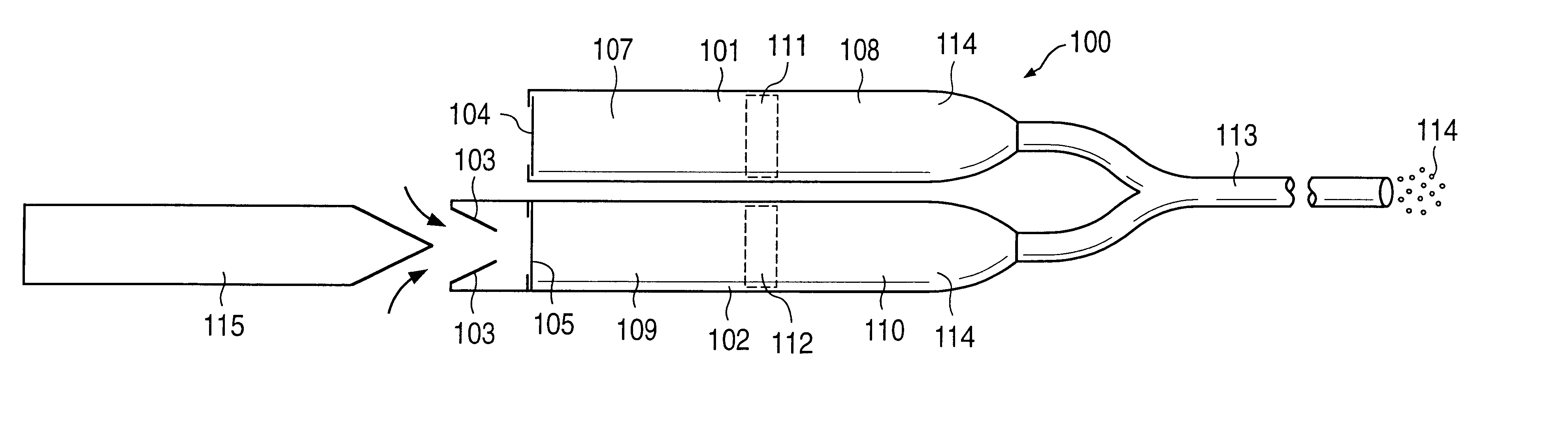
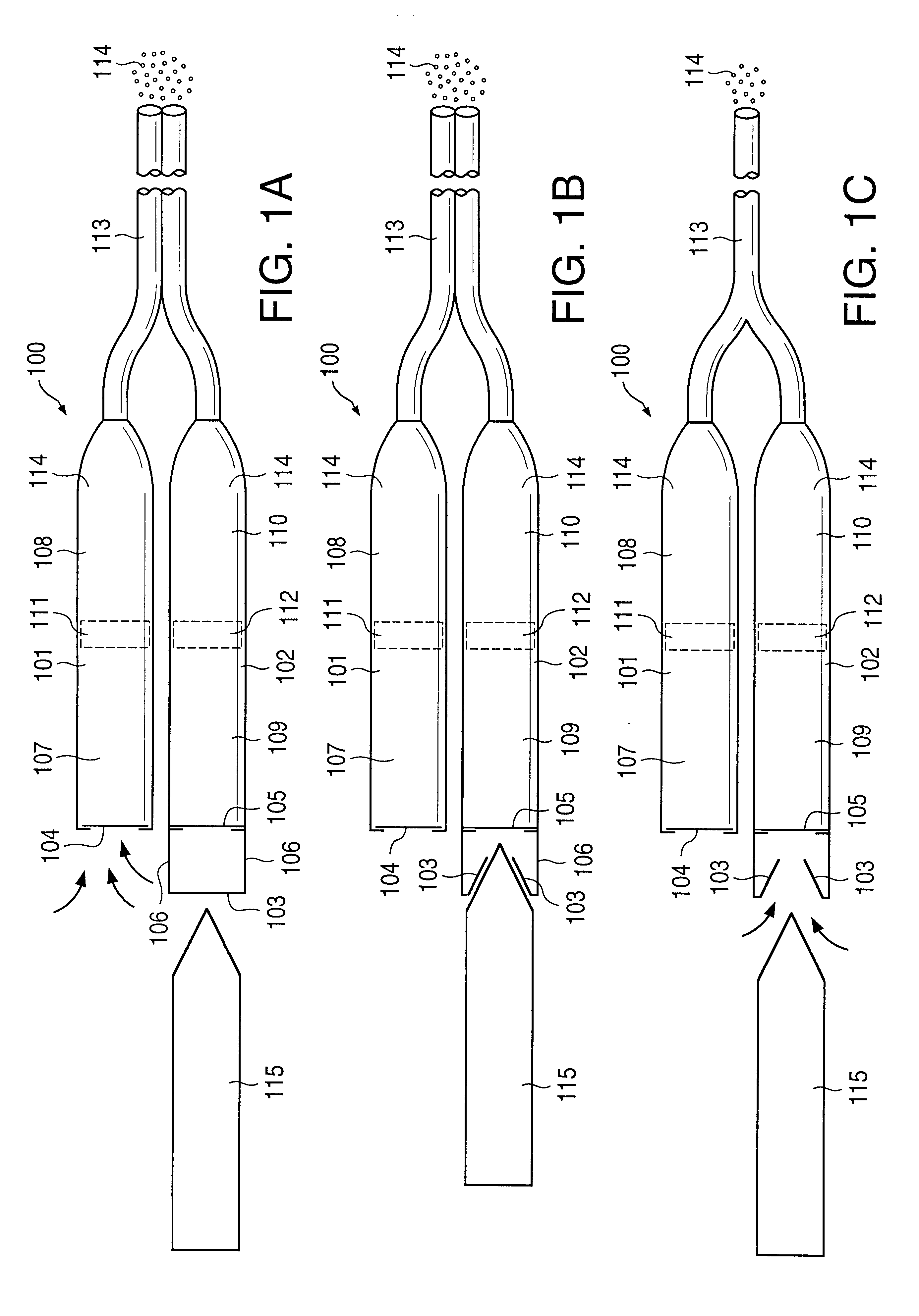
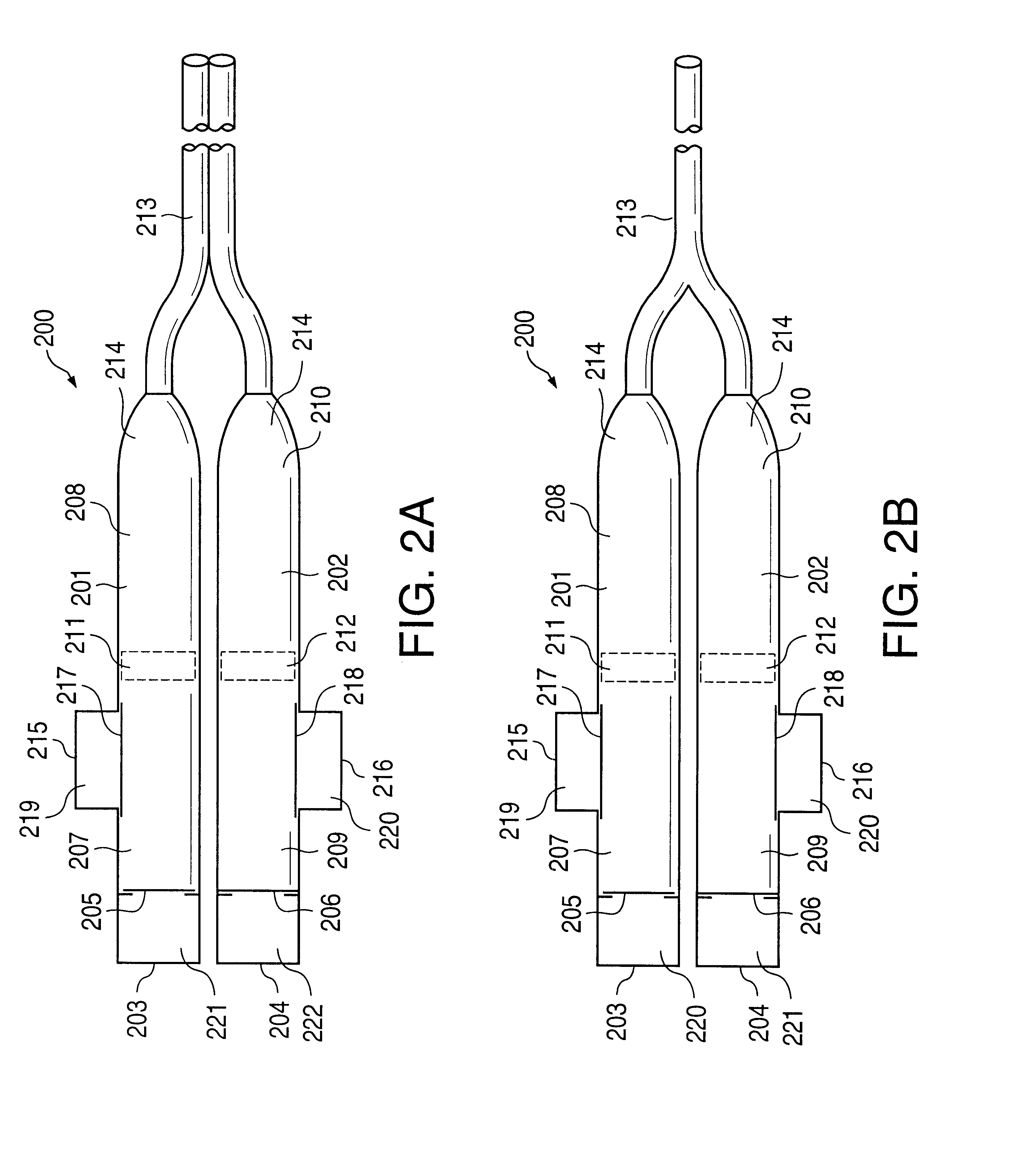
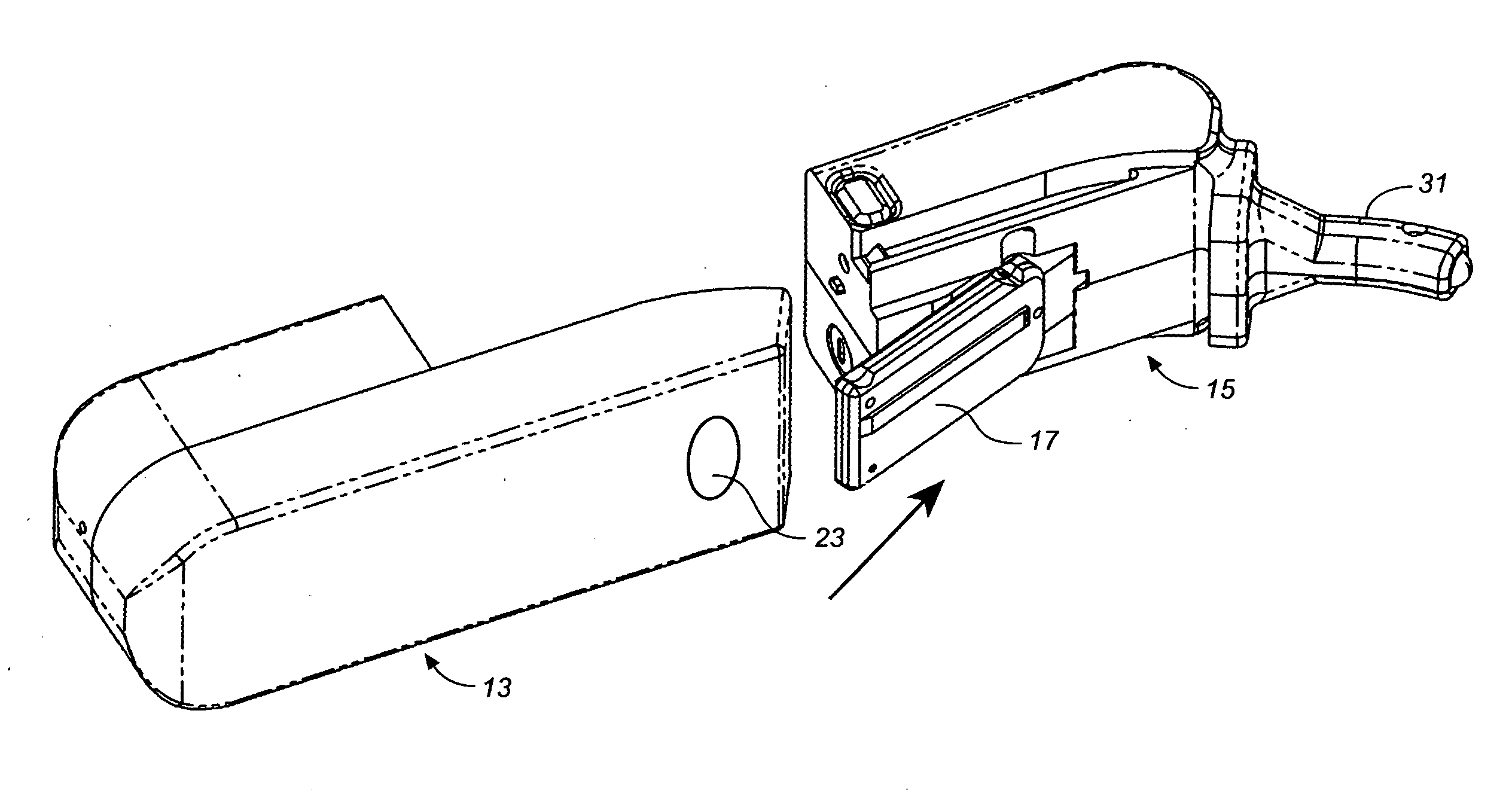
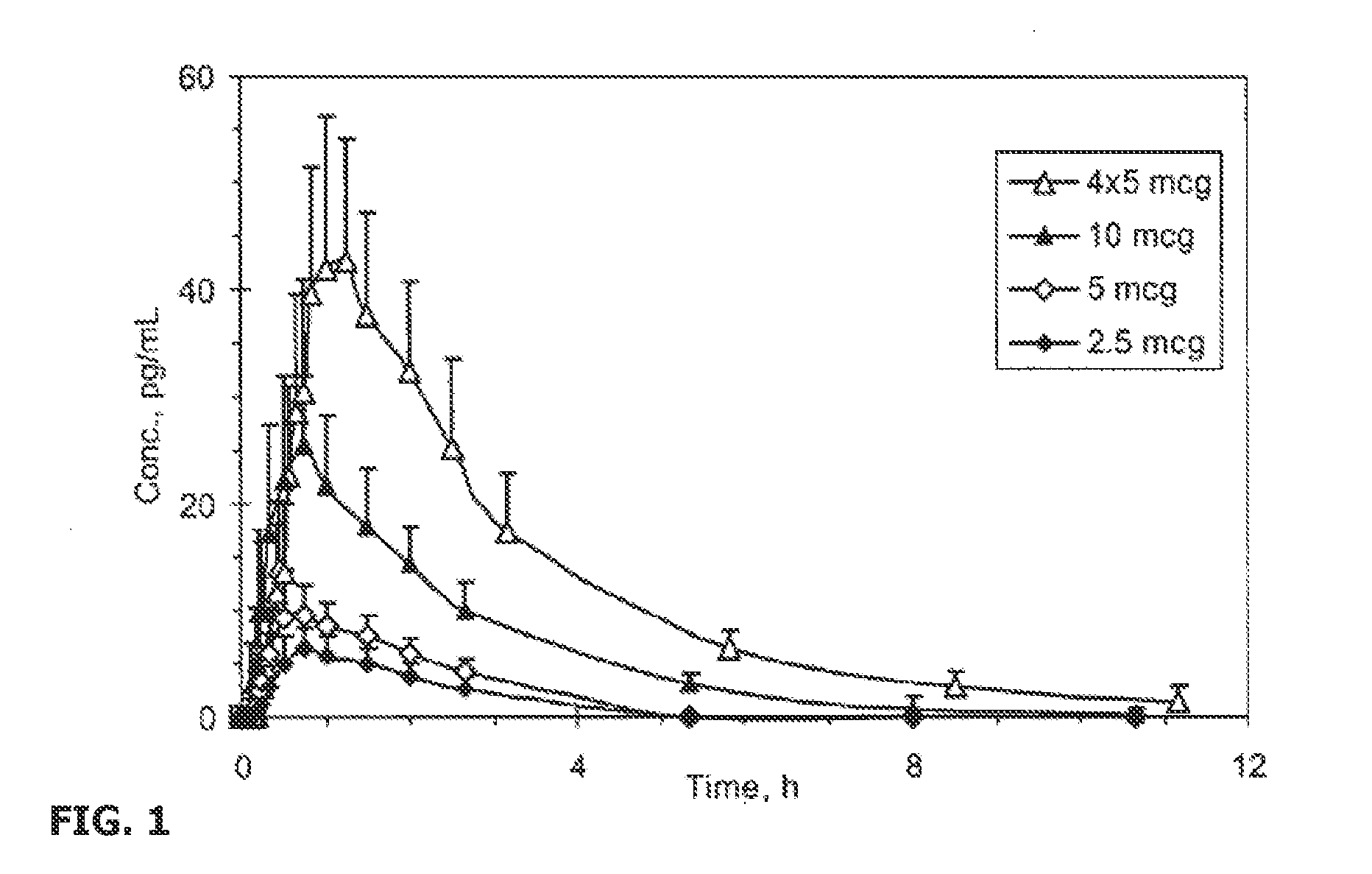
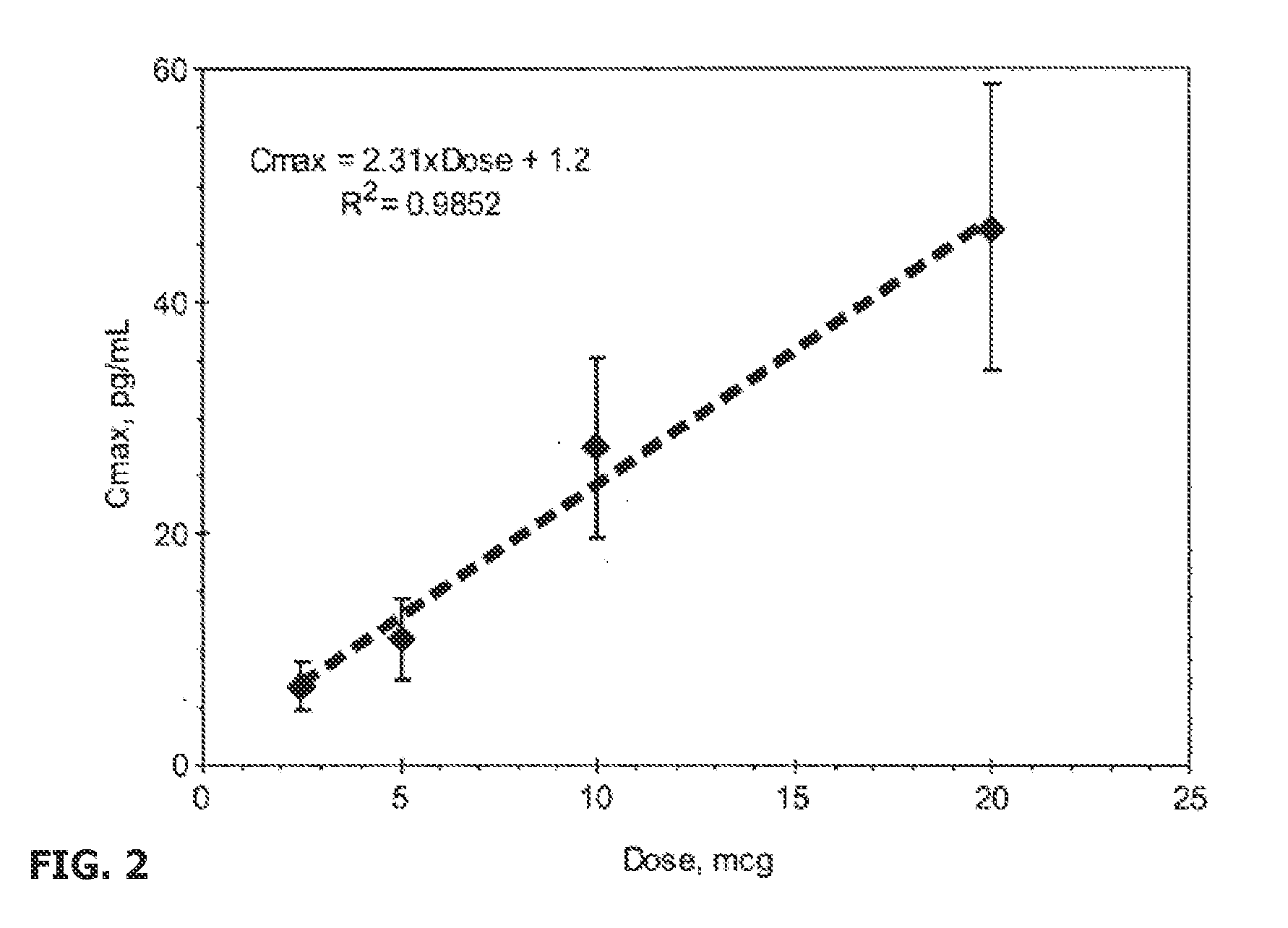
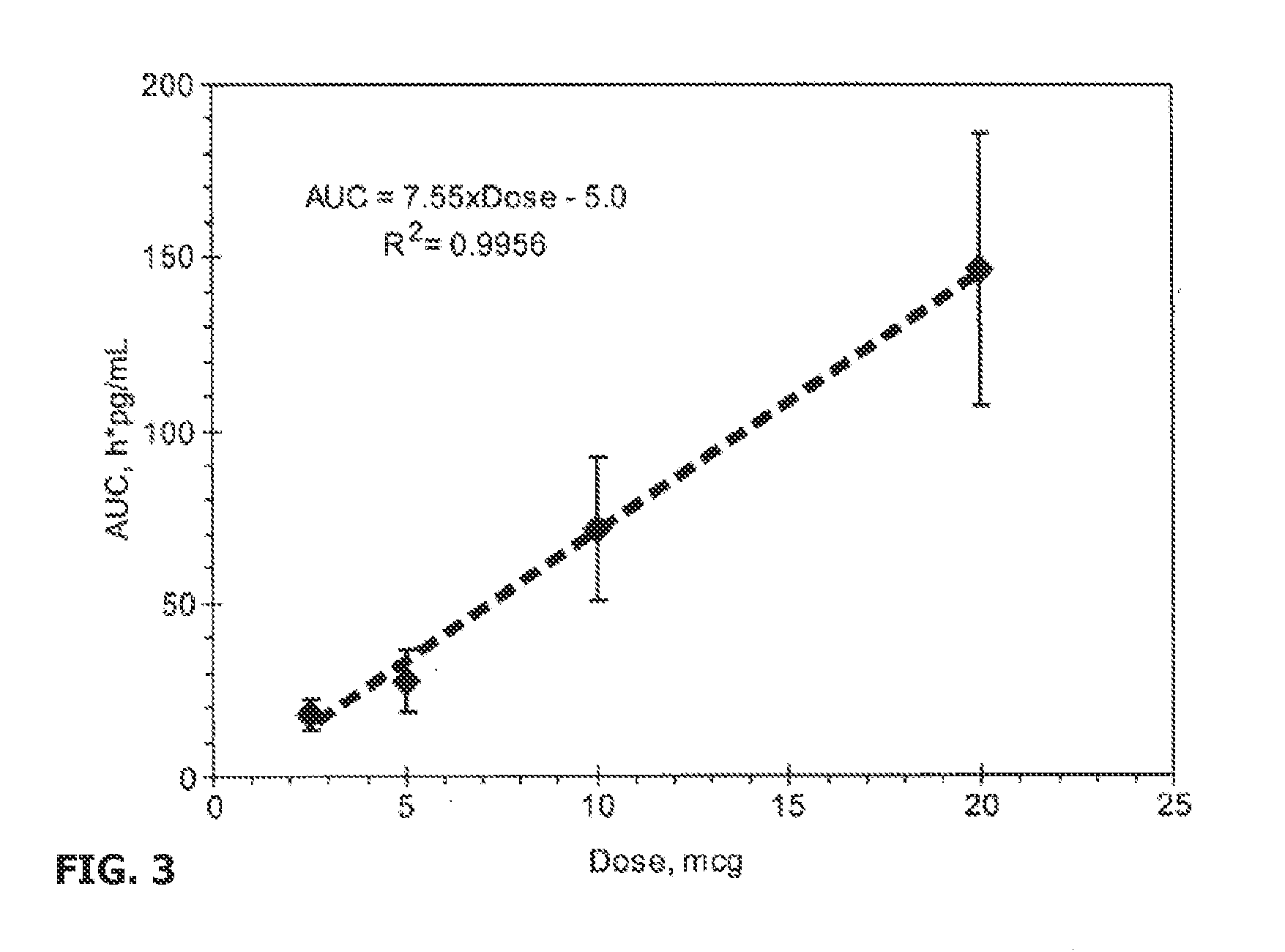
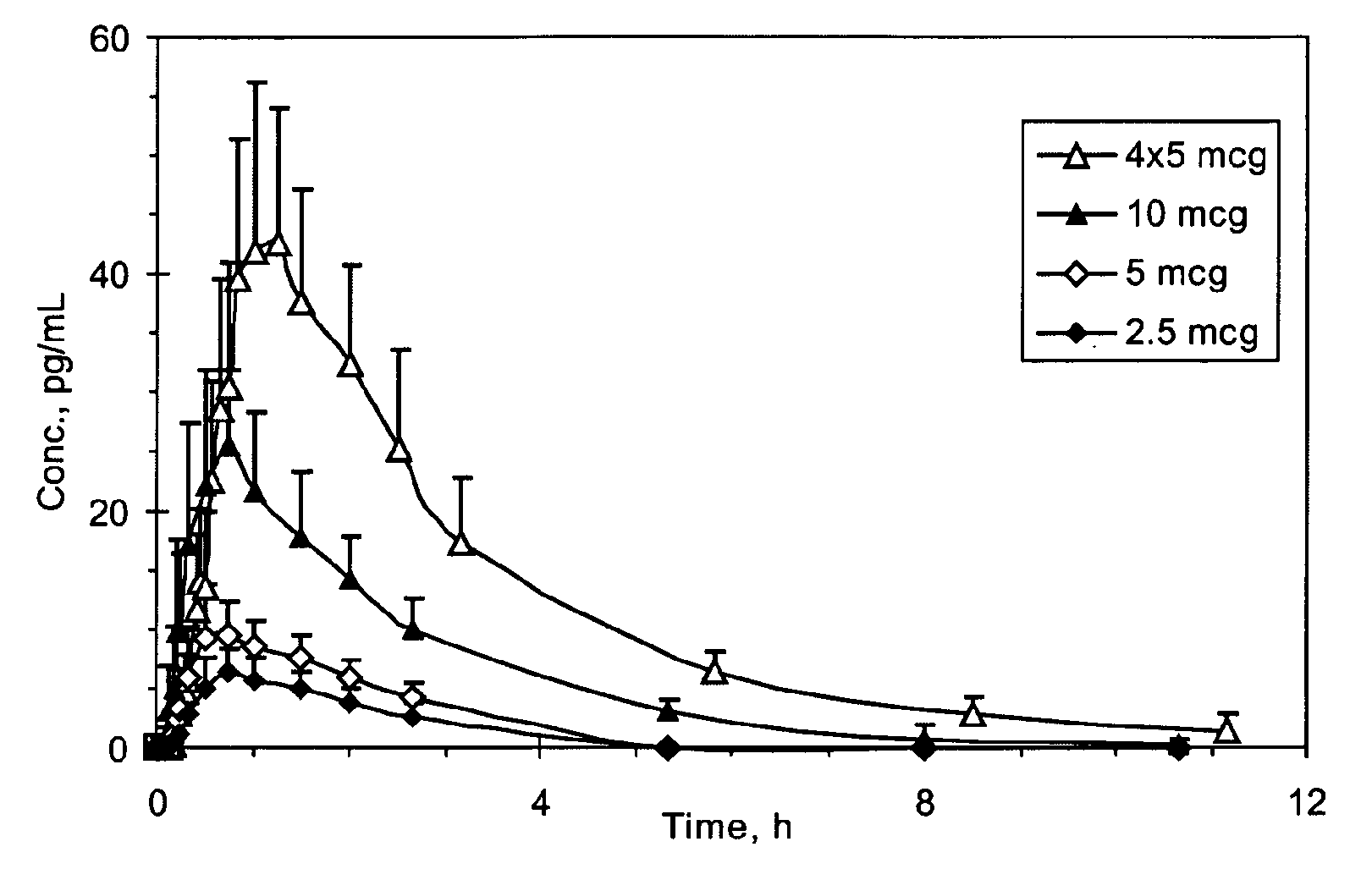
Patents
Literature
72 results about "Sufentanil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
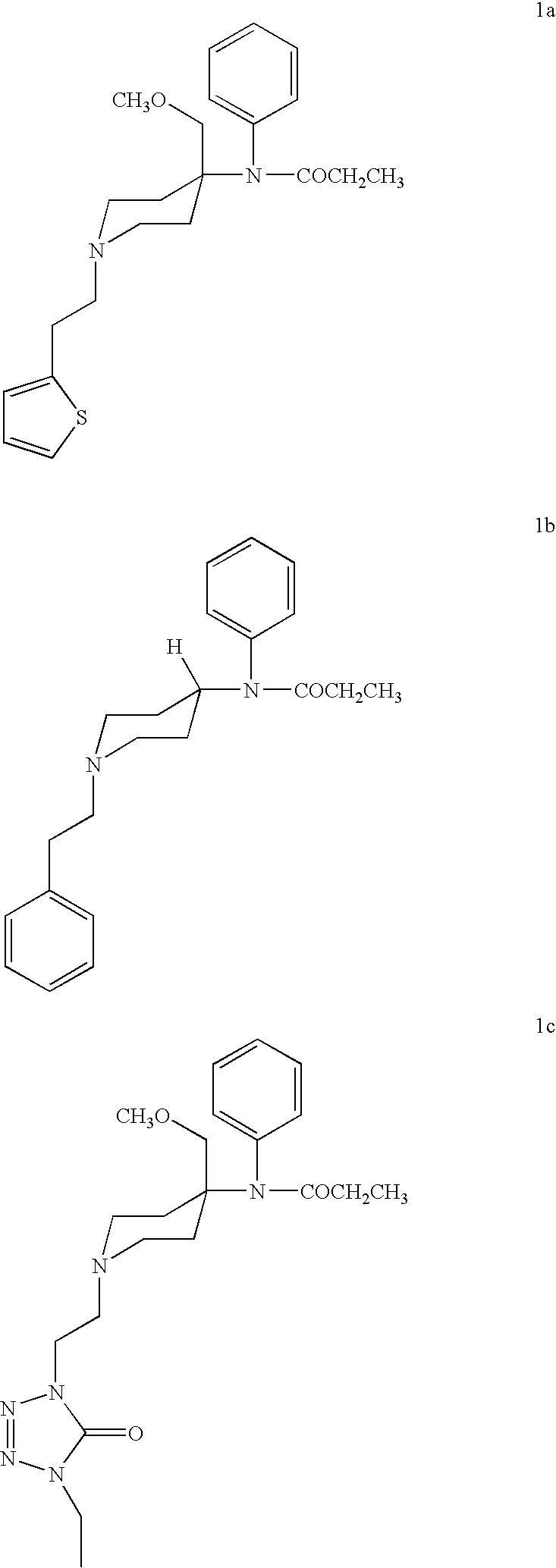
Sufentanil, sold under the brand names Dsuvia and Sufenta, is a synthetic opioid analgesic drug approximately 5 to 10 times as potent as its parent drug, fentanyl, and 500 times as potent as morphine. Structurally, sufentanil differs from fentanyl through the addition of a methoxymethyl group on the piperidine ring (which is believed to reduce duration of action), and the replacement of the phenyl ring by thiophene. Sufentanil first was synthesized at Janssen Pharmaceutica in 1974.
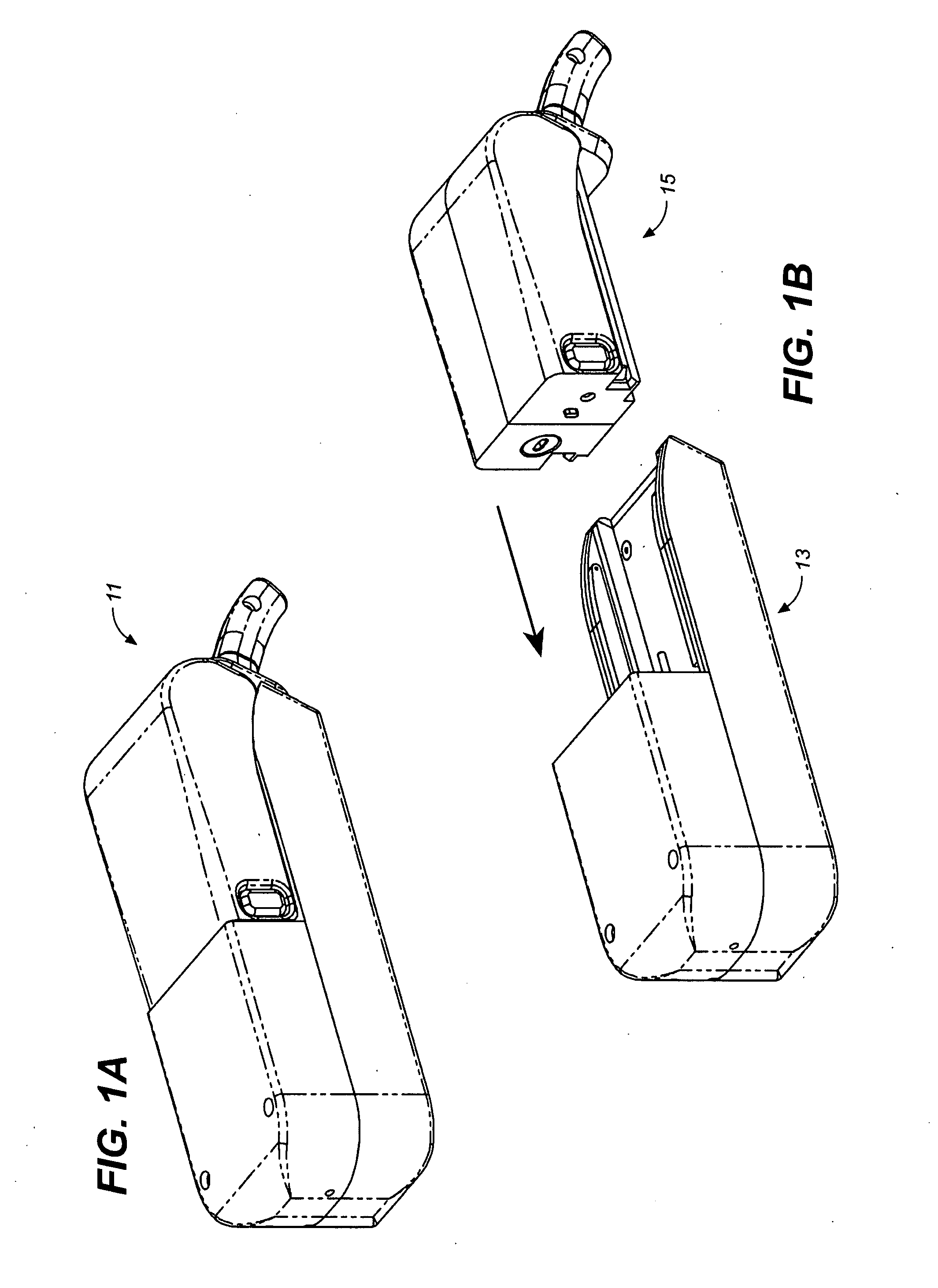
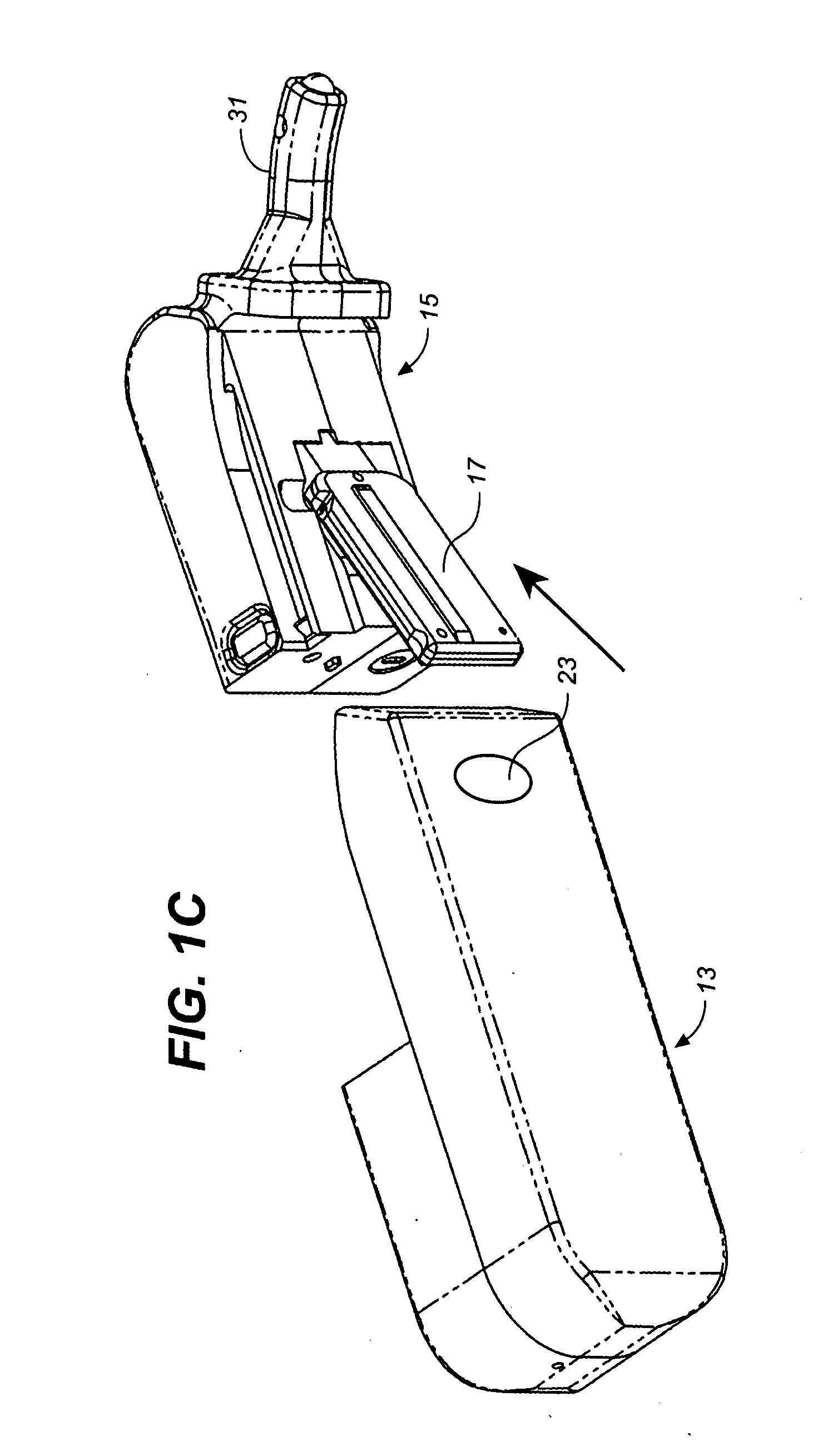
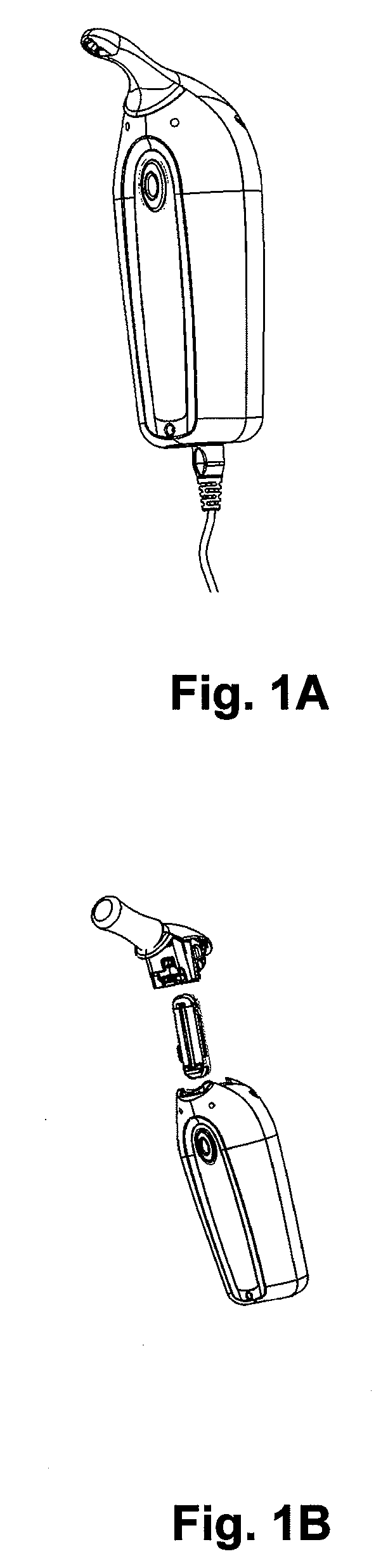
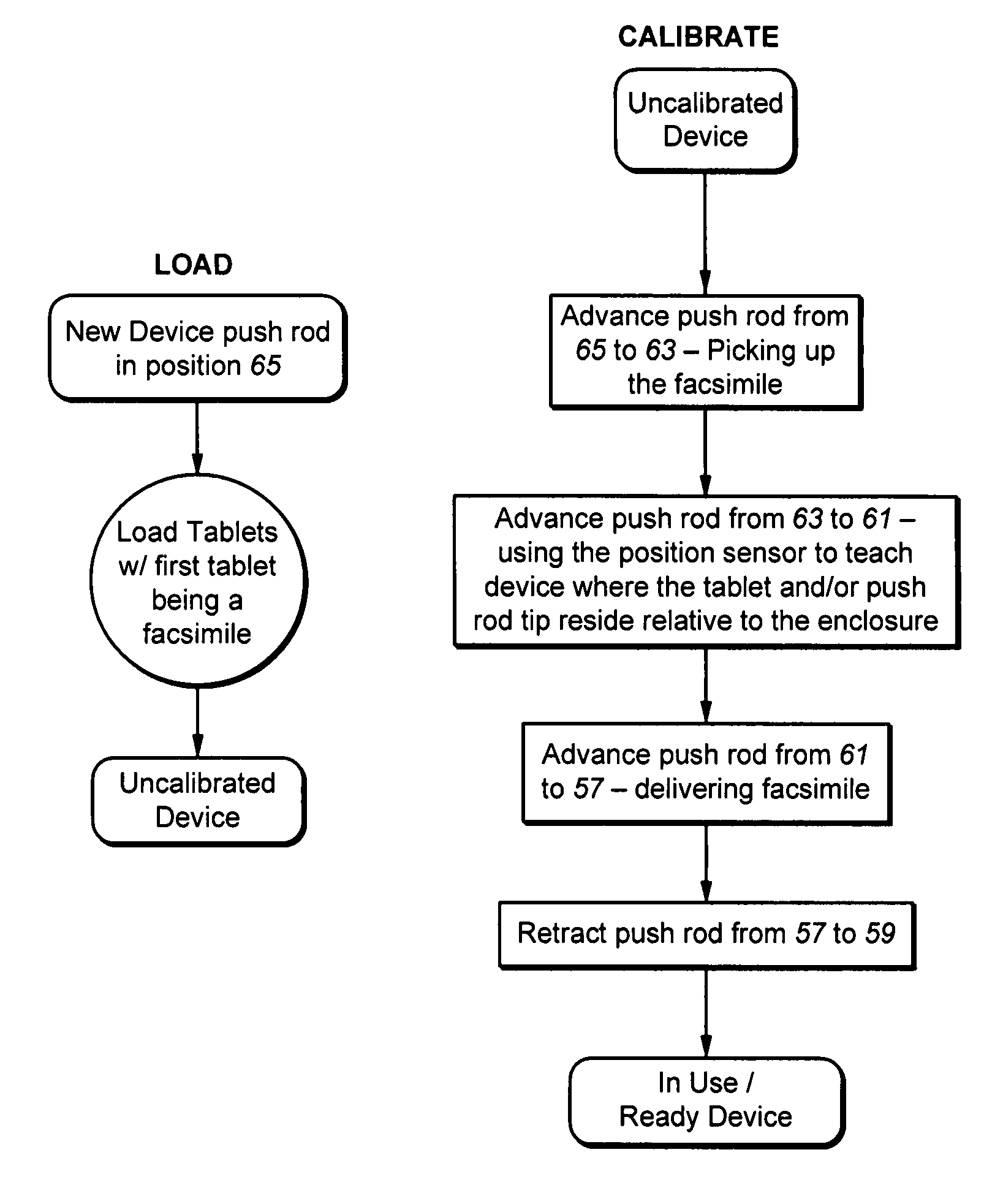
Osmotic pump drug delivery systems and methods
InactiveUS6471688B1Avoid mixingMedical devicesPharmaceutical delivery mechanismTreatment effectAnalgesics effects
Implantable osmotic pump devices and systems include multiple osmotic pumps and / or semipermeable membranes to extend the useful life cycle and functionality of the drug delivery system. Use of an implantable system including multiple implantable osmotic pumps allows different drugs to be administered from the same implanted system. One or more of the semipermeable membranes of the system may be initially sealed by an overlying impermeable membrane upon implantation of the system into the patient. When the patient develops a tolerance to a first drug or to a first dose of the first drug, the impermeable membrane may be breached, to expose the underlying semipermeable membrane to the osmotic pressure of the patient at the implant site. This causes the infusion rate to increase, thereby providing the patient with the needed relief and / or other desired therapeutic effect. In the case of a multiple pump system, breaching an impermeable membrane may cause the infusion of a second drug. The second drug may potentiate a therapeutic effect (such as an analgesic effect) of the first drug, as is the case with Sufentanil and Clonidine.
Owner:MICROSOLUTIONS
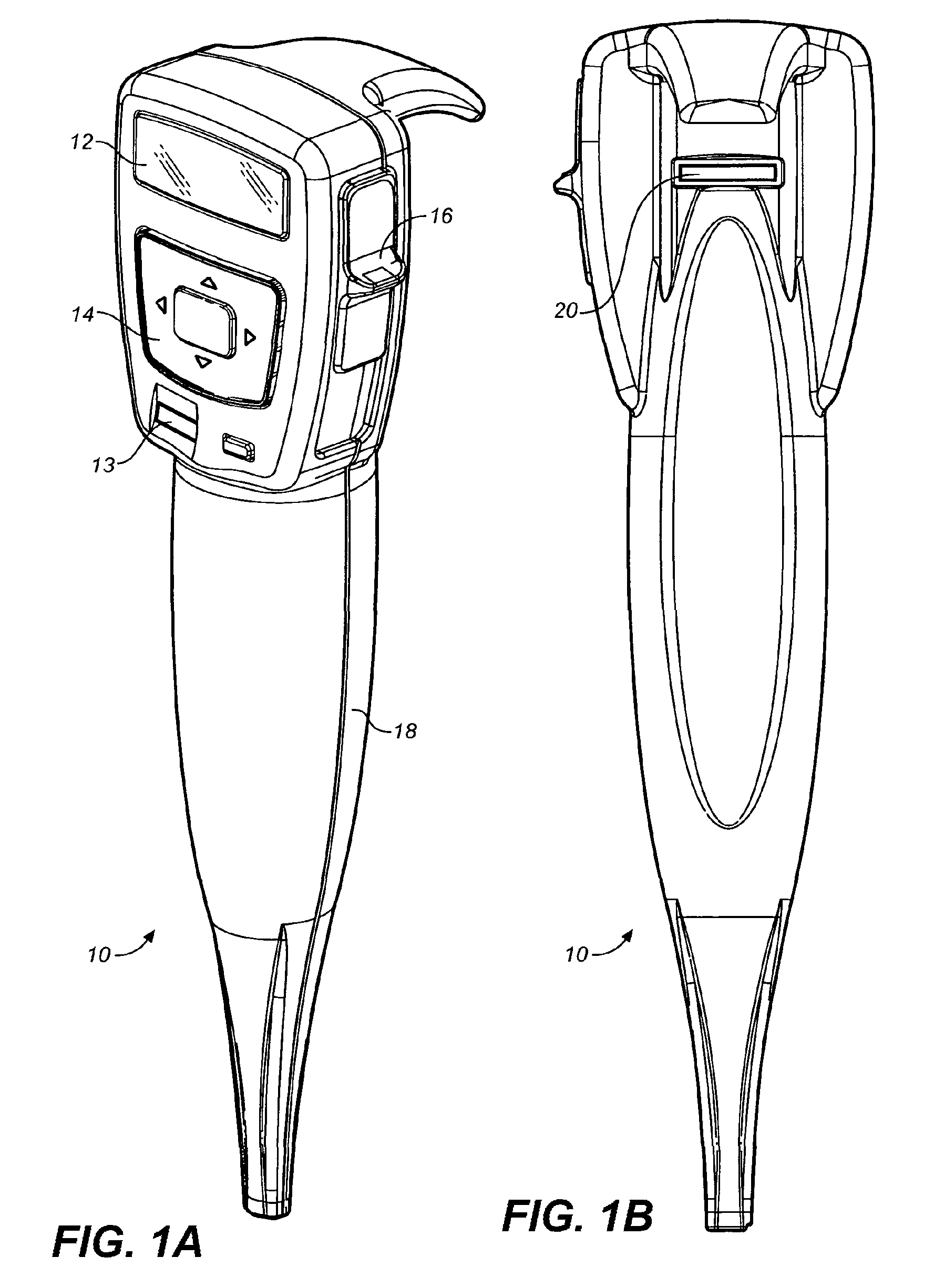
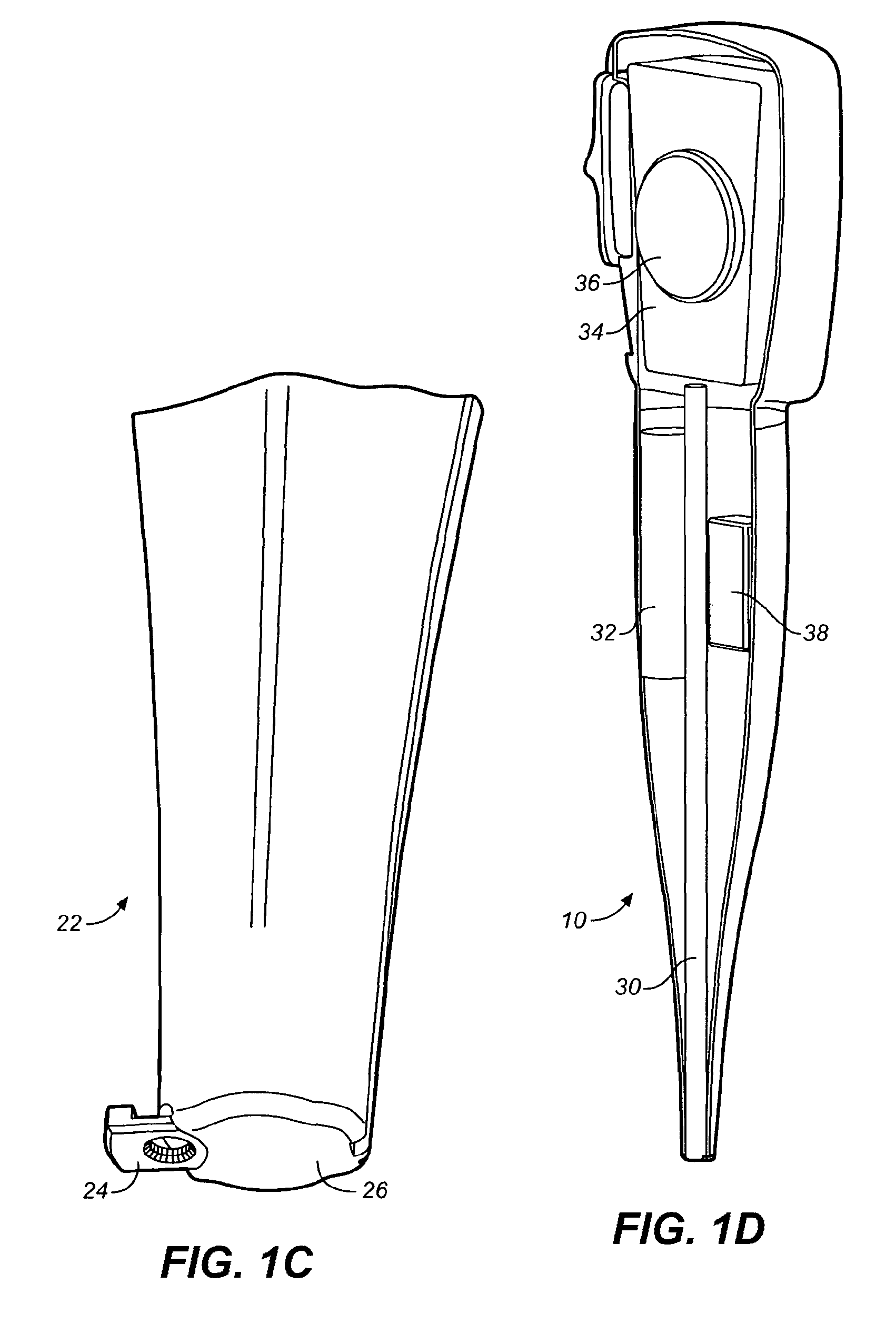
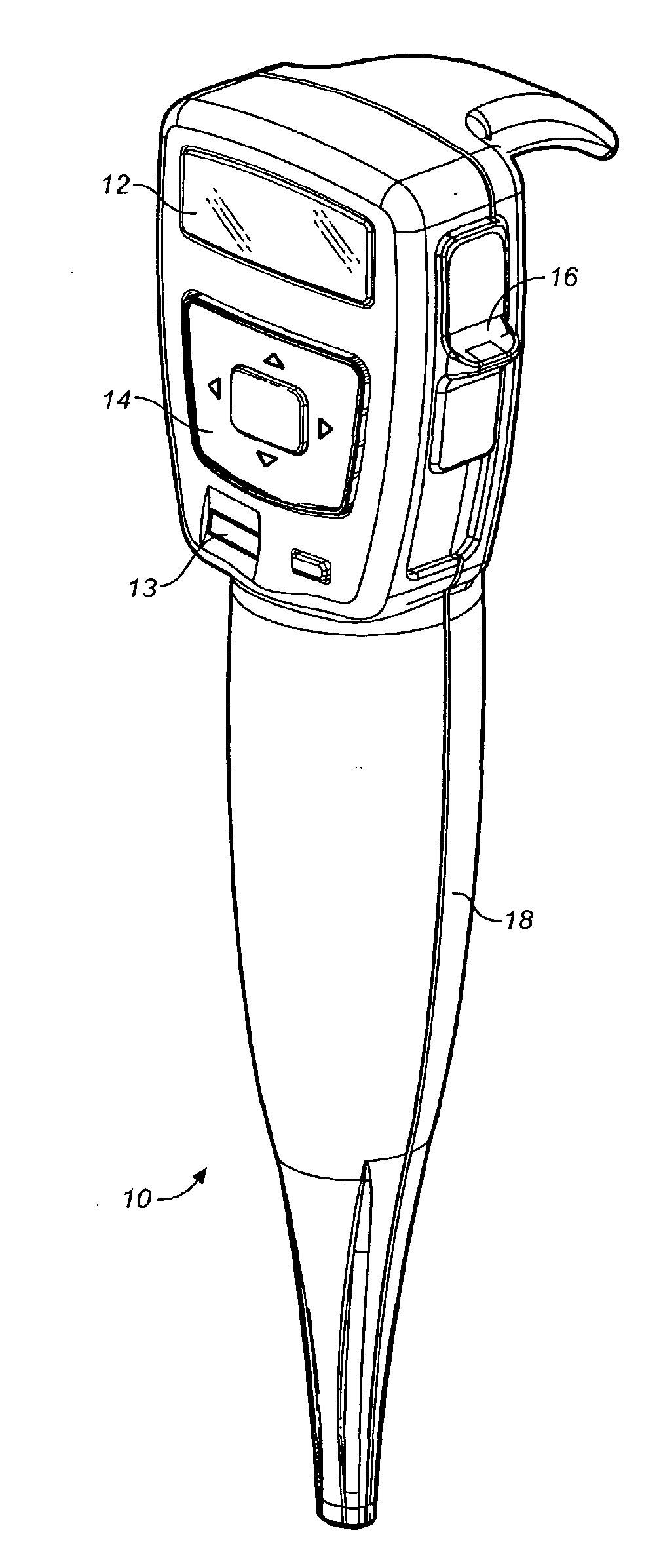
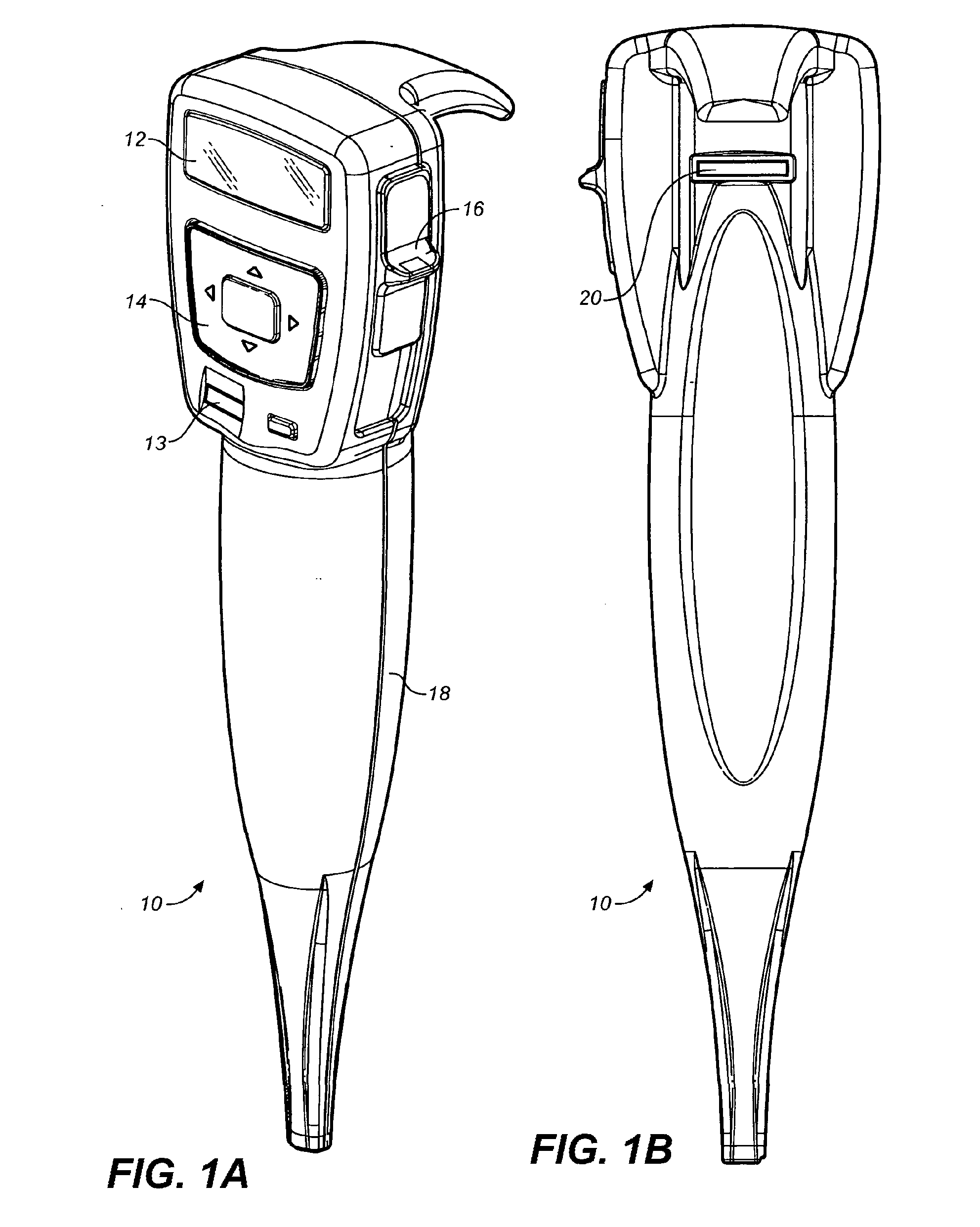
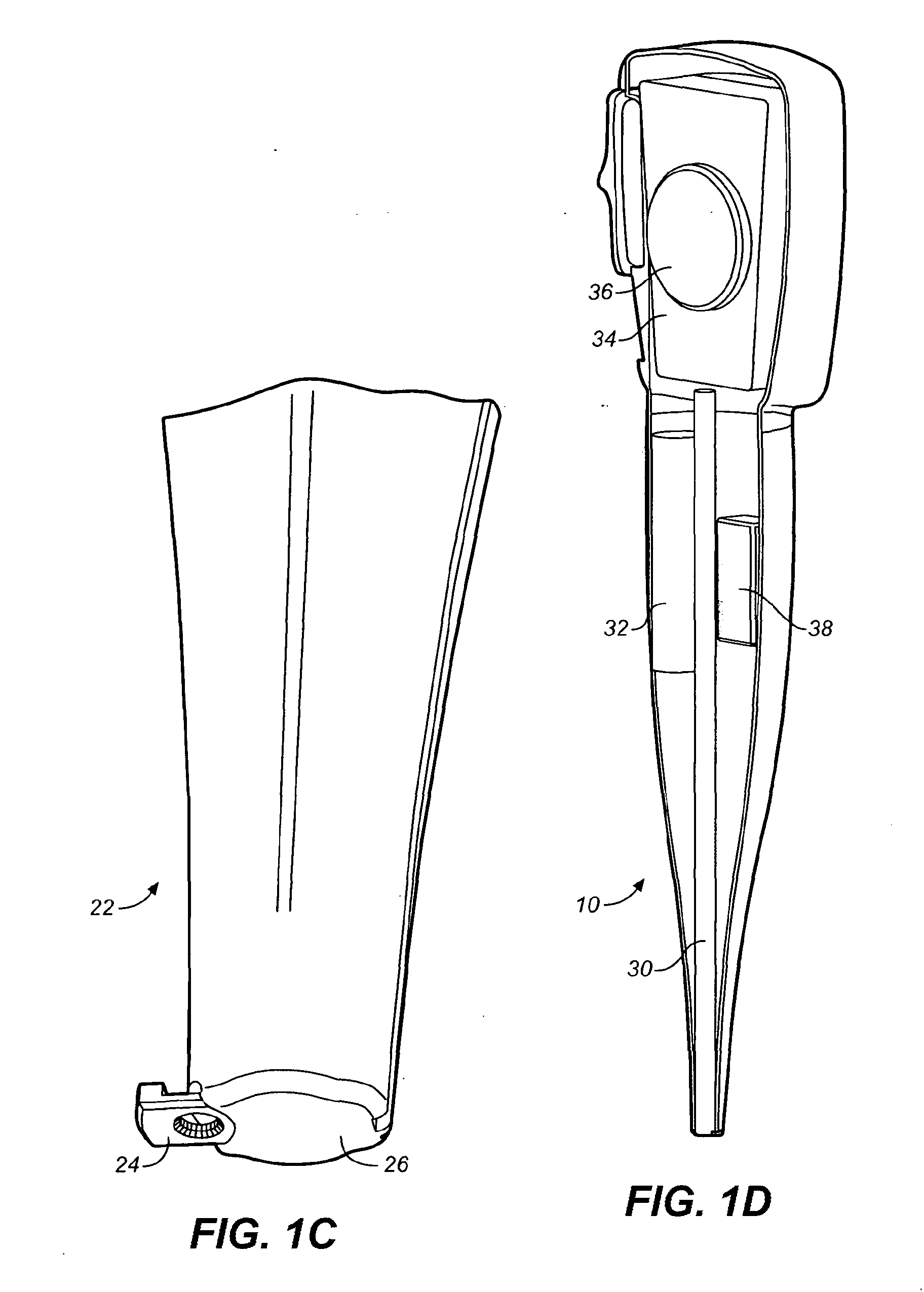
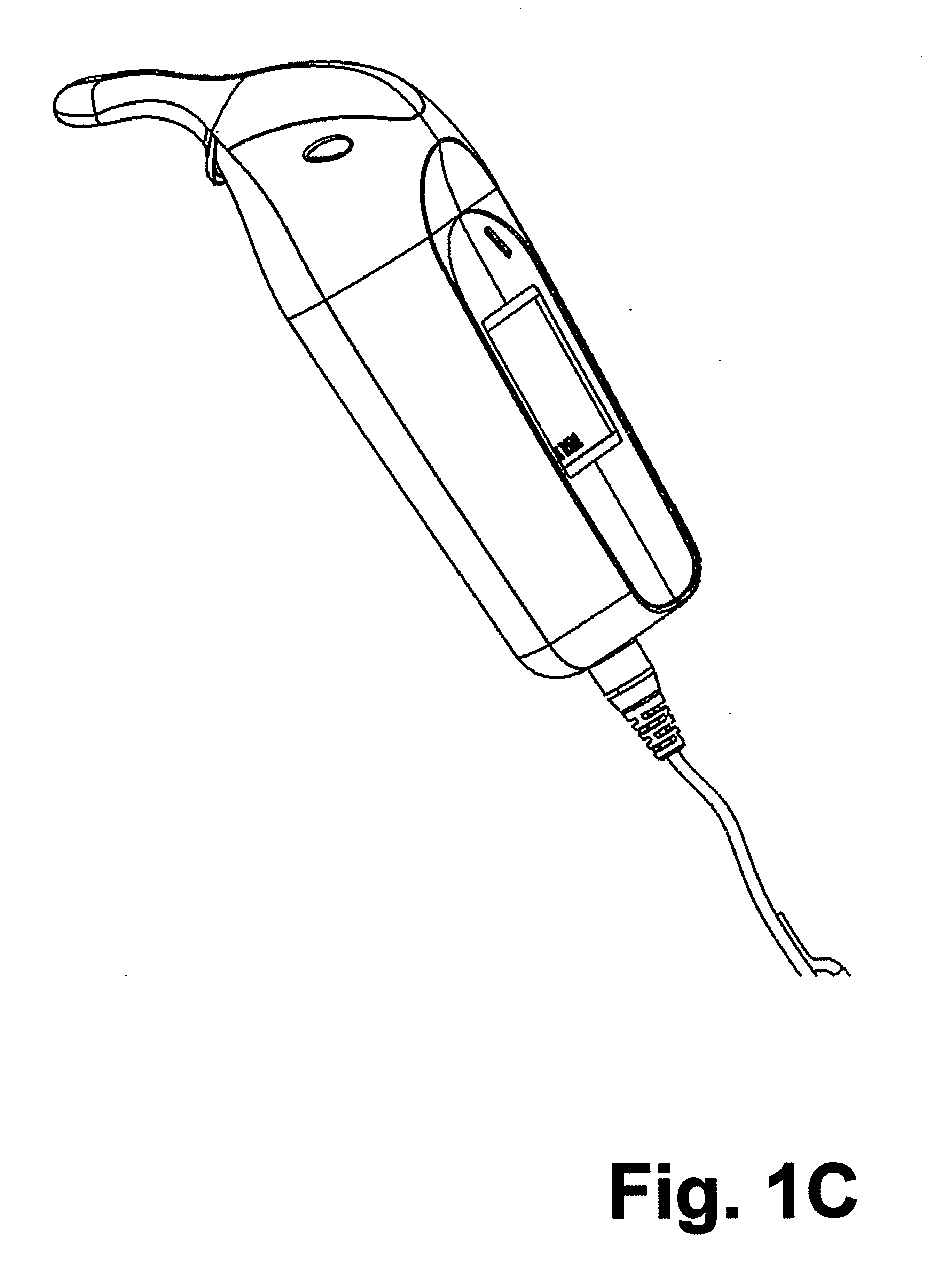
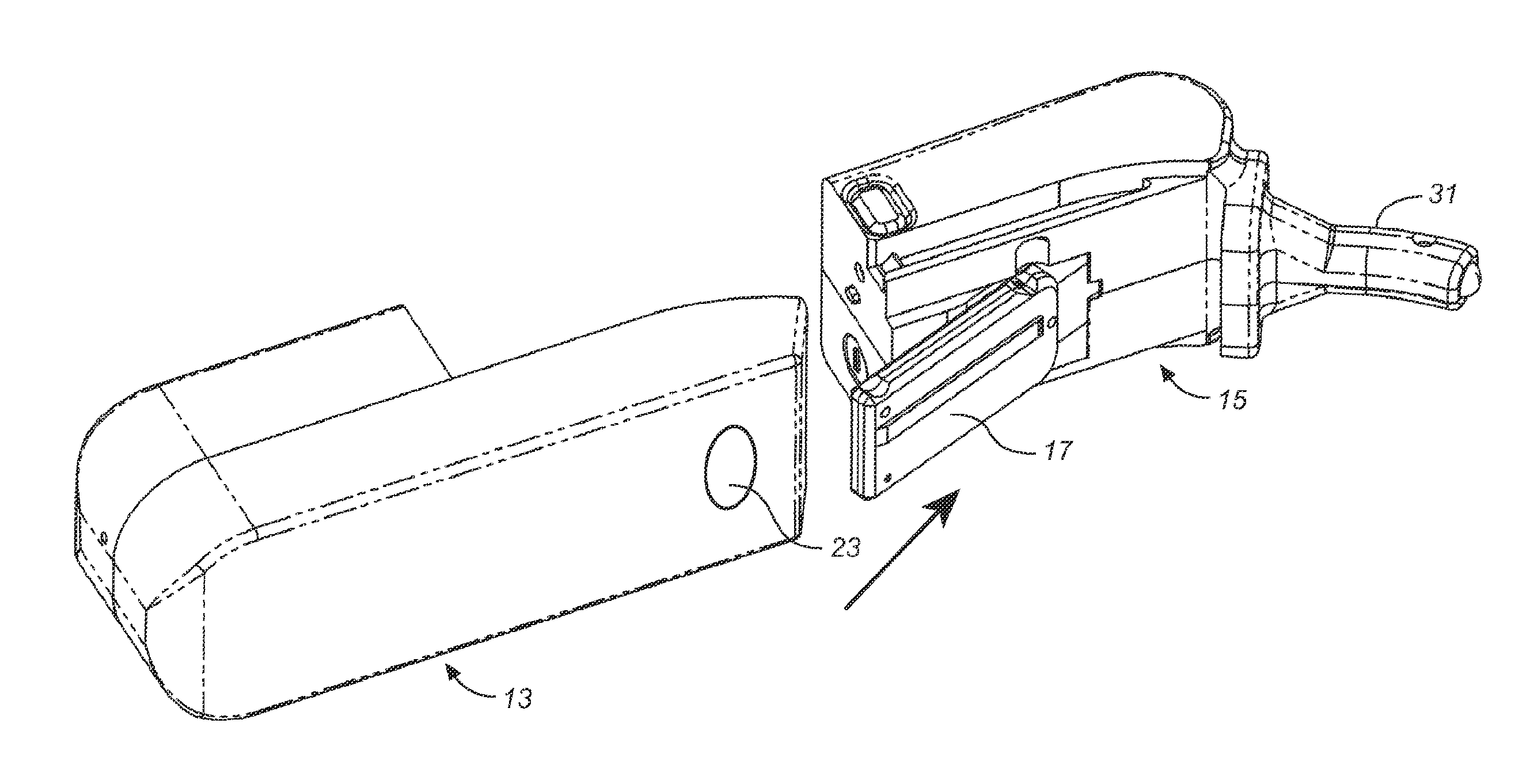
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Owner:ACEIRX PHARM INC
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Compositions, systems and methods for administration of small volume sufentanil drug dosage forms via the oral transmucosal route of a subject for treatment of pain.
Owner:VERTICAL PHARMA
Small Volume Oral Transmucosal Dosage Forms Containing Sufentanil for Treatment of Pain
Compositions, methods and systems for administration of small volume sufentanil-containing drug dosage forms to the oral mucosa of a subject are disclosed.
Owner:VERTICAL PHARMA LLC
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
Osmotic pump drug delivery systems and methods
InactiveUS20030032947A1Avoid mixingMedical devicesPharmaceutical delivery mechanismTherapeutic effectAnalgesics effects
Owner:MICROSOLUTIONS
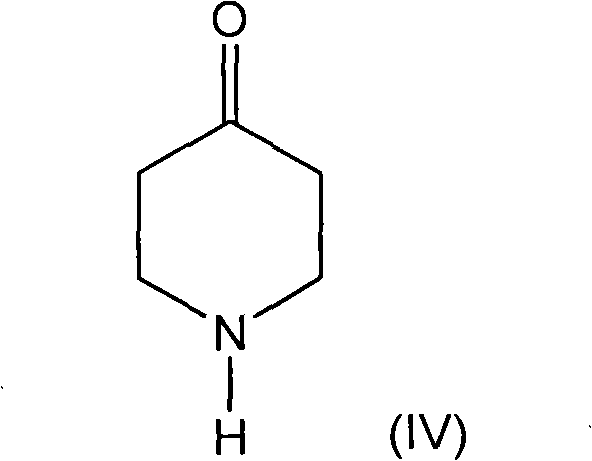
Methods for the syntheses of alfentanil, sufentanil and remifentanil
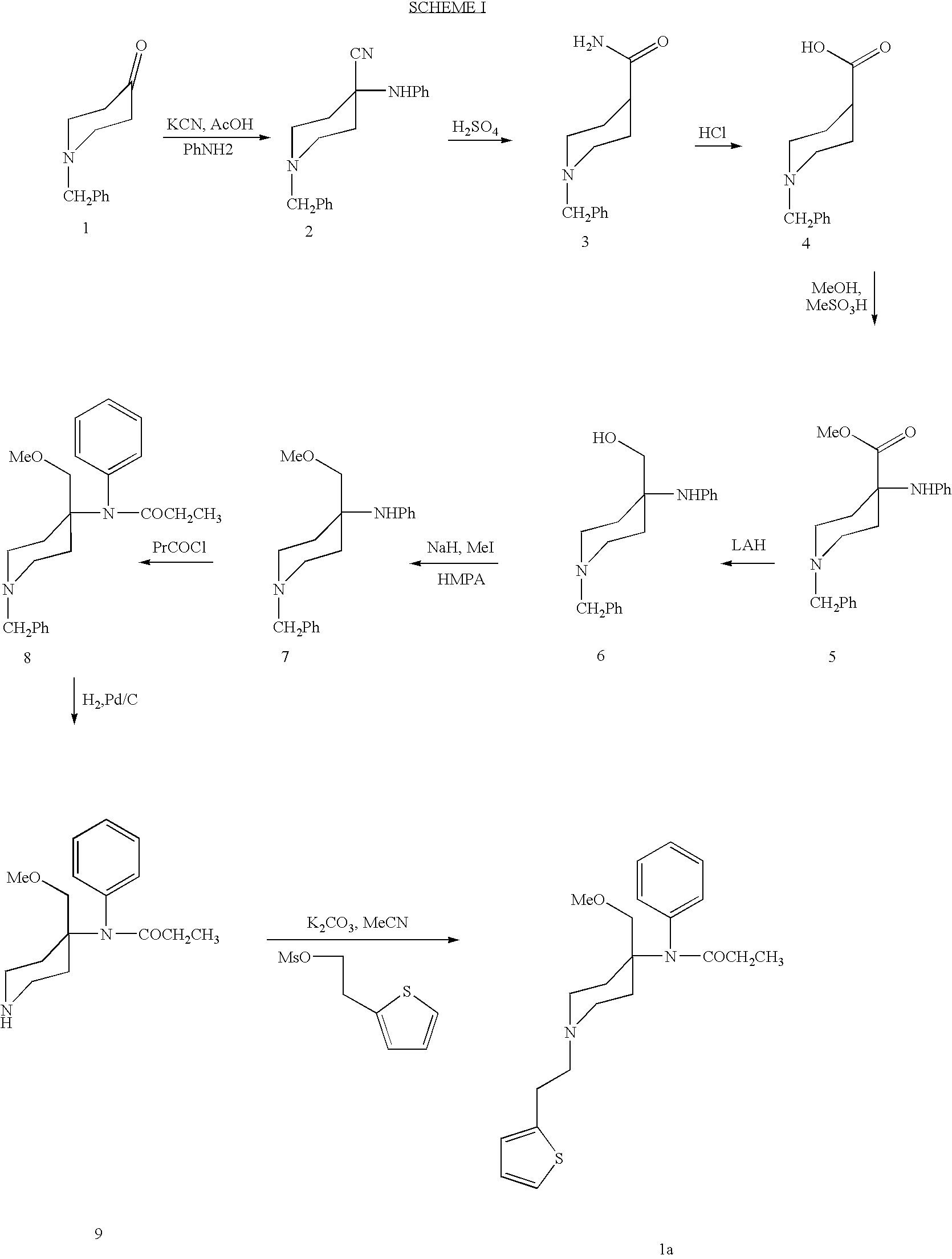
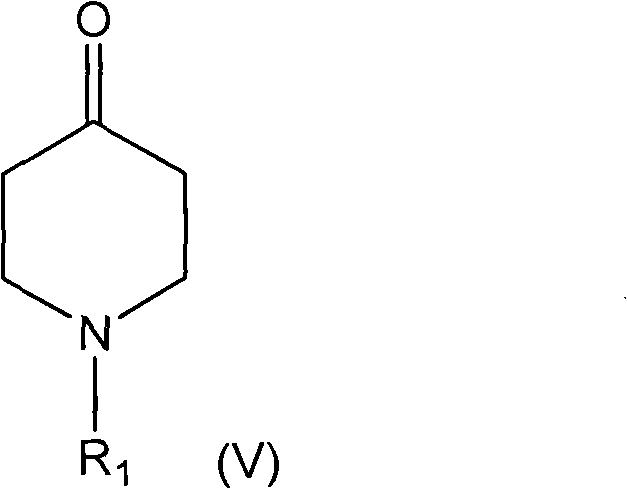
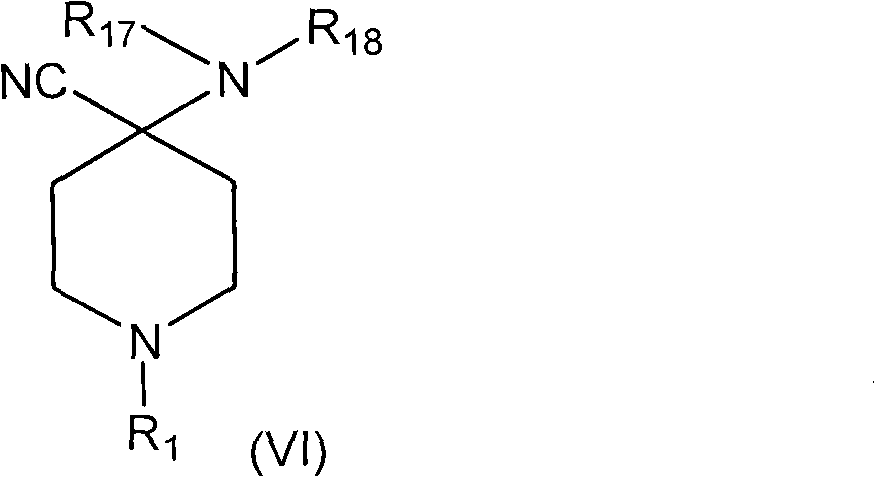
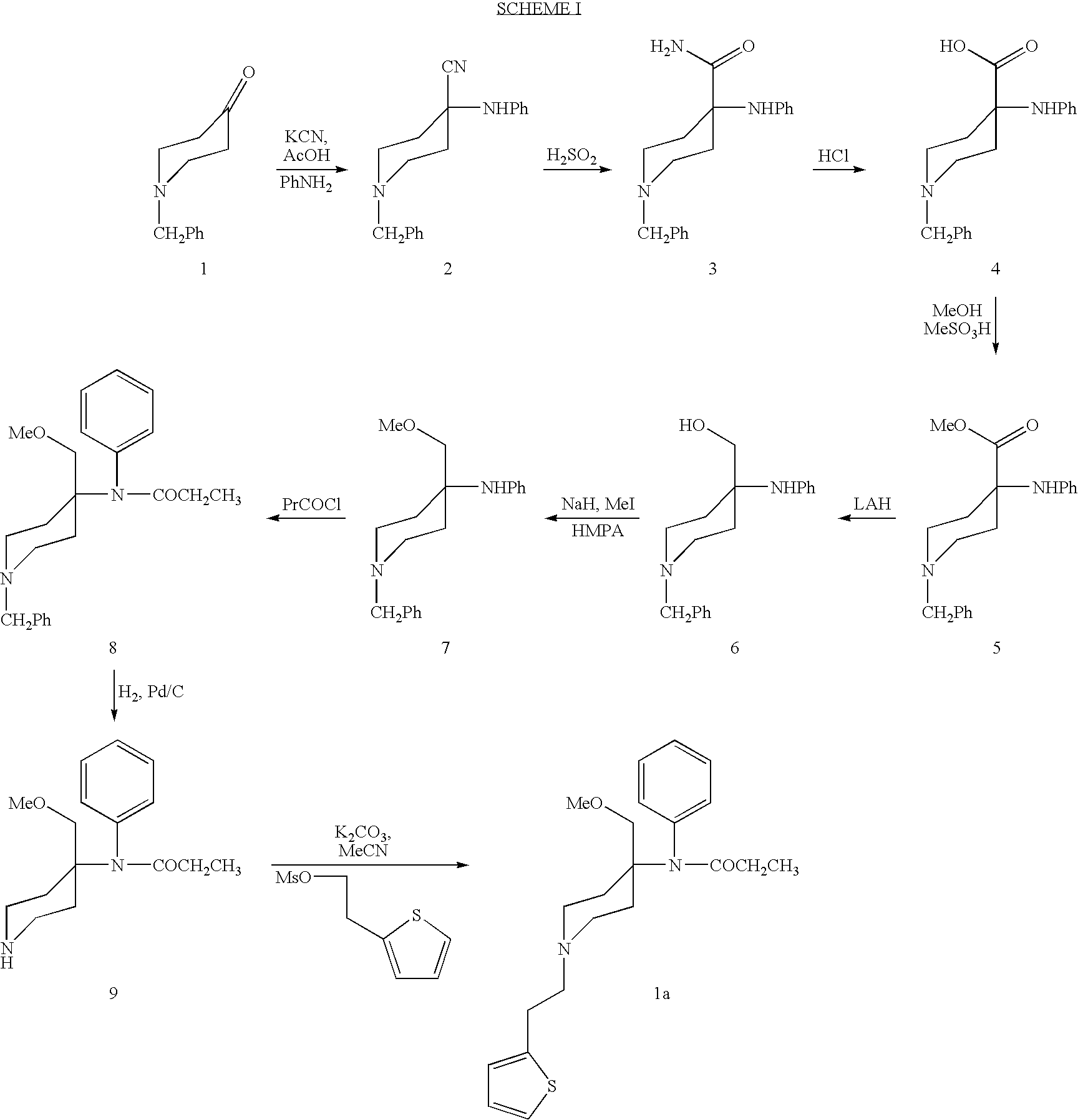
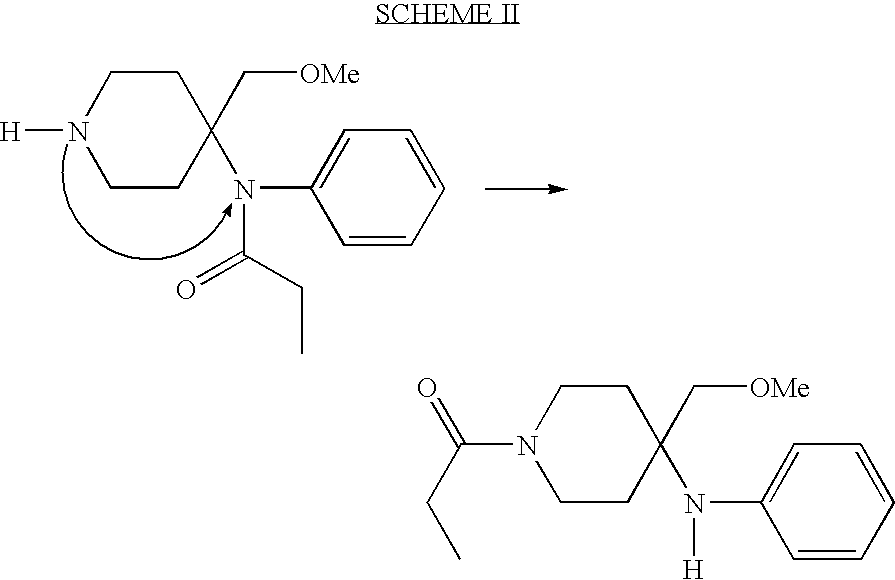
Synthetic pathways are disclosed for synthesizing derivatives or analogs of fentanyl. Specifically set out are pathways for synthesizing alfentanil, sufentanil and remifentanil. The disclosed methods require fewer steps and produce a greater yield of product than methods reported in the prior art. The pathways to all these compounds begin with a common pathway of condensing a piperidone with a primary amine so as to form a 4-amino carboxyamino-piperidine, wherein N of said piperidone is a —N—COO—(CH2)nCH3, alkylating an N of said primary amine which was condensed with said piperidone thereby producing an N-alkyl-anilide, and hydrolyzing said —COO—(CH2)nCH3? group of said 4-amino-4-carboxyamino-piperidine following the condensation reaction so as to form a piperidine hydrolysis product. This product can then be convened to remifentanil in a 4 step reaction. Also, this hydrolysis product can be treated with a hydride to yield a 4-hydroxymthyl-piperidine which can be converted to alfentanil in 3 further steps, to sufentanil in 3 more steps, or to a variety of remifentanil analogs in two steps.
Owner:MALLINCKRODT INC
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
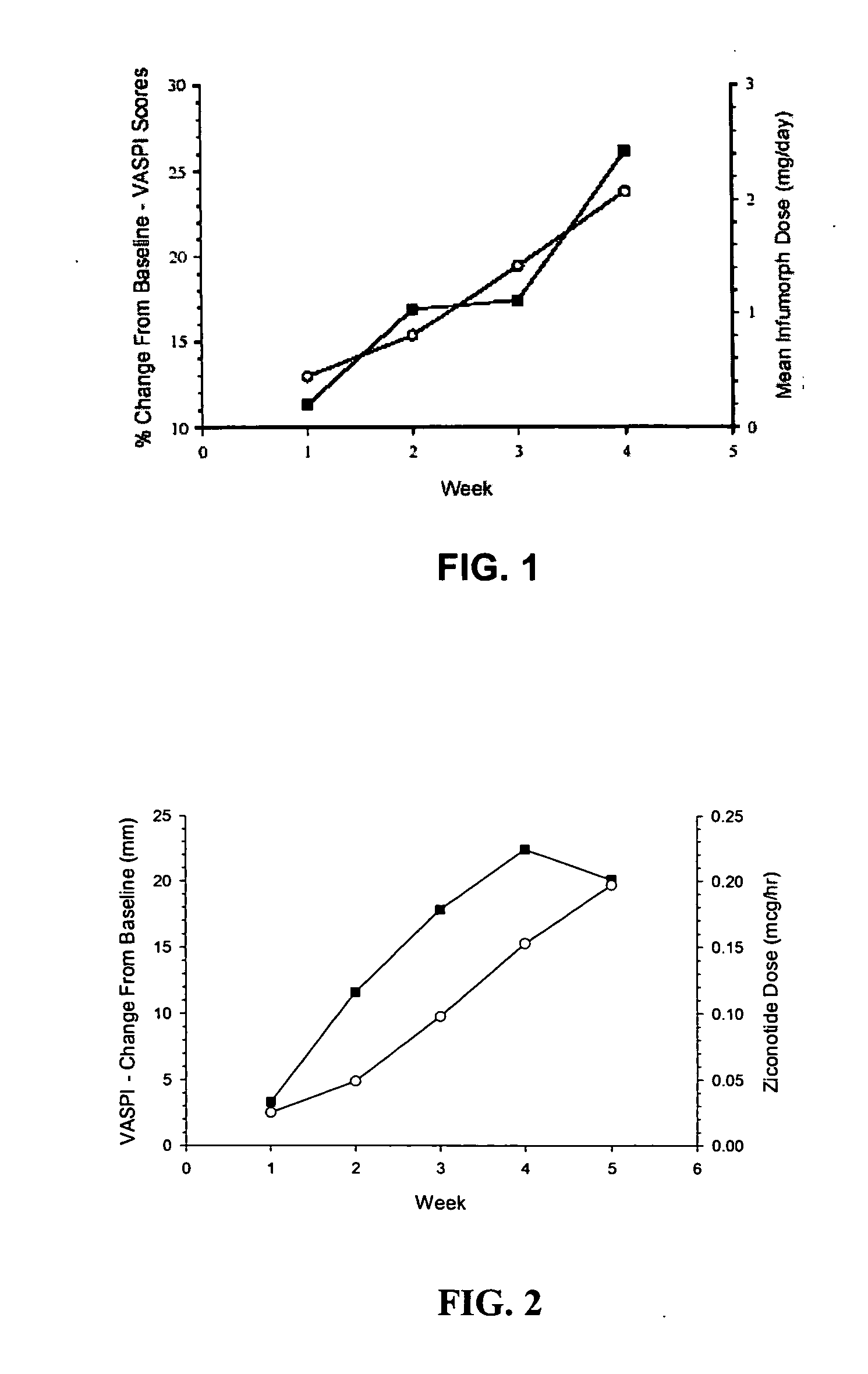
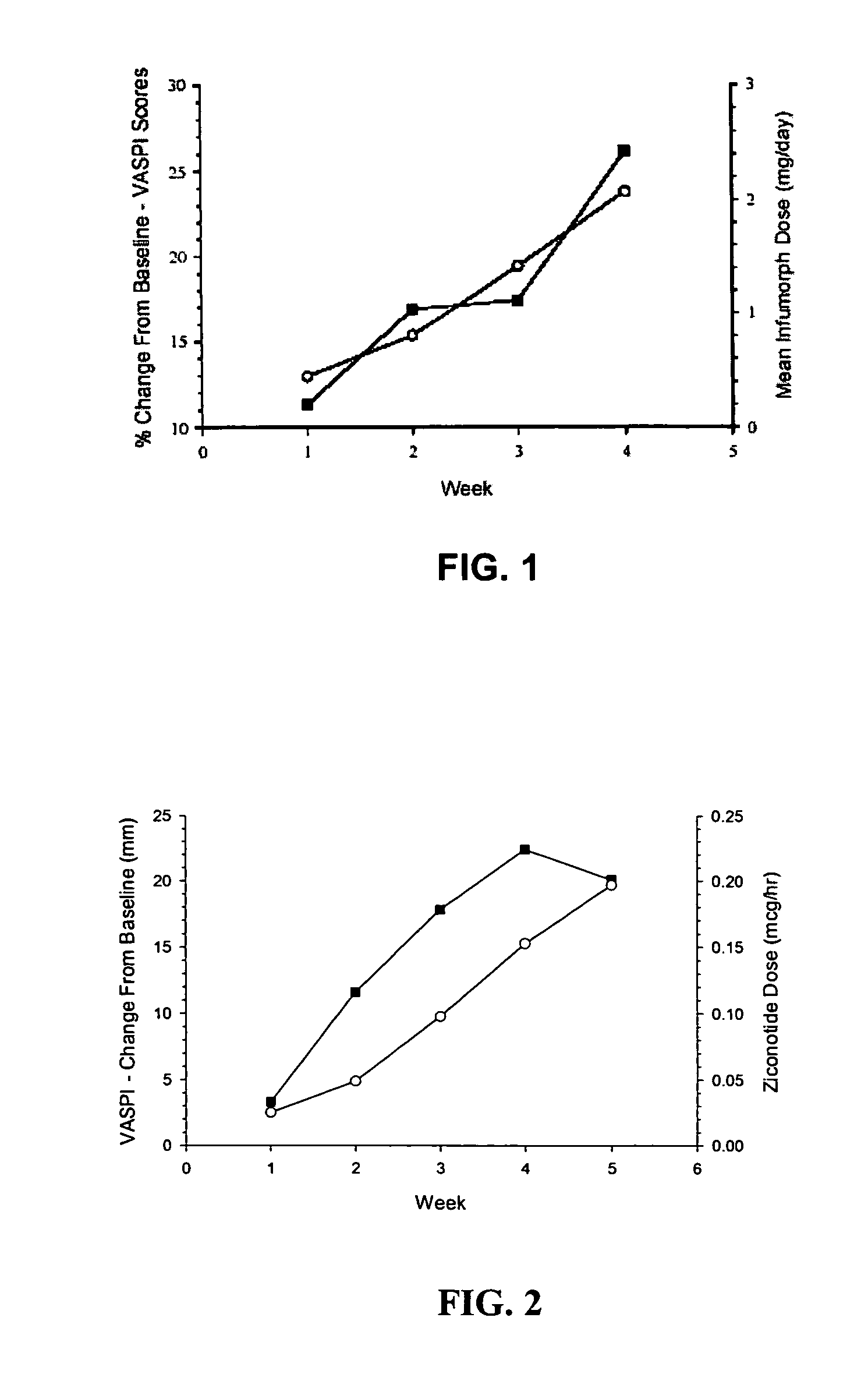
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Oral Transmucosal Administration of Sufentanil
Compositions and methods for administration of sufentanil-containing drug formulations to the oral mucosa of a subject are disclosed.
Owner:ACEIRX PHARM INC
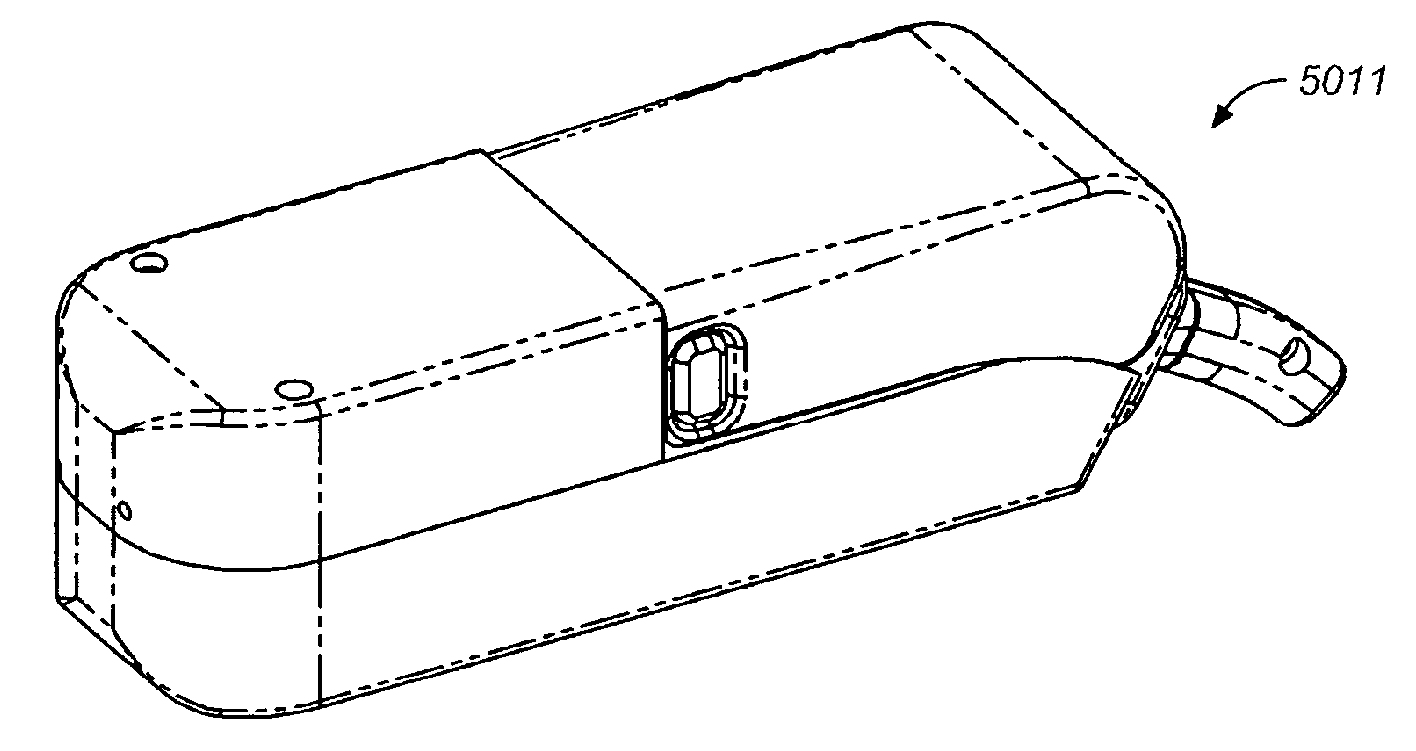
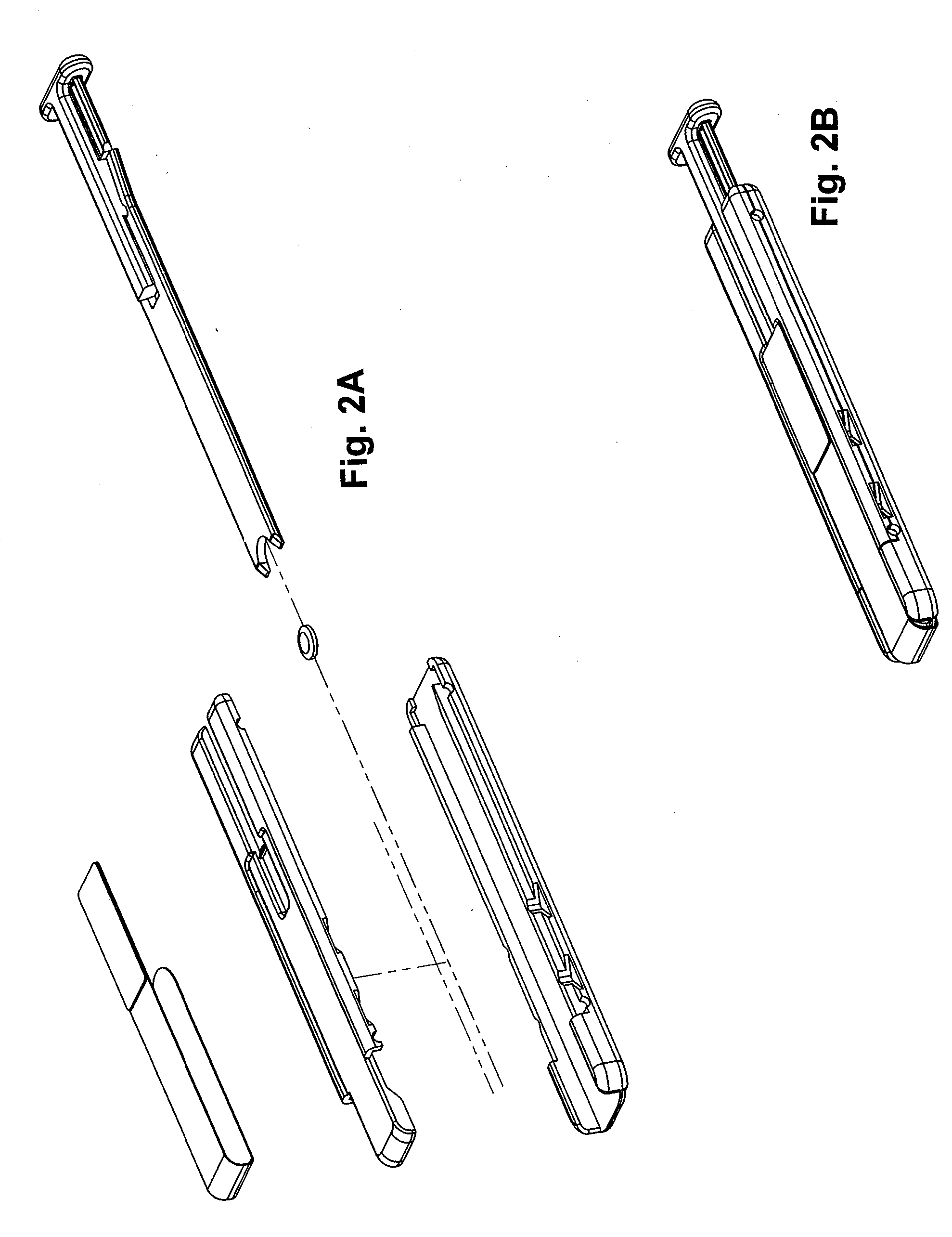
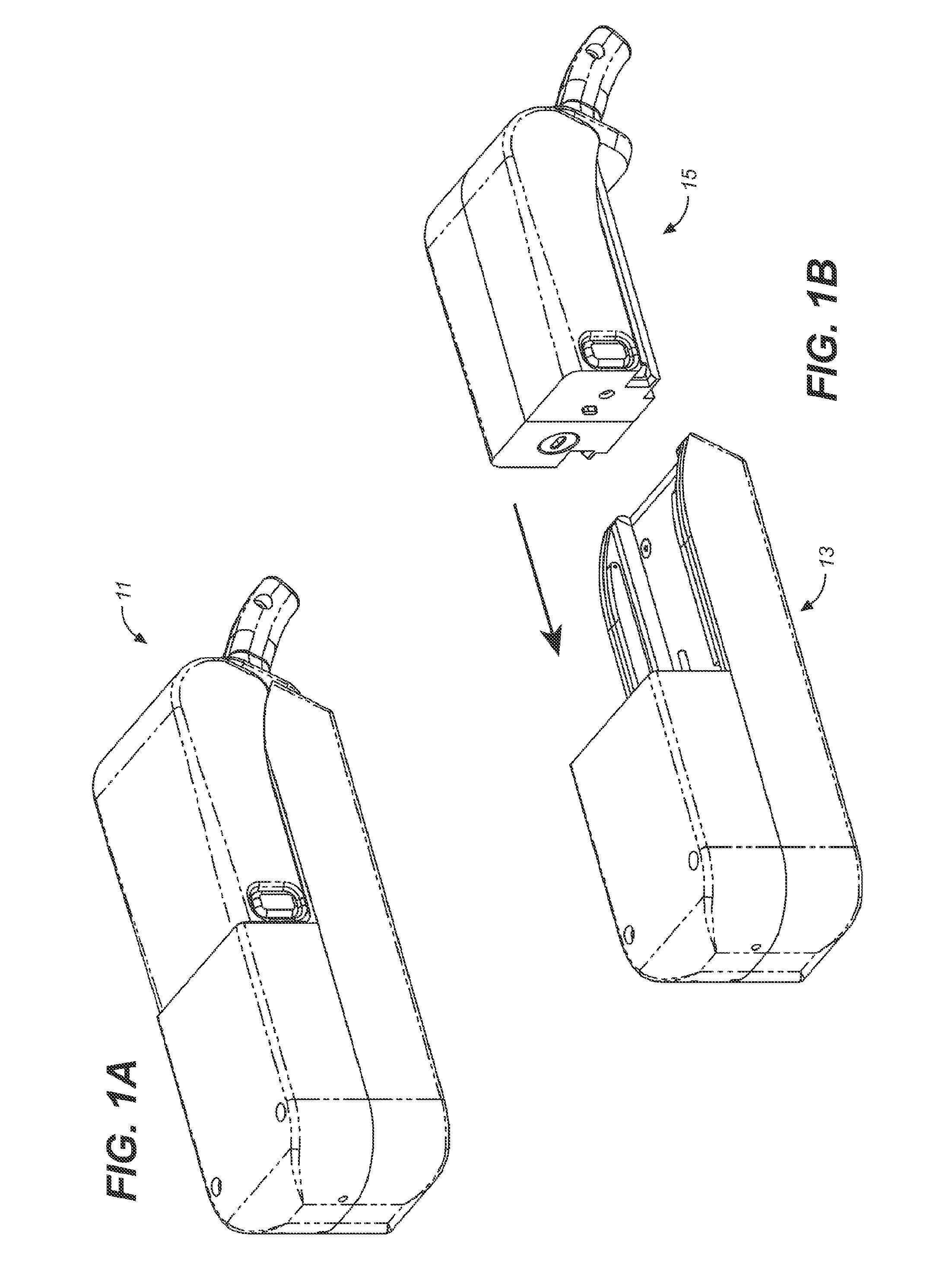
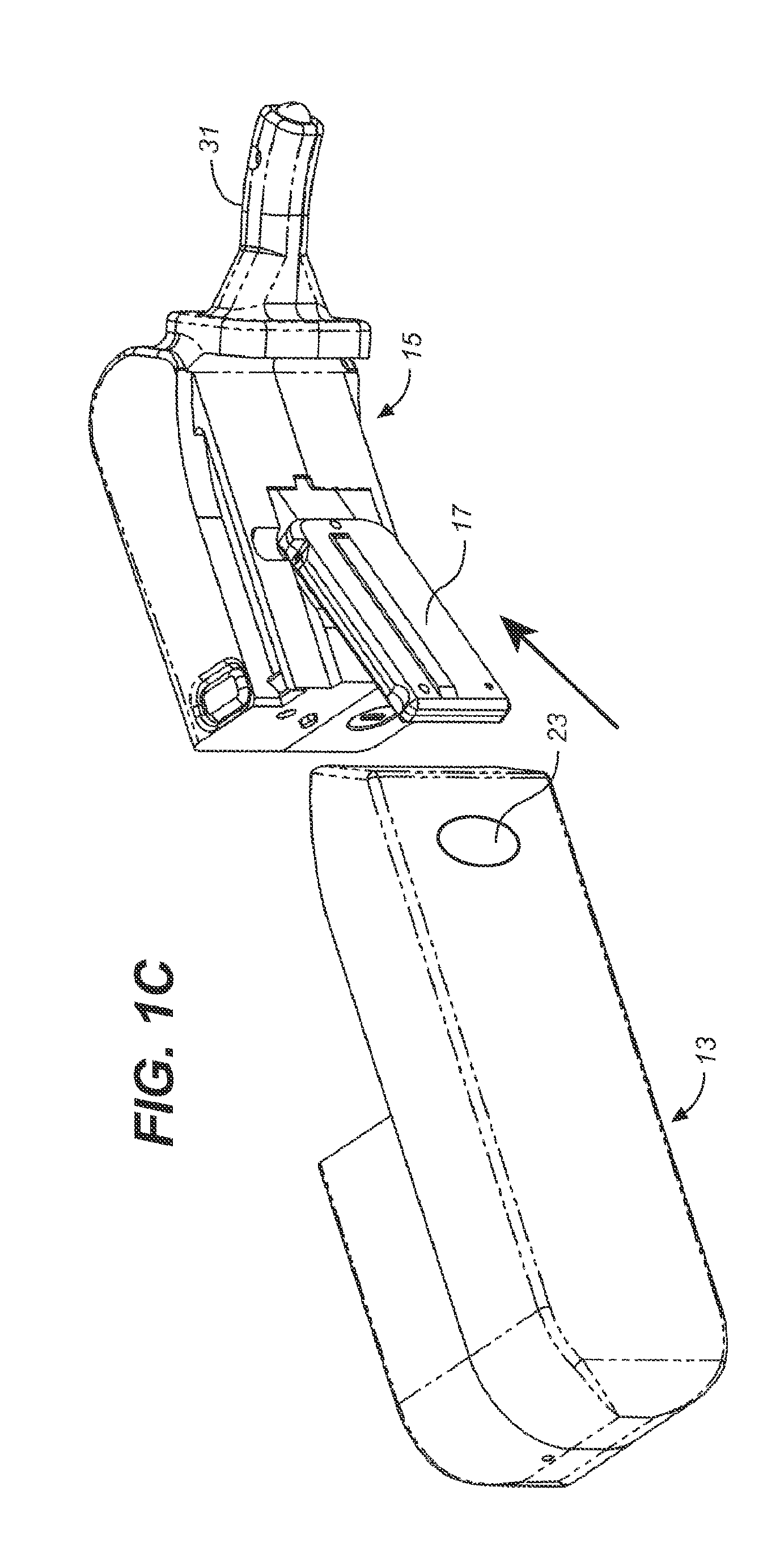
Storage and Dispensing Devices for Administration of Oral Transmucosal Dosage Forms
ActiveUS20100137836A1Organic active ingredientsPeptide/protein ingredientsDrug dispensingDosage form
Compositions, methods and systems for oral transmucosal administration of small volume sufentanil-containing drug dosage forms to a subject using a drug dispensing device are disclosed. The drug dispensing device may provide for administration of multiple doses, a single dose at a time or be a single dose applicator (SDA).
Owner:ACEIRX PHARM INC
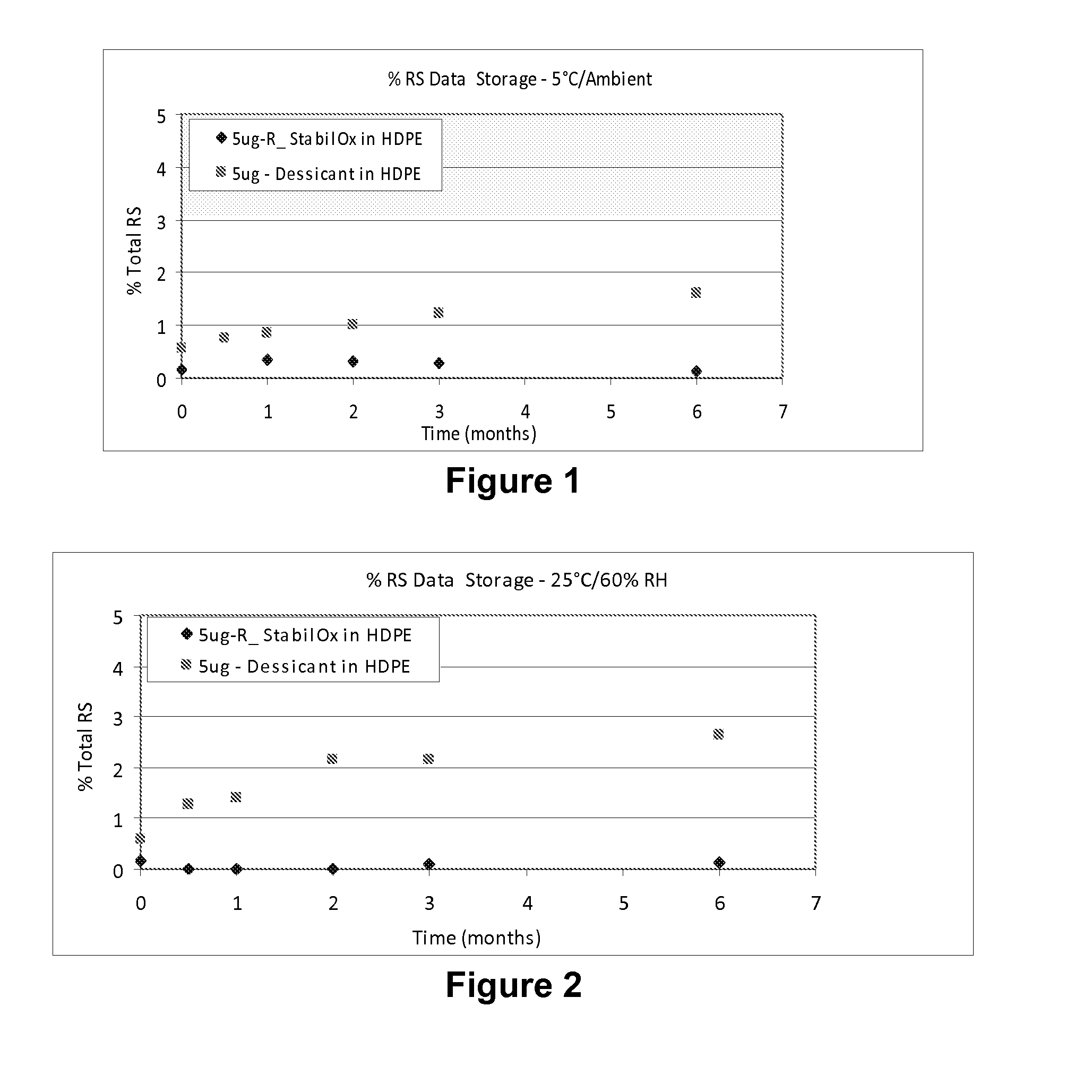
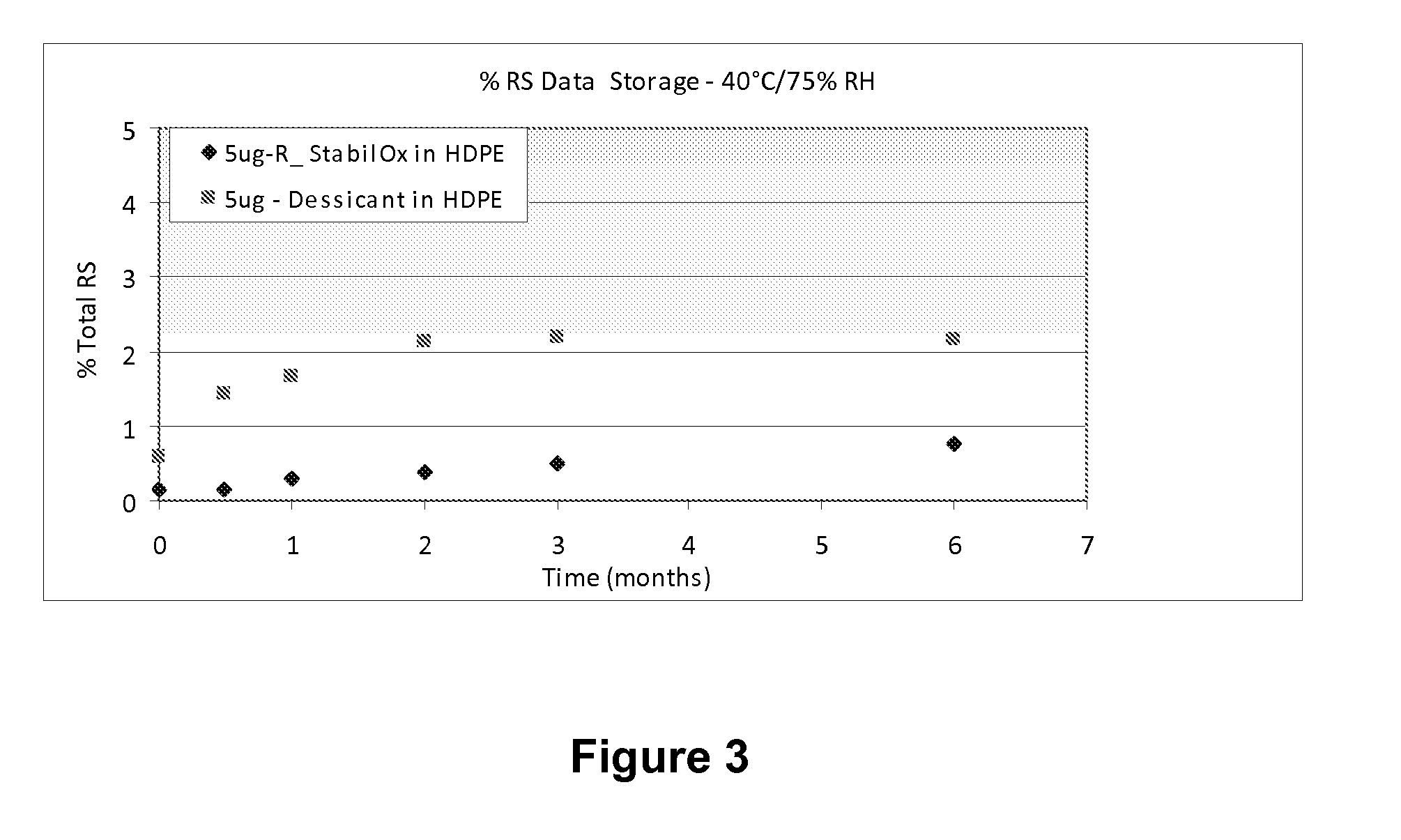
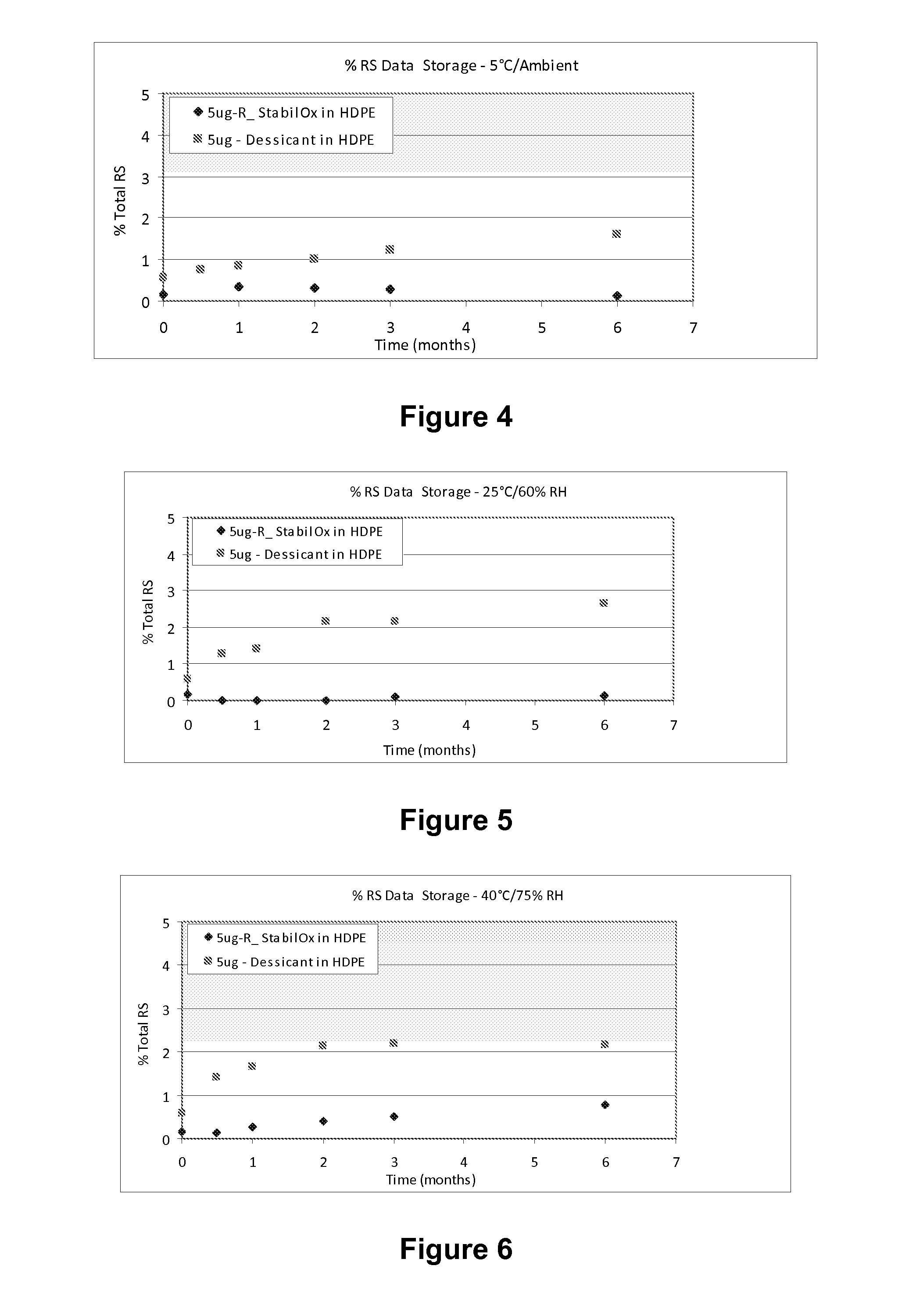
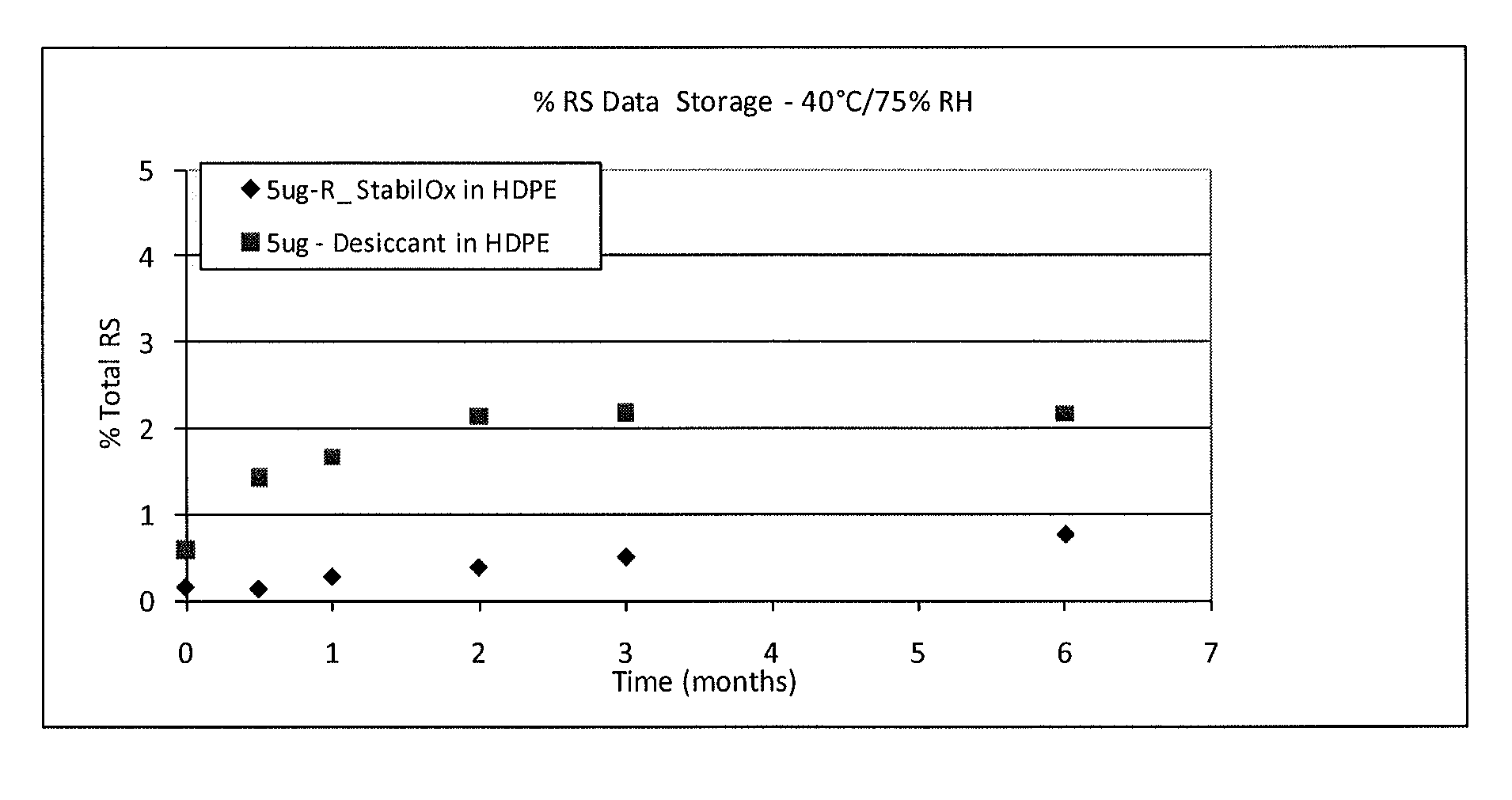
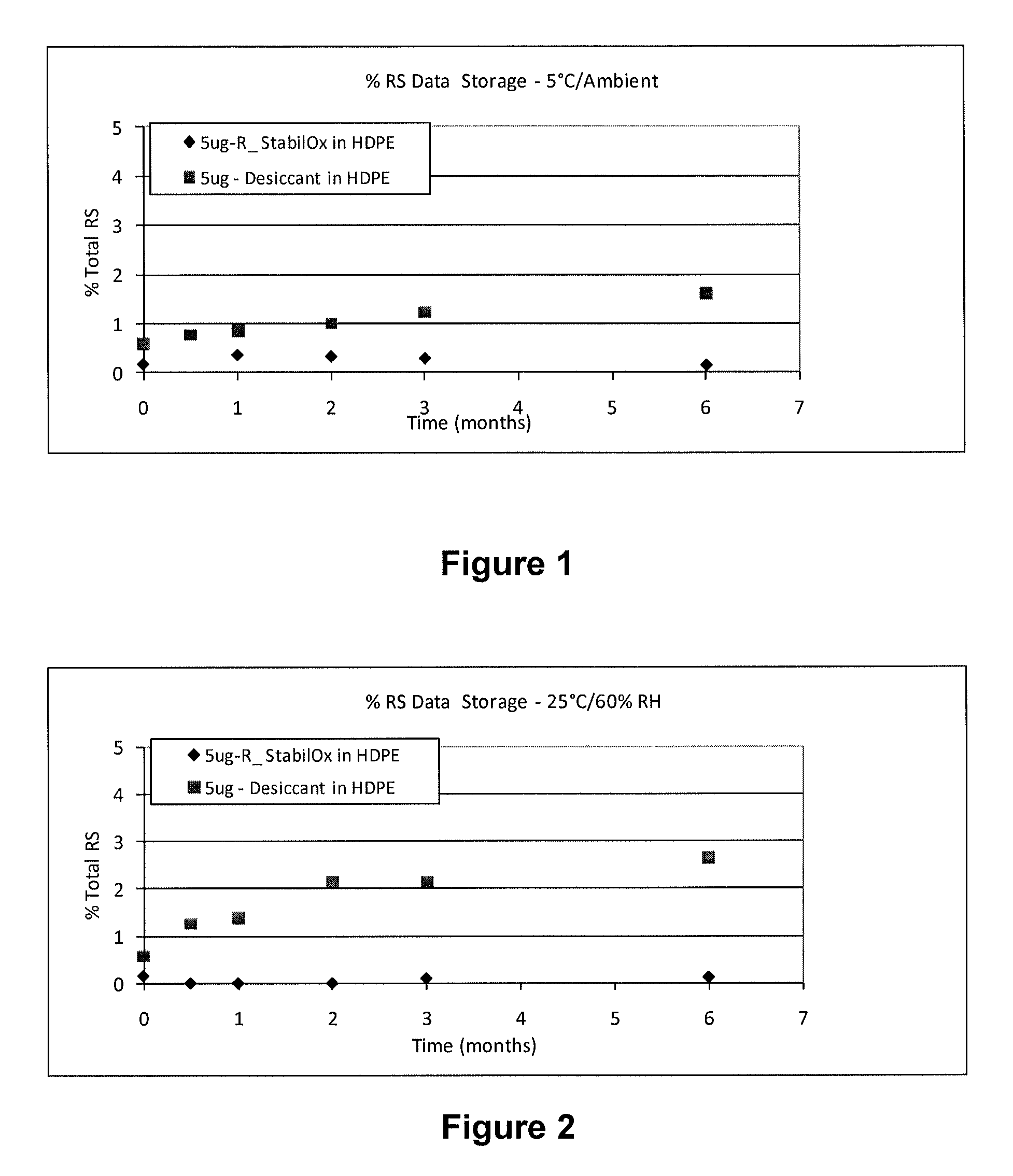
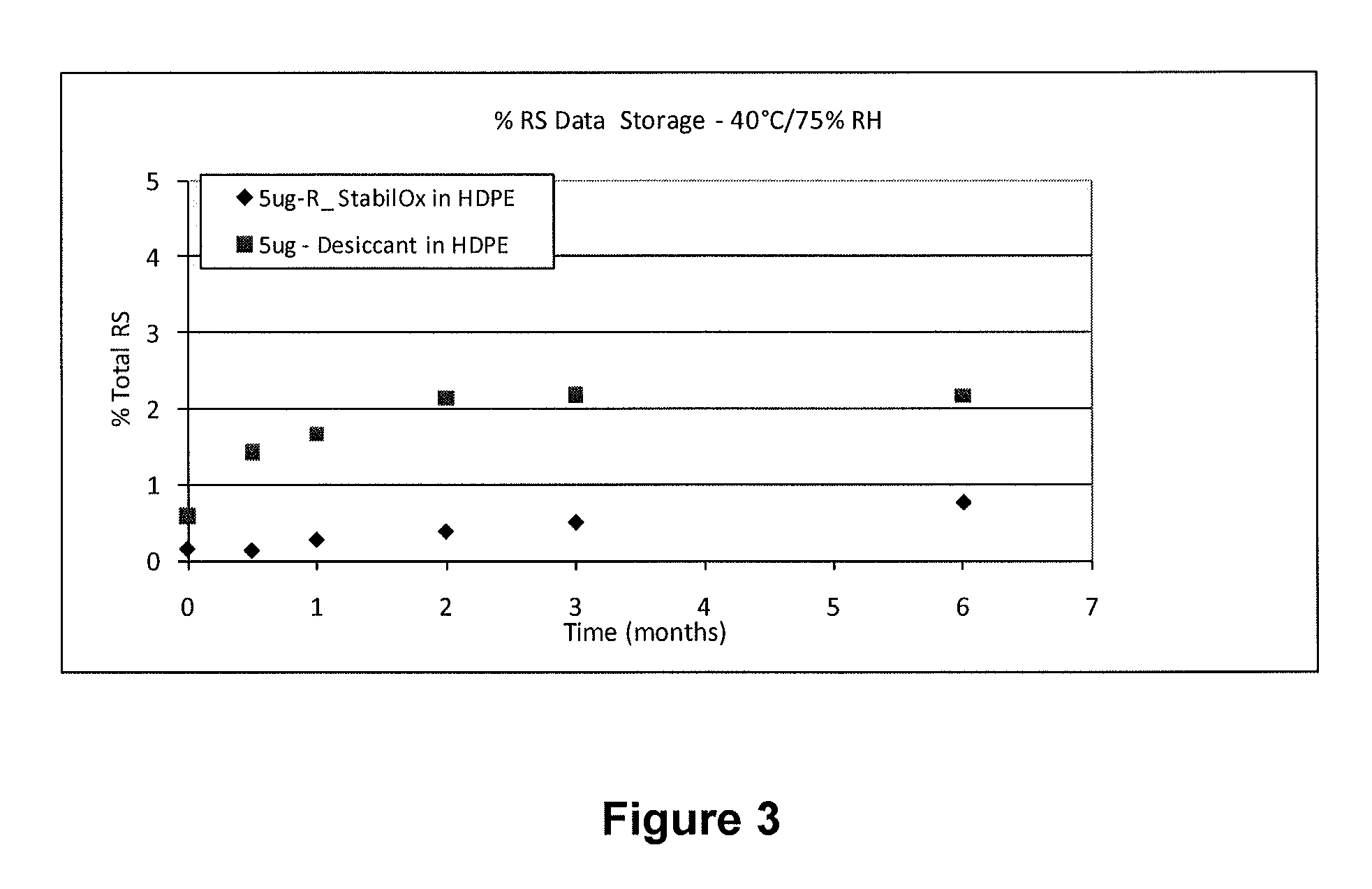
Sufentanil Solid Dosage Forms Comprising Oxygen Scavengers and Methods of Using the Same
ActiveUS20100130551A1Percentage of sufentanil oxidative degradation products isOxidative degradation can be preventedBiocidePill deliveryDosage formOxygen scavenger
Compositions and methods effective to minimize or eliminate the presence of oxidative degradation products in solid dosage forms comprising sufentanil are provided.
Owner:VERTICAL PHARMA
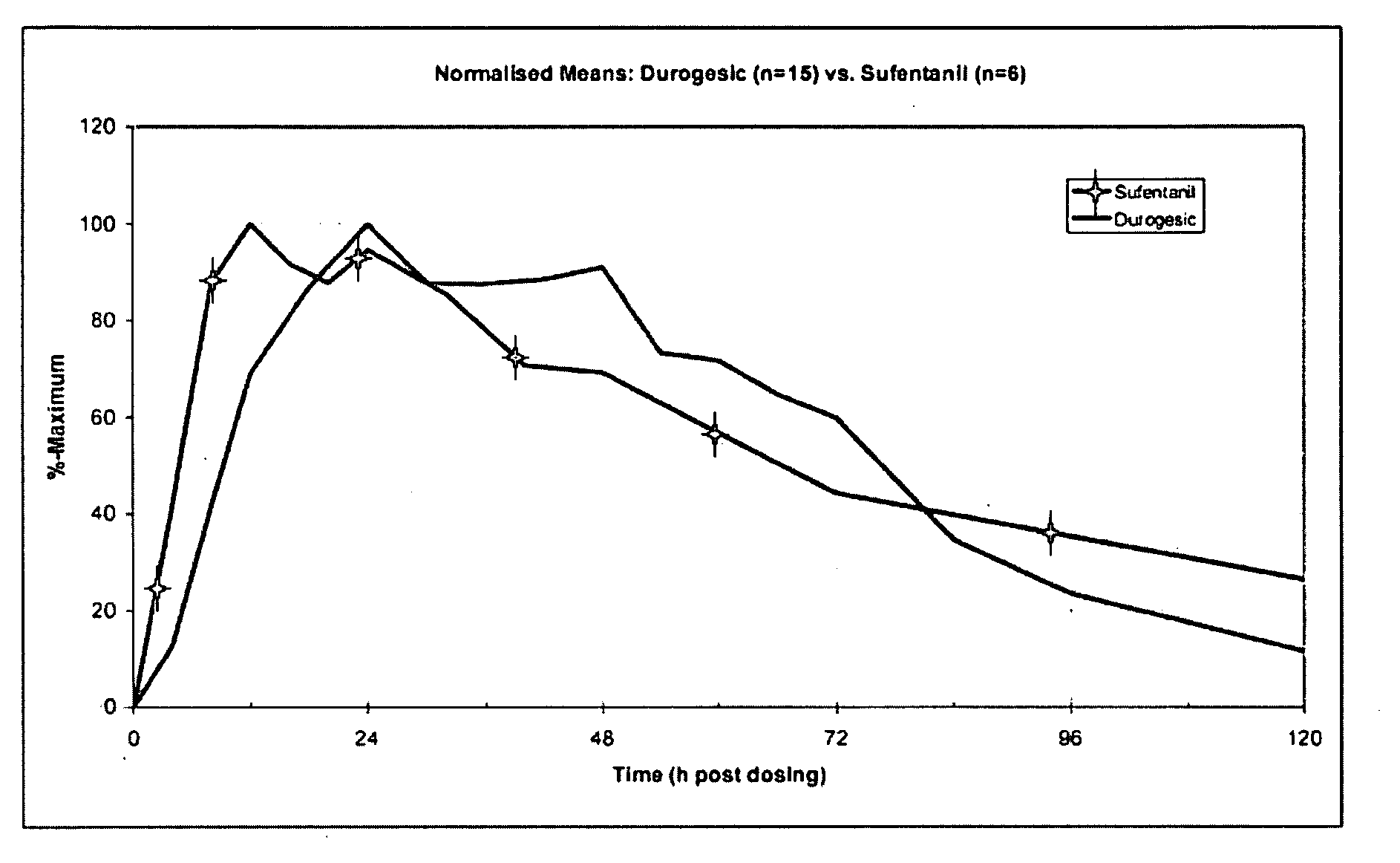
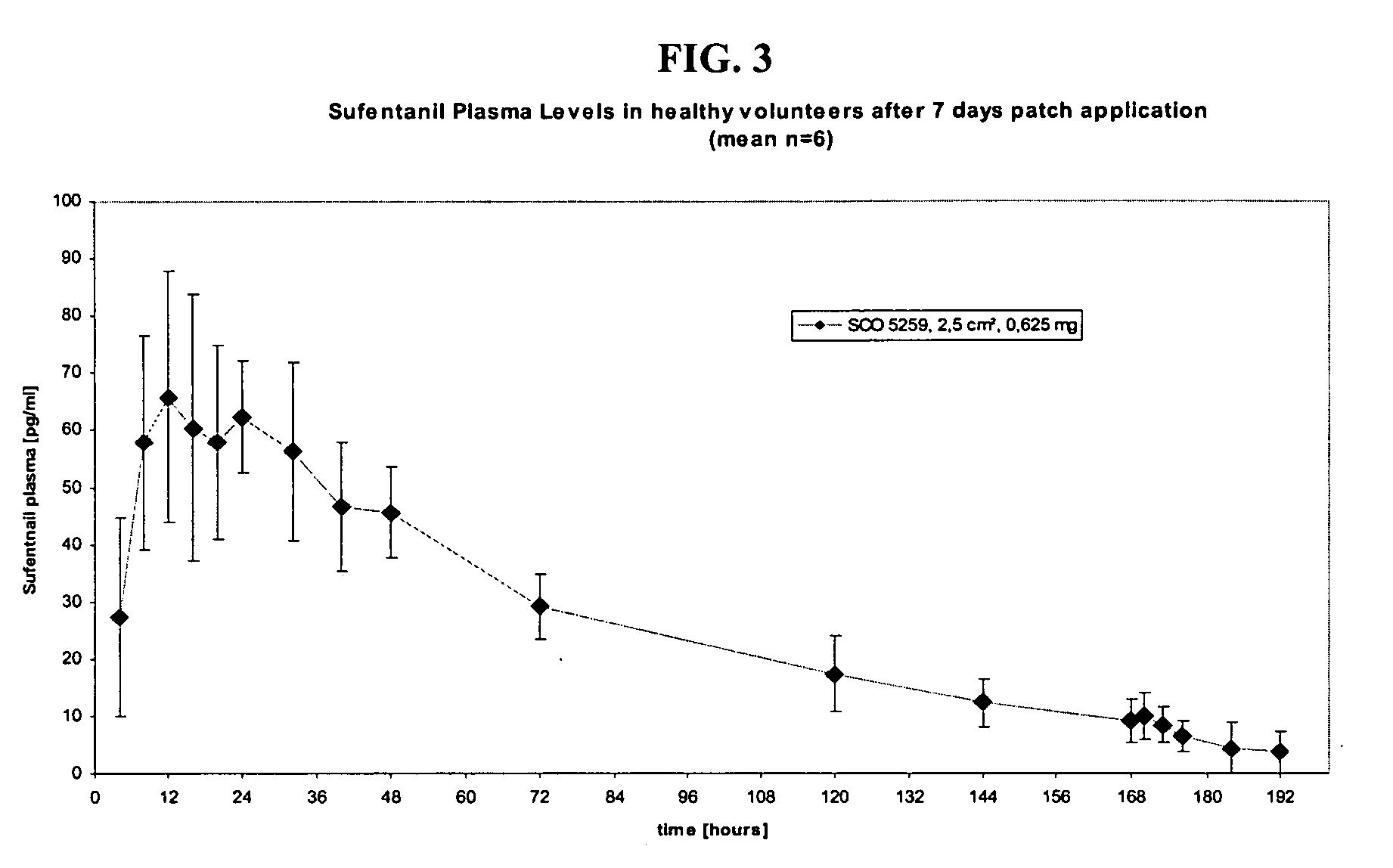
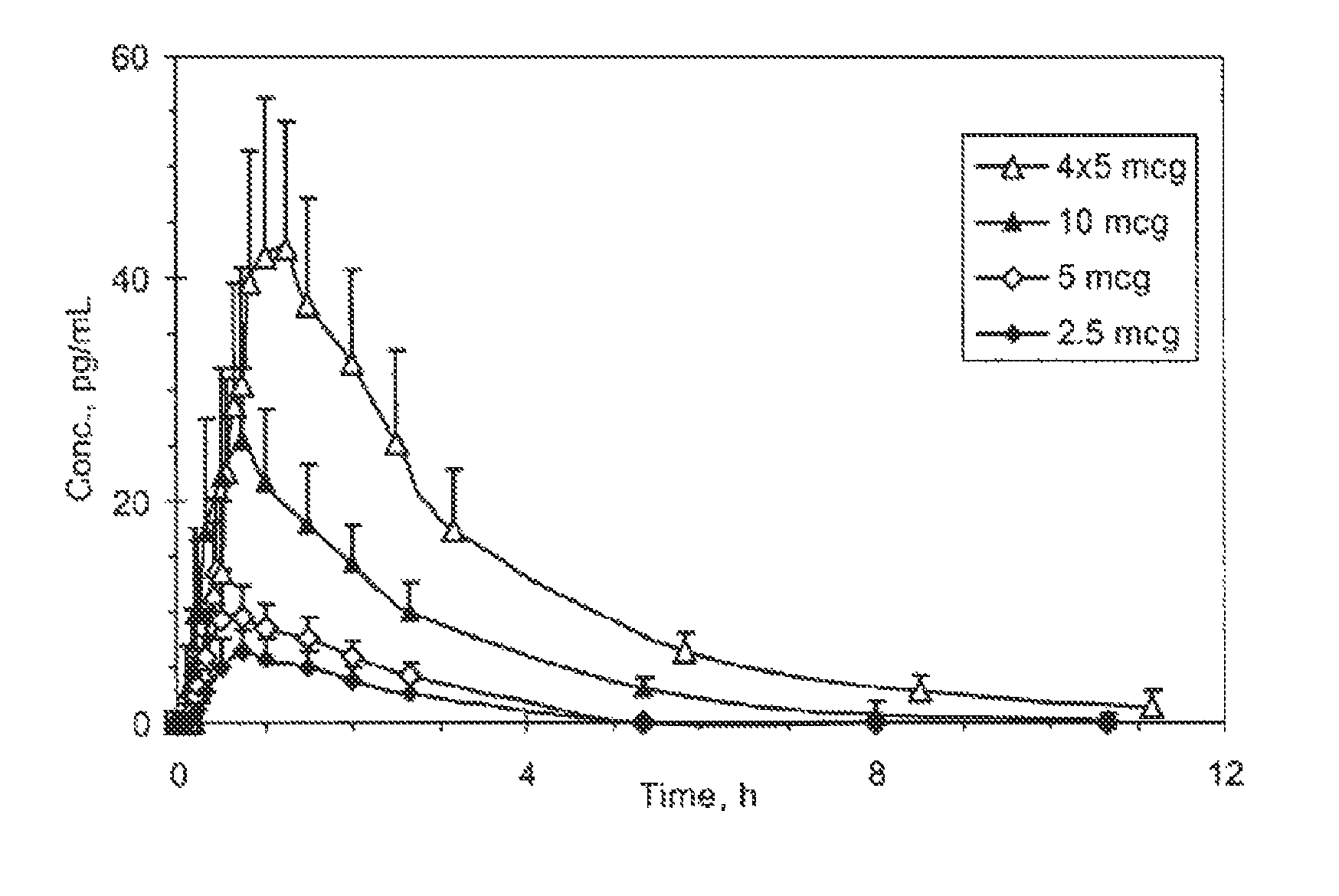
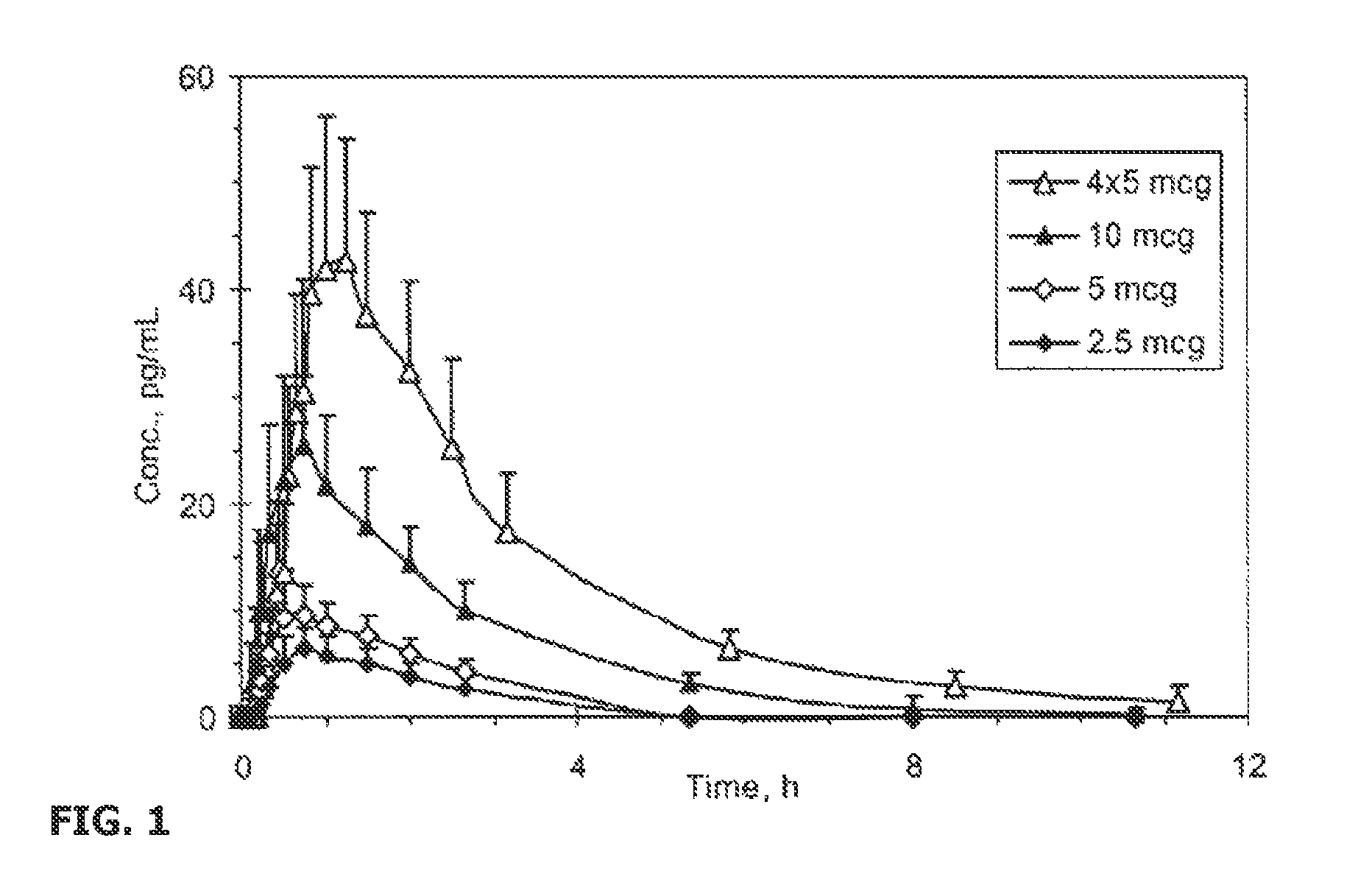
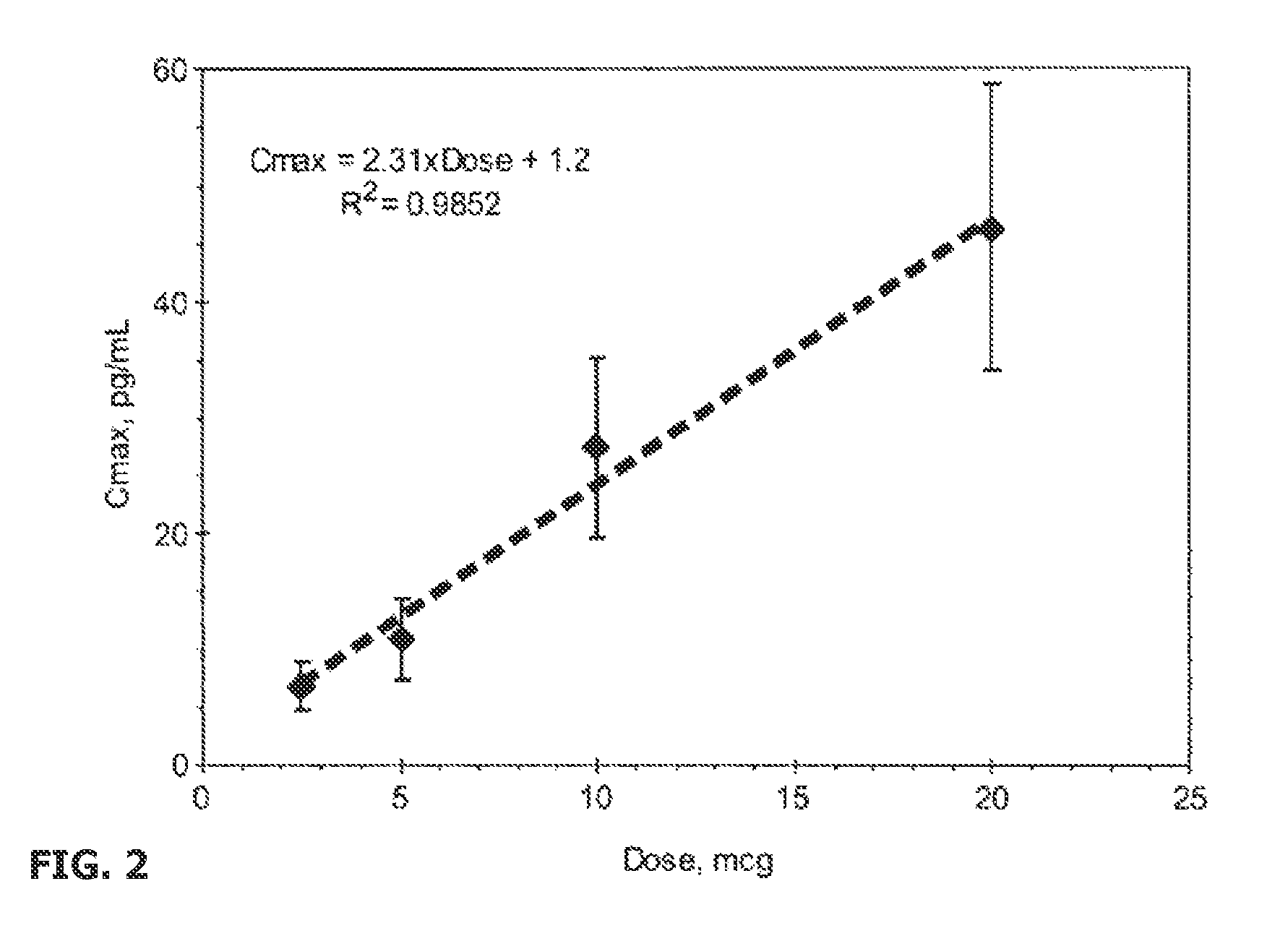
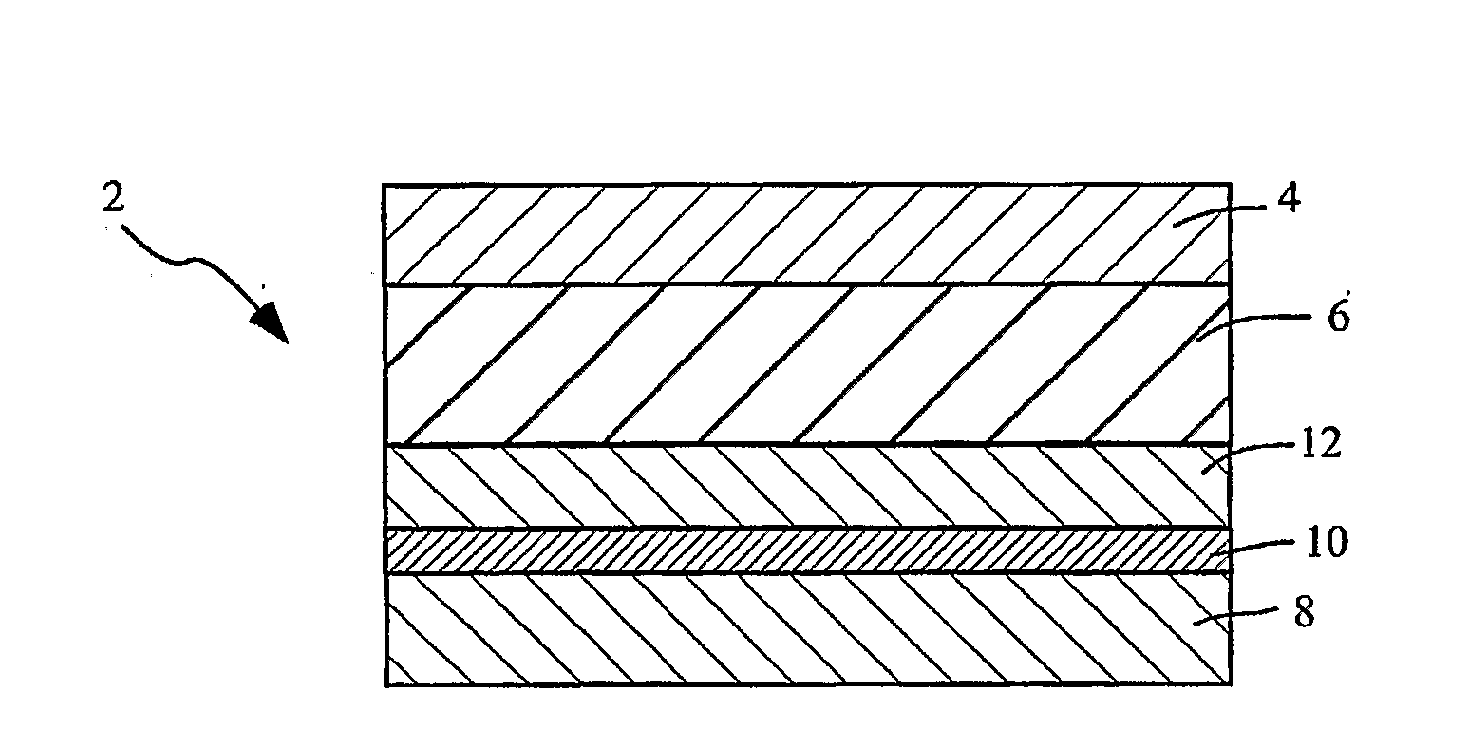
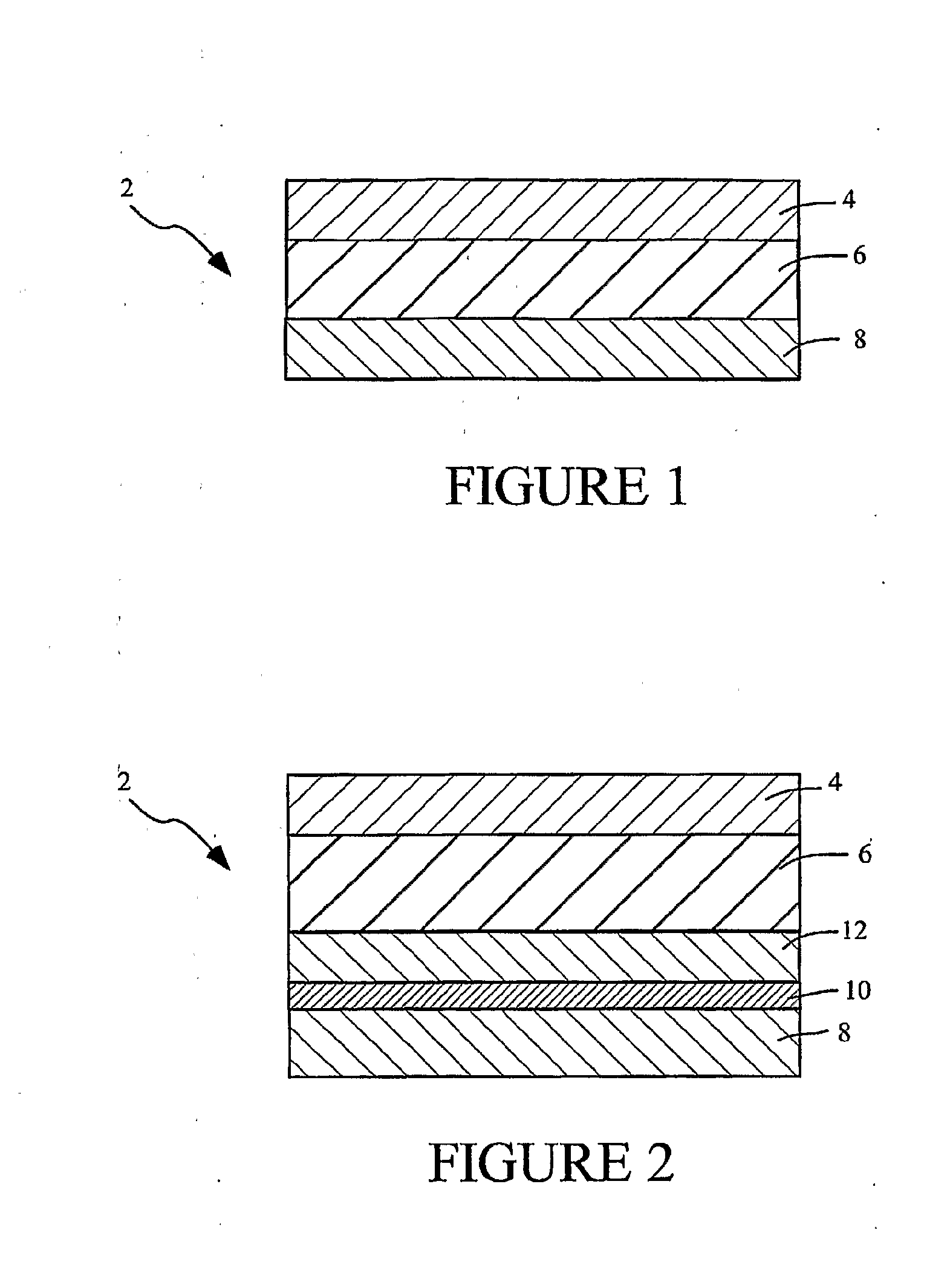
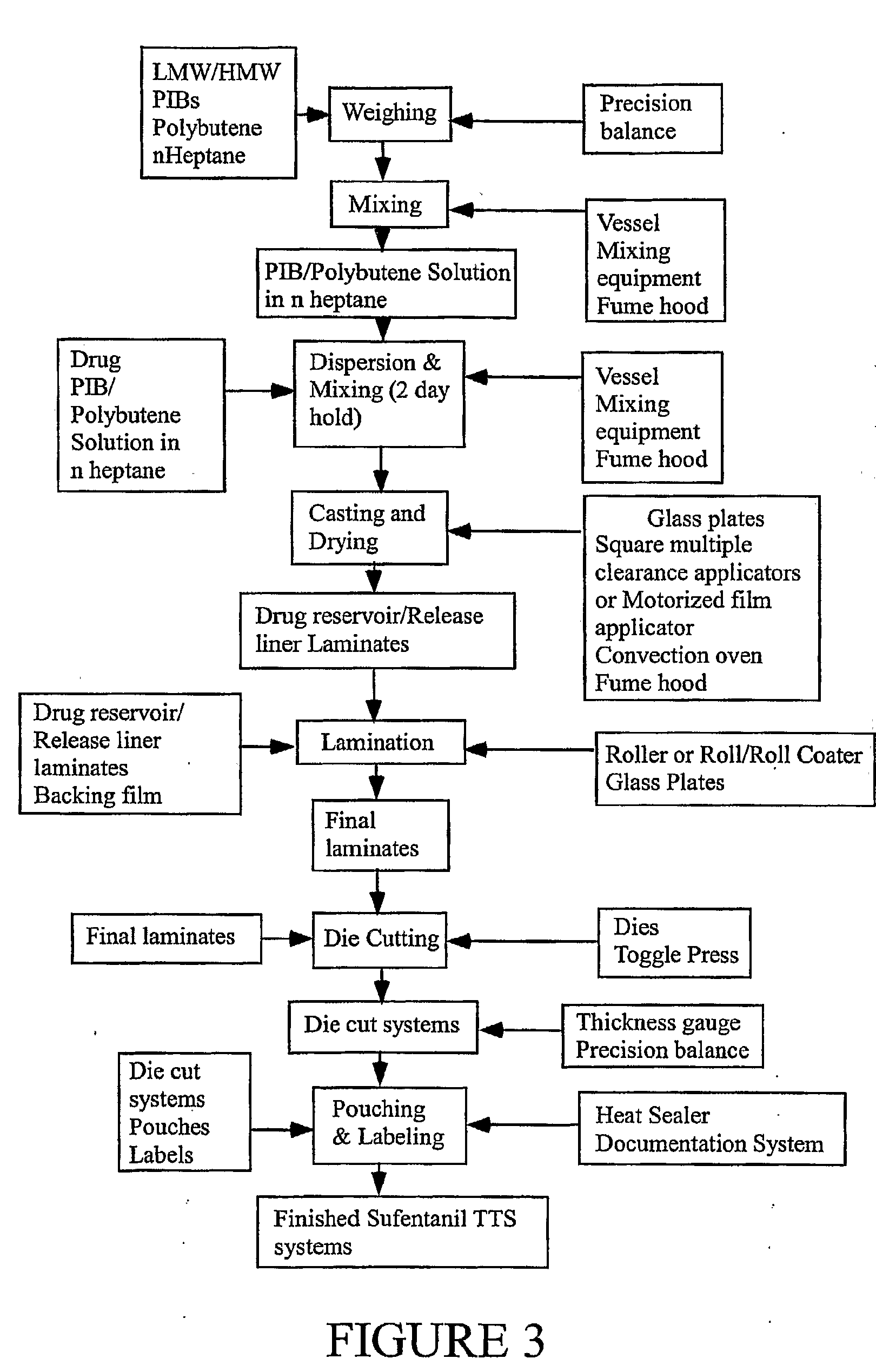
Transdermal System for the Delivery of Sufentanil and Its Analogs
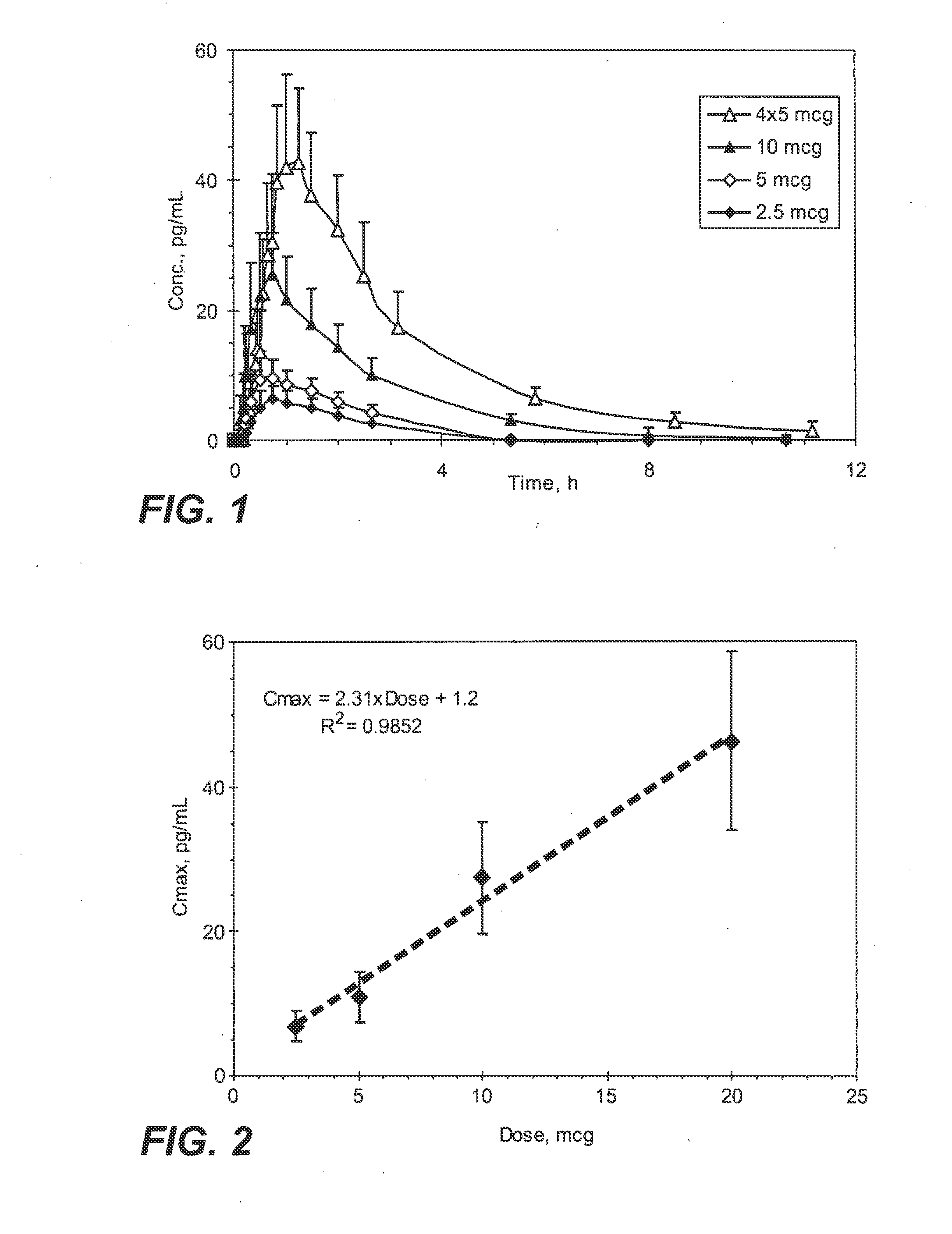
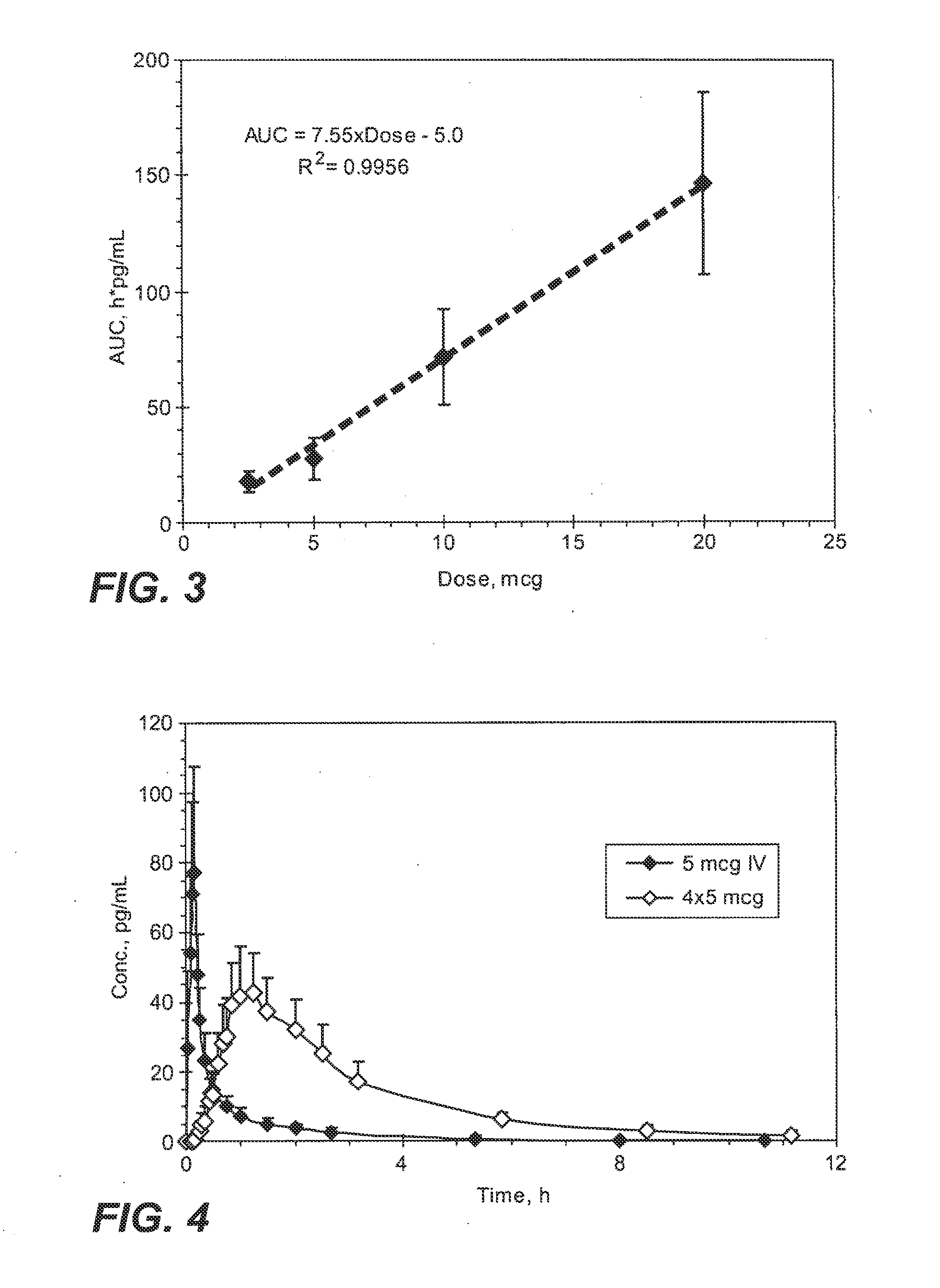
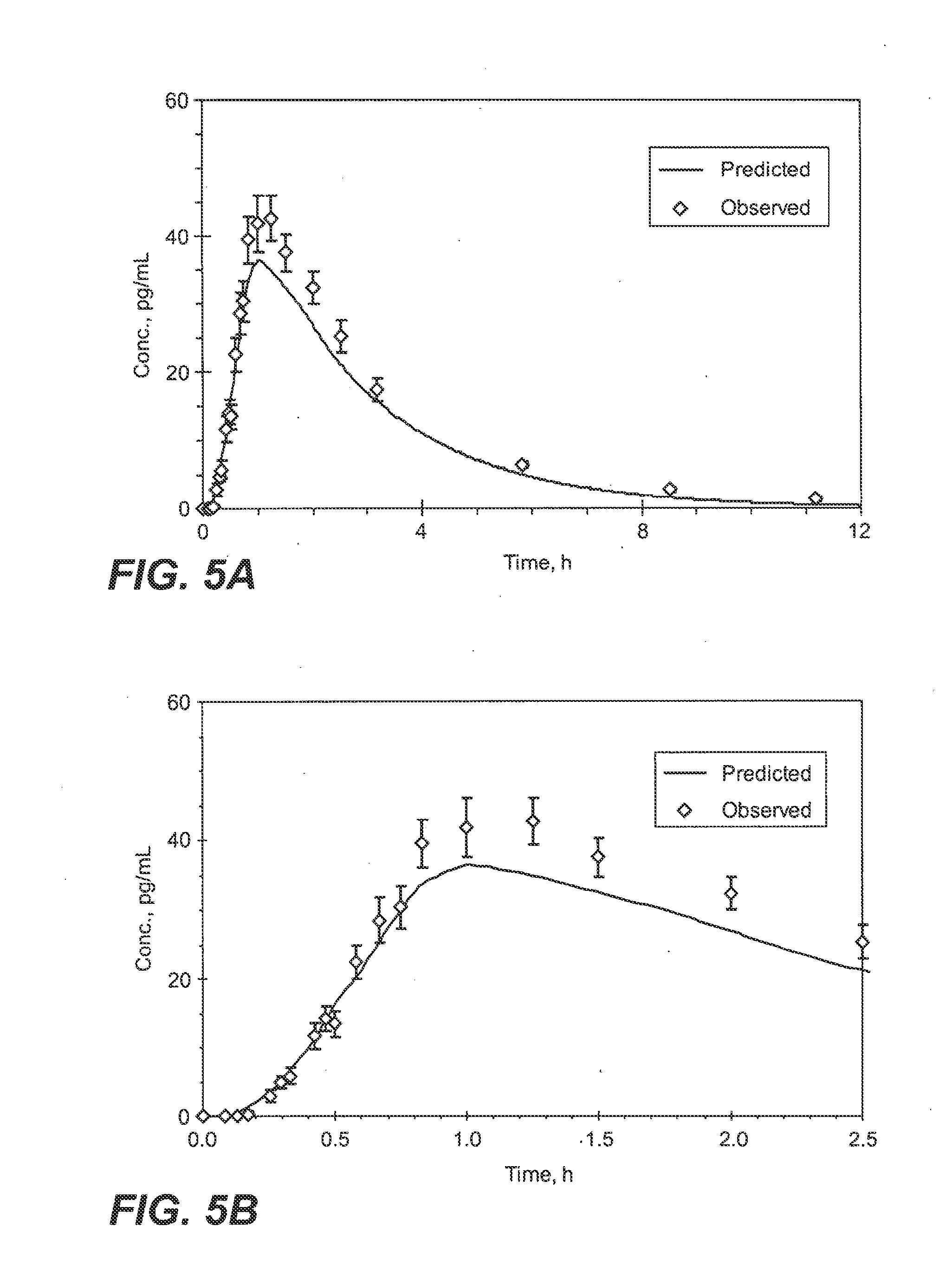
InactiveUS20090130190A1Reduce the amount requiredHigh CmaxBiocideAbsorbent padsPersistent painAcute pain
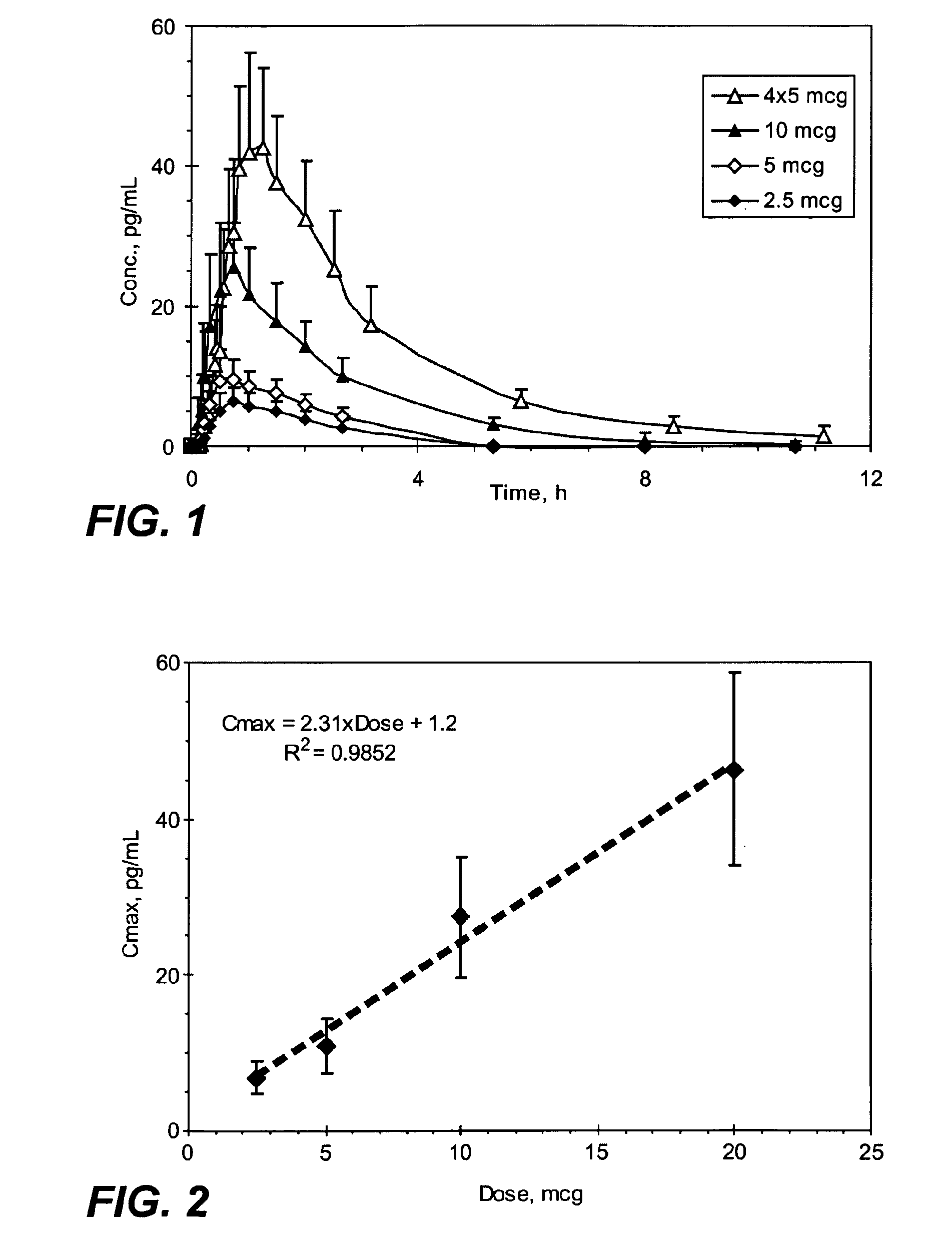
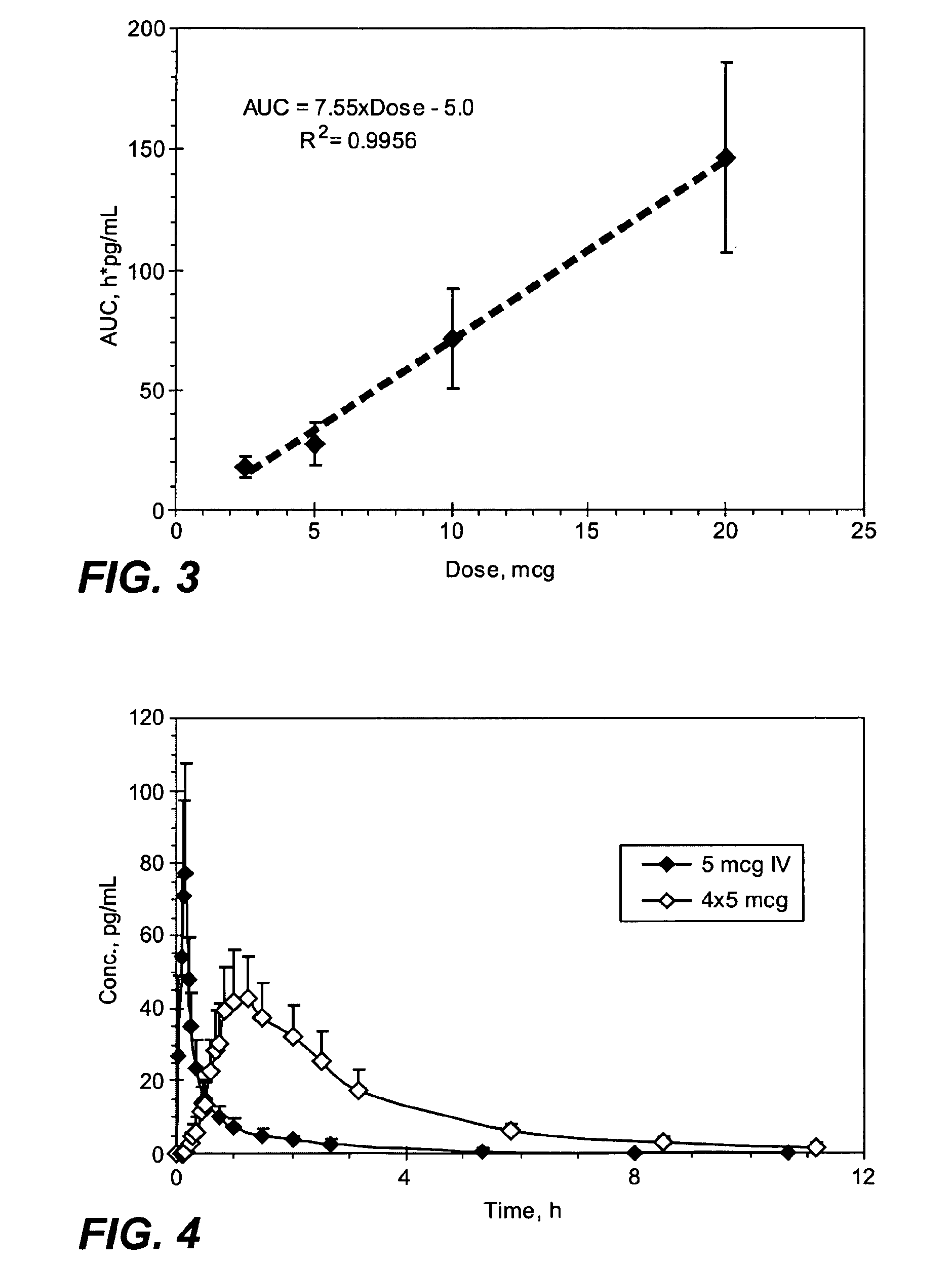
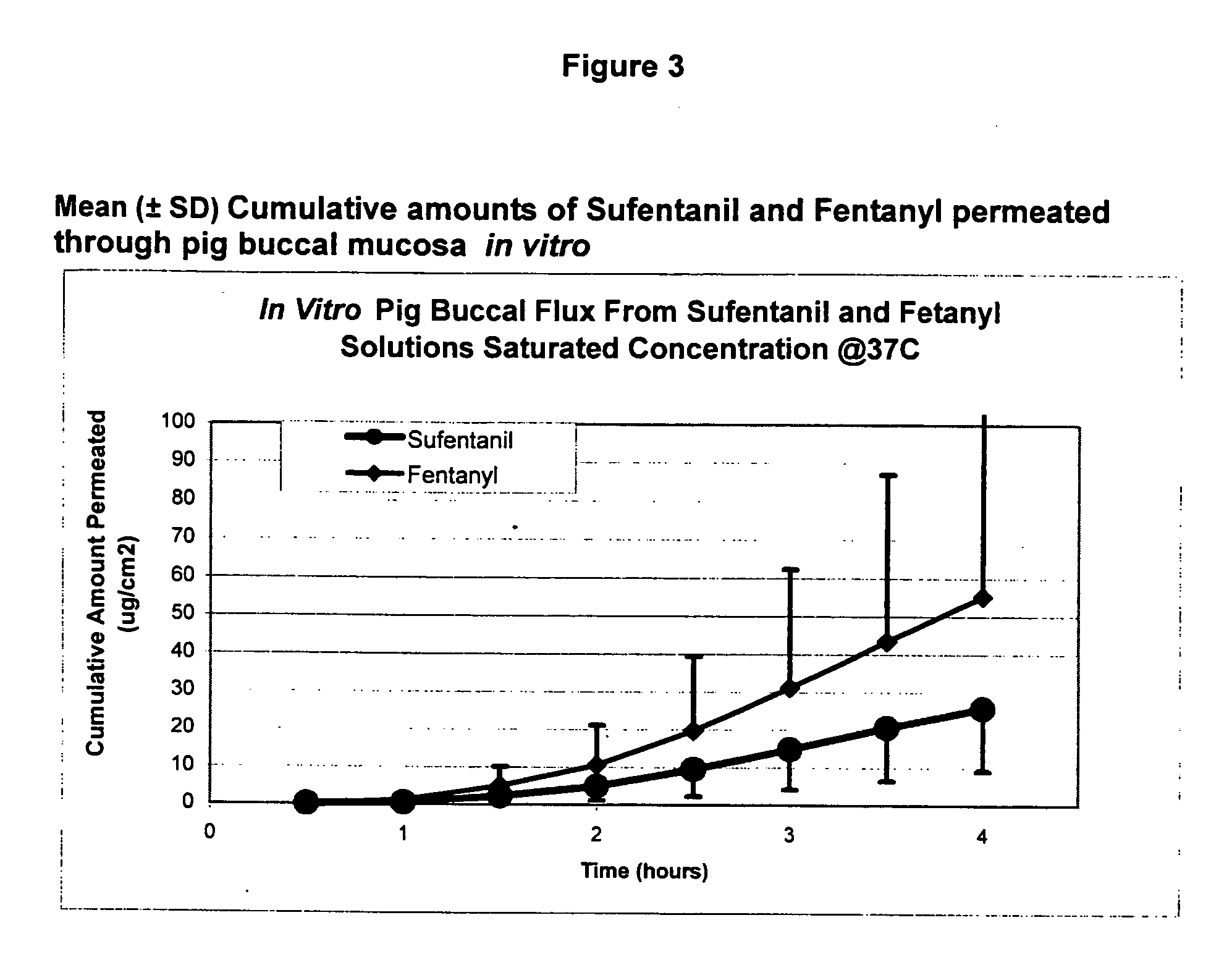
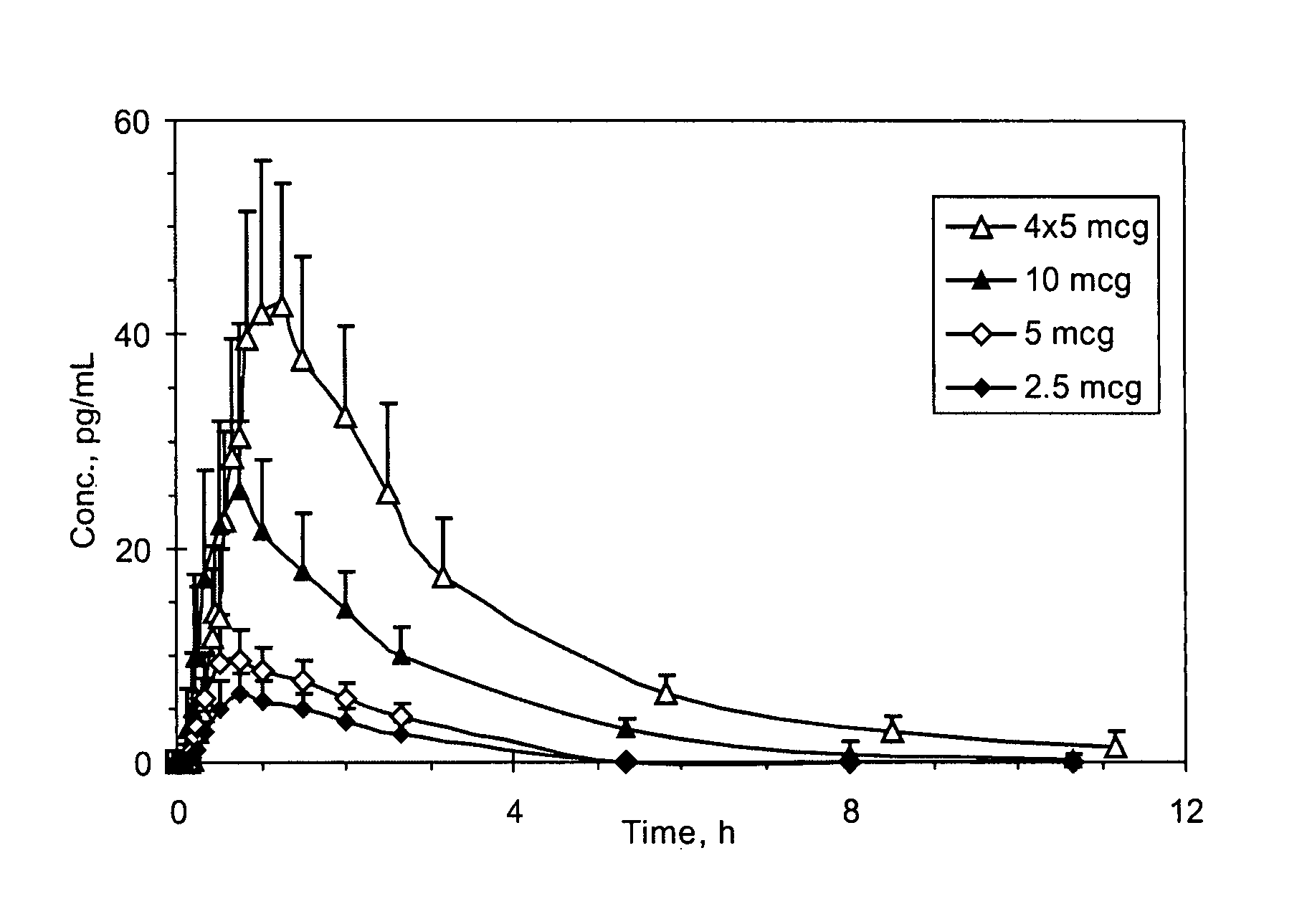
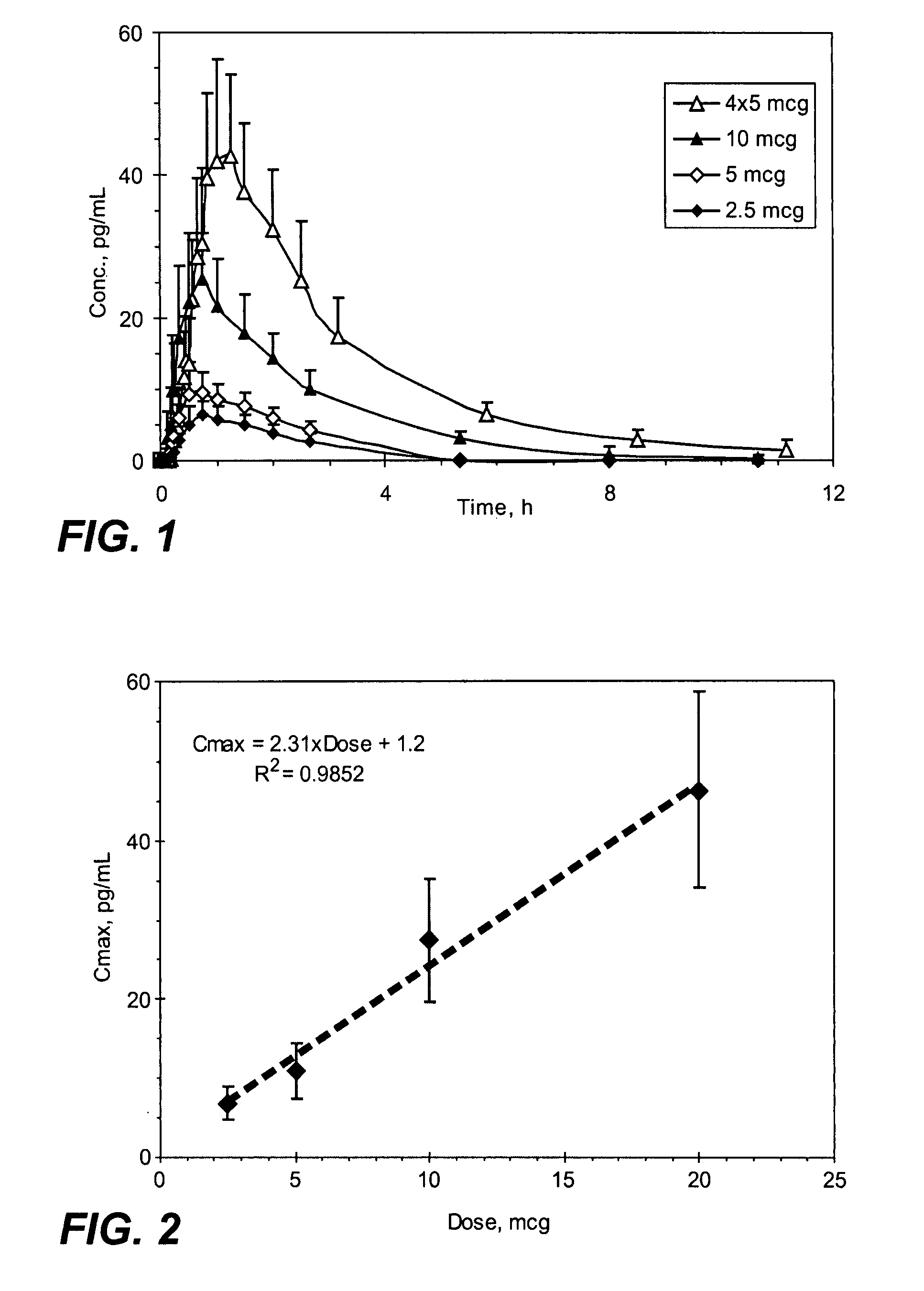
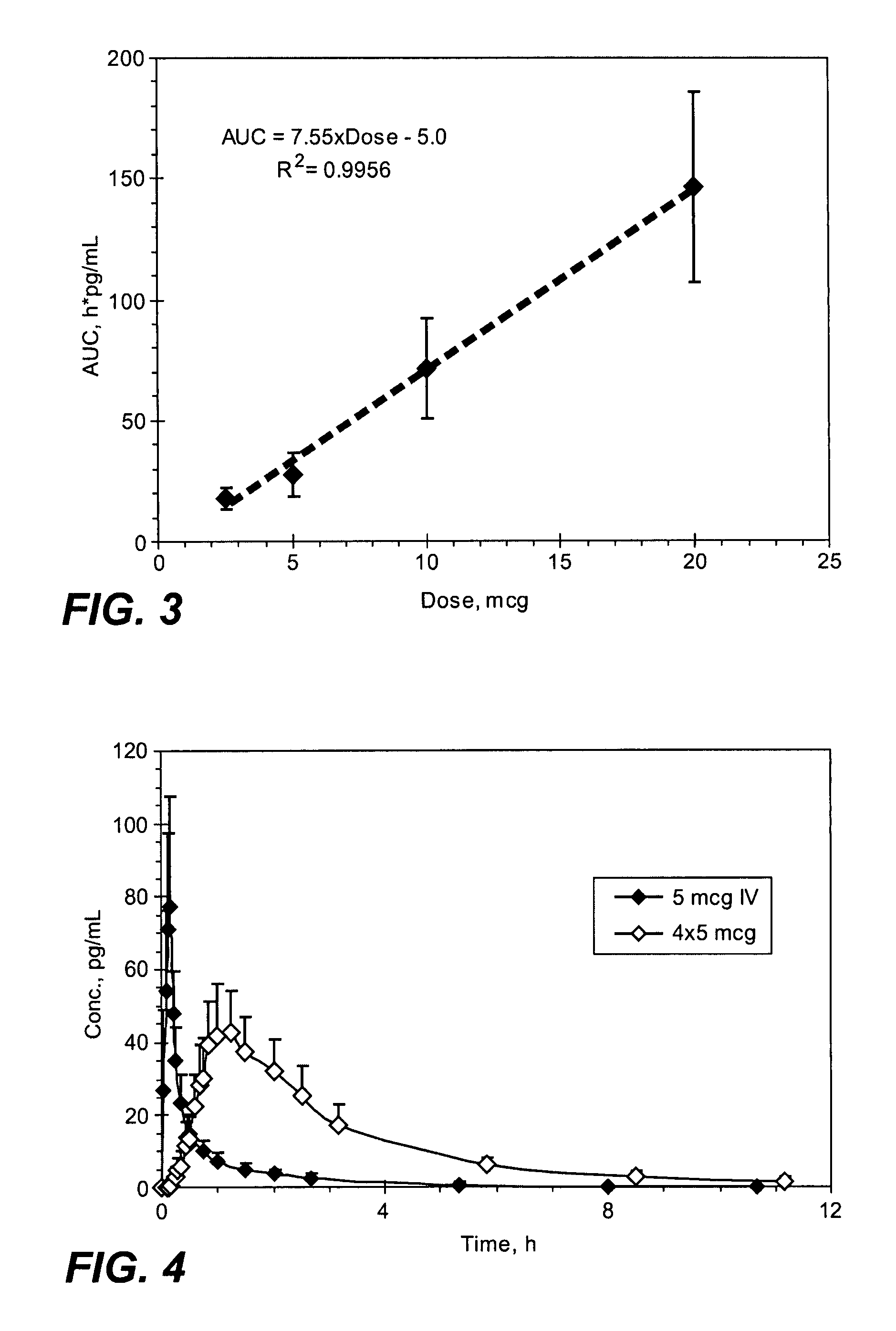
Methods and systems for the transdermal delivery of sufentanil and its analogs are described, from patches having a unique pharmacodynamic profile that can be used to treat persistent pain over extended periods and acute pain episodes of limited duration.
Owner:LABTEC
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Transdermal delivery systems
InactiveUS20080175890A1Easy constructionPreferential pharmacological release characteristicBiocideAdhesive dressingsPhysiologyPermeation
Transdermal delivery systems for administering sufentanil through the skin are provided. The systems contain a sufficient amount of sufentanil to induce and maintain a constant state of analgesia when applied to a subject. The systems are characterized as having one or more features including a high degree of dosage form rate control over flux of sufentanil from the system, a high net flux of sufentanil from the system through the skin, lack of a permeation enhancer, an adhesive member demonstrating superior shear time, a low coefficient of variation in the net flux of sufentanil from the system, a high delivery efficiency, and a substantially constant steady state net flux of sufentanil from the system. Methods of using the transdermal delivery systems to administer a sufficient amount of sufentanil to induce and maintain analgesia for extended periods when applied to a subject are also provided.
Owner:DURECT CORP
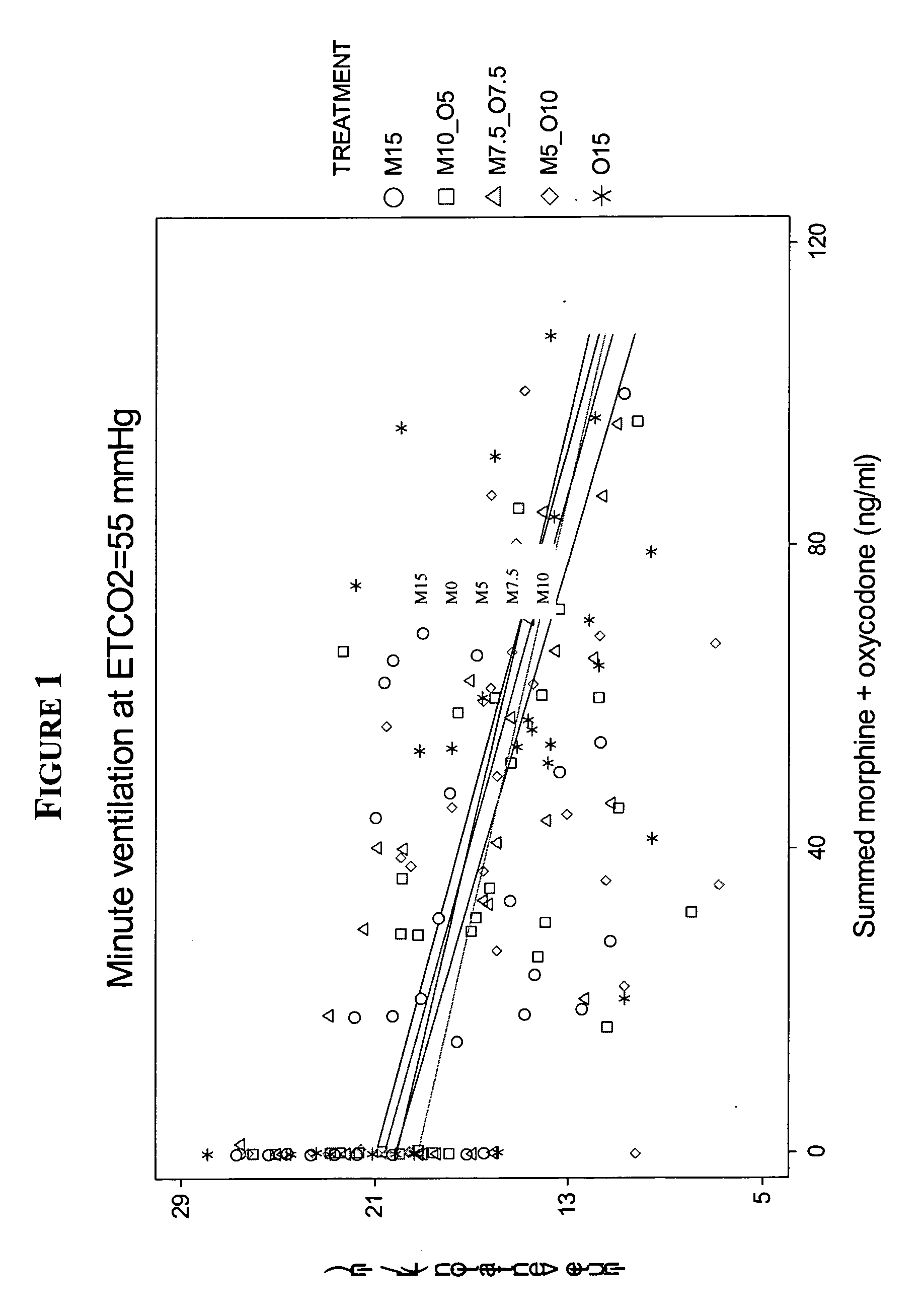
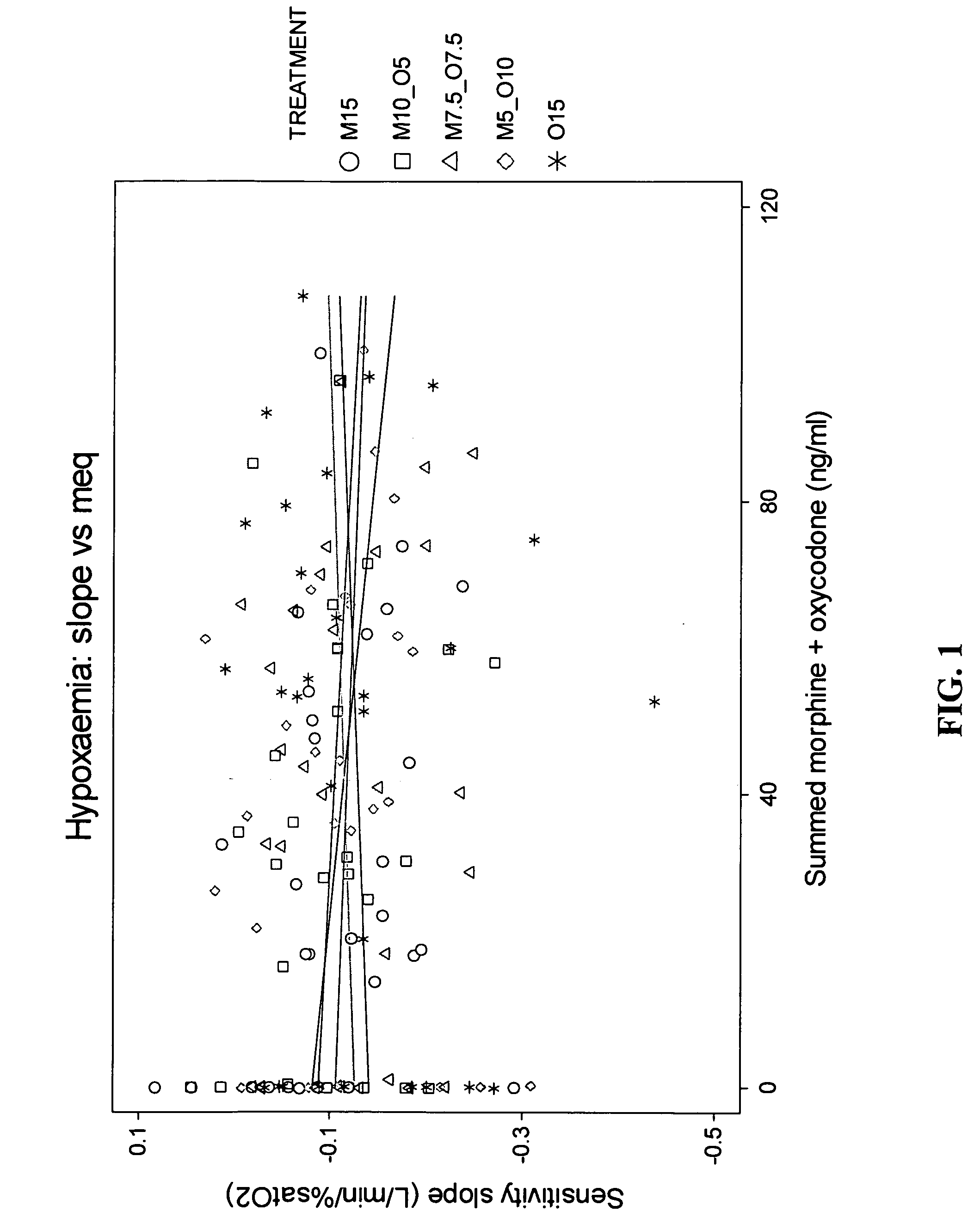
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a μ-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Compositions, systems and methods for administration of small volume sufentanil drug dosage forms via the oral transmucosal route of a subject for treatment of pain.
Owner:VERTICAL PHARMA
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Owner:ACEIRX PHARM INC
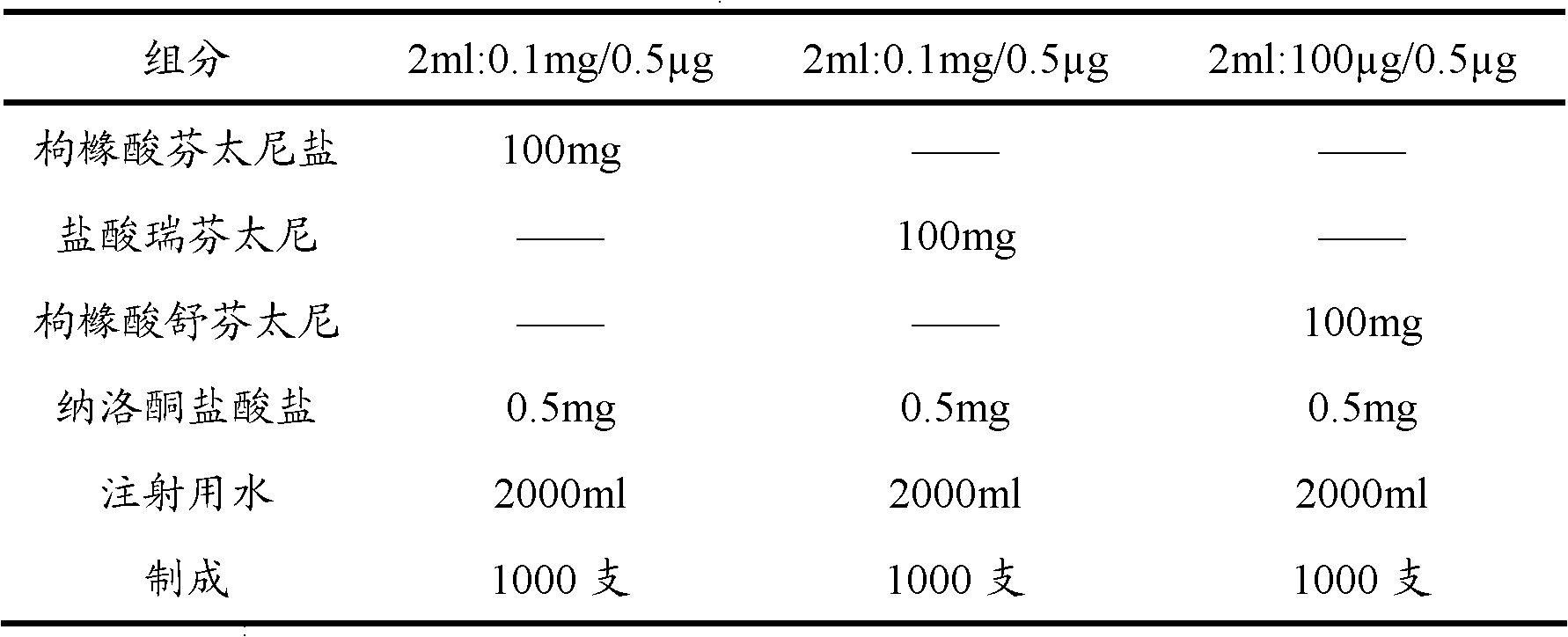
Opiates painkiller and opiate receptor antagonist-containing medicinal composition
ActiveCN102068697AGood analgesic effectPrevent and/or mitigate adverse effectsOrganic active ingredientsNervous disorderSide effectNK1 receptor antagonist
The invention provides an opiates painkiller and opiate receptor antagonist-containing medicinal composition. In the medicinal composition, an opiates painkiller is fentanyl, remifentanil, sufentanil, alfentanil and pharmaceutically acceptable salts thereof; and an opiate receptor antagonist is naloxone, naltrexone, nalmefene and pharmaceutically acceptable salts thereof. The medicinal composition has a pharmacological effect on analgesia. Compared with using the opiates painkiller singly, the composition can prevent and / or lighten side effects in pain treatment, reduce abuse and improve adaptability, and has a reinforcing effect on an analgesic effect of the opiates painkiller.
Owner:YICHANG HUMANWELL PHARMA
Transoral dosage forms comprising sufentanil and naloxone
InactiveUS20100010031A1Efficacy of treatmentIncreased safety marginBiocideNervous disorderMicrogramDosage form
The invention pertains to methods that include administering to a subject a transoral dosage form comprising a pharmaceutical carrier and sufentanil, and maintaining a mean pH ranging from about 3.5 to about 5.5 during a dosing period after administration of the transoral dosage form as determined using an in vitro donor media test. Related dosage forms are also disclosed. Also disclosed are transoral dosage forms and related methods, wherein a transoral dosage form may comprise: (1) about 5 to about 1000 micrograms of sufentanil; (2) about 50 micrograms to about 100 milligrams of naloxone; and (3) acidifying material in an amount sufficient to provide a mean pH ranging from about 3.5 to about 5.5 during a dosing period after administration of the transoral dosage form as determined using an in vitro donor media test; wherein the dosing period begins no earlier than about 1 minute after administration of the transoral dosage form, and ends no later than about 120 minutes after administration of the transoral dosage form.
Owner:YUM II SU +3
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Owner:VERTICAL PHARMA
Sufentanil solid dosage forms comprising oxygen scavengers and methods of using the same
ActiveUS8945592B2Percentage of sufentanil oxidative degradation products isOxidative degradation can be preventedBiocidePill deliveryDosage formSufentanil
Compositions and methods effective to minimize or eliminate the presence of oxidative degradation products in solid dosage forms comprising sufentanil are provided.
Owner:VERTICAL PHARMA
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a p-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
Transdermal Delivery Systems
InactiveUS20080206314A1Easy constructionPreferential pharmacological release characteristicBiocideNervous disorderPhysiologyPermeation
Transdermal delivery systems for administering sufentanil through the skin are provided. The systems contain a sufficient amount of sufentanil to induce and maintain a constant state of analgesia when applied to a subject. The systems are characterized as having one or more features including a high degree of dosage form rate control over flux of sufentanil from the system, a high net flux of sufentanil from the system through the skin, lack of a permeation enhancer, an adhesive member demonstrating superior shear time, a low coefficient of variation in the net flux of sufentanil from the system, a high delivery efficiency, and a substantially constant steady state net flux of sufentanil from the system. Methods of using the transdermal delivery systems to administer a sufficient amount of sufentanil to induce and maintain analgesia for extended periods when applied to a subject are also provided.
Owner:DURECT CORP
Process for synthesizing remifentanil
An improved process for synthesizing opiate or opioid analgesics and anesthetics, and intermediates thereof is provided. In particular, processes of synthesizing intermediates for use in the preparation of synthetic opiate or opioid compounds such as, for example, remifentanil, carfentanil, sufentanil, fentanyl, and alfentanil are disclosed. The preparation process requires fewer steps, and results in reduced costs and higher efficiency than processes known in the art for producing remifentanil and carfentanil.
Owner:MALLINCKRODT INC
New methods for the synthesis of alfentanil, sufentanil, and remifentanil
Synthetic pathways are disclosed for synthesizing derivatives or analogs of fentanyl. Specifically set out are pathways for synthesizing alfentanil, sufentanil and remifentanil. The disclosed methods require fewer steps and produce a greater yield of product than methods reported in the prior art.
Owner:MALLINCKRODT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com