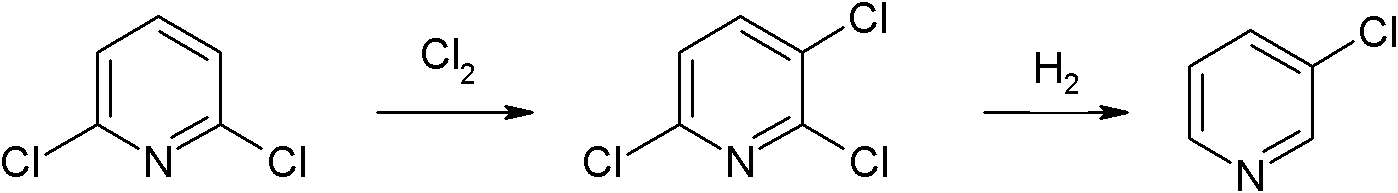
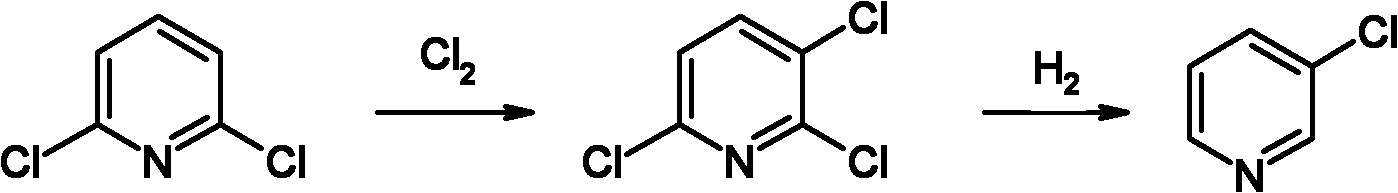
Preparation method of 3-chloropyridine
A technology of chloropyridine and trichloropyridine, which is applied in the field of preparation of important fine chemical intermediate 3-chloropyridine, can solve the problems of multiple synthesis steps, complex reaction types, and high raw material prices, and achieve high-efficiency separation and recovery technology and novel process routes simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Chlorination
[0033] Weigh 1480.0g of 2,6-dichloropyridine and anhydrous FeCl 3 89.2g was placed in a 2000ml four-necked flask and mixed and heated up. When the temperature reached 120~140℃, chlorine gas was introduced. After the reaction was complete, the temperature was lowered to 100℃, and vacuum distillation was carried out at -0.1MPa, the top temperature was 118~ The product fractions are collected at 124°C, and the low-concentration distillate produced during the rectification process can be applied to the next batch for re-reaction or applied to the next batch for re-rectification and purification. After the application, 1715.0 g of 2,3,6-trichloropyridine is finally obtained, the total yield after the application is 94.0%, and the purity is ≥99.5%.
[0034] Hydrogenation reaction
[0035] Put 557.8g of 2,3,6-trichloropyridine prepared in the chlorination reaction, 232.0g of triethylamine, 8.5g of palladium on carbon, and 1675g of toluene into the reactor at one time...
Embodiment 2
[0039] Chlorination
[0040] Weigh 299.2g of 2,6-dichloropyridine and 533.7g of the recovered fraction (of which 2,6-dichloropyridine accounts for 86.1%, 2,3,6-trichloropyridine accounts for 9%), and anhydrous AlCl 3 36.5g is placed in the reactor and mixed, and when the temperature is raised to 120~140℃, chlorine gas is introduced. After the reaction is completed, the temperature is lowered to 100℃, and vacuum distillation is carried out. The product fraction is collected at -0.1MPa and the top temperature is 118~124℃. , The low-concentration product steamed out can be applied to the next batch for re-reaction or applied to the next batch for re-rectification and purification; after the application, 890.7g of 2,3,6-trichloropyridine is finally obtained, and the total yield after application is 95.2%, with purity ≥99.5%.
[0041] Hydrogenation reaction
[0042] 1340g of the toluene solution containing 2,3,6-trichloropyridine recovered in Example 1 can be added with 410.5g of 2,3,6-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com