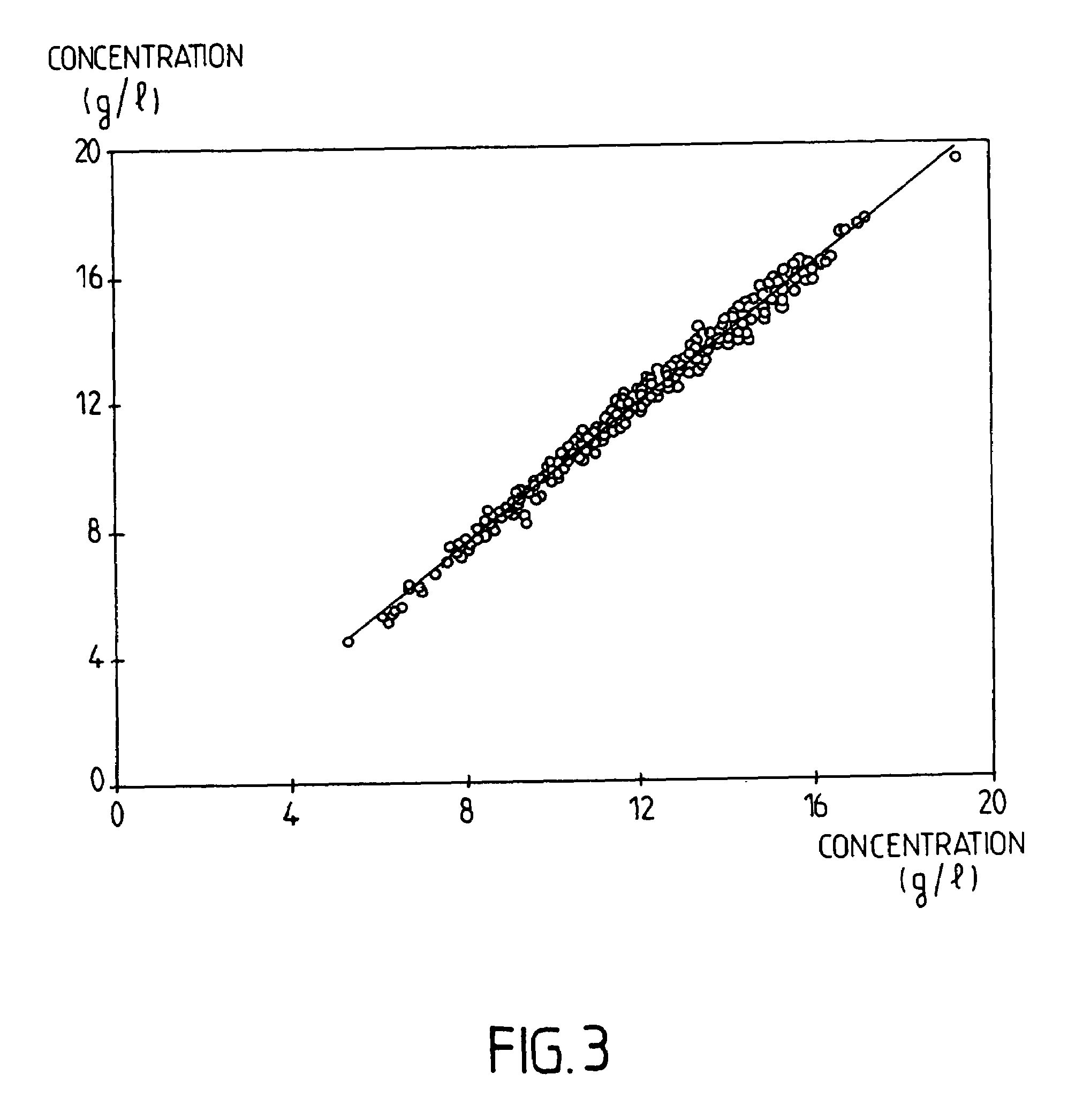
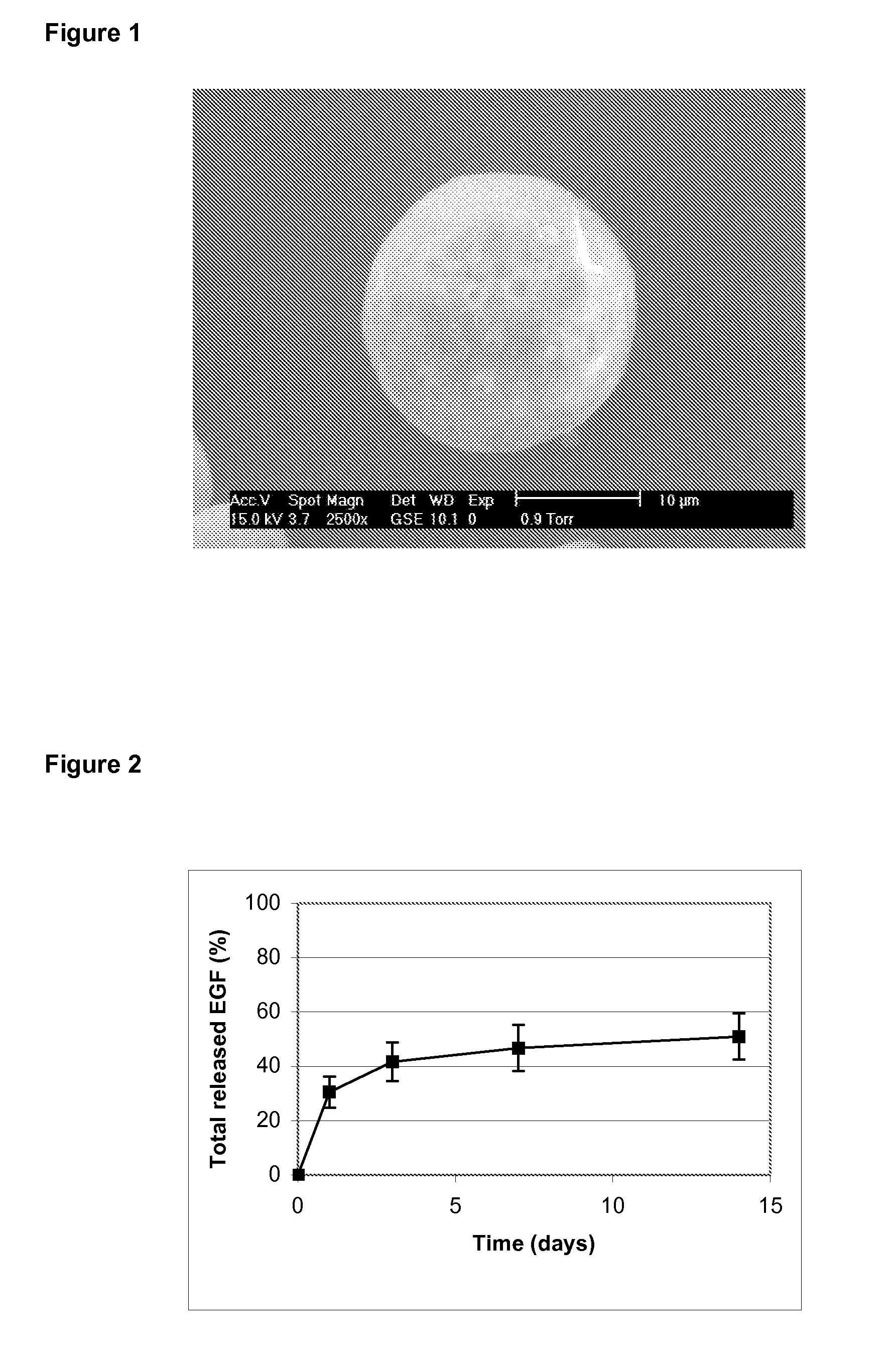
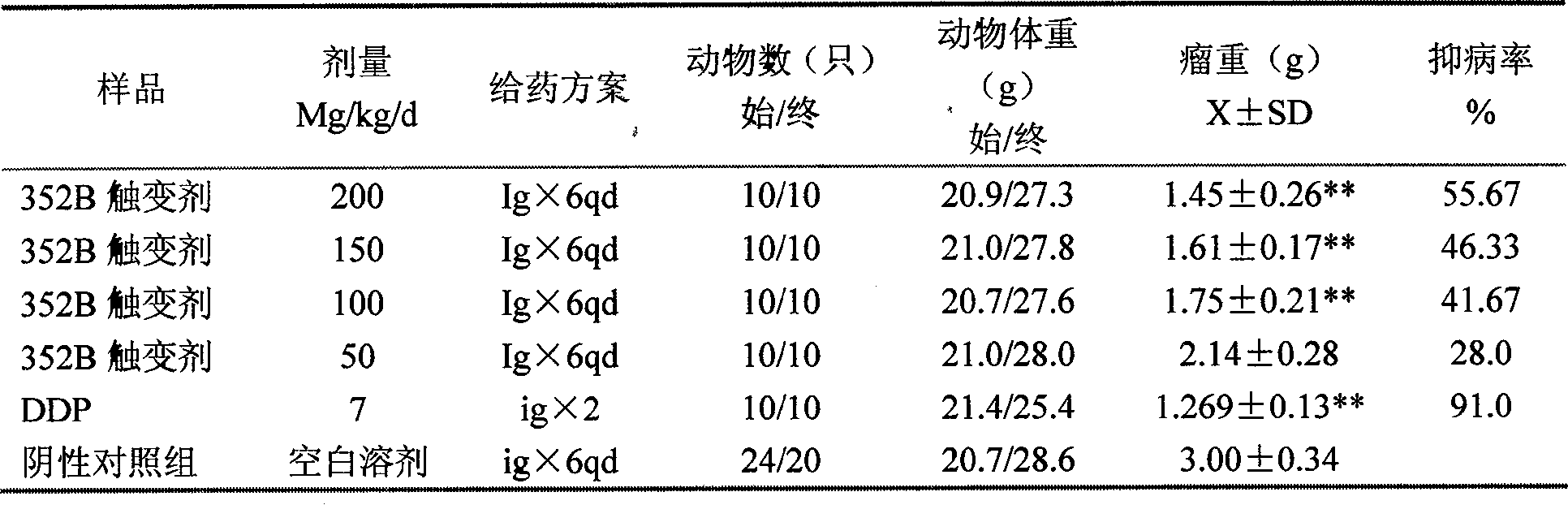
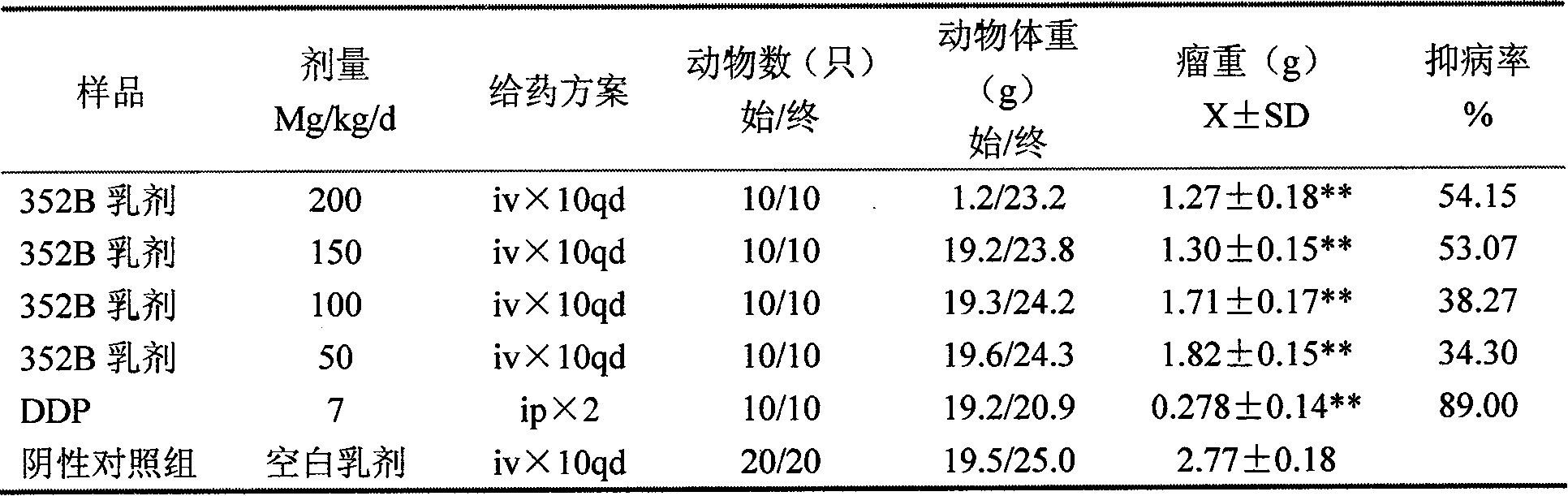
Patents
Literature
82 results about "Human medicine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
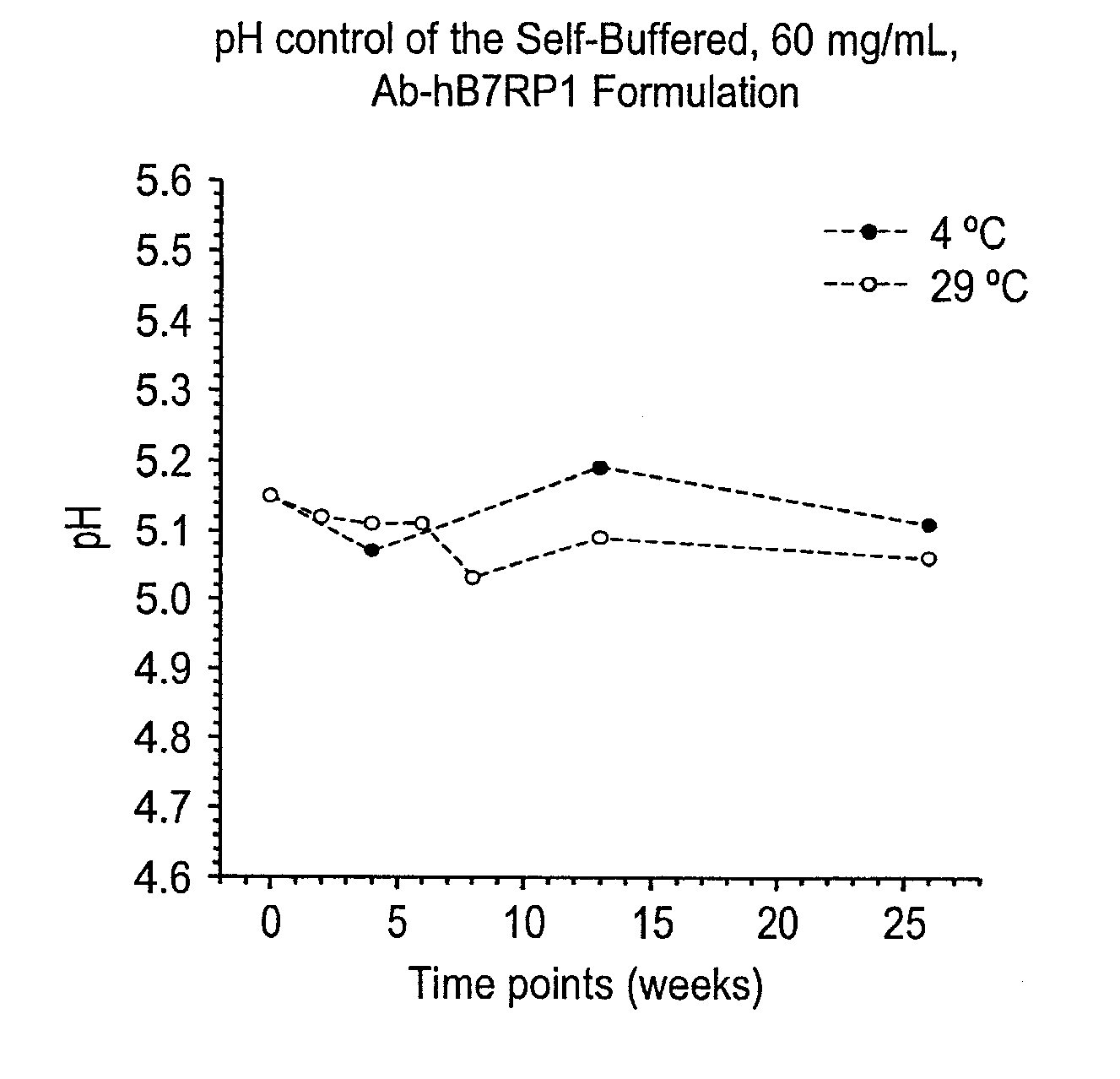
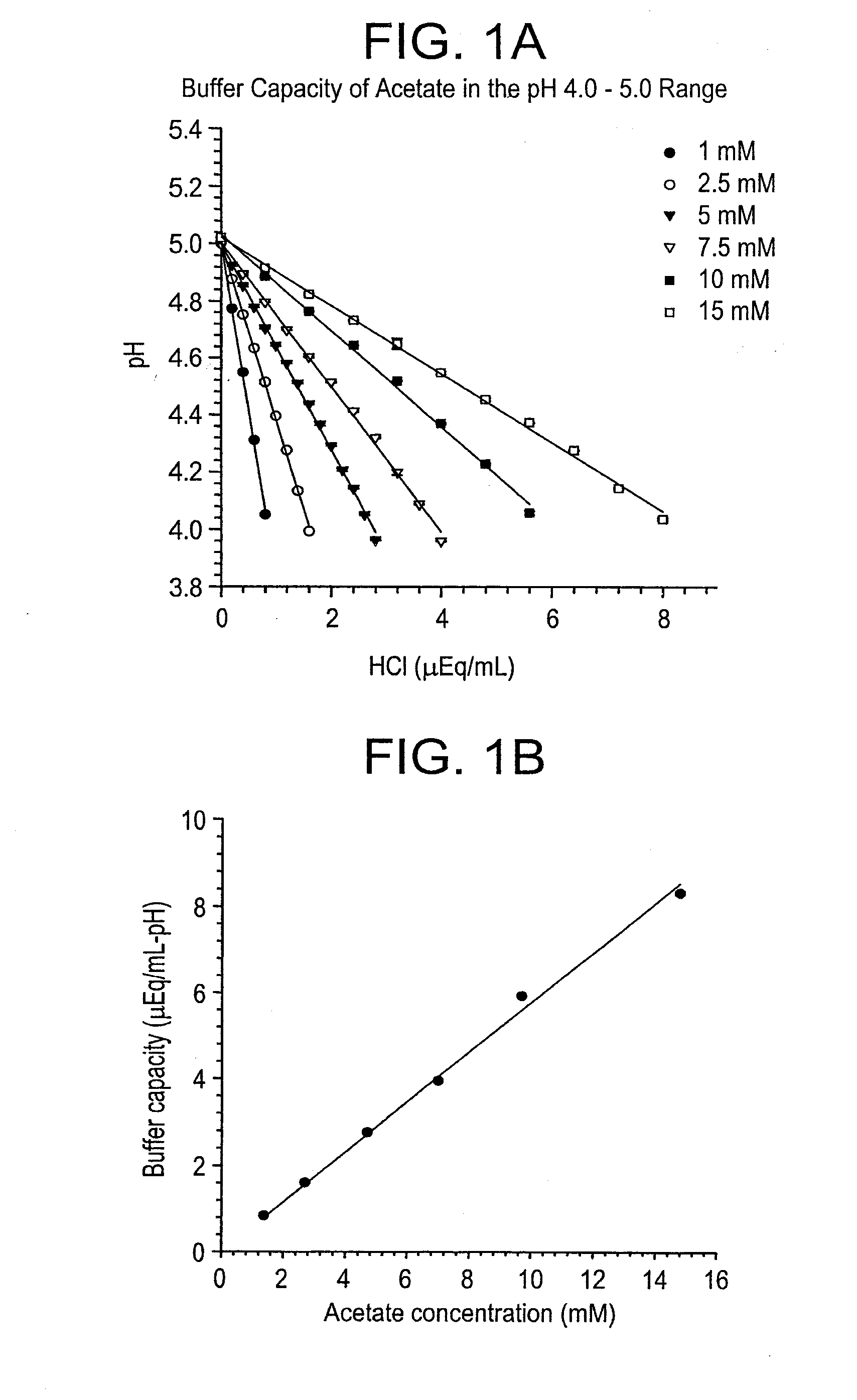
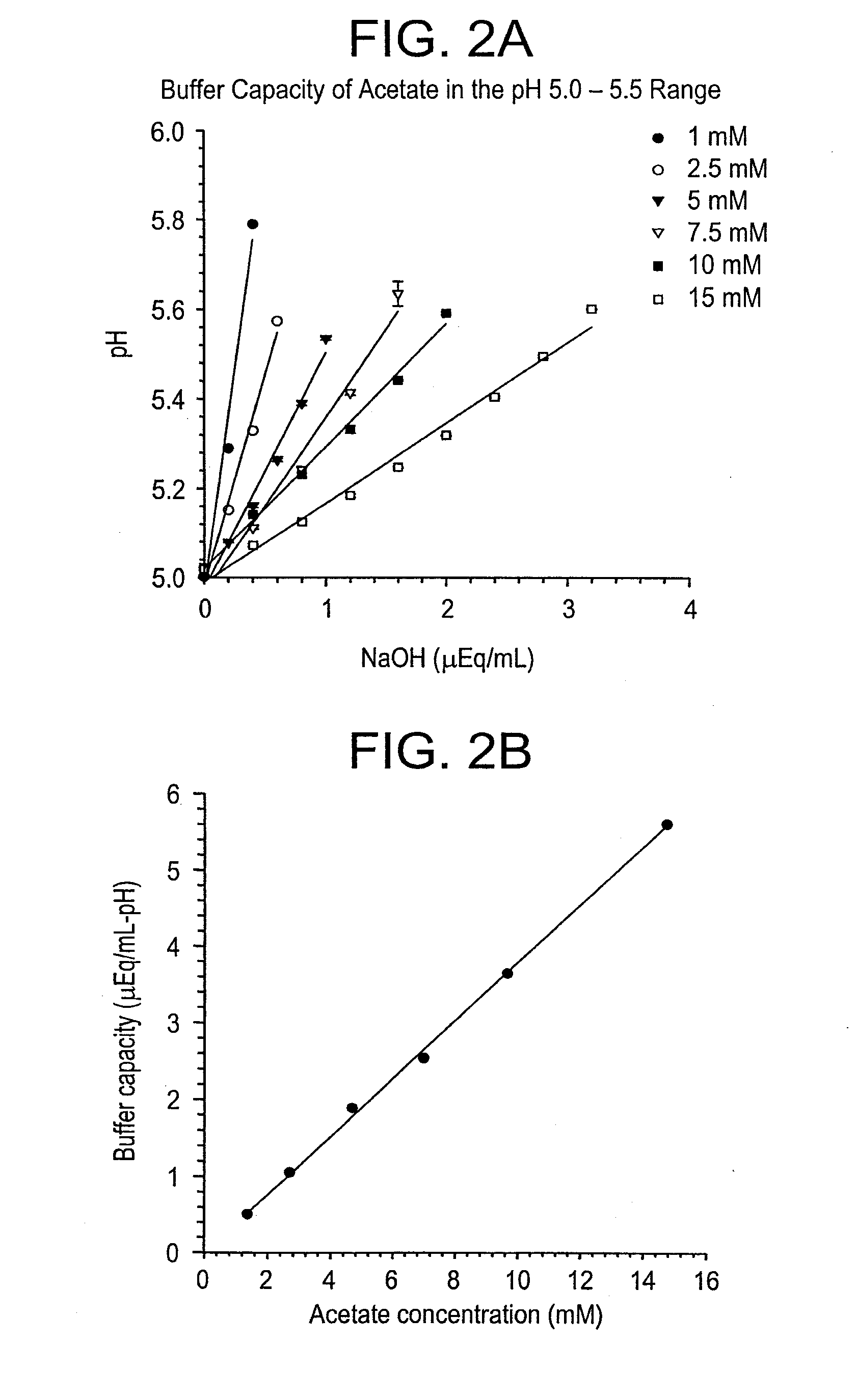
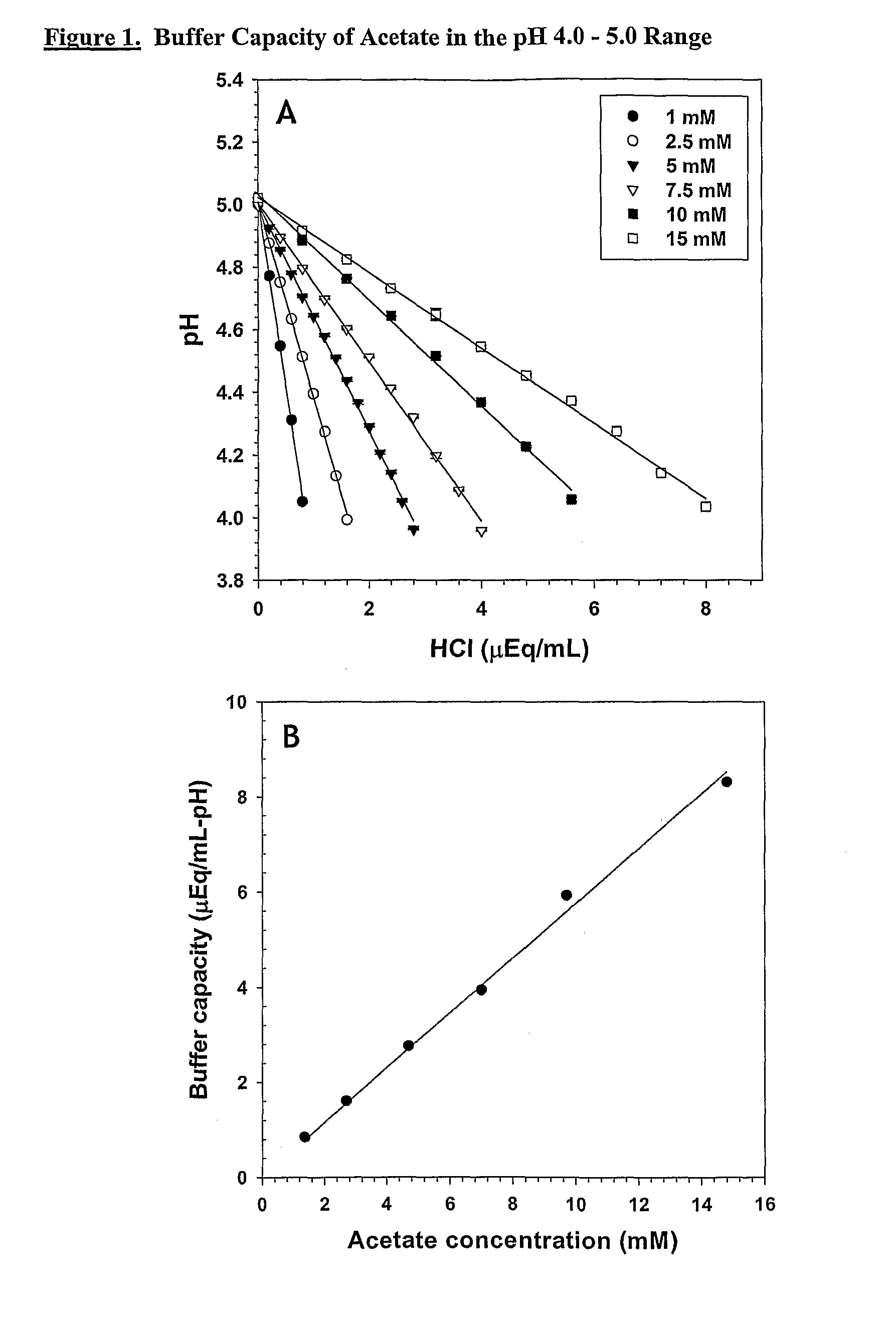
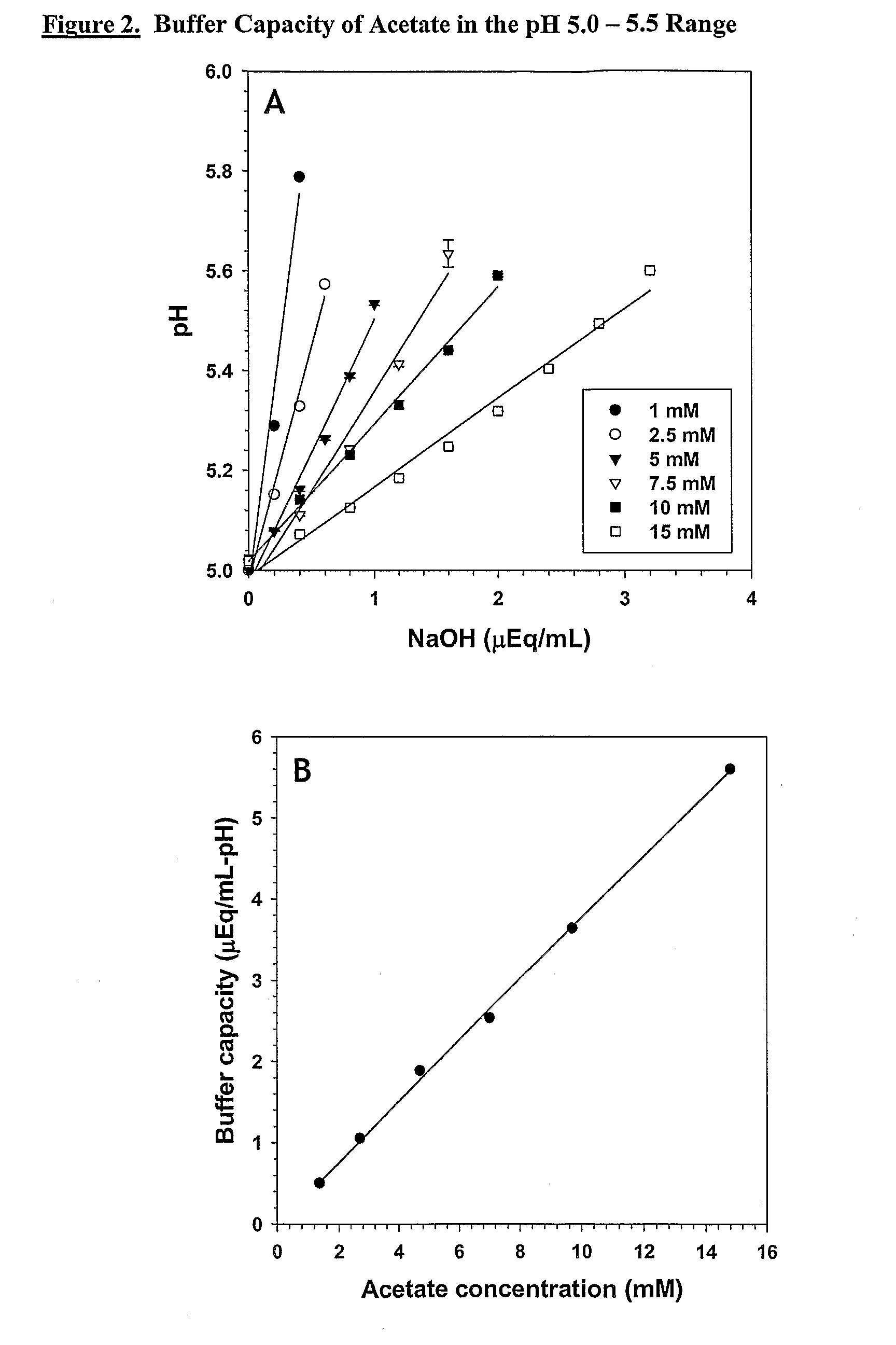
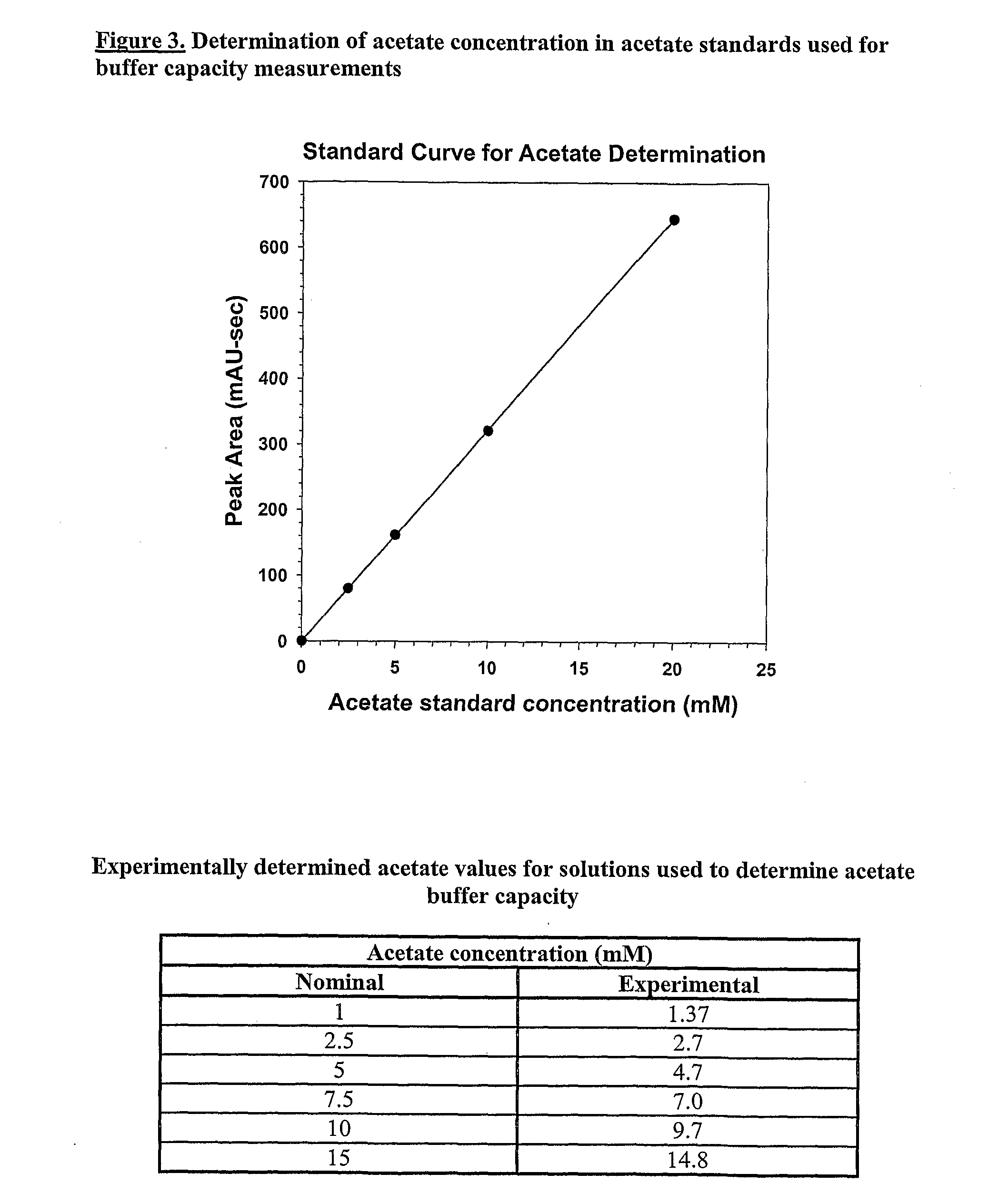
Self-Buffering Protein Formulations
InactiveUS20080311078A1Peptide/protein ingredientsInorganic non-active ingredientsDiseaseHuman medicine
The invention herein described, provides, among other things, self-buffering protein formulations. Particularly, the invention provides self-buffering pharmaceutical protein formulations that are suitable for veterinary and human medical use. The self-buffering protein formulations are substantially free of other buffering agents, stably maintain pH for the extended time periods involved in the distribution and storage of pharmaceutical proteins for veterinary and human medical use. The invention further provides methods for designing, making, and using the formulation. In addition to other advantages, the formulations avoid the disadvantages associated with the buffering agents conventionally used in current formulations of proteins for pharmaceutical use. The invention in these and other respects can be productively applied to a wide variety of proteins and is particularly useful for making and using self-buffering formulations of pharmaceutical proteins for veterinary and medical use, especially, in particular, for the treatment of diseases in human subjects.
Owner:AMGEN INC
Self-Buffering Protein Formulations
InactiveUS20120028877A1Peptide/protein ingredientsInorganic non-active ingredientsDiseaseHuman medicine
Owner:AMGEN INC
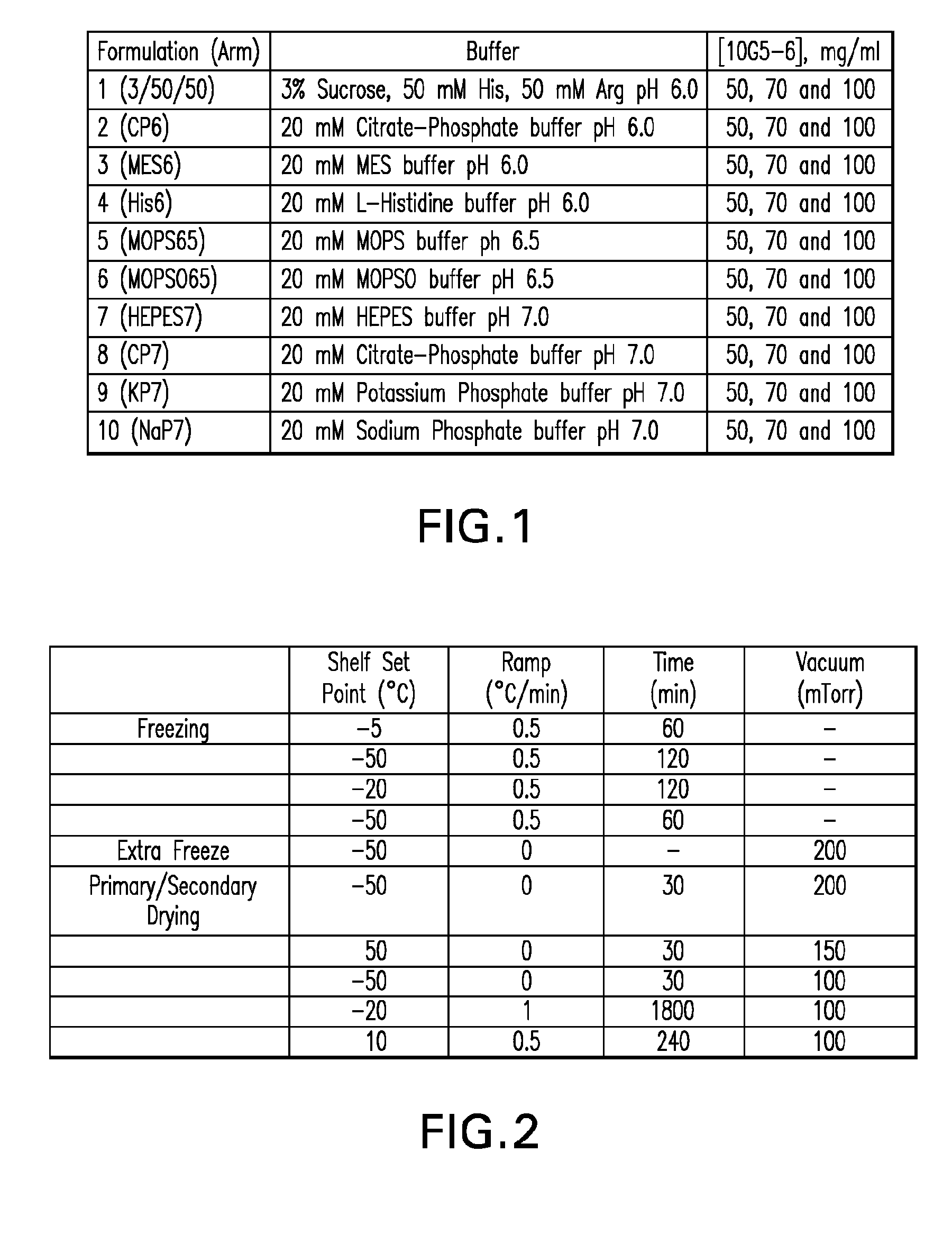
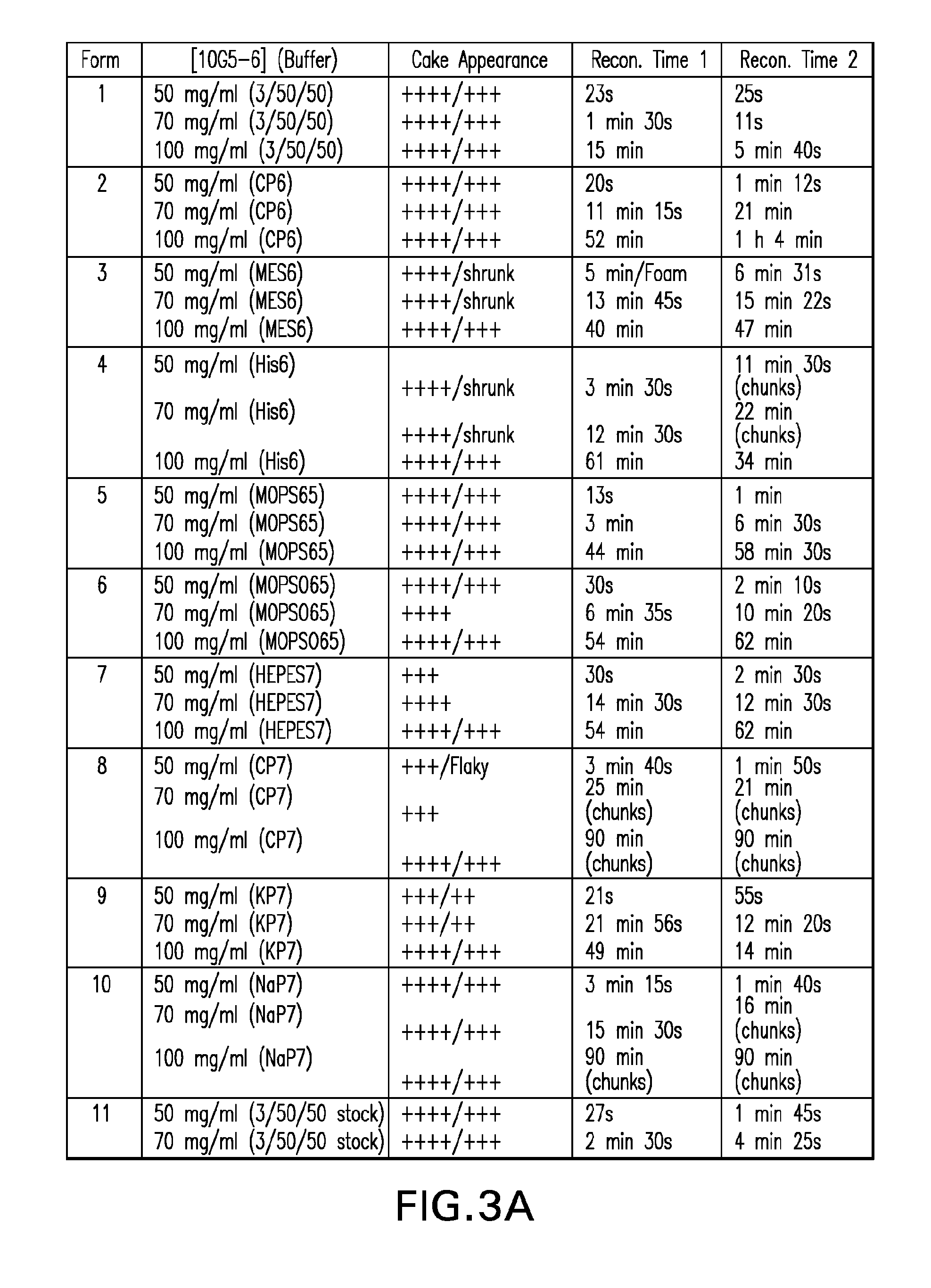
Methods for producing high concentration lyophilized pharmaceutical formulations
InactiveUS20120121580A1Contribute to physical structure and uniformity and stabilityPeptide/protein ingredientsImmunoglobulins against animals/humansHigh concentrationTherapeutic protein
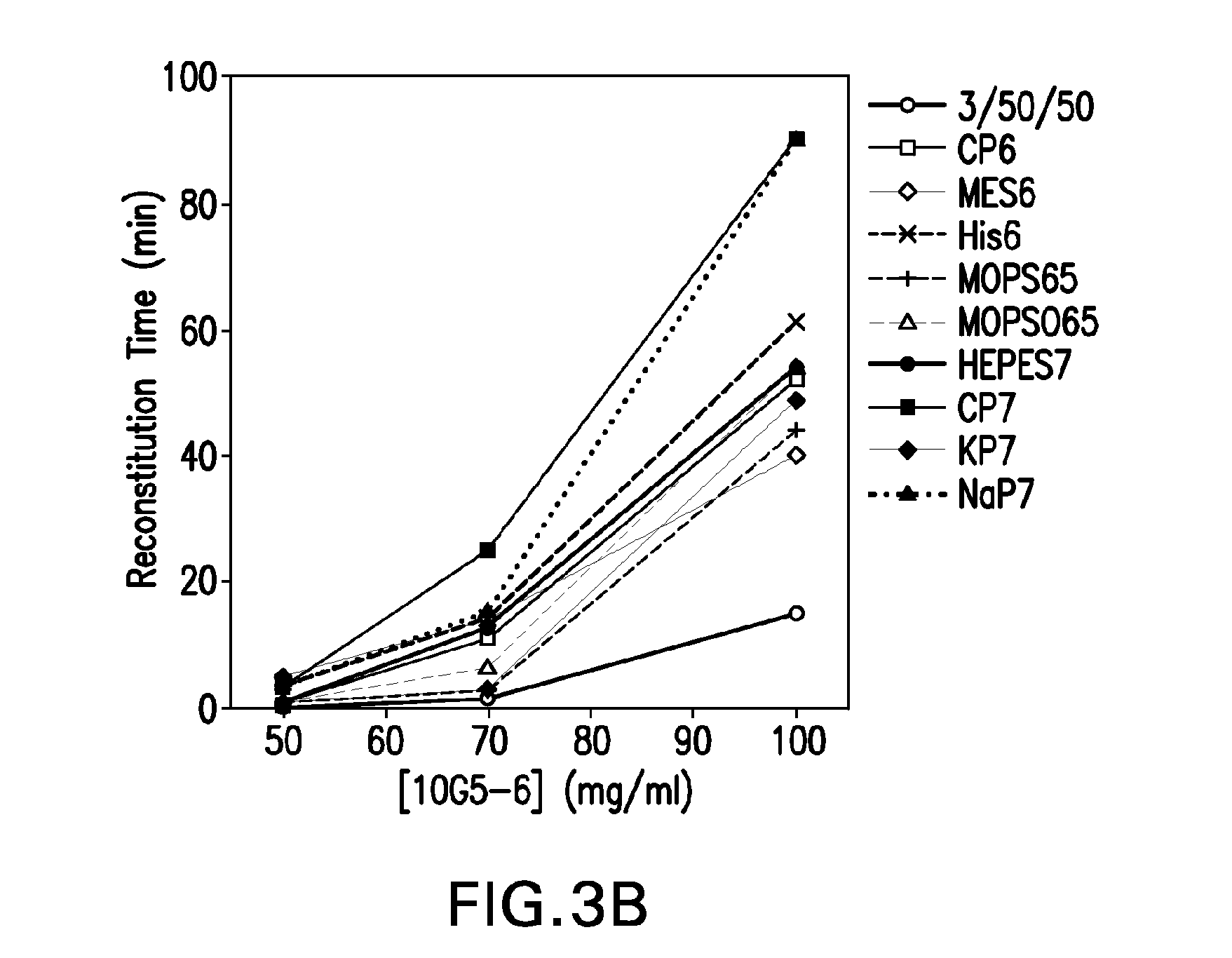
The present invention relates to methods of producing lyophilized pharmaceutical compositions comprising a high concentration of therapeutic protein or antibody prior to lyophilization, wherein the lyophilized formulation can be reconstituted with a diluent in about 15 minutes or less. The invention also relates to the high concentration lyophilized formulations produced by the methods described herein. The lyophilized formulations produced by the methods of the invention are stable and are suitable for veterinary and human medical use and are suitable for modes of administration including oral, pulmonary and parenteral, such as intravenous, intramuscular, intraperitoneal, or subcutaneous injection. Also provided by the invention are high concentration pharmaceutical compositions that have long term stability and can be reconstituted, following lyophilization, in a short period of time, preferably 15 minutes or less.
Owner:MERCK SHARP & DOHME CORP
Treatment of iatrogenic disease
InactiveUS20080194489A1Maintain securityTetrapeptide ingredientsTripeptide ingredientsGeneMedical treatment
The invention relates to the field of human or veterinary medicine and to the treatment of subjects (be it man or animal) that suffer from iatrogenic disease, i.e., experience problems or complications resulting from a medical treatment. Provided is a method for modulating an iatrogenic event in a subject, the method comprising: providing the subject with a gene-regulatory peptide or functional analogue thereof. Furthermore, provided is the use of an NF-kappaB down-regulating peptide or functional analogue thereof for the production of a pharmaceutical composition for the treatment of an additional pro-inflammatory cytokine response occurring after an iatrogenic event in a subject.
Owner:BIOTEMPT
Implantable medical device which may be controlled from central station
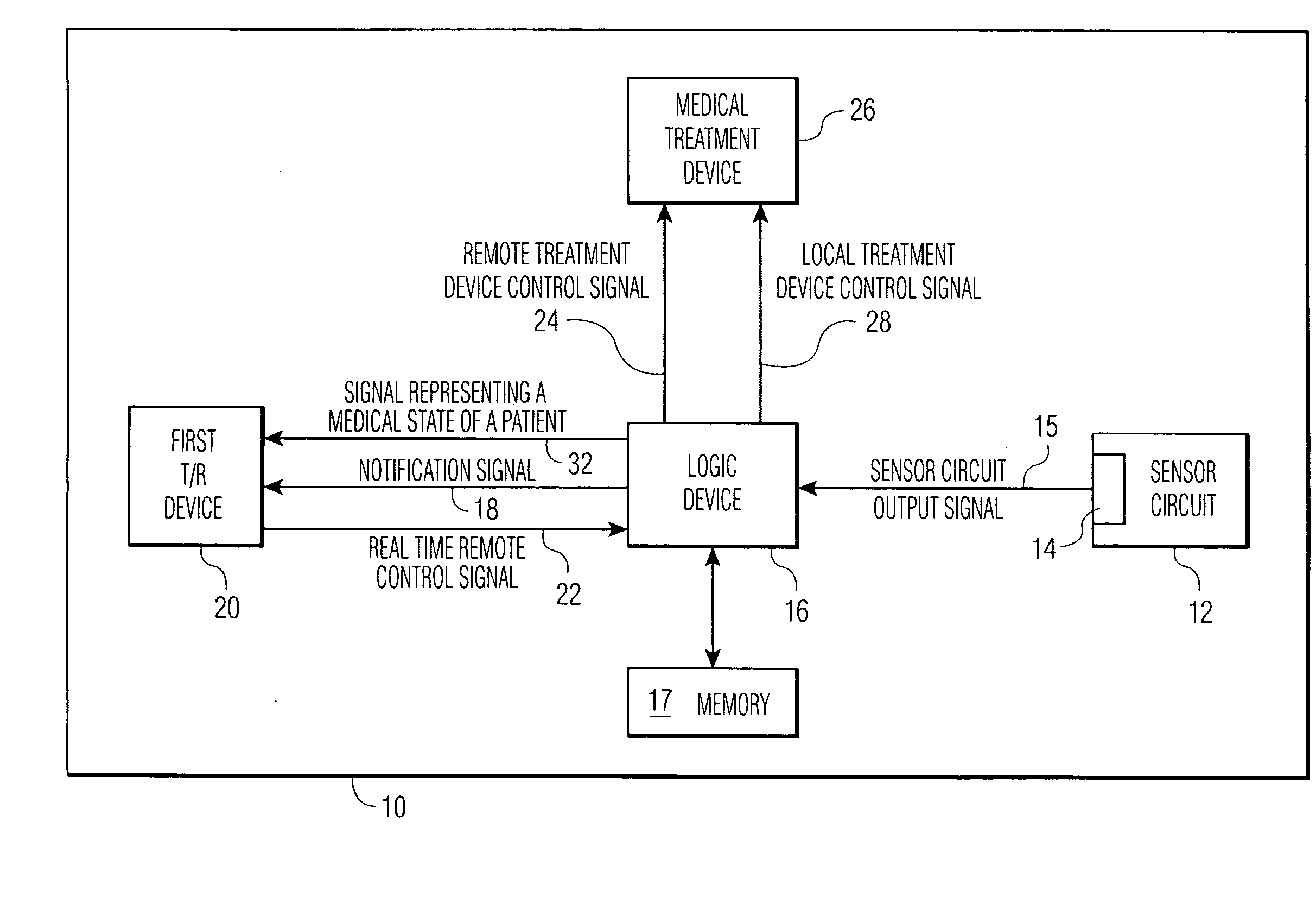
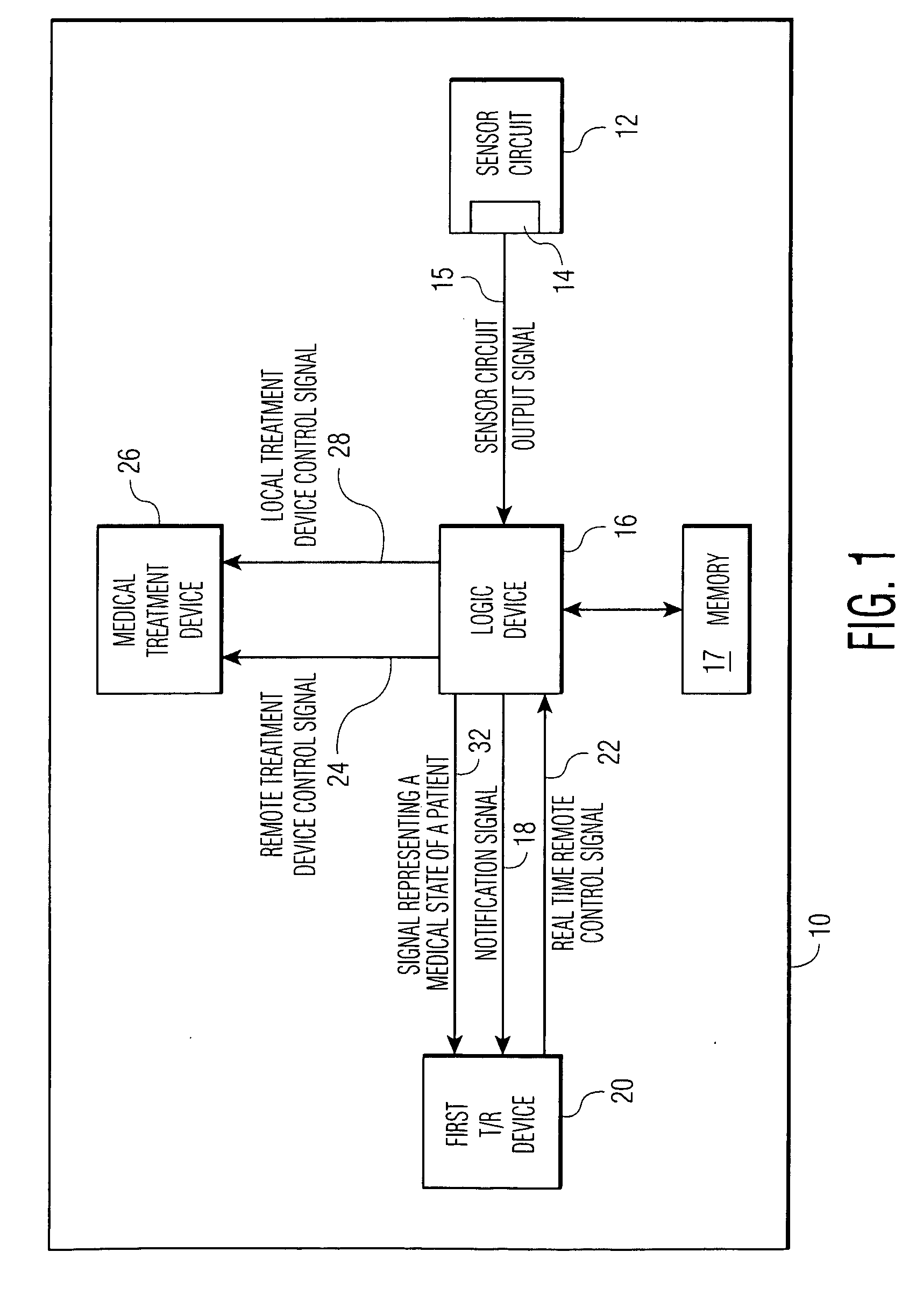
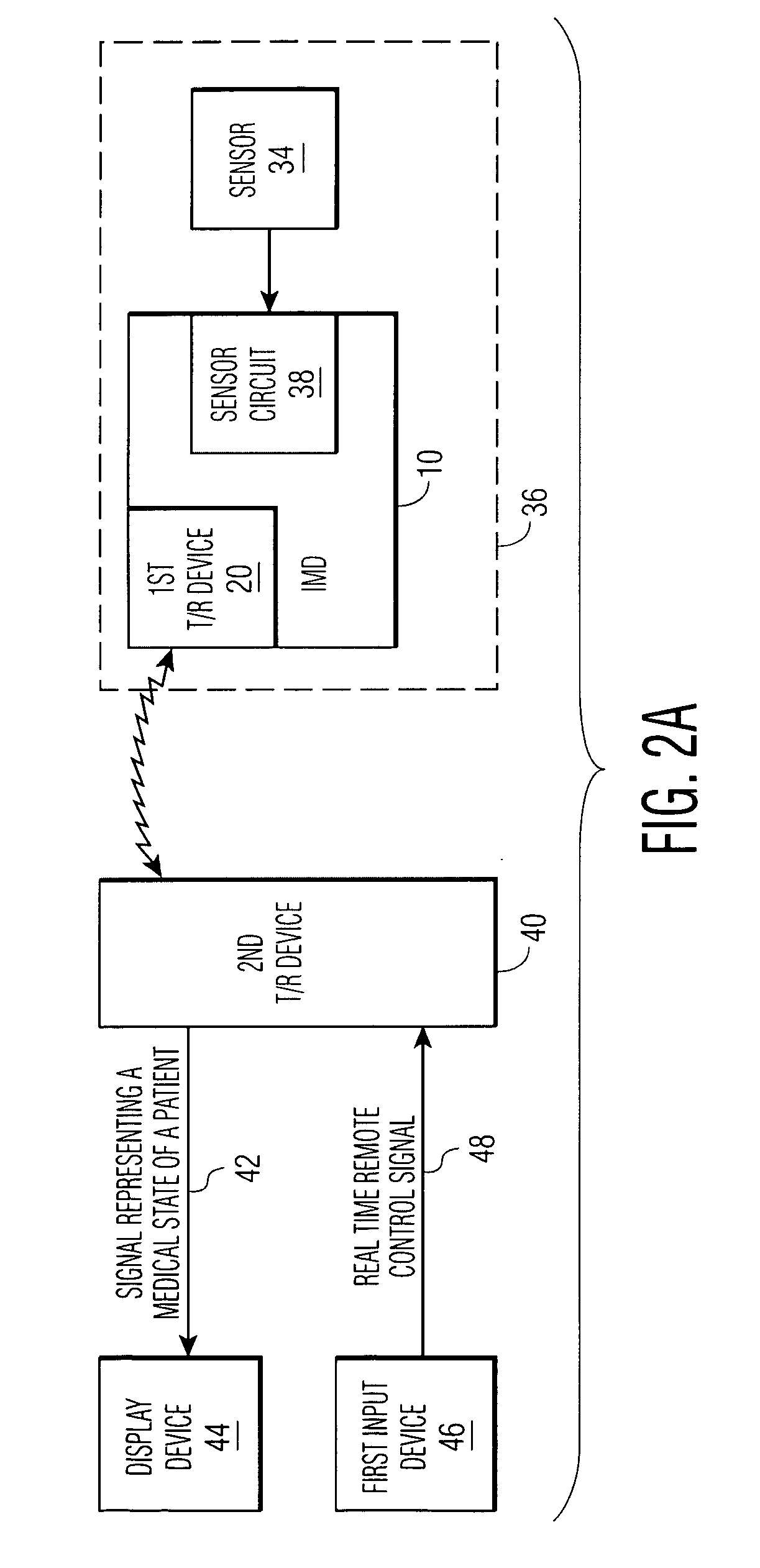
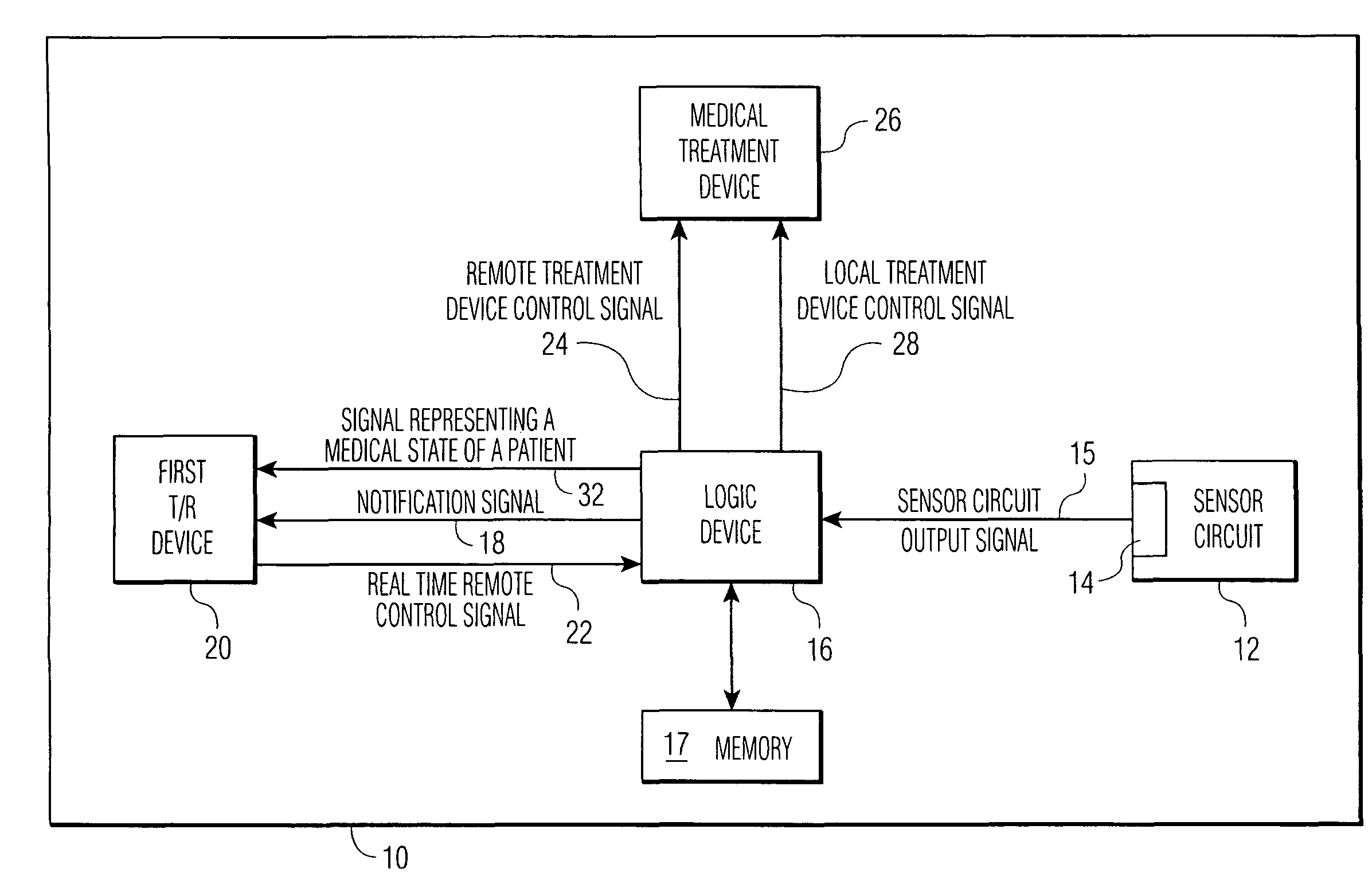
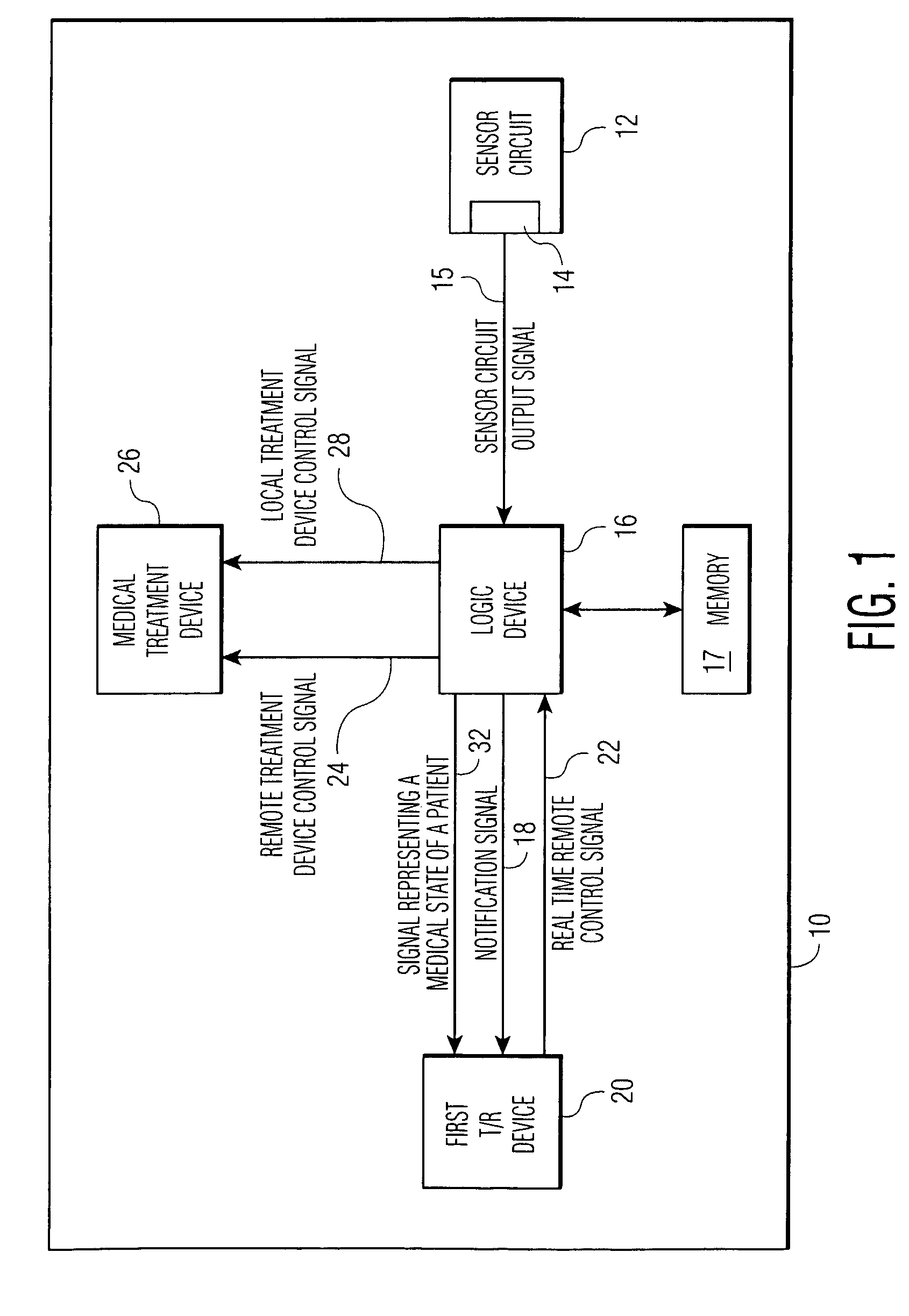
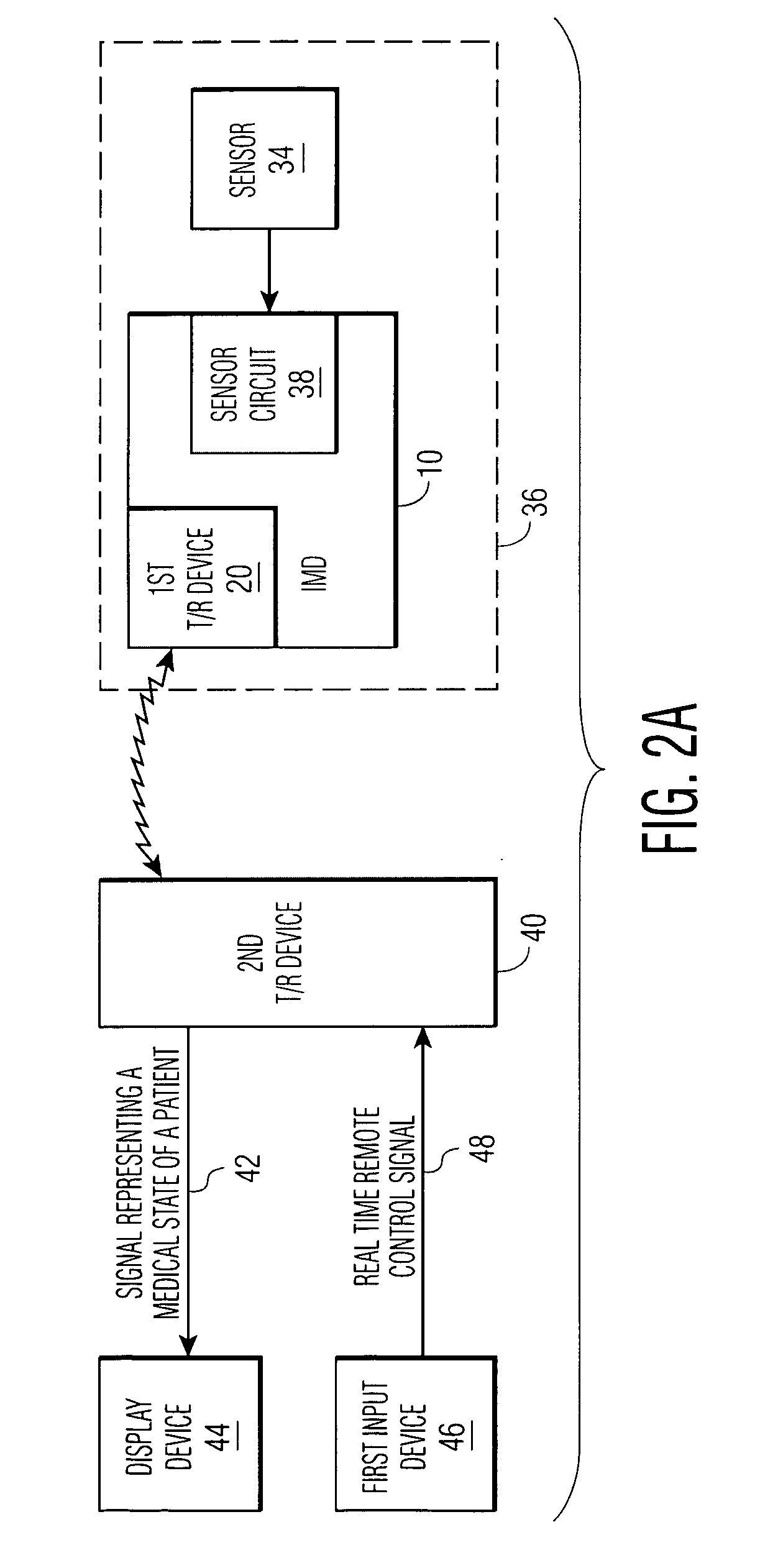
An implantable medical device (IMD) comprises a transmitting / receiving (T / R) device for transmitting medical data sensed from a patient to, and for receiving control signals from, a medical expert (a human medical professional and / or a computerized expert system) at a remote location; an electronic medical treatment device for treating the patient in response to control signals applied thereto; and a sensor circuit, having a sensor circuit output, for producing sensor circuit output signal(s) representing medical data sensed from the patient. The IMD also includes logic device which analyzes the sensor circuit output signal(s) to detect a medical abnormality which requires notification of the medical expert at the remote location, and generates notification signal(s) if required. T / R device transmits the notification signal(s) as well as signal(s) representing a medical state of said patient to the remote location. The logic device is also operative to generate local treatment device control signal(s) based on an analysis of the sensor circuit output signal(s) and to generate a remote treatment device control signal in response to real time remote control signal(s) received from the remote location. The medical treatment device of the IMD thus delivers therapy to the patient in response to either the local treatment device control signal(s) or the remote treatment device control signal(s), or both.
Owner:MATOS JEFFREY A
Method and pharmaceutical composition for disrupting lactation in a mammary gland and for treating and preventing mastitis
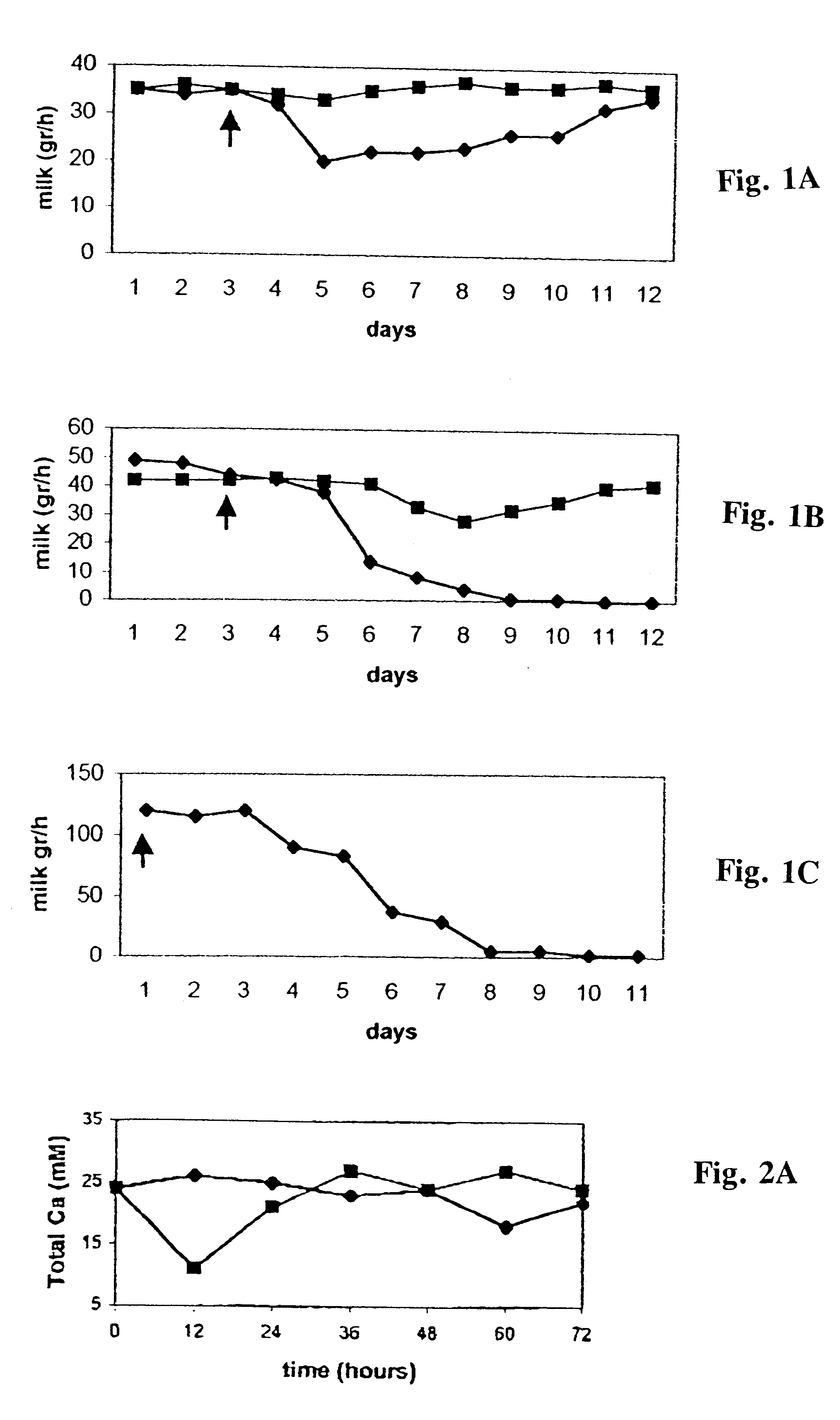
A method and pharmaceutical composition for ceasing milk production, for inducing involution, or for treating infection in a mammary gland of a lactating animal is described. The method is effected by direct administration of calcium chelators to the gland, or upon administration of enzymes which cause production of chelators in situ. The invention can be used to change the physiologic state of a single mammary gland of a lactating animal without significantly affecting the physiologic state of other mammary glands of the same animal. Changes resulting from use of the invention may be either transient or long lasting. The invention is expected to have uses in commercial agriculture and human medicine.
Owner:THE STATE OF ISRAEL MINIST OF AGRI & RURAL DEV AGRI RES ORG ARO VOLCANI CENT
Disinfectant and antiseptic composition
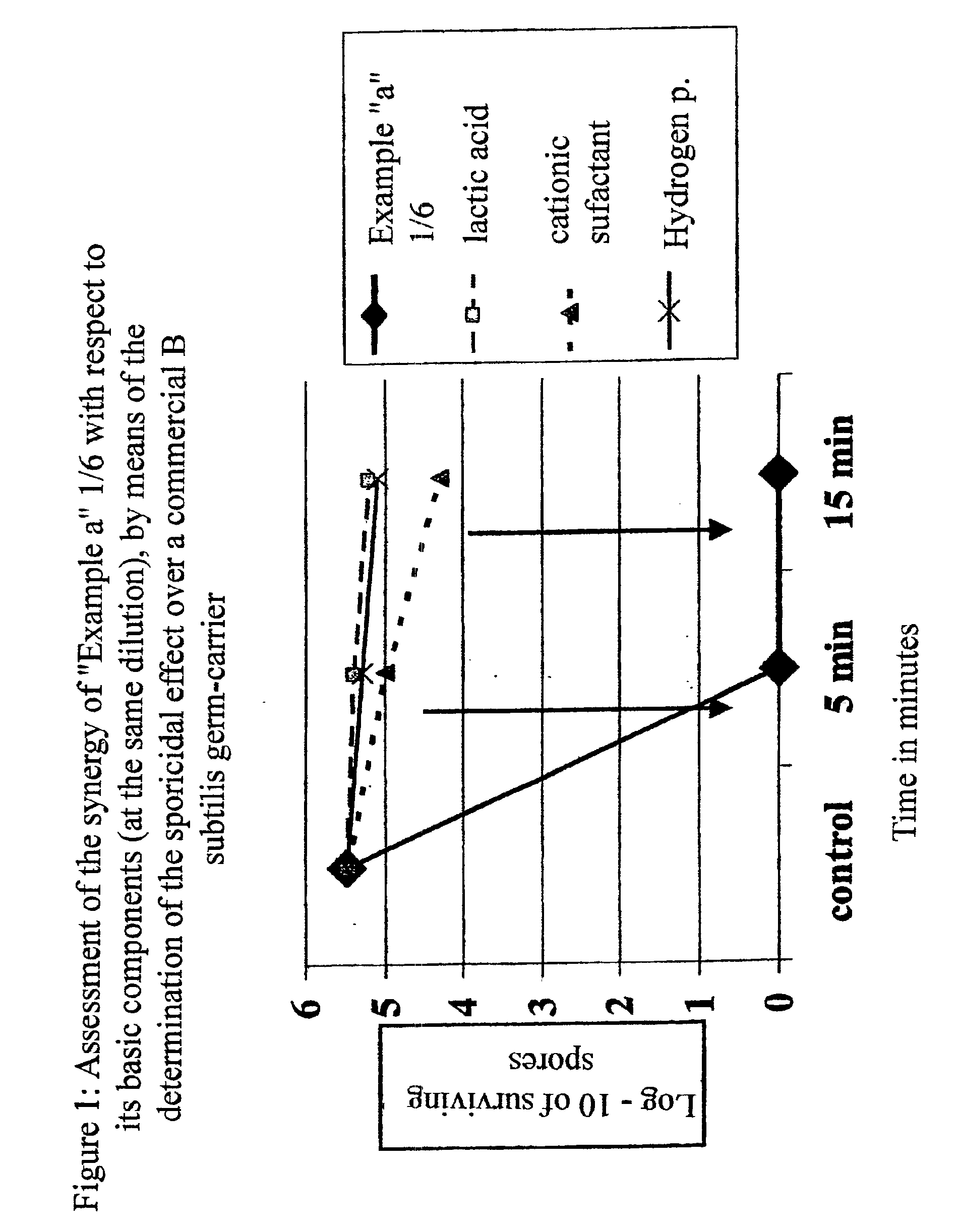
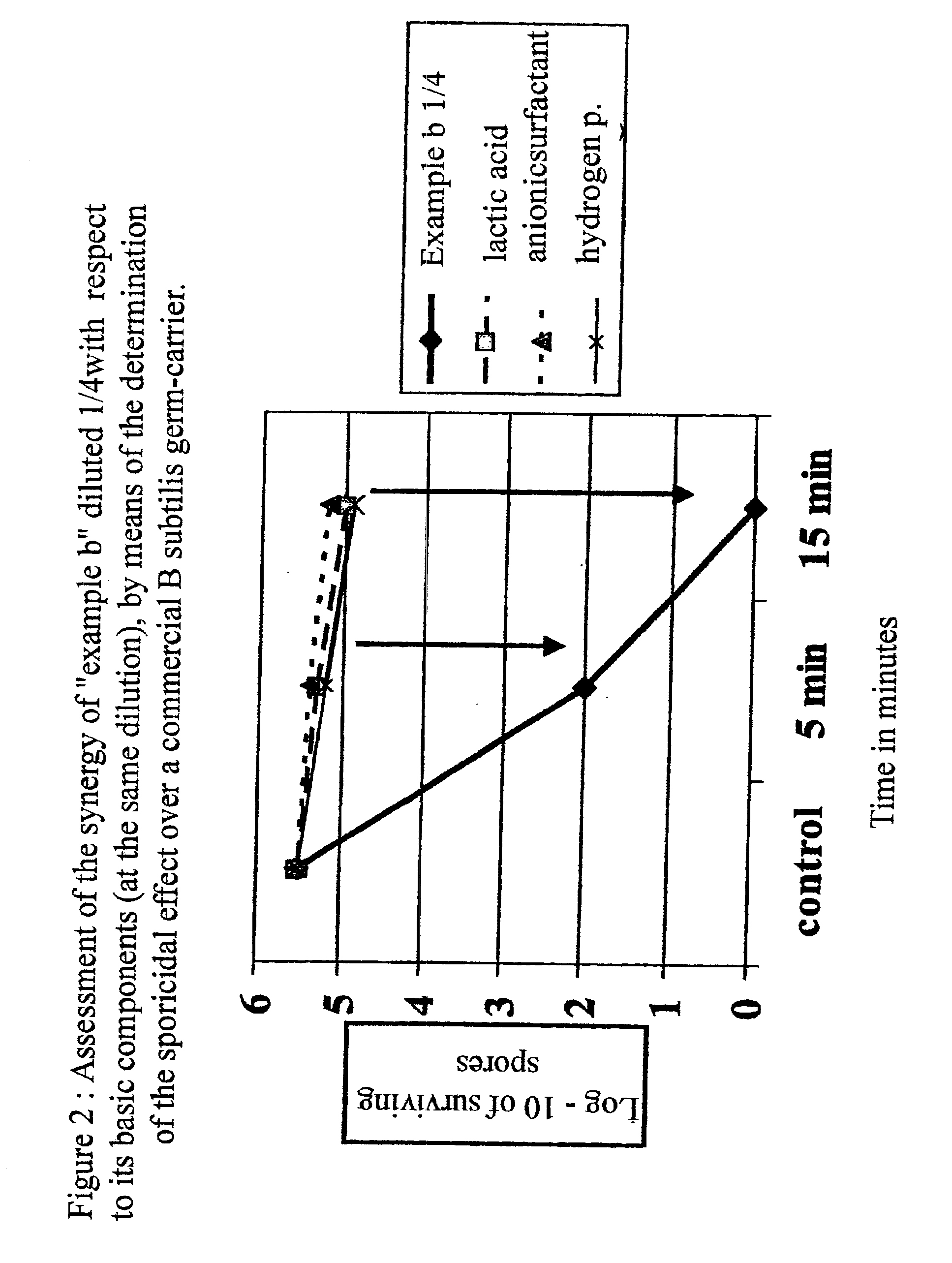
Wide spectrum disinfecting and antiseptic composition for use in the fields of human medicine, veterinary science and industry, characterized because it includes:Hydrogen peroxide, lactic acid and halogen salts (Br, I) and / or salts of heavy metals (for example, silver halides) with surfactant agents, either cationic, like chlorhexidine and / or quaternary ammonium salts, like didecyl-methyl-polyoxy-ethyl-ammonium propionate, chlorides of ammonium or compounds of ammonium propylamide or anionic, like lauryl sulphate, dodecyl sulphate or alkyl succinic salts, with suitable excipients, some of which may be ethyl or isopropyl alcohol, chlorhexidine, non-chlorinated quaternary ammonium salts, like didecyl-methyl-polyoxy-ethyl-ammonium propionate, combined or not with iodine, and / or its salts, together with excipients, some of which may be ethyl or isopropyl alcohol.
Owner:OFTRAI
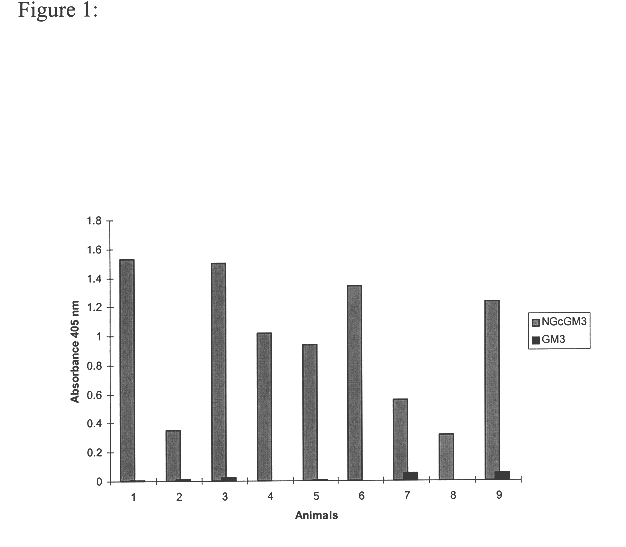
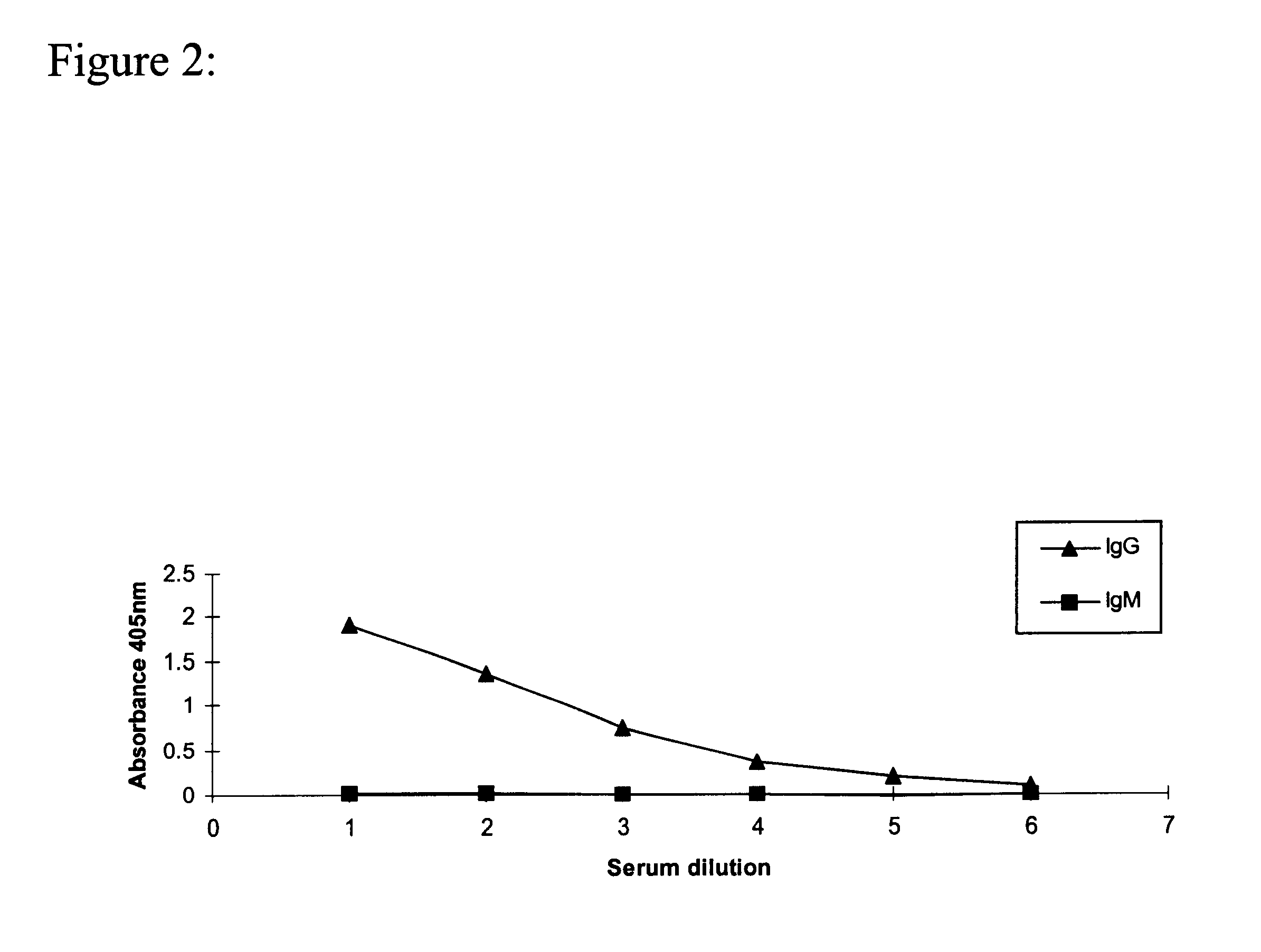
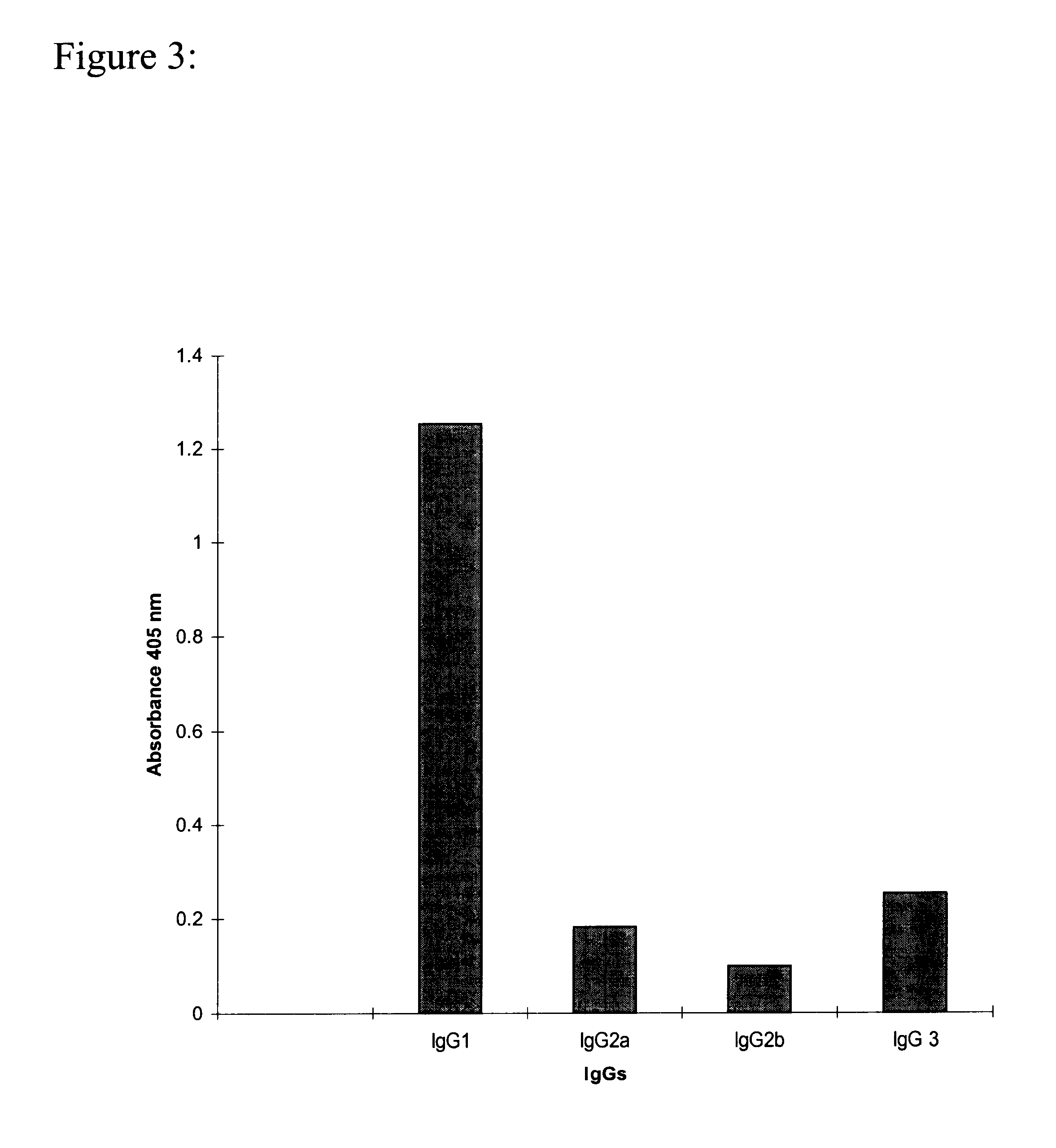
Monoclonal antibody which recognizes the oligosaccharide N-glycolylated-galactose-glucose sialic acid in malignant tumors, and composition containing it
InactiveUS6429295B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseMelanoma
The present invention is related with the field of immunology and human medicine, particularly with the generation and selection of a monoclonal antibody (Mab) that recognizes the N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence presents in malignant tumors.One of the objectives of this invention is to provide a Mab of the IgG1 type that has the characteristic of recognizing with high specificity N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence presents in malignant tissues of breast, melanomas and tumors of the liver, stomach, colon, rectum and kidneys. It also has the capacity of producing direct cytolysis of the tumoral cells bearing the N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence, thus can be used for the diagnosis and treatment of certain neoplasic diseases.Another objective of the present invention is to provide the hybridoma producing the referred Mab as well as the pharmaceutical composition containing it, for the treatment of neoplasic diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Composite Materials Based On Polysilicic Acid And Method For The Production Thereof
InactiveUS20070196419A1Positive change in the elasticity of the composite materialMore compression-elasticPharmaceutical delivery mechanismTissue regenerationCalcium biphosphateHuman medicine
The invention relates to composite materials based on polysilicic acid, said materials containing novel compositions which have improved material properties and can be in the form of dispersions, pastes, powders, granulated materials, layers or compact moulded bodies. The aim of the invention is to produce composite materials based on polysilicic acid with improved mechanical properties. To this end, the composite materials contain polysilicic acid, between 0.01 and 20 mass % of an organic polymer, more than 15 mass % of at least one calcium phosphate phase, and optionally a use-specific additive. The material produced according to the invention can be implanted or injected. The composition of the composite material with the resulting properties enables the composite material to be used for bone substitution and / or bone regeneration in both human medicine and animal medicine. The inventive material can also be used to heal wounds.
Owner:NEW YORK CITY DEPARTMENT OF TRANSPORTATION
Shaped bodies for use as implants in human medicine and method for the production of such shaped bodies
Implants are disclosed for use in humans having a rigidity comparable to human bone comprising polyurethane with hydrolysable ester linkages which are spaced apart to provide hydrolysis fragments which are sufficiently small to be resorbed in the human body, the polyurethane comprising a network polymer which is substantially free of urea groups. Methods for the production of such implants are also disclosed.
Owner:ARTIMPLANT
Reagent for determination of leucocytes and measurement of haemoglobin in a sample of blood
The invention concerns a reagent for determination of leucocytes and measurement of haemoglobin in a sample of blood. This reagent comprises a buffer system that is suited to adjust selectively the pH of the reagent to an acidic value, at least one detergent of cationic type, a nitrogenous compound and, optionally, at least one inorganic salt. This reagent can be used in haematological analyses in human medicine and also permits the identification of a leucocytic subpopulation, in particular the basophil polymorphonuclear leucocytes.
Owner:HORIBA ABX SAS
Use of epidermal growth factor for the morphofunctional restoration of peripheral nerves in diabetic neuropathy
ActiveUS20100136062A1Eliminate neuropathic painFree from painNervous disorderPeptide/protein ingredientsAnesthetic AgentMicrosphere
The invention relates to human medicine and to the use of epidermal growth factor (EGF) for preparing a pharmaceutical composition which is administered by infiltration into the periphery of nerve ganglia and / or trunks for the morphofunctional restoration of peripheral nerves in painful sensory-motor neuropathy as well as manifestations of ischemic neuritis. The invention also includes a composition containing EGF which can be formulated together with anaesthetics or analgesics or encapsulated in microspheres and to the use thereof for the morphofunctional restoration of peripheral nerves in painful sensitive-motor-type diabetic neuropathy and the manifestations of ischemic neuritis.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Application of triacontanol in preparing anti-cancer medicine
ActiveCN101190210AEnhance pharmacological effectsHydroxy compound active ingredientsNitro compound active ingredientsFreeze-dryingLung cancer
The invention relates to the application of the existing chemical substance in the preparation of human medicine. The invention relates to the application of triacontanol in the preparation of the medicine for treating liver cancer. The invention relates to the application of triacontanol in the preparation of the medicine for treating lung cancer. The invention develops a new medical application of the existing compound of triacontanol and exploits a new application field. The triacontanol of the invention is safe and nontoxic, strong in pharmacological action, which promises bright medical prospect. The medicine prepared by the triacontanol of the invention can be a plurality of dosage forms such as tablets, capsules, dropping pills, sustained release preparation, as well as injection, freeze-drying, suspensions, and emulsion used for injection.
Owner:KUNMING LONGJIN PHARMA
Medicine-food dual-purpose colorful vermicelli and preparation technology thereof
InactiveCN104187363AReduce destructive powerPromote digestion and absorptionFood ingredient functionsFood preparationFood additiveHuman medicine
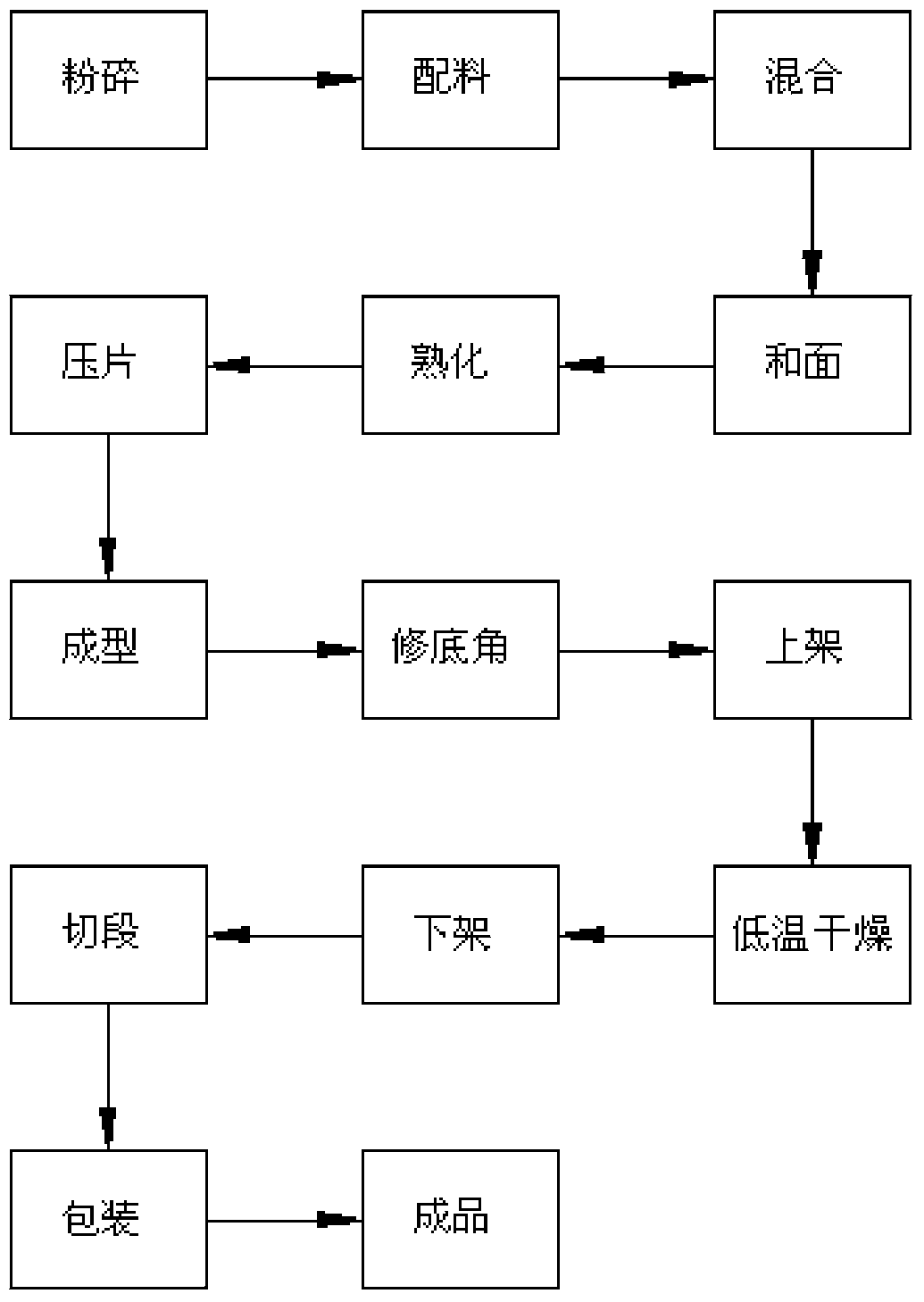
The invention discloses medicine-food dual-purpose colorful vermicelli which comprises the following raw materials by mass: 50 to 95% of refined wheat flour, 5-50% of cell wall breaking powder, 0.5-2% of salt, 3-12% of a natural food additive and the right amount of water. The cell wall breaking powder is prepared by low temperature superfine pulverization, and is realized through the technology of superfine powder. After cell wall breaking of medicine-food dual-purpose food materials, human medicine and food absorption rate can be increased, the bioavailability is improved, easy fracture of in the vermicelli production process can be effectively prevented, and the finished product rate can be improved. The preparation technology of the medicine-food dual-purpose colorful vermicelli is as follows: superfine grinding, dosing, homogeneous mixing, kneading dough, curing, pressing, molding, shelving, low temperature drying, off-shelving, section cutting, packaging to obtain the finished product, and can produce all new varieties of high content of colorful vermicelli.
Owner:杨义涛
Method for producing antibiotic preparation and its application
The present invention relates to a preparation method and application of an antibiotic preparation used in human medicine and veterinary medicine for treating local microbial infections of hard tissue and soft tissue. The preparation method of the antibiotic preparation of the present invention is as follows: from water and an amphoteric component such as an alkyl sulfate, and one or more antibiotics selected from the group consisting of aminoglycoside antibiotics, lincosamide antibiotics, and tetracycline antibiotics The components, an organic auxiliary component and / or an inorganic auxiliary component, and if necessary, at least one biologically active auxiliary component are mixed with each other, and can be further made into molded bodies, granules, and powders. , film, felt and thread.
Owner:HERAEUS KULZER
Cell DNA staining kit and its prepn process
InactiveCN101046434AEasy to manufactureEasy to useMicrobiological testing/measurementPreparing sample for investigationBiological cellHuman medicine
The present invention is cell DNA staining kit for use in life science and medicine, especially the sole staining of cell nucleus, and its preparation process. The kit includes DNA staining solution, DNA fixing solution and DNA rinsing liquid in the volume ratio of 1.05 to 2 to 1. It is prepared through the following steps: preparing DNA staining solution, preparing DNA fixing solution, preparing DNA rinsing liquid, preparing cell preserving solution, and setting slide glass, the DNA staining solution, the DNA fixing solution, the DNA rinsing liquid and the cell preserving solution into a box to constitute the cell DNA staining kit. The present invention facilitates cell DNA staining and raises the accuracy.
Owner:SOUTHEAST UNIV
Implantable medical device which may be controlled from central station
Owner:MATOS JEFFREY A
Pharmaceutical formulation having reverse thermal gelation properties for local delivery of nanoparticles
InactiveUS20170340756A1Reduce high temperatureDecreased critical micelle concentrationCompounds screening/testingPowder deliveryEpitopeAqueous buffer
The present invention refers to a pharmaceutical formulation for injection comprising fluorescent nanoparticles as in vivo diagnostics. The present invention relates to an injectable pharmaceutical formulation for human medicine and / or veterinary use, comprising 17% to 20% per weight of poloxamer 407 and 3%-15% per weight of poloxamer 188, 0.10 nM to 10.0 μM fluorescent nanoparticles and water or an aqueous buffer, wherein the pharmaceutical formulation is liquid at 4° C.-32° C. and forms a gel at about 37° C., their use as an in vivo marker and methods of their preparation. The inventive formulation is useful for local control and prevention of spreading / diffusion of nanoparticles, and thus allows full utilization of their quantum physics properties for example as a tool to enable surgical precision of tumor removal; even without tumor specific epitope binding antibodies.
Owner:EXCHANGE IMAGING TECH GMBH
Antibiotic-/ antibiotic-polymer compound
An antibiotic polymer combination / antibiotics polymer combination that ensures the continuous release of antibiotics over a period of several days under physiological conditions, and that can be used in human and veterinary medicine. Surprisingly, one or more antibiotic salts, which are sparingly soluble in water, from the groups comprising aminoglycoside antibiotics, lincosamide antibiotics, tetracycline antibiotics, glycopeptide antibiotics, quinolone antibiotics and chlorhexidine, are suspended in homogeneous polymer mixtures, which comprise one or more hydrophobic, nonionic polymers from the groups comprising poly(vinyl, chloride), post-chlorinated poly(vinyl chloride), poly(vinylidene chloride), poly(vinyl fluoride), poly(vinylidene fluoride) and copolymers comprising vinyl chloride and one or more nonionic monomers, and which comprise one or more hydrophilic polymers from the groups comprising polyethers, and this suspension forms composites that exhibit the release of an active ingredient over a period of days in an aqueous medium.
Owner:HERAEUS KULZER
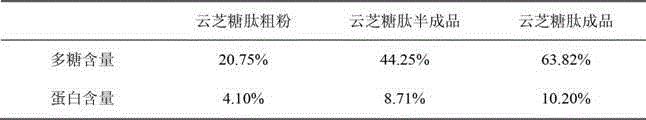
Preparation method and application of high-purity polysaccharopeptide
ActiveCN102942614ARaise antibody levelsImprove immunityPeptide preparation methodsAntibody medical ingredientsMedicineHuman medicine
The invention relates to a preparation method and application of high-purity polysaccharopeptide. The preparation method comprises extracting and purifying polysaccharopeptide coarse powder and obtaining the polysaccharopeptide with high polysaccharide content and high protein level. The polysaccharopeptide can improve antibody levels of chickens, accordingly improve immunity of the chickens, be used for preparing human medicines or beast medicines with immunity or / and physiological accommodation activity and particularly serve as vaccine immunopotentiator of beasts and birds.
Owner:重庆优宝生物技术股份有限公司
Inhalation support apparatus and method for inhalation support
The invention relates to an inhalation support apparatus, use thereof, and a method for supporting the inhalation of an inhalation mixture that is to be inhaled. The invention further relates to a device for the control of pressure changes, use thereof in the medical and non-medical fields and a method for detecting respiratory problems.
Owner:D·基尔施
Antibiotics-polymer composition
The present invention relates to an antibiotic-polymer complex that ensures continuous release of antibiotics for several days under physiological conditions and can be used in human and veterinary medicine. The antibiotic-polymer composite of the present invention is characterized in that it contains one or more hydrophobic polymers selected from the group consisting of polymethacrylates, polyacrylates, methacrylate-acrylate copolymers and a Or in a homogeneous mixture of polymers composed of hydrophilic polymers such as polyethers, one or more substances selected from the group consisting of aminoglycoside antibiotics, lincosamide antibiotics, tetracycline antibiotics, and quinolone antibiotics are suspended in water. A poorly soluble antibiotic, a water-soluble antibiotic selected from aminoglycoside antibiotics, lincosamide antibiotics, β-lactam antibiotics and tetracycline antibiotics as needed, and an organic auxiliary as needed material and causing the suspension to form a composite.
Owner:HERAEUS KULZER
External antibacterial traditional Chinese medicine oil component for treating rhinitis and application of component
InactiveCN107126462AIncrease profitAvoid side effectsAntibacterial agentsPharmaceutical delivery mechanismNasal cavitySinusitis
An antibacterial traditional Chinese medicinal oil component for external use and its application for treating rhinitis, comprising a mixed herbal oil formed by superimposing functional oil and base oil components; taking a sufficient amount of Xinyi flower 2-5%, Xanthium 2-5%, Angelica dahurica 1 ~4%, peppermint 2~6%, thyme 1~3%, lavender 2~3%, juniper 1~2%, tea tree oil 1~3%, and the balance is base oil, stir evenly, and pack 5ml to 30ml Store in ceramic or opaque glass bottles. The invention mainly uses magnolia flower, cocklebur, angelica dahurica, peppermint, thyme, juniper, lavender and tea tree oil as main ingredients for external use, adds base oil, and is formed by reasonable matching to form compatibility. It is a pure natural plant formula, which avoids the harm of western medicine to the human body, makes the medicine go directly to the focus, and is safe and reliable in clinical application. This invention is suitable for treating allergic rhinitis, sinusitis, nasal polyps, and hypertrophy of the nasal cavity. Healed from rhinitis.
Owner:王树军
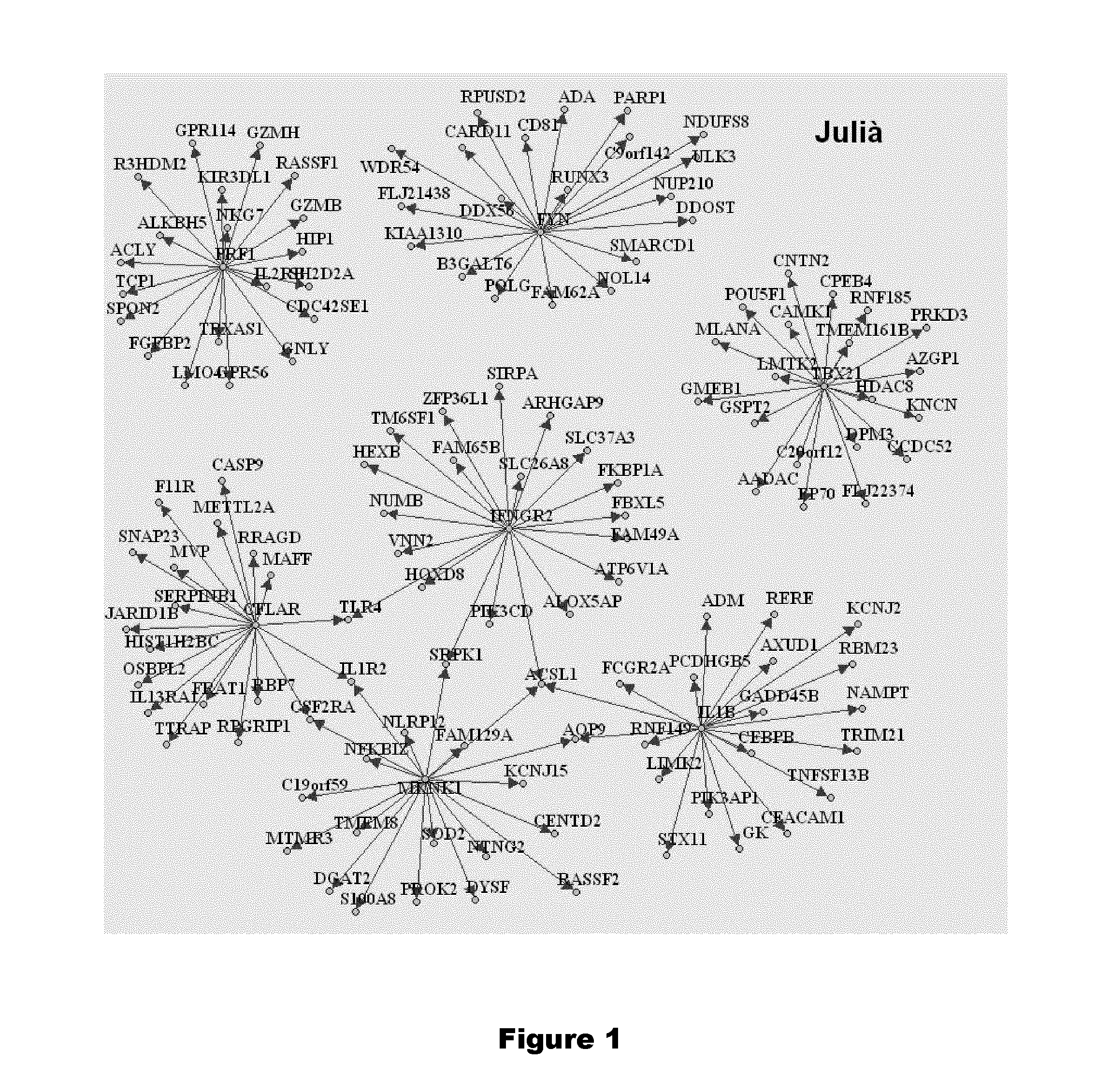
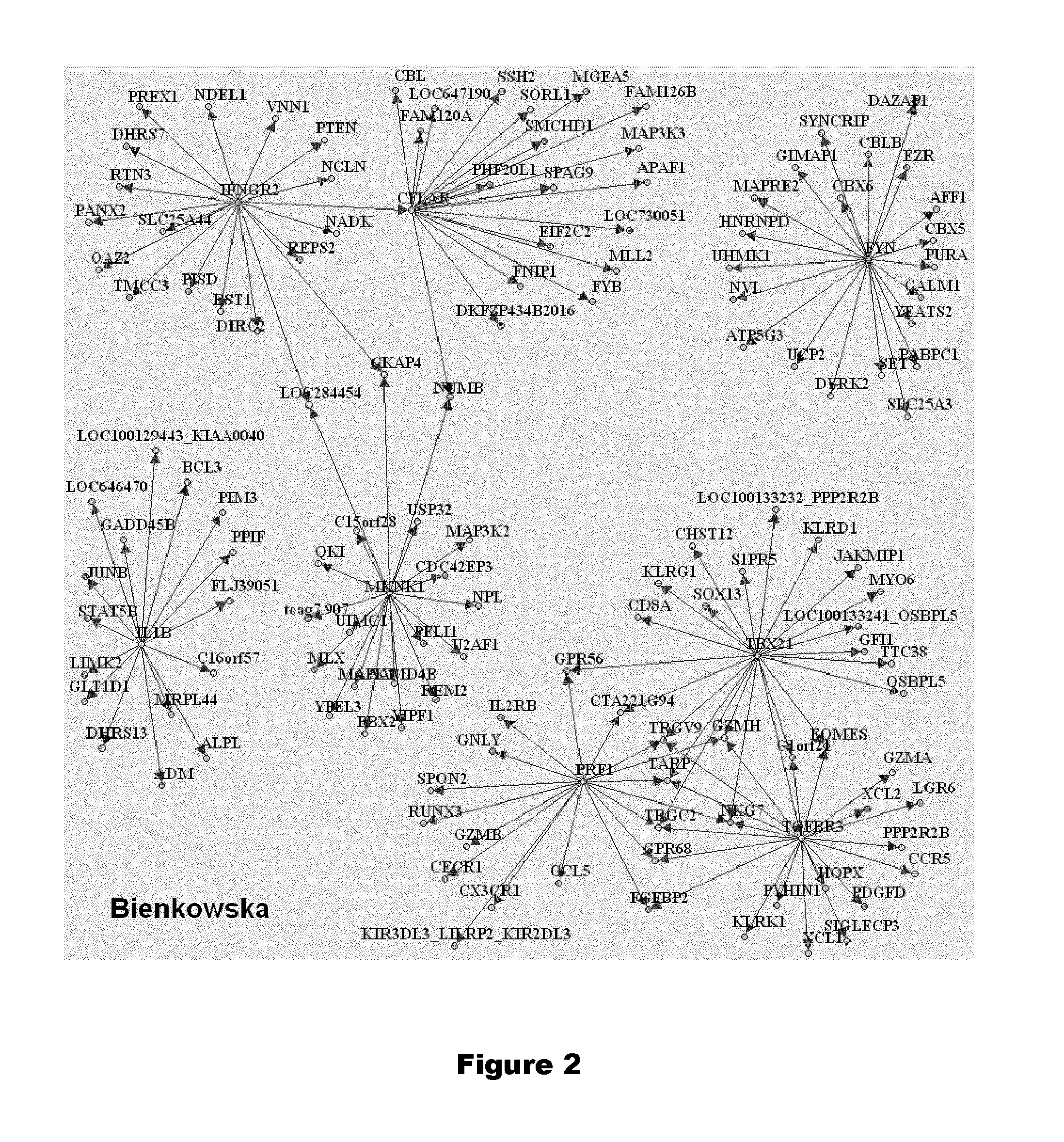
Genes and genes combinations based on gene mknk1 predictive of early response or non response of subjects suffering from inflammatory disease to cytokine targeting drugs (CYTD) or Anti-inflammatory biological drugs
InactiveUS20150240304A1Increase valueStrong specificityOrganic active ingredientsNucleotide librariesTBX21Cytokine
This invention refers to the field of human medicine and specifically to the diagnosis / prognosis of the responsiveness to a cytokine targeting drug (Cy TD) or an anti-inflammatory biological drug treatment of a subject suffering from an inflammatory disease. More precisely, the present invention concerns a method for the in vitrodiagnosis / prognosis of a Cy TD or an anti-inflammatory biological drug responsive or non-responsive phenotype, comprising: (a) determining from a subject biological sample an expression profile comprising the gene MKNK1; or of the genes MKNK1 and GNLY; or of the genes MKNK1, TBX21 and TGFBR3; or of the genes MKNK1, GNLY, and ADI1; or of the genes MKNK1, GNLY, ADI1, and IL1B; or of the genes MKNK1, GNLY, ADI1, IL1B, and IL1R1; or of the genes MKNK1, PRF1, TBX21, TGFBR3, IFNGR2, FYN, IL1B and CFLAR; or of the genes MKNK1, PRF1, TBX21, TGFBR3, IFNGR2, FYN, IL1B, CFLAR, MAPK14 and GNLY; or of the genes MKNK1, PRF1, TBX21, TGFBR3, IFNGR2, FYN, IL1B, CFLAR, CD14 and TGFBR2; or of the genes MKNK1, IFNGR2, IL1B, MAPK14, GNLY, and CD14; or of the genes MKNK1, PRF1, TBX21, TGFBR3, IFNGR2, IL1B, CFLAR, MAPK14, GNLY, CD14 and TGFBR2; or of all the 46 genes of following Tables 2, 3 and 4; or Equivalent Expression Profile thereof, provided that, in said Equivalent Expression Profile thereof, MKNK1 is not replaced by gene S 100A8 nor gene MAPK14, and (ii) optionally one or more housekeeping gene(s), (b) comparing the obtained expression profile with at least one reference expression profile, and (c) determining the responsive or non-responsive phenotype from said comparison. The present invention also relates to kits and nucleic acid microarrays for performing said method. The present invention also relates to methods of treatment of inflammatory disease-suffering patients.
Owner:CERVINO ALESSANDRA +2
Vaccine composition containing transforming growth factor alpha (TGFalpha). it use in malignant diseases therapy
InactiveUS20030054011A1Prevent proliferationAvoid resistanceBacteriaPeptide/protein ingredientsVaccinationImmunologic function
The present invention relates to the field of immunology and human medicine, in particular with a vaccine preparation able to provoke an immune-castration of self-TGFalpha. The object of this invention is to obtain a vaccine composition for the active immunotherapy of malignant tumors that depend of TGFalpha for its growth. As well as for the treatment of other TGFalpha depend diseases. Another important object of this invention is to obtain a vaccine composition containing a combination of TGFalpha with other EGF-R ligands, such as epidermal growth factor (EGF), able to inhibit the proliferation of tumors whose progression depends on these factors. In that way would be avoided the resistance generated by tumors vaccines containing each molecule for separate, developing tumorigenic phenotype that not depend on the growth factor used in the vaccination. These vaccine preparations are able to inhibit the proliferation of tumors with the characteristics mentioned before, and in this way to be useful in the treatment of malignant neoplasias. Therefore, the invention is also related with the field of specific active immunotherapy of cancer.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Nucleotide sequences coding for proteins involved in the biosynthesis of L-serine, an improved method for the microbial production of L-serine and a genetically modified microorganism suitable therefor
InactiveUS7083952B2Copy numberHigh expressionOrganic active ingredientsBacteriaProtein insertionCorynebacterium amycolatum
The present invention relates to nucleotide sequences of coryneform bacteria, coding for proteins involved in the bio-synthesis of L-serine and to methods for the isolation thereof. The invention further relates to an improved method for the production of L-serine. In addition, the present invention relates to the use of L-serine in the food, animal feed and / or pharmaceutical industries or in human medicine.
Owner:FORSCHUNGSZENTRUM JULICH GMBH
Method and apparatus for determining variation over time of a medical parameter of a human being
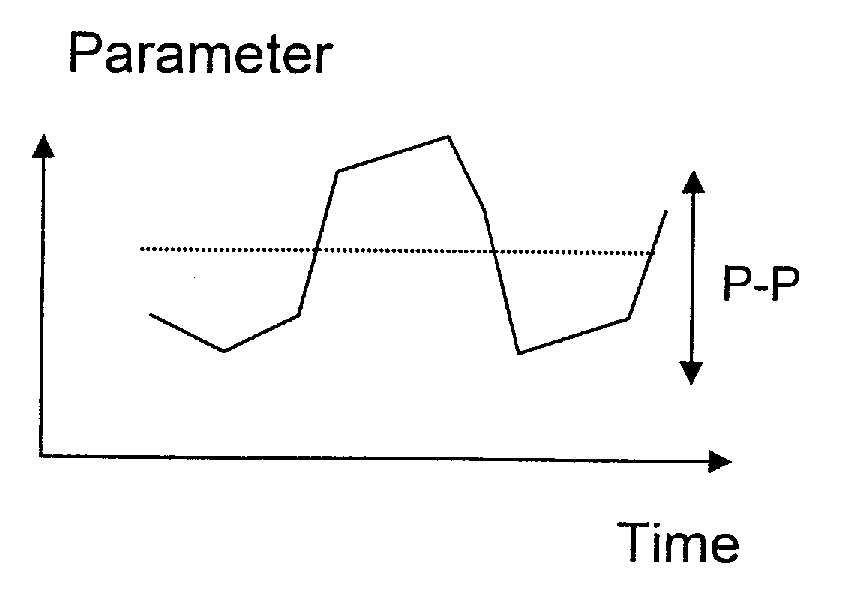
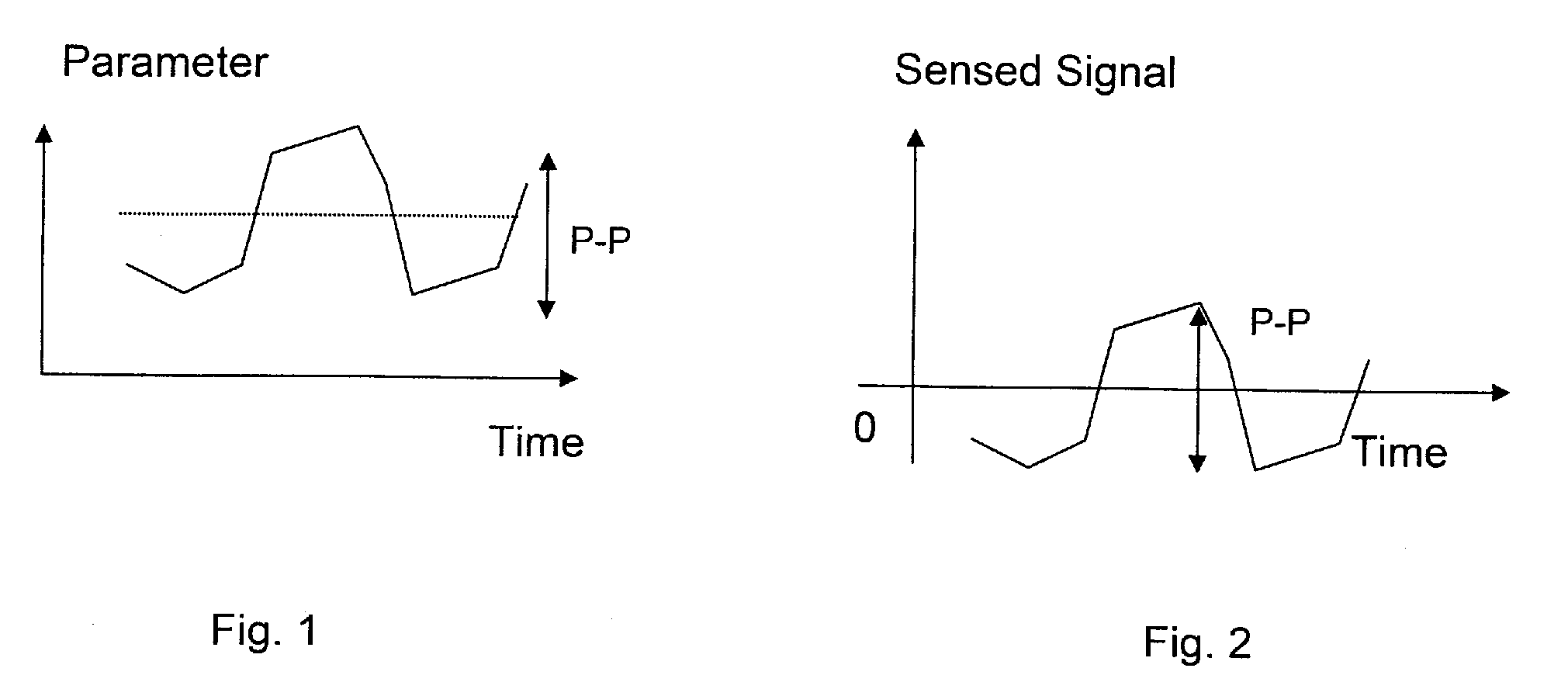
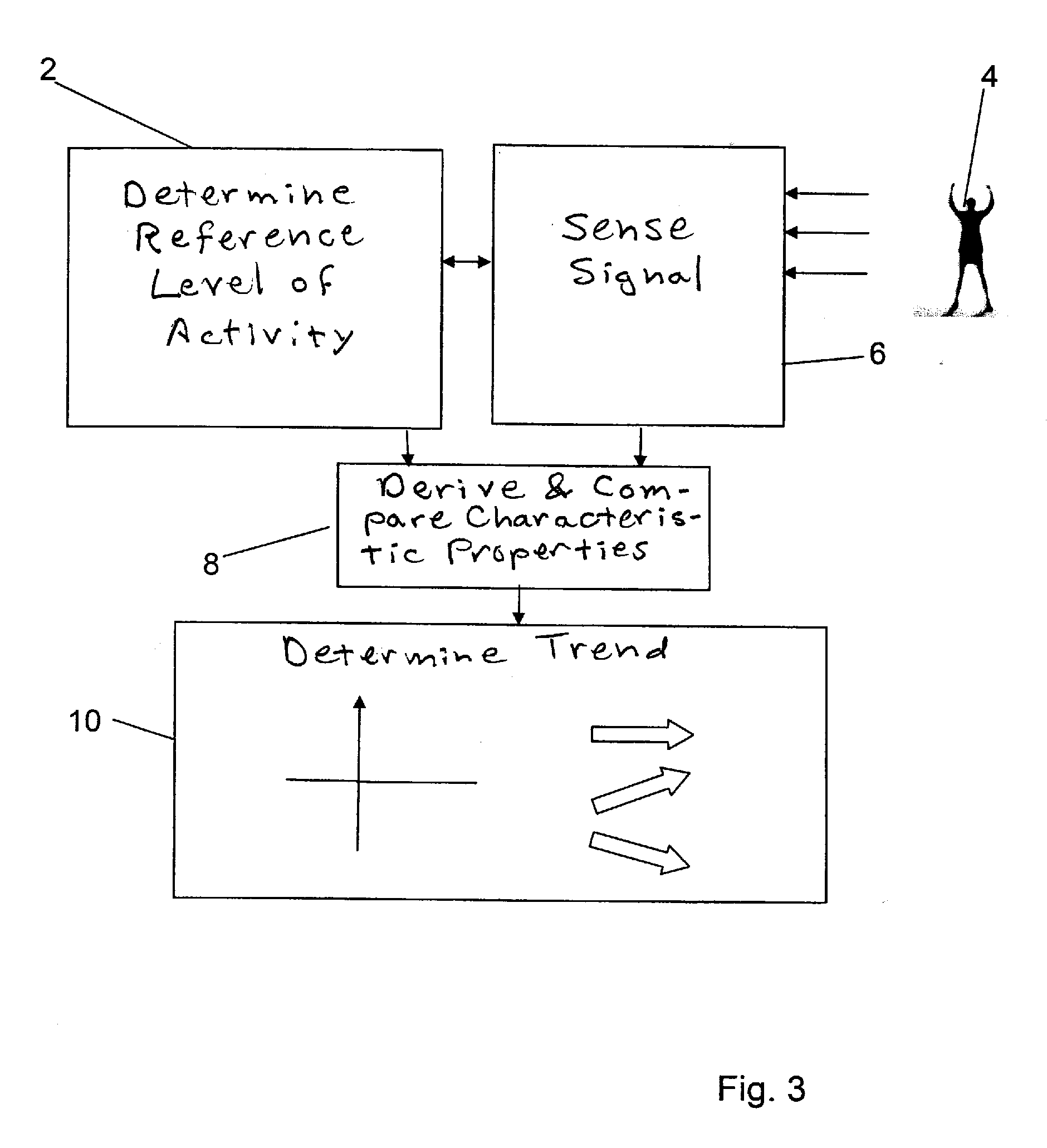
ActiveUS20100249864A1Quality improvementReduce impactPerson identificationCatheterHuman medicineBiomedical engineering
An apparatus for determining variation over time of a medical parameter of a human being obtained from a sensed signal has a sensor implantable in the human being for sensing the signal. A comparator compares at least one characteristic property, derived from the sensed signal obtained for at least one predetermined first level of activity of the human being, with corresponding reference property of a sensed reference signal, obtained for a predetermined reference level of activity of the human being, for determining a relation between the characteristic property of the sensed signal and the reference property. A trend determining unit determines trends in the medical parameter by analyzing the relation between the characteristic property of the sensed signal obtained at different times and the reference property. A corresponding method also function an implant for heart failure diagnostics also function as described. A sensor is then arranged to pick up dynamic mechanical information from the heart of the human being and generate a corresponding signal. A heart stimulator includes such an implant and a control unit arranged to control stimulation of the heart depending on determined trends in the medical parameter.
Owner:ST JUDE MEDICAL
Genes and genes combinations predictive of early response or non response of subjects suffering from inflammatory disease to cytokine targeting drugs (CYTD)
This invention refers to the field of human medicine and specifically to the diagnosis / prognosis of the responsiveness to a cytokine targeting drug (CyTD) treatment of a subject suffering from an inflammatory disease. More precisely, the present invention concerns a method for the in vitrodiagnosis / prognosis of a CyTD responsive or non-responsive phenotype, comprising: (a) determining from a subject biological sample an expression profile comprising the gene MAPK14; or the genes MAPK14 and S100A9; or the genes MAPK14 and GNLY; or the 6 genes of Table 2, or the gene S100A9; or the genes S100A9, IL2RB, and CASP5; or the genes S100A9, IL2RB, KLRK1, HCK, and GNLY; or the genes S100A9, IL2RB, KLRK1, HCK, GNLY, CTSZ, ARF5, and UTP14C, or Equivalent Expression Profile of anyone of the expression profiles of (i) and (ii), and optionally one or more housekeeping gene(s), (b) comparing the obtained expression profile with at least one reference expression profile, and (c) determining the responsive or non-responsive phenotype from said comparison. The present invention also relates to kits and nucleic acid microarrays for performing said method. The present invention also relates to methods of treatment of inflammatory disease-suffering patients.
Owner:TC LAND EXPRESSION
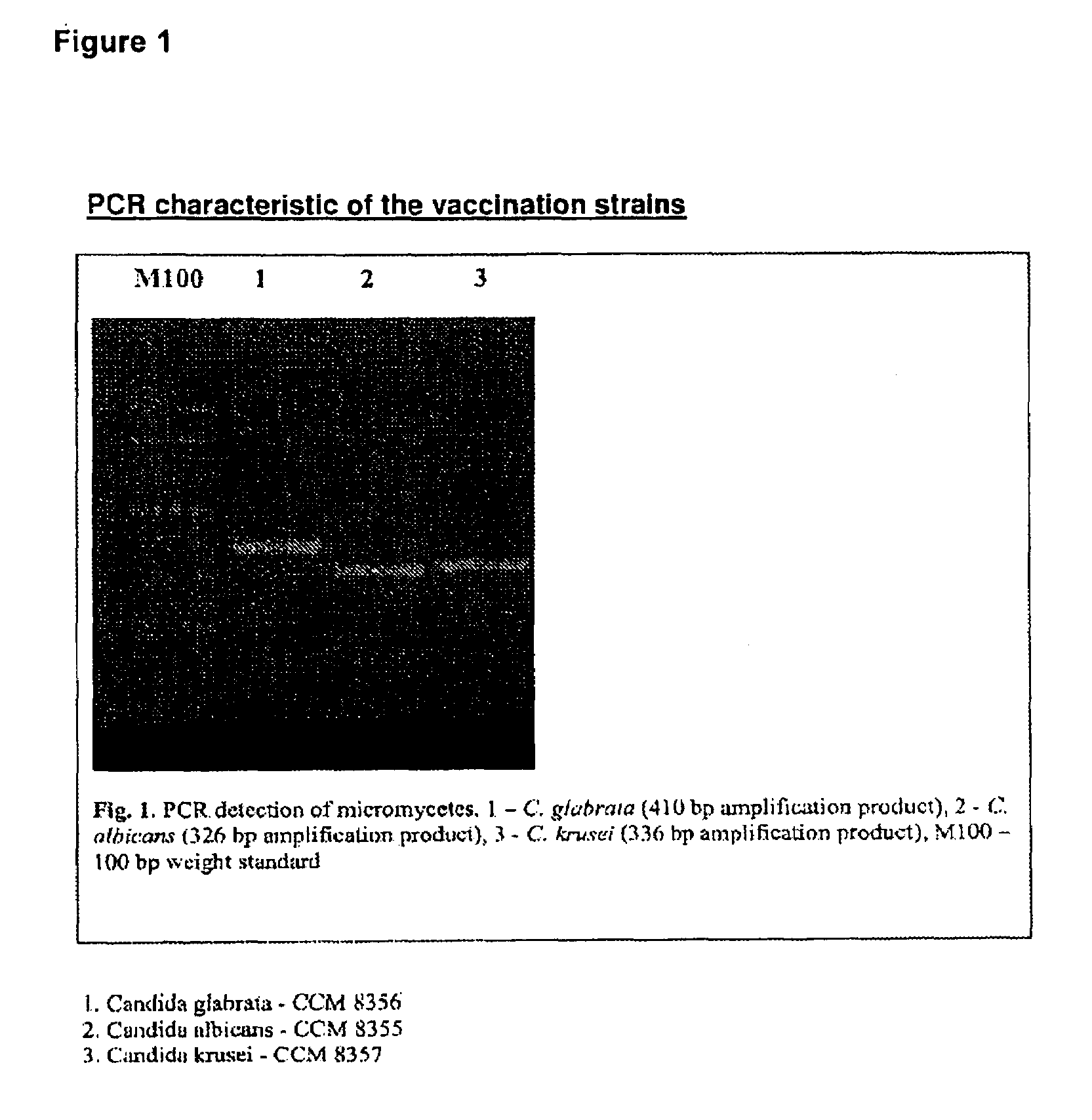
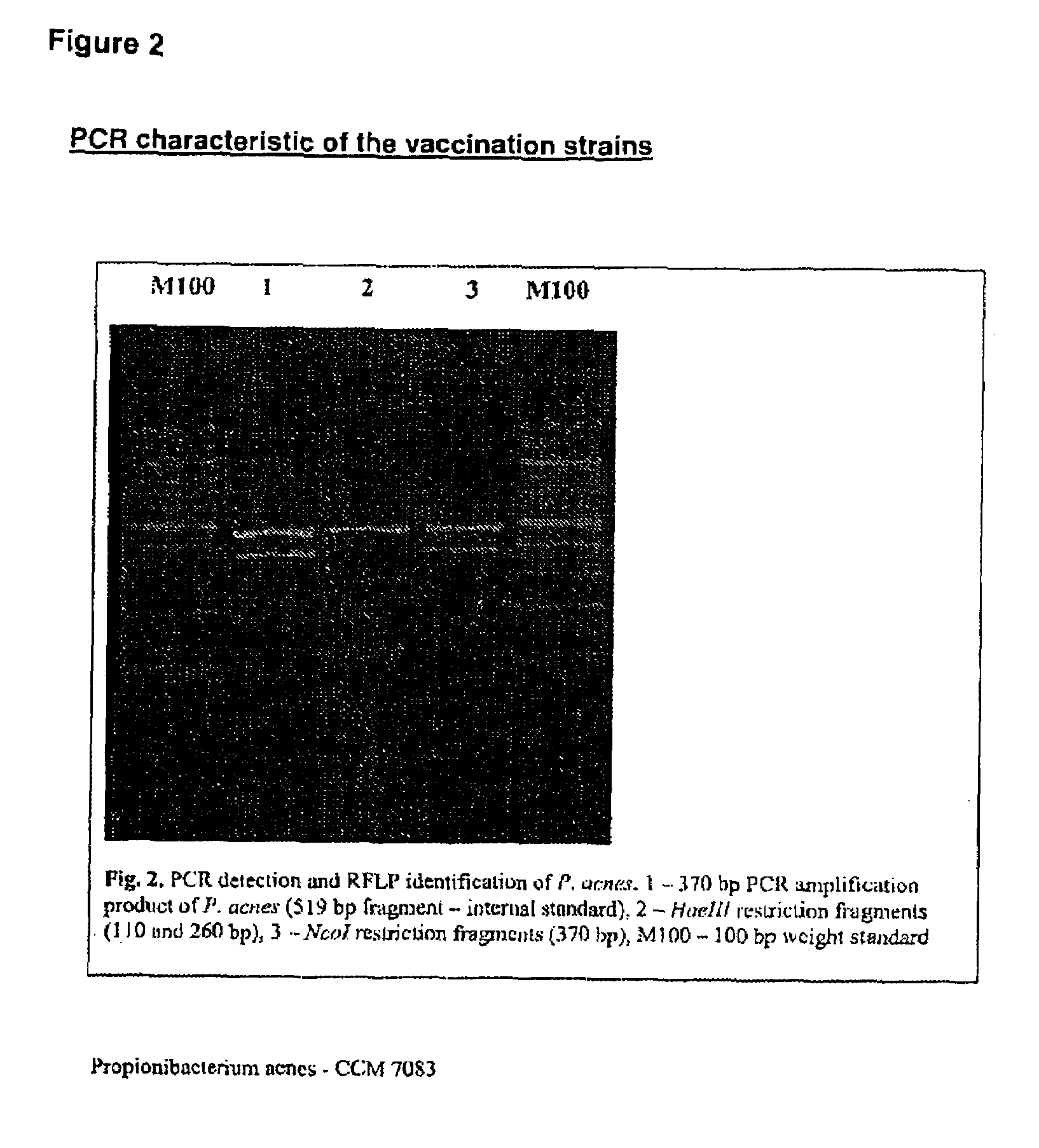
Vaccine for veterinary and human medicine prophylaxis and therapy
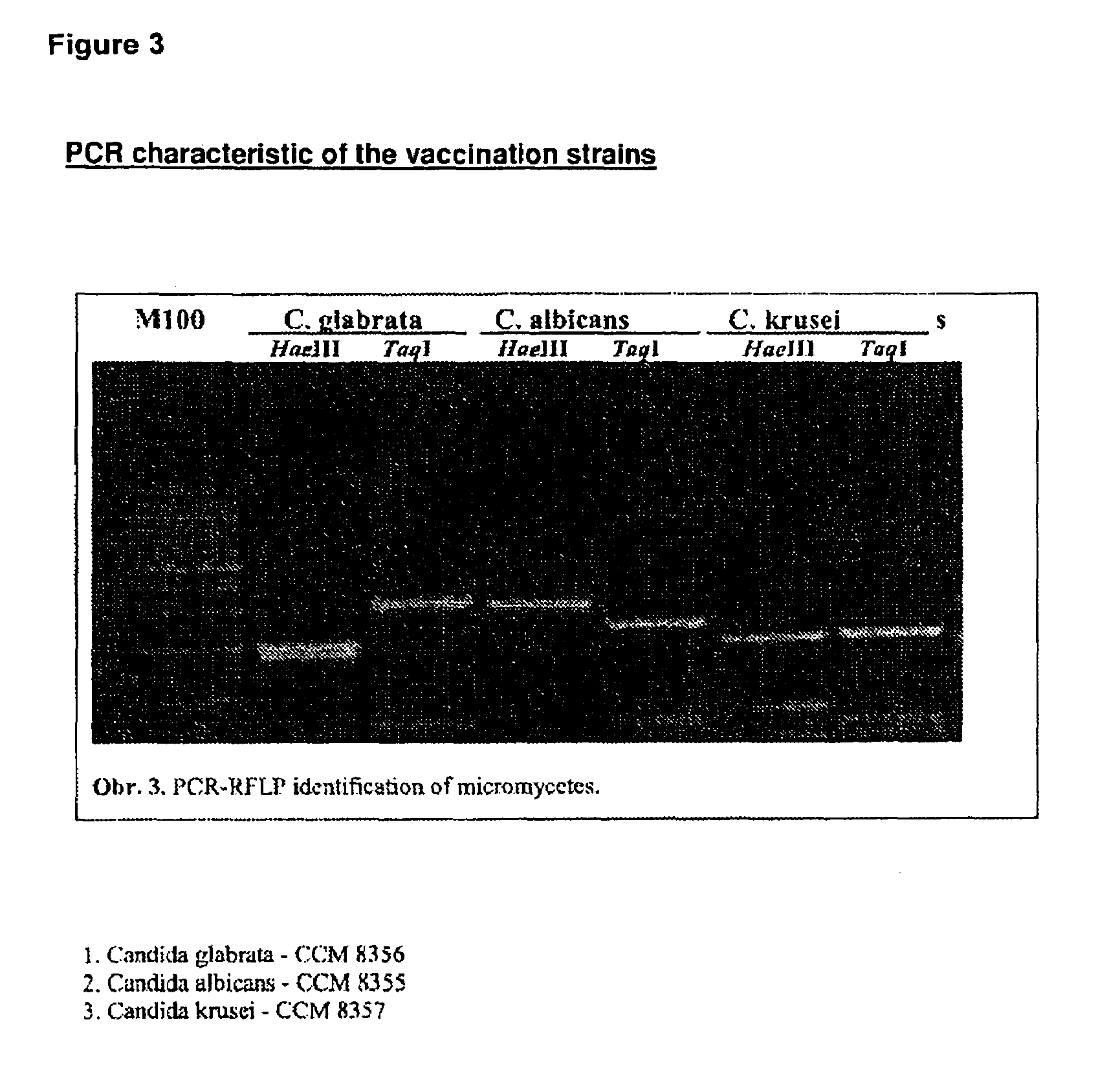
The present invention relates to a novel vaccine, its use for immunoprophylaxis and / or the treatment of candidamycoses in human and veterinary medicine as well as methods for its preparation, wherein said vaccine consists of the combination of the Candida strainsa1) Candida albicans CCM 8355a2) Candida glabrata CCM 8356a3) Candida krusei CCM 8357 anda4) an immunomudulating Propionibacterium acnes strain,and optionally pharmaceutically acceptable excipients, such as carriers, wherein the ratio of the components a1-a4 in the end product is a1:a2:a3:a4 is 10-20:10-20:10-20:40-70.
Owner:VRZAL VLADIMIR +1
Preparation method of human insulin analogue and usage thereof
InactiveCN101157725ANot easy to formLow self-integrationPeptide/protein ingredientsMetabolism disorderDipeptideBio engineering
The present invention pertains to the field of human medicine, which relates to a drug for the treatment of type I and type II diabetes and provides a preparation method and the usage of a human insulin analogue. The human insulin analogue of the present invention is characterized in that the human insulin analogue has the form as the right formula, wherein, R1 is any one amino acid residue of Pro, Ala, Gly, Ser, Val and Leu; R2 is any needed amino acid residue which can be encoded genetically; R3 is n paragraphs of R2-R1-dipeptide fragment, n is any one number of 0, 1, 2, 3 and 4. The human insulin analogue of the present invention is prepared by using a biological engineering method, the active insulin analogue has the advantages that the human insulin analogue is not easy to from the dimer and mainly exists by a monomer form after the subcutaneous injection, the local absorption is faster, the onset time is shorter, and the pharmacokinetics characteristics are more in line with the change spectra of physiological insulin and blood glucose.
Owner:江苏未名生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com