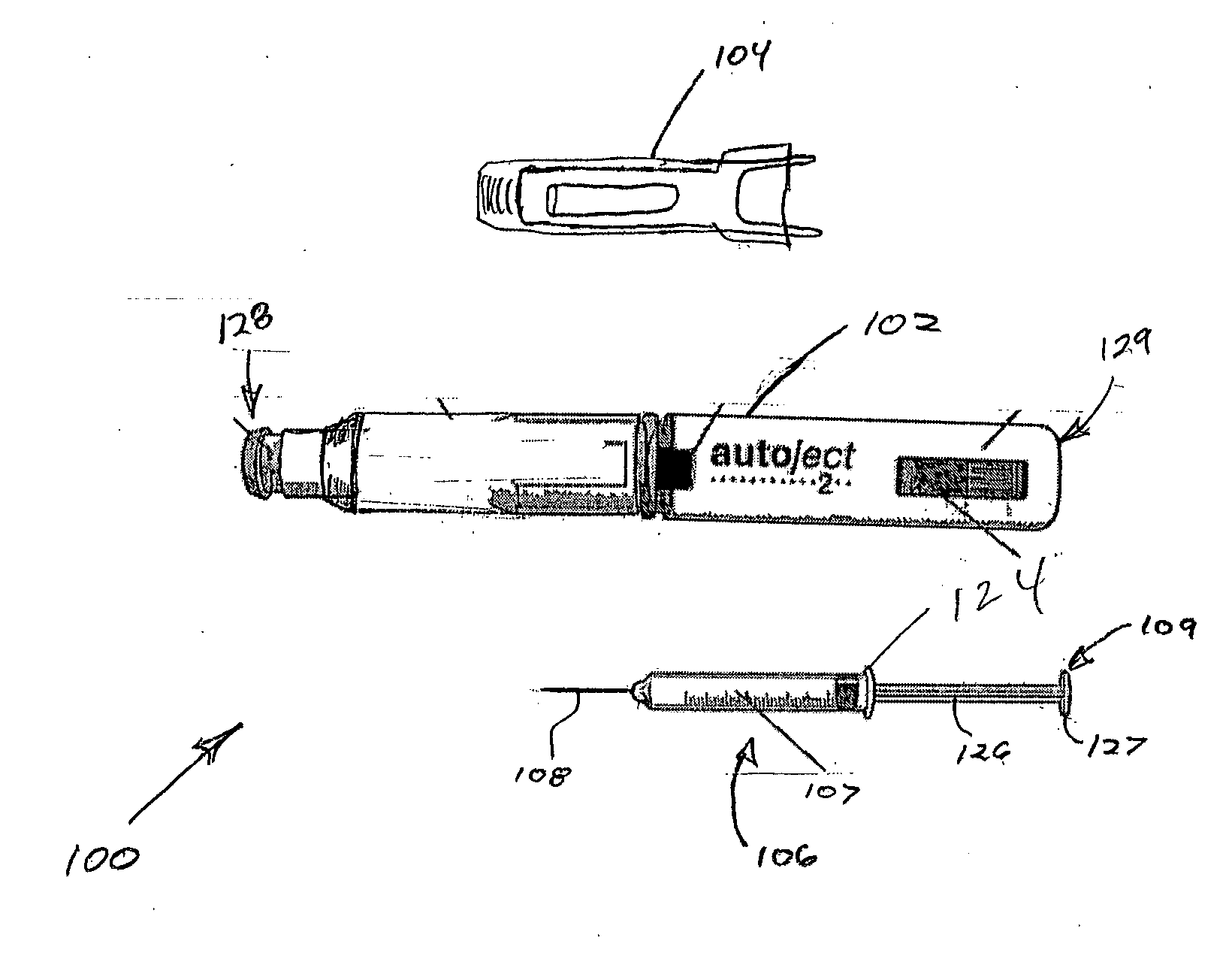
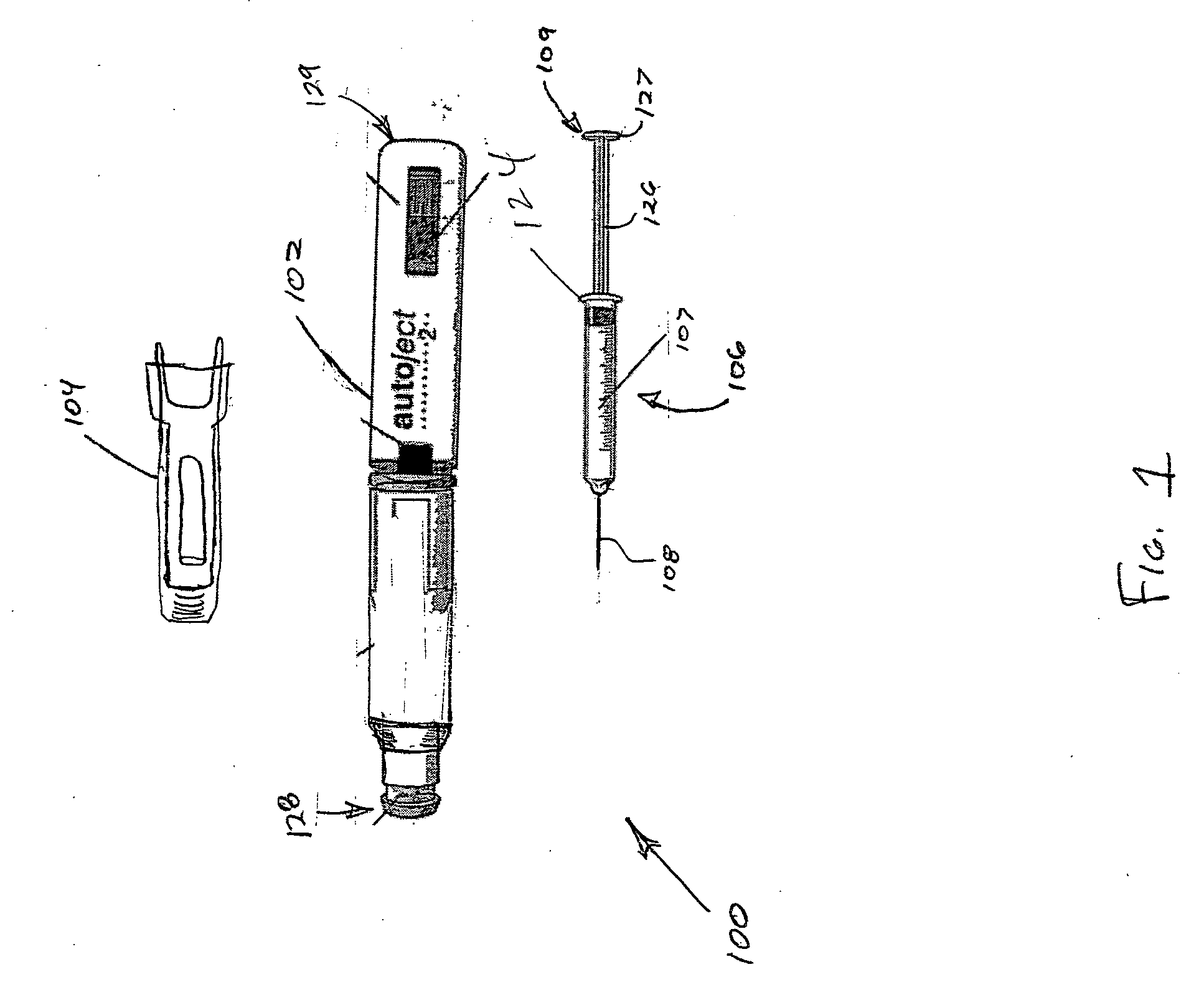
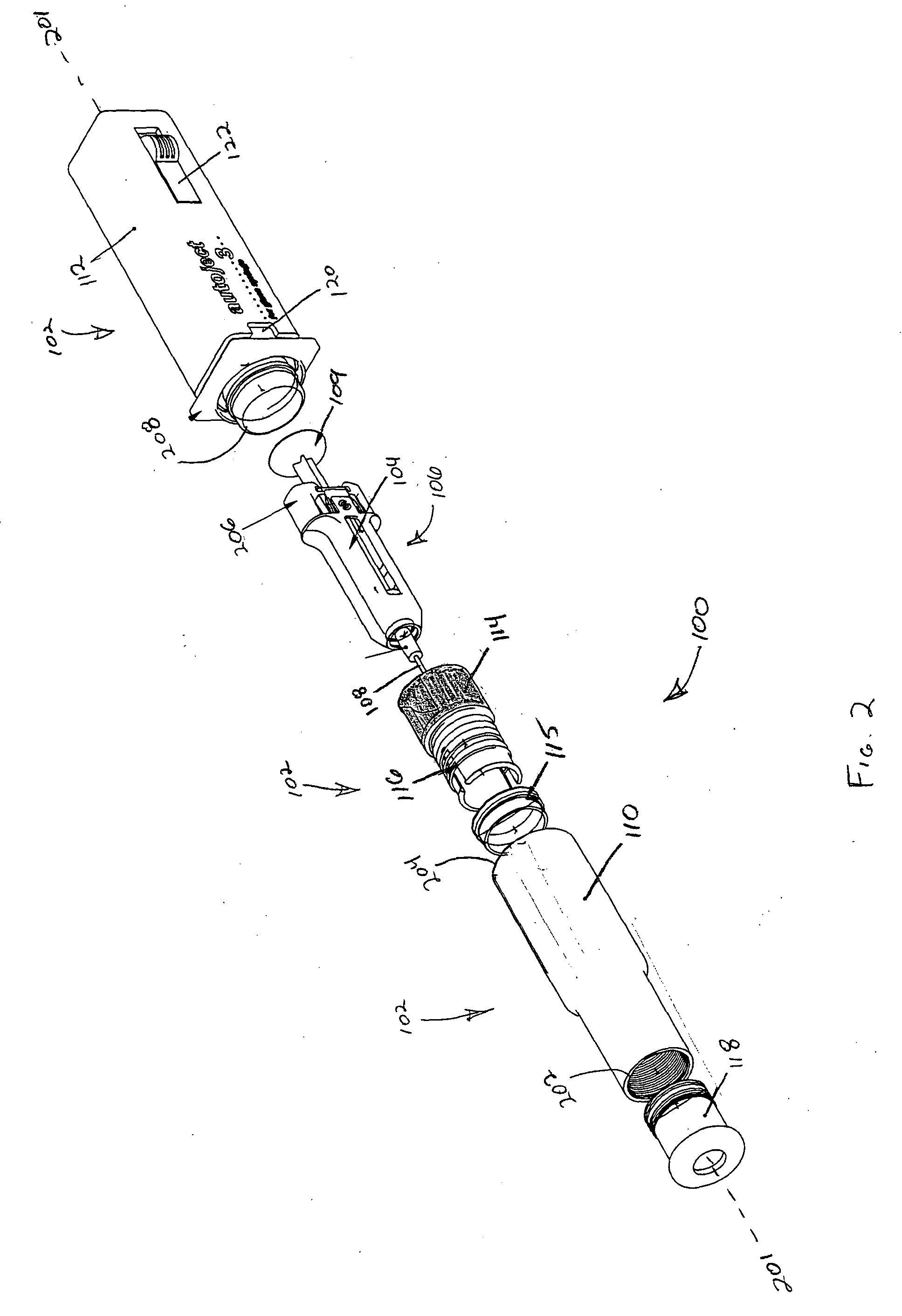
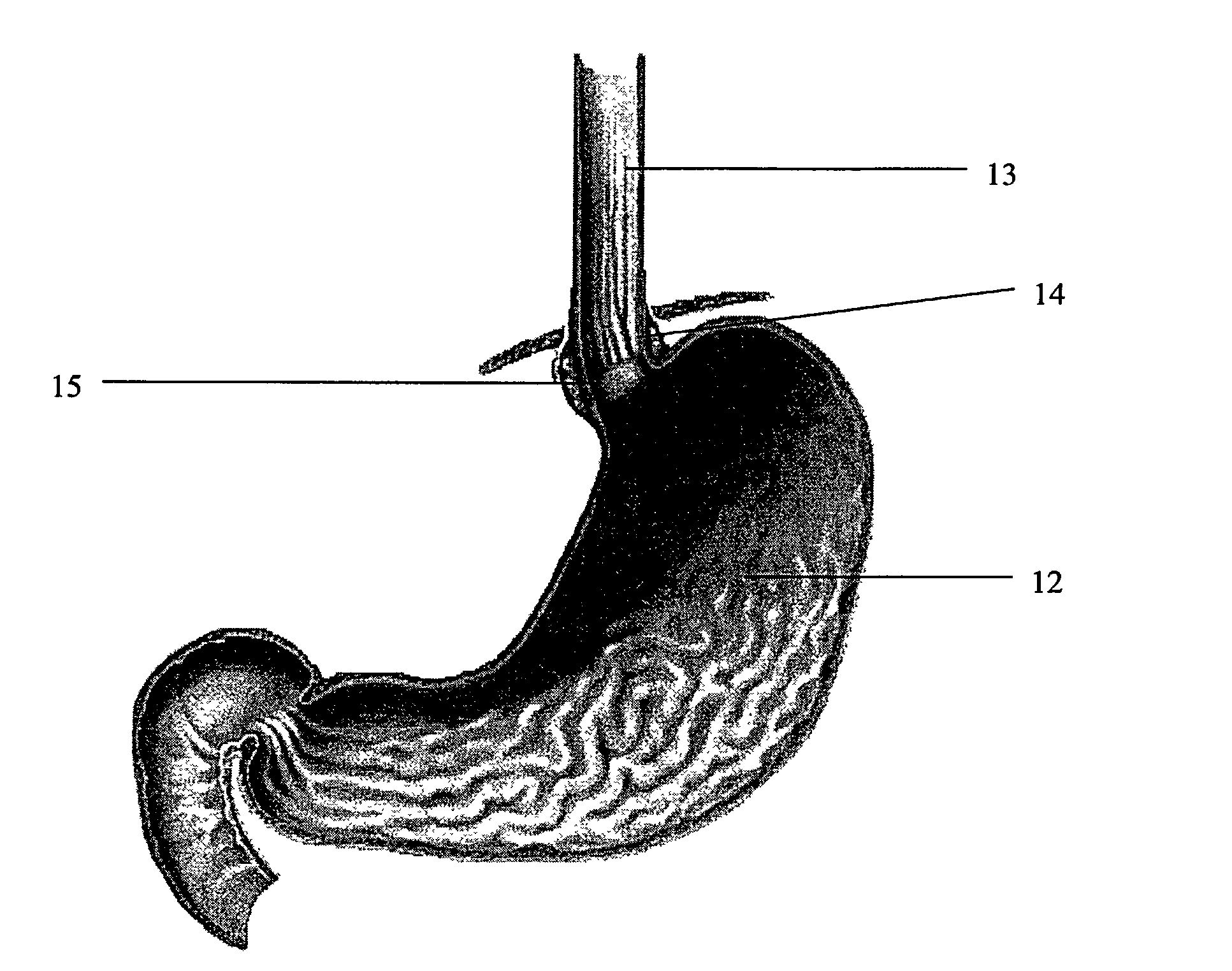
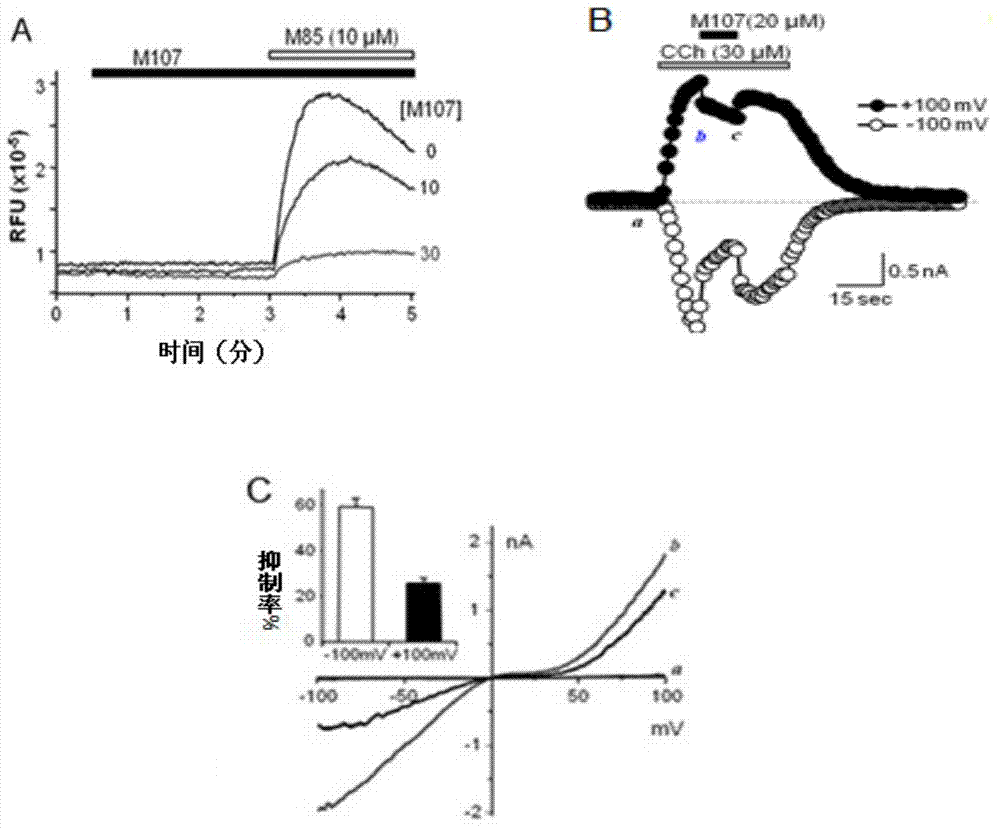
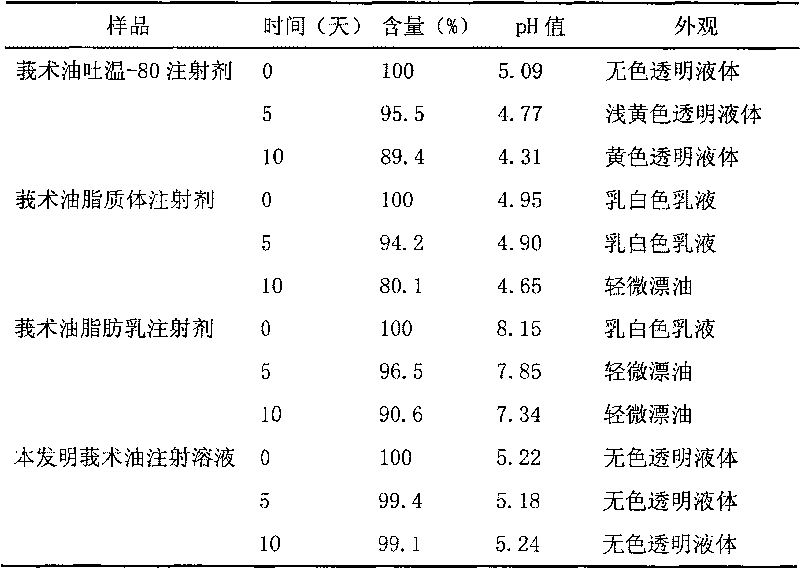
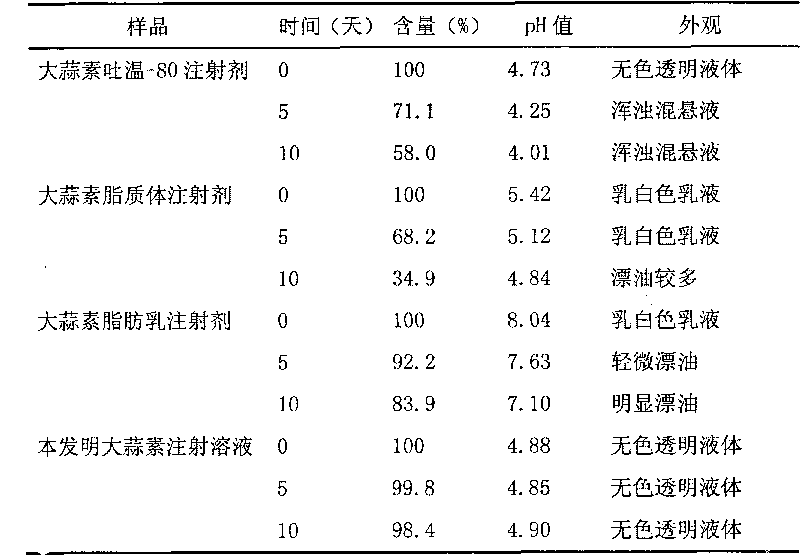
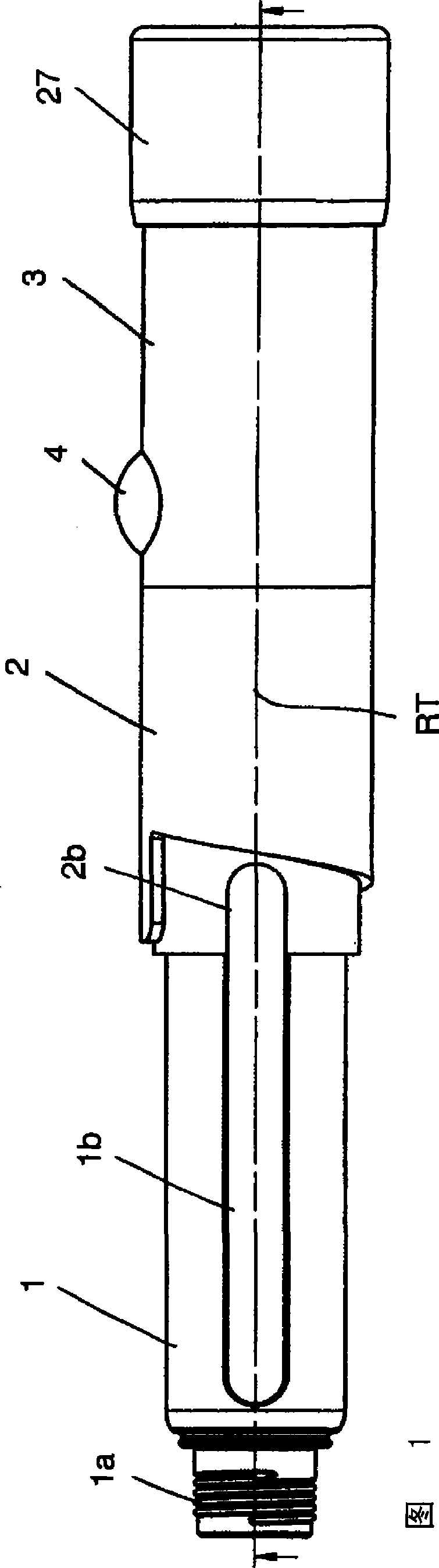
Patents
Literature
380 results about "Injectable Product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
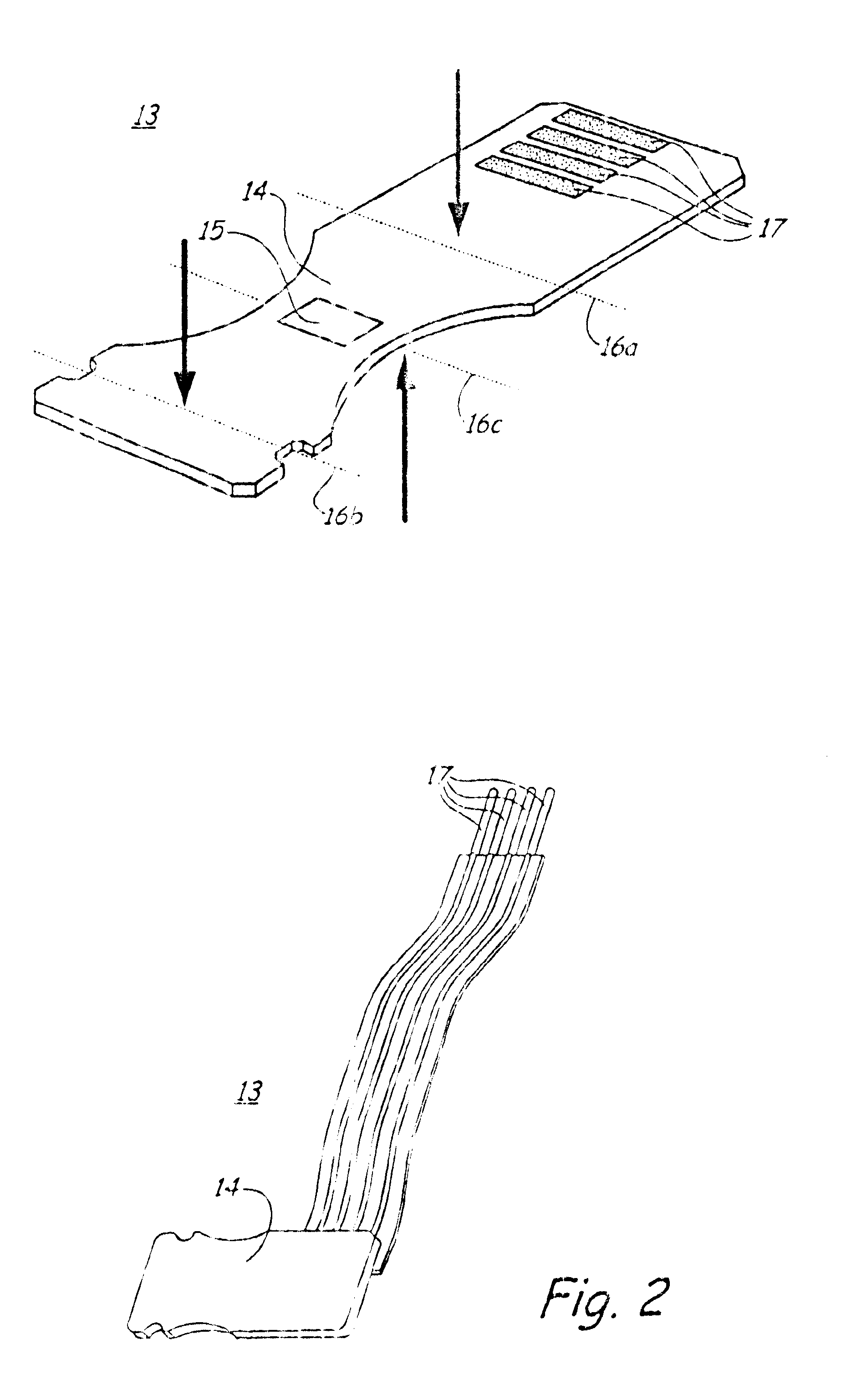
Systems and methods for automatic medical injection with safeguard
A medical injection system includes a needle guard and an automatic injection system. The needle guard has a cartridge housing coupled with a release mechanism and configured to house a medical cartridge having a needle, a plunger and a carrier configured to carry the dose. Coupled with the cartridge housing and the release mechanism is a shield that is extendable between a retracted and an extended position that substantially covers the needle. The release mechanism is engageable and configured to maintain the shield in the retracted position and allow the shield to extend upon engagement. The automatic injection system can be configured to house the needle guard and configured to inject the dose when activated. The automatic injection system has a drive system configured to depress the plunger and engage the release mechanism when the automatic injection system is activated, and an activation system configured to activate the automatic injection system.
Owner:SAFETY SYRINGES
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
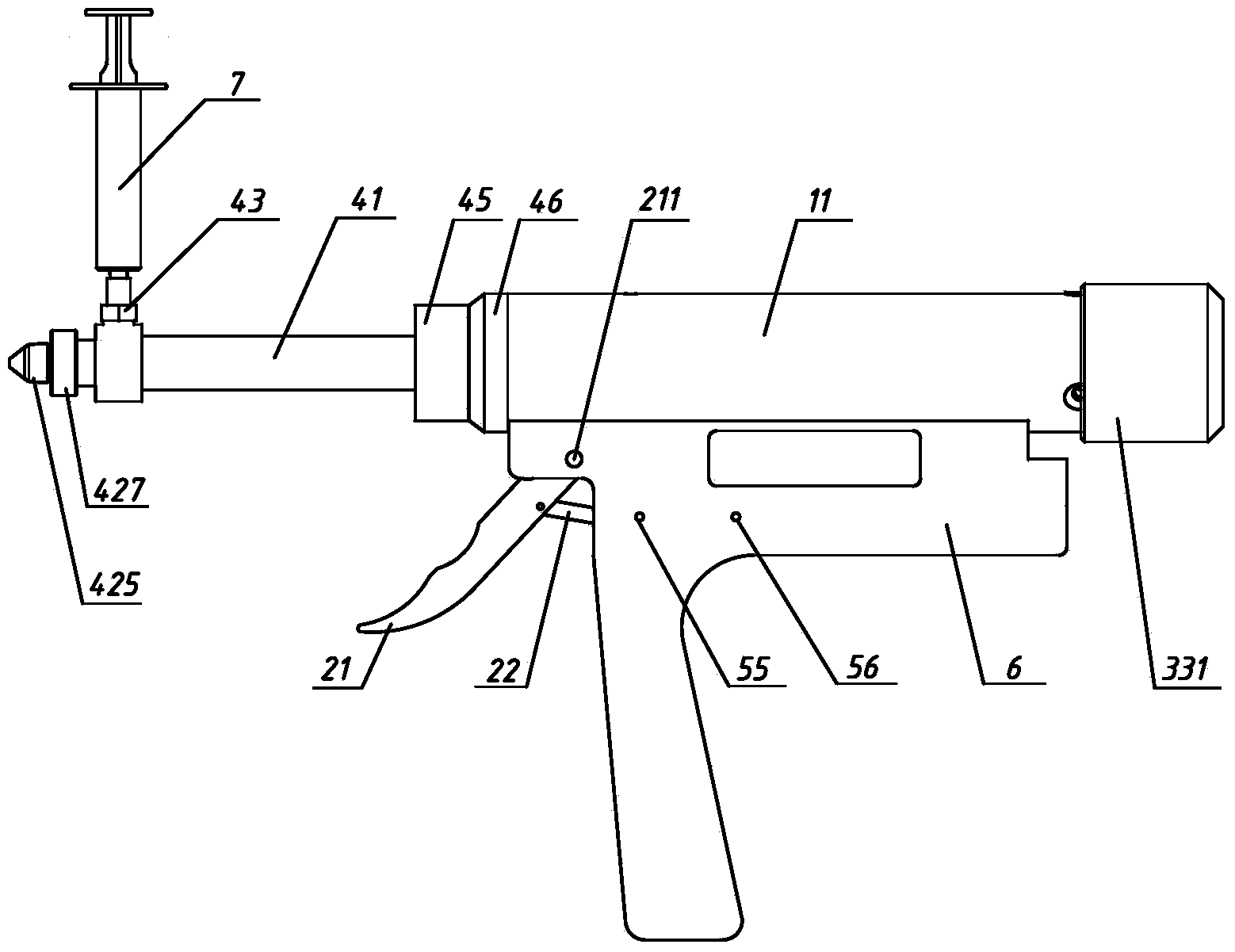
Device for administering an injectable product in doses
InactiveUS6878132B2Avoid spendingIncrease expensesAmpoule syringesOther blood circulation devicesEngineeringAlarm signal
The present invention provides a device for administering an injectable product in doses, the device including a casing, a container accommodated by the casing, a delivering appliance for delivering the product from the container, a drive for the delivering appliance, and a means for determining a malfunction of the device, wherein a vibrator motor is accommodated by the casing, the vibrator motor being triggered by the means for determining a malfunction such that it generates a vibrating alarm signal when a malfunction is determined.
Owner:ROCHE DIABETES CARE INC
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
Injection syringe including device for preparation of injection
An injection syringe including a device for preparation of an injection in situ, useful to the injection which is liable to suffer chemical changes if left for a long time in the state of solution or dispersion ready to inject. The injection syringe is portable by a patient who may prepare a necessary injection in situ with the use of a device included in the syringe which will automatically perform an injection with a prescribed dosage. The injection syringe includes a device for preparation of an injection, whereby mechanical impacts affecting the medicine and consequently, chemical changes with a medicine would be minimized during the step of dissolving the medicine. The syringe as noted is especially useful for preparation of injection and injection of human growth hormones, interferon and various polypeptides which are of an environmentally sensitive nature and liable to suffer chemical changes if left for a long time in the state of solution or dispersion.
Owner:JCR PHARMA
Aripiprazole complex formulation and method
An aripiprazole formulation is provided which includes the antipsychotic agent aripiprazole in the form of an inclusion complex in a β-cyclodextrin, preferably, sulfobutyl ether β-cyclodextrin (SBECD), which in the form of an injectable produces reversible generally minimal to mild irritation at the intramuscular injection site. A method for minimizing or reducing irritation caused by aripiprazole at an intramuscular injection site and a method for treating schizophrenia employing the above formulation are also provided.
Owner:OTSUKA PHARM CO LTD
Apparatus for subcutaneous administration of an injectable product
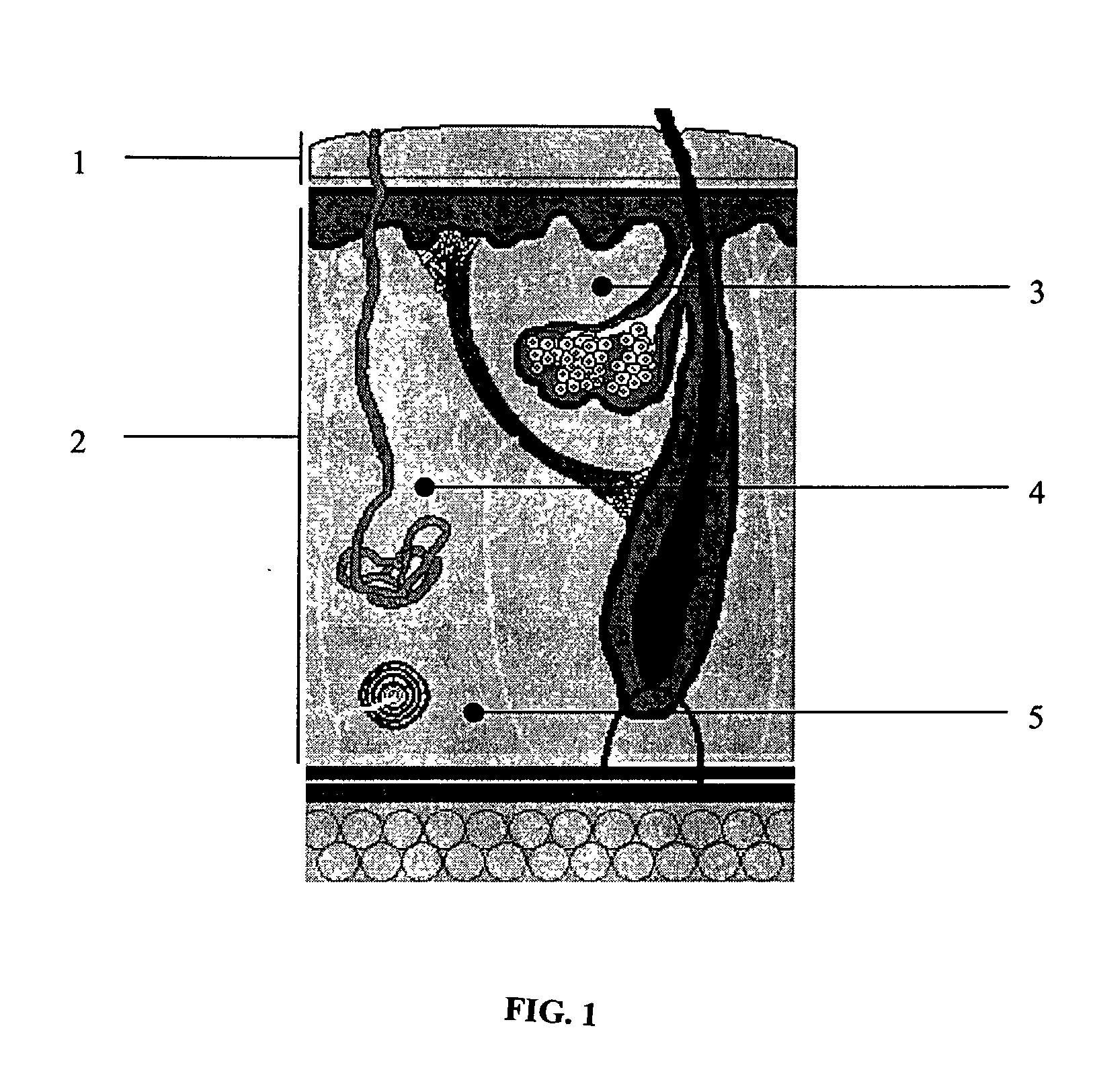
InactiveUS7074211B1Improve securityImprove user convenienceMedical devicesInfusion needlesHypodermoclysisInjectable Product
An apparatus for subcutaneous administration of an injectable product, including a housing, a container for the product to be injected, an injection needle and a needle protection sleeve surrounding the injection needle, wherein the apparatus also includes an indicator to tell a user when the needle protection sleeve has attained its maximum distal position.
Owner:TECPHARMA LICENSING AG
Novel drug delivery technology
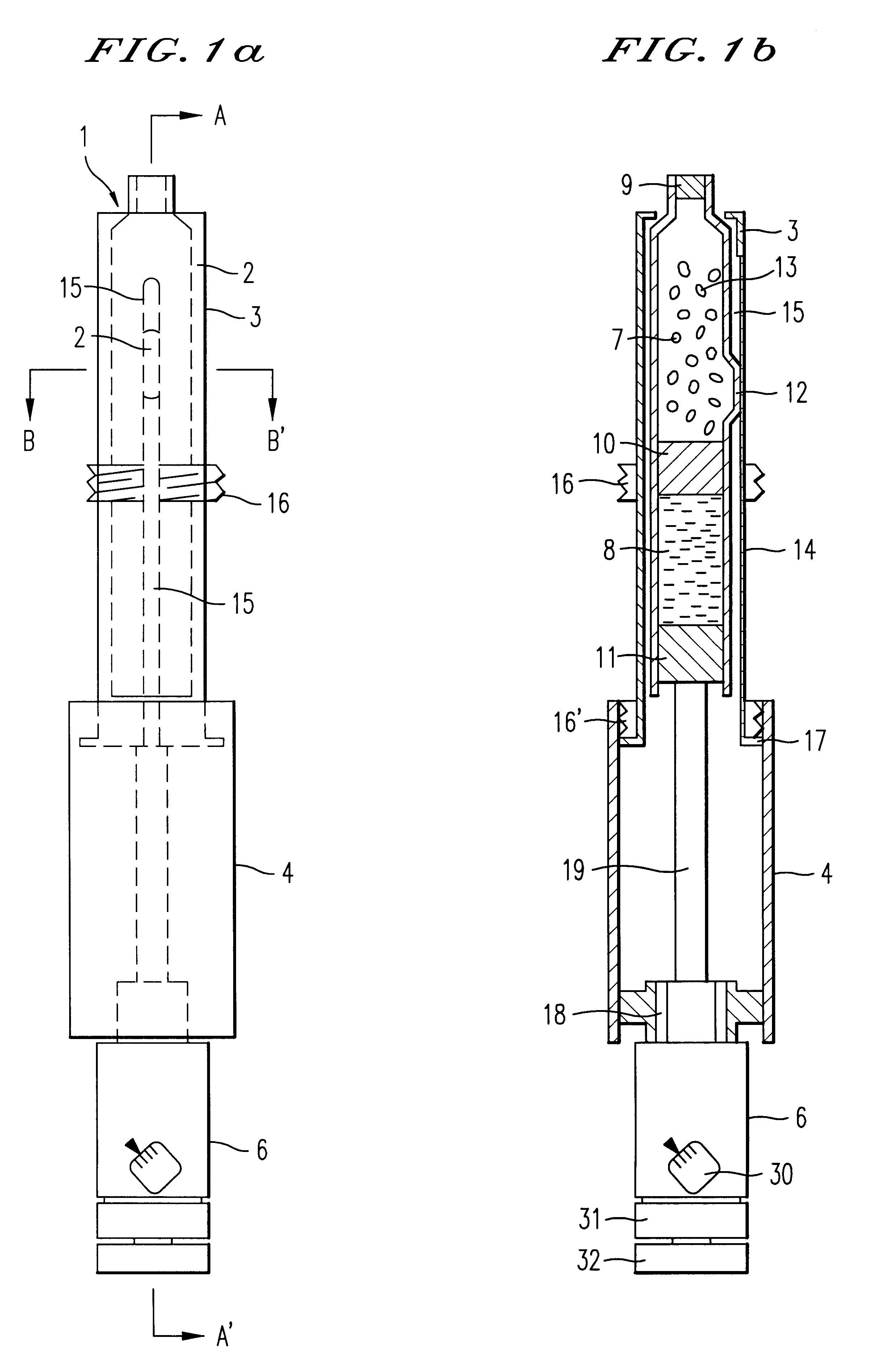
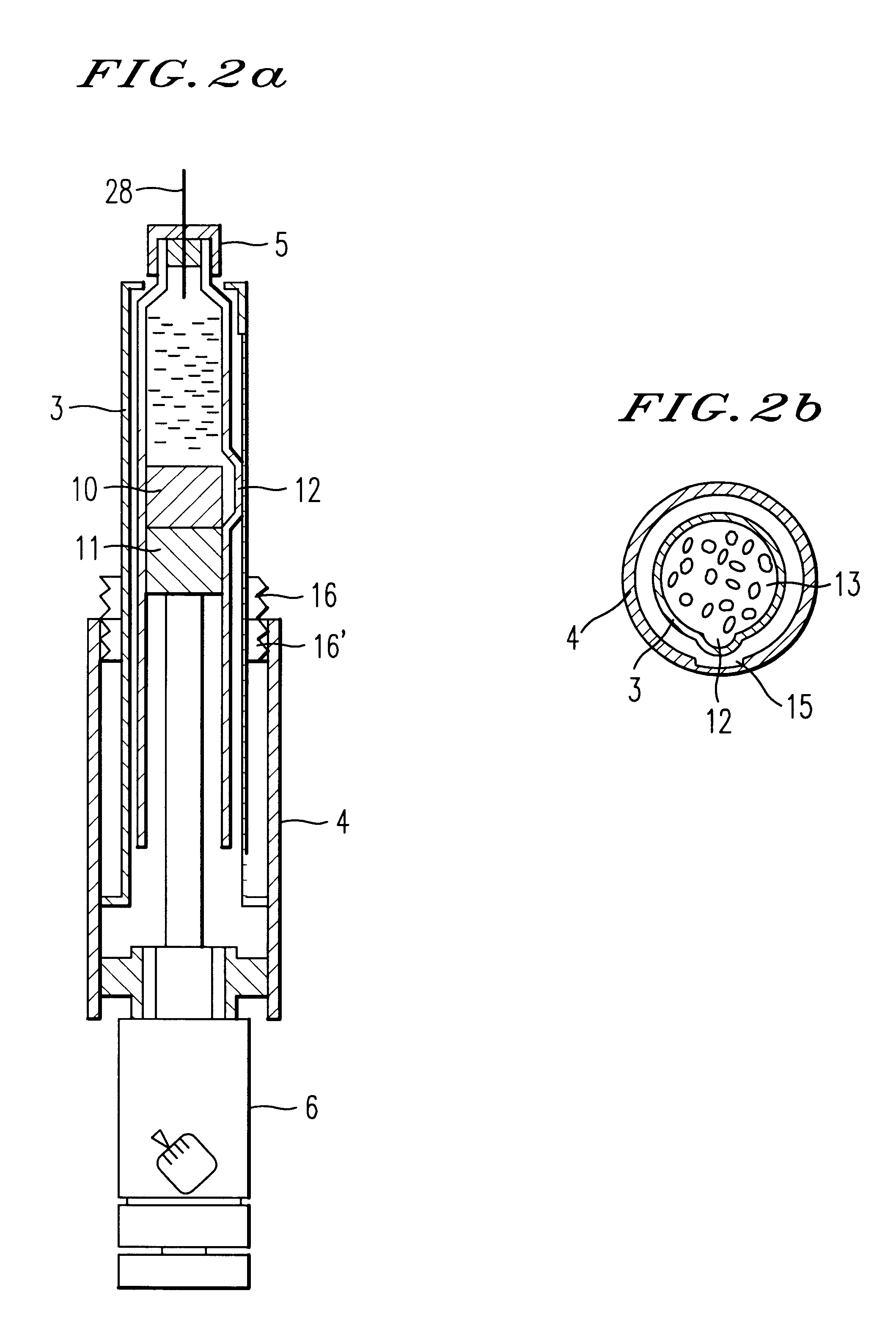
The invention relates to a novel drug delivery technology. More particularly the invention relates to a method of delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient; to a throwaway or reusable device for delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient in a manner as set out by the method; to a pioneer projectile for use in said method; to formulations for use in said method and to an injectate comprising a pioneer projectile and formulation. It also relates to a disposable component containing either a pioneer projectile or an injectate. The invention also relates to a throwaway or reusable device for delivering at least one therapeutic compound, or a formulation comprising the at least one therapeutic compound (hereafter drug) to a patient, and a method for administering a drug to a patient using said device. It also relates to a packaged drug for use with said device.
Owner:ENESI PHARM LTD
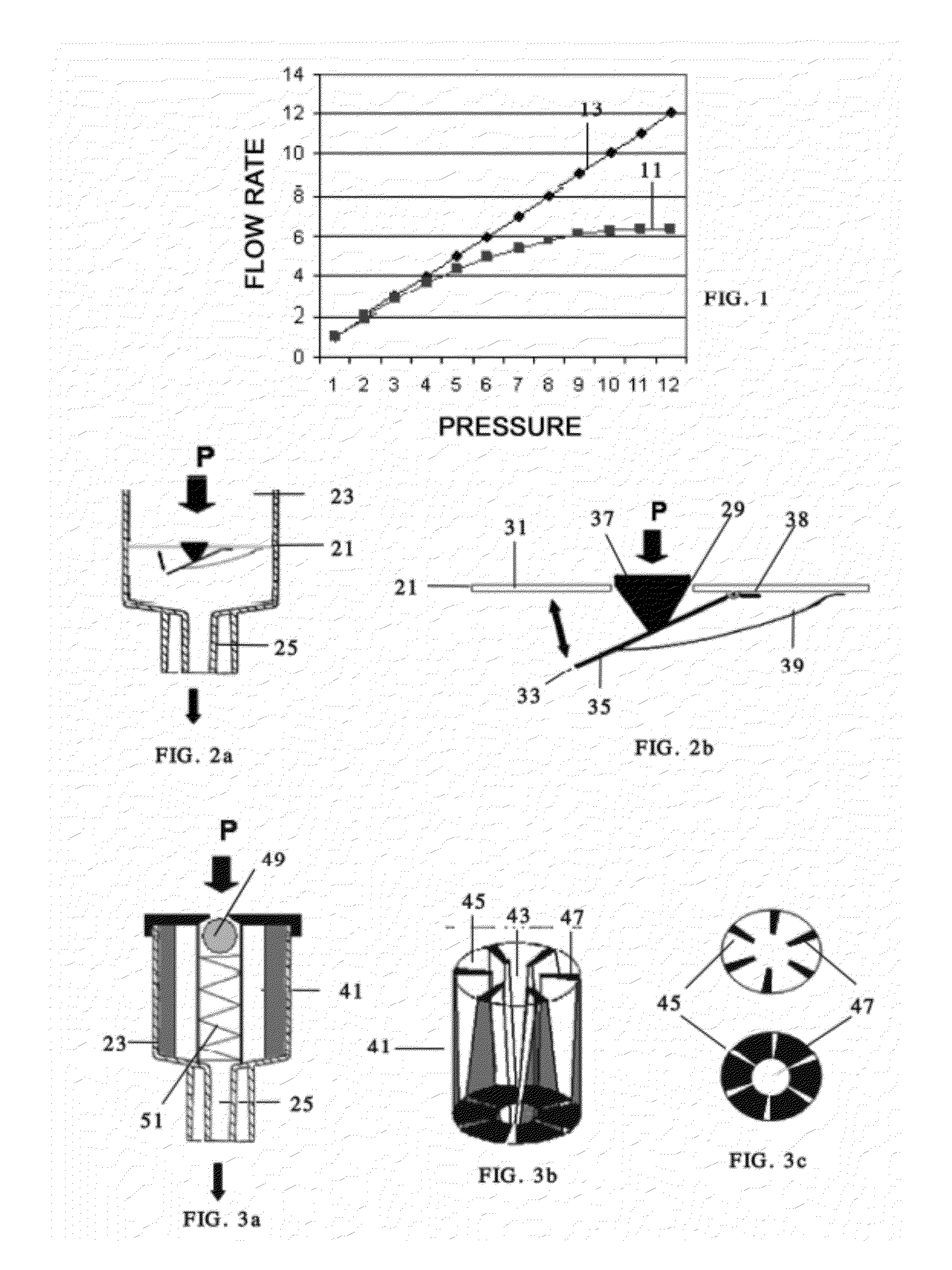
Device for the administration of injectable products with a controlled flow rate
A device is for the administration of injectable products with controlled flow rate, with a container (23) in the form of both a syringe and an ampoule preloaded with the injectable product, an injection port (25) and a control valve (21) for controlling the outlet rate and pressure arranged between the injectable products and the injection port (25). A device for reducing the outlet flow rate depends on the pressure increase applied during the administration of the injectable product, the administration being moderated by closing the valve (21) when the pressure exceeds a predetermined limit. A device prevents the backflow of fluids during the administration of the injectable product. The valve (21) can be positioned both in the container (23) and at an injection end (27) including the injection port (25) which is coupled to the container (23).
Owner:INNOVA SALUD DESARROLLOS SANITARIOS
Automated visual inspection system for the detection of microbial growth in solutions
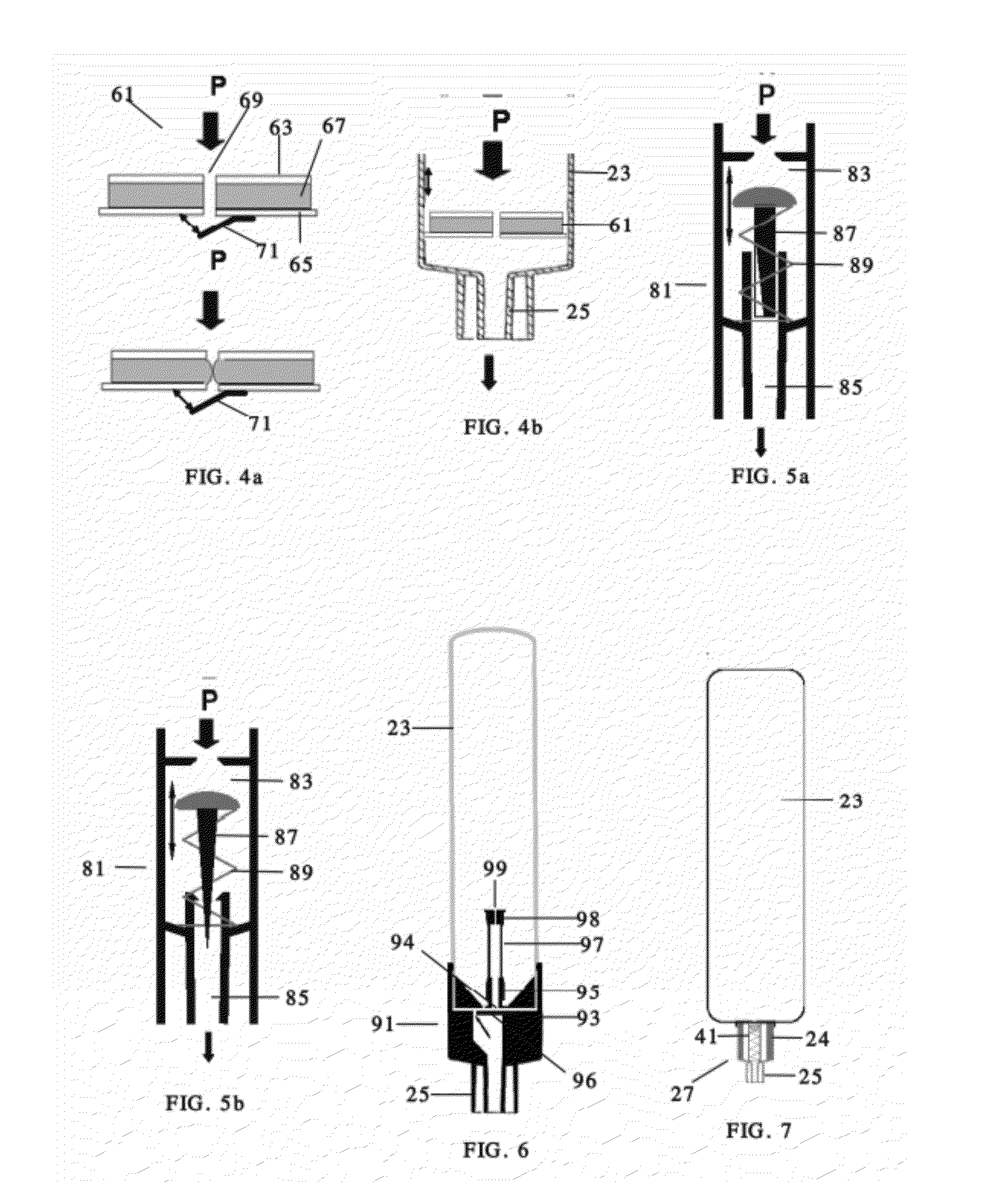
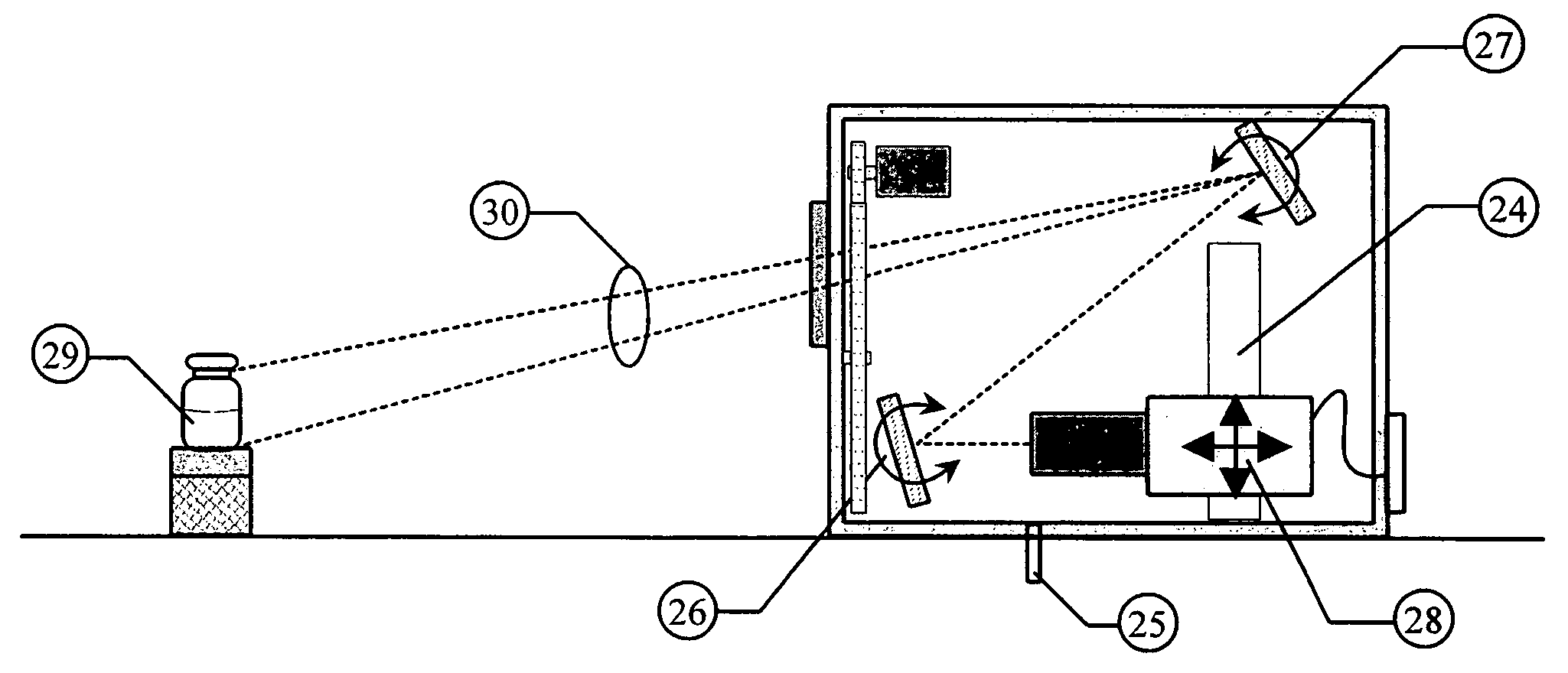
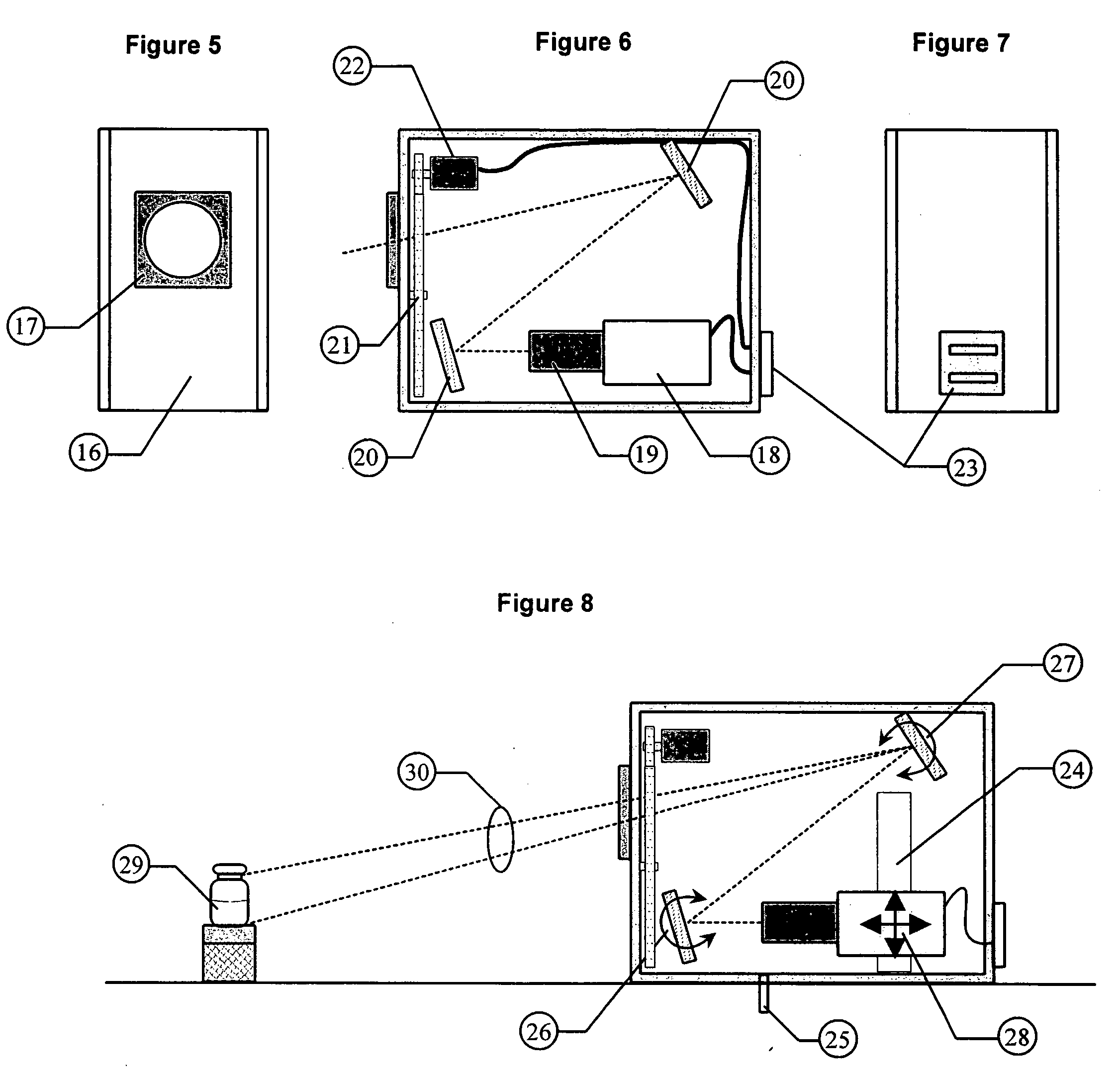
ActiveUS20060072111A1Easy to adjustLess optical densityOptically investigating flaws/contaminationParticle size analysisNon destructiveVisual inspection
Essential prerequisites for any injectable product are its sterility, its freedom from pathogens and its freedom from visible particle contamination . . . . These requirements must be satisfied prior to the release of an injectable product batch for sale and use. A major difficulty in responding to these assay requirements is the need for a size sensitivity difference of 100 or greater in determining the presence of viable pathogenic organisms and of non-viable random particle contaminants. The wide dynamic testing range cannot be satisfied in current art with a single non-destructive testing station. The present invention uses a special agitation procedure to generate separate liquid volumes containing the small viable and larger non-viable particle contaminants. This separation makes possible the introduction of sensing systems that have been optimized for each size range and that can operate in parallel without interference.
Owner:BUDD GERALD WALTER +1
Solid or semi-solid therapeutic formulations
InactiveUS20050064008A1High retention rateImprove delivery efficiencyBiocideGenetic material ingredientsEngineeringInternal medicine
An injectable or insertable dosage form comprising a biodisintegrable binder and an ablation agent in a concentration effective to cause tissue necrosis. The injectable dosage form is a solid or semi-solid dosage form. Due to the solid or semi-solid nature of the dosage form, retention at the site of injection or insertion is improved, thereby improving delivery efficiency of the ablation agents within the dosage form and / or reducing the nonspecific tissue damage associated with the dosage form.
Owner:BOSTON SCI SCIMED INC
Suspension of hyaluronic acid or salt thereof containing macromolecule hydrogel for injection and preparation method thereof
The invention relates to hyaluronic acid which contains giant molecule hydrogel and is applied to injection or turbid liquor of salt thereof and a preparation method thereof; the turbid liquor of the invention is characterized in that hyaluronic acid or isosmotic solution of salt thereof serves as a carrier; water-insoluble macromolecular compound aqueous gel particle fully swelling in isosmotic solution is added. In the invention, the preparation of the turbid liquor has the steps as follows: preparing cross linking macromolecule compound or polymer particle so as to facilitate the compound or polymer particle to fully swelling in the isosmotic solution to form gel particles, then being mixed with hyaluronic acid or salting liquid dissolved in the isosmotic solution. The hyaluronic acid containing giant molecule hydrogel or the turbid liquor of salt thereof are applied to preparing injection for beauty care or medical treatment, which has the characteristics of easy injection, long-term local effect, good plasticity and fine biocompatibility; in local injection part, the invention is used as an isolating and lubricating pad which enables surrounding tissues to be fully repaired; the characteristic is even more pronounced in treating arthritis by bone joint cavity injection.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Method and apparatus for adjusting the contents of a needle-less injector
InactiveUS7018356B2Lower the volumeJet injection syringesMedical devicesNeedle Free InjectionEngineering
A method and apparatus for adjusting the contents of a needle-less injector that contains an injectable product are described. A needle-less injector includes an adjustment switch in mechanical contact with the driver of a needle-less injector. A user may displace the adjustment switch to expel air or gas contained in the product section of the needle-less injector prior to administration of a needle-less injection with the same. The adjustment switch may alternatively or additionally be used to expel at least a portion of the injectable product contained in the needle-less injector to reduce the volume of the product to be injected with the needle-less injector.
Owner:WISE ROGER R +1
Epinephrine dosing regimens comprising buccal, lingual or sublingual and injectable dosage forms
The present invention relates to methods of administering a series of epinephrine doses for the treatment of allergic emergencies, including anaphylaxis, comprising buccal, lingual or sublingual epinephrine dosage forms and injectable epinephrine dosage forms. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
Device for administering an injectable product in doses
InactiveUS6837876B2Improve securitySimple and manual dosingAutomatic syringesMedical devicesPath lengthPiston
A device for administering an injectable product in doses, wherein the device includes a casing, a reservoir for the product, a piston, an advancing element which moves the piston a selected path length, and a dosing mechanism including a number of dosing bodies and a dosing element operable to move the dosing bodies, wherein the path length is selected by the dosing element moving at least one dosing body between the piston and the advancing element.
Owner:TECPHARMA LICENSING AG
Pyrazolopyrimidine compound and pharmaceutical composition thereof as well as pharmaceutical application of pyrazolopyrimidine compound
The invention provides a pyrazolopyrimidine compound shown as a structural formula (I), a pharmaceutical composition taking the pyrazolopyrimidine compound as an active component, a preparation method of the pyrazolopyrimidine compound and the pharmaceutical composition as well as an application of the pyrazolopyrimidine compound and the pharmaceutical composition in preparation of a TRPC6 (Transient Receptor Potential Channel 6) adjustor probe medicine and related medicines for preventing and treating glomerulopathy and myocardial hypertrophy. The pyrazolopyrimidine compound and derivatives provided by the invention can be used to prepare medical preparations in various forms which comprise oral liquids, injections, pulmonary inhalation preparations and transdermal preparations, specifically injections, oral liquids, troches, capsules, granules, aerosols, dry powder inhalation, patches and the like.
Owner:泸州天演生物医药科技有限公司
Chinese medicinal essential oil injection solution, injection and preparation method thereof
InactiveCN101708314AFix stability issuesSimple preparation processAntibacterial agentsSulfur/selenium/tellurium active ingredientsEmulsionSolvent
The invention relates to the technical field of medicaments, and discloses a Chinese medicinal essential oil injection solution, an injection thereof and a preparation method thereof. The invention provides a stable and safe Chinese medicinal essential oil injection without Tween-80, which consists of the Chinese medicinal essential oil injection solution and a dispersion medium, wherein the Chinese medicinal essential oil injection solution consists of a Chinese medicinal essential oil, a stabilizing agent, a pH value regulator and a solvent for injection; and the dispersion medium is an emulsion for injection. When in clinical medication, the Chinese medicinal essential oil injection solution is dispersed in the emulsion for the injection and is prepared into the Chinese medicinal essential oil injection, and then the intravenously administrable can be performed. In the invention, the Chinese medicinal essential oil injection solution and the emulsion for the injection are packaged and stored respectively so that the stability of a medicament stored for a long time can be improved. The invention provides a harmfulless, safe and conveniently-stored intravenous injection for the Chinese medicinal essential oil.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
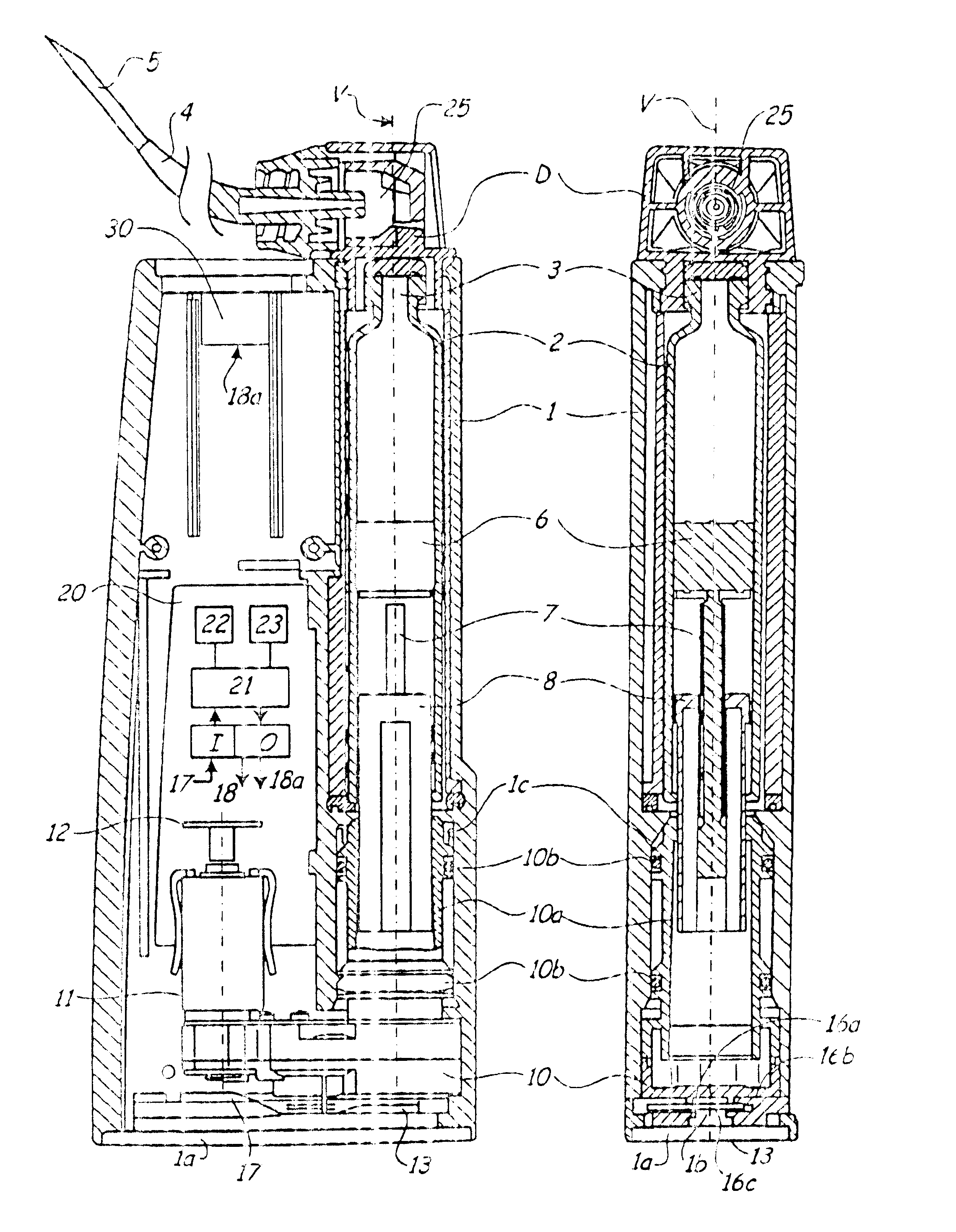
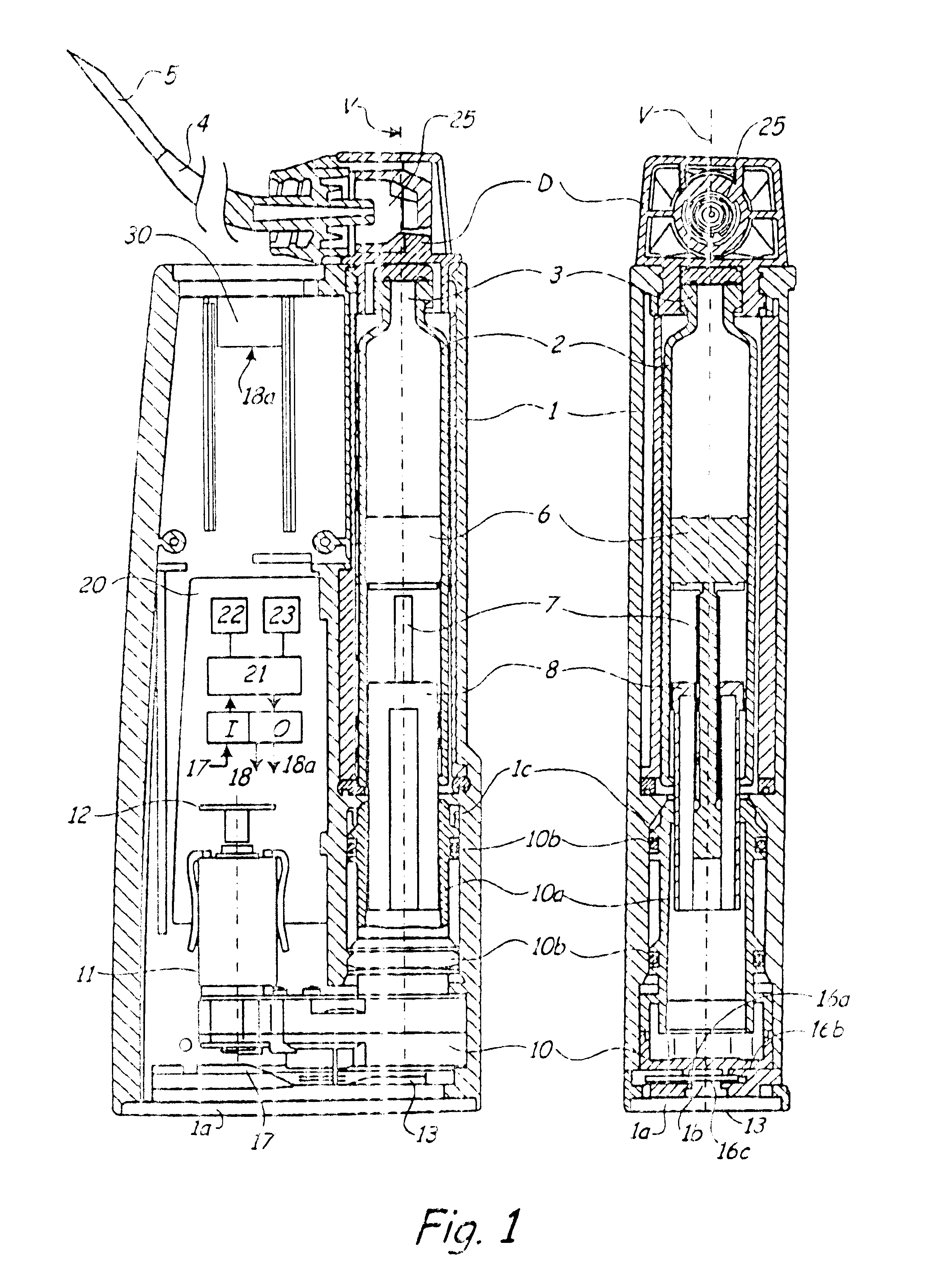
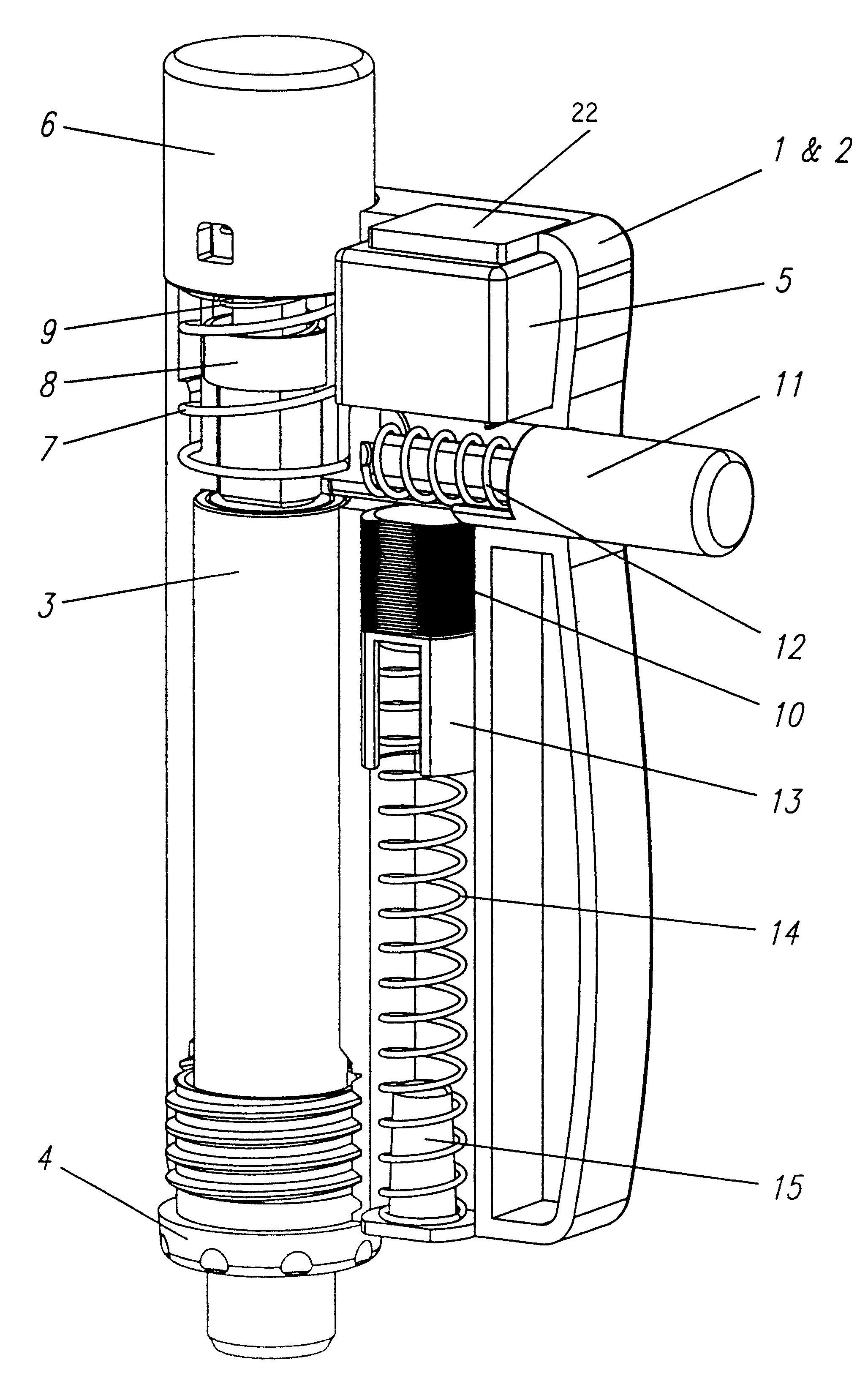
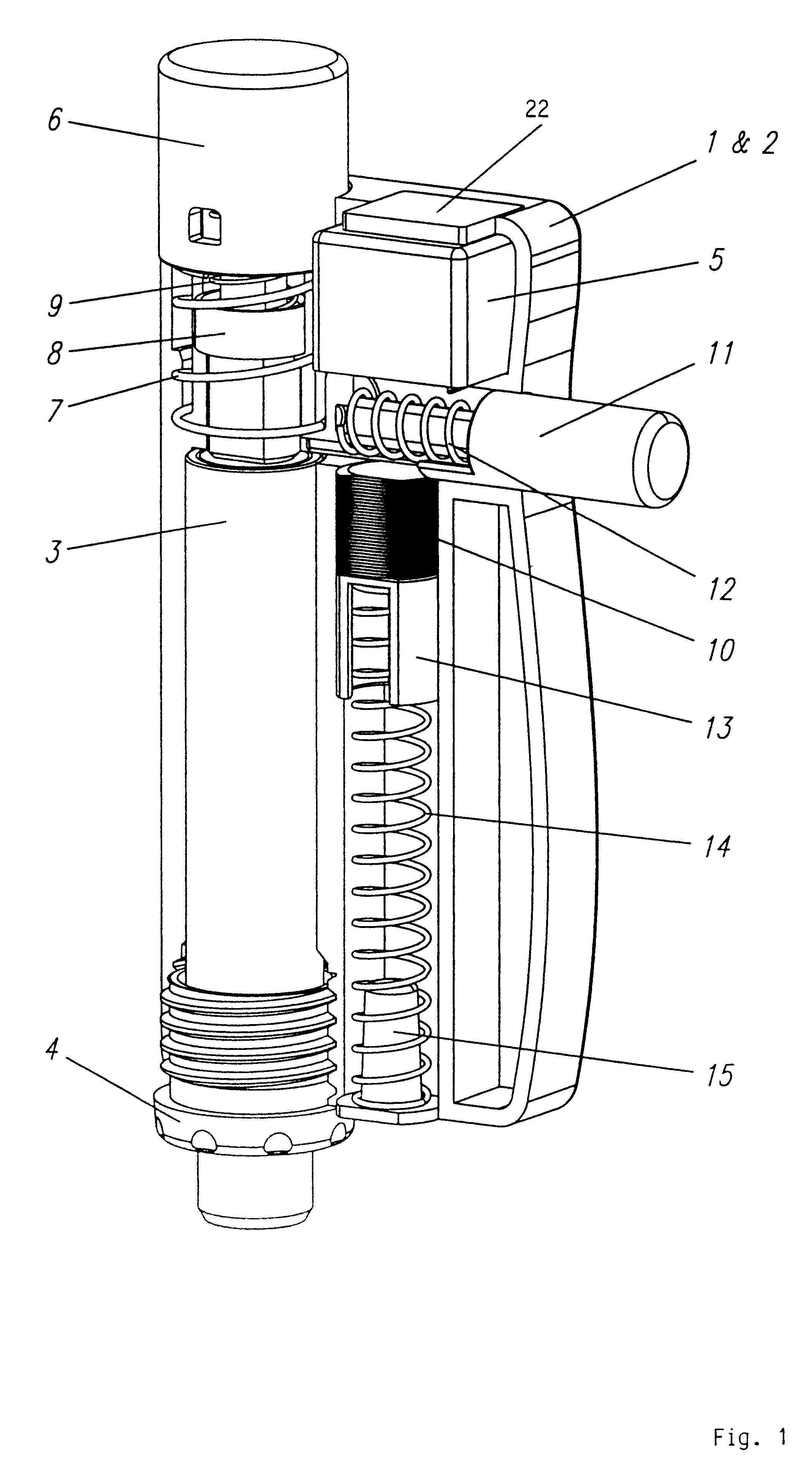
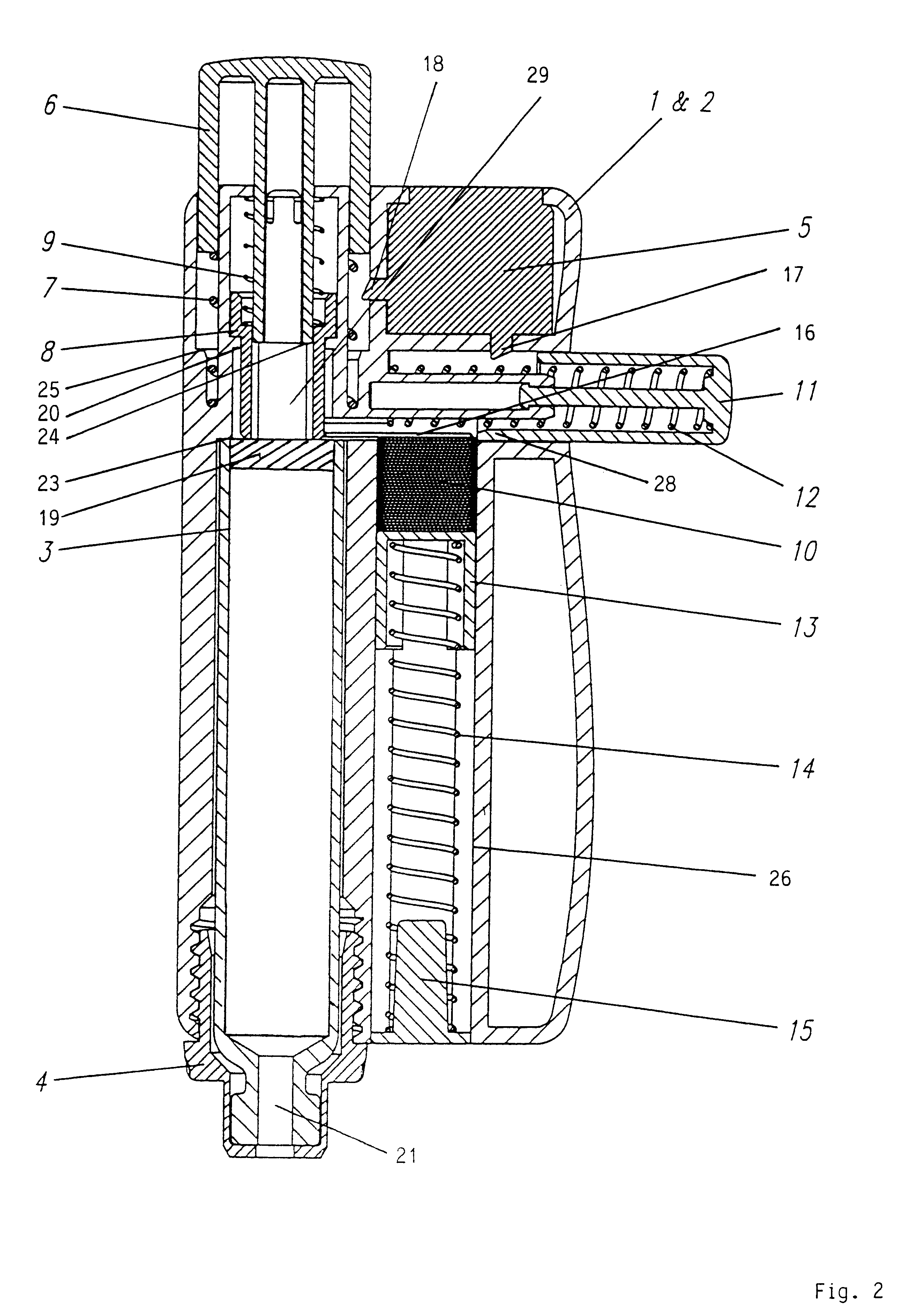
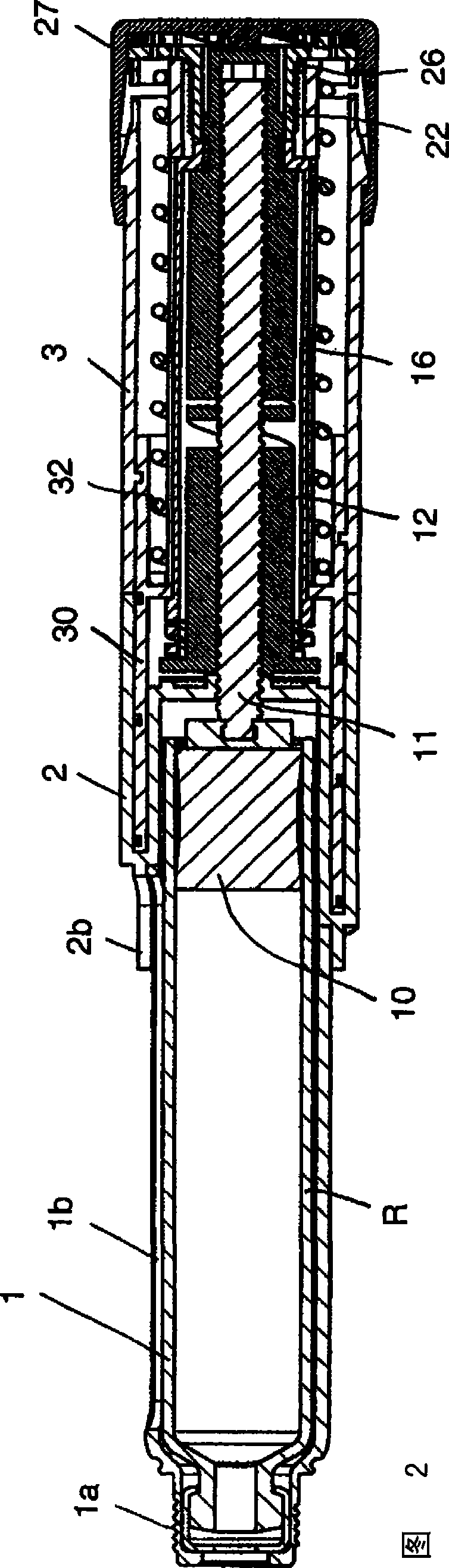
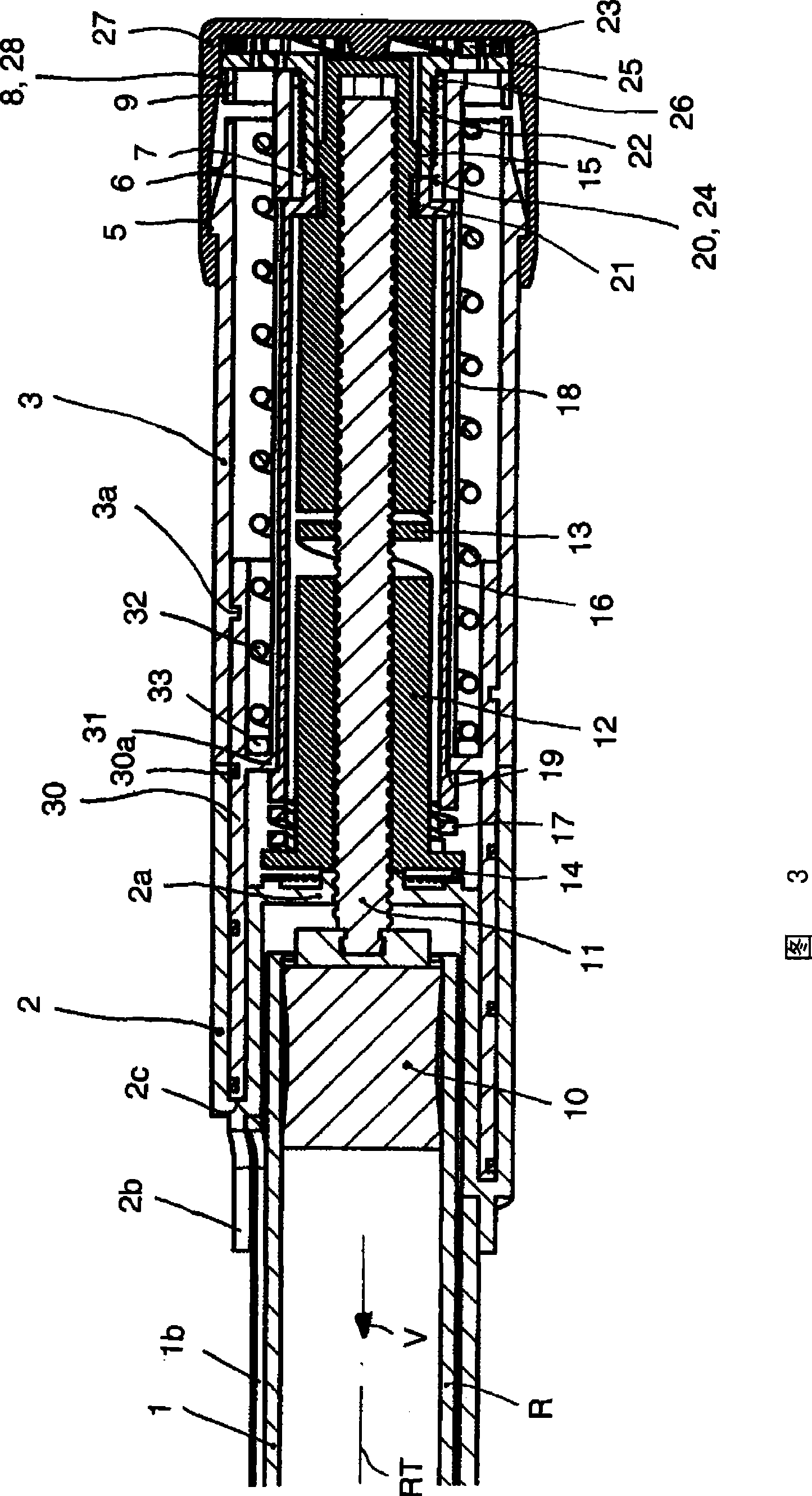
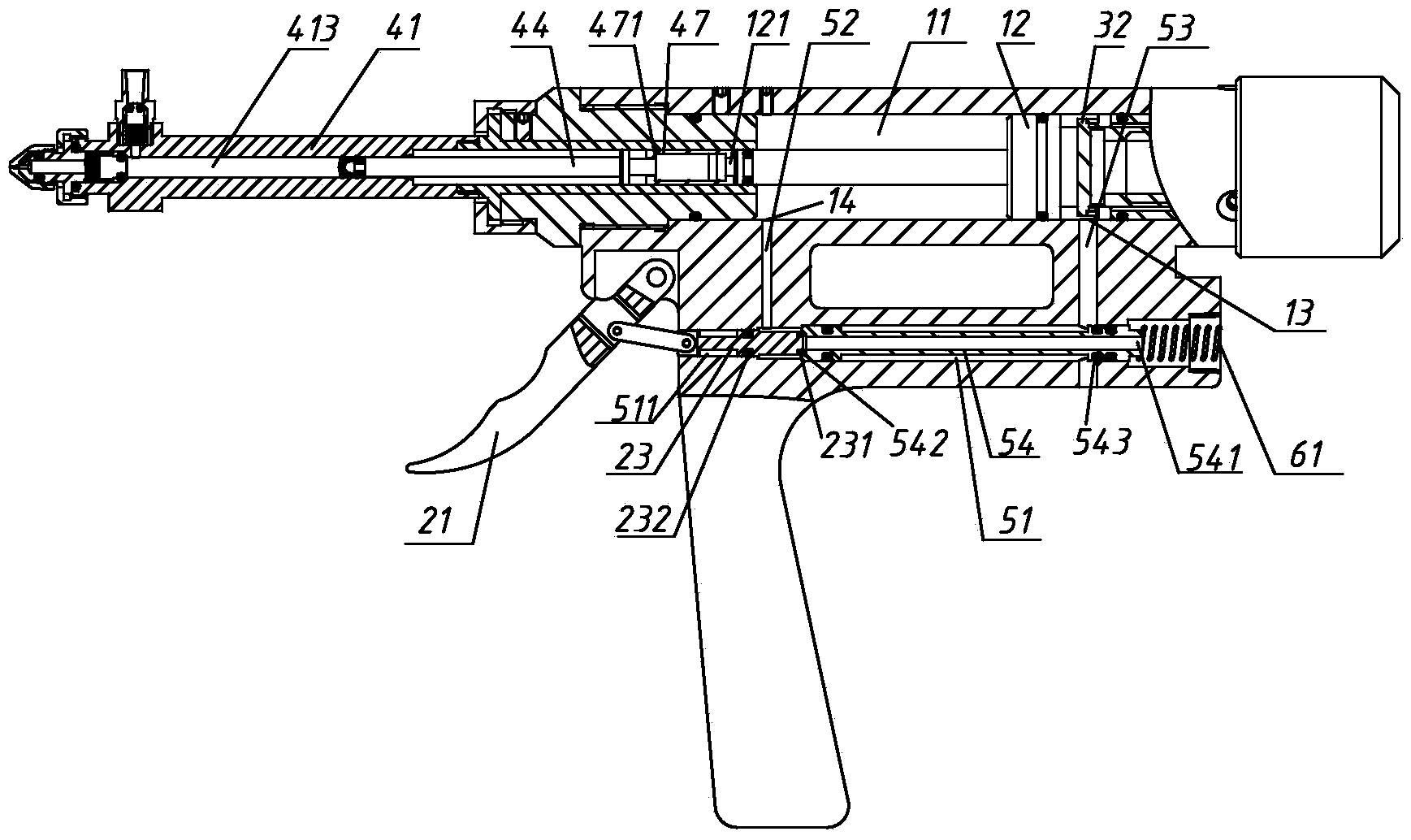
Injection device with axially overlapping dosing or display member
An injection device comprises: a) a housing (1-3) with a reservoir (R) for an injectable product, b) a plunger (10) which is axially movable in a direction of propulsion (V) in the reservoir (R), c) a dosing and propulsion means provided for the plunger (10) and arranged in an axial continuation of the reservoir (R), with d) a dosing or display member (30) which is moved in one axial direction relative to the plunger (10) when setting a product dose and is moved in the opposite direction when the dose is dispensed, e) and optionally a force-imparting member (32) which forces the dosing or display member (30) in the direction of an axial end position, f) wherein at least one out of the dosing or display member (30) and optional force-imparting member (32) axially overlaps the reservoir (R), at least with the dosing or display member (30) in the end position.
Owner:TECPHARMA LICENSING AG
Method and apparatus for adjusting the contents of a needle-less injector
InactiveUS20040087896A1Lower the volumeMaintaining the sterility of the adjustment slotJet injection syringesMedical devicesNeedle Free InjectionEngineering
A method and apparatus for adjusting the contents of a needle-less injector that contains an injectable product are described. A needle-less injector includes an adjustment switch in mechanical contact with the driver of a needle-less injector. A user may displace the adjustment switch to expel air or gas contained in the product section of the needle-less injector prior to administration of a needle-less injection with the same. The adjustment switch may alternatively or additionally be used to expel at least a portion of the injectable product contained in the needle-less injector to reduce the volume of the product to be injected with the needle-less injector.
Owner:WISE ROGER R +1
Calcium magnesium injectable bone cement and preparation method and application thereof
The invention discloses a product used for forming calcium magnesium injectable bone cement, comprising solid phase powder obtained by uniformly mixing composite phosphoric acid calcium salt and magnesium phosphate powder as well as curing solution obtained by dissolving dextrin and hydrophosphate in water. The invention also discloses a calcium magnesium injectable bone cement and preparation method and application thereof. The curing solution and solid phase powder in the product are uniformly mixed and blended into paste, thus obtaining water phase high efficiency scattering-resistant rapid curing bone cement capable of being injected with calcium magnesium and injectable product used for preparing bone tissue wound repairing. The invention is low in cost of raw materials, preparation method is simple, the obtained calcium magnesium injectable bone cement has strong scattering-resistance and rapid curing speed.
Owner:EAST CHINA UNIV OF SCI & TECH
Pneumatic needleless injector
ActiveCN103961768AReduced risk of being bentLow machining accuracy requirementsInfusion syringesIntravenous devicesNeedle Free InjectionNeedle free
The invention discloses a pneumatic needleless injector which comprises a base, an injection component, an air way component, a manual trigger component, an injection dosage regulating component and a needle cylinder component. An impacting piston of the injection component pushes liquid medicine in the needle cylinder component to achieve the purpose of injection. High-pressure air is led into the air way component to push the piston of the injection component to move. The manual trigger component makes contact with a valve element of the air way component through a trigger and a connecting rod to control opening and closing of the air way component. A threaded rod and a limiting stop block of the injection dosage regulating component are connected through threads, and the threaded rod is rotated to enable the limiting stop block to move in the axial direction, so that the initial position of the impacting piston of the injection component is limited, and the purpose of regulating injection dosage is achieved. The needle cylinder component is sealed through a plunger and a one-way valve structure of a nozzle, and is used for guiding liquid medicine in and accommodating liquid medicine.
Owner:JIANGXI SANXIN MEDTEC
Compositions and methods comprising collagen
InactiveUS20090312524A1Reduces eliminates potentialEasy to purifyConnective tissue peptidesPeptide/protein ingredientsCollagen spongeCollagen VI
In various embodiments, a collagen product is provided that is derived from an animal, the collagen product comprises precipitated collagen that is substantially pure. In various embodiments, the collagen is obtained from a marine animal and does not contain prions or viruses. In various embodiments, the collagen can be made or incorporated into collagen films, collagen membranes, cosmetic collagen masks, collagen sponges, gelatin, hemostasis sponges, lyophilized foams, collagen injections, artificial skins and dura, bones, cartilage, screws, shafts, stems, or tube guides.
Owner:ALTERNATIVE SOURCED COLLAGEN
Method for synthesizing multifunctional active targeted hyaluronic acid-polylactic acid carrier and preparing anti-tumor medicinal micelle of multifunctional active targeted hyaluronic acid-polylactic acid carrier
ActiveCN104056275AExtend cycle timeSmall toxicityPharmaceutical non-active ingredientsEmulsion deliverySolubilityPolyester
The invention belongs to the fields of polymer chemistry and medicinal preparations, and particularly relates to a method for synthesizing an active targeted hyaluronic acid-polylactic acid carrier, a method for preparing an anti-tumor medicinal micelle of the active targeted hyaluronic acid-polylactic acid carrier and an application thereof. By adopting a novel self-assembly technology, amphipathic PEG (polyethylene glycol) block polyester copolymer and tumor targeted ligand hyaluronic acid-polylactic acid copolymer are self-assembled by means of the electrostatic interaction to form a multifunctional composite micelle; the solubility of insoluble tumor medicaments and the drug loading capacity and encapsulation efficiency of water-soluble anti-tumor medicines can be remarkably improved by virtue of the anti-cancer drug-loaded micelle and composite micelle composition, the medicines can be biodegraded in a body, phagocytosis of a reticuloendothelial system (RES) and excretion of a kidney can be avoided. The active targeted hyaluronic acid-polylactic acid carrier has a long-circulating effect, the multifunctional composition has a prominent advantage of tumor active targeting effect, and parameters of pharmacodynamics in vitro and in vivo of the micelle are remarkably superior to those of common anti-tumor injections. Clinically acceptable administration means of the micelle includes injection administration or mucosal administration, and preparations of the micelle can be injection, transfusion, injection lyophilized powder injections or dry powder inhalation.
Owner:CHINA PHARM UNIV
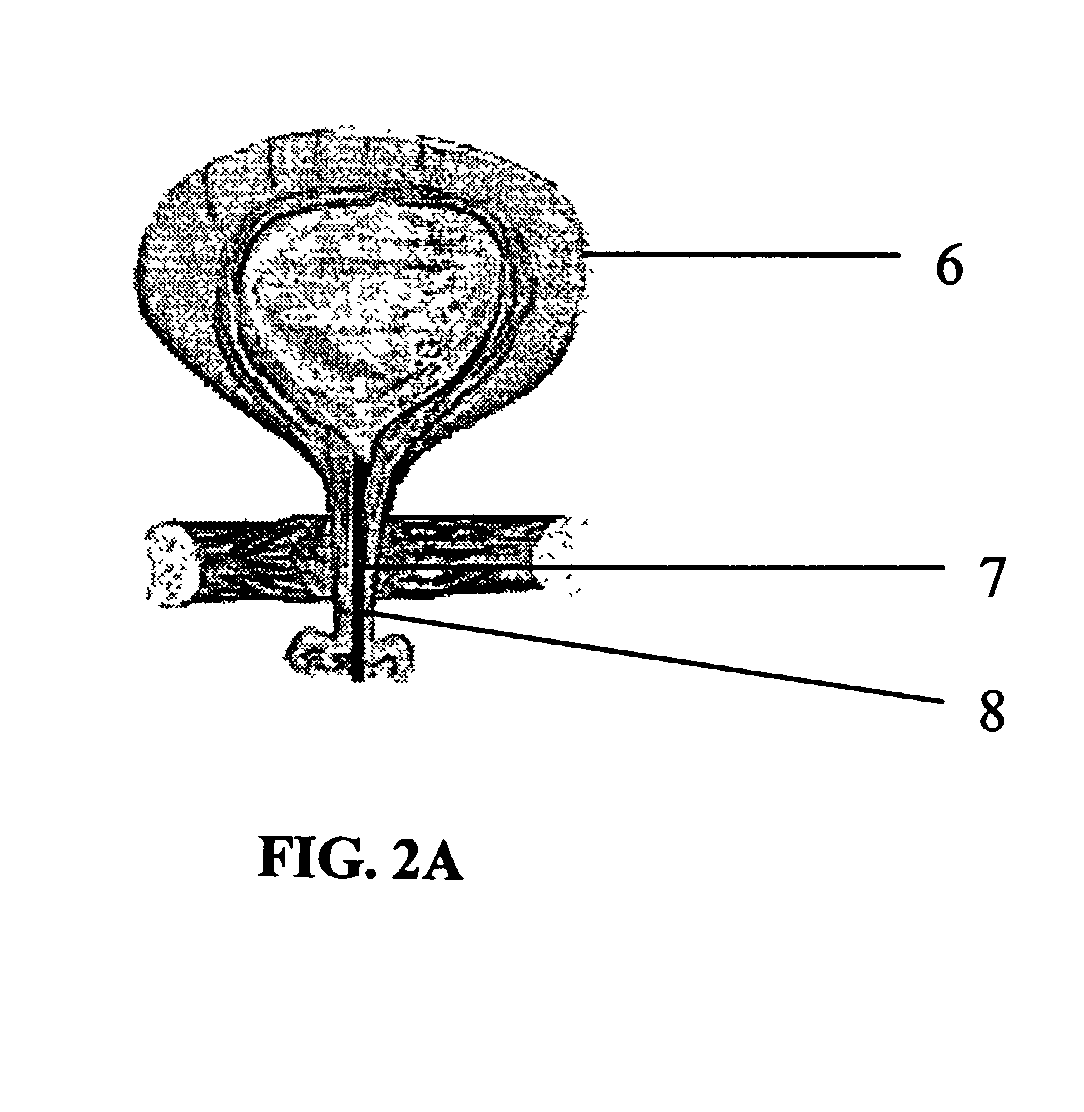
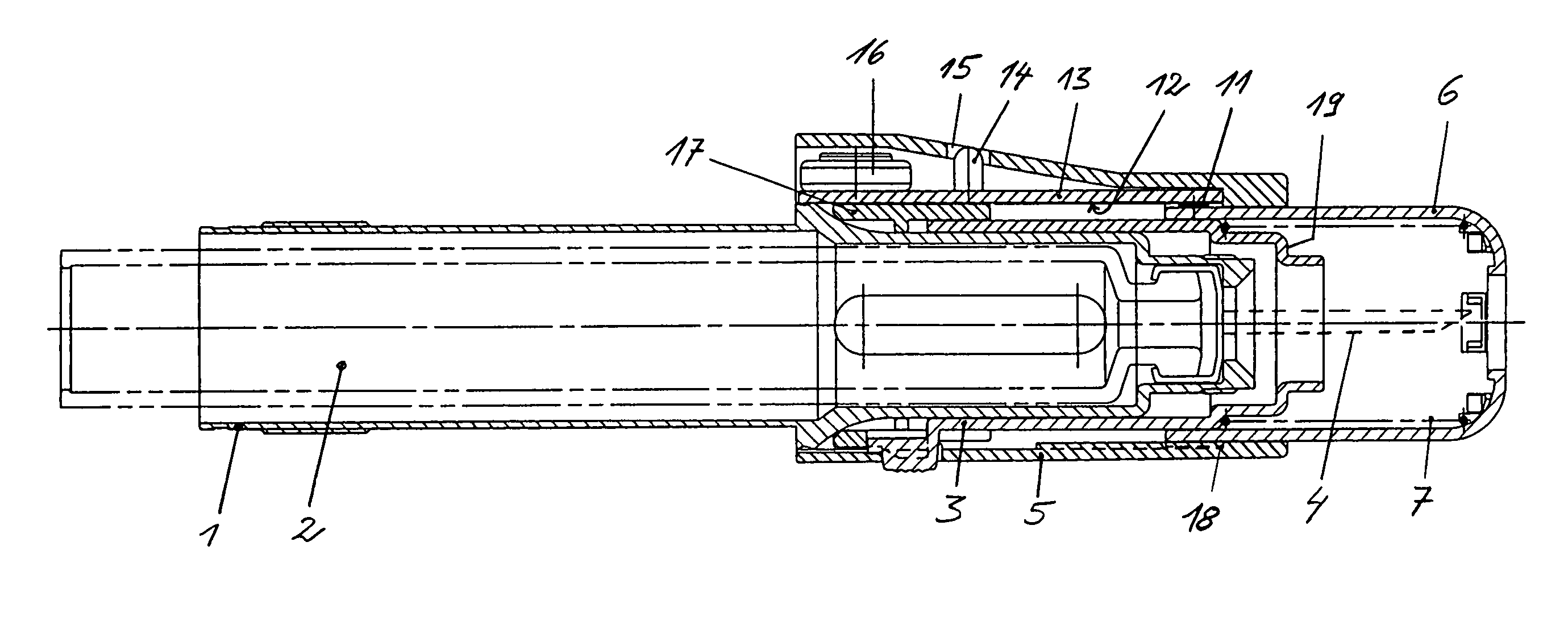
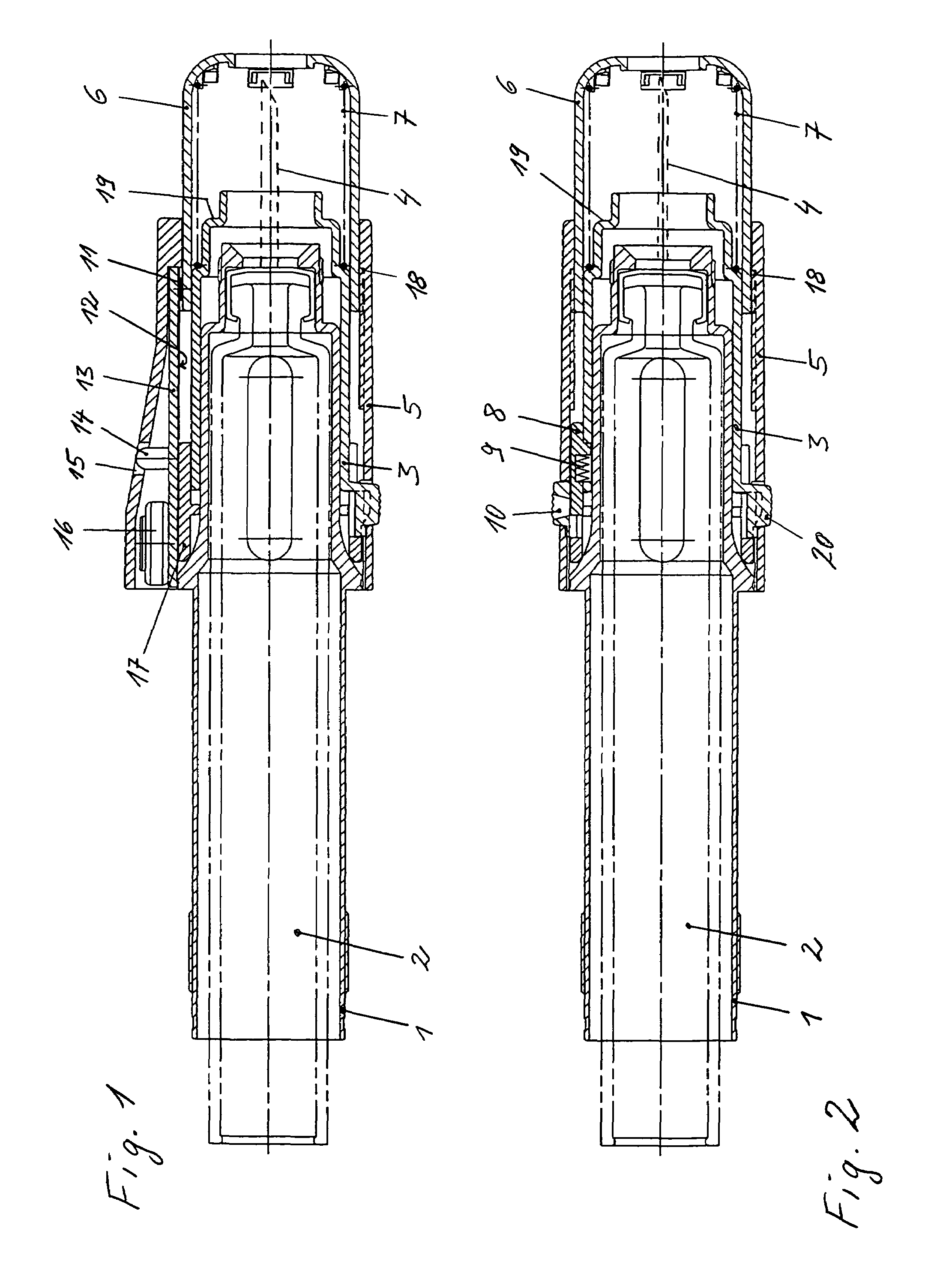
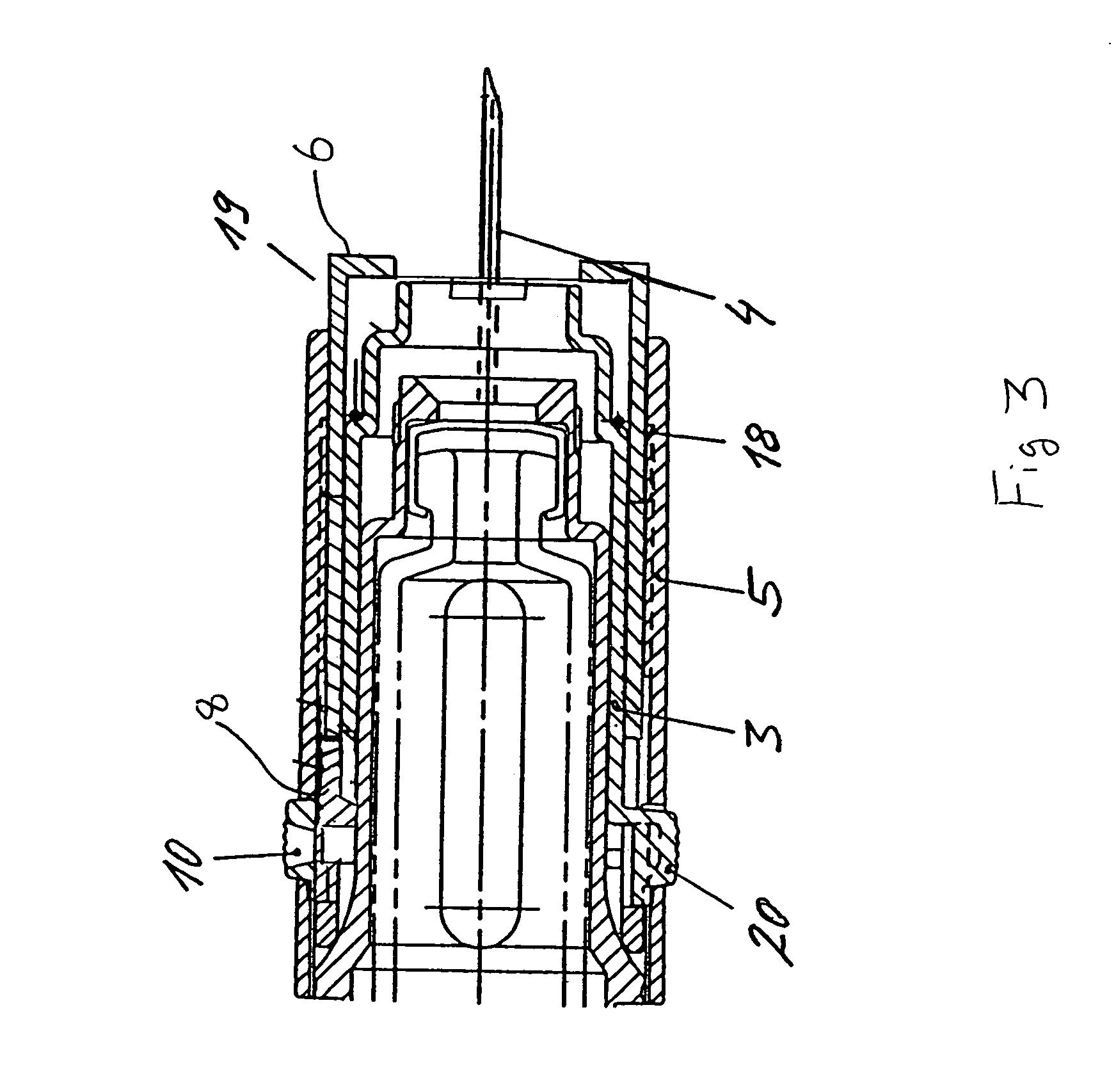
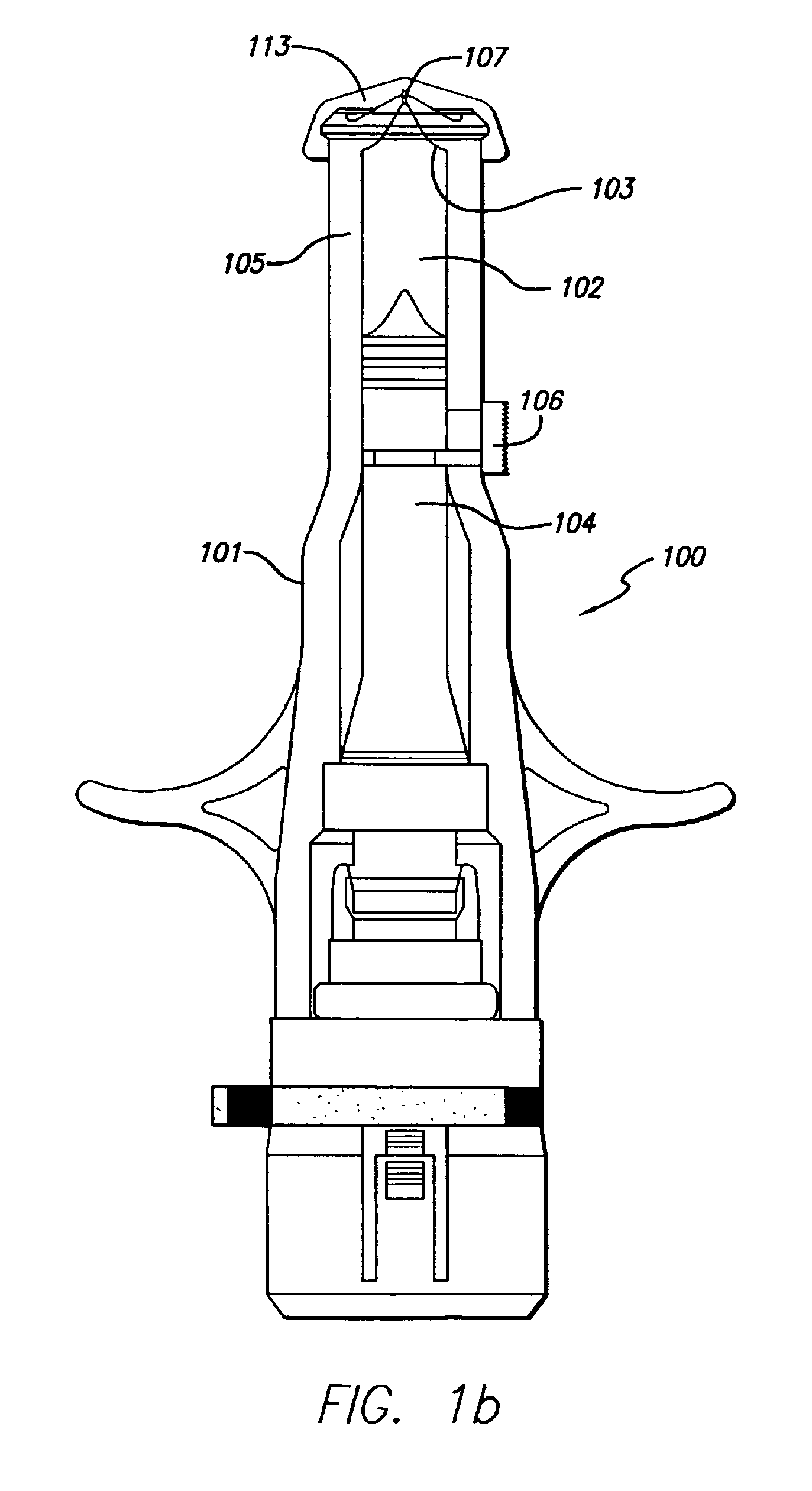
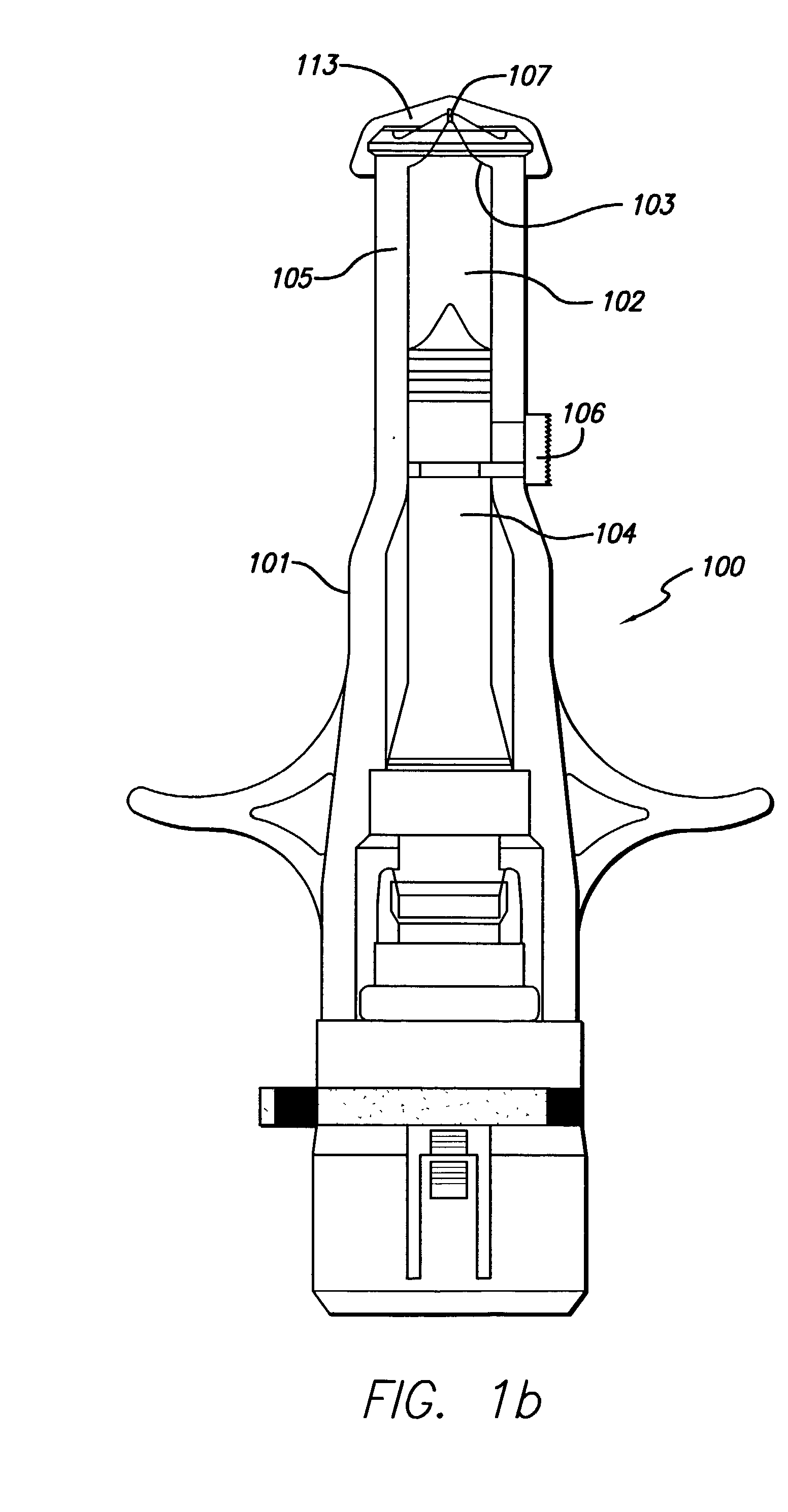
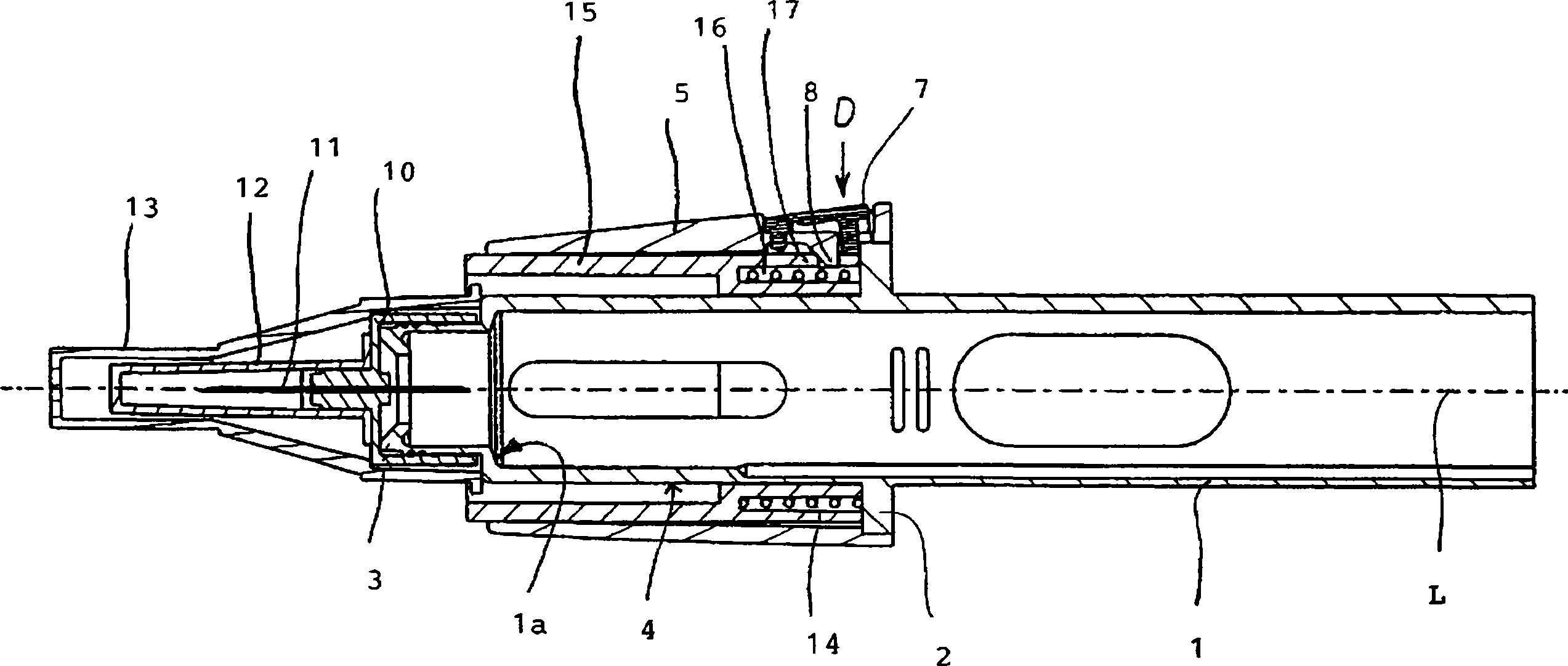
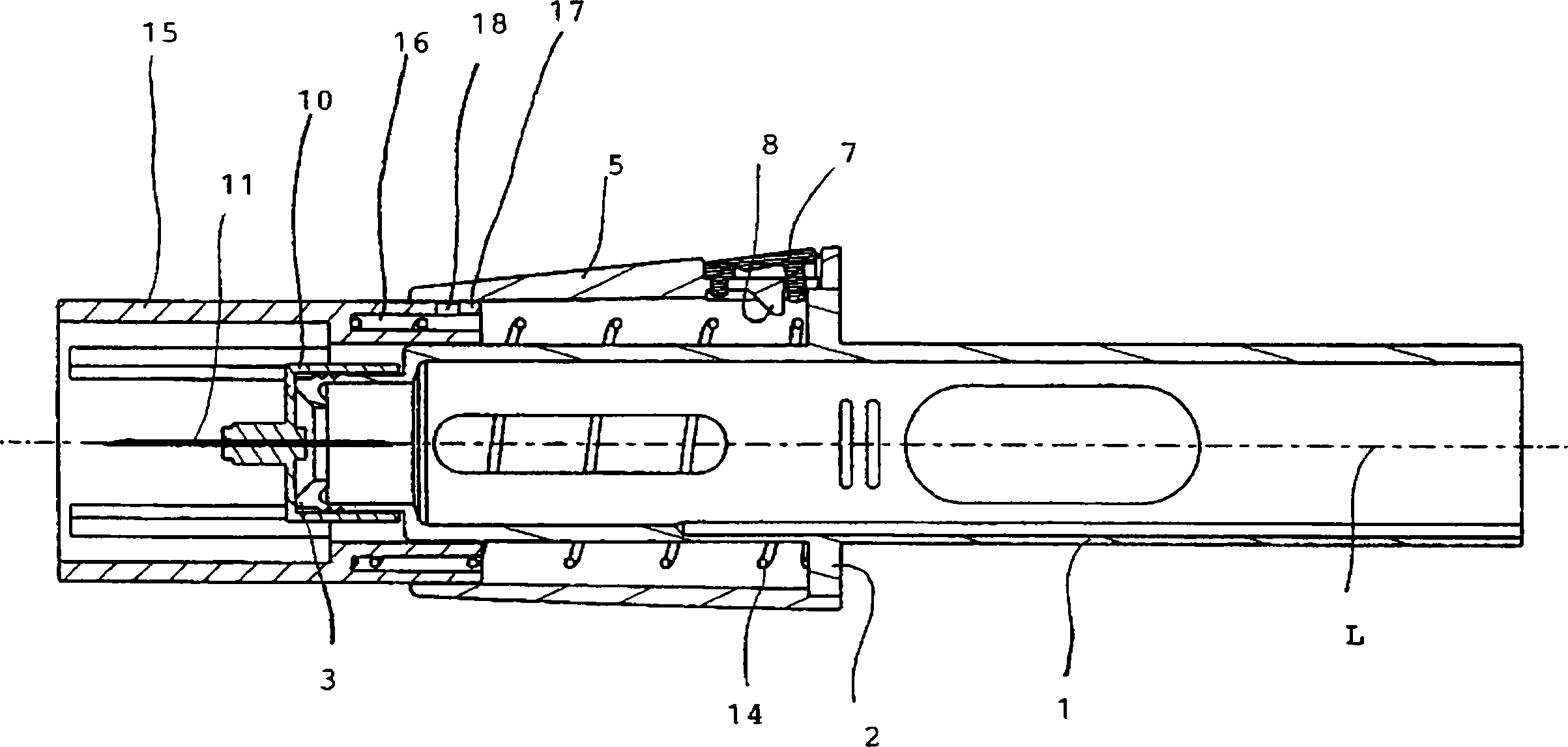
Injection device comprising a needle cover
An injection device comprising a needle cover (15), comprising: a housing (1, 5) forming a receptacle for receiving a container containing an injectable product or forming the container itself; Delivery device; a needle holder (10) comprising an injection needle (11) via which the needle holder (10) is fluidly connected or fluidically connectable to a container. The needle cover (15) is mounted by the housing (1, 5) in such a way that the needle cover (15) can move in the proximal direction against a restoring force, from the protection position through the injection position, moving to the assembly position. In the protected position, the needle cover (15) extends beyond the distal tip of the injection needle (11), while in the injection position, the needle cover (15) is shorter than the needle tip. In said assembled position, the needle cover (15) allows access to the needle holder (10), so that the needle holder (10) can be grasped and connected to the housing (1, 5) and / or removed from the housing ( 1, 5) on and off.
Owner:TECPHARMA LICENSING AG
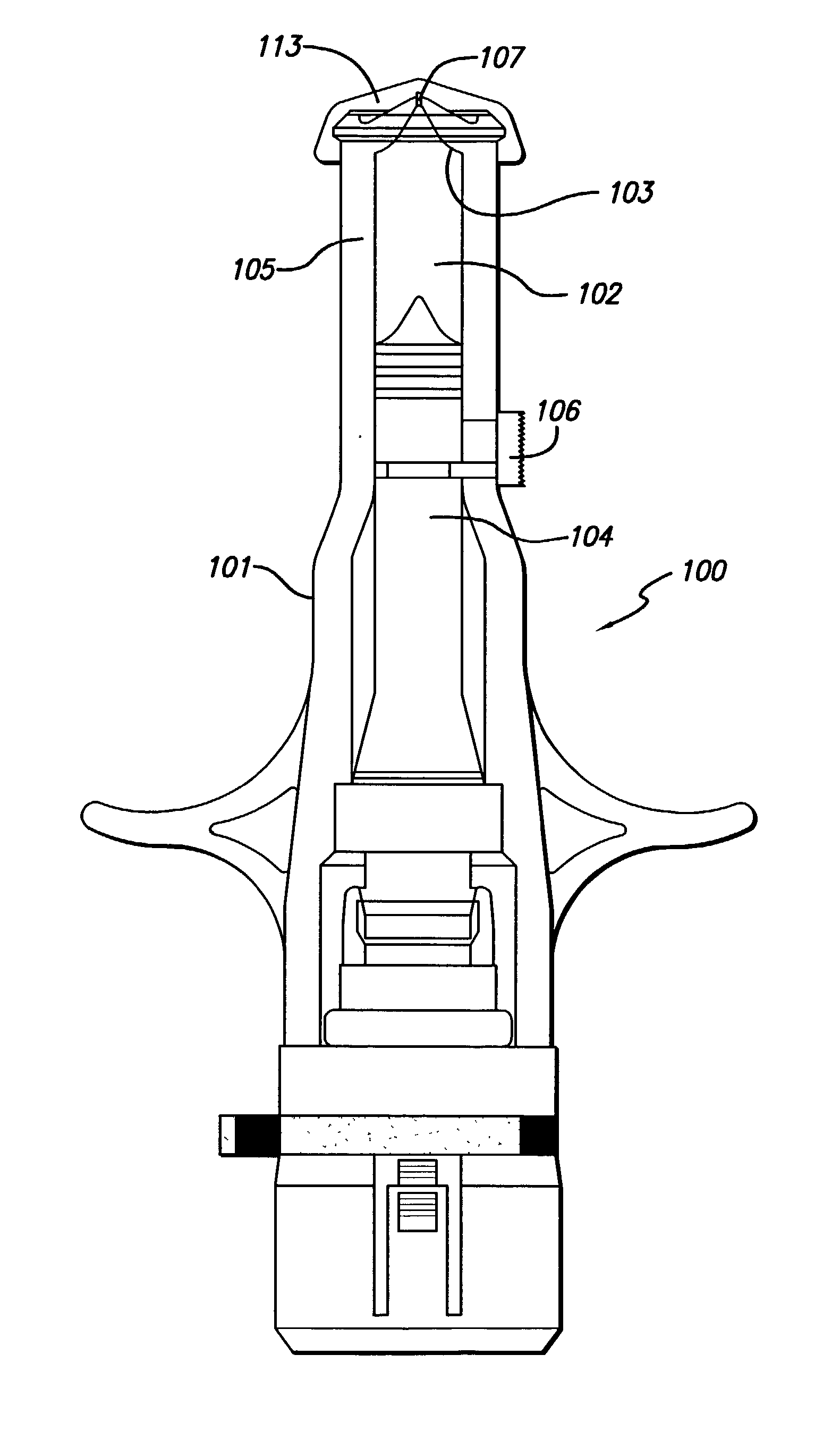
Device for injecting a product, in particular for medical use
ActiveUS20060111674A1Eliminate riskAmpoule syringesMedical devicesInjection productMedical treatment
This device comprises: a body housing a hollow injection needle and a container containing the injectable product; the needle is connected to the body but able to move relative to the latter between an injection position and a retracted position; a plunger that slides in the body and is displaceable relative to the latter to perform the injection; said container is able to move relative to the plunger between a position that enables the injection to be performed and a retracted position; means for keeping the needle and the plunger in injection position, which means can be released to free the needle and the plunger to move to said retracted position.
Owner:BECTON DICKINSON & CO
Use of beta-nicotinamide mononucleotide in preparation of Anti-aging drugs or health-care products
InactiveUS20170266213A1Good curative effectHighly safe as a drug or health-care productOrganic active ingredientsAntinoxious agentsNicotinamide mononucleotideBody weight
Disclosed is use of β-nicotinamide mononucleotide in the preparation of anti-aging drugs or health-care products. A single dose of the β-nicotinamide mononucleotide is 1-500 mg / Kg body weight / day, and the drug or health-care product is in the form of tablets, capsules, granules, aqueous solutions, enteric-coated preparations or injections.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
High-throughput automated cellular injection system and method
ActiveUS20080077329A1Easy to adaptReduce throughputBiological testingSpecial data processing applicationsHigh fluxGenetic Materials
An automated cell injection system and method are described, which can perform automatic, reliable, and high-throughput cell injection of foreign genetic materials, proteins, and other compounds. The system and method overcome the problems inherent in traditional manual injection that is characterized by poor reproducibility, human fatigue, and low throughput. The present invention is particularly suited for zebrafish embryo injection but can be readily extended to other biological injection applications such as mouse embryo, drosophila embryo, and C. elegans injections, capable of facilitating high-throughput genetic research at both academic and industry levels. A novel vacuum based cell-holding device is also provided.
Owner:SUZHOU BOUNDLESS MEDICAL TECH CO LTD
Injectable pharmaceutical formulation of melphalan
InactiveUS20130131174A1High viscosityBiocideOrganic active ingredientsPharmaceutical formulationOrganic solvent free
An injectable pharmaceutical formulation of Melphalan comprising a solid composition of melphalan hydrochloride lyophilized with a content of impurities up to 1.3% (p / p)and a pH buffer solution; a process to prepare said solid composition. Also a reconstituted solution of melphalan comprising a solid composition of melphalan lyophilized reconstituted wherein said solution is aqueous, a perfusion free of organic solvent and a kit.
Owner:ERIOCHEM SA
Drug delivery technology
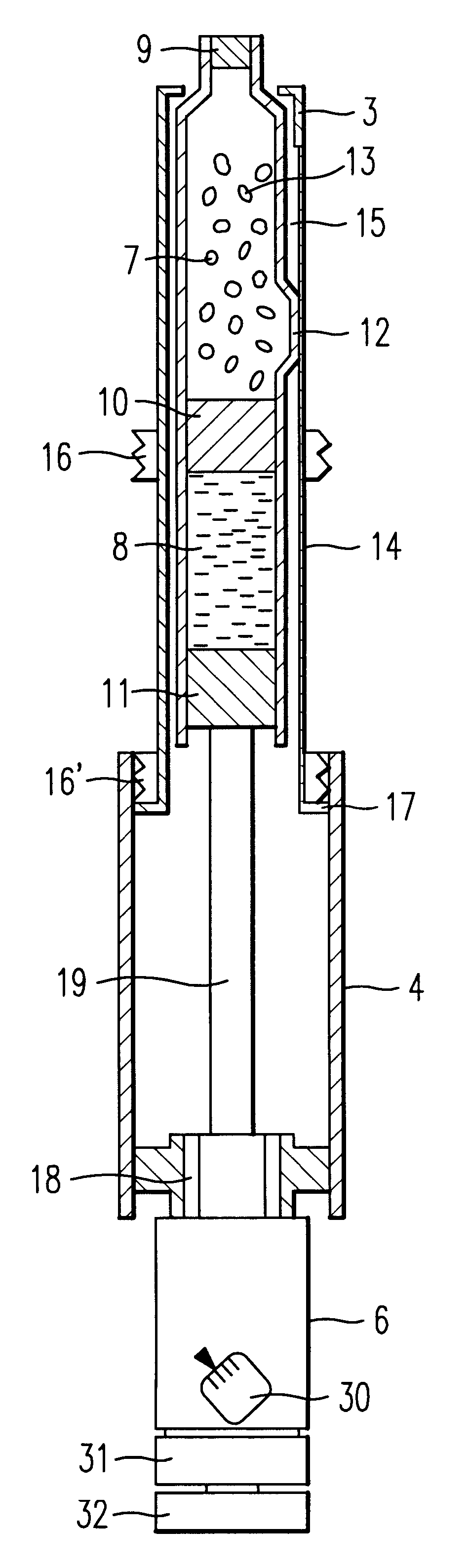
ActiveUS7615234B2Splash back and seepage can be avoidedControl moreAmmunition projectilesPowder deliveryProjectileInjectable Product
The invention relates to a novel drug delivery technology. More particularly the invention relates to a method of delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient; to a throwaway or reusable device for delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient in a manner as set out by the method; to a pioneer projectile for use in said method; to formulations for use in said method and to an injectate comprising a pioneer projectile and formulation. It also relates to a disposable component containing either a pioneer projectile or an injectate. The invention also relates to a throwaway or reusable device for delivering at least one therapeutic compound, or a formulation comprising the at least one therapeutic compound (hereafter drug) to a patient, and a method for administering a drug to a patient using said device. It also relates to a packaged drug for use with said device.
Owner:ENESI PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com