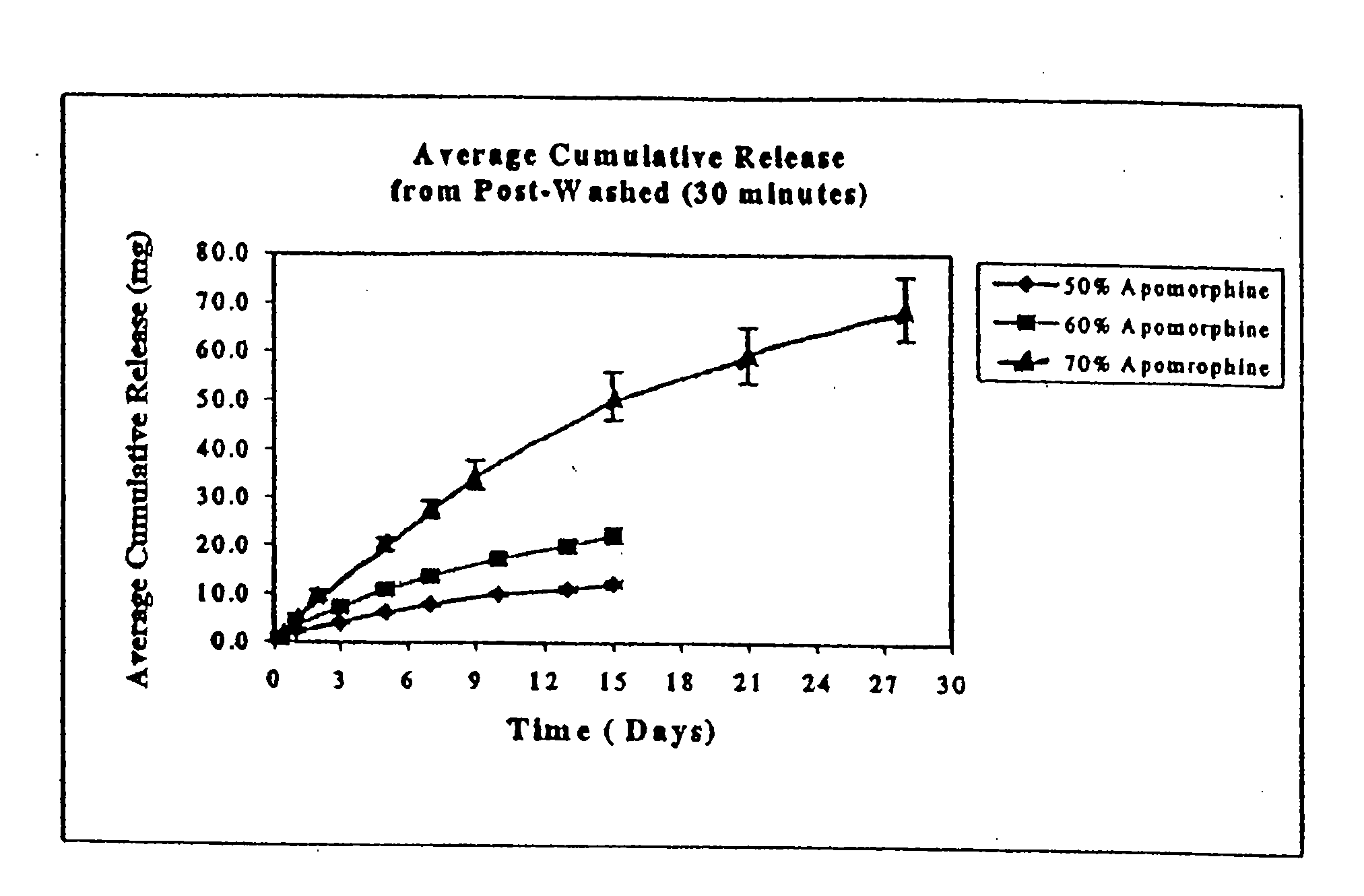
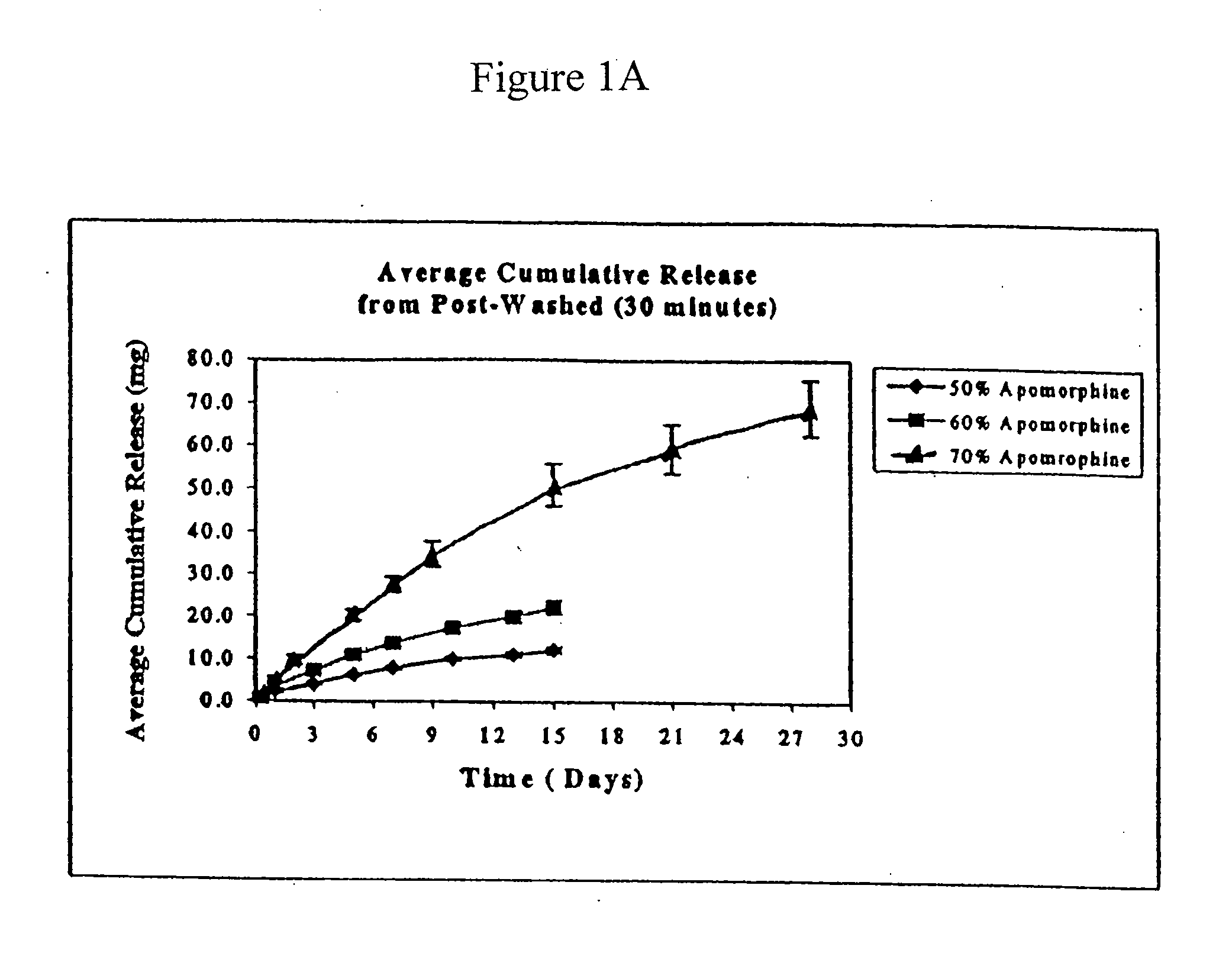
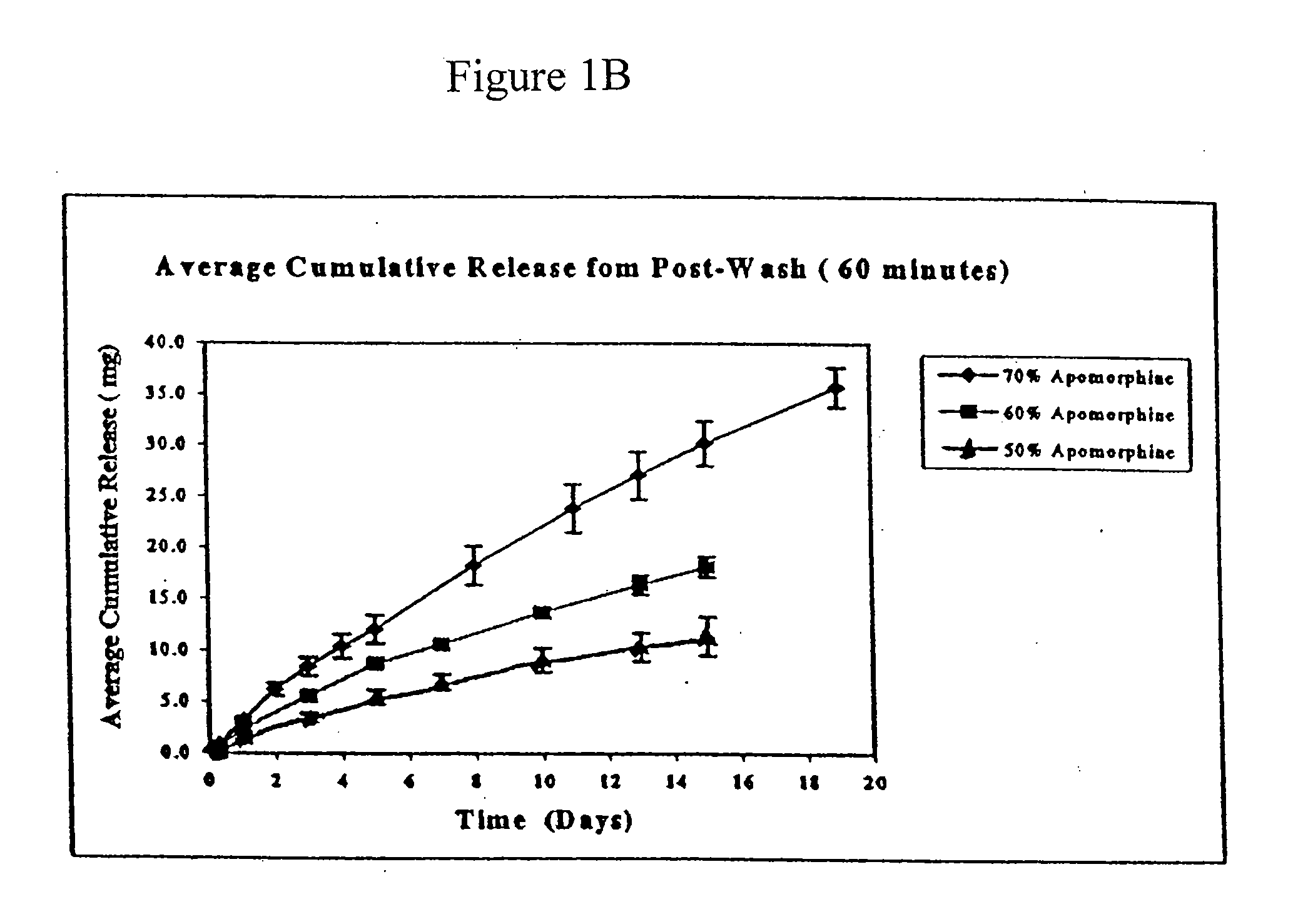
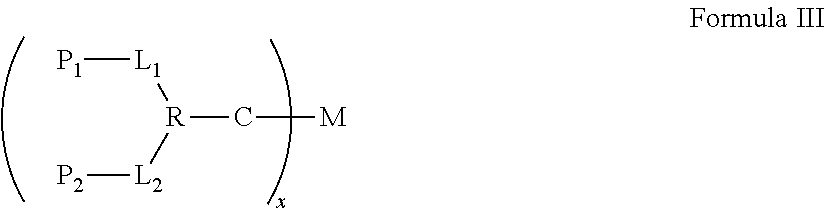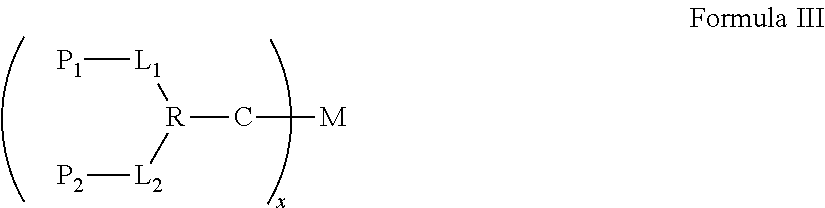Patents
Literature
235 results about "Continuous release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A continuous release may be a release that occurs 24 hours a day, such as a radon release from a stock pile. It may also be a release that occurs during a certain process, such as benzene released during the production of polymers, or a release of a hazardous substance from a tank vent each time...
Synergistic biomolecule-polymer conjugates
InactiveUS20130195799A1Promote excretionClear wellPeptide/protein ingredientsAntiviralsIn vivoContinuous release
The synergistic biomolecule-polymer conjugates are the long-acting, in vivo controlled continuous-release and hybrid synergy systems of biomolecules that provide increased biological activities and enhanced pharmacological properties for achieving greater therapeutic efficacies.
Owner:PEG BIOSCI
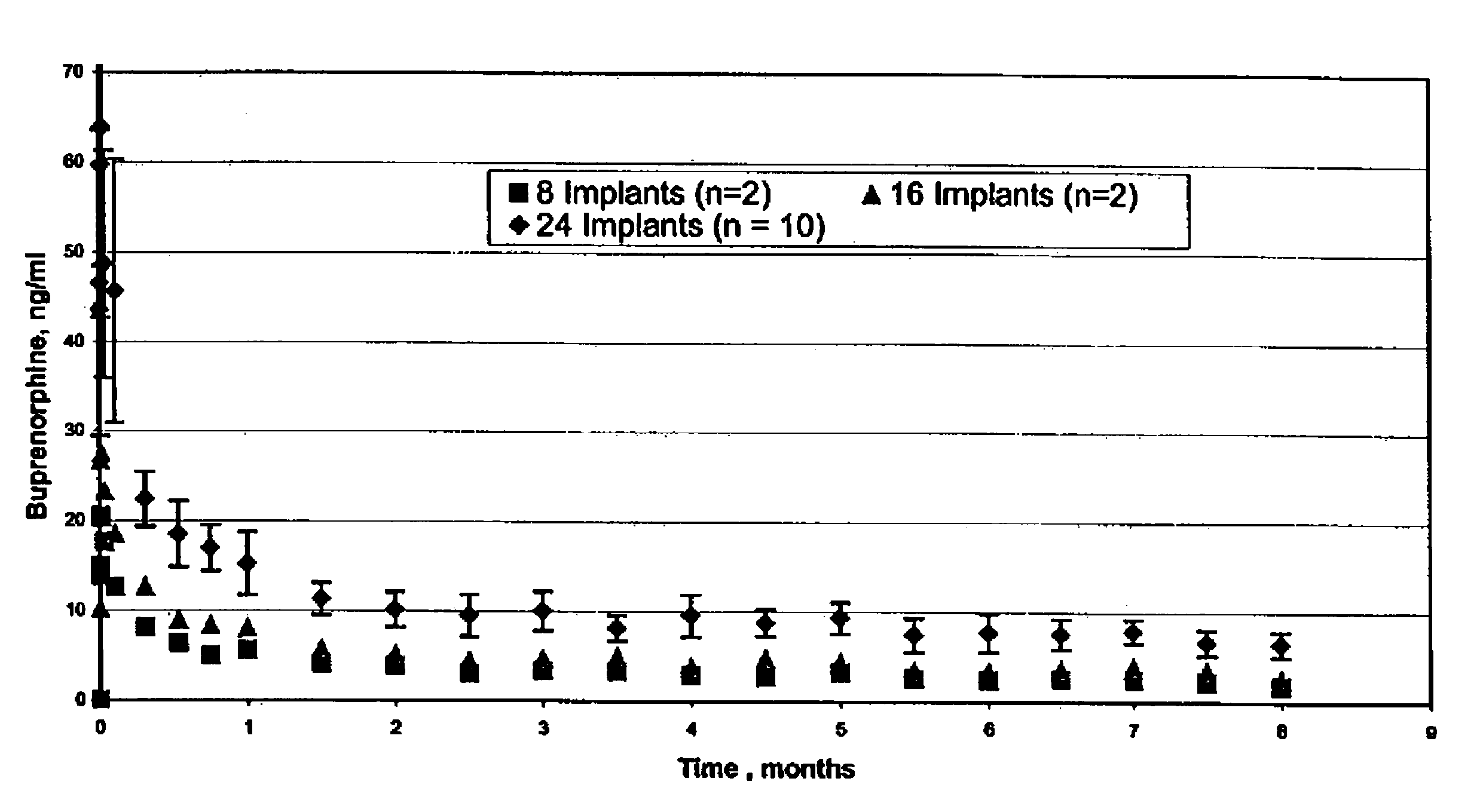
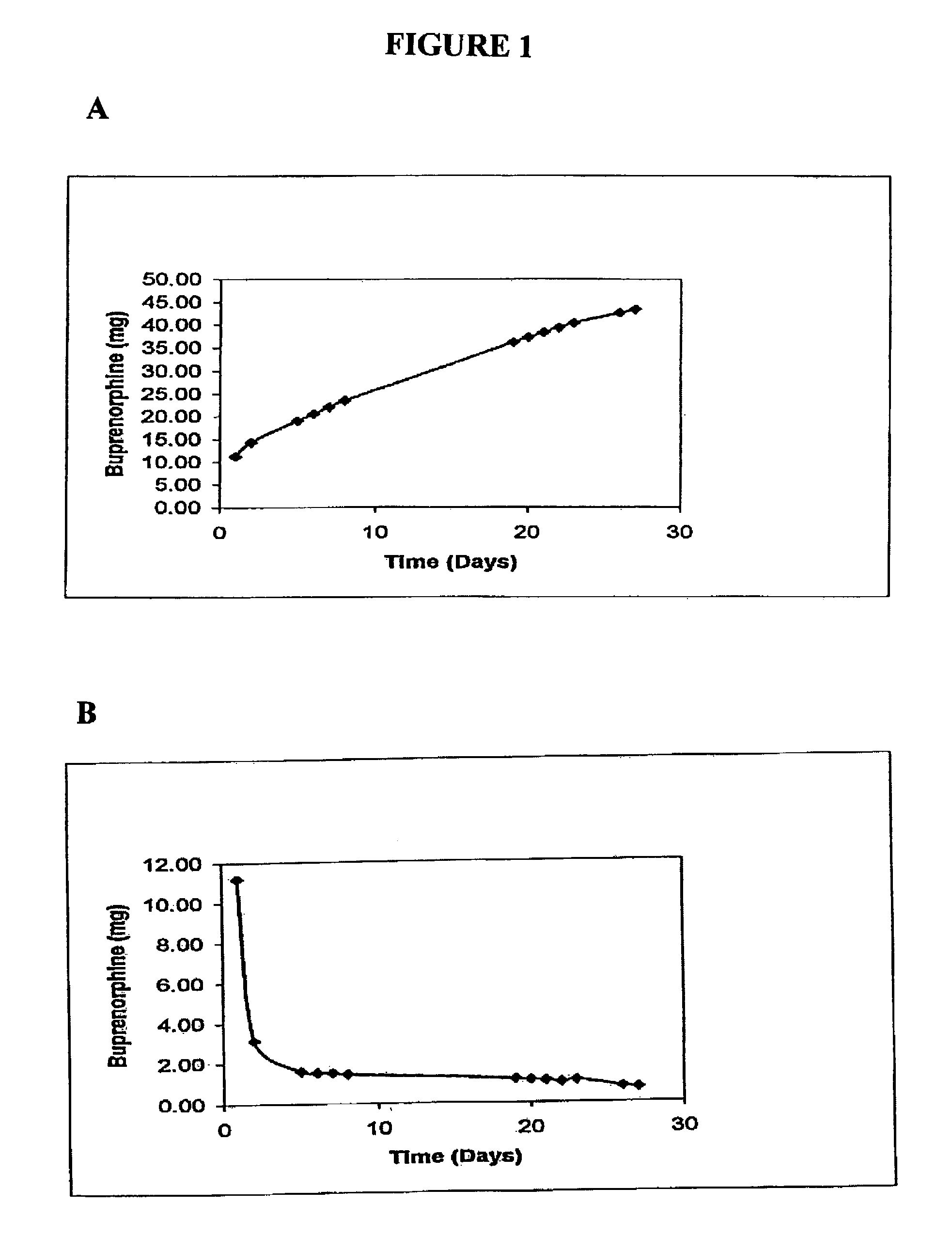
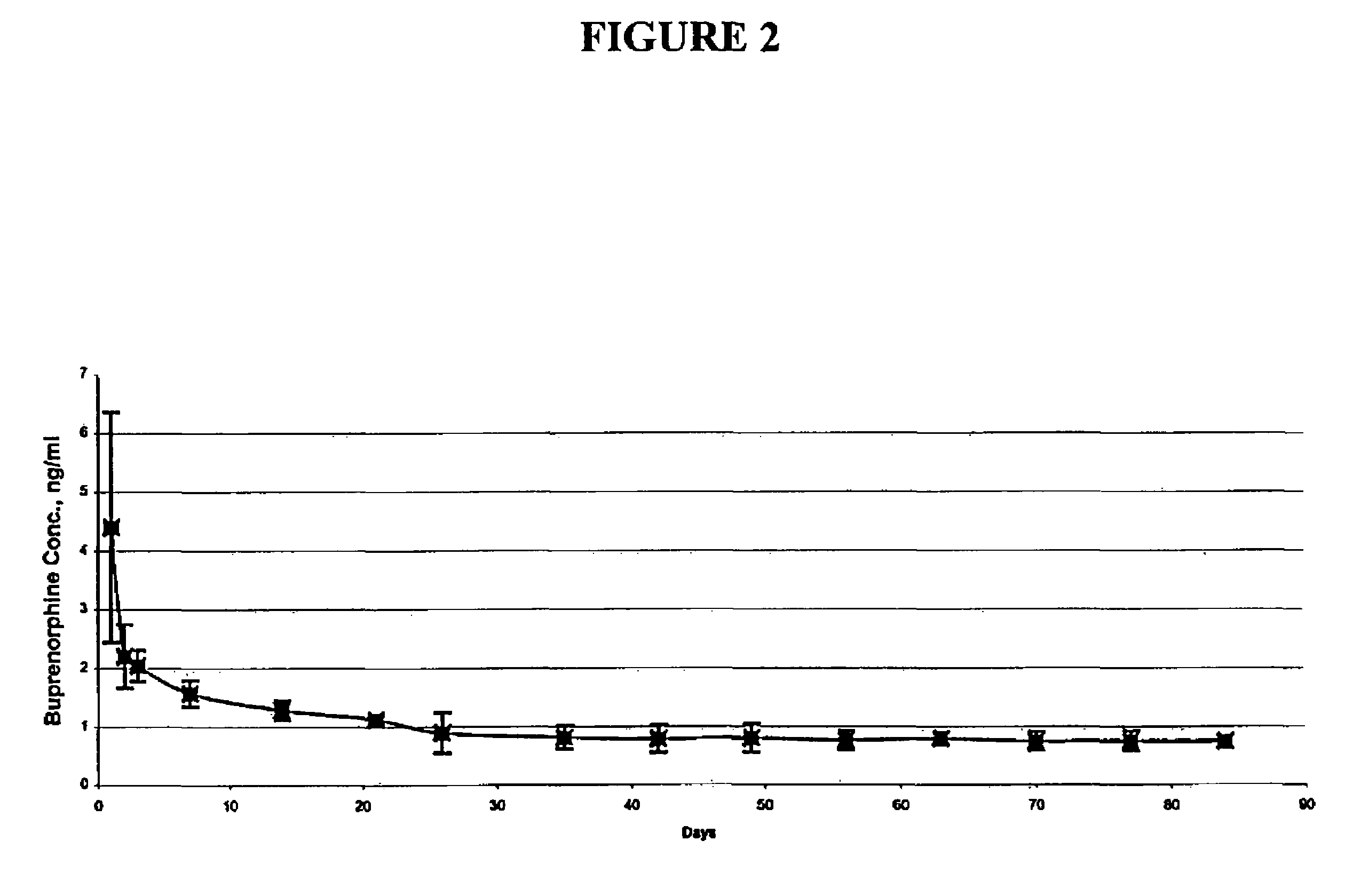
Implantable polymeric device for sustained release of buprenorphine
The present invention provides compositions, methods, and kits for treatment of opiate addiction and pain. The invention provides a biocompatible nonerodible polymeric device which releases buprenorphine continuously with generally linear release kinetics for extended periods of time. Buprenorphine is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with buprenorphine.
Owner:FEDSON INC
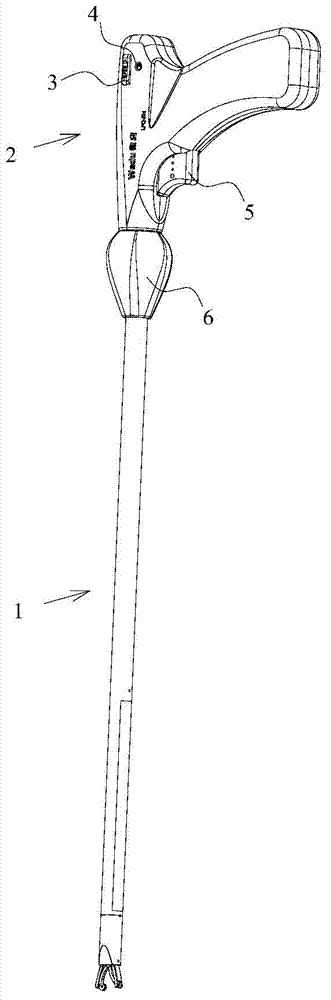
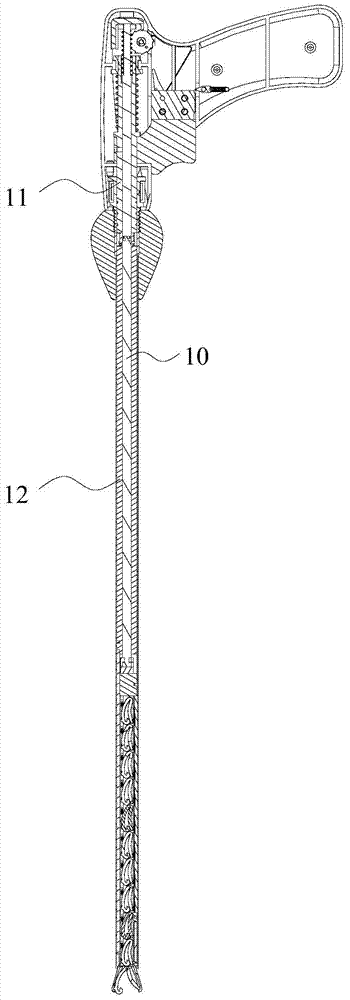
Clip applier capable of continuously releasing hemostatic clips
The invention discloses a clip applier capable of continuously releasing hemostatic clips. The clip applier comprises a clip tube and a handle, wherein a transmission component and a trigger extending into the clip tube are arranged in the handle; a magazine is fixedly arranged in the clip tube; a magazine clip in which a plurality of hemostatic clips are included is arranged in the magazine; the side wall of the magazine clip is provided with a hemostatic clip pushing groove; the end, away from the handle, of the clip tube is provided with a hemostatic clip release head; a conveying passage, which is mutually aligned for transferring the hemostatic clips, is formed among the magazine, the magazine clip and the release head; the part, which is positioned in the clip tube, of the transmission component is provided with an elastic poking sheet which extends into the hemostatic clip pushing groove to push the hemostatic clips in the magazine clip. When the clip applier is used, the hemostatic clips can be continuously released by continuously pulling the trigger; after the hemostatic clips are released, the old magazine clip is popped up, and then a new magazine clip is replaced for reuse.
Owner:ZHEJIANG APELOA JIAYUAN BIOMEDICAL MATERIAL
Preparation Method for Sustained Release Microspheres Using a Dual-Feed Nozzle
InactiveUS20070275082A1Peptide/protein ingredientsPharmaceutical product form changePresent methodMedicine
Disclosed is a method of preparing sustained release microspheres by spray-drying liquids with different compositions for preparation the sustained release microspheres through an ultrasonic dual-feed nozzle. Unlike conventional methods of preparing sustained release microspheres by spray-drying a single liquid containing a biodegradable polymer, a drug, an additive and a solvent through a single-feed nozzle, the present method is characterized by simultaneously spray-drying two liquids with different compositions for preparation of the sustained release microspheres respectively through internal and external channels of an ultrasonic dual-feed nozzle to coat sprayed droplets through the internal channel with other sprayed droplets through the external channel. The present method is effective in achieving a low initial release and a desired continuous release.
Owner:PEPTRON
Polymer coated controlled release fertilizer based on water-based reaction for forming films and preparation method thereof
ActiveCN101823917AImprove hydrophobicityHigh solid contentFertilizer mixturesWater basedPolymer science
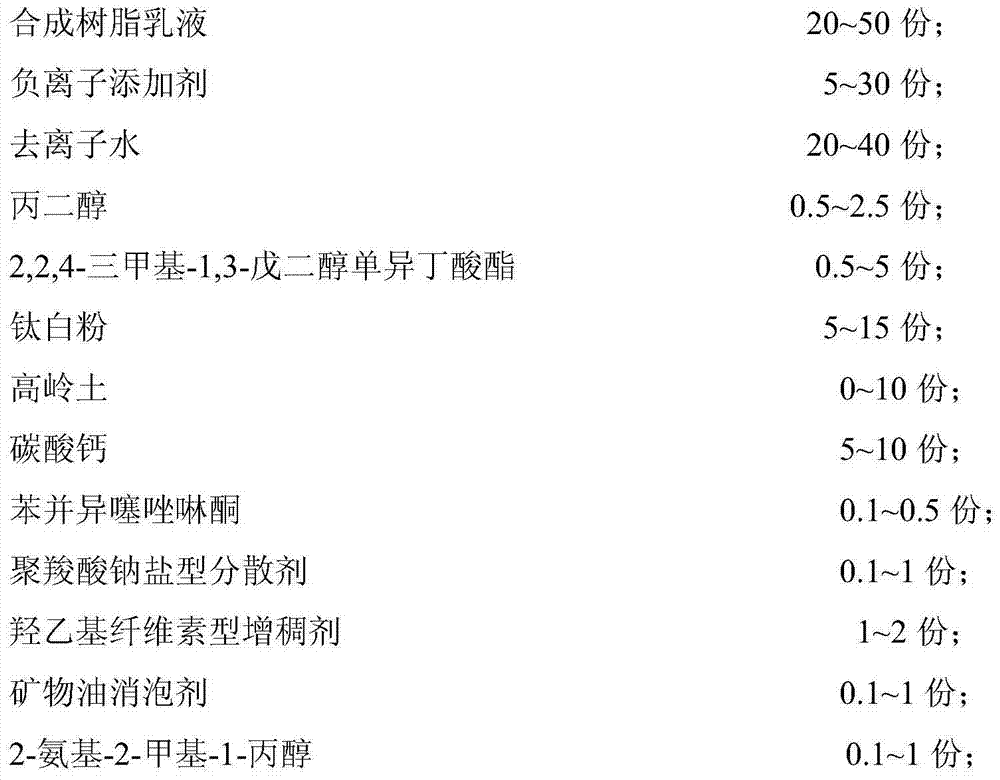
The invention provides a polymer coated controlled release fertilizer based on water-based reaction for forming films and a preparation method thereof. The polymer coated controlled release fertilizer comprises fertilizers and high polymer coating. The fertilizer is characterized in that the high polymer coating comprises the following raw materials in parts by mass: 100 parts of acrylic monomers, 10-16 parts of emulsifiers, 0.5-1.2 parts of initiators, 50-200 parts of water, 0-30 parts of organosilicon and 0-5 parts of crosslinking agents; and the raw materials of the high polymer coating account for 5-15% of the fertilizer by mass. The preparation process comprises preparation of coating mother liquid, preparation of coating liquid and preparation of the coated fertilizer. Release of the fertilizer nutrients is effectively controlled and the fertilizer nutrients can be continuously released for more than 30 days in the water at 25 DEG C. In the invention, the acrylic substance is used as the coated polymer body, water-based reaction for forming films is adopted to produce the polymer coating and secondary pollution caused by organic solvents does not exist in the production process. In addition, the hydrophobicity of the coated materials is effectively controlled and the controlled release effect is good.
Owner:INST OF SOIL SCI CHINESE ACAD OF SCI
System for testing operation of software
InactiveUS8479165B1Error detection/correctionSpecific program execution arrangementsStability indexContinuous release
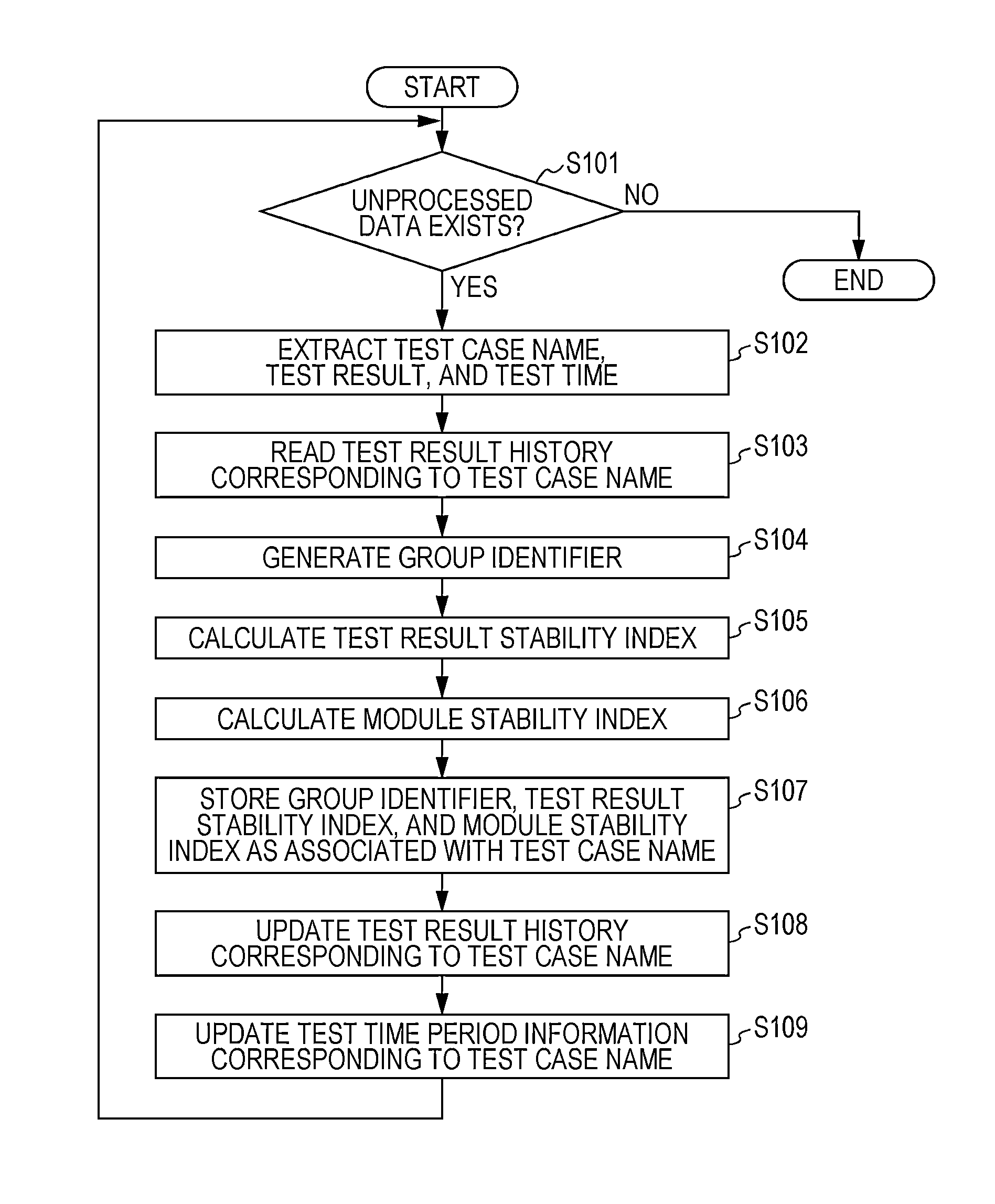
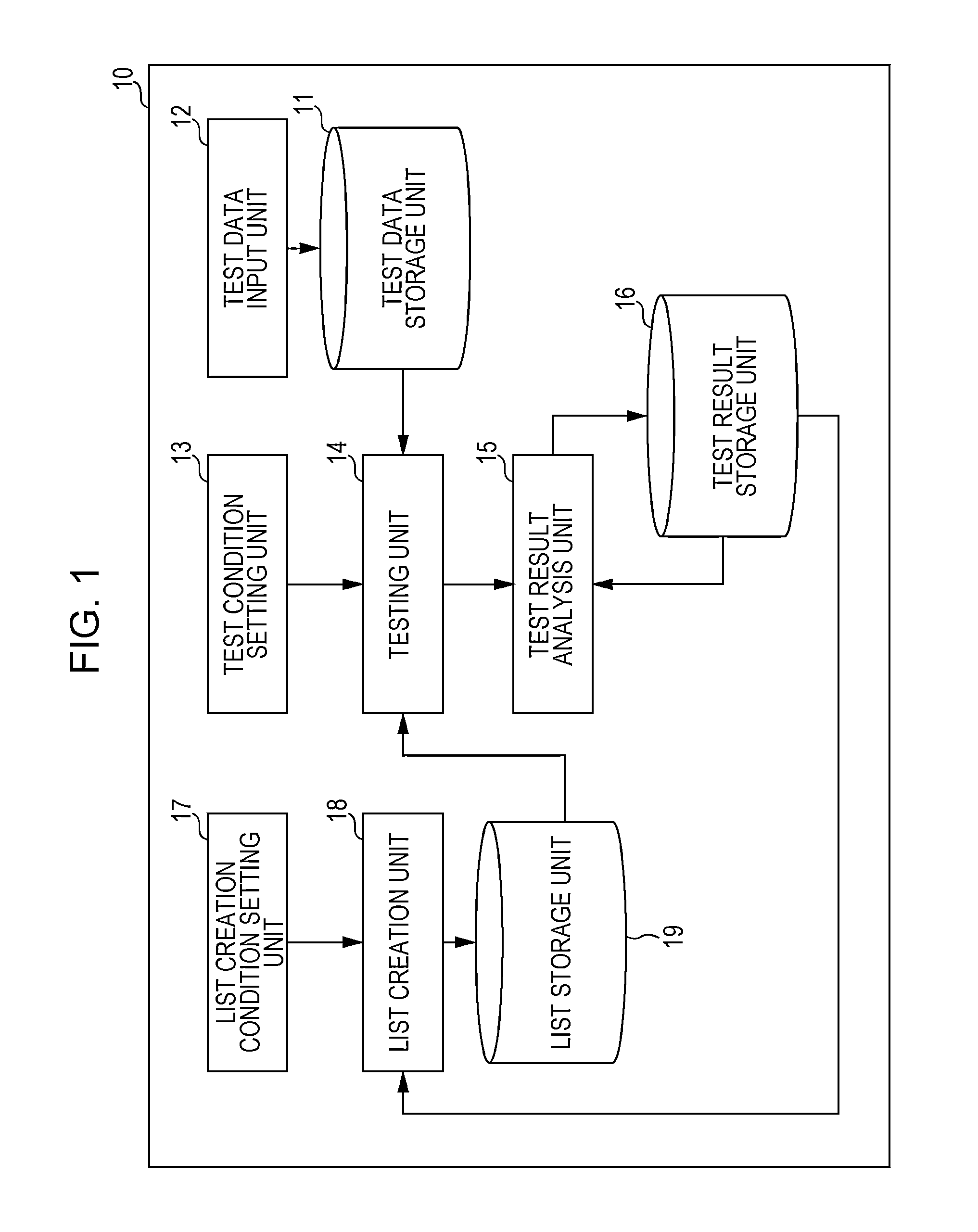
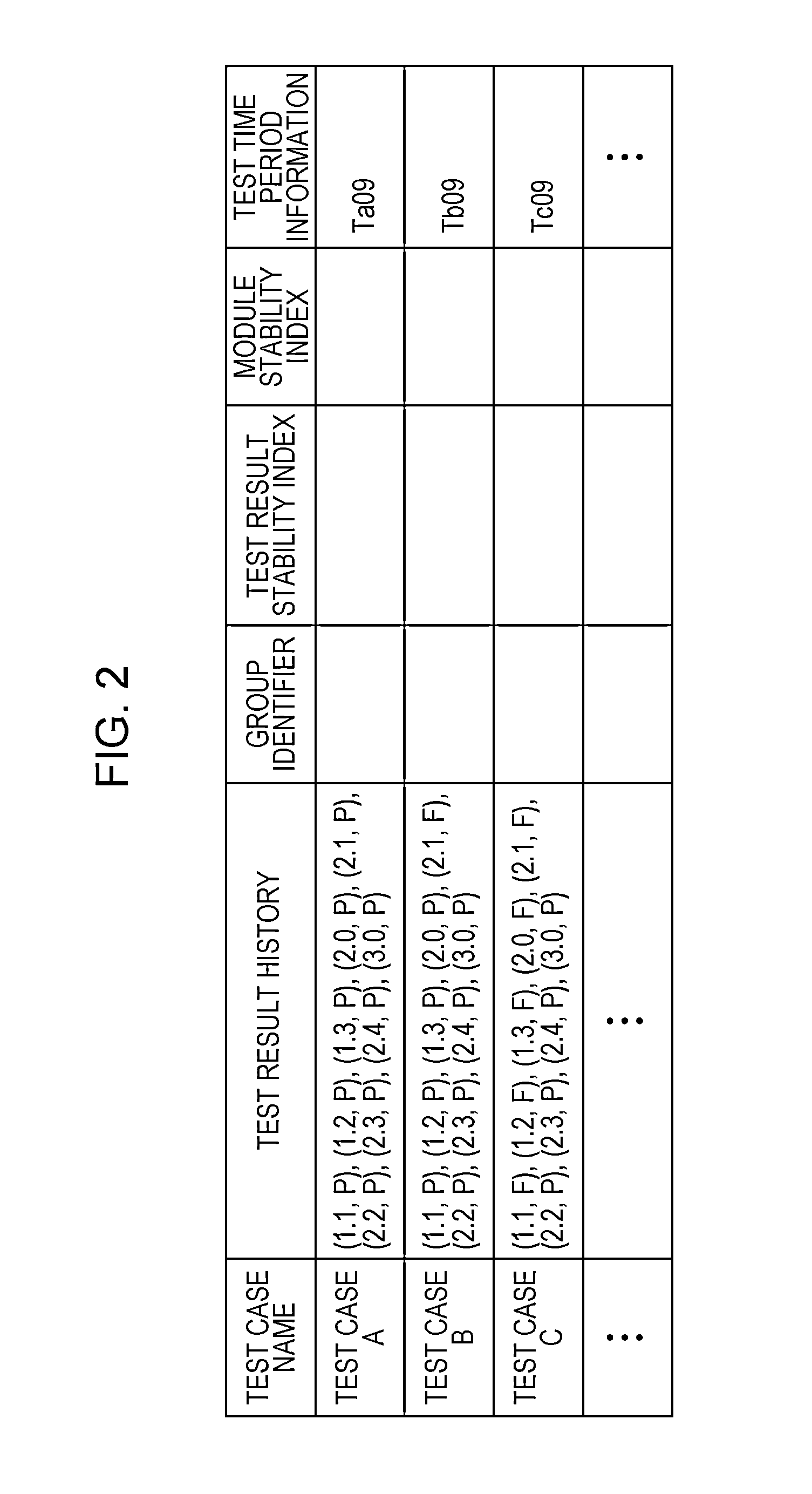
A system for implementing a method for processing test results from testing operation of software. A test result of pass, fail status, or unperformed is received for each test case of a test performed for each release of the software. A group to which each test belongs is ascertained. A test result stability index is calculated for each test case as being proportional to a total number of consecutive releases that include and are prior to the last release of the software such that the test result for each of the consecutive releases denotes a pass. A module stability index is calculated for each test case as being a summation over a product of a weight at each release and a binary stability indicator of 0 at each release for which the test result is changed from that of the immediately prior release and 1 at every other release.
Owner:KYNDRYL INC
Lithium secondary battery containing capsule for controlled-release of additives
ActiveUS7592095B2Minimizing adverse side reactionDeferred-action cellsCell electrodesLithiumControlled Release Capsule
Provided is a lithium secondary battery comprising a controlled-release capsule which continuously releases a desired amount of additives necessary for electrolytes or electrodes at a constant level and is included in an electrolyte and / or an electrode material, thereby providing inherent effects of additives while simultaneously minimizing adverse side reactions of surplus additives, consequently optimizing the battery performance.
Owner:LG ENERGY SOLUTION LTD
Negative ion-containing antibacterial haze-cleaning paint
InactiveCN104497685ALong-term antibacterial and antifungal effectsLong-lasting antibacterial and anti-mildew functionAntifouling/underwater paintsPaints with biocidesEmulsionMildew
The invention relates to the technical field of functional building paint and provides negative ion-containing antibacterial haze-cleaning paint. Through raw material grinding and mixing, the negative ion-containing antibacterial haze-cleaning paint is prepared from 15-40 parts by weight of a film-forming emulsion, 8-30 parts by weight of a negative ion additive, 0.5-20 parts by weight of a silver ion additive, 5-35 parts by weight of a pigment and a filler, 5-15 parts by weight of an assistant and 20-50 parts by weight of deionized water. Through use of the negative ion additive, the coating film has a negative ion continuous-release function and can purify air so that a PM 2.5 concentration is reduced. The silver ion additive has lasting antibacterial and mildew-resistant functions. The preparation method is simple and convenient and is conducive to reduction of an industrial production cost. The negative ion-containing antibacterial haze-cleaning paint has low VOC (volatile organic compound) content, can greatly reduce environmental pollution, is environmentally friendly, is especially suitable for occasions such as residential buildings, commercial buildings and common buildings and has a wide application prospect.
Owner:ZIGONG INNOVATION CENT OF ZHEJIANG UNIV
Mitomycin double sustained release film of implanted antineoplastic agents and its preparation method
InactiveCN101204382AGood physical and chemical propertiesHigh encapsulation efficiencyOrganic active ingredientsMacromolecular non-active ingredientsPolymer dissolutionSide effect
The invention discloses a double controlled release film agent of implanted antineoplastic agent mitomycin and a manufacturing method; the invention relates to a medicinal controlled release film agent. The invention provides a double controlled release film agent of implanted antineoplastic agent mitomycin, which relates to a medicinal controlled release film agent with high enveloping ratio, long continuous release duration, small incidence of burst release, few toxic and side effects, high medicine concentration on tumor lesions and low recrudescence probability of tumor, and a manufacturing method. The film agent includes mitomycin, polymer, lecithin, chitosan and collagen. The mitomycin and the lecithin are dissolved, lyophilized in order to obtain a colloidal mixture; the polymer is dissolved in organic solvent in order to obtain a polymer solution; the polymer solution is then added in the colloidal mixture in order to obtain a reverse micelle; the reverse micelle is further added to a polyvinyl alcohol solution, and an O / W latex is obtained by emulsification; the latex is lyophilized in order to obtain carrier micro-spheres; the collagen is dissolved in the acid solution in order to obtain a collagen solution; the chitosan is dissolved in the acid solution in order to obtain a chitosan solution; the collagen solution and the chitosan solution are mixed in order to obtain a collagen-chitosan mixture solution; finally the carrier micro-spheres are dispersed in the collagen-chitosan mixture solution which is then poured in a mould and dried.
Owner:XIAMEN UNIV
Minocycline hydrochloride microballoons and preparation method and application in pharmacy thereof
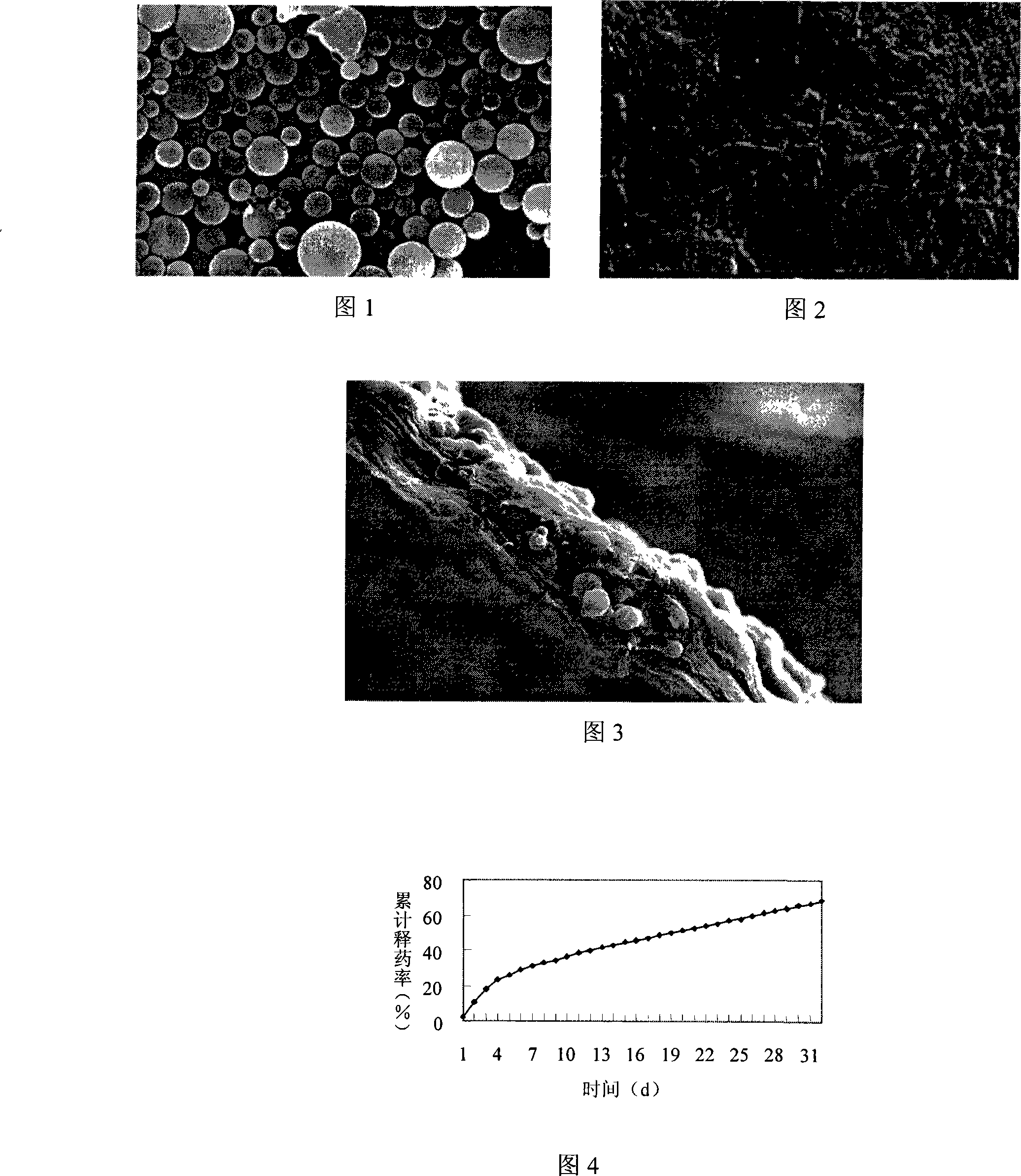
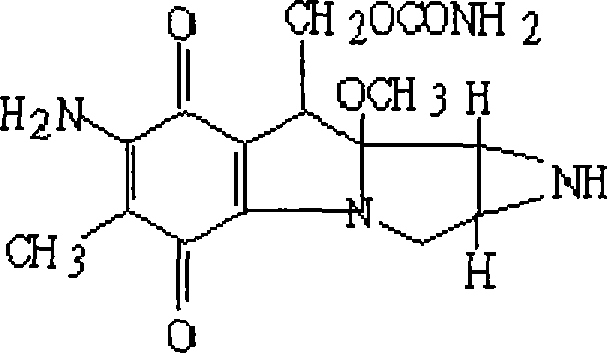
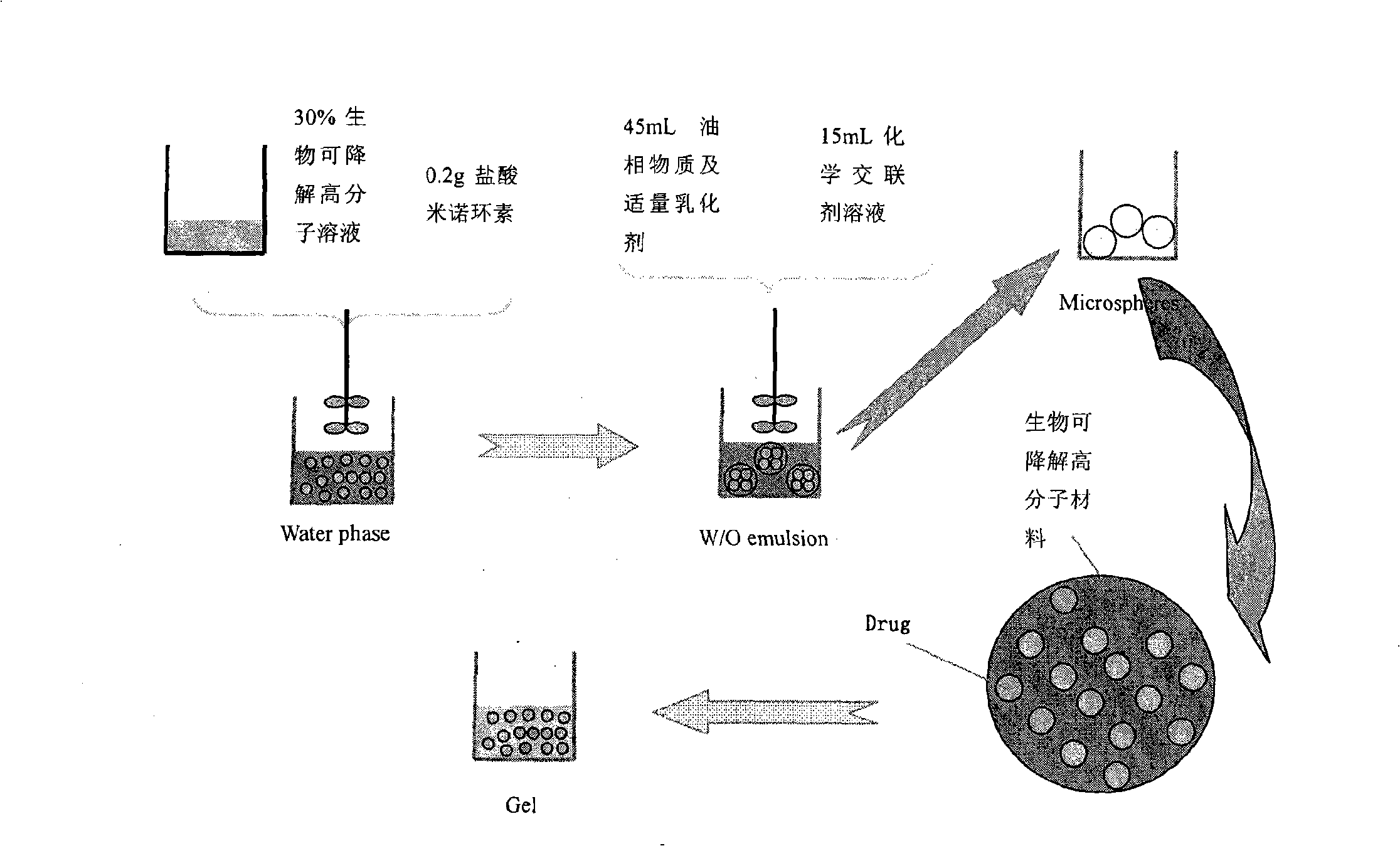
InactiveCN101288673AAvoid side effectsReduce the burden onTetracycline active ingredientsDigestive systemSide effectMicrosphere
The invention relates to a minocycline hydrochloride microsphere, a preparation method thereof and the application thereof in medicine preparation; the invention is technically characterized in that the components comprise minocycline hydrochloride drug, biodegradable polymer material, emulsifier and chemical cross-linking reagent; the weight percentage of the components is 10 percent to 20 percent (W / W) of minocycline hydrochloride, 15 percent to 40 percent (W / W) of biodegradable polymer material; the ratio between biodegradable polymer material and chemical cross-linking reagent is 1g : 5mL to 1g : 20mL; the volume ratio between aqueous phase and fat phase is 1 : 10 to 1 : 20 (V / V). The minocycline hydrochloride microsphere and the related preparation thereof (such as jellies) prepared by the invention not only has the remarkable advantages of avoiding the toxic and side effects resulted from systemic antibiotics use, being able to reach the depth of periodontal pocket, sustained-release of the medicine at the bottom (one dose can result in continuous release for 7 days), etc., but also greatly reduces the marketing price owing to the cost reduction resulted from totally using home-made excipients, which is beneficial for reducing the patient burden.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Medicinal composition for inhalation
InactiveUS7858650B2Prolonged bronchodilating and antiinflammatory effectInhibitionOrganic active ingredientsBiocideRESPIRATORY DISTRESS SYNDROME ADULTPulmonary Injury
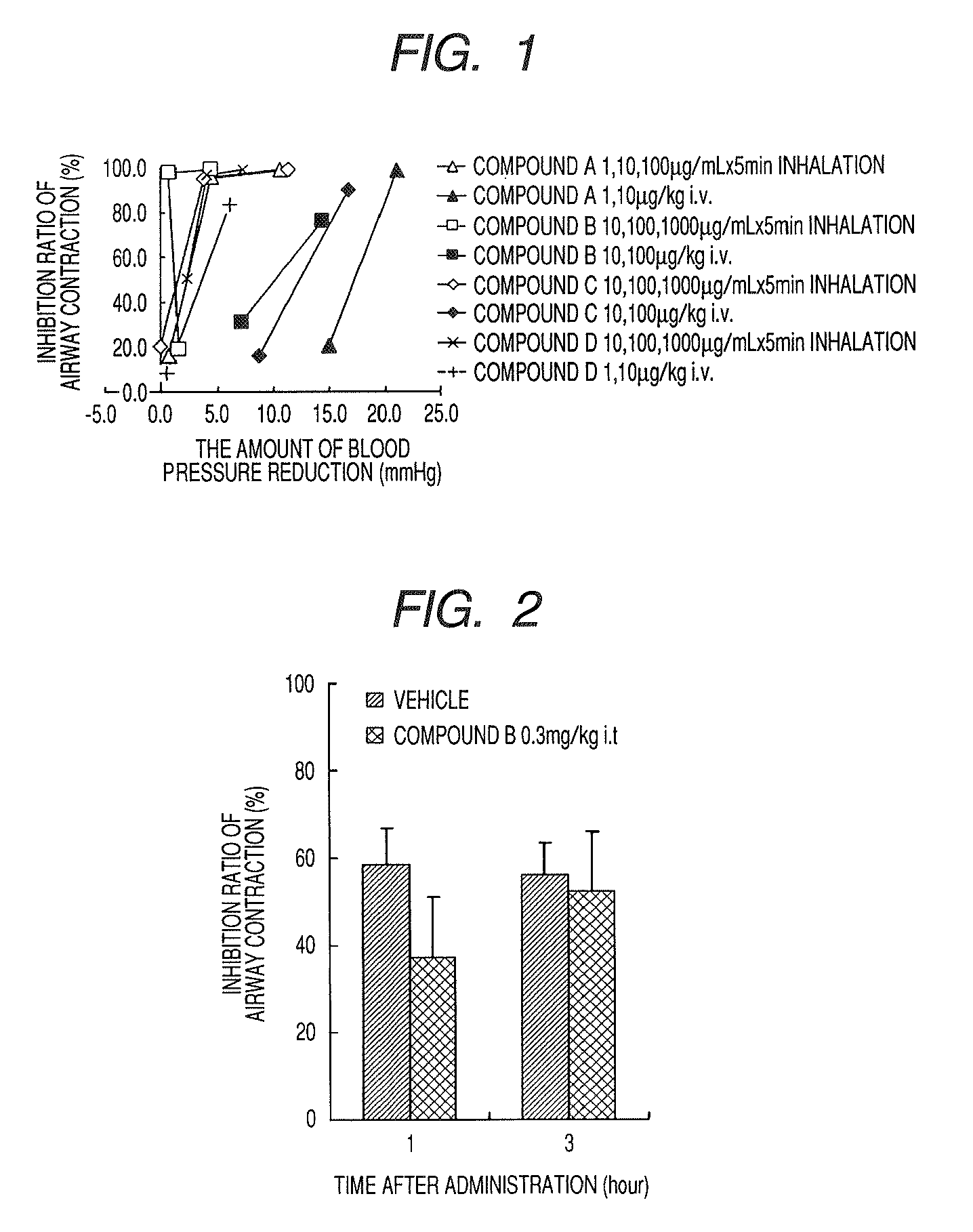
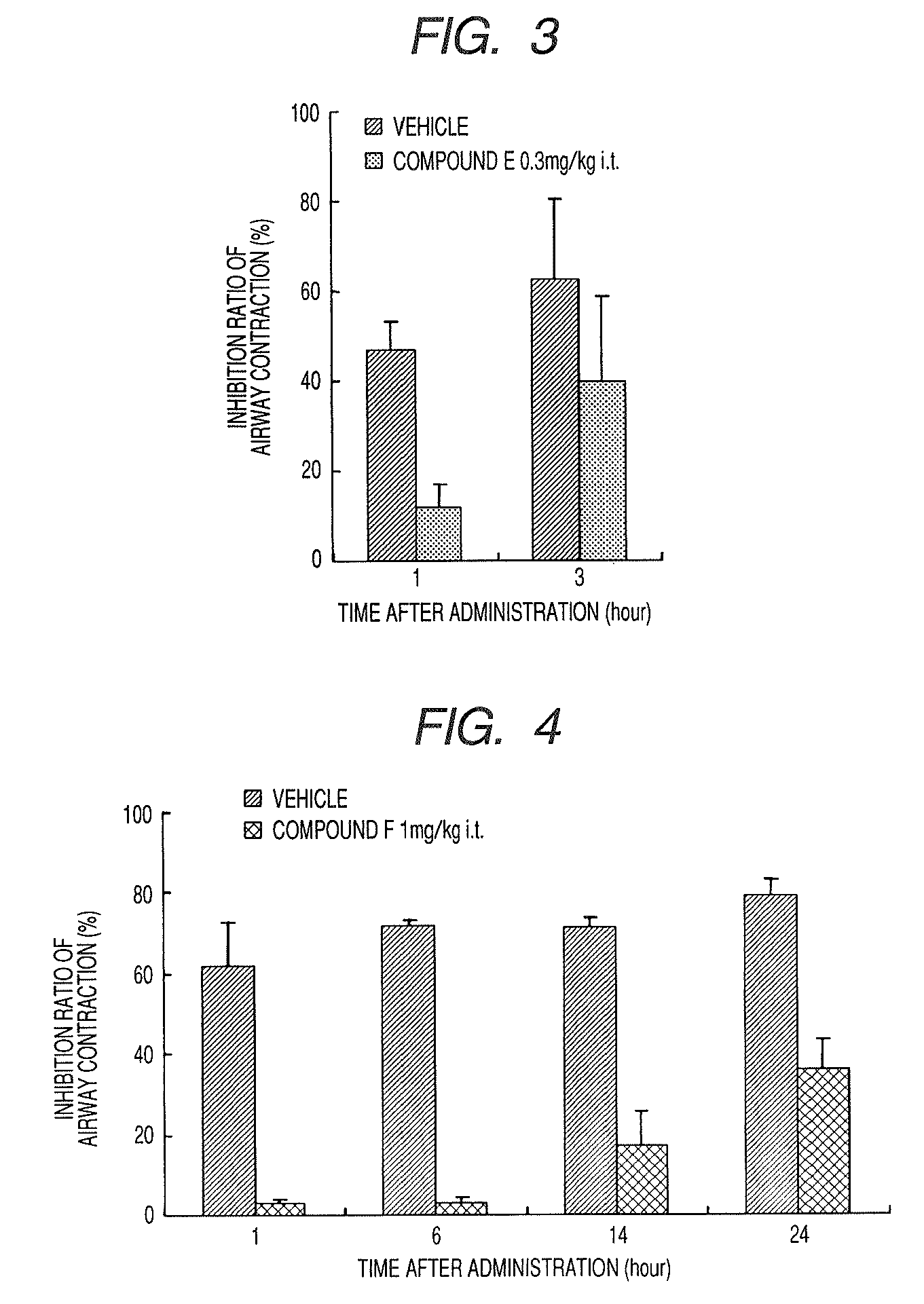
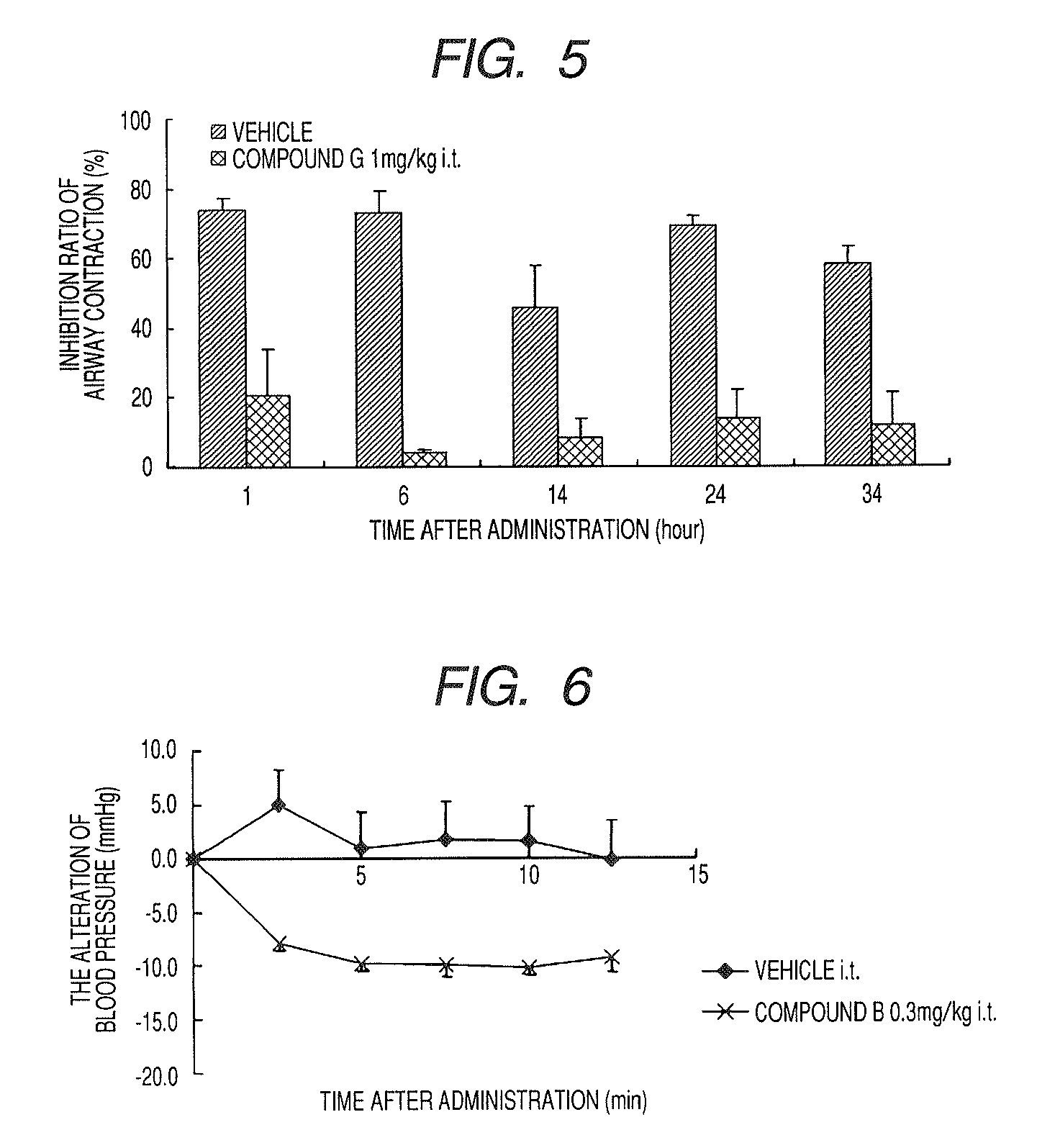
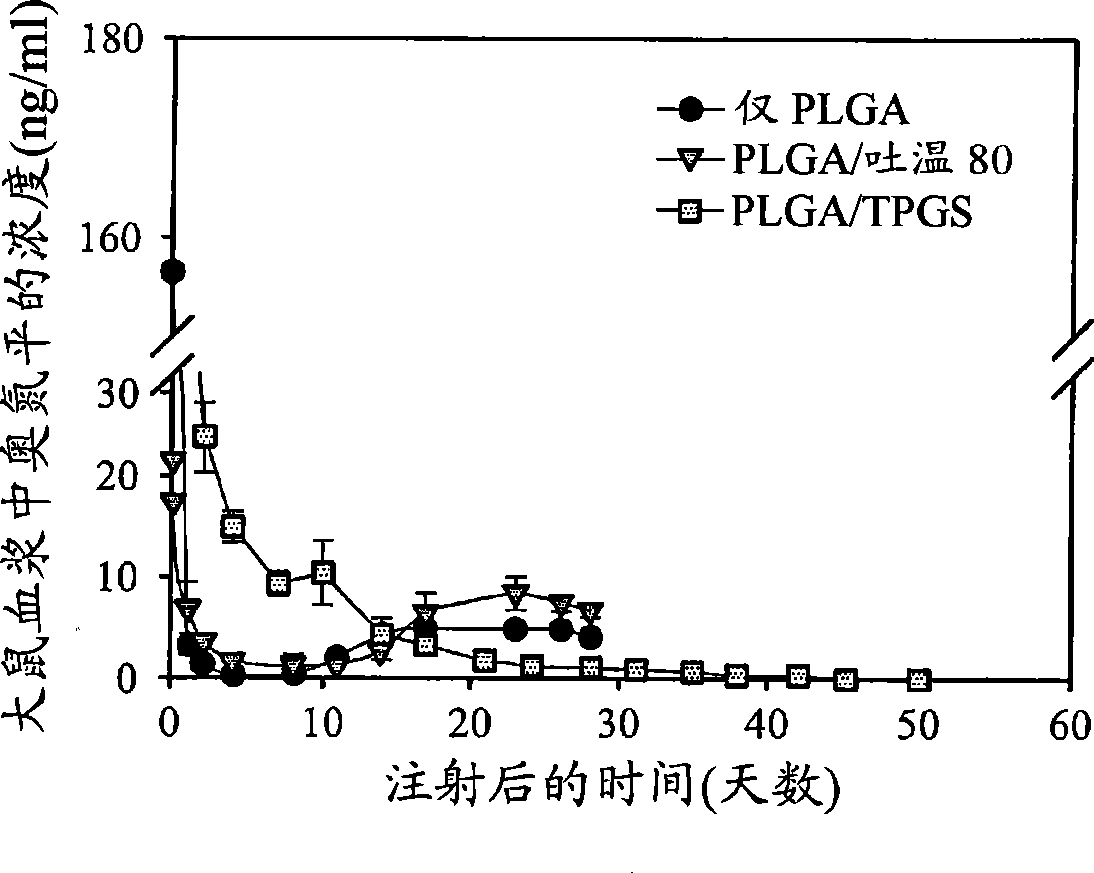
A medicinal composition for inhalation containing a continuous-release type prodrug of an EP2 agonist topically exhibits a prolonged bronchodilating and antiinflammatory effects. Namely, the medicinal composition for inhalation containing a continuous-release type prodrug of an EP2 agonist is useful as a safe preventive and / or a remedy for respiratory diseases (for example, asthma, pulmonary injury, pulmonary fibrosis, pulmonary emphysema, bronchitis, chronic obstructive pulmonary disease, adult respiratory distress syndrome, cystic fibrosis, pulmonary hypertension or the like) without causing any systemic effect such as lowering blood pressure. Thus, a safe and useful remedy for respiratory diseases is provided.
Owner:ONO PHARMA CO LTD
Sustained-release composition and method for preparing the same
ActiveCN101467959APharmaceutical delivery mechanismPharmaceutical non-active ingredientsControlled releaseActive agent
The present invention provides a continuous-release composition and a preparing method thereof. The continuous-release composition comprises a polymer, a bioactivator and a releasing rate control agent, wherein the releasing rate control agent is dispersed into the continuous-release composition for controlling the releasing of bioactivator. The preparing method of continuous-release composition comprises the following steps: providing an oil phase which comprises the bioactivator, the polymer and the releasing rate control agent; providing an aqueous phase which comprises a surfactant; and mixing the oil phase and aqueous phase for forming the continuous-release composition with release control function.
Owner:IND TECH RES INST
Risperidone or paliperidone implant formulation
The present invention is directed to an injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is risperidone and / or paliperidone or any pharmaceutically acceptable salt thereof in any combination, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and a DMSO solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 4 weeks and wherein the composition has a pharmacokinetic profile in vivo that makes it suitable to be administered each 4 weeks or even longer periods.
Owner:LAB FARM ROVI SA
Long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres, and preparation method thereof
InactiveCN102302458ASmall particle size distributionFlat surfaceAntibacterial agentsOrganic active ingredientsWound dressingMicrosphere
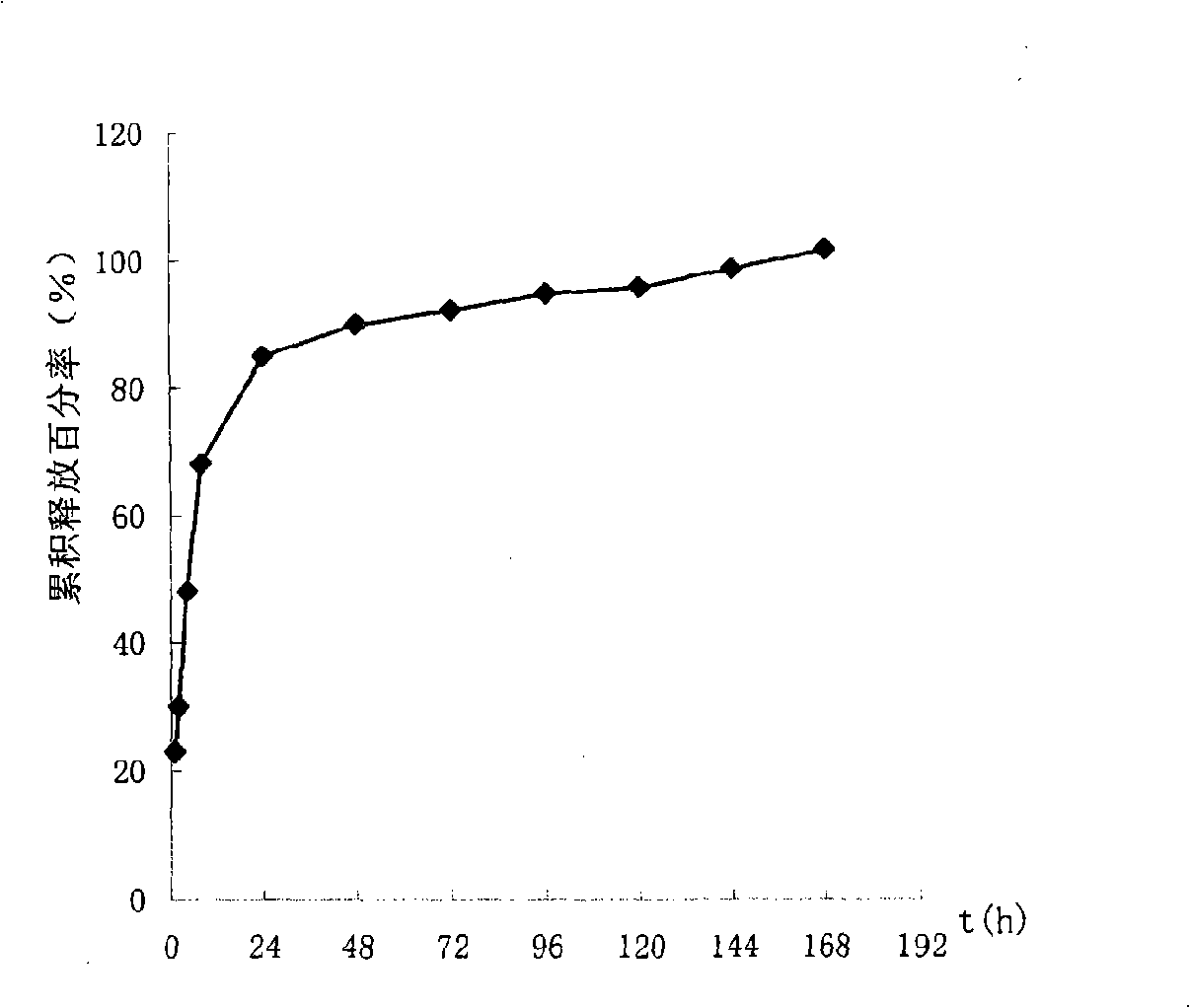
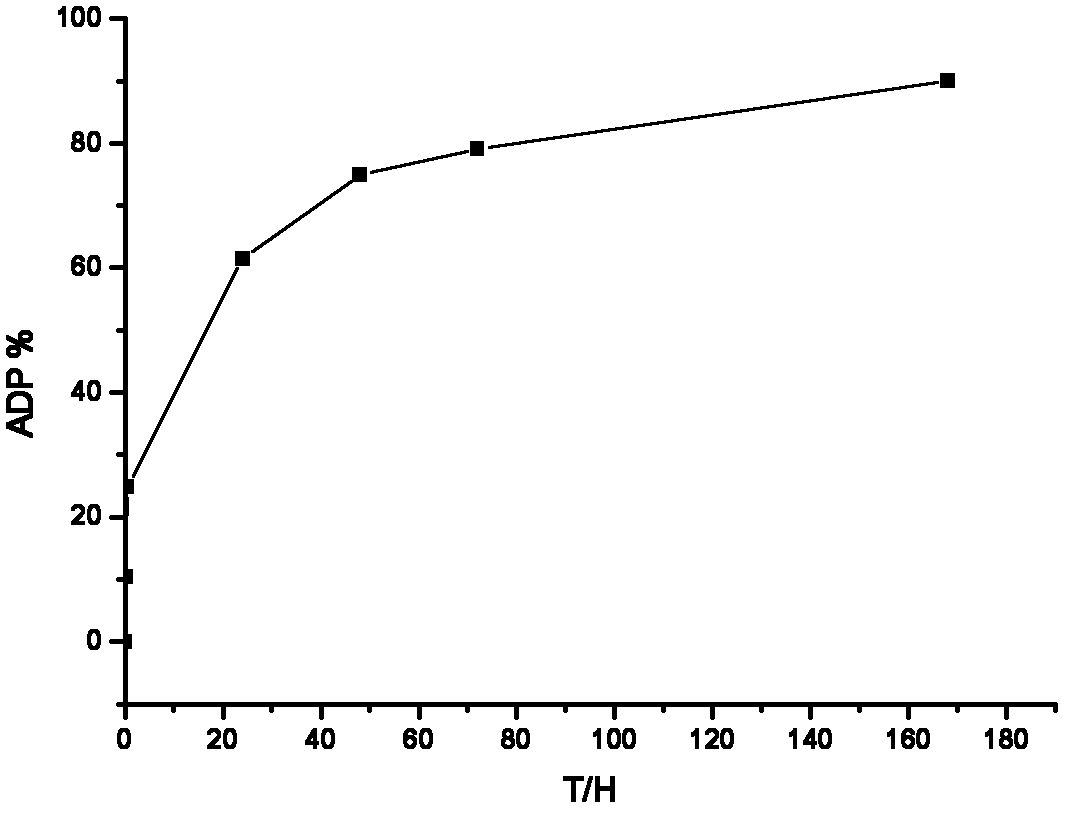
The invention discloses a long-acting sustained-release wound dressing containing levofloxacin sustained-release microspheres. According to the preparation method of the wound dressing, levofloxacin sustained-release microspheres with grain diameter between 10 and 20mu m are fixed in a composite medical non-woven fabric which mainly comprises chitosan fiber, alginate fiber, viscose fiber, and hydrophobic ethylene propylene fiber; and the long-acting sustained-release wound dressing is prepared by taking the levofloxacin sustained-release microspheres as a medicinal component. In a use process, the medicament quickly release levofloxacin after contacting the blood, tissues and surrounding skin of the wound, and can continuously release levofloxacin for a long time, and the effective release of levofloxacin accumulatively reaches 168h. The long-acting sustained-release wound dressing is applied to treatment of bacterium infection of burnt and scalded skin, and the microsphere state medicament can remarkably improve the bioavailability of effective components of the medicament; and the long-acting sustained-release wound dressing has excellent moisture penetrability, hygroscopicity, mechanical tensile property and biocompatibility, the frequency of dressing change can be reduced, and the curative effect and administration safety cam be enhanced.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
Coalbed methane high-power pulse fracturing developing device
InactiveCN101737028AThe role and effect are obviousQuick extensionFluid removalTime delaysEngineering
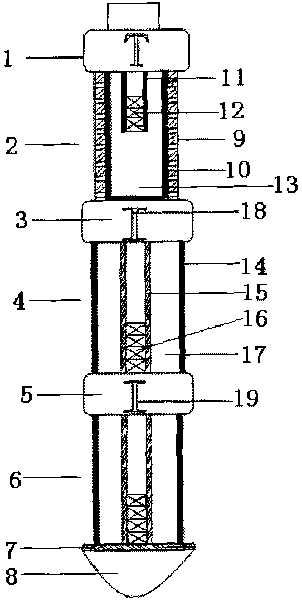
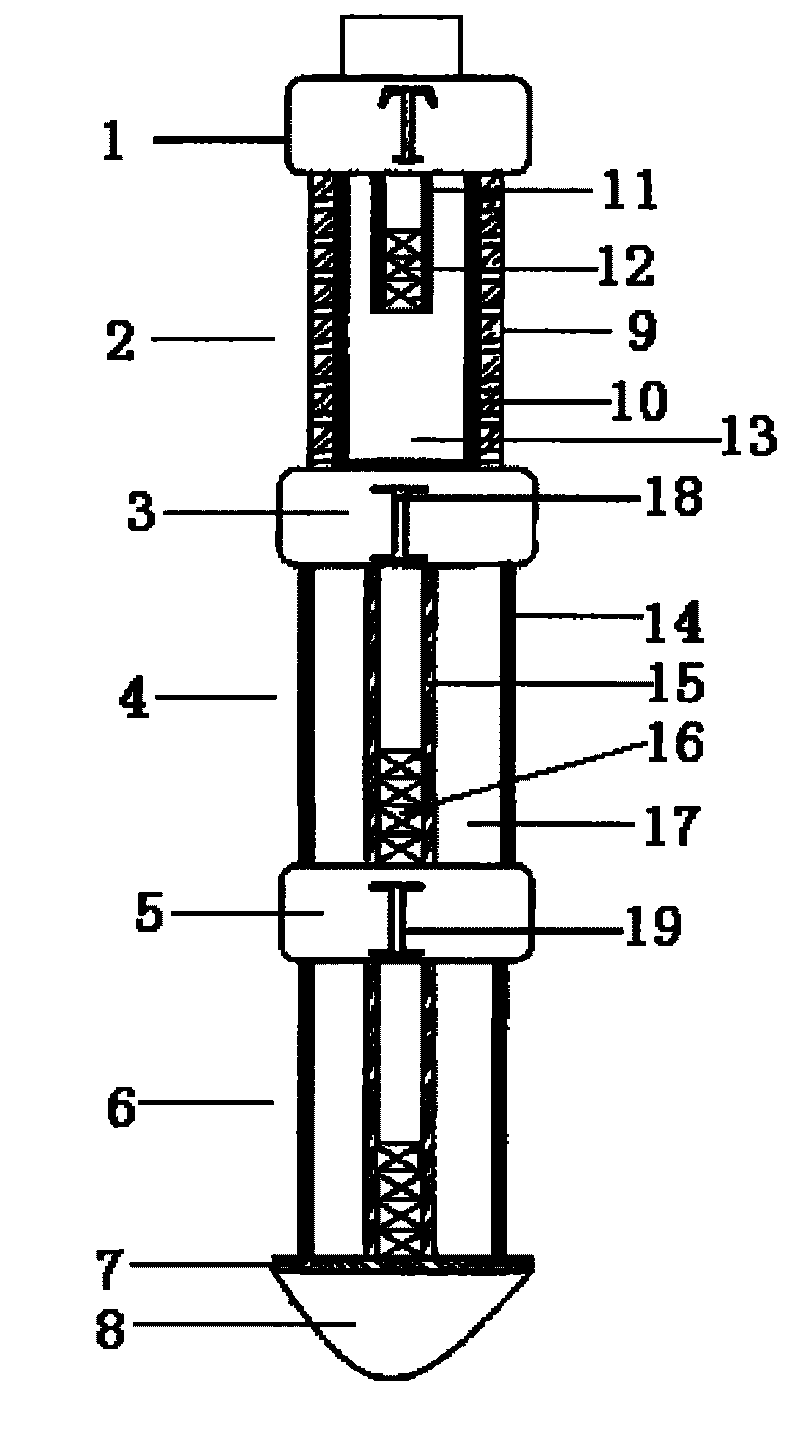
The invention relates to a coalbed methane high-power pulse fracturing developing device which comprises an explosion initiating device 1, wherein the explosion initiating device 1 is connected with a high pressure gas gun device 2; the high pressure gas gun device 2 is connected with a first bearing-type gas device 4 by a first switching connecting piece 3; the first bearing-type gas device 4 is connected with a second bearing-type gas device 6 by a second switching connecting piece 5; the end of the second bearing-type gas device 6 is provided with a combustible guide body 8; a combustible baffle 7 is positioned between the second bearing-type gas device 6 and the combustible guide body 8; fracturing explosive matching composition with higher burning speed and lower burning speed is adopted by the bearing-type gas devices which are controlled by time delay igniters inside the switching connecting pieces; and multi-stage high power pulse pressure is formed by continuously releasing high-temperature and high-pressure gas energy, and continuous fracturing is carried out on the coal stratum for a plurality of times, so that the fracture of the coal stratum is promoted to rapidly extend and expand, a longer multi-crack system is generated and formed, and the aim of increasing the yield of a coalbed methane well can be achieved.
Owner:XI'AN PETROLEUM UNIVERSITY
Method and system with contact lens product for treating and preventing adverse eye conditions
ActiveUS8440217B1Effective controlEffectively controlling distribution and dosagePowder deliveryEye implantsDrug releaseContinuous release
A contact lens product, a method and system for forming the contact lens product, and a method of using the contact lens product. The contact lens product includes a soft disposable contact lens loaded with a drug and the carriers which carry the drug. The lens has a mechanical and optical structure formed by the core polymer included within the lens. The contact lens product is configured to have the drug released from its carrier continuously into an eye of a mammal while the contact lens product is adhered to the eye of the mammal during a continuous period of time, the drug being configured to treat or prevent at least one adverse condition of the eye of the mammal during the continuous period of time. The mammal may be a human being or a veterinary animal.
Owner:EL NAGGAR MAWAHEB M +1
Use of a matrix for orally administering sustained release magnesium, and composition containing said matrix
A tablet for oral administration comprises a matrix of progressive and continuous released magnesium. For the administration of 90 to 110 parts by weight of magnesium, the matrix comprises 180 to 190 parts by weight of hydroxypropylmethylcellulose, 19.8 to 22.2 parts by weight of glyceryl behenate, 10 to 12 parts by weight of lactose and 10 to 12 parts by weight of colloidal silica. A non-enteric protective coating that slows down the gastric dissolution of the magnesium may comprise 15 to 75 parts by weight of shellac, cellulose ether or a mixture thereof. The tablet may be administered to patients in need thereof.
Owner:JOANNY FABIENNE
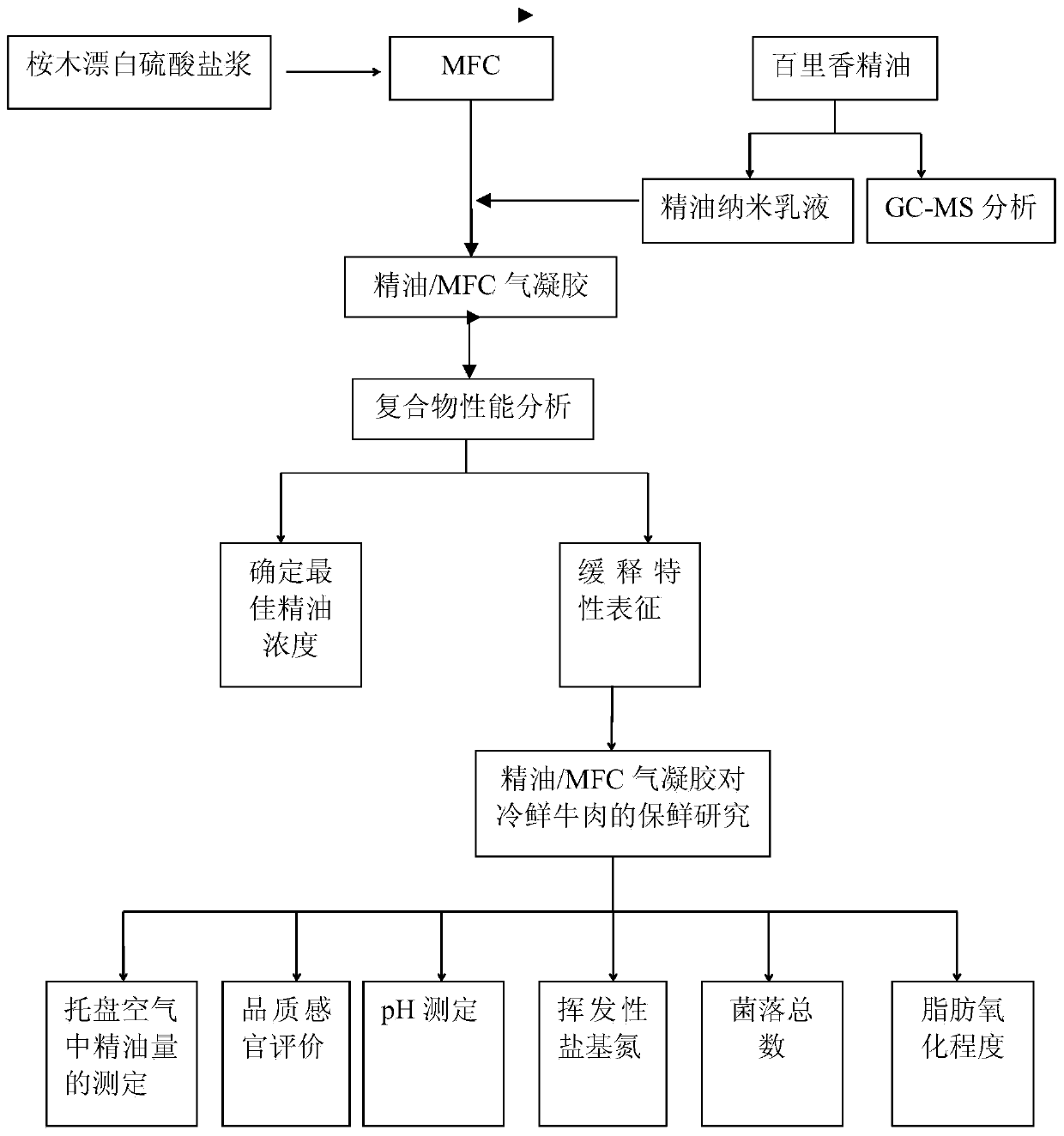
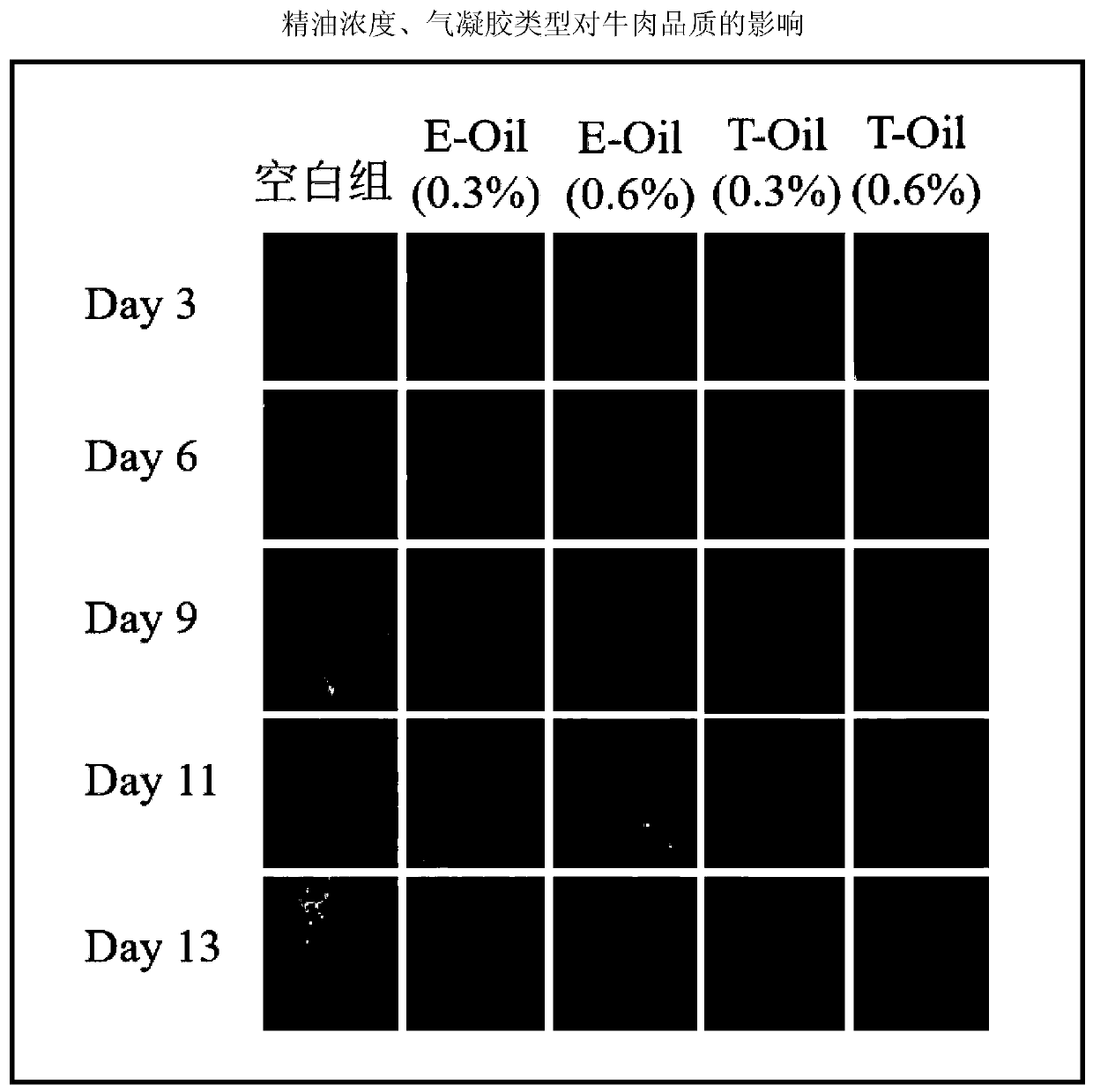
Preparation method of plant-source slow-release antibacterial aerogel
The invention relates to a preparation method of a plant-source slow-release antibacterial aerogel. The preparation method comprises following steps: an eucalyptus wood bleached sulfate pulp is takenas a raw material to prepare microfibrillated cellulose MFC, and ultrasonic emulsification technology is adopted to prepare thyme essential oil nanometer emulsion; a MFC water suspension and the thymeessential oil nanometer emulsion are combined and mixed to be uniform at a certain ratio, and freeze-drying is carried out so as to obtain the essential oil / MFC aerogel. The MFC aerogel is high in porosity, ultra low in density, and excellent in adsorption performance, so that absorption of the high-efficiency essential oil with broad-spectrum antibacterial performance by the internal part of theaerogel is realized, essential long term continuous release is realized, the concentration can be maintained at a certain range in a long time period, the plant-source slow-release antibacterial aerogel can be used in refrigerated food antibacterial package, the preparation method is simple and convenient, is economical, and is friendly to the environment, and refrigerated food service life can be prolonged effectively.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Device for dispensing granular products
InactiveUS20100084420A1Easy selectionSuccessive can be facilitatedSmall article dispensingRacksContinuous releaseBiomedical engineering
Dispensers for successive release of tablet-like or pill-like products, such as for instance sweets, sweeteners, medication, lozenges and so on are already known. A drawback of the known dispenser is that only tablet products of the same shape can be successively dispensed. The invention relates to an improved device for dispensing such products.
Owner:INDEL ONTWERPBUREAU HSM
Release liner for label laminate
InactiveUS20120231197A1Simple methodDielectric heatingInduction heatingContinuous releaseElectromagnetic induction
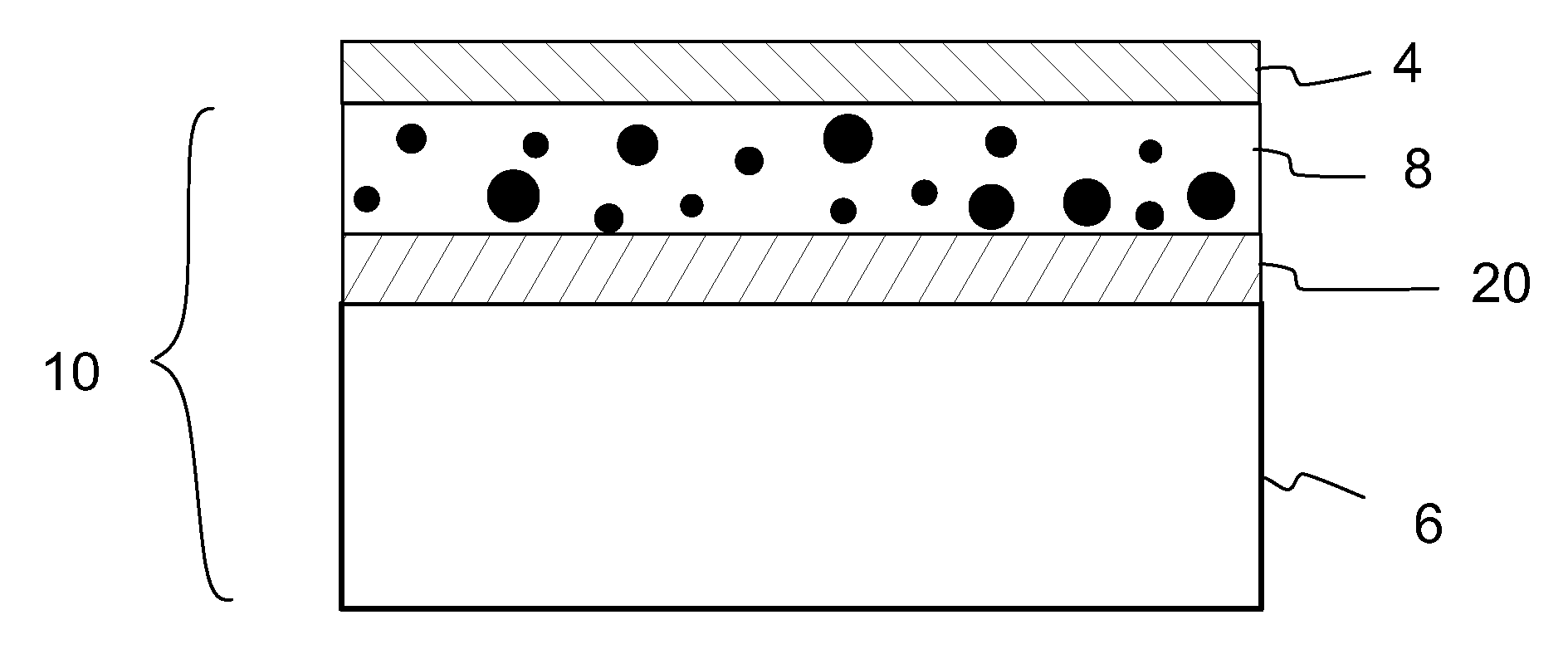
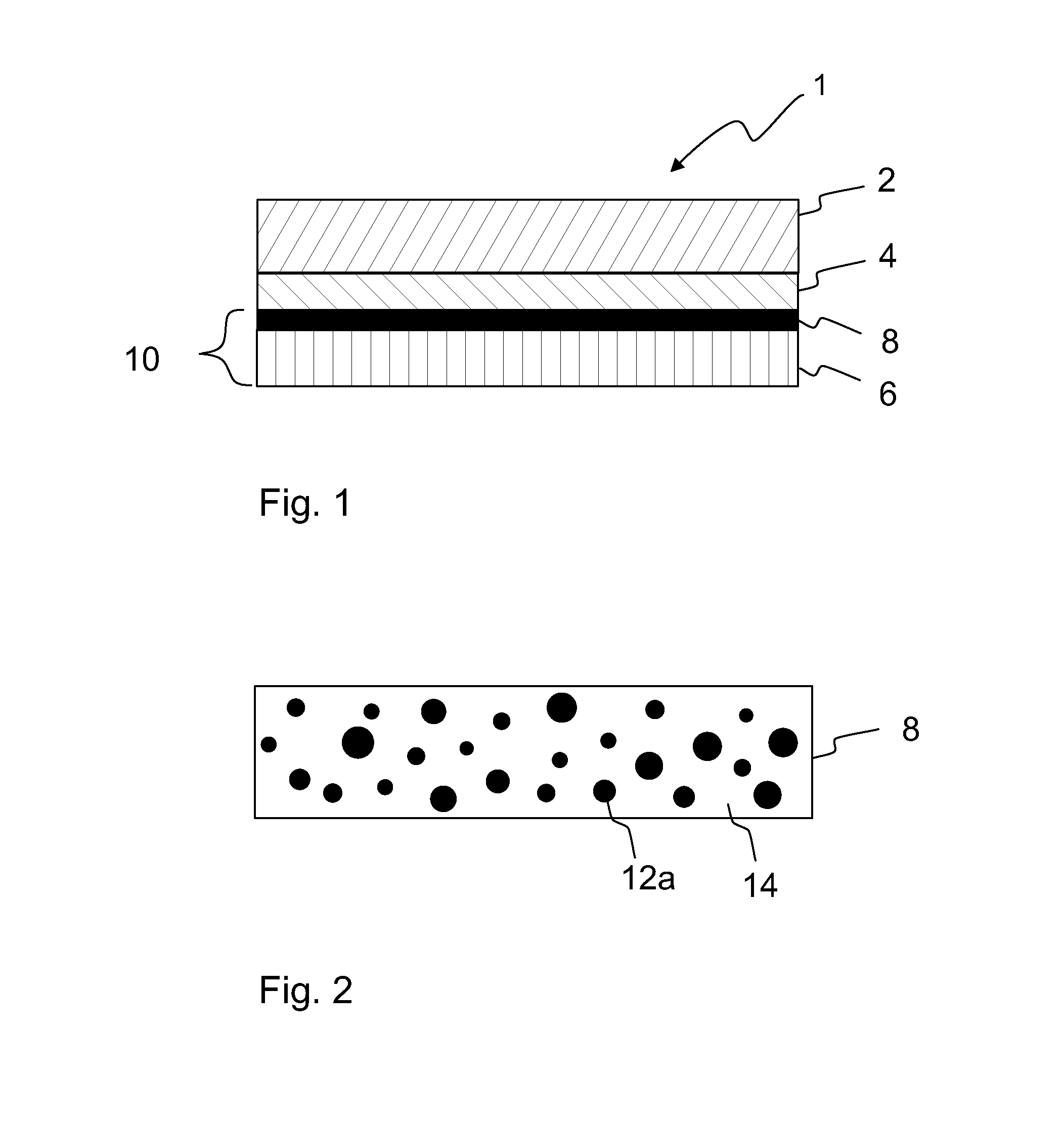
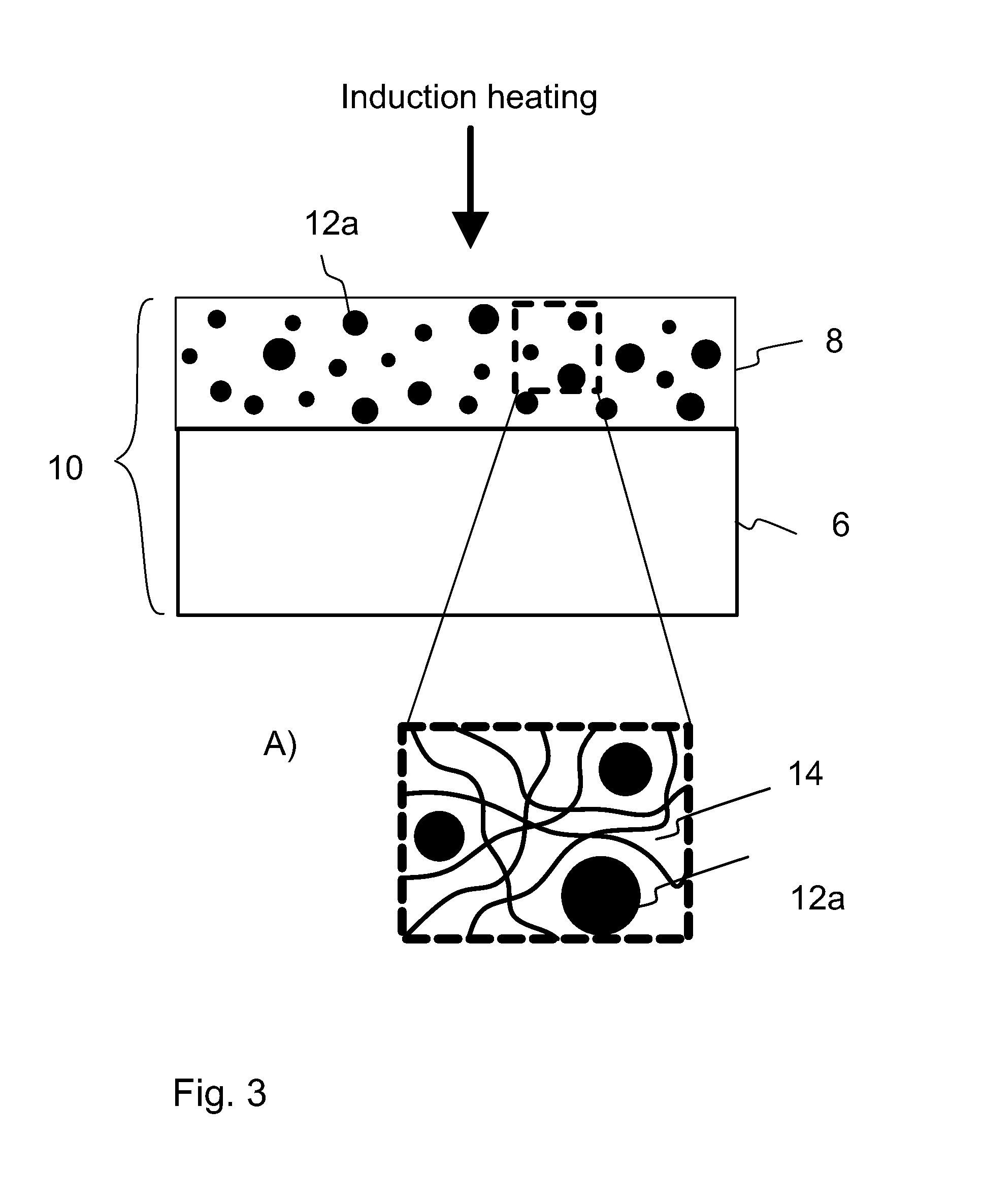
A method of preparing a release layer of a release liner for a label laminate. A substantially continuous release layer with controlled thickness is formed on a liner substrate from a composition including at least a release agent and an inductive filler material. The release layer is heated to cure the release agent. The heating is at least partly based on electromagnetic induction heating of the inductive filler material. Also a release liner and a label laminate.
Owner:UPM RAFLATEC OY
Methods for culturing undifferentiated cells using sustained release compositions
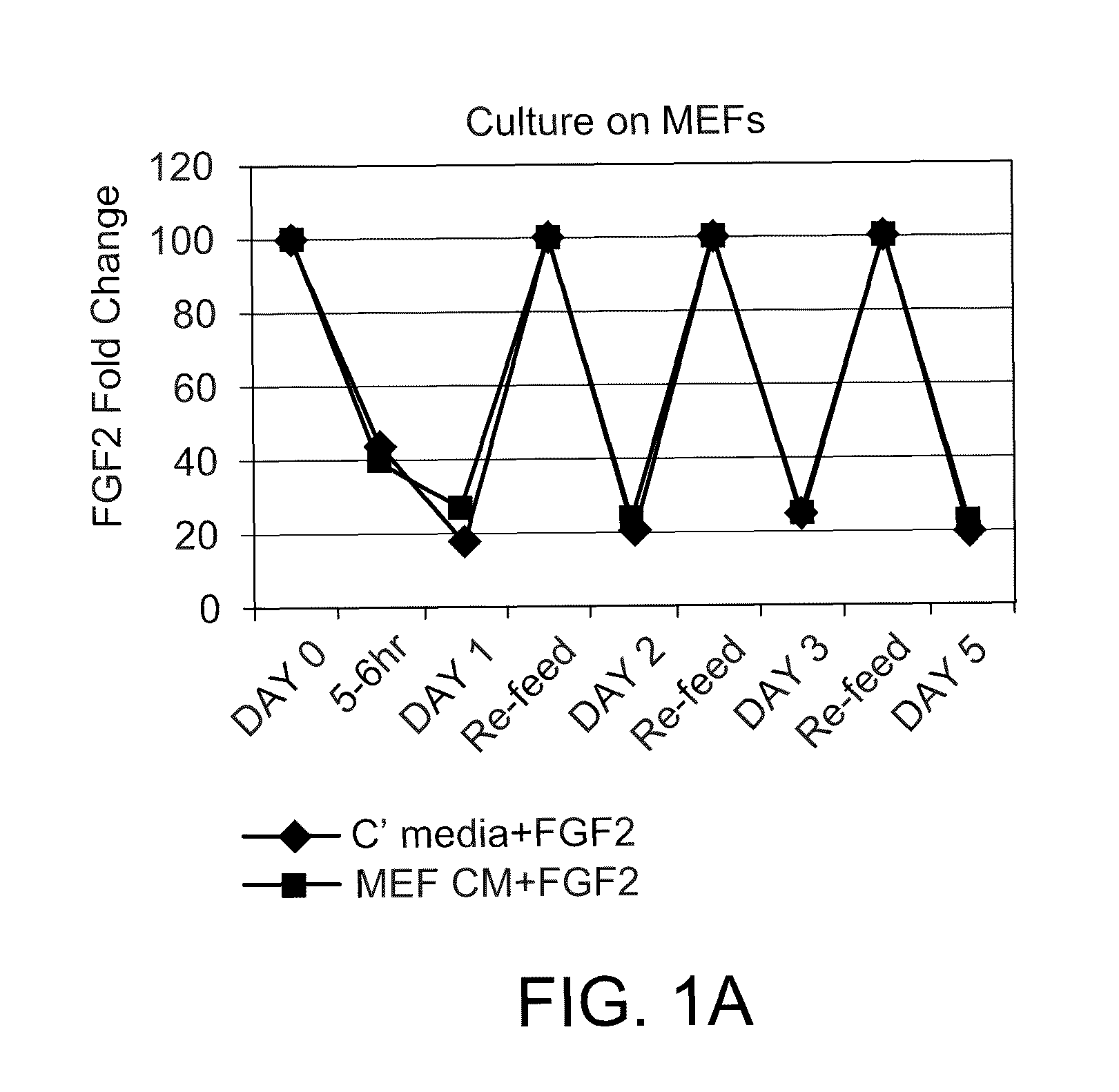
ActiveUS8481308B2Reduces and eliminates their spontaneous differentiationReduce expensesCulture processNervous system cellsProgenitorContinuous release
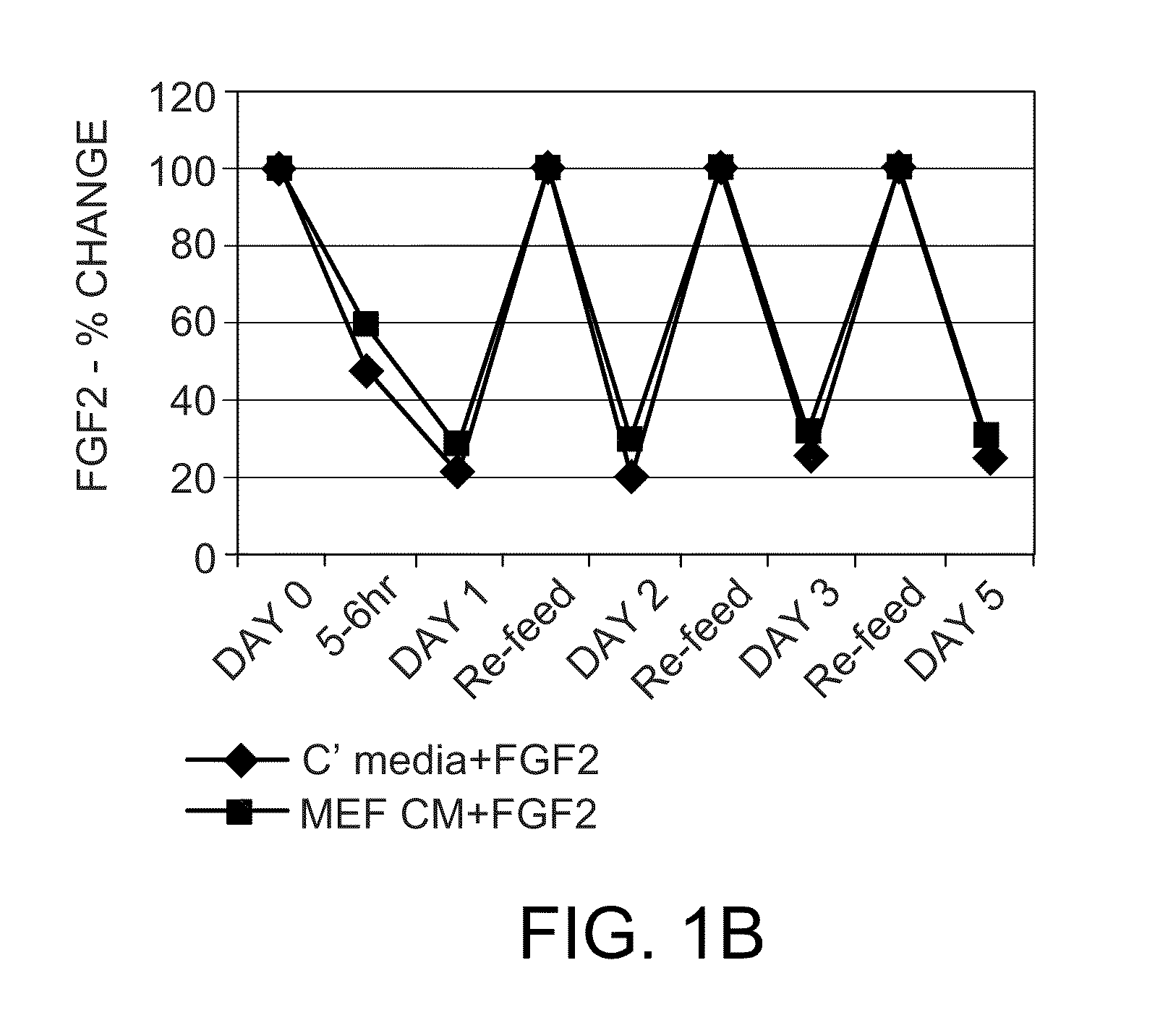
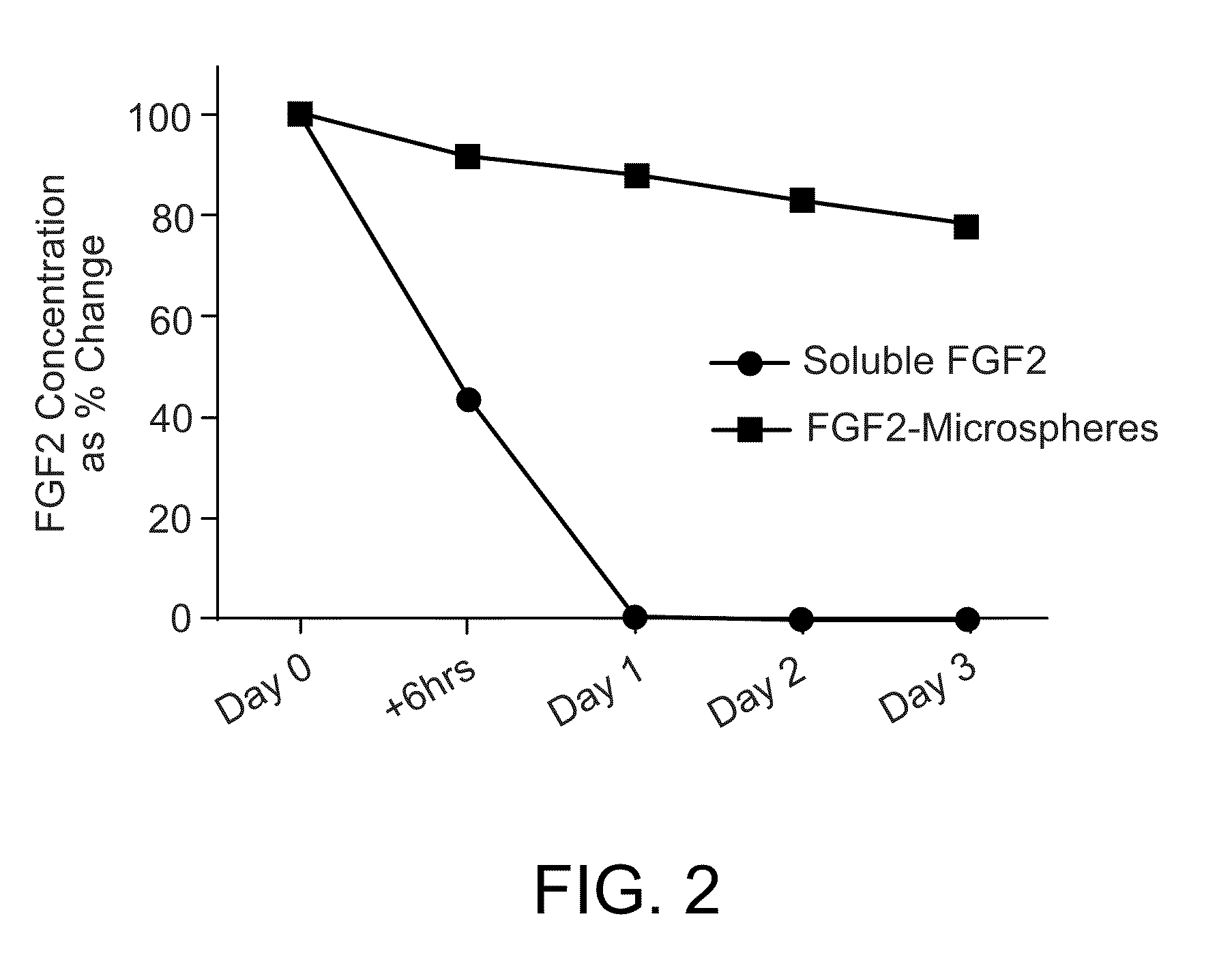
Methods for culturing undifferentiated mammalian cells, such as stem and progenitor cells, are provided. The methods involve incubating the cell in the presence of a sustained release composition containing at least one growth factor, wherein the sustained release composition continuously releases the growth factor(s), and wherein the presence of the sustained level of growth factor maintains the cell in an undifferentiated state.
Owner:REGENERATIVE RES FOUND
Negative ion texture paint
InactiveCN104497756ALow VOC (i.e. Volatile Organic Compound) contentReduce contentPolyurea/polyurethane coatingsEpoxy resin coatingsLacquerIon release
The invention relates to the technical field of functional building paint and provides negative ion texture paint. The negative ion texture paint is prepared from 20-40 parts by weight of negative ion basic paint, 60-80 parts by weight of quartz sand and 5-20 parts by weight of a thickening agent aqueous solution by mixing. The thickening agent aqueous solution contains 0.5-10 parts of solids and contains at least one of hydroxyethyl cellulose, an alkali swollen acrylic acid thickening agent and sodium-based bentonite. Through use of the negative ion additive, the coating film has a negative ion continuous-release function. The preparation method is simple and convenient and is conducive to reduction of an industrial production cost. The negative ion texture paint has low VOC (volatile organic compound) content, can greatly reduce environmental pollution, is environmentally friendly, has good negative ion release performances and decorativeness, is especially suitable for occasions such as residential buildings, commercial buildings and common buildings and has a wide application prospect.
Owner:ZIGONG INNOVATION CENT OF ZHEJIANG UNIV
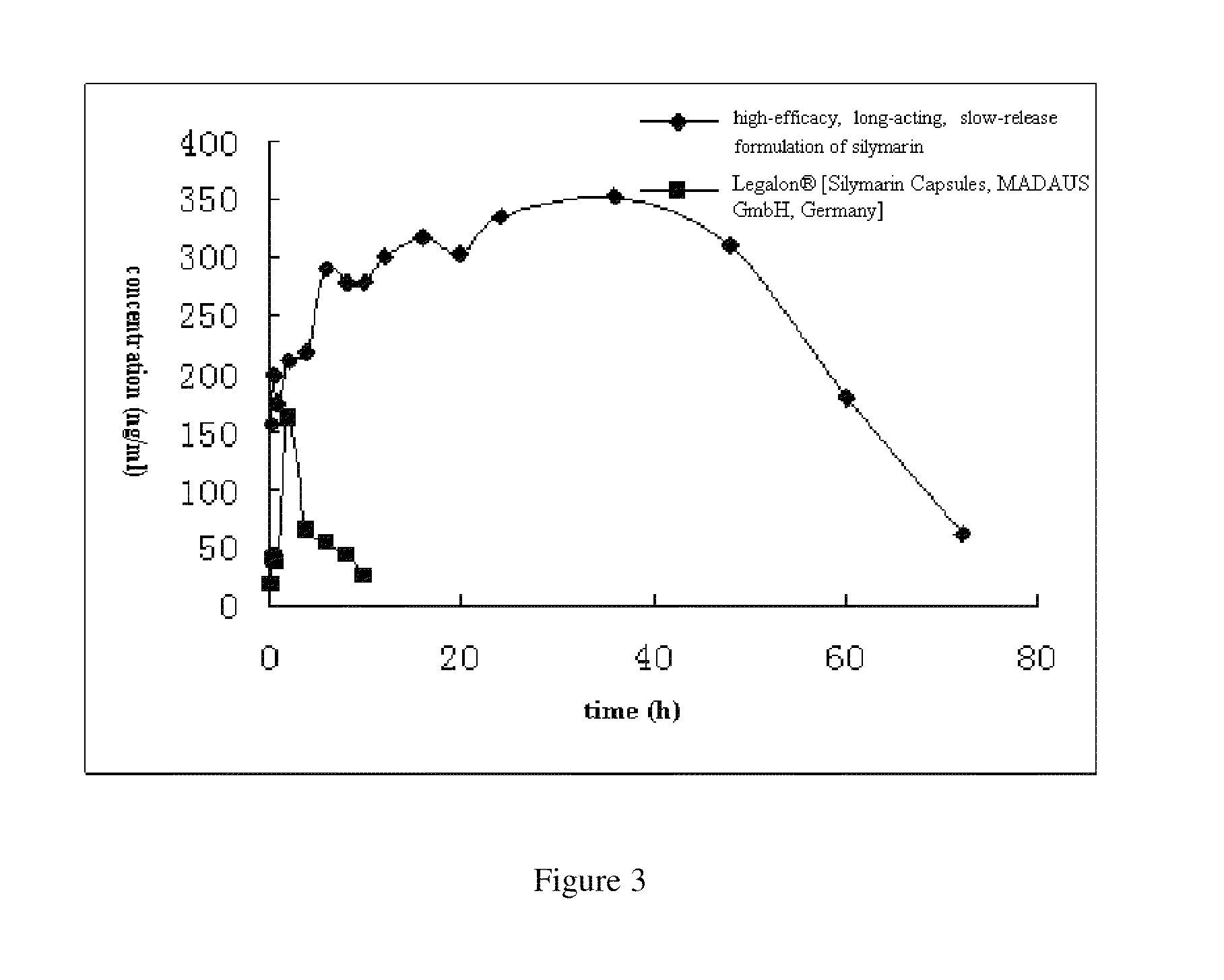
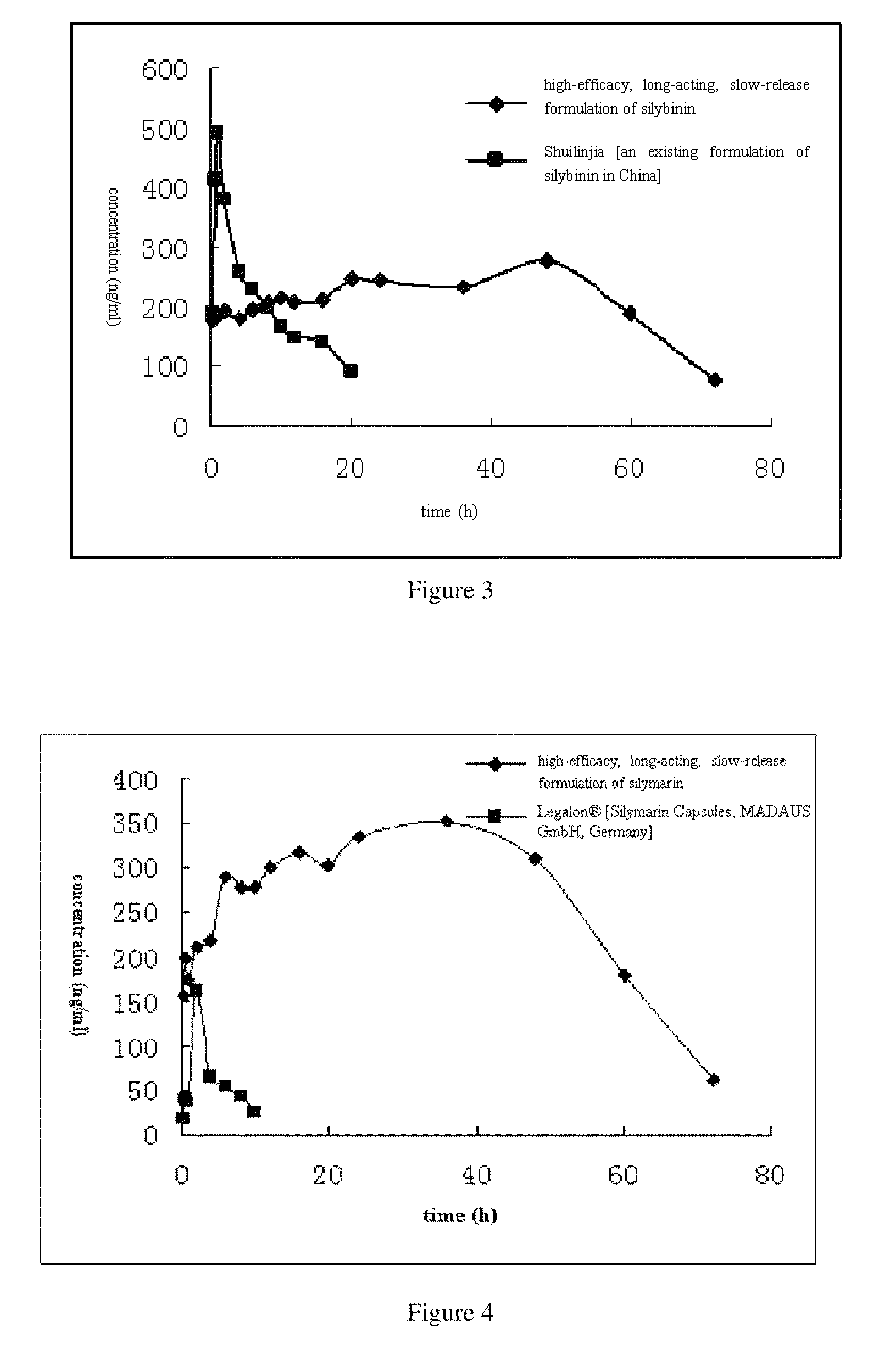
formulation of silymarin with high efficacy and prolonged action and the preparation method thereof
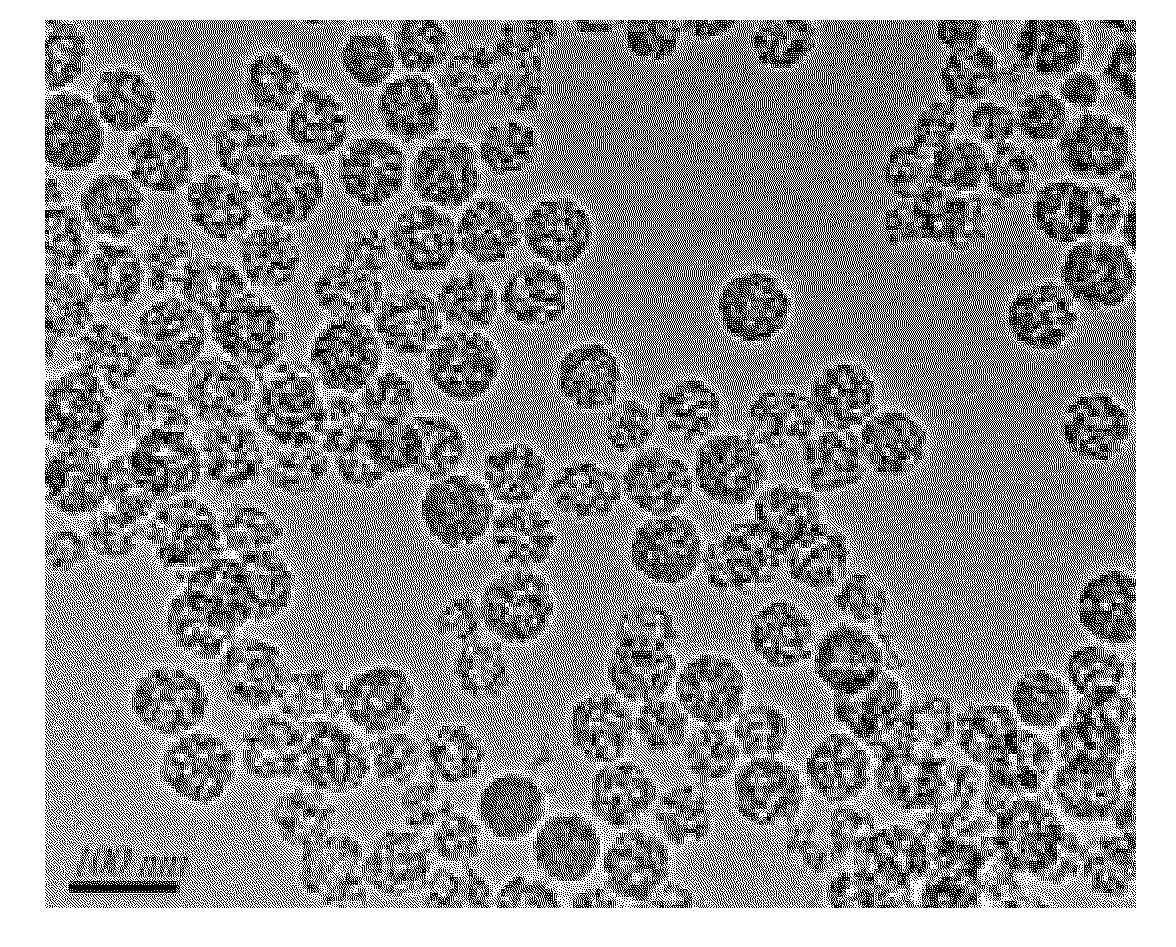
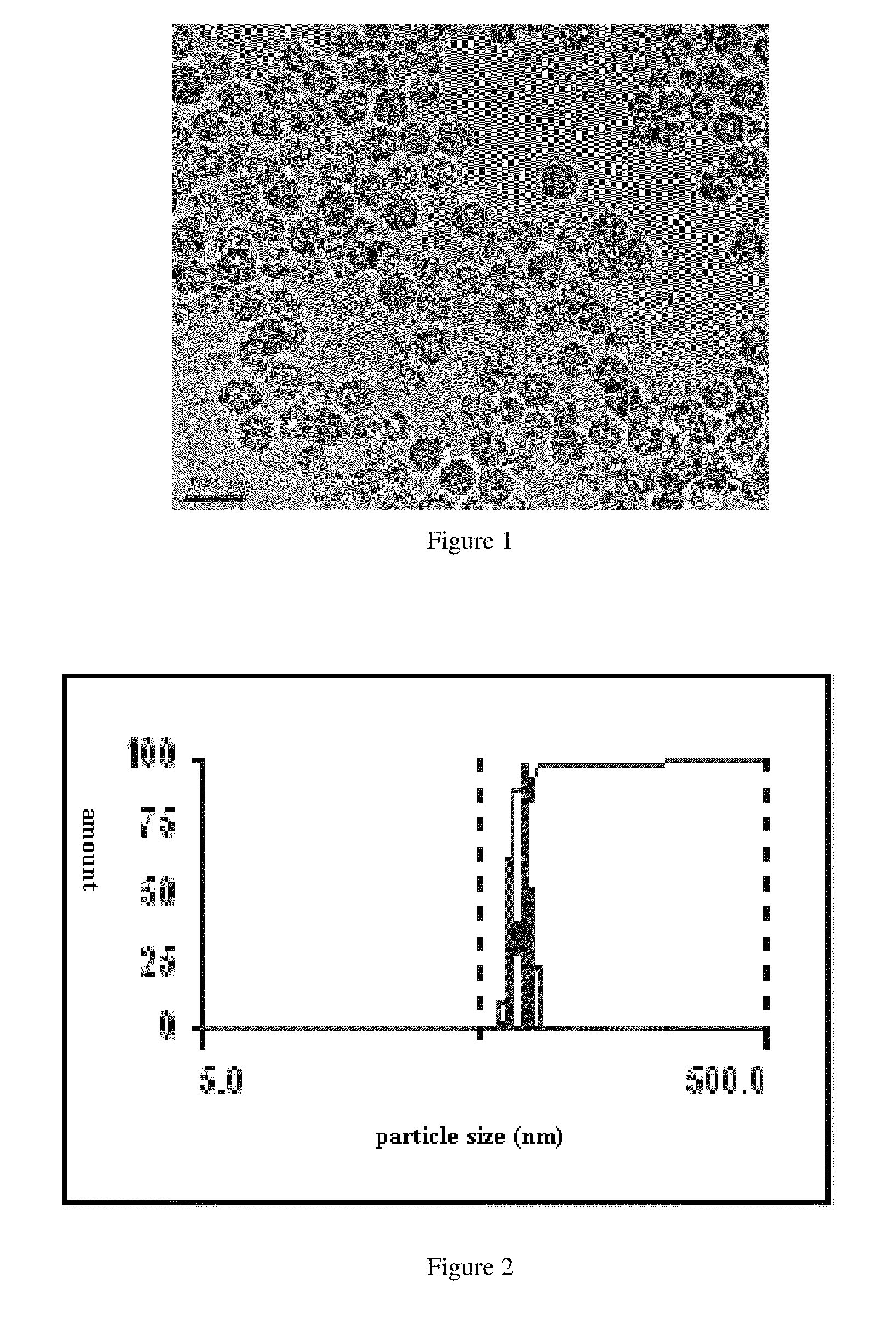
ActiveUS20110201680A1Promote absorptionLarge specific surface areaPowder deliveryBiocideAcrylic resinHalf-life
A high-efficacy, long-acting formulation of silymarin, comprising silymarin solid dispersion, silymarin-loaded silica nanoparticles, slow-release matrix material and release enhancer, wherein the mass ratio of these components is silymarin solid dispersion:silymarin-loaded silica nanoparticles:slow-release matrix material:release enhancer=1:0.5˜1.25:0.1˜0.3:0.1˜0.3; the drug loading rate of the said silymarin-loaded silica nanoparticles is 51.95%-52.87%; the said silymarin solid dispersion contains povidone K30, soybean lecithin and acrylic resin IV, and the mass ratio between silymarin and other medical accessories in silymarin solid dispersion is silymarin:povidone K30:soybean lecithin:acrylic resin IV=1:1˜3:0.3˜0.8:0.2˜0.5. Compared with the existing formulations, the half life of the high-efficacy, long-acting formulation of silymarin disclosed in this invention is 2.3 times longer while the mean residence time (MRT) of which is 9.94 times longer; when tested in vivo in Beagle dogs, this new formulation of silymarin presents a smoother concentration-time curve and reaches a continuous release for 72 hours. This invention discloses its preparation method.
Owner:JIANGSU UNIV
Highly efficient and long-acting slow-release formulation of poorly soluble drugs and preparation method thereof
ActiveUS20110250269A1Promote absorptionLarge specific surface areaBiocidePowder deliverySilica nanoparticlesHalf-life
A high-efficacy, long-acting, slow-release formulation of the poorly soluble drug, comprising solid dispersion of the poorly soluble drug, silica nanoparticles loaded with the poorly soluble drug, matrix material, and release enhancer, wherein the mass ratio of these components is solid dispersion of the poorly soluble drug: silica nanoparticles loaded with the poorly soluble drug: matrix material: release enhancer=1: 0.5˜1.25: 0.1˜0.3: 0.1˜0.3; the said solid dispersion of the poorly soluble drug contains povidone K30, soybean lecithin, and acrylic resin IV, wherein the mass ratio of the drug and the accessory materials is poorly soluble drug: povidone K30: soybean lecithin: acrylic resin IV=1: 1-3: 0.3˜0.8: 0.2˜0.5. Compared with the existing formulations, the in vivo half life of the high-efficacy, long-acting formulation of the poorly soluble drug disclosed in this invention is 2.3˜14.8 times longer while the mean residence time (MRT) of which is 7.94˜4.52 times longer; when tested in vivo in Beagle dogs, this new formulation of the poorly soluble drug presents a smoother concentration-time curve and reaches a continuous release for 72 hours. This invention discloses its preparation method.
Owner:JIANGSU UNIV
Slow-release tablets containing felodipine and metoprolo salt, and preparation method thereof
ActiveCN102727460ASimple processOrganic active ingredientsPharmaceutical delivery mechanismFluidized bedMedicine
The present invention provides slow-release tablets containing felodipine and a metoprolo salt, and a preparation method thereof. The slow-release tablets comprise a tablet core, a drug-containing layer, a slow-release coating layer and a film coating layer from the inside to the outside, wherein the tablet core is prepared by tableting a metoprolol salt, an insoluble slow-release framework material, and other pharmaceutically-acceptable excipients, and the drug-containing layer comprises micronized felodipine, a solubilizing agent and a binder. According to the present invention, the process is simple and feasible, the characteristic of 24-hour continuous release is provided, the direct tablet core coating manner is adopted to prepare the slow-release tablet, the production process can be completed in the traditional production workshop, and disadvantage of requirement of the fluidized bed and other equipment in the original process is overcome.
Owner:SHANDONG UNIV +1
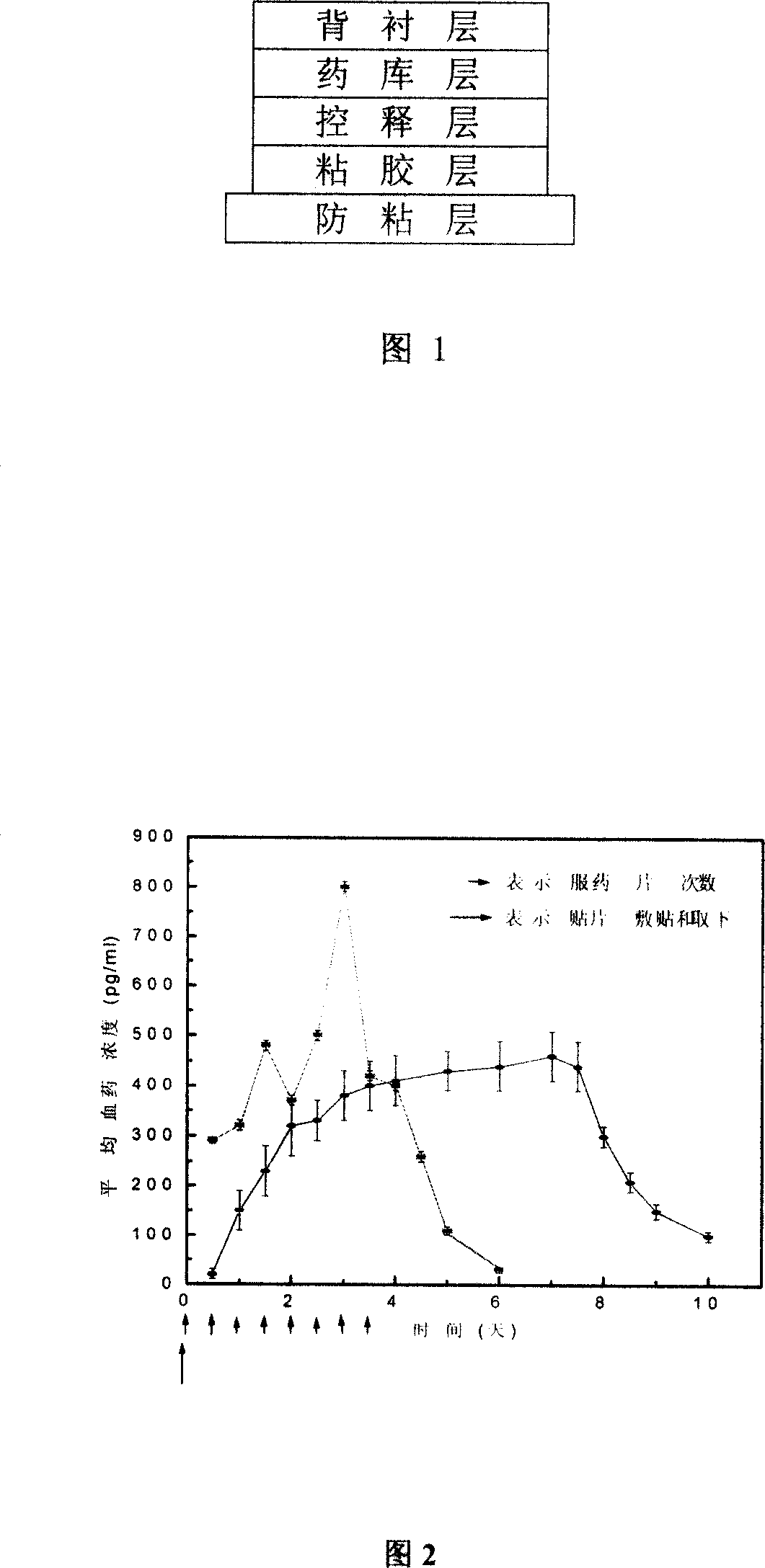
Clonidine paster for treating children's hyperkinetic symptom, twitch symptom and its preparation method
InactiveCN101011391AImprove bioavailabilityAvoid peaks and valleys in blood concentrationOrganic active ingredientsNervous disorderDrug contentControl layer
The invention relates to a cola stable paster which can treat hyperkinetic symptom of children, which comprises back liner, drug layer, release-control layer, paste layer, and anti-paste layer. The invention is characterized in that the back liner is aluminized film; the drug layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the release-control layer is modified EVA film or nuclear track porous film; the paste layer is cola stable drug and polyacrylacid ester pressure-sensitive gel; the cola stable drug content of drug layer is higher than the cola stable drug content of paste layer. The invention can avoid oral taking, with high biological utilization, stable effect, continuous release, and high safety. The invention also provides relative production.
Owner:北京克莱斯瑞控释药业有限公司
Bamboo fiber-contained non-woven fabrics containing rose essential oil capable of continuously releasing negative oxygen ion and preparation method thereof
InactiveCN106087250AEasy to useIncrease the function of absorbing odorConjugated cellulose/protein artificial filamentsConjugated synthetic polymer artificial filamentsPolyethylene glycolOxygen ions
The invention discloses a bamboo fiber-contained non-woven fabrics containing rose essential oil capable of continuously releasing negative oxygen ion and a preparation method thereof, and is characterized in that the bamboo fiber-contained non-woven fabrics comprises, by amounts by weight, the following raw materials: 4-9 parts of rose essential oil, 3-7 parts of bamboo fiber powder, 2-5 parts of wool fibre, 4-8 parts of nanometer tourmalinite powder, 4-9 parts of PAMAM solution, 5-10 parts of polylactic acid, 1-3 parts of mulberry silk, 1-2 parts of polyethylene glycol 200, 3-5 parts of active carbon, 4-8 parts of mint essence, 10-15 parts of sodium periodate solution, 50-70 parts of isotactic polypropylene, 3-6 parts of diatomite, 1-2 parts of sodium dodecanesulphonate, proper amount of sodium hydroxymethyl cellulose, and proper amount of water. The method comprises the step of adding nanometer tourmalinite powder in the non-woven fabrics to get the effect of releasing negative oxygen ion continuously. The non-woven fabrics has the advantages of calming, easing pain, releasing cough, relieving itching, raising appetite and reducing blood pressure when being used as clothes inner liner; and rose essential oil and pure natural flavoring agent are added. The fabrics is pollution-free.
Owner:安徽泽泓塑业股份有限公司
Silver containing antimicrobial fibre, fabric and wound dressing and its method of manufacturing
InactiveUS20150297411A1Durable and faster releaseLong efficiencyNon-adhesive dressingsPackage sterilisationFiberMicroorganism
The present invention relates to a silver containing antimicrobial fibre, fabric and wound dressing and its method of manufacturing. Silver ions are evenly distributed in the interior or on a surface of an antibacterial fibre structure. The silver content in the dressing is 0.01-10 weight %. As wound treatment dressing, the fibre wound dressing has a capability of continuously releasing a sufficient amount of silver, is particularly suitable for chronic wound treatment, can provide a long-term and effective antibacterial function, and can effectively prevent various bacteria or other microorganisms from infecting a wound.
Owner:FOSHAN UNITED MEDICAL TECH
Apigenin polylactic acid sustained release microsphere and preparation method thereof
ActiveCN103585113AOvercome water solubilityOvercome poor fat solubilityOrganic active ingredientsNervous disorderSolubilityBiocompatibility Testing
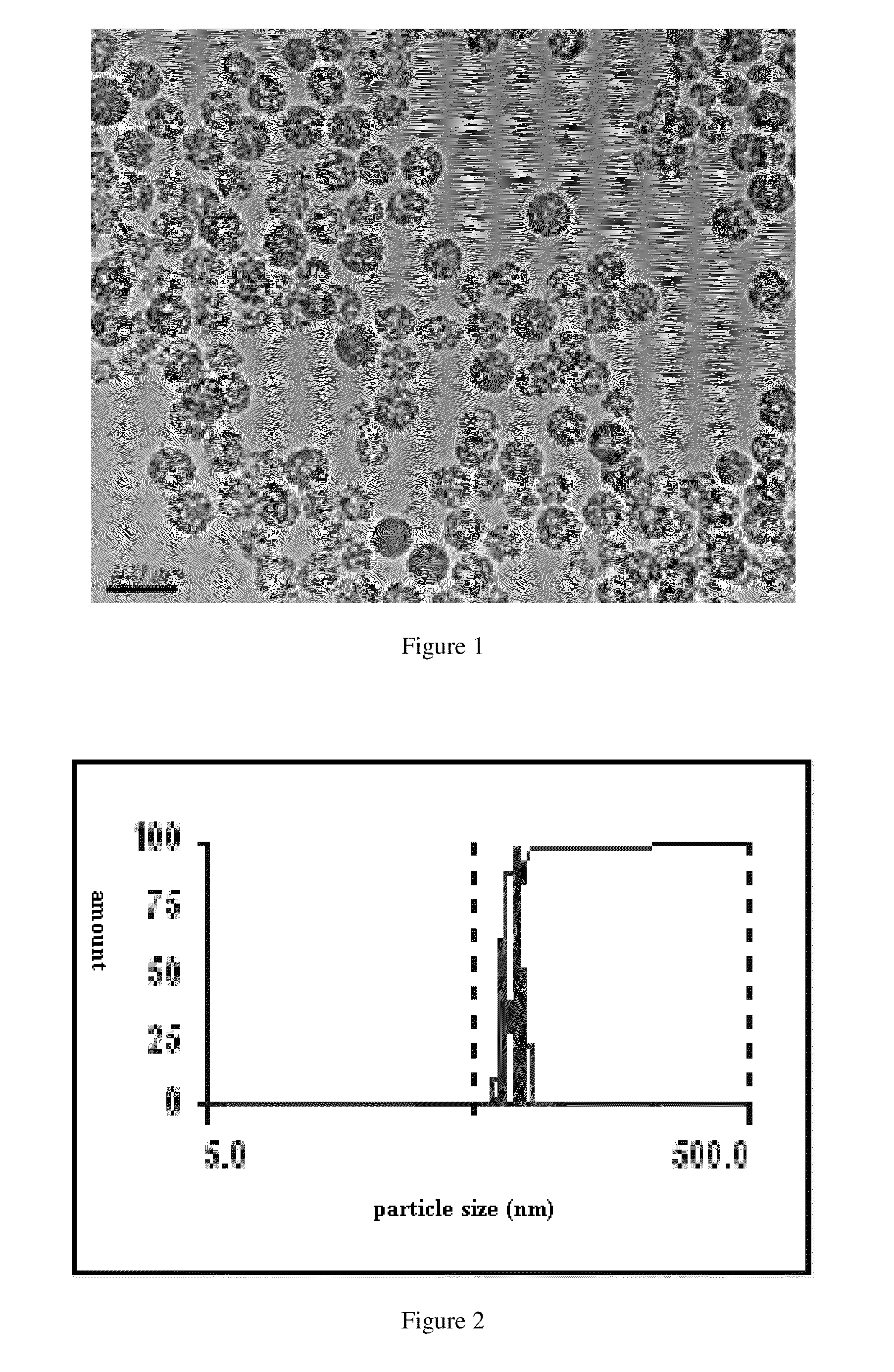
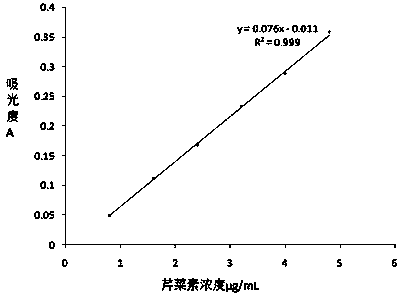
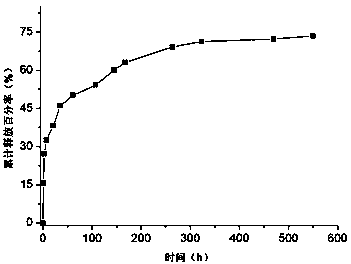
The invention discloses an apigenin polylactic acid sustained release microsphere and a preparation method of the apigenin polylactic acid sustained release microsphere. The preparation method comprises the following steps: dissolving hydroxyl-terminated racemic polylactic acid and soybean lecithin in an organic solvent; stirring the liquid, and slowly adding the apigenin mixed liquid subjected to ultrasonic treatment in an emulsifier aqueous solution to be emulsified; reducing the pressure, removing the solvent with steam, concentrating the volume; centrifuging at high speed, washing and drying the obtained mixed liquid to prepare the polylactic acid entrapped apigenin acid sustained release microsphere, wherein the microsphere particle size is 1-5 microns, the drug loading ratio is larger than 25%, the encapsulation efficiency is larger than 79%, and the sustained release time is more than 550 hours. According to the polylactic acid with good biocompatibility and biodegradability is taken as a clad material to prepare the apigenin polylactic acid sustained release microsphere, so that the microsphere is uniform in shape, smooth and adhesion-free on the surface, uniformly distributed in the particle size, and good in slow-release effect, can keep continuous release time in vitro 550 h above, conquers the defects that the drug is poor in water solubility and fat solubility, improves the oral administration availability, prolongs the drug action time and improves the drug therapeutic effect.
Owner:TAIYUAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com