Process for water soluble azole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Illustrates Prior Process of U.S. Ser. No. 60 / 177,169
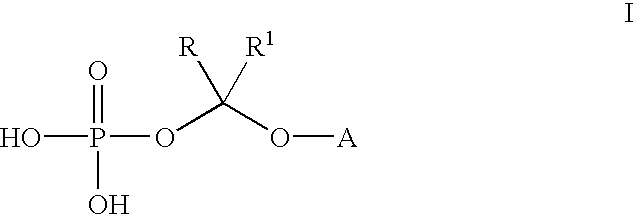
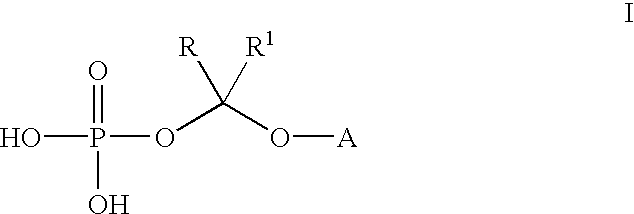
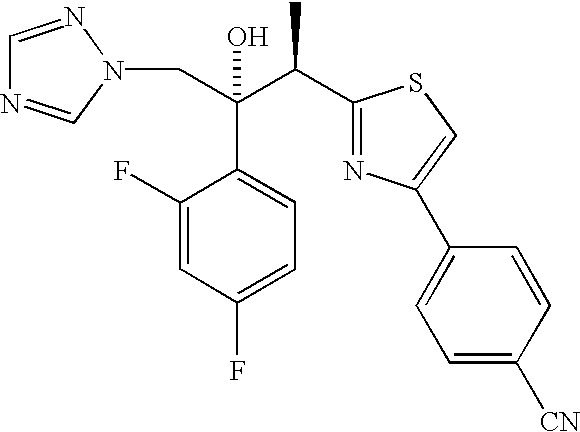
(2R,3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1 H-1,2,4-triazol-1-yl)-2-[(dihydrogen phosphonoxy)methoxy]butane, sodium salt
[0085] 25
A. (2R, 3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1H--1,2,4-triazol-1-yl)-2-[(di-tert-butyl phosphonoxy)methoxy]butane
[0086] 26
[0087] To a solution of (2R, 3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4--difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2-oI, II, (8.74 g, 20 mmol) in THF (40 mL) under a nitrogen atmosphere was added sodium hydride (0.80 g, 60% in oil, 20 mmol) at rt. The resulting mixture was stirred at rt for 0.25 h then di-tert-butyl chloromethyl phosphate, III (10.3 g, 40 mmol) was added. The reaction mixture was heated at 50.degree. C. for 16 h. The reaction mixture was then allowed to cool to rt and was concentrated under reduced pressure. The residue was dissolved in Et.sub.2O and was washed with H.sub.2O and brine. The organic layer was d...
example 2
(2R,3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1H-1,2-,4-triazol-1-yl)-2-[(dihydrogen phosphonoxy)methoxy]butane
[0100] 29
[0101] A. An oven dried, 1 L round-bottom flask equipped with a mechanical stirrer, nitrogen inlet adapter, pressure-equalizing addition funnel fitted with a rubber septum and temperature probe was charged with sodium hydride (2.89 g, 0.069 mol, 60%) and THF (50 mL). To this stirred suspension, (2R,3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluorophen-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol, II, (10 g, 0.023 mol) in 30 mL of THF was added dropwise over 20 minutes at room temperature. After stirring for 45 minutes, a solution of iodine (2.99 g, 0.0115 mol) in THF (30 mL)) was added dropwise over 10 minutes followed by dropwise addition of di tert butylchloromethyl phosphate, III (13.29 g, 0.035 mol, .about.68% purity) over 15 minutes. The reaction mixture was stirred for 4 h at about 41.degree. C. to complete the reaction. The completion of...
example 3
Bis lysine salt of (2R,3R)-3-[4-(4-cyanophenyl)thiazol-2-yl]-2-(2,4-difluo-rophenyl)-1-(1H-1,2,4-triazol-1-yl)-2-[(dihydrogen phosphonoxy)methoxy]but-ane
[0103] The above obtained title product from Example 2 was dissolved in methanol (75 mL) and to this L-lysine (1.8 g) was added and heated at 60.degree. C. for 4.5 h. The hot reaction mixture was filtered through a bed of Celite. The filtrate was concentrated to a volume of about 5 mL, mixed with ethanol (100 mL) and heated to 65.degree. C. to crystallize the bis lysine salt. The salt was collected on a Buchner funnel and dried under vacuum to afford 3.71 g as an off white crystalline solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com