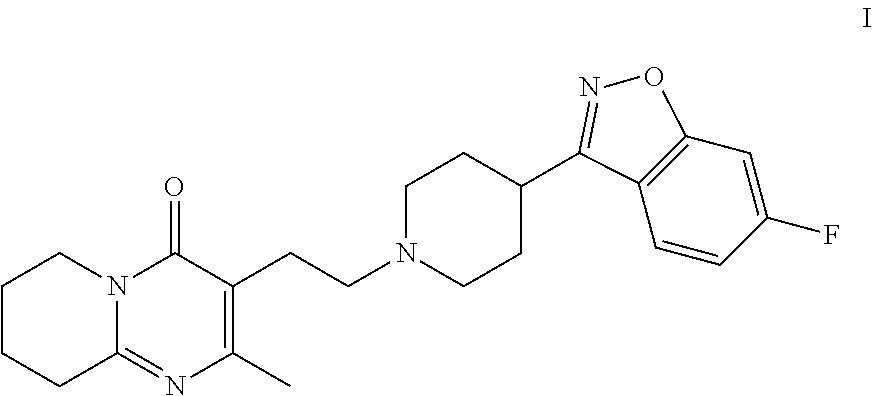
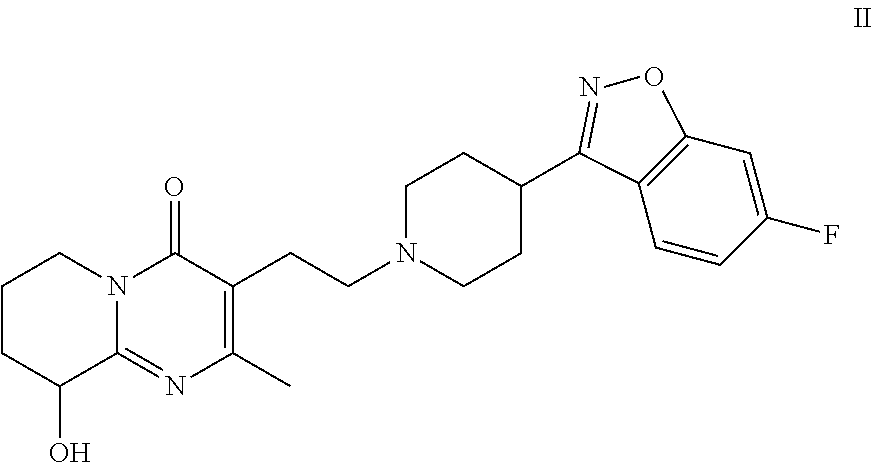
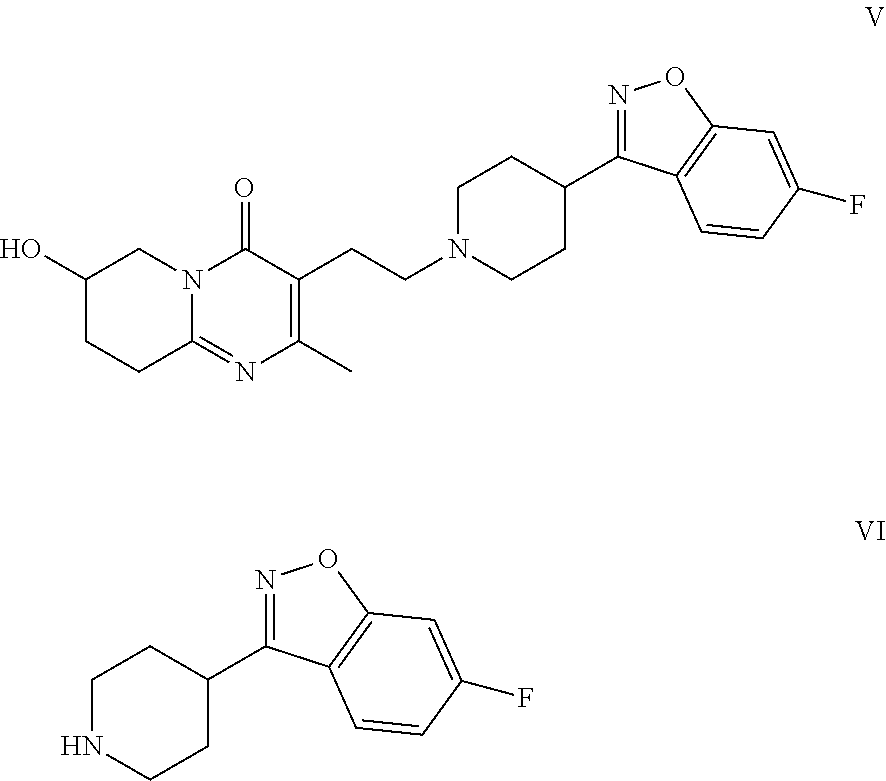
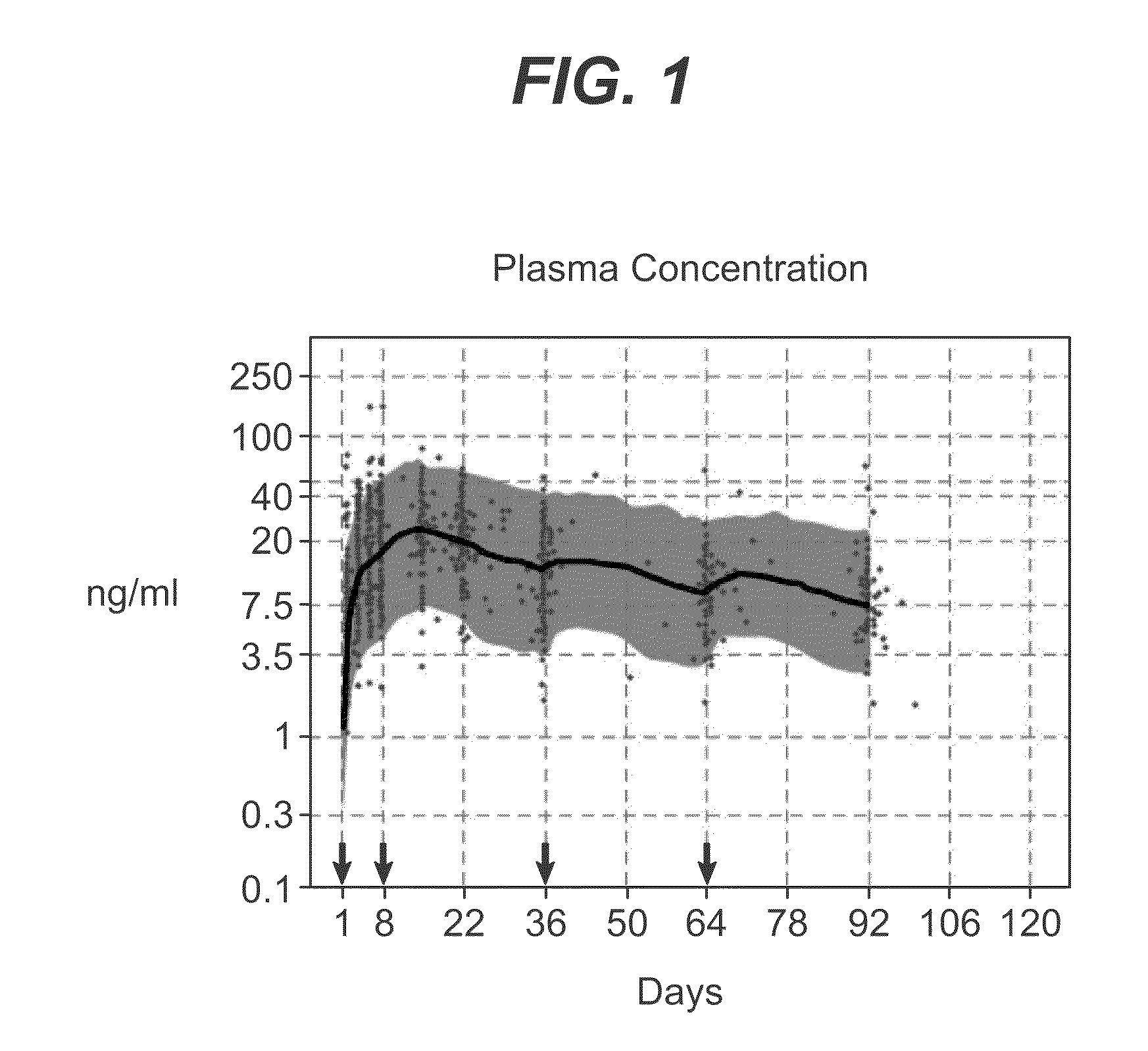
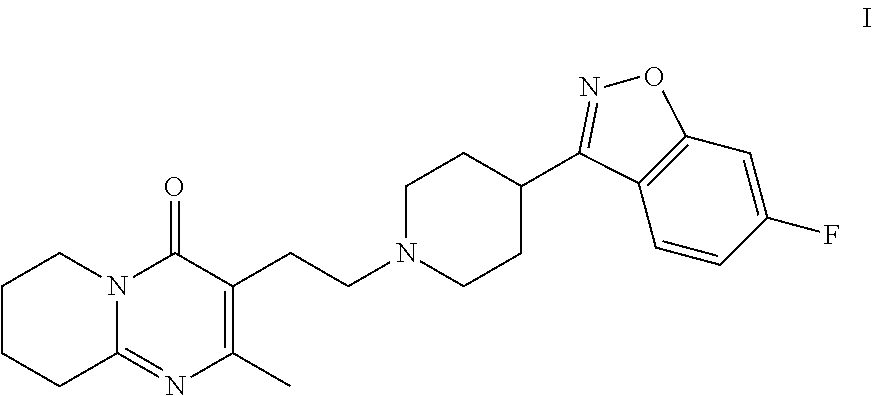
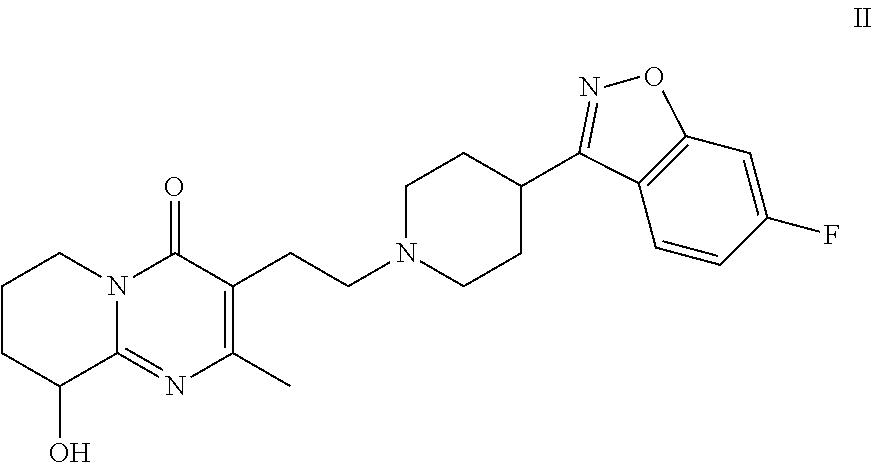
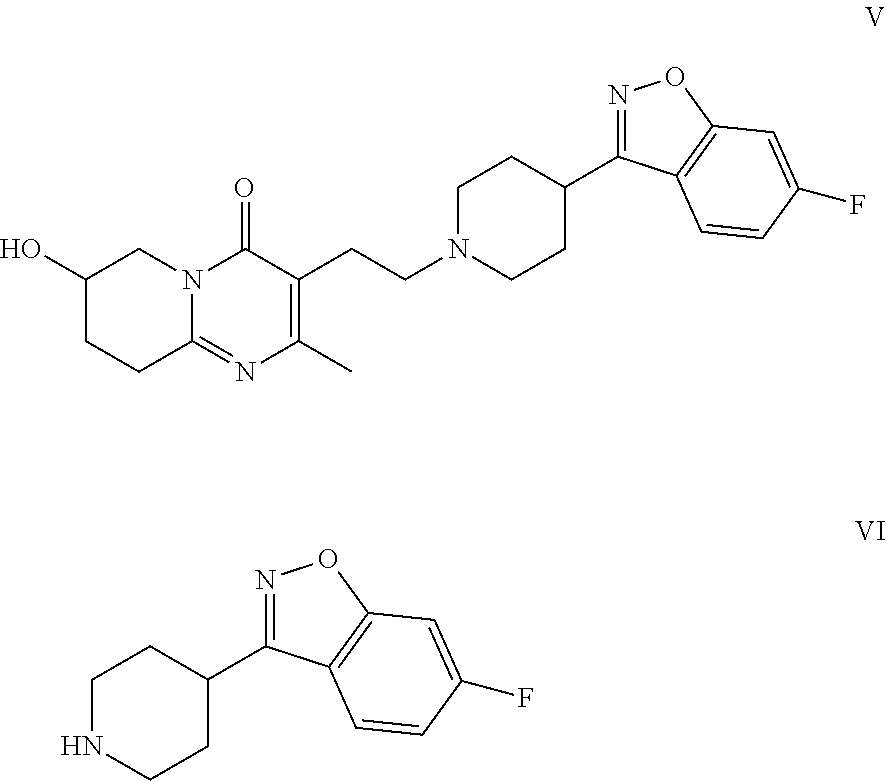
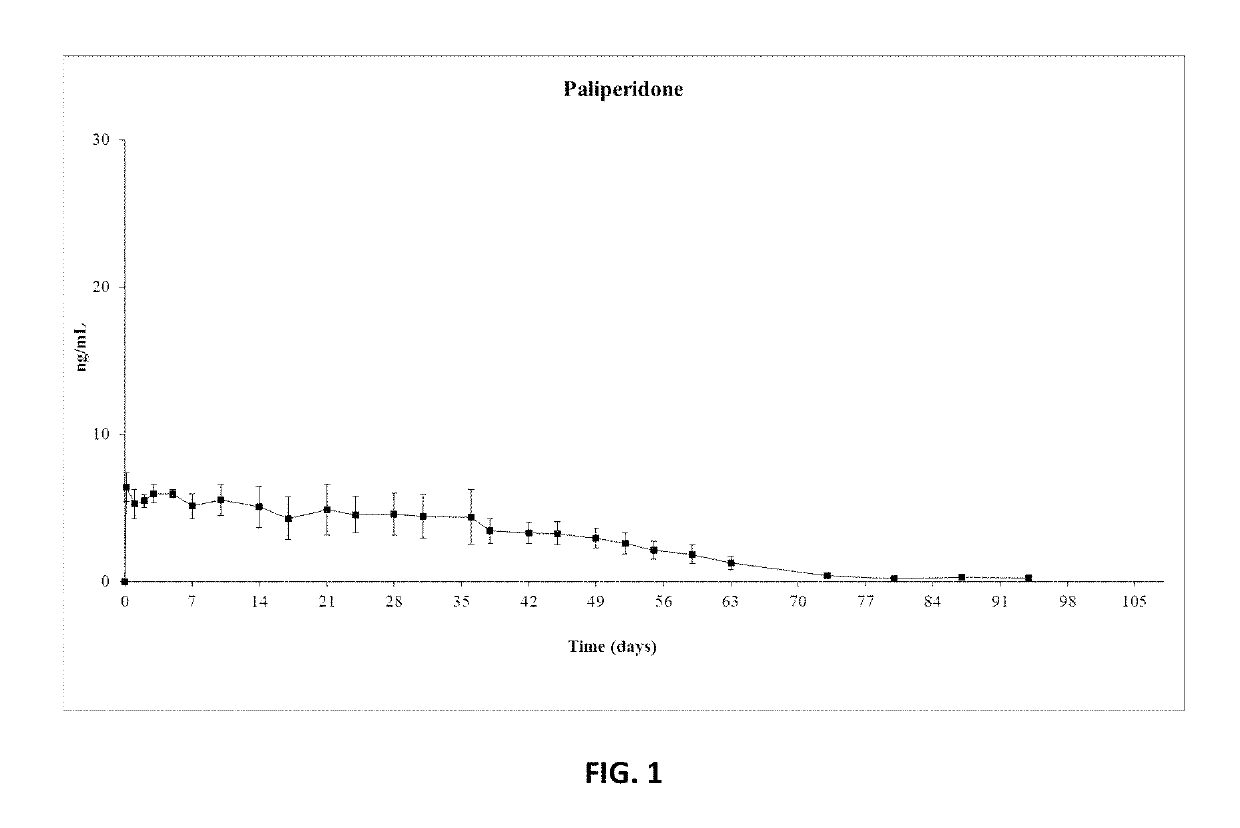
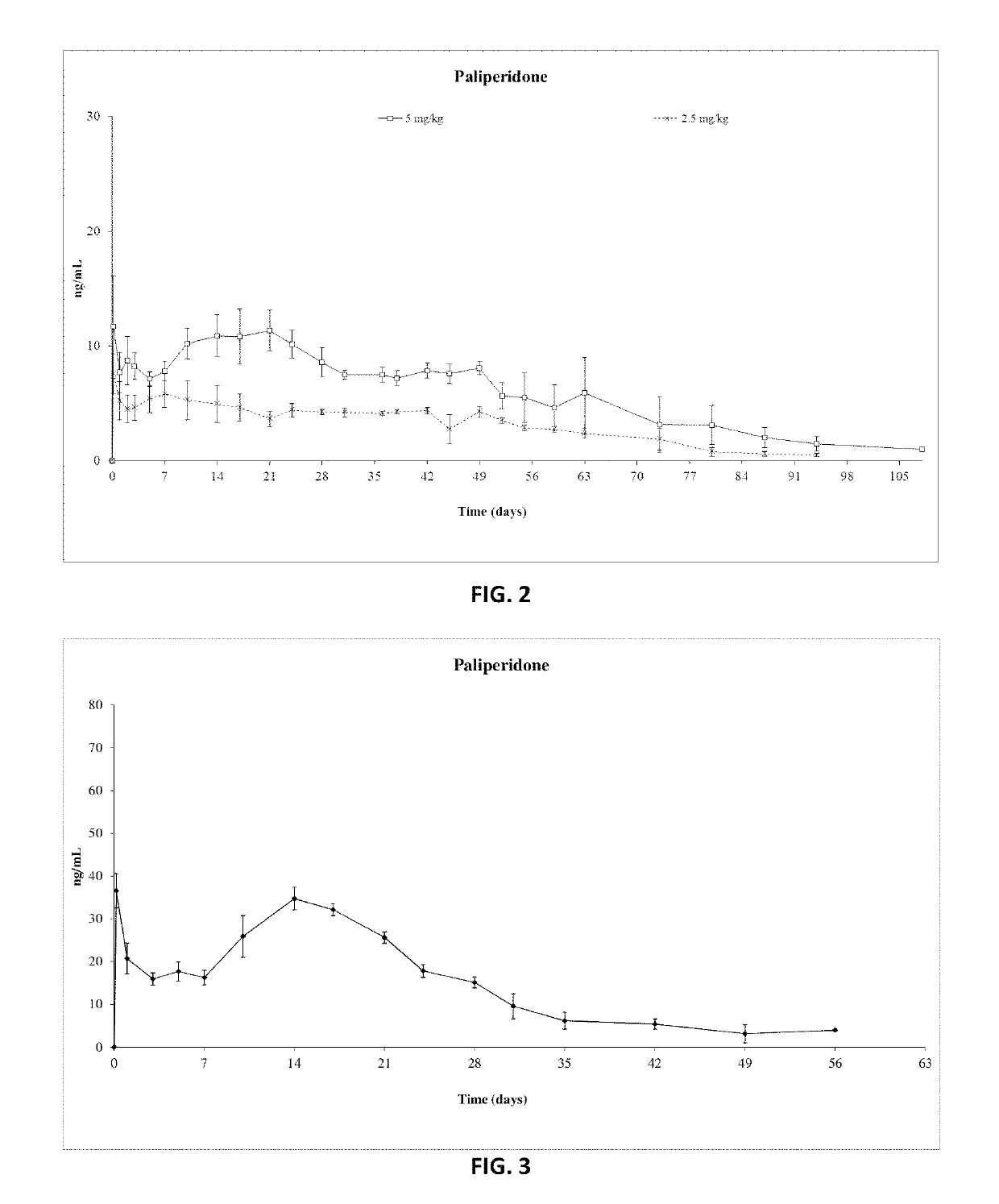
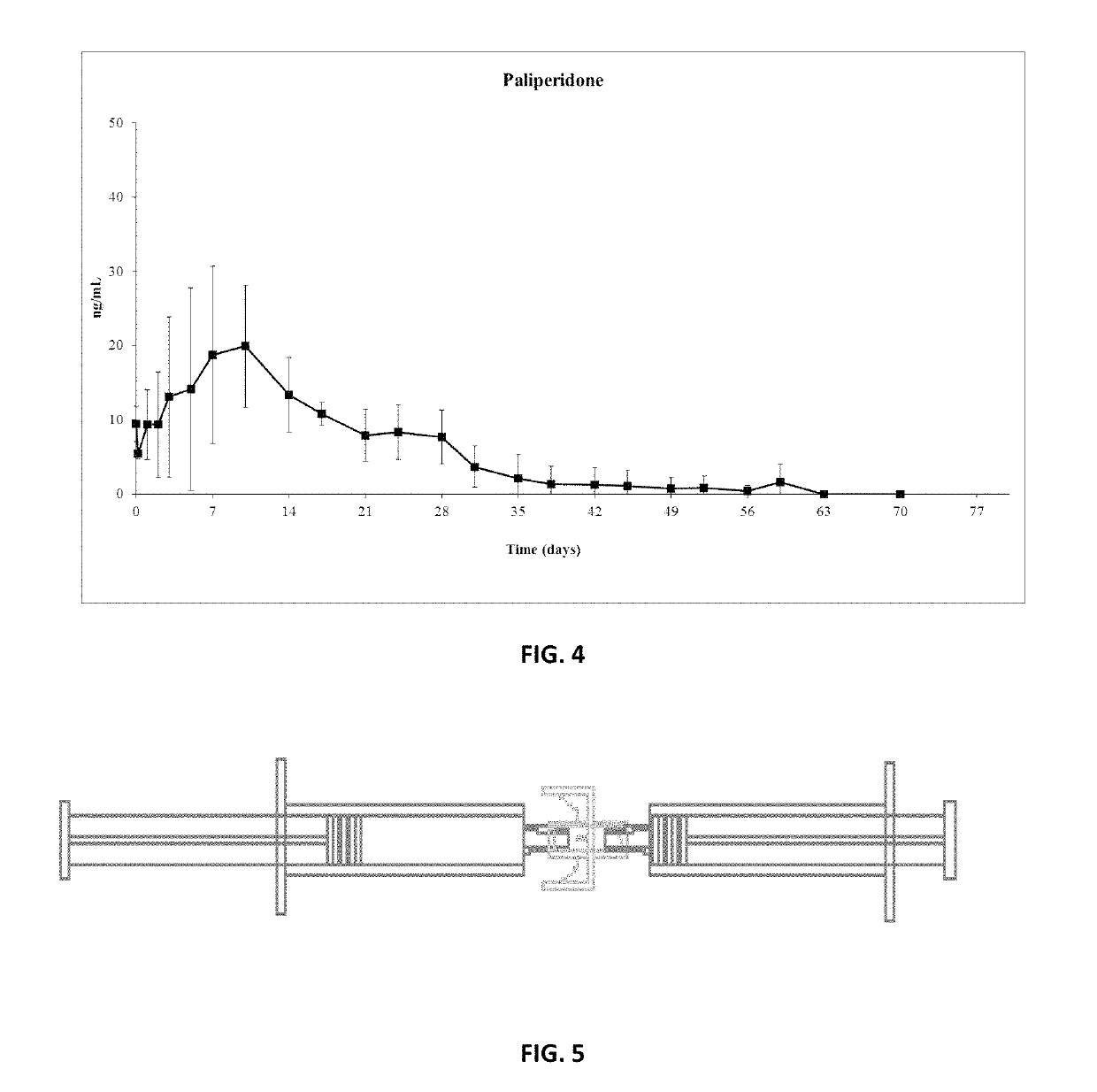
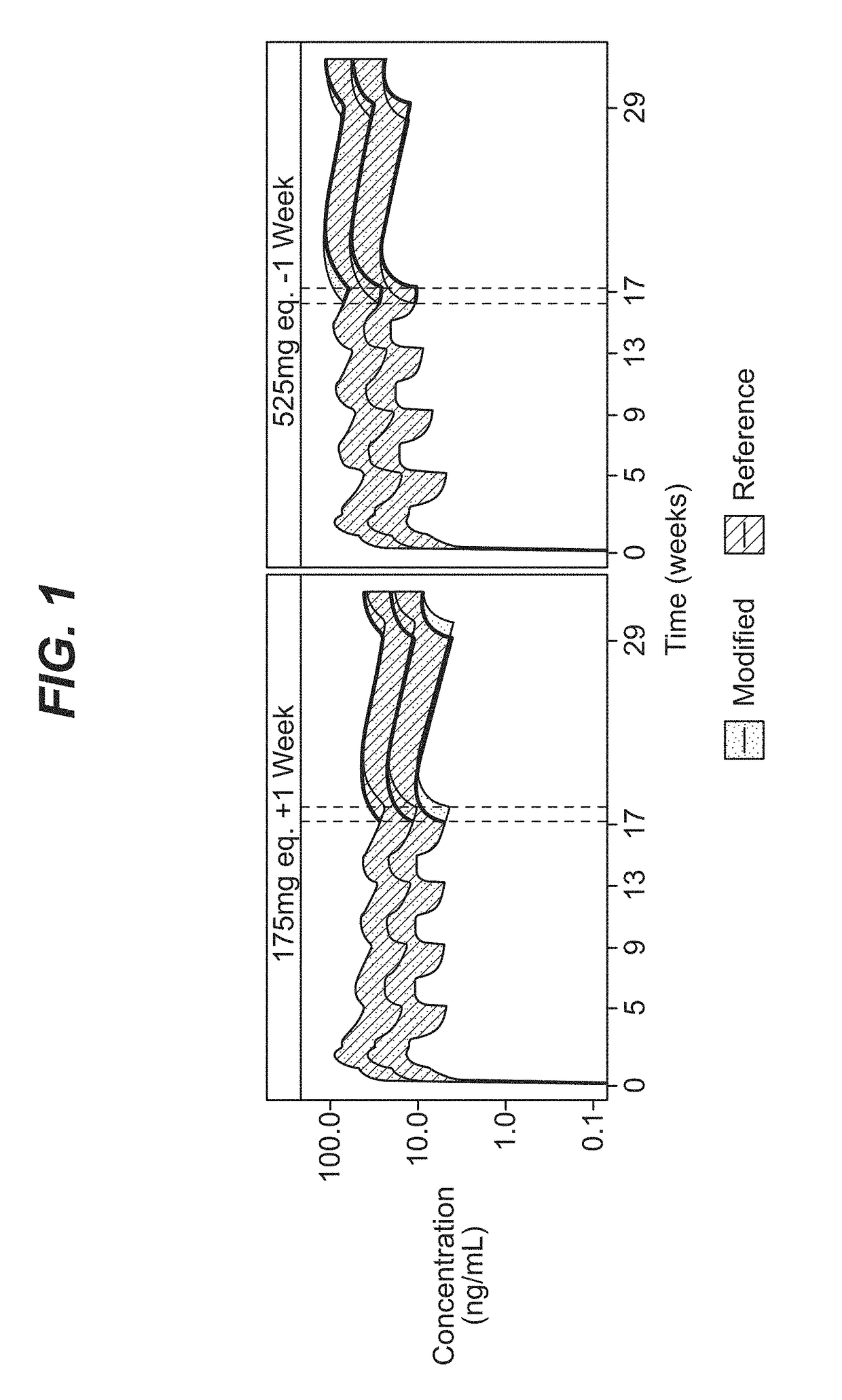
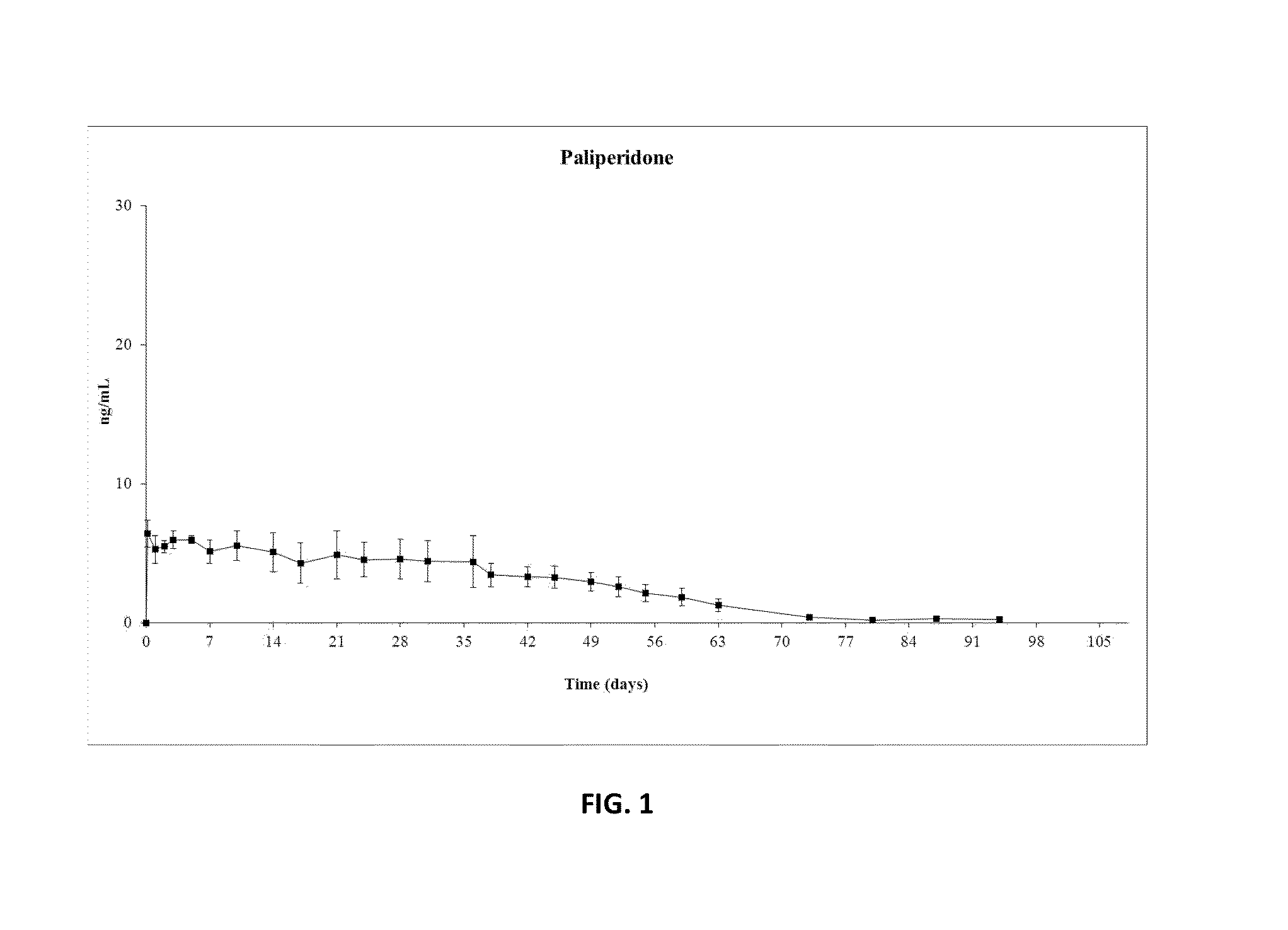
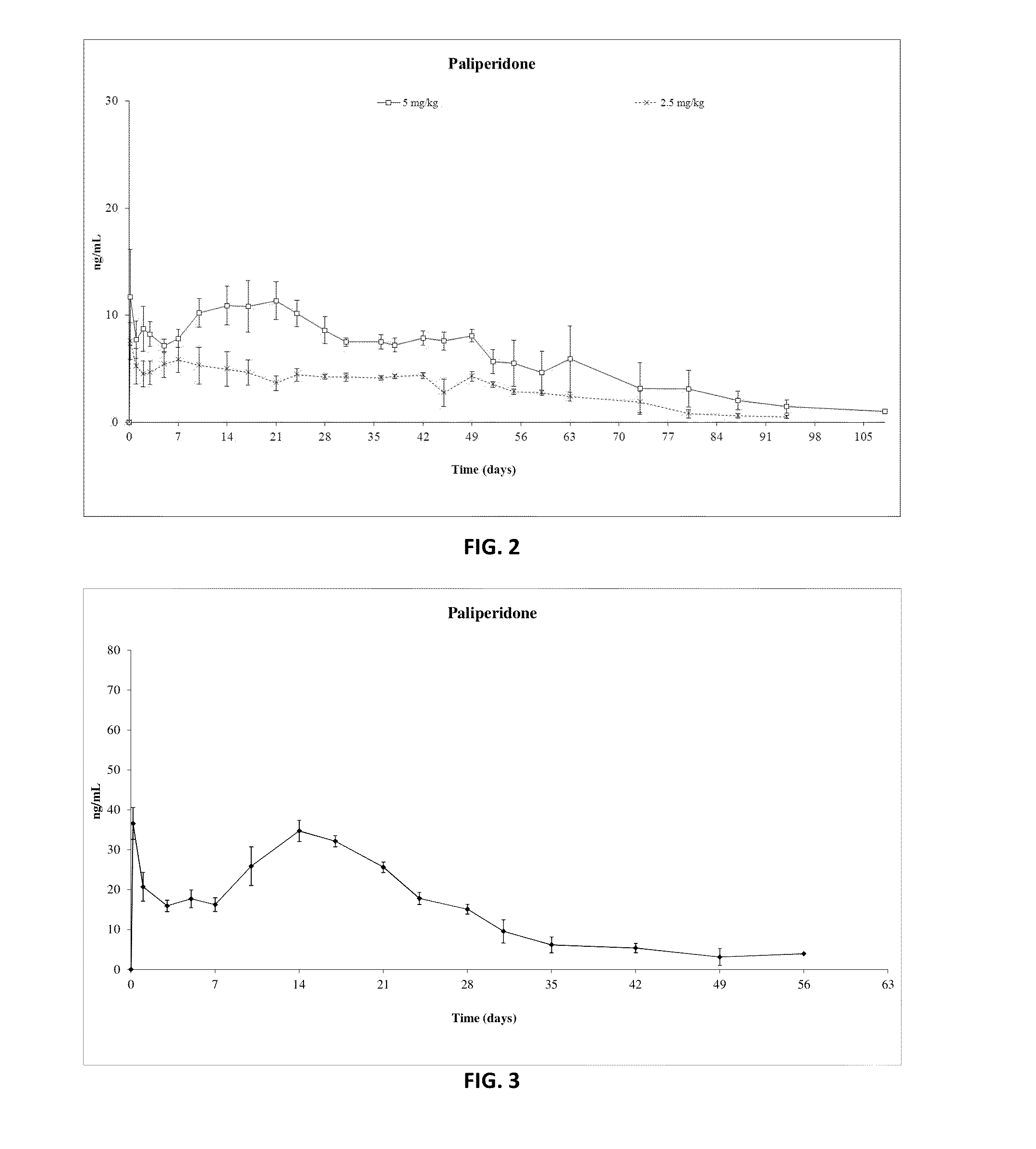
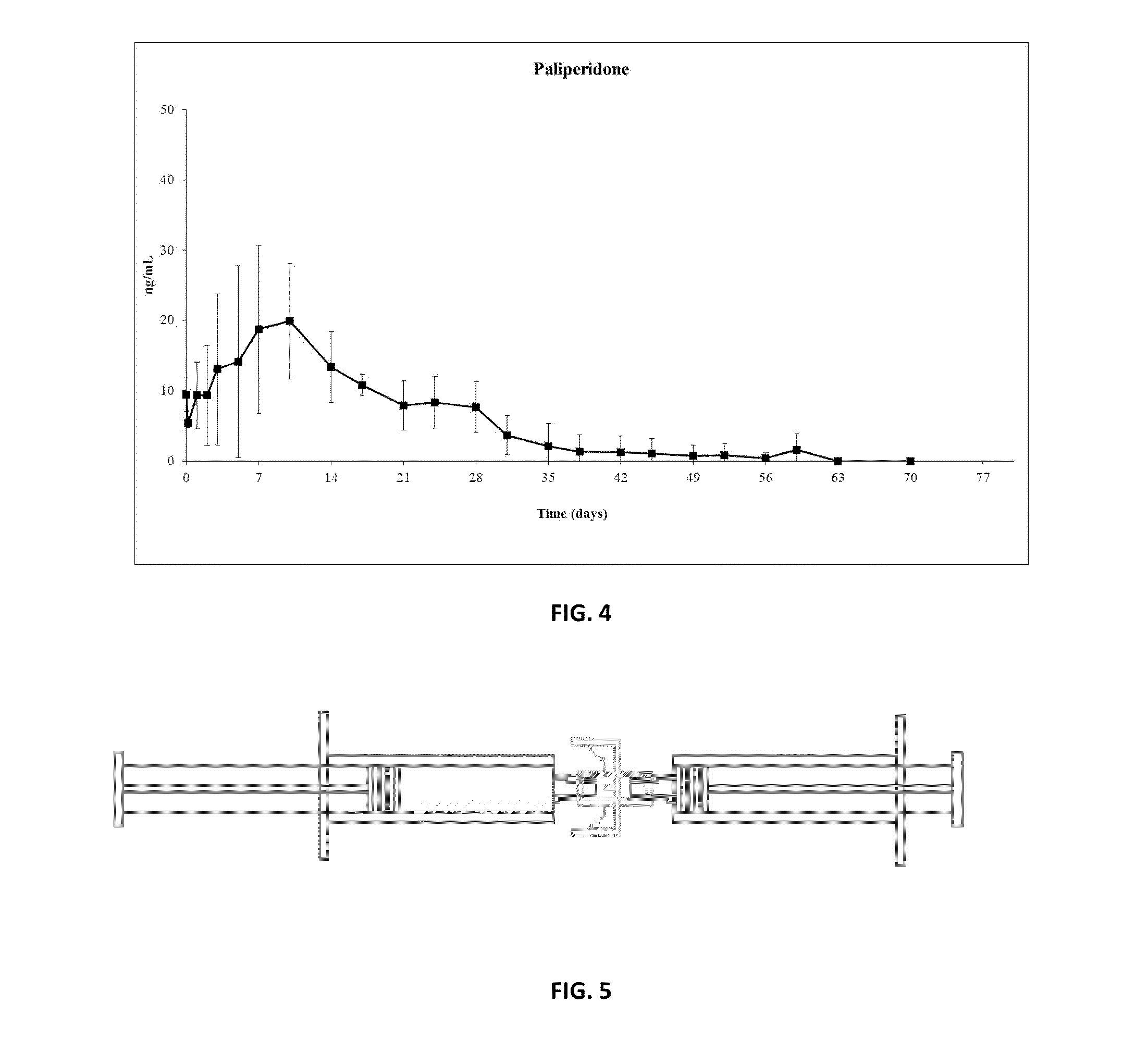
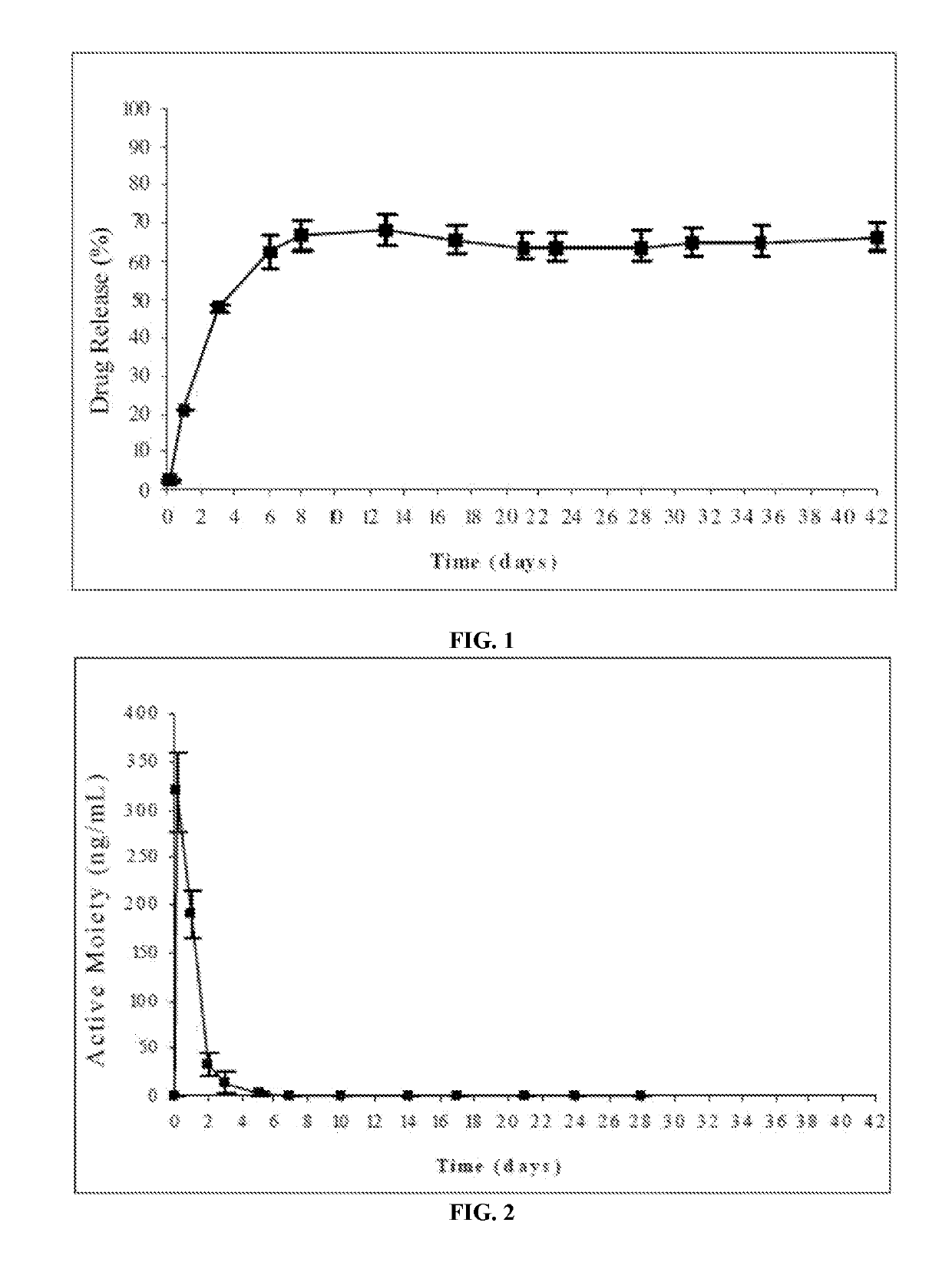
Patents
Literature
154 results about "Paliperidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
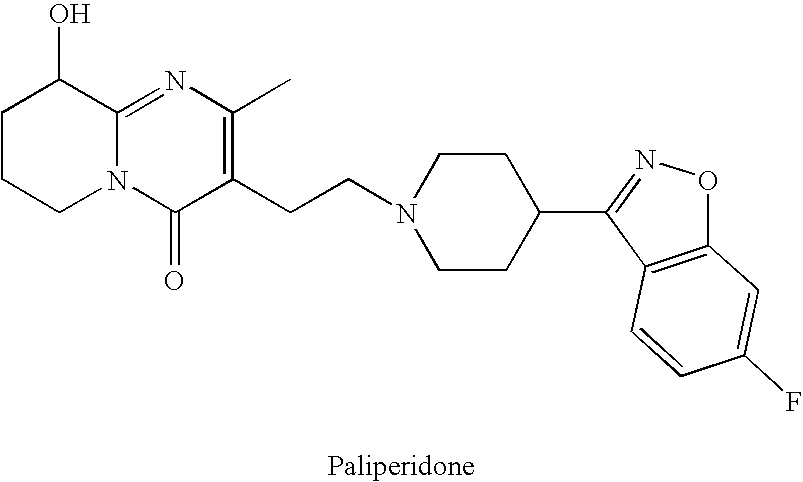
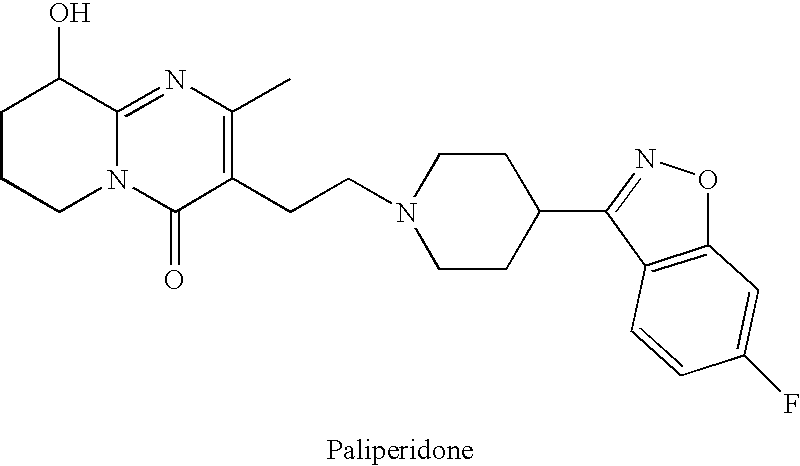
This medication is used to treat certain mental/mood disorders (such as schizophrenia, schizoaffective disorder).
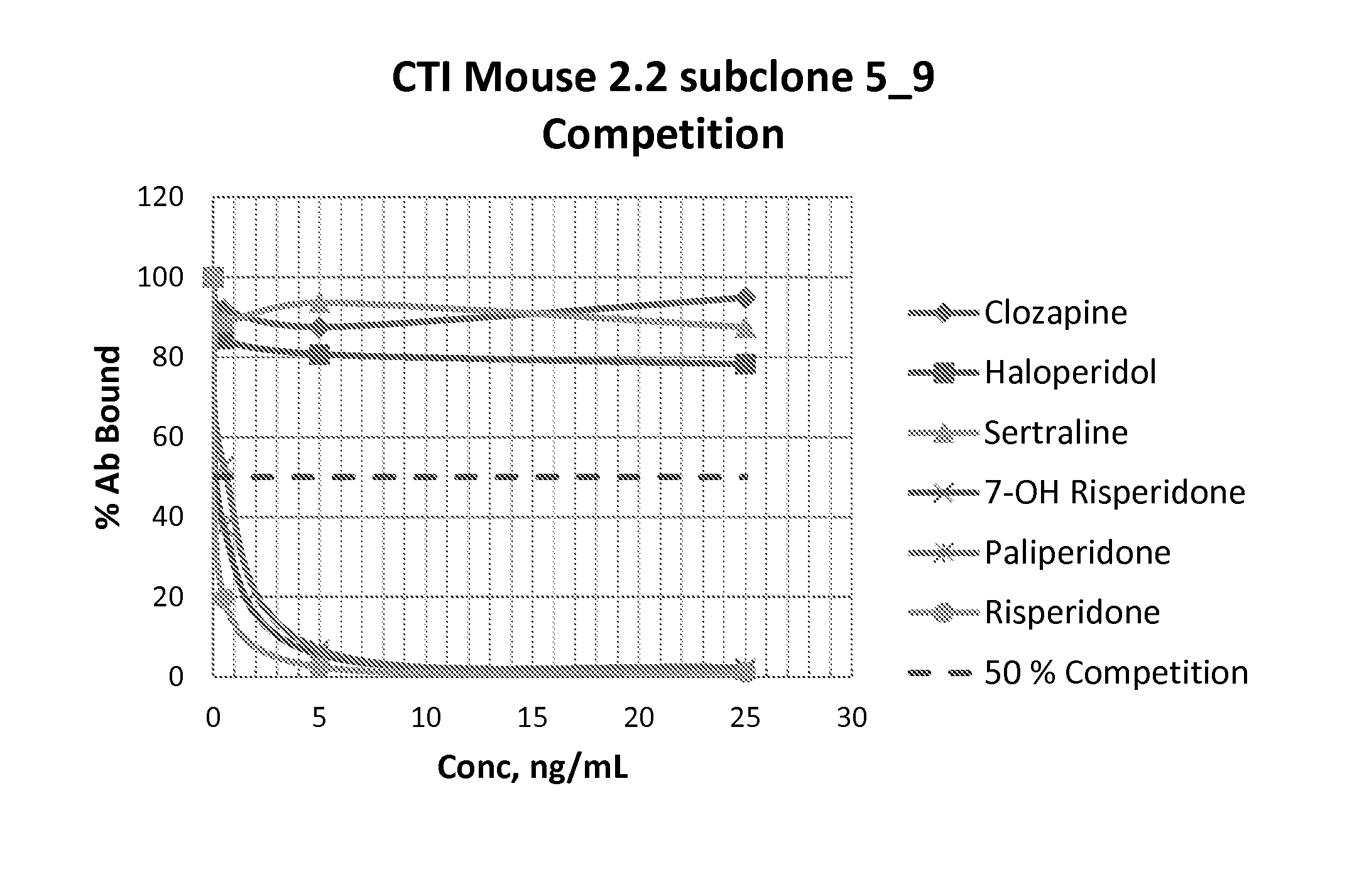
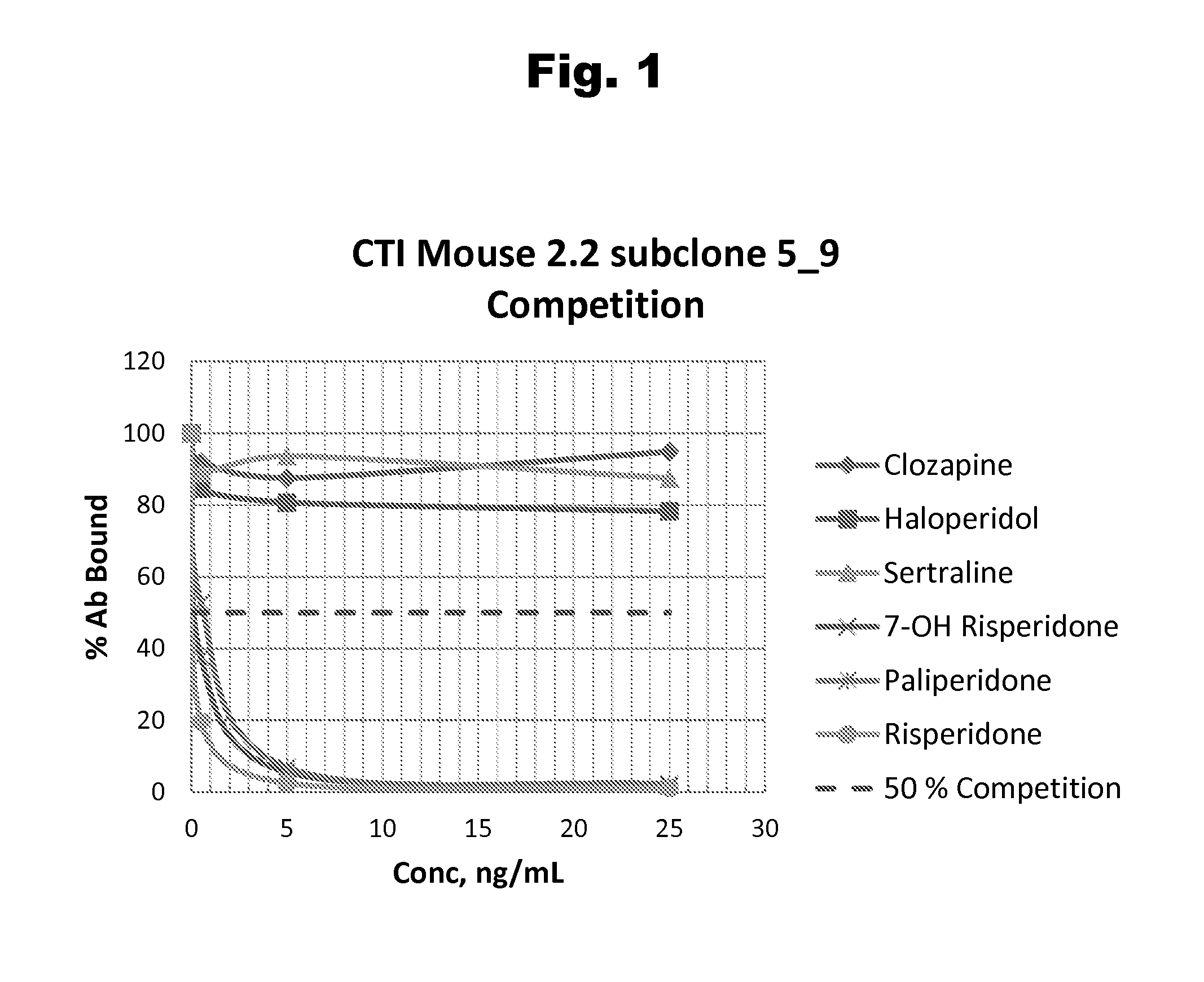
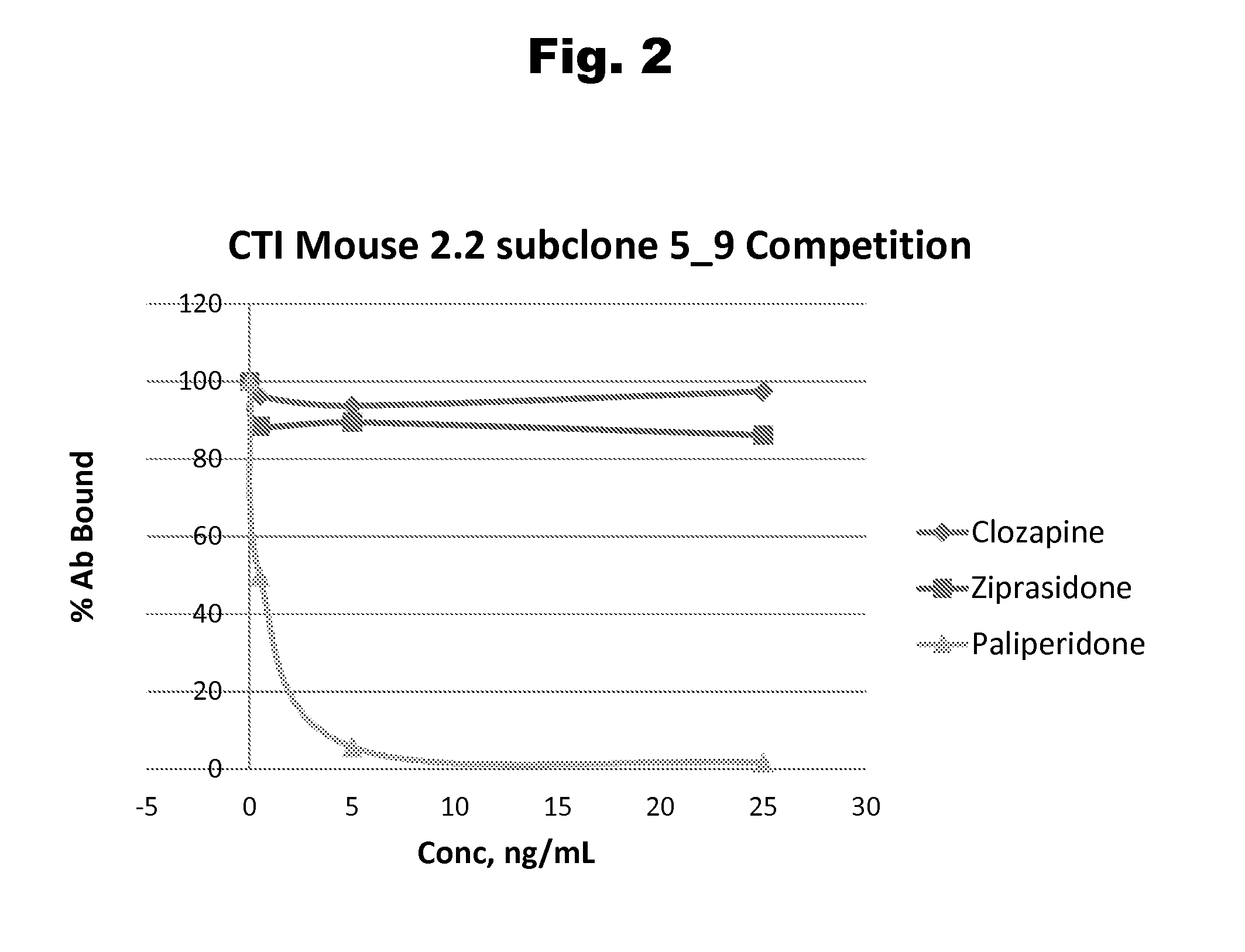
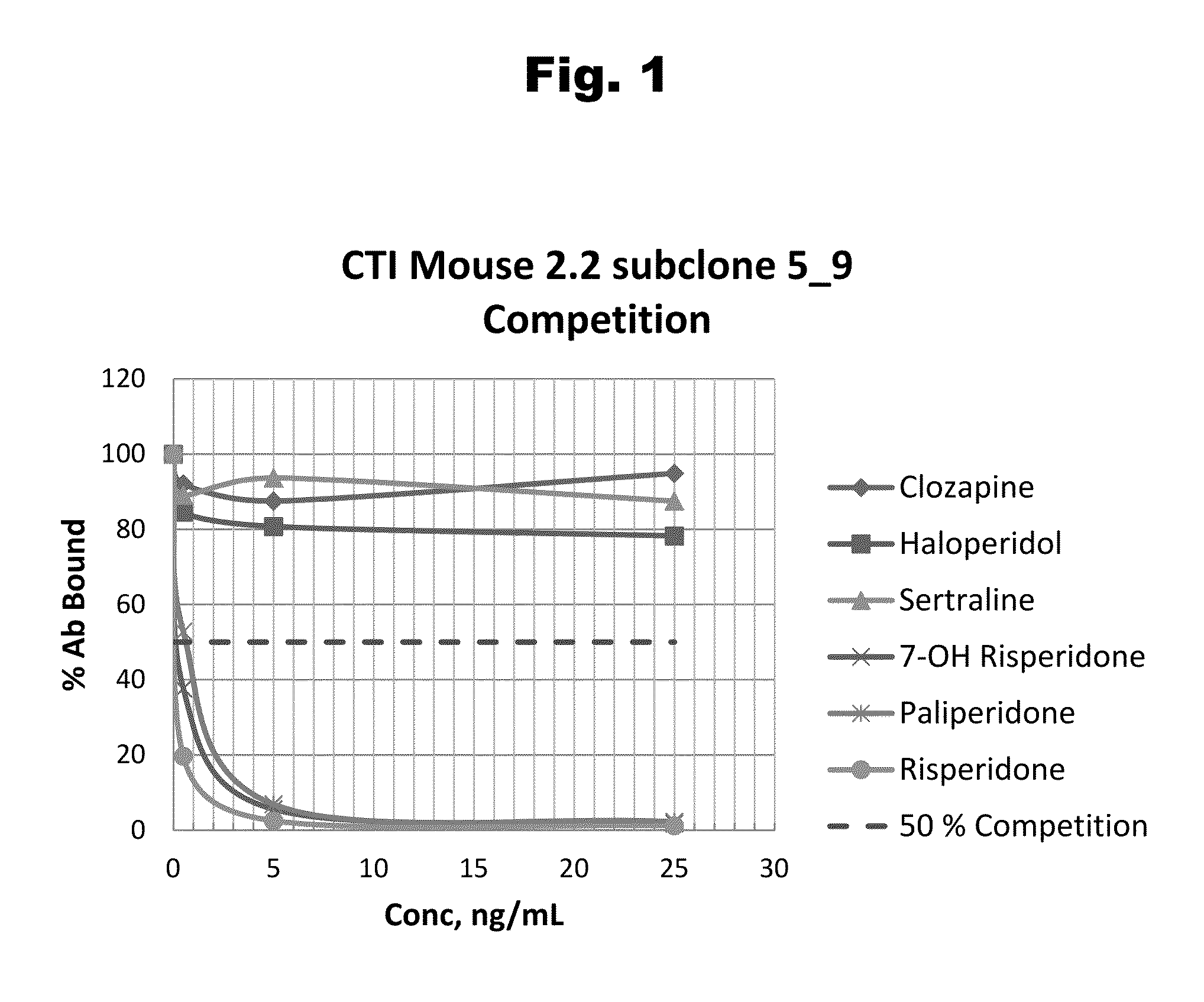
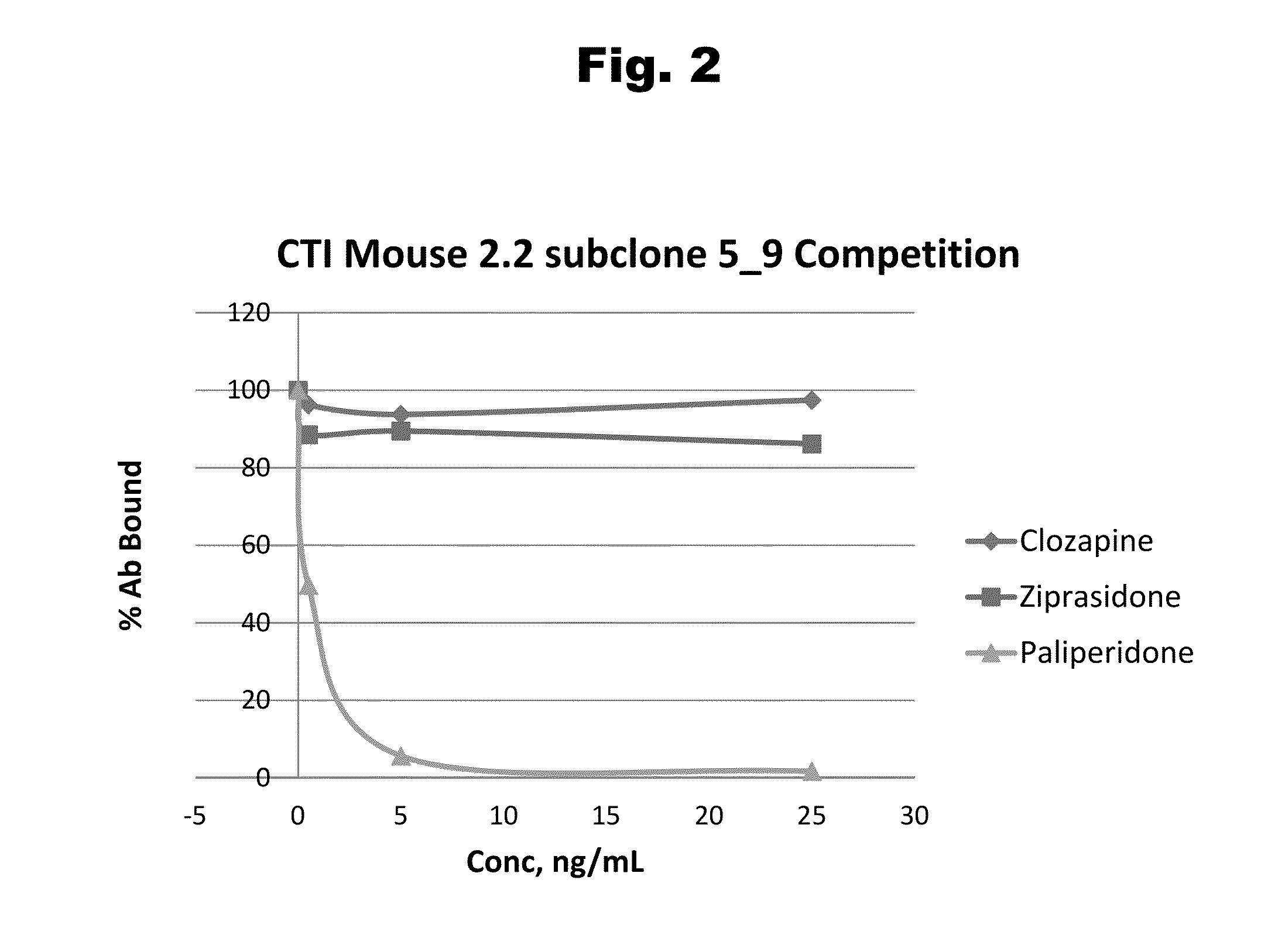
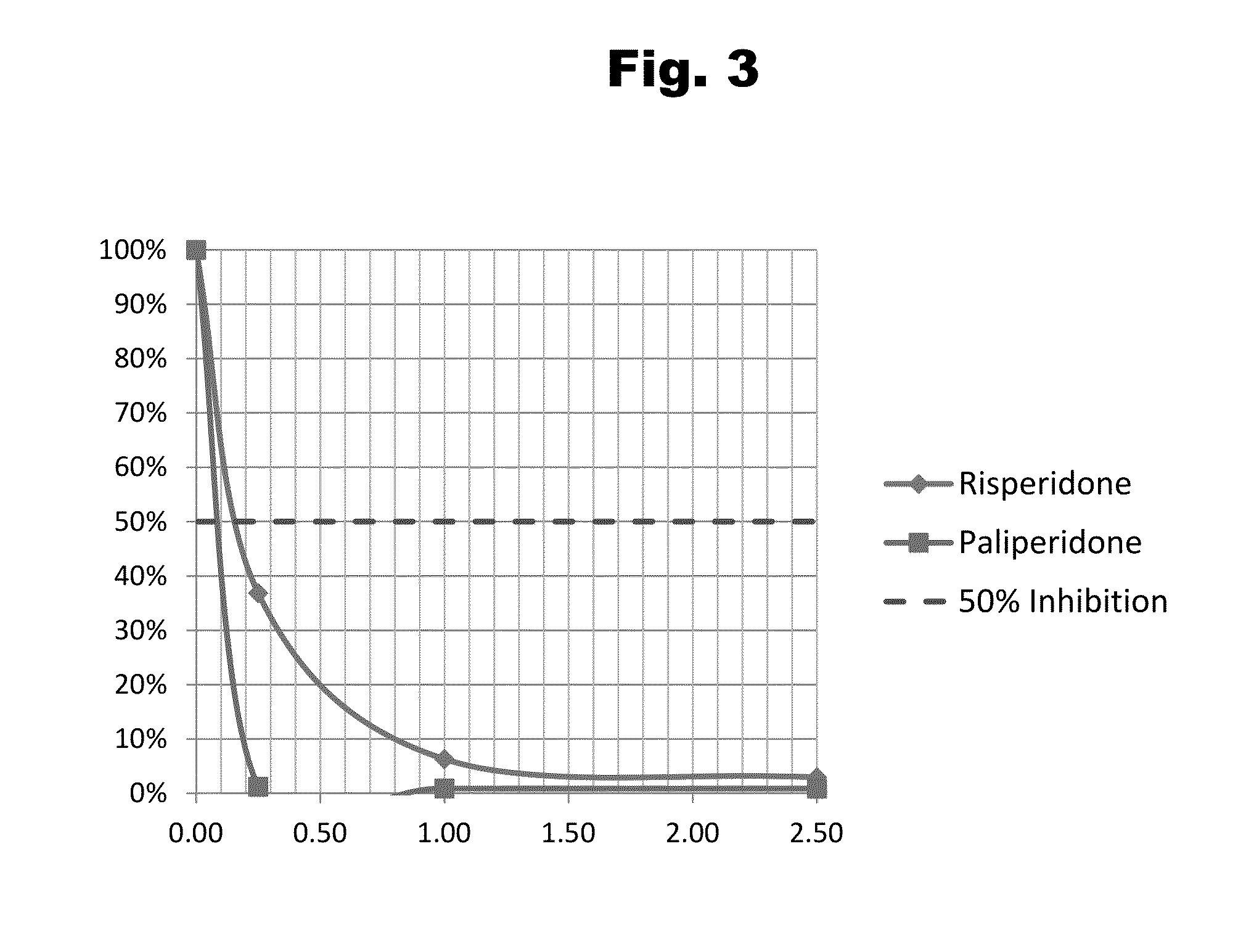
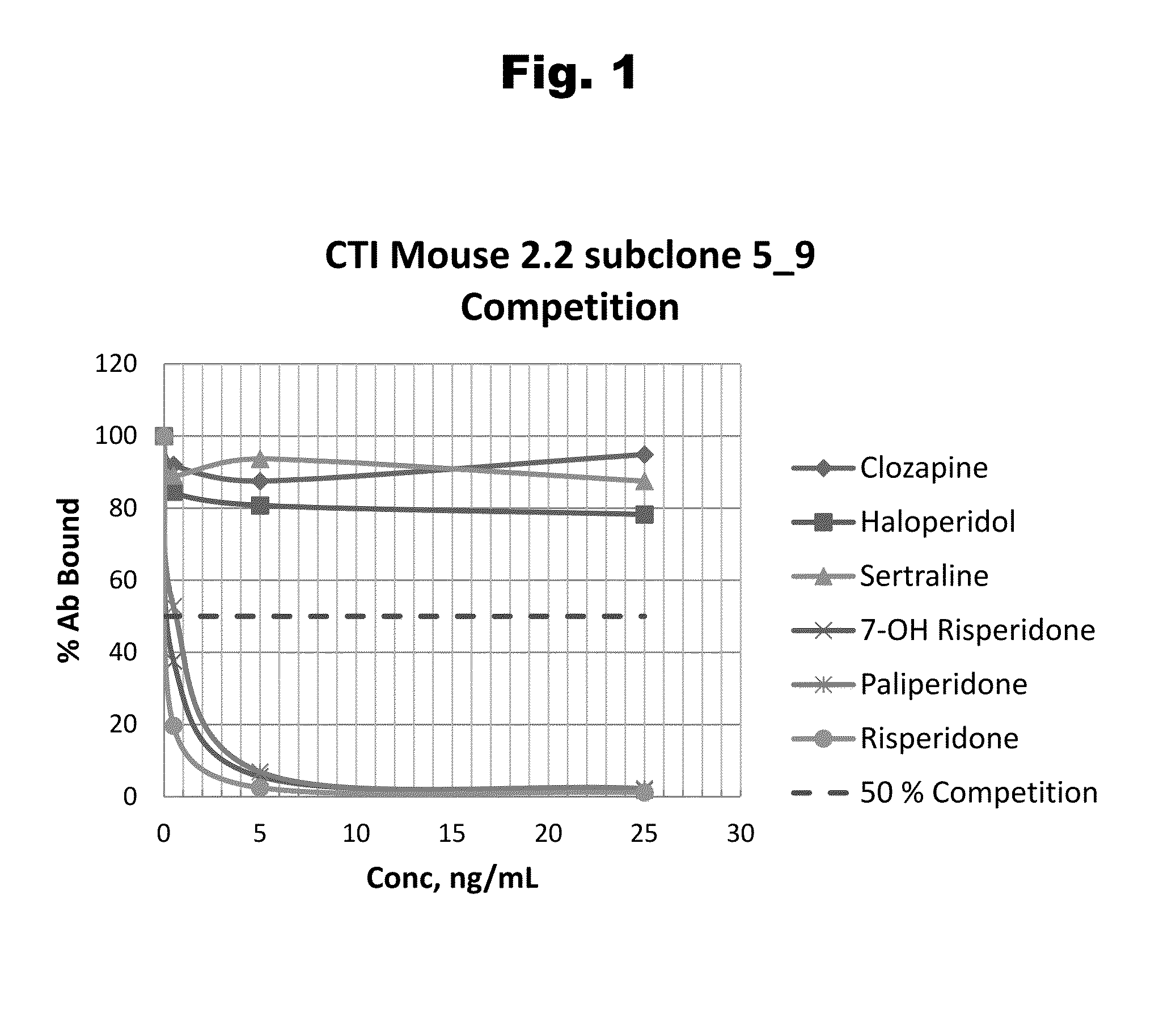
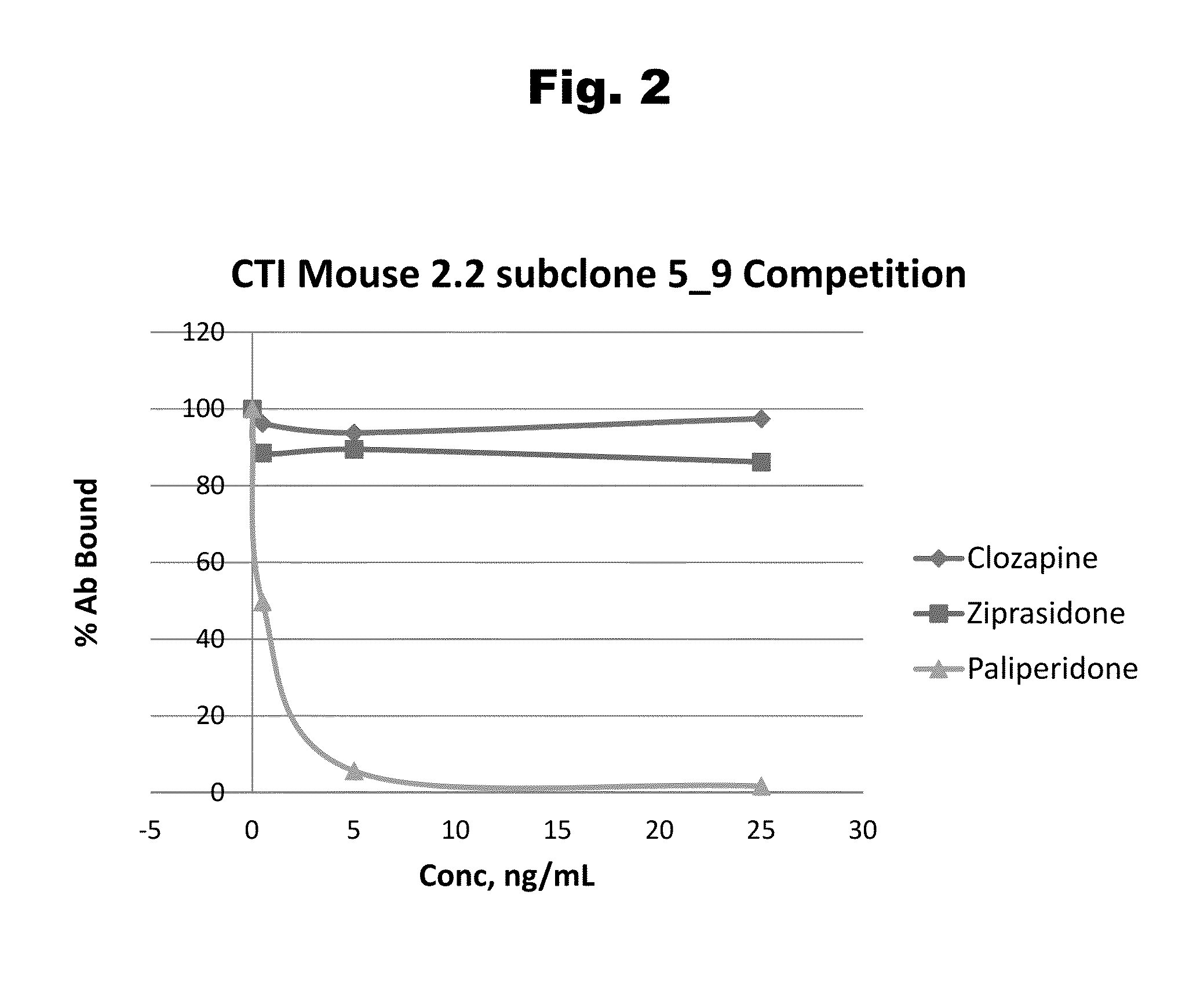
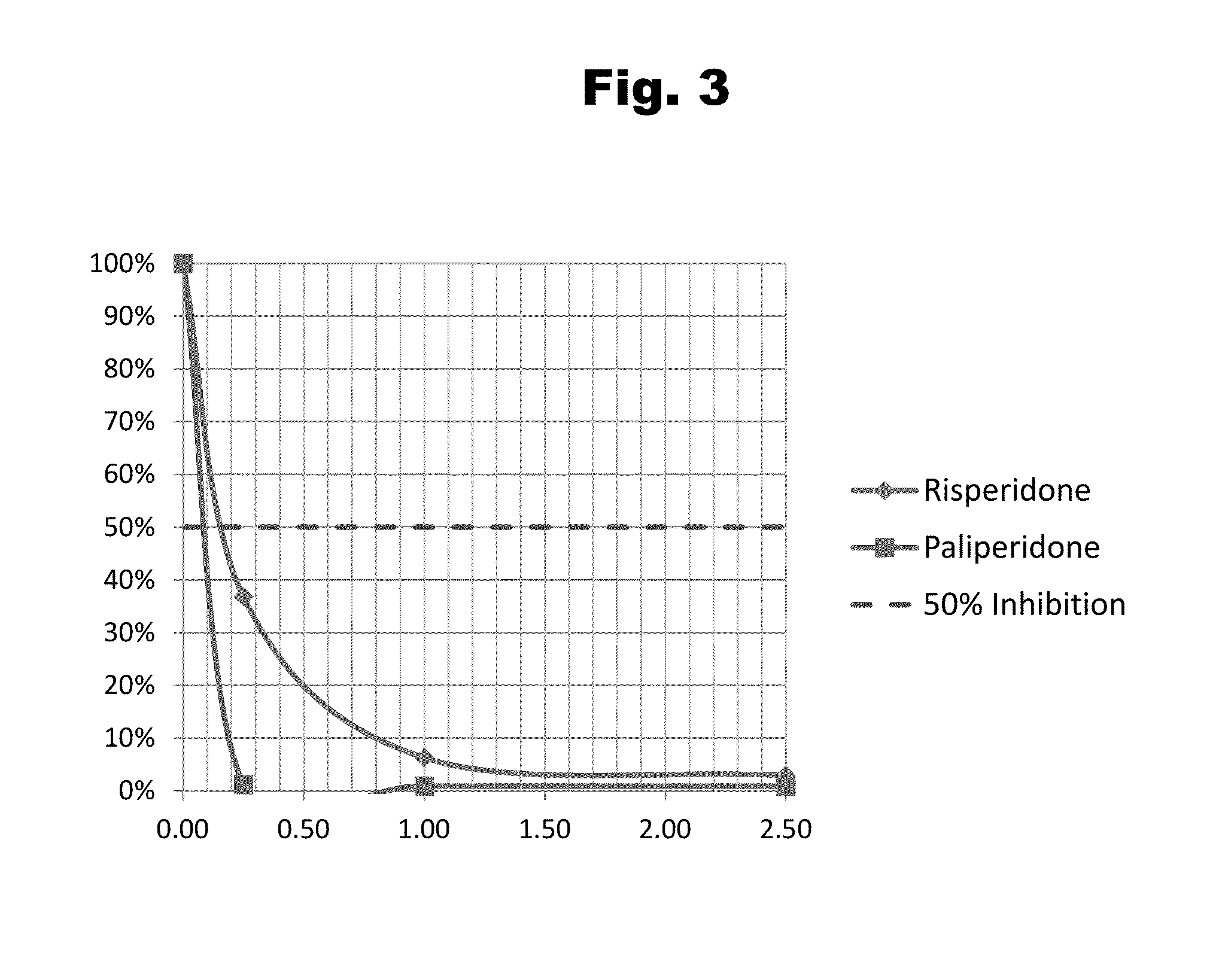
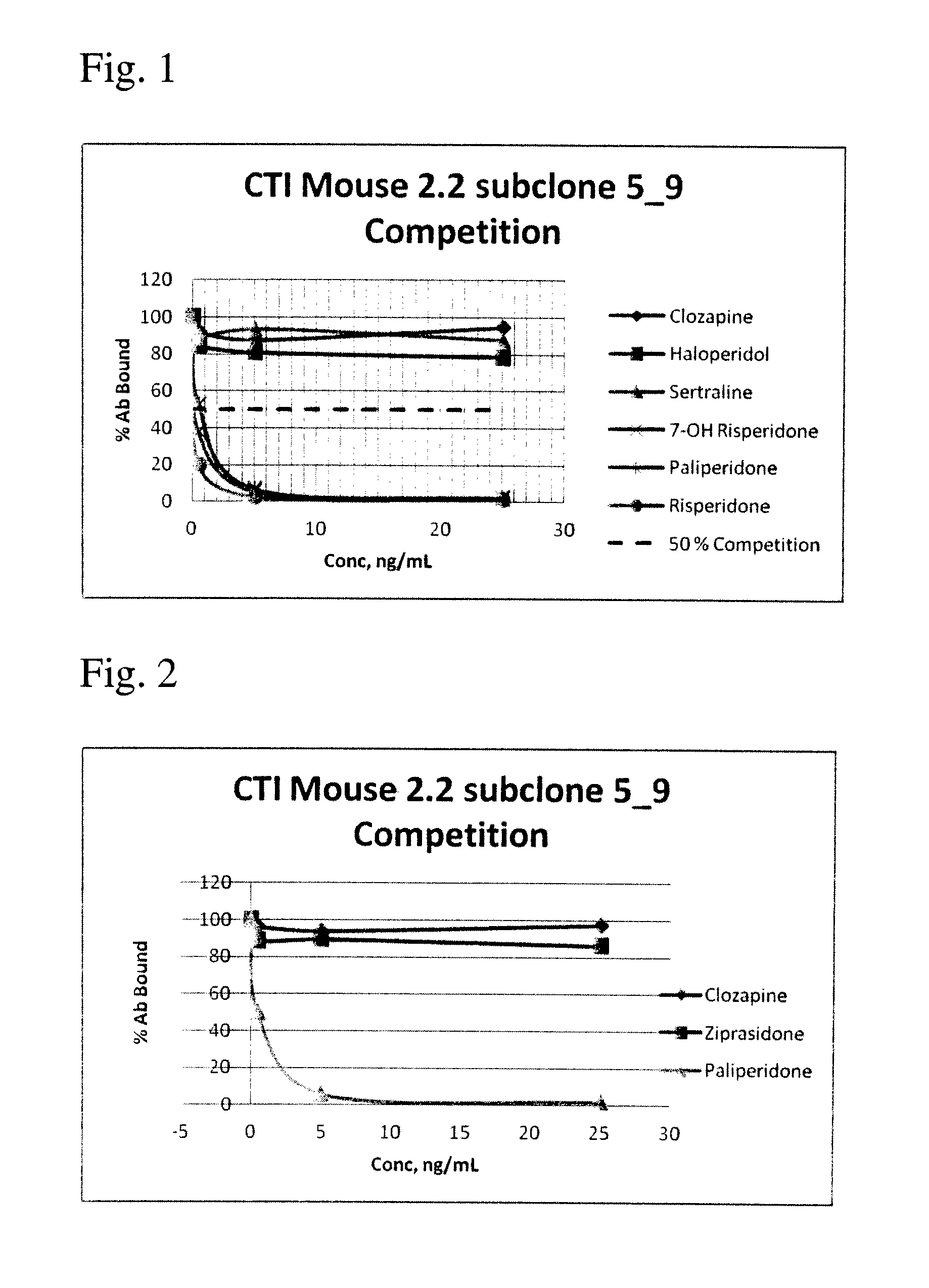
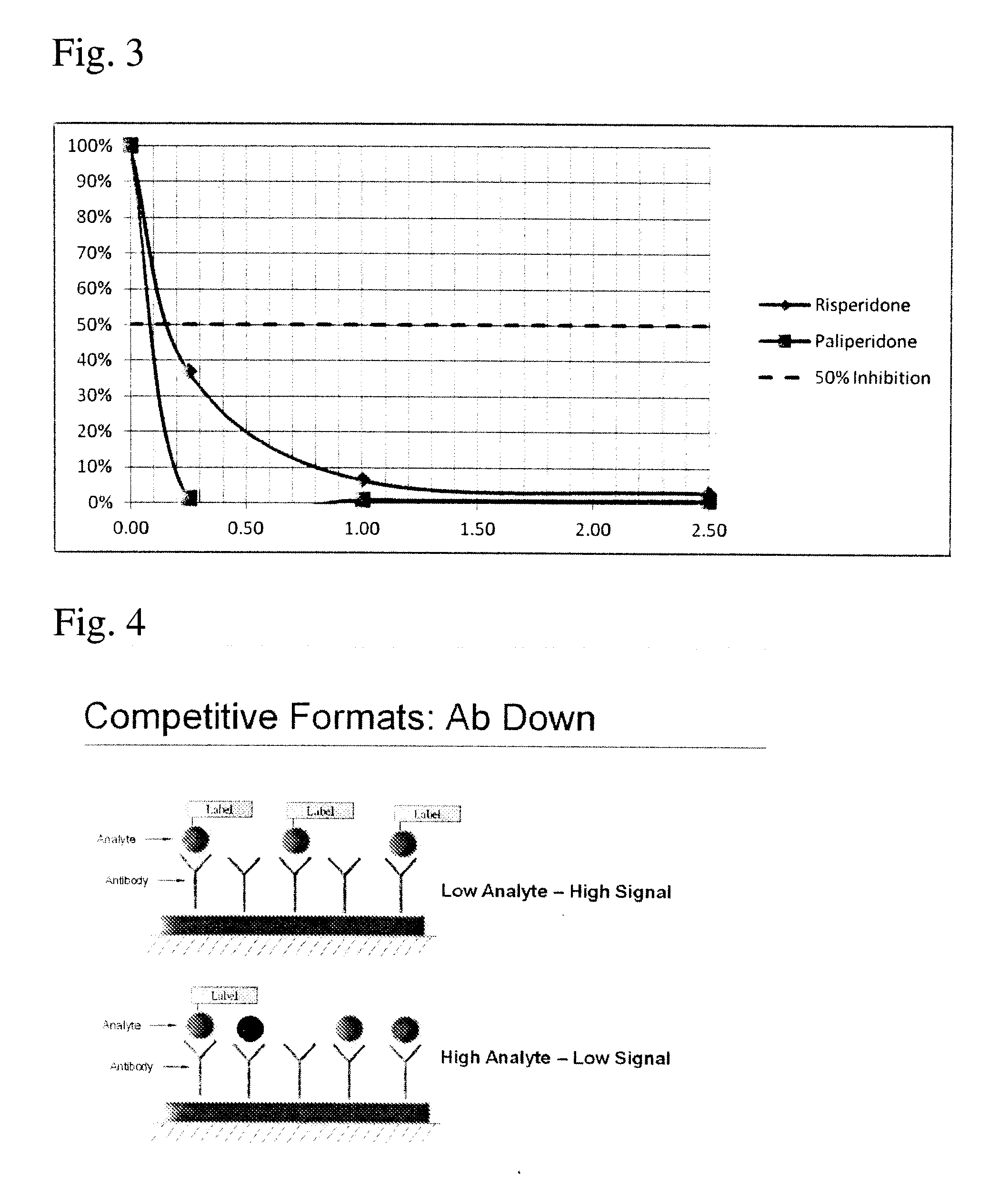
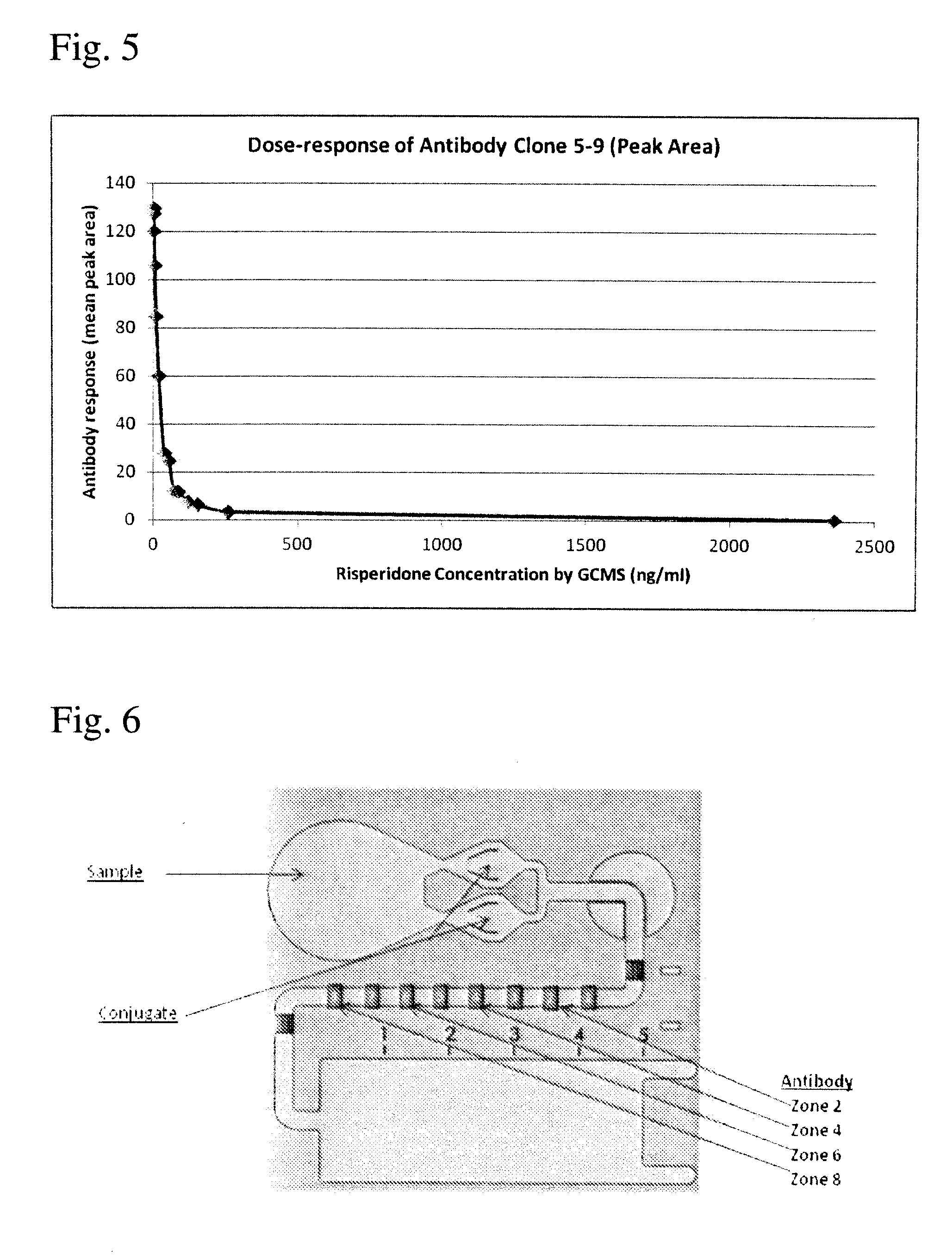
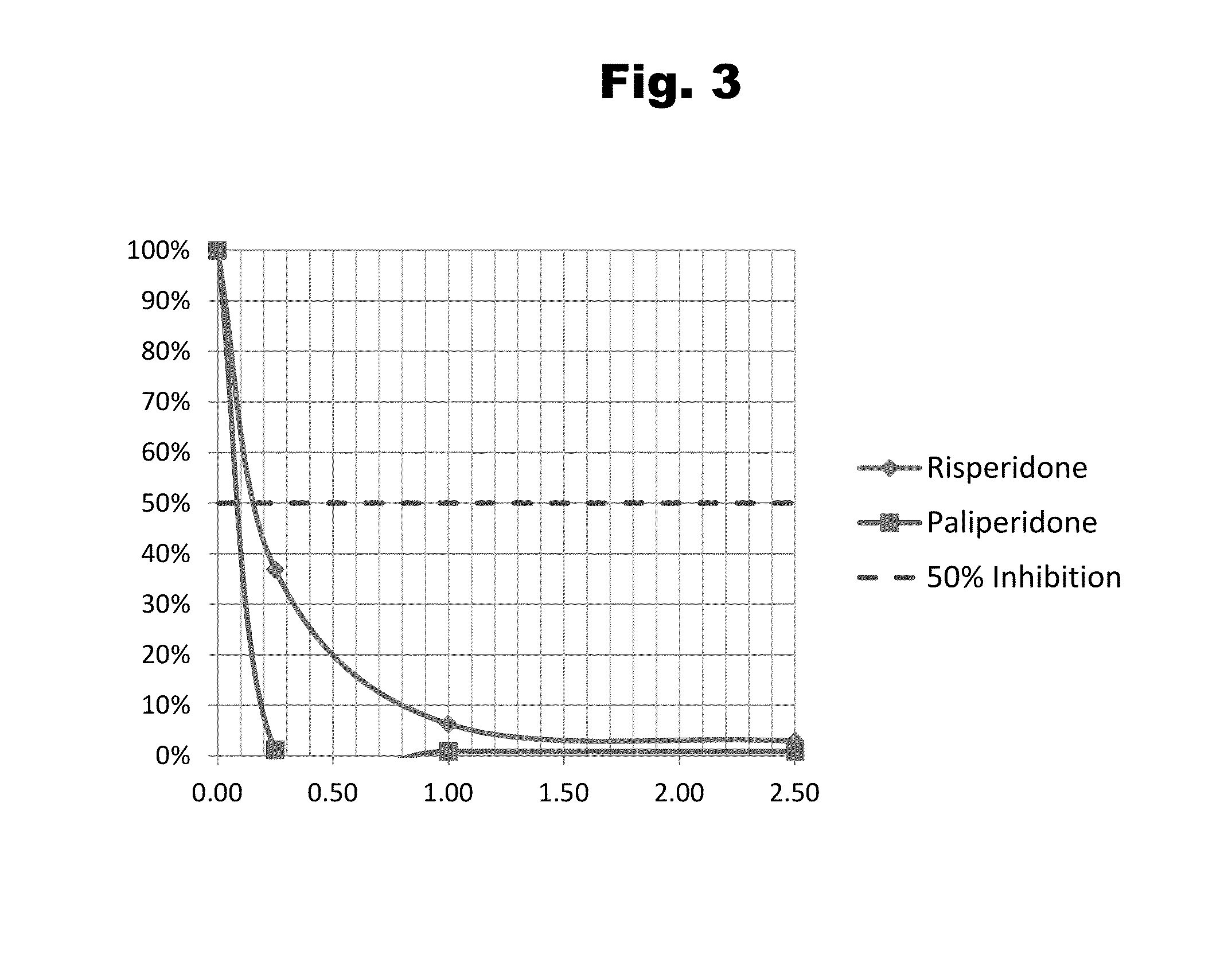
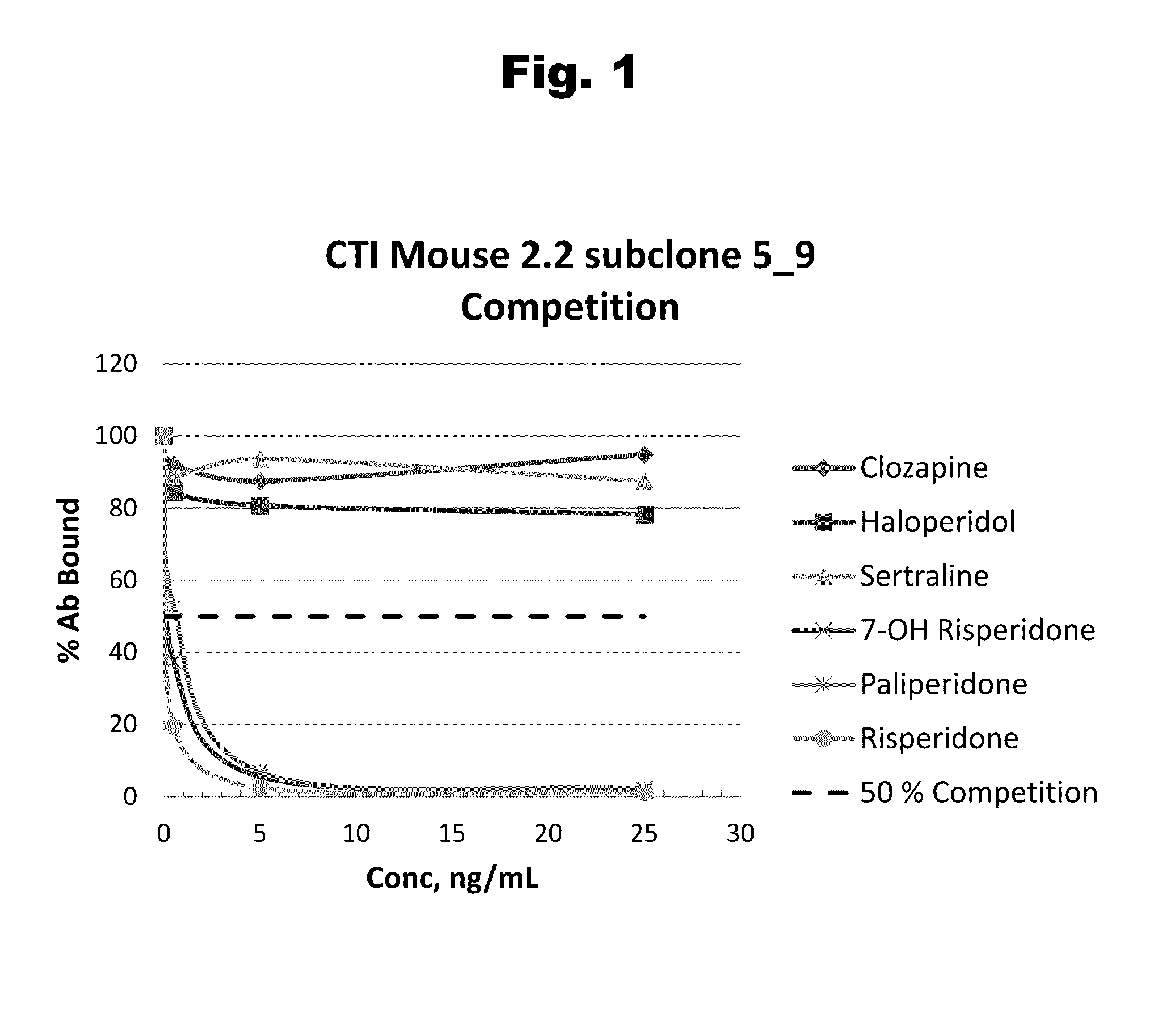
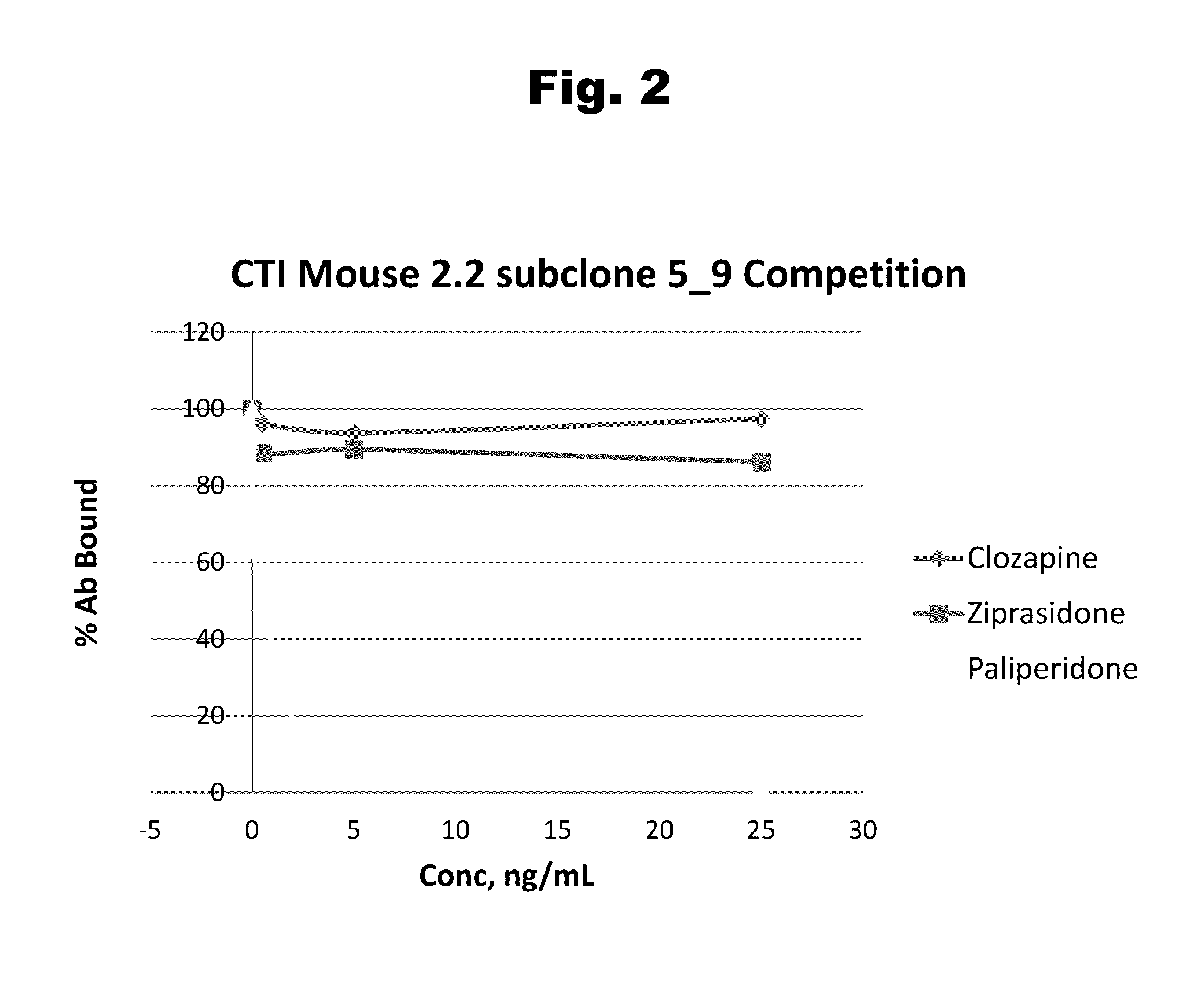
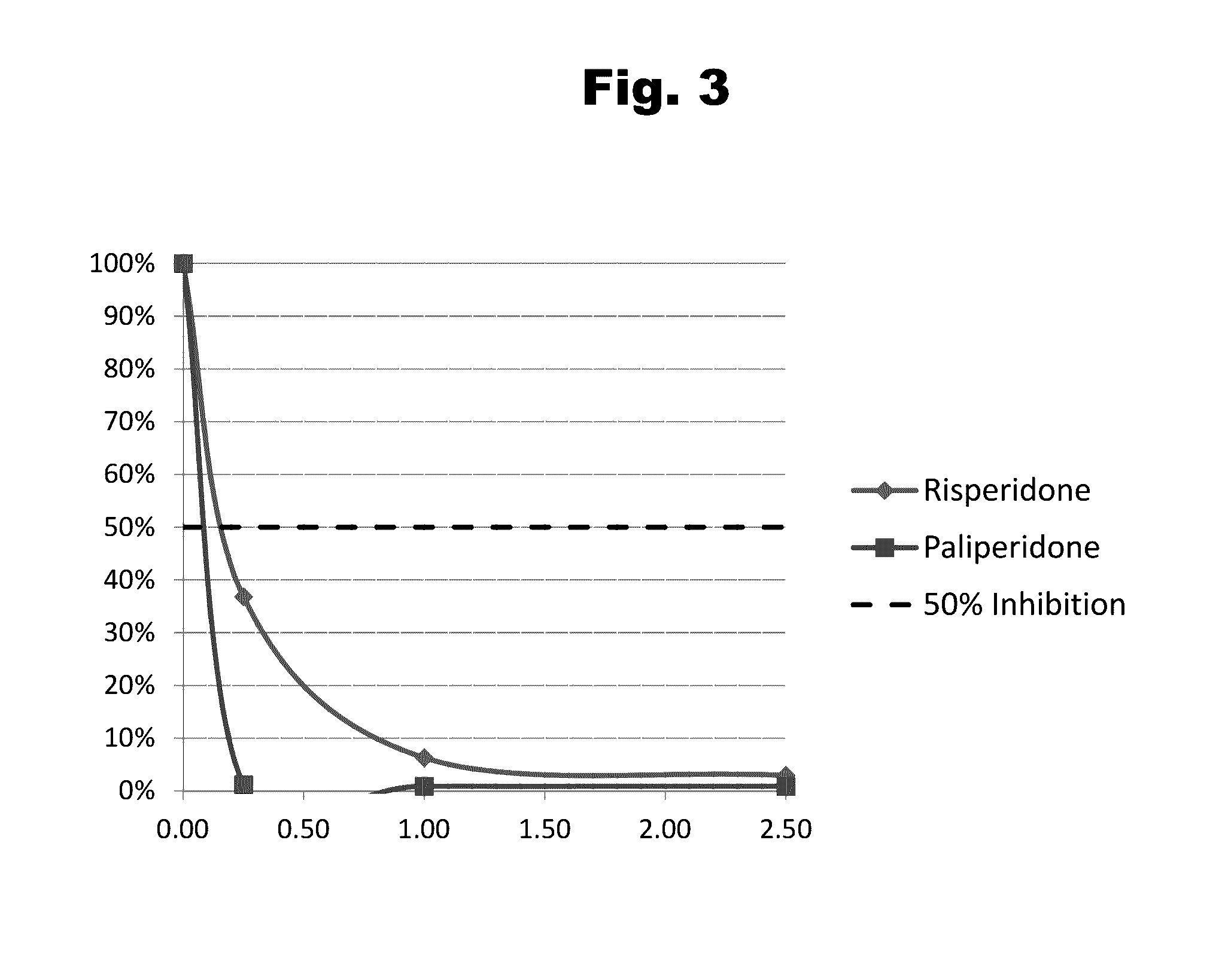
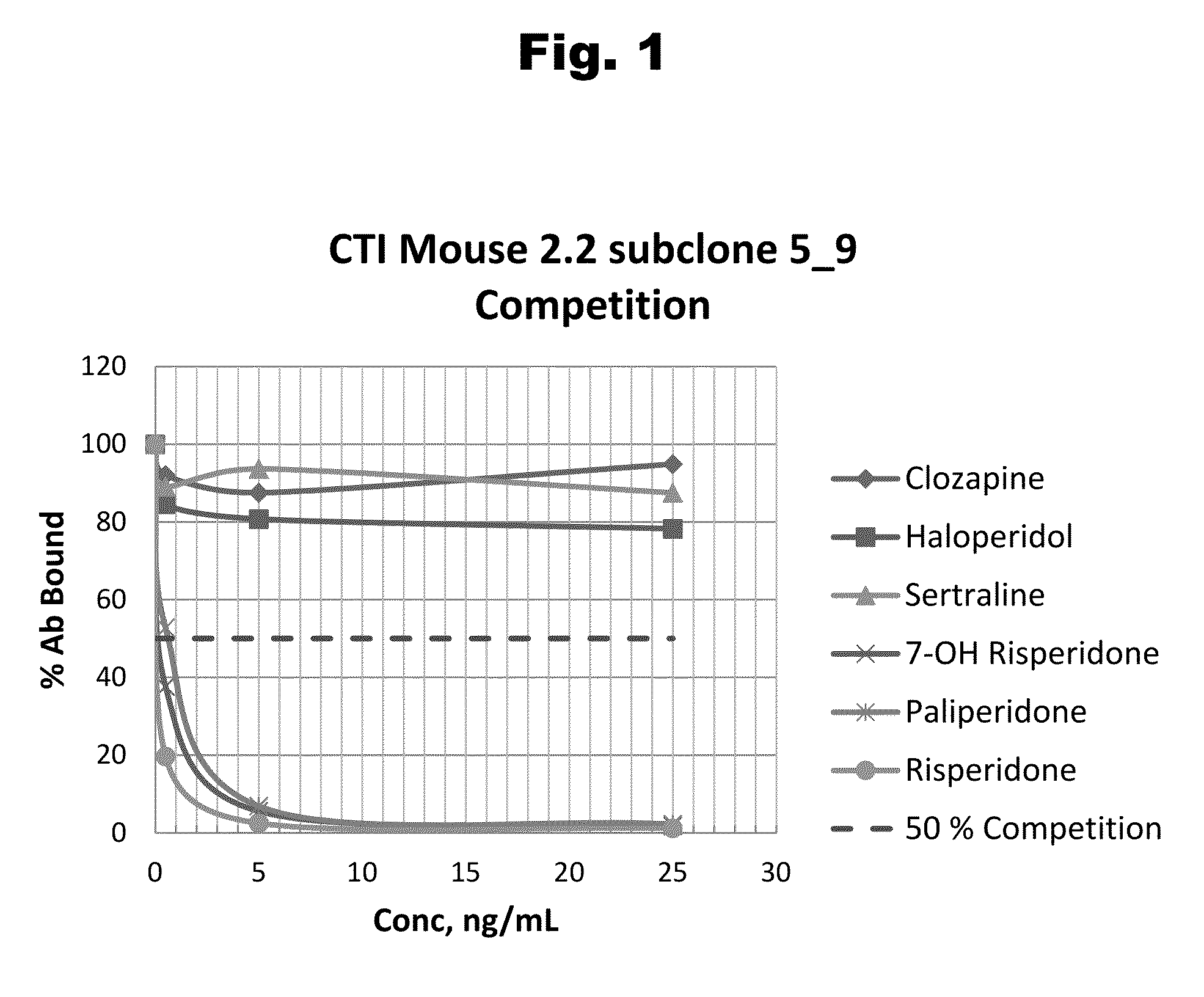
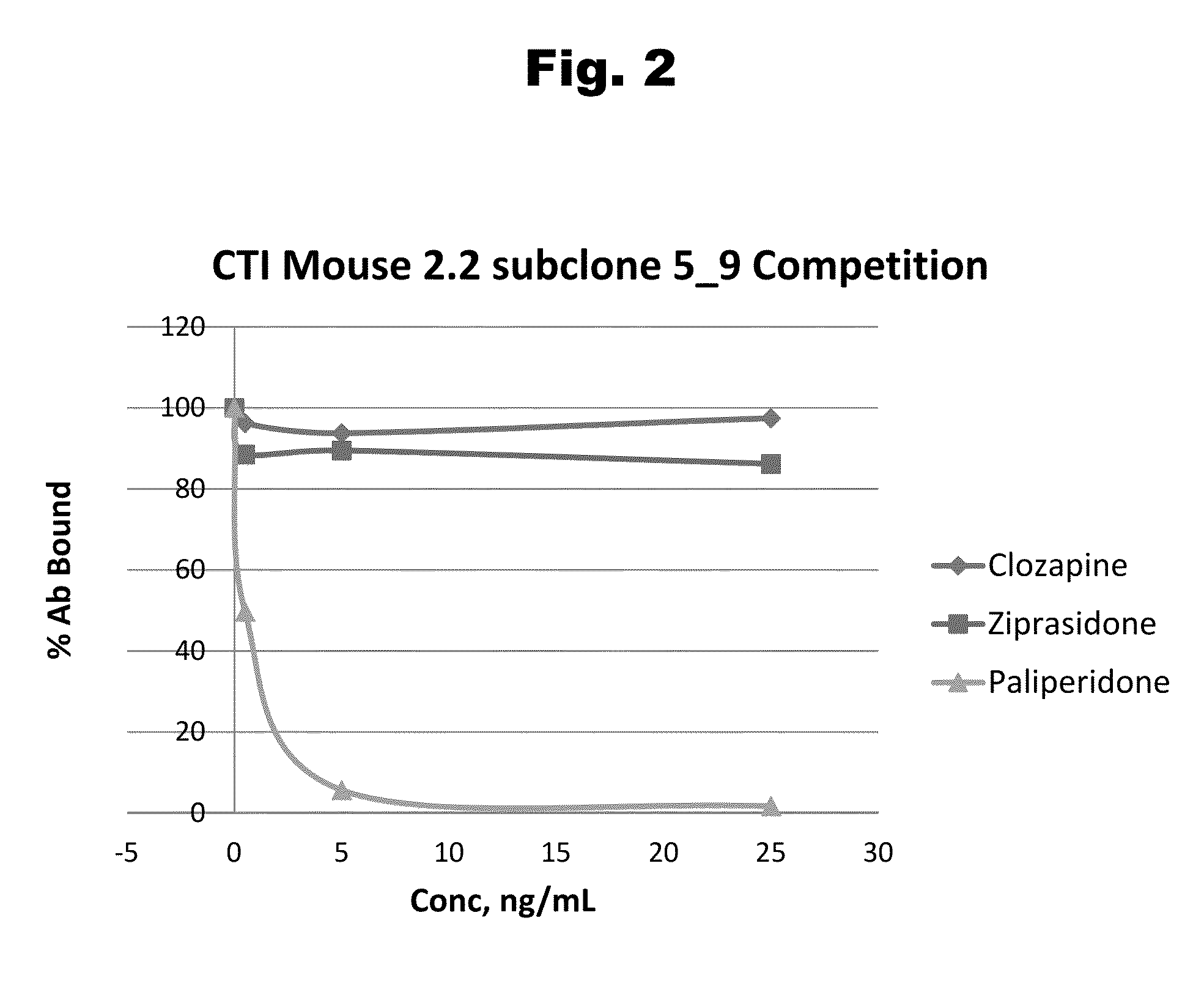
Risperidone immunoassay
Novel conjugates and immunogens derived from risperidone and antibodies generated by these immunogens are useful in immunoassays for the quantification and monitoring of risperidone and paliperidone in biological fluids.
Owner:SALADAX BIOMEDICAL INC
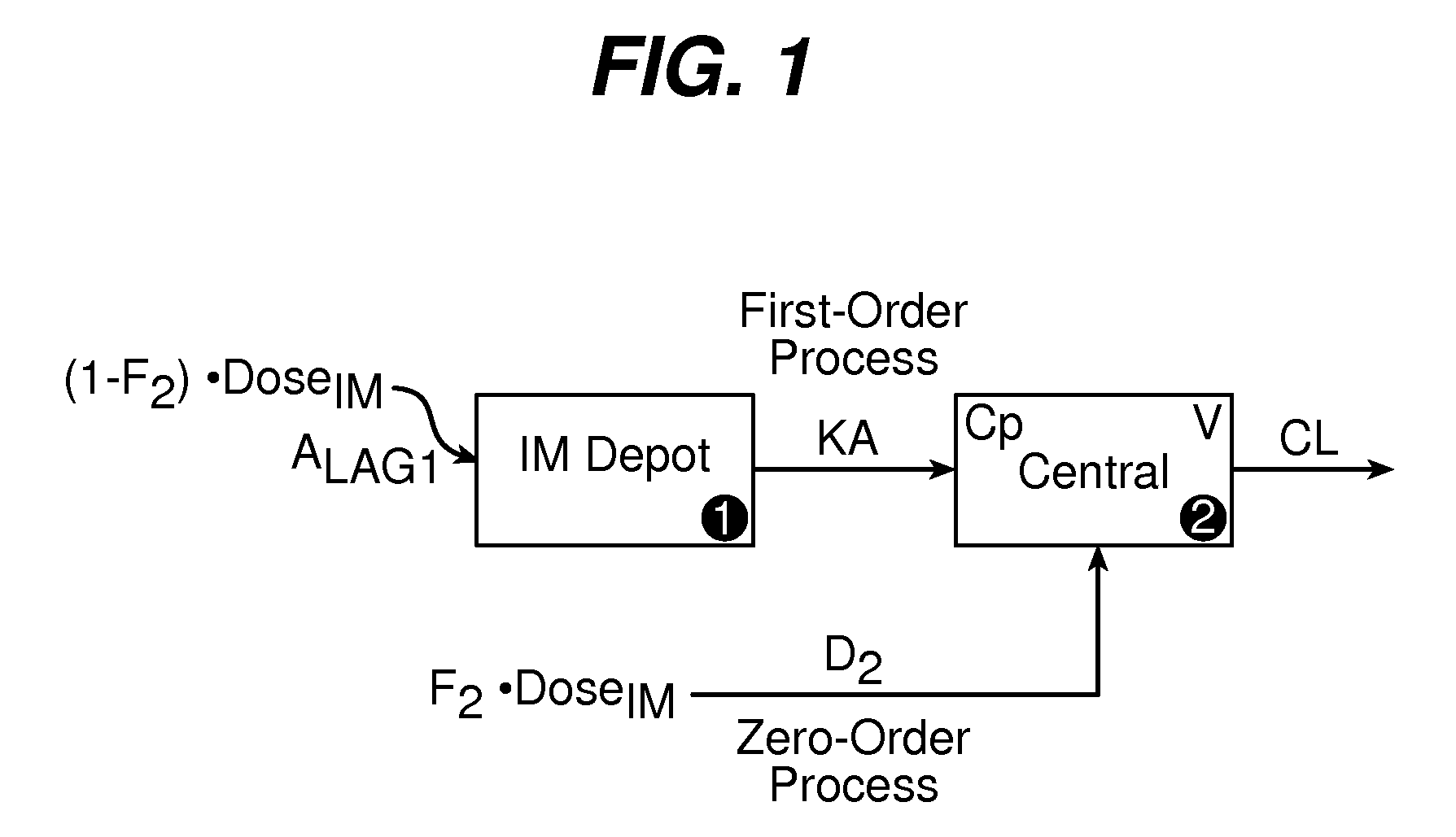
Dosing regimen associated with long acting injectable paliperidone esters
The present invention provides a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations.
Owner:JANSSEN PHARMA NV
Methods and dosage forms for controlled delivery of paliperidone and risperidone
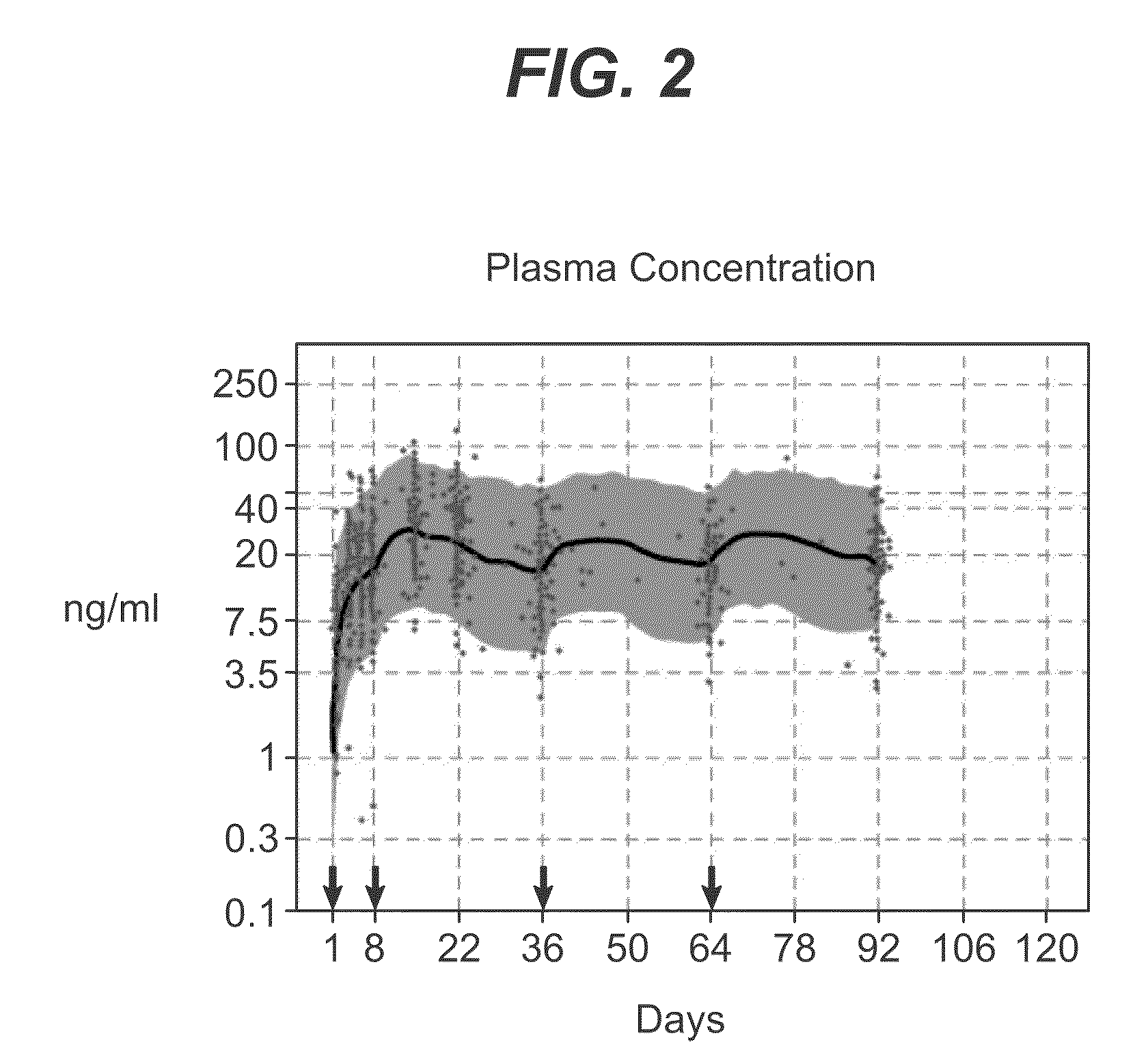
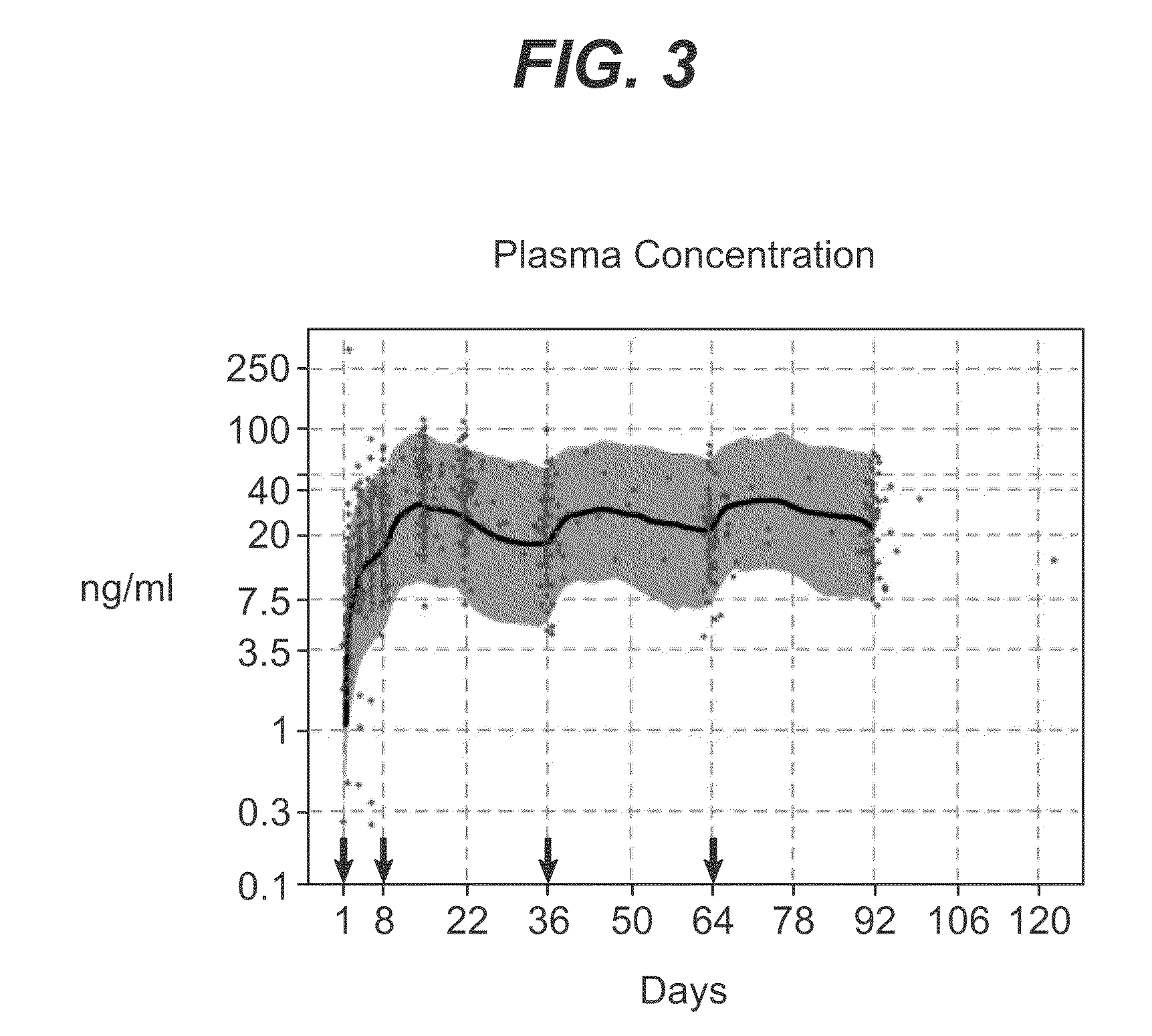
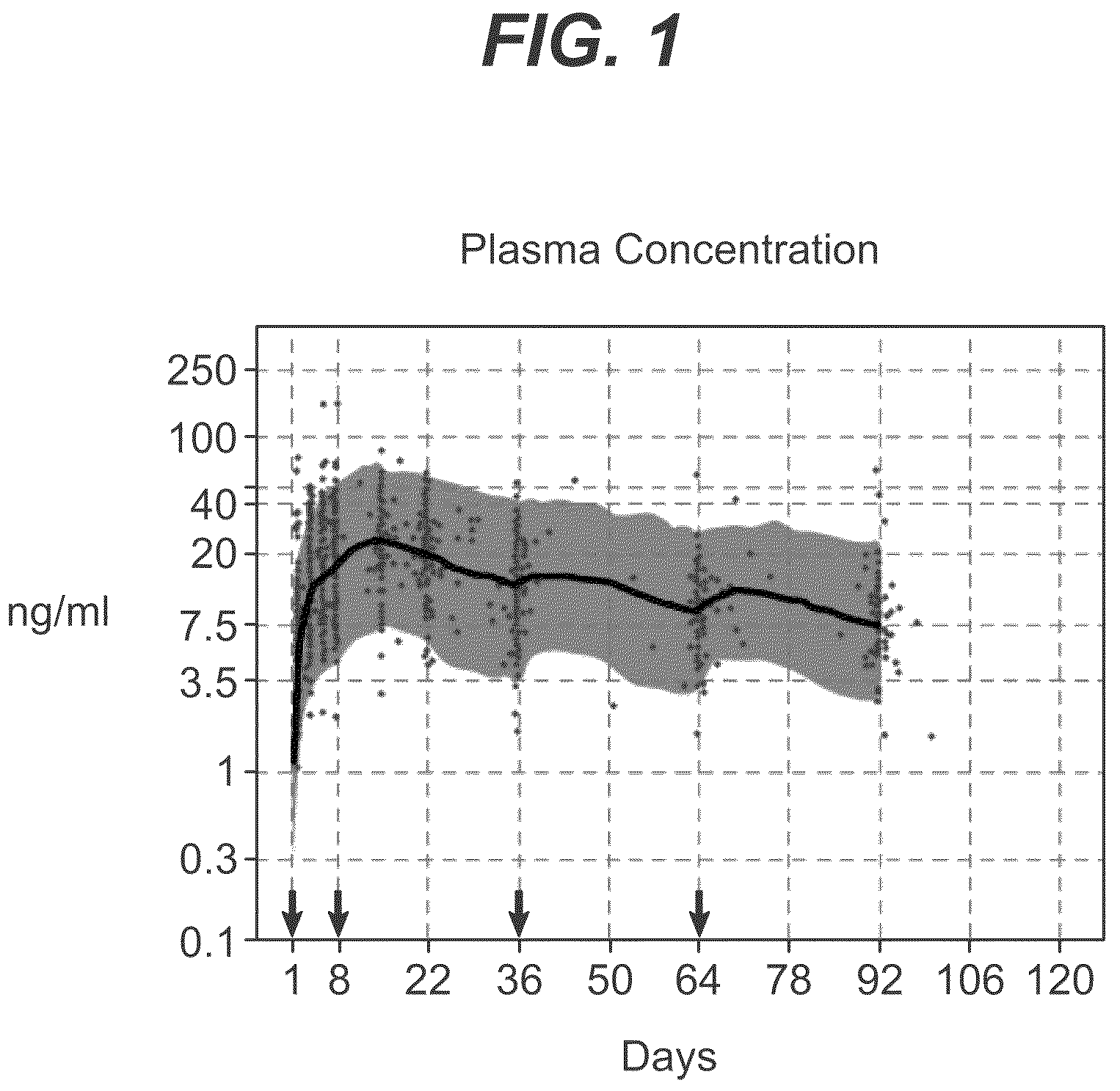
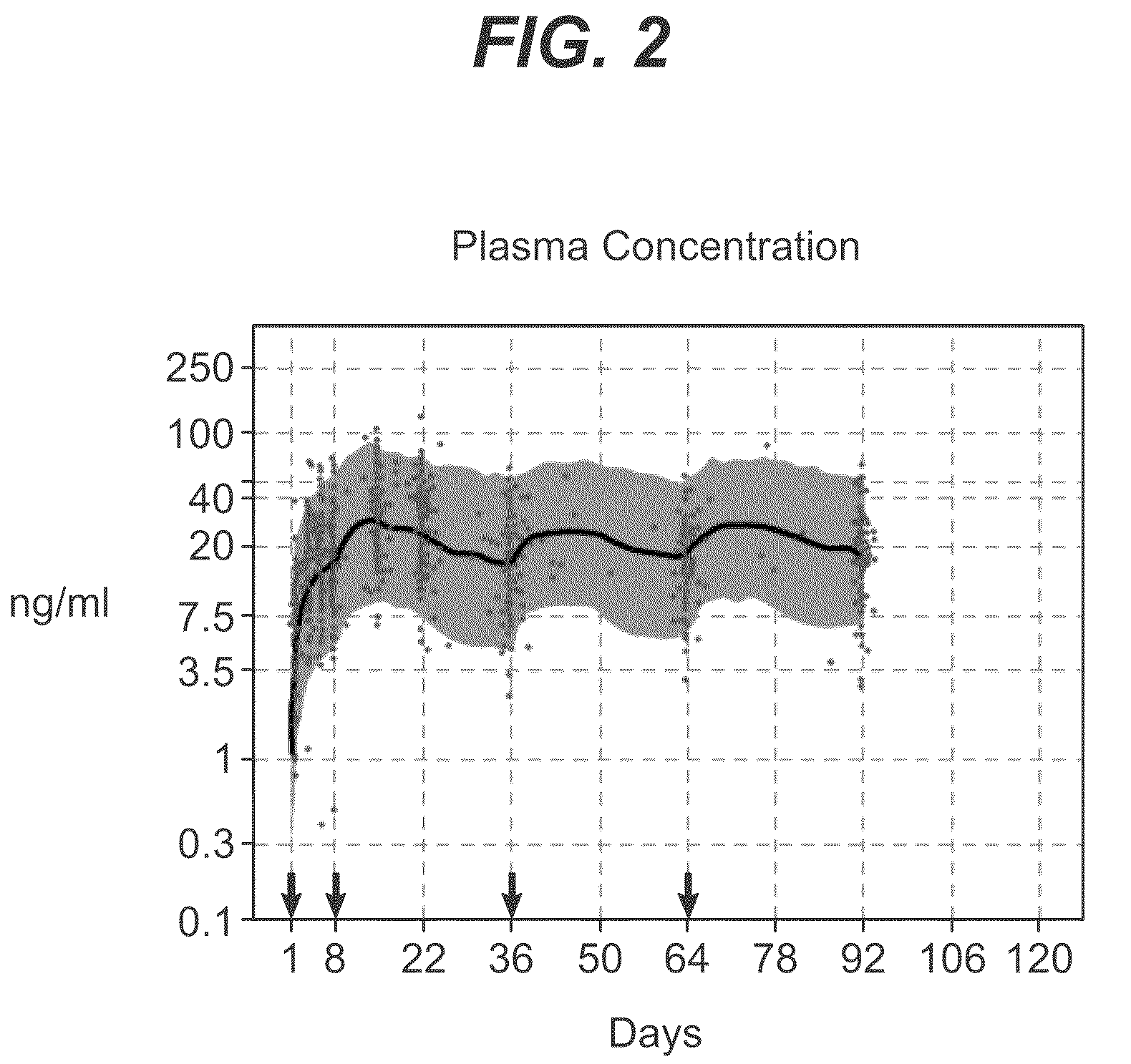
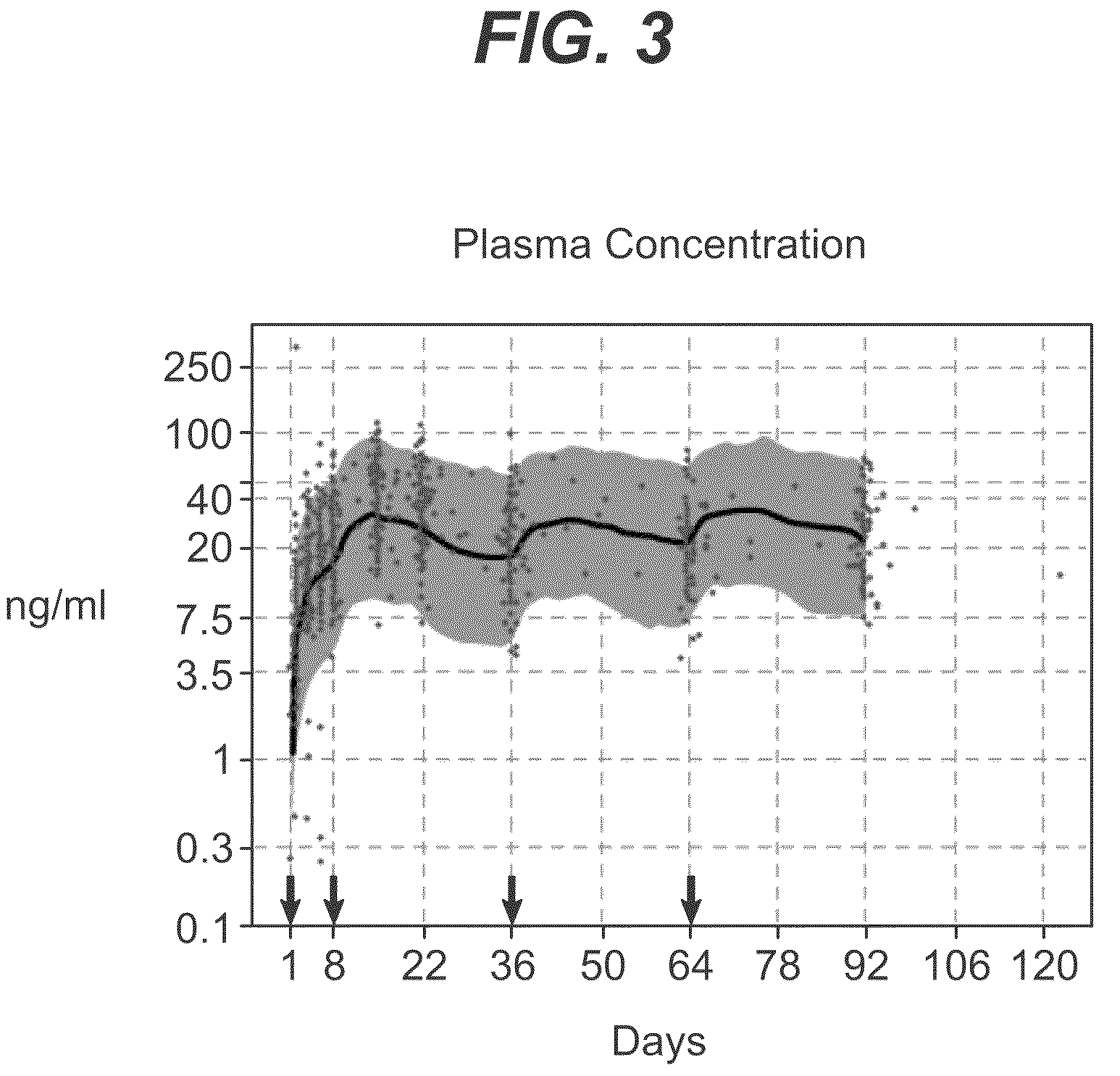
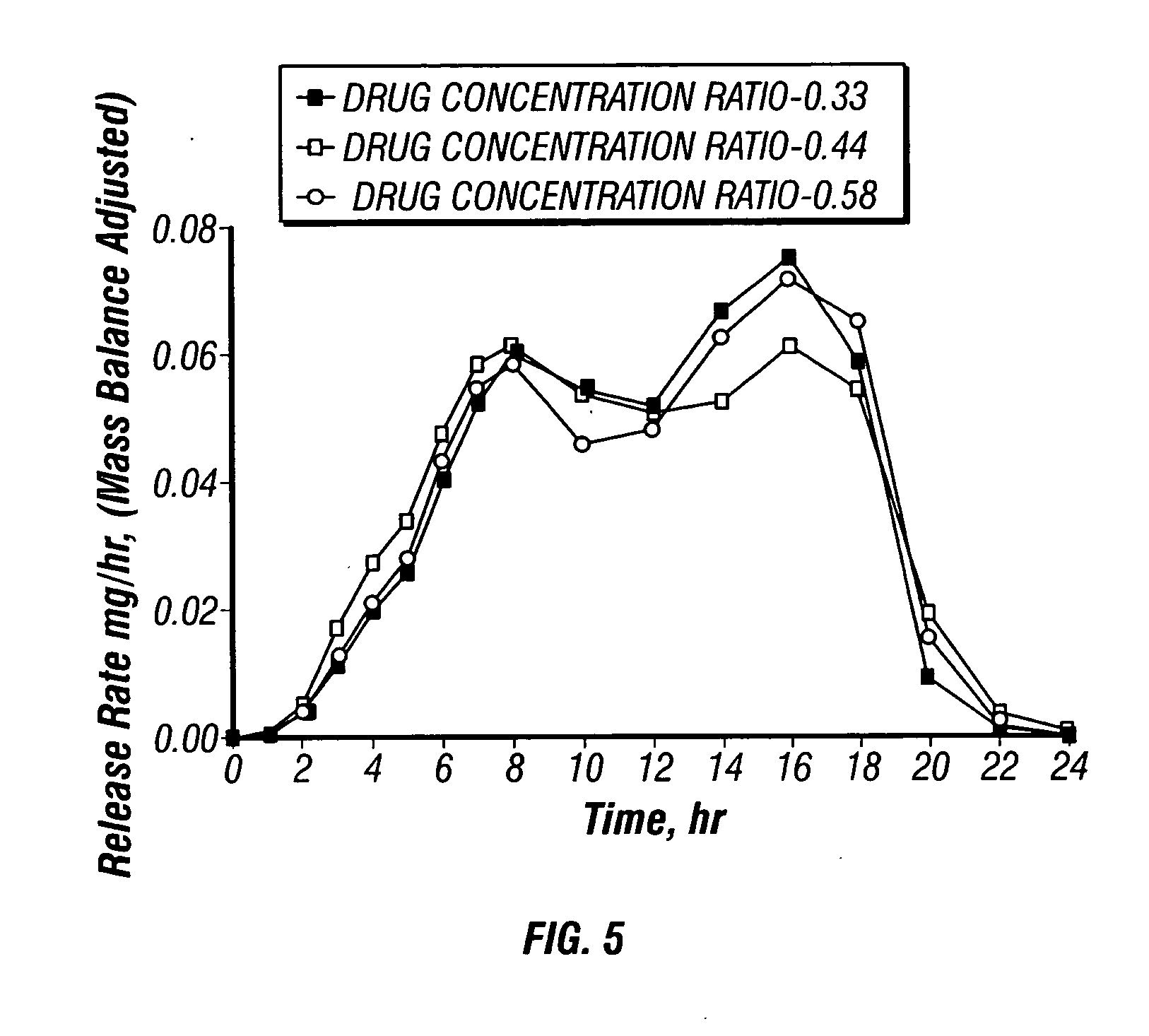
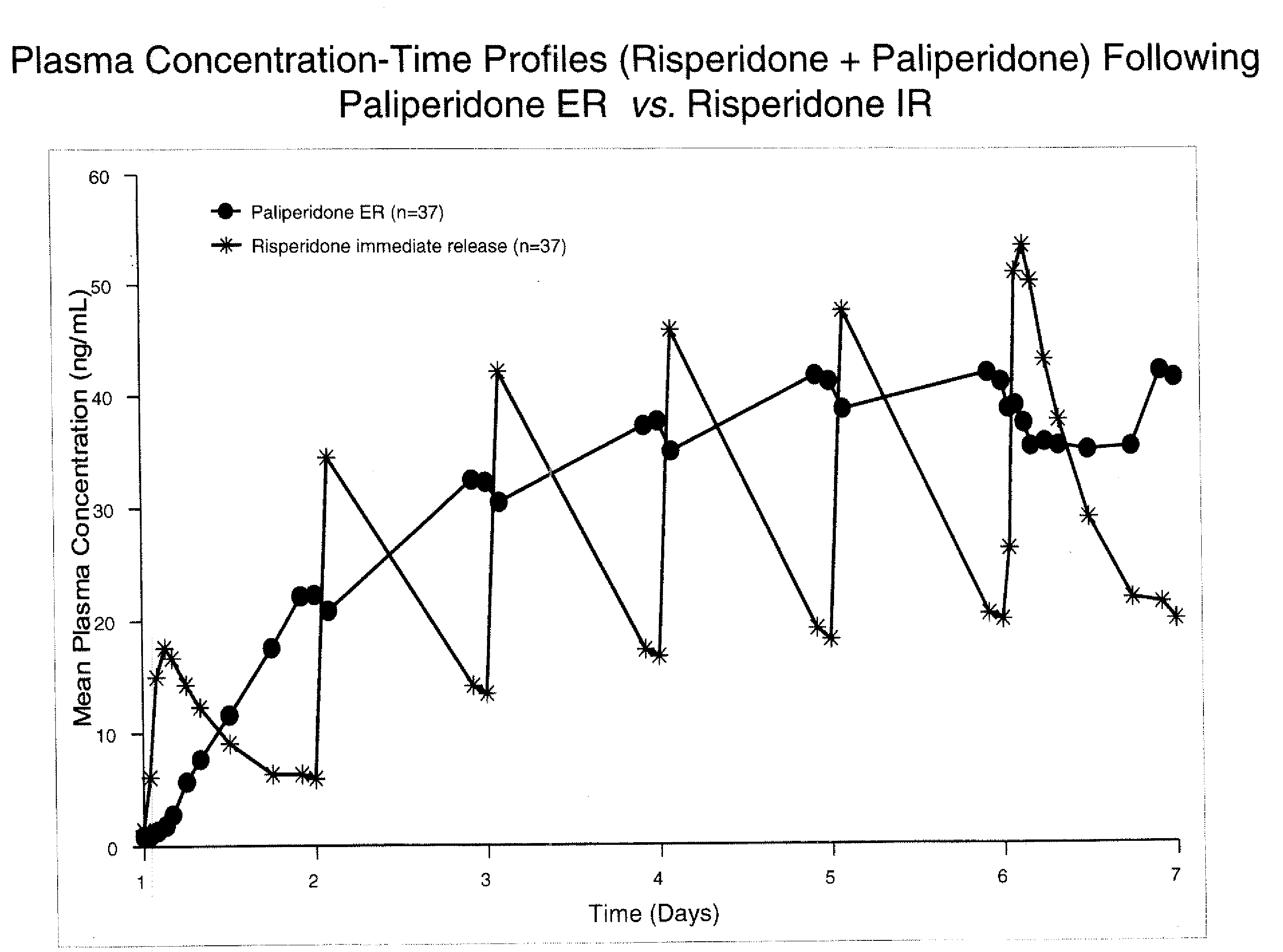
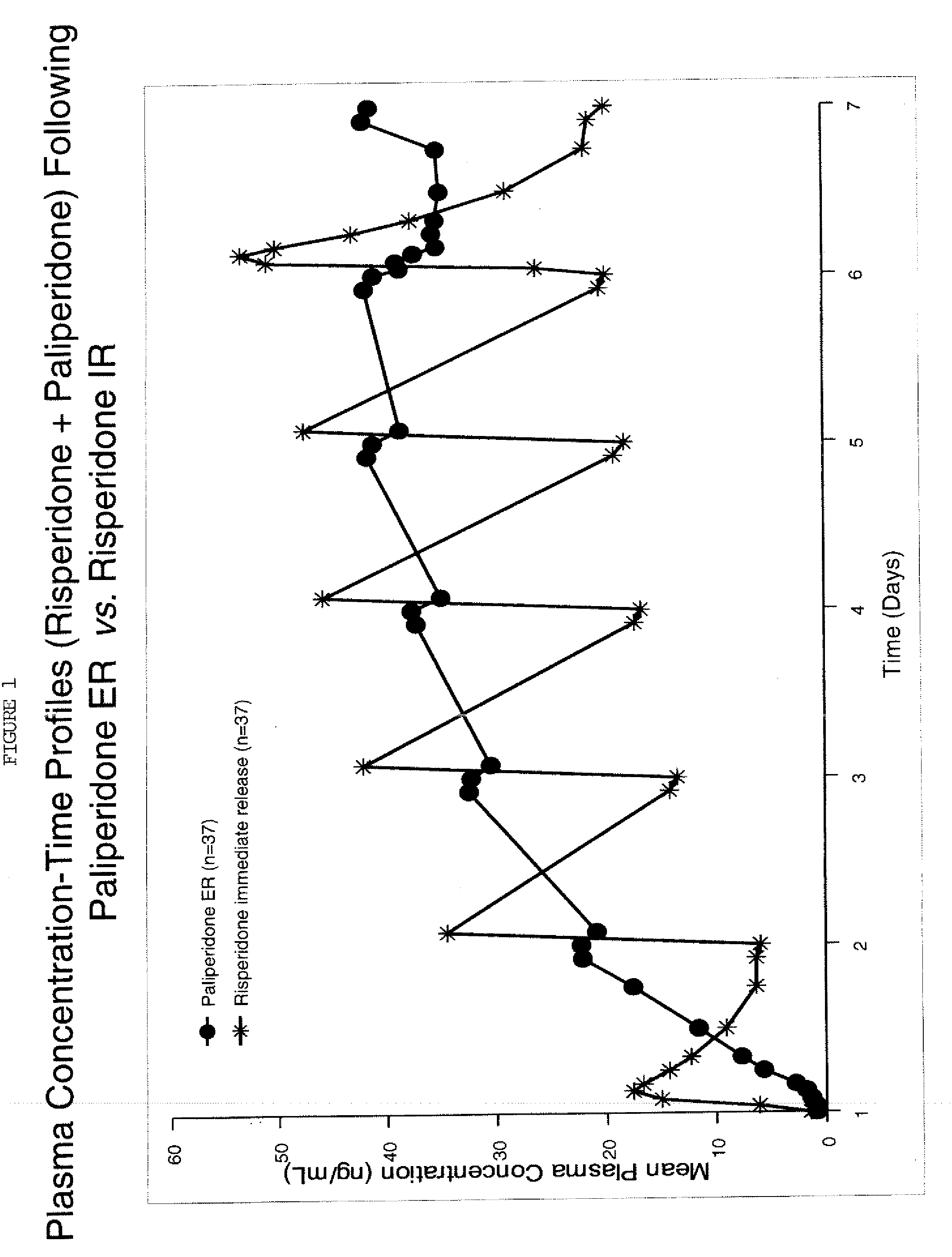
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
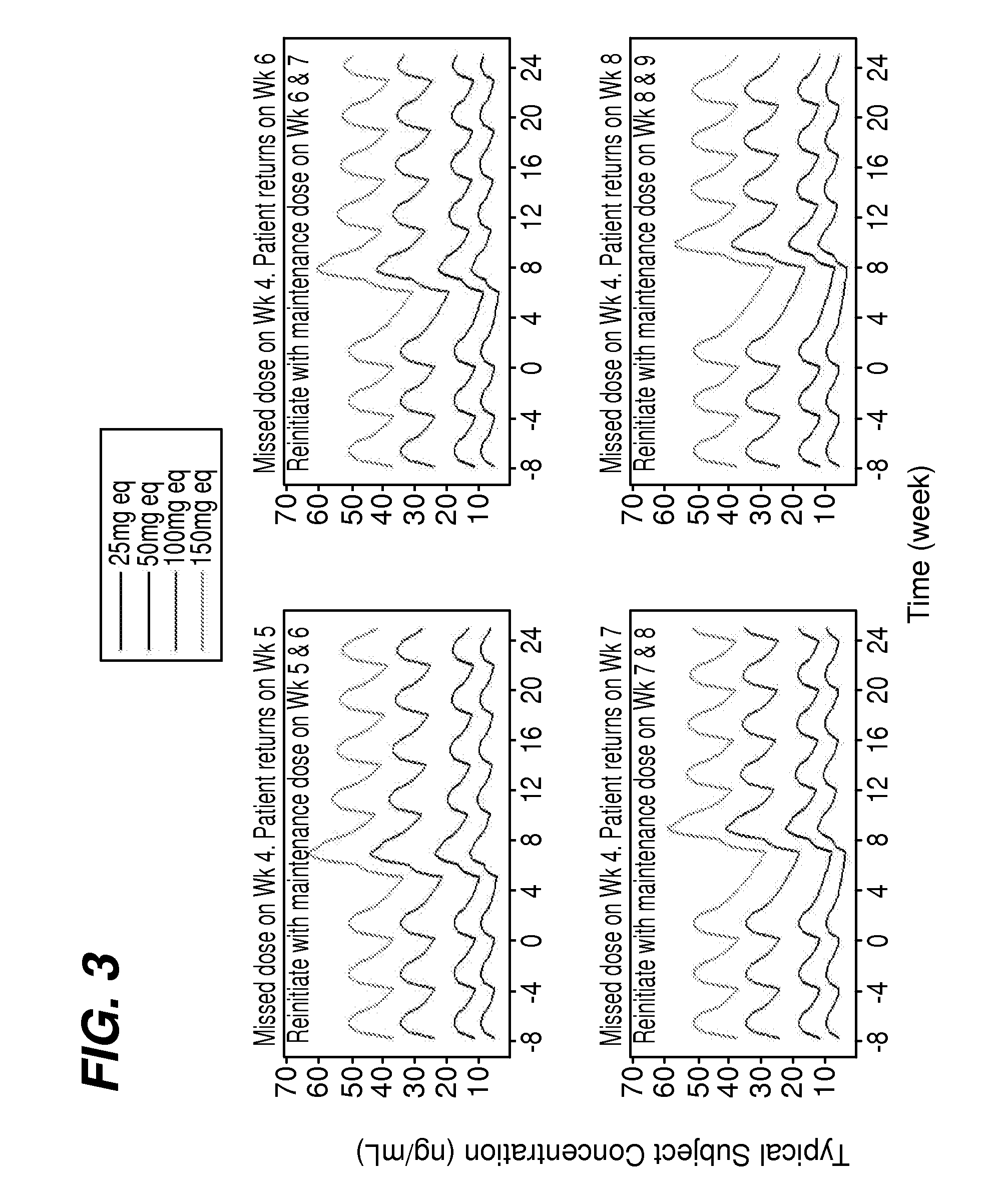
Dosing regimen associated with long-acting injectable paliperidone esters
The present application provides a method for treating patients in need of psychiatric treatment, wherein said patient misses a stabilized dose of a monthly maintenance regimen of paliperidone palmitate. The present application also provides a method for treating psychiatric patients in need of a switching treatment to paliperidone palmitate in a sustained release formulation.
Owner:JANSSEN PHARMA NV
Risperidone immunoassay
ActiveUS20110229979A1Reduced responseLittle and cross reactivityPeptide-nucleic acidsNervous disorderBiochemistryPaliperidone
Novel conjugates and immunogens derived from risperidone and antibodies generated by these immunogens are useful in immunoassays for the quantification and monitoring of risperidone and paliperidone in biological fluids.
Owner:SALADAX BIOMEDICAL INC
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
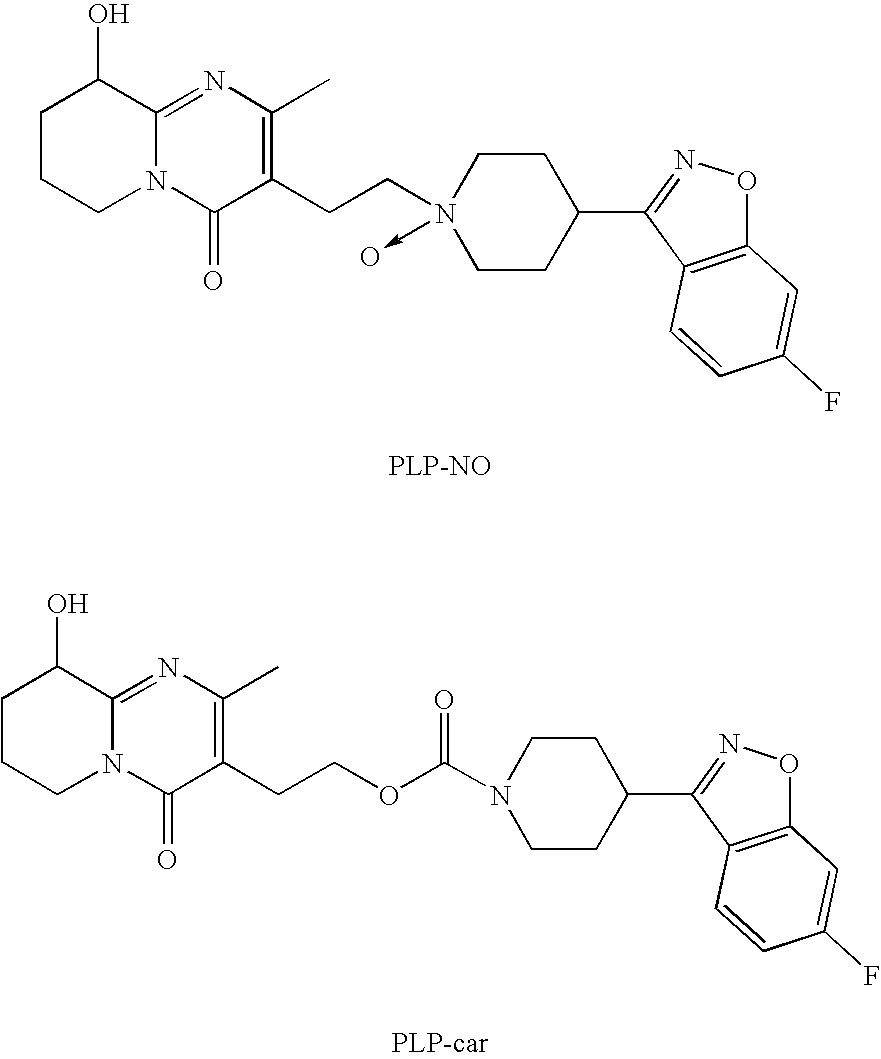
Haptens of risperidone and paliperidone
The invention relates to compounds of Formula I, wherein R1 and R2 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from risperidone and paliperidone. The invention also relates to conjugates of a risperidone or paliperidone hapten and a protein.
Owner:JANSSEN PHARMA NV
Haptens of paliperidone
The invention relates to compounds of Formula I, wherein L1, L2, and L3 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from paliperidone. The invention also relates to conjugates of a paliperidone hapten and a protein.
Owner:JANSSEN PHARMA NV
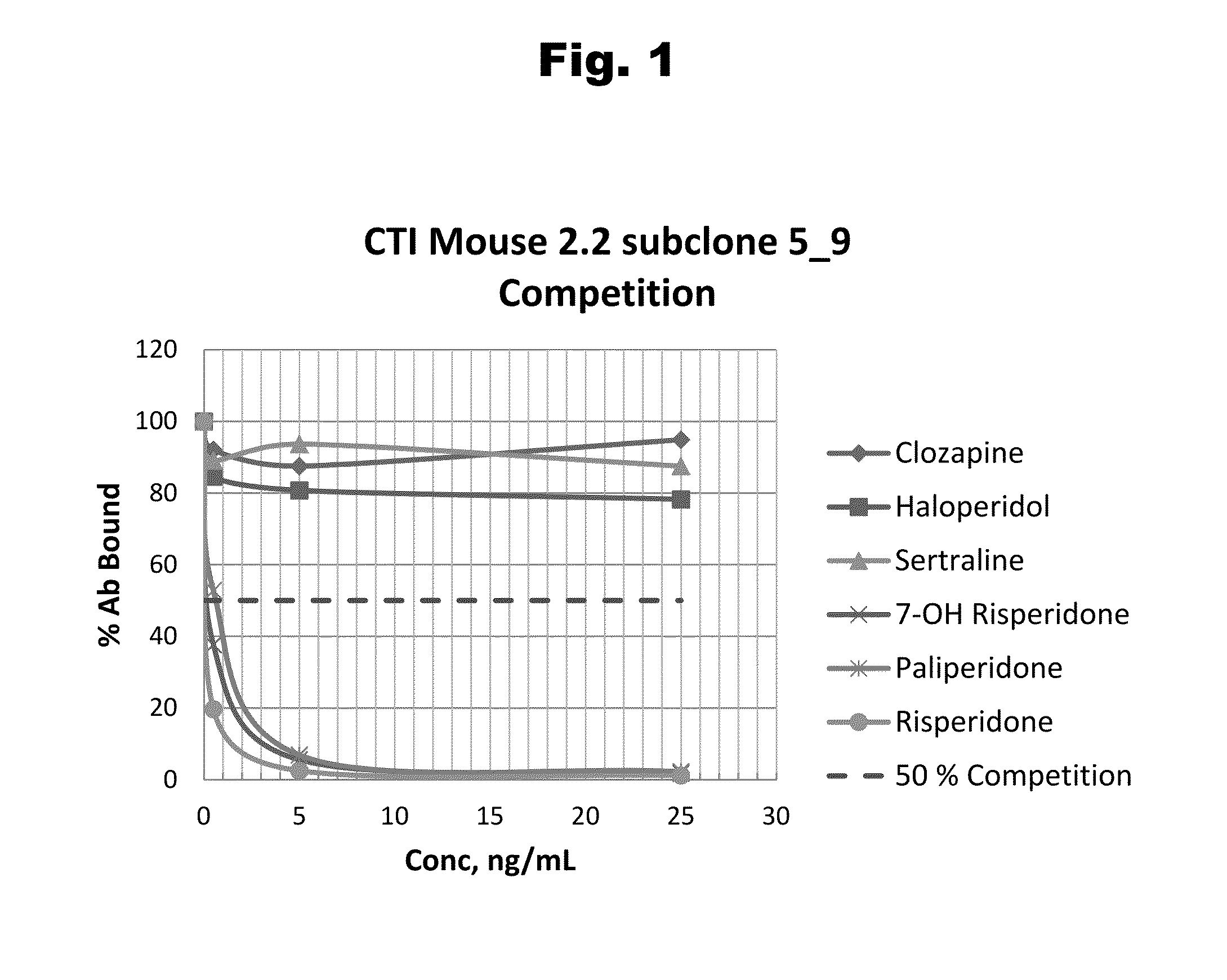
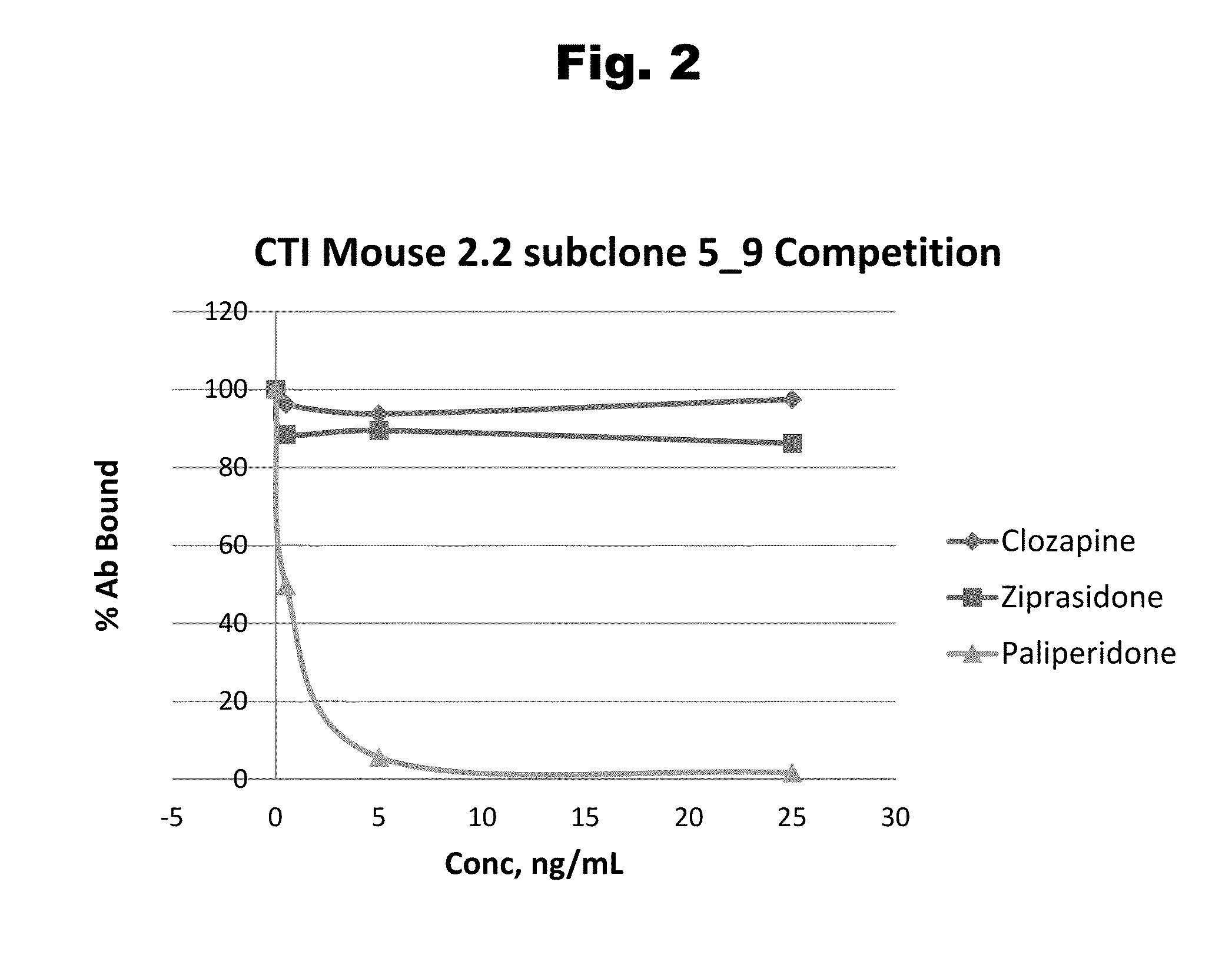
Antibodies to Paliperidone and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to Paliperidone Haptens and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, risperidone and paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Haptens of risperidone and paliperidone
The invention relates to compounds of Formula I, wherein R1 and R2 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from risperidone and paliperidone. The invention also relates to conjugates of a risperidone or paliperidone hapten and a protein.
Owner:JANSSEN PHARMA NV
Antibodies to paliperidone and use thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Dosing regimen associated with long acting injectable paliperidone esters
The present invention provides a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations.
Owner:JANSSEN PHARMA NV
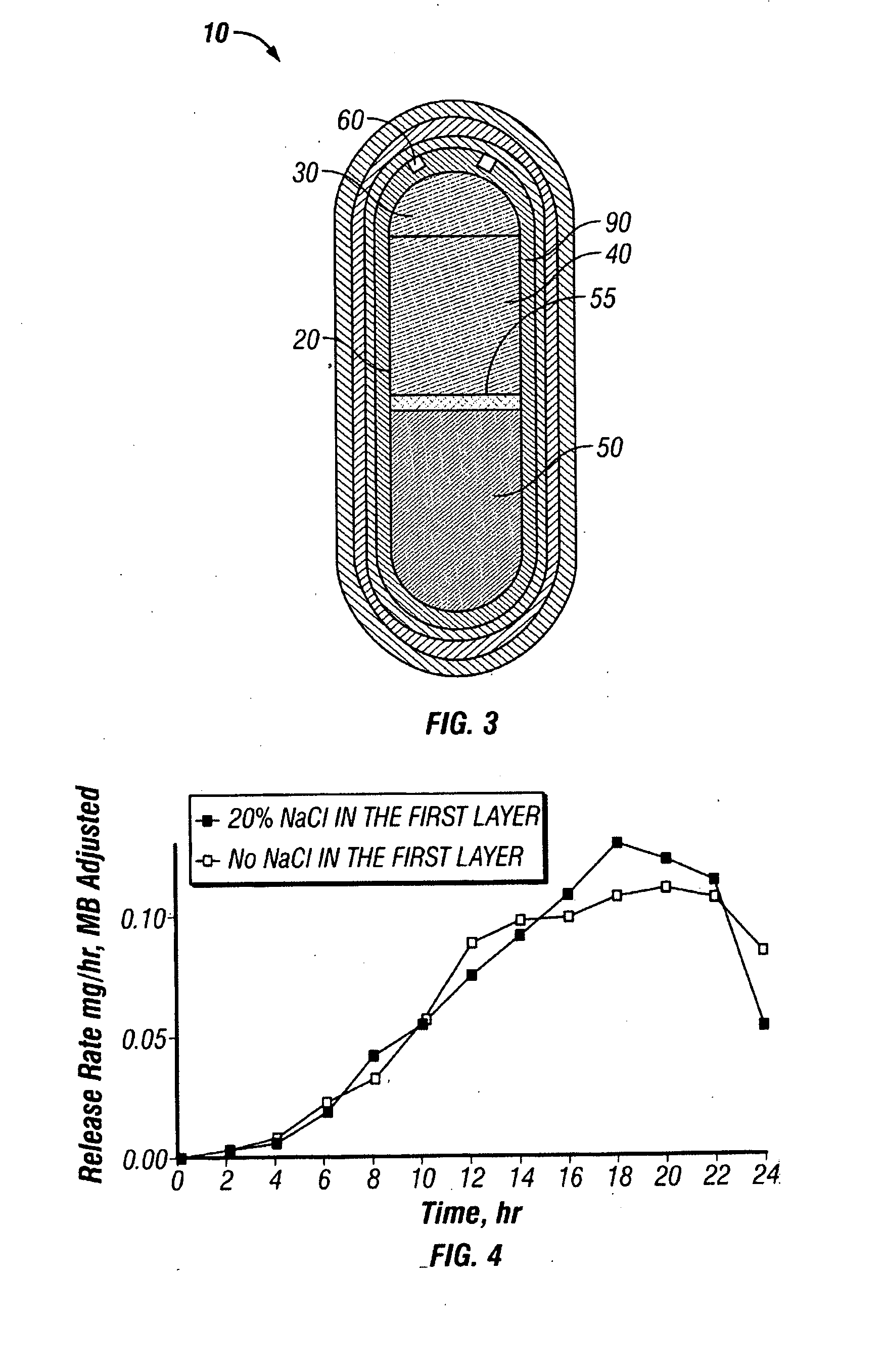
Paliperidone double-layered osmotic pump controlled release tablet and preparation method thereof
ActiveUS20120301547A1Increase release rateRapid onsetOrganic active ingredientsBiocideDrug release rateHydrophilic polymers
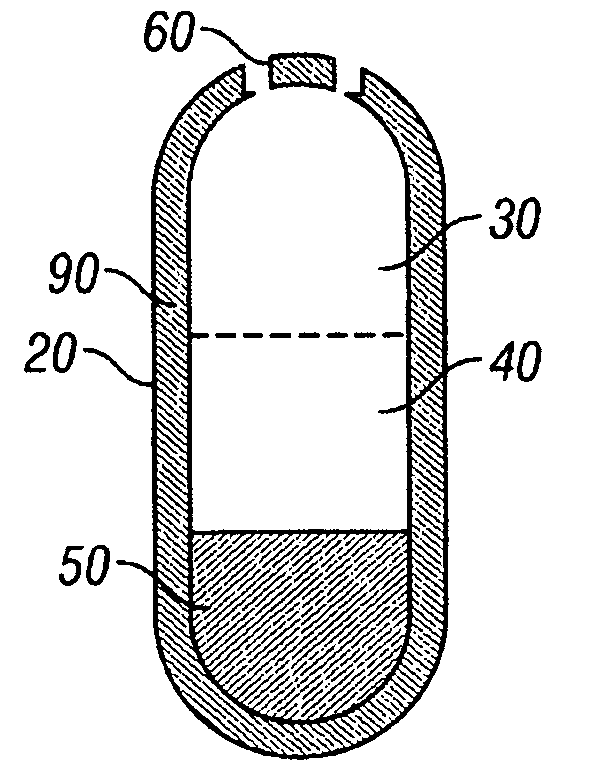
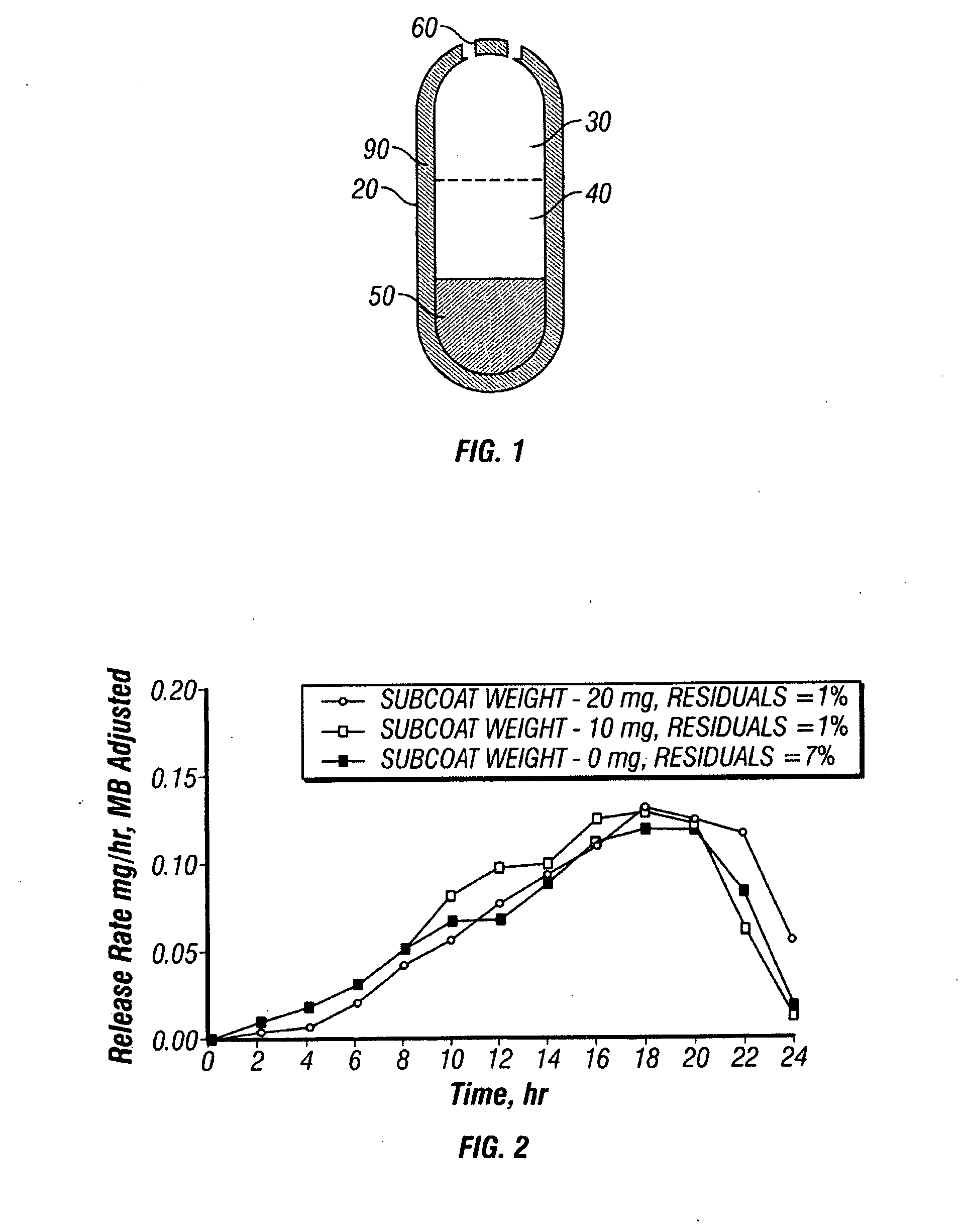
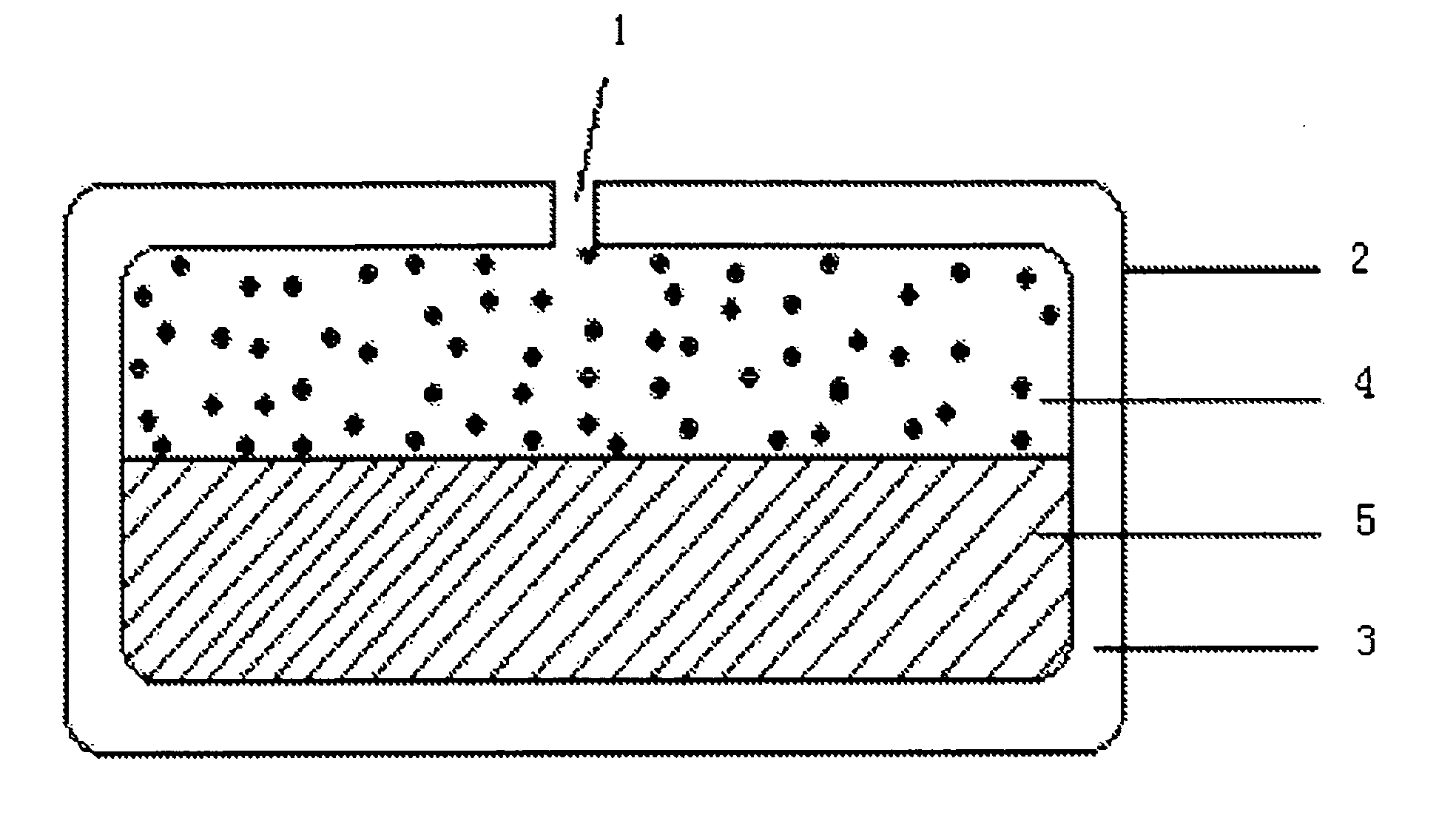
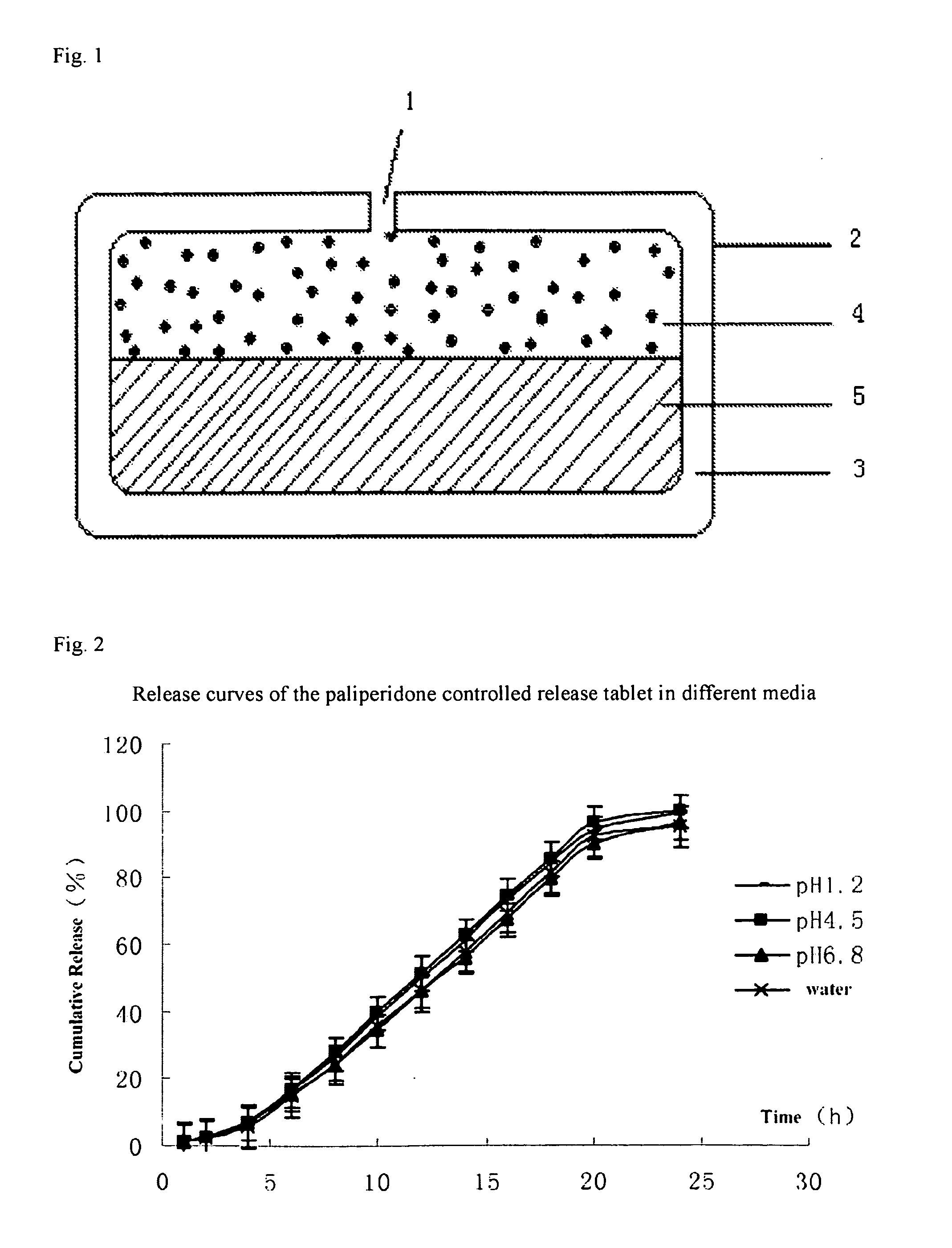
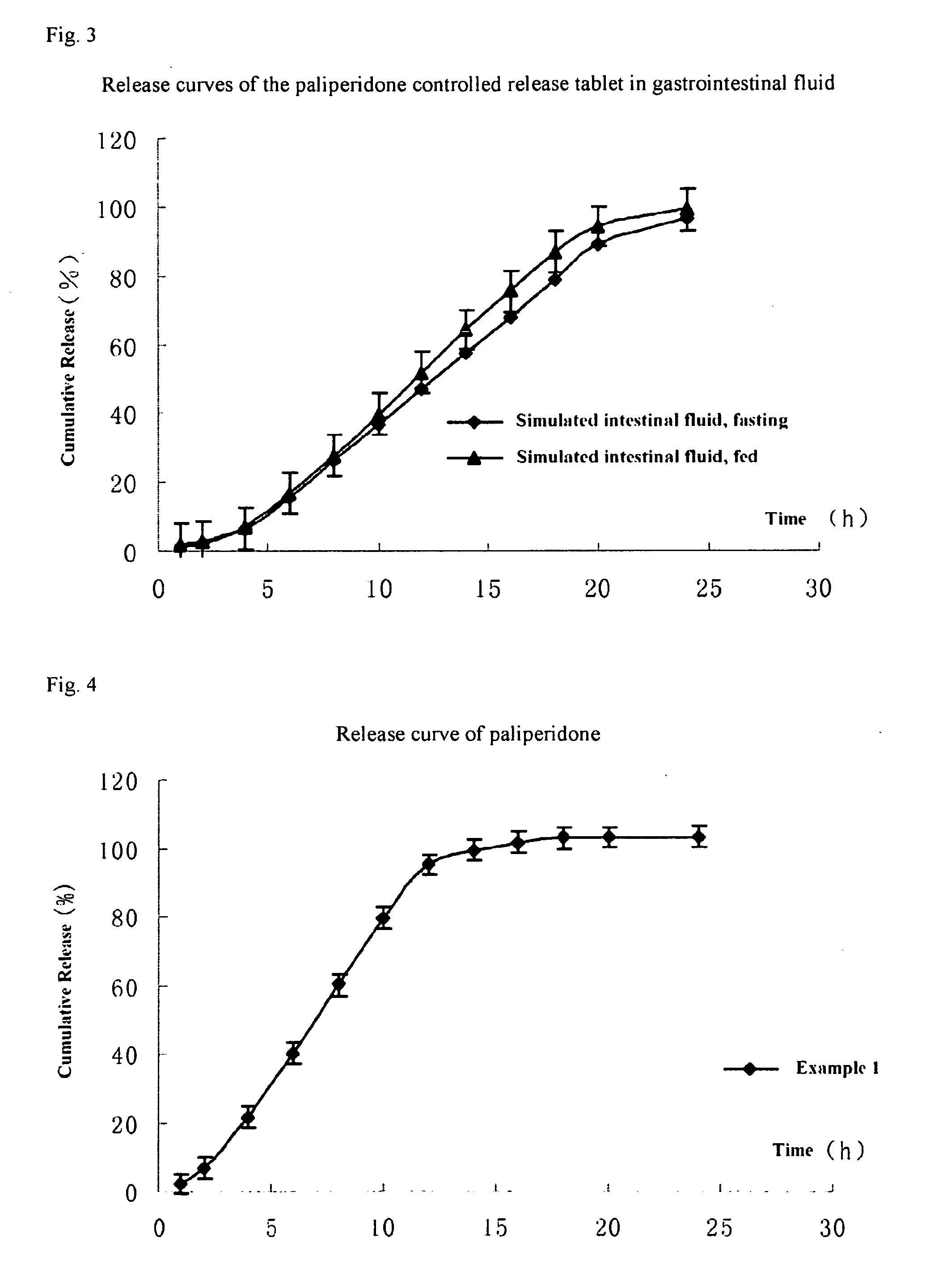
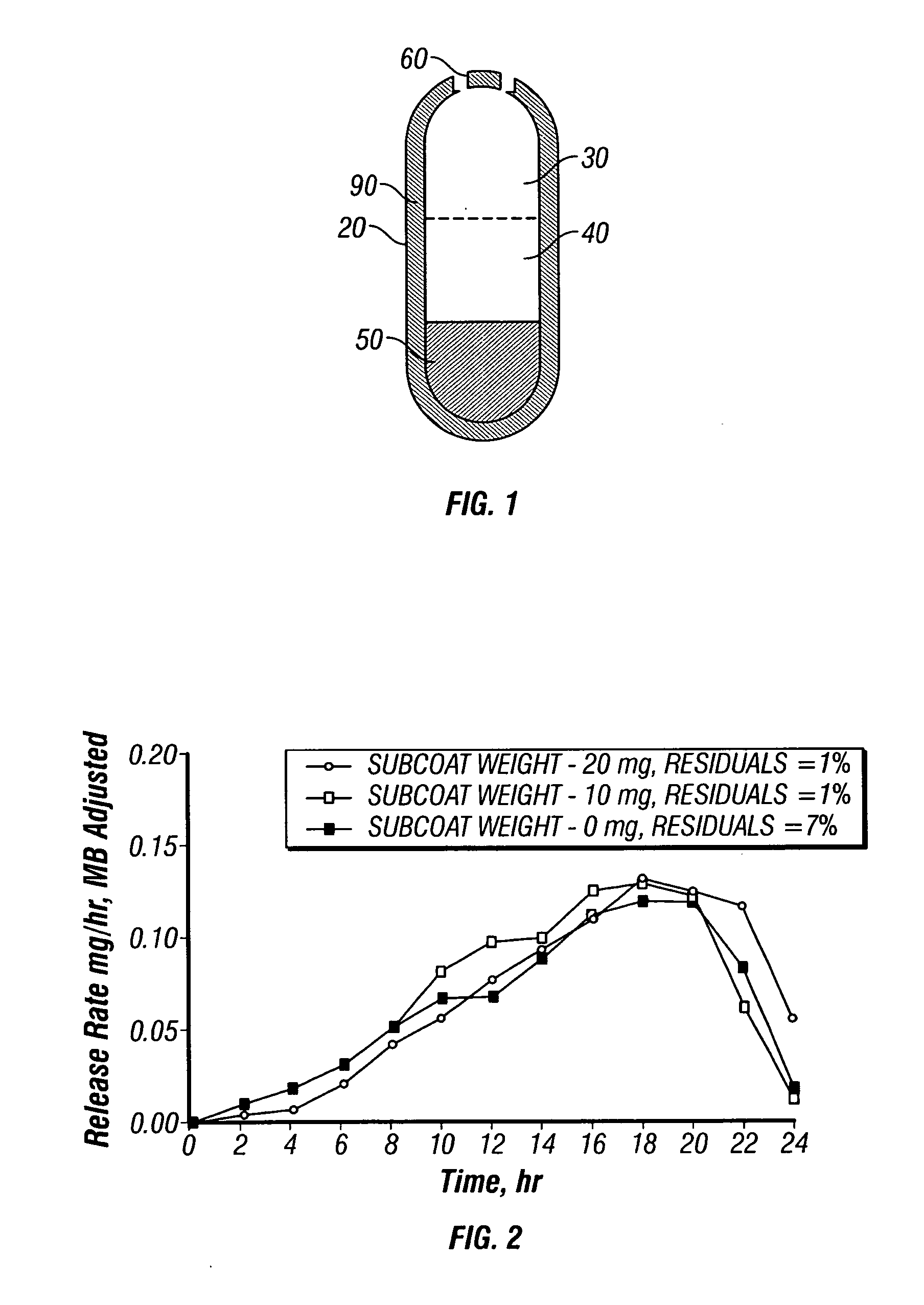
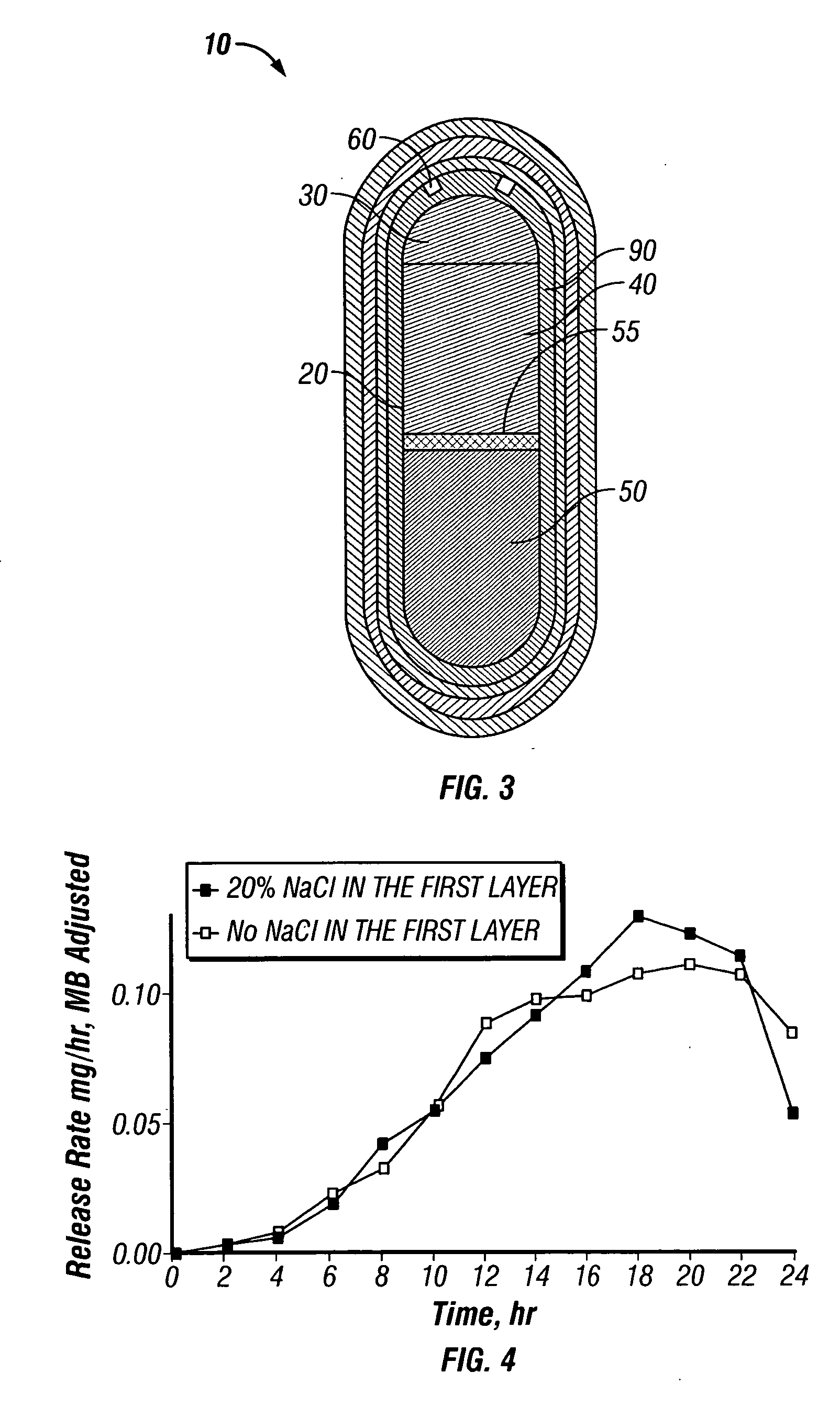
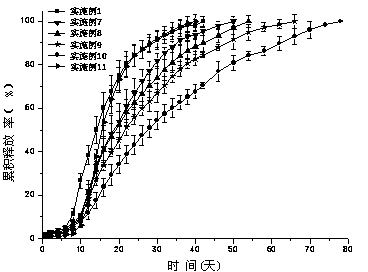
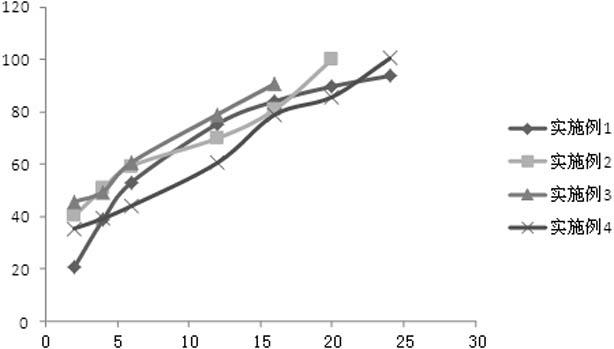
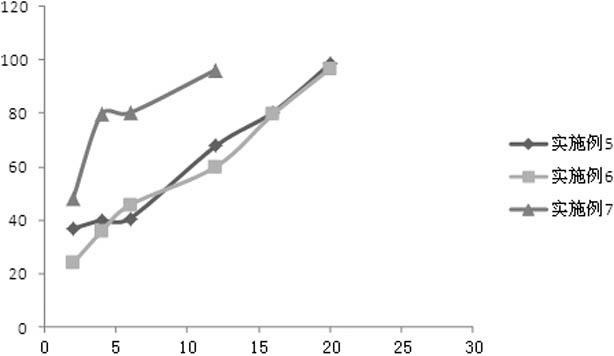
A paliperidone double-layered osmotic pump controlled release tablet and the preparation method thereof are disclosed. The double-layered osmotic pump controlled release tablet comprises a rigid membrane, a push layer, a drug layer, an isolation layer and an aesthetic coating, wherein the rigid membrane contains a semi-permeable polymer, a porogen and / or a plasticizer and has one or more drug release orifices on one end, the push layer comprises an expanding material, an osmotic agent, a binder, a colorant and a lubricant, the drug layer contains a pharmaceutically active ingredient, a hydrophilic polymer, an osmotic agent, a colorant, a lubricant and an antistatic agent, the isolation layer is located between the inner surface of the rigid membrane and the push layer, and contains a hydrophilic polymer. The paliperidone double-layered osmotic pump controlled release tablet shows an increasing drug release rate at early stage and keeps a constant drug release rate at later stage.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Haptens of paliperidone
The invention relates to compounds of Formula I, wherein L1, L2, and L3 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from paliperidone. The invention also relates to conjugates of a paliperidone hapten and a protein.
Owner:JANSSEN PHARMA NV
Method for synchronously detecting seventeen antipsychotics in blood sample
InactiveCN109668979ARealize detectionAchieving Simultaneous DetectionComponent separation9-HydroxyrisperidoneSolvent
The invention discloses a method for synchronously detecting seventeen antipsychotics in a blood sample. The method comprises the steps that the sample is extracted with a mixed solution of methanol and acetonitrile to obtain supernate, and after a solvent is removed from the supernate, the supernate is dissolved in a methanol aqueous solution and filtered to obtain a specimen; a high performanceliquid chromatography tandem mass spectrometry method is adopted to detect the specimen; the sample is serum or plasma, and the antipsychotics are amisulpride, aripiprazole, dehydro aripiprazole, chlorpromazine, clozapine, desmethylclozapine, fluphenazine, haloperidol, olanzapine, paliperidone, perphenazine, quetiapine, risperidone, 9-hydroxyrisperidone, sulpiride, ziprasidone and thioridazine; inthe high performance liquid chromatography, a mobile phase A is an aqueous solution of formic acid, and a mobile phase B is a methanol solution of the formic acid; and the mass spectrometry adopts amulti-ion reaction monitoring mode of positive ion electrospray ionization.
Owner:JINAN YING SHENG BIOTECH
Paliperidone implant formulation
ActiveUS10350159B2Organic active ingredientsPharmaceutical delivery mechanismGlycolic acidPharmaceutical drug
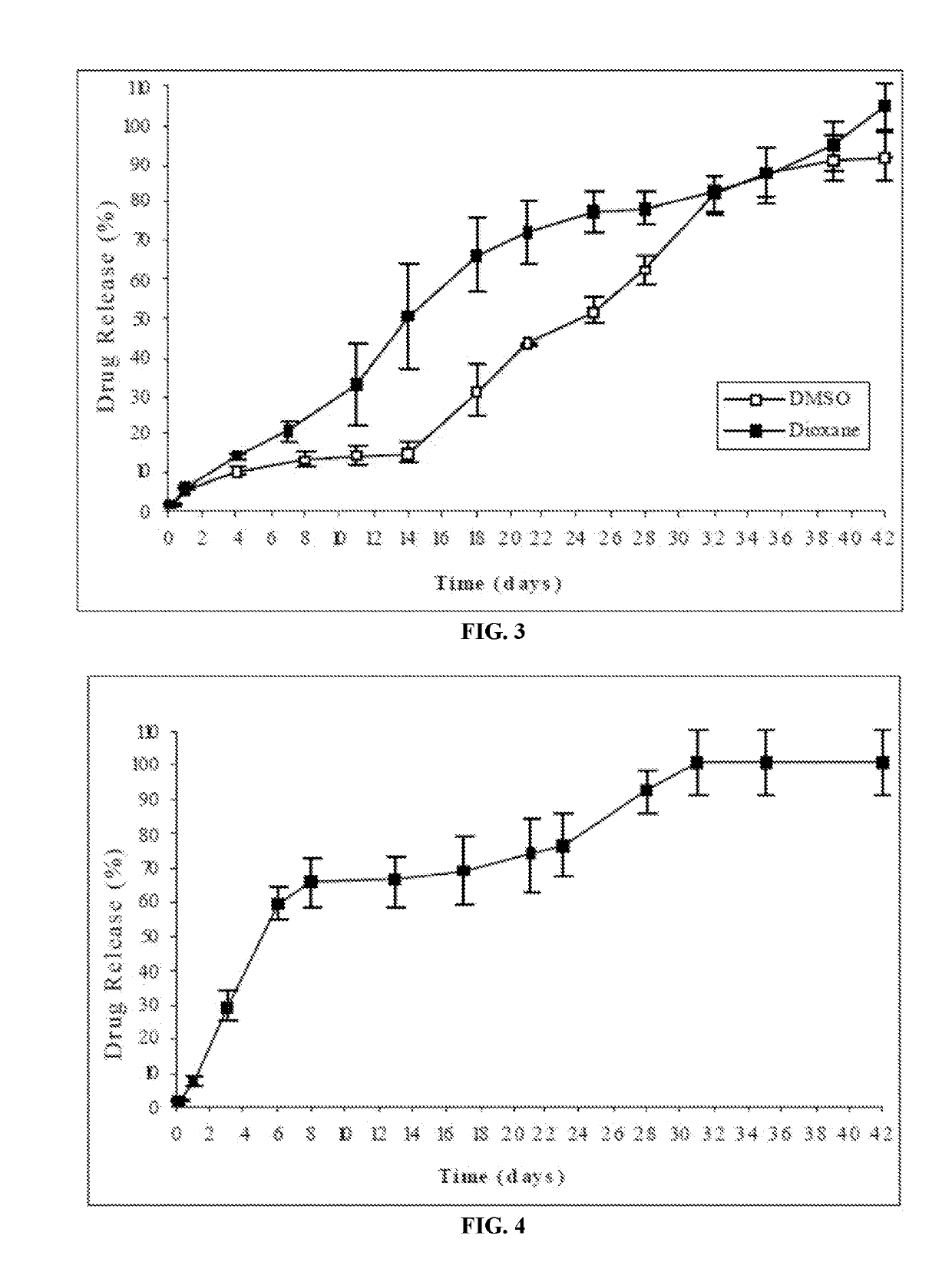
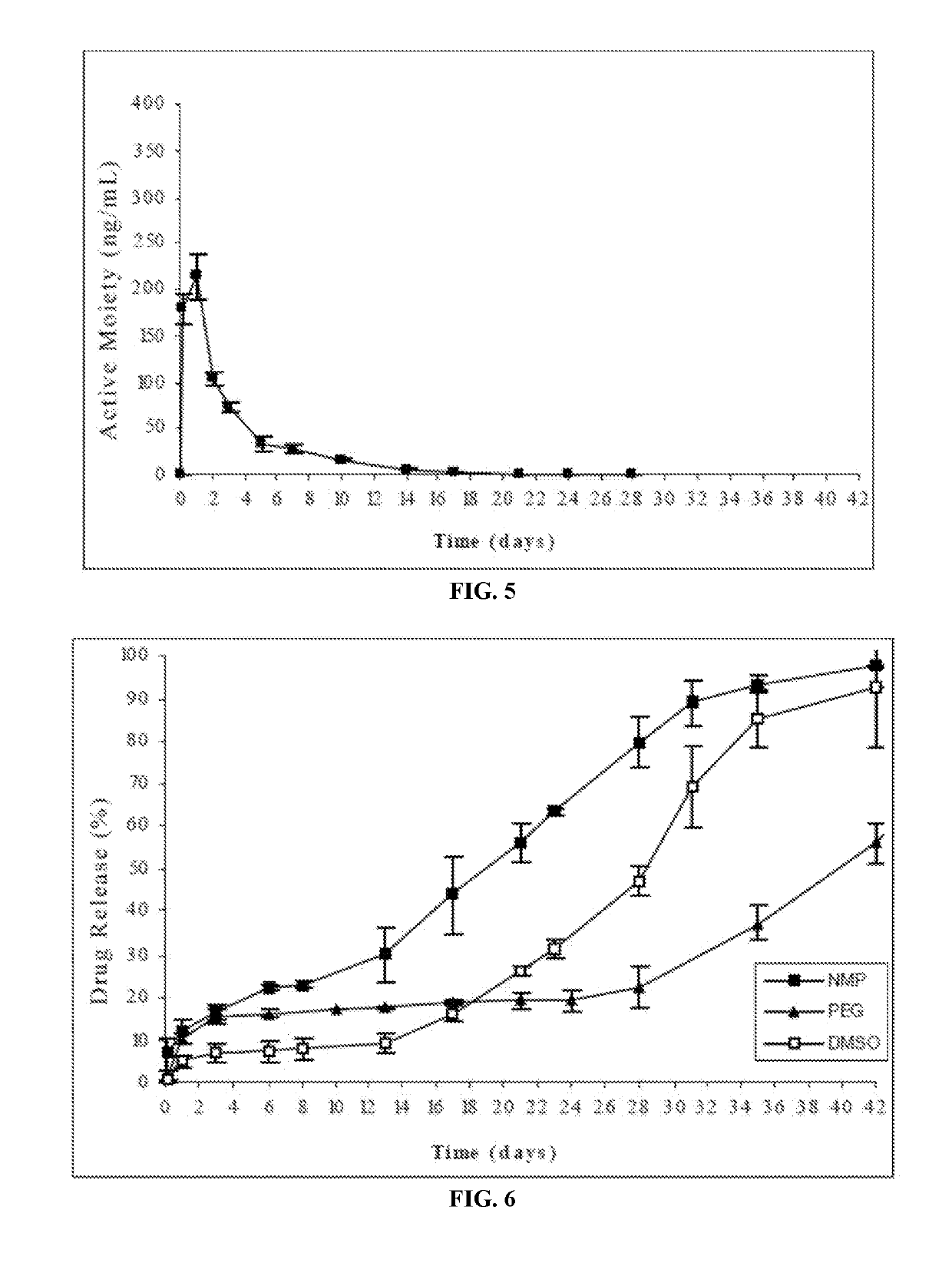
An injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is paliperidone and / or its pharmaceutical acceptable salts in any combination thereof, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and DMSO as solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 8 weeks and wherein the composition has a pharmacokinetic profile in vivo suitable for the formulation to be administered each 8 weeks or even longer periods.
Owner:LAB FARM ROVI SA
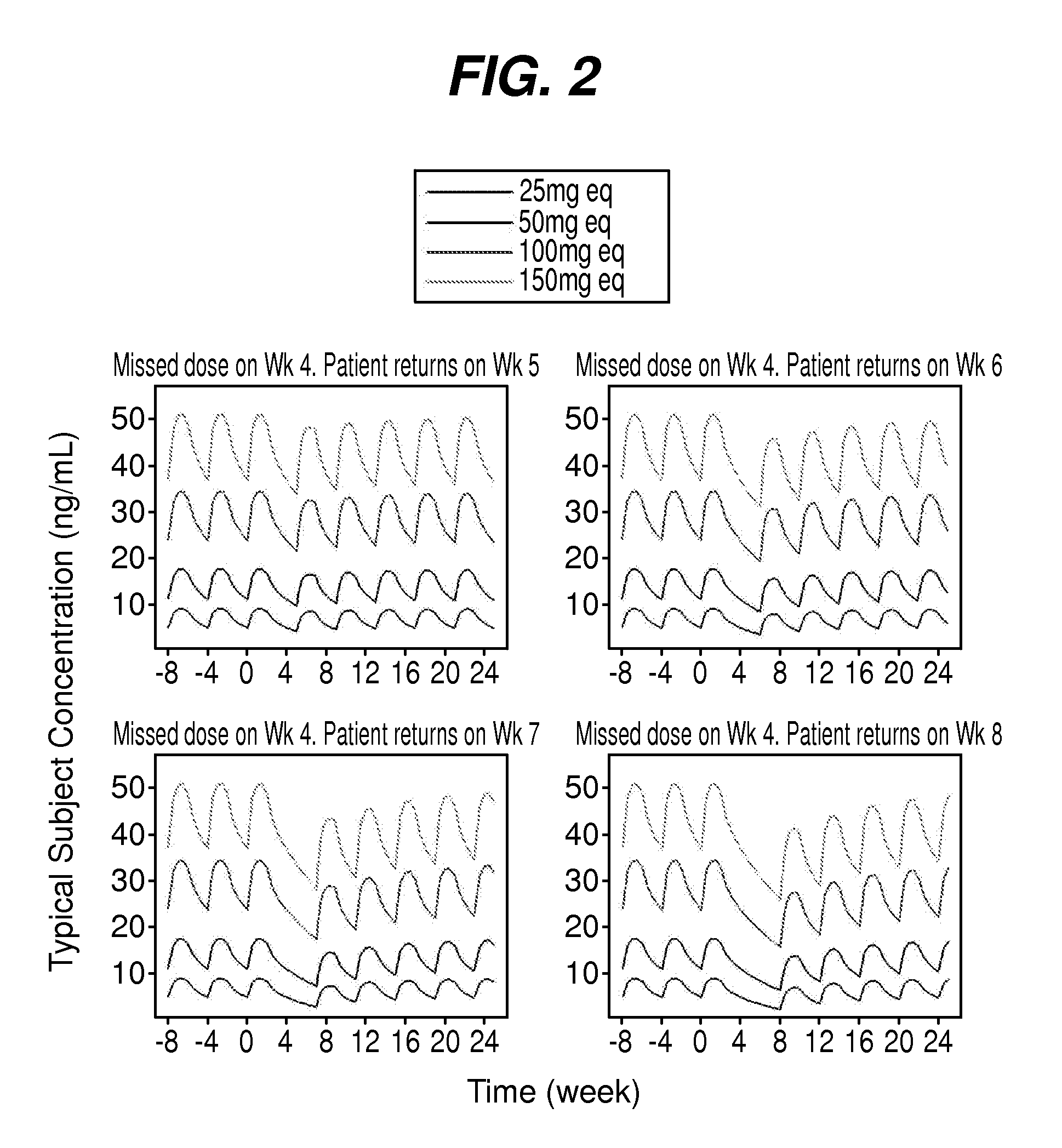
Dosing regimen for missed doses for long-acting injectable paliperidone esters
The present application provides a method for treating patients in need of psychiatric treatment, wherein said patient is being treated with the 3-month formulation of paliperidone palmitate and fails to take the next scheduled dose of the 3-month formulation of paliperidone palmitate.
Owner:JANSSEN PHARMA NV
Risperidone or Paliperidone Implant Formulation
The present invention is directed to an injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is risperidone and / or paliperidone or any pharmaceutically acceptable salt thereof in any combination, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and a DMSO solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 4 weeks and wherein the composition has a pharmacokinetic profile in vivo that makes it suitable to be administered each 4 weeks or even longer periods.
Owner:LAB FARM ROVI SA
Risperidone or paliperidone implant formulation
The present invention is directed to an injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is risperidone and / or paliperidone or any pharmaceutically acceptable salt thereof in any combination, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and a DMSO solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 4 weeks and wherein the composition has a pharmacokinetic profile in vivo that makes it suitable to be administered each 4 weeks or even longer periods.
Owner:LAB FARM ROVI SA
Paliperidone Implant Formulation
Owner:LAB FARM ROVI SA
Antipsychotic Injectable Depot Composition
ActiveUS10463607B2Simple methodAvoiding irregular initial burst release of drugOrganic active ingredientsPharmaceutical delivery mechanismMedicineSolvent
The present invention is directed to a composition that can be used to deliver an antipsychotic drug such as risperidone, paliperidone or a combination thereof, as an injectable in-situ forming biodegradable implant for extended release providing therapeutic plasma levels from the first day. The composition is in the form of drug suspension on a biodegradable and biocompatible copolymer or copolymers solution using water miscible solvents that is administered in liquid form. Once the composition contacts the body fluids, the polymer matrix hardens retaining the drug, forming a solid or semisolid implant that releases the drug in a continuous manner. Therapeutic plasma levels of the drug can be achieved from the first day up to at least 14 days or more even up to at least four weeks.
Owner:LAB FARM ROVI SA
Pure paliperidone and processes for preparing thereof
The present invention provides pure paliperidone as well as purification processes to obtain thereof.
Owner:TEVA PHARM USA INC
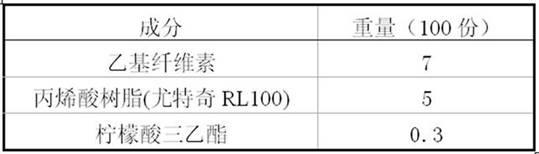
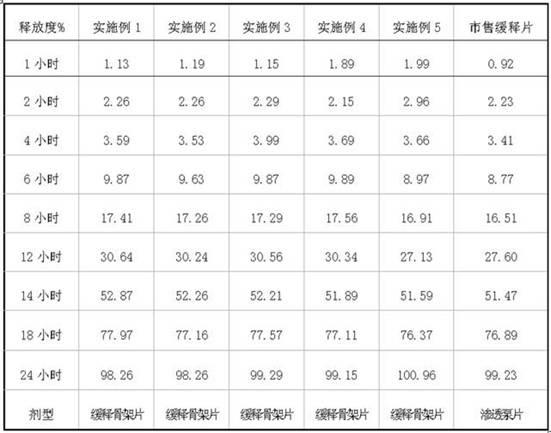
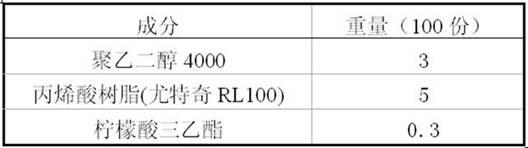
Paliperidone slow release formulation and preparation method thereof
InactiveCN102058517ADisadvantages of changing release instabilityEffective absorptionOrganic active ingredientsPharmaceutical delivery mechanismSlow Release FormulationPaliperidone
The invention relate to a paliperidone slow release formulation and a preparation method thereof. The slow release formulation comprises a slow release skeleton, a film controlling coating layer, a thinner, a binding agent and a lubricant. Because the slow release formulation can be released at a continuous constant rate within a long period of time, a stable blood medicament level is provided and maintained in a stable and efficient range, the administration frequency is reduced, and the medicament plays the role of long action.
Owner:泰州万全医药科技有限公司
Methods and dosage forms for controlled delivery of paliperidone and risperidone
InactiveUS20050232995A1Eliminate side effectsImprove development of toleranceOrganic active ingredientsBiocideDosing regimenRegimen
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Paliperidone derivative slow release microsphere preparation and preparation method thereof
InactiveCN104013578AGood treatment effectSmall toxicityOrganic active ingredientsNervous disorderUse medicationMicrosphere
The invention discloses a paliperidone derivative slow release microsphere preparation and a preparation method thereof. A hydrophobic aliphatic ester serves as an additive of the biological slow release microsphere preparation, and the problem that the paliperidone derivative cannot be stably and slowly released for one month in the preparation process of microspheres is solved by changing the microsphere structure and drug crystallinity and distribution in the microspheres. The drug can be slowly and stably released for one month, one month and a half even over two months, the drug administration frequency is greatly reduced, the bioavailability and treatment effect of the drug are improved, and the toxic and side effects are reduced, so that the pain of vast patients is greatly reduced, and the living quality is improved.
Owner:南京锐利施生物技术有限公司
Use of paliperidone for the treatment of sleep disturbances and/or excessive daytime sleepiness in psychiatric patients
InactiveUS20070232624A1Reduced daytime drowsinessReduced sleep disturbanceOrganic active ingredientsBiocideExcessive daytime sleepinessPharmaceutical drug
The present invention provides a pharmaceutical composition, and provides for the use thereof for the treatment of excessive daytime sleepiness and / or a sleep disturbance in a psychiatric patient in need thereof. The pharmaceutical composition comprises a therapeutically effective amount of paliperidone, its pharmaceutically acceptable acid addition salts, enantiomeric forms, and esters thereof, together with a pharmaceutical carrier, and provides a relatively low plasma concentration variation of paliperidone to the psychiatric patient in need of treatment. The present invention also provides for the use of a pharmaceutical composition as defined above, in the preparation of a medicament for the treatment of excessive daytime sleepiness and / or a sleep disturbance in a psychiatric patient in need thereof.
Owner:PALUMBO JOSEPH M +2
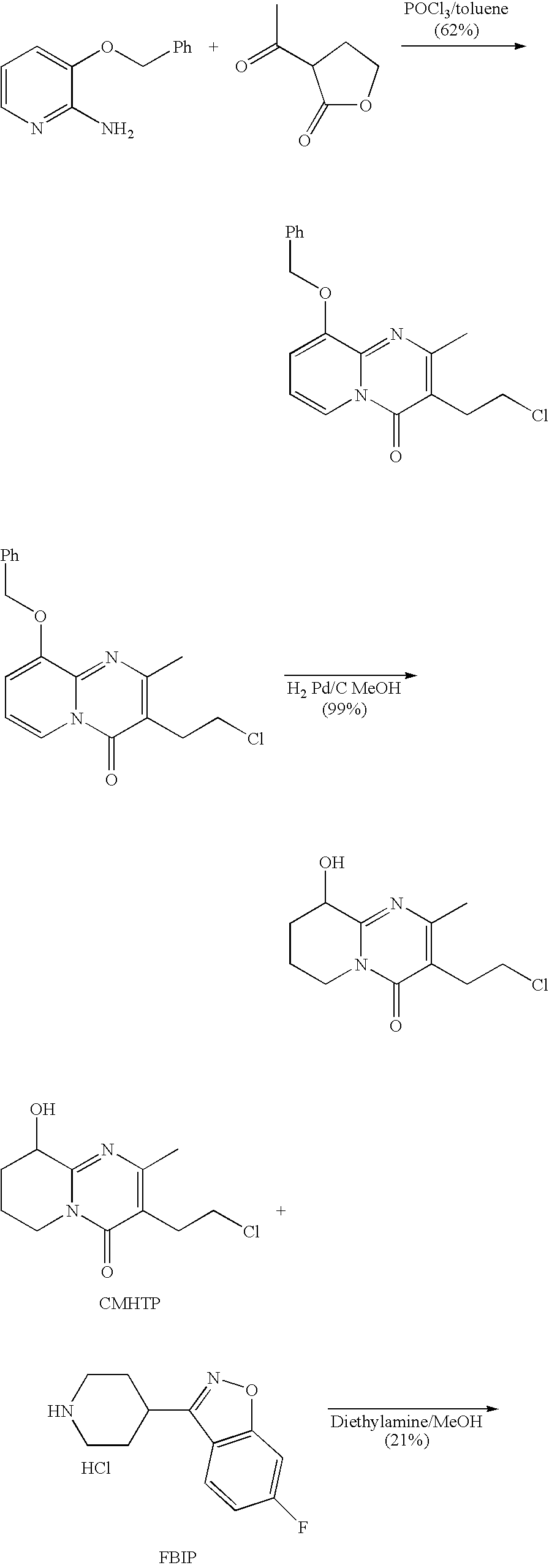
Process for the synthesis of 9-hydroxy risperidone (paliperidone)
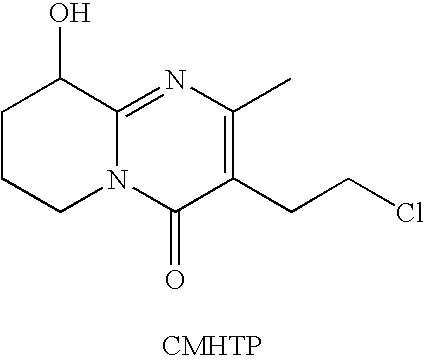
The present invention relates to a process for preparing paliperidone from its intermediate 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrrido[1,2-a]-pyrimidin-4-one.
Owner:TEVA PHARM USA INC
Novel paliperidone progressively-increased release osmotic pump preparation and preparation method thereof
ActiveCN103271889AAvoid titrationImprove complianceOrganic active ingredientsNervous disorderControlled Release TabletOsmotic pump
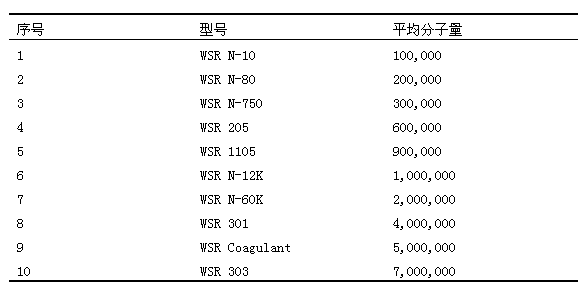
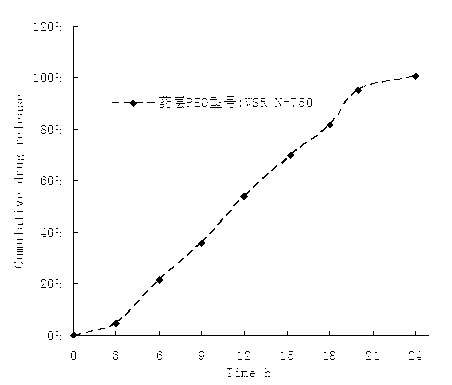
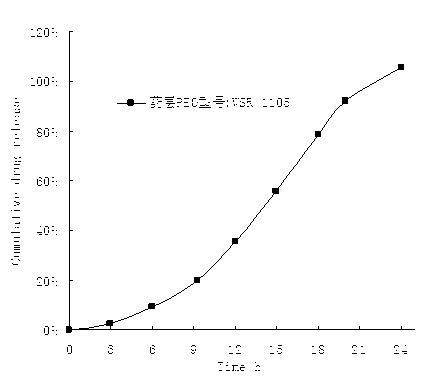
The invention belongs to the technical field of pharmaceutical preparations and discloses a paliperidone bi-layer osmotic pump progressively-increased controlled release tablet and a preparation method thereof. The osmotic pump preparation comprises a tablet core and a semipermeable coating film, wherein the tablet core comprises a medicine containing layer and a boosting layer, the semipermeable coating film envelops the tablet core and is provided with medicine release holes, the medicine containing layer contains middle molecular polyoxyethylene (average molecular weight is 900, 000-1, 000,,000) and an osmotic active substance in a certain proportion so that the hydration rate of the medicine containing layer is proper, the release of the active medicine basically presents a progressively-increased release trend and the progressively-increased release can be kept for 14-24 hours by using the bi-layer tablet core and the single-layer coating. By adopting the preparation, the adverse reaction of the medicine can be reduced greatly, the compliance of a patient can be improved, simultaneously, the preparation is simple to prepare, the preparation process of the progressively-increased osmotic pump preparation is simplified greatly, and the preparation is more conductive to industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY
Paliperidone extended-release mini pill
InactiveCN102614132AControlled release rateProlong the effective concentration time of blood drugsOrganic active ingredientsNervous disorderBiotechnologyProlonged-release tablet
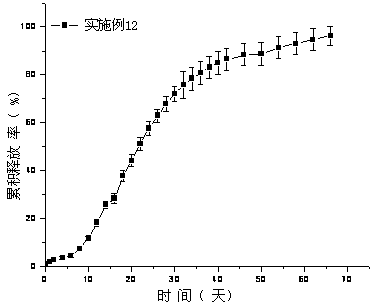
The invention provides a paliperidone extended-release mini pill, which solves the problems that a conventional extended-release tablet has a complex manufacturing process, high cost and side effects in taking. The paliperidone extended-release mini pill structurally comprises a pill core containing medicine and an extended-release coating layer from the inside out. The preparation method comprises the following steps: preparing a pill core containing medicine and wrapping the extended-release coating layer on the surface of the pill; and the grain diameter of the mini pill is 0.48 mm to 2.5 mm. The paliperidone extended-release mini pill can be released for 24 hours stably, lower than 30% of paliperidone extended-release mini pill is released within 2 hours, 40% to 60% of paliperidone extended-release mini pill is released within 12 hours, and more than 80% paliperidone extended-release mini pill is released at more than 20 hours. The paliperidone extended-release mini pill has the advantages of simple manufacturing process, low cost, uniform particle size, high yield and good release effect.
Owner:SICHUAN INDAL INST OF ANTIBIOTICS CHINA NAT PHARMA GROUP CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com