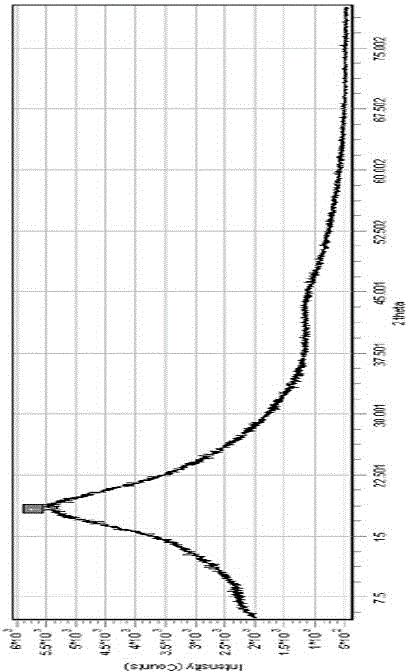
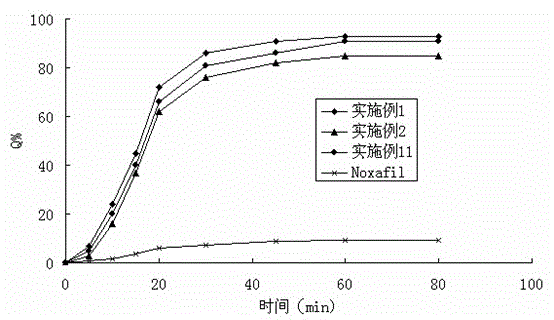
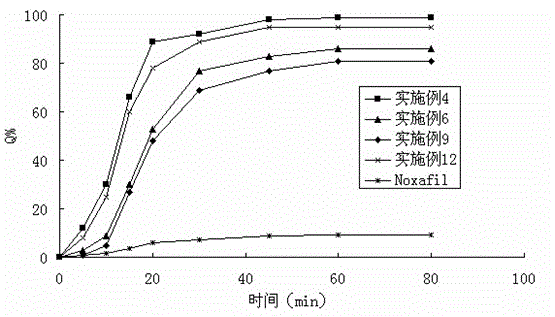
Solid dispersion of antifungal agent
A solid dispersion and dispersion technology, applied in antifungal agents, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of poor solubility in the small intestine and low bioavailability, and achieve stable preparation quality, simple preparation process, and improved solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of solid dispersion containing posaconazole and HP-β-CD (1:2)
[0050] Dissolve 3 g of posaconazole in 15 ml of methanol and HCl conc (10:1, v / v) mixed solution; Dissolve 6g HP-β-CD in 6ml purified water, then mix the two solvents, stir for 2h to get a clear solution, then pass the clear solution through 0.45μm polytetrafluoroethylene Filtrate with a filter (Rezist 30, Whatman, Dassel, Germany); cool in liquid nitrogen, and finally freeze-dry for 56 hours to obtain a white solid powder.
Embodiment 2
[0051] Example 2 Preparation of solid dispersion containing posaconazole and HBen-β-CD (1:4)
[0052] Dissolve 3 g of posaconazole in 15 ml of methanol and HCl conc (10:1, v / v); Dissolve 12g of HBen-β-CD in 12ml of purified water. Then the two solvents were mixed and stirred for 2 hours to obtain a clear solution, which was spray-dried with a spray dryer, the temperature at the inlet of the dryer was 115°C, and the temperature at the outlet was 65°C. After drying, the powder was collected and cooled to obtain a white solid powder.
Embodiment 3
[0053] Example 3 Preparation of solid dispersion containing posaconazole and SEB-β-CD (1:1)
[0054] Dissolve 3 g of posaconazole in 15 ml of methanol and HCl conc (10:1, v / v) mixed solution; 3g SEB-β-CD was dissolved in purified water to make a 50% SEB-β-CD solution. Then the two solvents were mixed, stirred for 2 hours to obtain a clear solution, and then the clear solution was filtered through a 0.45 μm polytetrafluoroethylene filter; cooled with liquid nitrogen, and finally freeze-dried for 56 hours to obtain a white solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com