Transdermal System for the Delivery of Sufentanil and Its Analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Determination of Minimum and Average Effective Plasma Levels for Sufentanil
[0088]Table A below summarizes the median sufentanil i.v. infusion rates, and resulting steady state plasma levels, from sufentanil infusion for intensive case applications and for sustained analgesia in post-surgical or chronic pain applications. In the intensive care unit (ICU), the goal is generally moderate patient sedation (somnolent but easily aroused) and effective analgesia. For sustained pain control, the goal is effective pain control with minimal to moderate sedation. In these studies, steady-state sufentanil infusions were generally combined with additional bolus injections of sufentanil as a pre-medication and as demanded by the patient through patient controlled analgesia (PCA) pumps.
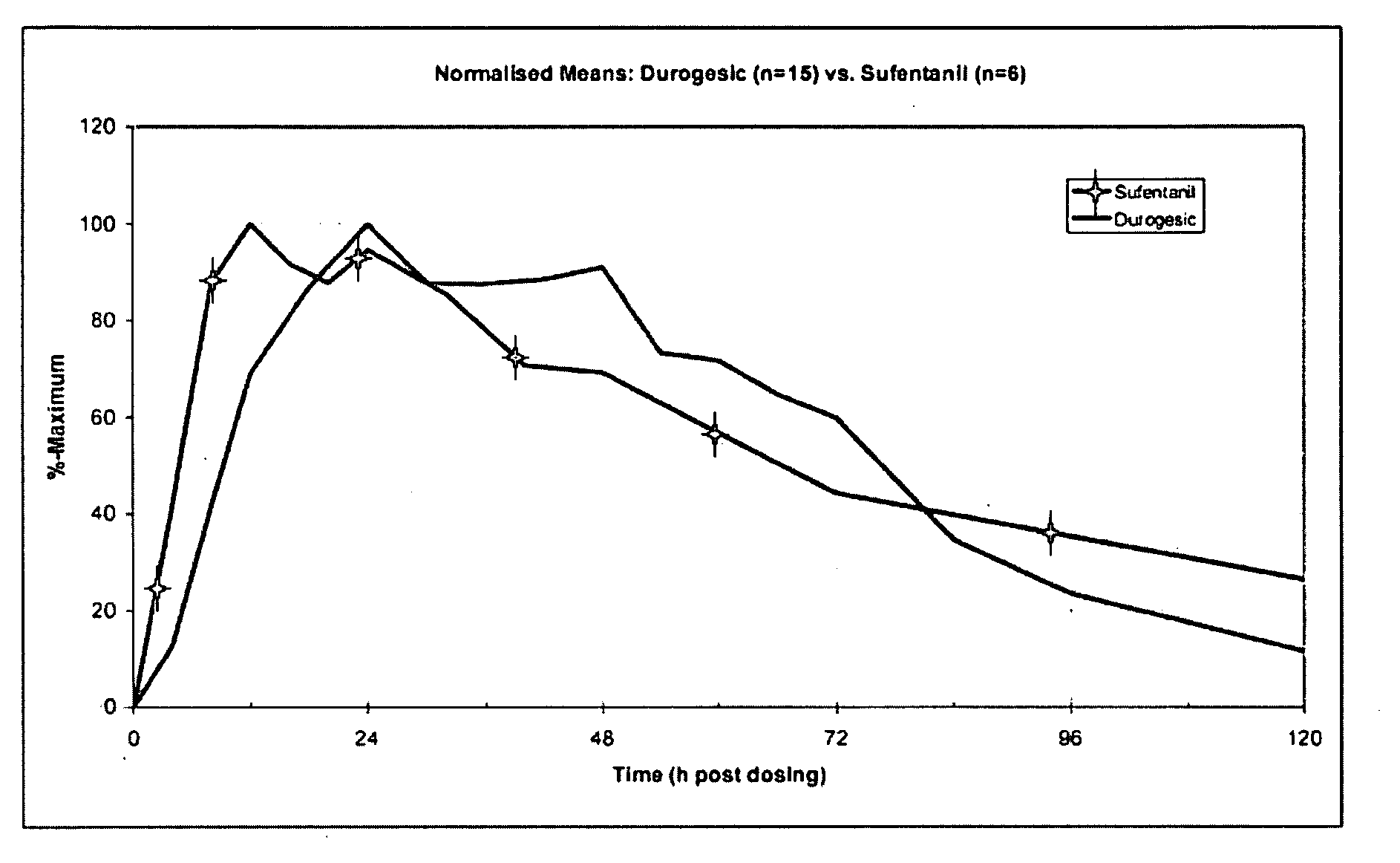
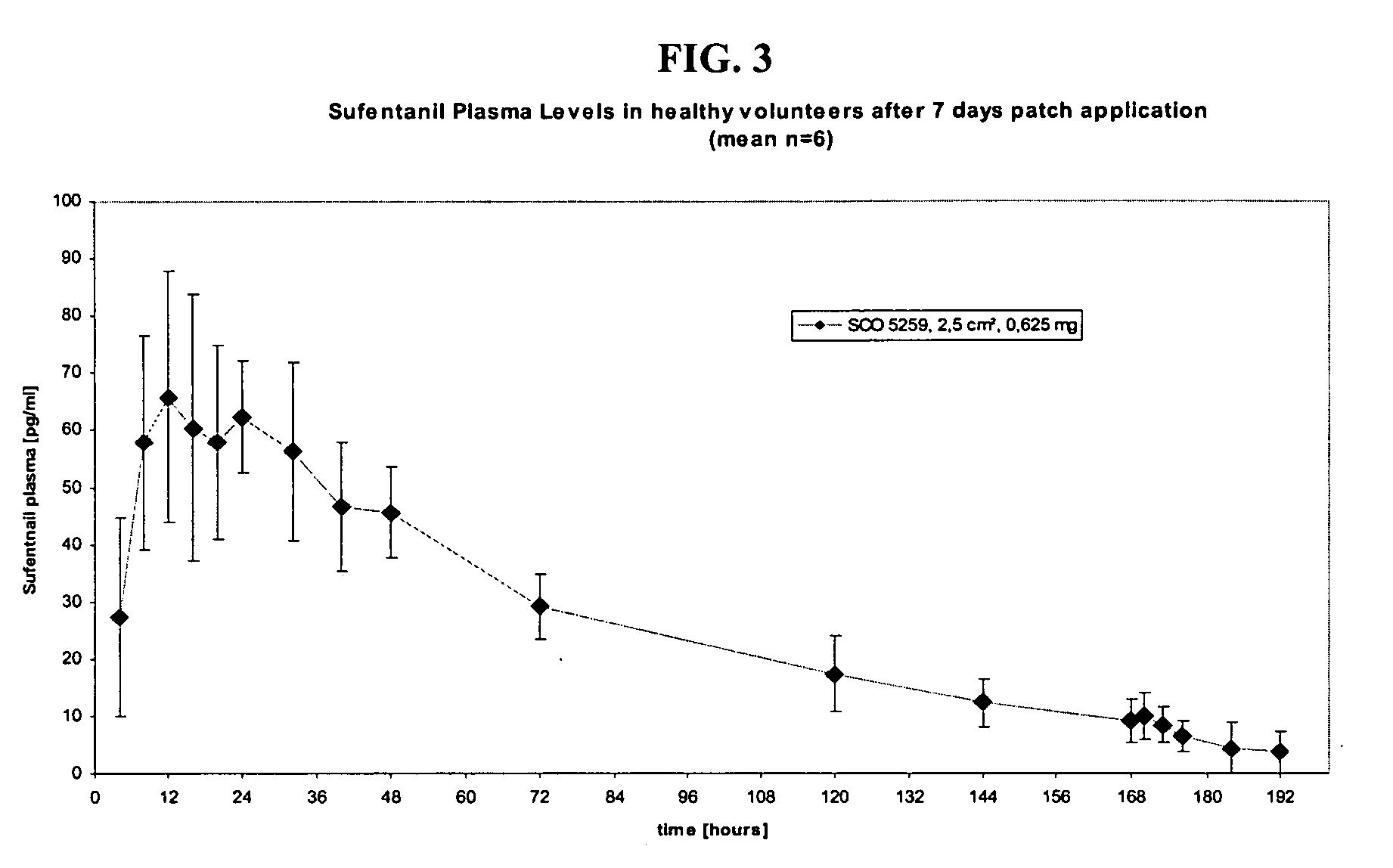
[0089]The “average intravenous infusion rate for sustained analgesia,” from six studies in Table A (using the midpoint of the range from Coda et al. (1997)) was 0.104 mcg / kg / h. The average of the mean or median plas...
example 2
Composition of Sufentanil Patch
[0091]Table C sets forth an exemplary composition for the patches of the present invention.
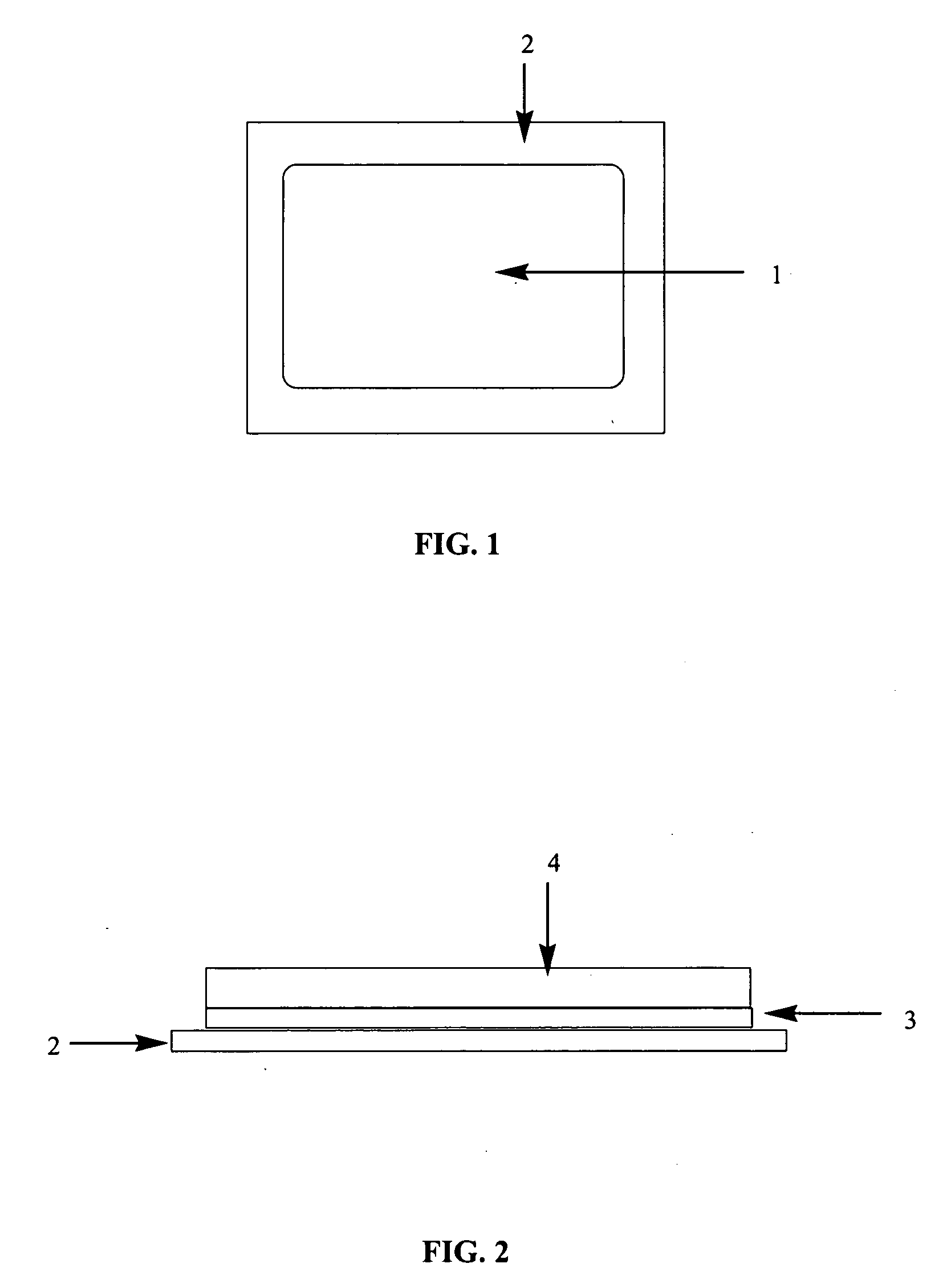
TABLE CComponentFunctionmg per sqcmSecuron PP ® (Corovin)Non-woven backing2.10Scotchpack 1022 ® (3M)Release Liner—(removed at application)Sufentanil BaseAPI0.25Calcium glycero phosphateSkin care agent0.25Oppanol ™ B100Adhesive polymer1.00(high molecular PIB)Oppanol ™ B10Adhesive polymer5.00(low molecular PIB)PolybuteneTackifier2.00Ethyl acetateSolventSolvent, not partof final product!Oppanol B100 is polyisobutylene with a molecular weight of > 1,000,000 g / mol.Oppanol B10 is polyisobutylene with a molecular weight of 40,000 g / mol.
example 3
Method of Making Sufentanil Patch
[0092]The required amount of the three excipients forming the PIB adhesive (Oppanol B100, Oppanol B10 and Parapol 920)—are weighed and dissolved in hexane under stirring. Sufentanil is dissolved in ethyl acetate. Calcium glycerol phosphate is added to the clear drug solution under stirring to yield a homogenous suspension. The adhesive solution is added slowly to the drug solution under stirring and stirred for an additional hour to yield a homogenous mixture without any air bubbles. In bench scale manufacturing this mixture is then treated with ultra-sound for 2 times 15 min (to remove bubbles if any).
[0093]The mixture is coated onto the release liner (the mixture has to be kept under constant stirring to avoid segregation of the dispersed calcium glycerol phosphate). The coated film is dried at room temperature for 10 min followed by 20 min at 750C. The backing foil is applied and the patches are punched out of the resulting laminate, followed by p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com