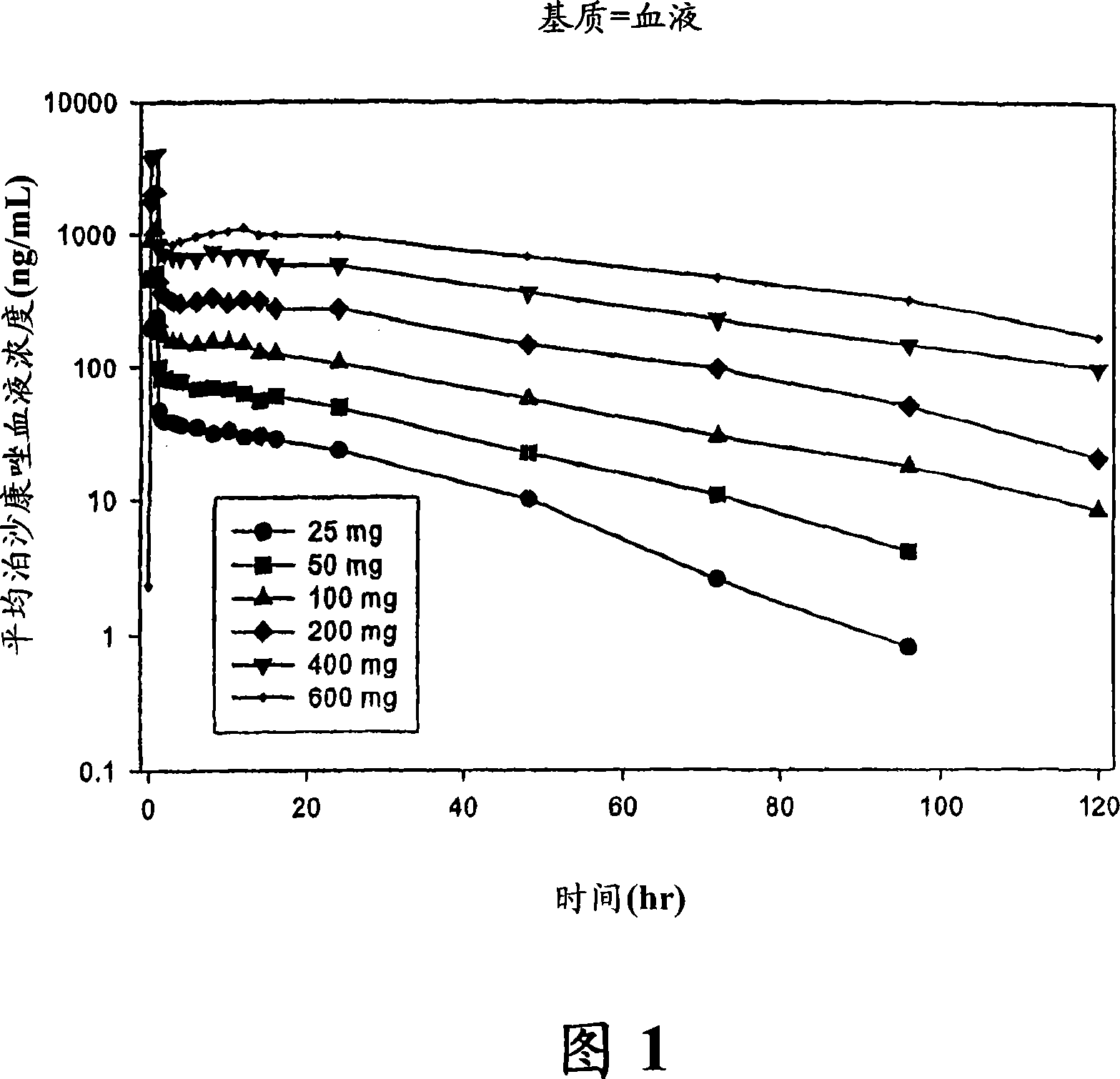
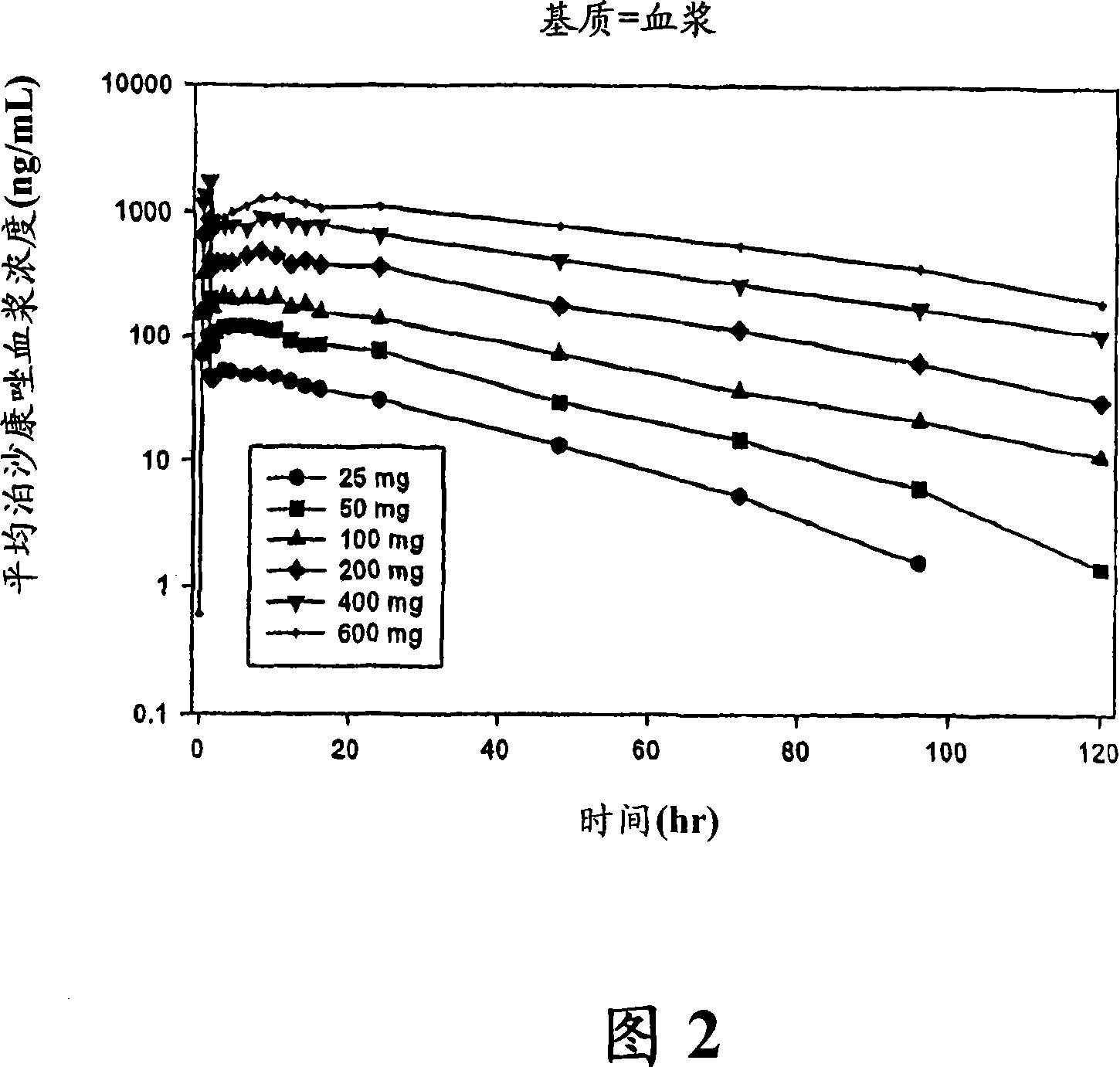
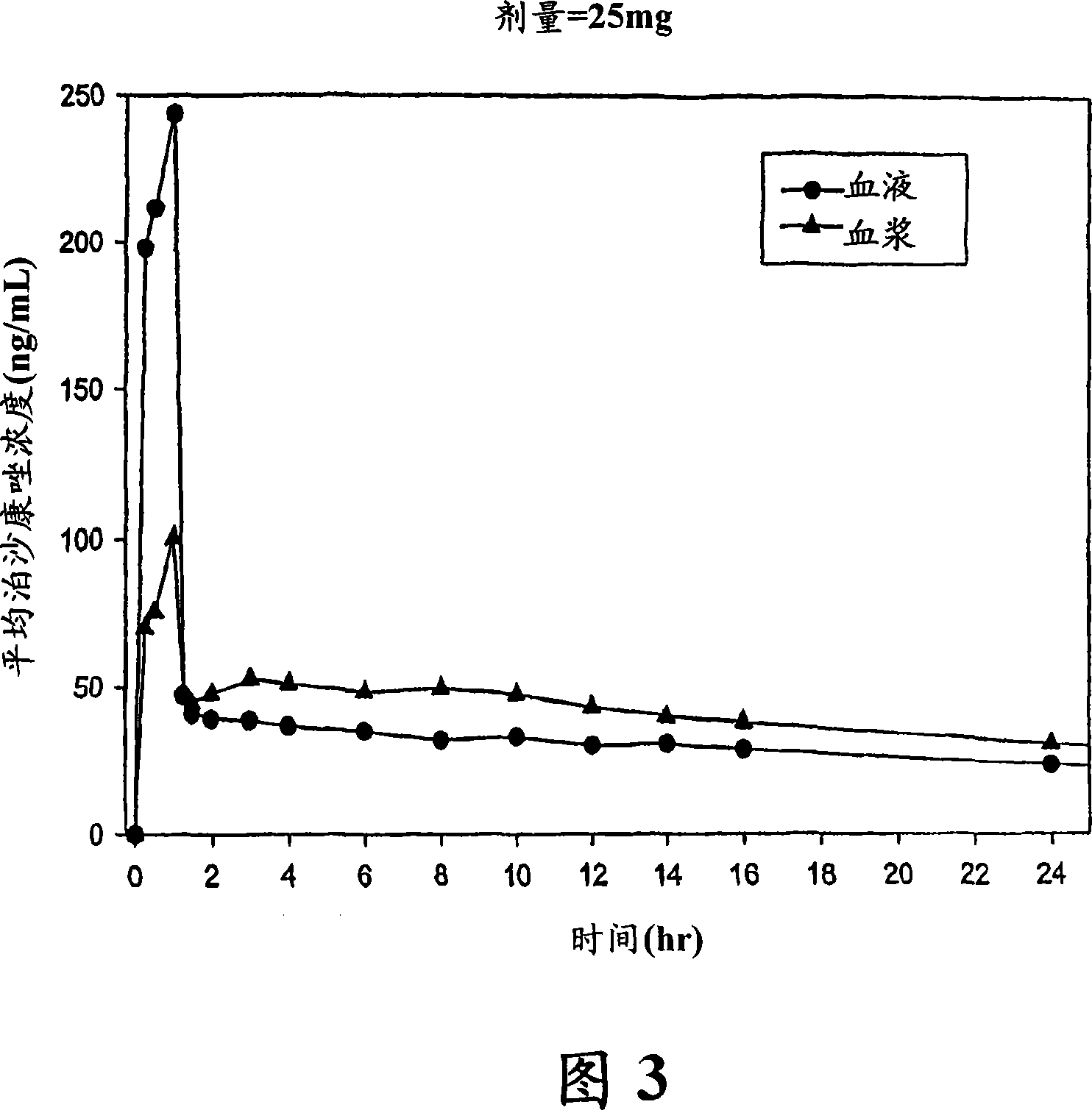
Injectable pharmaceutical suspension comprising posaconazole
A technology of posaconazole and medicine, applied in the field of preparations for treating infection, can solve the problems such as undisclosed posaconazole suspension for injection
Inactive Publication Date: 2007-06-27
SCHERING AG
View PDF10 Cites 18 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0006] However, none of the above references discloses a posaconazole injectable suspension that remains stable through terminal steam sterilization and throughout the shelf life of the product.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0220] Element
[0221] Note: In Example 1 the pH is 7.4.
Embodiment 2
[0223] Element
[0224] Note: In Example 2 the pH is 6.4.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient posaconazole in an injectable suspension that is stable when subjected to terminal steam sterilization.
Description
field of invention [0001] The present invention relates to formulations for the treatment of infections. In particular, these formulations include injectable suspensions of the active pharmaceutical ingredient posaconazole which are stable upon terminal steam sterilization and throughout the shelf life of the product. Background of the invention [0002] Posaconazole is an antifungal drug represented by the following chemical structure: [0003] [0004] It has been developed as an oral suspension (40 mg / ml) by Schering Corporation, Kenilworth, NJ. under the trade name NOXAFIL(R), see for example US Patent Nos. 5,703,079, 5,661,151, WO 02 / 80678 published October 17, 2002 and EP 1 372 394 published January 2, 2004. Additionally, other formulations of posaconazole have been disclosed. Solid formulations (capsules / tablets) of posaconazole are disclosed in US Patent Nos. 5,972,381 and 5,834,472. Finally, topical forms of posaconazole such as lotions, creams, ointments or ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/10A61K31/496A61P33/00A61P31/10A61K47/02A61K47/24A61K47/26
CPCA61K47/26A61K47/24A61K47/02A61P31/10A61P33/00A61P43/00A61K31/496
Inventor L·维奇-拉科什马南S·乌格伍V·桑德维斯C·赫达洛R·S·哈尔G·克里希纳Z·王M·塔格列提
Owner SCHERING AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com