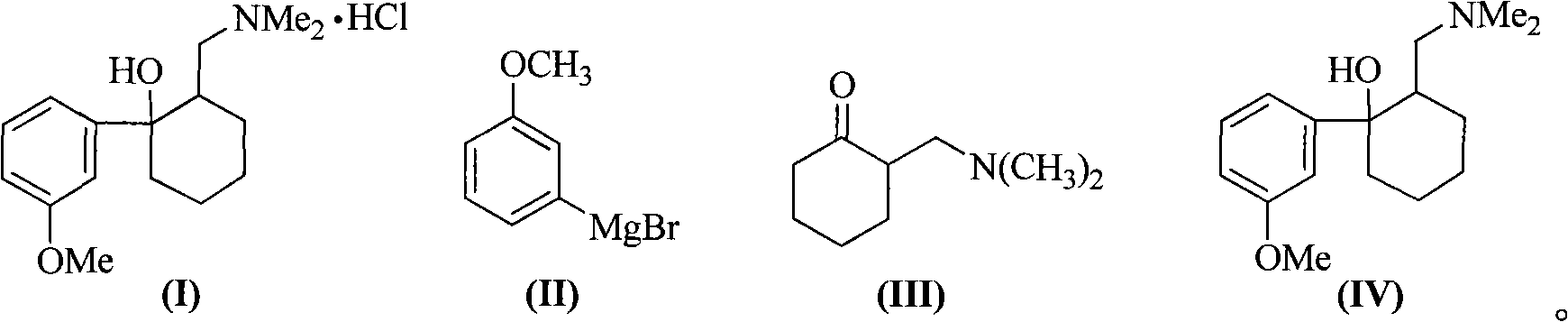
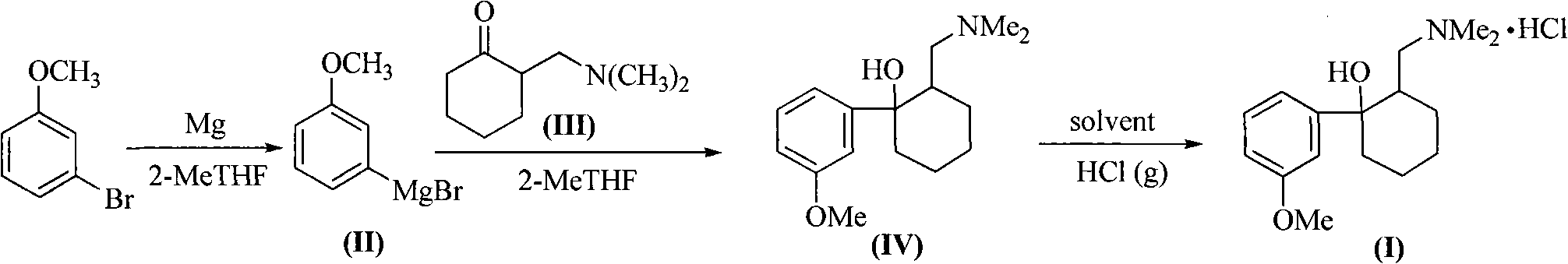
Method for synthesizing tramadol hydrochloride
A technology for the synthesis of tramadol hydrochloride and its synthesis method, which is applied in the field of synthesis of tramadol hydrochloride, which can solve the problems of flammability, anesthesia, explosion, etc., and achieve shortened reaction time, high reaction yield, and easy separation The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The amount ratio of the feed material is 2-dimethylaminomethylcyclohexanone: m-methoxybromobenzene: metal magnesium is 1.0: 1.0: 1.0, and the dropwise reaction method is to add 2-dimethylaminomethyl The 2-MeTHF solution of cyclohexanone (III) is dropped into the Grignard reagent solution, and the solvent is 2-MeTHF.
[0034] Add magnesium bar (2.4g, 0.1mol) and 2-MeTHF (8.6g, 10mL) in reaction bottle, N 2 Under protection, an appropriate amount of 1,2-dibromoethane was added to initiate the reaction, m-methoxybromobenzene (18.6g, 0.1mol) in 2-MeTHF solution (30.15g, 35mL) was slowly added dropwise, and the reaction was stirred at 80°C After 2 hours, the Grignard reagent (II) was prepared. After cooling to 0°C, a solution of 2-dimethylaminomethylcyclohexanone (15.5 g, 0.1 mol) in 2-MeTHF (23.25 g, 27 mL) was slowly dropped into the above reaction flask, and the reaction was kept for 4 hours after dropping. with saturated NH 4 The reaction was quenched with Cl, and the...
Embodiment 2
[0036] The amount ratio of the feed material is 2-dimethylaminomethylcyclohexanone: m-methoxybromobenzene: metal magnesium is 1.0: 1.5: 1.6, and the dropwise reaction method is to add 2-dimethylaminomethyl The 2-MeTHF solution of cyclohexanone (III) is dropped into the Grignard reagent solution, and the solvent is 2-MeTHF.
[0037] Add magnesium bar (3.84g, 0.16mol) and 2-MeTHF (8.6g, 10mL) in reaction flask, N 2 Under protection, an appropriate amount of 1,2-dibromoethane was added to initiate the reaction, m-methoxybromobenzene (27.9g, 0.15mol) in 2-MeTHF solution (30.15g, 35mL) was slowly added dropwise, and the reaction was stirred at 80°C After 2 hours, the Grignard reagent (II) was prepared. After cooling to 0°C, a solution of 2-dimethylaminomethylcyclohexanone (15.5 g, 0.1 mol) in 2-MeTHF (23.25 g, 27 mL) was slowly dropped into the above reaction flask, and the reaction was kept for 4 hours after dropping. with saturated NH 4Cl quenched the reaction, recovered 2-MeT...
Embodiment 3
[0039] The amount ratio of the feed material is 2-dimethylaminomethylcyclohexanone: m-methoxy bromobenzene: metal magnesium is 1.0: 1.2: 1.2, and the dropwise reaction method is to add 2-dimethylaminomethyl The 2-MeTHF solution of cyclohexanone (III) is dropped into the Grignard reagent solution, and the solvent is 2-MeTHF.
[0040] In the reaction flask, add magnesium bar (2.88g, 0.12mol), 2-MeTHF (8.6g, 10mL), N 2 Under protection, add an appropriate amount of iodine to initiate the reaction, slowly add m-methoxybromobenzene (22.32g, 0.12mol) in 2-MeTHF solution (30.15g, 35mL) dropwise, and stir the reaction at 80°C for 2 hours to obtain Grignard Reagent (II). 2-Dimethylaminomethylcyclohexanone (15.51 g, 0.1 mol) in 2-MeTHF (23.25 g, 27 mL) was slowly dropped into the above-mentioned reaction flask at 0° C., and the reaction was incubated for 4 hours after the dropping. with saturated NH 4 The reaction was quenched with Cl, and the separated 2-MeTHF layer was washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com