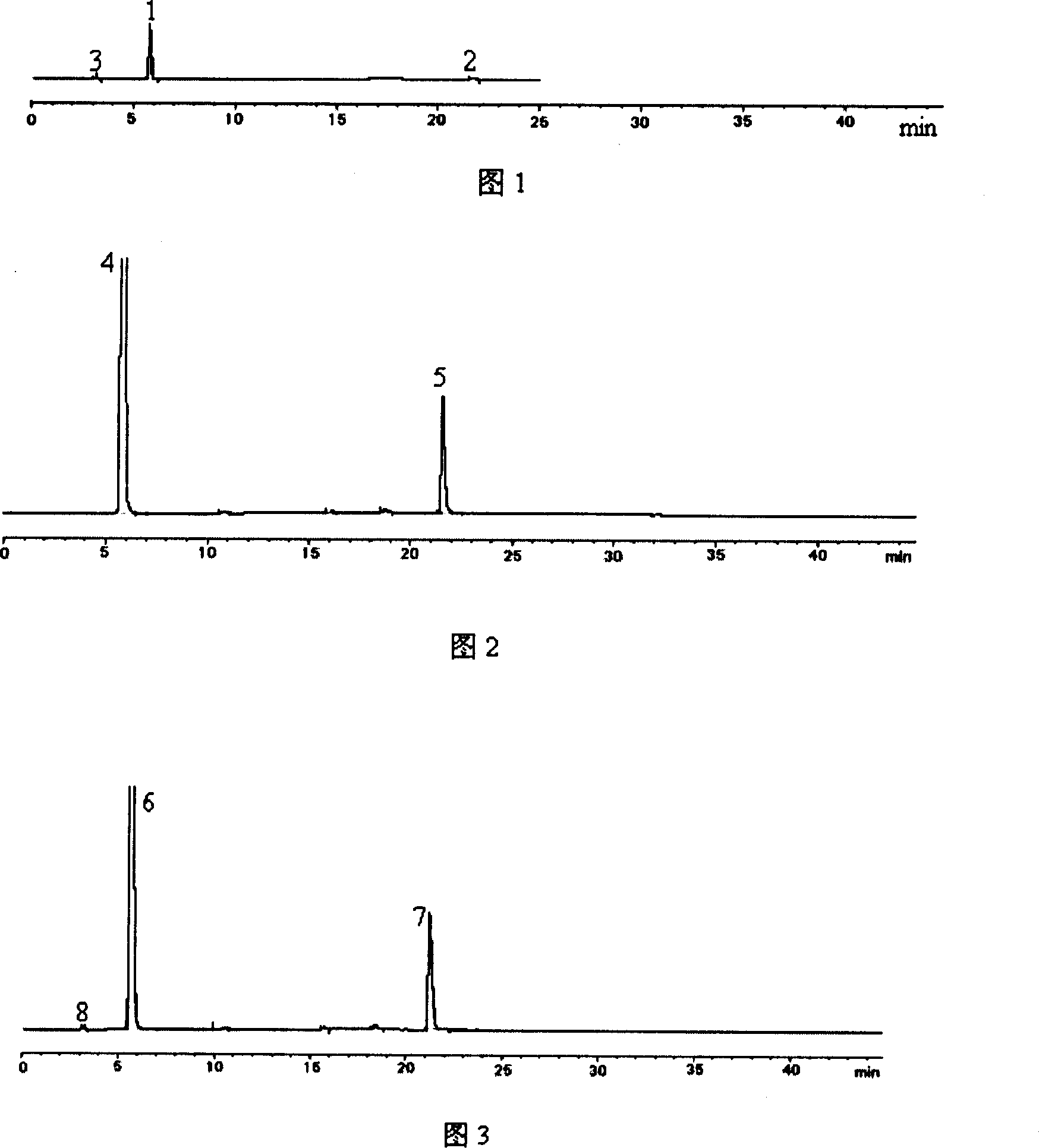
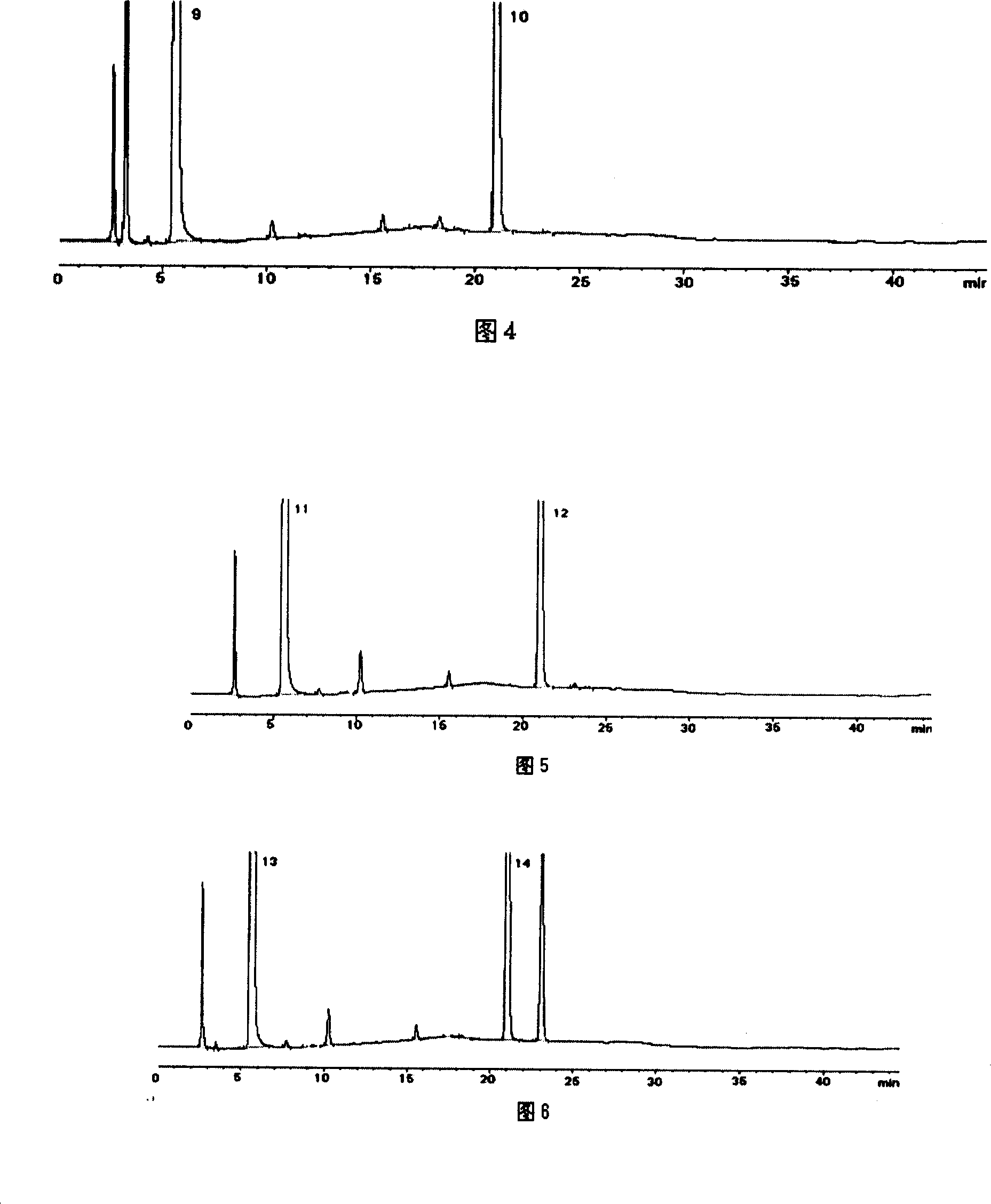
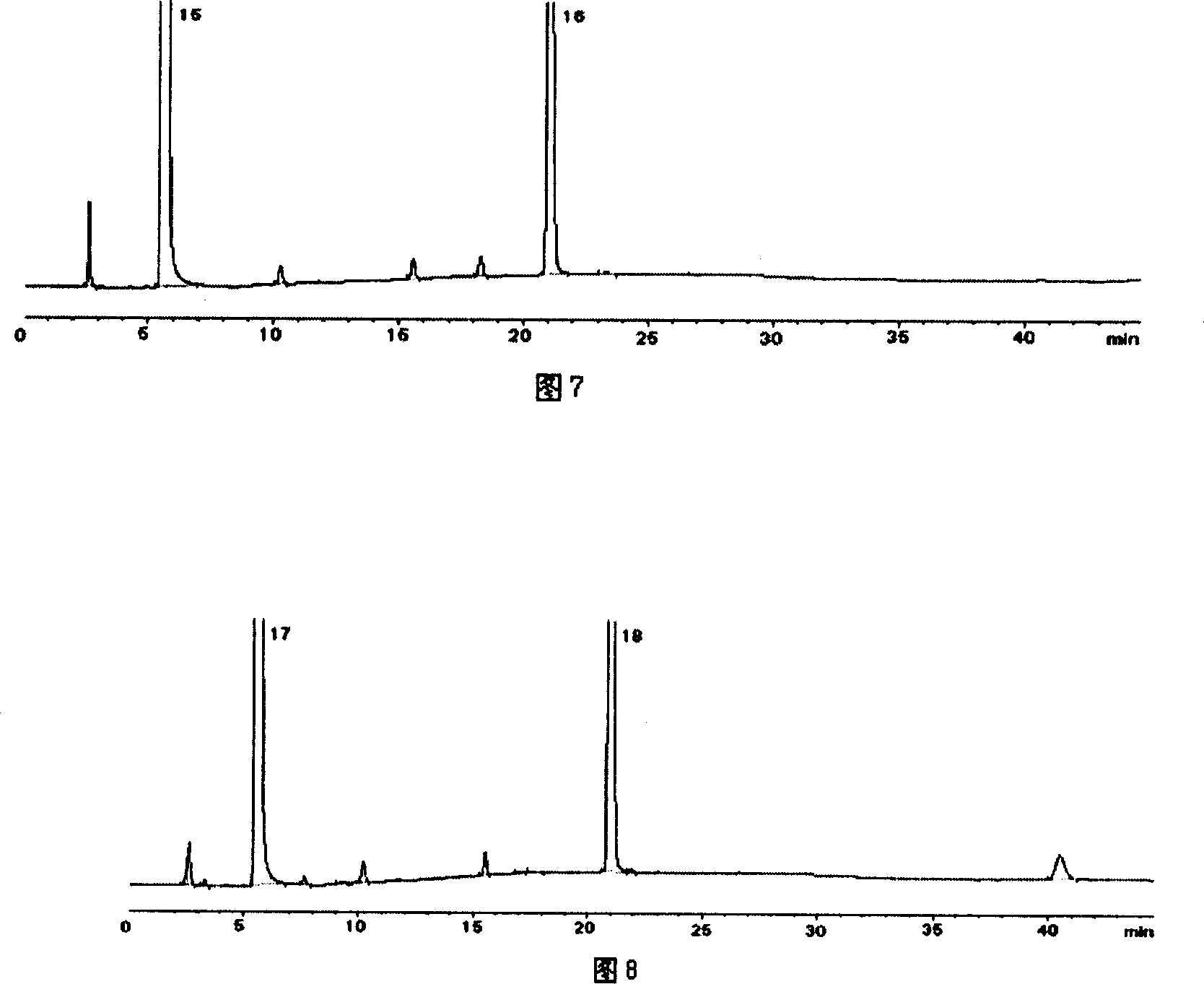
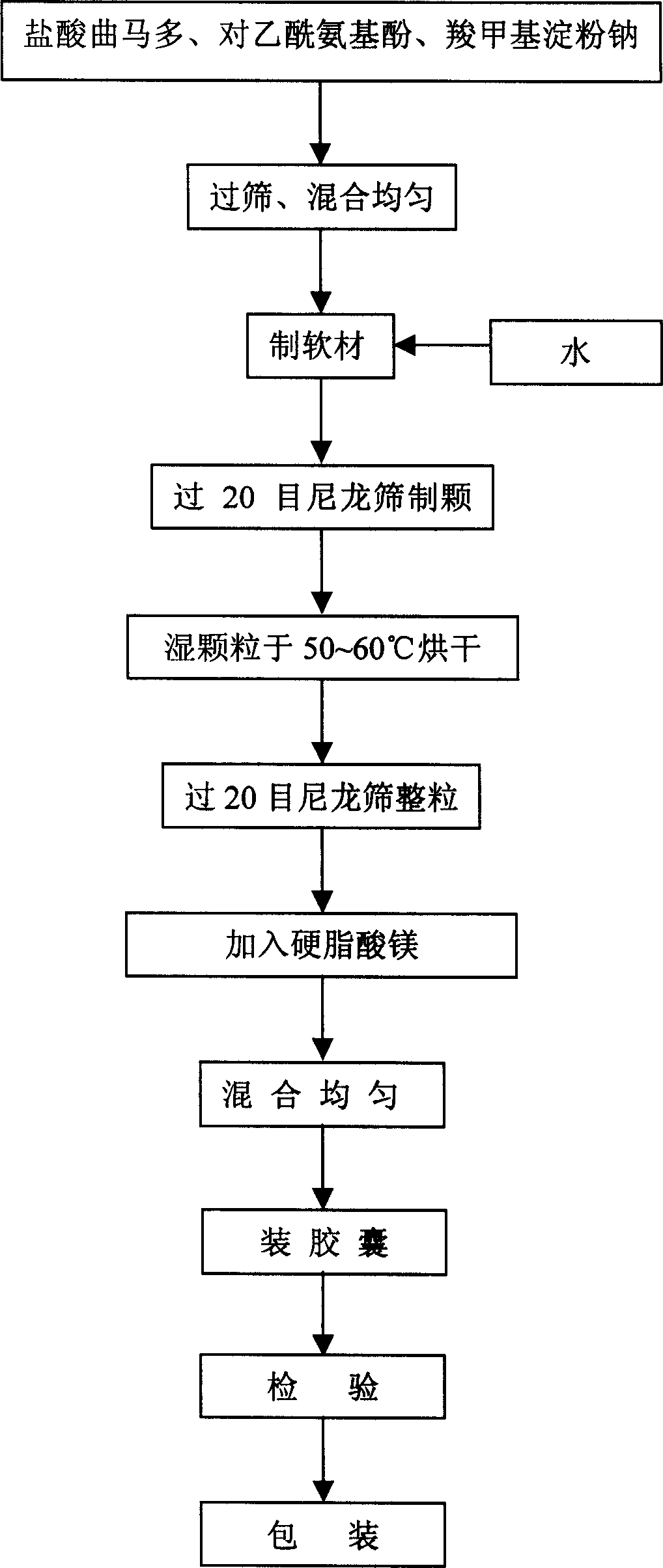
Patents
Literature
43 results about "Tramadol Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
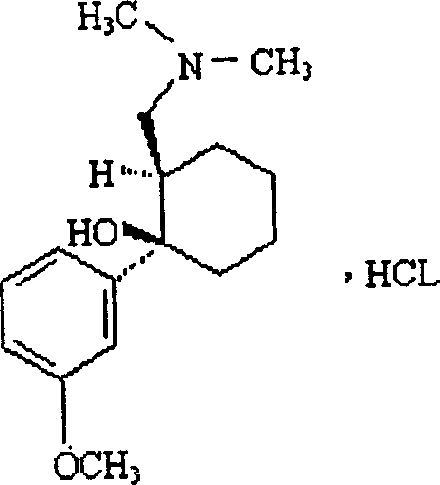
A synthetic codeine analogue, Tramadol Hydrochloride has central analgesic properties with effects similar to opioids, such as morphine and codeine, acting on specific opioid receptors. The hydrochloride salt of Tramadol and used as a narcotic analgesic for severe pain, it can be addictive and weakly inhibits norepinephrine and serotonin reuptake. (NCI04)
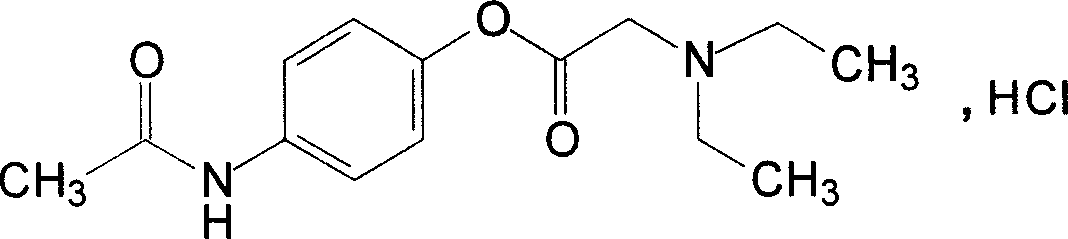
Direct bilirubin detecting kit
ActiveCN1967252AWide linear test rangeHigh precisionMicrobiological testing/measurementColor/spectral properties measurementsThioureaPhosphate
The invention discloses a direct bilirubin detects reagent kit, and the kit is liquid-type double-reagent comprising reagent 1 and reagent 2. The reagent 1 is: tartaric acid-NaOH buffer solution (pH 2.6~3.4) 7.5~37.5g / L, ethylenediamine tetraacetic acid disodium 0.2~1g / L, thiourea 0.1~1.4g / L, hydroxylammonium 0.2~1.2g / L, tramadol hydrochloride 1001.5~7.5 ml / L. the reagent 2 is: Na2HPO4 1~5.5g / L, potassium dihydrogen phosphate 0.25~1.5g / L, sodium nitrite 0.15~0.7g / L, ethylenediamine tetraacetic acid disodium 2~13g / L, NaCl 4~22.5g / L, thiomersal 0.04~0.3g / L. The reagent kit of the invention has the advantages of wide linear test range, high precision and accuracy, good interference-resistance, and high sensitivity, and it has greater clinical practical value.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Pharmaceutical composition for injection and its medicine box
InactiveCN101147735AImprove securityLow vasoirritant effectOrganic active ingredientsNervous disorderTramadol HydrochloridePropacetamol
The present invention relates to an injection medicine composition containing propacetamol hydrochloride and tramadol hydrochloride. It is characterized by that said injection medicine composition also contains sodium citrate so as to raise the safety of said injection medicine composition.
Owner:沈阳华泰药物研究有限公司
Analgesic tramadol hydrochloride oral disintegrating tablet and preparing method thereof
InactiveCN1528273AEasy to takeReduce adverse reactionsOrganic active ingredientsAntipyreticFreeze-dryingTramadol Hydrochloride
The present invention relates to an analgesic tramadol hydrochloride oral disintegrant tablet. It is made up by using main medicine tramadol hydrochloride and auxiliary material gelatin and / or mannitol and / or aspartame and / or glucose and / or microcrystal cellulose and / or crosslinked povidone and essence, etc. and adopting freeze-drying or coating, granulating and pressing processes. When it is taken in, it has no need of drinking water, it can be quickly disintegrated in oral cavity, and can be used for curing acute and chronic pain, moderate and light pain due to cancer, pain due to fracture and pain after various operations and toothache, and can obtain good therapeutic effect.
Owner:周宇
Method for determining impurities for paracetamol and tramadol hydrochloride preparation
ActiveCN101025409ADetection contentMeet the needs of relevant partiesComponent separationTramadol HydrochloridePhenol
The invention discloses a method to measure contaminant of acet amidogen phenol and tramadol hydrochloride preparation. Using this invention, it can be simple, rapid and accurate detect pair amidogen phenol content in the preparation, the detection error of only 0.4%, while it can detect others unknown impurity gross, the invention will be able to meet the needs of the parties concerned.
Owner:SHANGHAI HUA SURNAME PHARMA
Oral disintegrating tablet containing tramadol hydrochloride and acetaminopher, and its preparation method
ActiveCN1803128AMeet the requirement of rapid disintegrationImprove liquidityOrganic active ingredientsNervous disorderOrally disintegrating tabletTramadol Hydrochloride
The invention provides an orally disintegrating tablet containing tramadol hydrochloride and paracetamol, and the preparing process, wherein tramadol hydrochloride and paracetamol are dressed for mouth cavity use. During the dressing process, right amount of medicinal auxiliary materials are charged.
Owner:SHANGHAI SINE PHARMA LAB
Composite tramadol hydrochloride formulation and preparation process thereof
InactiveCN1762340AAchieve synergistic pain reliefReduce dosageOrganic active ingredientsNervous disorderCarboxymethyl starchTramadol Hydrochloride
The invention relates to a composite tramadol hydrochloride formulation and preparation process, wherein the compound preparation can be made into the forms of capsules or dispersing tablets, each 1000 capsules or 1000 dispersing tablets comprise tramadol hydrochloride 40-45g, acetaminopher 300-350g, sodium carboxymethyl starch 7.5-25g, and magnesium stearate 2.5-5g.
Owner:周卓和
Oral disintegration tablet of tramadol hydrochloride and preparation method
InactiveCN1660056AFast absorptionQuick effectOrganic active ingredientsNervous disorderTramadol HydrochlorideOrally disintegrating tablet
An orally disintegrating tablet of tramadol hydrochloride is prepared through preparing the dispersed solid from tramadol hydrochloride and dispersing solid carrier, mixing it with filler, disintegrant, flavouring and lubricant, stirring and tabletting.
Owner:HENAN UNIVERSITY +1
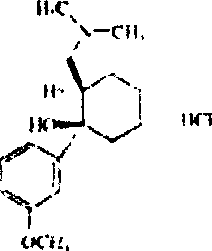
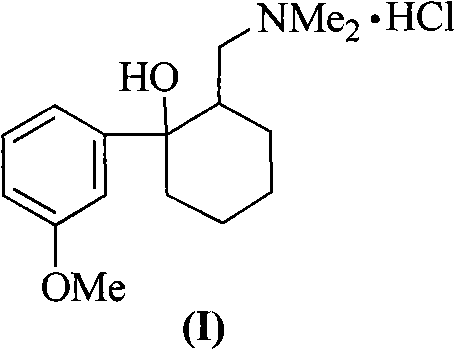
Tramadol, salts thereof and process for their preparation
Owner:RUSSINSKY
Tramadol hydrochloride and paracetamol combined injection
The present invention relates to an injection preparation formed from tramadol hydrochloride and paracetamol. It is a new analgetic medicine with the effect of quickly-stopping pain.
Owner:陈旭良
Method for determination of related substances of paracetamol and tramadol hydrochloride capsules
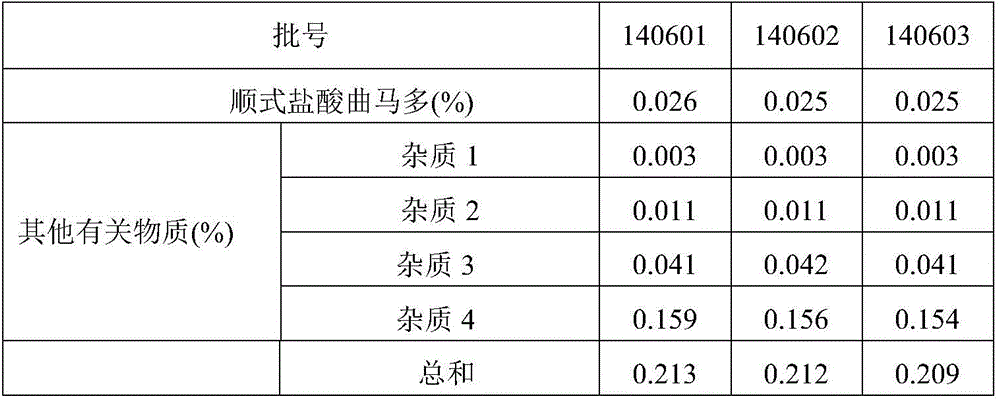
ActiveCN106525994AQuality improvementImprove detection abilityComponent separationFiller ExcipientTramadol Hydrochloride
The invention discloses a method for determination of related substances of paracetamol and tramadol hydrochloride capsules; a high performance liquid chromatography (HPLC) is adopted, a chromatographic column with octylsilane bonded silica gel as a filler is used, potassium dihydrogen phosphate and tetrahydrofuran are used as a mobile phase, chromatographic conditions are screened and improved, and an HPLC reference substance method is added for inspecting the content of cis-tramadol hydrochloride; the inspecting ability is improved, the limits of cis-ramadol hydrochloride and other related substances are formulated, the quality of the paracetamol and tramadol hydrochloride capsules is effectively controlled, and the safety and effectiveness of the paracetamol and tramadol hydrochloride capsules are ensured.
Owner:SHANXI GOODDOCTOR PHARMA CO LTD
Gel material for preventing intestinal adhesion
InactiveCN102793952ARelieve inflammationImprove isolationOrganic active ingredientsAerosol deliveryIsolation effectFibrin blood clot
The invention relates to a gel material for preventing intestinal adhesion, which is used for preventing peritoneal adhesion after operation. Hyaluronic acid crosslinking preparations for isolating damaged wounds can cause severe adverse reactions during application, such as abdominal pain, abdominal infection, and intestinal adhesion, and even reoperation is performed due to adverse reactions for some patients; the hyaluronic acid crosslinking preparations also have a dissociation phenomenon in the body, and the isolation effect is reduced. The gel material for preventing intestinal adhesion of the invention is a gel system formed by polyacrylamide, a hydrotalcite colloid material, iron ion crosslinked hyaluronic acid gel liquid, 2-deoxy-D-glucose (2-DG) with anti-inflammation and pain-relieving efficacy, tramadol hydrochloride, and acetaminophen. After operation, the gel material is coated directly on a wound; crosslinking effect is generated with fibrin blood clots on the wound; thus the gel material is firmly adhered to the wound; good isolation effect is reached. The medicinal components with anti-inflammation pain-relieving efficacy are directly applied to the wound; and inflammation caused by singly application of hyaluronic acid isolating substances is alleviated.
Owner:SHANDONG PROVINCIAL HOSPITAL
Compound Tramadol Hydrochoride dispersible Tablet
InactiveCN1666736AOrganic active ingredientsNervous disorderAdditive ingredientTramadol Hydrochloride
The invention relates to one agent type of compound tramadol hydrochloride. The provided technical project produces tramadol hydrochloride and acetyltropolone dispersible tablet with following components in weight fractions: tramadol hydrochloride in 37.5 deals; acetyltropolone in 325 deals; filling agent in 15-25 deals selected from lactose, compressible starch, white dextrine and / or starch; disintegrating adminicle in 50-70 deals selected from crosslinked polyvinyl pyrrolidone, microcrystalline cellulose, L-HPC, sodium carboxymethylstarch and / or compressible starch; lubricating agent in 1-2.5 deals selected from magnesium or / and talcum powder. The time of dispersing uniformly of provided dispersible tablet can reach 1 minutes and the time limit of disintegrating can reach 2 minutes.
Owner:黄本东
Chewing tablet of tramado hydrochloride and its preparation method
ActiveCN1732910AGreat tastePromote absorptionOrganic active ingredientsNervous disorderAdjuvantTramadol Hydrochloride
The invention provides a novel preparation using tramadol hydrochloride and paracetamol compositions as the main medicaments, i.e. aminophen tramadol chewing tablet. The weight ratio of tramadol hydrochloride and paracetamol is 0.1:1-0.2:1, and the adjuvant employs medicinal excipient, which includes 50.1-83.7% of water-soluble bulking agent, crumbling agent, lubricating agent, flavoring agent, moistening agent or binding agent.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD
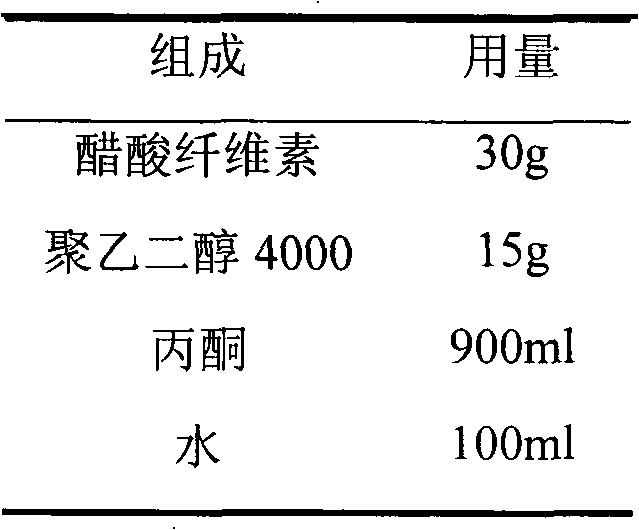
Transdermal plaster prepn of Tramadol hydrochloride
The present invention discloses one kind of transdermal plaster preparation of Tramadol hydrochloride. The plaster preparation of Tramadol hydrochloride is used in ultrasonic administrating system, ion introducing administrating system, electroporating administrating system and high pressure gas administrating system, to treat various kinds of chronic pain, post-operational pain and cancer pain. It may be also used in drug addiction eliminating treatment.
Owner:陶燃
Trimetazidine hydrochloride double-layer osmotic pump controlled-release tablet and preparation method
ActiveCN103735528BSmall toxicityAvoid peaks and valleys in blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectTramadol Hydrochloride
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Tramadol hydrochloride slow release dripping pill and its preparing method
InactiveCN1820738AGood analgesic effectLess drug dependenceOrganic active ingredientsNervous disorderTramadol HydrochlorideDrug product
The present invention relates to pain relieving medicine preparation, and is especially one orally taken slow released preparation with tramadol hydrochloride as main material. The present invention provides slow released tramadol hydrochloride dripping pill superior to available technology. The slow released tramadol hydrochloride dripping pill has the advantages of controllable releasing period, complete medicine release and high bioavailability.
Owner:北京博智绿洲医药科技有限公司
Tramadol hydrochloride drops and their preparation
InactiveCN1582908ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsNervous disorderTramadol HydrochloridePharmacology
A dripping pill of tramadol hydrochloride is prepared by ultrafine pulverizing and conventional process for preparing dripping pills.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
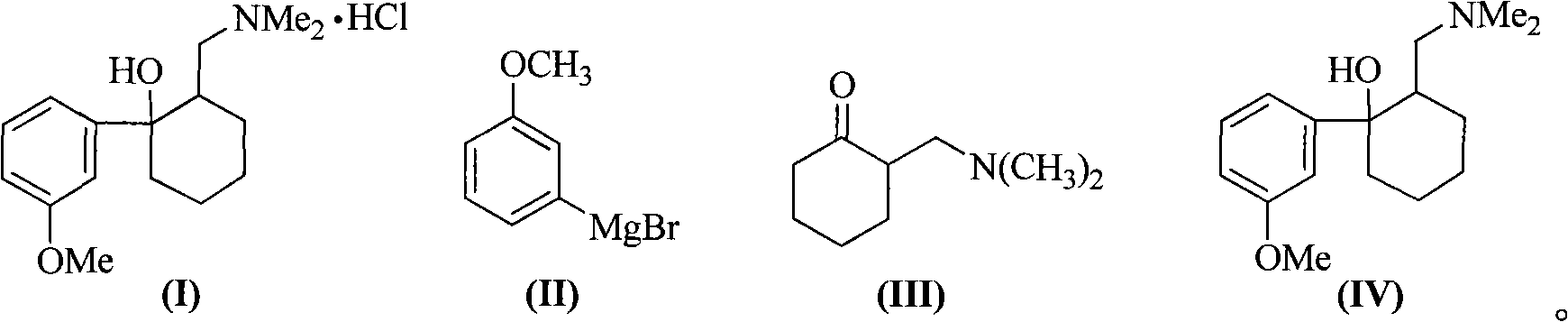
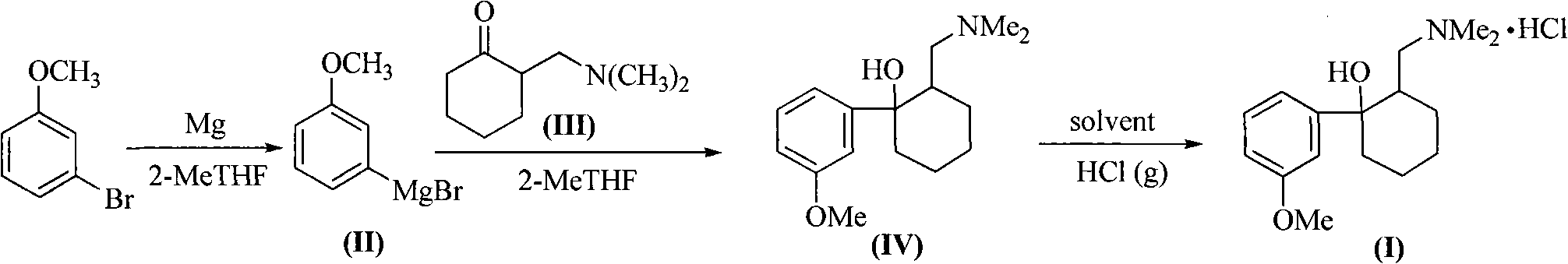
Method for synthesizing tramadol hydrochloride
ActiveCN101265201AEasy to recycleReduce solubilityNervous disorderOrganic compound preparationAlcoholTramadol Hydrochloride
The invention discloses a synthesis method of tramadol hydrochloride. The synthesis method comprises the steps as follow: Grignard reagent that is m-methoxy phenyl magnesium bromide is manufactured in 2-methyltetrahydrofuran that is green solvent, then the Grignard reagent reacts with 2-dimethylamino methyl cyclohexanone to obtain crude products which are salified in hydrochloric alcoholic solution, so as to manufacture the tramadol hydrochloride. Compared with the traditional technology, the synthesis method has the advantages that the 2-methyltetrahydrofuran that is green solvent is adopted in the Grignard reaction, the reaction yield rate is higher, products are easy to separate and purify, the solvent consumption is less and easy to be recycled and reused, the environmental pollution is small, and the synthesis method is suitable for industrialized production.
Owner:DUODUO PHARMA
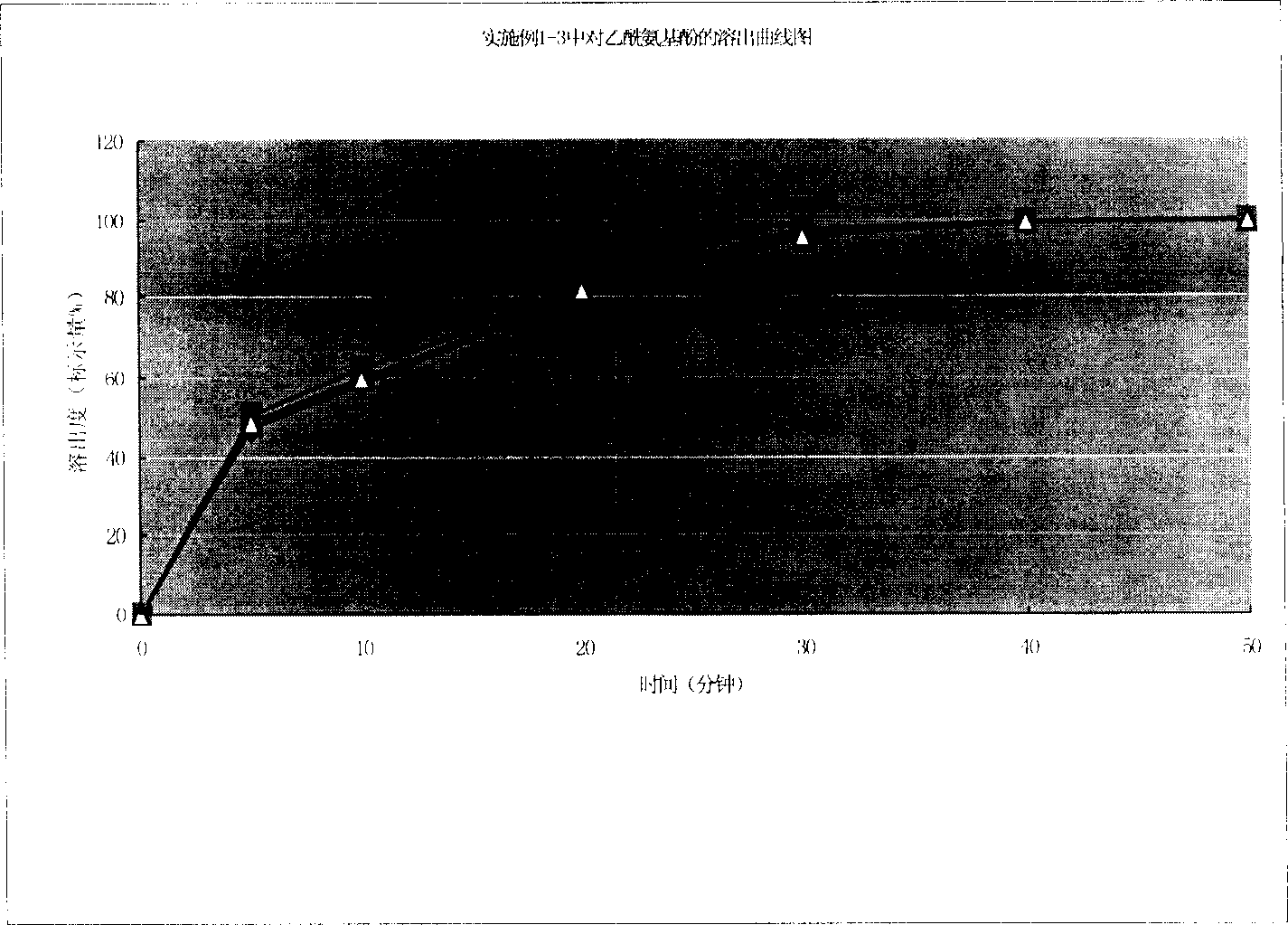
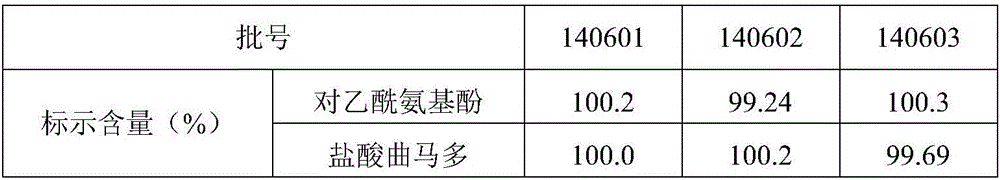
Quality standard for paracetamol and tramadol hydrochloride capsules
ActiveCN106353428AQuality standard scienceQuality Standard SpecificationComponent separationTramadol HydrochlorideDissolution
Paracetamol and tramadol hydrochloride capsules are a compound preparation made with paracetamol and tramadol hydrochloride and paracetamol. The invention discloses a quality standard for paracetamol and tramadol hydrochloride capsules. Based on a quality standard for paracetamol and tramadol hydrochloride and paracetamol, modifications are made to character indexes, related substance and paracetamol inspection, and dissolution detecting and content measuring method, a quality standard for paracetamol and tramadol hydrochloride capsules is established, and the quality of paracetamol and tramadol hydrochloride capsules is controlled scientifically, regularly and effectively.
Owner:SHANXI GOODDOCTOR PHARMA CO LTD
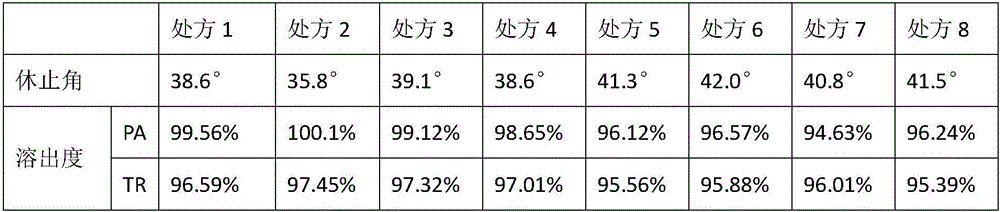
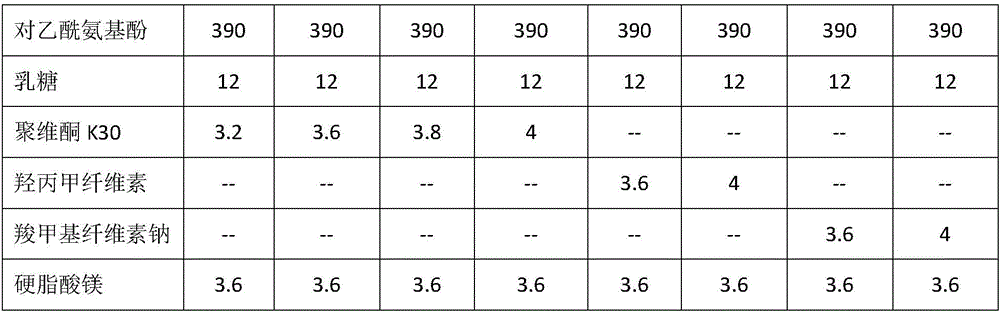
Preparation containing tramadol hydrochloride and paracetamol and preparation method of preparation
ActiveCN106214671AEasy to prescribeQuality improvementOrganic active ingredientsNervous disorderMaterials preparationTramadol Hydrochloride
The invention provides a preparation containing tramadol hydrochloride and paracetamol and a preparation method of the preparation. The preparation is prepared from, by weight, 45 parts of tramadol hydrochloride, 390parts ofparacetamol, 10-15parts oflactose, 3.2-4parts ofpovidone K30 and 3-4.2parts ofmagnesium stearate. The preparation method includes the steps of crushing, sieving, weighing, material preparation, granulating, whole mixing, intermediate inspection, filling, polishing or tableting, aluminum-plastic package and the like. The preparation is simple in prescription, the usage amount of the auxiliary materials is small, and it is proved through a long-term test and an acceleration test that the related substance content change in the preparation obtained by adopting the preparation process is small, the tramadol hydrochloride and paracetamol content is basically unchanged, and no p-aminophenol is detected out.
Owner:SHANXI GOODDOCTOR PHARMA CO LTD
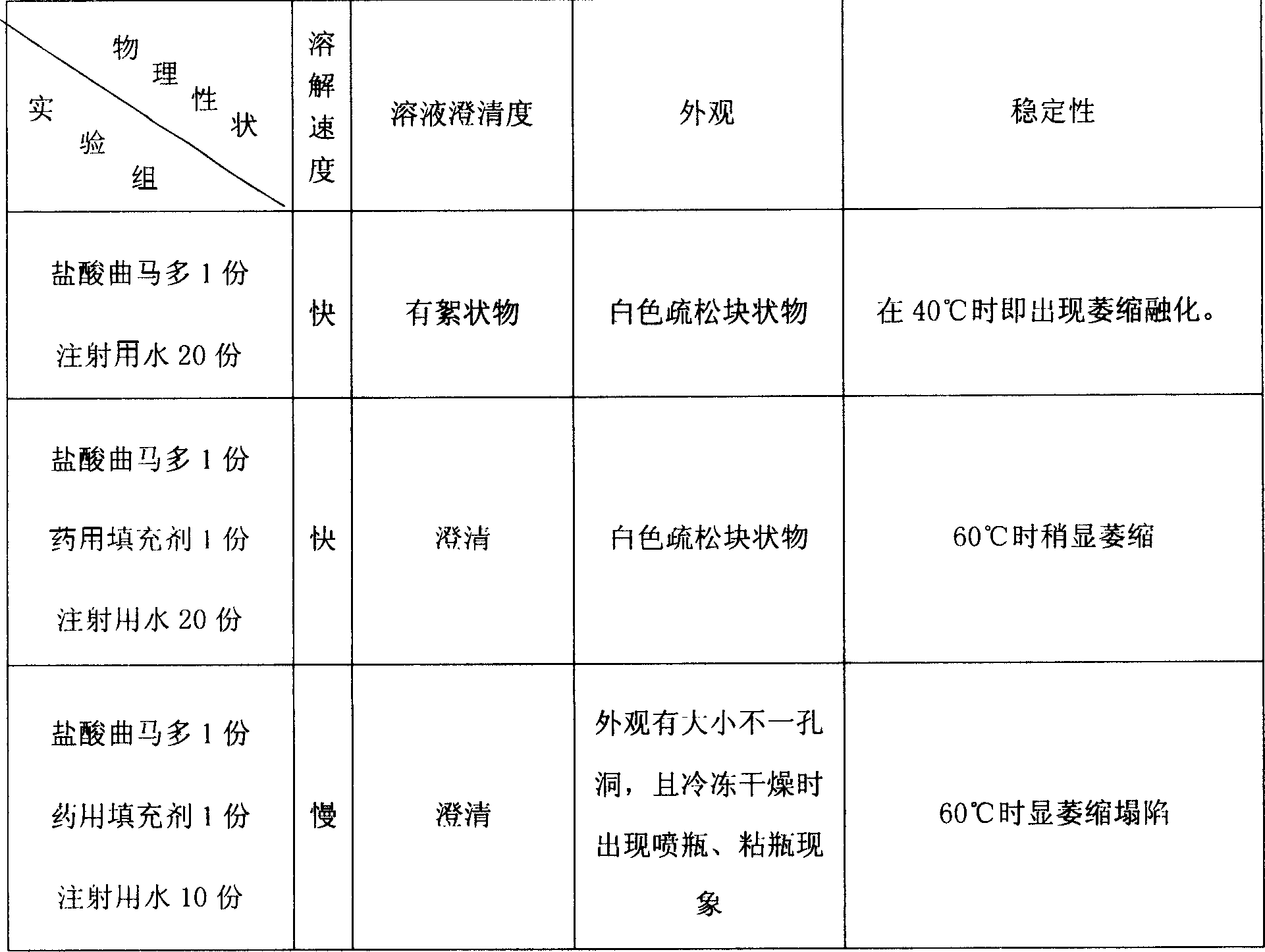
Freeze dried powder injection of tramadol hydrochloride and its preparation process
ActiveCN1736370AImprove stabilityStable storagePowder deliveryOrganic active ingredientsTramadol HydrochlorideFiller Excipient
The invention discloses a freeze dried powder injection of tramadol hydrochloride and its preparation process, wherein the powder injection contains effective amount of tramadol hydrochloride and corresponding medicinal filling agent and water for injection. The preparing process comprises the following steps, (1) dissolving tramadol hydrochloride raw material and filling agent with right amount of water for injection, filtering and loading, (2) pre-freezing to -45 to -40 deg C, thermally insulating 3 hours, slowly elevating temperature to 25 deg C, (3) thermally insulating 2-3 hours at 25 deg C., finally carrying out germ-free fusion sealing.
Owner:CSPC OUYI PHARM CO LTD
Compound anesthetic for cats and preparation method thereof
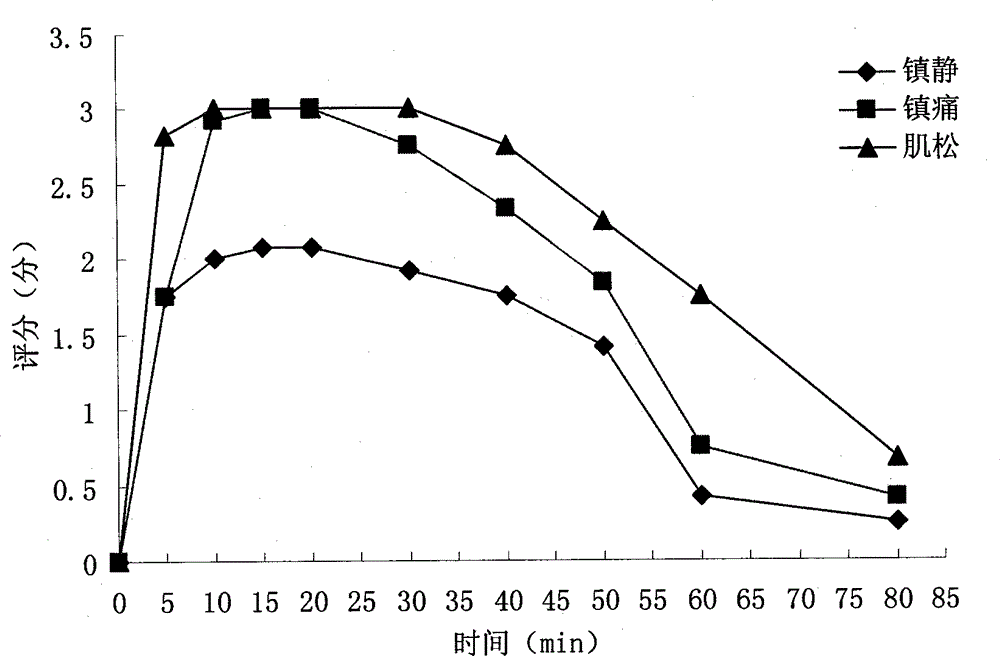
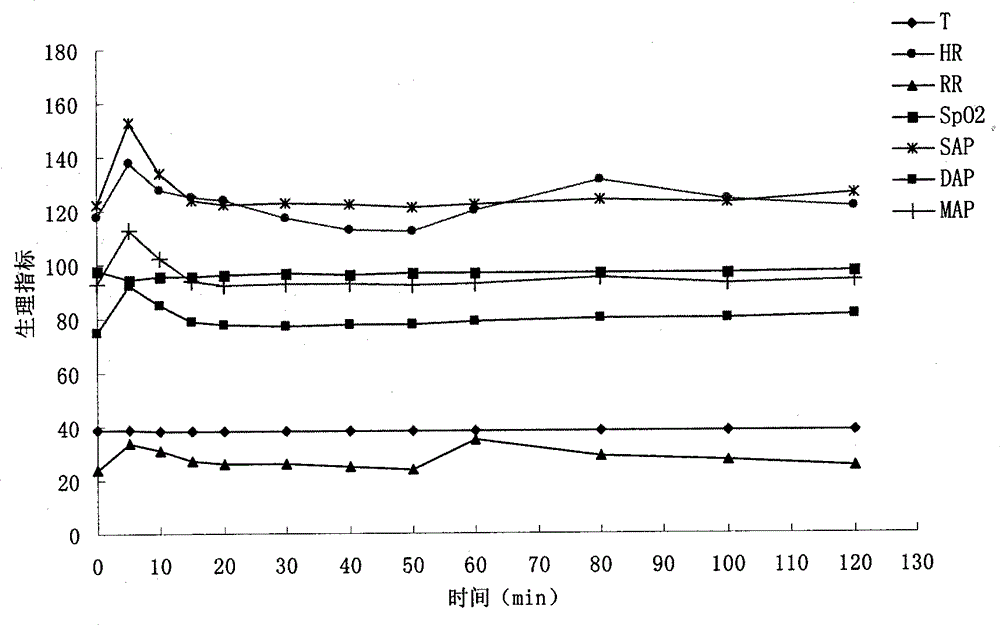
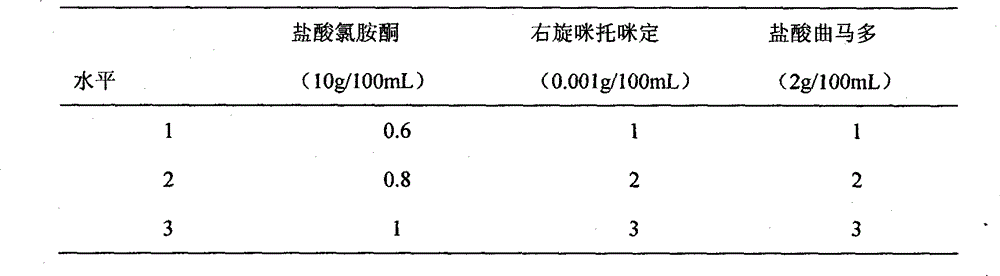
InactiveCN102920708BQuick effectFast redistributionOrganic active ingredientsNervous disorderSide effectTramadol Hydrochloride
The invention belongs to veterinary medicine preparation technology and provides a compound anesthetic for cats and a preparation method thereof. The compound anesthetic is formed by ketamine hydrochloride, dexmedetomidine, tramadol hydrochloride, pentobarbital sodium, sodium chloride, gelatin and water for injection. The ketamine hydrochloride, the dexmedetomidine, the tramadol hydrochloride, the pentobarbital sodium, the sodium chloride and the gelatin are dissolved in the water for injection in sequence, by means of 0.2 mole per litre of hydrochloric acid solution, potential of hydrogen (PH) is adjusted to 7.8, the water for injection is added again, after dilution and filtering, the obtained solution is poured into an ampoule bottle, and after sterilization, the compound anesthetic for the cats can be obtained. The compound anesthetic is rapid in anesthesia induction, good in anesthetic effects, safe and effective in the process of anesthesia, slight in influence on normal physiological and biochemical indexes, small in toxic and side effects, and the cats can revive stably, and the preparation method is simple and feasible.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Compound preparation for treating vertebra osteomyelitis and preparation method of compound preparation
InactiveCN106109481ASignificant effectPromote circulationOrganic active ingredientsPeptide/protein ingredientsSide effectTramadol Hydrochloride
The invention discloses a compound preparation for treating vertebra osteomyelitis and a preparation method of the compound preparation. The compound preparation consists of the following ingredients: amikacin, diacerein, onychin, eleutheroside, paracetamol glucoside, active protein peptide, chitosan, trace elements, vitamin D, methyl salicylate, arbutin, algae polysaccharide, tramadol hydrochloride, radix dipsaci alkali and gypenoside. The compound preparation for treating the vertebra osteomyelitis disclosed by the invention has functions of relieving pain, resisting inflammation and killing bacteria, and the compound preparation is capable of promoting blood circulation, promoting development of bones and strengthening tendons and bones; moreover, the compound preparation is capable of relieving apoptosis, enhancing resistance of organs and improving human metabolism; and clinical application verifies that the compound preparation is significant in curative effect on the vertebra osteomyelitis, and the compound preparation is safe and low in side effects.
Owner:孙鹏
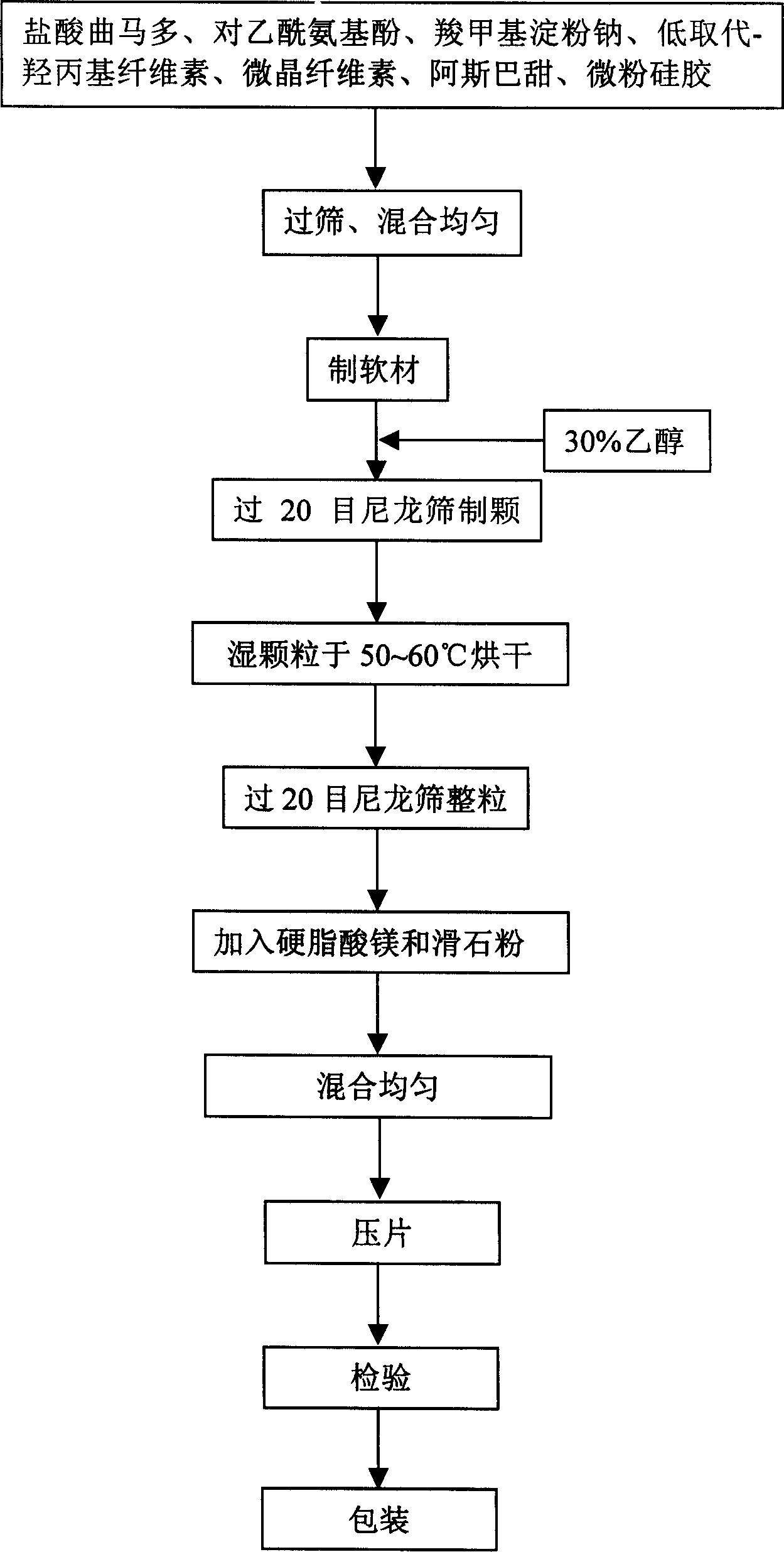
Preparation method for tramadol hydrochloride dispersible tablet
InactiveCN110101673AQuick effectGood dispersionOrganic active ingredientsAntipyreticTramadol HydrochlorideMagnesium stearate
The invention provides a preparation method for a tramadol hydrochloride dispersible tablet. The tramadol hydrochloride dispersible tablet is prepared from tramadol hydrochloride of the effective treatment dosage, pregelatinized starch, microcrystalline cellulose, polyvinylpolypyrrolidone, calcium carbonate and magnesium stearate. After partial tramadol hydrochloride and partial pregelatinized starch are adopted for sufficient mixing in the earlier stage, smashing is conducted, so that the mixture is uniformly mixed sufficiently, and the dispersion effect of the tramadol hydrochloride is improved; the quick effect is ensured. Then, the rest of tramadol hydrochloride, the polyvinylpolypyrrolidone and the calcium carbonate are mixed sufficiently, the adhesive characteristic of the polyvinylpolypyrrolidone is utilized, so that the partial tramadol hydrochloride is slowly released, and analgesic effect time is prolonged.
Owner:安徽金太阳生化药业有限公司
Novel pharmaceutical composition containing analgesic
The invention discloses a compound slow release preparation prepared from acetaminophen and tramadol hydrochloride for the first time, realizes taking medicine twice per day, and reduces non-indication of patients to the medicine, and the sustained medicine effect can achieve abirritation for a long time.
Owner:苏州世林医药技术发展有限公司
Oral disintegrating tablet containing tramadol hydrochloride and acetaminopher, and its preparation method
ActiveCN100404025CMeet the requirement of rapid disintegrationImprove liquidityOrganic active ingredientsNervous disorderOrally disintegrating tabletTramadol Hydrochloride
The invention provides an orally disintegrating tablet containing tramadol hydrochloride and paracetamol, and the preparing process, wherein tramadol hydrochloride and paracetamol are dressed for mouth cavity use. During the dressing process, right amount of medicinal auxiliary materials are charged.
Owner:SHANGHAI SINE PHARMA LAB
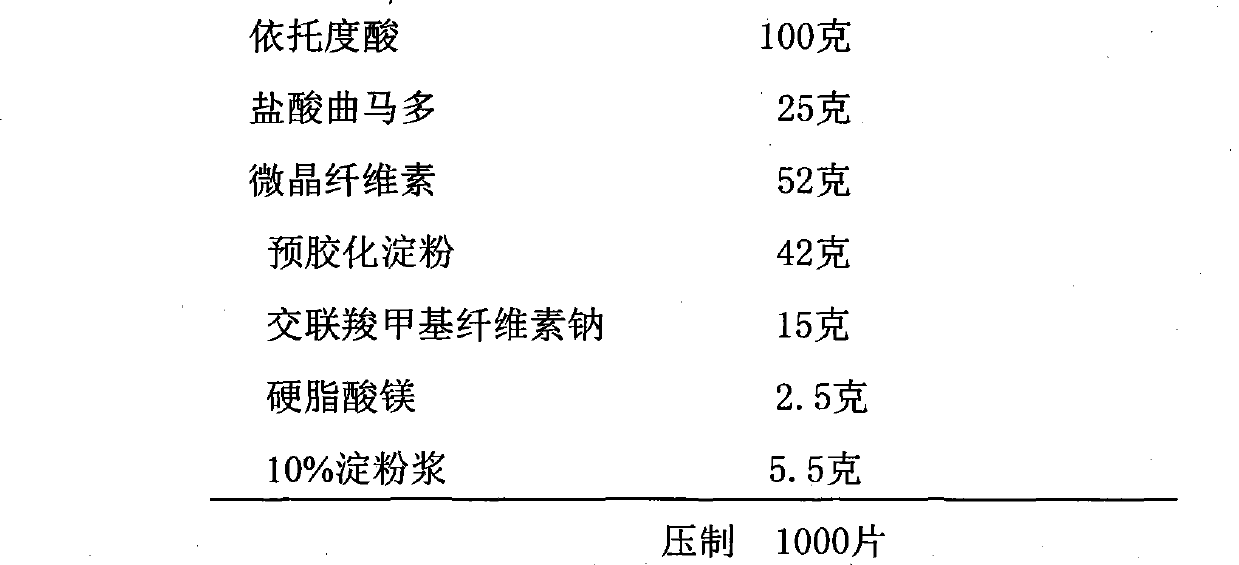
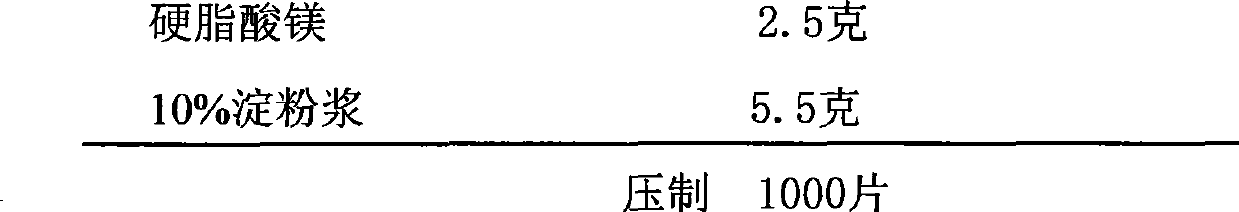
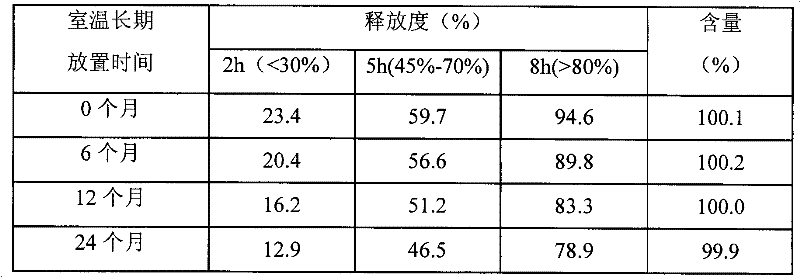
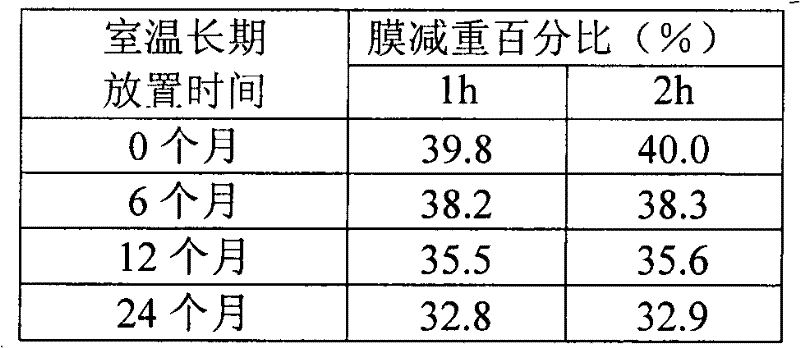
Pharmaceutical composition containing etodolac and tramadol hydrochloride and application thereof
InactiveCN103860551APain BenefitsGood treatment effectOrganic active ingredientsAntipyreticAnalgesics drugsTramadol Hydrochloride
The invention belongs to the field of medicine, and particularly relates to a pharmaceutical composition and an application thereof in preparation of analgesic drugs. The pharmaceutical composition takes etodolac and tramadol hydrochloride as active pharmaceutical ingredients, wherein the ratio of etodolac to tramadol hydrochloride is preferably 0.1-100:1, more preferably 4-8:1. The pharmaceutical composition can be made into an appropriate pharmaceutical preparation for drug administration, such as oral preparations. A large number of pharmacological experiments confirm that etodolac and tramadol hydrochloride have a significant synergistic effect in treatment of pains caused by various factors. The pharmaceutical composition is strong in analgesic effect, low in untoward effects, and suitable for long-term application for patients with pains.
Owner:LUNAN PHARMA GROUP CORPORATION
Method of preparing nano medical adhesive
InactiveCN108714242AHigh bonding strengthHigh compressive strengthSurgical adhesivesPharmaceutical delivery mechanismFibrin gluePolyvinyl alcohol
The invention discloses a method of preparing nano medical adhesive. The nano medical adhesive is made from the following parts, by weight, of 55-65 parts of polyvinylpyrrolidone, 8-15 parts of polyethersulfone ether ketone, 50-60 parts of polyvinyl alcohol, 25-40 parts of xanthan gum, 3-8 parts of agar, 10-15 parts of fibrin glue, 5-10 parts of sweet potato starch, 3-8 parts of ethyl acetate, 4-6parts of nano-calcium oxide powder, 2-6 parts of sodium glycocholate, 3-5 parts of triglyceride monostearate, 5-8 parts of tramadol hydrochloride, 10-15 parts of polyether triol, 3-5 parts of zinc oxide, 5-10 parts of sodium carboxymethyl cellulose, 15-20 parts of sodium alginate and 11-14 parts of cellulose. The prepared nano medical adhesive has the advantages of having high bond strength and compressive strength and little stimulation to organism tissues, adding no harmful reagents, having antibacterial hemostasis, and being suitable for use in surgery and especially suitable for the bonding of soft tissues.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Tramadol hydrochloride osmotic pump controlled release tablet
InactiveCN102670542APrevent agingOrganic active ingredientsNervous disorderTramadol HydrochlorideSemipermeable membrane
The invention provides a novel single-chamber tramadol hydrochloride osmotic pump controlled release tablet preparation. A semi-permeable membrane containing ethyl cellulose (EC) and polyvidone (PVP) is coated on the surface of a medicament table core, so that the phenomenon of ageing of the semi-permeable membrane can be avoided, and medicament residues are reduced. The invention further provides a method for improving the anti-ageing performance of a single-chamber tramadol hydrochloride osmotic pump controlled release tablet. The method is characterized in that: ethyl cellulose-polyvidone is taken as a semi-permeable membrane material. Moreover, the invention further provides an application of an ethyl cellulose-polyvidone composition to preparation of a single-chamber tramadol hydrochloride osmotic pump controlled release tablet with anti-ageing performance.
Owner:内蒙古天衡医院管理有限公司
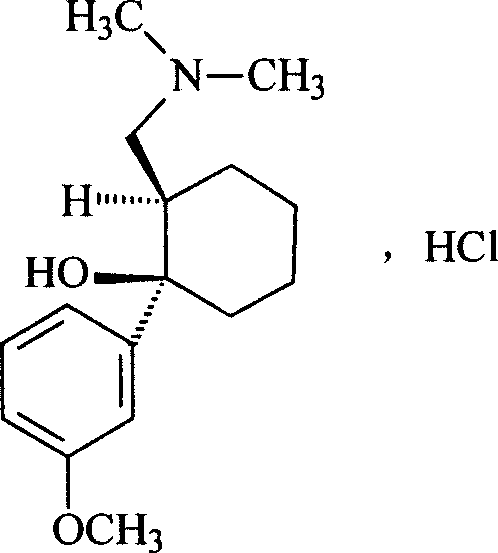
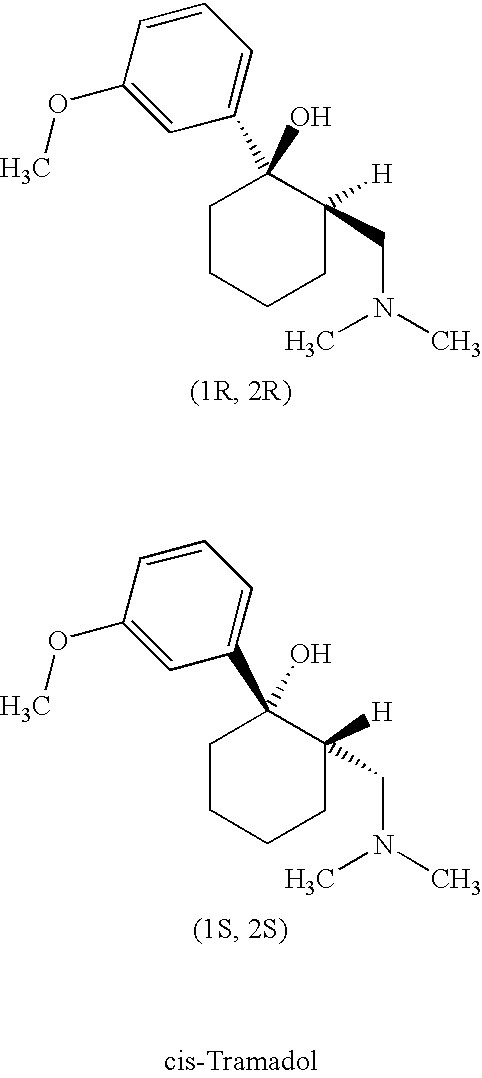
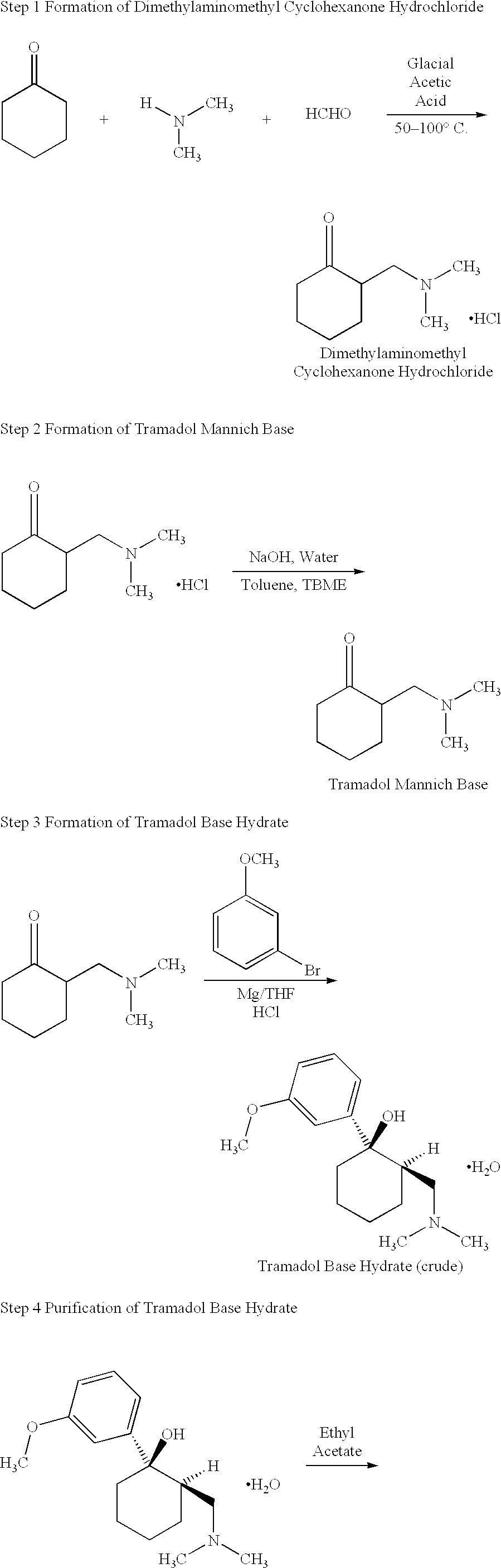
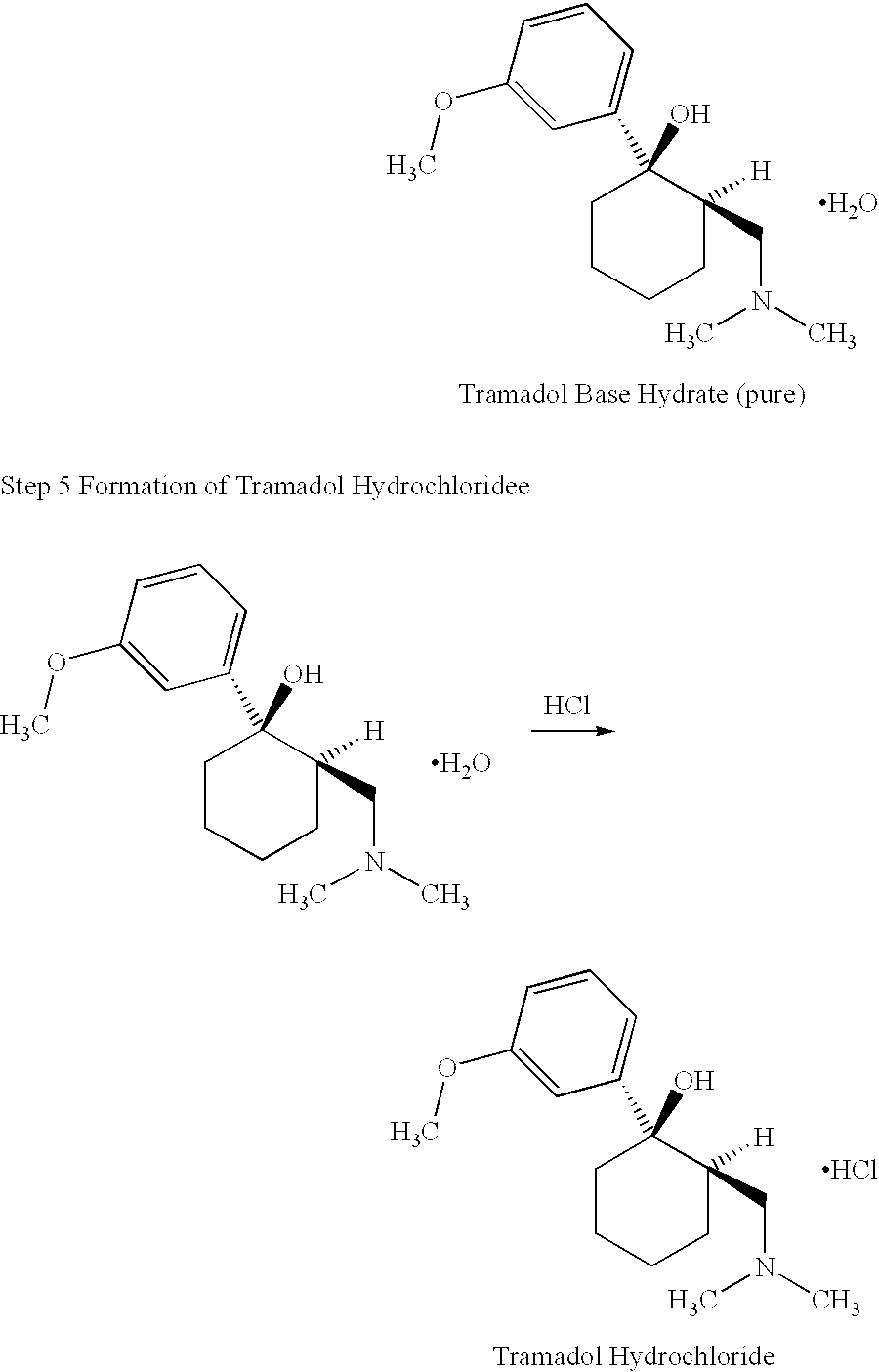
Tramadol, salts thereof and process for their preparation
Cis-Tramadol hydrochloride is prepared by forming a Mannich hydrochloride, liberating the Mannich base, reacting the Mannich base with a Grignard reagent to form a base hydrate of cis-Tramadol which is used to form pure cis-Tramadol hydrochloride. Also claimed is the base hydrate of cis-Tramadol per se and its use as a medicament.
Owner:RUSSINSKY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com