Method for preparing talipexole
An allyl and amino technology, which is applied in the field of preparation of talixol, can solve the problems of high cost, long steps, and difficulty in industrialization, and achieve the effects of low production cost, short reaction steps, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
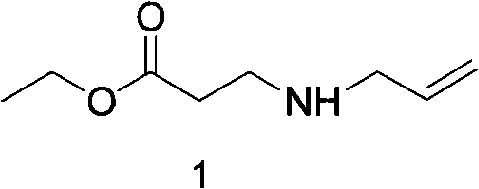
[0029] (1) Preparation of ethyl 3-allylaminopropionate
[0030] 100g (0.1mol) ethyl acrylate, 57g allylamine, dissolved in 500ml ethanol, reacted at 30°C for 2 hours, spin off the solvent, and distill under reduced pressure to obtain 130g of ethyl 3-allylaminopropionate with a yield of 84.2% .
[0031] 1 H NMR (300MHz, CDCl3) δ: 5.93-5.79 (m, 1H), 5.19-5.04 (m, 2H), 4.12 (q, J = 6.0 Hz, 1H), 3.25-3.22 (m, 2H), 2.85 ( t, J=6.3Hz, 2H), 2.49(t, J=6.3Hz, 2H), 1.22(t, J=6.0Hz, 3H)
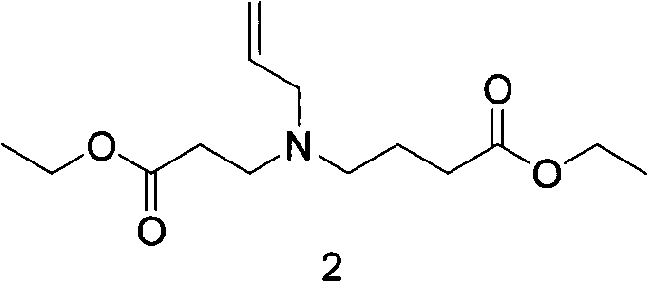
[0032] (2) Preparation of ethyl 4-(allyl(3-ethoxy-3-oxopropyl)amino)butyrate
[0033] 79g (0.5mol) ethyl 3-allylaminopropionate was dissolved in 300ml acetone, 138g (1mol) potassium carbonate was added, 115g ethyl 4-bromobutyrate was added at room temperature, and reacted at 40°C for 3 hours. Pour 3 liters of ice water, stir for 20 minutes, then add 2 liters of methyl tert-butyl ether for 3 extractions, wash with saturated brine, dry, and spin-dry to obtain 140 g, and distill under reduced pressure to obtain ...
Embodiment 2
[0044] (1) Preparation of ethyl 3-allylaminopropionate
[0045] 100g (0.1mol) ethyl acrylate, 57g allylamine, dissolved in 500ml ethanol, reacted at 60°C for 2 hours, spin-dried the solvent, and distilled under reduced pressure to obtain 140g ethyl 3-allylaminopropionate with a yield of 89.2% .
[0046] (2) Preparation of ethyl 4-(allyl(3-ethoxy-3-oxopropyl)amino)butyrate
[0047] 79g (0.5mol) ethyl 3-allylaminopropionate was dissolved in 300ml DMF, 138g (1mol) potassium carbonate was added, 115g ethyl 4-bromobutyrate was added at room temperature, and reacted at 80°C for 3 hours. Pour 3 liters of ice water, stir for 20 minutes, add 2 liters of methyl tert-butyl ether for 3 extractions, wash with saturated brine, dry, and spin-dry to obtain 140 g, and distill under reduced pressure to obtain 110 g, with a yield of 81.2%.
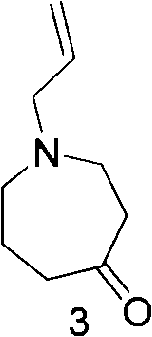
[0048] (3) Preparation of 1-allylazacycloheptan-4-one
[0049] Add 1 liter of toluene, add 85 g of potassium tert-butoxide, and heat to reflux until the potassium t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com