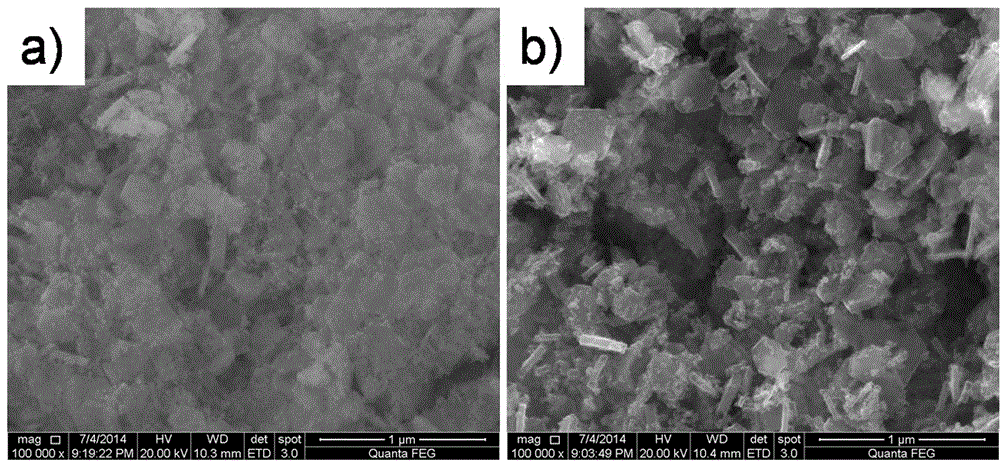
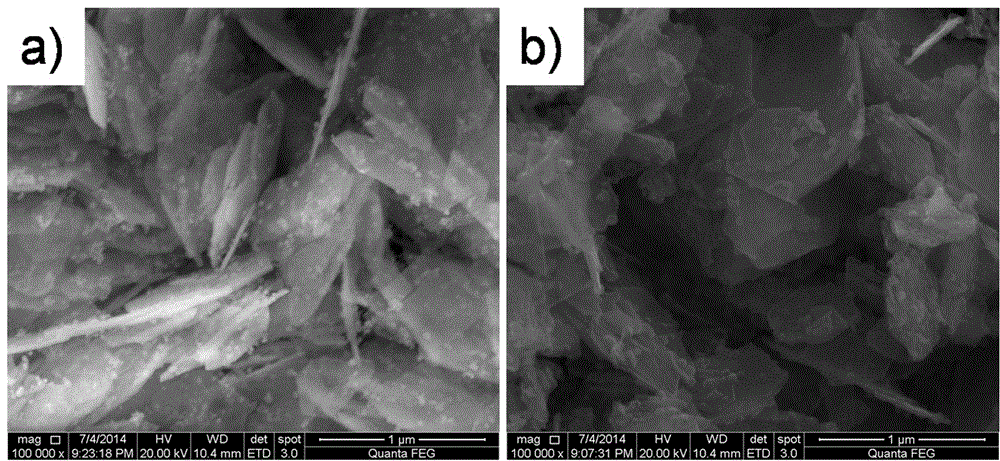
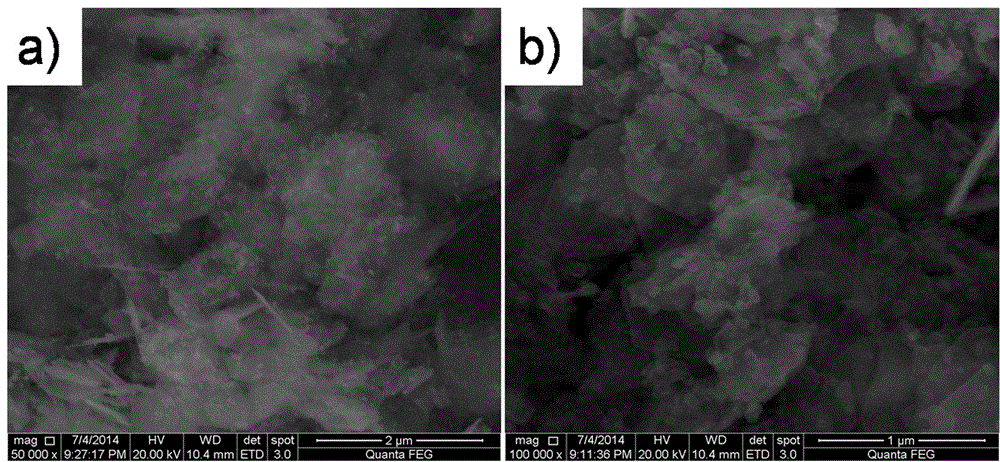
Preparation method of dye-sensitized solar battery cobalt-nickel sulfide counter electrode
A solar cell and cobalt-nickel sulfide technology, which is applied in the field of electrode preparation, cobalt, and nickel sulfide preparation, can solve the problems of complex preparation process and inability to prepare a large area, and achieve simple preparation process, high mechanical strength, and good electrocatalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Weigh 2.4mmol Ni(OAc) respectively 2 4H 2 O and 4.8 mmol CoCl 2 ·6H 2 O was put into 40 mL of deionized water, placed in a magnetic stirrer and stirred for 30 min to dissolve to prepare A solution. Weigh 24mmol NaOH into 40mL deionized water, place in a magnetic stirrer and stir to dissolve for 30min to prepare B solution. Under the condition of rapidly stirring solution B, slowly pour solution A into solution B, and continue stirring for 1 h after the addition. After that, transfer the mixed reaction suspension of A and B to a stainless steel reaction kettle with a polytetrafluoroethylene liner, seal it and put it in a hydrothermal furnace, slowly rise from room temperature to 160°C and react at 160°C for 10h . After the reaction, it was cooled to room temperature with the furnace and taken out, and the obtained brown-black cobalt-nickel precursor was centrifugally washed 3 times with deionized water.
[0023] Step 2: After washing, disperse it into 240mL...
Embodiment 2
[0027] Step 1: Weigh 2.4mmol Ni(OAc) respectively 2 4H 2 O and 4.8 mmol CoCl 2 ·6H 2 O was put into 40 mL of deionized water, placed in a magnetic stirrer and stirred for 30 min to dissolve to prepare A solution. Weigh 24mmol NaOH into 40mL deionized water, place in a magnetic stirrer and stir to dissolve for 30min to prepare B solution. Under the condition of rapidly stirring solution B, slowly pour solution A into solution B, and continue stirring for 1 h after the addition. Afterwards, transfer the mixed reaction suspension of A and B to a stainless steel reaction kettle with a polytetrafluoroethylene liner, seal it and put it in a hydrothermal furnace, slowly rise from room temperature to 160°C and react at 160°C for 20h . After the reaction, it was cooled to room temperature with the furnace and taken out, and the obtained brown-black cobalt-nickel precursor was centrifugally washed 3 times with deionized water.
[0028] Step 2: After washing, disperse it into 240mL...
Embodiment 3
[0037] Step 1: Weigh 2.4mmol Ni(OAc) respectively 2 4H 2 O and 4.8 mmol CoCl 2 ·6H 2 O was put into 40 mL of deionized water, placed in a magnetic stirrer and stirred for 30 min to dissolve to prepare A solution. Weigh 16mmolNaOH and 8mmolNa 2 CO 3 Put it into 40mL of deionized water, place it in a magnetic stirrer and stir and dissolve for 30min to prepare B solution. Under the condition of rapidly stirring solution B, slowly pour solution A into solution B, and continue stirring for 1 h after the addition. After that, transfer the mixed reaction suspension of A and B to a stainless steel reaction kettle with a polytetrafluoroethylene liner, seal it and put it in a hydrothermal furnace, slowly rise from room temperature to 160°C and react at 160°C for 10h . After the reaction, it was cooled to room temperature with the furnace and taken out, and the obtained brown-black cobalt-nickel precursor was centrifugally washed 3 times with deionized water.
[0038] Step 2: Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com