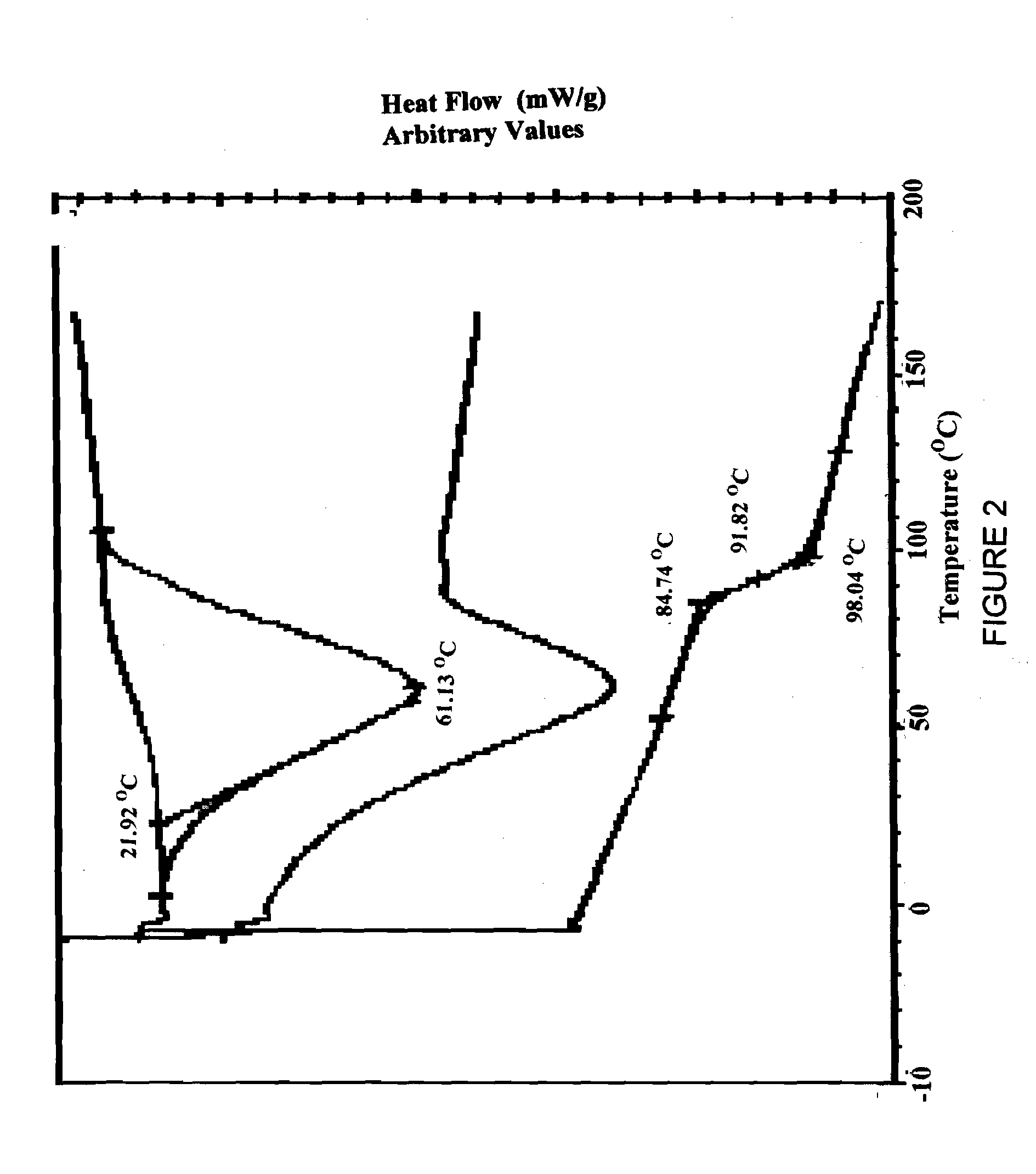
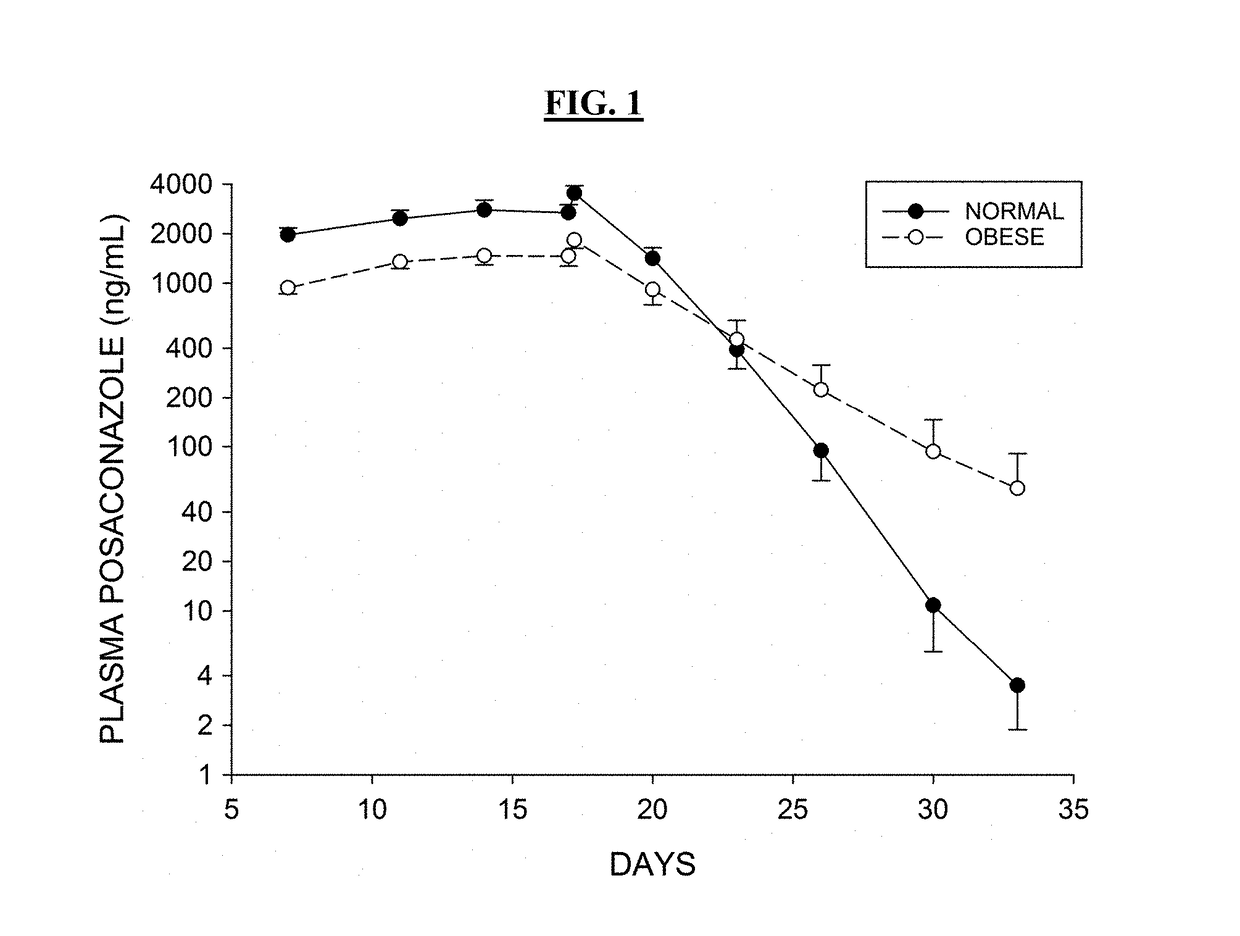
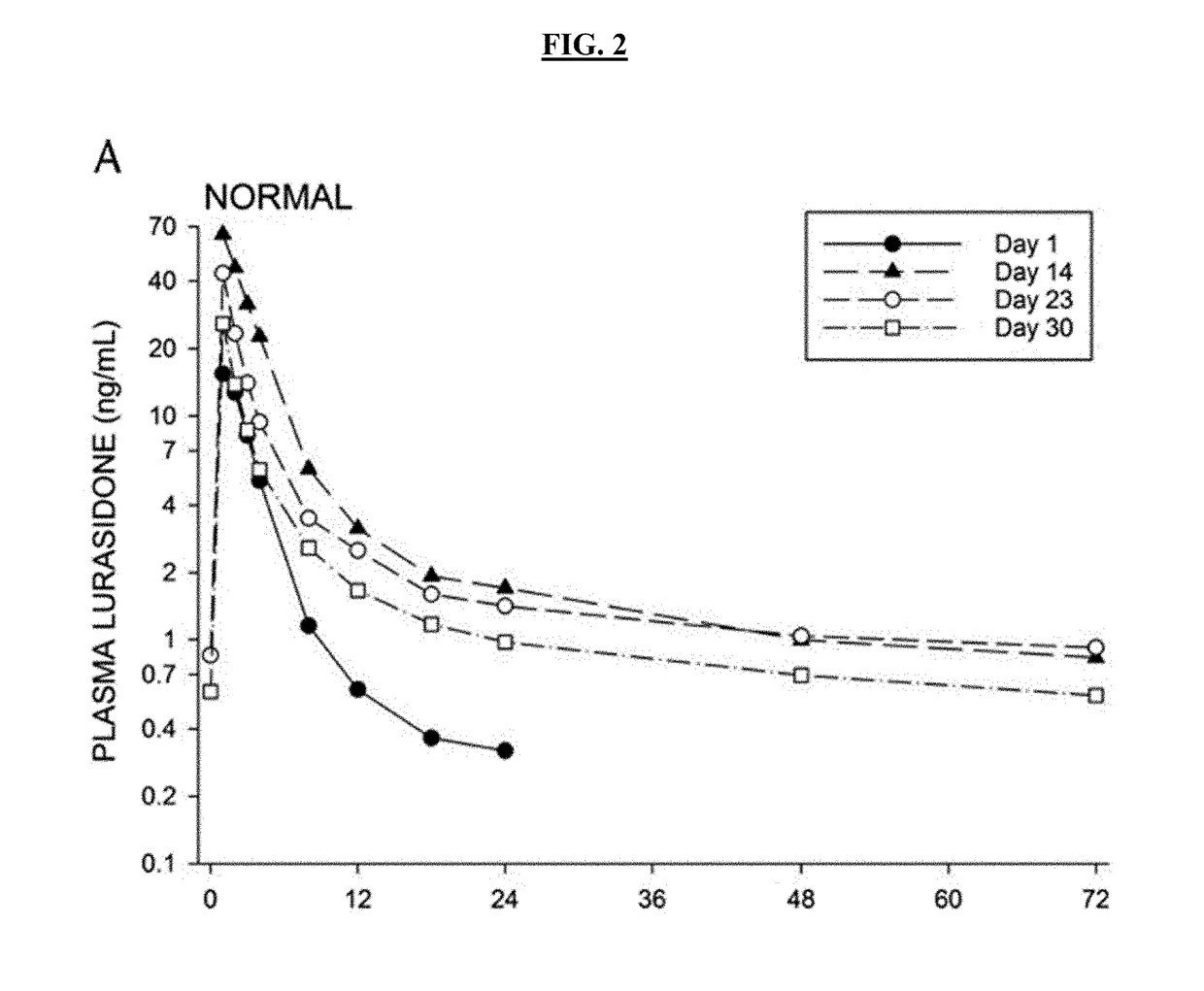
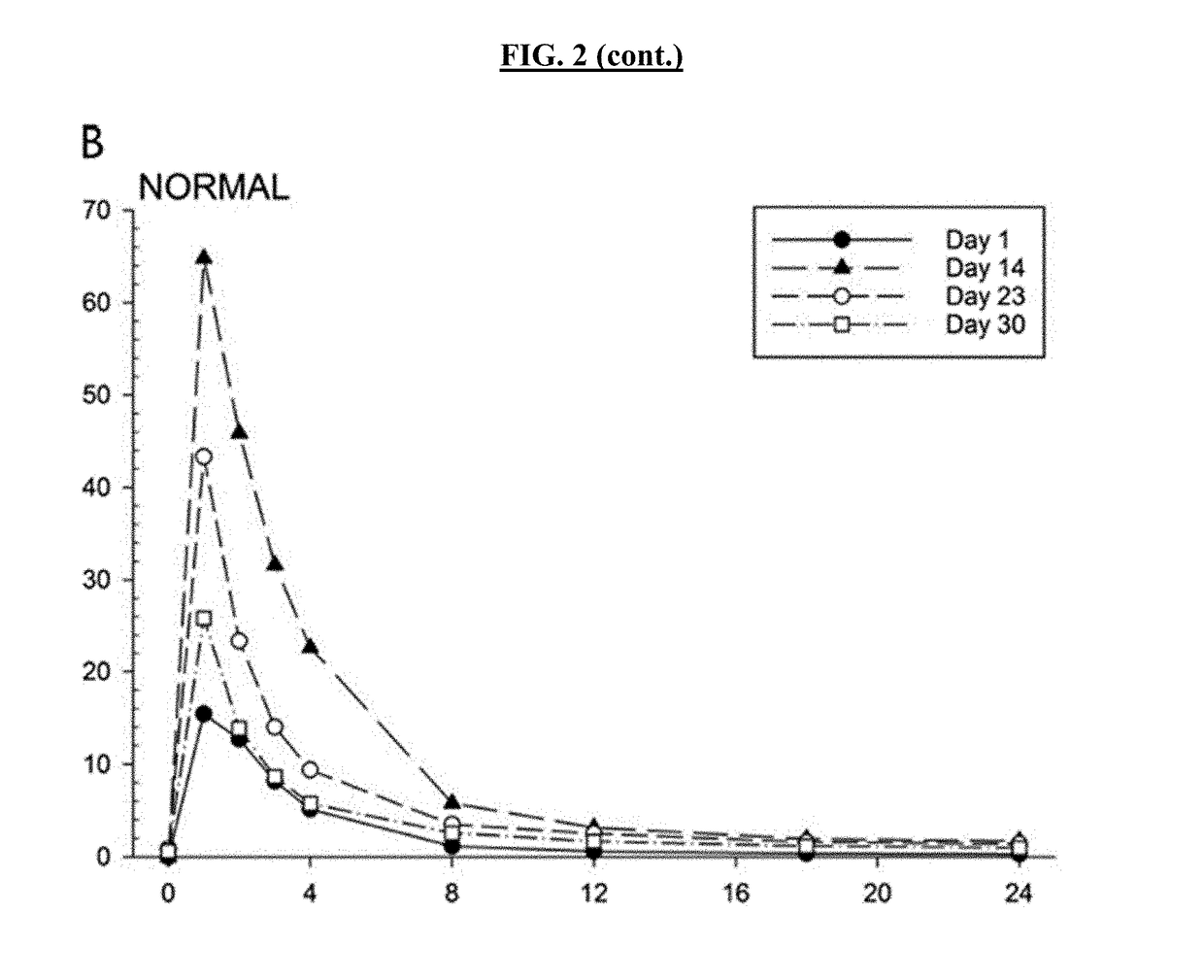
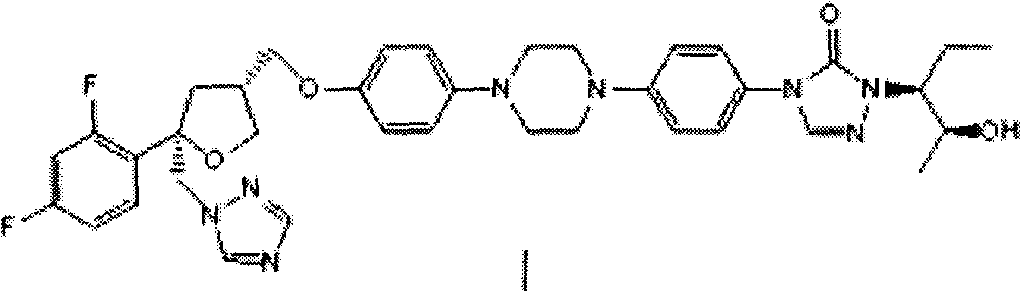
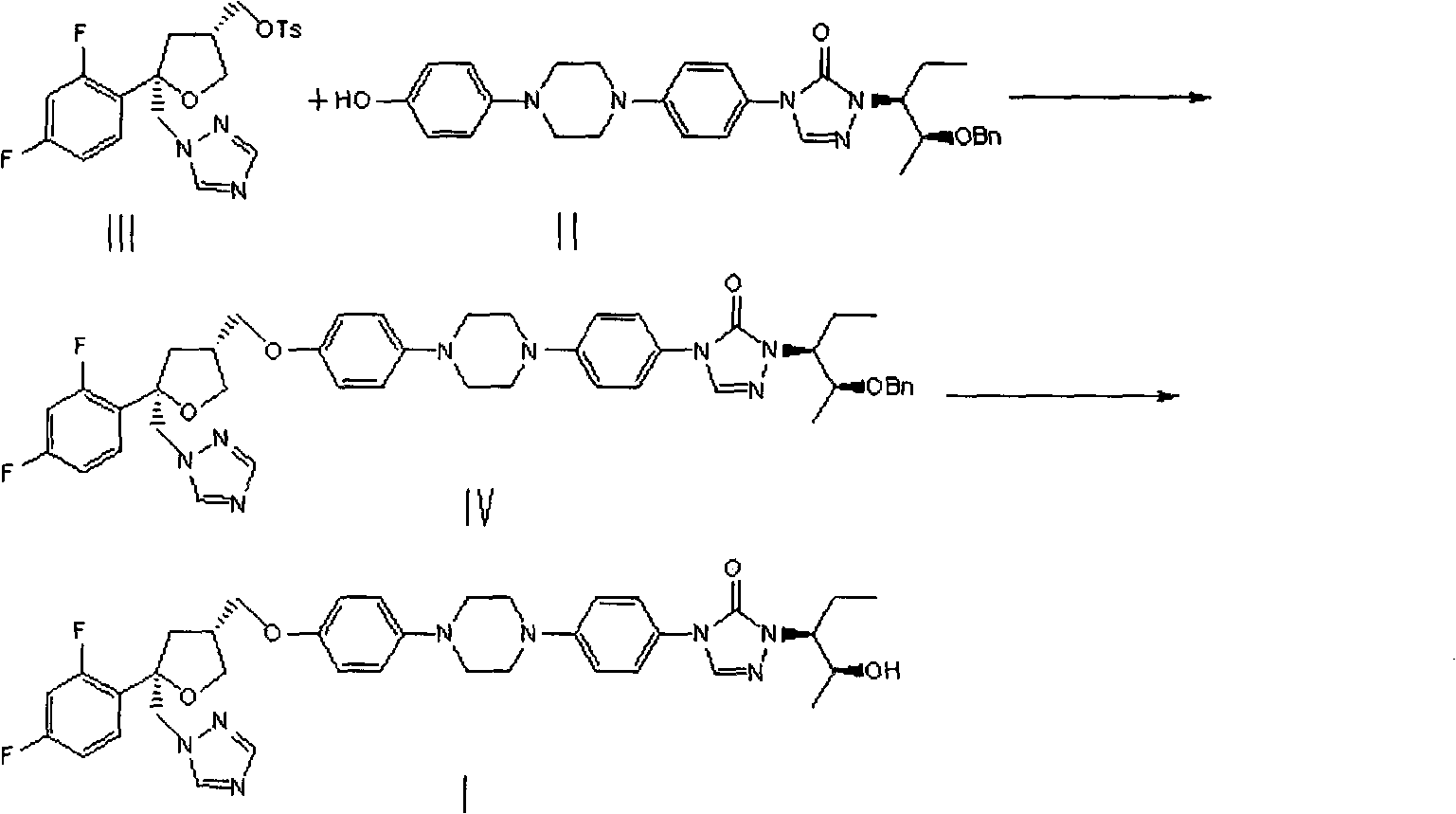
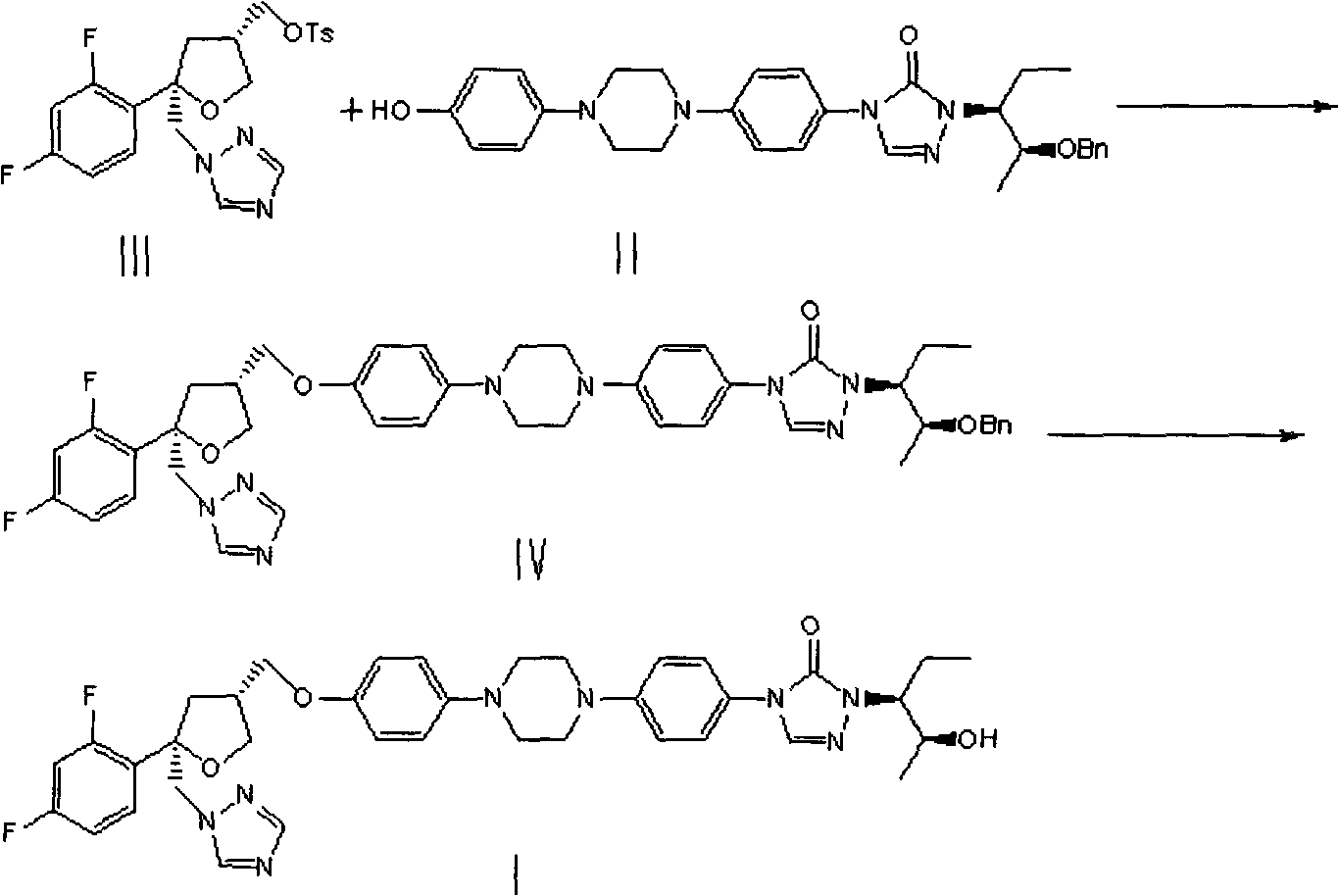
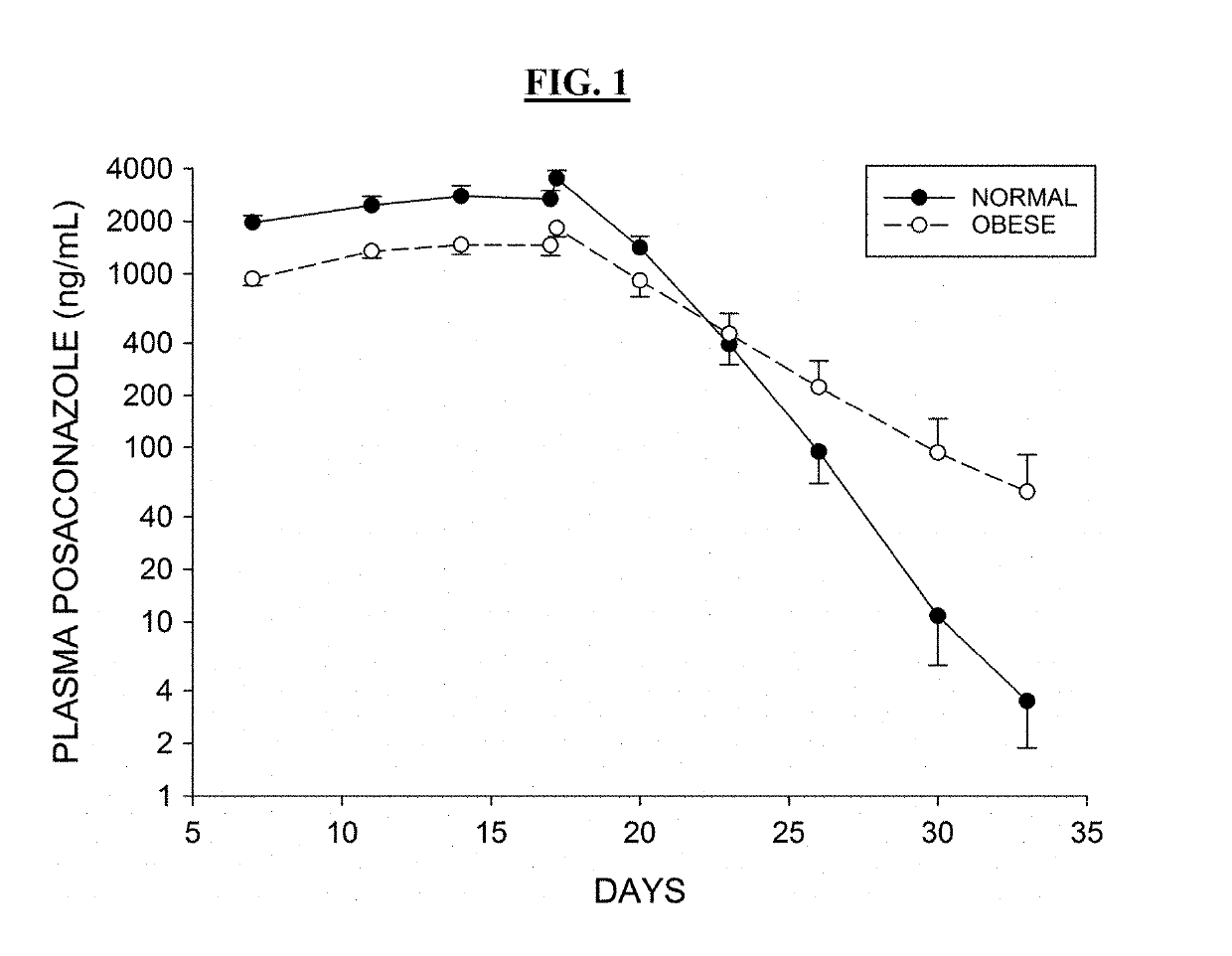
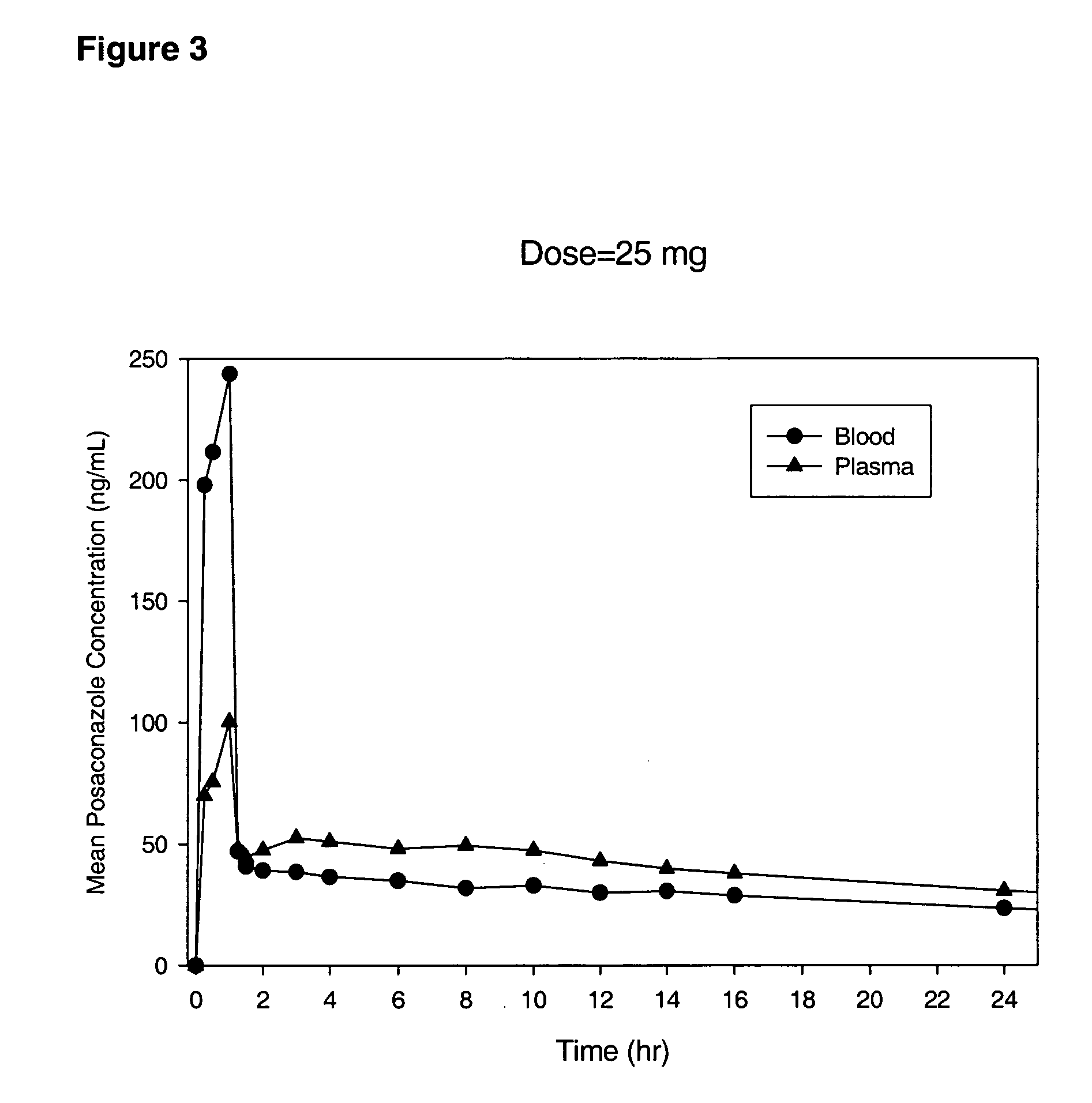
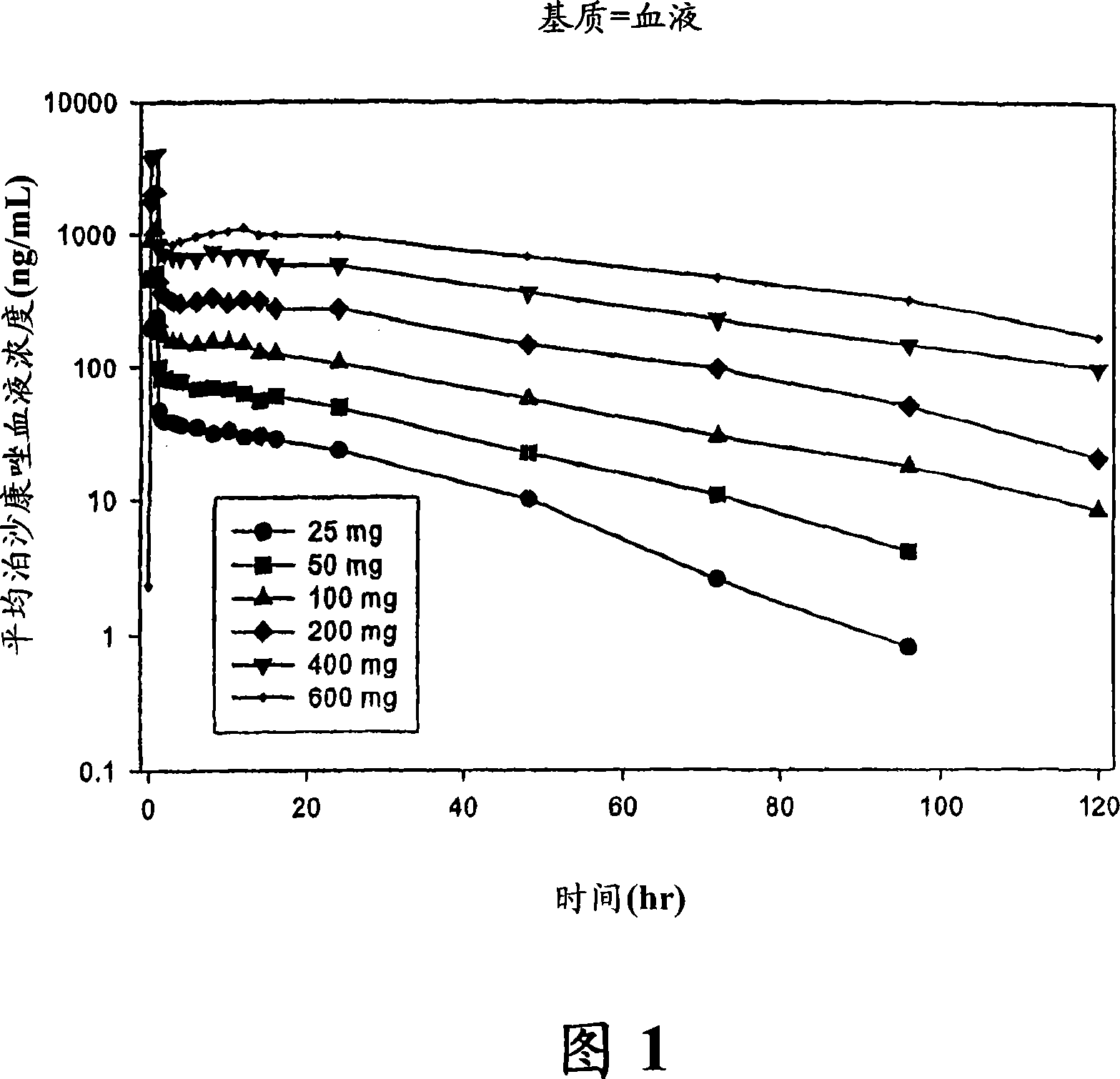
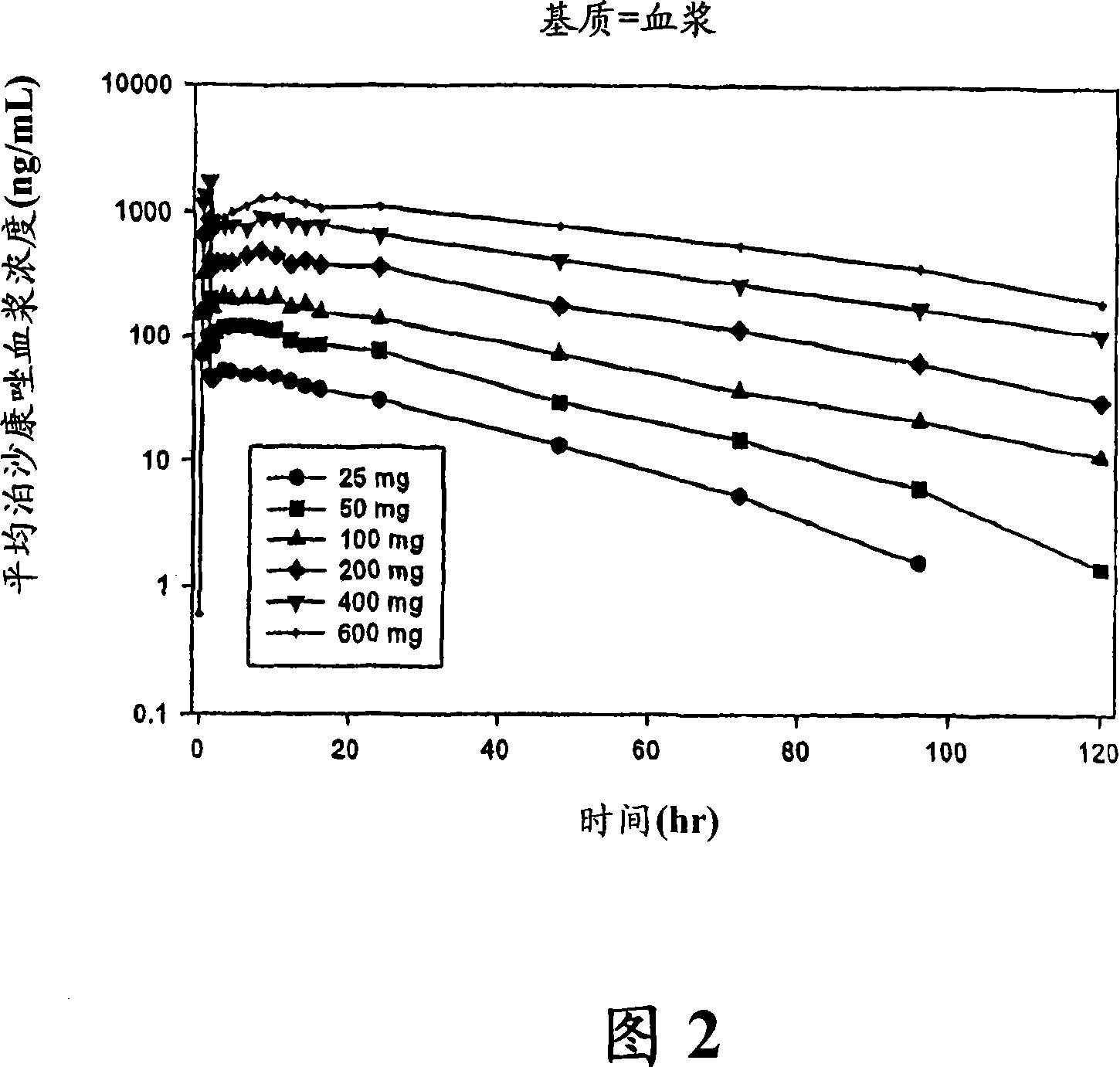
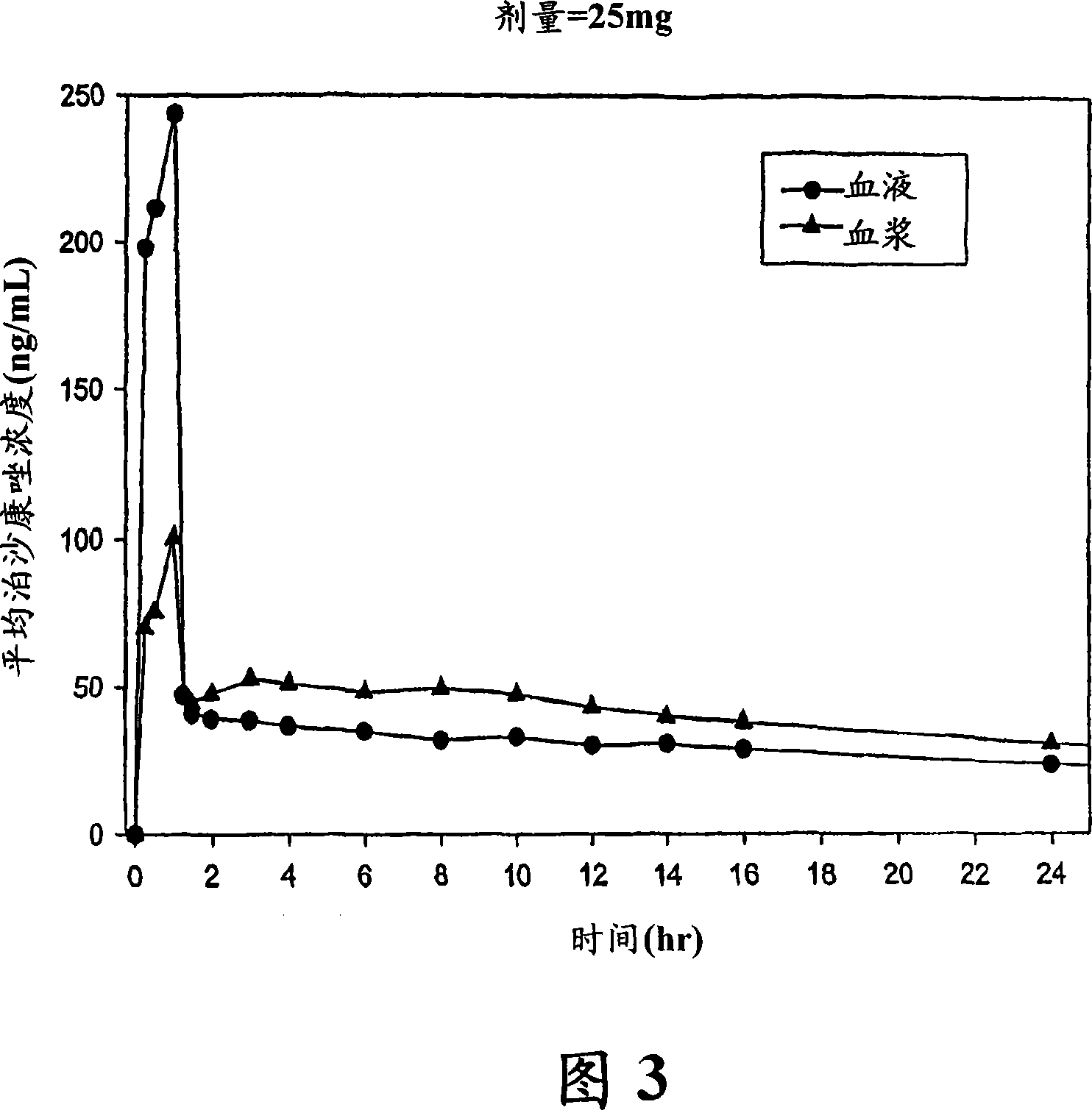
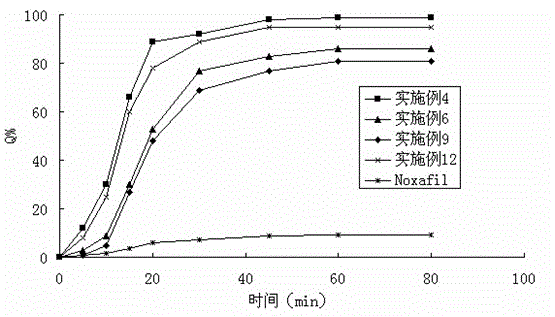
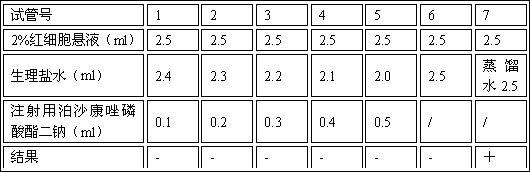
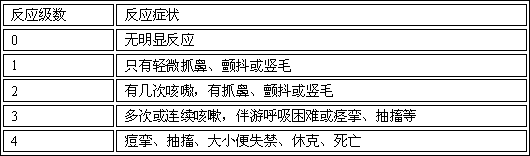
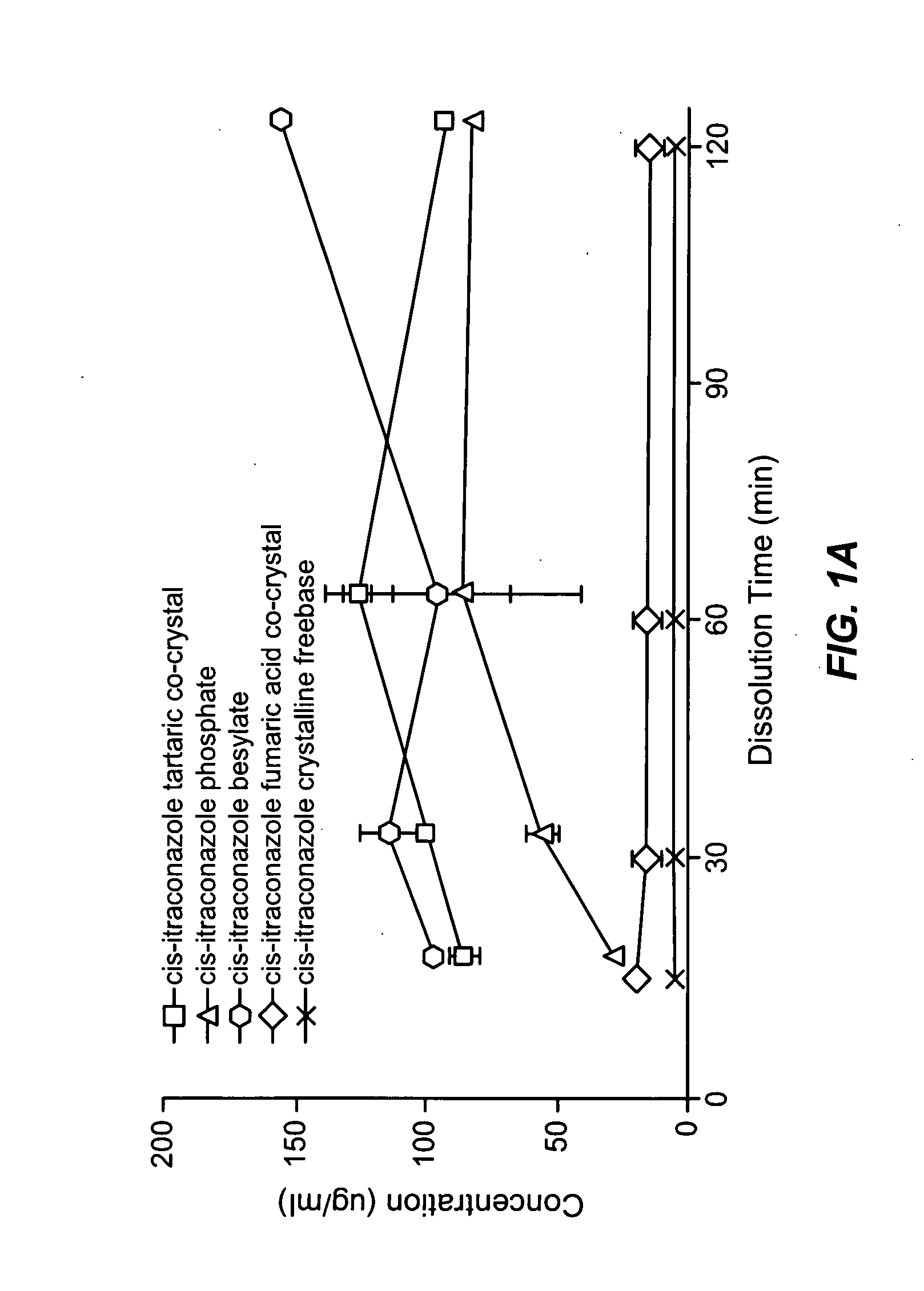
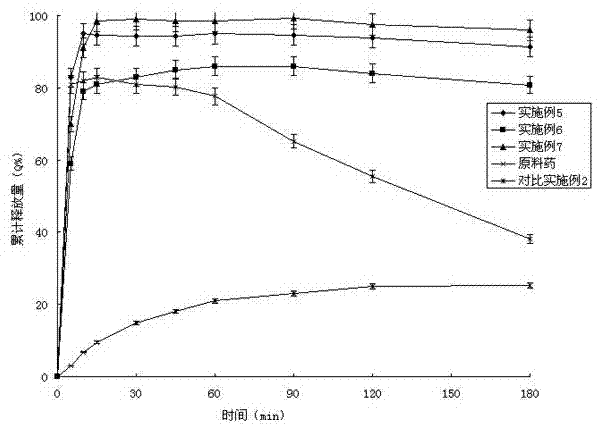
Patents
Literature
162 results about "Posaconazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
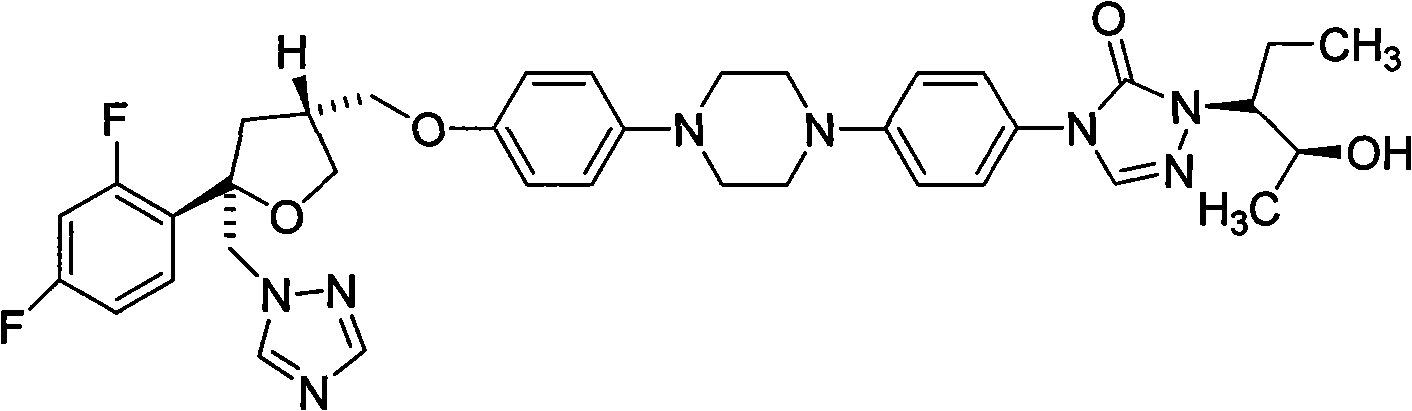
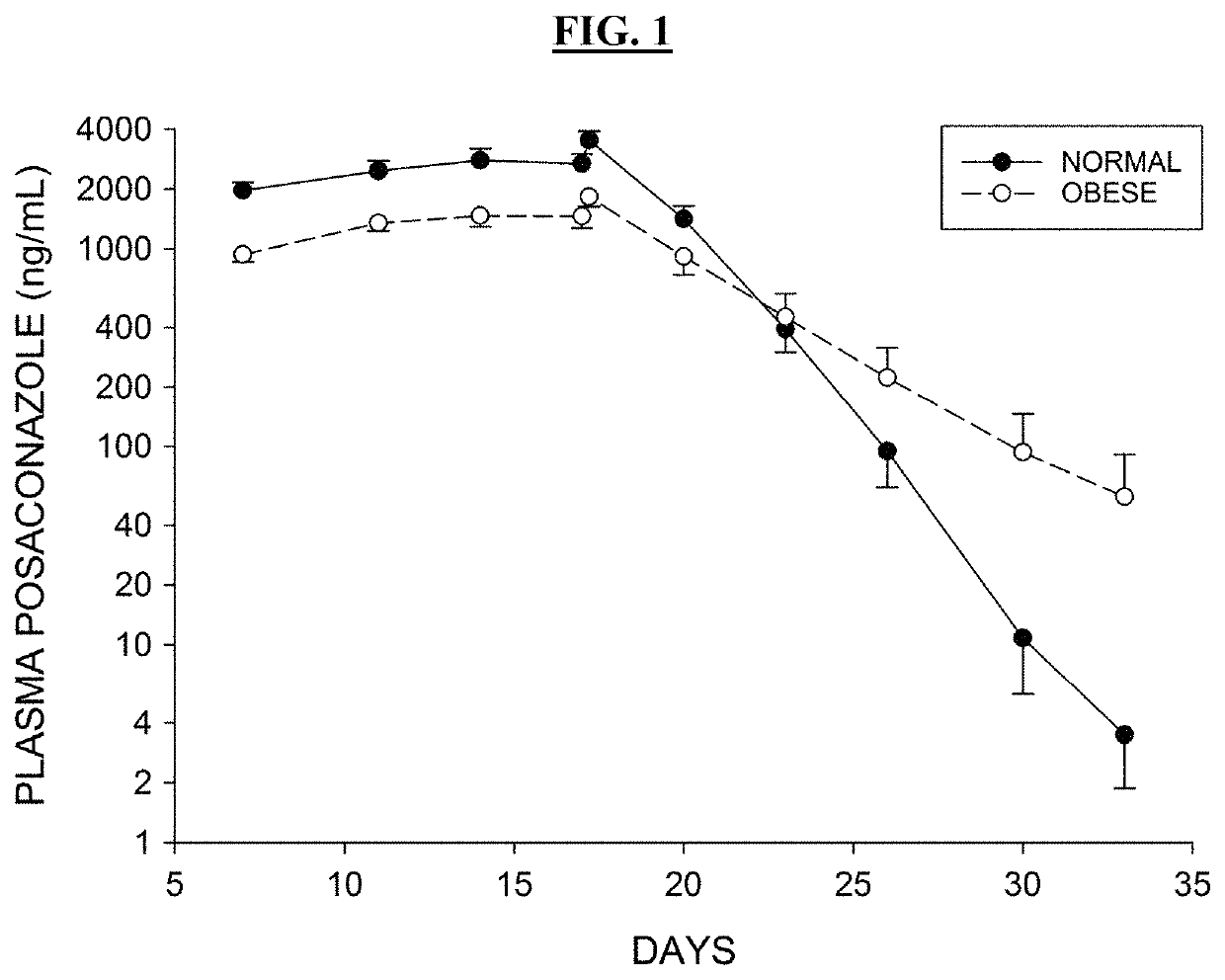
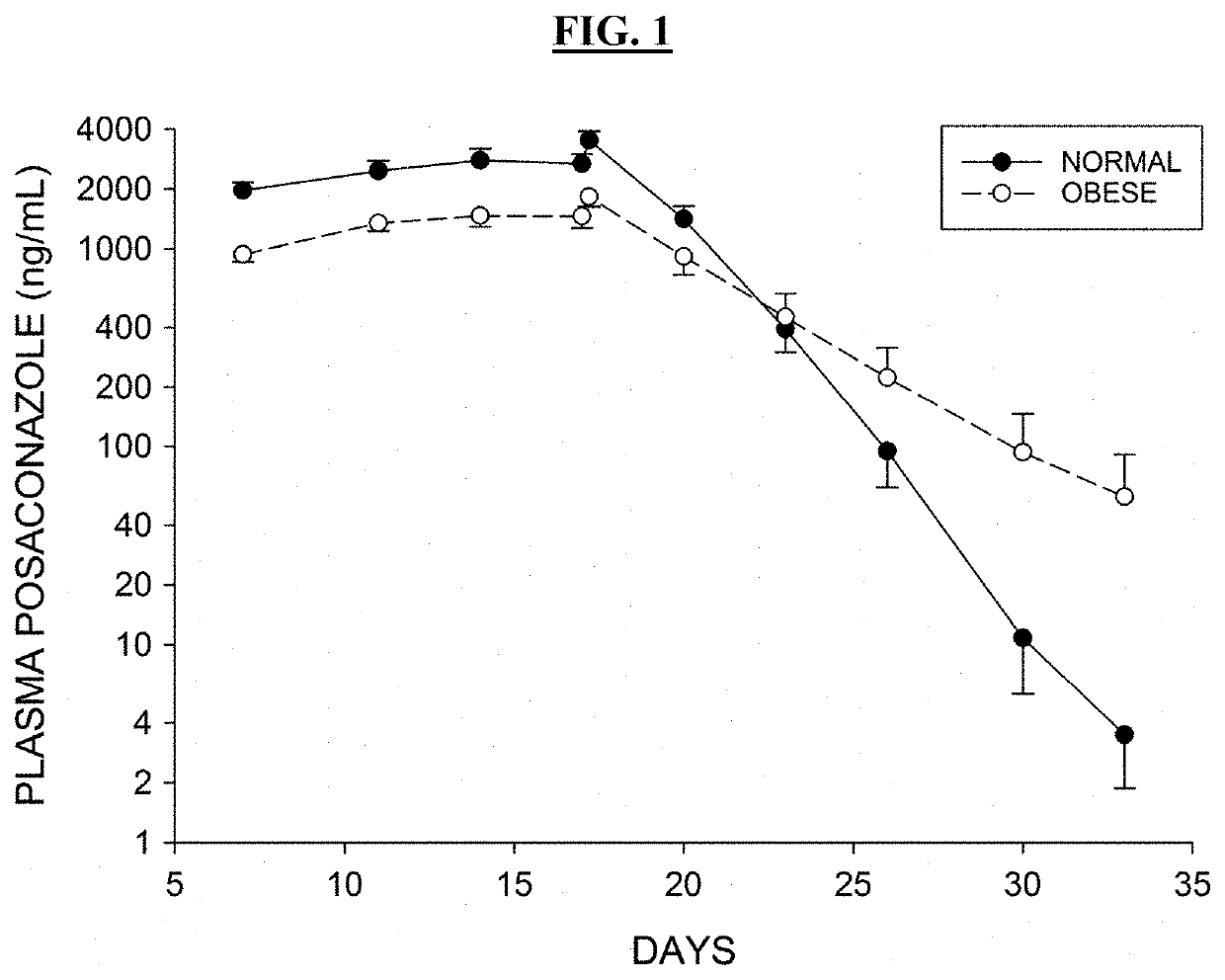
Posaconazole is used to prevent certain fungal infections in patients who have severely weakened immune systems (such as patients who have had chemotherapy).
Nanoparticulate posaconazole formulations
The invention is directed to compositions comprising a nanoparticulate posaconazole, or a salt or derivative thereof, having improved bioavailability. The nanoparticulate posaconazole particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of fungal infection and related diseases. The posaconazole particles may be formulated as a parenteral dosage form.
Owner:ALKERMES PHARMA IRELAND LTD
Particulate-stabilized injectable pharmaceutical compositions of Posaconazole
InactiveUS20060160823A1Reduce autoclave-induced particle size growthPromote growthOrganic active ingredientsAntimycoticsParticulatesAdditive ingredient
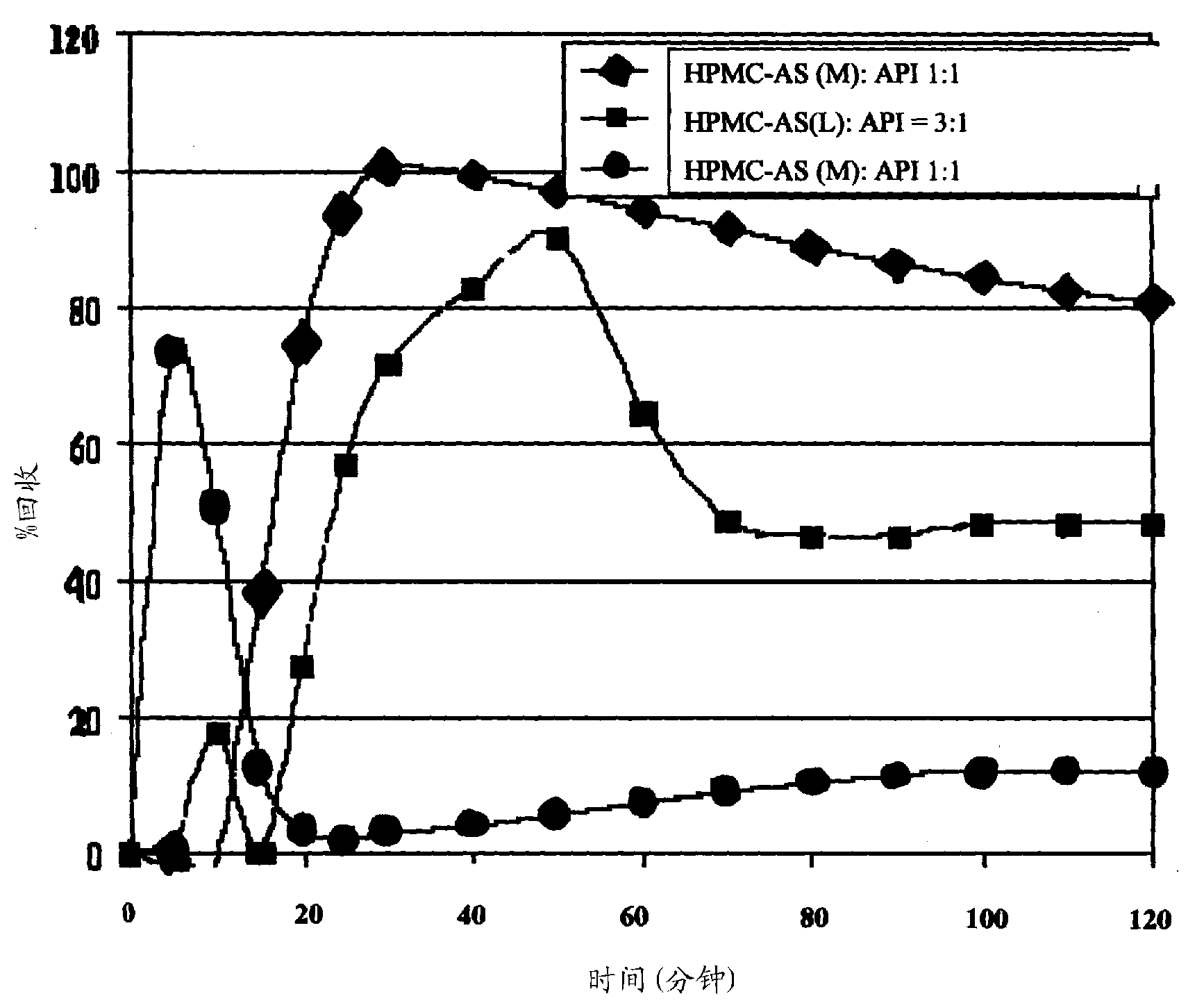
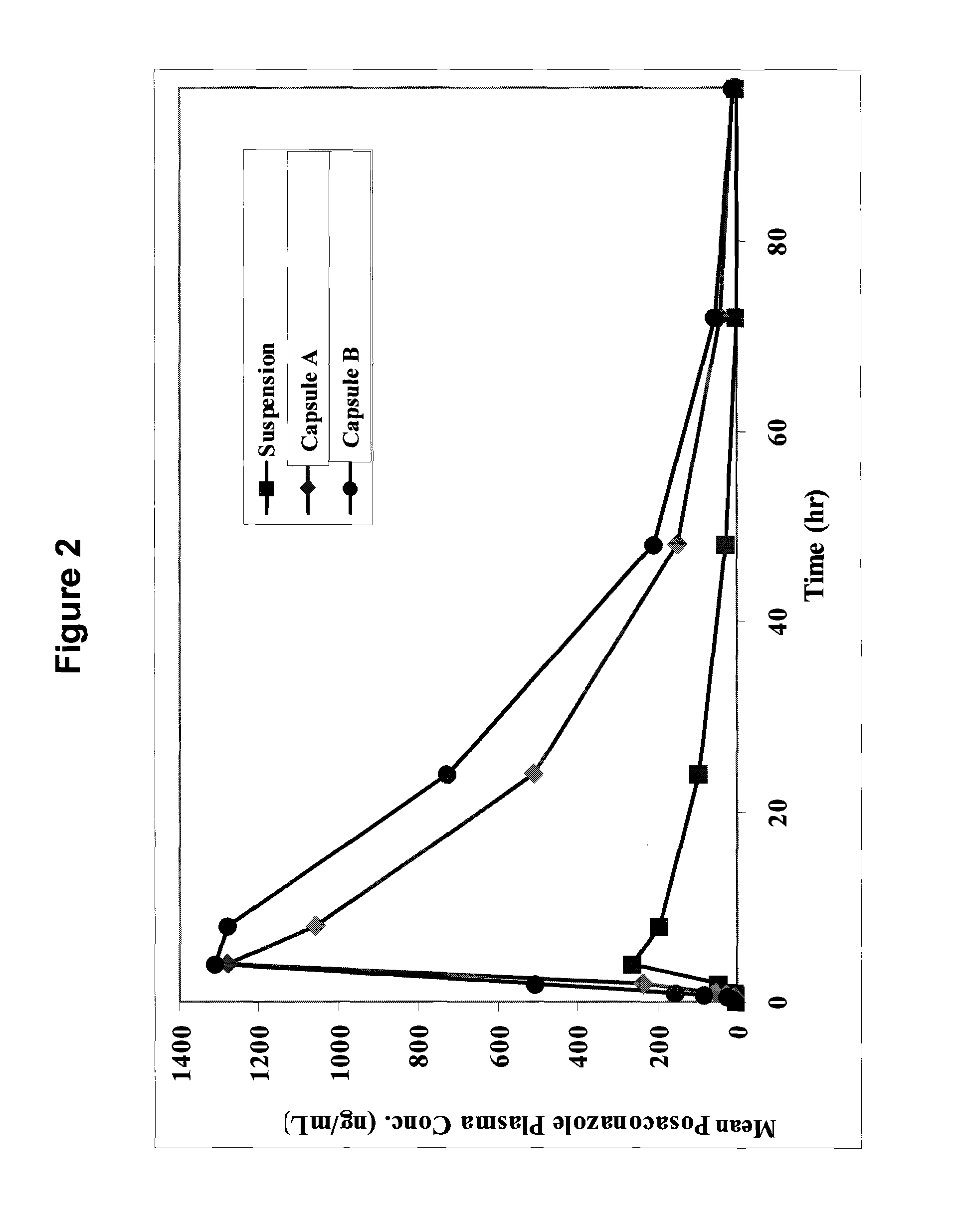
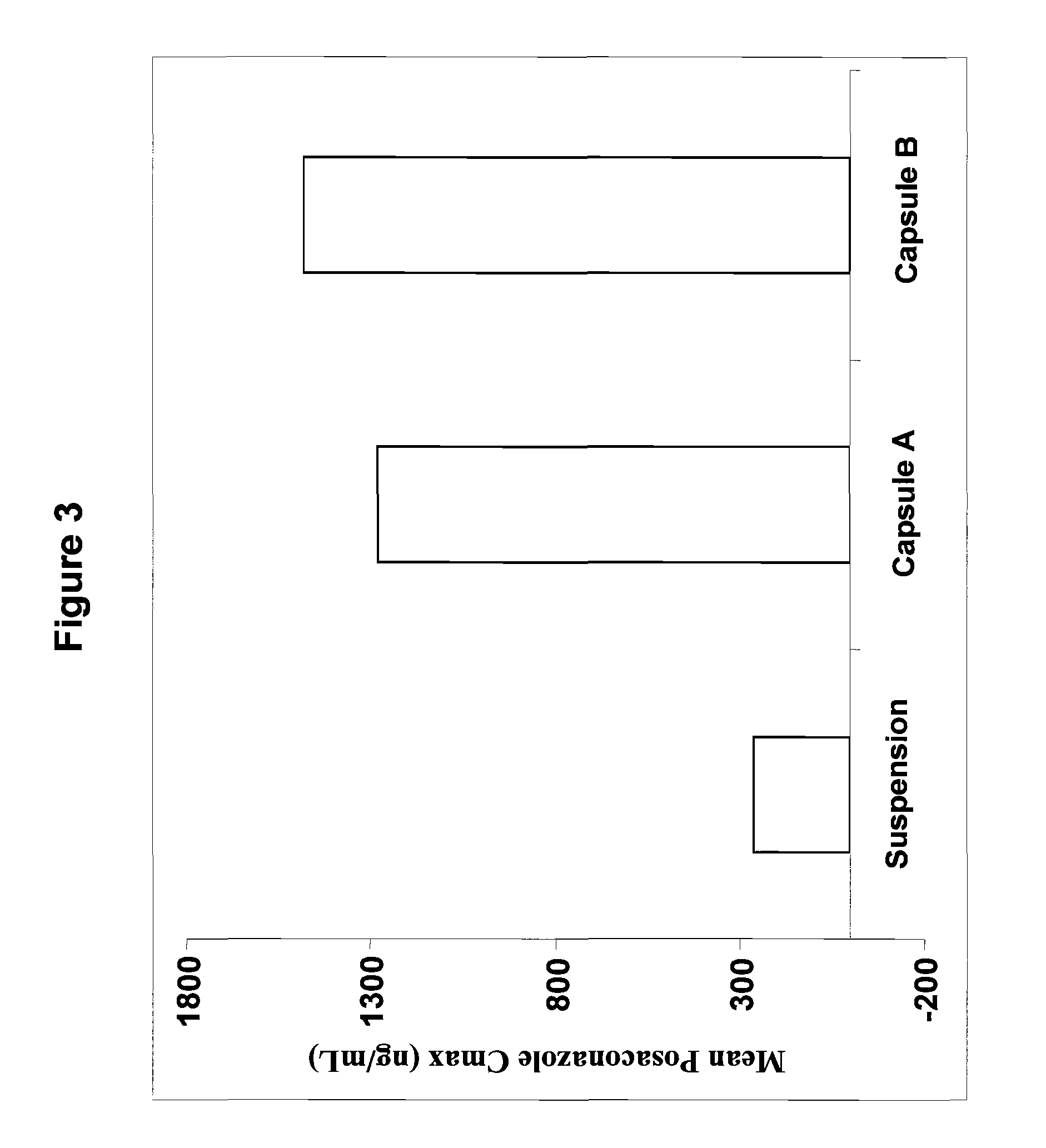
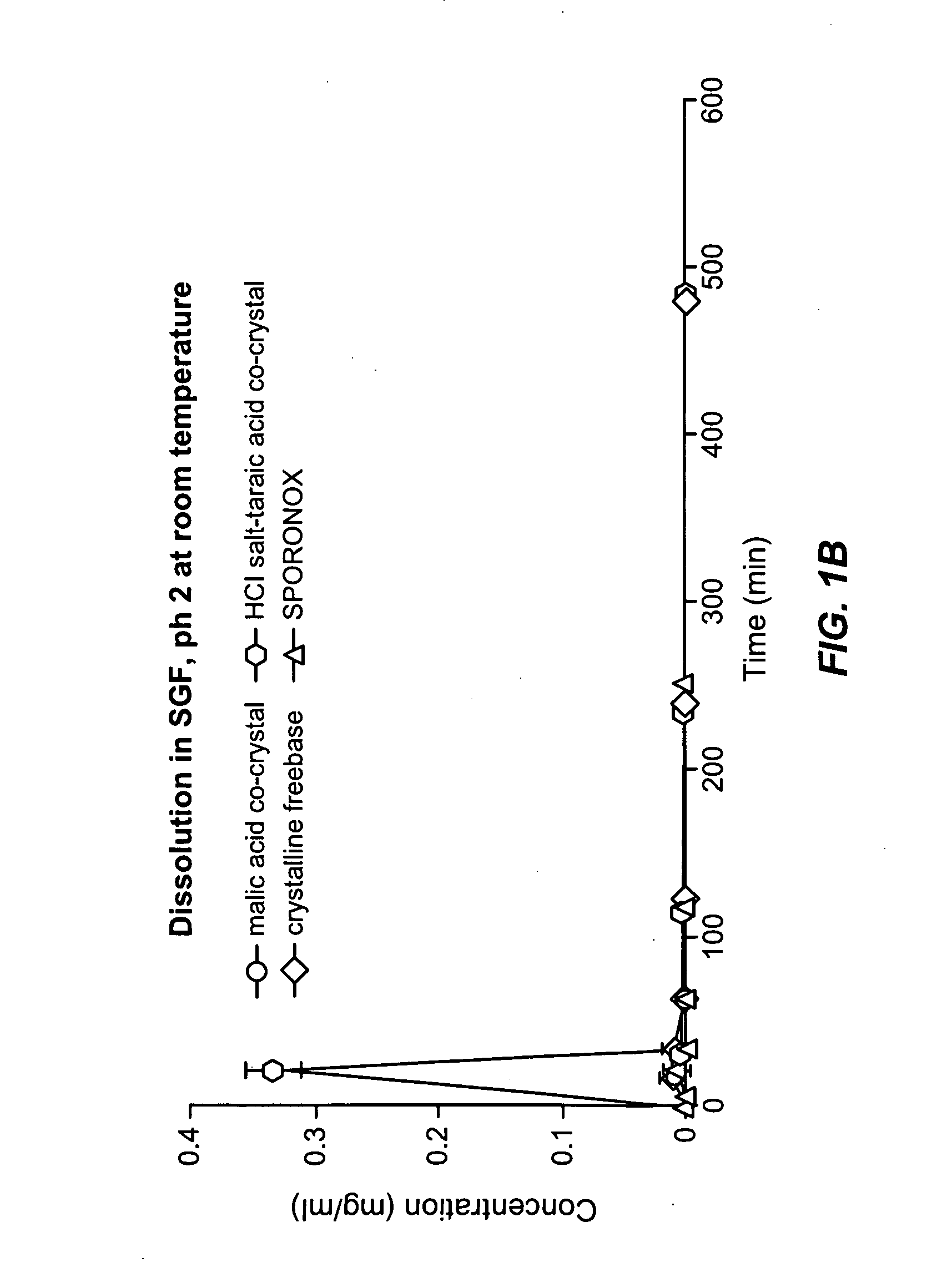
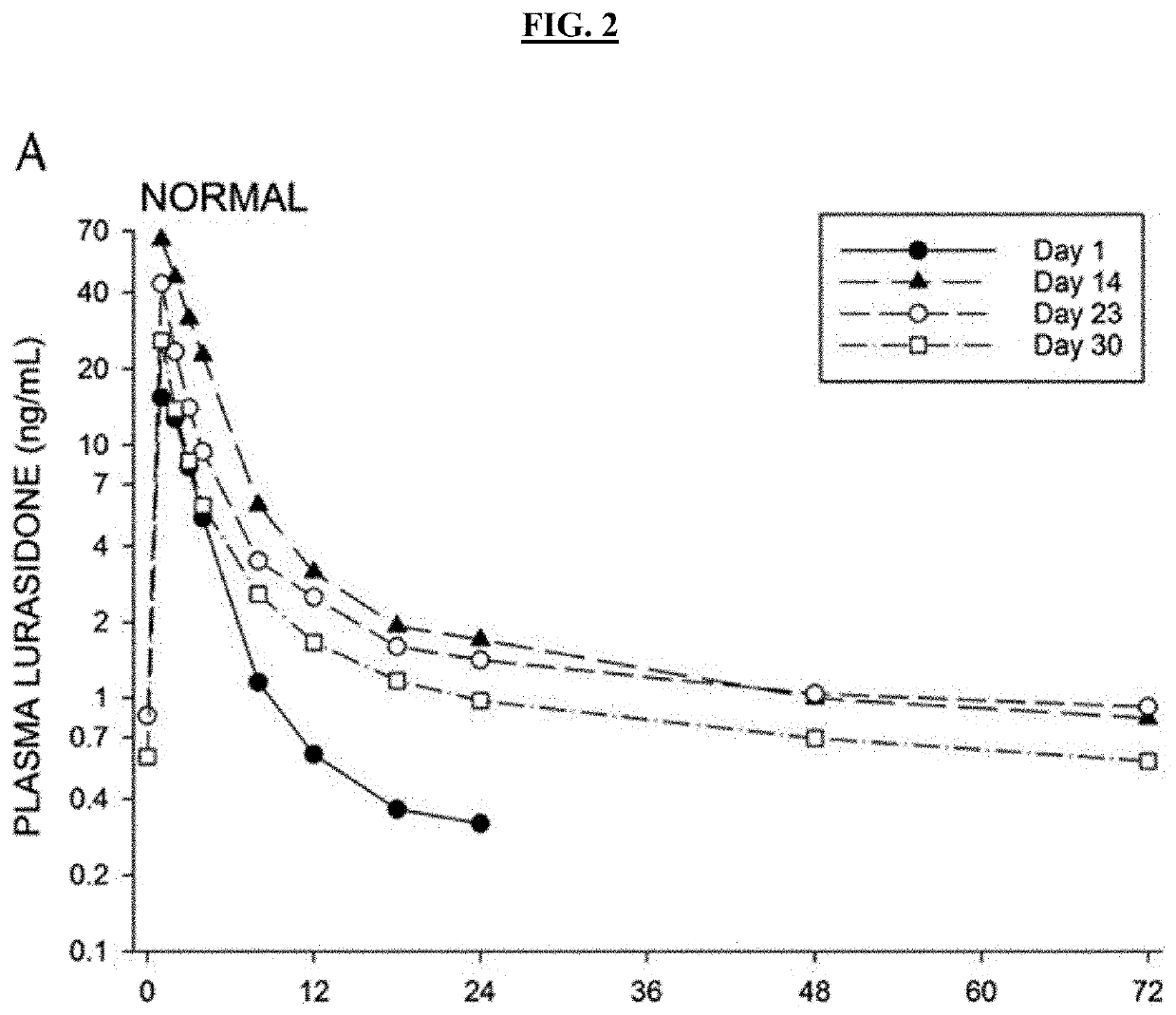
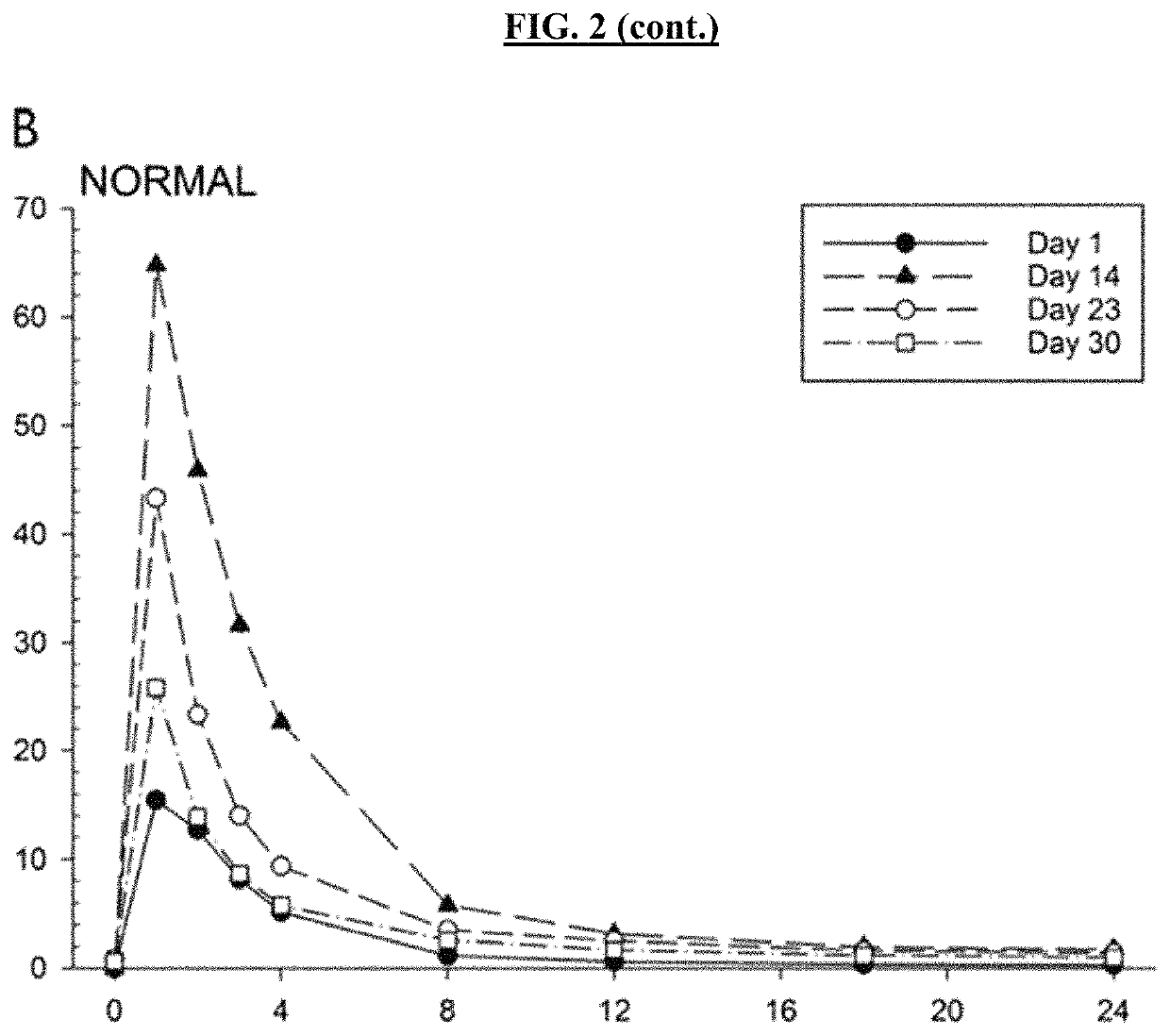
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient Posaconazole in an injectable suspension of particles that is stable when subjected to terminal sterilization. Preferred median particle sizes of between 1.5 and 3.0 microns are found to result in superior pharmacokinetic characteristics, such as those displayed below.
Owner:SCHERING CORP
Simple preparation method for posaconazole and piperazine intermediate thereof
InactiveCN101824009ASimple and fast operationHigh yieldAntimycoticsOrganic chemistryPosaconazolePiperazine
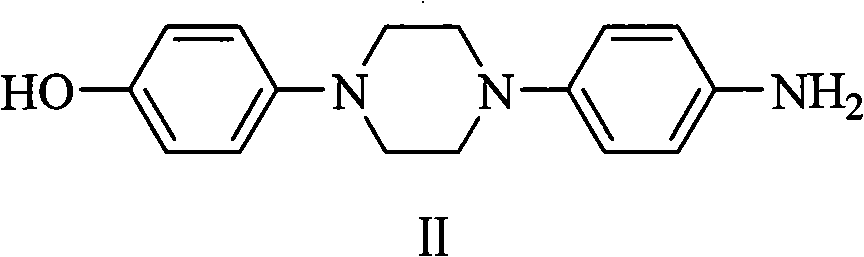
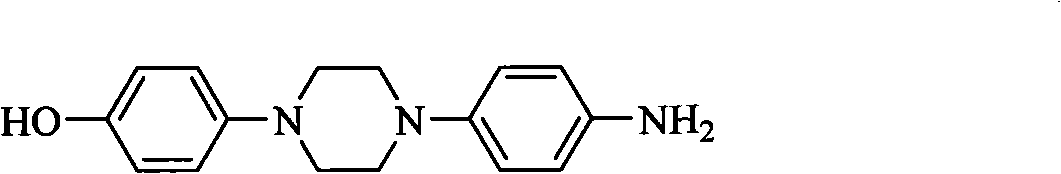
The invention discloses a method for preparing 1-(4-hydroxyphenyl)-4-(4-aminophenyl)piperazine intermediate. The piperazine intermediate has a structural formula II. The compound is a main intermediate of a novel medicament posaconazole for treating invasive fungal infection.
Owner:BEIJING D VENTUREPHARM TECH DEV
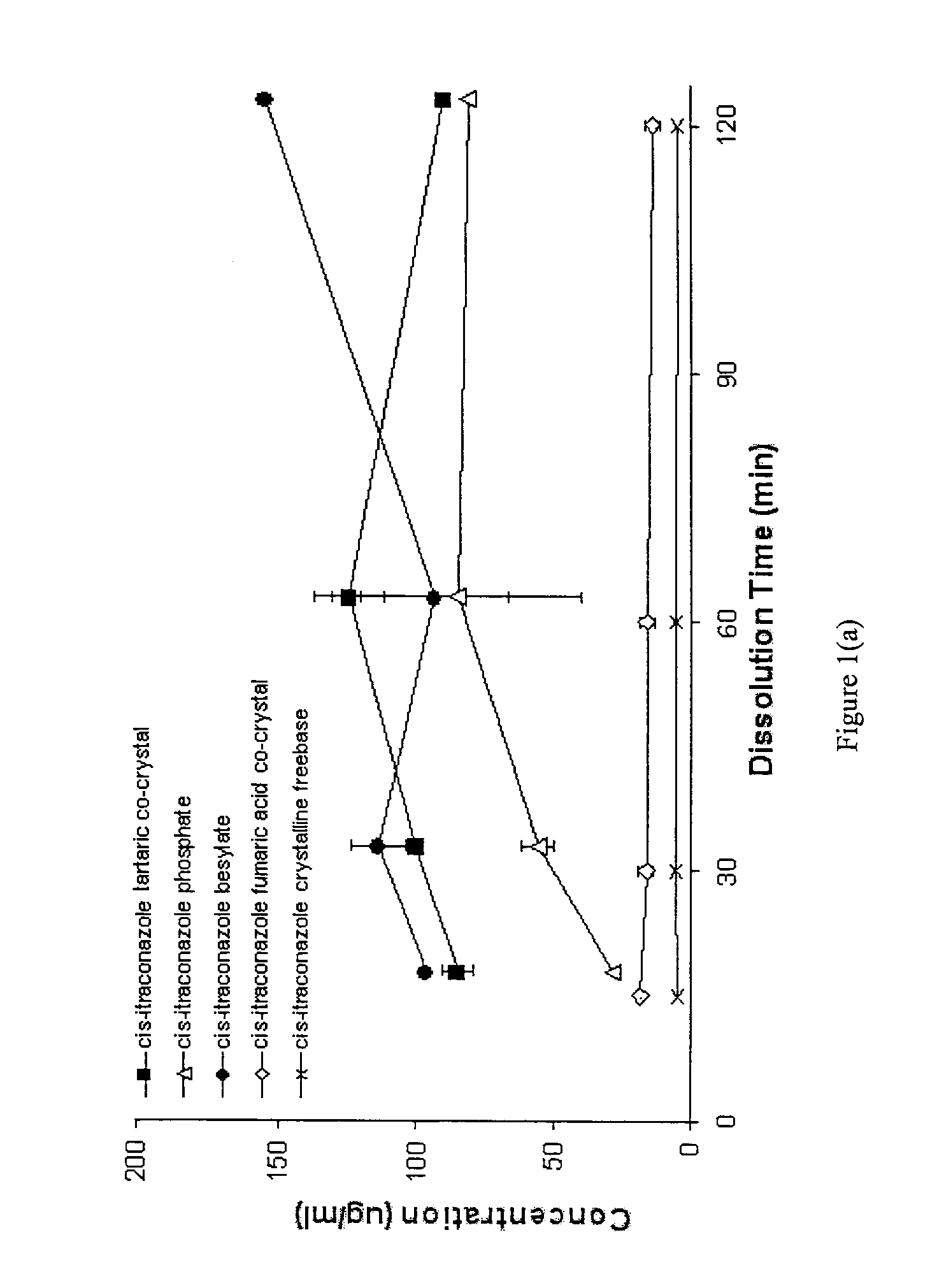
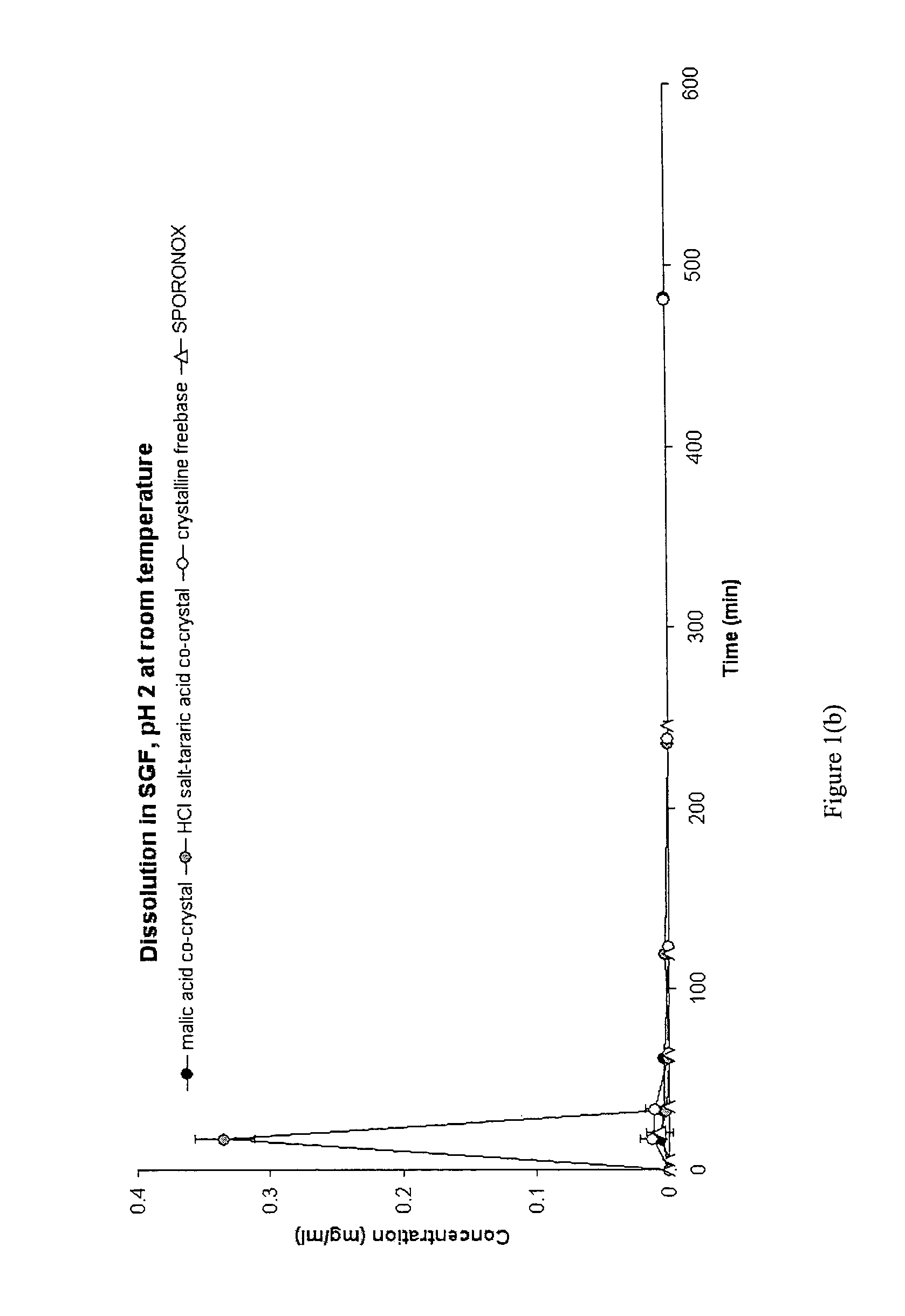
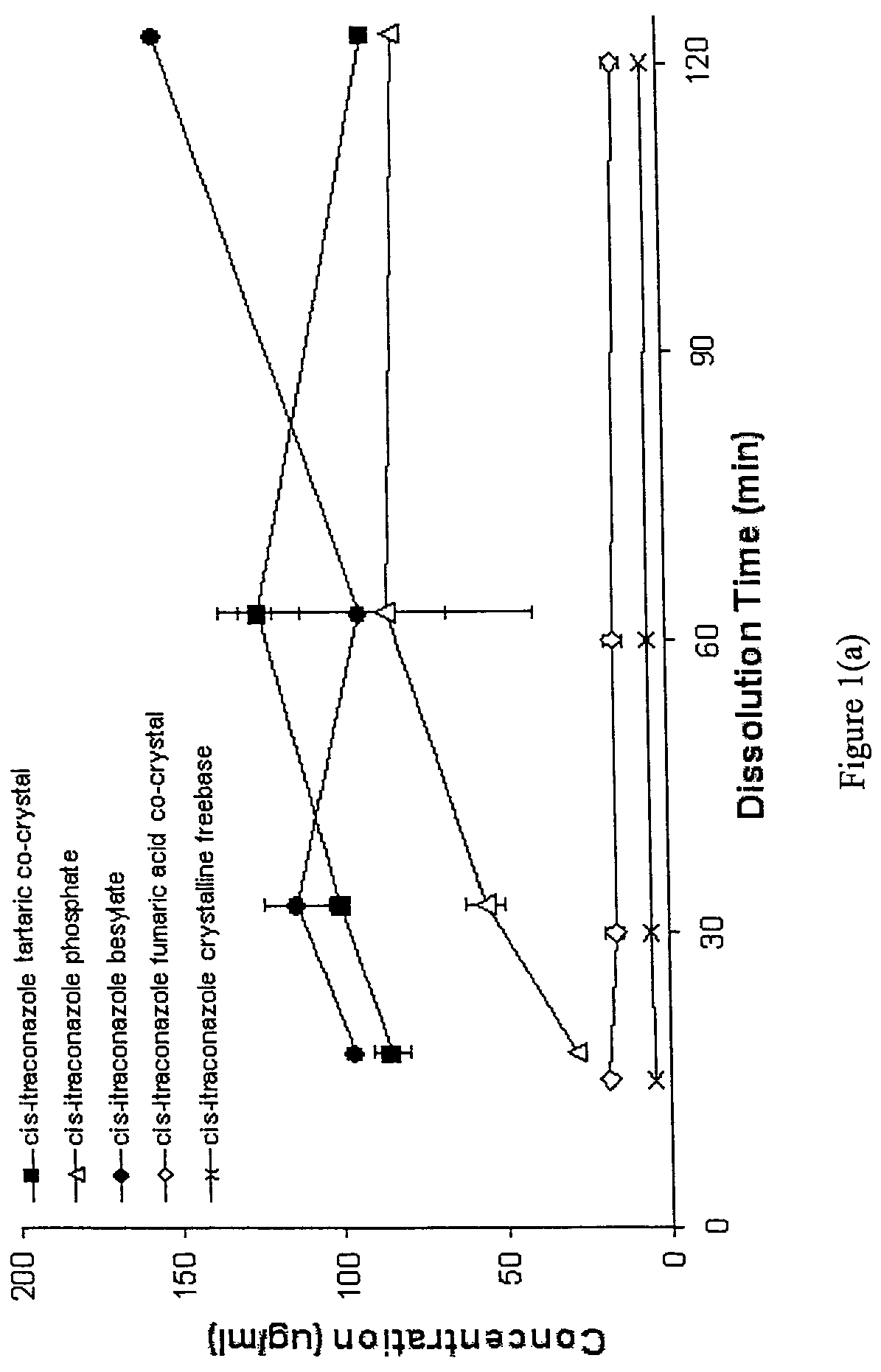
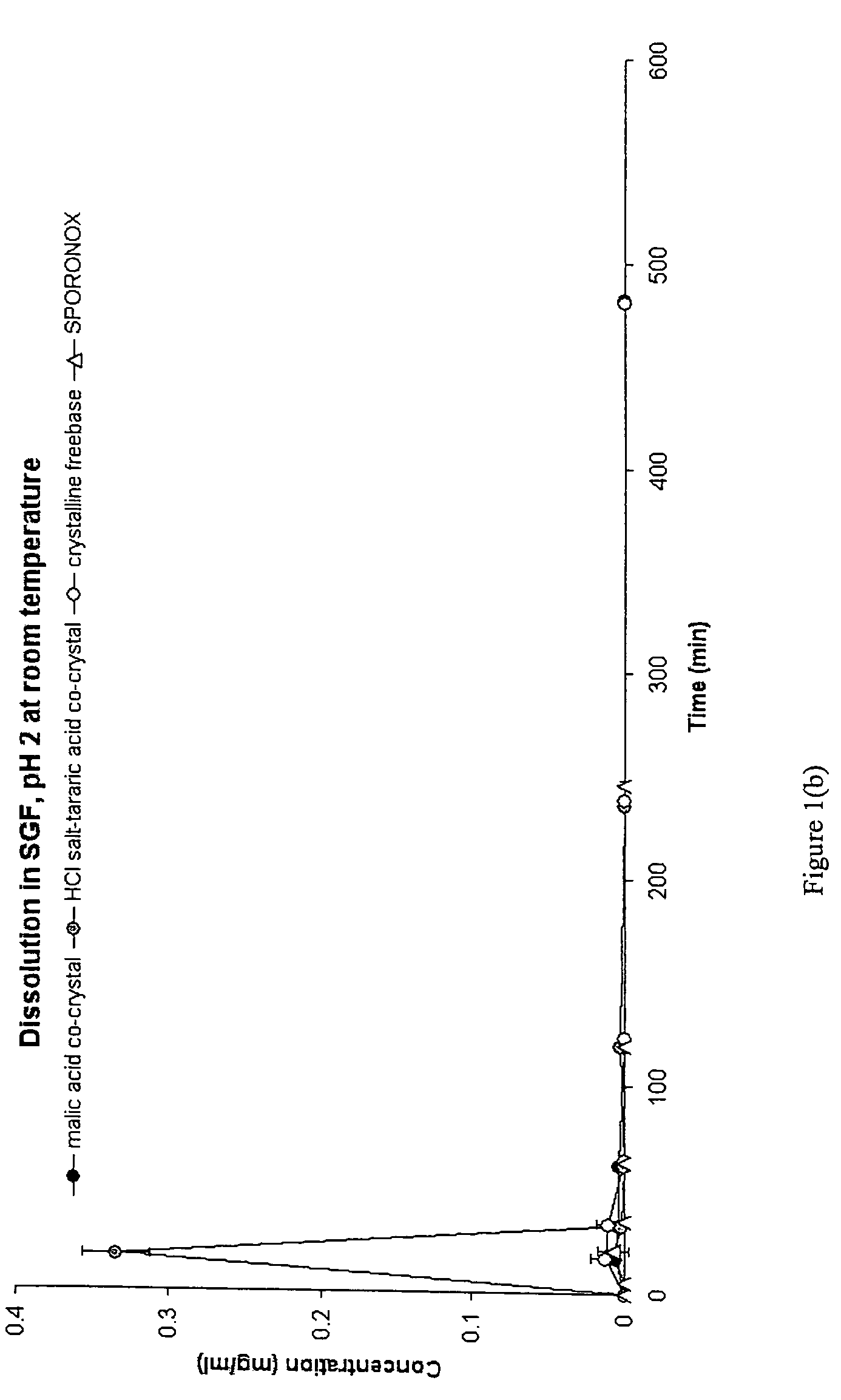
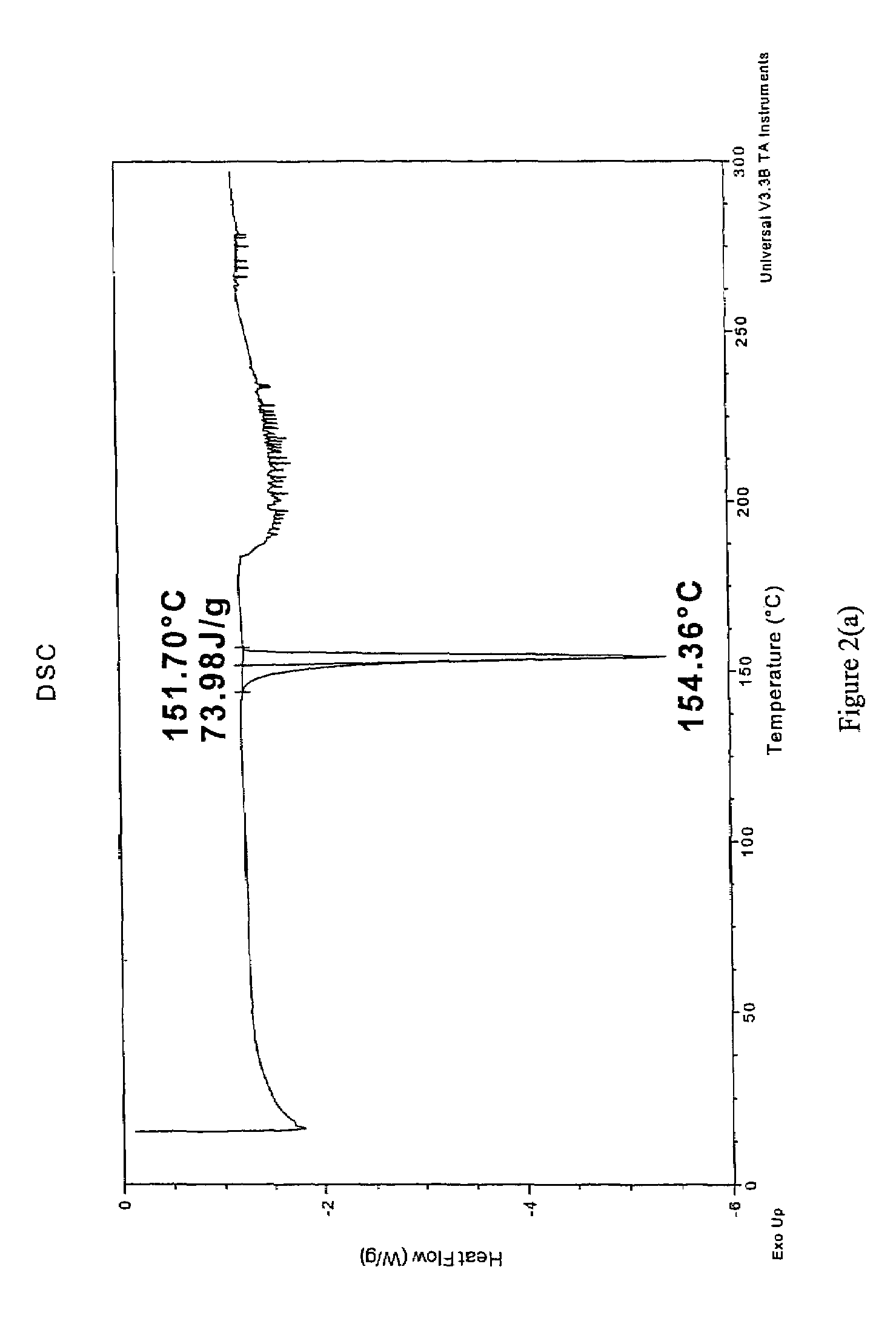
CIS-itraconazole crystalline forms and related processes, pharmaceutical compositions and methods
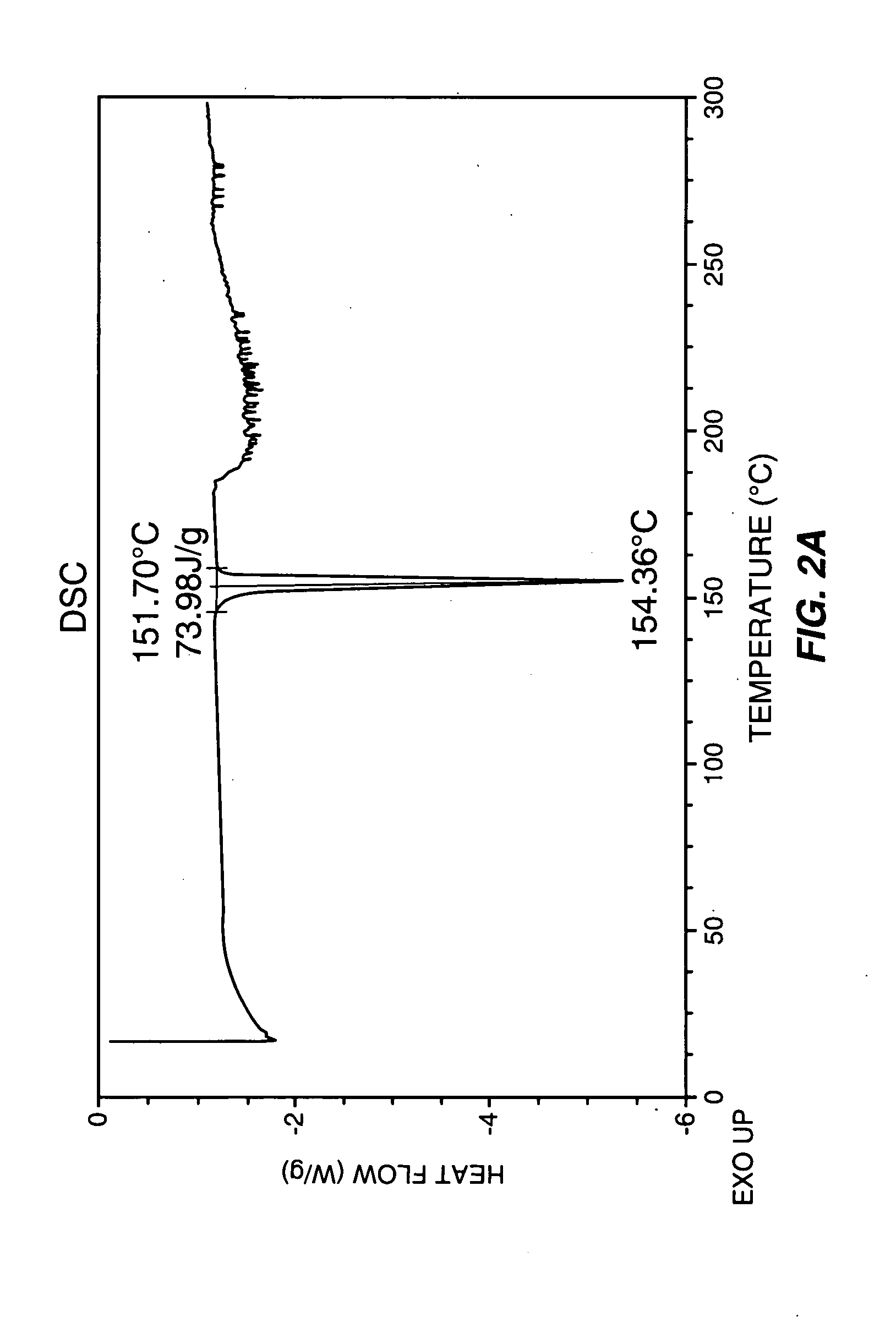
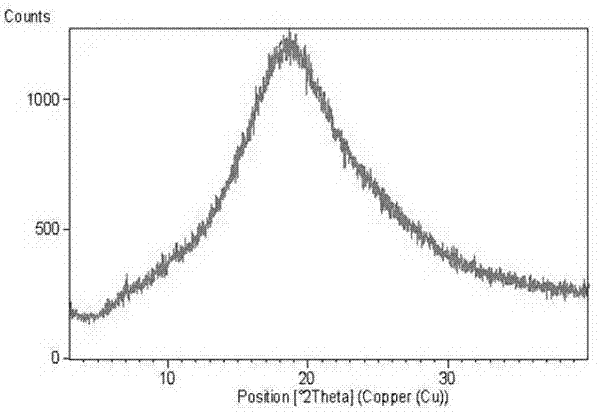
The invention provides novel soluble conazole crystalline forms (e.g. itraconazole, posaconazole and saperconazole) that include salts, co-crystals and related solvates useful as pharmaceuticals. The invention also provides pharmaceutical compositions comprising, and processes for making, these conazole crystalline forms. Methods of using such compositions for the treatment or prevention of systemic and local fungal, yeast, and dermatophyte infections are also provided.
Owner:TRANSFORM PHARMACEUTICALS INC
Method of treating a patient with a cyp3a4 substrate drug
ActiveUS20180333409A1Reduce morbidityAvoid and reduce incidenceNervous disorderHydroxy compound active ingredientsReduced dosePosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug contraindicated for concomitant administration with a strong CYP3A4 inhibitor, wherein the patient is treated with multiple doses of posaconazole, stops posaconazole treatment, and then is treated with the CYP3A4 substrate drug. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-21 after stopping posaconazole. In some embodiments, the patient is treated with or prescribed a reduced dose of the CYP3A4 substrate drug for about 2-21 after stopping posaconazole.
Owner:BOW RIVER LLC
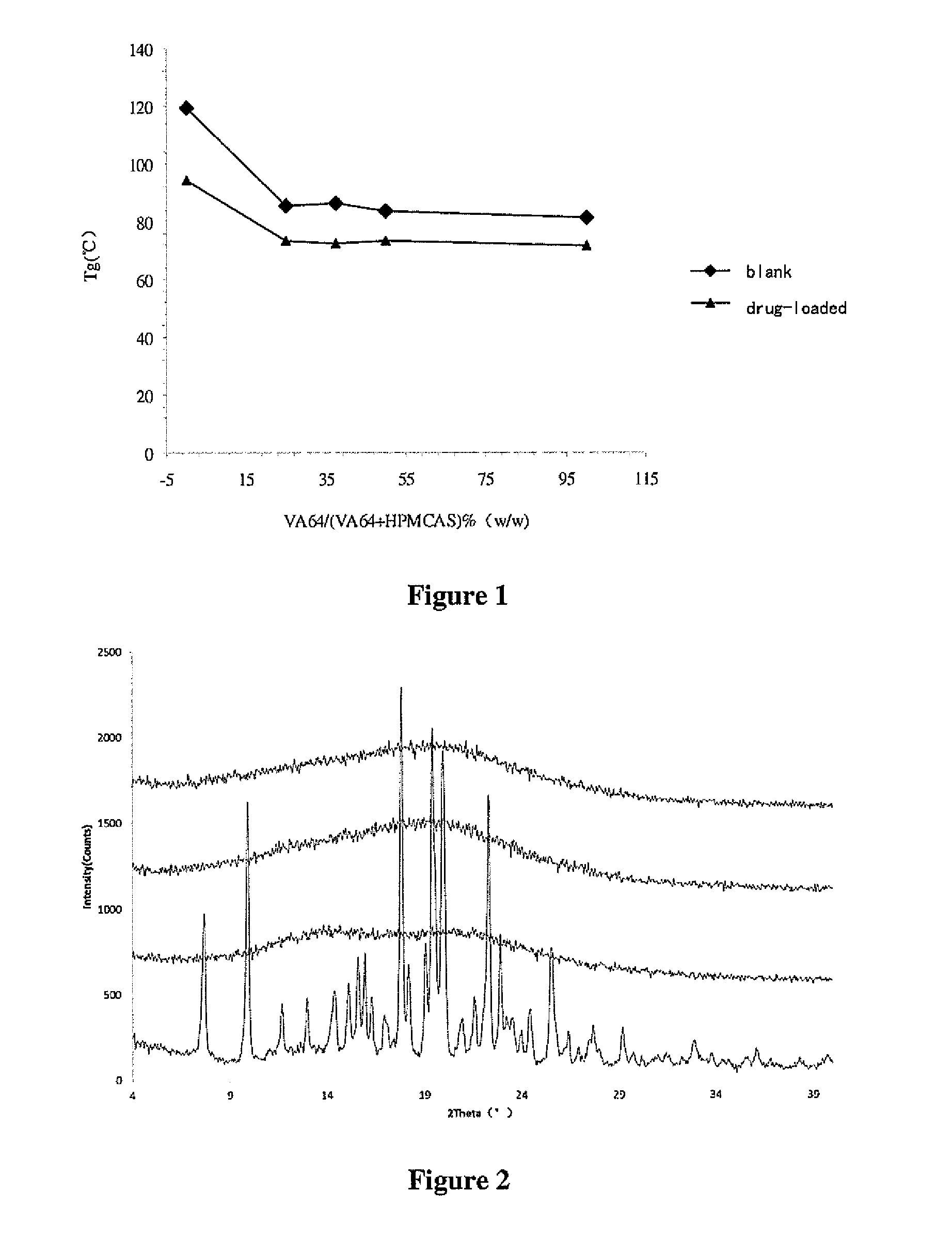
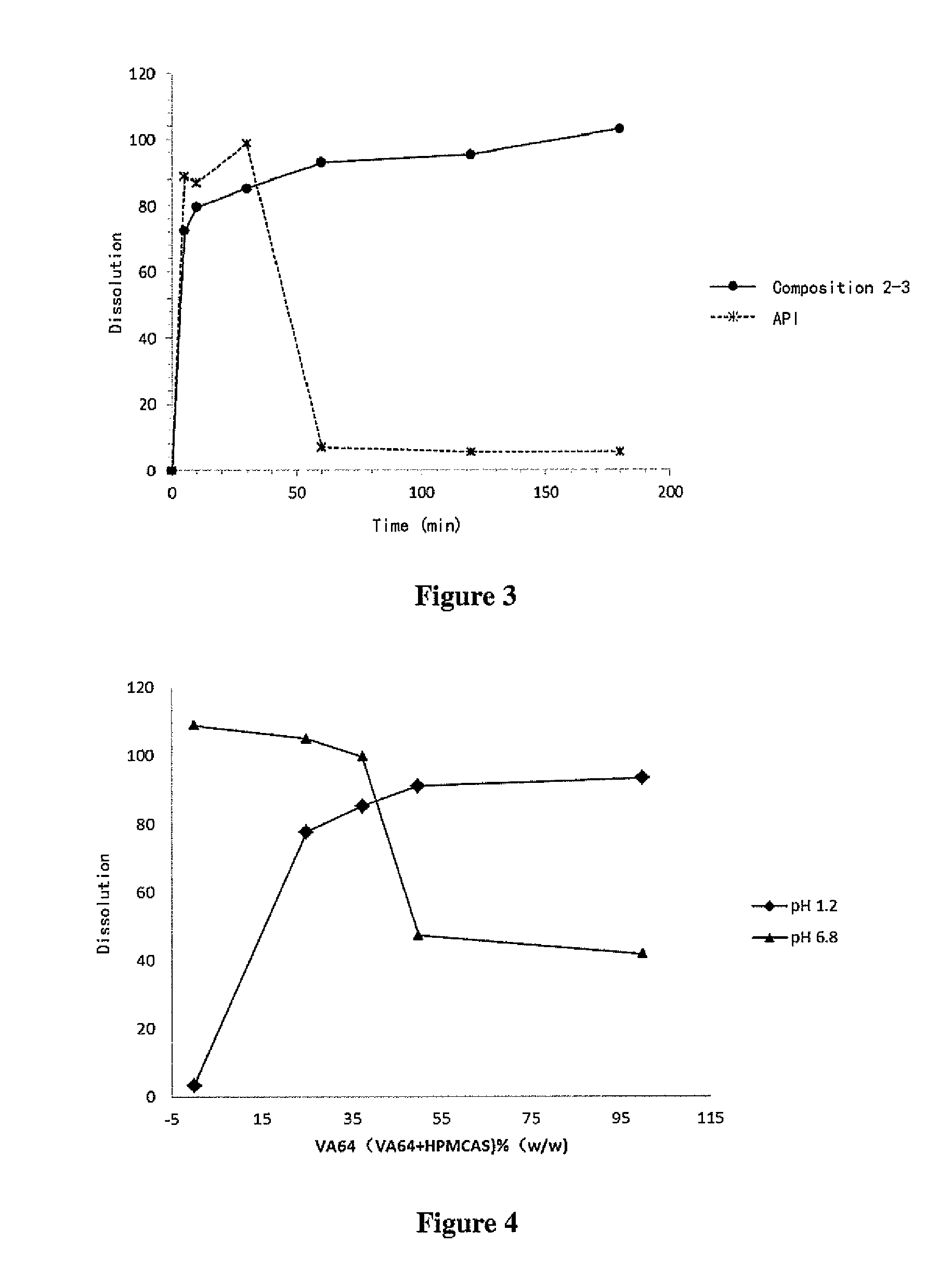
High density compositions containing posaconazole and formulations comprising the same
InactiveUS20110123627A1Improve bioavailabilityHigh plasma levelOrganic active ingredientsPowder deliveryHigh densityPosaconazole
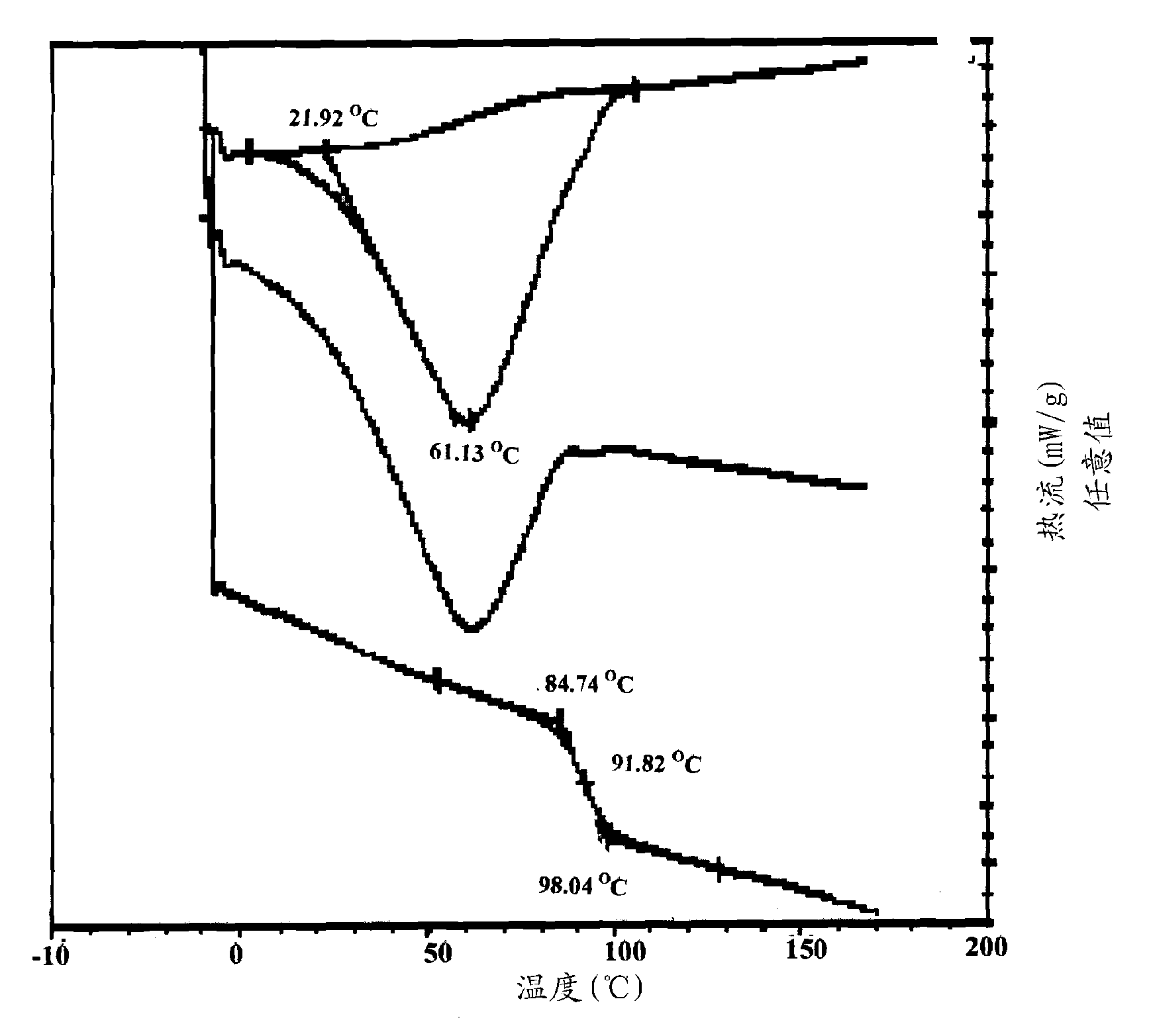
The present application provides novel compositions comprising posaconazole and a polymer wherein the composition has a glass transition temperature (Tg) of less than about 1100 C. The application also describes compositions comprising posaconazole and a polymer having a bulk density of greater than about 0.4 mg / mL. The application also describes compositions comprising posaconazole and a polymer which provide an exposure (AUCtf) of at least about 10,000 ng·hr / mL when administered to a patient in a fasted state. The application also describes a novel process for preparing these compositions. The preff erred polymer is HPMCAS. Preferably the composition is an extruded material.
Owner:MERCK SHARP & DOHME CORP
Methods of treatment with cyp3a4 substrate drugs
ActiveUS20190076425A1Avoid and reduce incidenceSafety managementOrganic active ingredientsPharmaceutical drugPosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Preparation method of posaconazole
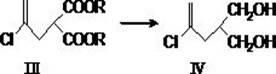
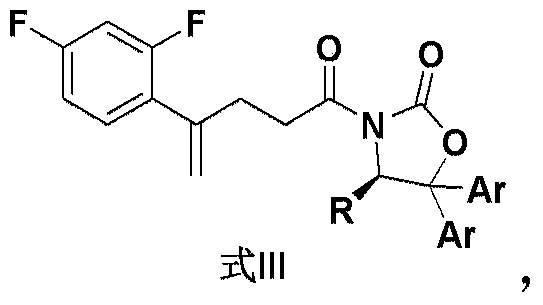
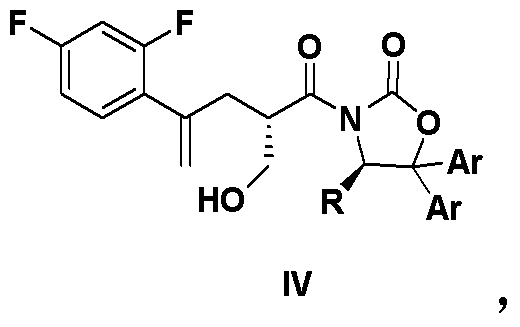
The invention belongs to the medicine pharmacy field, and relates to a preparation method of posaconazole, which comprises the following steps: adding a compound in a formula (II) in an aprotic solvent for stirring and dissolving, adding an alkaline aqueous solution, and adding a compound in a formula (III) for reacting to obtain an intermediate compound in a formula (IV); reacting a compound in a formula (IV) and hydrochloric acid to obtain a compound crude product in a formula (I); and performing recrystallization by a mixed solvent of acetone / methanol. The method of the invention has the advantages that the operation is safe, the reaction is simple, the post-treatment is convenient, and the method is suitable for industrial production.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Methods of treatment
ActiveUS20190262328A1Avoid and reduce incidenceSafety managementOrganic active ingredientsNervous disorderDose ReducedReduced dose
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Particulate-stabilized injectable pharmacutical compositions of posaconazole
InactiveUS20060009469A1Useful in treatmentOrganic active ingredientsAntimycoticsParticulatesAdditive ingredient
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient posaconazole in an injectable suspension that is stable when subjected to terminal steam sterilization.
Owner:SCHERING CORP
Injectable pharmaceutical suspension comprising posaconazole
The present invention provides formulations useful for treating infections, in particular, formulations that include the active pharmaceutical ingredient posaconazole in an injectable suspension that is stable when subjected to terminal steam sterilization.
Owner:SCHERING AG
Crystalline forms of conazoles and methods of making and using the same
InactiveUS7446107B2Decreased food effectReduce impactOrganic active ingredientsPowder deliveryYeastPosaconazole
Owner:TRANSFORM PHARMACEUTICALS INC
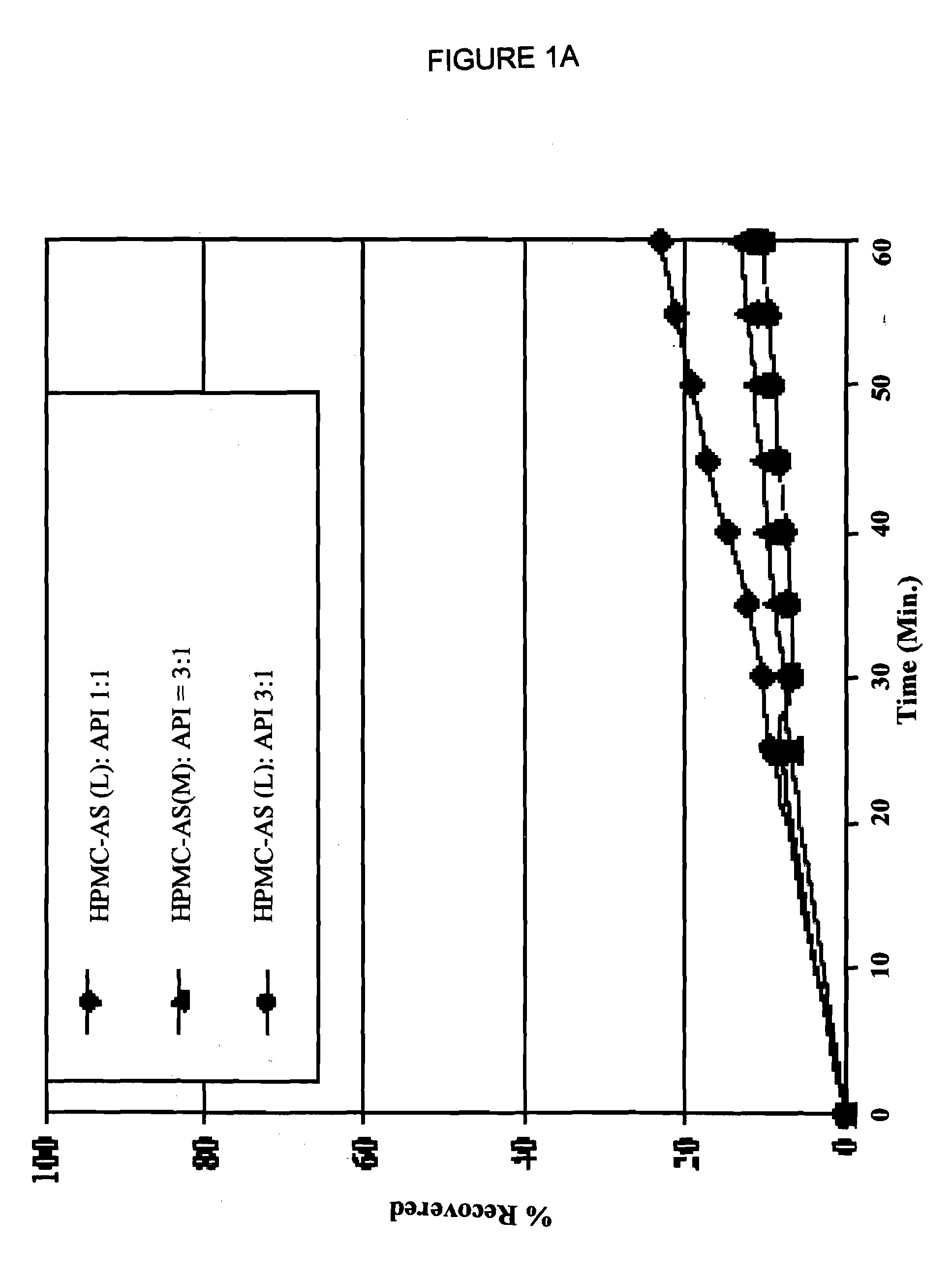
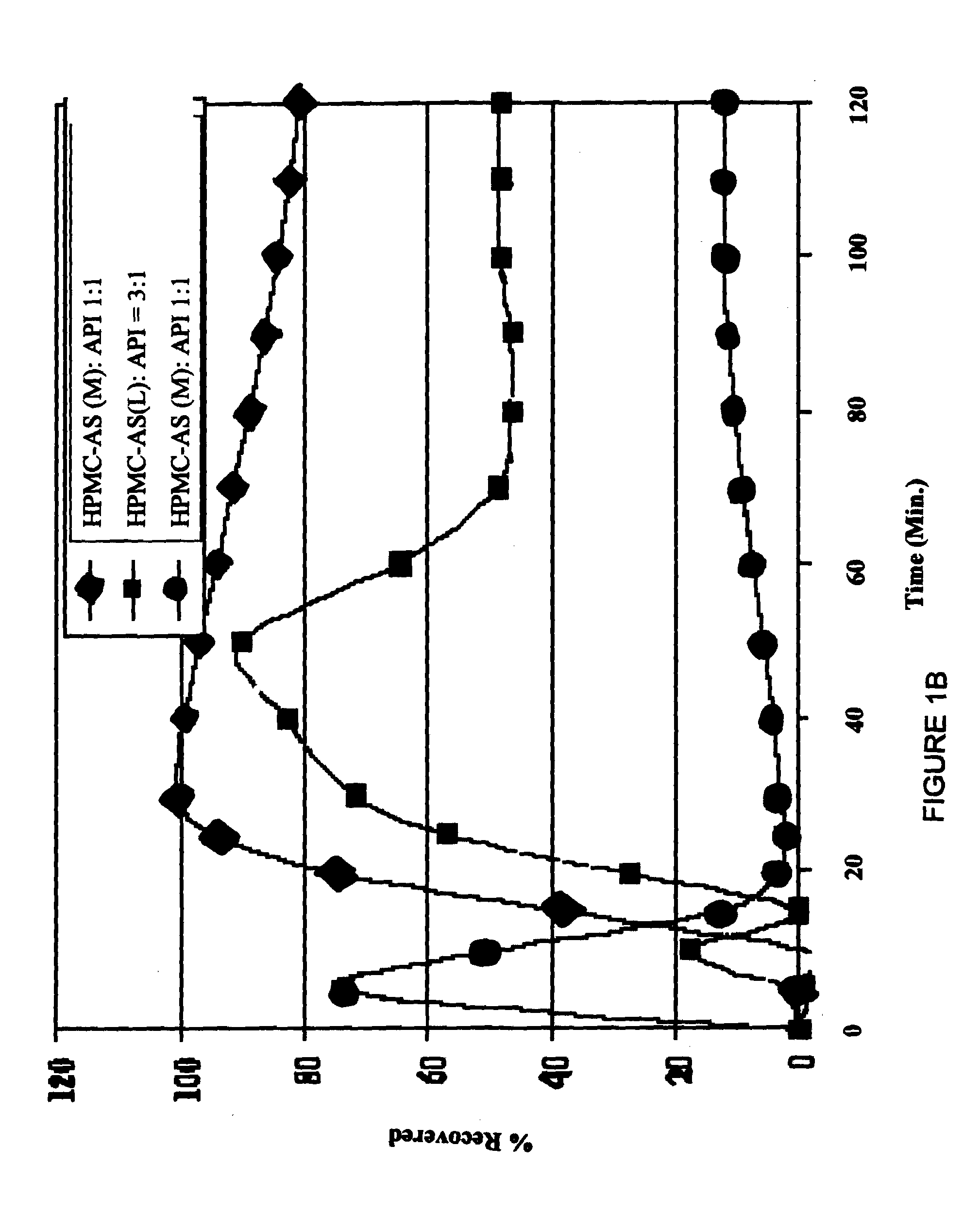
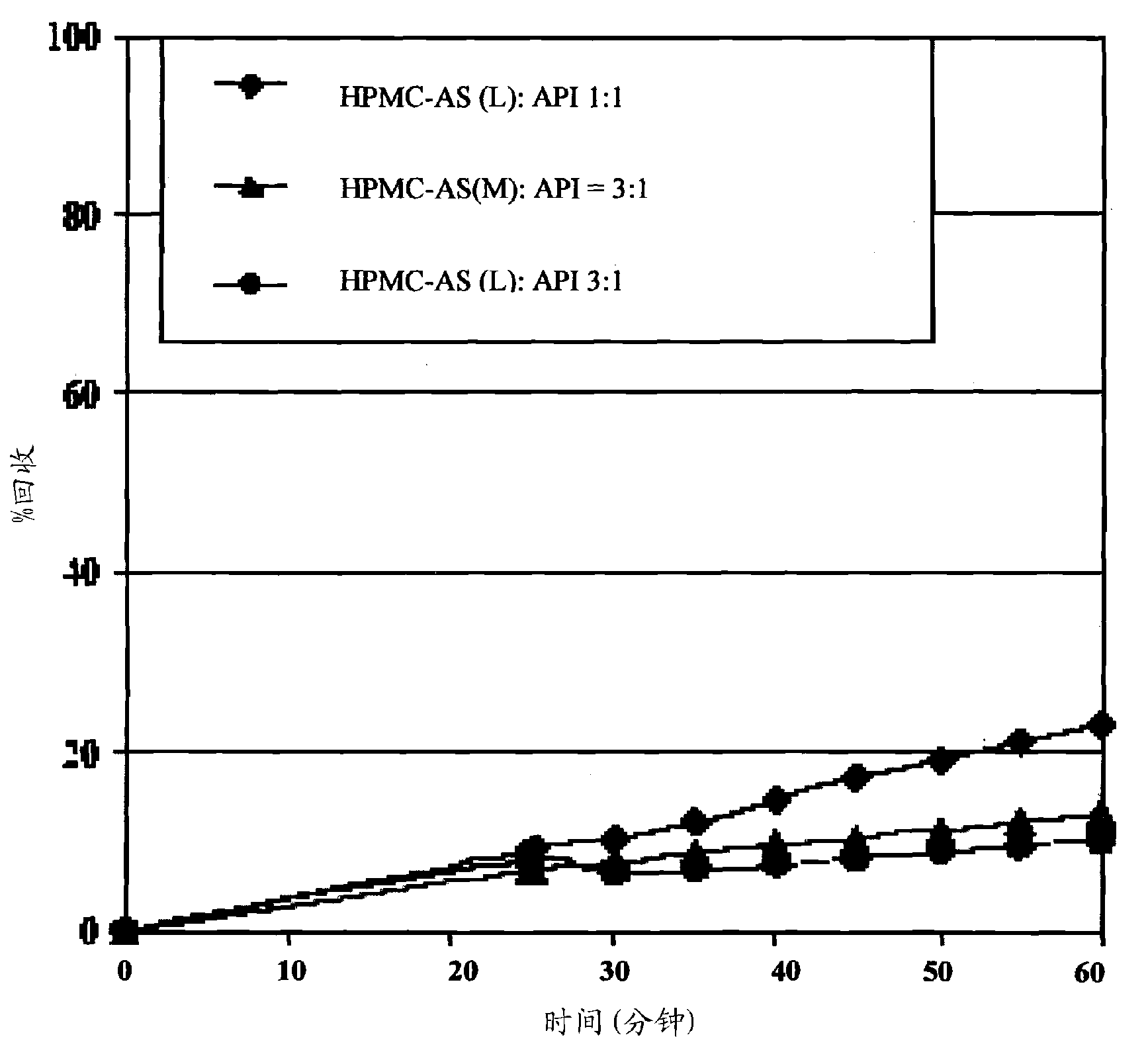
Oral pharmaceutical compositions in a solid dispersion comprising preferably posaconazole and HPMCAS
The present application provides novel compositions comprising posaconazole and a polymer wherein the composition has a glass transition temperature temperature (Tg) of less than about 110 DEG C. The application also describes compositions comprising posaconazole and a polymer having a bulk density of greater than about 0.4 mg / mL. The application also describes compositions comprising posaconazole and a polymer which provide an exposure (AUCtf) of at least about 10,000 ng.hr / mL when administered to a patient in a fasted state. The application also describes a novel process for preparing these compositions.
Owner:MERCK & CO INC
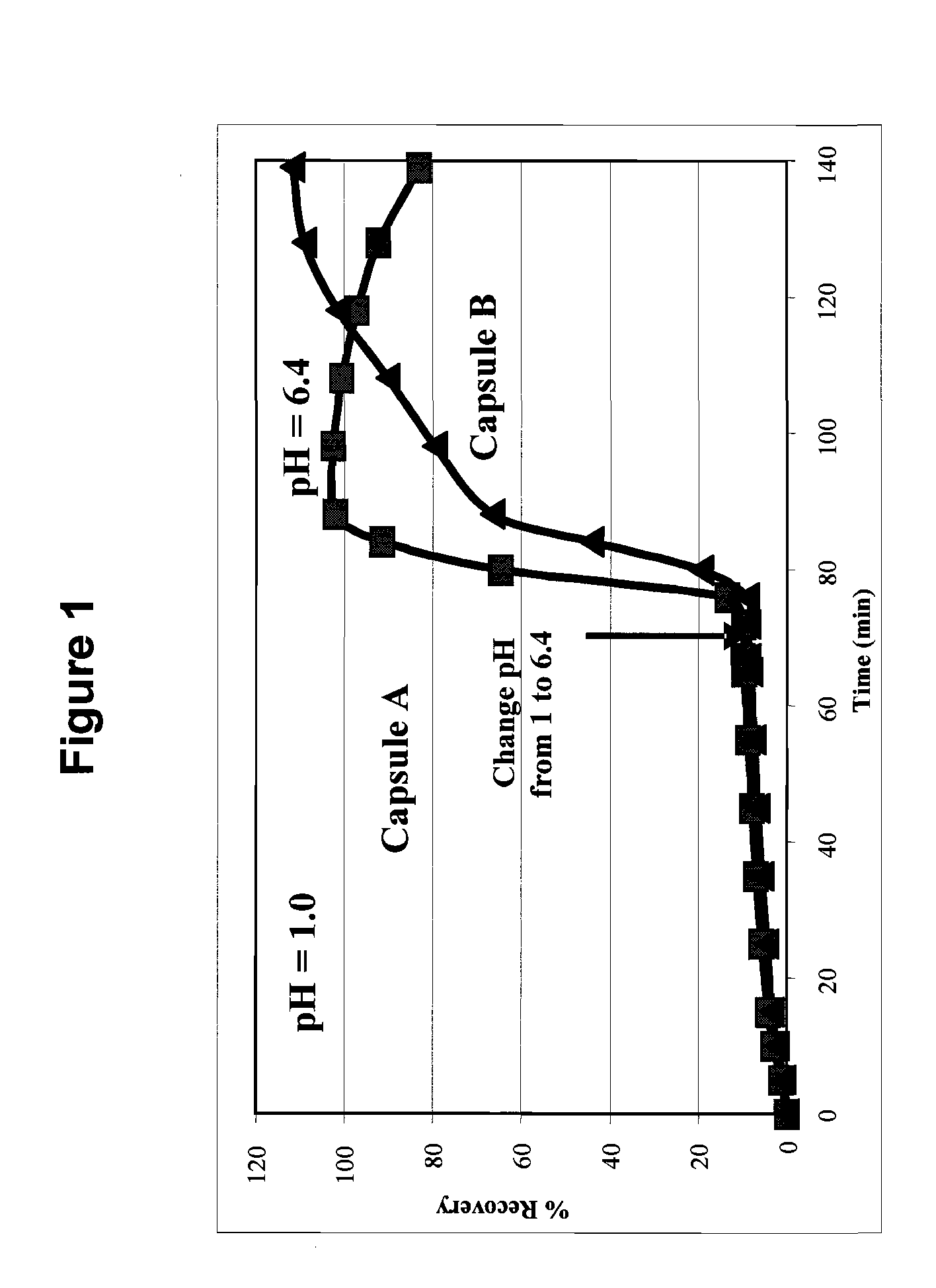
Delayed release posaconazole tablets
The present invention relates to a delayed release composition comprising Posaconazole dissolved or molecularly dispersed in a polymer other than a hydroxypropyl methylcellulose derived polymer; wherein the composition is prepared by hot melt extruding an admixture of Posaconazole and the polymer. The present invention also provides a process of preparing said composition.
Owner:CADILA HEALTHCARE LTD
Oral Pharmaceutical Compositions in a Solid Dispersion Comprising Preferably Posaconazole and HPMCAs
InactiveUS20110034478A1Low variabilityReduce the impactOrganic active ingredientsAntimycoticsPH-sensitive polymersWater soluble
The present invention provides a solid molecularly dispersed composition comprising a poorly water soluble and weakly basic azole antifungal compound and a pH sensitive polymer, pharmaceutical compositions, comprising the solid molecularly dispersed composition of the invention and methods of treating and / or preventing a fungal infection in a patient in need thereof comprising orally administering a pharmaceutical composition comprising a composition of the invention to a patient in need thereof. Preferably the antifungal, compound is posaconazole, and the pH sensitive polymer is HPMCAS.
Owner:MERCK SHARP & DOHME CORP
Method of treating a patient with a CYP3A4 substrate drug
ActiveUS10376507B2Reduce morbidityAvoid and reduce incidenceNervous disorderHydroxy compound active ingredientsReduced doseCYP3A4 Inhibitor
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug contraindicated for concomitant administration with a strong CYP3A4 inhibitor, wherein the patient is treated with multiple doses of posaconazole, stops posaconazole treatment, and then is treated with the CYP3A4 substrate drug. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-21 after stopping posaconazole. In some embodiments, the patient is treated with or prescribed a reduced dose of the CYP3A4 substrate drug for about 2-21 after stopping posaconazole.
Owner:BOW RIVER LLC
Solid dispersion of antifungal agent
InactiveCN104546724AImprove solubilityPromote absorptionOrganic active ingredientsPowder deliveryAntifungalSmall intestine
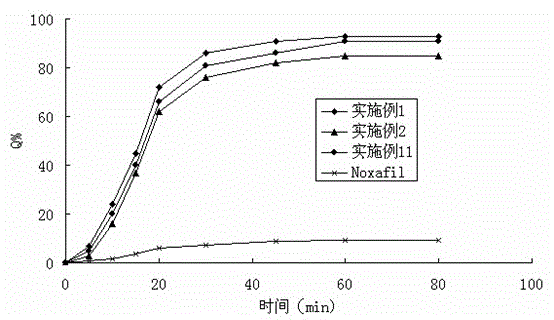
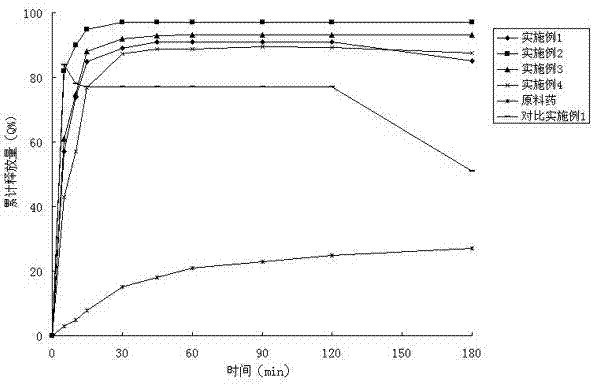
The invention belongs to the field of a medicinal preparation and specifically relates to a posaconazole solid dispersion and a preparation method of the solid dispersion and drug use. The invention provides a posaconazole solid dispersion and its drug use. The posaconazole solid dispersion contains posaconazole, a cyclodextrin derivative and a water-soluble or enteric carrier material, wherein mass ratio of posaconazole to the cyclodextrin derivative is 1:1-4 or mass ratio of posaconazole to the cyclodextrin derivative to the water-soluble or enteric carrier material is 1:1-4:0.001-3. The posaconazole solid dispersion can remarkably raise dissolution rate of the difficultly-soluble antifungal agent posaconazole in the small intestine and also can significantly enhance bioavailability of posaconazole. By the use of a dosage form prepared from the solid dispersion, the preparation technology is simple, and quality of the dosage form is stable.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Freeze-drying composition of posaconazole prodrug and preparation method and application of freeze-drying composition of posaconazole prodrug
InactiveCN105287403AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliverySolubilityCase fatality rate
The invention relates to a freeze-drying composition of a posaconazole prodrug and a preparation method and application of the freeze-drying composition of the posaconazole prodrug. The freeze-drying composition has the advantages that the freeze-drying composition is high in water solubility, and safety of the freeze-drying composition is guaranteed due to the fact that cyclodextrins auxiliary materials need not to be added during the preparation of the freeze-drying composition; the freeze-drying composition is suitable for being used for treating various amphotericin-intolerant or refractory adult invasive fungal infections; the freeze-drying composition is used as a preventive drug for high-risk patients, the freeze-drying composition is applicable to patients above 13 years old and with impaired immunity and especially applicable to patients who have graft versus host disease (GVHD) after hematopoietic stem cell transplant, patients with leukemia and patients with long-term leukopenia due to chemotherapy; compared with control drugs such as fluconazole and itraconazole, the freeze-drying composition can effectively prevent invasive aspergillosis and can lower the mortality related to the invasive fungal infections.
Owner:HC SYNTHETIC PHARMA CO LTD
Method of treating a patient with a cyp3a4 substrate drug
InactiveUS20180333410A1Reduce morbidityAvoid and reduce incidenceNervous disorderHydroxy compound active ingredientsReduced dosePosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug contraindicated for concomitant administration with a strong CYP3A4 inhibitor, wherein the patient is treated with multiple doses of posaconazole, stops posaconazole treatment, and then is treated with the CYP3A4 substrate drug. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-21 after stopping posaconazole. In some embodiments, the patient is treated with or prescribed a reduced dose of the CYP3A4 substrate drug for about 2-21 after stopping posaconazole.
Owner:BOW RIVER LLC
Novel crystalline forms of conazoles and methods of making and using the same
InactiveUS20090088443A1Decreased food effectReduce the impactOrganic active ingredientsPowder deliveryWhole bodyPosaconazole
The invention provides novel soluble conazole crystalline forms (e.g. itraconazole, posaconazole and saperconazole) that include salts, co-crystals and related solvates useful as pharmaceuticals. The invention also provides pharmaceutical compositions comprising, and processes for making, these conazole crystalline forms. Methods of using such compositions for the treatment or prevention of systemic and local fungal, yeast, and dermatophyte infections are also provided.
Owner:REMENAR JULIUS +4
Antifungal medicament solid dispersion
InactiveCN104721141APrevent crystallizationImprove stabilityPowder deliveryOrganic active ingredientsAntifungal drugPosaconazole
The invention provides an antifungal medicament solid dispersion, and in particular relates to a posaconazole solid dispersion dispersed in a polymer skeleton. The polymer skeleton comprises two polymers, a first polymer can realize uniform dispersion or molecular dispersion of posaconazole in the polymer skeleton, and a second polymer can construct a microenvironment for improving the dissolution rate of posaconazole in an aqueous environment. Further, the polymer skeleton preferably comprises Soluplus and EudragitE100 in the weight ratio of 1:1.5-4. The posaconazole solid dispersion provided by the invention has good stability, good solubility in the gastrointestinal tract, and high bioavailability.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
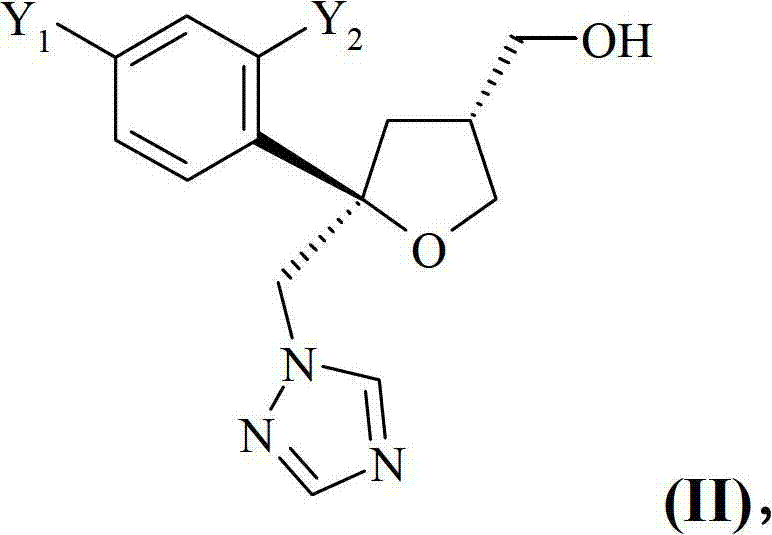
Preparation of posaconazole intermediates
The present invention relates to process for the preparation of a chiral compound of formula (IX) or a salt thereof, wherein Y1 and Y2 are independently F or C1, preferably F, the crystalline compound of formula (IX) as such, and its use for the preparation of an antifungal agent.
Owner:SANDOZ LTD
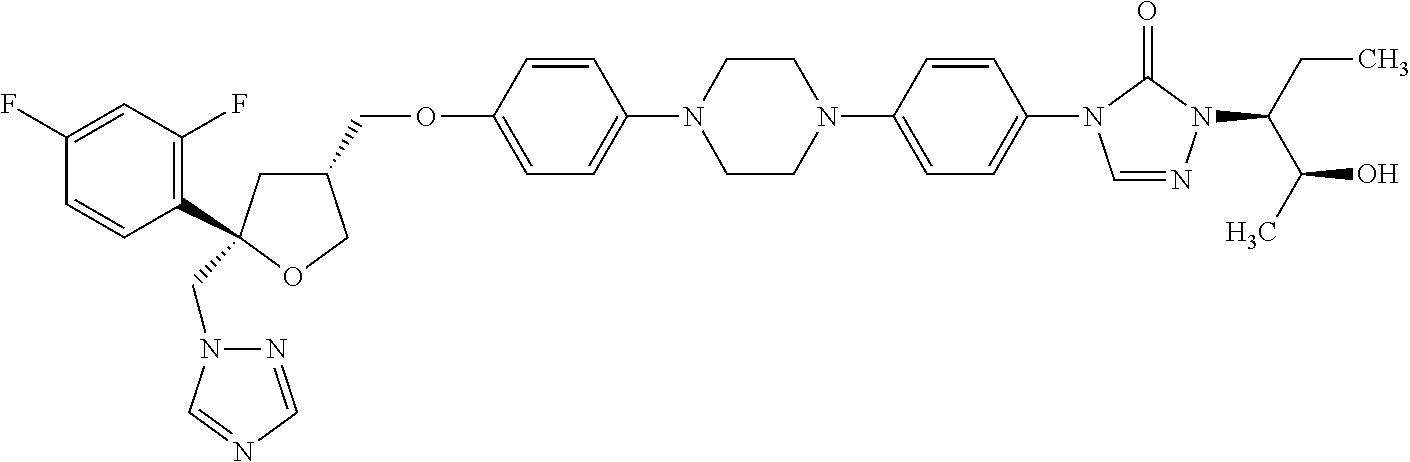
Posaconazole derivative, synthesis and application in prolonged action preparation thereof
InactiveCN106432338AOrganic active ingredientsAntimycoticsProlonged-Action PreparationsChemical compound
The invention relates to a posaconazole derivative, synthesis and an application in a prolonged action thereof. The invention relates to a formula of a compound and salt, N-oxide, quaternary ammonium and stereoisomer of the compound, wherein R1-R8 are defined according to what is claimed. The invention also relates to a preparation formula of an intermediate body and a method of the compound. The invention further relates to a formula of an application of the compound as a drug, especially the application in preventing or treating fungal infection. The detailed formulas are in the specification.
Owner:HC SYNTHETIC PHARMA CO LTD
Preparation method of posaconazole intermediate
InactiveCN102643194ANovel process routeReasonable process conditionsOrganic compound preparationCarboxylic acid esters preparationBiochemical engineeringPharmaceutical drug
The invention belongs to the technical field of pharmacochemistry and in particular relates to a preparation method of a posaconazole intermediate. The method comprises four steps of alkylation, reduction, acylation and cross-coupling, so that a target compound (I) is obtained. Compared with the existing synthetic method, the synthetic method provided by the invention has the characteristics of low price and easiness in obtainment of raw materials, simple and convenient technology, high yield and the like.
Owner:FUZHOU UNIV
Methods of treatment
ActiveUS10857144B2Avoid and reduce incidenceSafety managementOrganic active ingredientsNervous disorderPharmaceutical drugPosaconazole
The present disclosure provides for methods of treating a patient with a CYP3A4 substrate drug, wherein the patient is treated with posaconazole. In some embodiments, the patient stops posaconazole treatment, waits for at least 2 days, and then is treated with the CYP3A4 substrate drug as soon as it is safe to do so. In some embodiments, treatment with the CYP3A4 substrate drug is delayed for about 2-42 days after stopping posaconazole. In some embodiments, the patient is treated with a reduced dose of the CYP3A4 substrate drug for about 2-42 days.
Owner:BOW RIVER LLC
Methods of treatment with CYP3A4 substrate drugs
ActiveUS10835529B2Avoid and reduce incidenceSafety managementOrganic active ingredientsPharmaceutical drugPosaconazole
Owner:BOW RIVER LLC
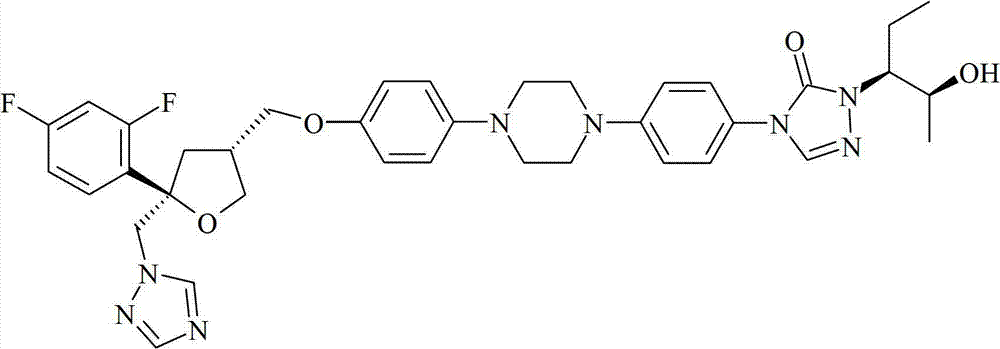
Posaconazole, composition, intermediate, preparation method and application thereof
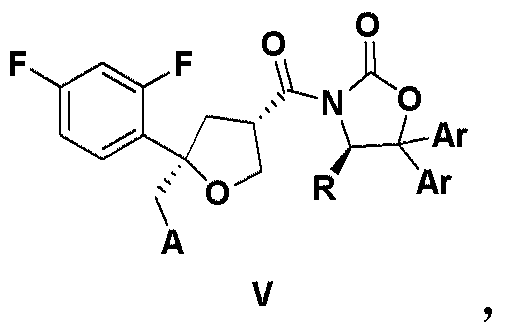
ActiveCN105503765AHigh purityFacilitate efficient separationOrganic chemistryArylCombinatorial chemistry
The invention relates to a compound as shown in the formula III, wherein R is selected from C1-C4 alkyl group, substituted or unsubstituted phenyl group and substituted or unsubstituted benzyl group, and is preferably selected from isopropyl group; and two Ar can be the same or different, respectively and separately selected from substituted or unsubstituted aryl group, and preferably selected from substituted or unsubstituted phenyl group, such as methoxyphenyl, etc. The compound is preferably in a solid form.
Owner:ZHEJIANG AUSUN PHARMA
Posaconazole solid dispersion and preparation method thereof
InactiveCN104510707ASimple preparation processImprove preparation qualityPowder deliveryOrganic active ingredientsAntifungalPharmacy
The invention belongs to the technical field of pharmacy, and specifically relates to a posaconazole solid dispersion and a preparation method thereof. The invention discloses a posaconazole solid dispersion which is composed of posaconazole and a water-soluble polymer according to a weight ratio of 1:0.5-10, and a pharmaceutical composition comprising the same. The provided posaconazole solid dispersion can prominently improve the dissolution rate of antifungal agent posaconazole, which is hard to dissolve, in small intestines, and is capable of prominently increasing the biological utilization rate of posaconazole. The pharmaceutical dosage forms prepared from the pharmaceutical composition comprising the provided solid dispersion has the advantages of simple preparation technology and stable quality.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Purification of posaconazole and posaconazole intermediates
The present invention relates to a process for the preparation of a hydrogen chloride (HC1) salt of a compound of formula (I) wherein Y1 and Y2 are independently F or C1, preferably F, said compound of formula (I) containing the cis-isomer and the trans-isomer, wherein the process comprises (1) providing the compound of formula (I) comprised in a first suitable solvent; and (2) treating the compound of formula (I) comprised in the first suitable solvent with HC1 comprised in a second suitable solvent to obtain the HC1 salt of the compound of formula (I).
Owner:SANDOZ LTD
Posaconazole pharmaceutical compositions and preparation methods, uses and pharmaceutical formulations thereof
ActiveUS20170027931A1Organic active ingredientsAntimycoticsPharmaceutical formulationEthylene glycol
The present invention relates to a pharmaceutical composition comprising posaconazole and a carrier material, wherein the carrier material comprises a vinylpyrrolidone-vinyl acetate copolymer or a polymer containing ethylene glycol units. The present invention also relates to a method for the preparation of the pharmaceutical composition, a method for the prevention and / or treatment of fungal infections and related diseases in a mammal using the pharmaceutical composition, and a pharmaceutical formulation comprising the pharmaceutical composition.
Owner:SINOTHERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com