Patents
Literature
681 results about "Clinical psychology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clinical psychology is an integration of science, theory, and clinical knowledge for the purpose of understanding, preventing, and relieving psychologically-based distress or dysfunction and to promote subjective well-being and personal development. Central to its practice are psychological assessment, clinical formulation, and psychotherapy, although clinical psychologists also engage in research, teaching, consultation, forensic testimony, and program development and administration. In many countries, clinical psychology is a regulated mental health profession.
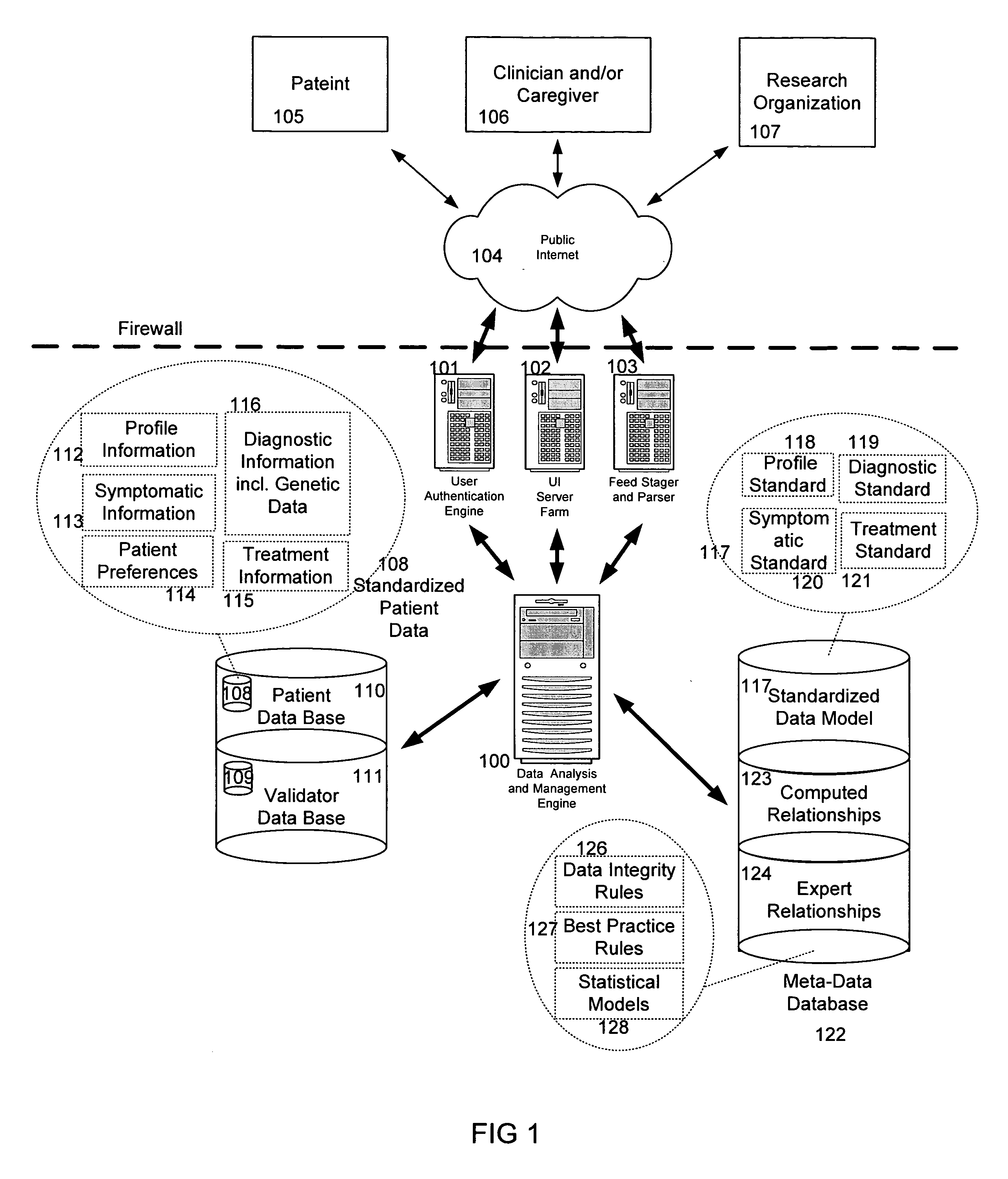
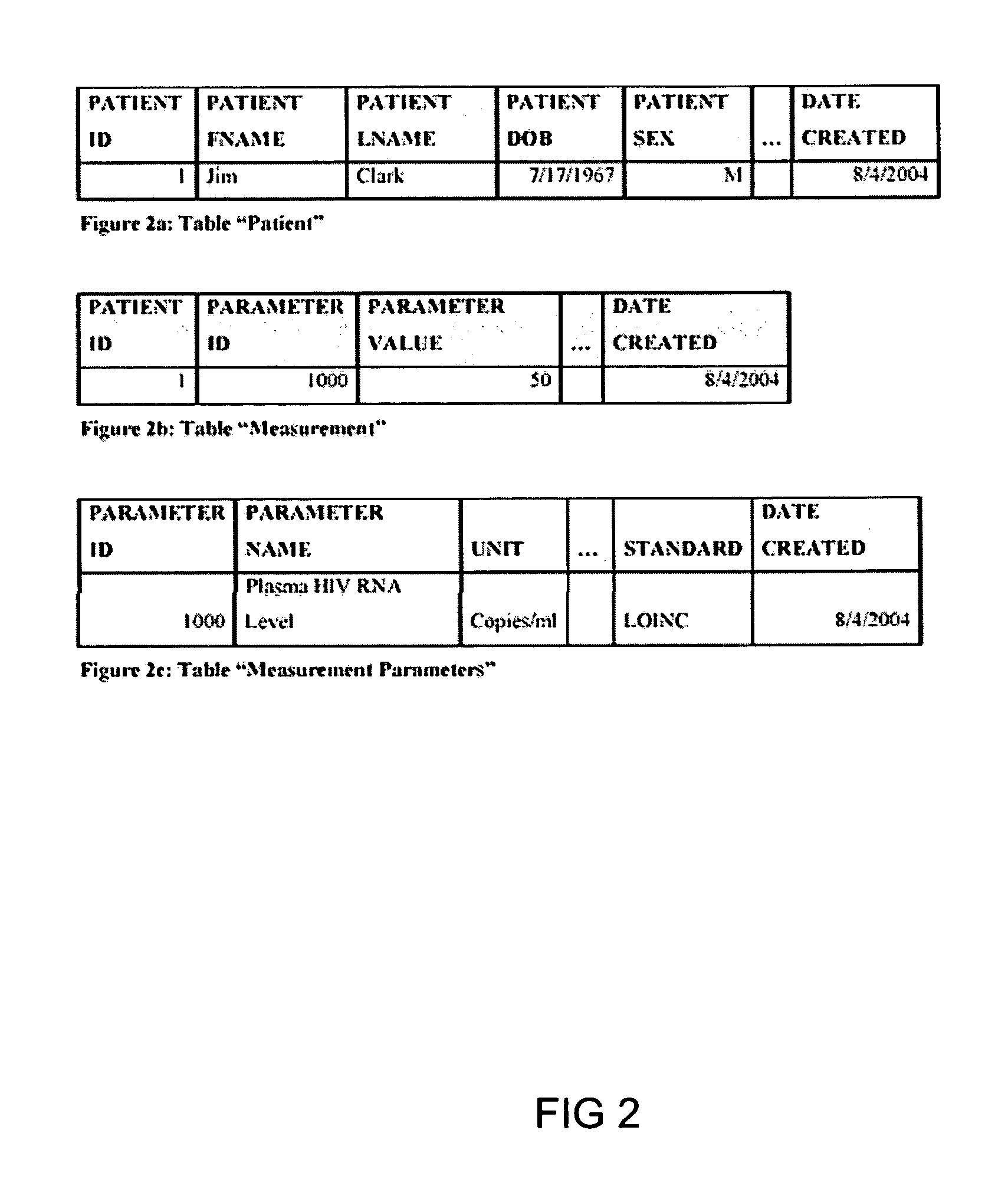
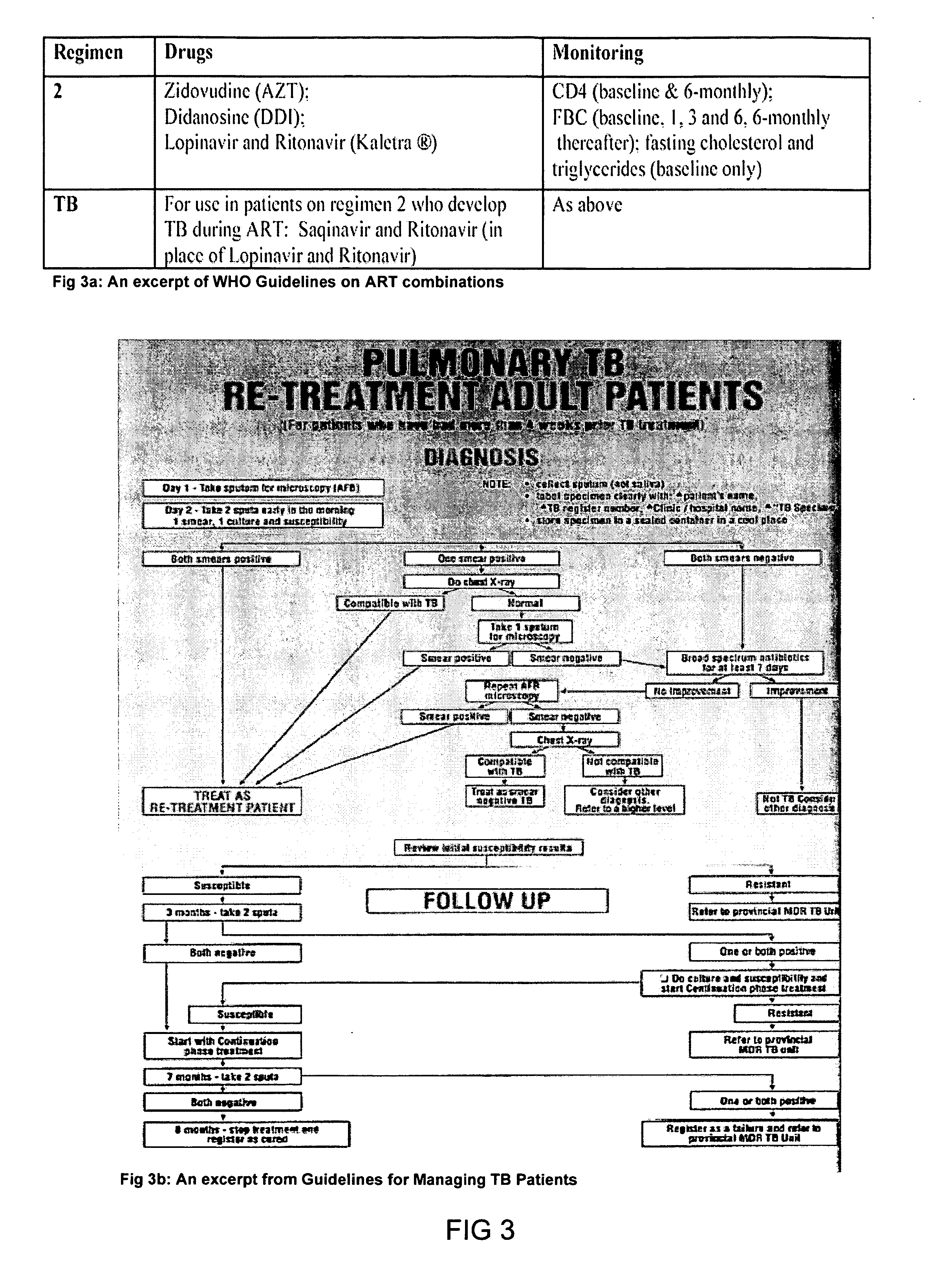
System and method for improving clinical decisions by aggregating, validating and analysing genetic and phenotypic data
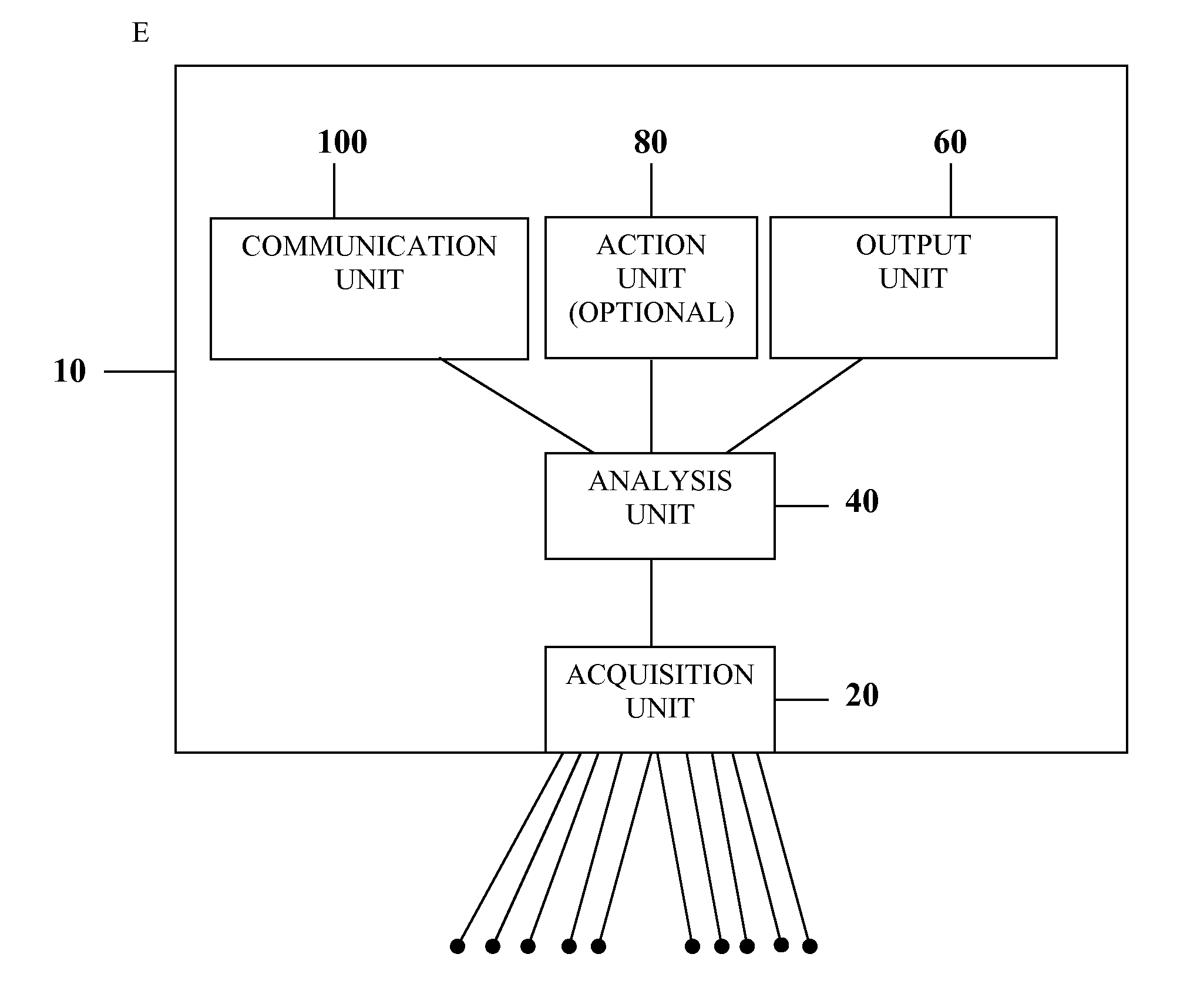
The information management system disclosed enables caregivers to make better decisions, faster, using aggregated genetic and phenotypic data. The system enables the integration, validation and analysis of genetic, phenotypic and clinical data from multiple subjects who may be at distributed facilities. A standardized data model stores a range of patient data in standardized data classes that encompass patient profile information, patient symptomatic information, patient treatment information, and patient diagnostic information including genetic information. Data from other systems is converted into the format of the standardized data classes using a data parser, or cartridge, specifically tailored to the source system. Relationships exist between standardized data classes that are based on expert rules and statistical models. The relationships are used both to validate new data, and to predict phenotypic outcomes based on available data. The prediction may relate to a clinical outcome in response to a proposed intervention by a caregiver. The statistical models may be inhaled into the system from electronic publications that define statistical models and methods for training those models, according to a standardized template. Methods are described for selecting, creating and training the statistical models to operate on genetic, phenotypic and clinical data, in particular for underdetermined data sets that are typical of genetic information. The disclosure also describes how security of the data is maintained by means of a robust security architecture, and robust user authentication such as biometric authentication, combined with application-level and data-level access privileges.
Owner:NATERA
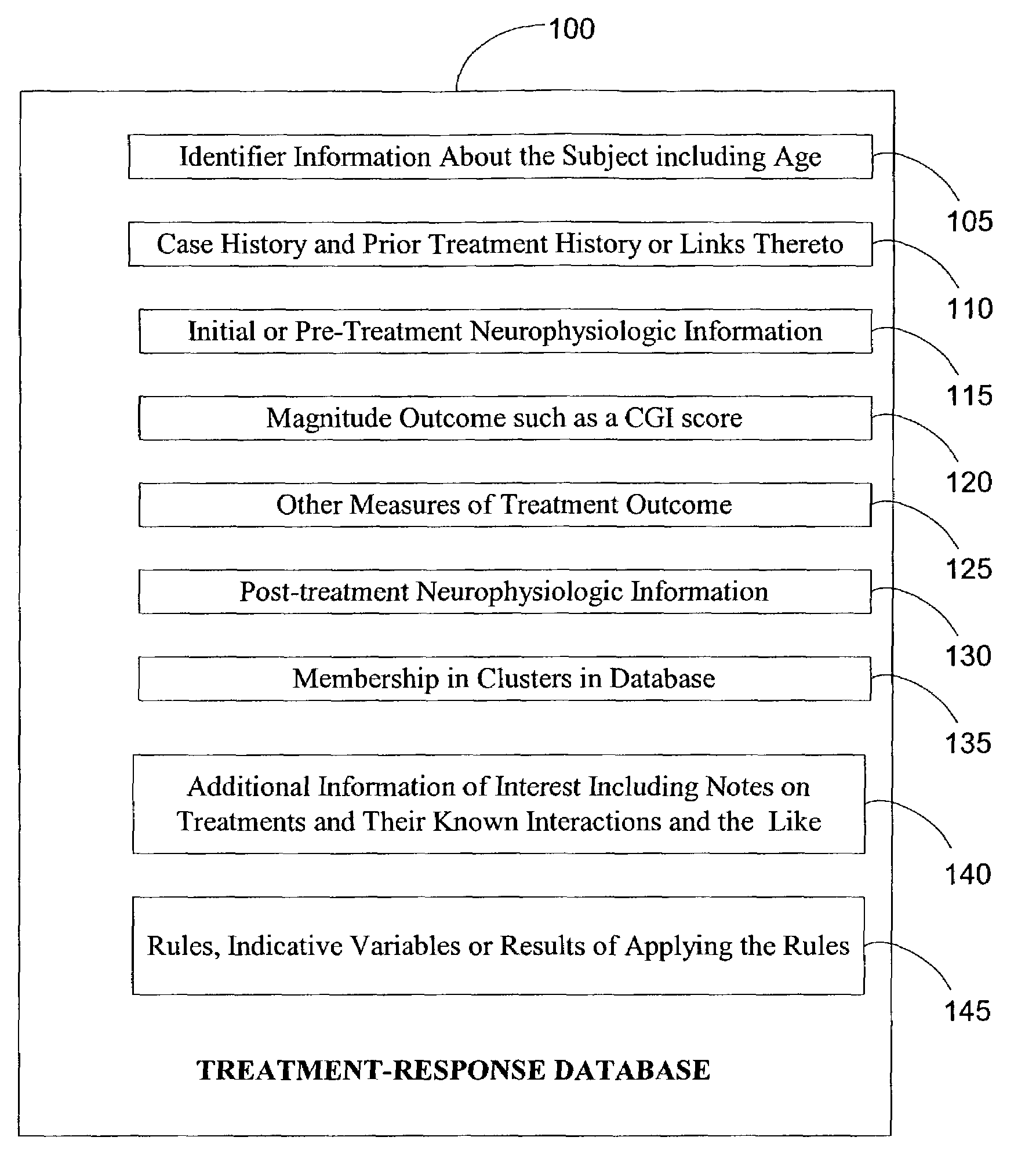
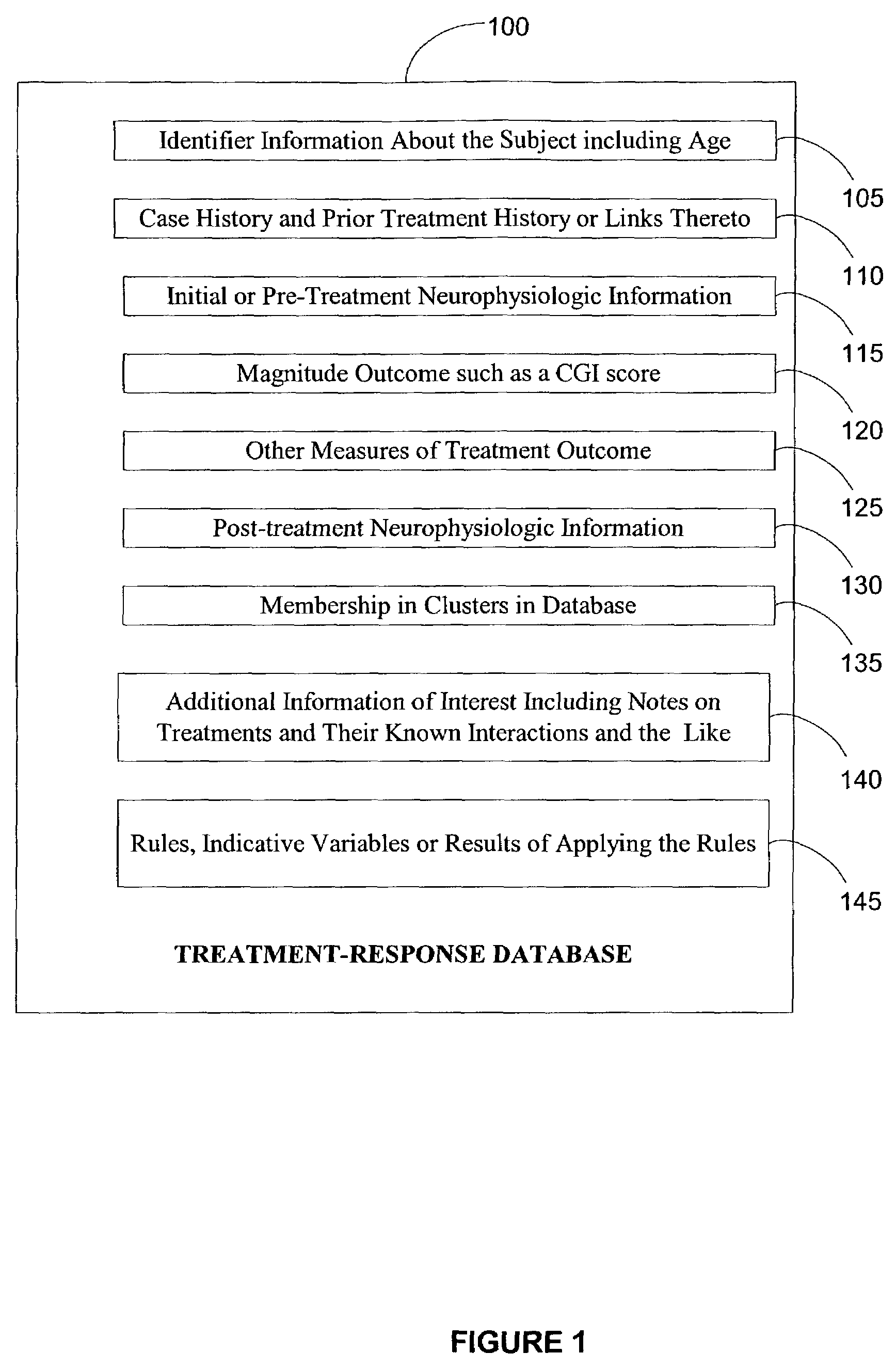
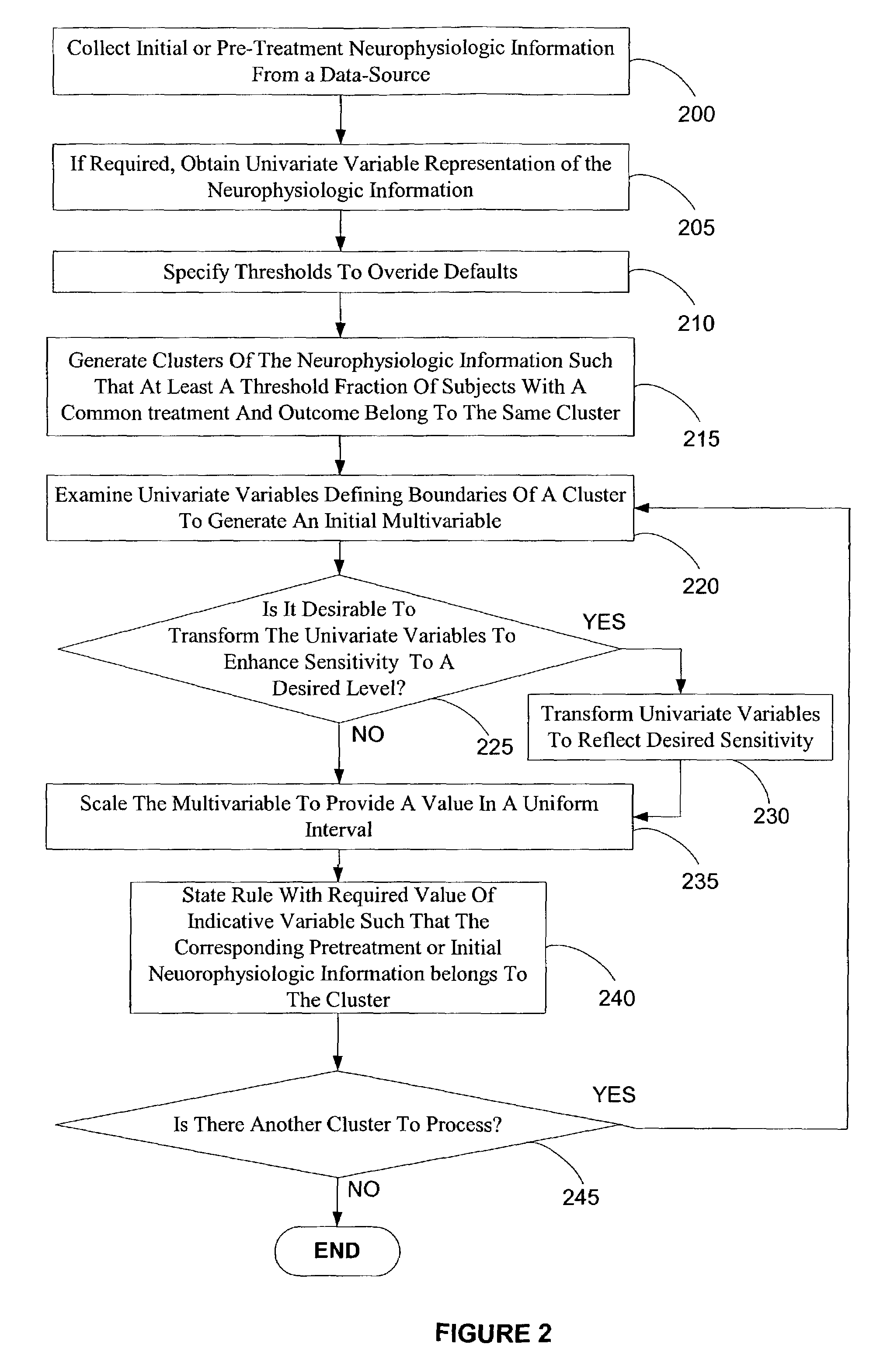
Electroencephalography based systems and methods for selecting therapies and predicting outcomes
InactiveUS7177675B2Quality improvementHigh-quality informationElectroencephalographyMedical data miningMode of actionClinical psychology
A method and system for utilizing neurophysiologic information obtained by techniques such as quantitative electroencephalography (QEEG), electrode recordings, MRI in appropriately matching patients with therapeutic entities is disclosed. The present invention enables utilization of neurophysiologic information, notwithstanding its weak correlation with extant diagnostic schemes for mental disorders, for safer and expeditious treatment for mental disorders, discovering new applications for therapeutic entities, improved testing of candidate therapeutic entities, inferring the presence or absence of a desirable response to a treatment, and deducing the mode of action of one or more therapeutic entities. In particular, methods for effectively comparing neurophysiologic information relative to a reference set are disclosed along with database-based tools for deducing therapeutic entity actions on particular patients such that these tools are readily accessible to remote users.
Owner:CNS RESPONSE
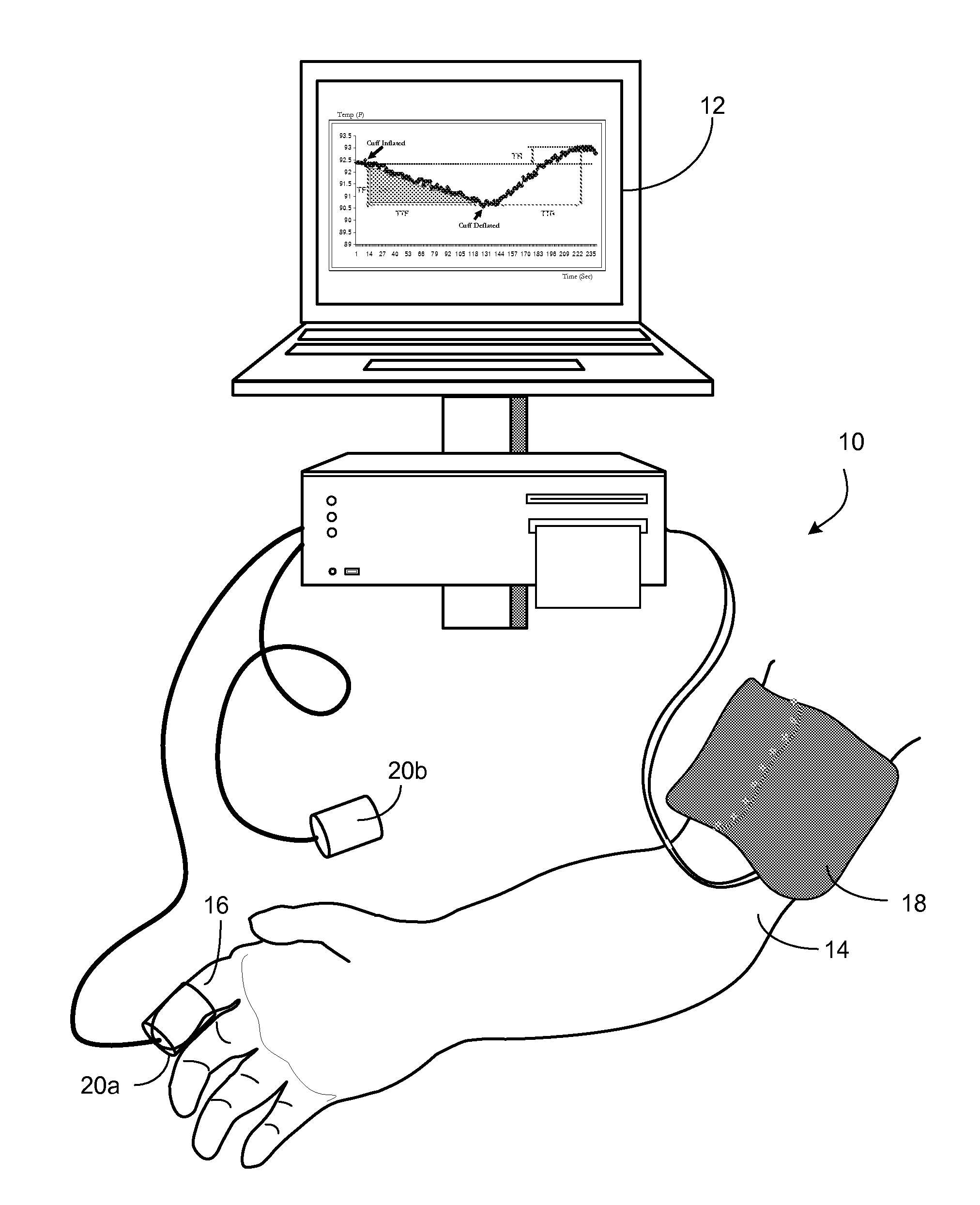
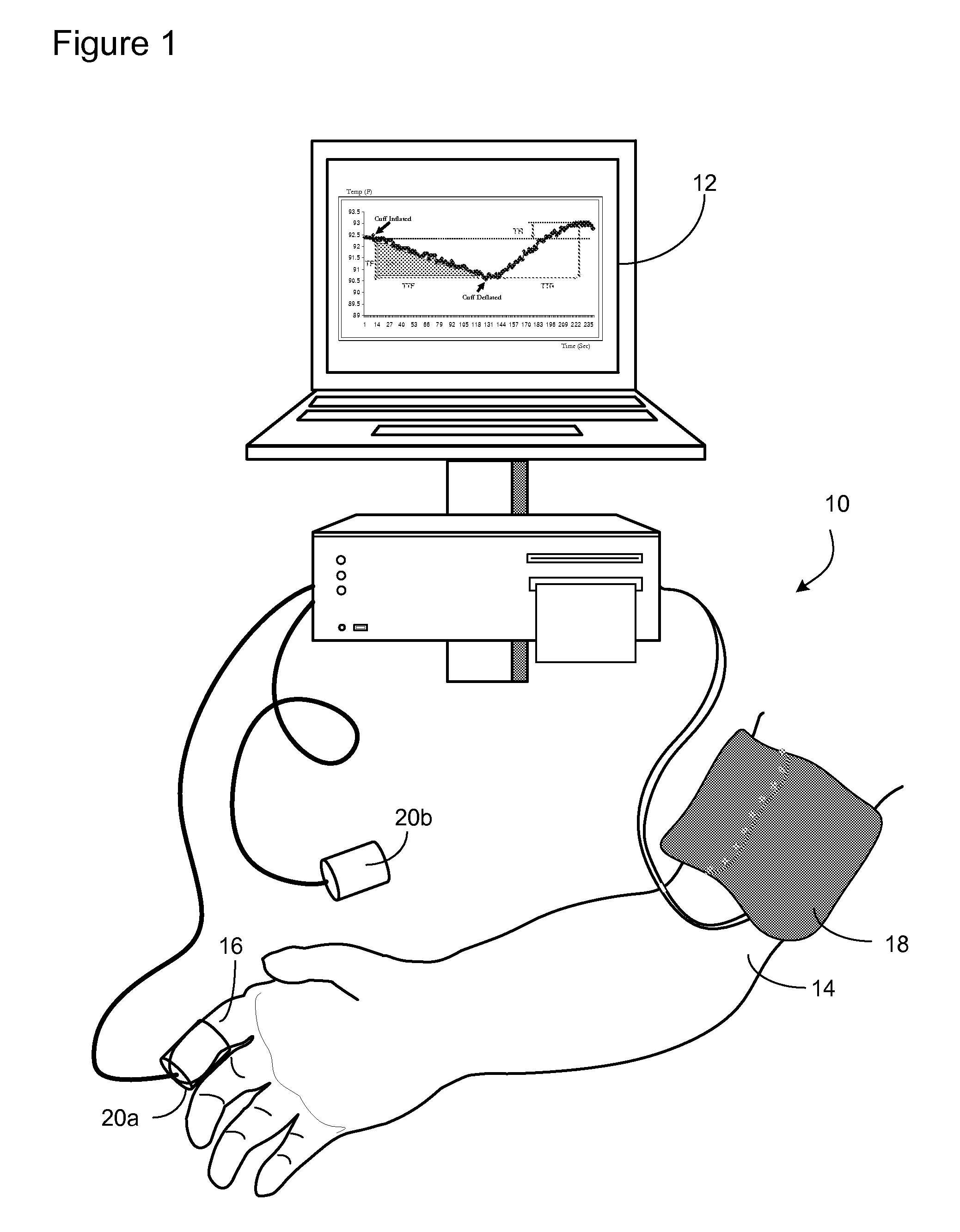
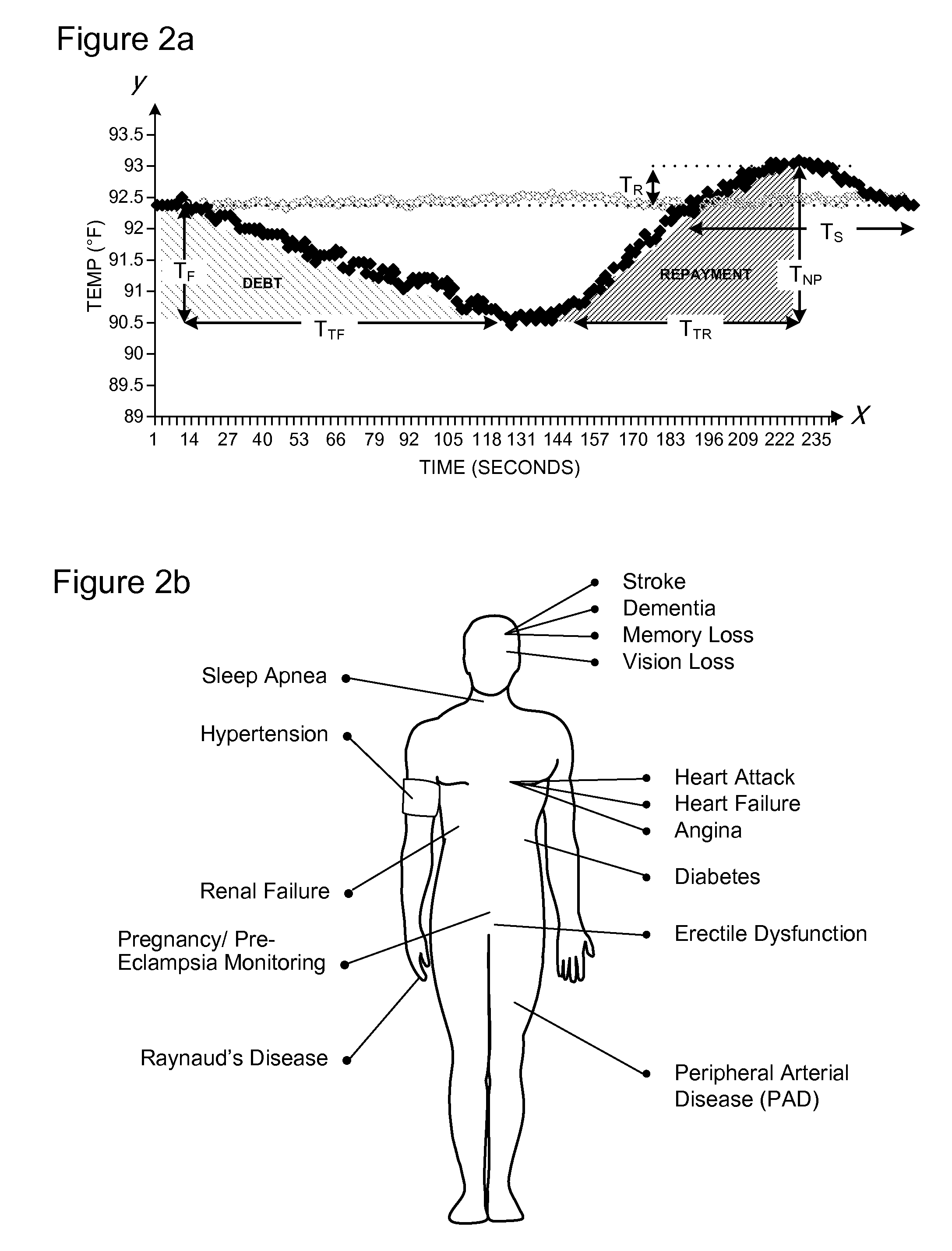
Methods and Apparatus for Profiling Cardiovascular Vulnerability to Mental Stress
Methods and apparatus are provided for determining individual responses to induced and actual mental stress and for identifying individuals susceptible to detrimental effects of mental stress on the cardiovascular system. The subjects may be at risk for chronic effects of mental stress by virtue of synergistic effects of mental stress and cardiovascular (CV) disease risk factors) or may be at risk, or vulnerable to, acute effects of mental stress by virtue of synergistic effects of mental stress and underlying hidden coronary atherosclerosis. The invention further provides methods and apparatus for assessing vascular reactivity in individuals under ambulatory conditions and relating mental stress responses to vascular reactivity.
Owner:ENDOTHELIX
Method for diagnosing, preventing, and treating neurological diseases
ActiveUS20120252775A1High activityLow systemic concentrationBiocideMicrobiological testing/measurementDiseaseClinical psychology
The invention provides for methods of treating autism associated with Desulfovibrio overgrowth in the gastrointestinal tract of a patient, said method comprising administering to the patient suffering from said autism a treatment course of aztreonam in an amount effective to treat autism in the patient, thereby treating autism.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
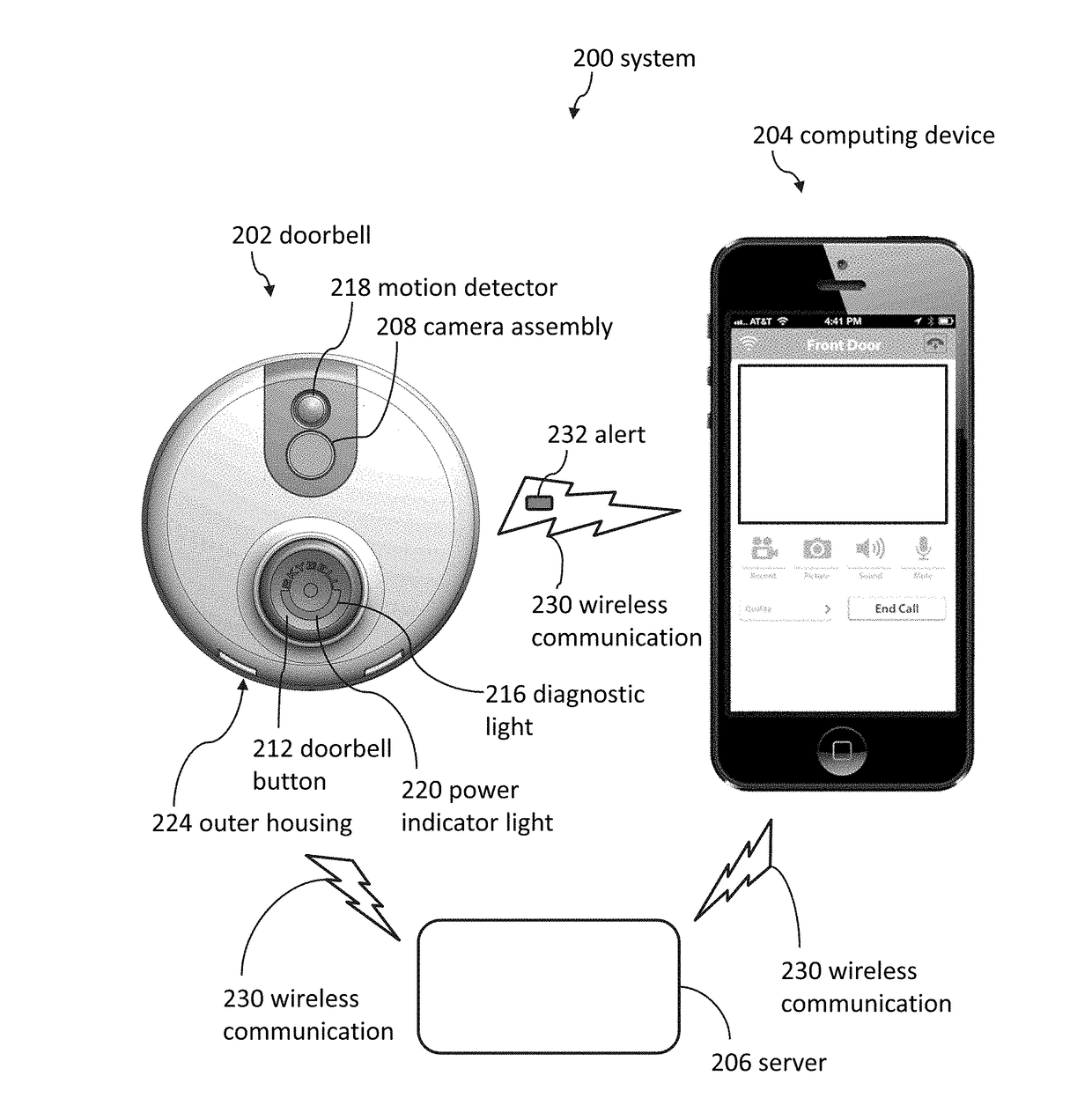
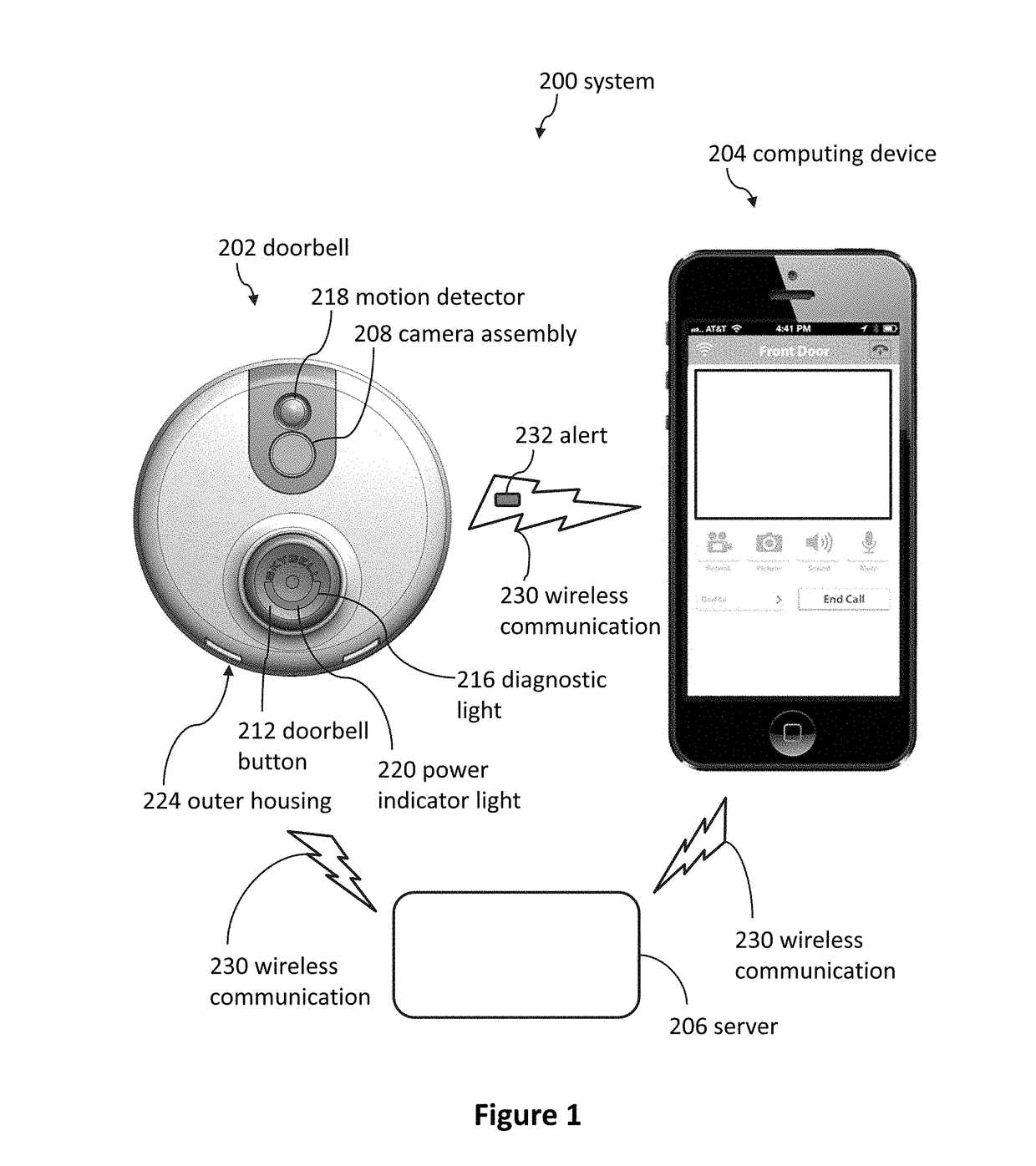
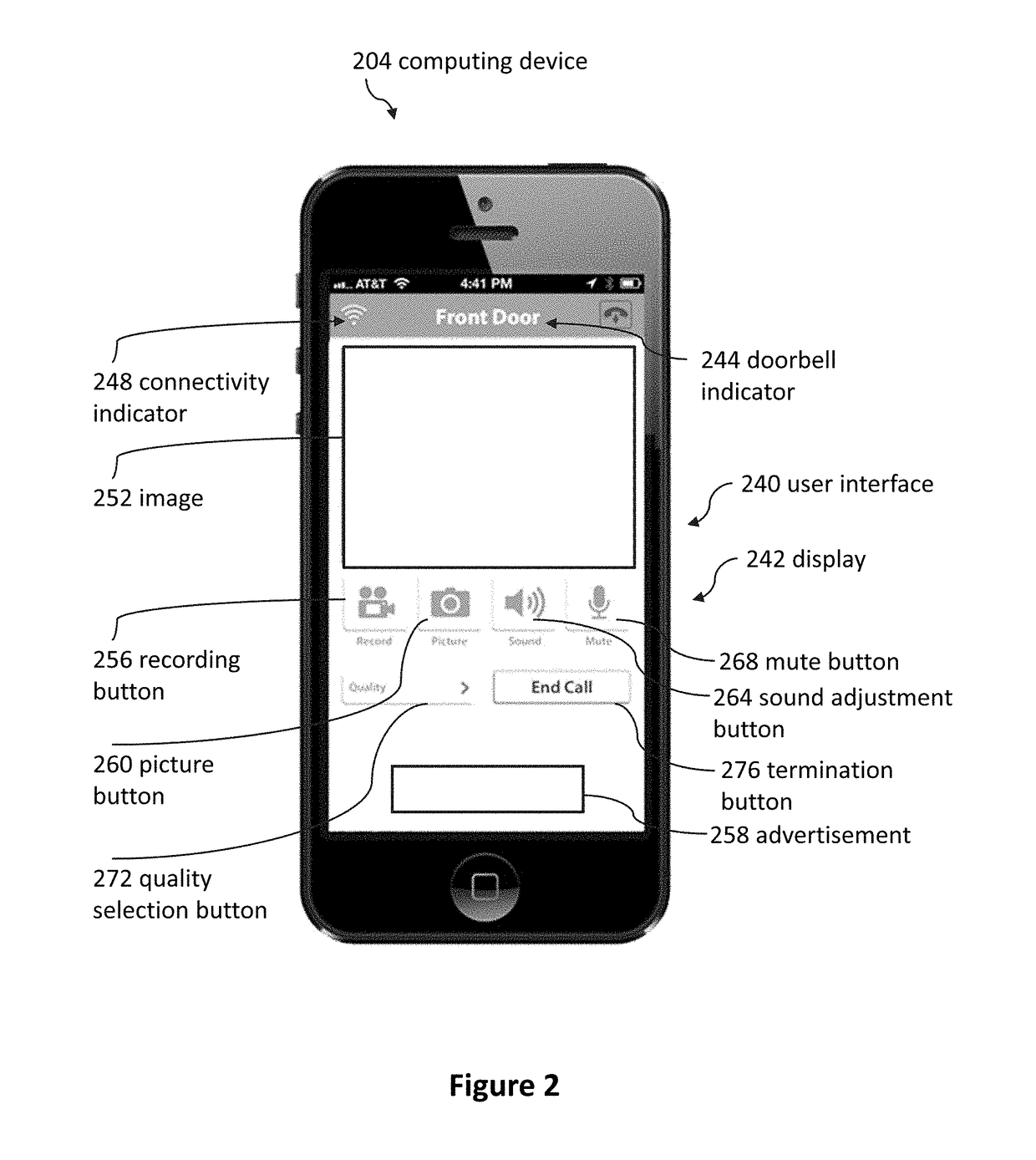
Doorbell chime systems and methods
ActiveUS9786133B2Lower the volumeTelephonic communicationSignalling system detailsDoorbellClinical psychology
Methods for using a doorbell chime system can include detecting, by a sleep detection system, that a person is asleep; receiving a first signal, by the doorbell or the doorbell chime, in response to the sleep detection system detecting that the person is asleep; and blocking the doorbell chime from emitting a first notification sound in response to detecting, by the sleep detection system, that the person is asleep.
Owner:SKYBELL TECH IP LLC
Motivation and enhancement of physical and mental exercise, rehabilitation, health and social interaction
InactiveUS20090233769A1Simple meansSimplified machine vision processingPhysical therapies and activitiesComputer controlPhysical disorderClinical psychology
The disclosed invention primarily relates to methods for assisting and motivating persons with respect to various exercise and rehabilitation regimens they might undertake, mentally as well as physically. The invention also has potential application to for diagnosis and / or treatment of certain mental and physical disorders, and in other situations where a form of companionship may be provided the user. In addition the invention herein provides an enjoyable means of social interaction with others providing further motivation for physical and mental activity represented.
Owner:MOTION GAMES
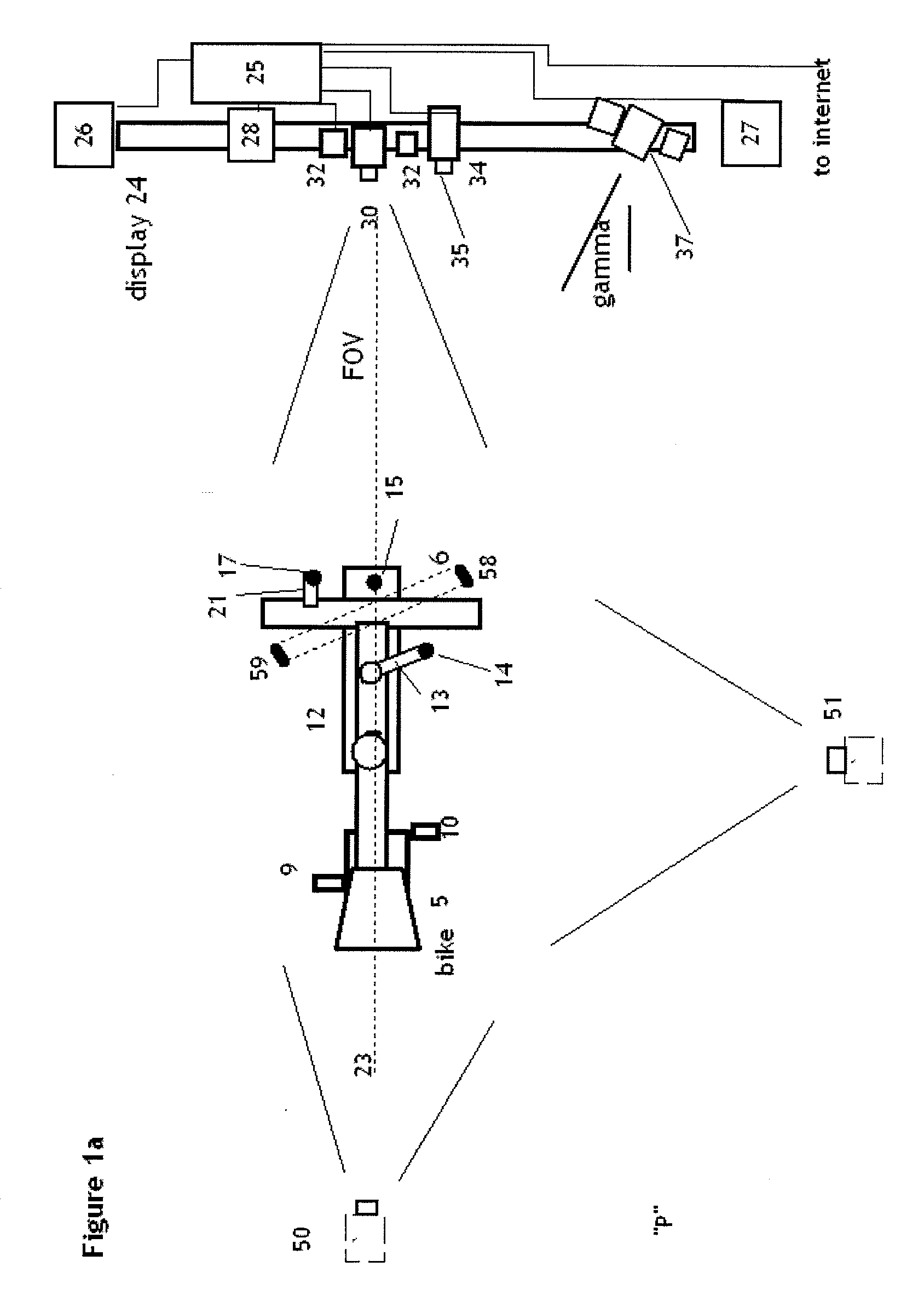
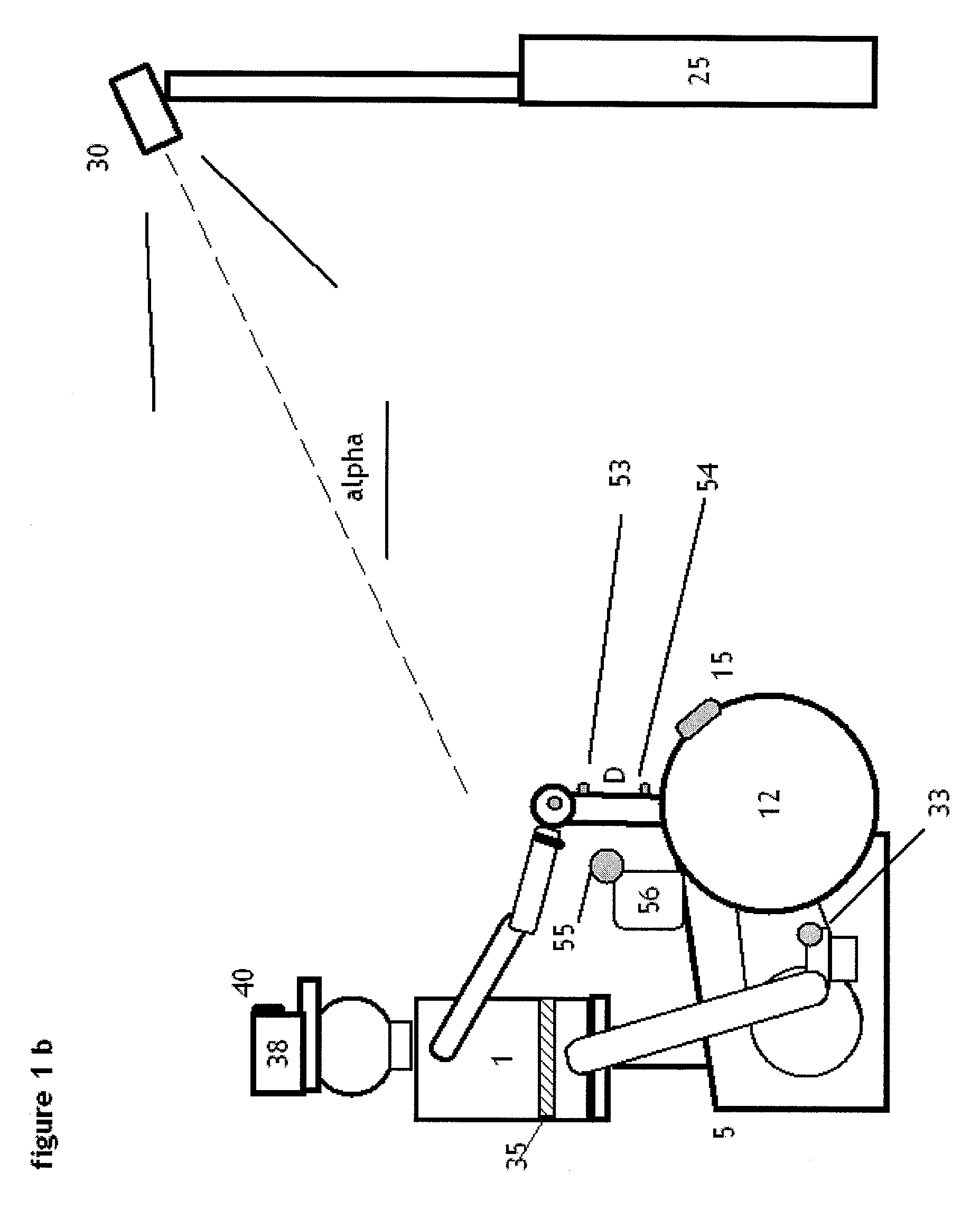
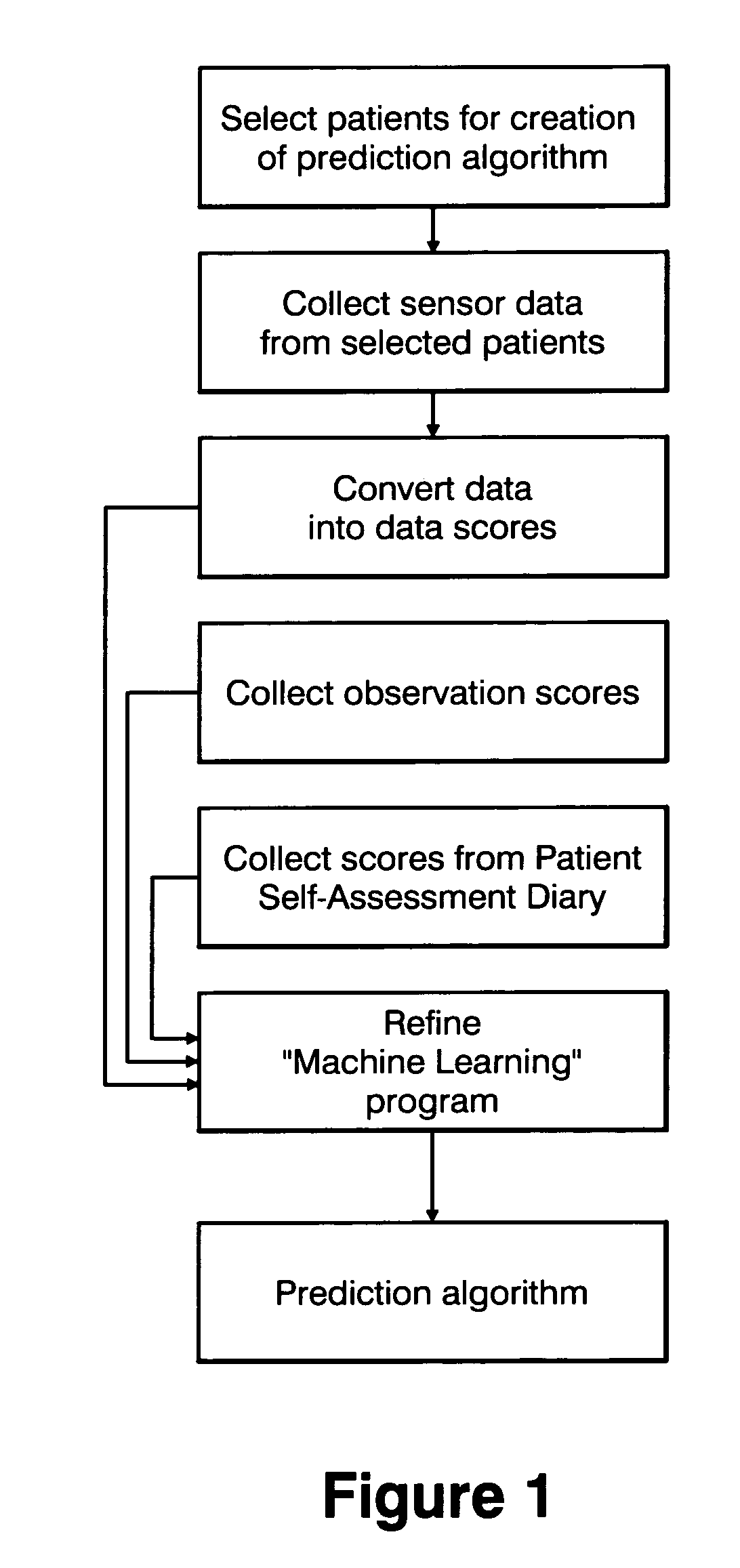
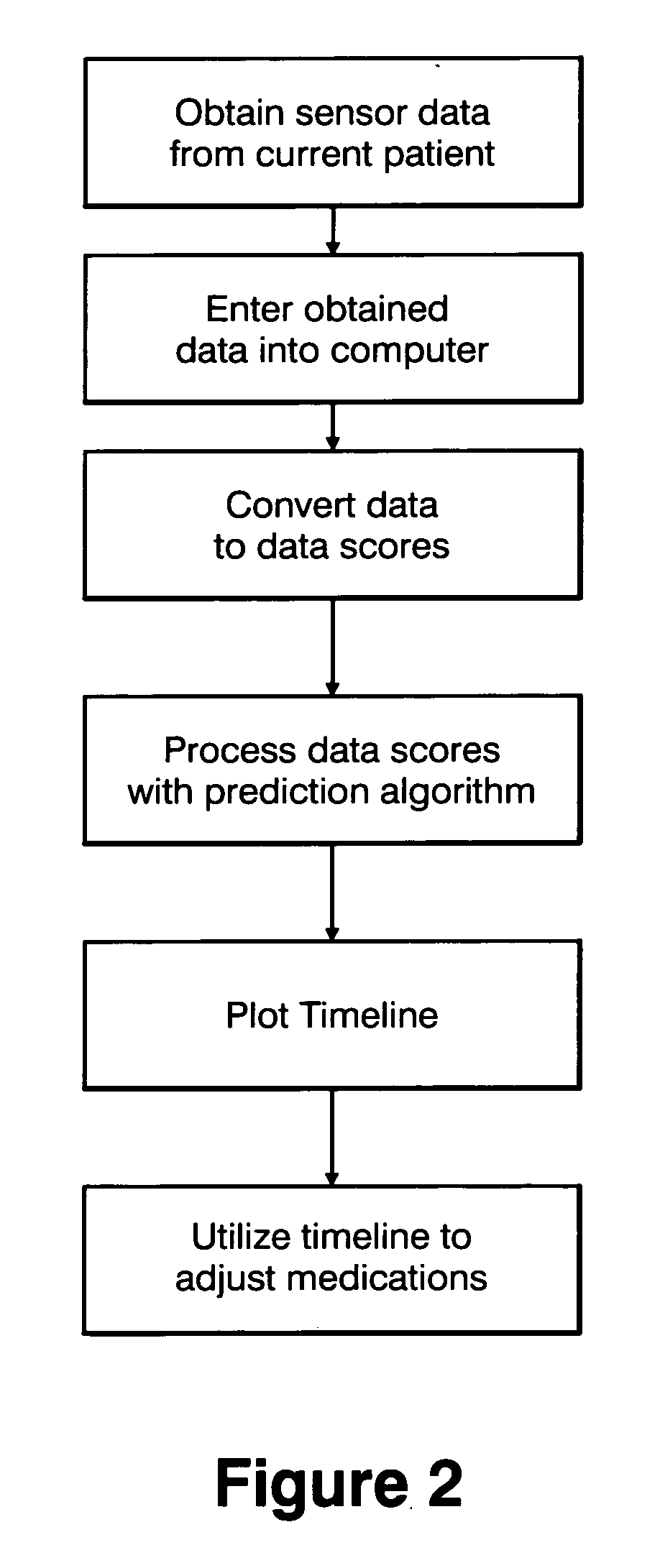
Method and apparatus for classification of movement states in Parkinson's disease
InactiveUS20050234309A1Easy to predictInertial sensorsMedical automated diagnosisAccelerometerClinical psychology
For Parkinson's patients to function at their best, their medications need to be optimally adjusted to the diurnal variation of symptoms. For this to occur, it is important for the managing clinician to have an accurate picture of how the patient's bradykinesia / hypokinesia and dyskinesia and the patient's perception of movement state fluctuate throughout the normal daily activities. The present invention uses wearable accelerometers coupled with computer implemented learning and statistical analysis techniques in order to classify the movement states of Parkinson's patients and to provide a timeline of how the patients fluctuate throughout the day.
Owner:PARK KINESIS INSTR
Computer-assisted means for assessing lifestyle risk factors
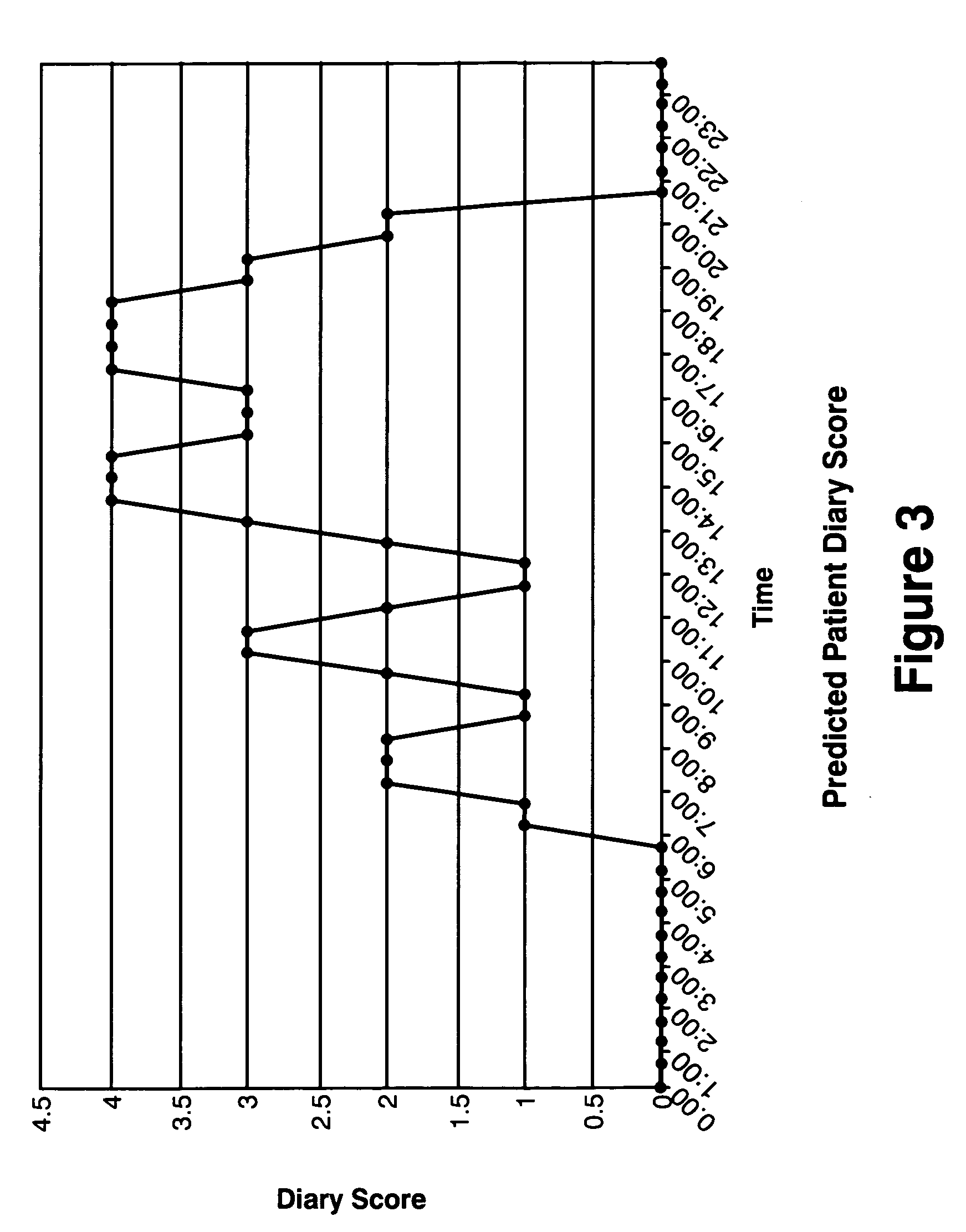
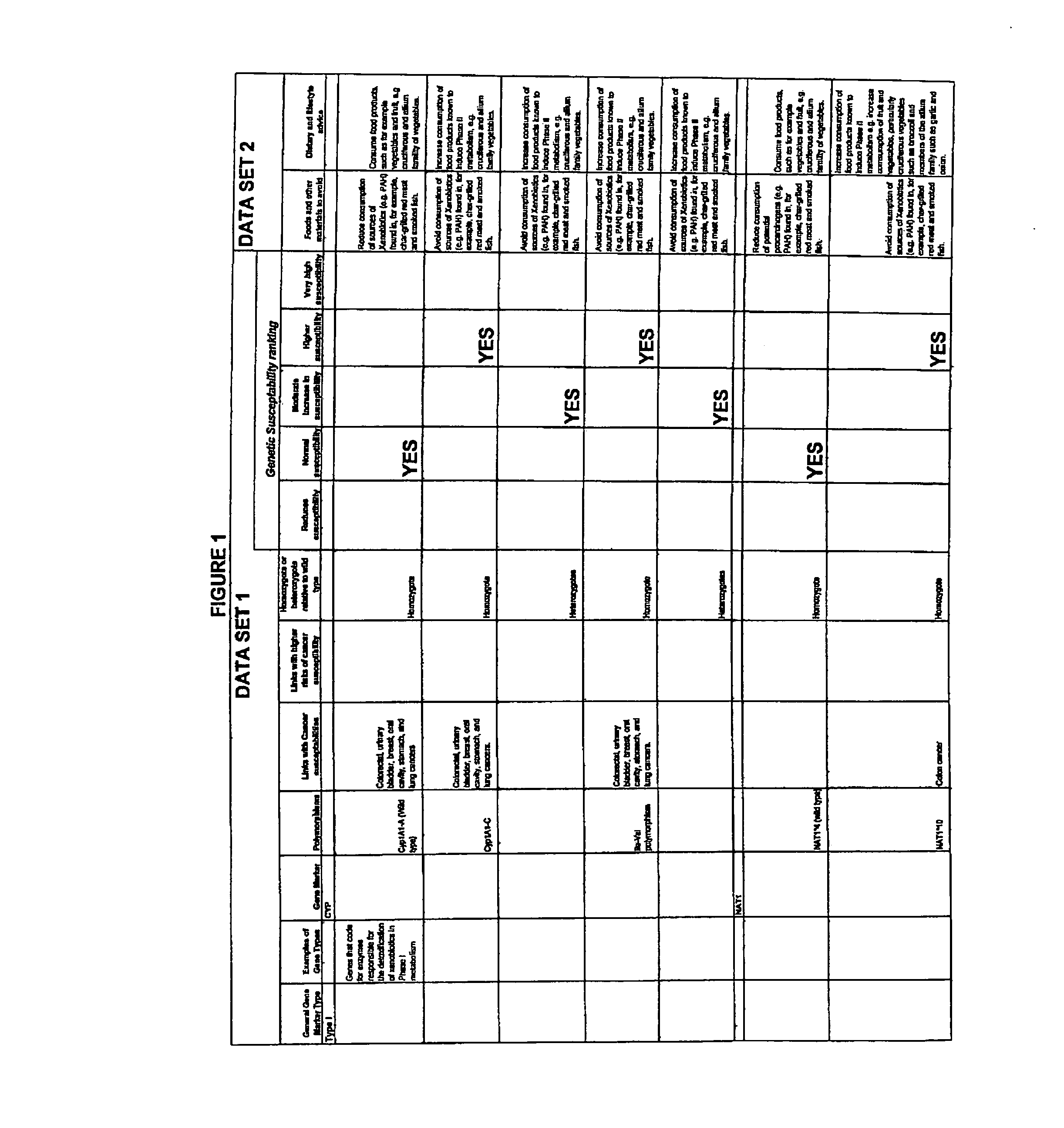
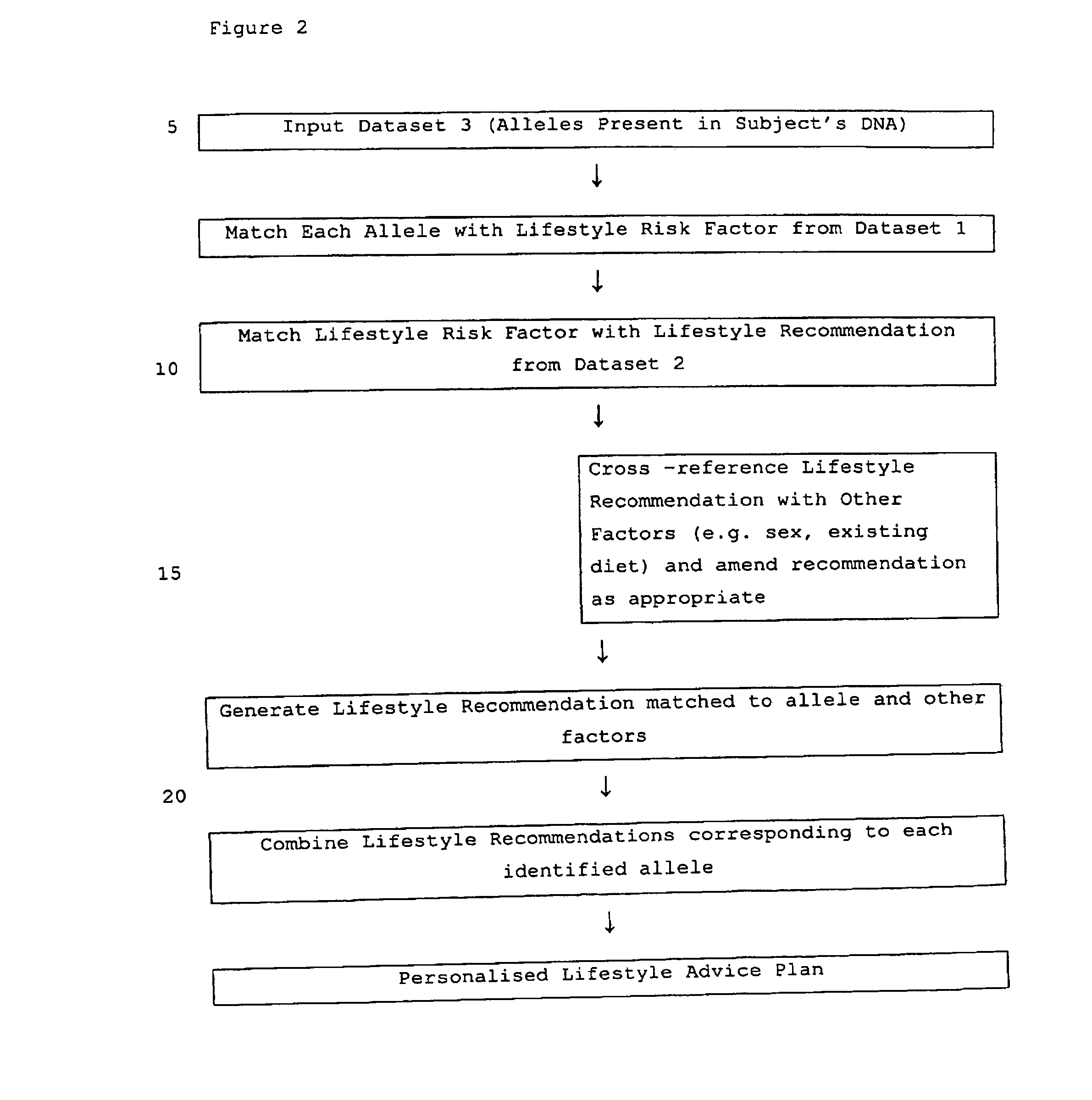
InactiveUS7054758B2Reduce disease riskProtect healthDigital data processing detailsBiostatisticsPersonalizationData set
The present invention relates to methods of assessing disease susceptibility associated with dietary and lifestyle risk factors. The invention provides for analysis of alleles at loci of genes associated with lifestyle risk factors, and the disease susceptibility profile of an individual is determined by reference to datasets which further match the risk factor with lifestyle recommendations in order to produce a personalized lifestyle advice plan.
Owner:BODYSYNC
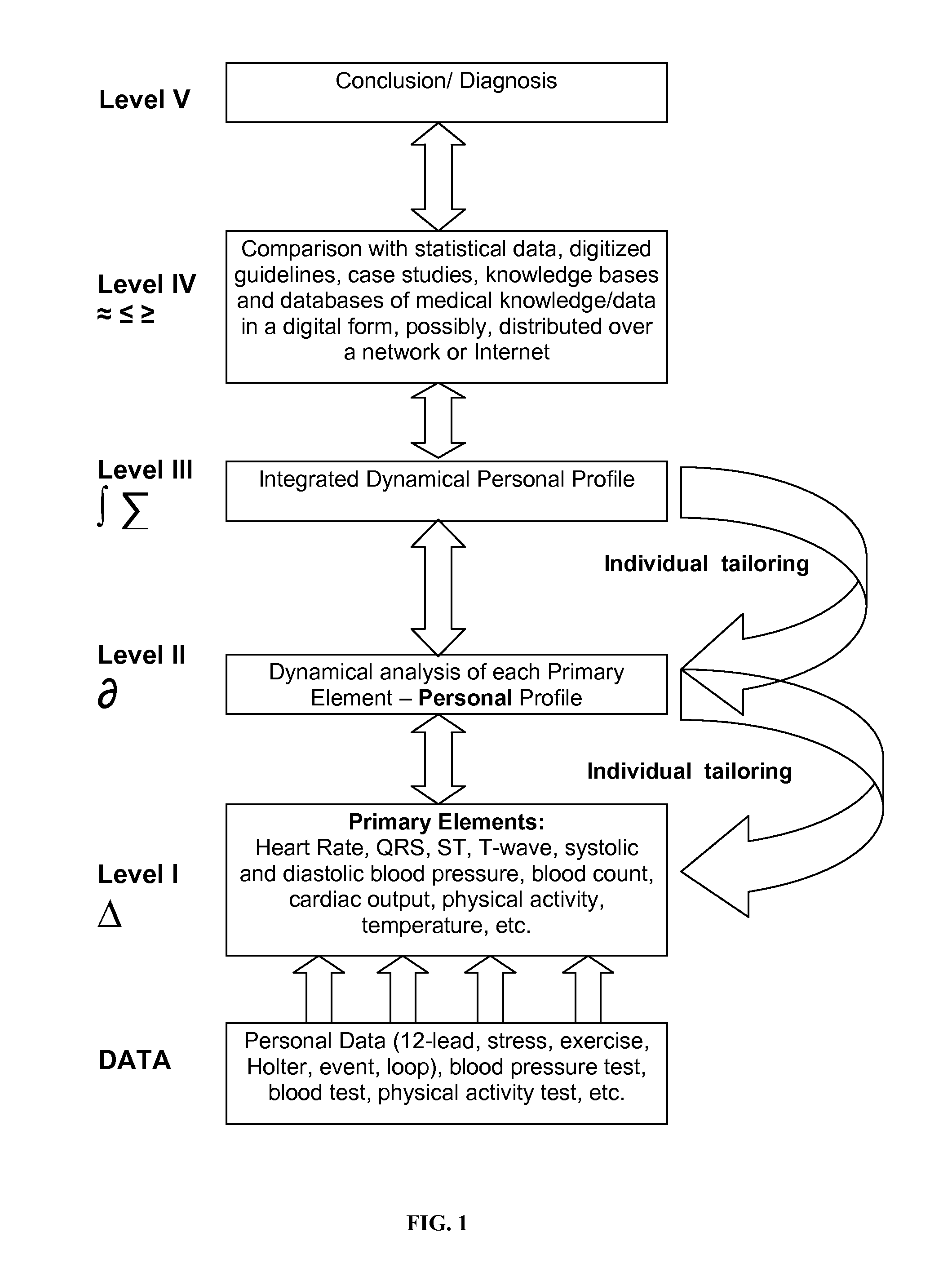
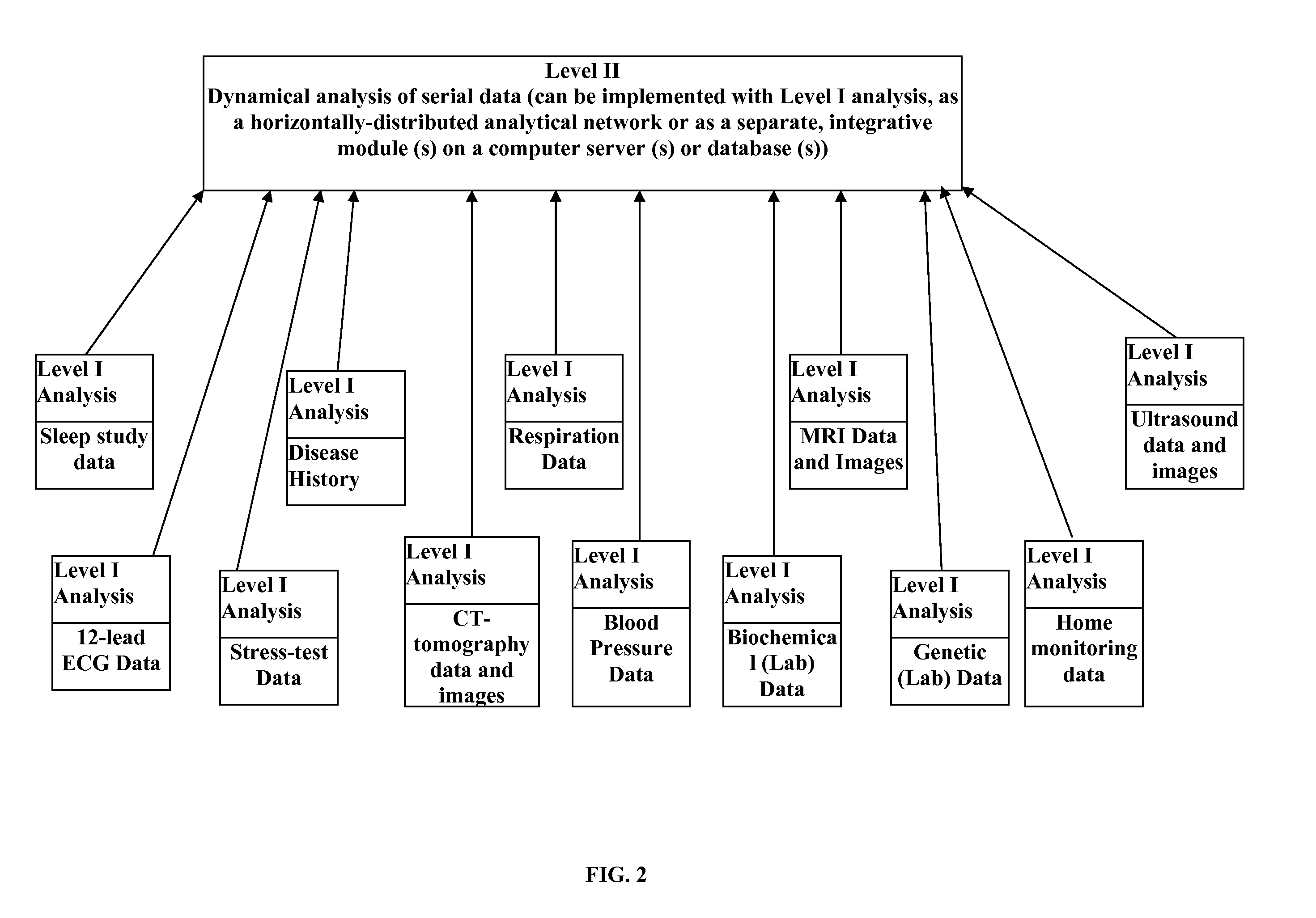
Personalized Monitoring and Healthcare Information Management Using Physiological Basis Functions
InactiveUS20110004110A1Accurate trackingAccurate classificationMedical data miningMedical automated diagnosisPersonalizationSide effect
Analysis of individual's serial changes, also referred to as the physiological, pathophysiological, medical or health dynamics, is the backbone of medical diagnosis, monitoring and patient healthcare management. However, such an analysis is complicated by enormous intra-individual and inter-individual variability. To address this problem, a novel serial-analysis method and system based on the concept of personalized basis functions (PBFs) is disclosed. Due to more accurate reference information provided by the PBFs, individual's changes associated with specific physiological activity or a sequence, transition or combination of activities (for example, a transition from sleep to wakefulness and transition from rest to exercise) can be monitored more accurately. Hence, subtle but clinically important changes can be detected earlier than using other methods. A library of individual's PBFs and their transition probabilities (which can be described by Hidden Markov Models) can completely describe individual's physiological dynamics. The system can be adapted for healthcare information management, diagnosis, medical decision support, treatment and side-effect control. It can also be adapted for guiding health, fitness and wellness training, subject identification and more efficient management of clinical trials.
Owner:SHUSTERMAN VLADIMIR
Diagnosis and treatment of autism spectrum disorder
ActiveUS20140065132A1Improve behaviorImprove performanceBiocideNervous disorderClinical psychologyAutism spectrum disorder
Disclosed herein are compositions, systems, and methods for diagnosing and treatment of subjects suffering from anxiety, autism spectrum disorder (ASD), or a pathological condition with one or more of the symptoms of ASD.
Owner:CALIFORNIA INST OF TECH
Method and system for providing dynamic orthodontic assessment and treatment profiles
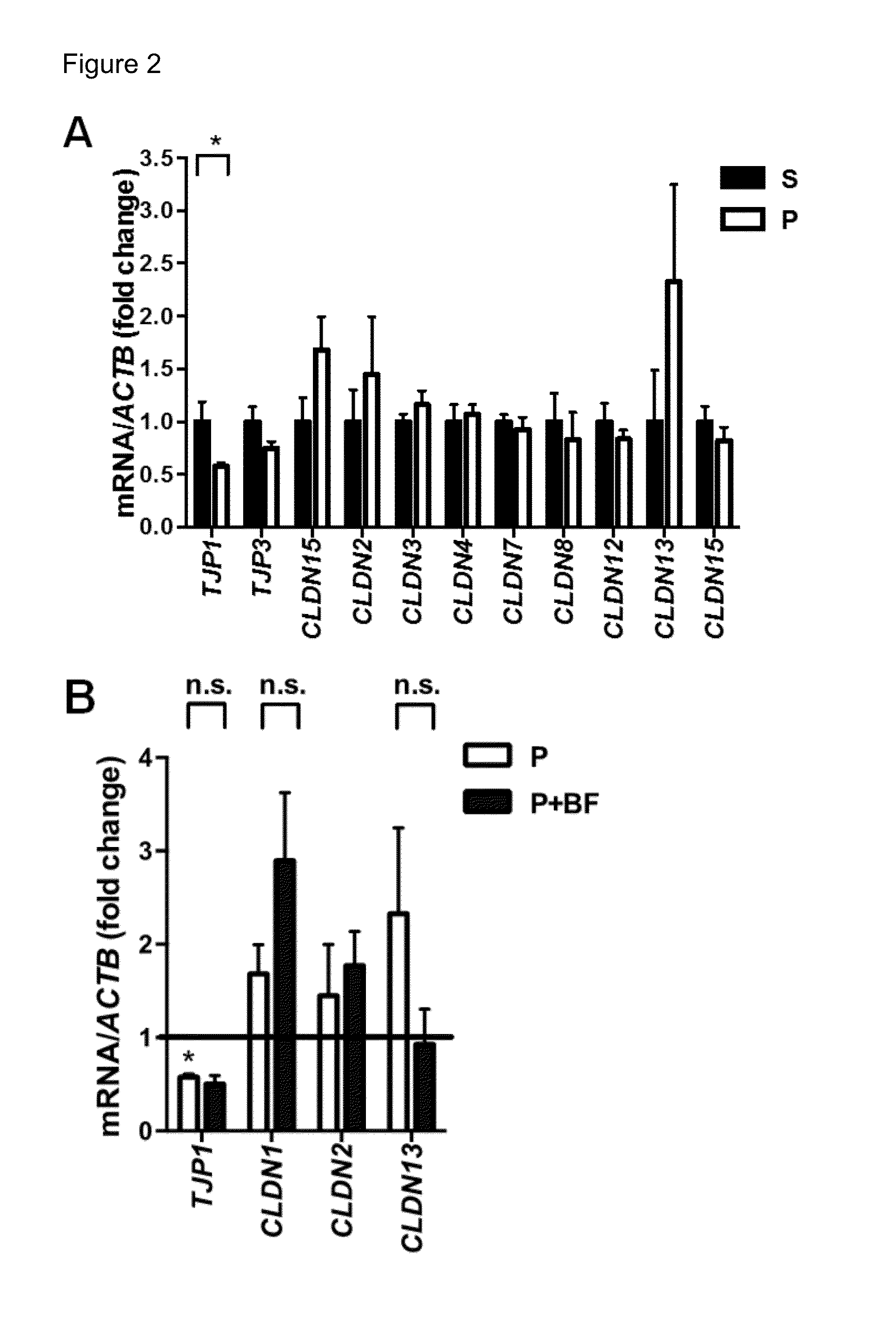
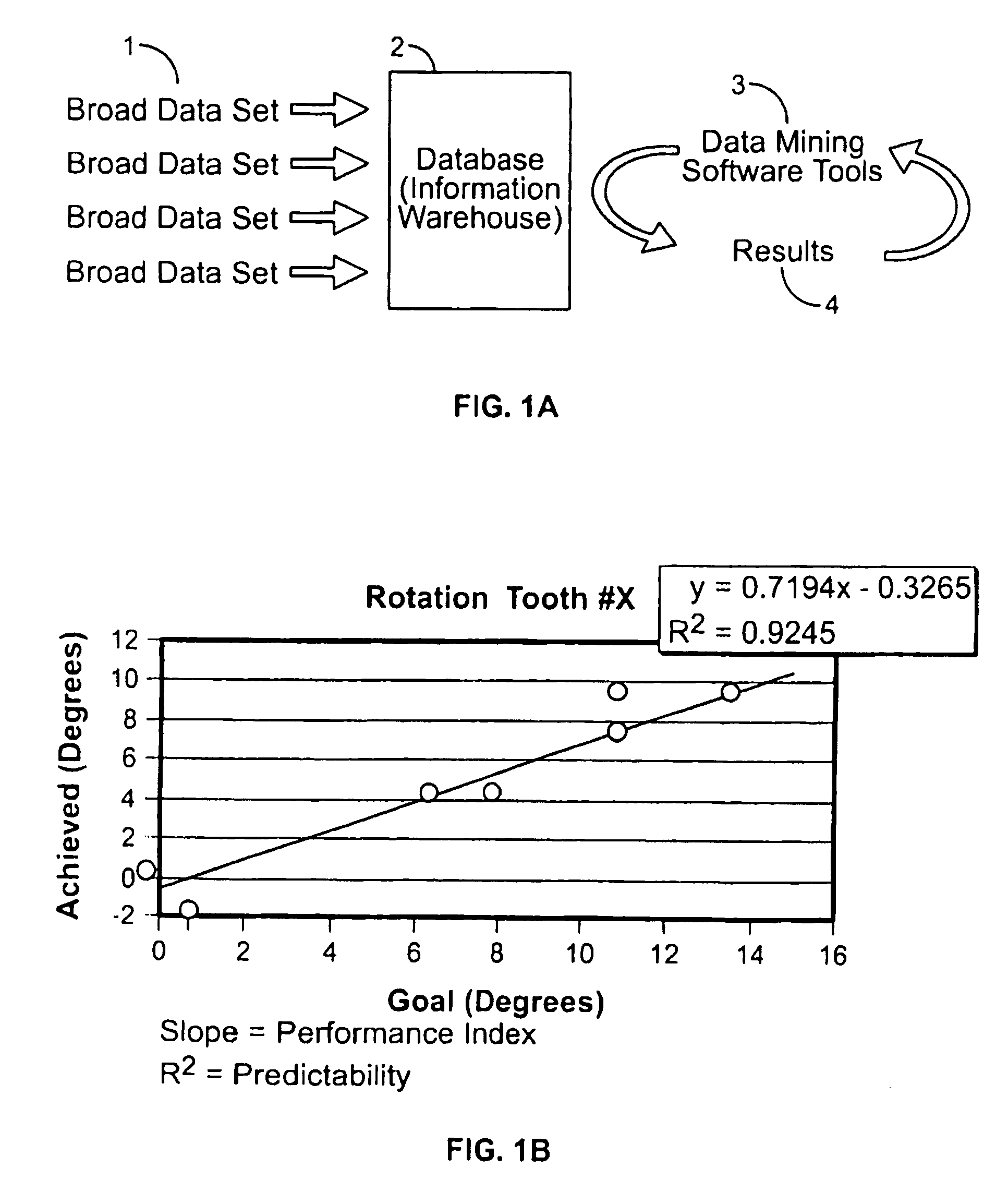
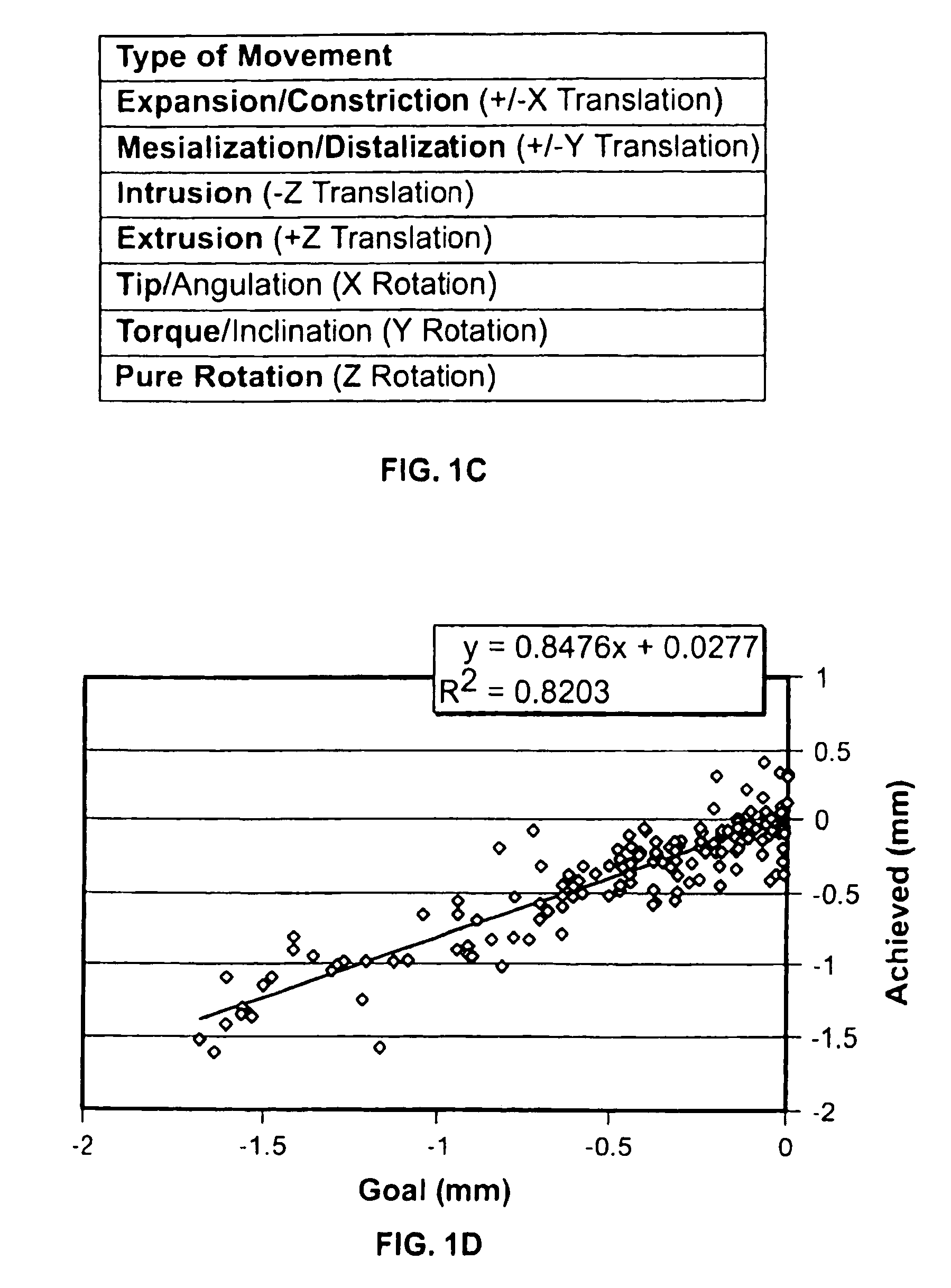
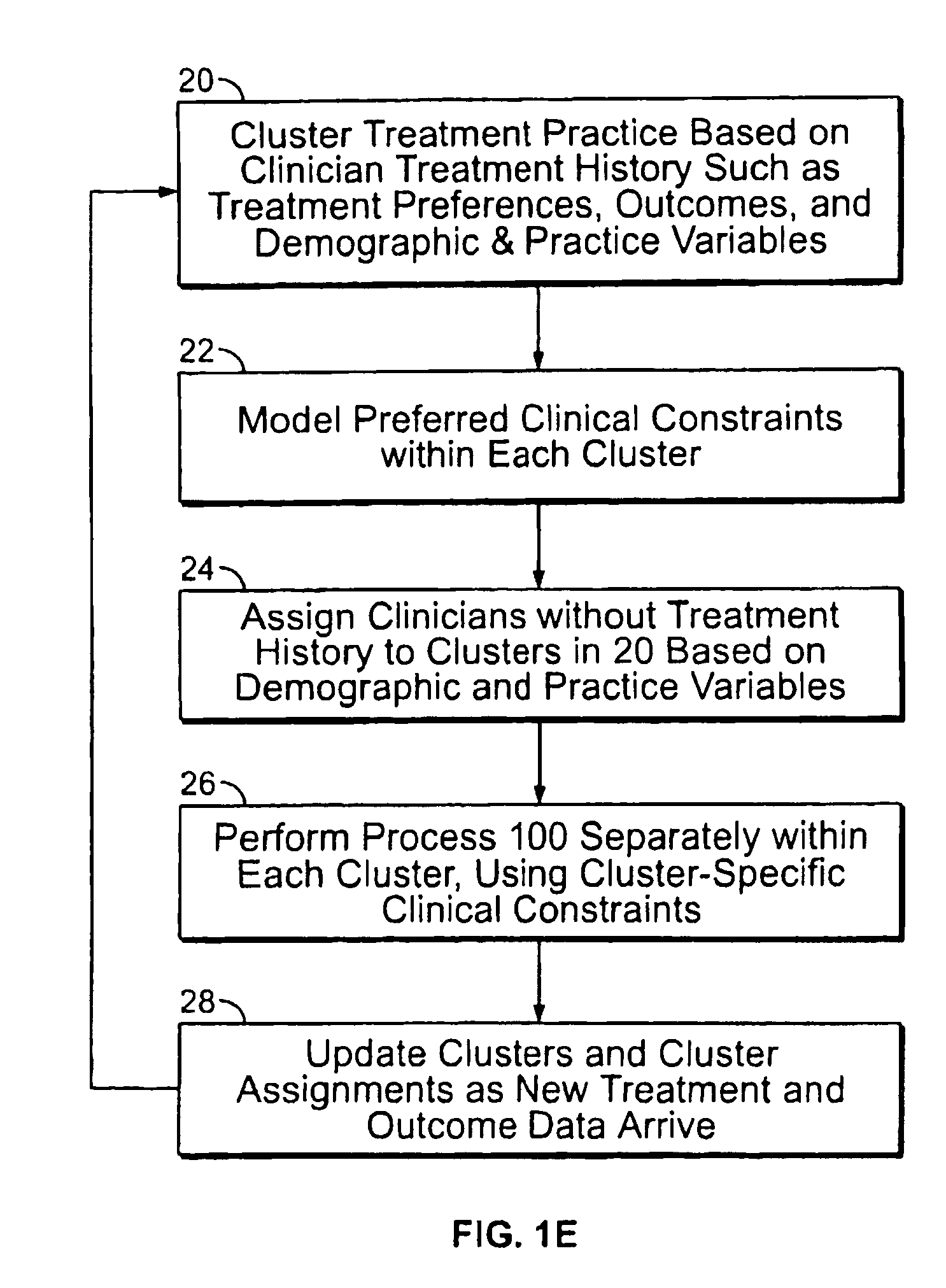
ActiveUS20070128574A1Medical data miningAdditive manufacturing apparatusClinical psychologyTreatment goals
Method and system for receiving one or more dentition conditions of a patient, associating a first set of one or more treatment goals based on the one or more dentition conditions, generating a case difficulty assessment based on the one or more treatment goals, detecting a modification to the case difficulty assessment, retrieving a second set of one or more treatment goals associated with the modified case difficulty assessment, and displaying the second set of one or more treatment goals are provided.
Owner:ALIGN TECH
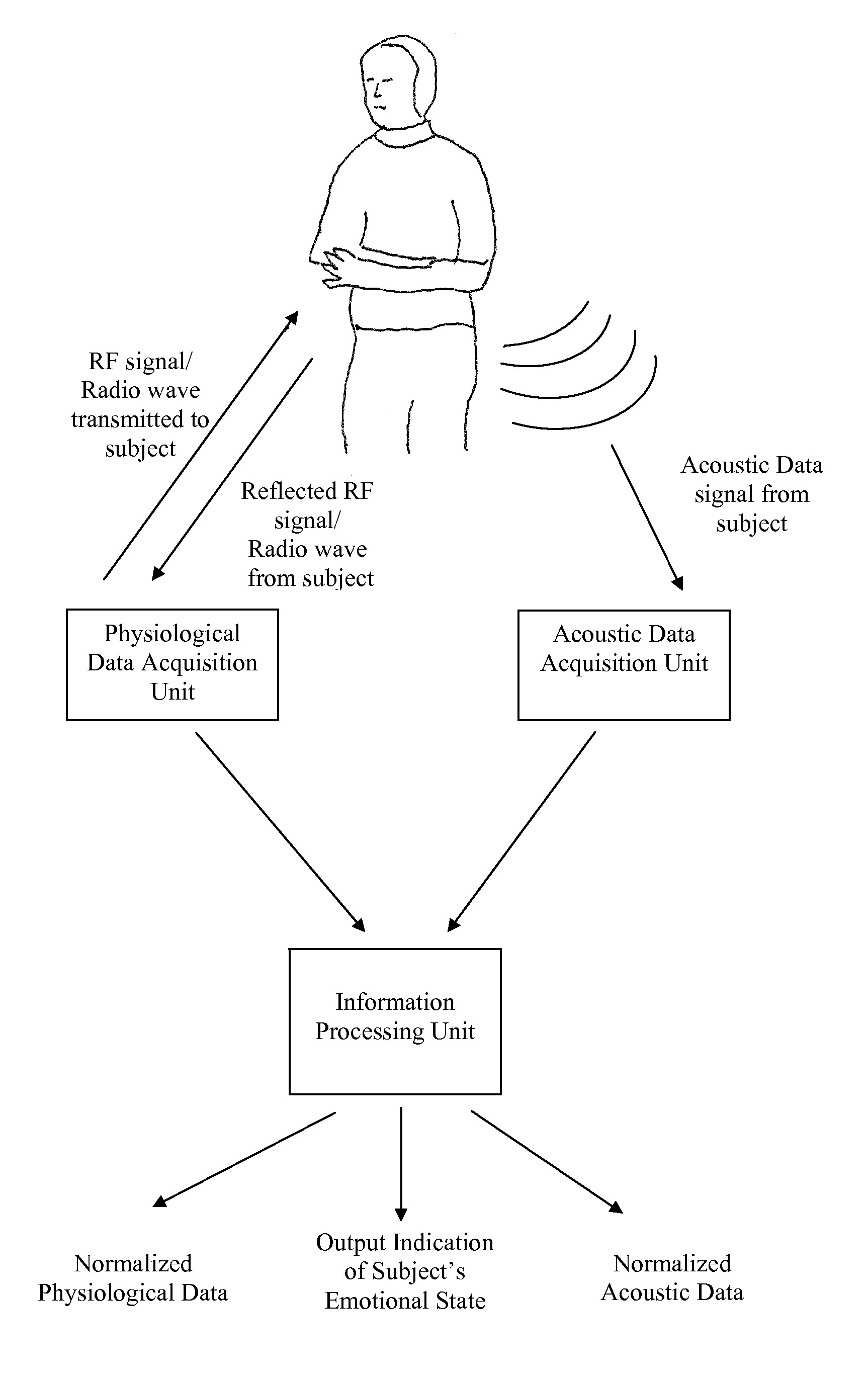
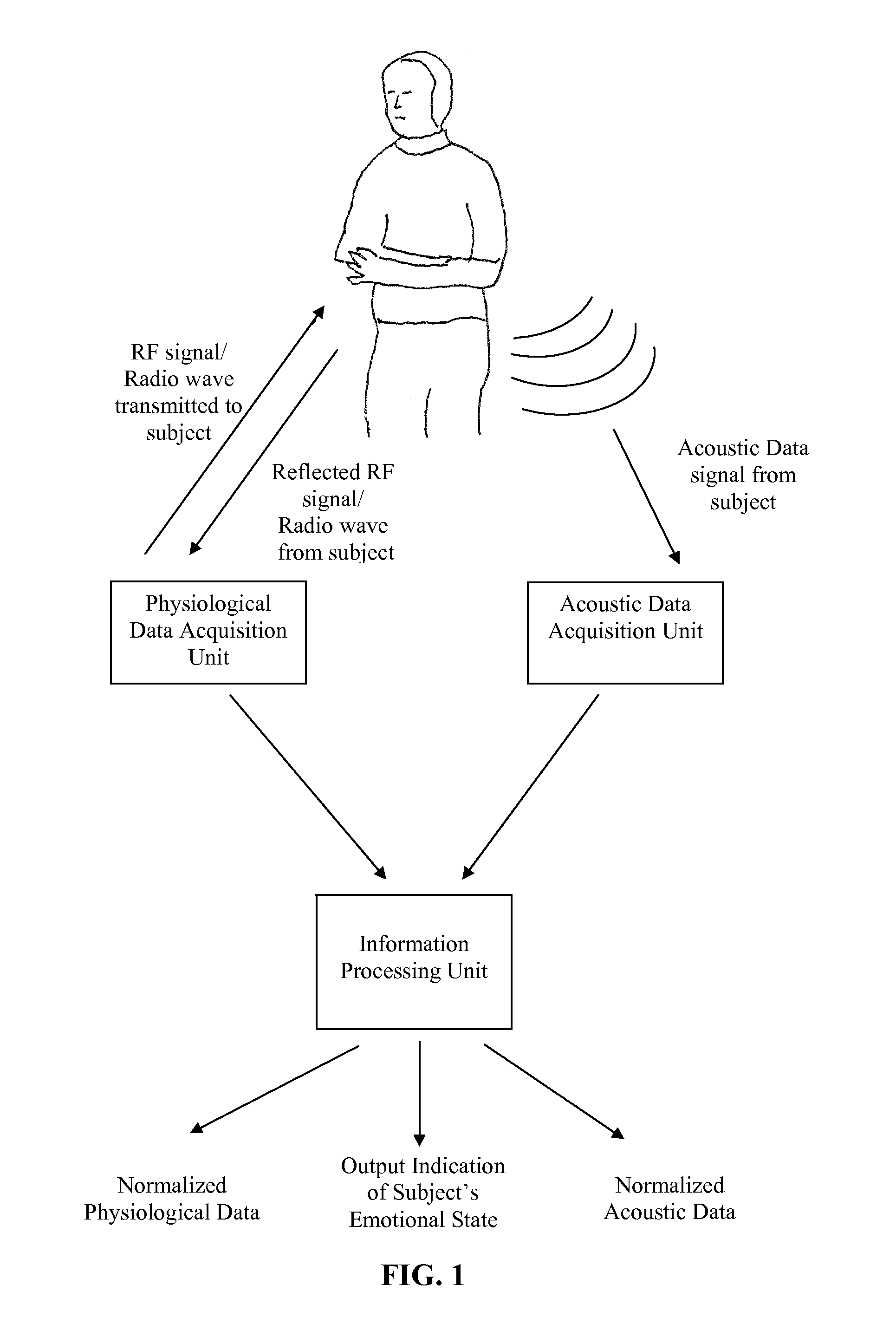
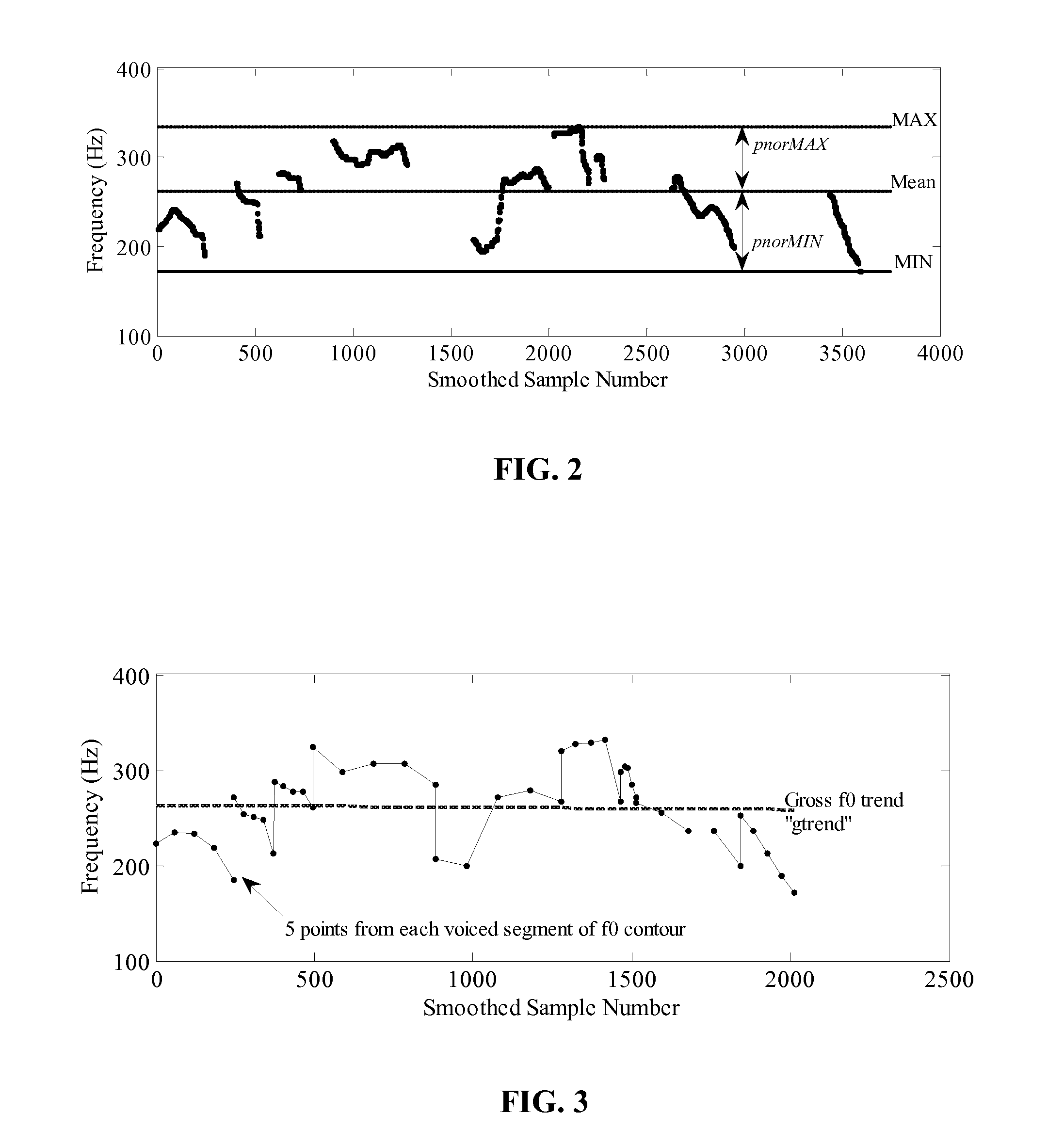
Method and apparatus for evaluation of a subject's emotional, physiological and/or physical state with the subject's physiological and/or acoustic data
Embodiments of the subject invention relate to a method and apparatus for remote evaluation of a subject's emotive and / or physiological state. Embodiments can utilize a device that can be used to determine the emotional and / or physiological state of a subject through the measurement and analysis of vital signs and / or speech. A specific embodiment relates to a device capable of remotely acquiring a subject's physiological and / or acoustic data, and then correlating and analyzing the data to provide an assessment of a subject's emotional and / or physiological state. In a further specific embodiment, the device can acquire such data, correlate and analyze the data, and provide the assessment of the subject's emotional state and / or physiological in real time.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
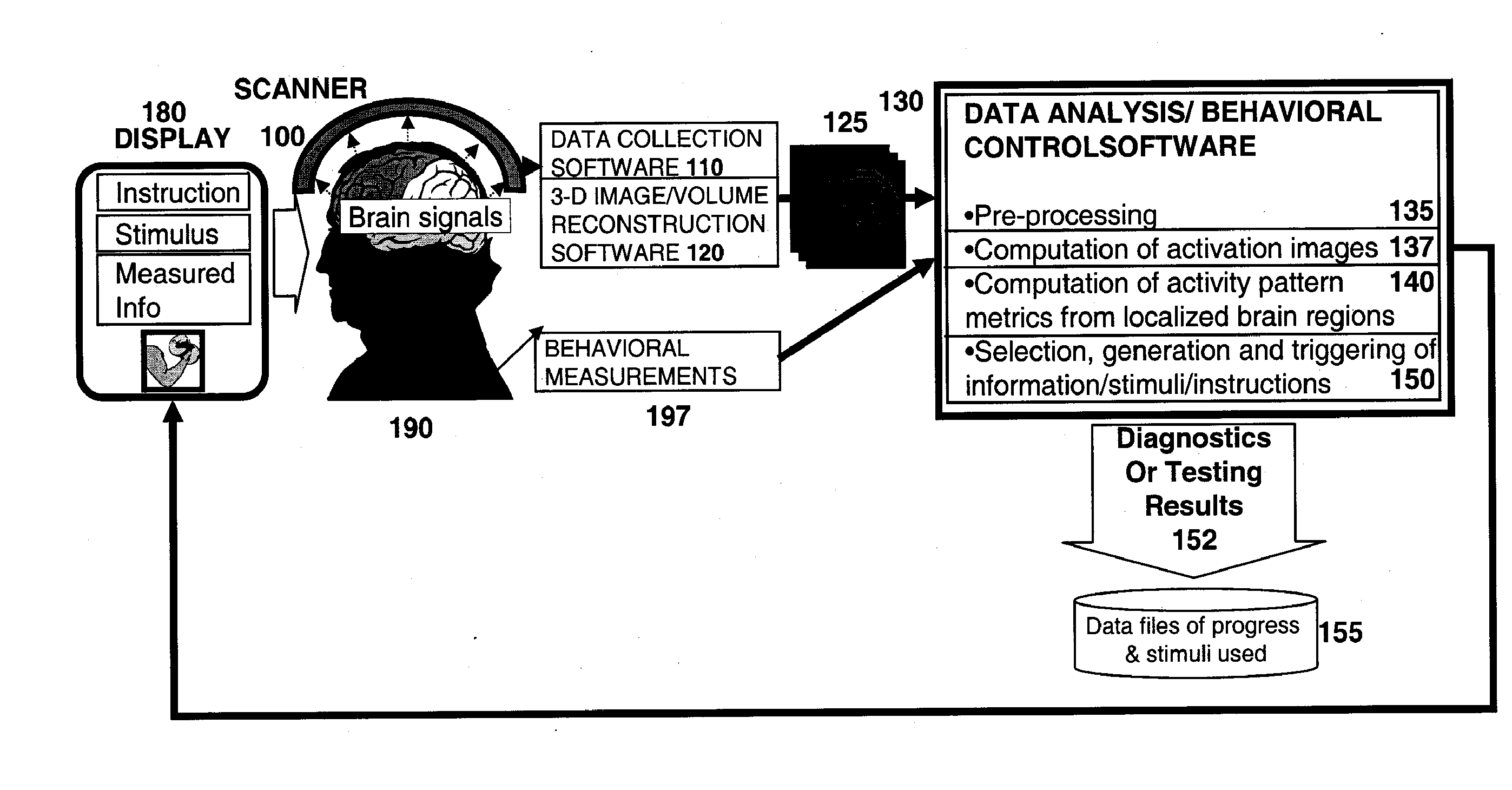
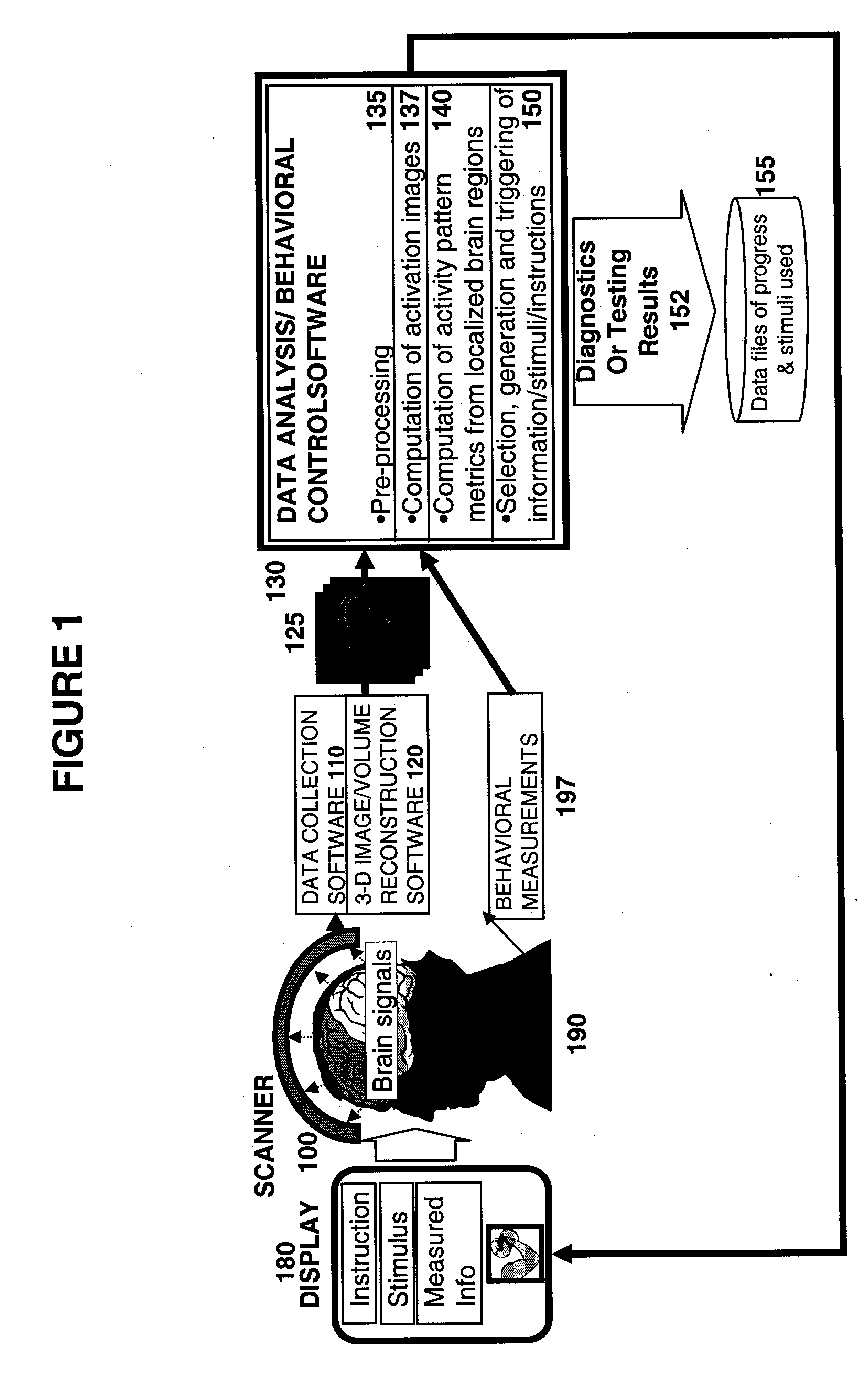
Methods for Measurement and Analysis of Brain Activity
InactiveUS20070191704A1Ultrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsClinical psychologyComputer-aided
A computer assisted method is provided for diagnosing the in condition of a subject associated with particular activation in one or more regions of interest, the method comprising: having the subject perform a behavior or have a perception adapted to selectively activate one or more regions of interest associated with the condition; measuring activity of the one or more regions of interest as the behavior is performed or the subject has the perception; diagnosing the condition associated with the one or more regions of interest based on the activity in response to the behavior or perception; performing an intervention; and repeating this process one or more times including repeating said behavior, said measuring of activity and said diagnosis at a later time; and observing changes between measurements that are associated with said intervention.
Owner:DECHARMS RICHARD CHRISTOPHER
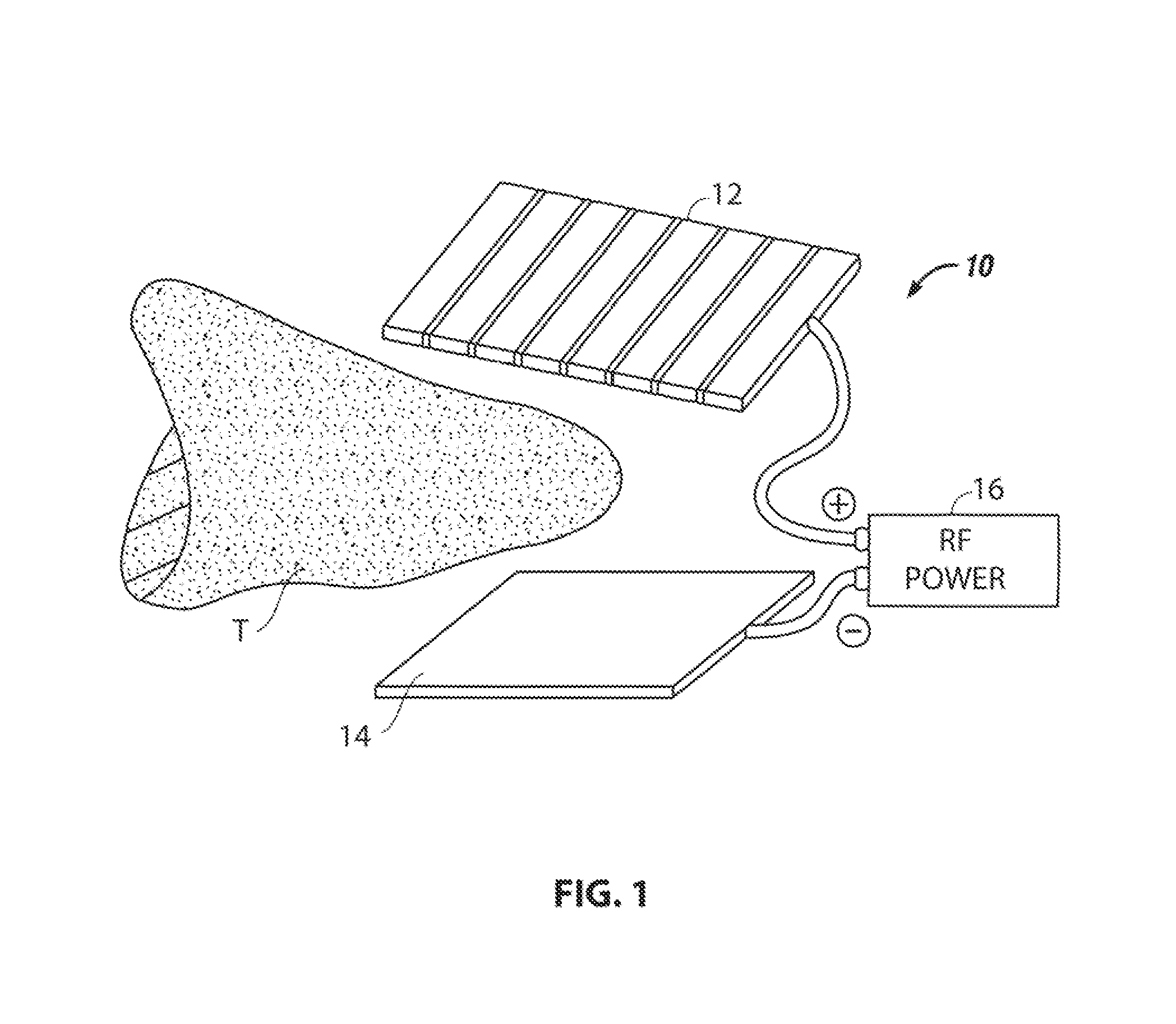
Surgical tool
ActiveUS8574229B2Facilitate tissue cauterizationEliminates, the bleedingDiagnosticsSurgical instruments for heatingClinical psychologyRadio frequency
The invention is concerned with cauterizing and resecting tissue. A pair of electrodes are placed on opposed tissue surfaces, and radio frequency power is applied through the electrodes to cauterizing a tissue mass therebetween. After cauterization has been effected, the tissue may be resected along a plane within the cauterized region with minimum or no bleeding. The tissue mass may then be removed.
Owner:AESCULAP AG
Method and apparatus for measuring indices of brain activity during motivational and emotional function
Owner:THE GENERAL HOSPITAL CORP
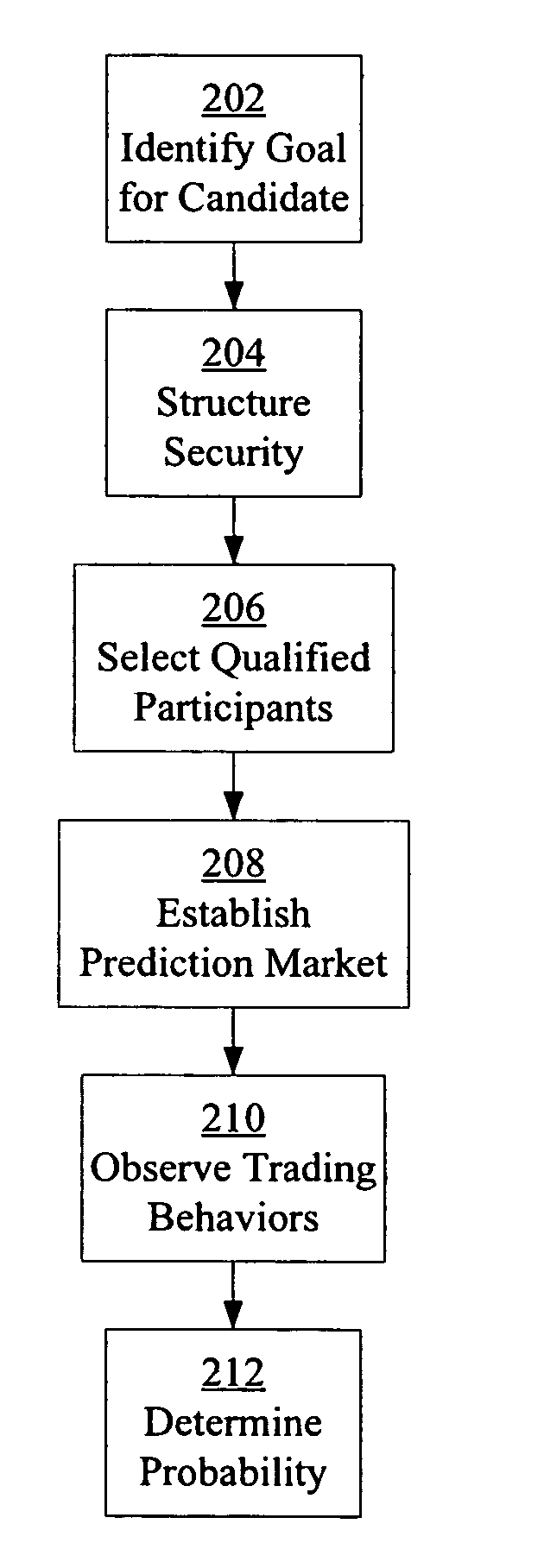
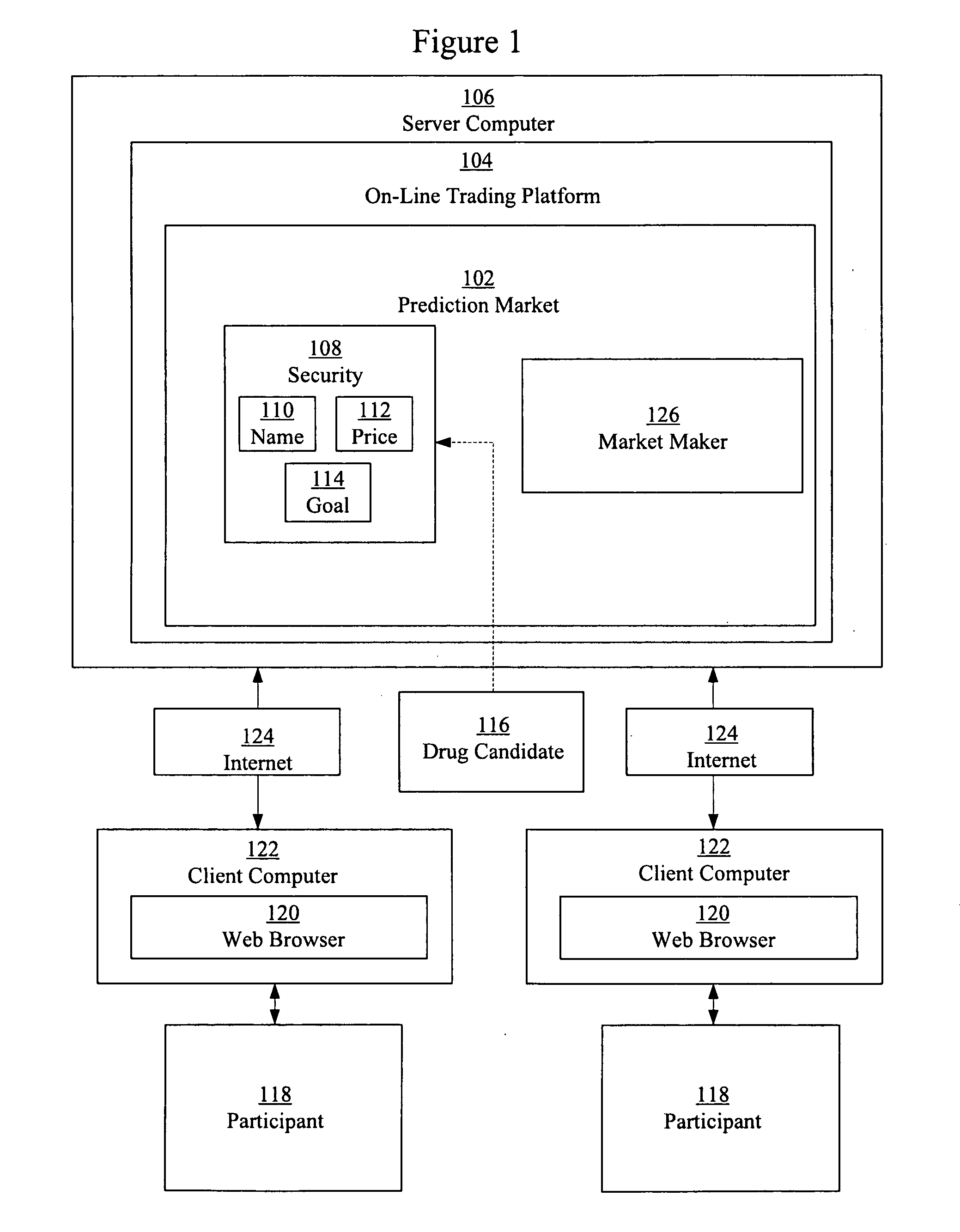
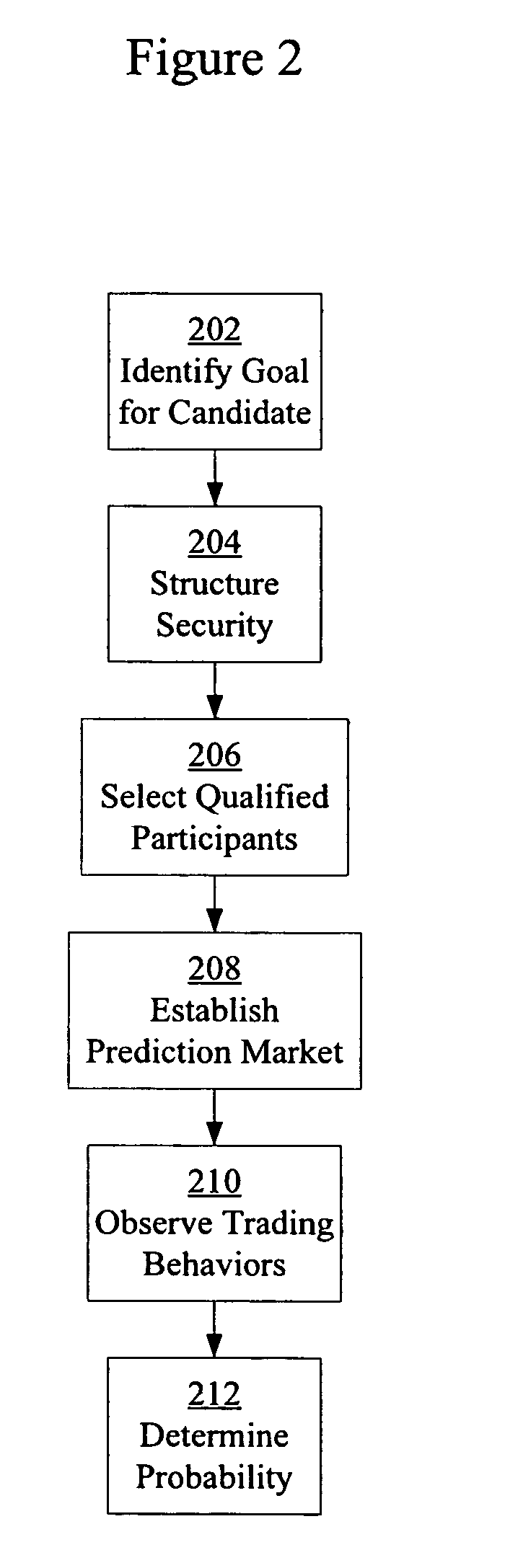
Prediction markets for assessing clinical probabilities of success
Prediction markets are used to determine the probability of an experimental therapeutic, diagnostic, or prophylactic candidate meeting clinical trial and post-trial goals, such as clinical trial endpoints and timelines. The prediction market processes buy and sell orders from market participants, while adjusting the prices of the securities according to the orders. The securities have specific meanings which correspond to goals in clinical trials or other outcomes in clinical candidate development. The price of a security determined by the market corresponds to the probability of the corresponding goal or outcome. The participants are selected for their expert knowledge of specific factors related to candidate development. Using appropriately selected securities and participants, the prediction market may be used to generate probabilities of success useful for long-range planning and valuation, determining production timelines and volumes, management of candidates in a development portfolio, and clinical management of patients by physicians.
Owner:CLINICAL FUTURES
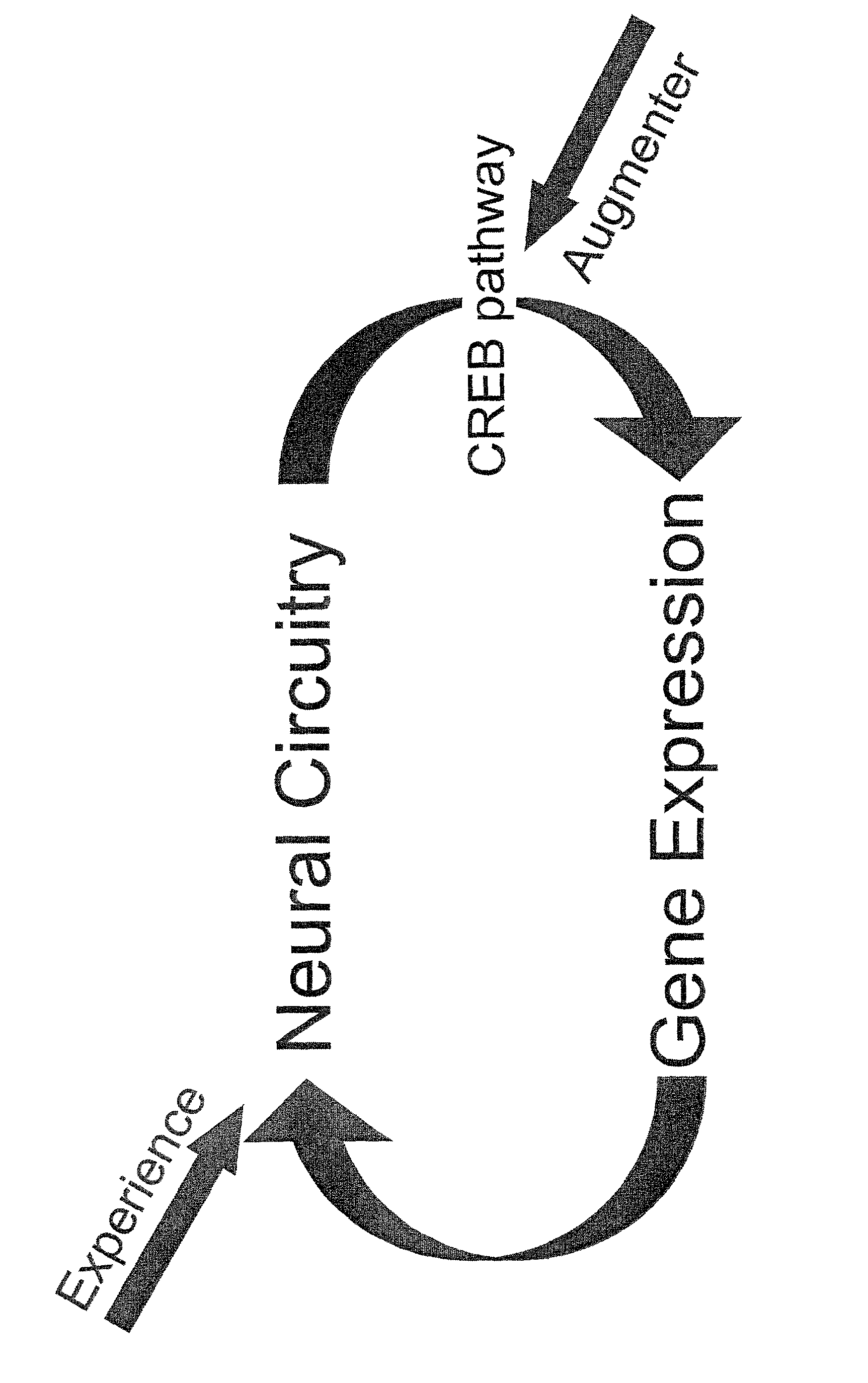
Augmented cognitive training
InactiveUS7947731B2Improve performanceNormally performanceBiocideNervous disorderNeuronal circuitsClinical psychology
The present invention provides methods of therapy of cognitive deficits associated with a central nervous system disorder or condition, methods of enhancing cognitive performance and methods for repeated stimulation of neuronal activity or a pattern of neuronal activity, such as that underlying a specific neuronal circuit(s). The methods comprise combining cognitive training protocols and a general administration of CREB pathway-enhancing agents.
Owner:COLD SPRING HARBOR LAB INC
Automated treatment system for sleep
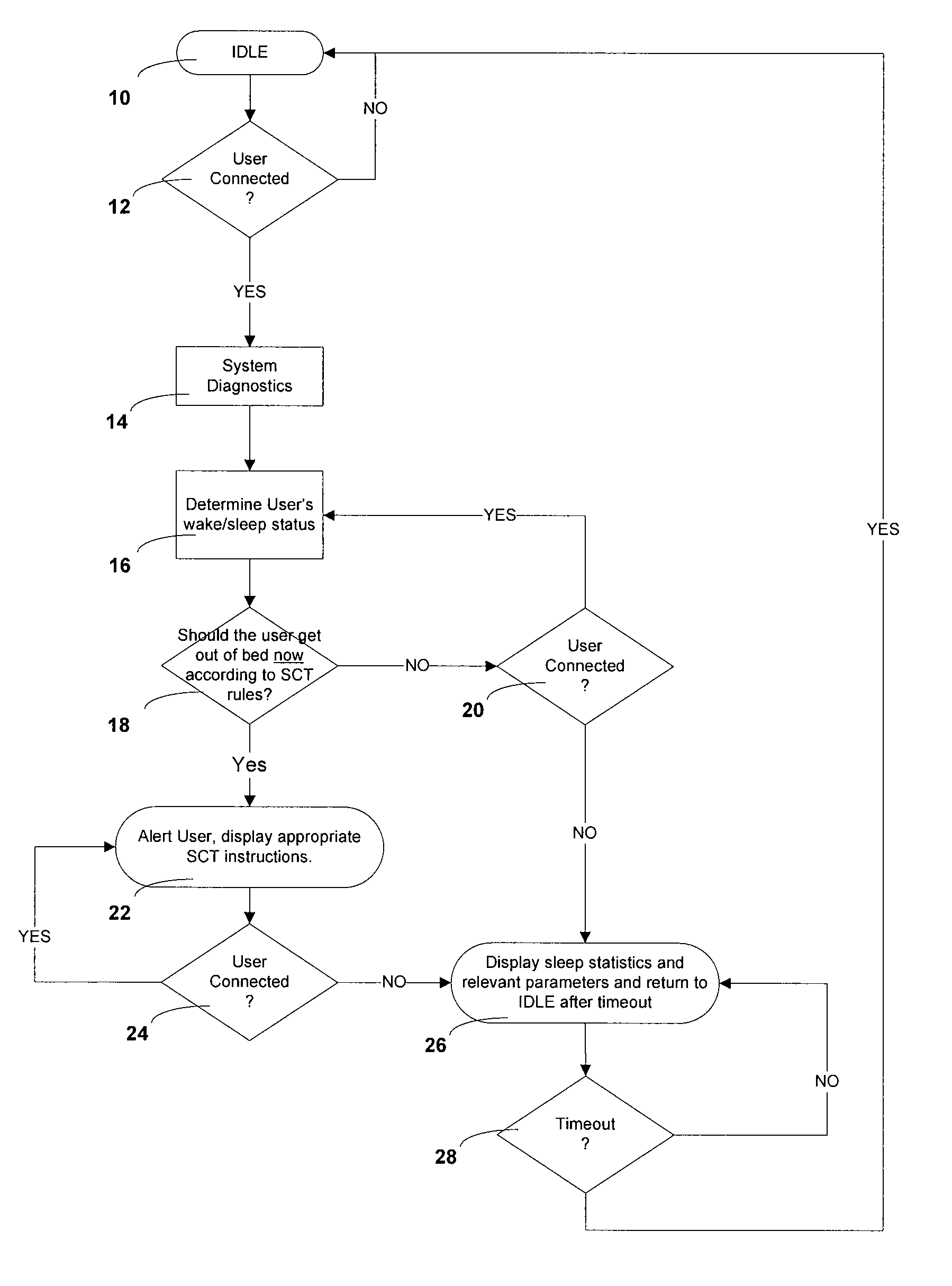
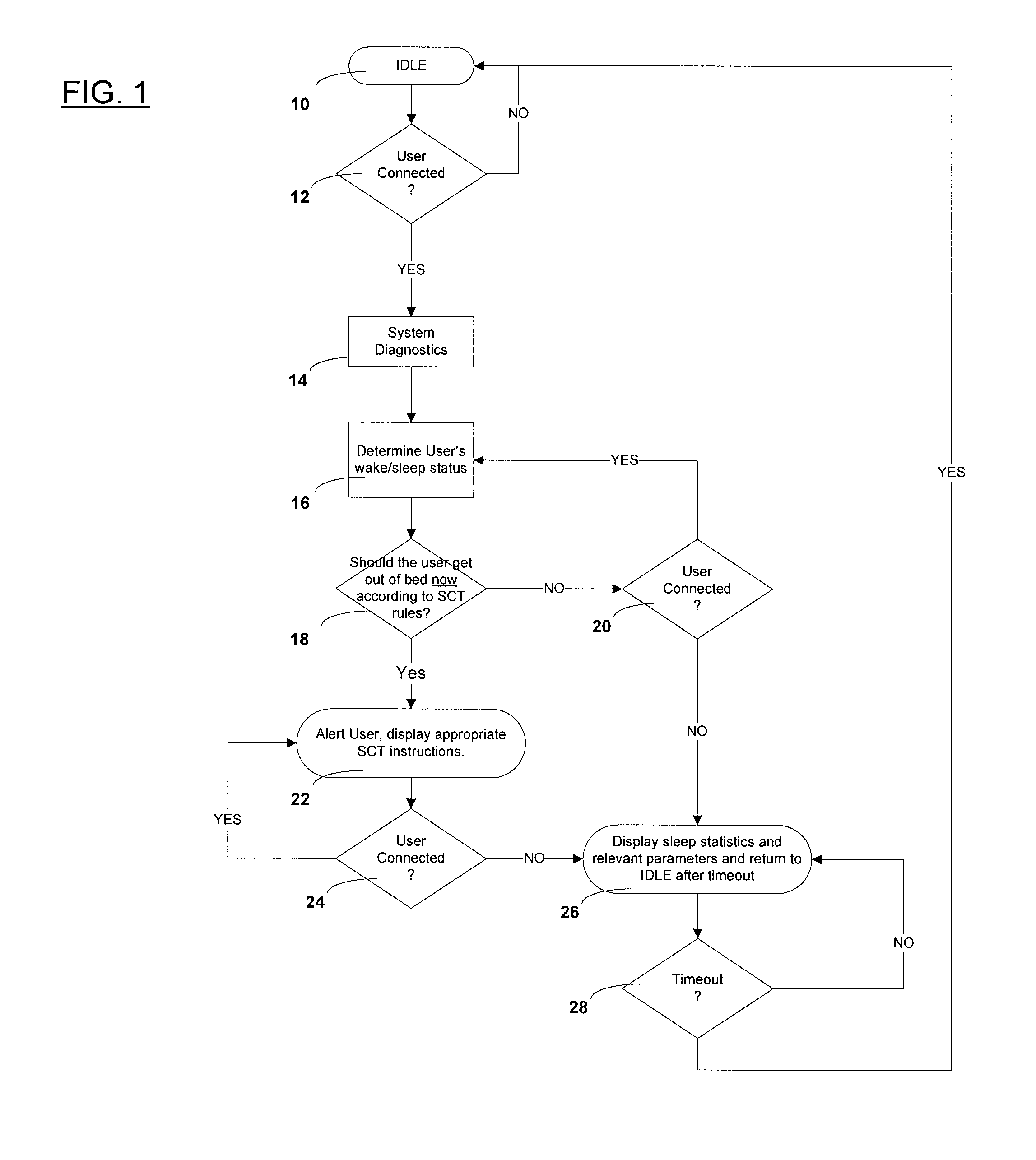
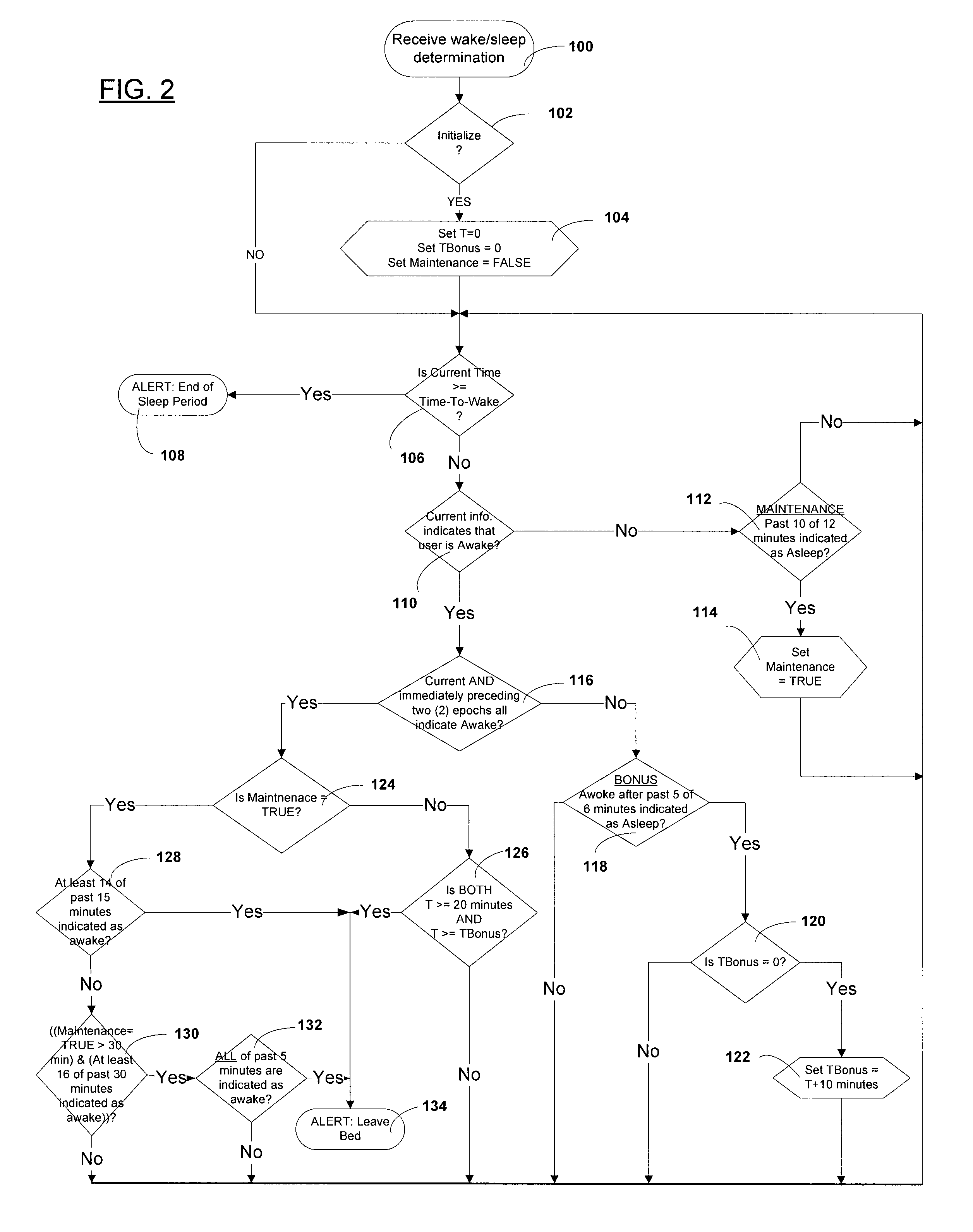
Automated behavioral methods and systems for treating Insomnia that use passive means for determining wake / sleep states.
Owner:CONSOL RES OF RICHMOND
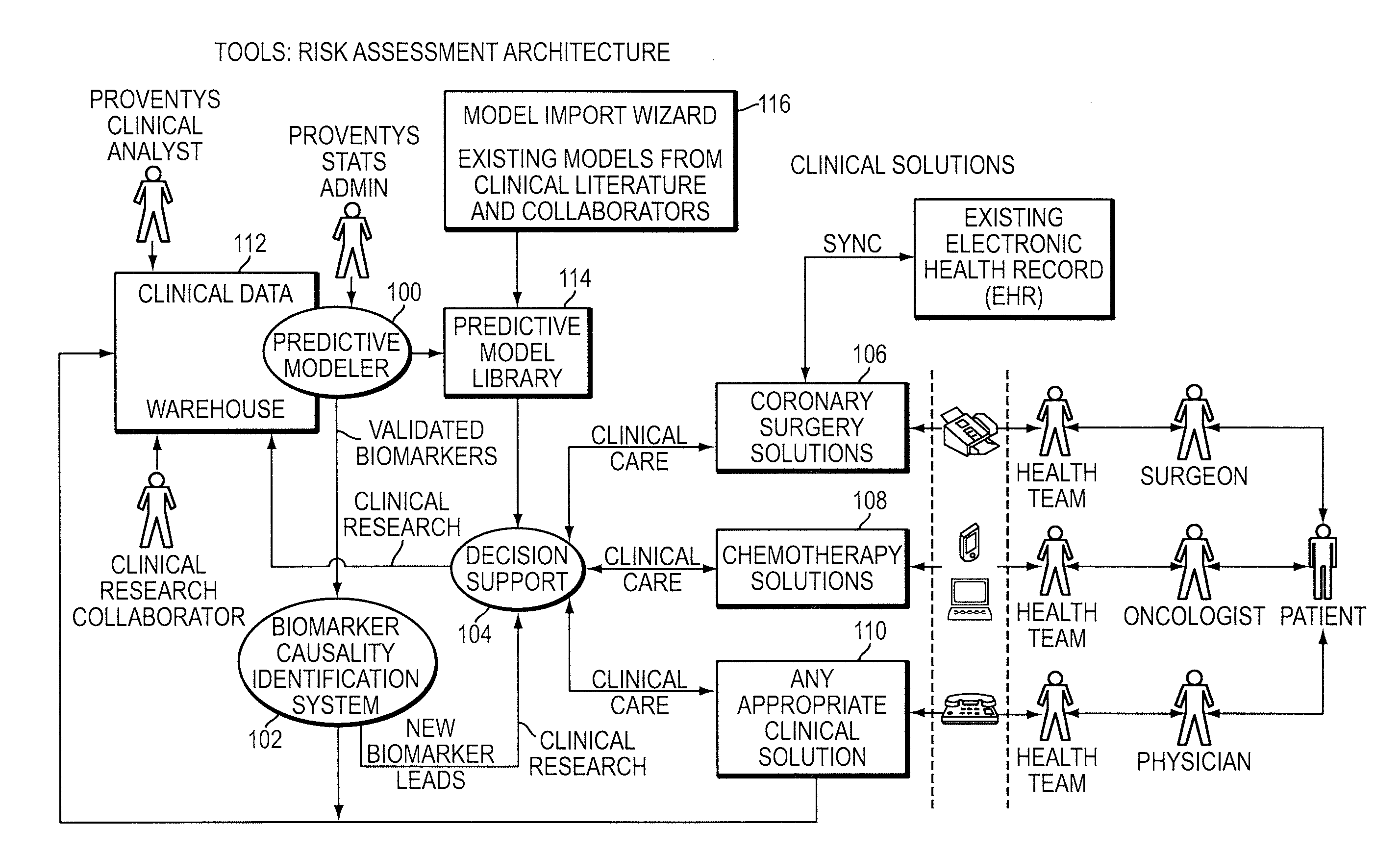
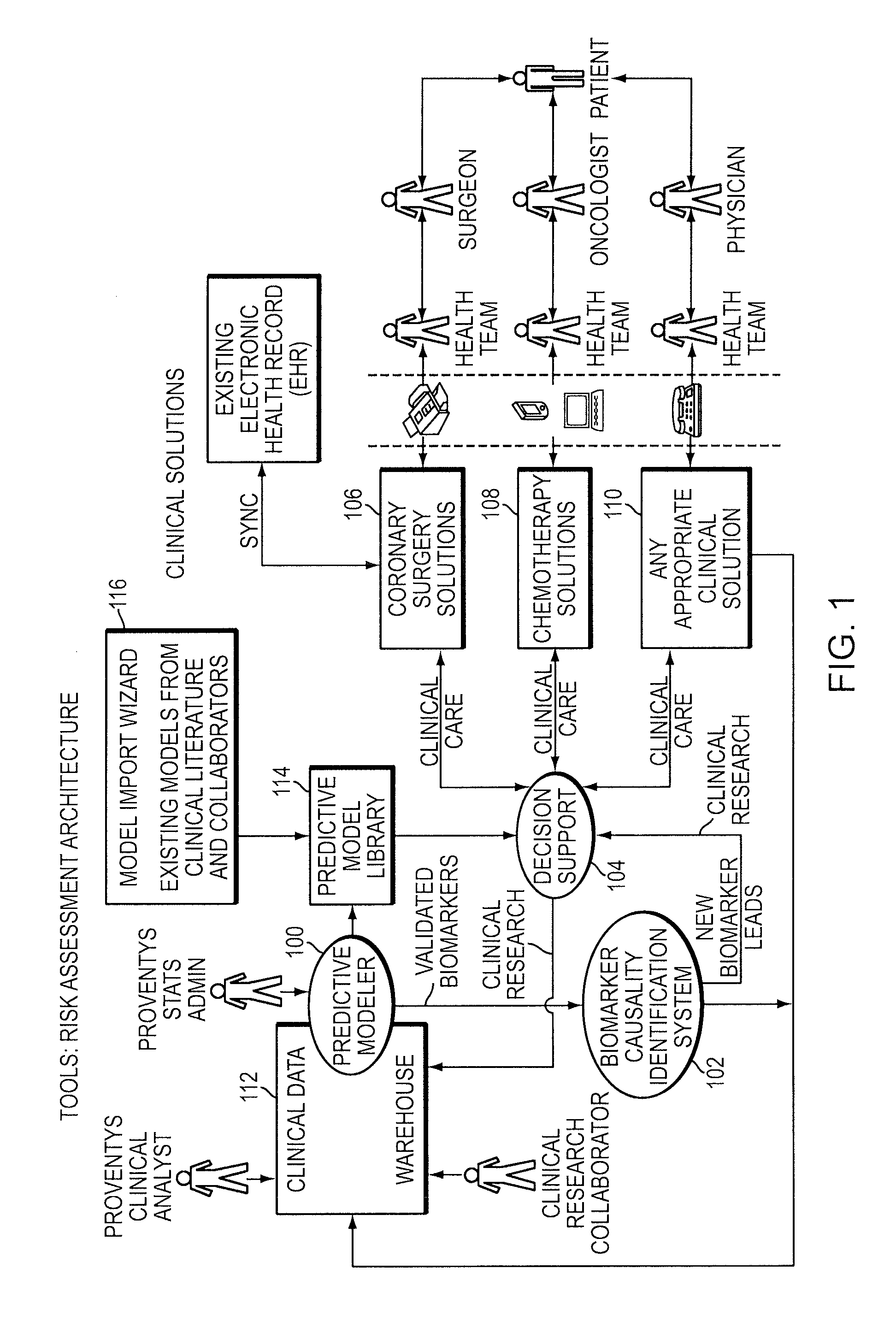
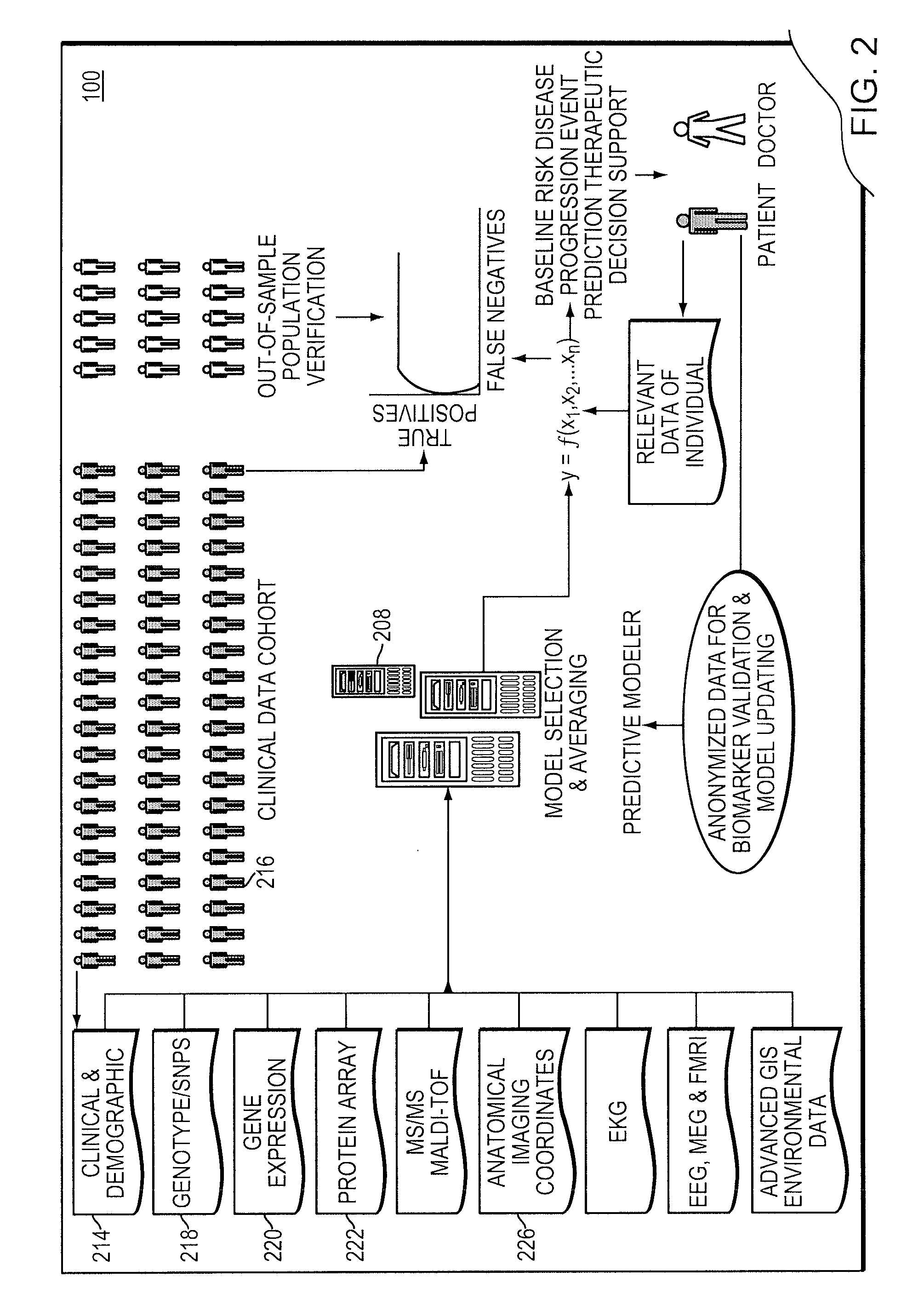
Integrated clinical risk assessment system
InactiveUS20090070138A1Minimize adverse outcomeMaximizing measurable deliveryData processing applicationsHealth-index calculationTherapeutic treatmentTherapy planning
A computer-implemented method is provided for analyzing patient risk and determining an individual therapeutic treatment plan for a cancer patient. The method includes entering patient medical information into a risk assessment tool; identifying any obtaining missing or out-of-date patient information; initializing the risk assessment tool based on the patient's demographics and cancer characteristics and determining a default treatment plan for the patient; modifying the default treatment plan by observing the modification of a risk score for the patient; and confirming a treatment order for the patient based on a balancing of the risk and treatment plan factors.
Owner:PROVENTYS
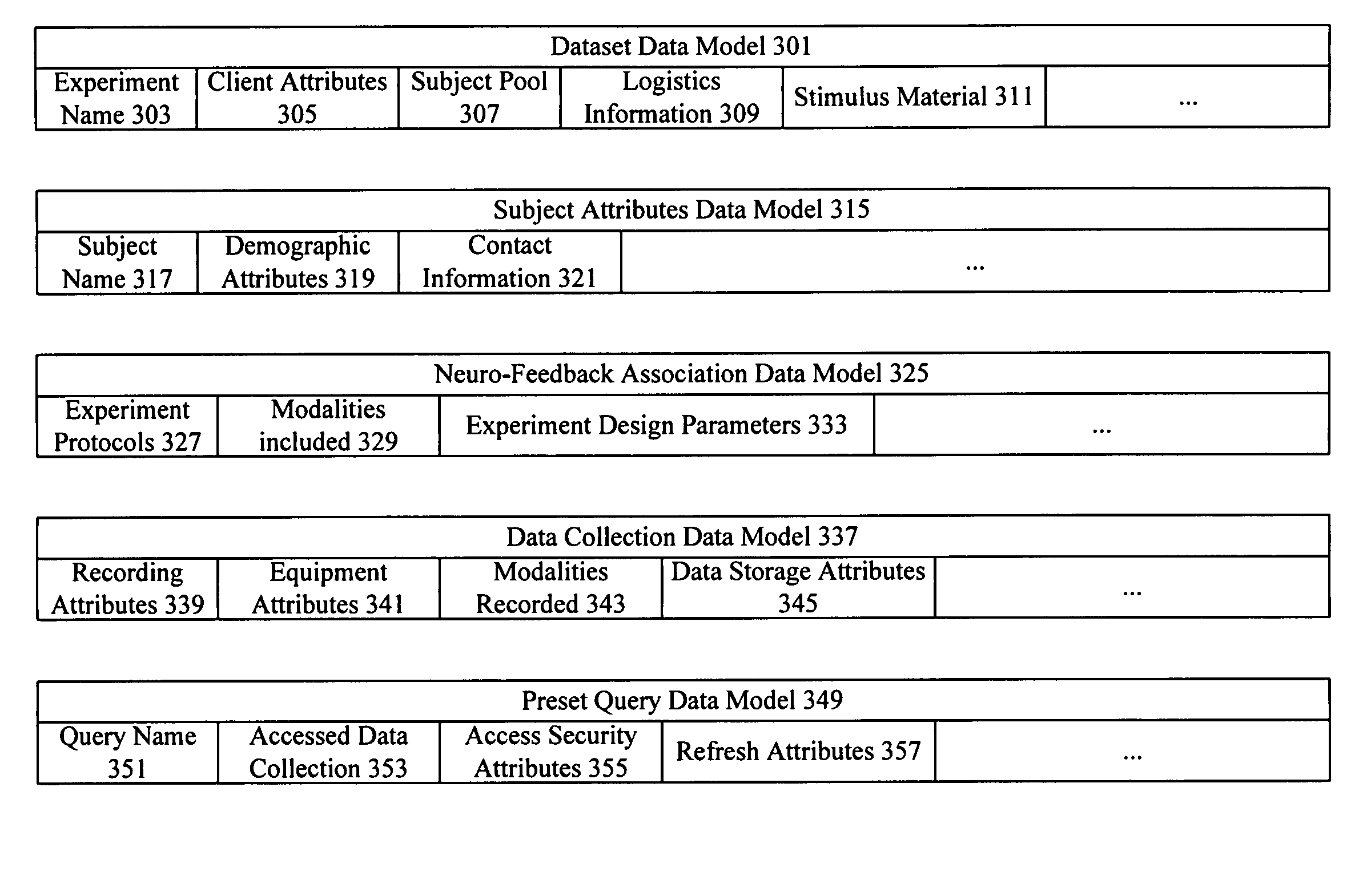
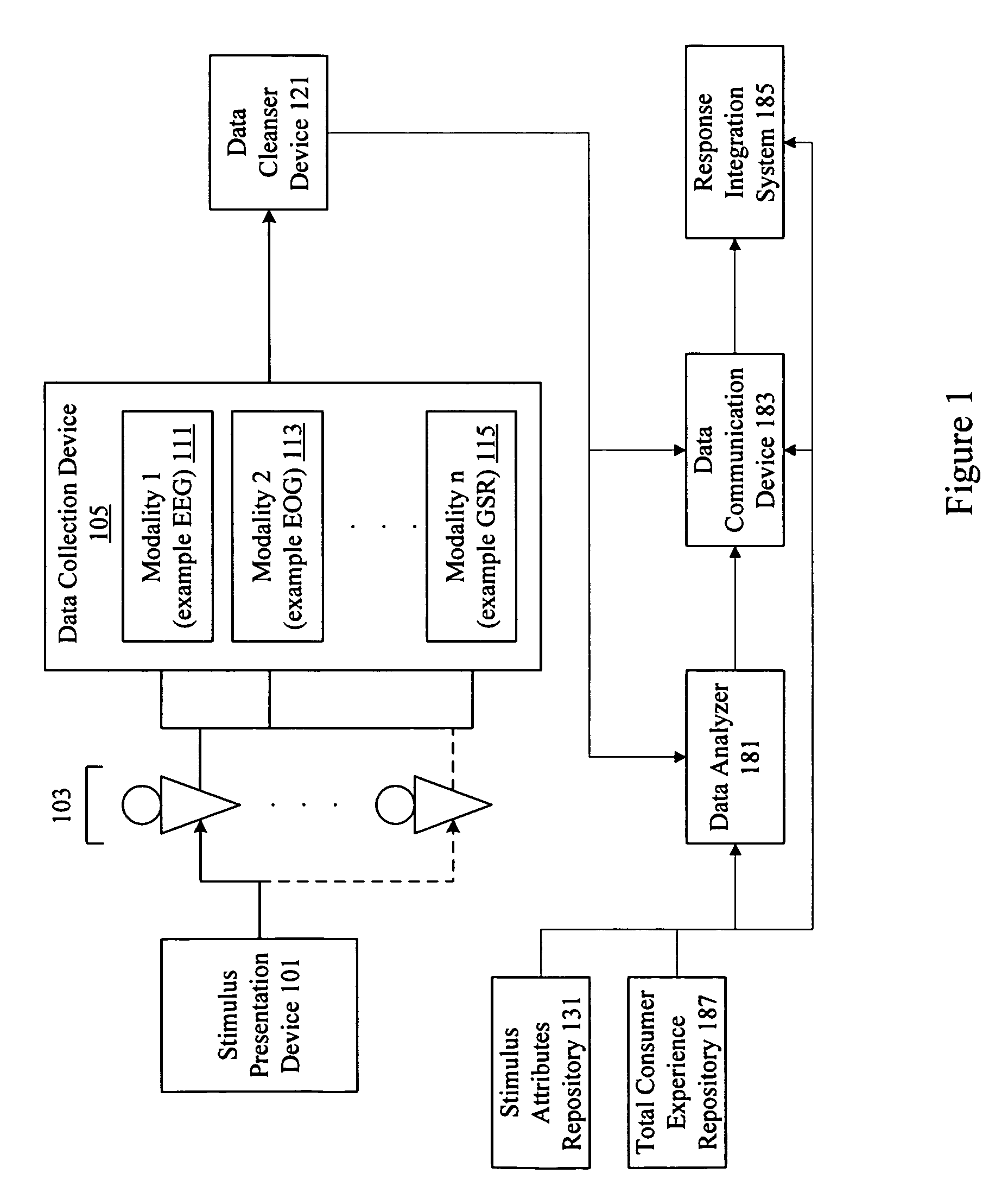
Consumer experience portrayal effectiveness assessment system
A system assesses consumer experience portrayal effectiveness by evaluating neuro-response measurements for a consumer directly experiencing stimulus and / or indirectly experiencing stimulus through observation of another. Neuro-response measurements are collected using multiple modalities to evaluate personal and observed experiences of products, services, offerings, and stimulus. Examples of neuro-response measurements include Electroencephalography (EEG), Galvanic Skin Response (GSR), Electrocardiograms (EKG), Electrooculography (EOG), eye tracking, and facial emotion encoding measurements. In many instances, neuro-response data is combined with other data and analyzed to assess total consumer experience portrayal effectiveness.
Owner:NIELSEN CONSUMER LLC
Mental balance or imbalance estimation system and method
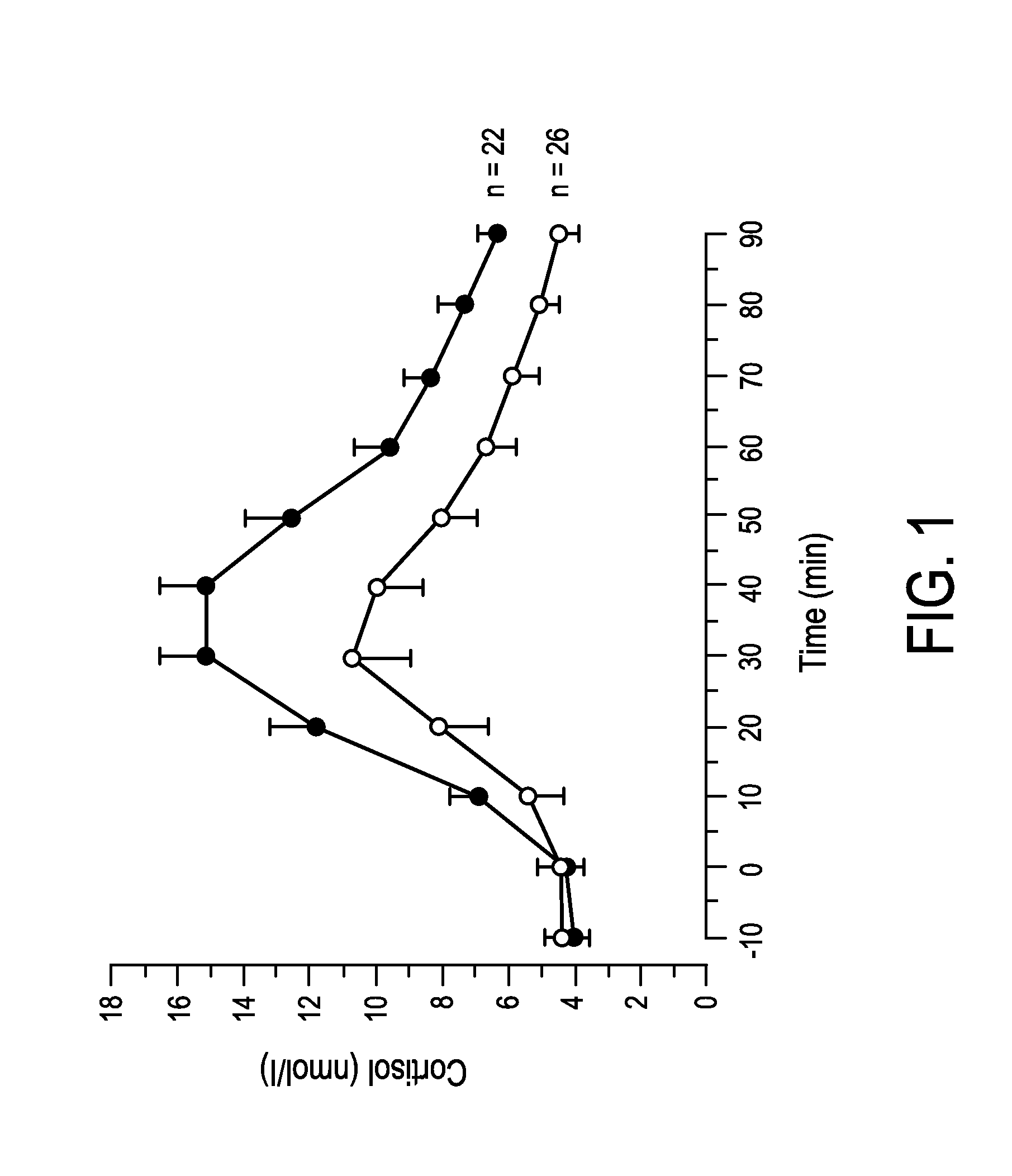
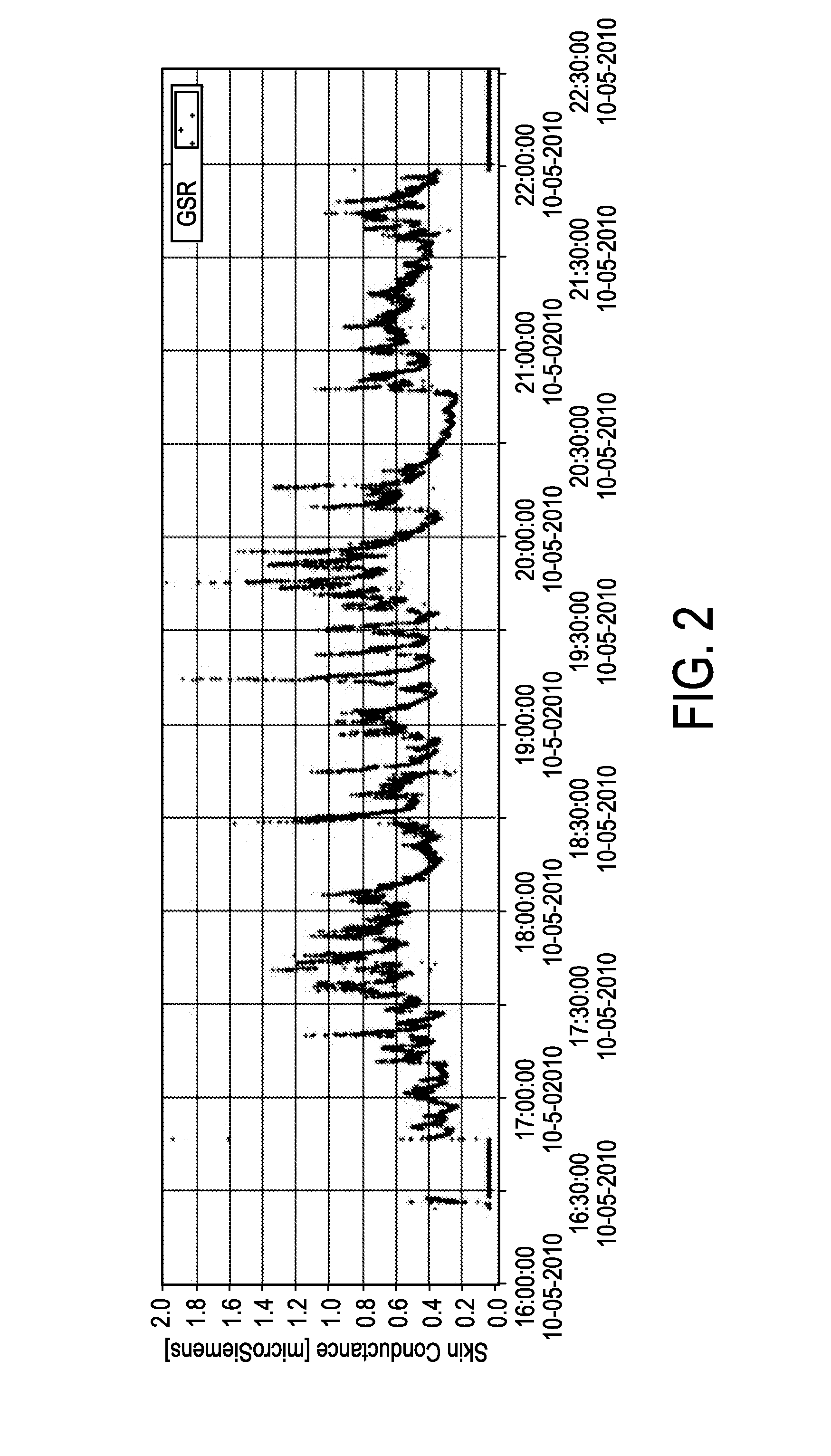
ActiveUS20140288401A1Improve balanceGood estimateHealth-index calculationMedical automated diagnosisClinical psychologyCortisol level
The present invention relates to a mental balance or imbalance estimation system (100) and method for estimating a level of mental balance or imbalance of a user (1). The system comprises a skin conductance sensor (20) for sensing the skin conductance of the user (1), the skin conductance over time forming a skin conductance trace (22). The system further comprises a processing unit (10) for receiving and processing the skin conductance trace (22), the processing unit configured to determine at least one stimulus response (24) in the skin conductance trace (22), to determine an estimated cortisol level trace (28) of the user (1) based on the determined at least one stimulus response (24), and to determine the estimated level of mental balance or imbalance of the user (1) based on the estimated cortisol level trace (28).
Owner:KONINKLJIJKE PHILIPS NV
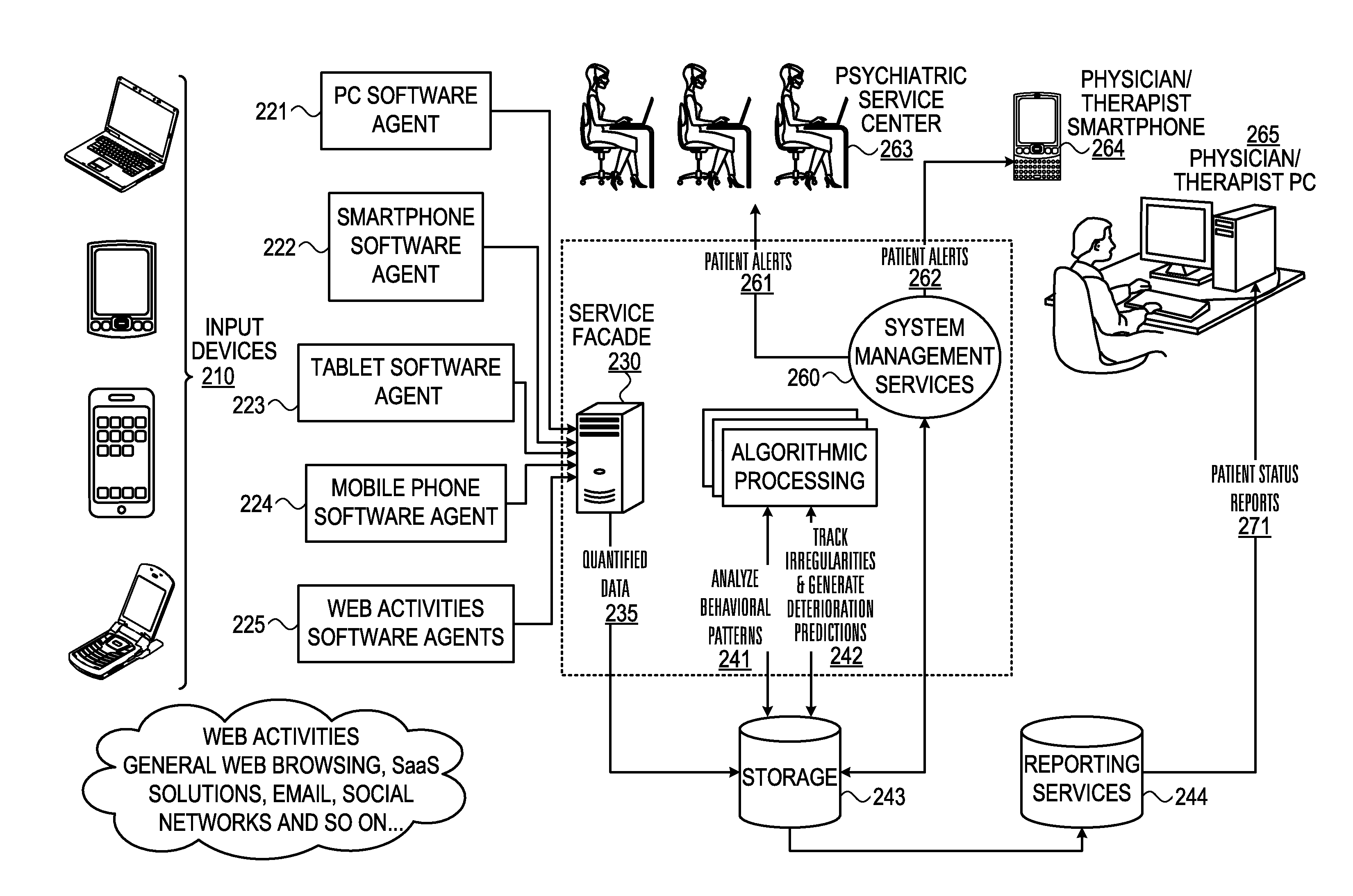
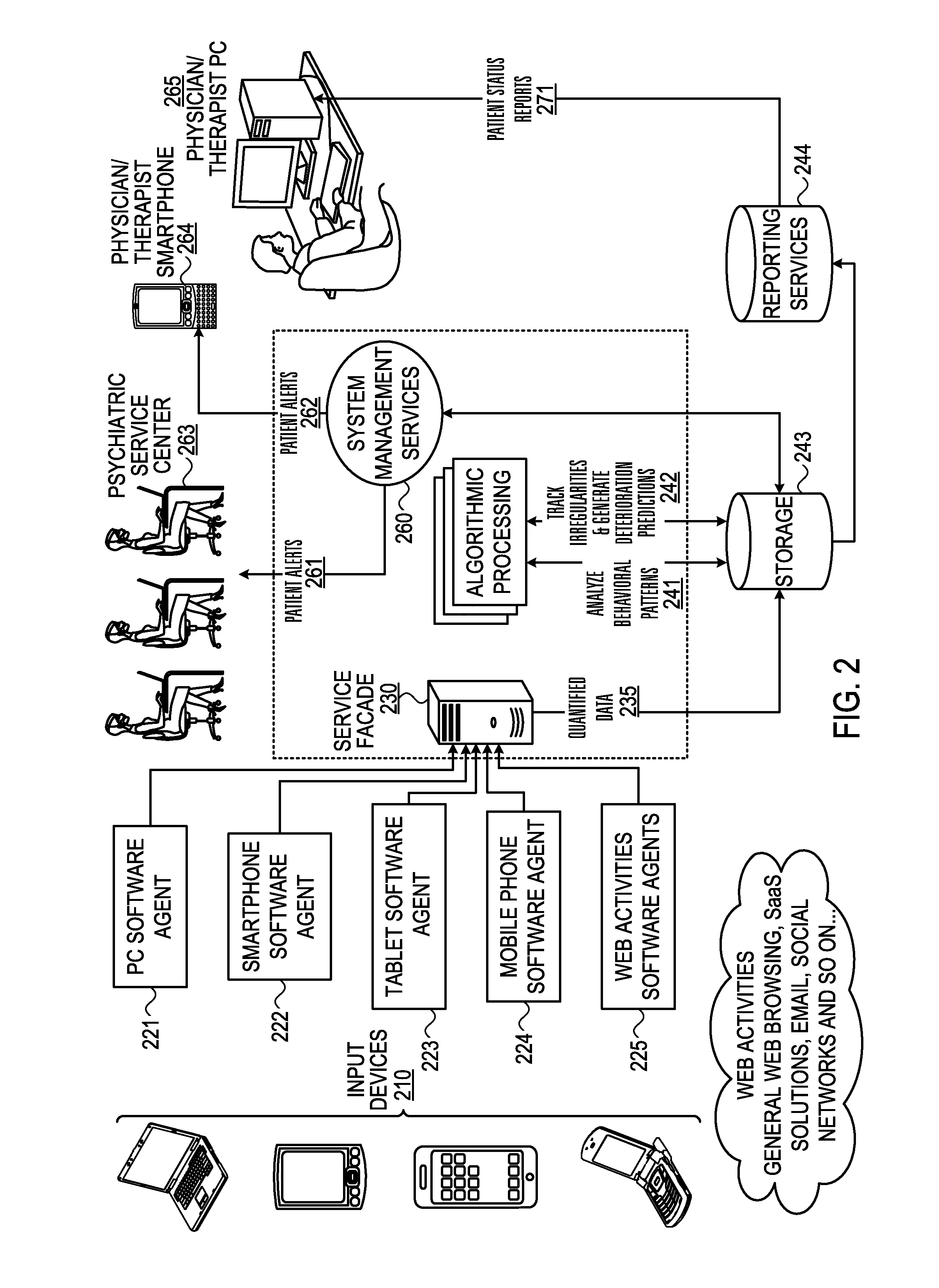
Mental health digital behavior monitoring support system and method
InactiveUS20130297536A1Digital computer detailsMedical automated diagnosisTablet computerSupporting system
A system and method for monitoring a user's mental health tor and collect data concerning. The user's use of electronic devices is tracked, such as usage of his mobile phone, tablet and his web activity. The invention “learns” each patient's unique behavioral patterns to be used as a “base line” representing the steady state (chronic phase) of the patient. The algorithmic processing unit detects any irregularities in a patient's behavioral patterns and produces a deterioration prediction. If it is determined that a threshold is exceeded, an alert is sent to a health professional.
Owner:ALMOSNI BERNIE +1
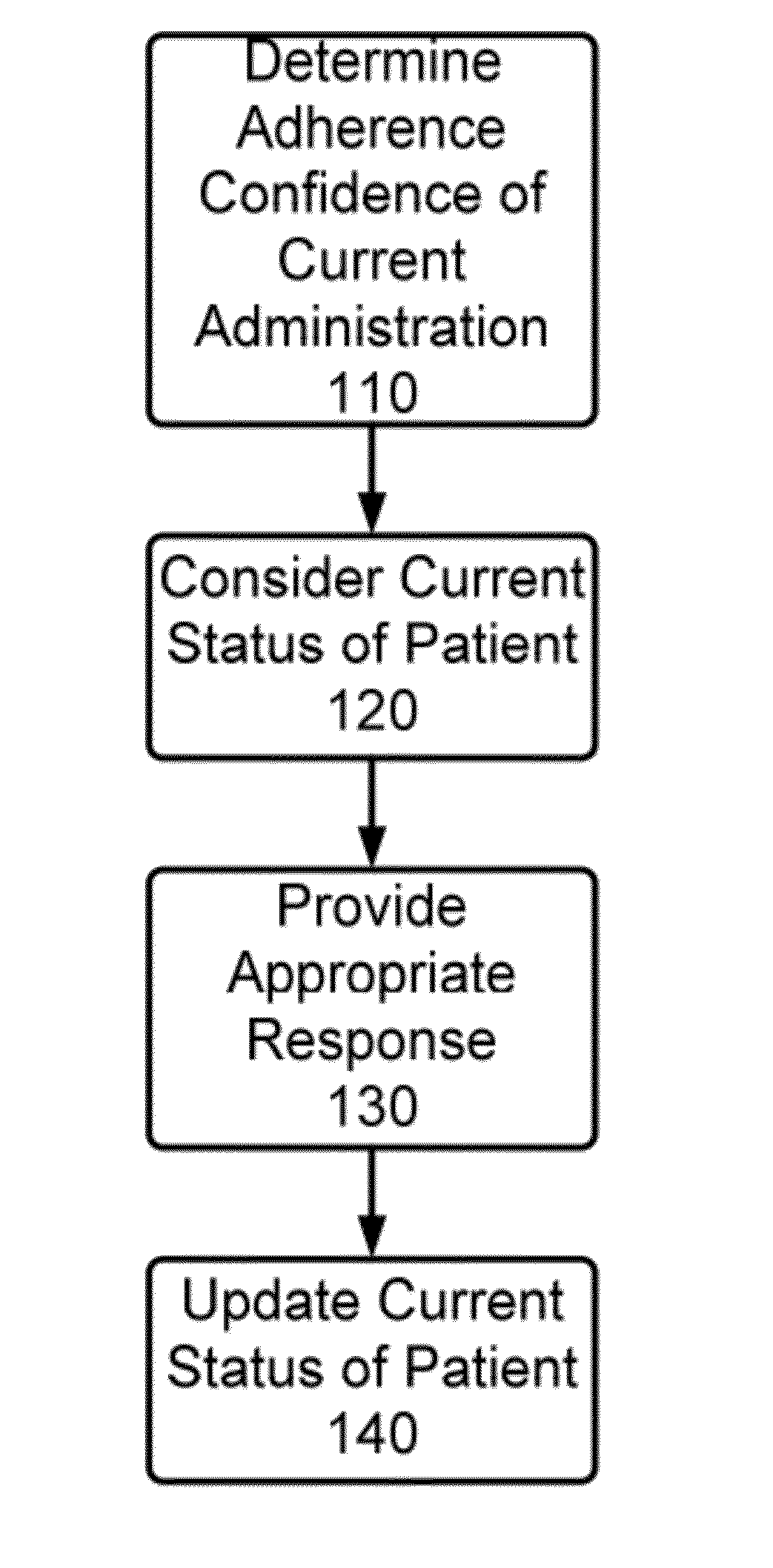
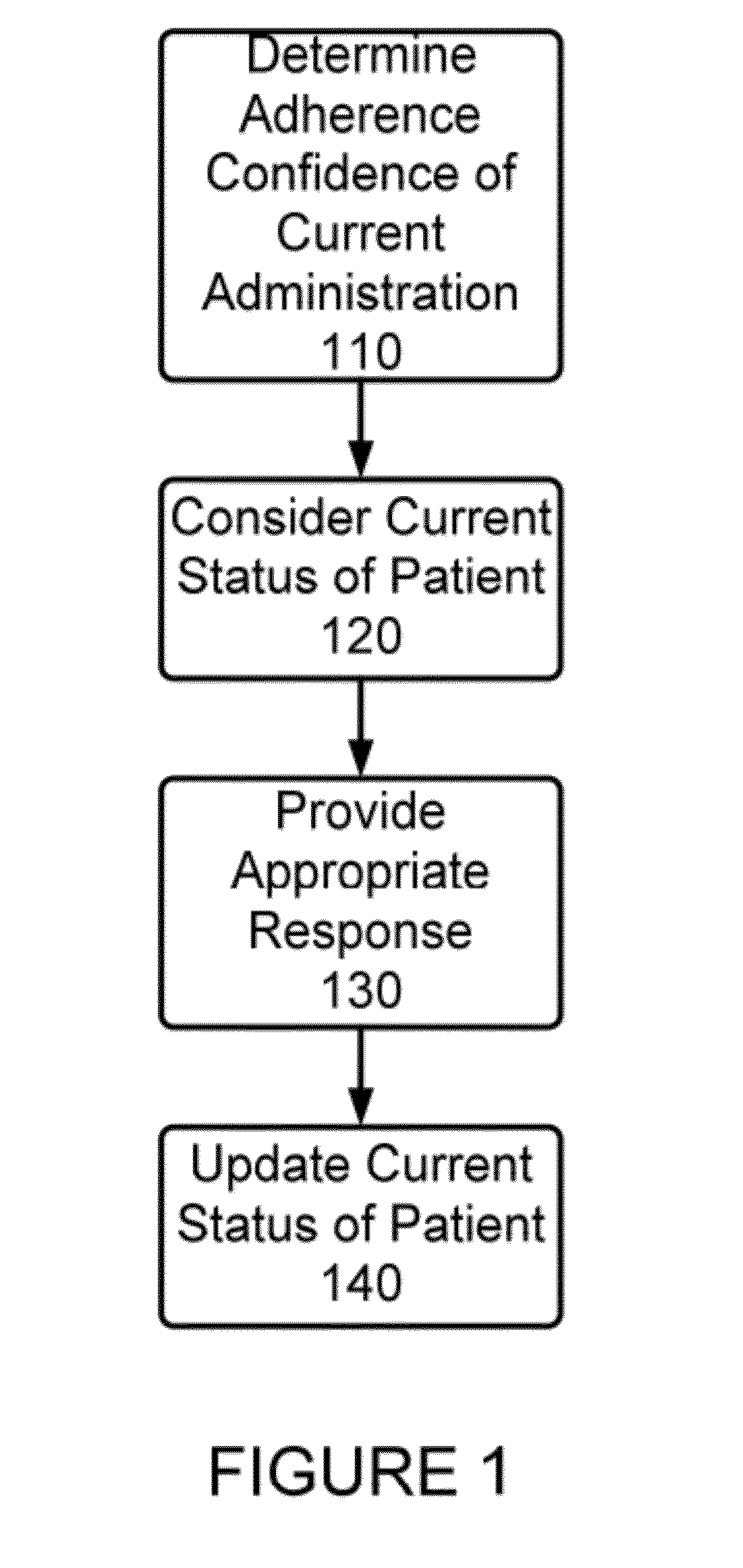
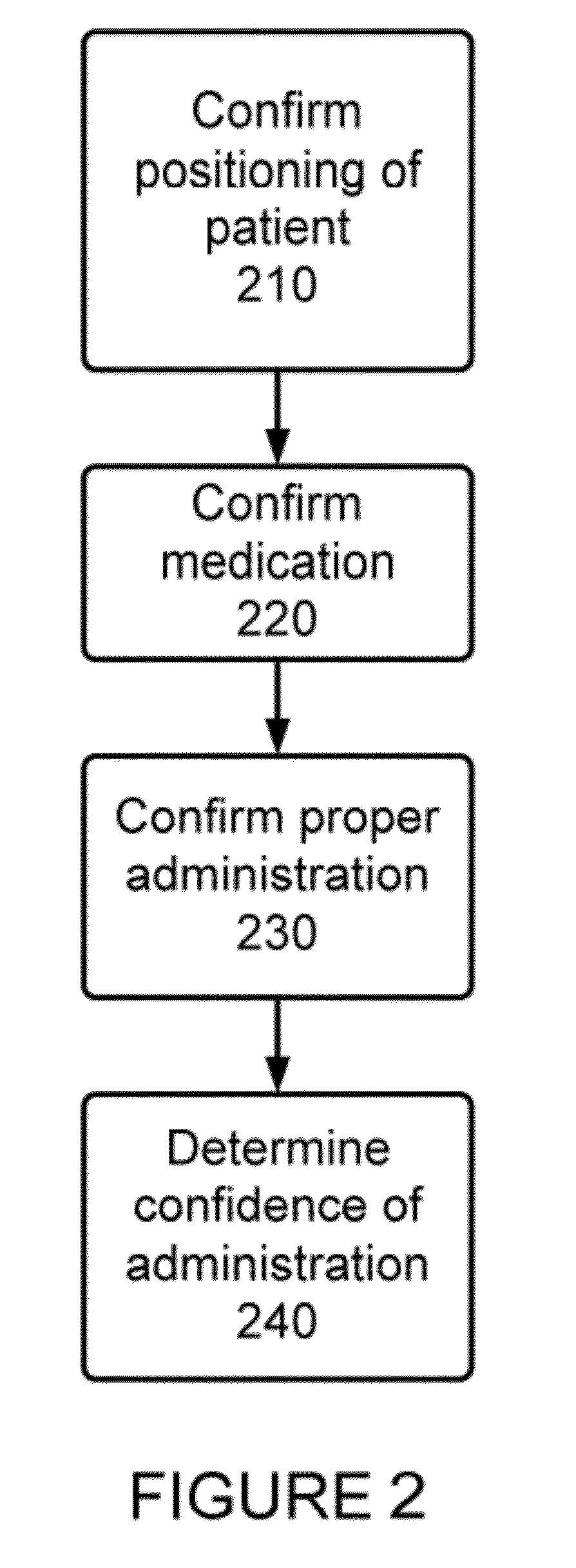
Method and Apparatus for Monitoring Medication Adherence
InactiveUS20120316897A1Quick switchReduce expensive hospital readmissionImage enhancementData processing applicationsMedication adherenceClinical psychology
A method and apparatus for monitoring medication adherence. The method includes the steps of determining a present adherence state of a patient, receiving video analysis information reporting on a medication administration session, and determining a next adherence state of a patient based upon the present adherence state of the patient and the video analysis information.
Owner:AIC INNOVATIONS GRP
Transgenic animal model for modelling pathological anxiety, a method for identifying compounds for treatment of diseases or disorders caused by pathological anxiety and a method for using wfs1 protein as a target for identifying effective compounds against pathological anxiety
InactiveUS20100146645A1Low environmental changeImprove anxietyCell receptors/surface-antigens/surface-determinantsBiological testingTolerabilityClinical psychology
The invention discloses the transgenic animal model for pathological anxiety, the method to generate this model, the method to test drugs and drug candidates for the treatment of pathological anxiety and the method to use Wfs1 as target for screening of new anxiolytic drugs to treat pathological anxiety. This animal model is useful to test potential drug candidates for the treatment of diseases caused by pathological anxiety and to screen therapeutic compounds for the psychiatric disorders caused by reduces stress-tolerance and deficiency in adaptation to environmental challenges.
Owner:TARTU ULIKOOL THE UNIV OF TARTU
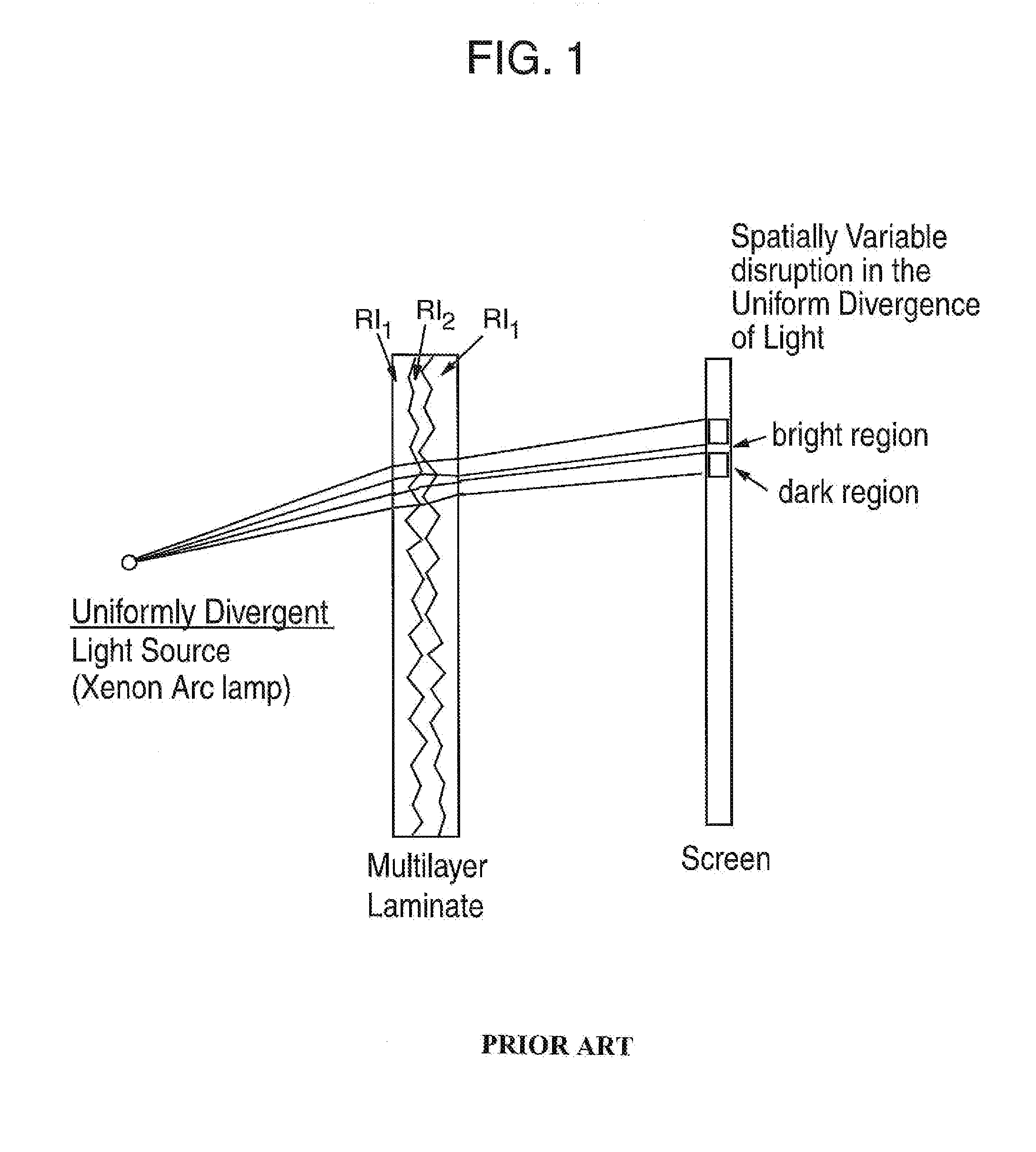
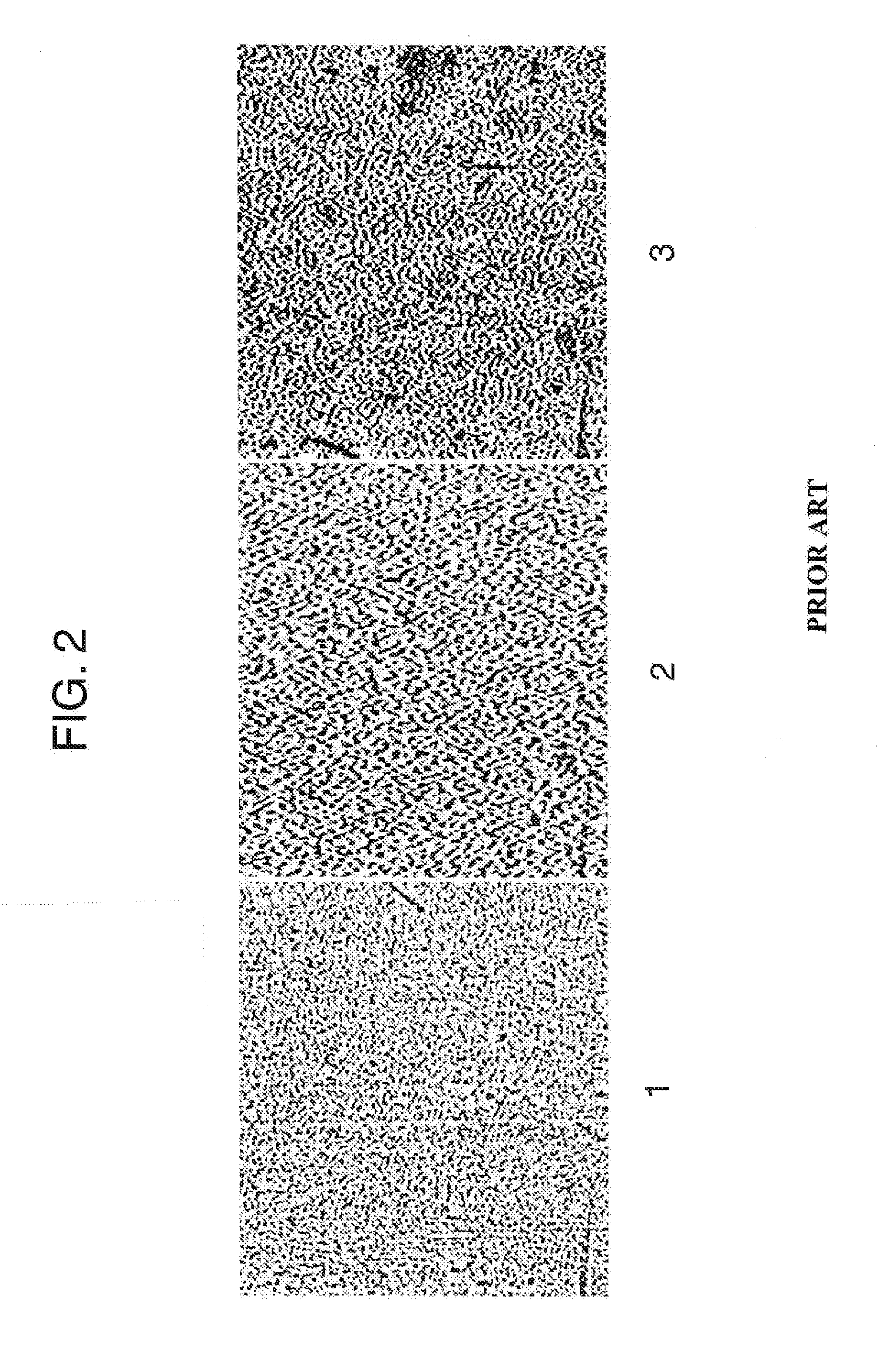
Clear Mottle Analyzer for Multilayer Laminates
This disclosure relates to systems, devices and methods utilized to quantitatively measure the level of mottle in multilayer laminates. The systems and method are generally free of the inconsistencies, variability and inherent biases of the traditional subjective process by which a person of ordinary skill in the art can assess and categorize the mottle of a multilayer laminate. In the method, variations in the images of mottle produced by a shadowgraph are quantified, measured and classified.
Owner:SOLUTIA INC
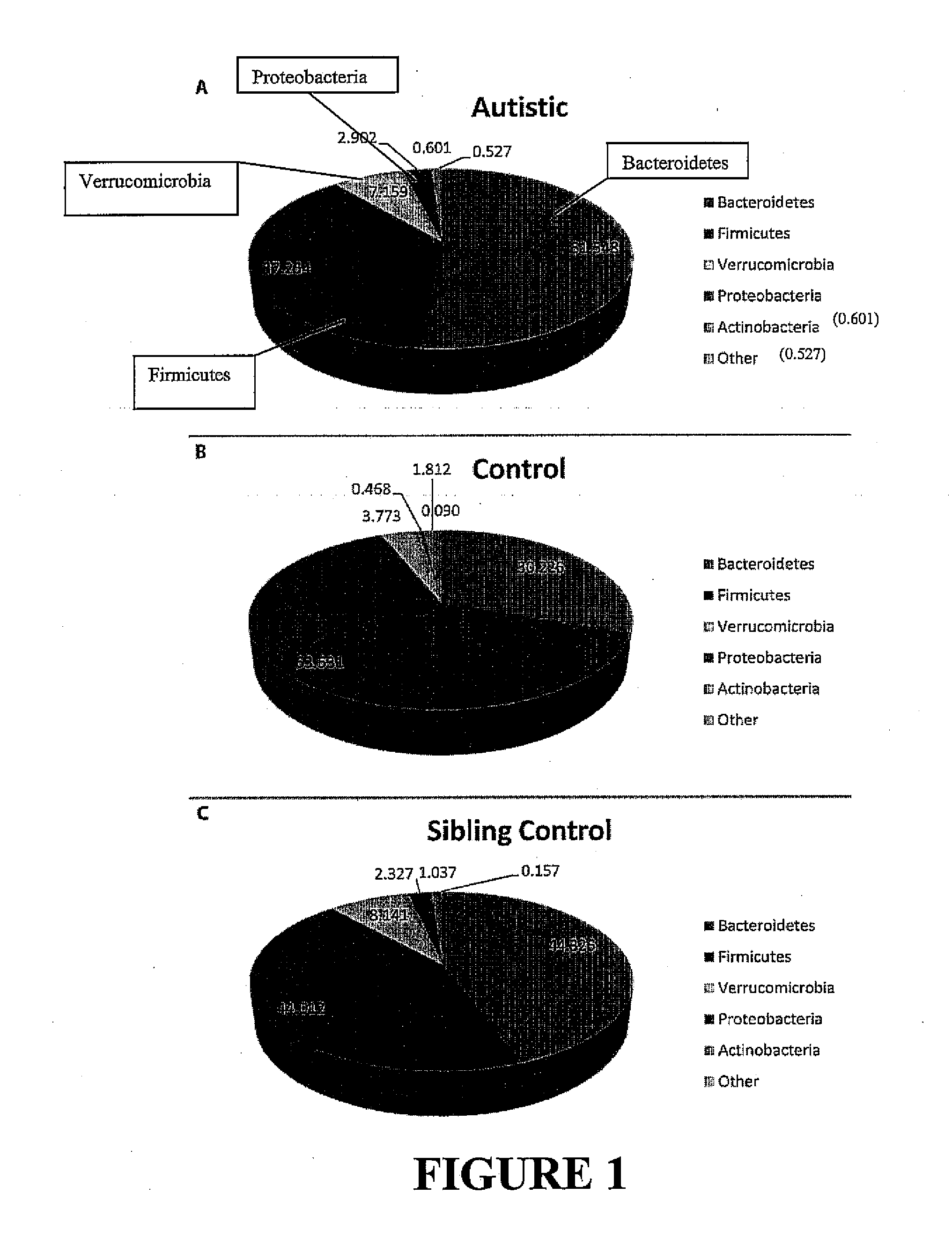
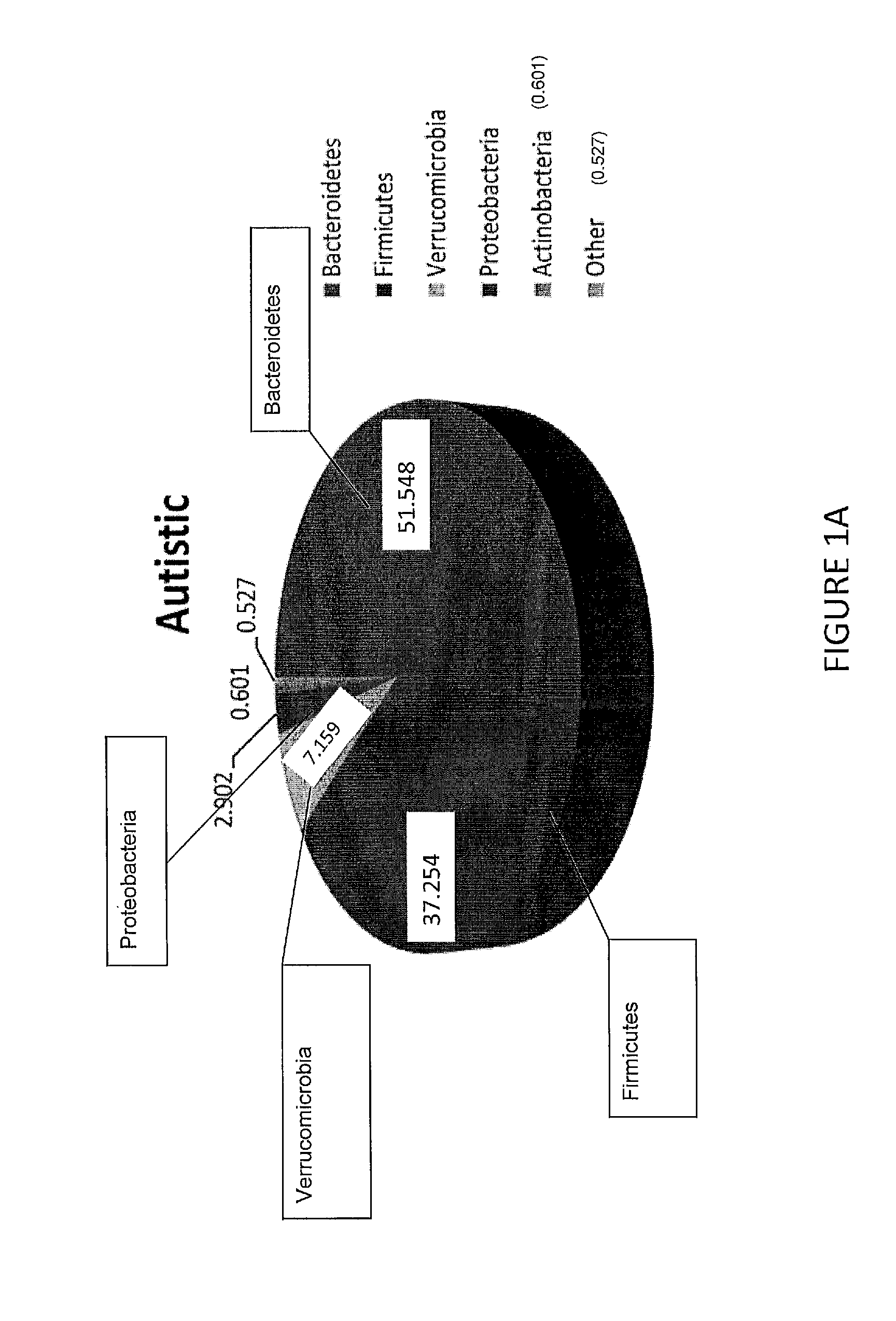
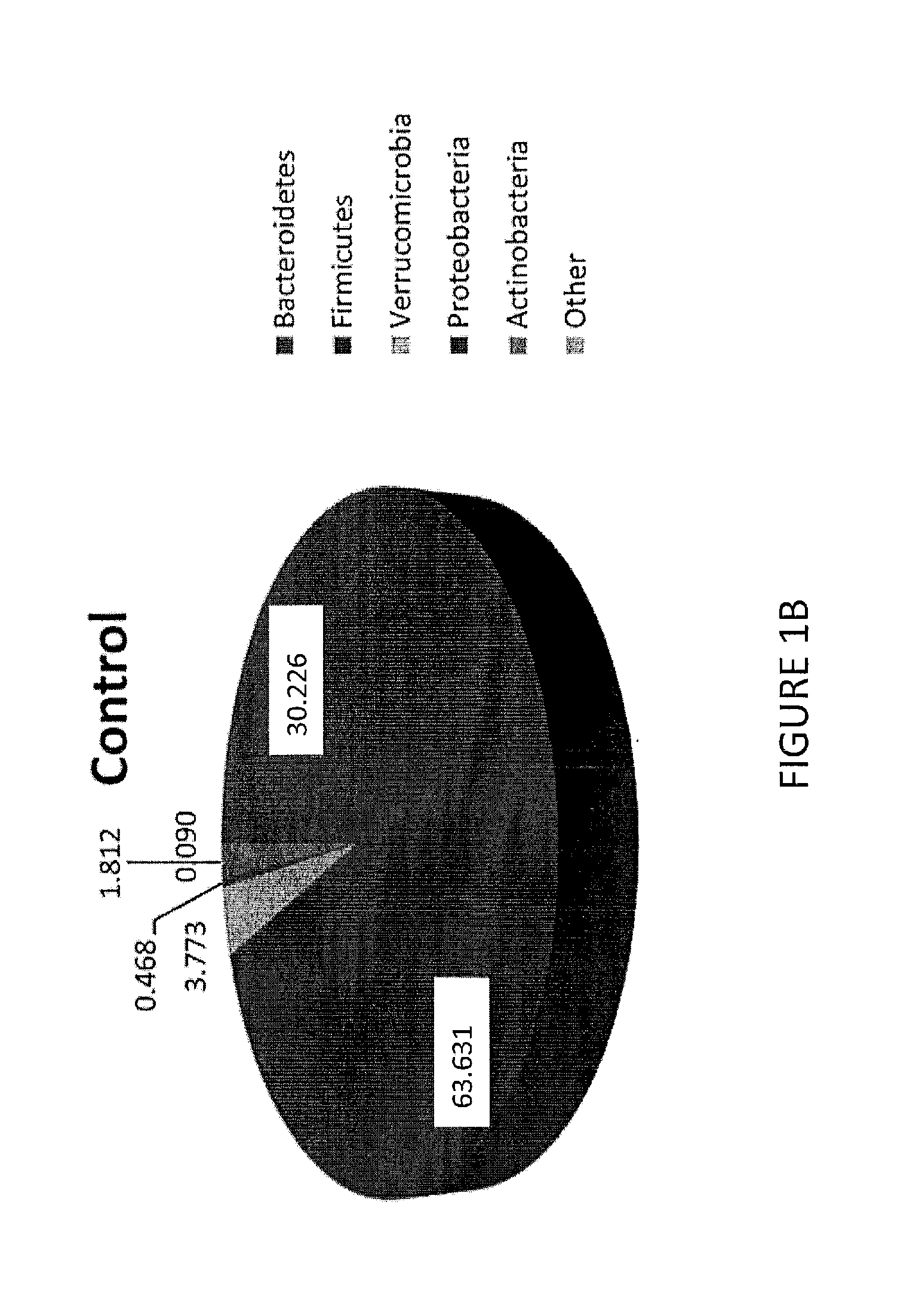
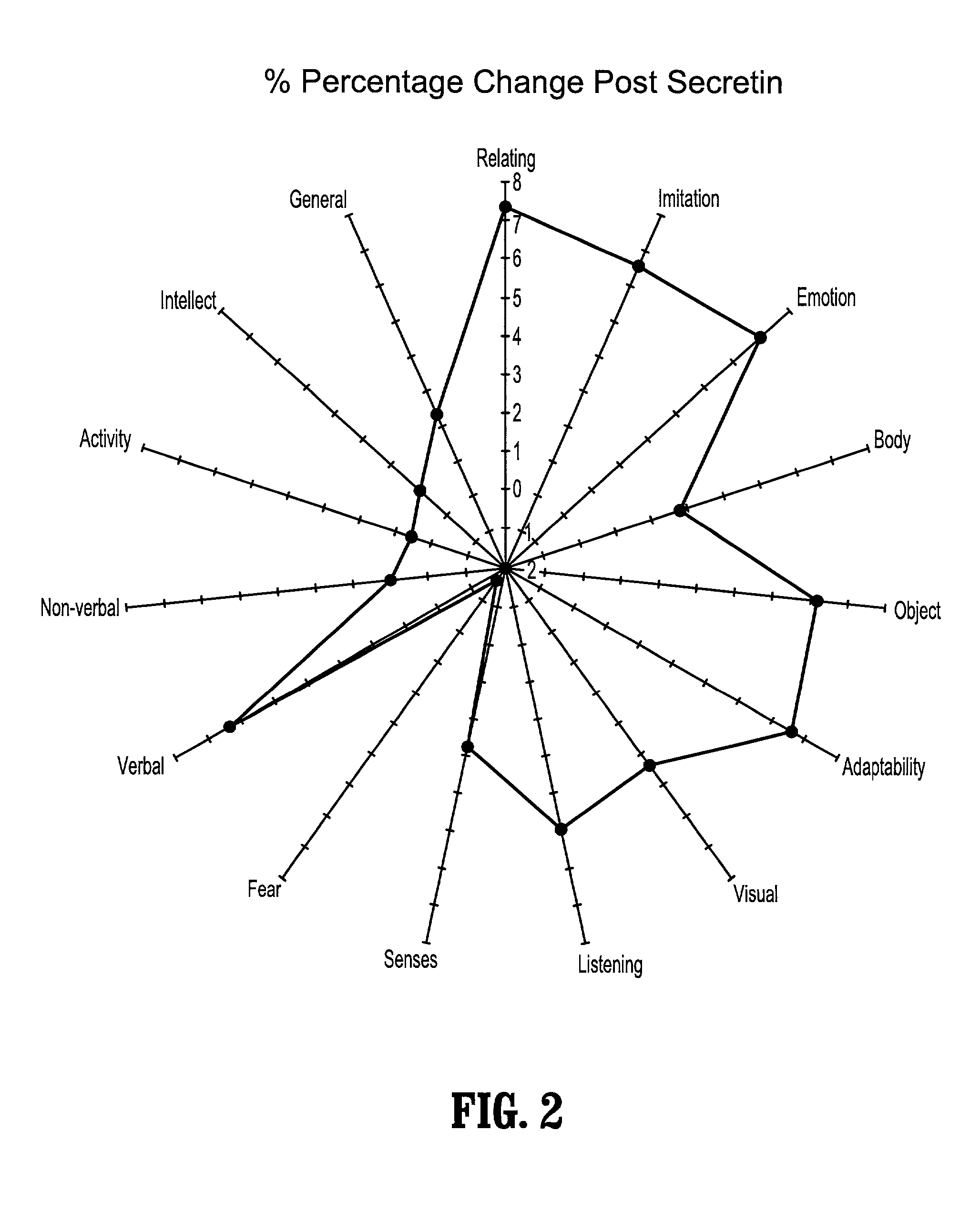
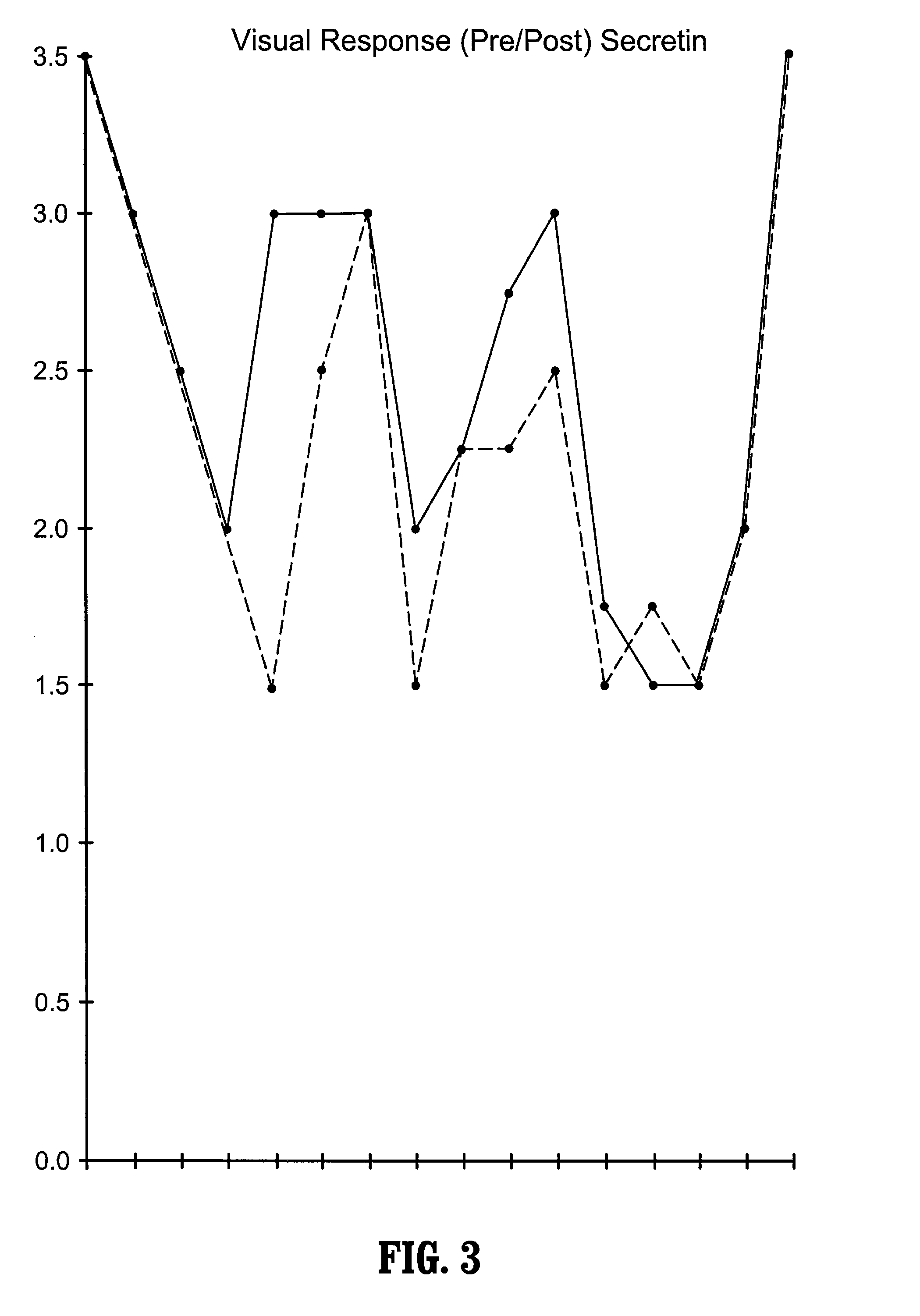
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Nutritional supplements for healthy memory and mental function
InactiveUS20080213401A1Optimizing mental energyImprove mental functionBiocideNervous disorderMental functionsMental nerve
Owner:APPLIED COGNITIVE SCI
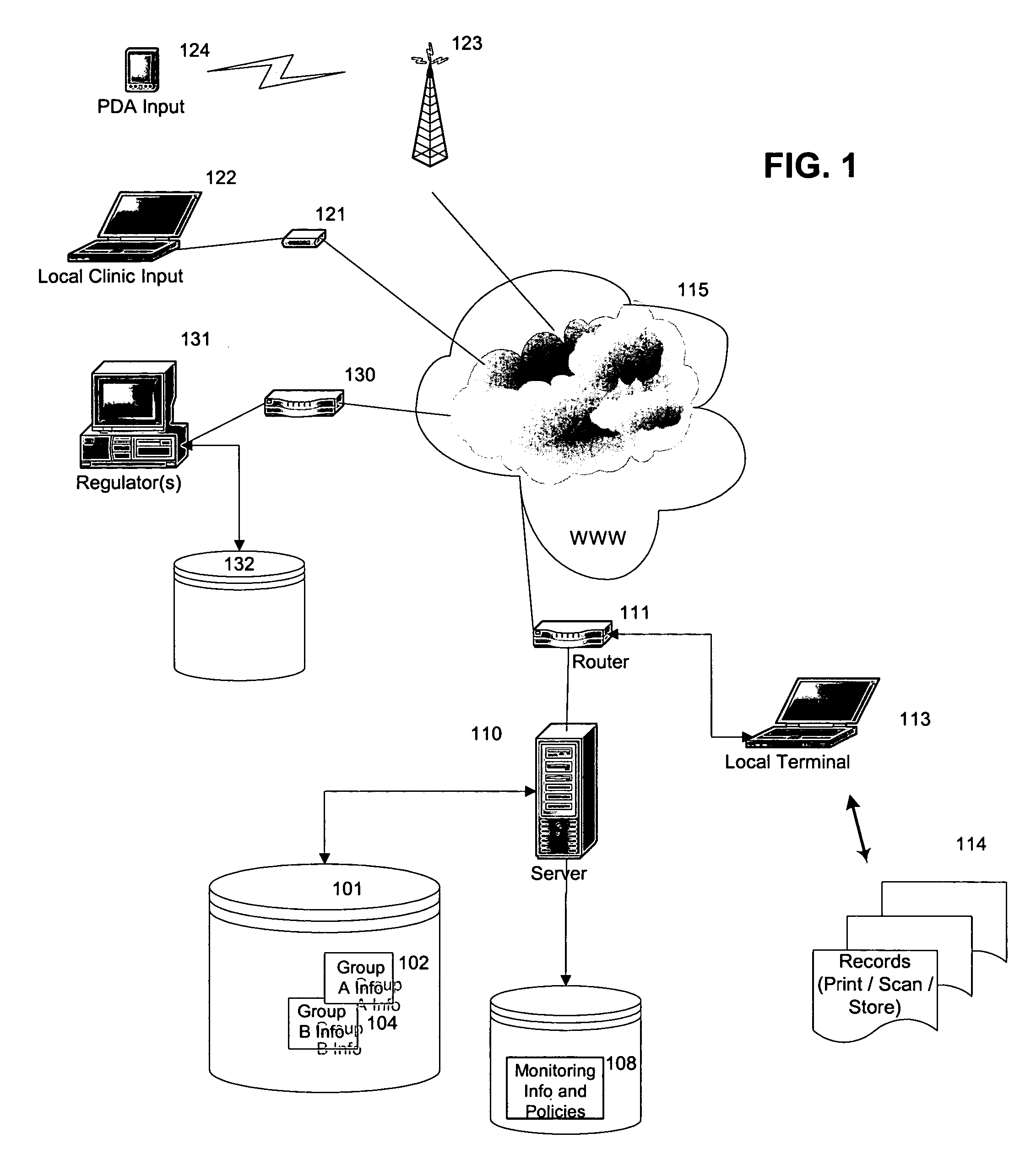
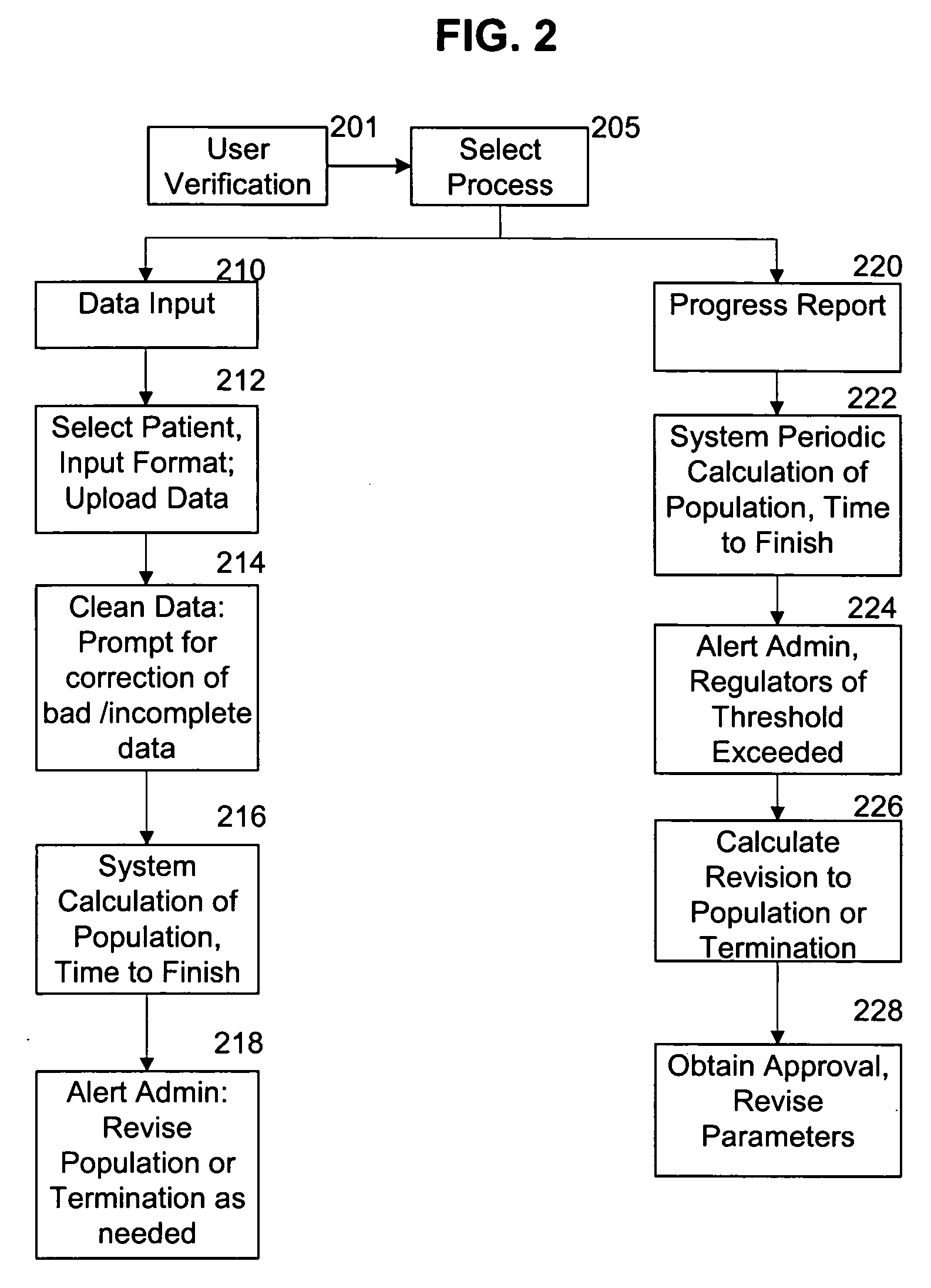
System for continuous outcome prediction during a clinical trial
InactiveUS20060129326A1Easy to controlData processing applicationsComputer-assisted medical data acquisitionClinical efficacyClinical psychology
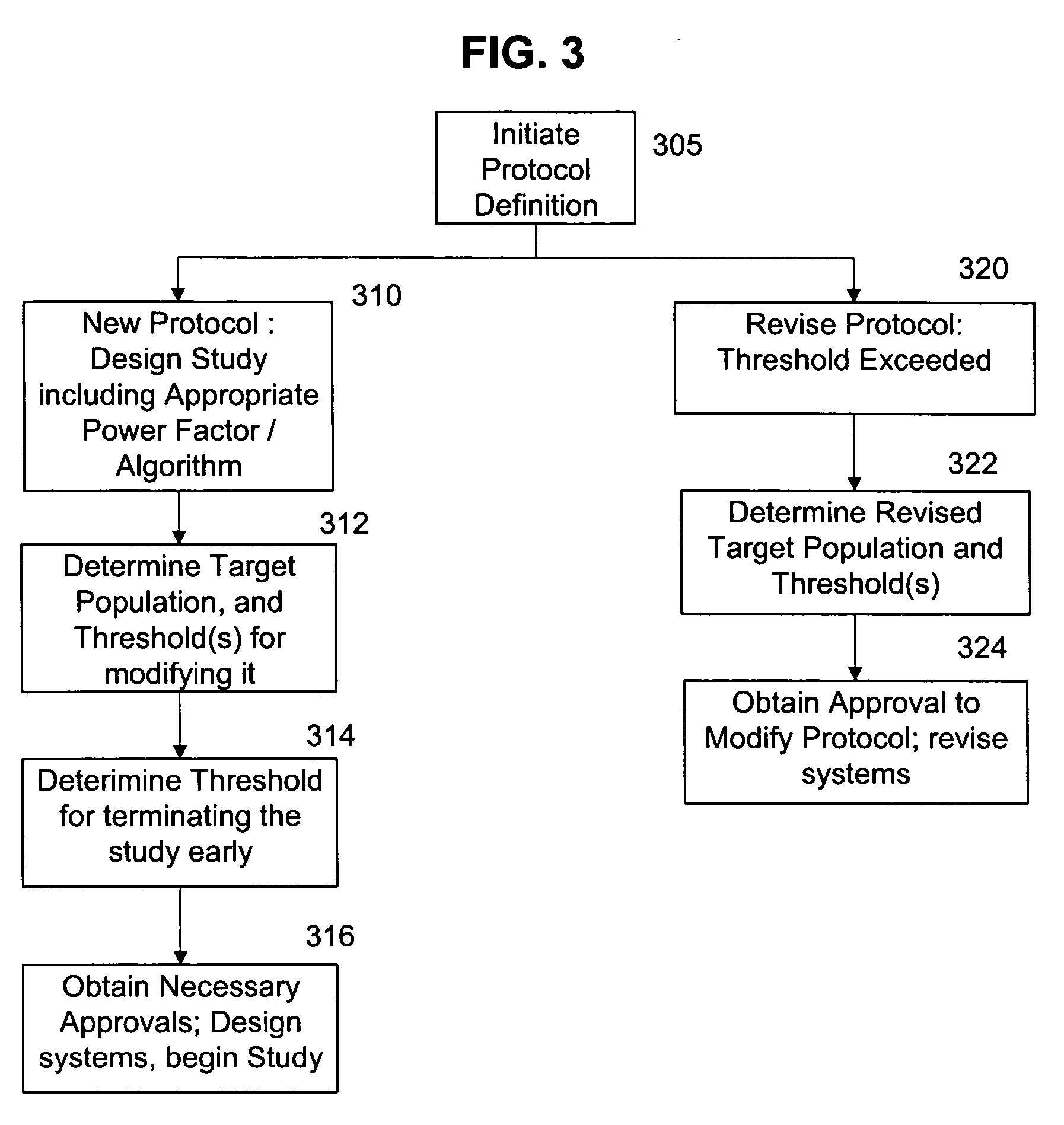
The present invention provides a method, apparatus, and computer instructions for improved control of clinical trials. In a preferred embodiment, after a clinical trial is initiated, data is regularly cleaned and processed to statistically analyze the data. The outcome includes a predictive measure of the timing and level by which the study will achieve one or more statistically significant levels, allowing mid-course modifications to the study (e.g., in population size, termination, etc.). Modification can be planned as part of the initial protocol, using thresholds or other appropriate criteria relating to the statistical outcome, making possible pre-approved protocol changes based on the statistical findings. This process has significant implications for the management of clinical studies, including ensuring the minimum possible time and number of patients are used in clinical studies to either prove (or disprove) the clinical efficacy of drugs or treatments.
Owner:AZERA RES
Psychotic manifestation and mental state evaluation apparatus and evaluation method
ActiveUS7942816B2Increase probabilityMedical data miningMental therapiesMental stateClinical psychology
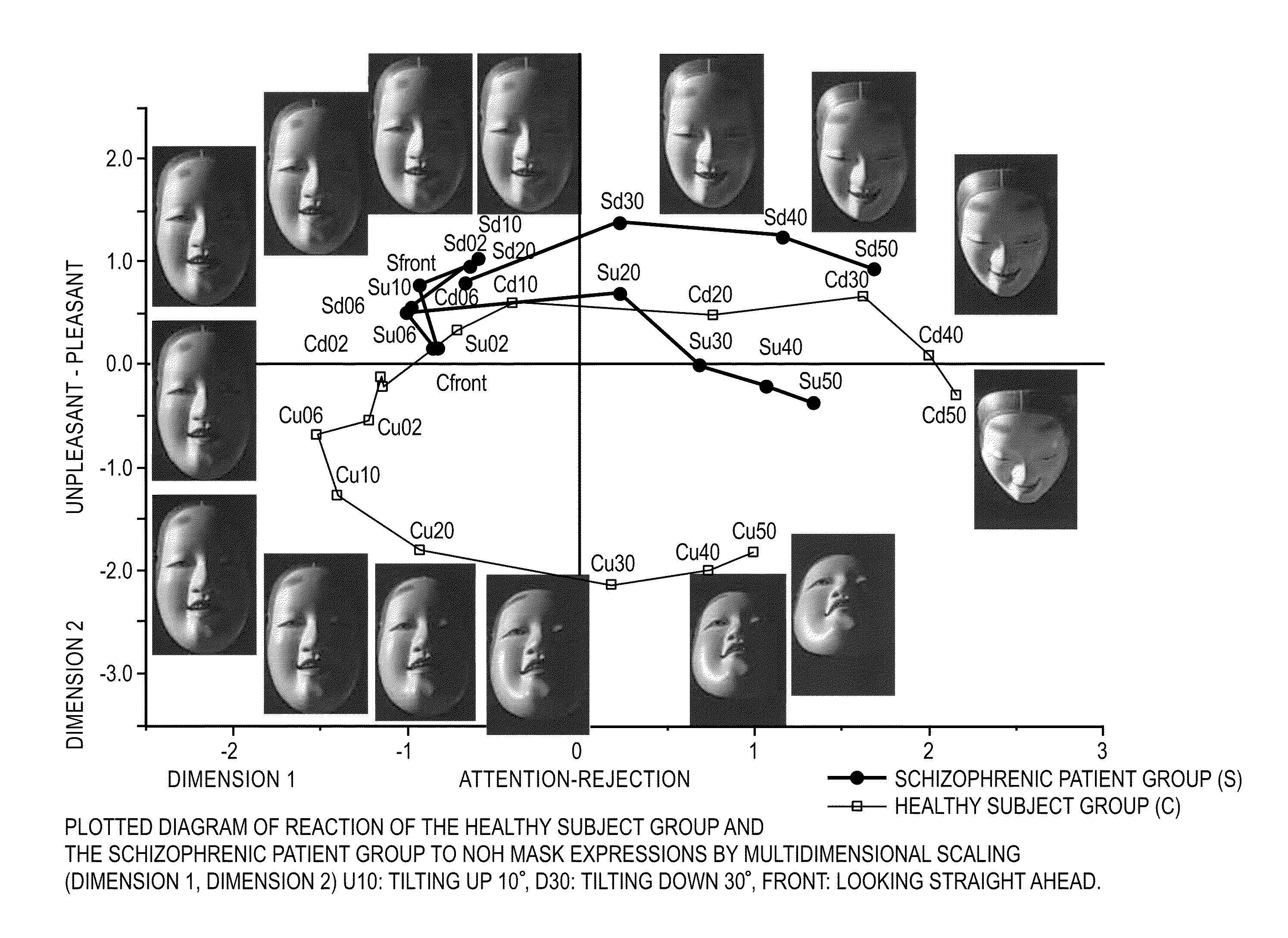
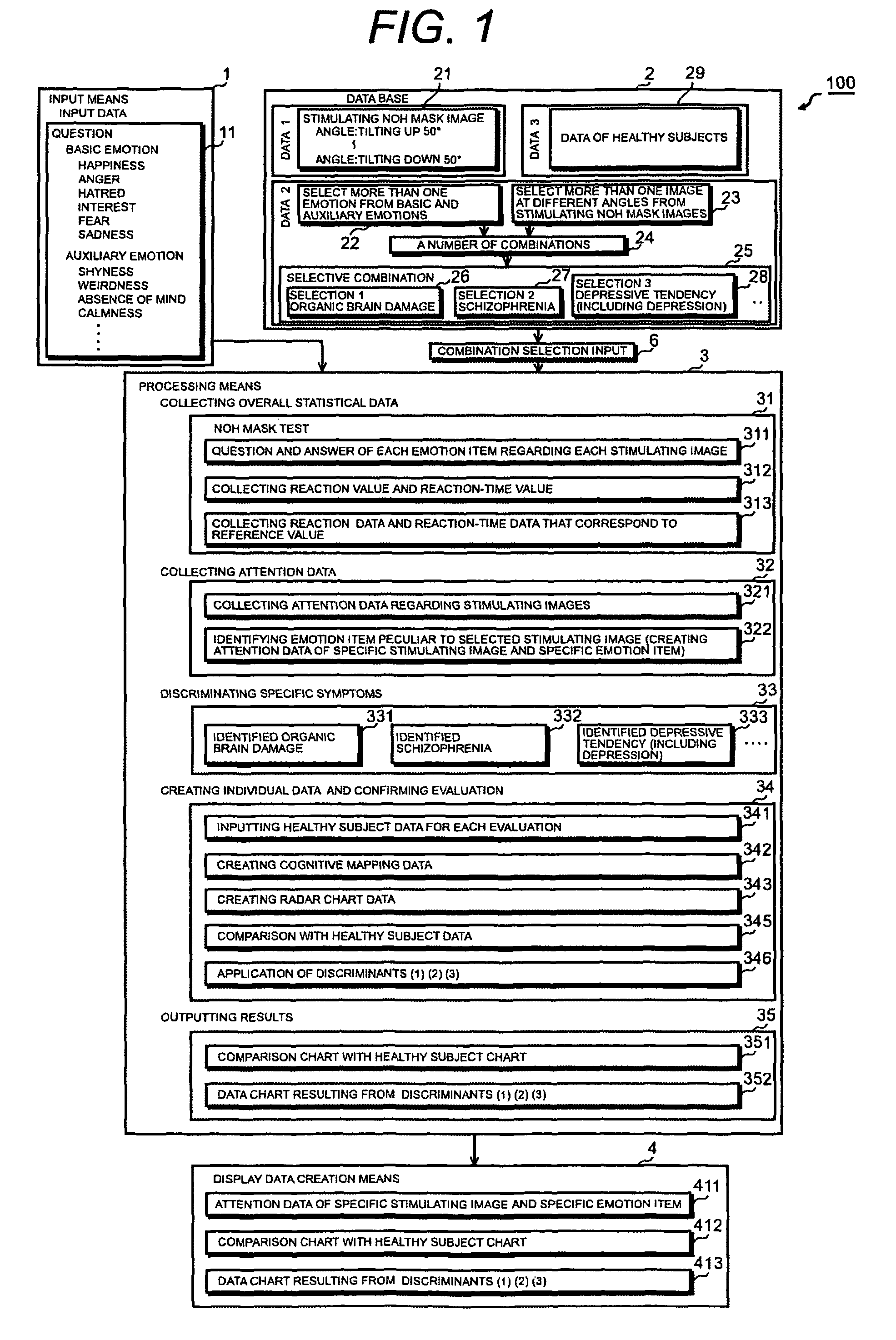
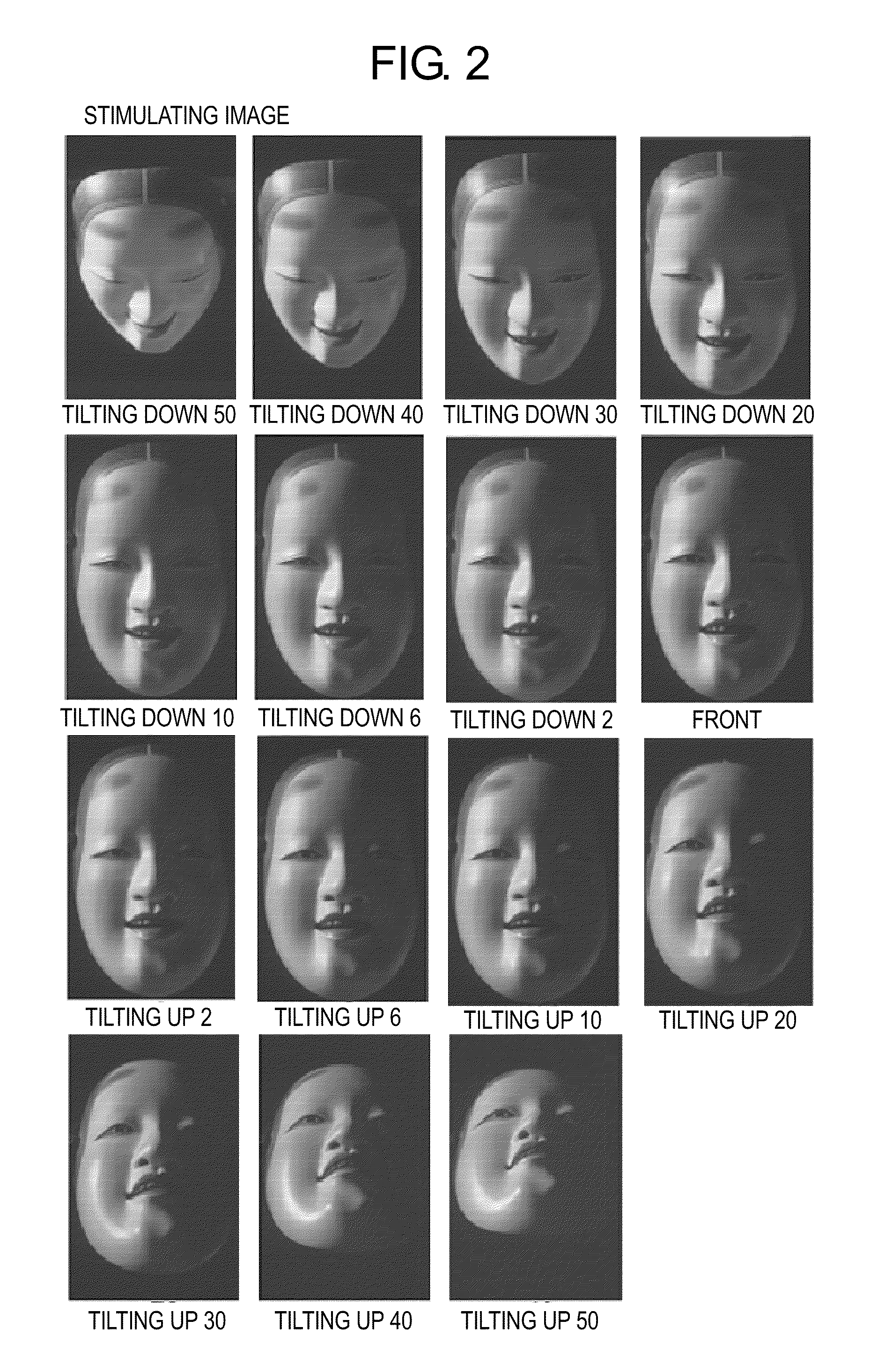
A psychotic manifestation and mental state evaluation apparatus and method which can individually discriminate among symptoms by taking advantage of stimulating Noh mask images and also evaluate with a high probability whether a person is suffering from a specific symptom at the time of diagnosing, clinical examination, assessment, or counseling.
Owner:SATOH SHINJI +1
Compositions and methods for the treatment of depression and other affective disorders
InactiveUS20050037983A1Reduce depressionTreatment alleviationSalicyclic acid active ingredientsBiocideClinical psychologyBiological activation
The present invention relates to compositions and methods for treating depression. The compositions and methods comprise the use of anti-inflammatory compounds alone or in combination with antidepressant compounds to down regulate HPA activation through the targeting of peripheral (non-CNS) cytokines.
Owner:NEUROCURE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com