Methods and dosage forms for controlled delivery of paliperidone and risperidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
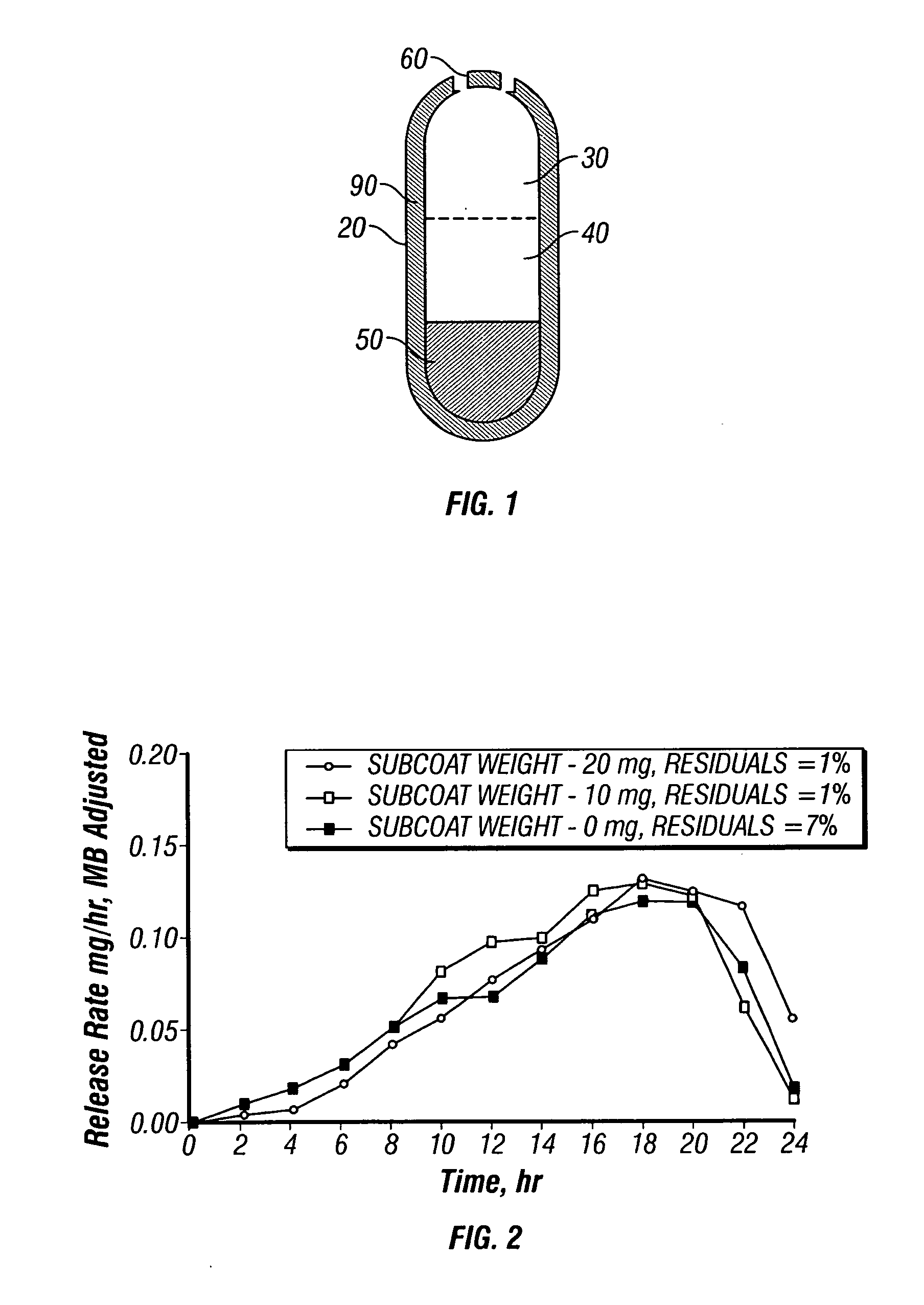
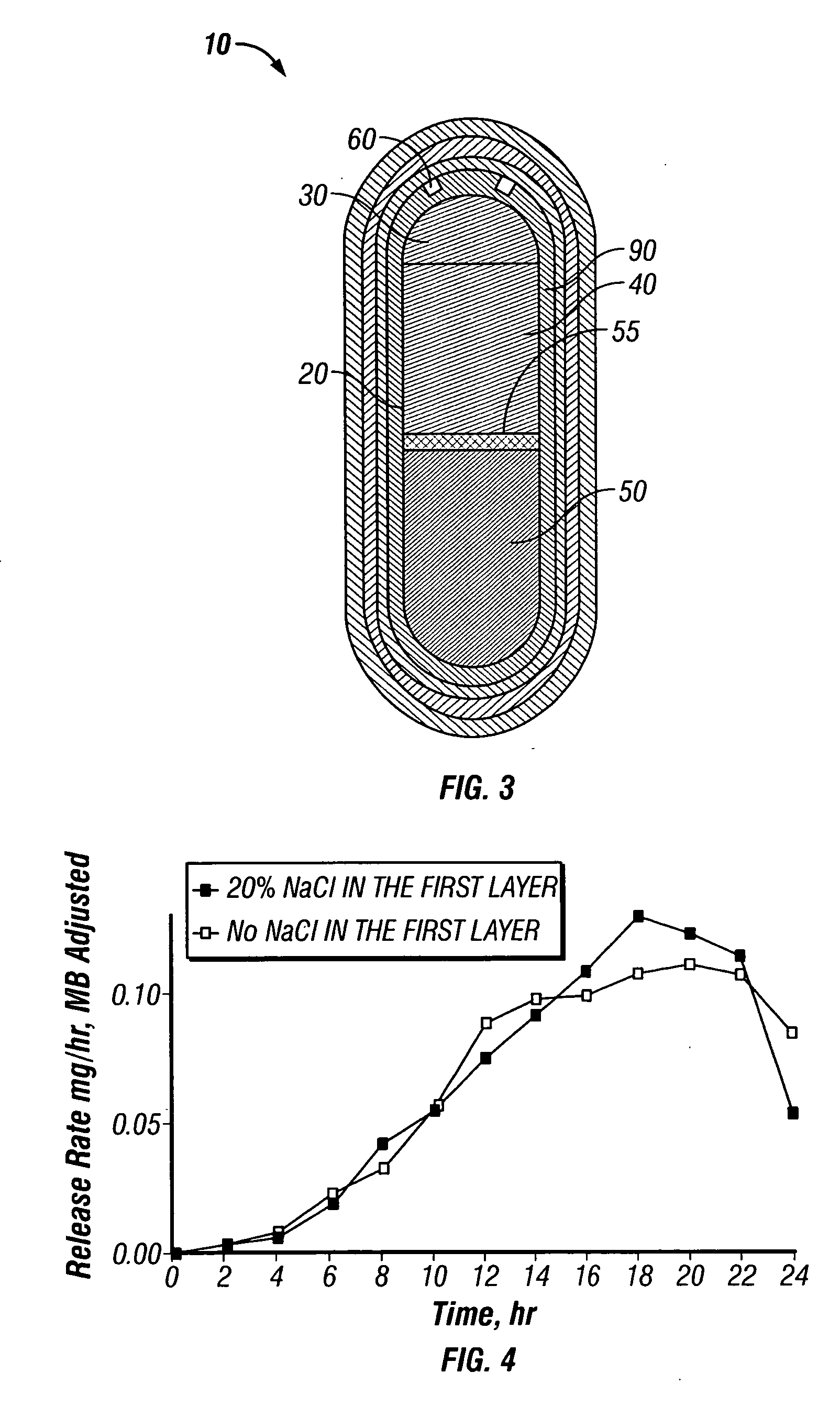
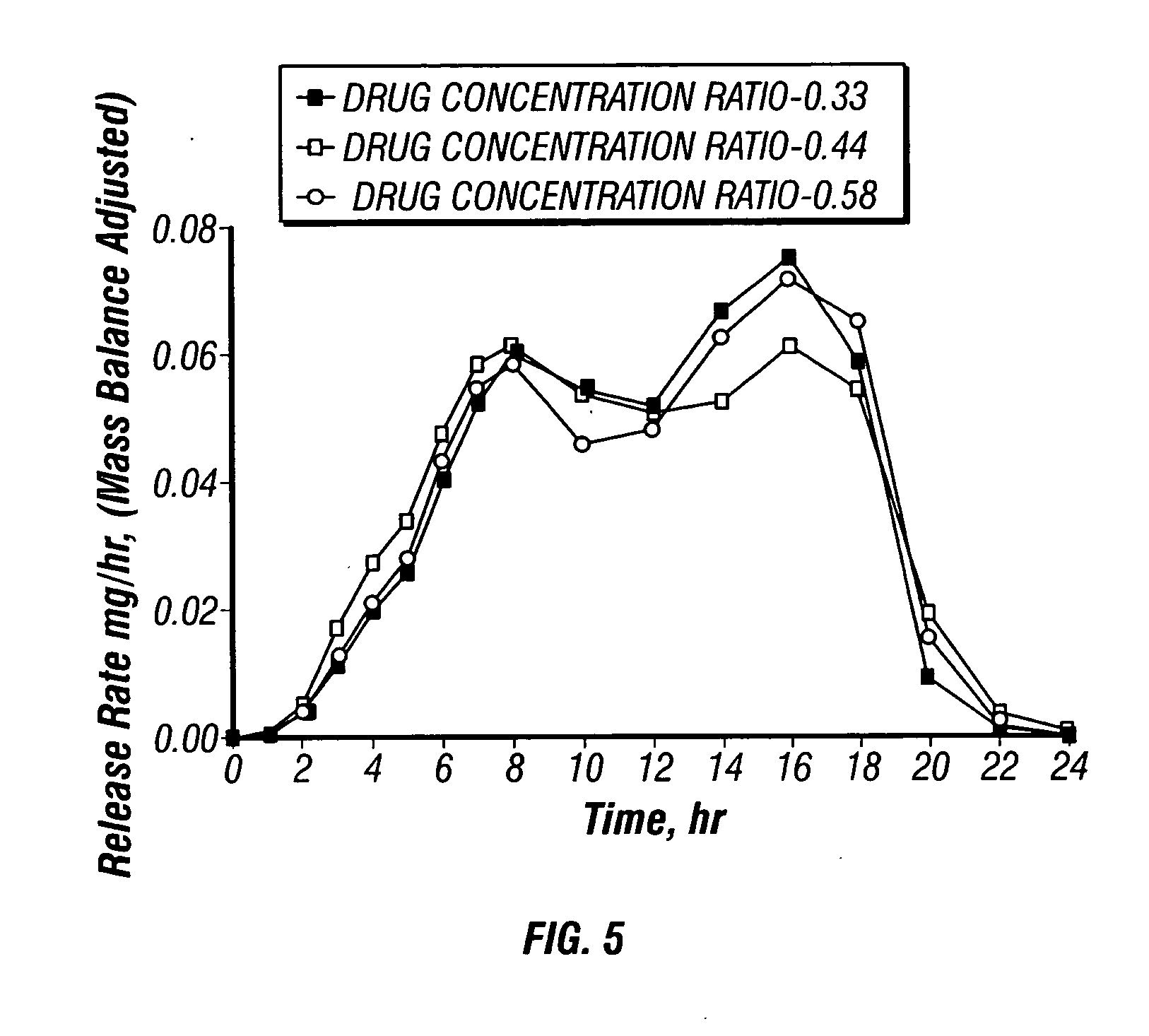
Image
Examples
example 1
Paliperidone Capsule Shaped Tablet, Trilayer 1.9 mg System
[0155] A dosage form adapted, designed and shaped as an osmotic drug delivery device is manufactured as follows: 100 g of paliperidone, 7345 g of polyethylene oxide with average molecular weight of 200,000, and 2000 g of sodium chloride, USP are added to a fluid bed granulator bowl. Next a binder solution is prepared by dissolving 800 g of hydroxypropylmethyl cellulose identified as 2910 having an average viscosity of 5 cps in 9,200 g of water. The dry materials are fluid bed granulated by spraying with 6750 g of binder solution. Next, the wet granulation is dried in the granulator to an acceptable moisture content, and sized using by passing through a 7-mesh screen. Next, the granulation is transferred to a blender and mixed with 5 g of butylated hydroxytoluene as an antioxidant and lubricated with 50 g of stearic acid.
[0156] Next, a second drug compartment composition is prepared as follows: 280 g of paliperidone and 9165...
example 2
Paliperidone Capsule Shaped Tablet, Trilayer 0.5 mg System
[0163] A dosage form adapted, designed and shaped as an osmotic drug delivery device is manufactured as follows: 25 g of paliperidone, 7420 g of polyethylene oxide with average molecular weight of 200,000, and 2000 g of sodium chloride, USP are added to a fluid bed granulator bowl. Next a binder solution is prepared by dissolving 800 g of hydroxypropylmethyl cellulose identified as 2910 having an average viscosity of 5 cps in 9,200 g of water. The dry materials are fluid bed granulated by spraying with 6750 g of binder solution. Next, the wet granulation is dried in the granulator to an acceptable moisture content, and sized using by passing through a 7-mesh screen. Next, the granulation is transferred to a blender and mixed with 5 g of butylated hydroxytoluene as an antioxidant and lubricated with 50 g of stearic acid.
[0164] Next, a second drug compartment composition is prepared as follows: 70 g of paliperidone and 9375 g...
example 3
Paliperidone Capsule Shaped Tablet, Trilayer 15 mg System
[0171] A dosage form adapted, designed and shaped as an osmotic drug delivery device was manufactured as follows: 900 g of paliperidone, 6544 g of polyethylene oxide with average molecular weight of 200,000, and 2000 g of sodium chloride, USP were added to a fluid bed granulator bowl. Next a binder solution was prepared by dissolving 800 g of polyvinylpyrrolidone identified as K29-32 having an average molecular weight of 40,000 in 9,200 g of water. The dry materials were fluid bed granulated by spraying with 6750 g of binder solution. Next, the wet granulation was dried in the granulator to an acceptable moisture content, and sized using by passing through a 7-mesh screen. Next, the granulation was transferred to a blender and mixed with 5 g of butylated hydroxytoluene as an antioxidant and lubricated with 50 g of stearic acid.
[0172] Next, a second drug compartment composition was prepared as follows: 2100 g of paliperidone ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com