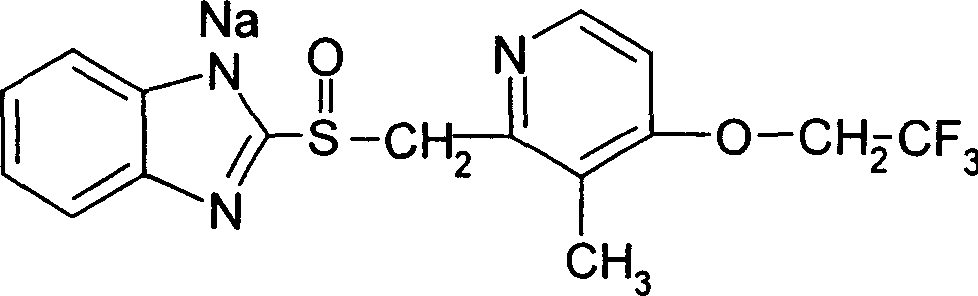
Freeze dried Lansoprazole sodium injection and its prepn process
A lansoprazole sodium and freeze-drying technology, which is applied in freeze-drying transportation, digestive system, powder transportation, etc., can solve the problems of increased pharmaceutical costs, difficult quality control, complicated process, etc., and achieve good chemical stability and easy operation , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Get lansoprazole sodium 15~30g and excipient 5~50g, described excipient is mannitol or meglumine, and lansoprazole sodium is dissolved in 200-400ml water for injection, and excipient Dissolve in 100-300ml of water for injection; combine the above two solutions, add water for injection to 2000ml-3000ml; add 0.5-1.5g of activated carbon for decolorization, filter the filtrate through a microporous membrane, fill in a vial; freeze under sterile conditions Dry, pre-freeze at -40~-45°C for 4~6 hours, then sublime for 28~36 hours, increase the temperature by 1~2°C per hour, to 35~45°C; out of the box, cover, that is, Lansoprazole Sodium lyophilized injection.
Embodiment 2
[0022] Get lansoprazole sodium 15g and excipient 50g, described excipient is mannitol or meglumine, lansoprazole sodium is dissolved in 200ml water for injection, and excipient is dissolved in 300ml water for injection ; Combine the above two solutions, add water for injection to 2000ml; add 0.5g of activated carbon for decolorization, filter the filtrate through a microporous membrane, fill in a vial; freeze-dry under sterile conditions, pre-freeze at -40~-45℃ for 4~ 6 hours, sublimated for 28 to 36 hours, and raised the temperature by 1 to 2 °C per hour to 35 to 45 °C; out of the box, capped, and the lyophilized injection of Lansoprazole Sodium was obtained.
Embodiment 3
[0024] Get lansoprazole sodium 30g and excipient 5g, described excipient is mannitol or meglumine, lansoprazole sodium is dissolved in 400ml water for injection, and excipient is dissolved in 100ml water for injection ; combine the above two solutions, add water for injection to 3000ml; add 1.5g of activated carbon for decolorization, filter the filtrate through a microporous membrane, fill in a vial; freeze-dry under sterile conditions, pre-freeze at -40~-45℃ for 4~ 6 hours, sublimated for 28 to 36 hours, and raised the temperature by 1 to 2 °C per hour to 35 to 45 °C; out of the box, capped, and the lyophilized injection of Lansoprazole Sodium was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com