Patents
Literature
340 results about "Control drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
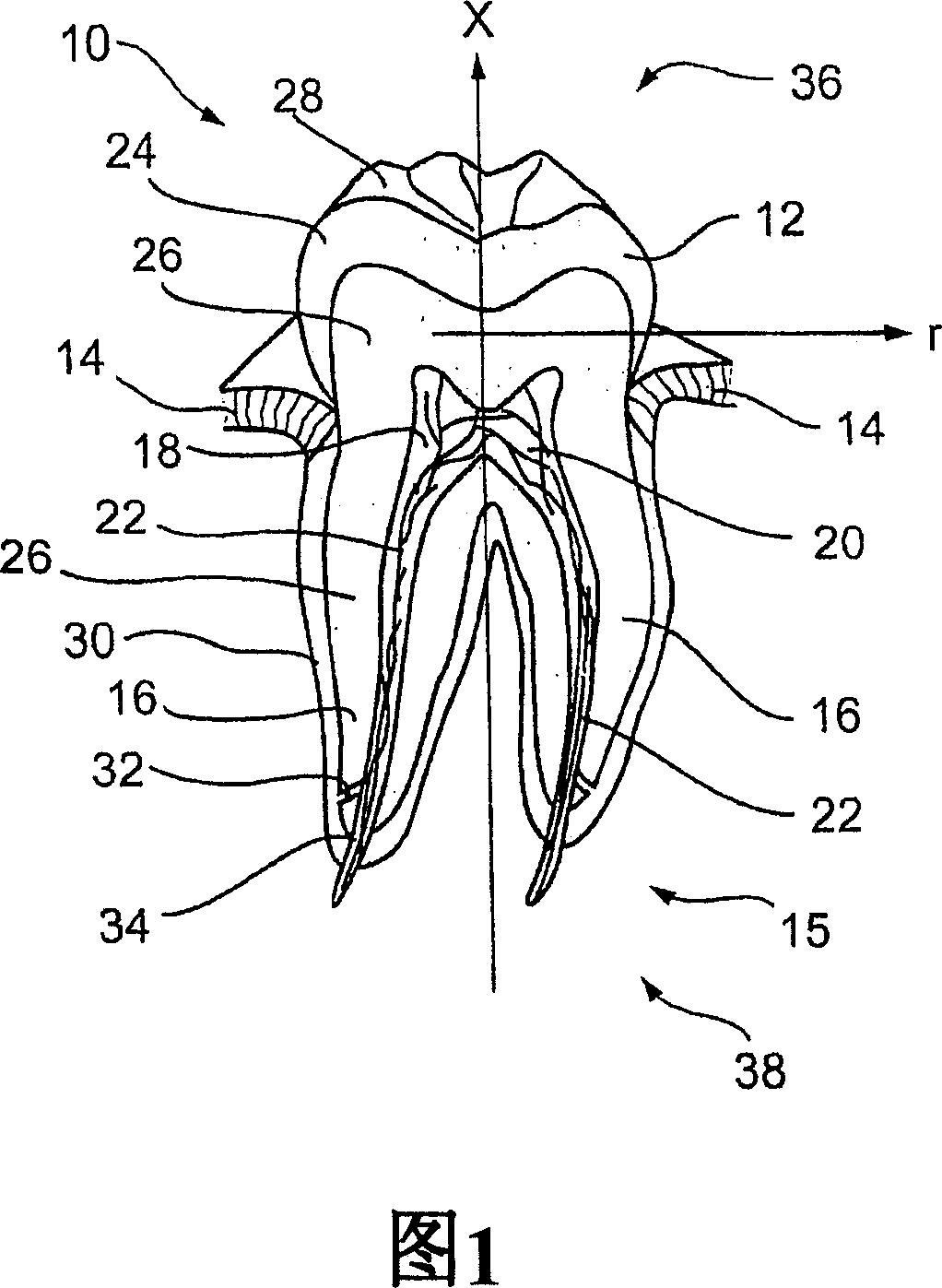
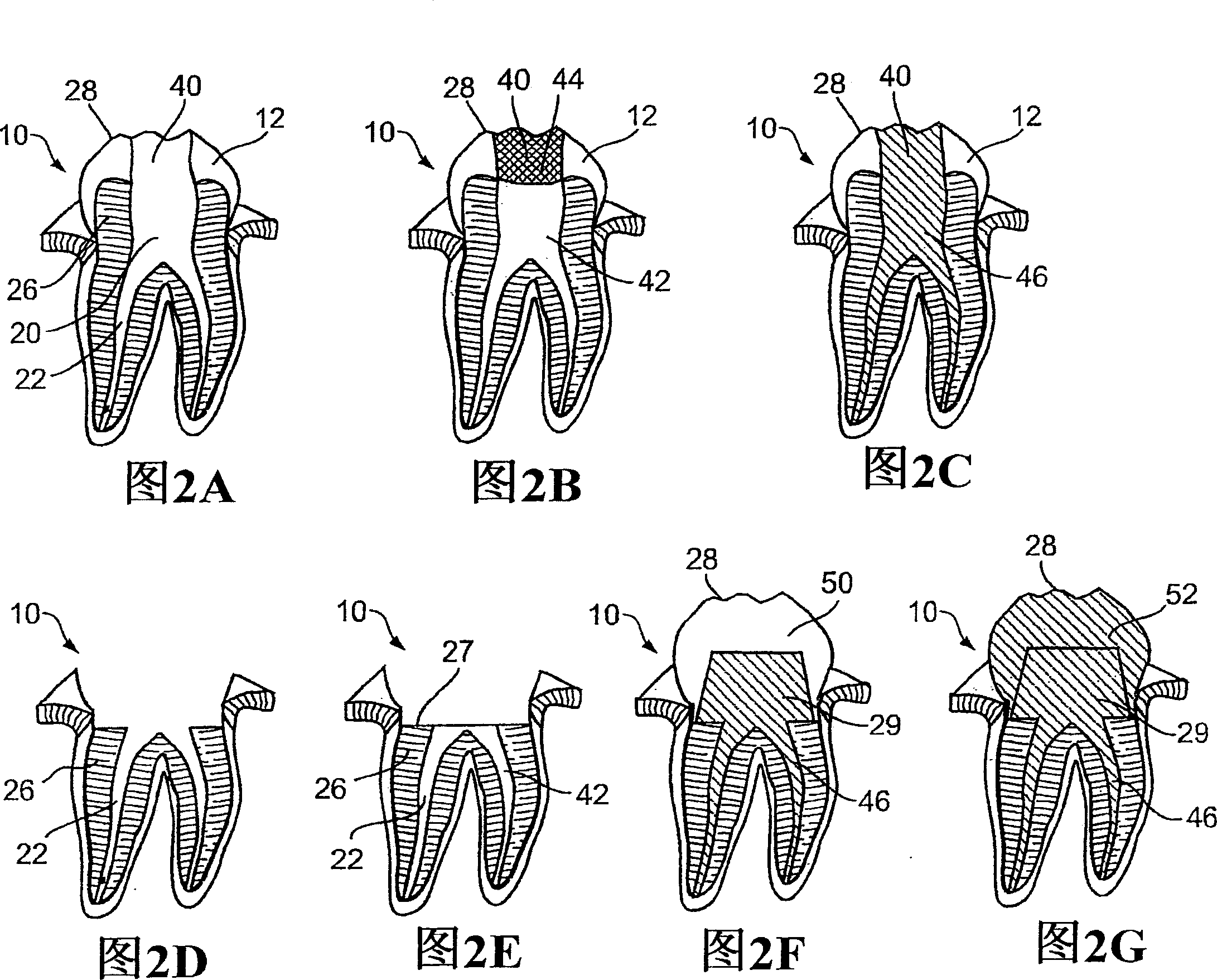
A controlled substance is simply a drug that is being controlled by the government. The government regulates the manufacture of the controlled substance; it regulates who can possess the drugs; and it also regulates who can use the drugs.
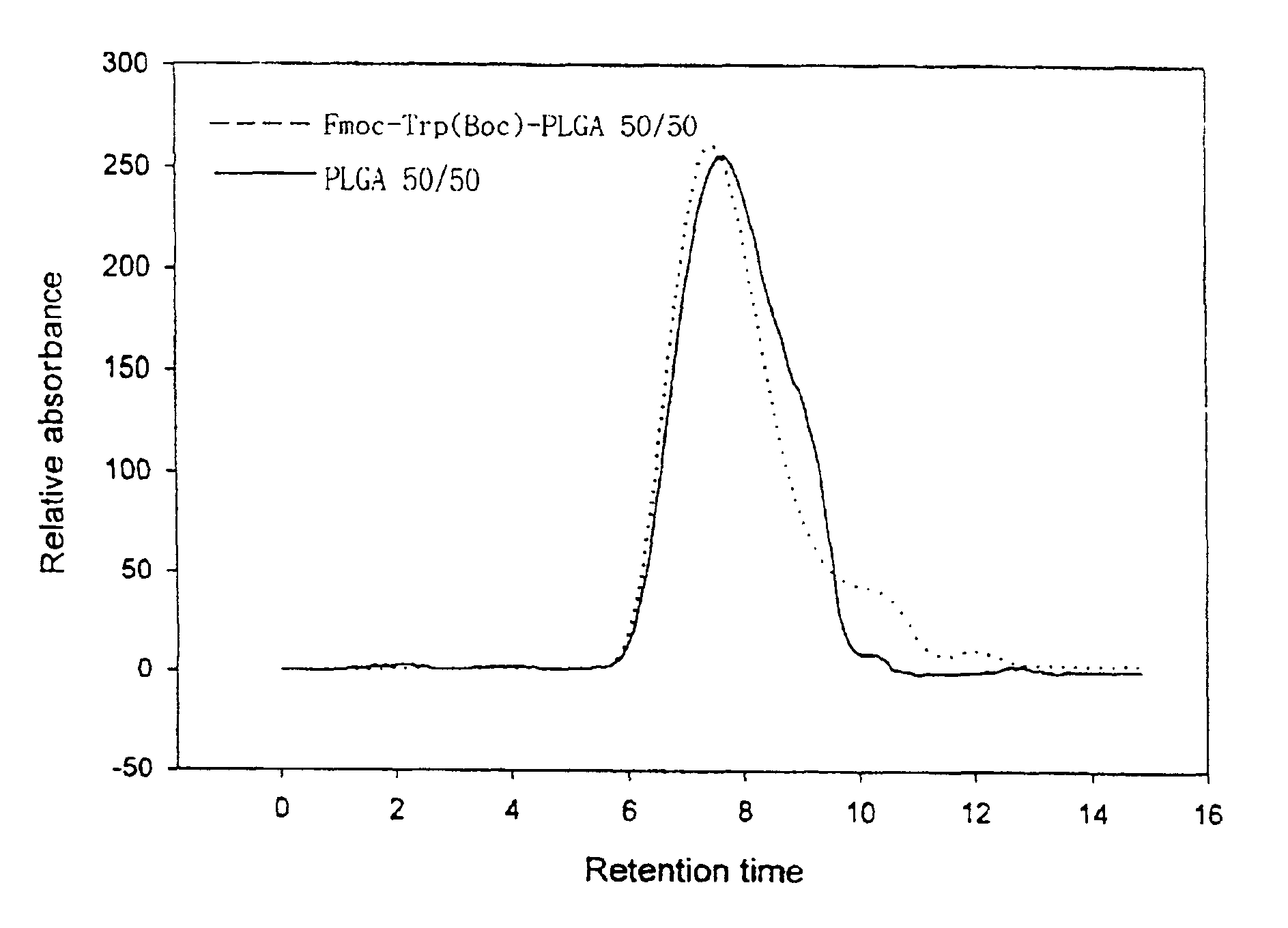
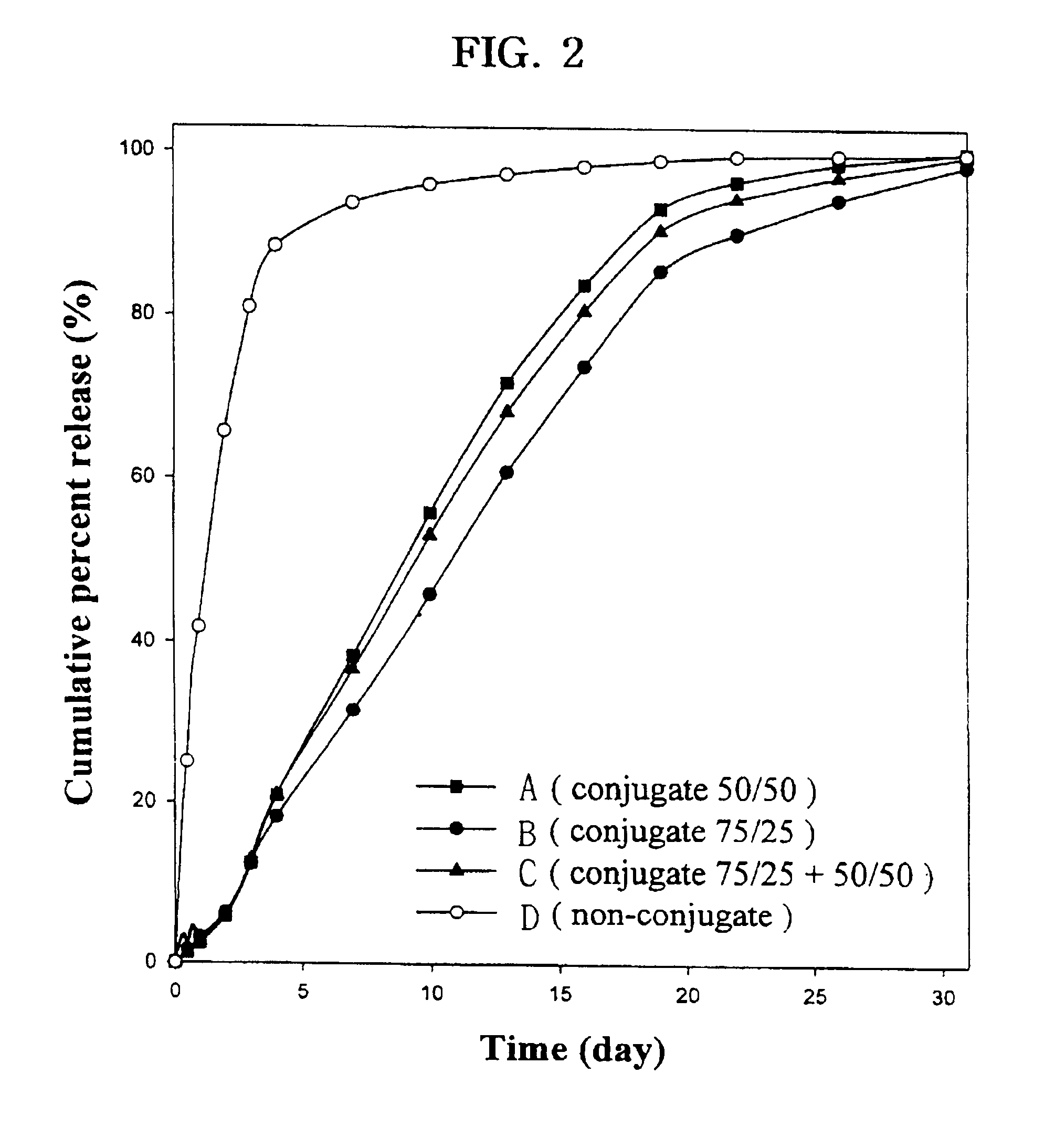
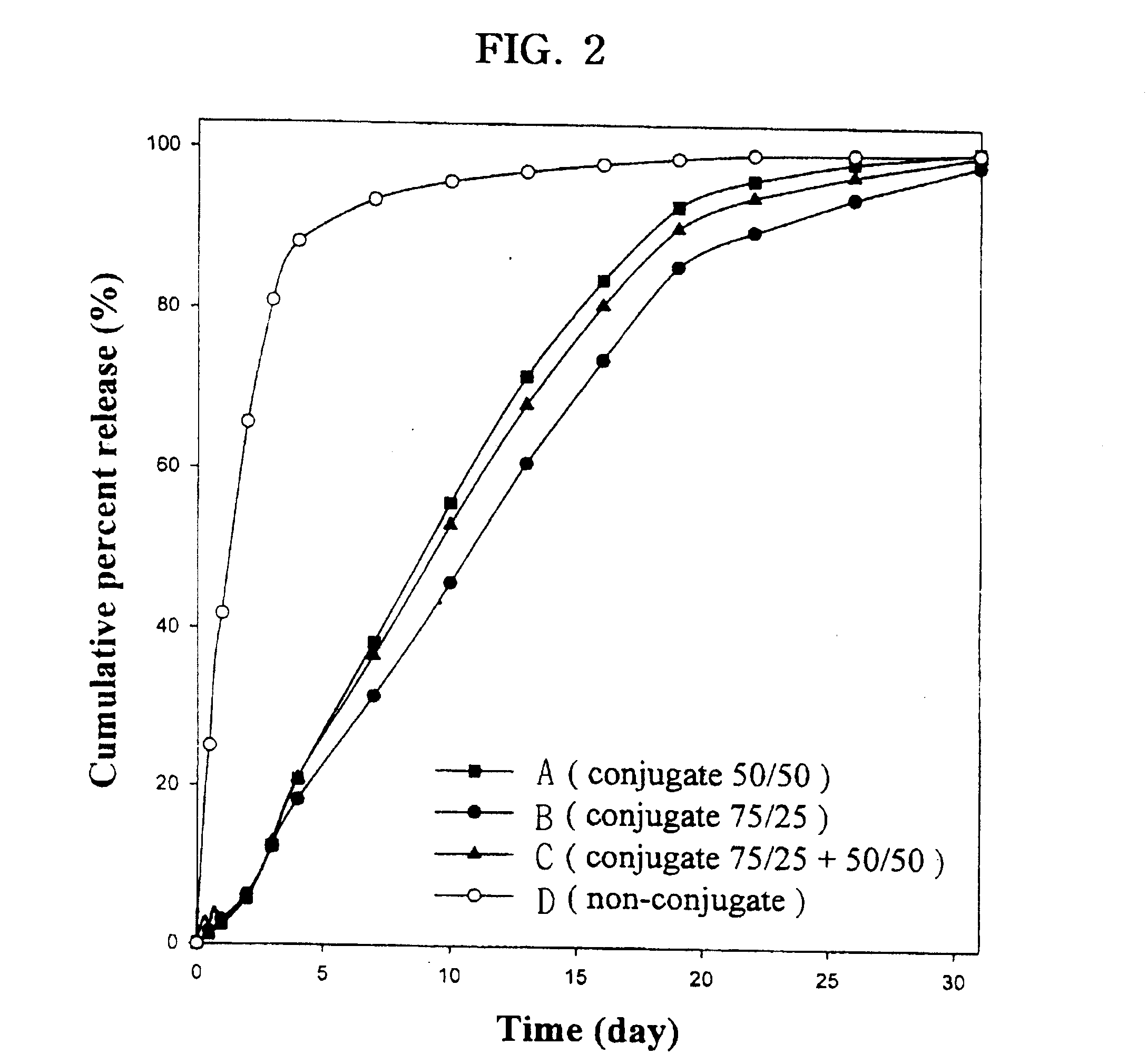
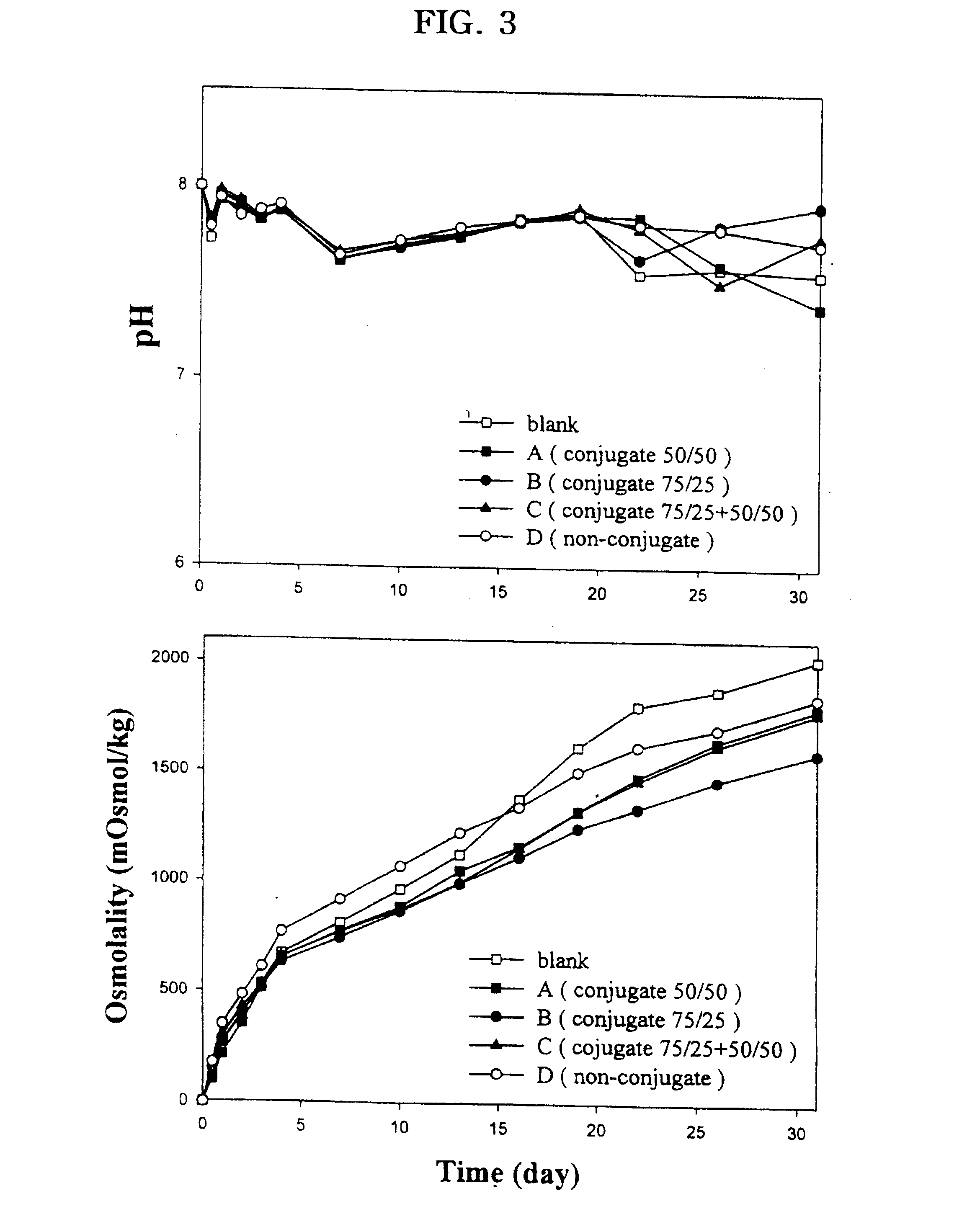
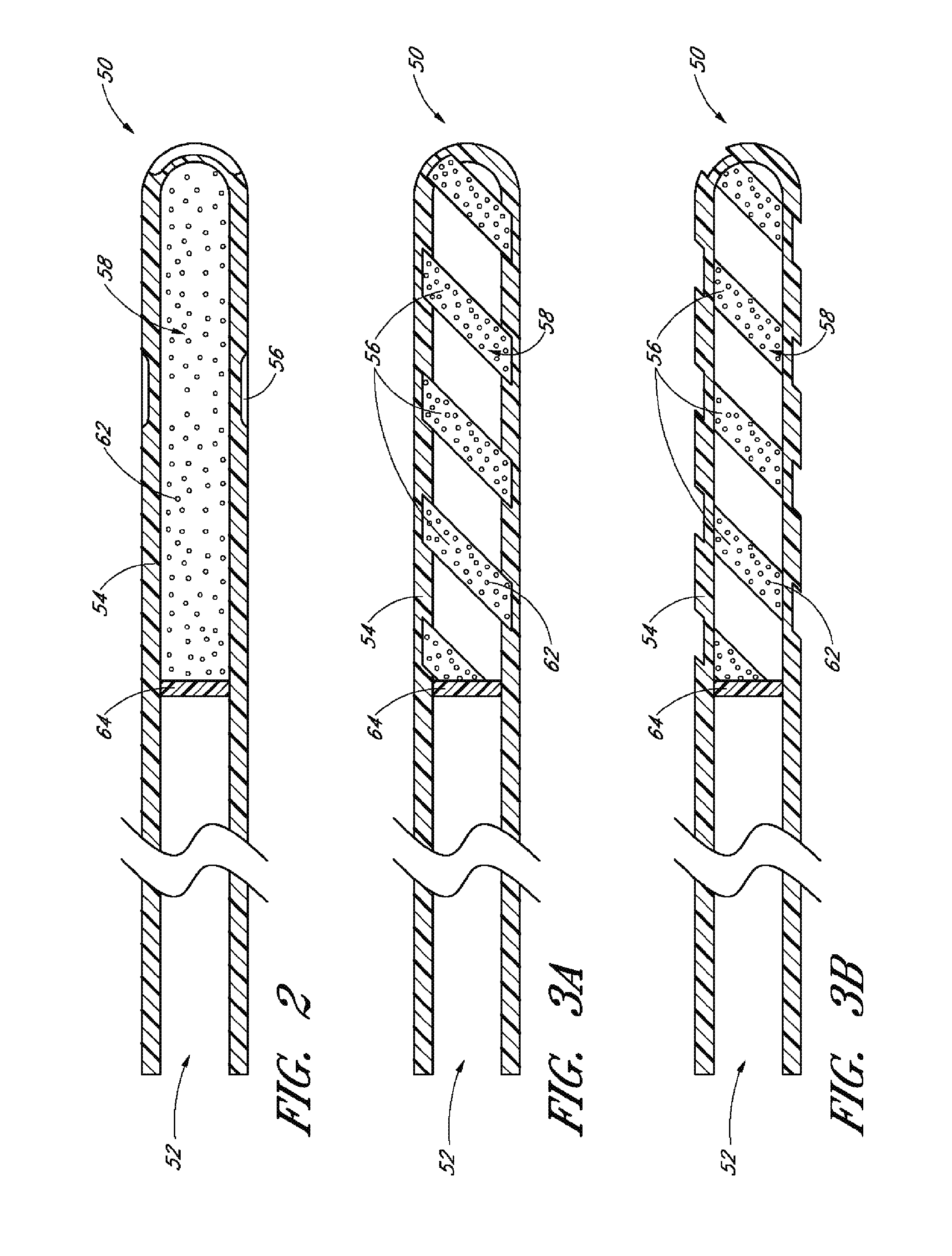
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
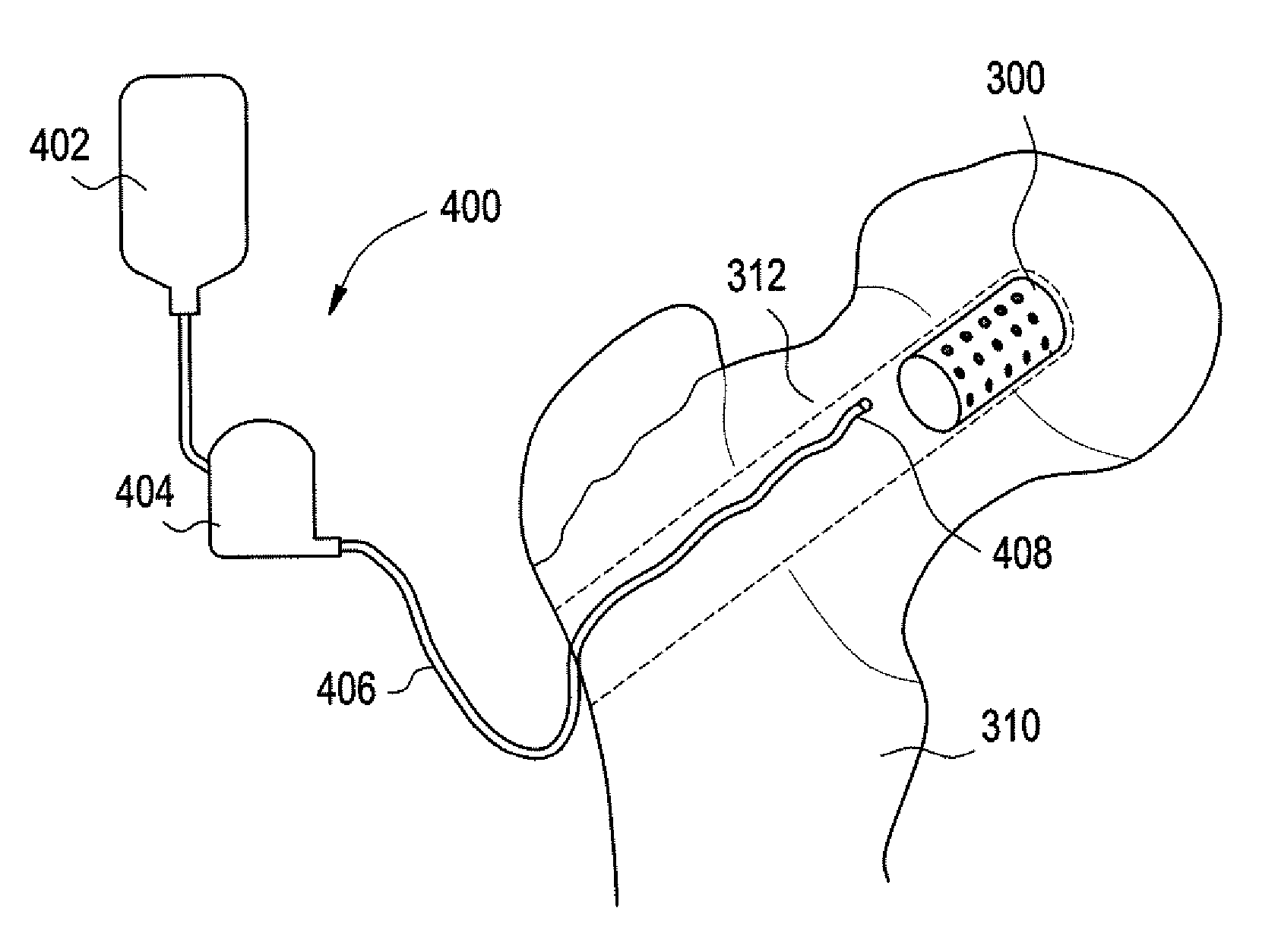
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
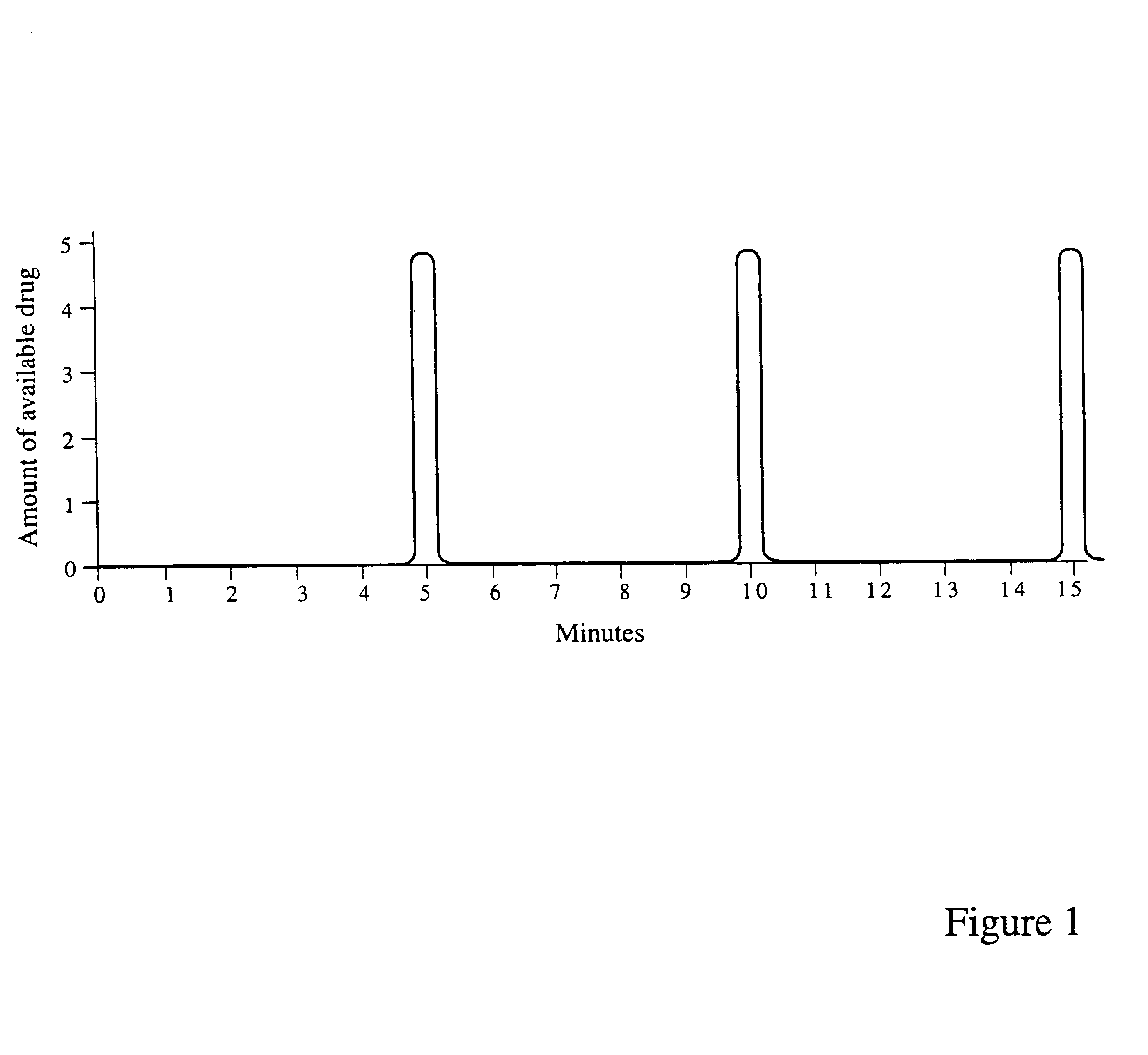
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
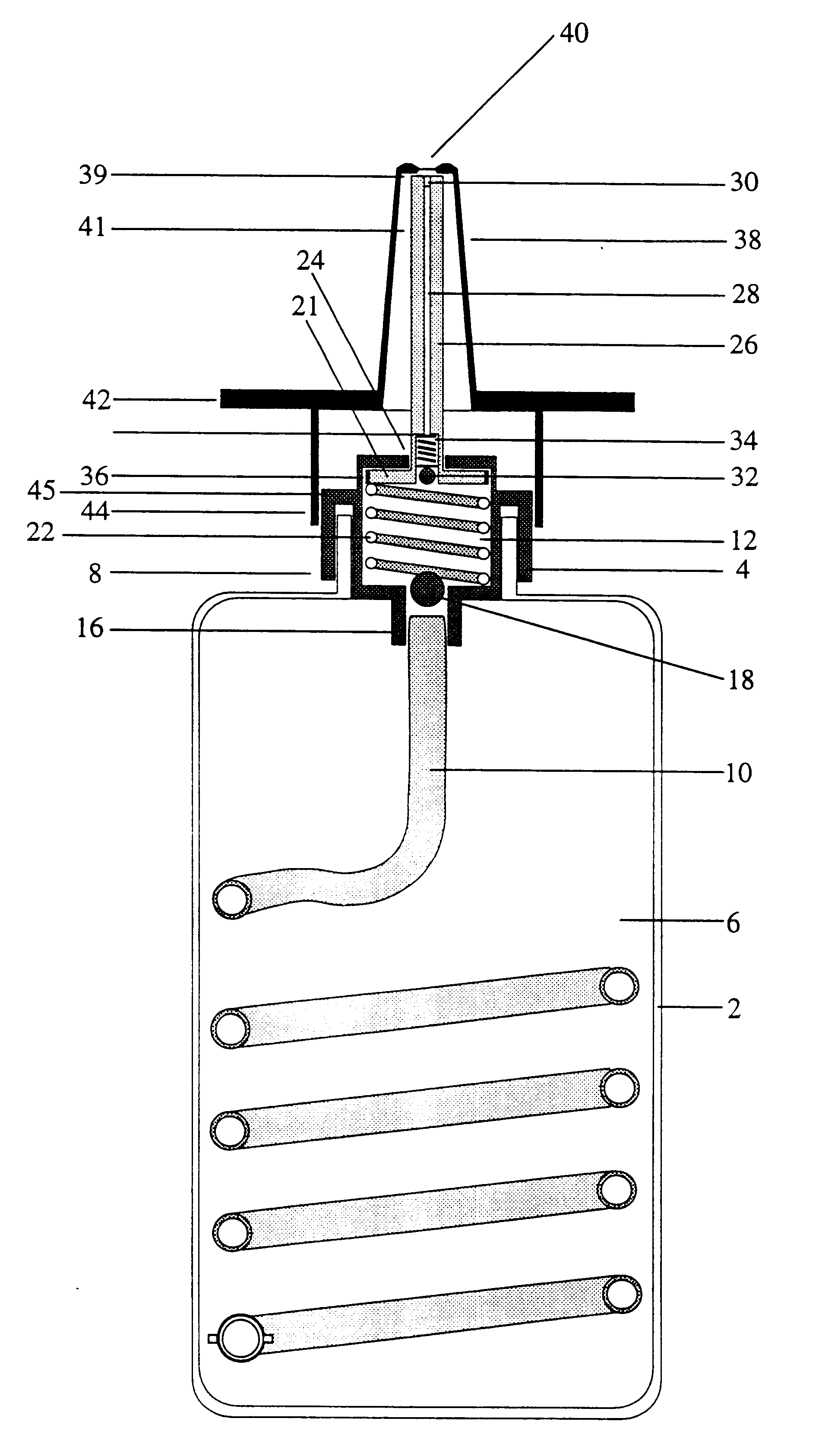
Implantable refillable and ported controlled release drug delivery device
InactiveUS6852106B2Prevent leakageEnhanced advantageSurgeryMedical devicesRate limitingDifferential pressure
An implantable, refillable, rate controlled drug delivery device is disclosed that includes a base structure having at least a first opening and a second opening, the base structure defining a chamber, a septum covering the first opening and configured to substantially prevent leakage from the first opening to an exterior of the device, a drug delivery tube comprising a first and second distal end, wherein the first distal end of the tube communicates with the chamber through the second opening, and at least one rate-limiting permeable membrane disposed across a passage between the base structure and the second distal end of the drug delivery tube, which membrane passively regulates drug delivery. The drug delivery device is used to provide controlled drug delivery to an internal portion of the body and is advantageously leak-proof and does not rely on a pressure differential to drive the drug from the device.
Owner:CONTROL DELIVERY SYST
Methods of manufacturing medical devices for controlled drug release
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Medical devices having porous polymeric regions for controlled drug delivery and regulated biocompatibility
The present invention relates to phase separated polymeric regions and to their use in conjunction with implantable or insertable medical devices. In some aspects of the invention, phase separated polymeric regions are provided that include (a) at least one biostable polymeric phase and (b) at least one biodisintegrable polymeric phase, which is of nanoscale dimensions and which undergoes biodisintegration such that the phase separated polymeric region becomes a nanoporous polymeric region in vivo. Other aspects of the invention are directed to methods of making implantable or insertable medical devices having at least one nanoporous polymeric region. These methods include (a) providing a phase separated polymeric region comprising a stable polymeric phase and a disintegrable polymeric phase of nanoscale dimensions, (b) selectively removing the disintegrable polymeric phase thereby producing the nanoporous polymeric region. In still other aspects, implantable or insertable medical devices are provided which have phase separated polymeric regions that include (a) at least one block copolymer having at least one biostable polymer block and at least one biodisintegrable polymer block and (b) at least one therapeutic agent which is released in vivo upon implantation or insertion of the medical device.
Owner:BOSTON SCI SCIMED INC
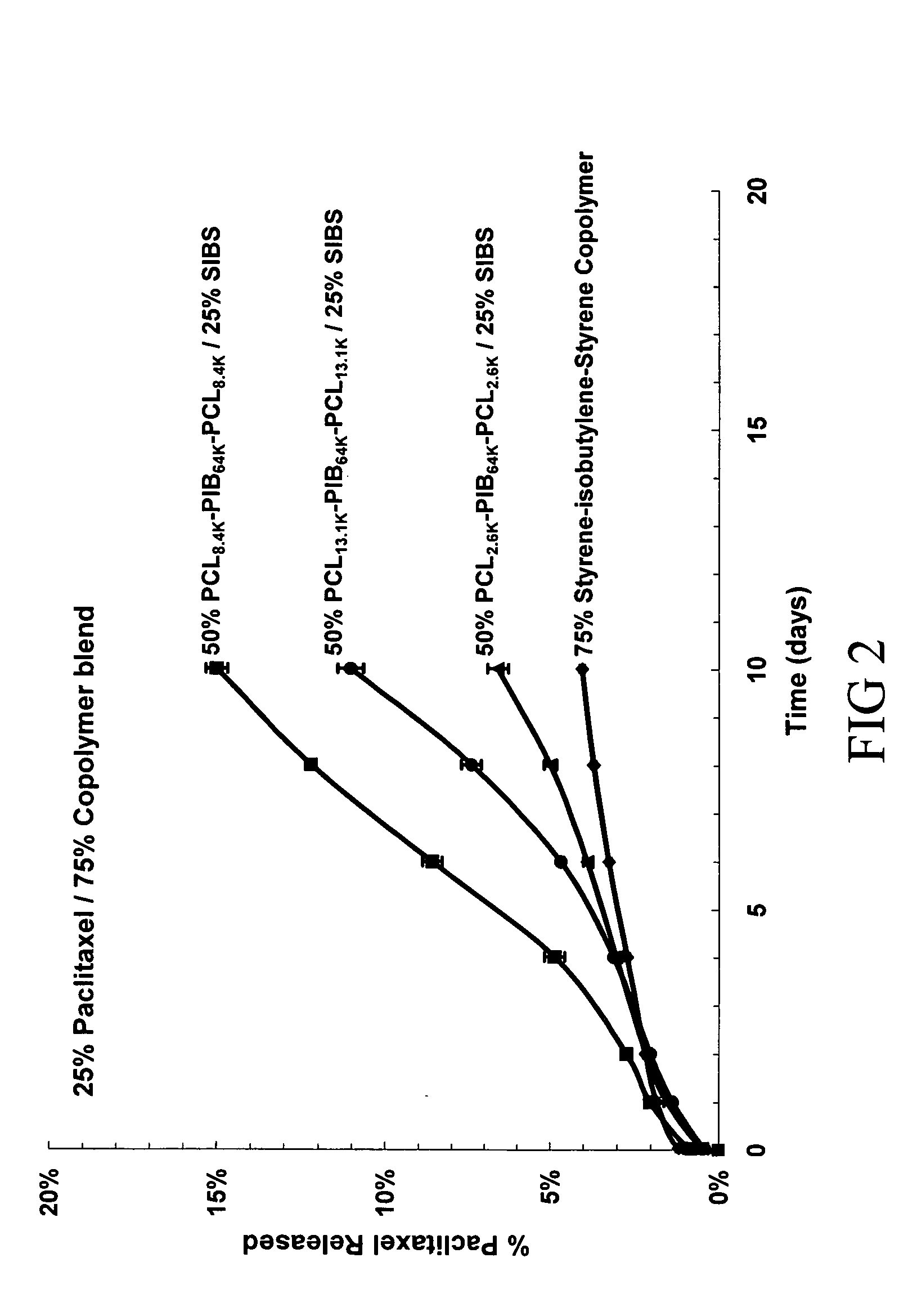
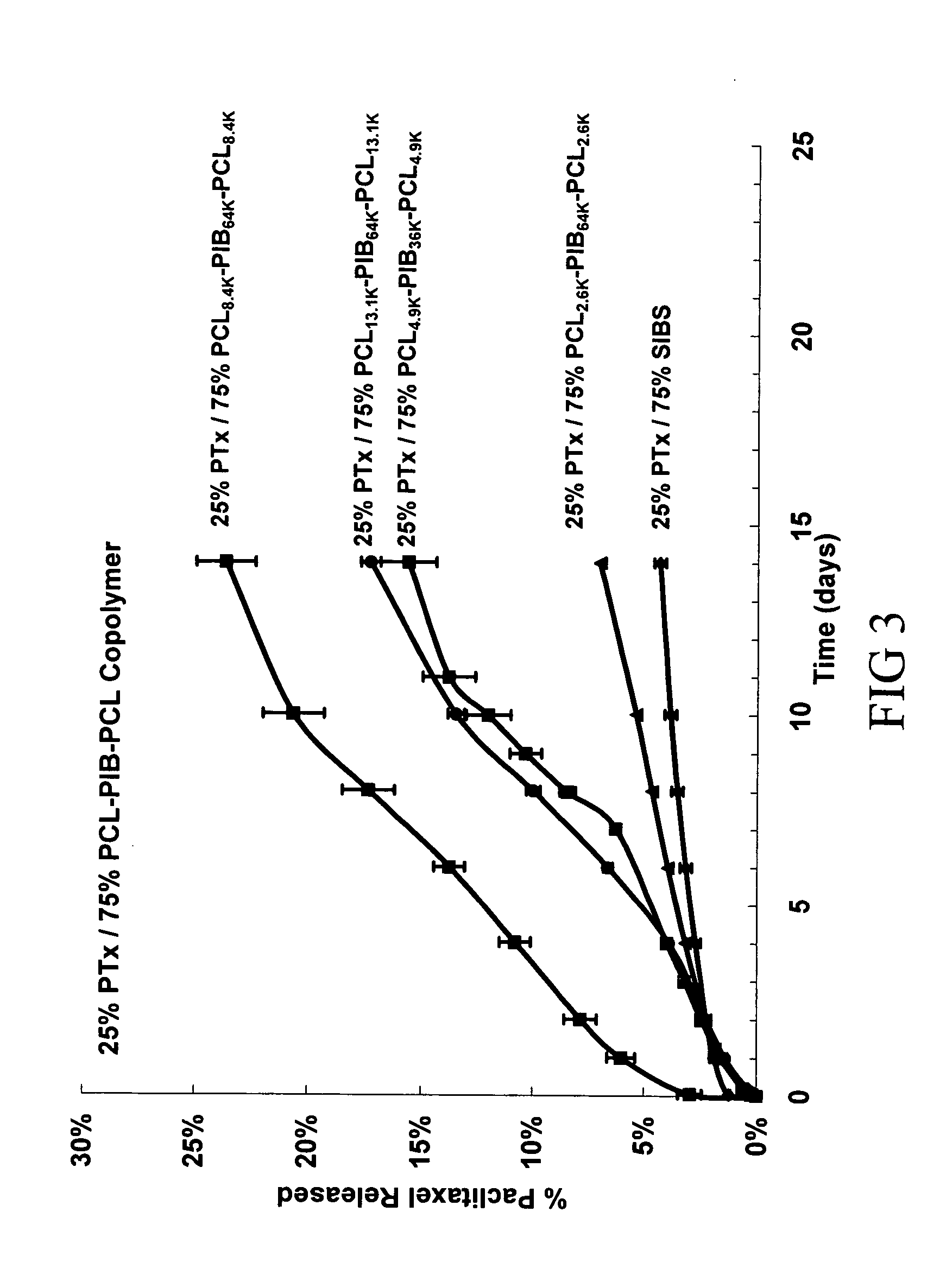
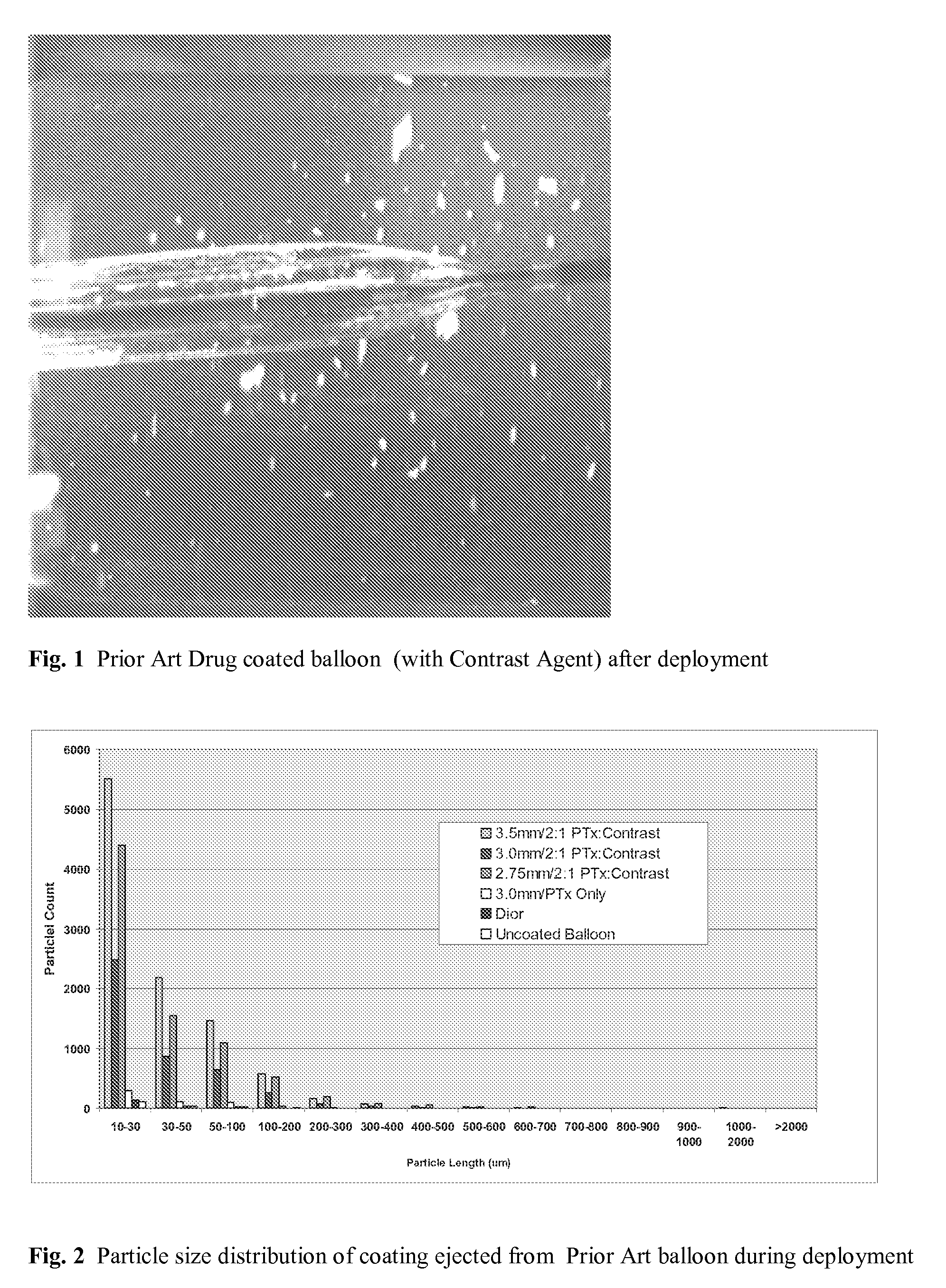
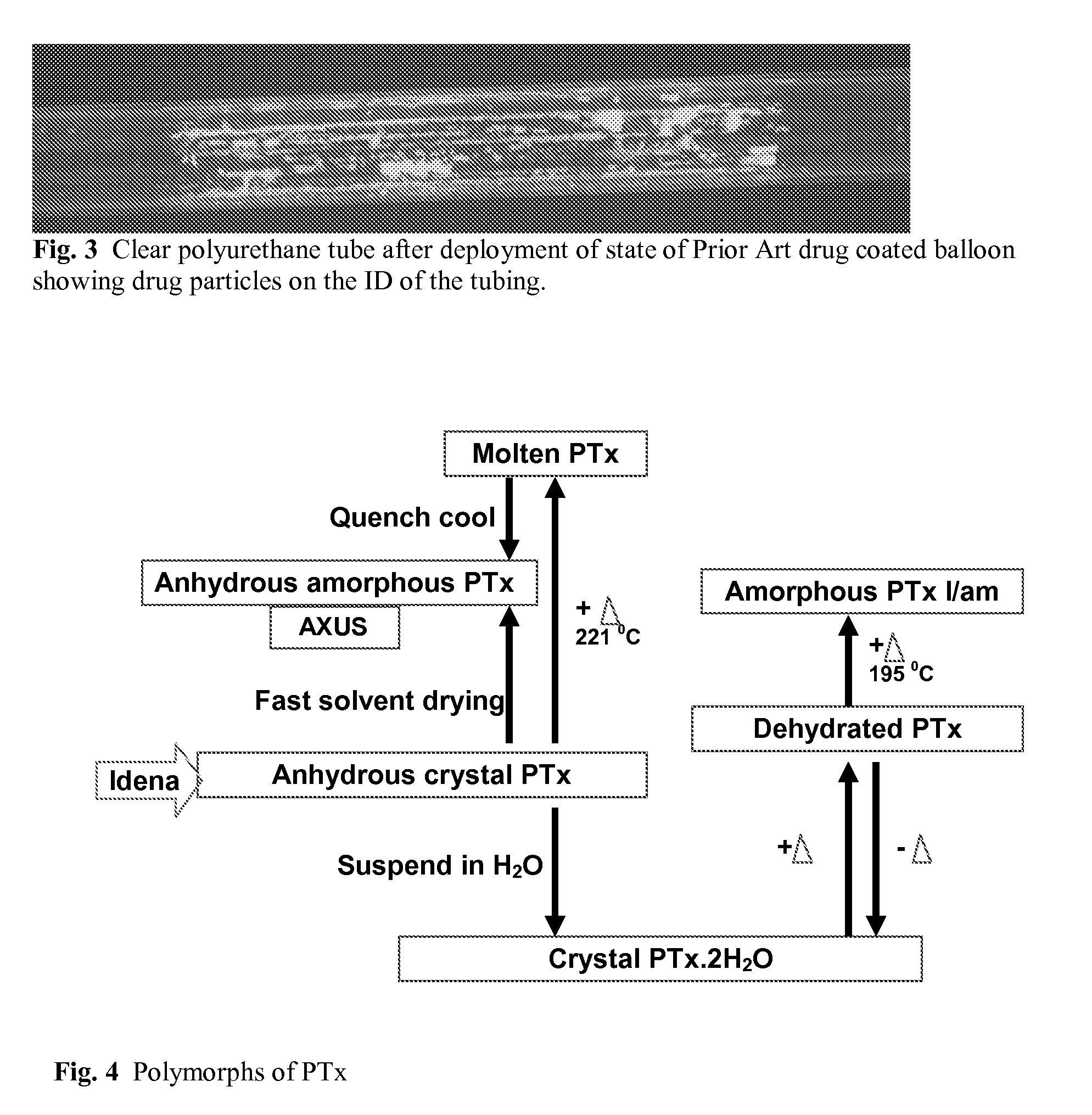
Use of Drug Polymorphs to Achieve Controlled Drug Delivery From a Coated Medical Device
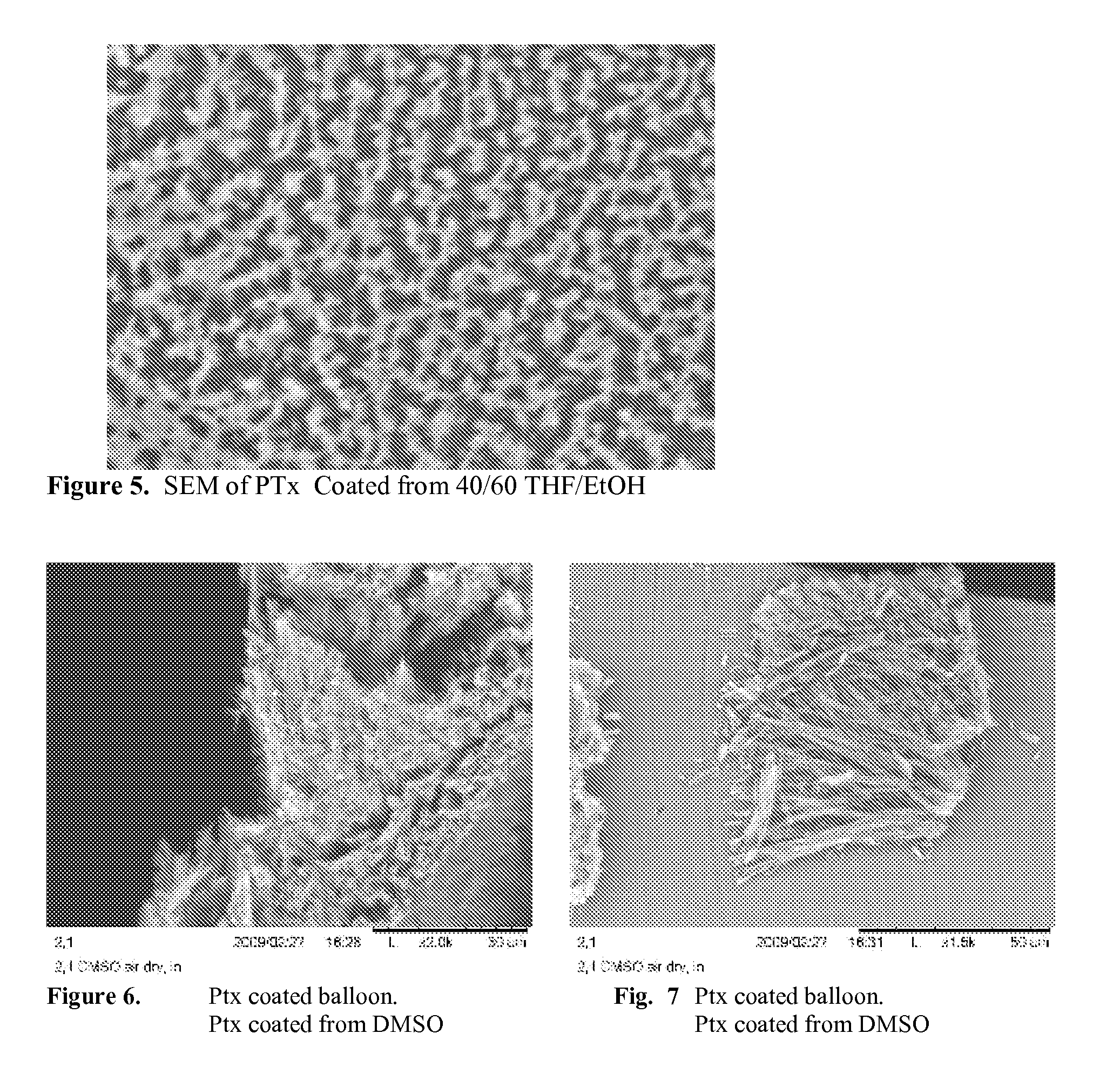
When making a medical device having a drug coating thereon, the drug having a plurality of characteristic morphological forms, the manufacturing process is controlled to produce a predetermined ratio of said morphological forms on the device. The process has application to drug coated balloons.
Owner:BOSTON SCI SCIMED INC
Combination x-ray radiation and drug delivery devices and methods for inhibiting hyperplasia
InactiveUS6537195B2Promote endothelializationReduced dosages/concentrationsStentsElectrotherapyInsertion stentPercent Diameter Stenosis
The present invention provides improved devices, methods, and kits for inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides controlled drug delivery in combination with x-ray radiation delivery to selected locations within a patient's vasculature to reduce and / or inhibit restenosis and hyperplasia rates with increased efficacy. In one embodiment, the combination radiation and agent delivery catheter for inhibiting hyperplasia comprises a catheter body having a proximal end and distal end, an x-ray tube coupleable to the catheter body for applying a radiation dose to a body lumen, and a porous material, matrix, membrane, barrier, coating, infusion lumen, stent, graft, or reservoir for releasing an agent to the body lumen.
Owner:XOFT INC +1
Method and device for controlling drug pharmacokinetics
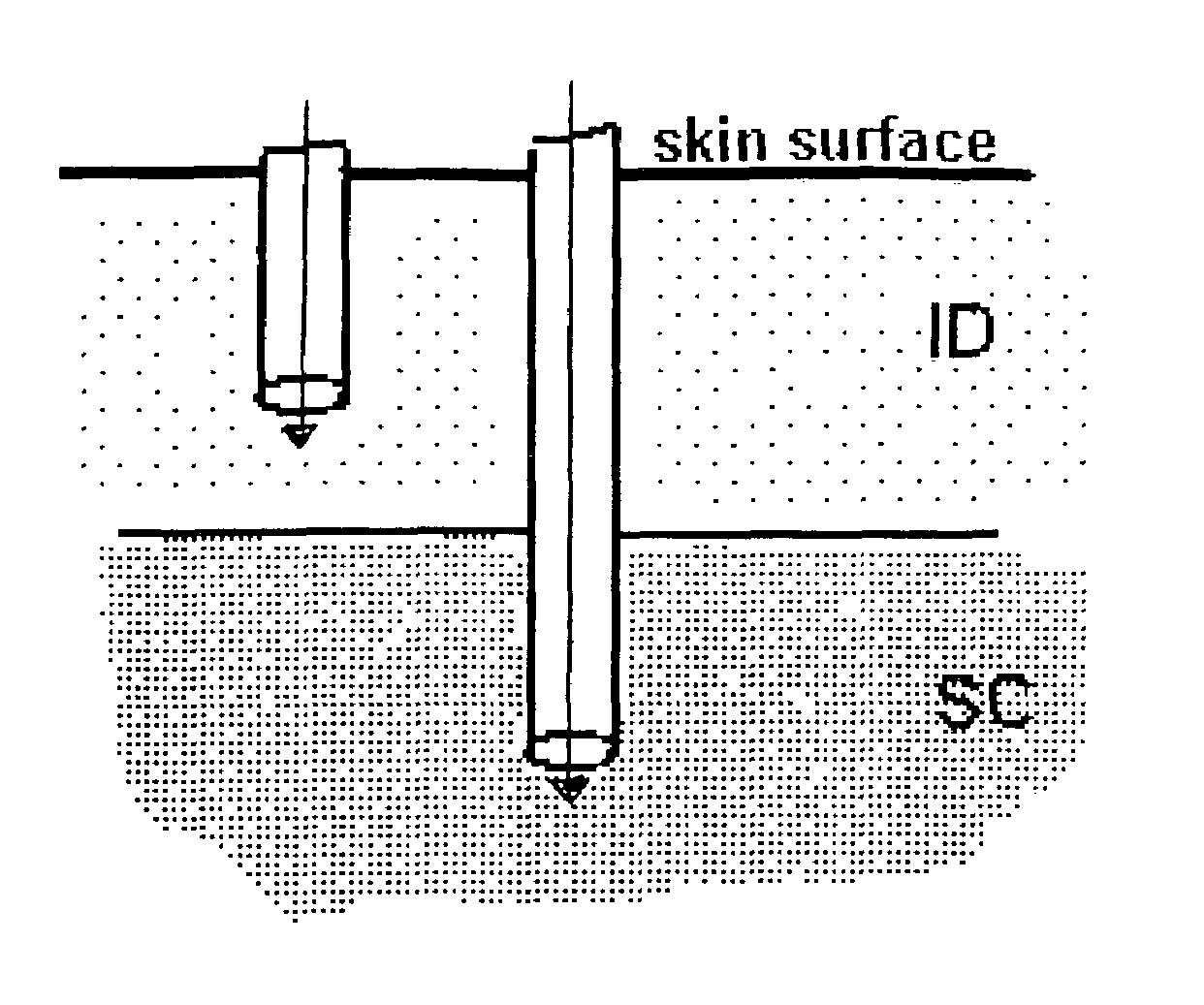
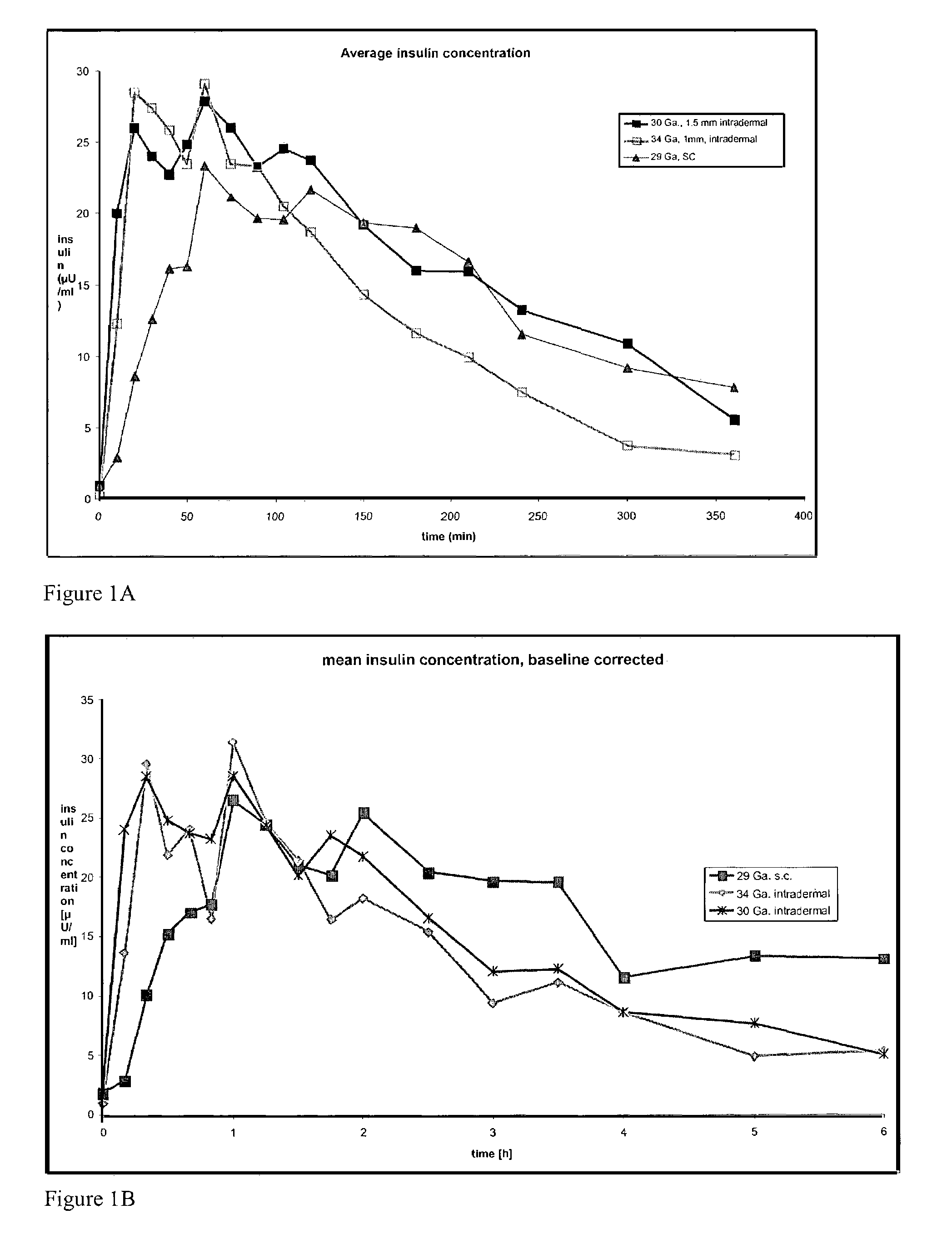
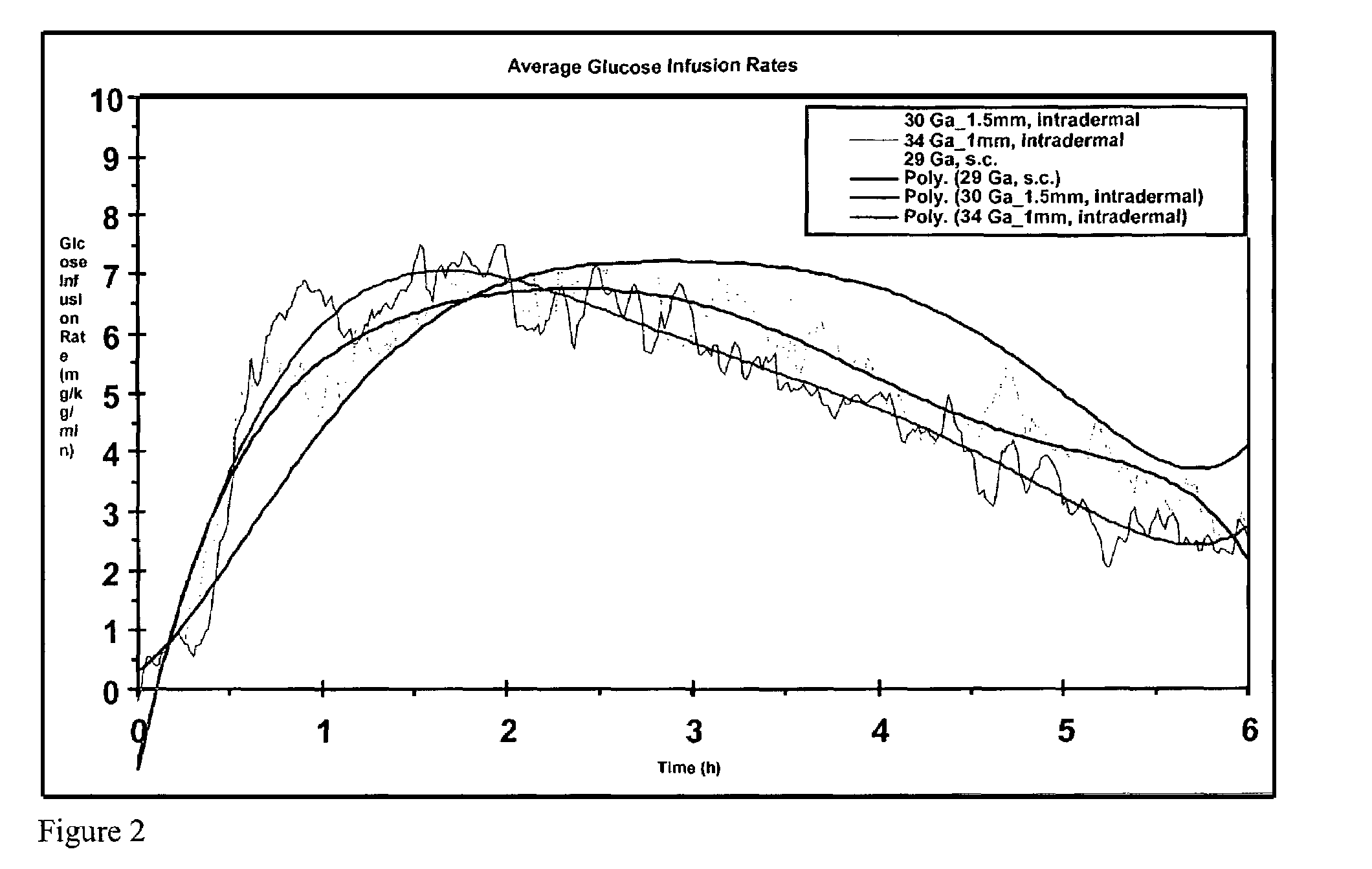
The invention pertains to methods and devices for controlling the pharmacokinetics of administered substances, particularly therapeutic substances by combining advantages of delivery to two or more compartments within the skin. The invention provides methods and devices for delivering substances to subcutaneous and intradermal compartments of the skin to achieve a hybrid pharmacokinetic profile that has a portion similar to that achieved by intradermal delivery, e.g., rapid and high peak onset levels of the substance, and a portion similar to that achieved by subcutaneous delivery, e.g., longer circulating levels of the substance.
Owner:BECTON DICKINSON & CO
Pharmaceutical composition for controlled drug delivery system
The present invention describes a novel controlled release multilayer composition that is capable of delivering a first active agent from one layer immediately followed by continuous controlled delivery of second active agent from matrix forming layer while the dosage form floats and is retained in the fluid of the environment. The floating bilayer system comprises of immediate release layer containing one active agent and a disintegrating agent whereas second floating matrix forming layer comprises a gas generating component, a gelling agent, and a second active agent. The present invention relates more particularly to a controlled release fluoroquinolone compositions, which maintain a therapeutically effective blood concentration of fluoroquinolone for duration with once a day administration.
Owner:J B CHEM & PHARMA
Drug delivery in association with medical or surgical procedures
InactiveUS20050112325A1Relieve painSafe controlDrug and medicationsMedical devicesData setConsciousness monitoring
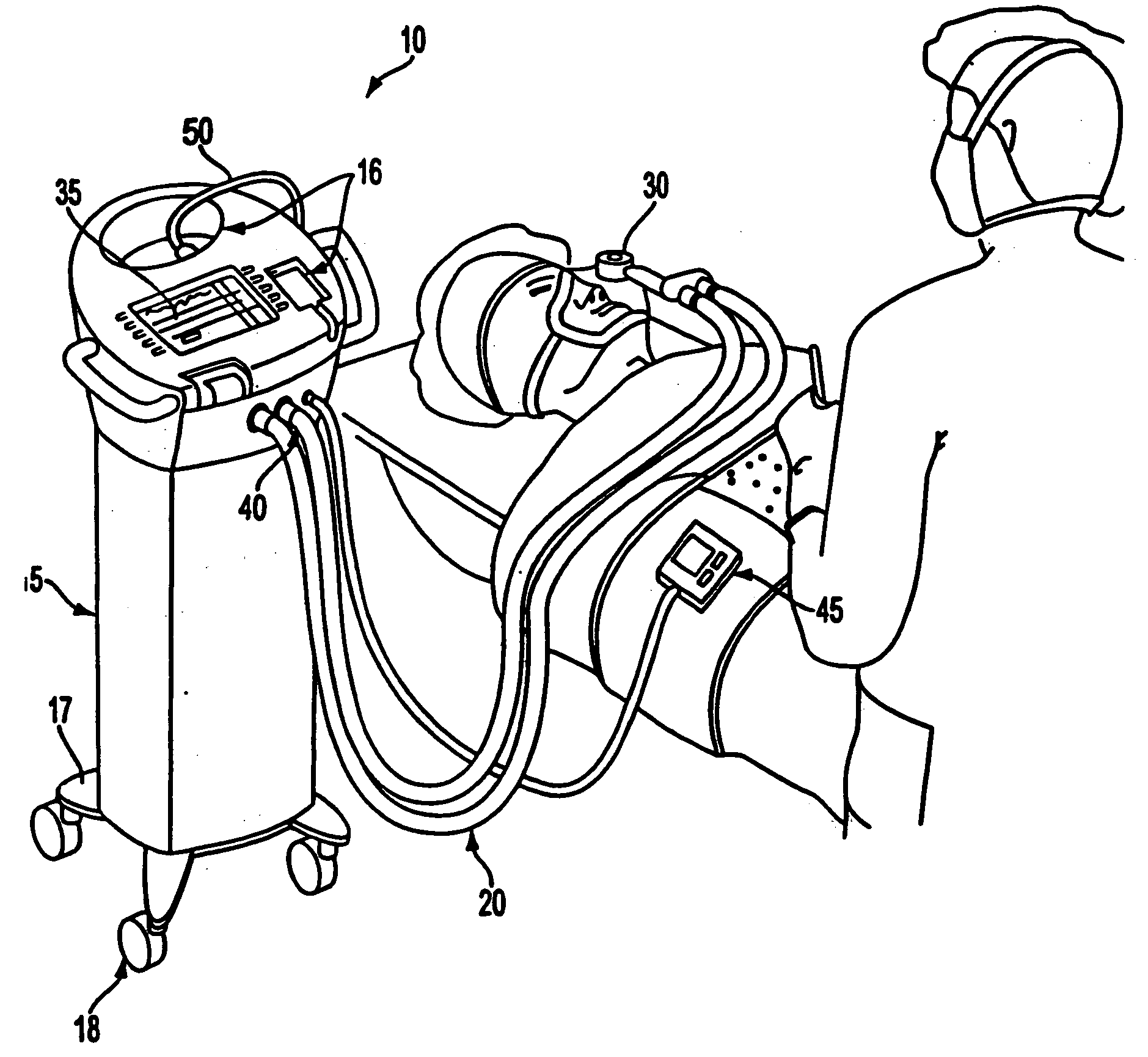
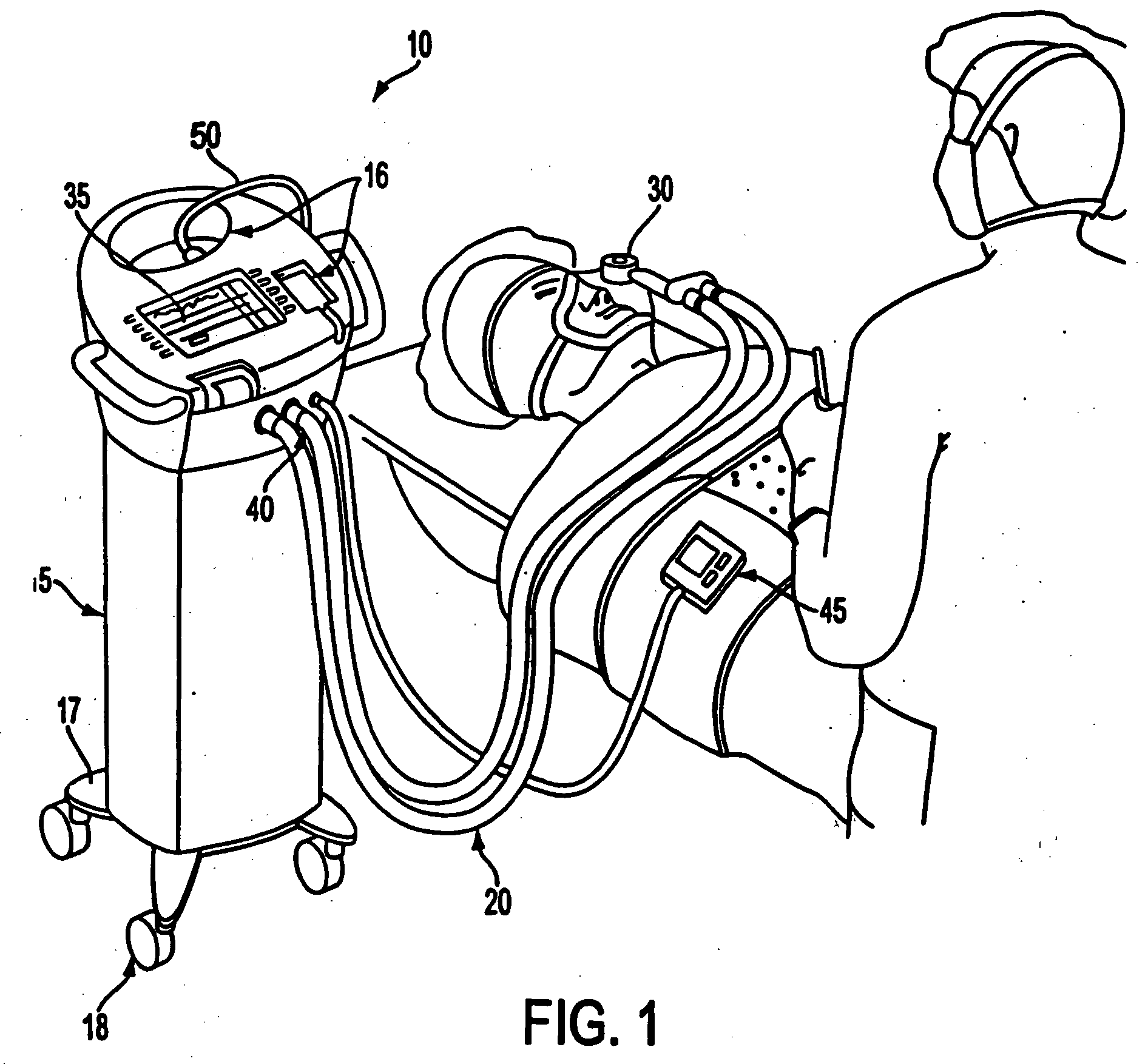
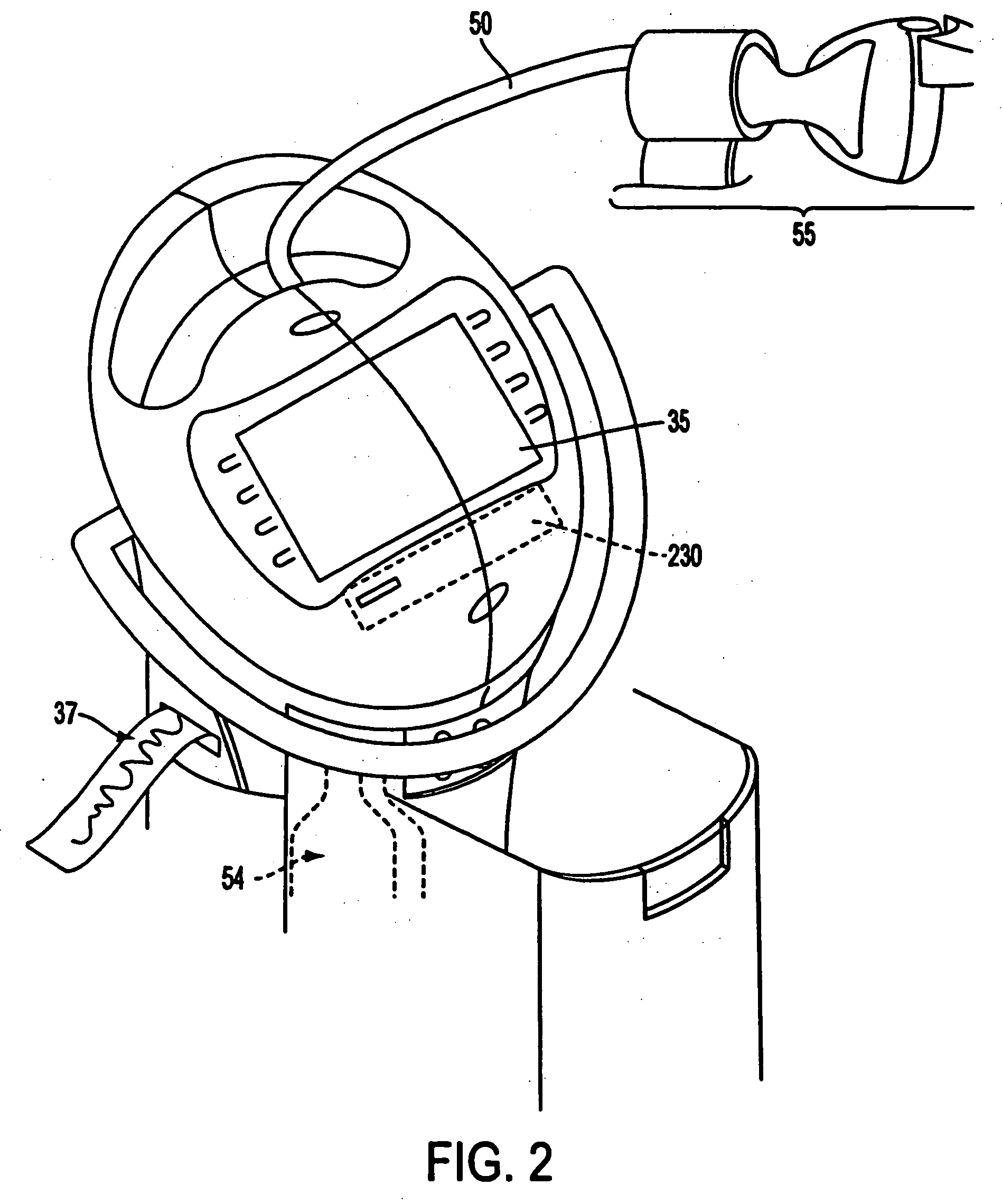
A care system and associated methods are provided for alleviating patient pain, anxiety and discomfort associated with medical or surgical procedures, the system comprising: at least one patient health monitor device coupled to a patient and generating a signal reflecting at least one physiological condition of the patient; a drug delivery controller supplying one or more drugs to the patient; a memory device storing a safety data set reflecting parameters of the at least one patient physiological condition; and an electronic controller interconnected between the patient health monitor, the drug delivery controller and the safety data set; wherein said electronic controller manages the application of the drugs in accord with the safety data set. In another aspect of the invention, the care system facilitates a procedural physician's safely and efficaciously providing conscious sedation to a patient by additionally providing a consciousness monitoring-system which monitors the consciousness of the patient and generates a value representing the level of patient consciousness. Methods for alleviating patient pain and anxiety in accordance with the invention comprise connecting a drug delivery device to a patient, such device having a drug delivery controller supplying one or more drugs and being coupled to an electronic controller; attaching at least one patient health monitor device to the patient; accessing a memory device which stores a safety data set reflecting parameters of at least one patient physiological condition; and delivering the drugs to the patient in accord with the safety data set. In further aspects of the invention, the consciousness monitoring system is an automated consciousness monitoring system which includes patient query and response devices. Additional embodiments of the system and methods are directed to alleviating patient pain or discomfort while enabling safe patient controlled drug delivery in correlation with the monitoring of patient health conditions.
Owner:SCOTT LAB
Compositions and methods for controlling drug loss and delivery in transdermal drug delivery systems
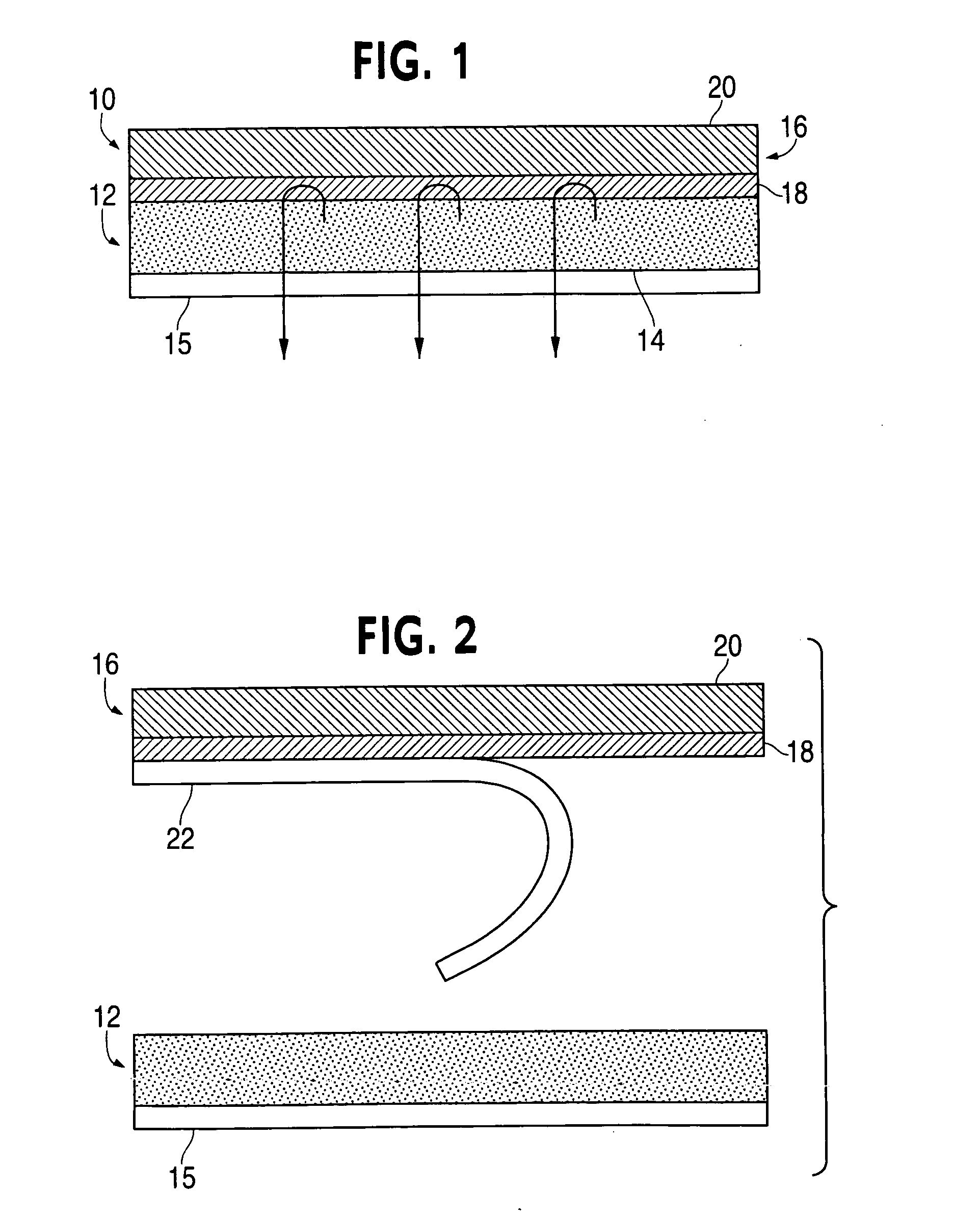
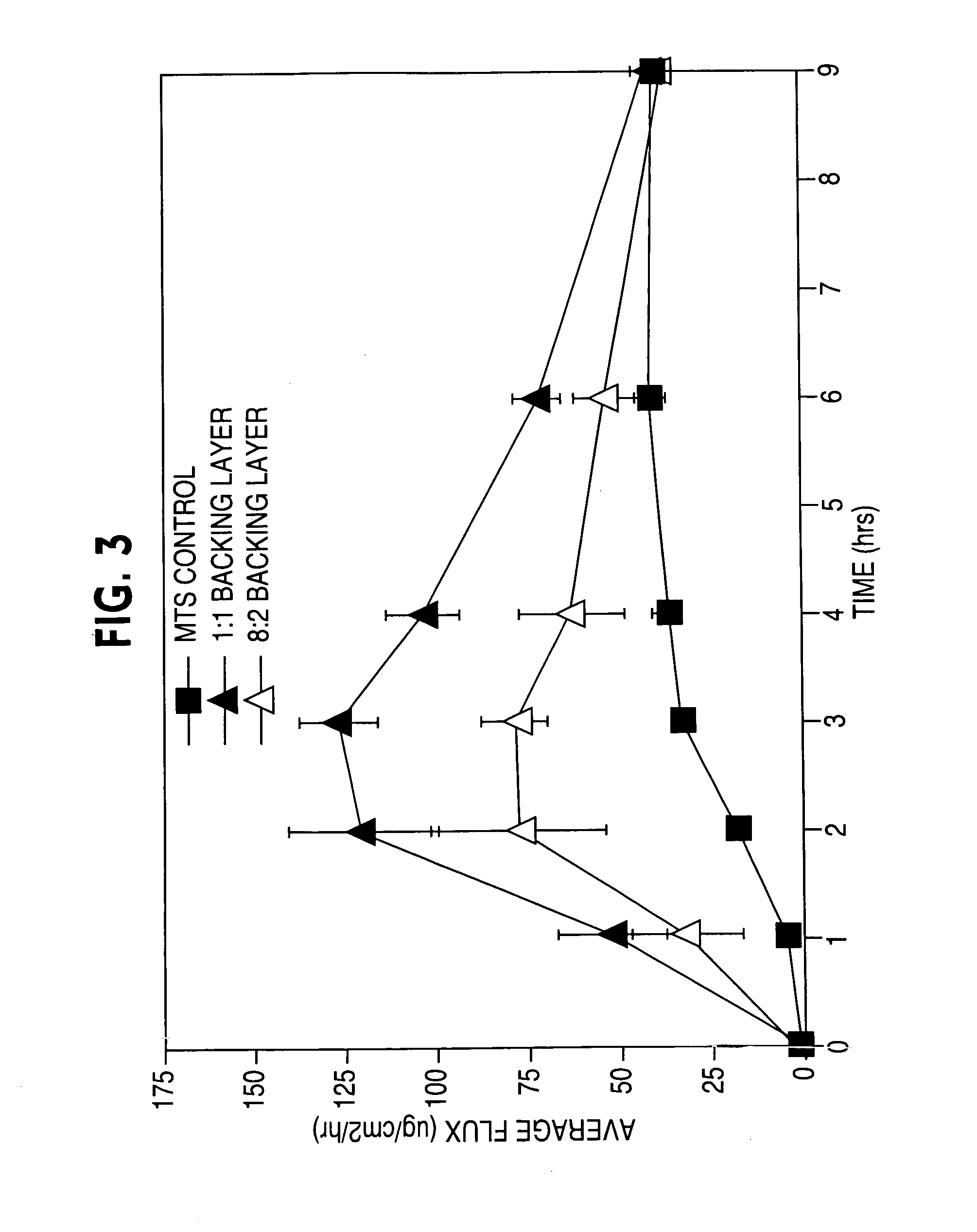
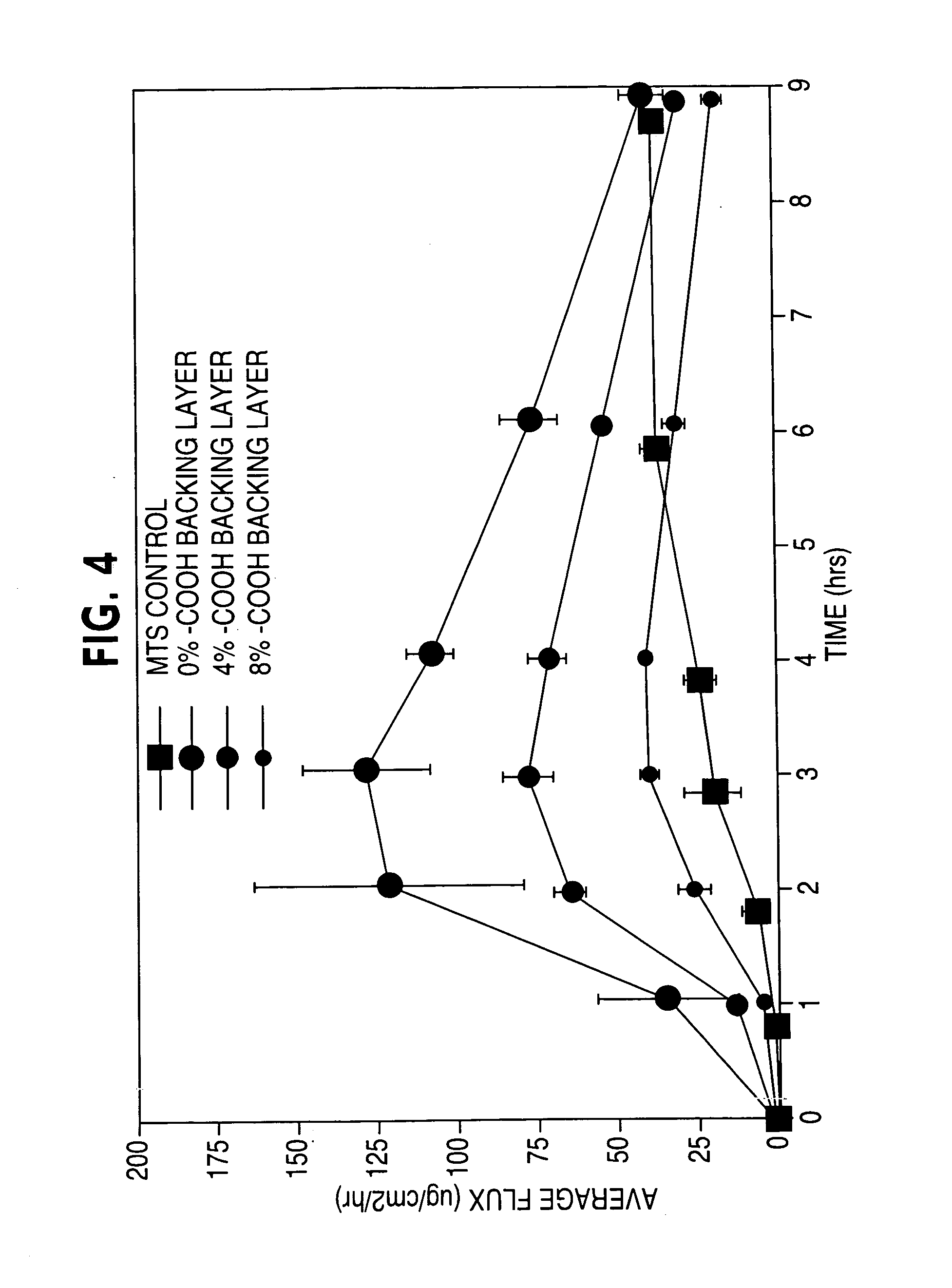
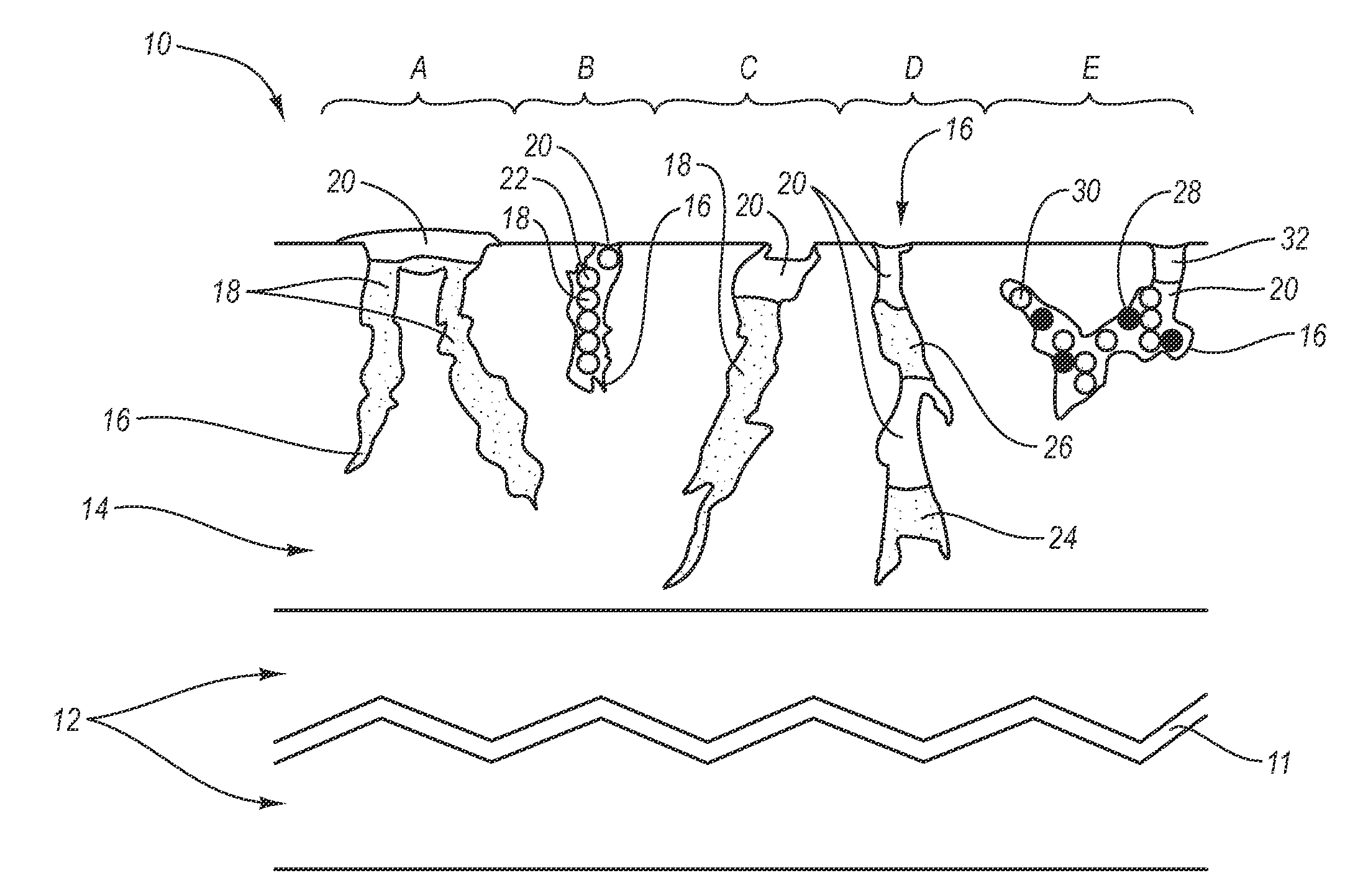
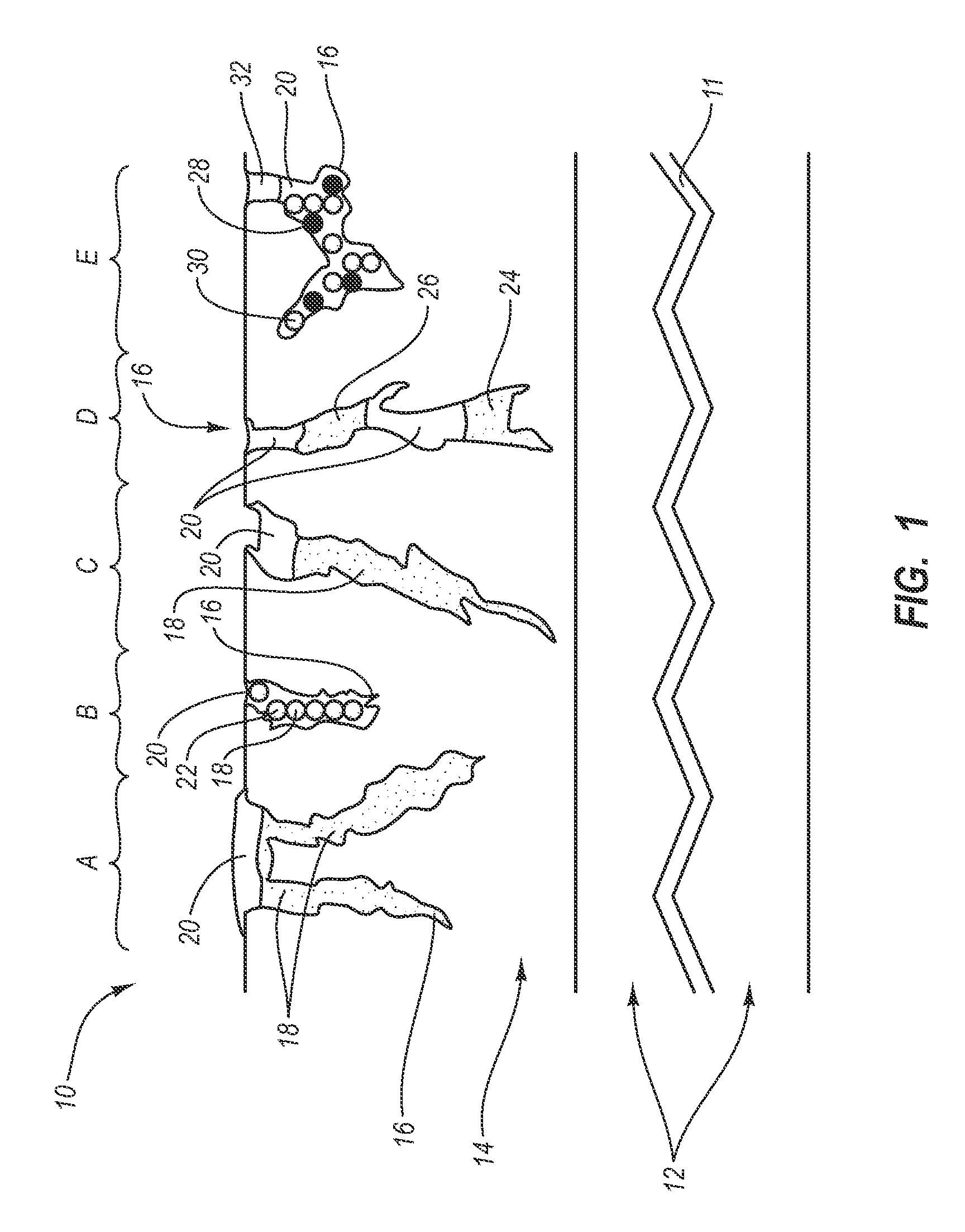
ActiveUS20050169977A1Simple and inexpensive to manufacturePreventing and minimizing drug lossOrganic active ingredientsMedical devicesActive agentDelivery system
A transdermal delivery system is provided for the topical application of one or more active agents contained in one or more polymeric and / or adhesive carrier layers, proximate to a non-drug containing polymeric and / or adhesive coating that is applied to either the transdermal system's backing or release liner. The transdermal delivery device is manufactured to optimize drug loading while providing desirable adhesion to skin or mucosa as well as providing modulation of the drug delivery and profile.
Owner:NOVEN PHARMA
Methods of using medical devices for controlled drug release
InactiveUS20080057103A1Treating and preventing diseaseStentsOrganic active ingredientsDiseaseVascular disease
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Medical devices for controlled drug release
The present invention is a medical device for controlling the release of an active agent. The medical device has a supporting structure having a porous body disposed therein. At least one elution rate controlling matrix containing an effective amount of at least one active agent is disposed within the pores of the porous body in a manner that protects the matrix from mechanical damage. The medical device may therefore be used for controlled drug release applications. Additionally, the present invention discloses a method for using the medical device for the treatment and prevention of diseases in mammals. This invention further relates to a method for using the medical device for treating and preventing vascular diseases.
Owner:ABBOTT LAB INC
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to the molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect. Moreover, the high loading efficiency of hydrophilic drugs can be achieved.
Owner:OH JONG EUN +3
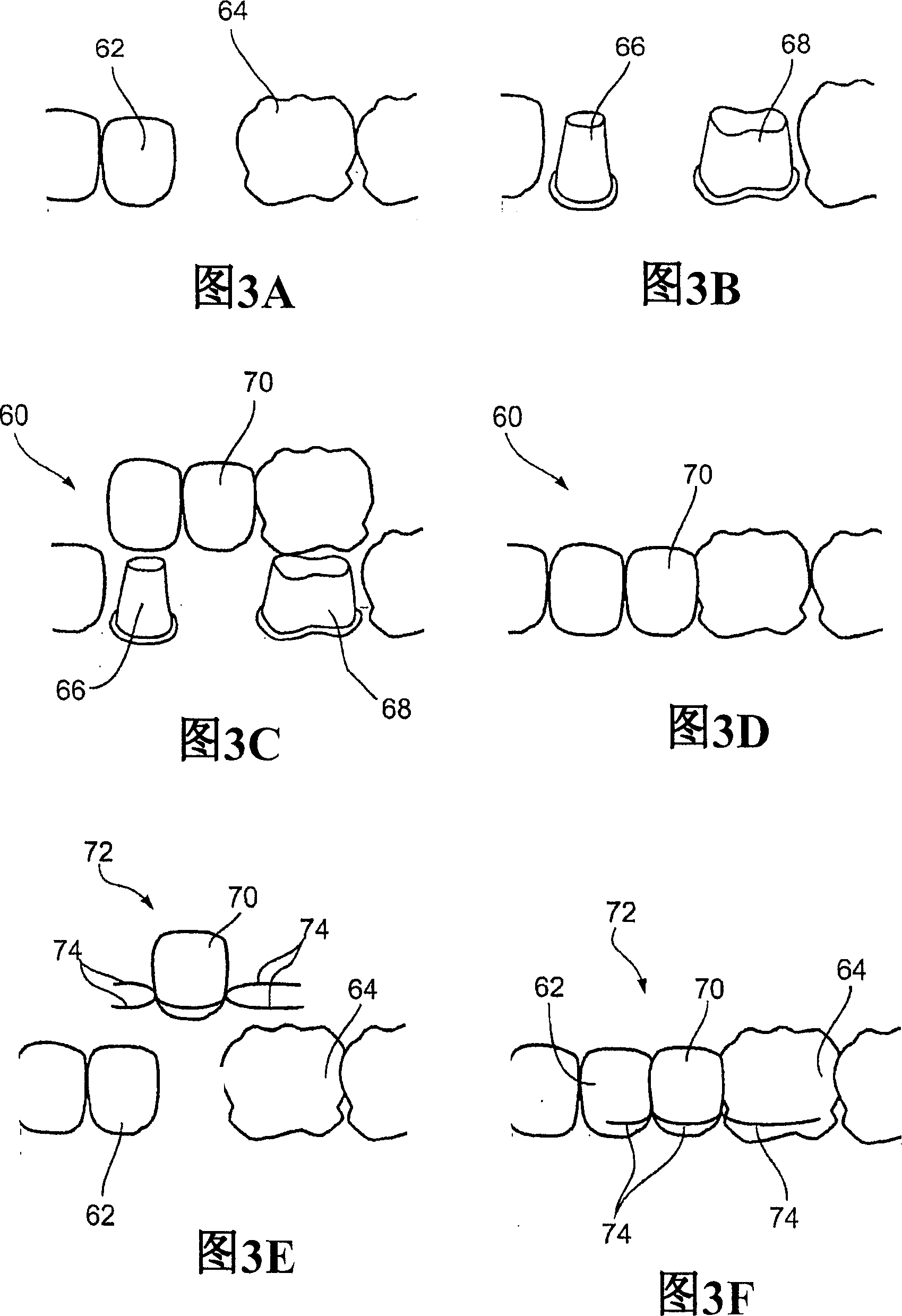
Patient controlled drug delivery device
InactiveUS6605060B1Simple and inexpensive to manufactureSimple and inexpensive to and useInfusion devicesLarge containersCatheterBiomedical engineering
A delivery device for patient-controlled infusion of a medicament (6), the delivery device comprising a reservoir (2) for the medicament (6) and a pump (4) having a predetermined delivery dose which is capable of displacing the medicament from the reservoir (2) and delivering it to a patient, wherein the pump (4) comprises a pumping means (14), a first conduit (10), capable of restricting flow rate, chosen in conjunction with the delivery dose of the pumping means (14) to define a predetermined maximum dosage rate, said conduit (10) connecting the reservoir (2) to a pumping means (14), a one-way valve (18) in fluid communication with the first conduit (10) and the pumping means (14) which permits medicament flow into the pumping means (14) but prevents reverse flow, a controlling means (32), and a second conduit (28) extending from the pumping means and having a distal end (30) through which, the medicament may be released, wherein the controlling means (32), (a) is in fluid communication with the pumping means (12) and the second conduit (28); (b) opens when pressure within the dose chamber (12) exceeds a predetermined minimum opening pressure for the controlling means (32); and, (c) is adapted to prevent the reverse flow of medicament and air into the pumping means.
Owner:ONEIL ALEXANDER GEORGE BRIAN & CHRISTINE ONEIL JOINTLY
Patient controlled drug administration device
ActiveUS6936035B2Without manipulationSafely self-administer the drugPressure infusionDetentEngineering
A patient controlled liquid drug administration device comprises a reciprocating pump having an inlet connectable to a source of a pressurized liquid drug, and an outlet connectable to the patient. A clamp rotates between closed and open positions respectively blocking and enabling pump outflow. A plunger above the pump has an end contacting a movable wall of the pump and is movable between raised and lowered positions. A button above the plunger moves between extended and depressed positions, and has a ledge that engages the clamp when the button is moved to the depressed position and moves the clamp to its open position. A compression spring is disposed between the plunger and the button. A spring catch engages a detent in the button when the button is moved to its depressed position and holds the button there until released by a catch release on the plunger that disengages the spring catch from the detent in the button when the plunger is moved to its lowered position. A removable tab permits easy, rapid priming of the pump by holding the clamp in its open position regardless of the position of the button, and by holding the button in its depressed position regardless of the position of the plunger.
Owner:AVENT INC
Composition comprising biodegradable hydrating ceramics for controlled drug delivery
ActiveUS8124118B2High X-ray and ultrasonic and magnetic resonance visibilityGood effectOrganic active ingredientsGenetic material ingredientsDrug carrierAqueous medium
The present invention relates to a drug carrier composition comprising i) one or more biodegradable hydrating ceramics ii) one or more expandable agents, and iii) sorbed aqueous medium which in solid form has a ruptured structure. The function of the expandable agent is to create a ruptured structure in the solidified composition, either a foam-like structure or a disintegrated structure where it is split into a large number of parts, particles, units, granules or pieces, so as to obtain an enlarged apparent surface area that is exposed to degradation or erosion upon administration. Suitable substances to obtain this surface enlarging effect are gas-forming agents or swelling agents, gelling agents or disintegrants, here referred to as expandable agents. The expandable agents may be bioresorbable or non-bioresorbable.
Owner:LIDDS AB
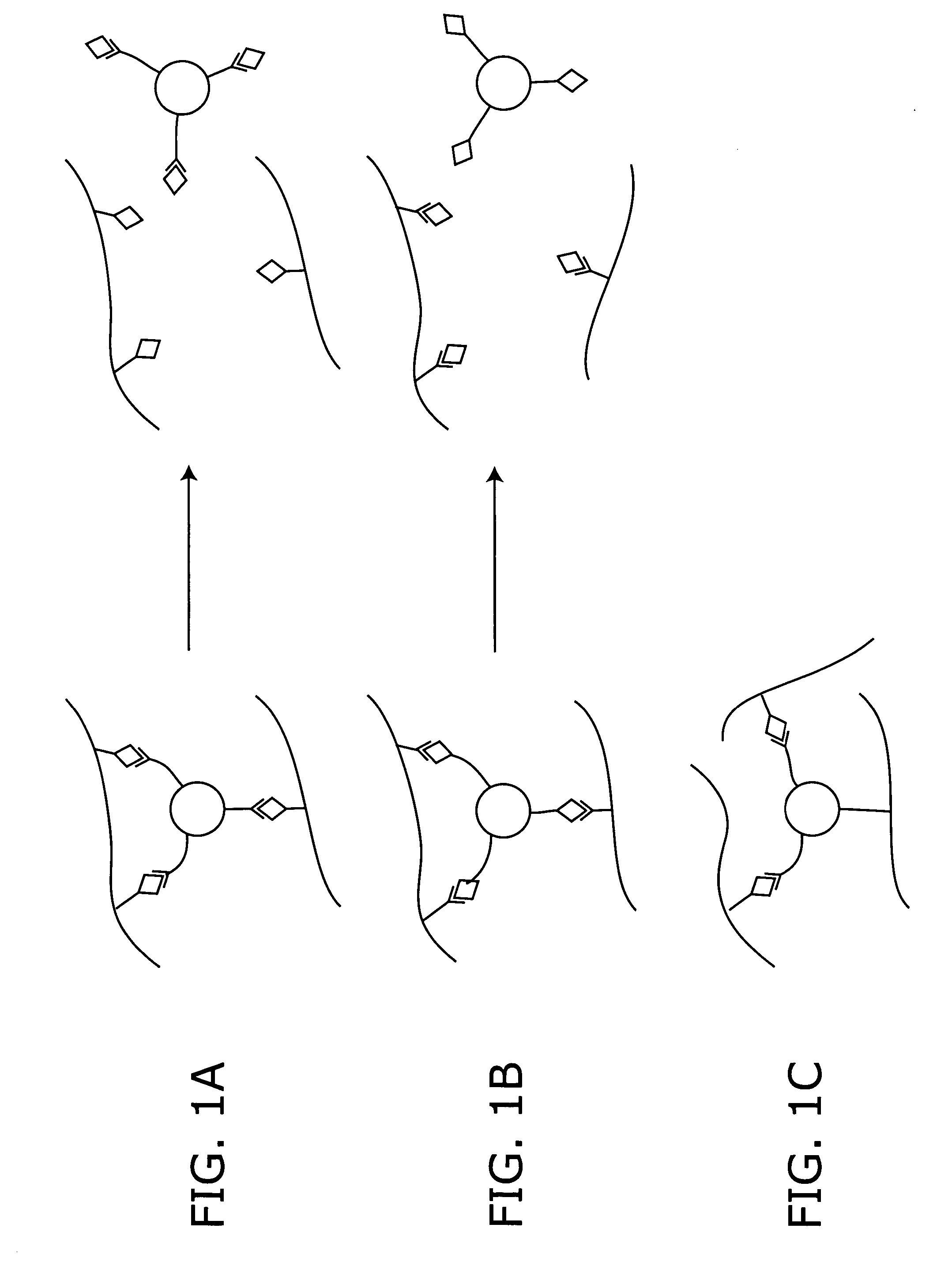
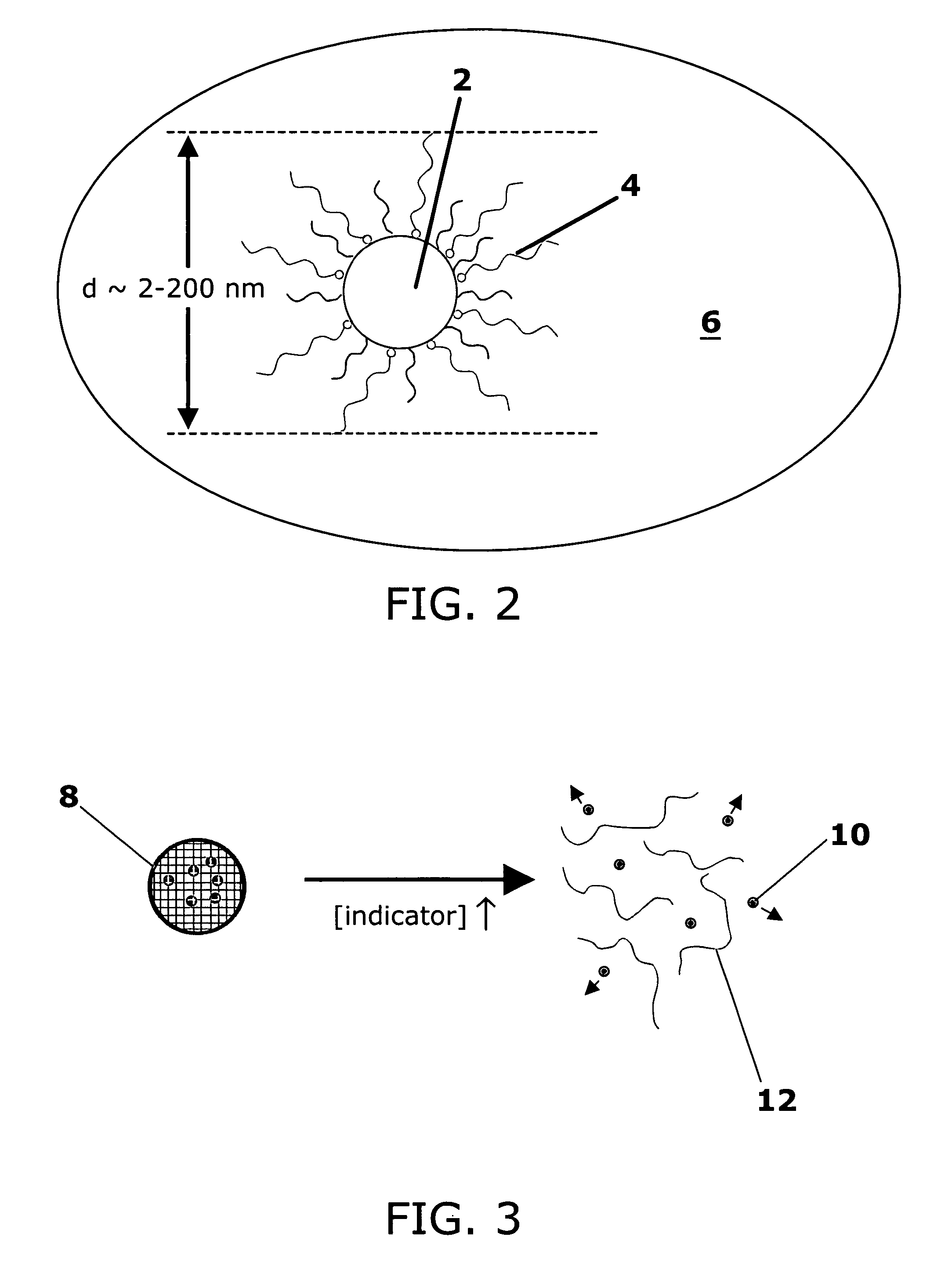
Stimuli-responsive systems for controlled drug delivery
A method of delivering a therapeutic agent by providing a cross-linked polymer encapsulating the therapeutic agent to a site in a patient. The degradation rate of the cross-linked polymer is correlated with a local concentration of an indicator, and the therapeutic agent is released as the cross-linked polymer degrades.
Owner:MASSACHUSETTS INST OF TECH
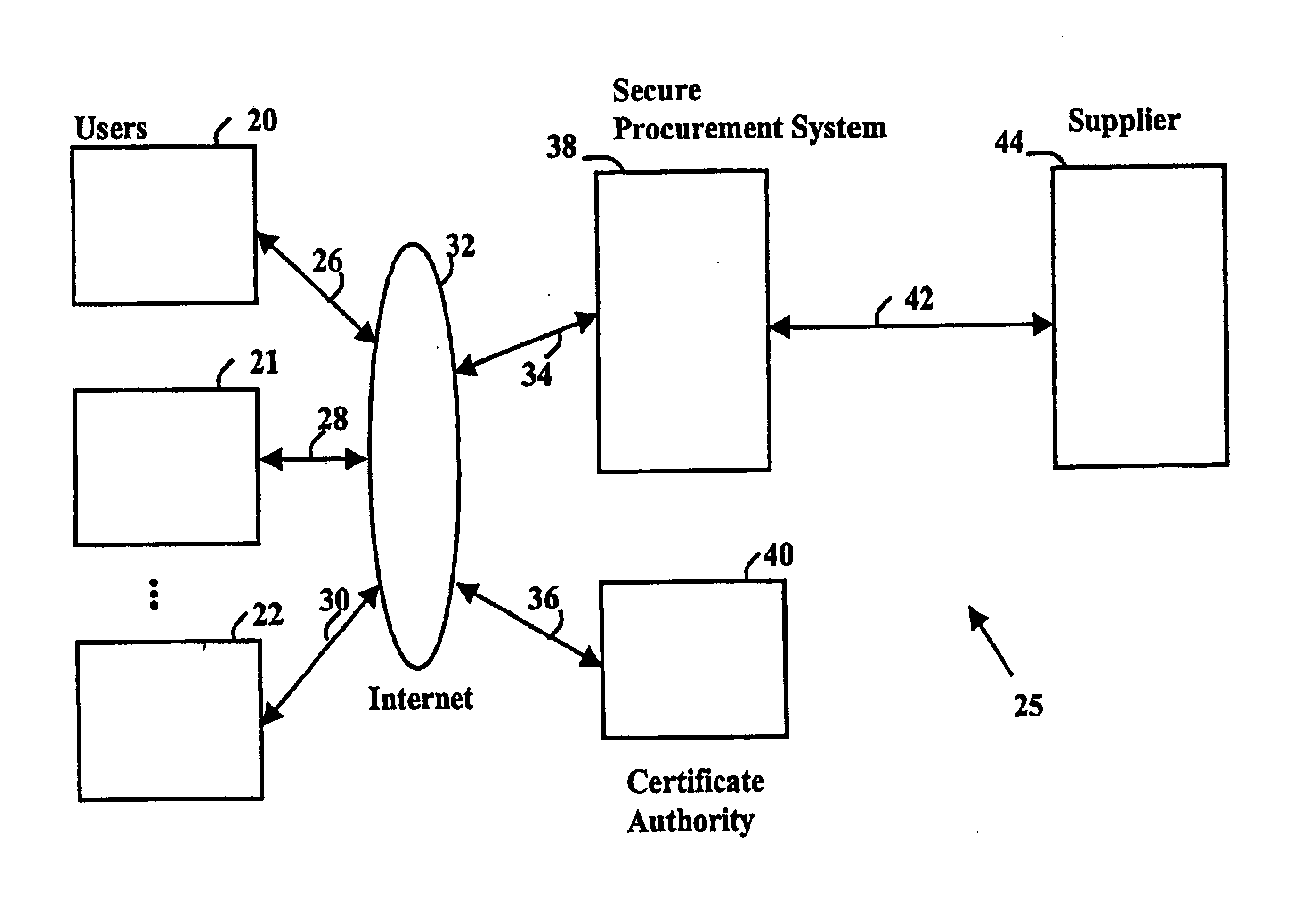
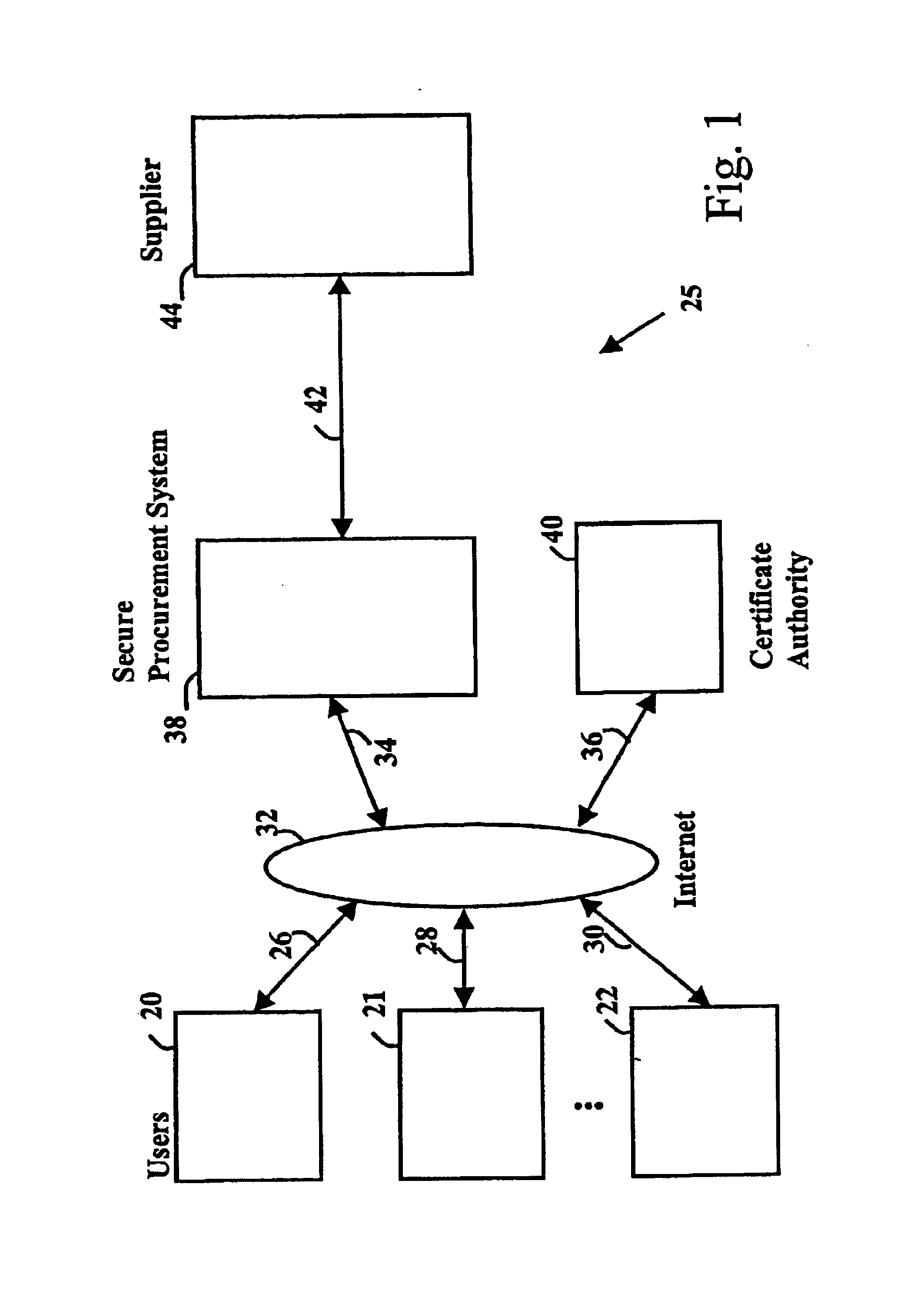
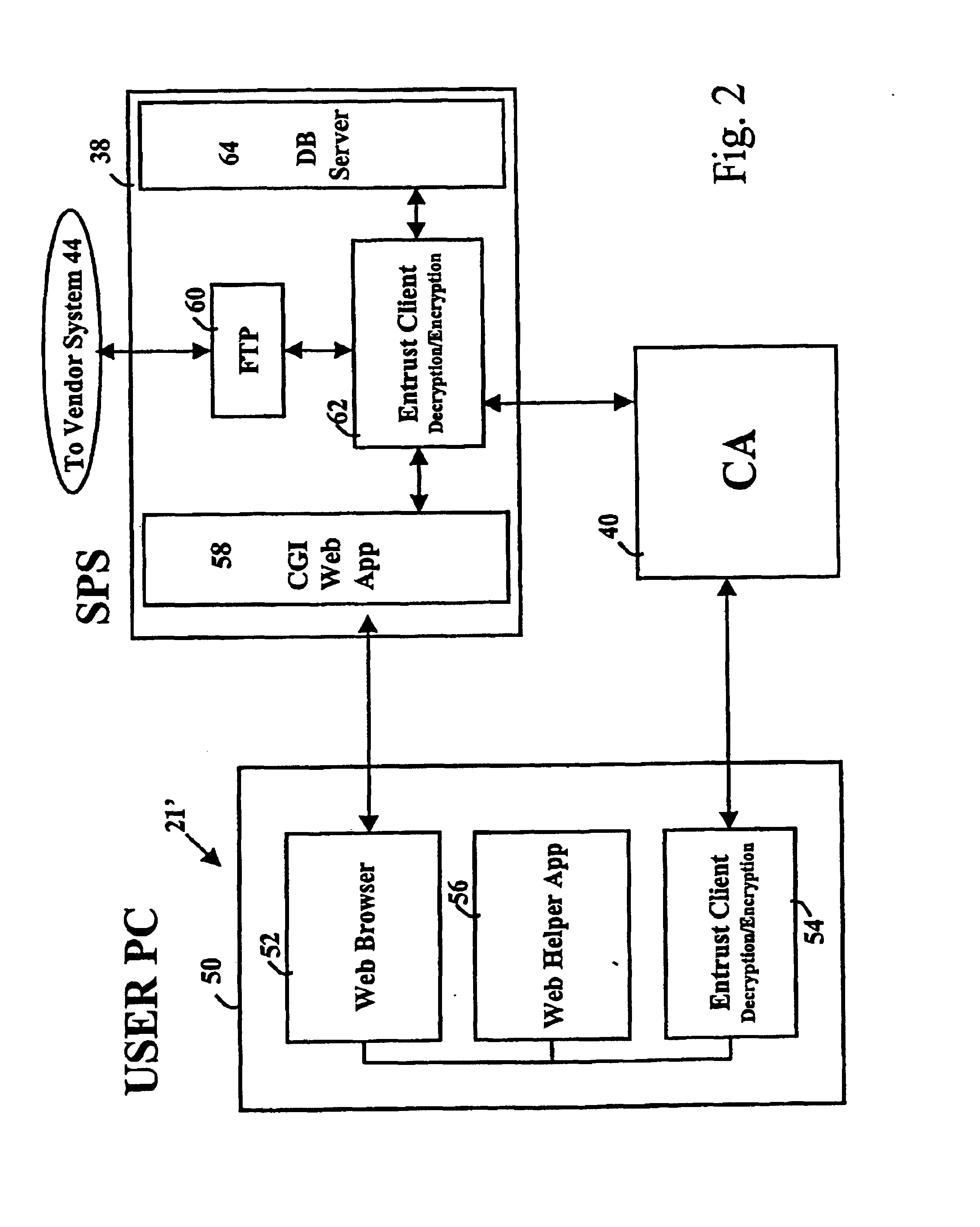
Secure electronic procurement system and method
InactiveUS7054844B2Reduce opportunity diversionReduce lossSpecial service provision for substationHand manipulated computer devicesUser deviceDigital signature
A secure electronic procurement system allows a user (e.g. pharmacist) to order and confirm receipt electronically over a network (e.g. Internet) goods normally subject to a verifiable chain of custody (e.g. narcotics, controlled drugs and substances, other public distribution regulated goods or valuables). The system includes hardware and software for a user device, a secure procurement system, a supplier system and a certificate authority to authenticate communications, particularly user orders and confirmed receipts. The supplier system may be a legacy EDI based order system. Copies of electronic orders, shipping notices and receipts confirming shipment received together with digital signatures are securely stored for audit trail purposes. Business rules within the secure procurement system operate to ensure proper adherence to the chain of custody.
Owner:DND CASH MANAGEMENT SOLUTIONS GP
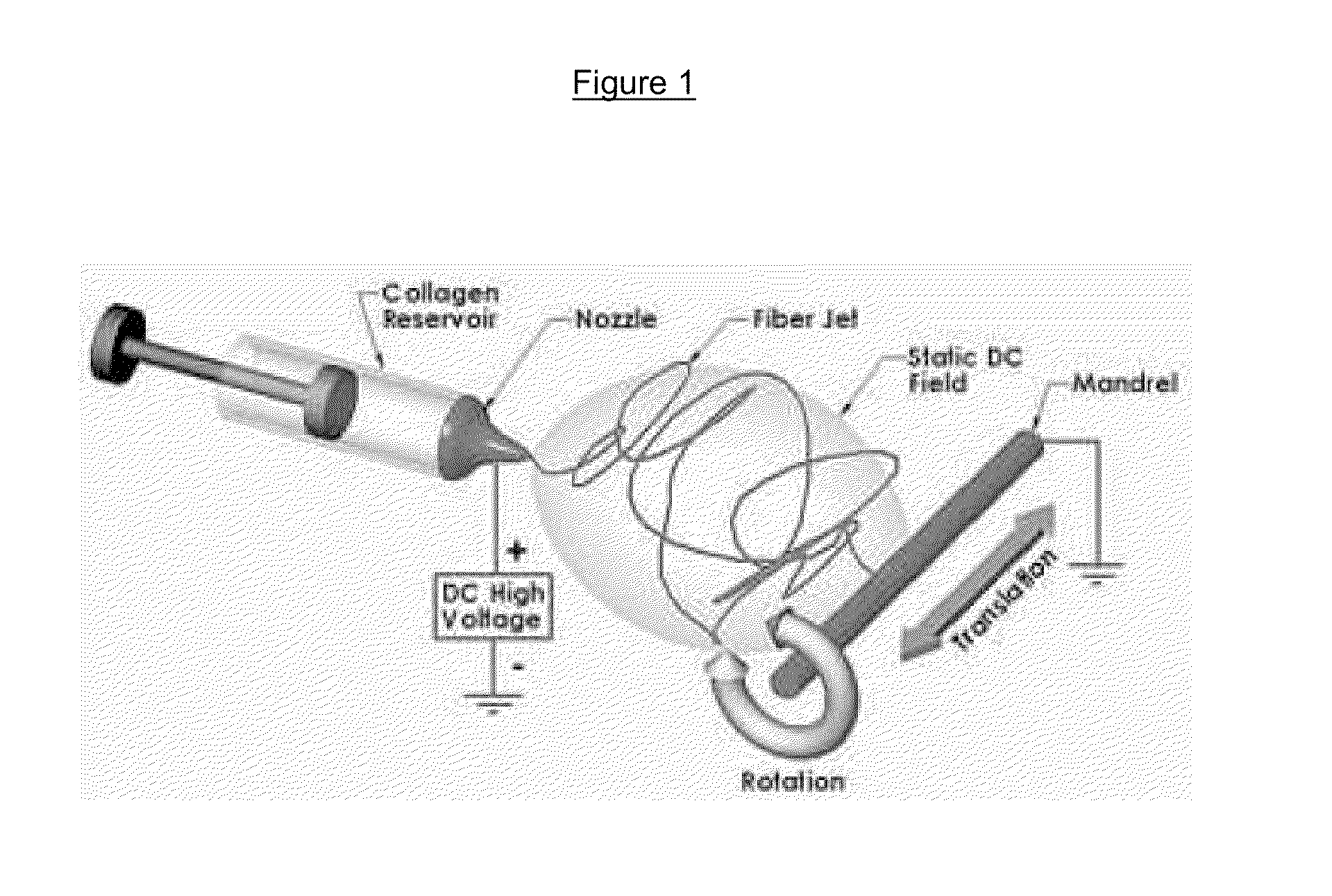
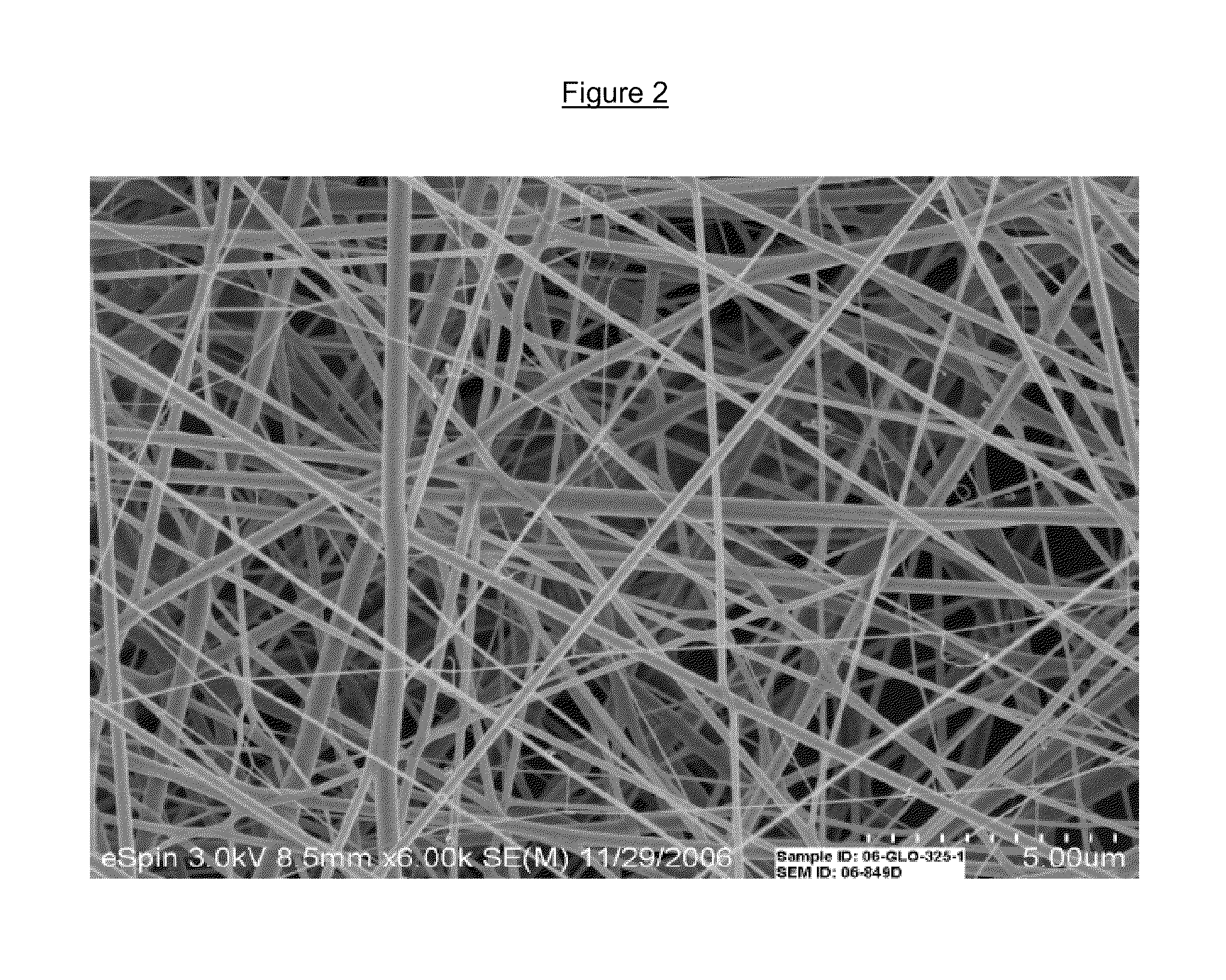
Drug delivery compositions and methods using nanofiber webs
Polymeric nanofibers have been developed which are useful in a variety of medical and other applications, such as filtration devices, medical prostheses, scaffolds for tissue engineering, wound dressings, controlled drug delivery systems, cosmetic skin masks, and protective clothing. These can be formed of any of a variety of different polymers, both non-biodegradable or biodegradable, and derived from synthetic or natural sources.The present invention discloses 1) the composition of fibrous articles and 2) methods for using these articles in medical applications.The biodegradable fibrous articles, which are preferably formed by electrospinning polymer solution of biodegradable fiberizable material with or in conjunction with medicinal agents and bioactive materials, comprise a composite (or asymmetric composite) of nanofibers with actives.Nanofibrous articles having specific medical uses include controlled drug delivery devices, glaucoma implants, tissue engineering, wound dressings, reinforcement grafts, corneal shields, and orbital blowout or sinus reconstructive materials.The methods include controlled drug delivery of a medicinal agent and providing treatment for inflammation, infection, trauma, glaucoma, and degenerative diseases.The drug delivery compositions and methods of this invention are directed towards improving the delivery of drugs to a target area of the body. These drug delivery compositions are nanofiber webs, mats, or whiskers which incorporate an active ingredient for delivery into a bodily fluid. The active ingredient is delivered in a controlled manner by placing the nanofiber web into the bodily fluid which allows the drug embedded in the nanofiber to be released in a controlled and longer lasting manner.
Owner:NOTUS LAB
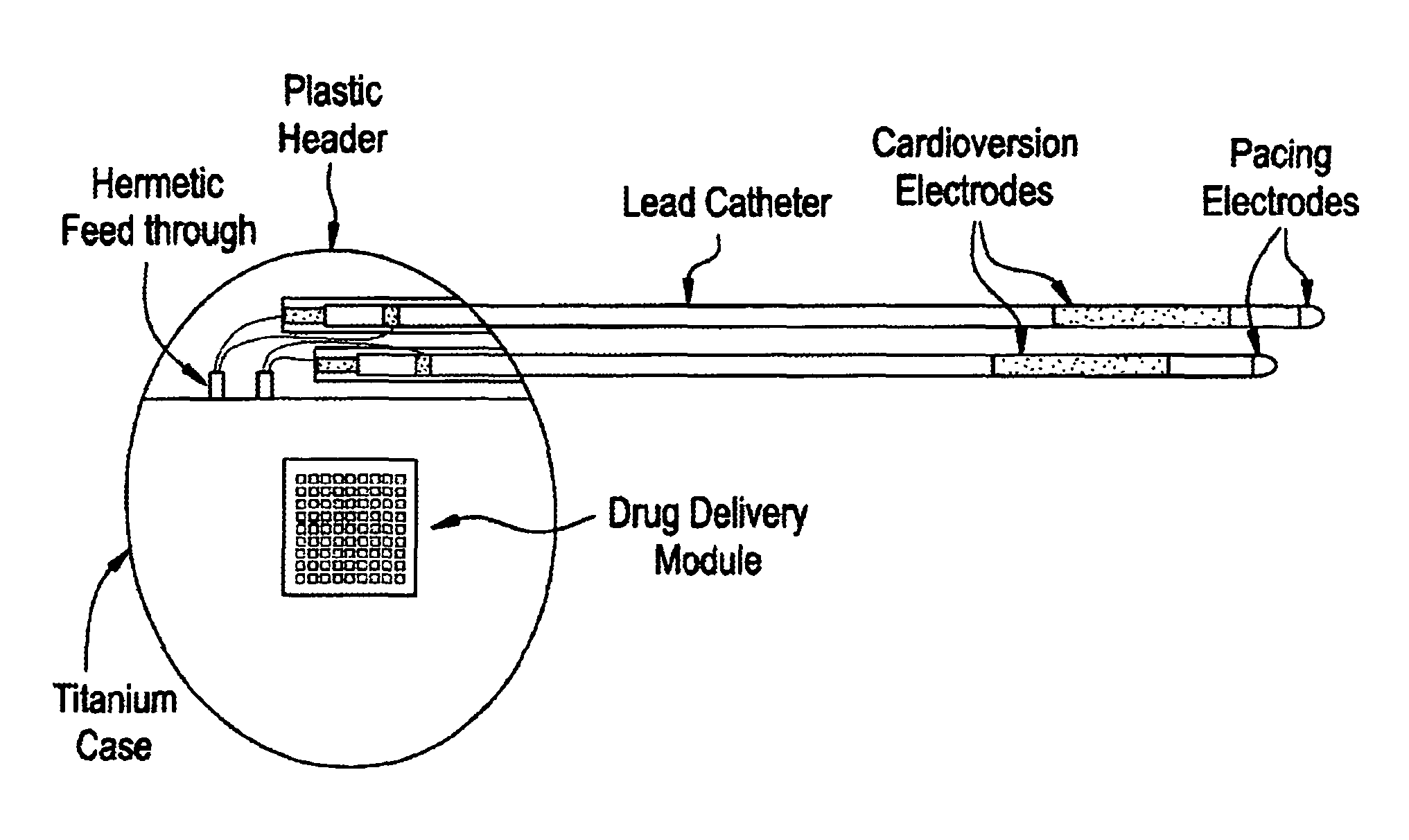
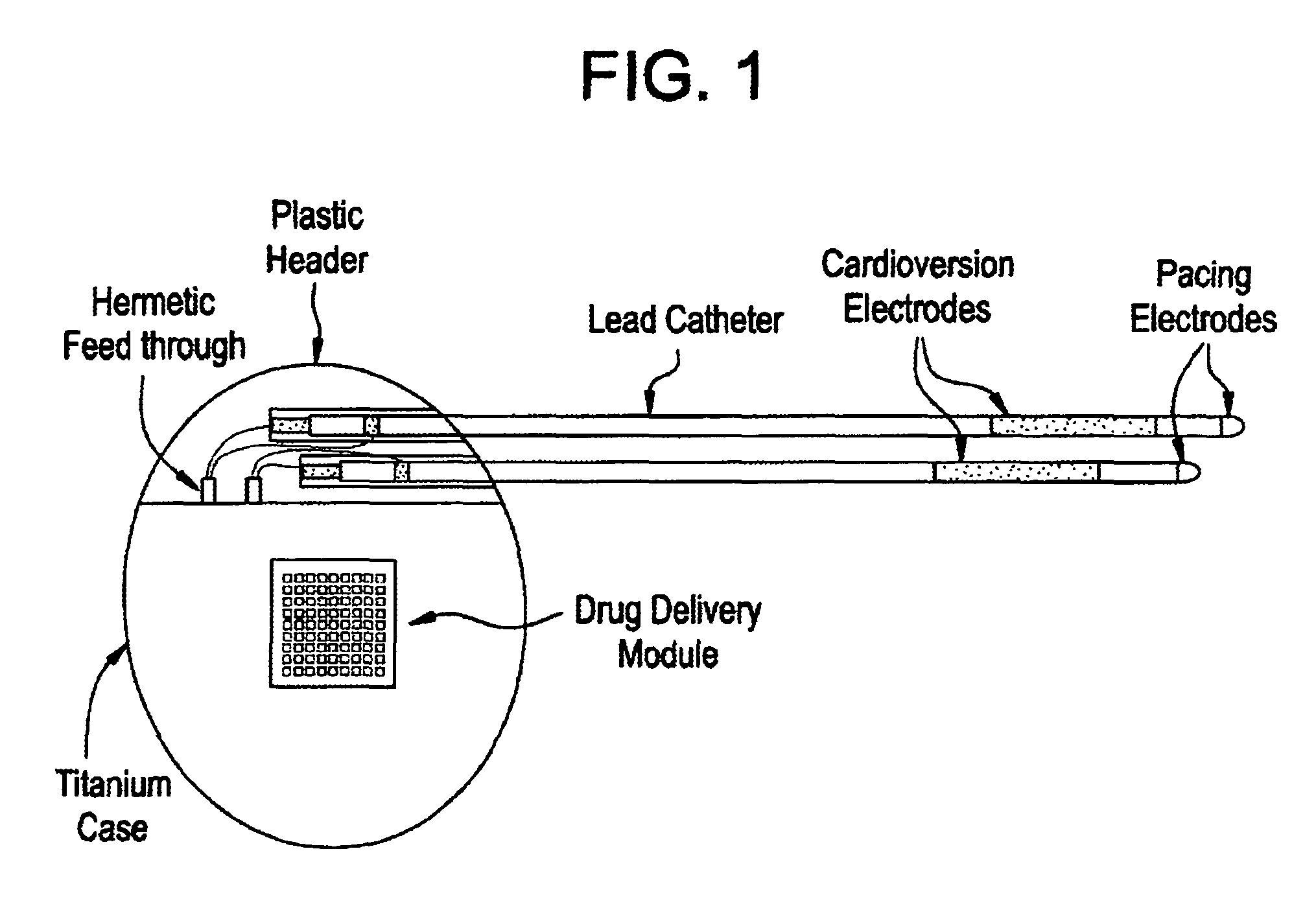
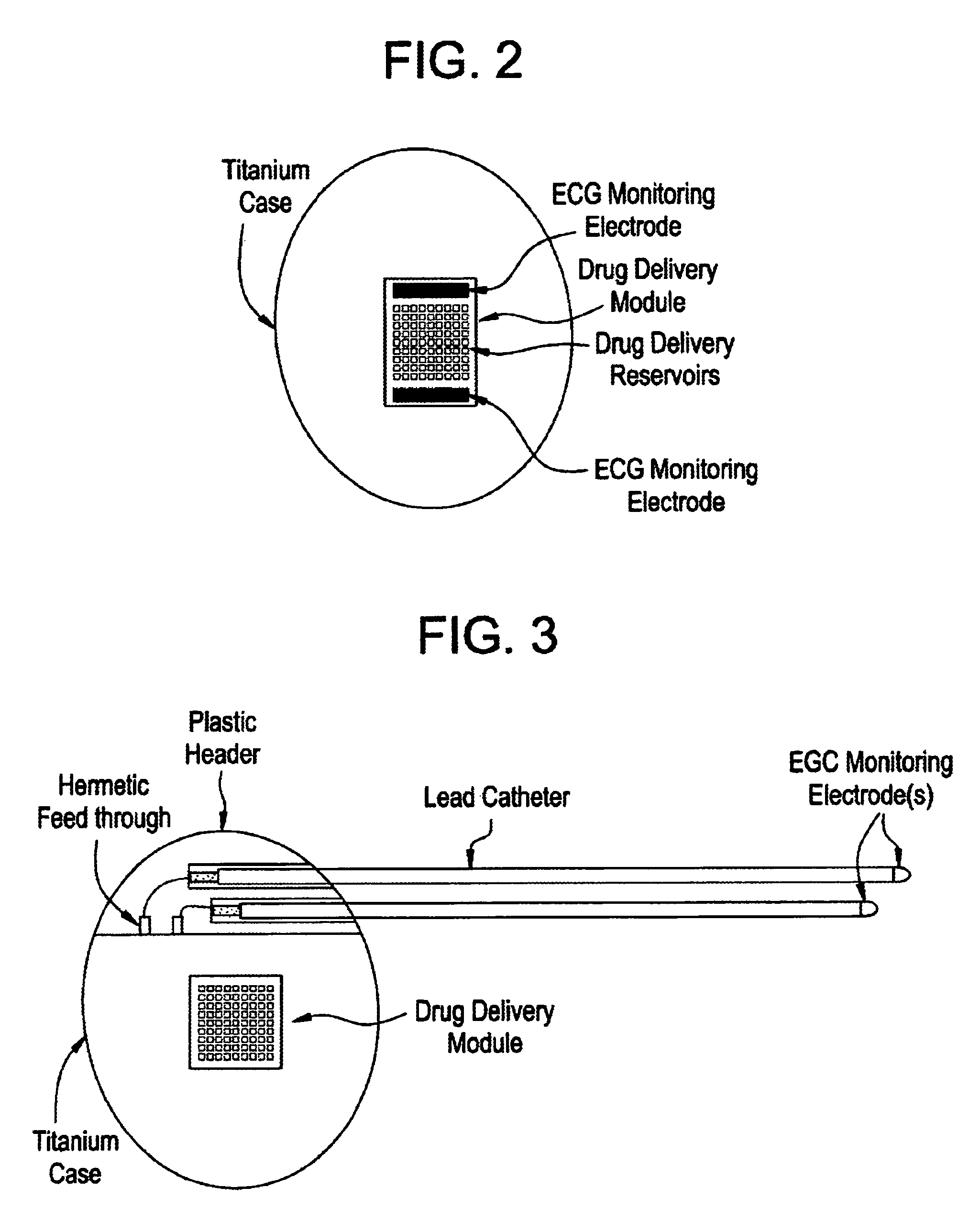
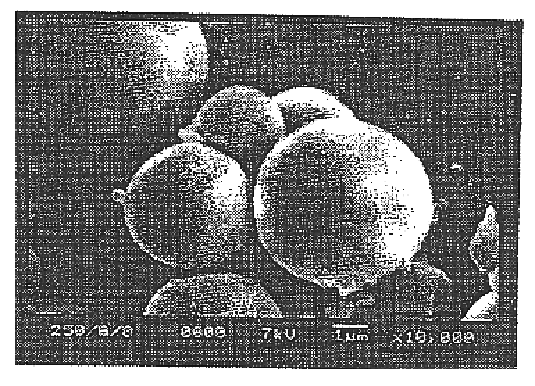
Medical device for controlled drug delivery and cardiac monitoring and/or stimulation
InactiveUS7917208B2Reduce frequencyHigh sensitivityElectrocardiographyHeart defibrillatorsMicrocontrollerCardioversions
Medical device and methods are provided for controlled drug delivery in a cardiac patient. The device includes an implantable drug delivery module comprising reservoirs containing a drug and a control means for selectively releasing an effective amount of drug from each reservoir; one or more electrodes or sensors for cardiac monitoring, stimulation, or both; and a microcontroller for controlling operational interaction of the drug delivery module and the cardiac electrode. The electrodes may comprise ECG monitoring, cardioversion, or cardiac pacing electrodes. A medical device also is provided for controlled delivery of drug to a patient having congestive heart failure, which includes an implantable drug delivery module comprising a natriuretic peptide and a release mechanism for selectively releasing a pharmaceutically effective amount of the natriuretic peptide into the patient; and a microcontroller for controlling the release mechanism, for example, in response to one or more monitored patient parameters.
Owner:MICROCHIPS BIOTECH INC
Controlled drug release system of retinoic acid
InactiveUS6764698B1Thorough understandingReduce inductionPowder deliveryHydroxy compound active ingredientsMicrosphereDrug release
The present invention relates to a controlled drug release system in which a certain ratio or retinoic acid is incorporated into a microsphere comprising biodegradable polymer and amphoteric block copolymer having both hydrophilic and hydrophobic groups.
Owner:GWANGJU INST OF SCI & TECH +1
Surface-modified hydrophobically modified drug-carried chitosan polymer micelle and method for preparing same
InactiveCN1883708AGood curative effectImprove instabilityPowder deliveryPharmaceutical non-active ingredientsPolymeric surfaceNanocarriers
Disclosed is a surfacely modified hydrophobic Chitosan oligosaccharide polymer drug-loaded colloidal cluster, obtained by grafting chitosan with a average molecular weight of 1.5kD-51kD and aliphatic acid of C10-C22, comprising based on hydrophobic modified Chitosan oligosaccharide polimer colloidal clusters, modifying amidogens or hydroxy groups of Chitosan oligosaccharide molecules on polimer surface with bi-functional small organic moleculers, forming chemical bond bridges between molecules on the surfaces of polymer colloidal clusters,to improve the instability of polymer colloidal cluster diluted, and to imorove the original loose structure of polimer colloidal cluster surfece to form a fine and close net-shaped structure, to decrease a sudden release of drugs, and to control drugs with slow release. The drug-loaded colloidal cluster provided is a nano-carrier with excellent organelle targeting, is applicated in life science field and pharmacy field.
Owner:ZHEJIANG UNIV
Controlling drug delivery apparatus
InactiveUS20050229931A1Reduces regulatory submissionEconomy of scaleData processing applicationsDrug and medicationsDrug treatmentControl drugs
A drug package comprising a plurality of drug vials containing drugs for delivery to a patient in a drug delivery device; and a data carrier including drug treatment information for use by the drug delivery apparatus.
Owner:RESPIRONICS
Implants with controlled drug delivery features and methods of using same
InactiveUS20150342875A1Reduces one or more side effectsLimits treatment-associated side-effectsOrganic active ingredientsEye surgeryAqueous humorControl release
Disclosed herein are drug delivery devices and methods for the treatment of ocular disorders requiring targeted and controlled administration of a drug to an interior portion of the eye for reduction or prevention of symptoms of the disorder. The devices are capable of controlled release of one or more drugs and may also include structures which allow for treatment of increased intraocular pressure by permitting aqueous humor to flow out of the anterior chamber of the eye through the device.
Owner:GLAUKOS CORP
Oral devices and methods for controlled drug delivery
Controlled-drug-delivery oral devices are implanted or inserted into an oral cavity, built onto a prosthetic tooth crown, a denture plate, braces, a dental implant, or the like. The devices are refilled or replaced as needed. The controlled drug delivery may be passive, based on a dosage form, or electro-mechanically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled delivery may be any one of the following: delivery in accordance with a preprogrammed regimen, delivery at a controlled rate, delayed delivery, pulsatile delivery, chronotherapeutic delivery, closed-loop delivery, responsive to a sensor's input, delivery on demand from a personal extracorporeal system, delivery regimen specified by a personal extracorporeal system, delivery on demand from a monitoring center, via a personal extracorporeal system, and delivery regimen specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted or induced by a transport mechanism, such as any one of, or a combination of iontophoresis, electroosmosis, electrophoresis, electroporation, sonophoresis, and ablation. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen. The oral devices and methods for controlled drug delivery apply to humans and animals.
Owner:安迪·沃尔夫 +1
Ambient temperature rapid self-polymerization compositions of high cross-linked or linear type beta-amino-ester alternative co-polymers and their applications
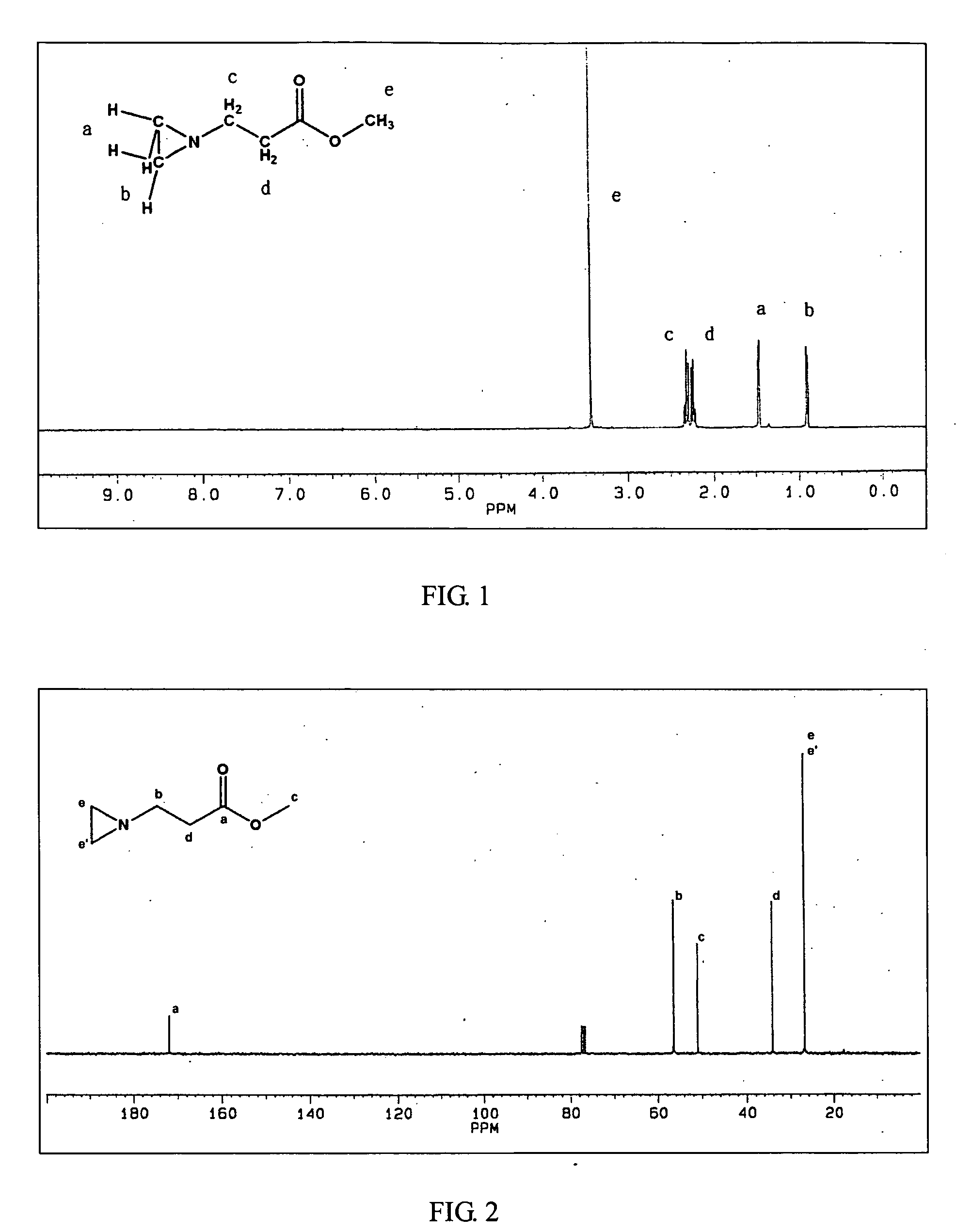
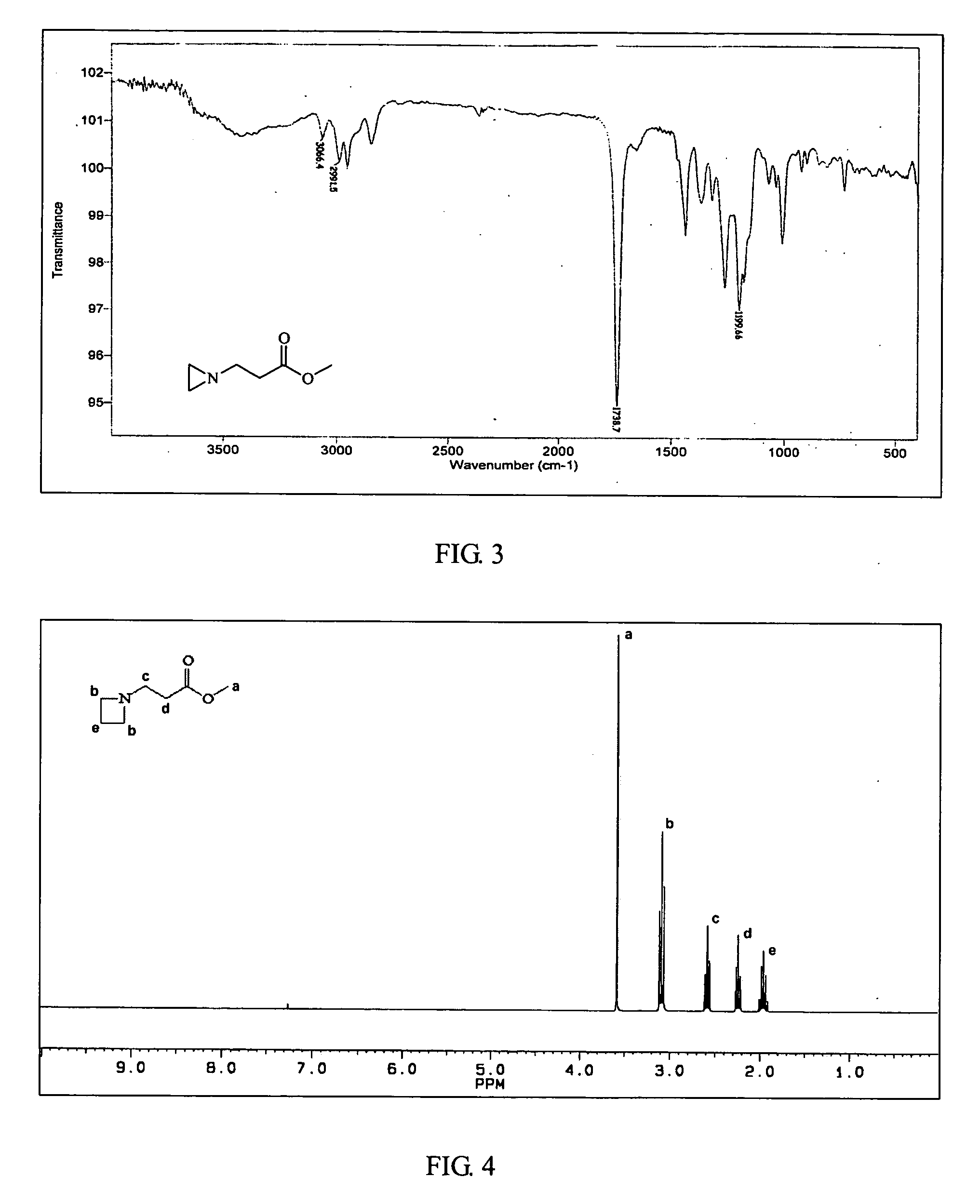
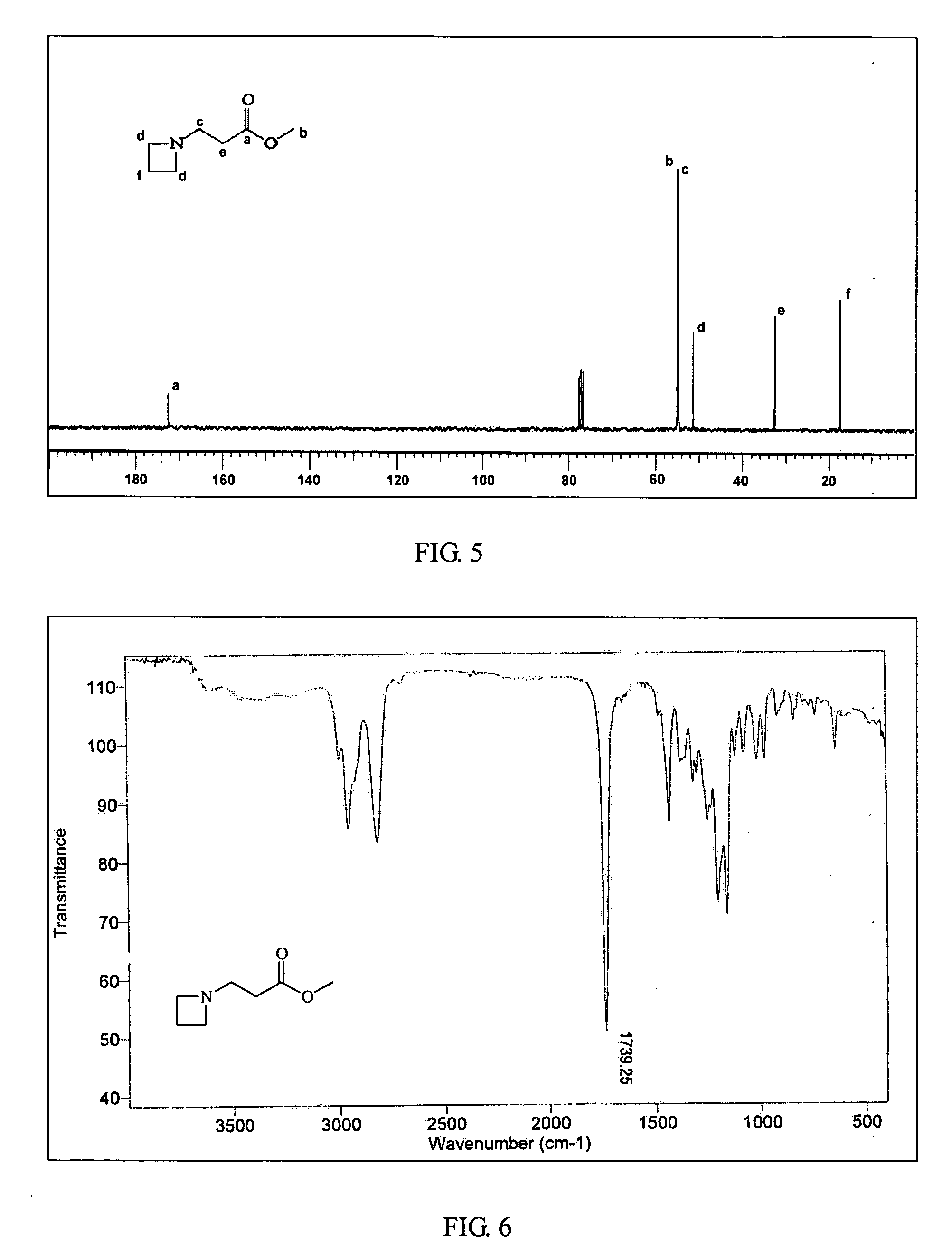
InactiveUS20070299211A1Self-polymerization can be very rapidSelf-polymerization rate can be controlledCross-linkAmino esters
Self-polymerization of mono-aziridine (or azetidine) and multi-aziridine (or azetidine) containing compounds with vinyl group containing organic acid, such as acrylic acid (AA), 2-methylenesuccinic acid, 2,3-dimethylenesuccinic acid and etc, at ambient temperature results in the new type of cross-linked and linear type copolymers, respectively.The polymerization of multi-functional aziridine (or azetidine) containing compounds with vinyl group containing organic acid results in the formation of high cross-linked polymers. The self-polymerization takes place at ambient temperature and the resultants, cross-linked polymeric networked materials, are solvent insoluble and potential for adhesive, composite matrix and other applications. These insoluble materials are hydrolyzed in an acidic or basic condition to form the water soluble β-amino acids.A linear poly(β-aminoester) is obtained from the self-polymerization of vinyl group containing organic acid with mono-aziridine (or azetidine) containing compound at ambient temperature. poly(β-aminoester) is applicable for gene transfer, controlled drug release and other applications. This self-polymerization process offers a convenient route for preparing poly(β-aminoesters).
Owner:TAMKANG UNIVERSITY
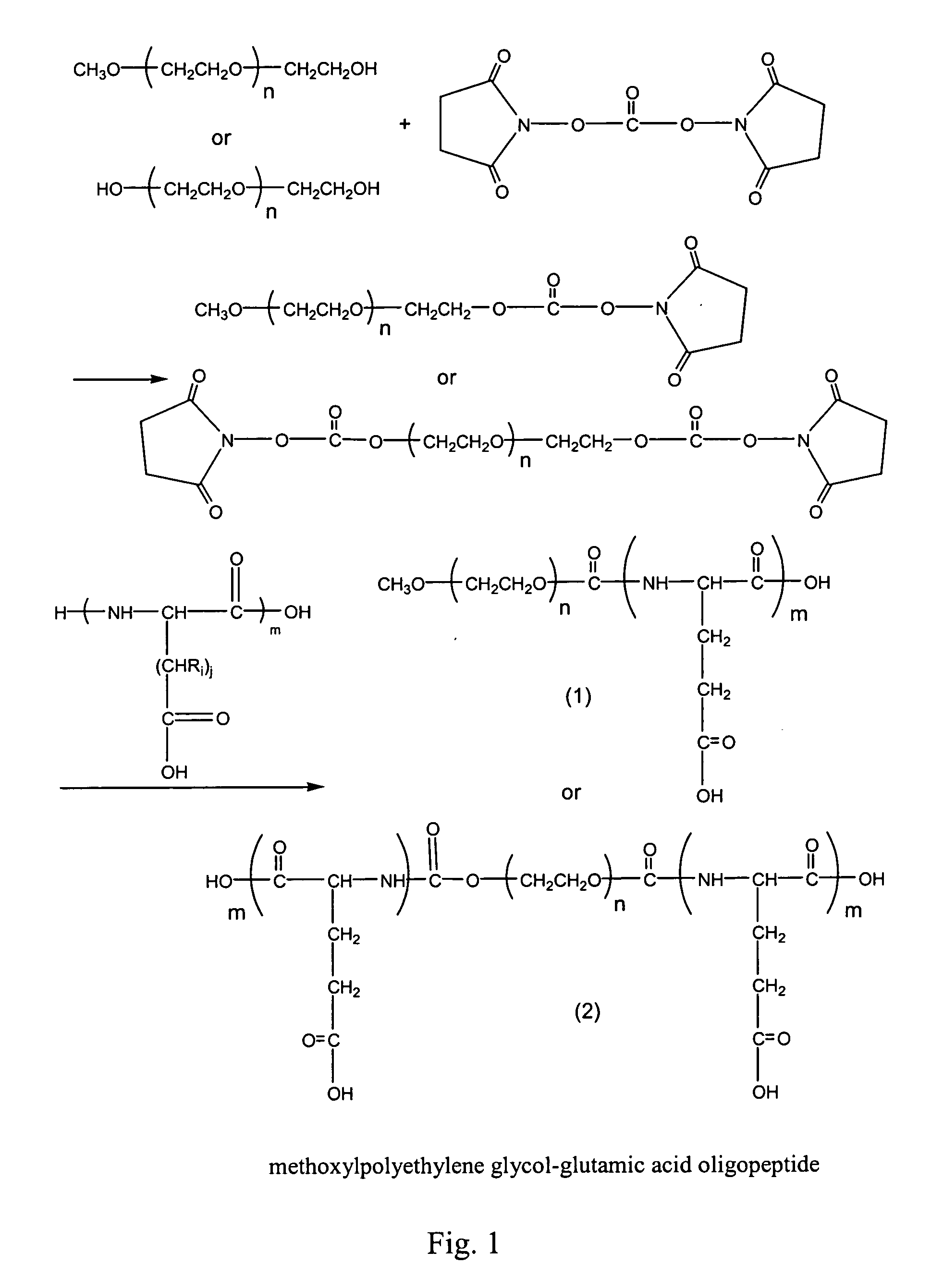
Compounds of hydrophilic polymer-polycarboxyl oligopeptide and medicines, medical composite comprising above compound and use of above compound in medicimes
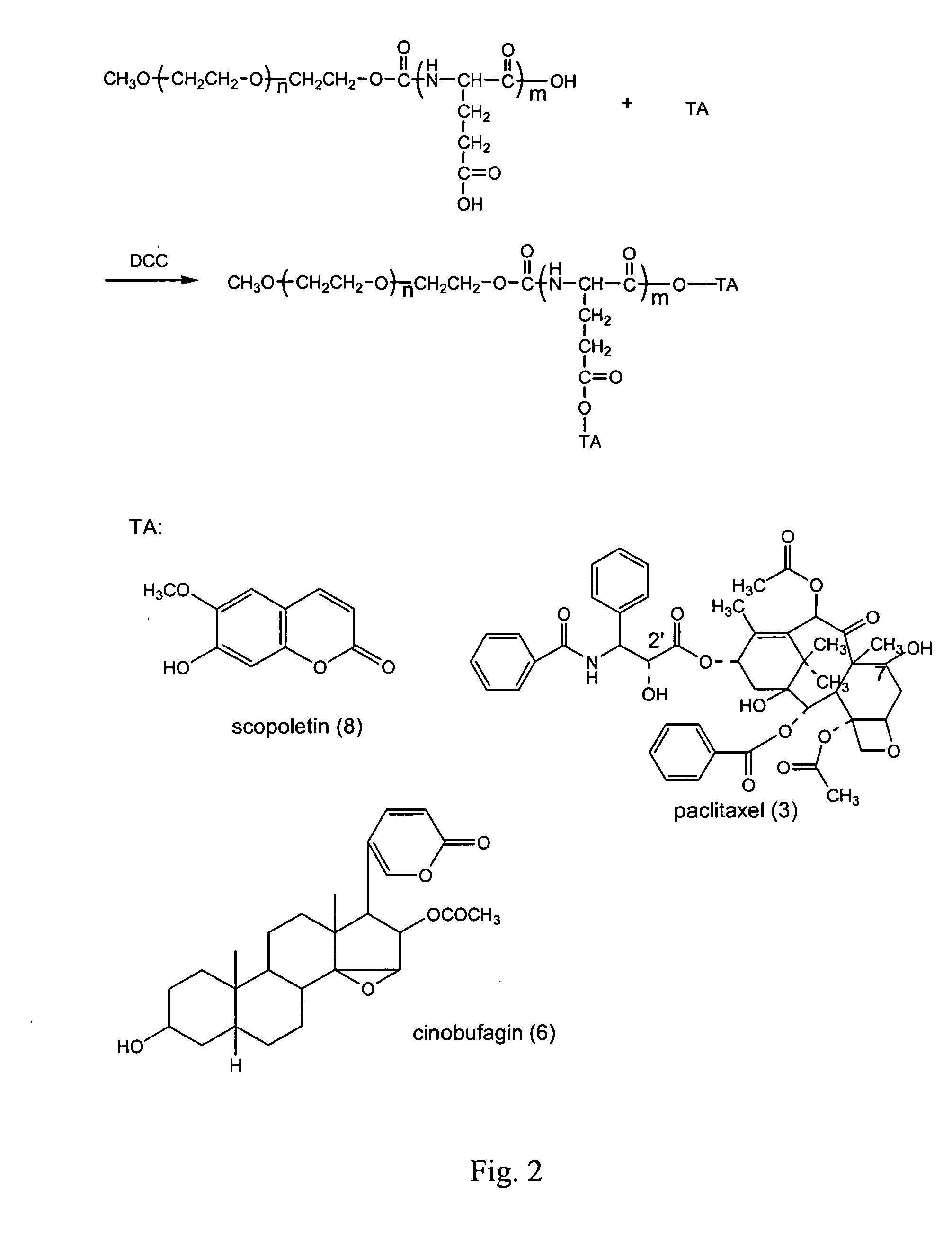
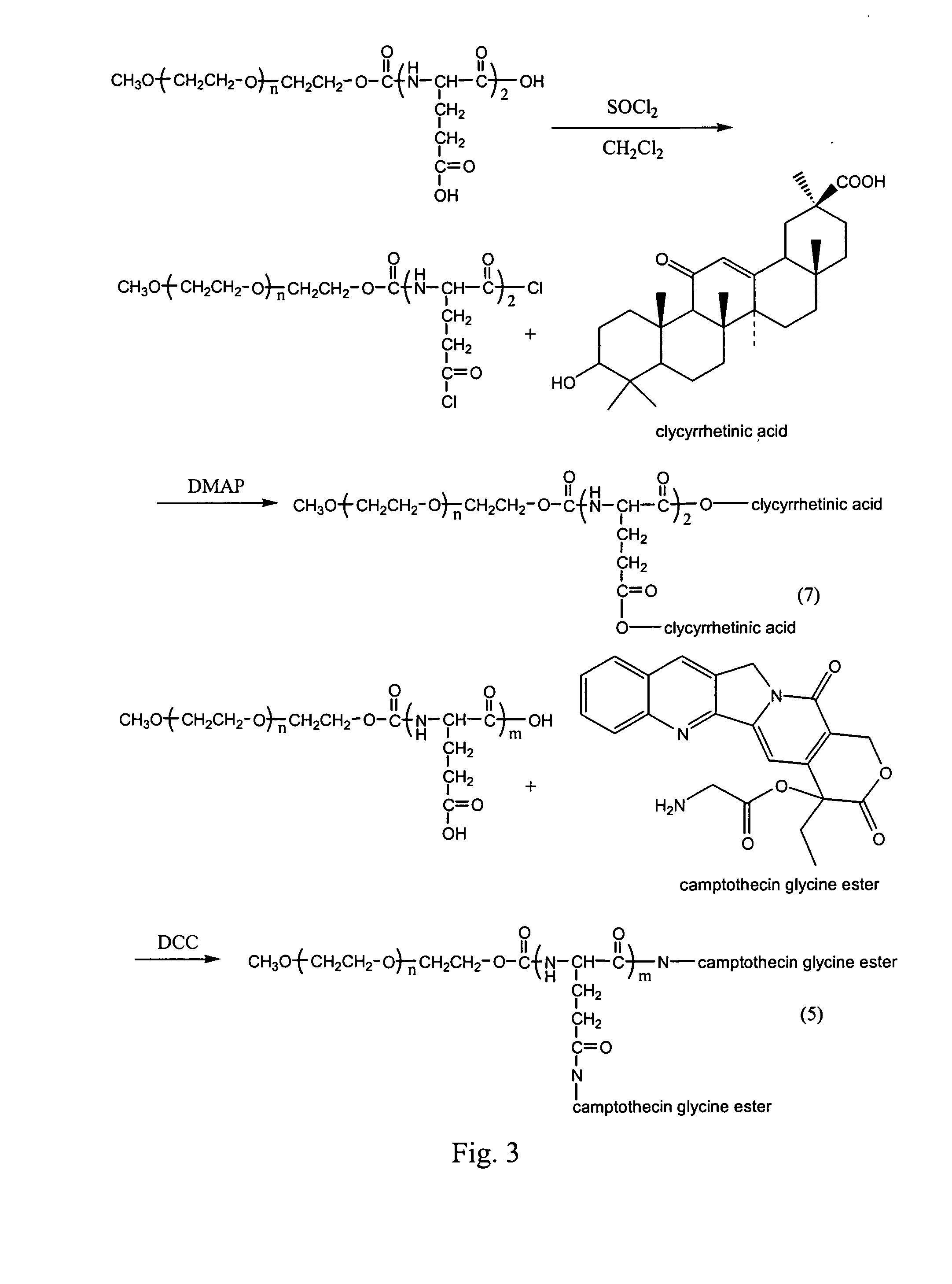
InactiveUS20050147617A1Promote absorptionExtended course of treatmentPowder deliveryDipeptide ingredientsSolubilityDrug release
The present invention relates to a conjugate of hydrophilic polymer-multicarboxyl oligopeptide and drug molecule of the following formula: wherein: P is a water soluble polymer; m is an integer of 2˜12; j is an integer of 1˜6; Ri is a group selected from H, C1-12 alkyl, substituted aryl, aralkyl, heteroalkyl and substituted alkyl; X and Z are linking groups; and TA is drug molecule. The conjugate has low toxicity and an ability to carry more than one drug molecule to improve solubility, sustain and control drug release, and has a remarkably enhancing effect especially to antitumor drug such as paclitaxel and camptothecin etc.
Owner:JENKEM TECH
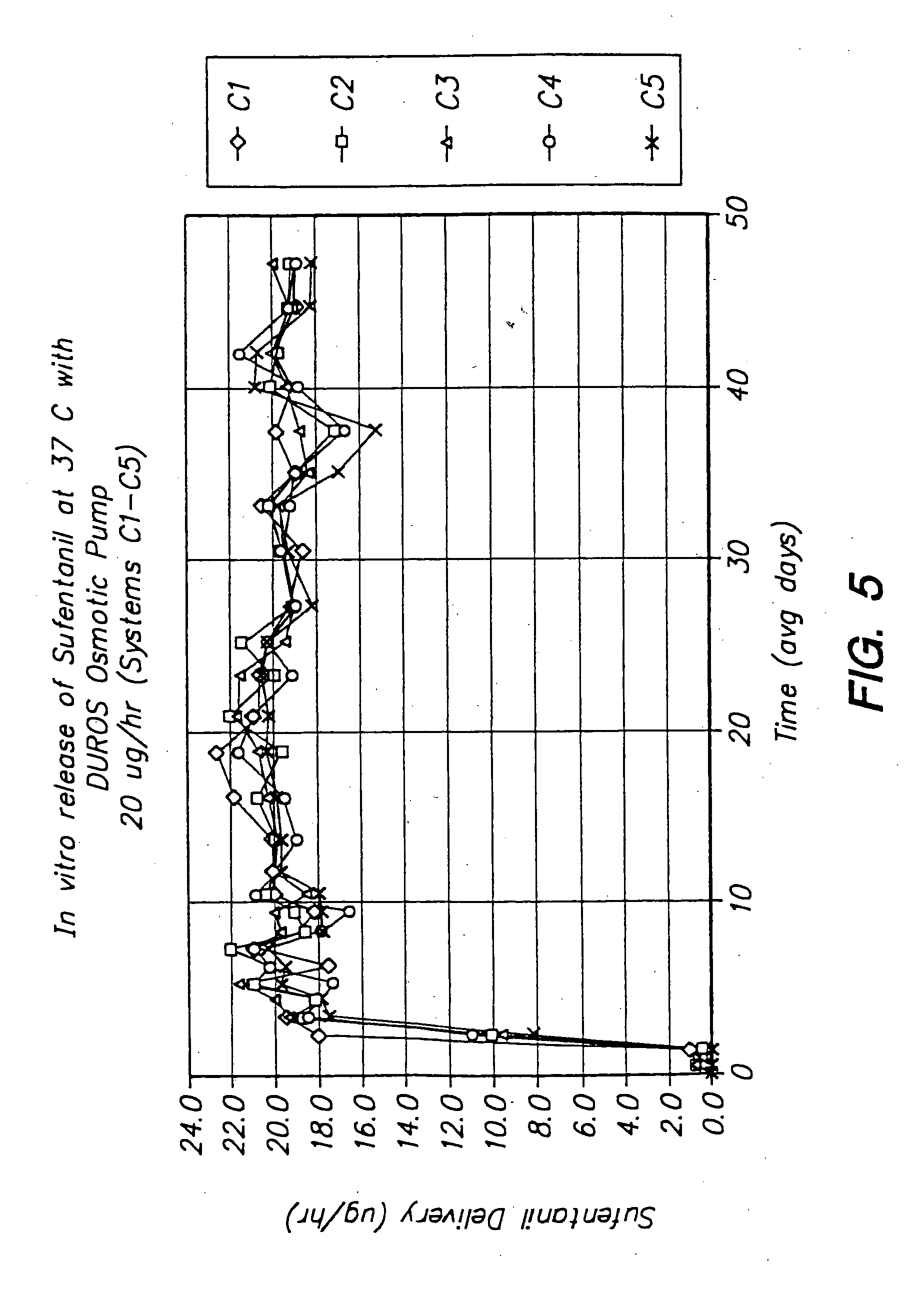
Devices and methods for pain management
InactiveUS20050129737A1Effective pain managementAdequate pain reliefBiocideNervous disorderPharmaceutical formulationControl drugs
The invention features devices and methods for the systemic delivery of fentanyl or a fentanyl congener (e.g., sufentanil) to treat pain. In the present invention, a drug formulation comprising fentanyl or a fentanyl congener is stored within a drug delivery device (e.g., contained in a reservoir or impregnated within a matrix within the controlled drug delivery device). The drug formulation comprises an amount of drug sufficient for treatment and is stable at body temperatures (i.e. no unacceptable degradation) for the entire pre-selected treatment period. The drug delivery devices store the drug formulation safely (e.g., without dose dumping), provide sufficient protection from bodily processes to prevent unacceptable degradation of the formulation, and release the drug formulation in a controlled fashion at a therapeutically effective rate to treat pain. In use, the drug delivery device is implanted in the subject's body at an implantation site, and the drug formulation is released from the drug delivery device to a delivery site. The delivery site may be the same as, near, or distant from the implantation site. Once released at the delivery site, the drug formulation enters the systemic circulation and is transported to the site of action in the body to modulate the pain response (e.g., the brain or other pain sensory location).
Owner:DURECT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com