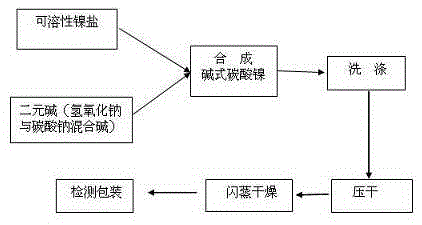
Process for synthesis of basic nickel carbonate from diacidic base
A technology of nickel carbonate and binary base, applied in the direction of nickel carbonate, etc., can solve problems such as non-recyclable, high volatility of ammonia, and hazards to the production environment, and achieve the effects of saving raw material costs and increasing OH- concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
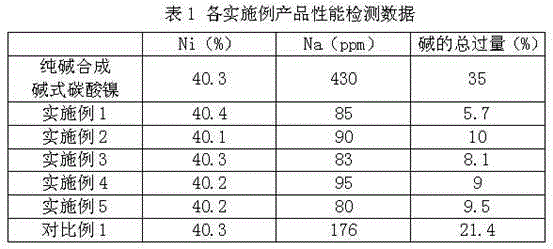
Examples
Embodiment 1
[0027] A process for synthesizing basic nickel carbonate with a binary base, the specific steps are:
[0028] (1) Configuration solution:
[0029] The nickel sulfate is configured into 2000L of an aqueous solution containing 60g / L of nickel and 3000L of mixed alkali, which contains 15g / L of sodium hydroxide and 56g / L of sodium carbonate. After the dissolution is complete, the two solutions will be filtered with a chamber filter respectively;
[0030] (2) Synthesis:
[0031] The two solutions of step (1) are synthesized by the method of adding the solutions. During the process, the pH is controlled to be 7.8, and when the synthesis is completed, the pH is 8.9 to obtain a basic nickel carbonate slurry. The synthesis reaction equation is:
[0032] ;
[0033] (3) Aging:
[0034] The basic nickel carbonate slurry obtained in step (2) continues to be stirred and aged for 1 hour;
[0035] (4) Washing:
[0036] Use a horizontal belt filter to wash the basic nickel carbonate obtained in step (3) to...
Embodiment 2
[0042] A process for synthesizing basic nickel carbonate with a binary base, the specific steps are:
[0043] (1) Configuration solution:
[0044] The nickel sulfate is configured into 1500L of an aqueous solution containing 80g / L of nickel and 3000L of mixed alkali, which contains 20g / L of sodium hydroxide and 53g / L of sodium carbonate. After the dissolution is complete, the two solutions will be filtered with a chamber filter respectively;
[0045] (2) Synthesis:
[0046] The two solutions in step (1) are synthesized by the method of adding the solution. During the process, the pH is controlled to be 8.0, and the pH is 8.6 when the synthesis is completed to obtain a basic nickel carbonate slurry. The synthesis reaction equation is:
[0047] ;
[0048] (3) Aging:
[0049] The basic nickel carbonate slurry obtained in step (2) is continuously stirred and aged for 1.5 hours;
[0050] (4) Washing:
[0051] Use a horizontal belt filter to wash the basic nickel carbonate obtained in step (3) t...
Embodiment 3
[0057] A process for synthesizing basic nickel carbonate with a binary base, the specific steps are:
[0058] (1) Configuration solution:
[0059] The nickel sulfate is configured into 1200L of an aqueous solution containing 100g / L of nickel and 2500L of mixed alkali, which contains 20g / L of sodium hydroxide and 67g / L of sodium carbonate. After the dissolution is complete, the two solutions will be filtered with a chamber filter respectively;
[0060] (2) Synthesis:
[0061] The two solutions in step (1) are subjected to a synthesis reaction. The solution adding method is parallel addition. During the process, the pH=8.3 is controlled, and the pH=8.9 when the synthesis is completed, to obtain the basic nickel carbonate slurry. The synthesis reaction equation is:
[0062] ;
[0063] (3) Aging:
[0064] The basic nickel carbonate slurry obtained in step (2) is continuously stirred and aged for 1.2 hours;
[0065] (4) Washing:
[0066] Use a horizontal belt filter to wash the basic nickel car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com