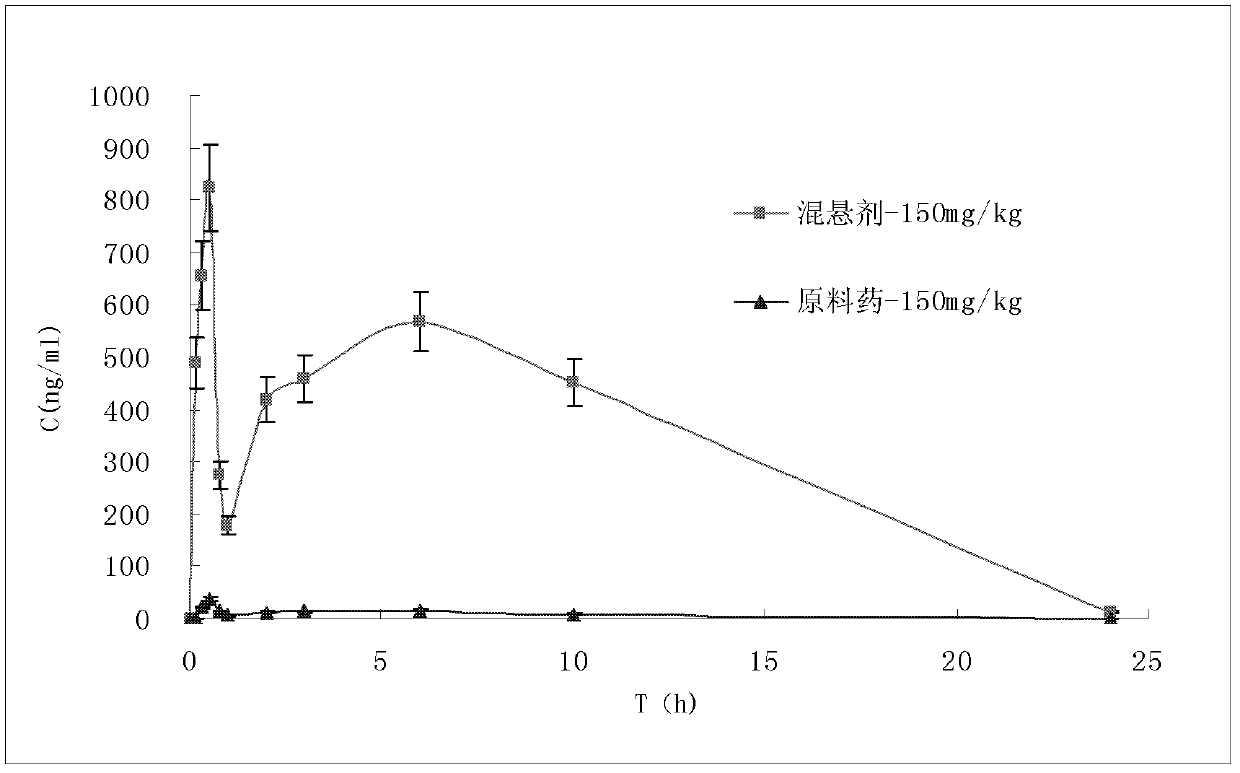
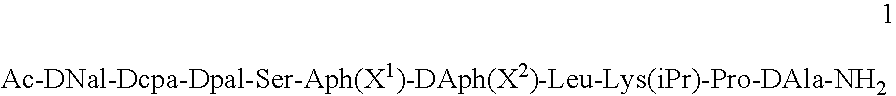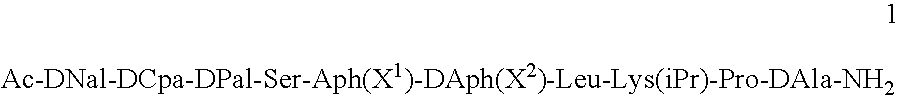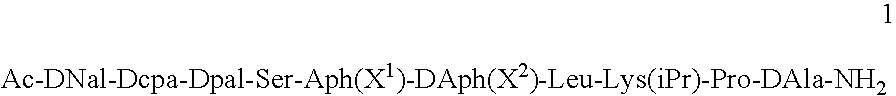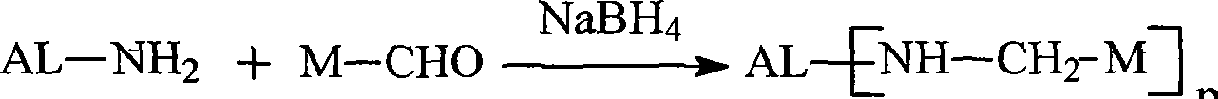Patents
Literature
741 results about "Im injections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
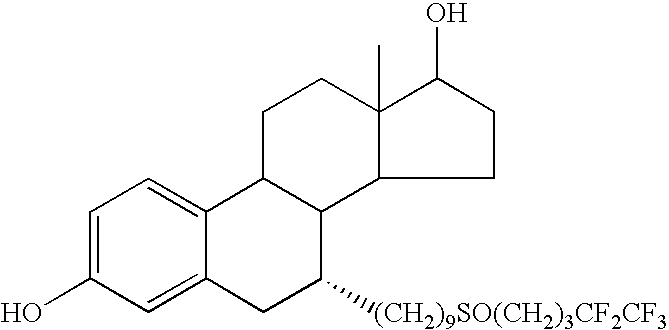
Intramuscular injection. Intramuscular (also IM or im) injection is the injection of a substance directly into muscle. In medicine, it is one of several alternative methods for the administration of medications (see route of administration).
Vegetable-based dog chew
A chew toy having a substantial component of vegetable matter wherein such vegetable matter, in dried and powdered or granulation form, is melted as it is injection molded. The process provides a chew toy with a consistency and chewability preferred by most dogs that is not attainable by known prior art baking or compression molding techniques.
Owner:T F H PUBLICATIONS
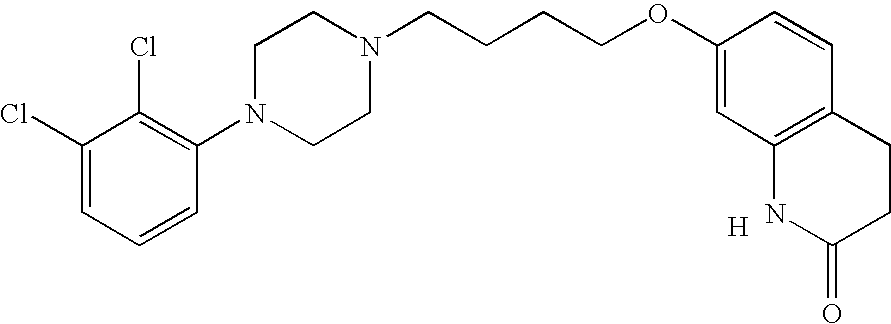
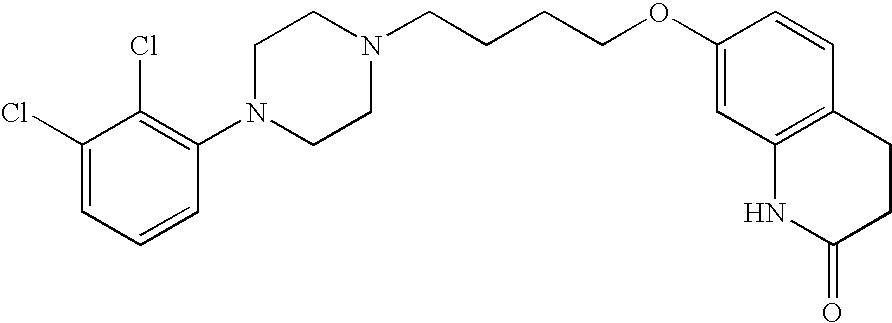
Aripiprazole complex formulation and method
An aripiprazole formulation is provided which includes the antipsychotic agent aripiprazole in the form of an inclusion complex in a β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBECD), which in the form of an injectable produces reversible generally minimal to mild irritation at the intramuscular injection site. A method for minimizing or reducing irritation caused by aripiprazole at an intramuscular injection site and a method for treating schizophrenia employing the above formulation are also provided.
Owner:OTSUKA PHARM CO LTD
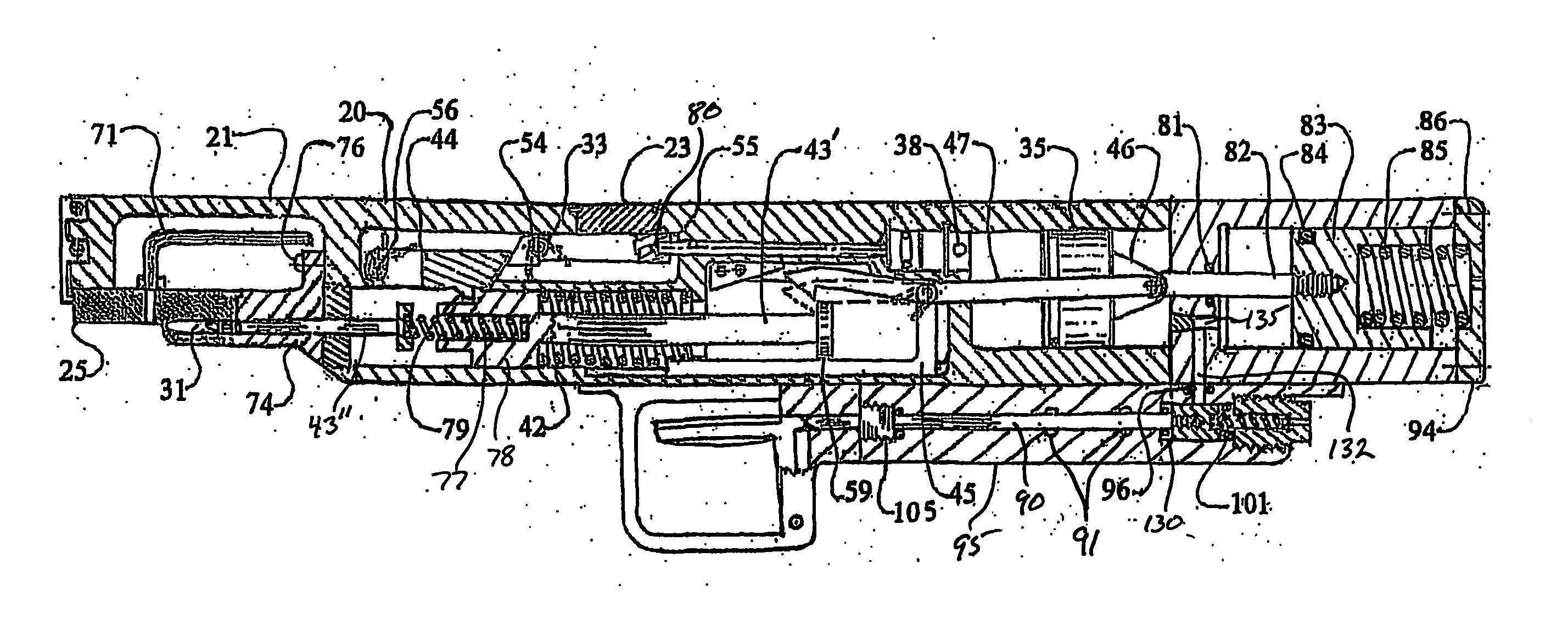
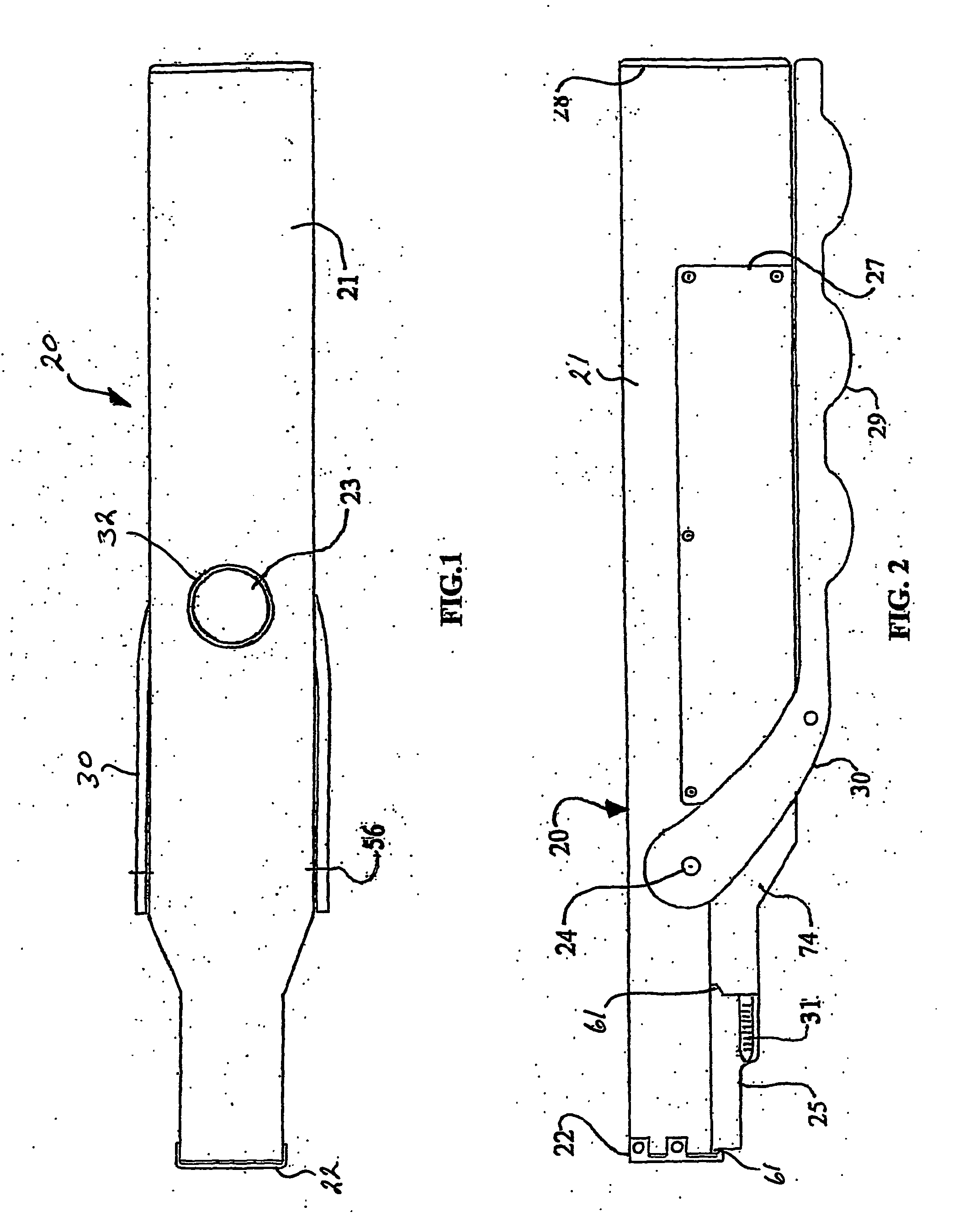
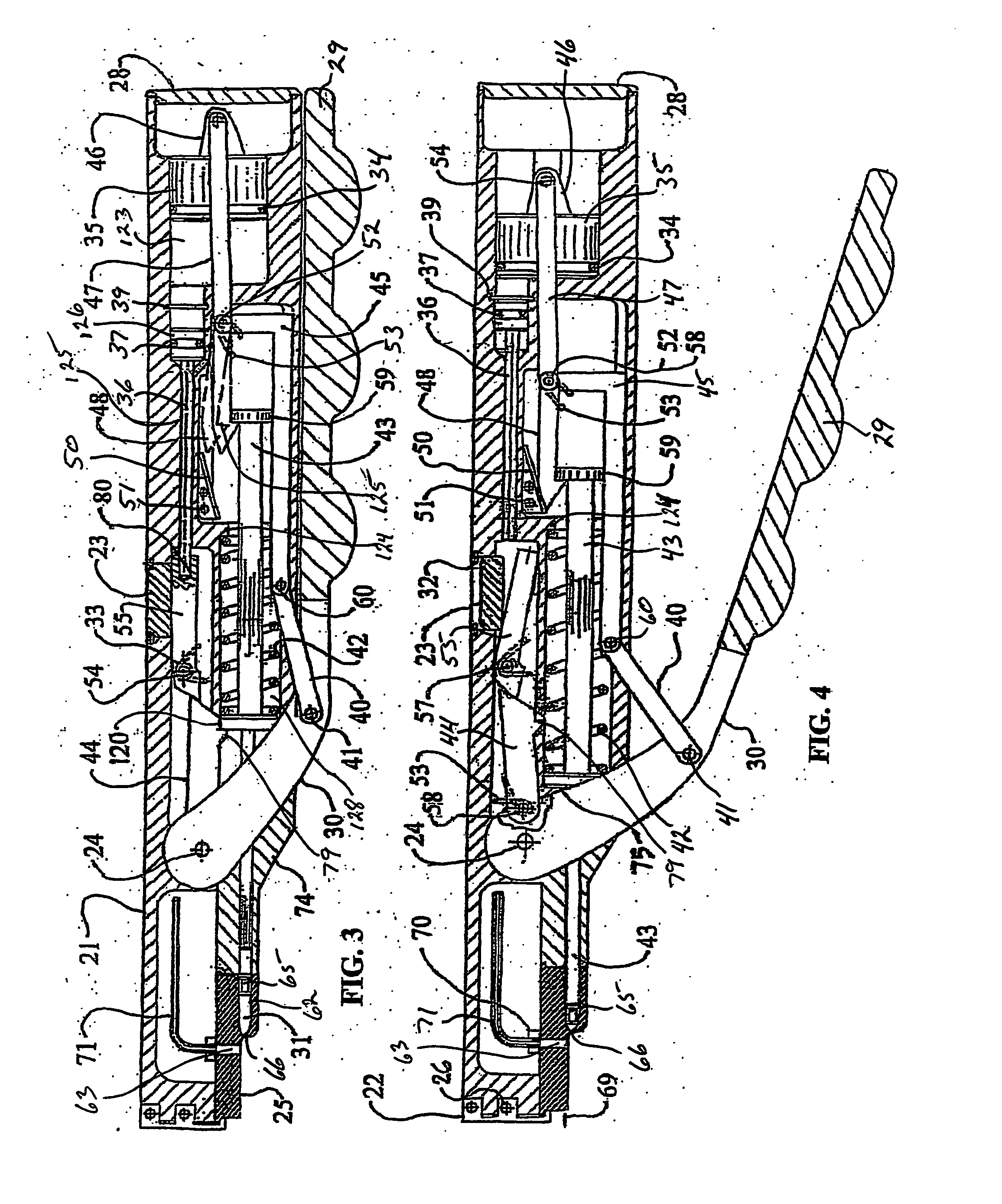
Needle free hypodermic injector and ampule for intradermal, subcutaneous and intramuscular injection
InactiveUS20070167907A1Reduce local pressureEliminate leaksJet injection syringesAutomatic syringesNeedle freeSuction force
A needle free hypodermic injector comprising a hand manipulatable elongate housing, an impact impulse injection mechanism within the housing, a suction generating means within the housing and cooperable with the impact impulse injection mechanism, a safety interlock mechanism, at least one medicament containing ampule cooperable with the impact impulse injection mechanism and the suction generating means and having a jet orifice through which medicament is injectable through a skin surface in response to an impulse placed on the medicament by the impact impulse injection mechanism, and means to receive and hold the ampule on the injector in registration with the impact impulse injection mechanism and in communication with the suction generating means; whereby the injector is adapted to expel the medicament from the ampule in a jet stream of sufficient velocity to penetrate skin tissue held against the orifice by the suction generating means and to deposit the medicament intradermally, subcutaneously or intramuscularly based on the position and angle of the jet orifice.
Owner:DESLIERRES JOHN +2
Gonadotropin releasing hormone antagonists in gel-forming concentrations
InactiveUS20050245455A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneActive agentGnRH Antagonist
Pharmaceutical compositions are provided for the treatment of steroid-dependent and other diseases. The compositions are solutions for subcutaneous or intramuscular injection, and the active agent is a GnRH antagonist peptide according to general formula (1): Ac-DNal-DCpa-DPal-Ser-Aph(X1)-DAph(X2)-Leu-Lys(iPr)-Pro-DAla-NH2 present at a concentration sufficient to from a gel following administration.
Owner:FERRING BV
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Nanocomposite temperature-sensitive gel and preparation method and application thereof
InactiveCN102525882AEasy to prepareSuitable for mass productionGenetic material ingredientsEmulsion deliveryHypodermic injectionLung cancer
The invention relates to a nanocomposite temperature-sensitive gel and a preparation method and an application thereof. The preparation method comprises the following steps of: coating antitumor active substances with a high molecular polymer serving as a carrier material to obtain nanoparticles; and adding a temperature-sensitive high molecular material to obtain the nanocomposite temperature-sensitive gel. The preparation method disclosed by the invention is simple and convenient, is suitable for large-scale production, and is particularly suitable for preparing medicaments or diagnostic reagents having the characteristics of long cycle, biodegradability, slow release, passive targeting, active targeting, active substance conveying function and tumor resistance. An antitumor medicament prepared with the method disclosed by the invention is suitable for ways such as intravenous injection, intramuscular injection, hypodermic injection, intradermal injection, intratumor injection, tumor-side injection, oral administration or transdermal medicament delivery and the like, is applied to treatment and diagnosis of pancreatic cancer, liver cancer, lung cancer, gastric cancer, colorectal cancer, esophageal cancer, prostatic cancer, uterine cancer and ovarian cancer, and has a good application prospect.
Owner:SHANGHAI INST OF ONCOLOGY
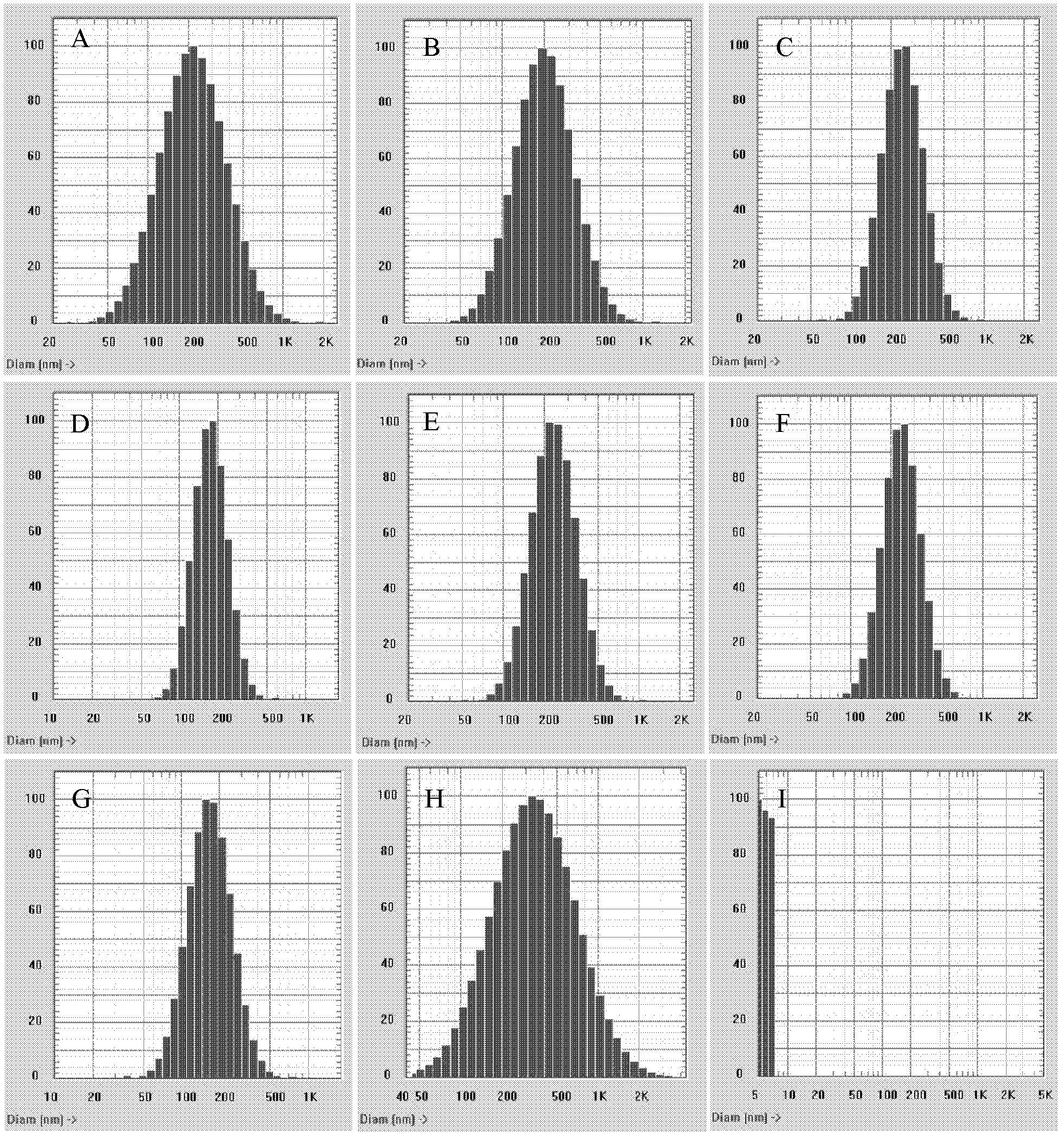
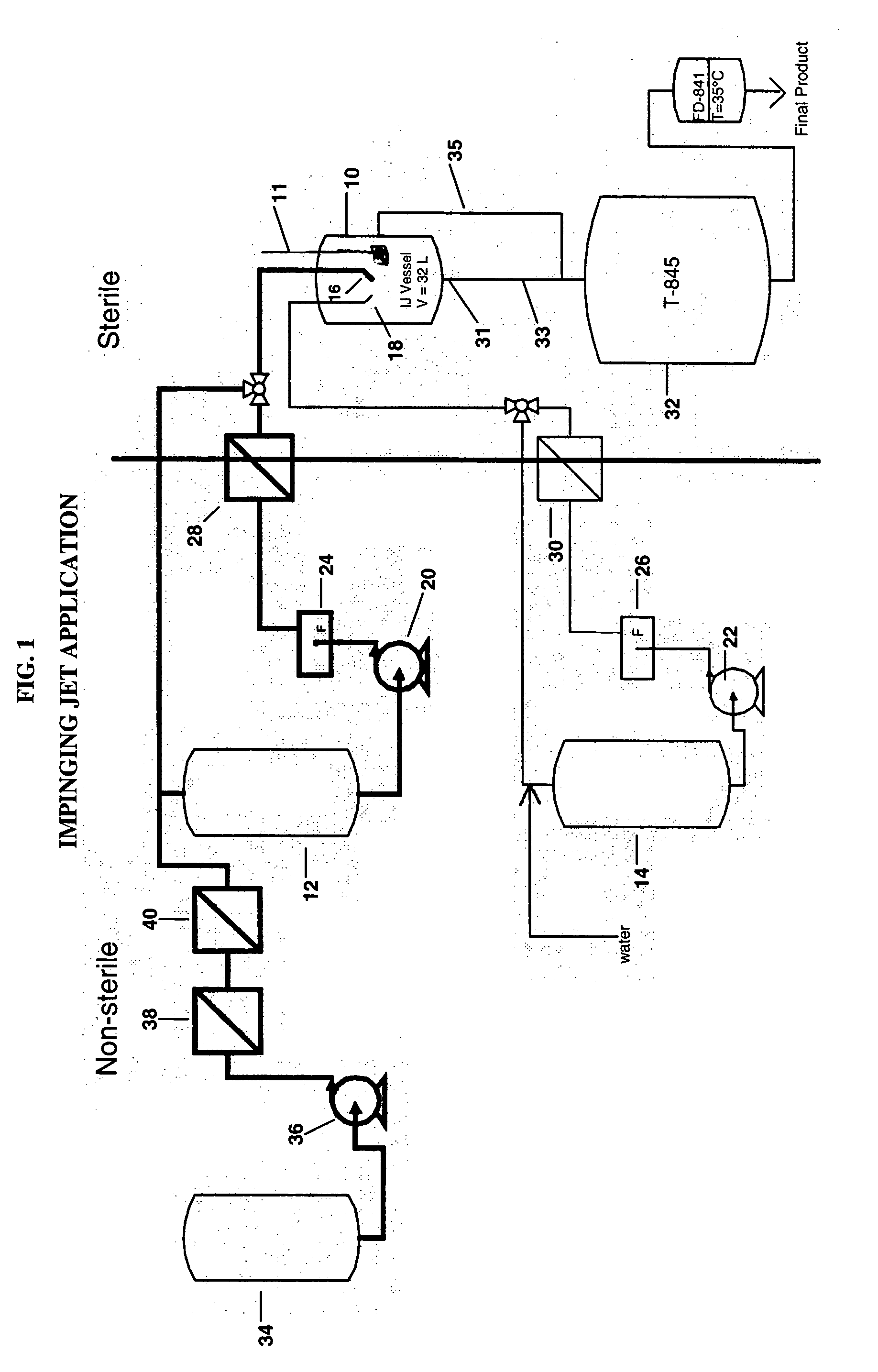
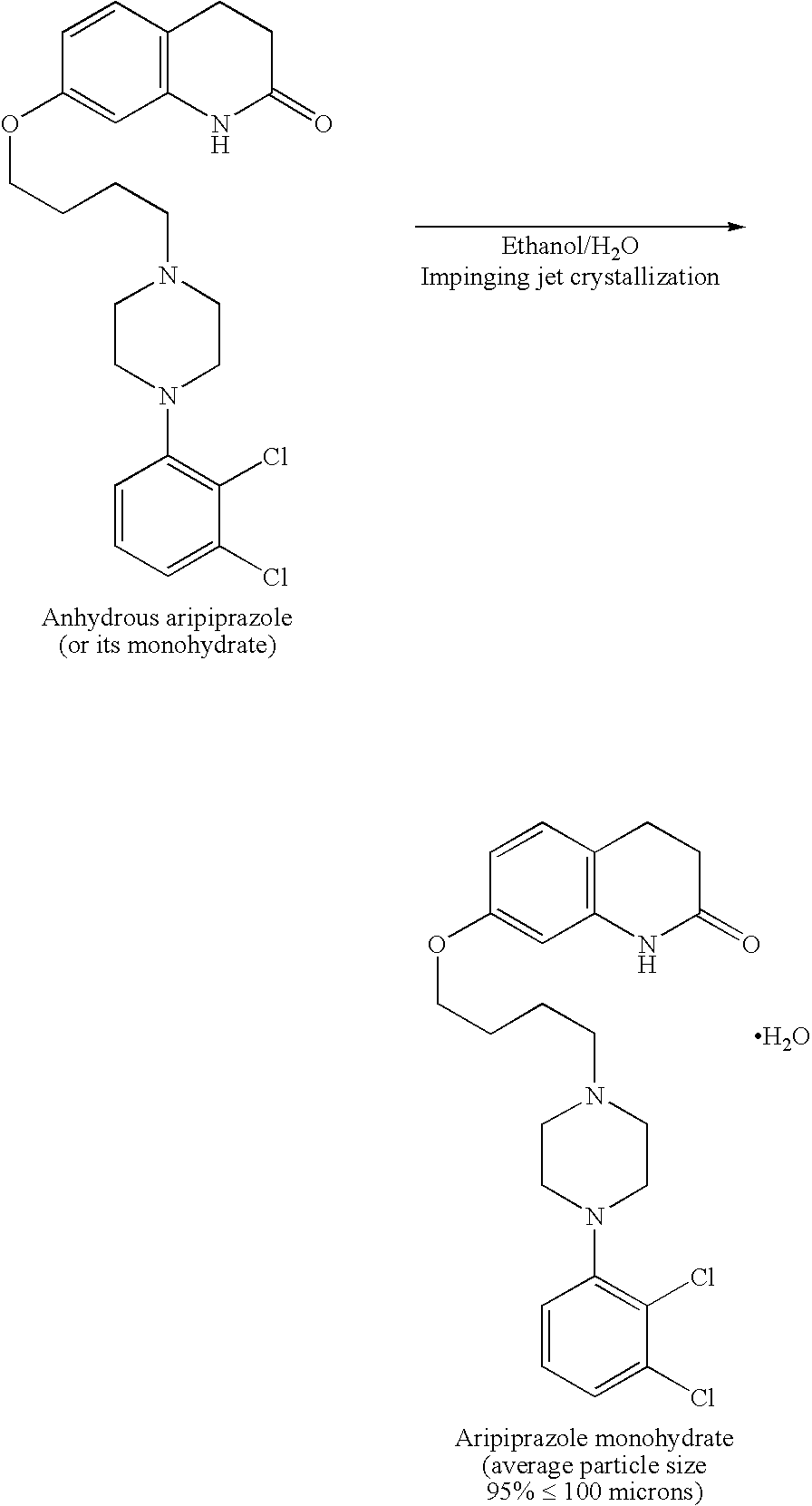
Process for making sterile aripiprazole of desired mean particle size
InactiveUS20050152981A1Enable formationHigh strengthOrganic active ingredientsPowder deliveryIntramuscular injectionFreeze-drying
A process is provided for making sterile aripiprazole having an average particle size less than 100 microns but preferably greater than 25 microns employing an impinging jet crystallization procedure. The resulting bulk aripiprazole of desired particle size may be used to form a sterile freeze-dried aripiprazole formulation, which upon constitution with water and intramuscular injection releases aripiprazole over a period of at least about one week and up to about eight weeks.
Owner:BRISTOL MYERS SQUIBB CO
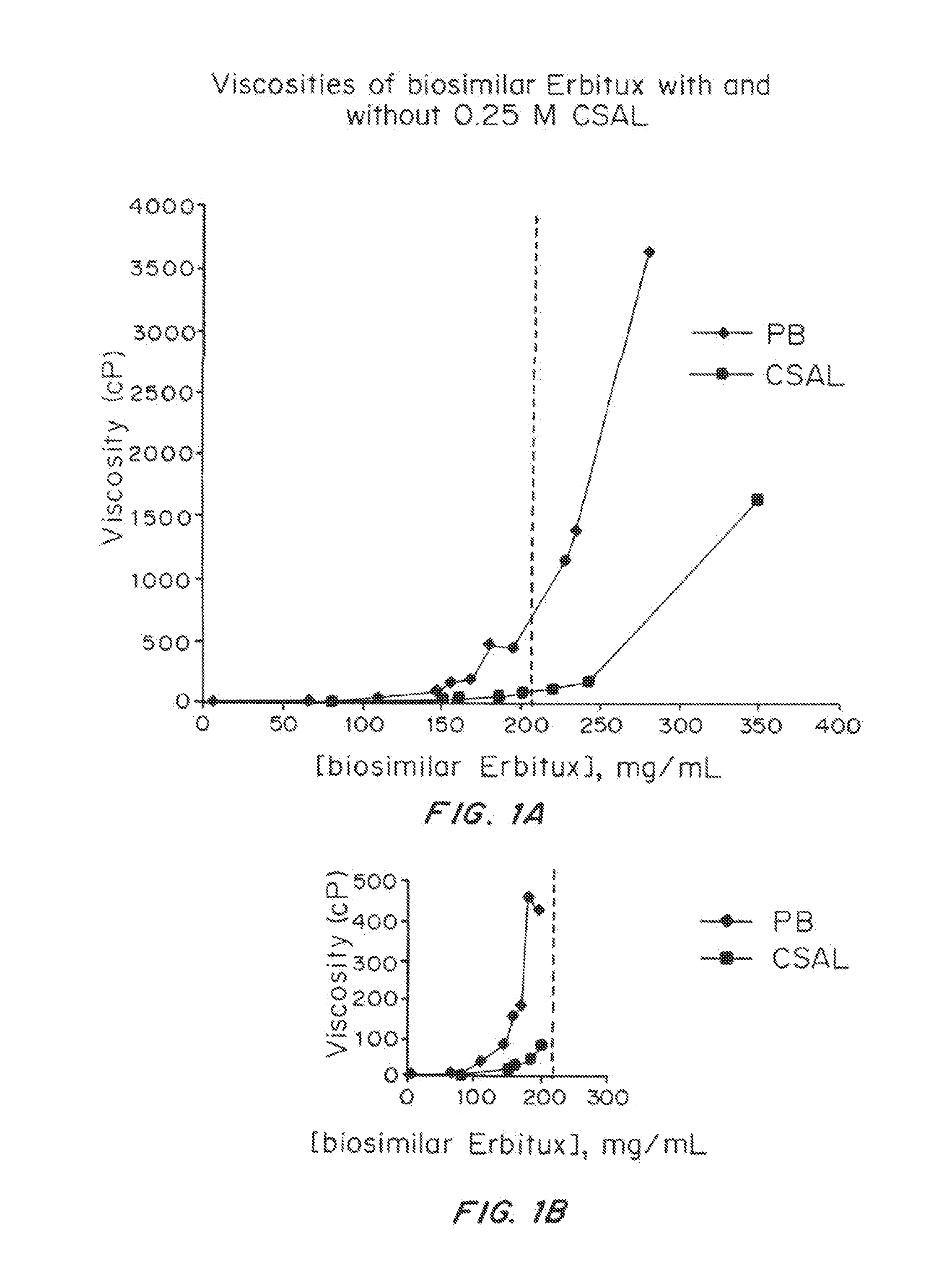
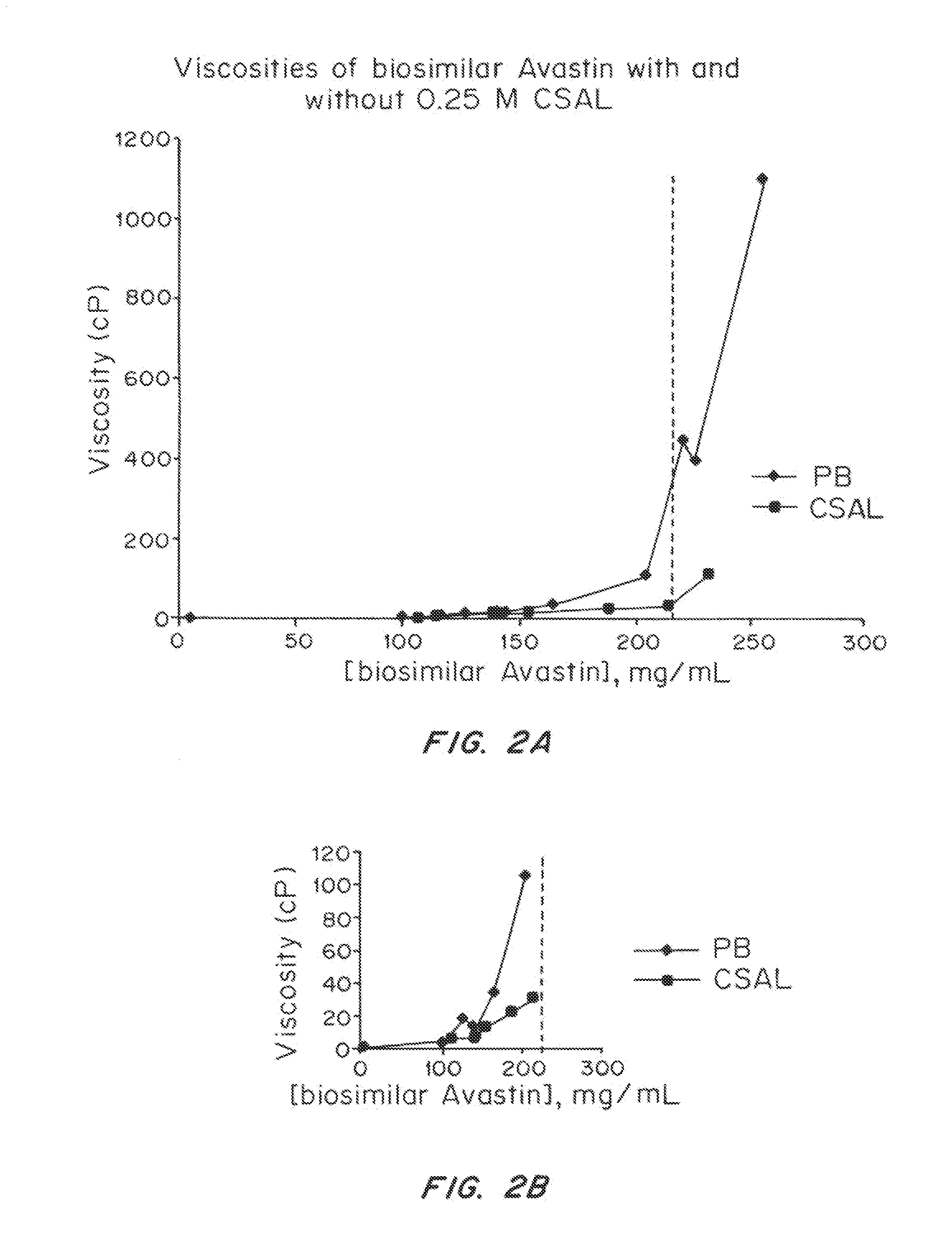
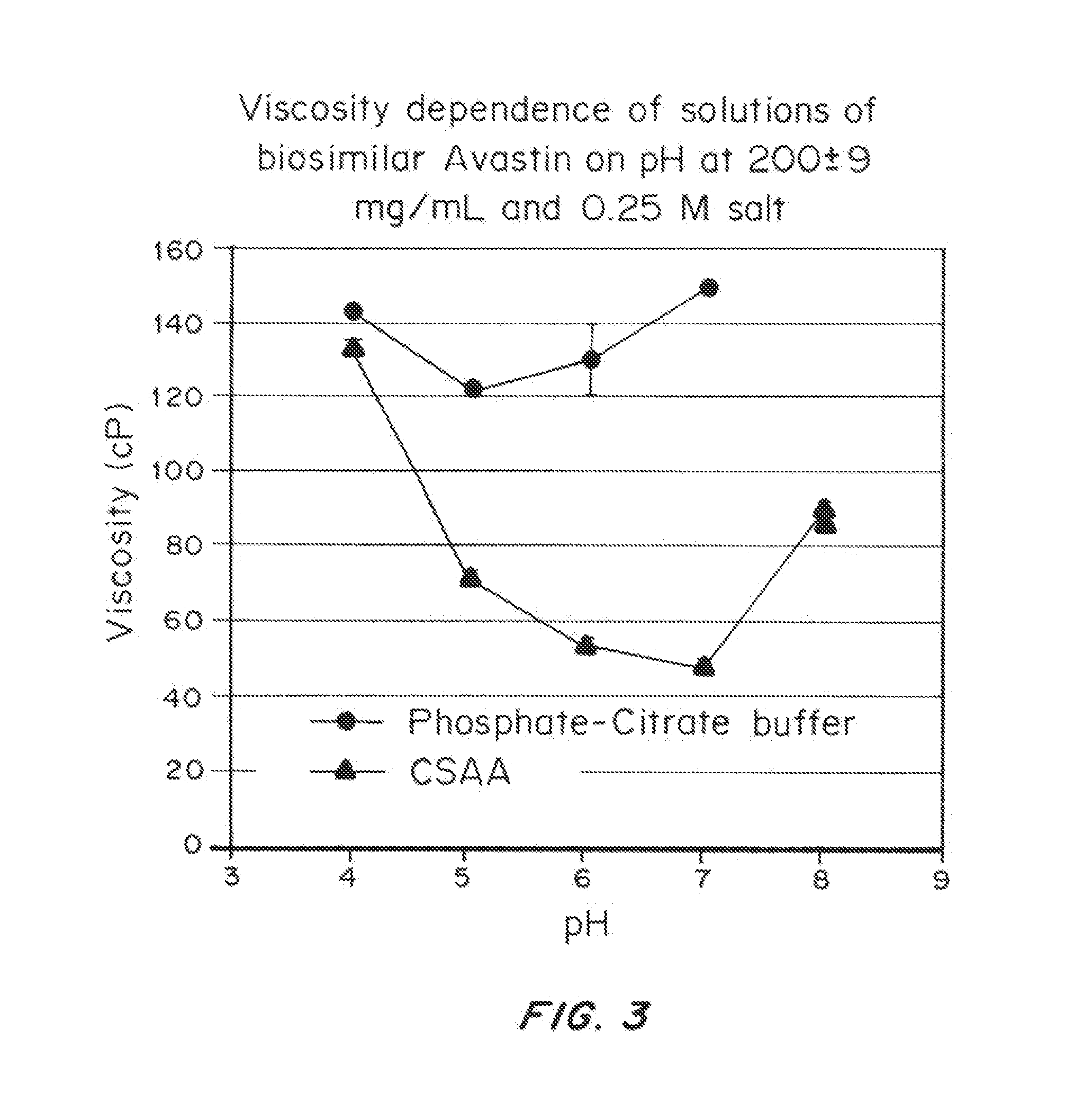
Liquid protein formulations containing viscosity-lowering agents
ActiveUS20150071925A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionAdditive ingredient
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering agents that are typically bulky polar organic compounds, such as many of the GRAS (US Food and Drug Administration List of compounds generally regarded as safe) and inactive injectable ingredients and FDA approved therapeutics.
Owner:EAGLE BIOLOGICS INC
Edaravone injection for treating acute cerebral thrombus and its prepn
InactiveCN1440749AAdjust detection sensitivityOrganic active ingredientsSolution deliveryEdaravone InjectionEmulsion
The Edaravone injection of the present invention is a kind of free radical eliminating medicine to treat acute cerebral infarction. It is injection prepared with Edaravone, additive and solvent and in the form of sterilized solution, emulsion or suspension. It may be small capacity sterilized injection, great capacity sterilized injection, bacteria-free powder preparation for injection, freeze dried powder preparation for injection, sterilized suspension injection or sterilized emulsion injection.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Use of a GnRH antagonist peptide in the treatment of sex hormone-dependent diseases
InactiveUS20090018085A1Peptide/protein ingredientsLuteinising hormone-releasing hormonePrecocious pubertyPhysiology
Owner:FERRING BV
Antimicrobial therapeutic compositions and method of use
InactiveUS6921539B2Enhanced broad based antimicrobial activityEasy to useBiocideHydroxy compound active ingredientsMicroorganismProcaine
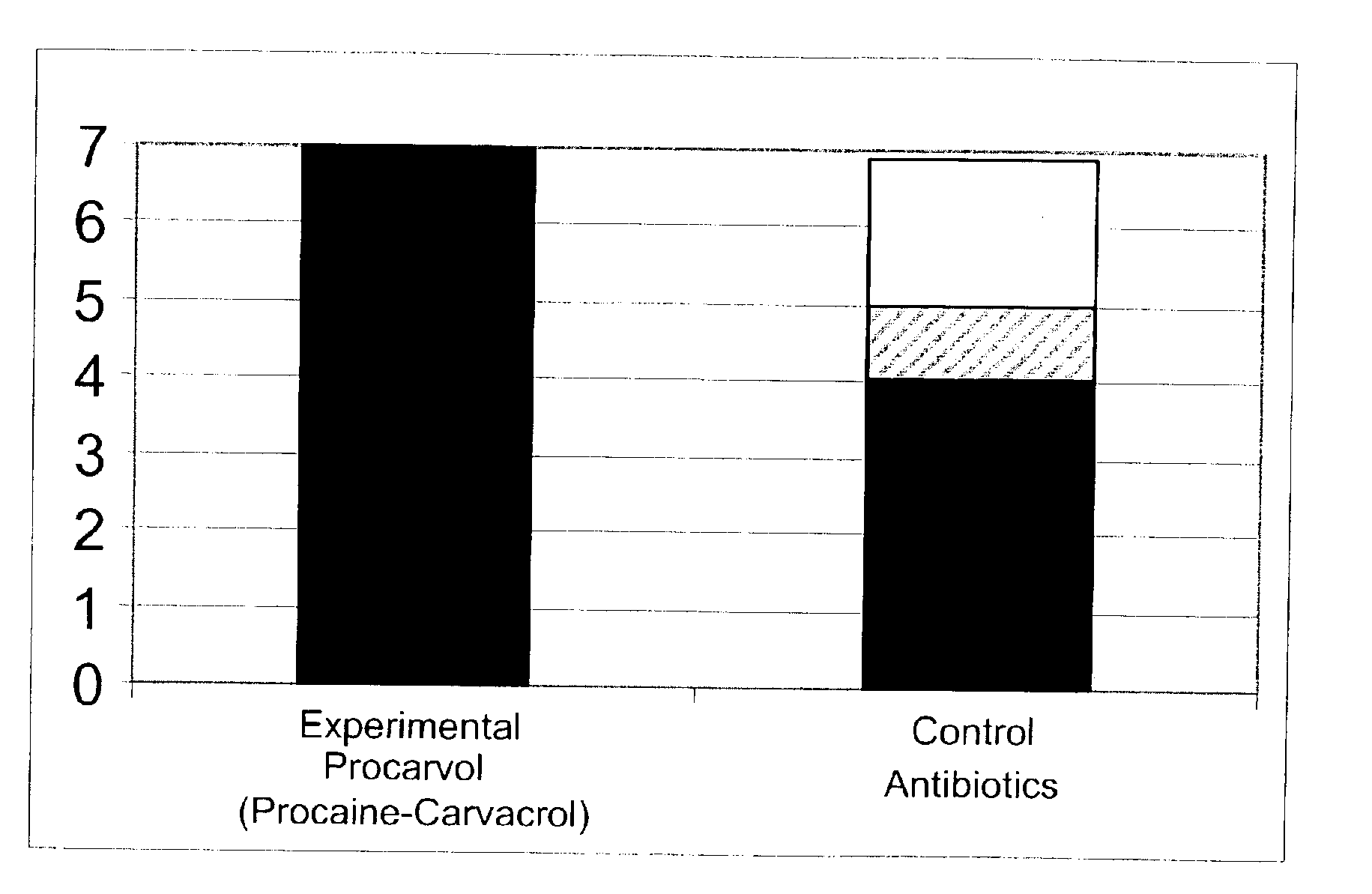
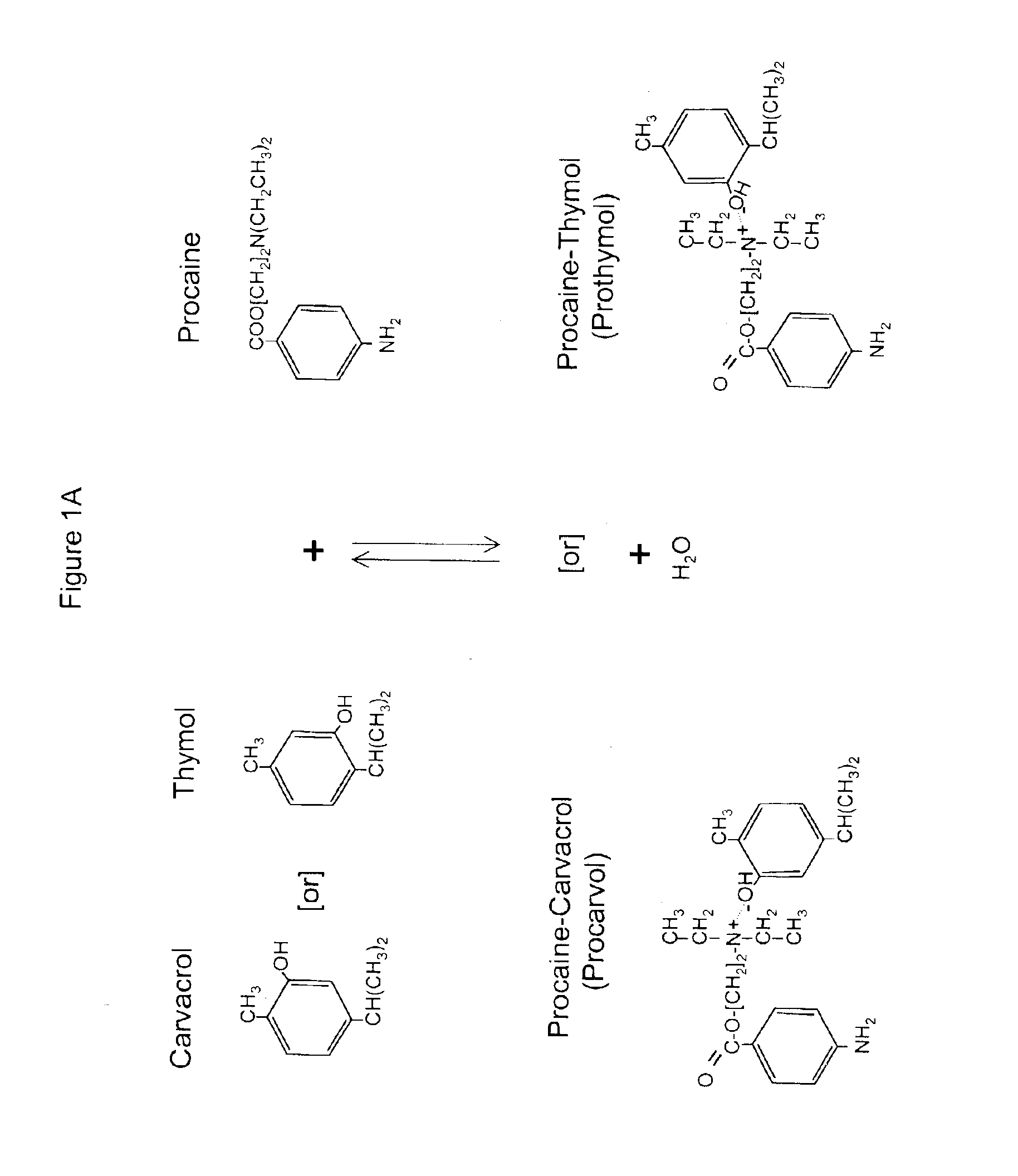
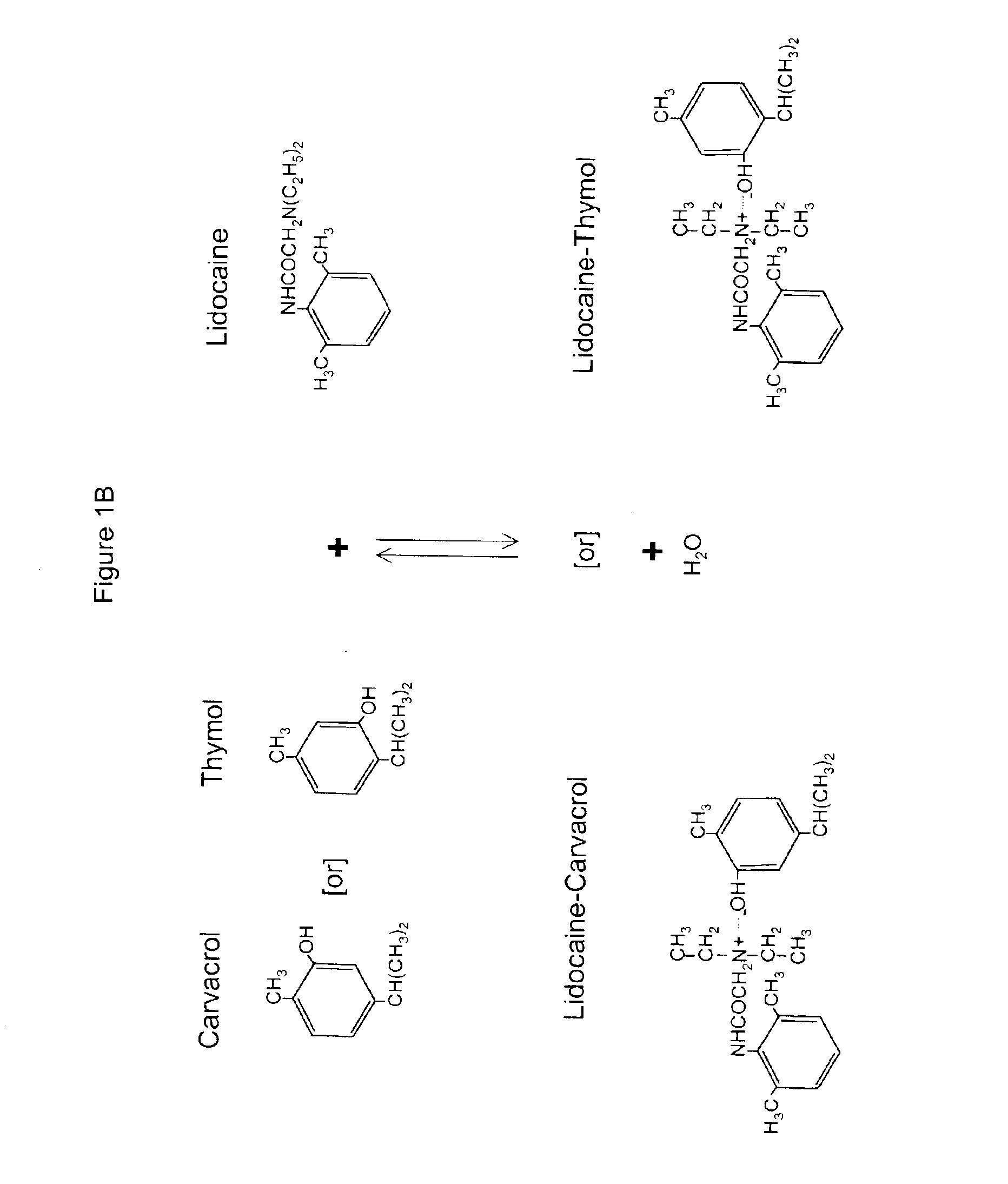
The invention provides therapeutic antimicrobial compositions and methods for their use based on natural organic phenolic compounds combined with pharmacological agents. The antimicrobial activities of each carvacrol and thymol are believed to be enhanced, while the pharmacological properties of procaine and related compounds are added to provide their unique properties to facilitate usefulness and effectiveness in humans. The therapeutic compositions are active against bacterial, fungal, and protozoan infections. The forms of the invention are intended to treat various internal infections through parenteral, subcutaneous, intradermal, intravenous, and intramuscular injections. They are also intended as useful agents to treat microbial infections that have become resistant to conventional anitibiotics as well as secondary opportunistic infections.
Owner:EUROVLOOT +2
Needle assisted jet injection administration of testosterone compositions
ActiveUS20130303985A1Minimize leak-backOrganic active ingredientsJet injection syringesJet injectionTestosterone
Owner:ANTARES PHARMA
Fulvestrant formulations
ActiveUS20090227549A1Lower the volumeBiocideOrganic active ingredientsIntramuscular injectionMedicine
Fulvestrant formulations suitable for intramuscular injection at concentration in excess of 40 mg / ml in the absence of castor oil and castor oil derivatives are disclosed.
Owner:EAGLE PHARMACEUTICALS INC
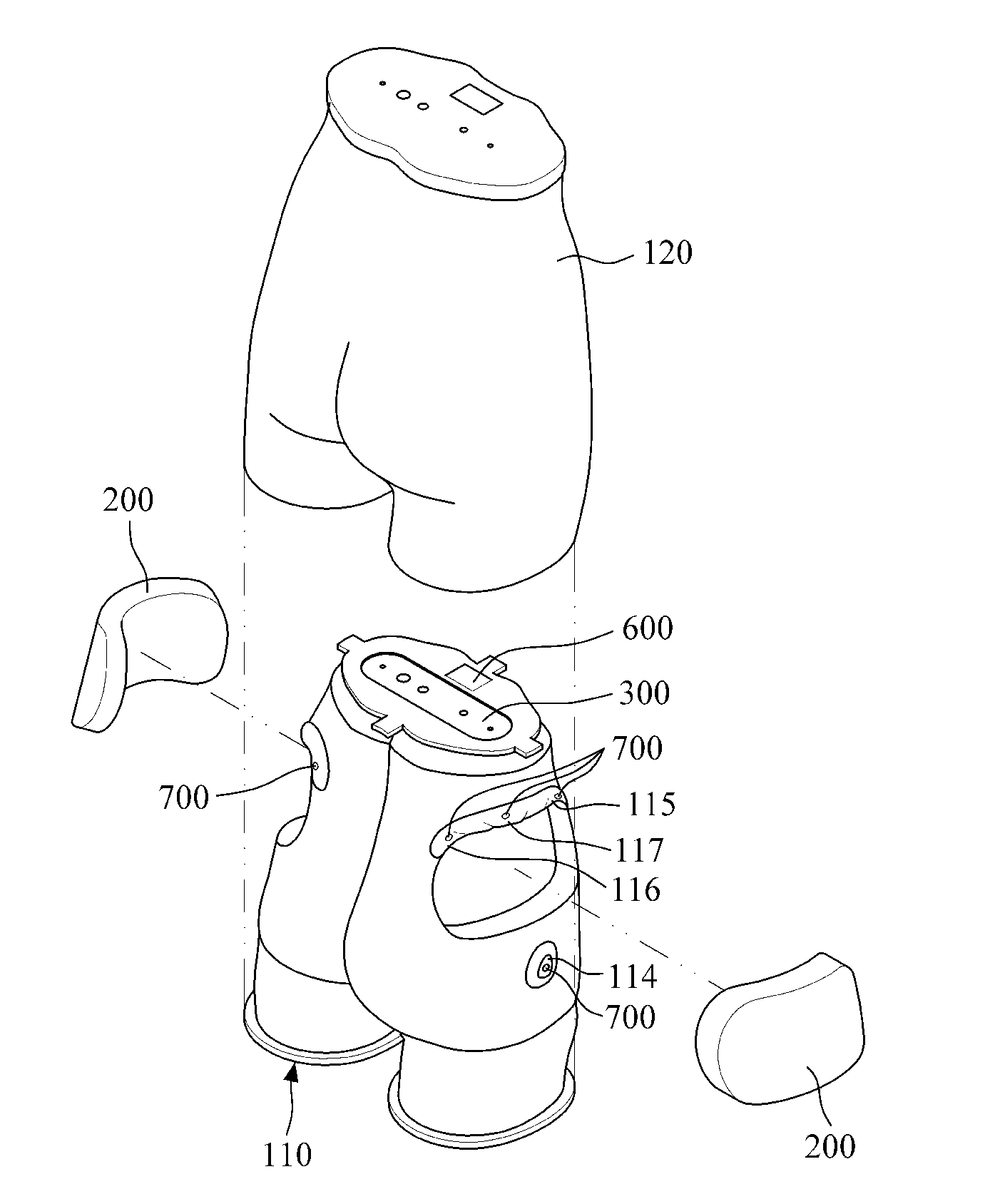
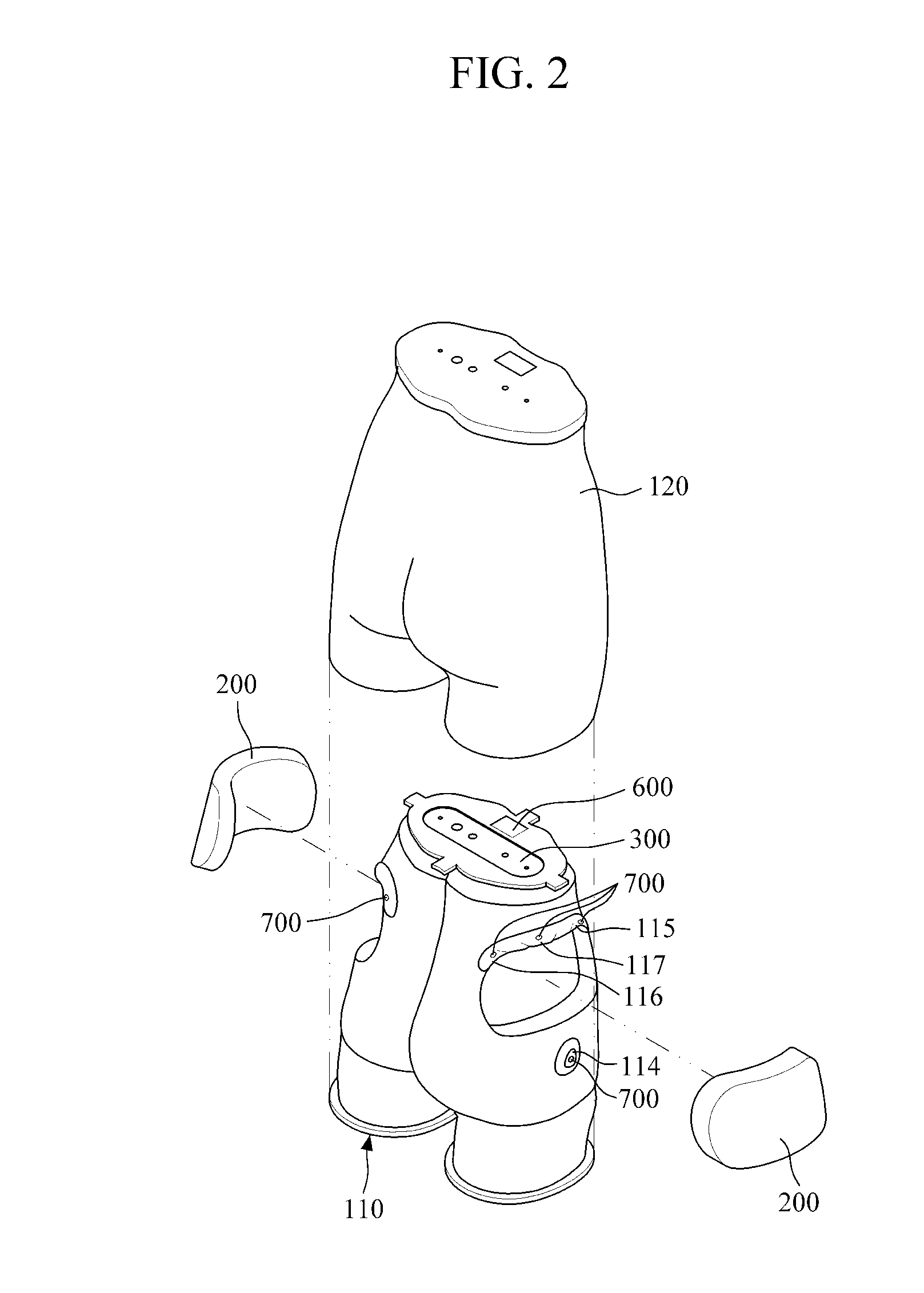
Intramuscular injection training model
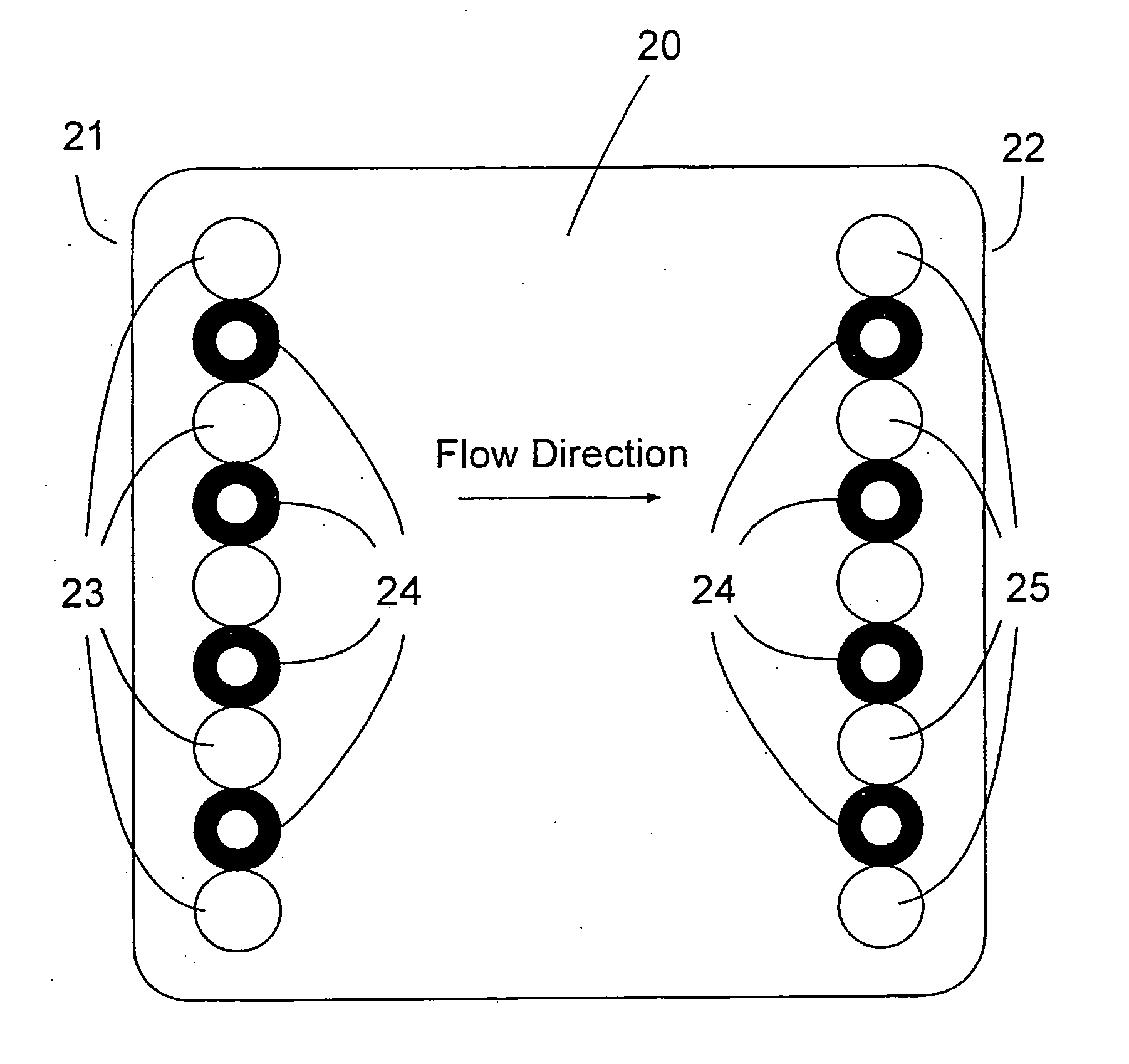
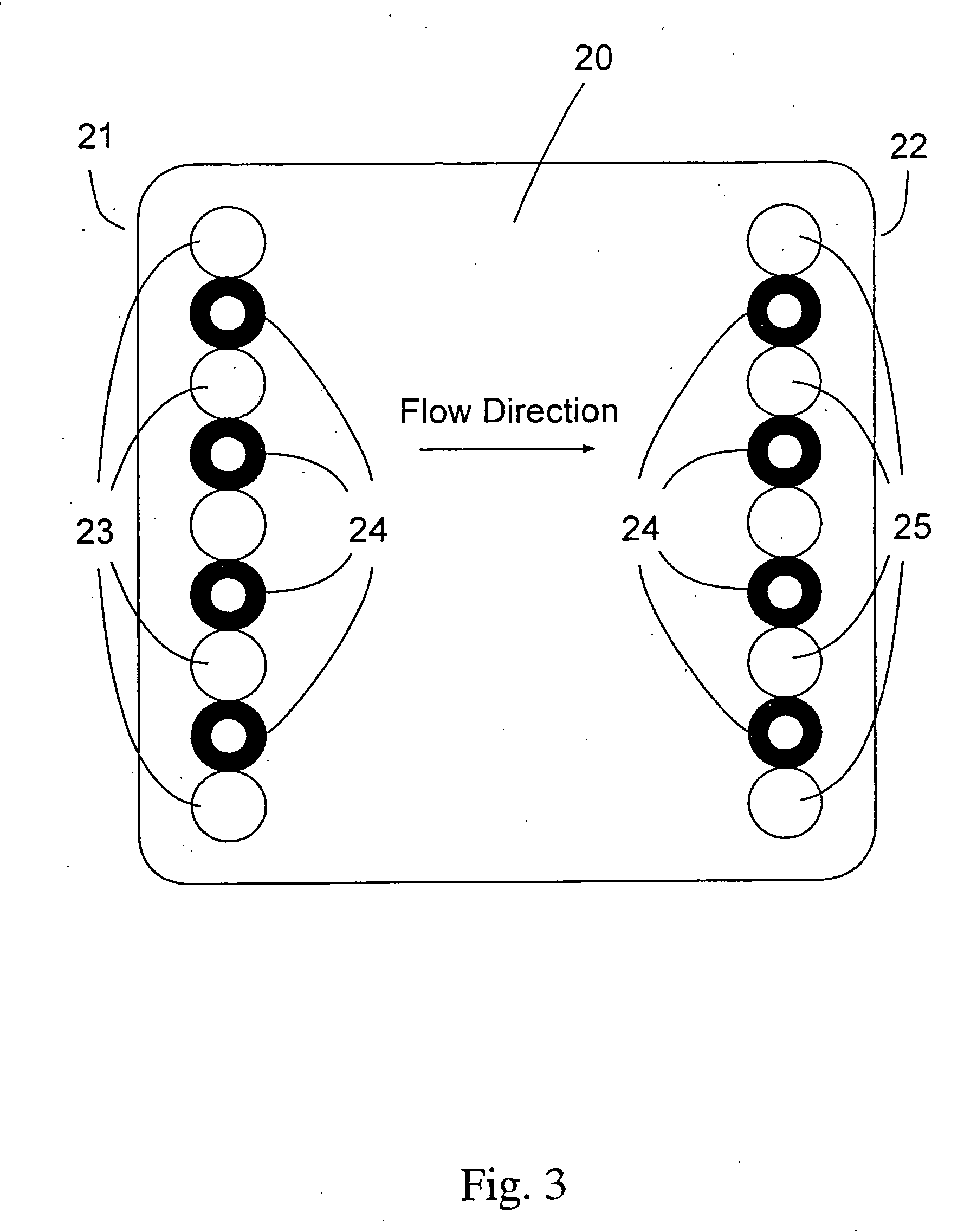
A buttocks intramuscular injection training model is provided. The buttocks intramuscular injection training model includes a hip model having a similar shape to a human hip; injection modules inserted into both sides of the hip model and each being configured to comprise electrode layers to detect a needle and a muscle layer into which injection liquid is injected; a controller connected to the electrode layers to detect a location of the needle; and an input and output device wired or wireless connected to the controller for bidirectional communication with the controller and configured to visibly output the location of the needle.
Owner:BT INC
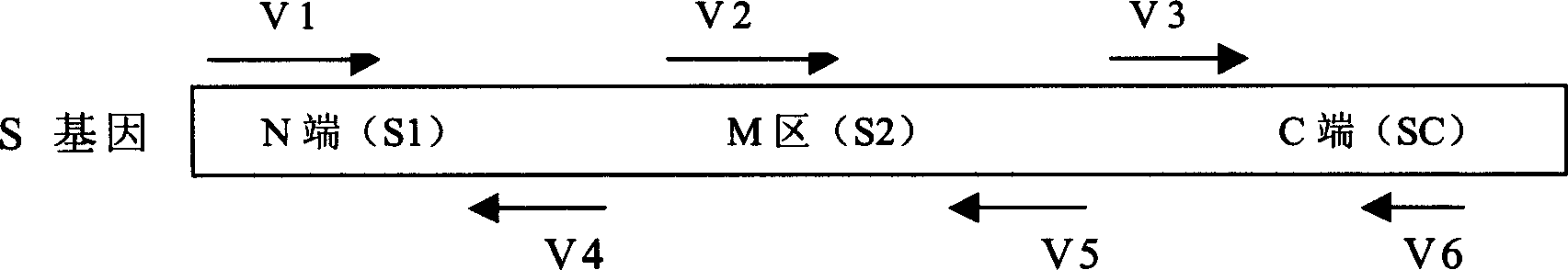
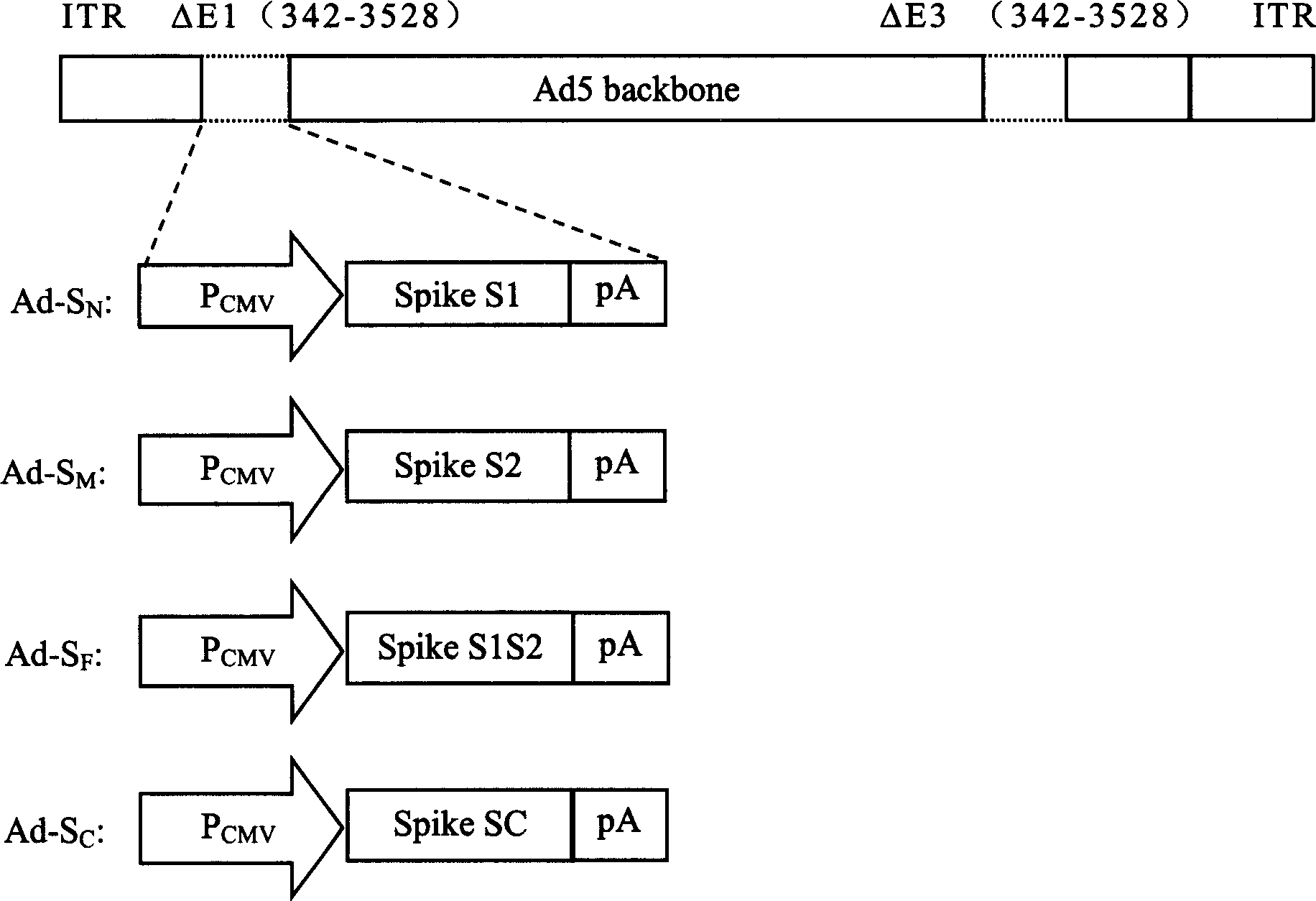
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
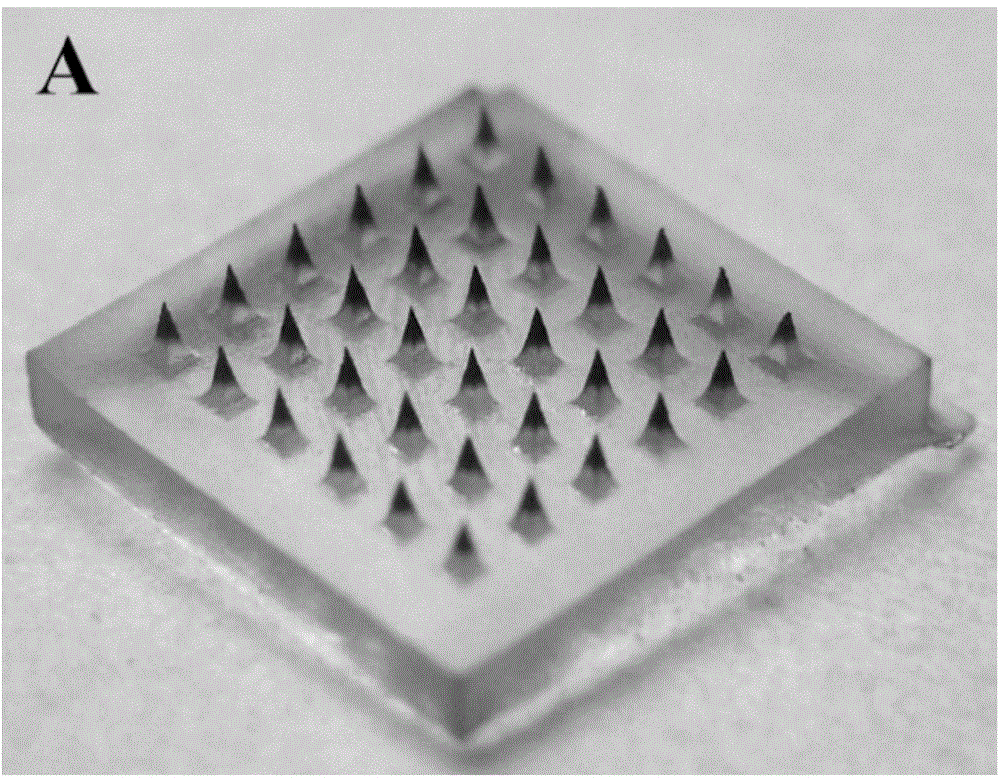
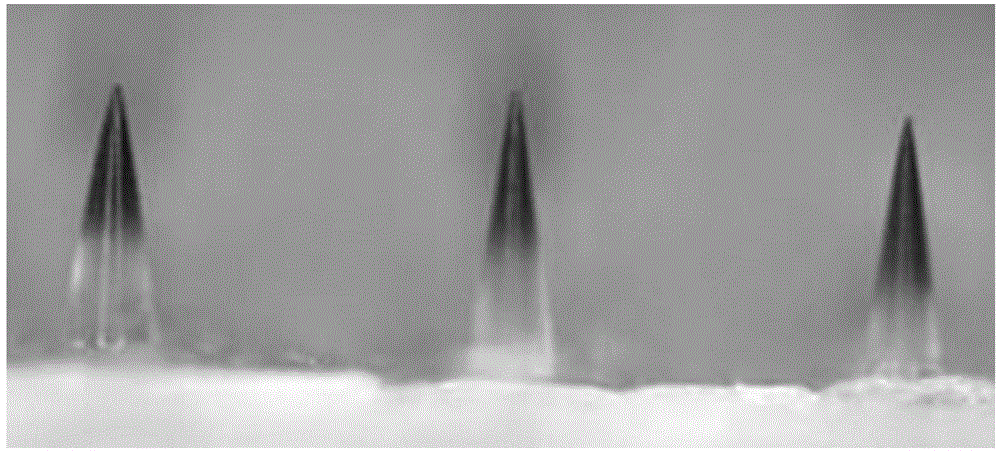
Autolytic microneedle transdermal patch and preparation method thereof
ActiveCN105311000AImproving immunogenicitySolve the problem that it is difficult to induce Th1 type cellular immunityPharmaceutical non-active ingredientsImmunological disordersTransdermal patchIntramuscular injection
The invention belongs to an autolytic microneedle in the technical field of transdermal drug delivery and especially relates to an autolytic microneedle transdermal patch and a preparation method thereof. In the invention, endogenic oligomerized hyaluronic acid and / or low-molecular-weight heparan sulfate are employed as a microneedle substrate material and a vaccine adjuvant at the same time to prepare the autolytic microneedle in which a vaccine is loaded, thereby preparing the autolytic microneedle transdermal patch in the invention. The autolytic microneedle transdermal patch can achieve effective transcutaneous immune of the vaccine and enhance humoral immune response of the vaccine. Compared with intramuscular injection immune and other autolytic microneedle transcutaneous immune in the prior art, the autolytic microneedle transdermal patch can significantly enhance cellullar immunologic response of the vaccine.
Owner:BEIJING CAS MICRONEEDLE TECH LTD
Nano aluminum-encapsulating carrier and application thereof
InactiveCN104055736AImprove stabilityEnhance internal and external stabilityAntiinfectivesEmulsion deliveryBiocompatibility TestingALUMINUM PHOSPHATE
The invention discloses a nano aluminum-encapsulating carrier and an application of the nano aluminum-encapsulating carrier in constructing a vaccine adjuvant transfer system. The nano aluminum is nanoparticles of aluminum phosphate, aluminum sulfate and aluminum hydroxide or a mixture of aluminum phosphate, aluminum sulfate and aluminum hydroxide, wherein the grain size is below 1 mu m; the carrier is a lipidosome, a lipoid, an emulsion, a nano-capsule or a micro-capsule. Construction of the vaccine adjuvant transfer system is the main purpose of the nano aluminum-encapsulating carrier. The application of the nano aluminum-encapsulating carrier in constructing the vaccine adjuvant transfer system comprises the following step: encapsulating vaccine components in the nano aluminum-encapsulating carrier; or adsorbing the vaccine components on the surface of the carrier; or purely mixing with the carrier to exert the vaccine adjuvant and the transfer function. The nano aluminum-encapsulating carrier disclosed by the invention has the advantages that the nano aluminum-encapsulating carrier is wide in application range, so that the nano aluminum-encapsulating carrier is suitable for antigens with different pathogens; the nano aluminum-encapsulating carrier is high in stability and can encapsulate antigens so as to enhance in vivo and in vitro stability; the nano aluminum-encapsulating carrier is high in safety, and the use materials have good biocompatibility; the nano aluminum-encapsulating carrier is many in inoculation and wide in way, and the carrier can be inoculated through track mucosa or subcutaneous, intracutaneous and intramuscular injection; the carrier is strong in immunosuppression induction potency.
Owner:ANHUI MEDICAL UNIV
In-situ phase change gel slow release system taking phospholipid as substrate and preparation method thereof
ActiveCN102526753AGood slow releaseImprove liquidityAerosol deliveryOintment deliveryIntramuscular injectionPhospholipid
The invention provides an in-situ phase change gel slow release system taking a phospholipid as a substrate, and provides a preparation method thereof. A high-concentration phospholipid slow release preparation is prepared from a high-concentration (50-85 percent) phospholipid, a bioactive ingredient, ethanol solutions of different concentrations and / or injection oil with a simple method, and has the characteristics of high biocompatibility, small untoward effect, remarkable slow release effect and suitability for various administration forms such as hypodermic injection, intramuscular injection, external administration and the like; the amount of a coated medicament can be conveniently adjusted according to the clinical dosage of a medicament; and the in-situ phase change gel slow release system has a wide application prospect.
Owner:成都师创生物医药科技有限公司
Multiple injection syringe holder
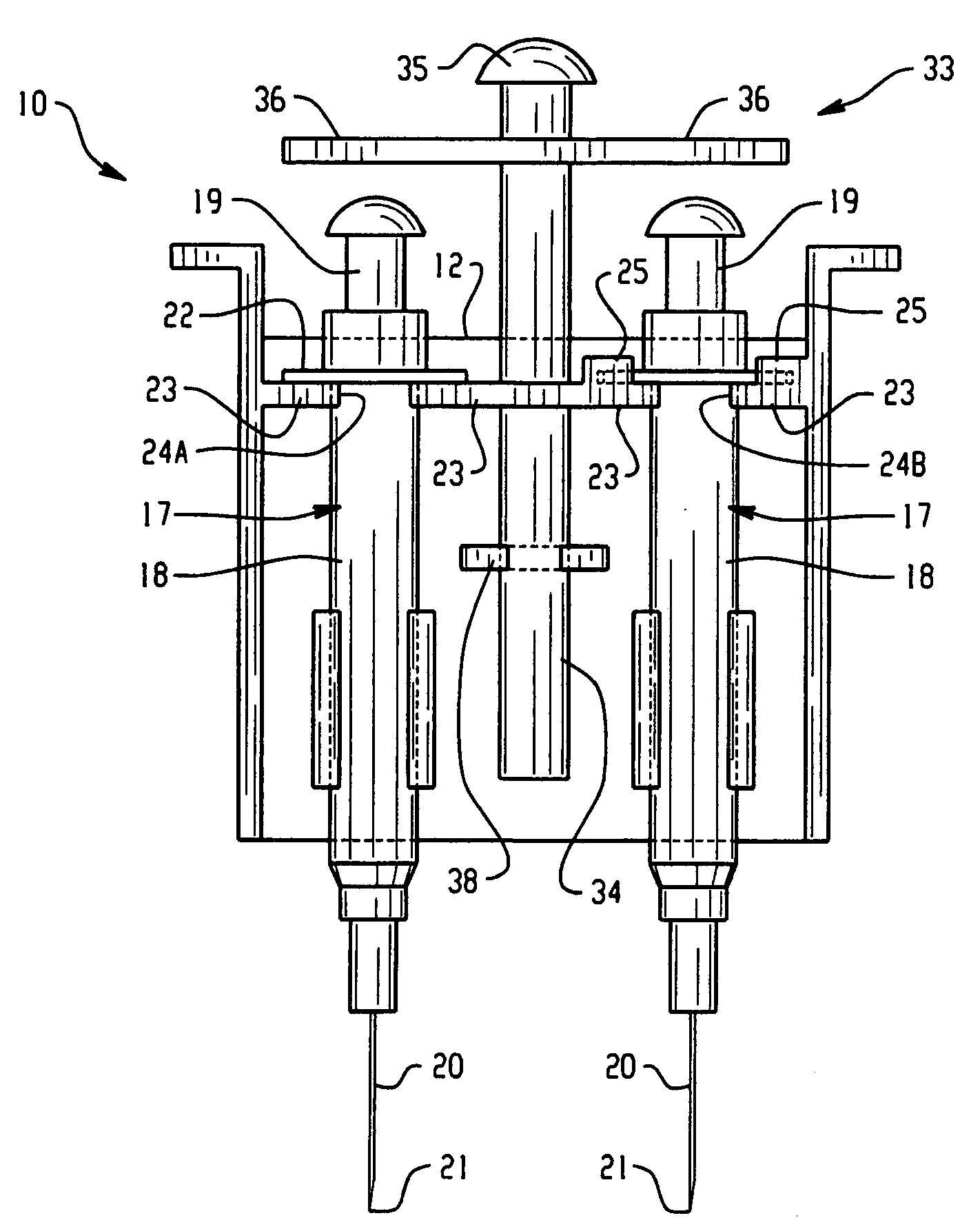
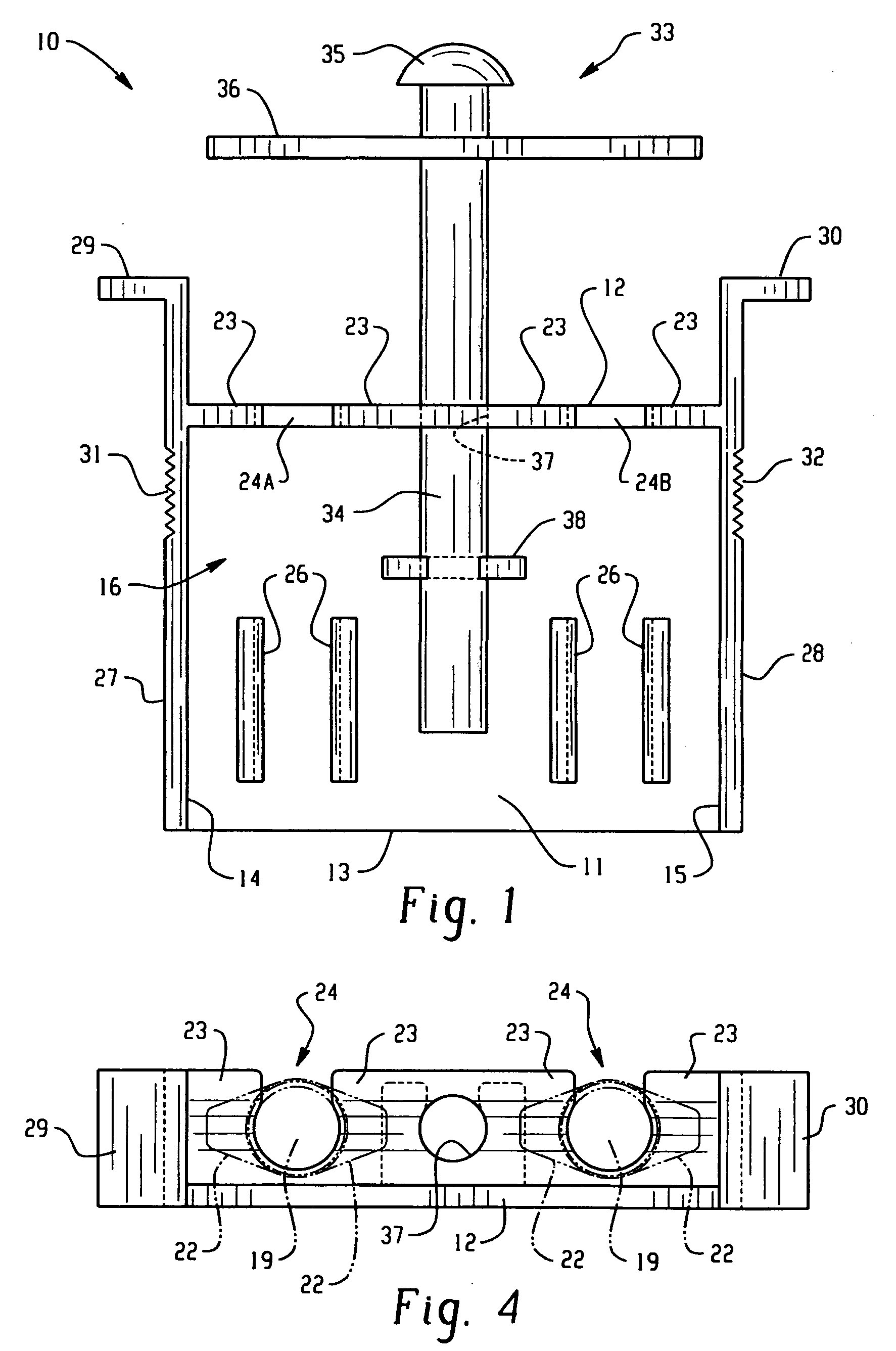
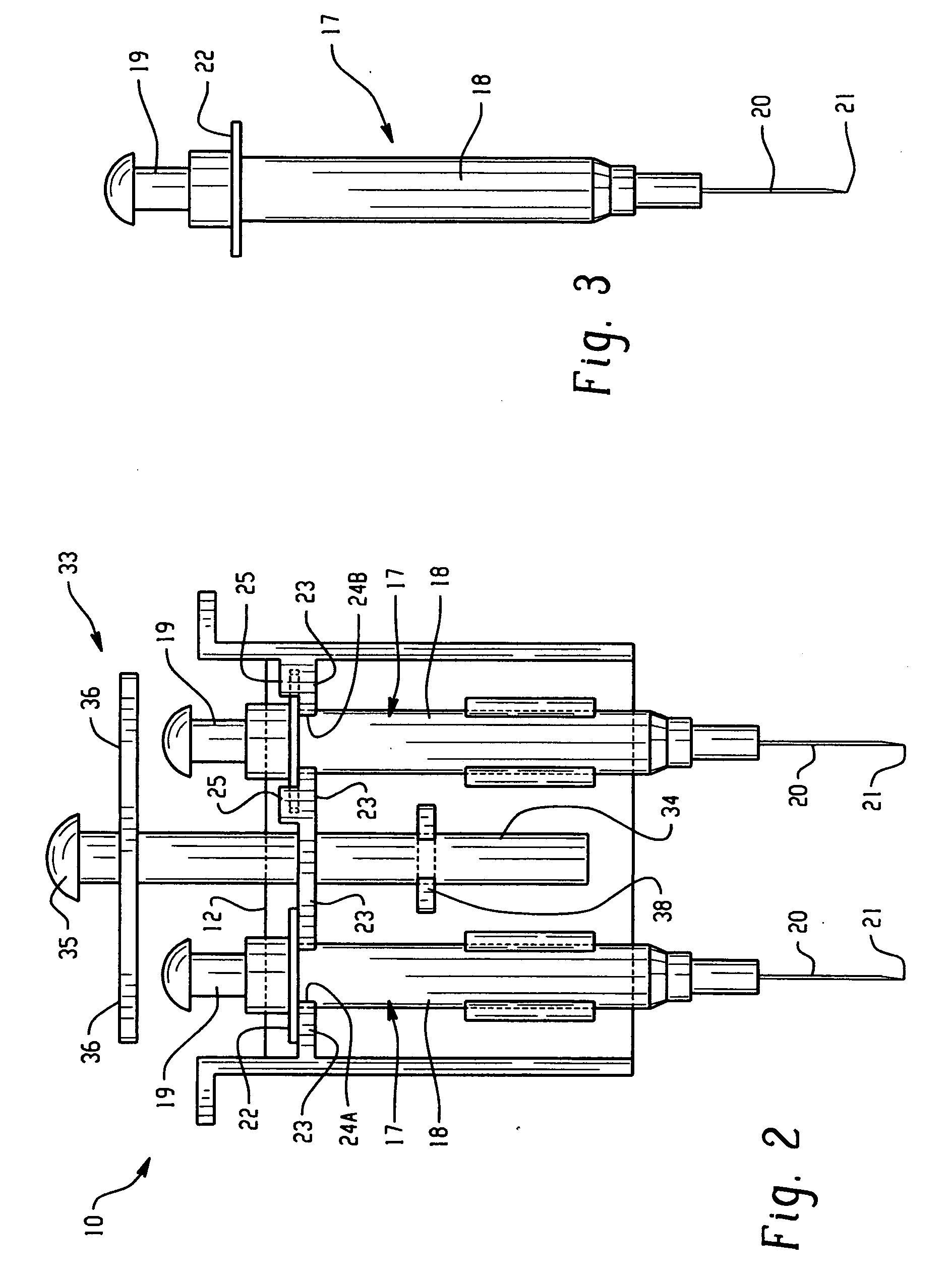
InactiveUS20080255520A1Easily cleanableEasily sterilizableAmpoule syringesIntravenous devicesMultiple injectionIntramuscular injection
The invention provides a lightweight, inexpensive syringe holder for substantially simultaneous multiple injections such as, but not limited to, intramuscular injections for immunizations. The syringe holder can be molded as a single unit, and can be disposable or reusable, and easily cleanable and / or sterilizable, as desired. The syringe holder can securely accommodate two or more syringes, and two or more substantially simultaneous injections can be performed easily by the administrator using only one hand. Thus, it is possible for the administrator to use two hands, and perform four or more injections that are substantially simultaneous, thus reducing pain, stress and trauma.
Owner:HENDERSON THOMAS D
Biphenyl diester liquid medicinal composition containing fat
InactiveCN1478465AOrganic active ingredientsDigestive systemBiphenyl dimethyl-dicarboxylateIntramuscular injection
A composite liquid biphenyl dimethyl dicarboxylate and its emulsion and oil injections for intravenous injection, intramuscular injection, or oral application are disclosed. Said emulsion has high stability and low granularity. Said solution-type oil has slow releasing effect.
Owner:佟丽 +1
Preparation and application of amphiphilic albumin derivative and pharmaceutical composition thereof
InactiveCN101543630AEvade captureHigh drug loadingPharmaceutical delivery mechanismMacromolecular non-active ingredientsCholic acidWater insoluble
The invention relates to a biodegradable amphiphilic albumin derivative and a pharmaceutical composition thereof. In the biodegradable amphiphilic albumin derivative, hydrophilic long-chain polyethyleneglycol and alkyl (acyl) group or (deoxidized) cholic acid are led to an albumin skeleton so that the amphiphilic albumin derivative is amphipathic and is self-assembled in water to form nano-micelle. The biodegradable amphiphilic albumin derivative is characterized in that medicament can be encapsulated through the double actions of a hydrophobic group and an albumin molecule chain with the medicament, and the capacity of the albumin encapsulating the medicament is markedly improved; in addition, a hydrophilic long-chain can reduce the immunogenicity of the albumin and improve the nano-micelle surface hydrophilicity so that the stability of the nano-micelle in aqueous medium can be improved and the nano-micelle has long-circulation characteristics in a body. The pharmaceutical composition of the biodegradable amphiphilic albumin derivative can be used as the carrier of organic medicament, water-insoluble or insoluble medicament and amphiphilic medicament, can be used for intravascular administration, intramuscular injection administration, oral administration, cavitary administration or external administration. The invention can be prepared with simple method and mature process and is suitable of large-scale continuous production.
Owner:CHINA PHARM UNIV
Liquid protein formulations containing water soluble organic dyes
ActiveUS20150071920A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionMedicine
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering water soluble organic dyes.
Owner:EAGLE BIOLOGICS INC
Andrographolide suspension and preparation method and medical application thereof
InactiveCN103371972AReduce stimulationGuaranteed validityAntibacterial agentsOrganic active ingredientsHypodermoclysisIntramuscular injection
The invention discloses an andrographolide suspension capable of obviously improving oral bioavailability and realizing slow release by intramuscular injection or hypodermic injection, a method for preparing the suspension and a medical application of the suspension. The preparation consists of a general suspension and a dry suspension and can be taken orally or subjected to hypodermic injection or intramuscular injection. The method is characterized in that the raw materials are processed to obtain micron-sized or nanometer-sized particles, a certain amount of auxiliary materials are added, thus the bioavailability is improved and the stability of the preparation is ensured. The whole preparation process is simple and controllable, and the industrial production can be realized. Compared with a preparation prepared from common raw materials, the preparation prepared by the preparation method, disclosed by the invention, has the advantages that the bioavailability can be obviously improved by taking orally; and the low release effect can be realized after intramuscular injection or hypodermic injection.
Owner:CHANGZHOU TARGET MEDICINE TECH CO LTD
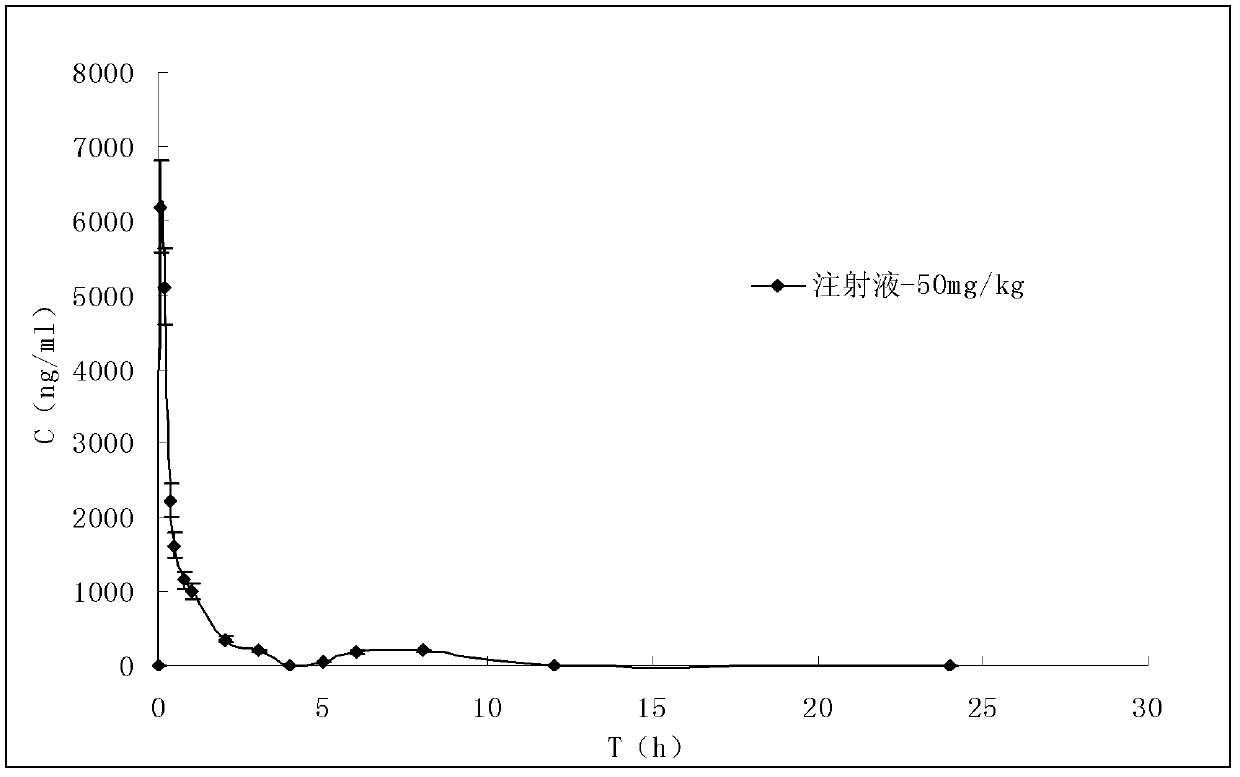
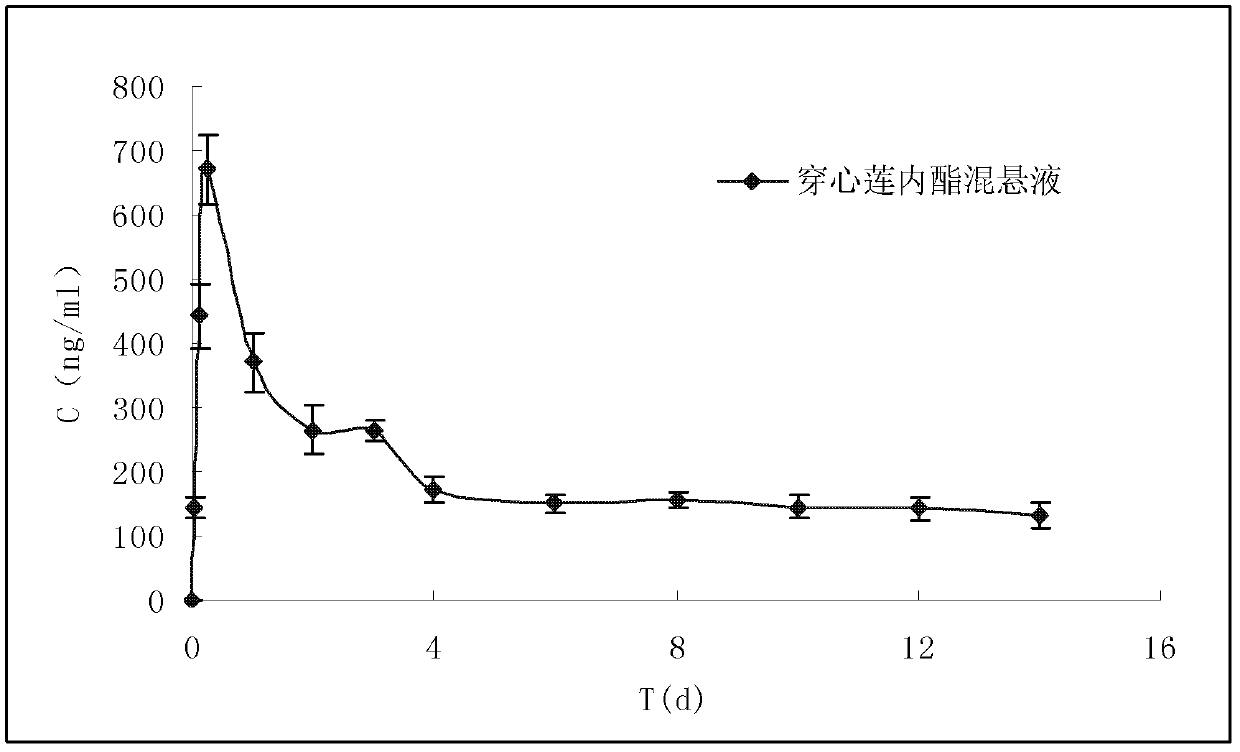
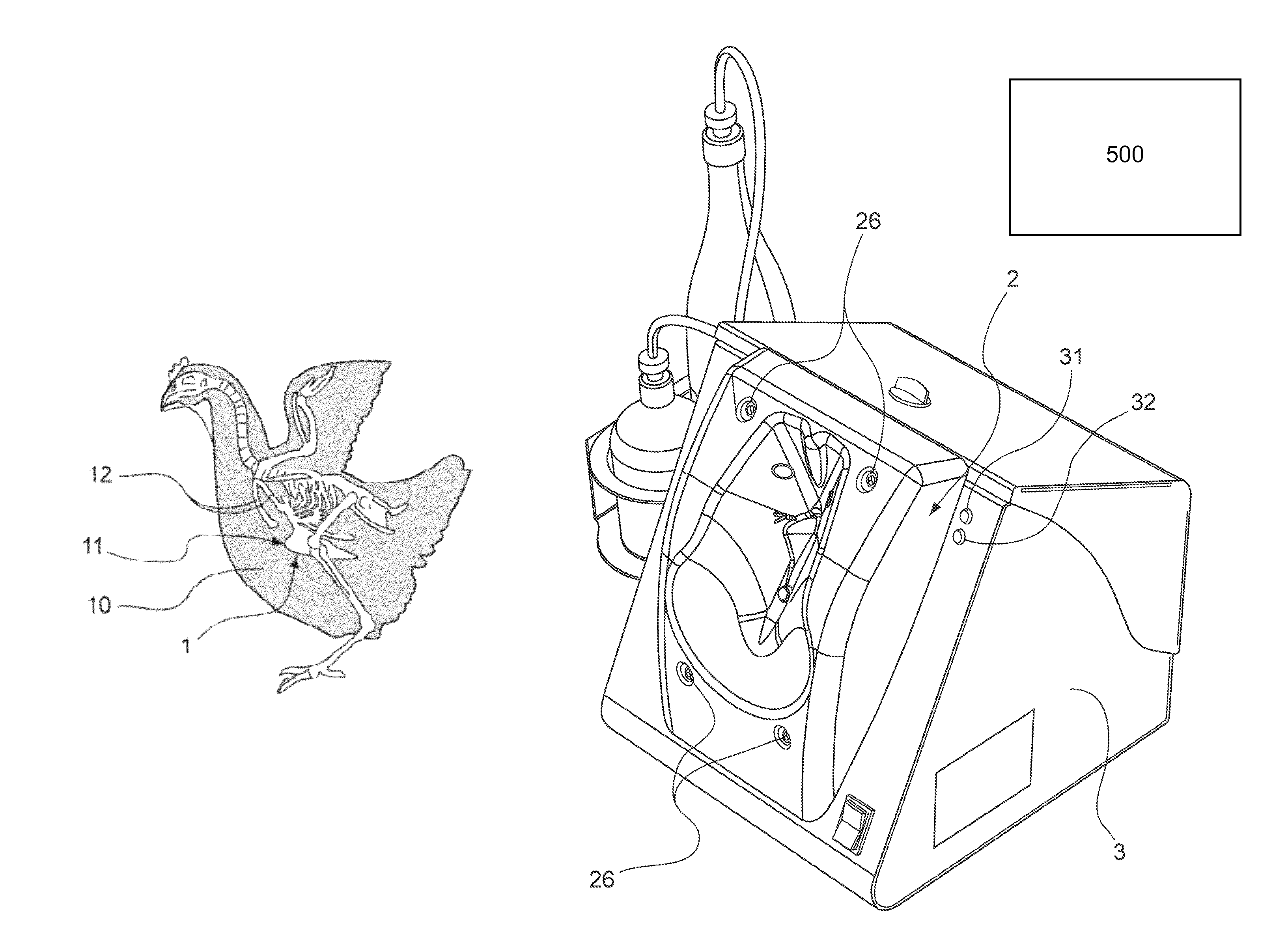
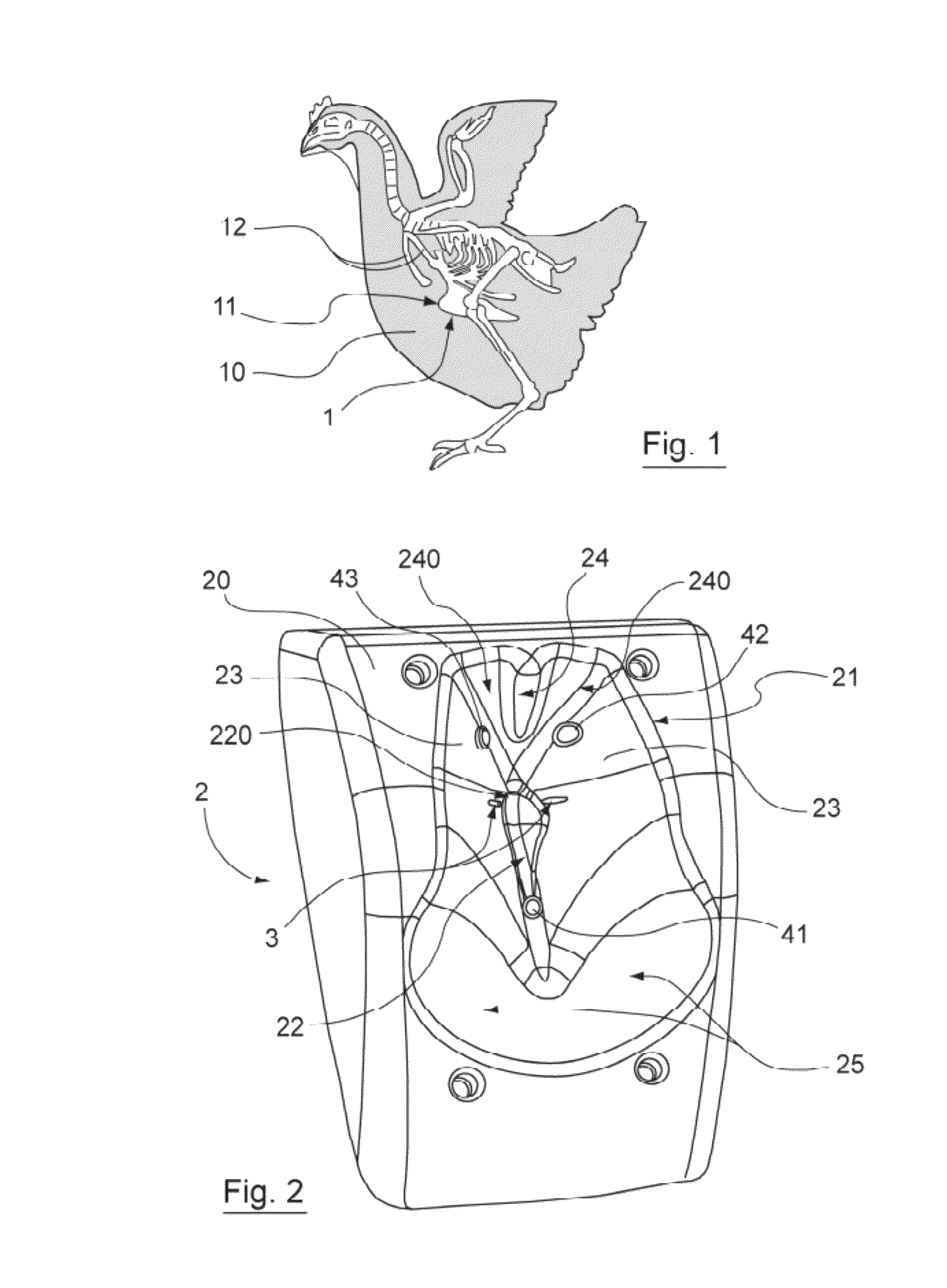
Device for injecting veterinary products to poultry including a retention member having an anatomic form with means for bracing a detectable bone
ActiveUS8211058B2Improve injection qualityAdvantageous for vaccinatingCannulasAutomatic syringesAnimal scienceIntramuscular injection
The invention relates to a device for injecting veterinary products to at least one bird by intramuscular injection, wherein said injection(s) can be carried out in the area of at least one muscle in the vicinity of a bone having a detectable shape in the body of said poultry, said device comprising: a retention member with means for bracing said detectable bone, at least one hole being formed in said retention member; at least one injection needle, said needle(s) being movable across said opening(s); characterized in that the retention member has: an anatomic shape that conforms to a portion of the body of said poultry and inside which is provided said means or bracing the detectable bone, said anatomic shape including a bearing surface for said body at said muscle; at least two contact sensors to be actuated by said poultry and provided on the anatomic shape with at least one on the bearing surface, said opening(s) being provided between said sensors.
Owner:DESVAC
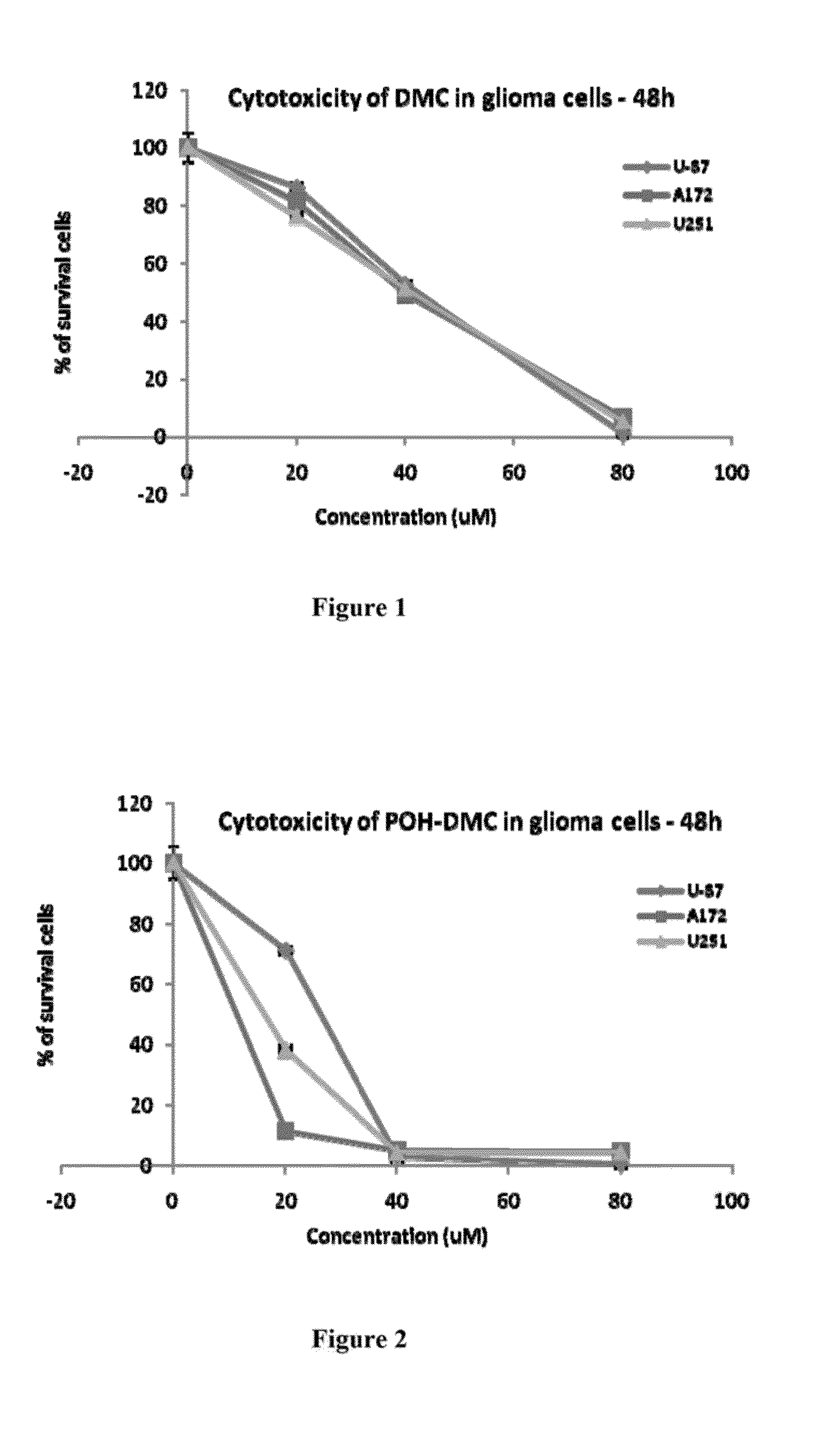
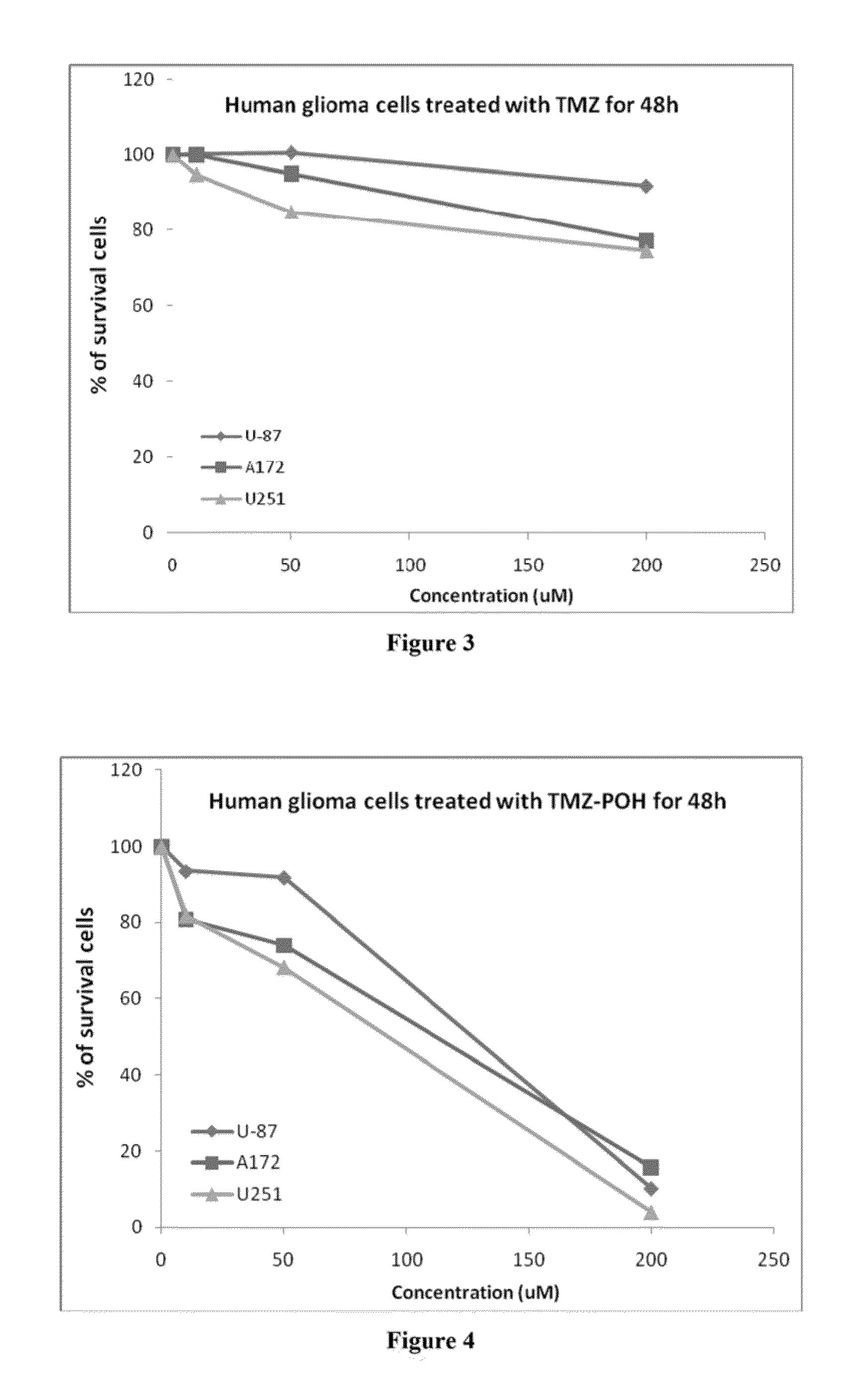
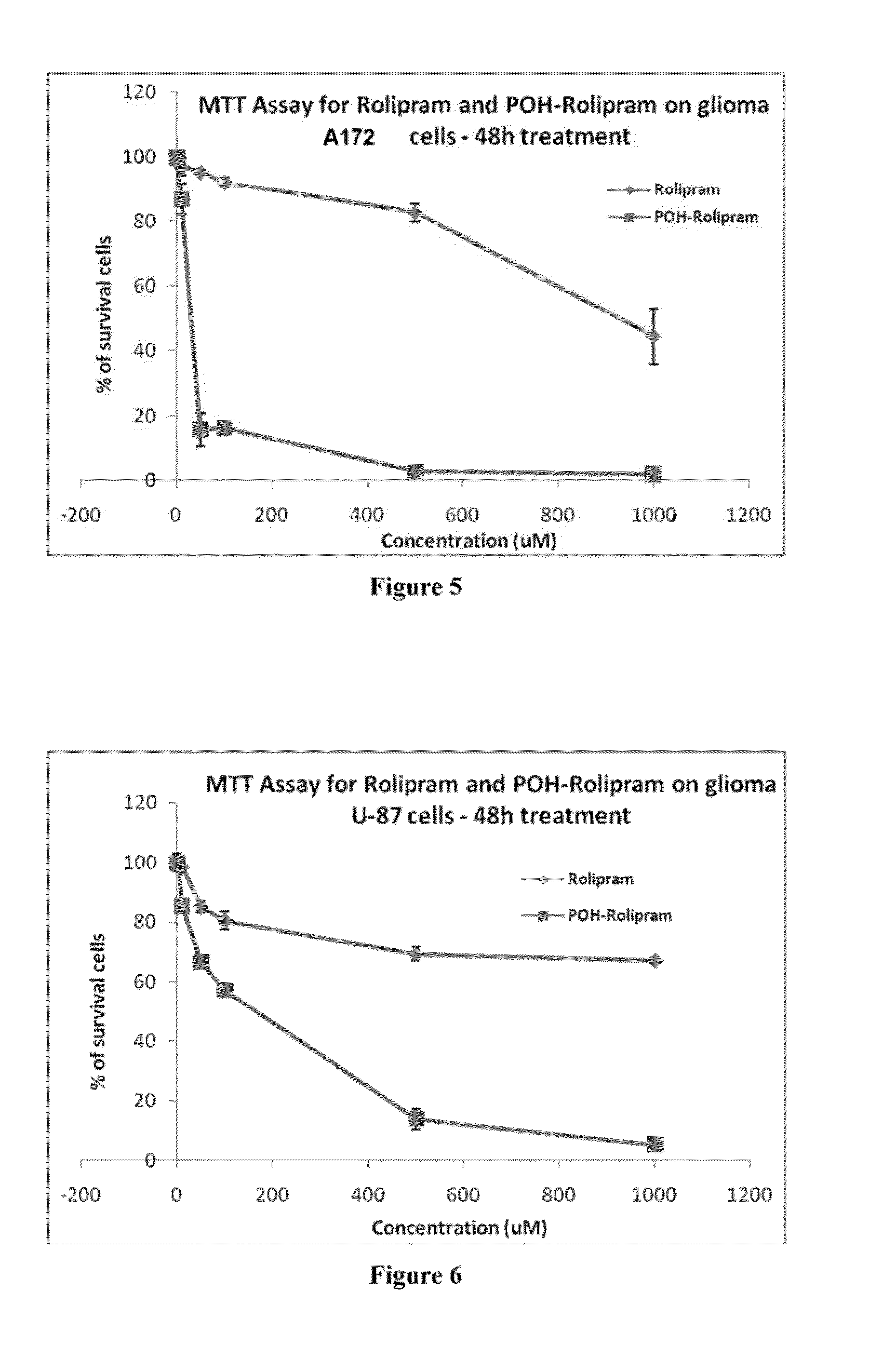
Pharmaceutical compositions comprising poh derivatives
The present invention provides for a derivative of monoterpene or sesquiterpene, such as a perillyl alcohol derivative. For example, the perillyl alcohol derivative may be a perillyl alcohol carbamate. The perillyl alcohol derivative may be perillyl alcohol conjugated with a therapeutic agent such as a chemotherapeutic agent. The present invention also provides for a method of treating a disease such as cancer, comprising the step of delivering to a patient a therapeutically effective amount of a derivative of monoterpene (or sesquiterpene). The route of administration may vary, and can include, inhalation, intranasal, oral, transdermal, intravenous, subcutaneous or intramuscular injection.
Owner:NEONC TECH
Sealing device for filtration devices
InactiveUS20060125187A1Improper alignmentImprove sealingSemi-permeable membranesMembranesFiltrationThermoplastic elastomer
The present invention relates to the formation of a gasket, sealing area or O-ring, such as a gasket on a screen for a filtration module such as a TFF or NF cassette or an O-ring on the outlet of a filter cartridge wherein the seal is proud of at least one surface of the screen. Preferably, the seal is molded to the filter component, more preferably it is injection molded to the component. The seal maybe formed of any elastomeric material such as thermoplastic, thermoplastic elastomers, thermosets and rubber, both natural and synthetic. The molded seal provides better sealing, allows for a variation in heights and geometries, and provides better cleanliness and lower extractables than the currently used adhesives or conventional gaskets or O-rings.
Owner:MILLIPORE CORP
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
InactiveCN101451145AActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsIntramuscular injectionTreating tuberculosis
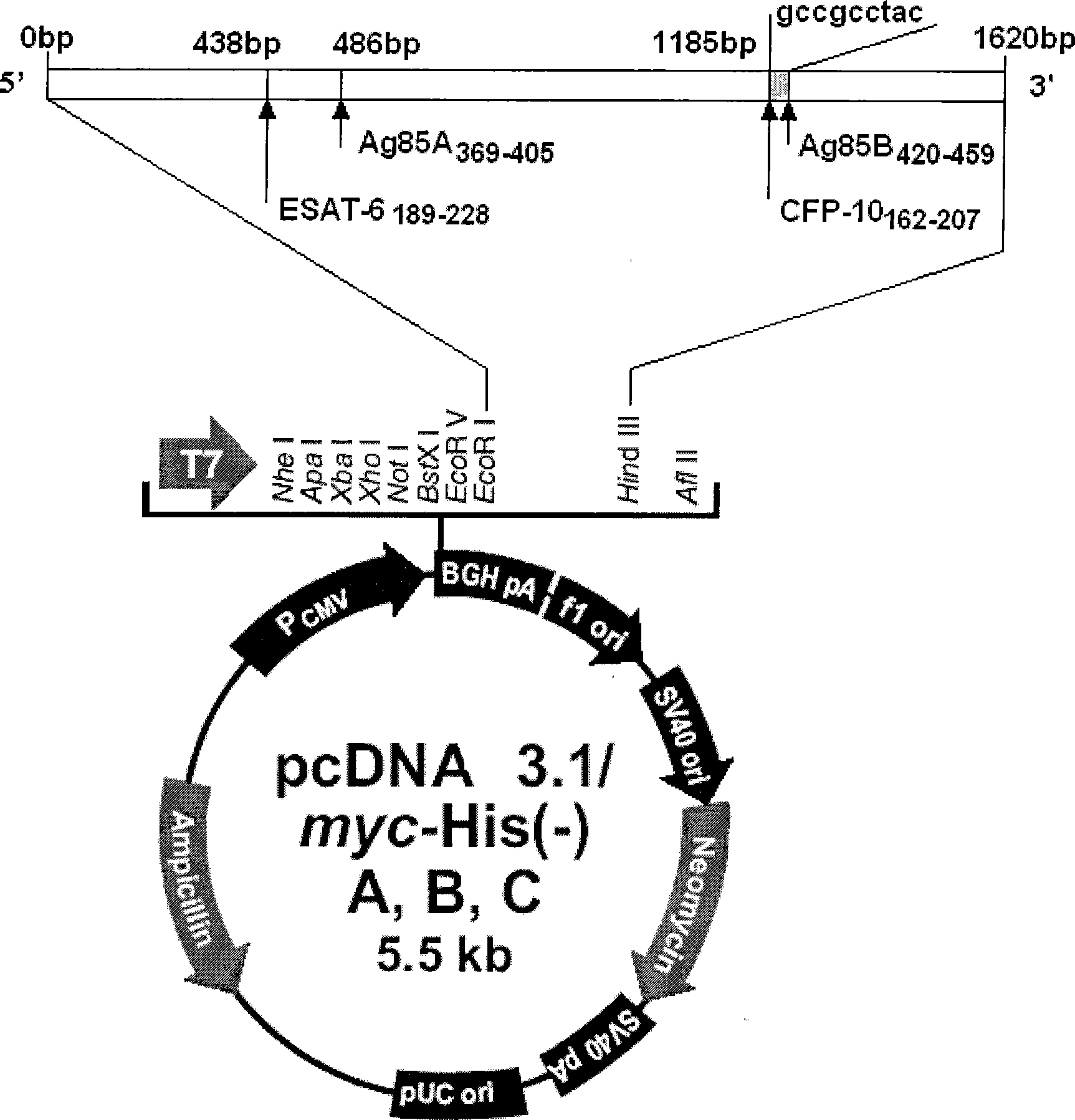
The invention discloses a tuberculosis gene vaccine based on T cell epitopes, wherein a full-length gene, embedded with four T cell epitope polypeptide genes which come from mycobacterium tuberculosis antigen, of mycobacterium tuberculosis heat shock protein is inserted into a vector. The invention also discloses a method for preparing the vaccine, which comprises the following steps: four T cell epitope genes, namely EAST-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 which come from the mycobacterium tuberculosis antigen are inserted into an HSP65 full-length gene. The invention also discloses application of an ECANS tuberculosis gene vaccine. Through the intramuscular injection of the gene vaccine into an immune mouse, the experiment proves that the vaccine can induce a specific antibody which aims at a plurality of tuberculosis antigens to response, can induce stronger tuberculosis specific killing response, can induce Th1 immune response at the same time, secrete high-level IFN gamma, and is a good vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
Injectable opioid partial agonist or opioid antagonist microparticle compositions and their use in reducing consumption of abused substances
InactiveUS20030152638A1Avoid prolonged useReadily injected intramuscularlyBiocidePowder deliveryOpioid antagonistControl manner
An injectable slow-release partial opioid agonist or opioid antagonist formulation is provided comprising a partial opioid agonist or opioid antagonist in a poly(D,L-lactide) excipient with a small amount of residual ethyl acetate. Upon intramuscular injection of the composition, a partial opioid agonist or opioid antagonist is released in a controlled manner over an extended period of time. The composition finds use in the treatment of heroin addicts and alcoholics to reduce consumption of the abused substances. Of particular interest are the drugs buprenorphine, methadone and naltrexone.
Owner:EVONIK CORP
Cyanocobalamin low viscosity aqueous formulations for intranasal delivery
ActiveUS7229636B1Prevent drynessAvoid stimulationBiocideIn-vivo radioactive preparationsIntramuscular injectionBioavailability
A stable pharmaceutical mercury-free aqueous solution of cyanocobalamin comprised of cyanocobalamin and water wherein said solution of cyanocobalamin is suitable for intranasal administration, has a viscosity less than about 1000 cPs, and wherein said solution of cyanocobalamin has a bioavailability of cyanocobalamin when administered intranasally of at least about 7% relative to an intramuscular injection of cyanocobalamin with the proviso that the solution is essentially free of mercury and mercury-containing compounds. The present invention is also directed towards a method for elevating the vitamin B12 levels in the cerebral spinal fluid (CSF) comprising administering intranasally a sufficient amount of a mercury-free cyanocobalamin solution so as to increase the average ratio of vitamin B12 in the CSF to that in the blood serum (B12 CSF / B12 Serum×100) to at least about 1.1 comprising intranasally administering an aqueous solution of a cyanocobalamin, wherein said solution of cyanocobalamin has a bioavailability of at least 7% relative to an intramuscular injection of a cyanocobalamin.
Owner:ENDO PHARMA INC
Freeze dried Lansoprazole sodium injection and its prepn process
InactiveCN1810244AAvoid destructionImprove efficacyOrganic active ingredientsPowder deliveryDiseaseFreeze-drying
The freeze dried Lansoprazole sodium sdinjection has Lansoprazole sodium as active component and mannitol or meglumine as excipient in the weight ratio of 15-30 g to 5-50 g. The preparation process of the freeze dried Lansoprazole sodium injection includes the following steps: dissolving Lansoprazole sodium in 15-30 g in injection water of 200-400 ml, dissolving mannitol or meglumine as excipient in 5-50 g in injection water of 100-300 ml, mixing the two kinds of obtained solutions and adding injection water to 2000-3000 ml, adding active carbon in 0.5-1.5 g, filtering with microporous filter membrane, packing in vial, freeze drying under bacteria-free condition, and pressing cap. The freeze dried Lansoprazole sodium injection is used clinically in intravenous drip or intramuscular injection to treat peptic ulcer and other diseases.
Owner:JINZHOU JIUTAI PHARML CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com