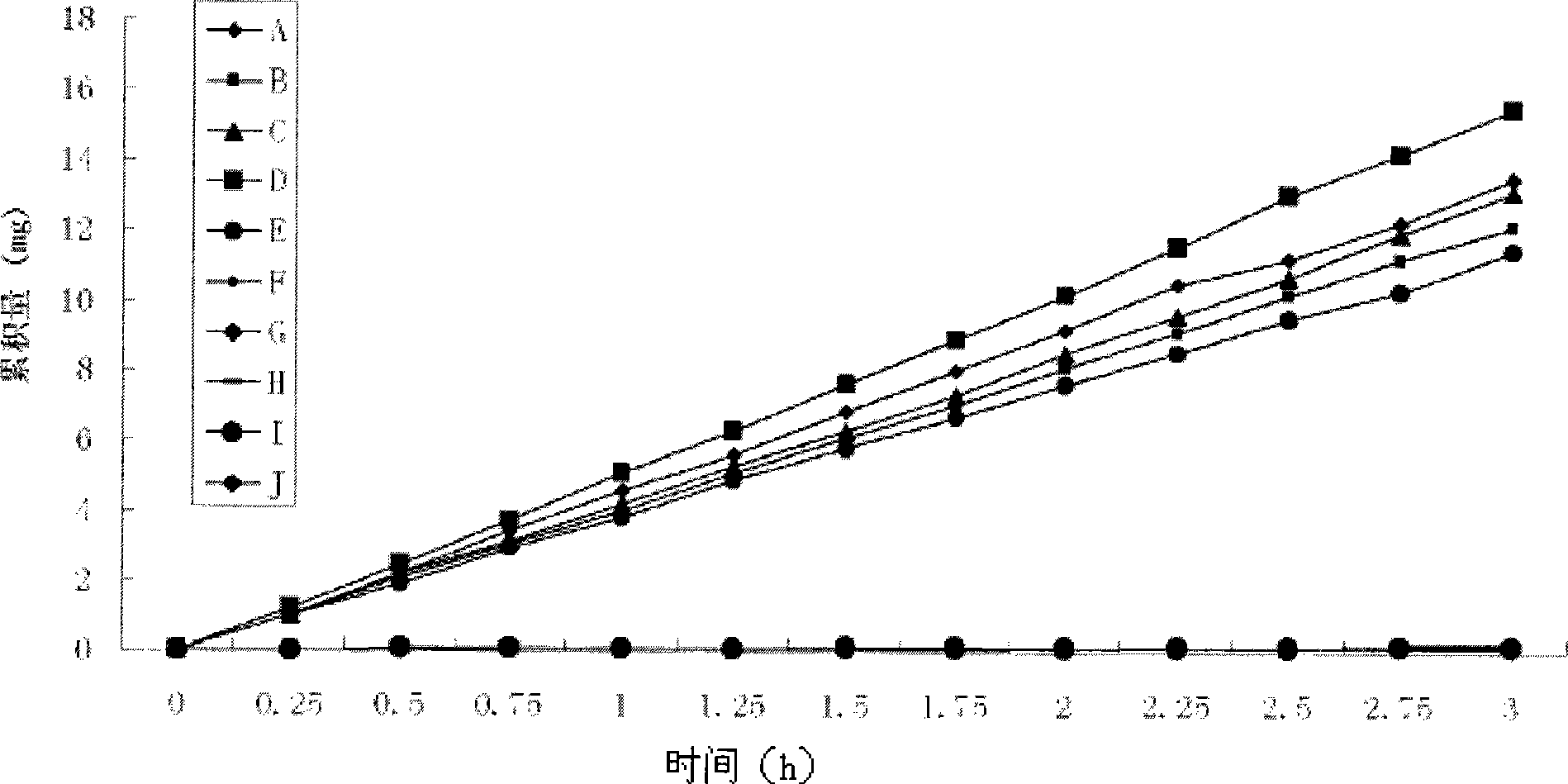
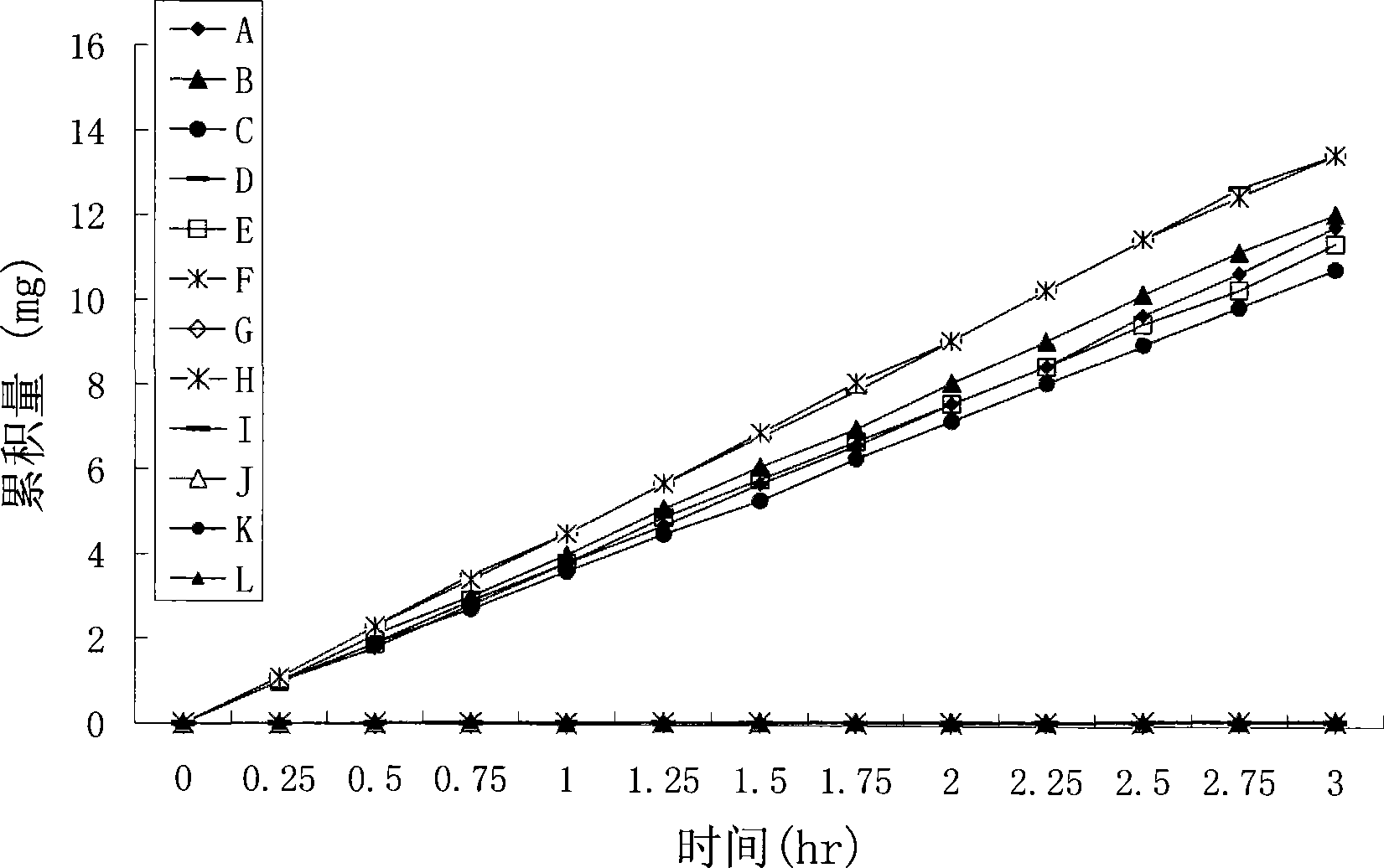
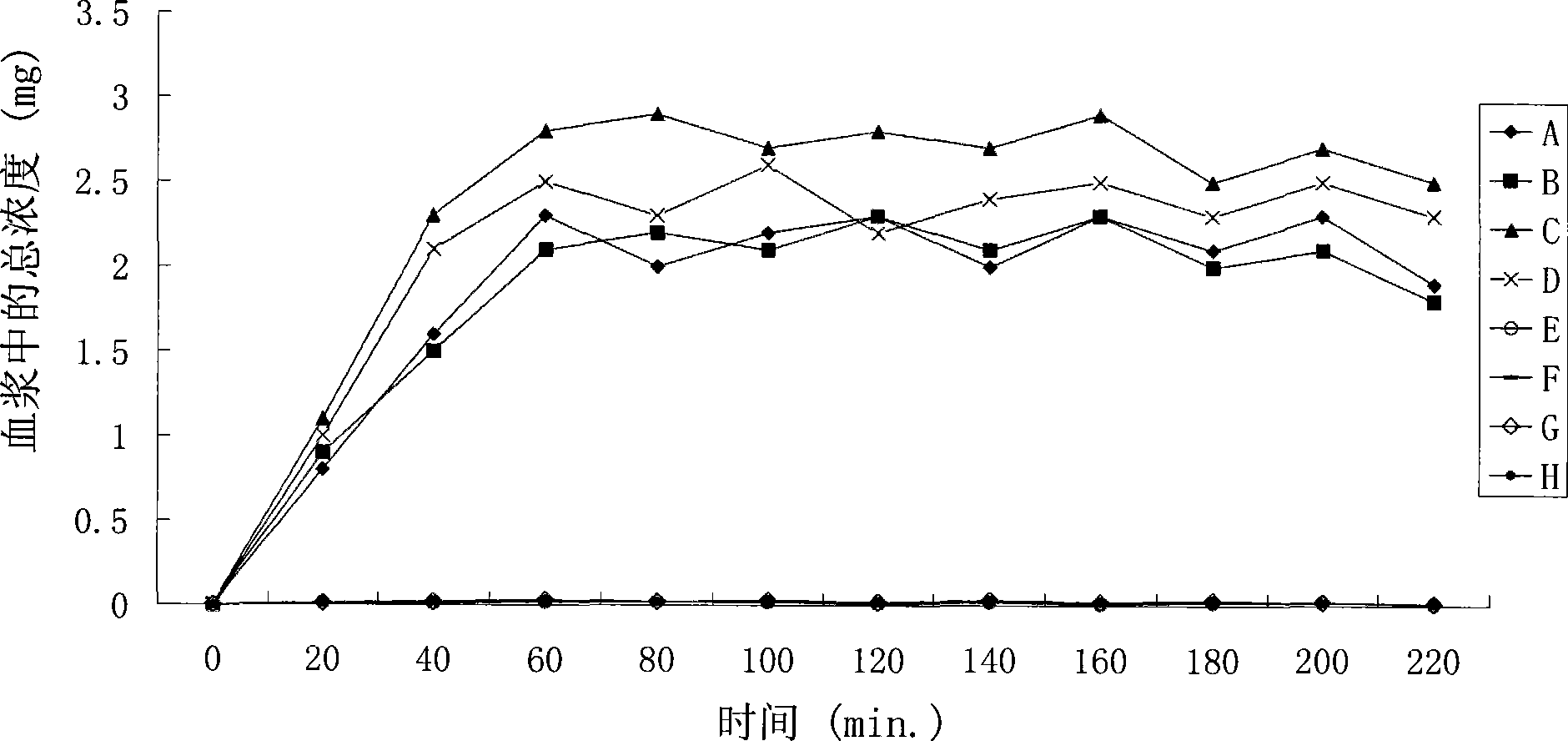
Positively charged water-soluble prodrugs of aryl- and heteroarylacetic acids with very fast skin penetration rate
An aryl-based and alkyl-based technology, which can be used in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., and can solve problems such as slow skin penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0084] Synthesis of 1-methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetic acid diethylaminoethyl acetate
[0085] 28.6 g (0.1 mol) of 1-methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetyl chloride was dissolved in 100 ml of chloroform. The mixture was cooled to 0 °C. 15 ml of triethylamine and 8.9 g (0.1 mol) of dimethylaminoethanol were added to the reaction mixture, and stirred at room temperature for 3 hours. The solvent was evaporated to dryness. The residue was dissolved in 300ml of methanol, 200ml of 5% sodium bicarbonate solution was added, and stirred for 3 hours. The mixture was evaporated to dryness. 300 ml of methanol were added to the residue with stirring. The solid was removed by filtration and washed with methanol. The filtrate was evaporated to dryness, and the residue was dissolved in 200 ml of chloroform. 6 g of acetic acid was added to the reaction mixture with stirring. The solids were removed by filtration. An additional 6 g of acetic acid was added to the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com