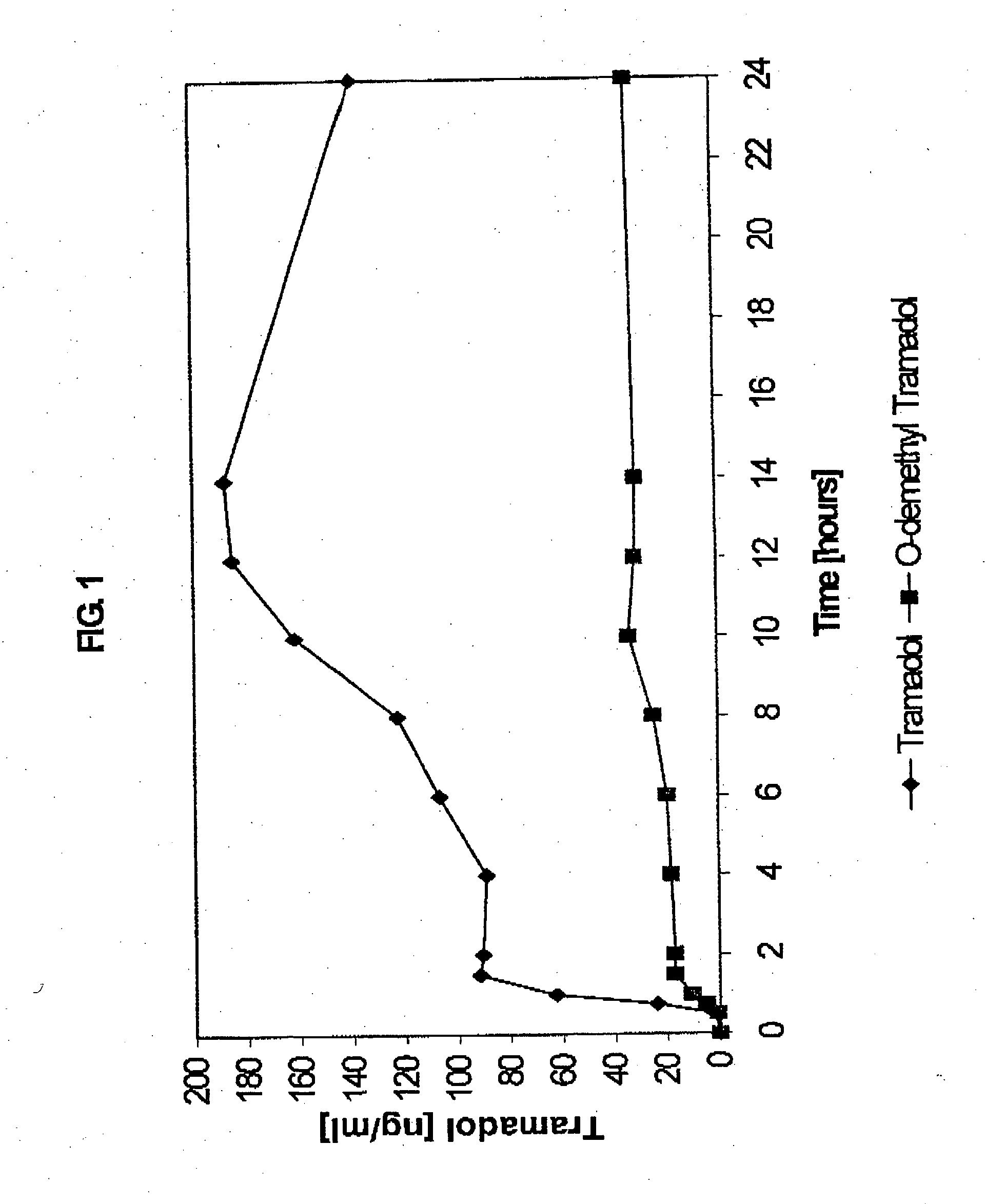
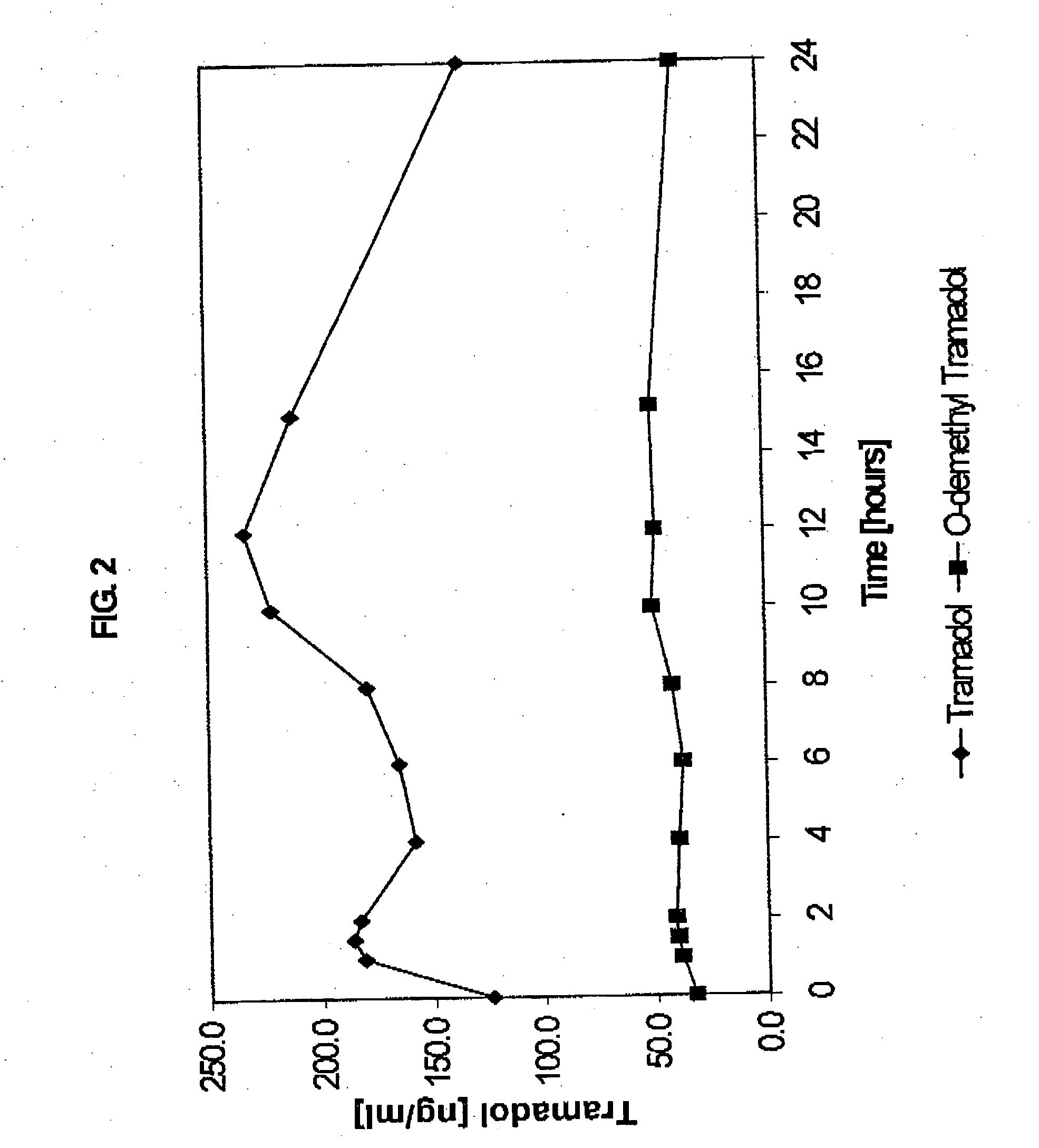
Extended release composition containing tramadol
a technology of extended release and tramadol, which is applied in the direction of drug compositions, biocides, microcapsules, etc., can solve the problems of increased plasma levels, increased risk of side effects, and increased pain in patients, so as to reduce pain, effectively control pain, and effectively reduce unwanted side effects of tramadol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Uncoated Beads
[0066]
Tramadol Hcl31.8 kgMicrocrystalline Cellulose (Avicel pH 101)21.0 KgSucrose Stearate (Crosdeta F160)2.17 kgPurified Water7.24 kg
[0067] In a planetary mixer collette of 160 liter capacity introduce the Tramadol Hcl, microcrystalline cellulose and the sucrose stearate, blend at speed 2 for about 15 min. Slowly add the purified water and continue mixing for an additional 15 minutes at speed 2 after all water is added. Extrude the blend through a Fuji Paudal extruder equipped with a 1 mm screen. Spheronize the extrudate during about 2 minutes and dry the beads in an oven at 50° C. for about 12 hours. The dried beads are screened though sieves of 1.4 and 0.7 mm. The yields of uncoated beads comprised between 0.7 and 1.4 mm was 44.0 kg (81%).
example 2
Coated Beads
[0068]
2a. Pre CoatingHydroxypropylmethylcellulose1.35 kgTalc5.40 kgPurified Water18.0 kg
[0069] 45 kg of sieved beads from Example 1 were placed in an fluidized bed coater (Aeromatic).
[0070] The beads were pre coated with 8.25 kg of the coating suspension.
2b. Sustained Release CoatingPolyacrylate (30%) Eudragit NE30D36.7 kgHydroxypropylmethylcellulose1.08 kgTalc1.08 kgPolysorbate 800.216 kg Simethicone0.540 kg Magnesium Stearate0.216 kg Purified Water18.0 kg
[0071] Immediately after the pre coatings 47.8 kg of the sustained release coating was applied onto the pre coated beads. Upon completion of the coating, the coated beads were placed onto trays in a drying oven for about 16 hours at 50° C. Upon cooling the coated beads were stored in containers for further use.
example 3
Tramadol Fast Release Tablet—30 mg
[0072]
Tramadol Hcl 3.00 kgLactose 2.63 kgAvicel pH 102 1.13 kgPovidone0.150 kgStarch0.525 kgMagnesium Stearate0.075 kg
[0073] Introduce a collette planetary mixer with a 20 liter bowl, all ingredients, except the magnesium stearate, are blend for 15 minutes at speed 2. Add the magnesium stearate and blend for about 1 minute at speed 1. The blend is compressed using dip cup punches of 5 mm diameter to produce 94,000 tablets of 75 mg containing 30 mg of Tramadol Hcl per tablet. The tablets at Example 3 were tested for release of Tramadol using USP Apparatus 1 (Paddles) in 900 mL of a buffer at pH 6.9 at 37° C. and at 50 rotations per minute.
Time [h]Tramadol Hcl Dissolved [%]0.2574.50.5098.60.75101.8
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com