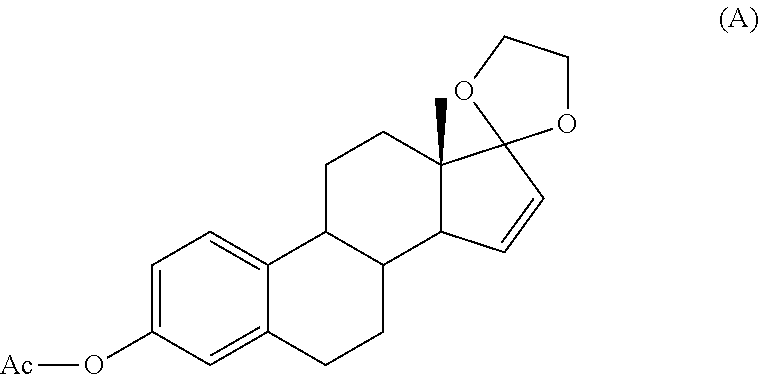
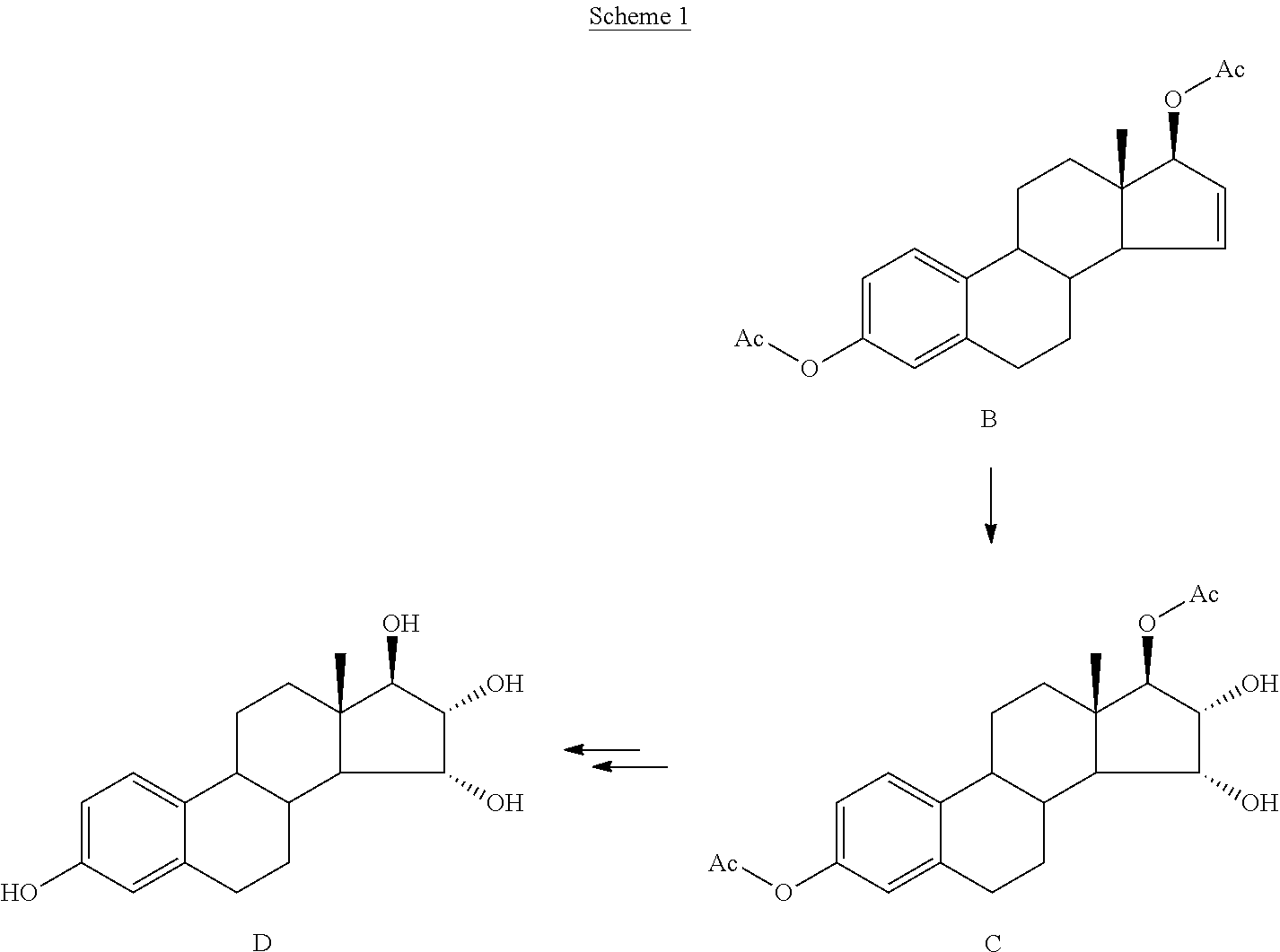
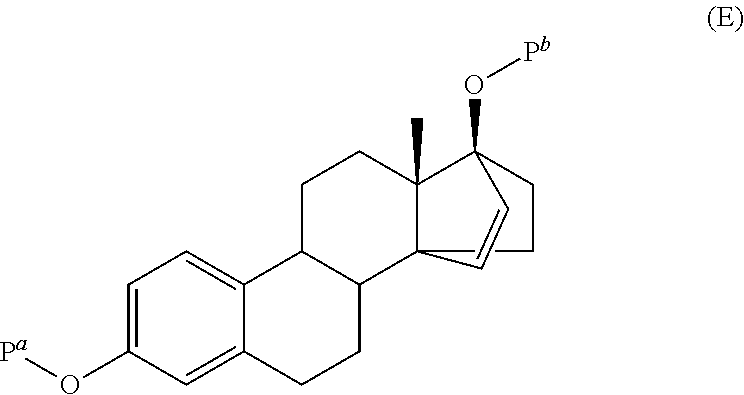
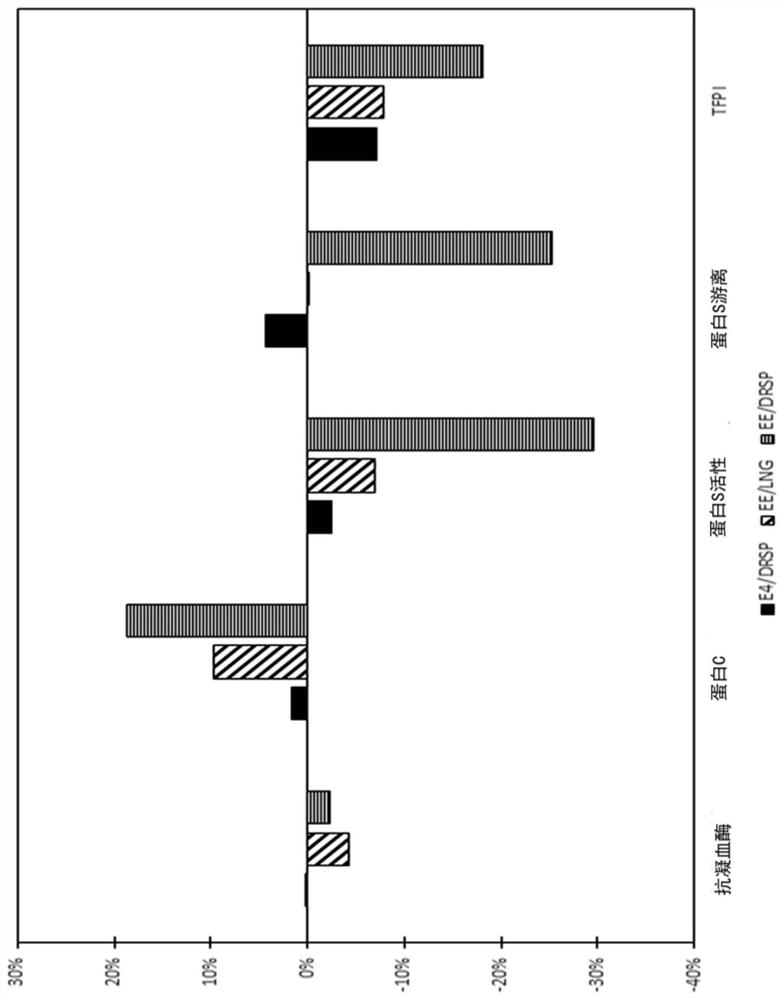
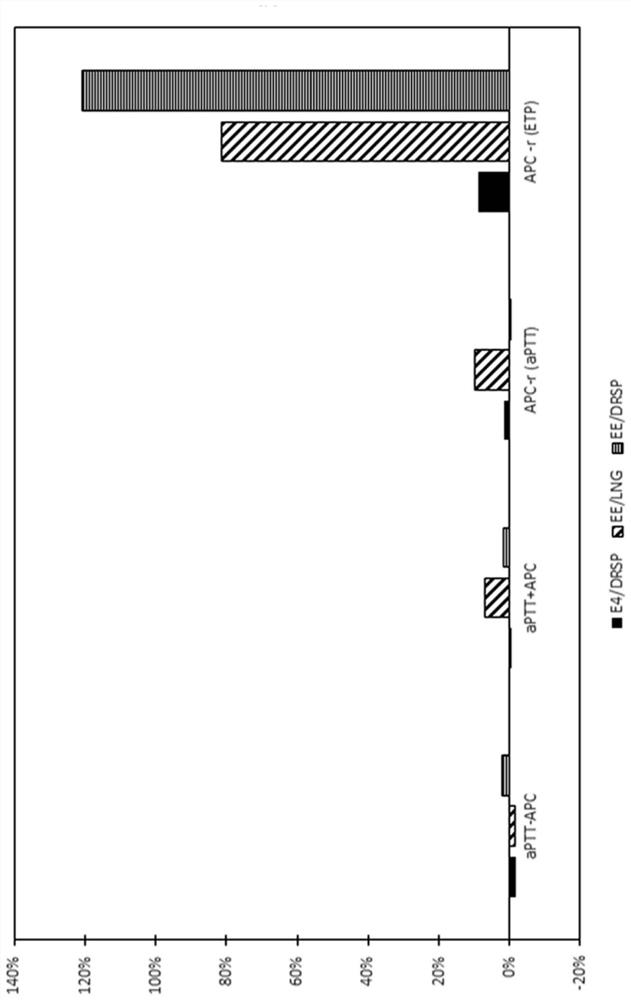
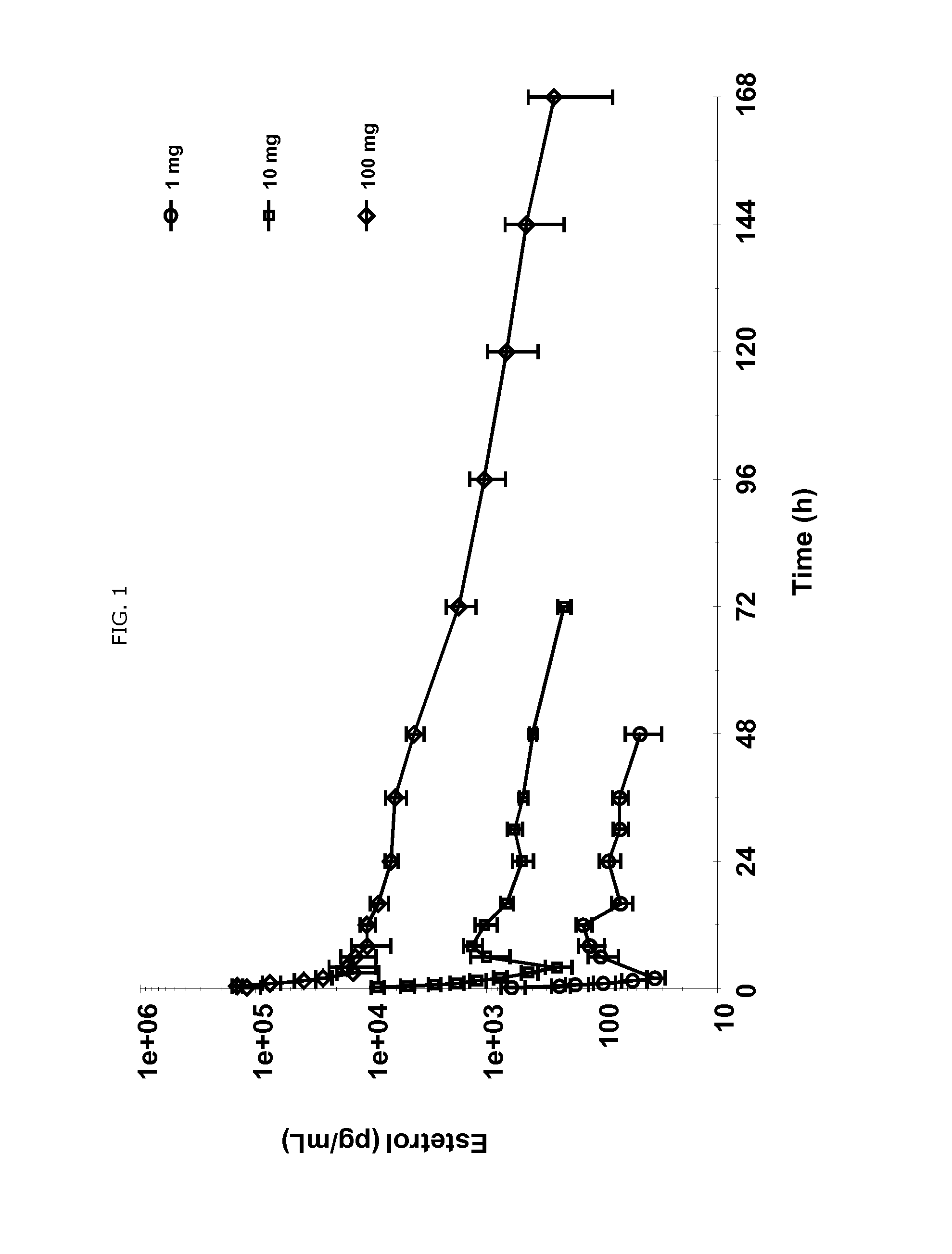
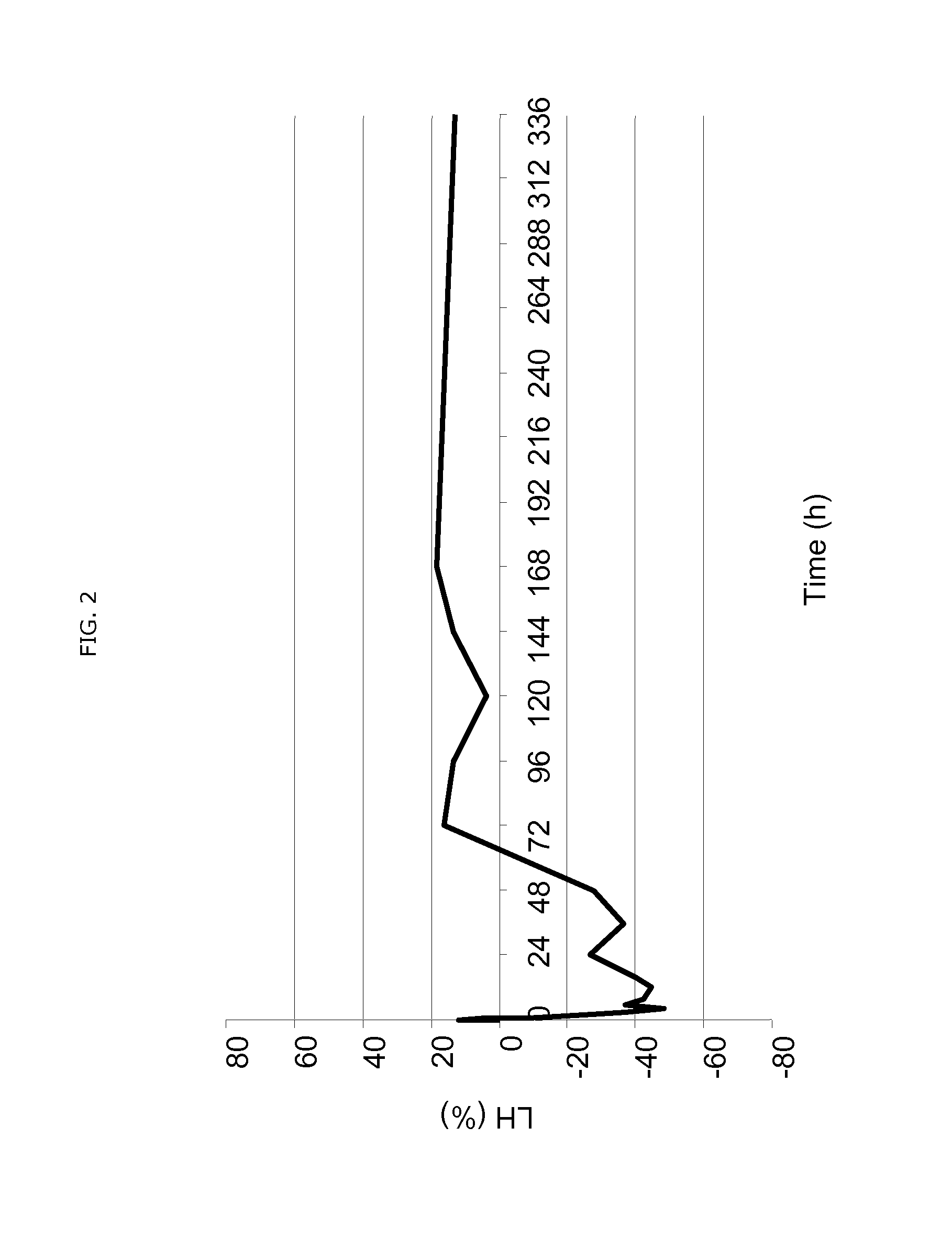
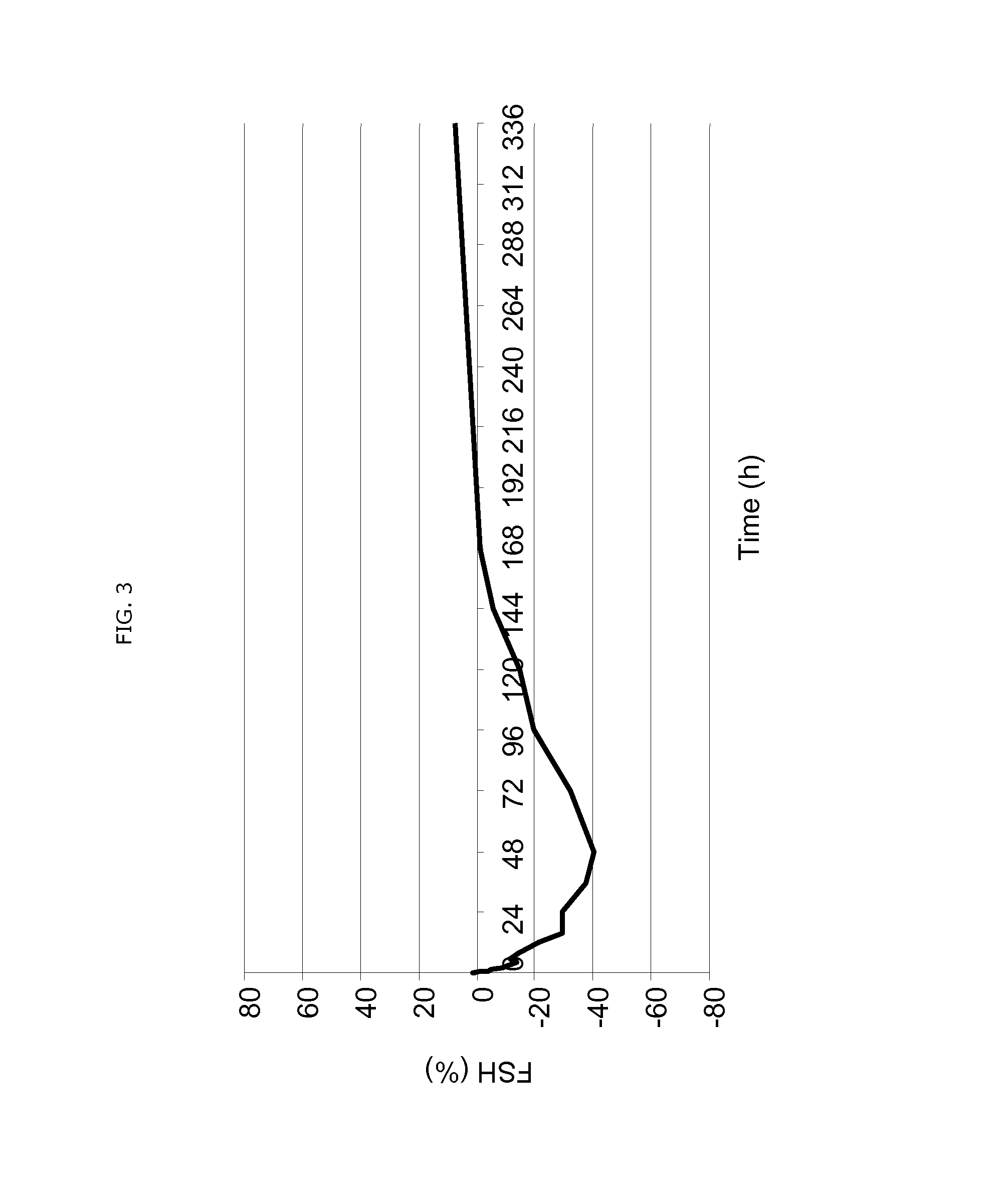
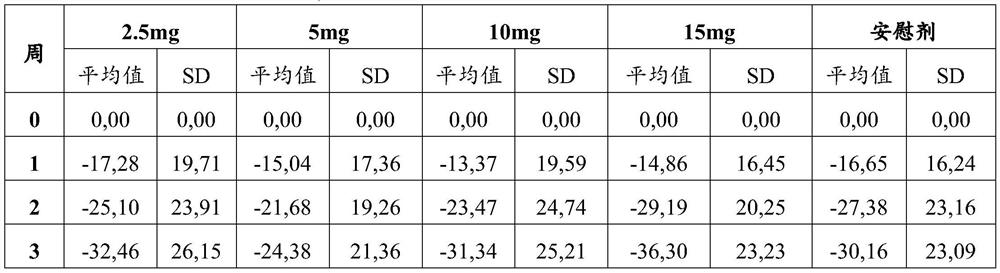
Patents
Literature
36 results about "Estetrol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
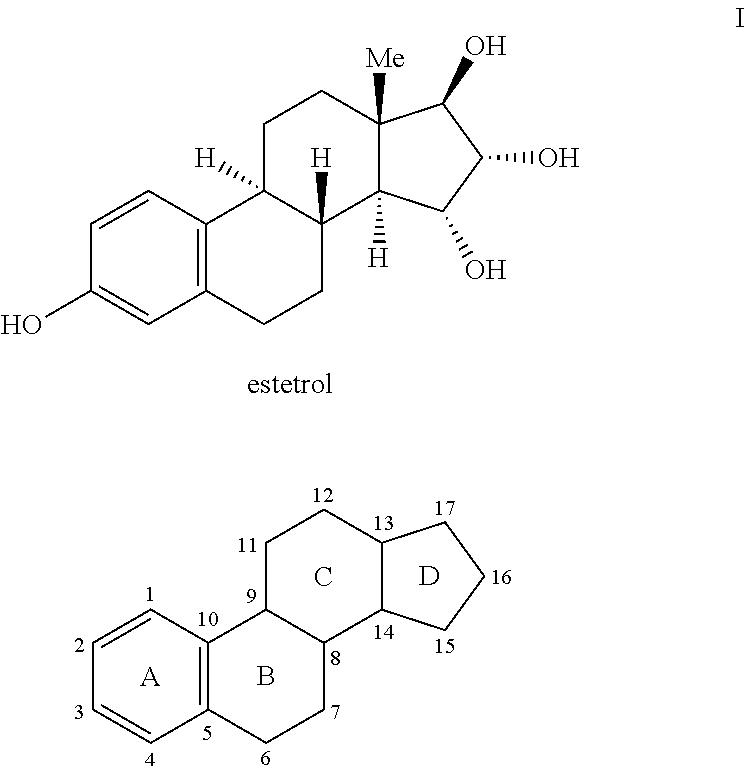
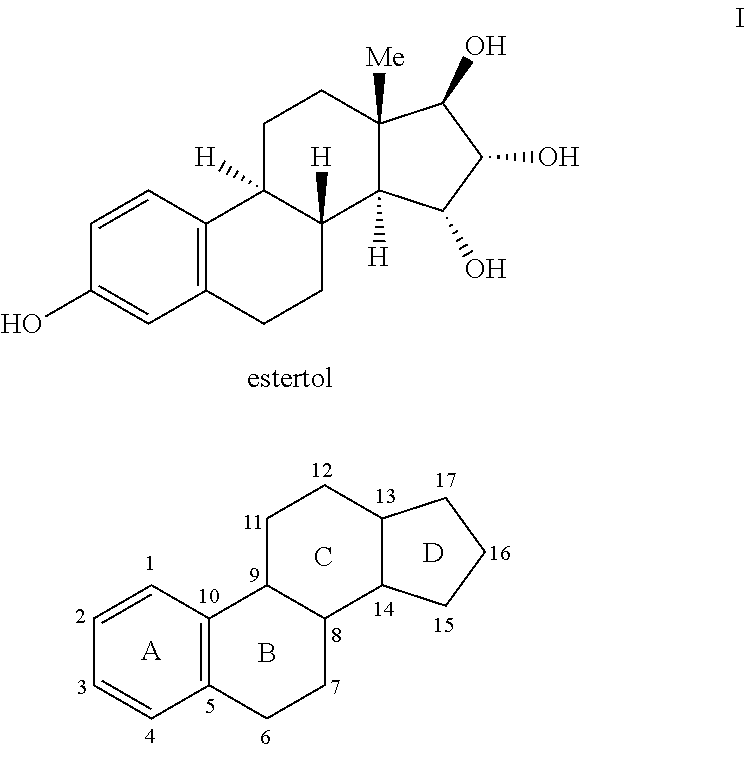
Estetrol (E4), or oestetrol, is a weak estrogen steroid hormone which is found in detectable levels only during pregnancy. It is produced exclusively by the fetal liver. Estetrol is closely related to estriol, which is also a weak estrogen that is found in high quantities only during pregnancy. Along with estradiol (E2), estrone (E1), and estriol (E3), estetrol is a major estrogen in the body, although only during pregnancy.
Pharmaceutical compositions comprising estetrol derivatives for use in cancer therapy
ActiveUS20060247221A1High affinityIncreased riskOrganic active ingredientsAntineoplastic agentsGynecologyPresent method
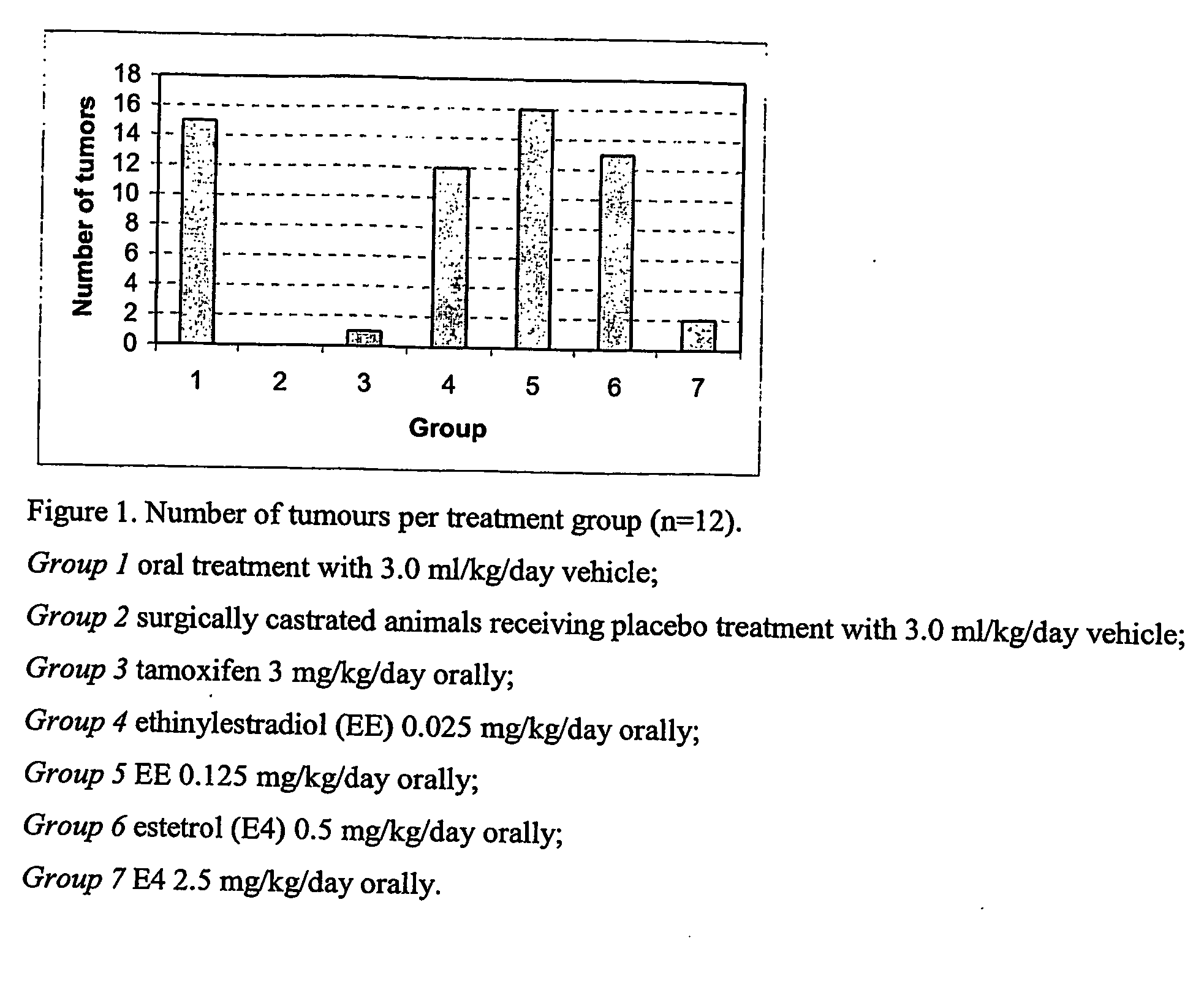
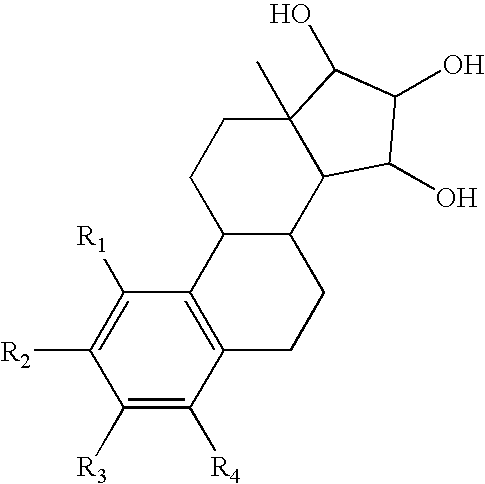
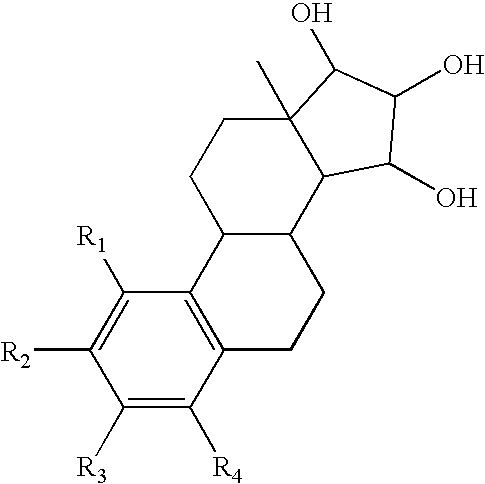
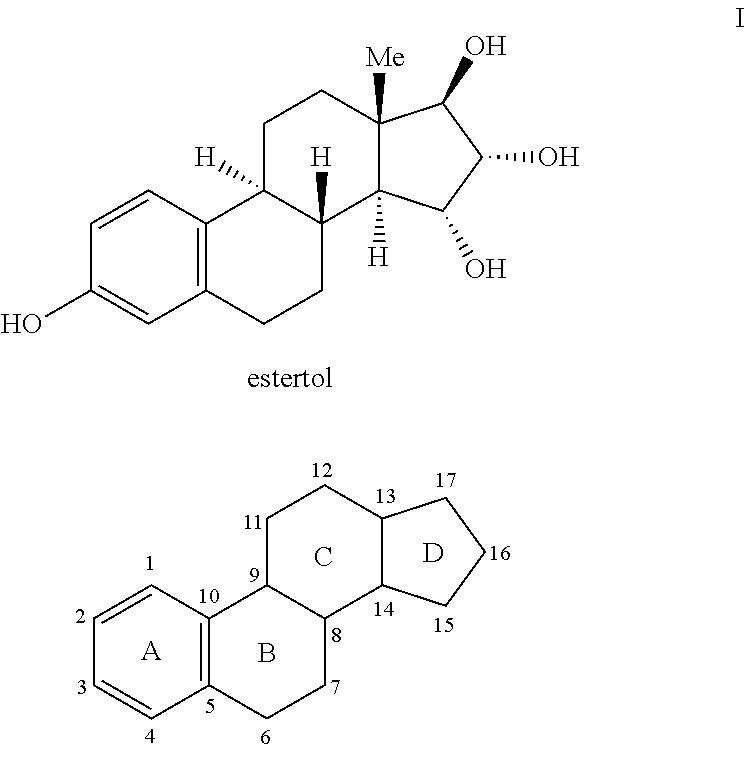
The present invention relates to a method of treating or preventing estrogen-suppressed tumours in a mammal, said method comprising the administration of a therapeutically effective amount of an estrogenic component to said mammal, wherein the estrogenic component is selected from the group consisting of: substances represented by the following formula (I) in which formula R1, R2, R3, R4, independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. The estrogenic component according to the invention is particularly useful in the treatment or prevention of colorectal and prostate cancer and, unlike commonly used estrogens, does not simultaneously enhance the risk of estrogen-stimulated cancers such as breast cancer.
Owner:ESTETRA SRL
Treatment of Meconium Aspiration Syndrome with Estrogens
ActiveUS20100184736A1Reduce incidenceReduce morbidityOrganic active ingredientsPharmaceutical delivery mechanismSuppositoryNon invasive
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
Treatment of meconium aspiration syndrome with estrogens
ActiveUS8367647B2Organic active ingredientsPharmaceutical delivery mechanismSuppositoryNewborn infant
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
Use of estetrol as emergency contraceptive
The present invention relates to a new use of tetrahydroxylated estrogens such as estetrol (1,3,5(10)-estratrien-3, 15α, 16α, 17β-tetrol), namely in a method of emergency contraception. The method of emergency contraception according to the invention comprises the oral administration of estetrol in a single dose within 120 hours of sexual intercourse.
Owner:ESTETRA SRL
Process for the preparation of estetrol
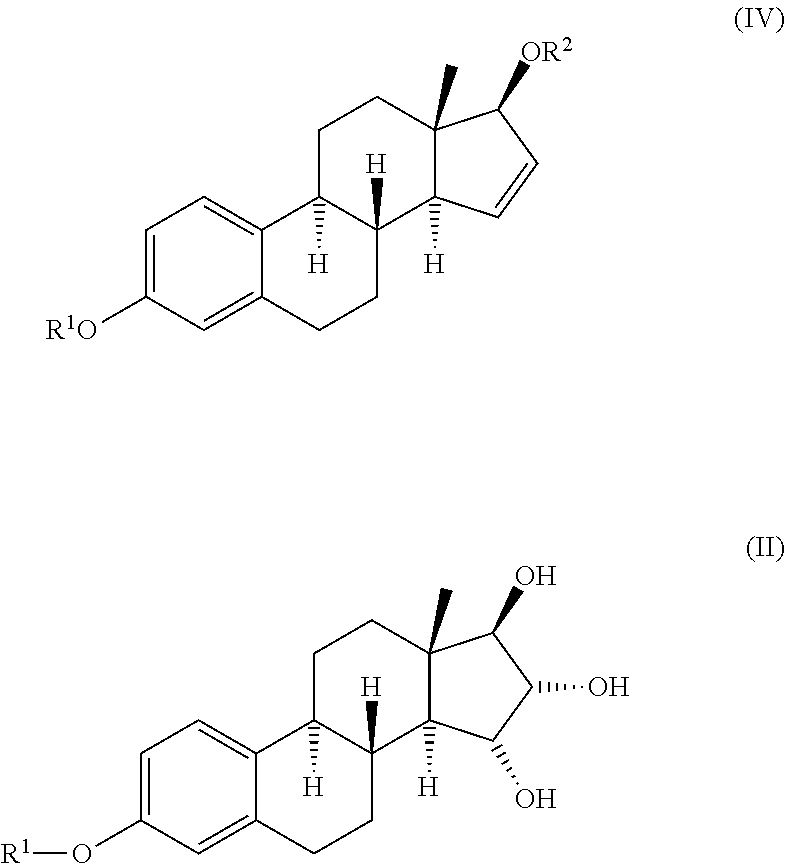
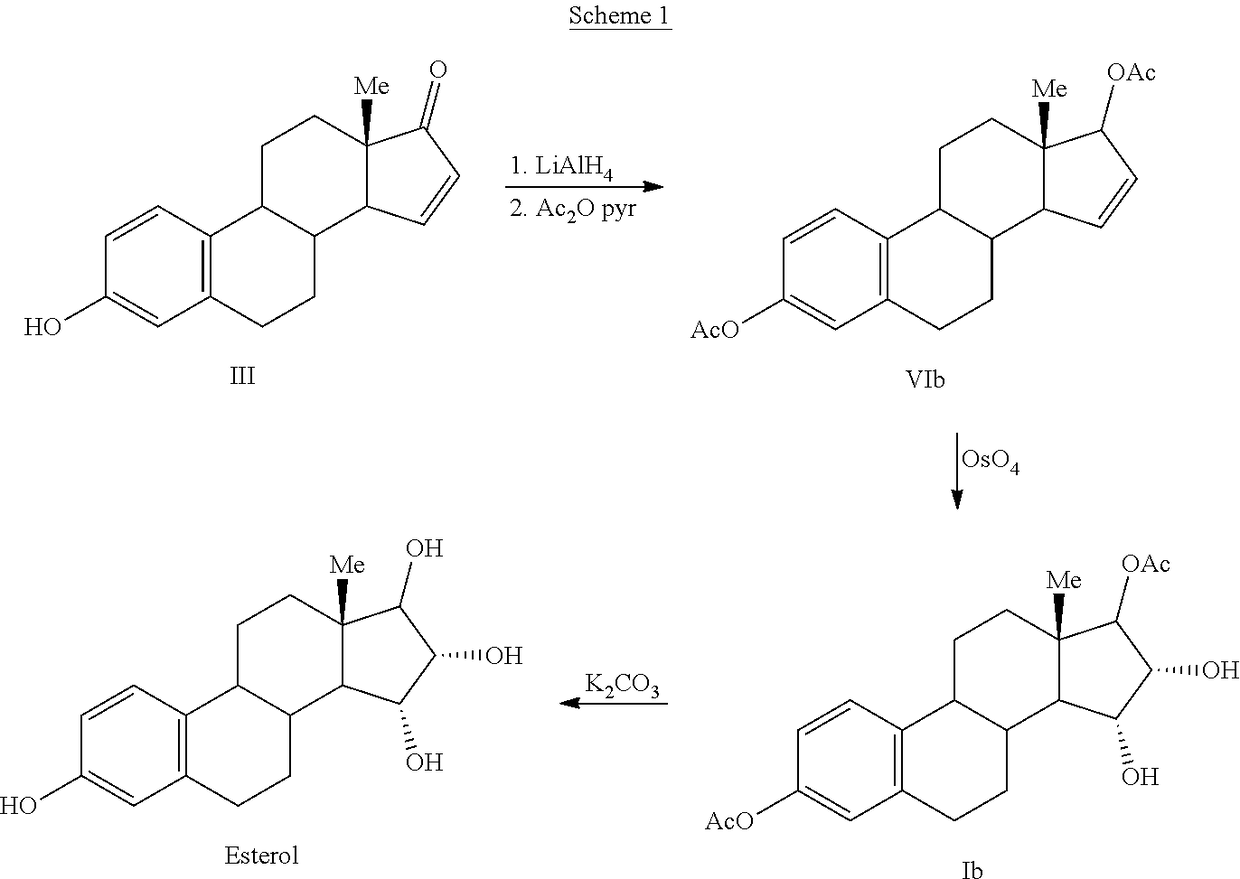
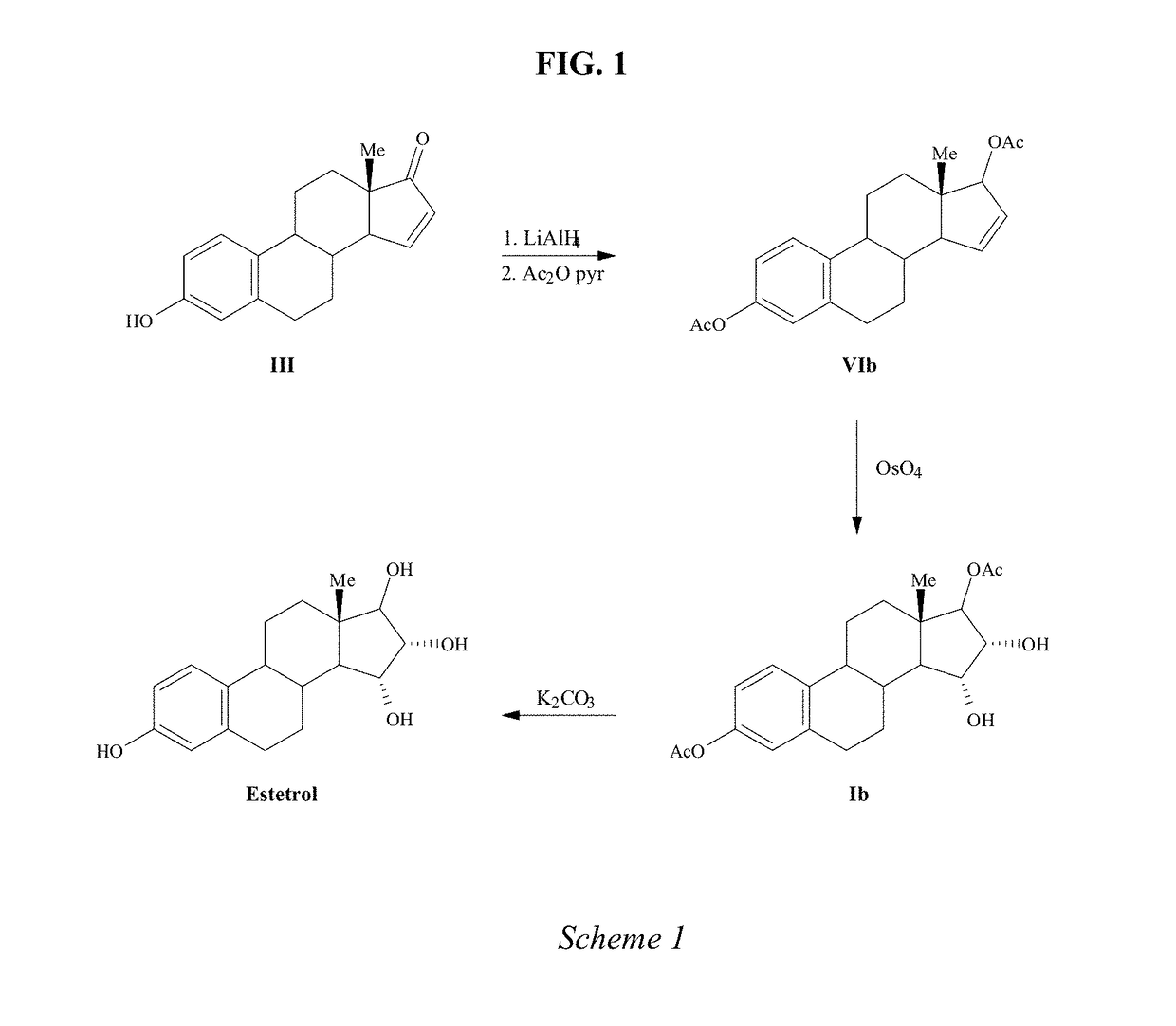
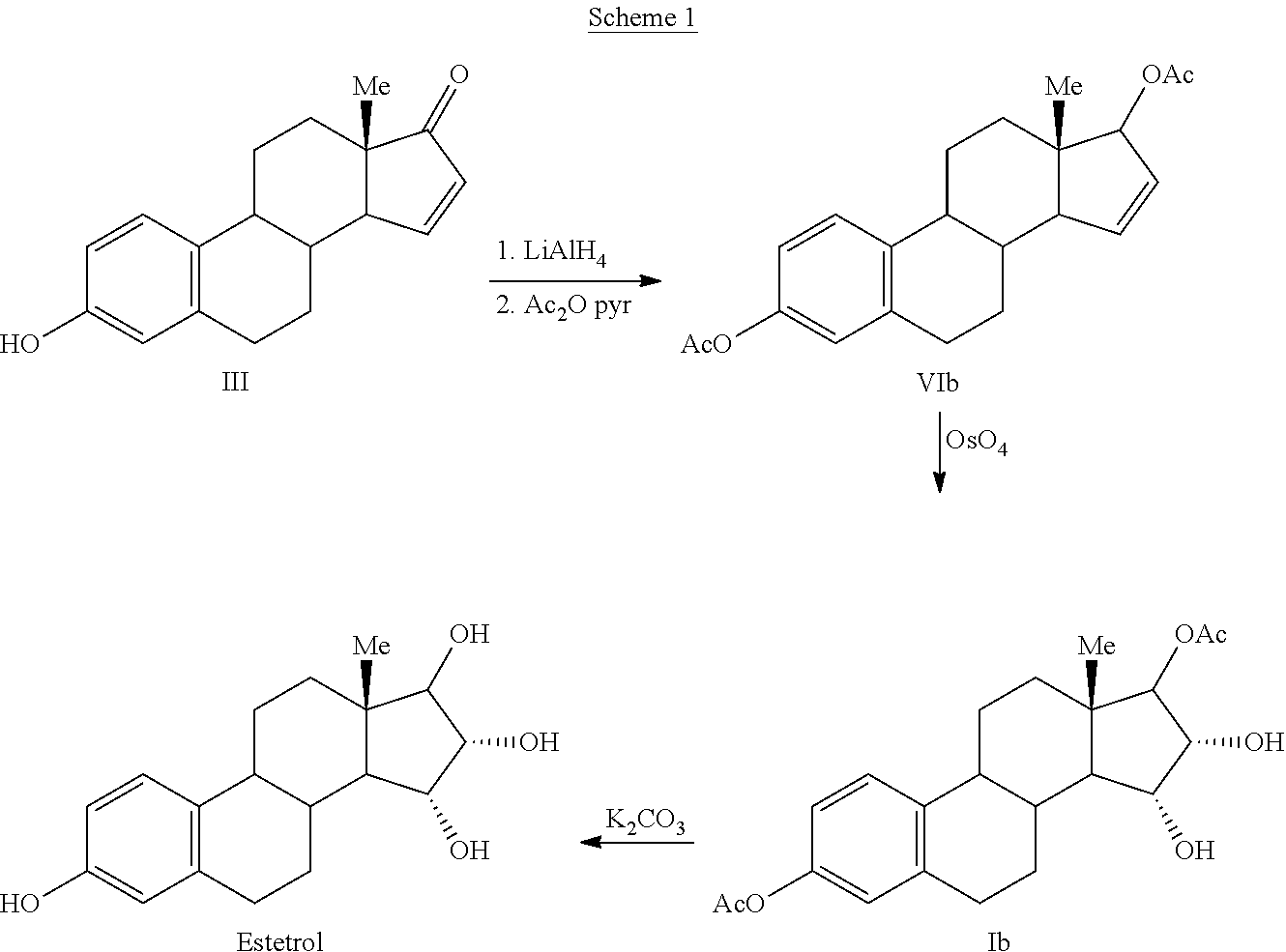
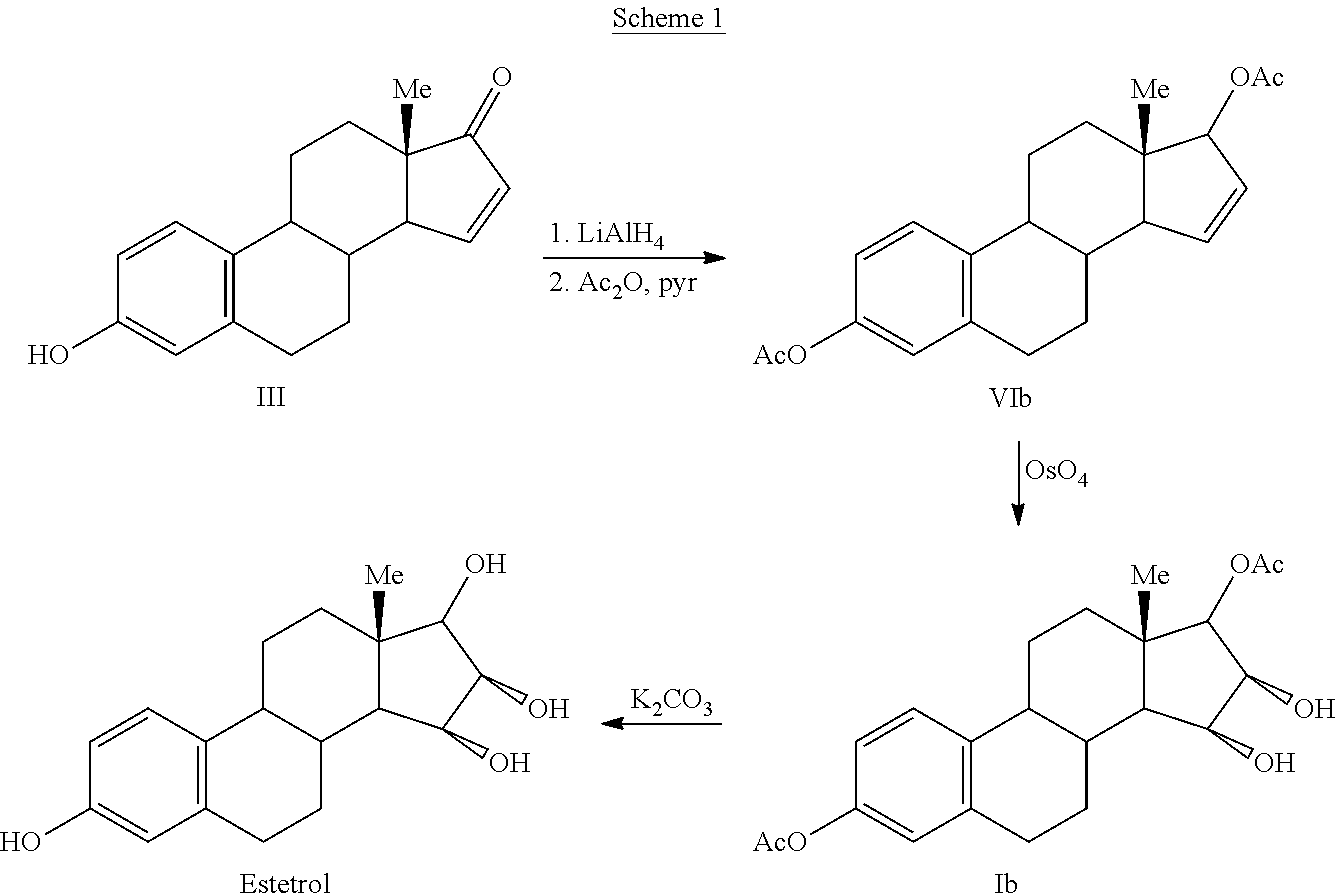
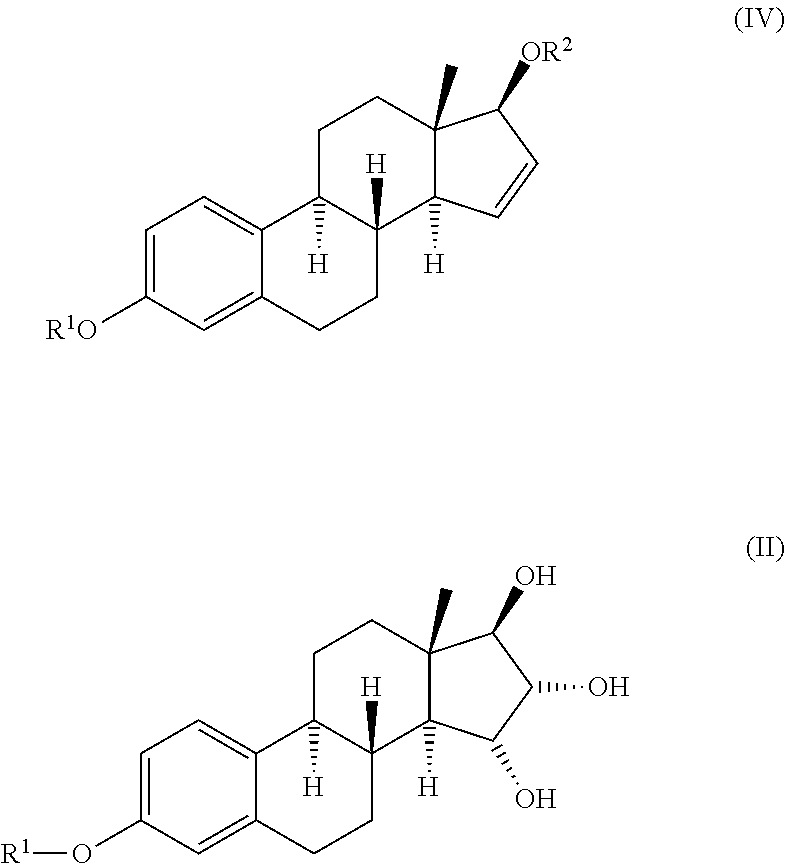
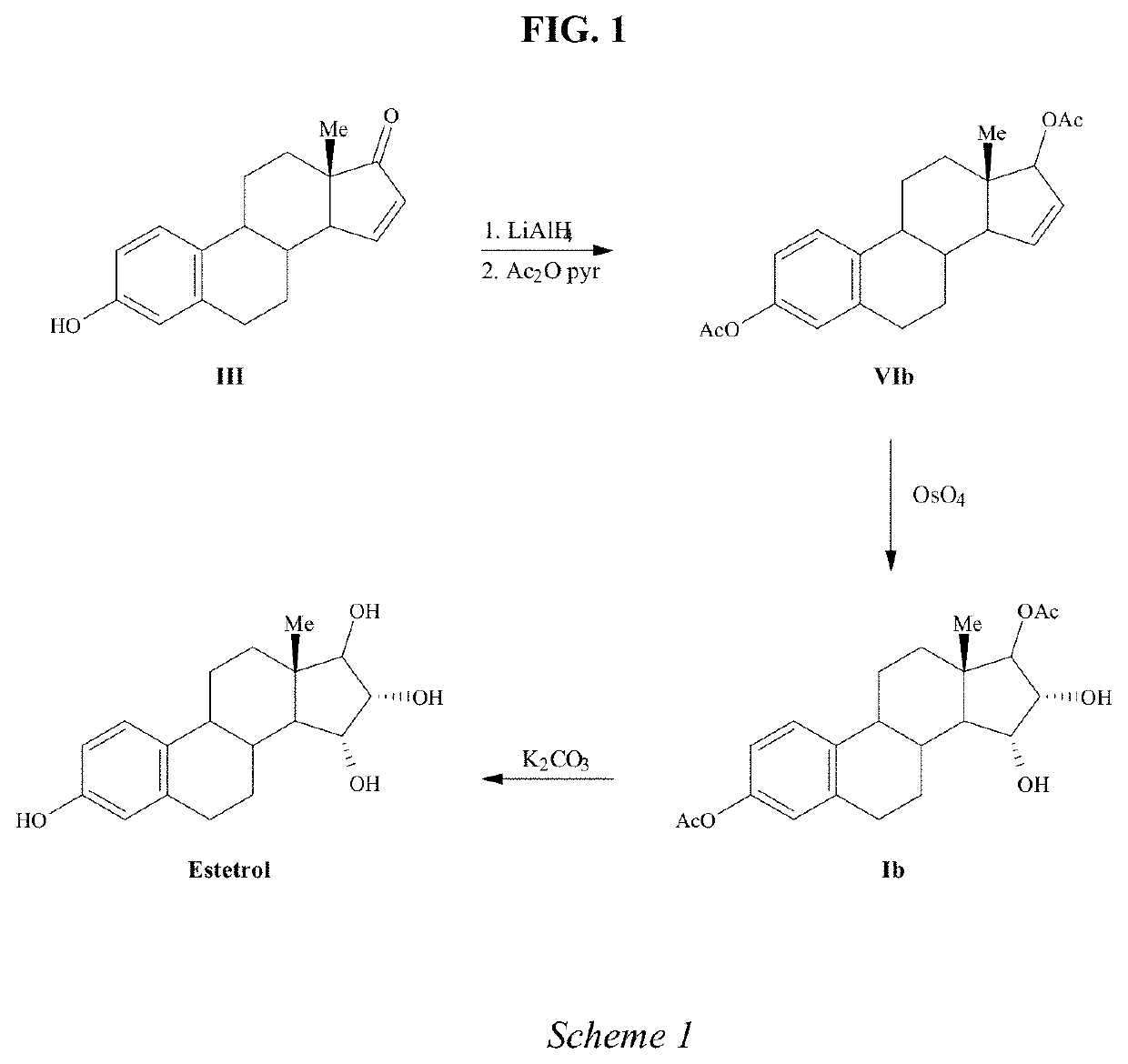
The invention relates to a process for obtaining Estetrol or a salt or solvate thereof, the process comprising: a) reacting a compound of formula (IV) or a salt or solvate thereof, wherein R1 is a hydroxyl protecting group selected from a silyl ether, an ether, an ester, a carbamate and a carbonate, and R2 is a hydroxyl protecting group selected from an ether, with an oxidizing agent selected from OsO4 or a source of osmium tetroxide to produce Estetrol or a compound of formula (II) or a salt or solvate thereof wherein R1 is as defined previously; and b) if a compound of formula (II) is obtained in step a), deprotecting said compound to produce Estetrol.
Owner:CRYSTAL PHARMA SA
Orally disintegrating solid dosage unit containing an estetrol component
ActiveUS9884064B2Easy to manufactureIncrease loadOrganic active ingredientsPharmaceutical non-active ingredientsSublabial administrationAdditive ingredient
Owner:ESTETRA SRL
Process for the preparation of estetrol
Owner:MITHRA R&D SA +1
Orodispersible dosage unit containing an estetrol component
InactiveUS20160367567A1Easy to manufactureQuick effectOrganic active ingredientsPill deliverySublabial administrationMedicine
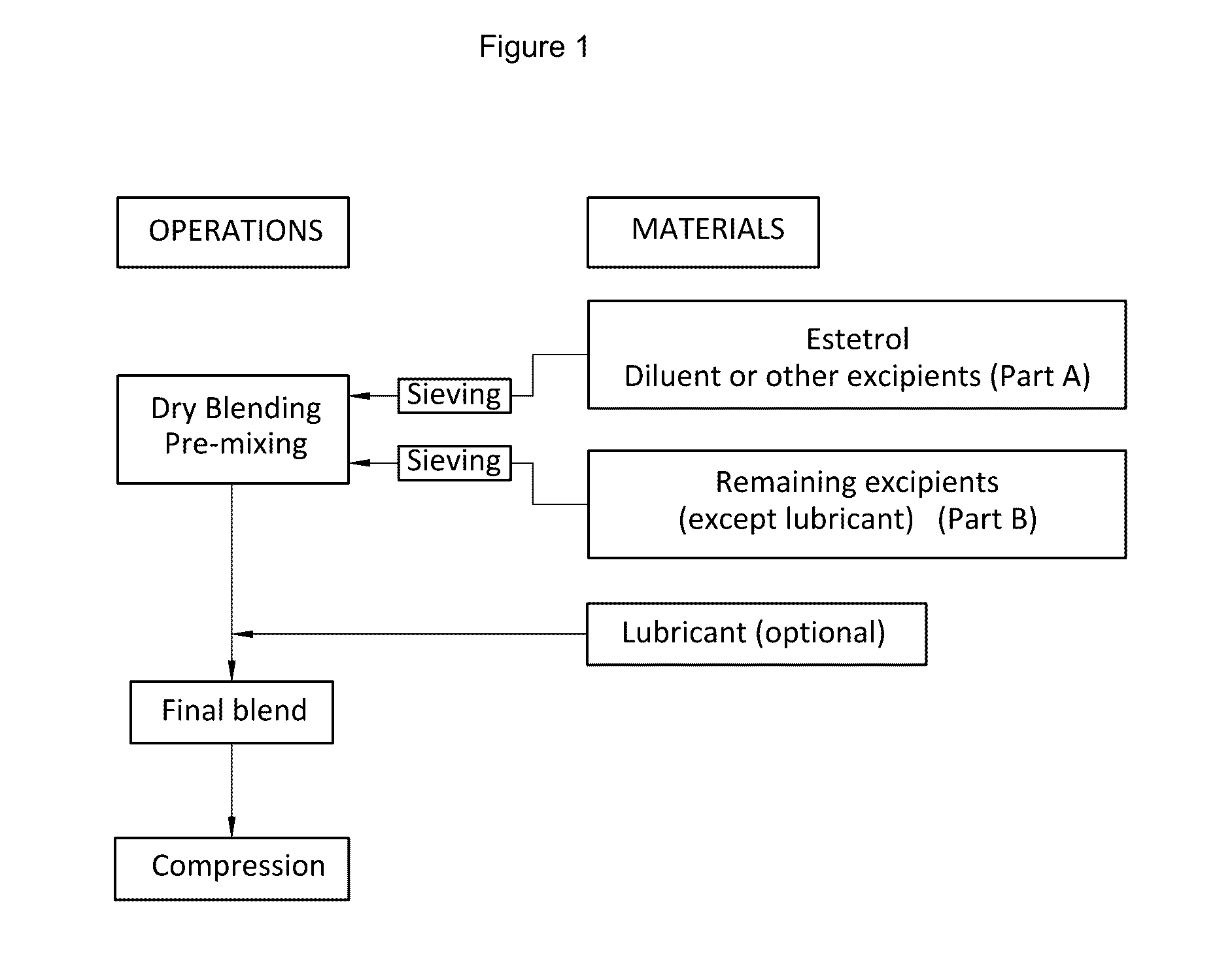
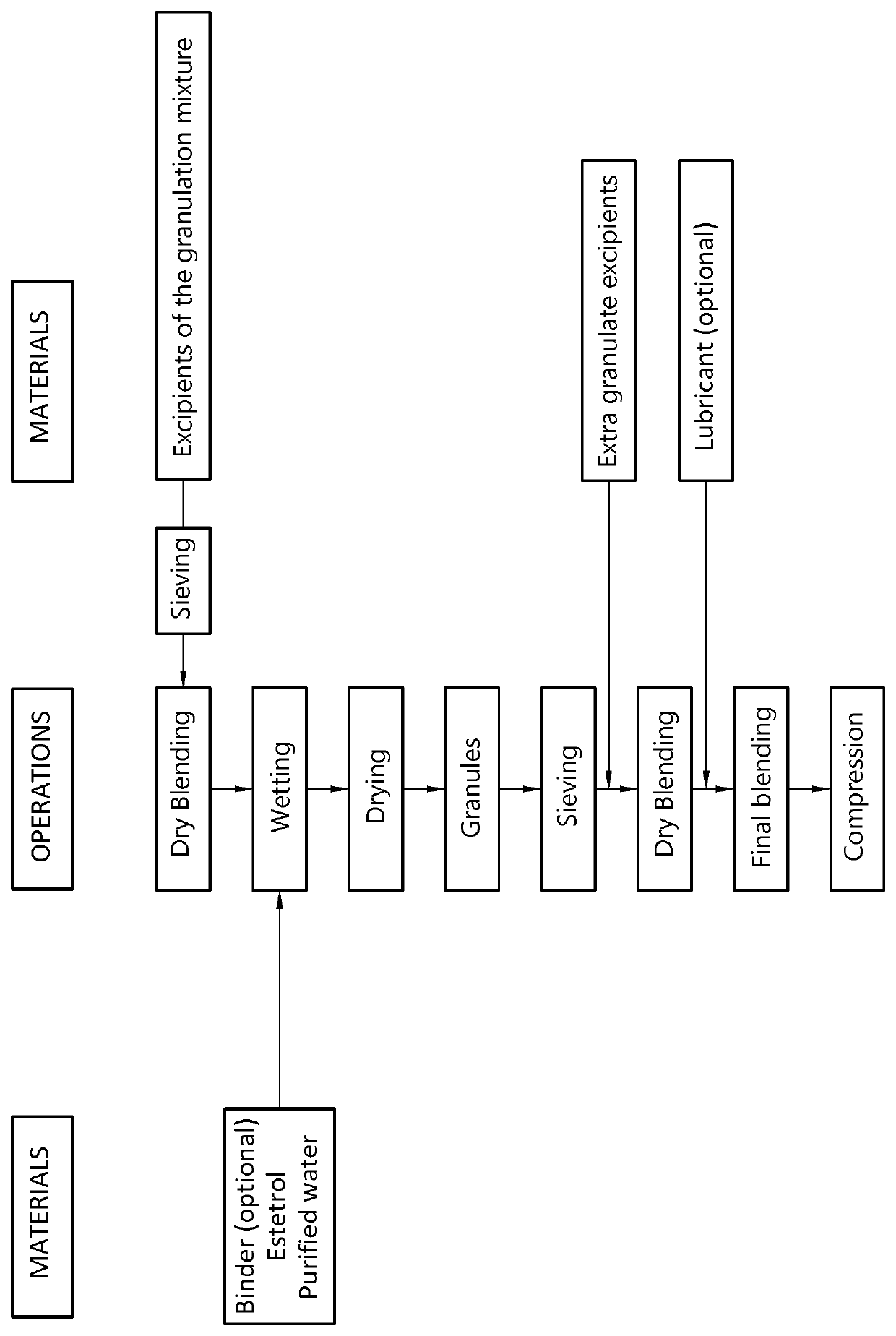
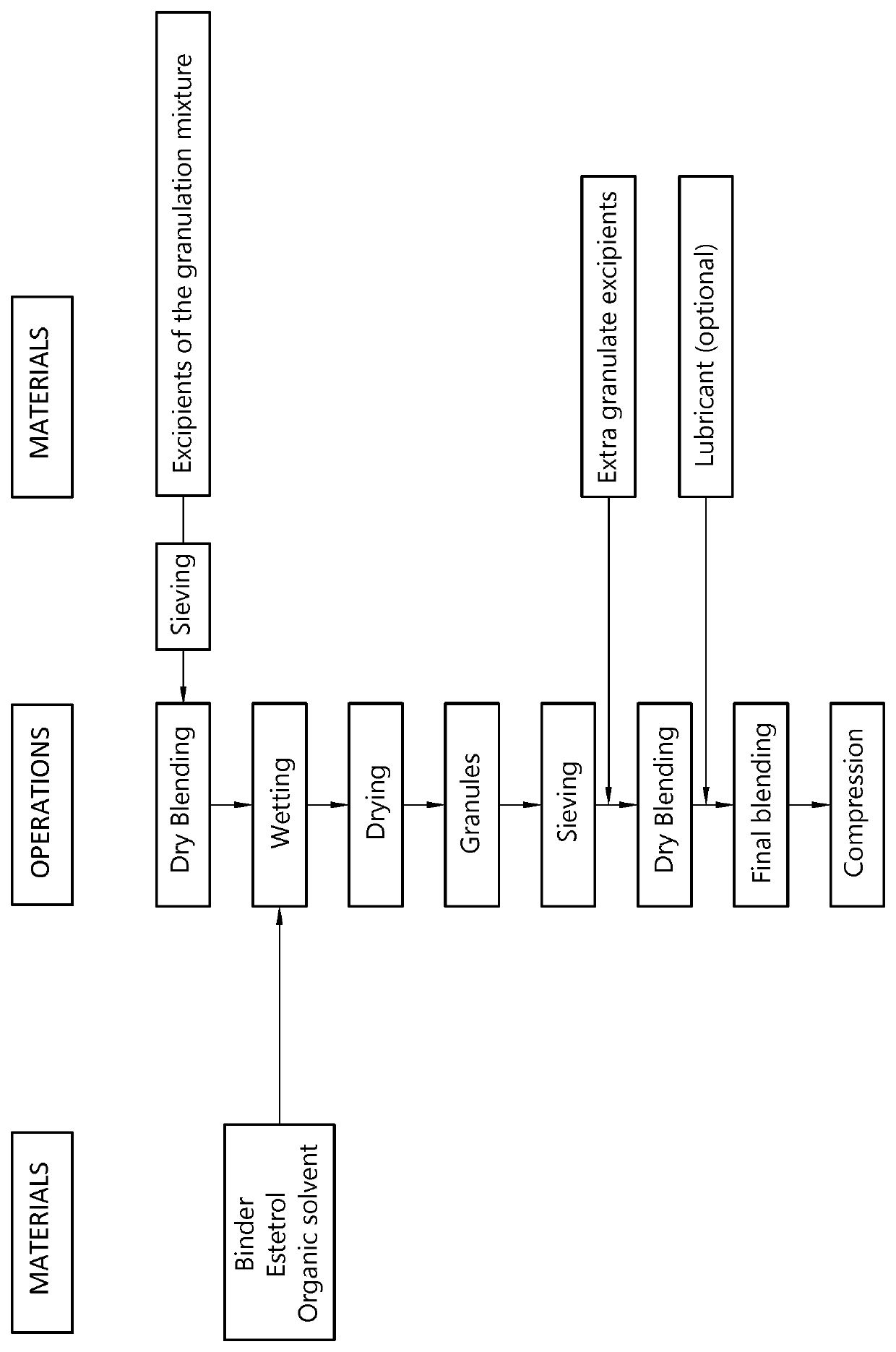
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit comprising:0.1-25 wt. % of estetrol particles containing at least 80 wt. % of an estetrol component selected from estetrol, estetrol esters and combinations thereof; and75-99.9 wt. % of one or more pharmaceutically acceptable excipients;the solid dosage unit comprising at least 100 μg of the estetrol component; and wherein the solid dosage unit can be obtained by a process that comprises compressing a dry blend of estetrol particles and one or more pharmaceutically acceptable excipients into a solid dosage unit.The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:MITHRA PHARMA SA
Process for the production of estetrol
ActiveUS8987484B2High yieldLow costSteroids preparationBulk chemical productionPhotochemistryEstetrol
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Process for the preparation of estetrol
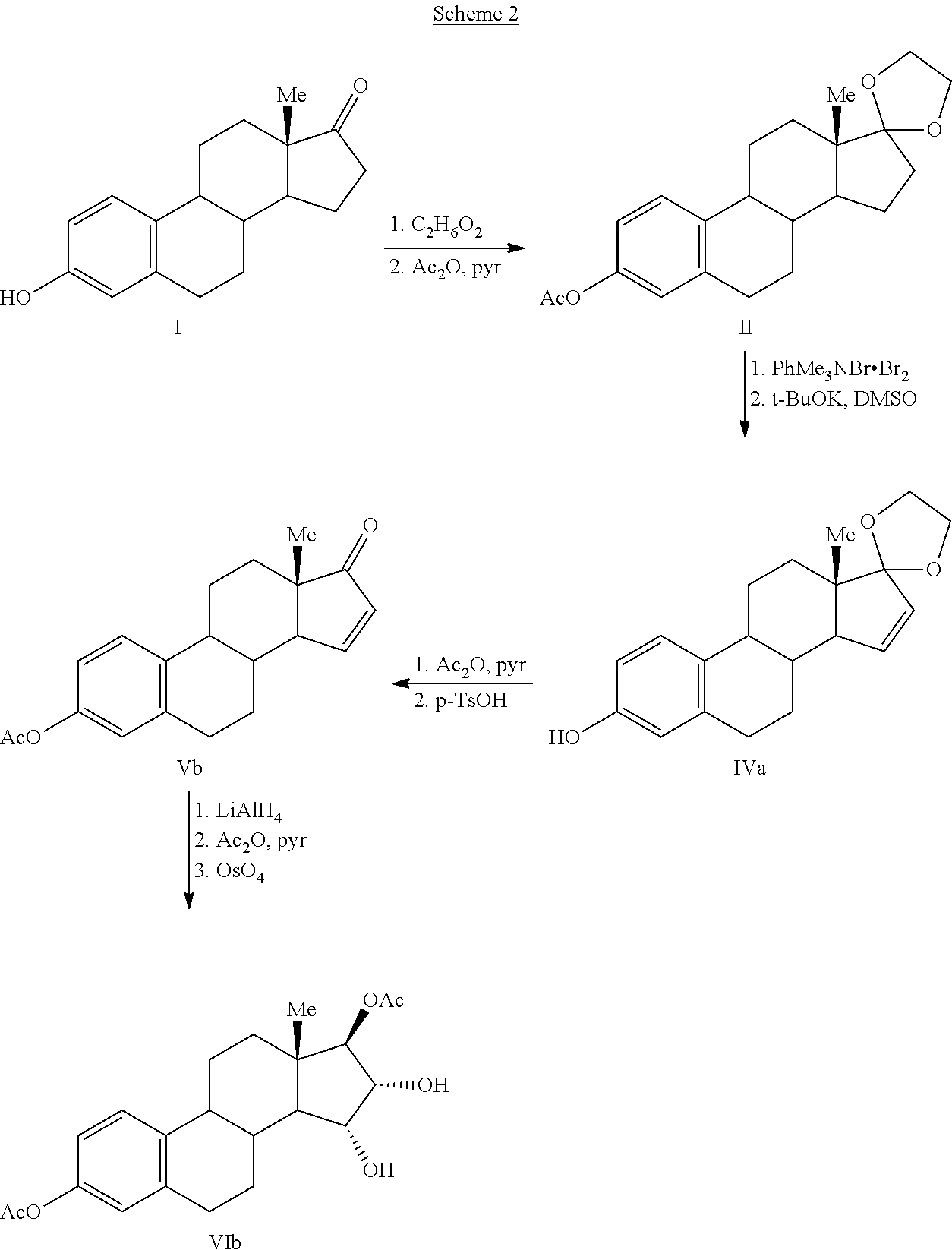
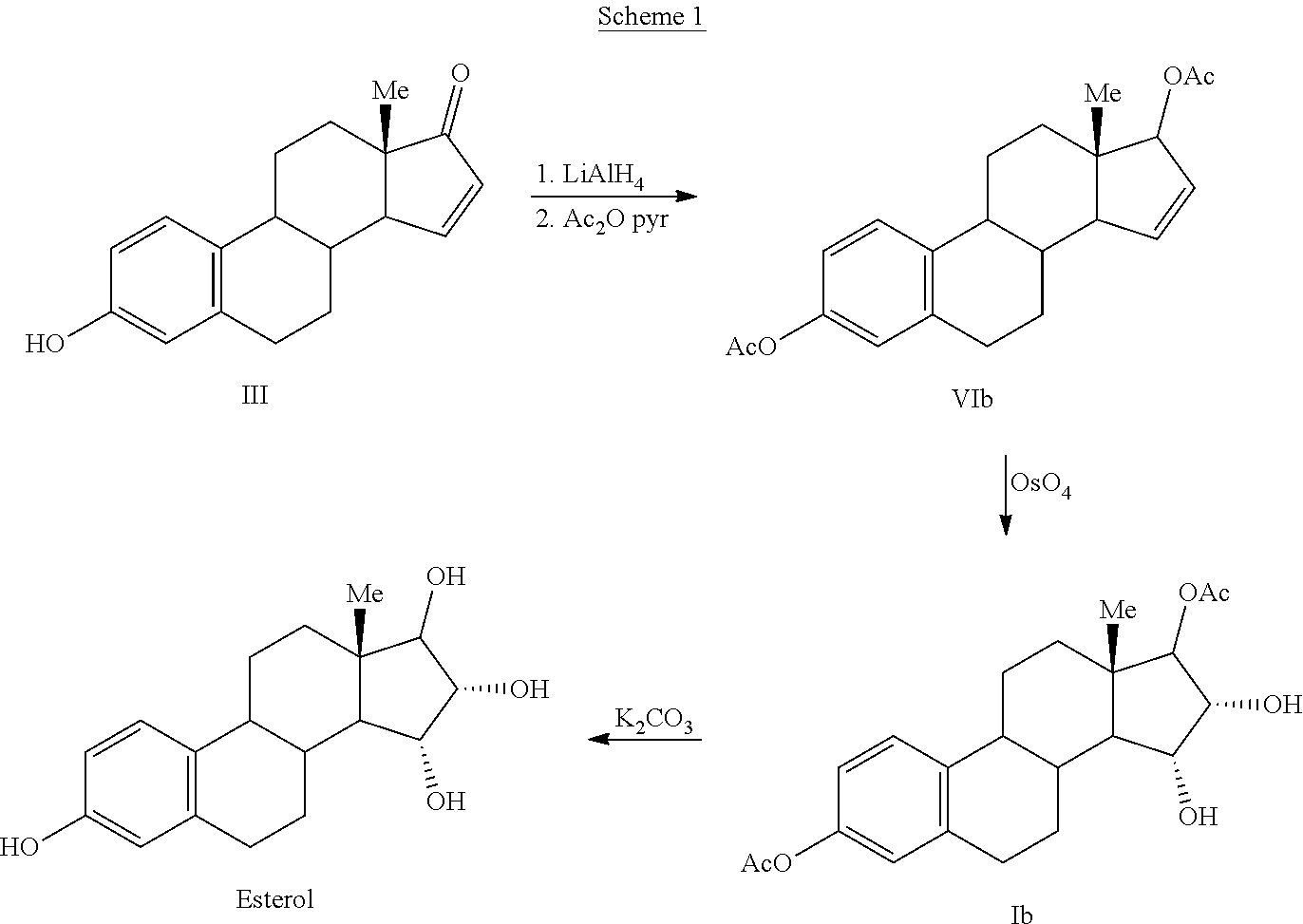
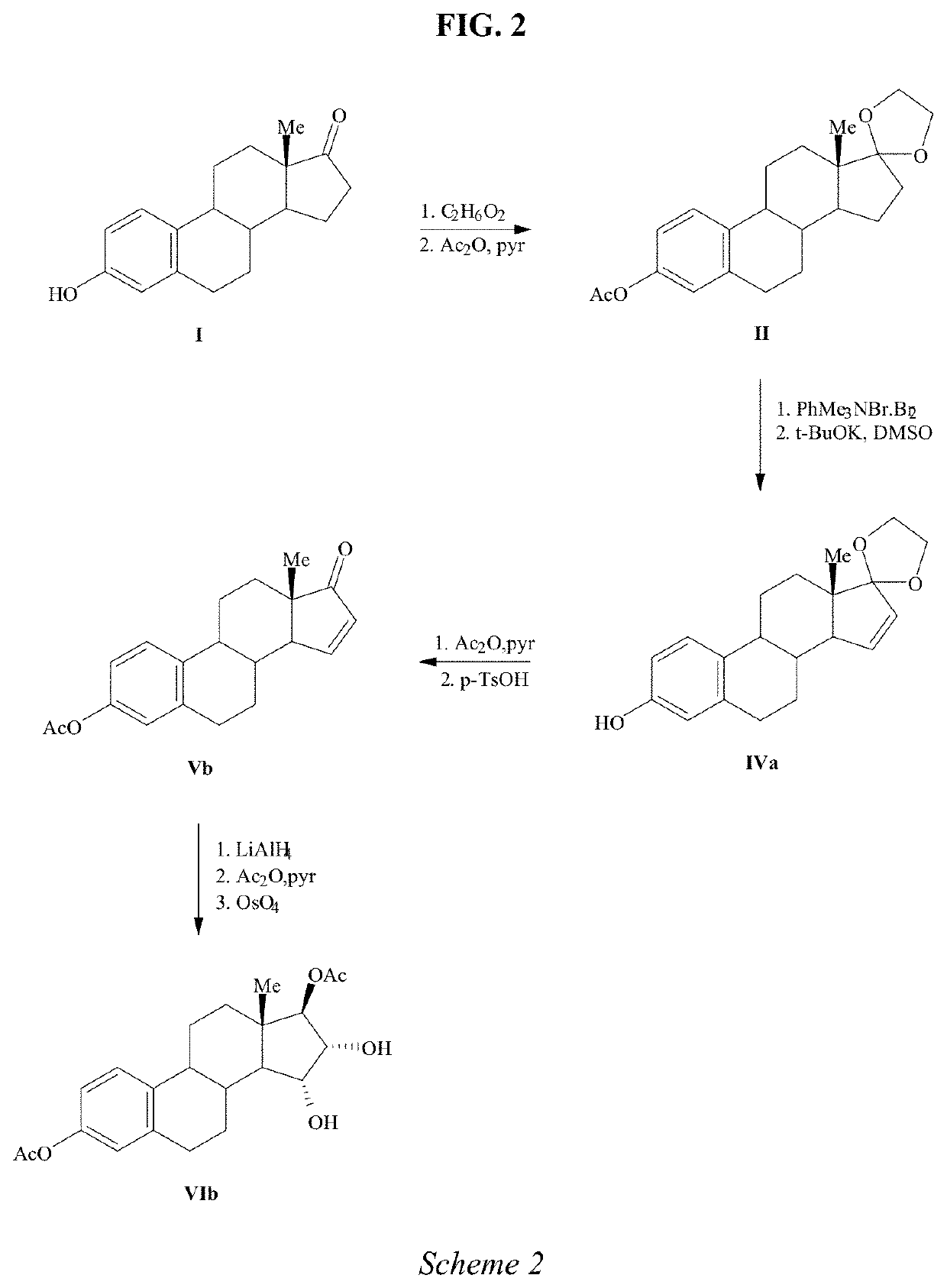
The present invention relates to a process for the preparation of estra-1,3,5(10)-trien-3,15α,16α,17β-tetraol (estetrol), via a silyl enol ether derivative 17-B-oxy-3-A-oxy-estra-1,3,5(10),16-tetraene, wherein A is a protecting group and B is —Si(R2)3. The invention further relates to a process for the synthesis of 3-A-oxy-estra-1,3,5(10),15-tetraen-17-one, wherein A is a protecting group, via said silyl enol ether derivative.
Owner:ESTETRA S P R L
Orodispersible dosage unit containing an estetrol component
ActiveUS20180169022A1Easy to manufactureAvoids first-pass liver exposureOrganic active ingredientsPharmaceutical non-active ingredientsSublabial administrationPharmaceutical drug
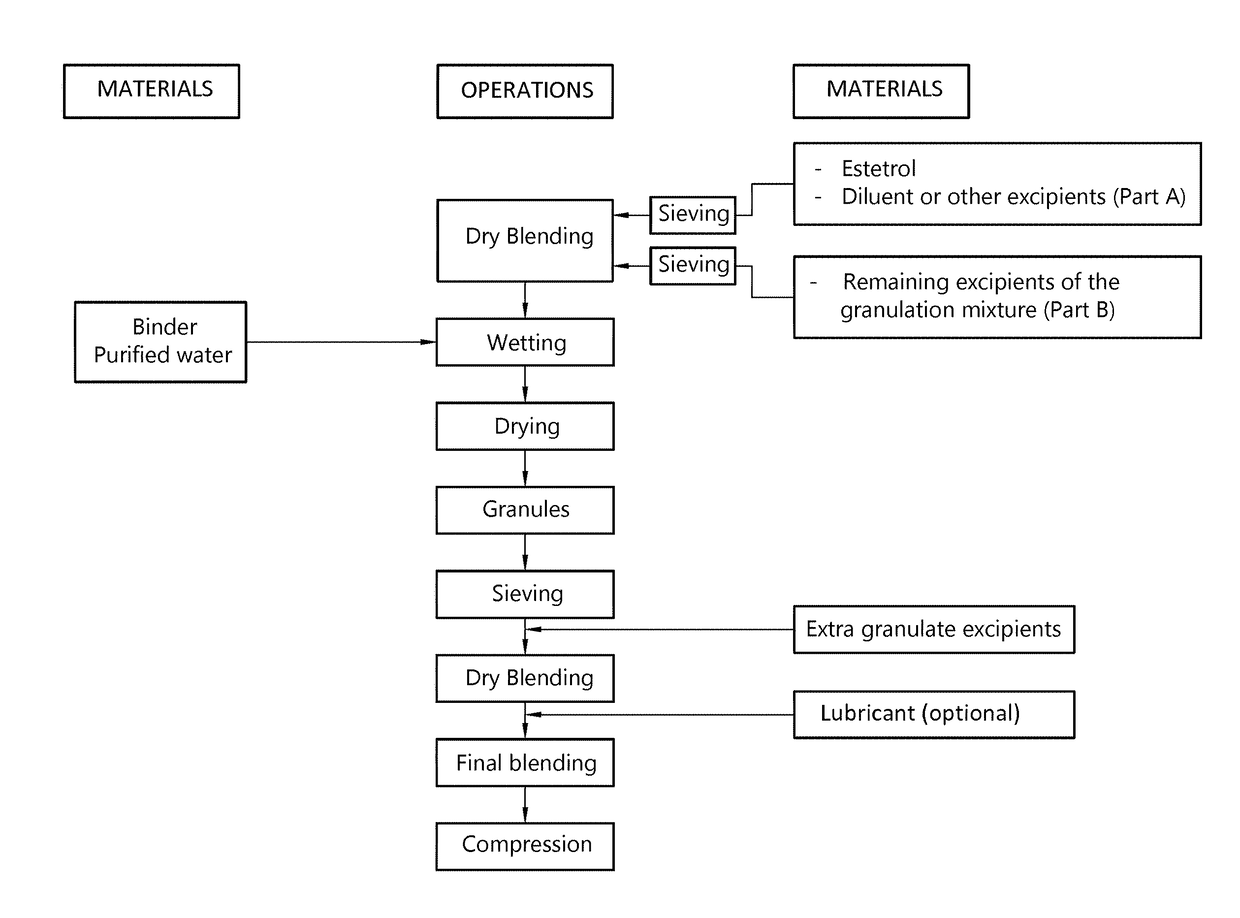
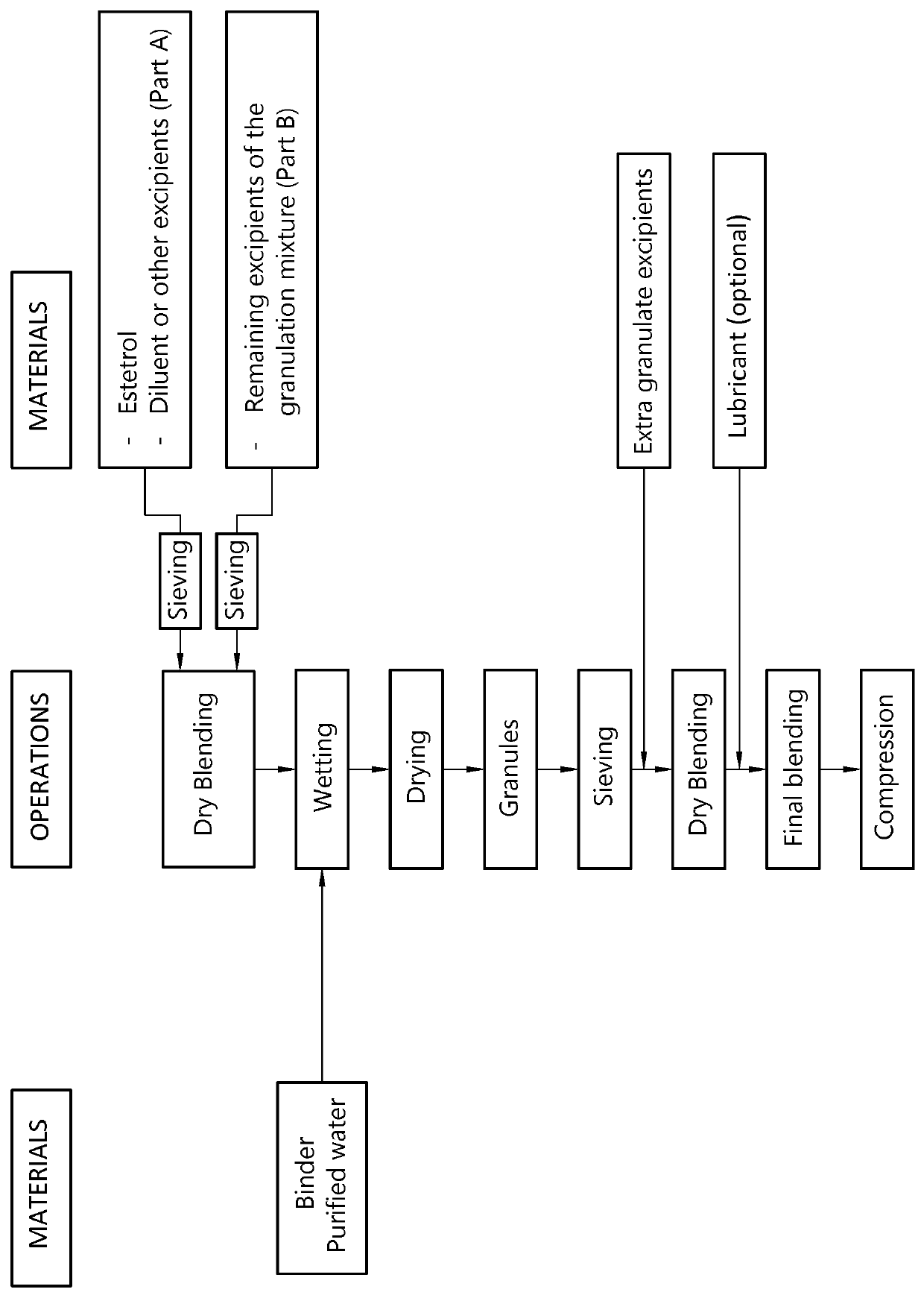
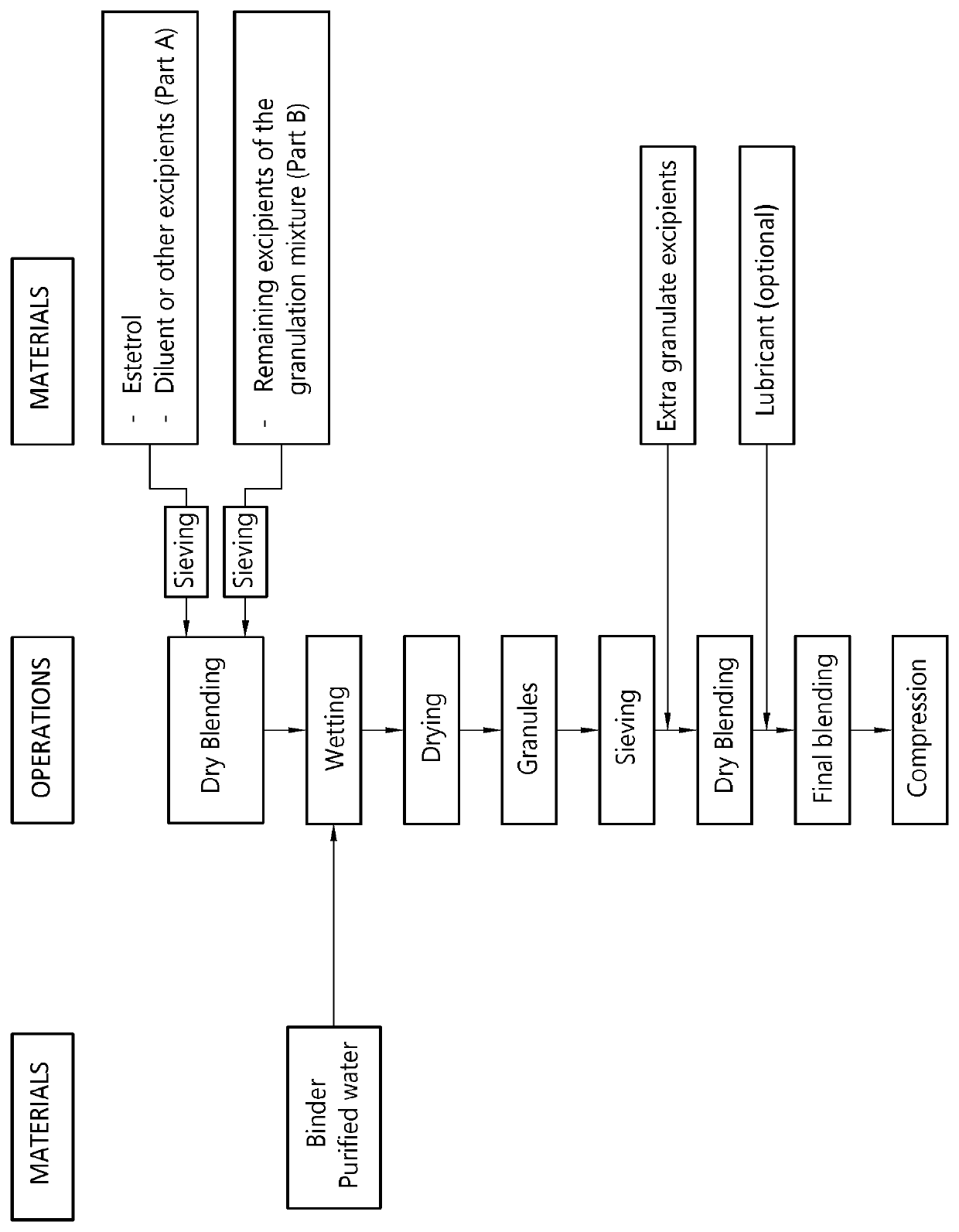
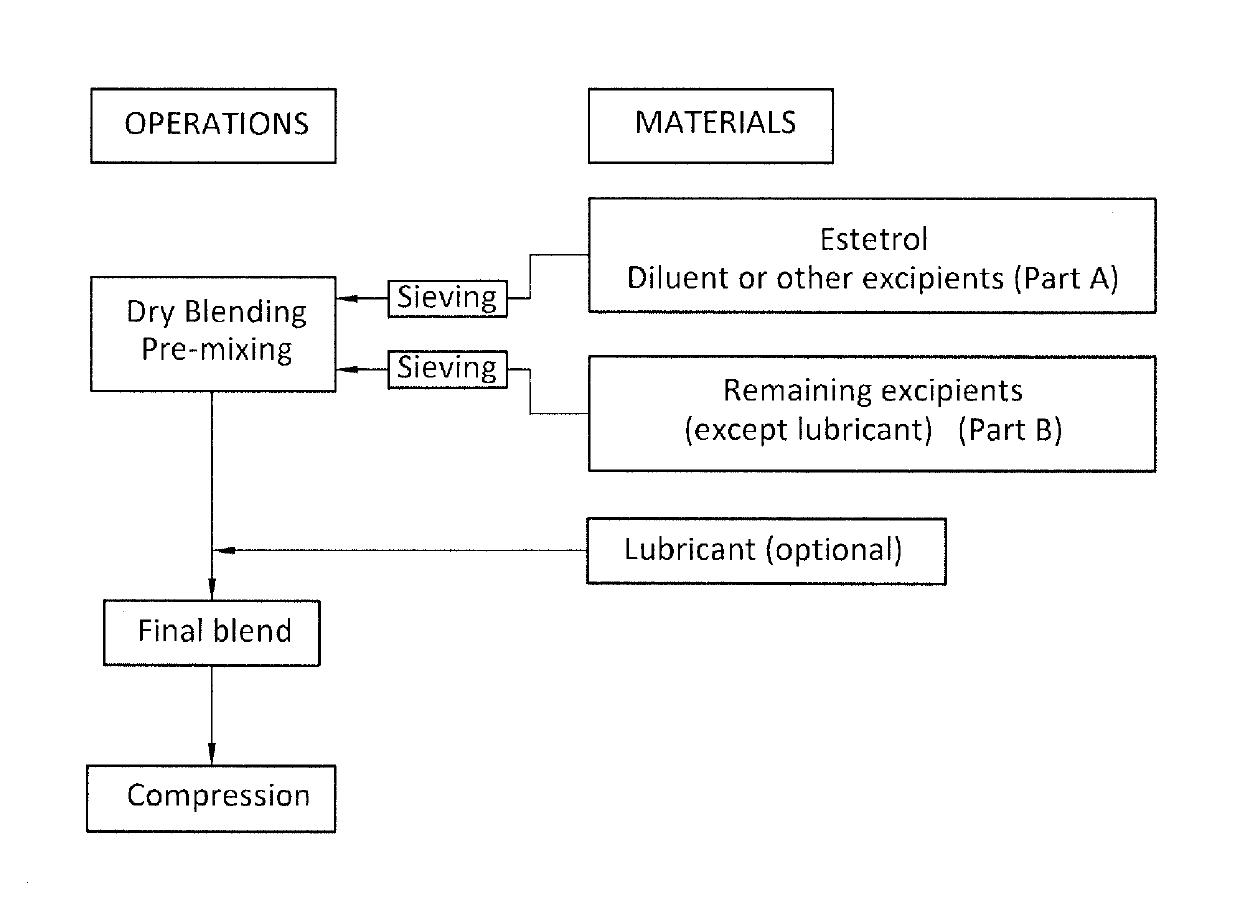
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit consisting of: 0.1-25 wt. % of estetrol particles containing at least 80 wt. % of an estetrol component selected from estetrol, estetrol esters and combinations thereof; and 75-99.9 wt. % of one or more pharmaceutically acceptable ingredients; the solid dosage unit comprising at least 100 μg of the estetrol component; and wherein the solid dosage unit can be obtained by a process comprising wet granulation of estetrol particles having a volume weighted average particle size of 2 μm to 50 μm. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Orodispersible tablet containing estetrol
ActiveUS10888518B2Easy to manufactureQuick effectOrganic active ingredientsPill deliverySublabial administrationPharmaceutical medicine
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process comprising: ⋅providing an aqueous liquid comprising water, estetrol component and optionally one or more other pharmaceutically acceptable ingredients; ⋅mixing 1 part by weight of the aqueous liquid with 0.5-20 parts by weight of the carrier particles to produce wet particles; ⋅removing water from the wet particles to produce loaded particles; ⋅optionally mixing the loaded particles with one or more tabletting excipients; and ⋅forming the loaded particles or the mixture of loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Orodispersible dosage unit containing an estetrol component
ActiveUS11147771B2Easy to manufactureQuick effectOrganic active ingredientsPill deliverySublabial administrationPharmaceutical drug
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit consisting of: 0.1-25 wt. % of estetrol particles containing at least 80 wt. % of an estetrol component selected from estetrol, estetrol esters and combinations thereof; and 75-99.9 wt. % of one or more pharmaceutically acceptable ingredients; the solid dosage unit comprising at least 100 μg of the estetrol component; and wherein the solid dosage unit can be obtained by a process comprising wet granulation of estetrol particles having a volume weighted average particle size of 2 μm to 50 μm. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Orodispersible dosage unit containing an estetrol component
PendingUS20220096385A1Easy to manufactureQuick effectOrganic active ingredientsPharmaceutical non-active ingredientsSublabial administrationPharmaceutical drug
Owner:ESTETRA SRL
Orodispersible dosage unit containing an estetrol component
ActiveUS20190125759A1Easy to manufactureQuick effectOrganic active ingredientsPill deliverySublabial administrationExcipient
An orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg is disclosed. The dosage unit comprises (a) 0.1-25 wt. % of estetrol particles containing at least 80 wt. % of an estetrol component selected from estetrol, estetrol esters and combinations thereof; and (b) 75-99.9 wt. % of one or more pharmaceutically acceptable excipients. The solid dosage unit comprises at least 100 μg of the estetrol component and can be obtained by a process that comprises compressing a dry blend of estetrol particles and one or more pharmaceutically acceptable excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Orodispersible tablet containing Estetrol
ActiveUS10894014B2Easy to manufactureQuick effectOrganic active ingredientsPill deliveryOrganic solventSublabial administration
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process that comprises: providing a loading liquid comprising organic solvent; estetrol component and optionally one or more other pharmaceutically acceptable ingredients; mixing 1 part by weight of the loading liquid with 0.5-20 parts by weight of carrier particles to produce wet particles; removing organic solvent from the wet particles to produce loaded particles; optionally mixing the loaded particles with one or more tabletting excipients; and forming the loaded particles or the mixture of the loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
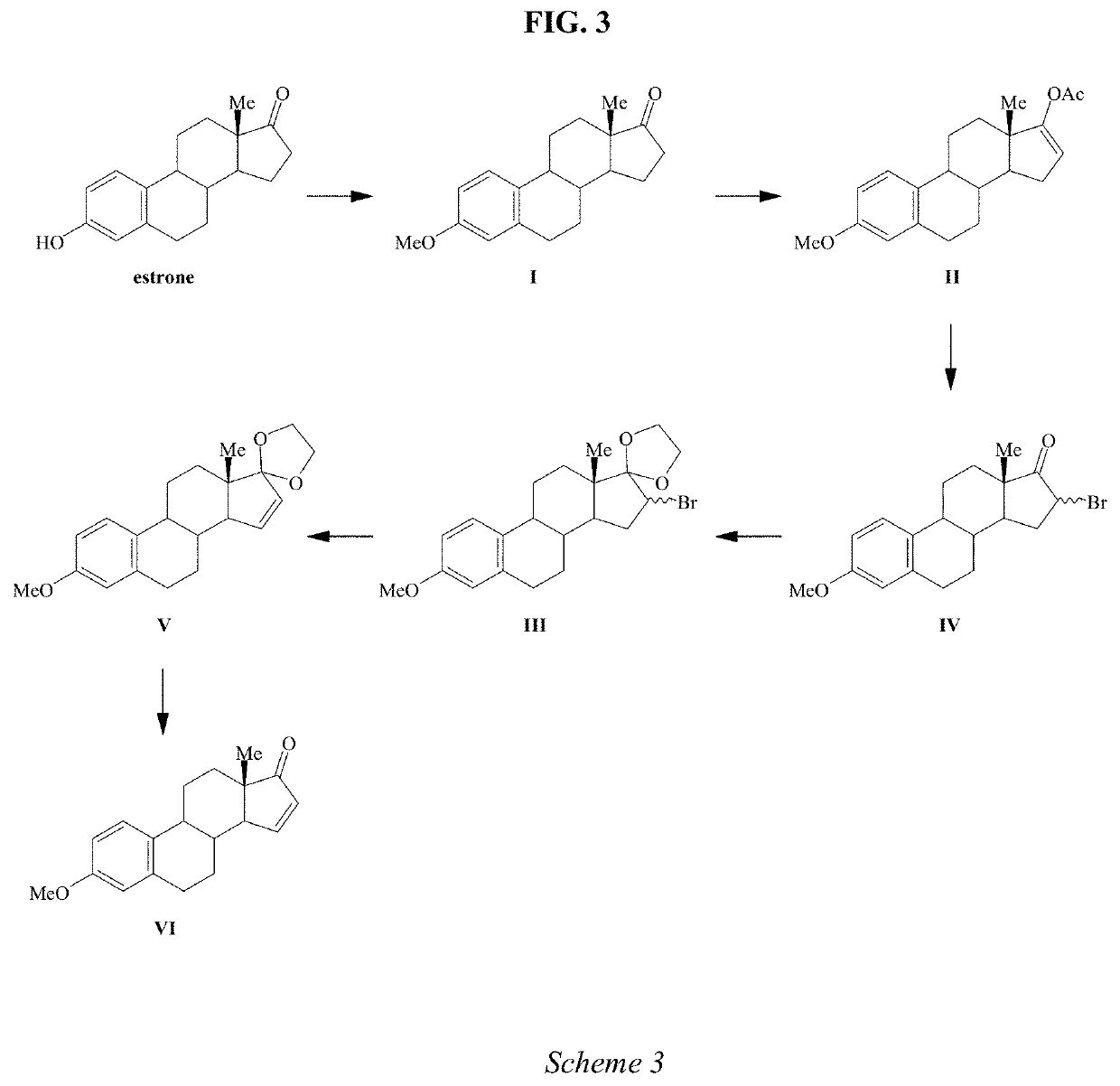
Synthesis of estetrol via estrone derived steroids
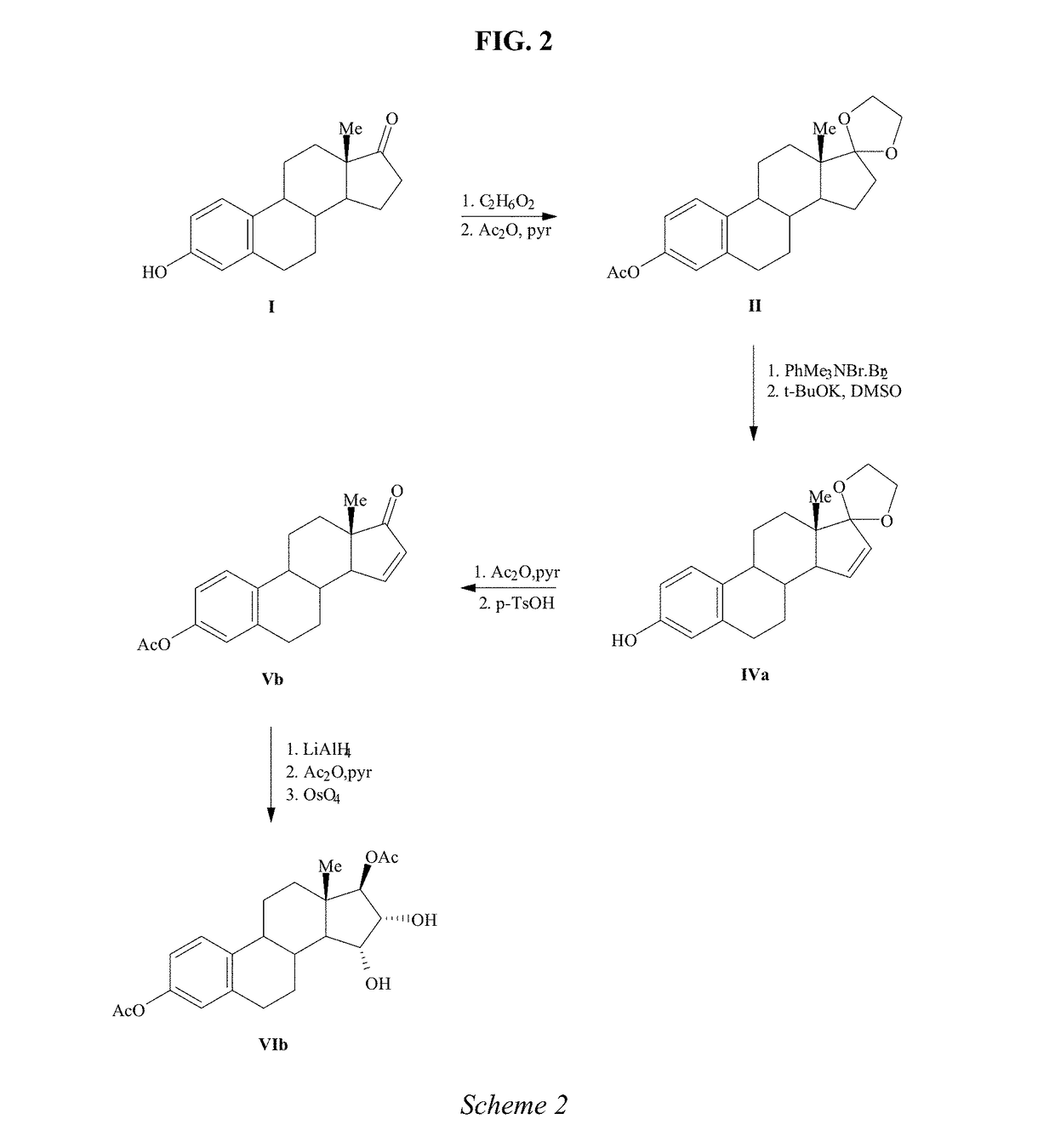
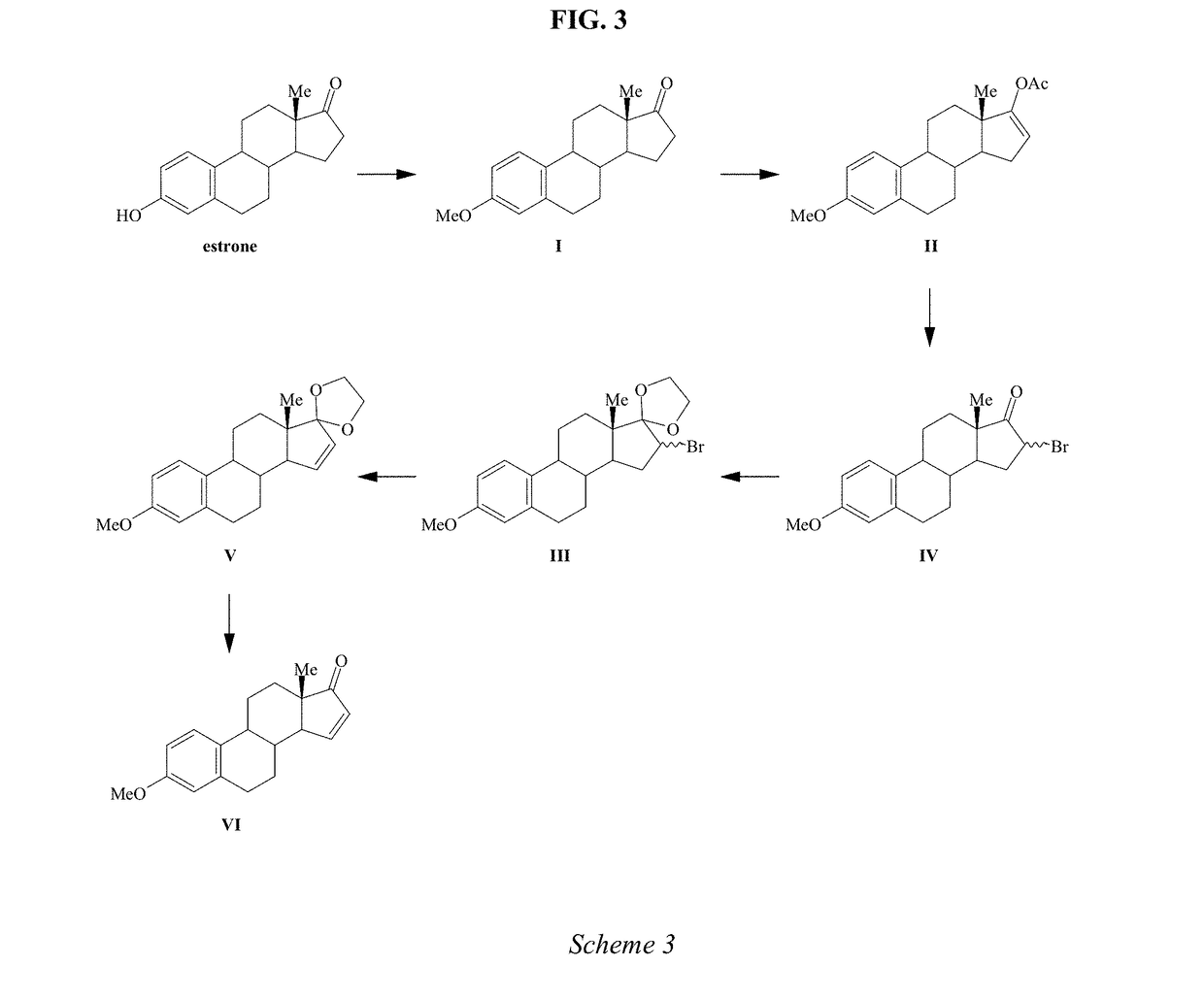
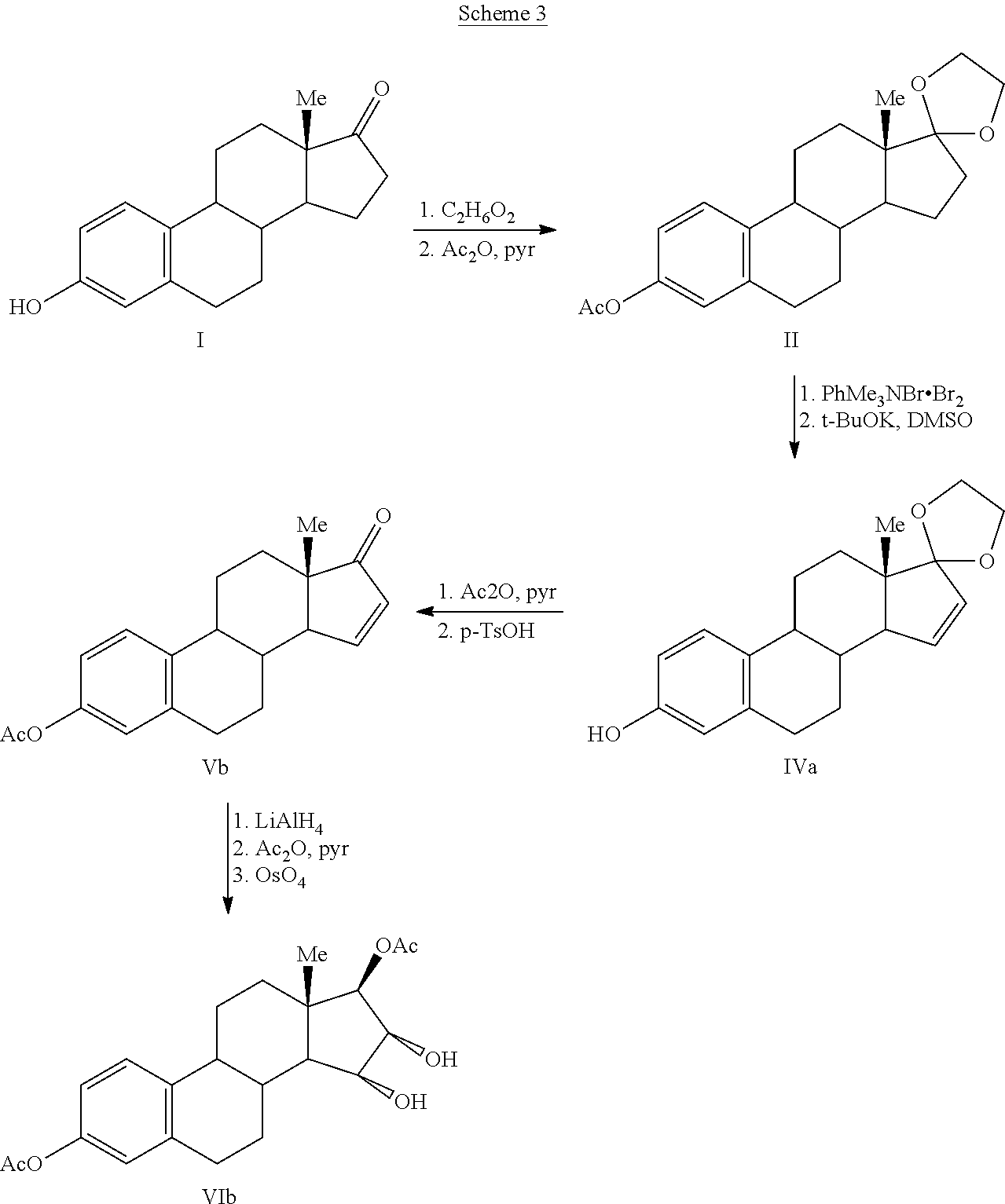
A process is provided for the making of estetrol starting from a 3-A-oxy-estra-1,3,5(10),15-tetraen-17-one, wherein A is an C1-C5 alkyl group, preferably a methyl group, or a C7-C12 benzylic group, preferably a benzyl group. This process is particularly suitable to industry.
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Process for the preparation of estetrol
The invention relates to a process for obtaining Estetrol or a salt or solvate thereof, the process comprising: a) reacting a compound of formula (IV) or a salt or solvate thereof, wherein R1 is a hydroxyl protecting group selected from a silyl ether, an ether, an ester, a carbamate and a carbonate, and R2 is a hydroxyl protecting group selected from an ether, with an oxidizing agent selected from OsO4 or a source of osmium tetroxide to produce Estetrol or a compound of formula (II) or a salt or solvate thereof wherein R1 is as defined previously; and b) if a compound of formula (II) is obtained in step a), deprotecting said compound to produce Estetrol.
Owner:CRYSTAL PHARMA SA
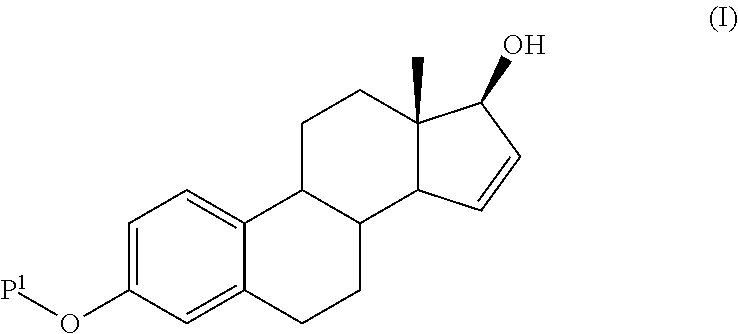
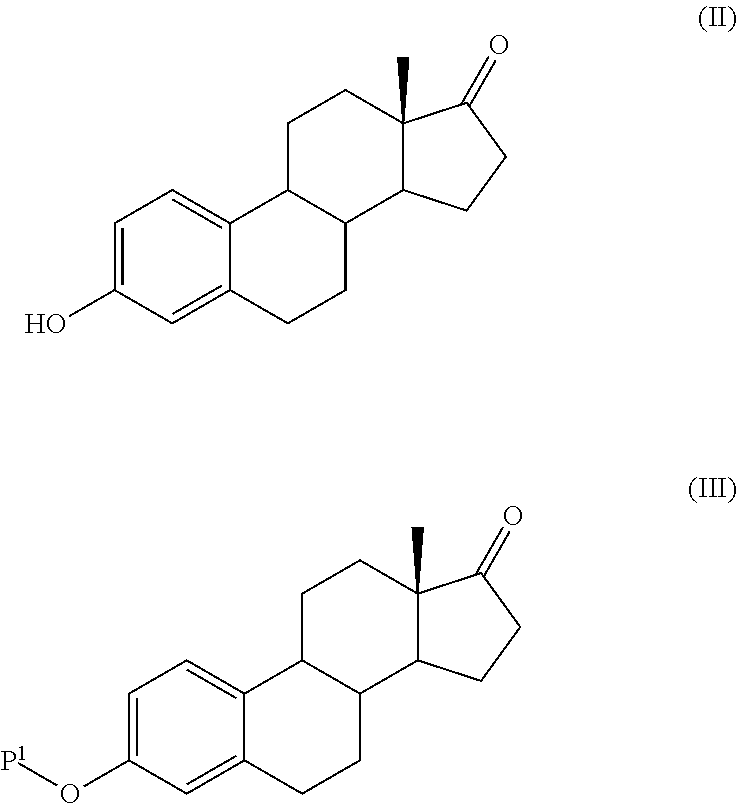
Process for the production of estetrol intermediates
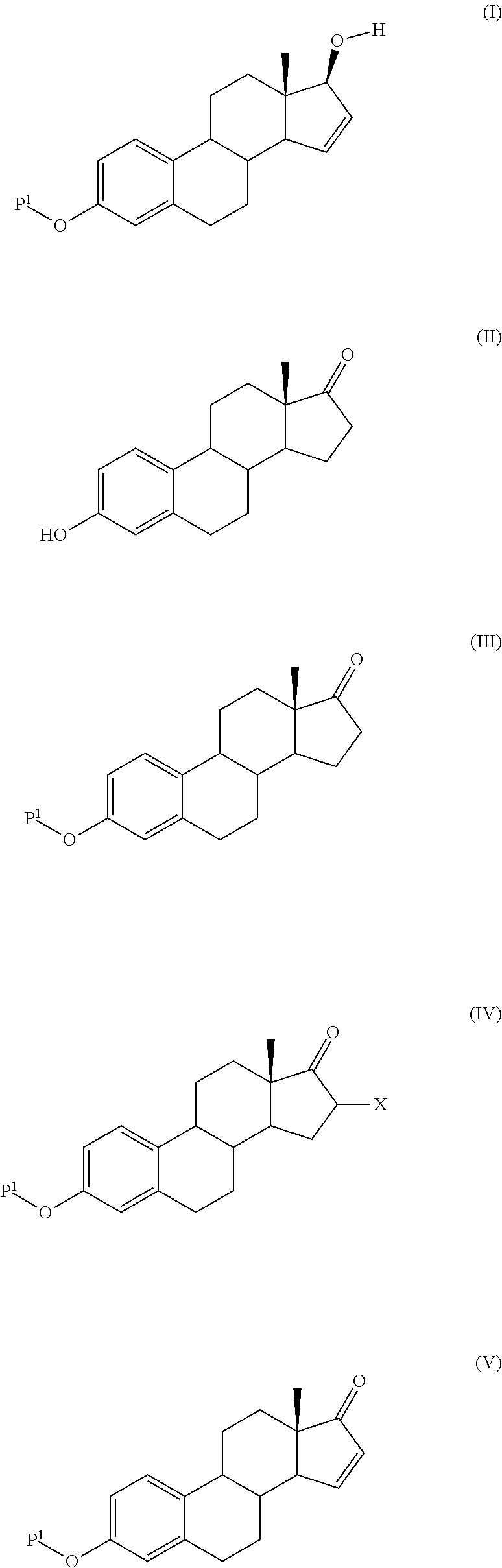
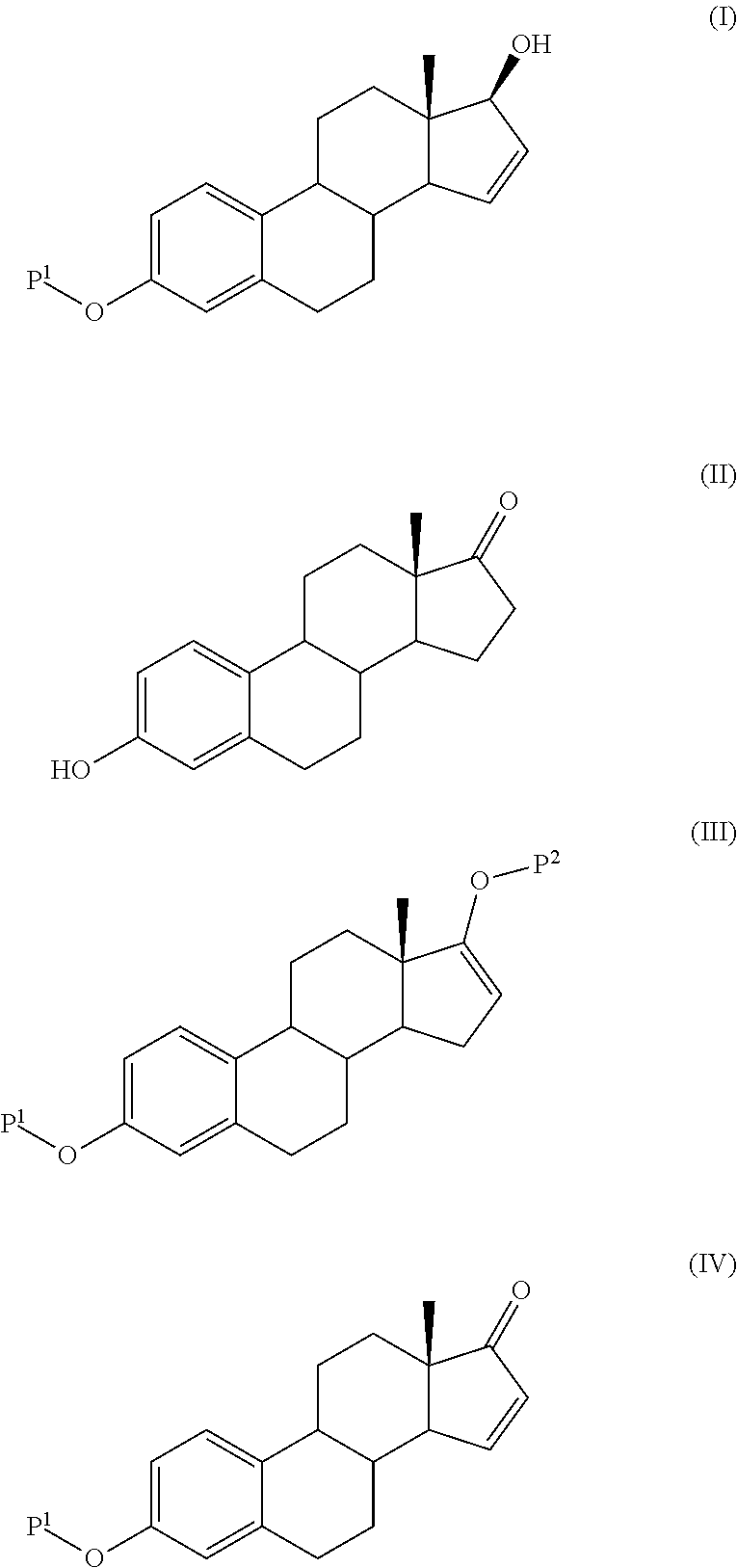
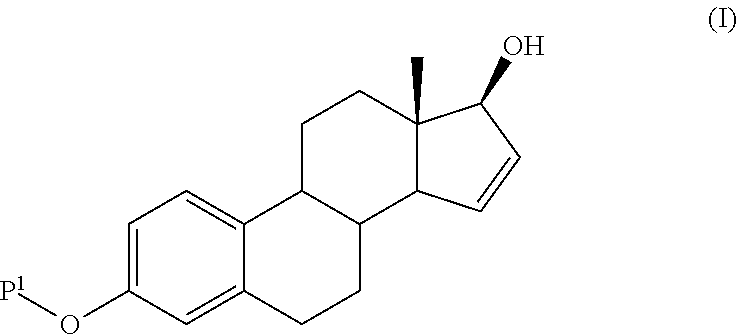
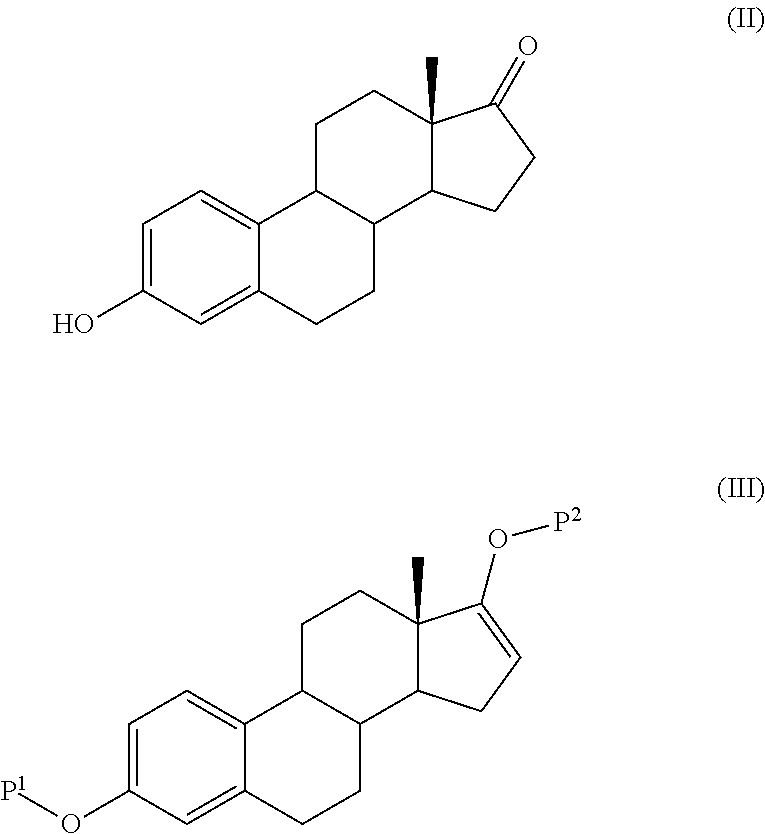
The present invention relates to a process for the preparation of a compound of formula (I) comprising the steps of a) reacting a compound of formula (II) with a silylating or an acylating agent to produce compound of formula (III), wherein P1 is a protecting group selected from R2—Si—R3R4 or R1CO—, R1 is a group selected from C1-6alkyl or C3-6cycloalkyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; R2, R3 and R4 are each independently a group selected from C1-6alkyl or phenyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; b) halogenation or sulfinylation of the compound of formula (III) to produce a compound of formula (IV); wherein X is halo, or —O—SO—R5, and R5 is a group selected from C6-10aryl or heteroaryl, each group being optionally substituted by one or more substituents independently selected from chloro or C1-4alkyl; c) dehalogenation or desulfinylation of the compound of formula (IV) to produce compound of formula (V); and d) reacting the compound of formula (V) with a reducing agent to produce compound of formula (I).
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Process for the Production of Estetrol
ActiveUS20140243539A1High yieldLow costSteroids preparationBulk chemical productionPhotochemistryEstetrol
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Industrial process for preparation of high purity estetrol
ActiveCN114302889AOrganic active ingredientsNervous disorderCombinatorial chemistryPerylene derivatives
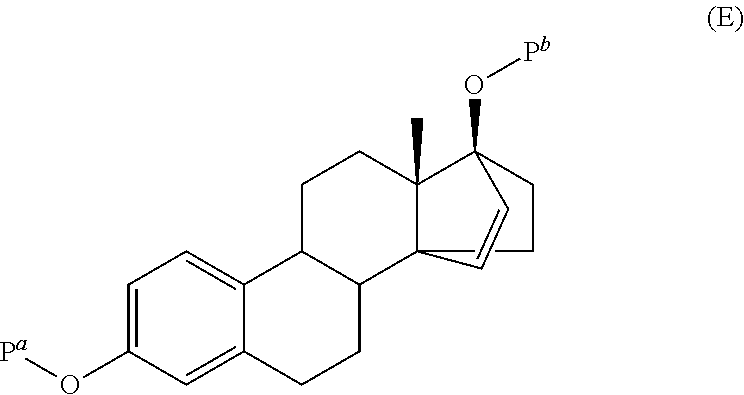
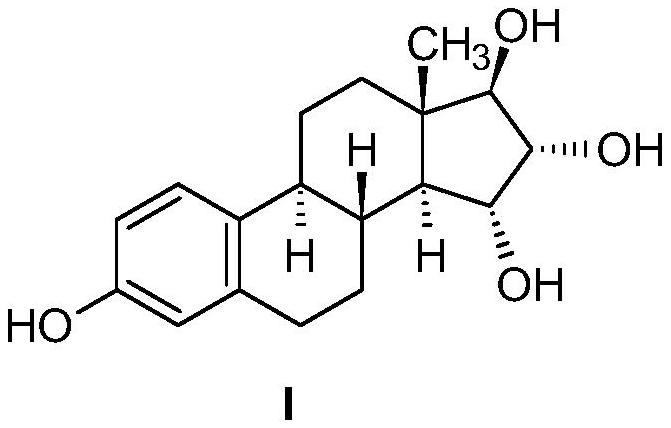
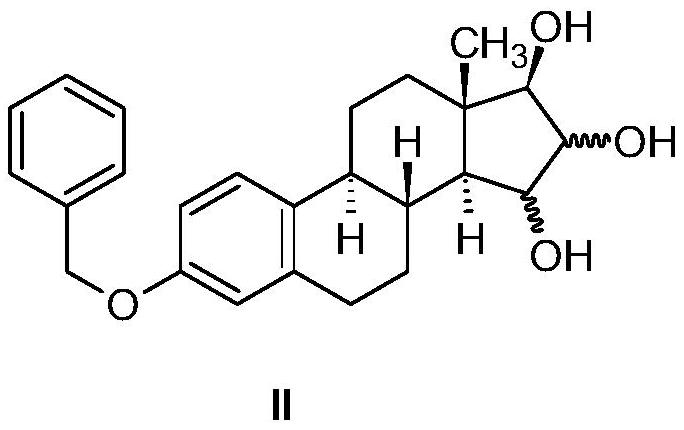
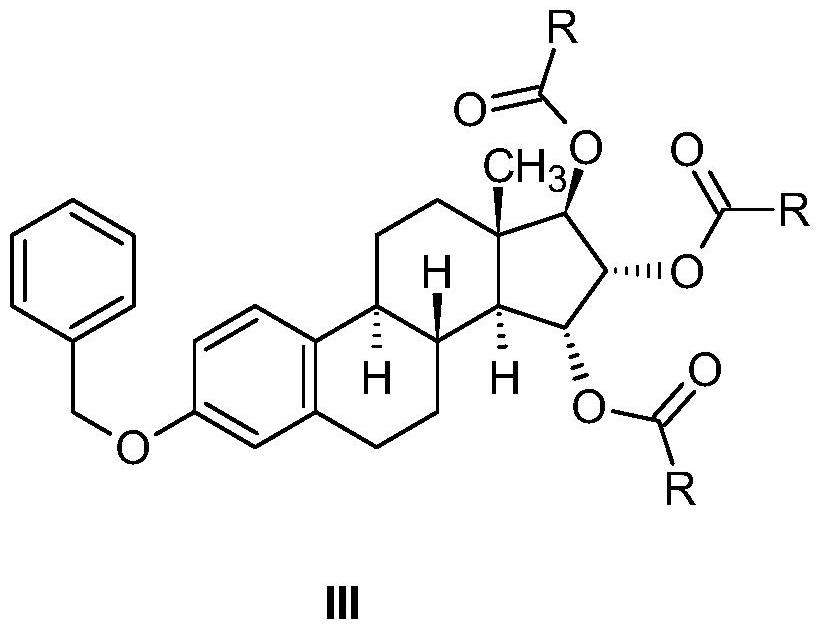
The invention relates to the preparation of estetrol of formula (I), its derivatives of general formula (III) protected at the 3, 15 alpha, 16 alpha, 17 beta-position, its 3-hydroxy derivatives of general formula (IV) protected at the 15 alpha, 16 alpha, 17 beta-position, and intermediates of general formulas (III) and (IV) for use in said preparation methods. A further aspect of the invention is the use of estetrol of formula (I) obtained by the process of the invention in the preparation of a pharmaceutical composition.
Owner:RICHTER GEDEON NYRT
Compounds and their uses for alleviating menopause-associated symptoms
ActiveUS11484539B2Quick effectAvoid exposurePharmaceutical delivery mechanismSexual disorderSide effectHormone replacement
The present invention relates to a hormone replacement therapy, to the associated compounds and to the associated packaging units, for alleviating menopause-associated symptoms which is based on the administration to a female mammal of an estetrol component at a specific daily dose, optionally in combination with a progestogenic component. The therapy enjoys a statistically significant efficacy combined with a favourable profile for side effects compared to currently available methods for alleviating menopause-associated symptoms.
Owner:ESTETRA SRL
Compounds and their uses for alleviating menopause-associated symptoms
The present invention relates to a hormone replacement therapy, to the associated compounds and to the associated packaging units, for alleviating menopause-associated symptoms which is based on the administration to a female mammal of an estetrol component at specified daily doses, optionally in combination with a progestogenic component. The therapy enjoys a statistically significant efficacy combined with a favourable profile for side effects compared to currently available methods for alleviating menopause-associated symptoms.
Owner:ESTETRA SRL
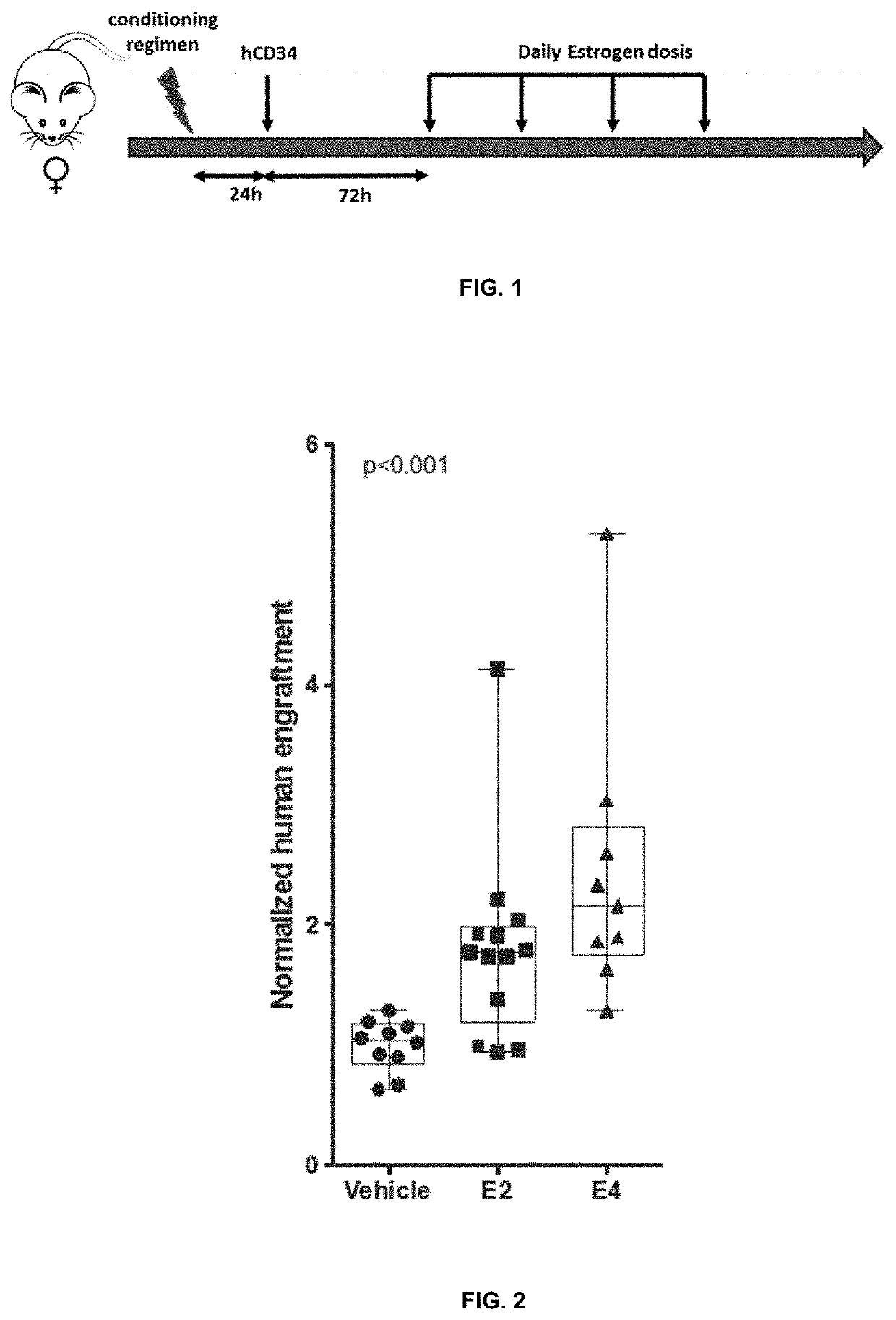
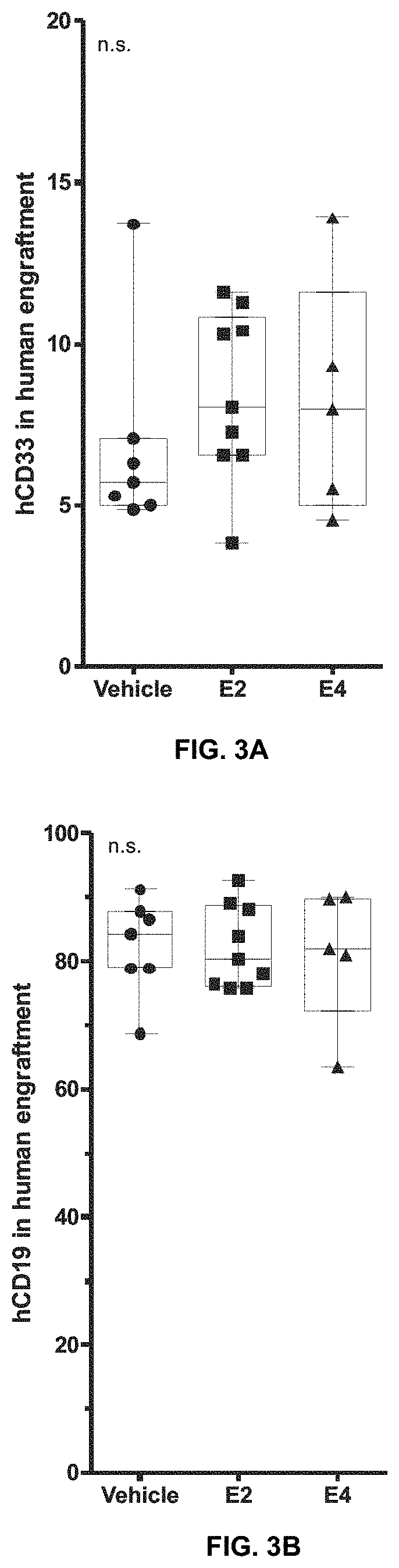
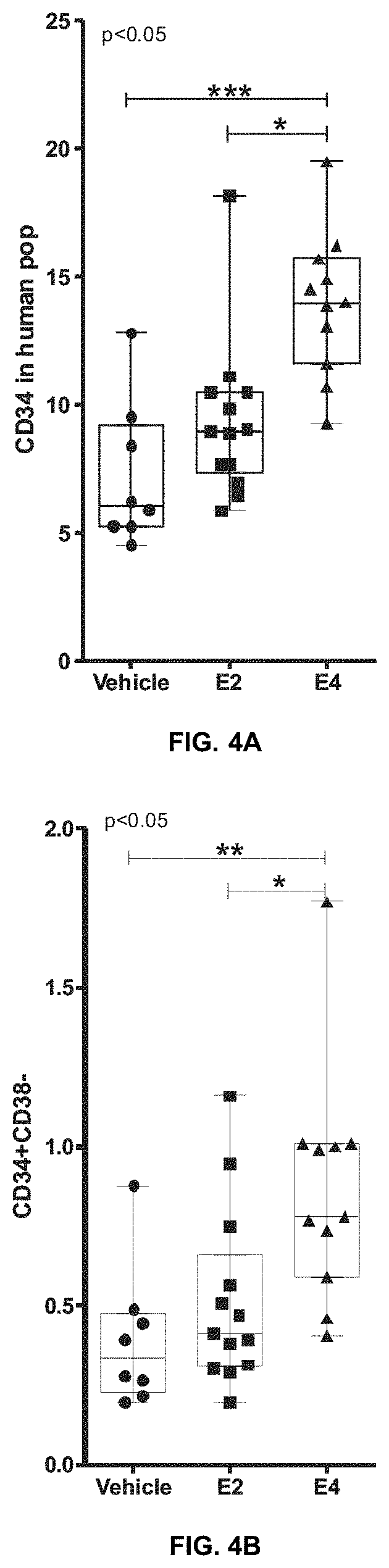
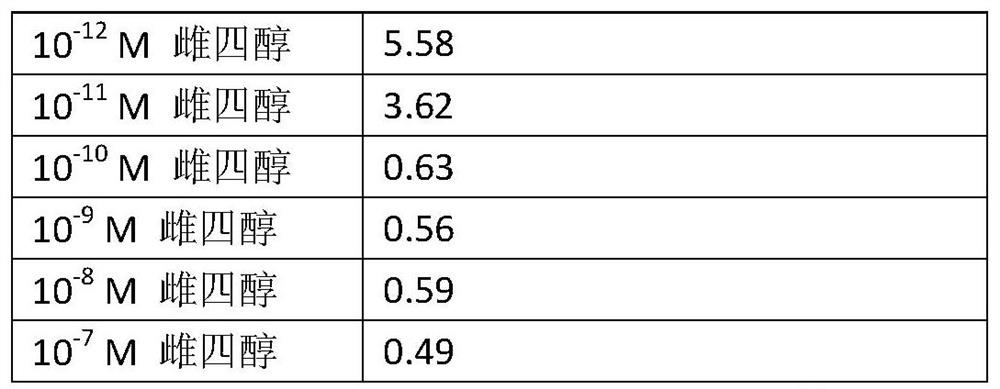
Improvements for performing and facilitating the recovery after hematopoietic stem cell transplantation
PendingUS20210386756A1Enhancing hematopoietic reconstitutionReduction of the apheresis cyclesOrganic active ingredientsSkeletal/connective tissue cellsHematopoietic cellHematopoietic progenitor
A method enhances hematopoietic reconstitution and recovery after hematopoietic stem cell transplantation, which is based on the administration of estetrol to the subject that has undergone the transplantation, because estetrol induced an increment in the percentage of hematopoietic cells derived from transplanted donor cells in the recipient. Additionally, estetrol increases the donor contribution in the hematopoietic stem cell compartment. A method also increases the number of hematopoietic progenitor or stem cells in a culture, based as well in the addition of estetrol to the culture. As the obtained hematopoietic progenitor or stem cells can also be transplanted, the method increases the availability of donor cells for transplantation. Thus, hematopoietic stem cell transplantation is improved in patients.
Owner:CENT DE INVESTIGACIONES ENERGETICAS MEDIOAMBIENTALES Y TECNOLOGICAS O A M P CIEMAT +2
Treatment of advanced estrogen receptor positive breast cancer
PendingCN112351782AOrganic active ingredientsPharmaceutical delivery mechanismEnzyme Inhibitor AgentAromatase inhibitor
The invention relates to the treatment of advanced estrogen receptor positive breast cancer in a subject who has been treated with an estrogen activity suppressor selected from a selective estrogen receptor modulator (SERM), an aromatase inhibitor and an anti-estrogen, said treatment comprising administration of an estetrol componentafter the treatment with an estrogen activity suppressor has beendiscontinued, said estetrol component being selected from estetrol, prodrugs of estetrol and combinations thereof.
Owner:ESTETRA S P R L
Process for the production of estetrol intermediates
ActiveUS11053274B2High yieldLow costOrganic active ingredientsEstrane derivativesAcetic acidChemical compound
The present invention relates to a process for the preparation of a compound of formula (I) said process comprising the steps of: a) reacting a compound of formula (II), with an acylating or a silylating agent to produce a compound of formula (III), wherein P1 and P2 are each independently a protecting group selected from R2—Si—R3R4, or R1CO—, wherein R1 is a group selected from C1-6alkyl or C3-6cycloalkyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; R2, R3 and R4 are each independently a group selected from C1-6alkyl or phenyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; b) reacting the compound of formula (III) in the presence of palladium acetate or a derivative thereof to produce compound of formula (IV); and c) reacting the compound of formula (IV) with a reducing agent to produce compound of formula (I).
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Contraceptive composition with reduced cardiovascular effects
The present invention relates to a contraceptive method with reduced cardiovascular effects, such as reduced thromboembolism risk, such as reduced venous thromboembolism (VTE) risk and reduced aorticthromboembolism (ATE) risk. The method of the invention comprises administering to a female mammal an effective amount of an estetrol component in combination with a progestogenic component. The method enjoys a favourable profile for thromboembolism compared to currently available methods which employs contraceptives from the so-called second, third or fourth generation.
Owner:ESTETRA S P R L
Use of estetrol as emergency contraceptive
ActiveUS20140200202A1Improve bioavailabilityHigh oral absorptionOrganic active ingredientsBiocideOral medicationEmergency Contraceptives
The present invention relates to a new use of tetrahydroxylated estrogens such as estetrol (1,3,5(10)-estratrien-3,15α,16α,17β-tetrol), namely in a method of emergency contraception. The method of emergency contraception according to the invention comprises the oral administration of estetrol in a single dose within 120 hours of sexual intercourse.
Owner:ESTETRA SRL
Compounds and their uses for alleviating menopause-associated symptoms
PendingCN112020360AAvoid exposureQuick effectPharmaceutical delivery mechanismSexual disorderSide effectHormone replacement
The present invention relates to a hormone replacement therapy, to the associated compounds and to the associated packaging units,for alleviating menopause-associated symptoms which is based on the administration to a female mammal of an estetrol component at specified daily doses, optionally in combination with a progestogenic component. The therapy enjoys a statistically significant efficacy combined with a favourable profile for side effects compared to currently available methods for alleviating menopause-associated symptoms.
Owner:ESTETRA S P R L
Process for the preparation of estetrol
ActiveUS10844088B2Estrane derivativesSteroids preparationCombinatorial chemistryPerylene derivatives
Owner:MITHRA R&D SA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com