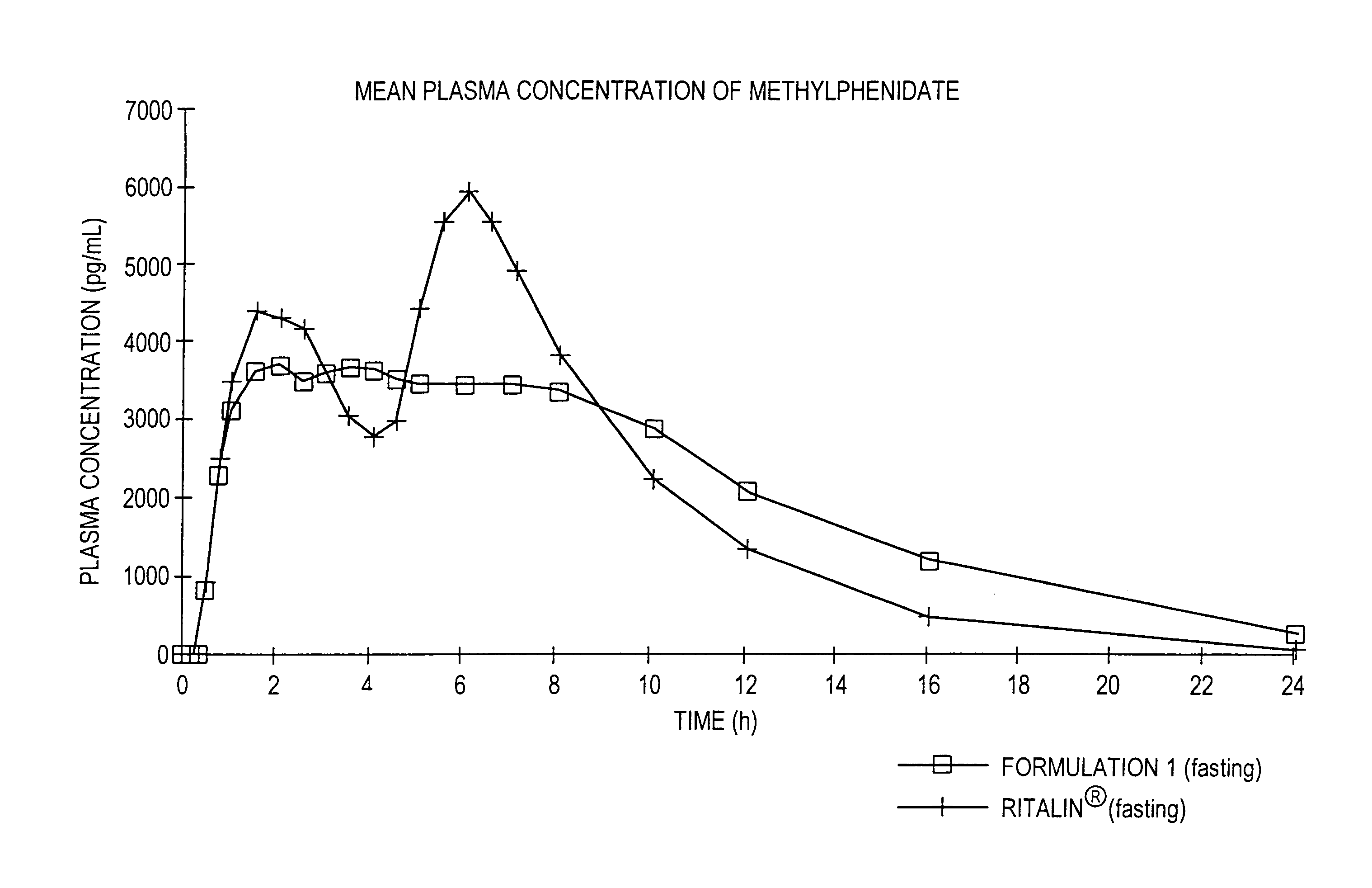Patents
Literature
118 results about "Peak concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peak concentration. the maximum amount of a substance or force, such as the highest concentration of a drug measured after it is administered.
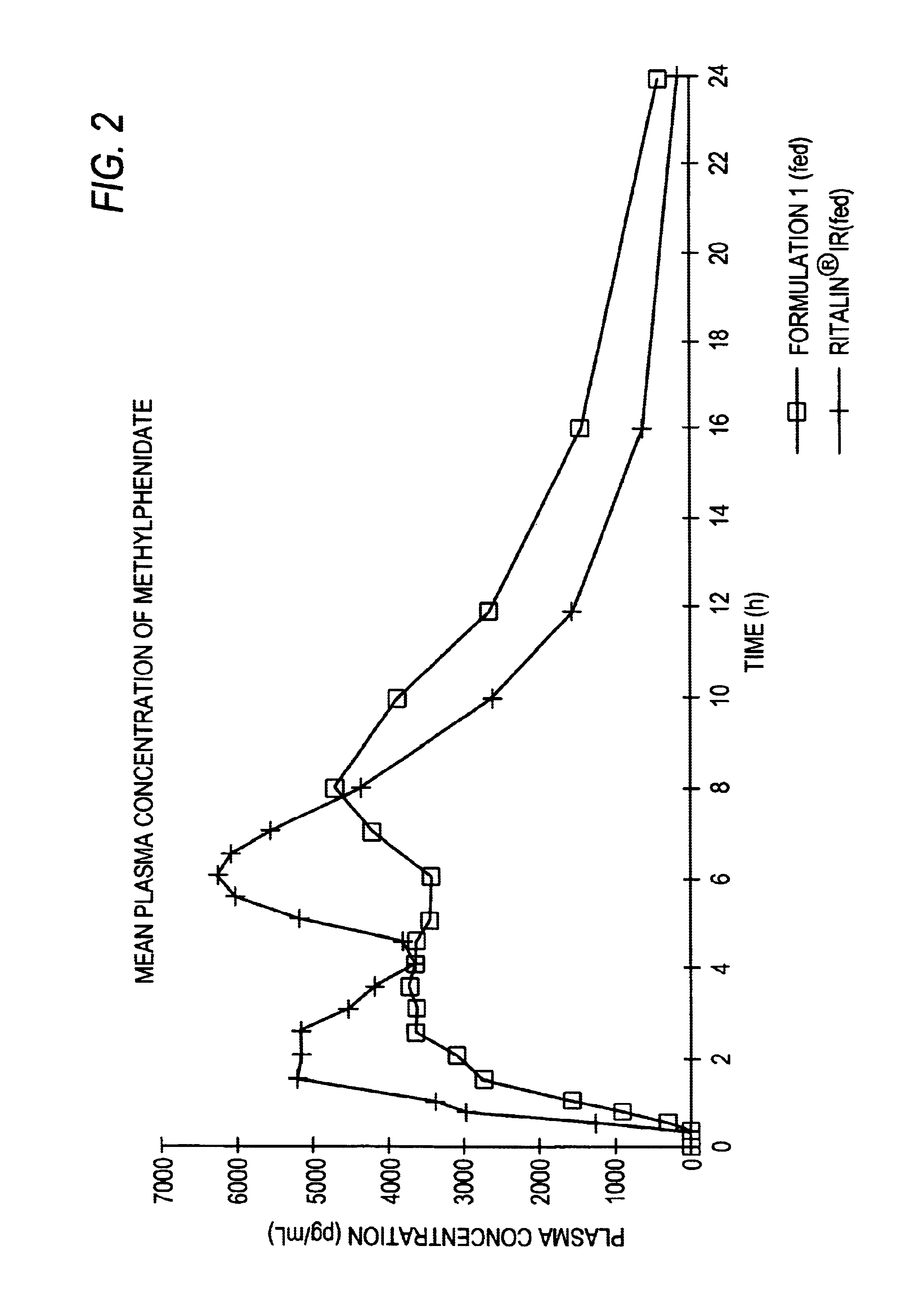
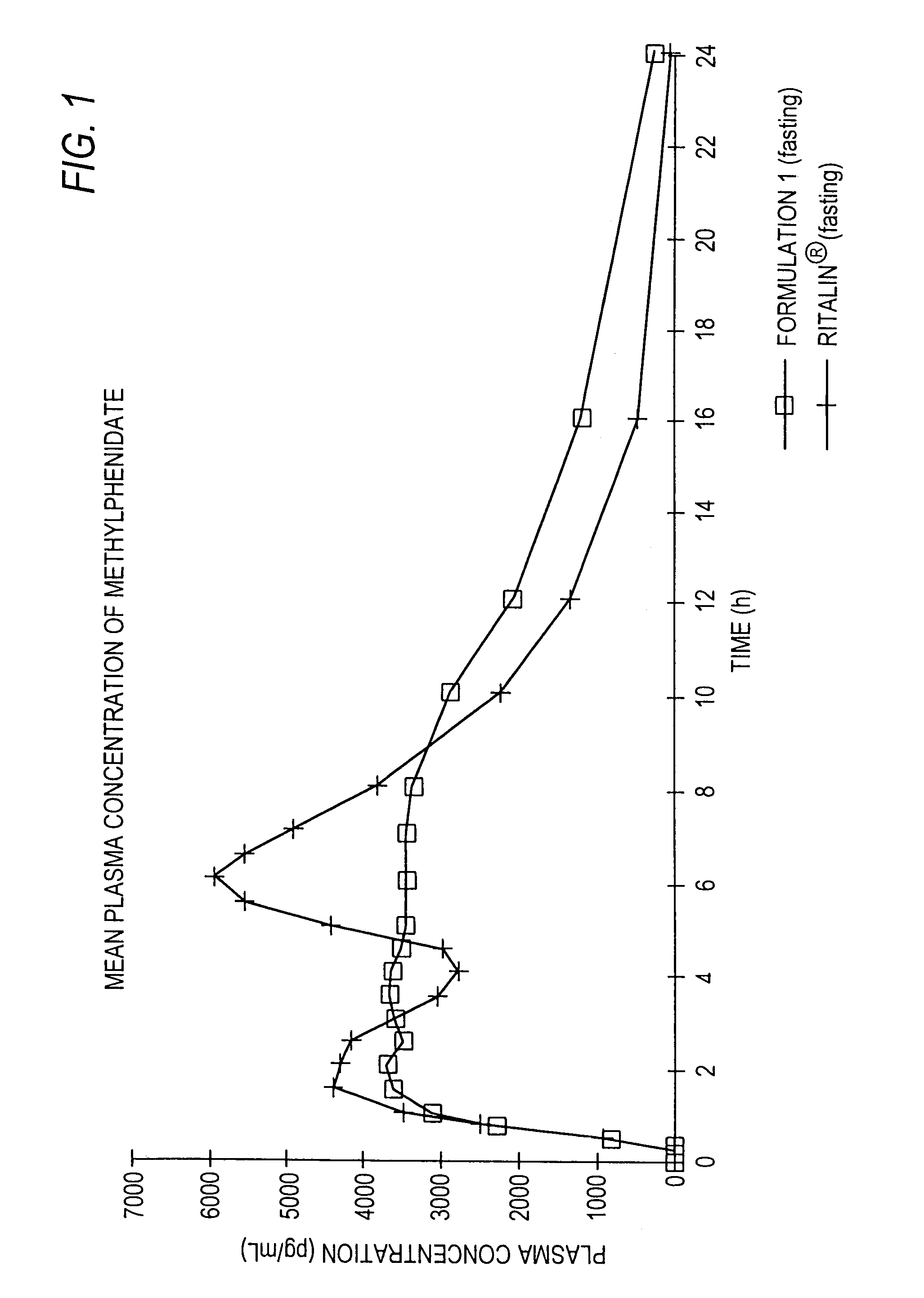
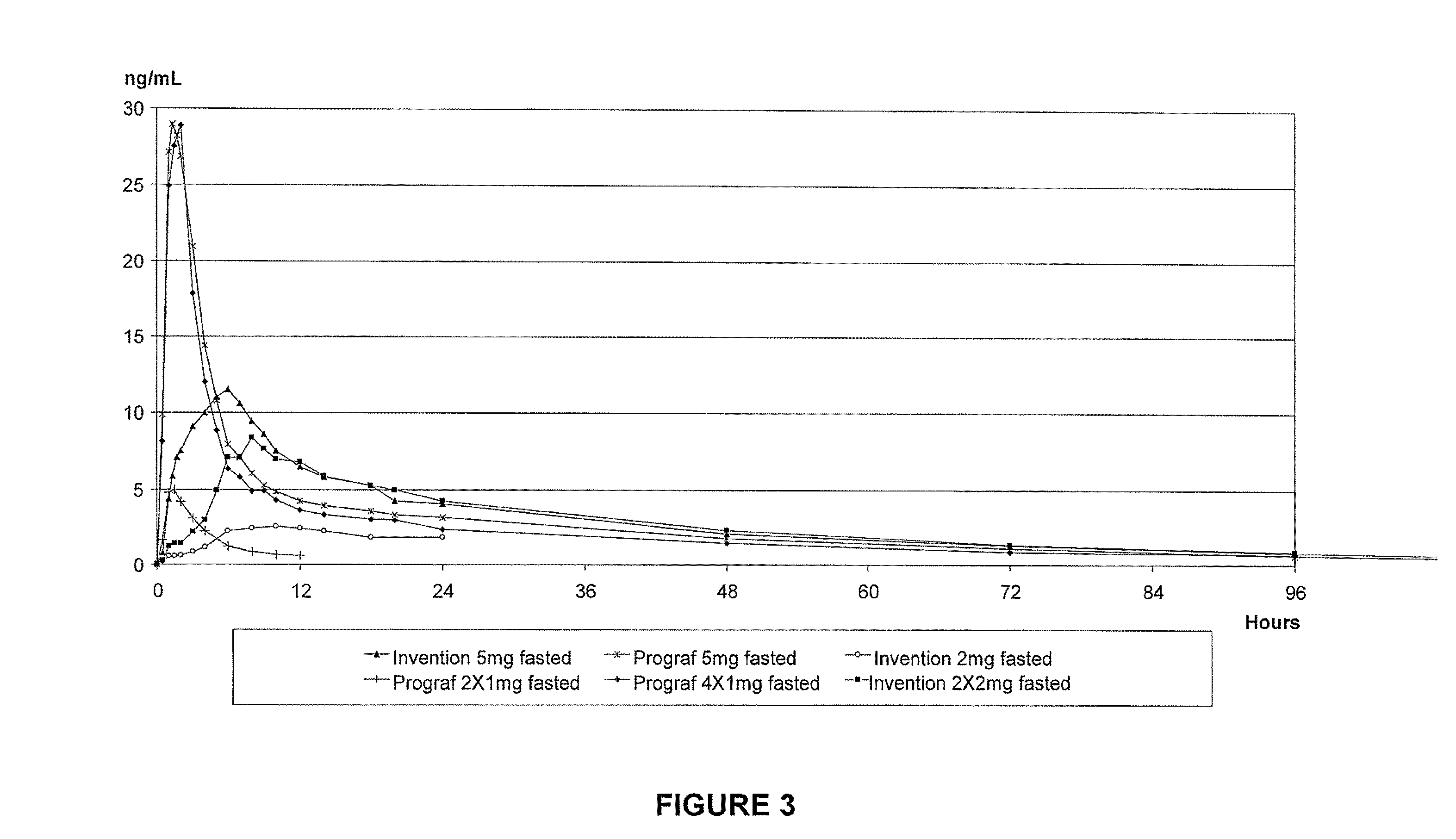
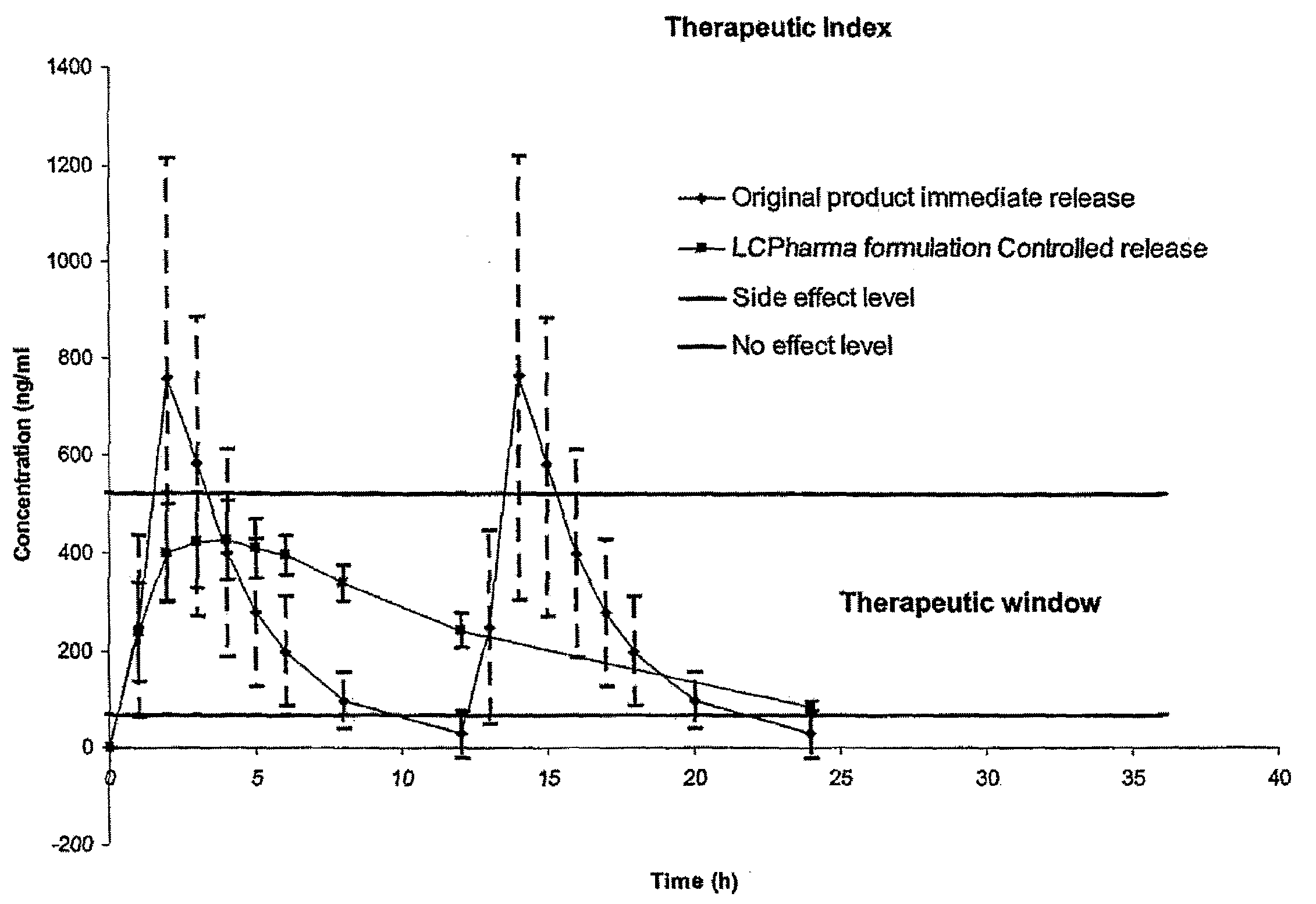
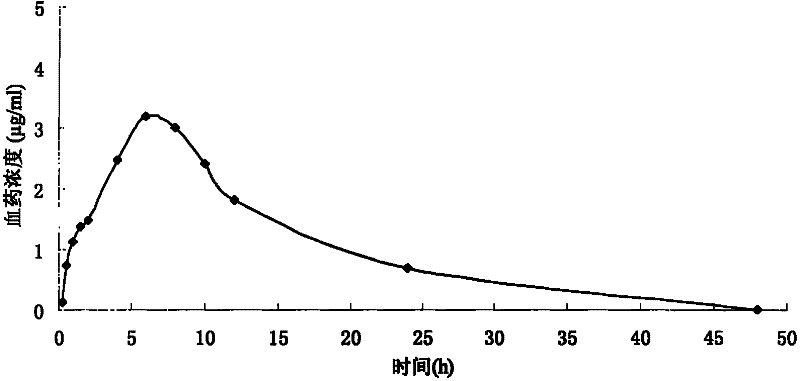
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
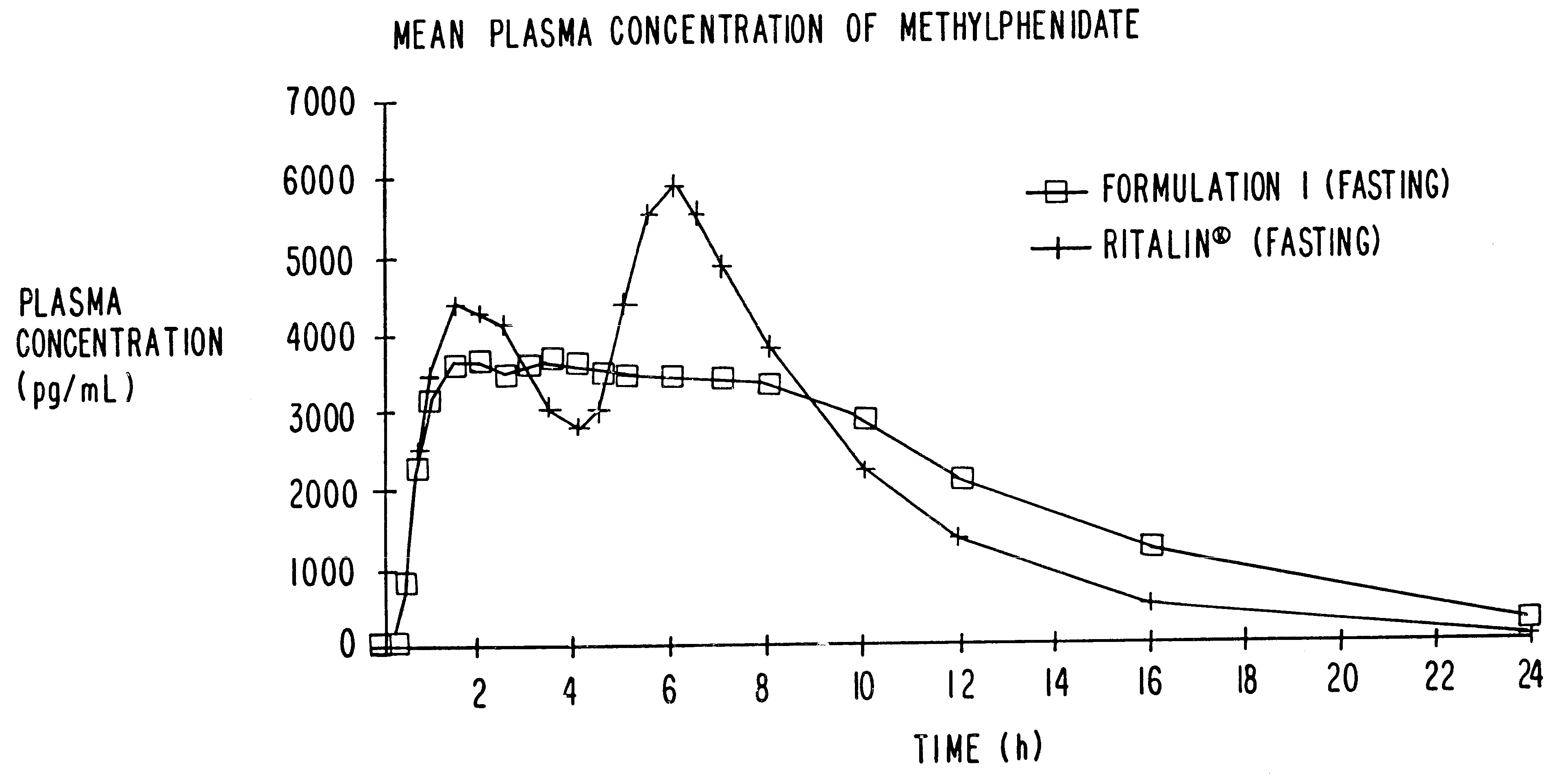
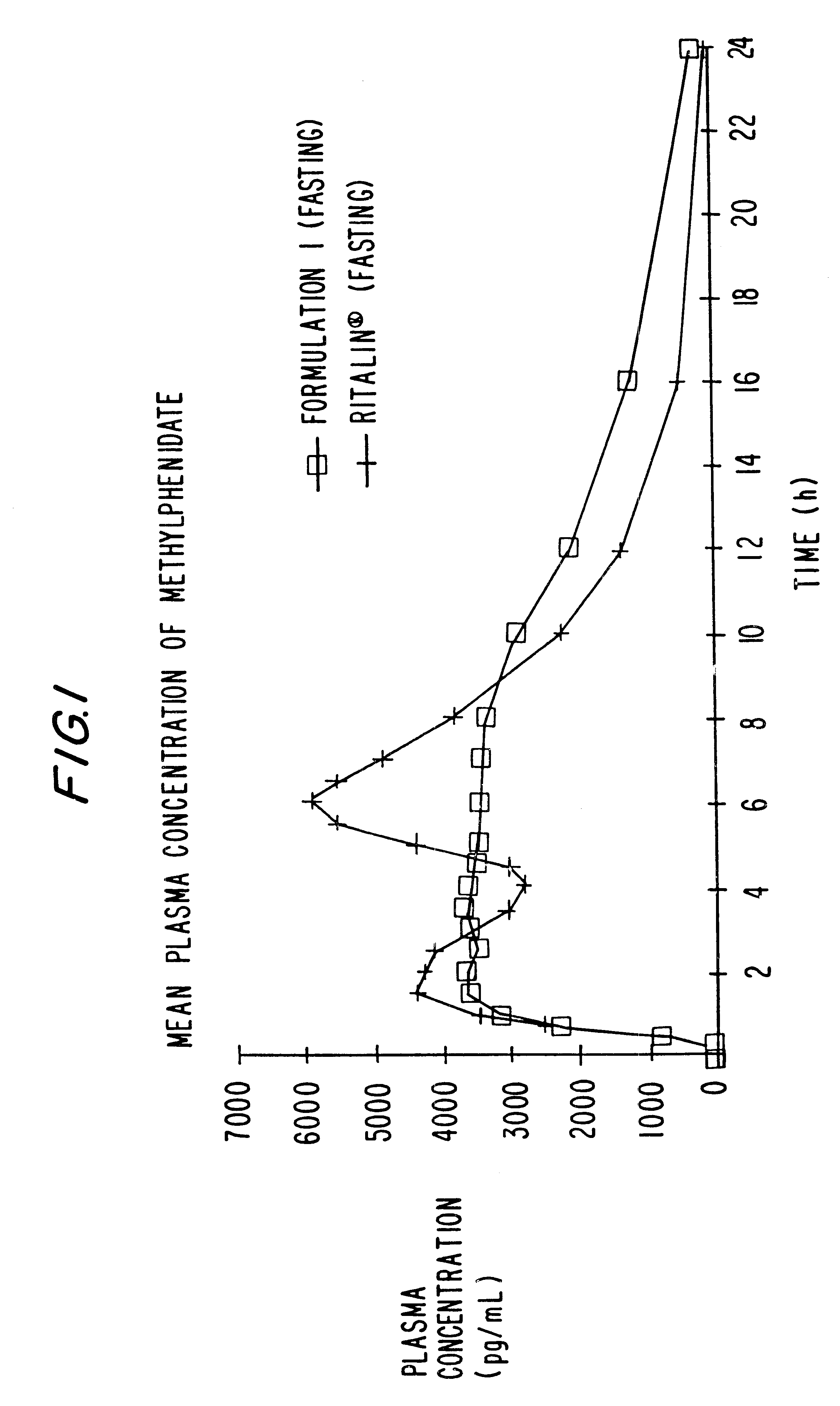
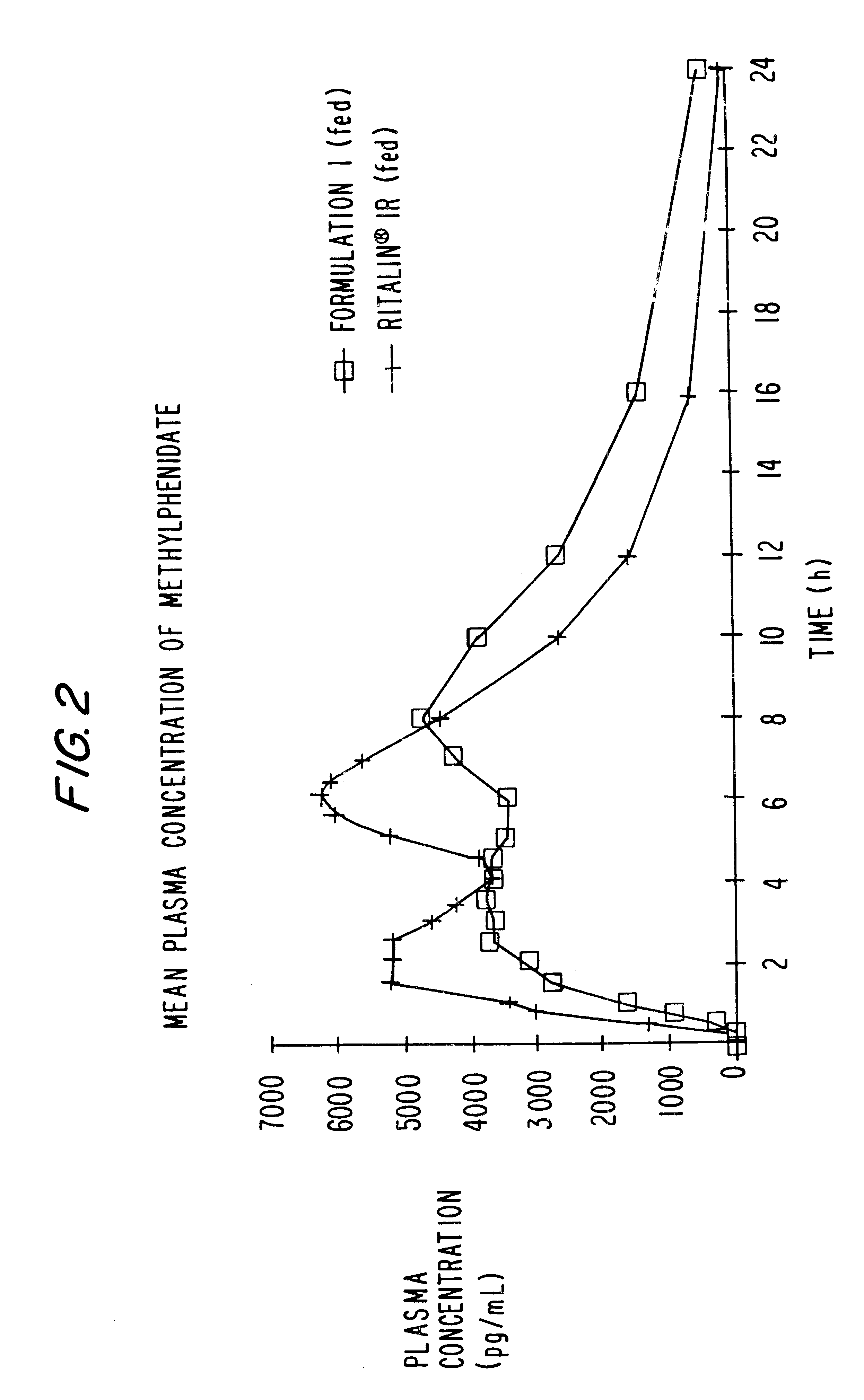
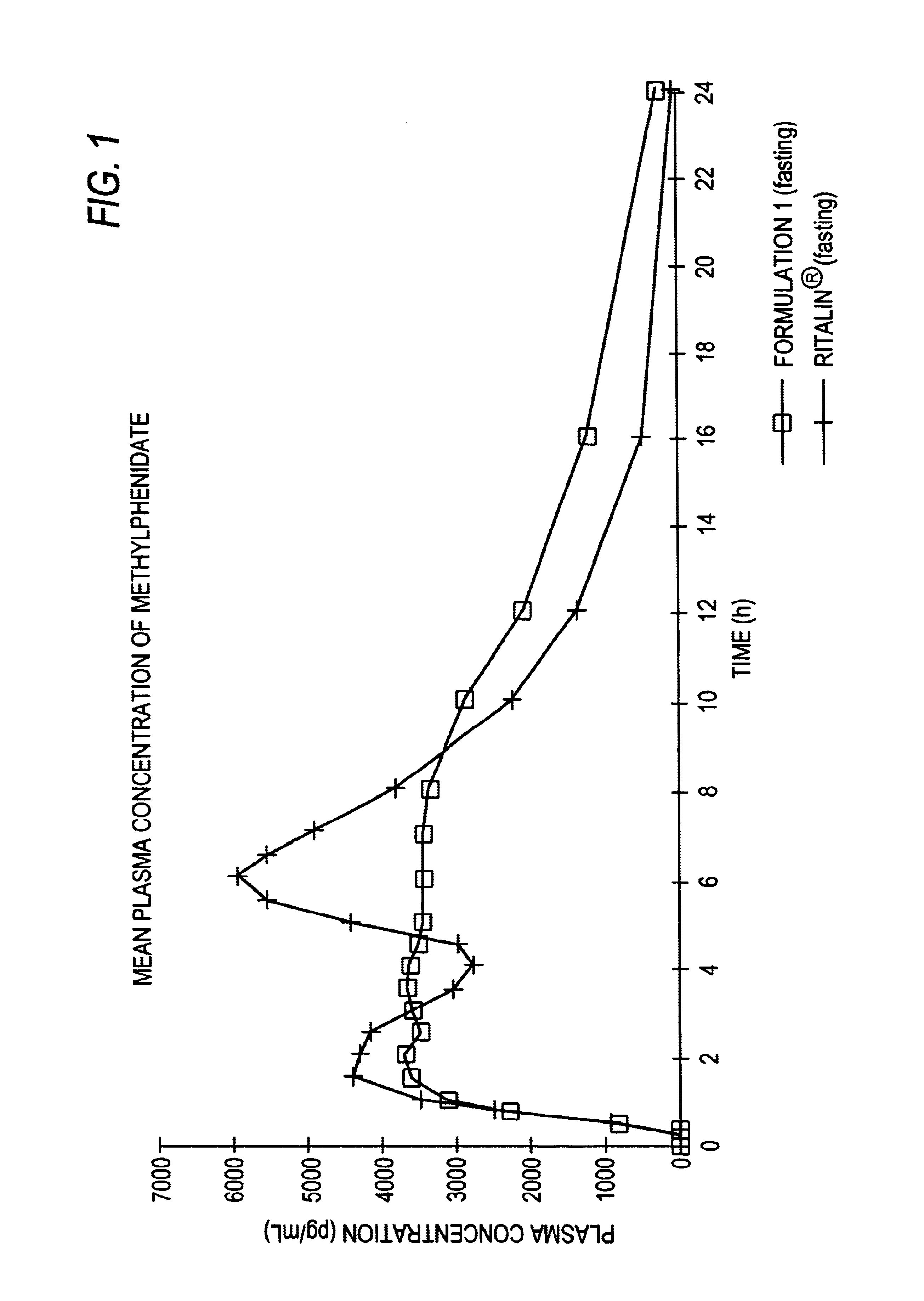
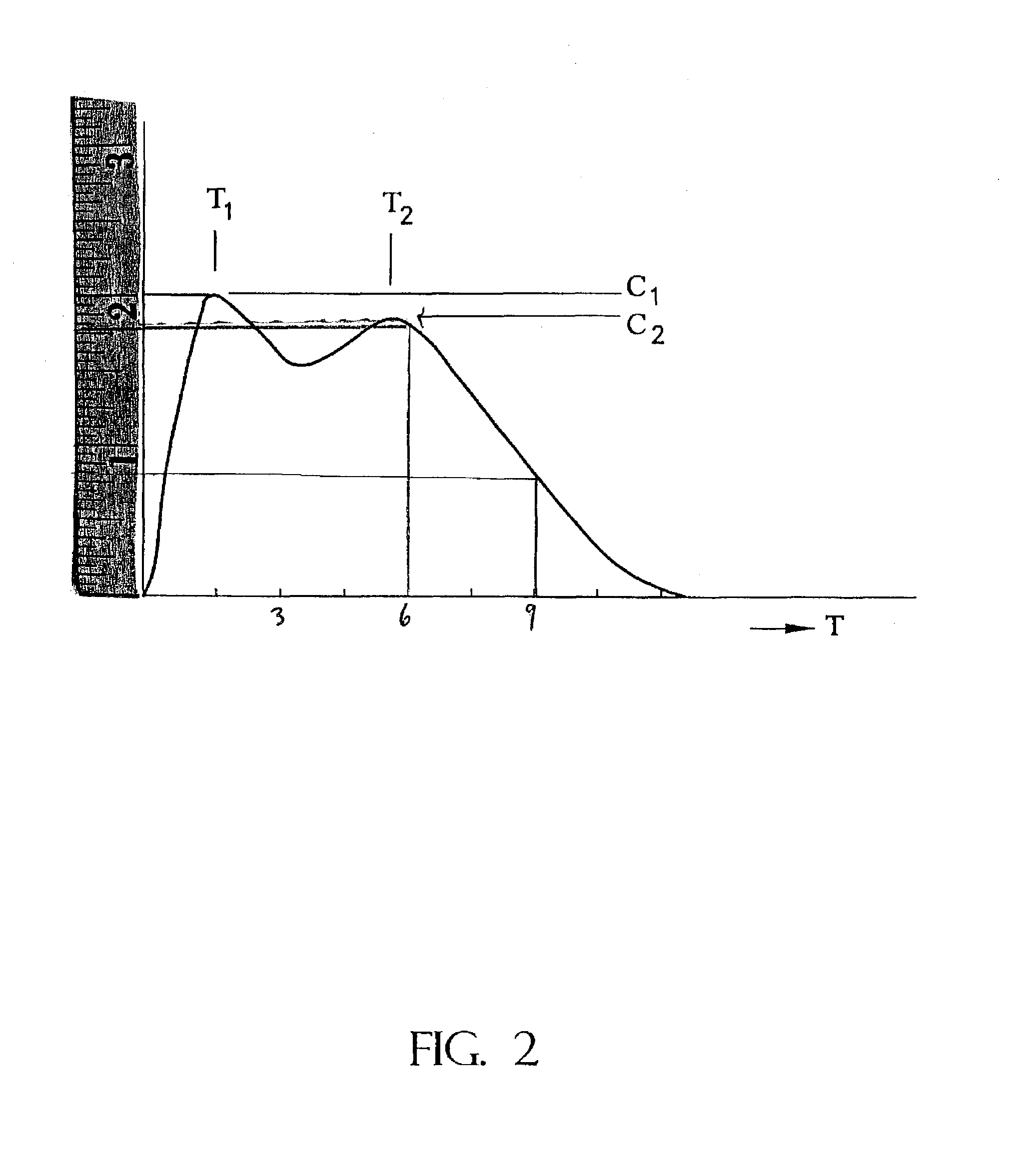
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
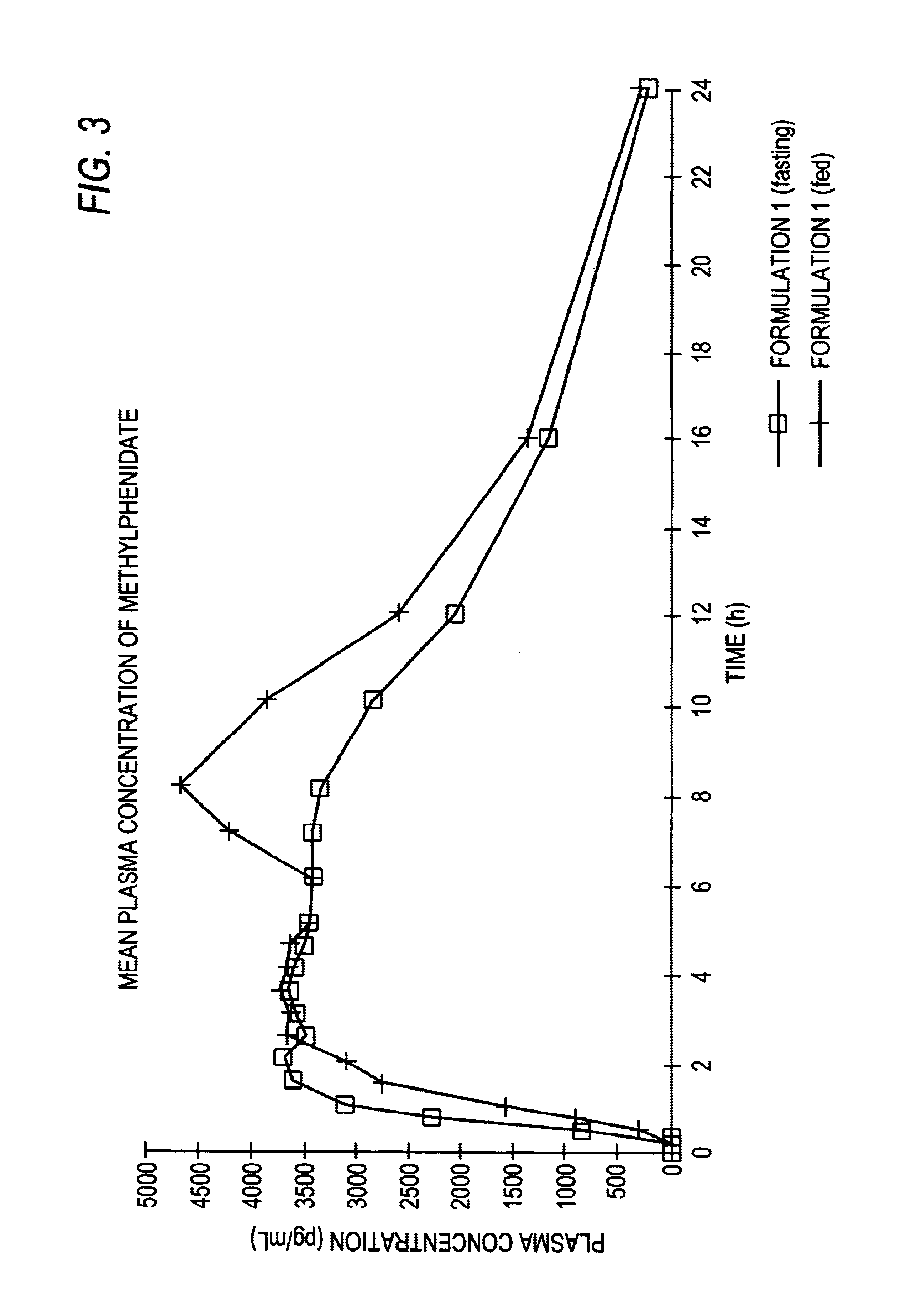
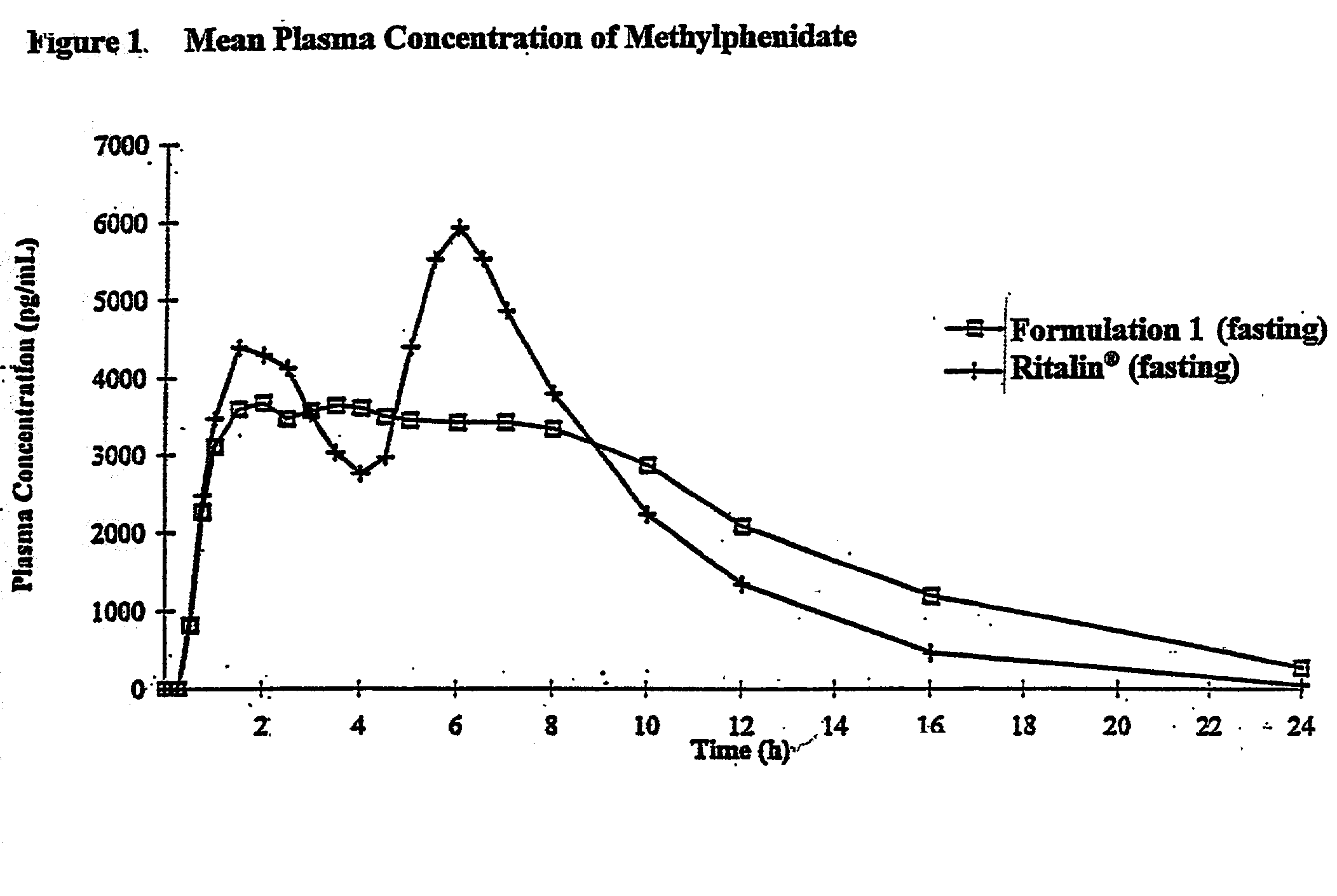
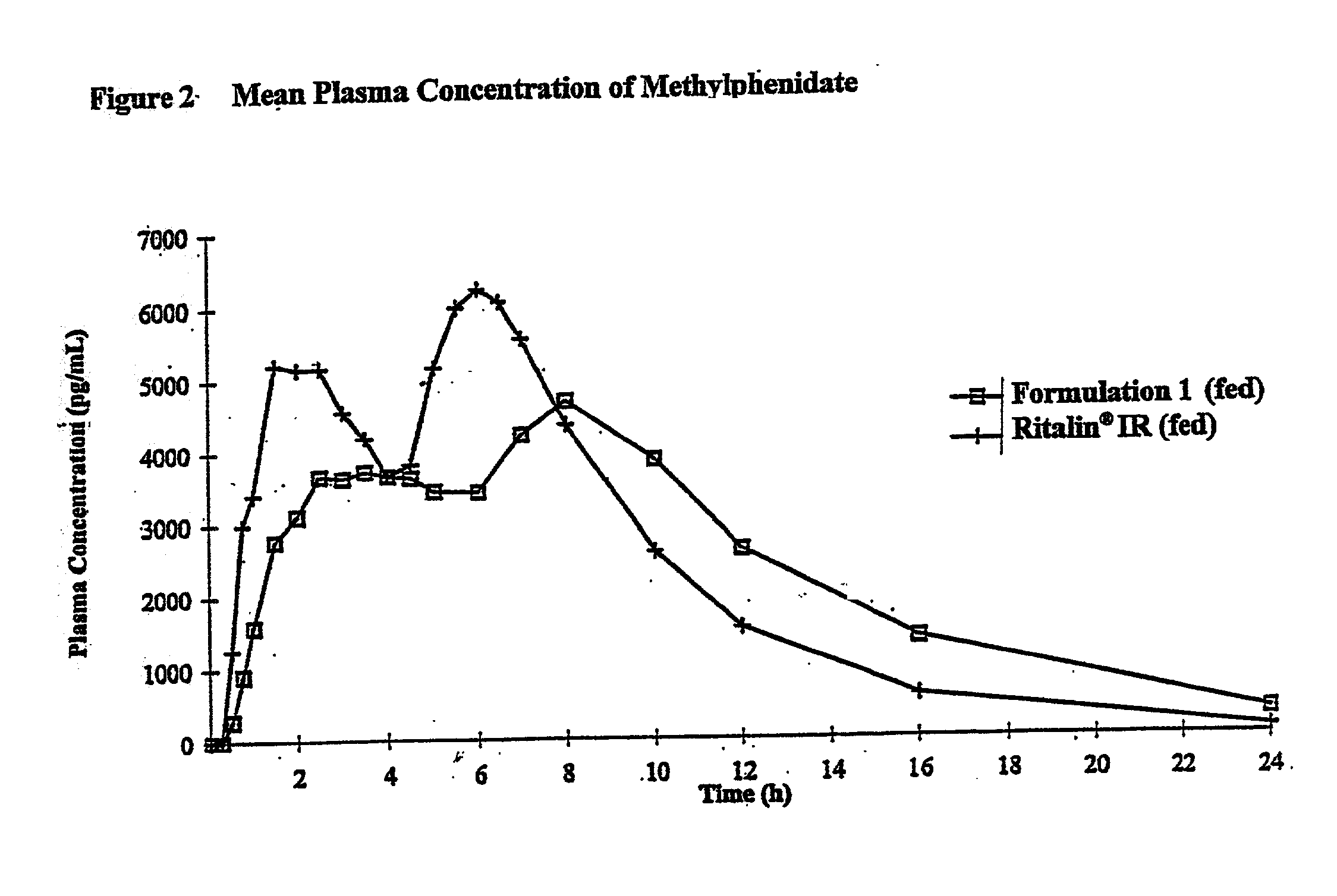
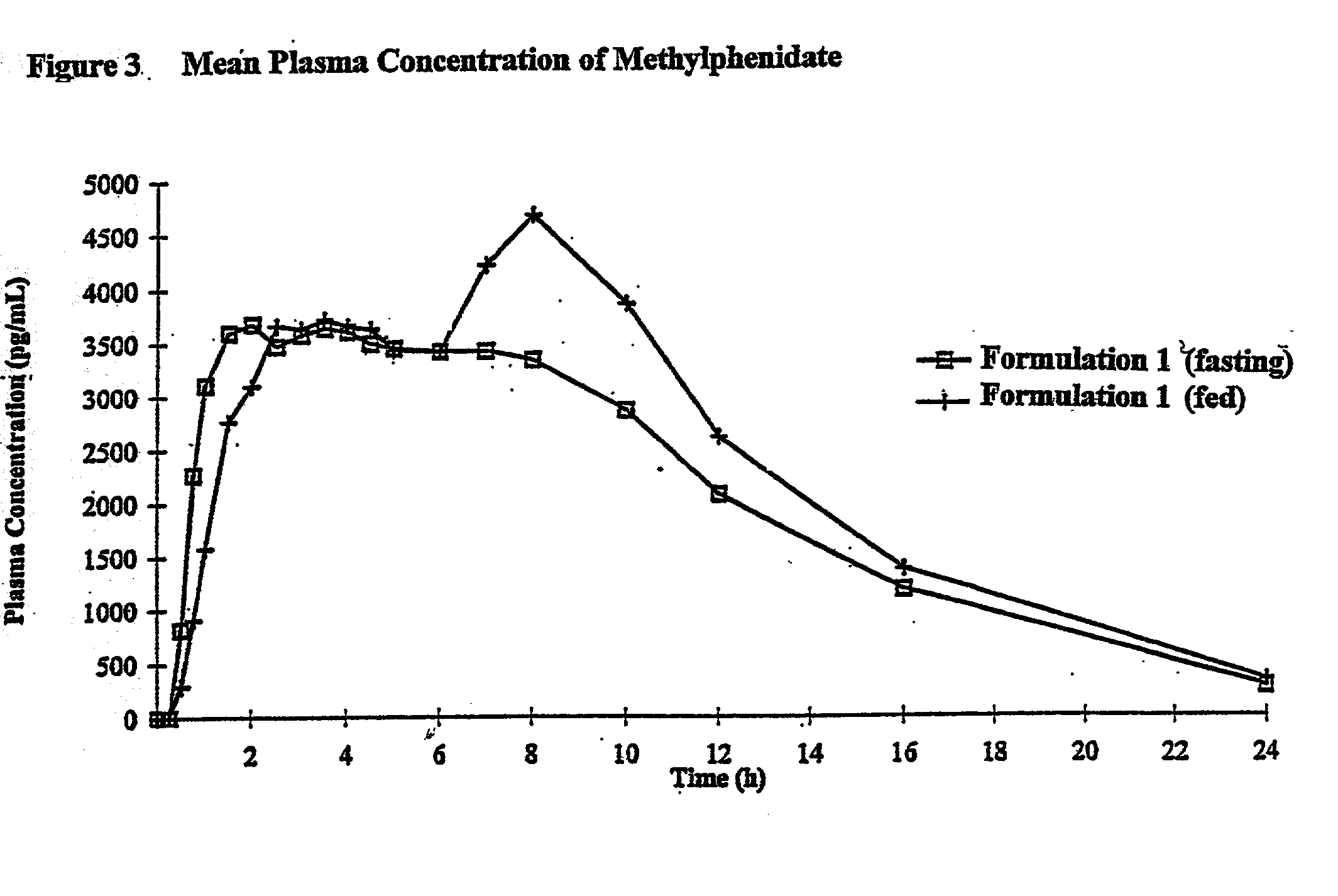
Controlled/modified release oral methylphenidate formulations
InactiveUS6673367B1Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Controlled/modified release oral methylphenidate formulations
InactiveUS7083808B2Patient compliance is goodPowder deliveryOrganic active ingredientsControlled releaseImmediate release
The invention is directed to oral modified / controlled release methylphenidate formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release methylphenidate formulations, and the duration of effect falls rapidly at the end of the dosing interval so as not to affect the appetite of the patient at dinner nor the patient's sleep thereafter.
Owner:RHODES PHARMA LP
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
InactiveUS20070155664A1Quick releaseSufficient amountOrganic active ingredientsPeptide/protein ingredientsRegimenBlood plasma
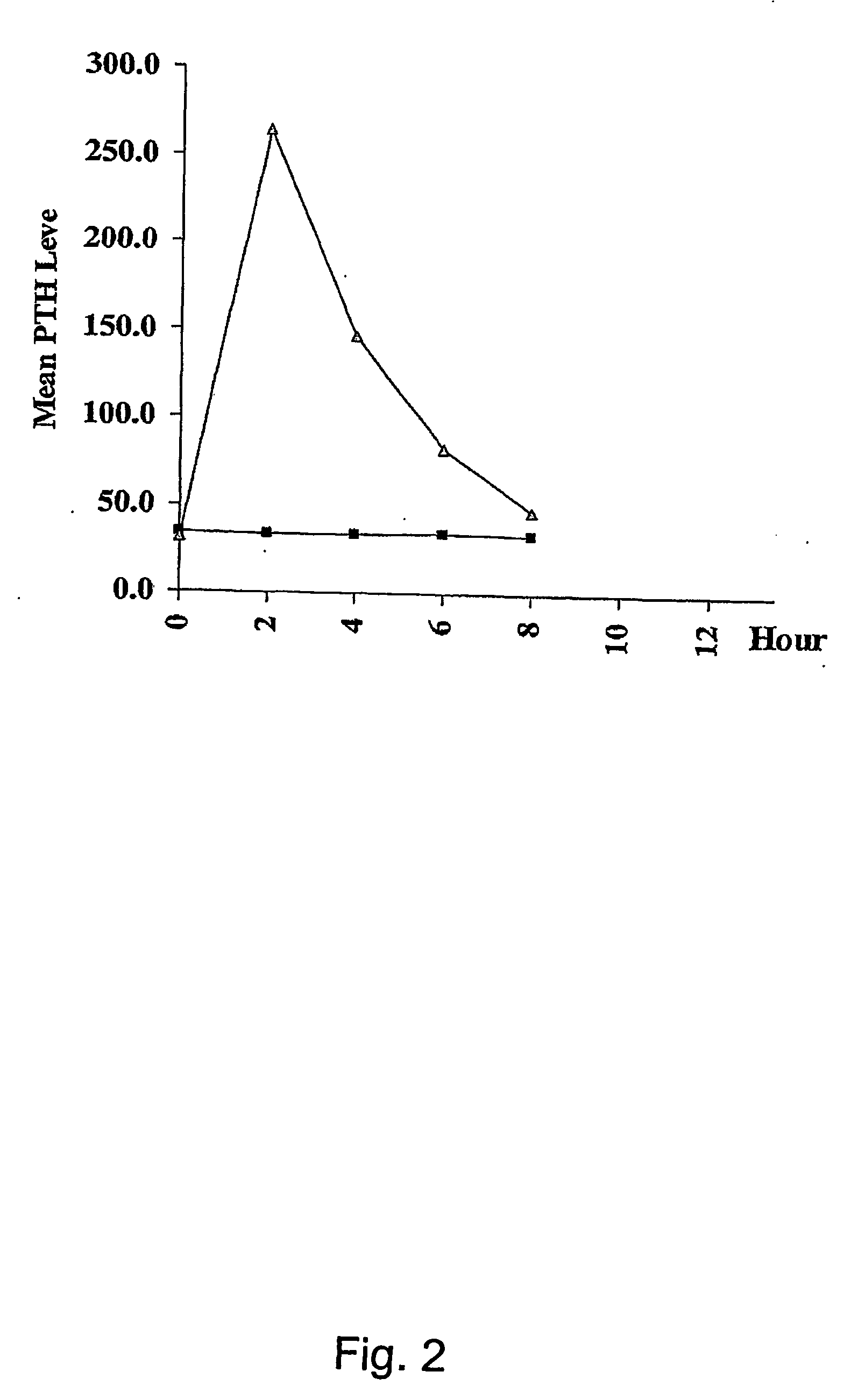
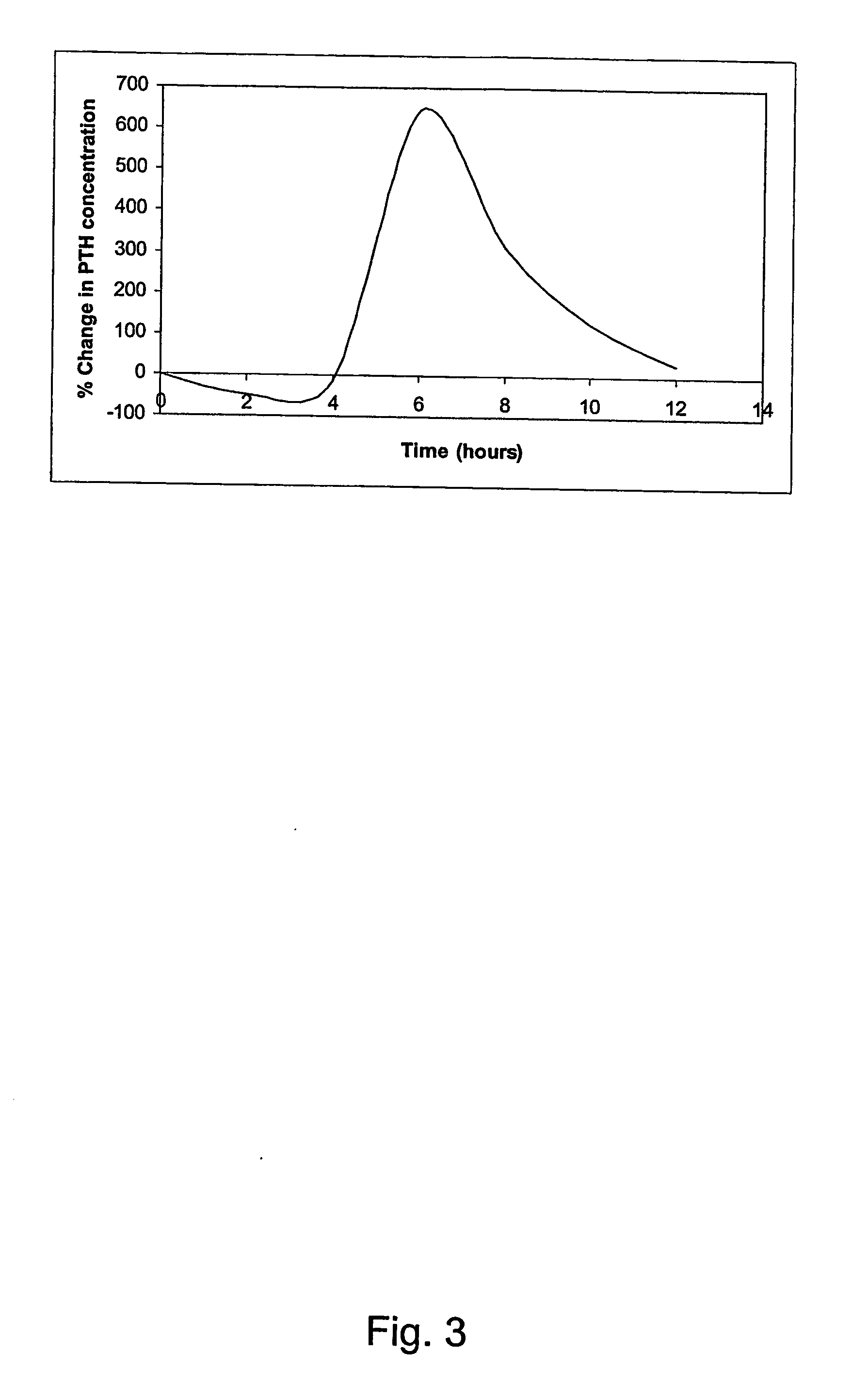
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of Pm once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
Compositions and methods for enhanced mucosal delivery of peptide YY and methods for treating and preventing obesity
InactiveUS7166575B2Improved pharmacokineticImproved pharmacodynamic resultPowder deliveryPeptide/protein ingredientsDiseaseHypodermoclysis
Pharmaceutical compositions and methods are described comprising at least one peptide YY compound and one or more intranasal delivery-enhancing agents for enhanced nasal mucosal delivery of the peptide YY, for treating a variety of diseases and conditions in mammalian subjects, including obesity. In one aspect, the intranasal delivery formulations and methods provide enhanced delivery of peptide YY to the blood plasma or central nervous system (CNS) tissue or fluid, for example, by yielding a peak concentration (Cmax) of the peptide YY in the blood plasma or CNS tissue or fluid of the subject that is 20% or greater compared to a peak concentration of the peptide YY in the blood plasma or CNS tissue or fluid of the subject following administration to the subject of a same concentration or dose of the peptide YY to the subject by subcutaneous injection.
Owner:MDRNA
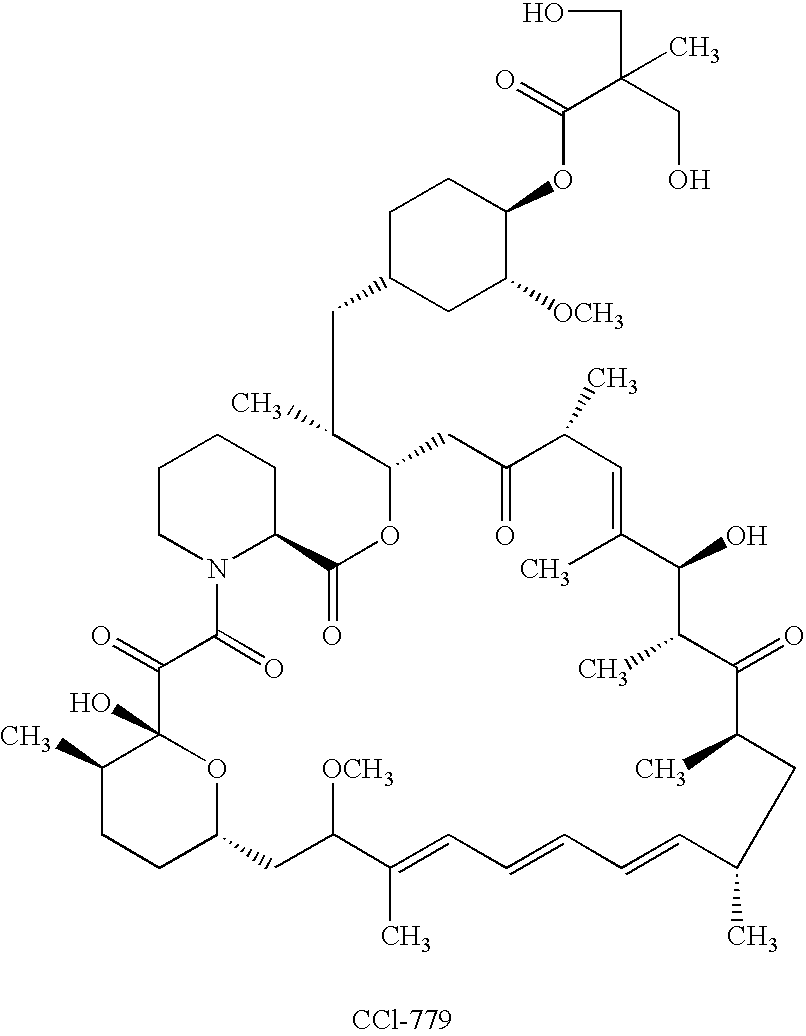
Orally bioavailable CCI-779 formulations
A CCI-779 oral dosage form is provided in which, after oral administration to a subject, the CCI-779 has a whole blood peak concentration (Cmax) of 5.4±1.8 ng / mL and an area under the curve (AUC) of about 66±about 22 ng-hr / ml and the sirolimus has a Cmax of 18.7±9.6 ng / mL and an AUC of about 600±about 228 ng-hr / ml, for a 25 mg unit dose of CCI-779. Another CCI-779 oral dosage form is provided which, after oral administration thereof to a subject, the CCI-779 has a Cmax of 5.7±1.7 ng / mL and an AUC of about 60±about 20 ng-hr / ml and the sirolimus has a Cmax of 17.1±8.1 ng / mL and an AUC of about 548±about 187 ng-hr / ml in whole blood, for a 25 mg unit dose of CCI-779. Products containing these oral dosage forms, and methods of use thereof, are also described.
Owner:WYETH LLC
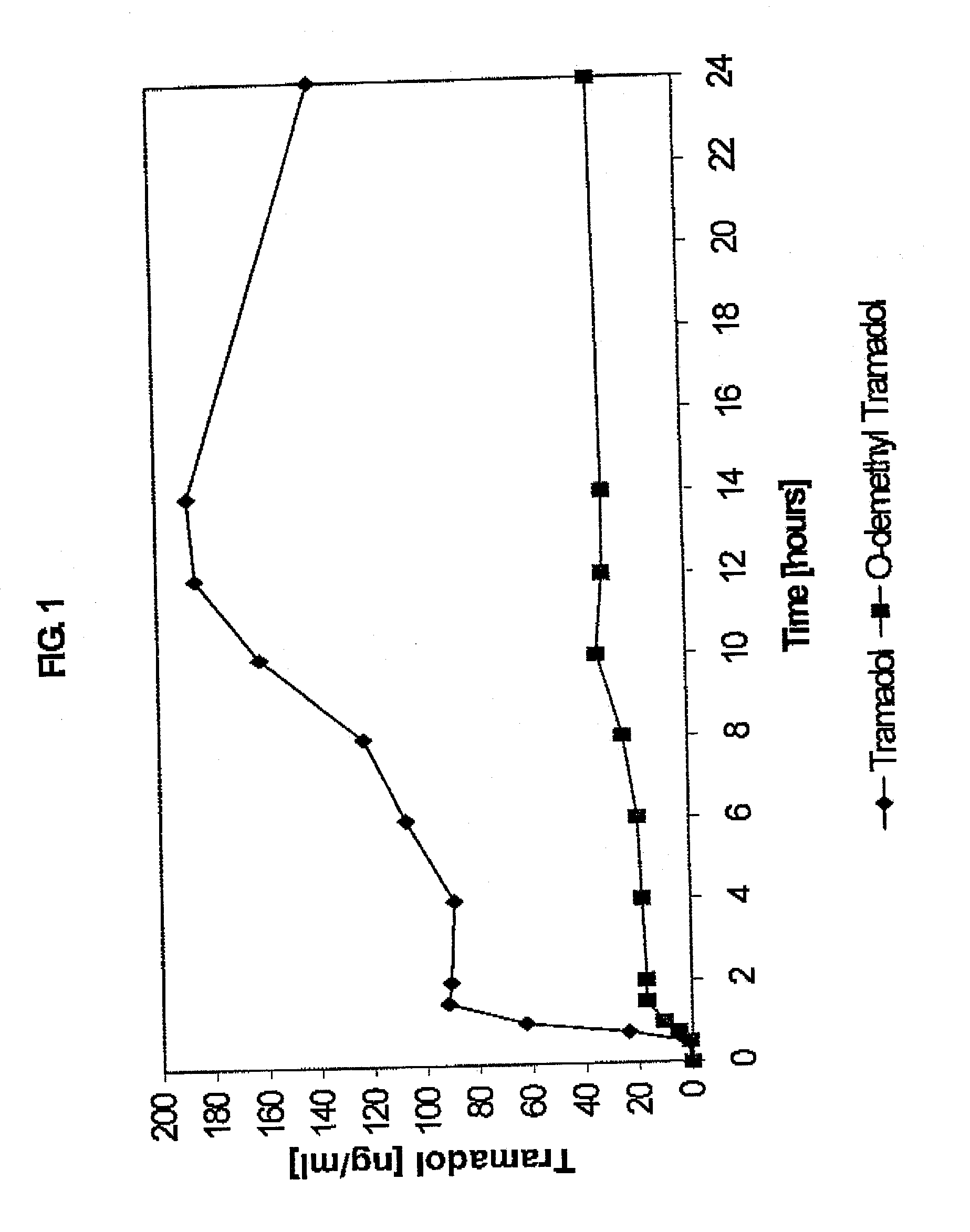
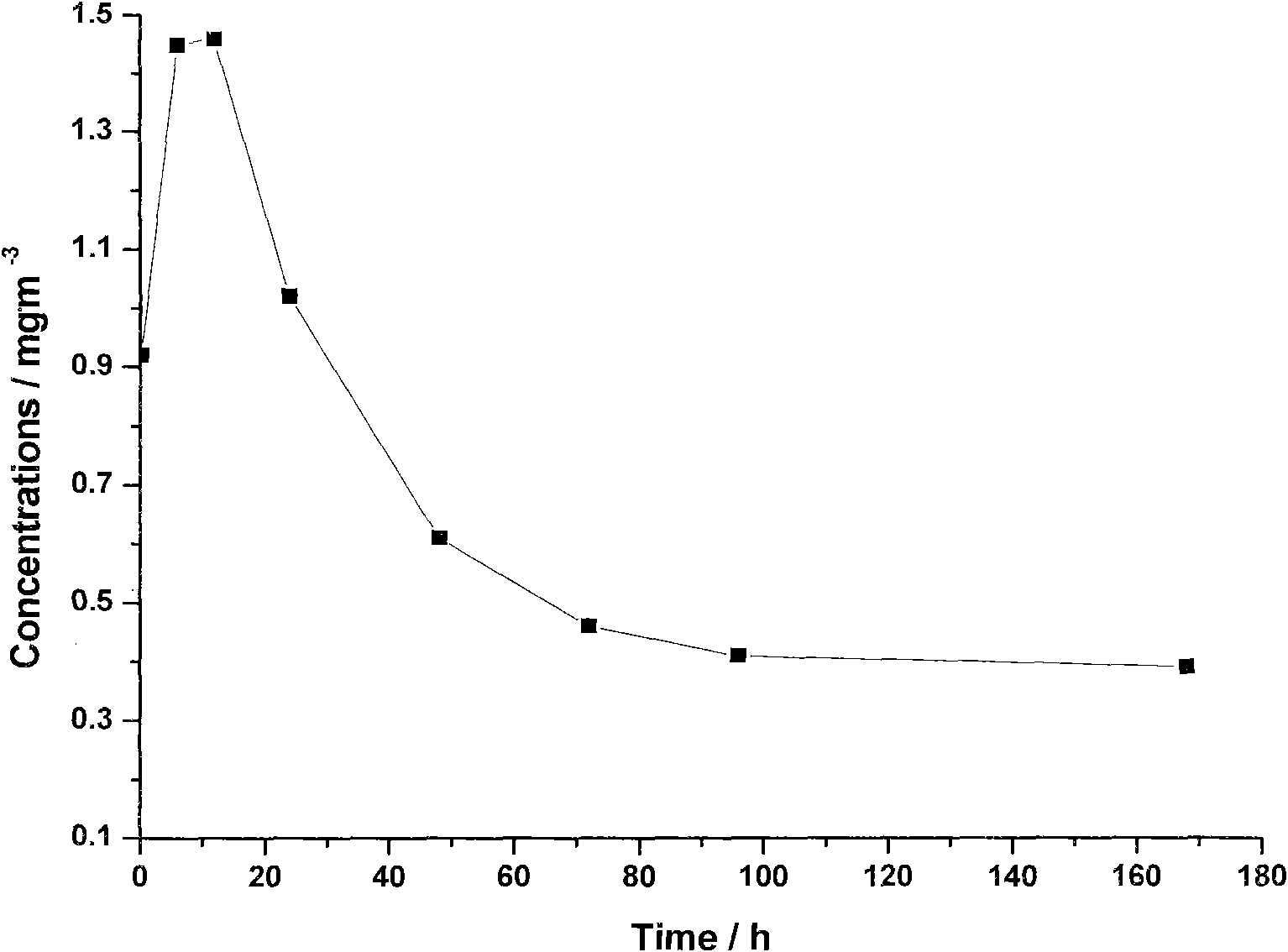
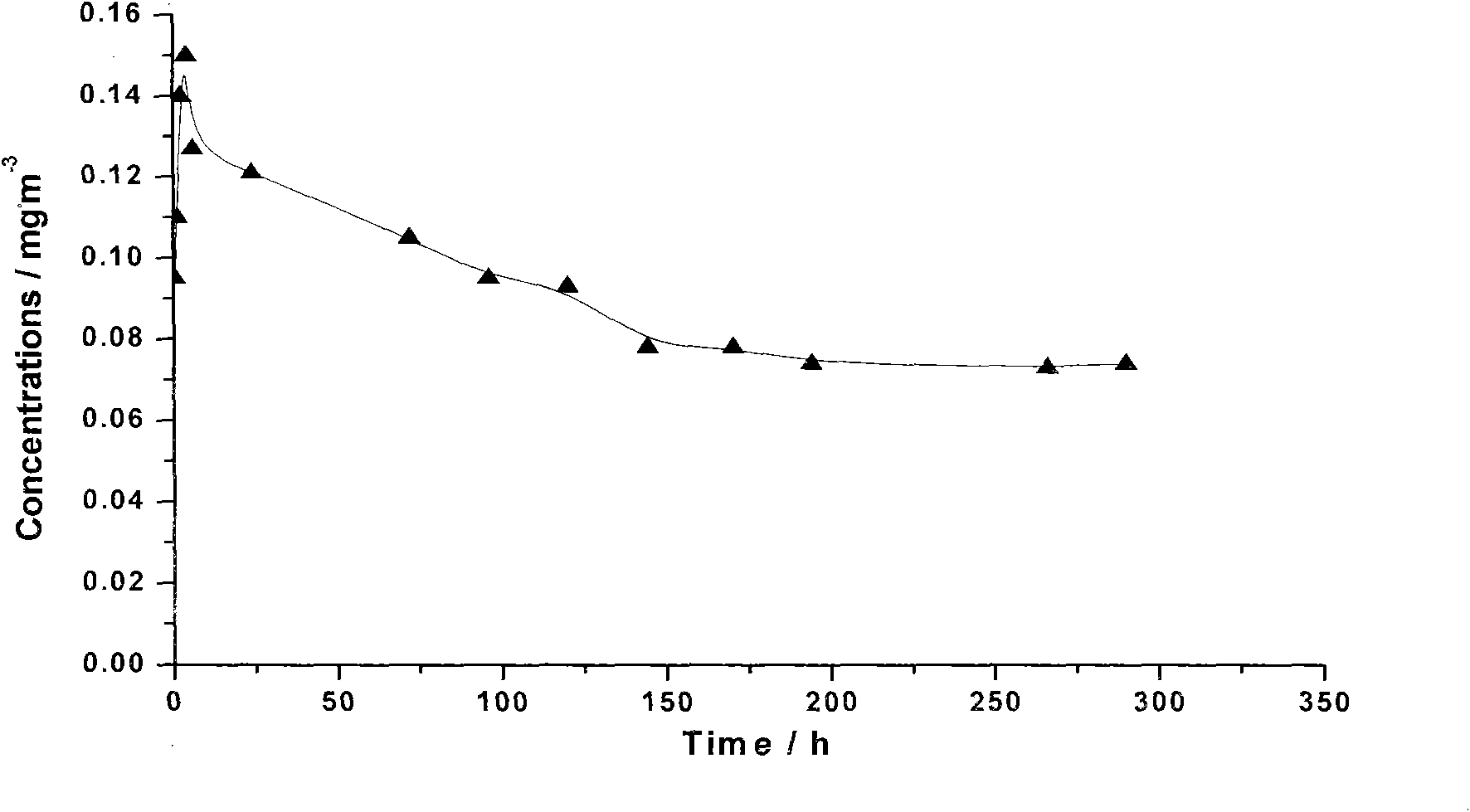
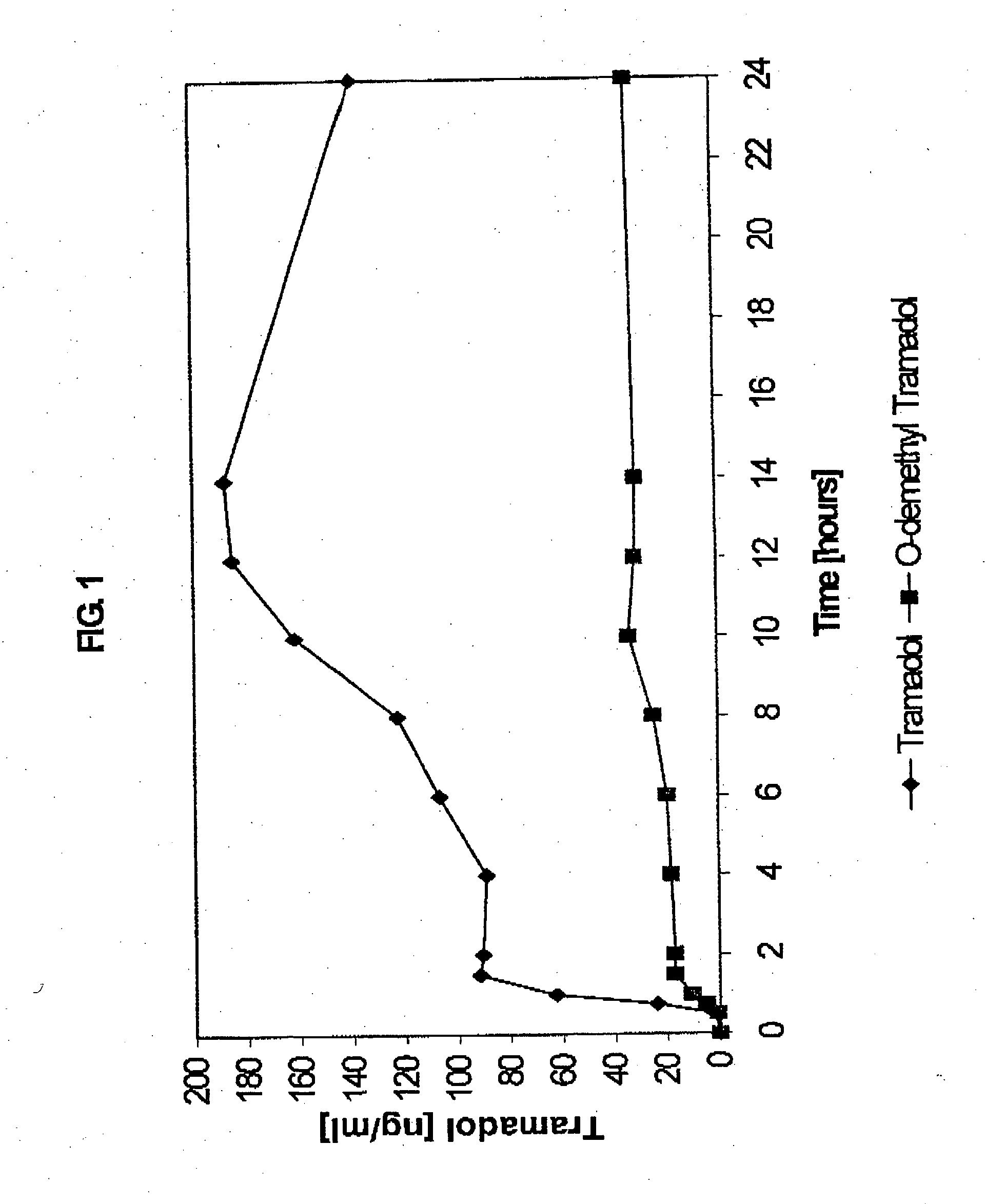
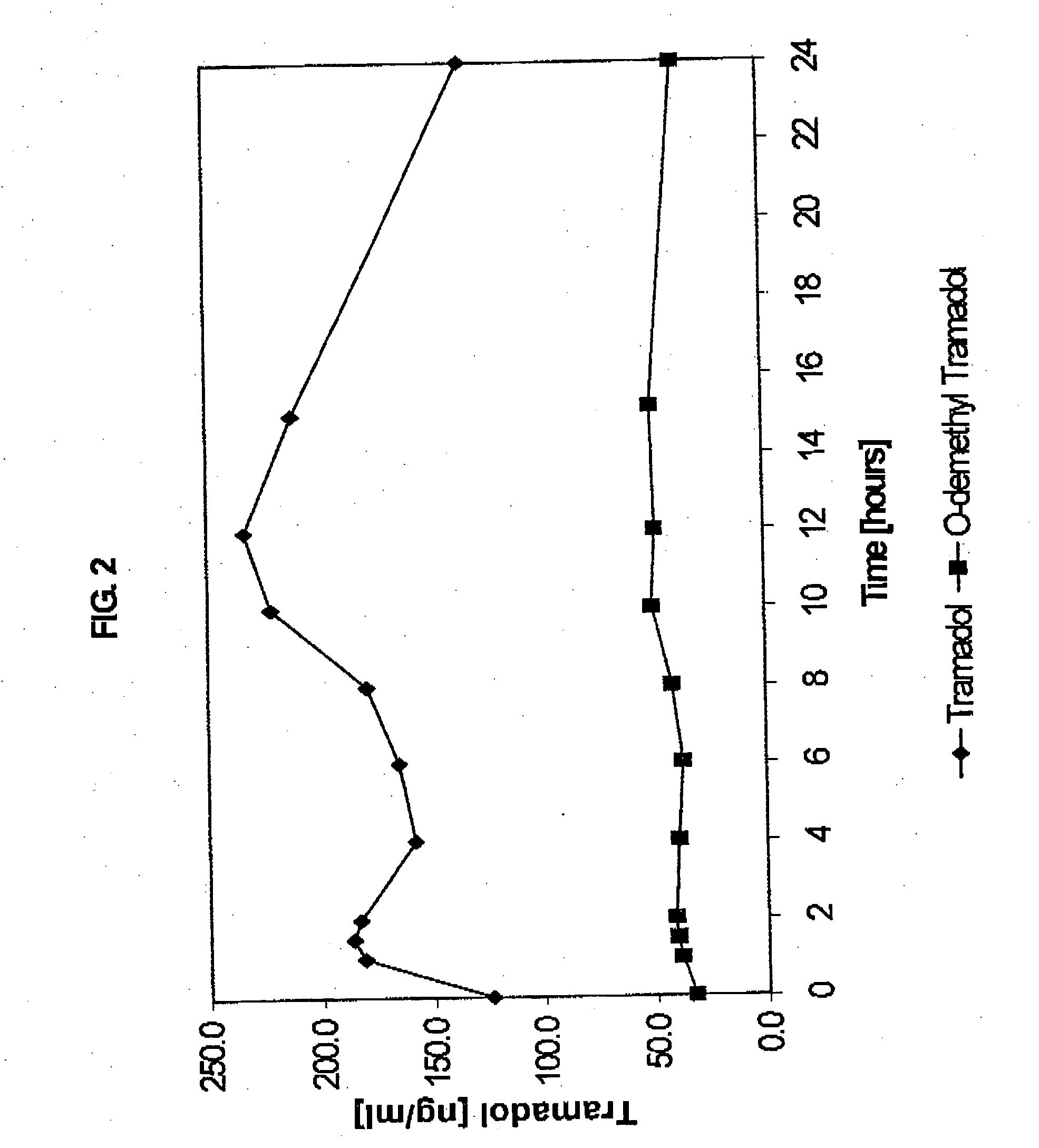
Extended release composition containing Tramadol
InactiveUS20030143270A1Effective controlRelieve painPowder deliveryBiocideBlood concentrationPeak concentration
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appear within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:GALEPHAR PHARMA RES
Low-voltage punch-through transient suppressor employing a dual-base structure
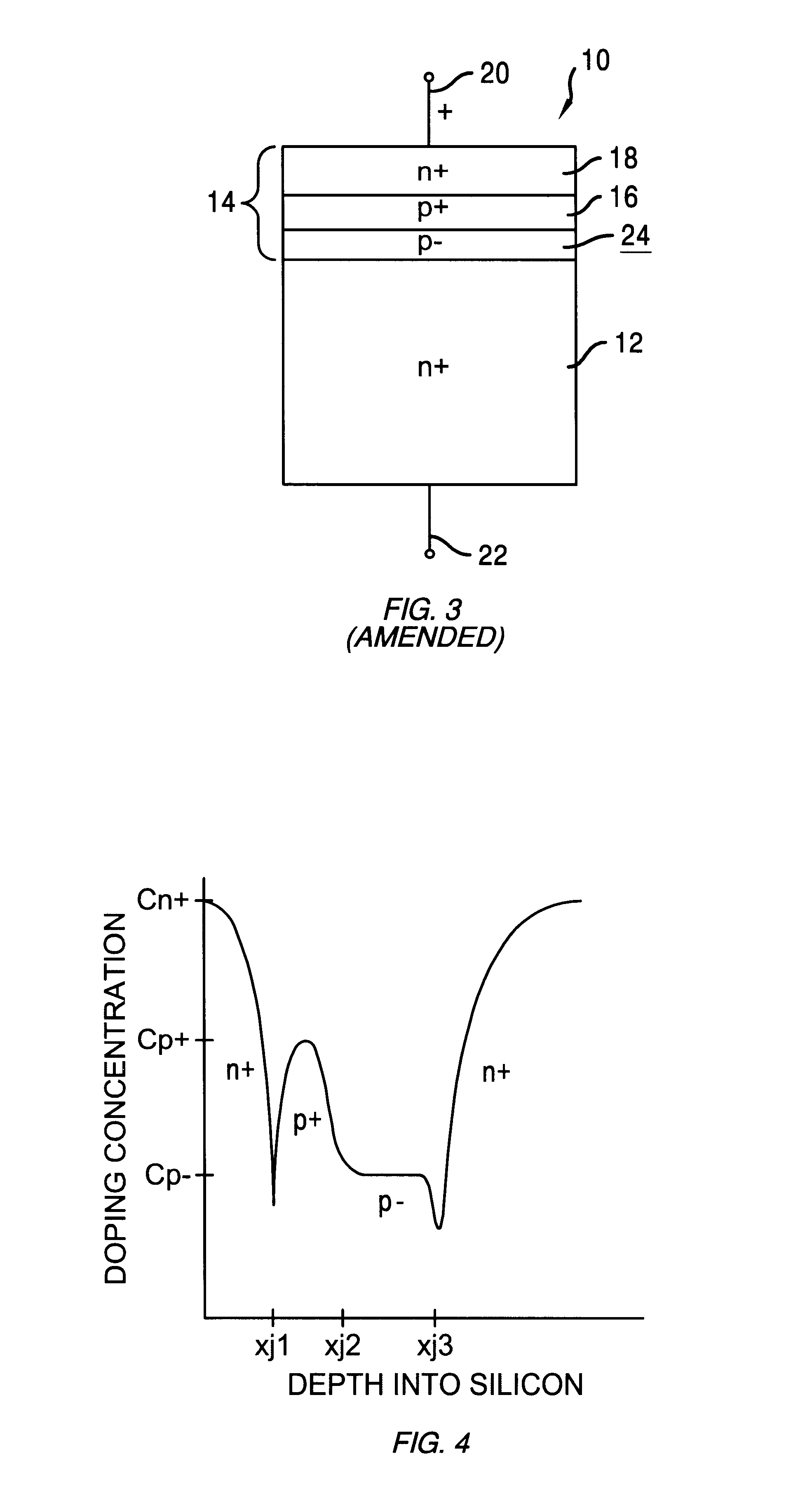
InactiveUS6015999AReduce leakage currentImprove featuresSemiconductor/solid-state device detailsSolid-state devicesDopantLow voltage
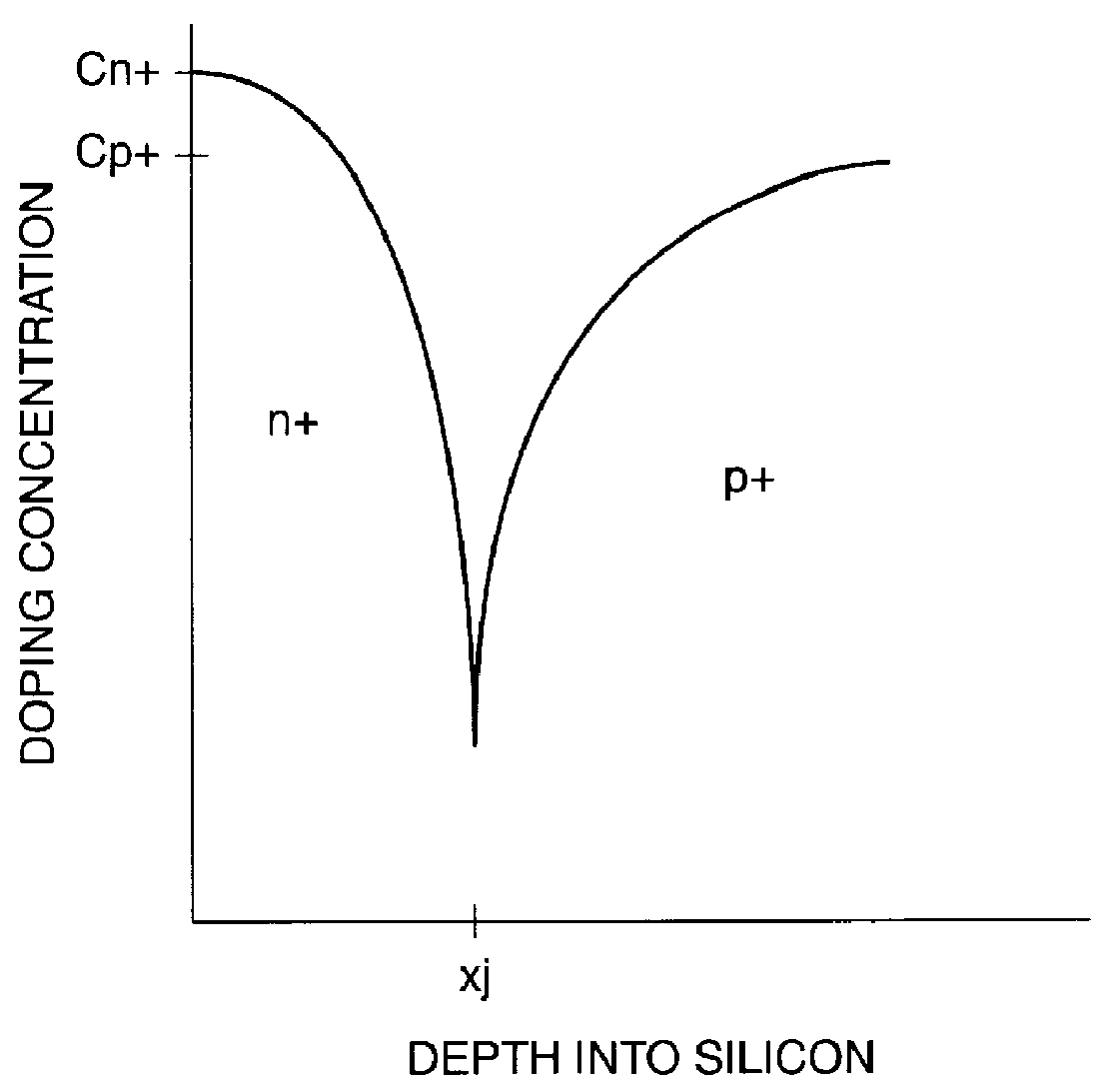
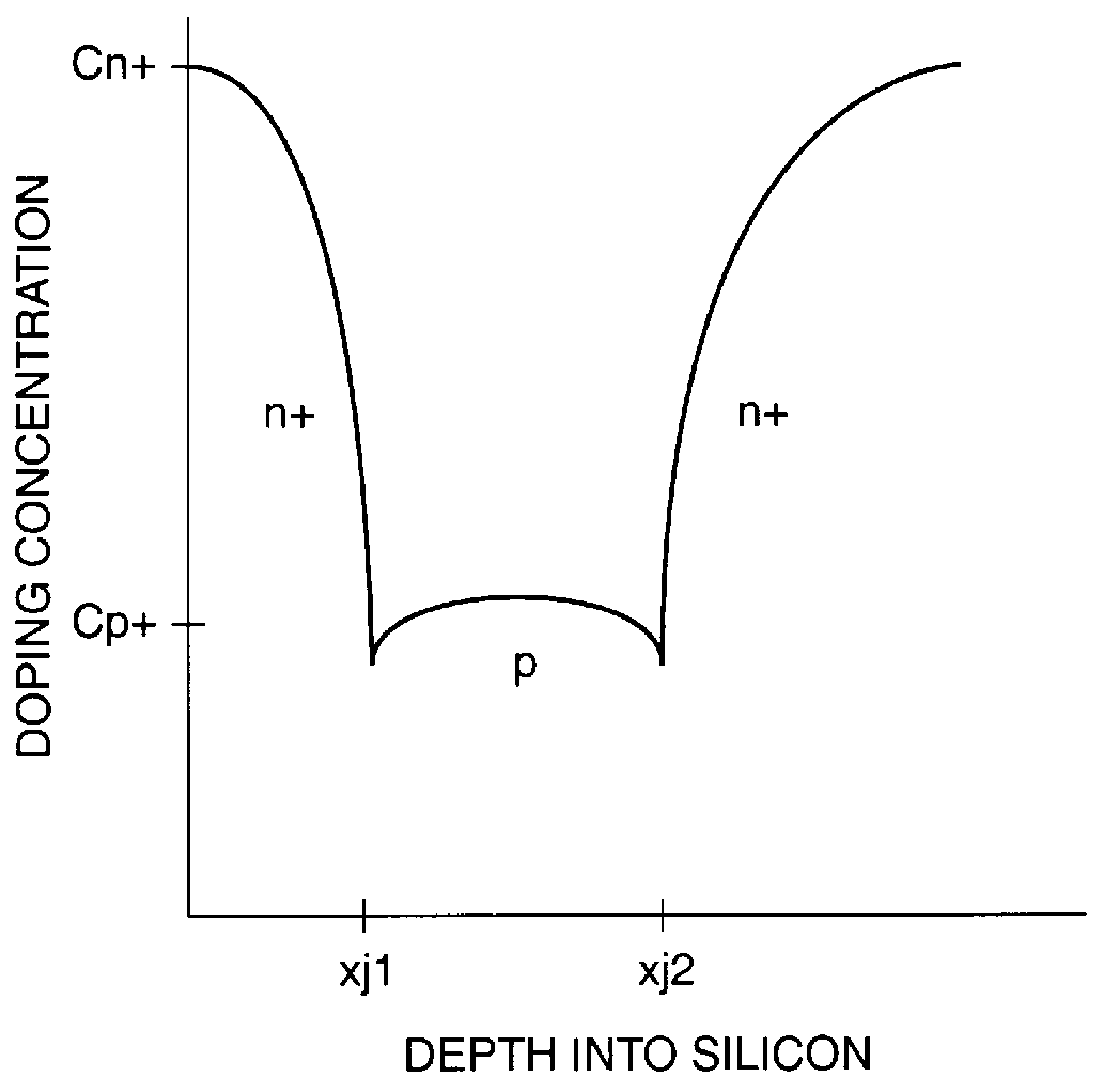
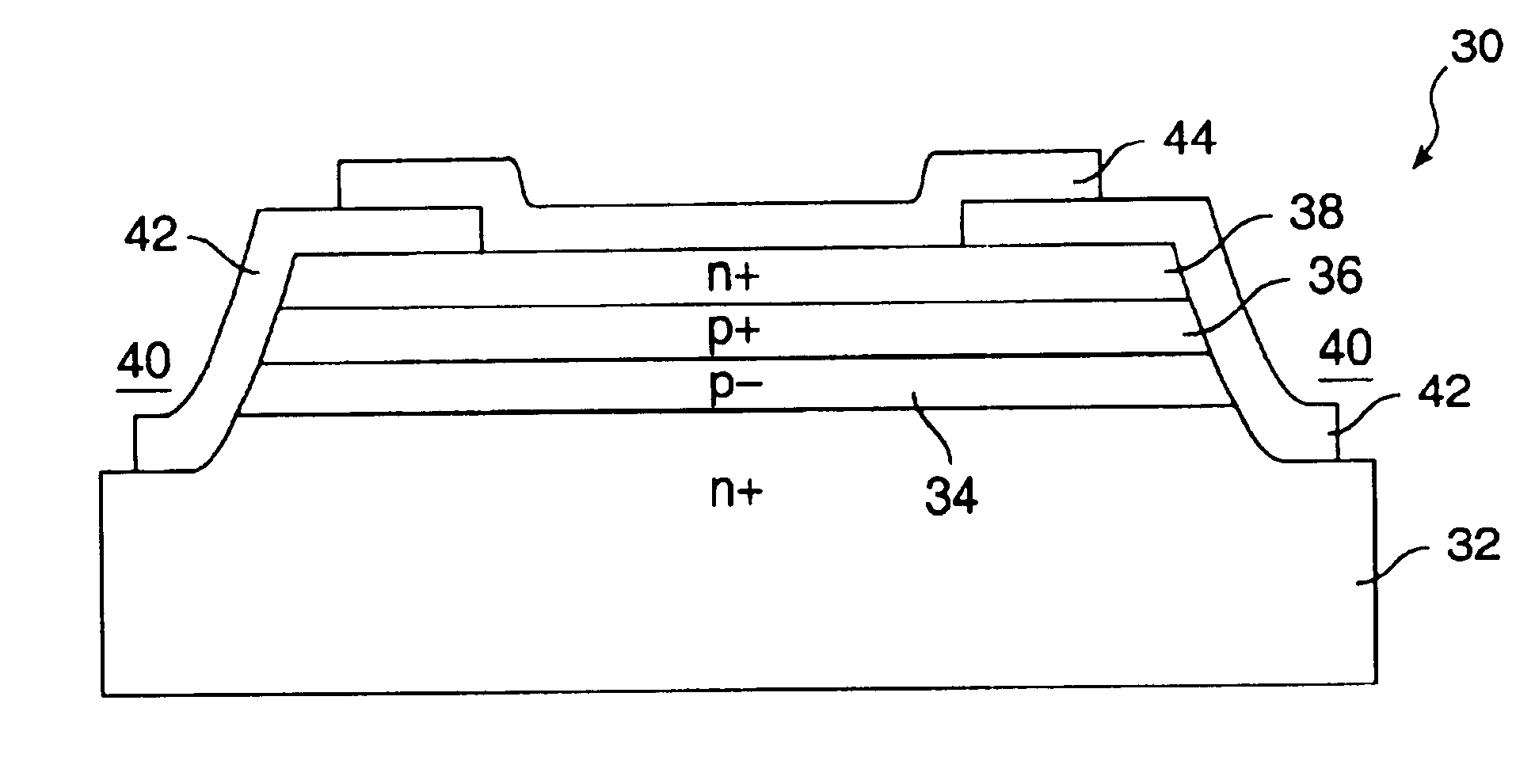
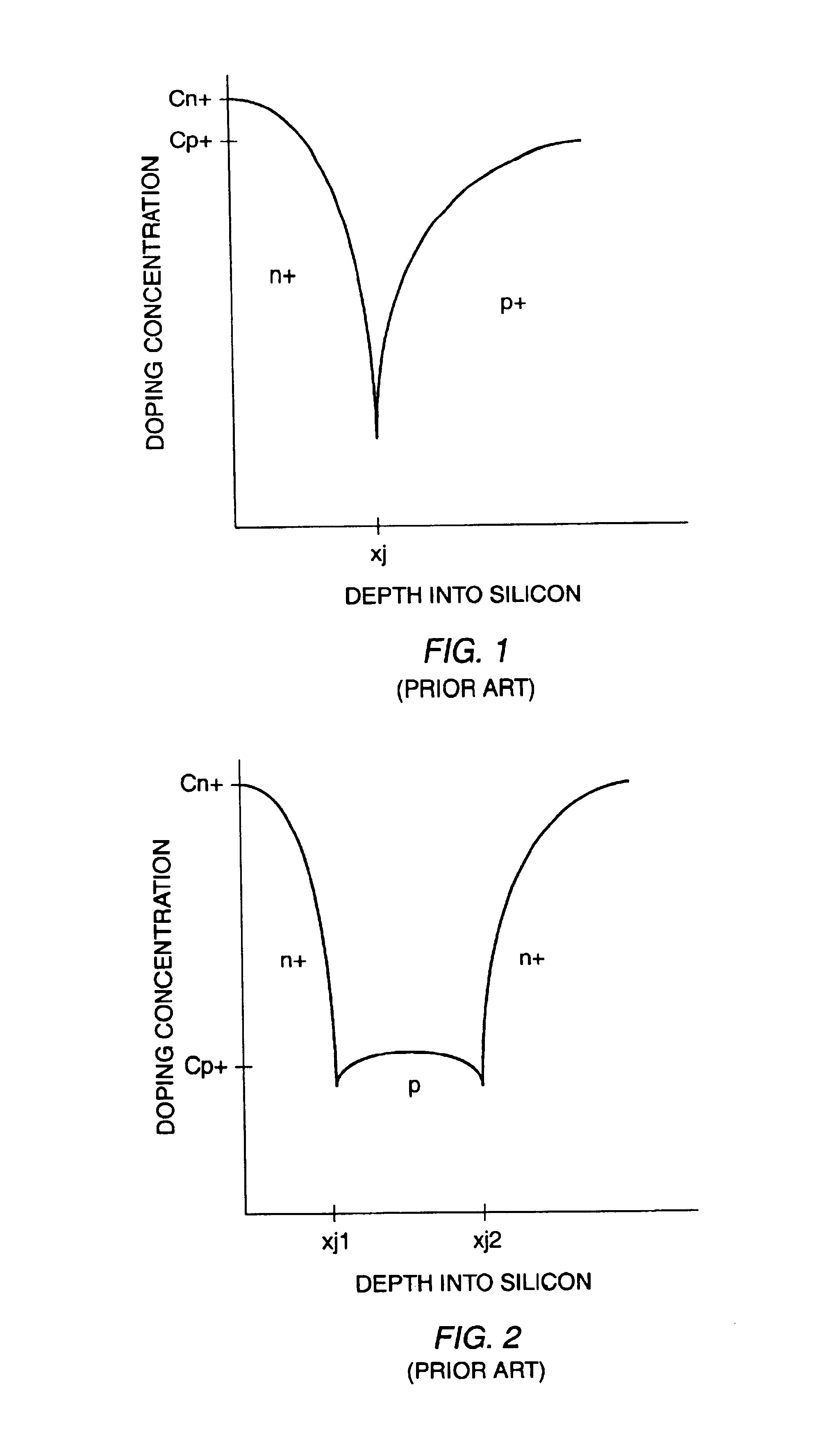
A punch-through diode transient suppression device has a base region of varying doping concentration to improve leakage and clamping characteristics. The punch-through diode includes a first region comprising an n+ region, a second region comprising a p- region abutting the first region, a third region comprising a p+ region abutting the second region, and a fourth region comprising an n+ region abutting the third region. The peak dopant concentration of the n+ layers should be about 1.5E18 cm-3, the peak dopant concentration of the p+ layer should be between about 1 to about 5 times the peak concentration of the n+ layer, and the dopant concentration of the p- layer should be between about 0.5E14 cm-3 and about 1.OE17 cm-3. The junction depth of the fourth (n+) region should be greater than about 0.3 mu m. The thickness of the third (p+) region should be between about 0.3 mu m and about 2.0 mu m, and the thickness of the second (p-) region should be between about 0.5 mu m and about 5.0 mu m.
Owner:SEMTECH CORP
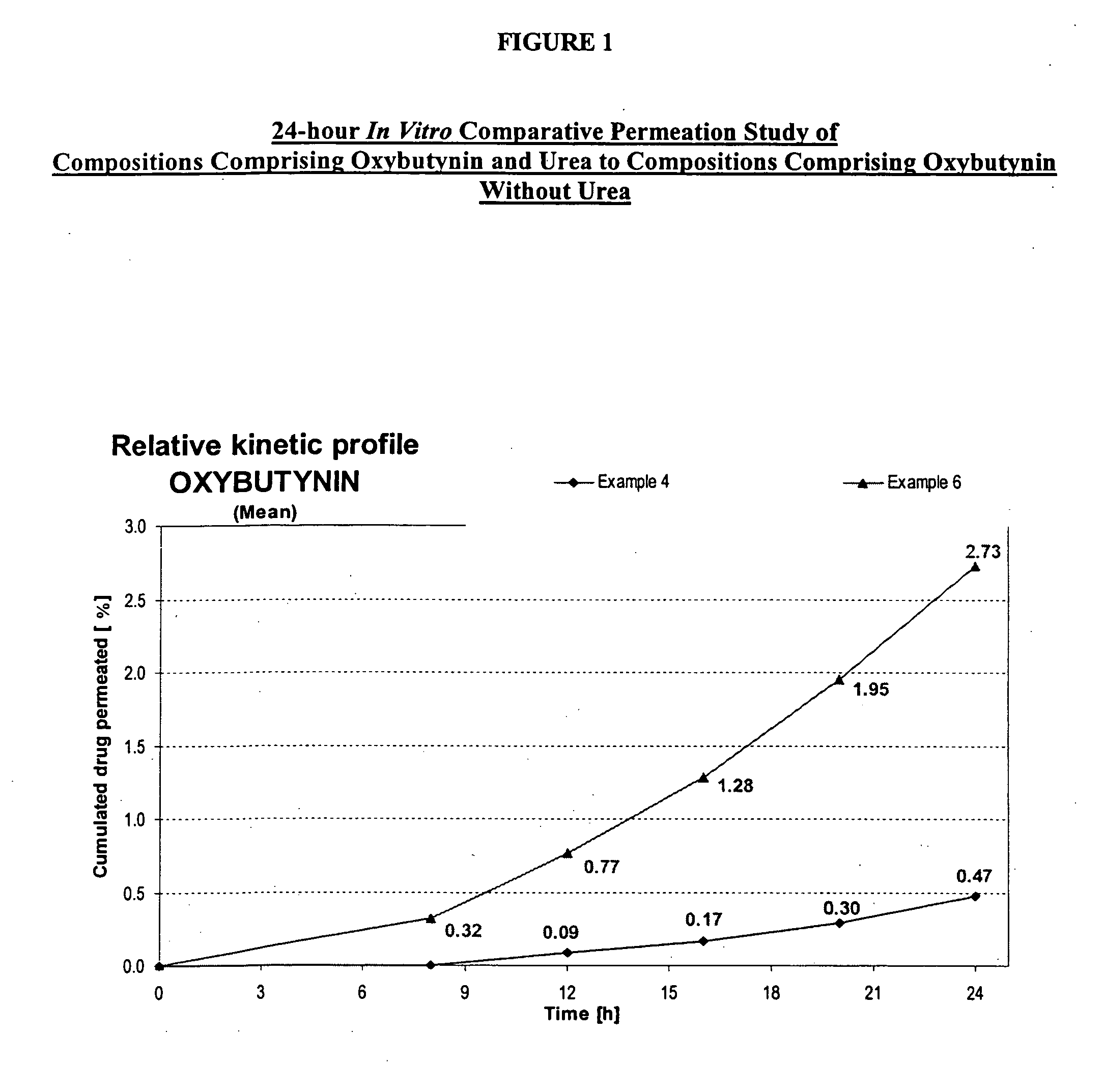
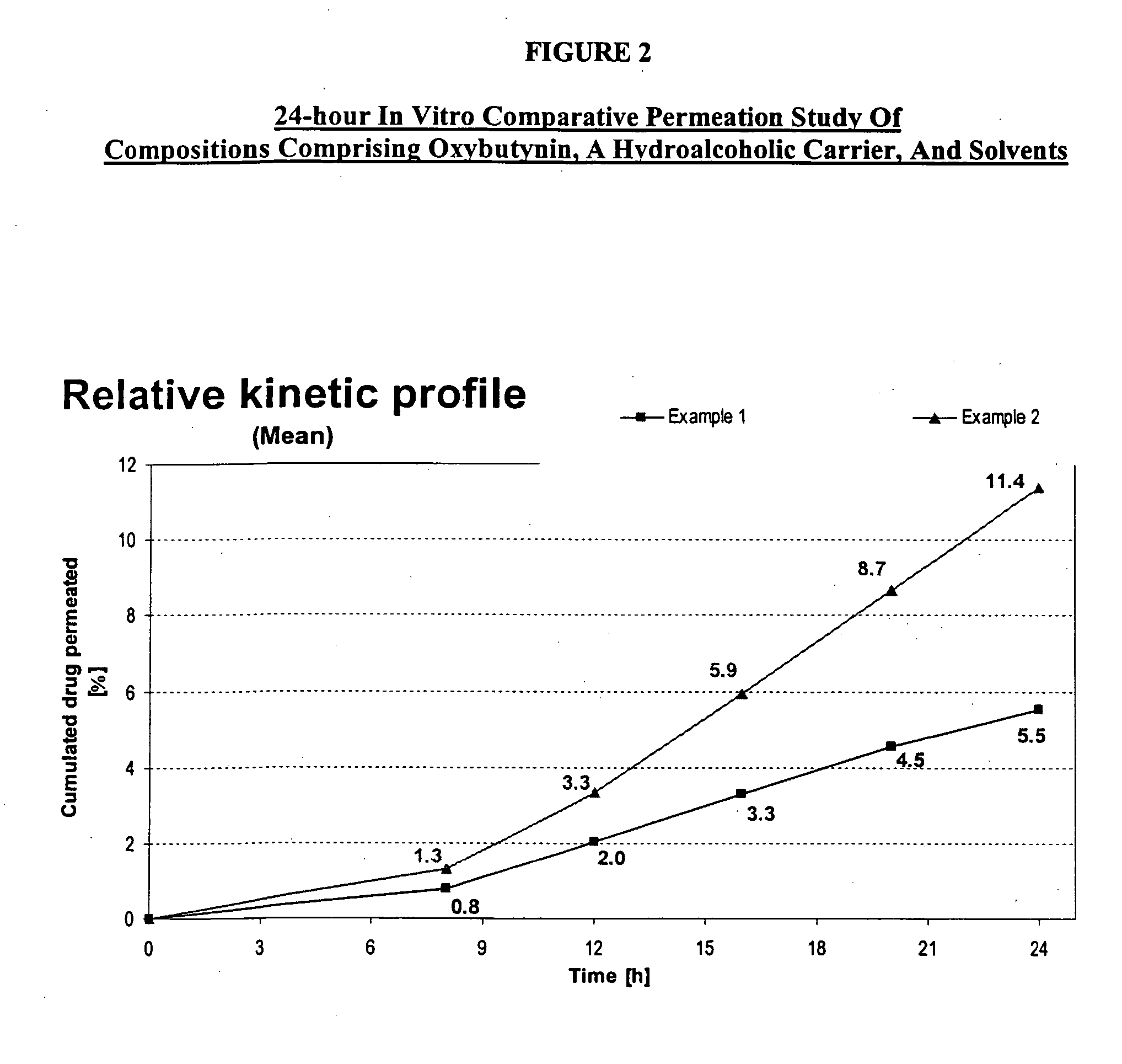
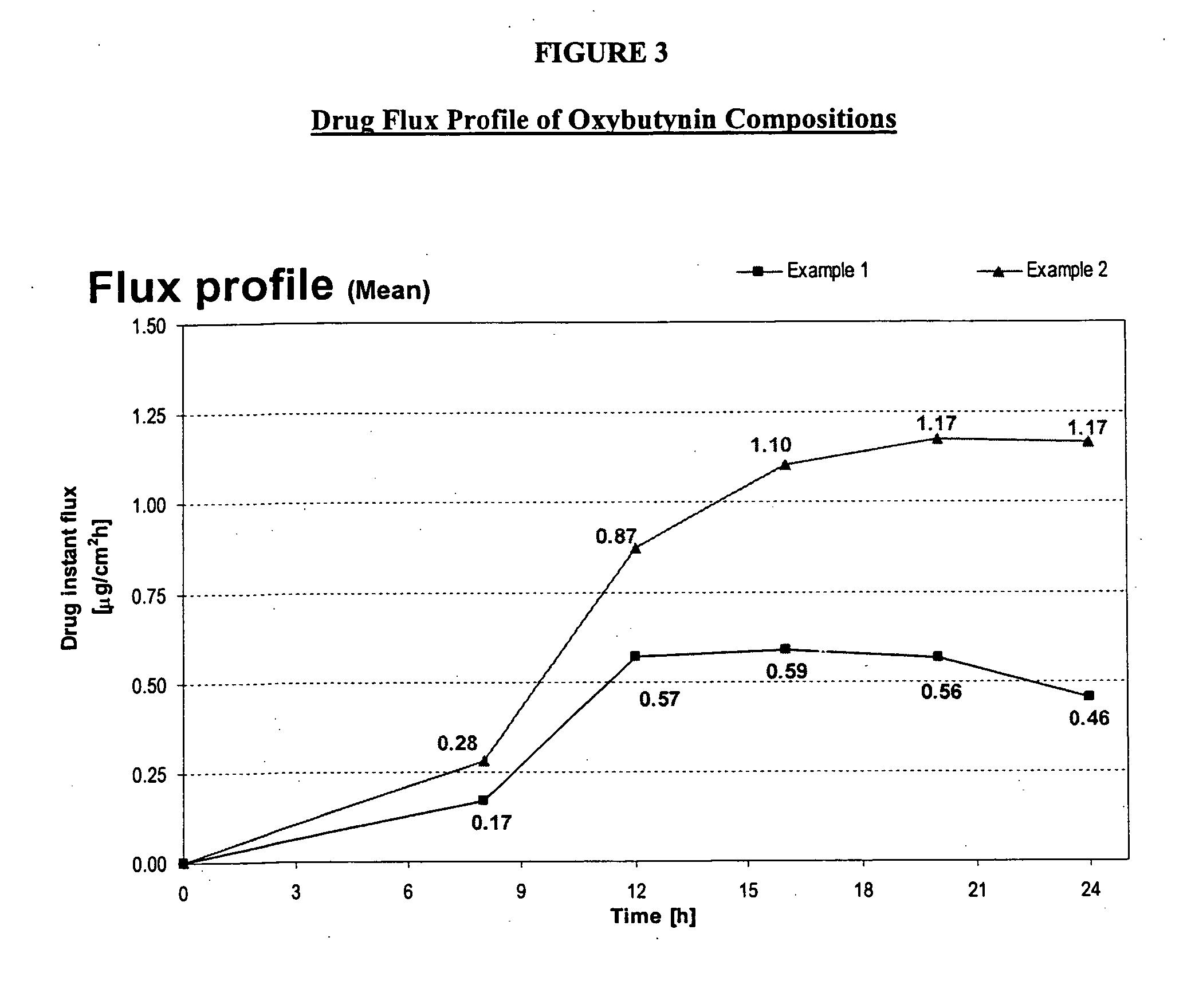
Transdermal compositions for Anti-cholinergic agents
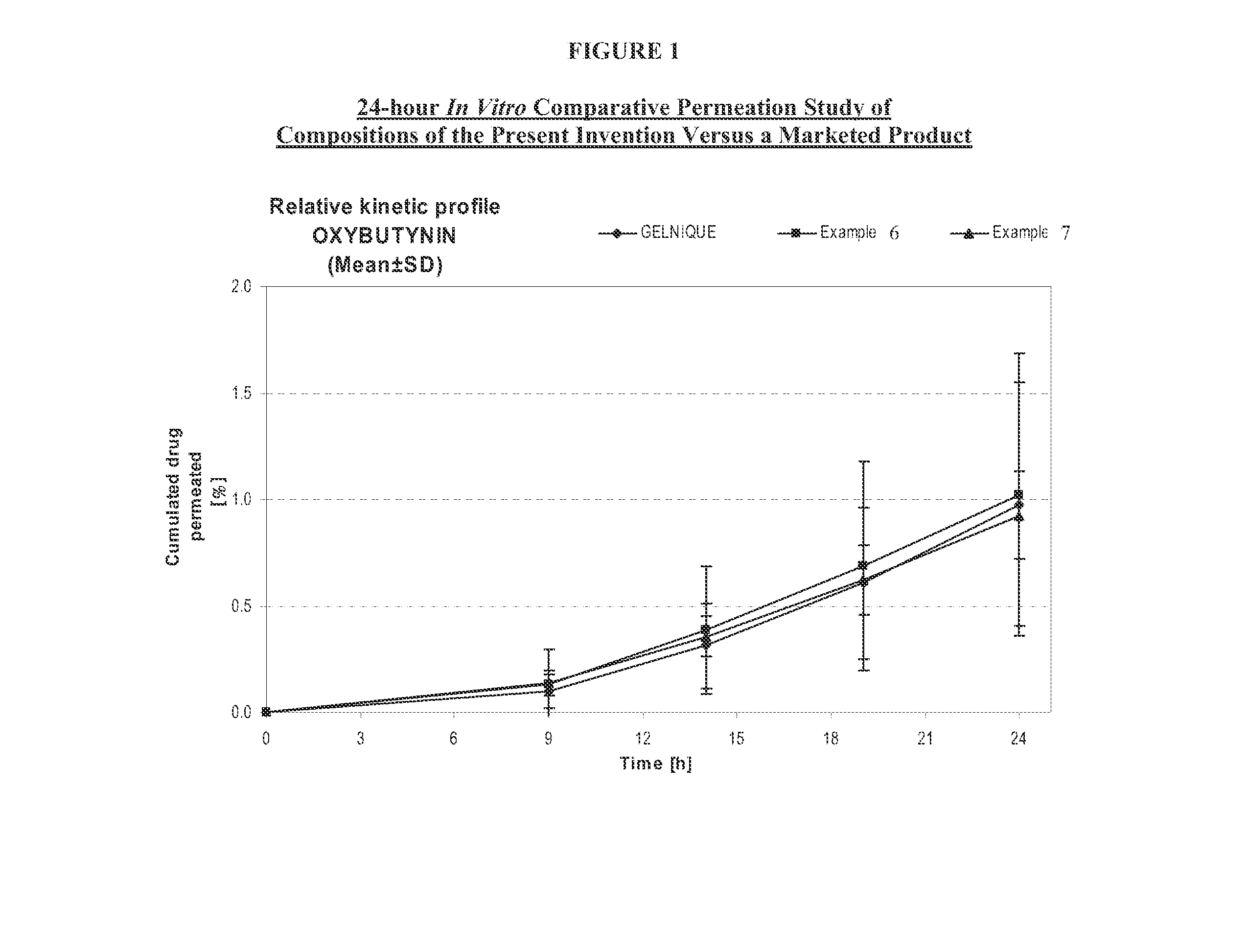
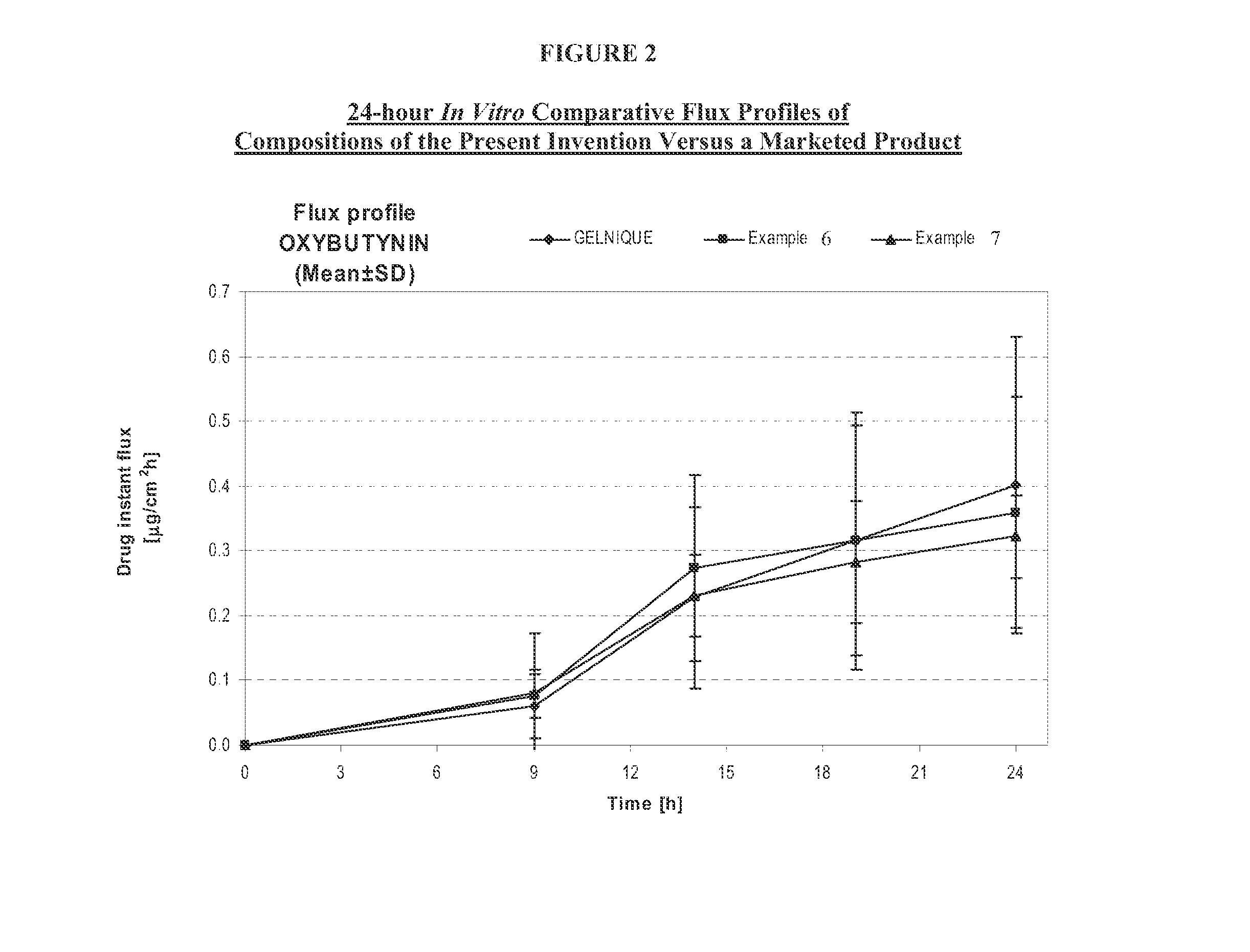
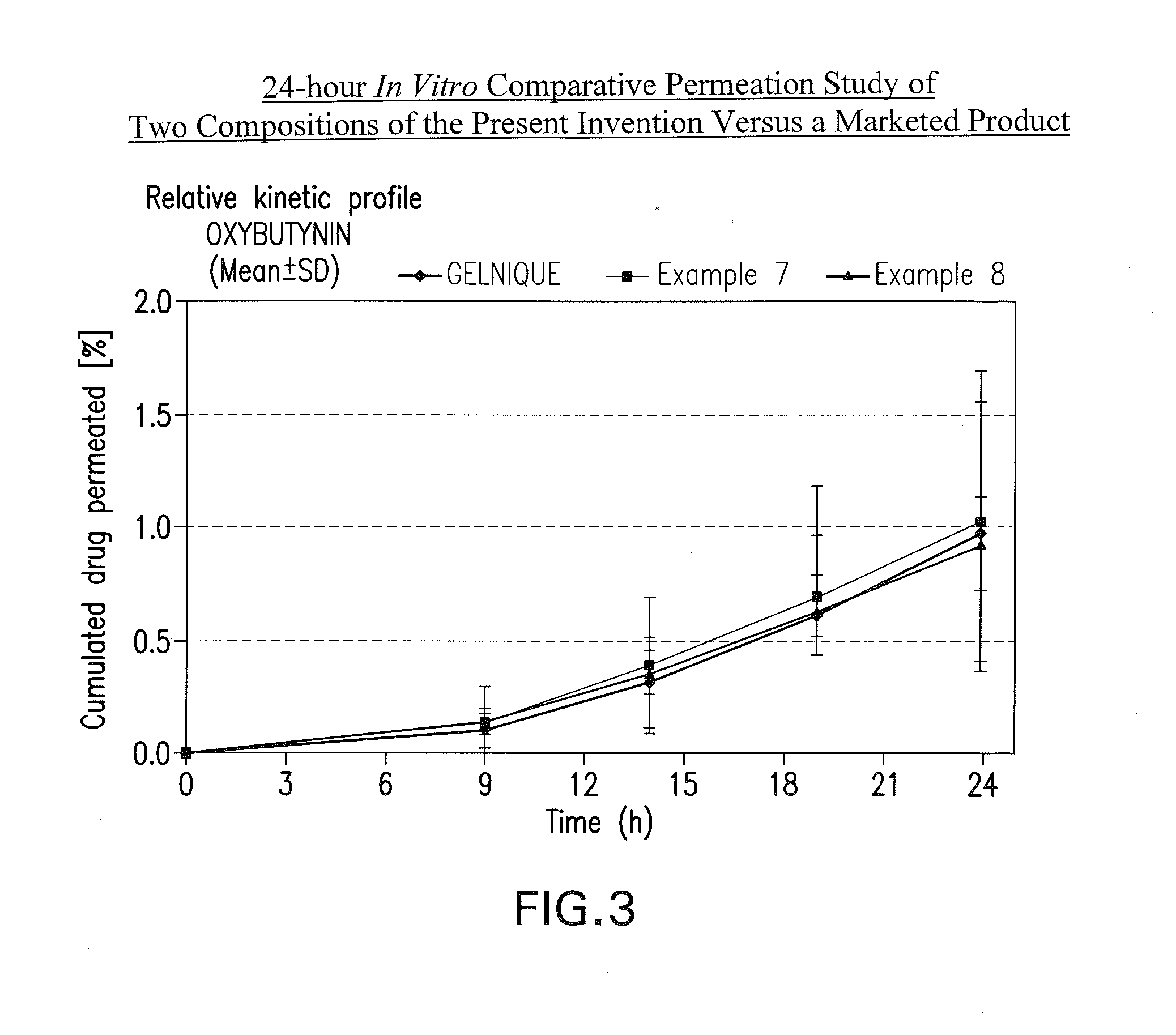
InactiveUS20140037713A1Reduce morbidityAvoids undesirable odor and irritation effectsCosmetic preparationsOrganic active ingredientsOxybutyninSide effect
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anti-cholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially live of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for hyperhidrosis with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anti-cholinergics.
Owner:ANTARES PHARMA IPL
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
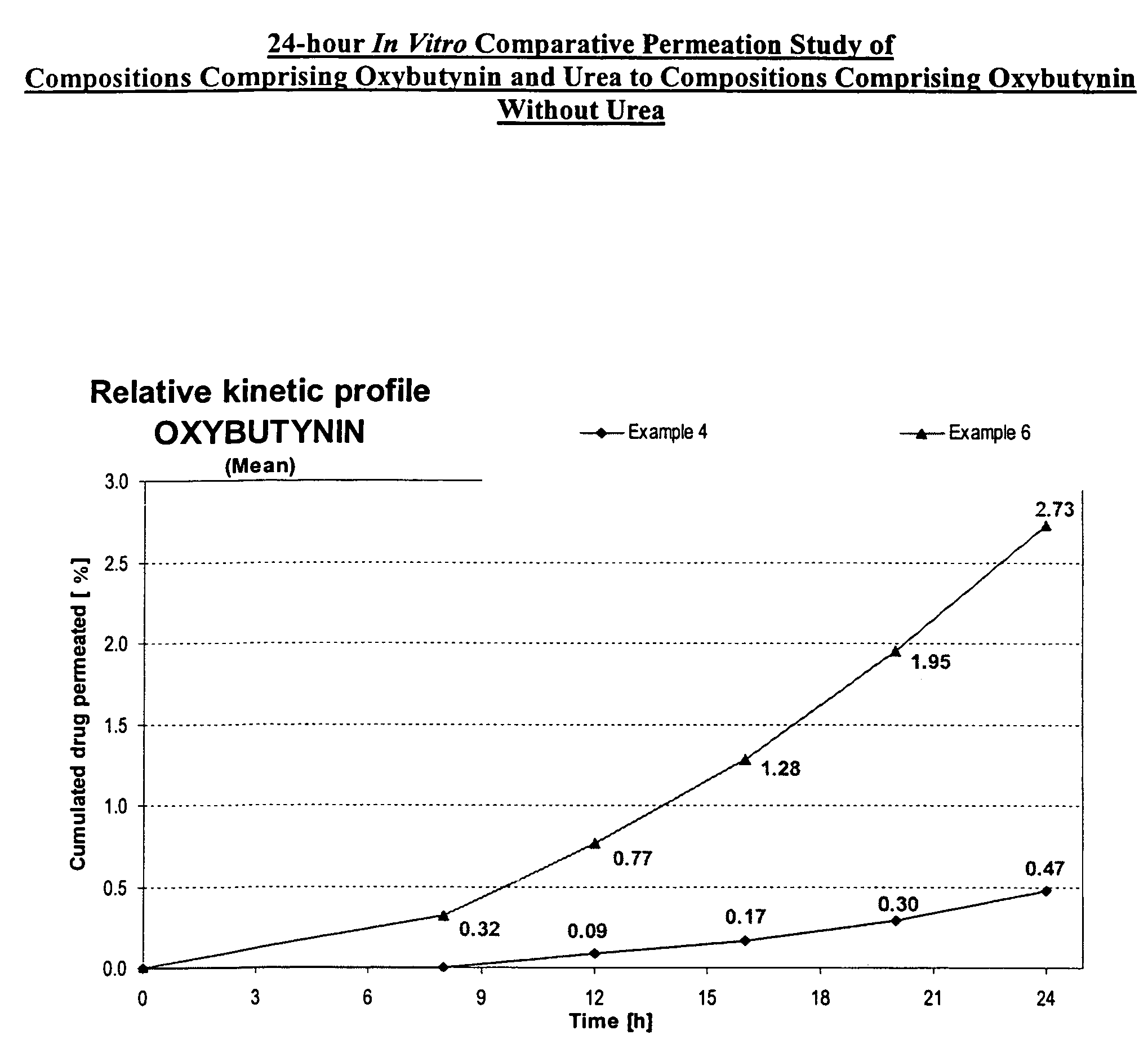
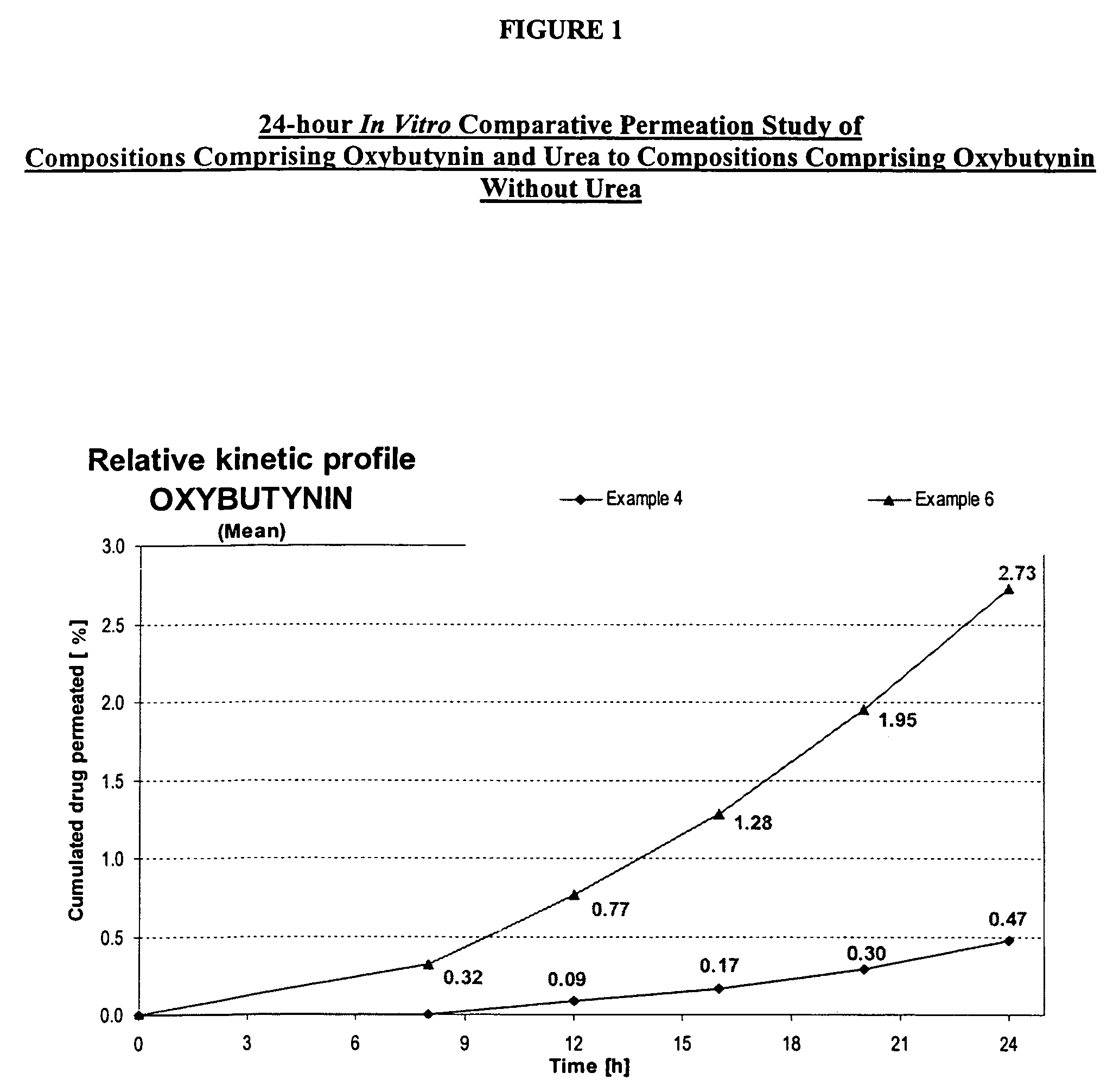
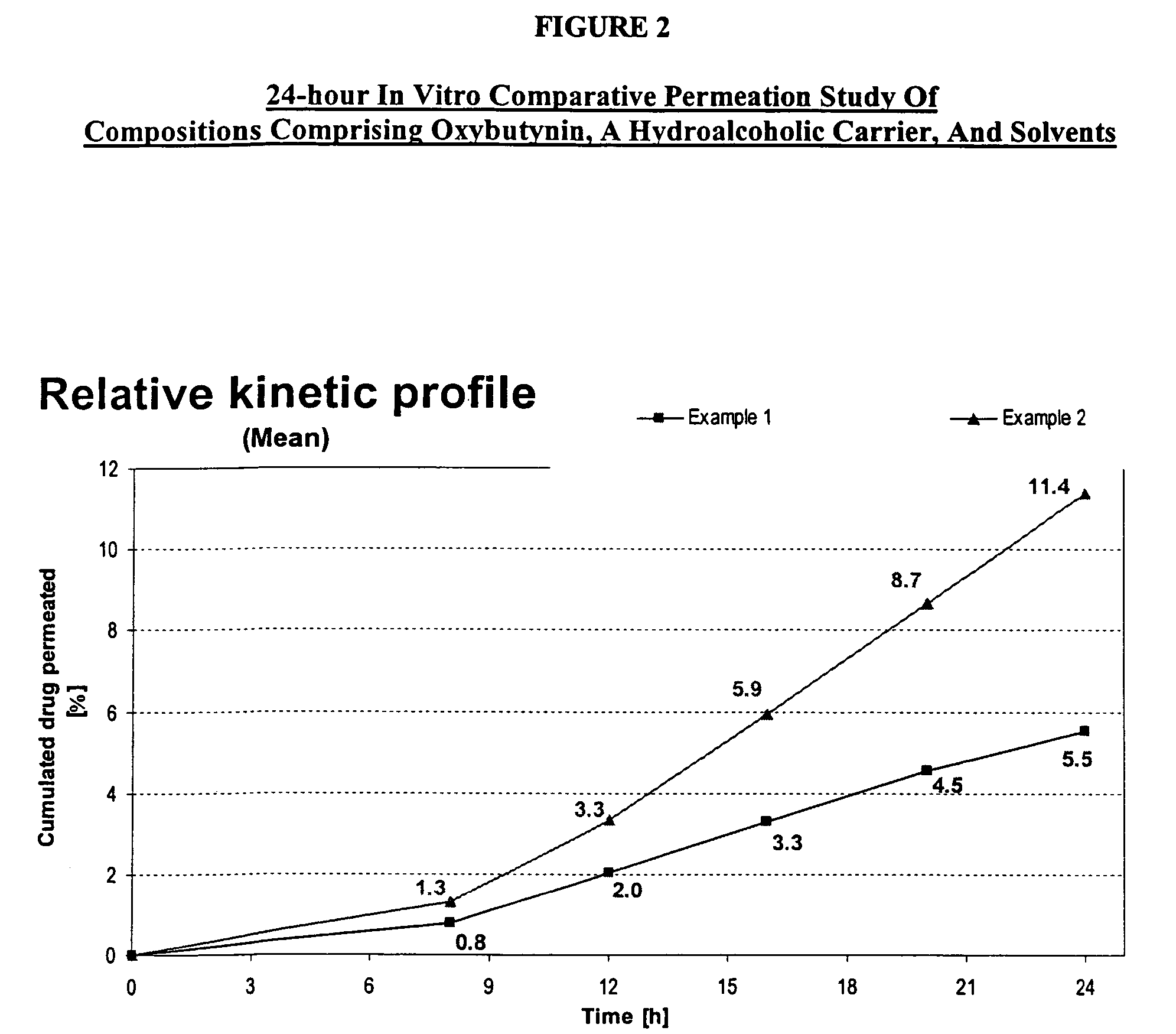
Permeation enhancing compositions for anticholinergic agents
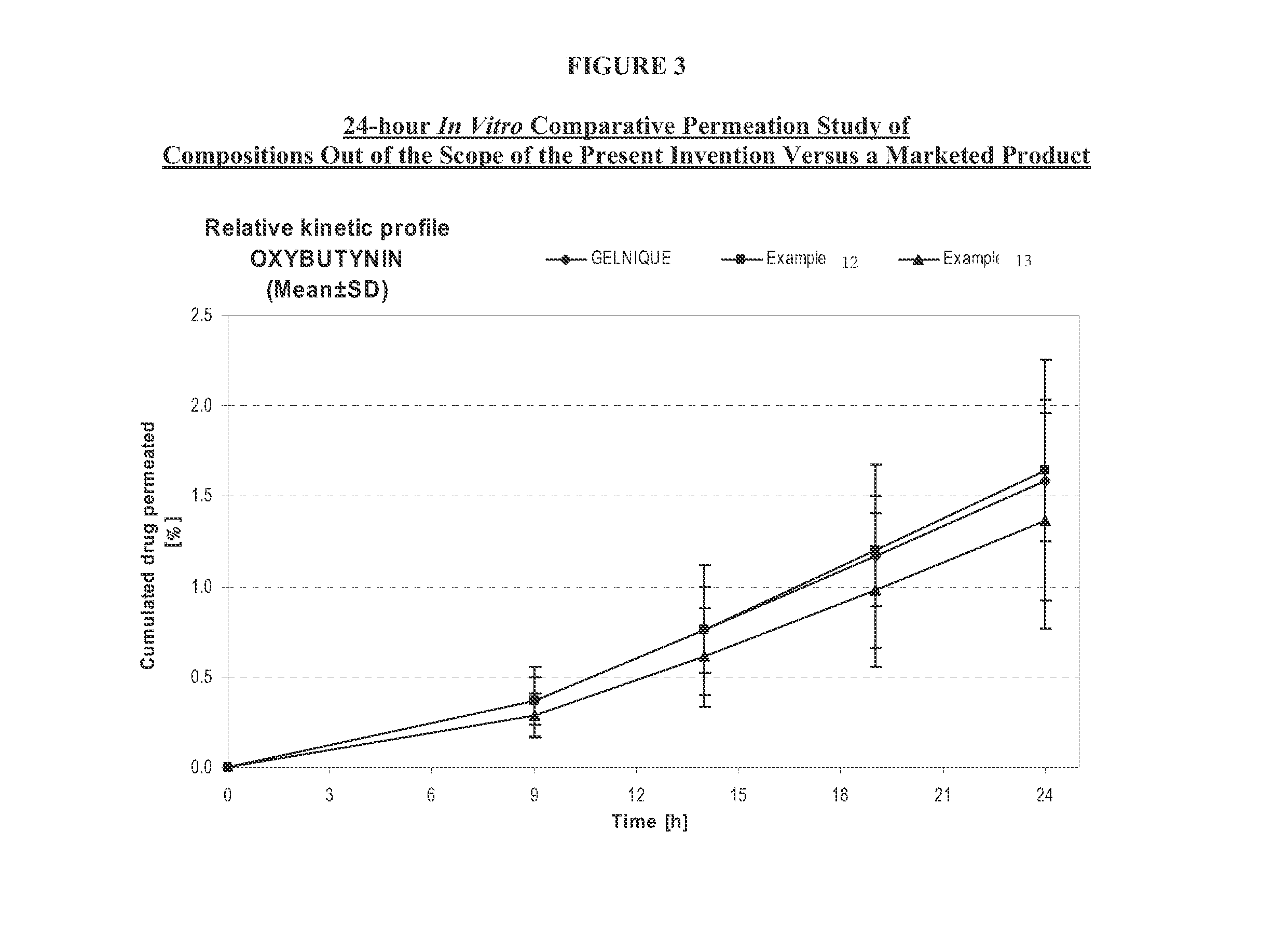
InactiveUS7425340B2Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
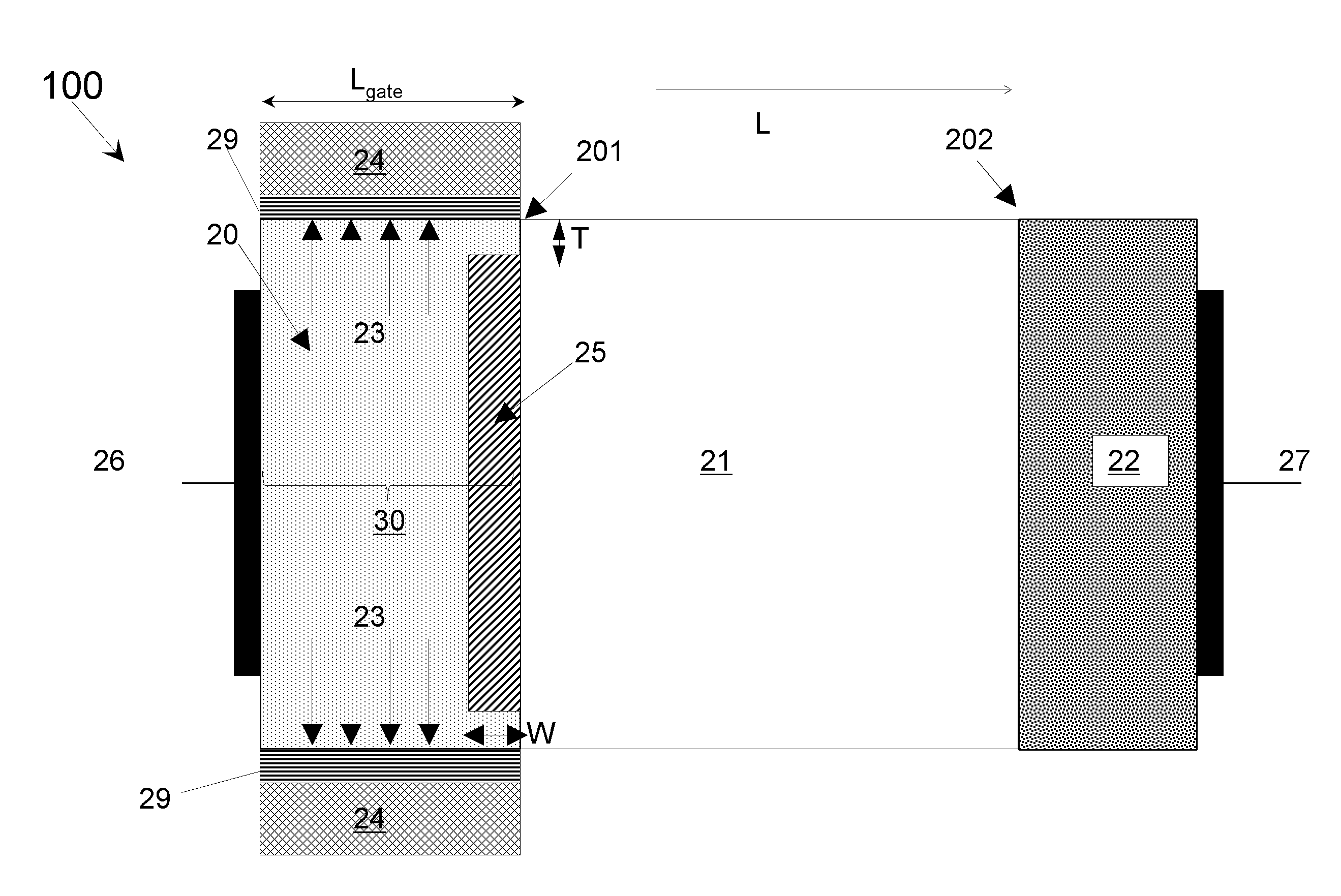
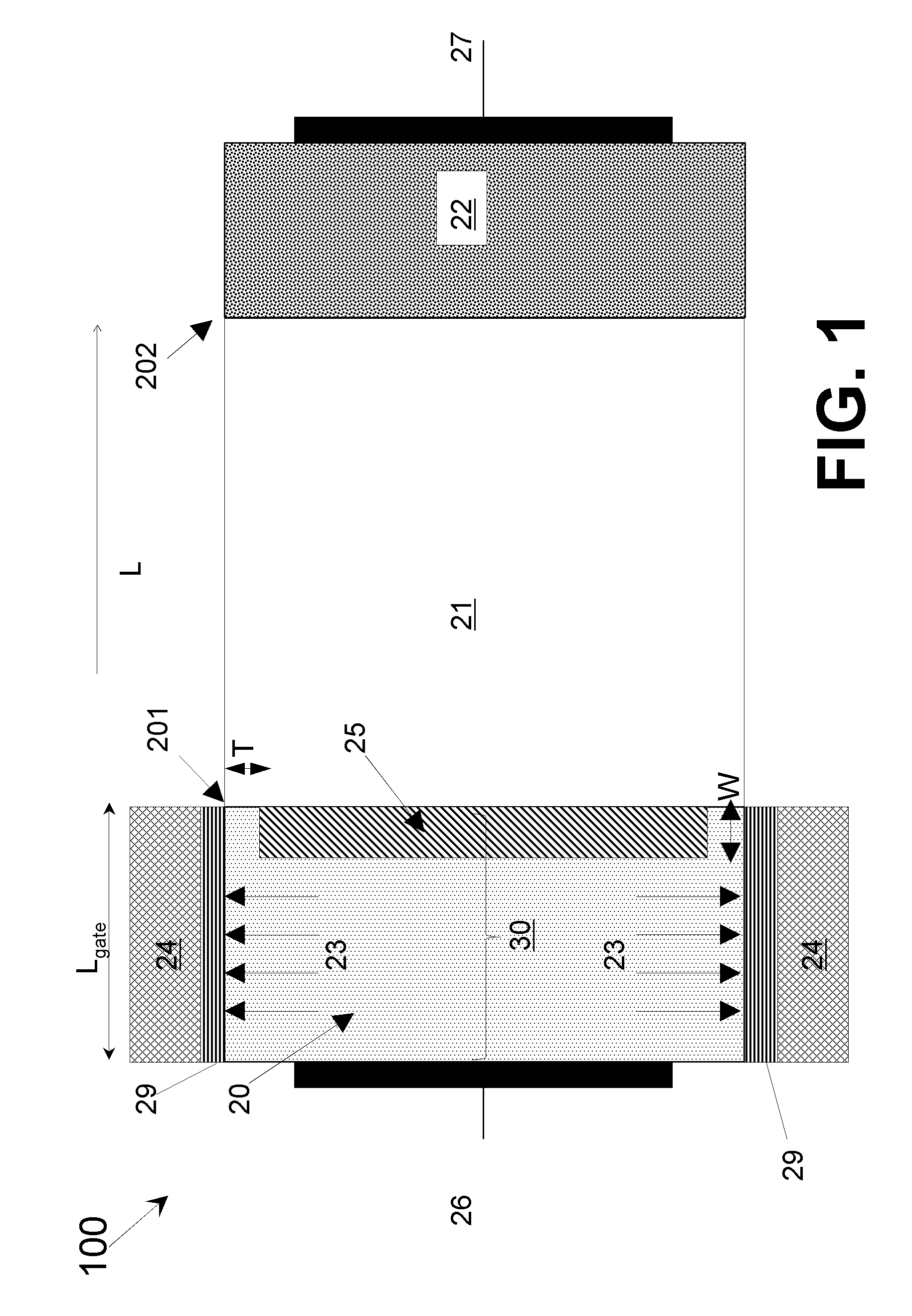
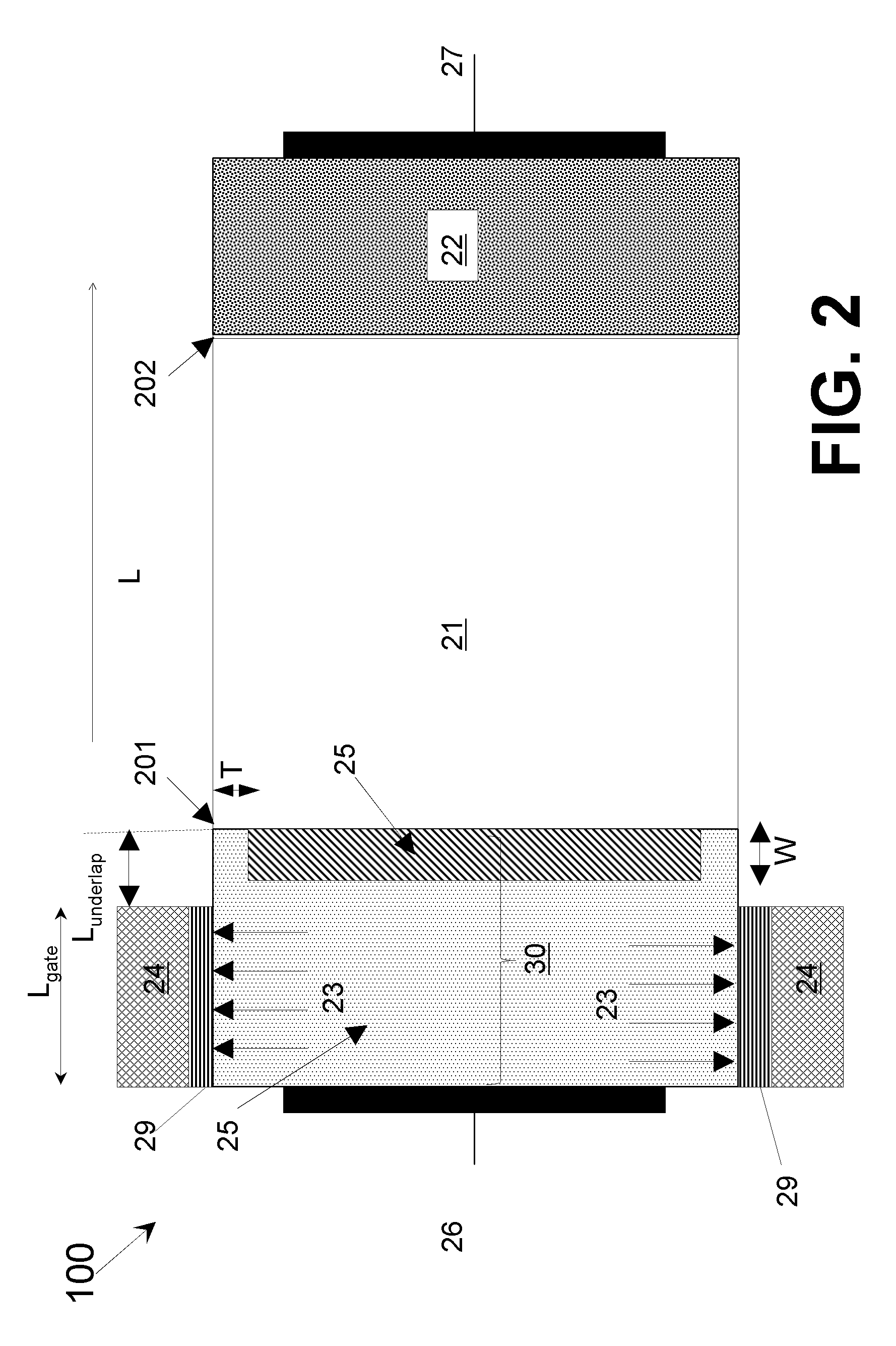
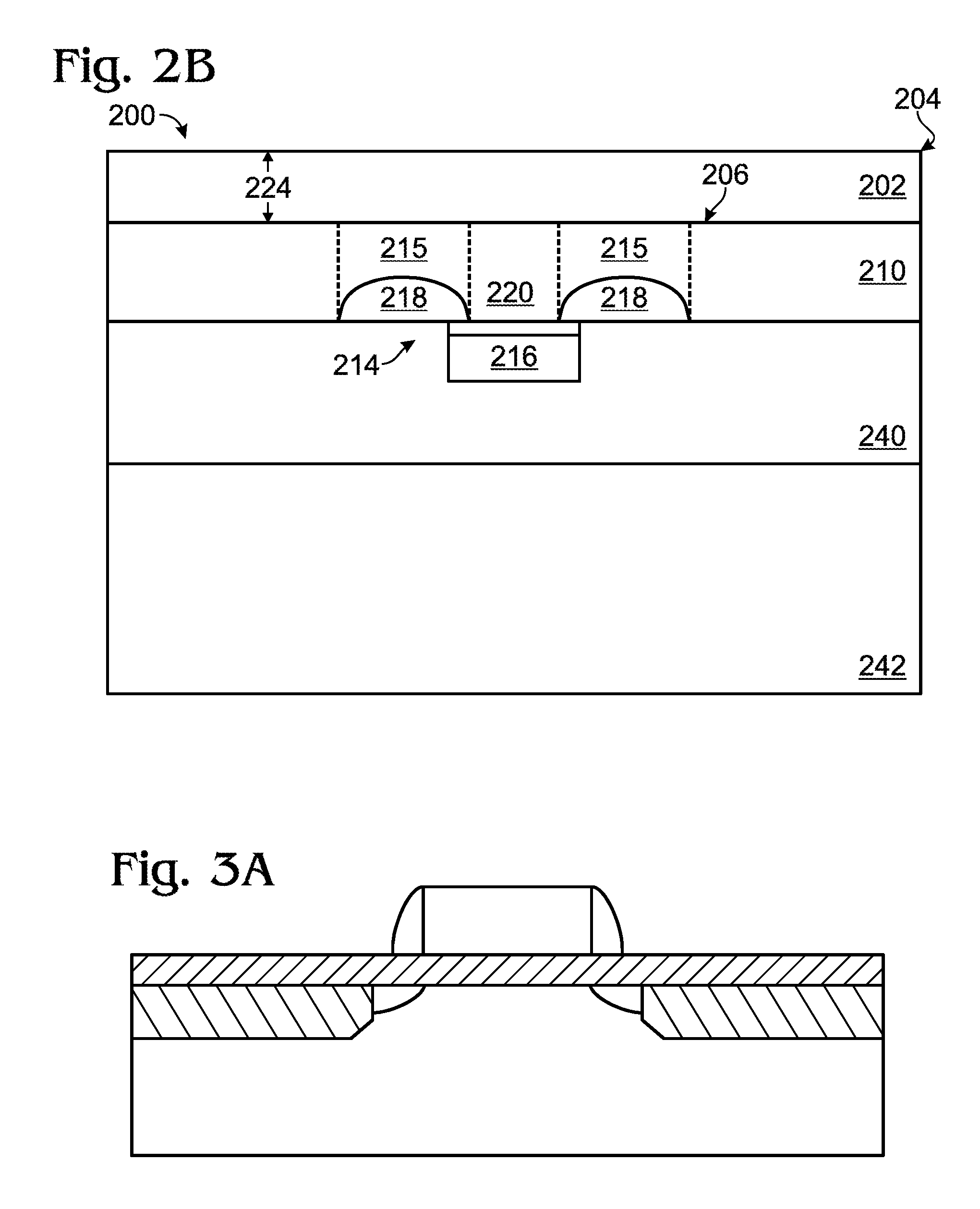
Line-tunneling tunnel field-effect transistor (TFET) and manufacturing method
InactiveUS20120298959A1Reduce variationNanoinformaticsSemiconductor/solid-state device manufacturingPeak concentrationCondensed matter physics
A tunnel field effect transistor (TFET) and method of making the same is provided. The TFET comprises a source-channel-drain structure and a gat electrode. The source region comprises a first source sub-region which is doped with a first doping profile with a dopant element of a first doping type having a first peak concentration and a second source sub-region close to a source-channel interface which is doped with a second doping profile with a second dopant element with the same doping type as the first dopant element and having a second peak concentration. The second peak concentration of the second doping profile is substantially higher than the maximum doping level of the first doping profile close to an interface between the first and the second source sub-regions.
Owner:INTERUNIVERSITAIR MICRO ELECTRONICS CENT (IMEC VZW) +2
Pharmaceutical Compositions Comprising Sirolimus and/or an Analogue Thereof
InactiveUS20080275076A1Improve safety/efficacy ratioReduce impactAntibacterial agentsBiocideParticulatesSide effect
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising sirolimus (rapamycin) and / or derivatives and / or analogues thereof. Compositions of the invention exhibit an acceptable bioavailability of sirolimus and / or a derivative and / or an analogue thereof. The pharmaceutical compositions of the invention are designed to release sirolimus in a controlled manner so that the plasma levels stays within the narrow therapeutic window that exist for this class of substances. An extended release profile, where the peak concentration has been reduced without loosing significant bioavailability, together with less variable absorption, is expected to improve the safety / efficacy ratio of the drug. Furthermore, compositions according to the invention provide for a significant reduced food effect and a delayed release of sirolimus is expected to reduce the number of gastro-intestinal related side effects.
Owner:LIFECYCLE PHARMA AS
Transdermal compositions for anticholinergic agents
InactiveUS20100216880A1Avoiding undesirable peak in drug concentrationReduce morbidityAntibacterial agentsBiocideOxybutyninLong chain fatty acid
The present invention relates generally to compositions or formulations for transdermal or transmucosal administration of anticholinergic agents such as oxybutynin. The invention utilizes a novel delivery vehicle and is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters. A method is disclosed for treating a subject for urinary incontinence with these formulations while reducing the incidences of peak concentrations of drug and undesirable side effects associated with oral anticholinergics.
Owner:ANTARES PHARMA IPL
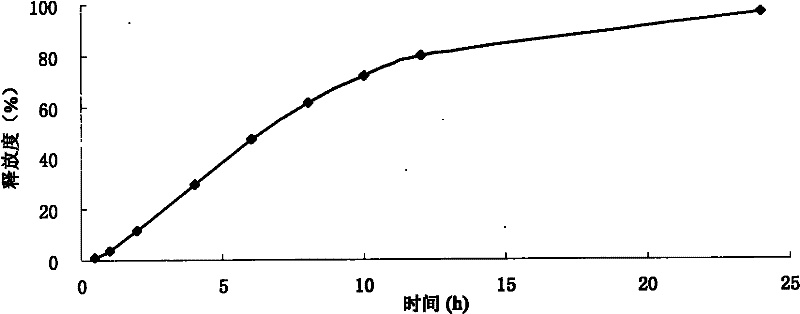
Method for measuring volatile substance release in interior wall coating
InactiveCN101832983AImprove pollutionPollution situation is intuitiveComponent separationColor/spectral properties measurementsEngineeringEnvironmental tests
The invention discloses a method for measuring volatile substance release in an interior wall coating, which comprises the following steps of: brushing the coating on two surfaces of a smooth inert substrate material twice according to the explanation of the using amount of the interior wall coating, immediately placing the material in an environmental test chamber with adjustable temperature, humidity and ventilation frequency, periodically extracting an air sample in the environmental test chamber according to the release rule of a volatile harmful substance, tracing and monitoring the release concentration of the volatile harmful substance in the sample, and making a release concentration-time curve to determine the release peak concentration and release steady-state concentration of the volatile harmful substance in the sample. The release concentration of a volatile organic compound (VOC) is detected by using gas chromatography, and the release concentration of formaldehyde is detected according to a phenol reagent method.
Owner:NANCHANG UNIV
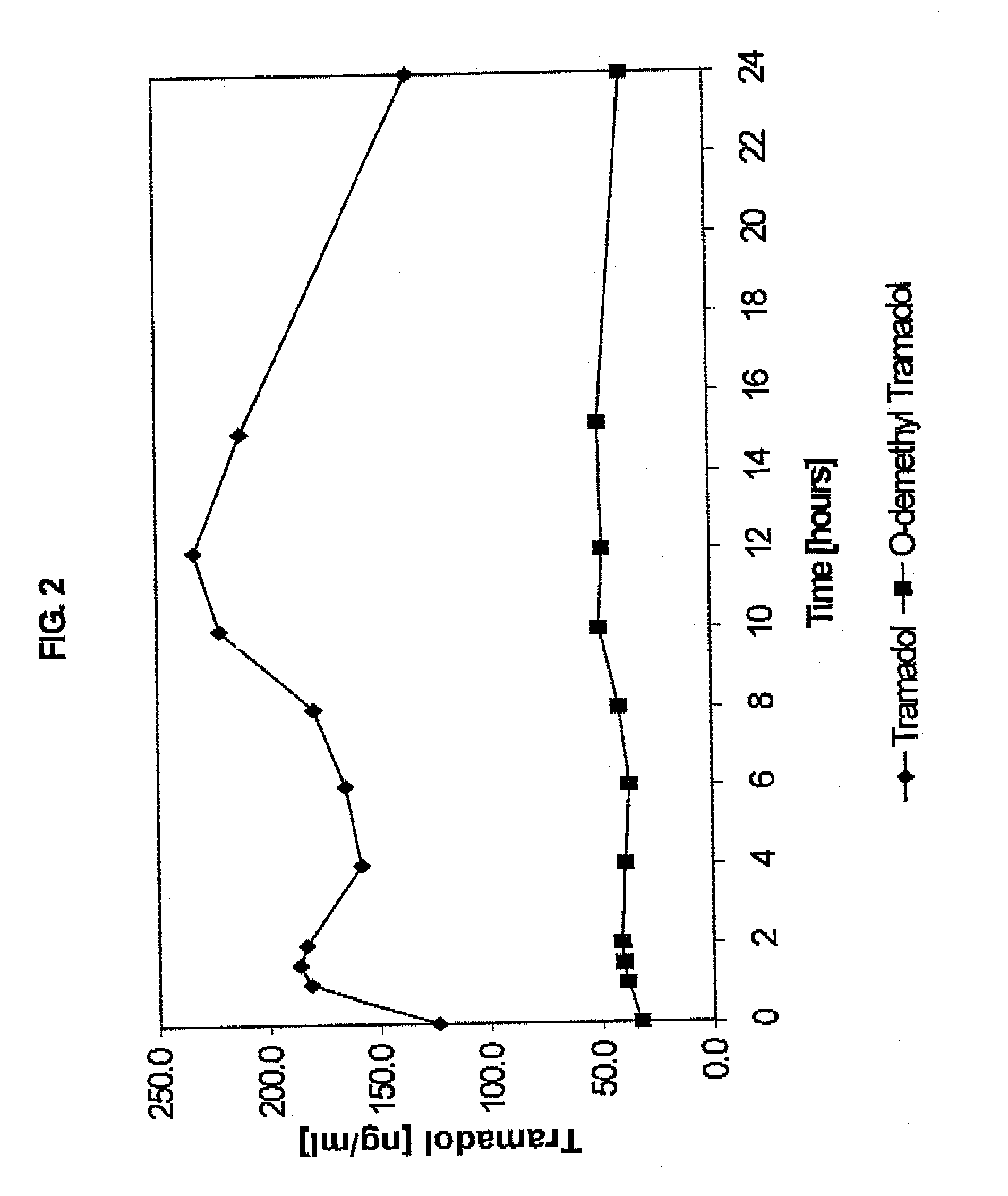
Extended release composition containing tramadol
InactiveUS20070122478A1Efficient ConcentrationBiocideOrganic active ingredientsBlood concentrationPharmaceutical formulation
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appears within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:DEBOECK ARTHUR M +2
Permeation enhancing compositions for anticholinergic agents
InactiveUS20050287194A1Improve permeabilityIncrease permeationBiocideNervous disorderOxybutyninSide effect
A transdermal or topical composition including anticholinergic agents, such as oxybutynin, a urea-containing compound and a carrier system. A method is disclosed for treating a subject for urinary incontinence while reducing the incidences of peak concentrations of drug and undesirable side effects.
Owner:ANTARES PHARMA IPL
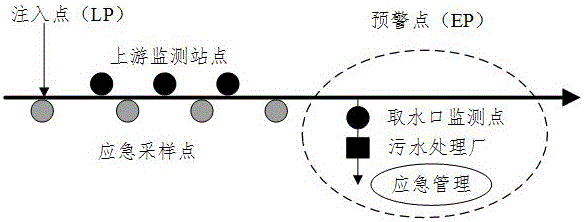
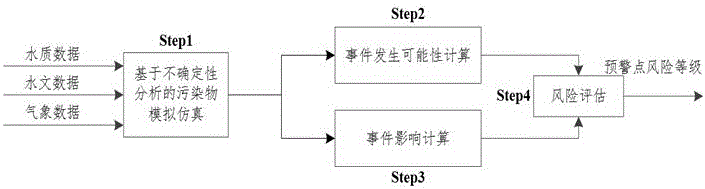
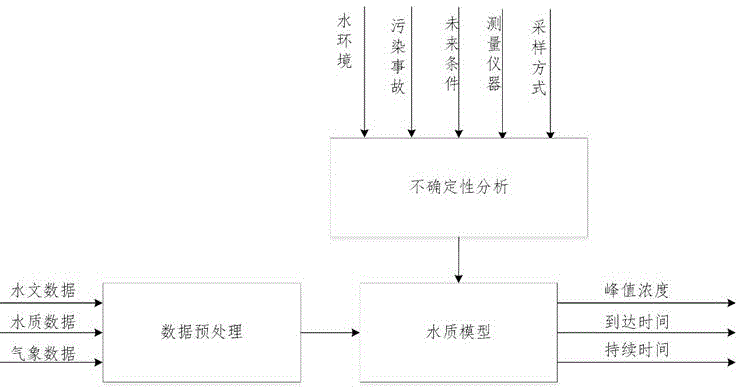
Sudden water pollution accident early warning method based on Monte Carlo and analytic hierarchy process
ActiveCN105095997AImprove accuracyGeneral water supply conservationForecastingWater qualityPeak value
The invention discloses a sudden water pollution accident early warning method based on Monte Carlo and an analytic hierarchy process. The method comprises the following steps: S1, pollutant simulation based on a Monte Carlo method, i.e., based on an uncertainty analysis, an uncertainty water quality model is constructed, and according to water quality, hydrology and meteorological data, time and space change rules of diffusion of pollutants are obtained through simulation and calculation by use of the Monte Carlo method; S2, calculation of a pollution accident occurrence probability, i.e., through defining occurrence conditions of downstream pollution accidents, through combination with a pollutant diffusion result, a probability density function between a pollutant peak concentration and a pollutant standard-exceeding duration is obtained, and the occurrence probability of the downstream pollutant accidents is further obtained; S3, calculation of pollution accident influences, i.e., by referring to the diffusion state of the pollutants, through combination with the analytic hierarchy process, sudden pollution accidents are evaluated, and health, economic, social and water supply system influences possibly caused by the pollution accidents are obtained; and S4, based on risk degree determination of a risk matrix, risks of early warning points are obtained comprehensively by use of a risk matrix method.
Owner:ZHEJIANG UNIV
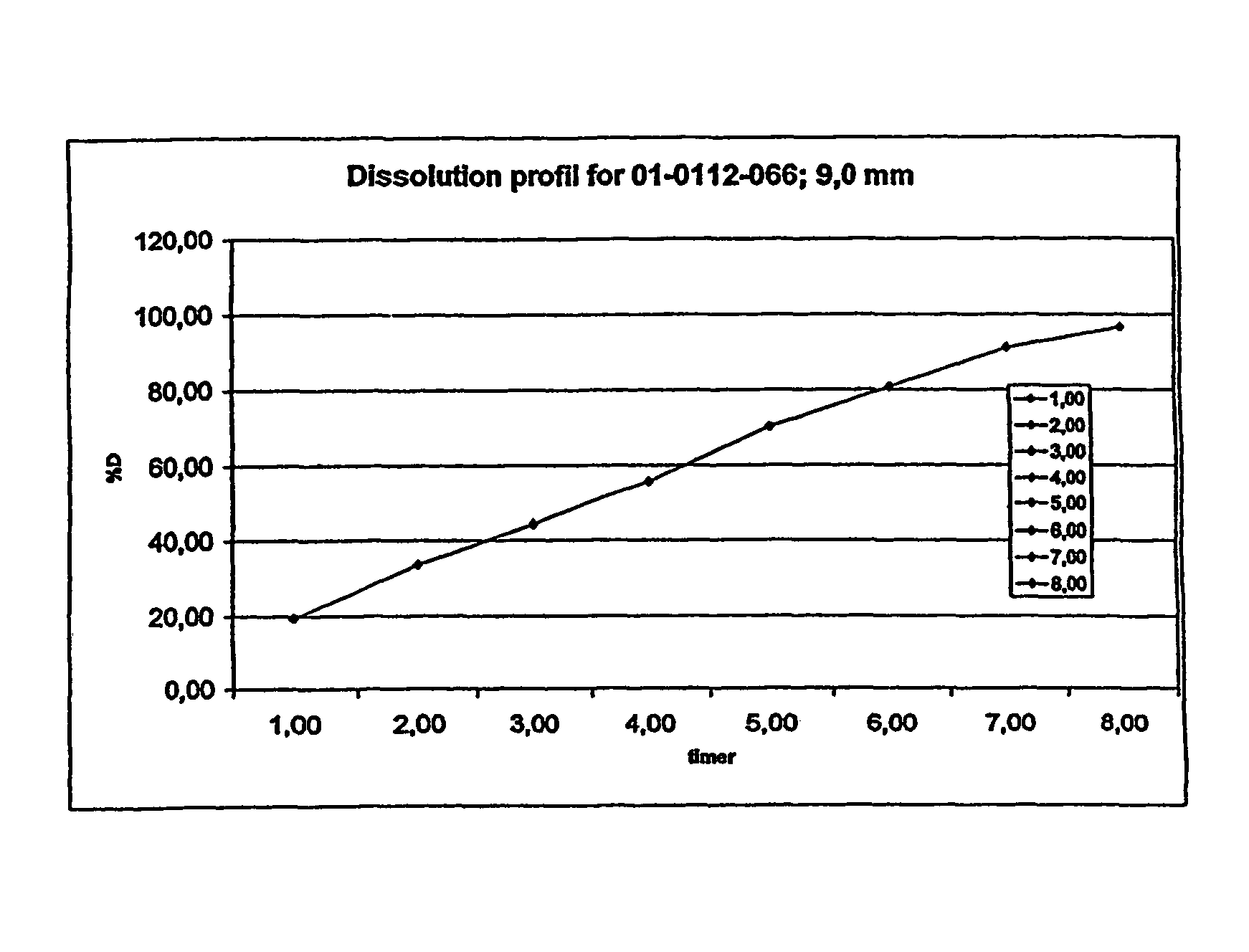
Morphine controlled release system
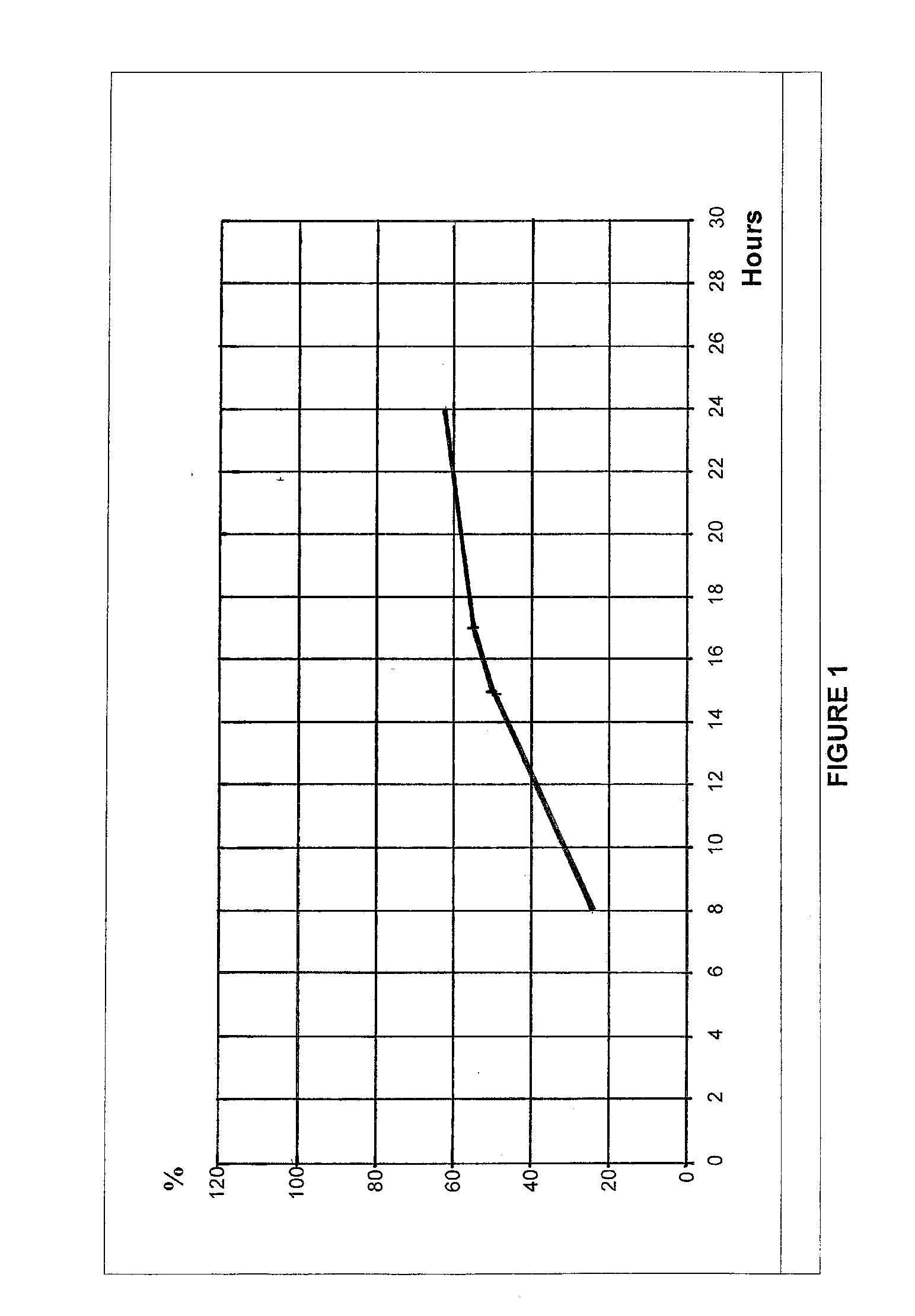
InactiveUS8877241B2Affecting extent of drug bioavailabilityReduce frequencyBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid—when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed peak concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Low-voltage punch-through transient suppressor employing a dual-base structure
InactiveUSRE38608E1Increase capacitanceImprove leakageSemiconductor/solid-state device detailsSolid-state devicesDopantLow voltage
A punch-through diode transient suppression device has a base region of varying doping concentration to improve leakage and clamping characteristics. The punch-through diode includes a first region comprising an n+ region, a second region comprising a p- region abutting the first region, a third region comprising a p+ region abutting the second region, and a fourth region comprising an n+ region abutting the third region. The peak dopant concentration of the n+ layers should be about 1.5E18 cm.sup.-3, the peak dopant concentration of the p+ layer should be between about 1 to about 5 times the peak concentration of the n+ layer, and the dopant concentration of the p- layer should be between about 0.5E14 cm.sup.-3 and about 1.OE17 cm.sup.-3. The junction depth of the fourth (n+) region should be greater than about 0.3 .mu.m. The thickness of the third (p+) region should be between about 0.3 .mu.m and about 2.0 .mu.m, and the thickness of the second (p-) region should be between about 0.5 .mu.m and about 5.0 .mu.m.
Owner:SEMTECH CORP
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS20030054033A1Patient compliance is goodOrganic active ingredientsNervous disorderImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
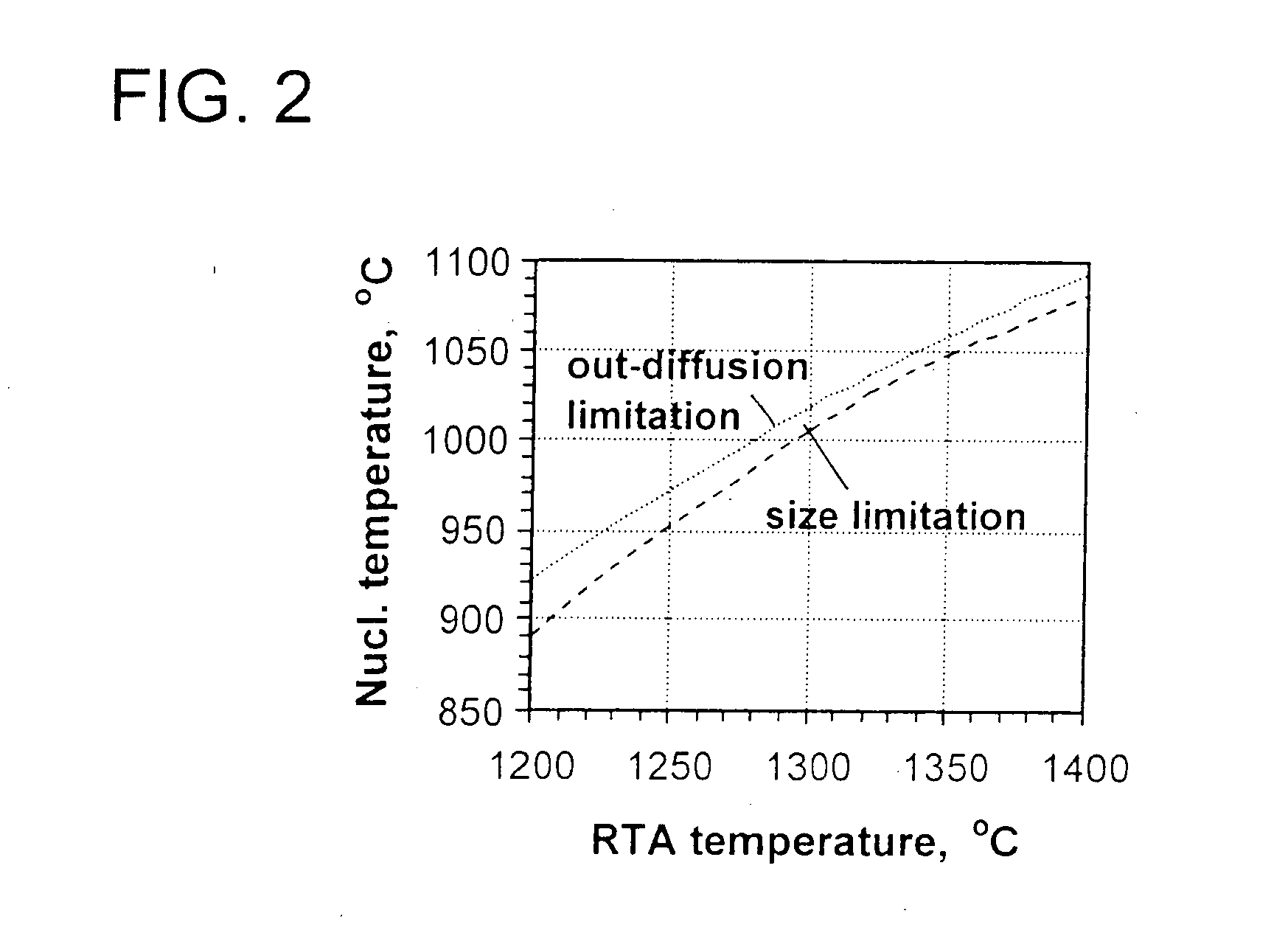
Process for preparing a stabilized ideal oxygen precipitating silicon wafer
ActiveUS20050005841A1Heating fastPolycrystalline material growthAfter-treatment detailsFree zoneSingle crystal
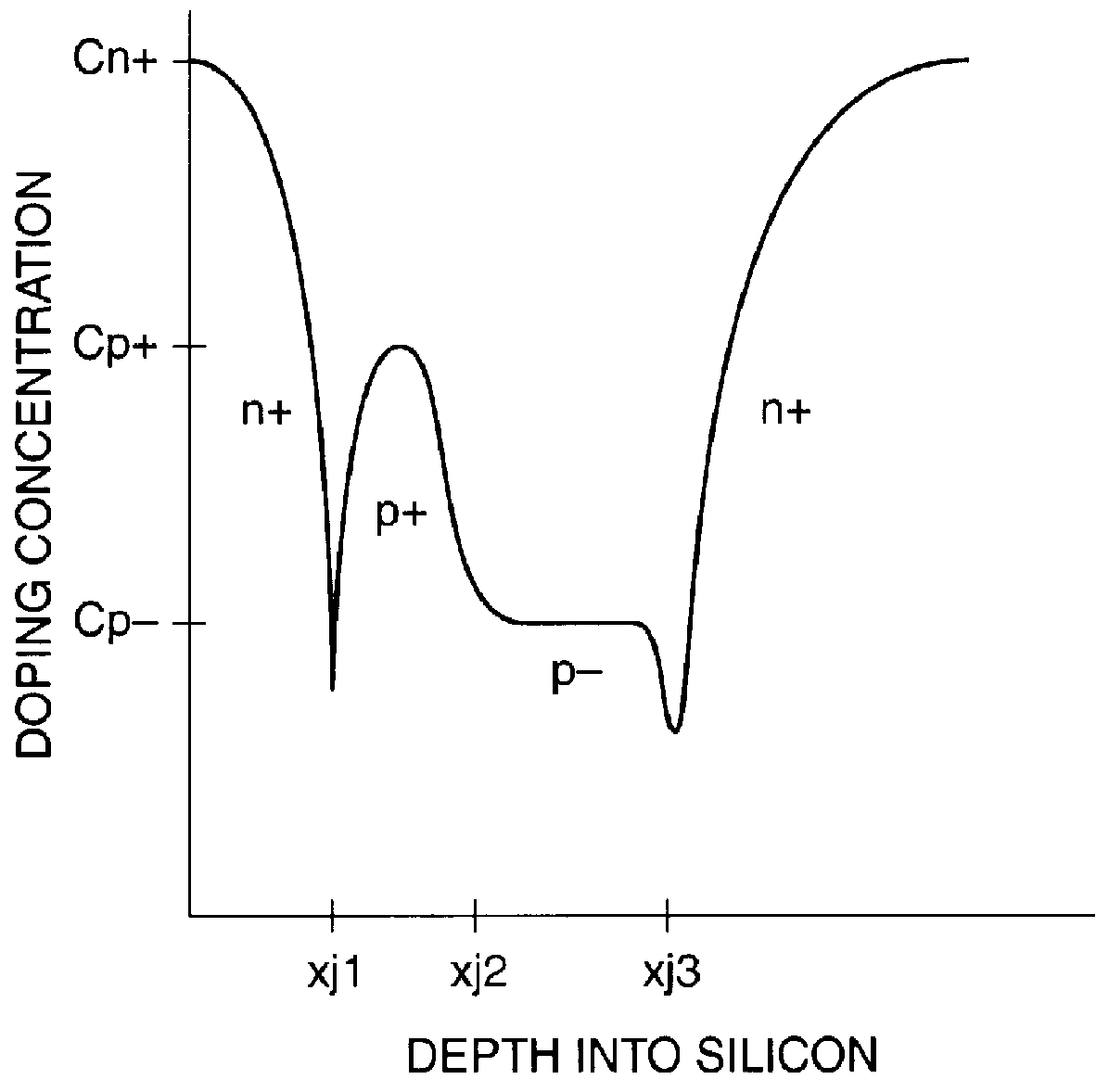
The present invention is directed to a single crystal Czochralski-type silicon wafer, and a process for the preparation thereof, which has a non-uniform distribution of stabilized oxygen precipitate nucleation centers therein. Specifically, the peak concentration is located in the wafer bulk and a precipitate-free zone extends inward from a surface.
Owner:GLOBALWAFERS CO LTD
Cleaved silicon substrate active device
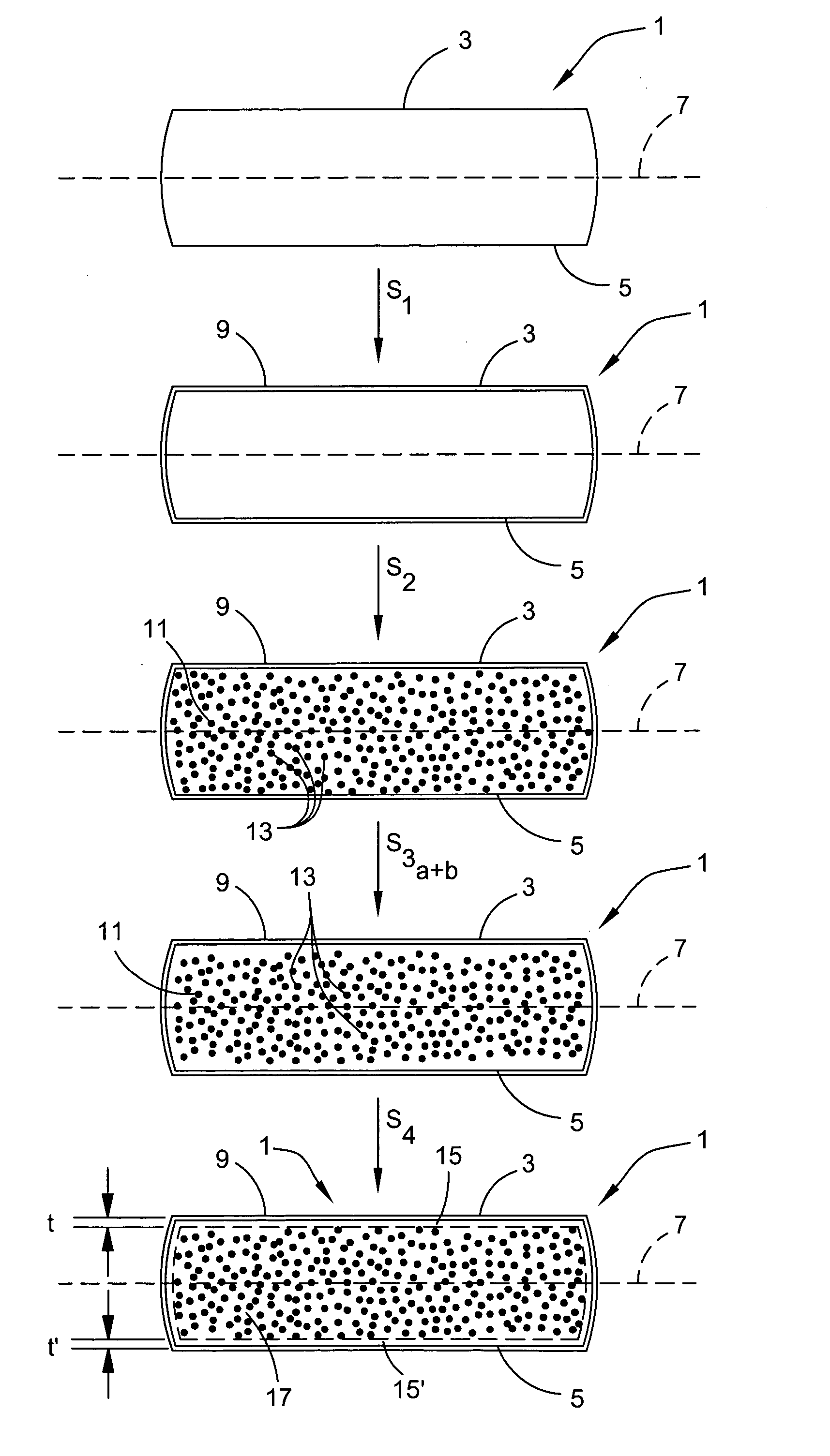
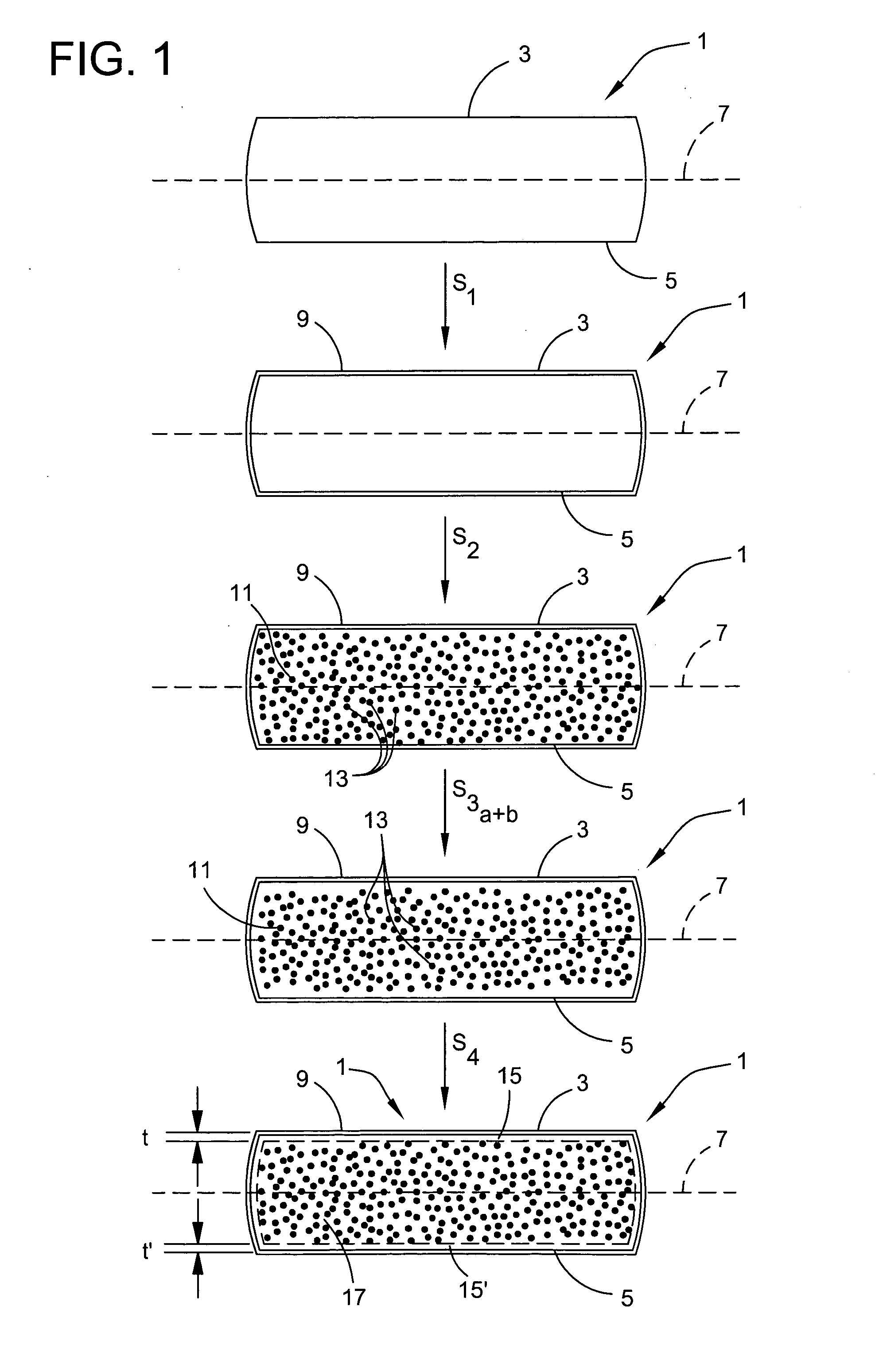
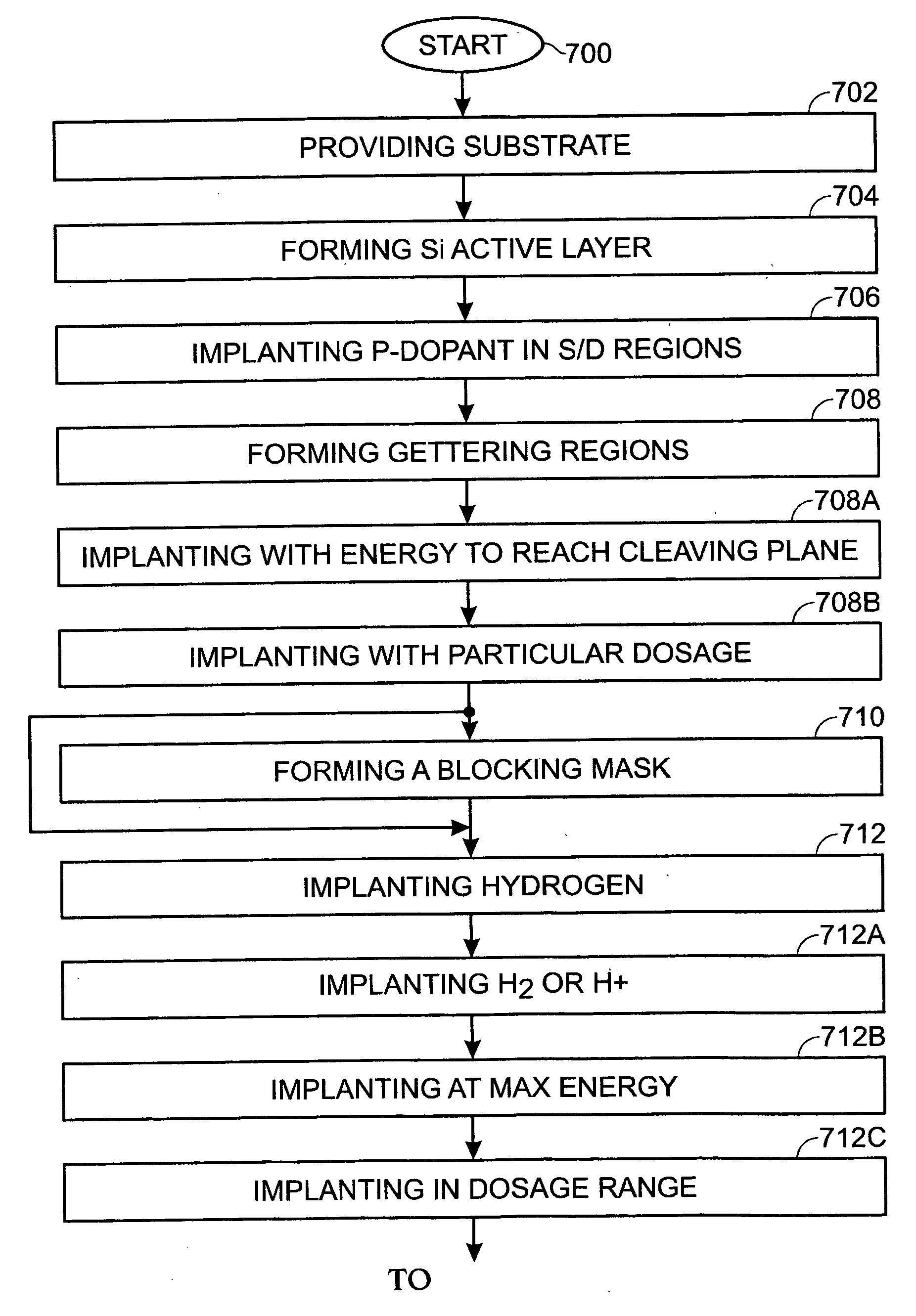
InactiveUS20070066035A1Narrow downReduce hydrogen concentrationSolid-state devicesSemiconductor/solid-state device manufacturingDopantHydrogen
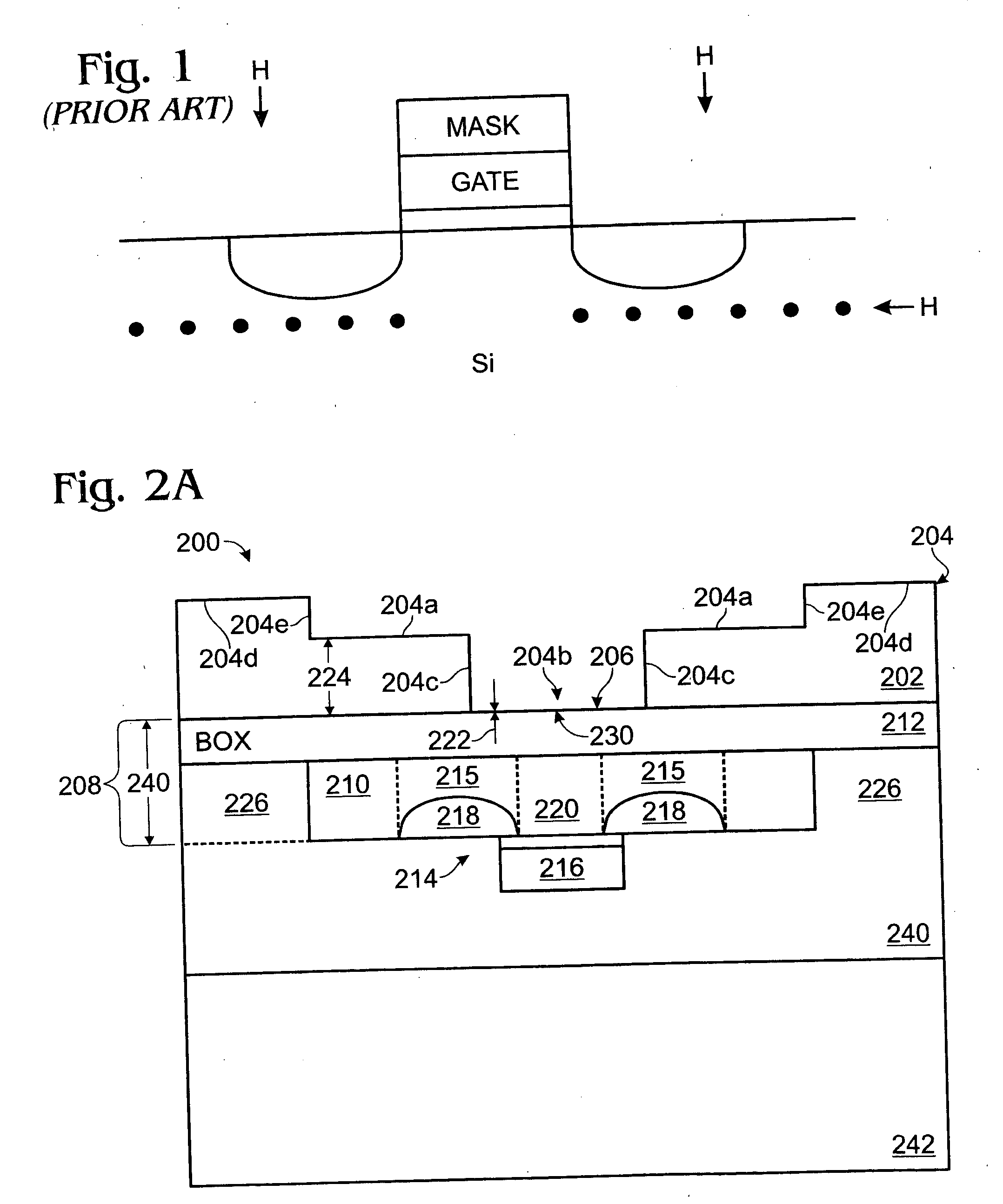
A hydrogen (H) exfoliation gettering method is provided for attaching fabricated circuits to receiver substrates. The method comprises: providing a Si substrate; forming a Si active layer overlying the substrate with circuit source / drain (S / D) regions; implanting a p-dopant into the S / D regions; forming gettering regions underling the S / D regions; implanting H in the Si substrate, forming a cleaving plane (peak concentration (Rp) H layer) in the Si substrate about as deep as the gettering regions; bonding the circuit to a receiver substrate; cleaving the Si substrate along the cleaving plane; and binding the implanted H underlying the S / D regions with p-dopant in the gettering regions, as a result of post-bond annealing.
Owner:SHARP KK
Cognitive Function
InactiveUS20120157445A1Improve performanceNormalizes performanceBiocideNervous disorderRegimenCognitively impaired
Provided herein are methods, drug formulations, and dosing regimens for improving cognitive function in a normal or cognitively impaired subject. For instance, methods provided herein comprise administering a GABAA receptor antagonist so that peak concentration of the GABAA receptor antagonist occurs when the subject is asleep.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
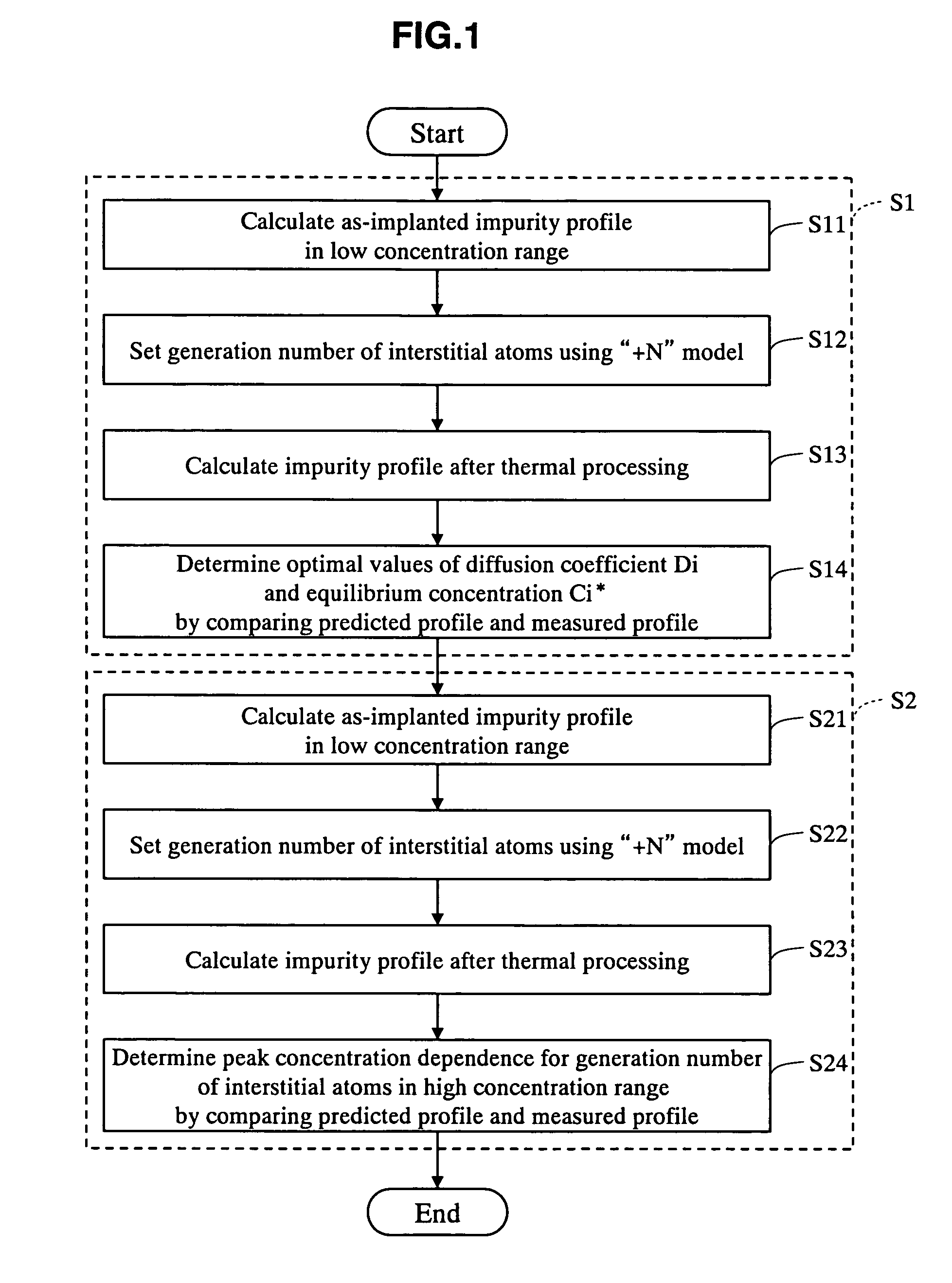
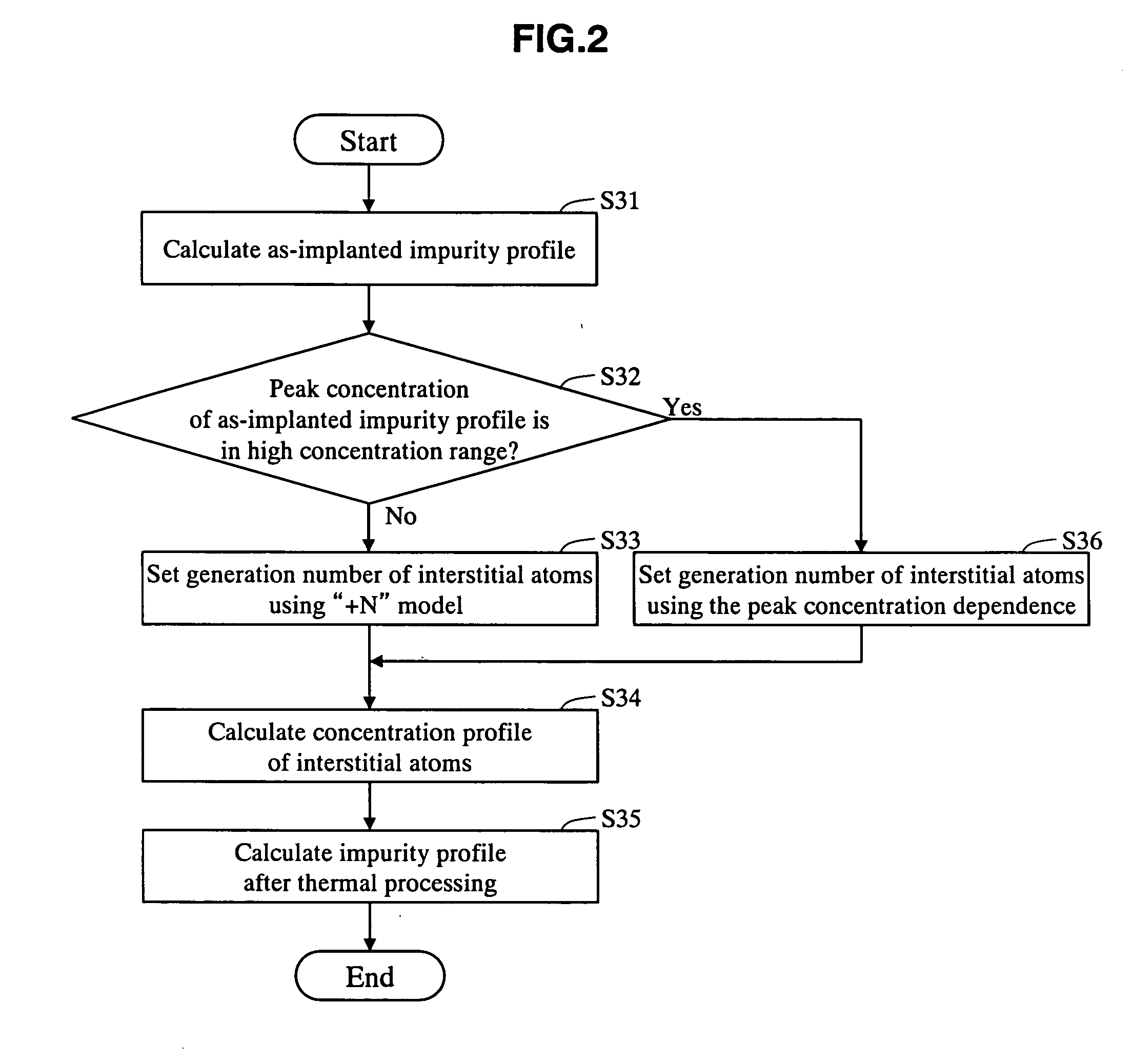
Impurity diffusion simulation method, impurity diffusion simulation apparatus, and impurity diffusion simulation program
InactiveUS20070026544A1Impurities increaseAccurate predictionSemiconductor/solid-state device testing/measurementSemiconductor/solid-state device manufacturingImpurity diffusionIon implantation
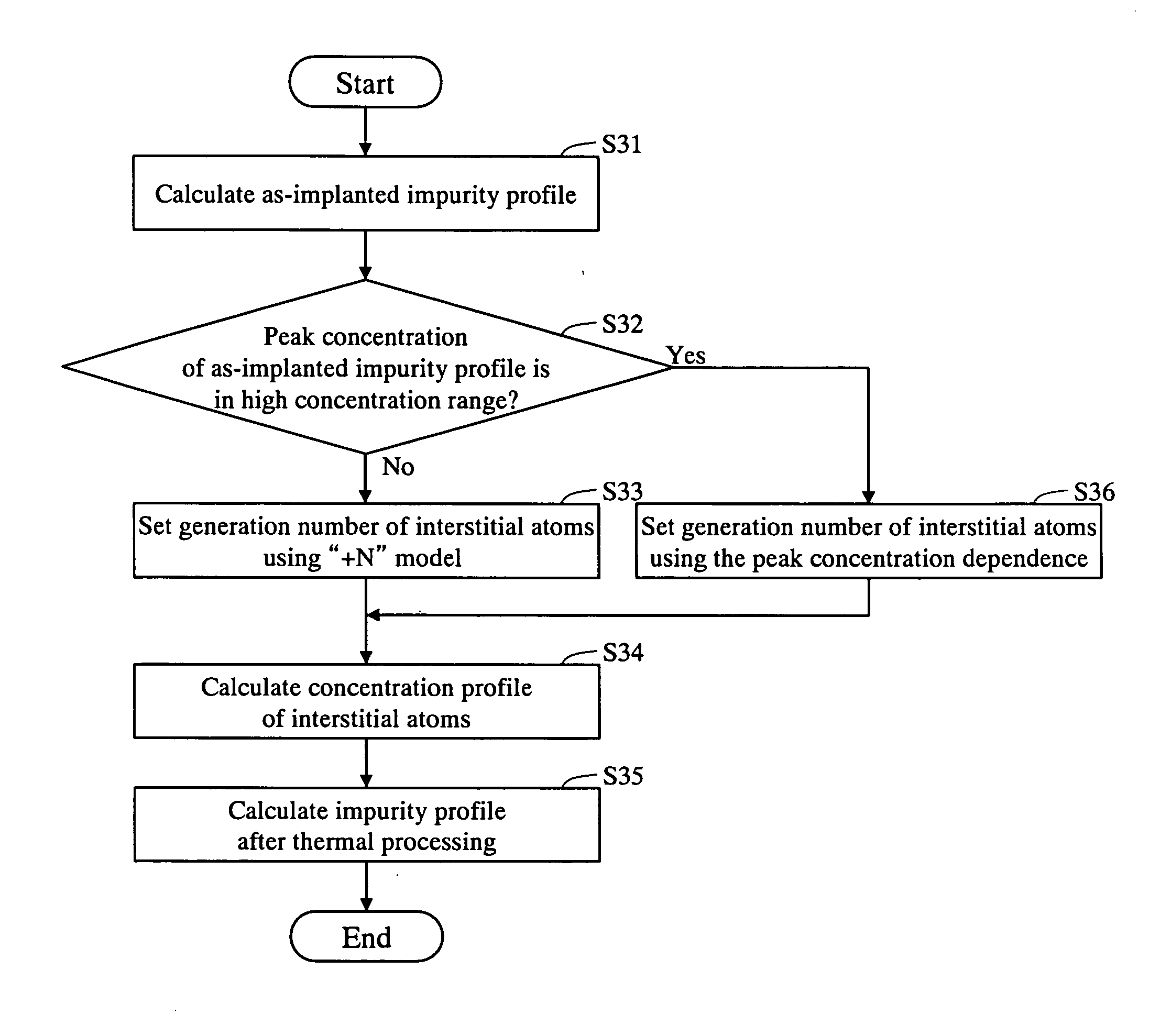
The as-implanted concentration profile of impurity atoms in the semiconductor substrate is calculated, and a number of interstitial atoms to be generated in the semiconductor substrate by one impurity atom implanted with the ion implantation is set based on a peak concentration of the calculated as-implanted concentration profile of impurity atoms. The concentration profile of interstitial atoms generated in the semiconductor substrate is calculated based on the calculated as-implanted concentration profile of impurity atoms and the set number of interstitial atoms, and the concentration profile of impurity atoms in the semiconductor after the thermal processing is calculated based on the calculated as-implanted concentration profile of impurity atoms and the calculated concentration profile of interstitial atoms.
Owner:PANASONIC CORP
Lansoprazole medicinal composition tablets and preparation method thereof
ActiveCN102198109AInhibition of secretionAvoid degradationOrganic active ingredientsDigestive systemSodium bicarbonateCoating drugs
The invention discloses Lansoprazole medicinal composition tablets and a preparation method thereof. The Lansoprazole medicinal composition tablets comprise the following components in part by mass: 20 to 30 parts of Lansoprazole medicinal composition tablets, 400 to 450 parts of sodium bicarbonate, 100 to 150 parts of calcium hydrophosphate, 600 to 700 parts of magnesium hydroxide, 250 to 500 parts of filler, 40 to 50 parts of disintegrating agent, 50 to 100 parts of flavoring agent, 80 to 100 parts of bonding agent and 20 to 25 parts of lubricating agent. In the Lansoprazole medicinal composition tablets disclosed by the invention, sodium bicarbonate, calcium hydrophosphate and magnesium hydroxide are used in place of enteric coating, the secretion of gastric acid can be inhibited, the Lansoprazole can be prevented from being decomposed by gastric acid, the medicines can be absorbed quickly, and peak concentration can be reached quickly.
Owner:MITSUBISHI PHARMA GUANGZHOU
Active Device on a Cleaved Silicon Substrate
InactiveUS20090045461A1Reduce hydrogen concentrationLower Level RequirementsSolid-state devicesSemiconductor/solid-state device manufacturingDopantHydrogen
A hydrogen (H) exfoliation gettering method is provided for attaching fabricated circuits to receiver substrates. The method comprises: providing a Si substrate; forming a Si active layer overlying the substrate with circuit source / drain (S / D) regions; implanting a p-dopant into the S / D regions; forming gettering regions underling the S / D regions; implanting H in the Si substrate, forming a cleaving plane (peak concentration (Rp) H layer) in the Si substrate about as deep as the gettering regions; bonding the circuit to a receiver substrate; cleaving the Si substrate along the cleaving plane; and binding the implanted H underlying the S / D regions with p-dopant in the gettering regions, as a result of post-bond annealing.
Owner:SHARP KK
Neostigmine bromide sustained-release tablet and preparation method thereof
InactiveCN102258492AImprove stabilityAvoid instabilityDigestive systemMuscular disorderSustained Release TabletSide effect
The present invention belongs to the technical field of medicinal preparation. The invention discloses a formula of a neostigmine bromide slow release preparation which can be taken once every day for treating myasthemia gravis, functional flatulence after being performed an operation and urinary retention, as well as its preparation method. The preparation is disclosed as a slow release tablet form composed of a skeleton core containing neostigmine bromide and the slow release preparation and a coating. The neostigmine bromide slow release preparation of the present invention is capable of overcoming the disadvantage of present medicament common tablets in the market, slowly releasing to keep stable blood and medicine concentration, acting for a longer period, possessing low toxicity andside effect and conveniently taking the preparation, and the slow release preparation keeps effective blood and medicine concentration for a long time, reduces the medicine taking frequency, raises the compliance of the patients and reduces the side-effect due to over peak concentration. The invention has the advantages of simple preparation technology, low cost, easy control and easy industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Creation of a polarizable layer in the buried oxide of silicon-on-insulator substrates for the fabrication of non-volatile memory
InactiveUS6551898B1Reduce harmHarm reductionSolid-state devicesSemiconductor/solid-state device manufacturingCelsius DegreeIon implantation
This invention concerns a process of forming a polarizable layer in a buried oxide layer of a silicon-on-insulator substrate for the fabrication of non-volatile memory. This process comprises implanting, through the active silicon layer, Si ions into the buried oxide layer at an ion implantation energy selected so that the implanted ion has its peak concentration between 5-50 nm from the silicon / buried oxide interface. The implantation step can occur while externally heating the silicon-on-insulator substrate at a temperature between 25-300 degrees Celsius. After implantation, an annealing step may be completed to repair any damage the implantation may have created in the silicon-on-insulator substrate.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
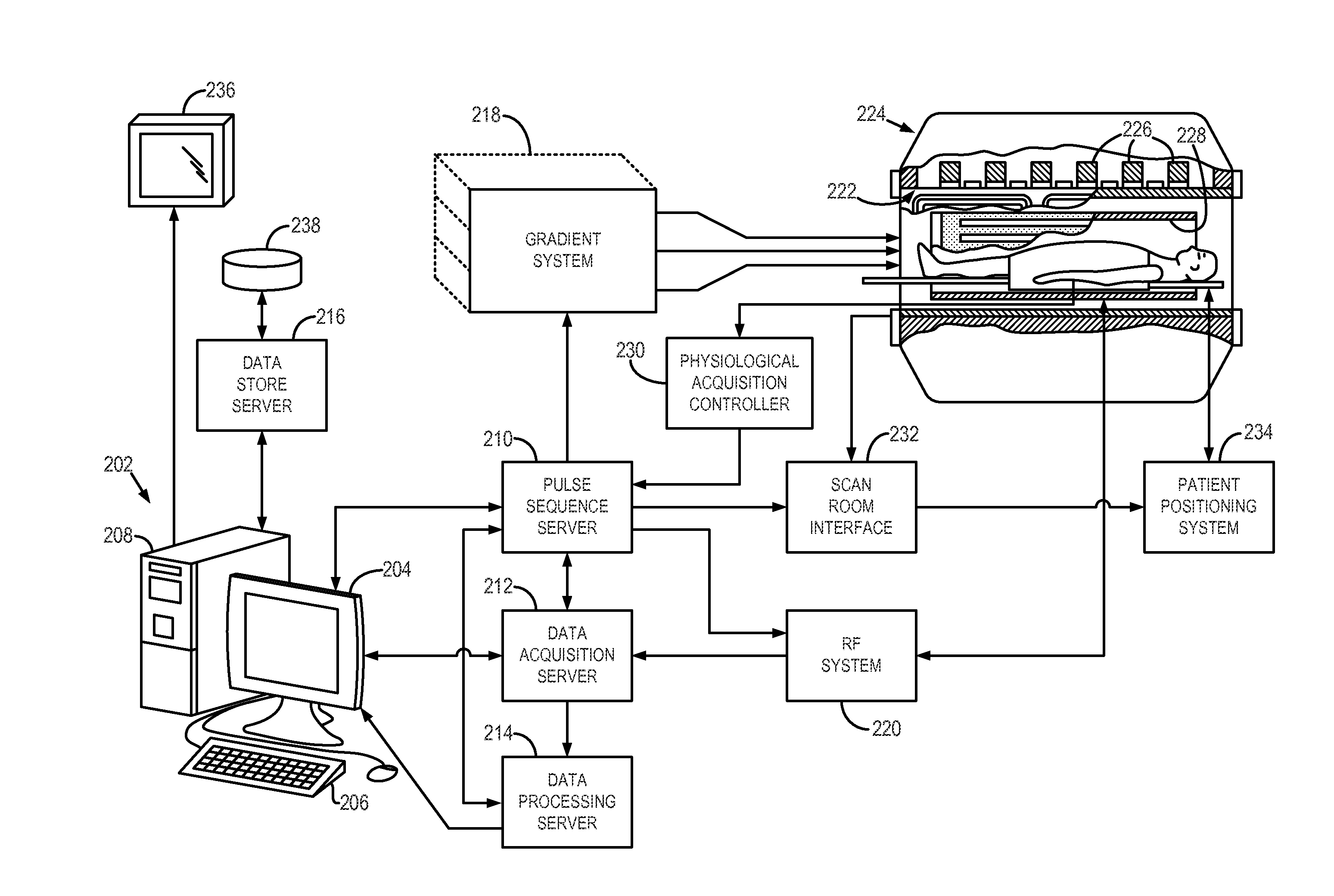
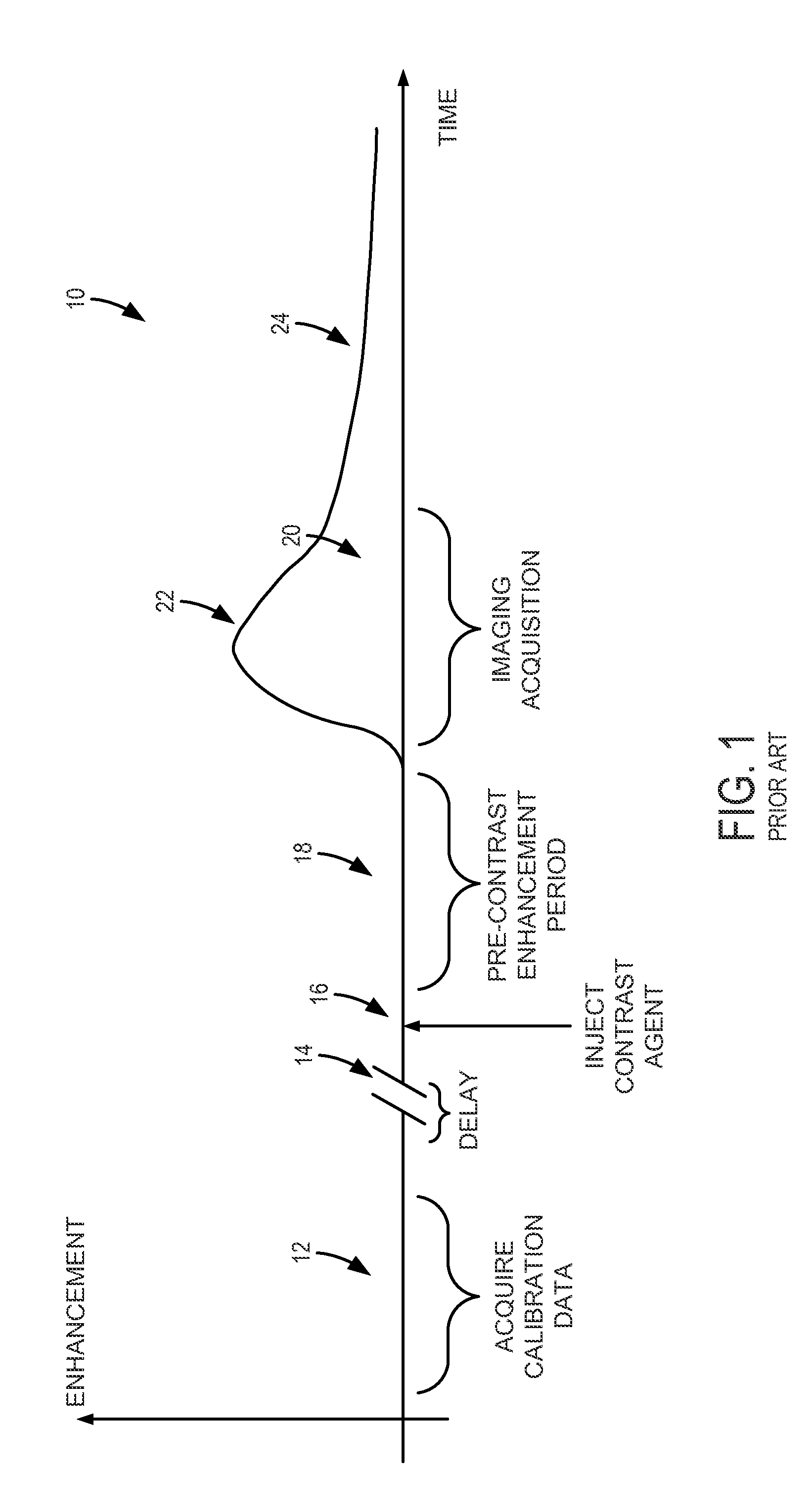
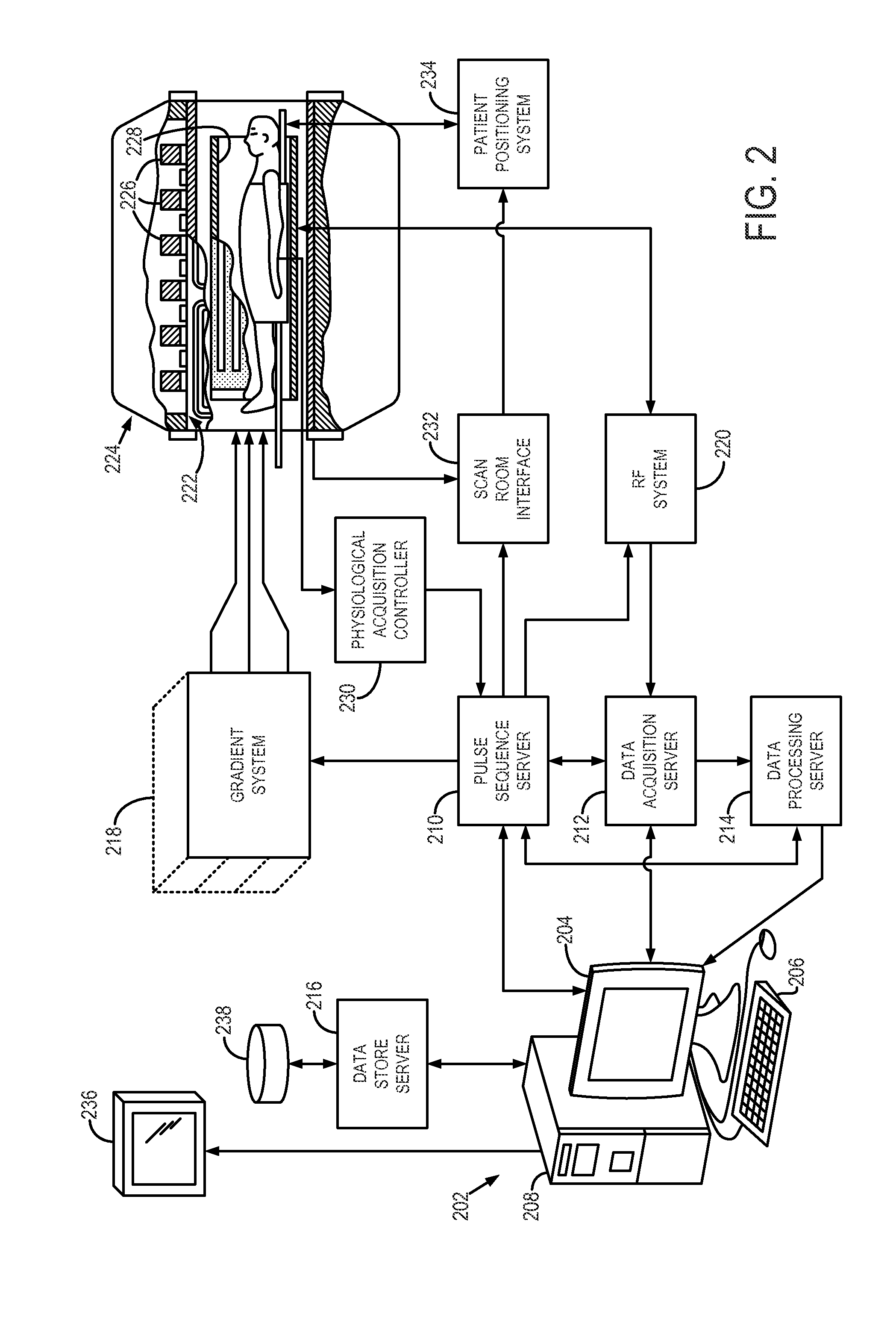
System and method for controlling calibration and delay phases of parallel, contrast-enhanced magnetic resonance imaging
ActiveUS20130063146A1Improves temporal scan requirementExaminationDiagnostic recording/measuringSensorsContrast-enhanced Magnetic Resonance ImagingParallel imaging
A system and method for performing parallel magnetic resonance angiography includes controlling operation of a magnetic gradient system and an RF system to perform a calibration data pulse sequence to begin acquiring calibration data for use in a parallel imaging reconstruction process after receiving an indication that the subject has received a dose of a contrast agent. The acquisition of the calibration data is discontinued before the contrast agent reaches a peak concentration within a region of interest (ROI) of the subject and operation of the magnetic gradient system and RF system is controlled to perform an imaging pulse sequence in accordance with a parallel imaging acquisition to begin acquiring image data from the ROI. The image data is reconstructed into an image of the ROI using the calibration data.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com