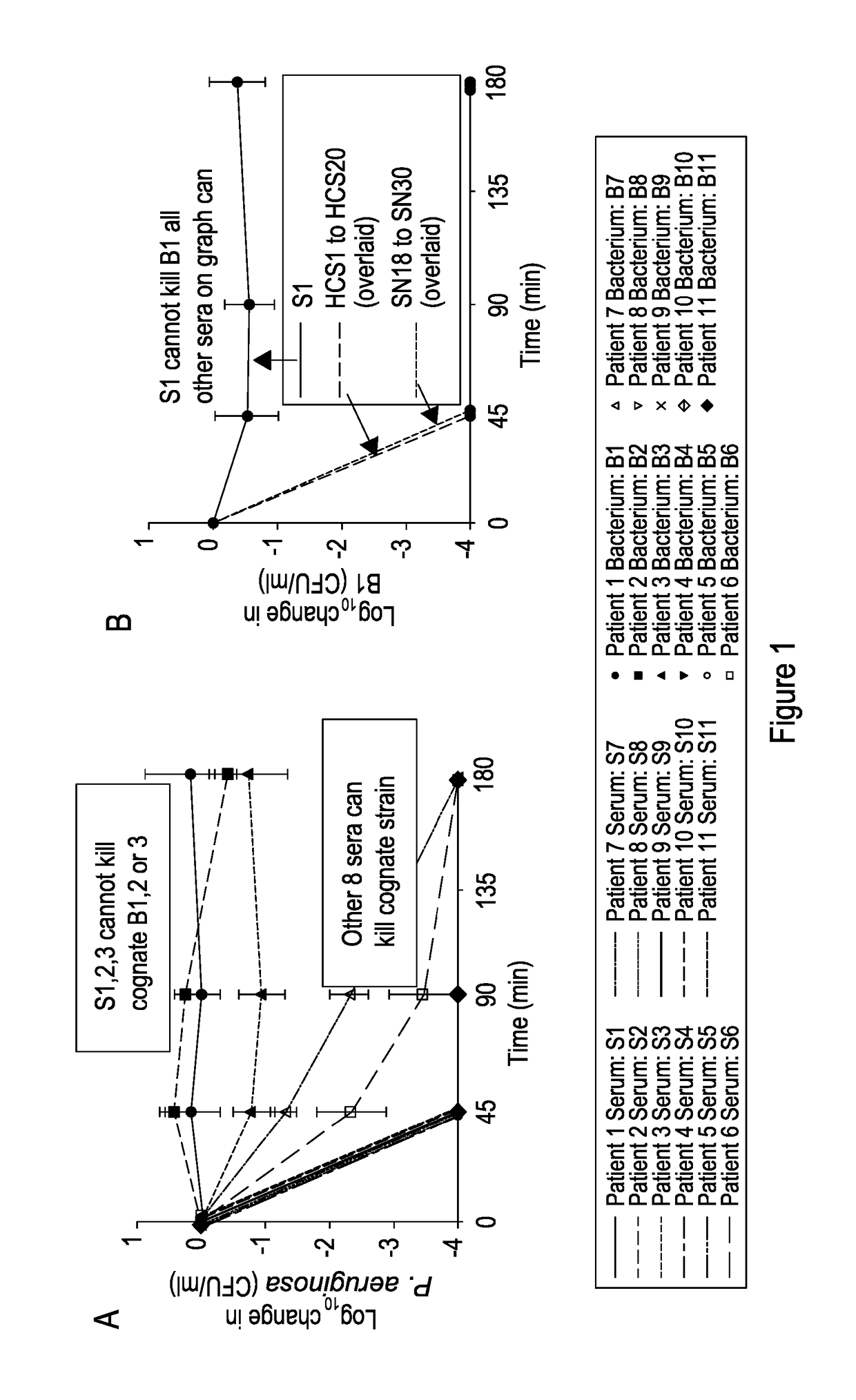
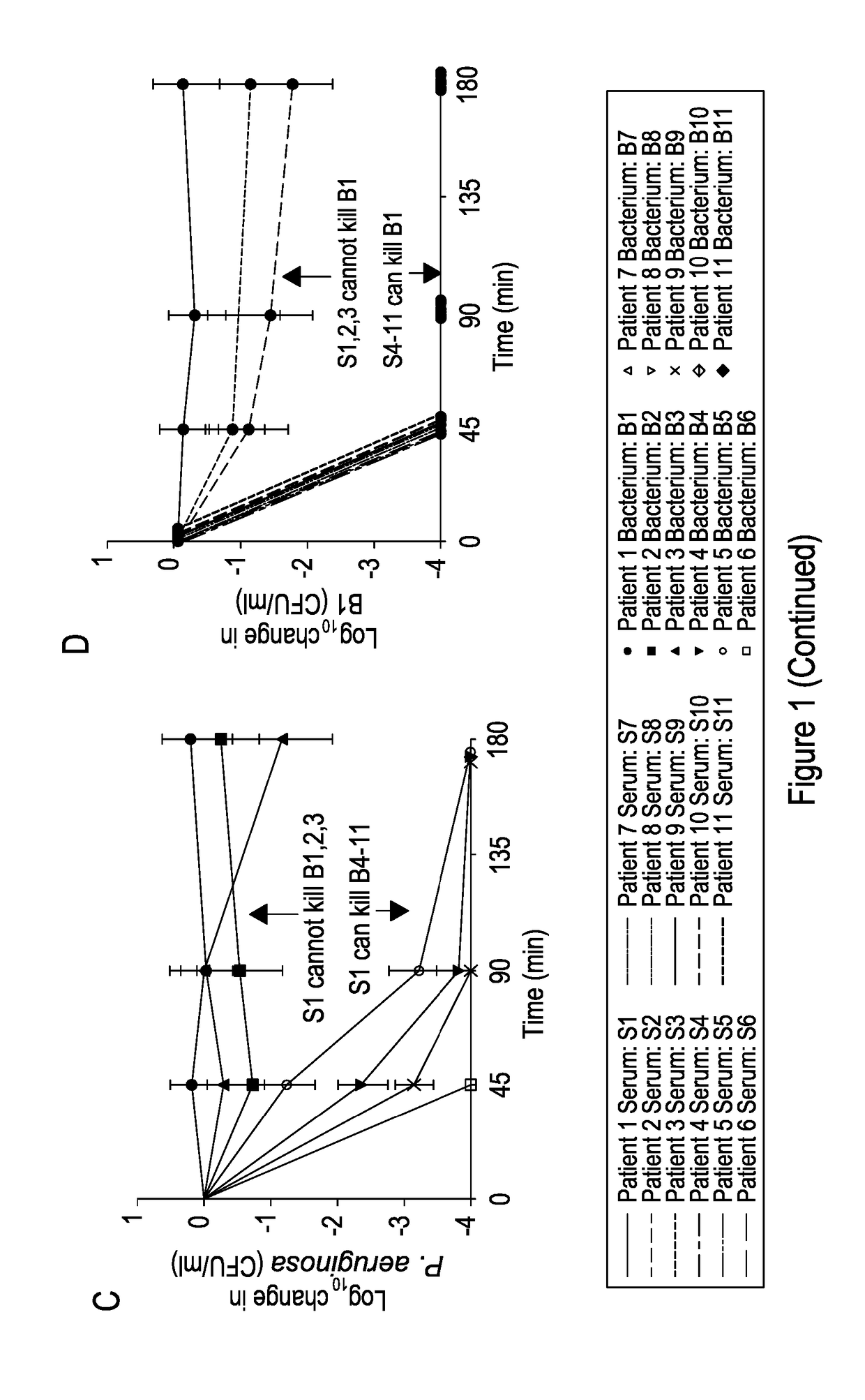
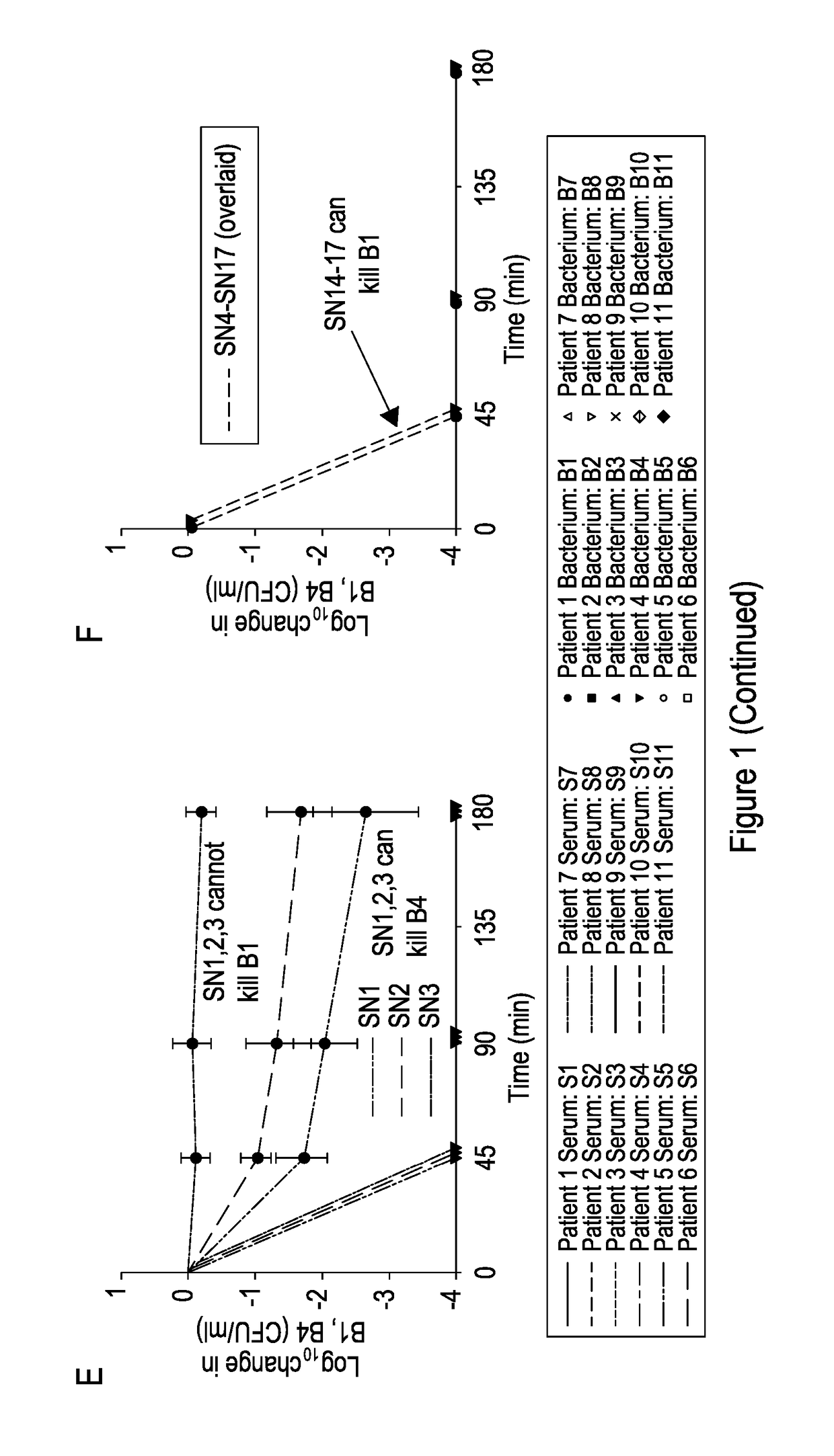
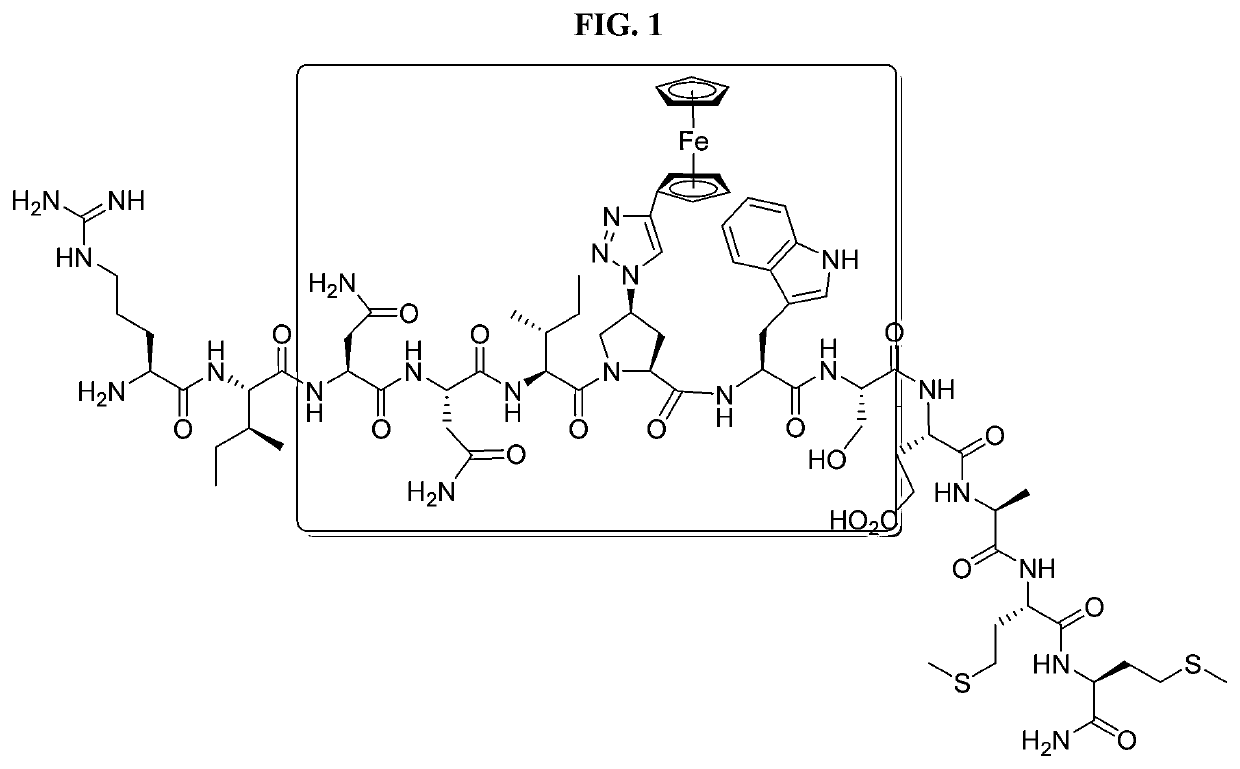
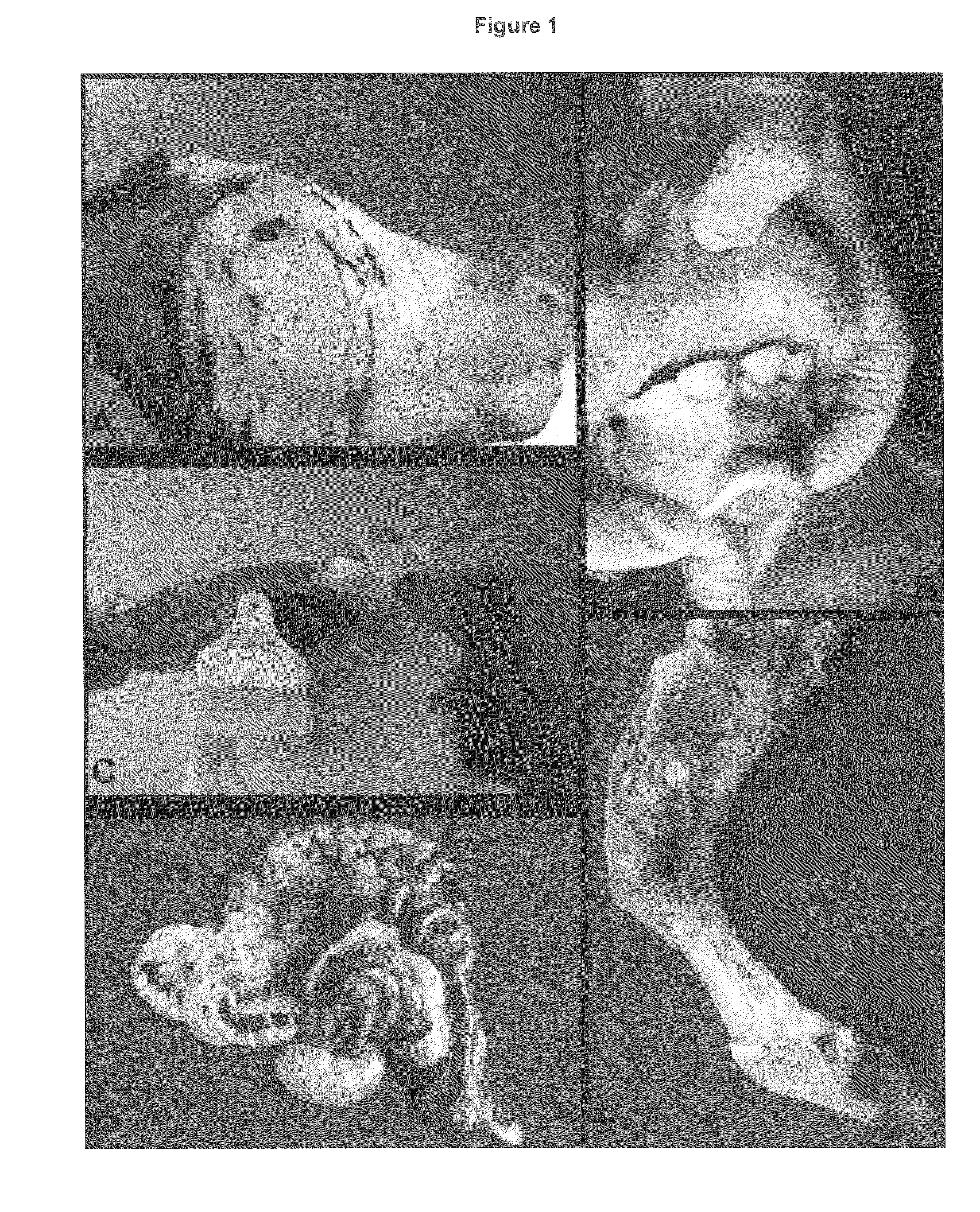Patents
Literature
30results about How to "Prevent and reduce infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Implantable medical device having biologically active polymeric casing
InactiveUS6968234B2Prevent and reduce infectionHeart defibrillatorsInternal electrodesPolyolefinActive agent
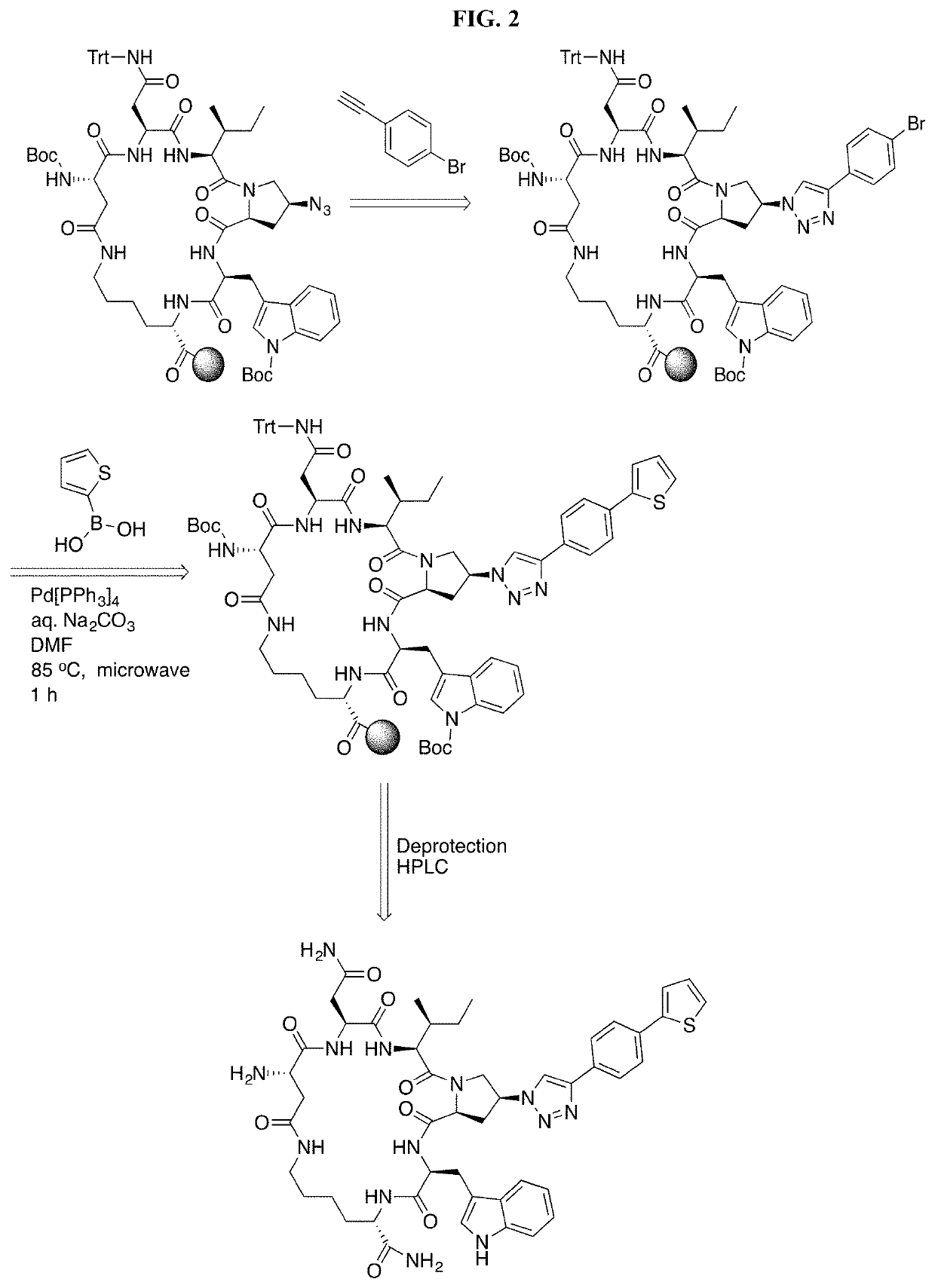
An implantable medical device has a medical unit, such as a pacemaker lead, and a casing at least partially enclosing the medical unit. The casing is formed of a base polymer such as a polyurethane, a polyurethane copolymer, a fluoropolymer and a polyolefin or a silicone rubber. The casing has biologically active agents covalently bonded to the base polymer. The biologically active agents can be attached to the base polymer as surface active end groups. As an alternative, the biologically active may be attached to a backbone the base polymer. As yet a further alternative, the biologically active agents may be attached to surface modifying end groups, which are in turn attached to the base polymer. Examples of suitable biologically active agents are microbial peptide agents, detergents, non-steroidal anti-inflammatory drugs, cations, amine-containing organosilicones, diphosphonates, fatty acids and fatty acid salts.
Owner:MEDTRONIC INC
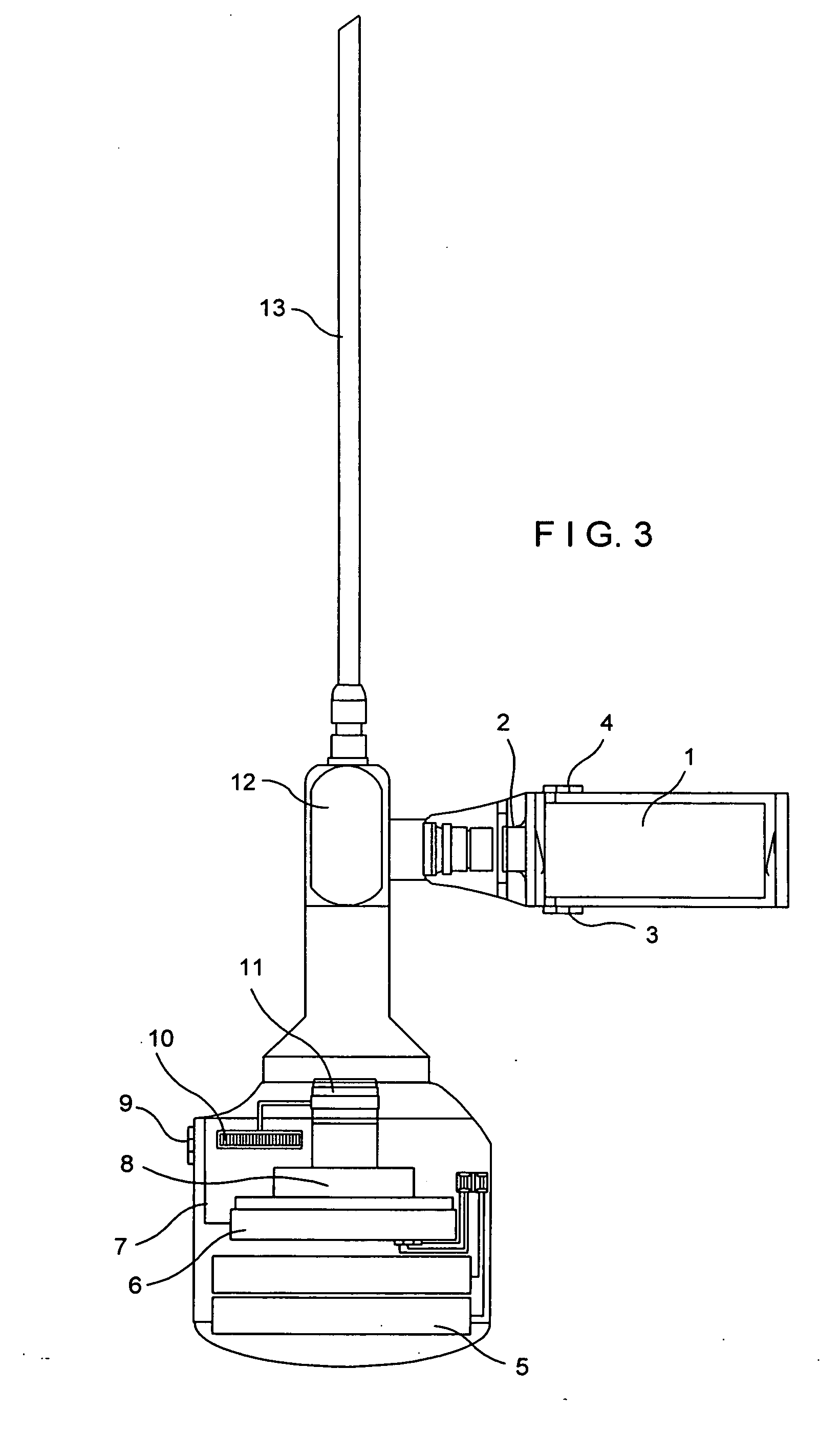
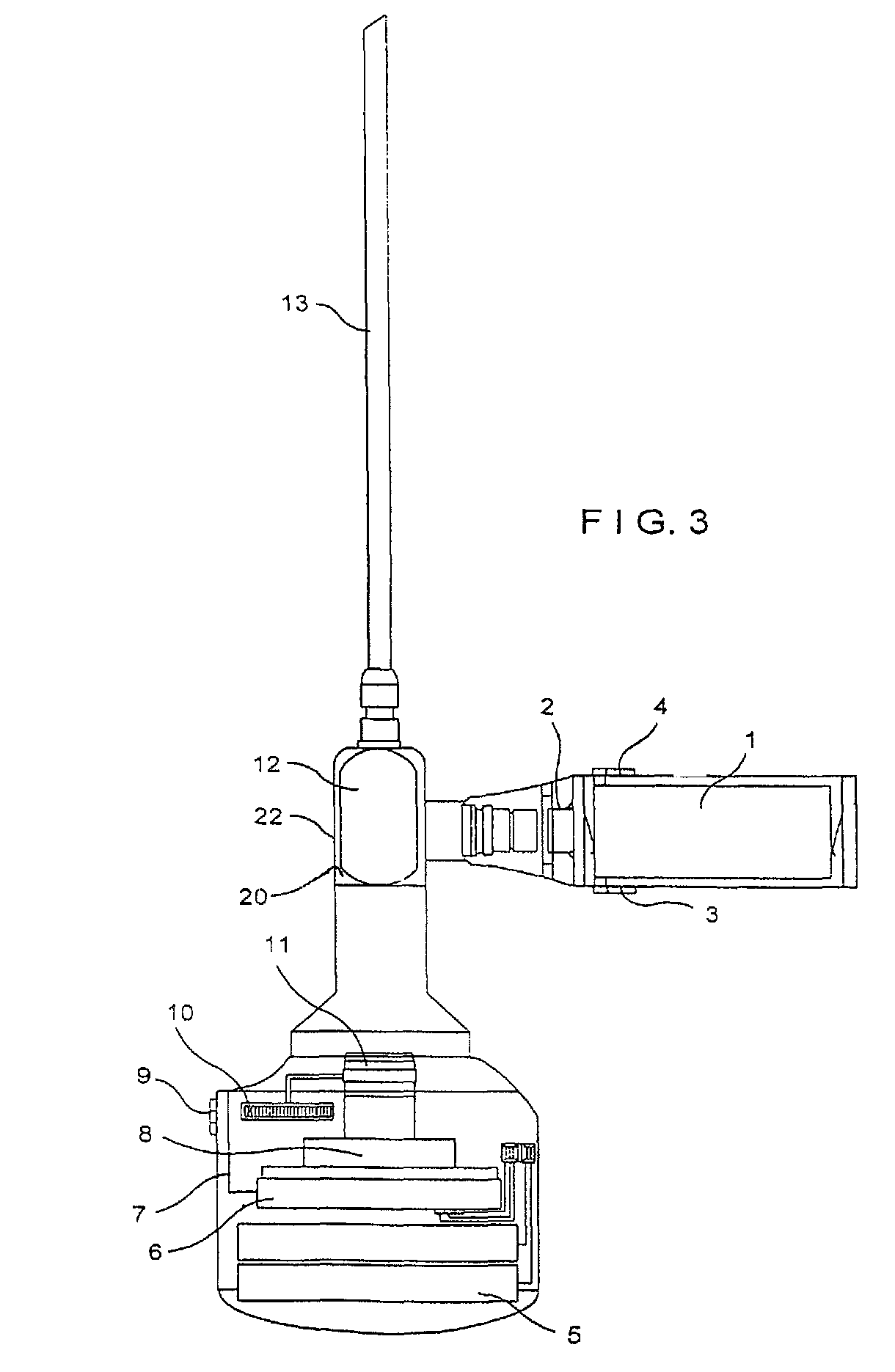
Cable-free arthroscopy
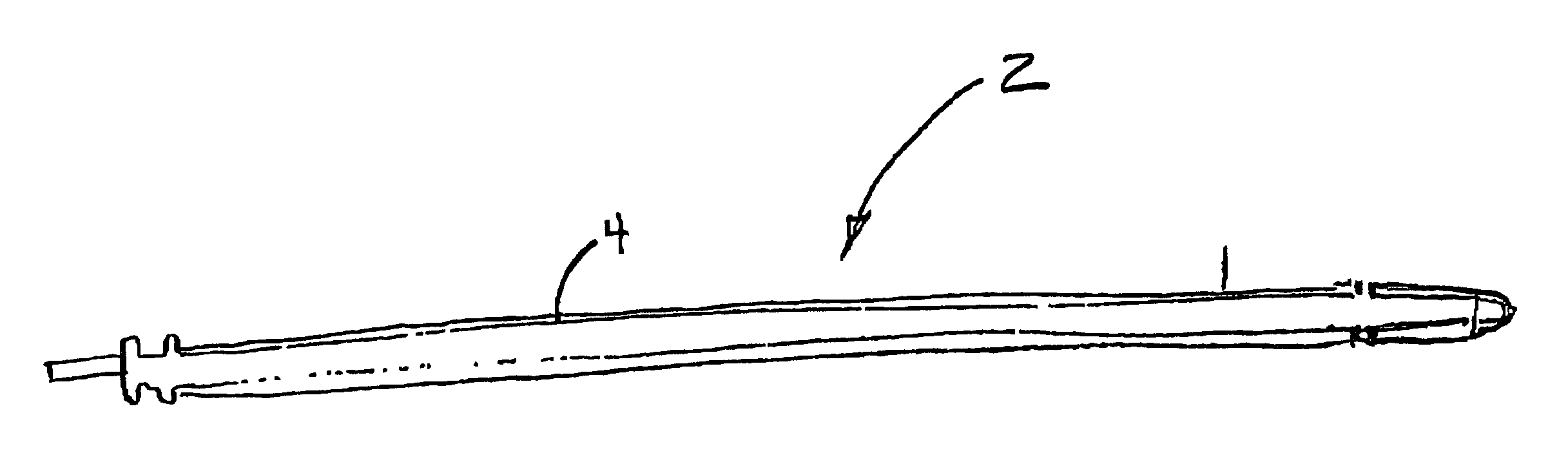
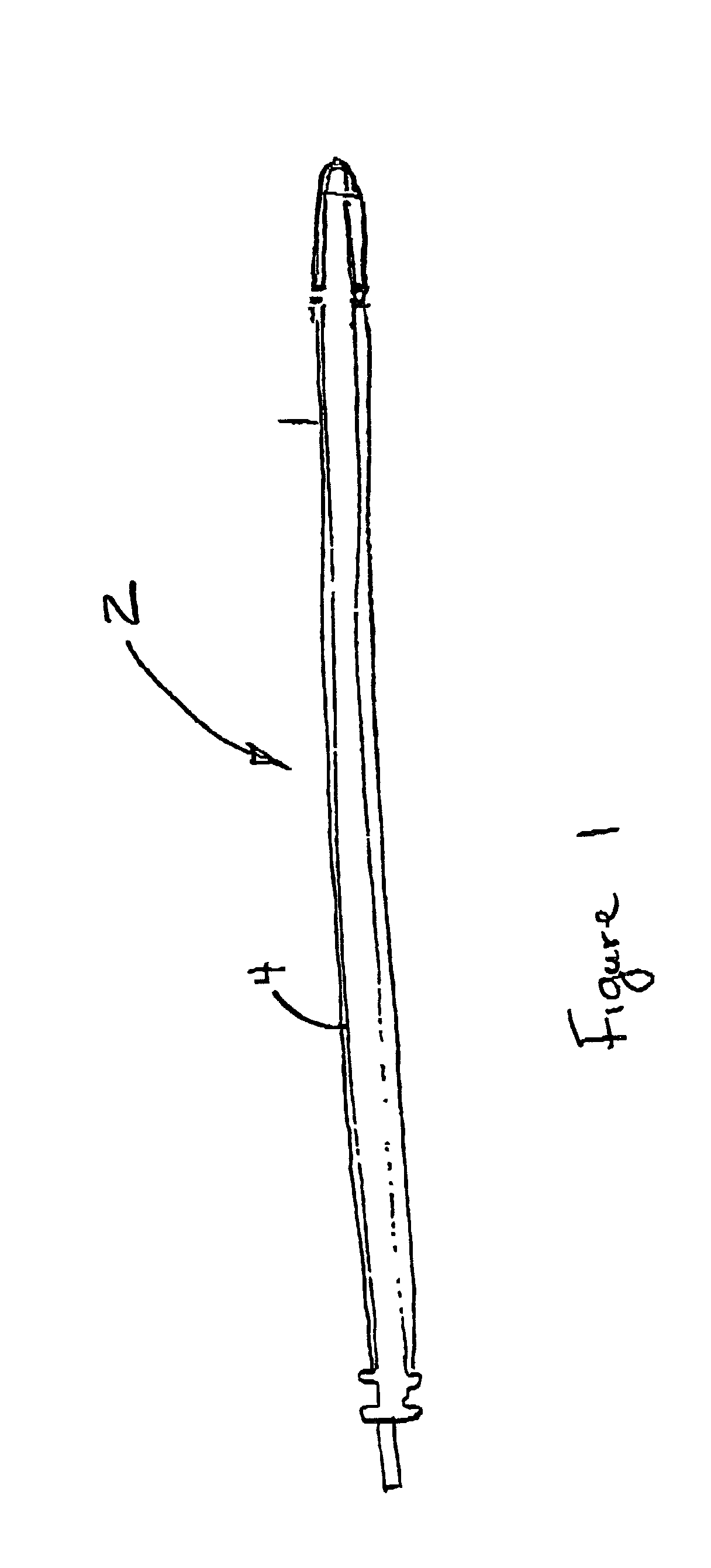
ActiveUS20080183028A1Reduce riskReduce the risk of infectionLaproscopesEndoscopesEngineeringArthroscopy
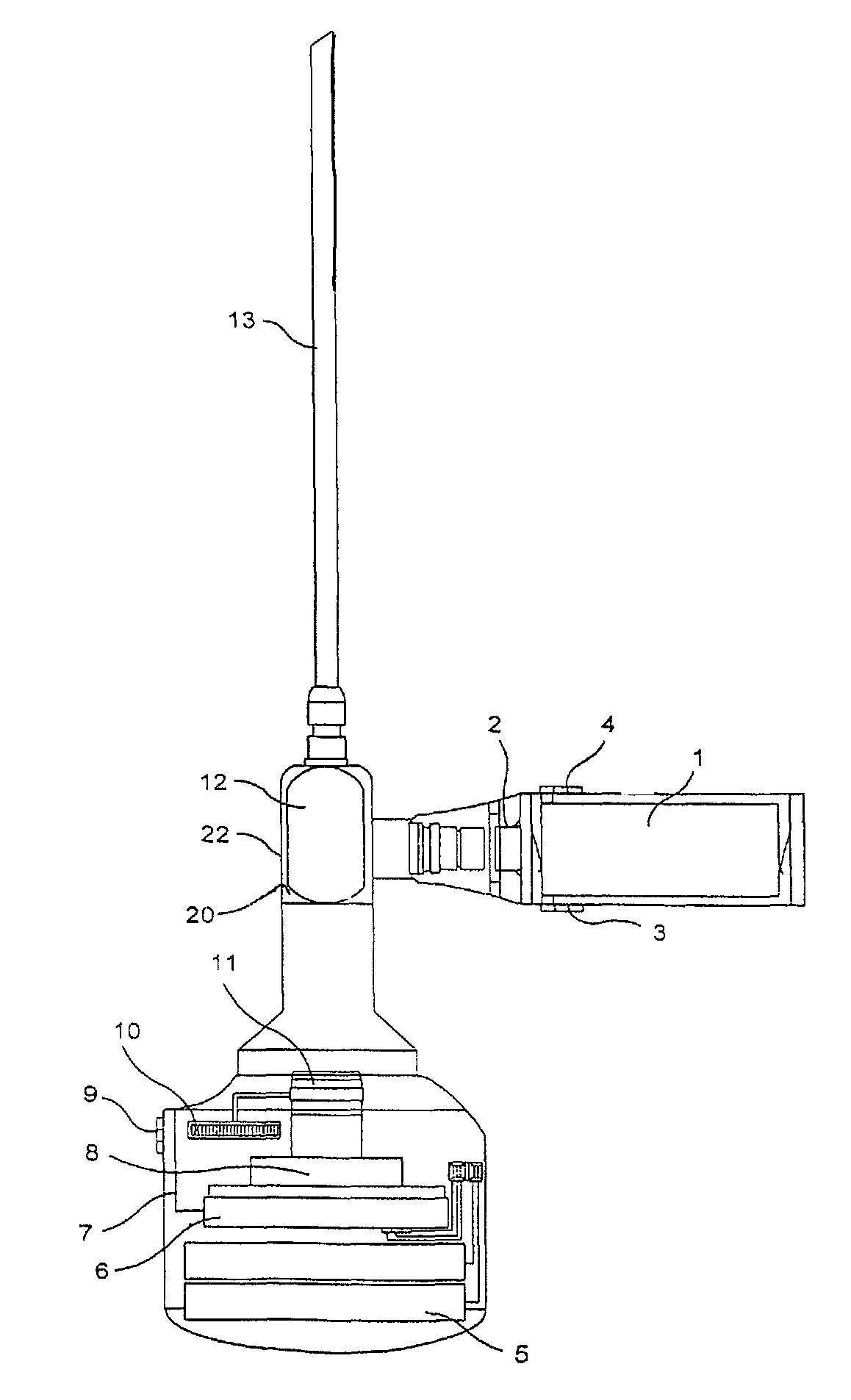
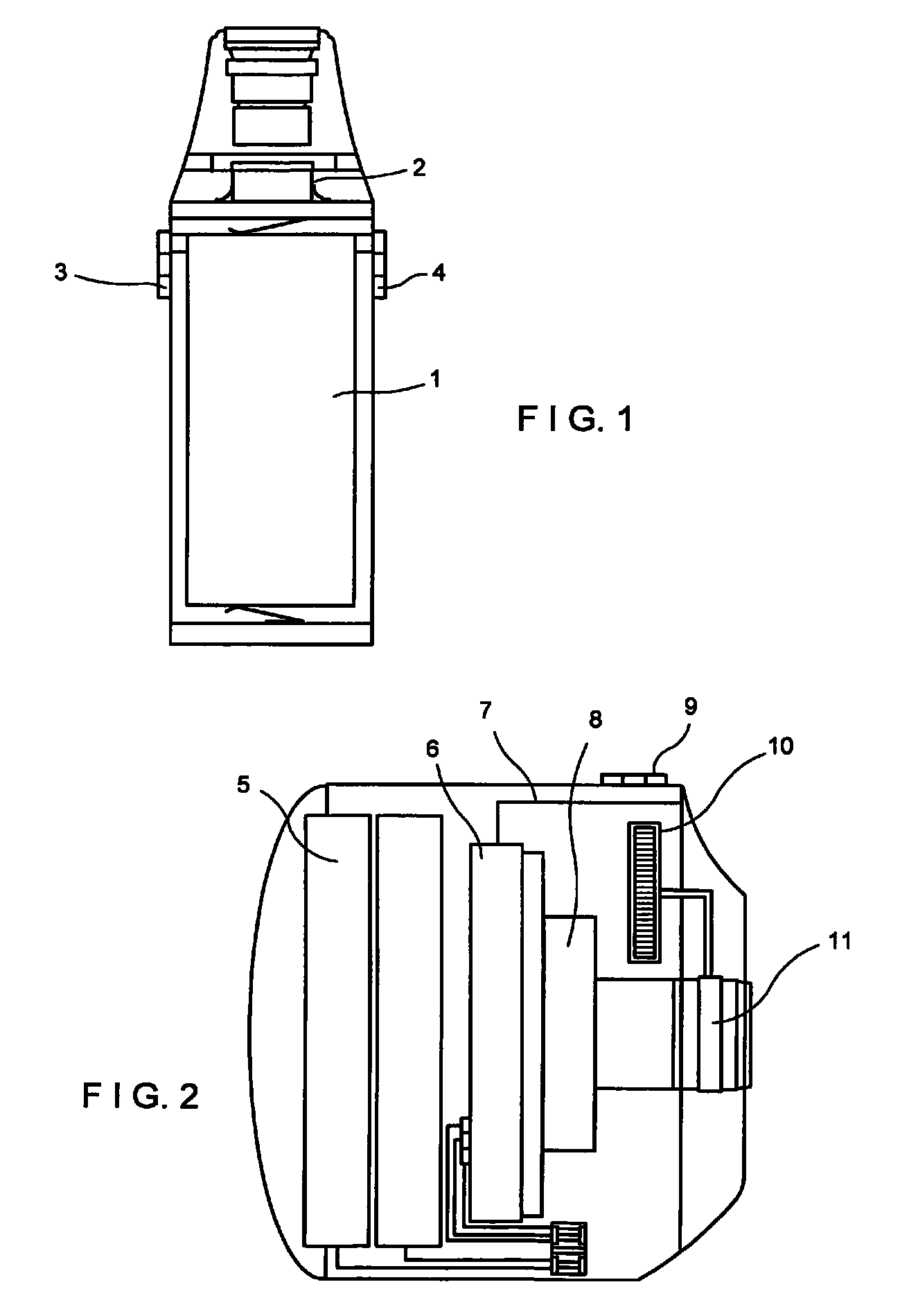
The present invention relates to an arthroscopy apparatus, comprising at least three elements selected from: a conventional arthroscopic lens (12), to which there is coupled a power supply device or capsule, in the inside of which is the power source (1), and a miniature camera (8), characterized by not comprising connecting cables.
Owner:GARCIA PEDRO GUILLEN
Deep-cleaning, antibacterial and tooth-protecting mouth wash based on chitosan derivatives and preparation method of mouth wash
InactiveCN106176546AWide range of raw materialsLow costCosmetic preparationsToilet preparationsOral diseasePropolis
The invention discloses a deep-cleaning, antibacterial and tooth-protecting mouth wash based on chitosan derivatives. The mouth wash is prepared from, by weight, 3% of chitosan derivatives, 5% of glycerol, 1.5% of xylitol, 2% of efficient solubilizer, 0.55% of citric acid, 0.3% of sodium citrate, 0.25% of malic acid, 0.12% of menthol, 0.15% of mint fragrance, 2% of propolis extracts, 3.5% of tea leaf extracts, 0.07% of caramel colour, 0.04% of saccharin sodium, 0.2% of chlorhexidine, 0.0003% of domiphen bromide and the balance deionized water. The invention provides the deep-cleaning, antibacterial and tooth-protecting mouth wash based on the chitosan derivatives and the preparation method of the mouth wash. The mouth wash prepared through the method has the functions of inhibiting bacteria, removing dental plaque and preventing oral ulcer, gingivitis and other oral diseases, and is good in antibacterial effect, quick in effect, free of toxic and side effects and comfortable in mouthfeel.
Owner:湖北立天生物工程有限公司
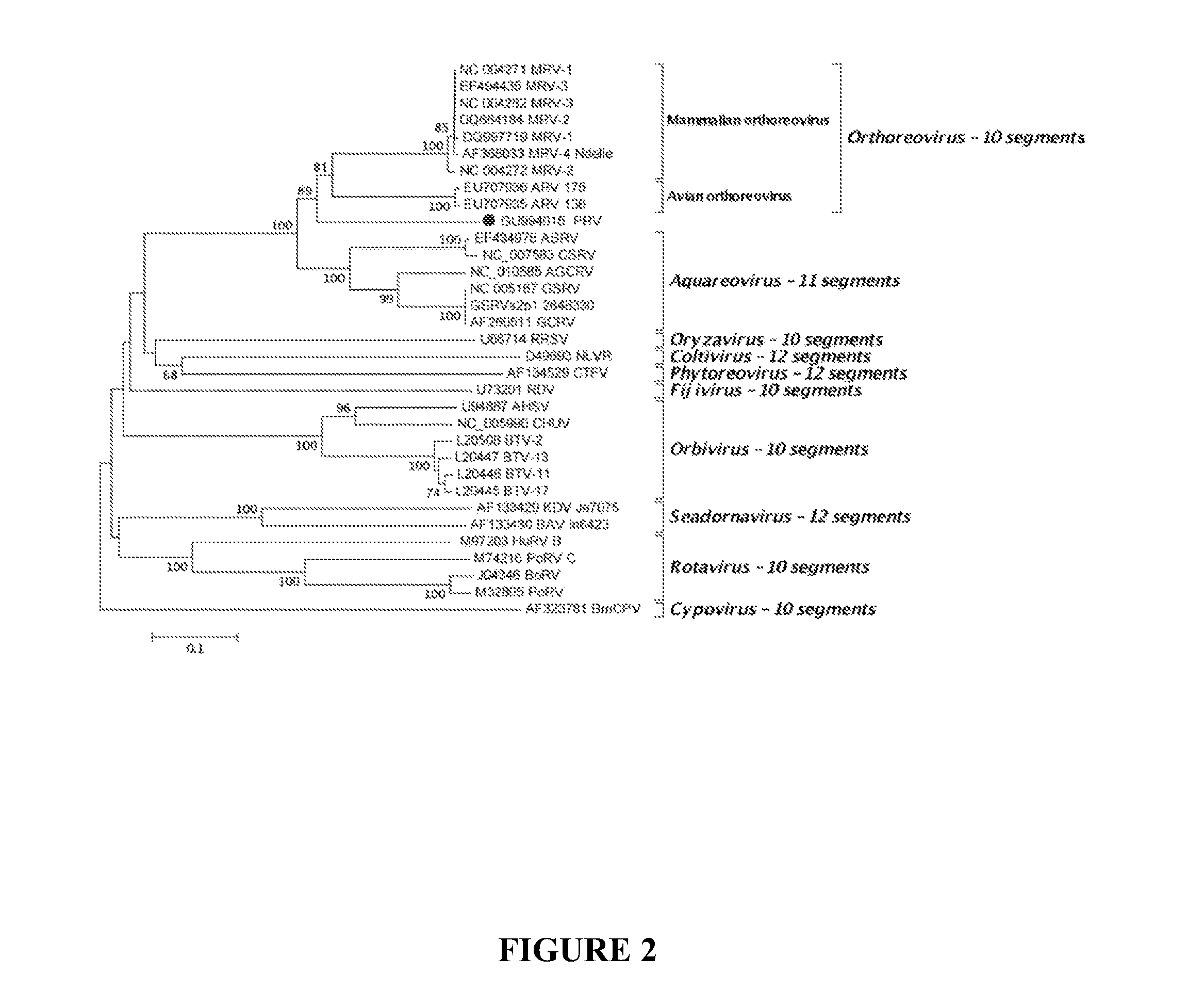
Piscine reovirus immunogenic compositions
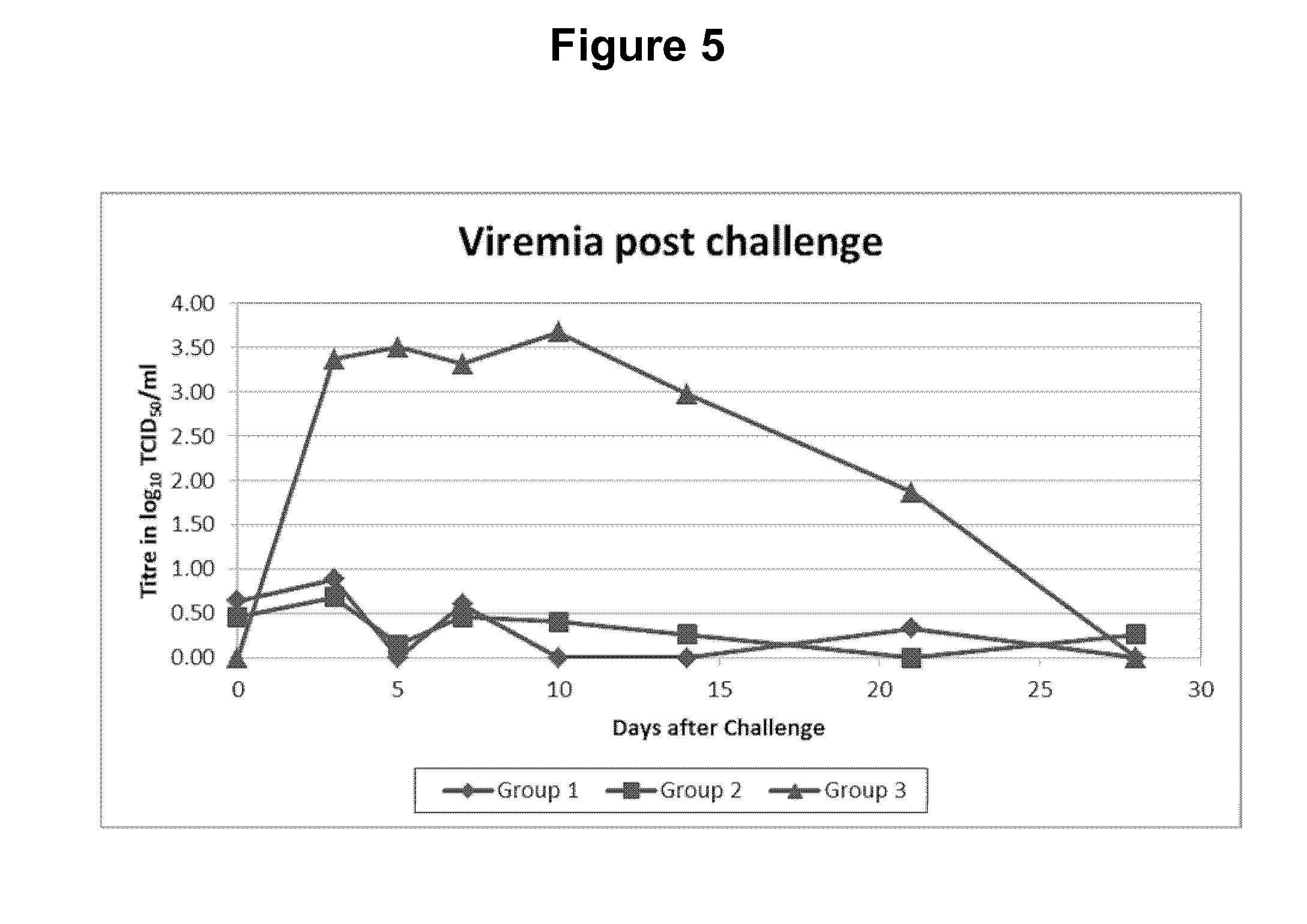
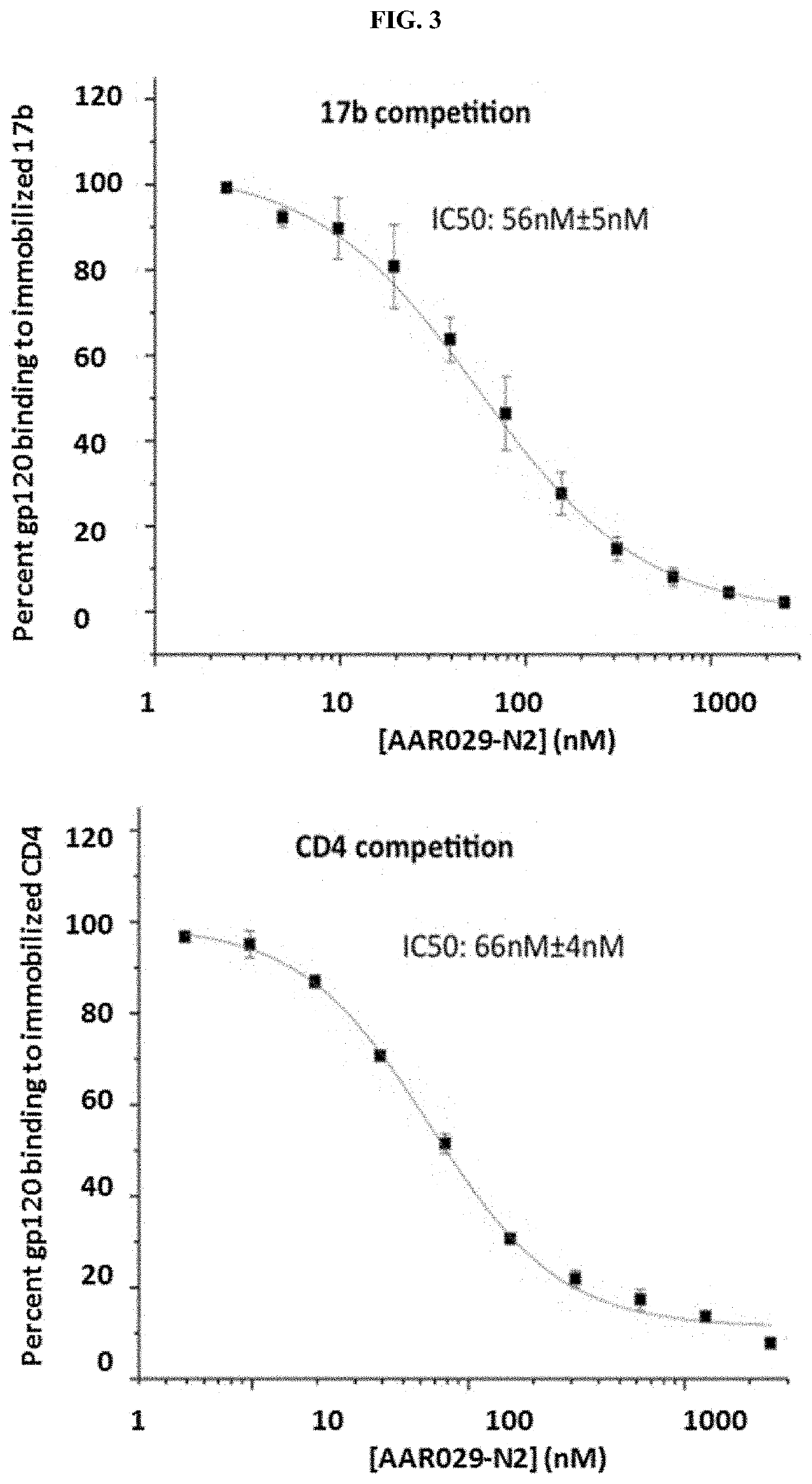
ActiveUS20130058968A1Prevent and reduce infectionOrganic active ingredientsVirus peptidesReovirus RNAImmunogenicity
Owner:THE NAT VETERINARY INST +1
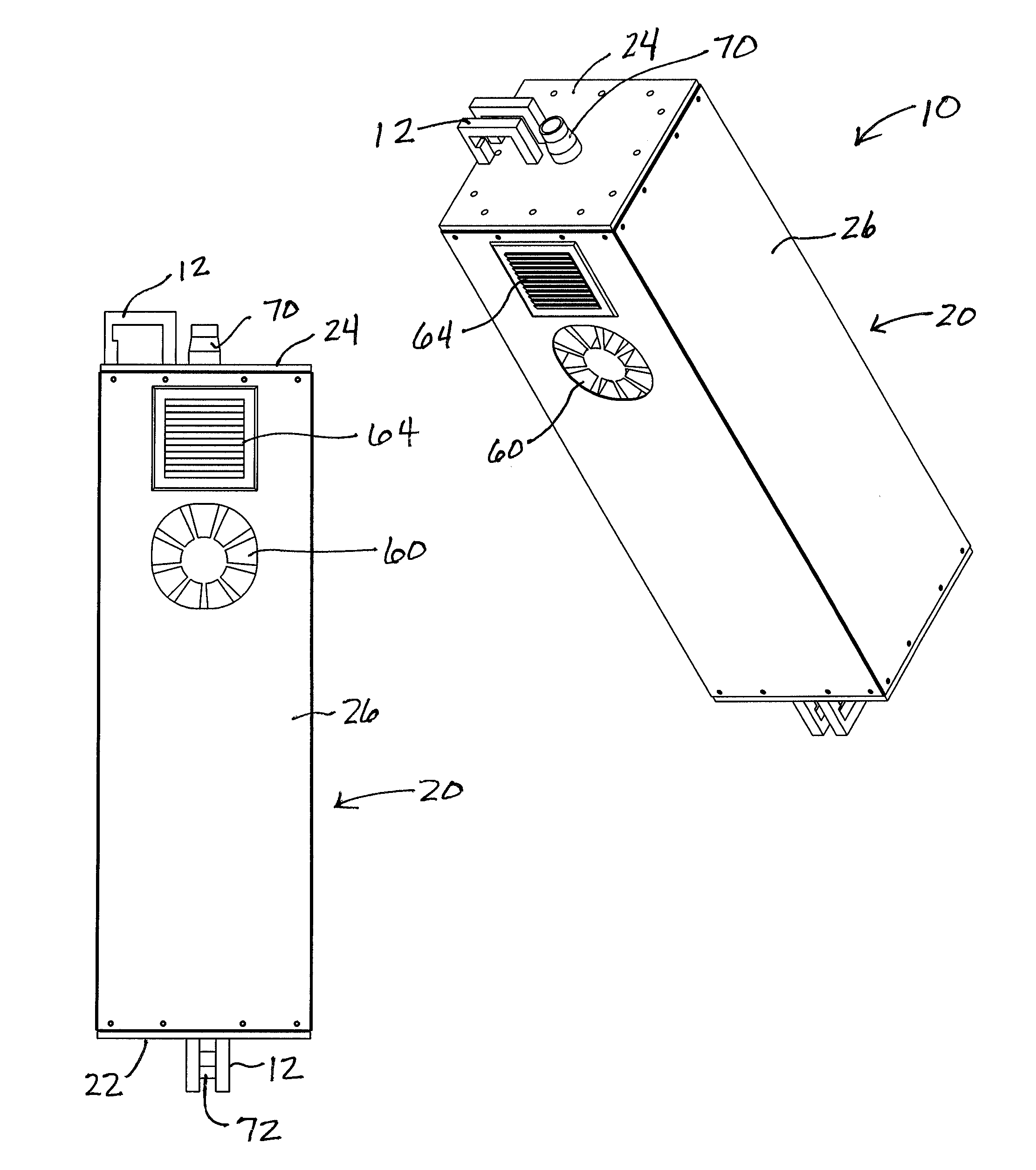
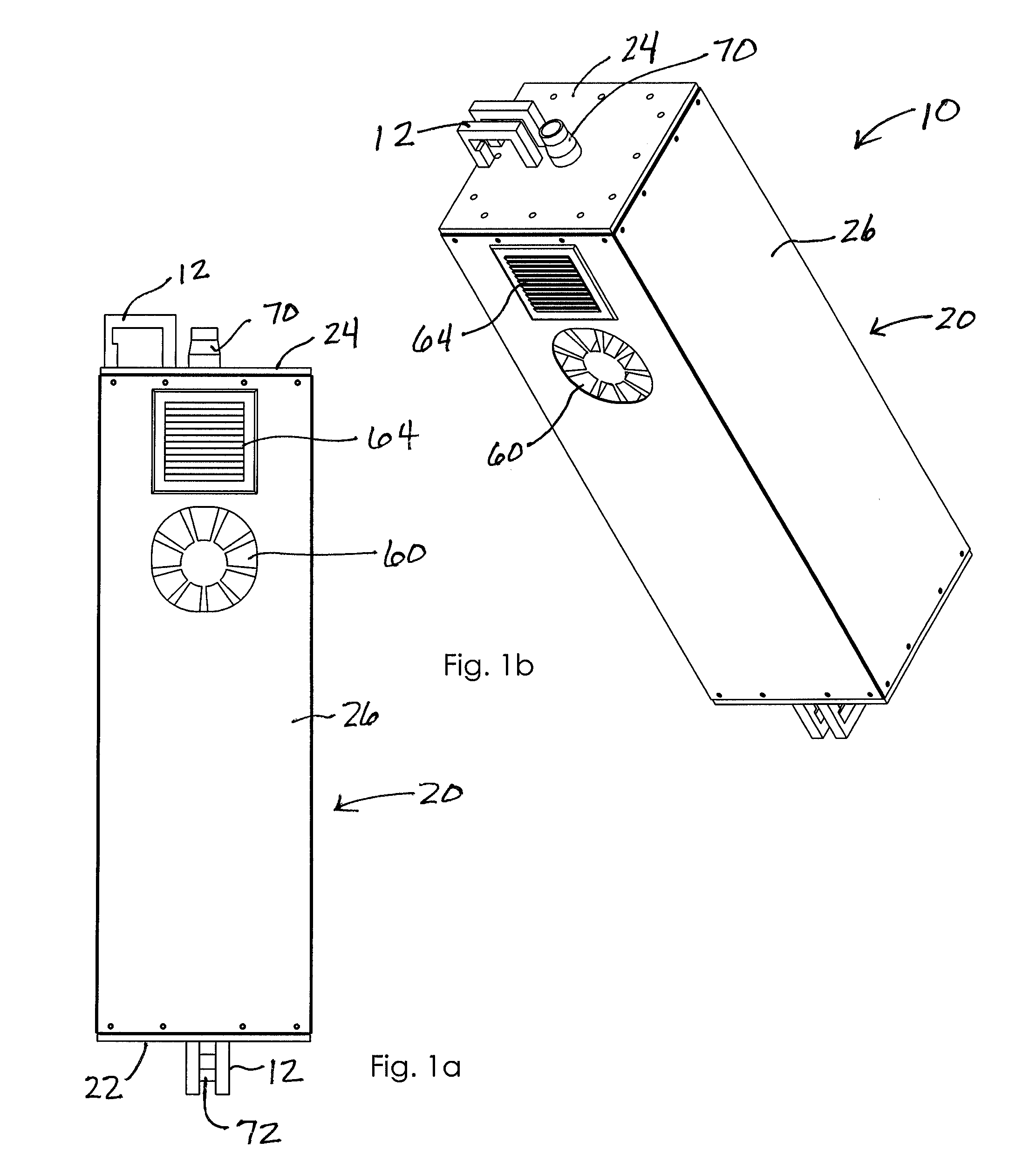
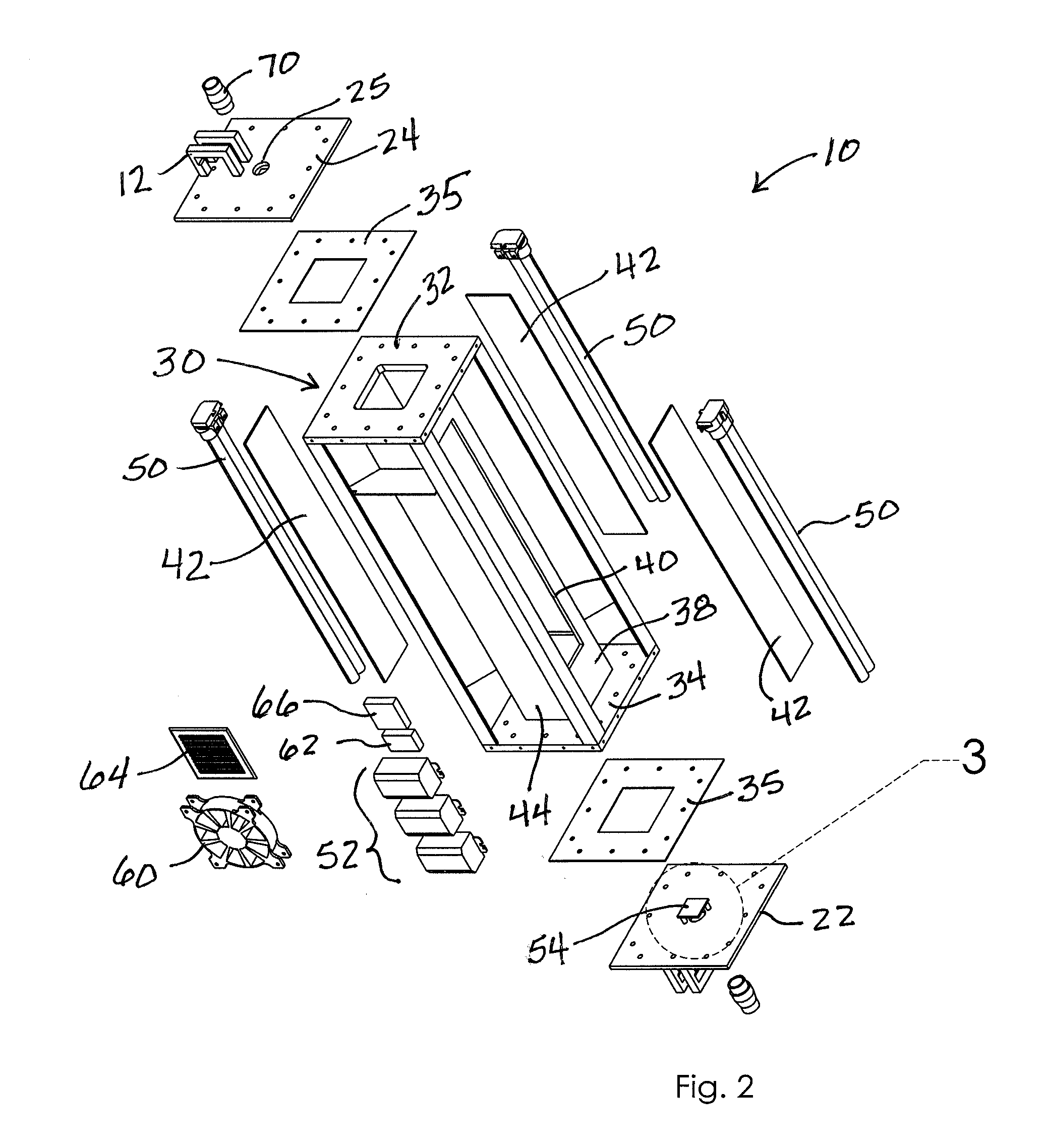
Ultraviolate light sterilization apparatus
InactiveUS20160271288A1Prevents and reduces future infectionPrevent and reduce infectionRespiratorsMedical devicesUltravioletMechanical engineering
A sterilization apparatus for sterilizing air includes an outer housing having side walls extending between opposed bottom and top walls that, together, define an outer chamber. An inner housing is situated in the outer chamber and includes opposed top and bottom ends and having a wall structure that defines a sealed inner chamber extending therebetween. The wall structure includes at least one side wall that defines a transparent window. An inlet port is in communication with one end of the inner chamber and that directs unsterilized air into the inner chamber. An ultraviolet (“UV”) light source is positioned outwardly adjacent the window and configured to emit UV light through the window and into the inner chamber when energized so as to sterilize air in the inner chamber. An outlet port is in communication with another end of the inner chamber that directs sterilized air downstream from the inner chamber.
Owner:DAVIS MATTHEW PHILLIP
Cosmetic freeze-dried powder preparation capable of beautifying features and caring skin and application of cosmetic freeze-dried powder preparation capable of beautifying features and caring skin to solutions
InactiveCN110721117ASmall molecular weightGood water solubilityCosmetic preparationsToilet preparationsAnti bacterialExcipient
The invention relates to a cosmetic freeze-dried powder preparation capable of beautifying features and caring skin and an application of the cosmetic freeze-dried powder preparation capable of beautifying features and caring skin to solutions. The cosmetic freeze-dried powder preparation capable of beautifying features and caring skin is prepared from the following raw material components in percentage by mass of 1%-5% of hyaluronic acid, 0.001%-0.005% of antibacterial peptide, 5%-15% of an excipient and 79.995%-93.999% of water. The cosmetic freeze-dried powder preparation capable of beautifying features and caring skin is prepared through the steps of stirring the raw materials, performing heating, and after the raw materials completely dissolve, performing sublimation through a freeze-drying technique to obtain the freeze-dried powder preparation. The cosmetic freeze-dried powder preparation capable of beautifying features and caring skin can be applied to raw liquor, essence, enzyme dissolving liquor or liquid dressing. Through the adoption of the cosmetic freeze-dried powder preparation capable of beautifying features and caring skin disclosed by the invention, the phenomenathat water-oil disequilibrium of skin and muscle appears, and pores are easy to block can be avoided, besides, the stability of the antibacterial peptide in the freeze-dried powder preparation can beimproved, the effects of the freeze-dried powder preparation for resisting bacteria, diminishing inflammation and preventing occurrence and development of acne can be exerted, and the function of repairing skin and muscle can be better realized.
Owner:广州一生美生物科技有限公司
Mammalian animal compositon
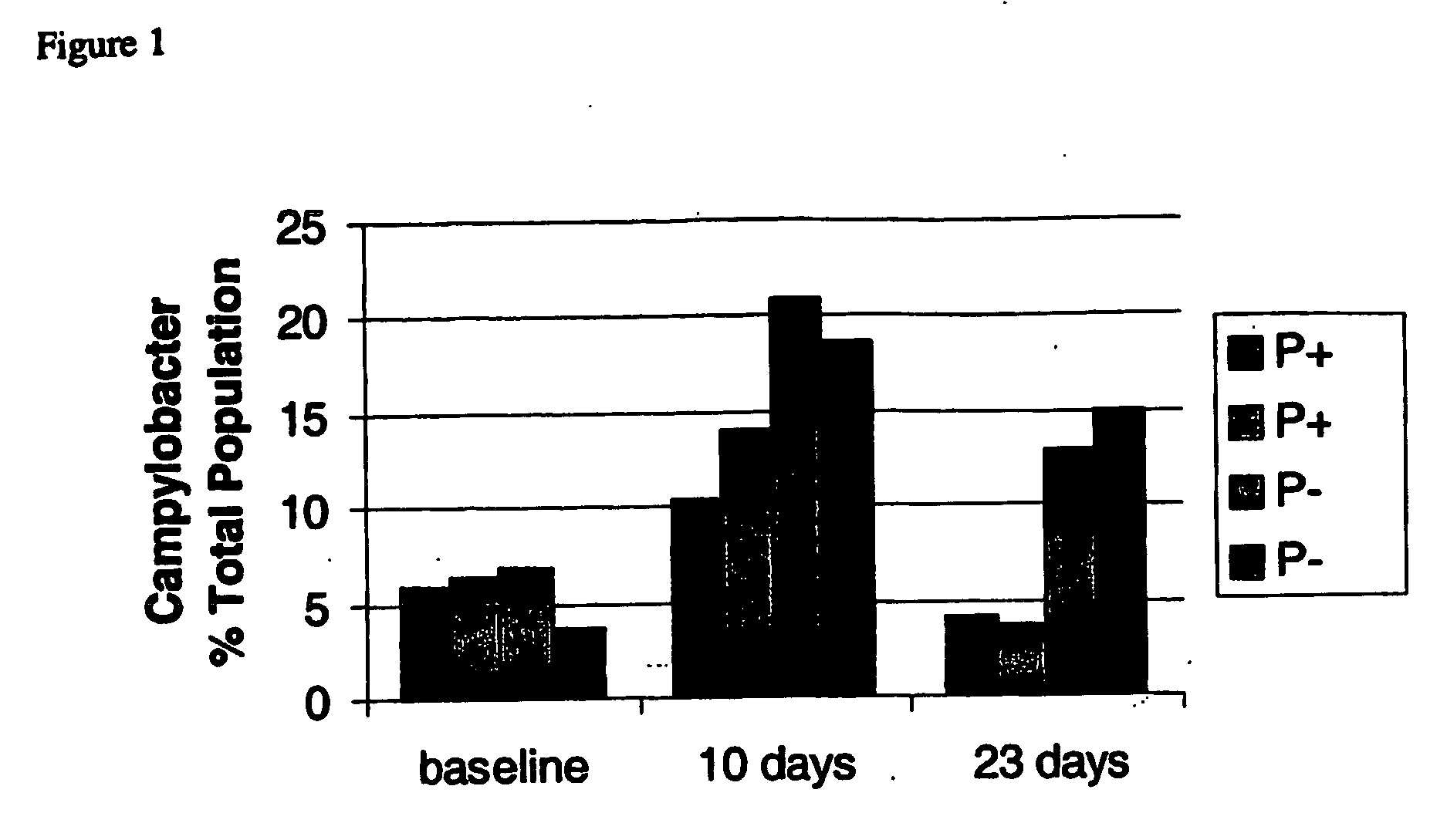
InactiveUS20060134082A1Reduce and prevent Campylobacter infectionReduces and prevents zoonotic riskBiocideBacteria material medical ingredientsMicroorganismMicrobiology
The present invention relates to the use of probiotic microorganism in the manufacture of a composition for the prevention or reduction of gastrointestinal Camplylobacter infection in a mammalian animal. It also relates to a method for the prevention or reduction of gastrointestinal Campylobacter infection in a mammalian animal, the method comprising administering to said animal, a probiotic microorganism. The invention also relates to a probiotic microorganism, for use in preventing or reducing gastrointestinal Campylobacter infection in a mammalian animal.
Owner:MARS INC
Methods And Compositions For Controlling Parasitic Infections Of Animals
ActiveUS20080008767A1Prevent and reduce harmful effectEffective effectBiocideOrganic active ingredientsZincYeast form
A composition for preventing or reducing harmful effects of protozoal infection is provided, comprising in one embodiment a yeast cell wall and a preparation derived from oregano. The composition may further include a mineral nutrient selected from selenium and / or zinc. The composition may also include a preparation derived from Yucca. Efficacy of the composition is shown against a variety of protozoal organisms.
Owner:ALLTECH CO LTD
Design of protective Anti-zikv vaccine without inducing cross-reactions with dengue
ActiveUS20190185520A1Efficiently colonizeInduces mucosalSsRNA viruses positive-senseBacteriaProtective antigenWhole body
The subject application provides a genetically modified recombinant facultative intracellular invasive bacterial pathogen (RFIIBP) or a recombinant attenuated Salmonella vaccine (RASV) strains encoding Zika virus (ZIKV) antigens. These strains exhibit high immunogenicity, complete safety, and attenuation, and are unable to persist or be shed in an infective or viable form. These RFIIBPs and RASVs also exhibit regulated delayed attenuation in vivo, regulated delayed in vivo synthesis of protective ZIKV antigens. Methods of inducing mucosal, systemic and cellular immunities in hosts are also provided and antibodies produced using the disclosed RFIIBPs and RASVs can comprise neutralizing antibodies against ZIKV that do not crossreact with Dengue virus (DENV).
Owner:UNIV OF FLORIDA RES FOUNDATION INC
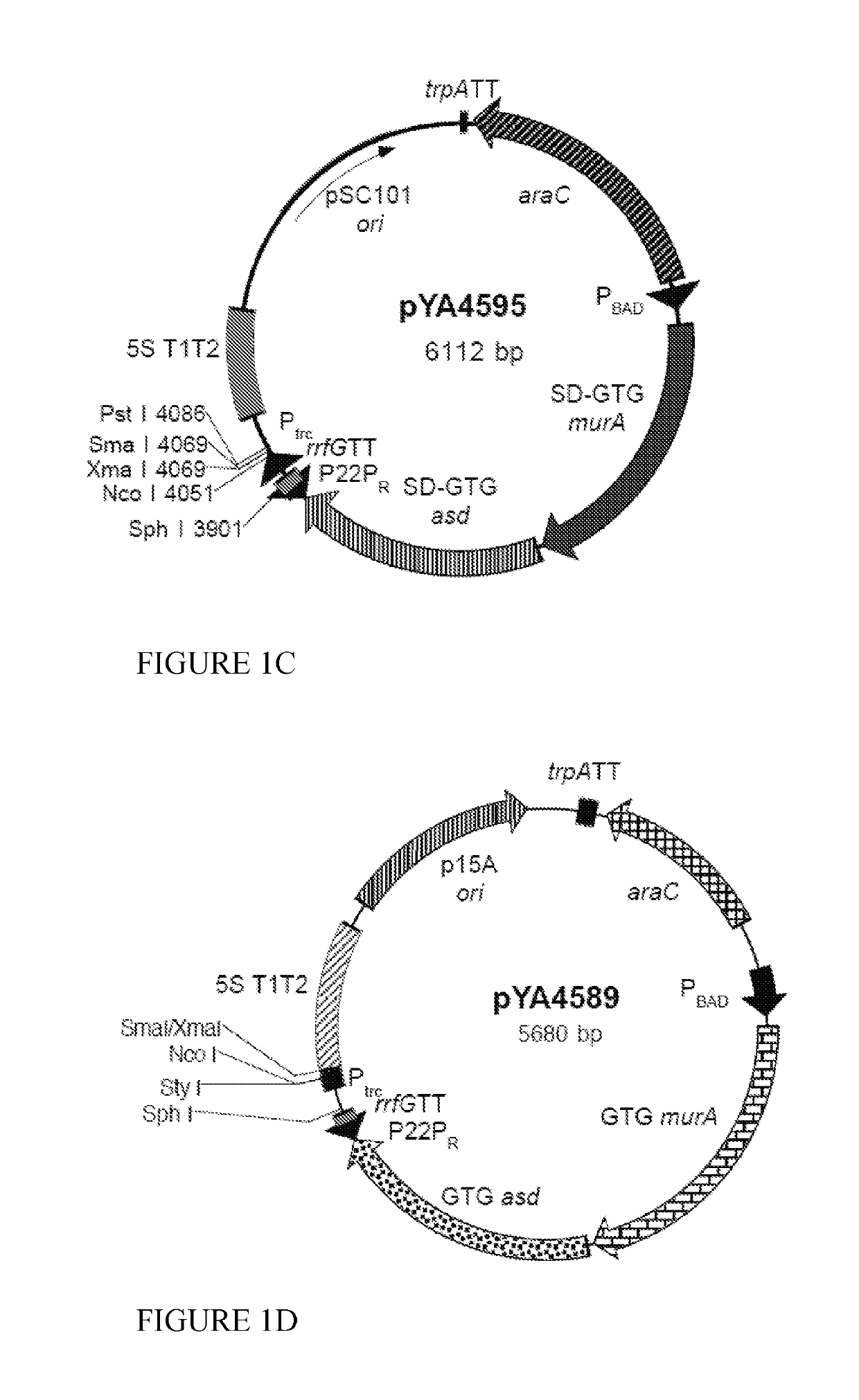
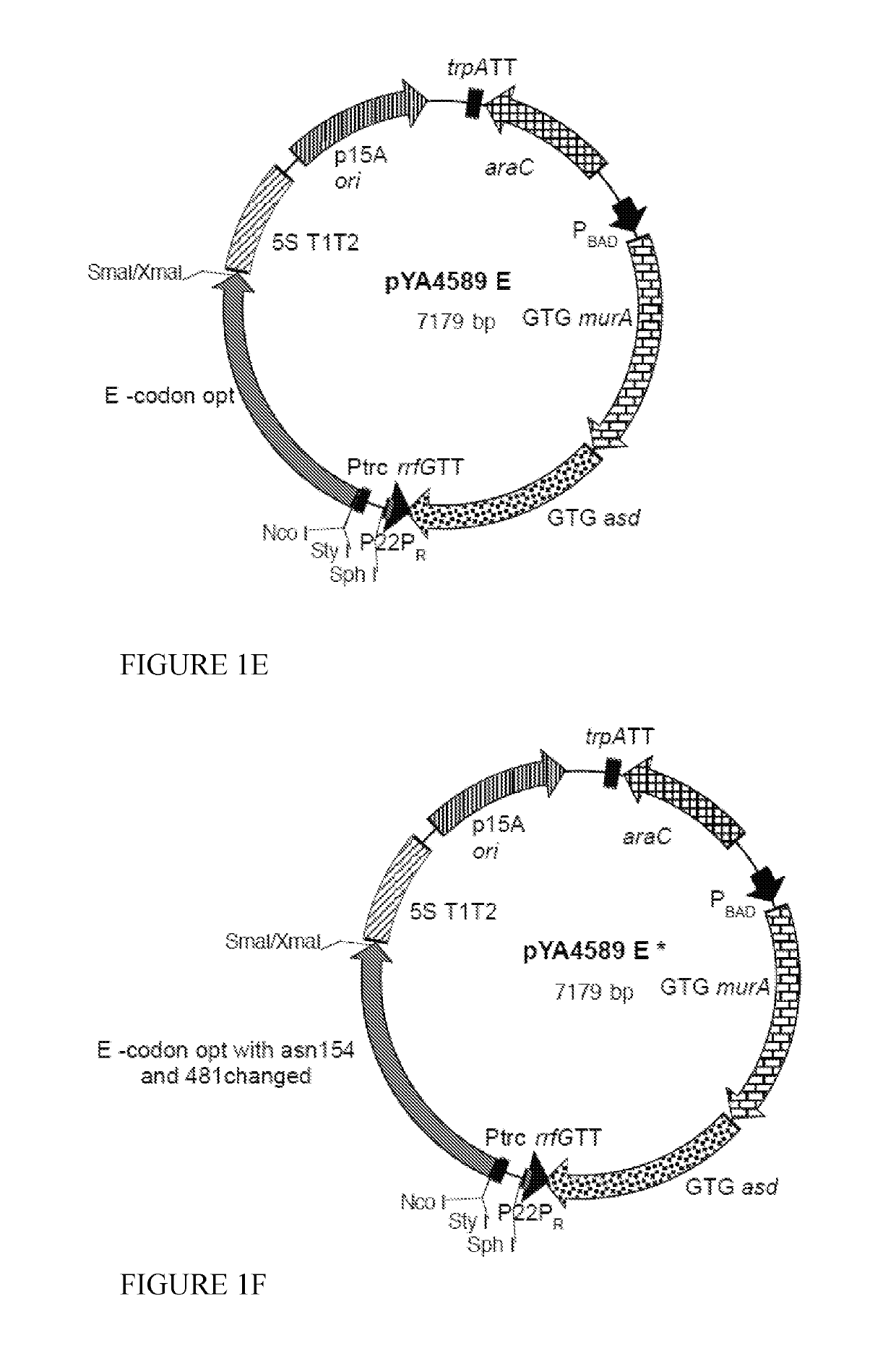
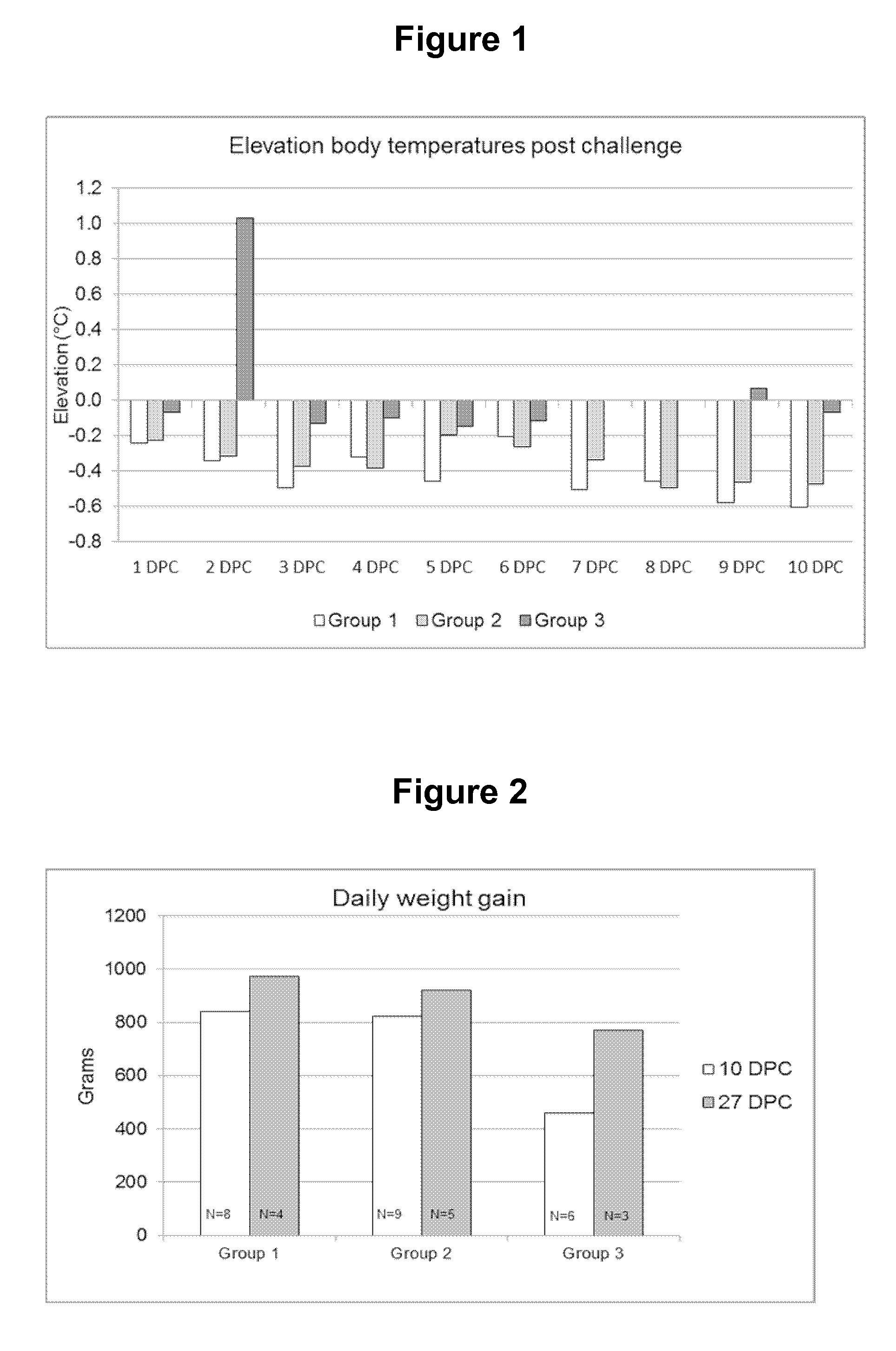
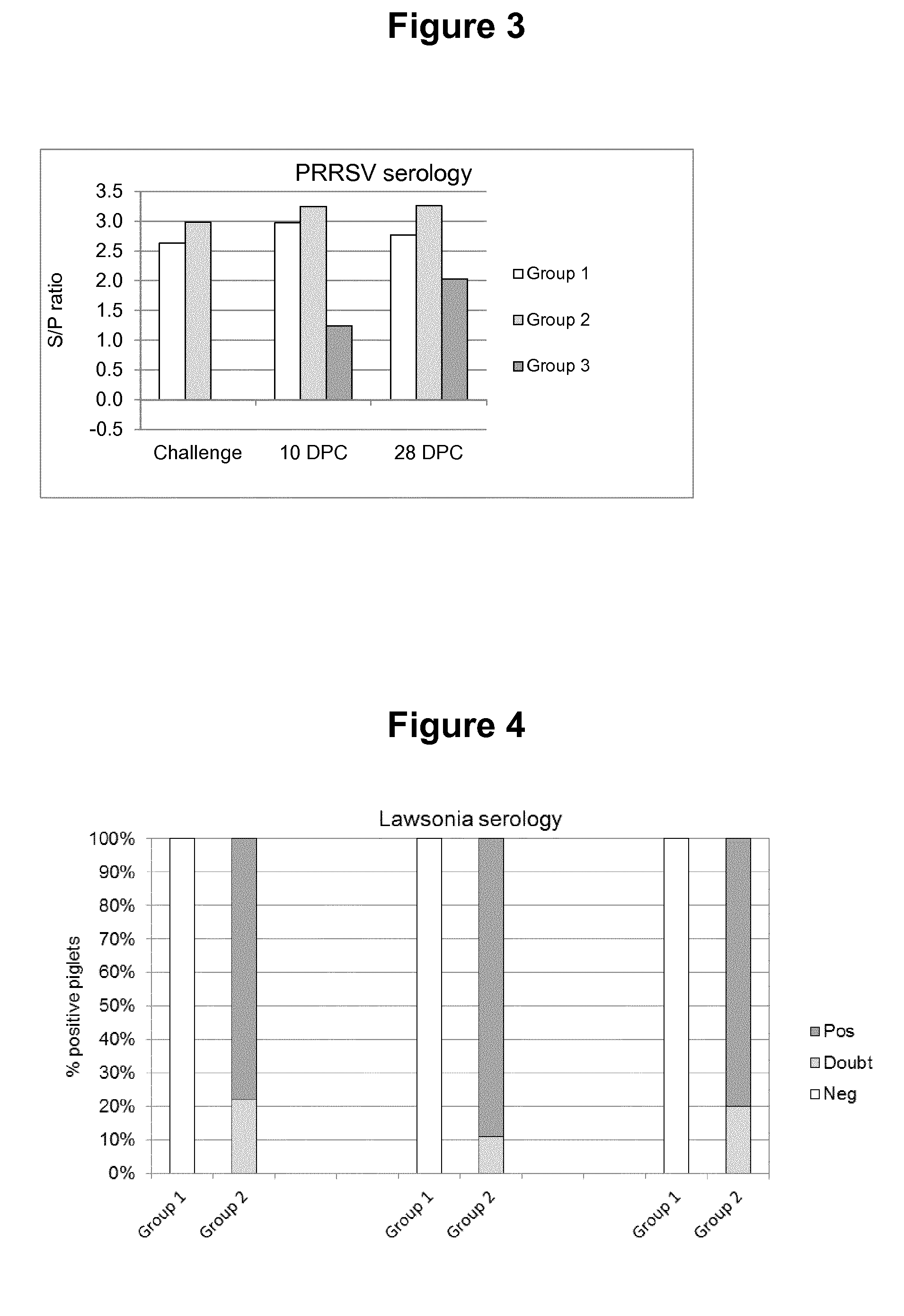
Swine Vaccine Against PRRS and Lawsonia Intracellularis
InactiveUS20160303219A1Less-safe and effectiveComplicate safetyAntibacterial agentsBacterial antigen ingredientsAntigenLawsonia intracellularis
The present invention pertains to a swine vaccine, in particular a vaccine comprising in combination live attenuated PRRS virus and inactivated Lawsonia intracellularis antigen, for the protection of a swine against an infection with PRRS virus and Lawsonia intracellularis bacteria. The invention also pertains to a method to protect a swine against an infection with PRRS virus and Lawsonia intracellularis bacteria using this vaccine.
Owner:INTERVET INC
Method for treating blood, blood products and organs
ActiveUS9591845B2Avoid infectionPrevent and reduce infectionNervous disorderPeptide/protein ingredientsAmyloid beta oligomerBlood product
The invention relates to the treatment of blood, blood products and organs for the removal and / or detoxification of amyloid-beta oligomers.
Owner:PRIAVOID GMBH
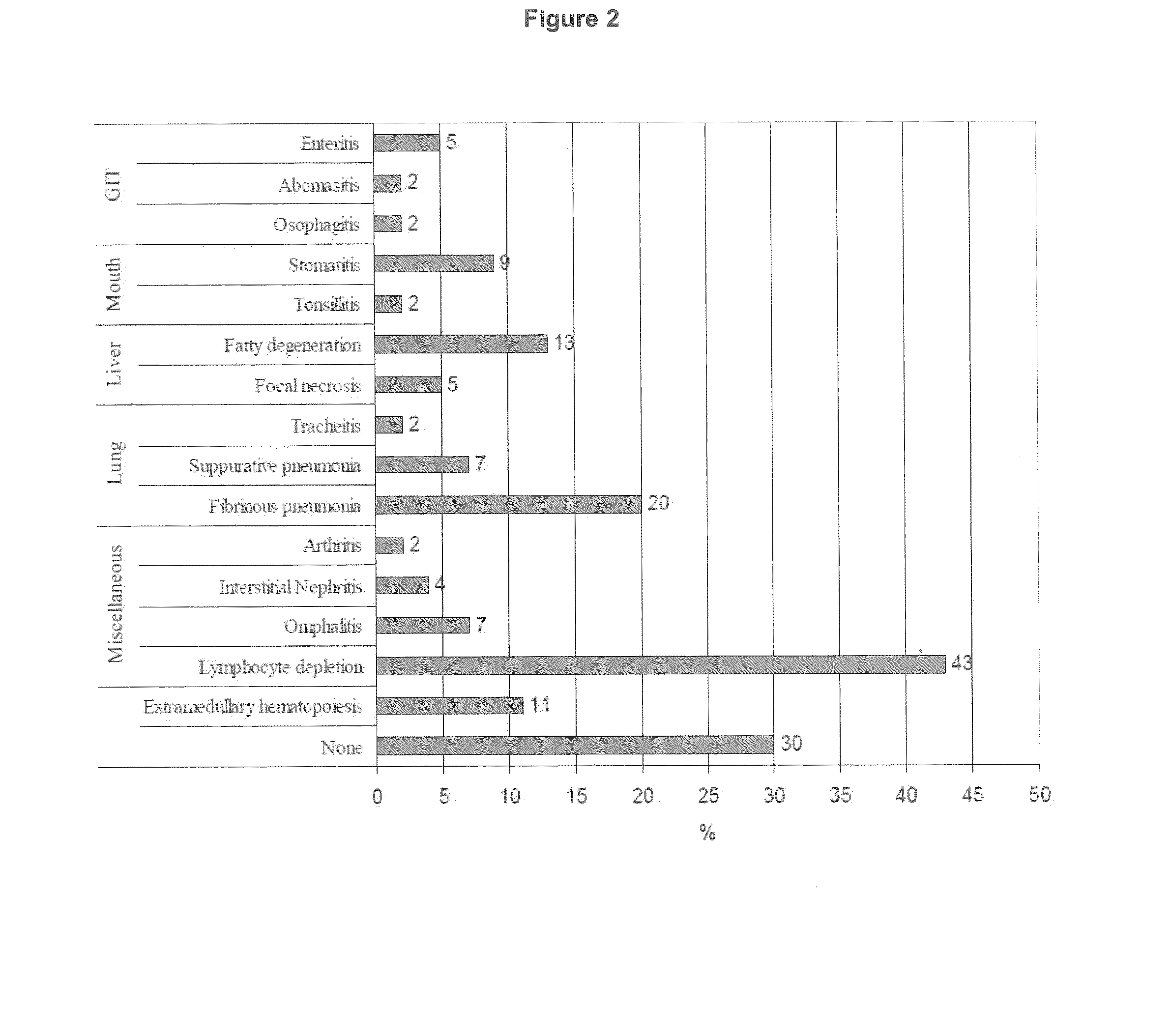
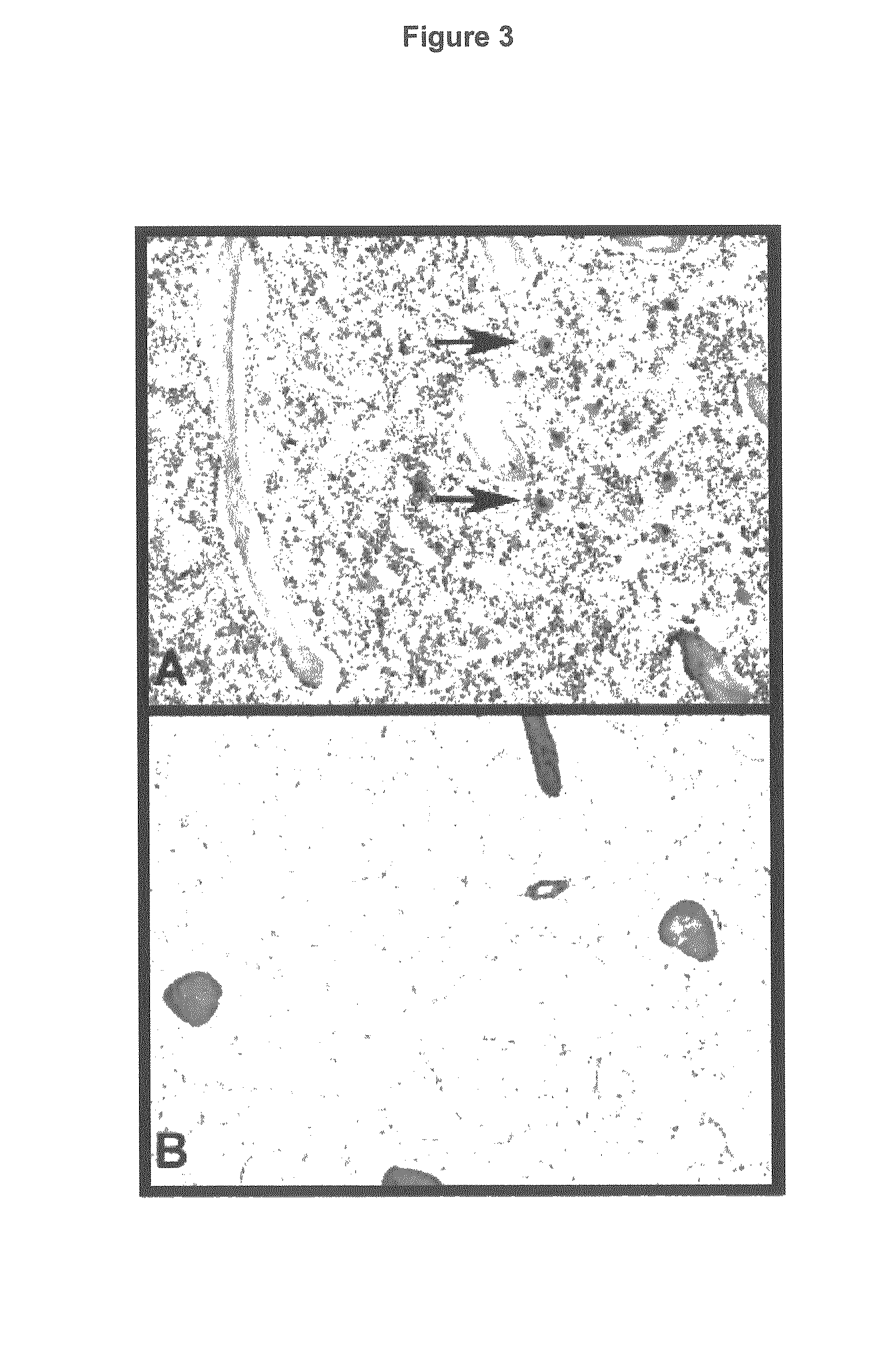
Bone marrow aplasia with haemorrhagic disease in calves caused by novel pathogen
InactiveUS20130251719A1Less reactiveReduce capacityPeptide/protein ingredientsVirus peptidesCircovirusBone Marrow Aplasia
The present invention refers to a novel circovirus as causative agent of bone marrow aplasia with haemorrhagic disease in cattle. The present invention provides novel nucleic acid and protein sequences for diagnostic and therapeutic uses.
Owner:UNIV LEIPZIG +1
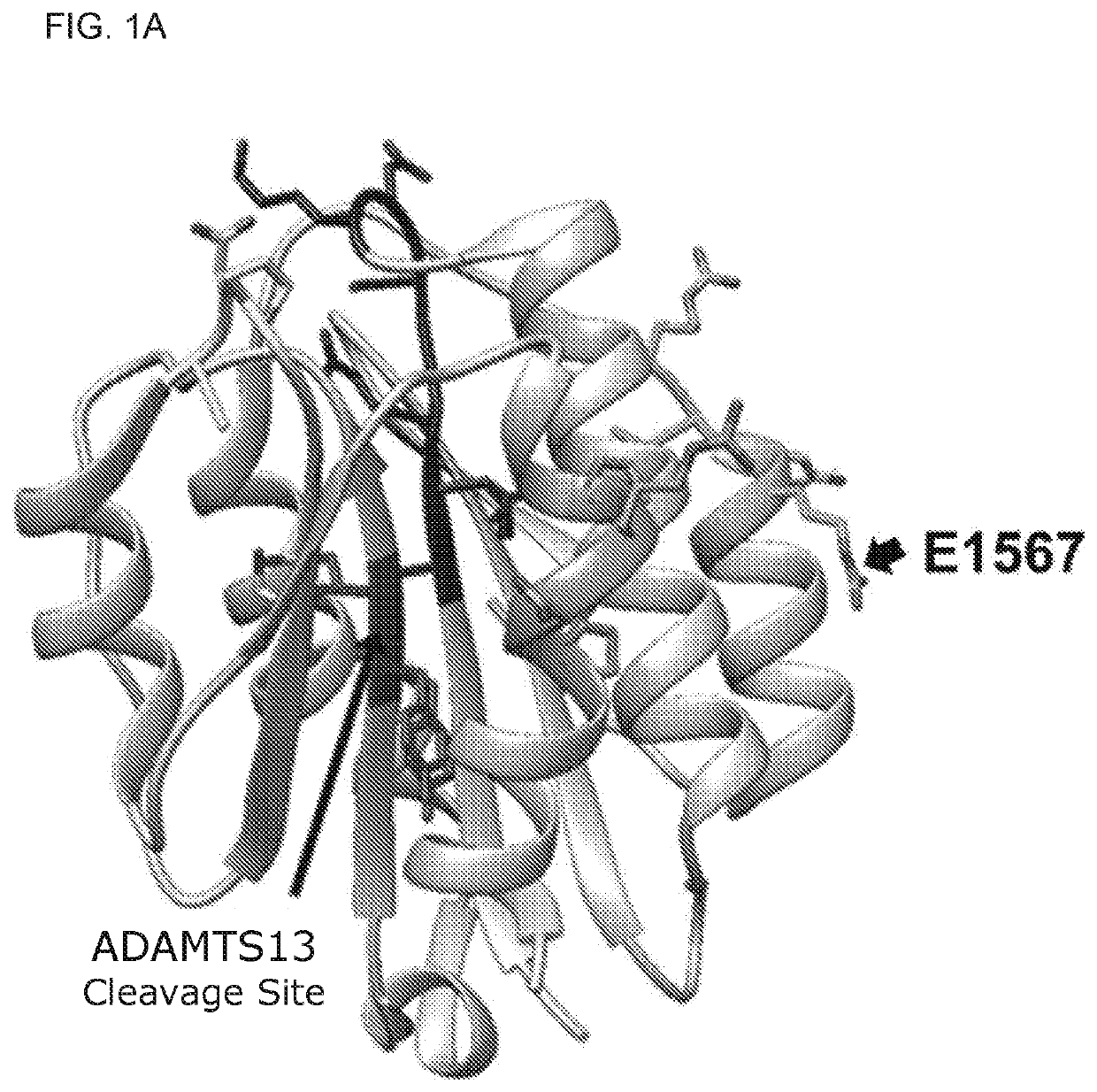
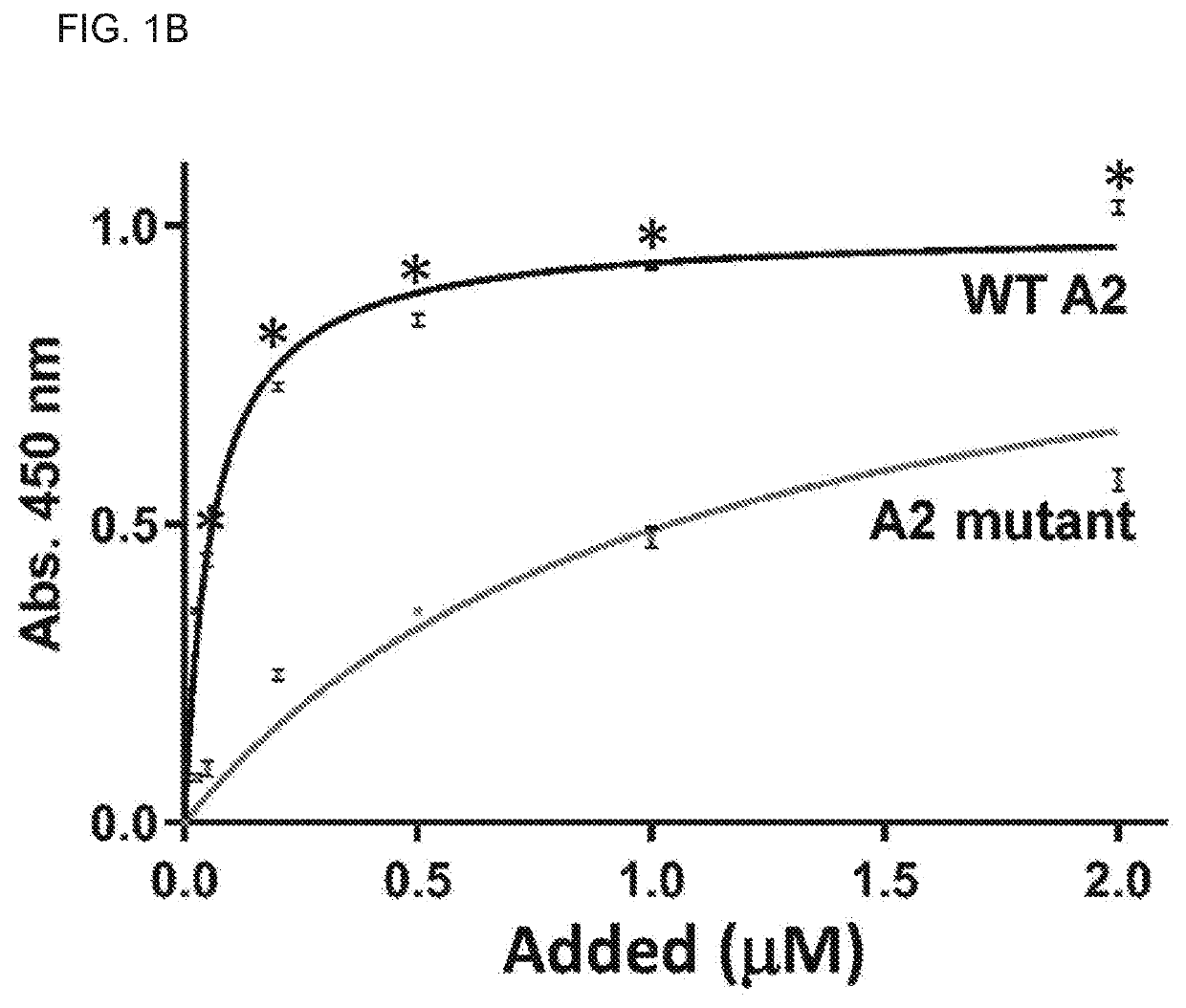
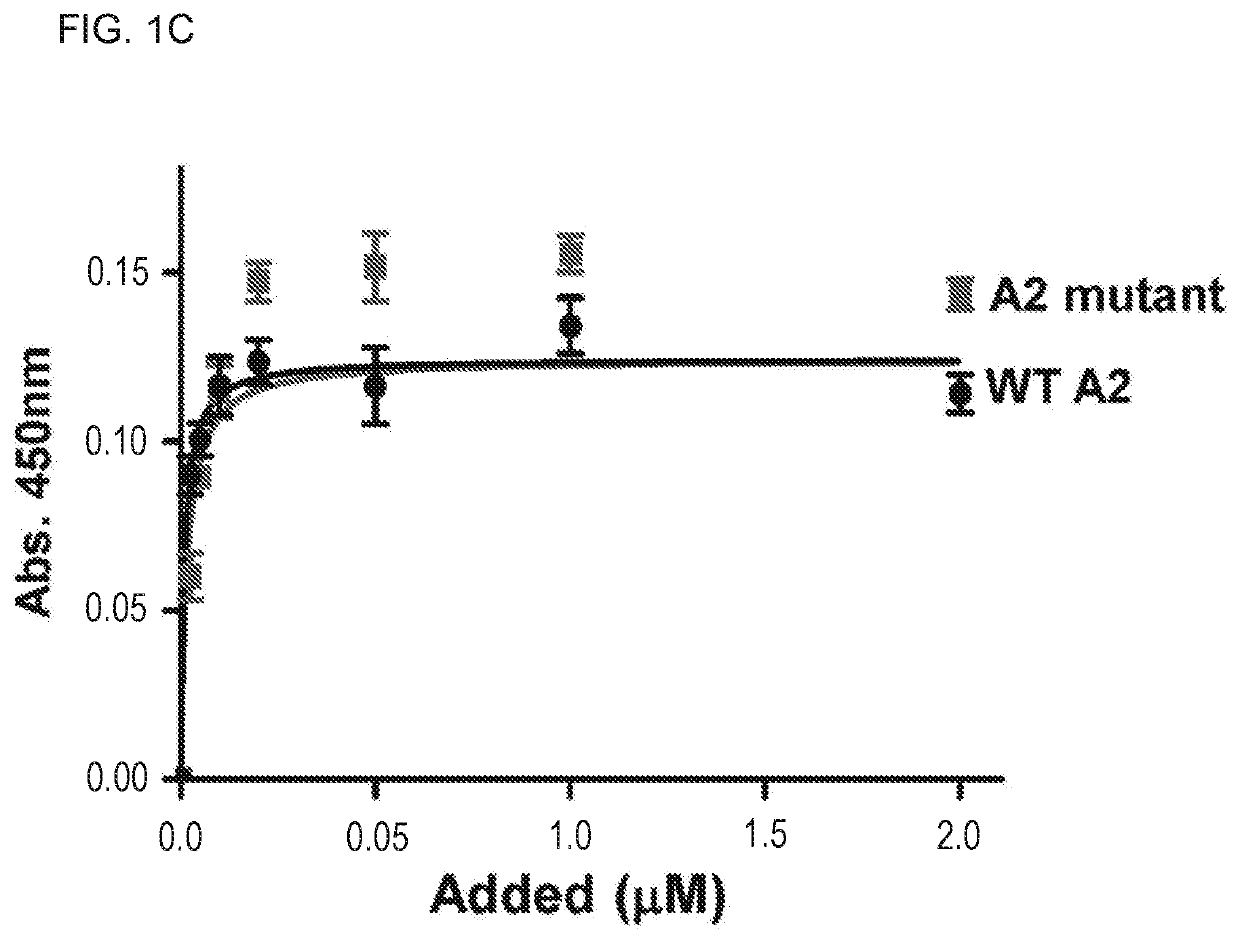
Uses of a2 domain of von willebrand factor
PendingUS20220193204A1Improve survivalImprove bleedingPeptide/protein ingredientsAntiviralsFactor VIII vWFVon willebrand
Embodiments of the disclosure encompass methods and compositions for maintaining a healthy fibrin network in an individual. The disclosure includes methods of targeting fibrin in an individual for the purpose of restoring fibrin that is subject to a level of fibrinolysis that is deleterious, such as excessive or reduced with respect to the general population. Such modifications of fibrin in an individual may include direct targeting of fibrin with the A2 domain of von Willebrand factor or a functional derivative or fragment thereof. In specific embodiments, the methods restore to a normal level any imbalance between coagulation and inflammation.
Owner:BAYLOR COLLEGE OF MEDICINE
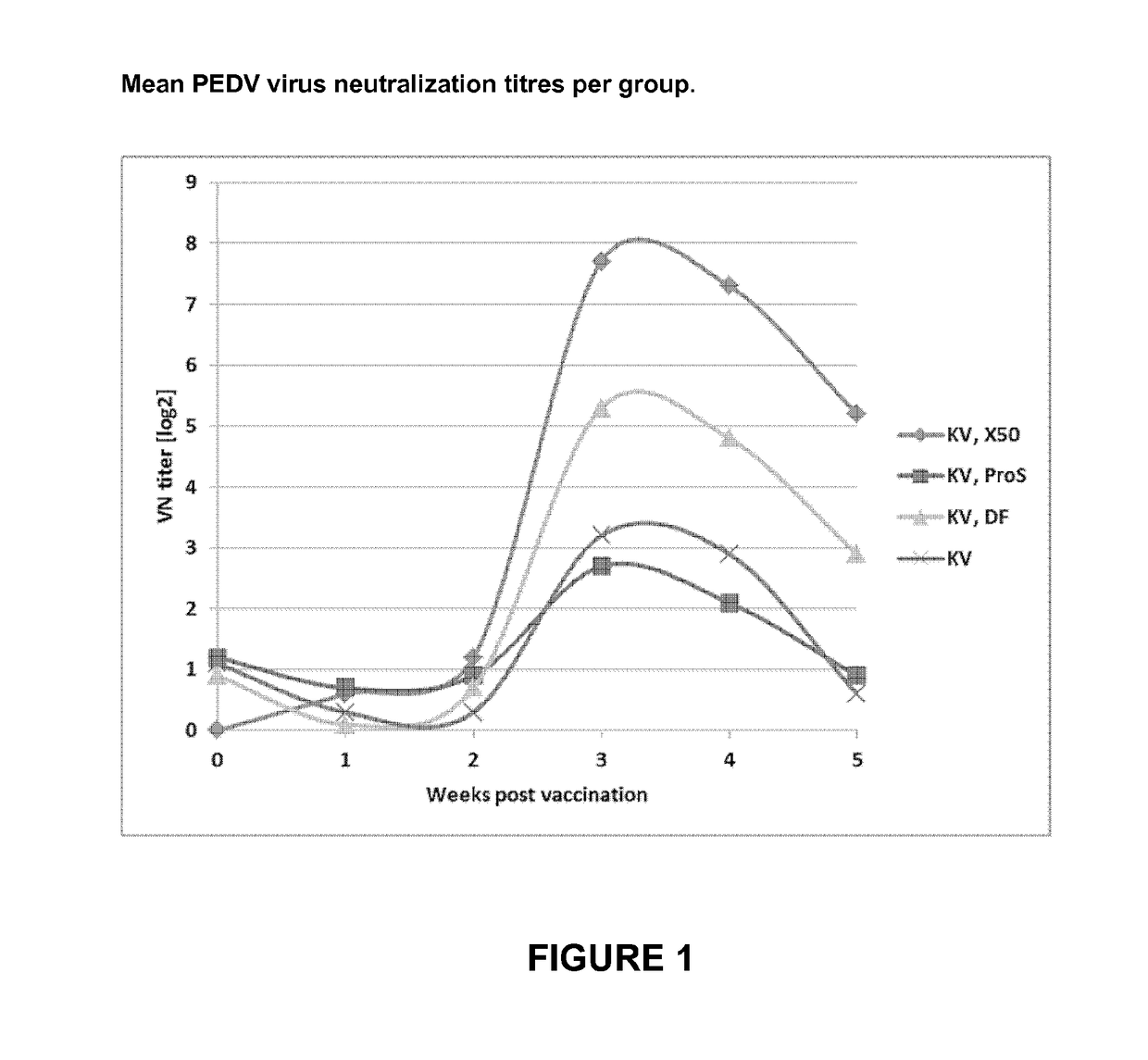
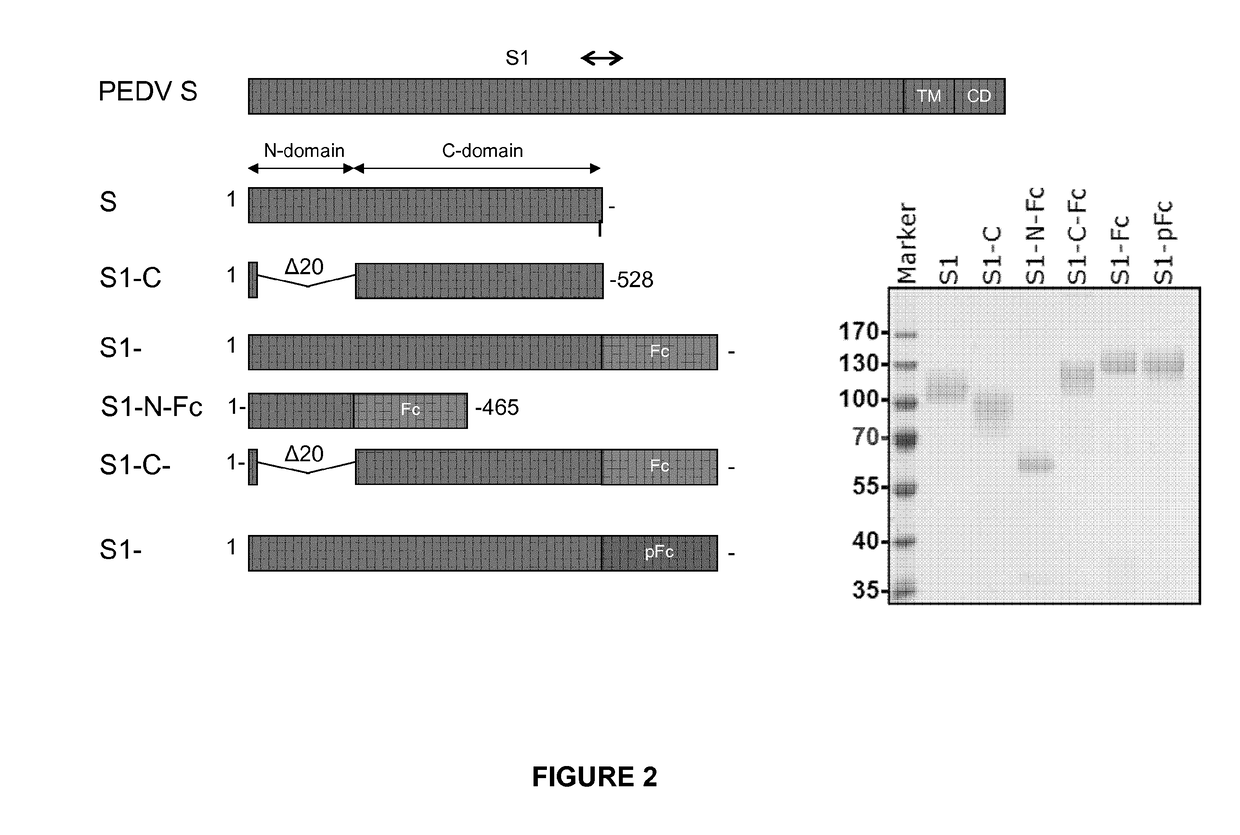
Vaccine for Use in Protecting Offspring of a Sow Against Porcine Endemic Diarrhea Virus
InactiveUS20170296648A1Safely immunizedHigh titres of VN antibodiesSsRNA viruses positive-senseViral antigen ingredientsAntigenAdjuvant
The present invention pertains to a vaccine for use in protecting offspring of a sow against an infection with porcine endemic diarrhea virus (PEDV), the vaccine comprising non-live PEDV antigen and an oil containing adjuvant, by administration of the vaccine to the pregnant sow at a dose of the antigen corresponding to at least 3.0E6 TCID50 killed whole PEDV. The invention also pertains to a method of protecting young piglets against an infection with porcine endemic diarrhea virus (PEDV).
Owner:UTRECHT UNIVERSITY +1
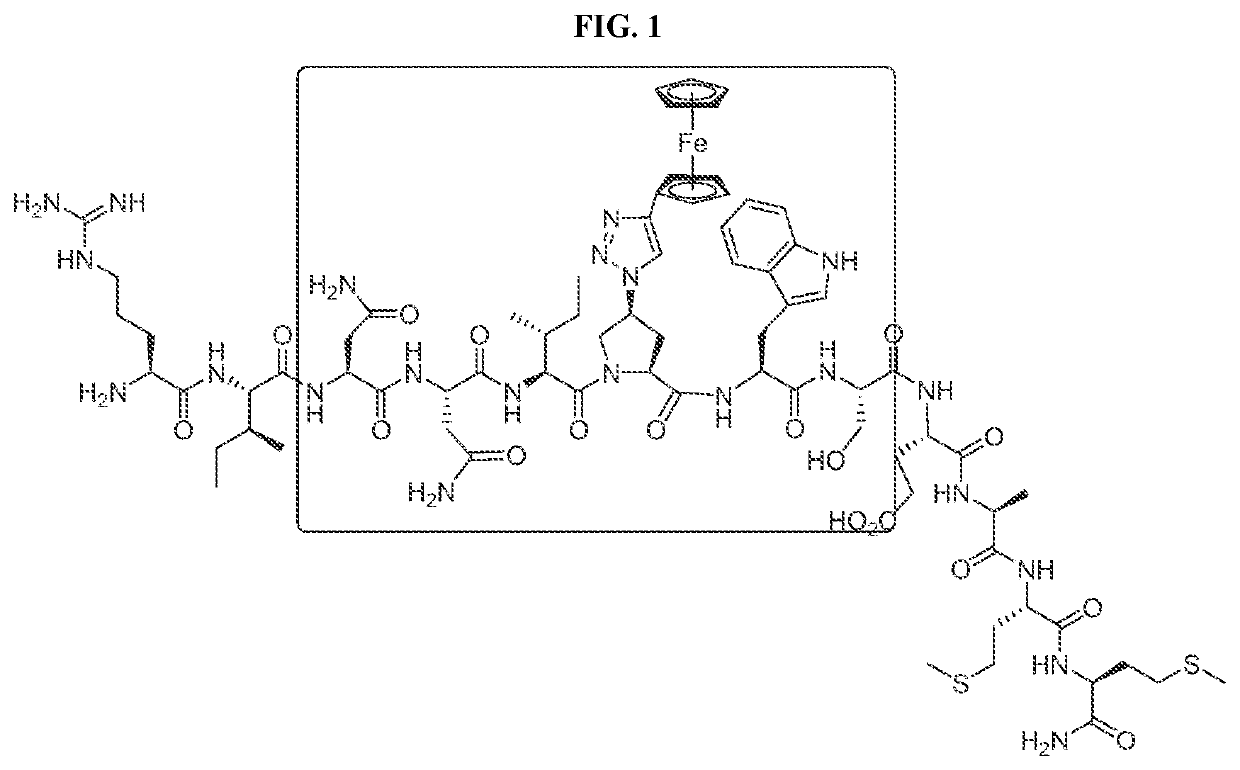
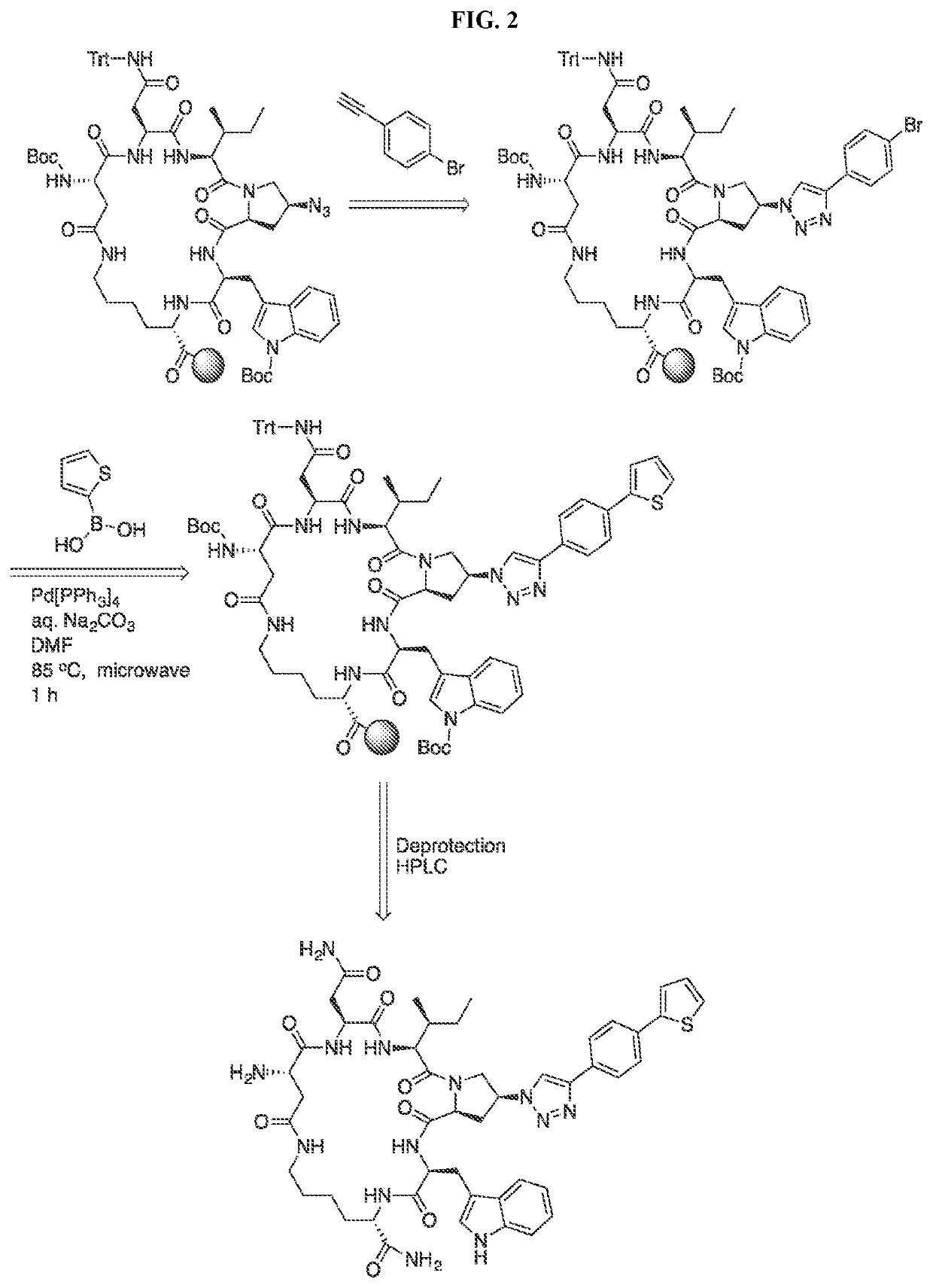
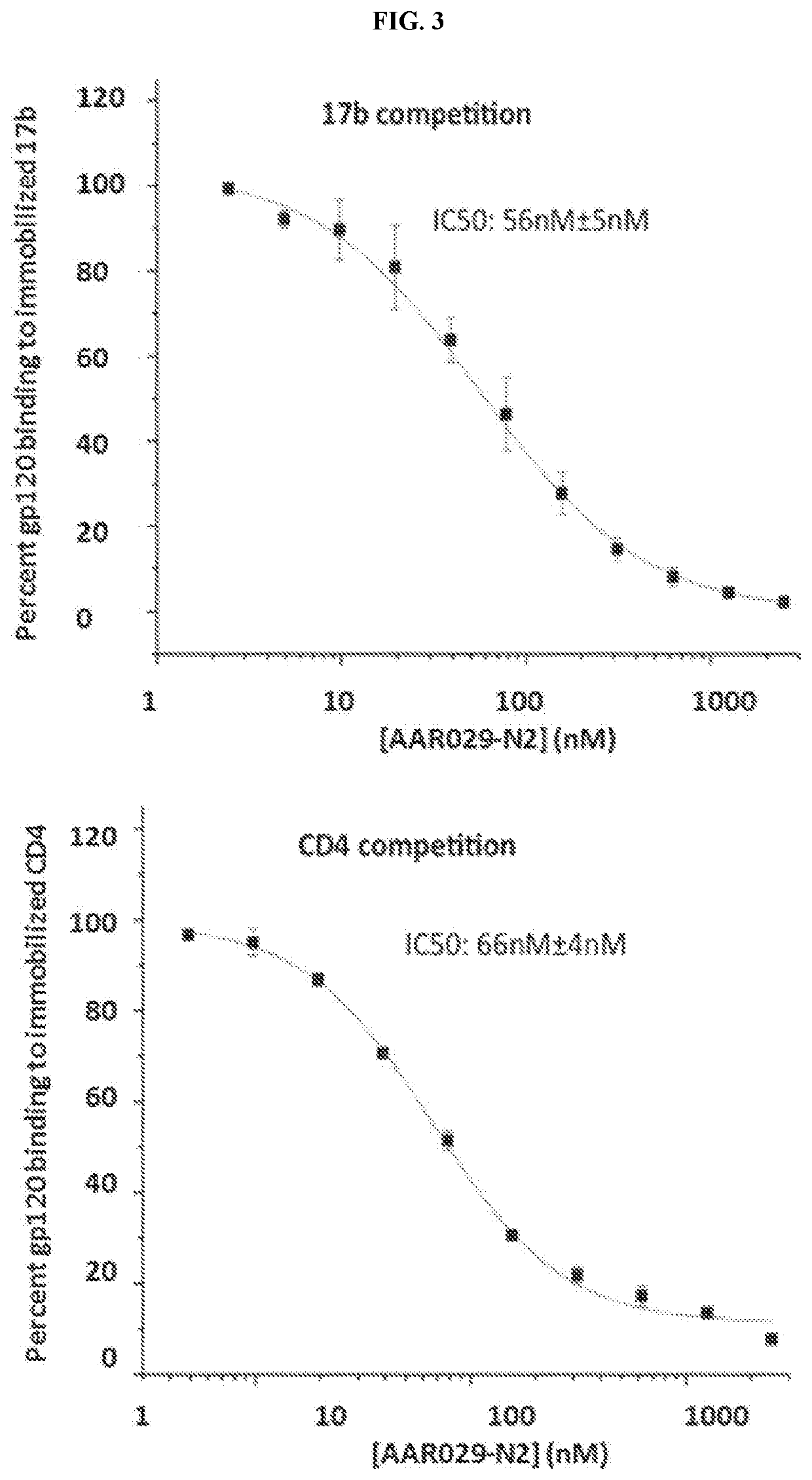
Cyclic Peptide Antiviral Agents and Methods Using Same
InactiveUS20190375792A1Reduce riskPromote virolysisPowder deliveryPeptide/protein ingredientsDrugHuman immunodeficiency virus (HIV)
The present invention includes novel cyclic peptides of formula (I). The present invention further includes novel cyclic peptides conjugated with a gold nanoparticle, and methods of using the same. The invention further provides a pharmaceutical composition comprising at least one pharmaceutically acceptable carrier and at least one cyclic compound of the invention. The invention further provides a method of treating, reducing, or preventing HIV-1 infection in a mammal in need thereof, the method comprising administering to the mammal a therapeutically effective amount of at least one cyclic compound of the invention.
Owner:DREXEL UNIV
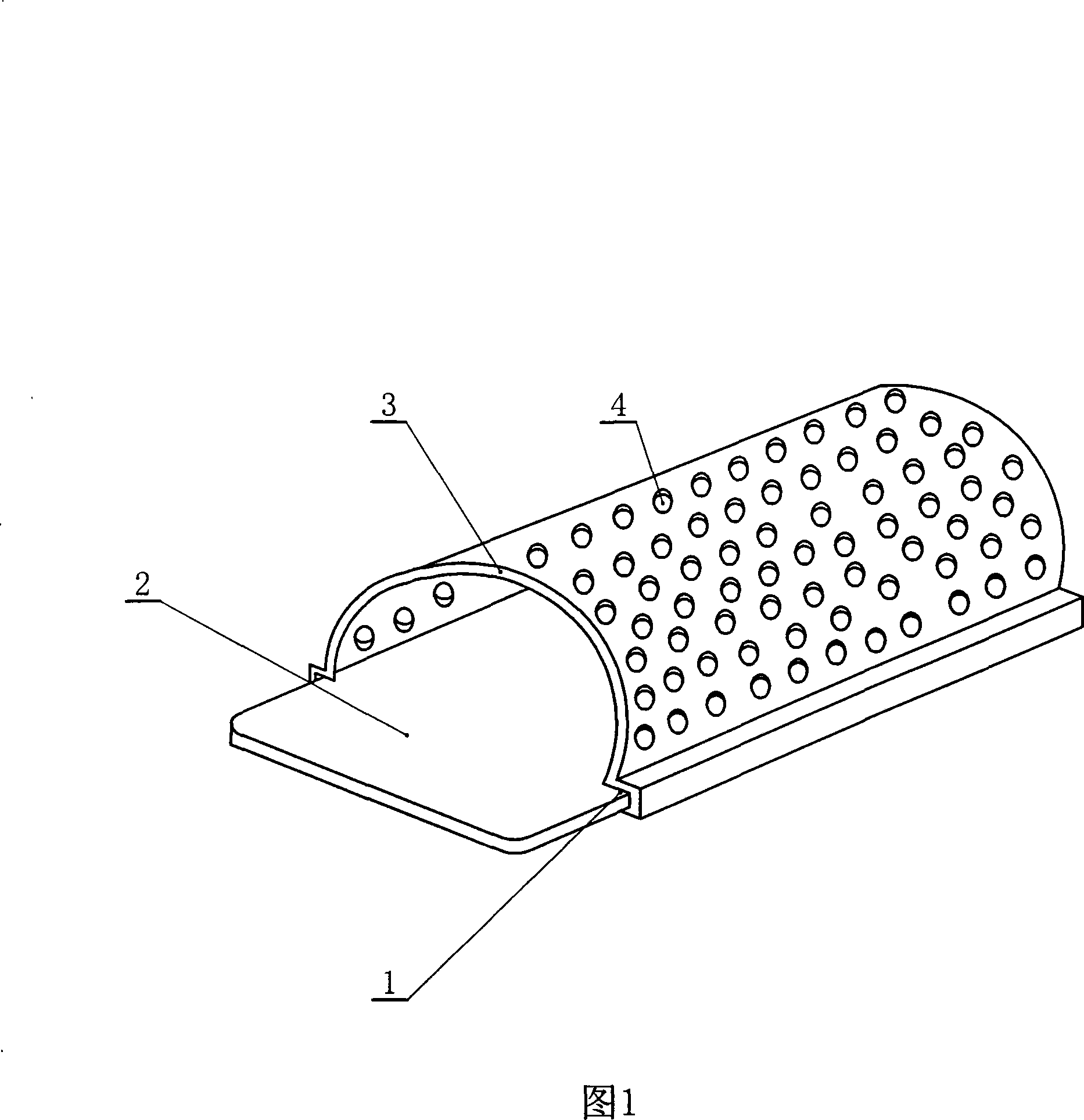
Hood for wound of limbs
Owner:王玉香
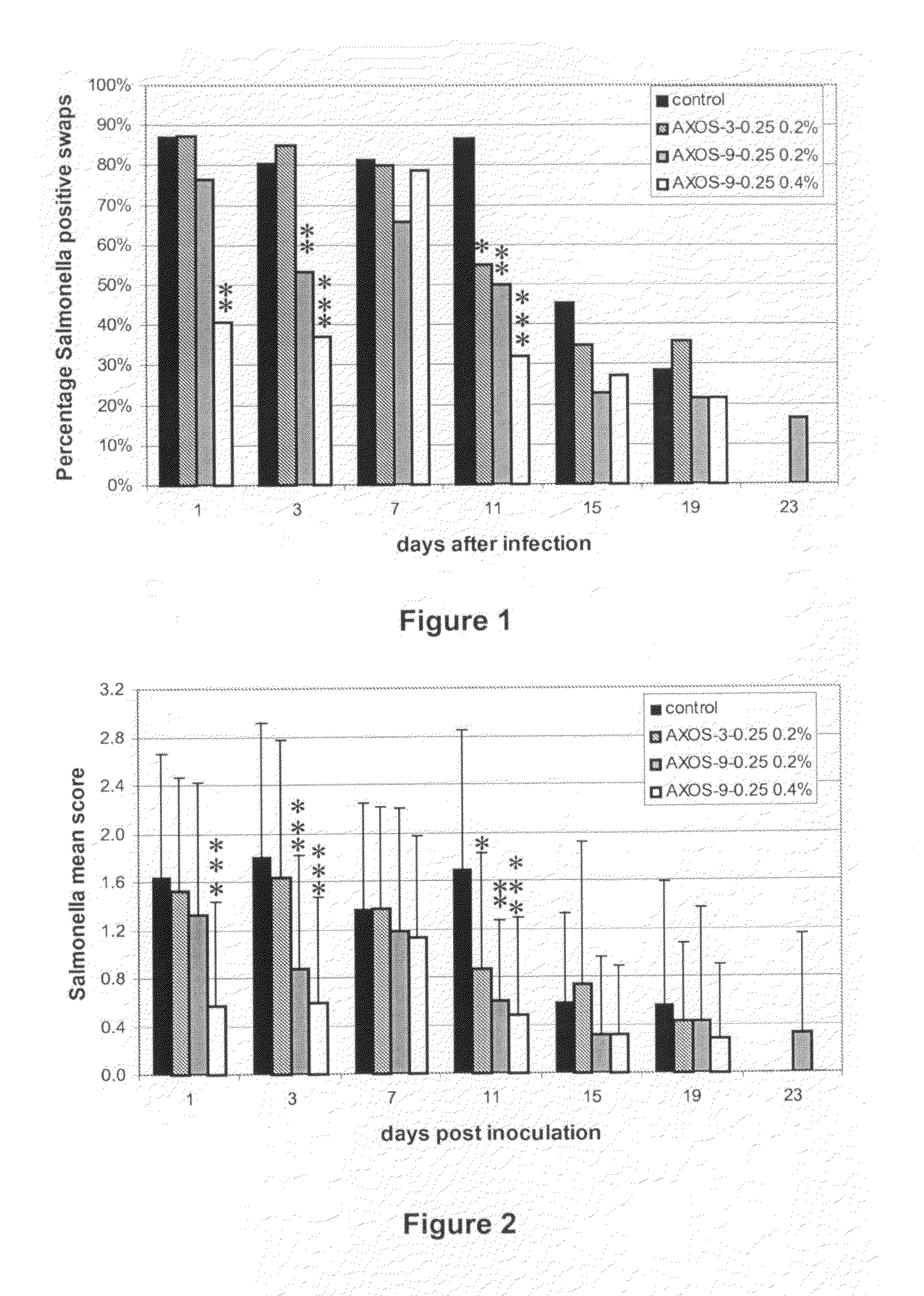
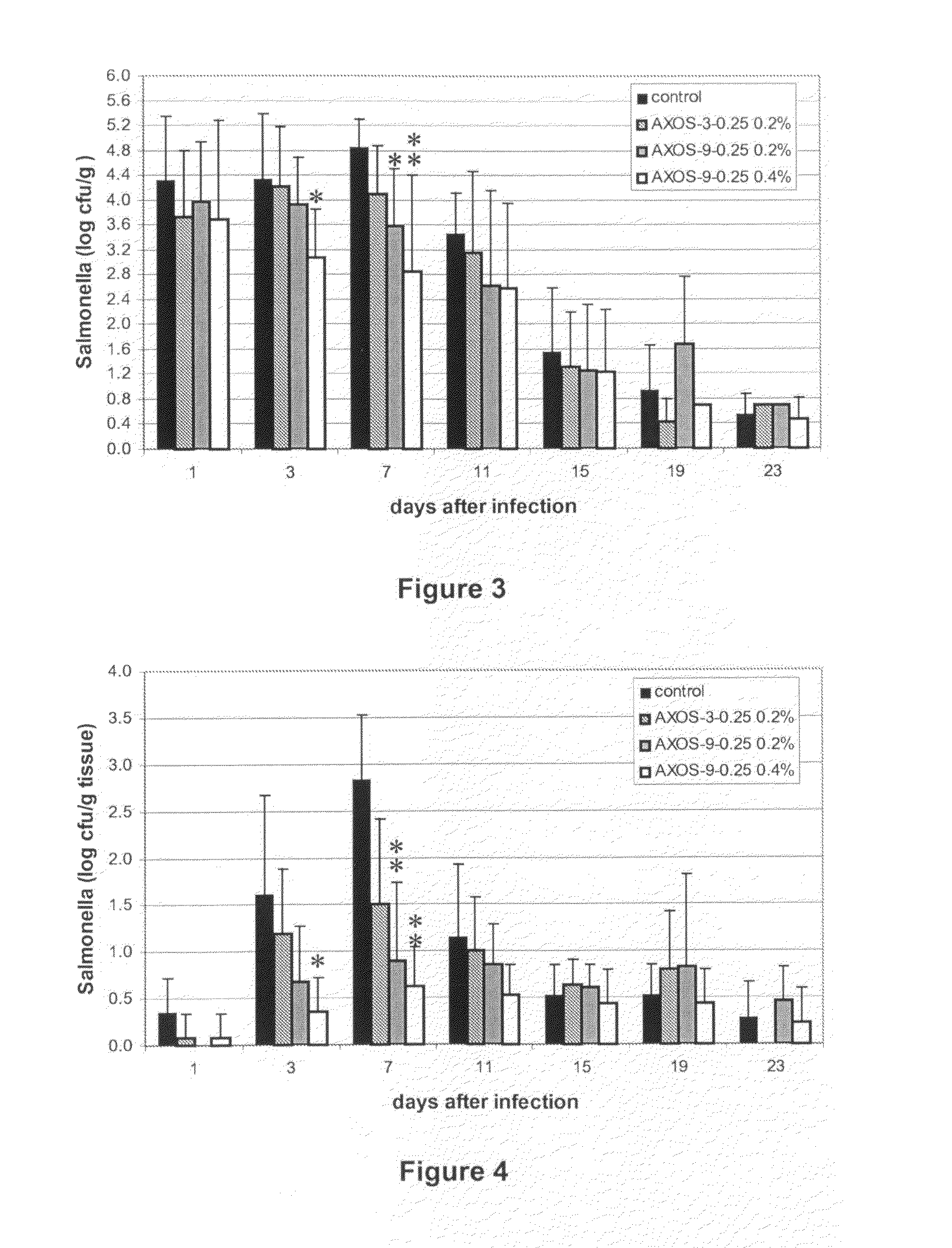
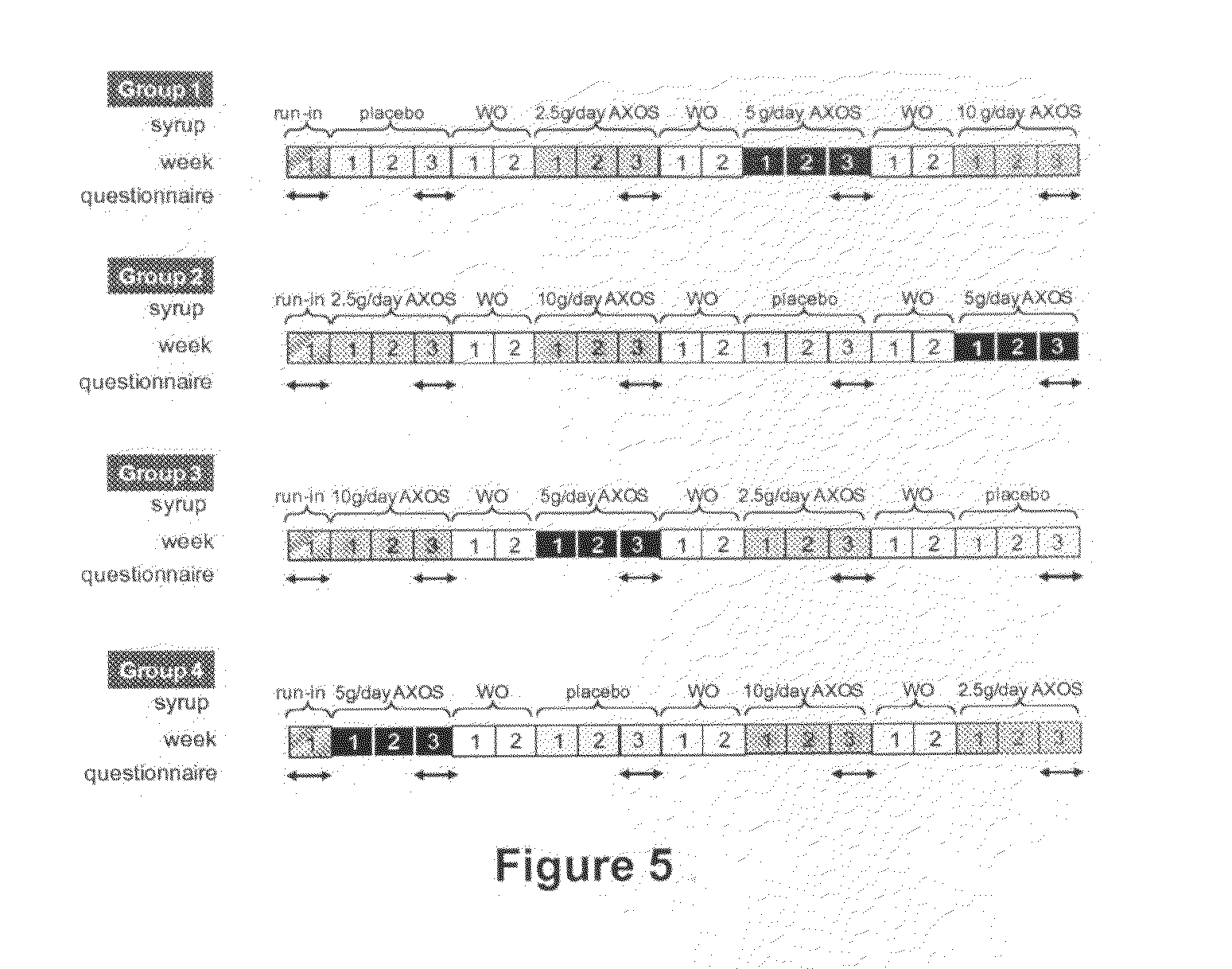
Arabinoxylo-oligosaccharides useful against gastrointestinal infections
InactiveUS20120015902A1Prevent and reduce infectionOrganic active ingredientsBiocideBacteroidesGastrointestinal infections
The present invention relates to the use of oligosaccharides derived from arabinoxylan for use in the prevention and treatment of gastrointestinal infection. More particularly the invention provides a method for preventing or reducing the gastrointestinal infection of a animal or human being with bacteria associated with gastroenteritis through the supplementation of their diets with the said oligosaccharides.
Owner:CARGILL INC
Method for inducing formation and proliferation of mucosal tissue resident memory T cells
PendingCN111012903APrevent and reduce infectionAntiinfectivesAntibody medical ingredientsPathogenMemory T cell
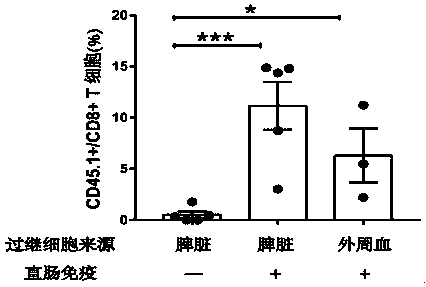
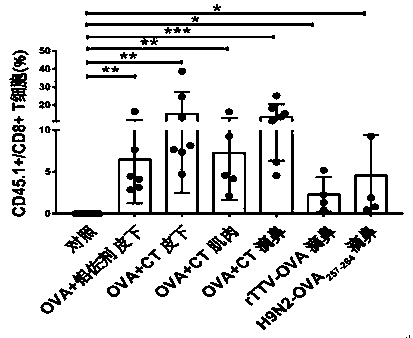
The invention relates to the field of mucosal immune cells, in particular to a method for inducing formation and proliferation of mucosal tissue resident memory T cells (TRM). The method at least comprises primary immunization of parts except intestinal tracts and enhanced immunization of the intestinal tracts, so that a large number of mucosal TRM are obtained and are used for preventing and treating mucosal invasion of pathogens. The primary immunization modes of the parts except the intestinal tracts at least comprise but are not limited to muscle inoculation, intracutaneous inoculation, subcutaneous inoculation, nasal drop inoculation, aerosol inhalation inoculation and sublingual inoculation. The immunity enhancing mode of the intestinal tract part comprises but is not limited to rectal intracavitary inoculation, and intestinal intracavitary inoculation is conducted through oral administration of enteric-coated vaccines.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
A vaccine for use against subclinical lawsonia infection in a pig
ActiveUS20180021422A1Reduce sheddingReduce negative impactAntibacterial agentsBacterial antigen ingredientsAntigenSubclinical infection
The present invention pertains to a vaccine comprising non live Lawsonia intracellularis antigen and a pharmaceutically acceptable carrier for use in a method to reduce in a pig the negative impact on average daily weight gain (ADWG) associated with subclinical infection with Lawsonia intracellularis, by systemic administration of the vaccine to the pig.
Owner:INTERVET INC
Vaccine for use against subclinical Lawsonia infection in a pig
ActiveUS10751405B2Reduce sheddingReduce negative impactAntibacterial agentsBacterial antigen ingredientsAntigenSubclinical infection
The present invention pertains to a vaccine comprising non live Lawsonia intracellularis antigen and a pharmaceutically acceptable carrier for use in a method to reduce in a pig the negative impact on average daily weight gain (ADWG) associated with subclinical infection with Lawsonia intracellularis, by systemic administration of the vaccine to the pig.
Owner:INTERVET INC
Smart composite with antibiofilm, mineralizing, and antiinfection therapeutic effects
PendingUS20220160464A1Promote bone growthPrevent and reduce infectionAntibacterial agentsDental implantsBiotechnologyTherapeutic effect
Owner:TEMPLE UNIVERSITY
Hood for wound of limbs
Owner:王玉香
A vaccine for use against subclinical lawsonia infection in a pig
ActiveUS20180000924A1Prevent and reduce infectionAvoid problemsAntibacterial agentsBacterial antigen ingredientsBacteroidesSubclinical infection
The present invention pertains to a vaccine comprising non live Lawsonia intracellularis antigen and a pharmaceutically acceptable carrier for use in a method to reduce in a pig the shedding of Lawsonia intracellularis bacteria associated with subclinical infection with Lawsonia intracellularis, by systemic administration of the vaccine to the pig.
Owner:INTERVET INC
Protective anti-ZIKV vaccine without inducing cross-reactions with dengue
ActiveUS11136354B2Development be complicatePrevent and reduce infectionSsRNA viruses positive-senseBacteriaProtective antigenIn vivo
The subject application provides a genetically modified recombinant facultative intracellular invasive bacterial pathogen (RFIIBP) or a recombinant attenuated Salmonella vaccine (RASV) strains encoding Zika virus (ZIKV) antigens. These strains exhibit high immunogenicity, complete safety, and attenuation, and are unable to persist or be shed in an infective or viable form. These RFIIBPs and RASVs also exhibit regulated delayed attenuation in vivo, regulated delayed in vivo synthesis of protective ZIKV antigens. Methods of inducing mucosal, systemic and cellular immunities in hosts are also provided and antibodies produced using the disclosed RFIIBPs and RASVs can comprise neutralizing antibodies against ZIKV that do not crossreact with Dengue virus (DENV).
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Methods And Compositions For Controlling Parasitic Infections Of Animals
ActiveUS20140147515A2Reduce harmful effectsEfficient infectionBiocideOrganic active ingredientsYeastBiotechnology
A composition for preventing or reducing harmful effects of protozoal infection is provided, comprising in one embodiment a yeast cell wall and a preparation derived from oregano. The composition may further include a mineral nutrient selected from selenium and / or zinc. The composition may also include a preparation derived from Yucca. Efficacy of the composition is shown against a variety of protozoal organisms.
Owner:ALLTECH CO LTD
Ultraviolate light sterilization apparatus
InactiveUS9457121B1Prevent and reduce infectionImprove efficiencyRespiratorsMedical devicesUltravioletEngineering
A sterilization apparatus for sterilizing air includes an outer housing having side walls extending between opposed bottom and top walls that, together, define an outer chamber. An inner housing is situated in the outer chamber and includes opposed top and bottom ends and having a wall structure that defines a sealed inner chamber extending therebetween. The wall structure includes at least one side wall that defines a transparent window. An inlet port is in communication with one end of the inner chamber and that directs unsterilized air into the inner chamber. An ultraviolet (“UV”) light source is positioned outwardly adjacent the window and configured to emit UV light through the window and into the inner chamber when energized so as to sterilize air in the inner chamber. An outlet port is in communication with another end of the inner chamber that directs sterilized air downstream from the inner chamber.
Owner:DAVIS MATTHEW PHILLIP
Inhibitory immunoglobulins
InactiveUS20180217144A1Easy to determineImpaired immune killing of Gram-negativeDisease diagnosisBiological testingAntigenCompound s
The present invention relates to methods for identifying the presence or elevated levels of IgG2 specific for O-antigen of Gram-negative bacteria in a subject. The method comprises providing a binding agent specific for said IgG2, contacting the binding agent with the sample, allowing the binding agent and IgG2 to form a complex and thereafter directly or indirectly detecting the complex. Also provided are methods for assessing the severity of infection and / or a worsening of a patient's condition. The present invention also relates to isolated O-antigens.
Owner:THE UNIV OF BIRMINGHAM
Cyclic Peptide Antiviral Agents and Methods Using Same
PendingUS20220204565A1Reduce riskPromoting virolysisPowder deliveryPeptide/protein ingredientsCyclic peptideNanoparticle
The present invention includes novel cyclic peptides, and methods of using the same. The present invention further includes novel cyclic peptides conjugated with a gold nanoparticle, and methods of using the same.
Owner:DREXEL UNIV
Vaccine for use against subclinical Lawsonia infection in a pig
ActiveUS10265392B2Reduce the shedding of <i>LawsoniaReduce negative impactAntibacterial agentsBiocideAntigenSubclinical infection
Owner:INTERVET INC
Cable-free arthroscopy
ActiveUS9089298B2Prevents and decreases riskReduce complexityLaproscopesEndoscopesArthroscopyTarsal Joint
The present invention relates to an arthroscopy apparatus, comprising at least three elements selected from: a conventional arthroscopic lens (12), to which there is coupled a power supply device or capsule, in the inside of which is the power source (1), and a miniature camera (8), characterized by not comprising connecting cables.
Owner:GARCIA PEDRO GUILLEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com