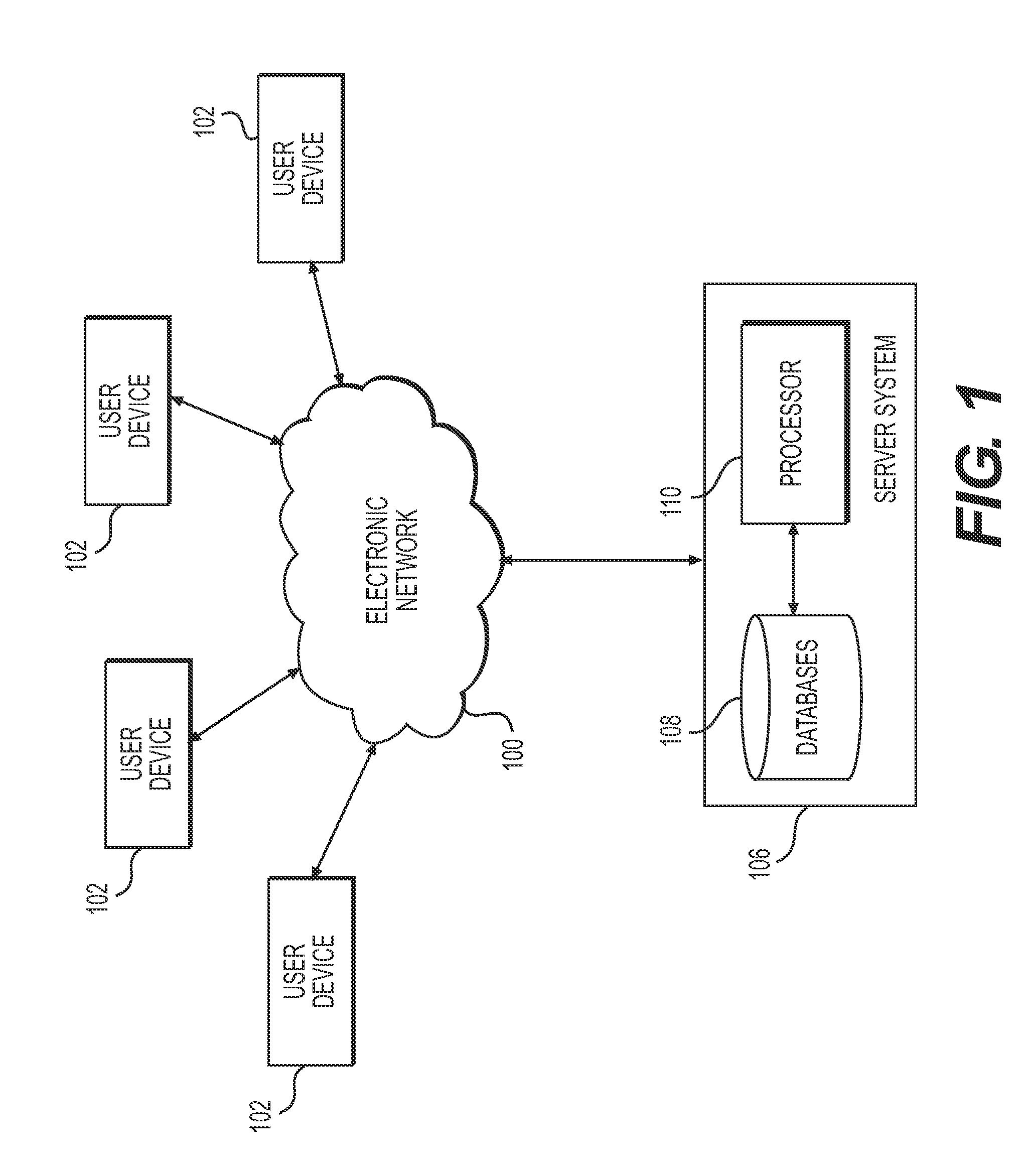
Patents
Literature
78 results about "Medication regimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
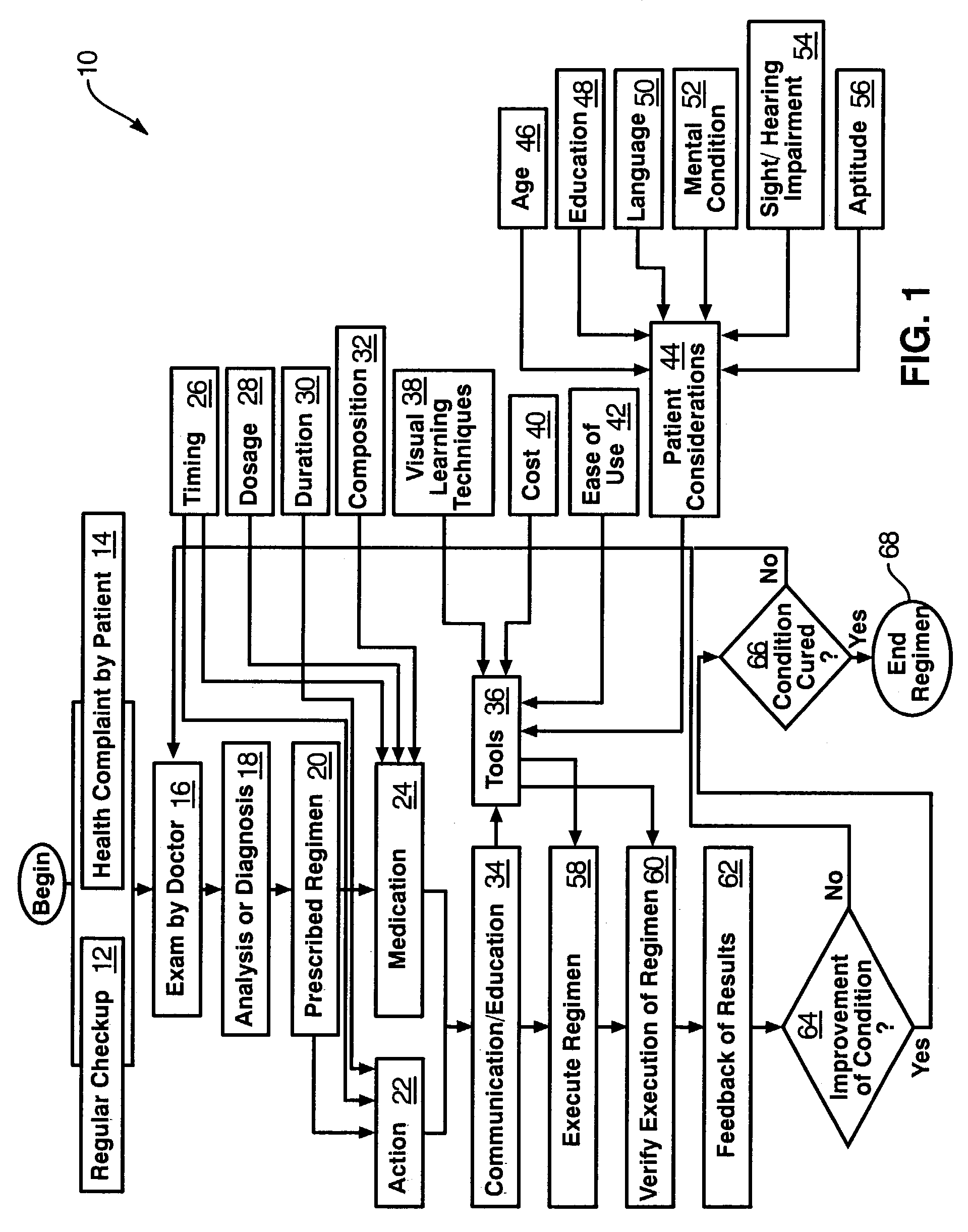
A regimen is a systematic approach to diet, medicine, or exercise. The word has other meanings, but this is its most common use.
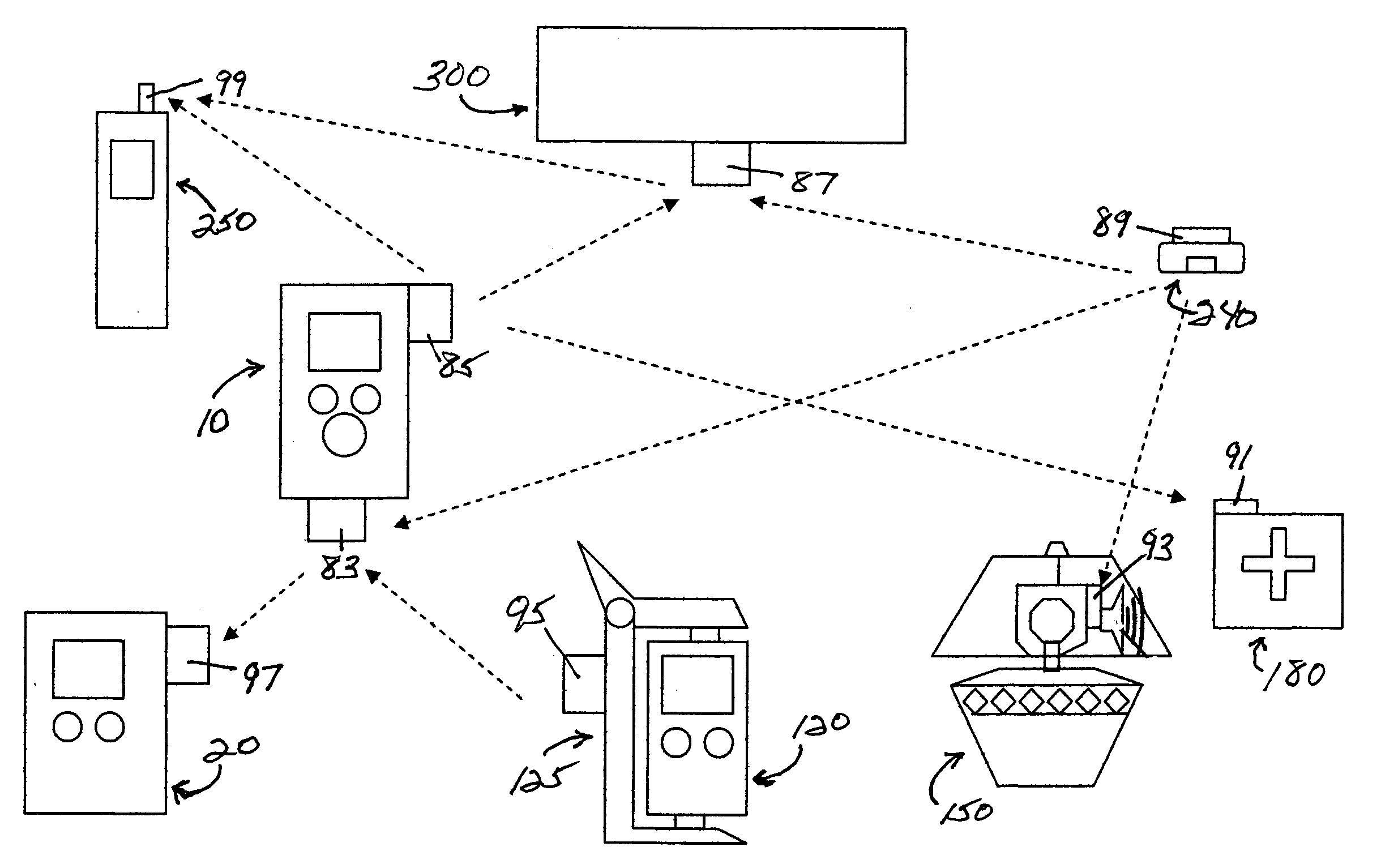
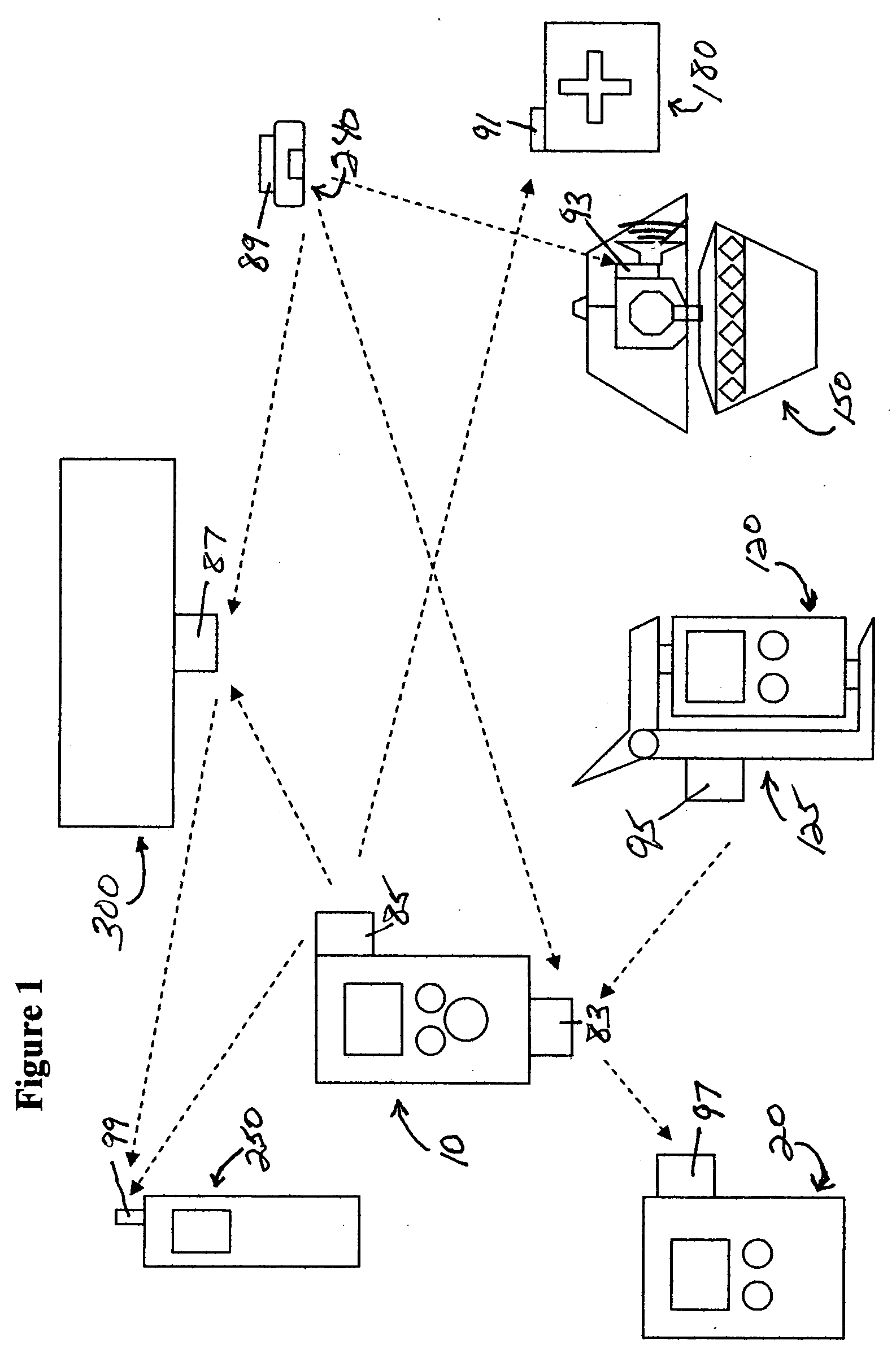
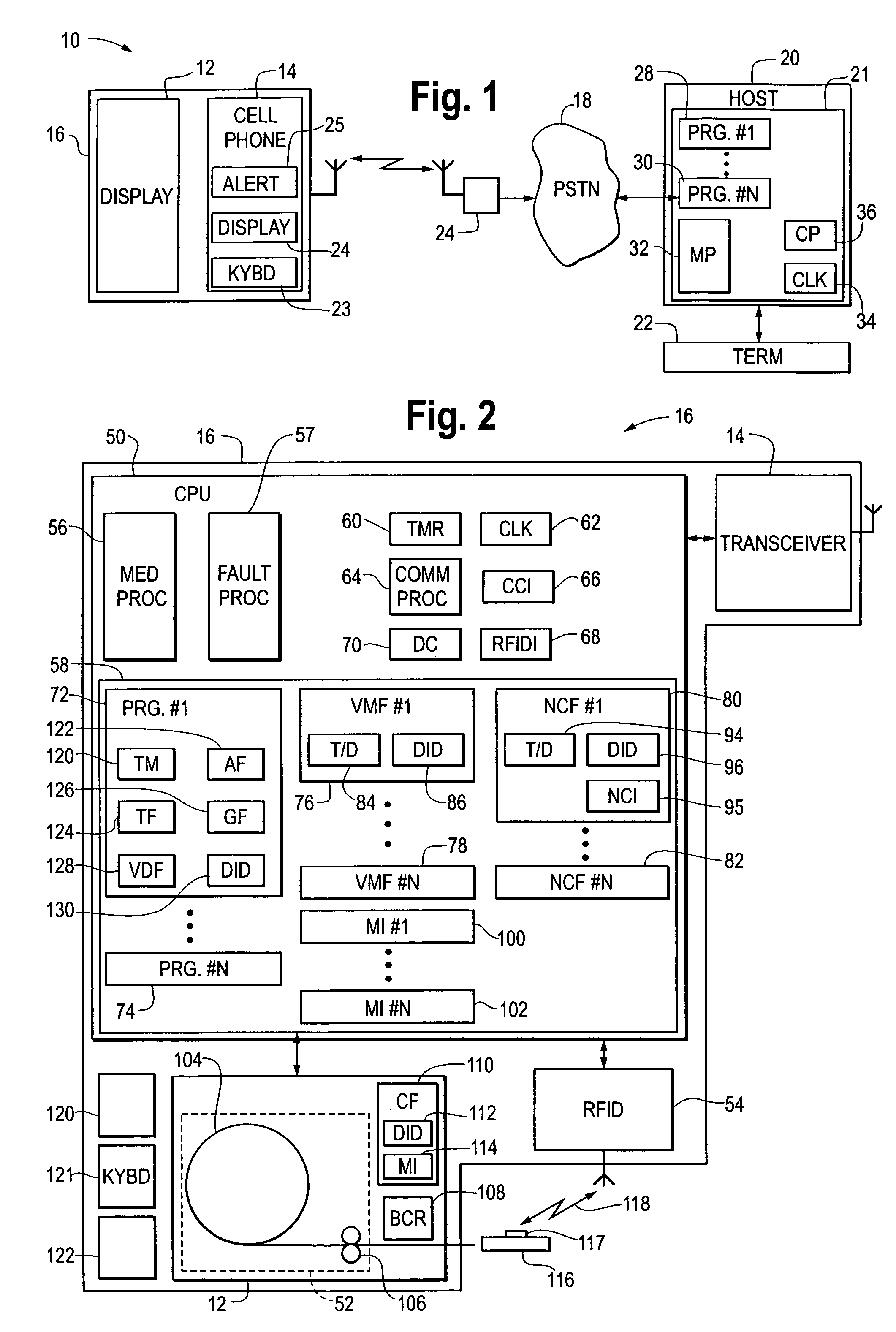
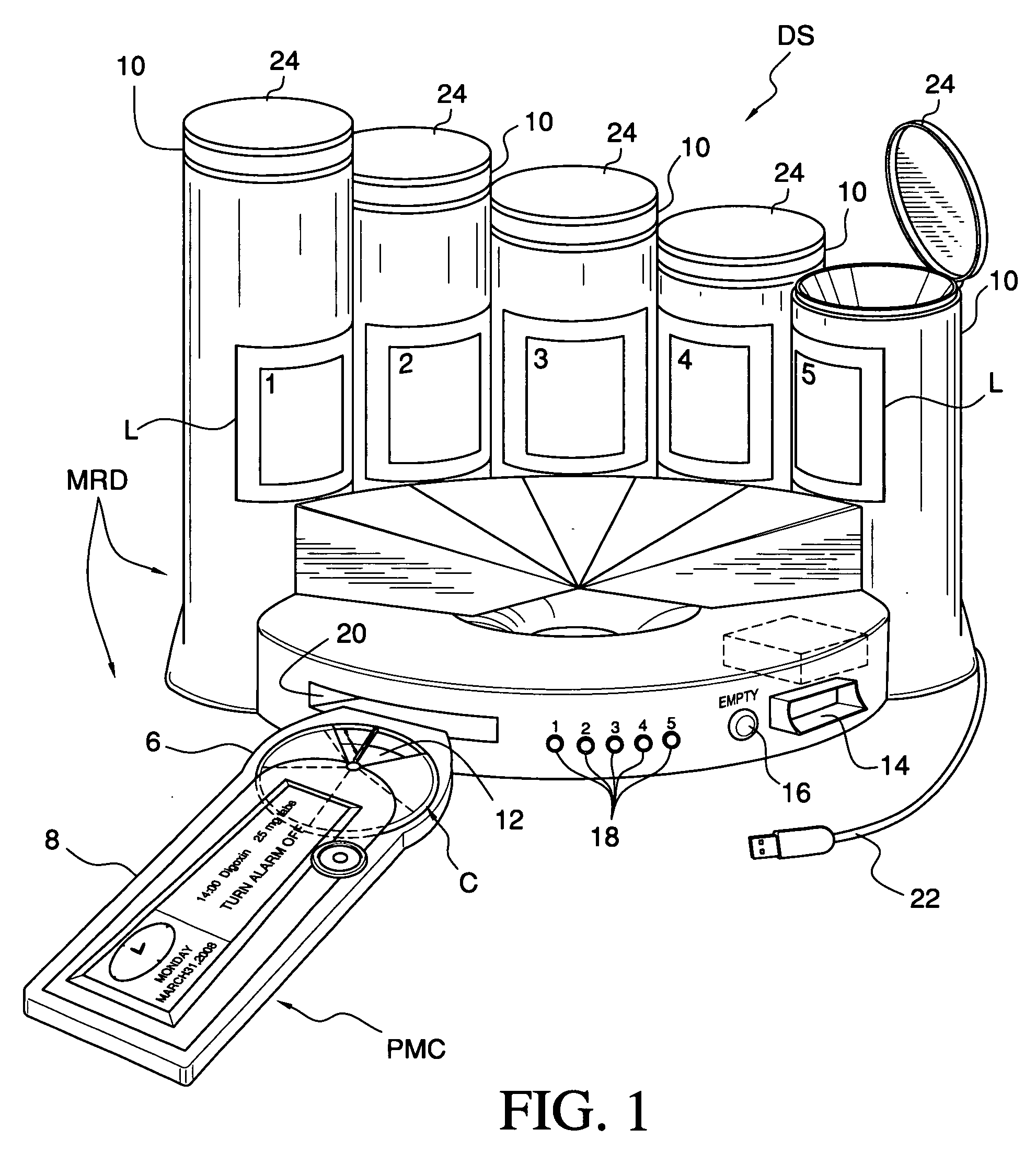
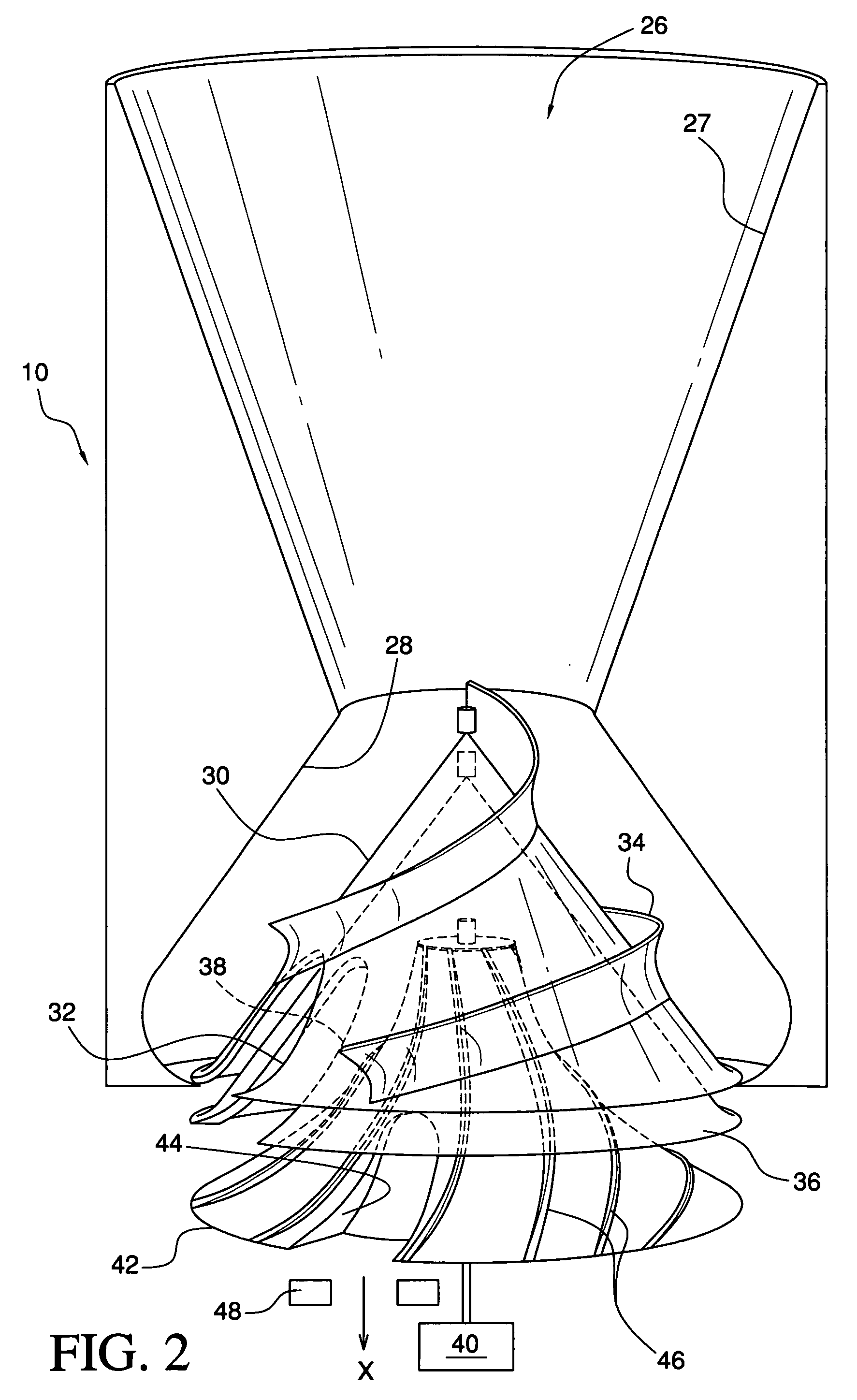
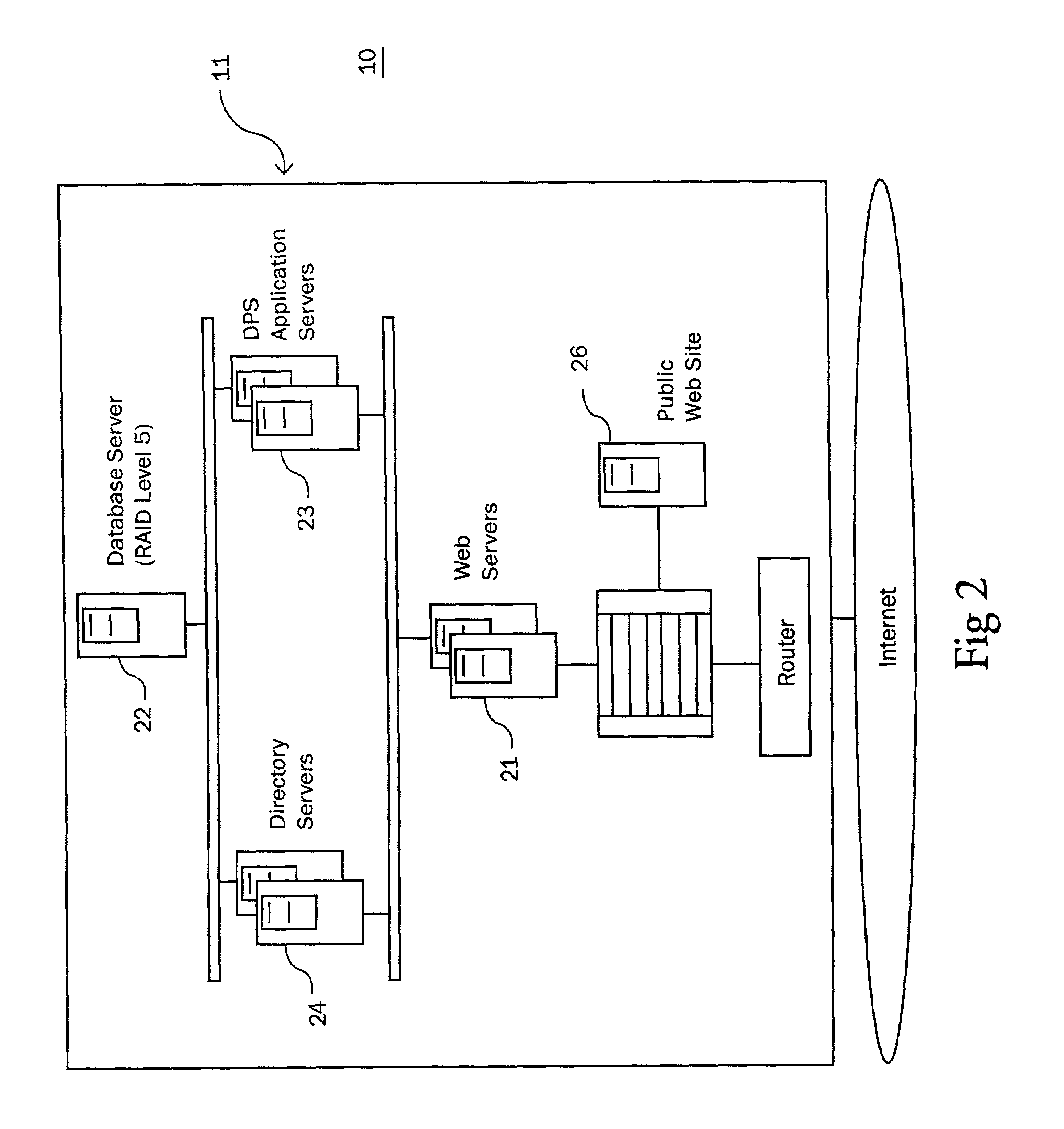
Medication & health, environmental, and security monitoring, alert, intervention, information and network system with associated and supporting apparatuses
InactiveUS20060154642A1Facilitate user and/or occupant well beingExtended stayDispersed particle filtrationDrug and medicationsNetworked systemHealth administration
Systems and apparatuses include devices, biosensors, environmental sensors, security related sensors, networked products, communications processors and components, alert and information components, processors, and software to support: 1) facilitating medication regimen and patient / user health administration, dosage control, tracking, compliance, information inquiry and presentation, reminder and notification; 2) providing monitoring, information, ordering, and intervention; 3) presenting the option of leveraging the preventative care, alert and notification components with other components to facilitate user or occupant well being, along with living, work area and dwelling environmental or security safety; and 4) enhancing the dwelling, living or work area with products that may be networked to support the widespread acceptance of these systems and apparatuses. The systems include a) processing, centralizing and communicating device commands and / or programs, e.g. a multifunctional device controller; b) device administration; c) patient / user information; d) dwelling environmental safety; e) security breach information; f) centralized and remote apparatus and system activations through primary component or at least one backup.
Owner:INSIGNIO TECH
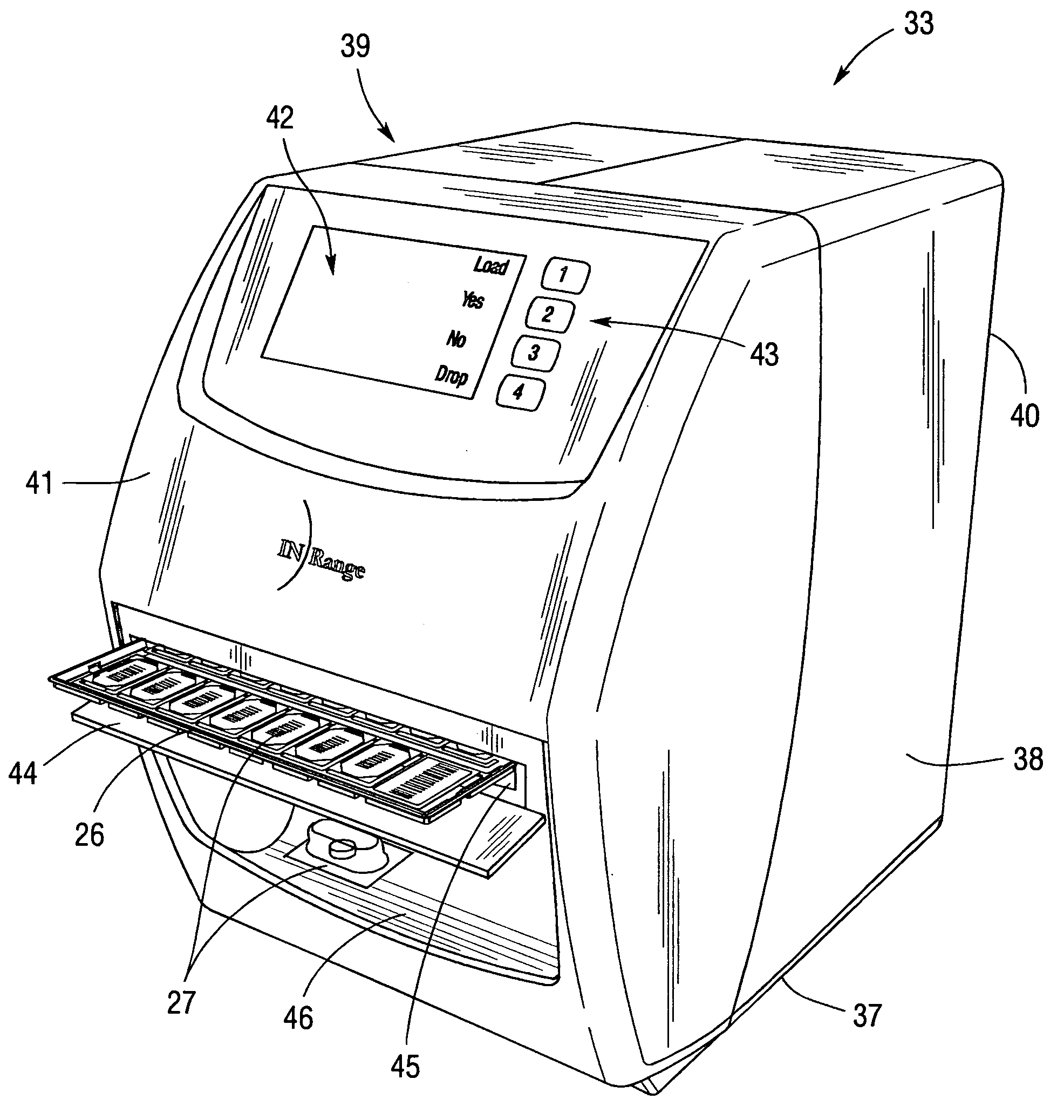
Integrated, non-sequential, remote medication management and compliance system
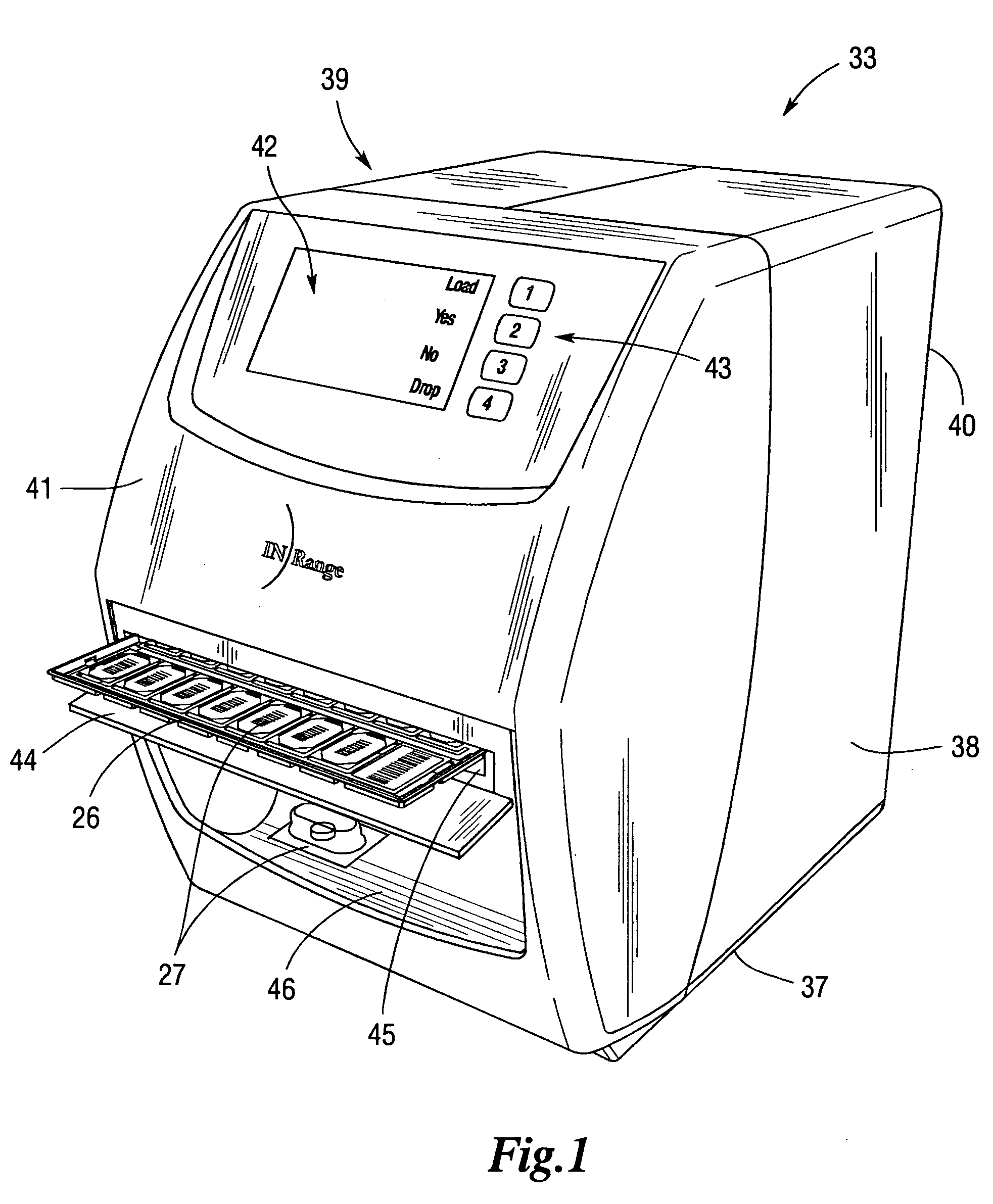
ActiveUS20050240305A1Precise deliveryMechanical clocksDrug and medicationsSecure communicationIndividual dose
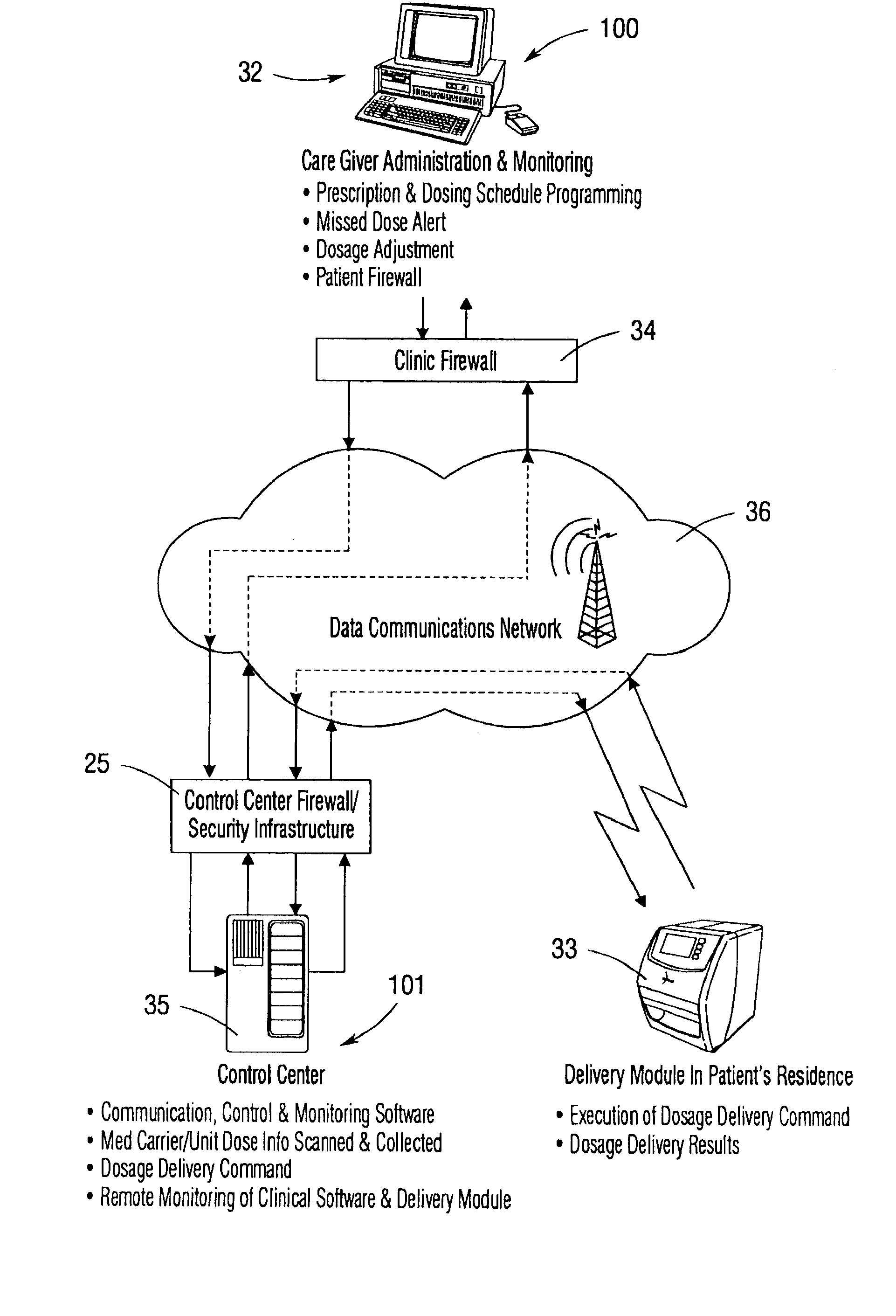
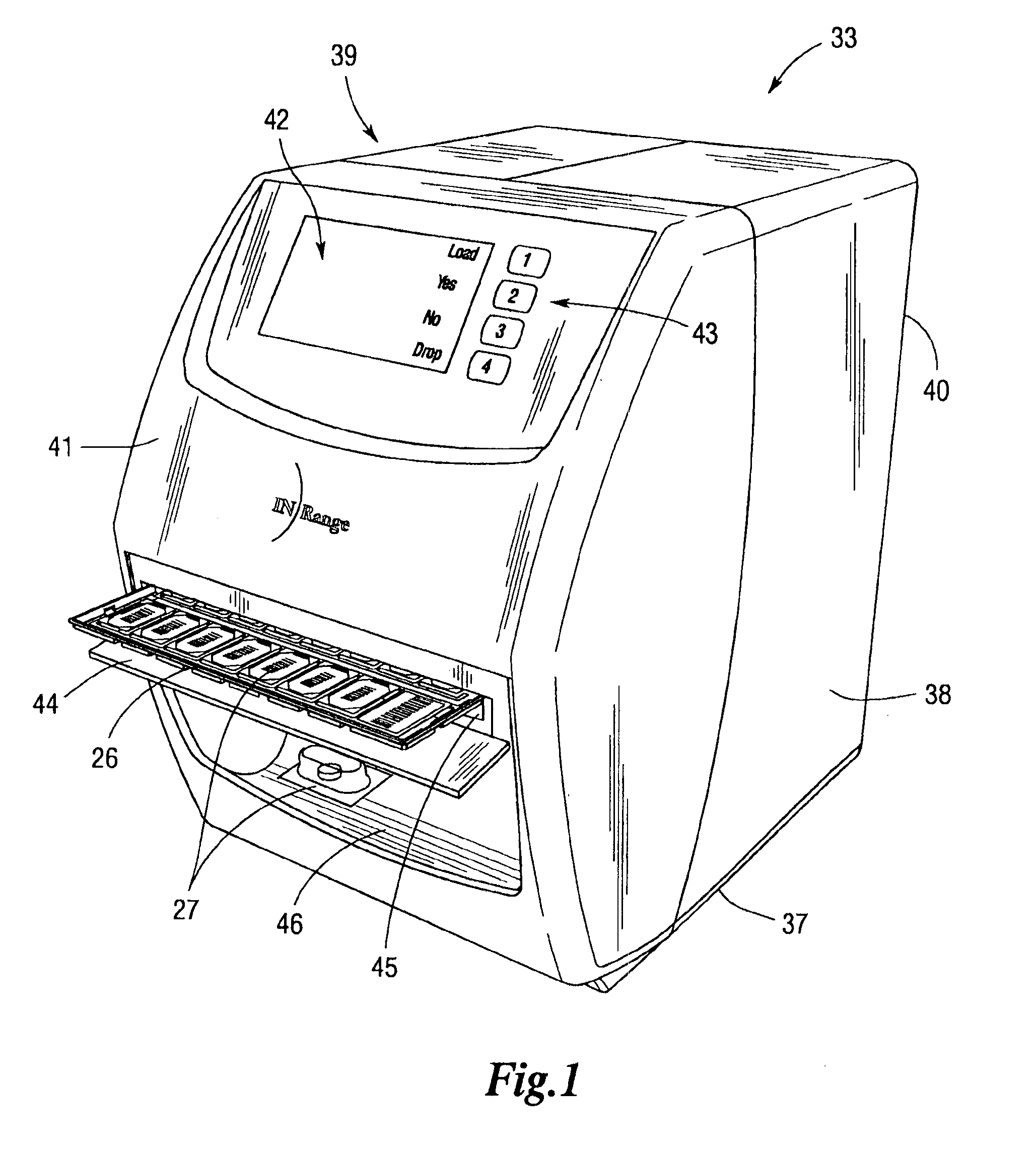
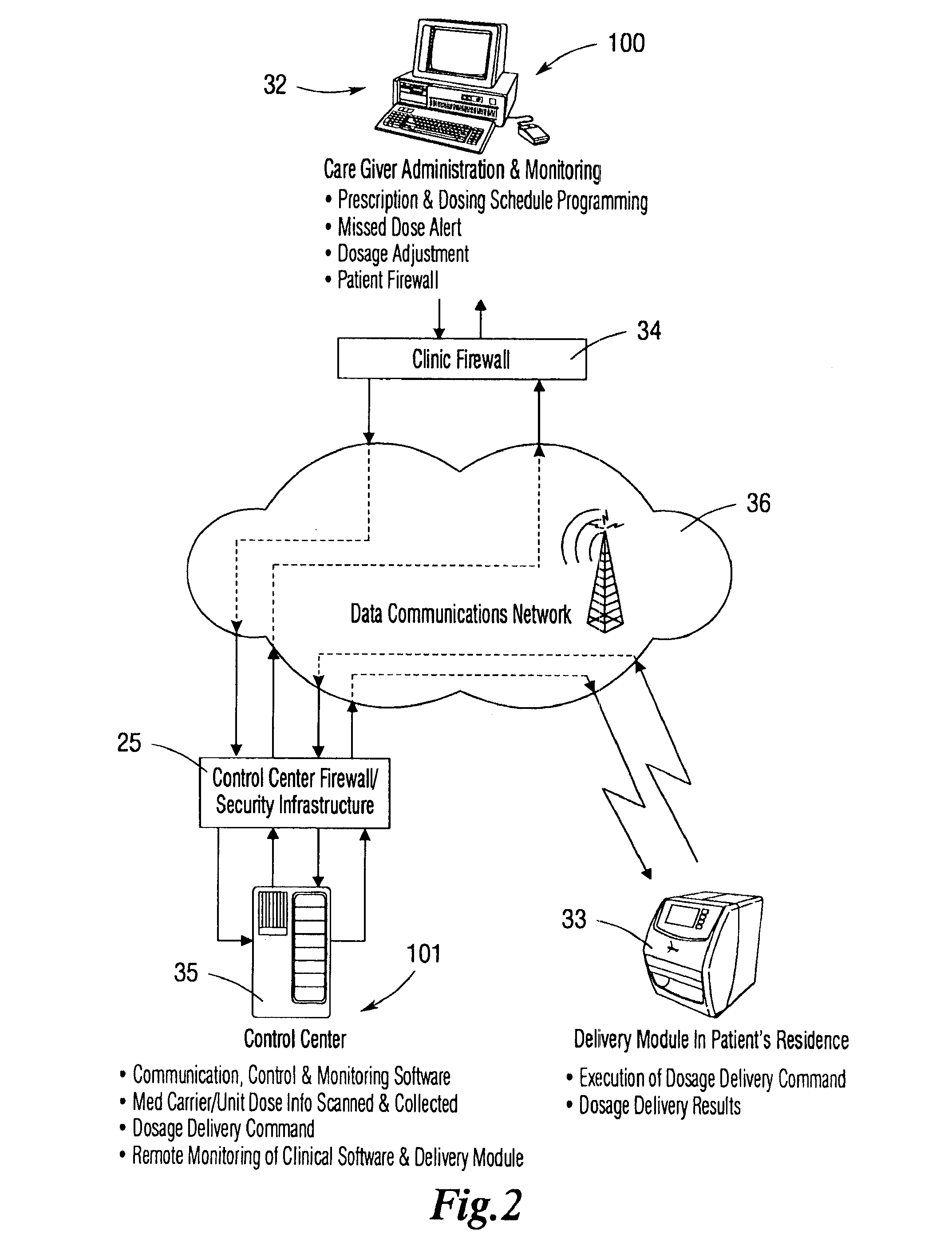
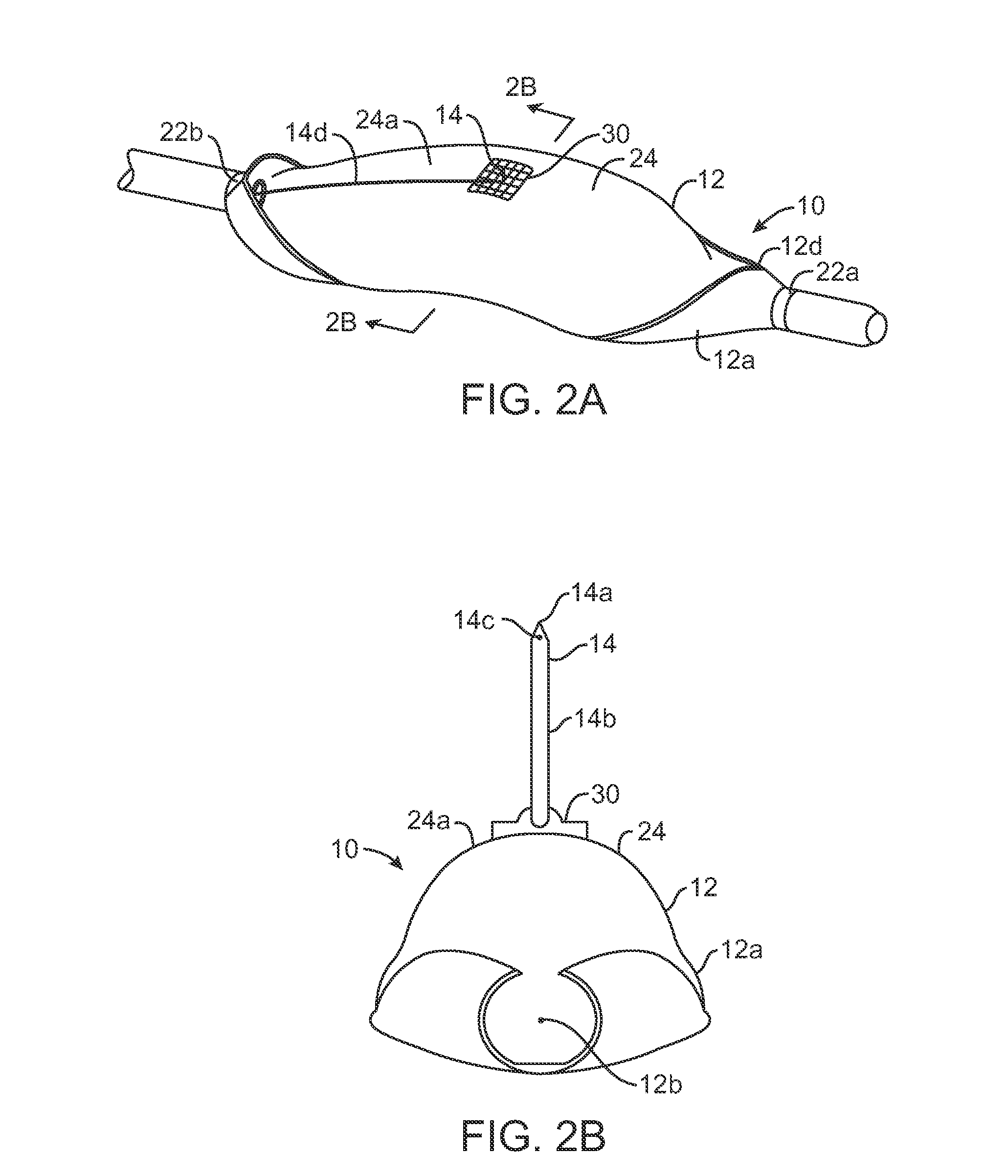
An integrated medication management and compliance system for enabling a care provider to remotely manage and deliver individual doses of therapeutic products to a patient, in a non-sequential fashion. The system includes delivery apparatus remotely located from the care provider, wherein the apparatus stores a plurality of sealed unit dose packages that are delivered to a patient at a scheduled dosing time. The delivery apparatus is coupled to a control facility and to a computer terminal of the care provider by way of a secure communications network. The system enables the patient's medication regimen to be remotely tailored in real-time to accommodate fluid medical conditions.
Owner:EMMA HEALTH TECH INC
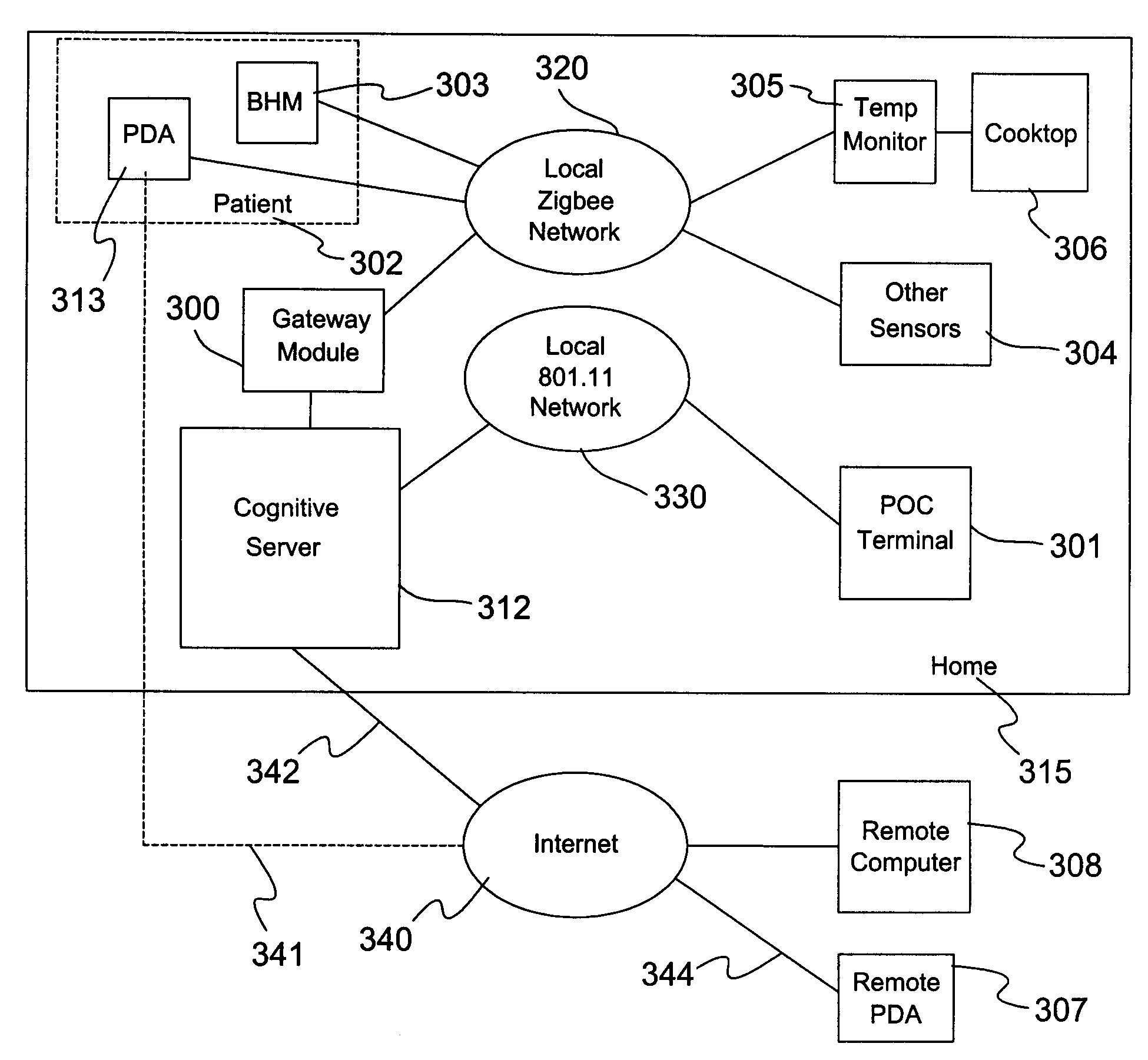
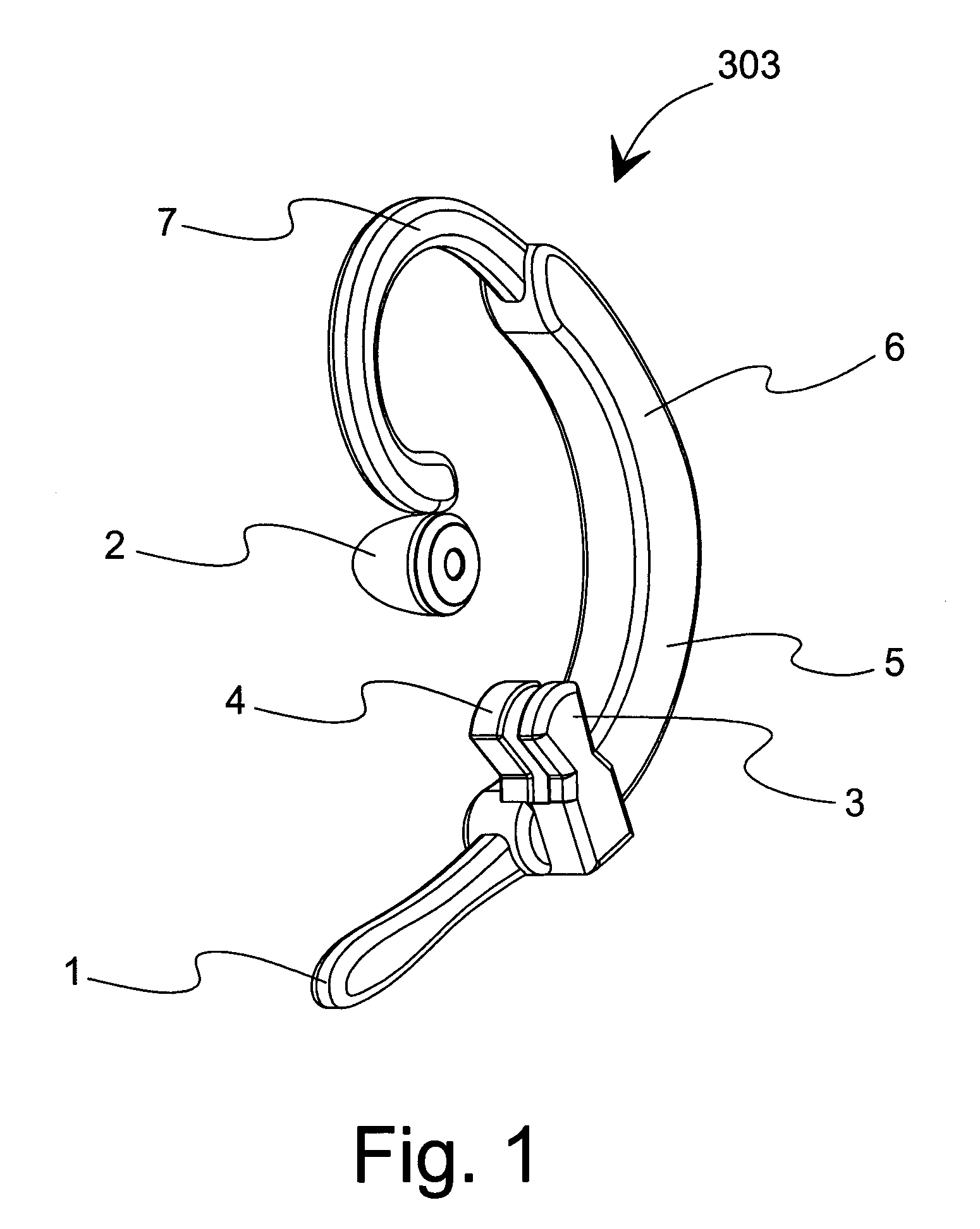
Wireless Health Monitor Device and System with Cognition
InactiveUS20070197881A1Easy to switchElectroencephalographyElectrocardiographyPoint of careCognitive capability
A home-based remote care solution provides sensors including a basic health monitor (BHM) that is a measurement and feedback system. The BHM operates with low power integrated communications combined with an in-home, low power mesh network or programmable digital assistant (PDA) with cell phone technology. A cognitive system allows remote monitoring of the location and the basic health of an individual. The BHM measures oxygen saturation (SaO2), temperature of the ear canal, and motion, including detection of a fall and location within a facility. Optionally, the BHM measures CO2, respiration, EKG, EEG, and blood glucose. No intervention is required to determine the status of the individual and to convey this information to care providers. The cognitive system provides feedback and assistance to the individual while learning standard behavior patterns. An integrated audio speaker and microphone enable the BHM to deliver audio alerts, current measurements, and voice prompts. A remote care provider can deliver reminders via the BHM. The device may be worn overnight to allow monitoring and intervention. Through the ability to inquire, the cognitive system is able to qualify events such as loss of unconsciousness or falls. Simple voice commands activate the device to report its measurements and to give alerts to care providers. Alerts from care providers can be in a familiar voice to assist with compliance to medication regimens and disease management instructions. Simple switches allow volume control and manual activation. The device communicates with a series of low-power gateways to an in-home cognitive server and point-of-care (POC) appliance (computer). Alone the BHM provides basic feedback and monitoring with limited cognitive capabilities such as low oxygen or fall detection. While connected to the cognitive server, full cognitive capabilities are attained. Full alerting capability requires the cognitive server to be connected through an Internet gateway to the remote care provider.
Owner:WOLF JAMES L +3
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
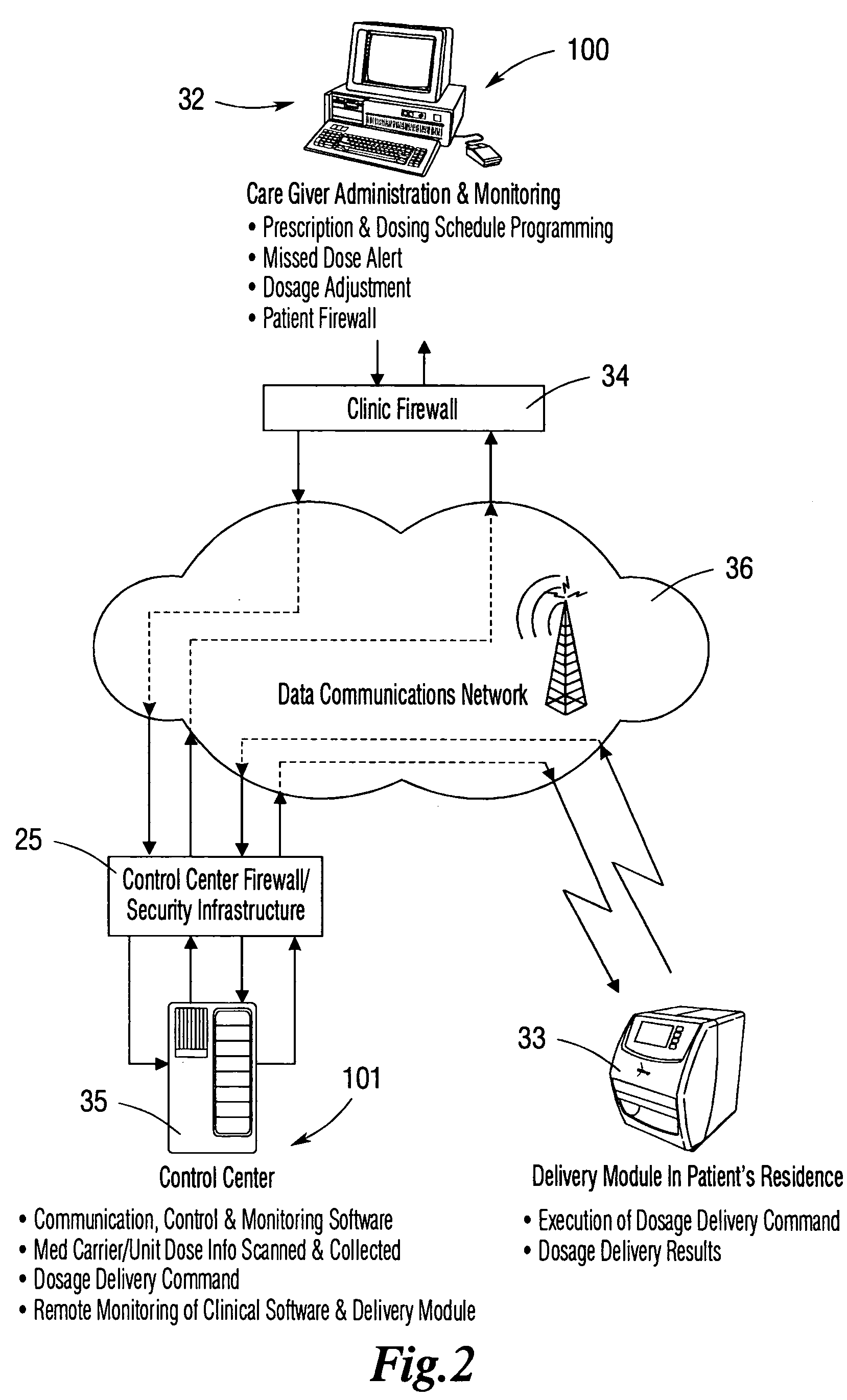
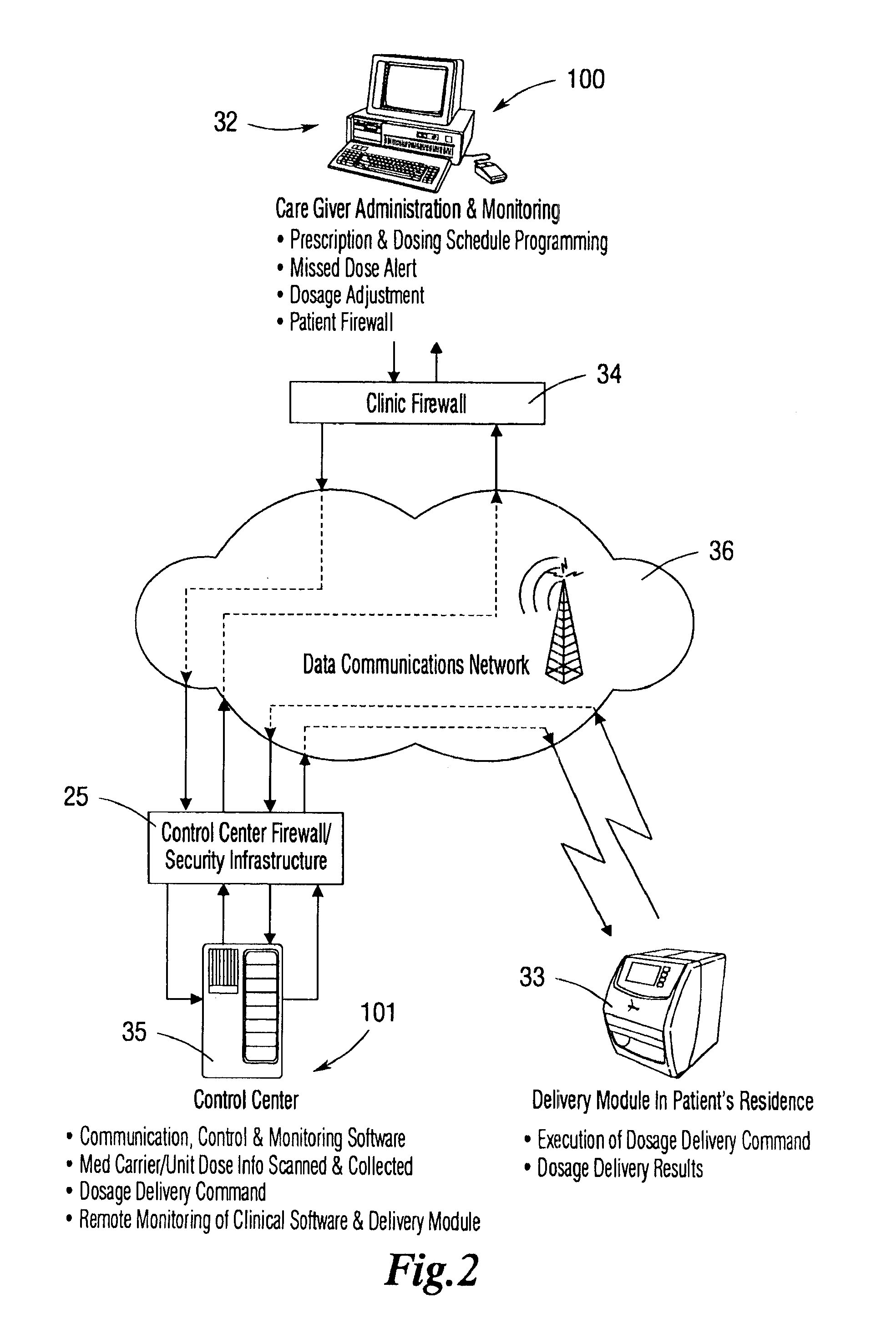
Operation Of A Remote Medication Management System
InactiveUS20080059228A1Data processing applicationsMechanical clocksSecure communicationIndividual dose
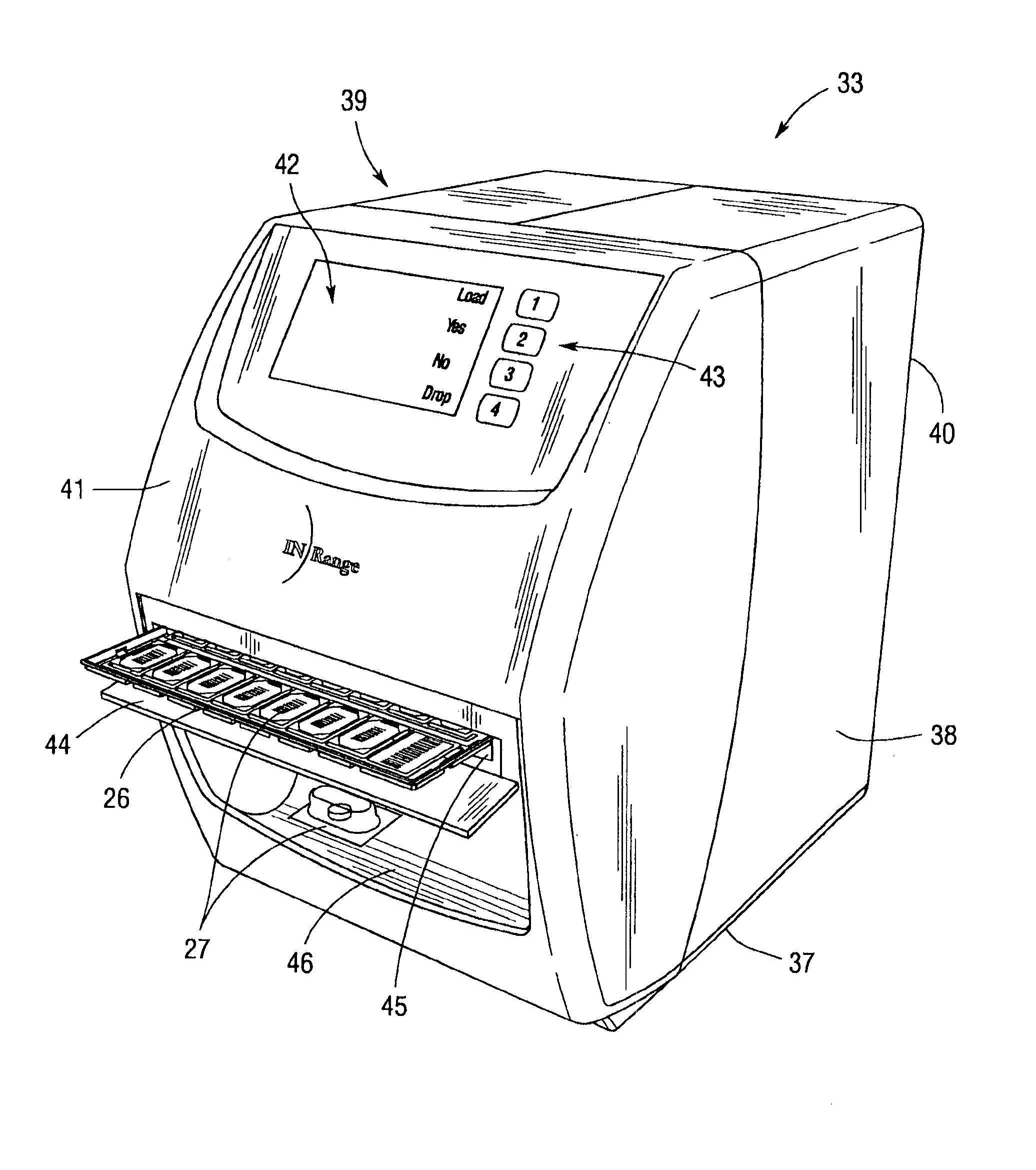
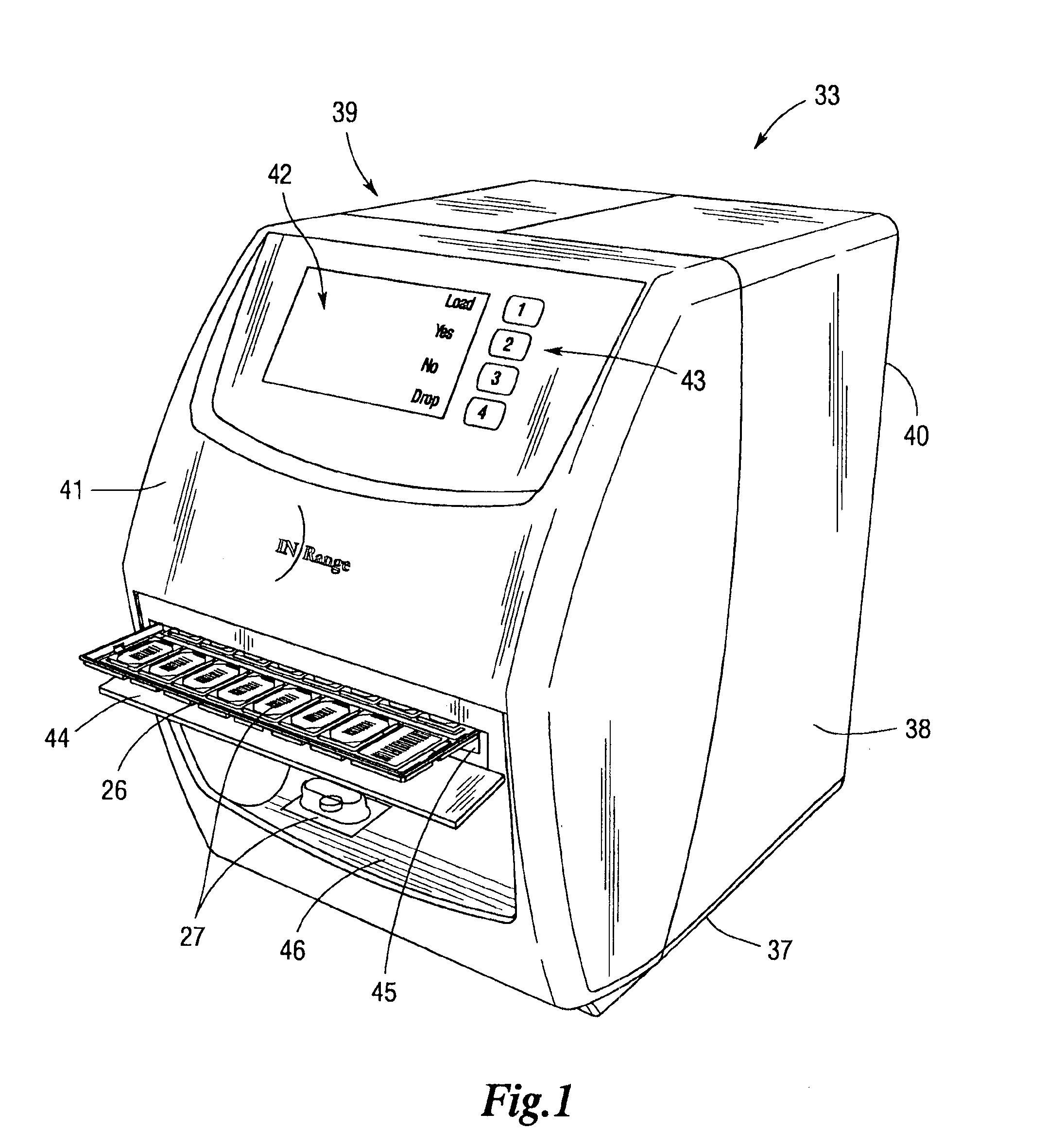
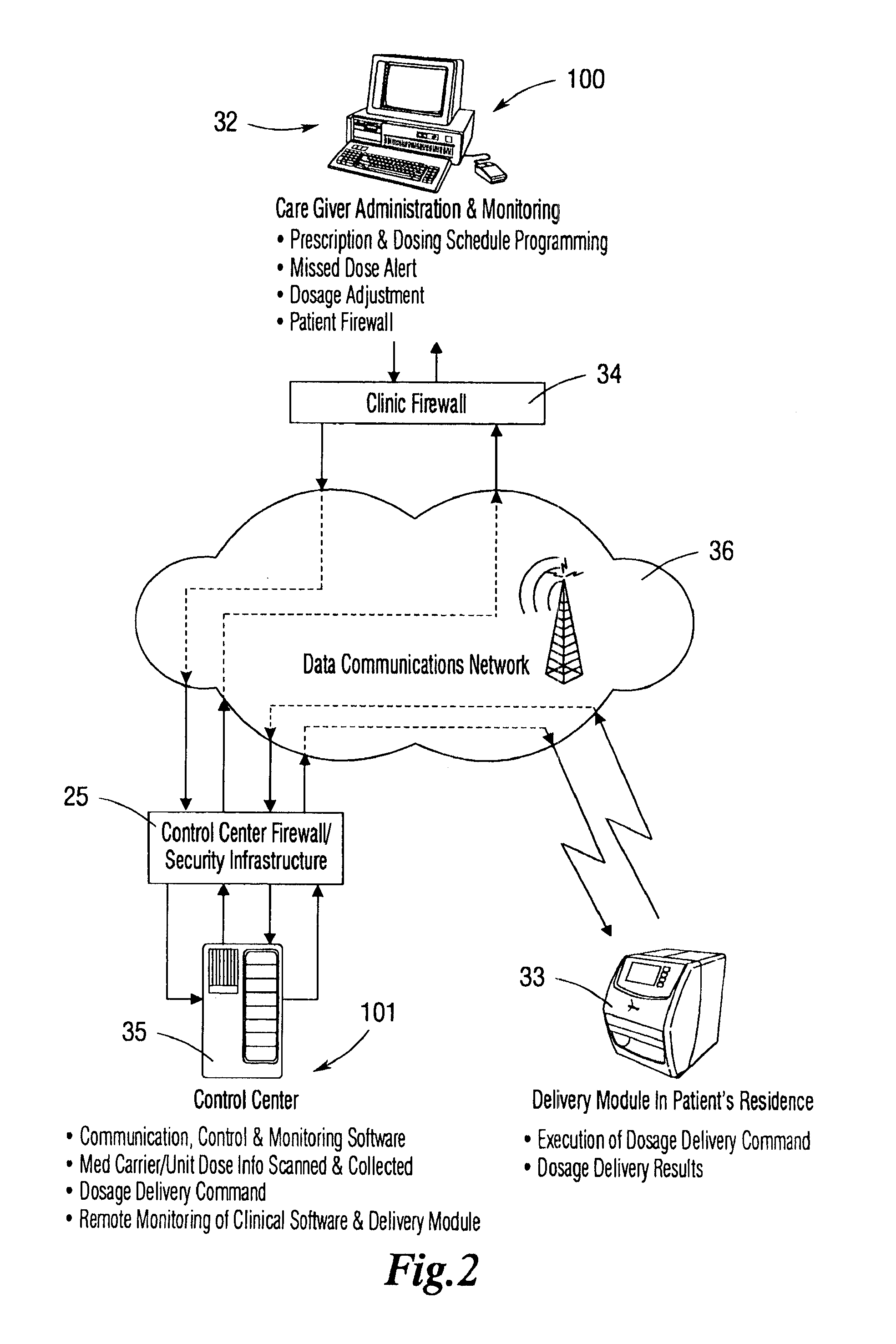
An integrated medication management and compliance system for enabling a care provider to remotely manage and deliver individual doses of medications to a patient, in a non-sequential fashion. The system includes delivery apparatus remotely located from the care provider, wherein the apparatus stores a plurality of sealed unit dose packages that are delivered to a patient at a scheduled dosing time. The delivery apparatus is coupled to a control facility and to a computer terminal of the care provider by way of a secure communications network. The system enables the patient's medication regimen to be remotely tailored in real-time to accommodate fluid medical conditions.
Owner:INRANGE SYSTEMS INC (A DELAWARE CORPORATION)
Medication compliance system
A method and apparatus are provided for facilitating compliance with a medication regimen by a user. The method includes the steps of providing the user with a medication dispensing unit for dispensing medication to the user and a wireless transceiver operatively coupled to a controller of the medication dispensing unit, downloading a set of instructions for controlling the medication unit from a server to the controller of the medication dispensing unit through the wireless transceiver and downloading a set of instructions that instruct the user on how to use the dispensed medication through the wireless transceiver to the user.
Owner:LOF LLC
Treatment of renal hypertension or carotid sinus syndrome with adventitial pharmaceutical sympathetic denervation or neuromodulation
ActiveUS20110104061A1Improve concentrationOrganic active ingredientsBacterial antigen ingredientsRenal HypertensionsCvd risk
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict medication regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
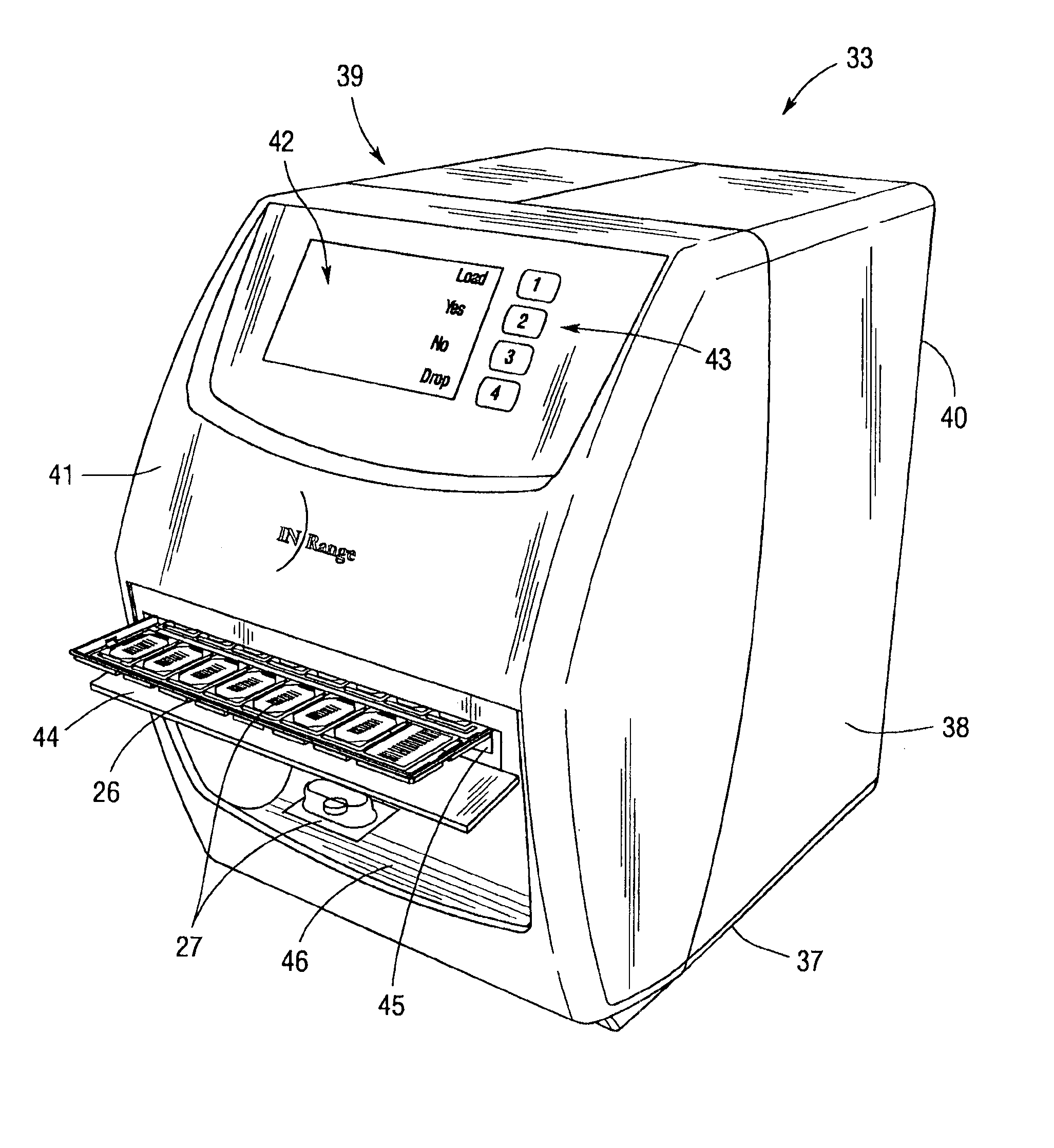
Remote Medication Management System
An integrated medication management and compliance system for enabling a care provider to remotely manage and deliver individual doses of medications to a patient, in a non-sequential fashion. The system includes delivery apparatus remotely located from the care provider, wherein the apparatus stores a plurality of sealed unit dose packages that are delivered to a patient at a scheduled dosing time. The delivery apparatus is coupled to a control facility and to a computer terminal of the care provider by way of a secure communications network. The system enables the patient's medication regimen to be remotely tailored in real-time to accommodate fluid medical conditions.
Owner:EMMA HEALTH TECH INC
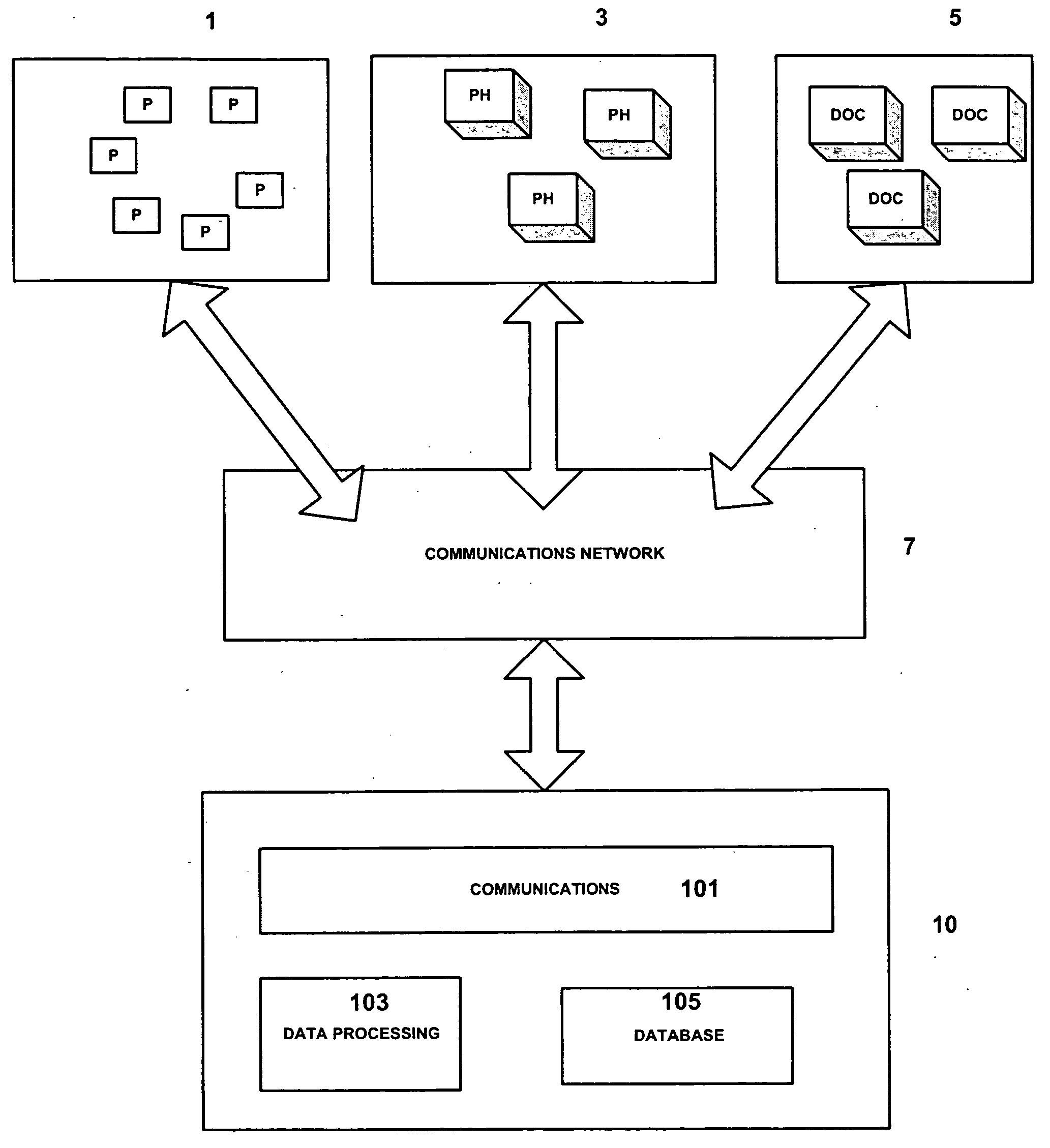
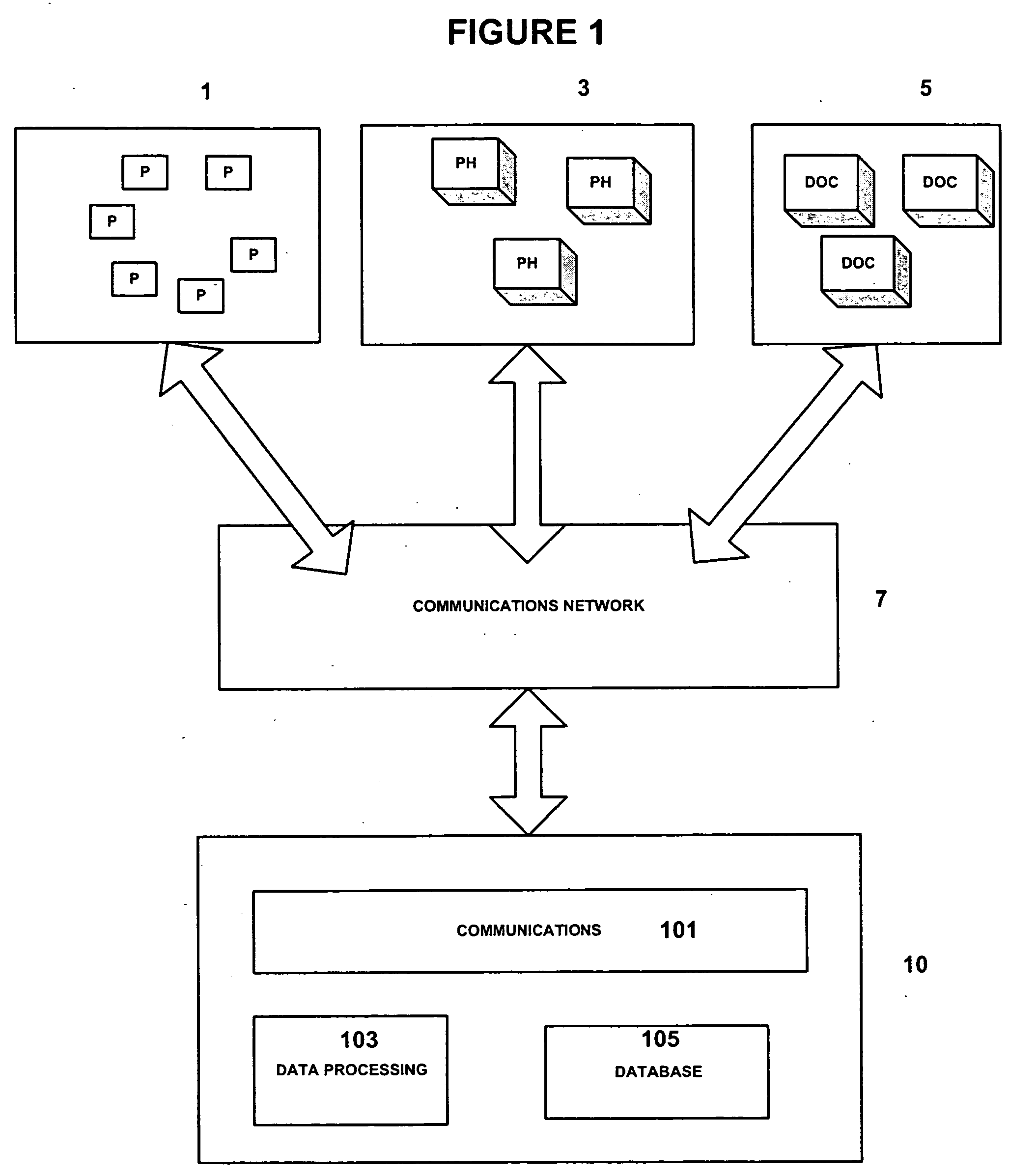
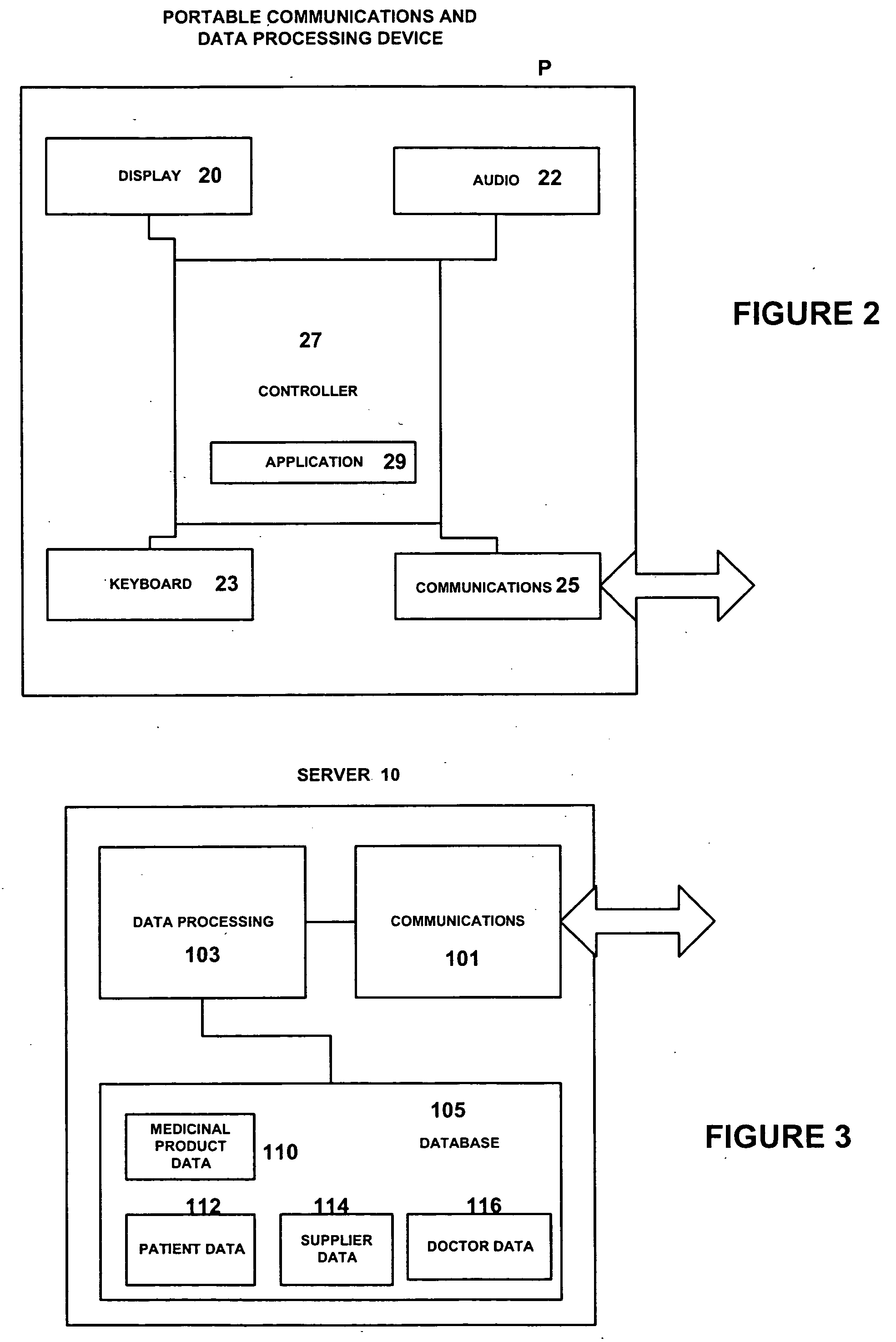
Medicinal product order processing system
InactiveUS20060136266A1Improve monitoring qualityProcess is repeatedDrug and medicationsPatient personal data managementRepeated prescriptionRegimen
A repeat prescription ordering system for allowing patients requiring resupply of medication or medical products to access a server using a portable communications and data processing device such as a smart phone or personal digital assistant. The server supplies to the patient a list of medication and medical products which they are authorized to order. The patient can select the products required, and the order is logged by the server and allocated to a supplier for completion of the order. The server maintains an estimate of the amount of medication or medical product held by the patient, this being based on the prescribed dosage regimen and information entered by the patient on their usage and, optionally, on checks on their own health. The patient may be alerted when the estimate indicates that their supplies are running low. The estimate is allowed to go below zero, this implying a possible departure from the prescribed medication regimen.
Owner:E SAN LTD
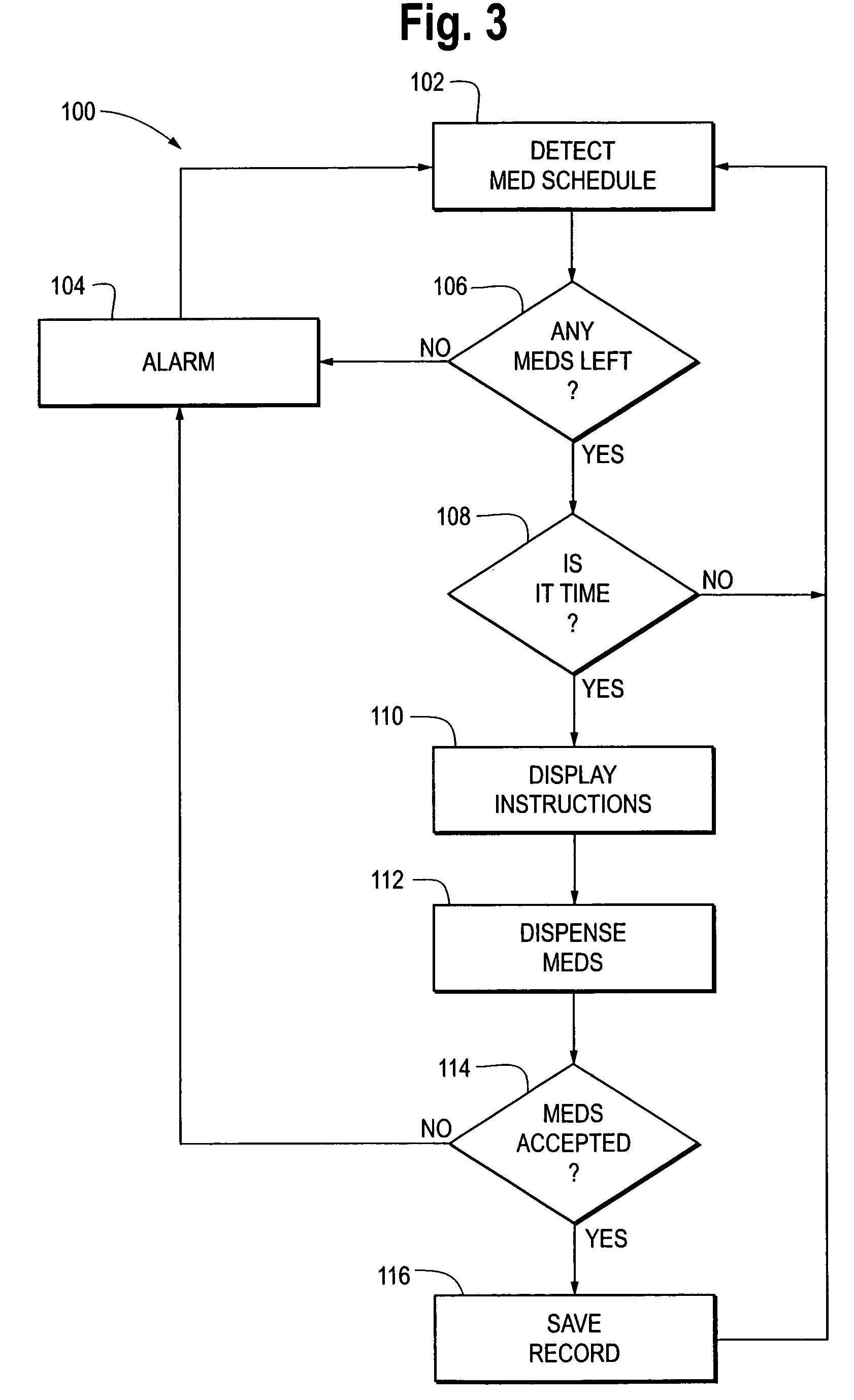
Automatic medication reminder and dispensing device, system , and method therefor
InactiveUS20090281657A1Improve complianceSmall article dispensingDrug and medicationsDocking stationMedicine
Owner:BAETA CORP
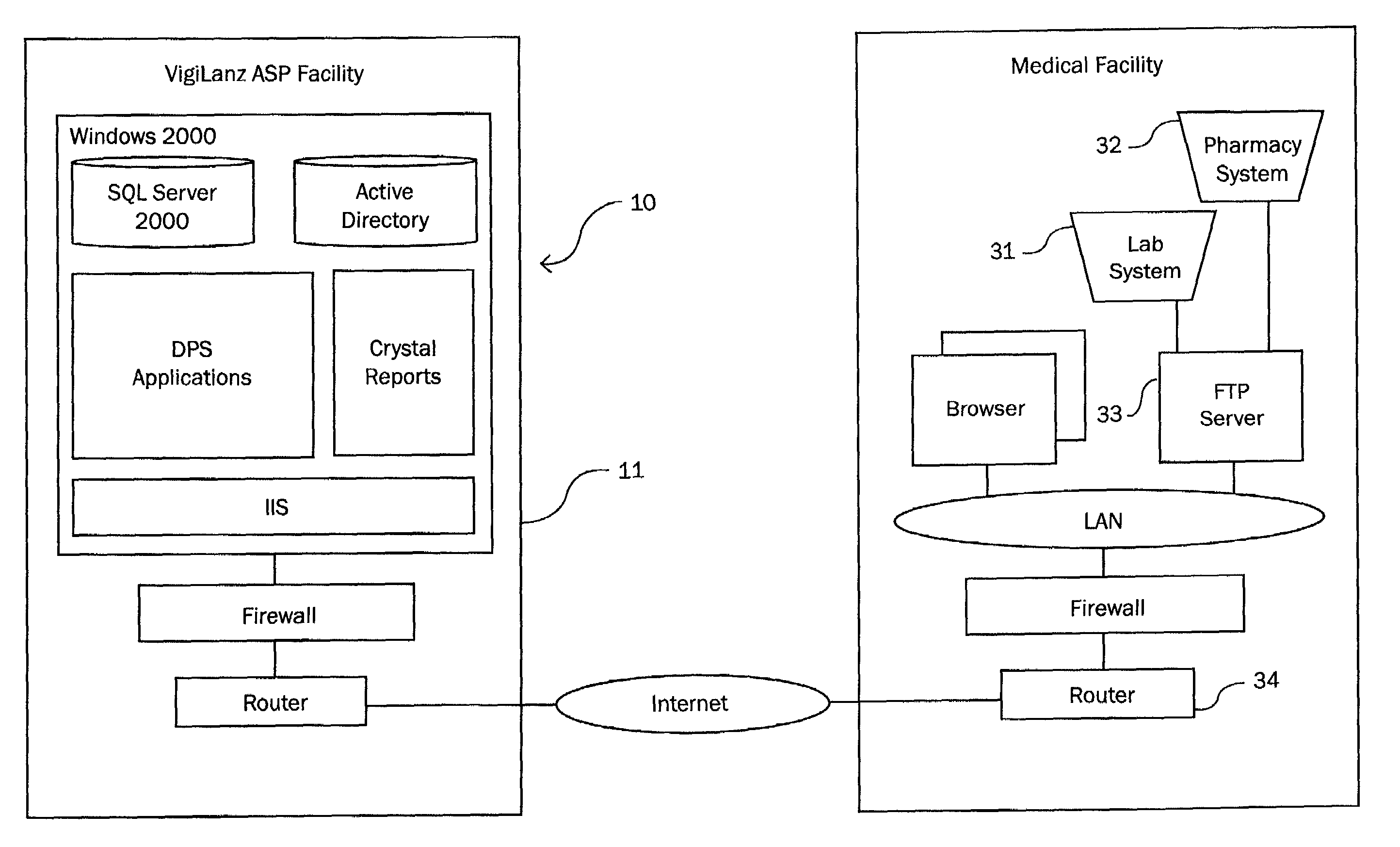
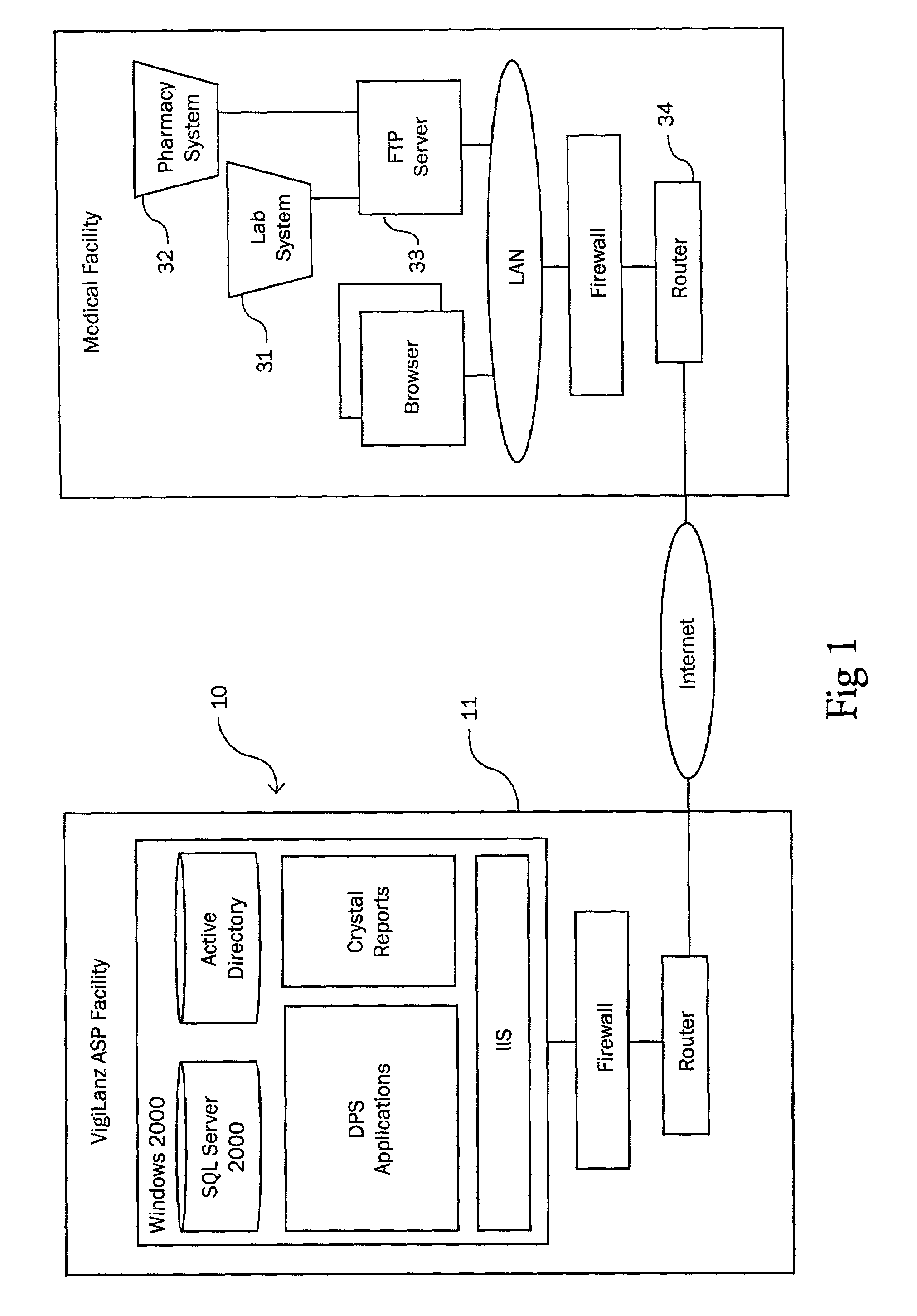
Method and system for identifying and anticipating adverse drug events
InactiveUS6993402B2Drug and medicationsComputer-assisted medicine prescription/deliveryPharmacyTechnical standard
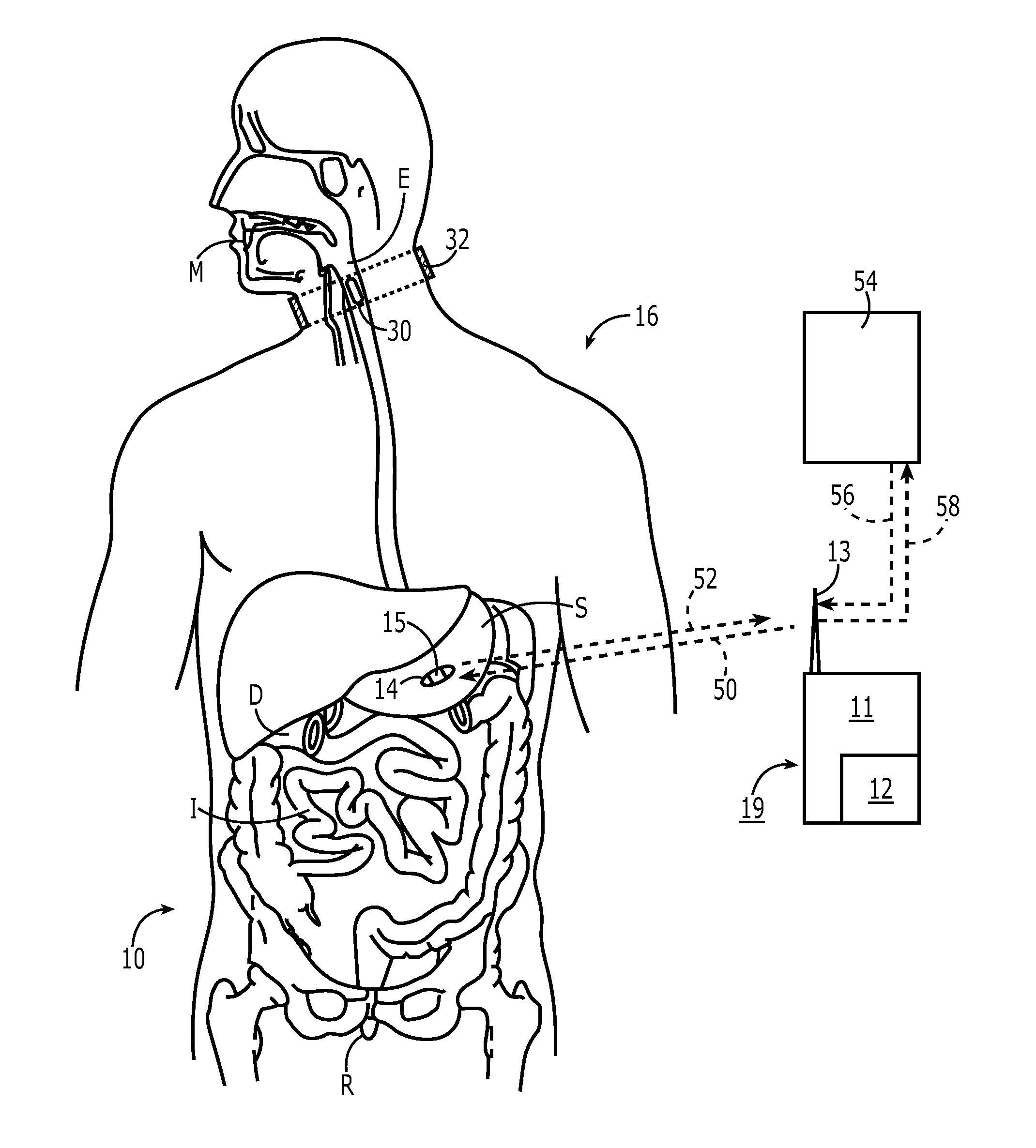
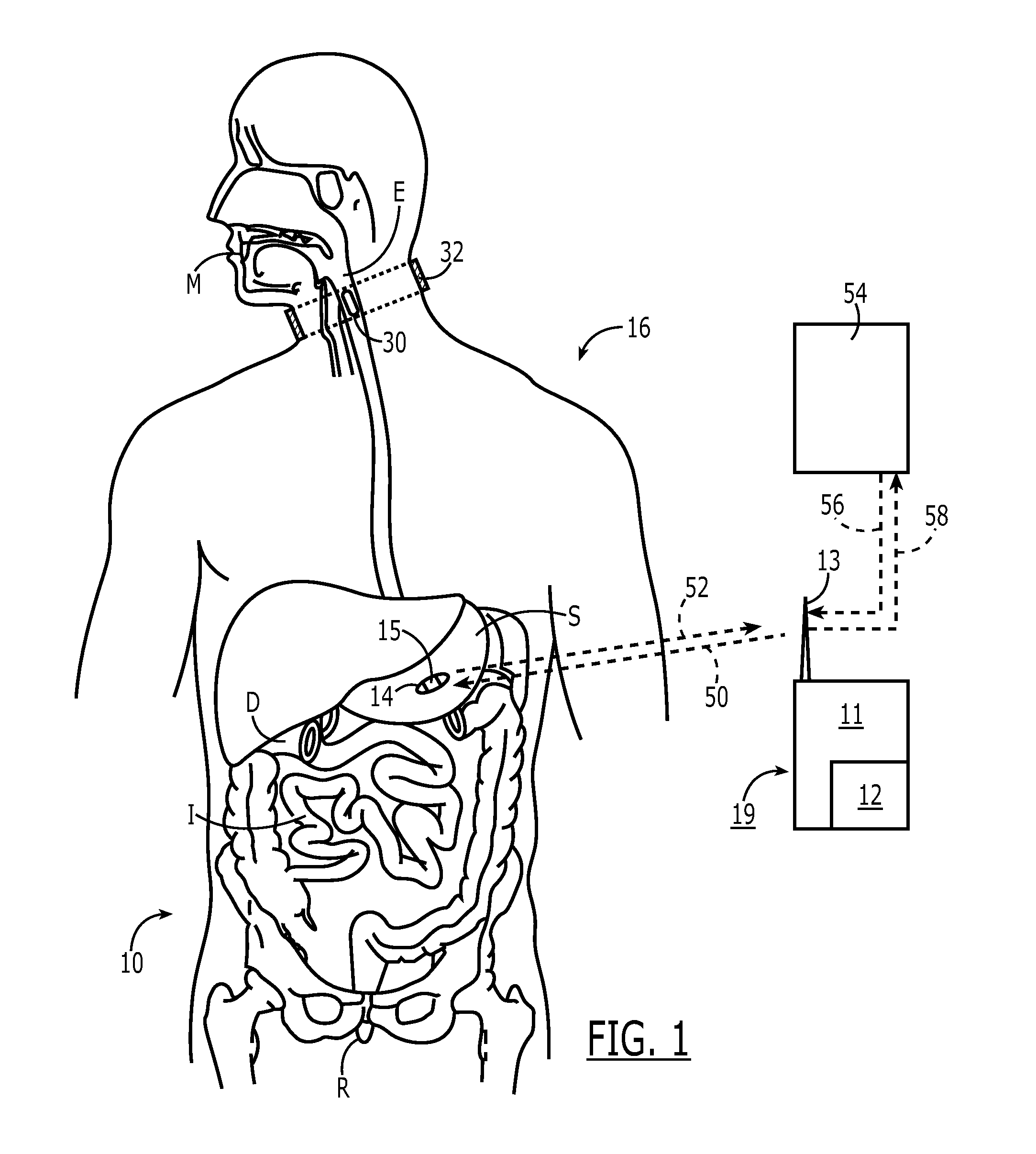
The present invention is a system and method for anticipating potential Adverse Drug Events (ADE) in a patient's medication regimen by integrating data typically located in laboratory and pharmacy information systems and filtering the data using predefined criteria. The present invention includes a system for anticipating a possible ADE through the use of a search engine that compares integrated data from laboratory and pharmacy information systems and compares it to predefined ADE rules defining normal ranges for a particular laboratory test. If an abnormal test value is received and a drug in the patient's medication regimen satisfies a drug included in an ADE rule then an alert procedure is triggered which allows for a period of time wherein the patient's lab and pharmacy data is monitored in order to determine if a proper corrective action is undertaken, and if no corrective action or an improper corrective action is taken within that period of time, the healthcare provider is warned of a potential ADE.
Owner:VIGILANZ CORP
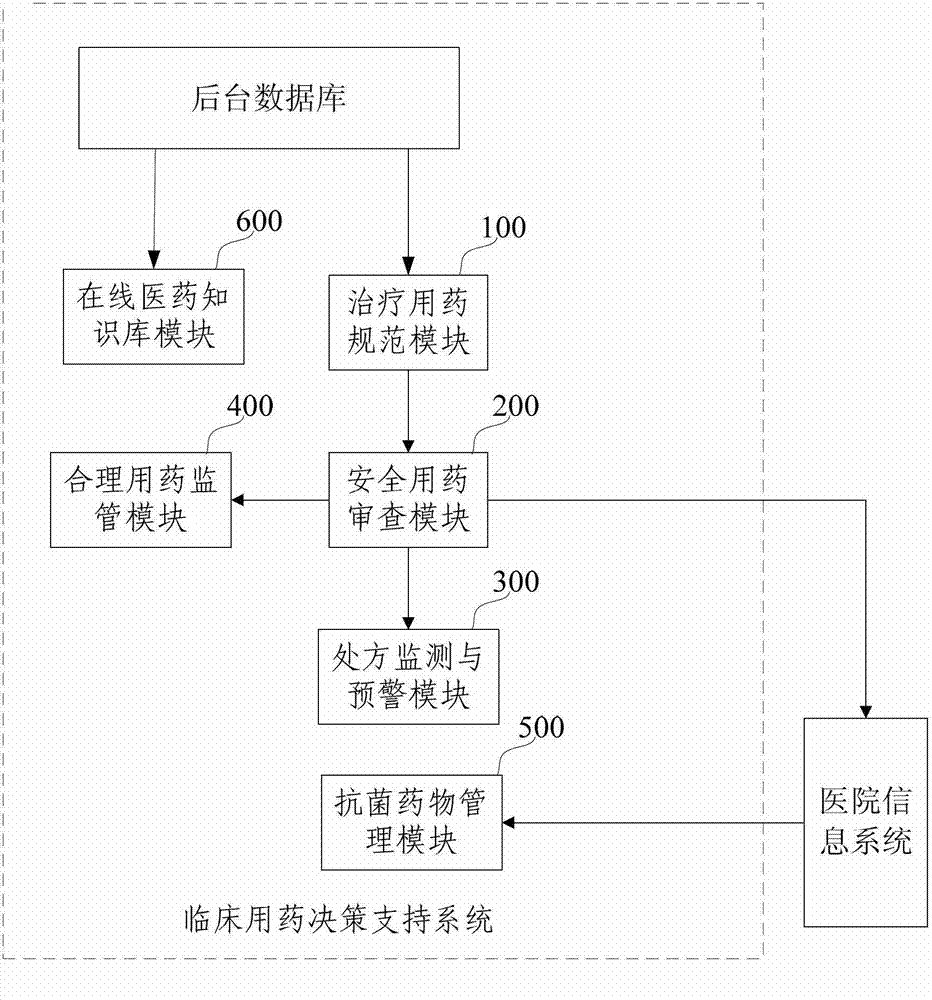
Clinical medication decision support system
ActiveCN102737165AImprove securityImprove normalizationSpecial data processing applicationsPrescription monitoringDecision taking
The invention discloses a clinical medication decision support system which relates to the field of medical systems. The system comprises a standard treatment medication module, a medication safety reviewing module and a prescription monitoring and warning module, wherein the standard treatment medication module is used for providing diagnosis and treatment reference information and a medication reference scheme according to pathology and physiology information of a patient, so that a doctor can make a prescription; the medication safety reviewing module is used for reviewing the medication safety condition of the patient according to the prescription and generating a reviewing log; and the prescription monitoring and warning module is used for estimating the prescription and generating warning information according to the reviewing log. The system can automatically provide the medication scheme according to the pathology and physiology information of the patient, assist in the doctor to make the prescription, review the safety of the prescription, estimate the prescription and conduct warning on the reviewing result, thus remarkably improving the security, normalization and standardization of clinical medication; and furthermore, by virtue of an antibacterial agent management module, the effective management and monitoring on antibacterial agents are realized, and thus the safety of the clinical medication is further improved.
Owner:BEIJING TYT SOFTWARE TECH
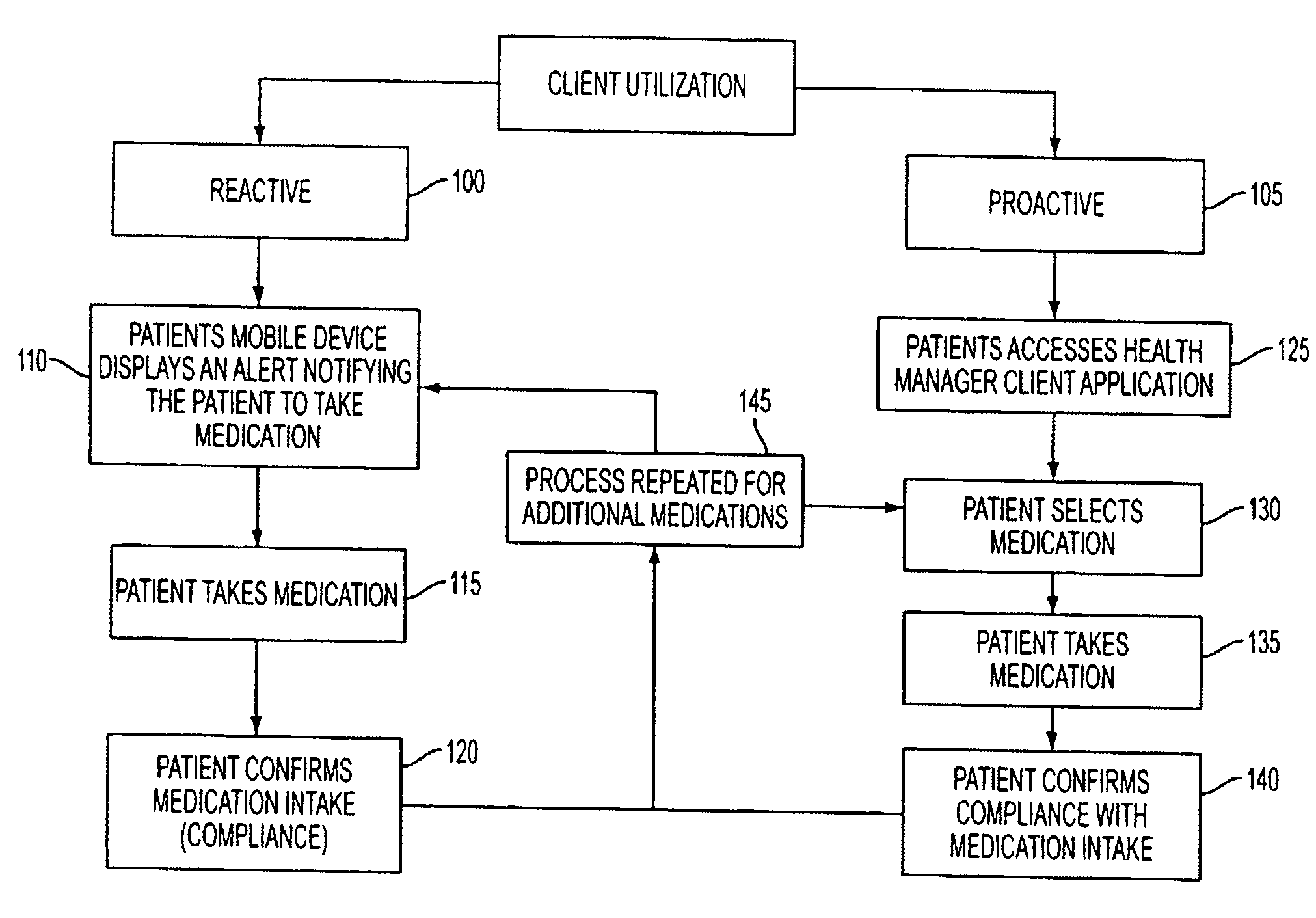
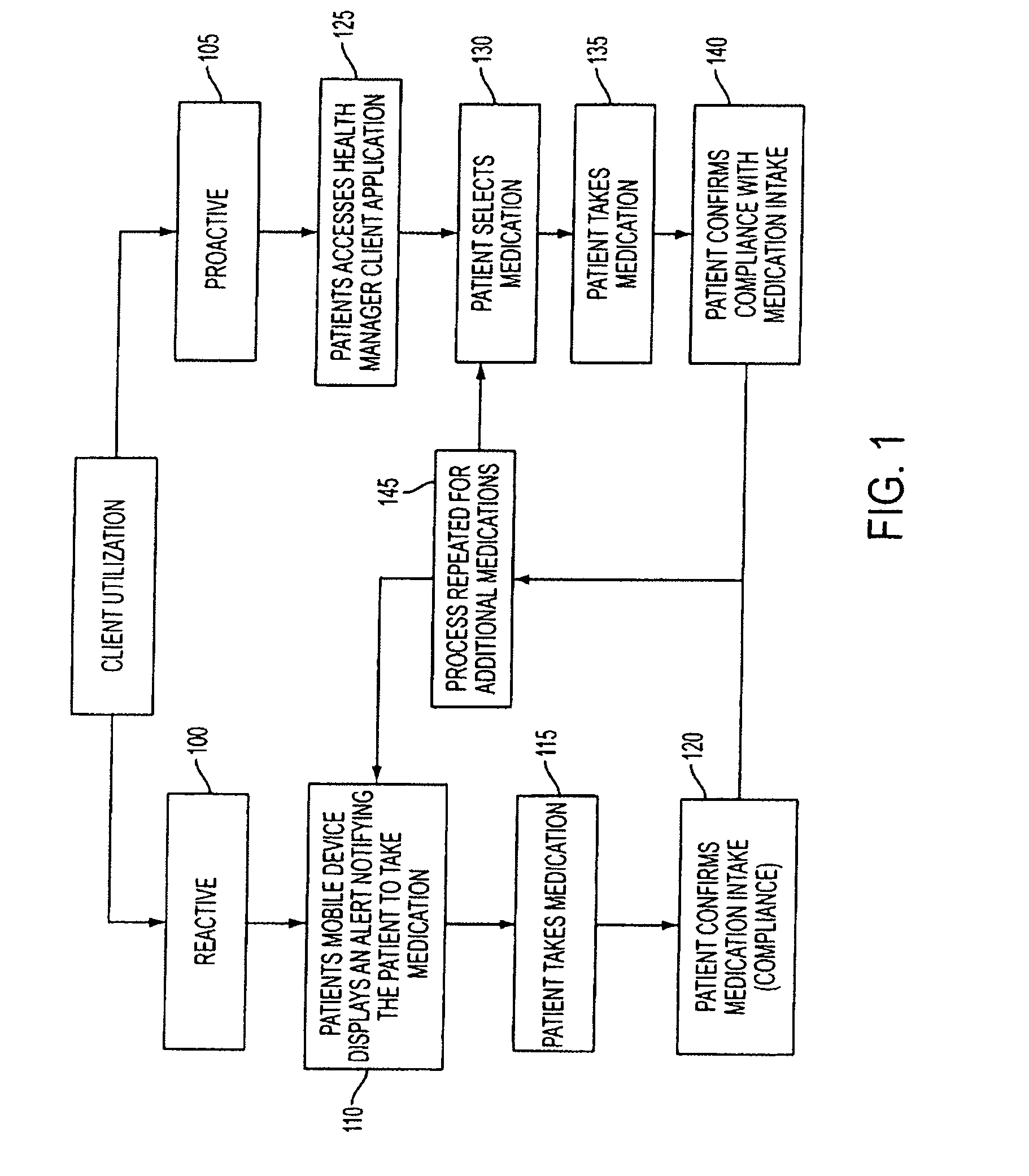
Methods and Systems for Medication Management
ActiveUS20080238666A1Data processing applicationsMechanical clocksCaregiver personMedical prescription
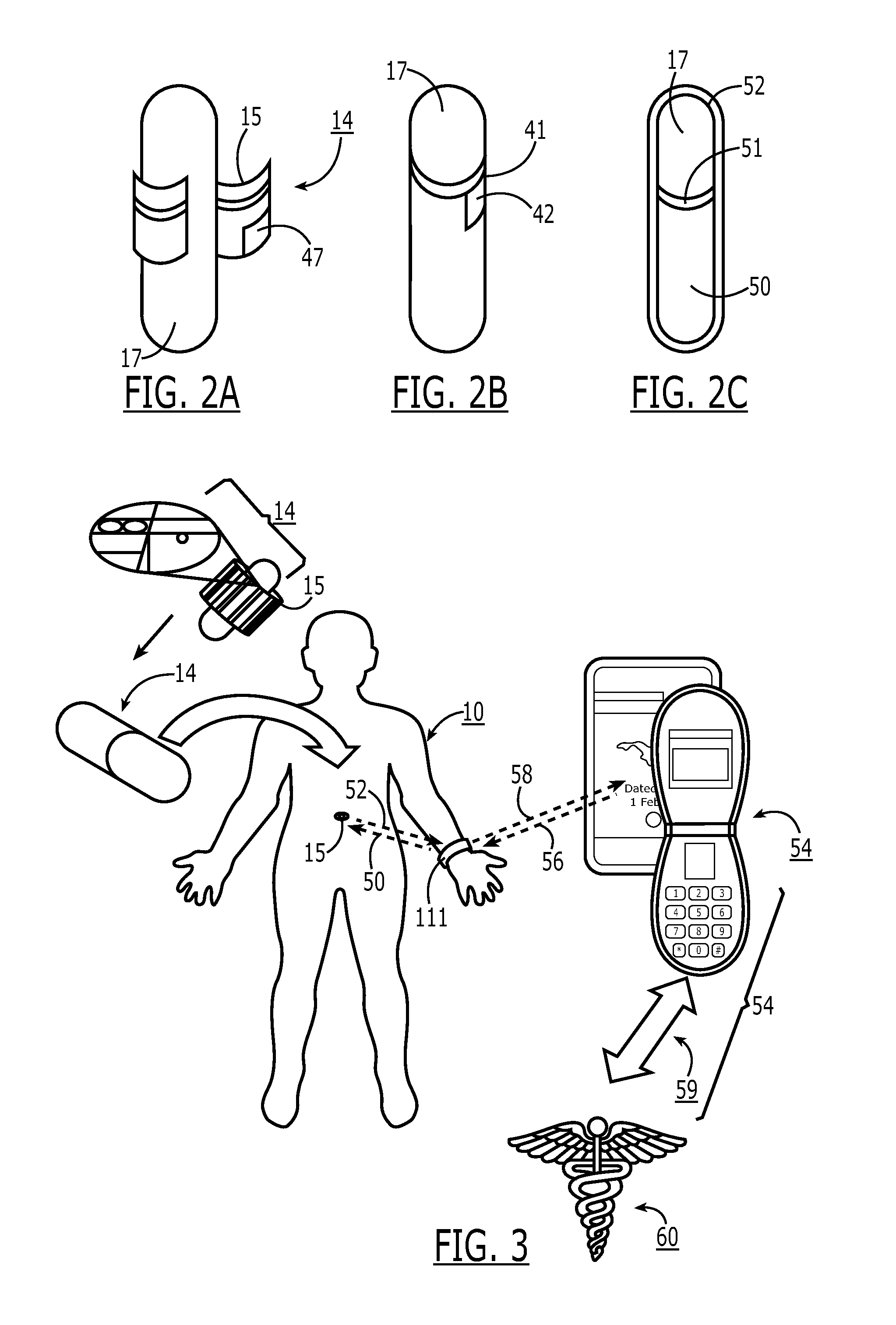
A method of monitoring a patient's compliance with a medication regimen may include identifying a patient, identifying a medication prescribed to the patient and determining one or more intake times associated with the medication. For each intake time, an alert reminding the patient to take the medication may be generated, and the alert may be transmitted to a mobile device associated with the patient. Receipt of the alert by the mobile device may trigger one or more of an audible alarm, a visual alarm and a tactile alarm on the mobile device. If an indication of compliance is not received from the mobile device within a predefined period of time, a caregiver associated with the patient may be notified.
Owner:CARESPEAK COMM
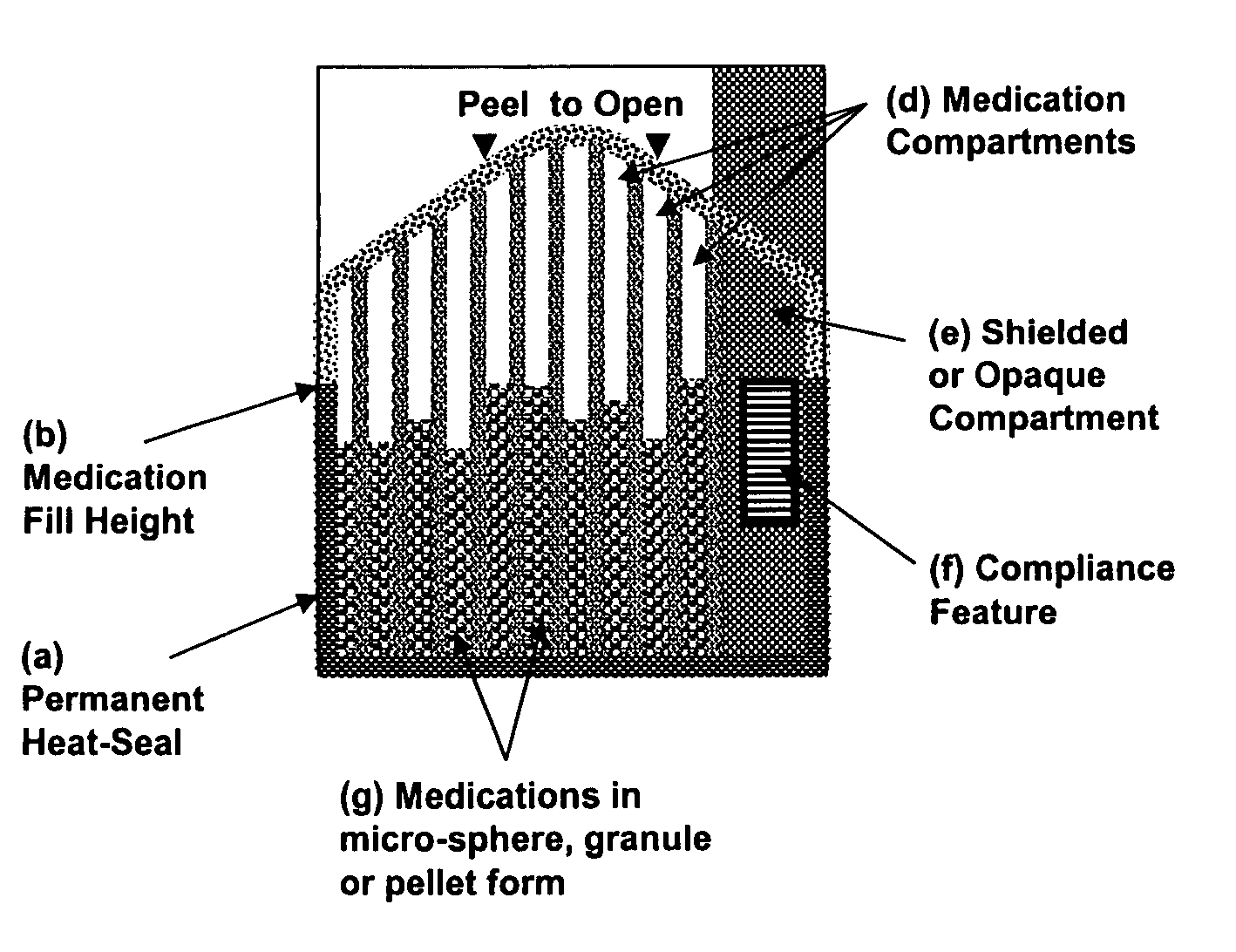
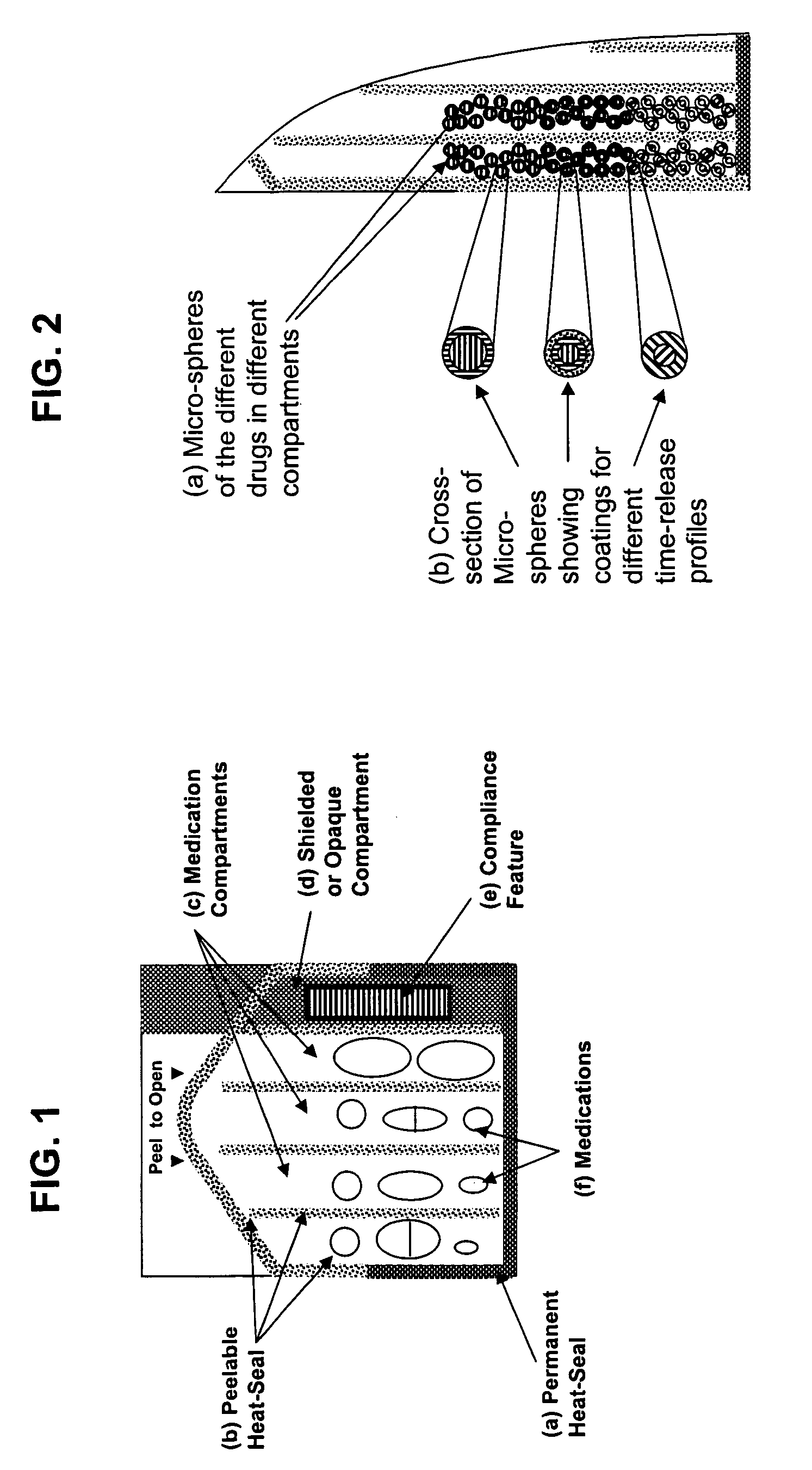
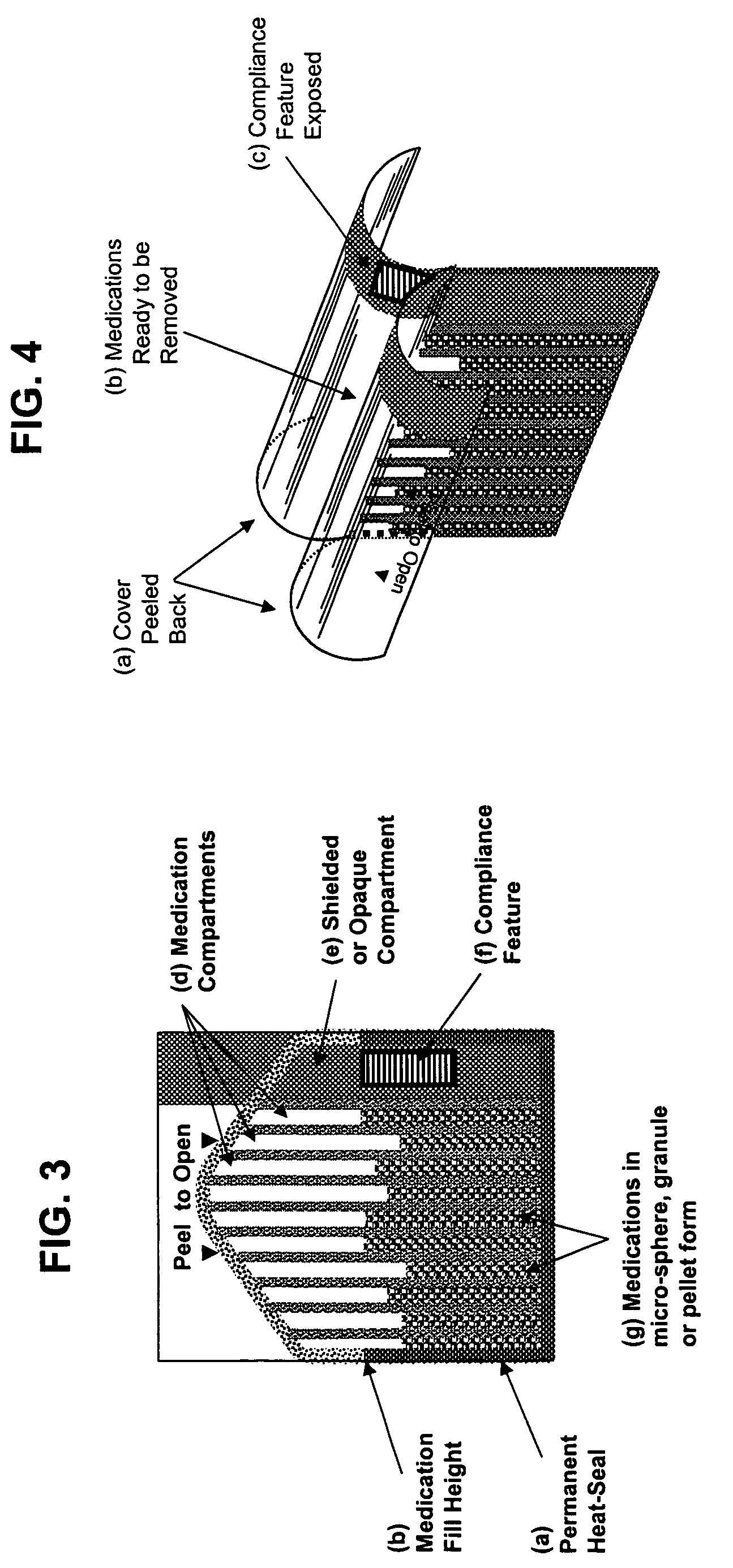
Unified ingestion package and process for patient compliance with prescribed medication regimen
Techniques for reducing non-compliance of medication use are described. The techniques involve generating and storing an identifier for a specific dosage instance of a specific patient, and creating a package that includes a mechanism for conveying the identifier. Once the package is created, a set of one or more medications that are prescribed to be taken by the specific patient at the specific dosage instance are placed in the package. The set of medications within the package in a manner that prevents the identifier from being perceived from the mechanism until the package is opened. Once opened, the identifier is perceived and communicated back to an automated compliance system.
Owner:RAMASUBRAMANIAN NARAYANAN +1
Remote medication management system
An integrated medication management and compliance system for enabling a care provider to remotely manage and deliver individual doses of medications to a patient, in a non-sequential fashion. The system includes delivery apparatus remotely located from the care provider, wherein the apparatus stores a plurality of sealed unit dose packages that are delivered to a patient at a scheduled dosing time. The delivery apparatus is coupled to a control facility and to a computer terminal of the care provider by way of a secure communications network. The system enables the patient's medication regimen to be remotely tailored in real-time to accommodate fluid medical conditions.
Owner:EMMA HEALTH TECH INC
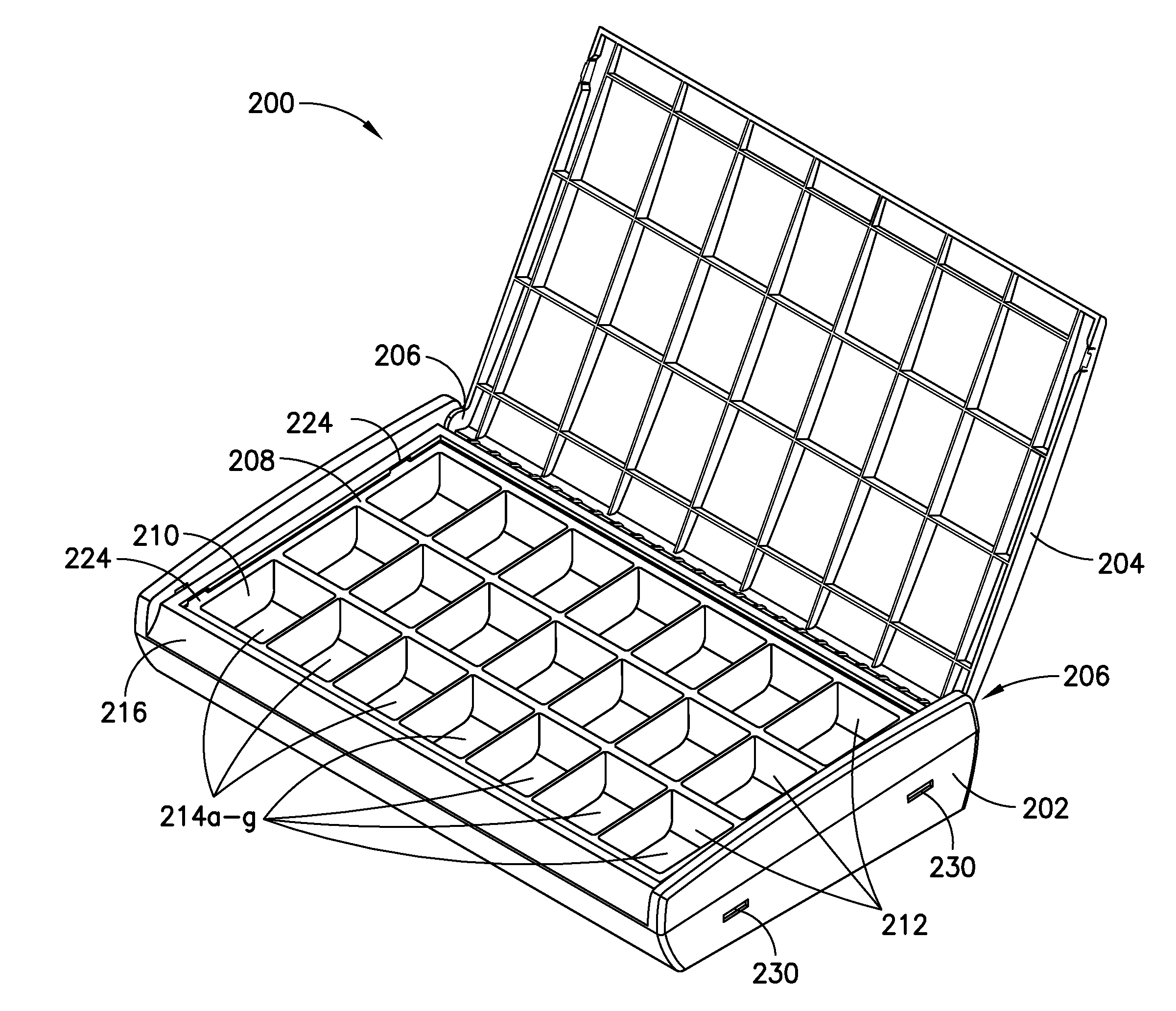
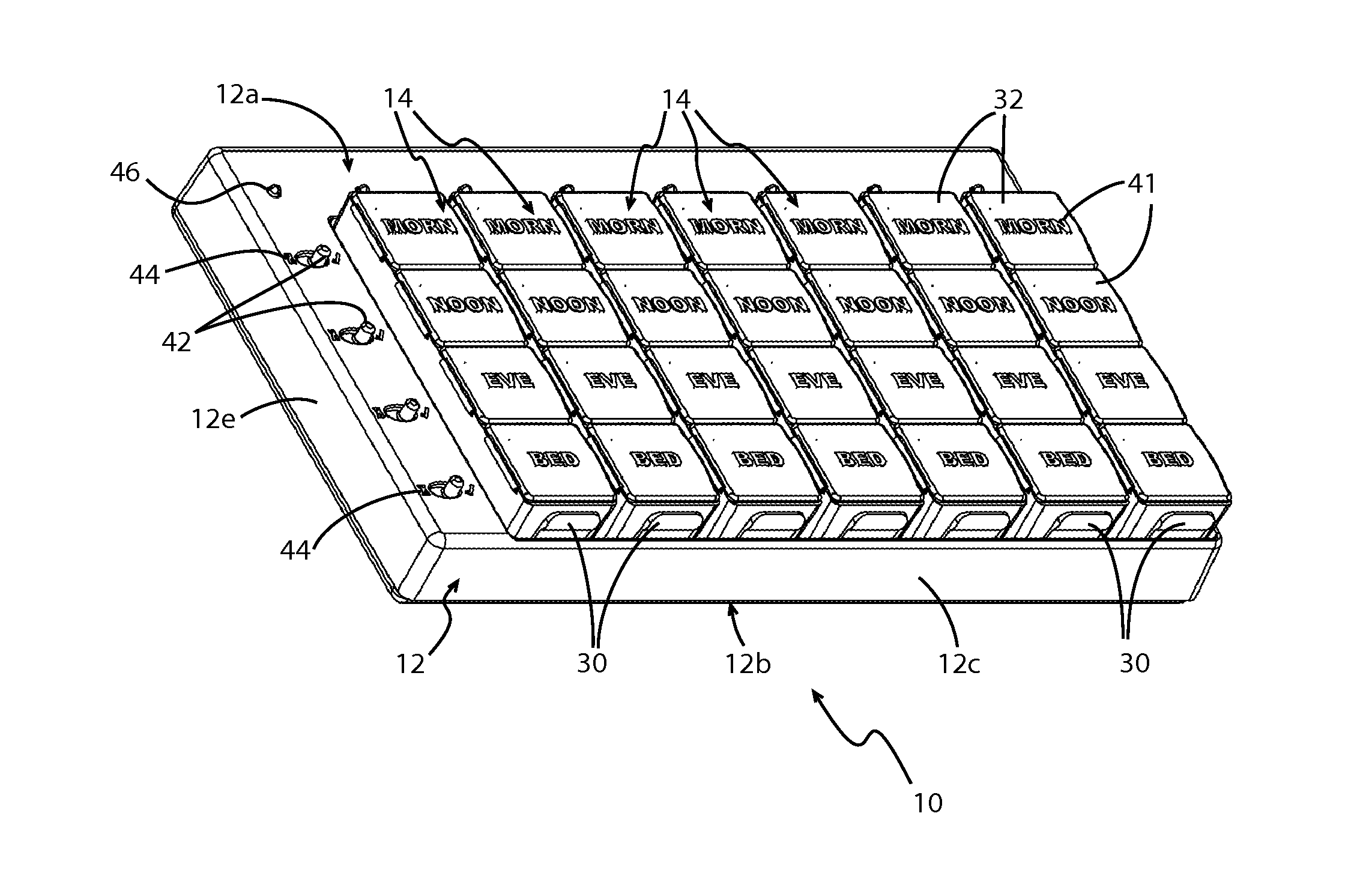
Apparatus and Associated Methods for Tracking and Increasing Medication Adherence for Patients
InactiveUS20150283036A1Simple loading processEasy to useSmall article dispensingPharmaceutical containersMedication adherenceBiomedical engineering
A medication container comprises a body portion and a grid coupled to the body portion. The grid and the tray each comprise a corresponding number of wells configured to contain medication. The tray is configured to be inserted into and removed from the body portion, above the grid. The tray may be configured to be connected to and disconnected from the grid, when the tray is inserted into the body portion. The wells of the grid may be configured to be manually loaded with medication by a user and the wells of the tray may be configured to be received by the user loaded with medication in accordance with a medication regimen. The first wells may be integral with the grid and the second wells may be integral with the tray.
Owner:TOWERVIEW HEALTH
Medication regimen compliance monitoring systems and methods
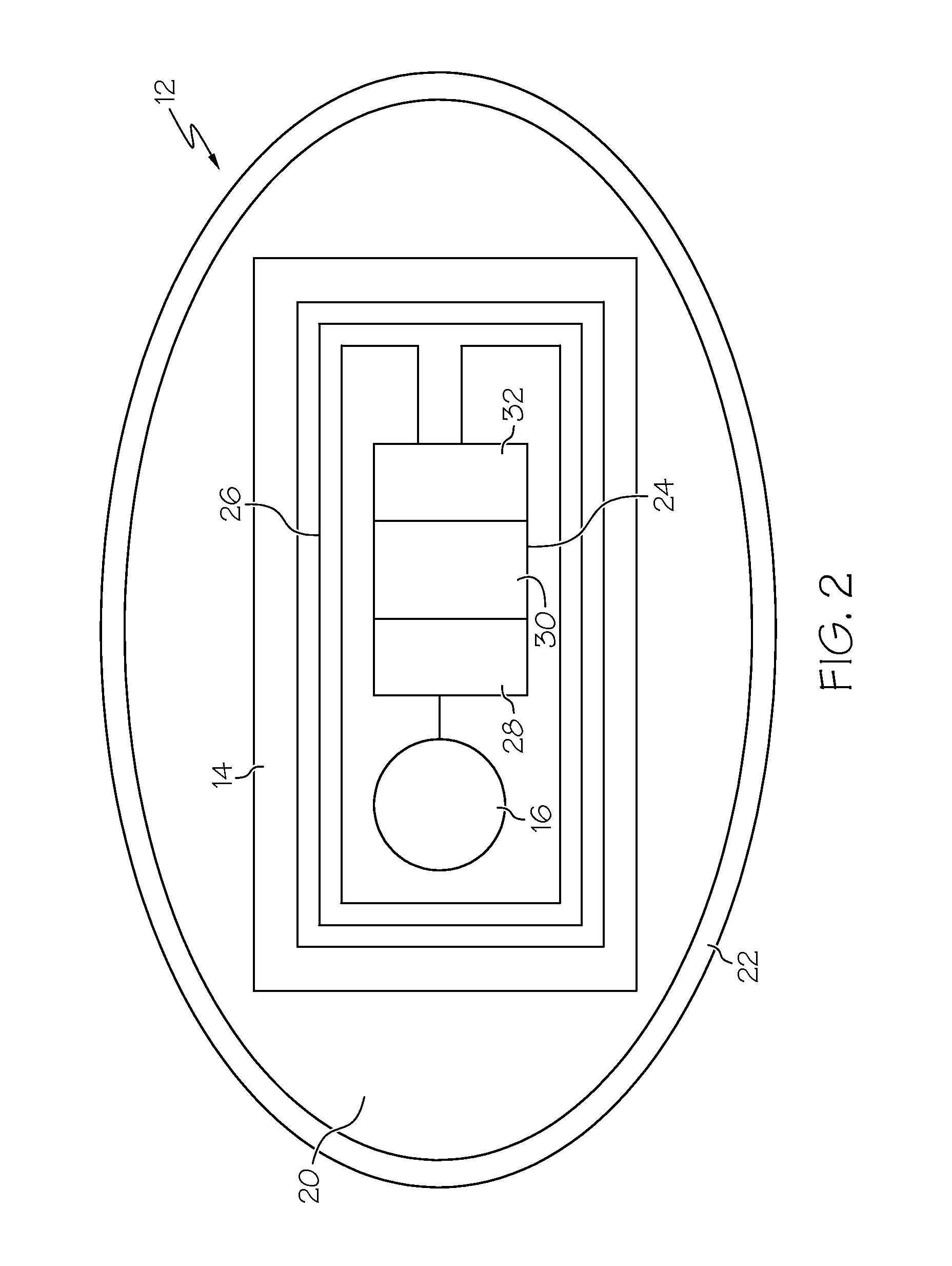
A system for monitoring compliance with a medication regimen comprises a patch. The patch includes an RFID apparatus and a sensor. The patch is configured to be removably securable to a body of a biological organism. The sensor is in electrical communication with the RFID apparatus and is configured to inspect a biological material of a biological organism for the presence of a detectable agent. The RFID apparatus is configured to communicate a result of the inspection to an RFID transceiver and / or a communication device. Methods are also provided.
Owner:AVERY DENNISON CORP
Electronic medication compliance monitoring system and associated methods
ActiveUS9047746B1Low costCorroborate complianceDrug and medicationsMedical devicesCompliance MonitoringElectronic tagging
A system and method for monitoring a patient's compliance with a medication regimen includes an electronic tag integral with or attached to a medicine delivery device such as a capsule, the tag having an antenna and a receiver / transmitter, the system also including a reader positioned externally for detecting the presence and location of the delivery device in the patient.
Owner:ETECTRX INC
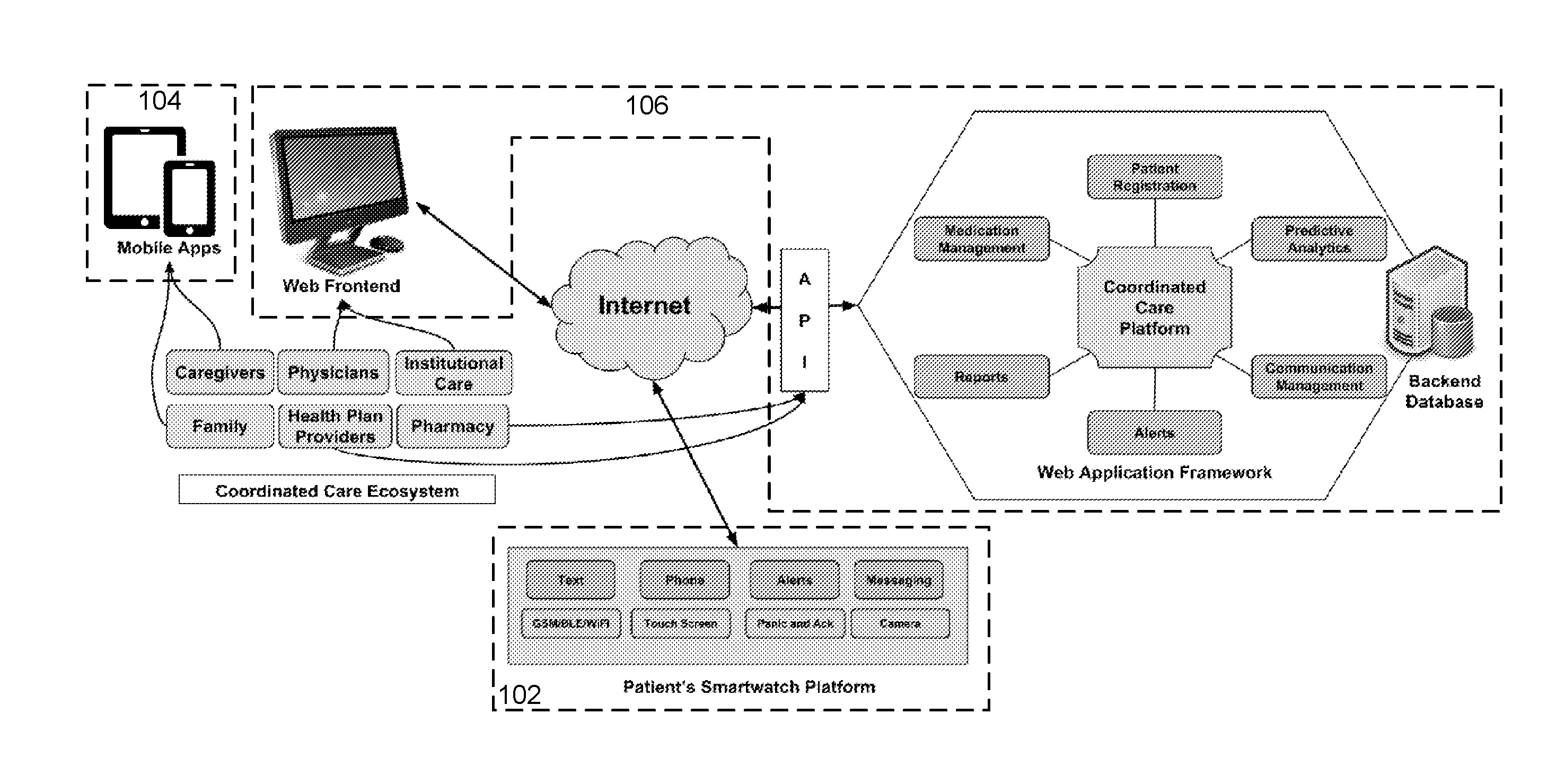
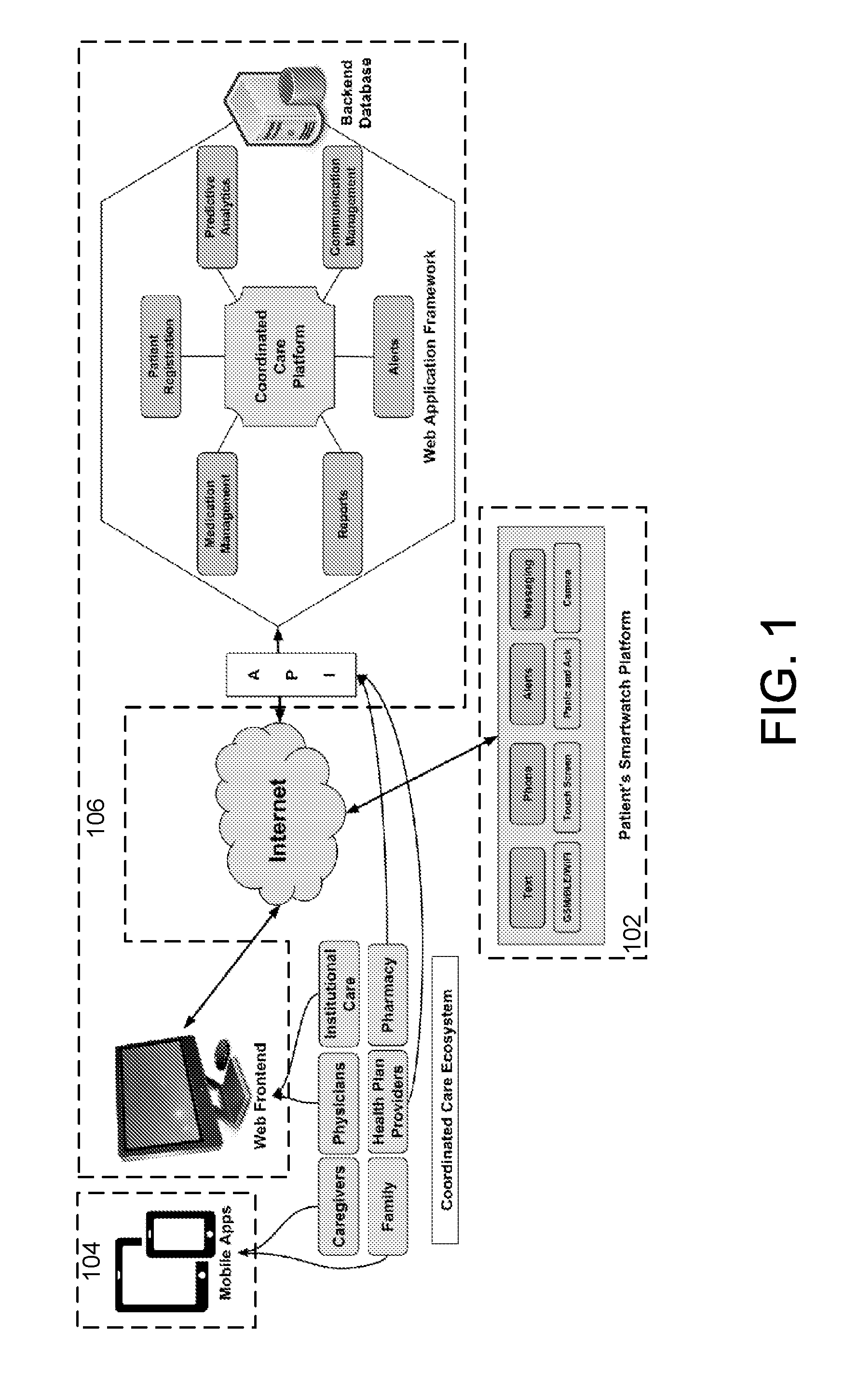
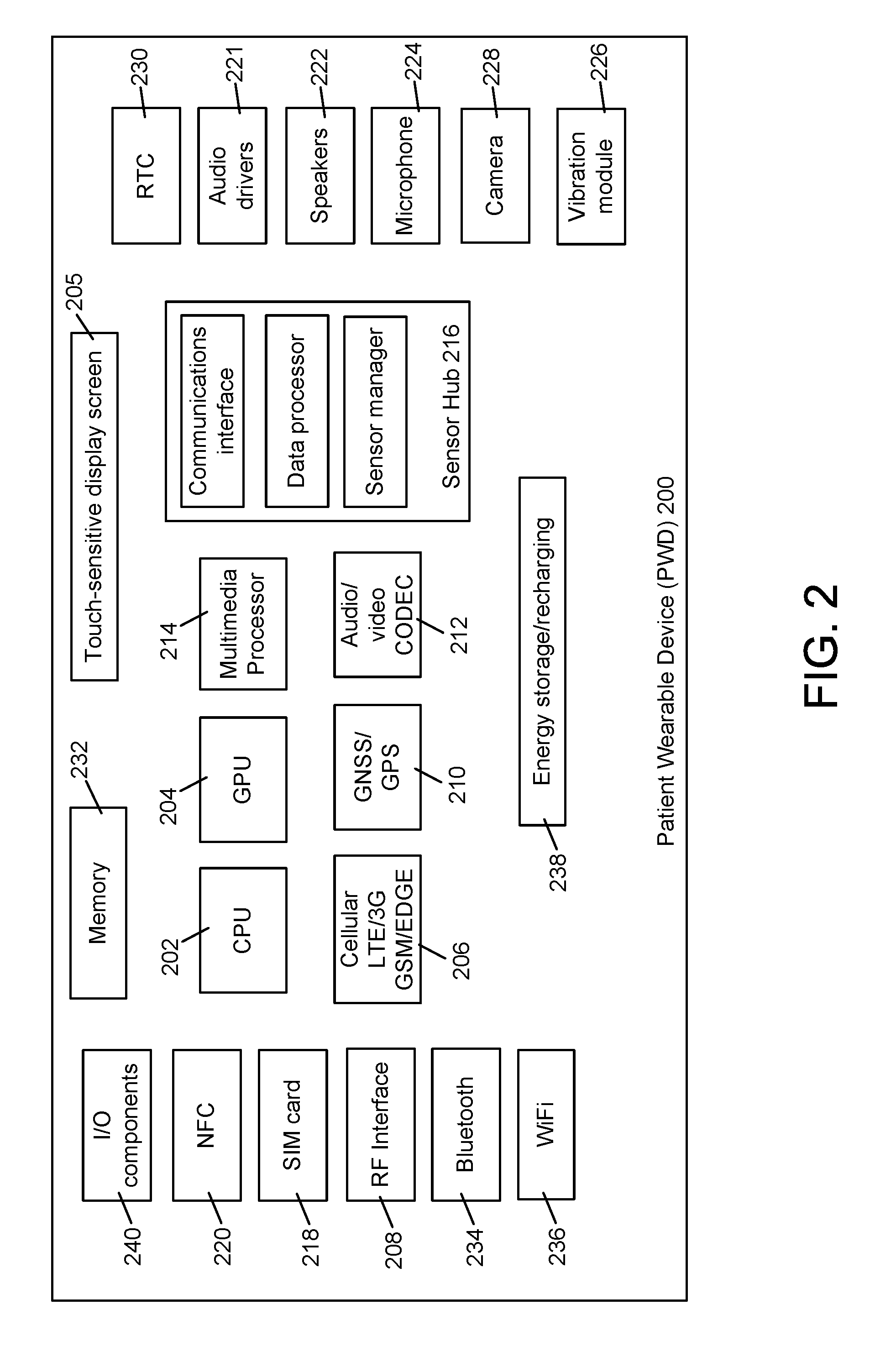
Medication adherence device and coordinated care platform
ActiveUS20160342767A1Constant connectivityConfirm its effectivenessLocal control/monitoringDrug and medicationsMedication adherenceMedication information
A method of supporting user adherence to a medication regimen includes storing received medication information associated with one or more medications using a patient wearable device and a coordinated care platform. The method also includes displaying identification and dosing information associated with the medications, generating a notification indicating that the identification information is being displayed, monitoring a response input indicating that the medications have been administered. For each of the one or more medications displayed, the method includes generating a medication status describing that either (i) the medication has been administered or (ii) the response has not been received. The method further includes generating a reminder notification when the medication status indicates that the response has not been received. The method may further include gathering behavioral data points associated with the user for submission to the coordinated care platform, which performs predictive analytics based on the data points.
Owner:WATCHRX INC
Intelligent medical cabinet
InactiveUS20060253096A1Drug and medicationsRegistering/indicating working of machinesMedical diagnosisDrug regimen
A method for dynamic control of medications provided to a patient at a remote location is disclosed. The method can include steps of selecting a customized set of medications for a patient, where the customized set of medications at least partially reflect a medical diagnosis of the patient, and providing the customized set of medications to the patient, where the customized set of medications are inaccessible to the patient without authorization from a medical care provider. Further steps of the method can include authorizing the patient to receive a first medication regimen from the customized set of medications, monitoring remotely at least one health parameter of the patient, and authorizing the first medication regimen to be modified to a second medication regimen based at least partially on the at least one health parameter.
Owner:HEWLETT PACKARD DEV CO LP +1
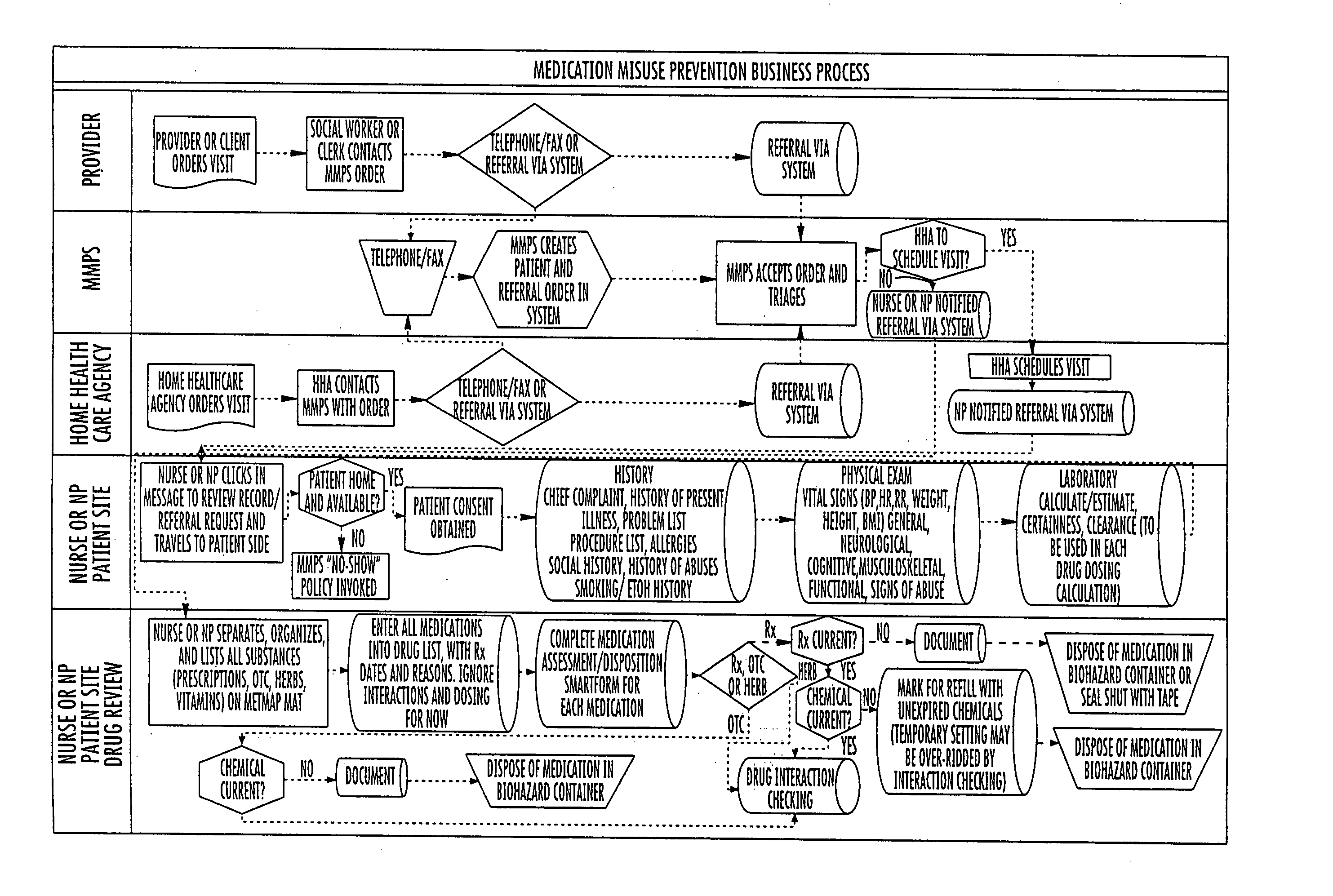
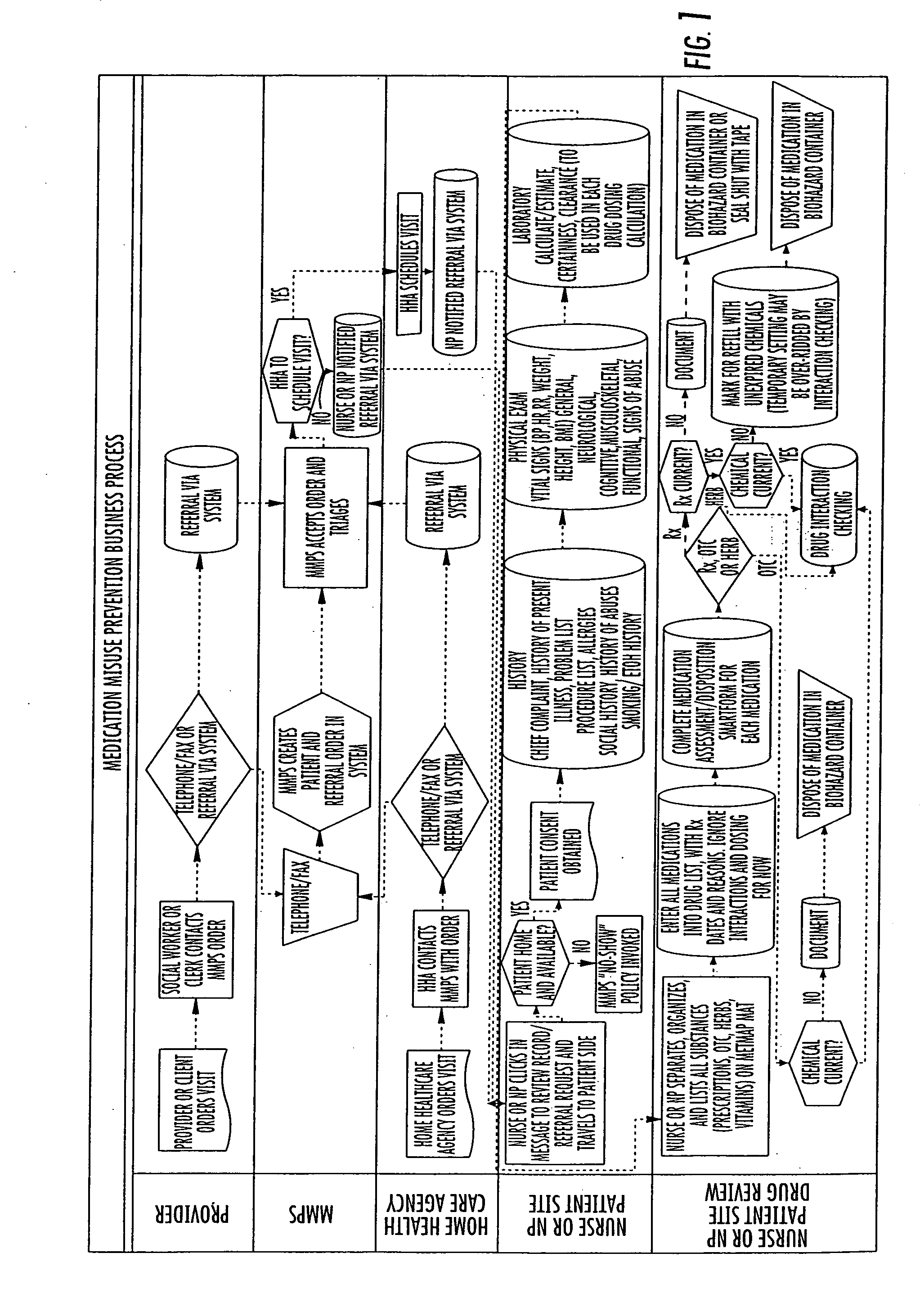
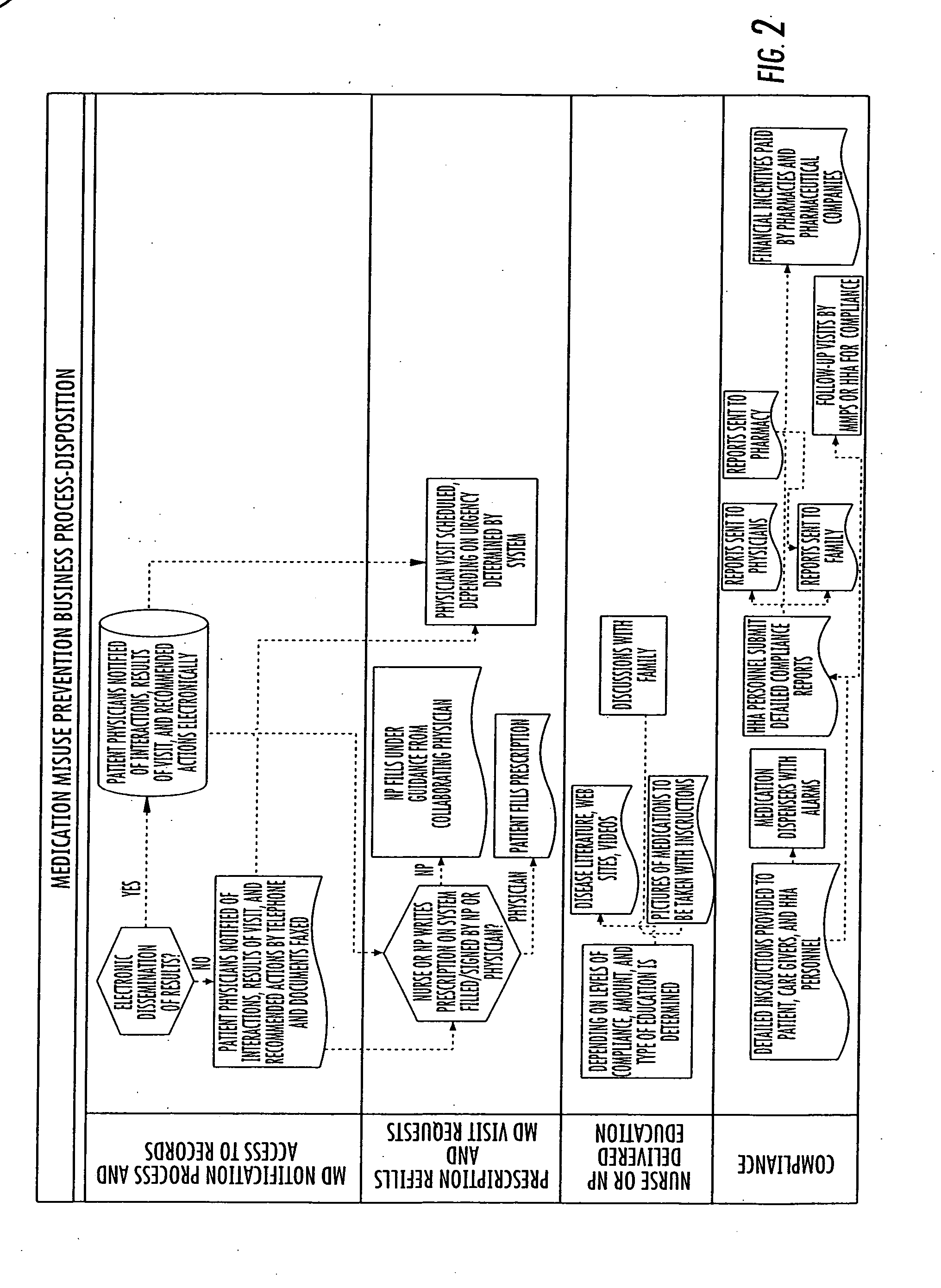
System and method for medication misuse prevention
InactiveUS20060224419A1Increased riskDrug and medicationsOffice automationSubstance use preventionRegimen
A system and method of identifying patients with an increased risk for medication misuse as a result of their physical condition or inability to follow a prescription drug regimen. This is accomplished by an onsite evaluation of the patient, their physical condition and medication usage, including prescriptions, over the counter medications, herbals and food interactions, by a qualified individual. The results of this evaluation are provided to a database that generates a report comprising a patient's vital signs, medication interactions, prescription changes, prescription renewals, discontinued medications, evaluation of a patient's compliance with their medication regimen and education approved for to assist the patient with their compliance for review by the patient's primary care physician, additional physicians treating the patient, authorized organizations, such as HMOs, gardians or other authorized individual. With this knowledge the physician can identify a current or potential problem and can recommend changes to a patient's prescription drug regimen or education regarding drug regimens to correct the problem.
Owner:MEDICATION MISUSE PREVENTION SERVICES
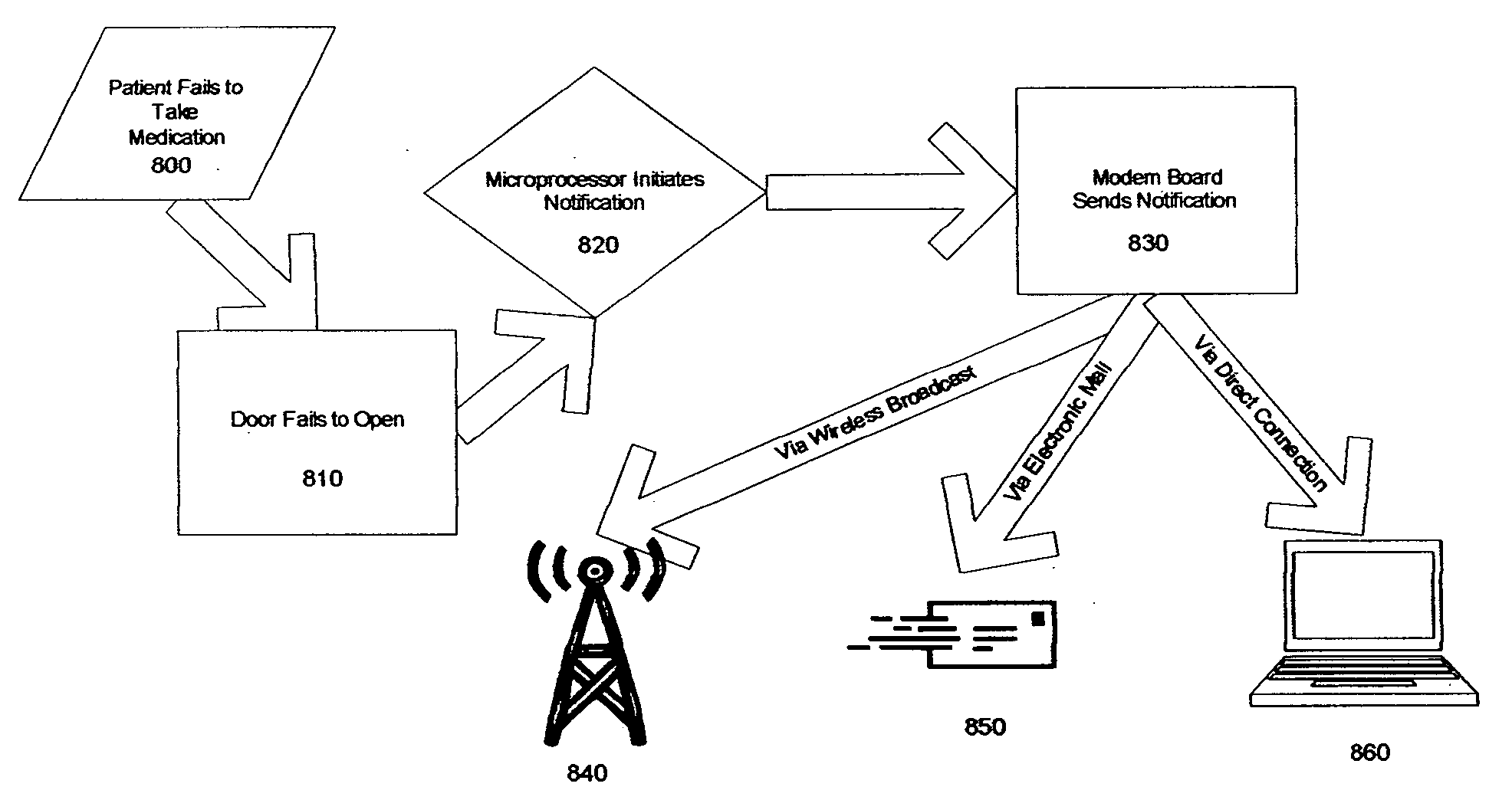
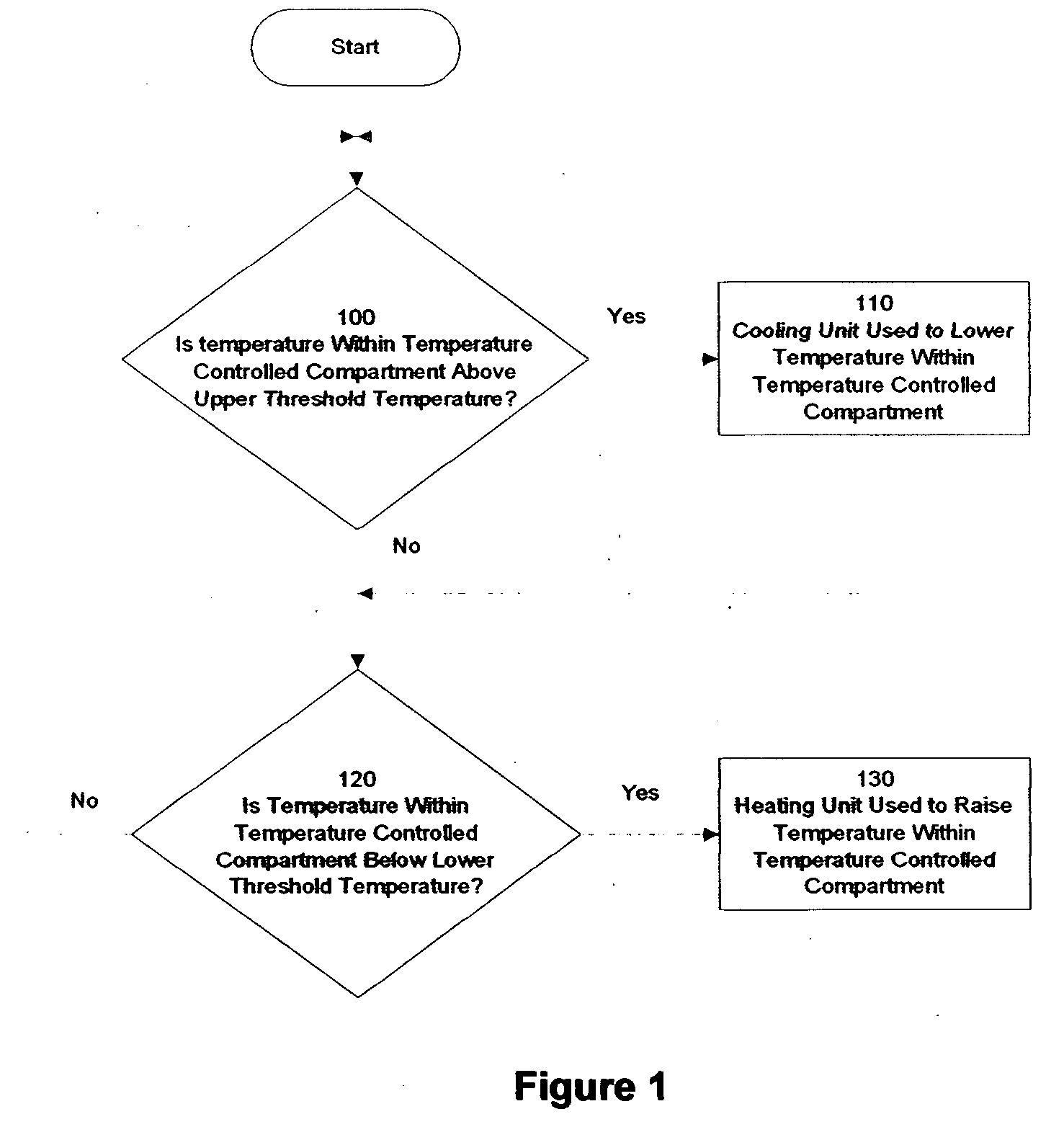
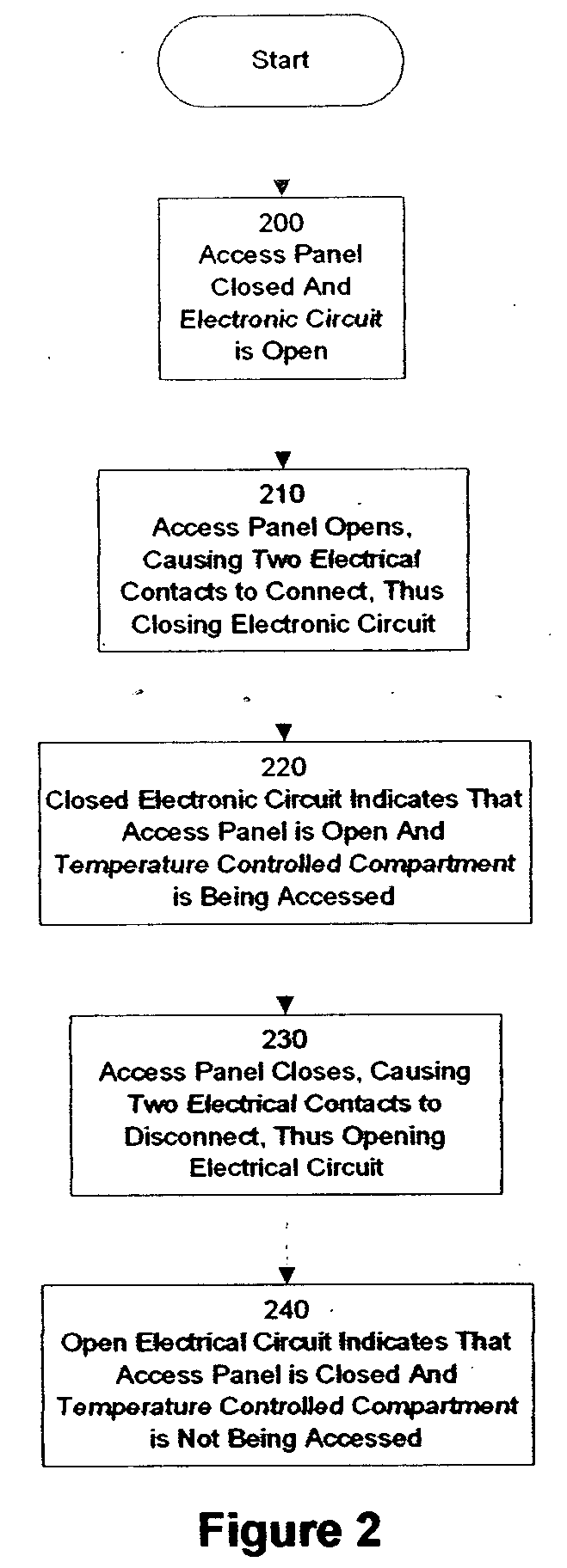
Method and apparatus for automatic health monitoring
InactiveUS20050240304A1Registering/indicating working of machinesTelemedicineTemperature controlComputer network
Embodiments of the present invention are directed to a method and apparatus for automatic health monitoring. In one embodiment, a regularly accessed device is equipped with an access detection unit. In one embodiment, a regularly accessed device is a temperature controlled compartment. In one embodiment, a timer is started when the access detection unit detects an access to the temperature controlled compartment. In one embodiment, a determination unit determines whether a second access is made within a time interval of a first access. In one embodiment, the time interval is determined using the patient's recommended medication regimen. In one embodiment, when a determination unit determines that a second access is not made within a time interval of a first access, a signal generator generates a signal. In one embodiment, the signal is transmitted to a healthcare provider. In one embodiment, the signal is transmitted via a computer network.
Owner:YORK MATTHEW +5
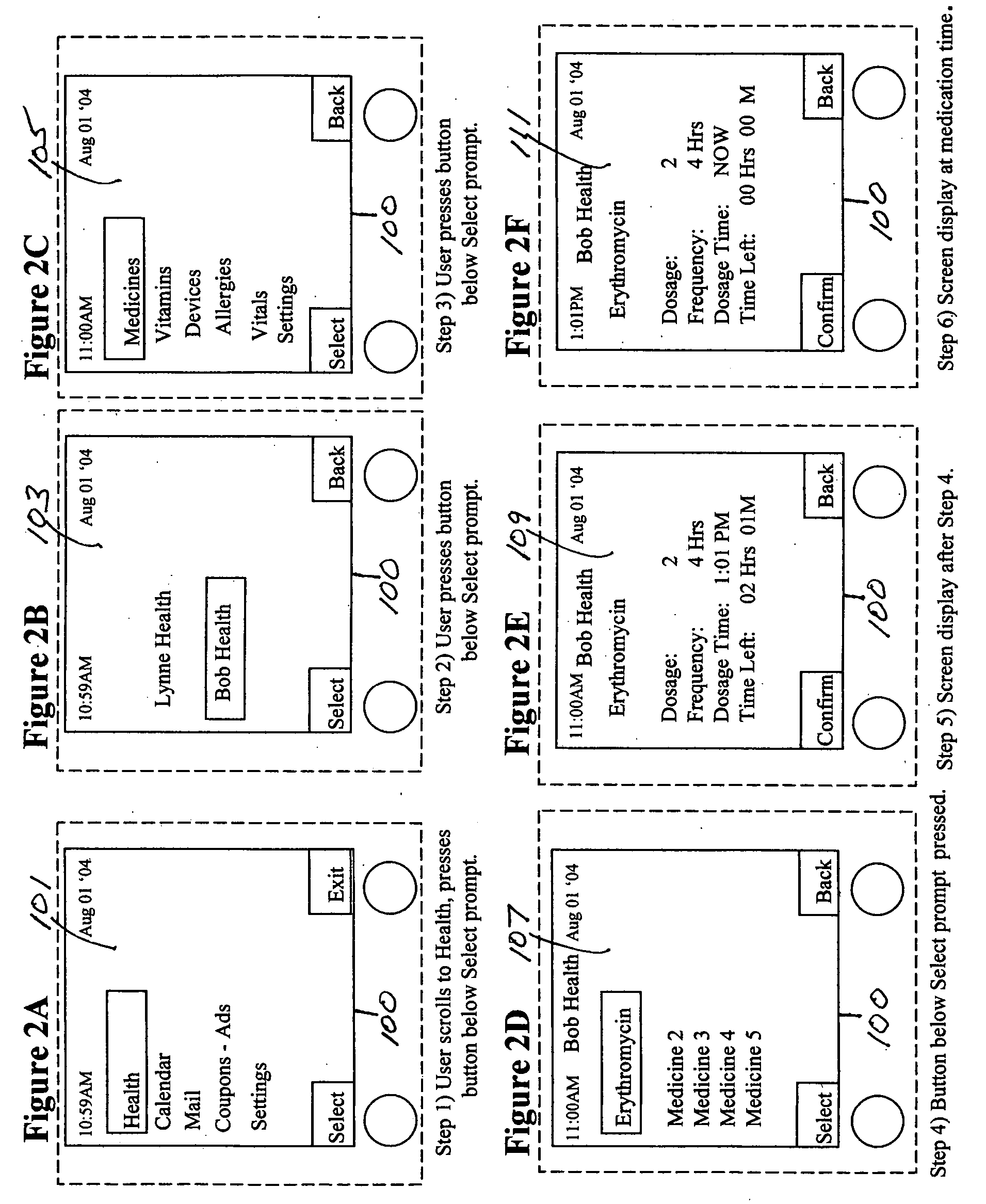
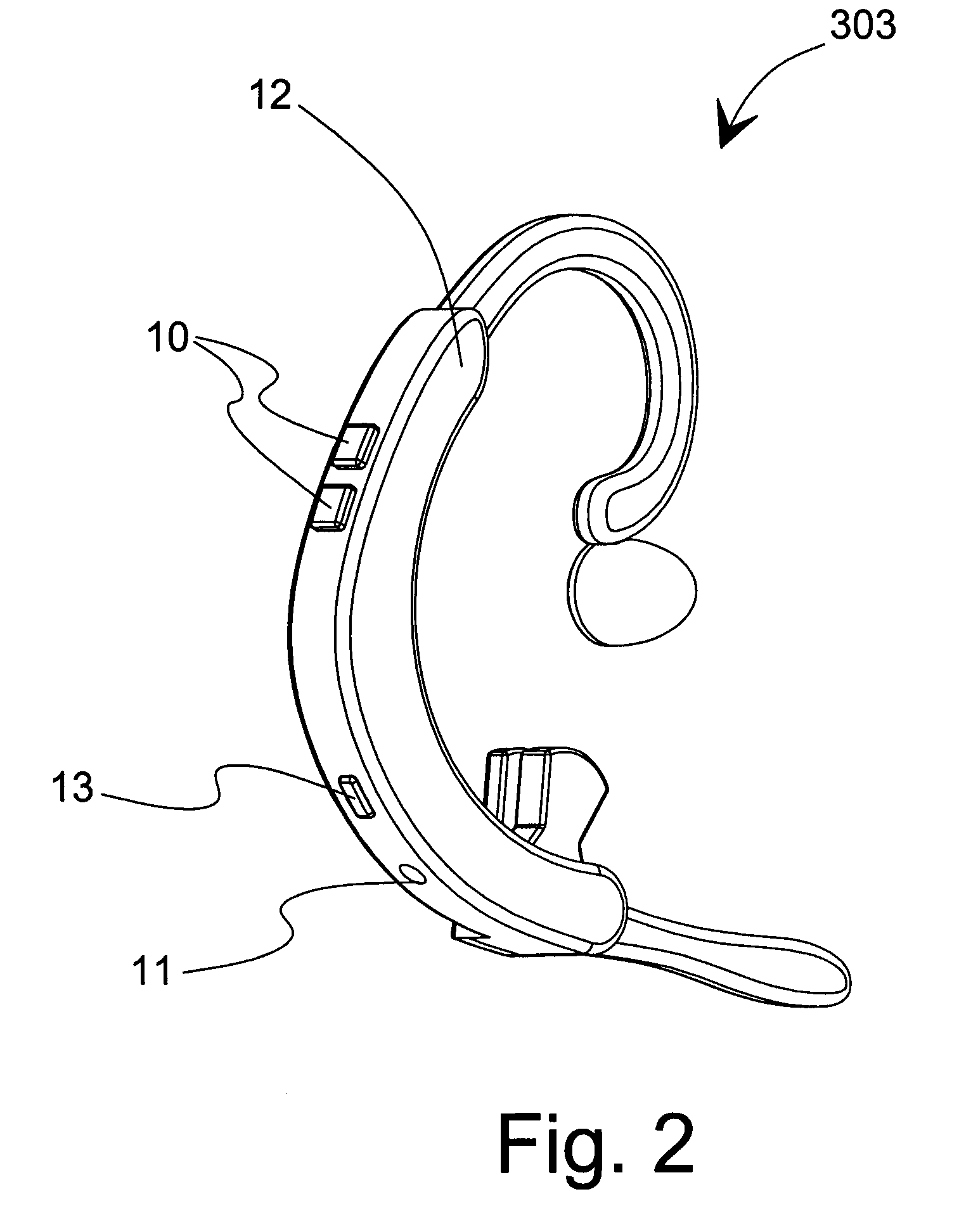
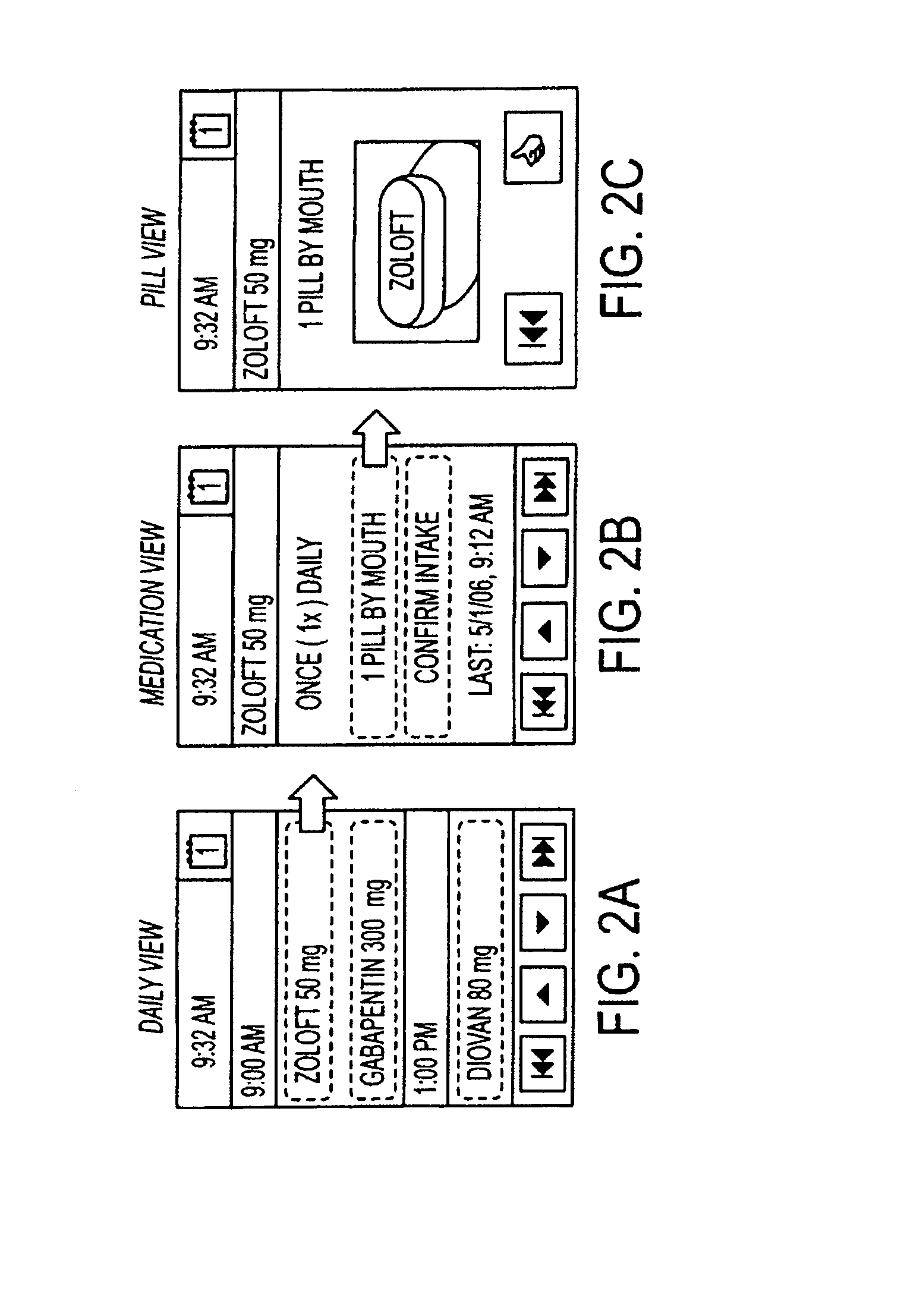
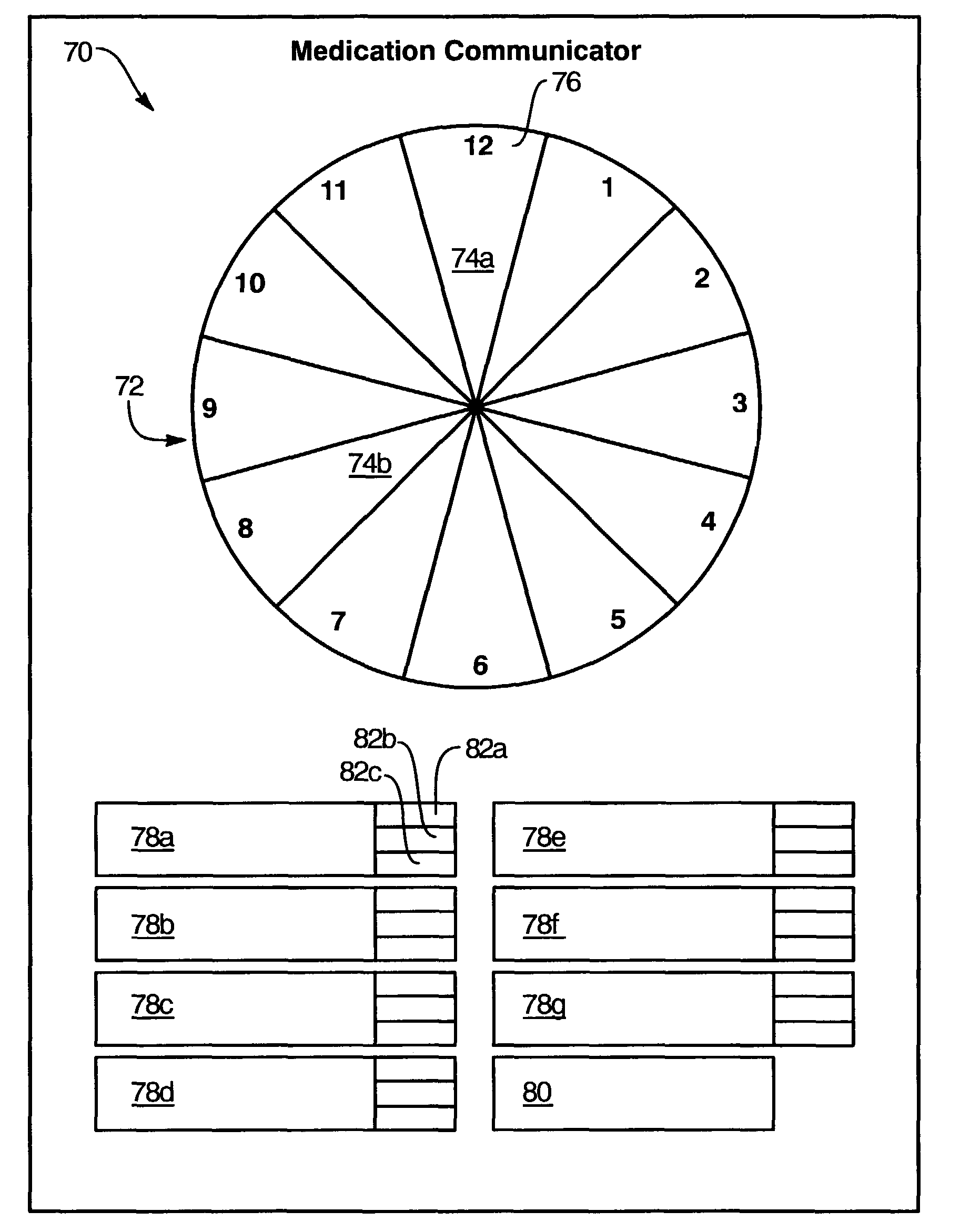
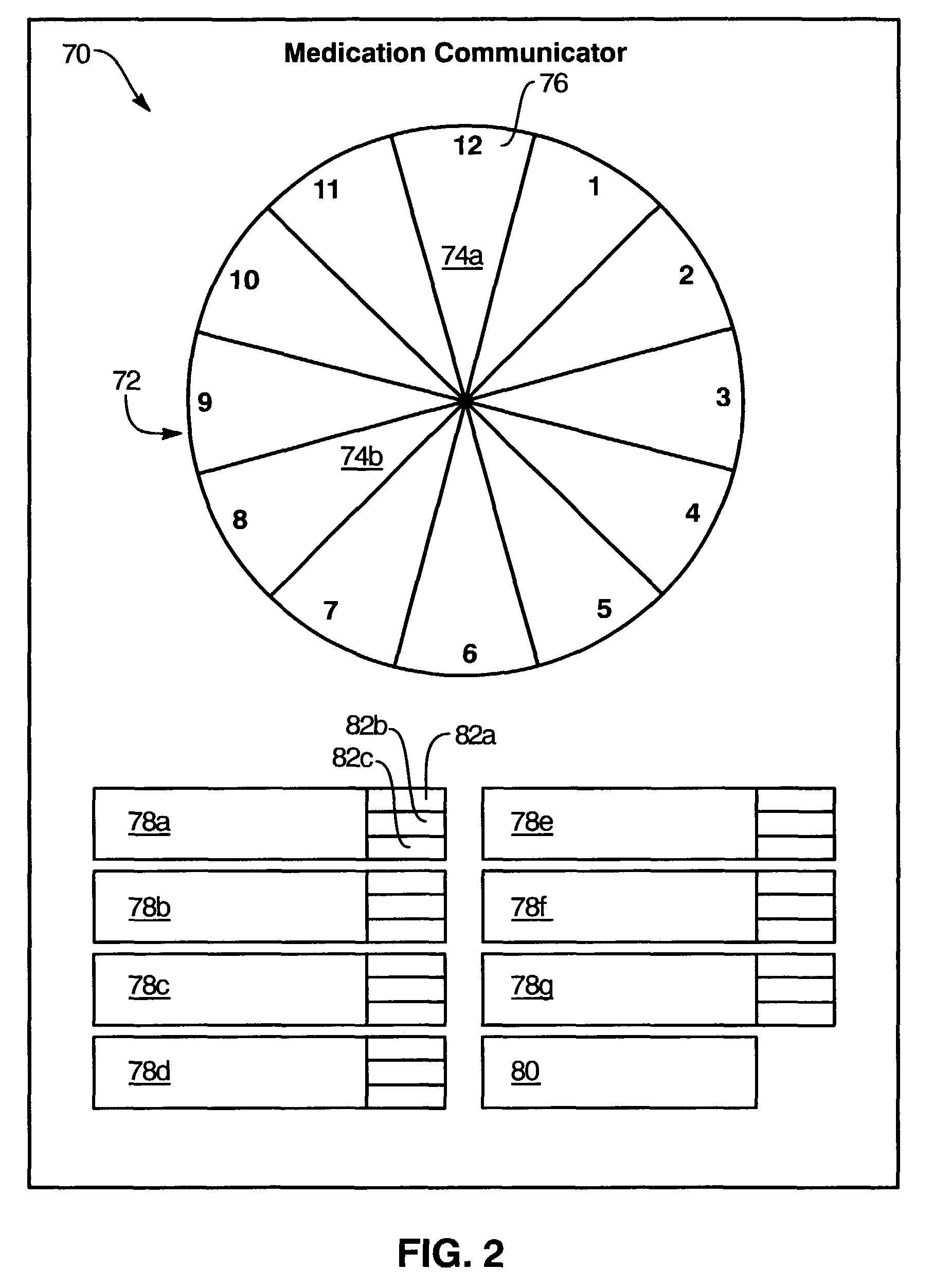
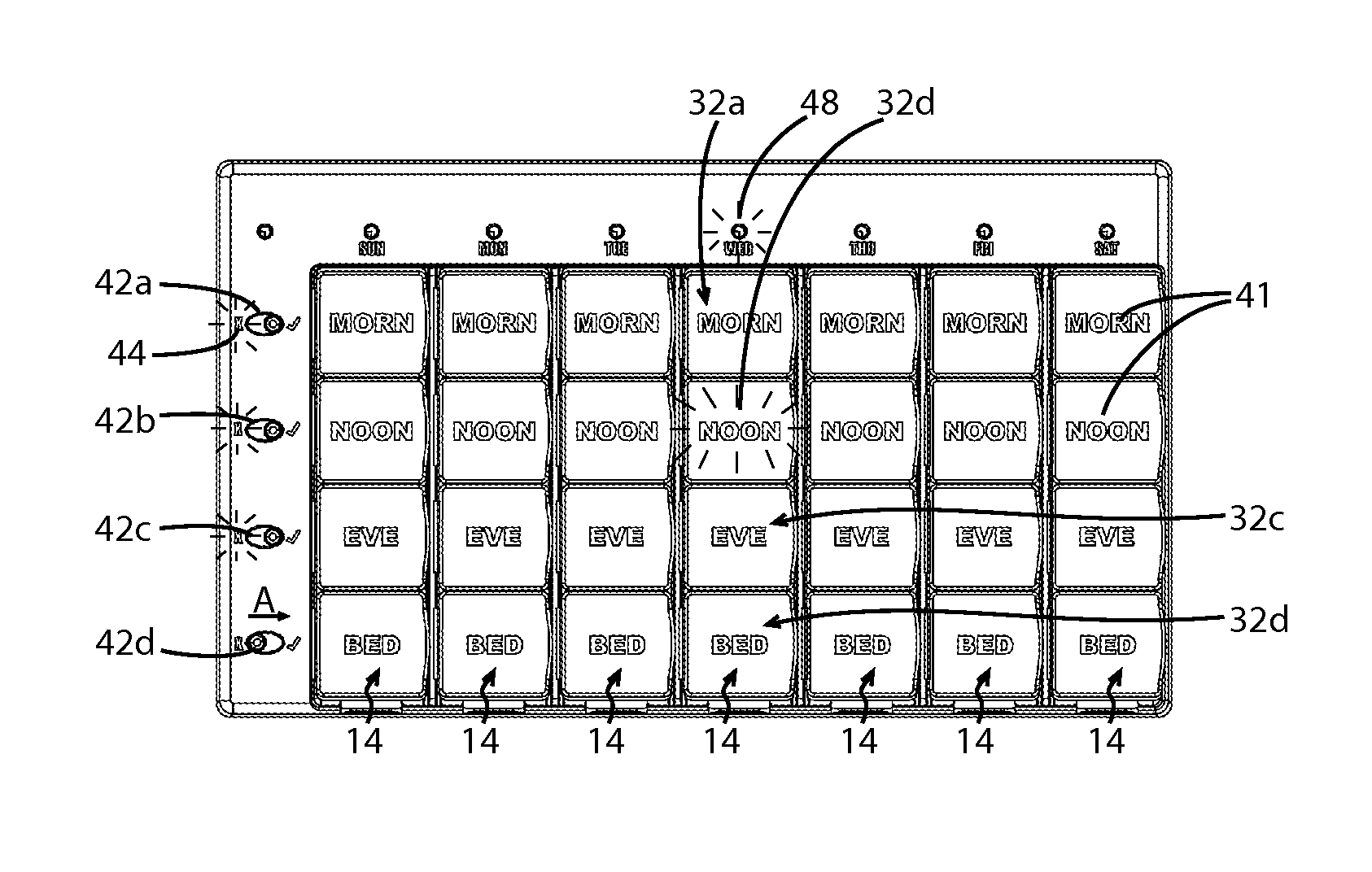
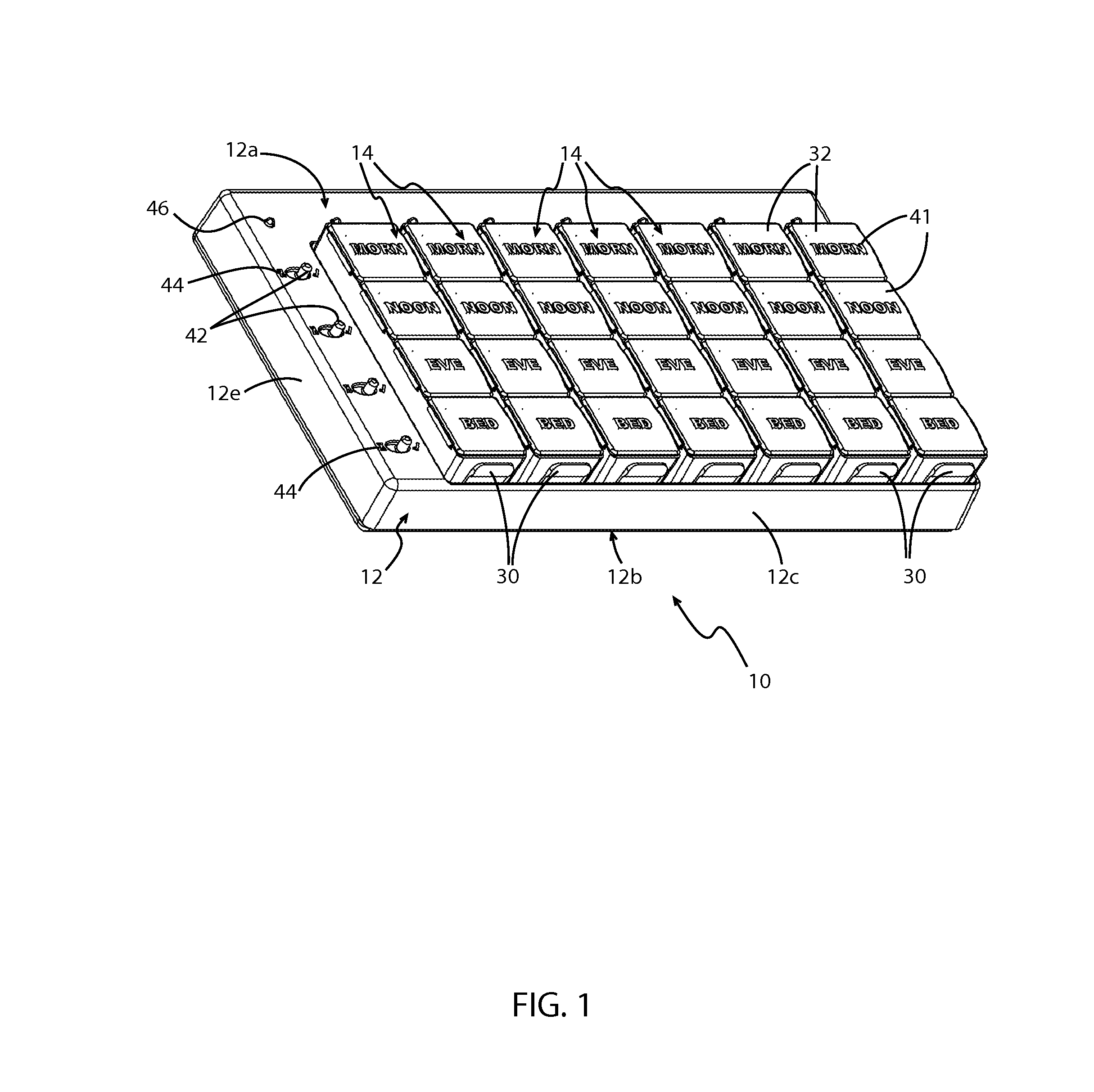
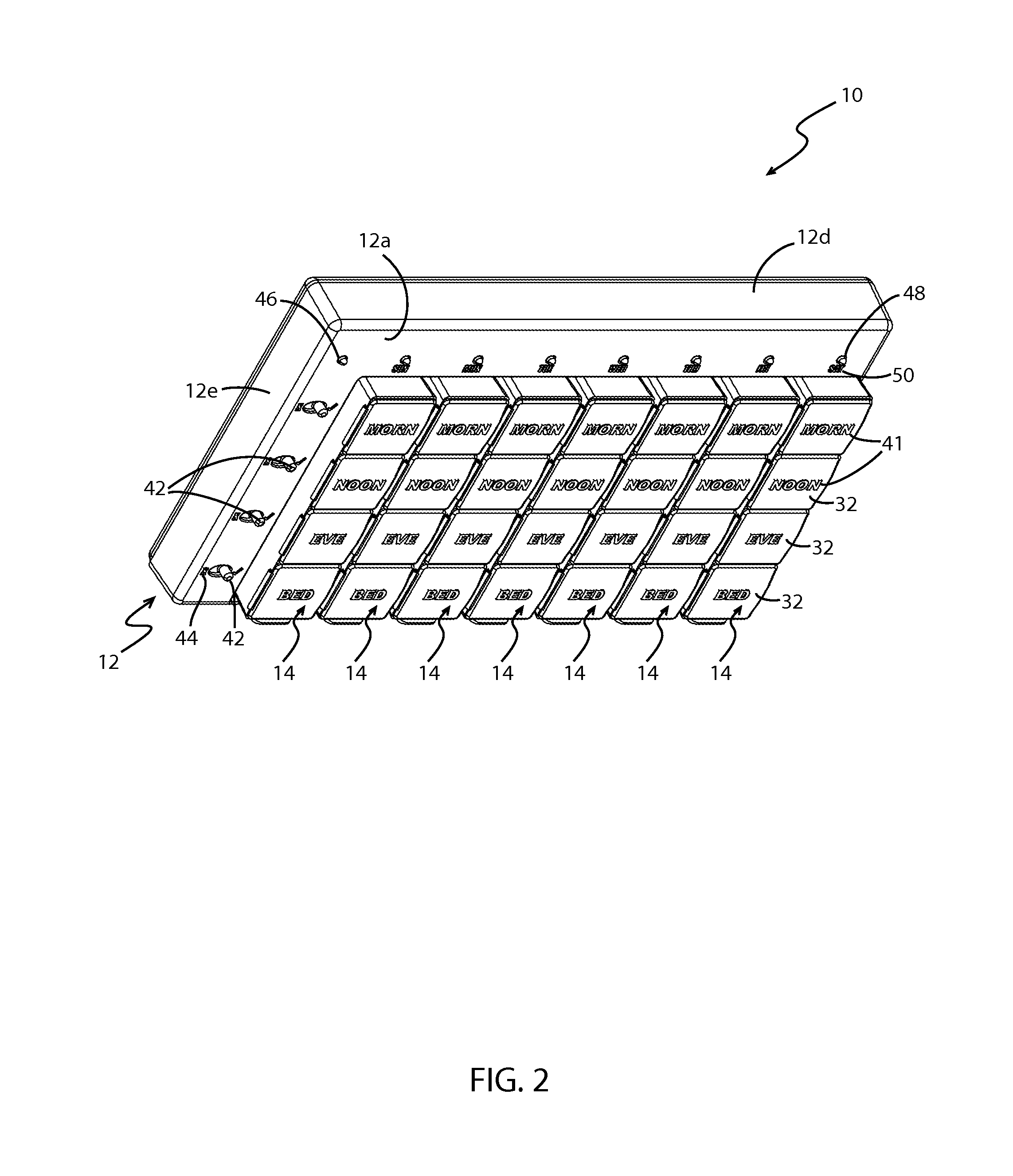
Medication regimen communicator apparatus and method
InactiveUS7773460B2Easy to understandImprove visualizationVisual indicationDrug and medicationsTeaching toolApplication time
A “medication communicator” chart is used as a teaching tool to educate patients and to schedule events corresponding to a prescribed medication regimen. The “medication communicator” chart includes a scheduling graph having the shape of a 12-hour or 24-hour analog clock. The scheduling graph is divided into regions corresponding to each hour of a day for scheduling event information. Fields, on the “medication communicator” chart, are receptive to labels communicating information corresponding to numerous medications in the medication regimen. Timing indicators may be applied to the regions of the scheduling graph to indicate consumption or application times of each of the medications.
Owner:HOLT LINDSAY
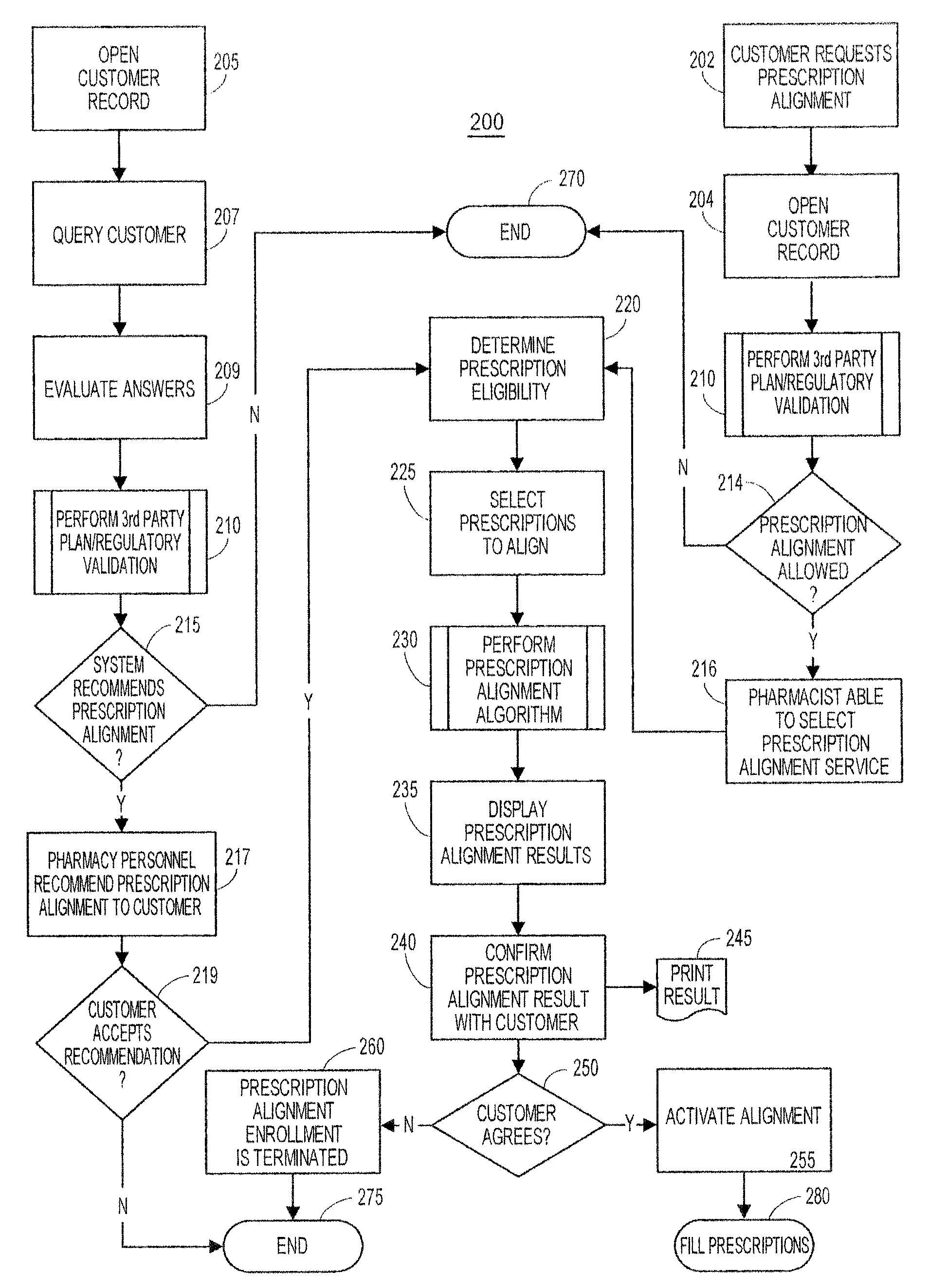
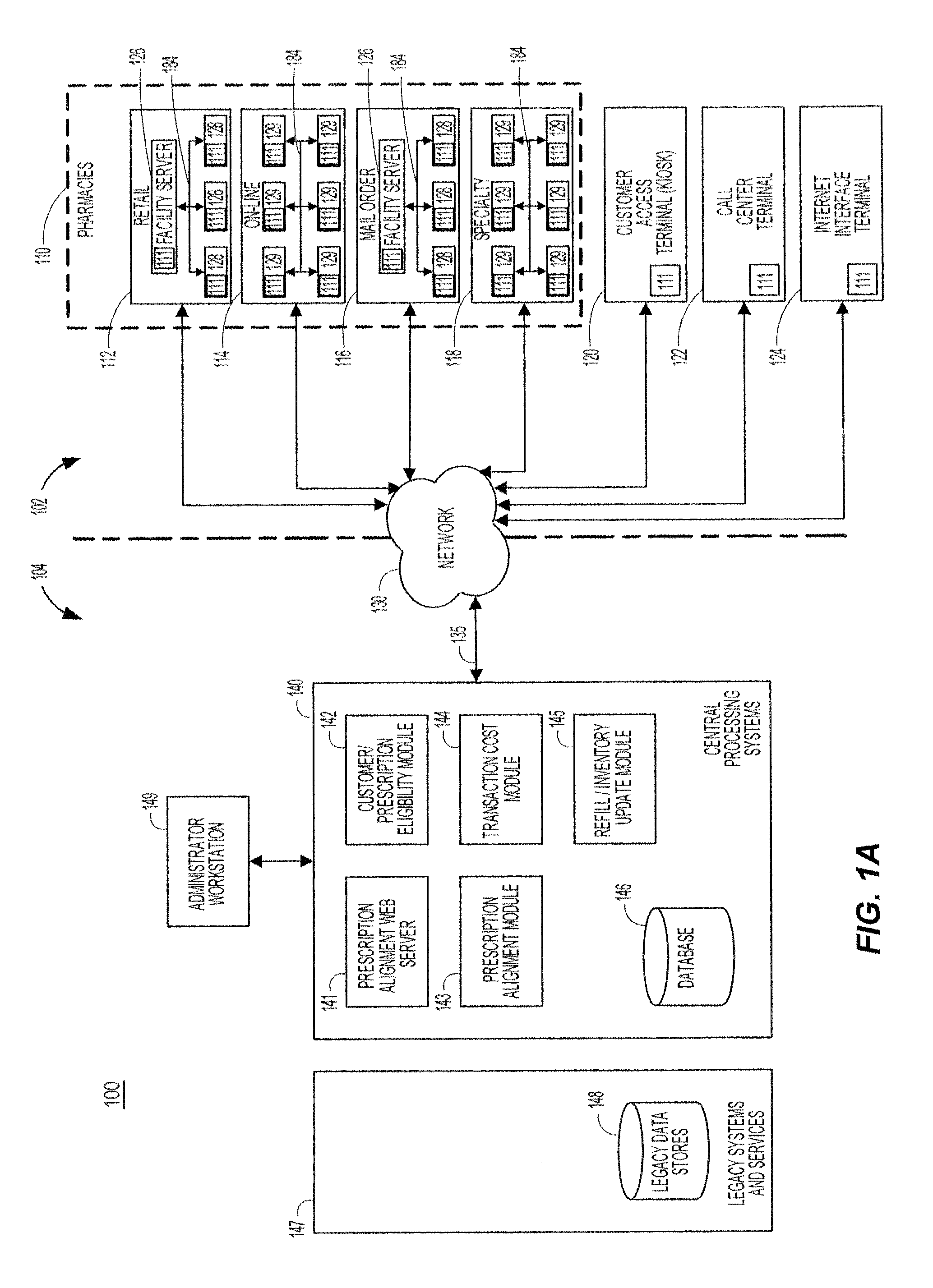
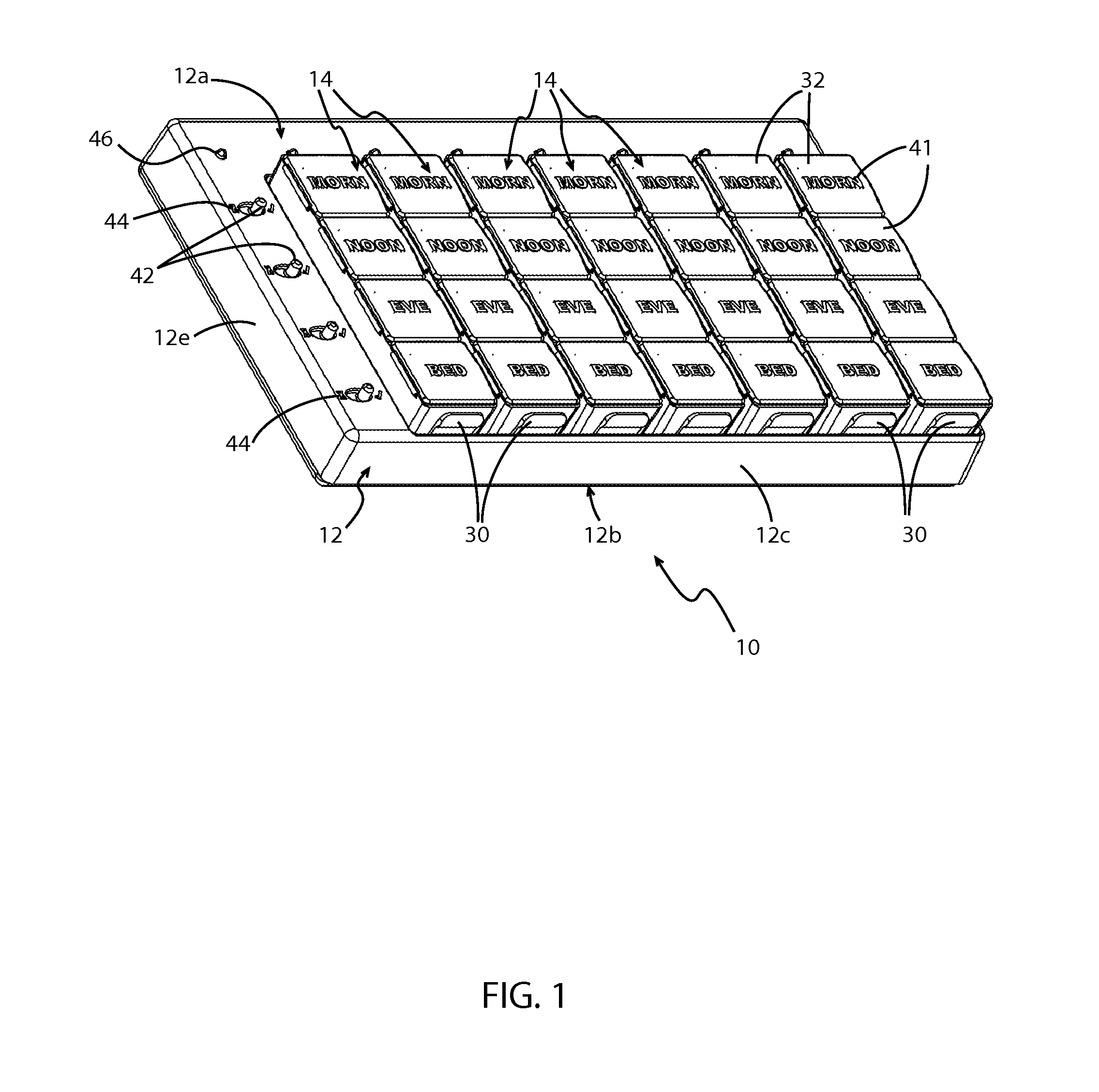
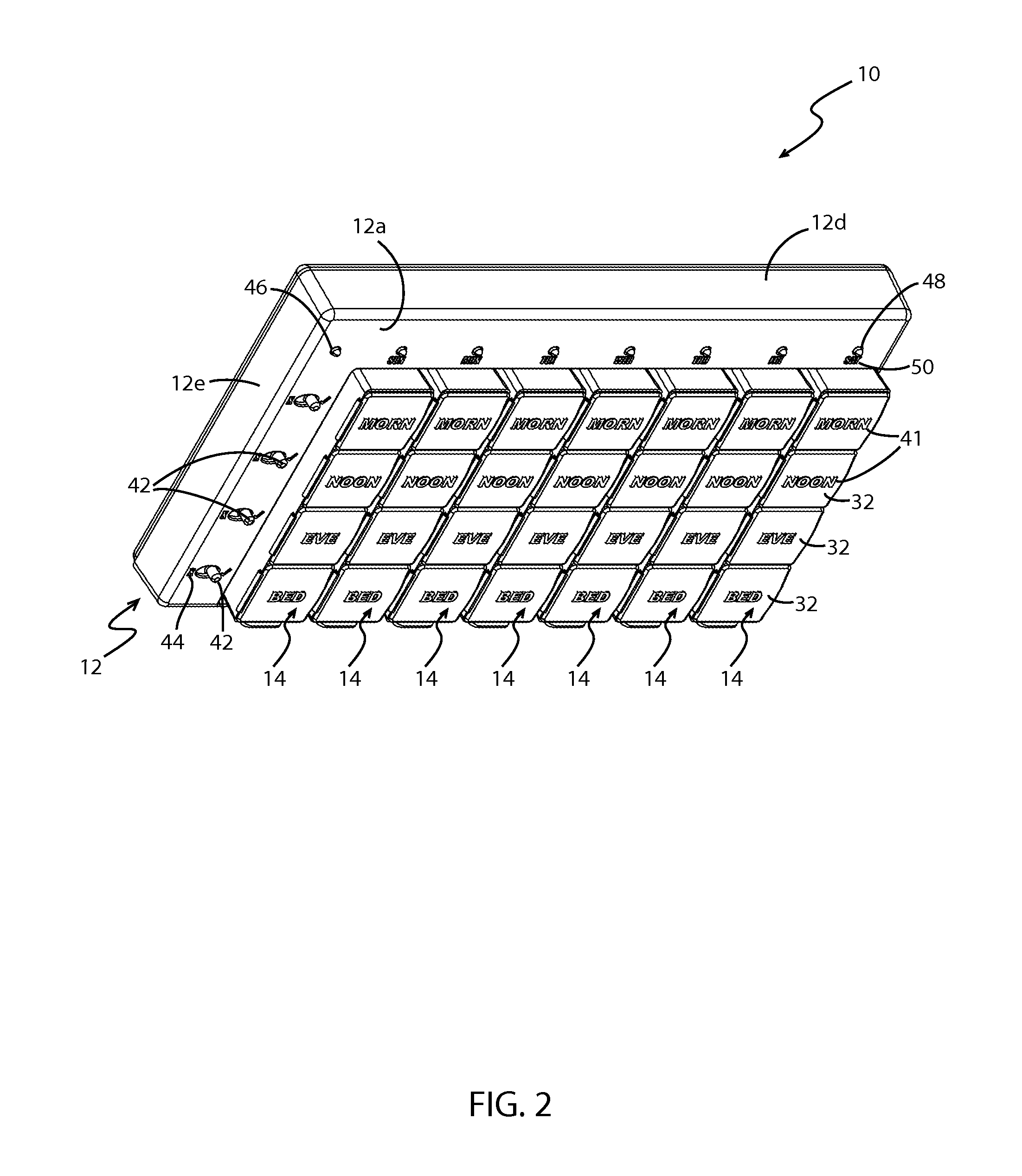
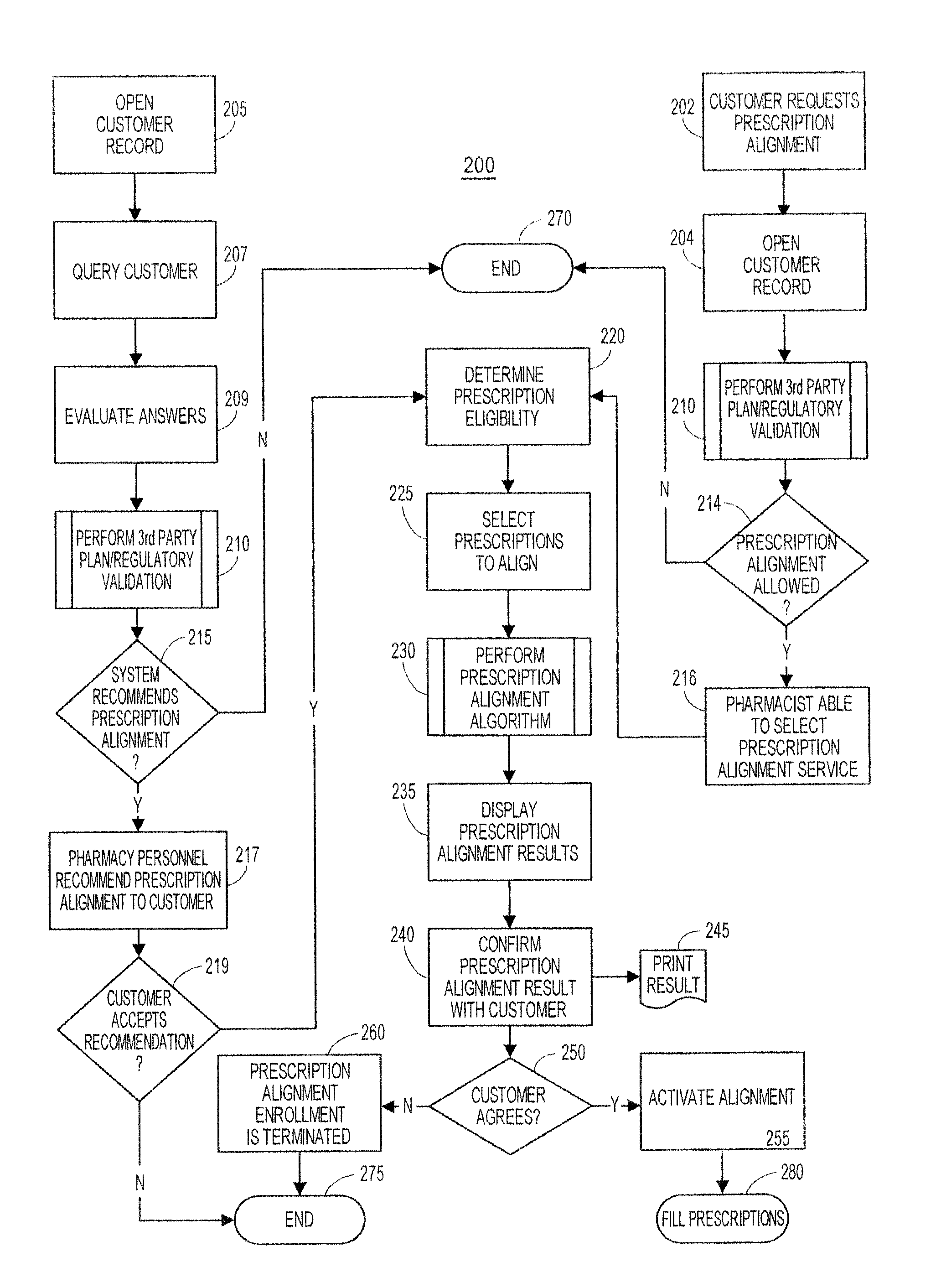
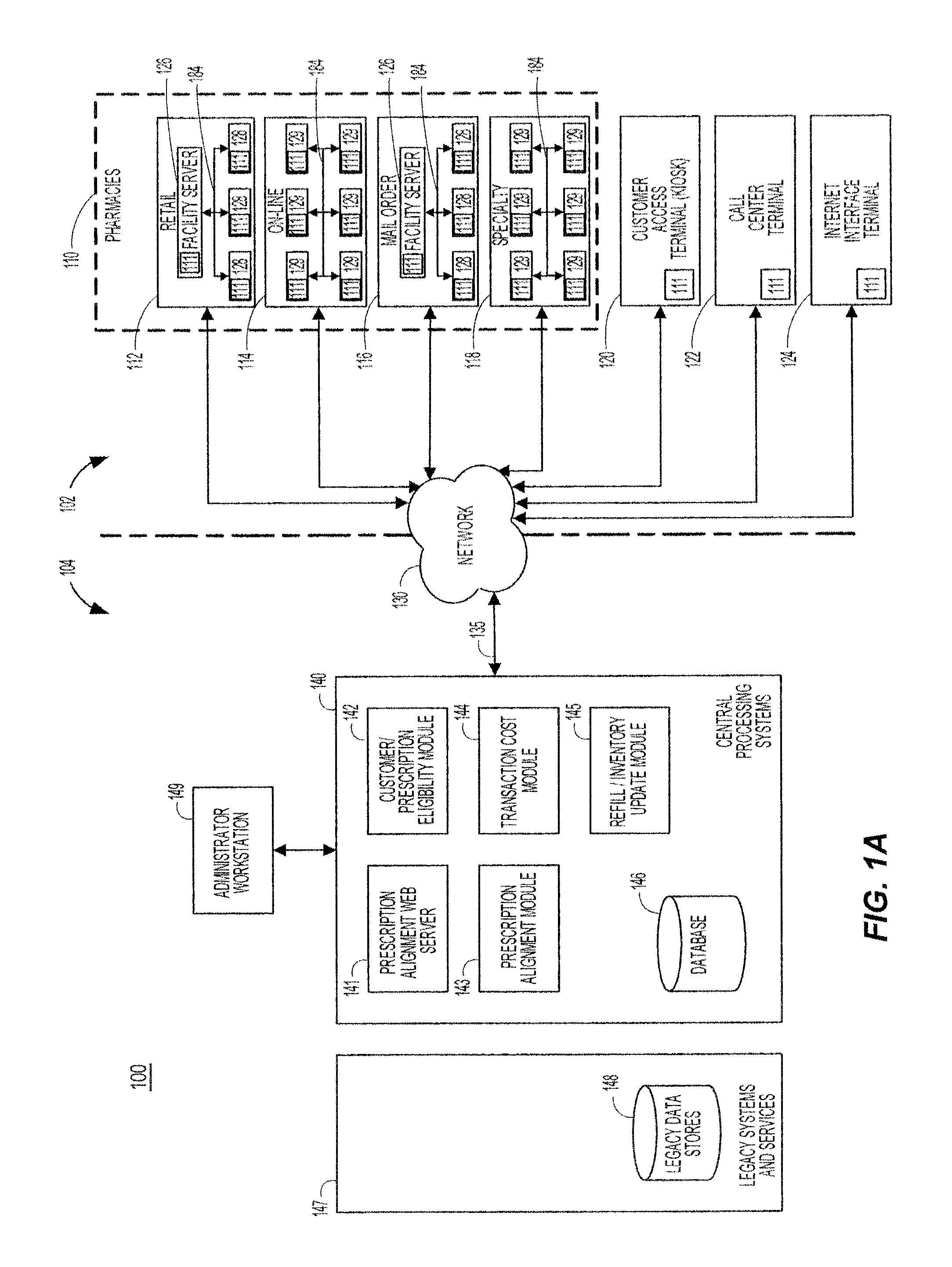
System and method of prescription alignment
ActiveUS20090030719A1Reduce effortImprove complianceFinanceDrug and medicationsPharmacyMedical prescription
The method, system and user-interface allows alignment of refill dates associated with a plurality of prescriptions, such that the plurality of prescriptions all require refills on the same date, thus limiting the number of occasions on which a customer must visit the pharmacy to retrieve refills of the aligned prescriptions, and increasing the likelihood that the customer will comply with the recommended medication regimen.
Owner:WALGREEN CO
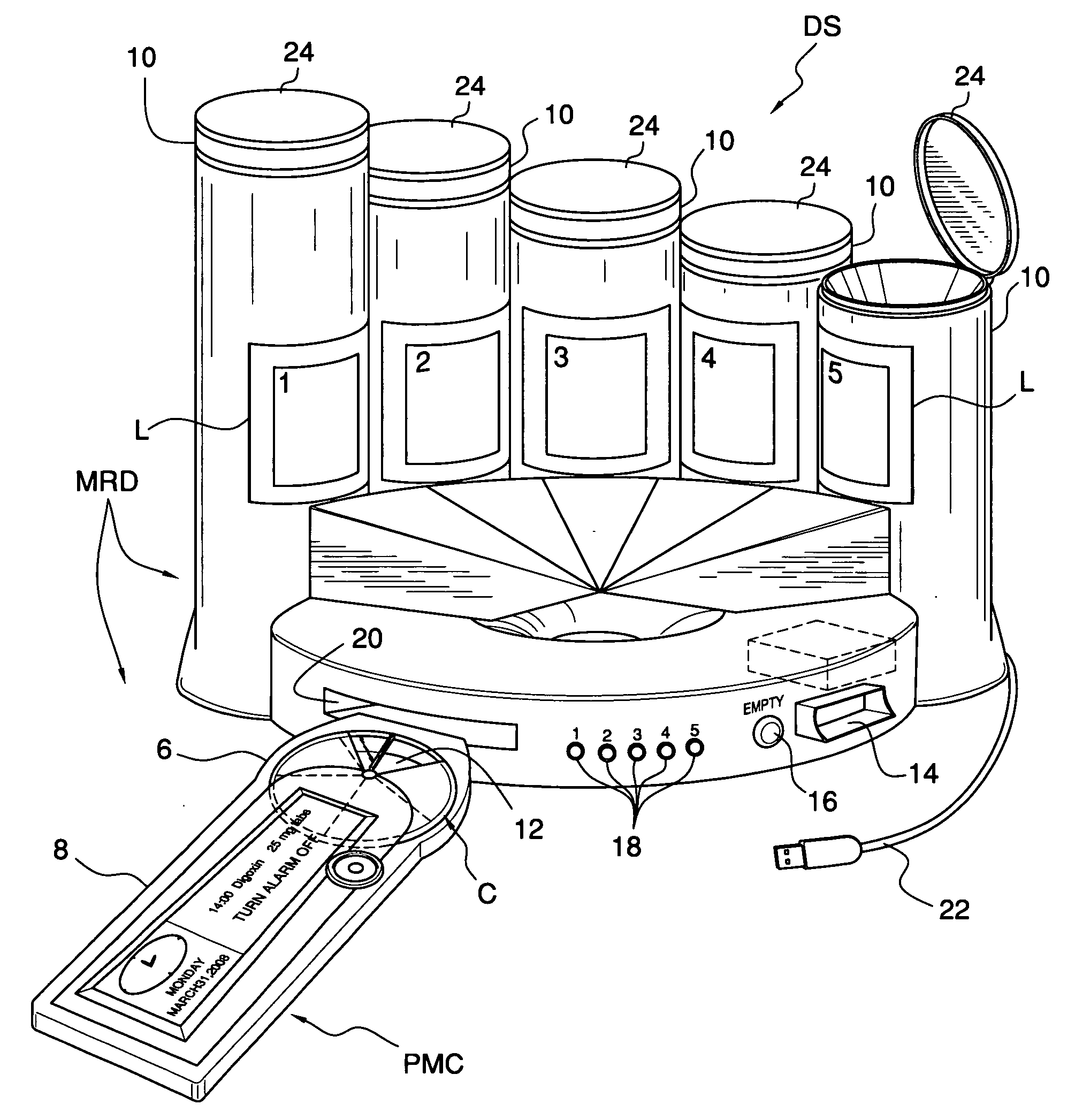
Method of using a medication reminder and compliance system including an electronic pill box
ActiveUS20150048100A1Easy to carryDrug and medicationsCoin-freed apparatus detailsGreen-lightMedical emergency
A medication reminder and compliance system including a pill box, a pill box and electronic device; or a pill box, electronic device and remote server. The pill box includes several detachable dosettes, each divided into several chambers having a door. A two-color LED is disposed beneath each chamber and is activated when a specific reminder time is reached. The LED initially illuminates the associated chamber with a green light but changes to a flashing red light if the door is not opened within a preset time. A switch engaged with the door deactivates the LED when the door is opened. The electronic device communicates with the pill box and alerts the patient when it is time to take medication. A remote server which communicates with both the pill box and electronic device can be programmed to control the reminder schedule and monitor the patient's compliance with a prescribed medication regimen.
Owner:NEXT PARADIGM
Method and system for aligning a plurality of prescription refills to multiple alignment dates
ActiveUS20090030725A1Reduce effortImprove complianceData processing applicationsDrug and medicationsEngineeringPrescription refills
The method, system and user-interface allows alignment of refill dates associated with a plurality of prescriptions to a plurality of alignment dates, such that a plurality of selected prescriptions require refills on each of the plurality of alignment dates, thus limiting the number of occasions on which a customer must visit the pharmacy to retrieve refills of the aligned prescriptions, and increasing the likelihood that the customer will comply with the recommended medication regimen.
Owner:WALGREEN CO
Oral devices and methods for controlled drug delivery
Controlled-drug-delivery oral devices are implanted or inserted into an oral cavity, built onto a prosthetic tooth crown, a denture plate, braces, a dental implant, or the like. The devices are refilled or replaced as needed. The controlled drug delivery may be passive, based on a dosage form, or electro-mechanically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled delivery may be any one of the following: delivery in accordance with a preprogrammed regimen, delivery at a controlled rate, delayed delivery, pulsatile delivery, chronotherapeutic delivery, closed-loop delivery, responsive to a sensor's input, delivery on demand from a personal extracorporeal system, delivery regimen specified by a personal extracorporeal system, delivery on demand from a monitoring center, via a personal extracorporeal system, and delivery regimen specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted or induced by a transport mechanism, such as any one of, or a combination of iontophoresis, electroosmosis, electrophoresis, electroporation, sonophoresis, and ablation. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen. The oral devices and methods for controlled drug delivery apply to humans and animals.
Owner:安迪·沃尔夫 +1
Electronic pill box and medication reminder and compliance system incorporating same
Owner:NEXT PARADIGM
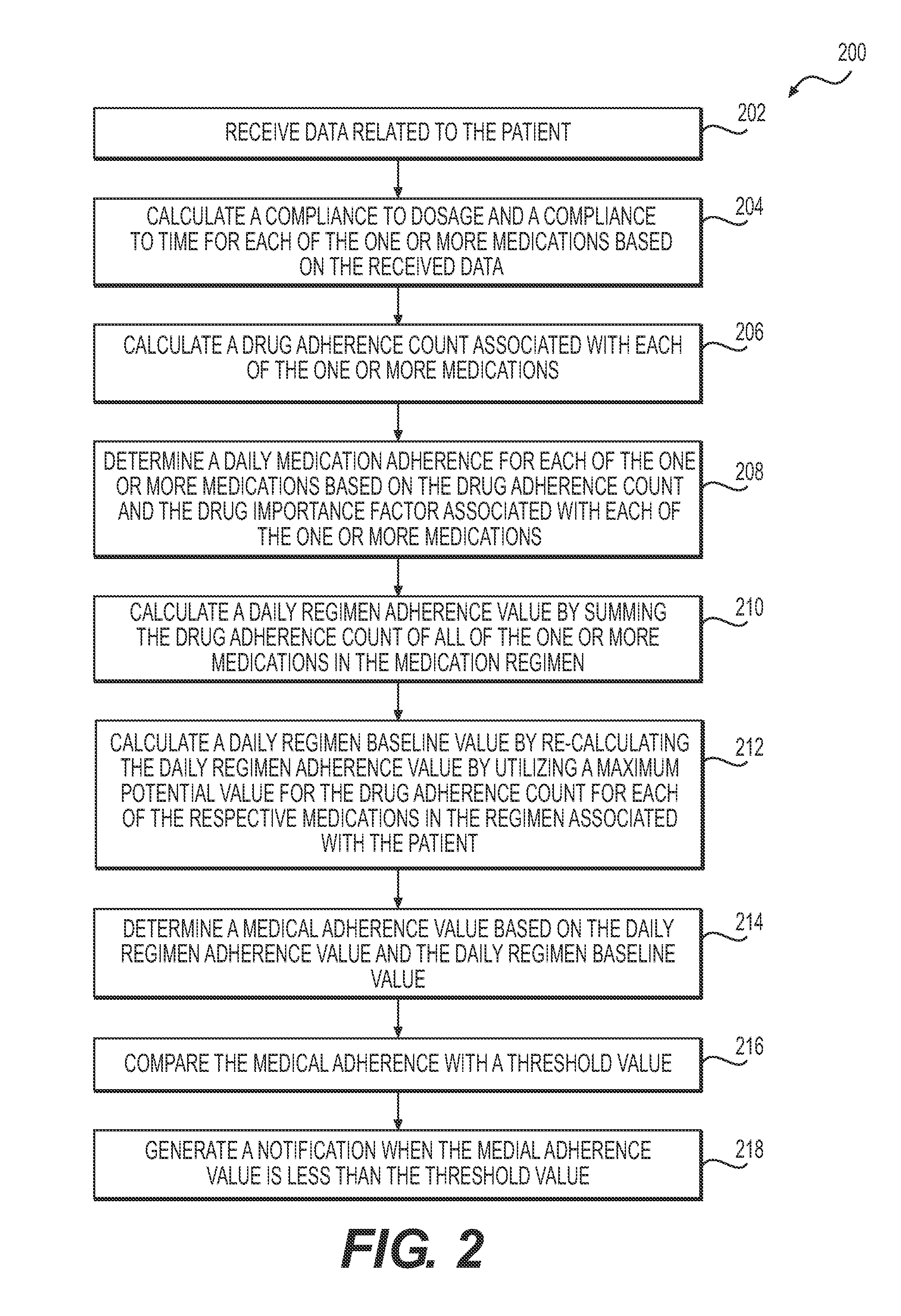
Systems and methods for managing medication adherence
Systems and methods are provided for managing medical adherence. An exemplary method may include managing medical adherence utilizing data aggregating and processing to determine impact on a user's health based on their behavior related to prescribed medication. The method may entail utilizing data related to a medication regimen and patient behavior to determine a patient's compliance to the regimen in terms of dosage and time. These values may be utilized to calculate a medical adherence value representing a patient's adherence to a prescribed regimen. Responsive to determining low medical adherence, a notification may be generated which may result in an intervention with the patient.
Owner:WELLDOC INC
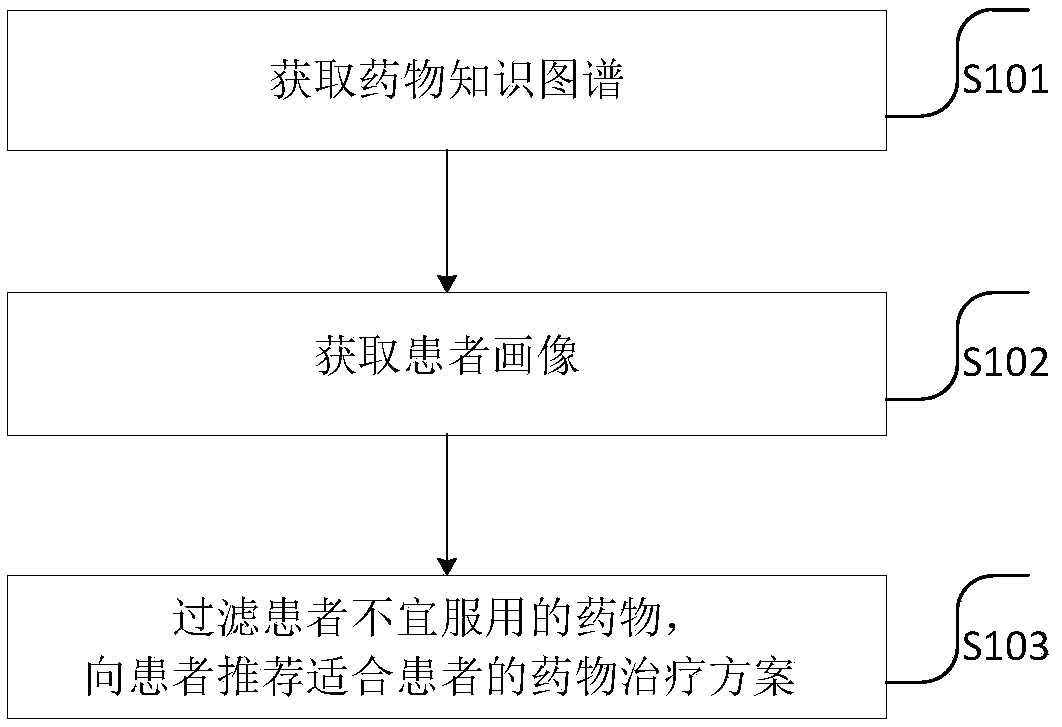
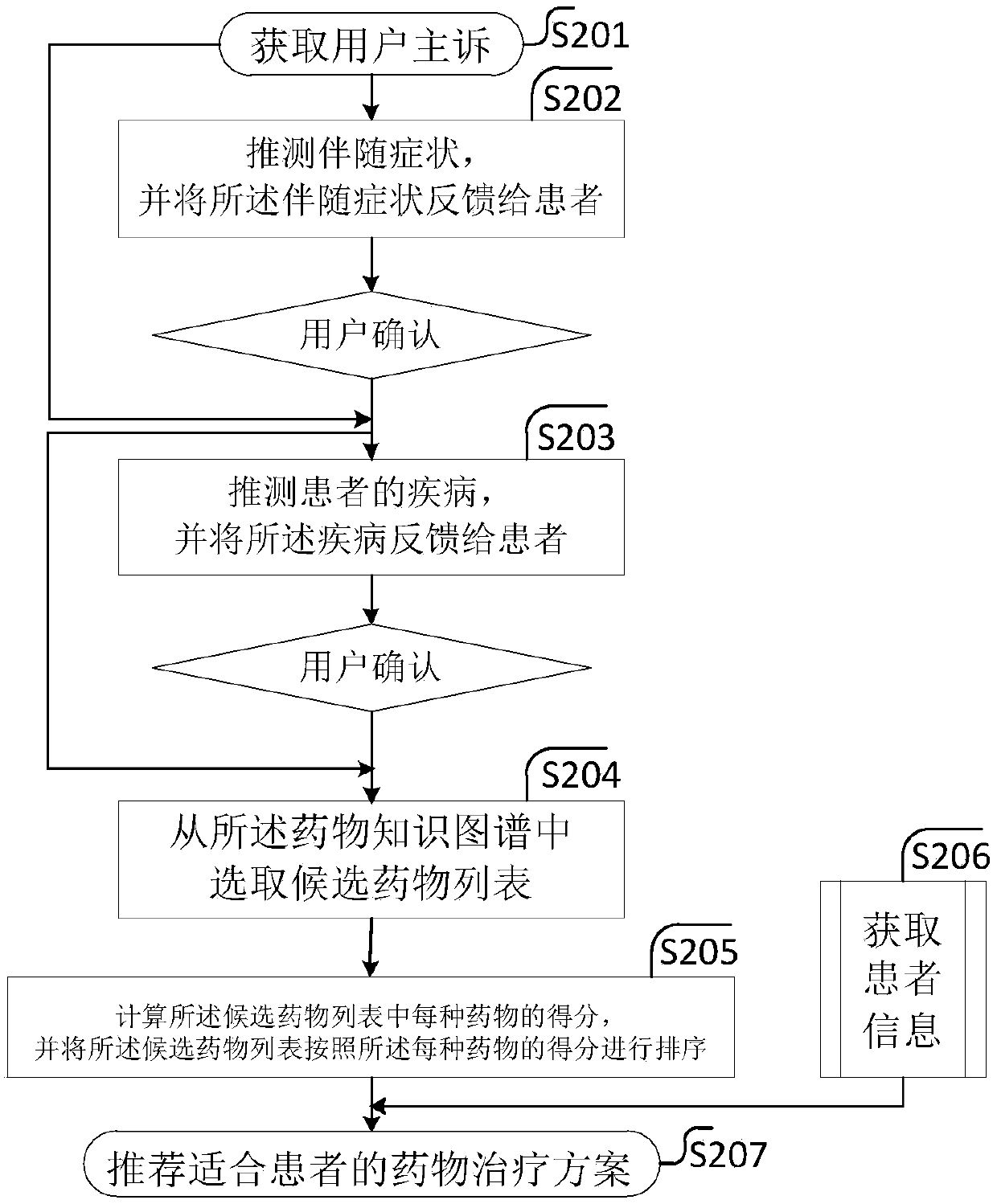
Drug recommendation method
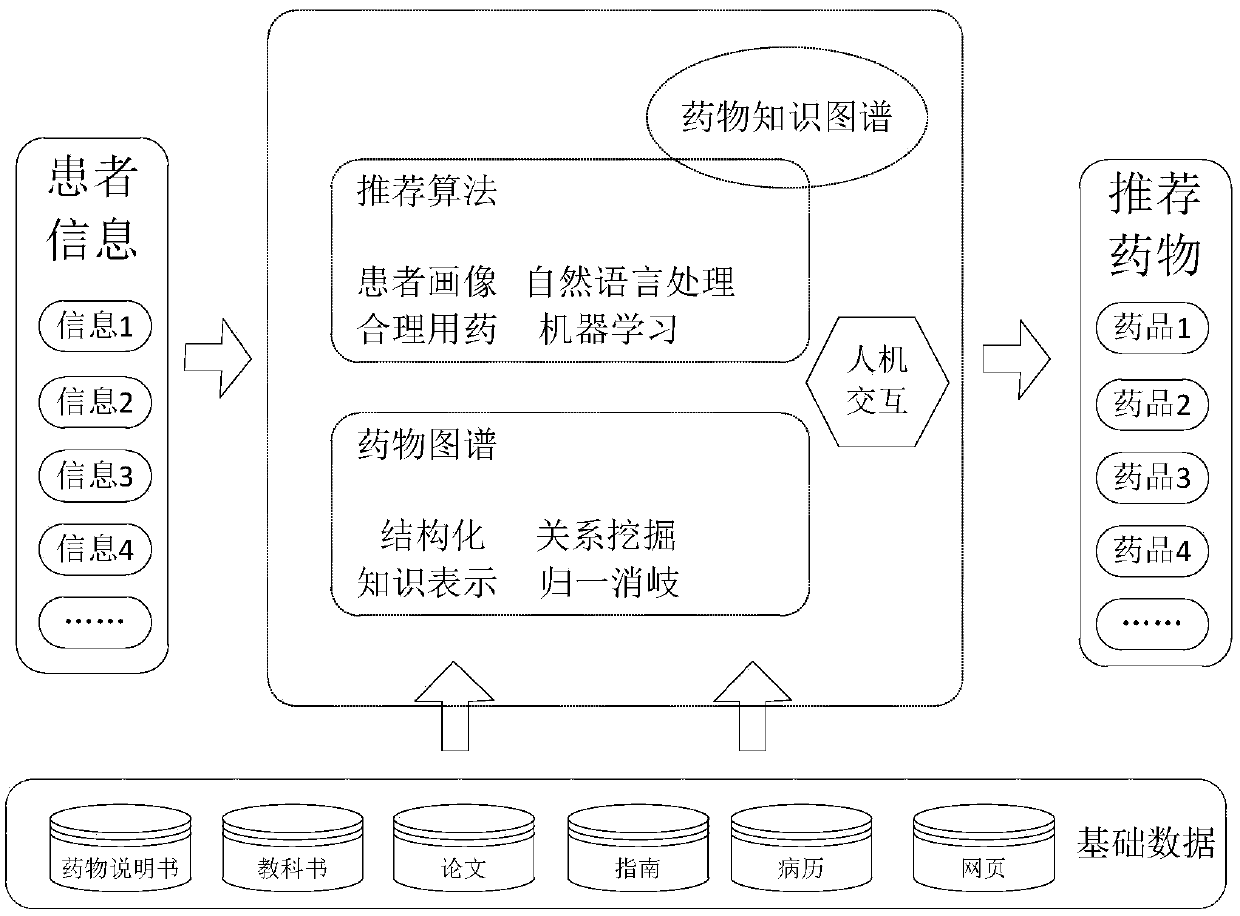
PendingCN109102855ASolve the problem of recommending only a large list of drug candidates with fewAvoid situations where users are contraindicatedMedical data miningDrug and medicationsMedical recordClinical psychology
The invention provides a drug recommendation method. The method comprises steps that the natural language processing technology is utilized to construct a drug knowledge map; the medical record structure technology is utilized to construct a patient portrait based on the patient information, drugs that the patient should not take are filtered according to the drug knowledge map, the patient portrait and the preset recommendation algorithm, and a patient-friendly medication regimen is recommended to a patient. The method is advantaged in that problems that the user information obtained throughthe word-matching-based search method is too small and only a relatively large drug candidate list is recommended are solved, the drugs that the patient should not take are filtered according to the patient portrait and the preset recommendation algorithm, the patient can be recommended for the drugs that are more suitable for the patient, and the situation that a recommended drug is just a contraindication to the user is avoided.
Owner:北京左医科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com