Preparation method of human insulin analogue and usage thereof
A technology of human insulin and analogs, applied in the field of human medicine, can solve the problems of inconvenience of patients, unable to effectively simulate the physiological secretion of insulin after meals, unable to effectively simulate the physiological secretion of insulin and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preparation method of this human insulin analog is completed by the following steps:
[0055] (1) construct a kind of reproducible expression vector, this vector comprises a section of DNA sequence encoding the human insulin analogue with amino acid sequence II:
[0056] Met-X 2 b -Y-R 3 -R 2 -R 1 -Phe-Val-Asn-Gln-His-Leu-Cys-Gly-Ser-His-Leu-Val-Glu-Ala-
[0057] Leu-Tyr-leu-Val-Cys-Gly-Glu-Arg-Gly-Phe-Phe-Tyr-Thr-Pro-Lys-Thr-X 1 a -Arg-Gly-
[0058] Ile-Val-Glu-Gln-Cys-Cys-Thr-Ser-Ile-Cys-Ser-Leu-Tyr-Gln-Leu-Glu-Asn-Tyr-Cys-Asn
[0059] II
[0060] where X 2 b is a peptide chain containing b amino acid residues, b is a non-negative integer; X 1 a is a peptide chain containing a amino acid residues, a is a non-negative integer; Y is Arg or Lys; R 1 Any amino acid residue in Pro, Ala, Gly, Ser, Val, Leu; R 2 is any desired genetically codable amino acid residue; R 3 It is an n segment-R2-R1-dipeptide fragment, n ta...
Embodiment 1
[0081] Example 1 Construction of FPPIns Recombinant Genetic Engineering Bacteria
[0082] 1. Acquisition of PPIns gene
[0083] Two primers P1 and P2 were designed by means of computer software, and the nucleotide sequences of the two primers were as follows:
[0084] P1: 5'ACAGGATCCAAGCGTCCGAAACCGTTTGTCAATCAGCACCTT 3'
[0085] P2: 5'TTAAAGCTTAGTTGCAGTAGTTCTCC 3'
[0086] A BamH I restriction site was introduced into primer P1, and a Himd III restriction site was introduced into primer P2. Primers were synthesized by Shanghai Yingjun Company.
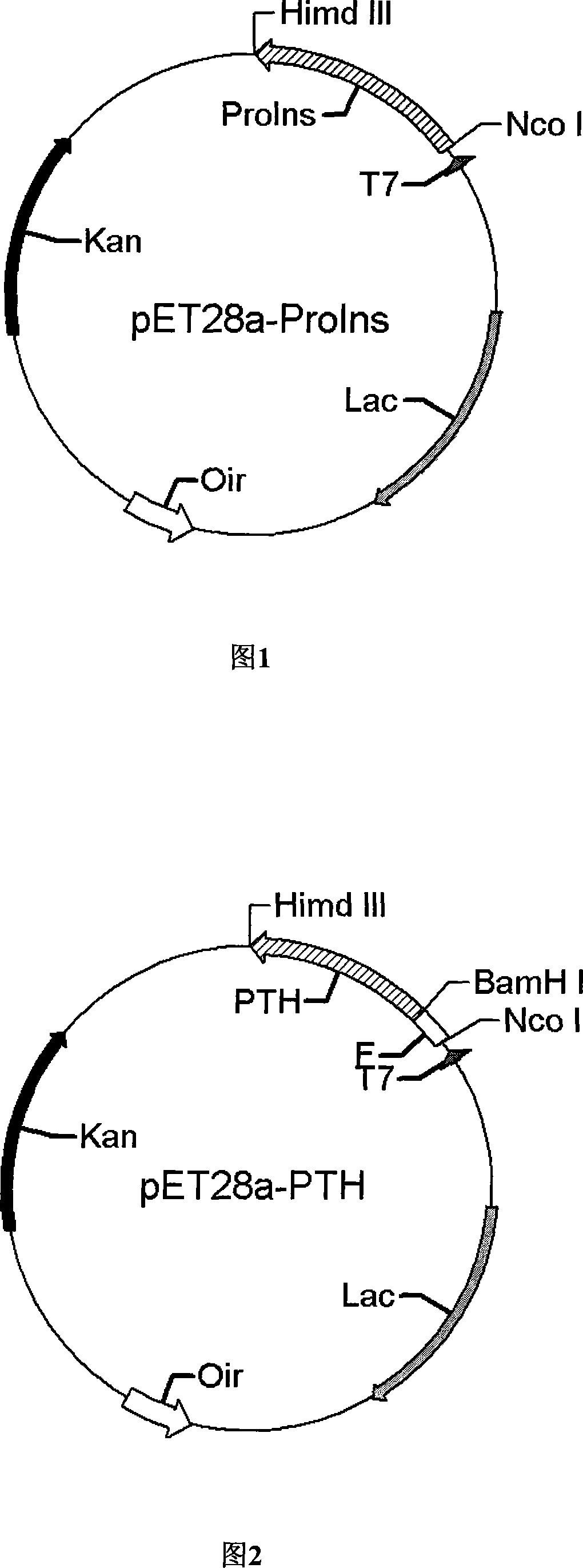
[0087] Using the pET28a-ProIns plasmid as a template, see Figure 1, and using P1 and P2 as upstream and downstream primers to perform PCR amplification to obtain the PPIns gene fragment.
[0088] PCR reaction system: 100 pmol P1, 100 pmol P2, 2 μl dNTP (10 nM), 5 units of PfuDNA polymerase, 0.5 μl of pET28a-ProIns plasmid, 5 μl 10x Pfu DNA polymerase reaction buffer, the total system is 50 μl.
[0089] PCR reaction conditions: 94°C...
Embodiment 2
[0094] Example 2 Expression of FPPIns gene in Escherichia coli
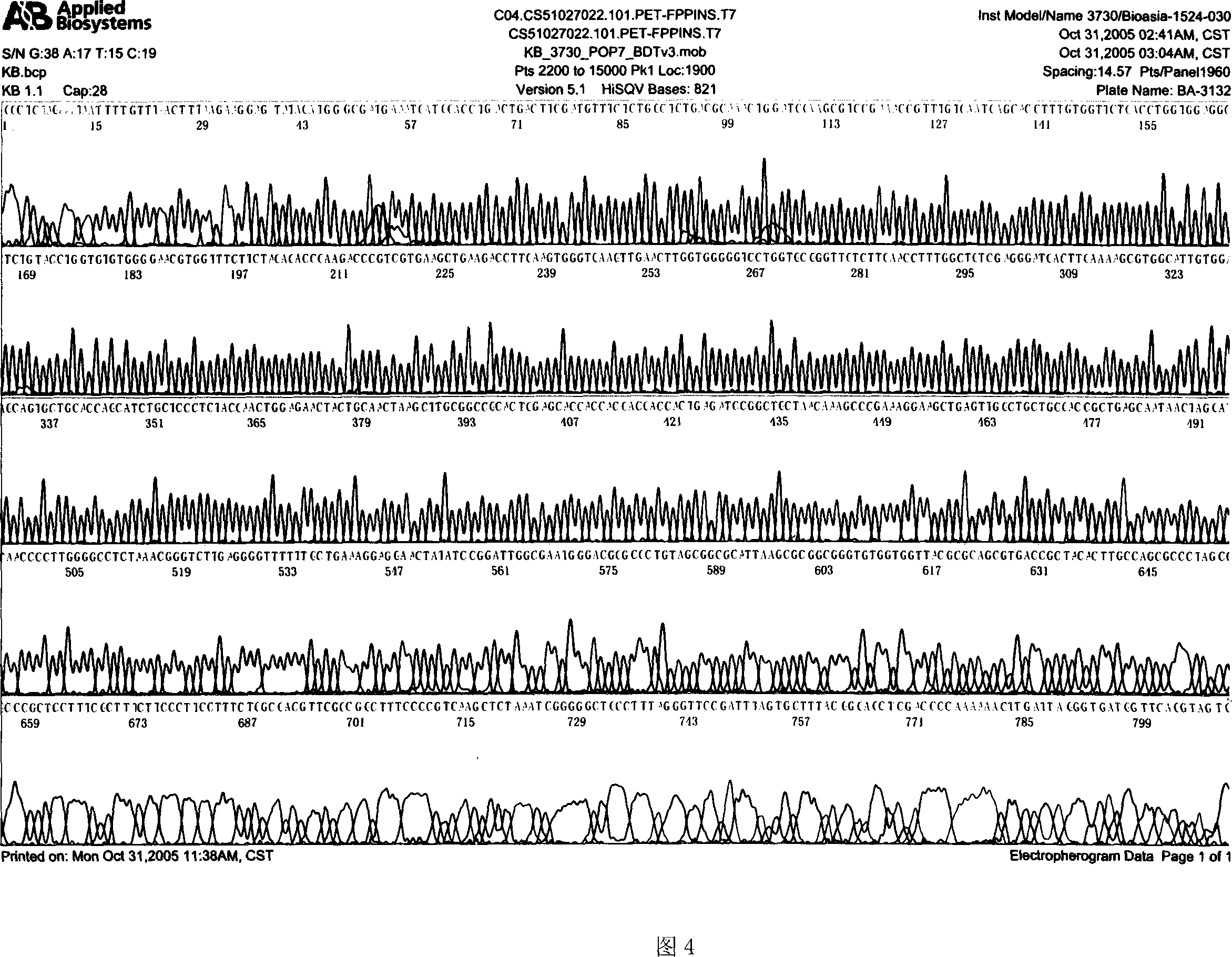
[0095]Pick a single colony from the culture plate of the pET28a-FPPIns recombinant genetically engineered bacteria, inoculate it in LB liquid medium containing 50 μg / ml kanamycin, culture it overnight at 37°C with constant temperature shaking, and inoculate it at a ratio of 1%. In LB liquid medium (50 μg / ml kanamycin), after culturing at 37°C for 4 hours, α-lactose at a final concentration of 0.5 mmol / L was added to induce Escherichia coli to express T7 RNA polymerase, and the culture was continued to express the fusion protein FPPIns. After 6 hours of induction, the cells were recovered by centrifugation, and SDS-PAGE electrophoresis and thin-layer scanning showed that the expression of the FPPIns precursor proinsulin protein gene had been realized, and the expressed protein accounted for about 40% of the total bacterial protein. The results are shown in Figure 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com