Patents
Literature
42 results about "Primary immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic polypeptide
InactiveCN103509107AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansPenicillinKeyhole-limpet haemocyanin
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic polypeptide. The method comprises the following steps: synthesizing a "CGAGAGSGAGAGS" polypeptide sequence by utilizing an Fmoc method, coupling the polypeptide with keyhole limpet hemocyanin (KLH) through the cysteine on the N terminus of the polypeptide so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain a primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antibody titer in the rabbit blood sample reaches 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate.
Owner:ZHEJIANG UNIV +1
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic dodecapeptide
InactiveCN103509108AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansKeyhole-limpet haemocyaninPrimary immunization
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic dodecapeptide. The method comprises the following steps: synthesizing a polypeptide with a "CGYGAGAGAGYGA" sequence, coupling the polypeptide with keyhole limpet hemocyanin (KLH) so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, carrying out an emulsion treatment so as to obtain primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then carrying out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antiserum titer of rabbit arrives at 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate. The antibody prepared by the invention has a strong specificity, and can be used for detection and analysis of silk fibroin in textile, and the like.
Owner:ZHEJIANG UNIV +1
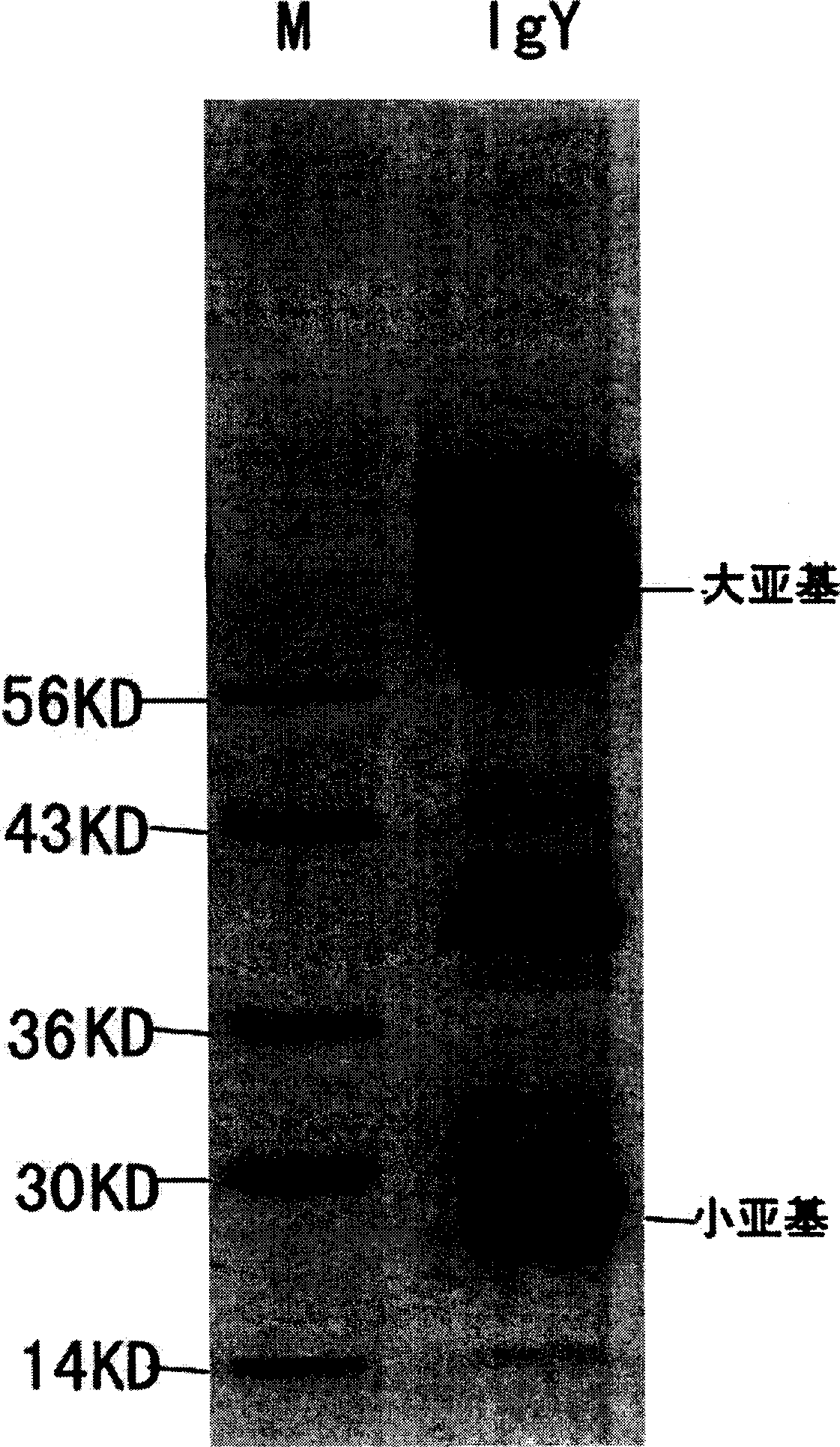
Yolk antibody of anti SARS coronavirus and its preparation method and liquid preparation
InactiveCN1556113AEasy to prepareFast preparation methodEgg immunoglobulinsImmunoglobulins against virusesYolkEpitope
A yolk antibody for SARS coronavirus is prepared through injecting the antigen, which may be recombinant genetic protein S, M, or E,or the antigen epitope for said protein able to represent SARS coronavirus, or the synthetic polypeptide of said protein, etc, in health hen for primary immunizing, booster immunizing, collecting its egg, extracting yolk, and extracting the yolk antibody from it. It can also be prepared to become liquid preparation. It can be used to prevent SARS.
Owner:BIOINFORBODY
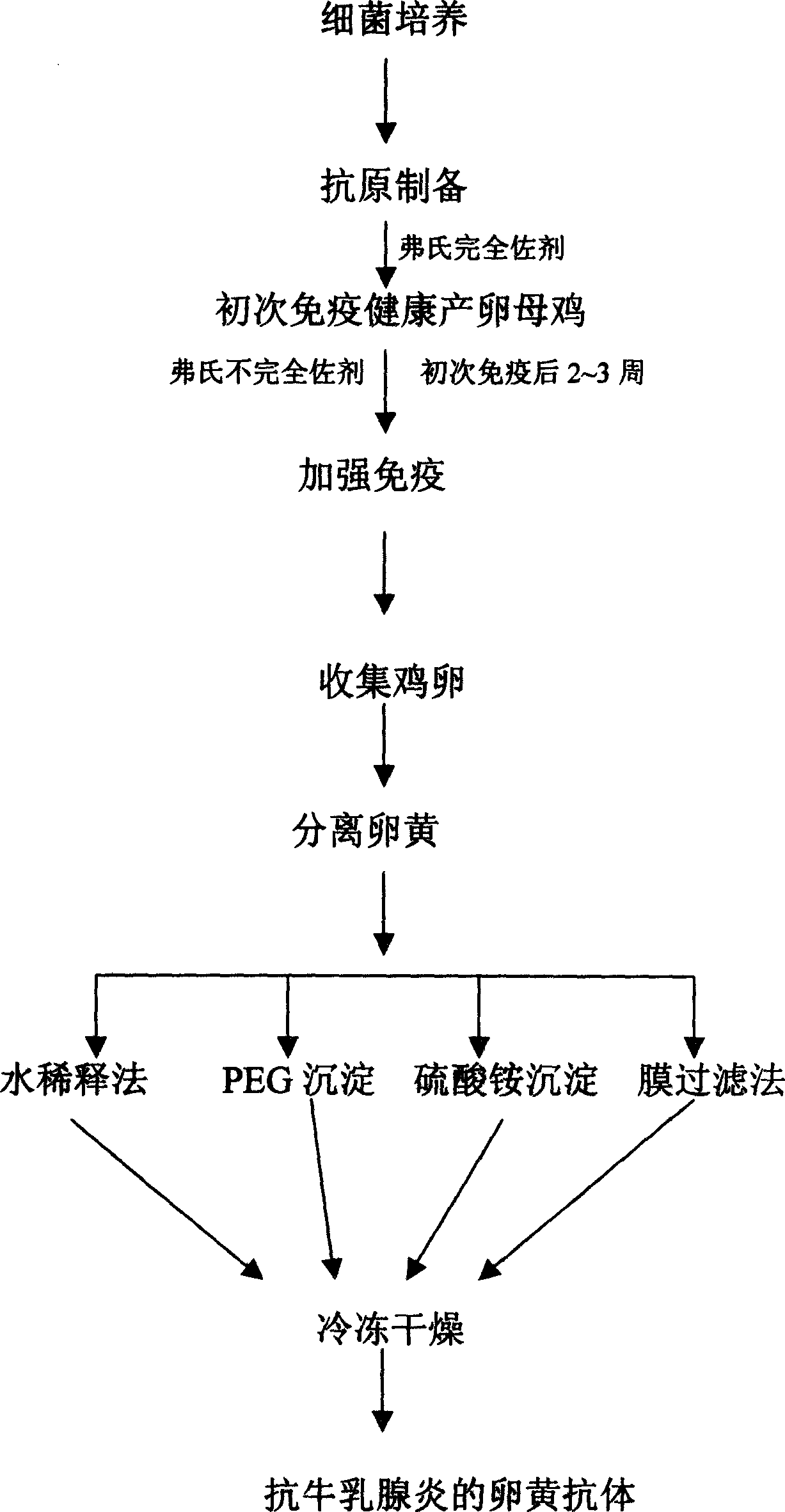
Bovine mastitis resistant yolk antibody and its preparation method and formulation
The invention discloses a vitelline antibody for resisting bovine mastitis, its preparing process and preparation thereof, wherein hens capable of healthy oviposition are used as immune animal, and pathogenic bacteria causing bovine mastitis diseases are used as antigens, the preparation process comprises the steps of first immunization, reinforced immunization, collecting eggs and extracting bovine mastitis resistant vitellus antibody from vitelline, the preparation includes liquid preparation, solid preparation and semi-solid ointment preparation. The vitelline antibody for resisting bovine mastitis and its preparation can be used in the prevention and cure of bovine mastitis caused by pathogenic bacteria.
Owner:BIOINFORBODY
Hybridoma cell strain secreting thiamethoxam monoclonal antibody and application thereof
InactiveCN108998422AHigh detection sensitivityImprove featuresMicroorganism based processesDepsipeptidesBALB/cIc50 values
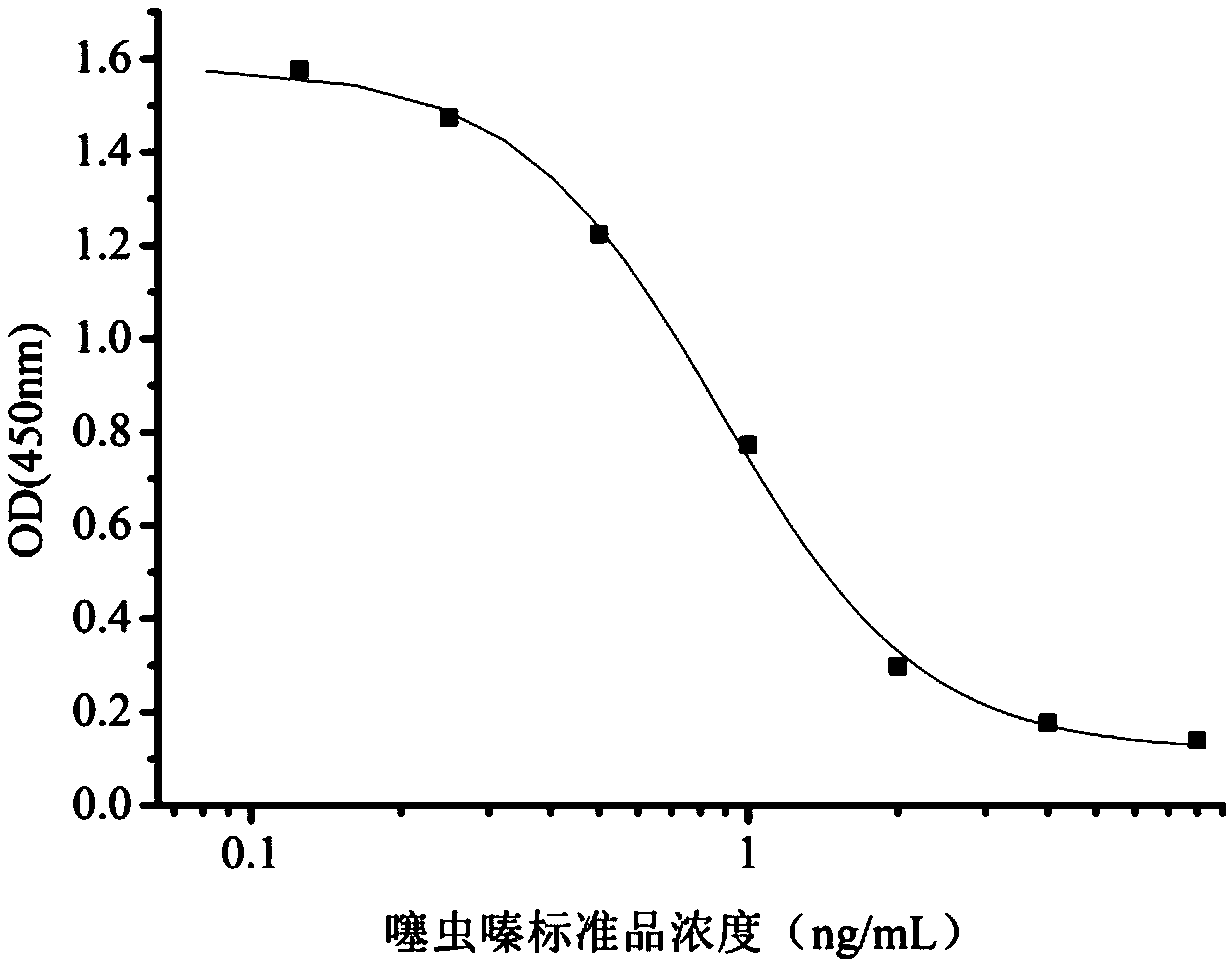
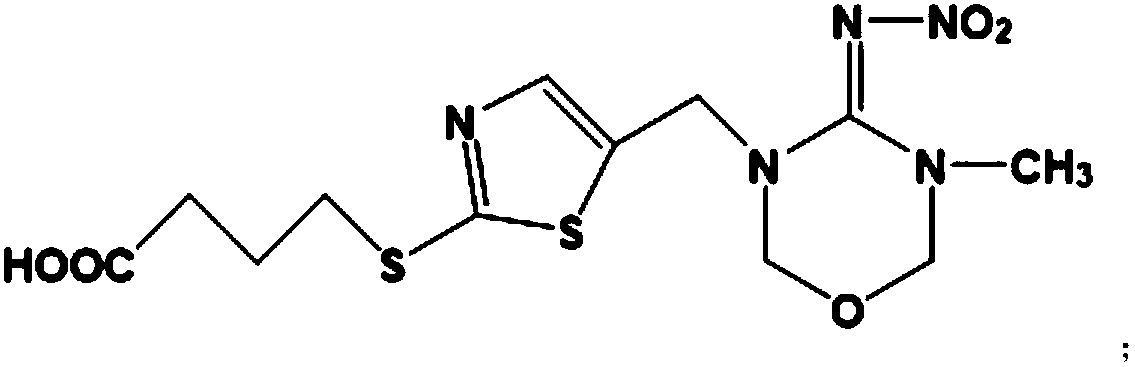
The invention relates to a hybridoma cell strain secreting thiamethoxacin monoclonal antibody and application thereof, belonging to the field of food safety immunodetection. The accession number of the hybridoma cell strain is CGMCC No. 14699. According to the invention, a complete Freund's adjuvant is used for primary immunization of a BALB / c mouse, then an incomplete Freund's adjuvant is used for booster immunization three times, and a thiamethoxam complete antigen containing no adjuvant is used for impact immunization once, so the BALB / c mouse is immunized; and then the high-titer low-IC50spleen cells of the immunized mouse are fused with mouse myeloma cells by using a PEG method, and then the cell strain is obtained through indirect competitive ELISA screening and subcloning three times. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (with an IC50 value of 0.81 ng / mL) to thiamethoxam and can be used for detection of thiamethoxamresidues in food.
Owner:JIANGNAN UNIV +1
Antigen-antibody complex for preventing and/or treating avian influenza
ActiveCN101732716AChange submission pathEnhance immune functionAntiviralsAntibody ingredientsAvian influenza virusOrganism
The invention provides an antigen-antibody complex for preventing and / or treating avian influenza, which comprises inactivation avian influenza totiviruses which are used as antigen and an immune body thereof, and the mass concentration ratio of the antigen and the immune body is more than 1. After entering organisms to perform initial immunization, the antigen-antibody complex stimulates the organisms again to induce immunoreaction, and immune response is quick in speed and strong in reaction; the antigen-antibody complex used as a carrier is more favorable for capturing and presenting antigen presenting cells and can also strengthen a breeder reaction of T cells effectively; purified totiviruses used as the antigen increase the molecular weight of the complex, are more favorable for ingestion of immunocyte of the organisms on the antigen, cause the more extensive immunoreaction quickly, and have a significance for preventing avian influenza viruses of which the antigen is easy for variation. A preparation of the antigen-antibody complex does not need to use solid carriers, does not cause intense stimulus on the organisms or initiates the organisms to generate an adverse reaction, and is simple to prepare and safe and convenient to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
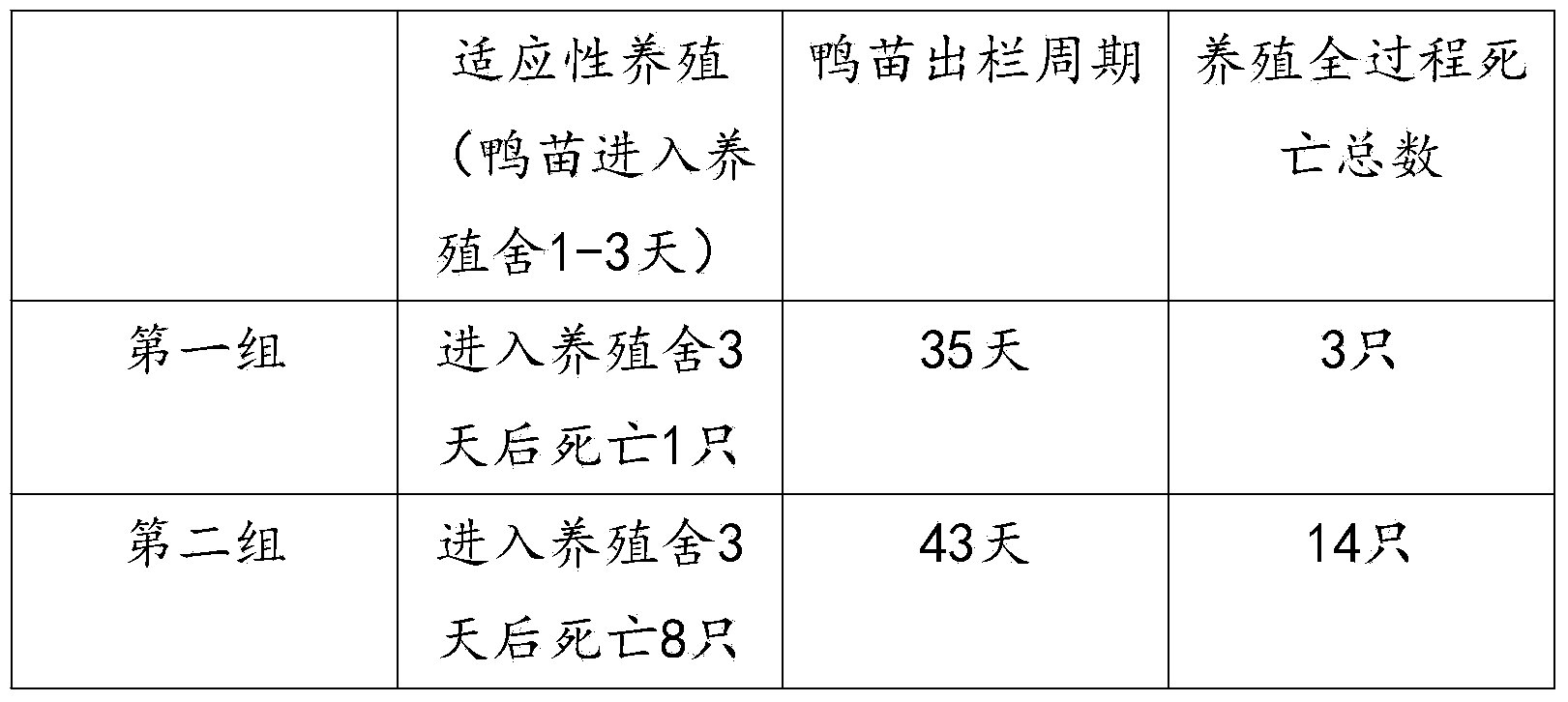
Meat duck online raising method
InactiveCN104137808AImprove the immunityReduce mortalityAnimal husbandryMaternal antibodyMortality rate
The invention provides a meat duck online raising method, and relates to the field of commercial duck raising. The method includes the following steps that according to a place where a farm is located, ducklings adapting to the local growing environment are purchased; when the ducklings just enter the farm, adaptive raising for 1 to 3 days needs to be conducted; after adaptive raising, the maternal antibody conditions of the ducklings are detected, accordingly, immunization of an avian influenza vaccine and feed preparation are conducted, and primary immunization time is determined according to a maternal antibody fading rule; after being vaccinated, the ducklings are placed in a mesh raising house to be raised, suspended meshes are horizontally arranged in the raising house, and meat ducks are raised on the meshes until being slaughtered. The temperature and the humidity of the environment where the ducklings are located are adjusted according to a steam fumigation method, essential oil separated out from traditional Chinese medicine is contained in steam, stress of the ducklings to the new environment can be reduced, immunity of the ducklings is improved, and therefore the death rate of the ducklings is decreased when the ducklings enter the new environment; compound feed is mixed with water extract of traditional Chinese medicine auxiliary materials, so that the purposes that immunity of the ducks is improved and the slaughter period is shortened are achieved.
Owner:安徽诺阳禽业有限公司
Egg yolk antibody mouth wash capable of preventing and treating ozostomia and preparation method thereof
InactiveCN106214510AInhibit the growth of bacteriaAvoid breedingAntibacterial agentsCosmetic preparationsNormal floraBooster immunizations
The invention belongs to the field of biotechnology and discloses an egg yolk antibody mouth wash capable of preventing and treating ozostomia and a preparation method of the egg yolk antibody mouth wash. The mouth wash comprises the following components in parts by weight: 1-10 parts of specific complex egg yok antibody preparation, 1-10 parts of a Chinese herb extract, and 100 parts of sterile water. The specific complex egg yolk antibody preparation is a complex antigen prepared from porphyromonas gingivalis, fusobacterium nucleatum and a bacterium internationally named Solobacterium moorei, a laying hen is primarily immunized and then immunized in a booster manner for five times all together, an egg is collected from the first booster immunization, an egg yolk liquid is obtained, separated and purified to obtain a specific complex egg yolk antibody. The egg yolk antibody mouth wash contains both the specific complex egg yolk antibody and an important extract, the breeding of microorganisms in an oral cavity is effectively inhibited, the ozostomia is prevented and treated, the normal flora of the oral cavity is not affected, and the mouth wash is safe and harmless.
Owner:GUANGDONG UNIV OF TECH
Influenza composition
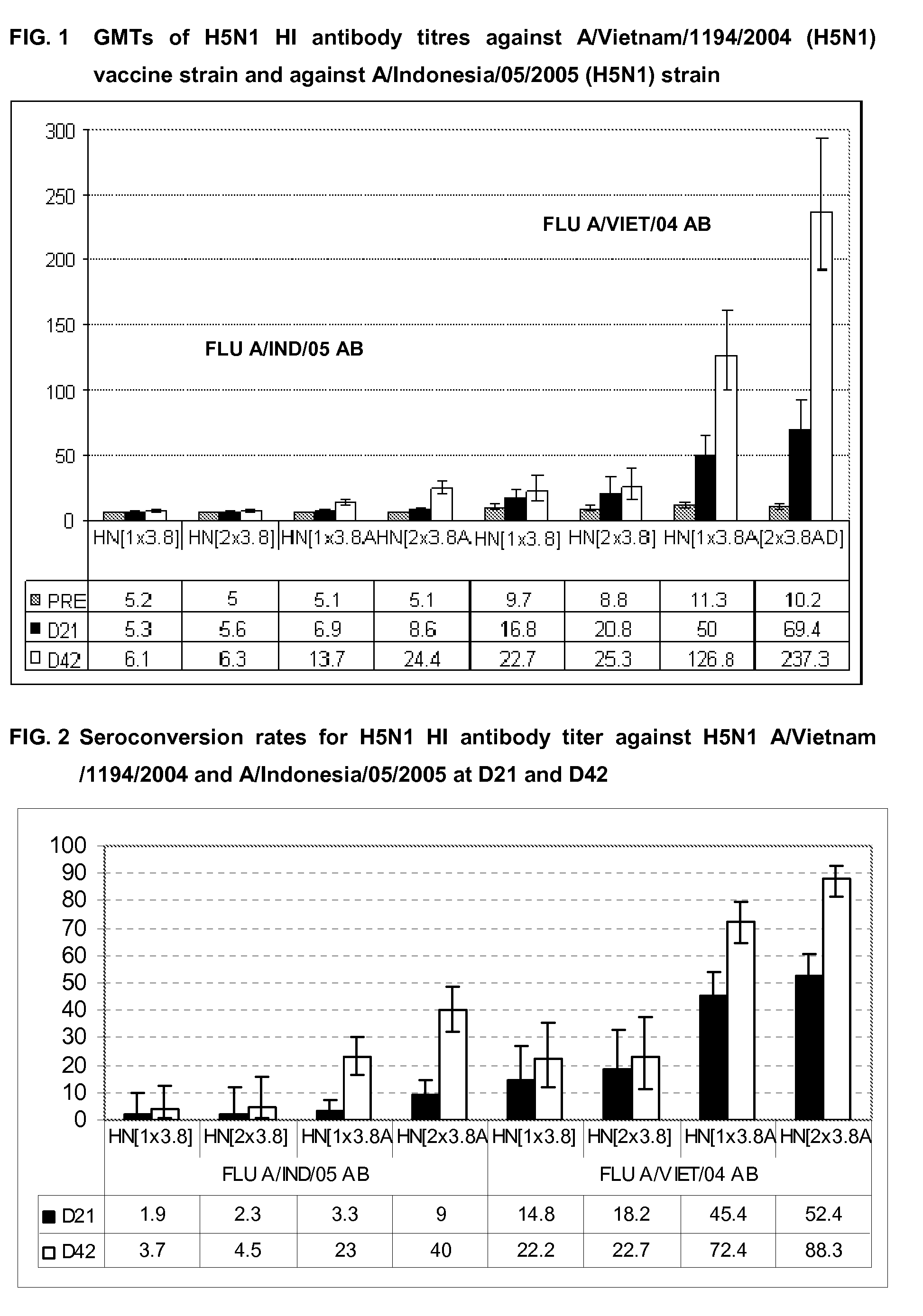
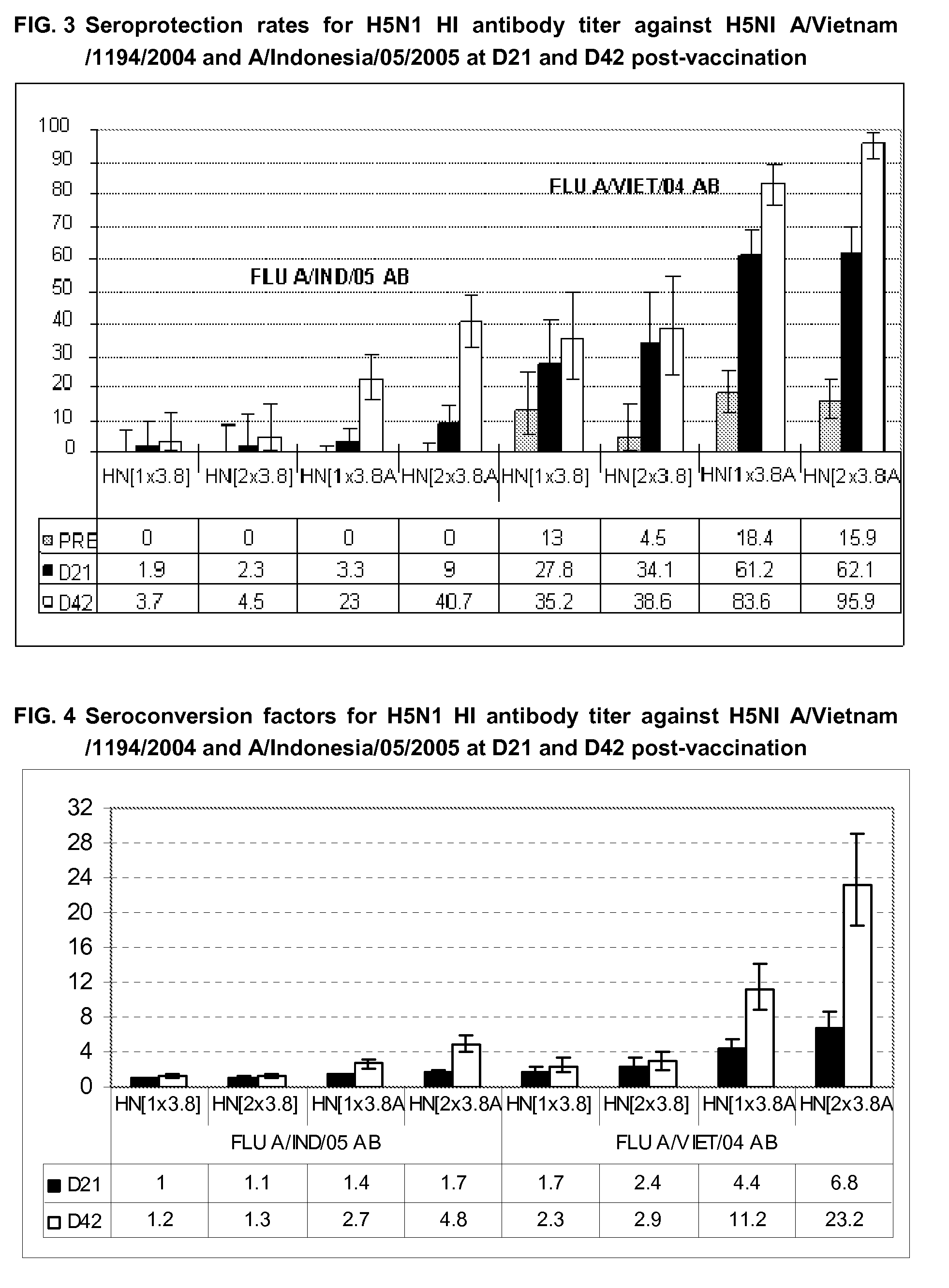
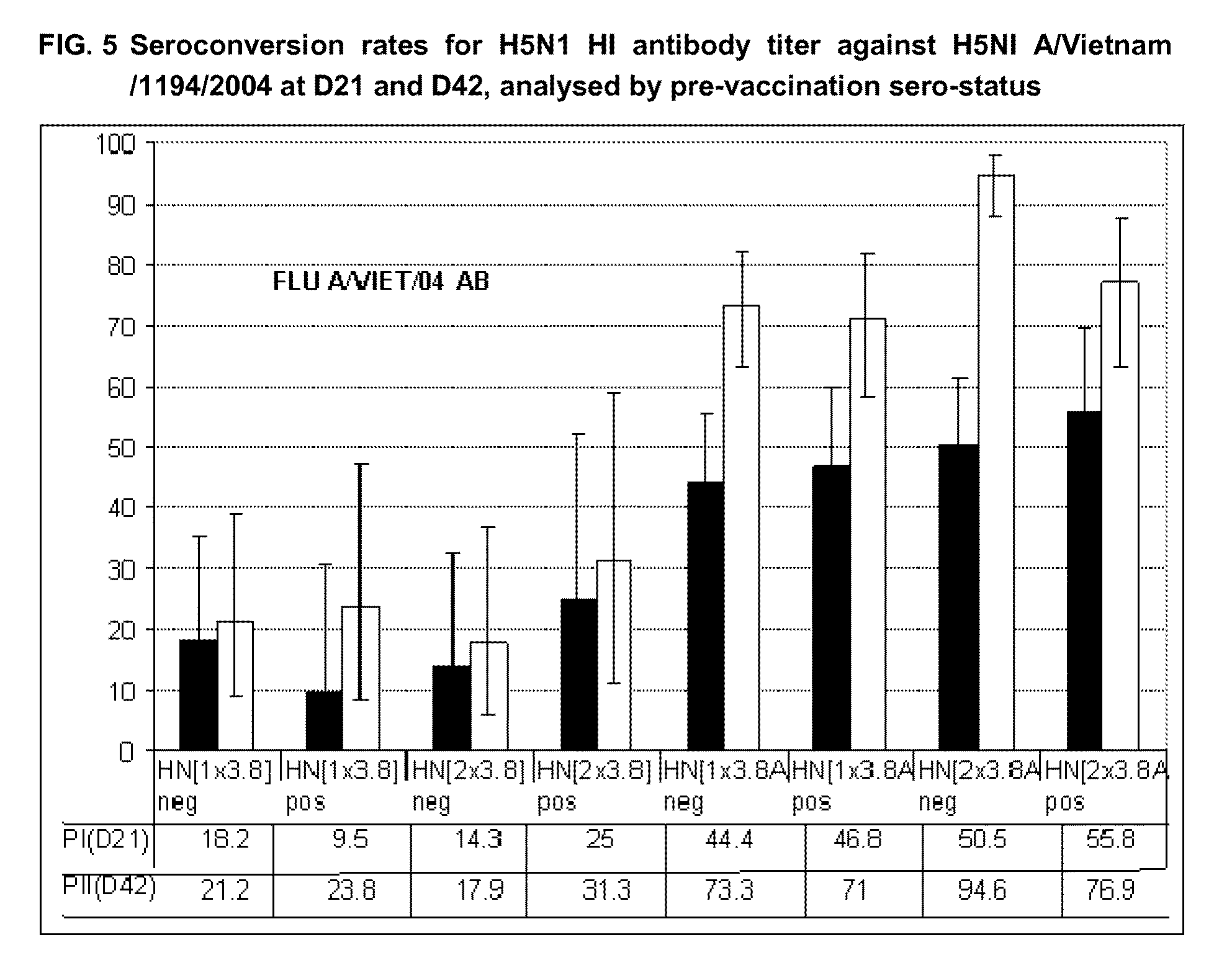
The present invention relates to influenza vaccine formulations and accelerating primary vaccination regimes for immunizing against influenza disease, their use in medicine, in particular their use in promoting effective immune responses to various antigens, and to methods of preparation. In particular, the invention relates to two-doses accelerated pandemic or seasonal pandemic primary immunization regimes with influenza immunogenic compositions comprising an influenza virus or antigenic preparation thereof in combination with an oil-in-water emulsion adjuvant, and to accelerated immunization regimes.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method for establishing Macaca fascicularis experimental autoimmune encephalomyelitis model and application thereof
InactiveCN103933550AIndividual smallReduce usagePeptide/protein ingredientsEmulsion deliveryDiseaseNODAL
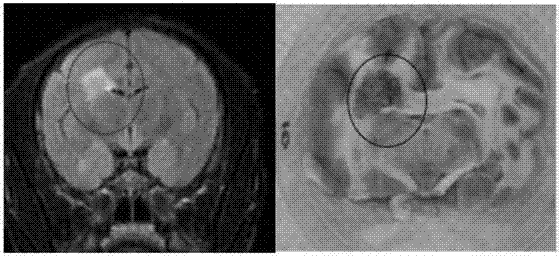
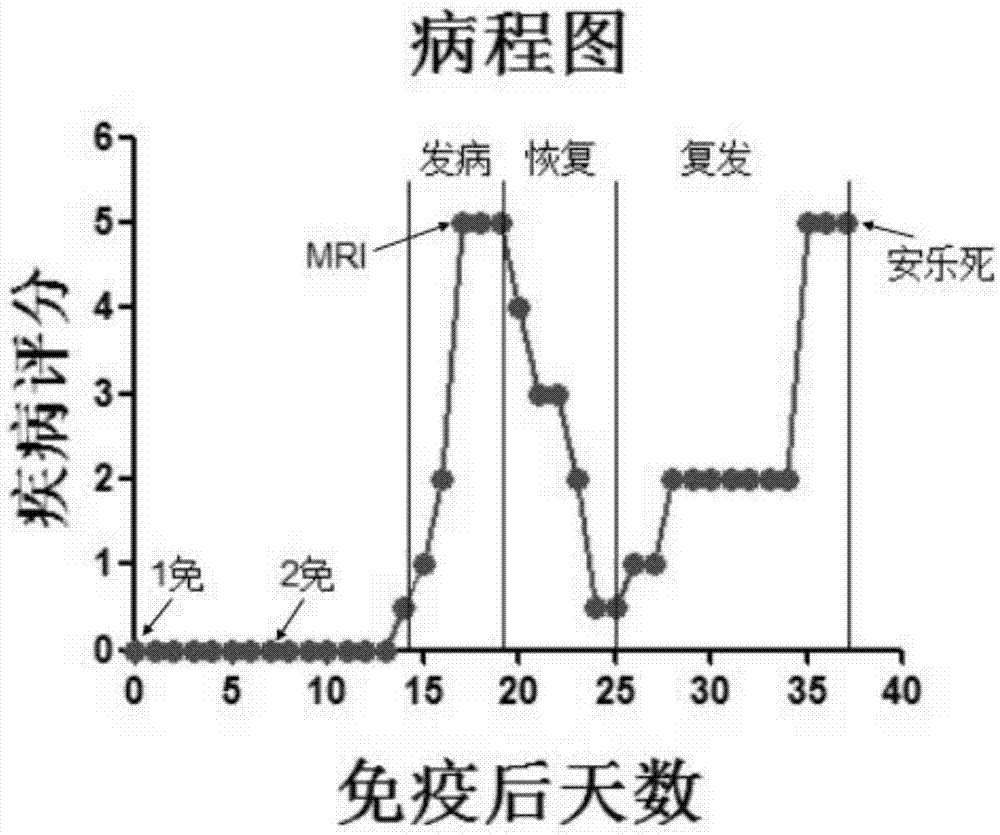
The invention discloses a method for establishing a Macaca fascicularis experimental autoimmune encephalomyelitis model and application thereof. According to a concrete technical scheme in the invention, the method comprises the following steps: preparing an emulsion (an MOG solution: CFA = 1: 1) from MOG 34-56 (100 mu g / ml); narcotizing Macaca fascicularis for experiments and injecting 1 ml of the prepared emulsion at 10 injection points; carrying out immunization injection of the emulsion (secondary immunization) according to the above-mentioned method and dose in 7 days after the primary immunization injection (primary immunization); carrying out clinical observation in one day after primary immunization and recording clinical scores; and determining pathogenic sites, degrees and the like by using an MRI iconographic method at pathogenic time nodes. The model established in the invention has an application value which cannot be achieved by other rodent models, has the characteristics of recurrence-alleviation type attacks, low cost, wide availability of Macaca fascicularis for experiments and the like compared with a Macaca rhesus model and has a wide application scope in fields related to multiple sclerosis diseases.
Owner:上海浦灵生物科技有限公司
A method for producing turbot immunoglobulin
InactiveCN102268088AOvercoming idiomsSimple structureImmunoglobulins against animals/humansAquatic animalBovine serum albumin
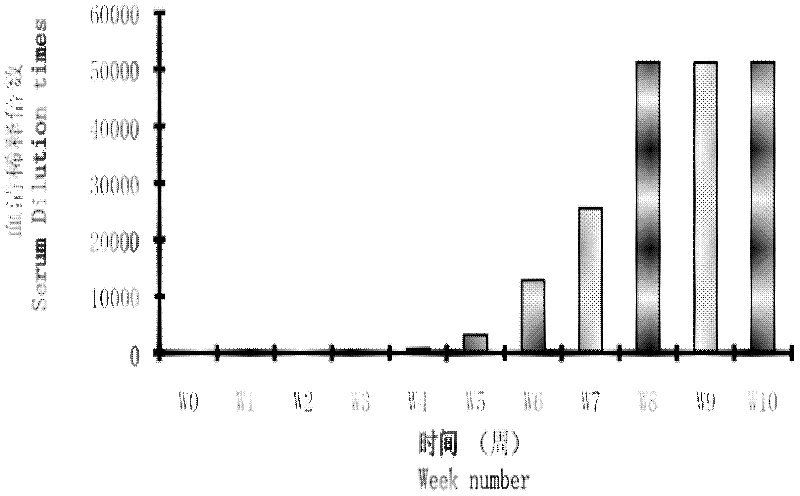
The invention relates to a method for producing turbot immunoglobulin, which is characterized in that the turbot is immunized by using bovine serum albumin BSA as an antigen; specifically, after three immunization operations, after the first immunization The anti-BSA immunoglobulin of turbot was obtained by affinity chromatography within 8 to 10 weeks. The invention overcomes the conventional usage of BSA in the field of biochemistry, and uses it as an antigen to immunize turbot through a specific immunization procedure, so as to obtain high titer anti-BSA specific antibody. The method of the invention solves the long-standing problem of lack of sufficient specific immunoglobulin in turbot immunization research. The BSA used at the same time has the advantages of simple structure, single component, not easy to interfere with immune results, and easy to obtain. BSA can be established as a model protein in aquatic animals, and a research model based on BSA can be formed.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Method for using characteristic hexapeptide for preparing tussah fibroin protein antibody
ActiveCN105504056AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansAdjuvantKeyhole-limpet haemocyanin
Owner:ZHEJIANG SCI-TECH UNIV
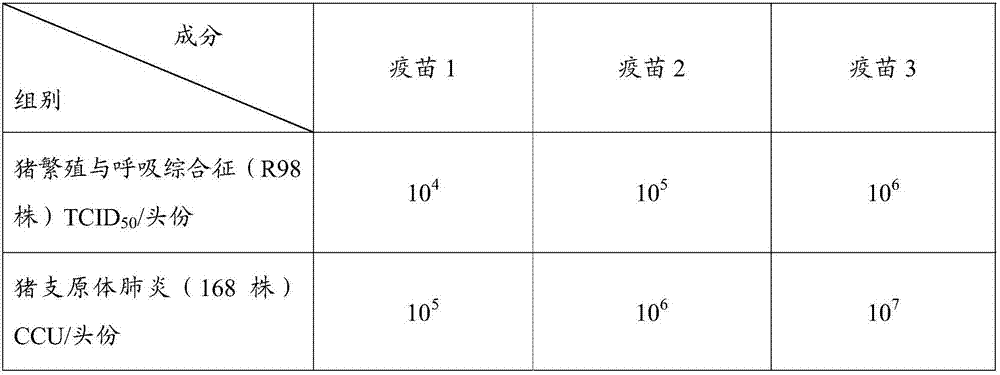
Porcine reproductive and respiratory syndrome, swine mycoplasmal pneumonia combined live vaccine and preparation method thereof
InactiveCN107349424ASecurity plusEasy to prepareAntibacterial agentsPowder deliveryFreeze-dryingVaccine antigen
The invention relates to porcine reproductive and respiratory syndrome, swine mycoplasmal pneumonia combined live vaccine and a preparation method thereof, which belong to the technical field of a veterinary biological product. The vaccine contains swine mycoplasmal pneumonia live vaccine antigen with immunization amount and porcine reproductive and respiratory syndrome with immunization amount, a freeze-drying protective additive is added, and a step of freeze drying is carried out. The antigenic component of the combined live vaccine are non-mutual interference or non-mutual influence, and mutually enhance the immunization effect, the primary immunization can reach the immunization protection, and the method has the advantages of good security, simple preparation method, convenient and fast immunization, and reduced immunization cost.
Owner:NANJING DAYAO NETWORK TECH CO LTD
Preparation method of BSA monoclonal antibody
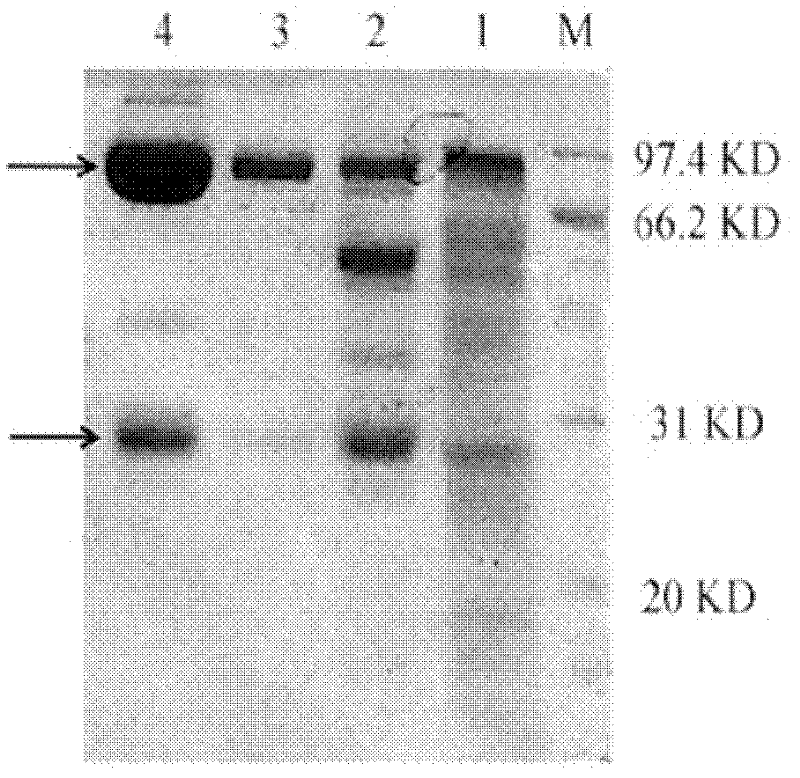
InactiveCN112694531AIncrease productionImmunoglobulins against animals/humansBiological material analysisPrimary immunizationBiochemistry
The invention provides a preparation method of a BSA monoclonal antibody. The method comprises the following steps: preparing polypeptide: firstly, preparing 10mg of polypeptide with the purity of 90%, and coupling 5mg of polypeptide with KLH; carrying out animal immunization: selecting five mice, firstly carrying out primary immunization, then carrying out secondary immunization, repeating the secondary immunization step to carry out third immunization on the mice, and then carrying out fourth immunization; carrying out cell fusion; screening: selecting monoclonal holes with high positive values; culturing and amplifying the selected monoclonal hybridoma cells and cryopreserving the cells; and obtaining purified monoclonal antibodies. A large number of monoclonal antibodies can be prepared, the prepared antibodies are stored at minus 20 DEG C after being subpackaged and stored at 2-8 DEG C in a short time, when the antibodies are used, the solution only needs to be diluted according to the needed concentration and prepared for immediate use, the antibody production amount is greatly increased, it is found through inspection that the monoclonal antibodies can be combined with BSA and cannot be combined with HAS, and a foundation is laid for future medical development.
Owner:TIANJIN AMCELLGENE ENG
Testing method for potency of inactivated vaccine against duck Tembusu viral diseases
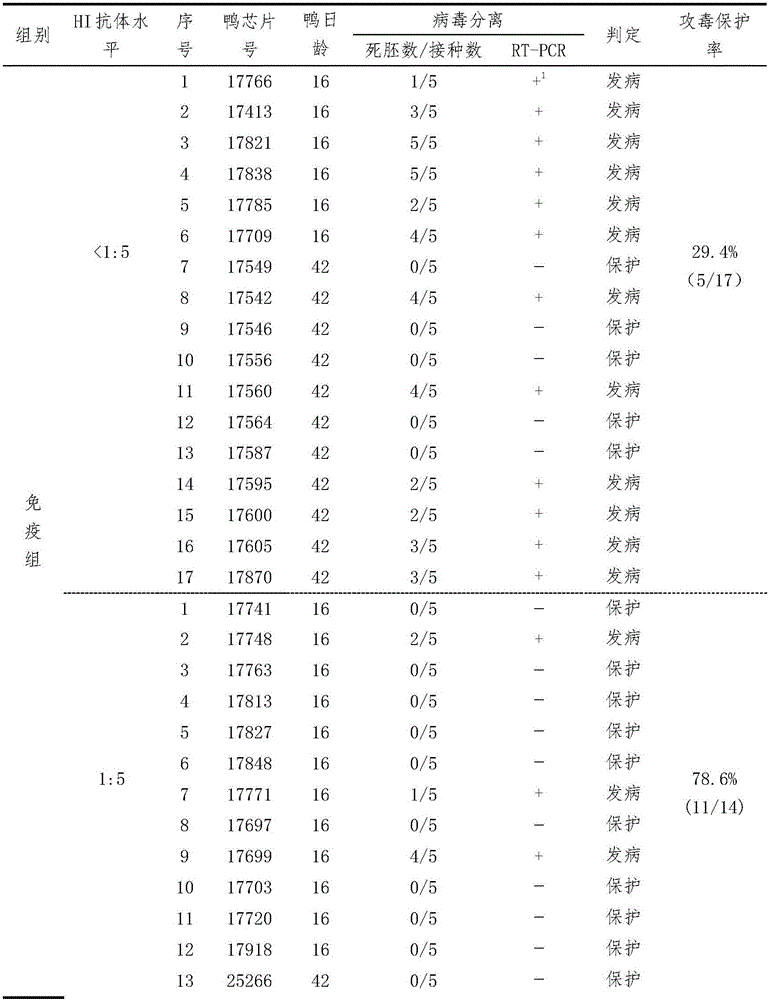
ActiveCN105866424ASolutionResolving Gaps in Judgment CriteriaCompounds screening/testingBiological testingTembusuPrimary immunization
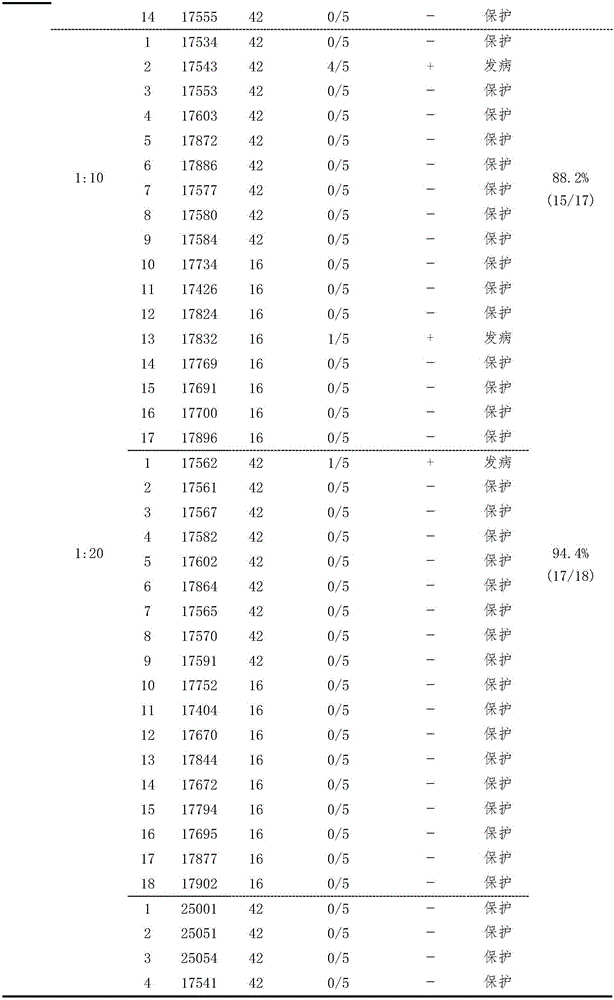
The invention provides a serological testing method for the potency of an inactivated vaccine against duck Tembusu viral diseases. According to the method, HI antibody-negative ducks are used and divided into two groups, i.e., an immunized group consisting of ten ducks and a control group consisting of five ducks; each duck in the immunized group receives hypodermic or intramuscular injection of a vaccine against duck Tembusu viral diseases; immunization with an inactivated vaccine is carried out twice, a dosage of 0.5 ml or 1.0 ml per duck is used each time, and secondary immunization is carried out in two weeks after primary immunization; for immunization with a live vaccine, each duck is immunized according to a dosage of a standard vaccine usage amount for poultry once; in 3 to 4 weeks after inoculation, blood is acquired, serum is separated and the titer of an HI antibody is determined; and when the titer of the HI antibody in the ducks of the control group is less than 1: 5 and the titer of the HI antibody in serum of at least seven ducks of the immunized group is no less than 1: 10, it is determined that the potency of the vaccine is qualified in testing. The serological testing method provided by the invention is convenient to operate and accurate in results and can be extensively applied to potency evaluation of vaccines against duck Tembusu viral diseases and formulation of immune procedure in primary-level organizations and vaccine development and examination units.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Hybridoma cell strain secreting acyclovir monoclonal antibody and preparation method
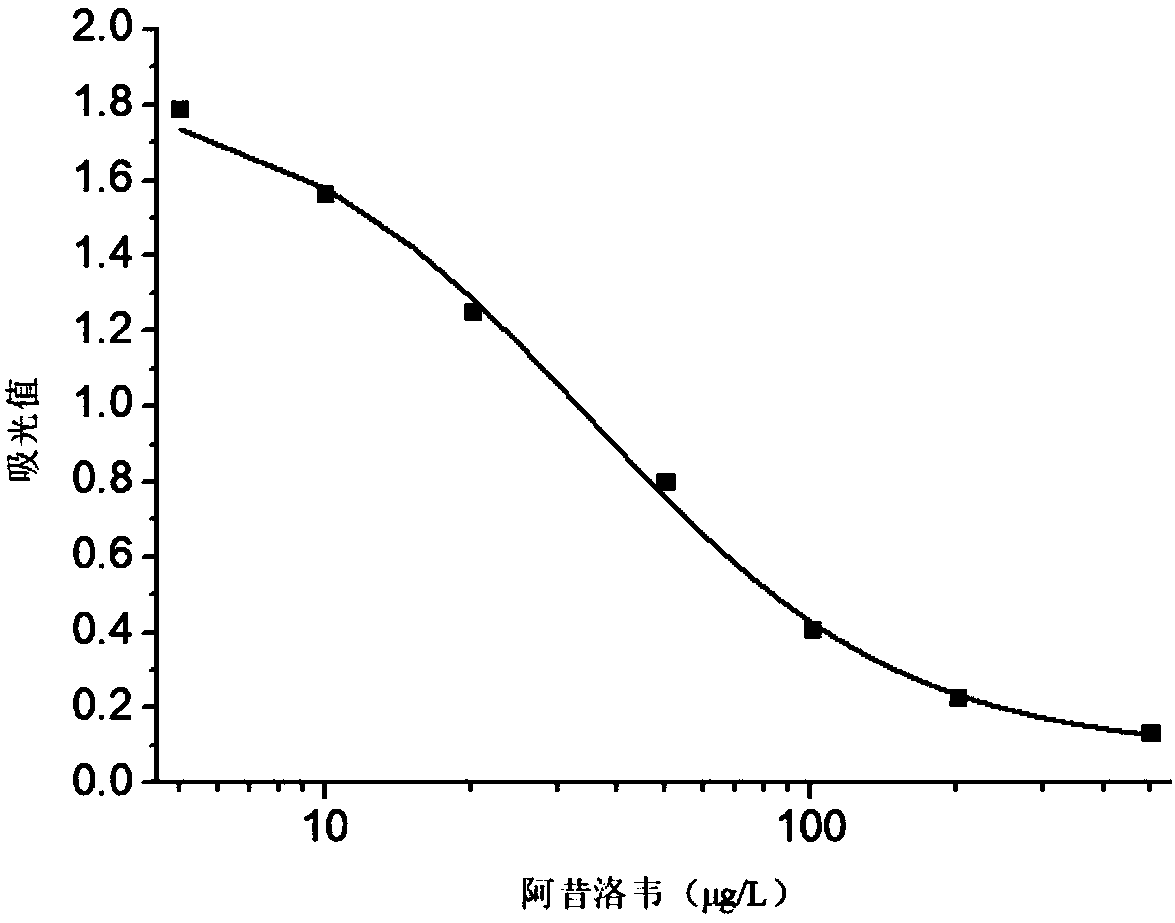
ActiveCN108330103AHigh detection sensitivityImprove featuresFused cellsImmunoglobulinsBALB/cPrimary immunization
The invention relates to a hybridoma cell strain secreting an acyclovir monoclonal antibody and a preparation method, and belongs to the field of food safety immunodetection. The preservation number of the hybridoma cell strain is CGMCC (China General Microbiological Culture Collection Center) No. 14695, a complete Freund's adjuvant for a BALB / c mouse is subjected to primary immunization, an incomplete Freund's adjuvant conducts immunization enhancement, an acyclovir complete antigen without containing the adjuvant conducts impact immunization, and thus the BALB / c mouse is immunized; an immunized mouse splenocyte with the high potency and low IC 50 (half maximal inhibitory concentration) is fused with a mouse myeloma cell through a PEG method, and the cell strain is obtained through indirect competition of ELISA (enzyme-linked immune sorbent assay) screening and three-time subcloning. The monoclonal antibody secreted by the cell strain has very good specificity and detection sensibility on acyclovir, and can be used for residue detection of acyclovir in food.
Owner:JIANGNAN UNIV +1
Somatostatin (SS) anti-idiotype monoclonal antibody vaccine and preparation method thereof
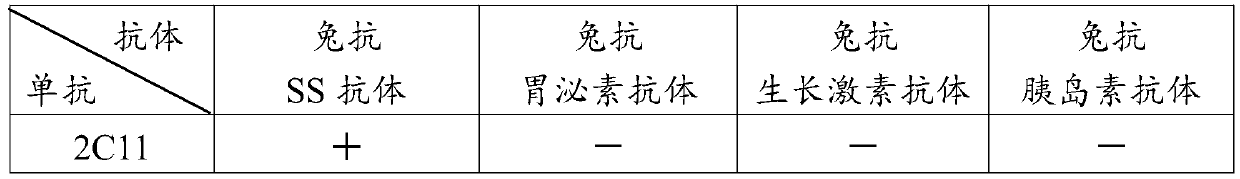
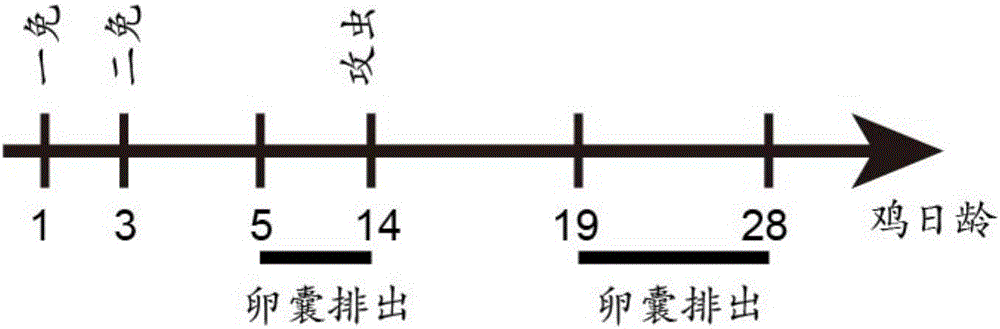
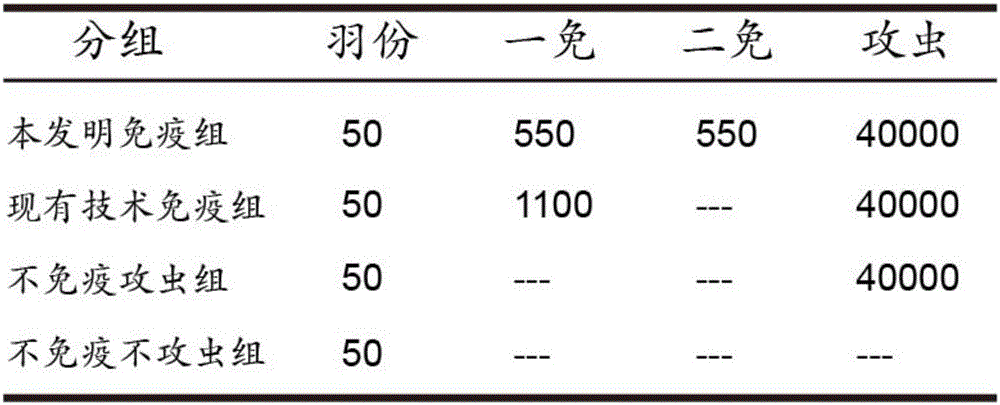
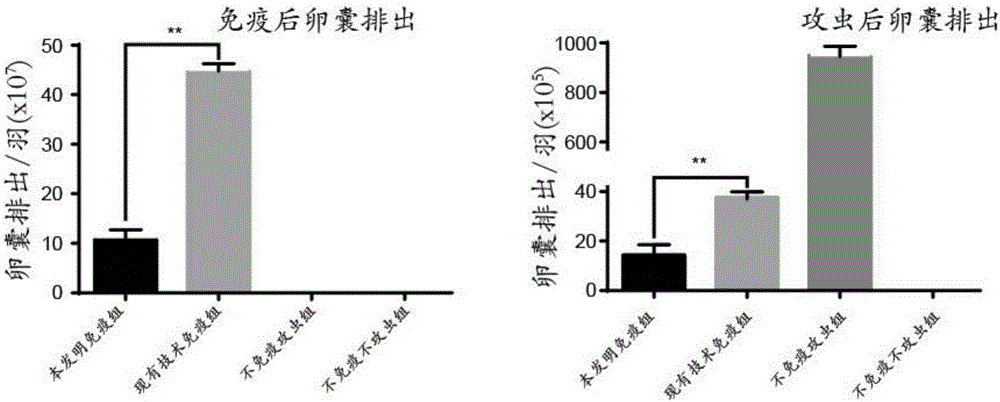
InactiveCN110283250APromote growthFacilitated releaseAntibody ingredientsImmunoglobulinsSomatotropic hormoneAntiendomysial antibodies
The invention provides a preparation method of an SS anti-idiotype monoclonal antibody, and belongs to the technical field of bioengineering and vaccine preparation. The method disclosed by the invention comprises the following steps: (1) coupling SS with a macromolecular protein carrier to obtain an SS immunogen complex; (2) carrying out primary immunization on animals with the SS immunogen complex to obtain an SS antibody; (3) carrying out secondary immunization on the animals which are the same or different from the animals in the step (2) with the SS antibody to obtain an SS anti-idiotype monoclonal antibody. The SS anti-idiotypic monoclonal antibody can be prepared by using the method disclosed by the invention. The SS anti-idiotypic monoclonal antibody prepared by the invention can be used as the vaccine to immunize the animals, so that the animals can produce the SS antibodies to immunize and neutralize SS in vivo, thereby promoting the release of growth hormones to promote the growth of the animals.
Owner:西安德轩驰生物科技有限公司 +1
Coccidiosis-in-chicken vaccine immune method
ActiveCN106492208AImprove uniformityReduce or eliminate usageProtozoaAntiparasitic agentsEconomic benefitsPrimary immunization
The invention relates to the field of biological immunity, and particularly discloses a coccidiosis-in-chicken vaccine immune method. The coccidiosis-in-chicken vaccine immune method includes the specific steps that one-day-age chickens are immunized coccidiosis vaccine through mouths for 1 day to 3 days, and then are immunized same coccidiosis vaccine. Coccidiosis-in-chicken vaccine immune is carried out with the method, the risk that oocysts are largely discharged after primary immunization to be eaten by chickens to break out coccidiosis can be effectively avoided, the uniformity coccidiosis vaccine immune can be increased to be 90% or above, the immune protection force can be also rapidly built, better protection is achieved to secondary coccidium infection, production performance reduction caused by vaccine immunity is retarded, and the culture benefits are increased. Meanwhile, the using amount of anti-coccidiosis medicine can be reduced and avoided, and safer and more environmentally friendly poultry and egg products are provided; in addition, the cost of the immune method is saved, and economic benefits are huge.
Owner:CHINA AGRI UNIV
Preparation method of rabbit anti-tree shew antiserum
InactiveCN103800921AReduce manufacturing costReduce mistakesBiological testingIn-vivo testing preparationsSerum igeEconomic benefits
The invention discloses a preparation method of rabbit anti-tree shew antiserum. The method comprises the steps of selecting an experimental animal, preparing serum, carrying out primary immunization and booster immunization and testing serum titer; the preparation method of the serum comprises the steps of collecting blood, stopping bleeding, preparing the serum and comparing the function of the serum; a bidirectional immune diffusion method is adopted by the test for the serum titer. According to the preparation method of the rabbit anti-tree shew antiserum, the content of tree shew uncoupling protein-1(UCP1) can be measured by the prepared antiserum, and the result error is generally within the range of 3-5%, so that the error range is reduced; after the steps of primary immunization and booster immunization are utilized, the production cost is lowered, the UCP1 content measurement effect is good, and the economic benefit is high.
Owner:YUNNAN NORMAL UNIV
Preparation and use method of ganoderan Chinese medicine immunoenhancer
ActiveCN108567793AImprove immunityPromote value-addedSsRNA viruses negative-senseOrganic active ingredientsPrimary immunizationCytokine
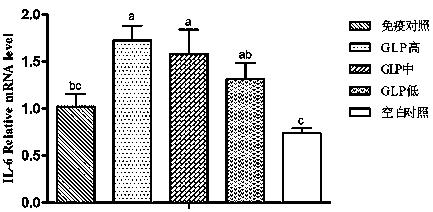
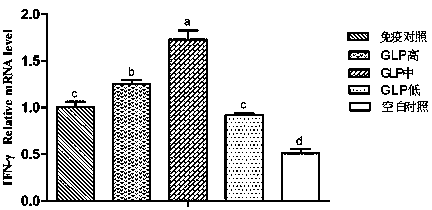
The invention provides a preparation and use method of a ganoderan Chinese medicine immunoenhancer. Polysaccharides in ganoderma lucidum sporocarp materials are extracted to prepare the Chinese medicine immunoenhancer, Newcastle disease IV nasal drip and eye droppings are used for 14-day-old non-immune chicken to realize primary immunization, secondary immunization is performed when the chicken are 28 days old, the chicken are orally drenched with ganoderan solution in each time of immunization, administration dosage is 12.5-50mg / kg of the body weight, and administration is performed once a day and continuously performed for three days. Measurement is performed in the 7th day, the 14th day, the 21st day and the 28th day of primary immunization and finds that ganoderan can obviously promoteperipheral blood and splenic lymphocytes proliferation of the chicken, ND-HI antibody level is improved, immune organ index is improved, the concentration of cell factors such as IL-4, IL-6 and IFN-gin serum is improved, the SIgA content of duodenums is increased, so that body immunity is improved, and the immune effect of vaccines is enhanced.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Method for immunizing cattle by brucella vaccine and application of method
InactiveCN108812548AReduce stress responseStrengthen immune memoryAnimal husbandryBrucella VaccineInfection risk
The invention provides a method for immunizing cattle by a brucella vaccine and an application of the method, and relates to the technical field of vaccines. The method for immunizing the cattle by the brucella vaccine comprises the following steps: firstly immunizing the cattle once by using a vaccine A, and immunizing the cattle at least once by using a vaccine B, wherein the vaccine A is a brucella live vaccine, and an immunizing amount is 60 hundred million to 150 hundred million CFU / each cattle; and the vaccine B includes at least one of an inactivated vaccine, subunit vaccine, recombinant protein vaccine, polypeptide vaccine and DNA vaccine of brucell. According to the method provided by the invention, in primary immunization, the live vaccine with a low dose is used to immunize andactivate humoral and cellular immunity of a body, then the non-live vaccine is used for secondary or more immunization of the body, so that immune memory of the body can be strengthened, a stress reaction of the cattle and infection risk of operators can be reduced while effective protection is provided, and the technical problems that the prior art lacks a method which is safe to humans and can effectively immunize the cattle for immunizing the cattle by the brucella vaccine are alleviated.
Owner:TECON BIOLOGY CO LTD
Preparation method of yolk antibody for preventing chronic periodontitis and application thereof
InactiveCN110128541AGrowth inhibitionGrowth does not affectAntibacterial agentsEgg immunoglobulinsYolkImmune effects
The invention discloses a preparation method of a yolk antibody for preventing chronic periodontitis. The ultrasonic fragmented porphyromonas gingivalis, tannerella forsythia and campylobacter rectusare mixed uniformly to prepare a composite antigen, and the laying hen is subjected to primary immunization and booster immunization for 6 rounds to collect eggs, and the specific yolk antibody is isolated and purified. The obtained yolk antibody has high titer and good immune effect, and can effectively inhibit the growth of porphyromonas gingivalis, tannerella forsythia and campylobacter rectus,has obvious effects on the treatment and prevention of chronic periodontitis, does not affect the growth of other oral unrelated flora, and is safe and harmless.
Owner:北京宇诚健康科技有限公司
Method for preparing tussah silk fibroin antibody using characteristic hexapeptide
ActiveCN105504056BStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansAdjuvantKeyhole-limpet haemocyanin
The present invention discloses a method for using a characteristic hexapeptide for preparing a tussah fibroin protein antibody, a synthetic sequence is a ''GSGAGG'' hexapeptide, the hexapeptide is coupled with keyhole limpet hemocyanin to obtain a full antigen; the full antigen is diluted with normal saline, the diluted full antigen is mixed evenly with Quick Antibody-Rabbit 5 w adjuvant, primary immunization is performed on a rabbit, then booster immunization is performed on the rabbit, reagents used for the booster immunization and the primary immunization are same; when titer of the antibody in a blood sample of the rabbit reaches 1: 10,000, blood of the immunized rabbit is collected, blood clots are fully contracted, antiserum is completely precipitated, then the antiserum is collected antiserum and centrifuged to obtain a supernatant, and the supernatant is sub-packed for standby use. The method is simple and quick, and the prepared antibody is highly specific and can be used to detect silk fibroin in textiles.
Owner:ZHEJIANG SCI-TECH UNIV
Foot and mouth disease virus-like particle antigen, vaccine composition, preparation method and application
ActiveCN111434677AHigh Titer Antibody TiterImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsParticulate antigenFoot mouth disease virus
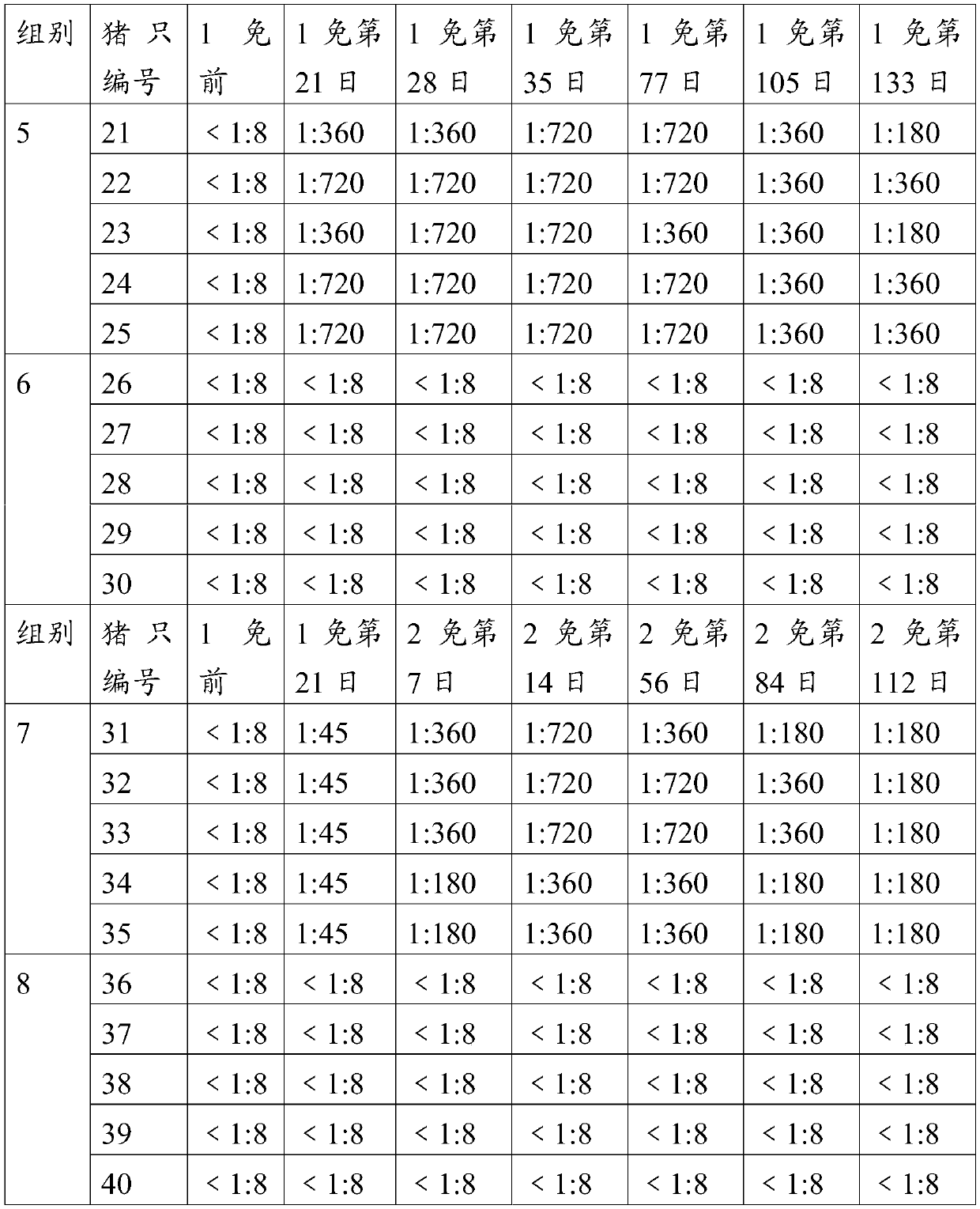
The invention provides an O-type foot and mouth disease virus-like particle antigen. The O-type foot and mouth disease virus-like particle antigen is a CATHAY-type O-type foot and mouth disease virus-like particle antigen and the CATHAY-type O-type foot and mouth disease virus-like particle antigen is composed from the composition of CATHAY-type O-type foot and mouth disease virus VP0, VP3 and VP1antigen proteins. The O-type foot and mouth disease virus-like particle antigen has good immunogenicity, a prepared vaccine can realize primary immunization, complete protection against O-type foot and mouth disease virus can be realized on the 14th day after immunization, the titer of a generated antibody is higher than the titer of a commercial inactivated vaccine, and the protective immune period can last for at least 133 days. The invention further relates to a prepared vaccine composition and a preparation method thereof and application.
Owner:PU LIKE BIO ENG
Hybridoma cell strain secreting meloxicam monoclonal antibody and application of hybridoma cell strain
ActiveCN108949699AHigh detection sensitivityImprove featuresOvalbuminMicroorganism based processesBALB/cAdjuvant
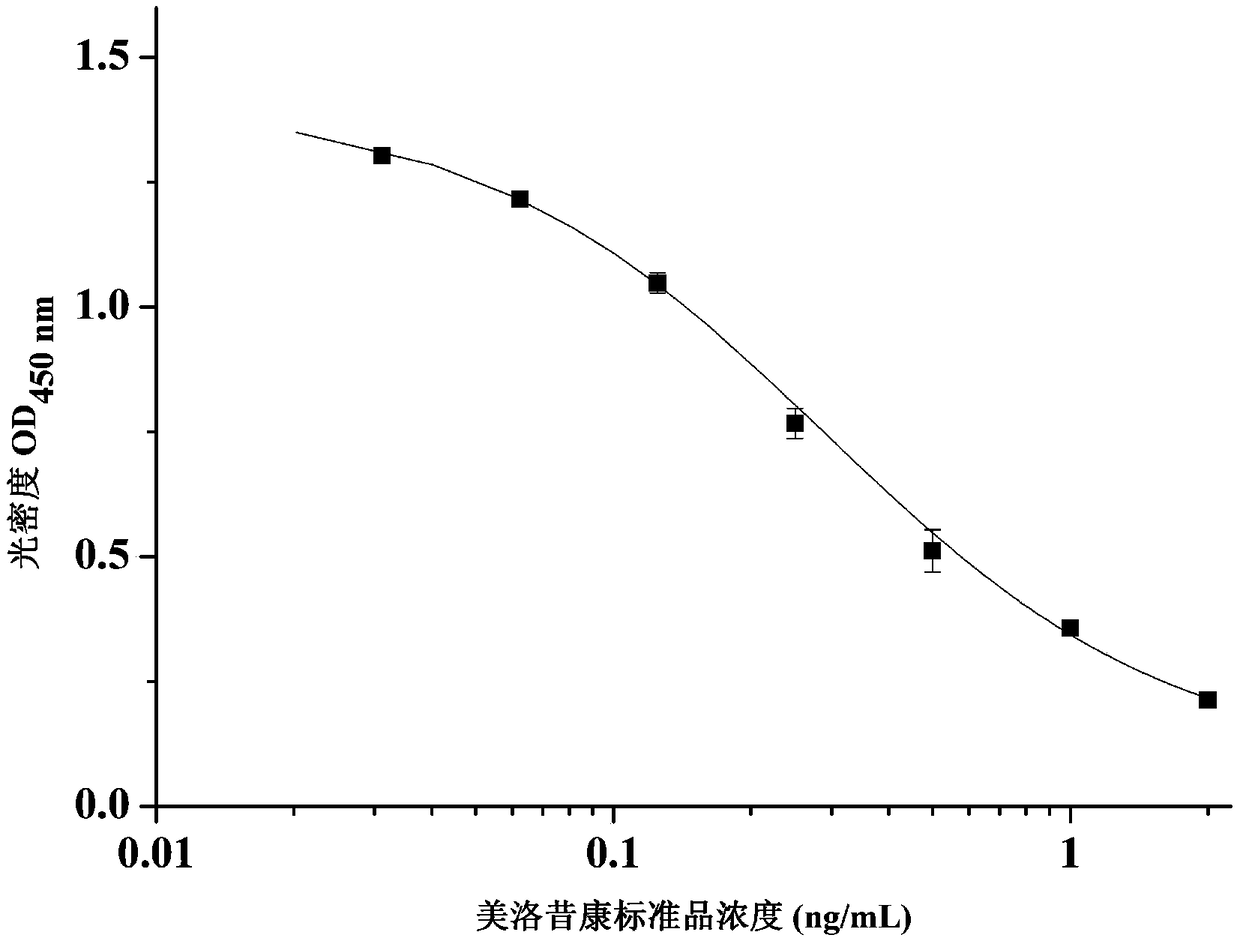
The invention relates to a hybridoma cell strain secreting meloxicam monoclonal antibody and a preparation method of the hybridoma cell strain and belongs to the field of food safety immunodetection.The hybridoma cell strain has a collection number of CGMCC No. 14700. The preparation method comprises the following steps: performing primary immunization on BALB / c mice with a complete Freund's adjuvant, performing booster immunization for three times by using an incomplete Freund's adjuvant, performing impact immunization once on an adjuvant-free meloxicam complete antigen, and enabling the BALB / c mice to be immunized; fusing high titer low-IC50 immunized mice spleen cells with mice myeloma cells by a PEG method, and performing indirect competitive ELISA (Enzyme-Linked Immuno Sorbent Assay)screening and three-times subcloning, thereby obtaining the cell strain. The monoclonal antibody secreted by the cell strain has excellent specificity and detection sensitivity (IC50 value of 0.1ng / mL) on meloxicam, and can be used for detecting residual meloxicam in foods.
Owner:JIANGNAN UNIV +1
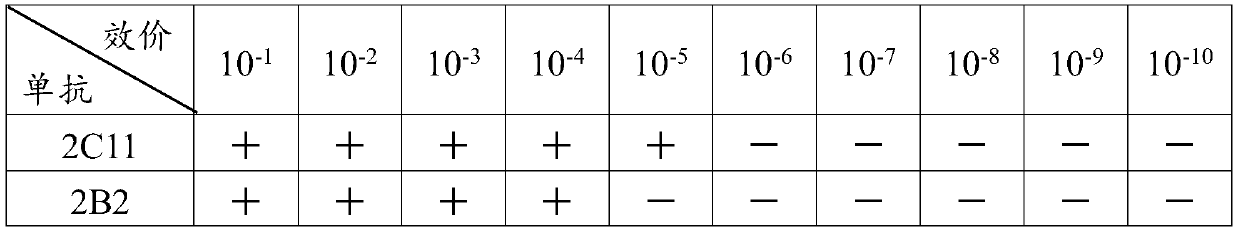
Agglutinative Moraxella catarrhalis monoclonal antibody as well as preparation method and application thereof
ActiveCN111157735AEasy to detectQuick checkMaterial analysisAntiendomysial antibodiesPrimary immunization
The invention relates to a preparation method of an agglutinative Moraxella catarrhalis monoclonal antibody, which comprises the following steps: (1) obtaining non-agglutinative Moraxella catarrhalis,carrying out conventional culture to obtain a bacterial solution, carrying out ultrasonic disruption on the bacterial solution in an ice bath, and collecting the supernatant to obtain a tolerant; (2)obtaining agglutinative Moraxella catarrhalis, carrying out conventional culture to obtain a bacterial solution, carrying out ultrasonic crushing on the bacterial solution in an ice bath, and collecting supernatant to obtain immunogen; (3) tolerance stage: carrying out primary immunization on the mouse by adopting the tolerance antigen, respectively injecting cyclophosphamide (Cy) into the abdominal cavities of the mouse within 10 + / -2 minutes, 24 + / -1 hours and 48 + / -1 hours, and measuring titer; and (4) an immunization stage: after the mouse is tolerated, continuing to immunize the mouse byadopting the immunogen and the immunologic adjuvant, determining titer, and carrying out hybridoma cell fusion to obtain the agglutinative Moraxella catarrhalis monoclonal antibody. The antibody is high in purity, strong in affinity and high in specificity.
Owner:GUANGZHOU WONDFO BIOTECH
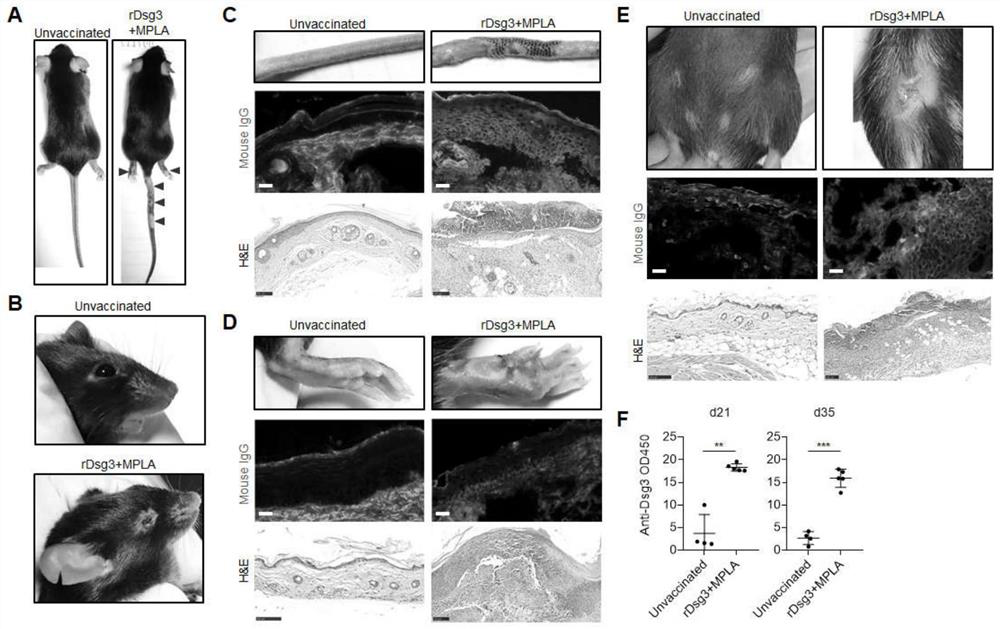
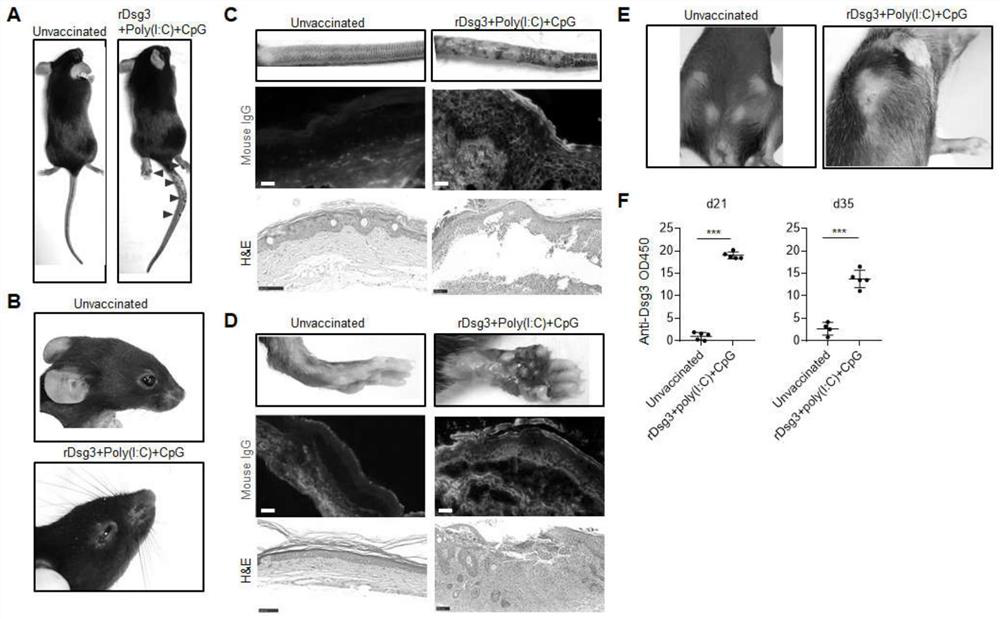
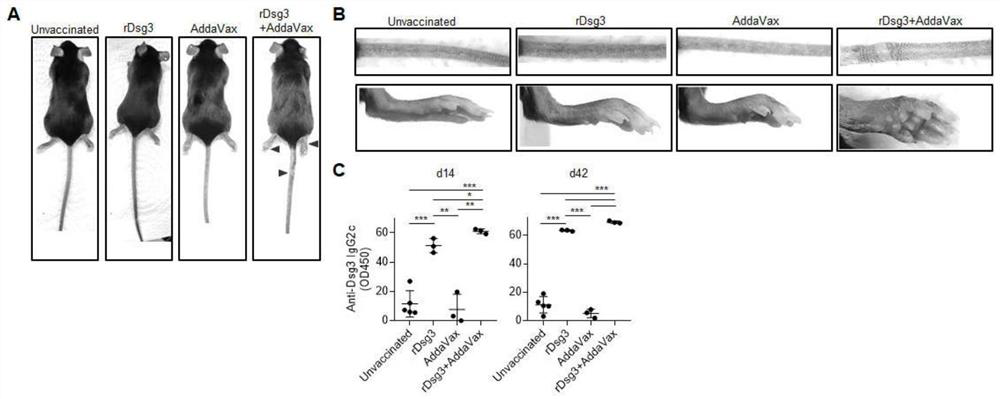
Method for constructing pemphigus vulgaris animal model
The invention discloses a pemphigus vulgaris animal model construction method, which comprises the following steps: carrying out primary immunization / booster immunization on a wild type mouse by using a vaccination technology and taking desmoplasmin 3 as a vaccine antigen, starting to appear phenotypes within 28-35 days, including blisters, epidermis exfoliation and skin ulceration on the rear feet and the tail of the mouse with the overall phenotype; a serum autoantibody; the pemphigus vulgaris immunotherapy kit has the advantages that pemphigus vulgaris changes such as epidermis spinous layer debonding and antibody deposition in histological examination are simulated, part of mice have disease phenotypes such as local skin unhairing, fester and canthus conjunctivitis, clinical symptoms of pemphigus vulgaris are simulated, and an important tool is provided for pemphigus vulgaris immunotherapy.
Owner:HOSPITAL OF DERMATOLOGY CHINESE ACAD OF MEDICAL SCI
Method for preparing tussah silk fibroin protein antibody using characteristic decapeptide
InactiveCN105504055BStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansSerum igeAdjuvant
Owner:ZHEJIANG SCI-TECH UNIV
Method for using characteristic decapeptidefor preparing tussah fibroin protein antibody
InactiveCN105504055AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansSerum igeAdjuvant
The present invention discloses a method for using a characteristic decapeptide for preparing a tussah fibroin protein antibody, a synthetic sequence is a ''AAAAAAAAAA'' polypeptide, the polypeptide is coupled with keyhole limpet hemocyanin to obtain a full antigen; the full antigen is diluted with normal saline, the diluted full antigen is mixed evenly with Quick Antibody-Rabbit 5 w adjuvant, primary immunization is performed on a rabbit, then booster immunization is performed on the rabbit, reagents used for the booster immunization and the primary immunization are same; when titer of the antibody in a blood sample of the rabbit reaches 1: 10,000, blood of the immunized rabbit is collected, blood clots are fully contracted, antiserum is completely precipitated, then the antiserum is collected antiserum and centrifuged to obtain a supernatant, and the supernatant is sub-packed for standby use. The method is simple and quick, and the prepared antibody is highly specific and can be used to detect silk fibroin in textiles.
Owner:ZHEJIANG SCI-TECH UNIV
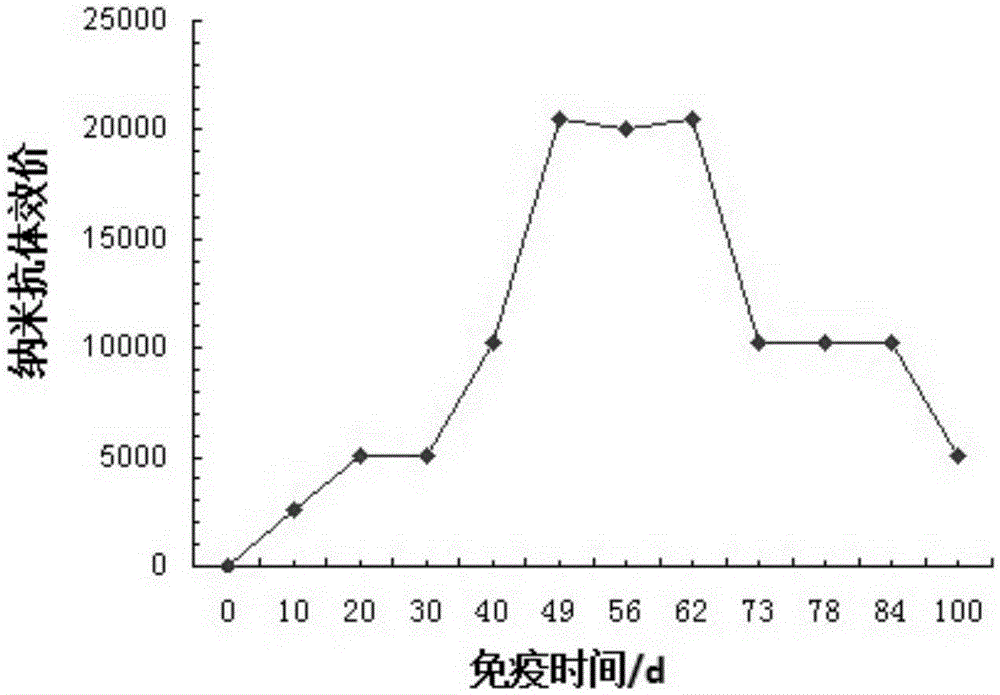
Hand-foot-and-mouth disease resistant specific nanobody and titer determination method thereof
InactiveCN105367655ASmall molecular weightGood water solubilitySerum immunoglobulinsImmunoglobulins against virusesSolubilityPhosphate
The invention discloses a hand-foot-and-mouth disease resistant specific nanobody and its titer determination method, and aims to provide a hand-foot-and-mouth disease resistant specific nanobody with small molecular weight, good water solubility, strong specificity and high stability and a titer determination method thereof. Technical points: the hand-foot-and-mouth disease resistant specific nanobody is prepared by the following steps successively: 1) preparing a soluble antigen by the use of inactivation purified EV71 type viral strain; 2) making the soluble antigen prepared in the step 1) into 0.8-1.2 mg / ml of an antigen solution by the use of 0.01 M of a phosphate buffer, and fully emulsifying the antigen solution by the use of a freund's complete adjuvant according to t two-humped camel he volume ratio of the antigen solution to the freund's complete adjuvant being 1:0.8-1.2 so as to prepare an immunizing antigen; 3) injecting 500-700 microliters of the immunizing antigen prepared in the step 2) at multiple sites under the skin of the back of each two-humped camel for 12-18 days of immunizing cycle, enhancing immunizing in the middle stage for three times, adopting the same primary immunization method and dosage, carrying out venous blood sampling after four immunizing cycles, collecting serum, and preserving in a refrigerator of 3-5 DEG C for standby; and 4) purifying the collected serum by a PEG three-step precipitation method and an improved water body comprehensive PEG method so as to obtain the nanobody. The invention belongs to the field of immunological technique.
Owner:GUANGDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com