Quality Standards for Tramadol Capsules
A technology of tramadol acetaminophen and quality standards, applied in the field of quality standards of tramadol acetaminophen capsules, achieving the effect of scientific quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Materials and reagents:
[0044] Instruments: Agilent 1200 high performance liquid chromatography, Mettler XS-205 electronic balance;
[0045] Reagents: Methanol and tetrahydrofuran are chromatographically pure, triethylamine, phosphoric acid and potassium dihydrogen phosphate are analytically pure, and water is self-made ultrapure water;
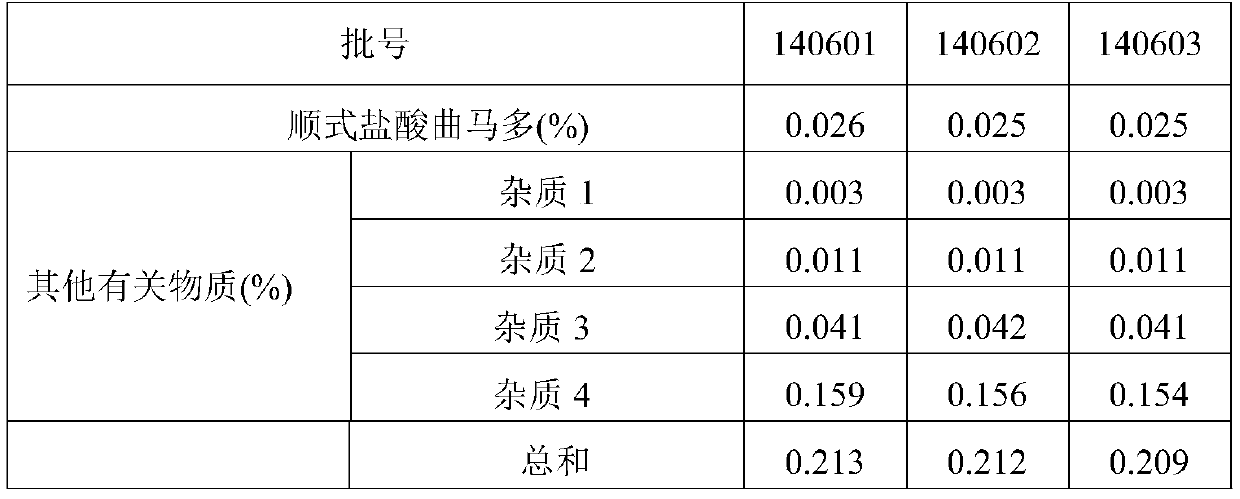
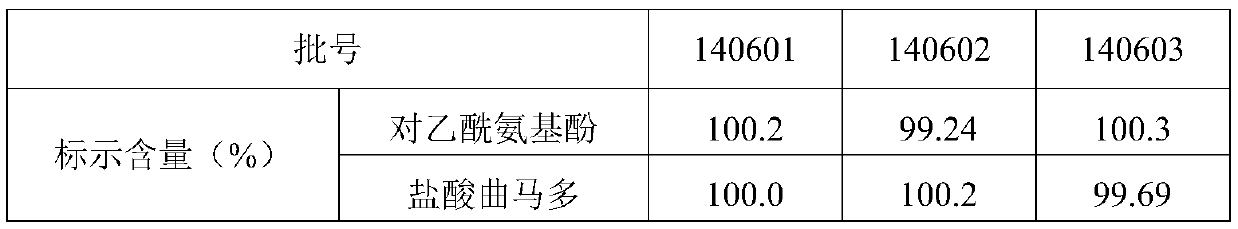
[0046] Sample: Tramadol paracetamol capsules (140601, 140602, 140603), provided by Shanxi Good Doctor Pharmaceutical Co., Ltd.;
[0047] Reference substances: paracetamol (batch number: 100018-200408), tramadol hydrochloride (batch number: 171242-201005), cis-tramadol hydrochloride (batch number: 171255-201103), p-aminophen (batch number: 100802-201002 ), from China National Institute for the Control of Pharmaceutical and Biological Products and China National Institute for Food and Drug Control.
[0048] 2. Experimental content:
[0049] 2.1 Characters:
[0050] Take a few capsules of the test product from three batches respect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com