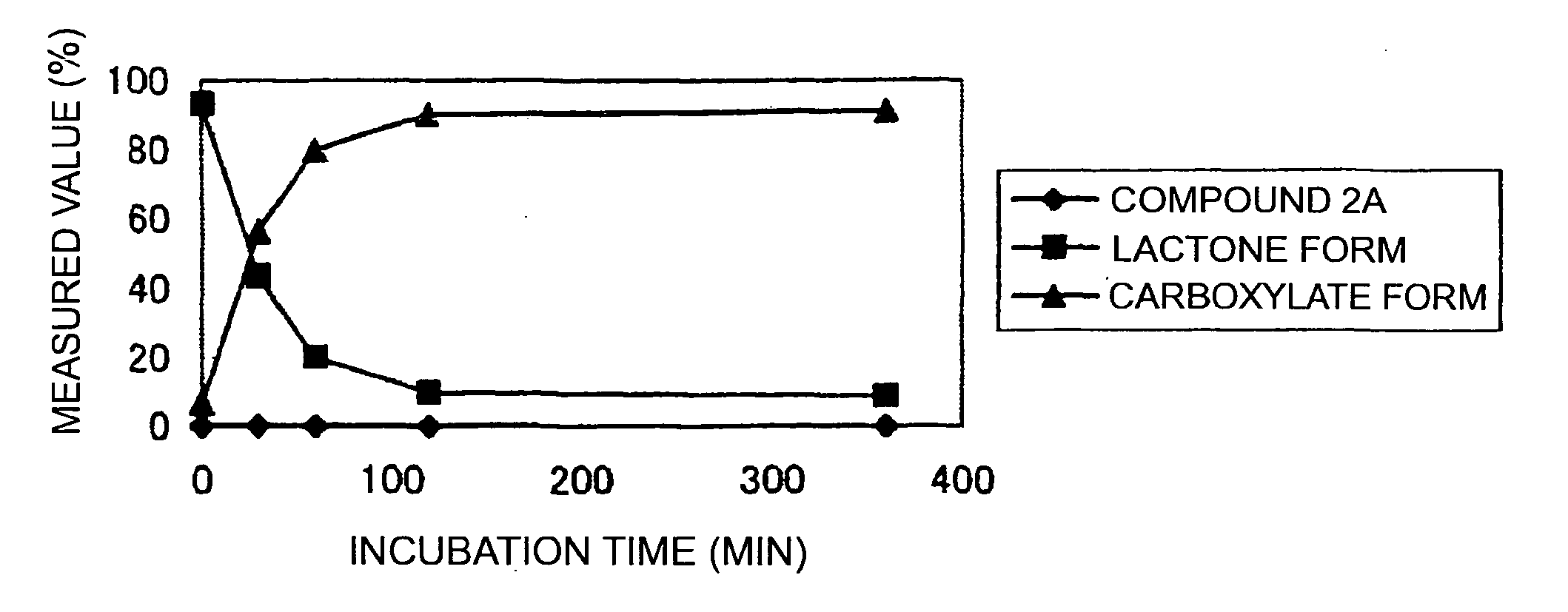
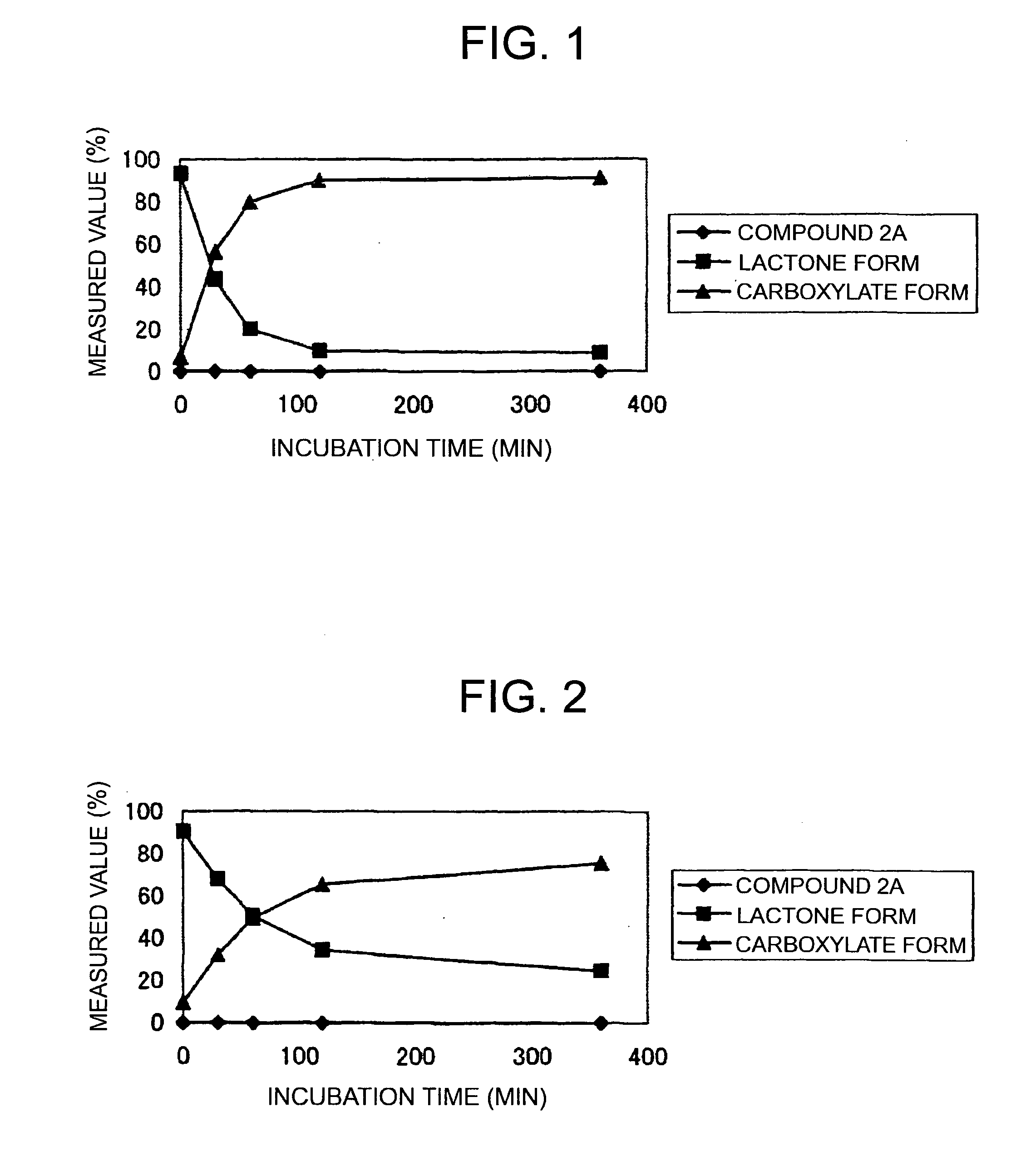
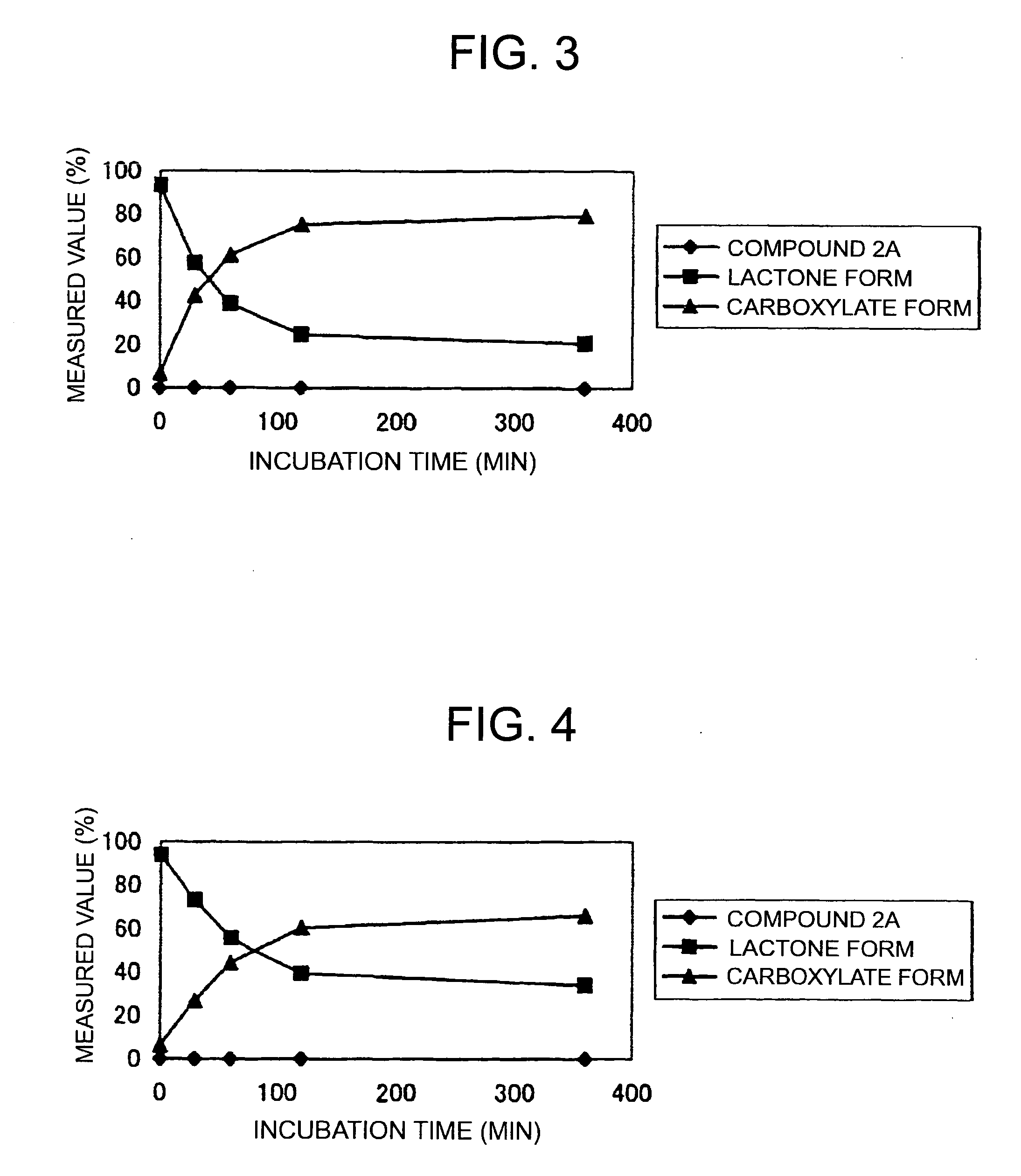
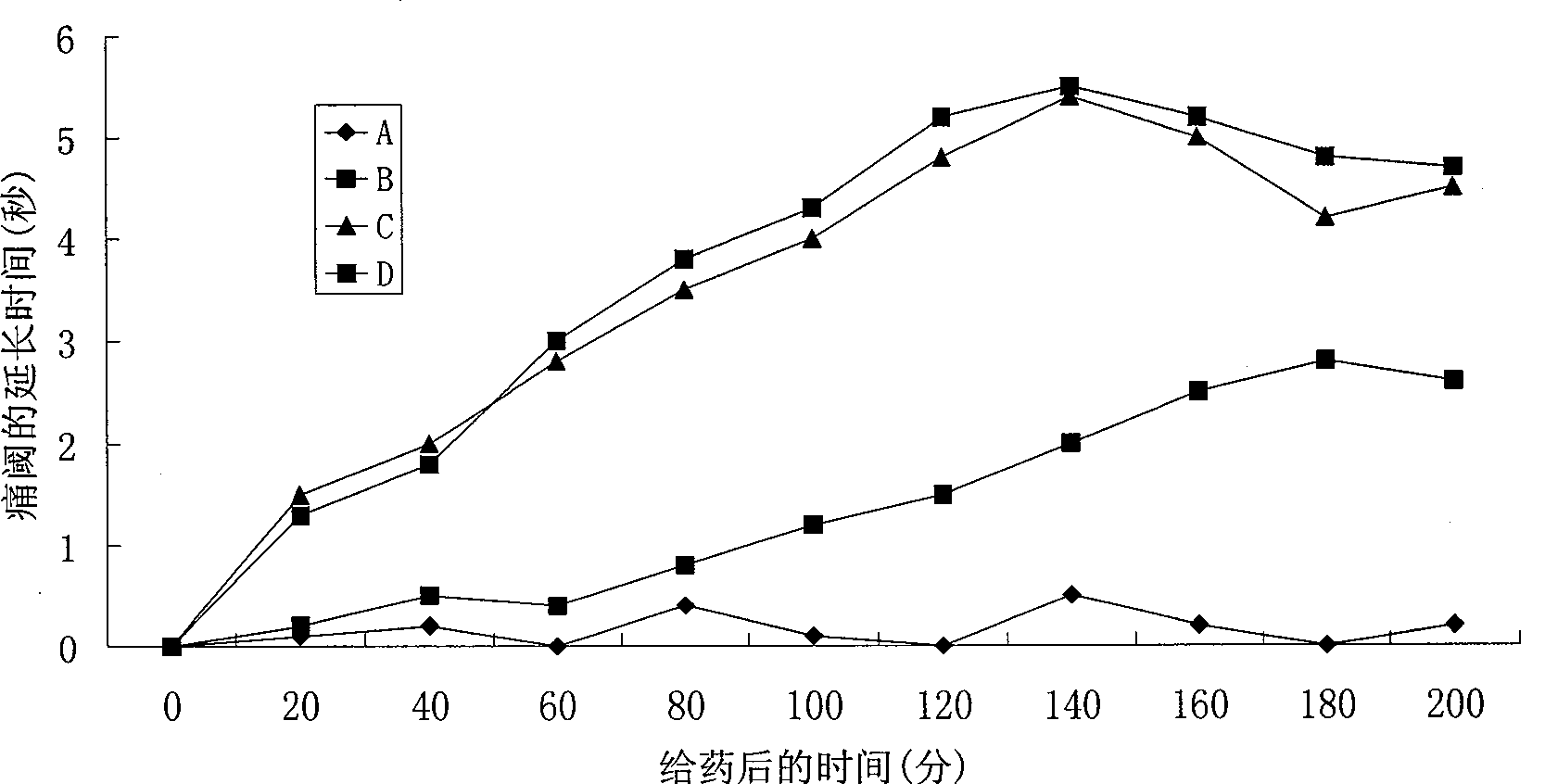
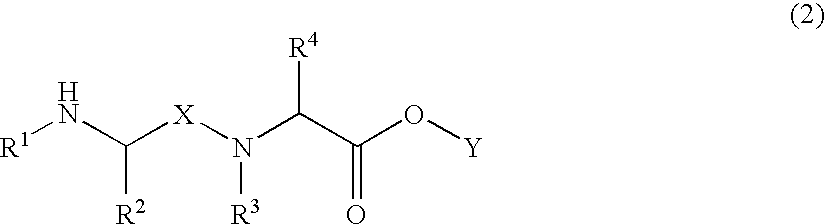
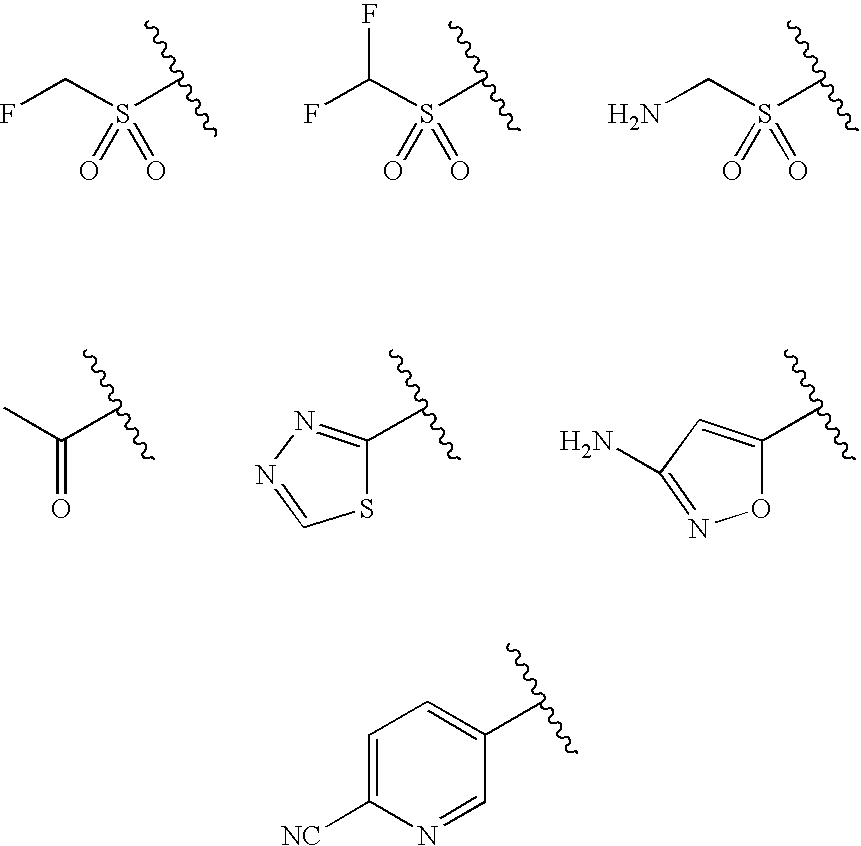
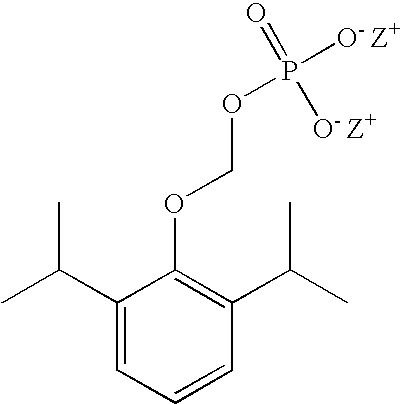
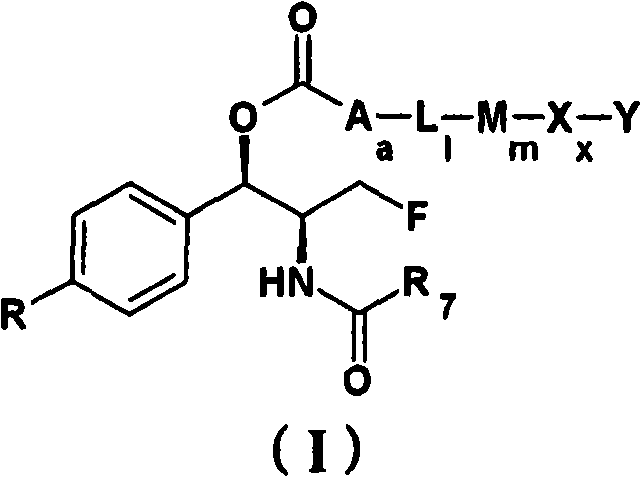
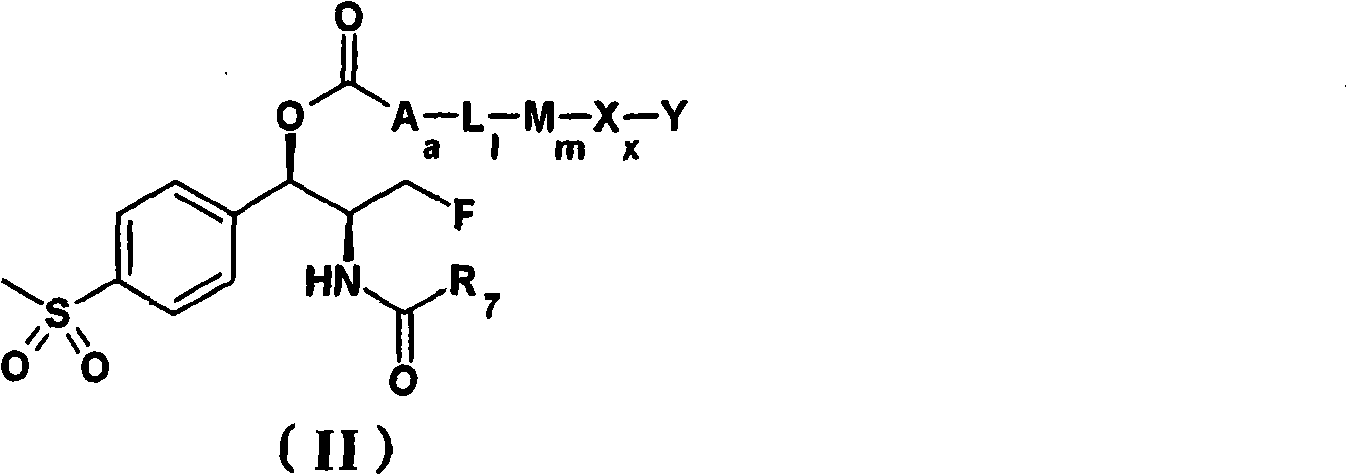Patents
Literature
79 results about "Water soluble prodrug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water Soluble Prodrugs • As the name suggests water soluble prodrugs are formulated using the aqueous solubility of the drug and for enhancing the oral drug delivery, generally includes the addition of an ionizable progroup to the parent compound (such as phosphate group).
Multi-arm polymer prodrugs
ActiveUS7744861B2Improve balanceHigh drug loadingPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsWater soluble prodrugPolymer science
Provided herein are water-soluble prodrugs. The prodrugs of the invention comprise a water-soluble polymer having three or more arms, at least three of which are covalently attached to an active agent, e.g., a small molecule. The conjugates of the invention provide an optimal balance of polymer size and structure for achieving improved drug loading, since the conjugates of the invention possess three or more active agents releasably attached to a multi-armed water soluble polymer. The prodrugs of the invention are therapeutically effective, and exhibit improved properties in-vivo when compared to unmodified parent drug.
Owner:NEKTAR THERAPEUTICS INC
Water soluble prodrugs of COX-2 inhibitors
Disclosed are water soluble compounds which are useful as prodrugs of COX-2 inhibitors, and pharmaceutical compositions comprising them.
Owner:MERCK & CO INC
Water-soluble prodrugs of azole compounds
Water soluble prodrugs of azole antifungal agents are provided by quaternizing a nitrogen atom of the azole ring with a phosphonooxymethyl group.
Owner:EISIA R&D MANAGEMENT CO LTD
Multi-arm polymer prodrugs
ActiveUS20050112088A1Improve balanceHigh drug loadingPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsWater soluble prodrugPolymer science
Provided herein are water-soluble prodrugs. The prodrugs of the invention comprise a water-soluble polymer having three or more arms, at least three of which are covalently attached to an active agent, e.g., a small molecule. The conjugates of the invention provide an optimal balance of polymer size and structure for achieving improved drug loading, since the conjugates of the invention possess three or more active agents releasably attached to a multi-armed water soluble polymer. The prodrugs of the invention are therapeutically effective, and exhibit improved properties in-vivo when compared to unmodified parent drug.
Owner:NEKTAR THERAPEUTICS INC
Process for water soluble azole compounds
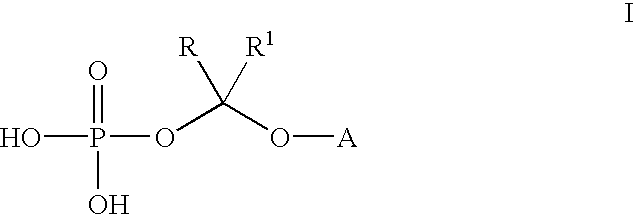
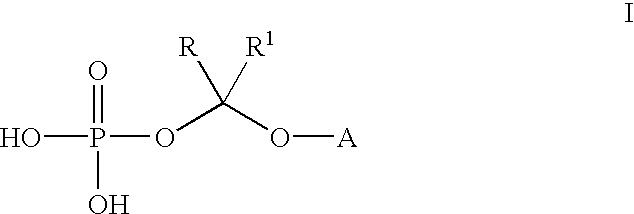
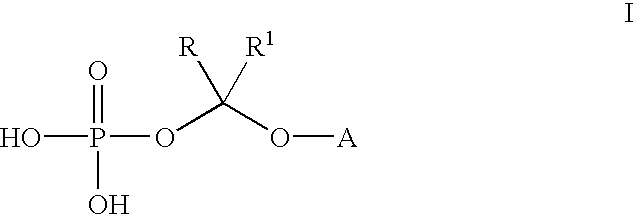
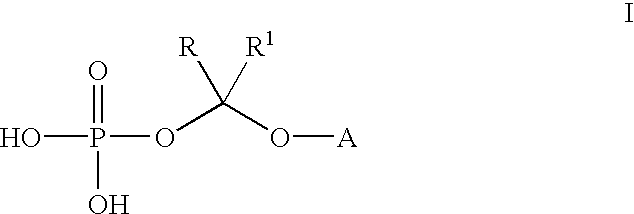
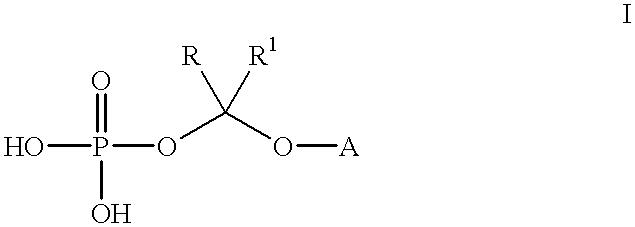
An improved process is provided for preparing water-soluble prodrugs of triazole antifungal compounds containing a secondary or tertiary hydroxyl group. More particularly, the improved process is directed toward preparation of water-soluble triazole antifungal compounds are provided having the general formulawherein A is the non-hydroxy portion of a triazole antifungal compound of the type containing a secondary or tertiary hydroxyl group and R and R1 are as defined in the specification.
Owner:EISIA R&D MANAGEMENT CO LTD
Multi-Arm Polymer Prodrugs
ActiveUS20090074704A1Improve balanceHigh drug loadingPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsWater soluble prodrugPolymer science
Provided herein are water-soluble prodrugs, compositions comprising such prodrugs, and related methods of making and administering the same. The prodrugs of the invention comprise a water-soluble polymer having three or more arms, at least three of which are typically covalently attached to an active agent, e.g., a small molecule. The conjugates of the invention provide an optimal balance of polymer size and structure for achieving improved drug loading, since the conjugates of the invention possess three or more active agents releasably attached to a multi-armed water-soluble polymer. The prodrugs of the invention are therapeutically effective, and exhibit improved properties in-vivo when compared to unmodified parent drug.
Owner:NEKTAR THERAPEUTICS INC
Water soluble prodrugs of azole compounds
Owner:EISIA R&D MANAGEMENT CO LTD
Hydrolytically degradable carbamate derivatives of poly(ethylene glycol)
InactiveUS6899867B2Facilitated releaseReduce deliveryAntibacterial agentsNervous disorderWater soluble prodrugCarbamate
Poly(ethylene glycol) carbamate derivatives useful as water-soluble prodrugs are disclosed. These degradable poly(ethylene glycol) carbamate derivatives also have potential applications in controlled hydrolytic degradation of hydrogels. In such degradable hydrogels, drugs may be trapped in the gel and released by diffusion as the gel degrades, or they may be covalently bound through hydrolyzable carbamate linkages. Hydrolysis of these carbamate linkages releases the drug at a controllable rate as the gel degrades.
Owner:NEKTAR THERAPEUTICS INC
Water soluble prodrugs of azole compounds
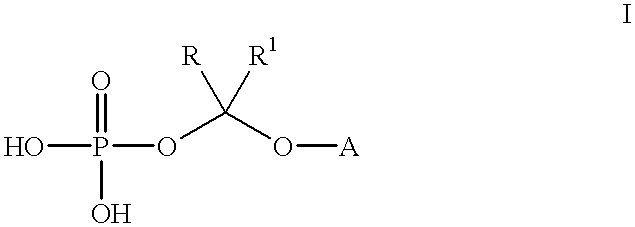
Water-soluble prodrugs of triazole antifungal compounds having a secondary or tertiary hydroxy group are provided. More particularly, new water-soluble triazole antifungal compounds are provided having the general formulawherein A is the non-hydroxy portion of a triazole antifungal compound of the type containing a secondary or tertiary hydroxyl group and n, R1 and R2 are as defined in the specification.
Owner:EISIA R&D MANAGEMENT CO LTD
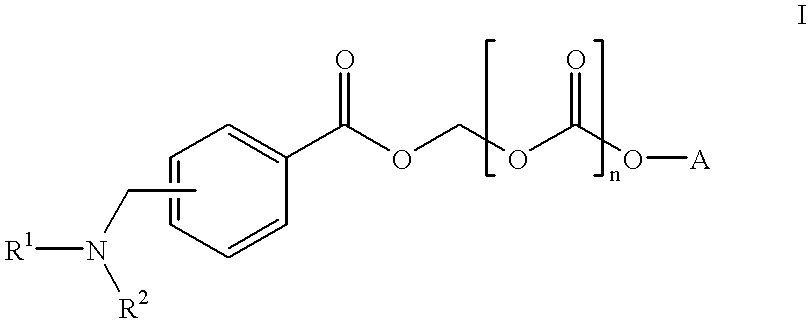
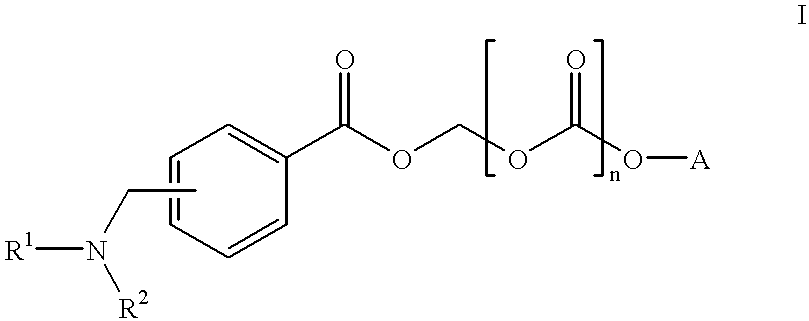
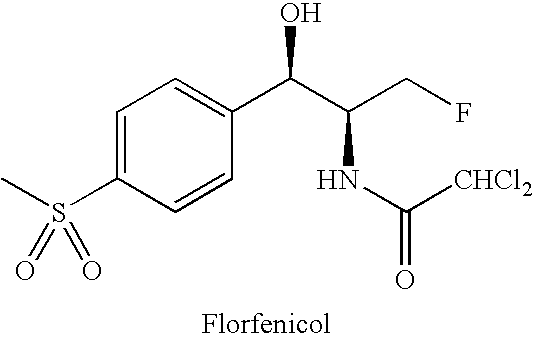
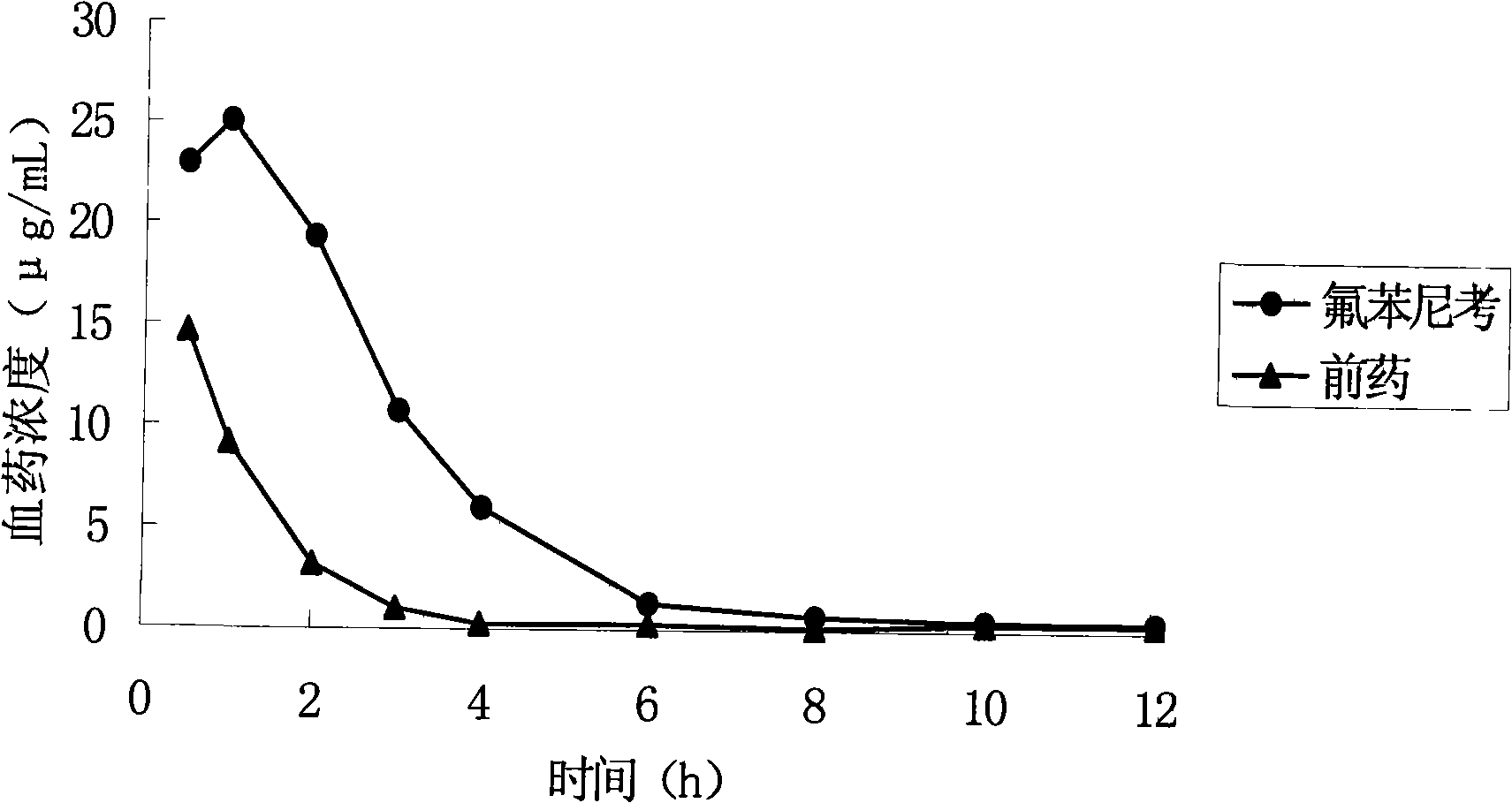
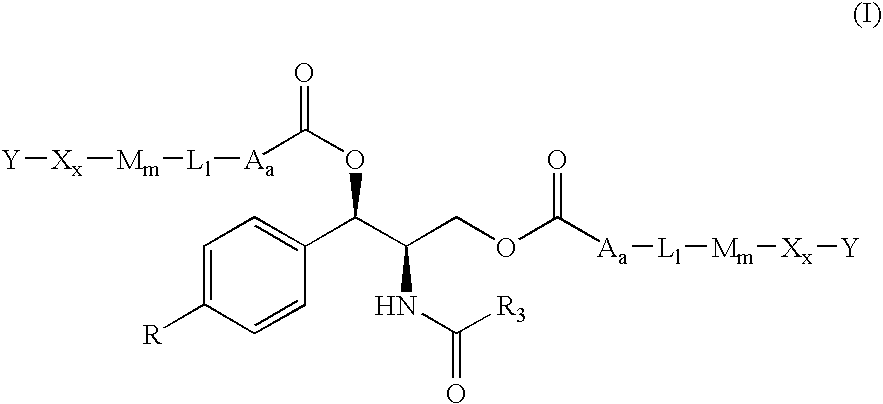
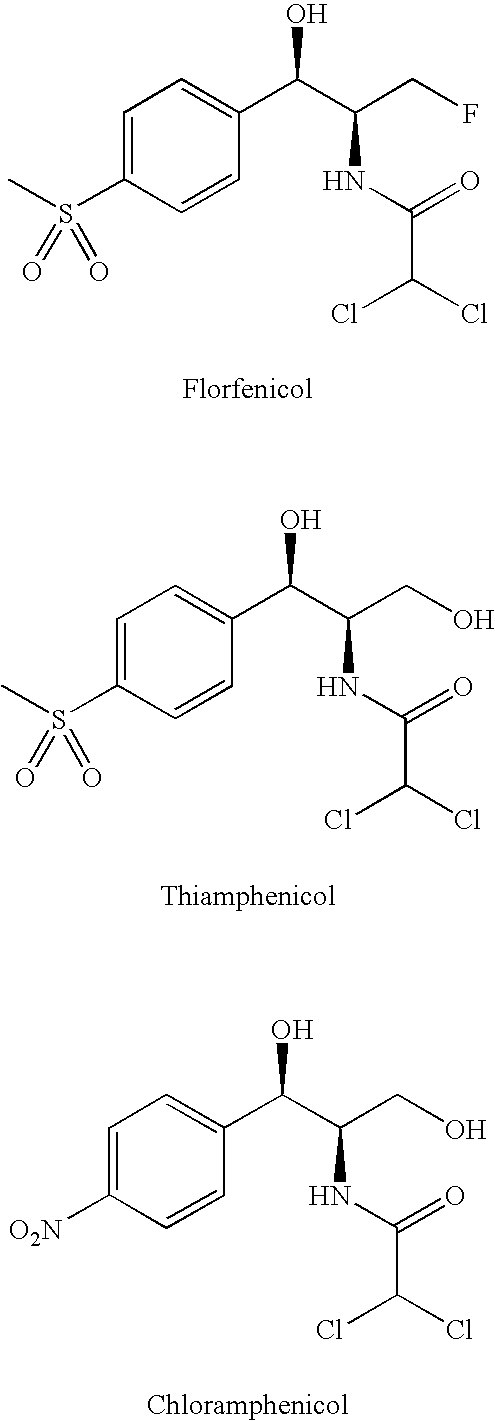
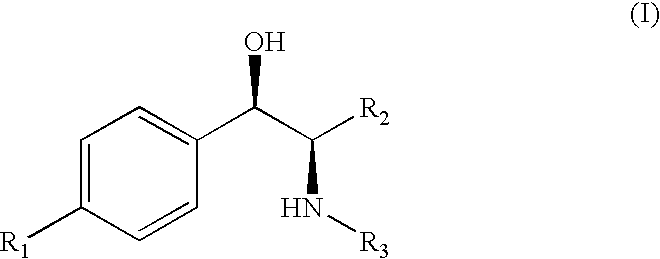
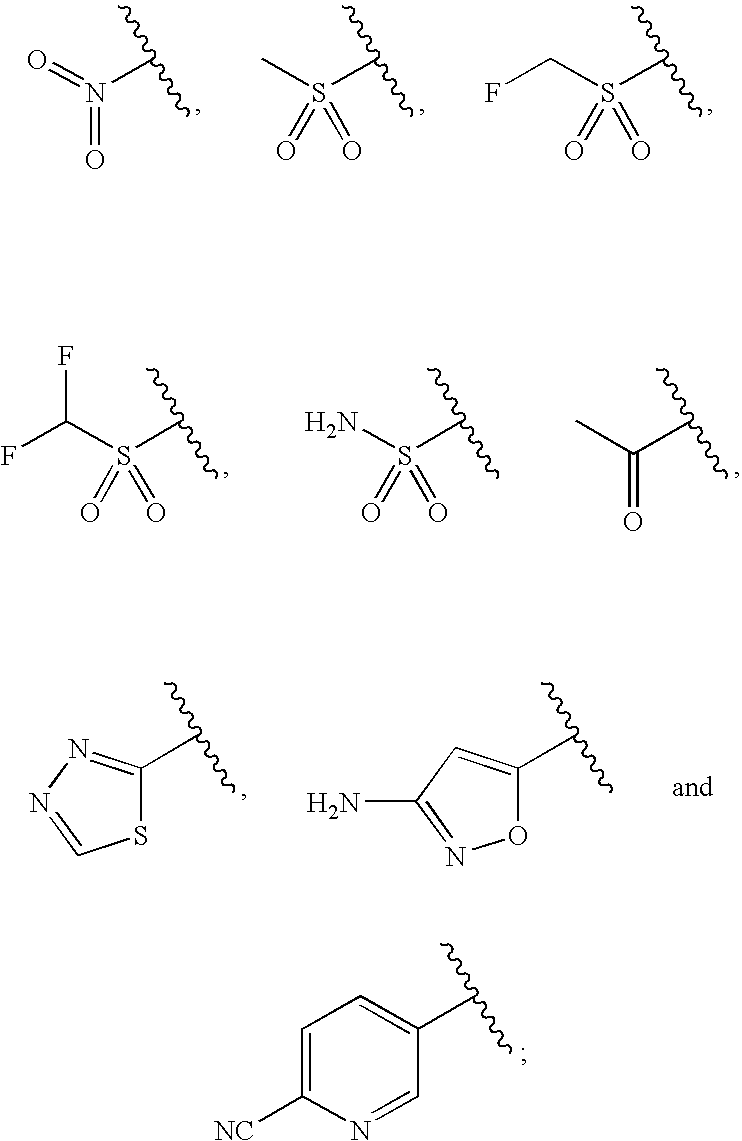
Water-Soluble Prodrugs of Florfenicol and its Analogs
The present invention discloses certain novel prodrugs of florfenicol and / or of florfenicol analogs, including prodrugs of salts pharmaceutically acceptable salts of florfenicol and its analogs, including nitrogen-containing esters of the secondary alcohol group of florfenicol and of its analogs, and pharmaceutically acceptable salts thereof, compositions containing them, and methods of administering them to subjects. In particular embodiments the prodrugs are sufficiently water-soluble to serve the functions needed of a water-soluble prodrug of florfenicol or of a water-soluble prodrug of a florfenicol analog. A certain subclass of the compounds also possesses the hydrolytic stability needed to maintain the prodrug in solution in the subject's system until appropriate conditions exist when the prodrug can hydrolyze, releasing florfenicol or the florfenicol analog in question.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
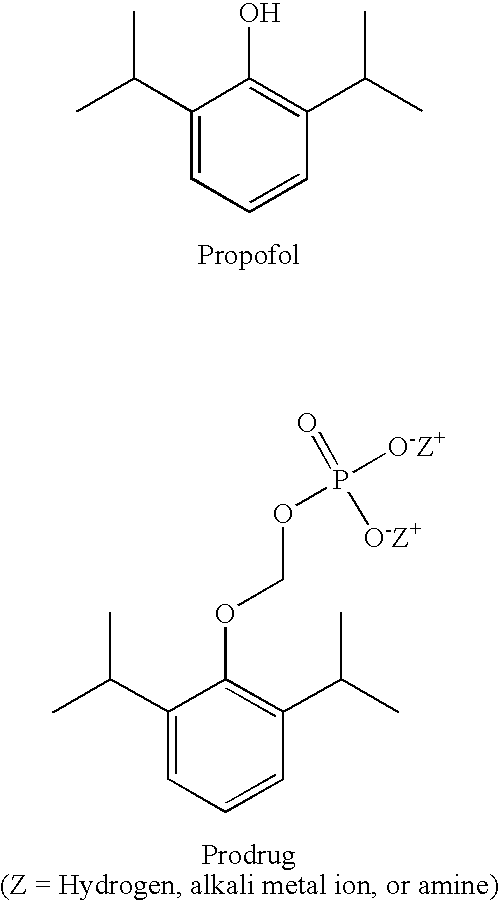
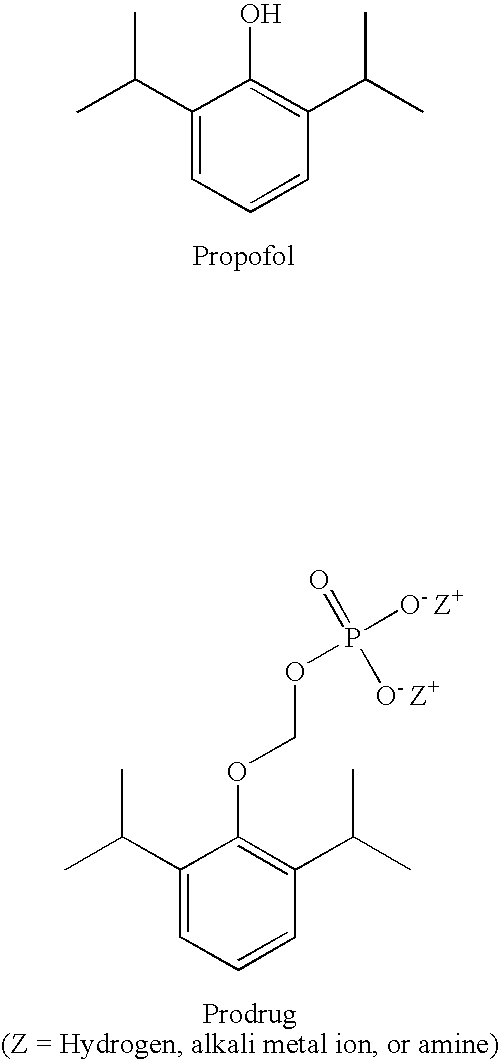
Watersoluble prodrugs of propofol
InactiveUS20050267169A1Good skin permeabilityEasy to refactorBiocideSugar derivativesWater soluble prodrugMammal
The present invention relates to propofol derivatives comprising a cyclic or linear amino acid, or a poly- or (oligo)saccharide moiety, a process for preparing said derivatives, a method for anesthetizing a mammal as well as a method for treating convulsions, migraine or related diseases, or for the inhibition of free radicals in a mammal to which said compounds are administered. Furthermore, the present invention relates to said compounds for use as a medicament and the use of said compounds for the preparation of a medicament for anesthetizing a mammal or for treating convulsions, migraine or related diseases, or for inhibition of free radicals in a mammal.
Owner:FRESENIUS KABI DEUT GMBH +1
Ethoxy Diphenyl Ethane Derivatives, Preparation Processes and Uses Thereof
ActiveUS20120046492A1Improve drug stabilityLow toxicityOrganic compound preparationGroup 5/15 element organic compoundsAmino acid side chainVascular endothelium
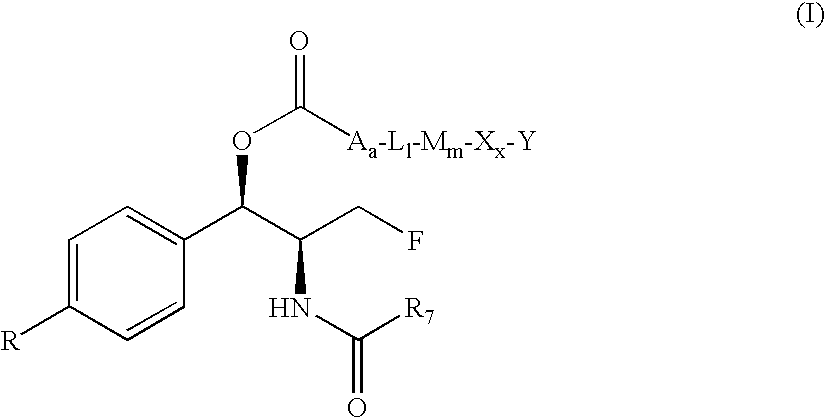
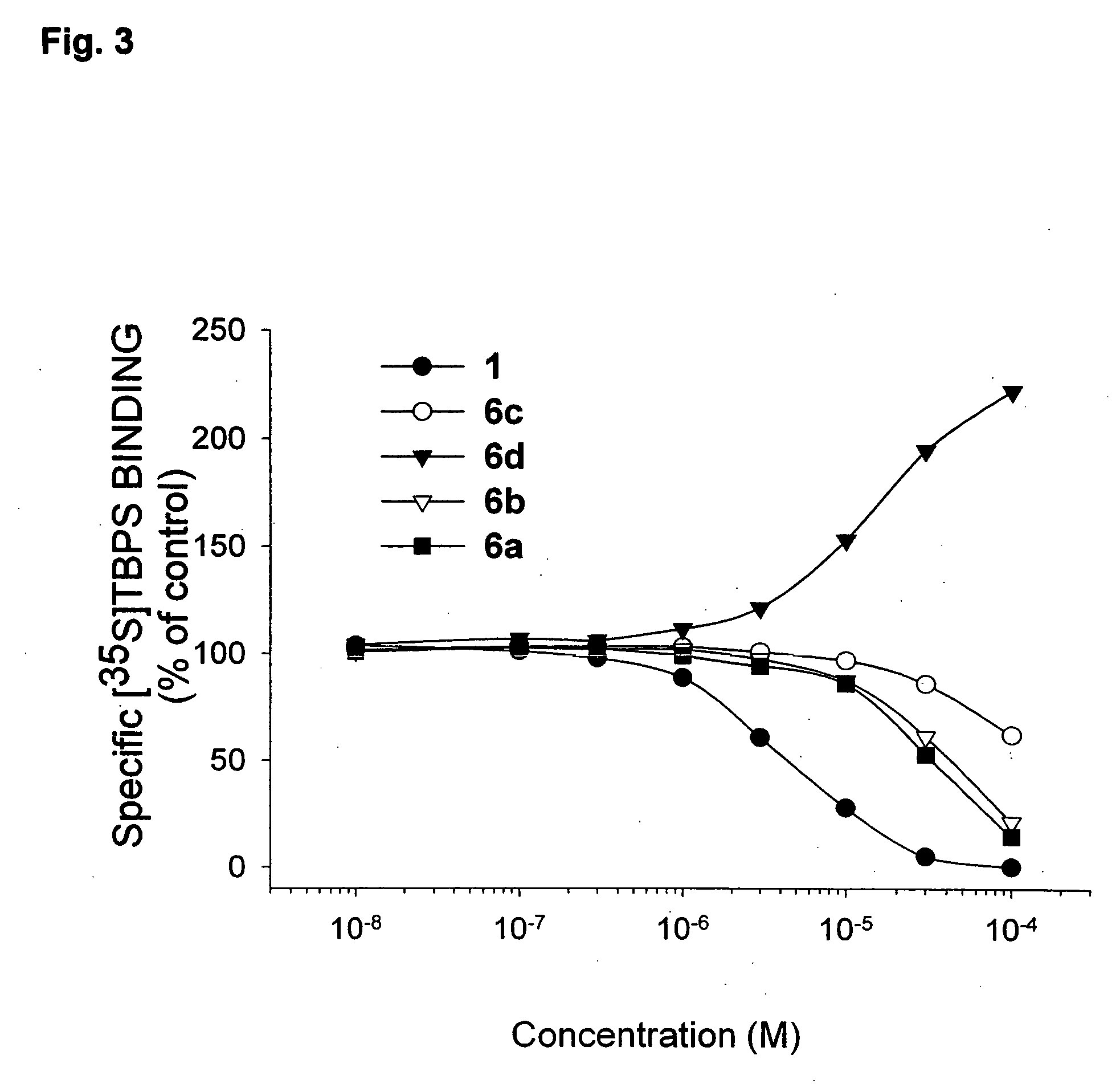
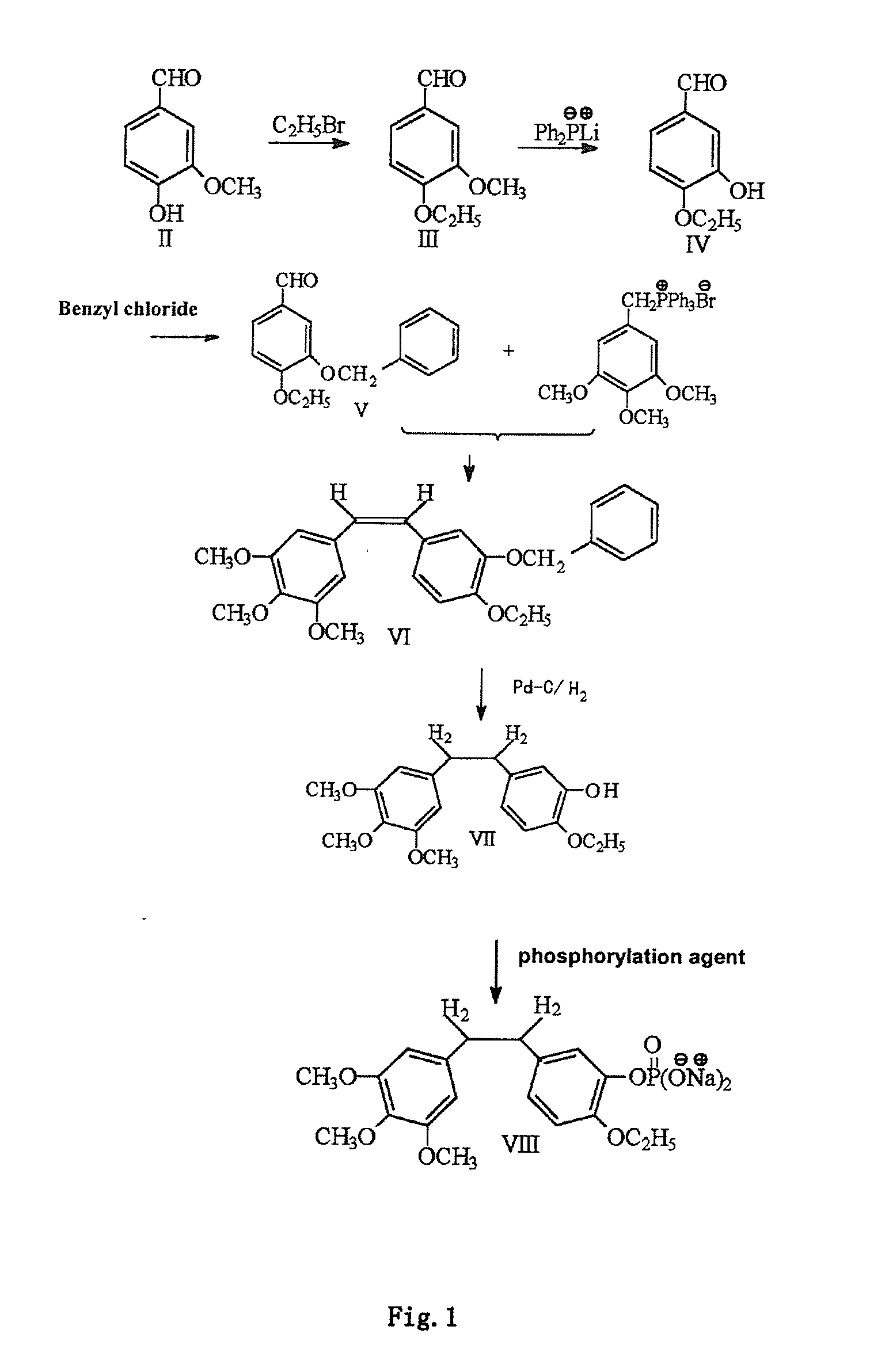
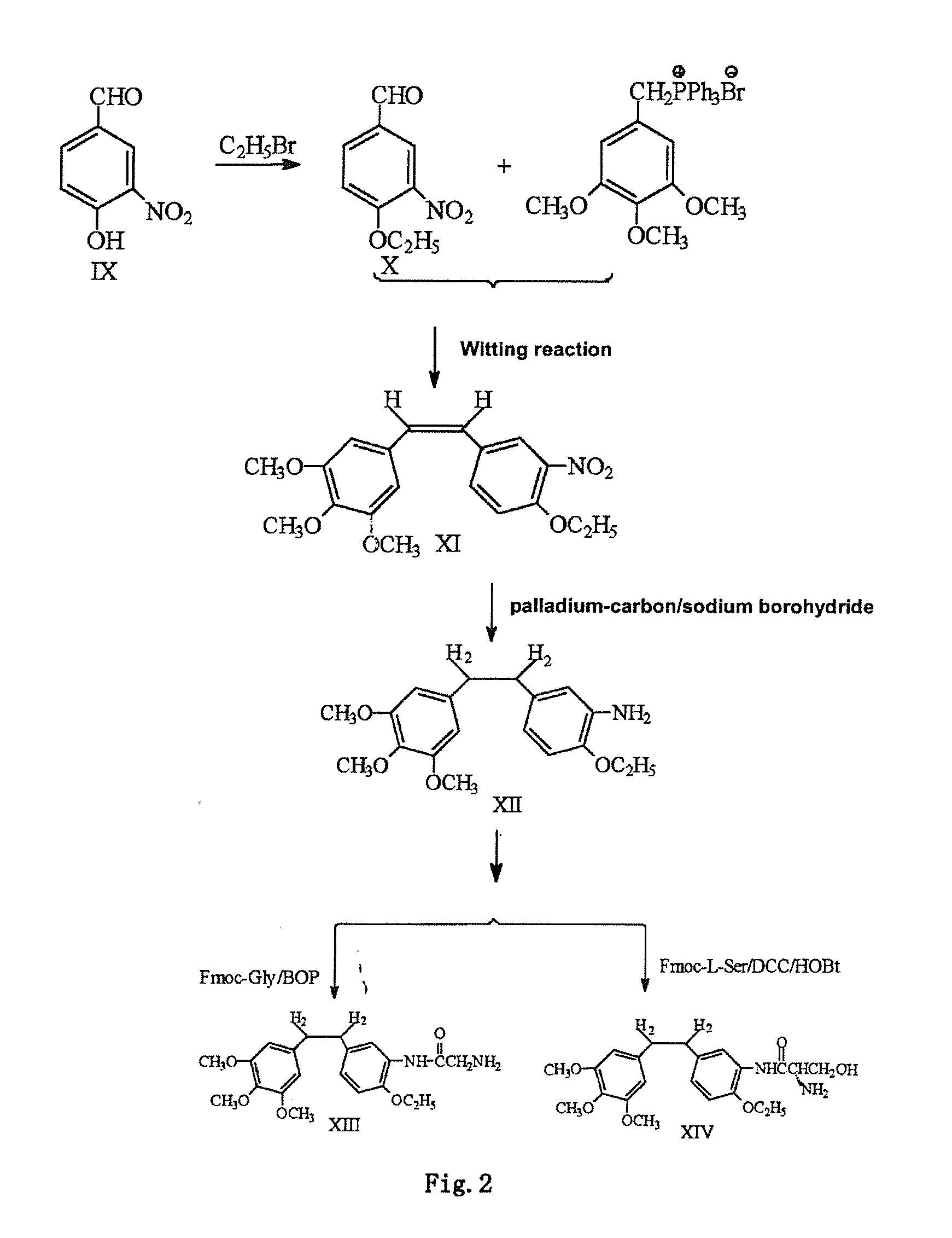
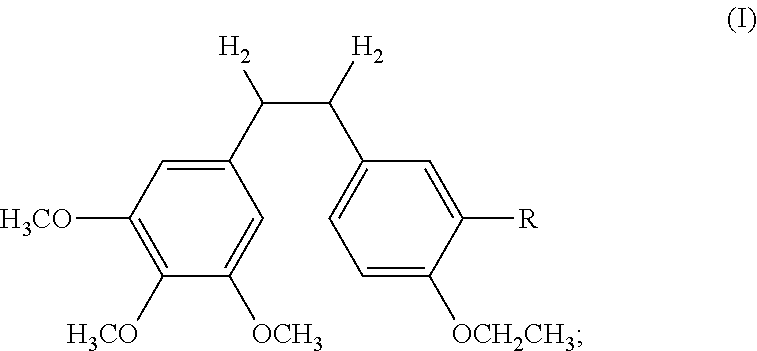
The invention discloses an ethoxydiphenylethane derivative and a synthetic method and uses thereof 4′ position of phenylethane B aromatic ring is chemically modified by ethoxy and hydroxy at position 3′ thereof is simultaneously modified to water soluble prodrug such as phosphate, and similarly, amino acid side chain is introduced to amino at position 3′ to form amino acid amide water soluble prodrug having the structure shown as formula (I)the ethoxydiphenylethane derivative and the prodrug thereof include strong tubulin aggregation inhibiting ability and obvious target damage effect for tumor vessels, selectively cause dysfunction and structural damage of tumor vessels and induce apoptosis of vascular endothelial cells in order to play the role of killing tumor cells or inhibiting tumor metastasis in case that the tumor cells are free from the support of nutrition and oxygen.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Novel Water-Soluble Prodrugs
An objective of the present invention is to provide water-soluble prodrugs that can be administered parenterally, and which show excellent water solubility and small interspecies or individual differences and are rapidly converted to the active form by chemical conversion. This invention provides water-soluble prodrugs represented by formula (1), or pharmaceutically acceptable salts, or hydrates or solvates thereof, (wherein, R1 represents a hydrogen atom, or C1-C6 alkyl group; W represents a divalent group comprising a tertiary amino group or sulfonyl group; and Y represents a residue of a compound represented by Y—OH comprising an alcoholic hydroxyl group).
Owner:CHUGAI PHARMA CO LTD
High-water-solubility florfenicol prodrug quickly released in vivo
InactiveCN101289416AThe synthesis process is simpleMedication convenienceOrganic chemistryOrganic compound preparationSolubilityArginine
The invention relates to a florenicol succinic acid monoester and a preparation method thereof, and the florenicol succinic acid monoester can be obtained by the esterification of the florenicol and succinic anhydride. Simultaneously, the invention also relates to acceptable salts and preparation methods thereof of the florenicol succinic acid monoester pharmacy, such as sodium salt, potassium salt, calcium salt and magnesium salt etc., and salts that are formed with basic amino acids and comprises lysine and arginine salts. By testing, the water-solubility of the corresponding salt is more than 500 mg / ml. The water-soluble derivatives disclosed by the invention quickly release the florenicol after being hydrolyzed in bodies and the derivatives can be used as high water-soluble prodrugs of the florenicol.
Owner:SOUTHWEST UNIV
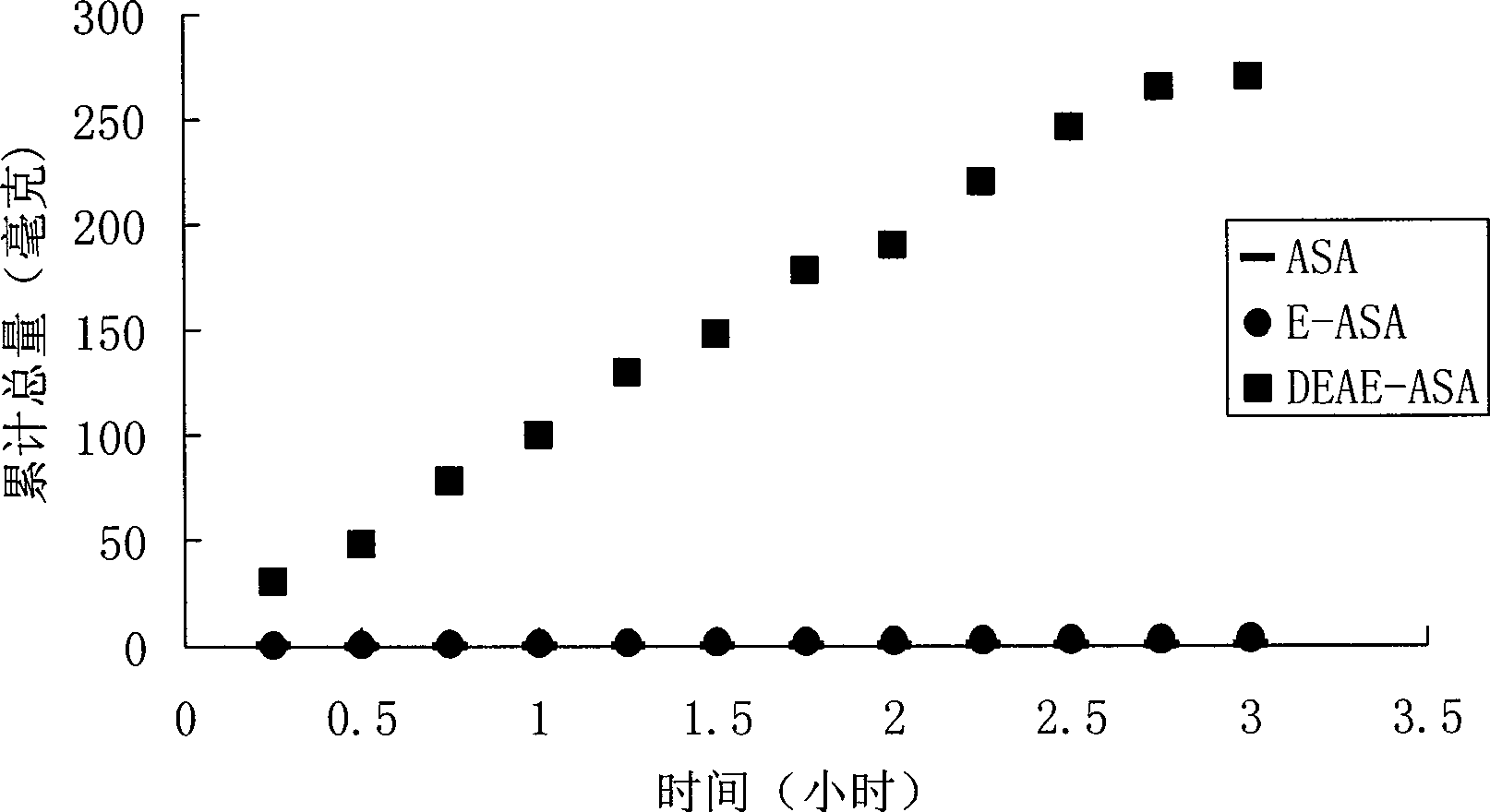
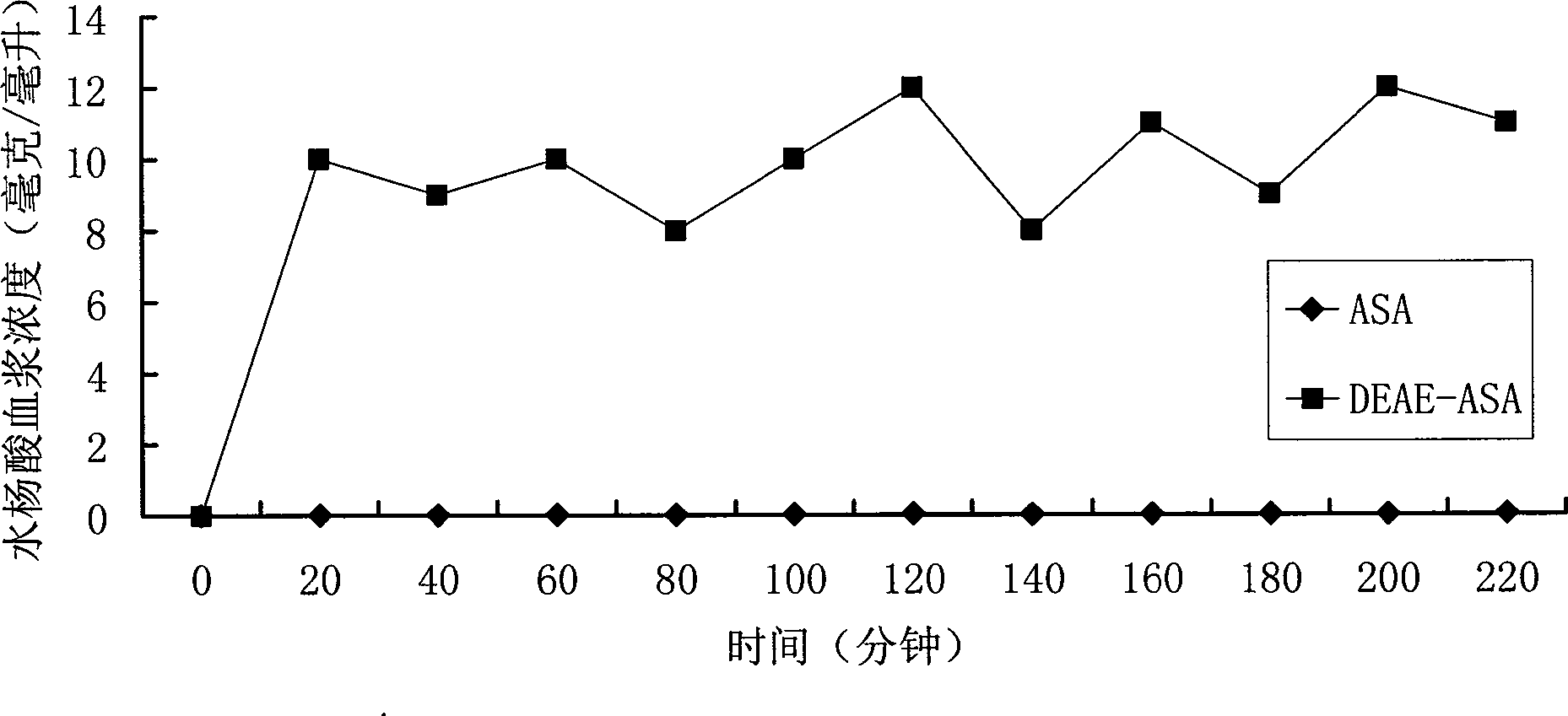
Positively charged water-soluble prodrugs of aspirin
InactiveCN101484415AFast in vivo penetrationAvoid side effectsSenses disorderNervous disorderSolubilityDisease
The novel positively charged prodrugs of acetylsalicylic acid and its analogues in the general formula(1) 'Structure 1' were designed and synthesized. The compounds of the general formula(1) 'Structure 1' indicated above can be prepared from functional derivatives of ASA or its analogues,(for example acid halides or mixed anhydrides), by reaction with suitable alcohols, thiols, or amines. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and push the pro-drug into the cytosol. The experiment results suggest that the pro-drug, diethylaminoethyl acetylsalicylate.AcOH, diffuses through human skin -400 times faster than acetylsalicylic acid itself and -100 times faster than ethyl acetylsalicylate. In plasma, 80% of these pro-drugs can change back to the drug in a few minutes. The pro-drugs can be used medicinally in treating any aspirin-treatable conditions in humans or animals and be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of aspirin, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis. Controlled transdermal administration systems of the prodrug enable the aspirin in the blood to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of aspirin.
Owner:于崇曦 +1
Preventive or Therapeutic Agents for Pancreatic Cancer, Ovarian Cancer, or Liver Cancer Comprising a Novel Water-Soluble Prodrug
InactiveUS20090118271A1Good water solubilityPrevention and therapyBiocideOrganic chemistryCamptothecinHydroxy compound
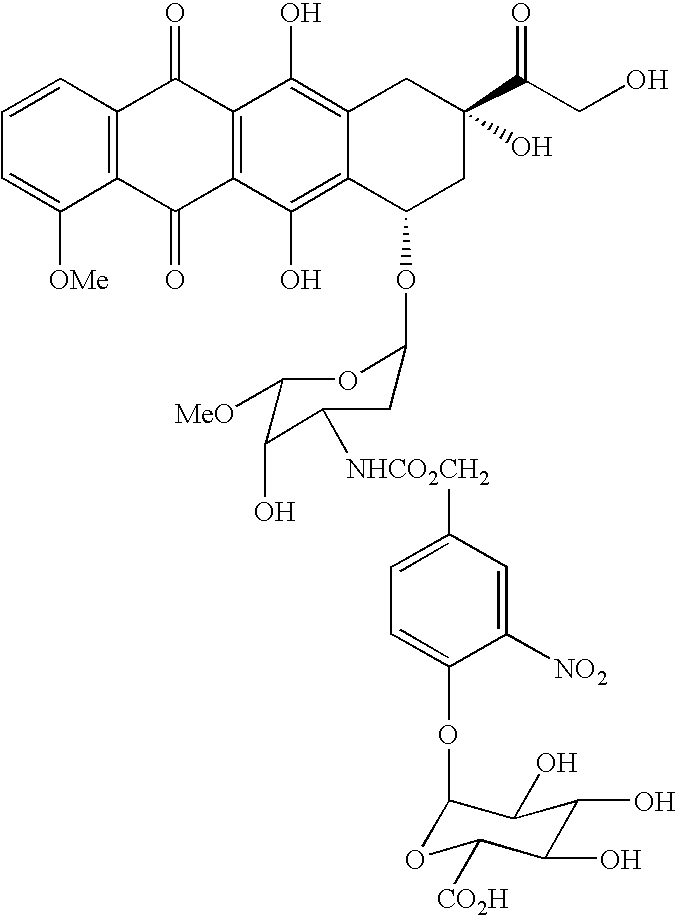
Preventive or therapeutic agents for pancreatic cancer, ovarian cancer, or liver cancer of the present invention comprise a water-soluble prodrug represented by formula 1 described below, or a pharmaceutically acceptable salt, or a hydrate or solvate of the prodrug or pharmaceutically acceptable salt,(wherein,R1 represents a hydrogen atom, or a C1-C6 alkyl group;W represents a divalent group comprising a tertiary amino group or a divalent group comprising a sulfonyl group, andY represents a residue of a compound represented by Y—OH comprising an alcoholic hydroxyl group, wherein said Y—OH is a camptothecin, a taxane, or an anticancer nucleotide).
Owner:CHUGAI PHARMA CO LTD
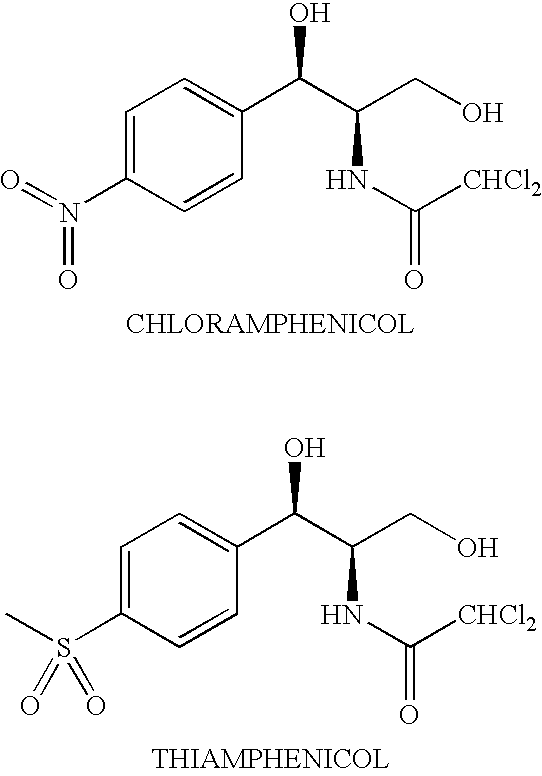
Water-Soluble Prodrugs of Chloramphenicol, Thiamphenicol, and Analogs Thereof
The present invention discloses certain novel prodrugs of chloramphenicol or thiamphenicol, or of an analog of either, including prodrugs of pharmaceutically acceptable salts of chloramphenicol or thiamphenicol or of their analogs, including nitrogen-containing esters of both alcohol groups of such compounds. In certain embodiments these novel prodrugs are sufficiently water-soluble to serve the functions needed of a prodrug of chloramphenicol or thiamphenicol or of an analog of either. In one embodiment, a certain subclass of the compounds also possesses the hydrolytic stability needed to maintain the prodrug in solution in the subject's system until appropriate conditions exist when the prodrug can hydrolyze, releasing the active compound in question.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
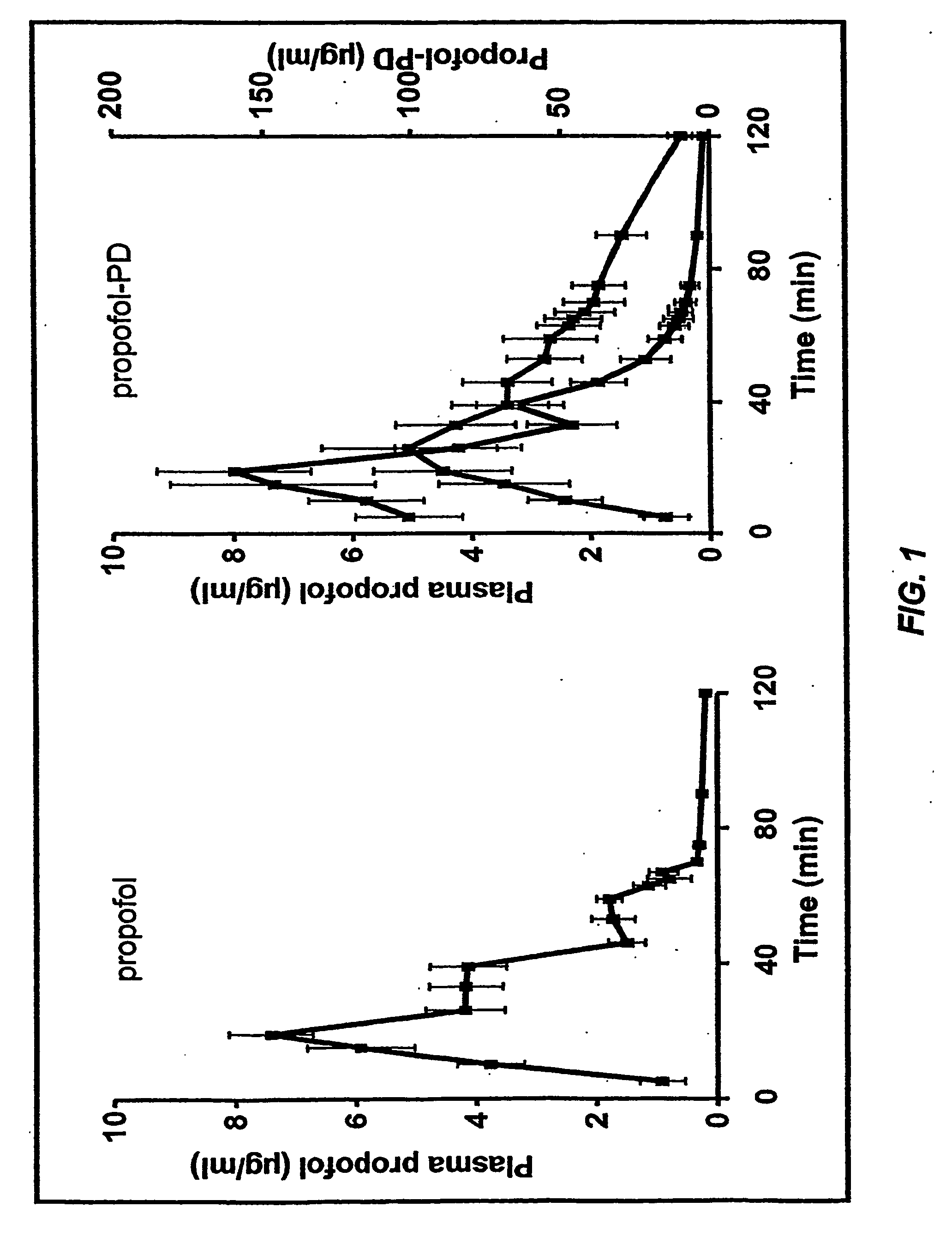
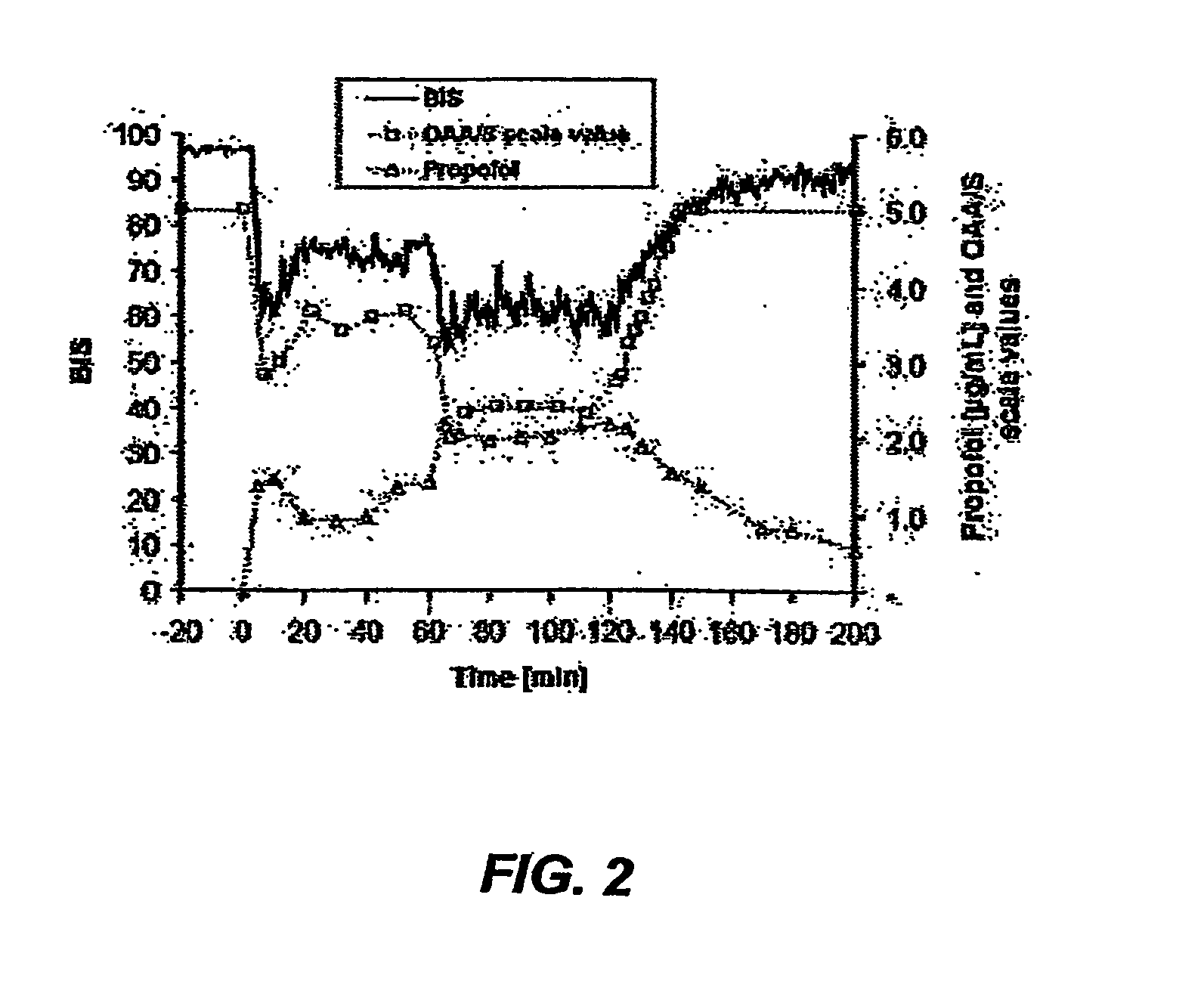
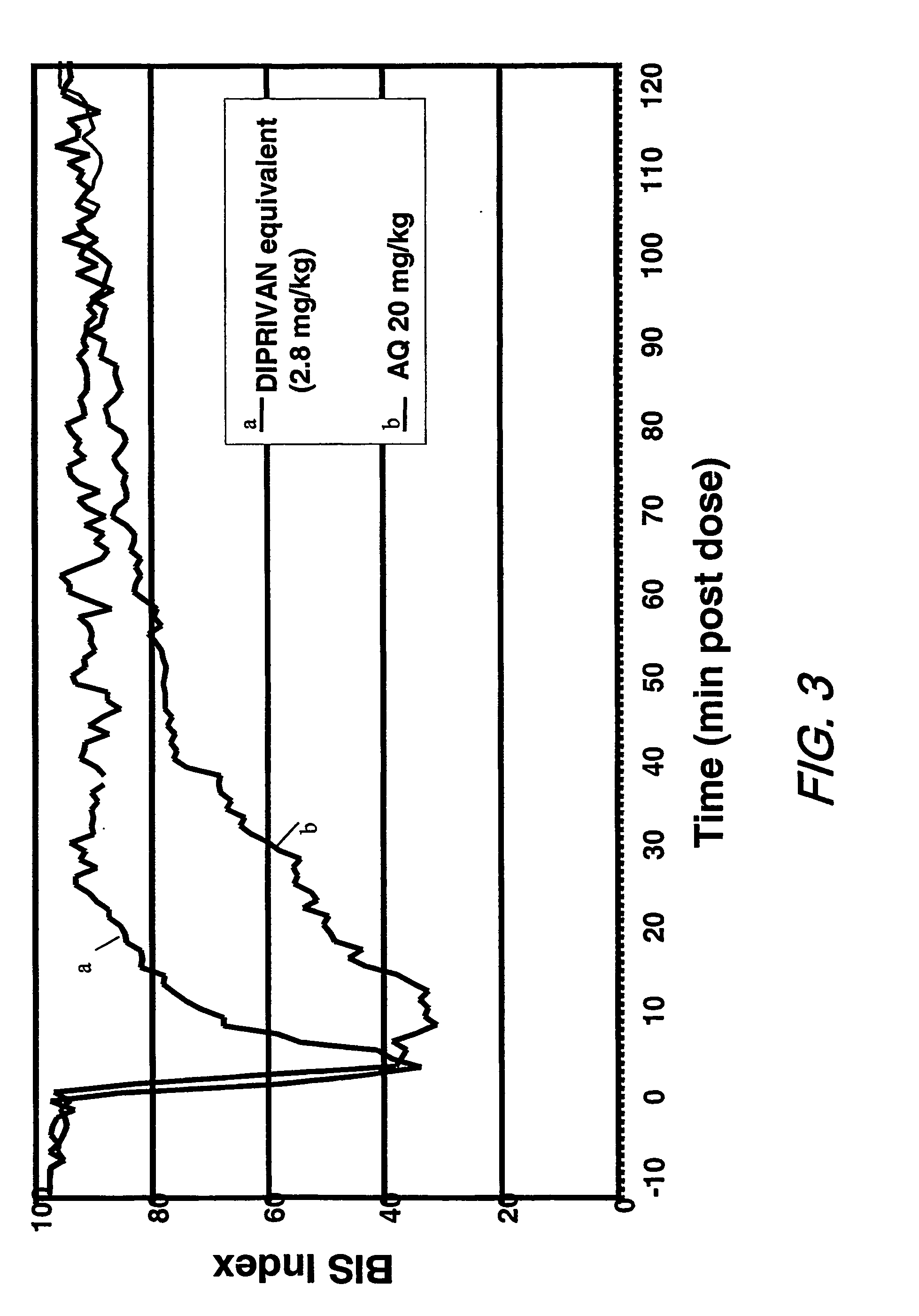
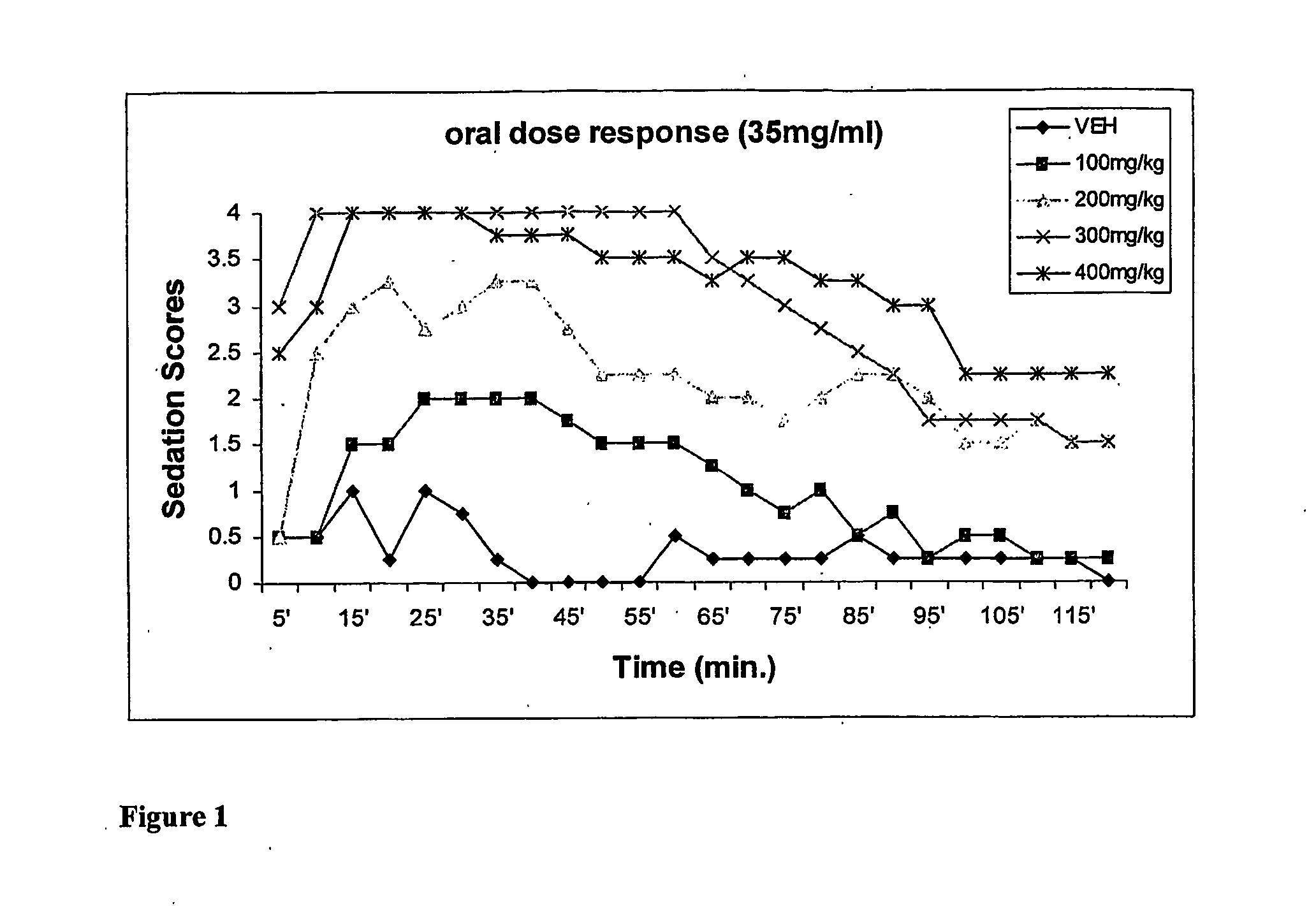
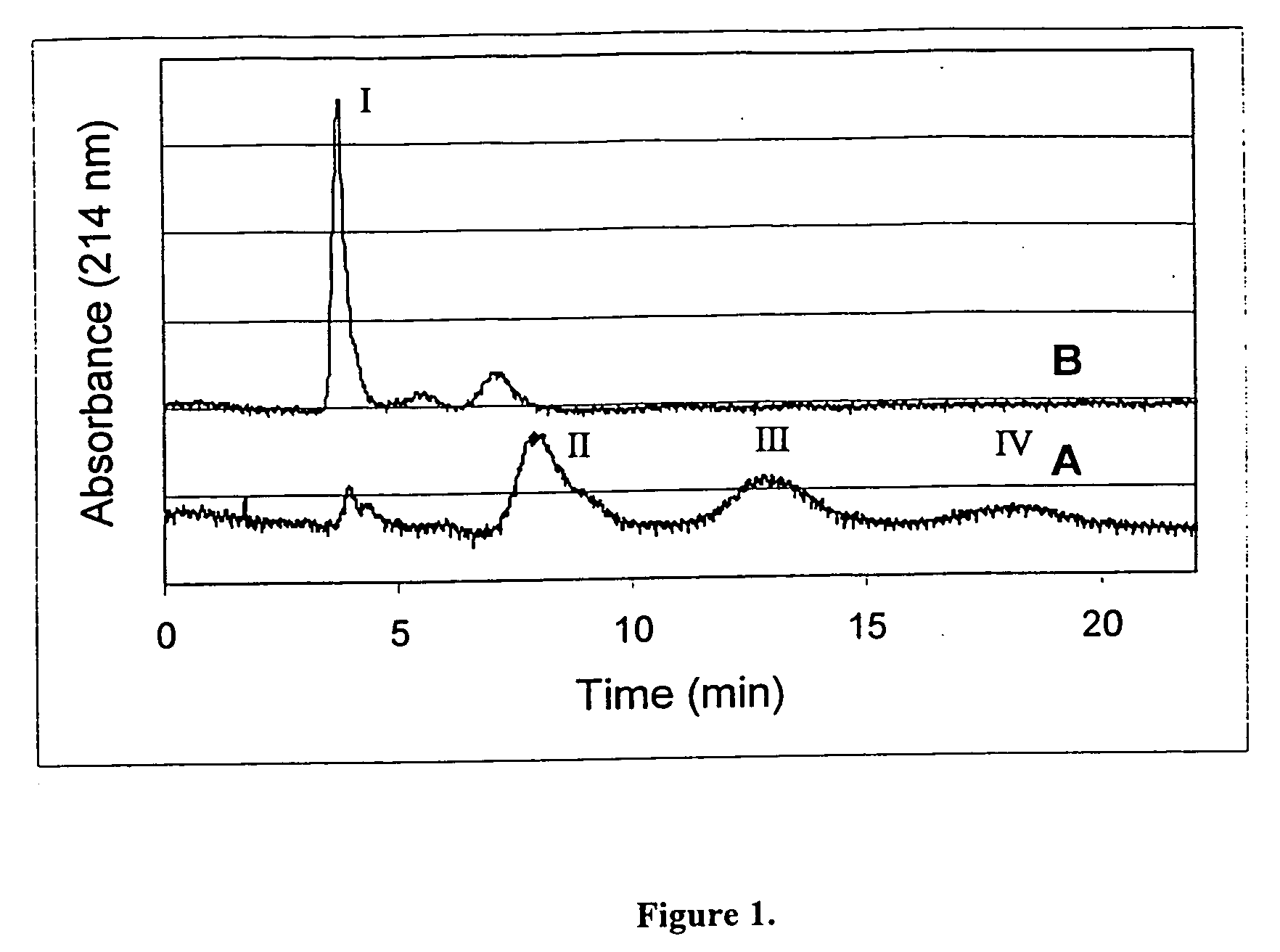
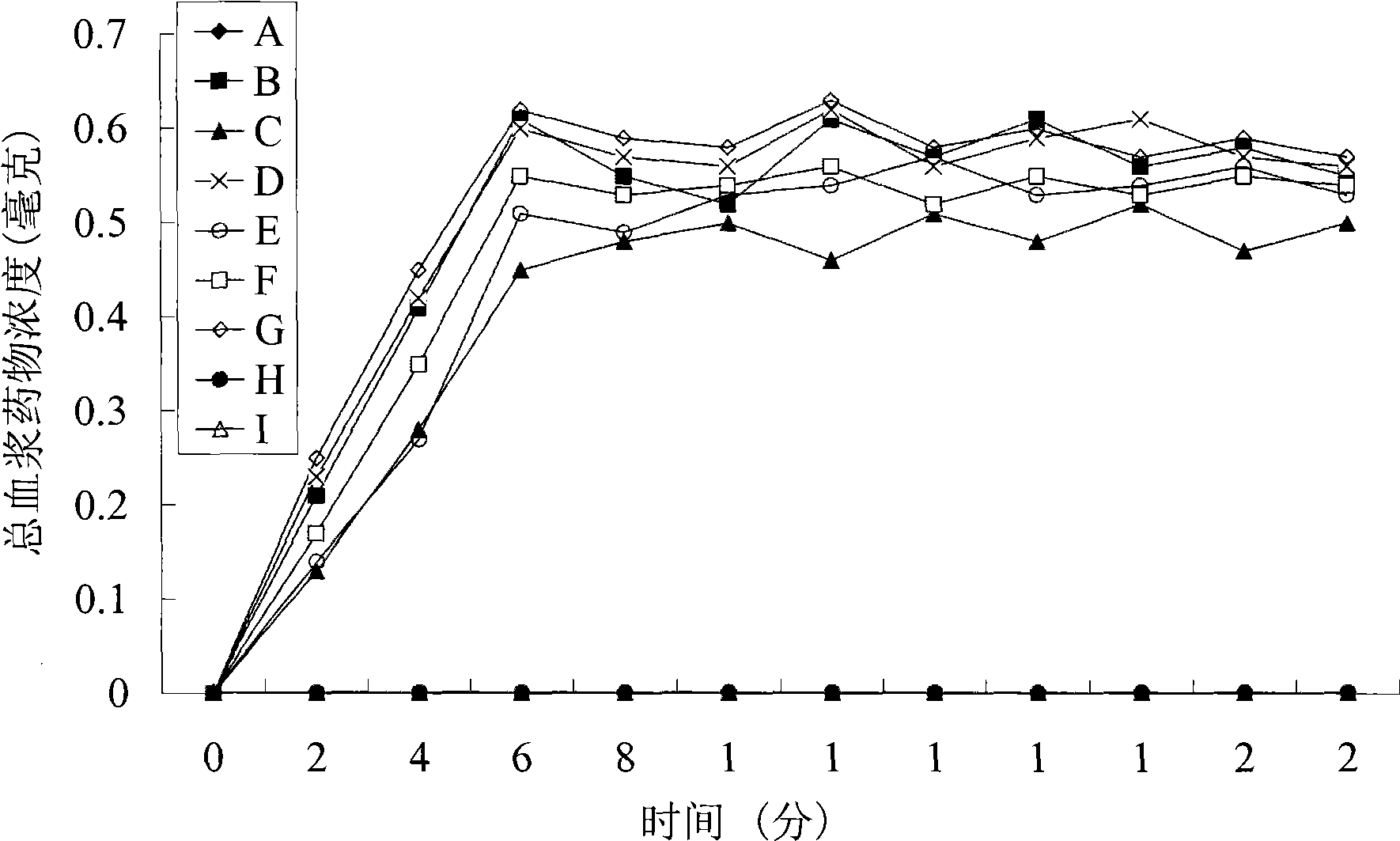
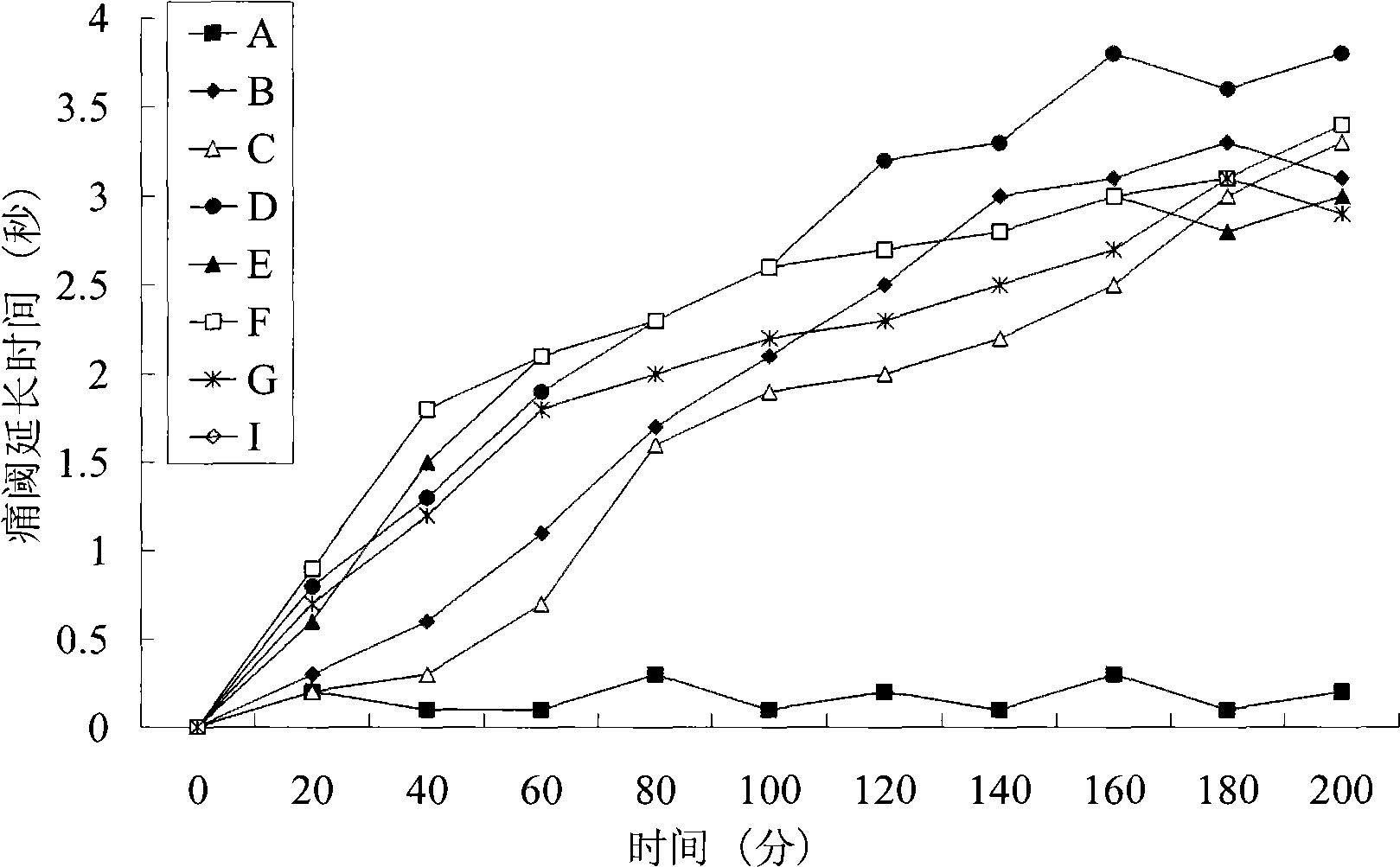
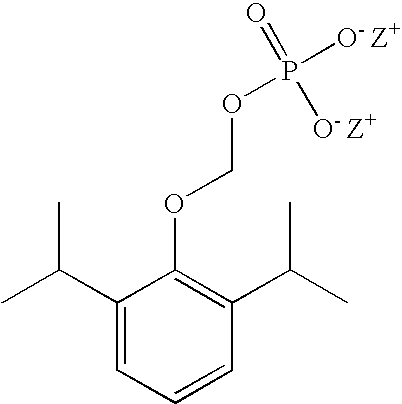
Pharmaceutical compositions containing water-soluble prodrugs of propofol and methods of administering same
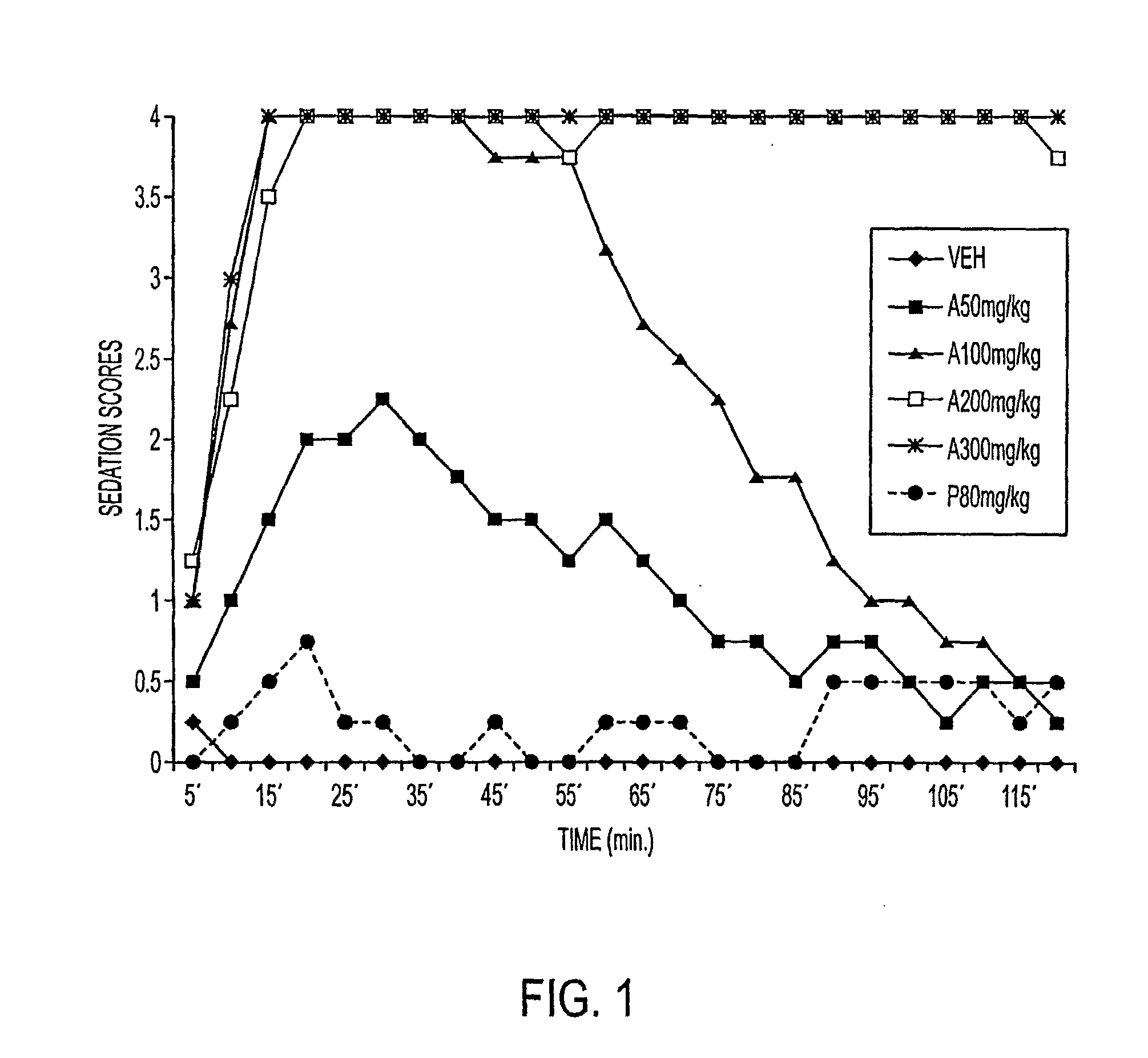
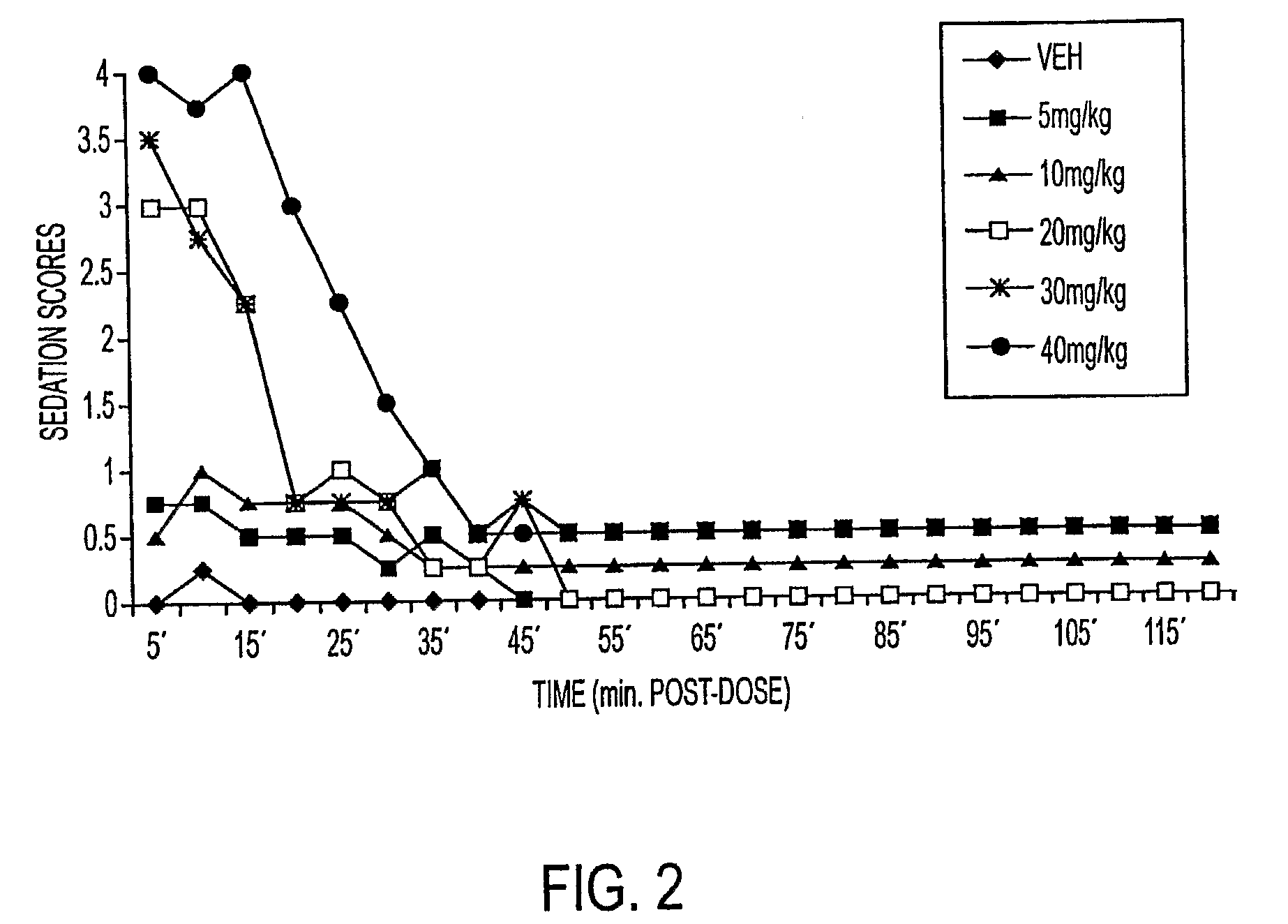
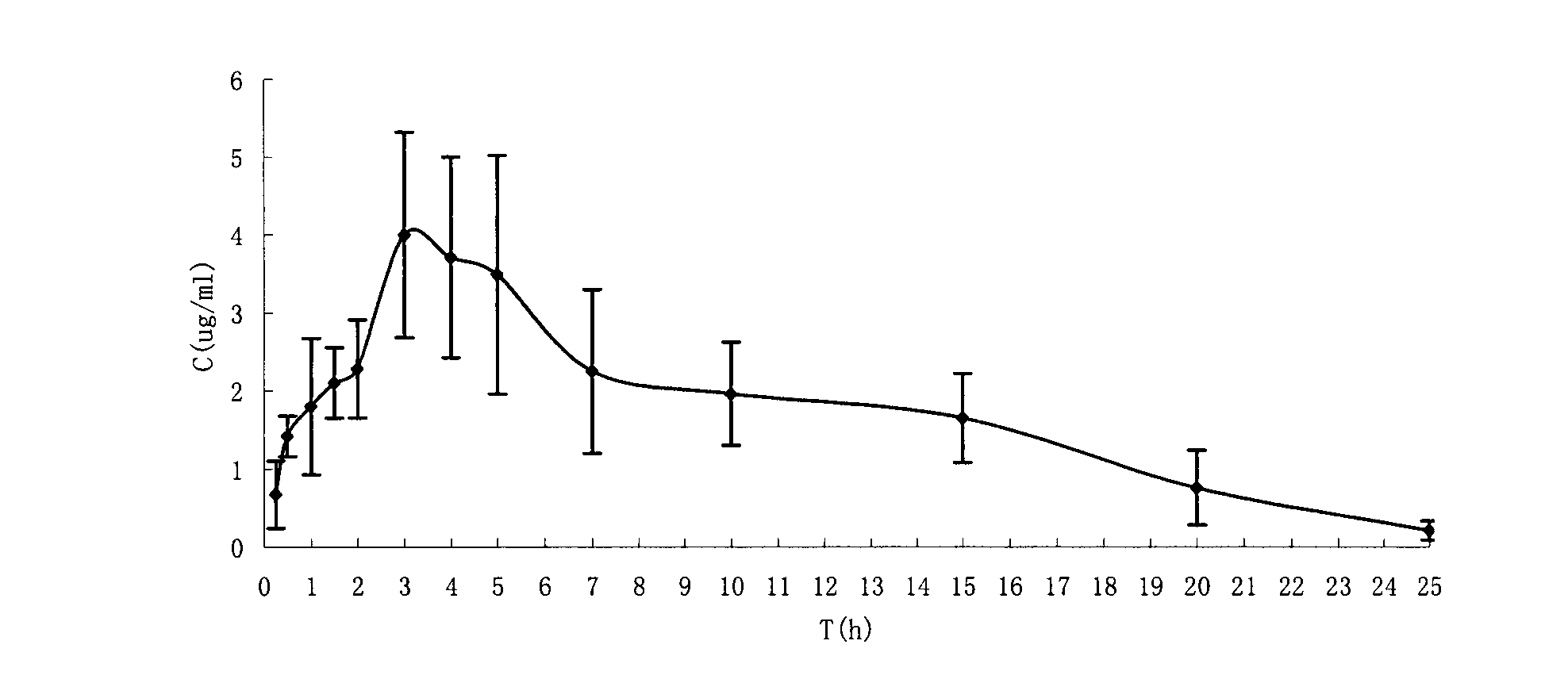
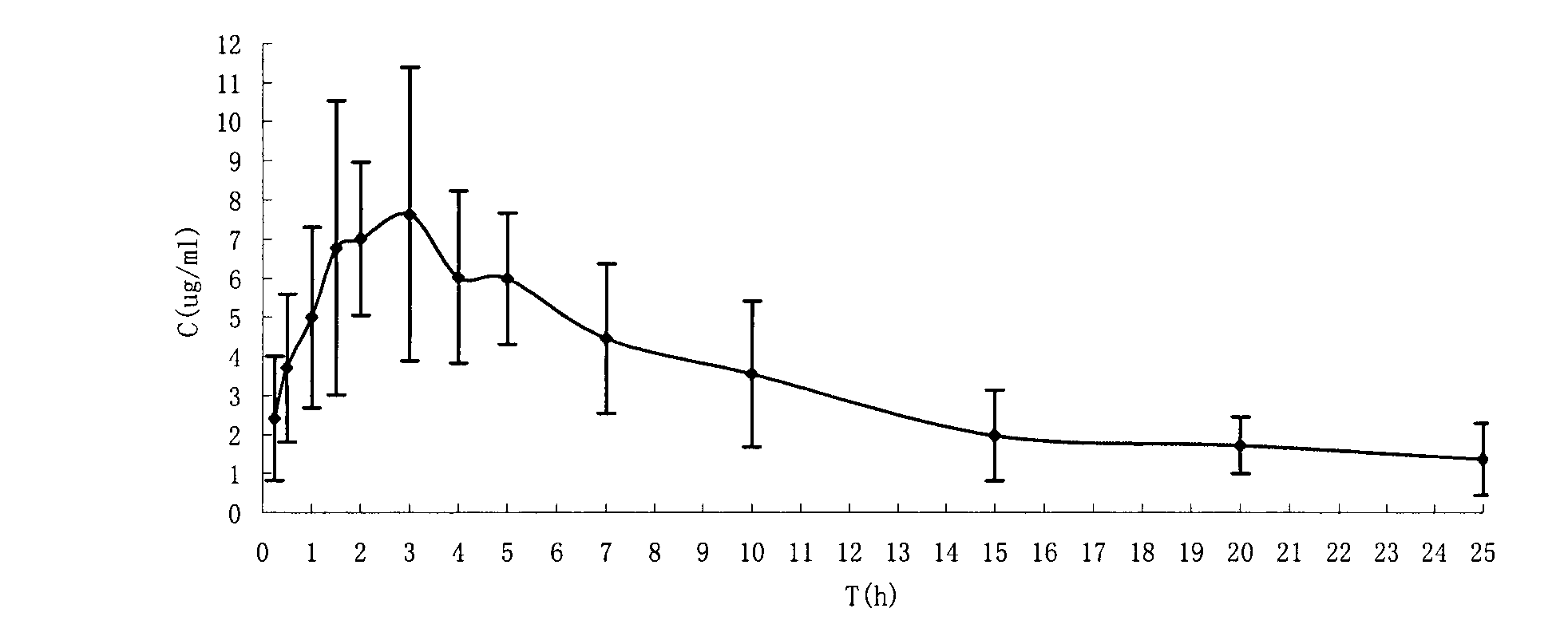
The present invention is directed to pharmaceutical compositions containing water-soluble prodrugs of propofol and methods of administering the prodrug. In one aspect, a method of inducing and / or maintaining a generalized anesthetic state comprises administering by parenteral infusion a prodrug of propofol in an amount sufficient to cause and / or maintain loss of consciousness. In another aspect, a prodrug of propofol is administered for producing a sedated state in a subject.
Owner:EISAI INC
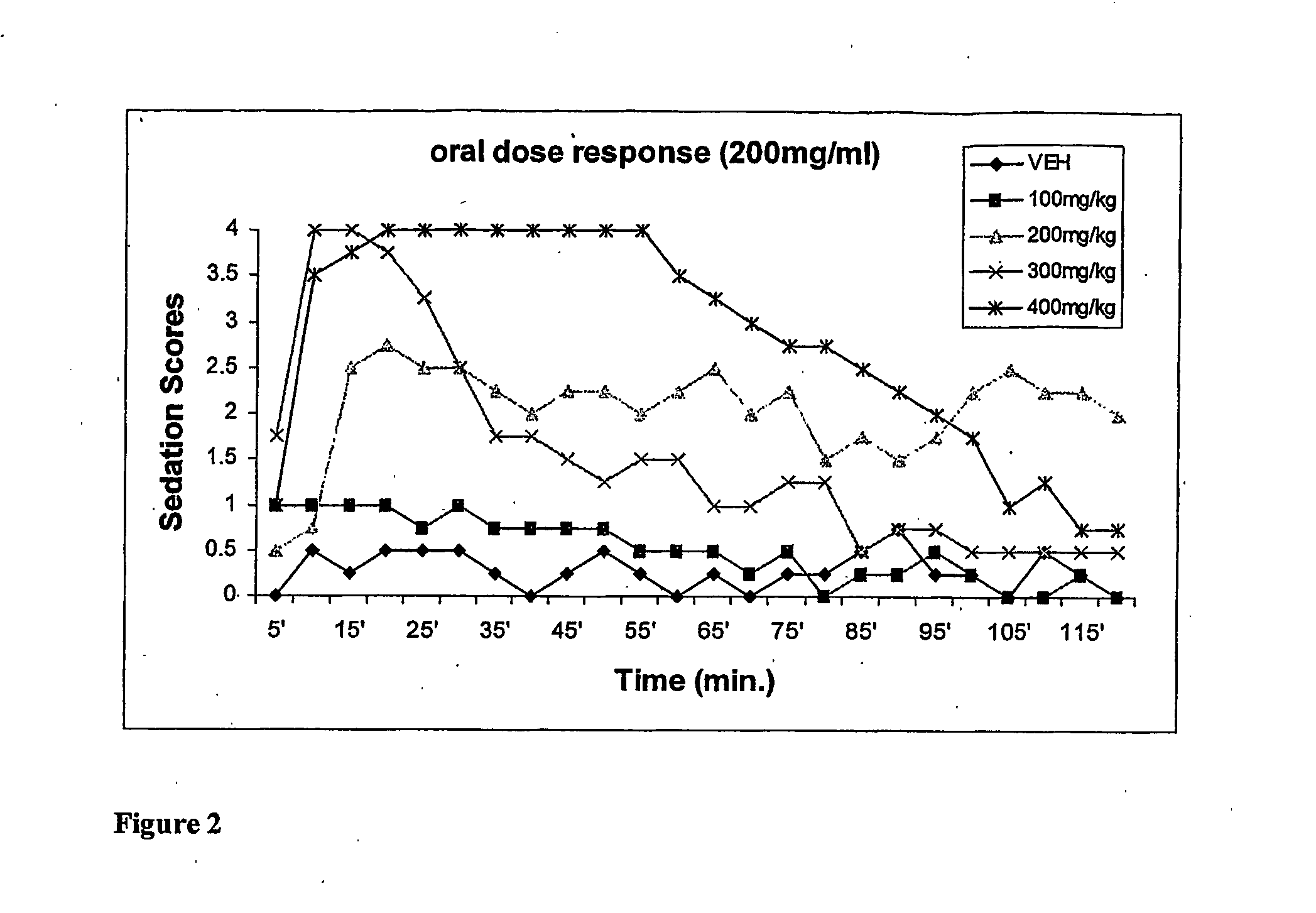
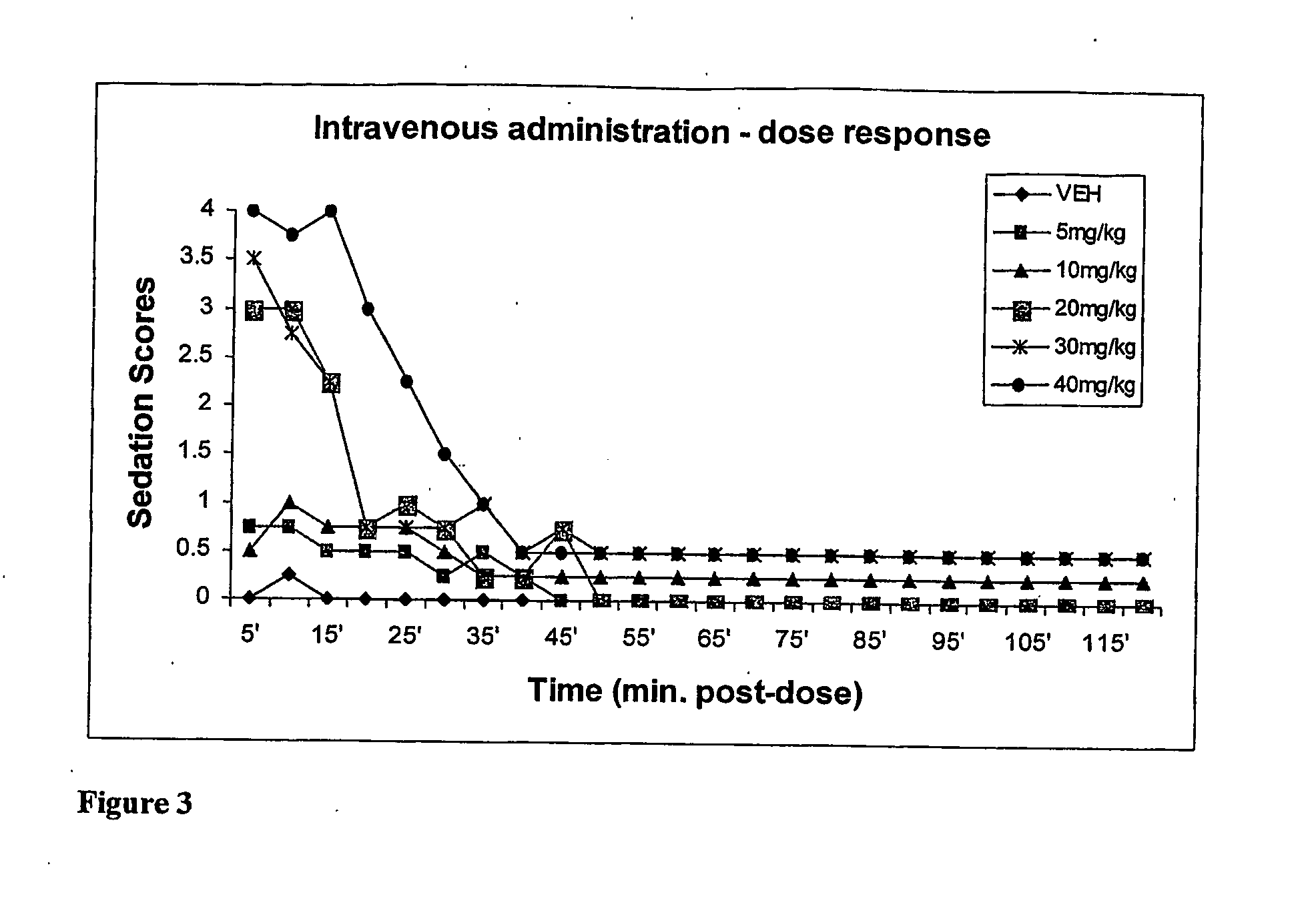
Methods Of Administering Water-Soluble Prodrugs Of Propofol For Extended Sedation
A method of administering a prodrug of propofol, preferably O-phosphonooxymethyl propofol disodium salt, comprises the oral, intragastric, or intraintestinal administration of the prodrug in amounts sufficient to induce or maintain a generalized anesthetized state, a conscious sedated state, or to treat insomnia, anxiety, nausea, vomiting, pruritus, epilepsy, and a range of pain syndromes and other medical conditions.
Owner:EISAI INC
Hydrolytically degradable carbamate derivatives of poly(ethylene glycol)
InactiveUS20050147583A1Facilitated releaseReduce deliveryAntibacterial agentsAntimycoticsWater soluble prodrugCarbamate
Owner:NEKTAR THERAPEUTICS INC
High water-soluble prodrug, and its preparing method and pharmaceutical use
InactiveCN1709516AGood water solubilityGuaranteed water solubilityPharmaceutical non-active ingredientsOrganic acidWater soluble prodrug
The present invention discloses a high water-solubility precursor medicine, its preparation method and application in pharmaceutical technology. Said invention provides a general formula accorded with said precursor medicine. Said precursor medicine can be made up by using parent medicine and organic aid and making them undergo a certain reaction process. Said invention also provides the concrete steps of its preparation method, and said precursor medicine can be used for preparing several medicines, and has the advantages of high water-solubility, good effect and lower toxic side-effect.
Owner:赵洪
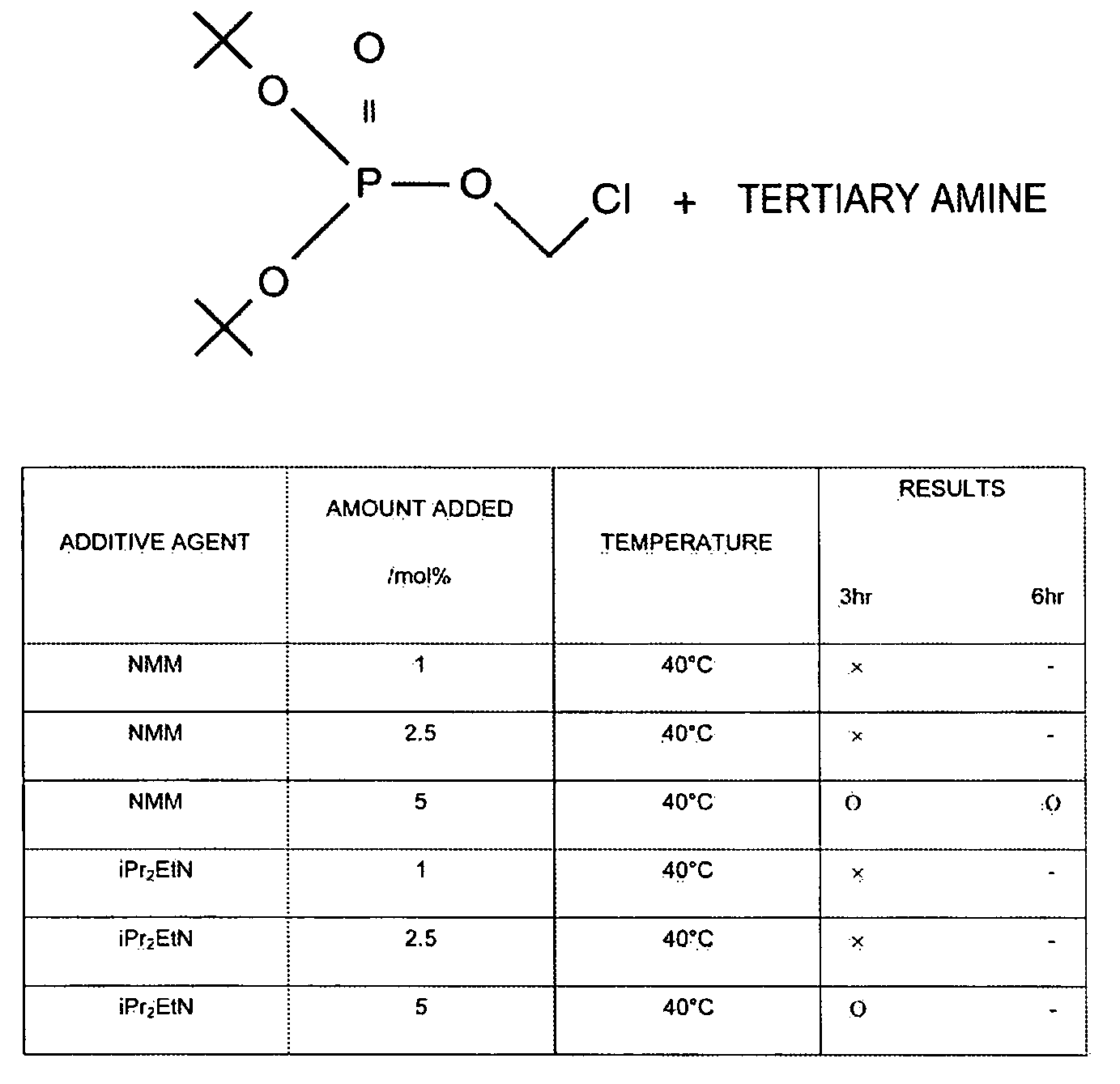
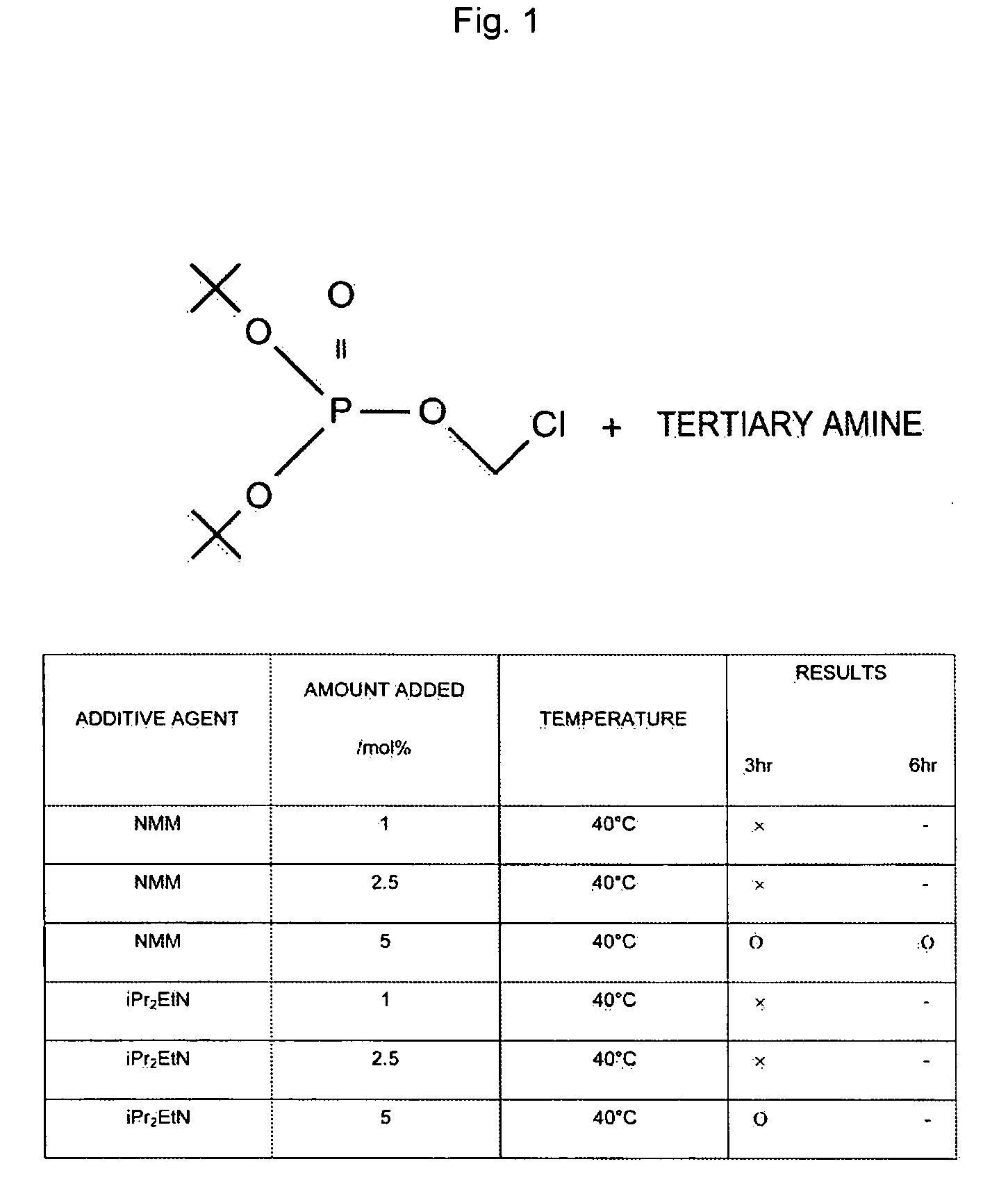
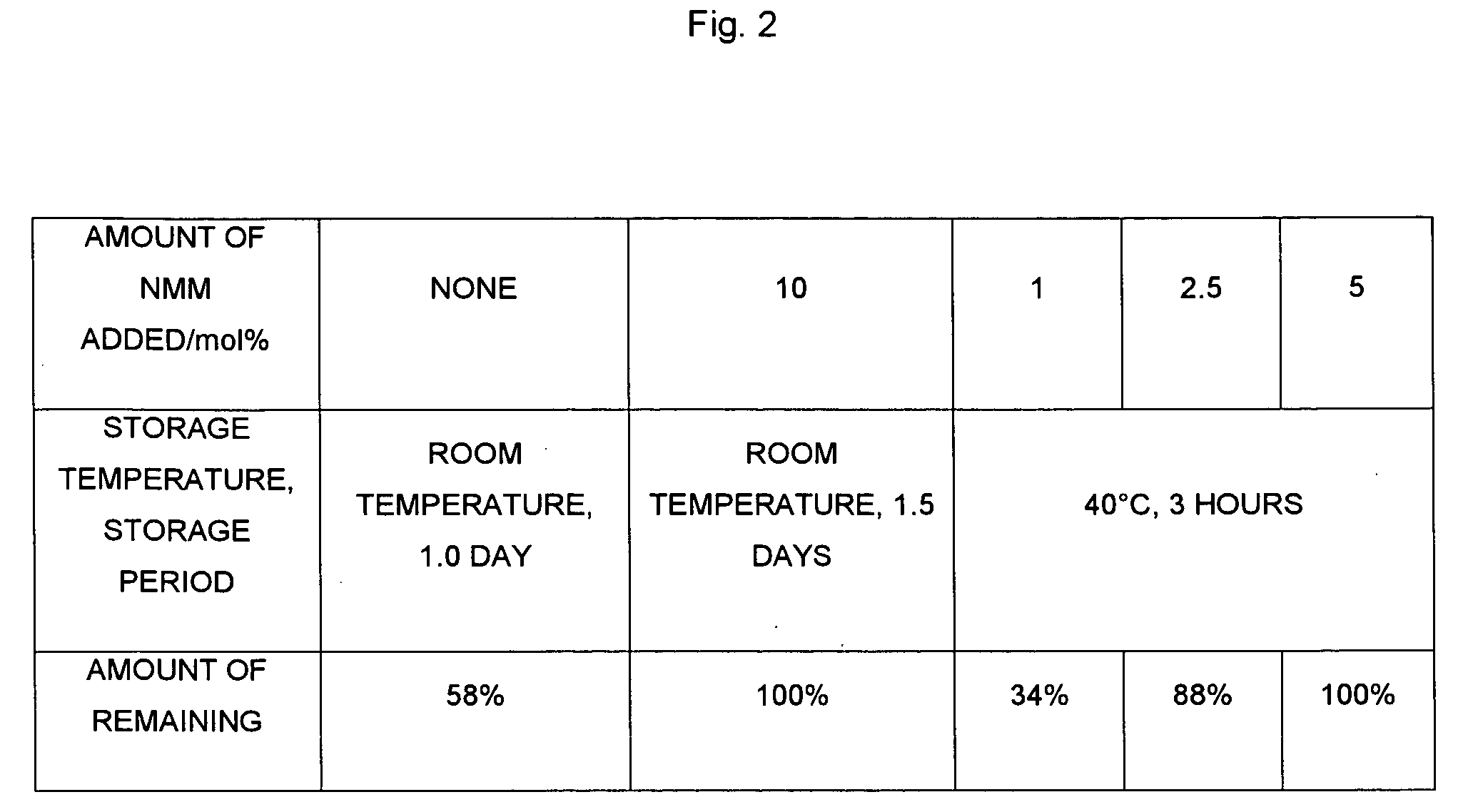
Composition Containing Stability-Improved Chloromethyl Phosphate Derivatve and Process for Producing Same
InactiveUS20090114877A1Easy to produceImprove workabilityOther chemical processesGroup 5/15 element organic compoundsArylWater soluble prodrug
The present invention provides a production process or the like, which is a process for producing a chloromethyl phosphate derivative useful for producing a water-soluble prodrug, and which is excellent from the points of view of workability, operativity and energy saving. According to the present invention, there is provided a process for producing a composition containing a compound represented by the following Formula (I) and a tertiary amine,(wherein R1 and R2 are identical or different from each other, and represent a C1-C6 alkyl group, a C2-C6 alkenyl group or a C6-C14 aryl C1-C6 alkyl group which may have a substituent, and R1 and R2 may together form a ring), the process comprising adding the tertiary amine to the compound represented by Formula (I).
Owner:EISIA R&D MANAGEMENT CO LTD
Methods of Administering Water-Soluble Prodrugs of Propofol
A method of administering a prodrug of propofol, preferably O-phosphonooxymethyl propofol disodium salt, comprises the subcutaneous or rectal administration of the prodrug in amounts sufficient to induce or maintain a generalized anesthetized state, a conscious sedated state, or to treat insomnia, anxiety, nausea, vomiting, pruritus, epilepsy, and a range of pain syndromes, including migraine pain, and other medical conditions.
Owner:EISAI INC
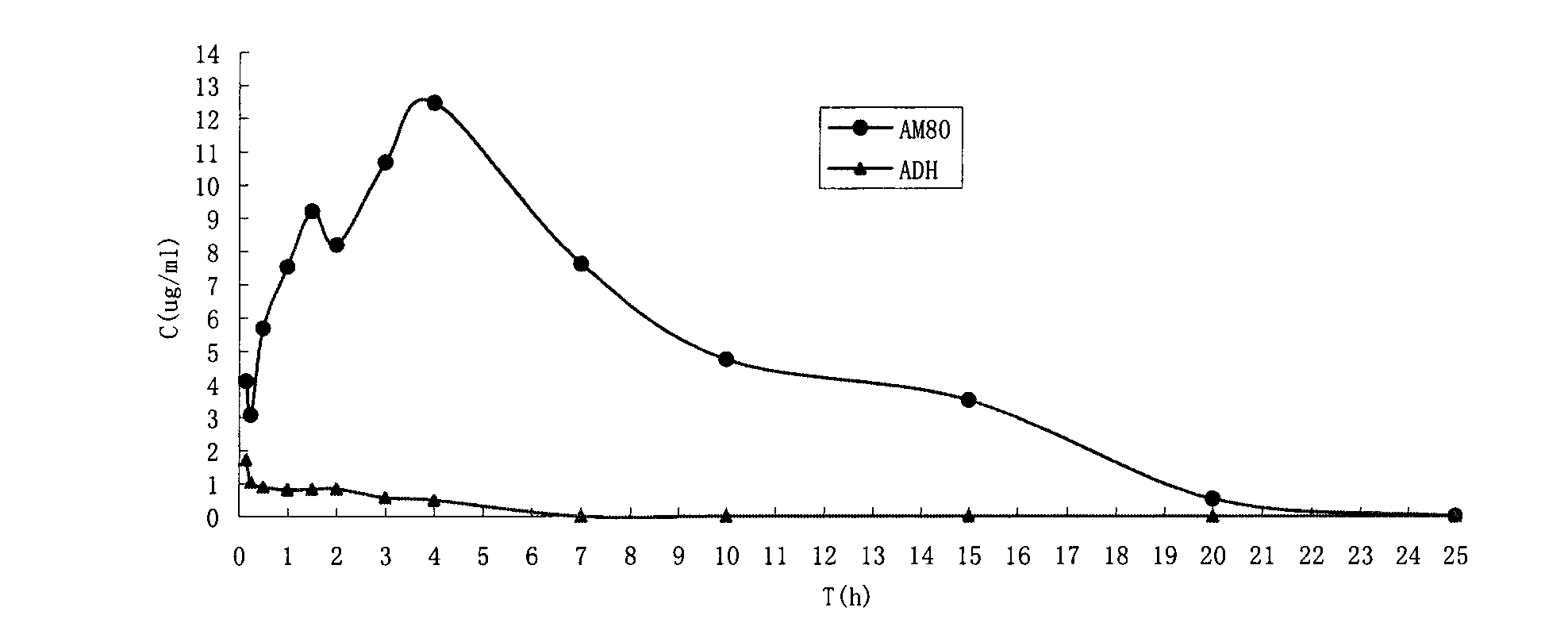
Water-soluble prodrug of tamibarotene, and preparation method and applications thereof
InactiveCN101665449AAppropriate drug crystal formGood water solubilityOrganic active ingredientsOrganic compound preparationSolubilityDrug crystals
The invention provides a water-soluble prodrug of tamibarotene, and a preparation method and applications thereof and belongs to the technical fields of organic compound synthesis and medical applications. The water-soluble prodrug of tamibarotene has favorable water solubility and suitable drug crystal form, and therefore, is suitable to be used as a raw material for medical preparations and especially suitable for preparing injections. Particularly, the invention provides medical acceptable salts of tamibarotene DMEA ester, which have the general formula (I), wherein HA is HCl, H2SO4, HNO3,H3PO4, HOAc, paratoluenesulfonic acid, maleic acid, succinic acid, citric acid or L(+)-tartaric acid.
Owner:SHANDONG UNIV
Process for water soluble azole compounds
An improved process is provided for preparing water-soluble prodrugs of triazole antifungal compounds containing a secondary or tertiary hydroxyl group. More particularly, the improved process is directed toward preparation of water-soluble triazole antifungal compounds are provided having the general formula wherein A is the non-hydroxy portion of a triazole antifungal compound of the type containing a secondary or tertiary hydroxyl group and R and R1 are as defined in the specification.
Owner:EISIA R&D MANAGEMENT CO LTD
Water soluble prodrugs of azole compounds
Water-soluble prodrugs of triazole antifungal compounds having a secondary or tertiary hydroxy group are provided. More particularly, new water-soluble triazole antifungal compounds are provided having the general formulawherein A is the non-hydroxy portion of a triazole antifungal compound of the type containing a secondary or tertiary hydroxyl group and R and R1 are as defined in the specification.
Owner:EISIA R&D MANAGEMENT CO LTD
Positively charged water-soluble prodrugs of oxicams and related compounds with very high skin penetration rate
InactiveCN101522692AAvoid side effectsImprove absorption rateSenses disorderNervous disorderSolubilitySide effect
The novel positively charged pro-drugs of oxicams and related compounds in the general formula (1) 'Structure 1' were designed and synthesized. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin approximately 100 times faster than do oxicams and related compounds. It takes 1-2 hours for oxicams and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about approximately 50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the parent drugs in a few minutes. The prodrugs can be used medicinally in treating any oxicams-treatable conditions in humans or animals. Second, the prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of oxicams. Controlled transdermal administration systems of the prodrugs enable oxicams and related compounds to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of oxicams and related compounds. Another great benefit of the transdermal administration of these pro-drugs is that administering medication, especially to children, will be much easier.
Owner:于崇曦 +1
Aqueous based pharmaceutical formulations of water-soluable prodrugs of propofol
InactiveUS20100311698A1Minimize degradationSuitable for packagingBiocideNervous disorderWater soluble prodrugAntioxidant
The present invention is directed to aqueous based formulations of water-soluble prodrugs of propofol. The formulations comprise in aqueous medium an effective amount of the water-soluble prodrug of propofol in the absence of an antioxidant. The formulations are particularly useful as intravenous injections. The formulations preferably are buffered to a pH suitable for minimizing degradation of the prodrug during storage. The formulations can be prepared without the use of harmful co-solvents or surfactants and are stable at room temperature over extended periods of time.
Owner:EISAI INC
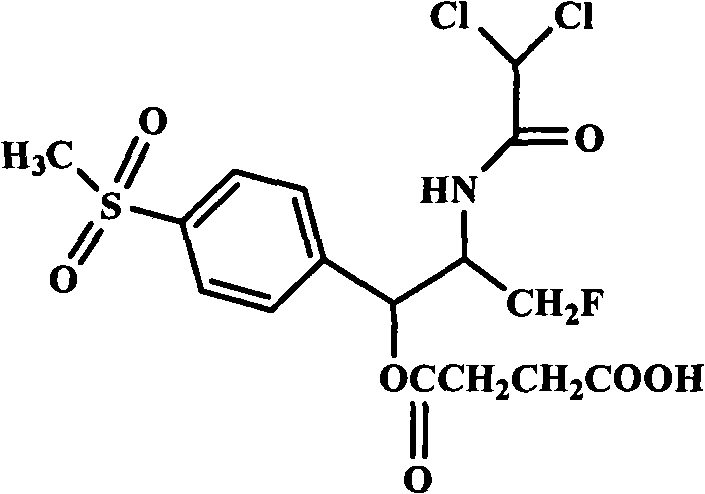
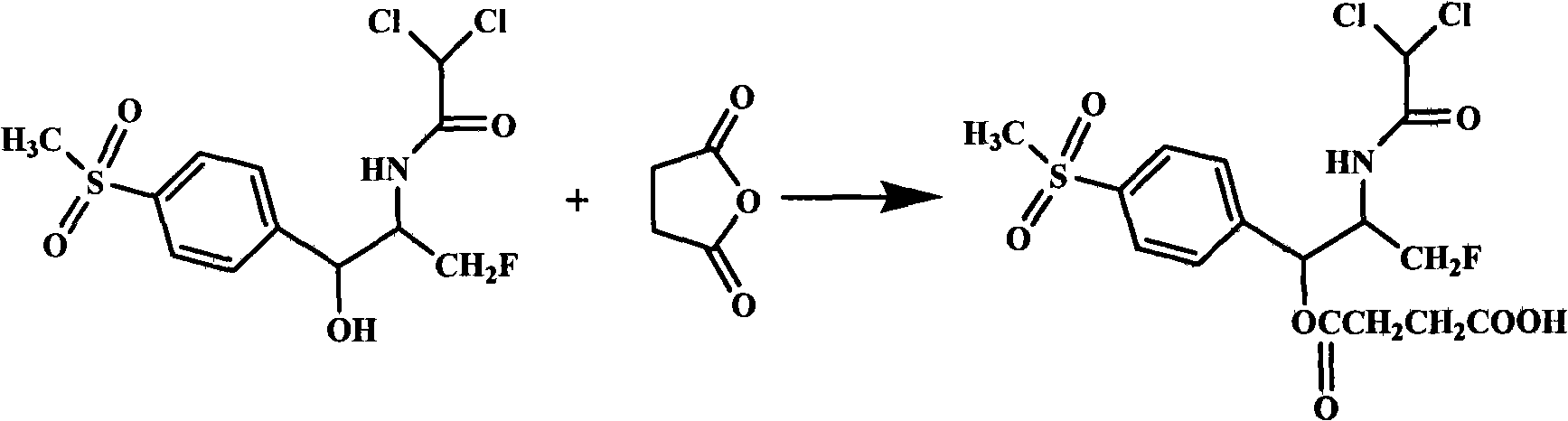
Water-soluble prodrugs of florfenicol and its analogs
The present invention discloses certain novel prodrugs of florfenicol and / or of florfenicol analogs, including prodrugs of salts pharmaceutically acceptable salts of florfenicol and its analogs, including nitrogen-containing esters of the secondary alcohol group of florfenicol and of its analogs, and pharmaceutically acceptable salts thereof, compositions containing them, and methods of administering them to subjects. In particular embodiments the prodrugs are sufficiently water-soluble to serve the functions needed of a water-soluble prodrug of florfenicol or of a water-soluble prodrug of a florfenicol analog. A certain subclass of the compounds also possesses the hydrolytic stability needed to maintain the prodrug in solution in the subject's system until appropriate conditions exist when the prodrug can hydrolyze, releasing florfenicol or the florfenicol analog in question.
Owner:SCHERING PLOUGH ANIMAL HEALTH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com