Preparation method of posaconazole intermediate
A technology for posaconazole and intermediates, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of expensive, complicated product separation, and low yield, and achieve the effects of low production cost, high reaction yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0058] Example 1 2-(2'-chloropropenyl)-synthesis of diethyl malonate (structural formula III, wherein R=C 2 h 5 )
[0059] Diethyl malonate (115 g, 715 mmol) and potassium iodide (30 mg) were added dropwise to ethanol (250 mL) containing 49.0 g (720 mmol) sodium ethoxide at room temperature. After refluxing for 10 min, 2,3-dichloropropene (78.0 g, 706 mmol) was added dropwise to the reaction solution, and the addition was completed within 2 h. Continue to reflux for 2 h and cool to room temperature. The solvent was distilled off under reduced pressure to obtain the residual oil, which was added with 300 mL of water and extracted with ethyl acetate (2?? 500 mL). The extracts were combined, washed with saturated sodium chloride (300 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the remaining oil was distilled under reduced pressure (bp: 94-100 °C / 3-4 mmHg) to obtain 2-(2'-chloropropenyl)-diethyl malonate ( (147 g, 89.1%...
example 2
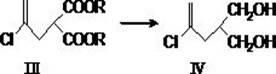
[0060] Example 2 Synthesis of 2-(2'-chloropropenyl)-1,3-propanediol (structural formula IV)
[0061] At 0??C, add 2-(2'-chloropropenyl)-diethyl malonate (30.0 g, 128 mmol) to a mixture containing 73 mL of tetrahydrofuran and 7.30 g (190 mmol) of lithium aluminum hydride ) in tetrahydrofuran (50 mL), dropwise over 30 min, stirred at this temperature for 10 min, and then refluxed for 8 h. After cooling with ice water, the reaction solution was slowly added to dilute hydrochloric acid (200 mL, prepared by mixing 170 mL ice water and 35 mL concentrated hydrochloric acid) while stirring. Diethyl ether (100 mL) was added to extract (2?? 100 mL), the combined extracts were washed with saturated brine, dried over anhydrous sodium sulfate, and filtered. The diethyl ether solvent was distilled off under reduced pressure to obtain a light yellow solid (17.6 g, 91.7%), which is the crude product of 2-(2′-chloropropenyl)-1,3-propanediol, which was directly used in the next step without fu...
example 3
[0062] Example 3 Synthesis of 2-(2'-chloropropenyl)-1,3-propanediol diacetate (structural formula V, wherein R 1 =CH 3 )
[0063] At room temperature, add dropwise Acetic anhydride (14.0 g, 138 mmol), stirred at room temperature for 4 h, then carefully added saturated sodium bicarbonate solution (150 mL). The layers were separated, and the organic phase was washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and filtered. The solvent was removed under reduced pressure to obtain the pale yellow title compound (15.1 g, 97.0%). 1 H NMR (500 MHz, CDCl 3 ) δ 5.24 (d, J = 1.3 Hz, 1 H), 5.18 (d, J = 1.1 Hz, 1 H), 4.10 (dd, J = 11.2, 4.6 Hz, 2 H), 4.05 (dd, J = 11.2, 5.6 Hz, 2 H), 2.46–2.40 (m, 3 H), 2.05 (s, 6 H); IR (KBr) = 3110, 2960, 1745, 1637, 1435, 1368, 1232, 1158, 1043, 890, 635 cm -1 ; MS (ESI + ) m / z : 235 [M+1] + , 257 [M+Na] + . Anal. Calcd for C 10 h 15 ClO 4 (234.68): C 51.18, H 6.44; Found: C 51.11, H 6.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com