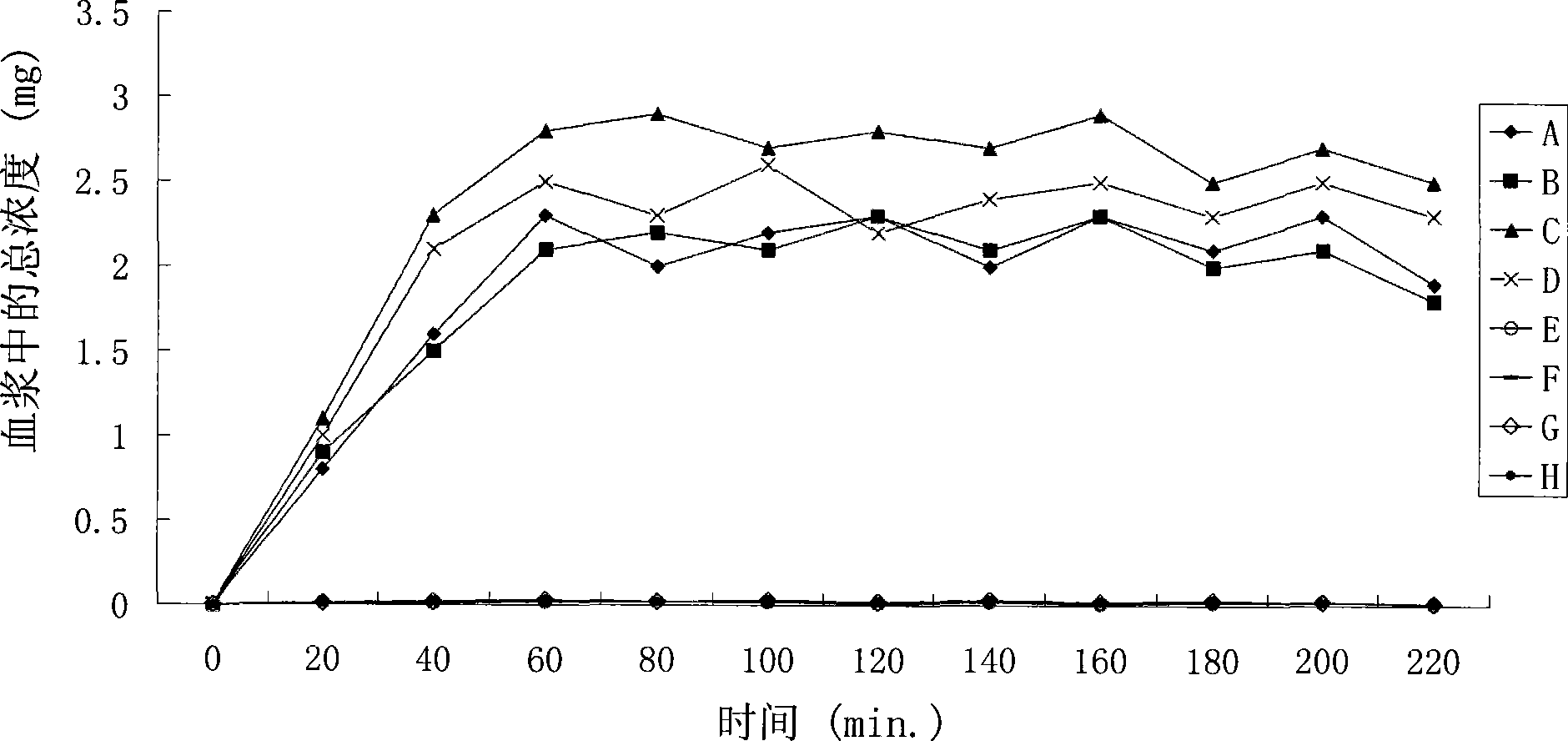
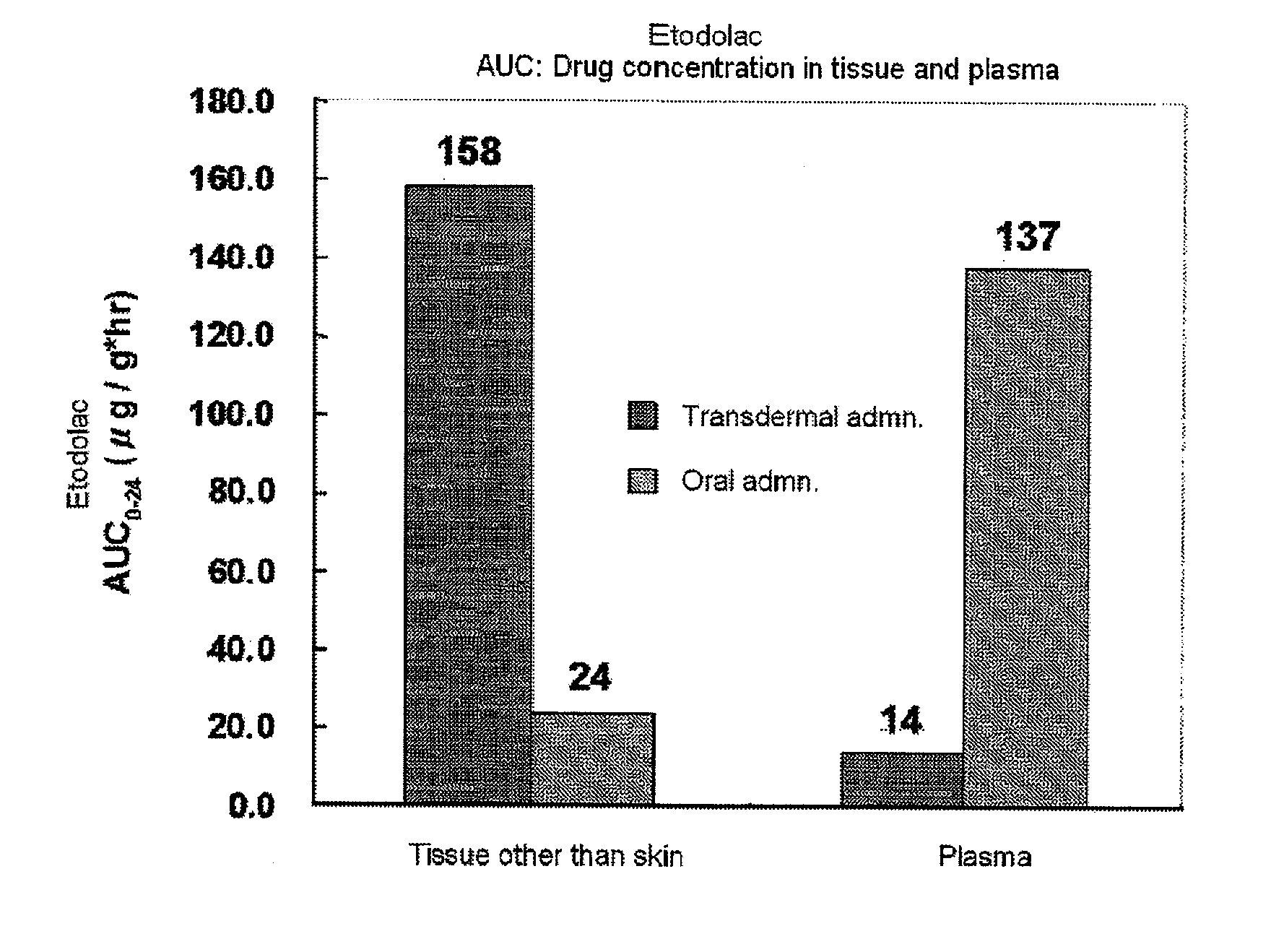
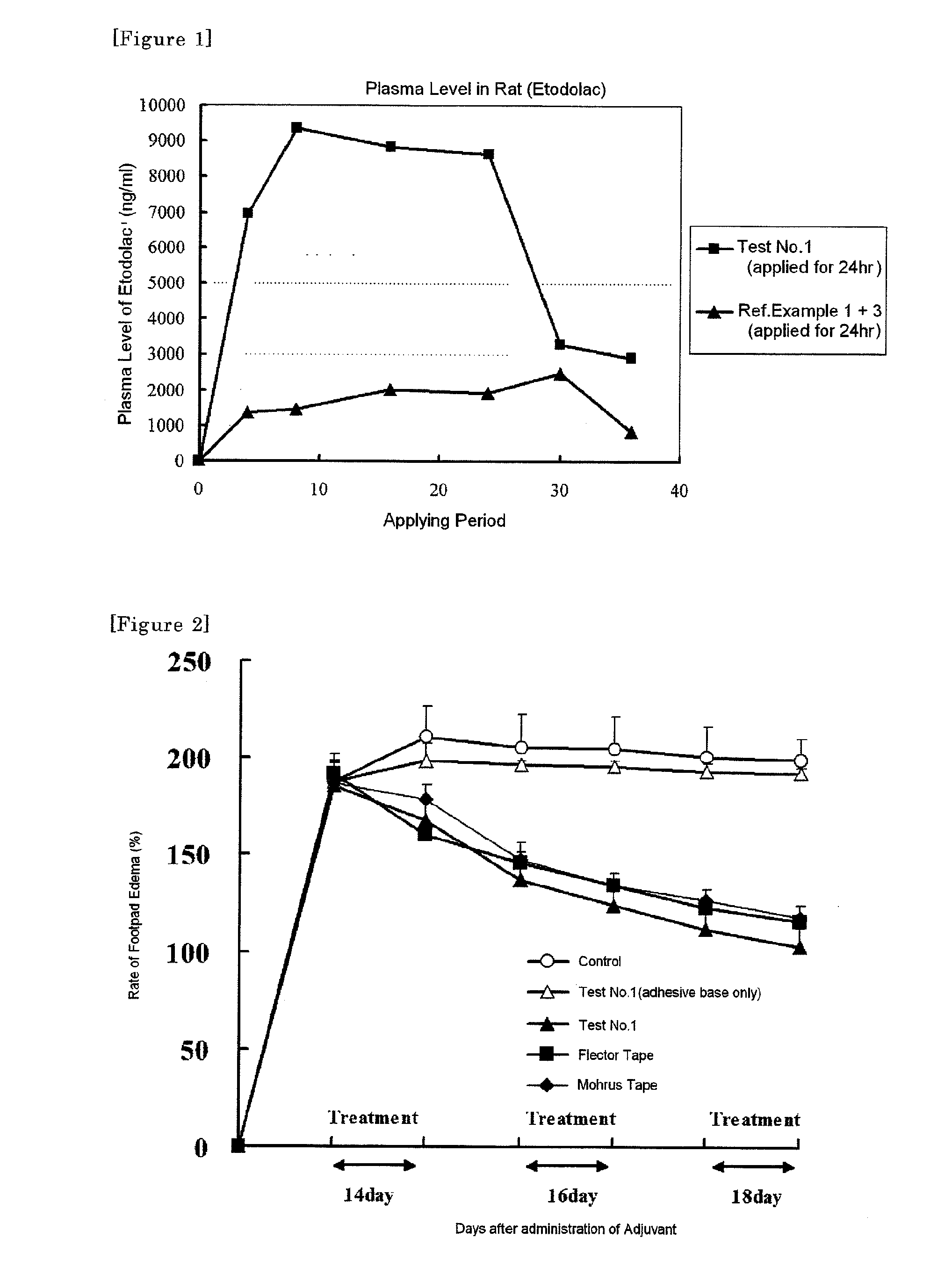
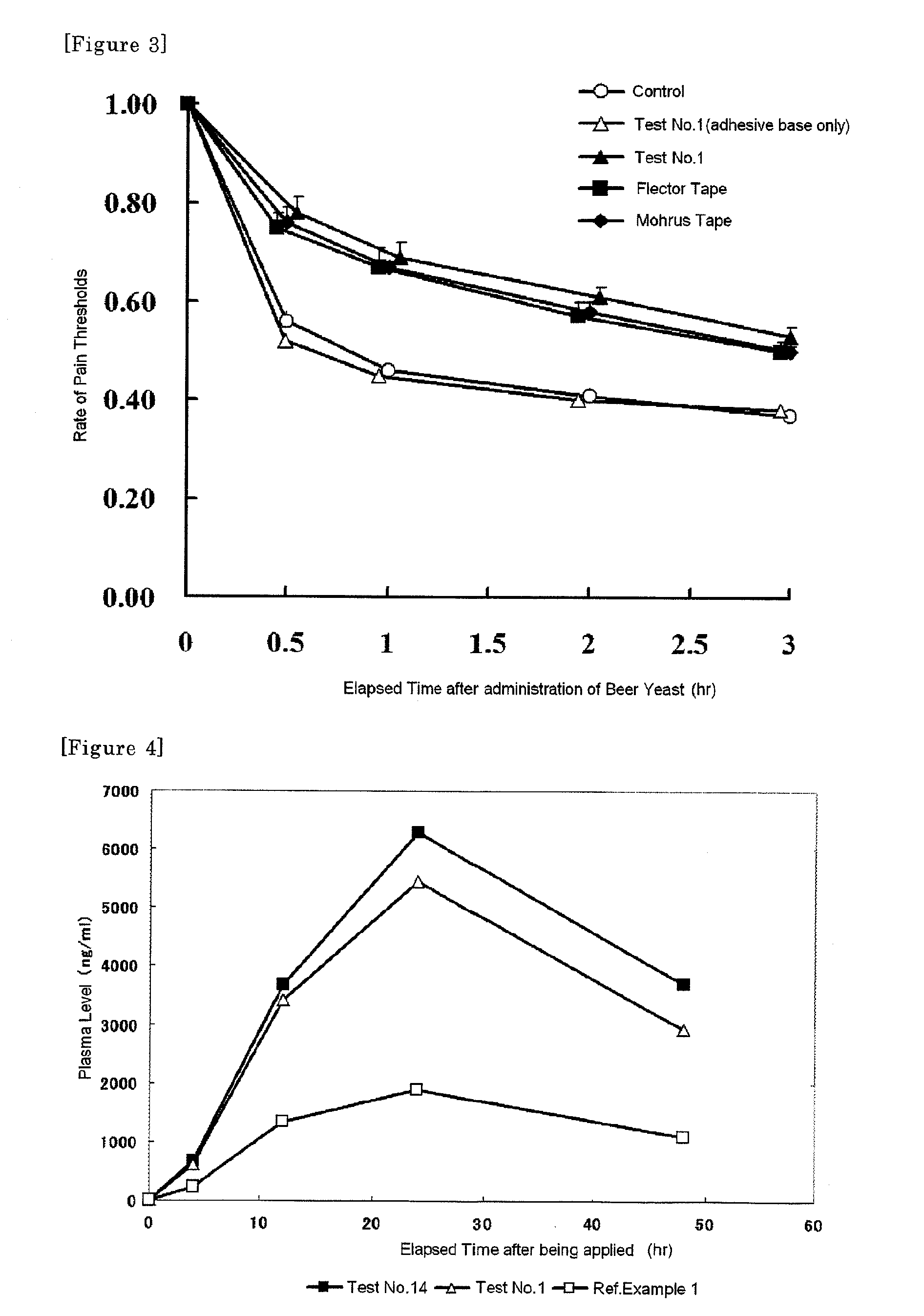
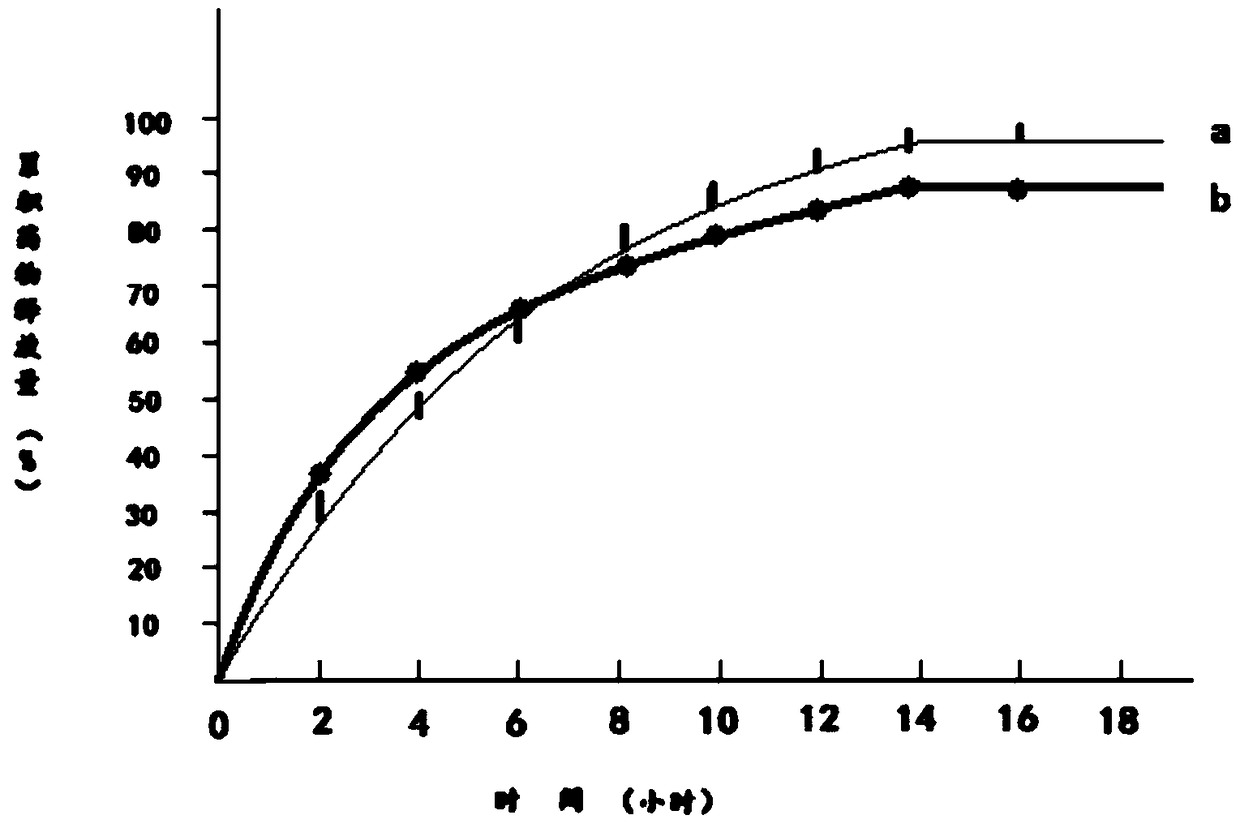
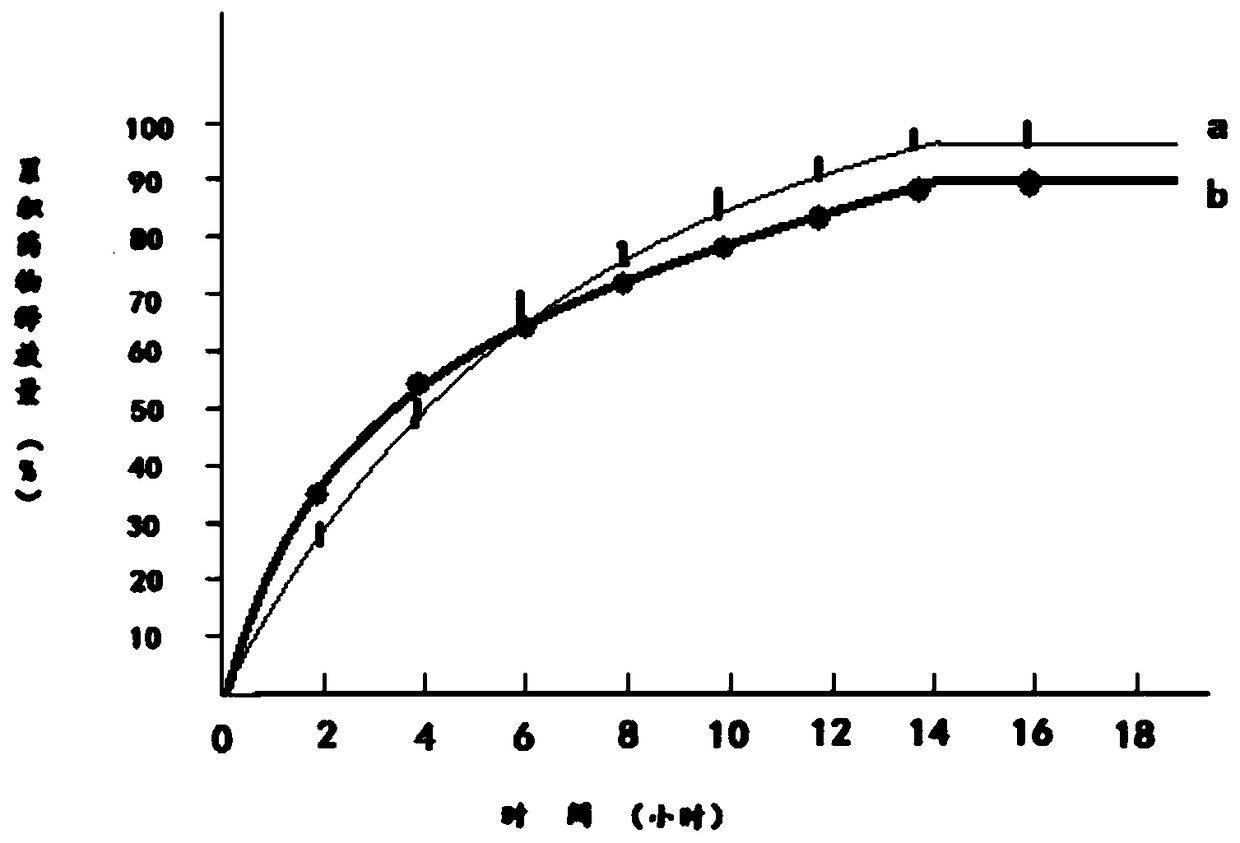
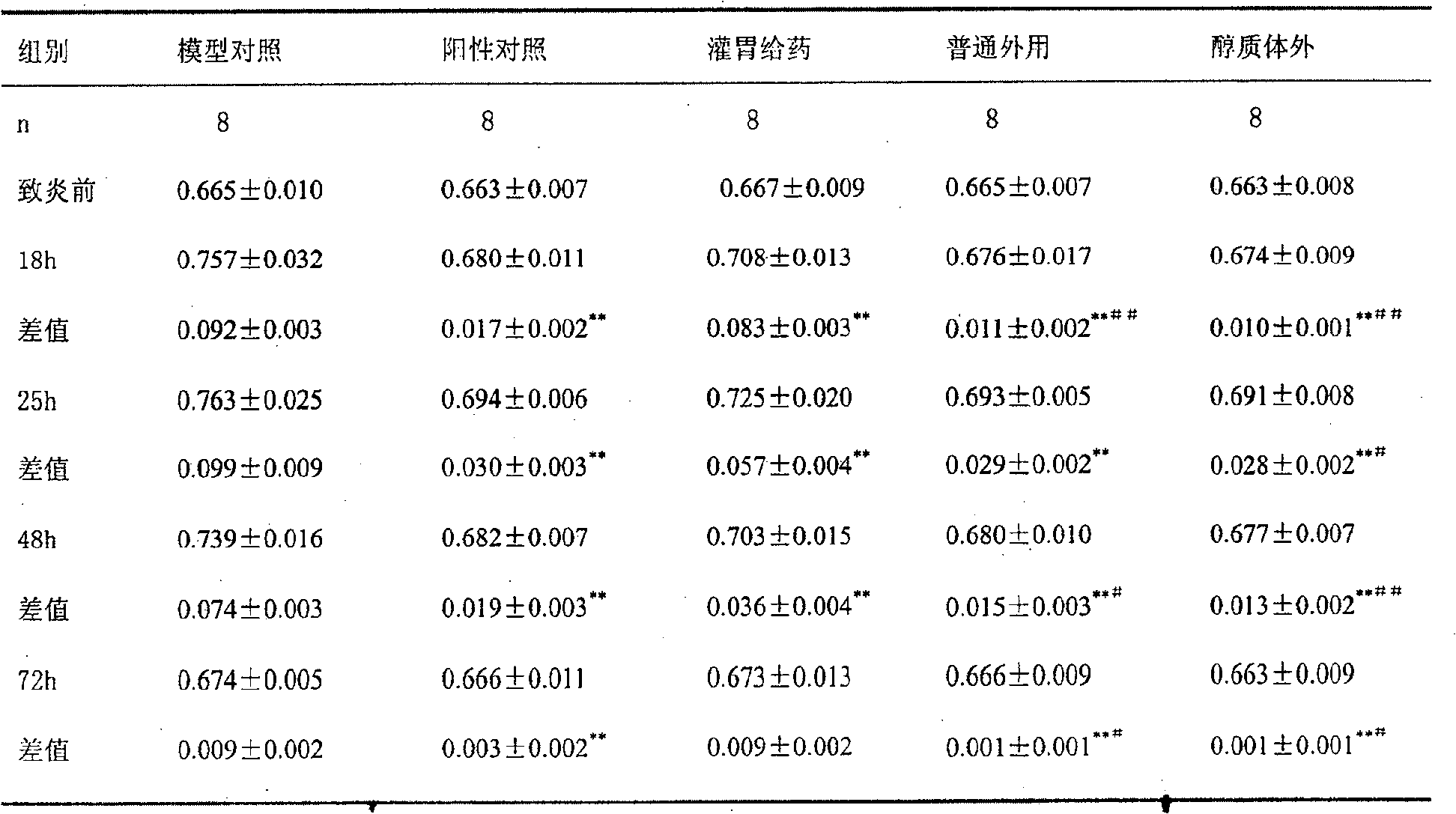
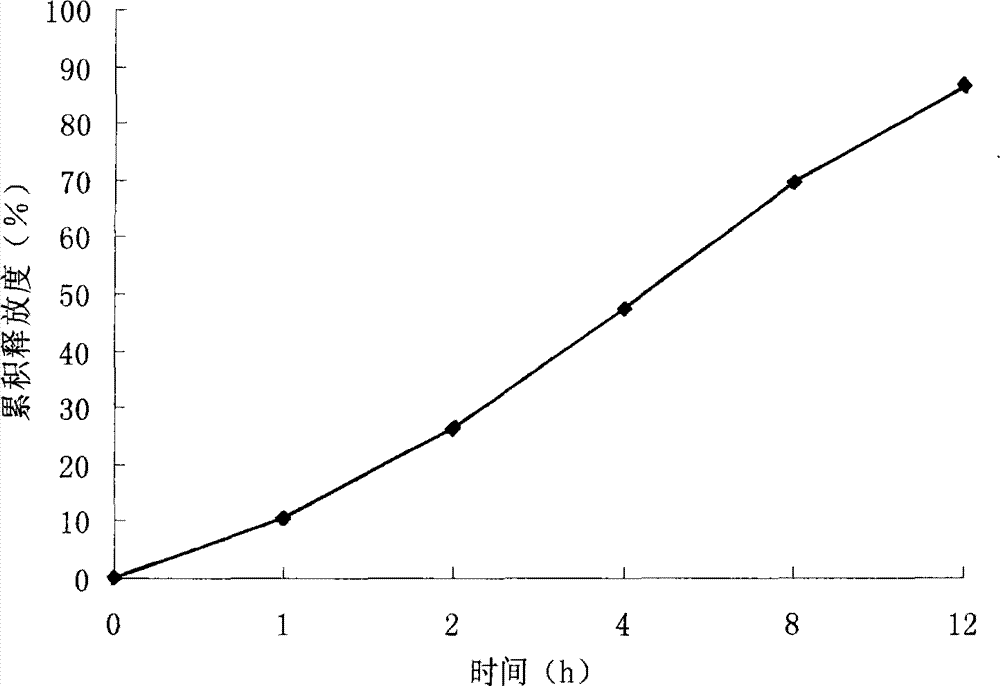
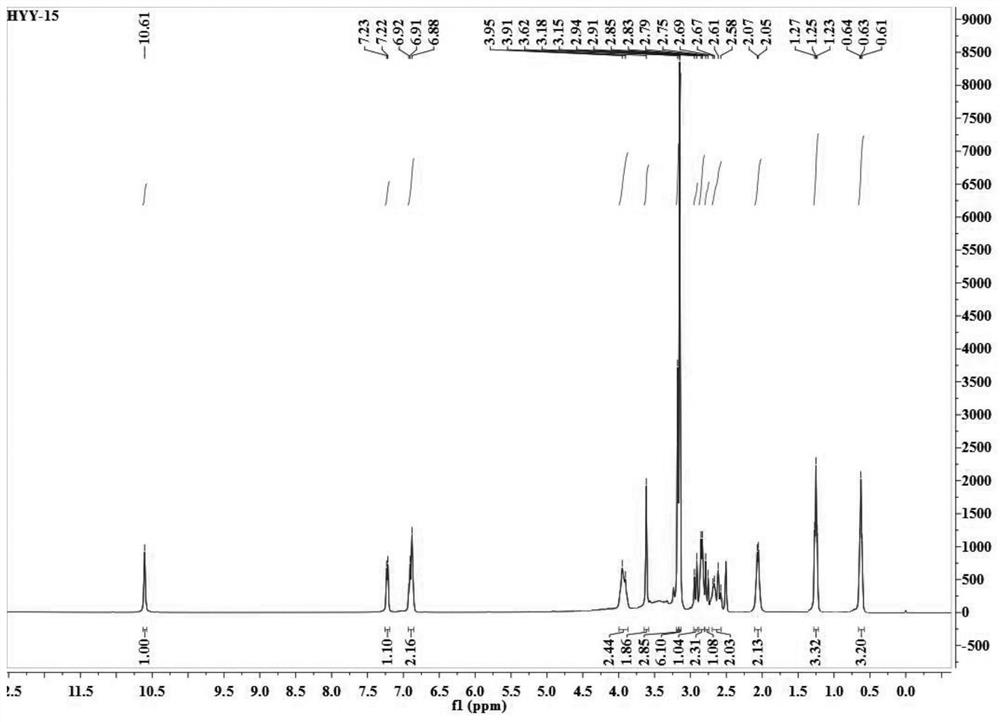
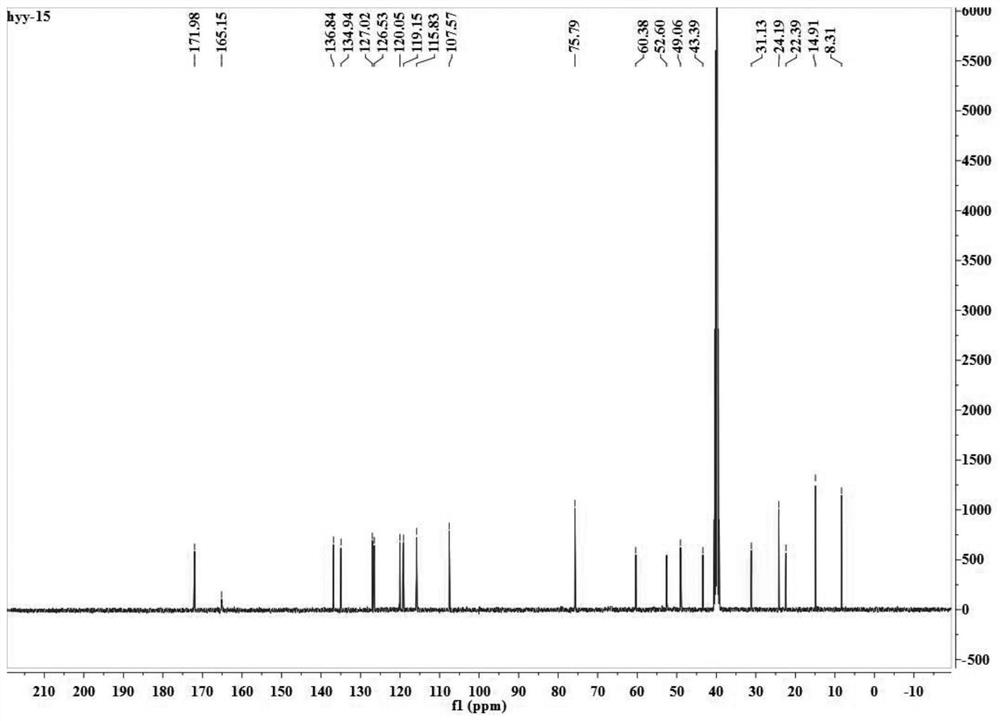
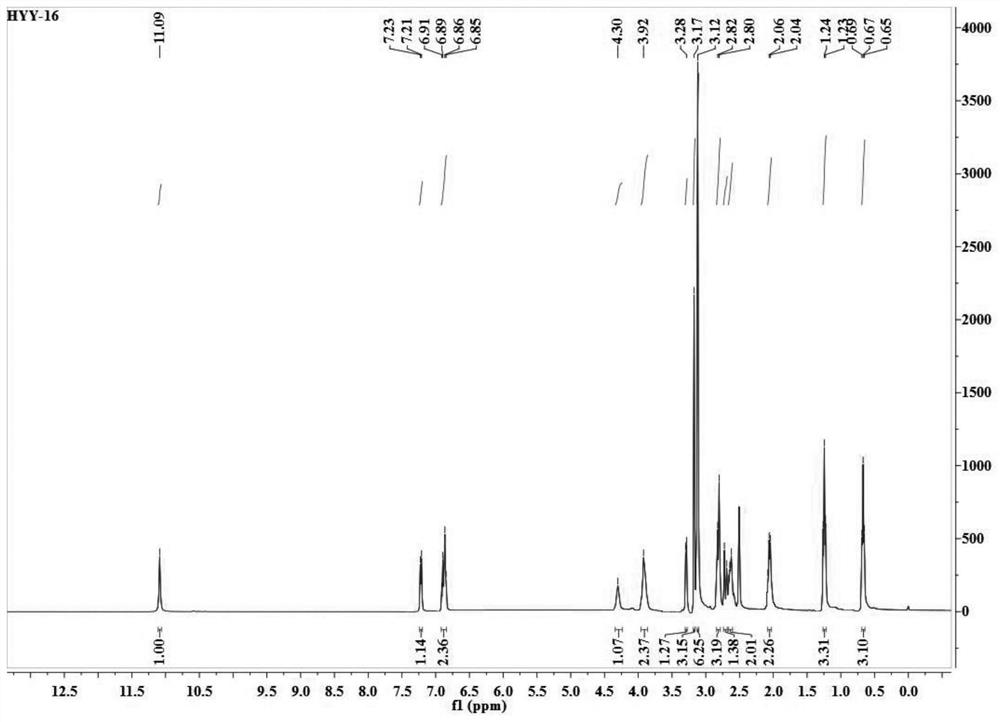
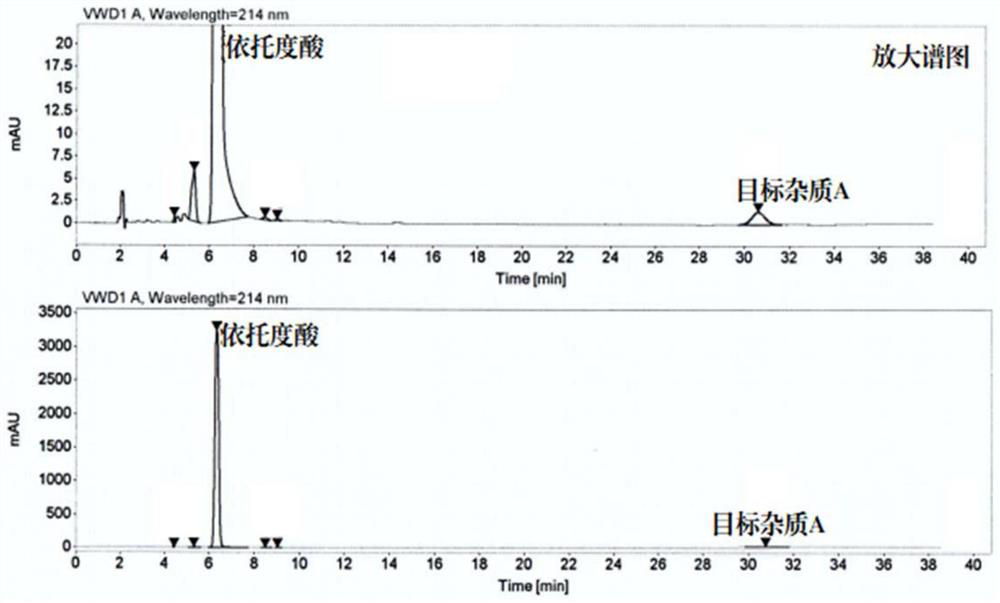
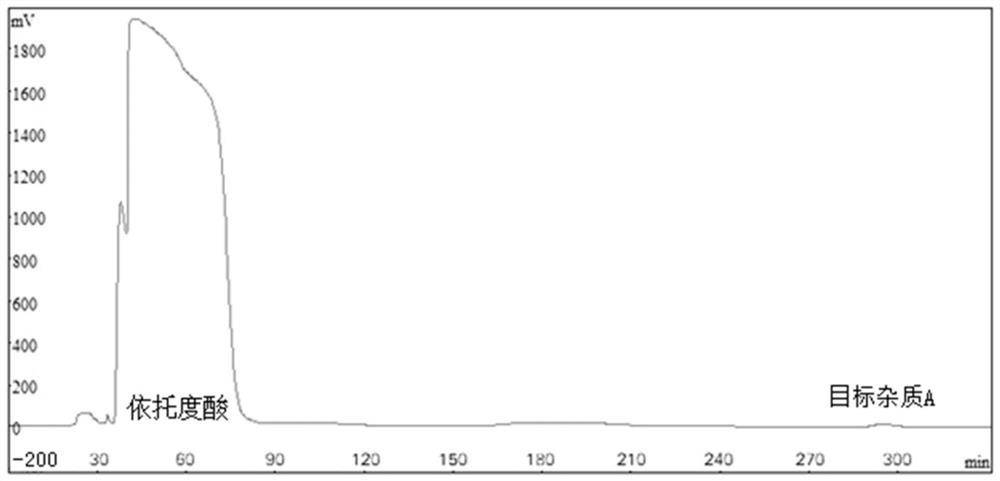
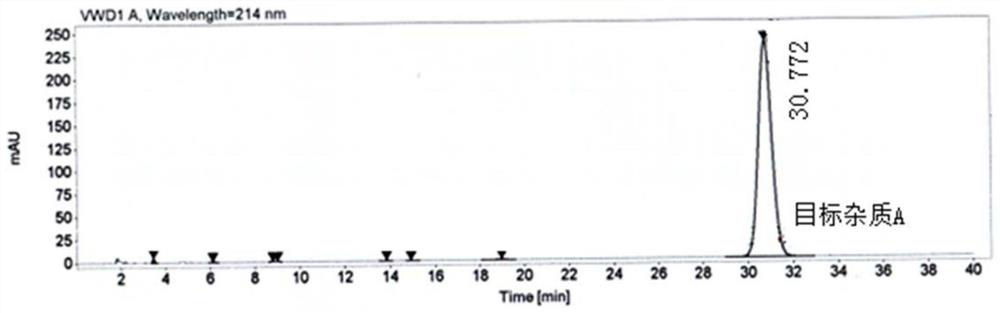
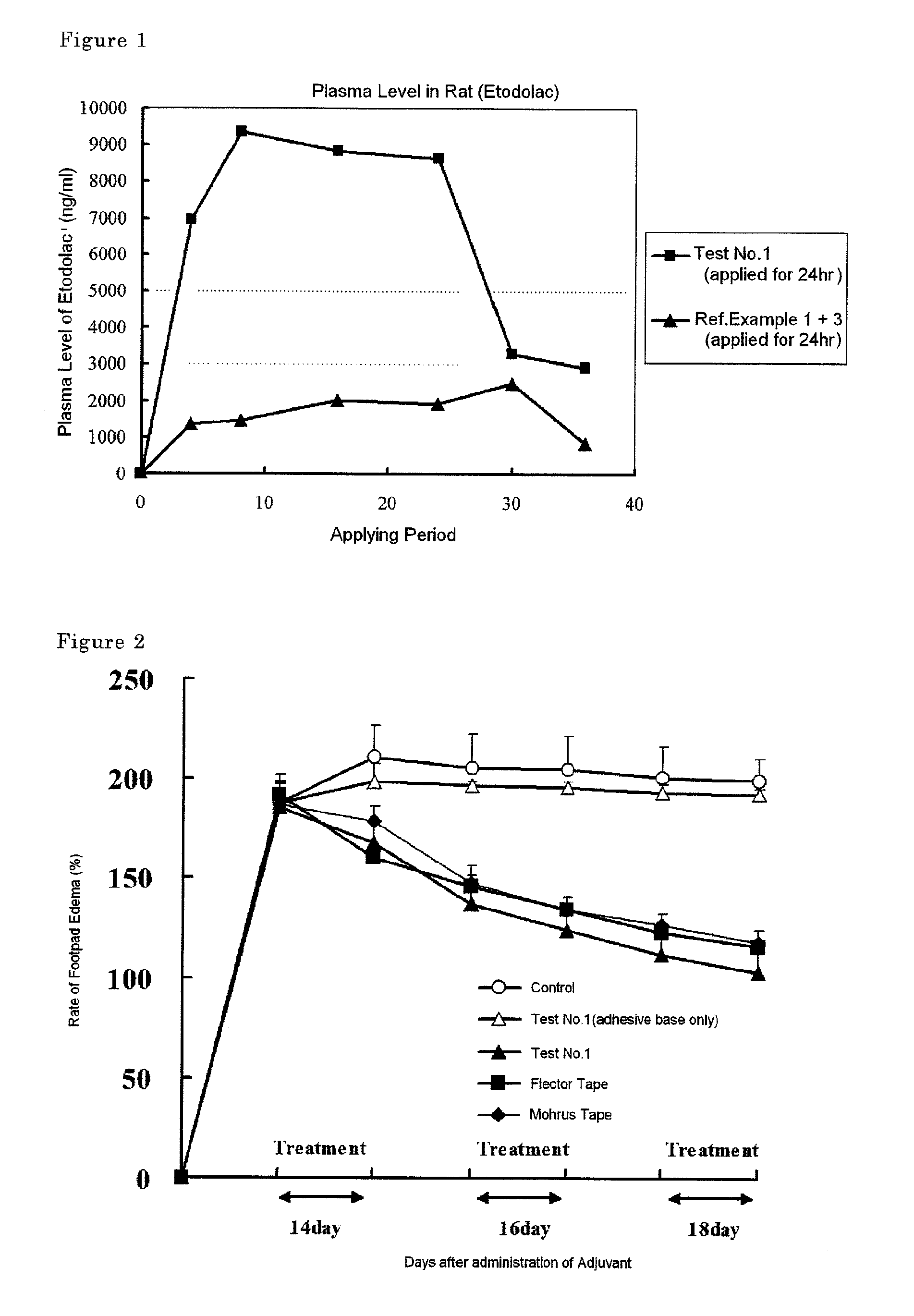
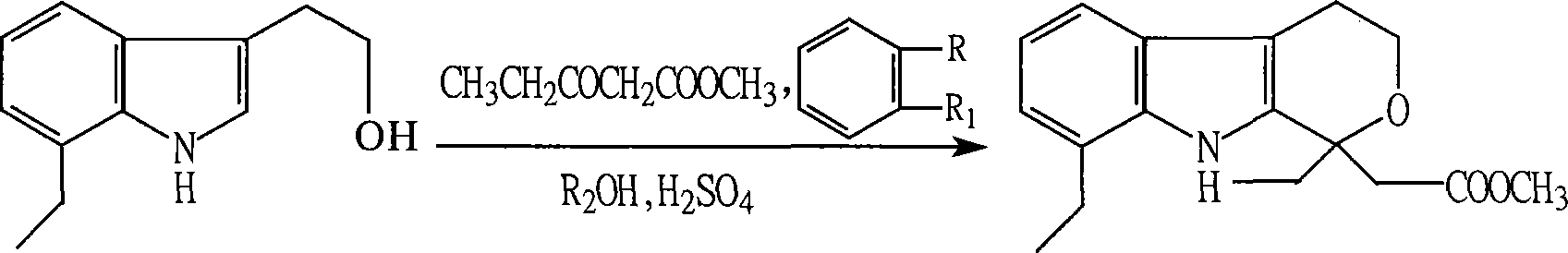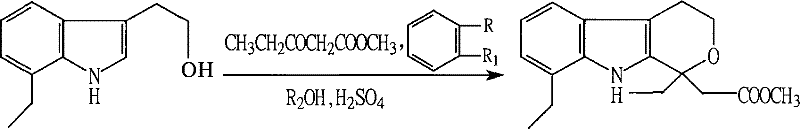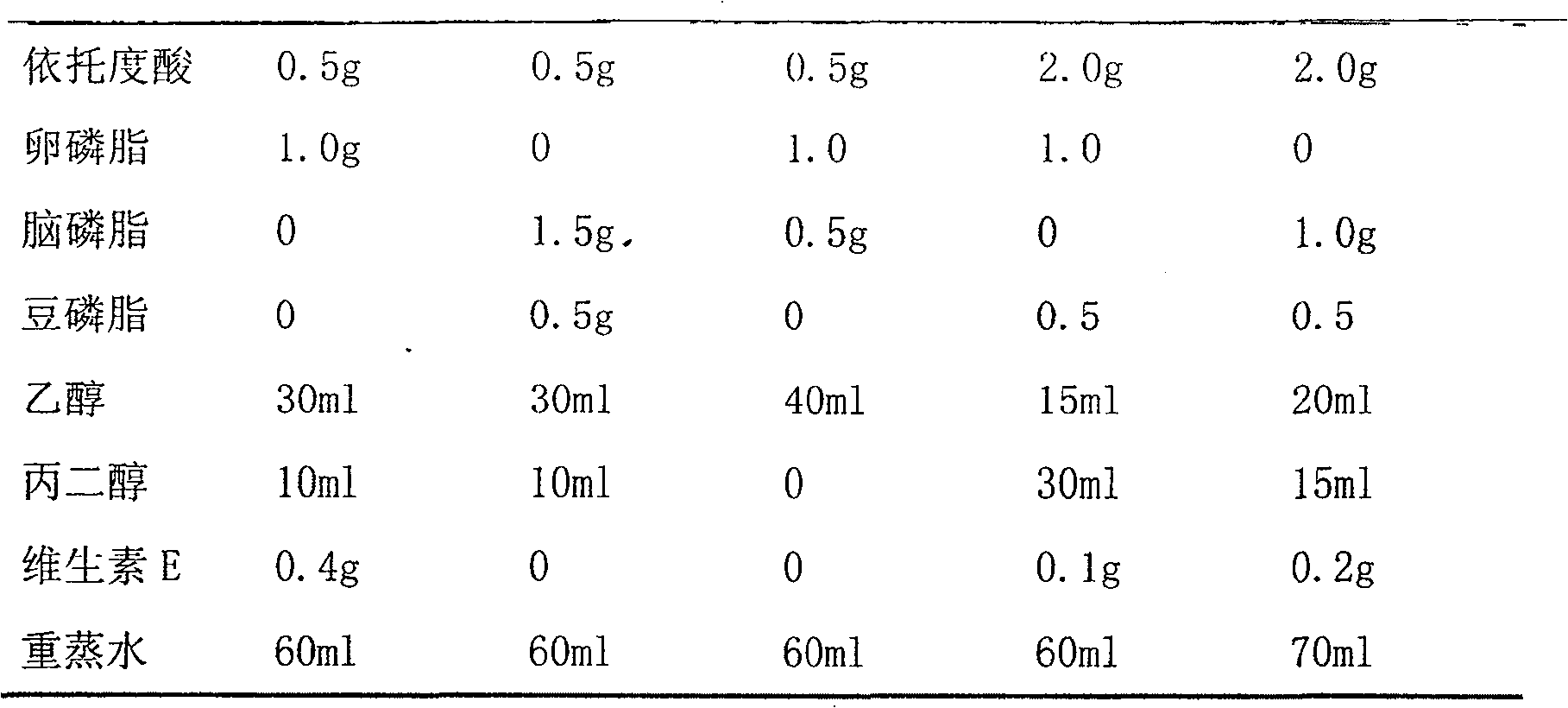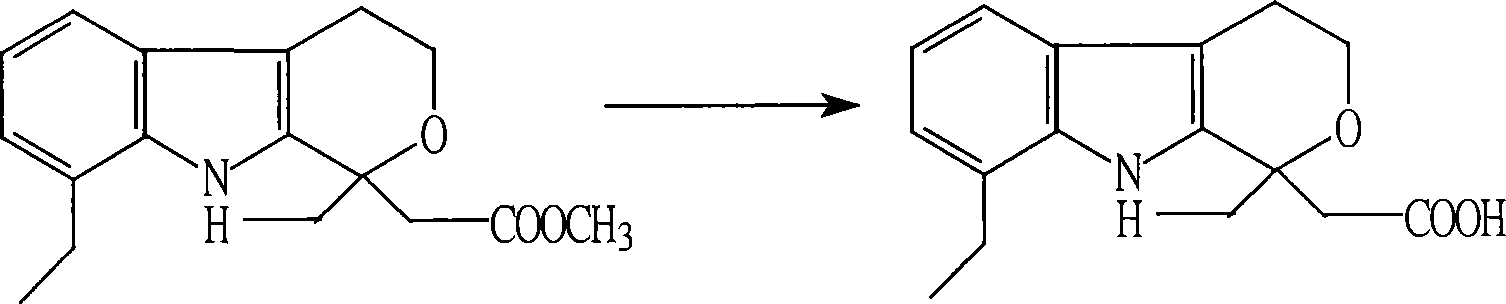Patents
Literature
34 results about "Etodolac" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
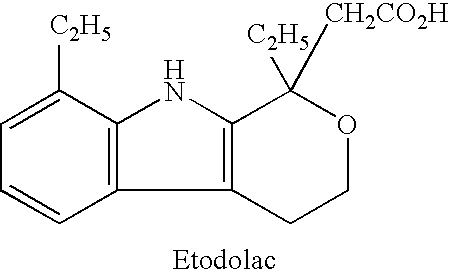
Etodolac is used to relieve pain from various conditions.
Use of etodolac for the treatment of prostate cancer
InactiveUS7105561B2Hinders viabilityIncreased susceptibilityBiocideDrug compositionsProstate cancerOncology
Owner:RGT UNIV OF CALIFORNIA
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
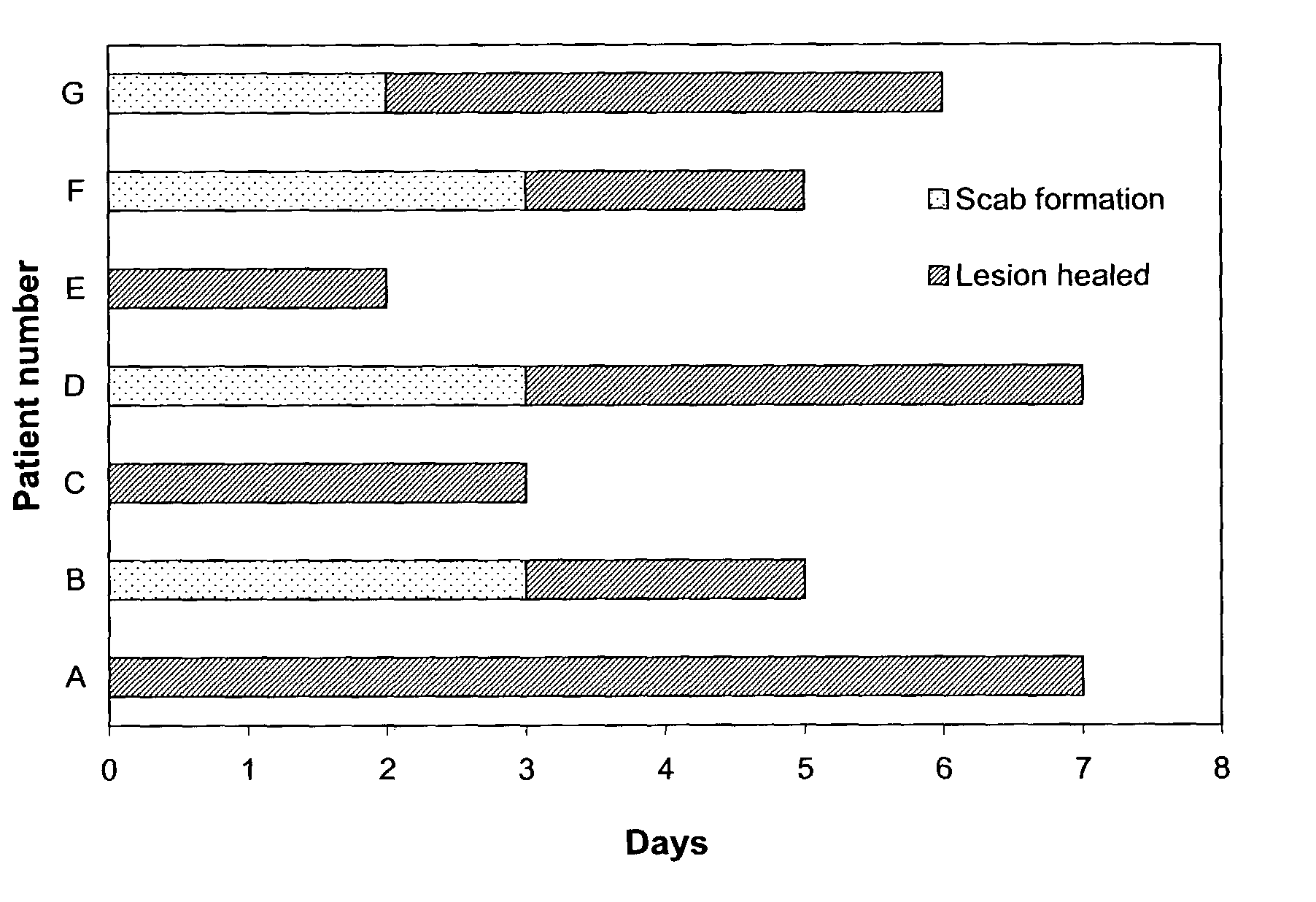
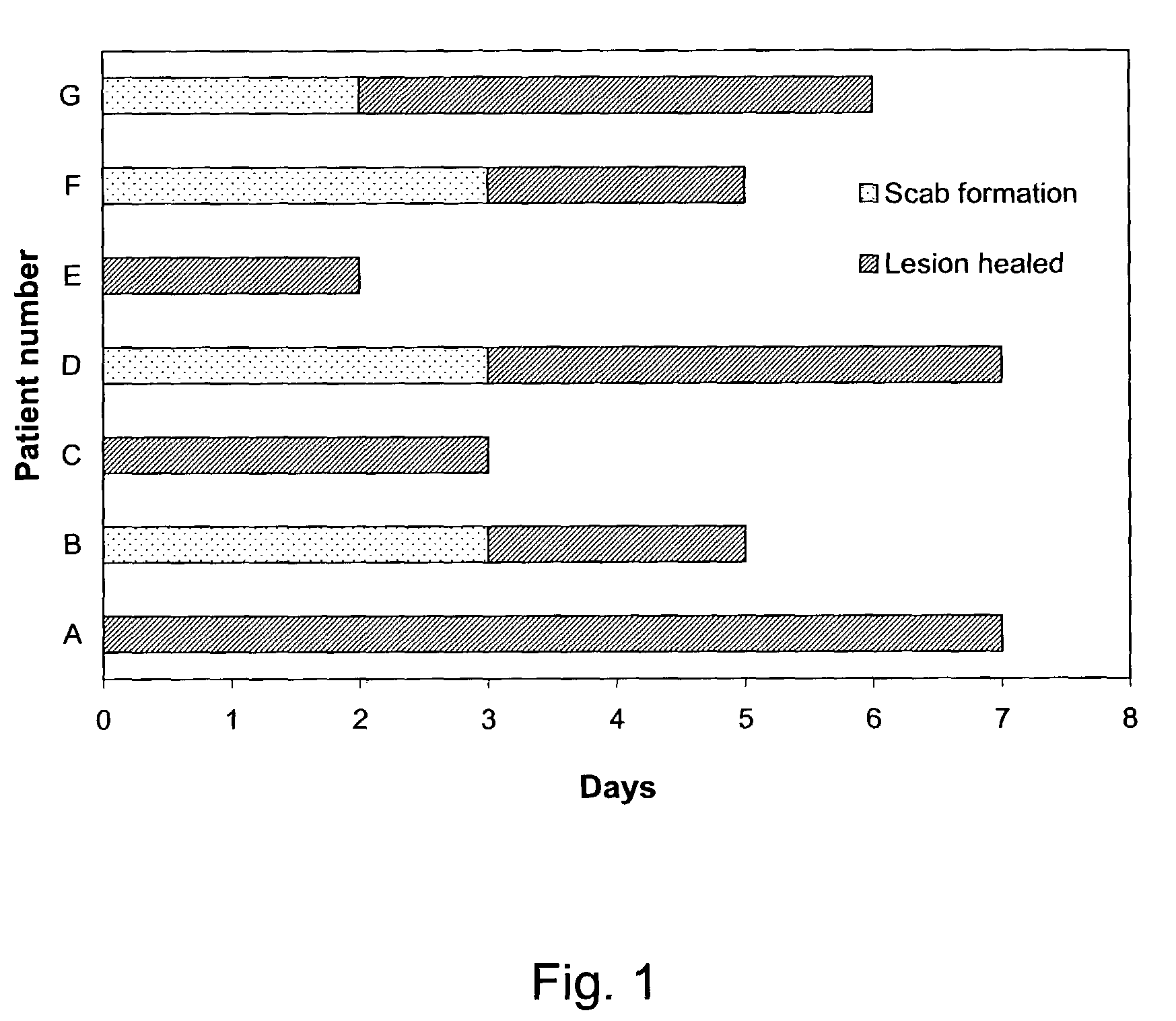
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Etodolac osmotic pump type controlled-release preparation and preparation thereof
InactiveCN101259113AAdjust the rate of constant releaseImprove complianceOrganic active ingredientsAntipyreticDiseaseSide effect
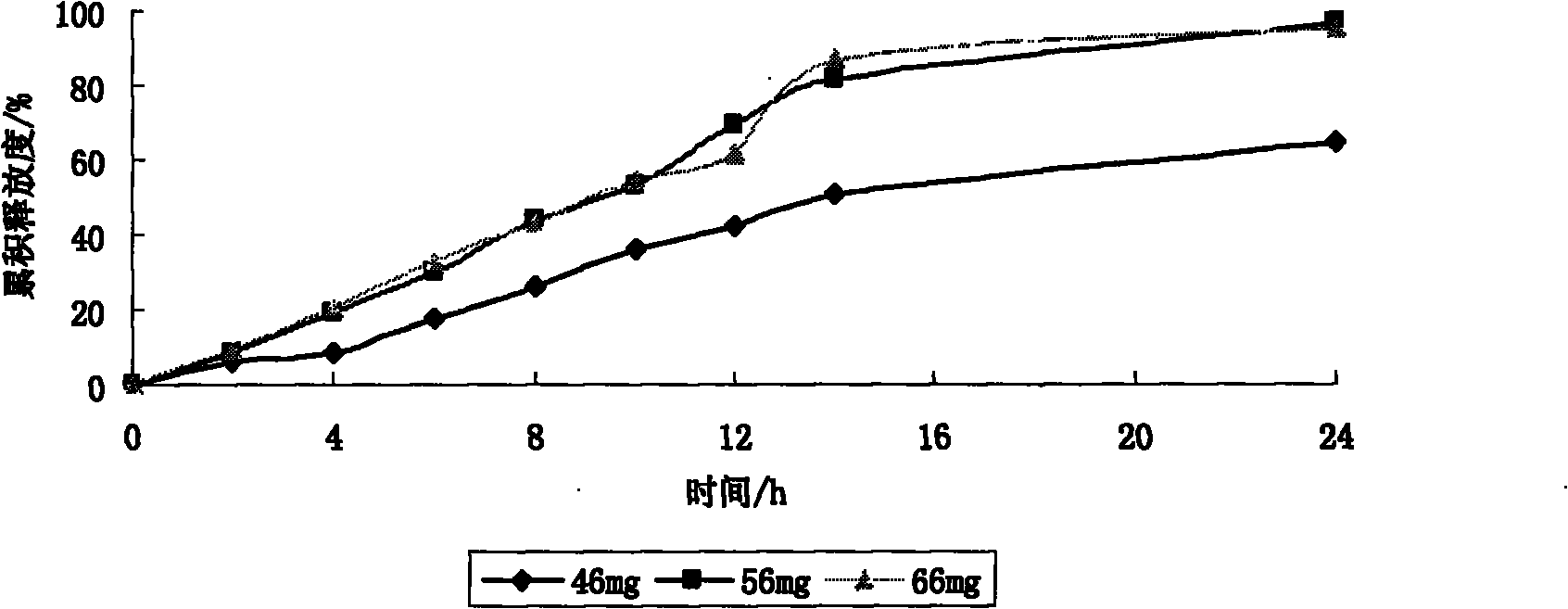
The invention belongs to the pharmaceutical preparation field and discloses an osmotic pump type controlled release preparation of etodolac and a preparation method thereof. An osmotic pump tablet comprises a tablet core and a controlled release semipermeable coating film that is coated outside the tablet core and with a hole for releasing drug. The tablet core comprises materials by following weight percentage: 60.9 to 65.5 percent of etodolac, 27.1 to 31.2 percent of osmotic pressure active material, 5.5 to 8.0 percent of an auxiliary material that can ensure the basic remedy to be releasedeasily and the rest is other auxiliary materials. The osmotic pump controlled release semipermeable coating film comprises by materials by following weight percentage: 71.4 to 76.9 percent of semipermeable high molecular coating material and 23.1 to 28.6 percent of pore-forming agent. The manners of pore-forming include a mechanical drilling and a laser boring. By adjusting the prescription of the tablet core and the coating film in the invention, the speed of constant release of drug can be effectively regulated so as to obtain a steadier, lasting and effective blood concentration, thus reducing the side effect of drug and times of dosage, and promoting the compliance of a sufferer. The osmotic pump type controlled release preparation of etodolac of the invention can be widely used for treating diseases like rheumatoid arthritis, arthritis deformans and osteoarthritisetc.
Owner:SHENYANG PHARMA UNIVERSITY
Anti-inflammatory analgesic for external use
InactiveUS20070054952A1Little to skinExcellent in penetratabilityBiocideAntipyreticSkin permeabilityMedicine
An objective of the present invention is to provide an anti-inflammatory analgesic for external use comprising etodolac as NSAID. The anti-inflammatory analgesic for external use is excellent not only in skin permeability but also in penetratability and diffusivity into tissues present in portions deeper than the skin, can act directly on the muscles or joint tissues with inflammation or pain, and is a little irritant to the skin. The anti-inflammatory analgesic for external use of the present invention is characterized by comprising etodolac and a local anesthetic.
Owner:MEDRX CO LTD
Anti-inflammatory analgesic for external use
InactiveUS7655687B2Excellent not only in skin permeabilityLittle to skinBiocideAntipyreticSkin permeabilityIrritation
An objective of the present invention is to provide an anti-inflammatory analgesic for external use comprising etodolac as NSAID. The anti-inflammatory analgesic for external use is excellent not only in skin permeability but also in penetratability and diffusivity into tissues present in portions deeper than the skin, can act directly on the muscles or joint tissues with inflammation or pain, and is a little irritant to the skin. The anti-inflammatory analgesic for external use of the present invention is characterized by comprising etodolac and a local anesthetic.
Owner:MEDRX CO LTD
Local preparation for remitting pains and inflammation caused by nerpes virus
A topical medicine for relaxing the pain and / or inflammation caused by herpes virus (HSV or VZV) contains diclofenac (or NSAID). The etodolac, ketoralac, ketoprofen, bromfenac, etc can be also contained. Its advantages are high curative effect and short course of treatment (7 days).
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
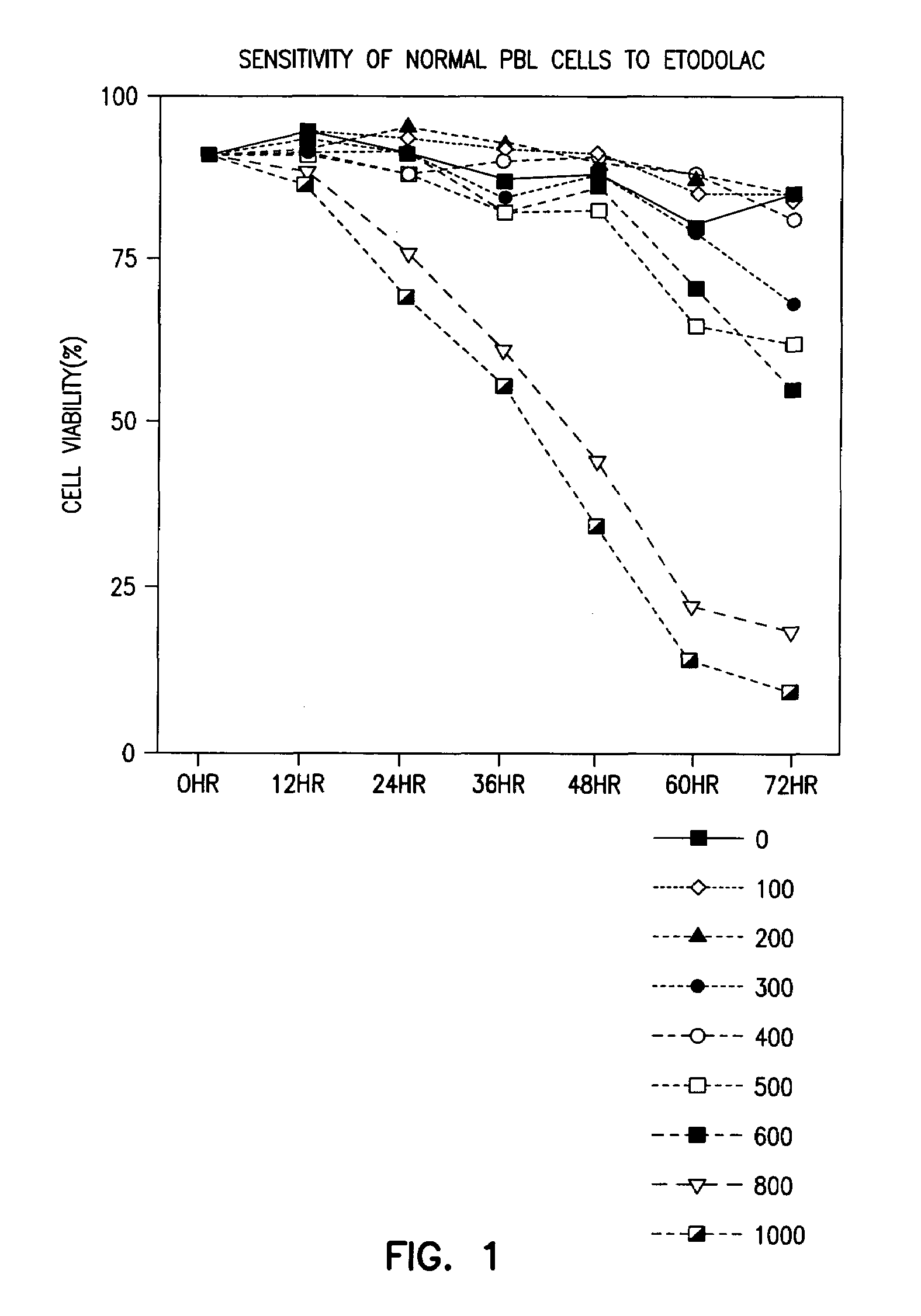
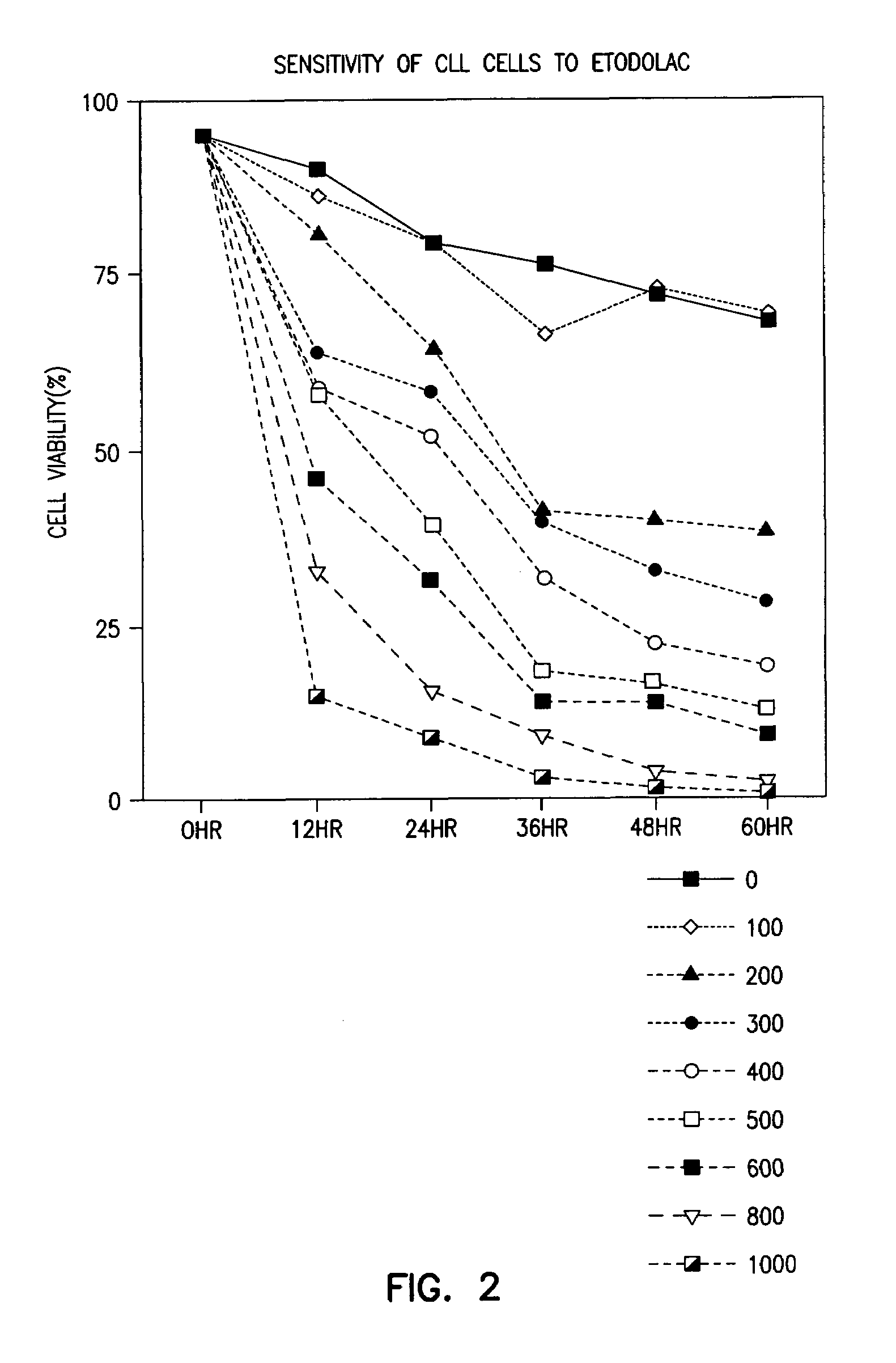
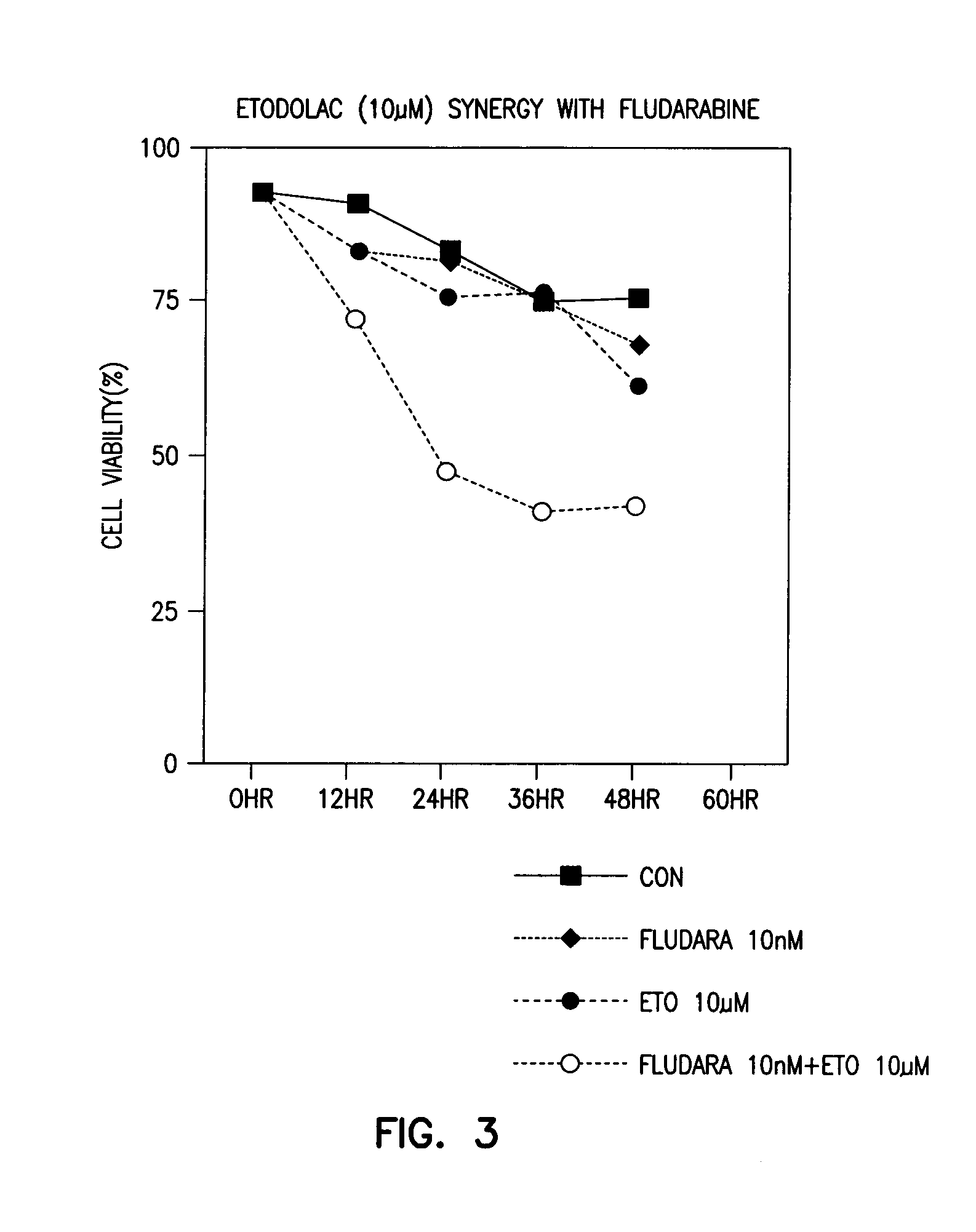
Methods and compositions for the treatment of chronic lymphocytic leukemia
The level of the leukemic lymphocytes in patients suffering from chronic lymphocytic leukemia (CLL) is reduced by the administration of certain indole or carbazole compounds, such as the nonsteroidal anti-inflammatory drug etodolac or related indole or carbazole compounds.
Owner:CEPHALON INC
Extended release formulation of etodolac
Owner:RANBAXY LAB LTD
Hard capsule containing etodolac
InactiveCN101390845ALow dissolution rateLow costOrganic active ingredientsAntipyreticHard CapsuleEtodolac
The invention relates to a hard capsule which contains etodolac, the substance in the capsule contains glutin as anti-crosslinking agent; the hard capsule which contains etodolac has the advantages of good stability, avoiding the crosslinking reaction on the capsule shell, being applicable to analgesia, diminishing inflammation and antipyretic.
Owner:BEIJING D VENTUREPHARM TECH DEV
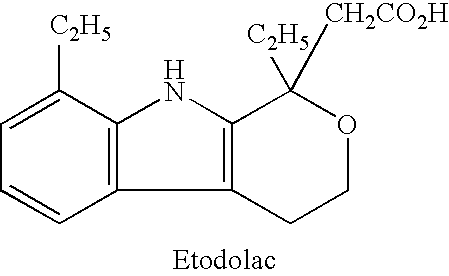
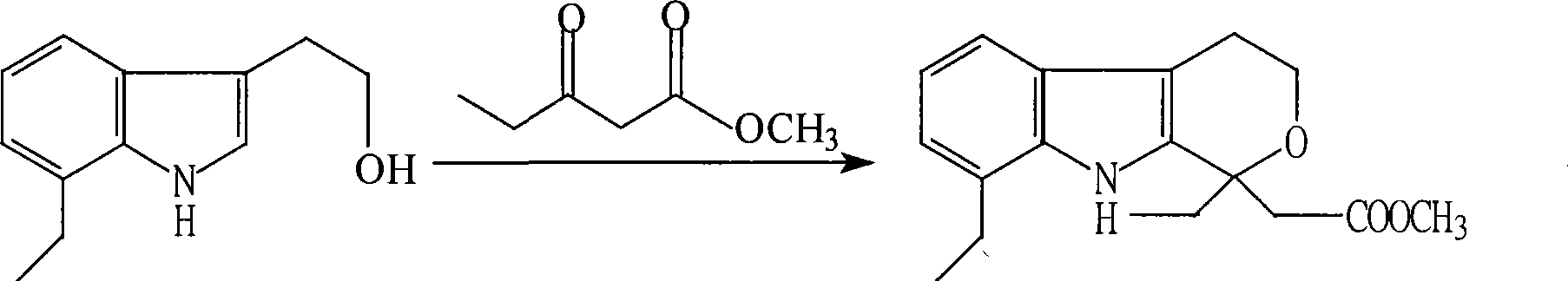
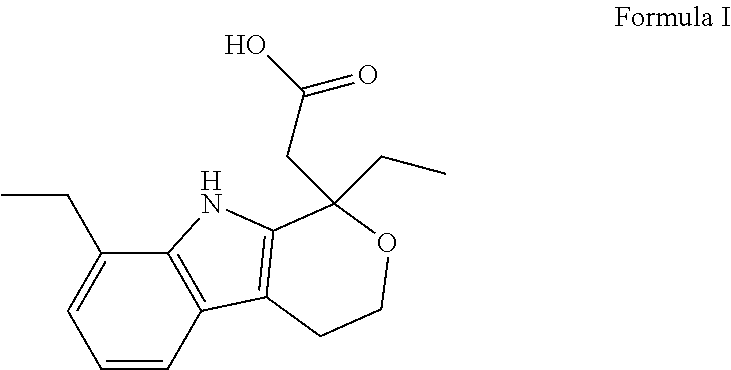
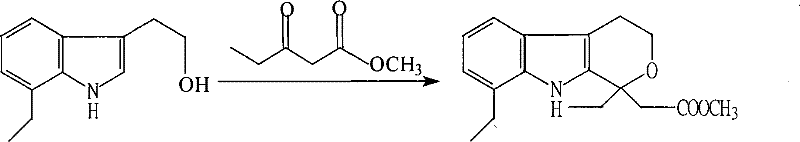
Method for preparing etodolac methyl ester
The present invention relates to new process of preparing methyl etodolate as the intermediate of non-steroid indole anti-inflammatory analgesic etodolic acid. The process includes the reaction between 7-ethyl tryptosol and 3-methyl oxy valerate inside mixed solvent of C1-C2 alcohol and benzene in the presence of acid catalyst, separating out acid layer after reaction, neutralizing the organic phase, concentrating, re-crystallizing. The process has high stability, high yield, high product quality, conversion rate near to 100 %, simple operation low cost, environment friendship and other advantages, and is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Positively charged water-soluble prodrugs of aryl- and heteroarylacetic acids with very fast skin penetration rate
InactiveCN101506160AGood absorption rateSignificant effectOrganic chemistryDipeptide ingredientsSolubilityZomepirac sodium
The novel positively charged pro-drugs of aryl- and heteroarylacetic acids in the general formula(1) 'Structure 1' were designed and synthesized. The compounds of the general formula(1) 'Structure 1' indicated above can be prepared from functional derivatives of tolmetin, zomepirac, etodolac, amfenac, bromofenac, alclofenac, fenclofenac, acemetacin, indomethacin, sulindac, fentiazac, lonazolac, bendazac, 6MNA, ibufenac, and related compounds, (for example acid halides or mixed anhydrides), by reaction with suitable alcohols, thiols, or amines. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin 100 times faster than does tolmetin, zomepirac, etodolac, amfenac, bromofenac, alclofenac, fenclofenac, acemetacin, indomethacin, sulindac, fentiazac, lonazolac, bendazac, or related compounds. It takes 2-4 hours for tolmetin, zomepirac, etodolac, amfenac, bromofenac, alclofenac, fenclofenac, acemetacin, indomethacin, sulindac, fentiazac, lonazolac, bendazac, 6MNA, ibufenac, and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about 40-50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the drug in a few minutes. The prodrugs can be used medicinally in treating any NSAIAs-treatable conditions in humans or animals. The prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of NSAIAs, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis.
Owner:于崇曦 +1
External applied preparation for etodolac
Osteoarthritis and rheumatoid arthritis are common chronic diseases, which have high morbidity and hard to heal, therefore a long period medicine treatment is required. Life of the patient can be threatened if the long period medicine treatment is not well insisted. Currently, among medicine for arthritis, nonsteroidal anti-inflammatory drugs are most important. But currently oral preparation account for the most portions, which has more side effects. Etodolac belongs to the indole nonsteroidal anti-inflammatory drugs, which play an important role in arthritis treatment. But the current oral preparation has a plurality of side effects, which brings trouble to the long period treatment in patients. Therefore the present invention develops an external preparation of Etodolac, which not only greatly reduces side effects of the drugs, but also applicable for long period treatment of arthritis particularly.
Owner:LUNAN PHARMA GROUP CORPORATION
Self-assembly system based on hydrophilic polymer and medicine and preparation method thereof
ActiveCN102091332AEasy to realize industrializationEasy to makeMacromolecular non-active ingredientsIndometacinPolymer science
The invention relates to a self-assembly system based on hydrophilic polymer and medicine and a preparation method thereof. The system consists of a hydrophilic polymer and a carboxyl-containing medicine, wherein the hydrophilic polymer is polymine or beta-cyclodextrin modified polymine; and the carboxyl-containing medicine is selected from ibuprofen, ketoprofen, fenoprofen, flurbiprofene, oxaprozin, naproxen, indometacin, sulindac, etodolac, diclofenac, pontal, meclofenamic acid, flufenamic acid, tolfenamic acid, lumiracoxib, licofelone, diflunisal and aspirin. The system is prepared by dissolving the hydrophilic polymer in a certain amount of water, dissolving the medicine in a water soluble solvent, slowly adding the organic solution of the medicine into the aqueous solution of the polymer under the action of ultrasound, placing the mixed solution in a dialysis bag, dialyzing in deionized water with magnetic stirring, replacing the deionized water at certain time interval, filtering dislysate after 5 to 48 hours, cooling and drying.
Owner:ARMY MEDICAL UNIV
Extended release formulation of etodolac
Owner:RANBAXY LAB LTD
Tape preparation comprising etodolac in ionic liquid form
InactiveUS20100280090A1Improve transdermal permeabilityExcellent tissue penetration abilityPowder deliveryBiocideSolventCervical syndrome
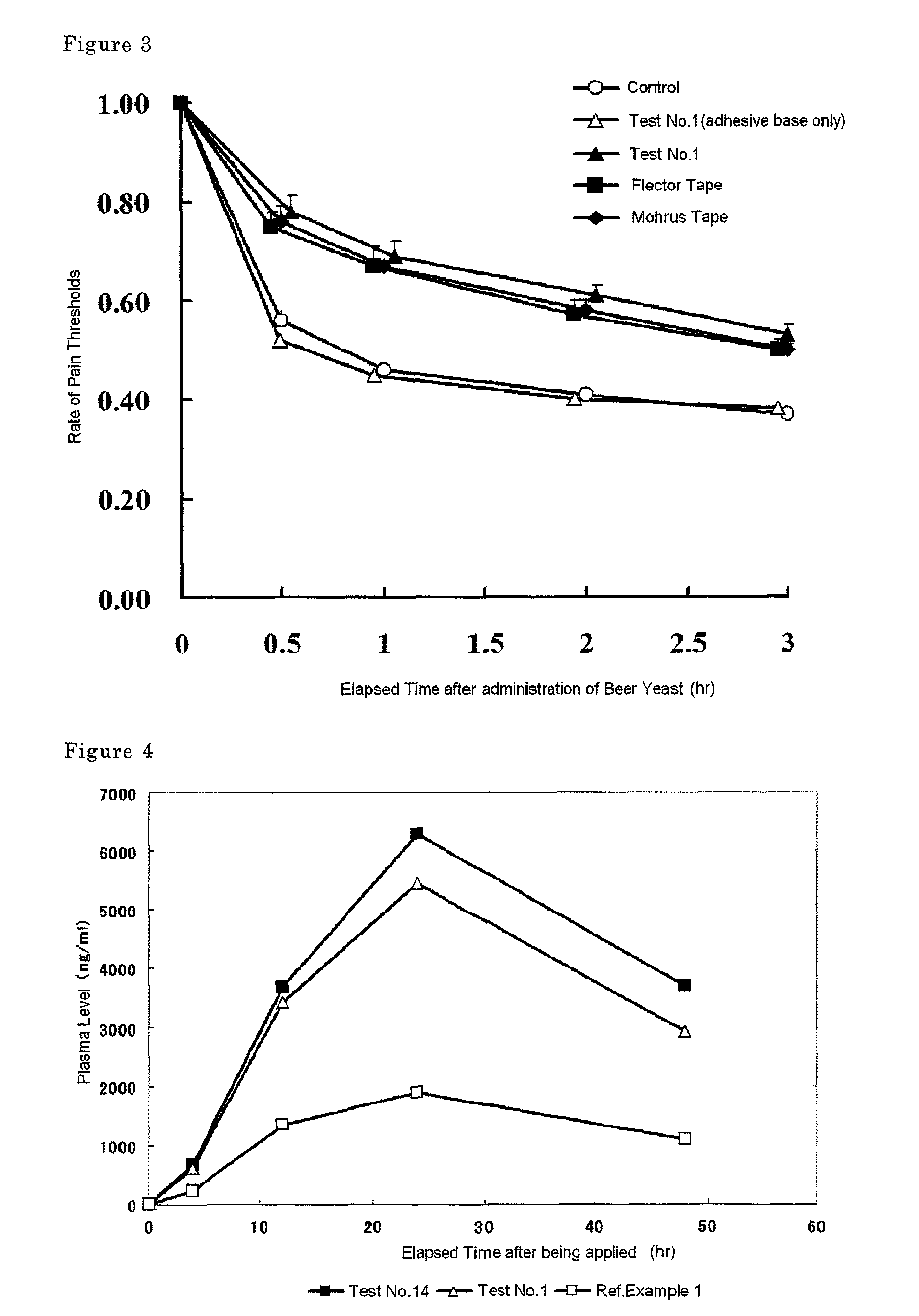
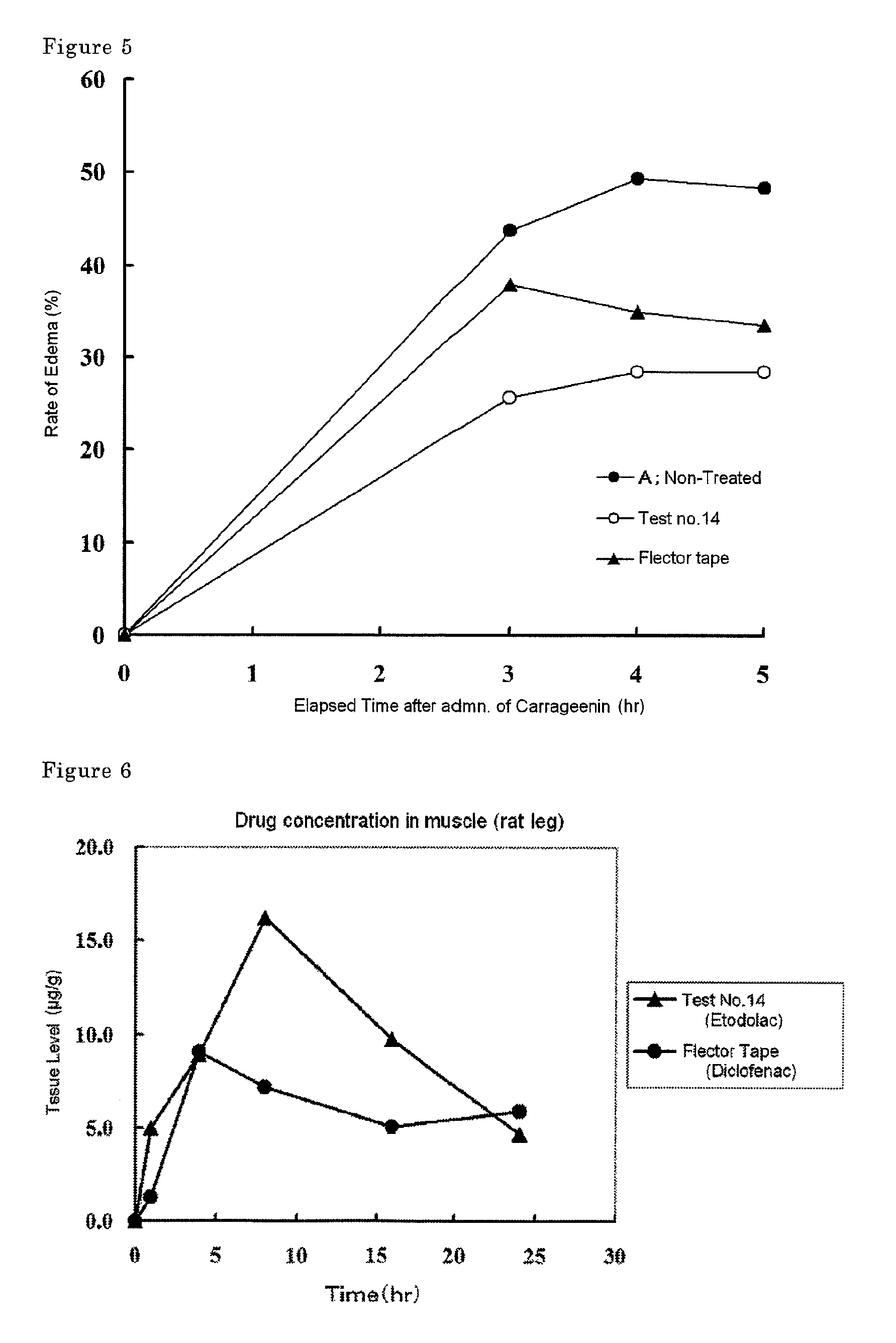
Disclosed is a tape preparation comprising etodolac in an ionic liquid form, which has high transdermal absorbability. Etodolac is reacted with an organic amine compound to produce an ionic liquid of etodolac. By using the ionic liquid, it becomes possible to increase the transdermal absorbability of etodolac. Further for the purpose of enhancing the transdermal absorbability and the tissue penetration ability of an ionic solution of etodolac, the composition of an organic solvent system for the ionic solution of etodolac is investigated, and it is found that a mixed solvent of an alcohol and an ester (1:2 to 2:1) is suitable as the organic solvent. Still further, an appropriate adhesion force can be achieved by properly selecting a softening agent. In this manner, a tape preparation having good transdermal absorbability can be prepared. The tape preparation can exert its pharmacological efficacy rapidly, and is therefore extremely effective for the teatment of a chronic pain such as rheumatoid arthritis, osteoarthritis and lumbago, an inflammatory diseases such as shoulder periarthritis and tendovaginitis, cervical syndrome, a pain induced by a surgery or an injury, or the like.
Owner:MEDRX CO LTD
Use of etodolac to treat hyperplasia
InactiveUS20070299042A1Inhibit and control growthReduced viabilityBiocideKetone active ingredientsNon malignantMedicine
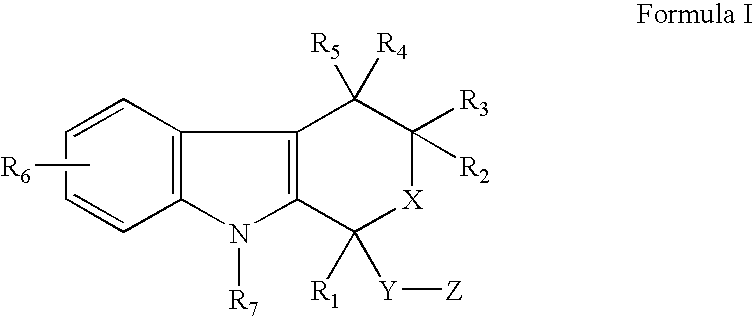
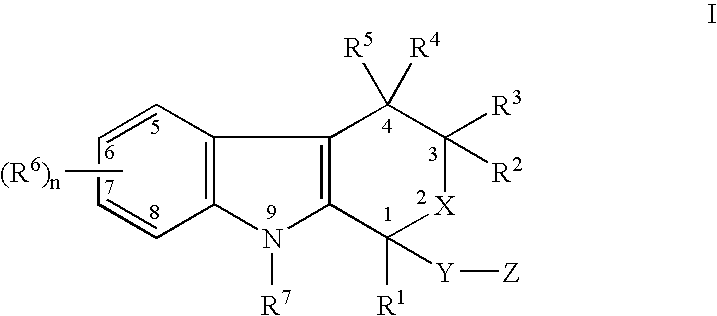
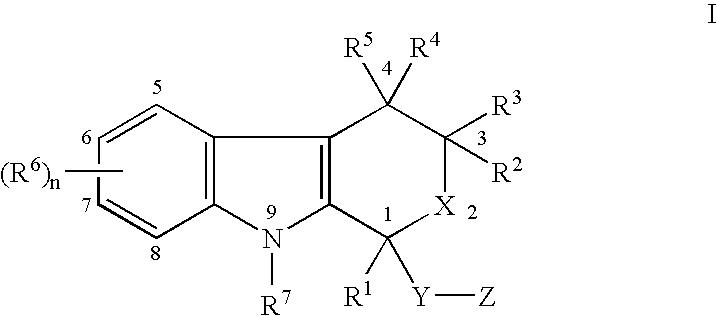
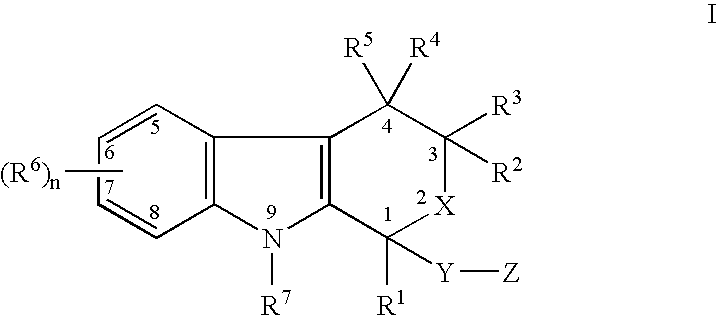
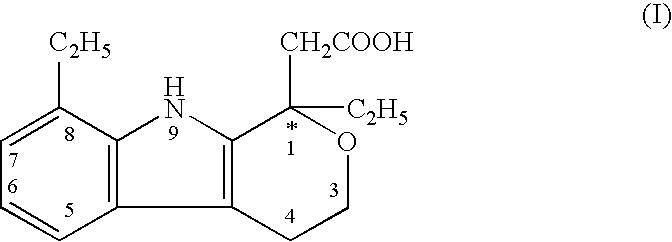
The present invention provides a therapeutic method to treat non-malignant diseases characterized by the excessive tissue growth, e.g., hyperplastic diseases, comprising administering to a mammal (e.g., human) afflicted with excessive tissue growth, an effective amount of a derivative of an indole compound of formula (I):formula (I): wherein R1 is lower alkyl, (hydroxy)lower alkyl, lower alkenyl, lower alkynyl, lower cycloalkyl, phenyl, benzyl or 2-thienyl; R2, R3, R4 and R5 are the same or different and are each hydrogen or lower alkyl; each R6 is individually hydrogen, lower alkyl, hydroxy, (hydroxy)lower alkyl, lower alkoxy, benzyloxy, lower alkanoyloxy, nitro or halo, R7 is hydrogen, lower alkyl or lower alkenyl, X is oxy and thio, Y is carbonyl, —(CH2)1-3—, —(C1-C3)alkyl(CO)—, or —(CH2)1-3SO2—; Z is hydroxy, lower alkoxy, (C2-C4)acyloxy, —N(R8)(R9), phenylamino, (ω-(4-pyridyl)(C2-C4 alkoxy), (ω-((R8)(R9) amino)(C2-C4 alkoxy), an amino acid ester of (ω-(HO)(C2-C4))alkoxy, —N(R8)CH(R8)CO2H, 1′-D-glucuronyloxy, —SO3H, —PO4H2, —N(NO)(OH), —SO2NH2, —PO(OH)(NH2), —OCH2CH2N(CH3)3+, or tetrazolyl; wherein R8 and R9 are each H, (C1-C3)alkyl or together with N are a 5- or 6-membered heterocyclic ring comprising 1-3 N(R8), S or nonperoxide O; n is 0, 1, 2, or 3; wherein R8 and R9 are each H, (C1-C3)alkyl or together with N are a 5- or 6-membered heterocyclic ring comprising 1-3 N(R8), S or nonperoxide O; each alkyl or phenyl group of R1, R2, R3, R4,R5, R6, R7 and Z is optionally substituted with 1, 2, or 3 (C1-C4)alkyl groups; or a pharmaceutically acceptable salt thereof.
Owner:CARSON DENNIS A +2
Pharmaceutical composition of etodolac and medical application thereof
InactiveCN105708837AHas therapeutic effectGood treatment effectAntipyreticAnalgesicsNatural productTreatment effect
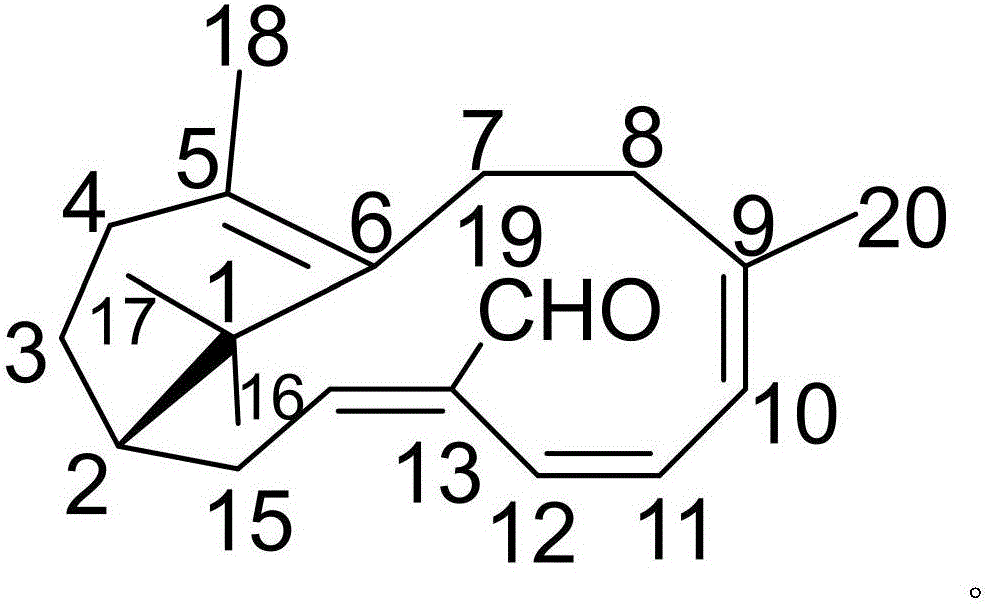
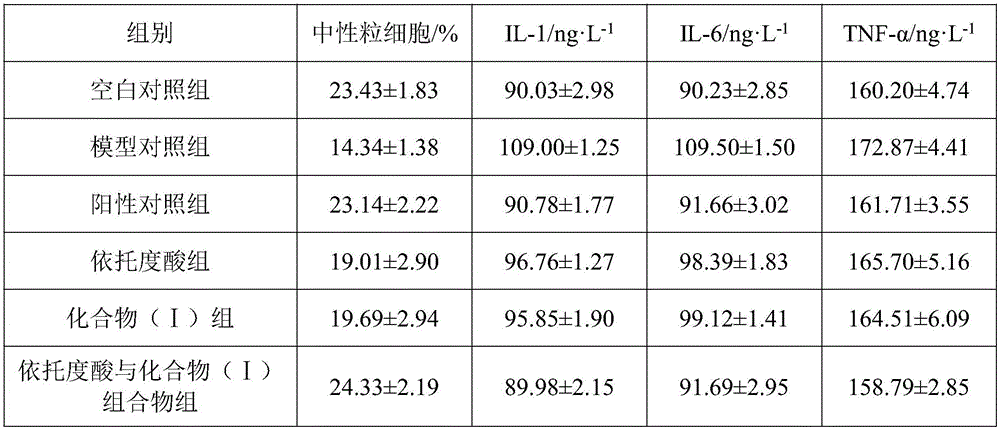
The invention discloses a pharmaceutical composition of etodolac and its medical use. The pharmaceutical composition of etodolac provided by the invention contains etodolac and a novel natural product compound (I), etodolac 1. When compound (I) acts alone, it has a therapeutic effect on acute pharyngitis; when etodolac and compound (I) act in combination, the therapeutic effect on acute pharyngitis is further improved, and it can be developed into a medicine for the treatment of acute pharyngitis. It has outstanding substantive features and notable progress compared with other products.
Owner:周俭
Etodolac capsule and preparation method thereof
ActiveCN104257625ASimple preparation processSuitable for industrial productionOrganic active ingredientsInorganic non-active ingredientsMedicinePotassium hydroxide
The invention discloses an etodolac capsule and a preparation method thereof. The etodolac capsule consists of etodolac acid, monopotassium phosphate, potassium hydroxide and a lubricant. Compared with the prior art, the etodolac capsule is simple in preparation process and suitable for industrial production, and has the advantages that the crosslinking of the etodolac acid and gelatin is avoided, the drug digestion is not remarkably changed after long-time and accelerated observation and the accessory type and dosage are less.
Owner:SHANDONG NEWTIME PHARMA
Etodolac double-layered osmotic pump controlled release tablets, and preparation method thereof
InactiveCN102335156AStable release rateEliminate side effectsOrganic active ingredientsAntipyreticSemipermeable membraneControlled Release Tablet
The invention belongs to the field of medicines, and specifically discloses etodolac double-layered osmotic pump controlled release tablets, and a preparation method thereof. The etodolac double-layered osmotic pump controlled release tablets provided by the invention are double-layered tablets with semi permeable membranes. The upper layers of the tablets are medicine-containing layers comprising medicines and auxiliary materials, and the lower layers are assisting layers composed of polymers and osmotically active substances. The etodolac double-layered osmotic pump controlled release tablets provided by the invention have advantages of stable in vivo blood-drug level, and long effective blood-drug level duration.
Owner:SHIJIAZHUANG UNIVERSITY
Topical pharmaceutical compositions comprising etodolac
The present invention relates to stable pharmaceutical compositions comprising nano size droplets of etodolac or salts thereof along with other pharmaceutically acceptable excipients. These compositions exhibit greater permeability, and improved bioavailability leading to enhanced therapeutic activity. The invention also relates to processes for the preparation of such compositions.
Owner:CADILA HEALTHCARE LTD
Method for preparing etodolac methyl ester
The present invention relates to a new process of preparing methyl etodolate as the intermediate of non-steroid indole anti-inflammatory analgesic etodolic acid. The process includes the reaction between 7-ethyl tryptosol and 3-methyl oxy valerate inside mixed solvent of C1-C2 alcohol and benzene in the presence of acid catalyst, separating out acid layer after reaction, neutralizing the organic phase, concentrating, re-crystallizing. The process has high stability, high yield, high product quality, conversion rate near to 100 %, simple operation low cost, environment friendship and other advantages, and is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
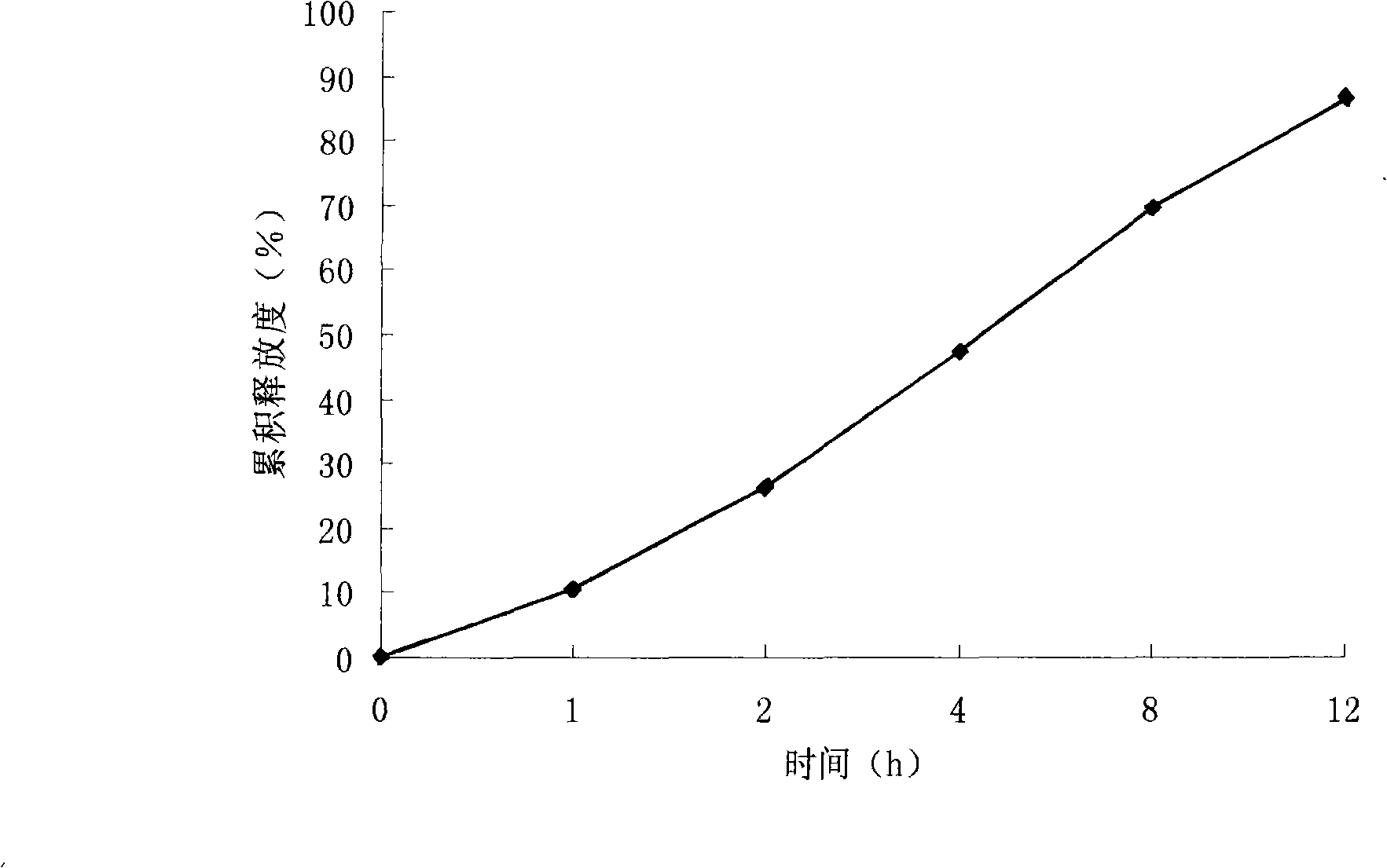
Preparation method of etodolac sustained release preparation
InactiveCN109010315AGood slow release functionRelease stabilityOrganic active ingredientsAntipyreticMANNITOL/SORBITOLPolyethylene glycol
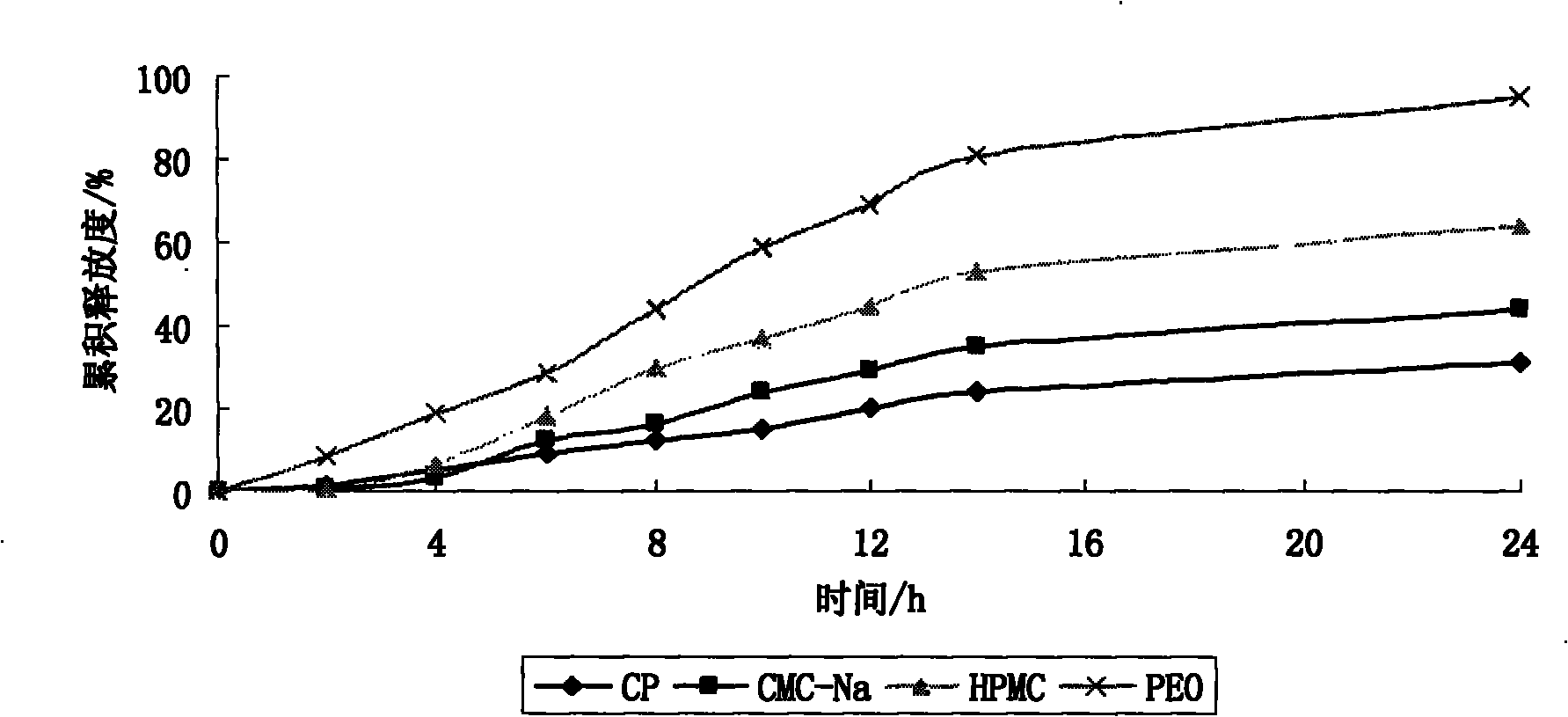
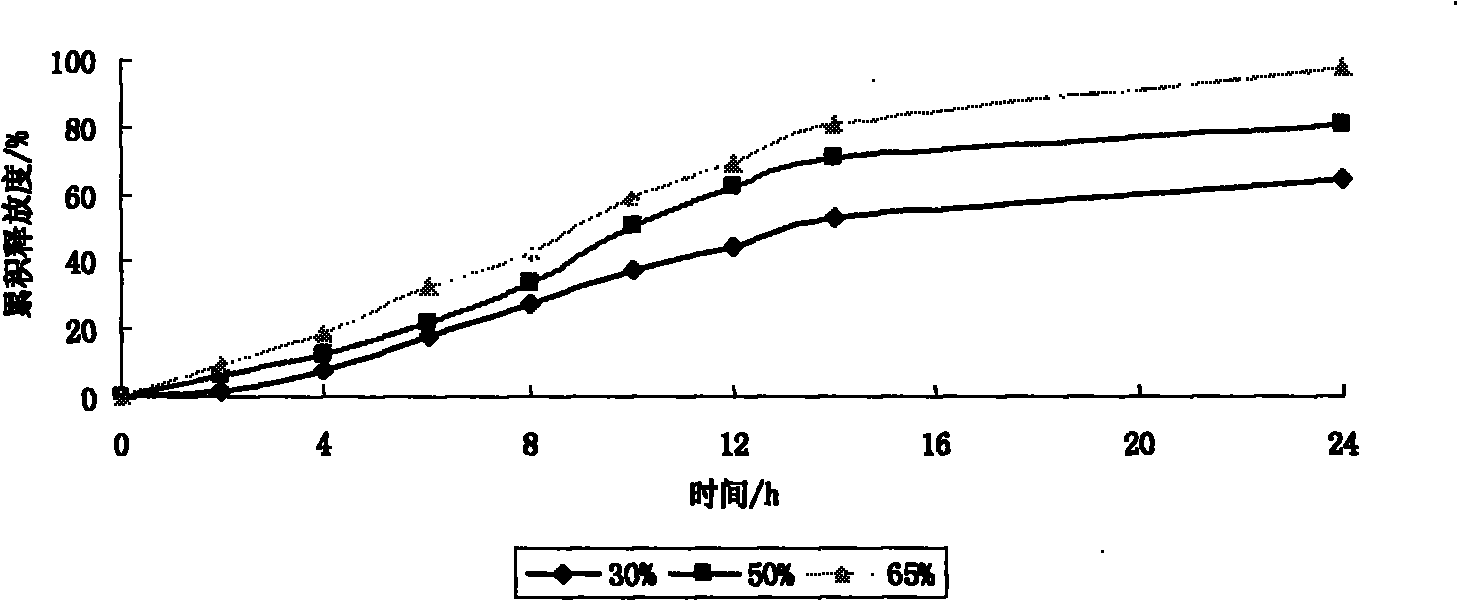
The invention provides a preparation method of an etodolac sustained release preparation. The preparation method is characterized in that the etodolac is dissolved in 95% ethanol to obtain a solutionA, and hydroxypropyl beta-cyclodextrin and mannitol are added in the solution A to obtain a solution B; polylactic acid and polyethylene glycol 200 are dissolved in acetone to obtain a solution C, thesolution B and the solution C are mixed to obtain a solution D, the solution D is transferred to a magnetic stirrer, the solution D is continuously stirred for 12 hours, the temperature of the solution D is lowered to 0 DEG C to 1 DEG C in 2 hours and the solution D is subjected to standing for 12 hours, and the temperature of the solution D is kept to 0 DEG C to 1 DEG C during a standing period;after standing for 12 hours, the solution D is heated, when the temperature of the solution D is increased to 15 DEG C to 18 DEG C, the solution D is continuously stirred, when the temperature of thesolution D is controlled at 15 DEG C to 18 DEG C while stirring, after the material is continuously stirred for 12 hours, the etodolac sustained release preparation is prepared by a low-temperature spray-drying method. The dosage of a capsule material is moderate, the drying temperature of the material is low, the obtained drug-loading preparation is uniform, the drug release is stable, and the etodolac sustained release preparation has sustained release characteristics.
Owner:刘丽
External applied preparation for etodolac
Osteoarthritis and rheumatoid arthritis are common chronic diseases with high incidence and difficult to cure in our country, requiring long-term medication. At present, non-steroidal anti-inflammatory drugs (NSAIDs) are the most important drugs for the treatment of arthritis, but most of them are oral preparations, which have serious adverse reactions. Etodolac belongs to indole non-steroidal anti-inflammatory analgesics and plays an important role in the treatment of arthritis, but the current oral dosage forms have many systemic adverse reactions, which bring difficulties to patients with long-term medication. Therefore, according to patients Need, the present invention develops etodolac external preparation, which can not only greatly reduce the adverse reactions of the drug, but also is especially suitable for the long-term drug treatment of arthritis.
Owner:LUNAN PHARMA GROUP CORPORATION
Etodolac osmotic pump type controlled-release preparation and preparation thereof
InactiveCN101259113BAdjust the rate of constant releaseImprove complianceOrganic active ingredientsAntipyreticDiseaseSide effect
The invention belongs to the pharmaceutical preparation field and discloses an osmotic pump type controlled release preparation of etodolac and a preparation method thereof. An osmotic pump tablet comprises a tablet core and a controlled release semipermeable coating film that is coated outside the tablet core and with a hole for releasing drug. The tablet core comprises materials by following weight percentage: 60.9 to 65.5 percent of etodolac, 27.1 to 31.2 percent of osmotic pressure active material, 5.5 to 8.0 percent of an auxiliary material that can ensure the basic remedy to be released easily and the rest is other auxiliary materials. The osmotic pump controlled release semipermeable coating film comprises by materials by following weight percentage: 71.4 to 76.9 percent of semipermeable high molecular coating material and 23.1 to 28.6 percent of pore-forming agent. The manners of pore-forming include a mechanical drilling and a laser boring. By adjusting the prescription of the tablet core and the coating film in the invention, the speed of constant release of drug can be effectively regulated so as to obtain a steadier, lasting and effective blood concentration, thus reducing the side effect of drug and times of dosage, and promoting the compliance of a sufferer. The osmotic pump type controlled release preparation of etodolac of the invention can be widely used for treating diseases like rheumatoid arthritis, arthritis deformans and osteoarthritisetc.
Owner:SHENYANG PHARMA UNIVERSITY
Etodolac ionic salt as well as preparation method and application thereof
PendingCN112079842AGood water solubilityPromote absorptionOrganic active ingredientsCosmetic preparationsFreeze-dryingAlkaloid
The invention belongs to the field of compounds for medicines and cosmetics, and discloses etodolac ionic salt as well as a preparation method and application thereof. The cations of the etodolac ionsalt are formed by alkaloid, and anions of the etodolac ion salt are formed by etodolac. The preparation method of the etodolac ion salt comprises the following steps: (1) in an inert gas atmosphere,adding etodolac and alkaloid into an organic solvent, and carrying out an ionization modification reaction to prepare an etodolac ion salt solution; and (2) concentrating the etodolac ion salt solution prepared in the step (1) under a vacuum condition, and freeze-drying to obtain the etodolac ion salt The etodolac ion salt completely retains main molecular skeletons and functional groups of cations and anions, has biological activities and effects of the two substances, and shows a good anti-inflammatory effect, water solubility and low cytotoxicity.
Owner:HARBIN INST OF TECH SHENZHEN GRADUATE SCHOOL
Method for separating and preparing trace impurity in etodolac bulk drug
InactiveCN112961164AHigh purityHigh recovery rateOrganic chemistryUltraviolet detectorsPhysical chemistry
The invention discloses a method for separating and preparing a trace impurity in an etodolac bulk drug. The molecular formula of the trace impurity is C29H34N2O3, and the trace impurity in the etodolac bulk drug is separated and prepared by adopting a gradient elution high-speed counter-current chromatography. A solvent system of the counter-current chromatography comprises normal hexane, ethyl acetate, ethanol and water. The elution mode is gradient elution. Online monitoring is performed through an ultraviolet detector, target fractions are collected and combined, and rotary evaporation is performed until the fractions are dry to obtain the trace impurity. The method provided by the invention enables the quality of etodolac series products to be easily controlled.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Tape preparation comprising etodolac in ionic liquid form
InactiveUS8697096B2Improve permeabilityRapidly exerts its pharmaceutical activityBiocidePowder deliveryPharmacologic actionSolvent
Disclosed is a tape preparation comprising etodolac in an ionic liquid form, which has high transdermal absorbability. Etodolac is reacted with an organic amine compound to produce an ionic liquid of etodolac. By using the ionic liquid, it becomes possible to increase the transdermal absorbability of etodolac. Further for the purpose of enhancing the transdermal absorbability and the tissue penetration ability of an ionic solution of etodolac, the composition of an organic solvent system for the ionic solution of etodolac is investigated, and it is found that a mixed solvent of an alcohol and an ester (1:2 to 2:1) is suitable as the organic solvent. Still further, an appropriate adhesion force can be achieved by properly selecting a softening agent. In this manner, a tape preparation having good transdermal absorbability can be prepared. The tape preparation can exert its pharmacological efficacy rapidly, and is therefore extremely effective for the teatment of a chronic pain such as rheumatoid arthritis, osteoarthritis and lumbago, an inflammatory diseases such as shoulder periarthritis and tendovaginitis, cervical syndrome, a pain induced by a surgery or an injury, or the like.
Owner:MEDRX CO LTD
Preparation method for etodolac
InactiveCN101085778AProcess stabilityMild reaction conditionsOrganic chemistryAnalgesics drugsEdetic Acid
Owner:SHANDONG XINHUA PHARMA CO LTD
Anti-inflammatory analgesic for external use
InactiveCN1909929AImprove permeabilityLess irritatingOrganic active ingredientsAntipyreticSkin permeabilityDermatomal
An objective of the present invention is to provide an anti-inflammatory analgesic for external use comprising etodolac as NSAID. The anti-inflammatory analgesic for external use is excellent not only in skin permeability but also in penetratability and diffusivity into tissues present in portions deeper than the skin, can act directly on the muscles or joint tissues with inflammation or pain, and is a little irritant to the skin. The anti-inflammatory analgesic for external use of the present invention is characterized by comprising etodolac and a local anesthetic.
Owner:MEDRX CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com