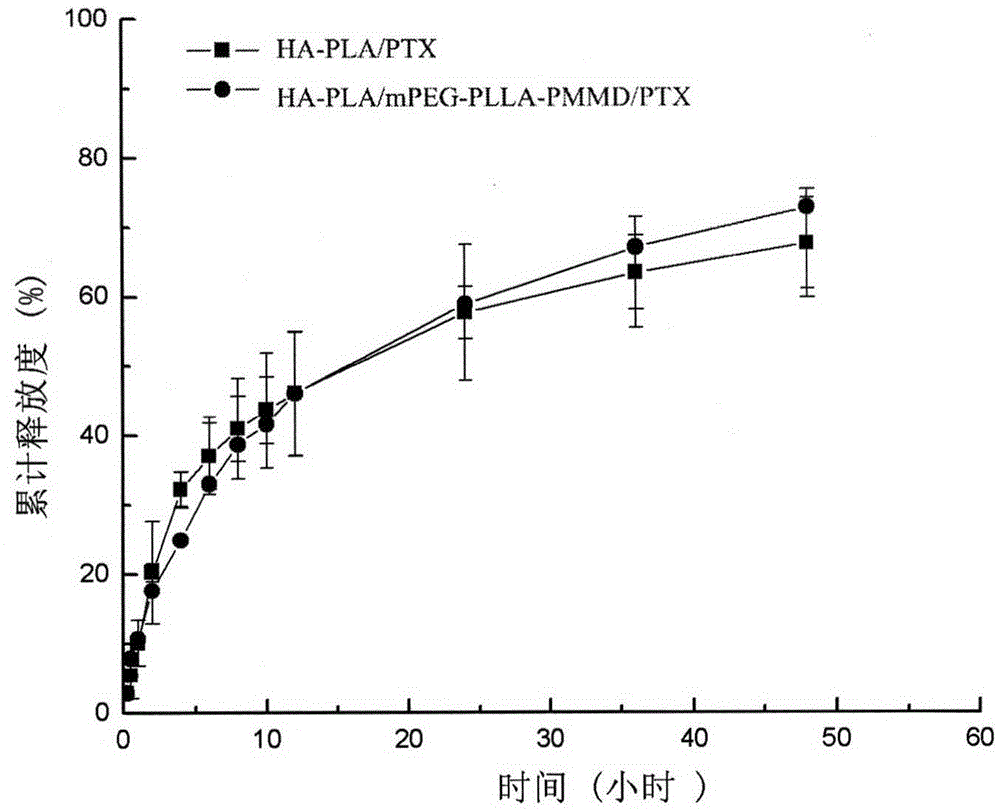
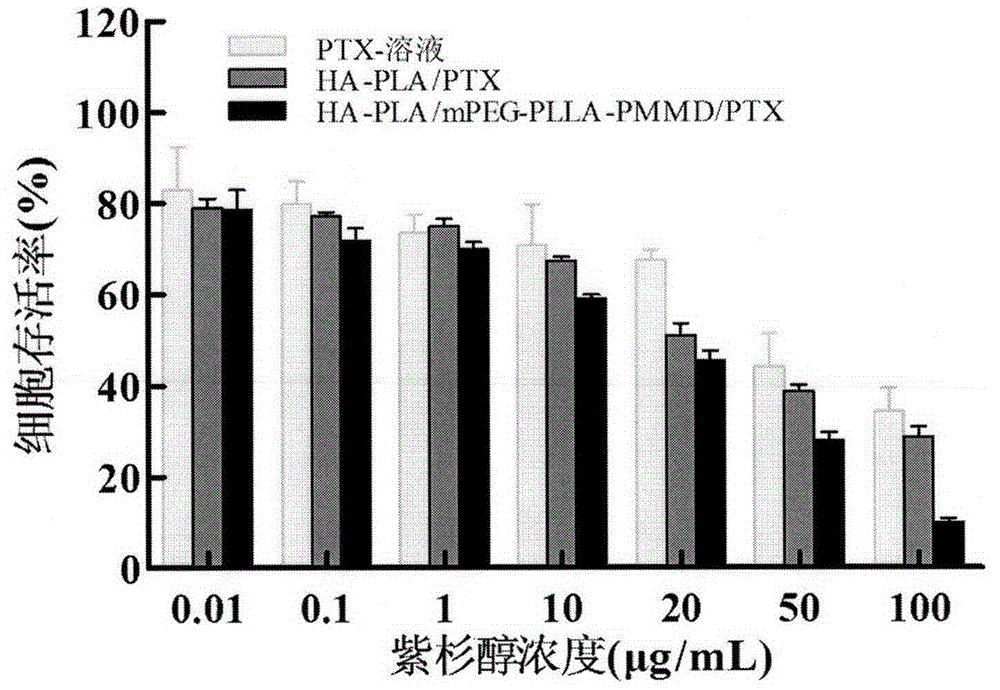
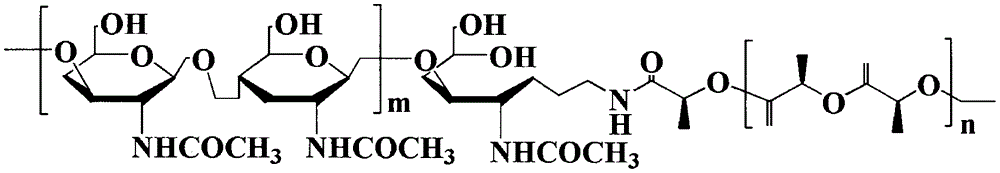
Method for synthesizing multifunctional active targeted hyaluronic acid-polylactic acid carrier and preparing anti-tumor medicinal micelle of multifunctional active targeted hyaluronic acid-polylactic acid carrier
An anti-tumor drug, hyaluronic acid technology, applied in the field of polymer chemistry and pharmaceutical preparations, can solve the problems of clinical application difficulties, poor biocompatibility of octadecanecarboxylic acid, etc., achieve active targeting of long tumors, avoid Effects of phagocytosis and excretion by the kidneys and prolongation of circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Synthesis and activation of polylactic acid (PLA)
[0039] Synthesis of polylactic acid (PLA): Using the ring-opening polymerization method, add 10 g D, L-lactide (69.4 mmol) into a single-necked round-bottomed flask, the temperature of the oil bath is 120 ° C, and after the lactide is melted, add the catalyst 1.8 mL Sn(Oct) 2 (5 mmol). The air in the container was purged with nitrogen gas, and the temperature was gradually raised to 160°C, and the polymerization reaction was carried out for 6 hours. Add an appropriate amount of ethyl acetate to dissolve, add ethanol to precipitate, filter with suction, wash with deionized water, and dry in vacuo to obtain a pale yellow PLA solid with a yield of 80.2%. The results of ring-opening polymerization reaction temperature and time on the molecular weight of polylactic acid are shown in Table 1.
[0040] The polylactic acid reaction formula is as follows:
[0041]
[0042] The molecular weight of PLA was det...
Embodiment 2
[0048] Example 2 Synthesis of hyaluronic acid-polylactic acid (HA-PLA)
[0049] (1) Terminal Amination Synthesis of Hyaluronic Acid
[0050] Amination of hyaluronic acid (Mn: 7800): adopt reductive amination reaction, dissolve 150mg N,N-diethylethylenediamine (1.3mmol) with pH9.5 phosphate buffer, weigh 9.5g hyaluronic acid ( HA 7800 ) and 100 mL of purified water to dissolve (1.2 mmol) was dropped into the above solution, and magnetically stirred for 4 h. Add 0.04 g of sodium cyanoborohydride (0.6 mmol), control the temperature at 8-20 ° C for 1 h, and react at room temperature for 12 h. Put the reactant into a dialysis bag (molecular weight cut-off is 8000-10000), and then dialyze with PBS buffer solution for two days. Freeze-dried and freeze-dried to obtain terminal amination-hyaluronic acid (HA-NH 2 ), yield 85.91%.
[0051] (2)HA 7800 -PLA 3500 Synthesis
[0052] Weigh 8.2g of HA-NH 2 (1.1mmol), add PBS (pH9.5) buffer solution 100mL, ultrasonically dissolve, add ...
Embodiment 3
[0055] Example 3 Structural characterization and property determination of hyaluronic acid-polylactic acid
[0056] (1) Specific steps for structural characterization
[0057] Infrared spectrum (FT-IR) characterization: take appropriate amount of PLA, HA and HA-PLA, mix with KBr, grind into powder, press into thin slices, use NICO LET-5700 Fourier transform infrared spectrometer to measure and analyze. The analysis from the FT-IR light diagram of HA-PLA is as follows: The characteristic absorption peak of HA is 3424.5cm -1 (υasOH and υasNH), 2892.8cm-1 (υC-H), 1628.7cm -1 (υasC-O); PLA characteristic absorption peak is 1758.1cm -1 (υC=O), 2995.8cm -1 (υasCH3), 1455.6cm -1 (δasCH3); 2892.8cm -1 The intensity of the peak increases, and through the analysis of the above results, it is confirmed that PLA is covalently linked to HA with a newly generated amide bond, and the synthetic product is determined to be a hyaluronic acid-polylactic acid (HA-PLA) block copolymer.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com