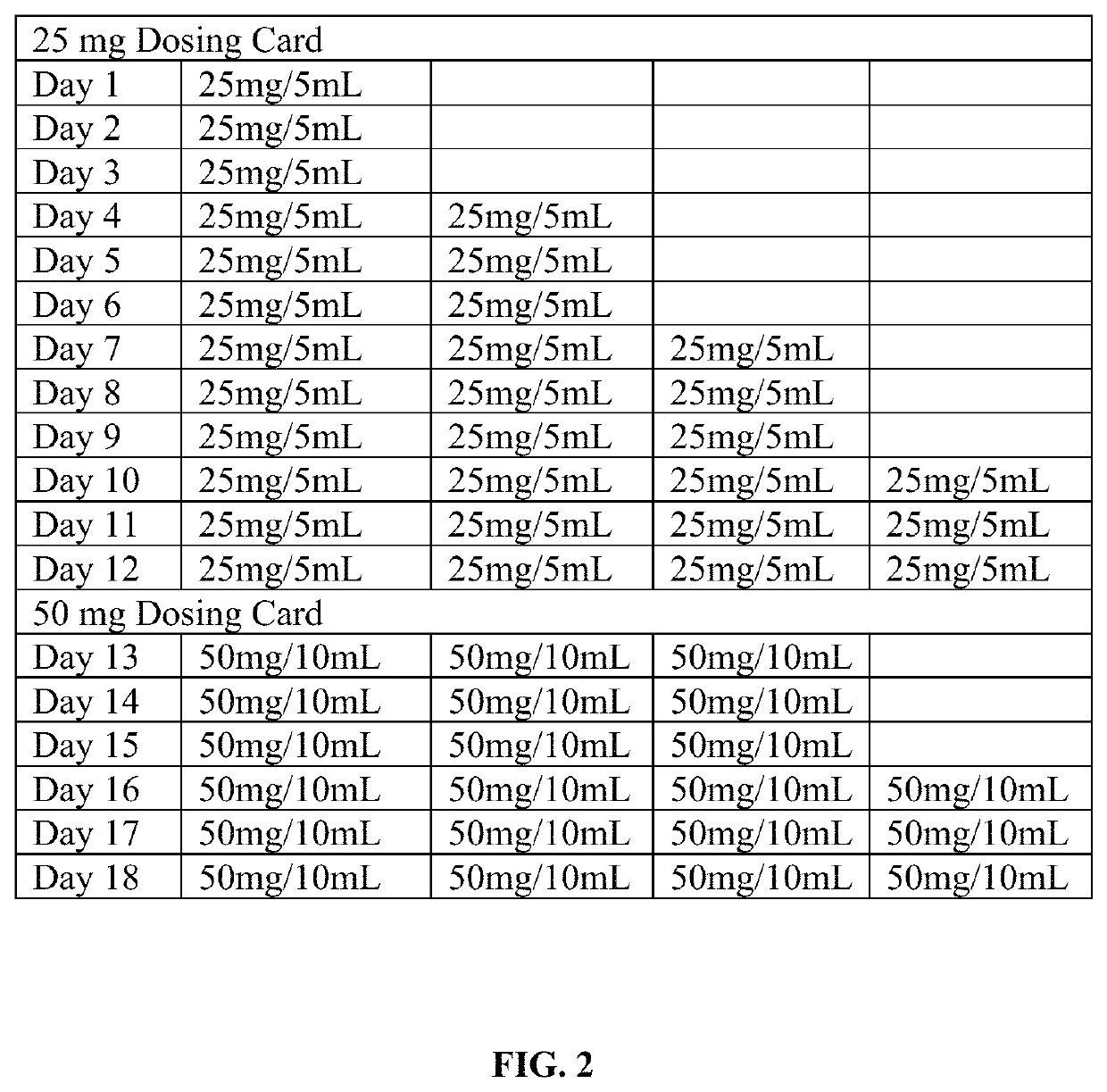
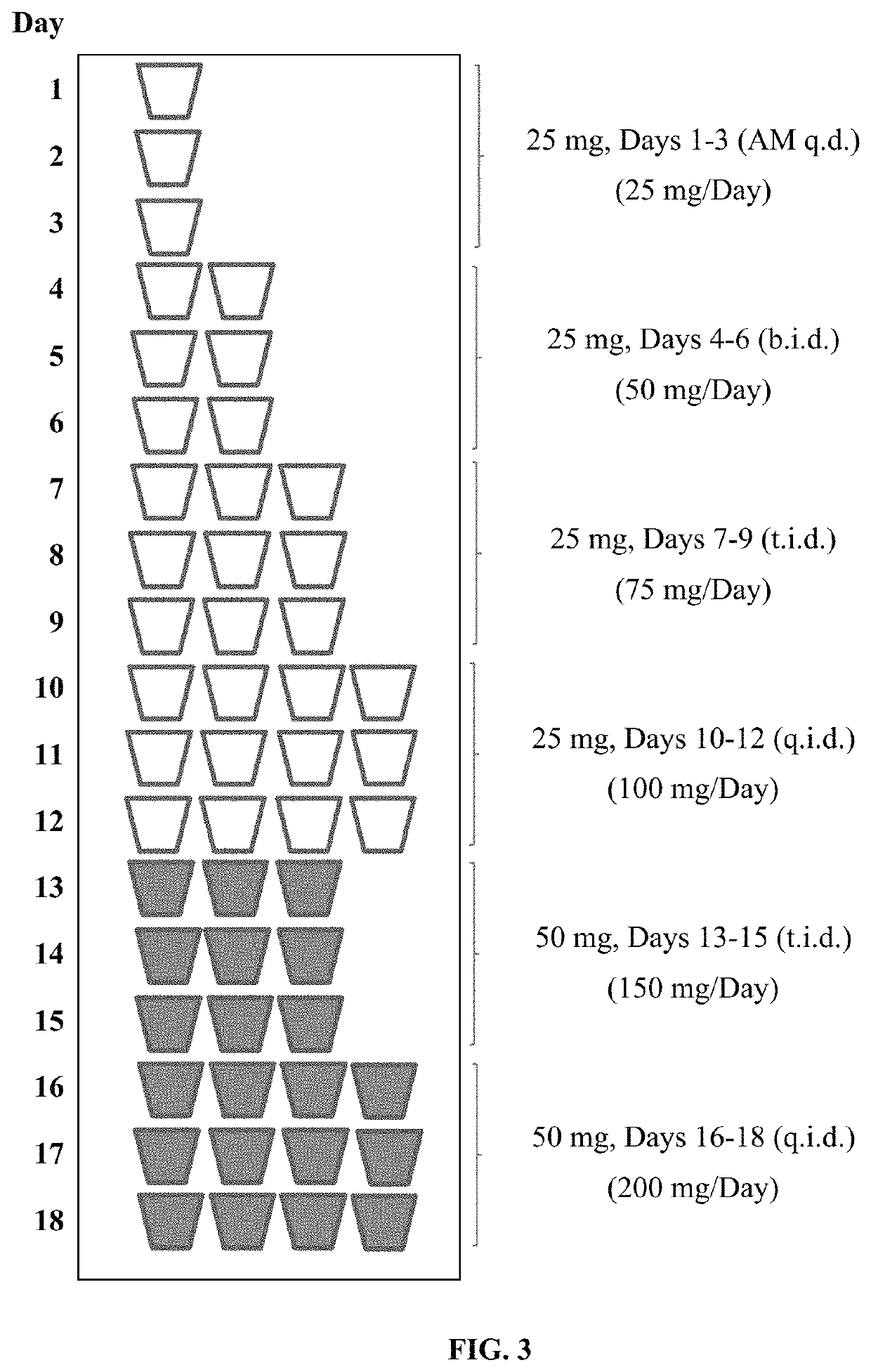
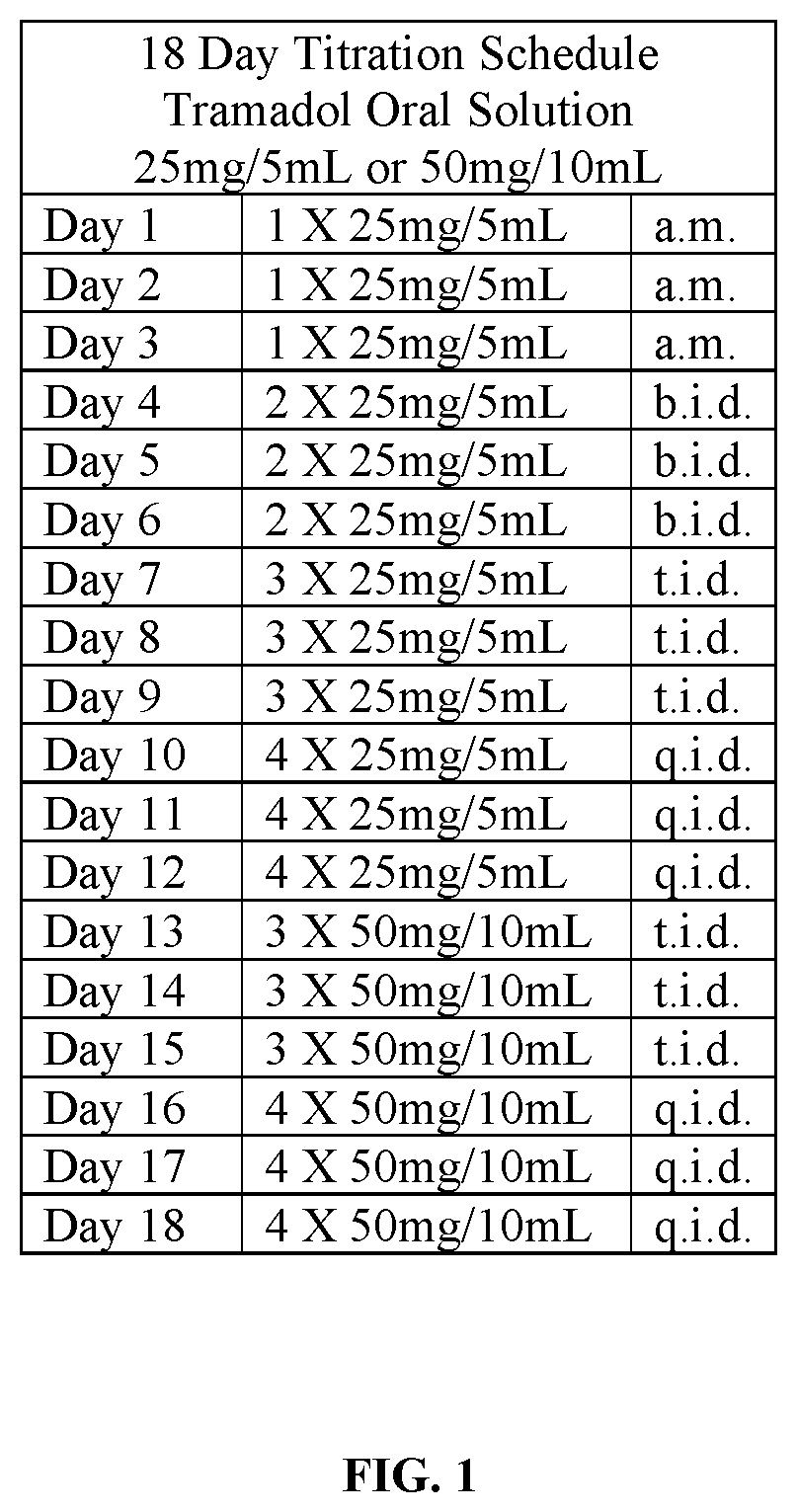
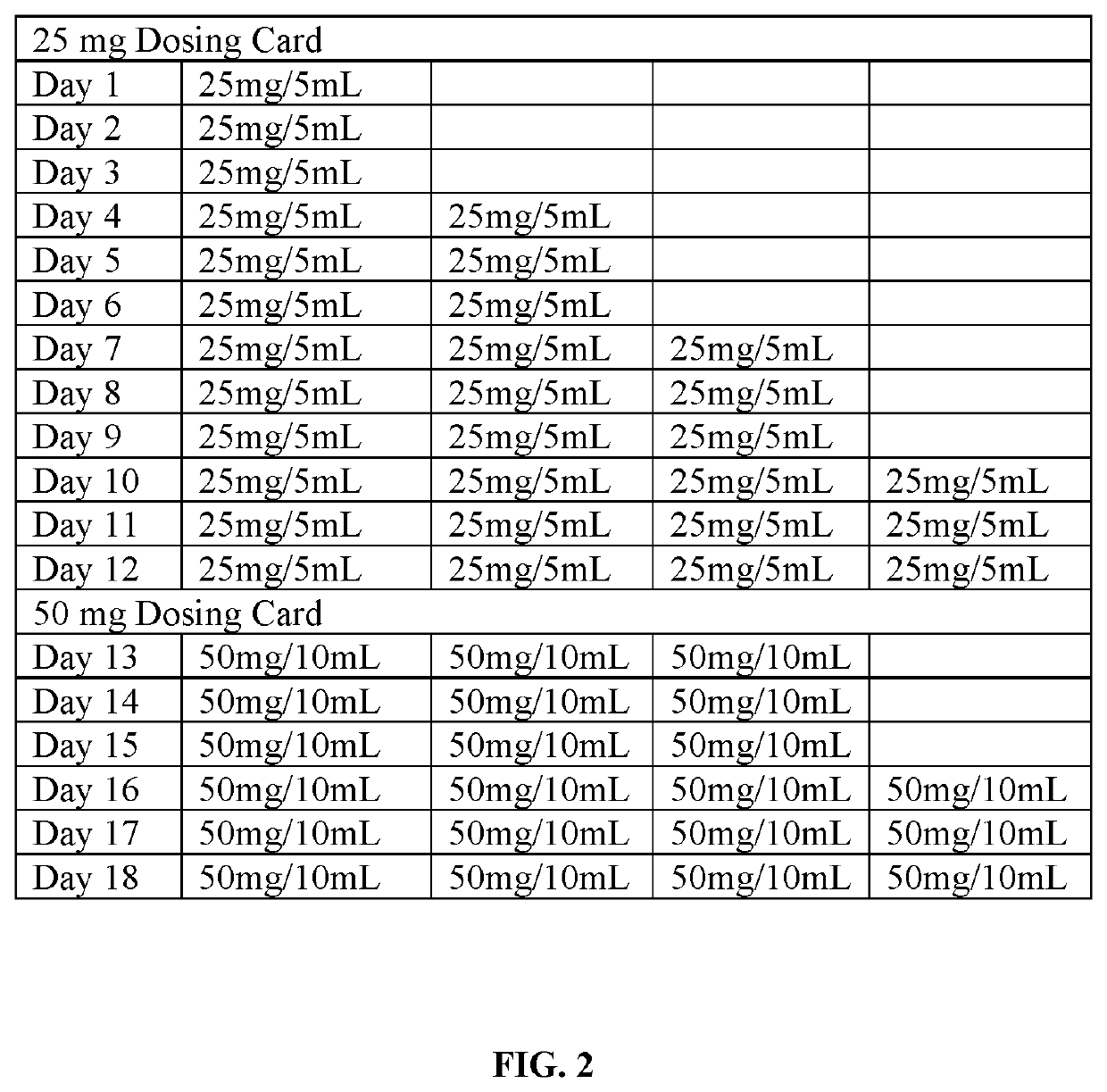
Patents
Literature
34 results about "Tramadol hcl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tramadol HCL. Tramadol is usually marketed as the hydrochloride salt (tramadol hydrochloride) and is available in both injectable (intravenous and/or intramuscular) and oral preparations. It is also available in conjunction with paracetamol (acetaminophen).
Sustained release formulations containing acetaminophen and tramadol
InactiveUS7374781B2Improve efficiency and qualityProvide clinical efficiencyOrganic active ingredientsNervous disorderDrugSustained Release Formulations
A sustained release formulation as a unit dose contains 100 mg-1000 mg of Acetaminophen and 15 mg-150 mg of tramadol hydrochloride, which comprises of 1) an immediate release portion comprising of 25%-75% of the total effective amount of drug in the dosage form and 2) a sustained release portion comprising of a) 25%-75% of the total effective amount of drugs in the dosage form; b) 6%-50% of gelling polymers of the total formulation, and c) optionally an enteric coating at a level of 5%-40% of the total formulation. The set forth formulation dissolves 25%-60% of the total drug in the first hour, 50%-90% of the total drug in the first four hours and not less than 80% of the total drug in the first 12 hours using USP dissolution method II at 50 rpm.
Owner:ZHANG SHUYI +1
Intravenous administration of tramadol
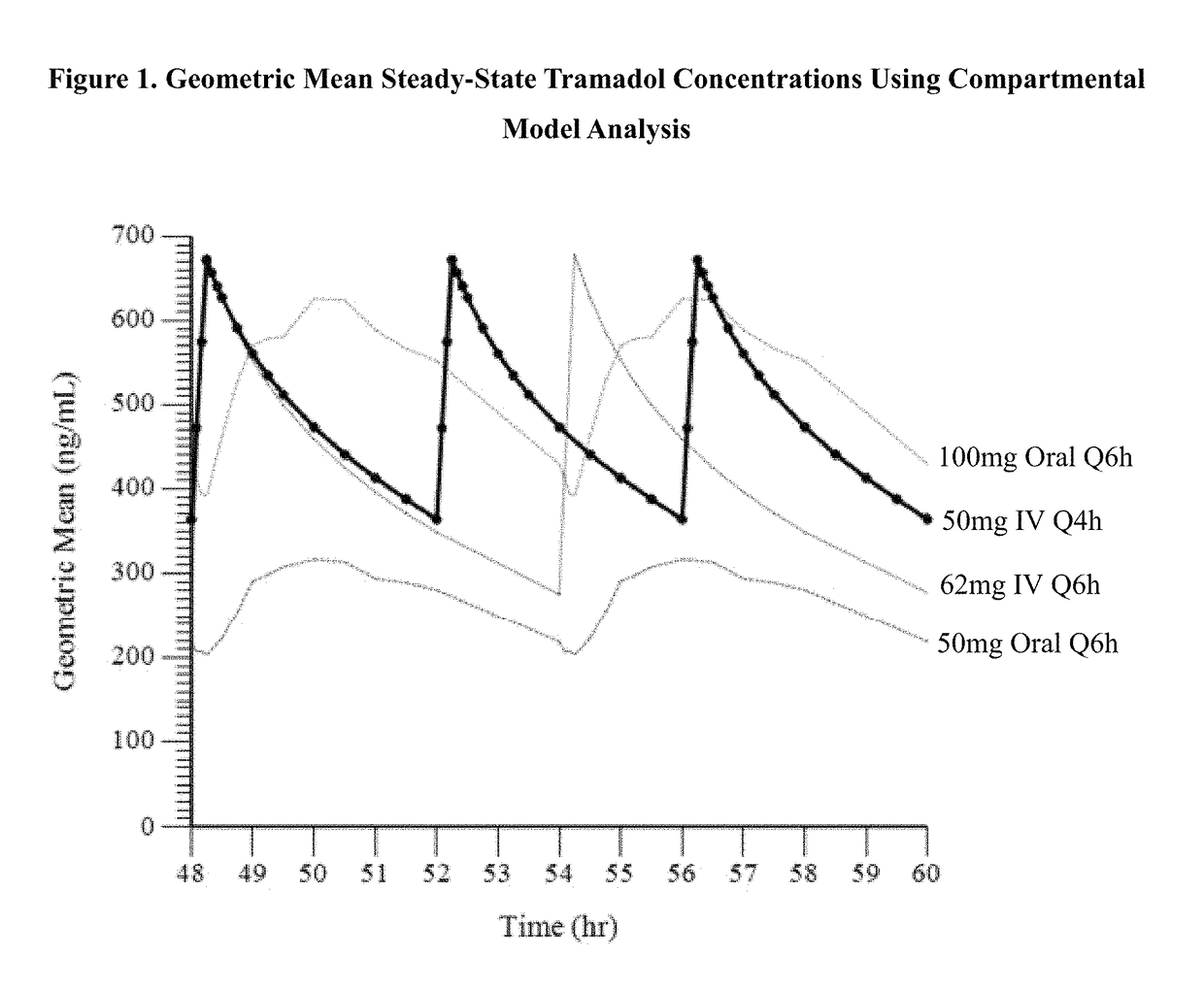
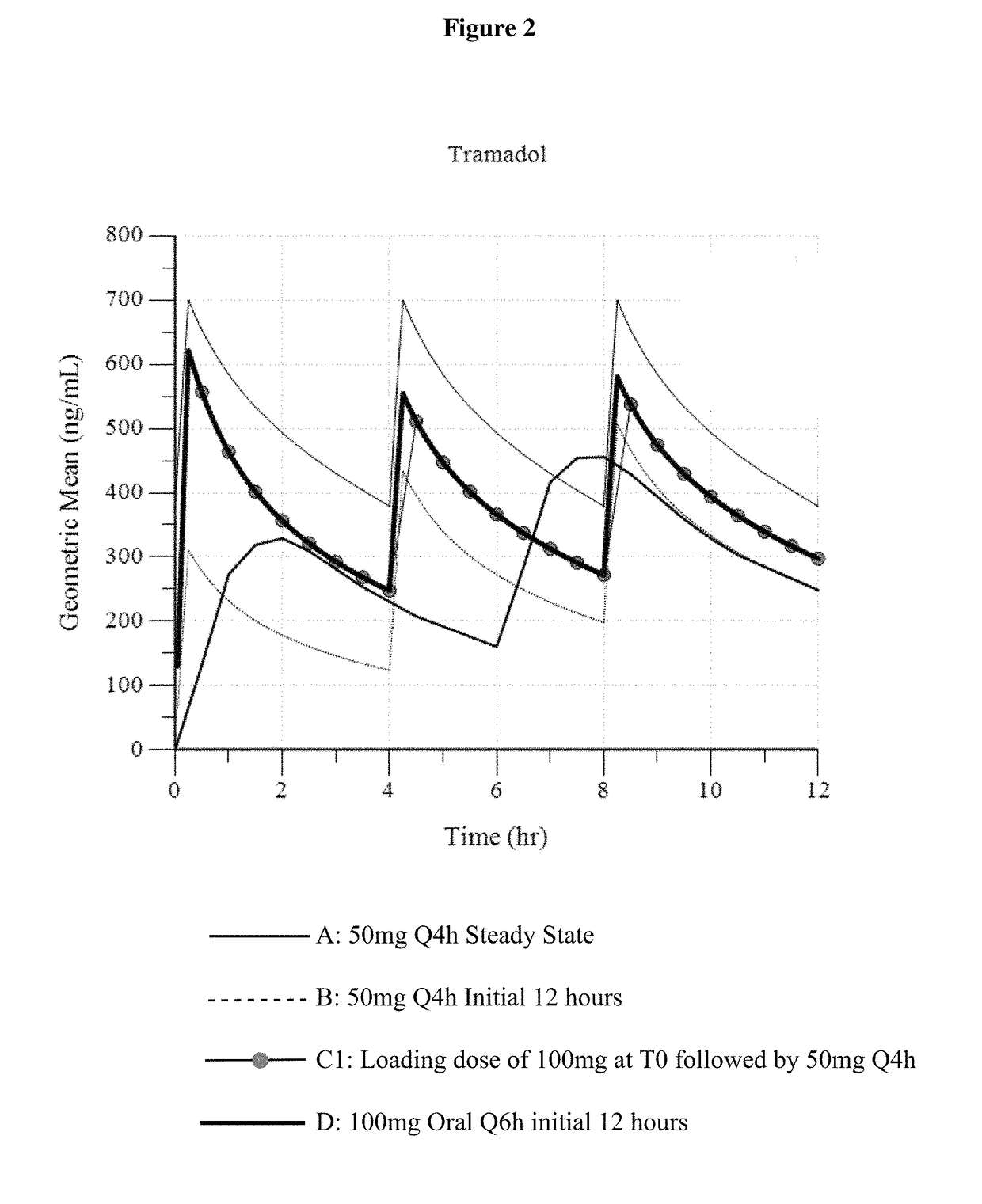
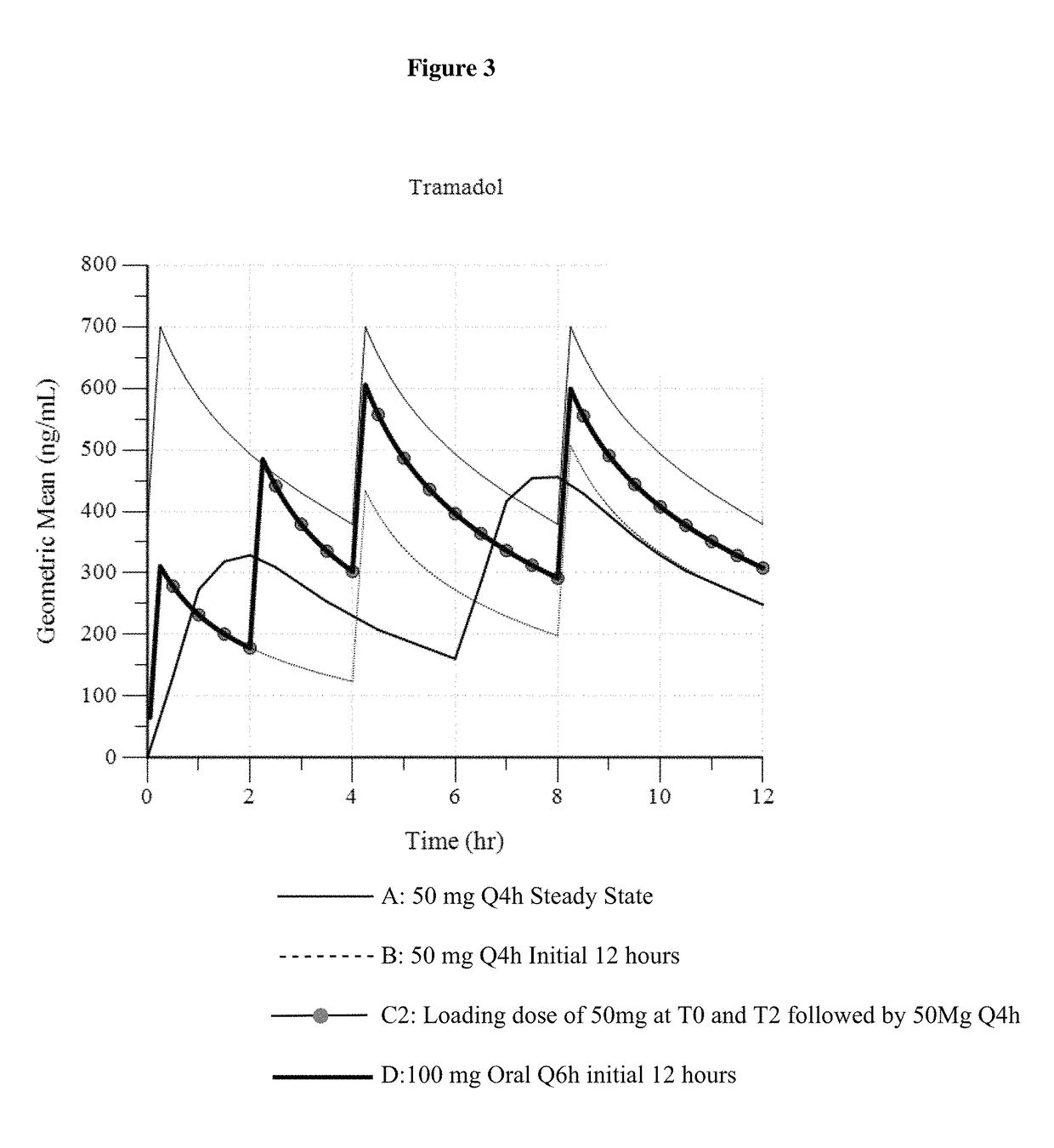
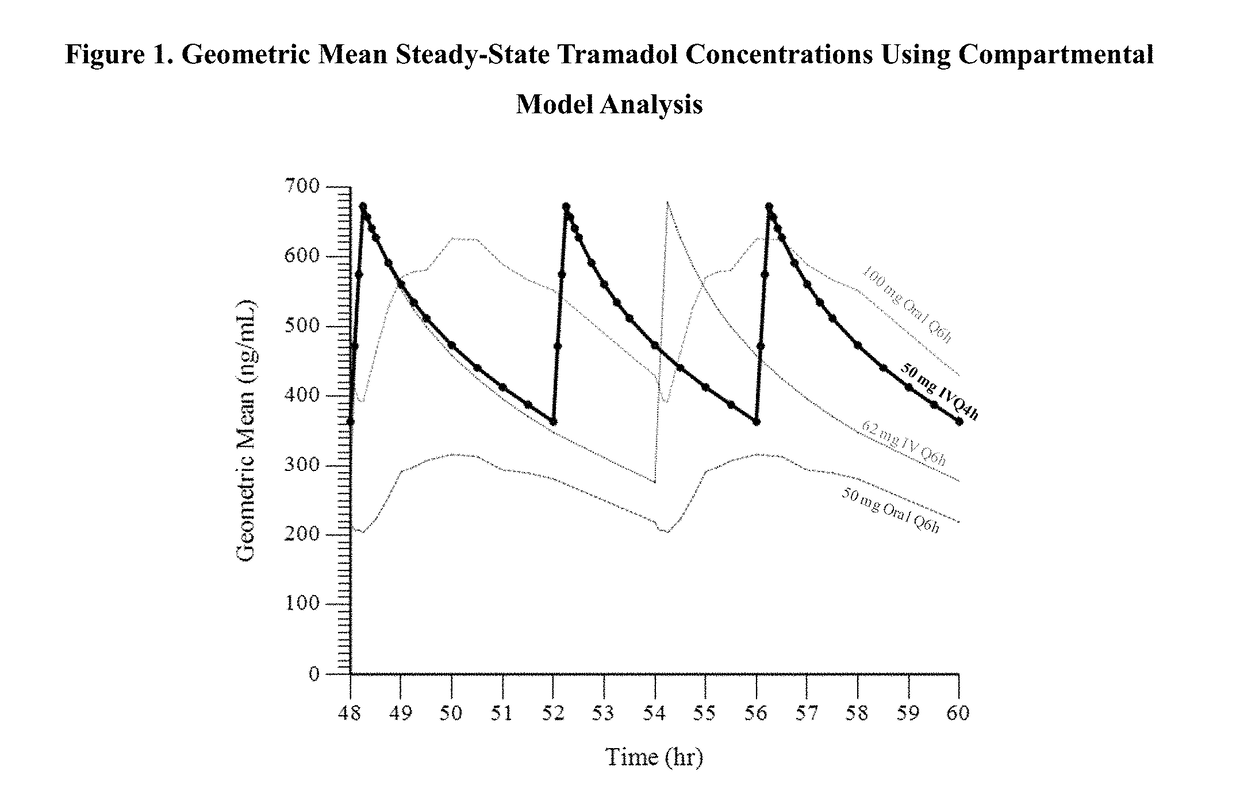
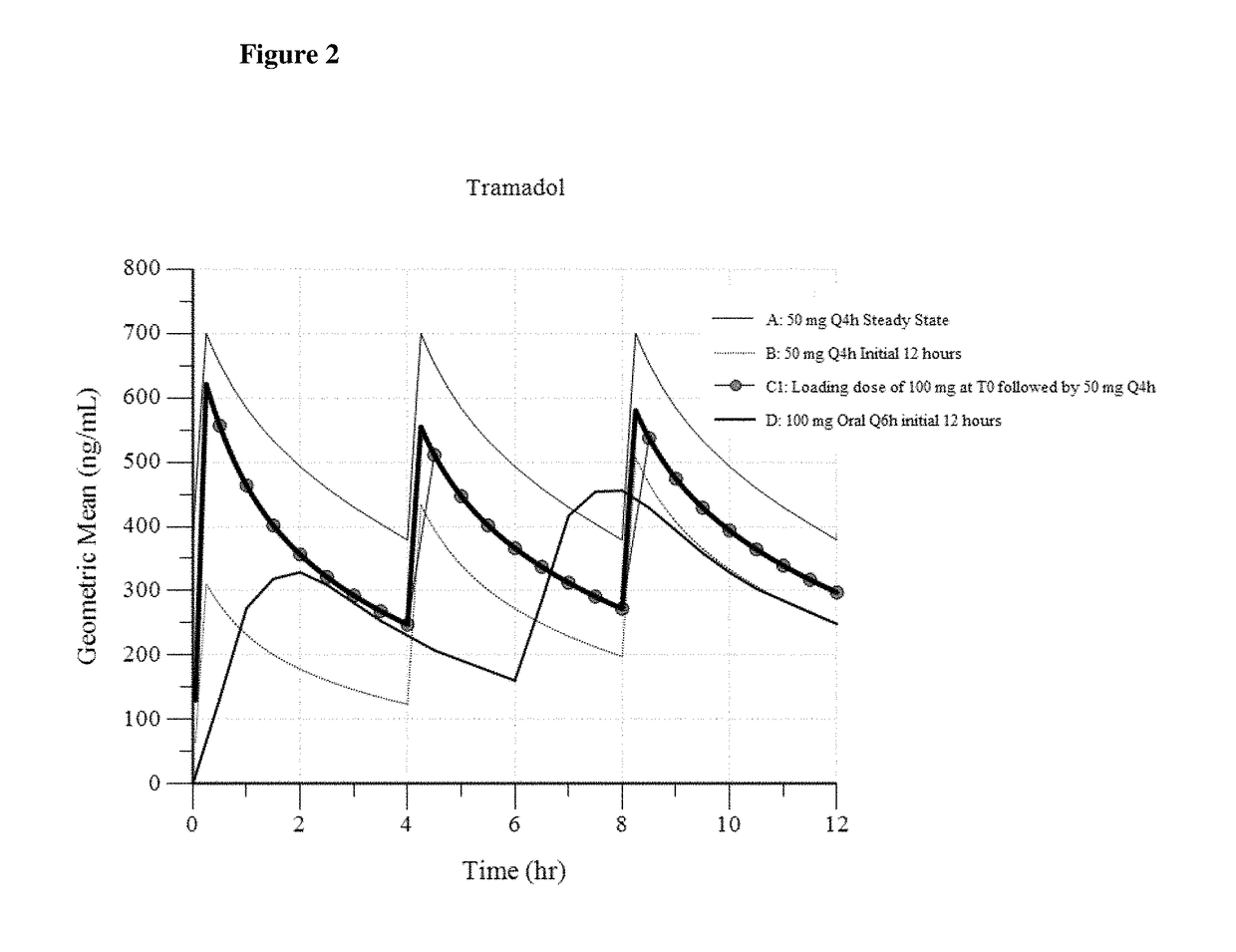
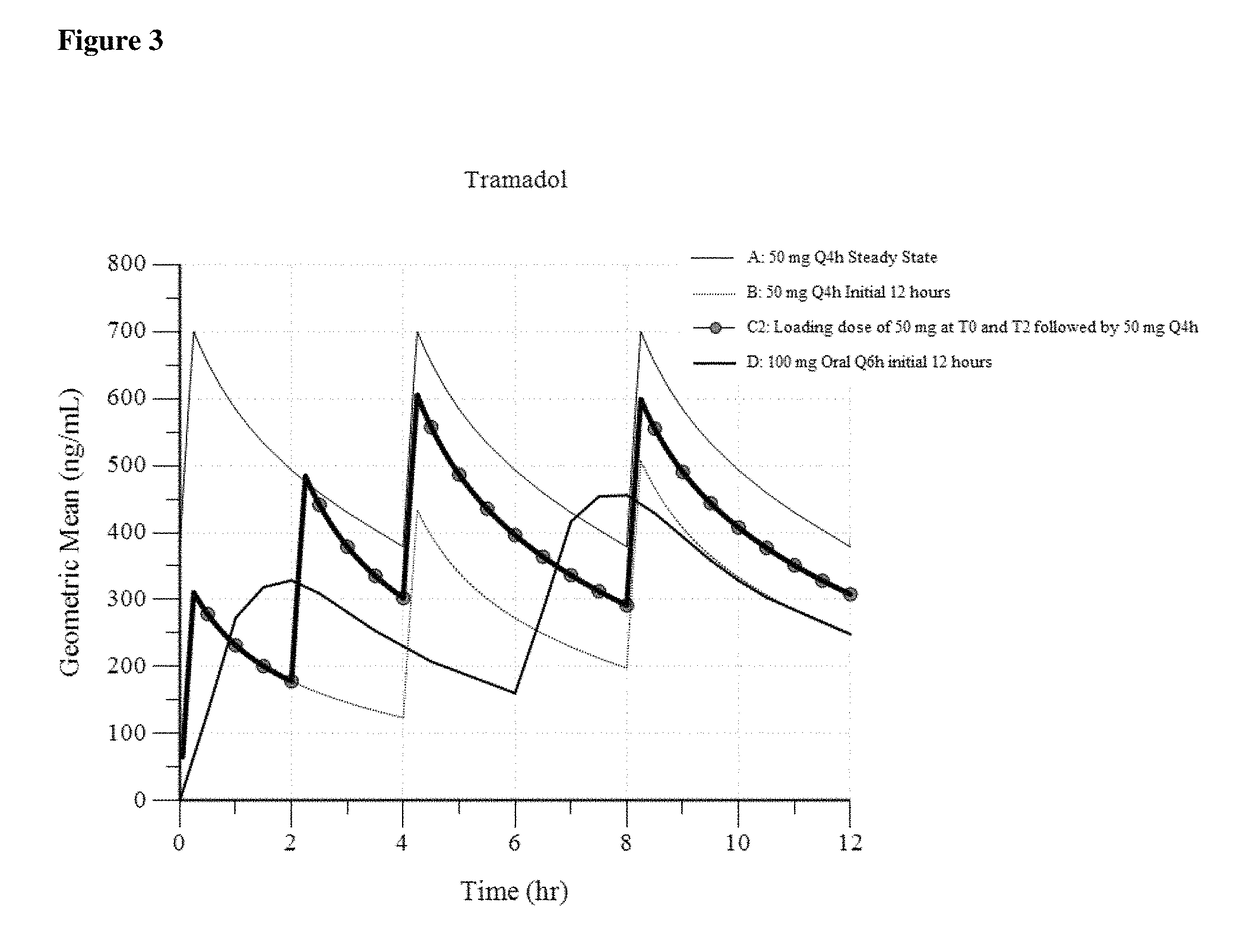
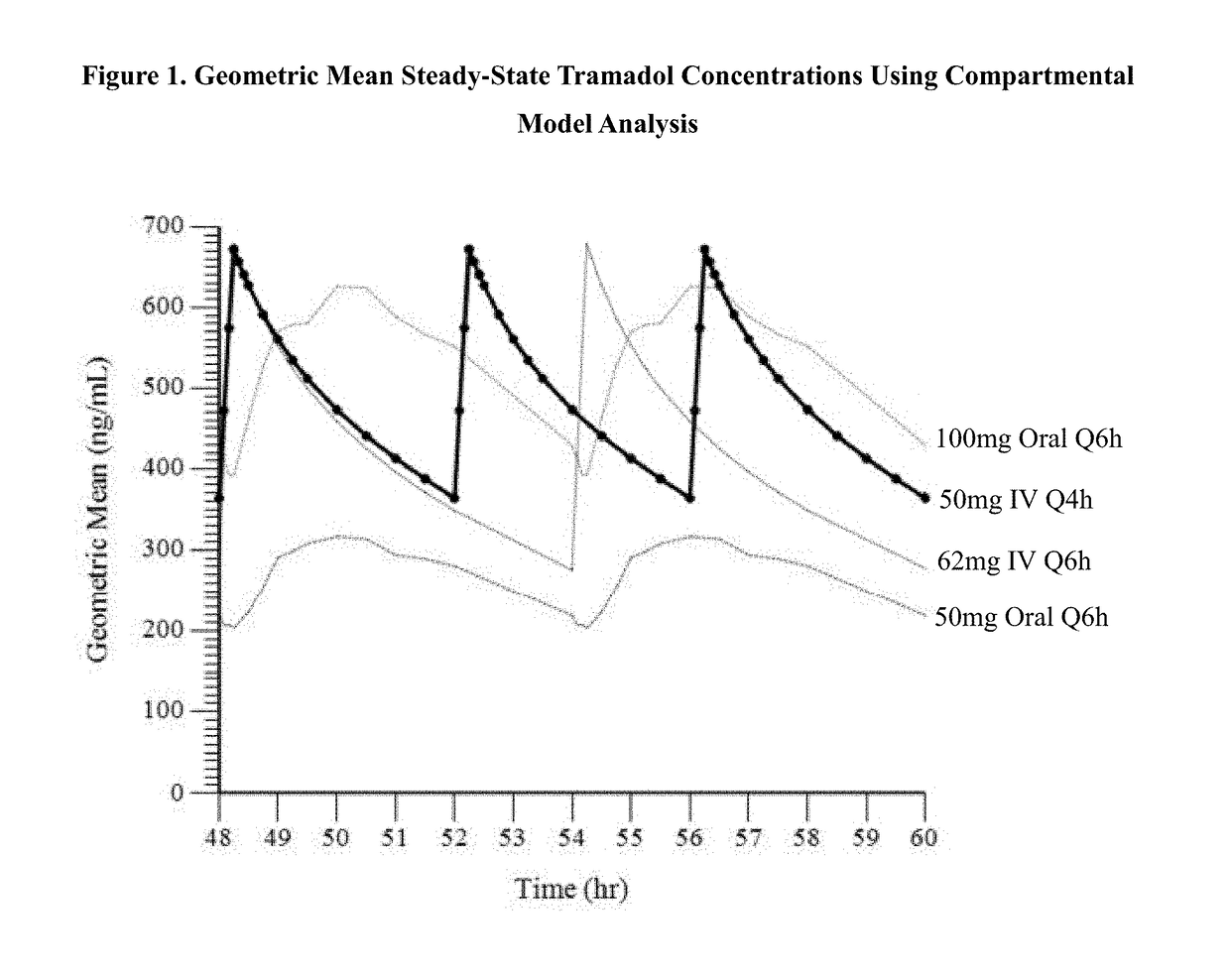
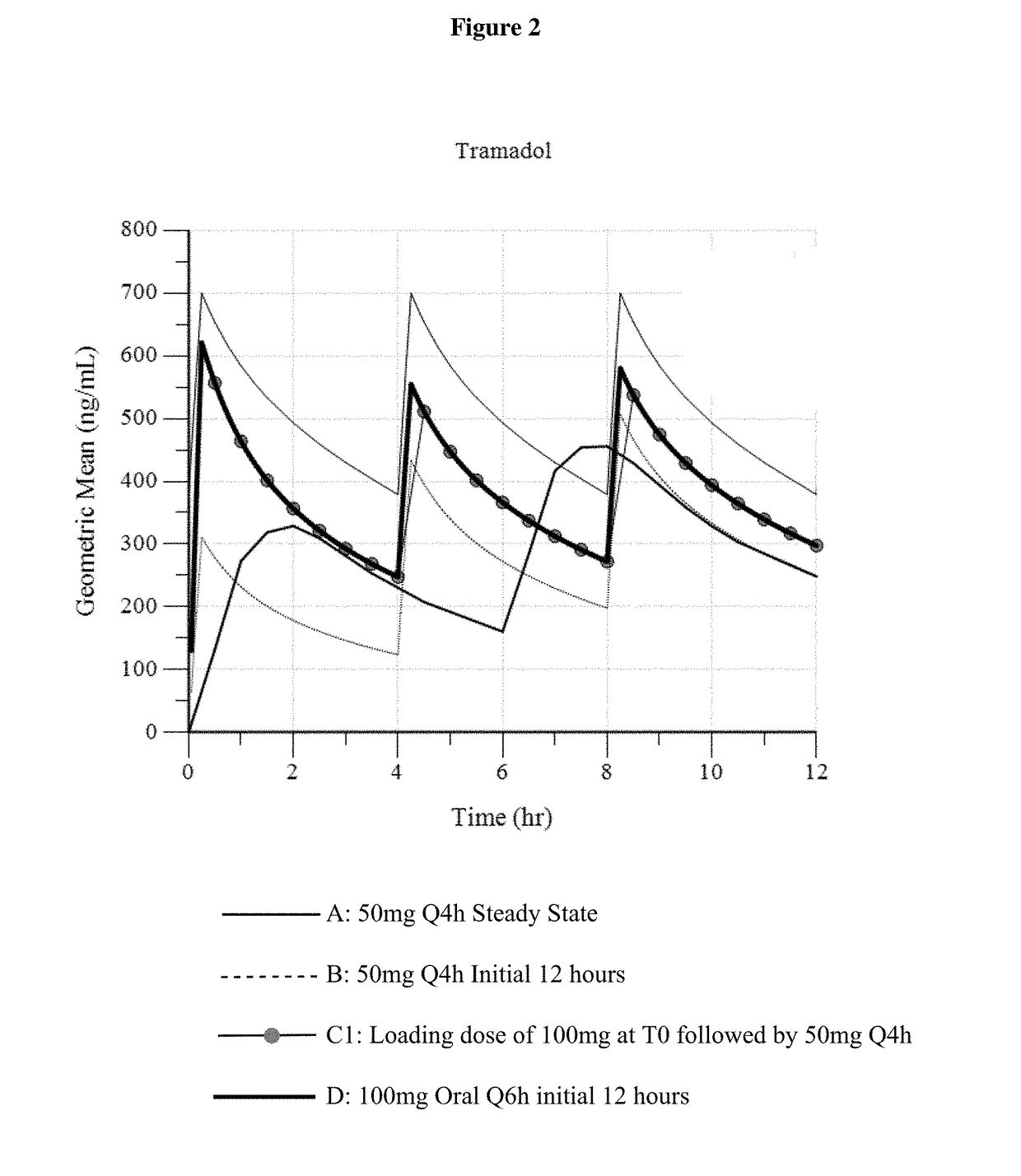
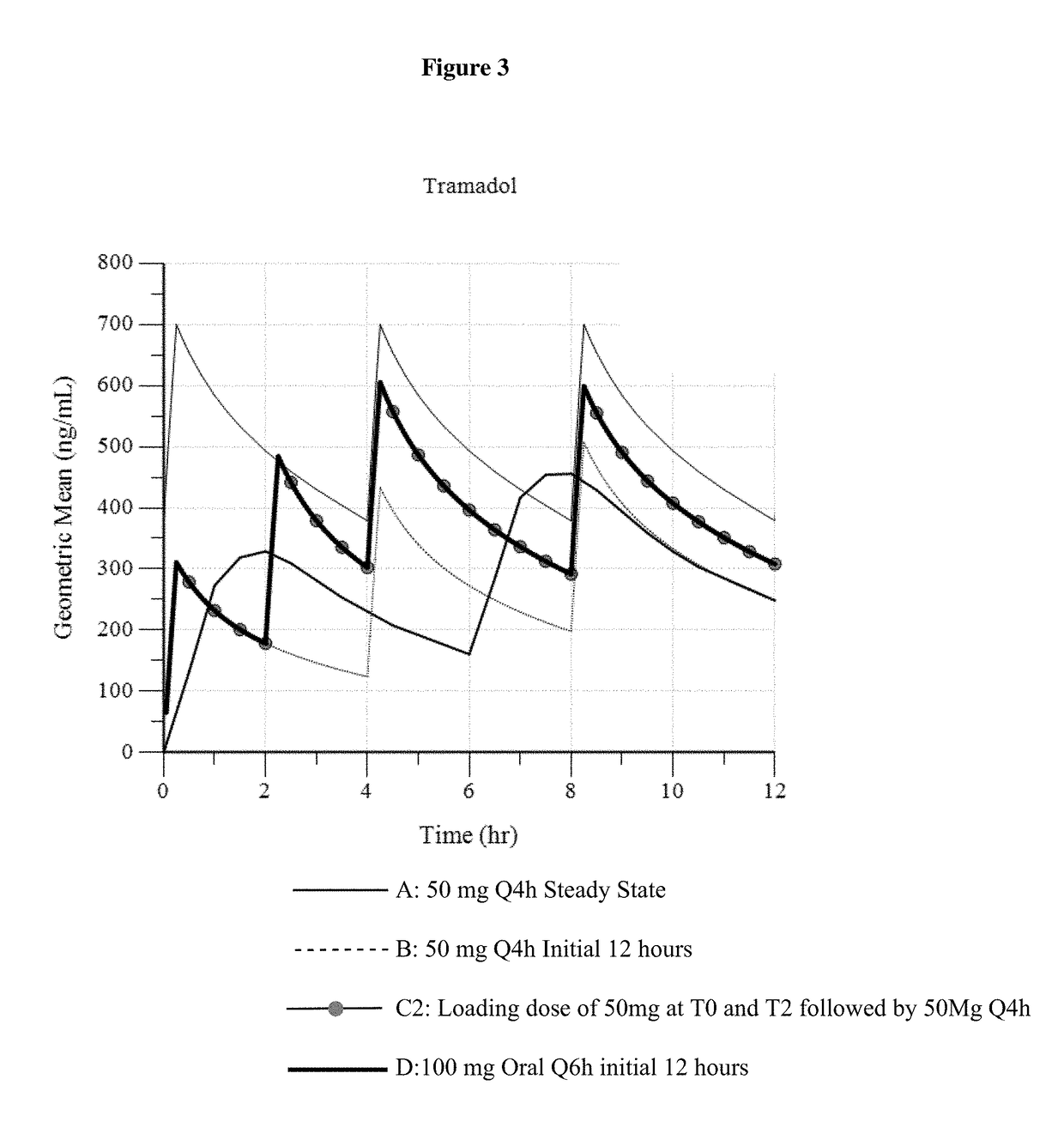
ActiveUS9693949B1InhibitionRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
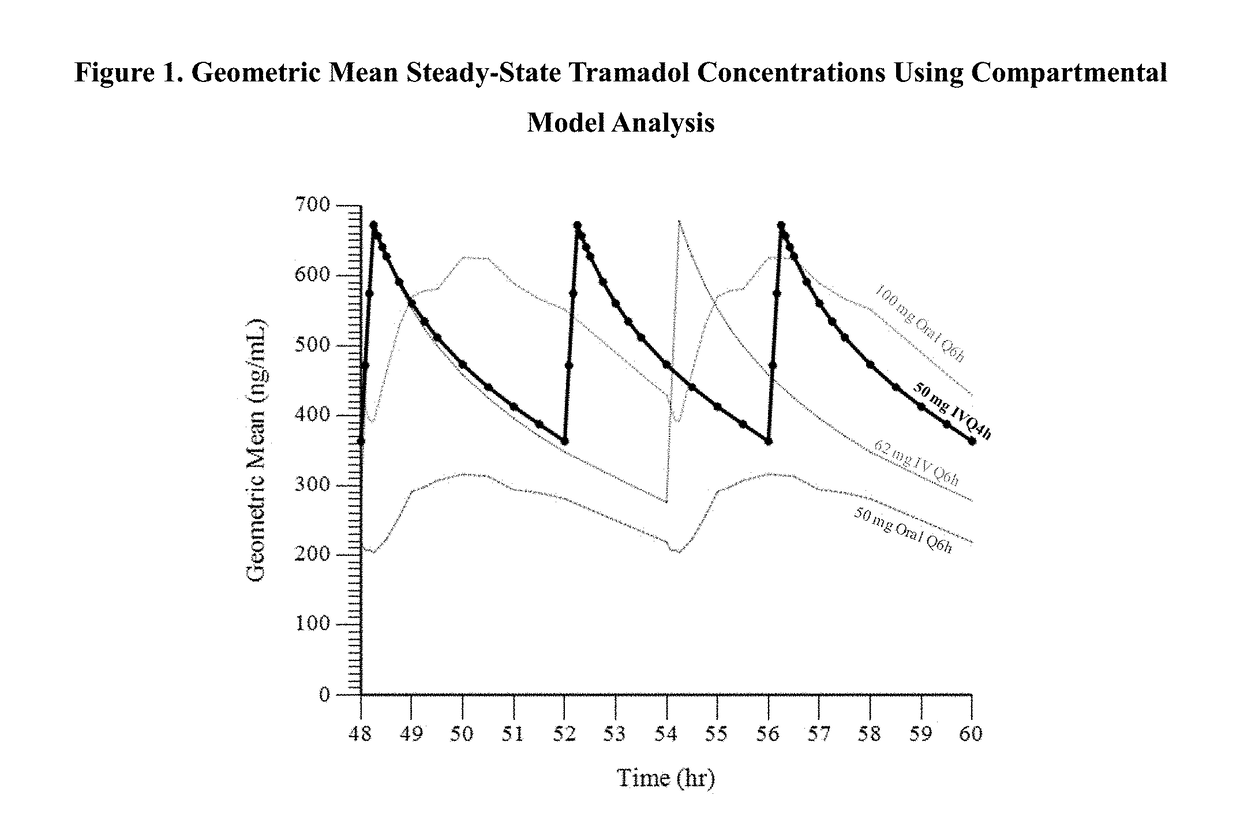
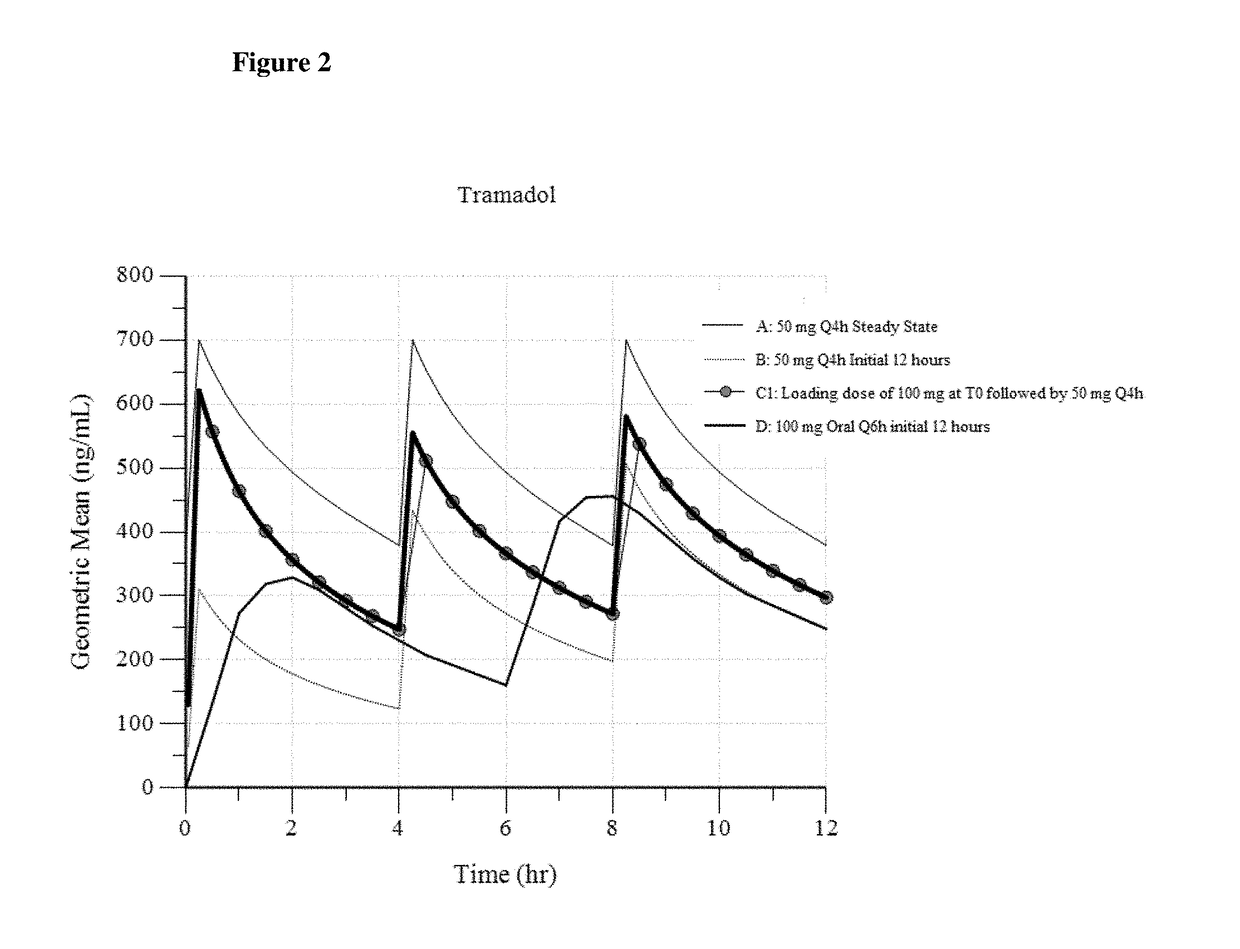
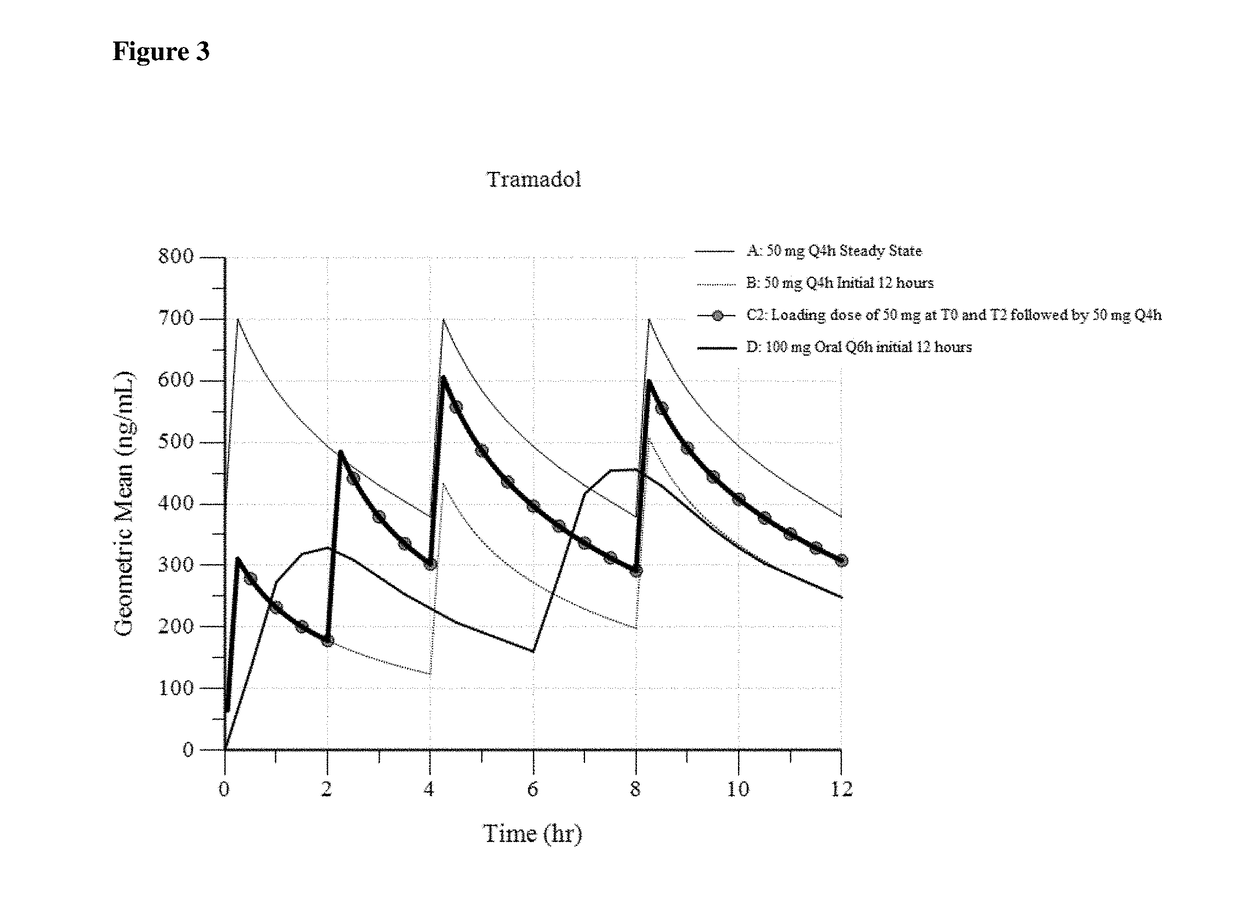
A method of treating pain, e.g., acute post-operative pain, by administering to a human patient(s) a therapeutically effective dose of tramadol intravenously in a dosing regimen which includes one or more loading doses administered at shortened intervals as compared to dosing at steady-state is disclosed. In certain embodiments, the dose of tramadol is from about 45 mg to about 80 mg and the second (and optionally) third doses are intravenously administered at intervals of from about 2 to about 3 hours, and thereafter the tramadol is intravenously administered at a dosing interval of about 4 to about 6 hours, until the patient no longer requires treatment with tramadol. In preferred embodiments, the intravenous dosing regimen provides a Cmax and AUC of tramadol is similar to the Cmax and AUC of an oral dose of 100 mg tramadol HCl given every 6 hours. In certain preferred embodiments, the dosing regimen comprises 50 mg IV tramadol at Hour 0, followed by 50 mg at Hour 2, 50 mg at hour 4, and 50 mg every 4 hours thereafter (e.g., until the patient no longer requires treatment with tramadol).
Owner:REVOGENEX IRELAND
Intravenous administration of tramadol
ActiveUS9980900B2Rapid onsetRelieve painOrganic active ingredientsNervous disorderDosing regimenRegimen
Owner:REVOGENEX IRELAND
Analgesic tramadol hydrochloride oral disintegrating tablet and preparing method thereof
InactiveCN1528273AEasy to takeReduce adverse reactionsOrganic active ingredientsAntipyreticFreeze-dryingTramadol Hydrochloride
The present invention relates to an analgesic tramadol hydrochloride oral disintegrant tablet. It is made up by using main medicine tramadol hydrochloride and auxiliary material gelatin and / or mannitol and / or aspartame and / or glucose and / or microcrystal cellulose and / or crosslinked povidone and essence, etc. and adopting freeze-drying or coating, granulating and pressing processes. When it is taken in, it has no need of drinking water, it can be quickly disintegrated in oral cavity, and can be used for curing acute and chronic pain, moderate and light pain due to cancer, pain due to fracture and pain after various operations and toothache, and can obtain good therapeutic effect.
Owner:周宇
Oral disintegration tablet of tramadol hydrochloride and preparation method
InactiveCN1660056AFast absorptionQuick effectOrganic active ingredientsNervous disorderTramadol HydrochlorideOrally disintegrating tablet
An orally disintegrating tablet of tramadol hydrochloride is prepared through preparing the dispersed solid from tramadol hydrochloride and dispersing solid carrier, mixing it with filler, disintegrant, flavouring and lubricant, stirring and tabletting.
Owner:HENAN UNIVERSITY +1
Co-crystals of tramadol and nsaids
ActiveCN102186465AImprove bindingConvenient treatmentOrganic active ingredientsNervous disorderPharmaceutical formulationCelecoxib
The present invention relates to co-crystals of tramadol and co-crystal formers selected from NSAIDs, processes for preparation of the same and their uses as medicaments or in pharmaceutical formulations, more particularly for the treatment of pain. In a preferred embodiment, the co-crystal is a co-crystal of (-) -tramadol and (S) -naproxen (1:2); (+) -tramadol and (R) -naproxen (1:2) or (rac) -tramadol-Hcl-celecoxib (1:1).
Owner:ESTEVE PHARMA SA
Chewing tablet of tramado hydrochloride and its preparation method
ActiveCN1732910AGreat tastePromote absorptionOrganic active ingredientsNervous disorderAdjuvantTramadol Hydrochloride
The invention provides a novel preparation using tramadol hydrochloride and paracetamol compositions as the main medicaments, i.e. aminophen tramadol chewing tablet. The weight ratio of tramadol hydrochloride and paracetamol is 0.1:1-0.2:1, and the adjuvant employs medicinal excipient, which includes 50.1-83.7% of water-soluble bulking agent, crumbling agent, lubricating agent, flavoring agent, moistening agent or binding agent.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD
Pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of pharmaceutical composition
ActiveCN112494486AImprove Medication AdherenceImprove experienceOrganic active ingredientsNervous disorderWithdrawal syndromeUse medication
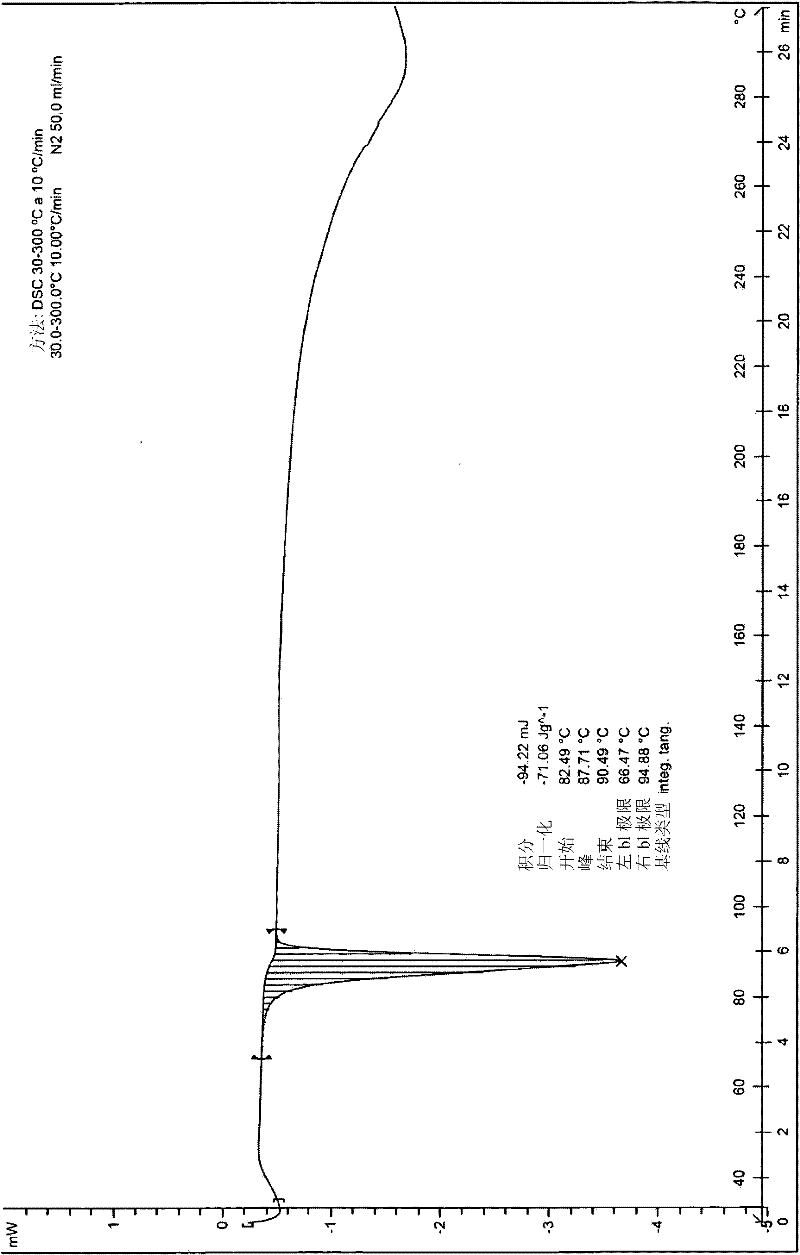
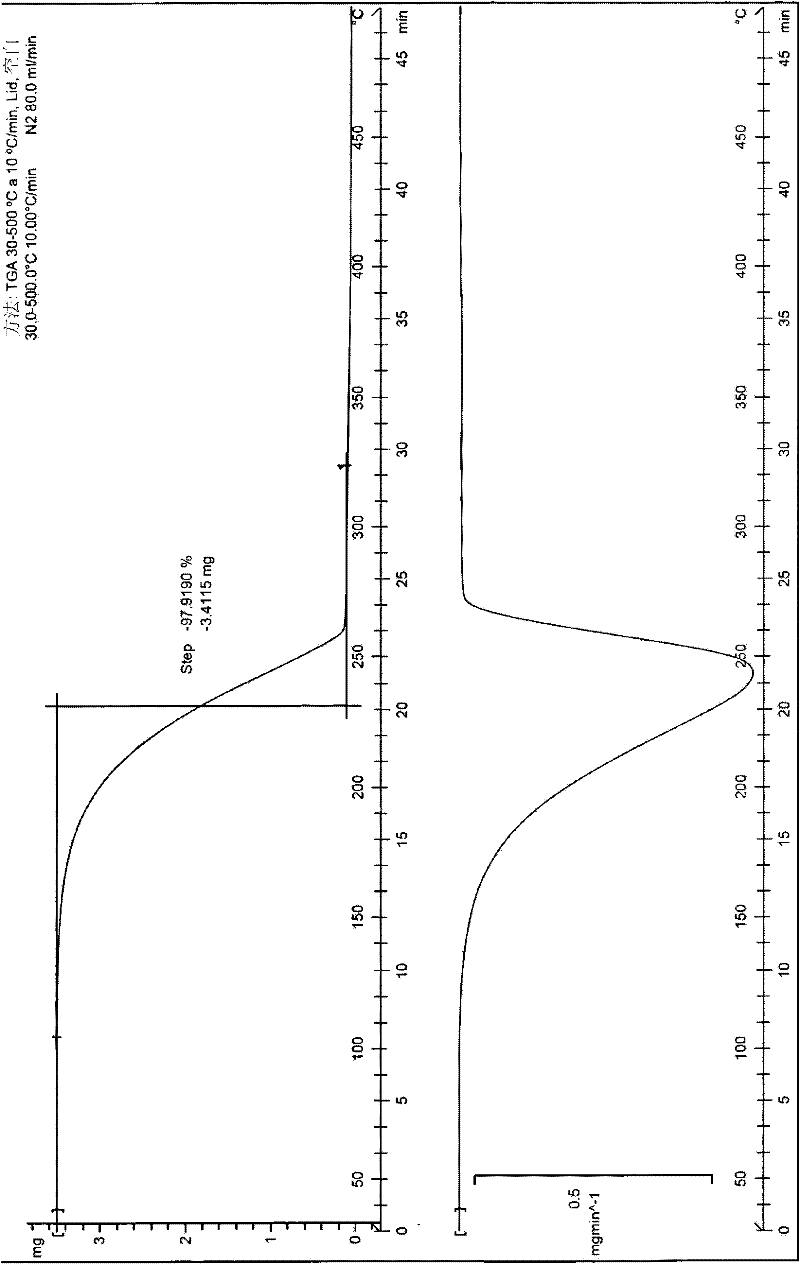
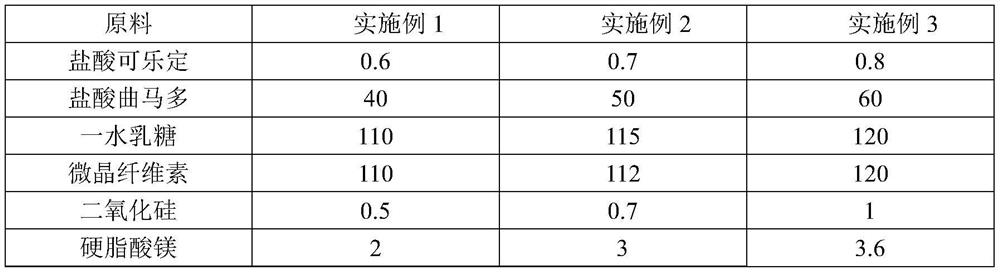
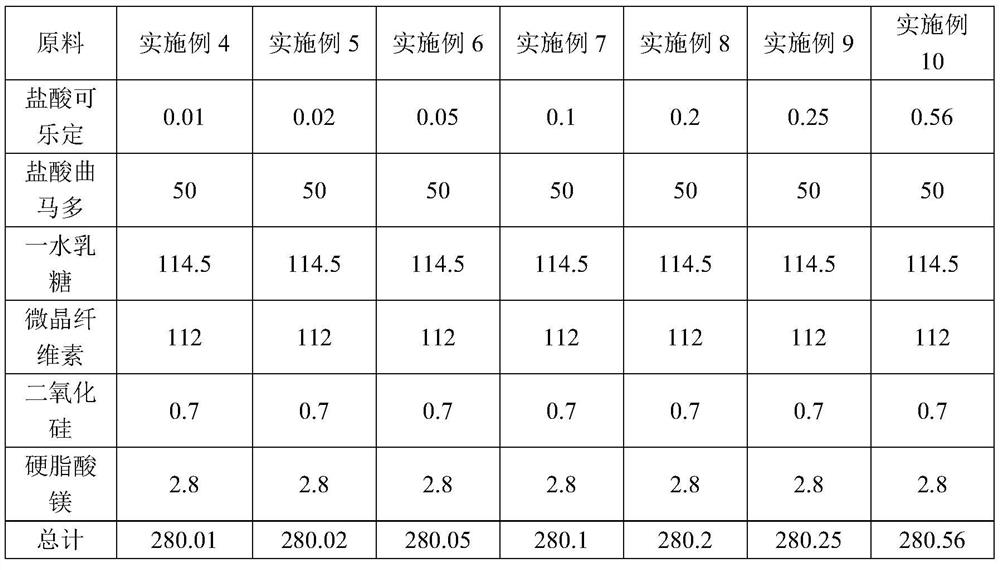
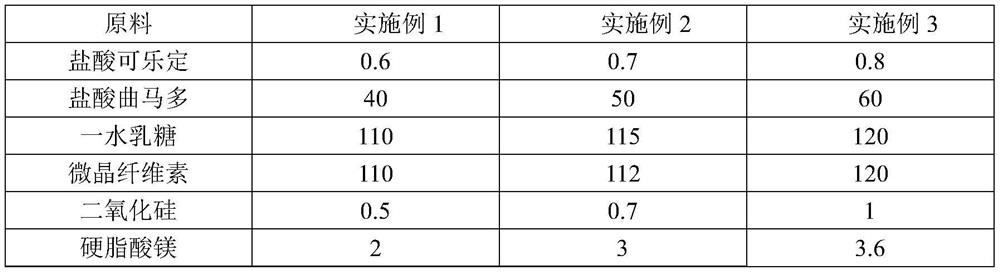
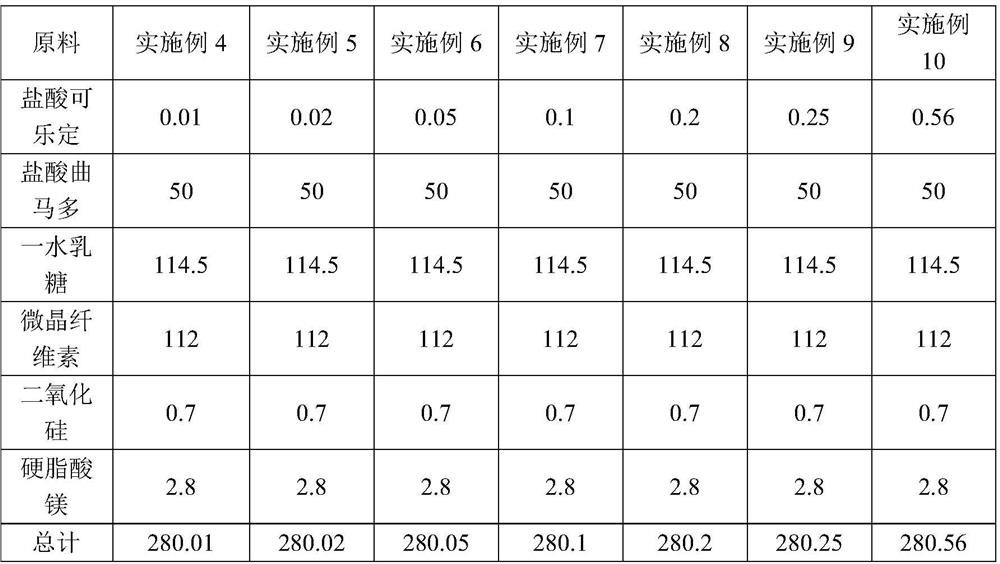
The invention discloses a pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of the pharmaceutical composition. The pharmaceutical composition comprises clonidine hydrochloride and tramadol hydrochloride in a mass ratio of (0.01-0.8):(40-60), and the application of the pharmaceutical composition in preparation of drugs for relieving or eliminating the protracted opioid abstinence syndrome is also disclosed. The pharmaceutical composition is more suitable for detoxification of opioid abuse patients, has the advantages of good effect, short time, completion in 3-5 days, safety, effectiveness and convenience in operation, has obvious clinical advantages and curative effects, can improve the medication compliance and medication experience of the patients, and prevents abuse.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Tramadol hydrochloride slow release dripping pill and its preparing method
InactiveCN1820738AGood analgesic effectLess drug dependenceOrganic active ingredientsNervous disorderTramadol HydrochlorideDrug product
The present invention relates to pain relieving medicine preparation, and is especially one orally taken slow released preparation with tramadol hydrochloride as main material. The present invention provides slow released tramadol hydrochloride dripping pill superior to available technology. The slow released tramadol hydrochloride dripping pill has the advantages of controllable releasing period, complete medicine release and high bioavailability.
Owner:北京博智绿洲医药科技有限公司
Compound consisting of parecoxib sodium and tramadol hydrochloride, and application of compound
InactiveCN107648228AImprove stabilityHigh encapsulation efficiencyPowder deliveryAntipyreticChemistryMechanism of action
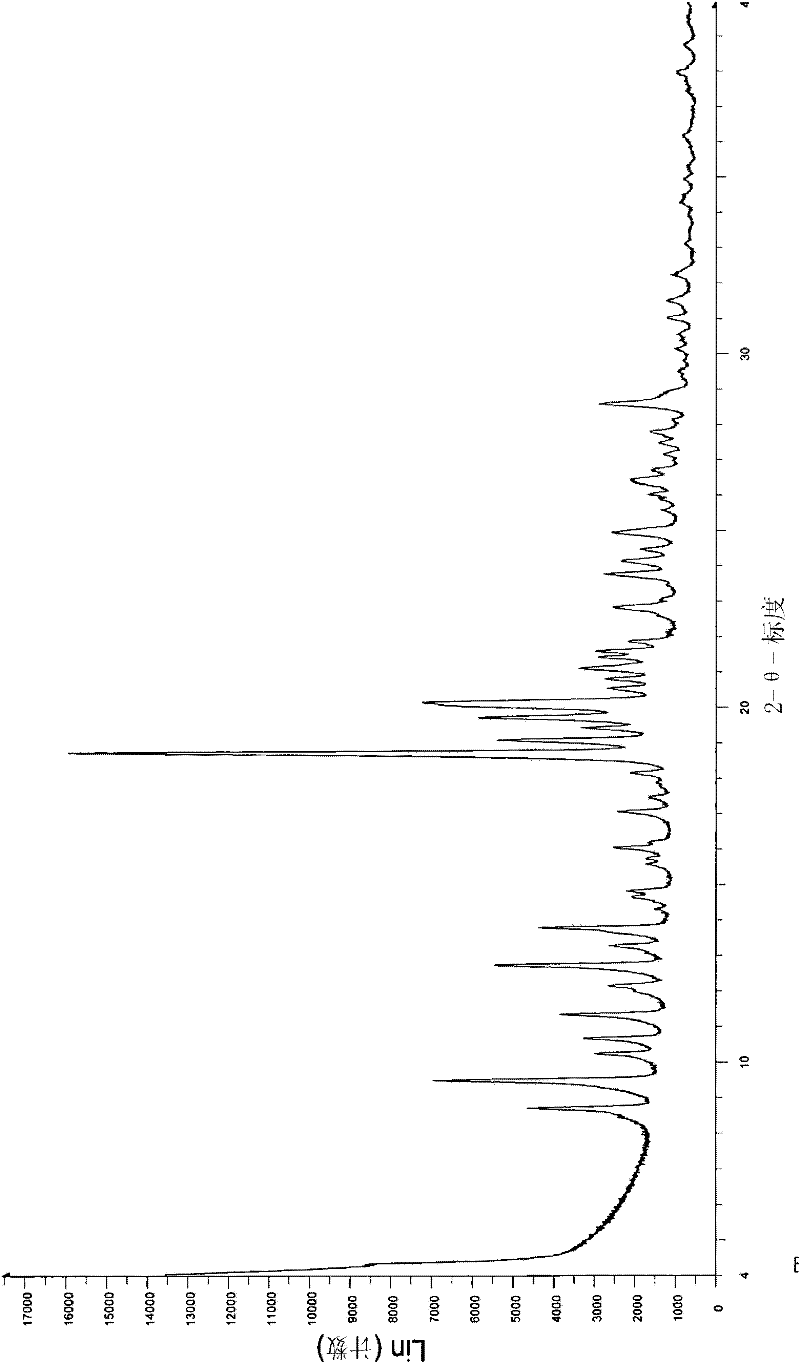
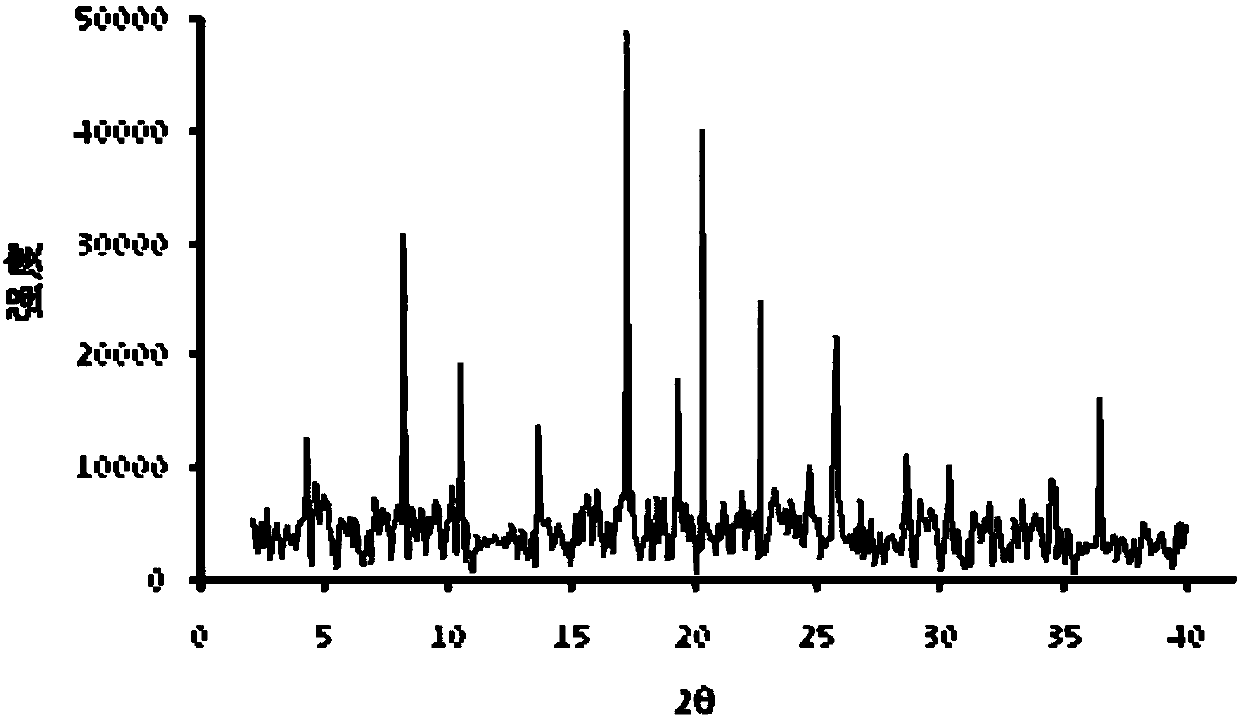
The invention provides a compound consisting of parecoxib sodium and tramadol hydrochloride, and application of the compound. The compound is a eutectic hydrate; the molecule number ratio of the parecoxib sodium to the tramadol hydrochloride to the water in the eutectic hydrate is 1:2:1; X-ray diffraction represented by 2theta has characteristic peaks at the positions of 8.2 degrees, 10.5 degrees,17.3 degrees, 20.4 degrees, 22.7 degrees and 25.8 degrees; the parecoxib sodium and the tramadol hydrochloride are combined, the combination mode is bound by hydrogen bonds, the eutectic hydrate formed by connecting the hydrogen bonds is more stable, and the pharmacokinetic property is obviously improved; and although the action mechanisms of the parecoxib sodium and the tramadol hydrochloride are different, the compound formed by the parecoxib sodium and the tramadol hydrochloride has an unexpected synergistic effect and has a positive application prospect in the field of treatment on pain caused by ankylosing spondylitis.
Owner:赛隆药业集团股份有限公司
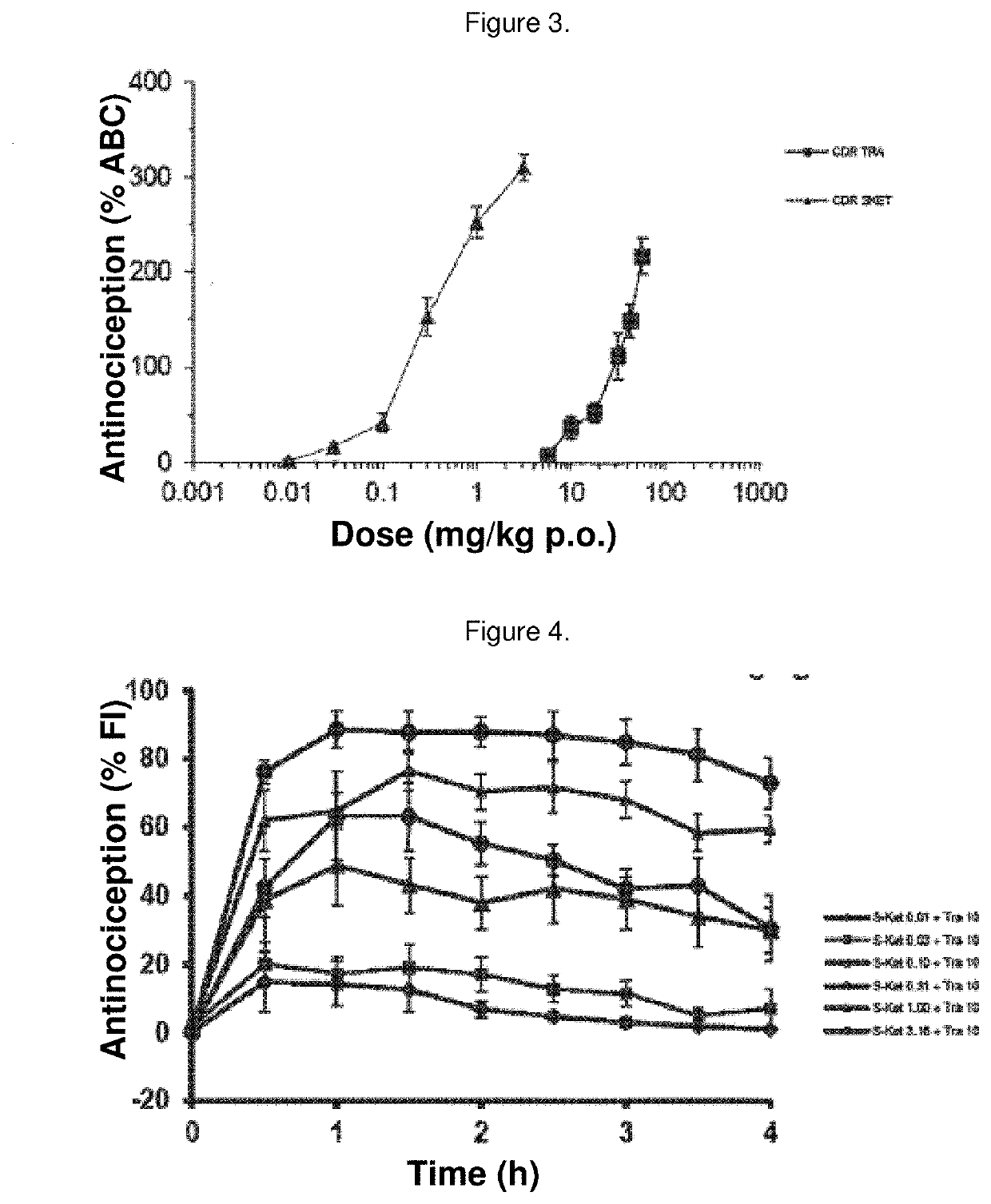
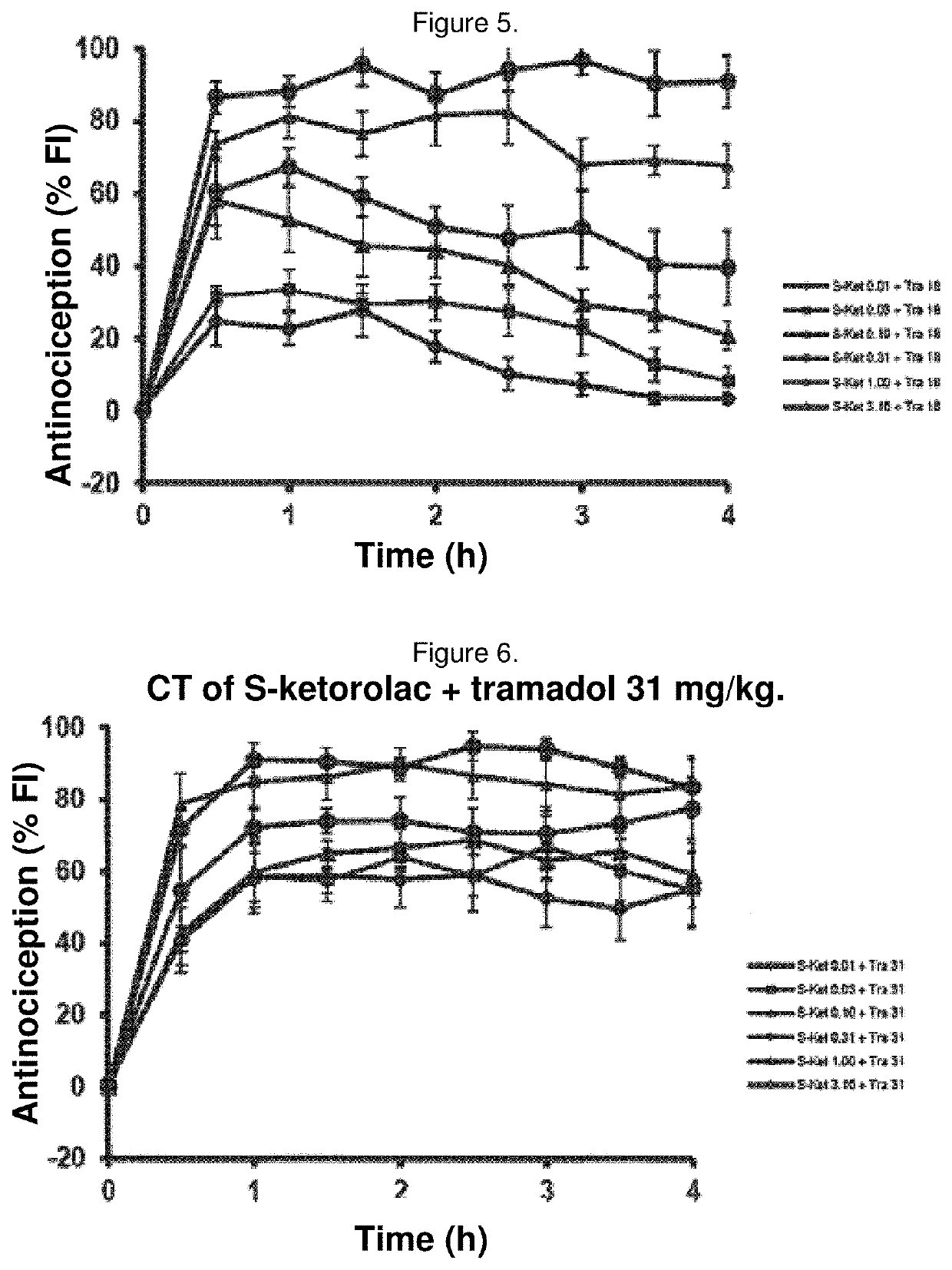
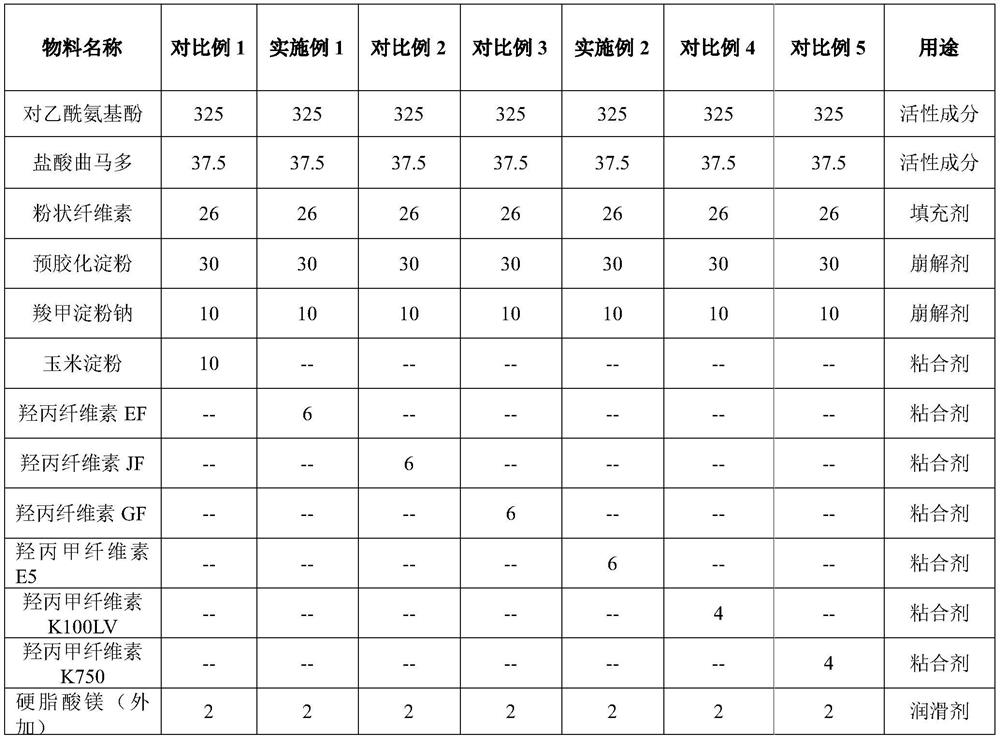
Synergistic pharmaceutical combination of the active enantiomer s-ketorolac tromethamine and tramadol chlorhydrate
PendingUS20210069151A1Decrease in symptomatologyImprove the quality of lifeOrganic active ingredientsNervous disorderOpioidergicNon steroid anti inflammatory drug
The present invention relates to a pharmaceutical composition comprising the synergistic combination of a non-steroidal anti-inflammatory drug (NSAID), such as the active ingredient s-ketorolac tromethamine, and an opioid agent, such as the active ingredient tramadol chlorhydrate, which ingredients are formulated with pharmaceutically acceptable excipients. The composition is used for the control and treatment of mild and / or moderate and / or severe pain.
Owner:AMEZCUA AMEZCUA FEDERICO +1
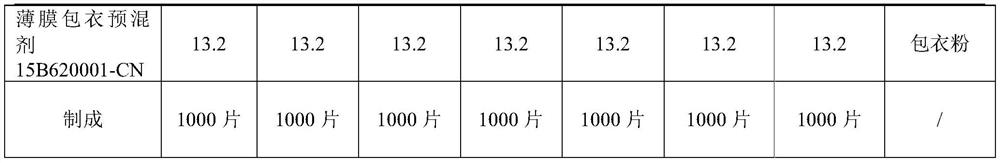
Preparation method of coated tablet containing acetaminophen and tramadol hydrochloride
ActiveCN113694051AHigh similarityThe granulation process is easy to operateOrganic active ingredientsAntipyreticCelluloseCoated tablets
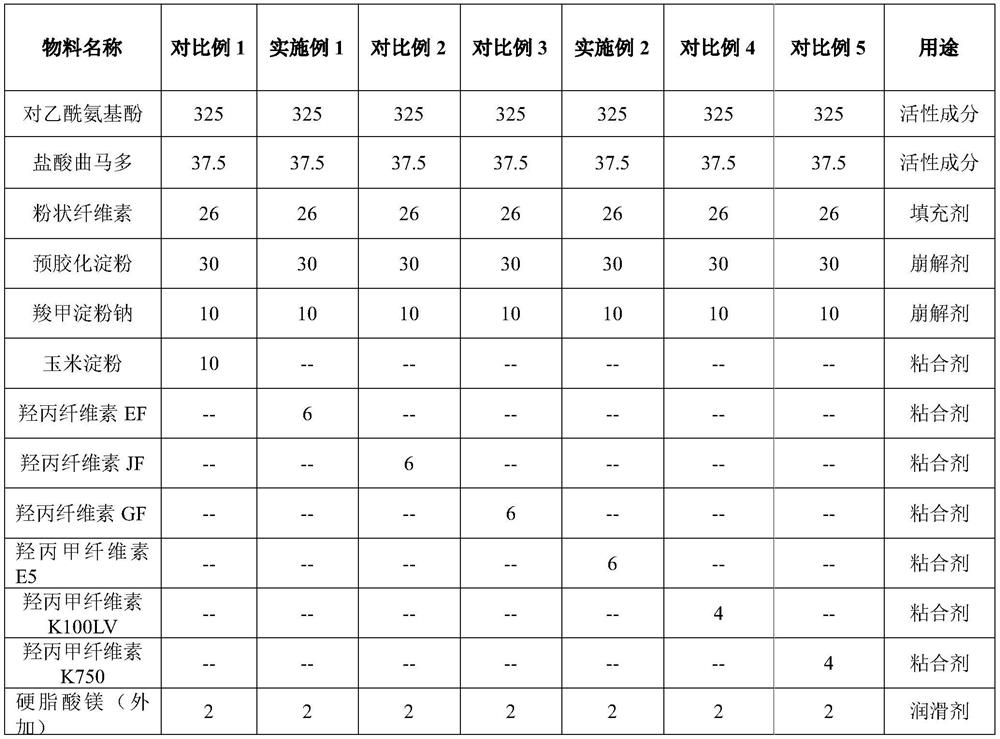
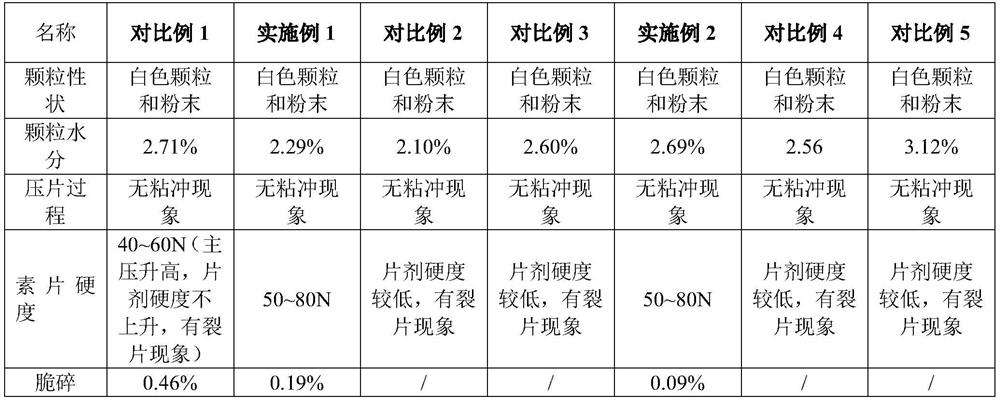
The invention discloses a preparation method of a coated tablet containing acetaminophen and tramadol hydrochloride. The preparation method comprises the following steps that S1, active component, a filling agent and a disintegrating agent are mixed to obtain premixed powder; the active components are the acetaminophen and the tramadol hydrochloride, and the mass ratio of the acetaminophen to the tramadol hydrochloride is 325: 37.5; S2, in the stirring process, an adhesive is added into the premixed powder, and wet particles are obtained through granulation; the adhesive is hydroxypropyl cellulose EF or hydroxypropyl methylcellulose E5; S3, the wet particles are dried and then subjected to size stabilization, then a lubricating agent is added for mixing, and total mixed particles are obtained; S4, the total mixed particles are tabletted to obtain tablets; and S5, the tablets are coated to obtain the tablet. According to the preparation method, the granulation process is simple and convenient to operate, the problems of poor compressibility, tablet cracking and the like of the acetaminophen are solved, the acetaminophen and the tramadol hydrochloride have high similarity, the preparation process is stable, the large-scale production is convenient, and the good bioavailability and the good quality safety are provided.
Owner:DUODUO PHARMA
Paracetamol and tramadol oral emulsion and preparation method thereof
ActiveCN111840227AGreat tasteAvoid degradation and oxidationOrganic active ingredientsNervous disorderSuccinic acidOil phase
The invention discloses a paracetamol and tramadol oral emulsion, which is an oil-in-water emulsion formed by uniformly dispersing an oil solution of p-acetamidophenol into an aqueous solution of tramadol hydrochloride. The invention also discloses a preparation method of the paracetamol and tramadol oral emulsion, which comprises the following steps: adding an oil-phase solution containing p-acetamidophenol into a mixed solution containing an octenyl succinic starch sodium emulsifier, and homogenizing in a water-phase solution containing tramadol hydrochloride. According to the paracetamol and tramadol oral emulsion, p-acetamidophenol is coated in the emulsion, degradation and oxidation of paracetamol are avoided, the bitter taste of paracetamol is covered, the covering effect of a tastecovering agent and a flavoring agent and the cross-linking effect of other components are combined, the bitter taste of tramadol hydrochloride is covered, the stability of the paracetamol and tramadoloral emulsion is improved, the taste of the paracetamol and tramadol oral emulsion is improved; the stable oil-in-water paracetamol and tramadol oral emulsion is prepared by adopting an emulsification coating method, and the process is simple.
Owner:陕西九州制药有限责任公司
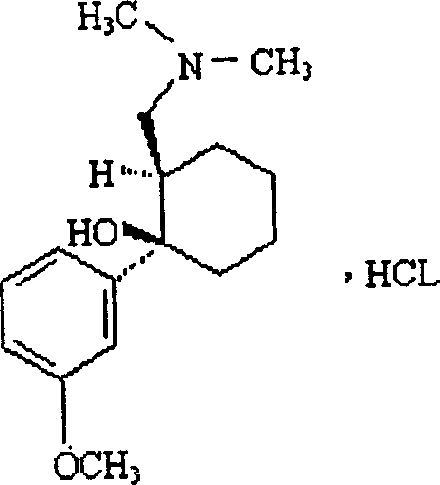
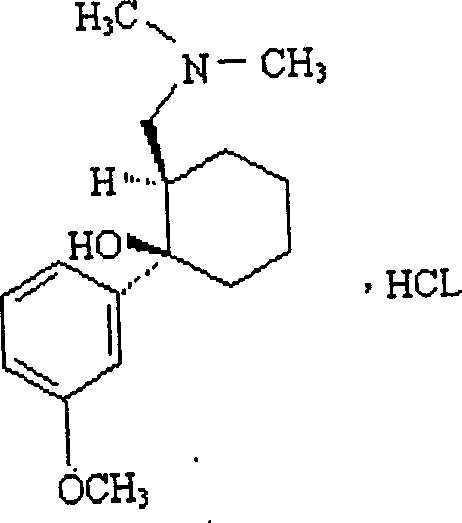
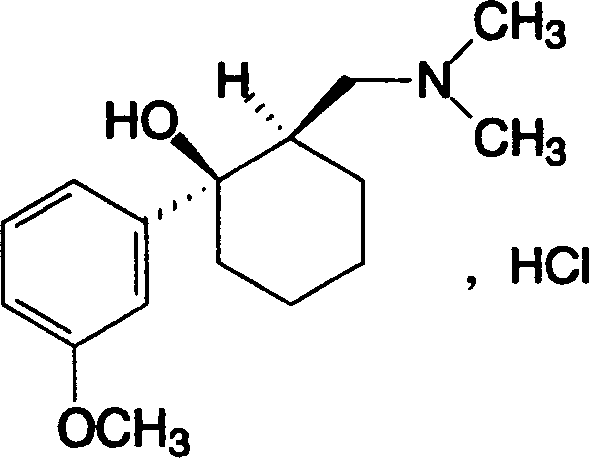
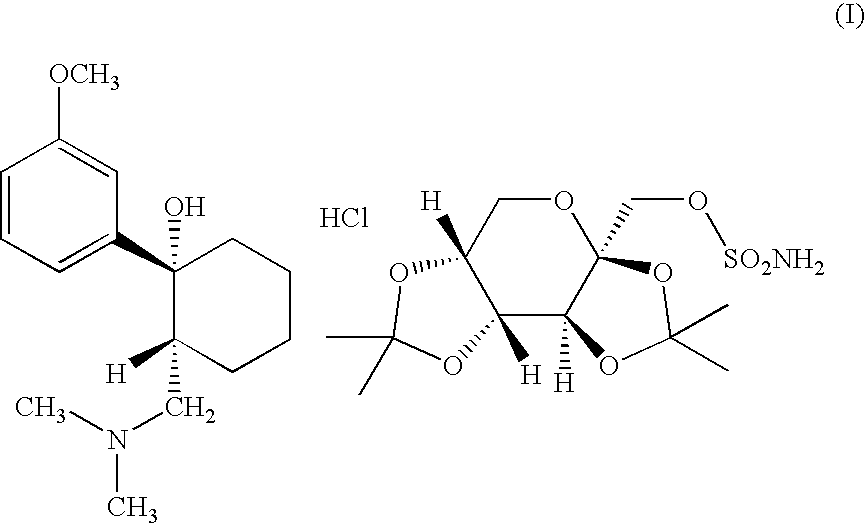
Adduct of topiramate and tramadol hydrochloride and uses thereof
This invention relates to a new pharmaceutically useful compound which is a stoichiometrically 1:1 adduct of tramadol hydrochloride and topiramate, to the manufacture and use thereof.
Owner:CILAG
Intravenous administration of tramadol
ActiveUS20170172914A1Improve securityImproving tolerabilityOrganic active ingredientsNervous disorderDosing regimenRegimen
A method of treating pain, e.g., acute post-operative pain, by administering to a human patient(s) a therapeutically effective dose of tramadol intravenously in a dosing regimen which includes one or more loading doses administered at shortened intervals as compared to dosing at steady-state is disclosed. In certain embodiments, the dose of tramadol is from about 45 mg to about 80 mg and the second (and optionally) third doses are intravenously administered at intervals of from about 2 to about 3 hours, and thereafter the tramadol is intravenously administered at a dosing interval of about 4 to about 6 hours, until the patient no longer requires treatment with tramadol. In preferred embodiments, the intravenous dosing regimen provides a Cmax and AUC of tramadol is similar to the Cmax and AUC of an oral dose of 100 mg tramadol HCl given every 6 hours. In certain preferred embodiments, the dosing regimen comprises 50 mg IV tramadol at Hour 0, followed by 50 mg at Hour 2, 50 mg at hour 4, and 50 mg every 4 hours thereafter (e.g., until the patient no longer requires treatment with tramadol).
Owner:REVOGENEX IRELAND
Oral disintegration tablet of tramadol hydrochloride and preparation method
InactiveCN100348180CFast absorptionQuick effectOrganic active ingredientsNervous disorderOrally disintegrating tabletTableting
An orally disintegrating tablet of tramadol hydrochloride is prepared through preparing the dispersed solid from tramadol hydrochloride and dispersing solid carrier, mixing it with filler, disintegrant, flavouring and lubricant, stirring and tabletting.
Owner:HENAN UNIVERSITY +1
Tramadol hydrochloride oral disintegration tablets, and prepn. method therefor
ActiveCN1279896CEasy to takeImprove complianceOrganic active ingredientsNervous disorderUse medicationOrally disintegrating tablet
The invention relates to a tramadol hydrochloride orally disintegrating tablet and a preparation method thereof, which comprises an effective amount of tramadol hydrochloride and a pharmaceutically acceptable excipient that can disintegrate rapidly in the oral cavity to release the drug, and the pharmaceutically acceptable excipient There are water-soluble fillers, disintegrants, lubricants, and wetting agents or binders. The tablet disintegrates quickly and takes effect quickly; the intestinal residue is small, the absorption is sufficient, and the side effects are low; it is convenient to use and has a good taste .
Owner:CSPC OUYI PHARM CO LTD +1
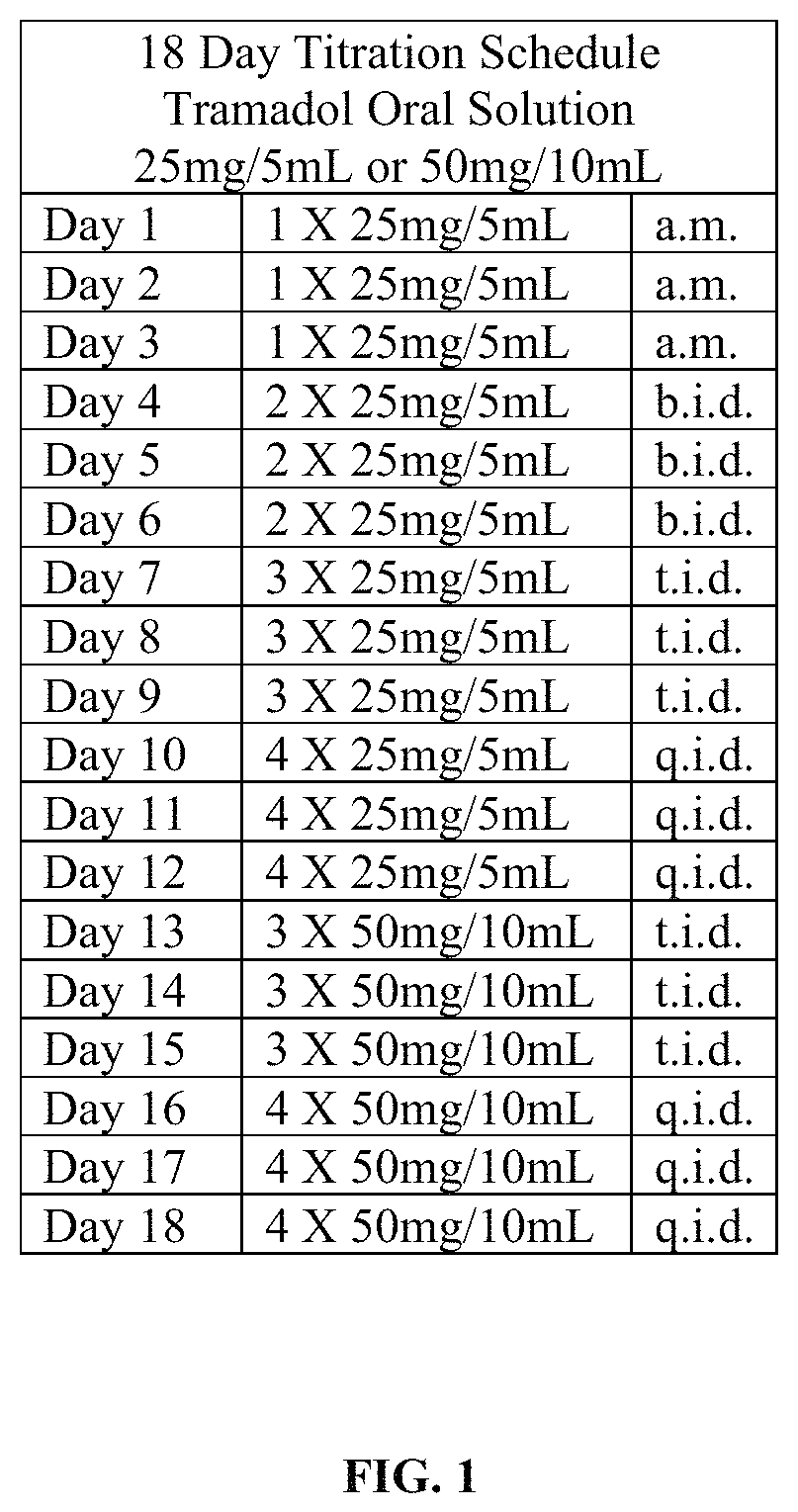
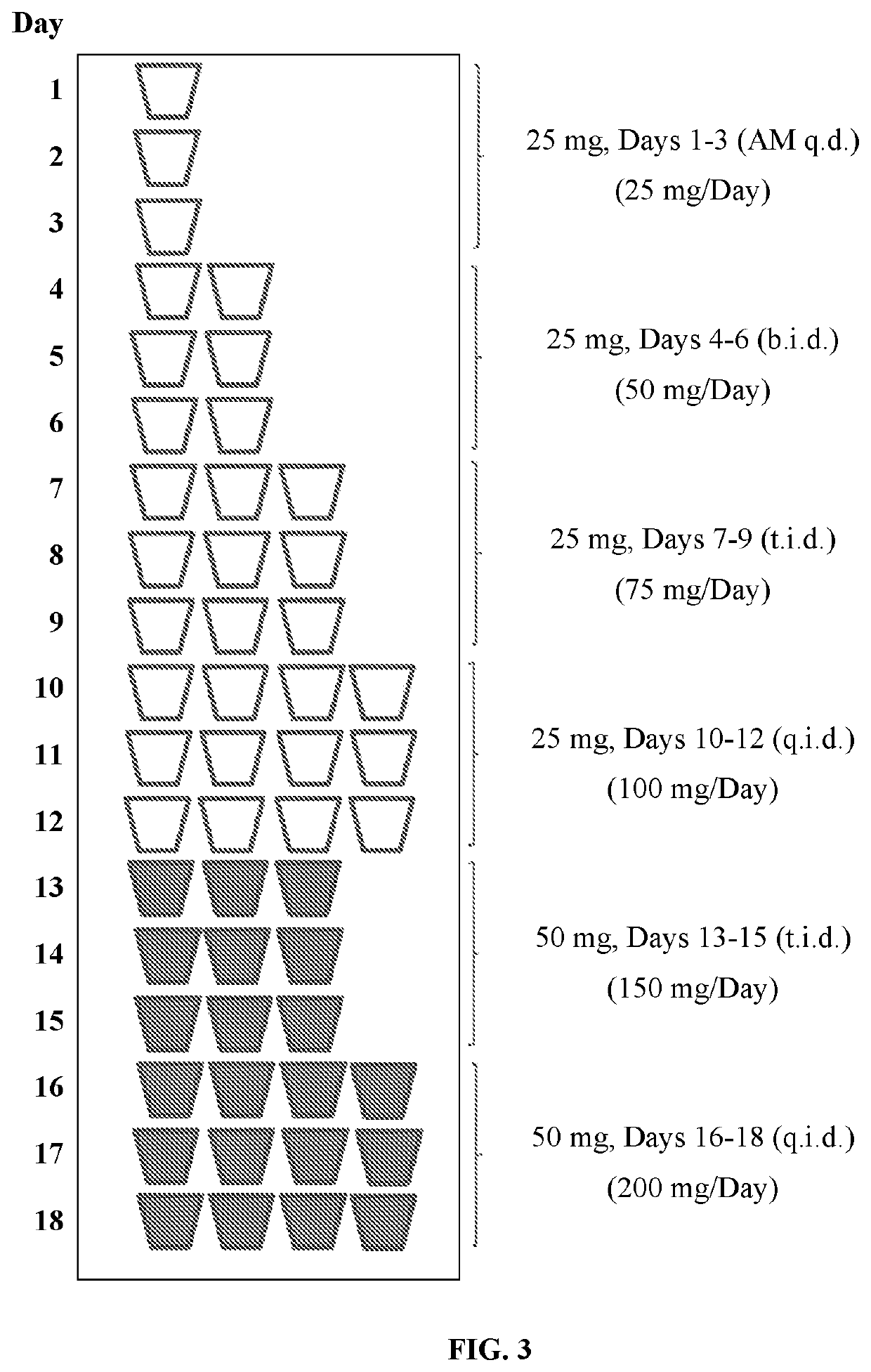
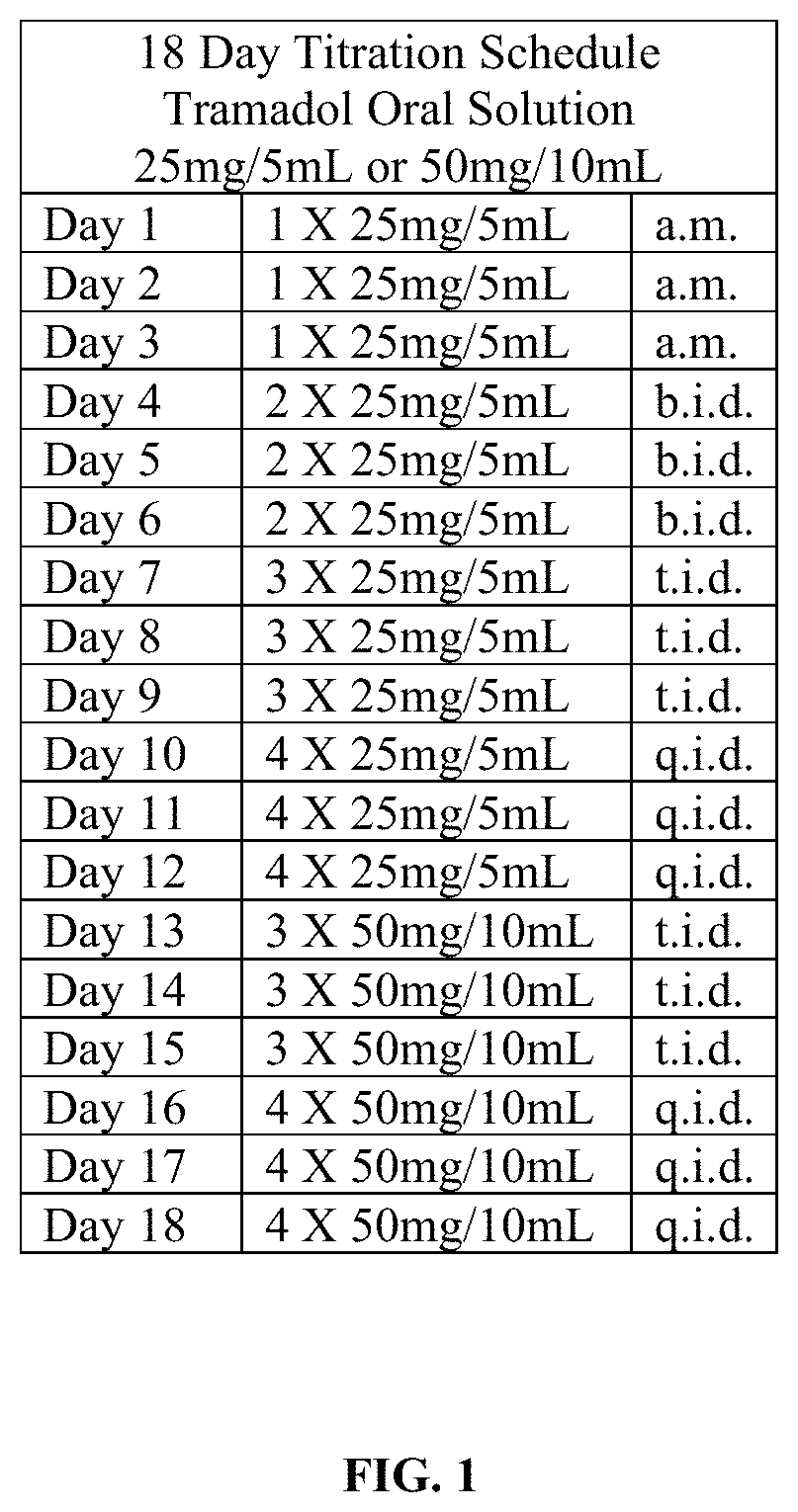
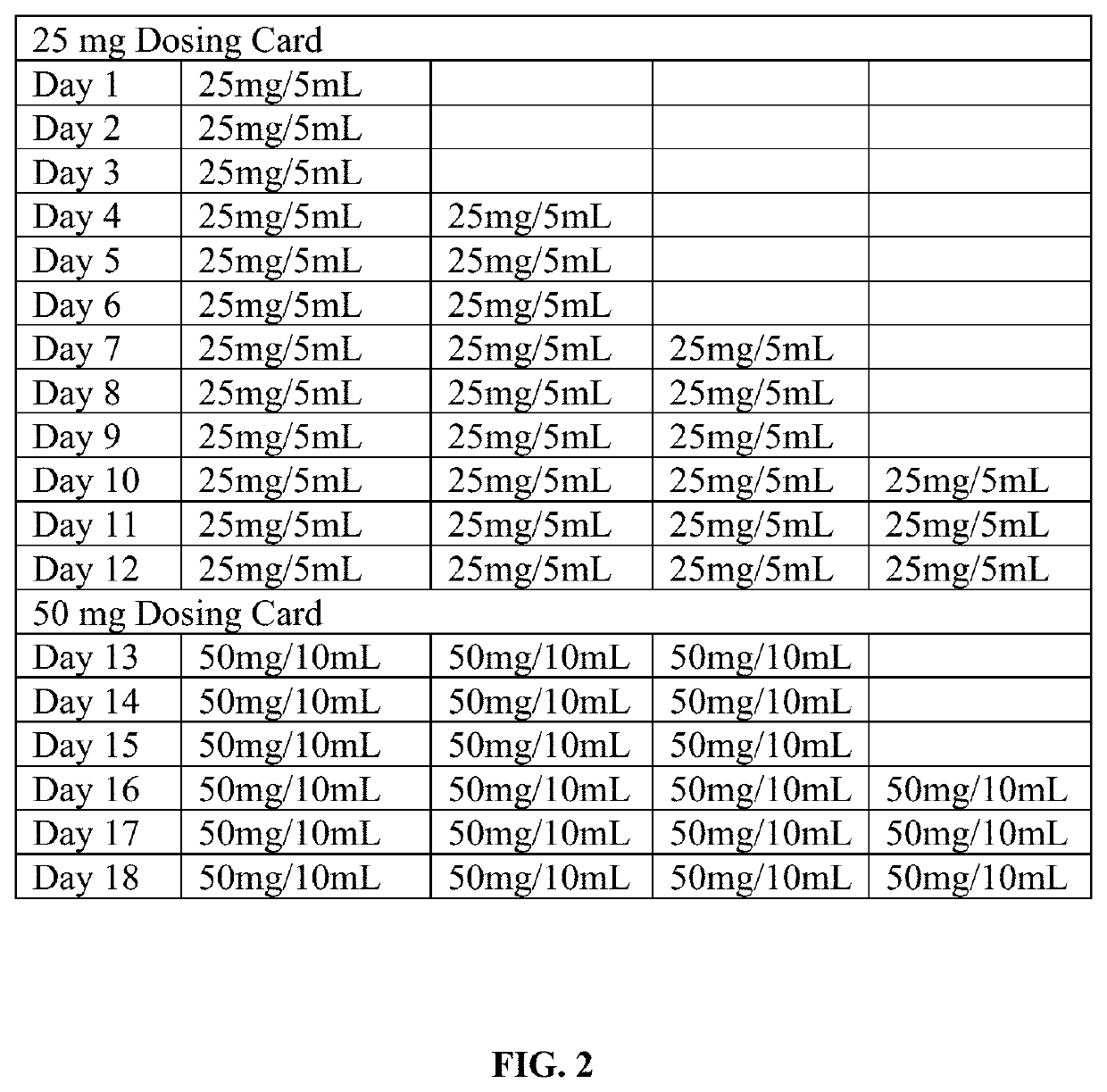
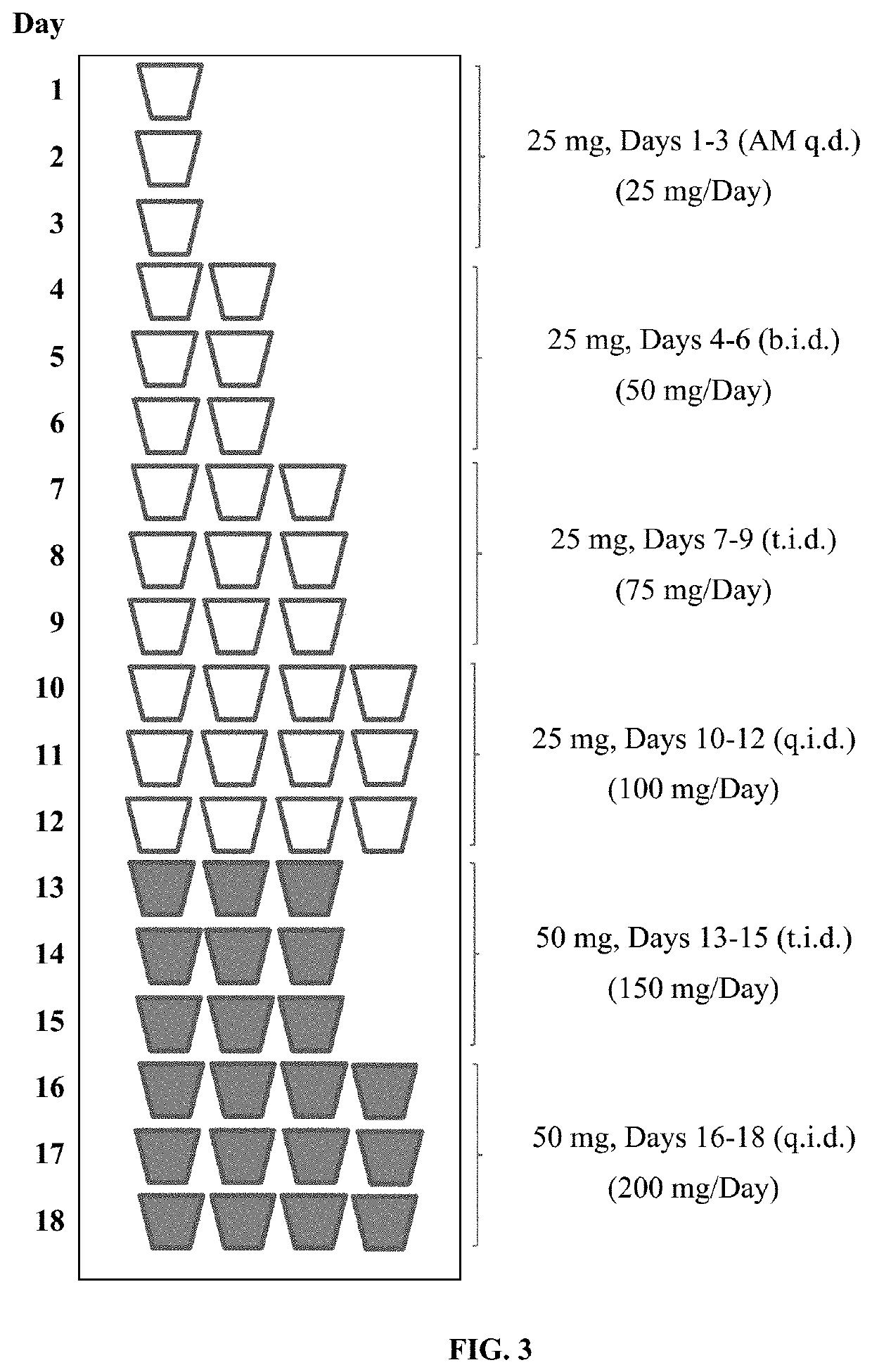
Tramadol hydrochloride solution
ActiveUS11103452B2Tolerability of solution can be improvedImprove toleranceOrganic active ingredientsDispersion deliveryDosing regimenRegimen
Disclosed herein is an analgesic solution for the treatment of pain comprising a pain-relieving effective amount of tramadol or a pharmaceutically acceptable salt thereof, a method of treating pain by administering said analgesic solution to a subject in need thereof, a kit that includes containers of the analgesic solution, and a dosing regimen for the analgesic solution.
Owner:ATHENA BIOSCIENCE LLC
Tramadol hydrochloride solution
ActiveUS20210299045A1Tolerability of solution can be improvedImprove toleranceOrganic active ingredientsDispersion deliveryDosing regimenRegimen
Disclosed herein is an analgesic solution for the treatment of pain comprising a pain-relieving effective amount of tramadol or a pharmaceutically acceptable salt thereof, a method of treating pain by administering said analgesic solution to a subject in need thereof, a kit that includes containers of the analgesic solution, and a dosing regimen for the analgesic solution.
Owner:ATHENA BIOSCIENCE LLC
Tramadol Hydrochloride Solution
ActiveUS20210137835A1Tolerability of solution can be improvedImprove toleranceOrganic active ingredientsDispersion deliveryDosing regimenRegimen
Disclosed herein is an analgesic solution for the treatment of pain comprising a pain-relieving effective amount of tramadol or a pharmaceutically acceptable salt thereof, a method of treating pain by administering said analgesic solution to a subject in need thereof, a kit that includes containers of the analgesic solution, and a dosing regimen for the analgesic solution.
Owner:ATHENA BIOSCIENCE LLC
A kind of pharmaceutical composition and application for alleviating or eliminating opioid withdrawal syndrome
ActiveCN112494486BImprove Medication AdherenceImprove experienceOrganic active ingredientsNervous disorderWithdrawal syndromeUse medication
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Paracetamol and tramadol oral emulsion and preparation method thereof
ActiveCN111840227BGreat tasteAvoid degradation and oxidationOrganic active ingredientsNervous disorderSodium starchSuccinic acid
The invention discloses an oral emulsion of paracetamol tramadol, which is an oil-in-water emulsion formed by uniformly dispersing an oil solution of paracetamol in an aqueous solution of tramadol hydrochloride; the invention also discloses an oral emulsion of paracetamol A preparation method of tramadol oral emulsion, the method firstly adds the oil phase solution containing paracetamol to the mixed solution containing starch sodium octenyl succinate emulsifier, and then adds it to the aqueous phase solution containing tramadol hydrochloride Homogenize in. The present invention avoids the degradation and oxidation of paracetamol and covers its bitter taste by covering the paracetamol in the emulsion, and combines the masking effect of the taste masking agent and the corrective agent and the crosslinking effect of other components to cover the The bitter taste of tramadol hydrochloride improves the stability of oral milk of tramadol and improves the mouthfeel of oral milk of tramadol and enhances its bioavailability; the invention adopts an emulsification encapsulation method to prepare stable oil-in-water Tramadol-type paracetamol oral emulsion, the process is simple.
Owner:陕西九州制药有限责任公司
Adduct of topiramate and tramadol hydrochloride and uses thereof
This invention relates to a new pharmaceutically useful compound which is a stoichiometrically 1:1 adduct of tramadol hydrochloride and topiramate, and to the manufacture and use thereof.
Owner:CILAG GMBH INTERNATIONAL
Preparation method of tramadol hydrochloride sustained-release preparation
InactiveCN108992425AGood slow release functionRelease stabilityAntibacterial agentsOrganic active ingredientsTramadol HydrochloridePolyethylene glycol
The invention provides a preparation method of a tramadol hydrochloride sustained-release preparation. The preparation method comprises the following steps: dissolving tramadol hydrochloride in 95% ethanol to obtain a solution A, and adding hydroxypropyl beta-cyclodextrin and mannitol into the solution A to obtain a solution B; dissolving polylactic acid and polyethylene glycol 200 in acetone to obtain a solution C, mixing the solution B with the solution C to obtain a solution D, transferring the solution D to a magnetic stirrer, continuously stirring the solution D for 12 hours, lowering thetemperature of the solution D to 0-1 DEG C within 2 hours, allowing to stand still for 12 hours, and maintaining the temperature of the solution D at 0-1 DEG C during the standing period; heating thesolution D after standing till for 12 hours, continuously stirring when the temperature of the solution D is raised to 15-18 DEG C, controlling the temperature of the solution D at 15-18 DEG C when stirring, and preparing the tramadol hydrochloride sustained-release preparation with a low-temperature spray drying method after continuously stirring for 12 hours. According to the invention, the dosage of a capsule wall material is moderate, the drying temperature of materials is low, and the prepared drug-loading preparation is uniform in size and stable in drug release, and has the characteristic of slow release.
Owner:刘丽
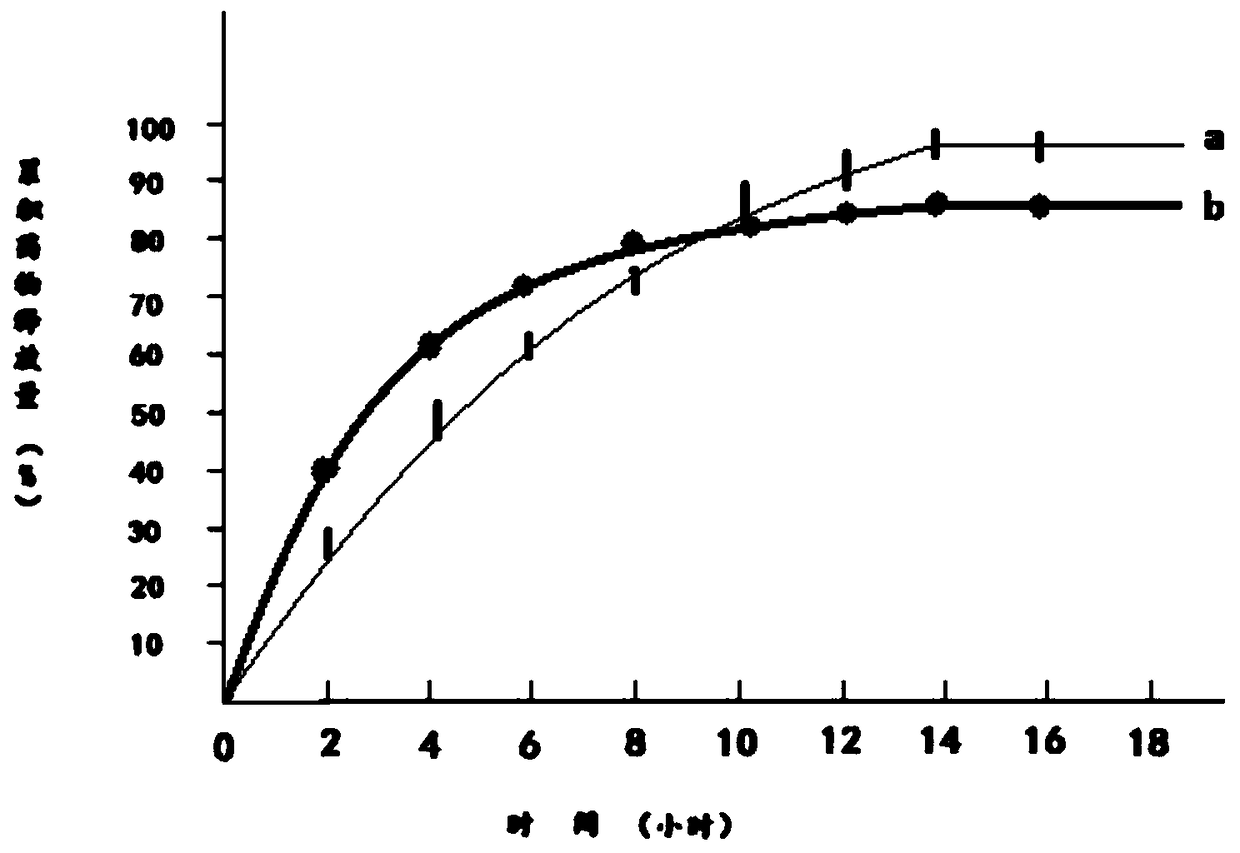
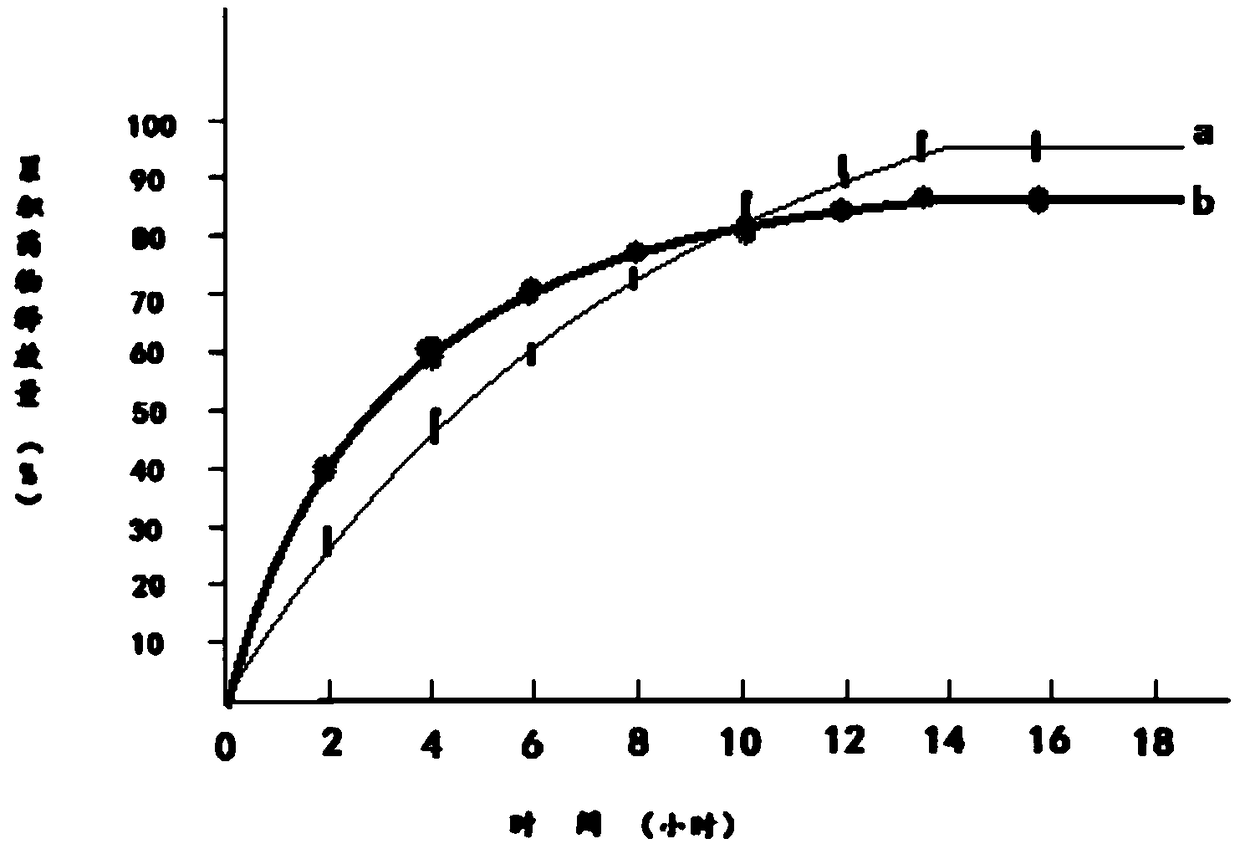
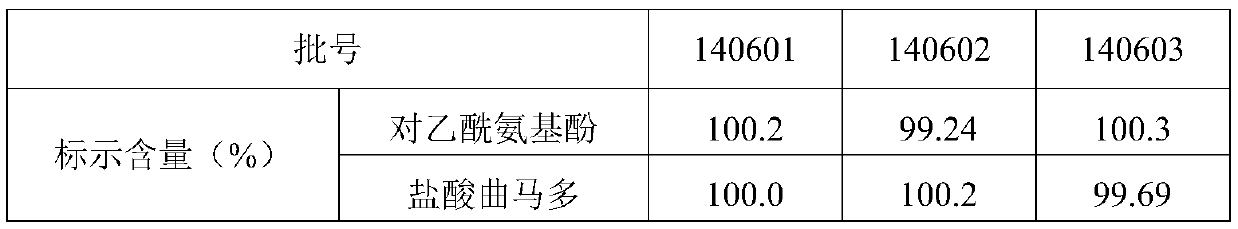
Quality Standards for Tramadol Capsules
ActiveCN106353428BQuality standard scienceQuality Standard SpecificationComponent separationMedicinal chemistryPolypill
Paracetamol and tramadol hydrochloride capsules are a compound preparation made with paracetamol and tramadol hydrochloride and paracetamol. The invention discloses a quality standard for paracetamol and tramadol hydrochloride capsules. Based on a quality standard for paracetamol and tramadol hydrochloride and paracetamol, modifications are made to character indexes, related substance and paracetamol inspection, and dissolution detecting and content measuring method, a quality standard for paracetamol and tramadol hydrochloride capsules is established, and the quality of paracetamol and tramadol hydrochloride capsules is controlled scientifically, regularly and effectively.
Owner:SHANXI GOODDOCTOR PHARMA CO LTD
Intravenous administration of tramadol
ActiveUS20170281533A1Improve securityImproving tolerabilityOrganic active ingredientsNervous disorderDosing regimenRegimen
Owner:REVOGENEX IRELAND
Combination of ibuprofen and tramadol for relieving pain
The present invention relates to the combination of ibuprofen in the form of a pharmaceutically acceptable salt, and tramadol, or a pharmaceutical acceptable salt thereof, for use in the treatment of pain in humans, wherein the dosage of ibuprofen in the combination is comprised between 350 mg and 450 mg and the dosage of tramadol is comprised between 35 mg and 40 mg, expressed as equivalent weight of tramadol hydrochloride. The combination is suitable for the treatment of moderate to severe pain, of chronic or acute origin, and is particularly effective for those patients suffering from more intense pain. It also relates to a pharmaceutical composition comprising said fixed-dose combination of ibuprofen and tramadol.
Owner:FARMALIDER
A kind of preparation method of the coated tablet containing acetaminophen and tramadol hydrochloride
ActiveCN113694051BHigh similarityThe granulation process is easy to operateOrganic active ingredientsAntipyreticCoated tabletsBULK ACTIVE INGREDIENT
The invention discloses a preparation method of a coated tablet containing acetaminophen and tramadol hydrochloride. The preparation method includes the following steps: S1, mixing the active ingredient, filler and disintegrant to obtain a premixed powder; the active ingredient is acetaminophen and tramadol hydrochloride, and the mass ratio of the two is 325:37.5; S2. During the stirring process, add a binder to the premixed powder, and granulate to obtain wet granules; the binder is hypromellose EF or hypromellose E5; S3, the wet granules are dried and sized , and then adding a lubricant for mixing to obtain the blended granules; S4, compressing the blended granules to obtain tablets; S5, coating the tablets to obtain the final product. The granulation process of the present invention is easy to operate, solves the problem of poor compressibility of paracetamol, tablet fragments, etc., and at the same time makes paracetamol and tramadol hydrochloride have a higher similarity, stable preparation process, and convenient for large-scale production , has good bioavailability and quality safety.
Owner:DUODUO PHARMA
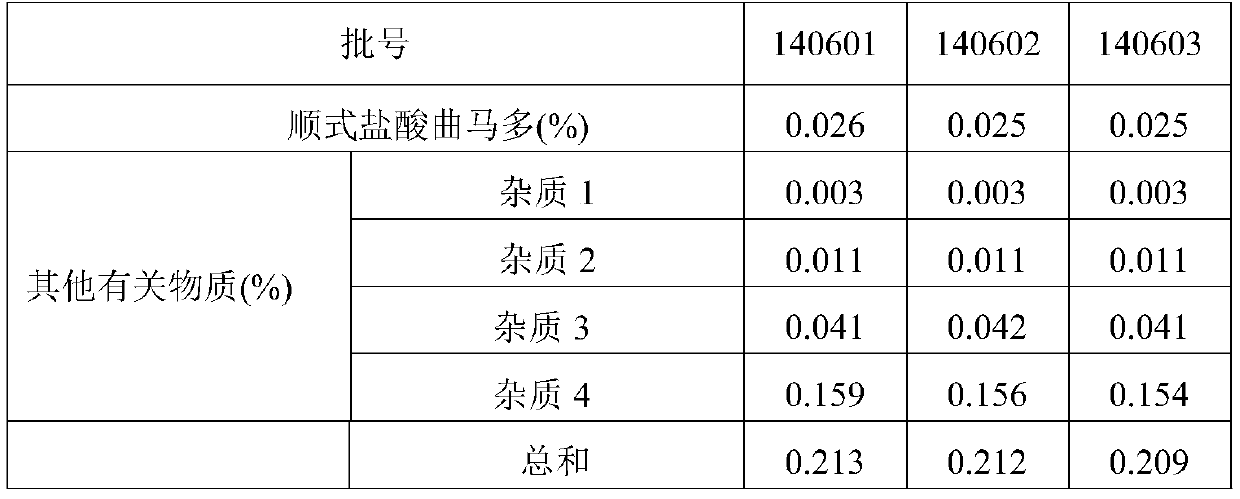
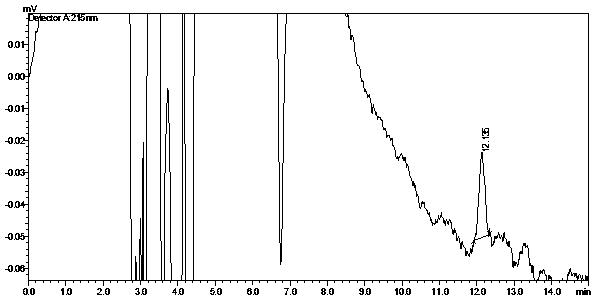
A kind of determination method of related substances of paracetamol tramadol capsules
ActiveCN106525994BQuality improvementImprove detection abilityComponent separationFluid phaseMonopotassium phosphate
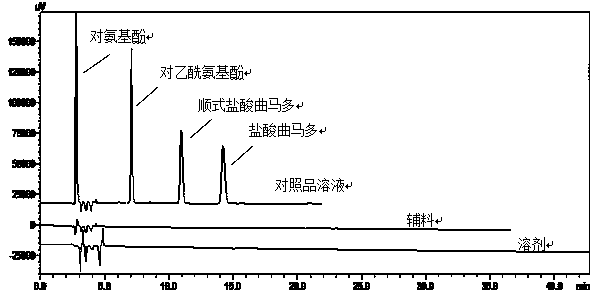
The invention discloses a method for determination of related substances of paracetamol and tramadol hydrochloride capsules; a high performance liquid chromatography (HPLC) is adopted, a chromatographic column with octylsilane bonded silica gel as a filler is used, potassium dihydrogen phosphate and tetrahydrofuran are used as a mobile phase, chromatographic conditions are screened and improved, and an HPLC reference substance method is added for inspecting the content of cis-tramadol hydrochloride; the inspecting ability is improved, the limits of cis-ramadol hydrochloride and other related substances are formulated, the quality of the paracetamol and tramadol hydrochloride capsules is effectively controlled, and the safety and effectiveness of the paracetamol and tramadol hydrochloride capsules are ensured.
Owner:SHANXI GOODDOCTOR PHARMA CO LTD
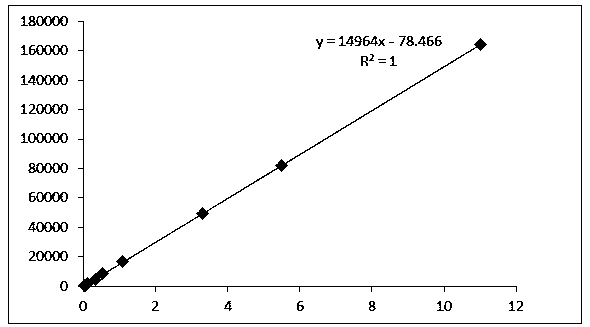
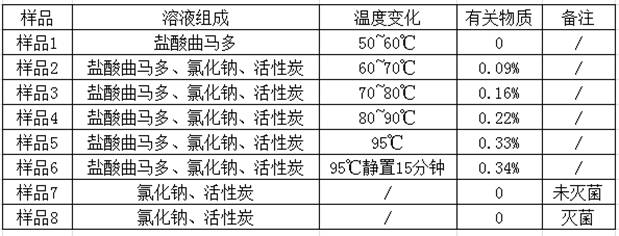
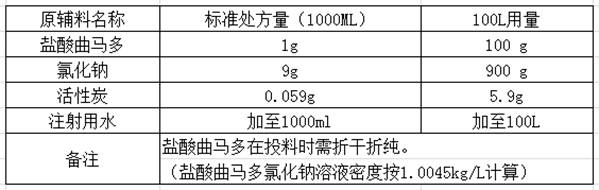
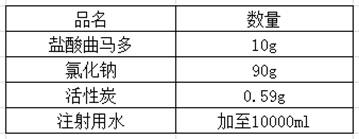
Preparation method of tramadol hydrochloride sodium chloride injection
InactiveCN112353758AAvoid direct contactLow content of related substancesOrganic active ingredientsNervous disorderActivated carbonSodium Chloride Injection
The invention discloses a preparation method of tramadol hydrochloride sodium chloride injection, and the method comprises the following steps: weighing tramadol hydrochloride, sodium chloride and activated carbon according to the preparation amount and the amount after drying and purification, adding a proper amount of water for injection into a concentrated preparation tank, adding the weighed sodium chloride, performing stirring until the sodium chloride is uniformly dissolved, adjusting the pH value of the solution in the concentrated preparation tank to 4.5-5.5 by using 10% diluted hydrochloric acid, adding weighed activated carbon into the concentrated preparation tank, performing heating to boil to 95 DEG C or above, standing and adsorbing for 10-20 minutes, circularly performing filtering and decarbonizing into a diluted preparation tank, finally adding weighed tramadol hydrochloride into the diluted preparation tank, fixing the volume, and performing stirring until the tramadol hydrochloride is completely dissolved; and circularly performing filtering to obtain the product tramadol hydrochloride sodium chloride injection. According to the tramadol hydrochloride sodium chloride injection produced by the process, the content of related substances is obviously reduced and can be as low as 0.1% or below, and the content of the related substances is basically kept unchangedin each stage of acceleration test in the later period.
Owner:BIOZEN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com