Clonidine paster for treating children's hyperkinetic symptom, twitch symptom and its preparation method
A technology for children with ADHD and tics, which is applied in the directions of medical preparations with non-active ingredients, medical preparations containing active ingredients, and sheet delivery, etc., can solve problems such as restricting the treatment of children's diseases, and avoid peaks and valleys of blood drug concentration. phenomenon, improving treatment adherence, reducing impulsivity and hyperactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
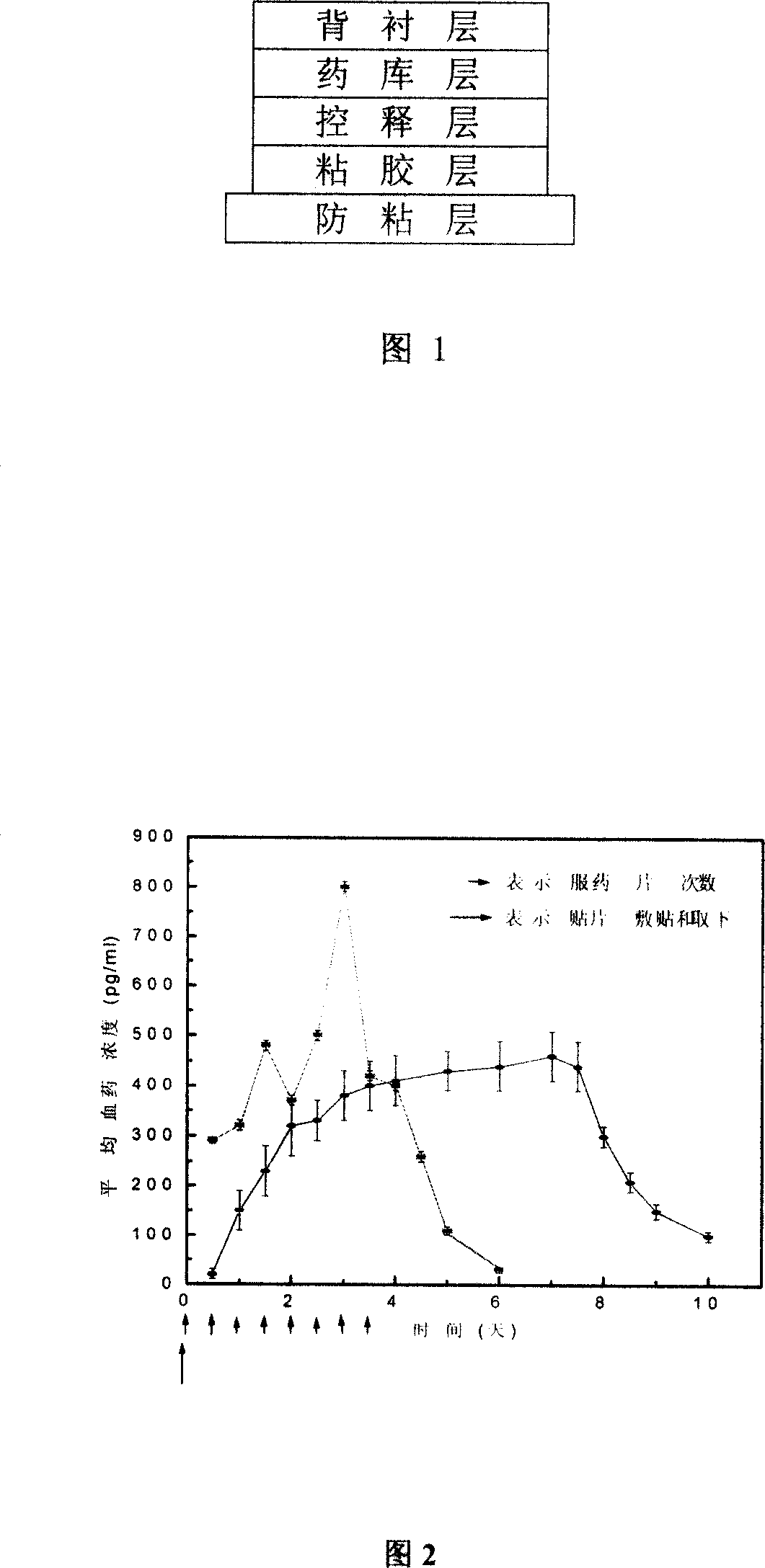
[0162] The clonidine patch for treating ADHD and tic disorder in children comprises five layers of a backing layer, a drug storehouse layer, a controlled-release layer, an adhesive layer, and an anti-adhesive layer in sequence, and is characterized in that the backing layer is an aluminized film, and the drug The reservoir layer is clonidine and polyacrylate pressure-sensitive adhesive, the release-controlling layer is modified EVA film, and the adhesive layer is clonidine and polyacrylate pressure-sensitive adhesive. Clonidine microparticles are 2 microns. The content of clonidine in the drug storehouse layer in every 1000 patches is 1.4g, the content of polyacrylate pressure-sensitive adhesive is 15g, and the content of clonidine in the sticking layer is 0.1g, and the content of polyacrylate pressure-sensitive adhesive is 15g .
[0163] The preparation method of the clonidine patch for the treatment of ADHD and tic disorder in children is characterized in that it comprises ...
Embodiment 2
[0170] The clonidine patch for treating ADHD and tic disorder in children comprises five layers of a backing layer, a drug reservoir layer, a controlled release layer, an adhesive layer, and an anti-adhesive layer in turn. The backing layer is an aluminized film, and the drug reservoir layer is Clonidine and polyacrylate pressure-sensitive adhesive, the controlled-release layer is modified EVA film, the adhesive layer is clonidine and polyacrylate pressure-sensitive adhesive, and the clonidine particle size is 30 microns. The content of clonidine in the medicine storehouse layer in every 1000 patches is 3.2g, the content of polyacrylate pressure-sensitive adhesive is 35g, and the content of clonidine in the described pasting layer is 0.3g, and the content of polyacrylate pressure-sensitive adhesive is 35g .
[0171] A preparation method of a clonidine patch for treating ADHD and tic in children.
[0172] Take the amount of preparing 1000 clonidine patches as an example to ill...
Embodiment 3
[0178] The clonidine patch for treating ADHD and tic disorder in children consists of backing layer, drug storage layer, controlled release layer, adhesive layer, and anti-adhesive layer. The backing layer is aluminized film, and the drug storage layer is clonidine and polyacrylate pressure-sensitive adhesive, the controlled-release layer is a nuclear track microporous membrane, the adhesive layer is clonidine and polyacrylate pressure-sensitive adhesive, and the clonidine particle size is 20 microns.
[0179] The content of clonidine in the drug storage layer in every 1000 patches is 2g, the content of polyacrylate pressure-sensitive adhesive is 20g, and there is also 5g of ethyl acetate in the drug storage layer. The content of clonidine in the adhesive layer is 0.2g, the content of polyacrylate pressure-sensitive adhesive is 20g, and the content of ethyl acetate is 10g.
[0180] The preparation method of the clonidine patch for the treatment of ADHD and tic disorder in chil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com