METAL AND TIN ALLOY HAVING LOW alpha-RAY EMISSION, AND METHOD FOR PRODUCING SAME
A manufacturing method, α-ray technology, applied in the manufacture of tools, metal processing, metal processing equipment, etc., can solve the problems of unguaranteed, unrealizable, and increased occurrence of soft errors, and achieve the effect of reliable precipitation removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach >
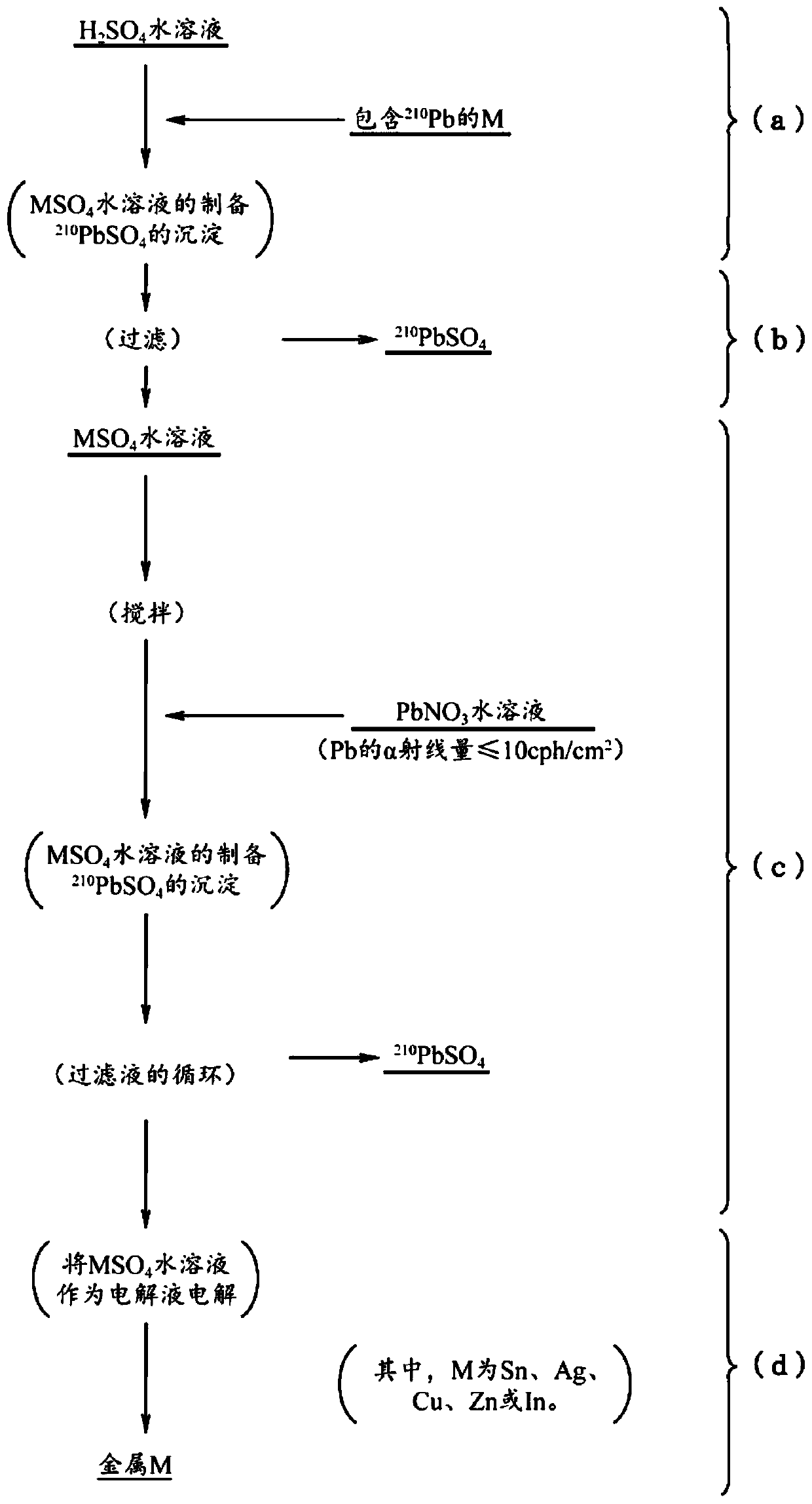
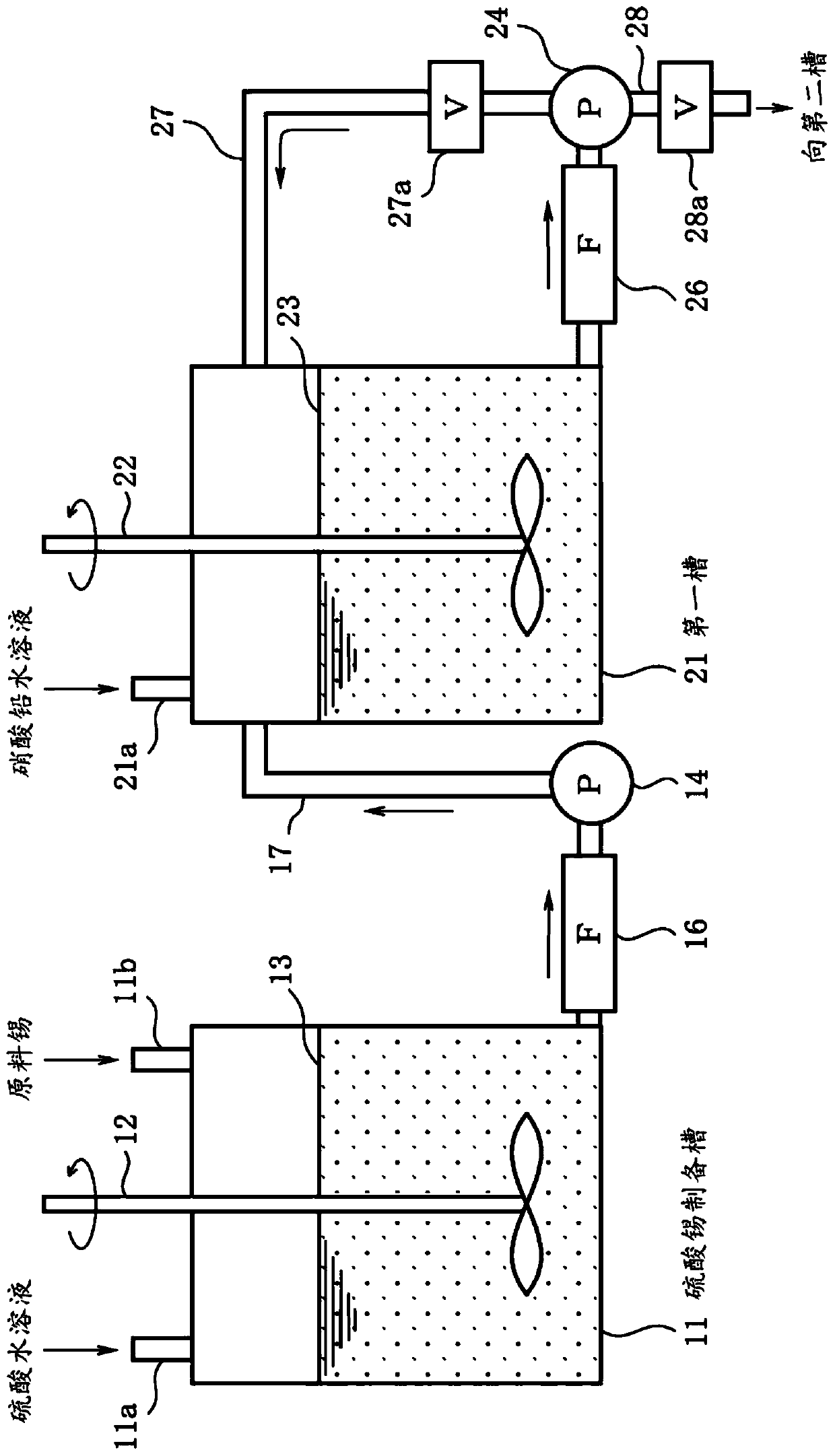
[0056] First, follow the figure 1 sequence of steps shown and according to image 3 The production apparatus shown is a description of the production method of any one of tin, silver, copper, zinc, and indium (metal material for solder material) with low α-ray emission according to the first embodiment of the present invention.
[0057]
[0058] 〔Metal raw material〕
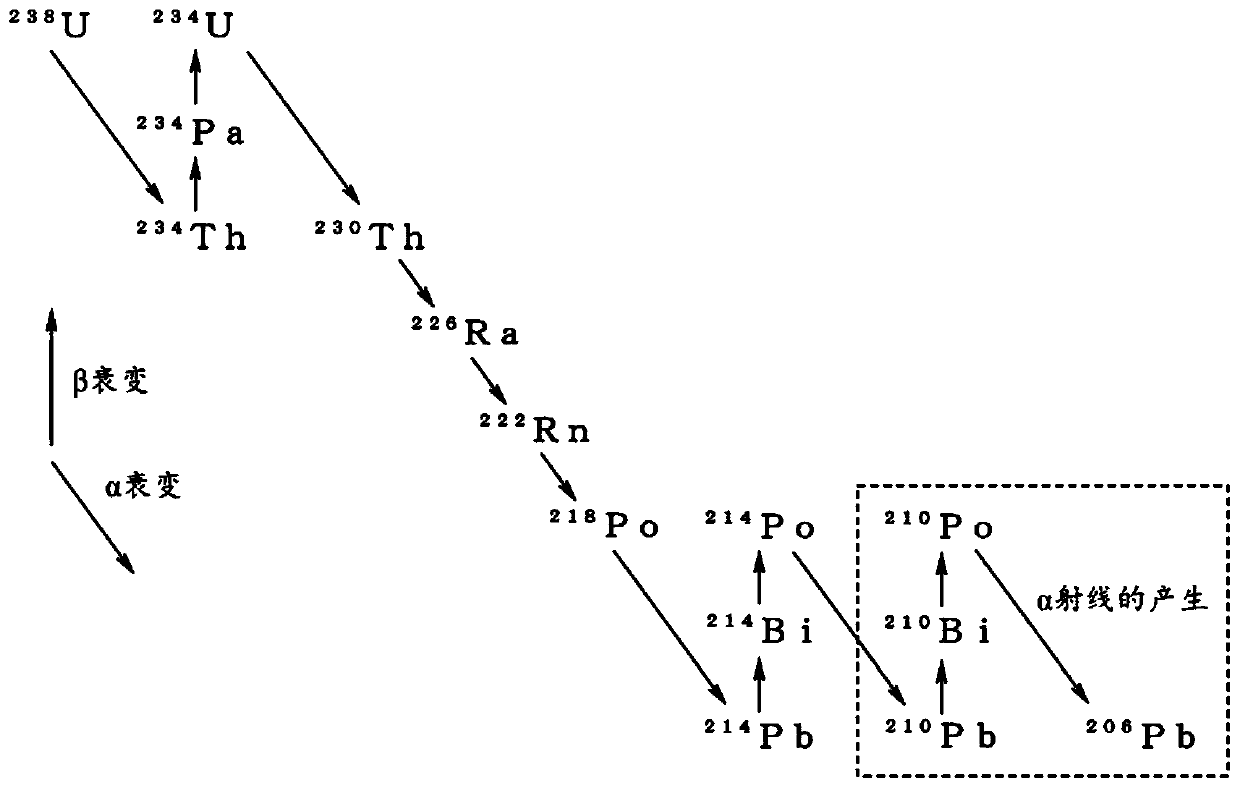
[0059] Regarding any one of tin, silver, copper, zinc and indium used to obtain the low α-ray emission of the first embodiment ( figure 1 Indicated by M in ), the selection of metal raw materials is not limited according to the Pb content of impurities or the amount of α-ray emission.
[0060] For example, even if the concentration of Pb is about 320 mass ppm and the amount of α-ray emission based on Pb is 9 cph / cm 2 A commercially available tin metal of about 100°C is used as the metal raw material, and in the above-mentioned metal finally obtained by using the manufacturing method and manufacturing apparatus...
no. 2 Embodiment approach >
[0090] Next, a method for producing a low-α-ray-emitting tin alloy according to a second embodiment of the present invention will be described.
[0091] In this manufacturing method, the metal tin (Sn) with low α-ray emission obtained in the first embodiment and one or more than two selected from silver, copper, zinc, indium, bismuth, nickel, and germanium metals are cast to make tin alloys.
[0092] Here, as the metal that forms an alloy with metal tin, when a tin alloy is used as solder, silver, copper, zinc, and indium are preferable from the viewpoint of the melting point and mechanical properties of the solder. In order to realize the purpose of the present invention, the α-ray emission of silver, copper, zinc, indium, bismuth or nickel alloyed with metal tin is 0.002cph / cm 2 the following.
[0093] In addition, in this embodiment, casting can utilize the furnace normally used for casting, such as a high-frequency induction melting furnace, for example. In addition, exam...
Embodiment
[0096] Next, examples of the present invention will be described in detail together with comparative examples.
[0097]
[0098] As a metal raw material, use an alpha ray emission of 10.2cph / cm 2 A commercially available Sn powder with a Pb concentration of 15 ppm was added and mixed to an aqueous sulfuric acid solution with a concentration of 130 g / L stored in a tin sulfate preparation tank, and dissolved at 50° C. to prepare 1 m 3 200g / L tin sulfate aqueous solution. Thereby, Pb contained in the tin of the metal raw material is precipitated as lead sulfate. The tin sulfate aqueous solution was filtered through a membrane filter (pore size: 0.2 μm) manufactured by Yuasa Membrane Systems Co., Ltd. to remove lead sulfate.
[0099] Next, in the first tank, while stirring the tin sulfate aqueous solution from which lead sulfate has been removed at a speed of 100 rpm, add a solution containing α-ray emission amount of 5cph / cm 2 lead nitrate aqueous solution of Pb (lead nitra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com