Testing method for potency of inactivated vaccine against duck Tembusu viral diseases
A duck Tembusu virus and inactivated vaccine technology is applied in the direction of material inspection products, pharmaceutical formulas, preparations for in vivo tests, etc., to achieve accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
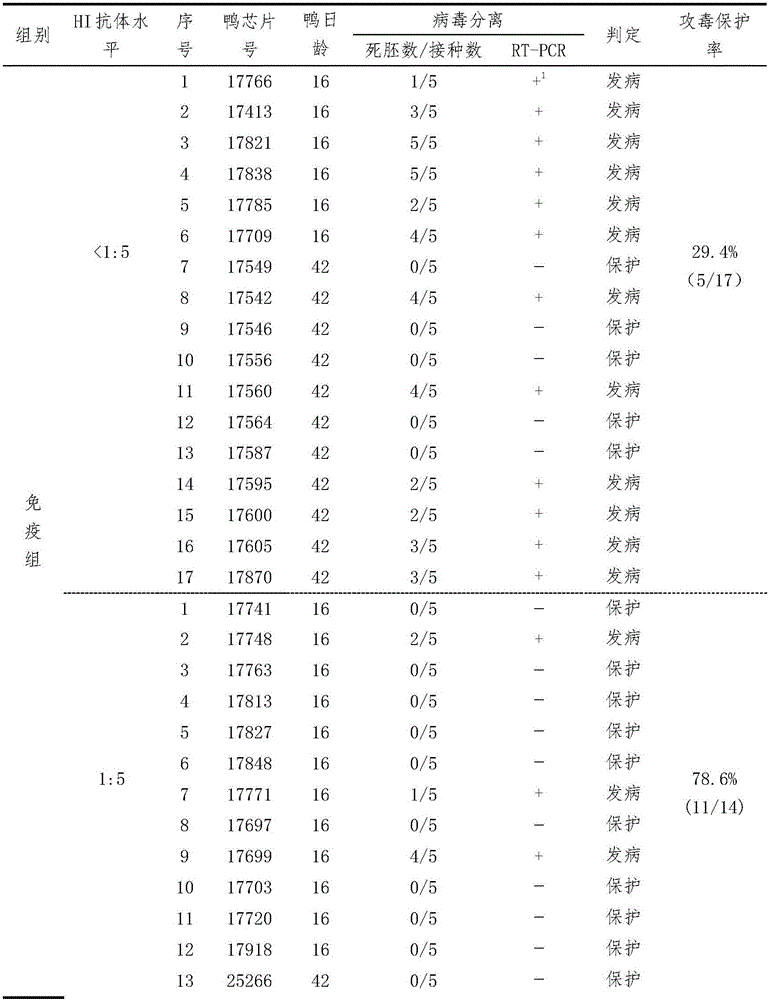
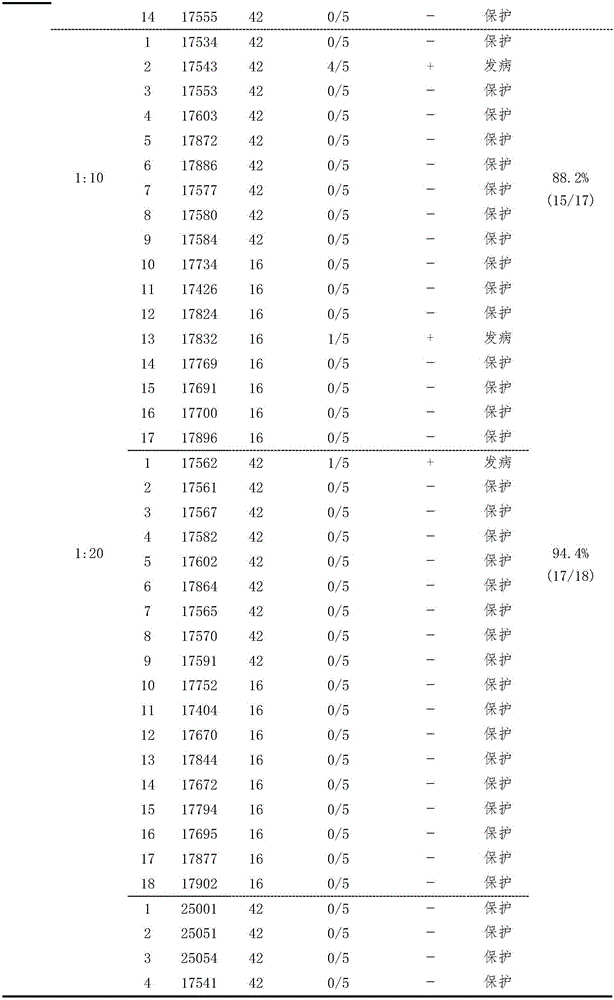
[0033] Example 1 Establishment of Serological Effectiveness Test Method for Duck Tembusu Virus Disease Inactivated Vaccine
[0034] Three batches (201201, 201202, 201203) of duck Tambusu virus disease (HB strain) inactivated vaccines were used to immunize 16- and 42-day-old Peking ducks for the second time at doses of 0.11, 0.33 and 1.0ml / bird, respectively. Blood was collected on the 28th day after the second immunization, and the HI antibody of each duck serum was measured, and 0.5ml / only (containing 100DID) 50 ) dose to attack the virus. Two days after the challenge, blood was collected, serum was separated, and virus isolation was carried out. According to HI antibody titer (<1:5, 1:5, 1:10, 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640), divide each test Corresponding analysis of HI antibody titer and virus isolation results of ducks, analysis of the correlation between HI antibody titer and challenge protection of each group, and formulation of qualified standards for vacci...
Embodiment 2
[0072] Example 2 Duck Tambusu Virus Disease Inactivated Vaccine (HI Antibody Assay) Efficiency Test Age in Days, Immunization Dose, Sampling Time of Ducks (1)
[0073] Inactivated duck Tembusu virus disease vaccine (batch number 201201, developed by the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry and Ruipu (Baoding) Biopharmaceutical Co., Ltd.) was used to immunize 60-day-old duck Tembusu virus HI antibody-negative shelduck ducks 10 rats, 0.5ml / rat, intramuscularly injected into the chest, and 5 non-immune controls were set at the same time. Two weeks after the first immunization, the second immunization was carried out according to the same dose and the same route, and the serum of the ducks was collected 4 weeks after the second immunization, and the serum of the immune ducks was detected using the annotated HI antibody detection method. The HI antibody titers of 5 non-immunized ducks were all less than <1:5, and 9 of 10...
Embodiment 3
[0074] Example 3 Duck Tambusu Virus Disease Inactivated Vaccine (HI Antibody Assay) Efficacy Test Age in Days, Immunization Dose, Sampling Time of Ducks (2)
[0075] Inactivated duck Tembusu virus disease vaccine (batch number 201202, developed by Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences and Ruipu (Baoding) Biopharmaceutical Co., Ltd.) was used to immunize 42-day-old Peking ducks negative for HI antibody of duck Tembusu virus 10 rats, 1.0ml / rat, intramuscularly injected into the chest, and 5 non-immune controls were set at the same time. Two weeks after the first immunization, the same dose and the same route were used for the second immunization, and the serum of the ducks 4 weeks after the second immunization was collected, and the serum of the immune ducks was detected by the HI antibody detection method in the note. The HI antibody titers of 5 non-immunized ducks were all <1:5, and 8 of 10 immunized ducks h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com