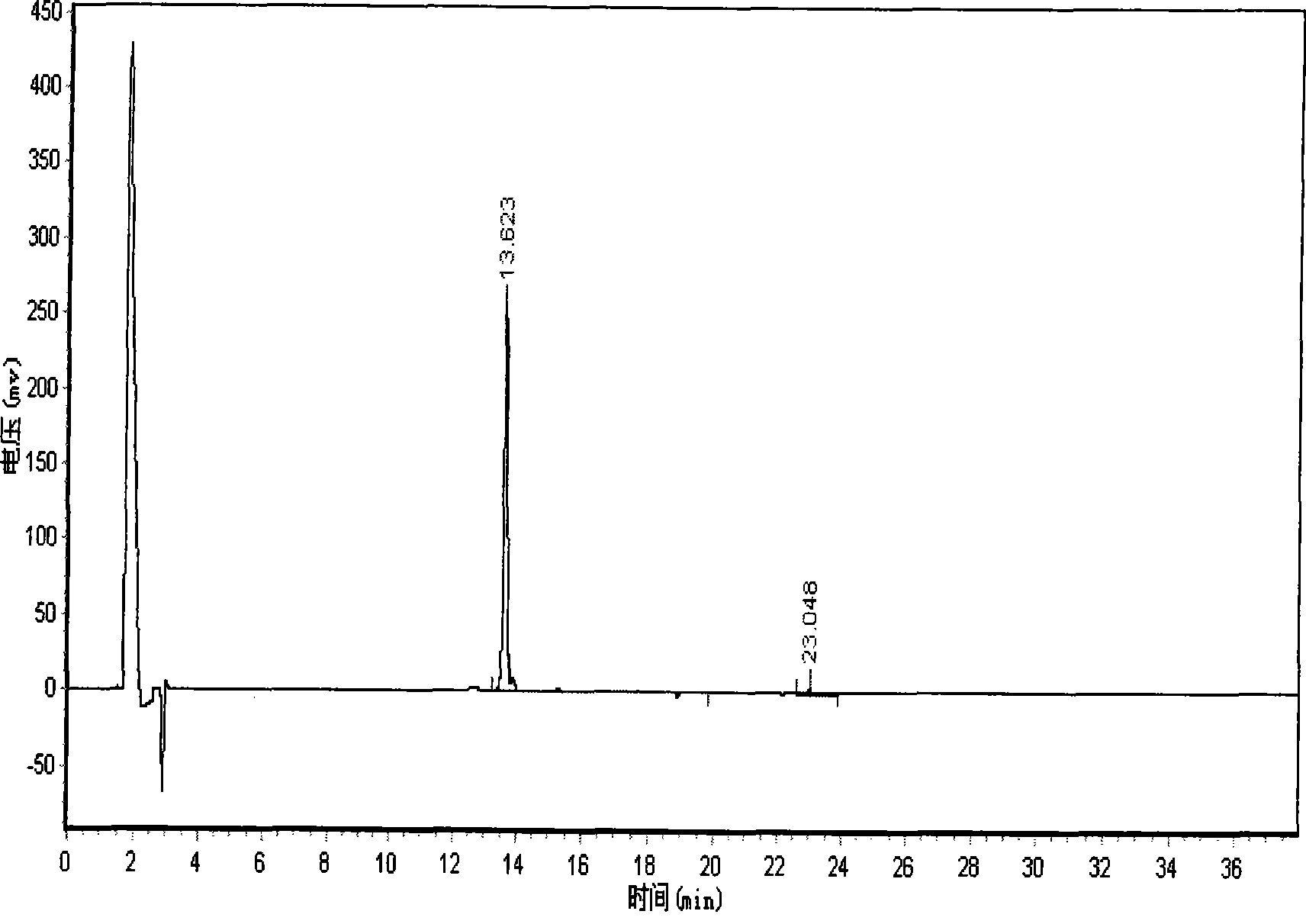
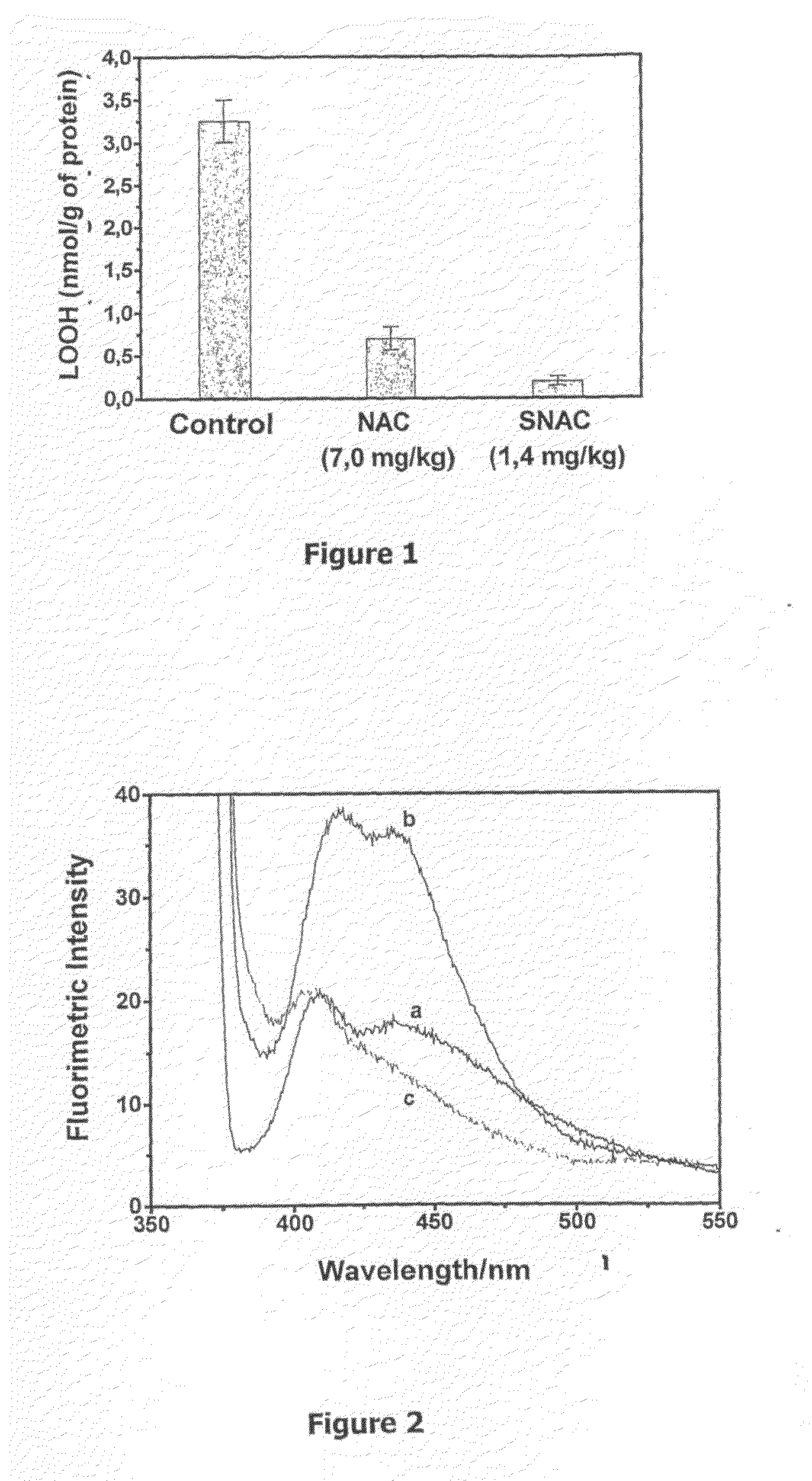
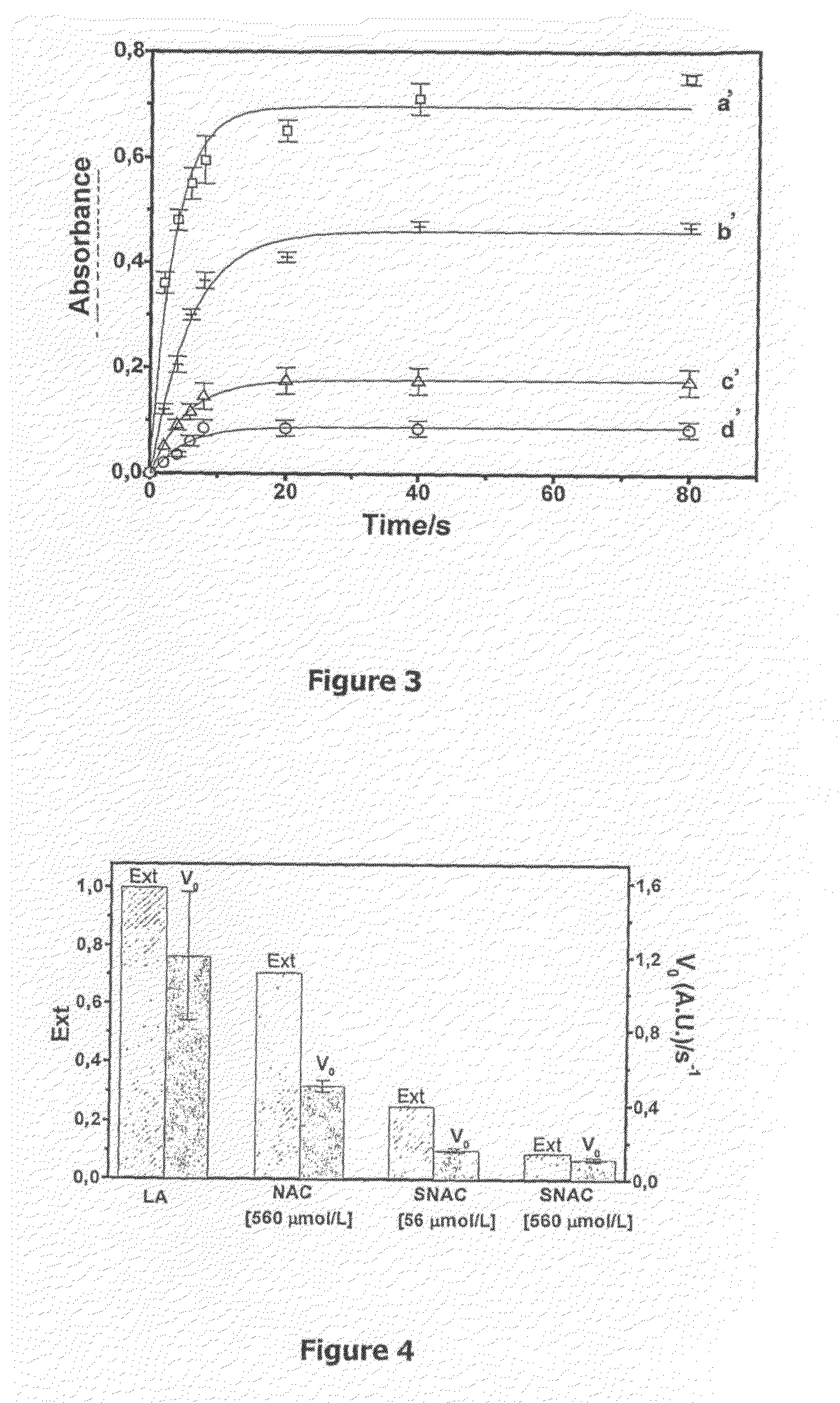
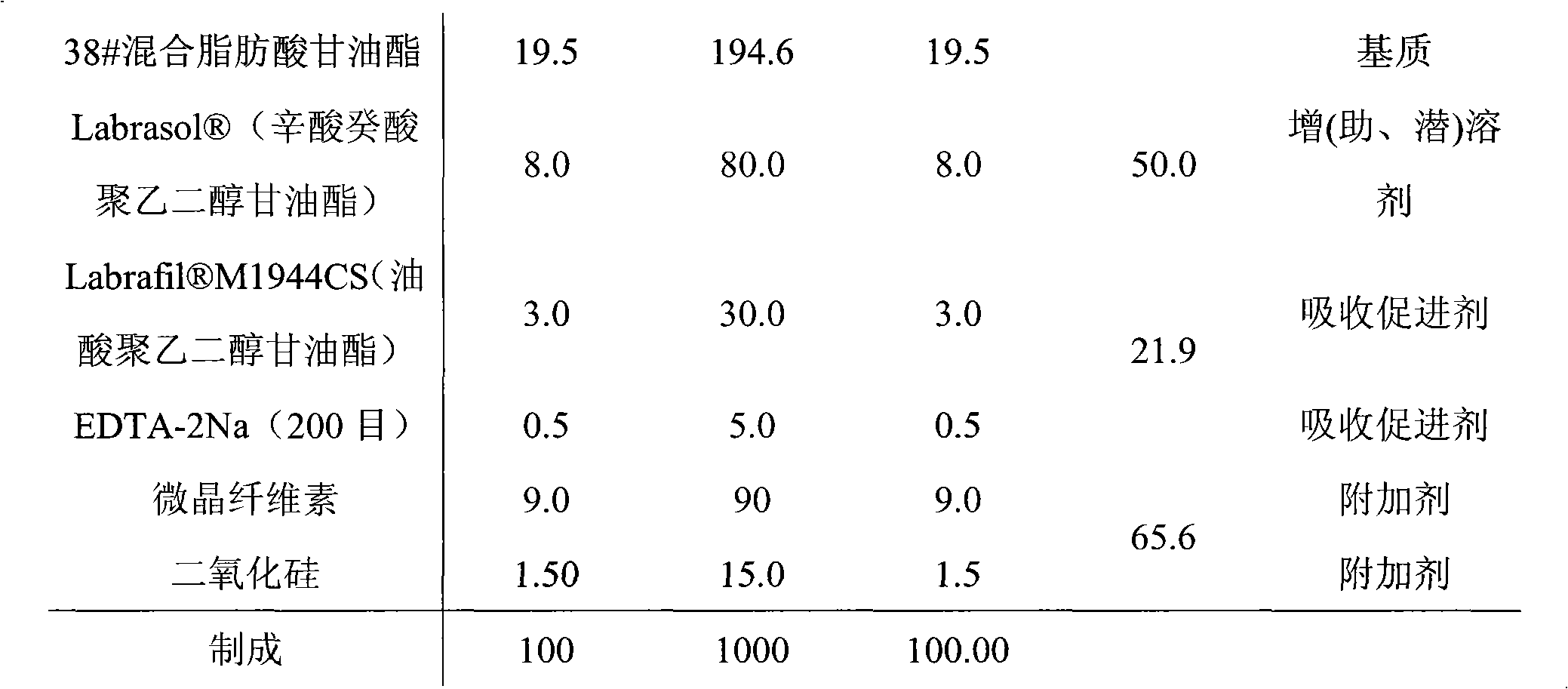Patents
Literature
238 results about "Rectal administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rectal administration uses the rectum as a route of administration for medication and other fluids, which are absorbed by the rectum's blood vessels, and flow into the body's circulatory system, which distributes the drug to the body's organs and bodily systems.
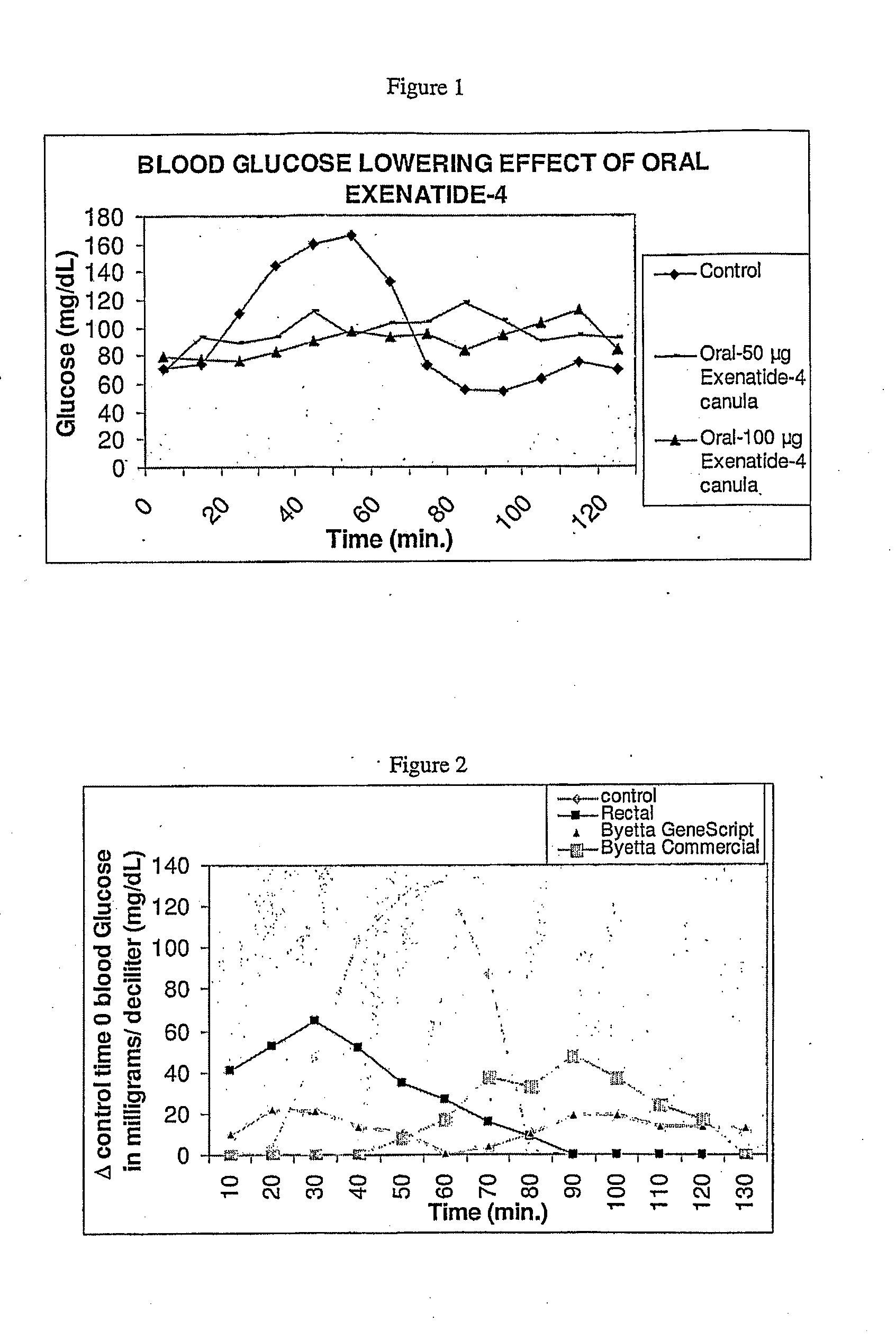
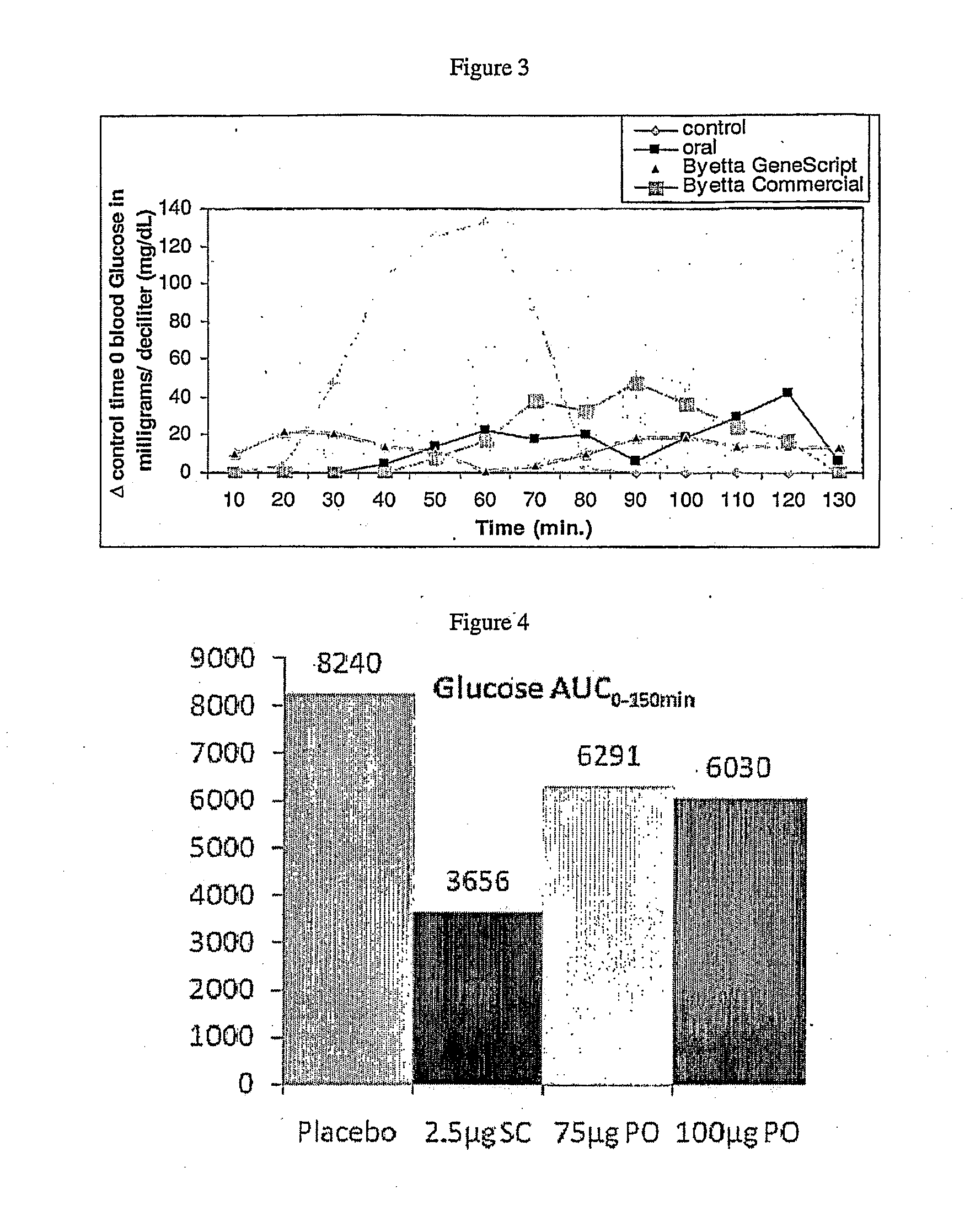
Methods and compositions for oral administration of exenatide
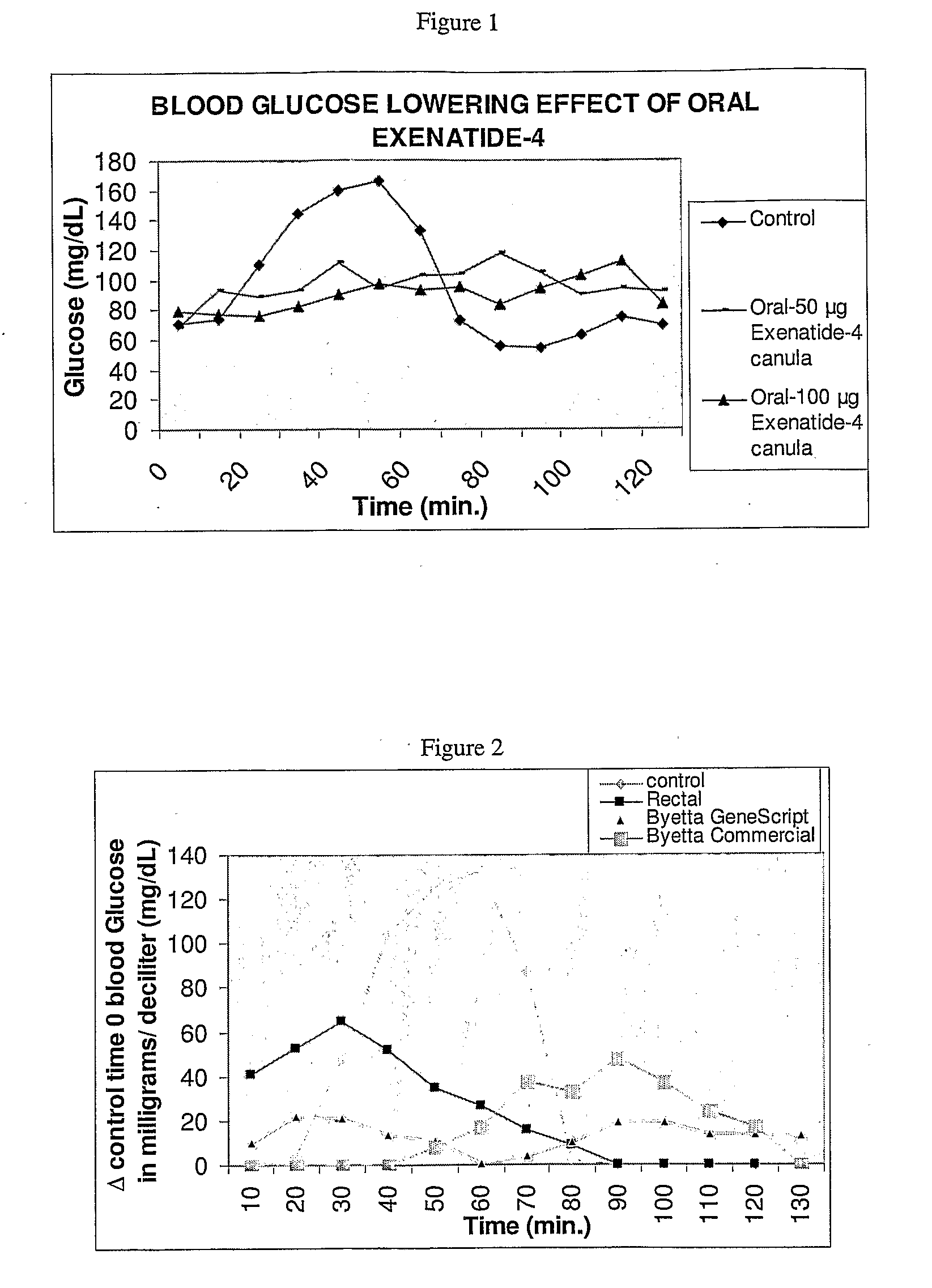
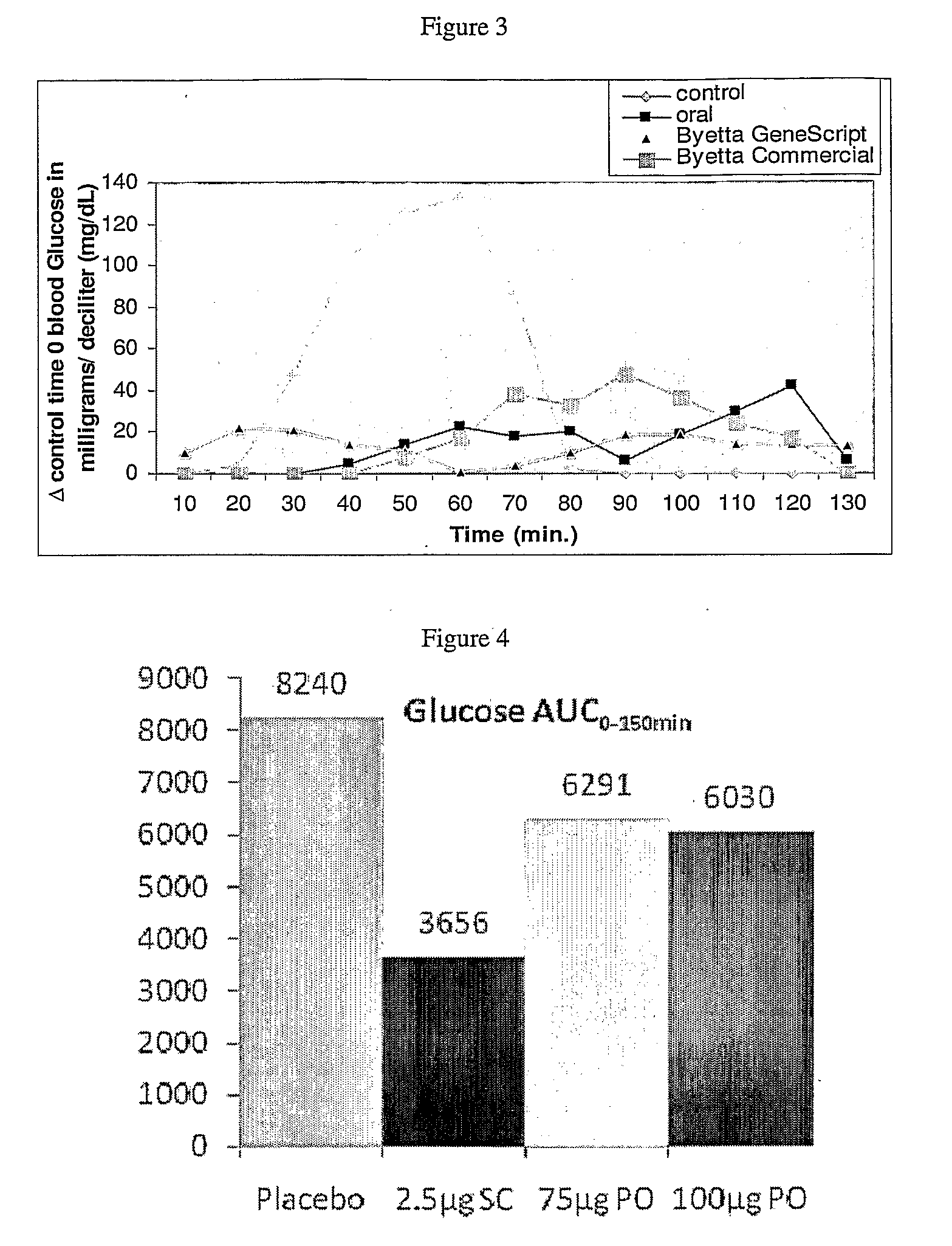
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Rapid acting drug delivery compositions
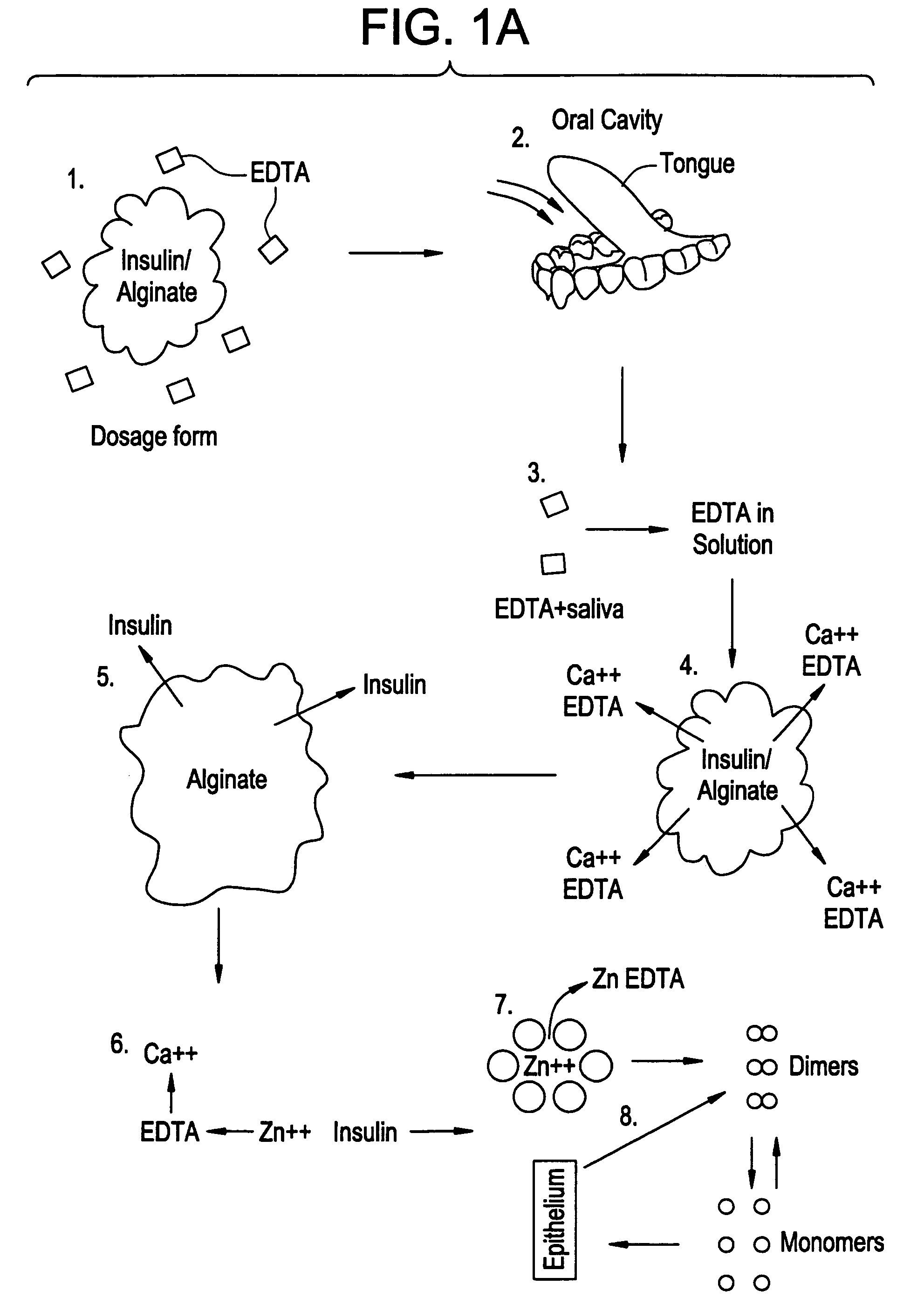
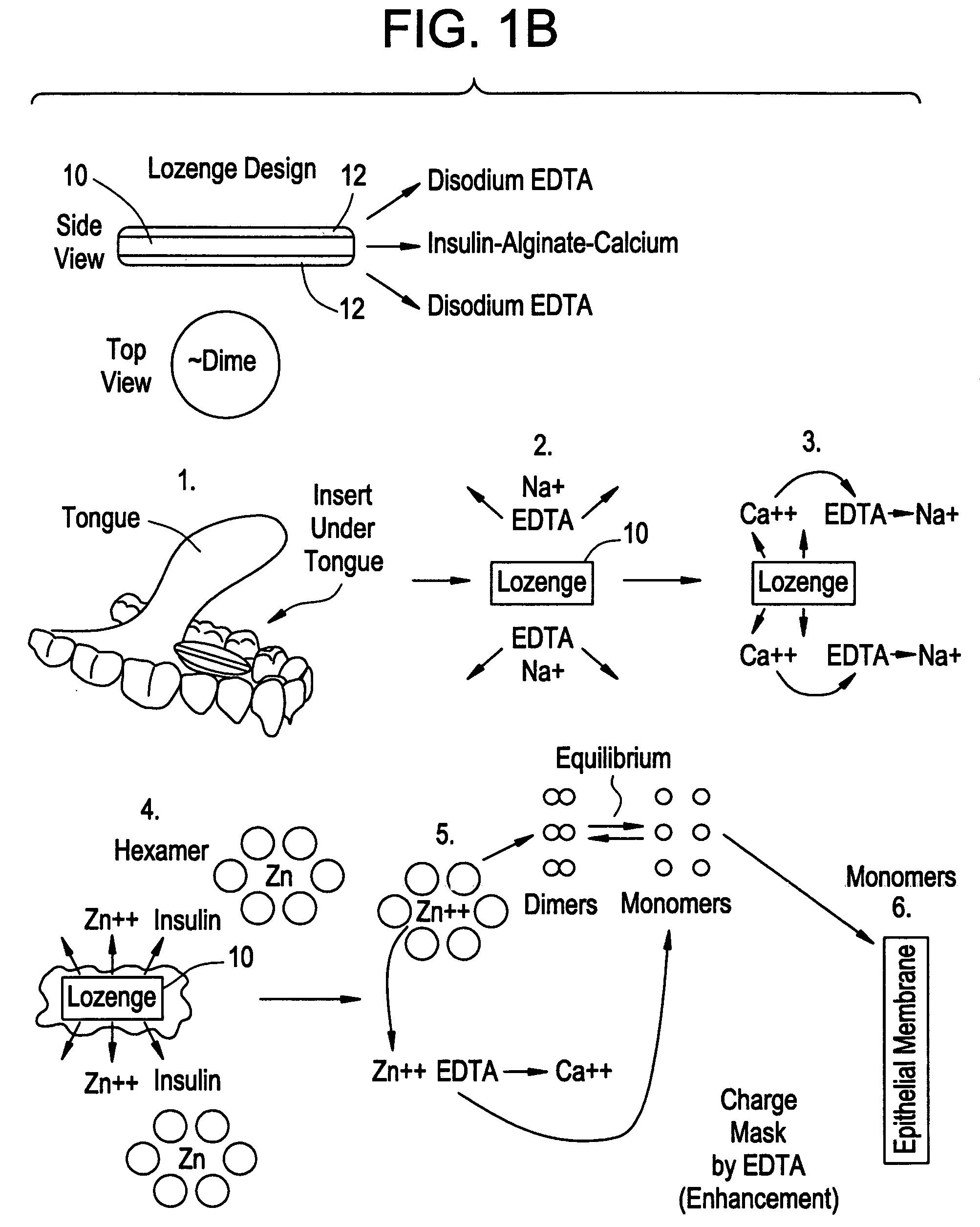
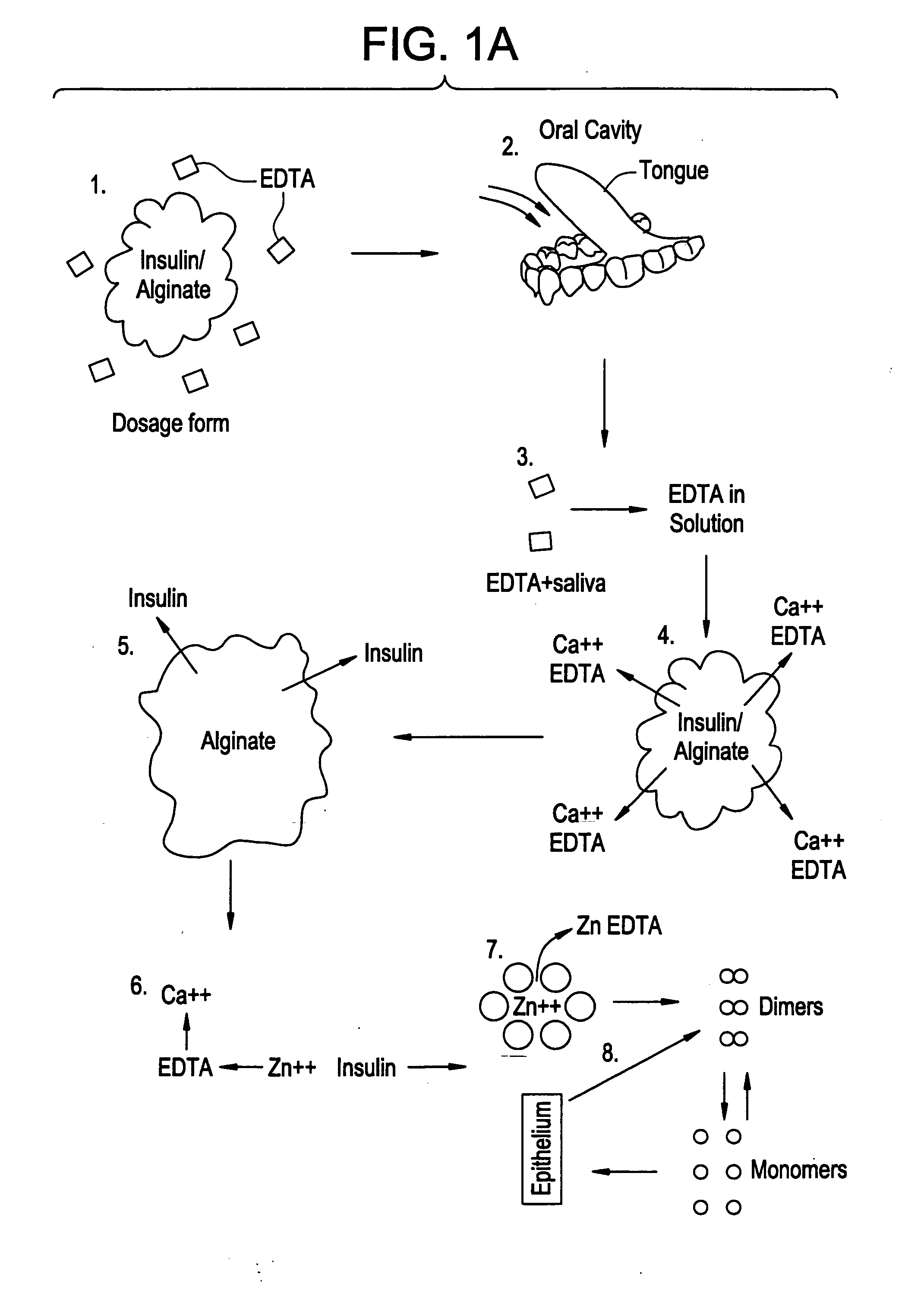
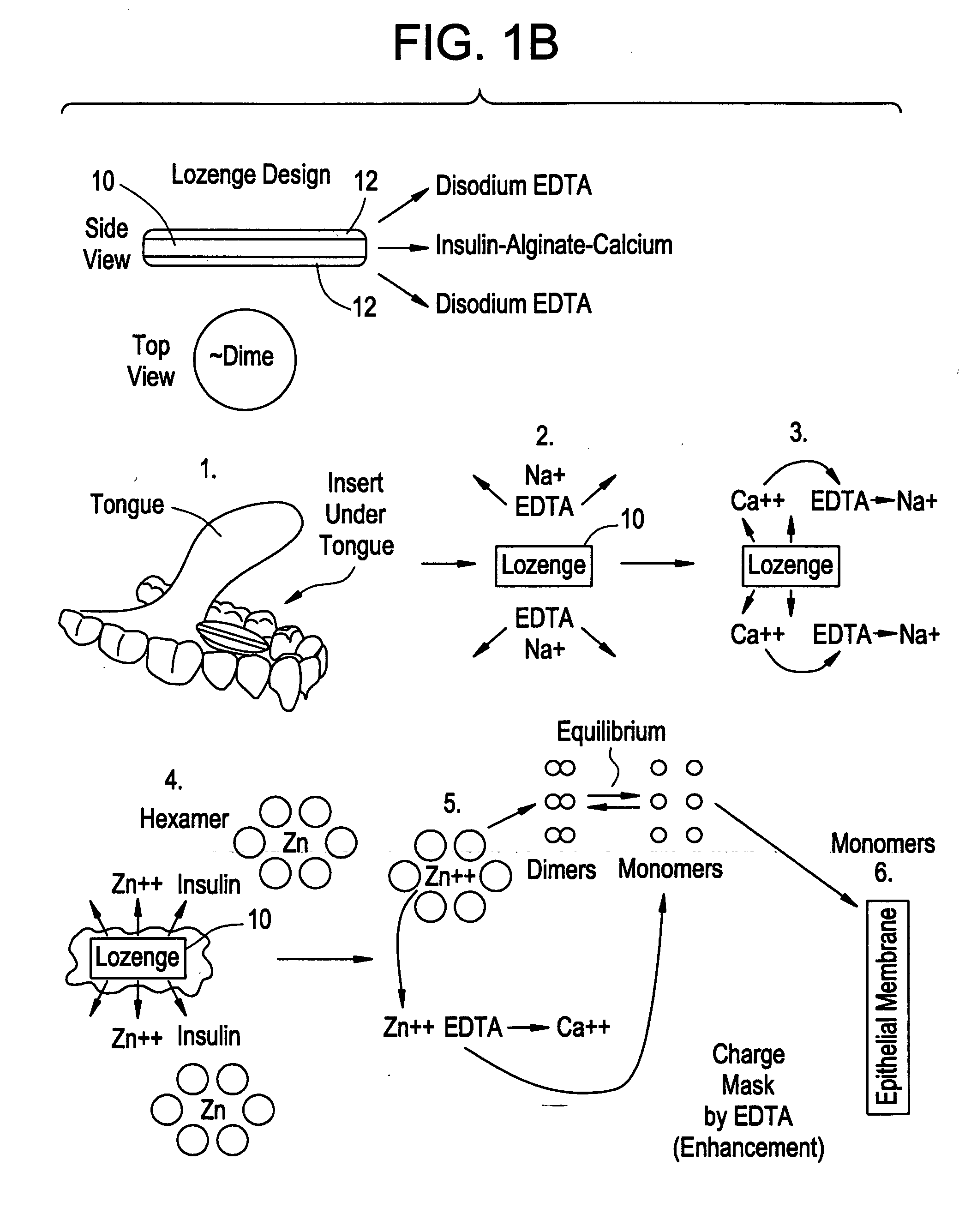
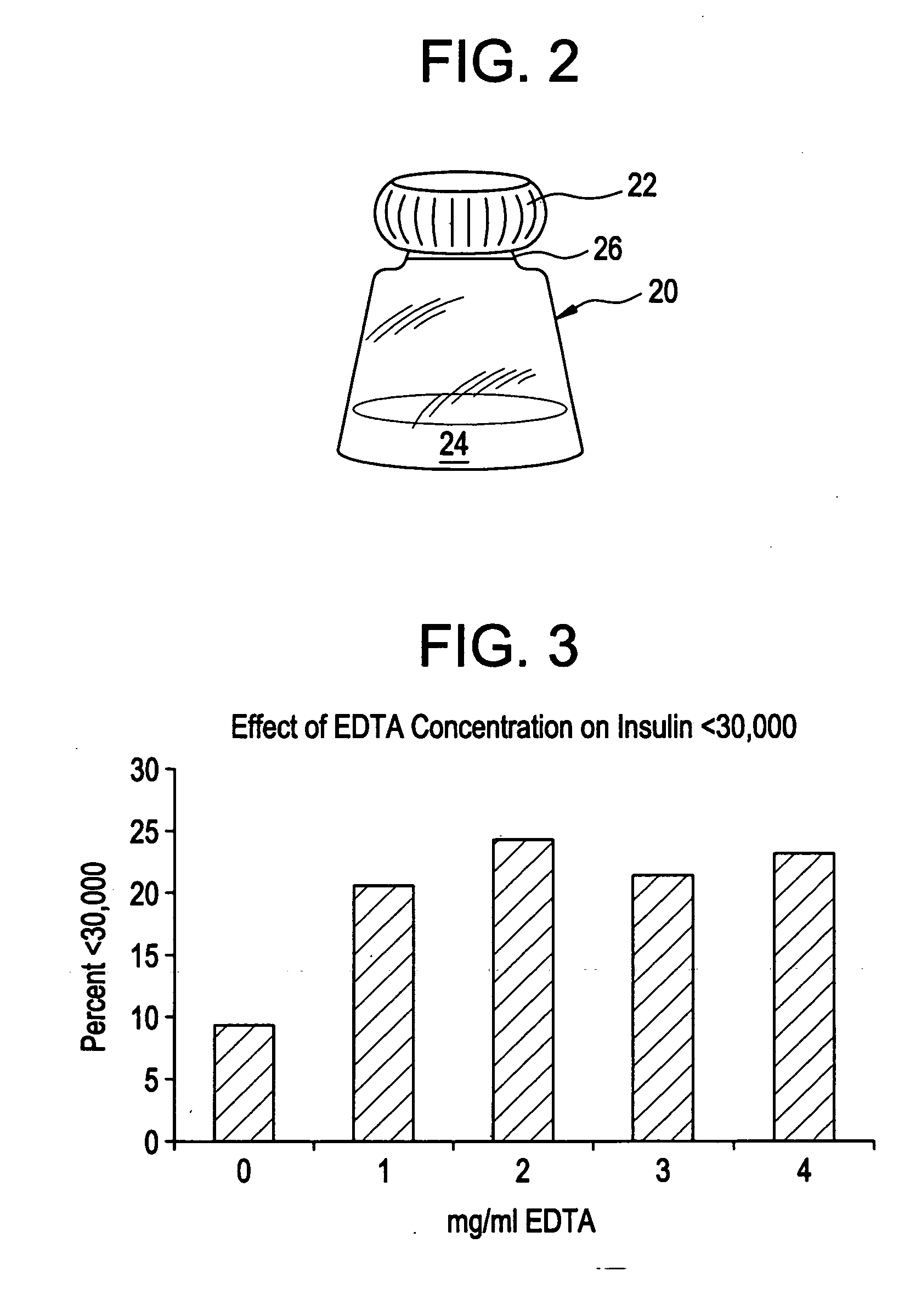
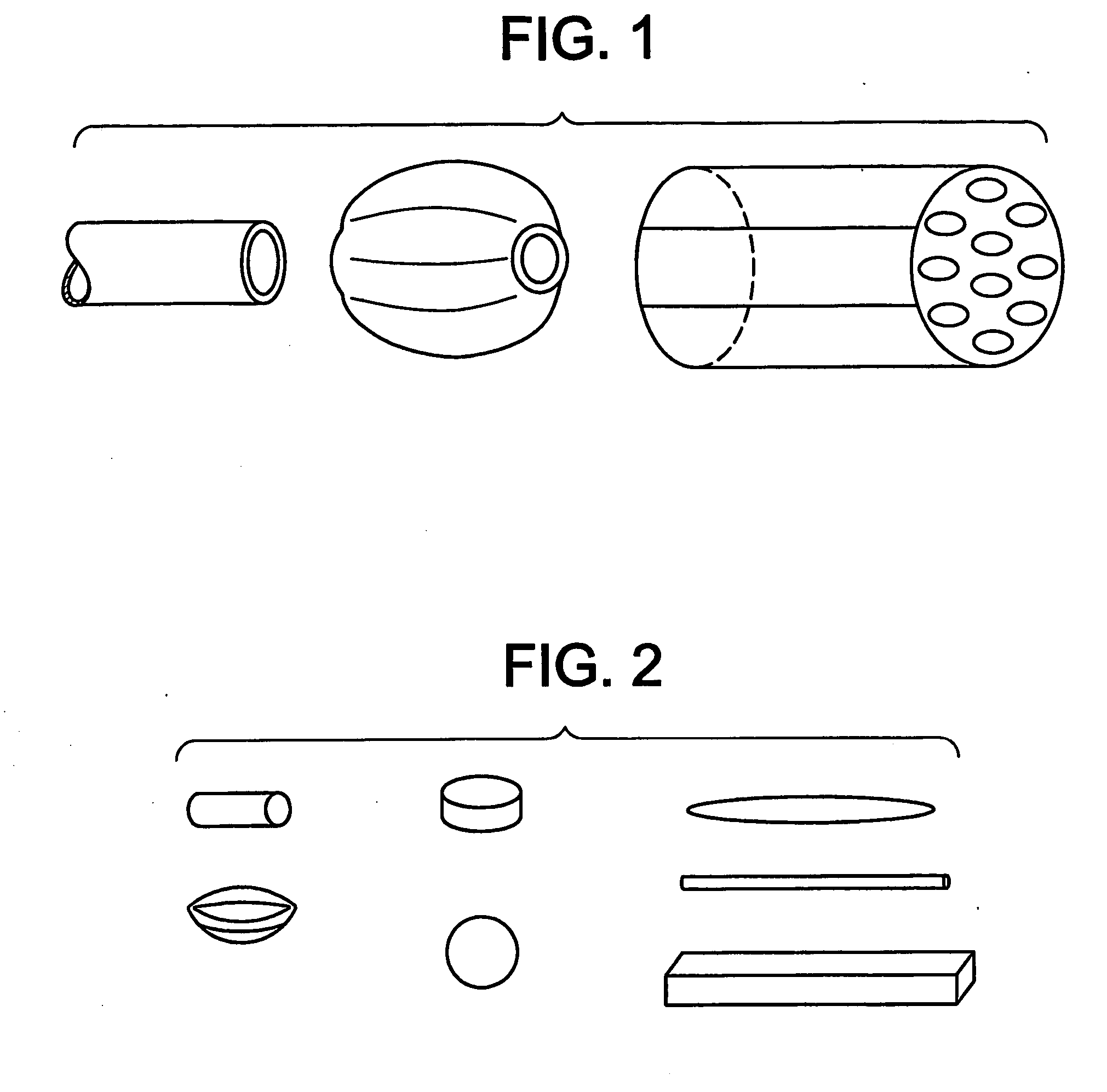
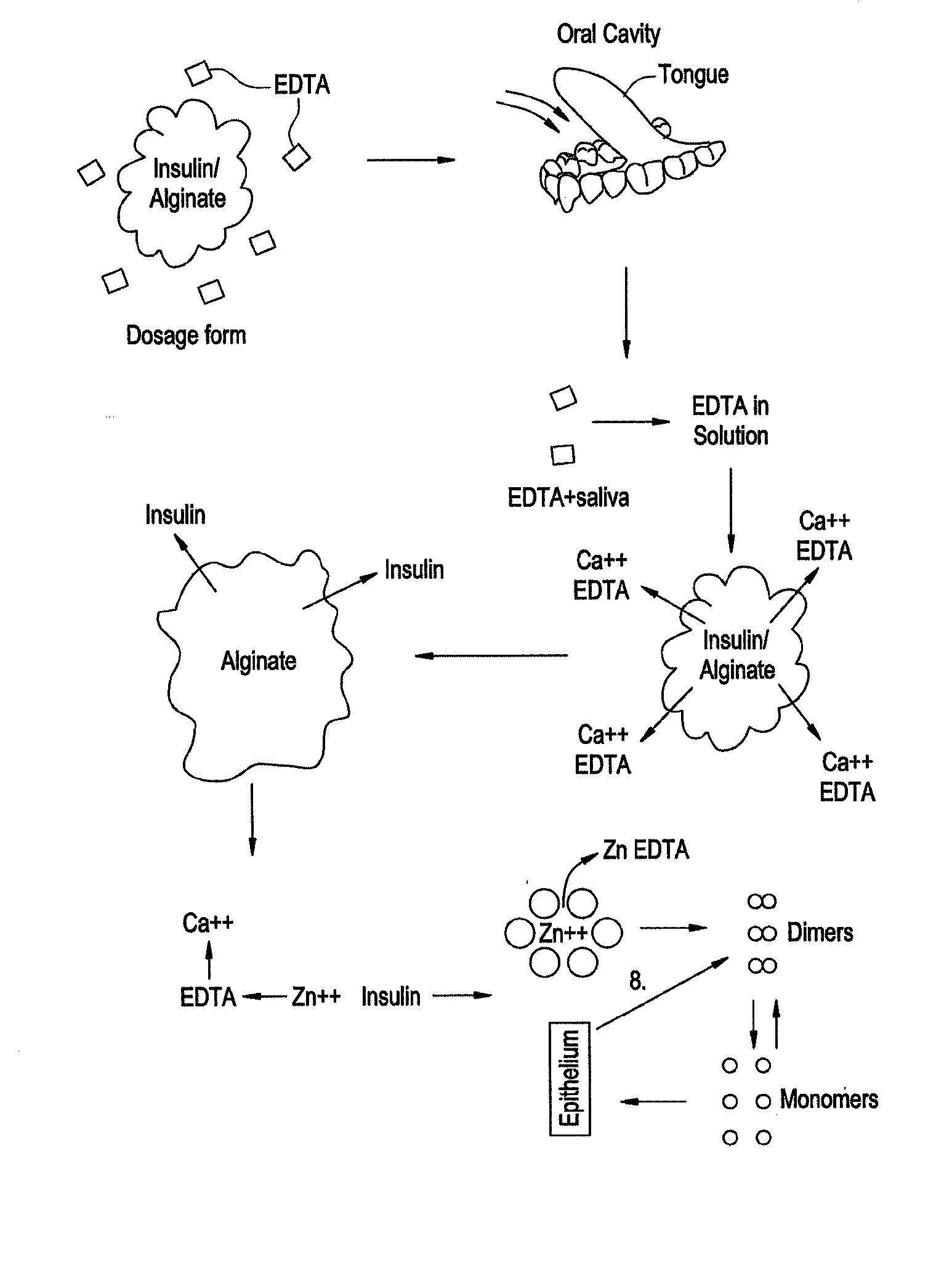
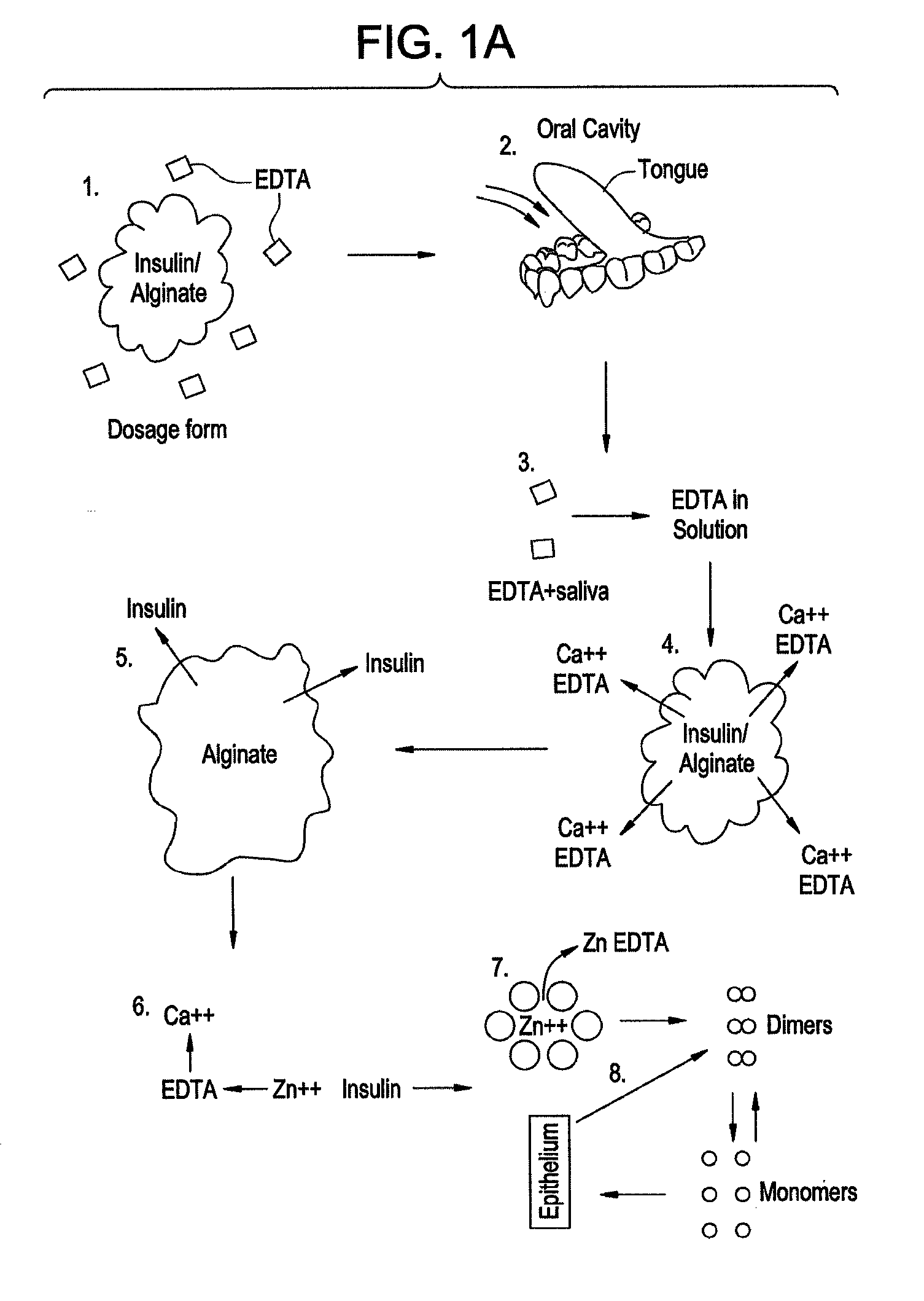
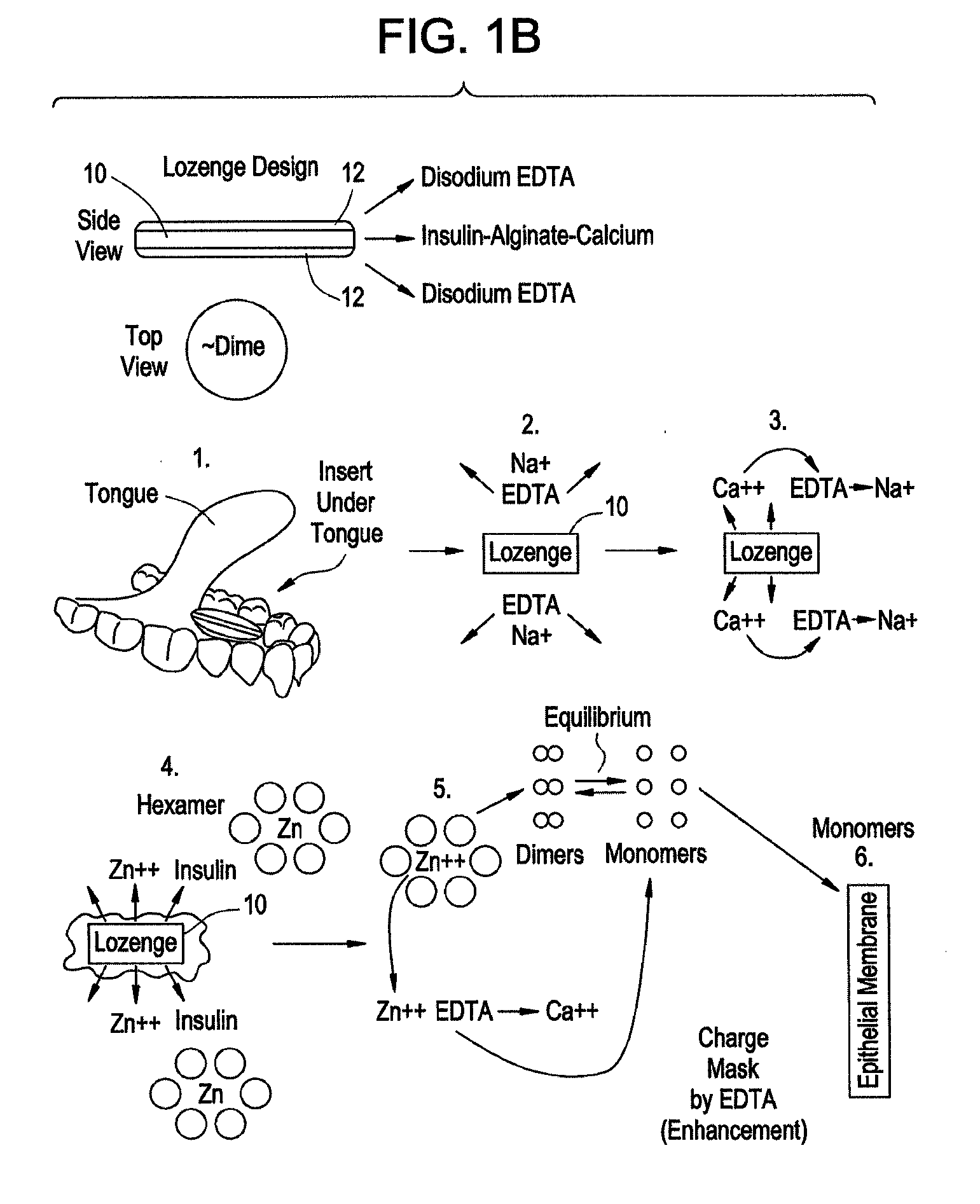
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Composition and method for inhibiting platelet aggregation
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Self-expanding device for the gastrointestinal or urogenital area
InactiveUS20060142794A1Lower the volumeLose weightSurgeryDilatorsIntestinal structureReproductive tract
Devices for treatments of diseases and disorders associated with the gastrointestinal tract, especially the stomach, or urinogenital tract are described herein. Initially, the device is in a temporary form which is suitable for oral or rectal administration. After exposure to a stimulus, such as a temperature or pH change, the device changes shape to a permanent form, which allows it to become mechanically fixed in the stomach, esophagus or intestine. In one embodiment, the device is used to reduce the volume of the stomach, esophagus or intestine without interfering with the flow of the food through the gastrointestinal tract. The device may be used to help overweight patients lose weight and to deliver drugs to treat disorders and diseases in the in the stomach or intestine. The devices are manufactured from a stimuli-sensitive polymeric material, which is biocompatible and primarily adapted to the mechanical properties and geometry in the area to which it is applied. In the preferred embodiment, the material is a shape memory polymer. Depending on the desired application, the polymer may be either biodegradable or non-degradable.
Owner:MINEMOSCIENCE GMBH
Treatment for joint inflammation
The invention provides the use of a glucocorticoid substance which has a minimal systemic effect in the manufacture of a medicament for oral or rectal administration for non-topical use in the treatment of joint inflammation.
Owner:ASTRAZENECA AB
Chinese herbal medicine combination for treating disease of disorder of bowels's function and its product
A Chinese medicine for treating irritable bowel syndrome (IBS) is prepared from 6 Chinese-medicinal materials including bupleurum root, white peony root, Chuan-xiong rhizome, liquorice root, etc. It may be particles, capsules, tablets, oral liquid injection, etc.
Owner:NANJING NORMAL UNIVERSITY
Rapid Acting Drug Delivery Compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:BIODEL INC
Treatment of Meconium Aspiration Syndrome with Estrogens
ActiveUS20100184736A1Reduce incidenceReduce morbidityOrganic active ingredientsPharmaceutical delivery mechanismSuppositoryNon invasive
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
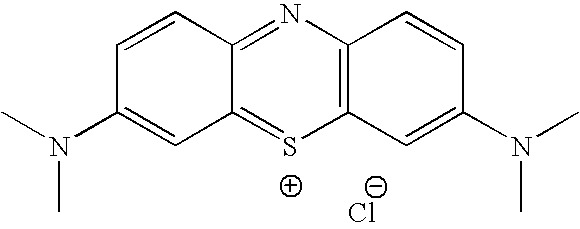
Methylene Blue Derivatives
InactiveUS20070116757A1Facilitated releaseRapid uptakeOrganic chemistryPill deliveryImmediate releaseFatty acid
Pharmaceutical compositions comprising a fatty acid salt, a dicarboxylic acid salt, an alkyl sulfate salt, an aryl sulfate salt or an alkyl aryl sulfonate salt of methylene blue or a derivative of methylene blue are described herein. The compositions are preferably administered orally and can be administered as tablets, soft or hard shell capsules (e.g. soft gelatin capsules), suspensions or solutions. The composition can also be formulated as a suppository or enema or rectal administration. The compositions further comprise a pharmaceutically acceptable carrier and optionally one or more pharmaceutically acceptable excipients. Suitable excipients include diluents, binders, plasticizers, lubricants, disintegrants, colorants, stabilizers, surfactants, and combinations thereof. The fatty acid salts, alkyl sulate salts, aryl sulfate salts or alkyl aryl sulfonate salts can be co-mixed or co-melted with one or more fatty acids to make more hydrophobic compositions, which may result in less staining formulations. The compositions can be formulated for immediate release, controlled release such as extended release, delayed release, and pulsatile release, or combinations thereof. In one embodiment, the derivative of methylene blue is methylene dodecylsulfate.
Owner:COLLEGIUM PHARMA INC
Methods and compositions for oral administration of exenatide
ActiveUS20130195939A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsSkeletal disorderDiabetes mellitusOral medication
Owner:ORAMED
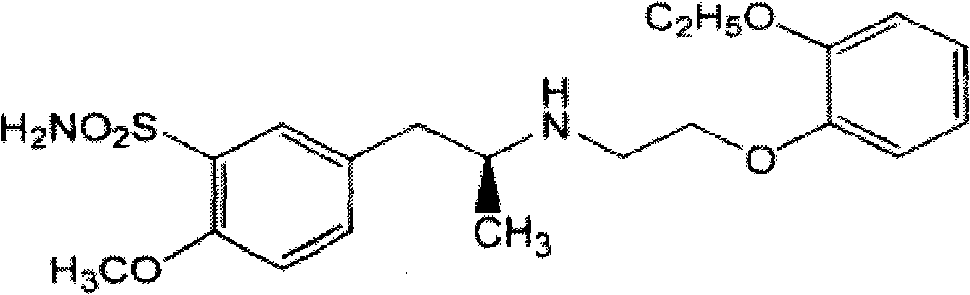
Compound of losartan compound or its medical salt and calcium channel blocker or its medical salt
InactiveCN101347427ALittle side effectsGood curative effectOrganic active ingredientsCardiovascular disorderCarboxylic acidLosartan
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Extracting method of high-purity cobratoxin and pharmaceutical composition containing high-purity cobratoxin
ActiveCN104327176AOvercoming Transgender DisadvantagesOvercoming the possibility of transgenderNervous disorderPeptide/protein ingredientsTherapeutic effectControllability
In consideration of physical and chemical properties that the cobratoxin is complex in component and cobratide is basic polypeptide, has molecular weight of about 7000 Dalton ad is relatively resistant to acid and alkali, and by considering the separating mechanism, the application characteristic, the long-term use stability, the cost controllability and the achievement of an extracting goal of each chromatographic column packing, the invention provides an extracting method of high-purity cobratoxin. The extracting method disclosed by the invention is stable in process, easily available in chromatography packing, low in comprehensive cost, good in operation condition adaptability and high in yield of neurotoxin. The invention further provides high-purity cobratoxin obtained by the method provided by the invention. An injection cobratide preparation prepared by the high-purity cobratoxin is less in impurities irrelevant with the treatment effect, quick to become effective in treatment effect and stable in curative effect. The invention further provides several pharmaceutical compositions taking the high-purity cobratoxin as an effective constituent formulated with preparation auxiliary materials needed for preparing a preparation as well as cobratide preparations with different dosage forms, which are suitable for being orally taken, absorbed by an oral cavity or rectal administration.
Owner:张庆宇
Medicinal composition containing insoluble medicament
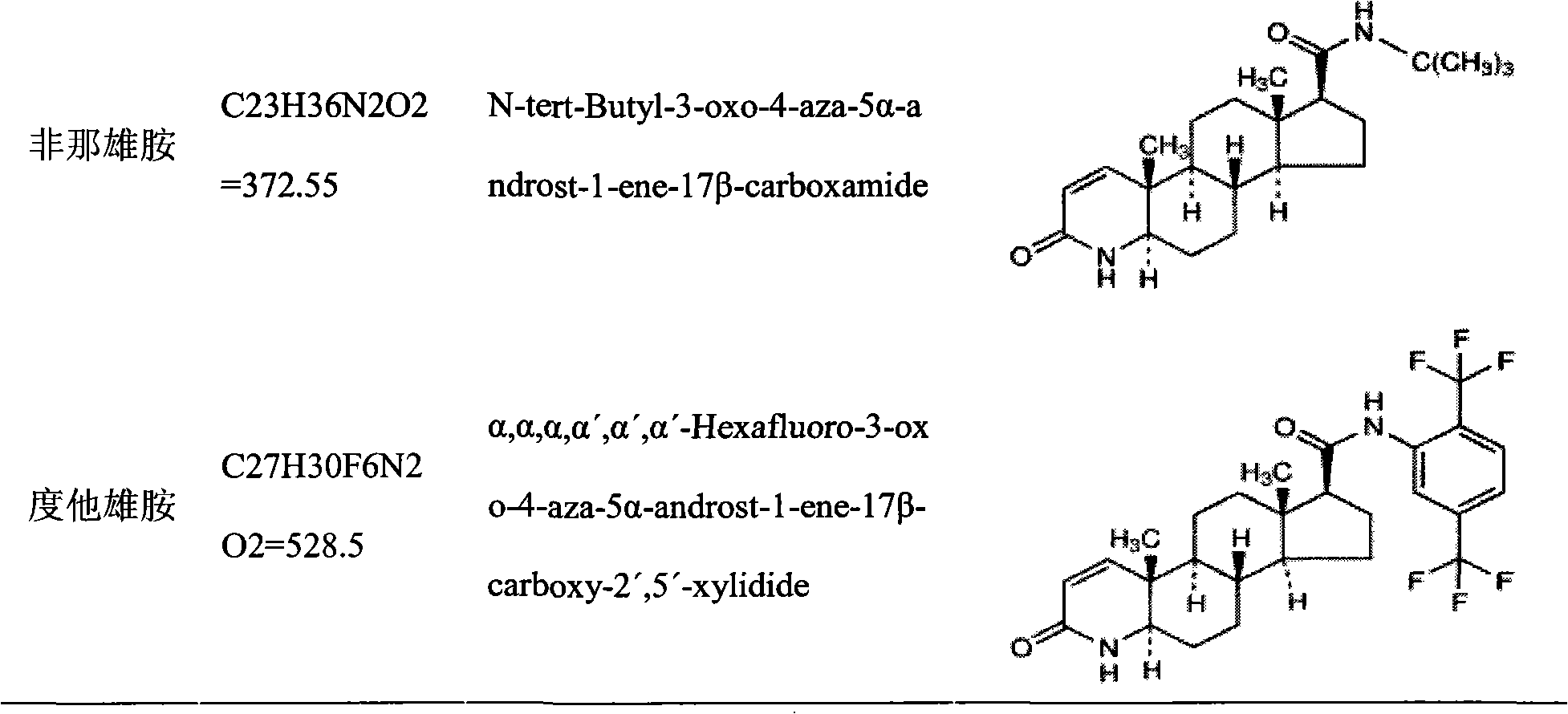
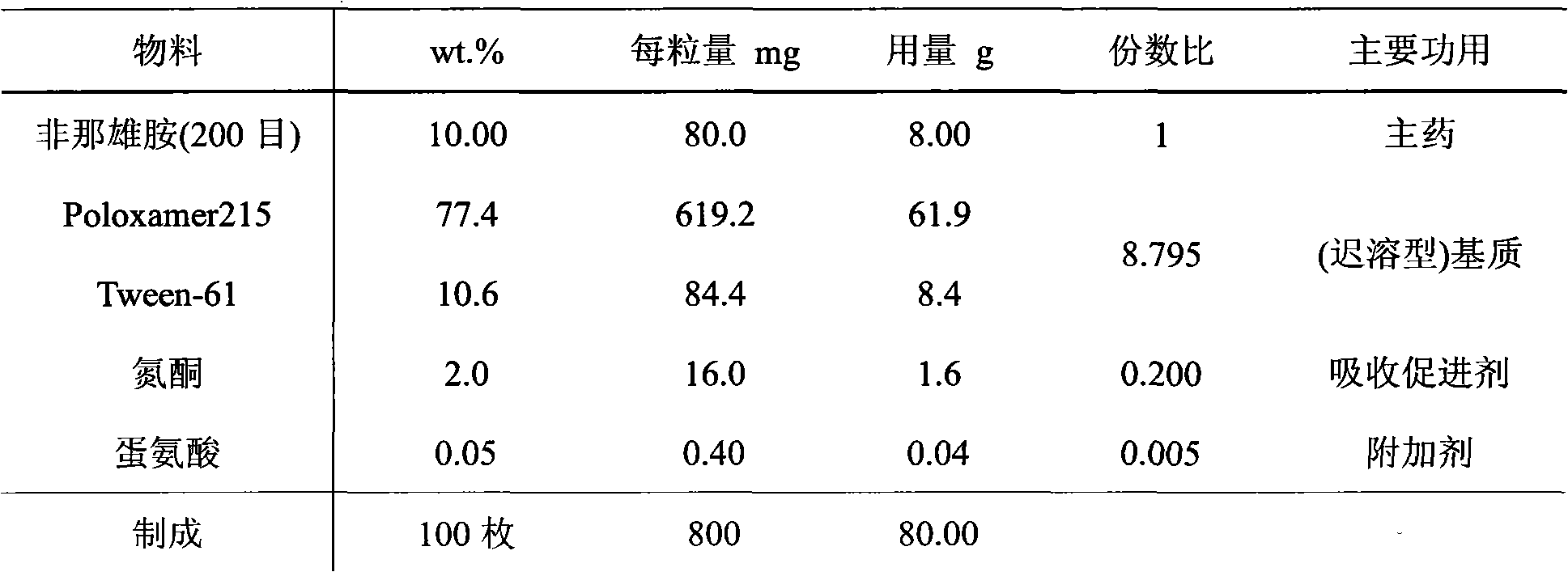
InactiveCN102145003AAvoid degradationAvoid Absorption IrregularitiesUrinary disorderAmide active ingredientsPoor complianceSide effect
The invention discloses a medicinal composition containing an insoluble alpha-receptor retardant and / or 5alpha-reductase inhibitor with an effective dose, which comprises the following components of: principal ingredients, a substrate, a solubilizer, a sorbefacient and an additive in a weight ratio of 1:(8-7,000):(0-460):(0-150):(0-150), wherein the medicinal composition at least contains one of the solubilizer and the sorbefacient. The effective dose of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor is in 0.05 to 80 milligrams of parent compounds of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor, the weight of a preparation per unit is between 0.8 and 4.2 grams. The medicinal composition can be subjected to oral administration or rectum administration, so the defects of poor curative effect and large toxic and side effect in the conventional oral administration and systemic administration of injection and large side effect and poor compliance of patients in local injection administration can be overcome, the lasting time of the medicinal effect can be increased, and better treatment means can be provided for medical care personnel and patients; and a product process is simple and is suitable for industrial batch production.
Owner:张立英
Cobratide extraction method, cobratide extracted thereby and formulation containing cobratide
ActiveCN101381408AHigh puritySimple methodNervous disorderPeptide/protein ingredientsFreeze-dryingUltrafiltration
The invention relates to a method for extracting cobratide, a medicine used to treat chronic pain, the cobratide extracted by the method and a preparation containing the cobratide. The method for extracting the cobratide comprises the following steps: firstly, a stock solution of snake poison of a cobra is pretreated with ammonium sulphate to remove partial other compositions in the snake poison,is subjected to desalination and concentration through ultrafiltration, dialysis or gel chromatography, and after the concentration, is purified by a cation column, and finally is subject to freeze-drying preservation after the concentration. The cobratide preparation comprises an enteric preparation, a rectal administration preparation, a colon-specific administration preparation and so on. Withcobratide extraction and purification processes provided by the method, the purity of the extracted cobratide is more than 95 percent, and the yield reaches 70 percent; and the method is simple and reliable, and saves cost and time.
Owner:BEIJING SAISHENG PHARMA
S-Nitrosothiols Containing Composition for the Treatment of Fatty Liver Diseases, Obesity and Other Diseases Associated with the Metabolic Syndrome and the Use of Such Compositions
InactiveUS20100062059A1Avoid developmentEasy to returnAntibacterial agentsBiocideFatty liverRectal administration
The present invention refers to pharmaceutical compositions containing S-nitrosothiols as active principle. The referred compositions are intended for the treatment of the fatty liver disease and other diseases associated with the metabolic syndrome. The composition is administered either orally or rectally.
Owner:PROTAGEN
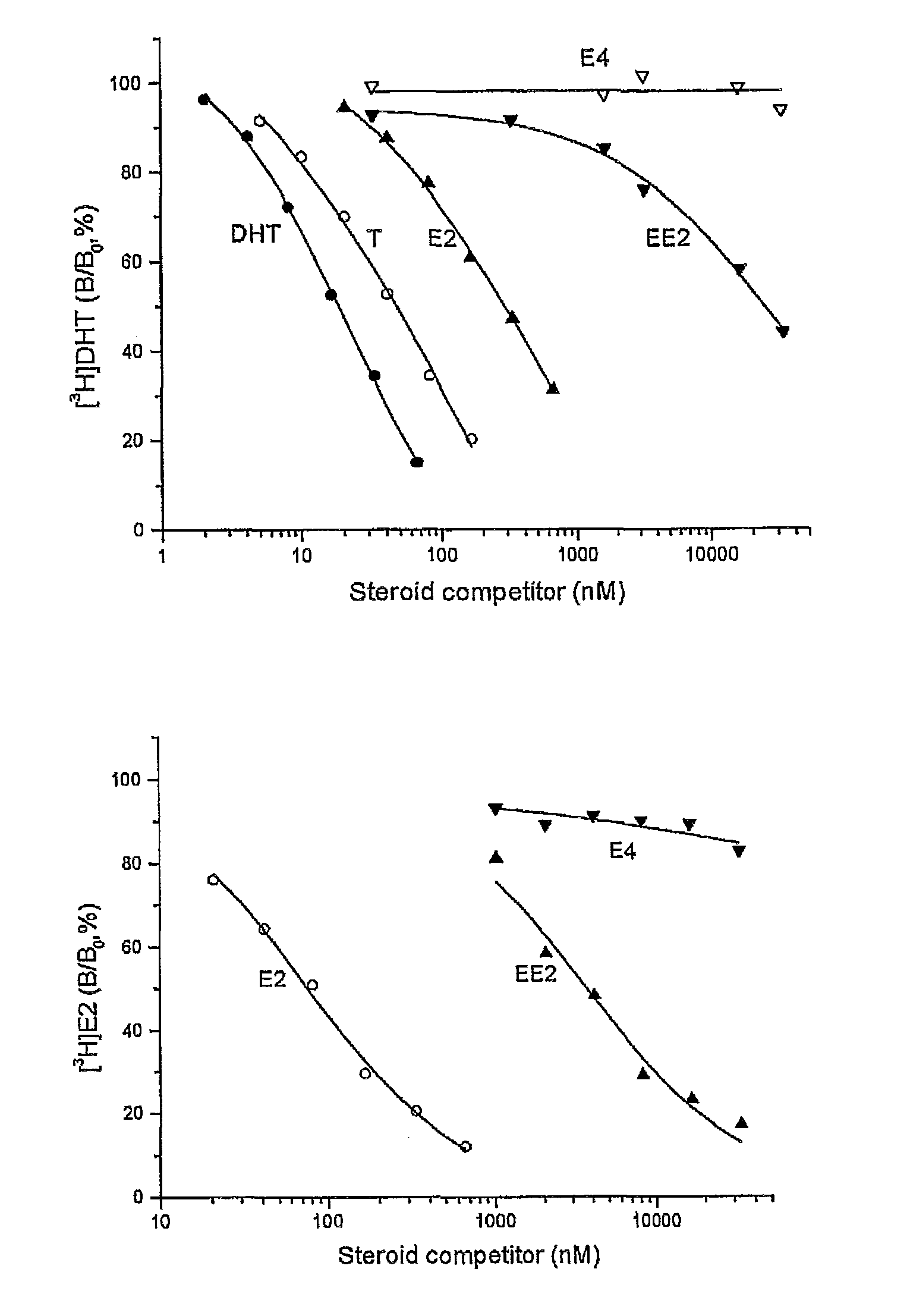
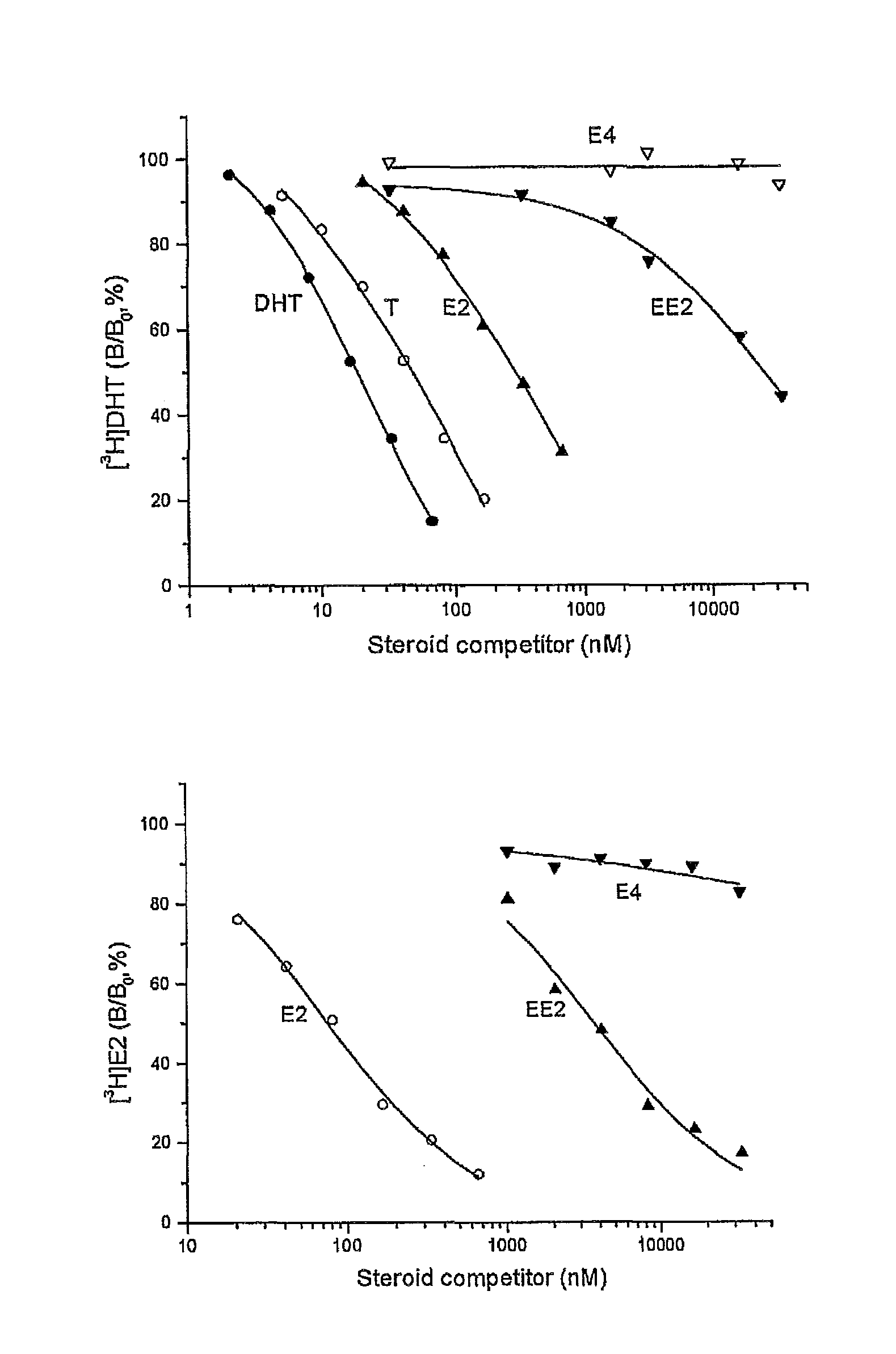
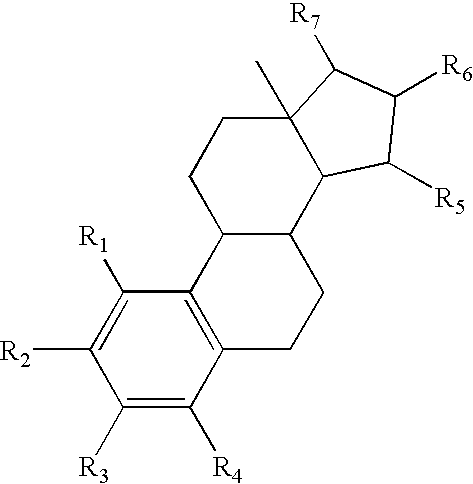
Drug delivery system comprising a tetrahydroxylated estrogen for use in hormonal contraception
ActiveUS7871995B2Improve reliabilityReliable efficacyOrganic active ingredientsBiocidePresent methodPhysiology
A method of contraception in mammalian females, which method comprises the parenteral or rectal administration of an estrogenic component and a progestogenic component to a female of childbearing capability in an amount effective to inhibit ovulation, wherein the estrogenic component is selected from the group consisting of substances represented by the following formula (1)in which R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a drug delivery system for parenteral or rectal administration that contains the aforementioned estrogenic component and a progestogenic component, said drug delivery system being selected from the group consisting of suppositories, systems for intravaginal delivery, inhalers, nasal sprays and transdermal delivery systems.
Owner:ESTETRA SRL
Compositions and methods for the treatment of multiple sclerosis
The present invention relates to compounds of Formula I and Formula II or its pharmaceutical acceptable salts, as well as polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprising an effective amount of compounds of Formula I and Formula II; and formulated to treat an underlying etiology by oral administration, delayed release or sustained release, transmucosal, syrup, topical, parenteral administration, injection, subdermal, oral solution, rectal administration, buccal administration or transdermal administration.
Owner:CELLIXBIO PTE LTD
Treatment of meconium aspiration syndrome with estrogens
ActiveUS8367647B2Organic active ingredientsPharmaceutical delivery mechanismSuppositoryNewborn infant
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
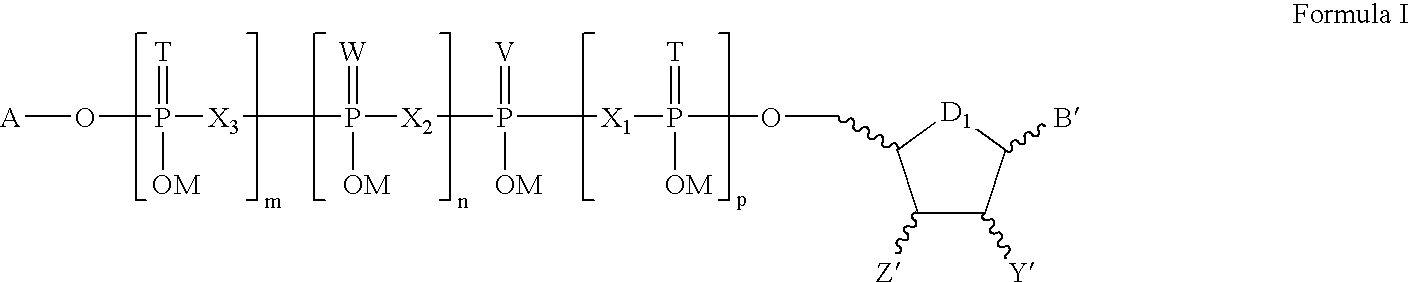
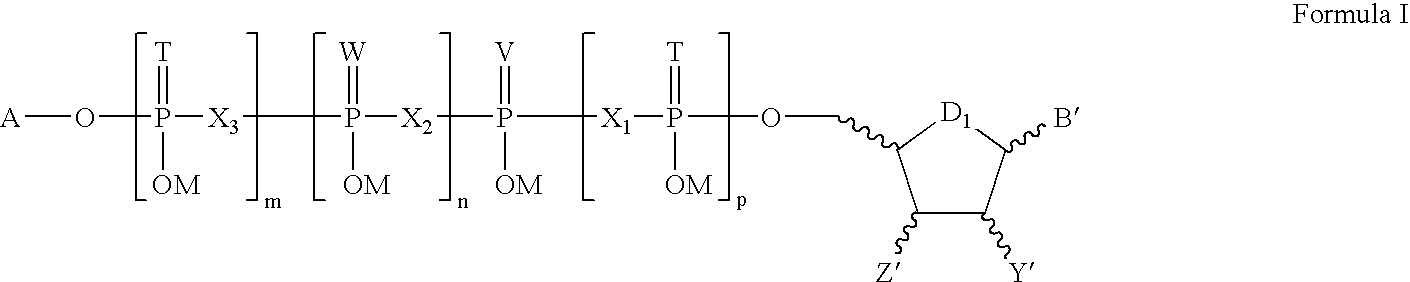
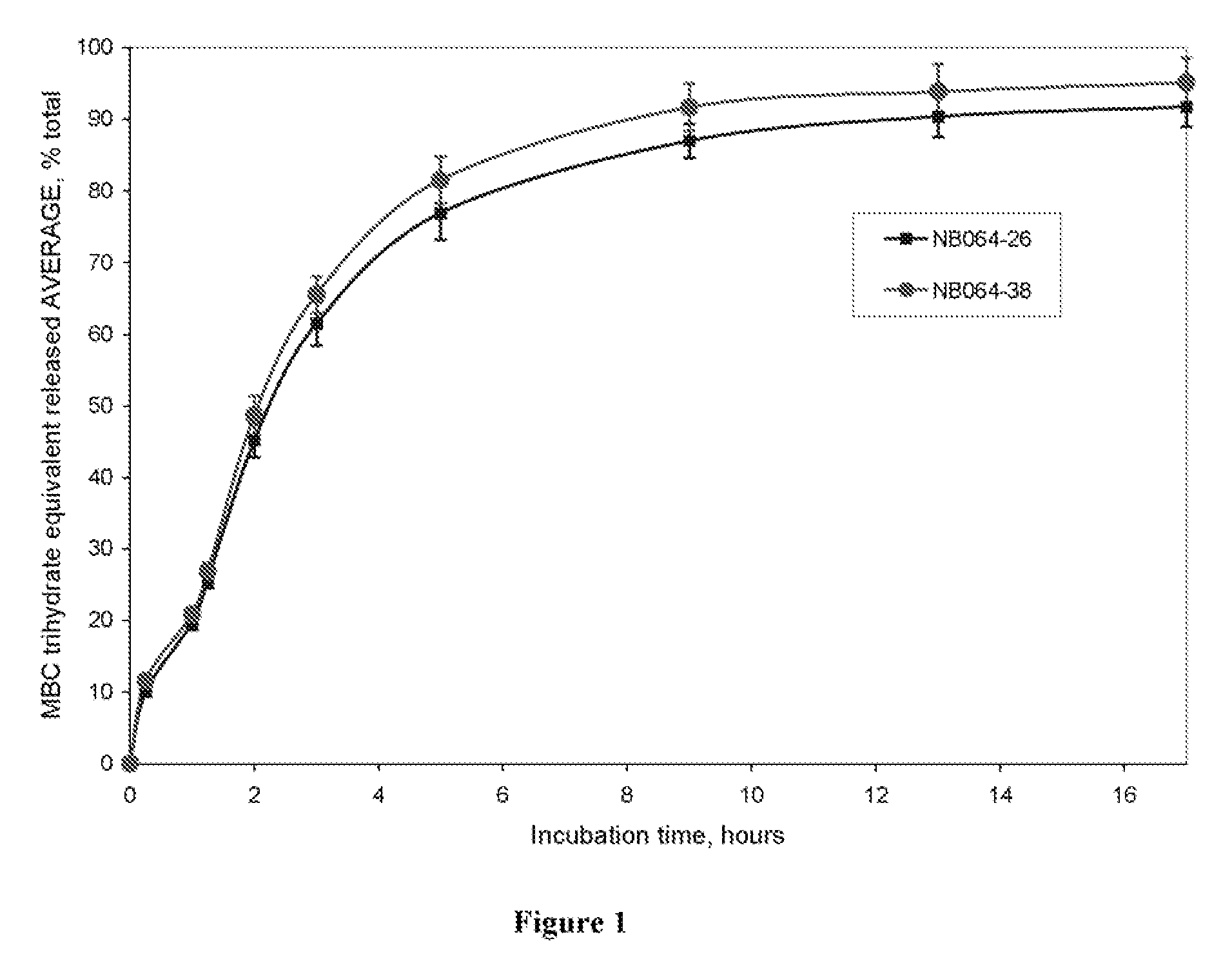
Pharmaceutical compositions for rectal administration
InactiveUS20140256661A1Avoiding unwanted side effectSymptoms improvedBiocideTetracycline active ingredientsRectal useBuccal administration
The present invention relates to a pharmaceutical composition, in particular a composition formulated for enema administration, wherein the composition comprises metronidazole or a pharmacologically acceptable derivative thereof in an amount to effectively treat both acute and chronic pouchitis and / or proctitis.
Owner:ARMSTRONG DAVID NIGEL
Chinese medicine preparation for treating chronic prostatitis and preparation method thereof
InactiveCN102058715AIncrease concentrationAvoid adverse reactionsUrinary disorderPlant ingredientsMyrrhRectal administration
The invention relates to a Chinese medicine preparation for treating chronic prostatitis, which is characterized by comprising the following raw medicines: radix rehmanniae preparata, cynomorium songaricum rupr, radix astragali, rhizoma corydalis, herba epimedii, dodder, herba ecliptae, schisandra chinensis, hedyotis diffusa, safflower, radix polygoni multiflori preparata, fructus ligustri lucidi, salvia miltiorrhiza, radix paeoniae alba, myrrh, prunella vulgaris, fructus cnidii, herba patriniae, scutellaria baicalensis georgi, angelica and cortex moutan. In the invention, Chinese herbal medicines for promoting blood circulation and removing blood stasis are adopted, the permeability of capillaries can be improved, inflammation reaction can be alleviated, the extinction and the absorption of inflammation focus can be promoted, the metabolism of connective tissues can be improved, the transformation and the absorption of hyperplastic lesions can be promoted, and atrophic connective tissues can recover. When the Chinese medicine preparation for treating chronic prostatitis is applied through rectums, the medicines can be absorbed in the rectums, enema or suppository enables the medicines to be absorbed through rectum mucosa to directly reach affected parts; and the Chinese medicine preparation can reach higher consistency in diseased prostate and has the advantages of ideal treating effect, low price and simple, convenient and practical preparation method.
Owner:郭瑶
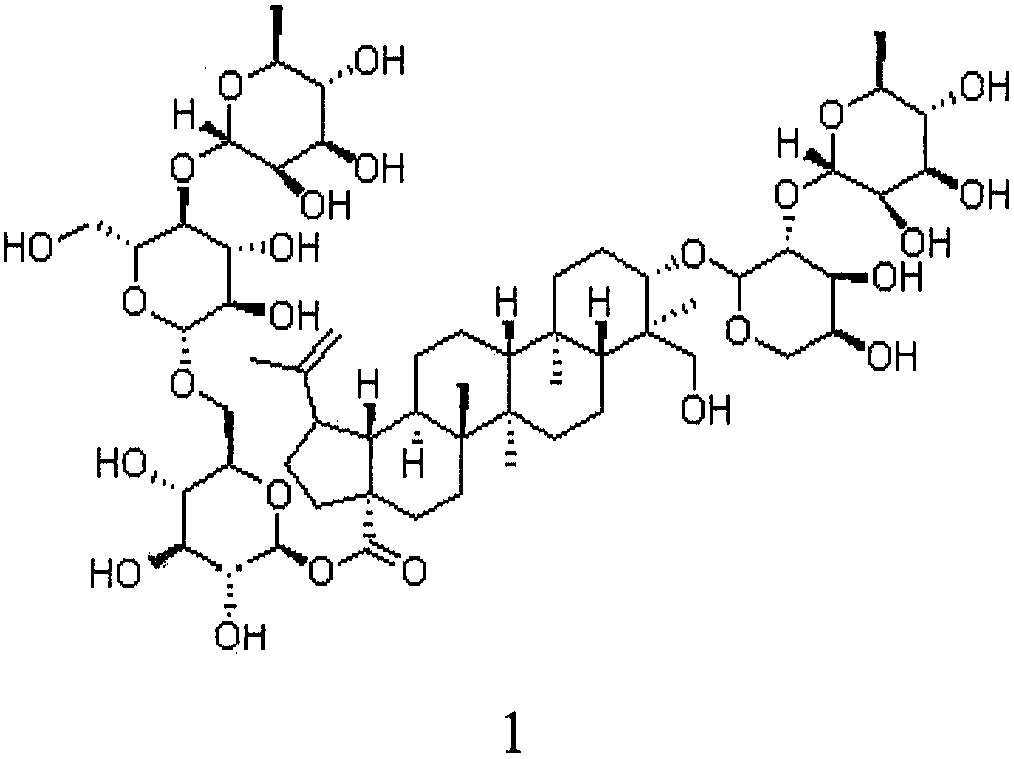
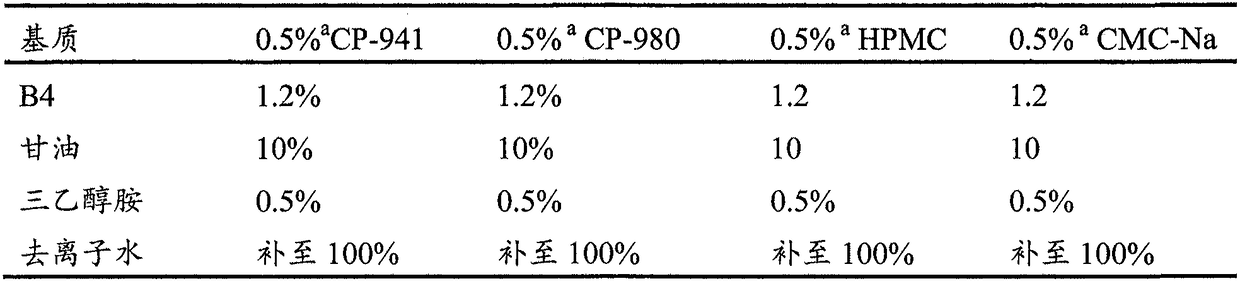
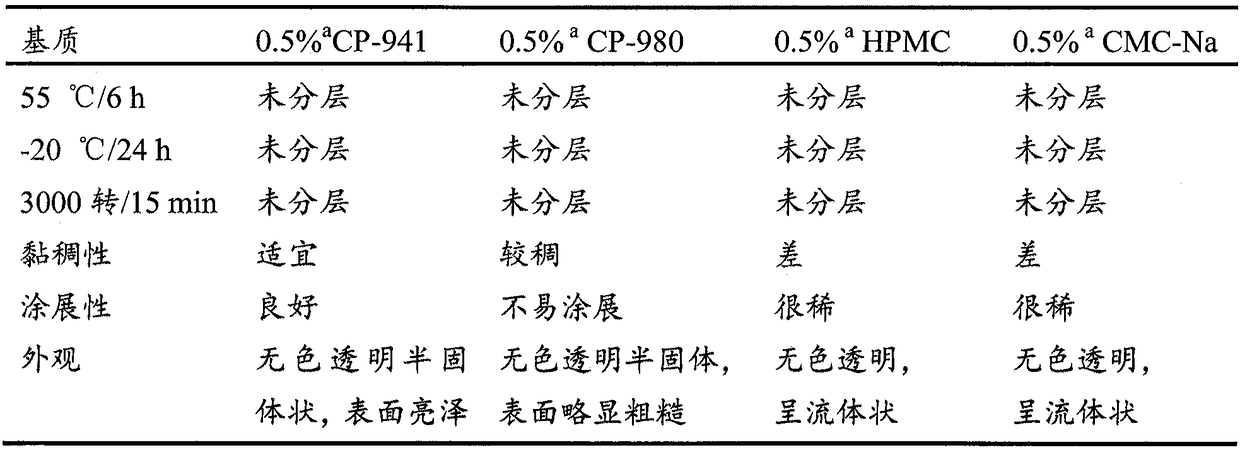
Preparation of anemoside B4 for rectal mucosal administration and preparation method thereof
The invention relates to a preparation of anemoside B4 for rectal mucosal administration, comprising anemoside B4 and a pharmaceutically acceptable matrix; the preparation herein is selected from oneor two of rectal gel and rectal suppository. The invention also specifically provides rectal gel of anemoside B4, rectal suppository of anemoside B4, and their preparation methods. The preparation ofanemoside B4 for rectal mucosal administration provided herein requires less dosage than oral administration and has better effect under the same the dosage.
Owner:刘琦
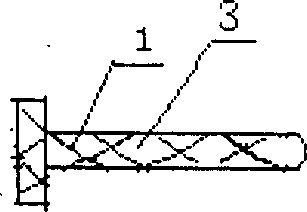
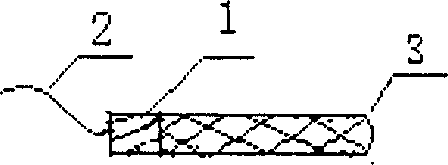
Carrying suppository for cavity/canal drug administration
the invention disclose a carrying suppository for cavity / canal drug administration, and includes suppository with cartilage used for vagina, rectum, nasal cavity drug administration, and novel suppository fit for rectum drug administration and special mold caddy for molding, packing, vivo imbedding. Wherein the suppository with cartilage is comprised by suppository core 1, pull wire 2, drug-containing carrier stroma and adhesive agent and / or developing agent; The rectum drug administration suppository is comprised by drug-containing carrier stroma, isolated layer, and adhesion coat; The special mold caddy is comprised by hollow suppository push rod and mainbody mold, wherein the mainbody mold includes case cap 5, upper cavity mold 6 in the case, lower mold 7 connected together with the case, wherein the upper mold can be integrated or two-body, i.e. a central mold 8 is added. By using the provided suppository, focus can fully contact with the drug, and the drug will not flow, and has longer active time and good positioning property, so that the suppository can realize drug administration against focus local.
Owner:丛繁滋
Qingkailing suppositorium
ActiveCN101327262AQuick effectLong duration of actionOrganic active ingredientsNervous disorderHydrolysateThird generation
The invention discloses a Qingkailing suppository, relates to a Chinese medicine compound preparation, the raw materials of which mainly come from plants and animals, in particular to a novel formulation of Qingkailing preparation. The Qingkailing suppository provided by the invention for supplying rectum with medicine adopts the following components of cholic acid, hyodeoxycholic acid, baicalin, buffalo horn, the hydrolysate of mother-of-pearl, gardenia, banlangen, the water purified extract of honeysuckle as Qingkailing combination. The suppository which is 1-3g weight is made from the raw materials and supplementary materials with the following weight proportions of 8-13 proportions of Qingkailing combination, 5-50 proportions of suppository base, 0.1-5 proportions of surfactant, 0.05-3 proportions of sorbefacient, and 0-3 proportions of additional agent. The Qingkailing suppository of the invention not only can compensate the defects that the oral taking preparation is slow to take effect and injection preparation has great toxicity, but also can prolong the duration of the medicine, provides a wider selection range for clinics and sufferers, has simple production technology, is suitable for industrialized batch production, and is suitable to be taken by infants.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
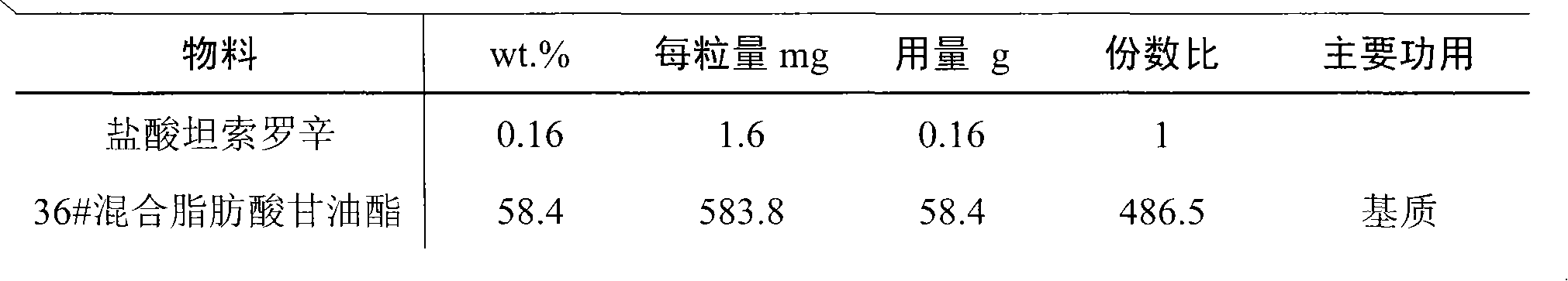
Rectal administration composition containing tamsulosin
InactiveCN101889996AImprove solubilityGood curative effectSuppositories deliveryOrganic non-active ingredientsSide effectWhole body
The invention relates to a rectal administration composition containing tamsulosin, which comprises effective quantities of tamsulosin or pharmaceutically acceptable salts or derivatives thereof, solubilizer and sorbefacient, wherein the effective quantity of tamsulosin or pharmaceutically acceptable salts or derivatives thereof is metered according to the equivalence tamsulosin hydrochloride; the content of the tamsulosin or pharmaceutically acceptable salts or derivatives thereof is equivalent to 0.025-1.6 mg of tamsulosin hydrochloride; and the preparation per unit weighs 0.8-4 g. The rectal administration composition containing tamsulosin can overcome the defects of poor curative effect and great toxic or side effect in oral administration and systemic injection administration, and the defects of great side effect and poor patient dependence in partial injection administration, and can prolong the duration of drug actions, thereby providing a better treatment means for medical care personnel and patients. The production technology is simple and suitable for industrial mass production.
Owner:张立英 +1
Therapeutic antisense oligonucleotide composition for the treatment of inflammatory bowel disease
InactiveUS20090275631A1Improve responseMinimal systemic exposureOrganic active ingredientsPeptide/protein ingredientsTolerabilityUlcerative colitis
Disclosed herein is a method for the sustained amelioration and / or treatment of ulcerative colitis comprising rectal administration of a compound comprising an antisense oligonucleotide having the sequence 5′-GCCCAAGCTGGCATCCGTCA-3′, ISIS 2302. The method results in a decrease in the indications of ulcerative colitis for an extended period (greater than 90 days) after the conclusion of the administration of the composition. The composition is well tolerated and systemic exposure is minimal.
Owner:ATLANTIC TECH VENTURES
Chinese medicine enema for treating acute pancreatitis
InactiveCN1857429APromote peristalsisStrong choleretic effectDigestive systemSulfur/selenium/tellurium inorganic active ingredientsVeinMedicine
The Chinese medicine enema for treating acute pancreatitis consists of rhubarb decoction and Glauber salt in certain weight ratio. It is rectum administrated to reach blood medicine concentration the same as that of vein administration but no influence of digestive enzyme and gastric acid. The medicine may be used to combine with Western medicine in treating acute pancreatitis to reach high curative rate.
Owner:何元仲
Application of dragon blood and dragon-blood extract
ActiveCN101559149APrevent or mitigate damageGood radiation protectionDermatological disorderPlant ingredientsRectal useOral solutions
The invention relates to application of dragon blood and dragon blood extract, which belongs to the field of Chinese medicaments. In particular, the invention relates to the application of dragon blood raw medicinal materials, dragon blood total phenols and dragon blood total flavonoids in preparing medicaments for preventing or treating radiation damage. In order to achieve the application of the invention, the application of the dragon blood or the dragon blood extract via or not via gastrointestinal tract includes but is not limited to oral, subcutaneous, intramuscular, intravenous, transdermal, nasal and rectal administration. Pharmaceutical compositions prepared from the dragon blood or the dragon blood extract and medically acceptable pharmaceutical excipient include but are not limited to various types of tablets, capsules, dropping pills, soft capsules, oral solution, pills, powder, granules, lozenges, decoction paste, syrup, liquid extract agent, extract agents, injections, as well as other clinically appropriate dosage forms. A preparation contains necessary additives, and the additives include but are not limited to fillers, wetting agents, adhesives, disintegrants, lubricants or pH regulators.
Owner:北京理工亘舒科技有限公司
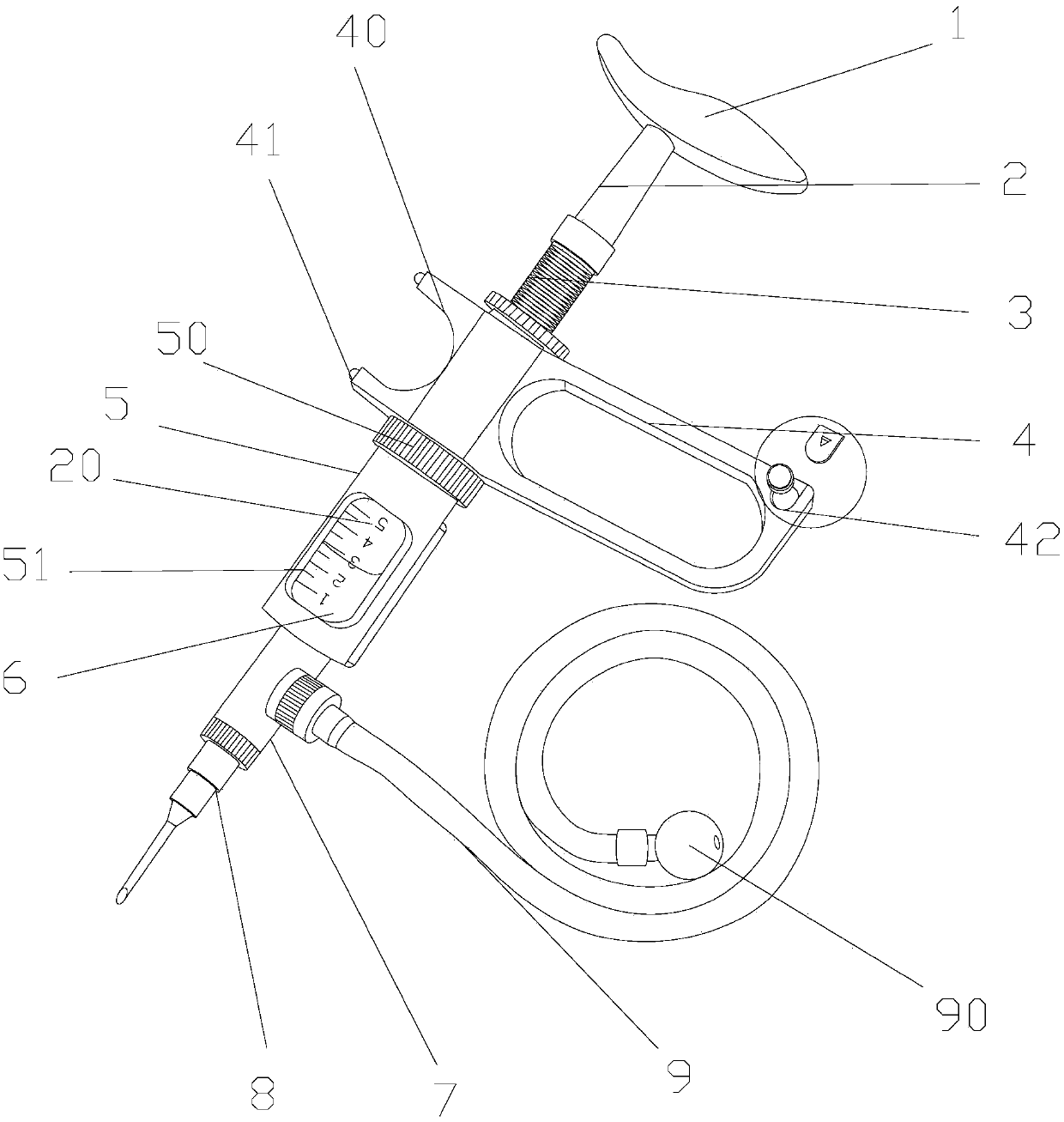
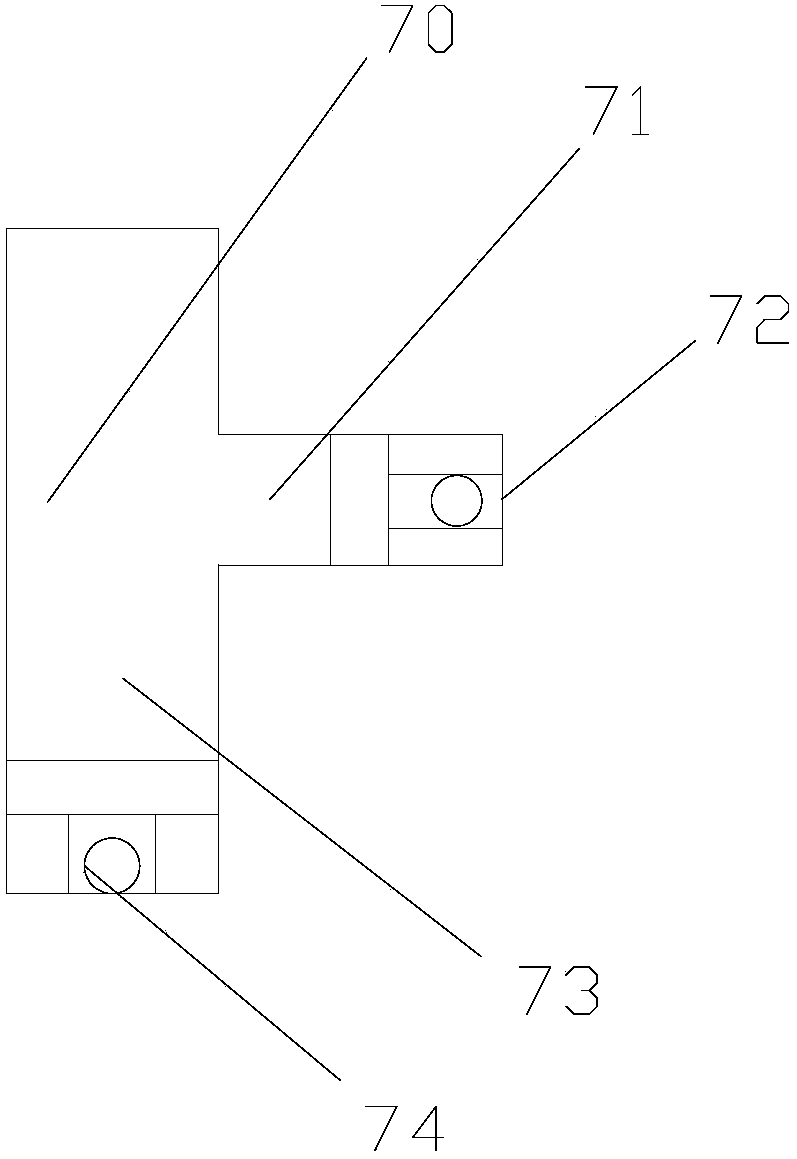
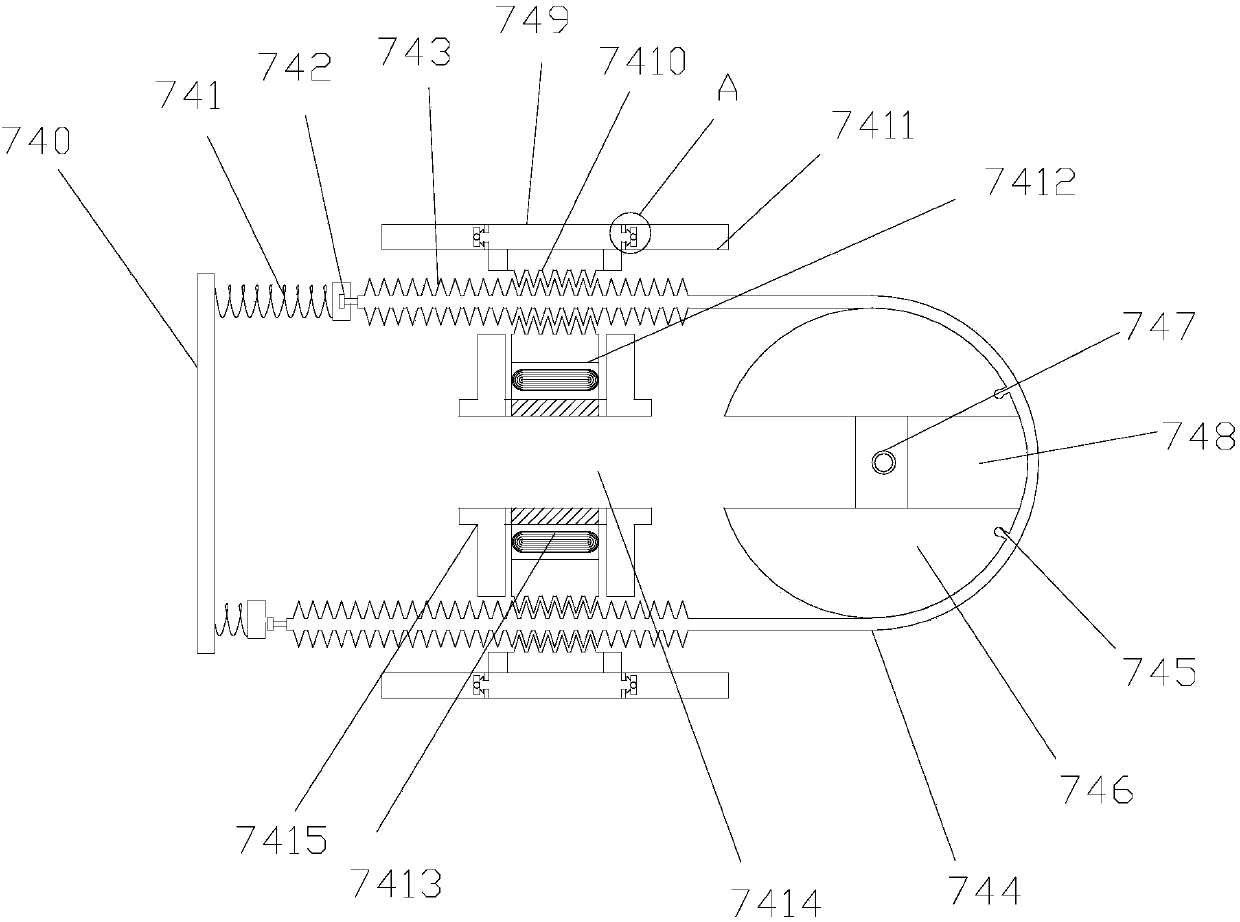
Rectal drug delivery device with electric regulation function in gastroenterology department
InactiveCN107802948AEasy Control of InjectionEasy to switchMedical devicesElectric controlRectal use
The invention discloses a rectal drug delivery device for gastroenterology with an electric adjustment function, which comprises a pressure pad, a pressure rod, a pressure rod positioning sleeve, a handle, a drug cartridge, an inner cavity, a liquid separator, an injection needle, and an enema tube , a rectal drug delivery device for gastroenterology with electric adjustment function of the present invention, when in use, the worm gear of the ring motor drives the rack to move back and forth, so that the pull bar connected to the rack pulls the ball valve to rotate along the ball valve shaft pin, and the two ends The return spring is tensioned to keep the through hole of the ball valve in an upward damping state. When the motor is started, the through hole of the ball valve becomes horizontal and the channel is connected; through the cooperation of the electric control valve and the liquid dispenser, the The drug delivery device can be easily controlled to inject with a needle or an enema tube, and the switch is convenient and quick, reducing the time for changing tools and working heads.
Owner:陈双
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com