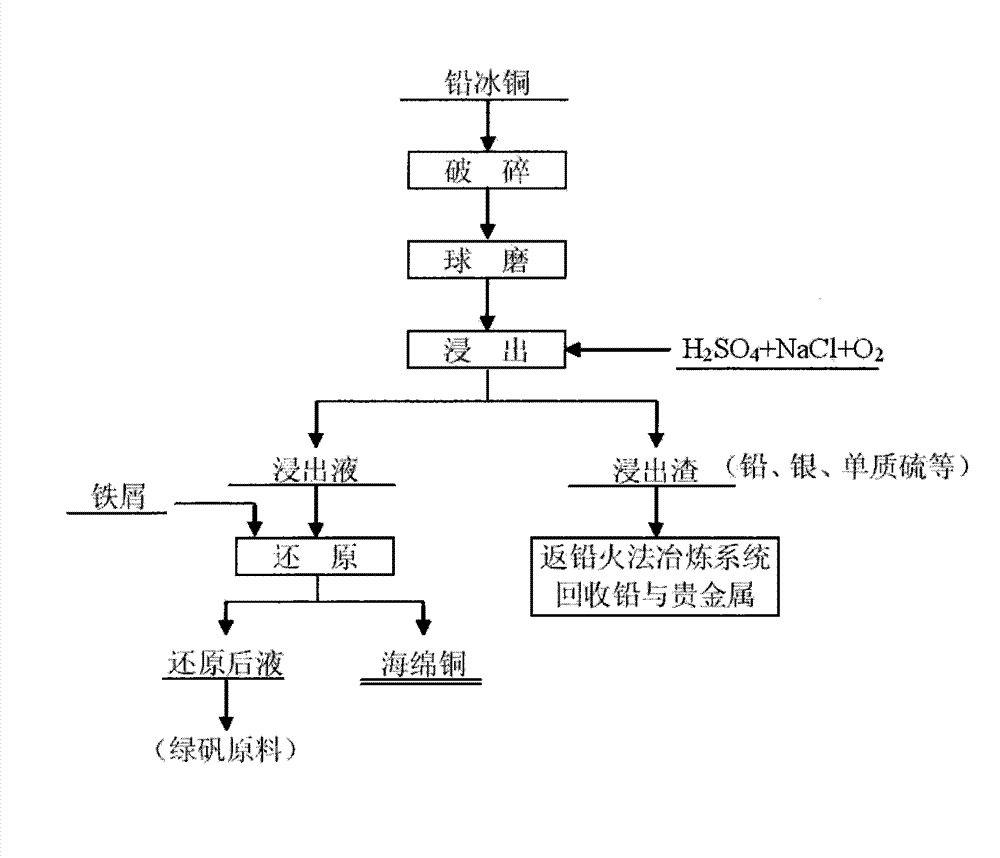
Technology for recovering metallic copper from high-lead copper matte
ActiveCN102994747ANo external emissionsHigh recovery ratePhotography auxillary processesProcess efficiency improvementPregnant leach solutionHydrometallurgy
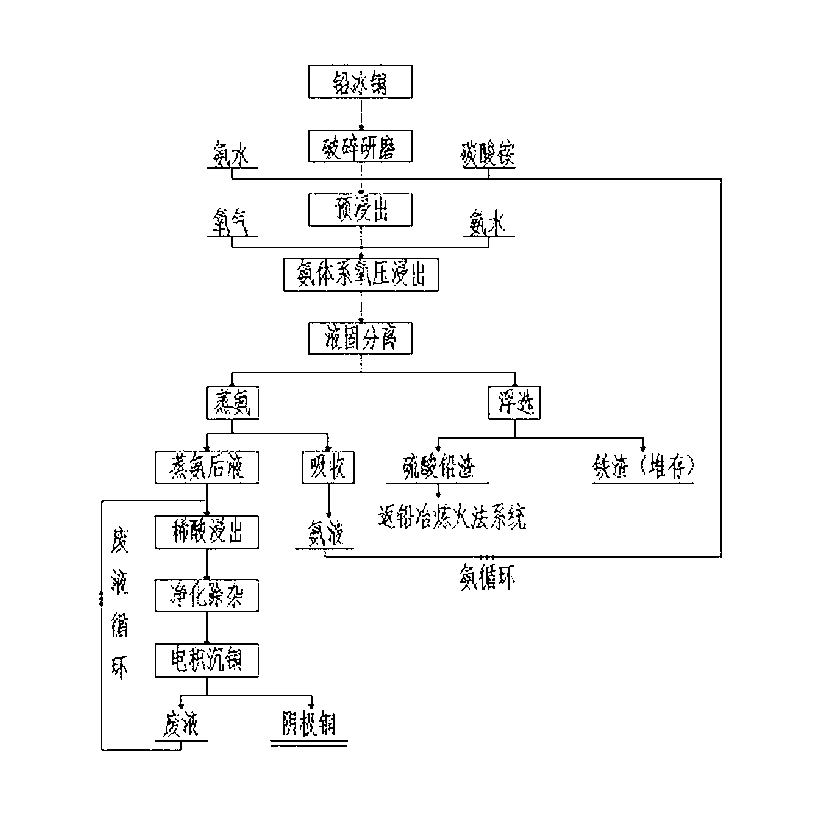
The invention relates to a technology for recovering metallic copper from high-lead copper matte, and belongs to the nonferrous metallurgy and wet metallurgy fields. The technology comprises the following steps: breaking and grinding high-lead copper matte to below 100 meshes, mixing the ground high-lead copper matte with an ammonium carbonate solution to prepare a pulp, adding a proper amount of ammonia water, and pre-leaching under a controlled pH value condition; pumping the ore pulp obtained after the above reaction into an autoclave, and adjusting the liquid-solid ratio to 6-10:1; letting in ammonia and high-pressure oxygen, and controlling the oxygen pressure between 0.1MPa and 1.2MPa and the total pressure between 1.0MPa and 3.7MPa; carrying out high-pressure ammonia system oxidizing leaching at a leaching temperature of 160-240DEG C; carrying out liquid-solid separation, and allowing the obtained solution to undergo ammonia steaming in order to recover ammonia and carbon dioxide; floating the obtained filter residues to recover lead sulfate; and sending the precipitate obtained after the ammonia steaming operation to a solution tank, carrying out dilute acid leaching treatment to recover copper sulfate in the precipitate, purifying to remove impurities, sending to an electro-deposition system, and recovering to obtain a product cathode copper.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Process for efficiently and selectively separating copper in lead copper matte
InactiveCN104017991AAdaptableHigh recovery ratePhotography auxillary processesProcess efficiency improvementPregnant leach solutionLead smelting
The invention discloses a process for efficiently and selectively separating copper in lead copper matte. The process comprises the following steps: smashing and grinding the lead copper matte taken as a raw material and screening to reach below 80 meshes; sending the lead copper matte which is ground and screened into a leaching tank and leaching by using a sulfuric acid; reacting for 3 to 5 hours at a normal pressure under the conditions that the oxidation potential of the solution is controlled within 450mV to 800mV, the concentration of sodium chlorate is controlled within 200g / L to 500g / L, the liquid-solid ratio is controlled within (5-15):1, the temperature is controlled within 70 DEG C to 100 DEG C and the concentration of the sulfuric acid is controlled within 1.0mol / L to 1.5mol / L; and leaching the copper under an acid condition by taking the sodium chlorate as an oxidizing agent. During the oxidizing and leaching process, sulfur in the lead copper matte is oxidized into elemental sulfur which is transferred into slags, wherein copper which is oxidized enters the solution in a form of copper ions while lead remains in the slags in a form of lead sulfate together with gold and silver. After the leaching process is finished, the solid-liquid separation is carried out, so that the primary separation of the copper and other valuable elements is realized; a certain amount of scrap iron is added into the leaching solution rich in the copper so as to replace the deposited copper, so that the spongy copper, which is a primary product, can be obtained; the leaching slags are sent into a pyrogenic lead smelting system, so that the valuable elements such as Pb and Ag are comprehensively recycled.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Processing method for waste liquid from stripping tin scolding
ActiveCN103031437AReduce wasteReduce pollutionLead halidesMultistage water/sewage treatmentWater chlorinationLead(II) sulfate
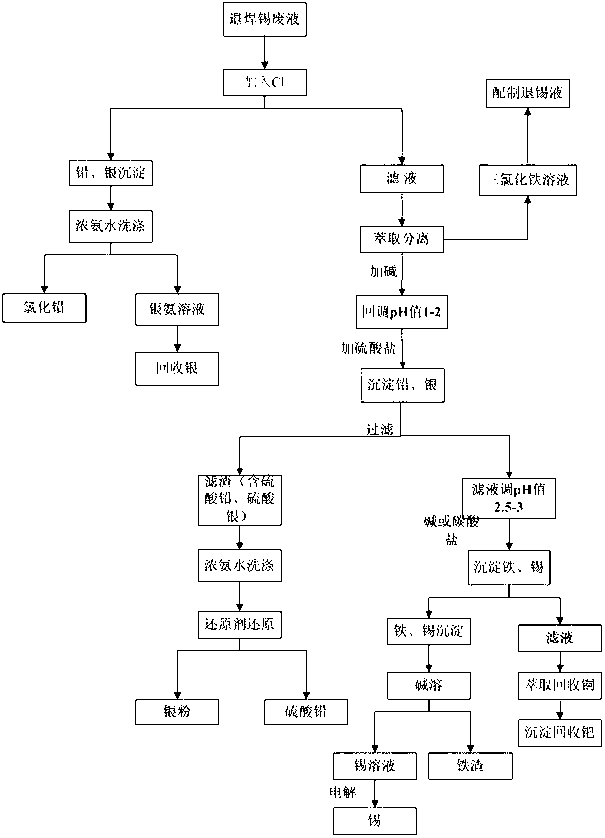
The invention provides a processing method for waste liquid from stripping tin scolding, which comprises the following steps: in the waste liquid from stripping tin scolding, adding a chlorate for reacting, then filtering the solution, washing the filter residue by a concentrated ammonia liquor for dissolving the silver chloride sediment, recovering silver by a washing lotion to acquire pure lead chloride, extracting a filtrate by an extractant to obtain ferric iron, wherein a strip liquor is a ferric trichloride solution which can be prepared to a tin stripping liquid, adding sulfate for depositing lead and silver, wherein the reacted filter residue is a mixed sediment of lead sulphate and silver sulfate, washing by the concentrated ammonia liquor, reducing to obtain a silver powder and lead sulphate; filtering and depositing lead and silver, depositing tin and iron, filtering to obtain the sediment and dissolving by alkali, and then electrolyzing to obtain tin, electrolyzing the residual filtrate to recovery copper, and depositing palladium in the solution to obtain the sediment for recovering palladium. The processing method has reasonable and useful process flow, and performs comprehensive recovery and utilization on valuable metal in the waste liquid from stripping tin scolding, the resource waste is reduced, and the environmental pollution is reduced.
Owner:JIANGXI GREEN ECO MFG RESOURCE CYCLE
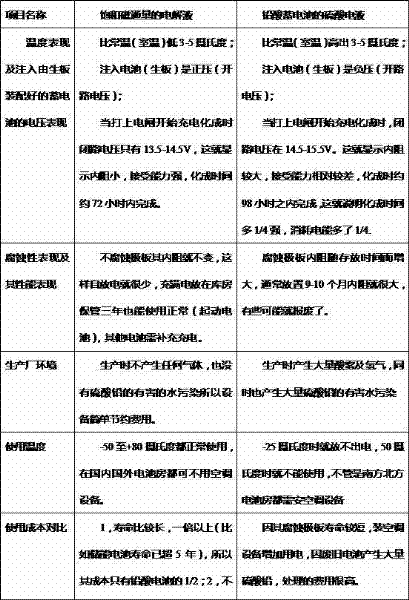
Colloid electrolyte capable of improving service life of lead-acid cell
InactiveCN104218261APrevent gelAvoid reunionLead-acid accumulatorsElectrolyte immobilisation/gelificationElectrochemical responseElectrolytic agent
The invention discloses a colloid electrolyte can improve the service life of a lead-acid cell, and the colloid electrolyte comprises the following raw materials by weight: 0.1-0.5% of polyethylene glycol, 0.1-1% of stannous sulfate, 0.5-1.5% of sodium sulfate, 0.5-1% of phosphoric acid, 0.05-2% of organosilicon polymer, 0.5-8% of fumed silica, 35-45% of sulfuric acid, and balanced of deionized water. The polyethylene glycol as a colloid stabilizer can prevent the occurrence of colloid coacervation or agglomeration before colloid is poured into the cell, colloidal stability is enhanced, and a stable three-dimensional network structure is formed; the stannous sulfate can be oxidized to tetravalent tin, the active material electrical conductivity and recharge ability after the cell is discharged can be improved, the sodium sulfate in the electrolyte can provides a certain amount of sulfate radicals for the electrochemical reaction, the degree of supersaturation of lead sulfate crystals can be reduced, formation of a lead sulfate layer with poor electrical conductivity can be prevented; the phosphoric acid can refine the grain diameter of the lead sulfate crystals; and silicon oxygen bonds and hydrogen bonds in the organosilicon polymer can effectively reduce the active material softening speed, inhibit the surface active material passivation, and improve the service life of the cell.
Owner:ZHEJIANG TIANNENG BATTERY JIANGSU NEW ENERGY
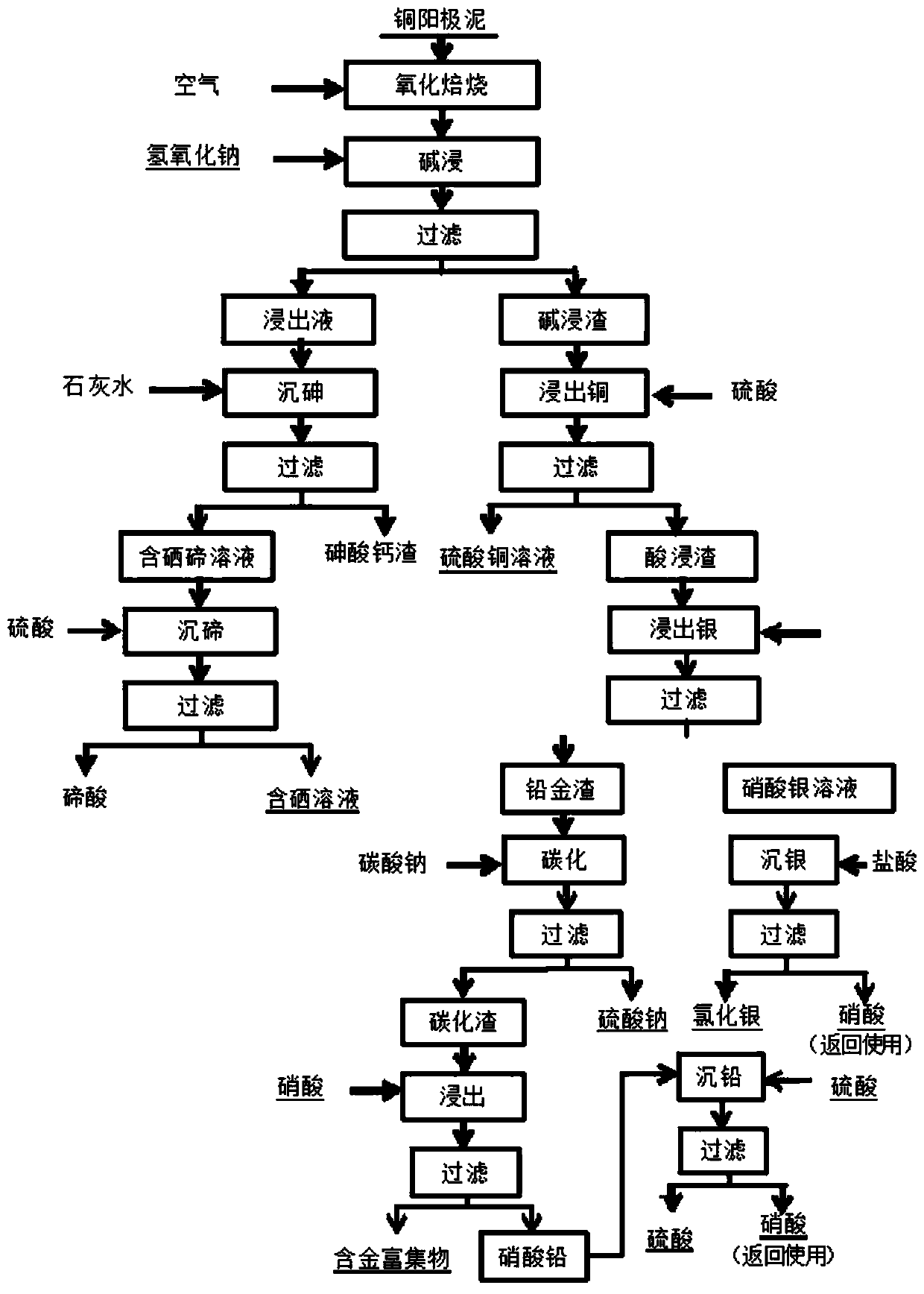
Method for separating selenium, tellurium, arsenic, copper, lead and silver and enriching gold from copper anode mud
ActiveCN111575483AAvoiding the problem of hard-to-leach telluriumAchieve recyclingProcess efficiency improvementSelenium/tellurium oxyacid saltsSlagLead nitrate
The invention discloses a method for separating selenium, tellurium, arsenic, copper, lead and silver and enriching gold from copper anode mud, and relates to the technical field of rare and preciousmetal metallurgy. The method comprises the following steps that roasted products obtained through low-temperature oxidization roasting of the copper anode mud and sodium hydroxide react to obtain selenium, tellurium and arsenic containing leaching liquor and alkaline leaching slag; the leaching liquor and whitewash react to obtain a selenium and tellurium containing solution and calcium arsenate slag; the alkaline leaching slag and sulfuric acid react to obtain copper sulfate and acid leaching slag; sulfuric acid and the selenium and tellurium containing solution react to obtain telluric acidand a selenium containing solution; the acid leaching slag and nitric acid react to obtain a silver nitrate solution and lead and gold slag; silver nitrate and hydrochloric acid react to obtain silverchloride and nitric acid; the lead and gold slag and a sodium carbonate solution react to obtain carbonization slag and a sodium sulfate solution; the carbonization slag and nitric acid react to obtain a lead nitrate solution and gold containing enrichment; and the lead nitrate solution and sulfuric acid react to obtain a lead sulfate and sulfuric acid solution. The method aims to solve the problems that existing methods for recycling metal from copper anode mud, the cost is high, recycled metal is single, and the comprehensive recovery effect is poor.
Owner:KUNMING BOREN PRECIOUS METALS +1
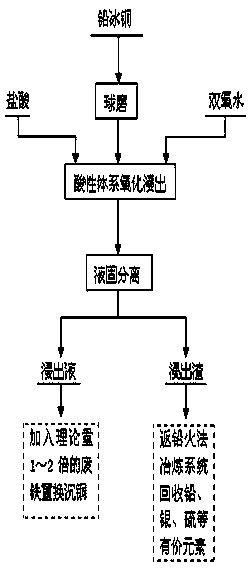
Method for separating copper from lead matte and comprehensively utilizing lead matte
InactiveCN104004907AReduce pollutionLow equipment requirementsProcess efficiency improvementSulfate radicalsRefining (metallurgy)
The invention relates to a method for separating copper from lead matte and comprehensively utilizing lead matte, and belongs to the field of wet metallurgy of non-ferrous metals. The process is characterized by adding hydrogen peroxide under a hydrochloric acid system for oxidization leaching. The method is as follows: ball-milling lead matte blocks until grain size is lower than 100 meshes, and feeding the ball-milled lead matte into a leaching slot for carrying out oxidization leaching, wherein HCl concentration is controlled to 1mol / L-6mol / L, concentration of hydrogen peroxide is controlled to 0.5mol / L-3.5mol / L, a liquid-solid ratio is controlled to (3-10):1, a temperature is controlled to 60-90 DEG C and reaction time is controlled to 1-2 hours. Under a hydrochloric acid condition, hydrogen peroxide is utilized and used as an oxidant for leaching sulfide; in the oxidization leaching process, sulfur in the lead matte is oxidized into elemental sulfur or sulfate radical, copper is oxidized to enter liquor in the form of ion, and lead is left in slag with gold and sliver in the form of lead chloride or lead sulfate. After the leaching process is completed, solid-liquid separation is carried out to realize preliminary separation of copper and lead. Copper-rich leachate can replace deposited copper by adding scrap iron, and leaching residue is returned to a pyrogenic process lead refining system for recycling valuable elements such as lead, sliver, elemental sulfur, and the like.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
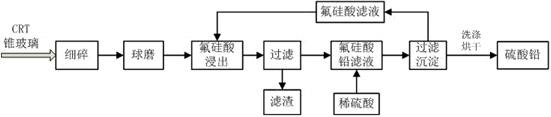
Method for extracting lead from waste CRT (Cathode Ray Tube) cone glass
InactiveCN102676826AEfficient extractionReduce pollutionProcess efficiency improvementLead smeltingEngineering
The invention relates to a method for extracting lead from waste CRT (Cathode Ray Tube) cone glass, comprising the specific steps of: (A) mechanically and roughly crushing the waste CRT cone glass and then finely crushing the waste CRT cone glass into 100-200 meshes; then mechanically milling for 4-12 hours to obtain a reaction raw material; (B) taking fluosilicic acid as an extracting agent at the temperature of 90-100 DEG C and continuously strengthening and leaching for 4-12 hours; and cooling and filtering to obtain a lead fluorosilicate solution; (C) slowly adding a pre-prepared dilute sulfuric acid solution into the lead fluorosilicate solution to form lead sulfate sediment; adjusting pH (Potential of Hydrogen) to complete precipitate; and (D) cooling and filtering; repeatedly washing a filter cake with de-ionized water for 3-5 times; and drying a white lead sulfate filter cake at 80 DEG C for 2-8 hours in vacuum to obtain a lead sulfate product with a higher purity, wherein the collected filtering solution is a regenerated fluosilicic acid solution and can be recycled and reused. The process does not generate secondary pollution; a waste liquid closed loop is realized in the whole process; and compared with a pyrogenic process lead smelting technology, the method has the advantages of low cost, simple technology and high production addition value.
Owner:TONGJI UNIV
Method for comprehensively recovering selenium, mercury, lead and silver from acid mud
InactiveCN111926187ARealize phase reconstructionAchieve recyclingProcess efficiency improvementElemental selenium/telluriumSlagWater chlorination
The invention relates to a method for comprehensively recovering selenium, mercury, lead and silver from acid mud, and belongs to the technical field of metallurgical acid mud treatment. The method comprises the steps that carbonate is added to subjected to phase transformation reaction with the acid mud to obtain a transformation solution and transformation slags; dilute acid is added to selectively leach the lead in the transformation slags, and sodium chloride is added after leaching to obtain lead-containing leachate and selenium-mercury-silver enriched slags correspondingly so as to realize separation of lead from the selenium, the mercury and the silver; an oxidizing agent and acid are added to chloridize and leach selenium-mercury enriched slags to obtain selenium-mercury leachate and silver-containing slags; sulfur dioxide is introduced into the selenium-mercury leachate for reduction, and crude selenium and a mercury-containing solution are obtained; sulfuric acid is added into the lead-containing leachate for a reaction to generate lead sulfate and the dilute acid, the dilute acid and the pure lead sulfate are obtained, and the dilute acid is recycled; and a vulcanizing agent is added into the mercury-containing solution for reaction to obtain mercury sulfide and vulcanized residual liquid. The method for comprehensively recovering the selenium, the mercury, the leadand the silver from the acid mud treats the smelting acid mud through a full-wet method, is low temperature and environmentally friendly, the mercury yield in the whole process is larger than 99.5%, the lead yield is larger than 95.0%, the selenium yield is larger than 98.0%, related treatment equipment is mature and easy to engineer, and the application prospect is good.
Owner:CHUXIONG DIANZHONG NON FERROUS METALS LLC +1
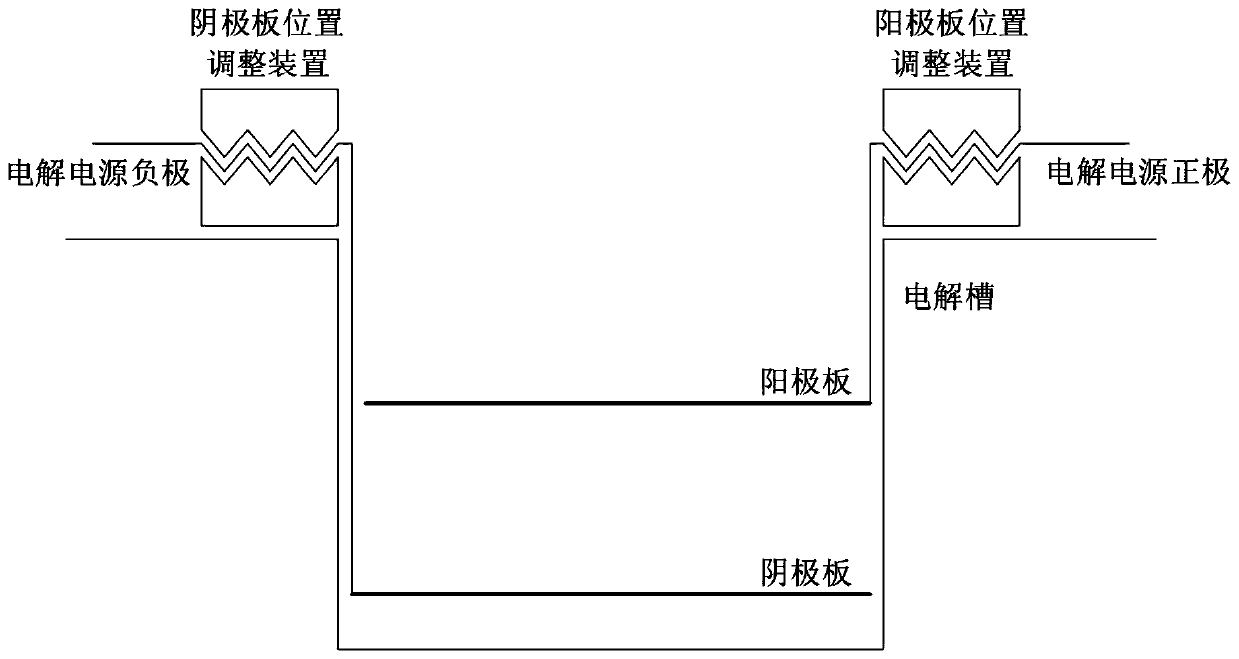
Method for recovering lead from waste lead paste by solid phase electrolysis method
ActiveCN111455404AReduce consumptionSimple processPhotography auxillary processesWaste accumulators reclaimingElectrolytic agentLead dioxide
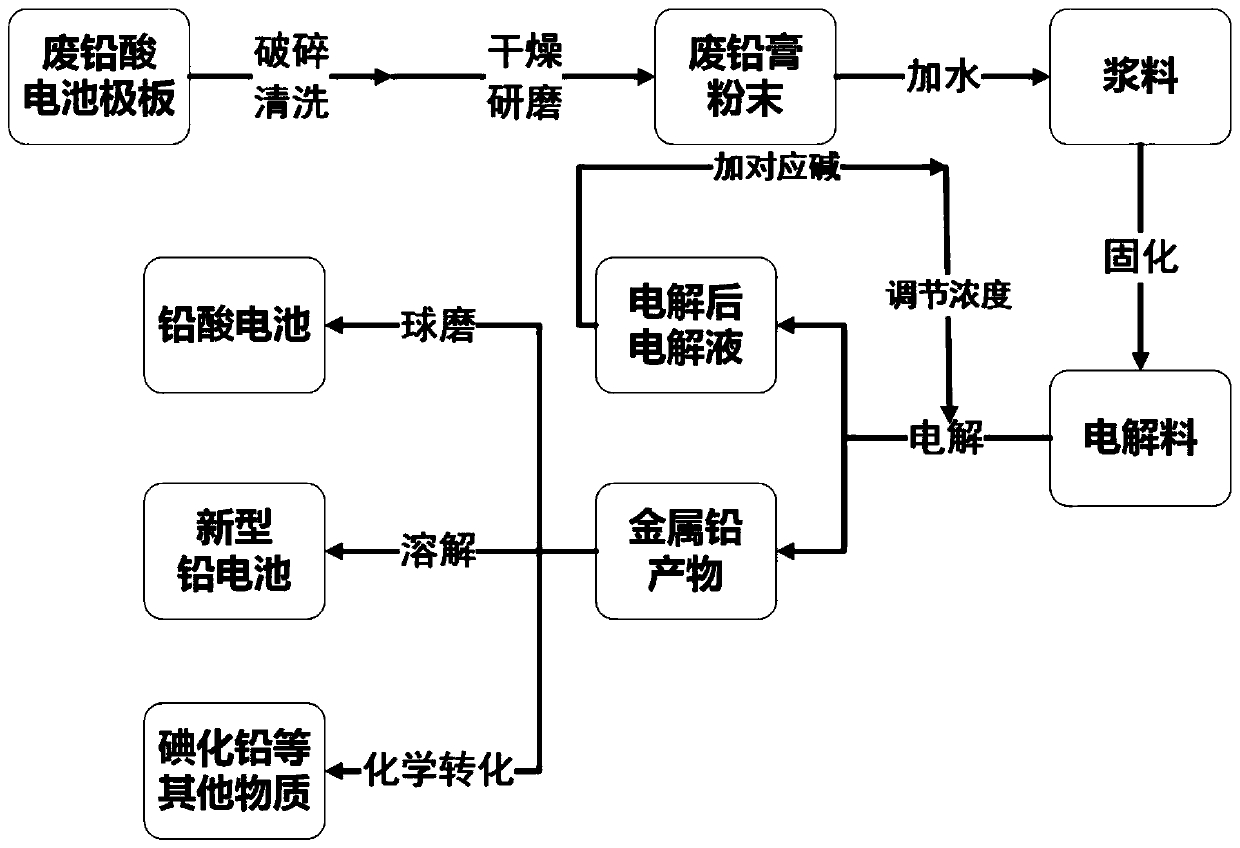
The invention discloses a method for recovering lead from waste lead paste by a solid phase electrolysis method, and belongs to the technical field of lead resource recovery. The method for recoveringthe lead from the waste lead paste by the solid phase electrolysis method comprises the steps that the waste lead paste is first made into a paste waste lead paste electrolytic material and a cathodeplate is coated with the waste lead paste electrolytic material; electrolyte is added to an electrolytic cell; the cathode plate coated with the electrolytic material is inserted into the electrolyte, and an anode is inserted into the electrolyte; the anode is an electrode with oxygen evolution electrocatalysis; the cathode plate and the anode are connected with a negative electrode and a positive electrode of a direct current power supply separately to make electrolysis in the electrolytic cell; and water at the anode loses electrons and precipitates oxygen, the electrolytic material on thecathode plate obtains the electrons and is reduced in situ to a lead single substance and attached to the cathode plate, that is, the lead is recovered from the waste lead paste. The method for recovering the lead from the waste lead paste by the solid phase electrolysis method directly electrolyzes the waste lead paste after simple pulping, lead sulfate, lead dioxide and lead oxide in the waste lead paste obtain the electrons and form in situ the lead simple substance, a large number of pretreatment processes are saved, the consumption of reagents is reduced, the process is simple, and the cost is reduced.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for recycling lead from lead-containing waste material
ActiveCN110857454AConforming to the characteristics of an economic responseEliminate secondary pollutionPhotography auxillary processesProcess efficiency improvementElectrolytic agentLead salt
The invention discloses a method for recycling lead from a lead-containing waste material. The method comprises the following steps of: (1) making the lead-containing waste material and a complexing agent water solution react to make sure that lead oxide and / or lead sulfate in the lead-containing waste material are / is completely dissolved, and separating a lead-ion-containing liquid supernatant from the precipitates containing no lead salt after reaction is ended; (2) making the liquid supernatant and a precipitant react so that the lead ions in the liquid supernatant are completely precipitated, and separating the lead-salt-containing precipitates from a regenerated complexing water solution after reaction is ended; and (3) making the lead-salt-containing precipitates and an electrolyte Awater solution have dissolution reaction to make sure that the lead-salt-containing precipitates are completely dissolved and a lead-containing electrolyte B and a precipitant are generated after reaction is ended, recycling the precipitant, and making the lead-containing electrolyte B have electrolytic reaction to obtain metallic lead, oxygen and a regenerated electrolyte A. according to the method disclosed by the invention, the complexing agent, the precipitant and the electrolyte A used in a recycling process can be repeated recycled; the method satisfies the characteristics of atomic economic reaction, so the method realizes zero consumption and zero emission and lowers the production cost.
Owner:BEIJING UNIV OF CHEM TECH
Method for recycling metal lead from lead-containing material such as lead sulfate slag
ActiveCN110923468AEfficientWith energy savingPhotography auxillary processesProcess efficiency improvementMethane sulfonic acidSlag
The invention provides a method for recycling metal lead from a lead-containing material such as lead sulfate slag. The problems that when metal lead is recycled from a lead-containing material such as lead sulfate slag in the prior art, energy consumption is high, cost is high, pollution is severe, the application range is limited, potential safety hazards exist, and metal cannot be sufficientlyutilized are solved. According to the method, the lead in the lead-containing material such as the lead sulfate slag is recycled in the following steps of (1) a chlorination leaching process; (2) a sodium carbonate conversion process; (3) a methane sulfonic acid leaching process; (4) an electrodeposition process; and (5) a melting-casting process
Owner:赵坤
Method for preparing tetrabasic lead sulfate with small grain size with sol-gel method
ActiveCN105958077AAvoid reunionHigh activityLead-acid accumulator electrodesLead sulfatesWater bathsAir atmosphere
The invention relates to a method for preparing tetrabasic lead sulfate with small grain size with a sol-gel method. The method comprises the following steps: under a water-bath stirring condition, taking lead acetate and a complexing agent to be dissolved in distilled water, and then adding a sulfuric acid solution and a dispersing agent to obtain a lead oxide / lead sulfate sol; continuously stirring the lead oxide / lead sulfate sol in water bath, after drying by distillation, carrying out low-temperature presintering and high-temperature sintering respectively in an air atmosphere so as to obtain tetrabasic lead sulfate powder, grinding, and sieving to obtain the tetrabasic lead sulfate with small grain size. The method has the advantages that the preparation process, condition and equipment are relatively simple, and the synthetic tetrabasic lead sulfate with small grain size has excellent performance as a positive active substance additive, and the specific capacity can be improved while the cycle life of a positive active substance is prolonged.
Owner:BOHAI UNIV
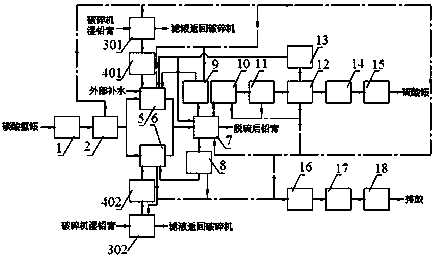
Regenerative lead-ammonium-method desulphurization and ammonium sulfate preparation process and device
PendingCN108654333AReduce evaporationSolve the problem of desulfurizationDispersed particle separationAmmonium sulfatesAmmonium sulfateEngineering
The invention discloses a regenerative lead-ammonium-method desulphurization and ammonium sulfate preparation process and device. The device comprises a loading machine, an unpacking machine, pre-desulphurization lead paste press filter, a dry lead paste conveyor, a primary desulphurization device, a secondary desulphurization device, a post-desulphurization lead paste press filter, a primary desulphurization filtrate storage tank, a lead paste rinsing solution storage tank, a secondary desulphurization filtrate storage tank, an ammonium sulfate filtrate purification device, an MVR crystallization drying device, a distilled water storage tank, a packing device, and a stacking machine and the like, and all devices are connected reasonably to form a system. By adopting the process and device, the desulphurization problem of waste battery lead paste can be solved, the filtrate is secondarily desulphurized, the concentration of the ammonium sulfate is improved, and the evaporation amount of the water is reduced, so that the production cost of the lead paste desulphurization can be significantly reduced; the degree of automation of the system is high, the labor strength of worker and the labor cost can be reduced, the production efficiency is improved, and the lead sulfate is directly converted to the ammonium sulfate, the quality of the ammonium sulfate can reach or exceed the grade-A quality of the national standard, and the economical benefit is good.
Owner:HUNAN JIANG YE MECHANICAL & ELECTRICAL TECH CO LTD
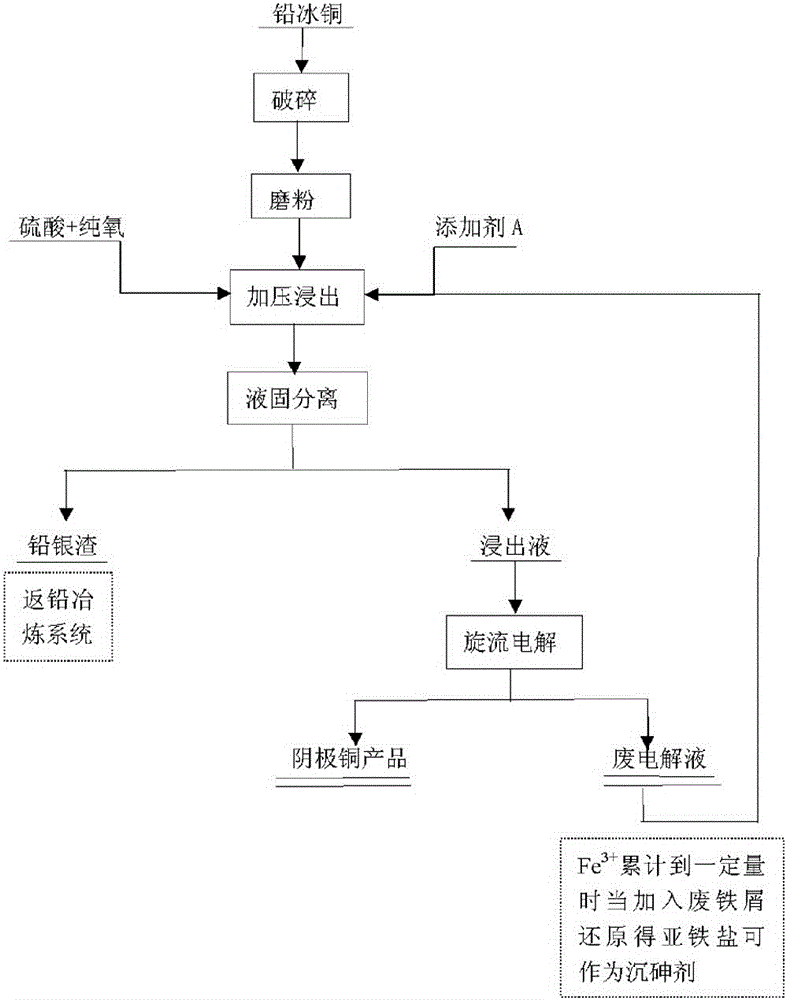
Wet process for treating lead copper matte
ActiveCN102051478BEasy to settleHigh recovery rateProcess efficiency improvementLead(II) sulfatePyrometallurgy
The invention relates to a wet process for treating lead copper matte, and belongs to the field of non-ferrous metal hydro-metallurgy. In the process, the lead copper matte is treated by a normal-pressure leaching wet process; and the lead copper matte is leached by sulfuric acid and sodium oxide, and oxygen is continuously introduced in the leaching process, wherein copper is leached, and the lead in forms of lead sulfate and lead chloride is retained in residue. After the leaching process is finished, liquid-solid separation is carried out and primary separation of metals is implemented; copper-containing leachate is reduced by scrap iron to form copper sponge, and solution after reduction serves as a raw material for recycling iron; and the leaching residue returns to a lead pyrometallurgy system, and valuable elements such as lead, silver, simple sulfur substance and the like are recycled. In the wet process, solution is not discharged, the environment is protected, and the wet process belongs to clean metallurgy; the process is simple and practicable, and is convenient to operate; and the cost is low and the comprehensive recoverability is high.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Concrete impact-resistant modifier
InactiveCN104591596AImprove stress resistanceImprove flexural strengthZinc hydroxideCellulose acetate
A disclosed concrete impact-resistant modifier is prepared from the following raw materials in parts by weight: 1.5-4.7 parts of tribasic lead sulfate, 2.5-4.5 parts of cellulose acetate, 1.2-3.3 parts of rosin-polythylene oxide ester, 1.2-1.9 parts of sodium methyl silicate, 1-3 parts of sodium poly(methylidyne naphthalenesulfonate), 2-3.5 parts of artificial ceramic particle, 0.5-1.3 parts of zinc hydroxide, and 5-10 parts of deionized water. Through the combined effect of the two flexible materials tribasic lead sulfate and cellulose acetate, the concrete possesses high compression resistance, high bending-resistant strength and high impact-resistance flexibility, the elastic modulus of concrete is effectively reduced, the damping ratio of concrete is improved, possibility is provided for vibration damping and noise reduction of concrete pavements, durability of flexible concrete is improved, and the service life of the structure is prolonged.
Owner:QINGDAO DESHENGTAI CONSTR INSTALLATION ENG
Tetrabasic lead oxide and lead monoxide composition to be used in lead-acid batteries and it's method of production
This invention relates to a new battery oxide mixture for use in lead acid batteries and / or battery plates, specifically tetrabasic lead sulphate and lead oxide mixture that is derived through basic reactions from lead sulphate and the method utilized to obtain the said tetrabasic lead sulphate and lead oxide mixture by basic reactions starting by lead sulphate as initial substance. Additionally, this invention relates to battery plates produced wherein the paste made of tetrabasic lead sulphate and lead oxide mixture is plastered on lead battery grids.
Owner:KARABACAK ENVER
Electrolyte for manufacturing emulsion storage battery and manufacturing method thereof
The invention relates to electrolyte for manufacturing an emulsion storage battery and a manufacturing method thereof. The electrolyte for manufacturing the emulsion storage battery in the invention comprises a mother liquid consisting of the following components in parts by weight: 1-1.3 parts of SiO2.nH2O with 30-45wt% SiO2, 7-11 parts of diluted sulphuric acid or carbonic acid, and 1.1-2.5 parts of pure water, wherein the mother liquid is treated by a high-pressure magnetic field to prepare the electrolyte containing saturated magnetic flux. The electrolyte containing the saturated magnetic flux in the invention has the characteristics of small internal resistance, low generation temperature, reduced losses, electricity saving performance and short formation time; when the emulsion storage battery manufactured by adopting the electrolyte is used, the electrolyte does not corrode polar plates, the emulsion storage battery is free from boosting charge after long-term storage and can still be started normally, and no acid mist and no pollution of harmful water containing sulfate are generated in a production process; in addition, the emulsion storage battery manufactured by adopting the electrolyte has the advantages of large applicable temperature range and long service life, cannot result in environment pollution after being used and is a truly environment-friendly and energy-saving product.
Owner:冯艺峰
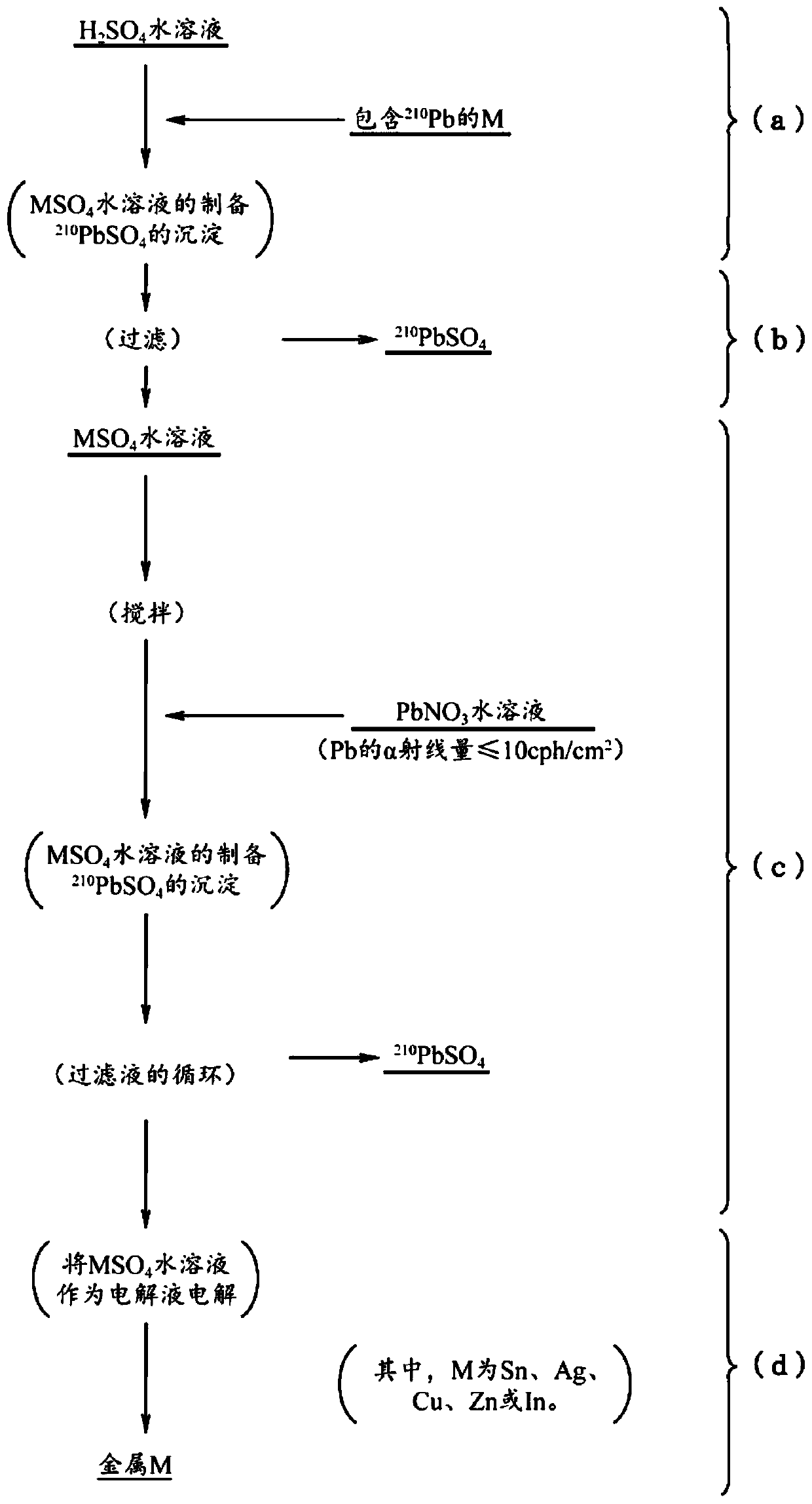
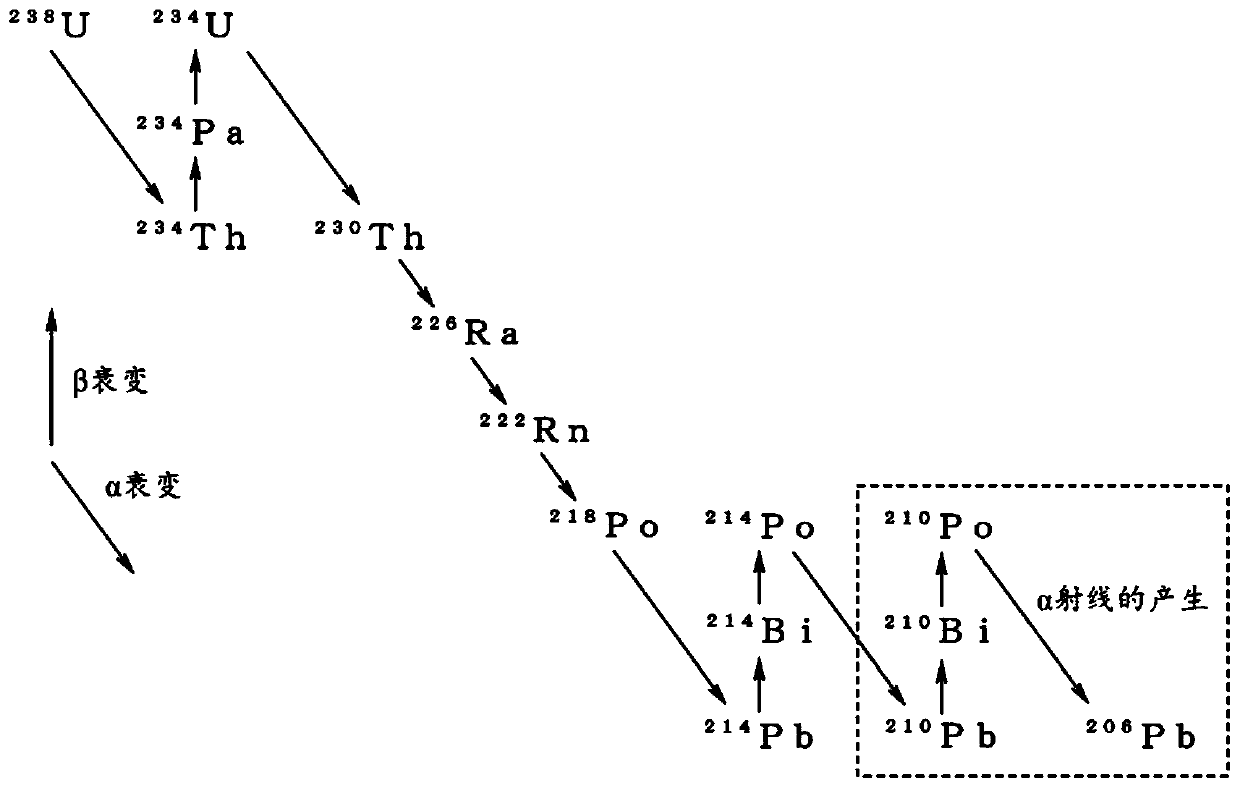
METAL AND TIN ALLOY HAVING LOW alpha-RAY EMISSION, AND METHOD FOR PRODUCING SAME
ActiveCN111032921AReduce releaseLow soft errorPhotography auxillary processesWelding/cutting media/materialsElectrolytic agentIndium
Provided is a metal which is one of tin, silver, copper, zinc, or indium, and has low alpha-ray emission, wherein the alpha-ray emission of the metal after being heated in air at 100 DEG C for 6 hoursis 0.002 cph / cm<2> or less. A metal selected from among tin, silver, copper, zinc, and indium, each of which contains lead as an impurity, is dissolved in an aqueous sulfuric acid solution to preparean aqueous solution of a sulfate of the metal, and lead sulfate in the aqueous solution is removed by precipitation. While stirring the aqueous sulfate solution from which lead sulfate has been removed, an aqueous lead nitrate solution containing lead having an alpha-ray emission of 10 cph / cm<2> or less is added to the aqueous sulfate solution to precipitate lead sulfate in the aqueous sulfate solution, and the aqueous sulfate solution is circulated while removing lead sulfate. The metal is electrolytically collected using the aqueous sulfate solution as an electrolytic solution.
Owner:MITSUBISHI MATERIALS CORP
Method for determining content of lead in silver alloy
InactiveCN108593839ASimple methodWide measuring rangeChemical analysis using titrationChemical technologyPrecious metal
The invention relates to a method for determining the content of lead in a silver alloy and belongs to the technical field of analytical chemistry. The method comprises dissolving a sample through nitric acid, adding sulfuric acid into the solution so that the lead forms lead sulfate precipitates and is separated, and carrying out titration in an acetic acid-sodium acetate buffer solution to meteron the lead content of the alloy through EDTA. The method is simple and rapid, has a wide measuring range, realizes accurate measurement, compensates for the blank of high-content lead analysis in the silver alloy, provides the technical support for analysis and determination of silver products in the precious metal field, is suitable for testing and analysis of large-volume samples, and can be widely applied in the ore test and analysis field.
Owner:CHANGCHUN GOLD RES INST
Method of recovering valuable metal in waste lining brick of gold silver smelting furnace
InactiveCN100554443CReduce pollutionRealize comprehensive utilizationProcess efficiency improvementEtchingBrick
A method to recover valuable metal from abandoned bricks by lines of gold and silver smelting, whose craft steps are as follows: de-magging from extraction of sulfuric acid, deprivation of lead sulfate etching by alkali, silver etching by nitric acid and gold etching by aqua regia. The process of de-magging from extraction of sulfuric acid is that: 200kg bricks powder of abandoned magnesia-chrome from lines of gold and silver smelting is stirred with 1350kg dilute sulfuric acid. The mixture reacts for 2 to 3 hours, and discharges clear MgSO4 strong solution. 100kg water is filled into extraction slot. Based on stirring, 25ppm flocculant is added and stewed for 10 minutes. After discharging MgSO4 solution, 1000kg is filled into extraction slot and filtrated to get MgSO4 wet materials. Then, it is neutralized with light-burned magnesia to achieve magnesium sulphate. Valuable metals including magnesium, lead, silver, gold and copper etc. can be recovered from abandoned bricks by lines of gold and silver smelting. Deposits including Cr(OH)3, Fe(OH)3, Cu(OH)2 and Al(OH)3 can be gathered for producing refractory materials, which realizes salvaging and reduces environmental pollution.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY +1
Modified asphalt capable of prolonging service life of asphalt pavement and preparation method of modified asphalt
PendingCN114133755AImprove water absorptionImproves UV resistanceBuilding insulationsSURFACTANT BLENDPotassium titanate
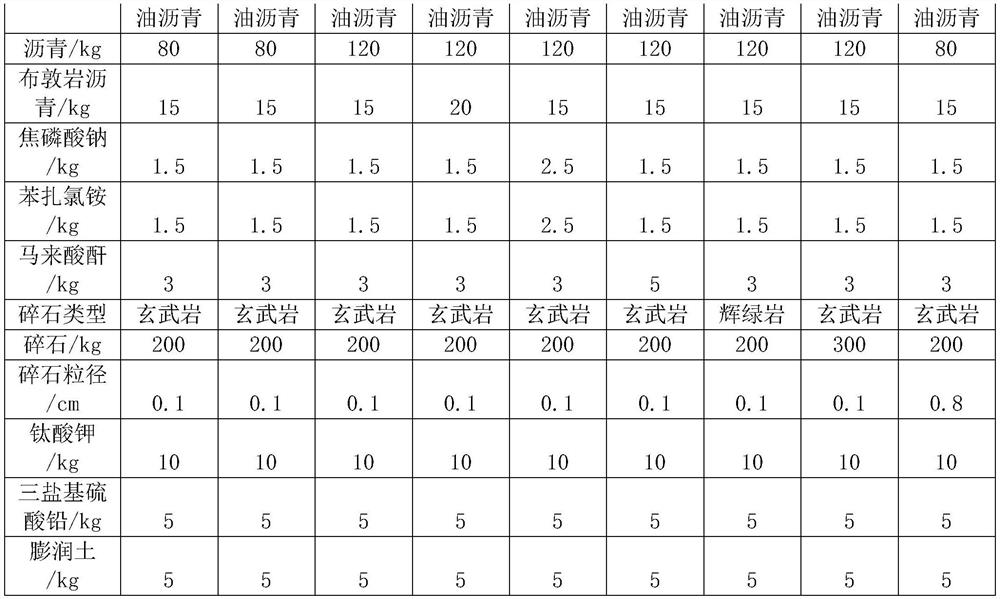
The invention relates to the field of asphalt, and particularly discloses modified asphalt capable of prolonging the service life of an asphalt pavement, and the modified asphalt is prepared from the following raw materials in parts by weight: 80-120 parts of matrix asphalt, 15-20 parts of buton rock asphalt, 3-5 parts of a surfactant, 3-5 parts of a compatilizer, 10-15 parts of potassium titanate, 5-7 parts of lead sulfate tribasic and 5-7 parts of bentonite. The preparation method comprises the following steps: S1, mixing 80-120 parts of matrix asphalt and 15-20 parts of buton rock asphalt, heating to 180 DEG C, shearing for 2 hours, and uniformly stirring; s2, continuously adding 3-5 parts of a surfactant, 3-5 parts of a compatilizer, 10-15 parts of potassium titanate, 5-7 parts of lead sulfate tribasic and 5-7 parts of bentonite, and uniformly stirring to obtain a modified asphalt finished product; the preparation method provided by the invention has the advantage of improving the anti-aging performance of the asphalt.
Owner:太仓市路桥工程有限公司
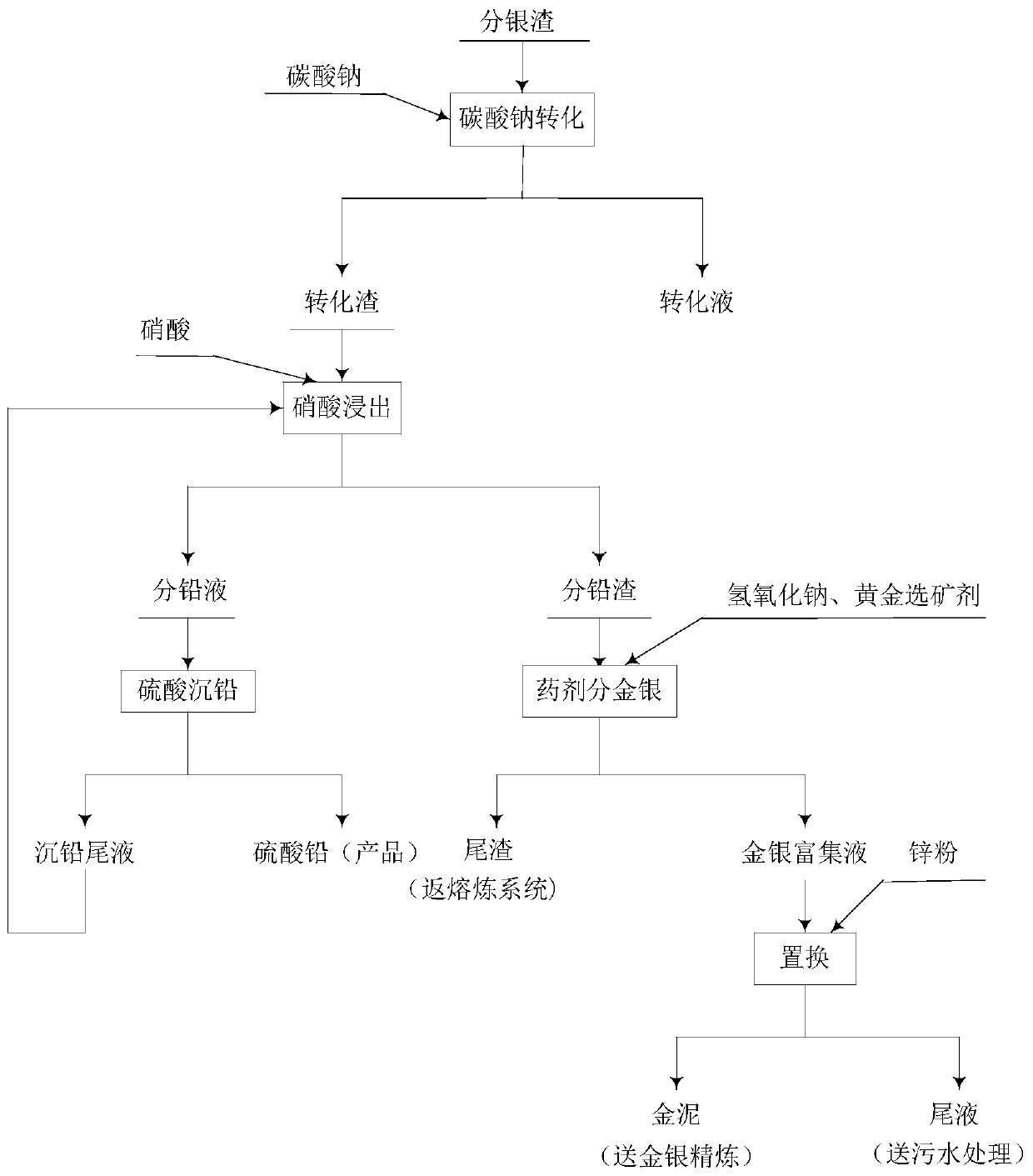
Method for recovering lead, gold and solver from silver separation slag
The invention discloses a method for recovering lead, gold and solver from silver separation slag. Lead in the silver separation slag is separated out in a form of lead sulfate precipitate through carbonate conversion, nitric acid leaching and sulfuric acid precipitation, so that the recovery rate of lead is high, and the purity of lead sulfate is high to meet the sale purity requirement. Gold andsolver in the silver separation slag are reduced into gold and silver simple substances by zinc powder after being enriched by a gold beneficiation agent, so that the recovery rate of gold and silveris high. The method recovers lead, gold and silver from the silver separation slag to achieve high economical value; the method optimizes process parameters to reach an optimal direct yield of lead,gold and silver; and the method implements a reaction under normal-temperature and normal-pressure condition, and is simple in process operation, low in recovery cost and free of causing environmentalpollution.
Owner:烟台国润铜业有限公司
Low-foaminess PVC section bar used for door and window frames
The invention discloses a low-foaminess PVC section bar used for door and window frames. The low-foaminess PVC section bar comprises following ingredients, by mass, 100 parts of PVC, 2 to 6 parts of titanium dioxide, 1 to 3 parts of AC foaming agent, 0.1 to 1 part of UV-9, 2 to 5 parts of paraffin, 10 to 15 parts of CPE, 0.1 to 0.5 part of PE wax, 2 to 5 parts of tribasic lead sulfate, 2 to 4 parts of dibasic lead phosphate, 8 to 10 parts of a modifier, 5 to 10 parts of calcium carbonate, 0.5 to 1 part of calcium stearate, and 0.5 to 2.5 parts of lead stearate. Product relative density ranges from 0.75 to 0.85, tensile strength ranges from 30 to 40MPa, bending elasticity modulus range from 35 to 40MPa, bending rupturing load ranges from 14000 to 15000MPa, bending strength ranges from 35 to 45MPa, and elongation ranges from 25 to 30%. The low-foaminess PVC section bar possesses light resistance and ultraviolet irradiation resistance, and can be used for replacing existing materials continuously; and large-scaled production can be realized.
Owner:WUJIANG DONGXIN PLASTIC PACKAGING PLANT
Soundproof and smoke eliminating sealing PVC strip and preparation method therefor
The present application discloses a soundproof and smoke eliminating sealing PVC strip and a preparation method therefor. The method comprises: weighing out the following materials in parts by weight: PVC, tribasic lead sulfate, ferric oxide powder, dibasic lead phosphite, zinc stearate, organic montmorillonite, a plasticizer, ferrocene, lead stearate, tricresyl phosphate, a flame retardant, CPE, calcium carbonate, dioctyl sebacate and paraffin; mixing the substances; extruding the mixture to perform tab-pulling and granulation. The oxygen index is 32-34%, and the elongation at break is 280-320%; the tensile strength is 20-30 MPa, and the cold resistance ensures that cracking does not occur at the temperature of minus 30 DEG C; and the Shore hardness is 58-62, the tear strength is 15-35 kN / m, and the compression set is 25-45%.
Owner:苏州市德莱尔建材科技有限公司
A comprehensive recovery process for selectively and efficiently extracting copper from lead matte
ActiveCN104789783BNo pollution to the environmentNo pollution in the processPhotography auxillary processesProcess efficiency improvementElectrolytic agentLead(II) sulfate
The invention discloses a process for selective efficient copper extraction and comprehensive recovery from lead copper matte and belongs to the field of wet processes of non-ferrous metal metallurgy. According to the process disclosed by the invention, the lead copper matte is taken as a raw material, and the process comprises the following steps: crushing, grinding and screening the lead copper matte, performing pulp conditioning on the lead copper matte after screening and sulfuric acid (or a waste electrolyte), then pouring into a high-pressure kettle, performing leaching, adding an adjusting agent A and introducing pure oxygen; controlling technical conditions, oxidizing sulfur in the lead copper matte to elemental sulfur in the oxidization and leaching processes, and transferring into slag; oxidizing copper to enable the copper to enter a solution in the form of copper ions and enabling lead to leave in the slag in the form of lead sulfate with gold and silver; and enabling most of iron to enter into the slag in the forms of hematite and yellow calcium iron vitriol under high-temperature, high-pressure and high-acid conditions. After the completion of the leaching process, liquid-solid separation is performed to realize primary separation of the copper and other valuable elements; after acid adjustment of a leachate, cyclone electrolysis is directly performed to extract the copper in the leachate, and then a cathode copper product which is in line with a national standard can be obtained; and leaching residues are sent into a lead pyrometallurgy system for comprehensively recovering Pb, Ag, Au and other valuable elements.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
A method for electrodepositing flake zinc in alkaline medium
ActiveCN109609978BImprove precipitation qualityHigh hardnessElectroforming processesLead carbonatePentaerythritol
The invention discloses a method for obtaining flaky zinc through electro-deposition in an alkaline medium. By the adoption of the method for obtaining flaky zinc through electro-deposition in the alkaline medium, the flaky zinc is obtained under the alkaline medium condition in an electro-deposition manner through the synergistic effect of compound additive and stirred-liquid-flow mass transferring. The compound additive is composed of 0.1-10 g / L of gelatin, 0.1-10 g / L of EDTA, 0.1-10 g / L of DPE and 0.1-10 g / L of additive A. The additive A is any one or more of lead oxide, lead sulfate and lead carbonate. In the electro-deposition process, mechanical stirring at the speed of 50-150 r / min is adopted to form liquid flow, and the flaky metal zinc can be obtained. The formed flaky zinc is easy to flush and convenient to transport, and the formed flaky zinc can be more easily molten to form ingots.
Owner:SHANGHAI SECOND POLYTECHNIC UNIVERSITY
High-strength life buoy and preparation method thereof
The invention relates to a high-strength life buoy. The life buoy is prepared from the following raw materials in parts by weight: 60-110 parts of polypropylene, 0.8-1.2 parts of a cross-linking agent, 22-30 parts of a foaming agent, 1.5-3.5 parts of zinc oxide, 0.12-0.18 part of tribasic lead sulfate, 0.15-0.19 part of a demolding agent stearic acid and 30-46 parts of calcium carbonate. The preparation method comprises the following steps: (1) respectively weighing the materials in the formula, adding the weighed materials into a mixer, stirring at 50-60 DEG C for 3-4 minutes, transferring into a mixing mill, and mixing at 110-120 DEG C for 4-6 minutes; (2) putting the mixed material into a normal-temperature double-roller open mill, and rolling into sheets, or extruding into sheets by anextruder; and (3) cutting a certain amount of the material sheets as required, putting the cut material sheets into a mold, carrying out hot pressing in a press vulcanizer at 170-180 DEG C under a pressure of 8-10 MPa for 8-10 min, opening the mold, cutting edges, and carrying out frosting treatment.
Owner:WUXI XINGTAI SHIPPING EQUIP
A kind of preparation method of lead alloy surface anti-corrosion composite coating
The invention discloses a preparation method of an anti-corrosion composite coating on the surface of a lead alloy. The composition of the electrolytic solution for chemical deposition includes 5-30 mmol / L cerium ammonium sulfate, 1-5 g / L polyvinylpyrrolidone, and 1-8 mol / L sulfuric acid; the process conditions for electrochemical deposition are: the temperature is 25-60 ° C, the number of scanning cycles is 50-300, the potential range is ‑0.9-2V, and the deposition time is 10-50min. The main component of the passivation film formed on the surface of the lead alloy by the method of the invention is a composite film layer containing lead sulfate and a small amount of cerium element and organic matter, which is not only flat and dense, but also effectively enhances the corrosion resistance of the lead alloy; the method of the invention has a simple process, and Time-consuming.
Owner:XIHUA UNIV
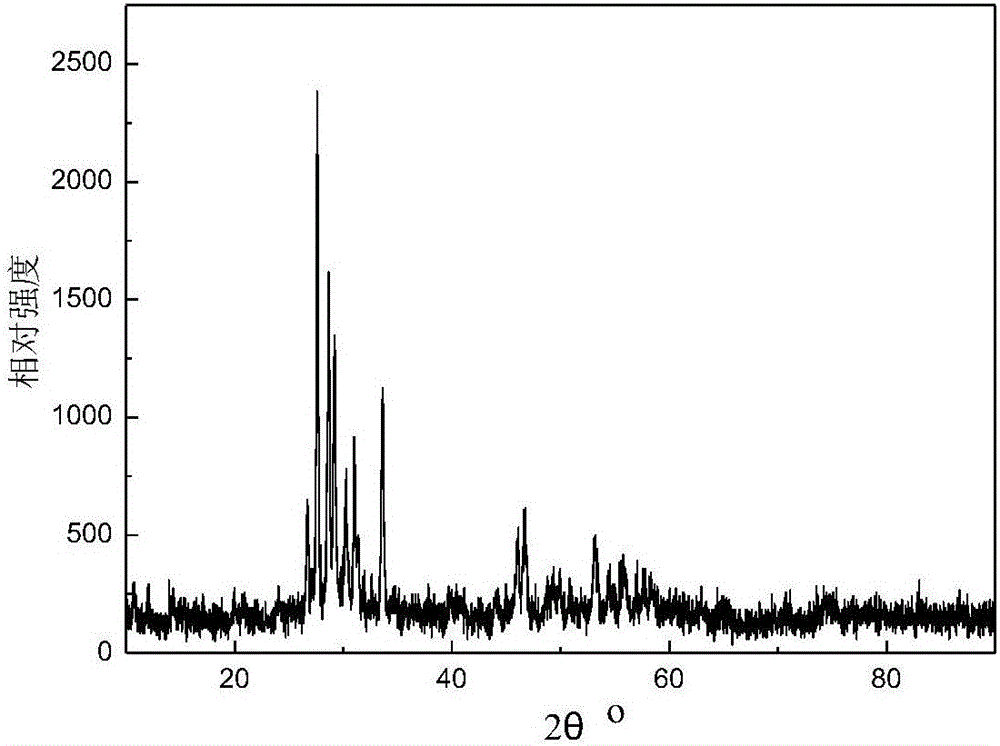
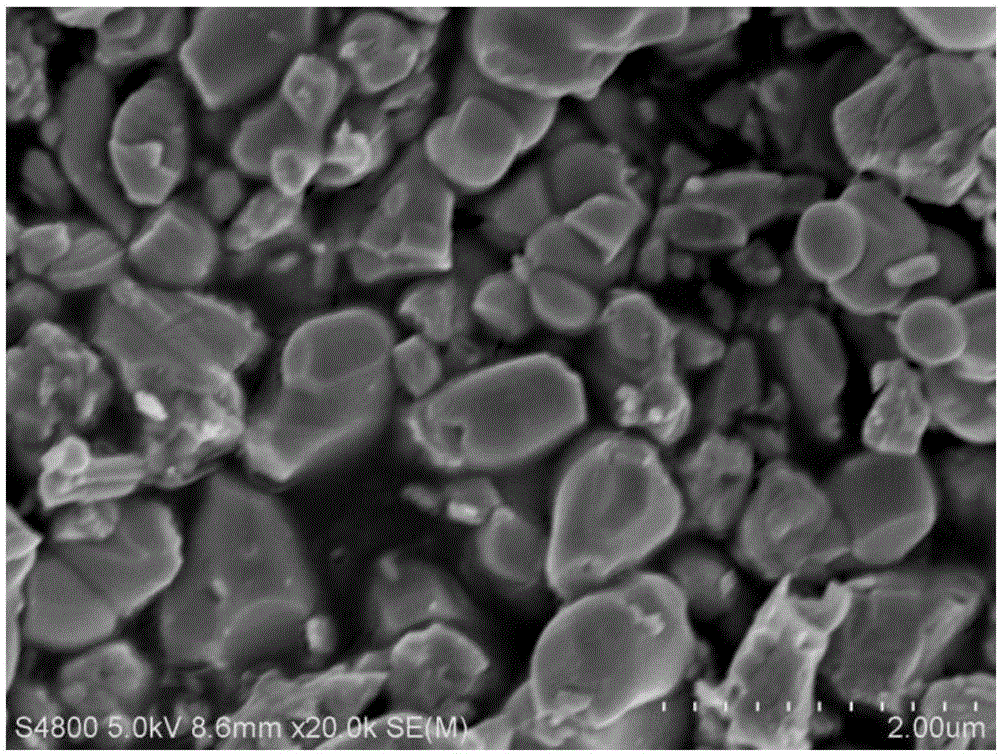
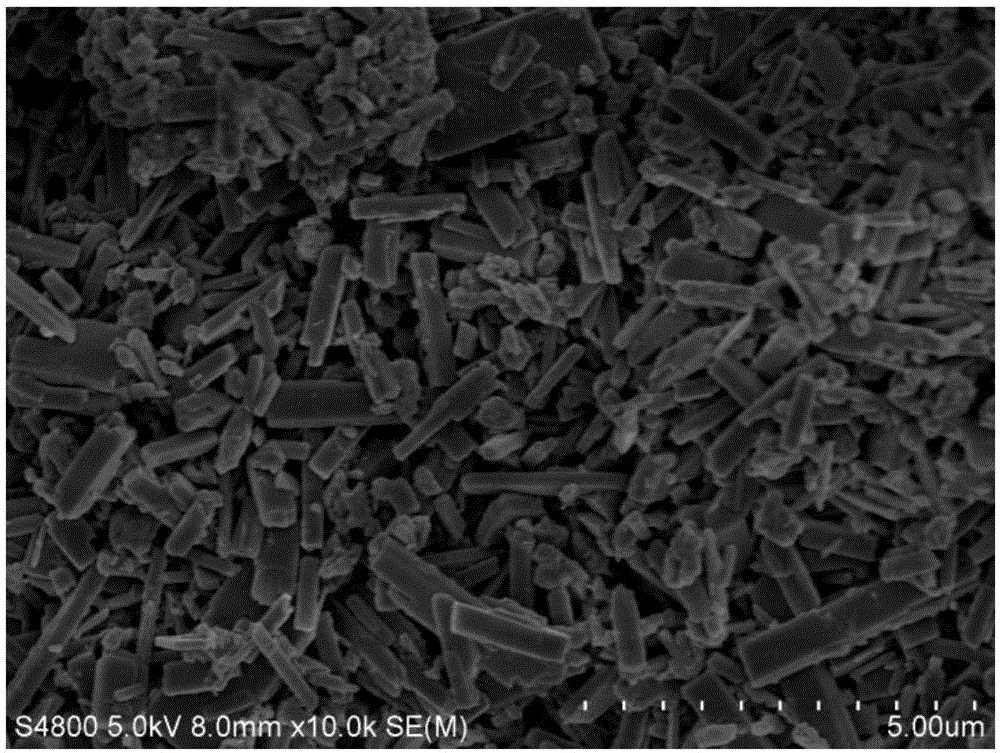
Preparation method of basic lead sulfate
The invention relates to the technical field of comprehensive utilization of lead resources, and discloses a preparation method of basic lead sulfate. The method comprises the following steps: addinga desulfurizing agent to lead sulfate to react for desulfurization, adding a regulator for hydration treatment to induce crystal nucleus formation, filtering a hydrated mixture, and conducting heatingand decomposition to obtain the basic lead sulfate. The method can be used for preparing different types of basic lead sulfate by controlling the addition amount of the desulfurizing agent and the desulfurizing ratio.
Owner:XIANGTAN UNIV
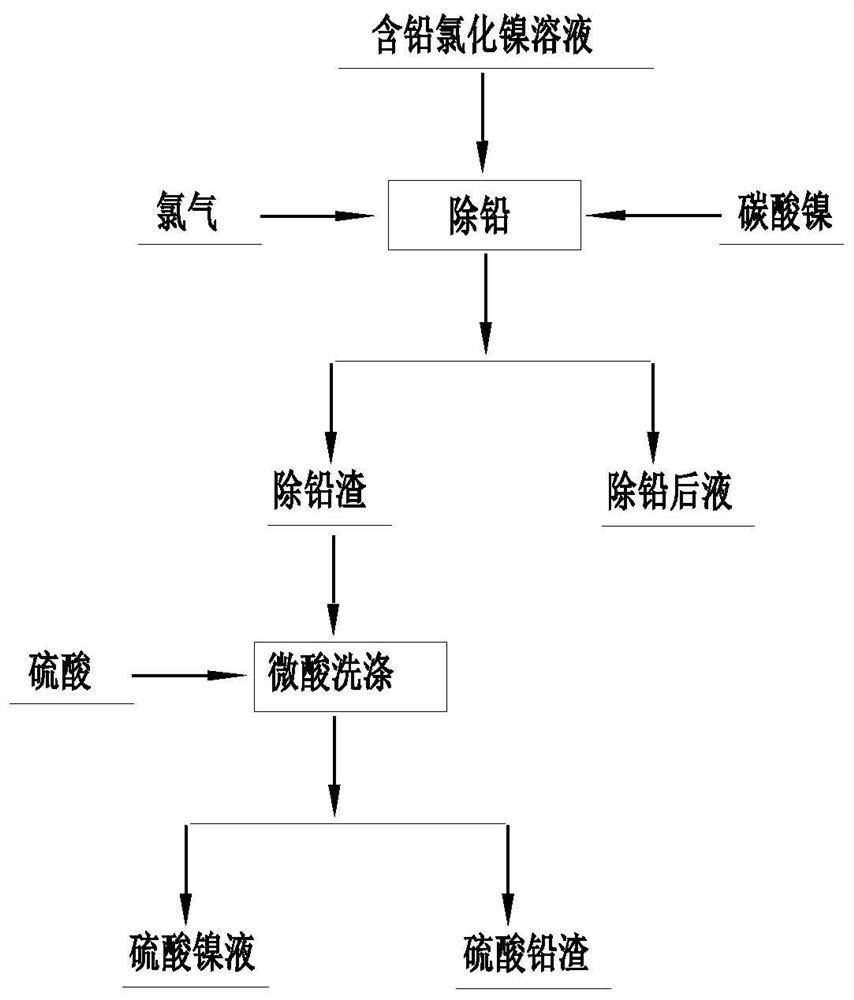
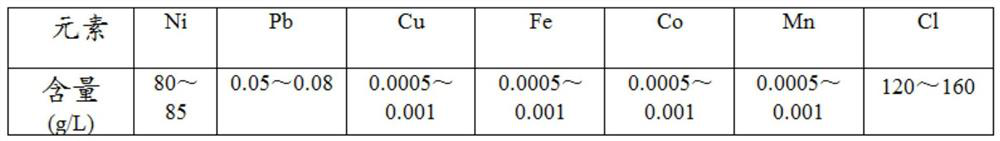
Lead removal method for nickel chloride solution
The invention discloses a lead removal method for a nickel chloride solution, relates to the technical field of lead removal, and aims to solve the problems of long flow, low efficiency, high cost and insufficient lead removal depth in the process of removing lead in the nickel chloride solution in the prior art. The nickel chloride solution is subjected to oxidation-reduction potential increasing and neutralized with nickel carbonate, the pH value of the neutralized solution is stabilized, and after the reaction is finished, a lead-removed solution and lead-containing slag are obtained after liquid-solid separation; and a lead slag removing and washing step: washing the lead-containing slag with sulfuric acid-containing water, filtering after washing, enabling the washing liquid to enter a nickel production system, and converting the washing slag into lead sulfate. According to the method, the technological process is shorter, the cost is lower, the lead removal efficiency is higher, the lead content of the solution obtained after lead removal is smaller than 0.00005 g / L, the Ni9999 high-quality electric nickel production requirement is met, and the produced lead slag is lead sulfate to be sold as a commodity.
Owner:JINCHUAN GROUP LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com