Patents
Literature
62 results about "Injectable Suspension" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Advertisement. Triamcinolone acetonide injectable suspension is a prescription medication used to treat a variety of conditions, depending on where the injection is administered.
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
Method of embolization using polyvinyl alcohol microspheres
The present invention relates to microspheres useful for embolization which comprises polyvinylalcohol. The present invention also relates to an injectable suspension suitable for embolization which comprises the polyvinylalcohol microspheres and a suitable liquid carrier. The present invention further relates to a method for prophylactic or therapeutic embolization which comprises administering to a mammal an injectable suspension containing the polyvinylalcohol microspheres and a suitable liquid carrier. Finally, the present, invention relates to a process for producing the polyvinylalcbhol microspheres.
Owner:BIOSPHERE MEDICAL SA (FR)
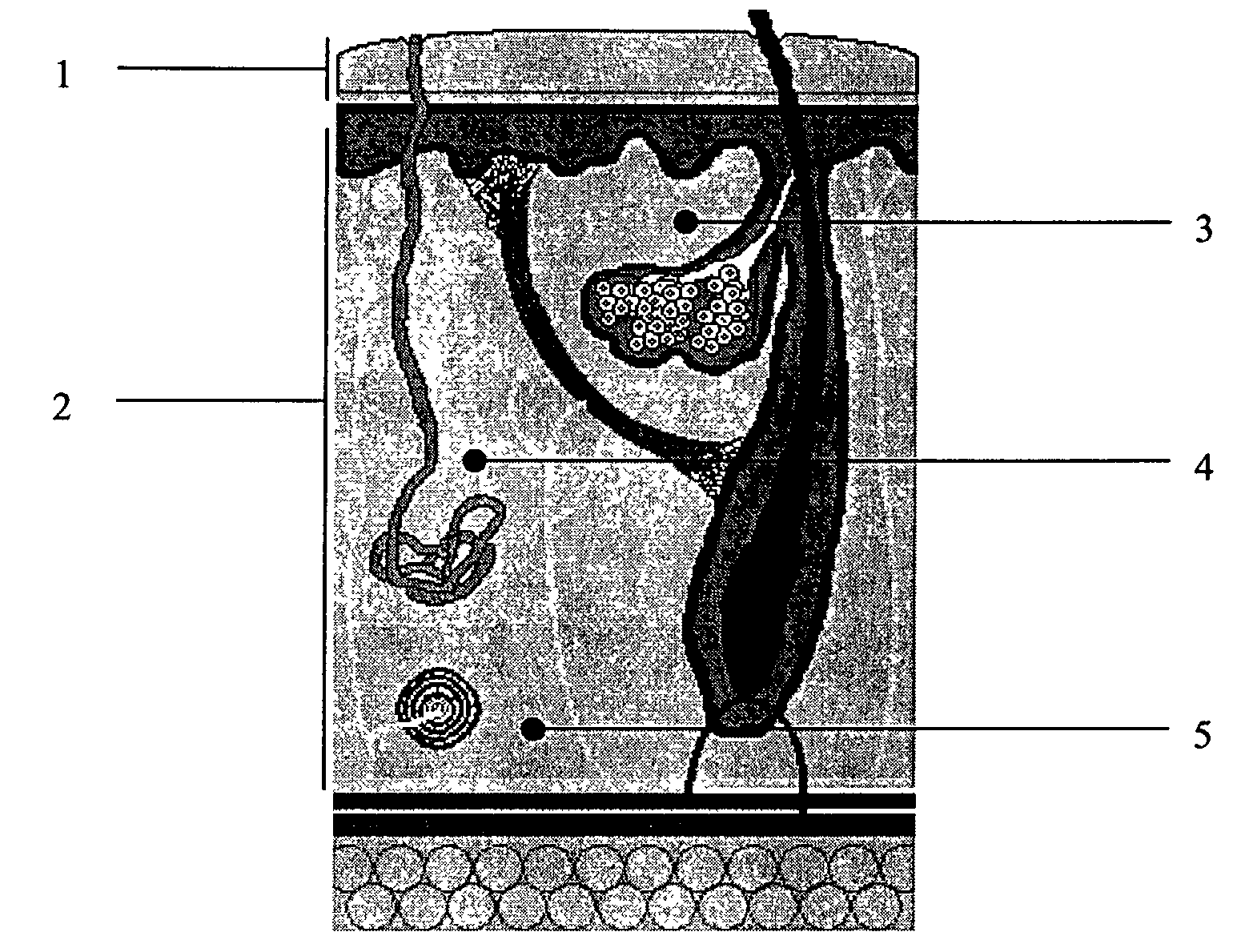
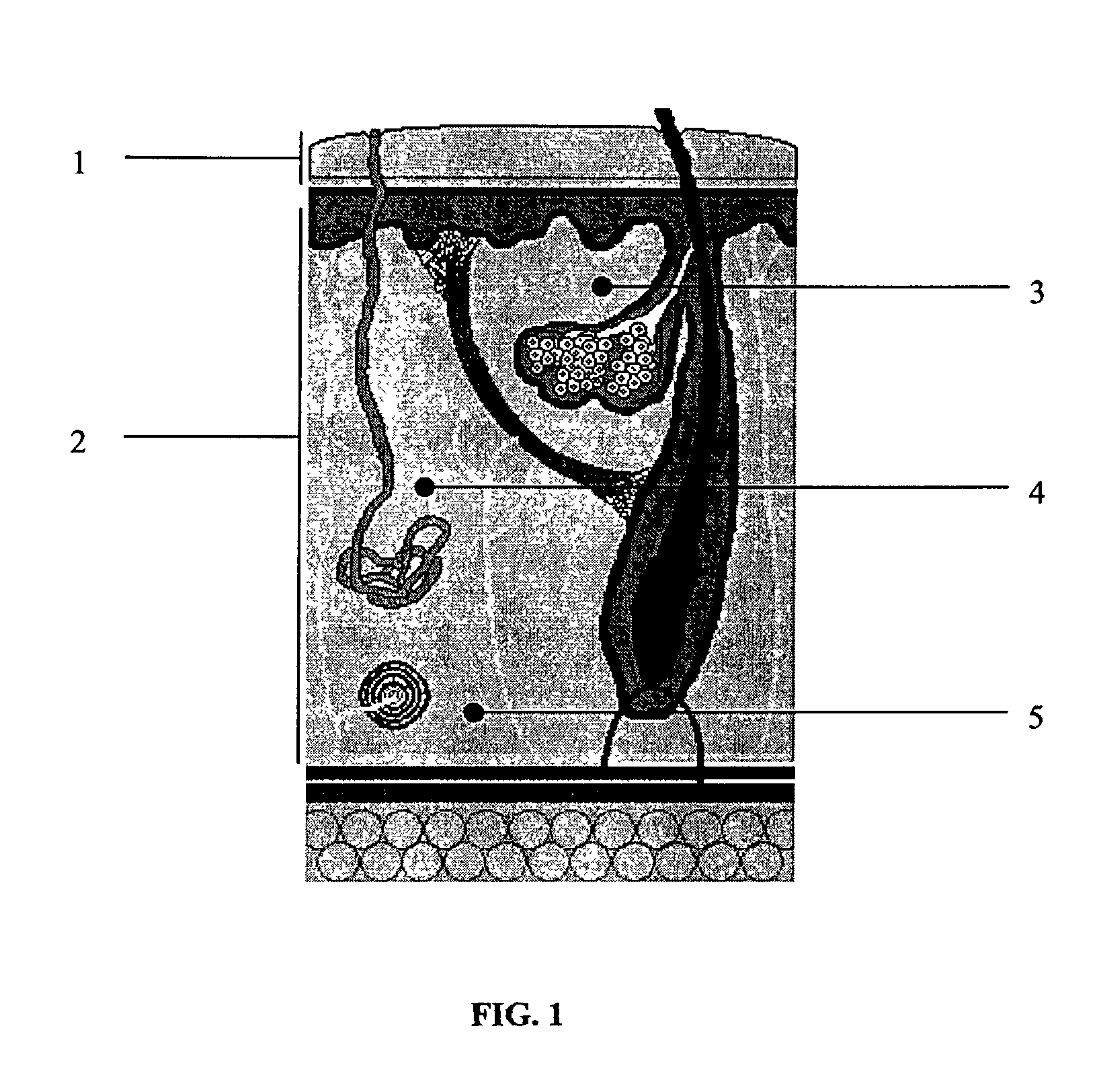
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20090110736A1Reduce wrinklesReduce scarsPowder deliveryCosmetic preparationsFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Polyvinyl alcohol microspheres, and methods for making and therapeutic uses of the same
The present invention relates to microspheres useful for embolization which comprises polyvinylalcohol. The present invention also relates to an injectable suspension suitable for embolization which comprises the polyvinylalcohol microspheres and a suitable liquid carrier. The present invention further relates to a method for prophylactic or therapeutic embolization which comprises administering to a mammal an injectable suspension containing the polyvinylalcohol microspheres and a suitable liquid carrier. Finally, the present invention relates to a process for producing the polyvinylalcohol microspheres.
Owner:BIOSPHERE MEDICAL SA (FR)
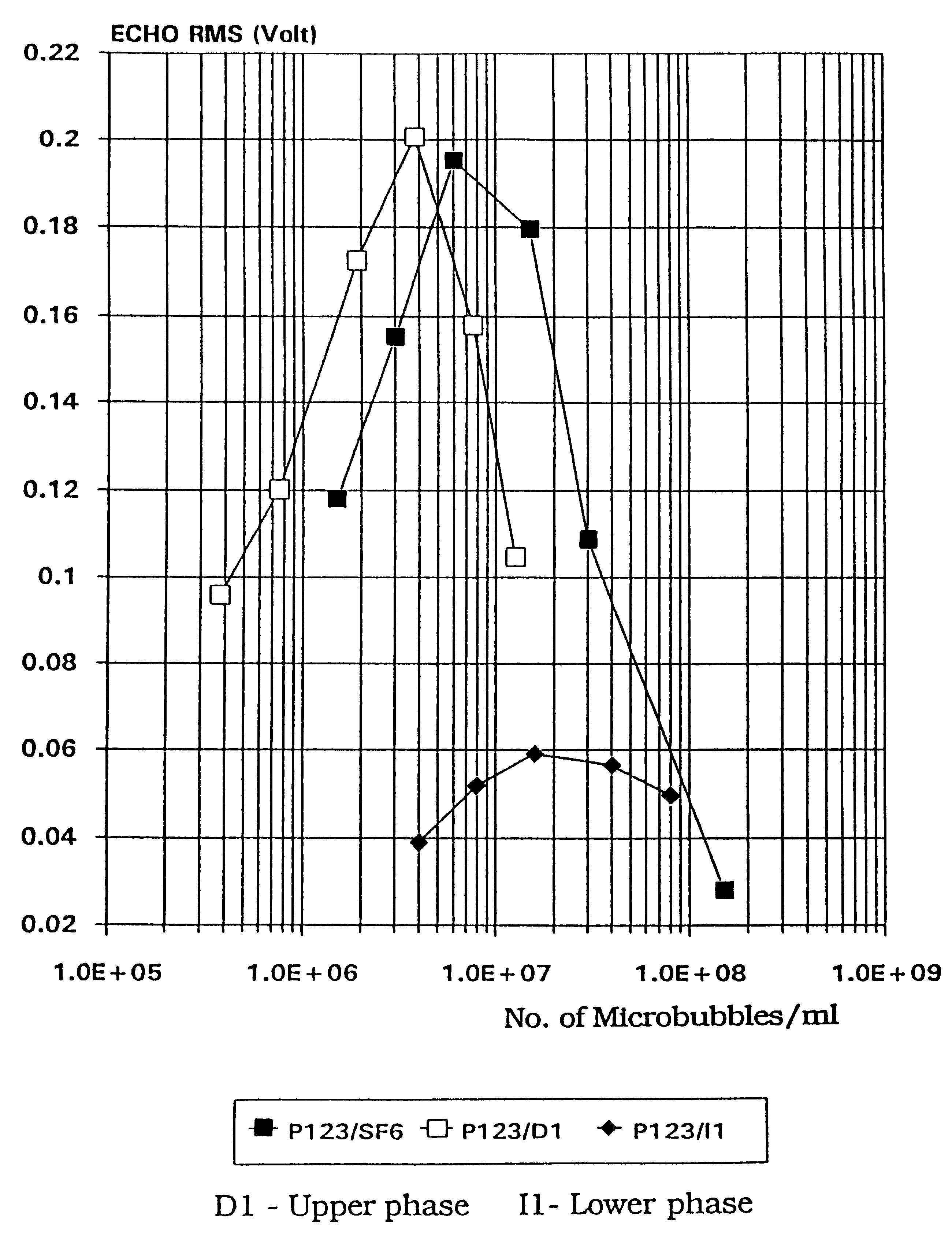
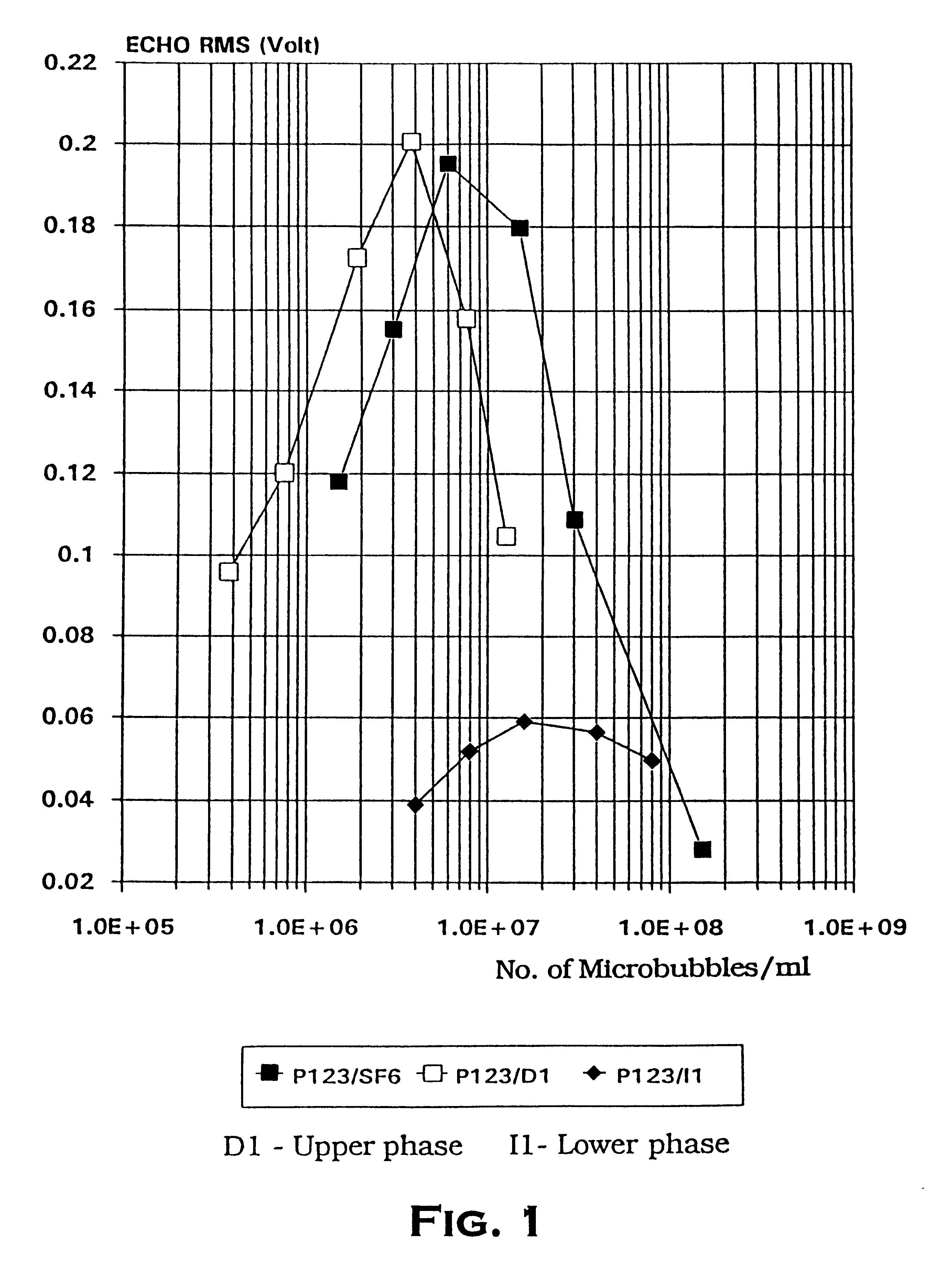
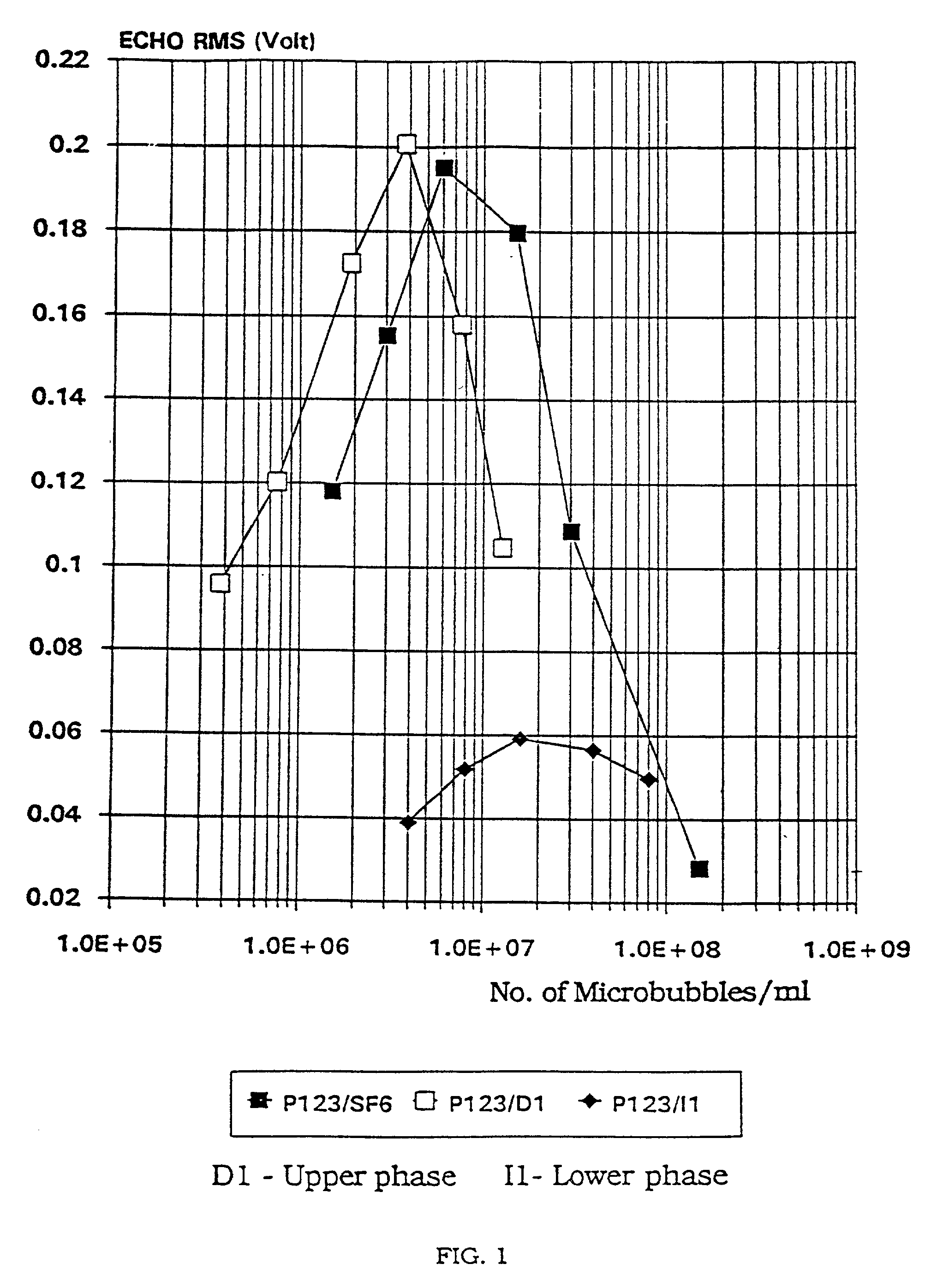
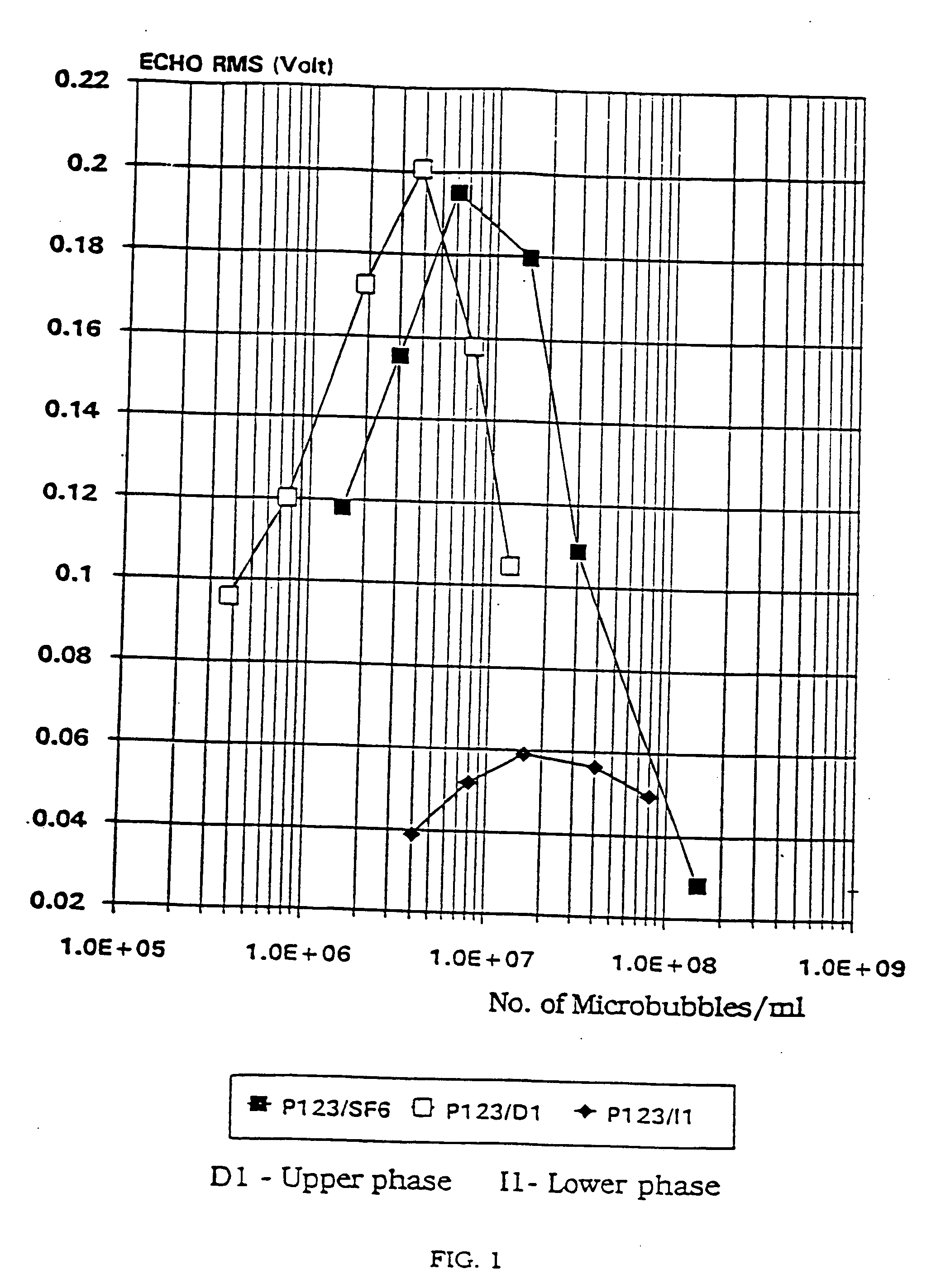
Stable microbubble suspensions as enhancement agents for ultrasonic echography
Disclosed are injectable suspensions of gas filled microbubbles in an aqueous carrier liquid usable as contrast agents in ultrasonic echography. The suspensions comprise amphipathic compounds of which at least one may be a laminarized phospholipid as a stabilizer of the microbubbles against collapse with time and pressure. The concentration of phospholipids in the carrier liquid is below 0.01% wt but is at least equal to or above that at which phospholipid molecules are present solely at the gas microbubble-liquid interface. Also disclosed is a method of preparation of the stable suspensions of air or gas filled microbubbles.
Owner:BRACCO INT BV
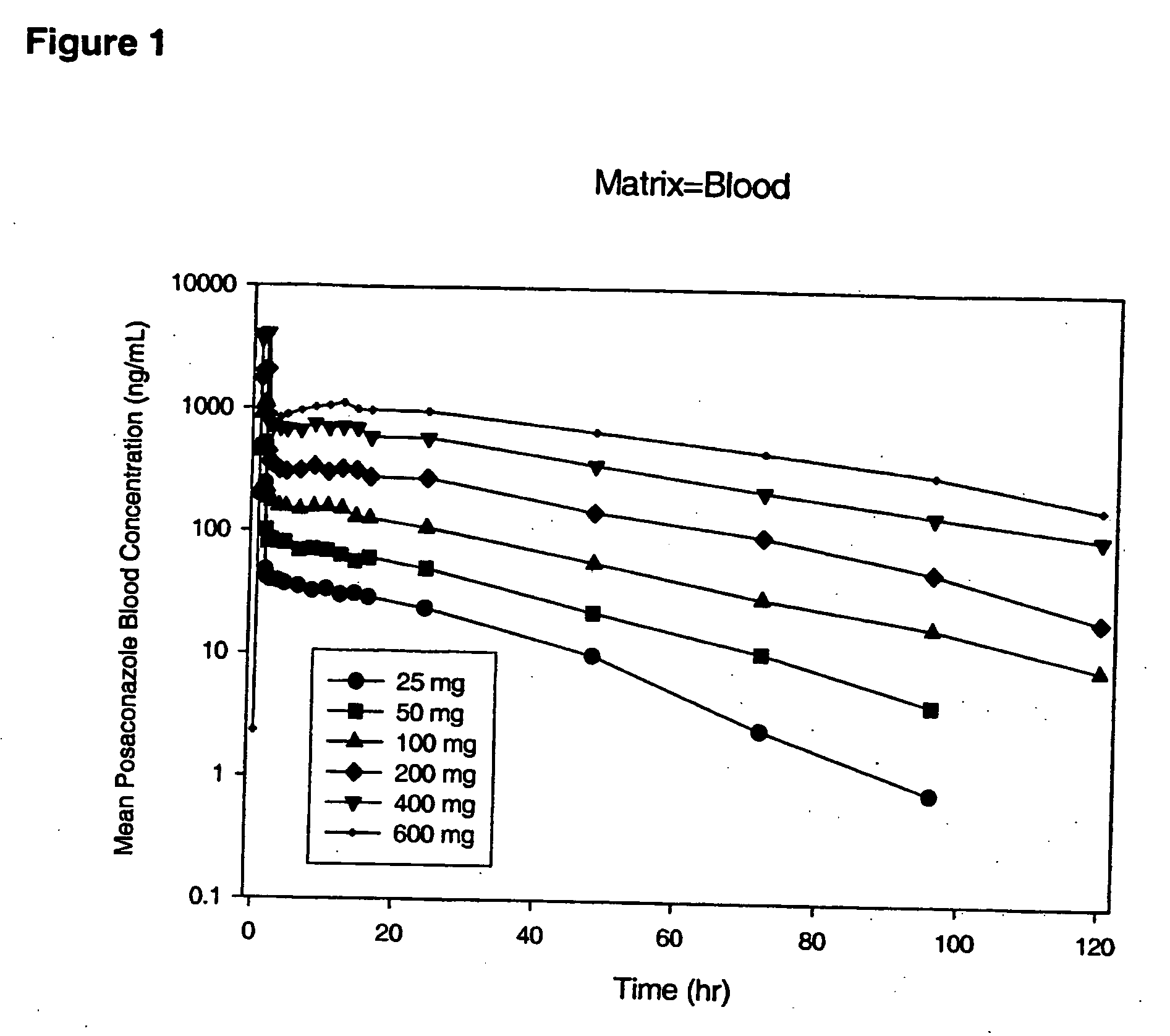
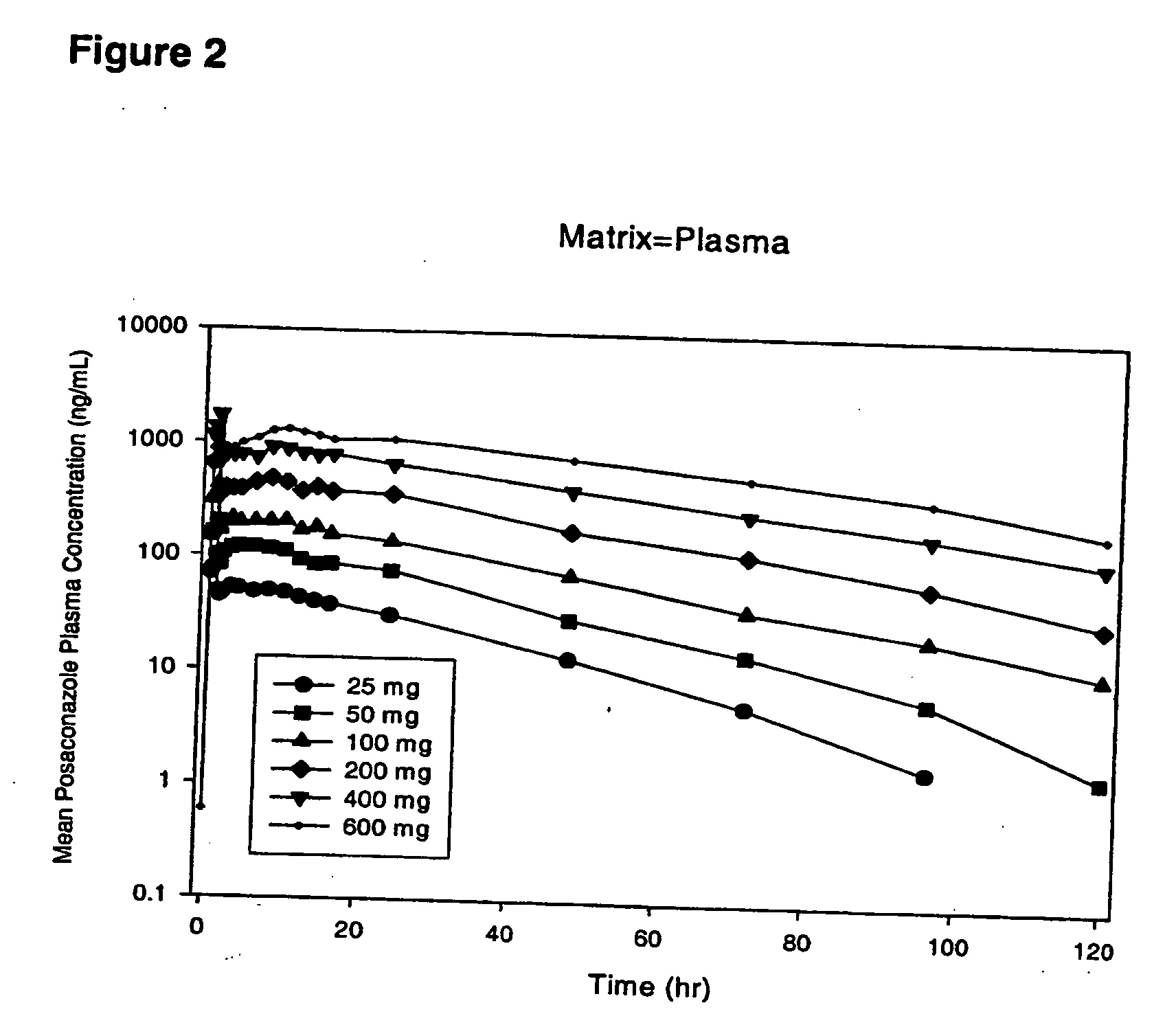
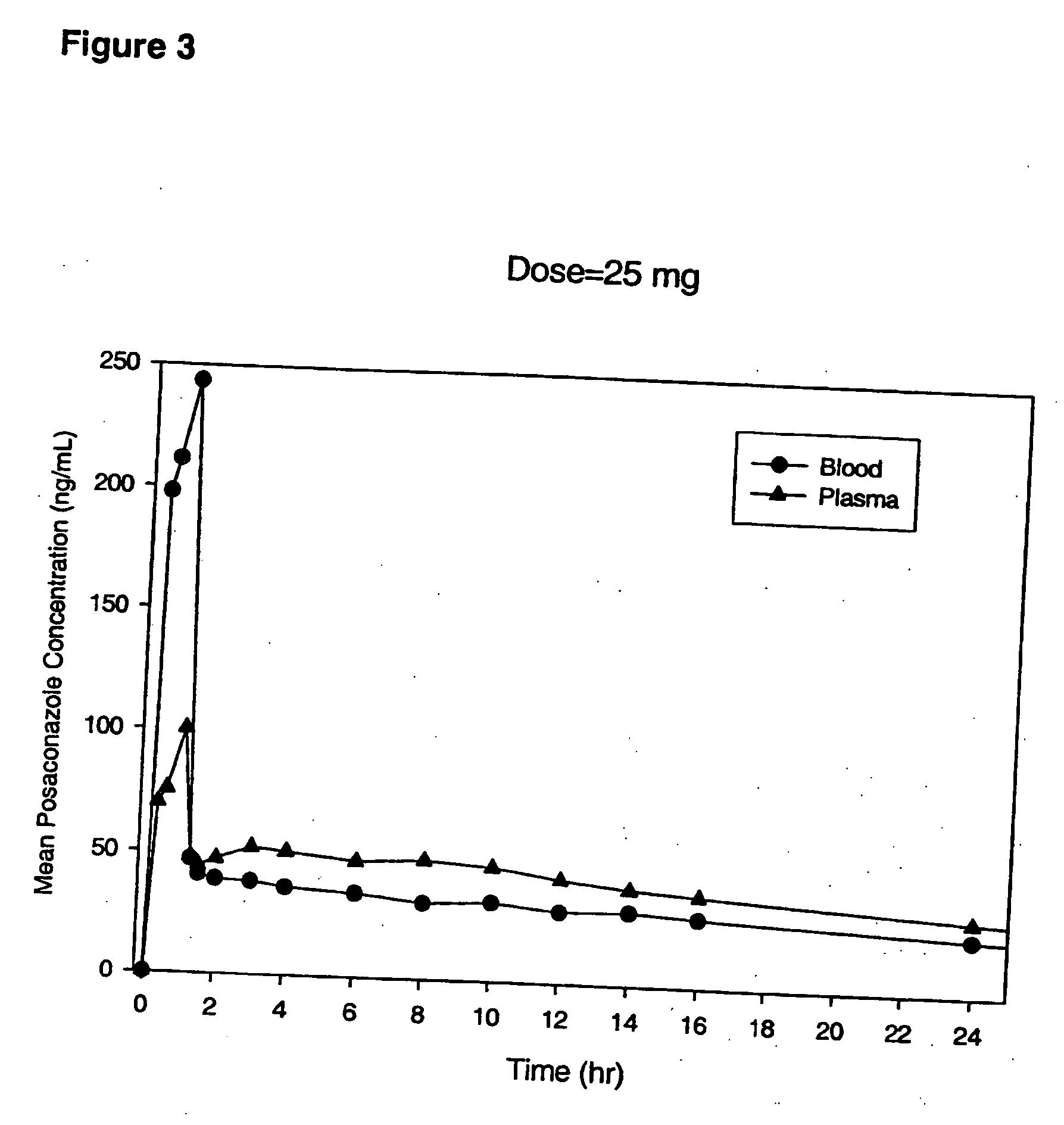
Particulate-stabilized injectable pharmaceutical compositions of Posaconazole
InactiveUS20060160823A1Reduce autoclave-induced particle size growthPromote growthOrganic active ingredientsAntimycoticsParticulatesAdditive ingredient
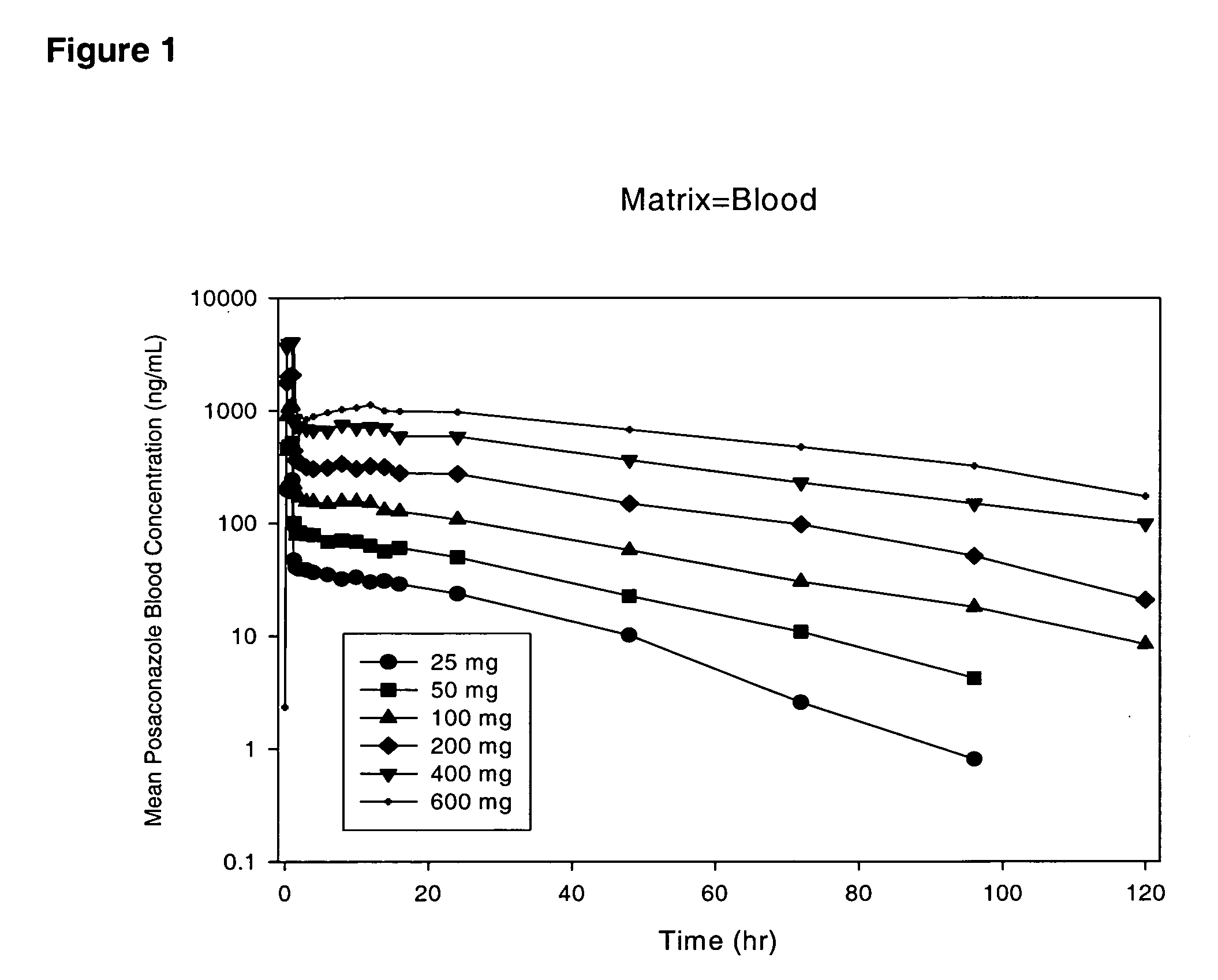
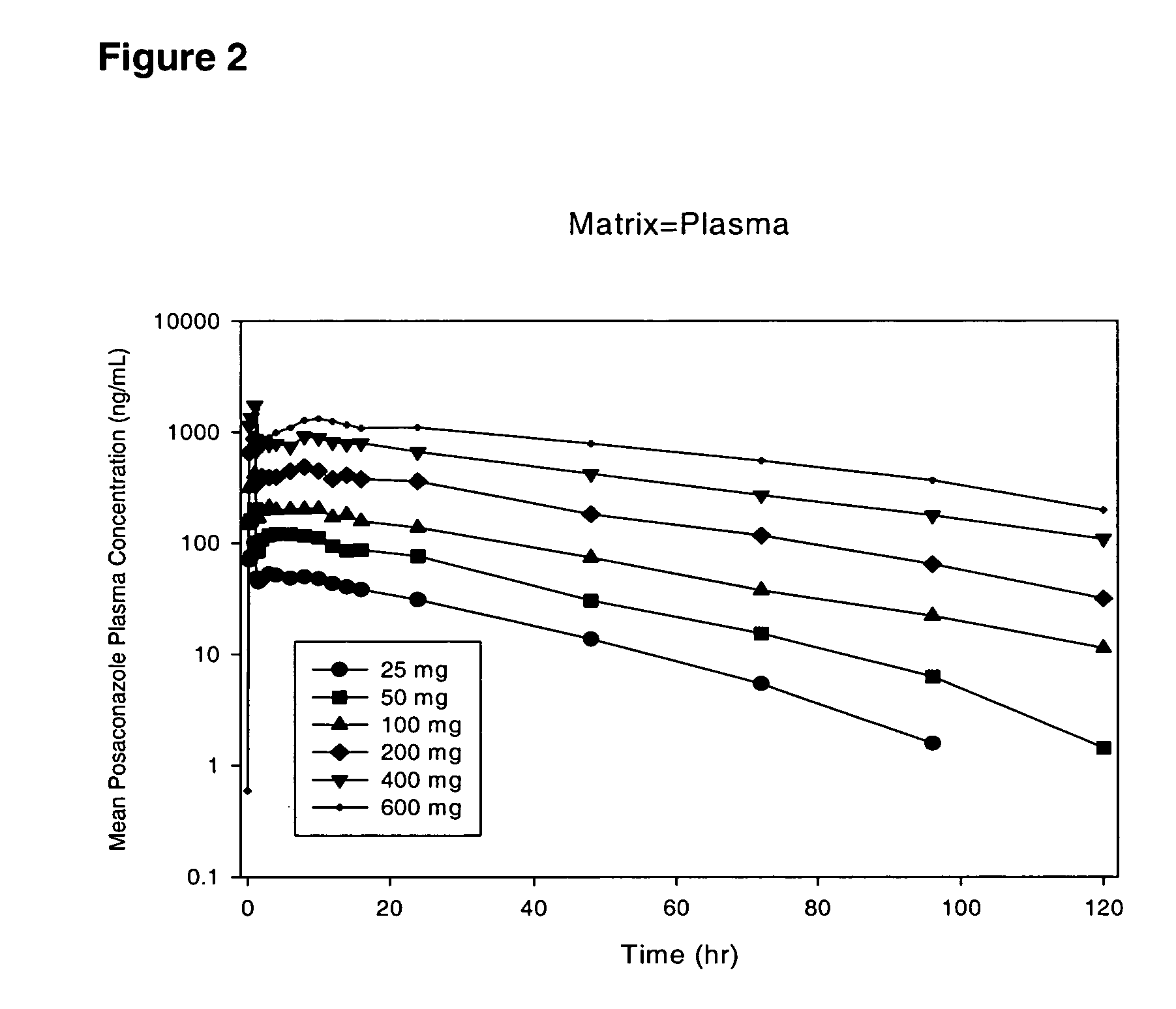
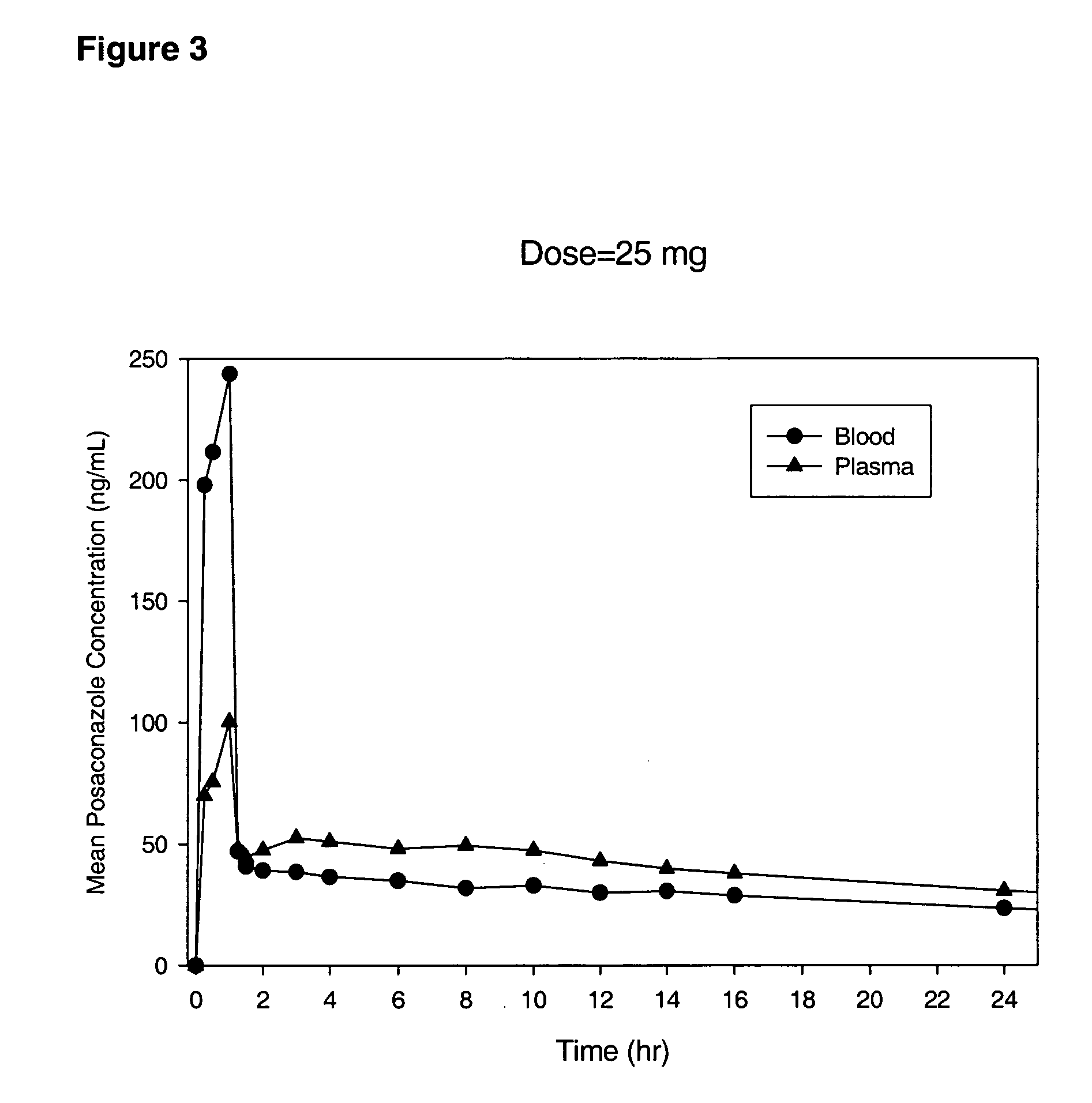
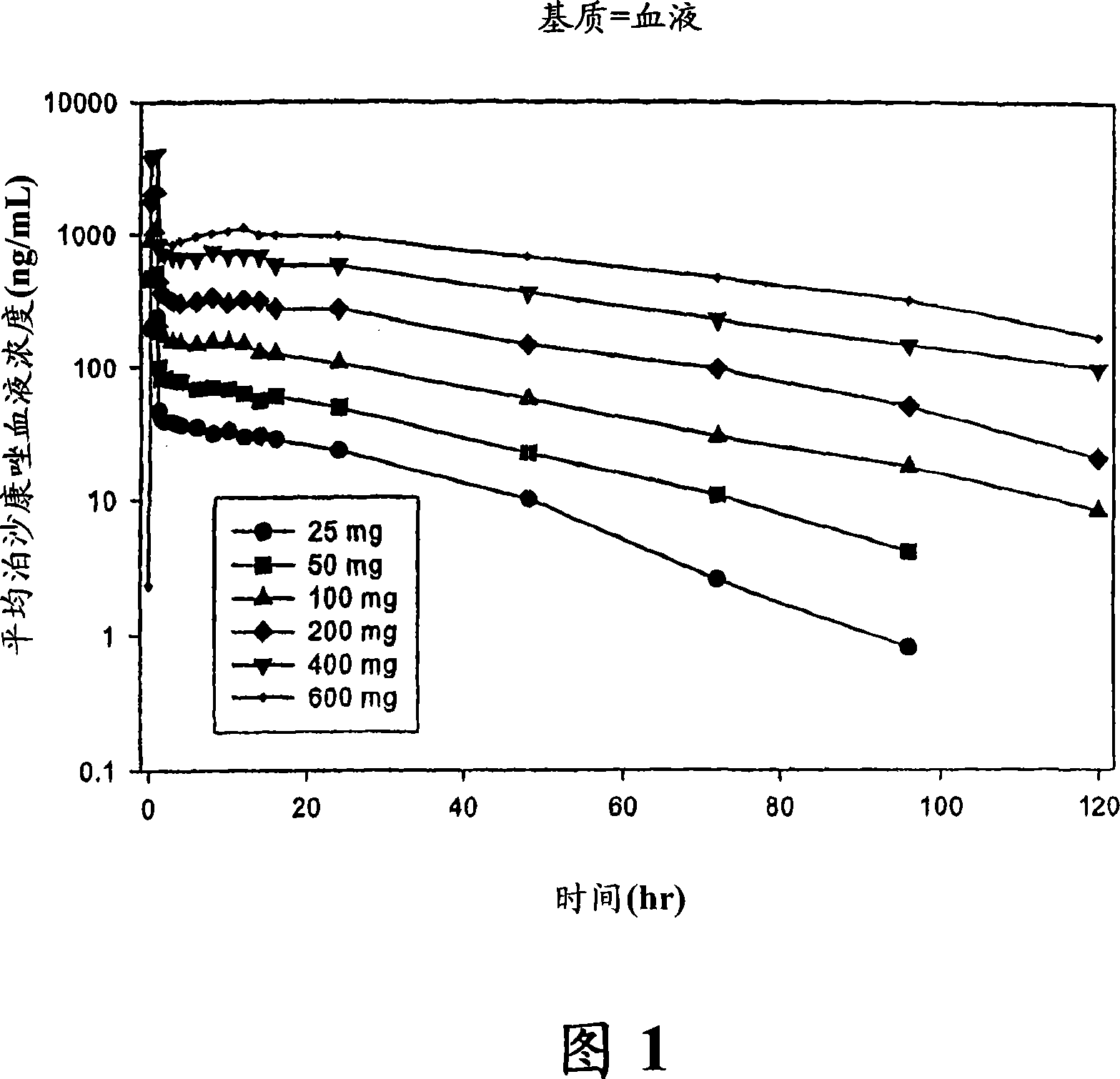
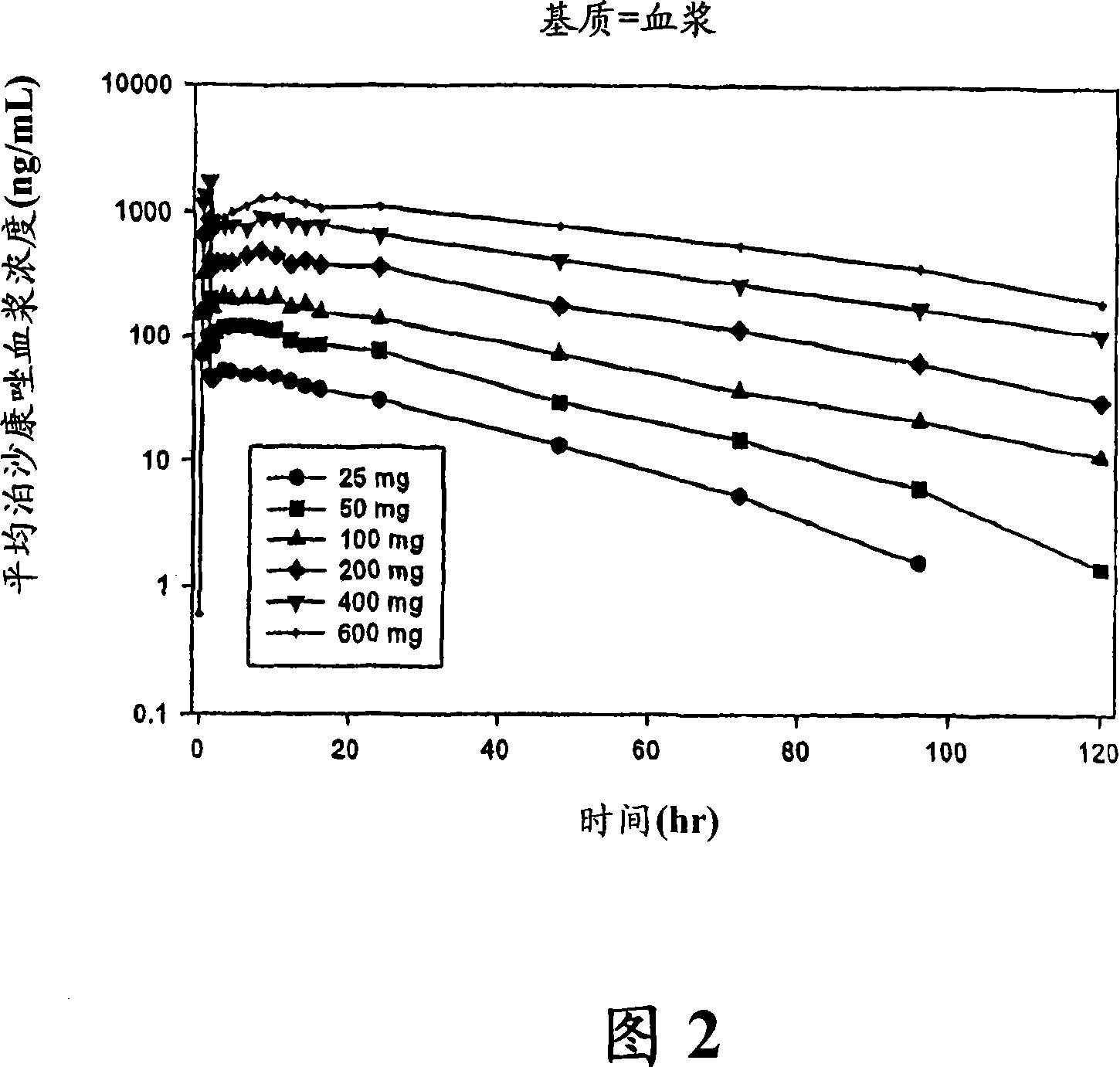
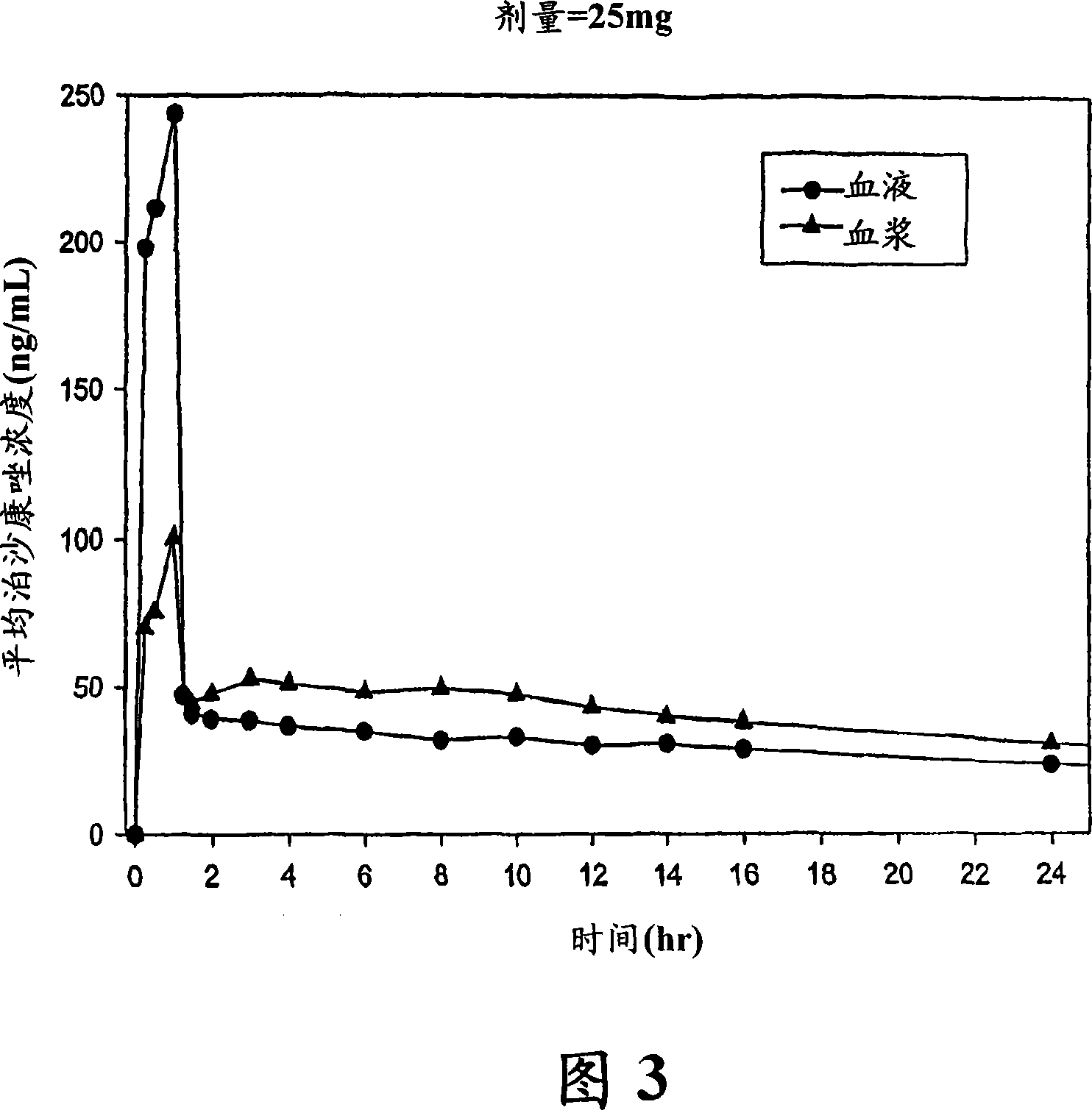
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient Posaconazole in an injectable suspension of particles that is stable when subjected to terminal sterilization. Preferred median particle sizes of between 1.5 and 3.0 microns are found to result in superior pharmacokinetic characteristics, such as those displayed below.
Owner:SCHERING CORP
Preparation of injectable suspensions having improved injectability
InactiveUS20030113380A1Nervous disorderInorganic non-active ingredientsFluid phaseInjection suspension
Injectable compositions having improved injectability. The injectable compositions include microparticles suspended in an aqueous injection vehicle having a viscosity of at least 20 cp at 20° C. The increased viscosity of the injection vehicle that constitutes the fluid phase of the suspension significantly reduces in vivo injectability failures. The injectable compositions can be made by mixing dry microparticles with an aqueous injection vehicle to form a suspension, and then mixing the suspension with a viscosity enhancing agent to increase the viscosity of the fluid phase of the suspension to the desired level for improved injectability.
Owner:ALKERMES PHARMA IRELAND LTD
Supercritical fluid facilitated particle formation in microfluidic systems
The use of supercritical fluids in the production of particles in microfluidic systems is generally described. Small particles with narrow particle size distributions are useful in a wide range of applications. Submicron and micron-sized organic particles may exhibit enhanced properties such as, for example, increased dissolution rates, enhanced pharmaceutical efficacy, and ease of suspension in a carrier medium. Small organic particles may be particularly useful in drug delivery, exhibiting enhanced performance as inhalation aerosols, injectable suspensions, controlled release dosage drugs, transdermally delivered drugs, and the like.Supercritical fluids exhibit unique transport properties such as the ability to simultaneously diffuse through solids (e.g., like a gas) and dissolve materials (e.g., like a liquid). Moreover, supercritical fluids are generally low in viscosity, enabling an enhanced ability to mix with other fluids, for example, upon transitioning from a supercritical to a non-supercritical state. The inventors have unexpectedly discovered that, when used in combination with microfluidic systems, supercritical fluids may be used to continuously and controllably nucleate particle precursor materials to produce, in some embodiments, nano- and microscale particles.
Owner:MASSACHUSETTS INST OF TECH
Embolization using poly-4-hydroxybutyrate particles
Absorbable particles which comprises poly-4-hydroxybutyrate and / or its copolymers are formulated in injectable suspension suitable for prophylactic or therapeutic embolization, which comprises administering to a human or animal the injectable suspension process for producing particles of the poly-4-hydroxybutyrate and / or its copolymer.
Owner:TEPHA INC
Particulate-stabilized injectable pharmacutical compositions of posaconazole
InactiveUS20060009469A1Useful in treatmentOrganic active ingredientsAntimycoticsParticulatesAdditive ingredient
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient posaconazole in an injectable suspension that is stable when subjected to terminal steam sterilization.
Owner:SCHERING CORP
Injectable pharmaceutical suspension comprising posaconazole
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient posaconazole in an injectable suspension that is stable when subjected to terminal steam sterilization.
Owner:SCHERING AG
Alloplastic injectable dermal filler and methods of use thereof
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Alloplastic injectable dermal filler and methods of use thereof
InactiveUS20100322982A1Reduce wrinklesReduce scarsCosmetic preparationsPowder deliveryFiller ExcipientReticular Dermis
A composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent is provided. A method of making a composition comprising an alloplastic injectable suspension for use as a dermal filler comprising a biocompatible and pliable material and a physiologically acceptable suspending agent, said method comprising admixing a biocompatible and pliable material with a physiologically acceptable suspending agent, is also provided. A method of augmenting soft tissue to provide long-term reduction of a skin defect, said method comprising stimulating collagen beneath the skin defect is further provided. In an embodiment of the method of augmenting soft tissue, the stimulation of collagen production is effected by injecting into the deep reticular dermis an a dermal filler, said dermal filler being an alloplastic injectable suspension and comprising a biocompatible and pliable material and a physiologically acceptable suspending agent.
Owner:BOUTROS AYMAN
Stable microbubble suspensions as enhancement agents for ultrasound echography and dry formulations thereof
InactiveUS20010024638A1Ultrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsMicrobubblesInjection suspension
Disclosed are injectable suspensions of gas filled microbubbles in an aqueous carrier liquid usable as contrast agents in ultrasonic echography. The suspensions comprise amphipathic compounds of which at least one may be a laminarized phospholipid as a stabiliser of the microbubbles against collapse with time and pressure. The concentration of phospholipids in the carrier liquid is below 0.01% wt but is at least equal to or above that at which phospholipid molecules are present solely at the gas microbubble-liquid interface. Also disclosed are methods of preparation of the stable suspensions of gas filled microbubbles, as well as dry formulations which, upon reconstitution in an aqueous carrier liquid, will form the injectable suspensions of gas filled microbubbles of the invention and methods of making and using the same The preferred dry formulations are stable when stored over time and at temperatures above ambient temperature and further comprise a lipid acid preserving agent additive.
Owner:BRACCO INT BV
Netilmicin sulfate lyophiled powder injection and preparation method thereof
ActiveCN102525963AGuaranteed singleImprove bioavailabilityAntibacterial agentsOrganic active ingredientsSodium bicarbonateBletilla striata
The invention discloses netilmicin sulfate lyophiled powder injection and a preparation method of the netilmicin sulfate lyophiled powder injection. Netilmicin sulfate and additives are added with water to be prepared into netilmicin sulfate injectable suspension, and the netilmicin sulfate lyophiled powder injection is obtained through freeze drying, wherein the additives are inclusion moleculesand pH conditioning agents, the inclusion molecules are selected from bletilla striata polysaccharide, beta-cyclodextrin, urea, thiourea, beta-cyclodextrin derivatives, deoxycholic acid and starch, and the pH conditioning agents are selected from sodium carbonate, sodium bicarbonate, ammonia water, sulfuric acid and phosphoric acid. Required auxiliary materials are few, the preparation process issimple, the freeze drying process is particularly screened and optimized, and the netilmicin sulfate lyophiled powder injection and the preparation method has the characteristics that the stability is high, the biological availability is high, the production cost is low, the quality is safe and reliable, and the like.
Owner:WUHAN PUSHENG PHARMA
Powder manufacturing method suitable for inhalation-type drug administration
The invention relates to a powder manufacturing method capable of accurately controlling aerodynamic force size of power particles and is suitable for inhalation-type drug administration. In the method, after 3,6-double (4-double fumaryl aminobutyl)-2,5-diketopiperazine or salt substituent thereof is dissolved into alkaline solution, drug is directly added into the solution, or the alkaline solution and the acid solution are mixed under high shearing environment to form injectable suspension, and then drug is added; and the solution or the injectable suspension is dried to prepare drug particles suitable for aerodynamic force size (0.5-10 micrometers). The drug particles are transported to positions, such as patient nasal cavity, bronchus, lungs and the like by a dry powder inhaler; the characteristics that drug particles are quickly dissolved in human body environment with the pH value of 6.4-8 and drug particles are stable when pH value is less than or equal to 6.4 or more than 8 are utilized to realize quick and effective adsorption of drug.
Owner:于清
Nano-micro structure silibinin drug composite powder and preparation method thereof
ActiveCN101780047AGood redispersibilityEvenly dispersedOrganic active ingredientsPowder deliveryMicro structureSolubility
The invention relates to a nano-micro structure silibinin drug composite powder and a preparation method thereof. The silibinin drug is dissolved into an organic solvent which is mutually soluble with the water, the mixture is added into a water solution containing water-solubility pharmaceutical excipients to obtain nano silibinin drug particle injectable suspension, the injectable suspension is atomized and dried to obtain the nano-micro structure silibinin drug composite powder. The composite powder has good redispersibility and is dispersed in the water to obtain the uniform injectable suspension with the grain diameter of the drug being less than 1000 nm. The method has simple operation, low cost and promising industrialized production prospect. The powder has fast dissolution rate and high biological utilization rate, and can be used for preparing tablet, capsule, granules or suspensions.
Owner:BEIJING UNIV OF CHEM TECH
Veterinary aqueous injectable suspensions containing florfenicol
The present invention relates to pharmaceutical compositions in the form of aqueous injectable suspensions for veterinary use, comprising sterile and micronized florfenicol or a substantially water-insoluble complex in a concentration up to 500 mg / ml. , co-crystalline or salt form of florfenicol. The suspension can achieve parenteral antibacterial treatment of animals with excellent overall and local tolerability through a limited number of injections. The suspension had limited settling on standing and after transport, was easily resuspended, and had good injectability.
Owner:CALLUNA PHARMA
Compound preparation for treating diseases caused by poultry sensitive bacteria and preparation method thereof
InactiveCN101987105ALess irritatingProlong the action timeAntibacterial agentsTetracycline active ingredientsEscherichia coliAntioxidant
The invention relates to a compound preparation for treating diseases caused by poultry sensitive bacteria and a preparation method thereof. The compound preparation is a long-acting injectable suspension prepared from florfenicol and occrycetin as main medicines and comprises 2.0-20.0% (W / V) of florfenicol, 5.0-30.0% (W / V) of occrycetin, 0.05-0.40% (W / V) of antioxidant, 1-10% (W / V) of antiallergic factor synergist, 0.01-0.04% (W / V) of complexing agent, 0.01-15% (W / V) of suspending agent, 1.0-10.0% (W / V) of flocculating agent, 0.01-0.5% (W / V) of preservative and 60-100% (W / V) of water for injection. The liquid medicine of the invention has stable performance and can effectively overcome the defects of instable performance, easy color change, precipitation and failure and the like of injections. The compound preparation has small irritativeness, low cost and simple process and can prolong the residence time of medicine in body and increase the bioavailability of the medicine. The compound preparation can be used for preventing and treating infectious diseases caused by poultry sensitive bacteria, such as pasteurellosis, colibacillosis, salmonellosis, contagious pleuropneumonia, mycoplasmlpneumonia of swine, acute respiratory infection and the like.
Owner:TIANJIN RINGPU BIO TECH
Preparation method of calamine lotion
InactiveCN102379897AImprove textureSlow stratificationInorganic active ingredientsPharmaceutical delivery mechanismOver the counter drugsGlycerol
The invention discloses a preparation method of a calamine lotion, and the product belongs to the field of pharmacy. The calamine lotion is the over the counter drug of dermatological pharmacy, is the light pink injectable suspension in appearance, is precipitated after being placed, but is the uniform injectable suspension after being shaken out. Calamine and zinc oxide which are contained in the product have the constriction and protection effects, and also have weaker antiseptic function, so the calamine lotion can be applied in acute pruritus skin diseases and various rashes, such as urticaria, prickly heat, eczema and the like. The product is produced from the raw materials of: calamine powder, zinc oxide powder, glycerol, polysorbate, bromogeramine and distilled water. The calamine lotion which is prepared by the method is fine, has low layered sedimentation velocity, is not easy to block after long-term storage, and is the calamine lotion with good quality.
Owner:殷峰林
Iodine, selenium and cobalt composite package for animal feed and preparation method and application method thereof
ActiveCN102422985AReduce generationReduce mutual antagonismAnimal feeding stuffSelenium CompoundInjectable Suspension
The invention provides an iodine, selenium and cobalt composite package for animal feed and a preparation method and an application method thereof, wherein the preparation method comprises but is not limited to the following steps: weighing the powder of iodine compound, selenium compound and cobalt compound according to the requirement of an animal, and uniformly mixing the compounds with water to obtain injectable suspension; and performing spray drying on the injectable suspension to obtain the iodine, selenium and cobalt composite package. By the spray drying, the iodine, selenium and cobalt composite package prepared by the method has regular particles and no powder on surface so as to reduce the dust during premixing feed additives and the loss of the product when the feed additivesare added into the animal feed, and maintain and optimize the production environment of production staff, and improve the mixing uniformity of the feed and the iodine, selenium and cobalt composite package in the processing process of the animal feed.
Owner:XINGJIA BIO ENG CO LTD
Stable microbubble suspensions as enhancement agents for ultrasound echography and dry formulations thereof
InactiveUS20040180004A1Ultrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsMicrobubblesChemical compound
Disclosed are injectable suspensions of gas filled microbubbles in an aqueous carrier liquid usable as contrast agents in ultrasonic echography. The suspensions comprise amphipathic compounds of which at least one may be a laminarized phospholipid as a stabiliser of the microbubbles against collapse with time and pressure. The concentration of phospholipids in the carrier liquid is below 0.01% wt but is at least equal to or above that at which phospholipid molecules are present solely at the gas microbubble-liquid interface. Also disclosed are methods of preparation of the stable suspensions of gas filled microbubbles, as well as dry formulations which, upon reconstitution in an aqueous carrier liquid, will form the injectable suspensions of gas filled microbubbles of the invention and methods of making and using the same The preferred dry formulations are stable when stored over time and at temperatures above ambient temperature and further comprise a lipid acid preserving agent additive.
Owner:BRACCO INT BV
Combination therapy
The invention relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof, and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof. The invention also relates to pharmaceutical compositions comprising: (a) at least one angiotensin receptor blocker or a pharmaceutically acceptable salt thereof; and (b) at least one chemokine receptor pathway inhibitor or a pharmaceutically acceptable salt thereof which inhibits a component of the chemokine receptor pathway other than the chemokine receptor. Oral sustained release pharmaceutical compositions comprising the pharmaceutical composition, as well as injectable sustained release pharmaceutical compositions comprising the pharmaceutical composition are described. The invention further relates to tablets, capsules, injectable suspensions, and compositions for pulmonary or nasal delivery comprising the pharmaceutical composition. Also described are: methods for assessing the efficacy of the pharmaceutical composition; methods for assessing the inhibition or partial inhibition activity of the pharmaceutical composition; methods for the treatment, amelioration or prevention of a condition or disease comprising administering to a subject a therapeutically effective amount of the pharmaceutical composition; and the use of the pharmaceutical composition for the manufacture of a dosage form for the treatment of a disease.
Owner:DIMERIX BIOSCIENCES PTY LTD
Preparation method and application of magnetically loaded ionic liquid microsphere immobilized cells
ActiveCN108949738AGood sphericityImprove mass transfer effectOn/in organic carrierOn/in inorganic carrierContinuous fermentationMicrosphere
The invention discloses a preparation method and application of magnetically loaded ionic liquid microsphere immobilized cells. The preparation method comprises the following steps of: mixing sodium alginate, a Fe3O4 / CS magnetic material and 1-butyl-3-methylimidazolium hexafluorophosphate to obtain a mixed solution; mixing the mixed solution with cell suspension to obtain injectable suspension; and immobilizing the injectable suspension by using CaCl2 as a crosslinking agent to obtain the magnetically loaded ionic liquid microsphere immobilized cells. As the immobilized cells prepared by the method have the magnetism, the separation and continuous fermentation of immobilized cells are facilitated, and ionic liquid loaded on a magnetic material greatly improves the catalytic activity of theimmobilized cells.
Owner:JIAXING UNIV
Suspension composition containing doxycycline and florfenicol for injection and technique for preparing the same
InactiveCN101502487ALower doseReduced responseAntibacterial agentsTetracycline active ingredientsHigh concentrationOrganic solvent
The invention relates to a high-concentration injectable suspension combination containing doxycycline and florfenicol and a preparation process thereof. The suspension combination comprises the following components: 4.0 % to 30.0% (W / V) of florfenicol, 4.0 % to 30.0% (W / V) of doxycycline, 0.05% to 0.40% (W / V) of anti-oxidant, 0.01% to 0.04 % (W / V) of complexing agent, 0.01% to 15% (W / V) of suspending agent, 1.0% to 10.0% (W / V) of flocculating agent, 0.01% to 0.5% (W / V) of antiseptic agent and 60% to 100% of water for injection. The invention has the advantages that the component content of the drug is high, the administration volume is small, animal suffers less pain and irritation, adverse reactions, safety accidents and the like caused by the organic solvent are reduced, the preparation cost is low and the preparation is simple. The invention is applicable in preventing and treating the mixed bacterial infection diseases of domestic animals and the like.
Owner:TIANJIN RINGPU BIO TECH
Preparation method of small unilamellar vesicle liposome of ivermectin
InactiveCN101664389AEvenly distributedImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolEvaporation
The invention discloses a preparation method of small unilamellar vesicle liposome of ivermectin, and relates to a preparation method of ivermectin liposome, which solves the problems that the existing ivermectin preparation has short effective date, and needs to deliver drug repeatedly and the sustained release macro-pill of the ivermectin has high toxicity. The method comprises the following steps of: adding raw ivermectin powder in organic solution of soya bean lecithin and cholesterol, then carrying out pressure reduction, rotation and evaporation to the obtained injectable suspension tilla layer of thin film is formed at the inner wall of a reactor, then dissolving the thin film in PBS solution, adding glass beads into a rotary-type oscillator, shaking to wash the film for 1 hour, then standing for 1 hour, and carrying out ultrasonic oscillation so as to obtain the small unilamellar vesicle liposome of ivermectin. The liposome obtained by the preparation method has similar structure as a biofilm, can be biodegraded in the organism, does not generate toxic substances, and can be slowly and continuously released as embedded drug in a storage mode, thus being capable of prolonging effective horizontal period of the drug and reducing drug delivery frequency; and the packet rate of the small unilamellar vesicle liposome of ivermectin is 89.5%.
Owner:INST OF ANIMAL SCI & VETERINARY TIBET ACADEMY OF AGRI & ANIMAL HUSBANDRY SCI
Coating material for complex tablets and preparation method thereof
ActiveCN102526743APrevent precipitationSolving Film Coating ProblemsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAcrylic resinGlycerol
The invention relates to a coating material for complex tablets and a preparation method thereof. The coating material is composed of following raw materials by weight: 3 parts to 8 parts of acrylic resin solution, 12 parts to 20 parts of red iron oxide injectable suspension, 1 part to 5 parts of glycerol, 1 part to 5 parts of talcum powder and 60 parts to 80 parts of purified water. The complex tablet thin membrane coated tablet made of the coating material is even in tablet surface color and strong in coating membrane adhesion. Accelerated tests and long term experiments show that the coating material is good in humidity resistance, can effectively prevent volatile element borneol from sublimating and dissolving out, and guarantees quality stability of products in an efficacy period.
Owner:ZHENGZHOU RUILONG PHARMA
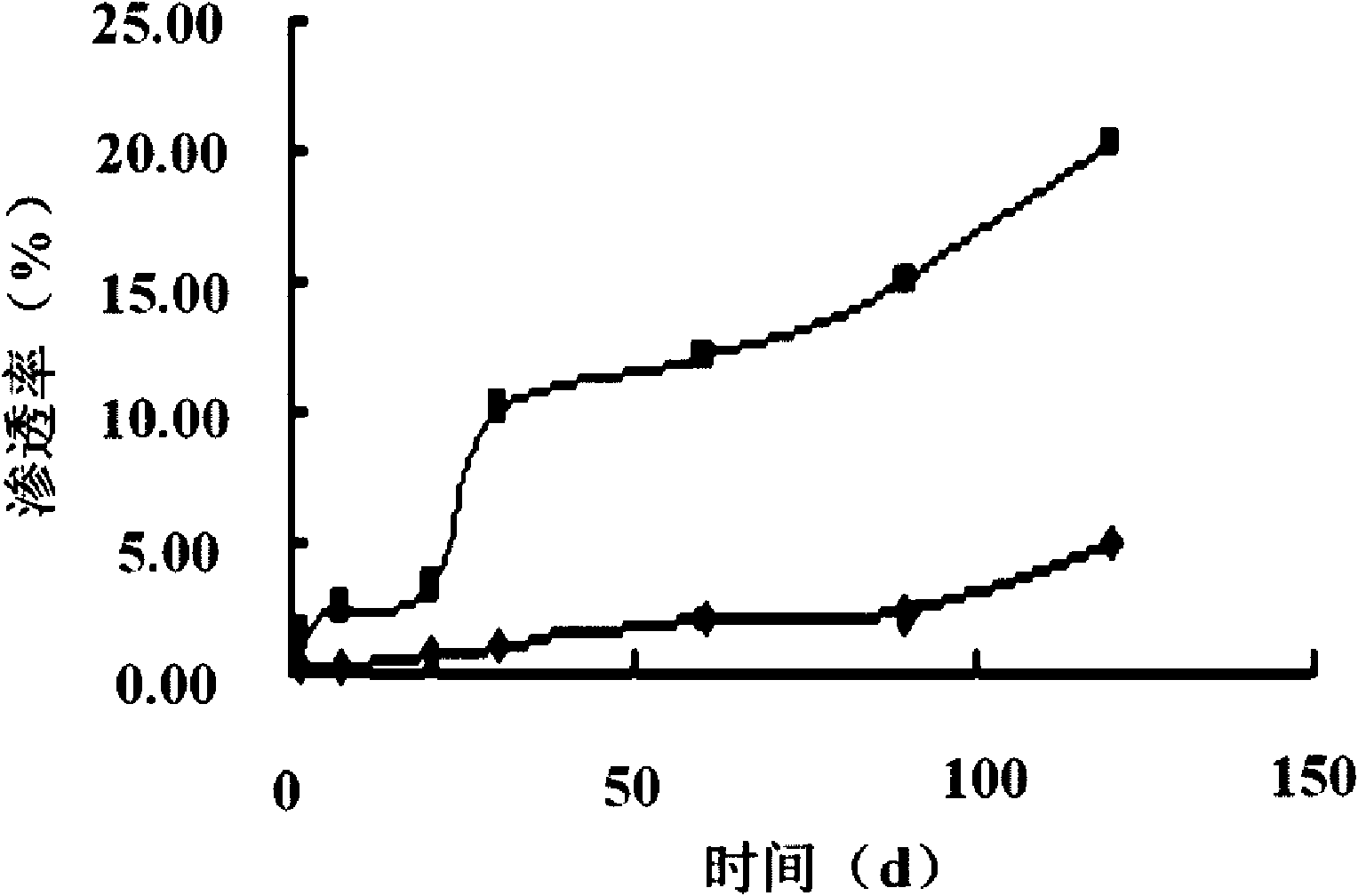
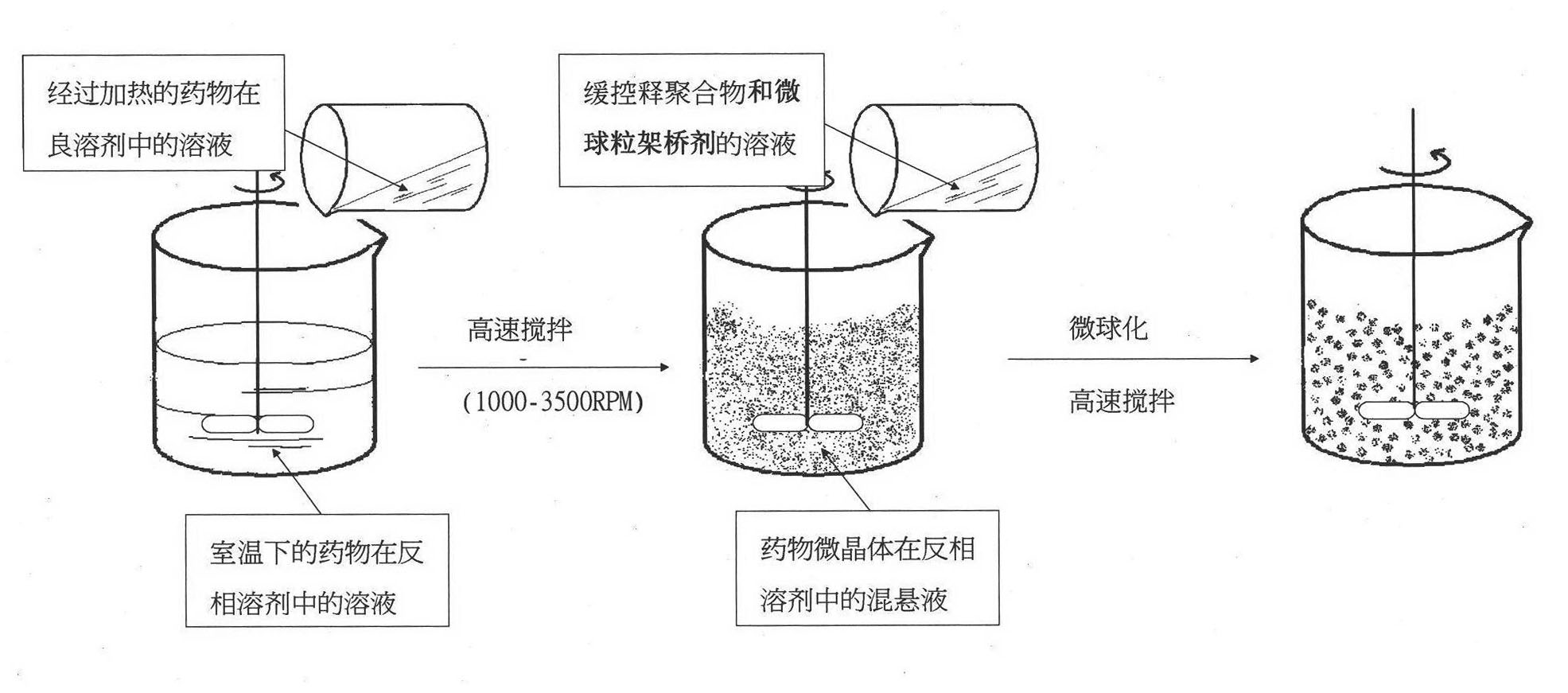
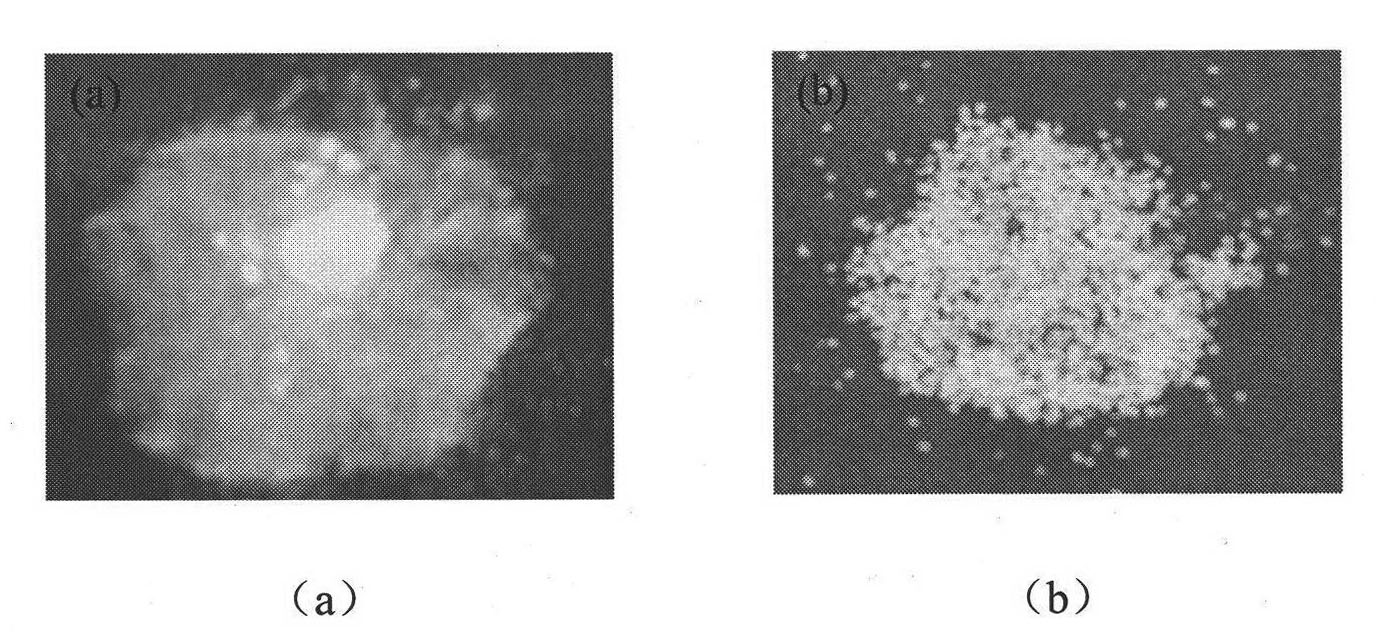
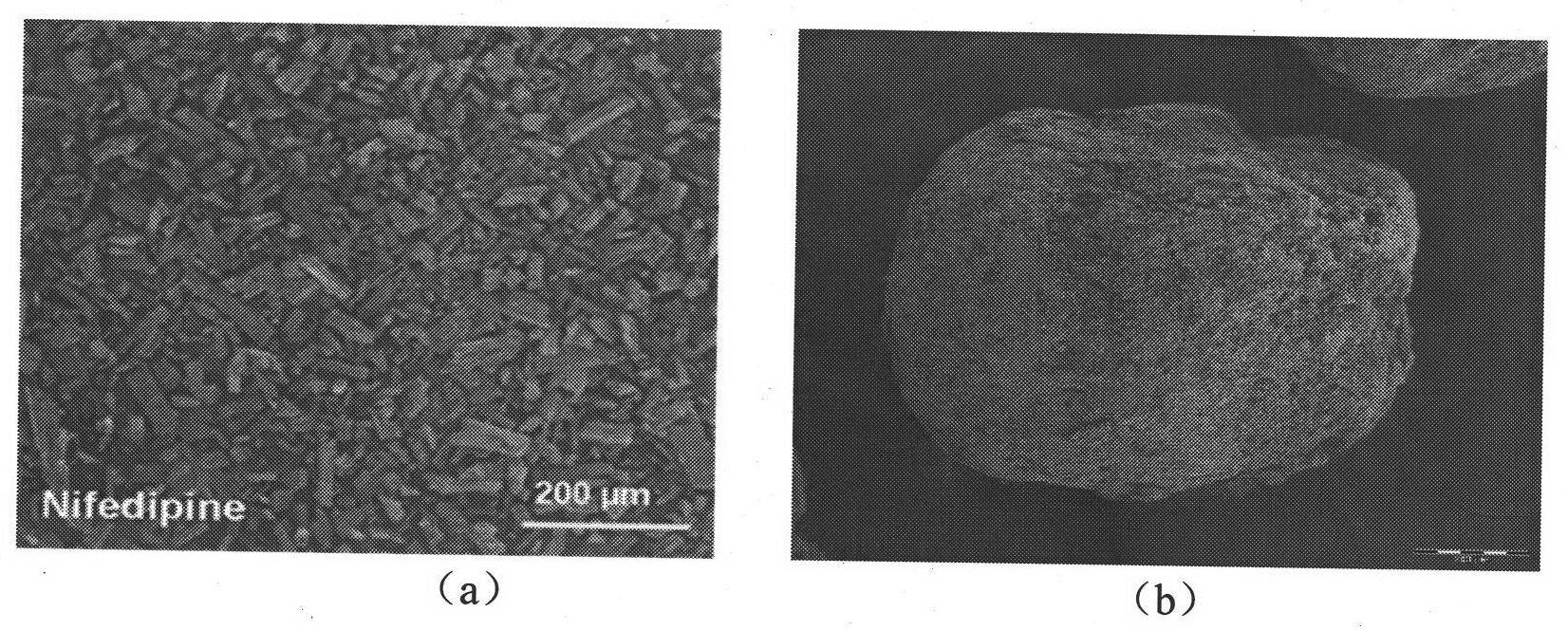
Crystal separating drug sustained-release microspherule and preparation method thereof
ActiveCN101874784AShorten the timeImprove dry powder flowabilityOrganic active ingredientsGranular deliveryDrugs solutionInjectable Suspension
The invention discloses a crystal separating drug sustained-release microspherule comprising the following components in parts by weight: 4-9 parts of water-insoluble drug, 0.1-2 parts of sustained-release polymer and 1-4 parts of microspherule bridging agent. In addition, the invention also discloses a preparation method of the crystal separating drug sustained-release microspherule, comprising the following steps: 1) dissolving water-insoluble drug into good solvent to form drug solution; 2) adding the drug solution into the opposite phase solvent of the drug, and stirring; 3) after the injectable suspension of drug microcrystal in the opposite phase solvent is completely formed and stabilized, adding solution containing the sustained-release polymer, the microspherule bridging agent and the solvent, and stirring; and 4) after the drug microspherule is completely formed and the solvent for dissolving the sustained-release polymer and the microspherule bridging agent is completely volatilized, stopping stirring, and filtering and drying the drug microspherule. The method can obtain the granule diameter with wider range, more regular and smooth surface and more controllable inner and outer releasing rate of drug.
Owner:PIVOT PHARMA TECH SHANGHAI
Sea cucumber rose liquor and preparation method thereof
InactiveCN101760381APromote absorptionImprove immunityAlcoholic beverage preparationInjectable SuspensionSea cucumber
The invention provides sea cucumber rose liquor and a preparation method thereof. The preparation method is characterized by comprising the following steps: taking high-quality food wine as substrate, matching with injectable suspension of sea cucumber and rose, and then carrying out the process steps of soaking, emulsifying and homogenizing, high-precision filtering, seasoning, encapsulating and the like. The sea cucumber rose liquor comprises the following components by weight percent: 70-90% of food wine and 15-35% of injectable suspension of sea cucumber and rose. In the invention, the compatibility of raw material components of the sea cucumber and rose is scientific, namely, the outer part can be nourished by the inner part, just like double swords in combination; and the liquor can relieve fatigue, strengthen human immunity and build the body after being drunk in a proper amount for a long time.
Owner:段茂宏
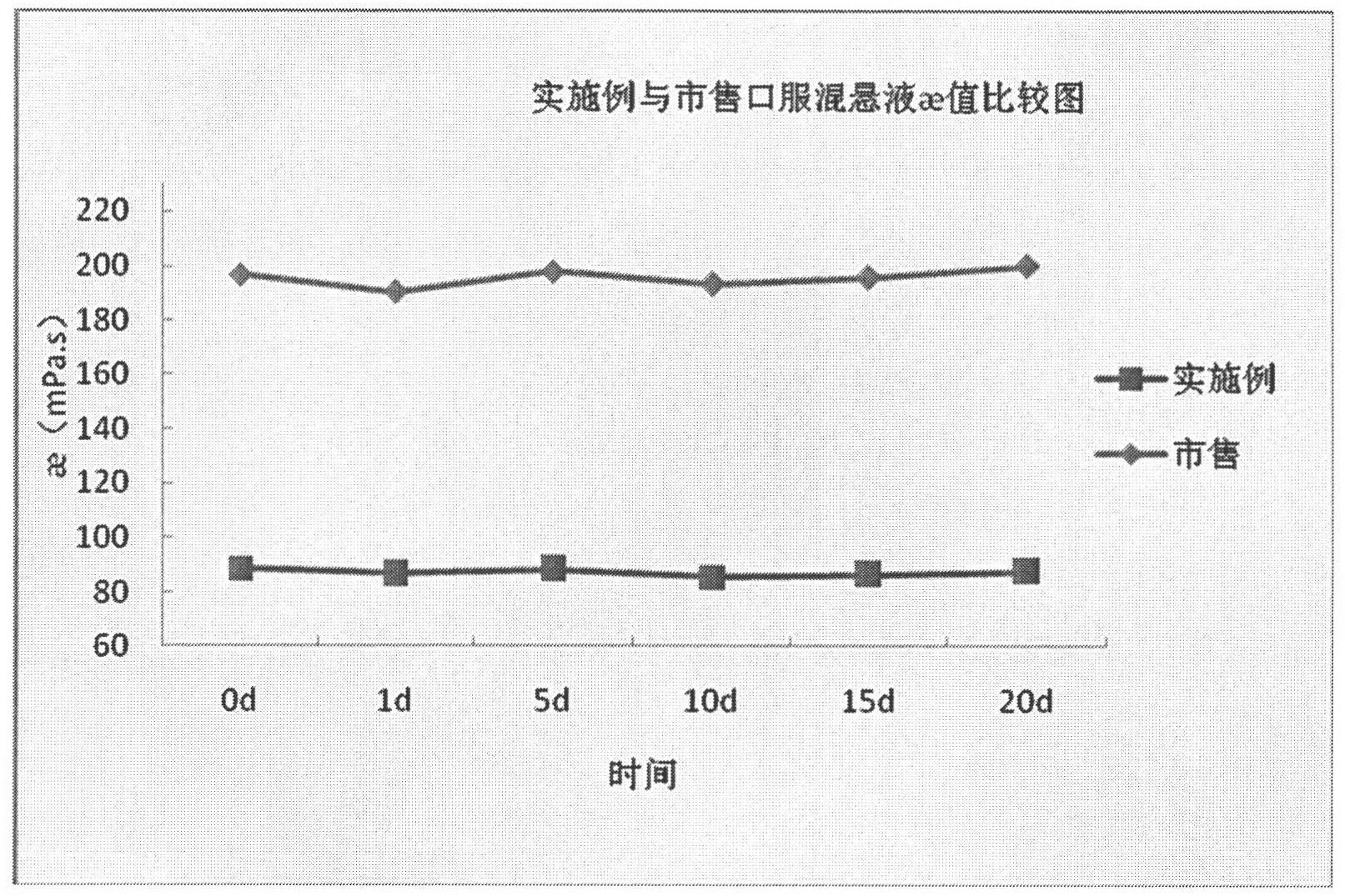
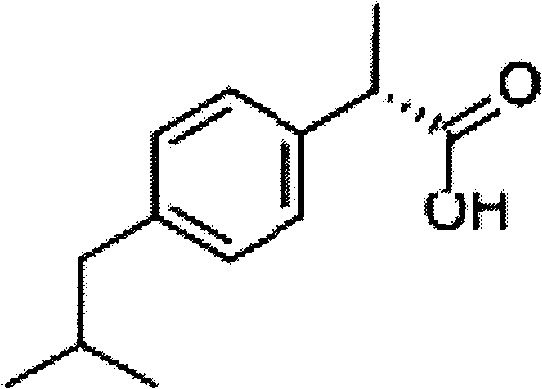
Dex-ibuprofen injectable suspension and preparation method thereof
The invention discloses a dex-ibuprofen injectable suspension and a preparation method thereof. The preparation consists of dex-ibuprofen, low-acyl gellan gum, carrageenan, pectin and a colloidal property reinforcing agent. The dex-ibuprofen injectable suspension prepared with the method is a thixotropic liquid, and has the characteristics of low viscosity, high suspension supporting property and permanent lamination resistance, so that the sizes of dex-ibuprofen particles can be kept stable during long-term storage.
Owner:陆荣政
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com