Method for continuously compounding high-purity alkali nickel carbonate
A technology of nickel carbonate and soda ash, applied in the direction of nickel carbonate, etc., can solve the problems such as difficult removal of impurities, high content of impurities, unsatisfactory, etc., and achieve the effect of fewer defects, high purity of finished products, and complete structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
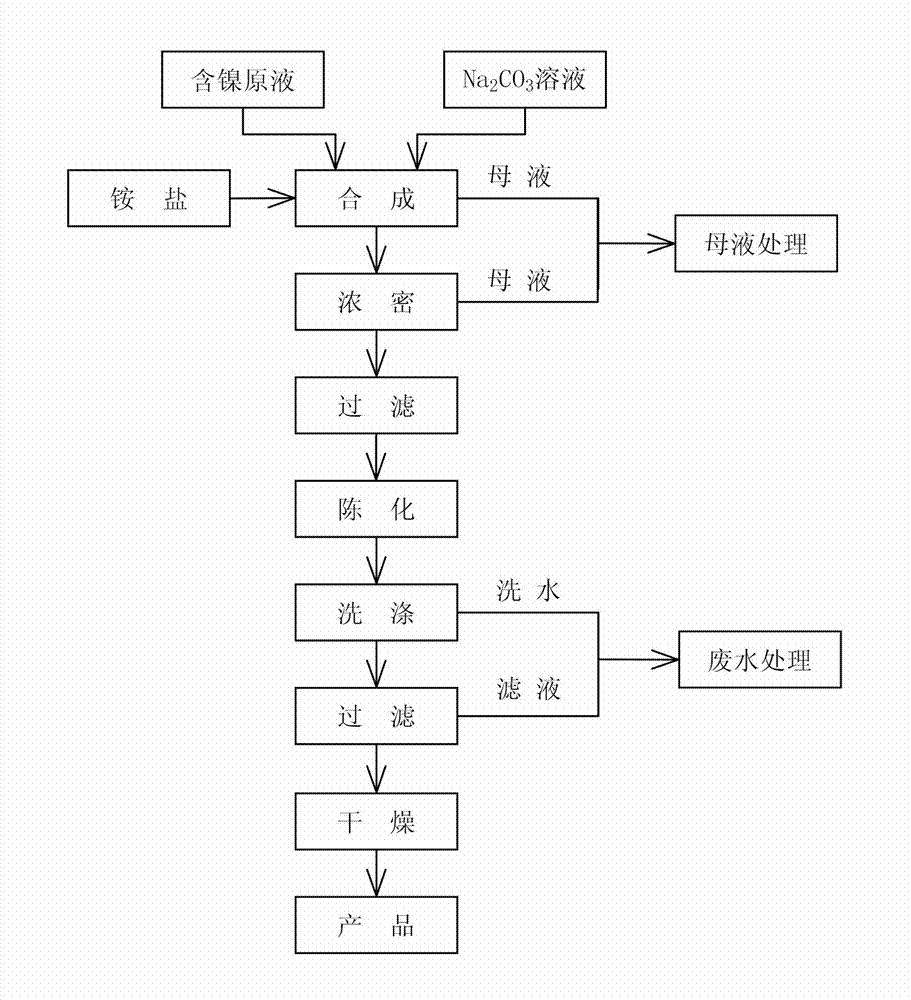
[0026] a kind of like figure 1 Shown the method for the continuous synthesis of high-purity basic nickel carbonate of the present invention, may further comprise the steps:
[0027] (1) Select nickel electrolyte to prepare nickel-containing stock solution, and Ni in the nickel-containing stock solution 2+The concentration is controlled at 50g / L, and the Cl in the nickel stock solution - and SO 4 2- The molar ratio is controlled to be 1:1.18 (see Table 1); select industrial grade Na 2 CO 3 (the purity of which is not less than 98.8%) is formulated into Na with a concentration of 120g / L 2 CO 3 Solution; the prepared nickel-containing stock solution, ammonium salt (at least one of ammonium chloride, ammonium sulfate, ammonium nitrate) and precipitant Na 2 CO 3 Continuously transported to the multifunctional reactor for continuous synthesis reaction, the addition flow rate of the nickel-containing stock solution during continuous transport was 500.0 mL / h, Na 2 CO 3 The a...
Embodiment 2
[0036] a kind of like figure 1 Shown the method for the continuous synthesis of high-purity basic nickel carbonate of the present invention, may further comprise the steps:
[0037] (1) Select nickel electrolyte to prepare nickel-containing stock solution, and Ni in nickel-containing stock solution 2+ The concentration is controlled at 70g / L, and the Cl in the nickel stock solution - and SO 4 2- The molar ratio of the control is 1:1.22 (see table 3 below); another choice of industrial grade Na 2 CO 3 (the purity of which is not less than 98.8%) is formulated into Na with a concentration of 130g / L 2 CO 3 Solution; the prepared nickel-containing stock solution, ammonium salt (at least one of ammonium chloride, ammonium sulfate, ammonium nitrate) and precipitant Na 2 CO 3 Continuously transported to the multifunctional reactor for continuous synthesis reaction, the addition flow rate of the nickel-containing stock solution during continuous transport was 500.0 mL / h, Na 2...
Embodiment 3
[0046] a kind of like figure 1 Shown the method for the continuous synthesis of high-purity basic nickel carbonate of the present invention, may further comprise the steps:
[0047] (1) Select nickel electrolyte to prepare nickel-containing stock solution, and Ni in nickel-containing stock solution 2+ The concentration is controlled at 70g / L, and the Cl in the nickel stock solution - and SO 4 2- The molar ratio is controlled at 1:1.40 (see Table 5 below); another industrial grade Na 2 CO 3 (the purity of which is not less than 98.8%) is formulated into Na with a concentration of 100g / L 2 CO 3 Solution; the prepared nickel-containing stock solution, ammonium salt (at least one of ammonium chloride, ammonium sulfate, ammonium nitrate) and precipitant Na 2 CO 3 Continuously transported to the multifunctional reactor for continuous synthesis reaction, the addition flow rate of the nickel-containing stock solution during continuous transport was 700.0 mL / h, Na 2 CO 3 The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com