Preparation method of microporous ultrafine high activity nickel carbonate
A microporous, nickel carbonate technology, applied in the direction of nickel carbonate, to achieve the effect of narrow particle size distribution and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 drum type reaction tank
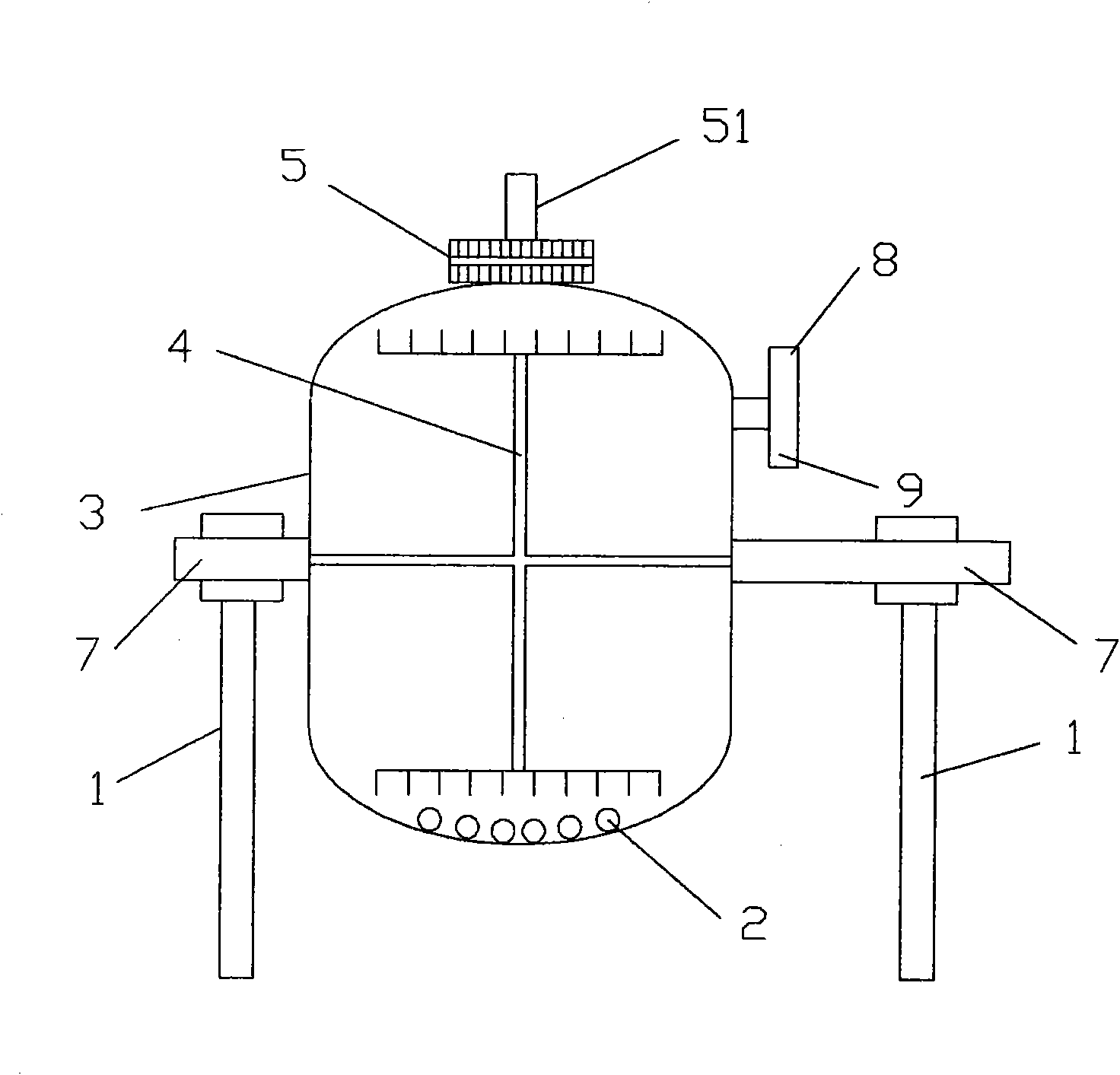
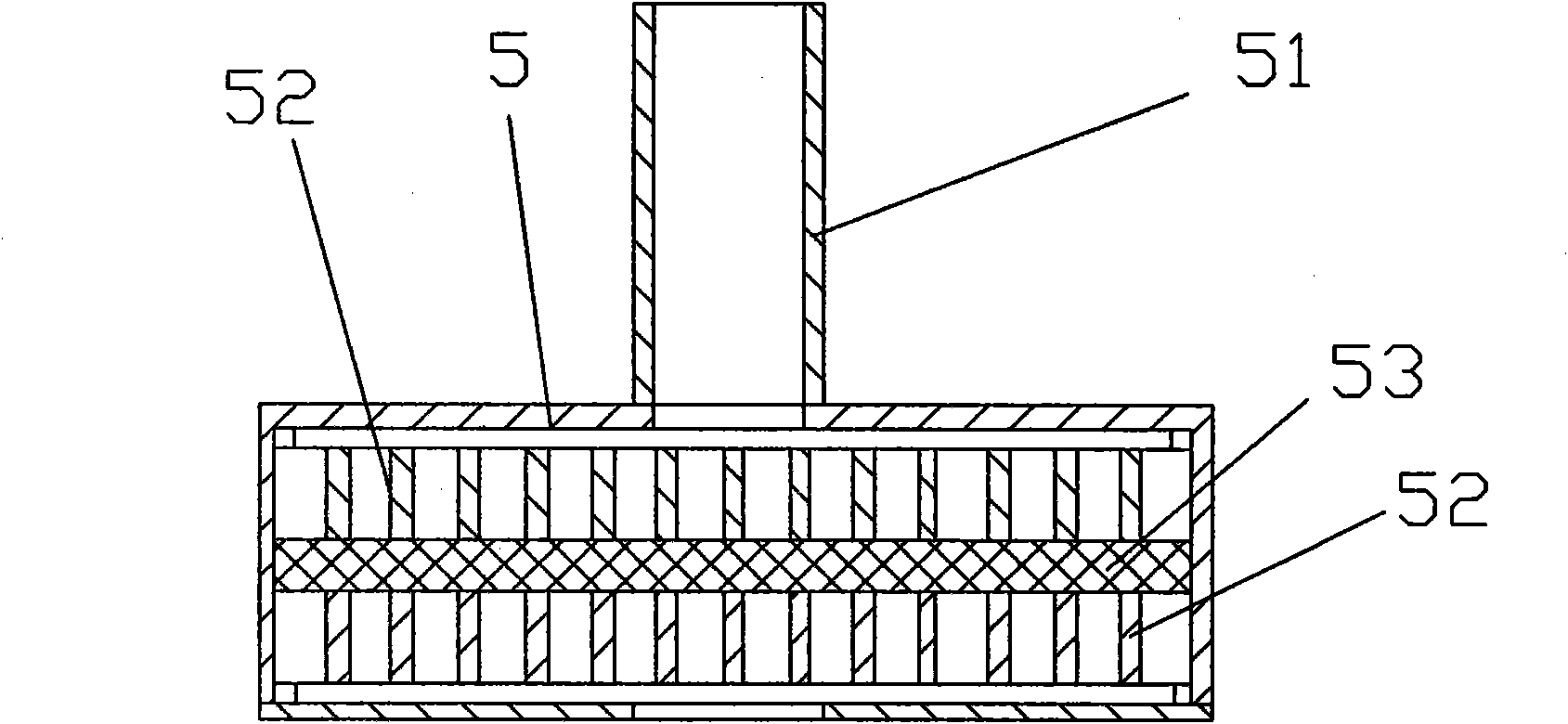
[0040] see figure 1 , 2 , a drum-type reaction tank, comprising: a reaction tank body 3, a horizontal bar 7, a support frame 1, a tank cover 5, a feed port 8 with a valve, the body 1 is circular, and the horizontal bar 7 is integrated with the reaction tank body 3, The horizontal bar 7 is fixed on the support frame 3, the tank cover 5 is on the top of one end of the reaction tank body 3, the horizontal bar is connected with the driving device, the reaction tank body 3 is equipped with a fixed comb-type plow stirring device 4, and the body 3 is also equipped with There is an air inlet 9 with a valve, and a filter device is provided in the tank cover; zirconia balls 2 are also housed in the reaction tank; the tank cover 5 is provided with a discharge port 51 with a valve for discharging, and the filter device is two A filter cloth 53 is sandwiched between the layers of screen cloth 52 .
Embodiment 2
[0041] Example 2 Drying and pulverizing machine
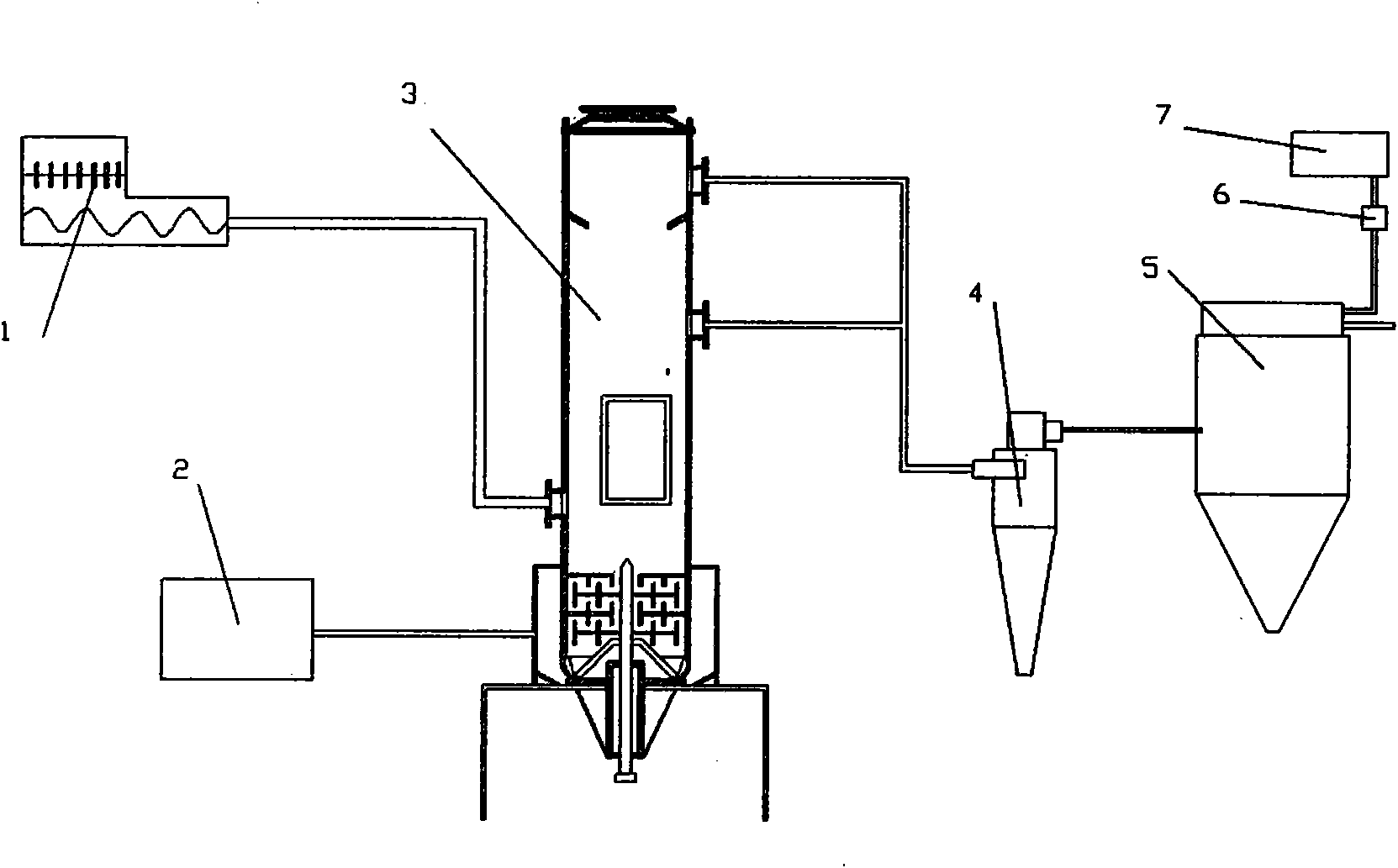
[0042] Such as image 3 , 4 As shown in , 5, the drying and crushing integrated machine is composed of a feeder 1, a hot air blower 2, a cyclone drying and crushing tower 3, a cyclone collecting tower 4, a dust collecting tower 5, an induced draft fan 6, a heat exchanger 7, and a tower base 9 Composition, the described whirlwind drying crushing tower 3 is made up of feed inlet 315, hot air pressure stabilizing chamber inlet 38, several hot air inlets 37, hot air agitator, discharge port 312 and discharge port 313, and the hot air pressure stabilizing chamber is The cavity formed around the base of the body 31 of the cyclone drying and crushing tower 3, the feed port 315 is located in the middle of the body 31 of the cyclone drying and crushing tower 3, above the agitator, connected with the feeder 1, and the hot air inlet 38 is located on the body On the bottom side wall of 31, it is connected with hot air blower 2, and disch...
Embodiment 3
[0045] see figure 1 , 2 ;
[0046] 1. According to the ratio of pure nitric acid, sulfuric acid and hydrochloric acid by weight: 4~8:2~6:1~3, the mixed acid solution prepared is then dissolved in the mixed acid solution to prepare the composite nickel salt;
[0047] 2. Mix uniformly according to the ratio of sodium carbonate, ammonium carbonate or ammonium bicarbonate and sodium hydroxide by weight: 5.0-8.5: 1.0-4.5: 0.05-0.1, and prepare a mixed precipitant;
[0048] 3. Ratio by weight: composite nickel salt: mixed precipitant=1: 1.1-1.3 ratio, with a concentration of 4-12% composite nickel salt aqueous solution and a concentration of 8-17% mixed precipitant aqueous solution, through the feeder with valve Port 8, parallel flow pump into the reaction tank, close the feeding port 8, and feed CO at high pressure through the gas filling port 9 2 At the same time, after opening the discharge port 51 with valve to discharge the air, close the discharge port 51 with valve. Under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com