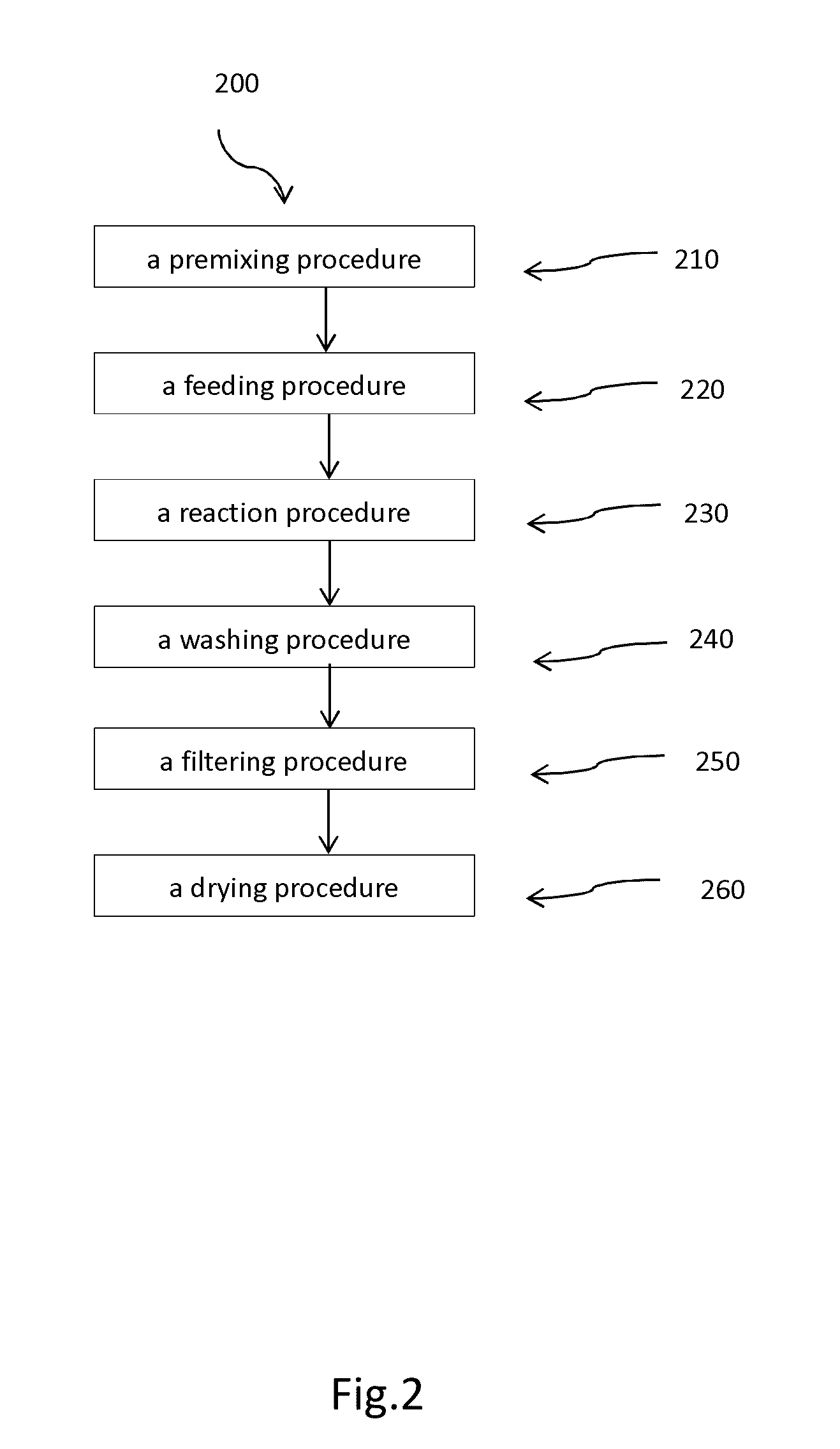
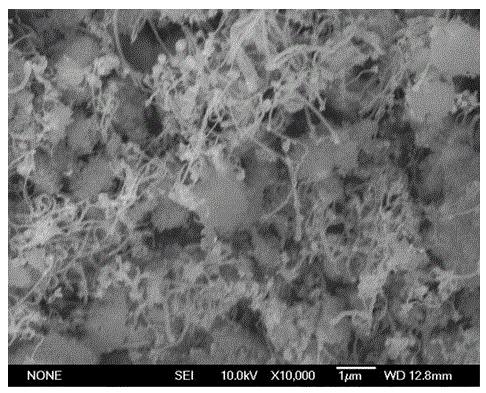
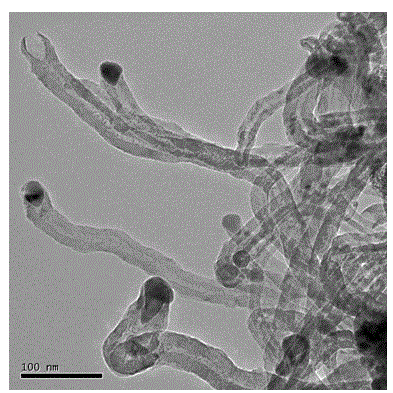
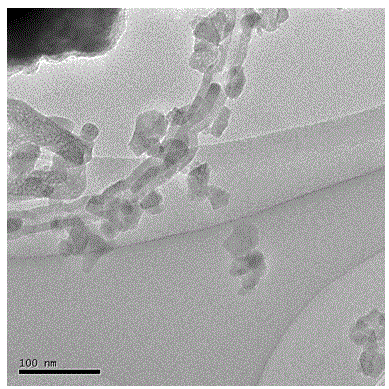
Patents
Literature
389 results about "Nickel Carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A light green, crystalline inorganic compound that produces toxic gases upon heating. Nickel carbonate is used in electroplating, to prepare nickel monoxide, to make colored glass and as a catalyst in the treatment of wastewater. Exposure to this substance can cause severe dermatitis, skin and asthma-like allergies and affects the lungs, kidneys, gastrointestinal tract and neurological system. Nickel carbonate is a known carcinogen and is associated with an increased risk of developing lung and nasal cancers. (NCI05)
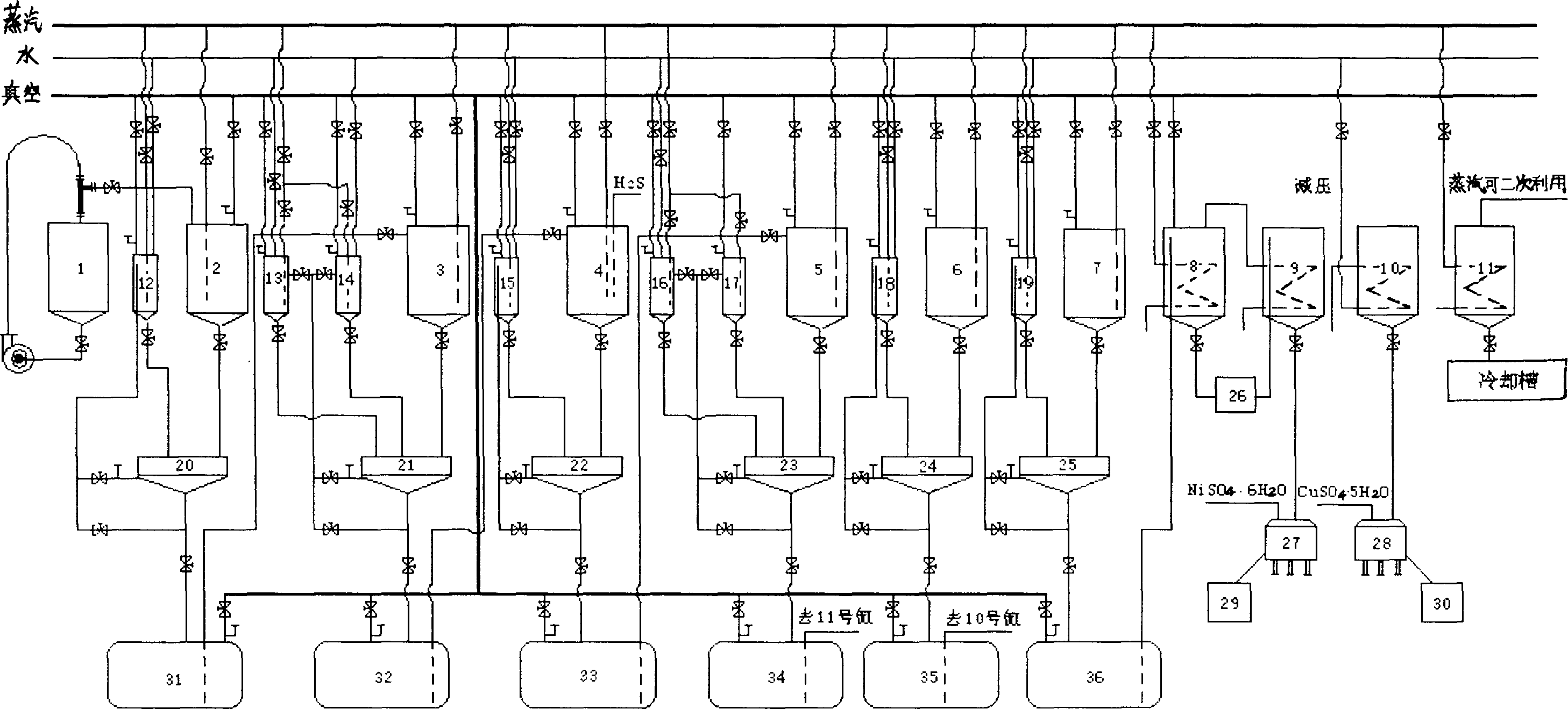
Magnesium alloy non cyanogen plating copper chemical plating nickle and its plating process
InactiveCN1598059AImprove bindingUniform coating thicknessSuperimposed coating processCopper platingChemical plating
The invention discloses a method of plating Nickel with magnalium alloy without cyanogen and the plating technique. The direction for the plating solution is: 20 to 80g.dm-3 of copper charred phosphate; one or several of the 60 to 320g.dm-3 of sodium charred phosphate or Potassium charred phosphate or 60 to 250g.dm-3 of sodium citric acid, 5 to 20g.dm-3 of Potassium sodium tartaric acid, 60 to 250g.dm-3 of HEDP and 60 to 250g.dm-3 of amine ethylene; one or several of the 5 to 20g.dm-3 of hydrogen amine di-fluorin or 5 to20 g.dm-3 sodium fluoride or 5 to 20g.dm-3 of Potassium fluoride or 5 to 20g.dm-3 of lithium fluoride. The plating solution chooses the nickel sulfate or alkali type nickel carbonate or nickel acetic acid as the main salt and adds the reducing agent, combination agent and stabilization agent. The plating technique adopts the acid plating copper-plating three nickel-plating chromium. The invention has little pollution to the environment, high binding power and erosion-proof property.
Owner:GCI SCI & TECH +1
Nickel metal compositions and nickel complexes derived from basic nickel carbonates
ActiveUS20110196168A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDicarbonateMetallurgy
Nickel-metal-containing solids for use in manufacturing nickel metal complexes are disclosed. The nickel-metal-containing solids are made by reducing basic nickel carbonates. By varying the molar ratios of carbonates and bicarbonates to nickel salts, the methods provide basic nickel carbonates that produce superior nickel metal-containing solids that react more effectively with phosphorous-containing ligands. The phosphorous containing ligands can be both monodentate and bidentate phosphorous-containing ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Method of recovery copper, nickel and noble metal in waste water and slag by combined technology of wet method and fire method
A process for recovering the copper, Ni and noble metals from the sewage and dregs by the combination of wet method and fire method includes the wet process including extracting, Cu-Ni separation, removing Fe, extracting, and refining nickel carbonate, and the fire process including sintering and smelting to produce nickel matte and black copper. Its advantages are high recovery rate of Ni, Cu, Au, Ag and Pd, and no secondary pollution.
Owner:孙涛 +1
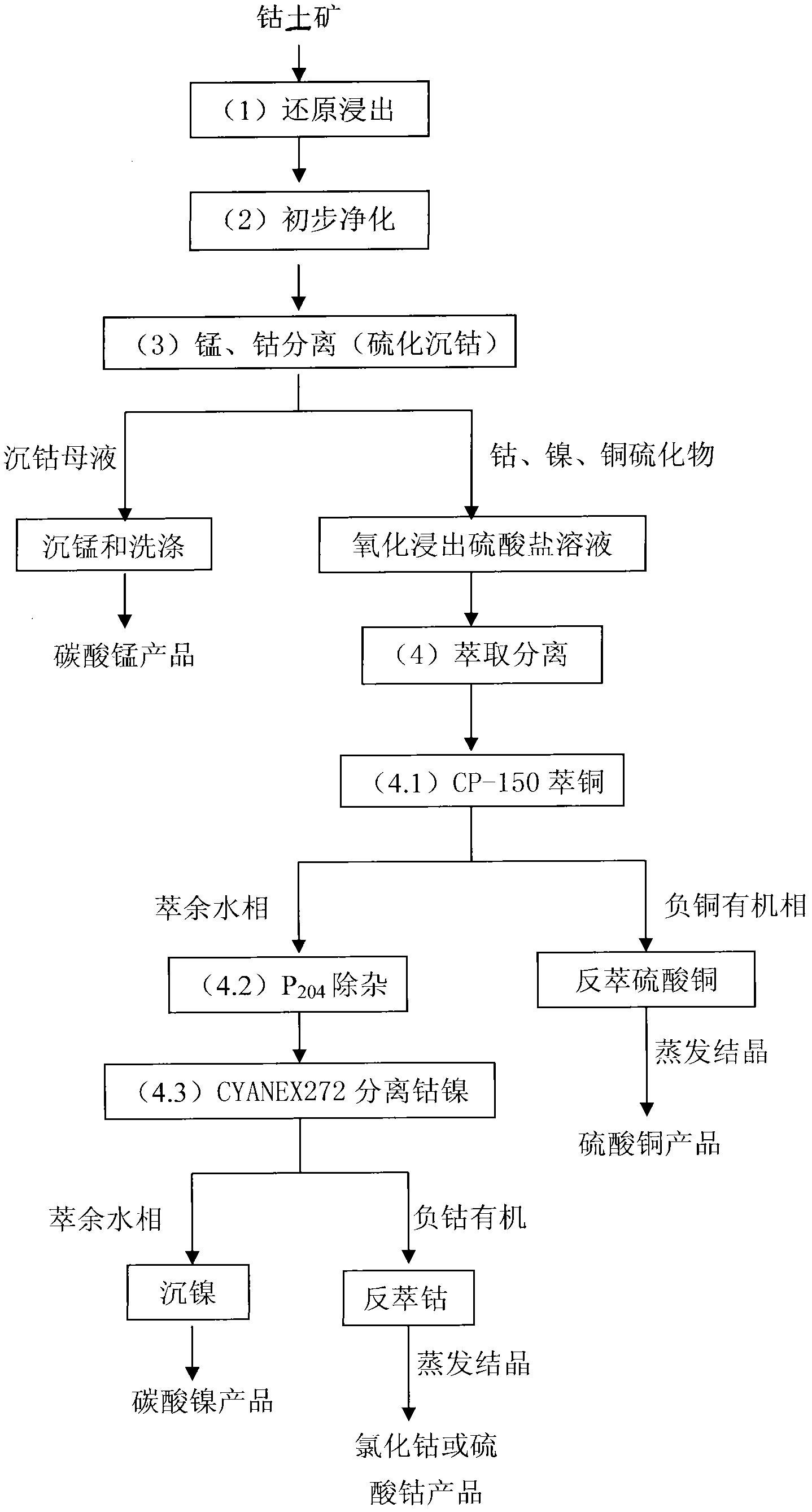
Processing method for comprehensively recovering high manganese asbolite
InactiveCN102021331AIncrease costLow recovery rateProcess efficiency improvementManganeseHydrometallurgy
The invention discloses a processing method for comprehensively recovering high manganese asbolite, belonging to the field of hydrometallurgy. In the processing method, the high manganese asbolite is processed through four processing steps comprising (1) reduction leaching, (2) primary cleaning, (3) manganese and cobalt separation and (4) extraction separation to obtain a product manganese carbonate, copper sulfate, nickel carbonate or cobaltous sulfate. The processing method has the advantages that the source of raw materials is wide, the process and equipment are simple, the operation is stable, the energy consumption is low, the production cost is low, the practicability is wide, and the economic effect and the society effect are obvious.
Owner:HAINAN ZHONGDAO ENERGY DEV
Process for production of nickel and cobalt using metal hydroxide, metal oxide and/or metal carbonate
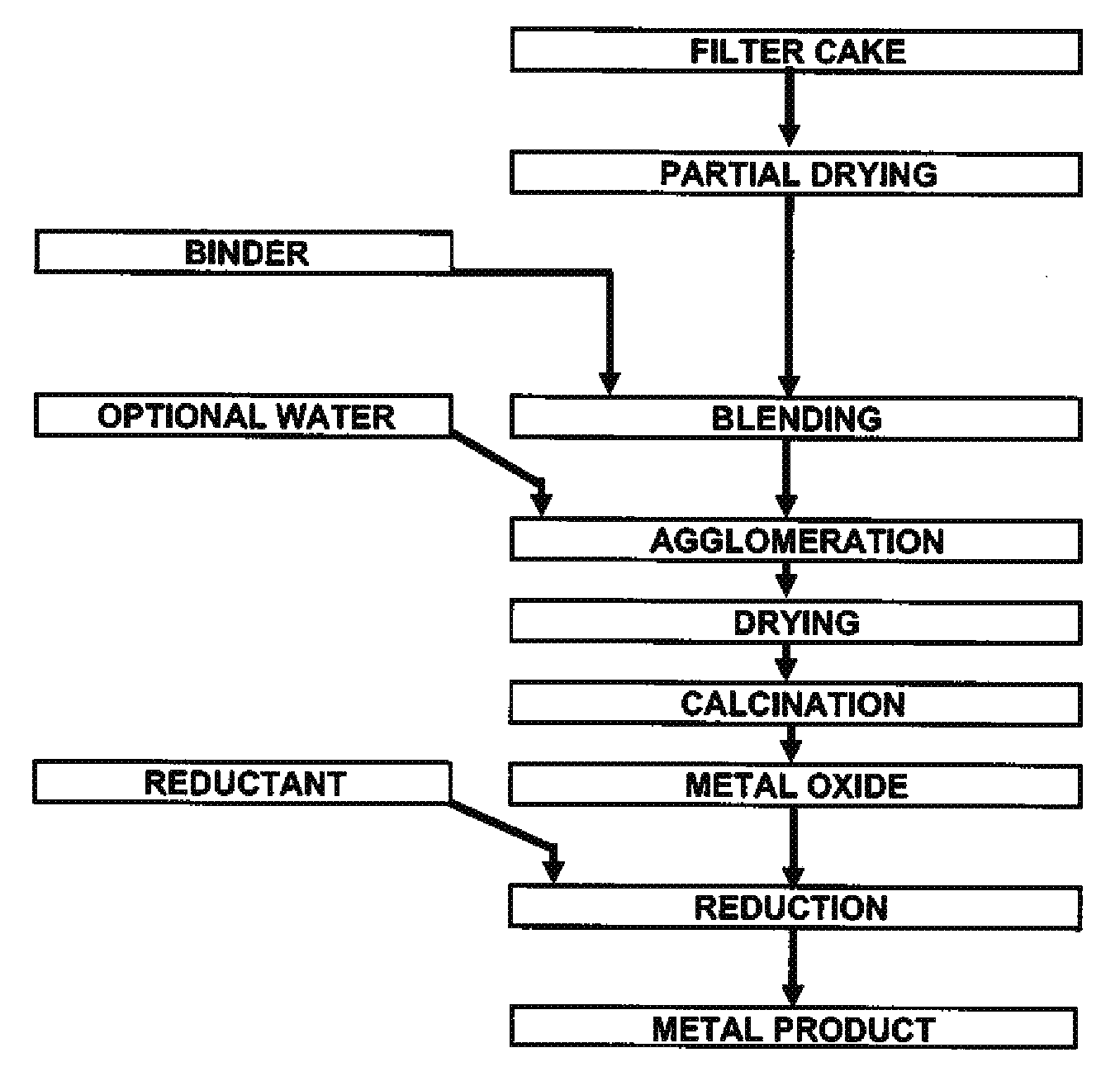
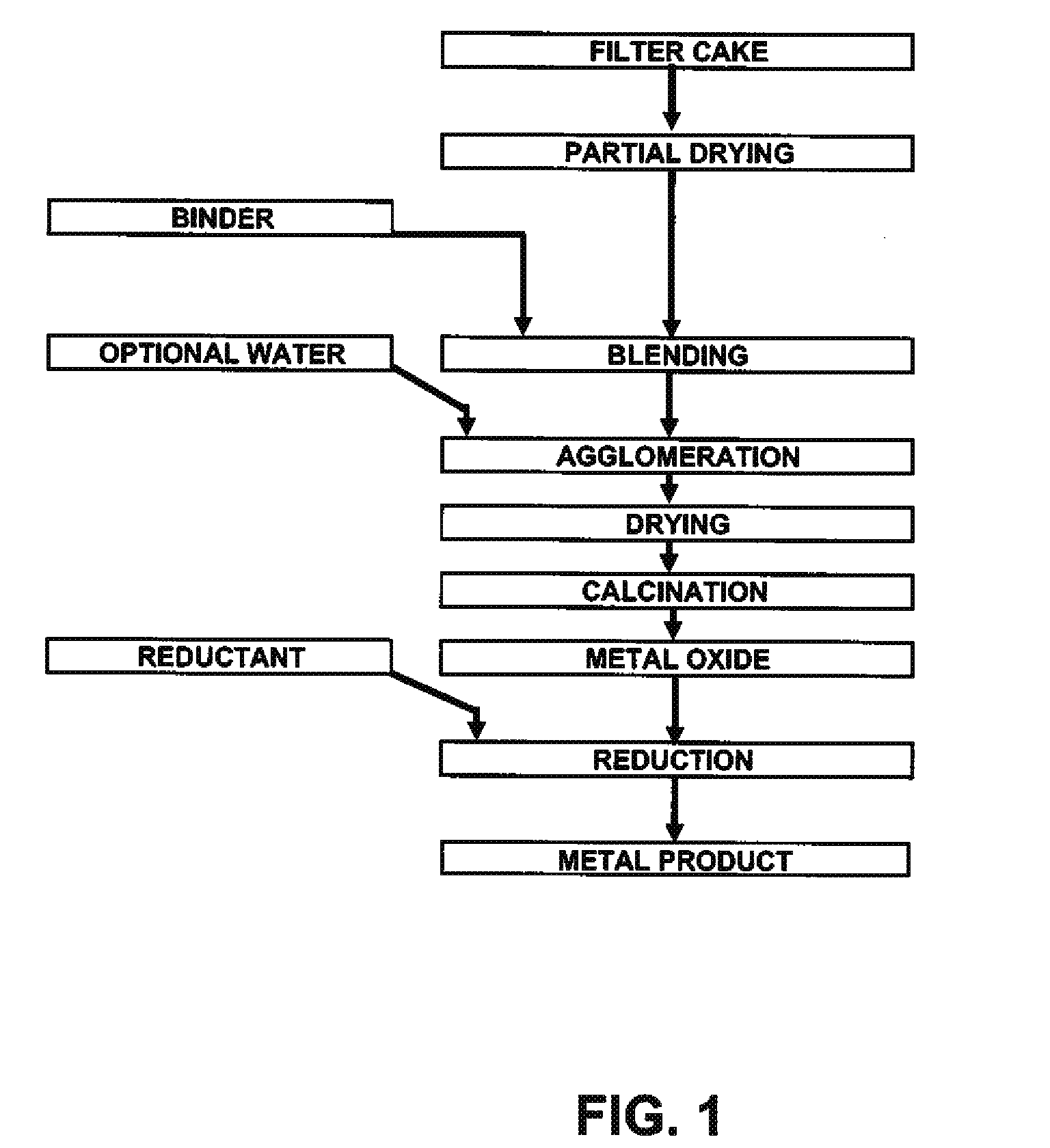
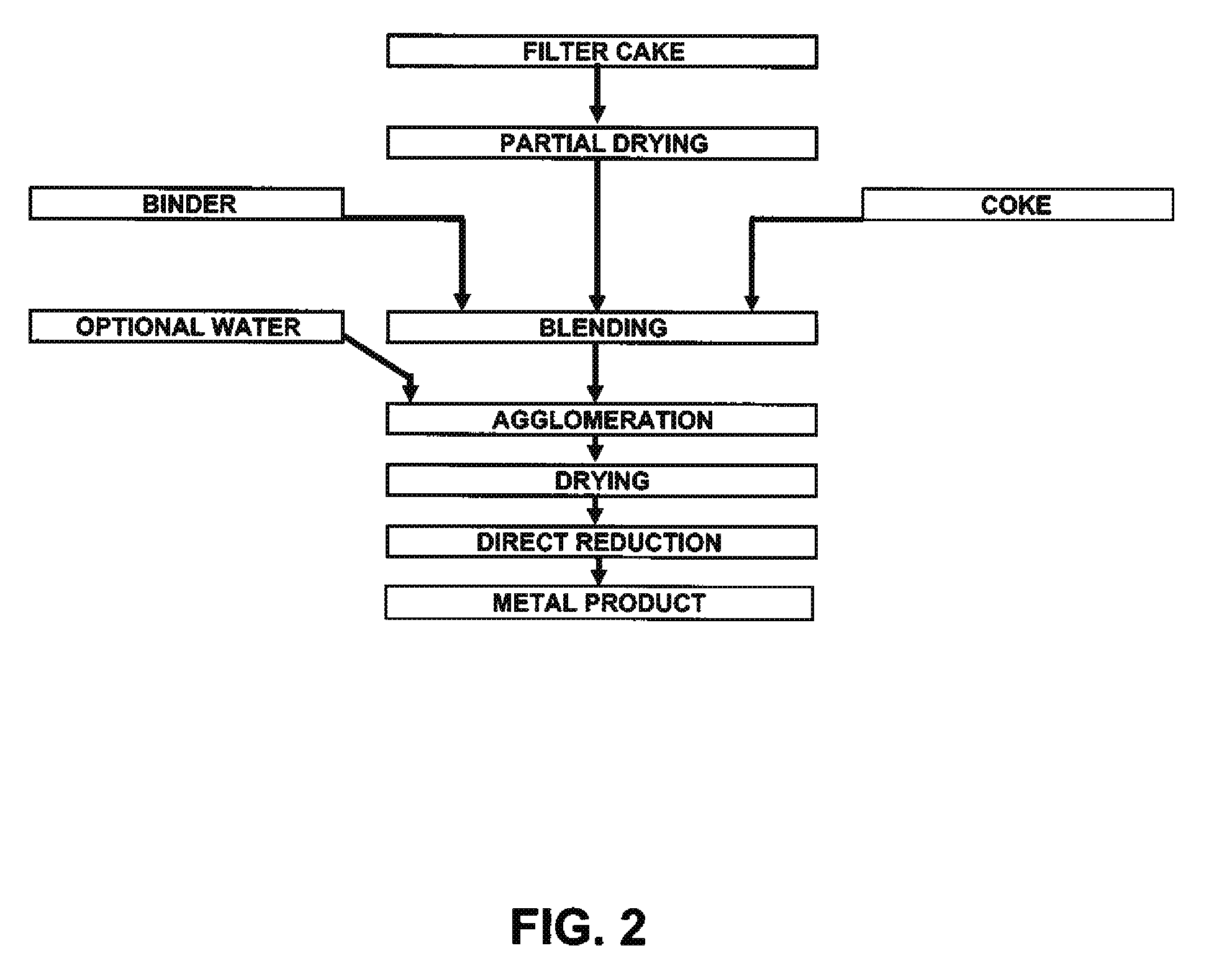
A method for producing metal oxide from a metal salt selected from nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof includes providing a mixture of the metal salt, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof, forming the mixture into agglomerates, and calcining the agglomerates to produce metal oxide. A method for making metallic nickel or cobalt includes providing a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate and combinations thereof, mixing the metal salt with a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof to form a mixture, optionally adding water, forming the mixture into agglomerates, drying the agglomerates, adding an effective reducing amount of coke and / or coal and directly reducing the dried agglomerates with an effective amount of heat to produce metallic nickel and / or cobalt. Coke particles may be added to the mixture prior to agglomeration. An agglomerate includes a metal salt selected from the group consisting of nickel hydroxide, cobalt hydroxide, mixed nickel-cobalt hydroxide, nickel carbonate, cobalt carbonate, mixed nickel-cobalt carbonate and combinations thereof; and a binder selected from the group consisting of inorganic binder, organic binder and combinations thereof.
Owner:VALE CANADA
Method of preparing electronic grade nickel carbonate by sodium carbonate deposition
The invention discloses a manufacturing method of electron-grade nickel carbonate through sedimenting sodium carbonate, which is characterized by the following: adopting soluble nickel salt as nickel source and sodium carbonate as sediment; co-flowing nickel salt solution and sodium carbonate solution; controlling pH value, temperature and aging condition; washing; drying; washing again; drying again; grinding into product.
Owner:JINCHUAN GROUP LIMITED +1
Method for directly producing high-purity electronic level cobaltous sulfate by using cobalt-containing waste
ActiveCN102061390AMeet high purity requirementsImprove overall recoveryProcess efficiency improvementTotal recoveryGoethite
The invention provides a method for directly producing high-purity electronic level cobaltous sulfate by using cobalt-containing waste, in particular a process for producing cobaltous sulfate by using cobalt-containing waste. The method comprises the steps of: checking and classifying raw materials, wet-milling and size-mixing, acid-decomposing, filtering, washing, separating, and extracting copper sponge. The method is characterized by also comprising the steps of: removing iron with a goethite process, extracting P2O4 and removing impurities, separating nickel from cobalt, extracting N235, purifying, concentrating and crystallizing. The high purity electron level cobaltous sulfate is directly regenerated by using various kinds of cobalt wastes, the requirement of the modern high-technology industry on high purity of the cobaltous sulfate is met; the total recovery of the cobalt is higher than or equal to 98 percent; various usable elements can be comprehensively recycled, and coppersponge, tungsten carbide, iron hydroxide and nickel carbonate can be regenerated while the electronic level cobaltous sulfate as a main product is regenerated. The invention has the advantages of comprehensively utilizing waste cobalt resources, recycling the wastes, improving the enterprise benefit, and being beneficial to the development of energy conservation, emission reduction, environment protection and circular economy.
Owner:HUNAN JINYUAN NEW MATERIALS CO LTD
Method for separating and recovering nickel, cobalt, magnesium, iron and silicon from nickel-bearing laterite
InactiveCN101525690AImprove leaching rateHigh dissolution rateProcess efficiency improvementSlagLaterite
A method for separating and recovering nickel, cobalt, magnesium, iron and silicon from nickel-bearing laterite is disclosed; nickel, cobalt, magnesium and iron therein are leached out by using high-temperature peracid, leachate is pre-neutralized via serpentine powder, after the pre-neutralization, the leachate is neutralized by magnesite powder for de-ironing, and the scum is delivered for ironmaking after being dewatered by smoke gases in a fluidized bed furnace for making sulfuric acid, the de-ironed clear nickel liquid uses magnesite powder to precipitate nickel carbonate, and the precipitated liquid is concentrated and crystallized to obtain magnesium sulfate heptahydrate. The leached slag mainly contains silicon and can be used for making white carbon black. The invention can sufficiently recover and utilize nickel, cobalt, magnesium, iron and silicon in the nickel-bearing laterite, and the invention is simple in technology, low in energy consumption and pollution-free on environment.
Owner:广西冶金研究院有限公司 +1
Magnesium alloy direct chemical plating NI-P-SiC plating solution formula and plating process
InactiveCN101665930AEasy to prepareFormulated stableLiquid/solution decomposition chemical coatingChemical platingSurface-active agents
The invention provides a magnesium alloy direct chemical plating NI-P-SiC plating solution formula and a plating process. The formula consists of chemical plating Ni-P plating solution and SiC dispersing solution, wherein the chemical plating Ni-P plating solution comprises 20-30g / l of nickel sulfate, 20-30g / l of sodium hypophosphite, 15-30g / l of complexing agent, 15-25g / l of sodium acefate, 10-20g / l of fluoride, 1-2mg / l of stablizer, appropriate amount of PH value modifier and the balance of water; the SiC dispersing solution is formed by stirring 10-80mg / l of surface active agent and 1-8g / lof micron SiC by magnetic force; and after pretreatment of the magnesium alloy, a Ni-P-SiC composite plating layer with thickness being 20-50Mum is obtained under the process conditions that the temperature is 80-90 DEG C, the PH value is 4.8-5.6, and the plating time is 60-120 minutes. In the invention, basic nickel carbonate is replaced by nickel sulfate which is introduced as main salt; and themicron SiC is added to conduct plating directly; on the base of keeping the excellent performance of magnesium alloy chemical plating Ni-P alloy, the rigidity and abrasive resistance of the magnesiumalloy chemical plating layer are greatly improved; the problem of lower abrasive resistance of magnesium alloy nickel-plating layer is solved; and the preparation of the plating solution is convenient, the cost is low, the plating solution is stable and the deposition rate is quick.
Owner:CHONGQING UNIV OF TECH
Methods of improving surface roughness of an environmental barrier coating and components comprising environmental barrier coatings having improved surface roughness
Methods for improving surface roughness of an environmental barrier coating including providing a component having a plasma sprayed environmental barrier coating; applying a slurry to the environmental barrier coating of the component, the slurry being a transition layer slurry or an outer layer slurry; drying the environmental barrier coating having the applied slurry; and sintering the component to produce a component having an improved surface roughness where the slurry includes a solvent; a primary transition material, or a primary outer material; and a slurry sintering aid selected from iron oxide, gallium oxide, aluminum oxide, nickel oxide, titanium oxide, boron oxide, alkaline earth oxides, carbonyl iron, iron metal, aluminum metal, boron, nickel metal, iron hydroxide, gallium hydroxide, aluminum hydroxide, nickel hydroxide, titanium hydroxide, alkaline earth hydroxides, iron carbonate, gallium carbonate, aluminum carbonate, nickel carbonate, boron carbonate, alkaline earth carbonates, iron oxalate, gallium oxalate, aluminum oxalate, nickel oxalate, titanium oxalate, solvent soluble iron salts, solvent soluble gallium salts, solvent soluble aluminum salts, solvent soluble nickel salts, solvent titanium salts, solvent soluble boron salts, and solvent soluble alkaline earth salts.
Owner:GENERAL ELECTRIC CO
Method for recycling copper, zinc, cobalt and nickel from various kinds of nonferrous metal containing waste
The invention discloses a method for recycling copper, zinc, cobalt and nickel from various kinds of nonferrous metal containing waste. The method comprises the steps that nonferrous metal containing dead catalysts, waste battery materials and other waste raw materials are subjected to leaching dissolution by adding acid and an oxidizing agent, a solution containing nickel, cobalt, copper and zinc is subjected to copper extraction after filter pressing, and reverse extraction is carried out on the copper solution for electro deposited copper production; and P204 extraction is carried out after the raffinate is subjected to deironing treatment, reverse extraction galvanizing zinc is obtained, a zinc carbonate product is obtained through settlement and roughening, P507 nickel and cobalt separation is carried out on the raffinate, and a cobalt salt solution and a nickel salt solution are obtained and used for producing cobalt sulphate, nickel carbonate, electrolytic nickel and other products. Leaching residues and deironining slag obtained after filter pressing are dissolved and enter a waste slag removal treatment workshop for sintering treatment.
Owner:ZHEJIANG XINSHIDAI ZHONGNENG RECYCLING TECH CO LTD
Method for separating and reclaiming metal nickel and tin from waste materials containing nickel and tin
InactiveCN101824540AGood choiceReduce recycling costsProcess efficiency improvementTin dioxideNickel oxide hydroxide
The invention discloses a method for separating and reclaiming metal nickel and tin from waste materials containing nickel and tin. The method is characterized by comprising the following steps of: leaching the waste materials containing the nickel and tin with acid; adjusting pH value of the materials by using an alkali substance to ensure that impurity iron and a valuable component tin are precipitated; filtering the solution to obtain nickel-containing solution and tin-containing precipitate; extracting the nickel-containing solution for impurity removal and purification, wherein purifying fluid can be directly crystallized to prepare nickel sulfate, can be precipitated and degraded to prepare nickel hydroxide, nickel carbonate and nickel oxalate powder materials, and can be further degraded or reduced to prepare nickel oxide powder and metal nickel powder; and adding sodium hydroxide solution into the tin-containing precipitate, dissolving the tin out and allowing the tin to enter the solution, recrystallizing the solution to prepare a sodium stannate product, or further crystallizing and degrading the solution to prepare a tin dioxide powder material. Acid and alkali in low price are consumables in the whole reclaiming process, and product variousness is provided, so the method has significant economic value and market competitiveness.
Owner:GUANGDONG BRUNP RECYCLING TECH
Ni-pt alloy and target comprising the alloy
InactiveUS20070098590A1Easily perform cool rollingGood effectPhotography auxillary processesVacuum evaporation coatingActivated carbon filtrationFiltration
The present invention provides a Ni—Pt alloy superior in workability containing Pt in a content of 0.1 to 20 wt % and having a Vickers hardness of 40 to 90, and a target comprising the Ni—Pt alloy. The present invention also provides a manufacturing method of Ni—Pt alloy superior in workability comprising a step of subjecting a raw material Ni having a purity of 3N level to electrochemical dissolution, a step of neutralizing the electrolytically leached solution with ammonia, a step of removing impurities through filtration with activated carbon, a step of blowing carbon dioxide into the resultant solution to form nickel carbonate and exposing the resultant product to a reducing atmosphere to prepare high purity Ni powder, a step of leaching a raw material Pt having a purity of 3N level with acid, a step of subjecting the leached solution to electrolysis to prepare high purity electrodeposited Pt, and a step of dissolving the resultant high purity Ni powder and high purity electrodeposited Pt. The foregoing method enables the rolling of the Ni—Pt alloy ingot upon reducing the hardness thereof, which results in the stable and efficient manufacture of a rolled target.
Owner:JX NIPPON MINING& METALS CORP
Penicillium, as well as preparation method and application
InactiveCN101434909AReach pollutionReduce pollutionFungiMicroorganism based processesEcological environmentPhosphate
The invention discloses a Penicillium, a preparation method and applications thereof, Penicillium fungus PSM11-5 is separated from a vanadium ore sample; insoluble tricalcium phosphate, sodium metavanadate, cobalt hydroxide and basic nickel carbonate are taken as indicating compounds; and a fungal strain is screened by testing the capability of decomposing the tricalcium phosphate, the sodium metavanadate, the cobalt hydroxide and the basic nickel carbonate. The Penicillium PSM11-5 is Penicillium sp.PSM11-5 CCTCCM208207. The strain is utilized for carrying out biological leaching of phosphorus and biological metallurgy, metals of phosphorus, vanadium, nickel, cobalt and the like are leached from lean ores, discarded ores, submarginal ores, difficult-to-mine ores, difficult dressing ores and refractory ores, thereby fully utilizing the mineral resources, reducing the metallurgical costs and protecting the ecological environment. The PSM11-5 is utilized for leaching the phosphorus from low-grade phosphate rock powder, a biological fertilizer is prepared to be applied to the soil, thereby leading the soil to contain higher content of soluble phosphorus which can be utilized by crops; the strain further leaches insoluble phosphorus which is deposited in the soil before, thereby reducing phosphorus fertilizer and reducing gas pollution caused by the phosphorus fertilizer and water pollution caused by the phosphorus fertilizer.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Process for rapidly producing basic nickel carbonate or basic cobaltous carbonate
InactiveCN101708868AReduce pollutionRapid productionNickel carbonatesCobalt carbonatesChlorideUltimate tensile strength
The invention discloses a process for rapidly producing basic nickel carbonate or basic cobaltous carbonate, comprising the following steps of: dissolving, synthetizing, filtering, water scrubbing and drying. The dissolving proportion by weight of nickel chloride or cobalt chloride in the dissolving process is NiCl2:H2O=1:4-8, CoCl2:H2O=1:4-8, and the dissolving proportion by weight of sodium carbonate is Na2CO3:H2O=1:4-8. Aqueous alkali and a nickel chloride or cobalt chloride solution are added by adopting a spray mode in the synthetic process. The synthetic reaction time is 2-4 hours. The invention not only has simple process, short production cycle, low labor intensity and arbitrary enlargement of production scale but also has simple and practicable operation, low cost, stable quality and high efficiency. The process has the characteristics of fast production, high quality of products and high efficiency; products can be turned out after water scrubbing at one time, and therefore, electricity, water, labor and time are saved. The products have even and superfine granules without crushing and sieving, thereby being beneficial to application.
Owner:江西核工业兴中科技有限公司
Chemical nickel plating liquid and preparation method
The invention provides chemical nickel plating liquid. The chemical nickel plating liquid is prepared from the following components: nickle salt, a reducing agent, a complexing agent, a buffering agent, a stabilizing agent, a surfactant and deionized water, wherein, the chemical nickel plating liquid is specially prepared from the components in parts by weight: 1000 parts of deionized water, 20 to35 parts of nickel salt, 15 to 32 parts of a reducing agent, 10 to 30 parts of a complexing agent, 2 to 12 parts of a buffering agent, 1 to 15 parts of a stabilizing agent, and 0.01 to 0.2 part of asurfactant. The nickel salt is one or more of nickel sulfate, nickel chloride, nickel acetate, nickel carbonate and nickel aminosulfonate.
Owner:WINSTAR CHEM SHANGHAI
Method for producing fine powder of metallic nickel comprised of fine spherical particles
A process for the production of fine powder of metallic nickel which comprises a first step of dissolving nickel carbonate and / or nickel hydroxide in aqueous ammonia or in an aqueous solution of ammonia and at least one selected from the group consisting of ammonium carbonate, ammonium hydrogencarbonate, a carbonate of an alkali metal and a hydrogencarbonate of an alkali metal to prepare an aqueous solution of a nickel salt; converting the aqueous solution of a nickel salt to a W / O emulsion, and then removing volatile components including ammonia from the droplets to form precipitates of nickel carbonate in the droplets, thereby providing fine spherical particles of nickel carbonate; and a second step of heating the particles of nickel carbonate in the presence of a fusion preventive agent that is a compound of at least one element selected from the group consisting of alkaline earth elements, aluminum, silicon and rare earth elements in an atmosphere of hydrogen, thereby reducing the nickel carbonate to metallic nickel.
Owner:SAKAI CHEM IND CO LTD
Process for synthesis of basic nickel carbonate from diacidic base
InactiveCN105384199ACreate pollutionIncrease concentrationNickel carbonatesNickel saltSodium acid carbonate
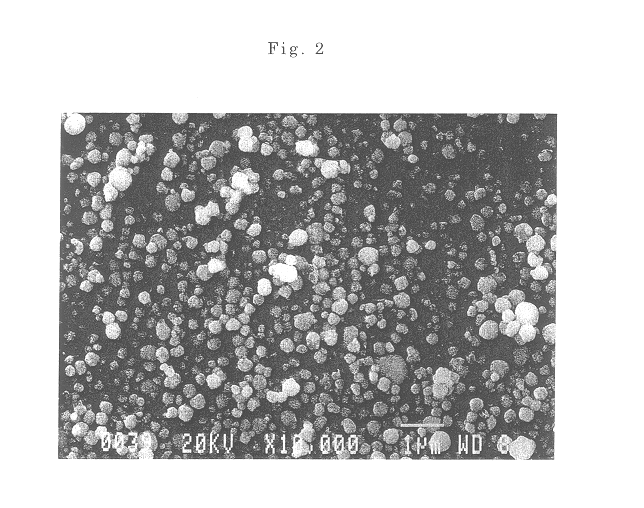
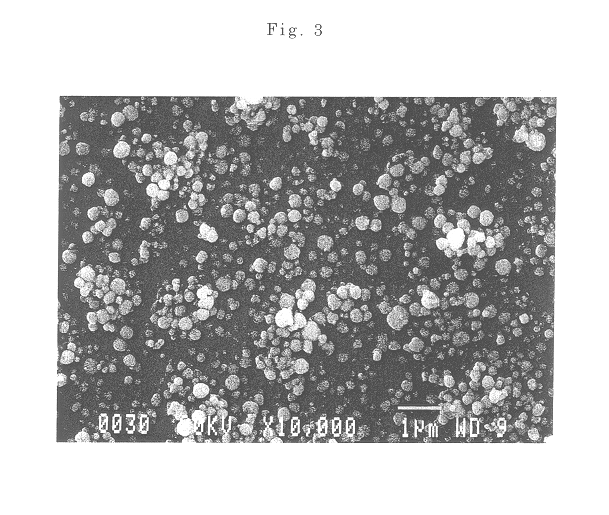
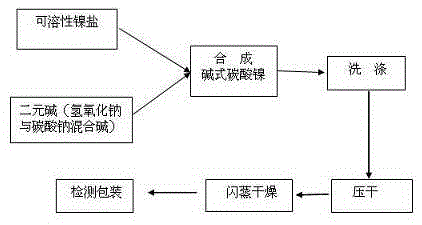
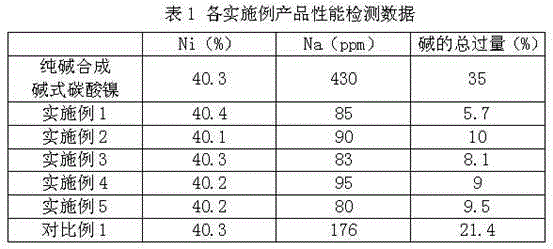
The present invention discloses a process for synthesis of basic nickel carbonate from a diacidic base, according to the process, a sodium carbonate and sodium hydroxide mixed diacidic base is used for precipitation reaction with a soluble nickel salt, the reaction is one-step room temperature reaction, operation is easy, the base dosage is reduced by 20 to 30% compared with a pure soda base synthesis process, the environment is not polluted, and Na in the obtained basic nickel carbonate product (containing 40% of Ni) can be 100ppm or less.
Owner:JIANGXI NUCLEAR IND XINGZHONG NEW MATERIALS
Method for preparing large-specific-surface-area porous nickel oxide microspheres
The invention discloses a method for preparing large-specific-surface-area porous nickel oxide microspheres. The method comprises the following steps of: preparing and synthesizing a mixed solution by taking nickel nitrate and urine as raw materials, taking hexadecyl trimethyl ammonium bromide as a surfactant, taking absolute ethanol and deionized water as washing agents and refining raw materials; heating in a reaction kettle for preparing hydrogen nickel carbonate; and washing, performing suction filtration, drying in vacuum, and baking to obtain large-specific-surface-area porous nickel oxide microspheres. The preparation method has the advantages of advanced process, short process flow, small using amount of raw materials, detailed and accurate data, high product yield which can be up to 95 percent and high product purity, which can be up to 98.5 percent; the specific surface area of the product, i.e., nickel oxide microspheres is 748.25m<2> / g, microsphere particles are less than or equal to 10 mum, irregular hexagonal nano-sheets are distributed on the surfaces of the microspheres and are less than or equal to 40 nanometers in diameter, and the microspheres can be matched with a plurality of chemical substances; and the method is very ideal method for preparing large-specific-surface-area porous nickel oxide microspheres.
Owner:TAIYUAN UNIV OF TECH
Method for forming Basic Nickel Carbonate
ActiveUS20160090311A1Alleviate volume expansionImprove conversion rateChemical/physical/physico-chemical processesNickel oxides/hydroxidesParticulatesPotential market
The present disclosure provides a novel method to fabricate the basic nickel carbonate particulates. The nickel content in the basic nickel carbonate particulates fabricated by this invention (51-53 mass %) is higher than the present commercialized products (44-46 mass %). Basic nickel carbonate is an important intermediate to prepare NiO and pure Ni particles, and NiO and pure Ni particles are important materials in electronic industrial. Therefore, basic nickel carbonate has its potential market.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY
Preparation method of carbon nanotube reinforced hydroxyapatite composite material
The invention discloses a preparation method of a carbon nanotube reinforced hydroxyapatite composite material, relating to a composite material for prosthesis materials. According to the preparation method, a carbon nanotube is synthesized in hydroxyapatite powder, the carbon nanotube is subjected to surface modification by using hydroxyapatite, and the carbon nanotube reinforced hydroxyapatite composite material is further prepared. The preparation method comprises the following steps of: firstly preparing carbon nanotube-hydroxyapatite powder from nickel carbonate and the hydroxyapatite powder, then preparing hydroxyapatite-modified carbon nanotube-hydroxyapatite powder, and finally preparing the carbon nanotube reinforced hydroxyapatite composite material. The preparation method disclosed by the invention overcomes the defects that the carbon nanotube is difficult to disperse in a hydroxyapatite matrix, the wettability and the interfacial strength between the carbon nanotube and the hydroxyapatite matrix are low, the hydroxyapatite powder of which the surface is loaded with the carbon nanotube is difficult to form and the biocompatibility of the composite material is poorer in the prior art.
Owner:HEBEI UNIV OF TECH
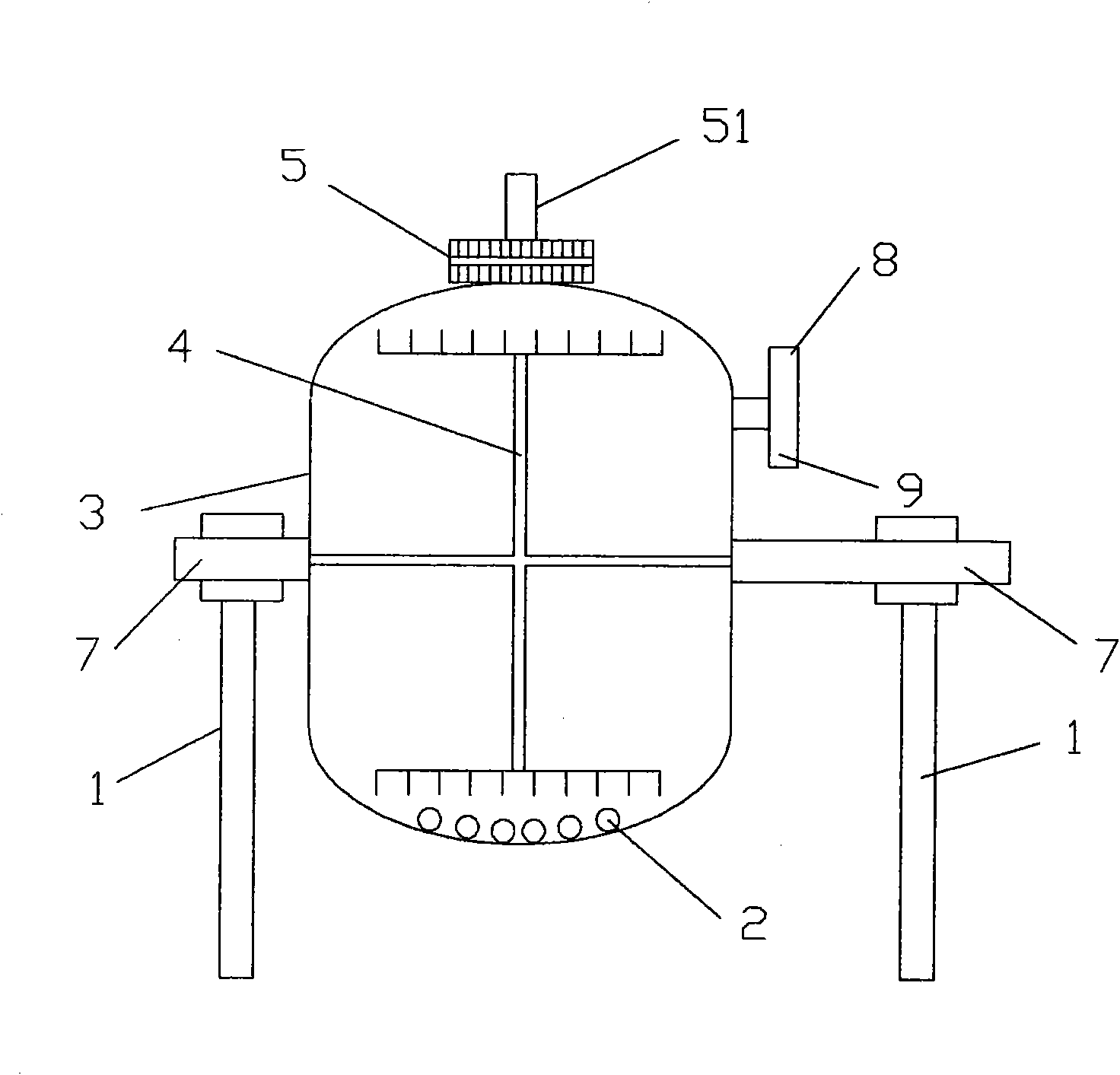
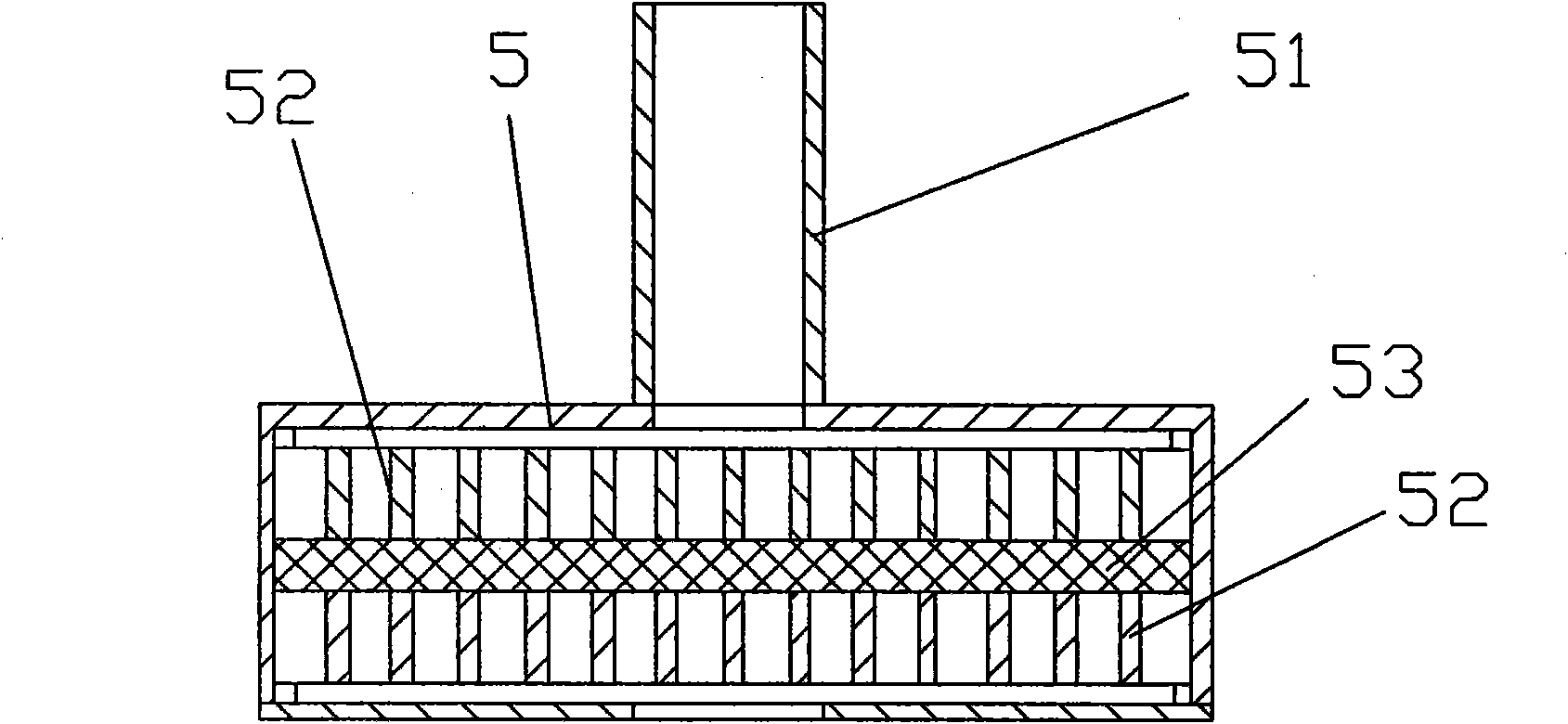
Method for continuously compounding high-purity alkali nickel carbonate
ActiveCN102923794ALow impurity contentFlat surfaceNickel carbonatesTemperature controlReaction temperature
The invention discloses a method for continuously compounding high-purity alkali nickel carbonate. The method comprises the steps as follows: a nickeliferous stock solution, ammonium salt and a precipitant Na2CO3 are continuously conveyed to a multifunctional reactor for carrying out continuous compounding reaction, and resultant slurry is continuously discharged from a material outlet; during the continuous compounding reaction, the reaction temperature is controlled within 50-80 DEG C with a real-time monitoring system, the reaction pH value is controlled within 7.5-8.5, and the average residence time of a reaction system in the multifunctional reactor is controlled within 20 h-30 h; and a reaction discharging is filtered, filter residue is aged, and then the reaction discharging enters into a multi-level washing tower to be washed, when the flow conductance value of the multi-level washing tower bottom is less than 600 ms / cm, a washed product is filtered and dried to obtain the high-purity alkali nickelous carbonate. The invention has the advantages of simple process, low equipment investment, environment protection, complete product structure, good quality and the like.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
Preparation method of dye-sensitized solar battery cobalt-nickel sulfide counter electrode
InactiveCN105719836AHigh mechanical strengthNo sheddingLight-sensitive devicesPhotovoltaic energy generationNickel saltSlurry
The invention discloses a method for preparing a cobalt-nickel sulfide counter electrode. The steps include: first step, hydrothermally reacting cobalt salt, nickel salt and excess sodium hydroxide or sodium carbonate at 120-200°C to generate hydrogen Oxycobalt nickel or basic cobalt nickel carbonate; the second step, mix the hydroxide cobalt nickel or basic cobalt nickel carbonate with a certain concentration of sulfur source aqueous solution, and heat it at 140-230 ° C; the third step is to generate the sulfur Cobalt nickel is made into a slurry and coated on a conductive substrate, and dried at room temperature. The electrode prepared by the invention does not need to be sintered under vacuum or protective atmosphere, and the preparation process is simple; the prepared cobalt-nickel sulfide counter electrode has high mechanical strength and no shedding phenomenon; Better electrocatalytic activity; Dye-sensitized solar cells assembled with cobalt-nickel sulfide electrodes obtained higher photoelectric conversion efficiency than dye-sensitized solar cells assembled with pyrolyzed Pt electrodes.
Owner:CHINA THREE GORGES UNIV
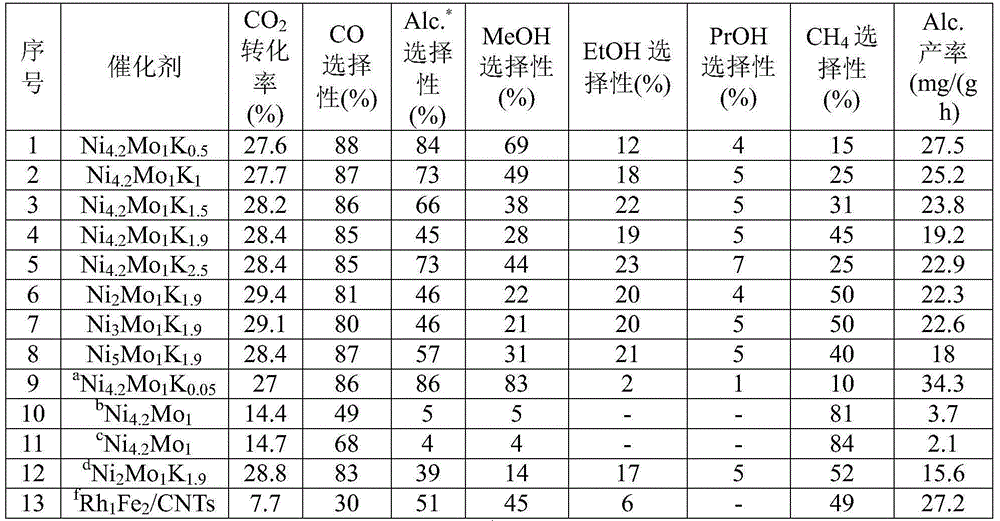
Catalyst for synthesis of low carbon alcohol by hydrogenation of carbon dioxide as well as preparation method and application thereof
ActiveCN106311281AHigh selectivityLow costOrganic compound preparationHydroxy compound preparationNickel saltAlcohol fuel
The invention relates to a catalyst for synthesis of low carbon alcohol by hydrogenation of carbon dioxide as well as a preparation method and an application thereof, and more specifically relates to a ternary metal sulfide catalyst with a layered structure as well as a preparation method and an application thereof. The catalyst employs basic nickel carbonate which is prepared by coprecipitation of nickel salt and base as a template, an ion exchange method is used for introducing transition metal molybdenum, alkali metal potassium is dipped, and sulfuration is carried out in order to obtain the catalyst. Hydrogenation for carbon dioxide is carried out on the catalyst in order to directly obtain ethanol, propanol and other alcohol fuel with high added values, and proportioning of each metal in the catalyst can be changed in order to adjust distribution of alcohol products. In an optimum condition, the mol fraction of ethanol in total alcohol reaches 43% and far exceeds the mol fraction of ethanol in total alcohol which is synthesized on a rhodium-based catalyst. Compared with a traditional coprecipitation immersion method, raw material loss due to different pH conditions of nickel and molybdenum precipitation is avoided, and utilization rate of raw material is substantially improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Yellow pigment able to reflect near infrared rays and preparation method thereof
InactiveCN105542516AStrong coloring powerStrong covering powerPigment physical treatmentSolid-phase synthesisHigh heat
The invention discloses a yellow pigment able to reflect near infrared rays and a preparation method thereof. The pigment is prepared from a main phase, a doping phase and a mineralizing agent in a mass ratio. The main phase is titanium dioxide with a molar percentage content of 80-98%, the doping phase is 1-10% of basic nickel carbonate and 1-10% of antimony oxide, or 1-10% of iron oxide and 1-10% of magnesium oxide. The mineralizing agent is boric acid or boric acid and ammonium chloride, and accounts for 0.5-3wt% of the total mass of the main phase and the doping phase. The preparation method comprises: batching, ball milling, grinding, high temperature solid phase synthesis, grinding, sieving and other processes. The pigment product is an anatase phase structure, and the obtained product has very good thermal stability. The method provided by the invention is a high temperature solid phase synthesis process, and the equipment and operation are simple and practicable, thus being convenient for large-scale and industrialized production.
Owner:SHAANXI UNIV OF TECH
Technique for producing high activity nickel cake with regeneration of waste material containing nickle as raw material
ActiveCN101063210AIncrease lytic activityReduce voltagePhotography auxillary processesProcess efficiency improvementHigh current densityHigh activity
The invention discloses a preparing method of high activity nickel cake, which comprises the following steps: 1 choosing nickel regenerating waste as raw material; dissolving nickel; 2 filtering selectively; 3 oxidizing Fe2+ to Fe3+; adjusting pH value of system to 4. 0-6. 0 with base; depositing ferric; separating; removing; 4 adjusting pH value of system to 1. 0-6. 0; adding into decoppering agent at 0-60 deg. c; depositing ferric; separating; removing; 5 removing zinc with active nickel carbonate; removing impurity with organic solvent extraction method; óÌ adding soda solution into nickel solution; producing nickel carbonate; ó adding into amido sulfonic acid; allocating electrolytic solution; óÓ adding the electrolytic solution into electrolyser; getting the product. This product possesses high active, which can increase availability ratio of resource.
Owner:XIANGYANG HUATONG CHEM
Hollow porous spherical mixed oxide for lithium ion battery negative electrode and preparation method of hollow porous spherical mixed oxide
ActiveCN104466108AEasy to prepareConducive to industrial mass productionCell electrodesNanotechnologyManganeseSpherical shaped
The invention relates to hollow porous spherical mixed oxide for a lithium ion battery negative electrode and a preparation method of the hollow porous spherical mixed oxide. The material is a uniform nano-mixture of Mn2O3 and NiMn2O4, and a specific chemical formula is NixMn<1-x>O<1.5-0.5x> (x is more than 0 and less than 1 / 3). The preparation method of the hollow porous spherical mixed oxide comprises the steps that based on complexing action of ammonia water and nickel ions, the precipitation speed of nickel carbonate is reduced, so that a spherical structure of the manganese carbonate cannot be destroyed by the nickel carbonate and has a certain modification function on a spherical structure of the manganese carbonate to form a uniform spherical mixture (NixMn<1-x>CO3, x is more than 0 and less than 1 / 3) of the nickel carbonate and the manganese carbonate; the prepared hollow porous spherical mixed oxide for the lithium ion battery negative electrode is obtained by using a high-temperature segmental roasting process. Compared with the prior art, the method is easy to operate and suitable for industrial large batch production; by utilizing the hollow porous spherical mixed oxide, the large-current charging / discharging performance of the lithium ion battery negative electrode can be effectively improved, and the and the cycle life of the lithium ion battery negative electrode can be effectively prolonged.
Owner:SHANGHAI JIAO TONG UNIV
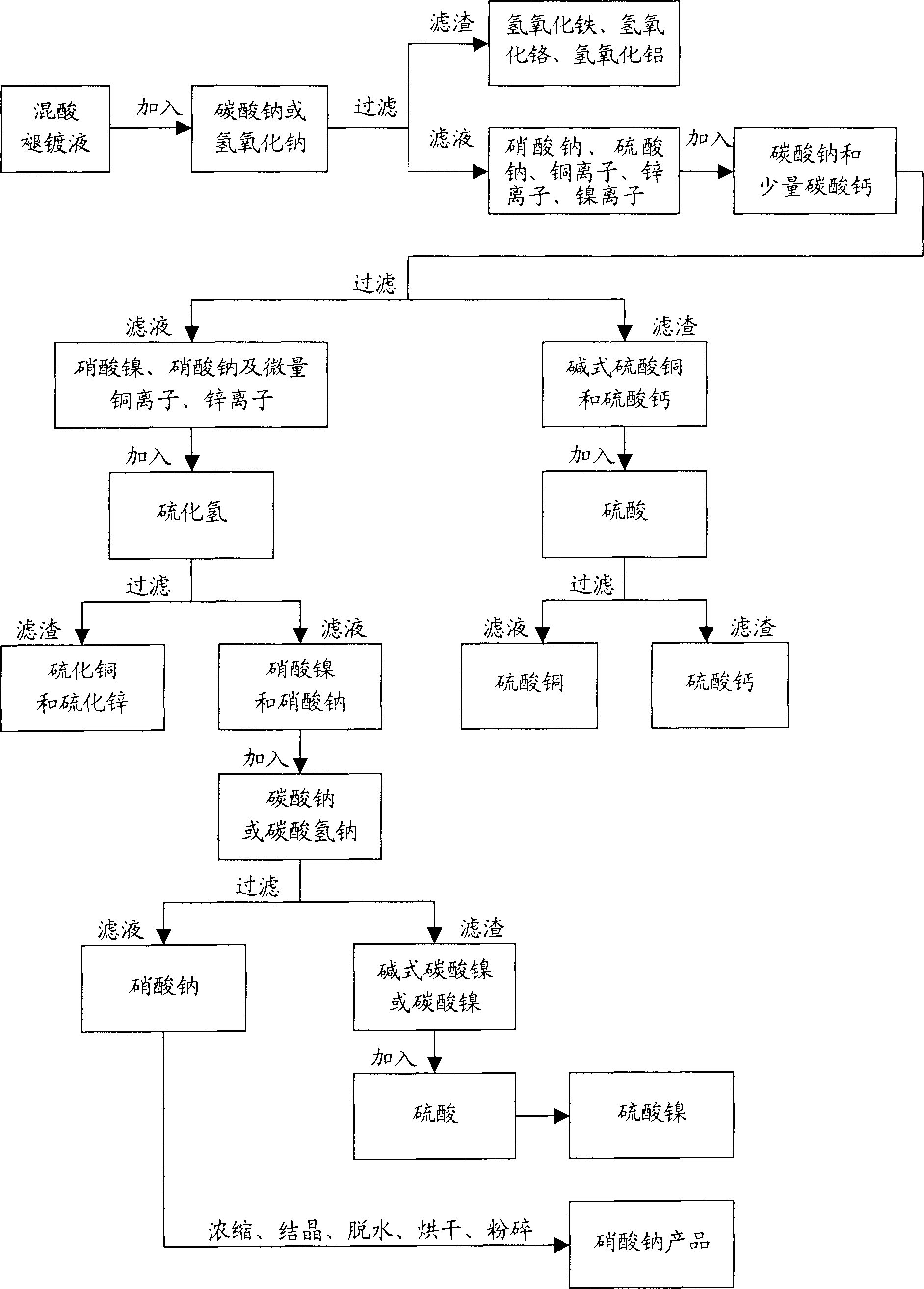
High-efficiency environment-friendly acid-mixed plating removal liquor
InactiveCN1803660AQuality improvementEfficient use ofWaste water treatment from metallurgical processWater/sewage treatment by neutralisationSodium bicarbonateSlag
The invention relates to a high-efficiency environmental-friendly utilization method for acid-mixed removal plating liquid, which comprises: putting the liquid into reaction kettle, adding Na2CO3 or NaOH to neutralize free HNO3 and H2SO4, separating the micro impurity of iron, chrome and aluminum as hydroxide precipitate, and controlling the pH value; filtering, adding Na2CO3 saturated solution for neutralization, and adding a little CaCO3 to deposit copper as CuSO4 and excessive SO4- as CaSO4; filtering, preparing CuSO4 with slag and H2SO4, separating CuS and ZnS with added H2S gas; filtering again, adding Na2CO3 or NaHCO3 to deposit the nickel as basic nickel carbonate or nickel carbonate; filtering, preparing nickel sulfate with slag and sulfuric acid, and preparing NaNO3 product as normal technique. This invention makes full use of the sewage.
Owner:常州市裕和金属材料有限公司
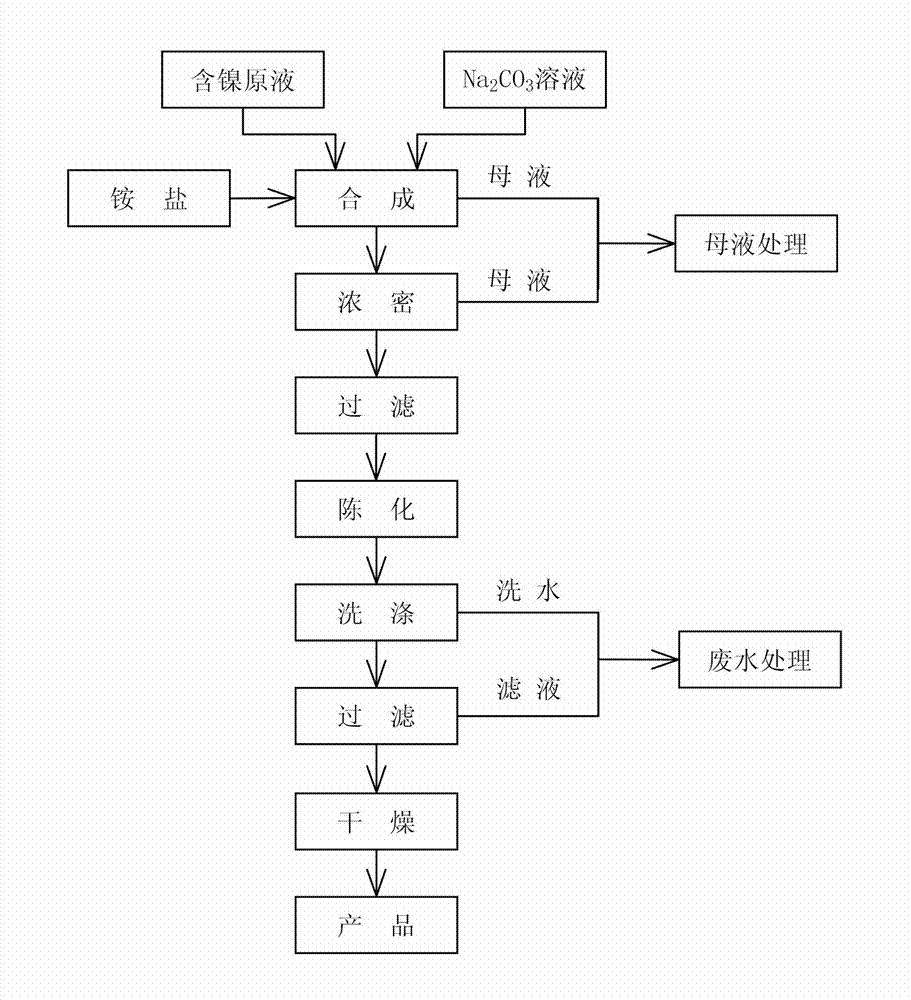
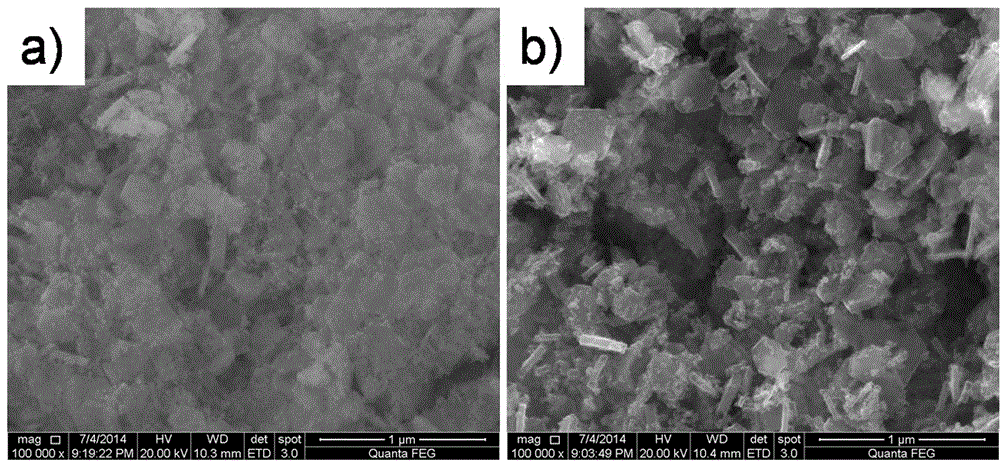
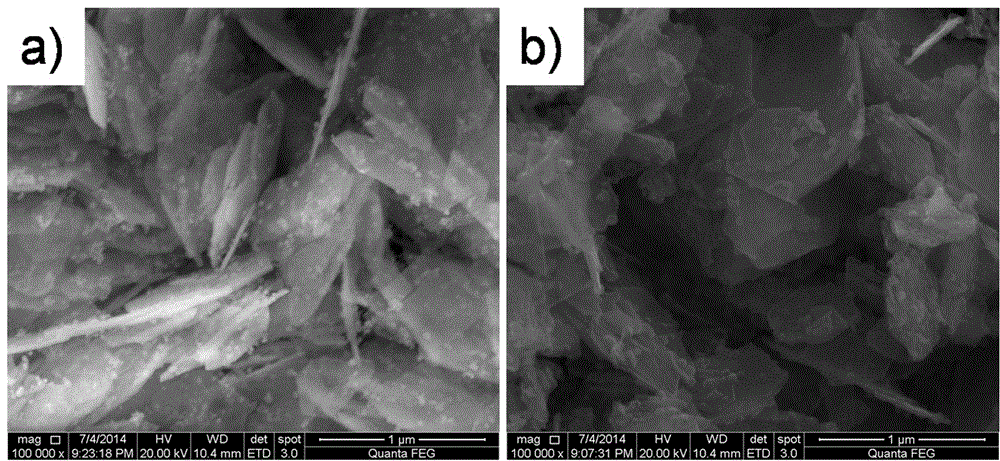
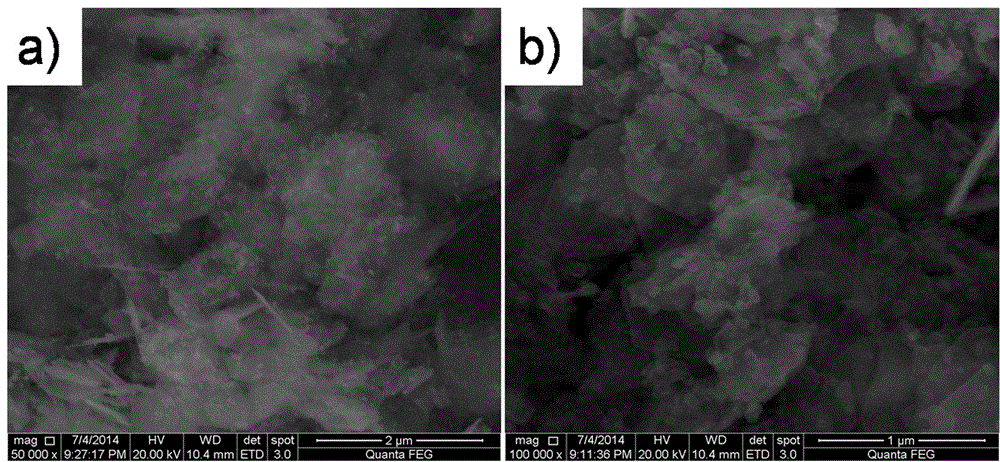
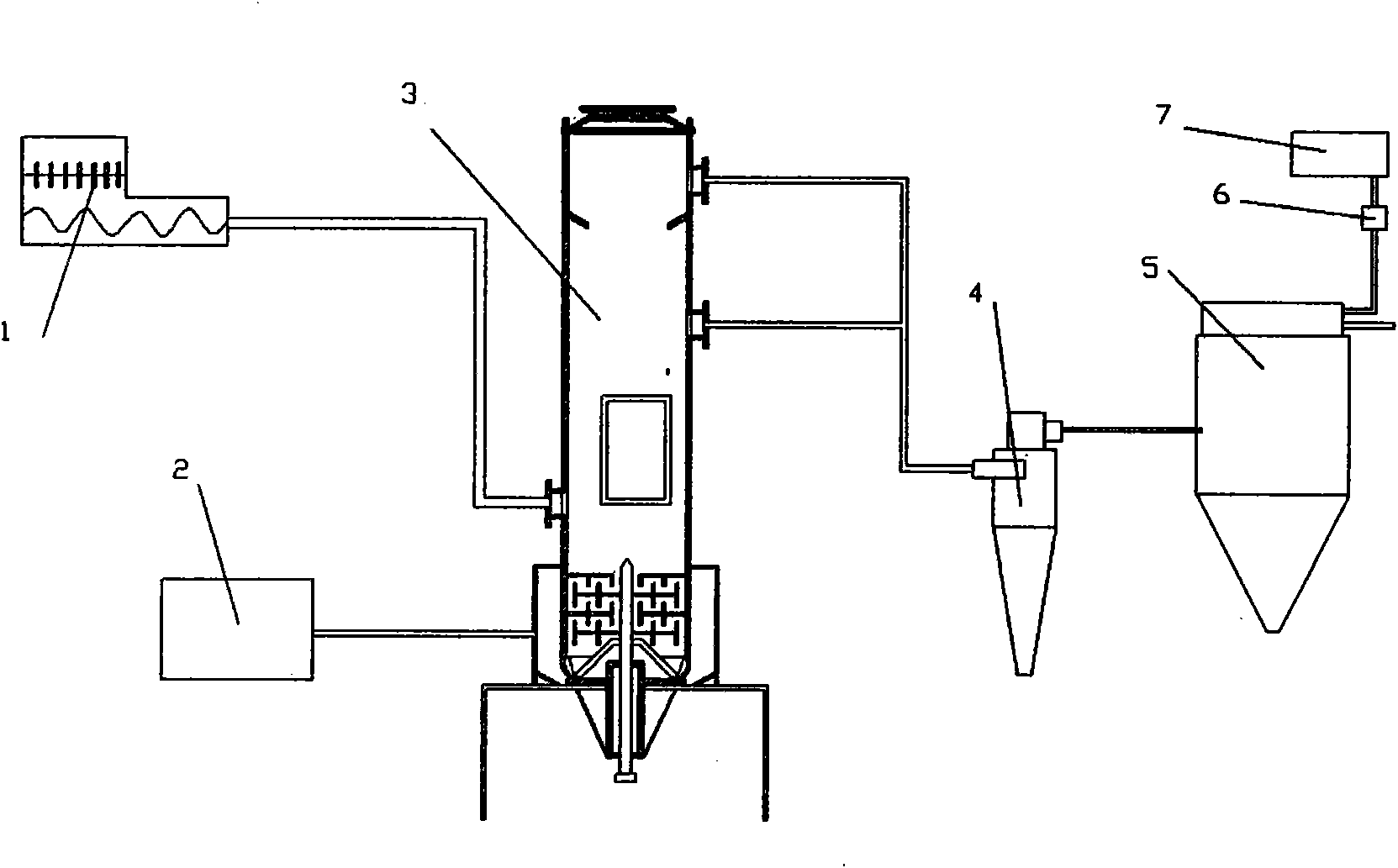
Preparation method of microporous ultrafine high activity nickel carbonate
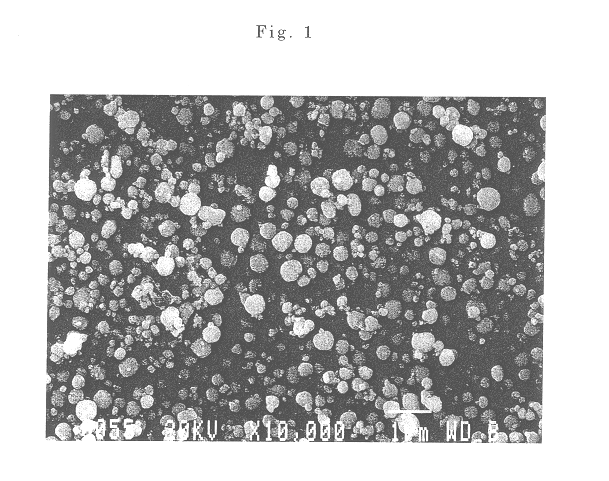
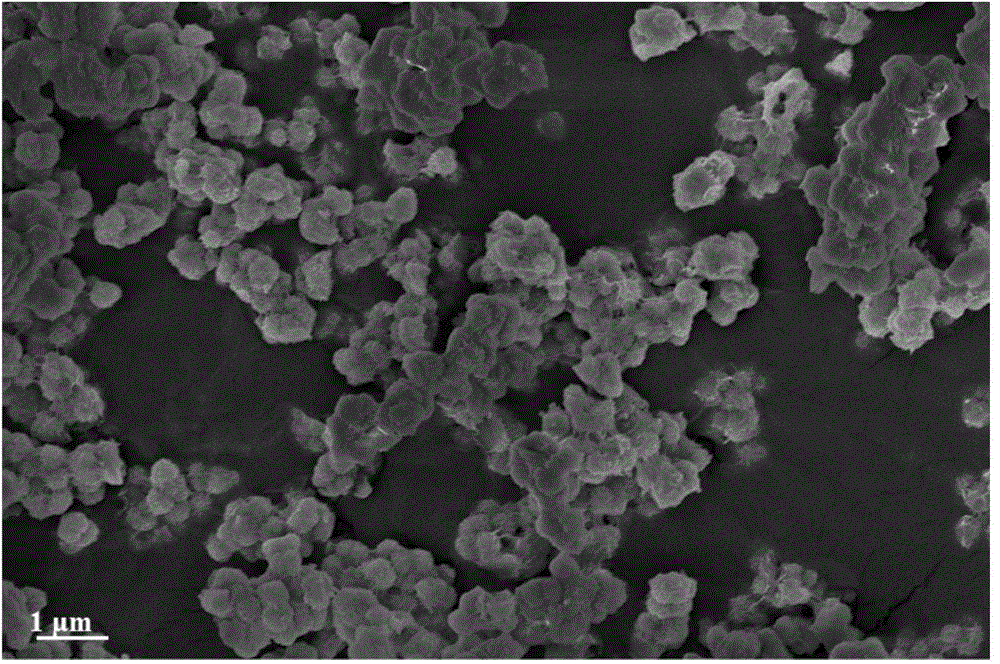
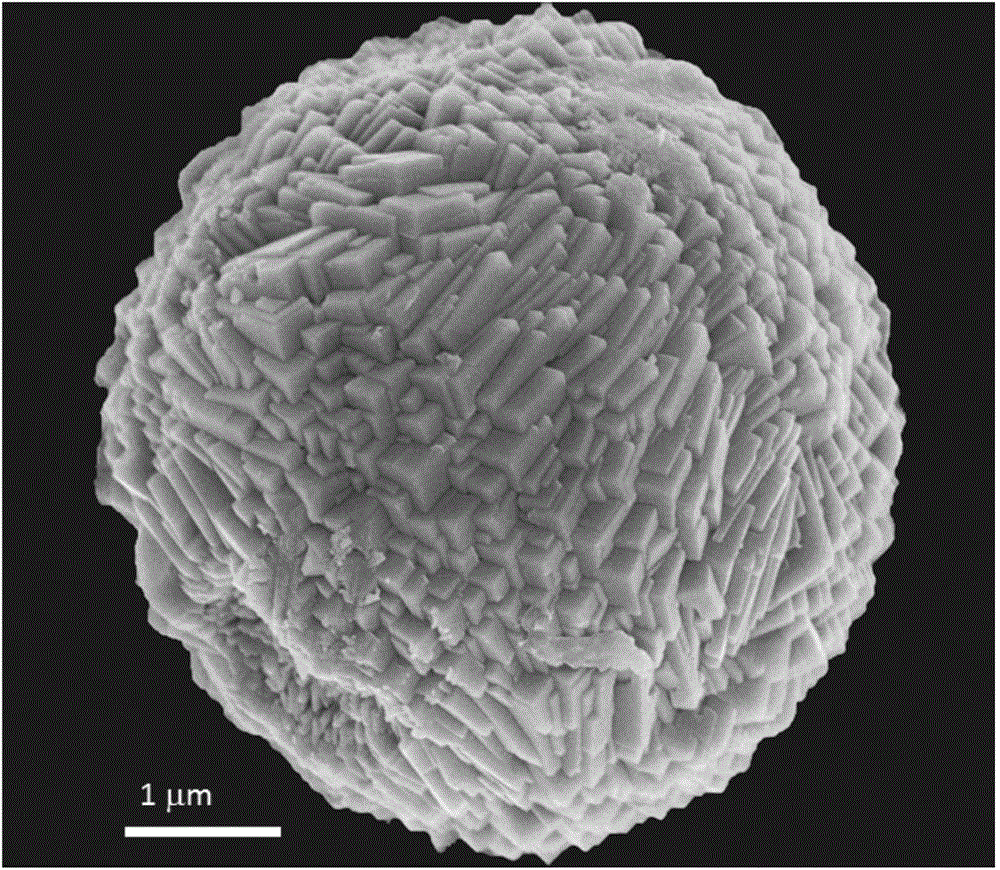
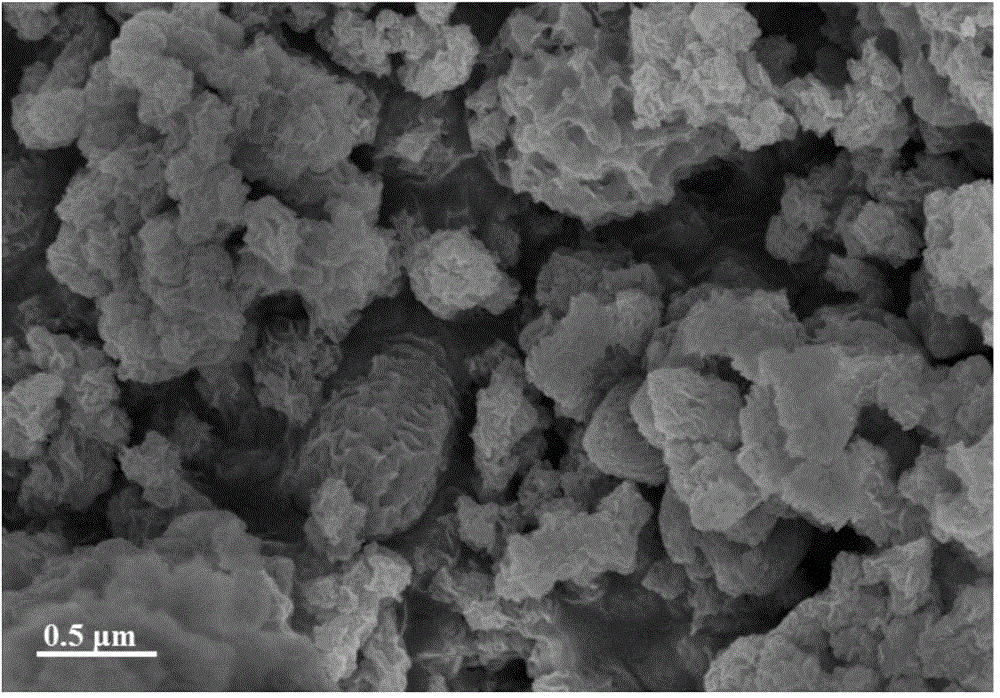
InactiveCN101863520AFine granularityNarrow and uniform particle size distributionNickel carbonatesSem micrographsHigh pressure
The invention discloses a preparation method of microporous ultrafine high activity nickel carbonate, comprising the following steps of adopting mixed acid solution prepared from nitric acid, sulfuric acid and hydrochloride to dissolve metal nickel to prepare composite nickel salts used as raw materials; carrying out precipitation reaction with the composite nickel salts by using mixed percipitant of sodium carbonate, ammonium carbonate or ammonium bicarbonate and sodium hydroxide in a sealed drum type reaction pot under the action of high pressure CO2; and preparing the nickel carbonate by crushing and drying precipitates by adopting an integrative machine of crushing and drying after washing the precipitates. Shown through a particle image analyzer, a full-automatic specific surface area voidage analyzer and an SEM (Scanning Electron Microscope) photograph result, the nickel carbonate has fine size, the particle size of D50 can achieve 1-5 microns, the particle size specific surface area can achieve more than 300m2 / g, the content of carbon dioxide is larger than 20 percent, the nickel carbonate has multiple and small micropores, the powder nickel carbonate with the nickel content of 49-51 percent has narrow and uniform particle size distribution, wherein the particle size of D10 is 0.93 microns, the particle size of D50 is 1.81 microns and the particle size of D90 is 2.82 microns, and the dispersion of the powder nickel carbonate is favorable.
Owner:郑景宜
Fluoride-modified nickel-enriched ternary composite electrode material and preparation method thereof
ActiveCN108807950AAvoid corrosionImprove cycle stabilityCell electrodesSecondary cellsManganeseDissolution
The invention relates to a fluoride-modified nickel-enriched ternary composite electrode material. The fluoride-modified nickel-enriched ternary composite electrode material comprises a nickel-enriched ternary composite electrode material and a modifying layer, wherein the nickel-enriched ternary composite electrode material is prepared from the following raw materials: lithium carbonate, nickel carbonate, cobalt carbonate and manganese carbonate; the molar ratio of the lithium element, the nickel element, the cobalt element and the manganese element is (1.0-1.1):(0.6-0.8):(0.2-0.1):(0.2-0.1);the modifying layer is metal fluoride; the metal fluoride accounts for 1-5% of the mass percentage of the total electrode material. The invention further relates to a preparation method of the composite electrode material. After the nickel-enriched ternary electrode material is modified by the fluoride, dissolution of metal ions in an active material can be prevented, corrosion of the active material by an electrolyte can be resisted, the surface impedance can be reduced, the cycle stability of the material can be improved, and the problems of poor stability and fast capacity attenuation of the nickel-cobalt-manganese ternary electrode material can be solved.
Owner:ENERGY RESOURCES INST HEBEI ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com