Patents
Literature
102results about "Estrane derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzopyran-containing compounds and method for their use
InactiveUS6060503AAvoid conversionEasy to synthesizeBiocideOrganic compound preparationDiseaseBenzopyran
Certain benzopyran antiestrogens are disclosed for treating estrogen sensitive diseases such as breast cancer. Prodrug forms provide ease of manufacturing, good shelf life, and bioavailability, and preferred stereoisomers are shown to be more effective than racemic mixtures.
Owner:ENDORES & DEV
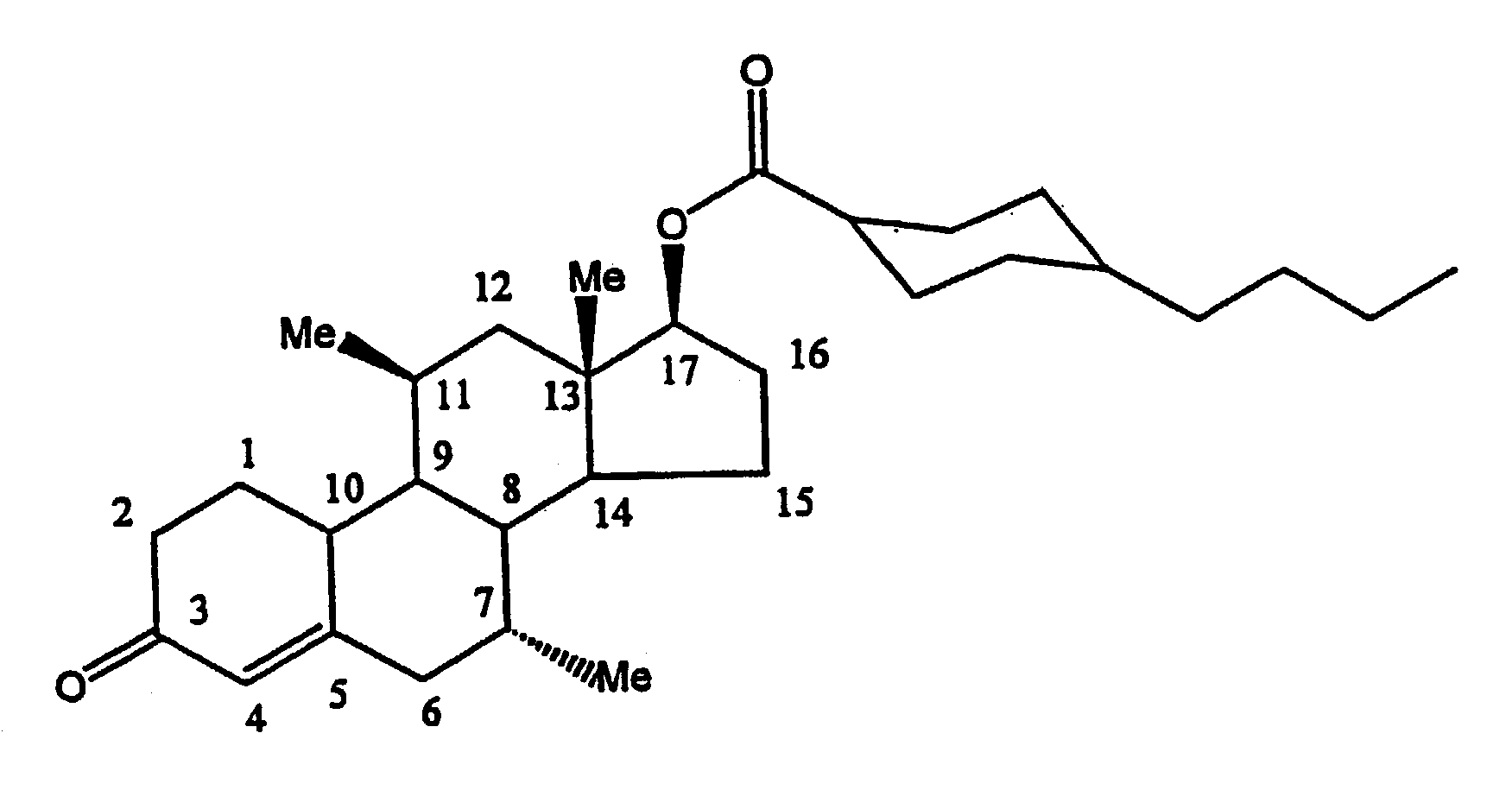
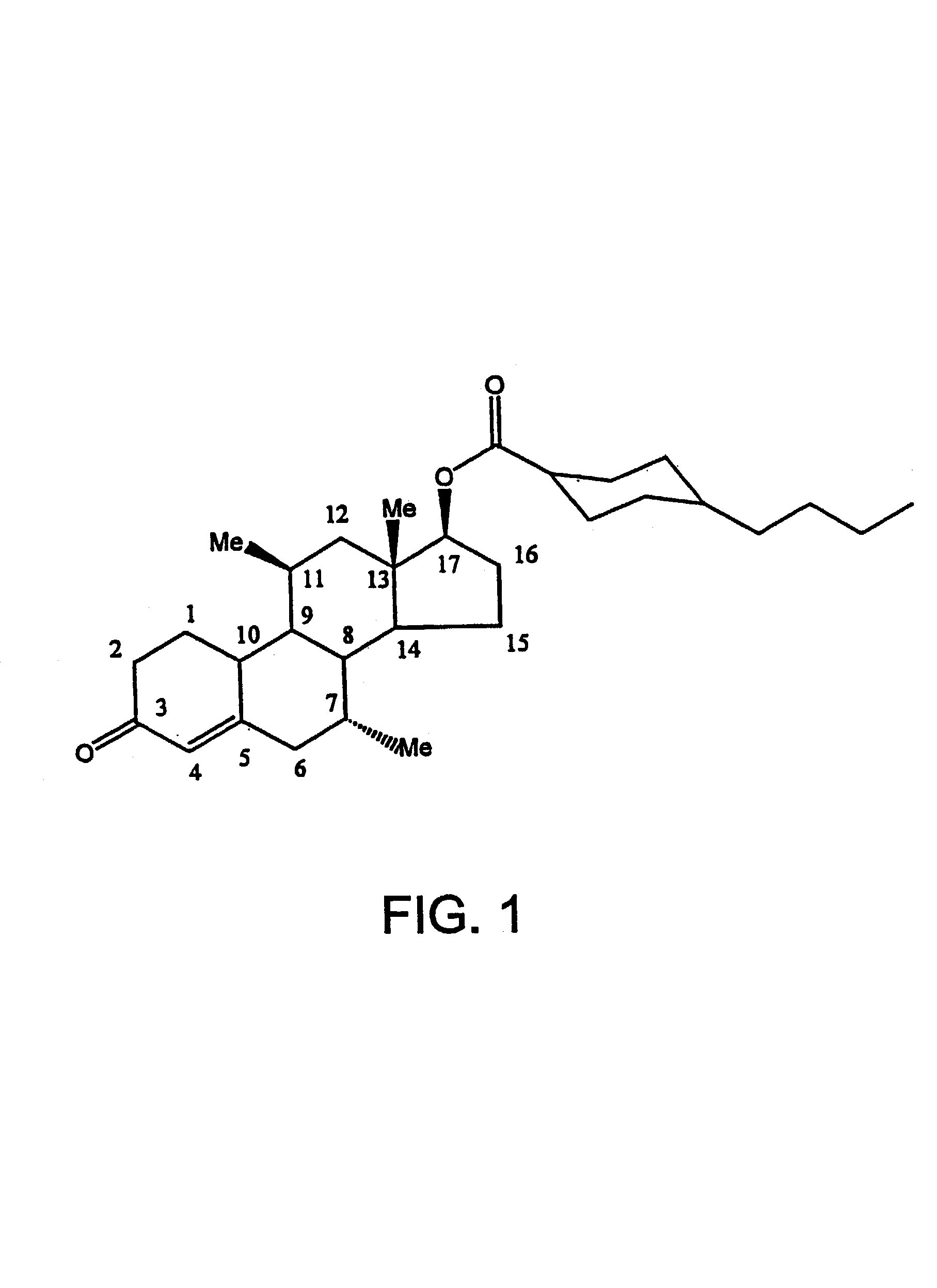
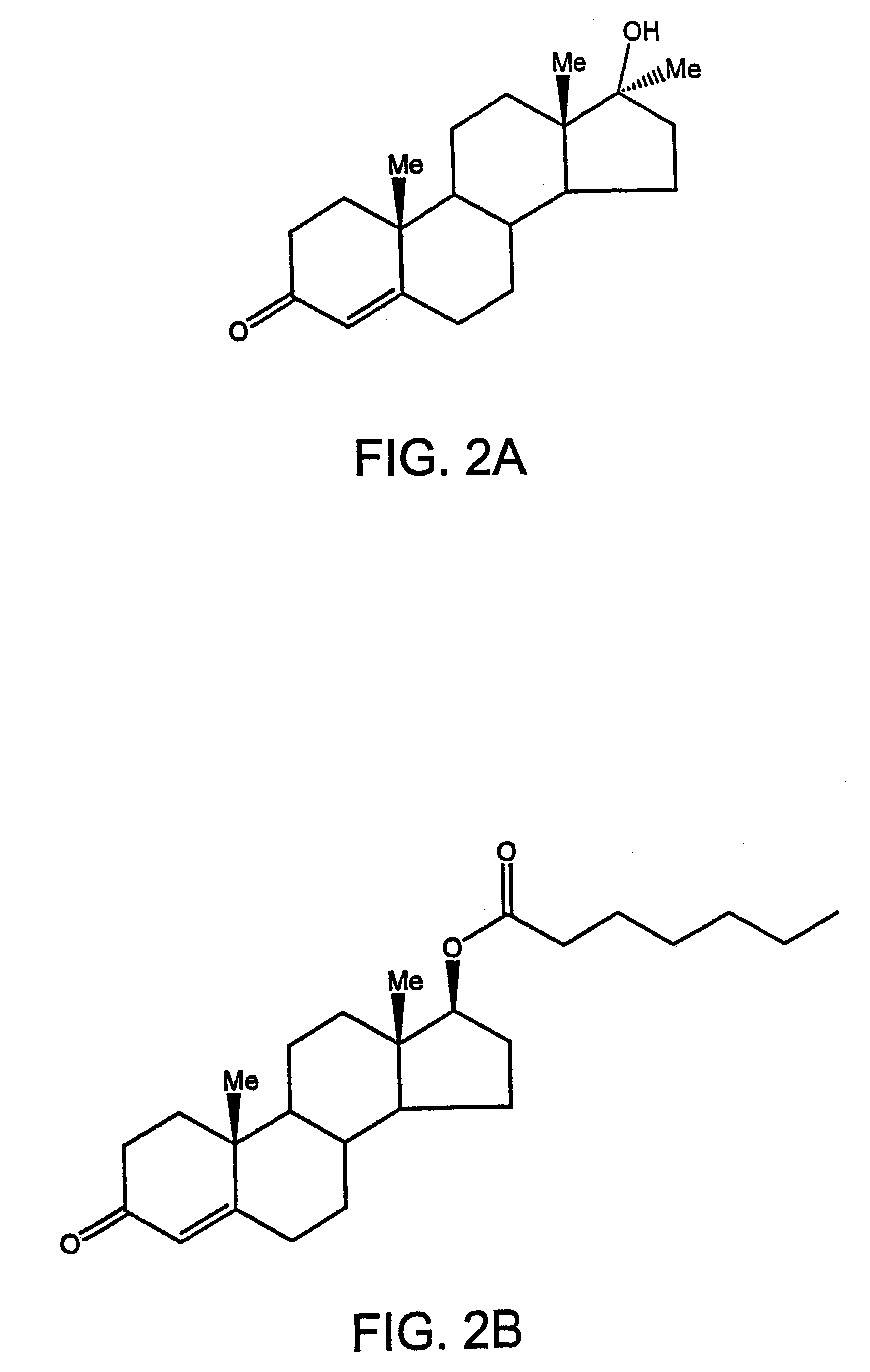
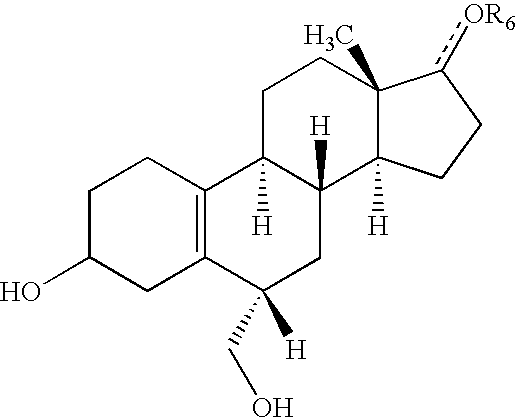
Methods of making, using and pharmaceutical formulations comprising 7alpha, 11beta-dimethyl-17beta-hydroxyestra-4, 14-dien-3-one and 17 esters thereof
InactiveUS7196074B2High yieldPositively affectingBiocideOrganic active ingredientsParenteral Dosage FormKetone
Owner:US DEPT OF HEALTH & HUMAN SERVICES
19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof
ActiveUS9512165B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderSubstance abuserWithdrawal syndrome
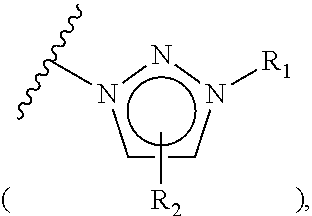
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Androgen derivatives for use in the inhibition of sex steroid activity
InactiveUS6110906AUseful in treatmentInhibiting sex steroid activityBiocidePhosphorous compound active ingredientsDiseaseAndrogen synthesis
Methods for treating sex steroid-dependent diseases by inhibiting sex steroid activity include administration of novel compounds which include an androgenic nucleus substituted at a ring carbon with at least one substituent specified herein. Such compounds may function by inhibiting sex steroid synthesis (both estrogen and androgen synthesis) and / or by antagonistically blocking androgen receptors.
Owner:ENDORES & DEV
19-nor c3, 3-disubstituted c21-n-pyrazolyl steroids and methods of use thereof
ActiveUS20160108080A1Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderWithdrawal syndromeSubstance abuse disorder
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein-, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful NI for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
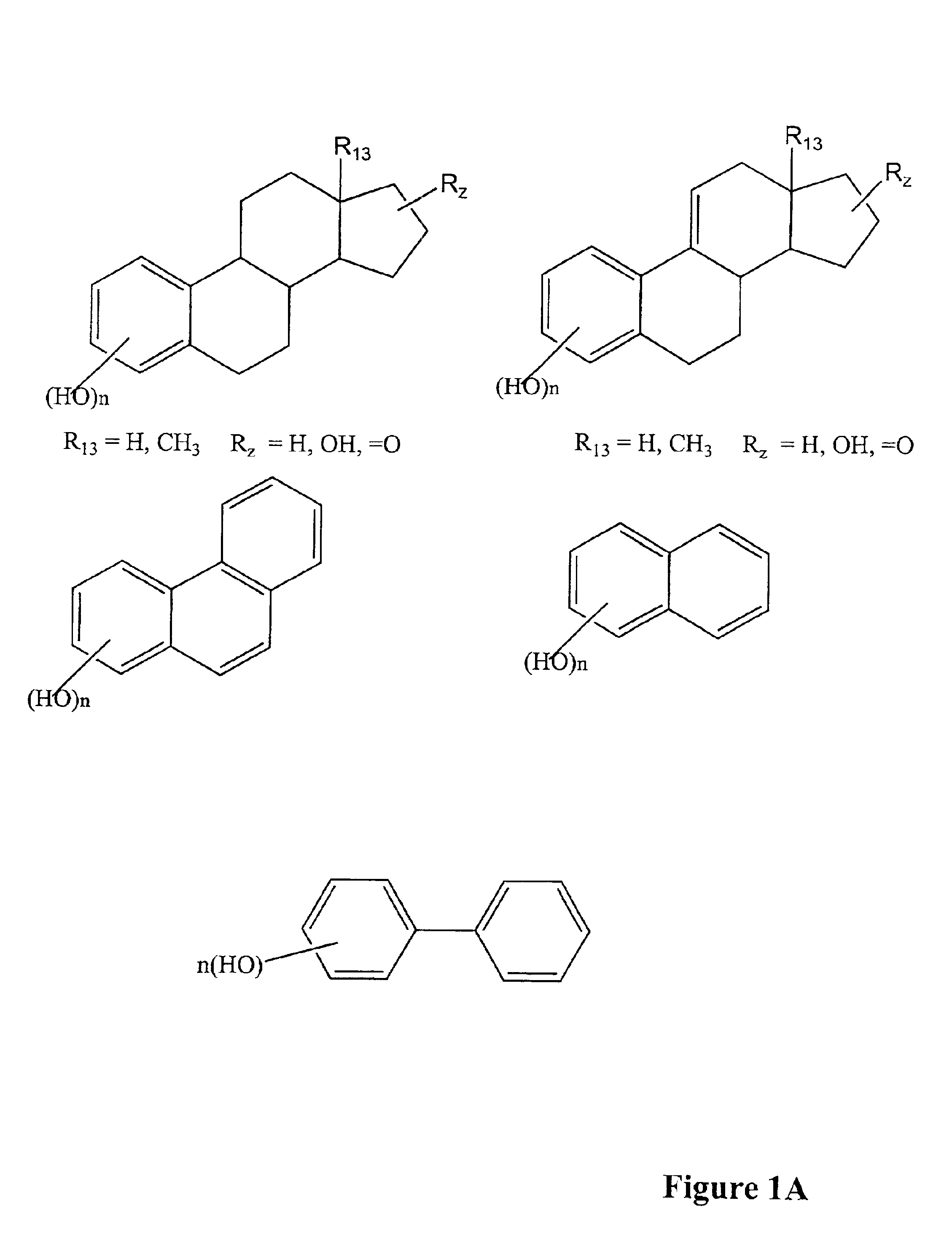
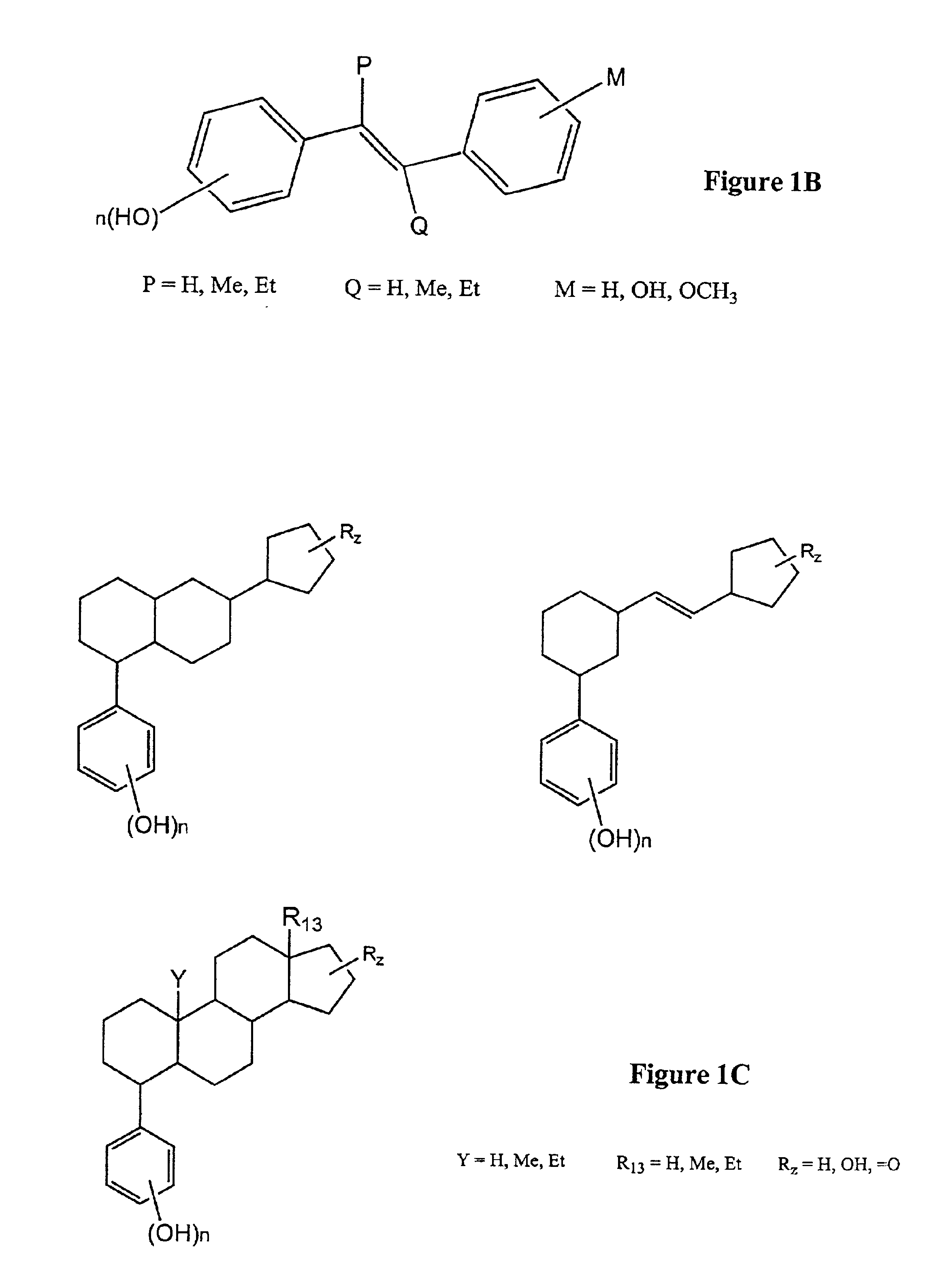
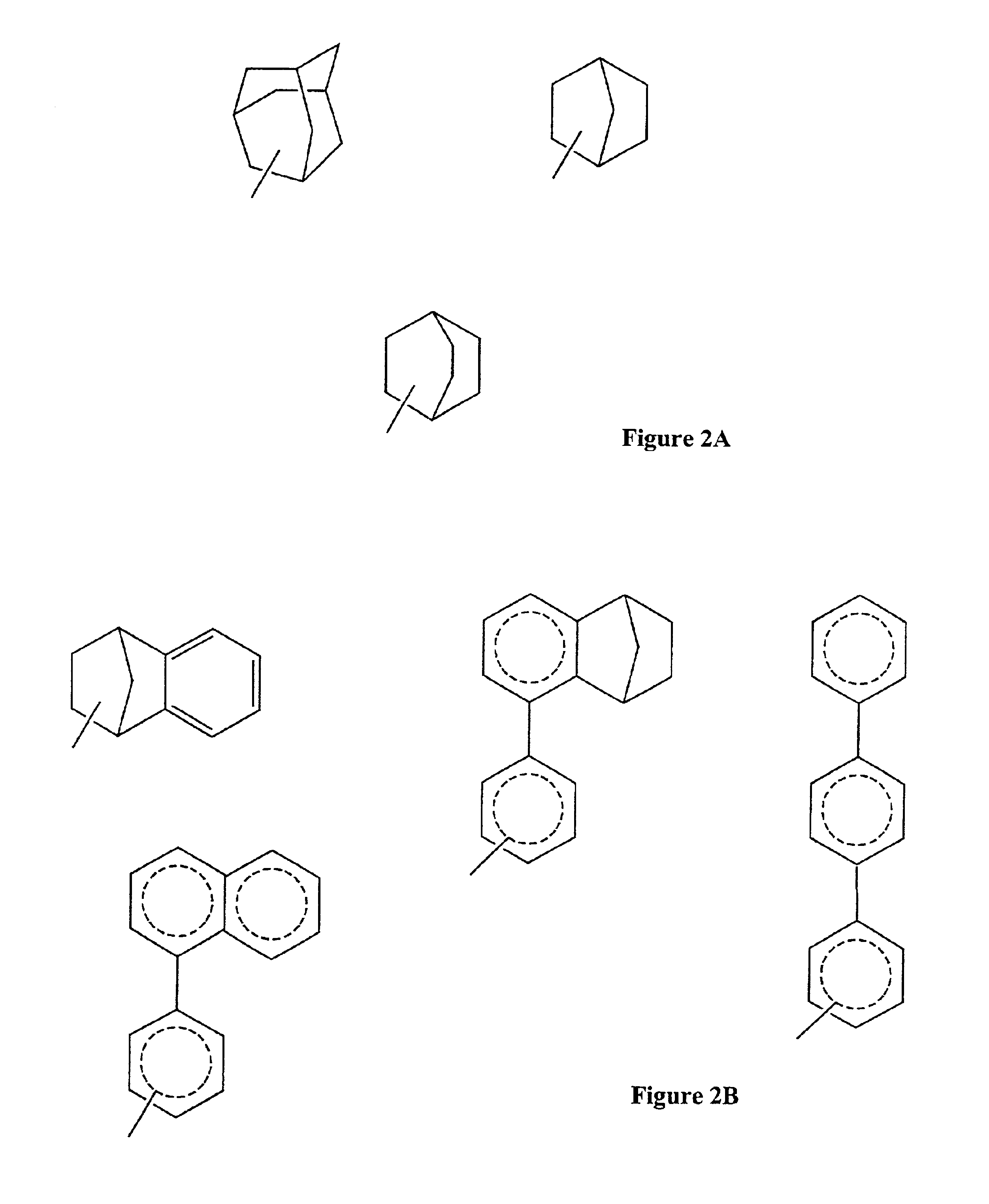
Modified, hydroxy-substituted aromatic structures having cytoprotective activity
The present invention is directed to modified, hydroxy-bearing aromatic ring structures having cytoprotective activity. More specifically, in a first embodiment the present invention is directed to phenolic compounds, and in particular steriods (e.g., estrogens), wherein a non-fused polycyclic, hydrophobic substituent is attached to the hydroxy-substituted A-ring thereof. The present invention is further directed to a process for conferring cytoprotection to a population of cells involving the administration of the compound.
Owner:WASHINGTON UNIV IN SAINT LOUIS
19-nor C3, 3-disubstituted C21-C-bound heteroaryl steroids and methods of use thereof
ActiveUS9725481B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsNervous disorderSubstance abuserWithdrawal syndrome
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5-to6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
19-nor neuroactive steroids and methods of use thereof
InactiveUS20160068563A1Eliminate potential for oxidationImprove bioavailabilityNervous disorderEstrane derivativesSubstance abuserWithdrawal syndrome
Provided herein are 3,3-disubstituted 19-nor-steroidal compounds according to Formula (I): and pharmaceutical compositions thereof. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, tinnitus, status epilepticus.
Owner:SAGE THERAPEUTICS
6-substituted estradiol derivatives and methods of use
InactiveUS20100130463A1Improved prognosisImprove complianceOrganic active ingredientsEstrane derivativesEnantiomerPhosphate
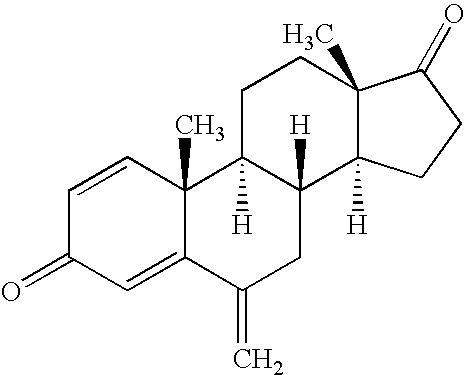
Disclosed are compounds of the formula:wherein R1, R2, R3 and R4 are independently hydrogen, C1-C6 alkyl, halo, a sulfate, a glucuronide, —OH, a bulky group, aryl, cycloalkyl, heteroaryl, heterocycloalkyl, —N(CH2)n; a phosphate group, and a phosphinate group; R9 is hydrogen, halogen or alkyl; R11 is selected from the group consisting of H, C1-C6 alkyl, halogen, a sulfate, a glucoronide, —SO2NH2, —COOH, —CN, —CH2CN—, —NHCN—, —CHO, ═CHOCH3, —COO salt, —OSO2alkyl, —NH2, and —NHCO(CH2)n; R12 is selected from the group consisting of H, a C1-C6 alkyl, a sulfate, a glucoronide, a bulky group, aryl, cycloalkyl, heteroaryl and heterocycloalkyl; X is selected from the group consisting of C1-C12 alkyl, C2-C12 alkenyl, C2-C12 alkynyl, halogen, a glucoronide, —NH2, —SO2NH2, —COOH, —CN, —CH2CN, —NHCN, —CHO, —COOsalt, —OSO2alkyl, —SH, —SCH3, —CH[(CH2)nCH2]COOCH2, —(CH2)mCOOCH3, —(CH2)m—O—CH3, —(CH2)m—O—(CH2)nCH3, (CH2)m—S—CH3, —(CH2)m—S—(CH2)nCH3, —(CH2)m—NH—(CH2)nCH3, —C2-C8 alkenyl-O—(CH2)nCH3, —C2-C8 alkenyl-S—(CH2)nCH3, —C2-C8 alkenyl-N—(CH2)nCH3, —C2-C8 alkynyl-O—(CH2)nCH3, —C2-C8 alkynyl-S—(CH2)nCH3, —C2-C8 alkynyl-N—(CH2)nCH3, —(CH2)m—OH, —(CH2)m—O—NH2, —(CH2)m—S—NH2, —NH(CH2)mCH3, —NH(CH2)mOCH3, —NH(CH2)mCHOH—COOH, —N(CH3)2, —(CH2)m(NH)CH2OH, —NHCOOH, —(CH2)mNHCOOH, —NO2, —SCN, —SO2alkyl, —B(OH)2, —(CH2)m N(CH3)—SO2—NH3, —(CH2)m—NH—SO2—NH2, —NHC(═S)CH3, and —NHNH2; and Y is selected from hydrogen, ═O, —OCO(R6) and —OH; wherein m is an integer between 0-20, n is an integer between 0-8, the symbol represents either a single or a double bond capable of forming a keto group at position 3 or 17; and the symbol represents any type of bond regardless of the stereochemistry; and the respective enantiomers, other stereochemical isomers, hydrates, solvates, tautomers and pharmaceutically acceptable salts of said compounds. The compounds are useful in the treatment of various types of cancer.
Owner:ENDECE LLC
Compositions comprising purified 2-methoxyestradiol and methods of producing same
2-methoxyestradiol having greater than 98% purity is obtained by synthetic or purification methods. This highly pure 2-methoxy estradiol, lacking estrogenic components, is particularly suitable for clinical use in humans. The purification methods of the invention involve the use of liquid-solid chromatography (LSC) to separate 2-ME2 from other compounds. The chromatographic media is preferably silica. The solvent system comprises a non-polar solvent, such as chloroform, and a polar solvent, such as methanol.
Owner:CASI PHARMA
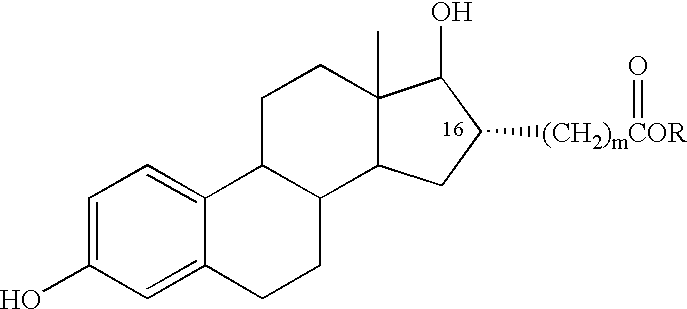
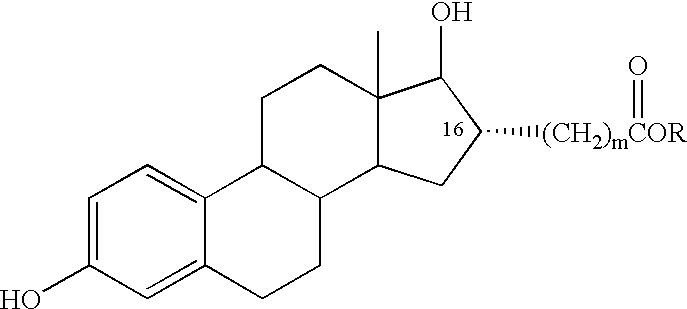
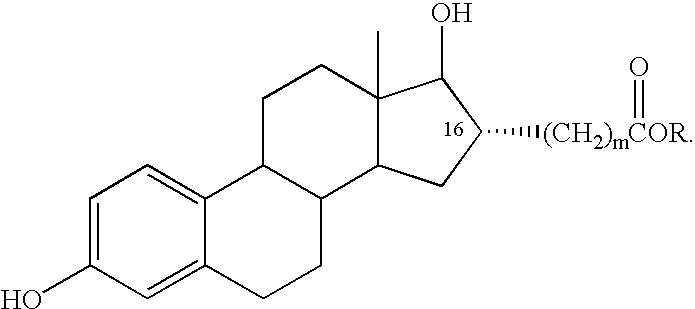
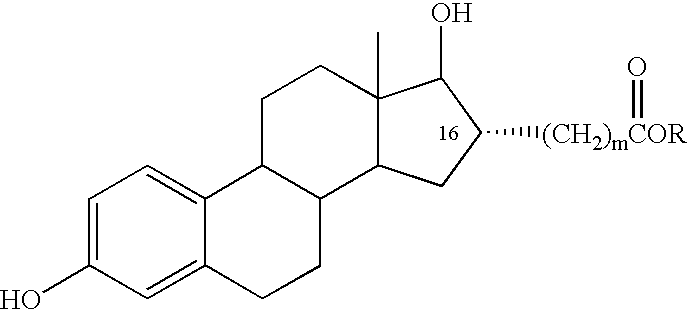
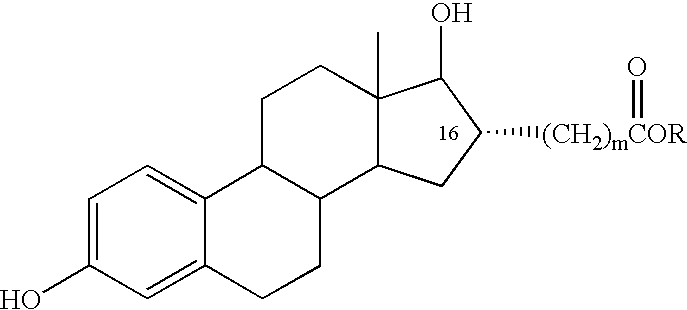
Estradiol-16alpha-carboxylic acid esters as locally active estrogens
InactiveUS6476012B2Limiting deleterious effectBig advantageOrganic active ingredientsBiocideCarboxylic acidDrug biological activity
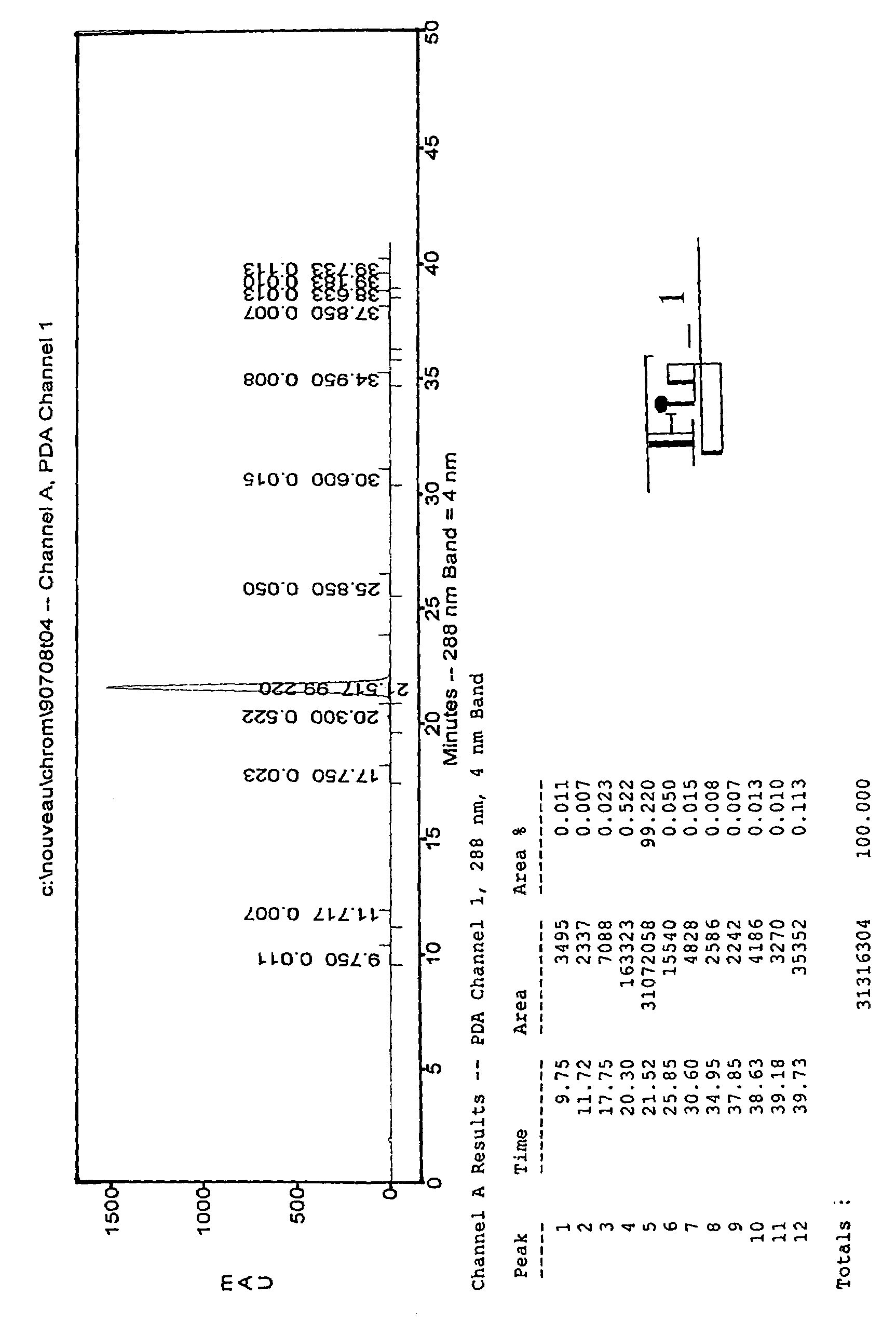
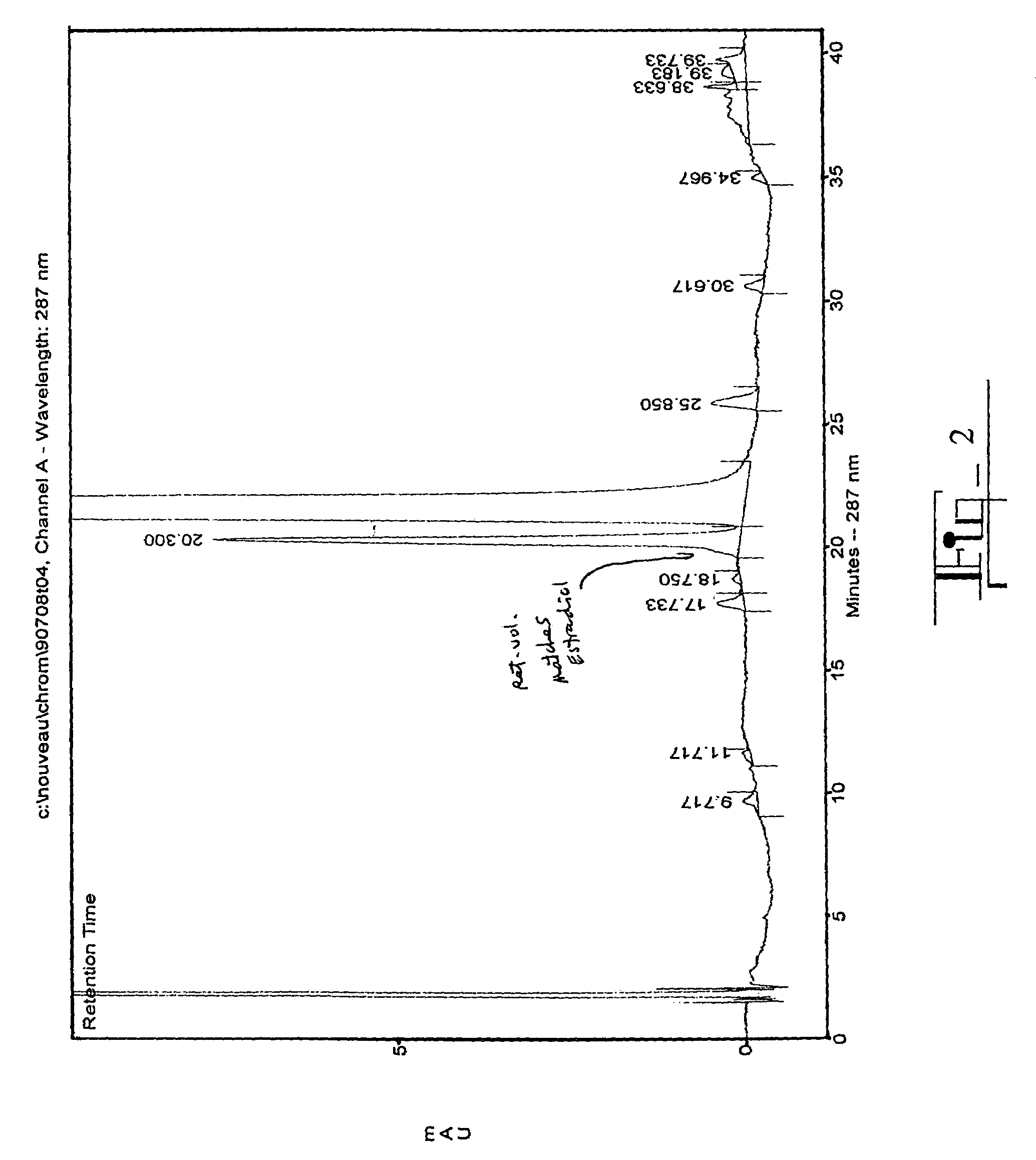
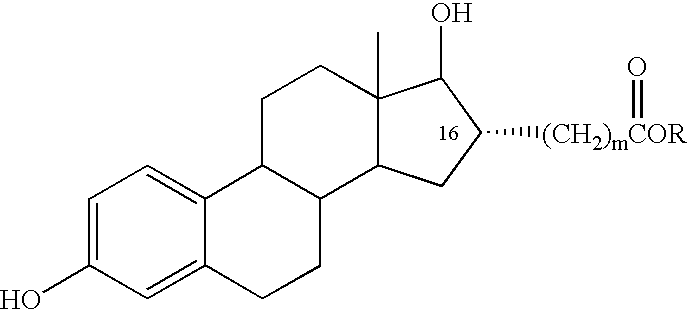
The present invention relates to analogs of estradiol, which, in their most preferred embodiment, act as locally active estrogens without significant systemic action. A series of 16alpha-carboxylic acid substituted steroids and their esters is presented which exhibit excellent biological activity for use in pharmaceutical compositions for the treatment of symptomology associated with menopause. The present invention is therefore directed to compounds according to the structure:Where R is H, a C1 to C5 alkyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3; andm is from 0-2, or a pharmaceutically acceptable salt thereof. Preferably, R is methyl, ethyl, propyl, iso-propyl, butyl, iso-butyl, pentyl, neo-pentyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0. More preferably, R is methyl, ethyl, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0.
Owner:YALE UNIV
Methods of using 2-methoxyestradiol of high purity
2-methoxyestradiol having greater than 98% purity is obtained by synthetic or purification methods. This highly pure 2-methoxy estradiol, lacking estrogenic components, is particularly suitable for clinical use in humans. The purification methods of the invention involve the use of liquid-solid chromatography (LSC) to separate 2-ME2 from other compounds. The chromatographic media is preferably silica. The solvent system comprises a non-polar solvent, such as chloroform, and a polar solvent, such as methanol.
Owner:CASI PHARMA
19-nor c3, 3-disubstituted c21-c-bound heteroaryl steroids and methods of use thereof
ActiveUS20160083417A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsNervous disorderDiseaseSubstance abuser
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5- to 6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
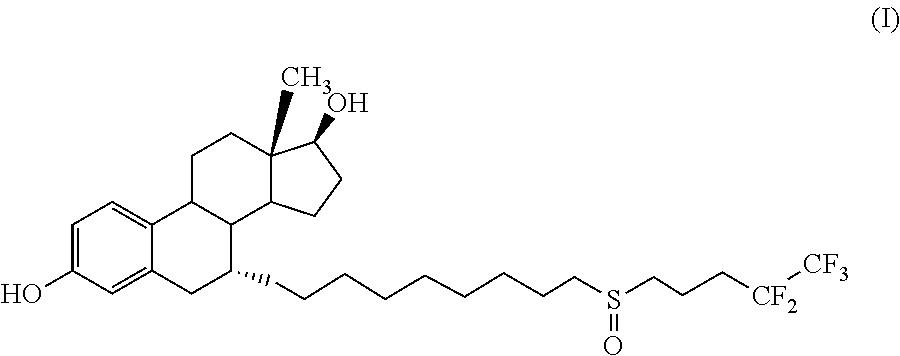
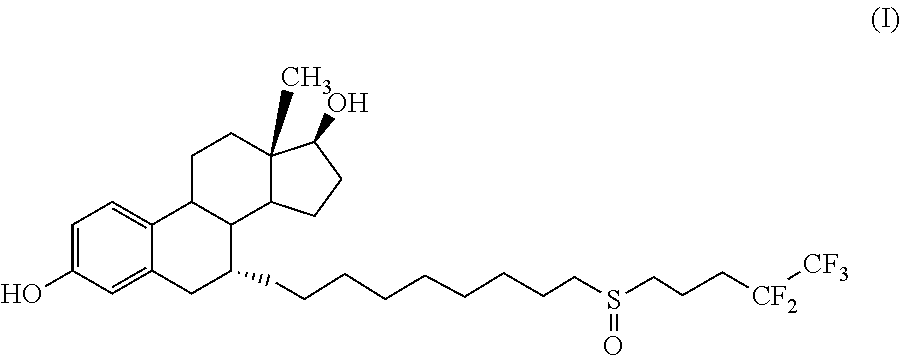
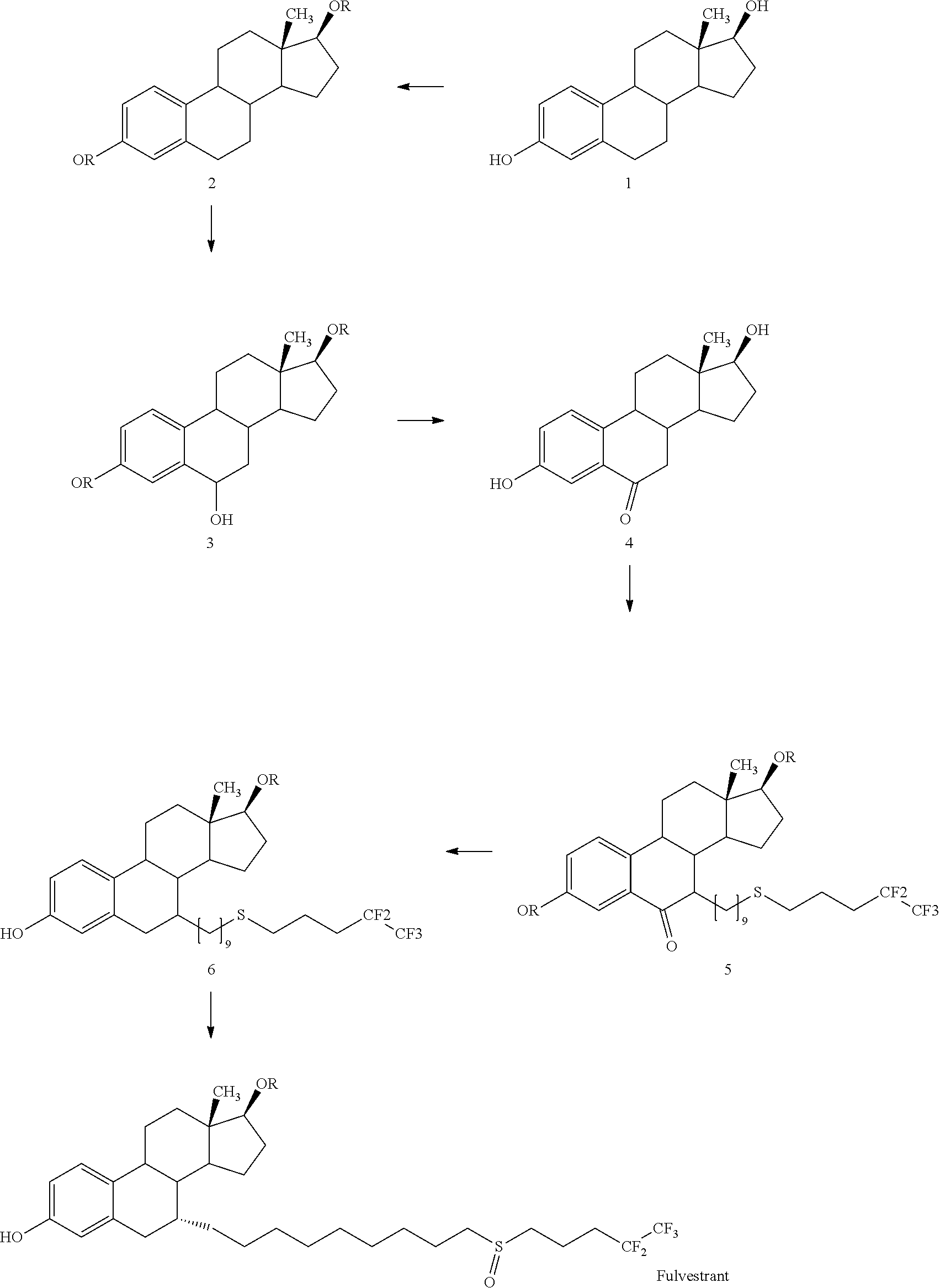
Process for the preparation of fulvestrant
The present invention relates to an improved process for the preparation of Fulvestrant (I). Also, provided is novel intermediate of Fulvestrant and a process for the preparation of the same.
Owner:INTAS PHARM LTD
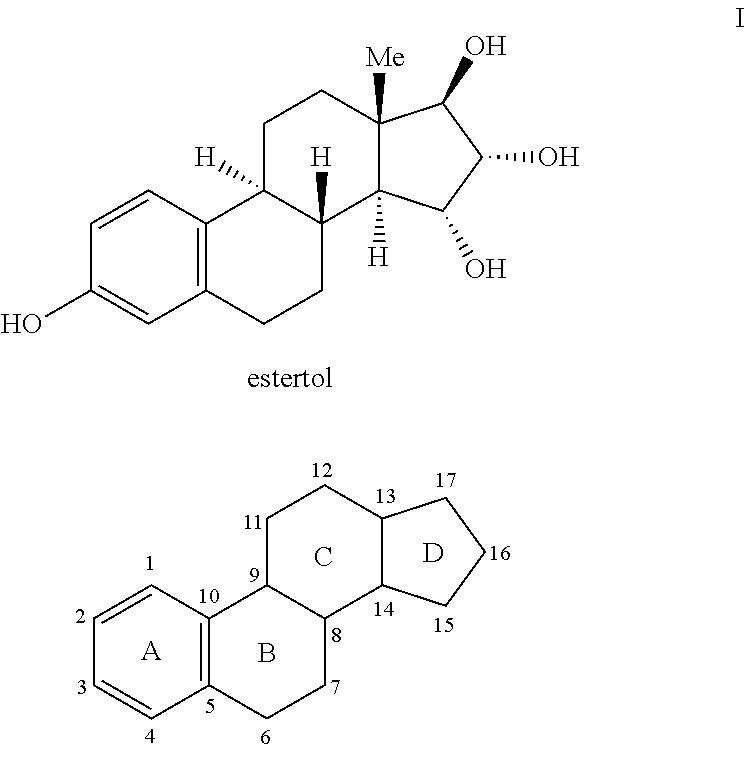
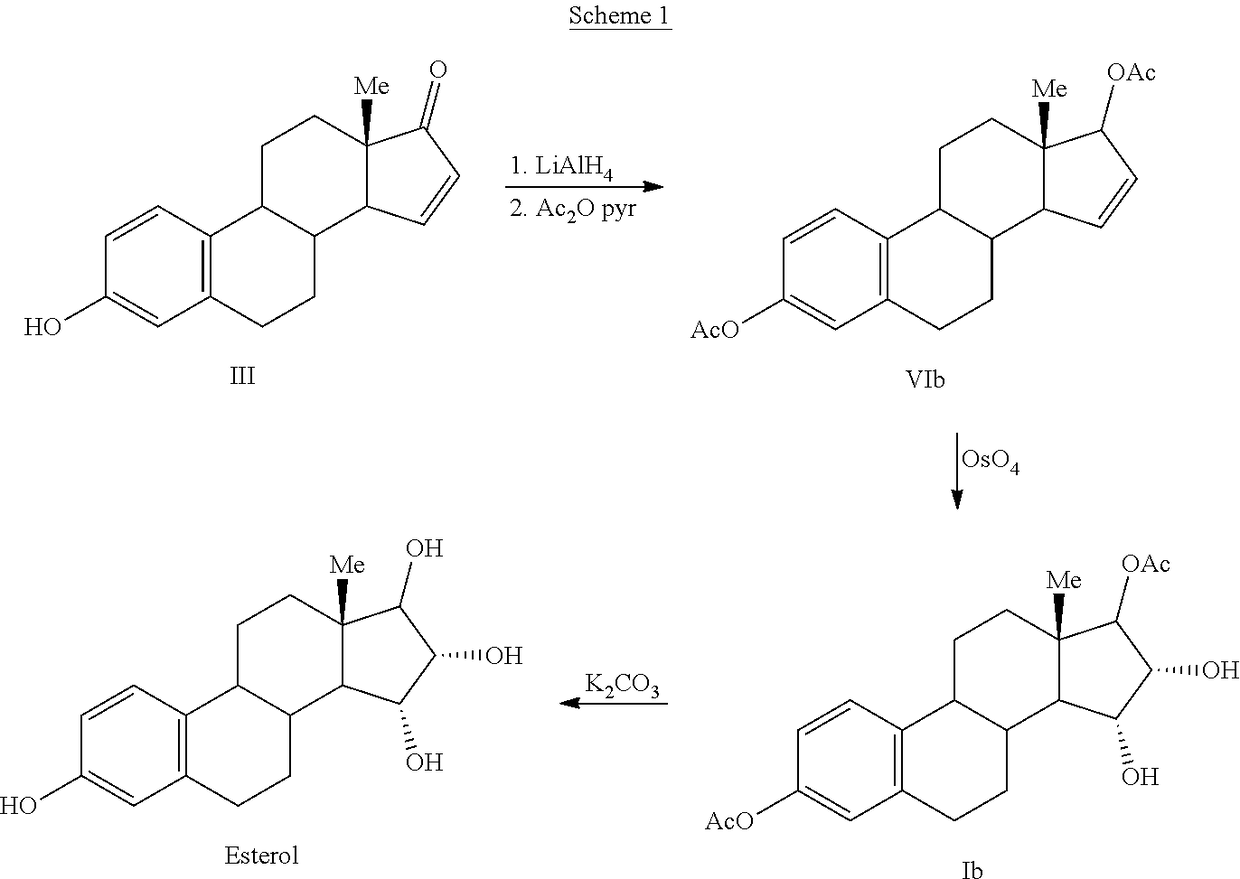
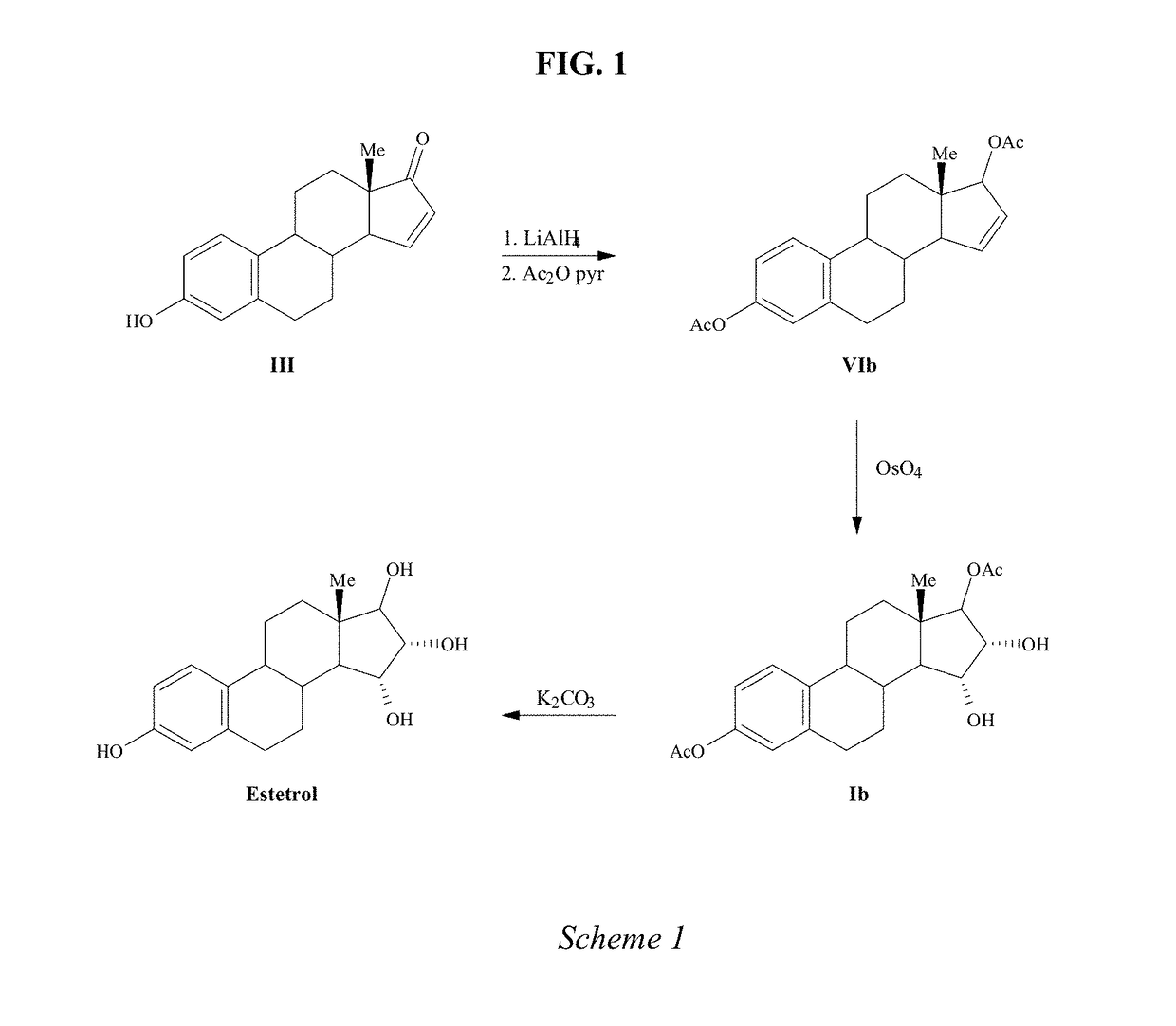
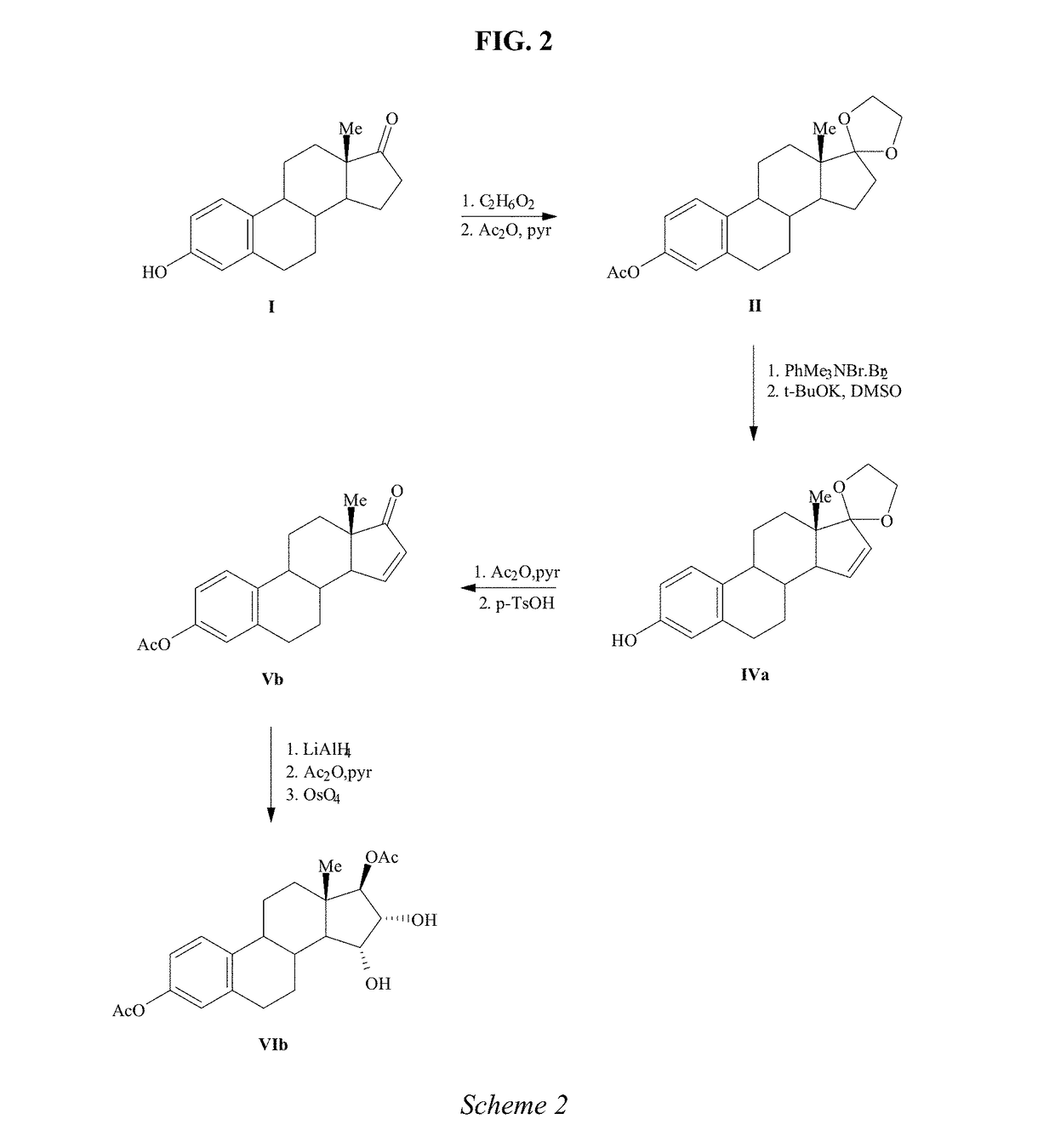
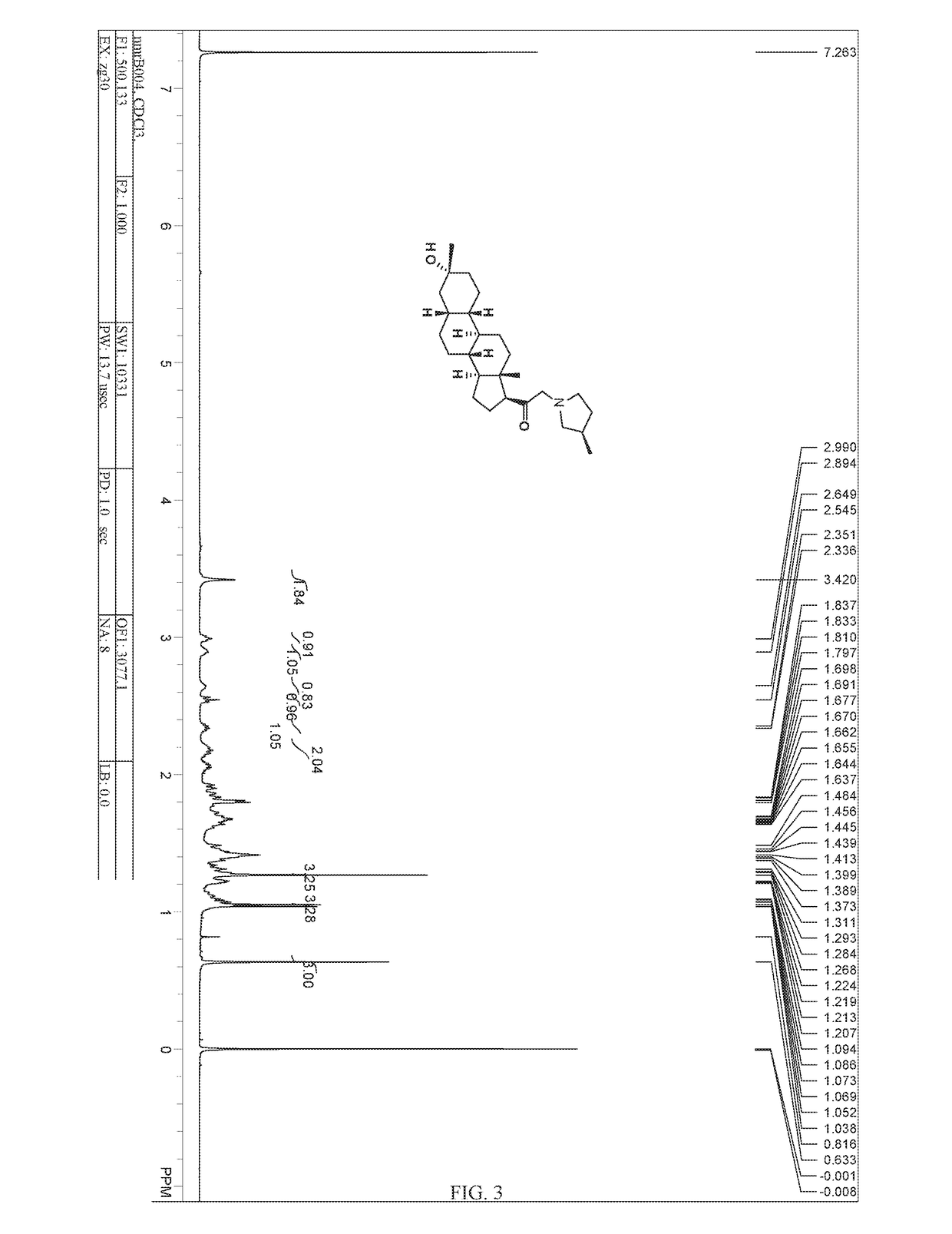
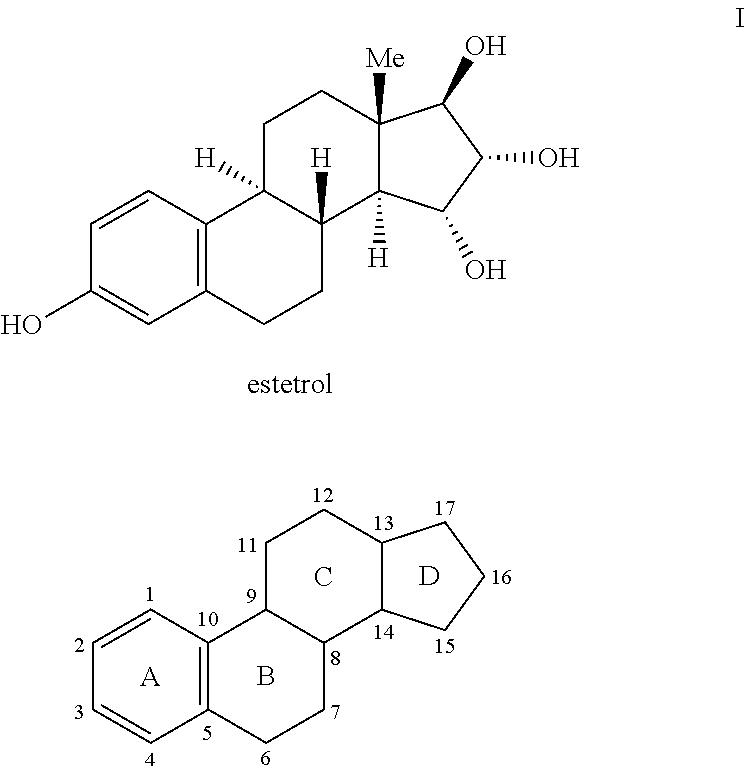
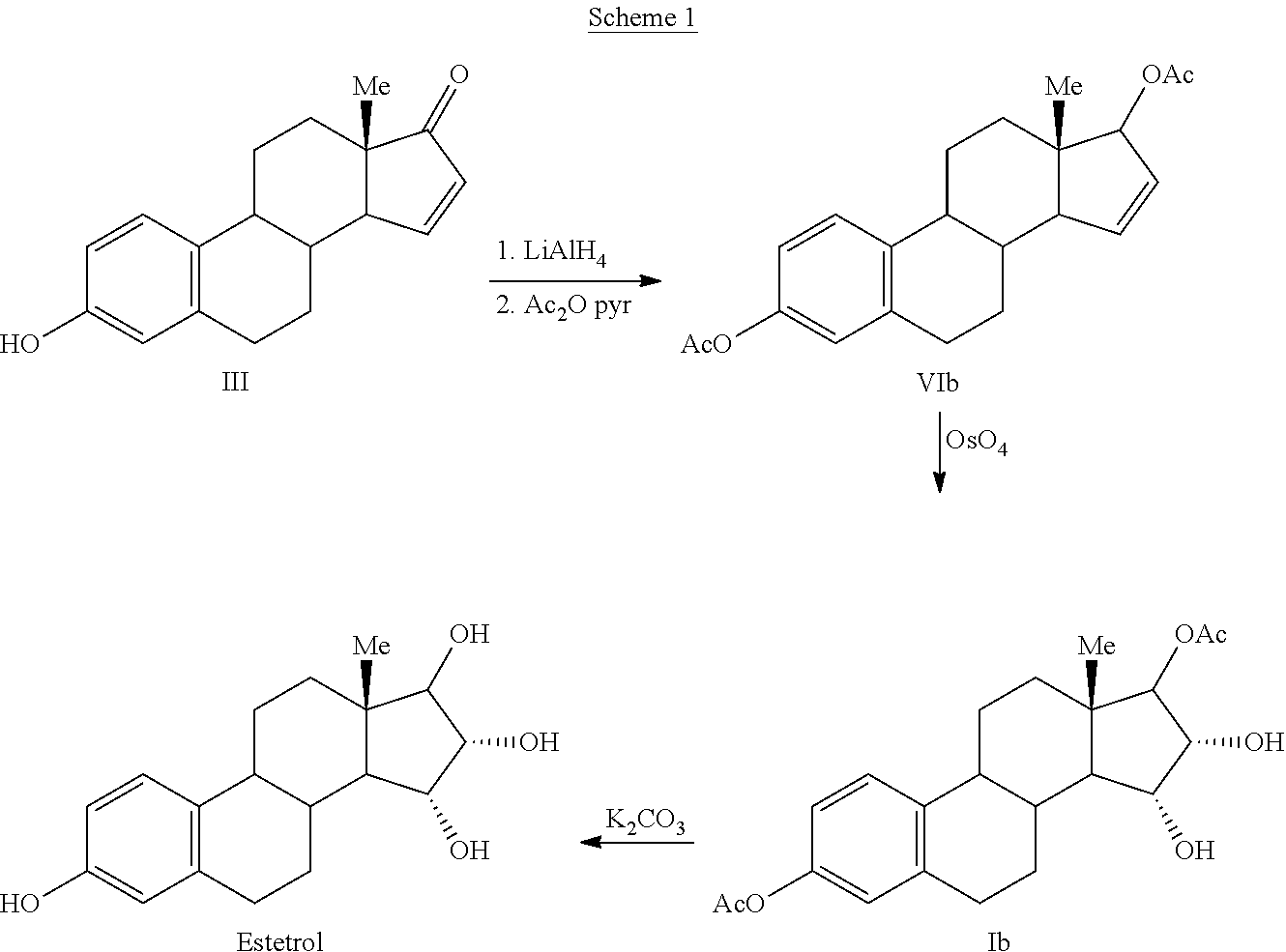
Process for the preparation of estetrol
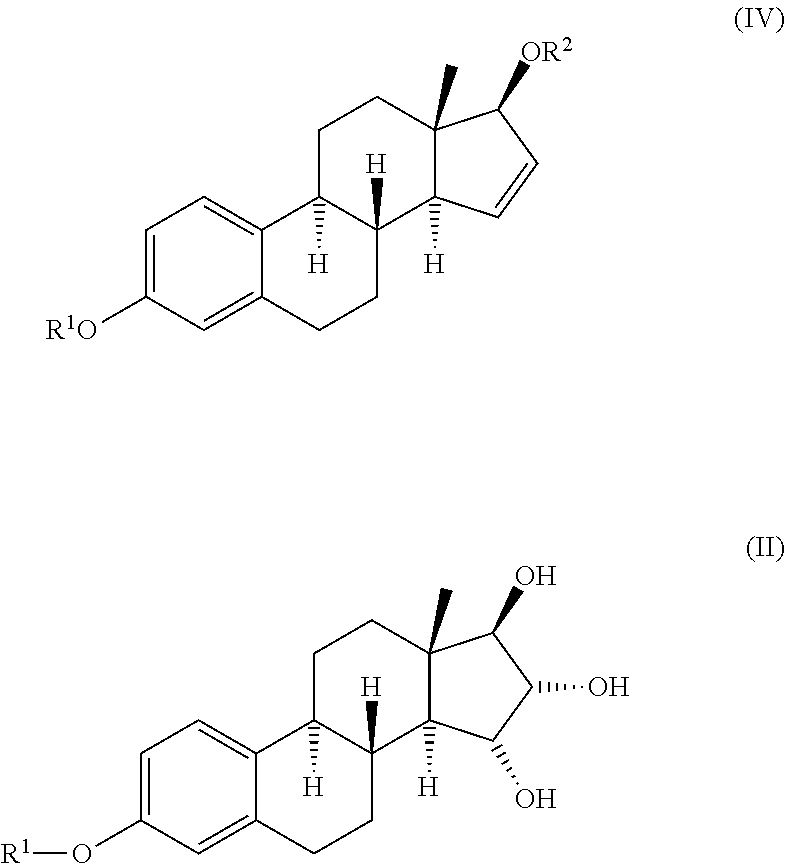
The invention relates to a process for obtaining Estetrol or a salt or solvate thereof, the process comprising: a) reacting a compound of formula (IV) or a salt or solvate thereof, wherein R1 is a hydroxyl protecting group selected from a silyl ether, an ether, an ester, a carbamate and a carbonate, and R2 is a hydroxyl protecting group selected from an ether, with an oxidizing agent selected from OsO4 or a source of osmium tetroxide to produce Estetrol or a compound of formula (II) or a salt or solvate thereof wherein R1 is as defined previously; and b) if a compound of formula (II) is obtained in step a), deprotecting said compound to produce Estetrol.
Owner:CRYSTAL PHARMA SA
Process for the preparation of estetrol
Owner:MITHRA R&D SA +1
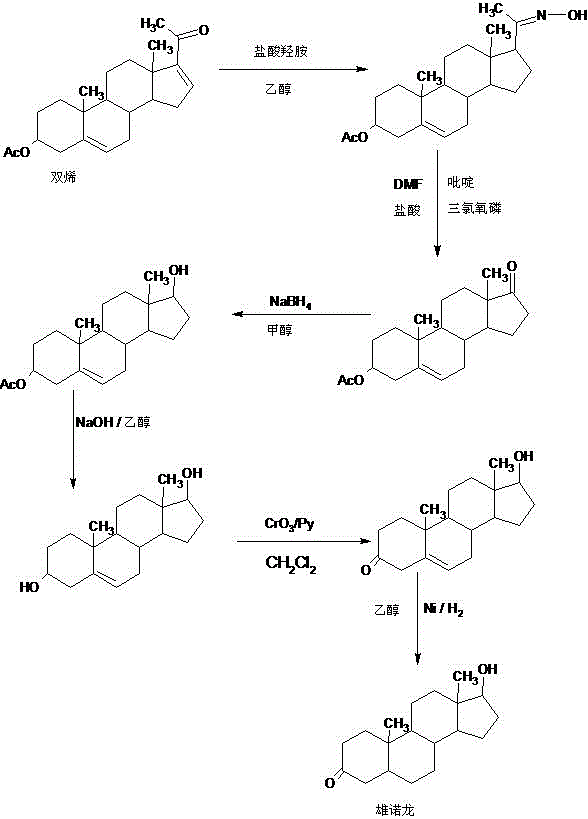
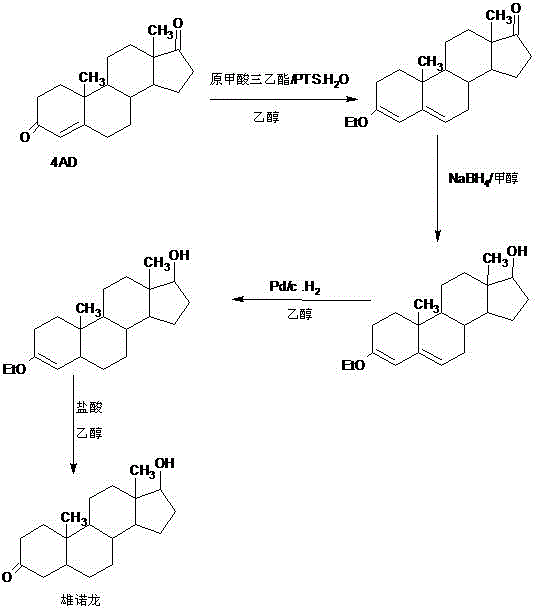
Preparation method of stanolone
InactiveCN106496297AWide variety of sourcesReduce manufacturing costEstrane derivativesDouble bondHydrolysis
A preparation method of stanolone comprises the following steps: with 4-androstenedione (shortened as 4AD) as a raw material, firstly performing an acid-catalyzed reaction on the 4AD and triethyl orthoformate in an organic solvent to obtain etherate 3-ethoxy-androst-3,5-diene-17-one; then, adding the etherate into the organic solvent, adding metal borohydride as a reducing agent, and reducing a 17-one group in a molecule of the etherate into a hydroxyl group to obtain the 3-ethoxy-androst-3,5-diene-17-ol; then, dissolving a reduced product into the organic solvent, adding a hydrogenation reaction catalyst, selectively catalyzing a 5-position double bond in the molecule of the reduced product to obtain hydride 3-ethoxy-androst-3-ene-17-ol; finally, performing acid-catalyzed hydrolysis on the hydride in the organic solvent to obtain the stanolone. Compared with the conventional production method, the preparation method provided by the invention has the advantages as follows: the raw material source is wide, the synthesis route is short, the preparation method is simple and environment-friendly in process, the product yield is high, the preparation method is economical and environment-friendly, the production cost is reduced by 35-40%, and the preparation method is very conductive to industrial production.
Owner:HUNAN KEREY BIOTECH
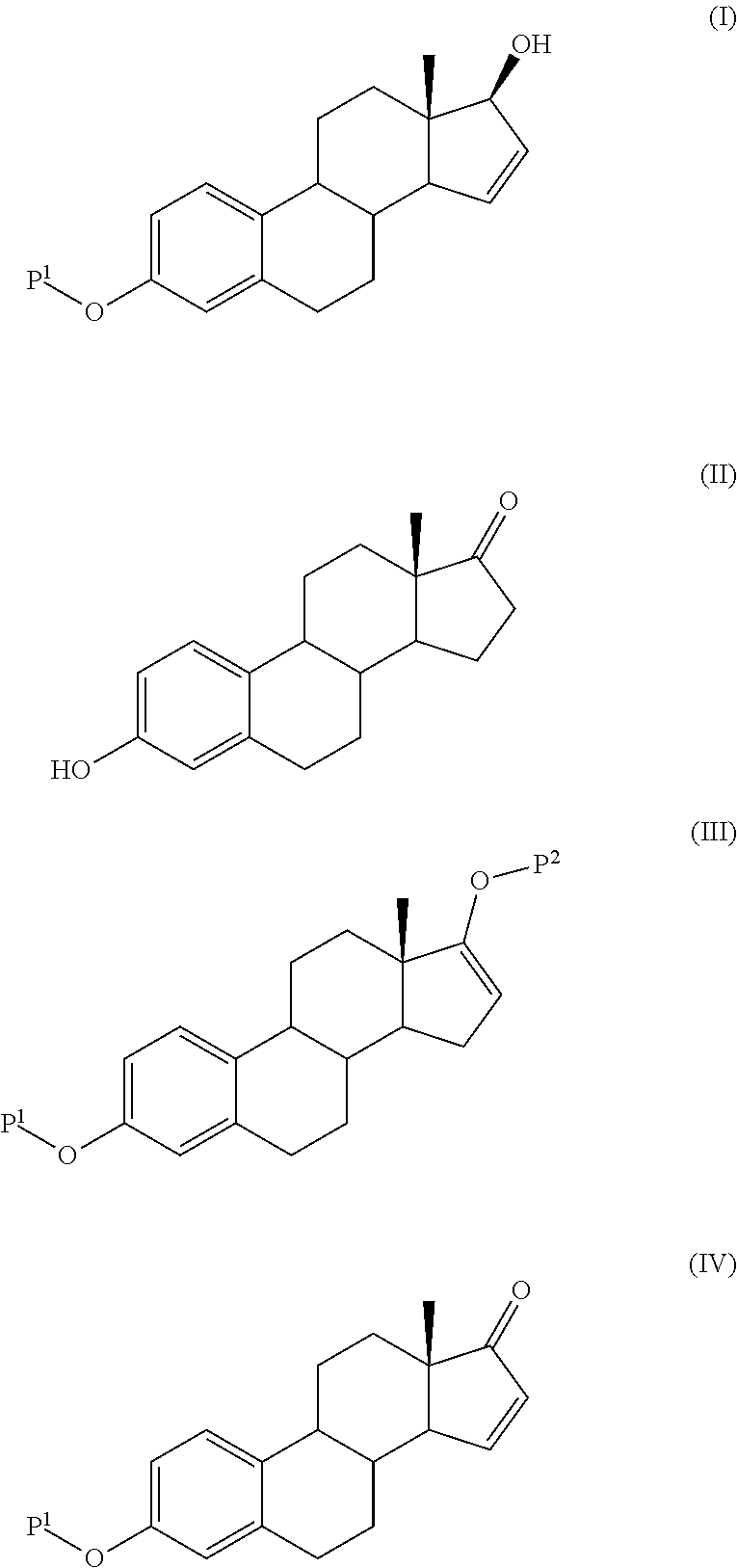
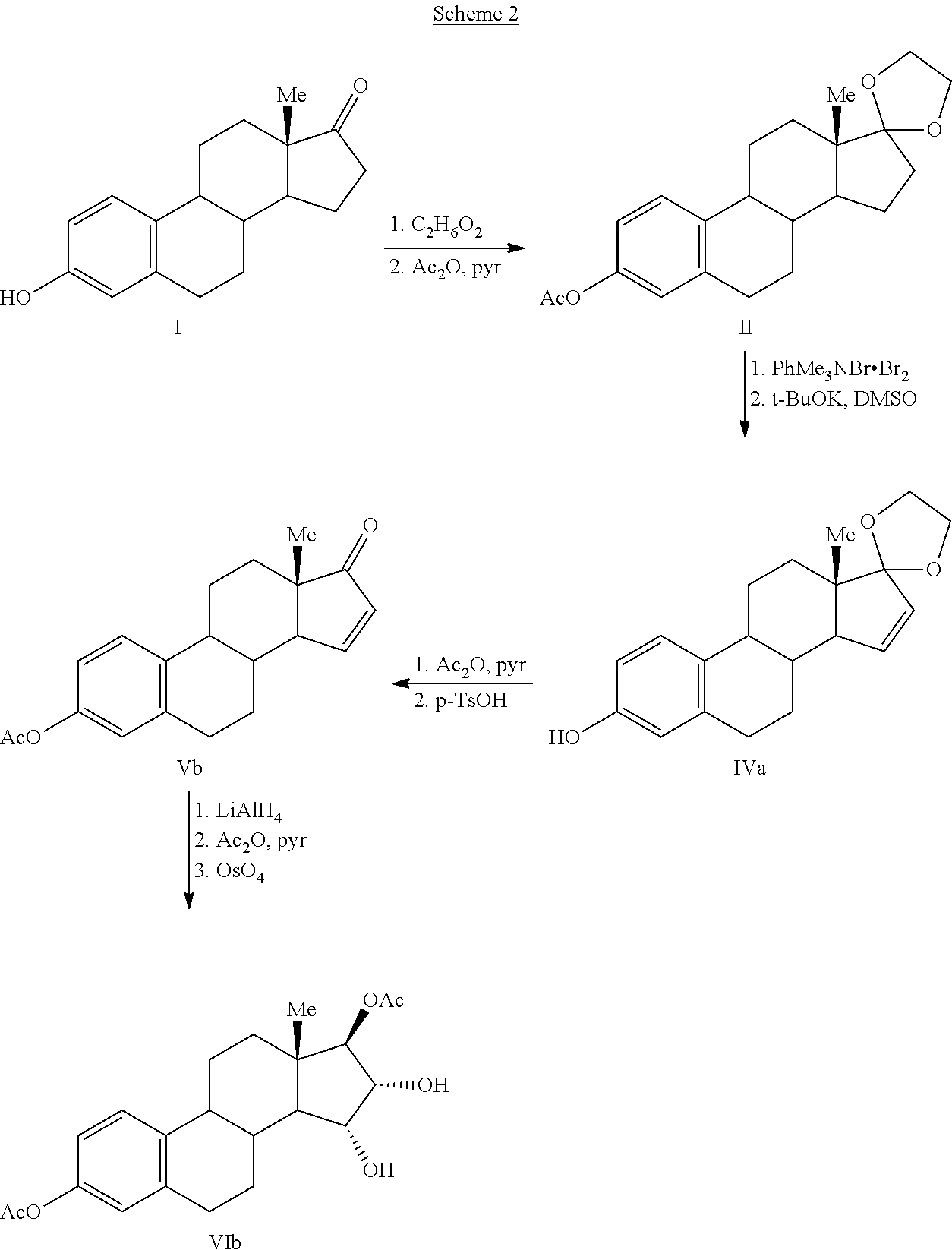
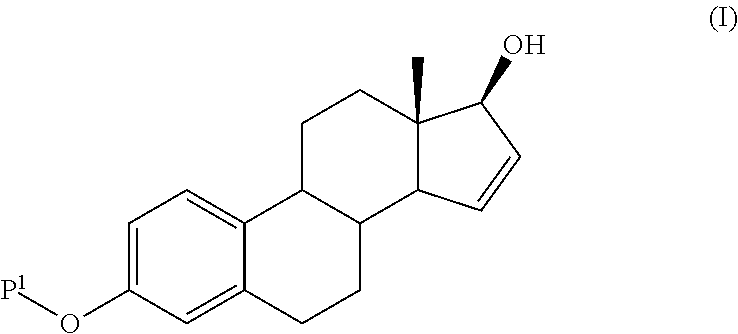
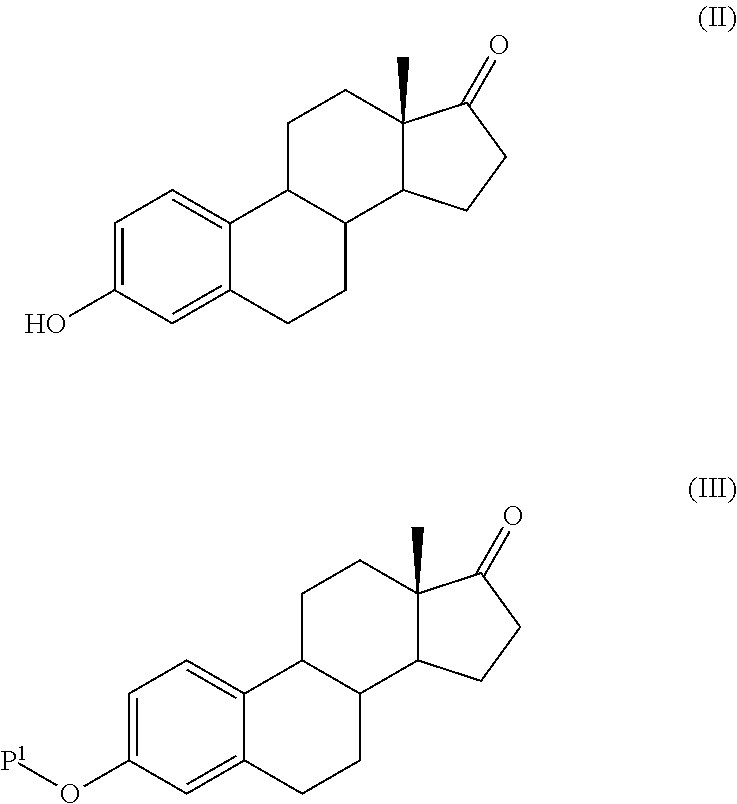
Process for the production of estetrol intermediates
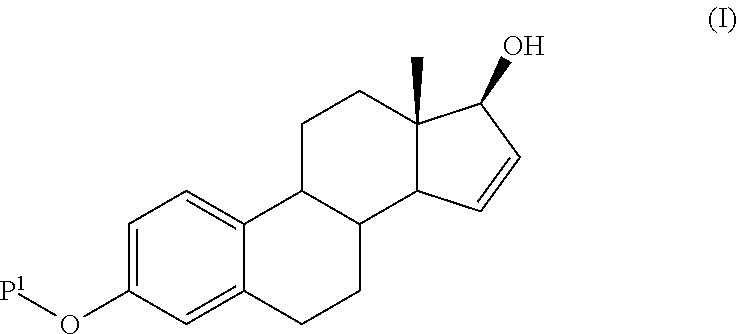
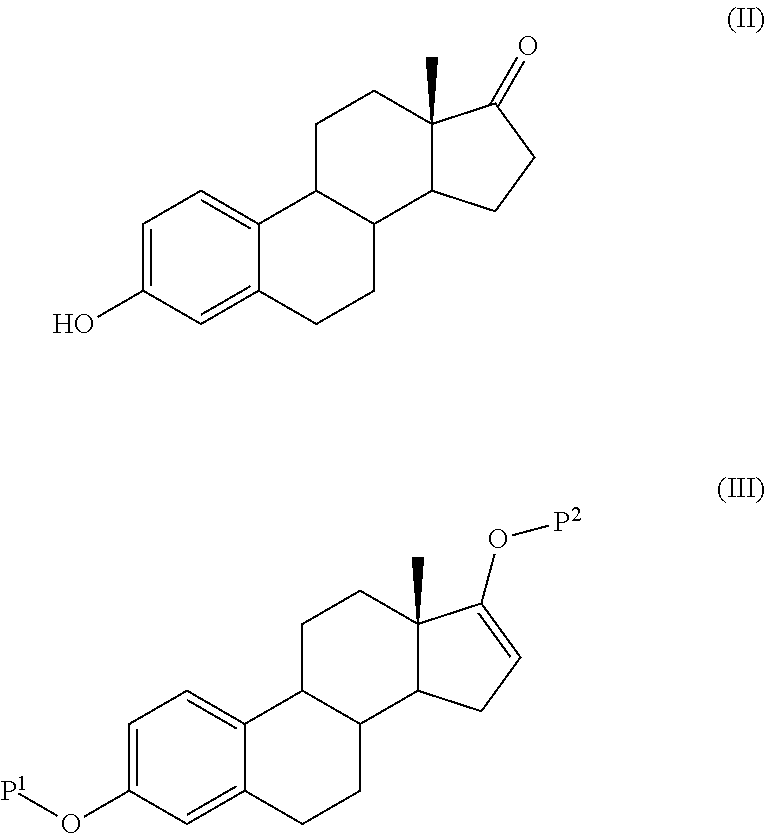
ActiveUS20140107358A1High yieldLow costOrganic active ingredientsEstrane derivativesSilylationProtecting group
The present invention relates to a process for the preparation of a compound of formula (I) said process comprising the steps of: a) reacting a compound of formula (II), with an acylating or a silylating agent to produce a compound of formula (III), wherein P1 and P2 are each independently a protecting group selected from R2−Si—R3R4, or R1CO—, wherein R1 is a group selected from C1-6alkyl or C3-6cycloalkyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; R2, R3 and R4 are each independently a group selected from C1-6alkyl or phenyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; b) reacting the compound of formula (III) in the presence of palladium acetate or a derivative thereof to produce compound of formula (IV); and c) reacting the compound of formula (IV) with a reducing agent to produce compound of formula (I).
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
19-nor neuroactive steroids and methods of use thereof
ActiveUS20170233432A1Eliminate potential for oxidationImprove bioavailabilityNervous disorderEstrane derivativesWithdrawal syndromeSubstance abuser
Owner:SAGE THERAPEUTICS
Process for the preparation of estetrol
The present invention relates to a process for the preparation of estra-1,3,5(10)-trien-3,15α,16α,17β-tetraol (estetrol), via a silyl enol ether derivative 17-B-oxy-3-A-oxy-estra-1,3,5(10),16-tetraene, wherein A is a protecting group and B is —Si(R2)3. The invention further relates to a process for the synthesis of 3-A-oxy-estra-1,3,5(10),15-tetraen-17-one, wherein A is a protecting group, via said silyl enol ether derivative.
Owner:ESTETRA S P R L
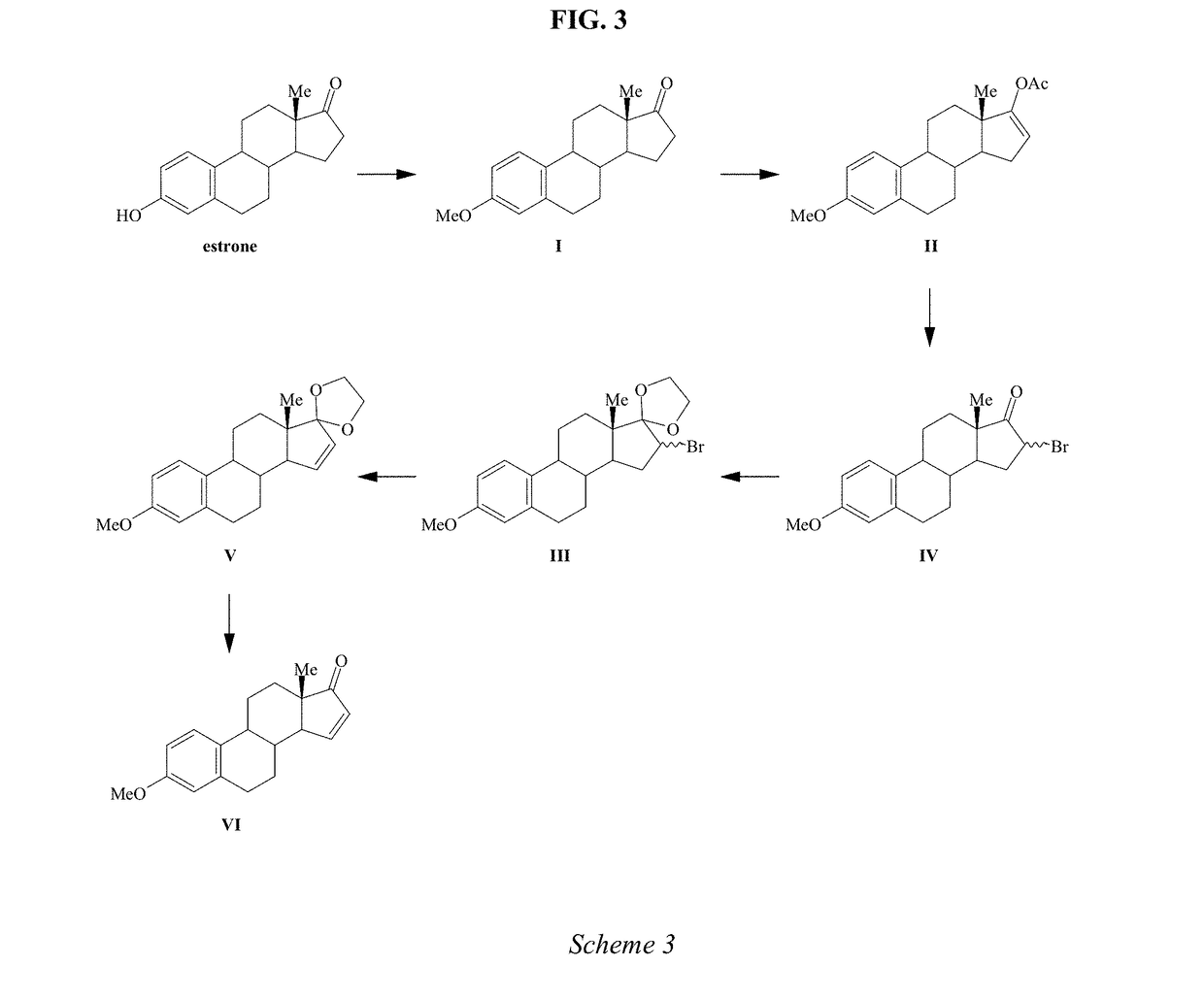
Synthesis of estetrol via estrone derived steroids
InactiveUS20060211669A1Resist formationMore practicalOrganic active ingredientsEstrane derivativesSteroid CompoundEstrone
A process is provided for the making of estetrol starting from a 3-A-oxy-estra 1,3,5(10),15-tetraen-17-one, wherein A is a C1-C5 alkyl group, preferably a methyl group, or a C7-C12 benzylic group, preferably a benzyl group. This process is particularly suitable to industry.
Owner:DONESTA BIOSCI
Process for the production of estetrol intermediates
The present invention relates to a process for the preparation of a compound of formula (I) comprising the steps of a) reacting a compound of formula (II) with a silylating or an acylating agent to produce compound of formula (III), wherein P1 is a protecting group selected from R2Si—R3R4 or R1CO—, R1 is a group selected from C1-6alkyl or C3-6cycloalkyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; R2, R3 and R4 are each independently a group selected from C1-6alkyl or phenyl, each group being optionally substituted by one or more substituents independently selected from fluoro or C1-4alkyl; b) halogenation or sulfinylation of the compound of formula (III) to produce a compound of formula (IV); wherein X is halo, or —O—SO—R5, and R5 is a group selected from C6-10aryl or heteroaryl, each group being optionally substituted by one or more substituents independently selected from chloro or C1-4alkyl; c) dehalogenation or desulfinylation of the compound of formula (IV) to produce compound of formula (V); and d) reacting the compound of formula (V) with a reducing agent to produce compound of formula (I).
Owner:MITHRA RECHERCHE & DÉVELOPPEMENT SA
Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride
ActiveUS20170197912A1Isotope introduction to sugar derivativesIsotope introduction to heterocyclic compoundsSulfonyl chlorideEvaporation
The present disclosure relates to the methods for the preparation of reactive [F-18]fluoride in a form of [F-18]sulfonyl fluoride suitable for efficient radiolabeling without an azeotropic evaporation step by the use of anion exchange resin and sulfonyl chloride, and its applications in the manufacturing of PET radiopharmaceuticals.
Owner:WASHINGTON UNIV IN SAINT LOUIS
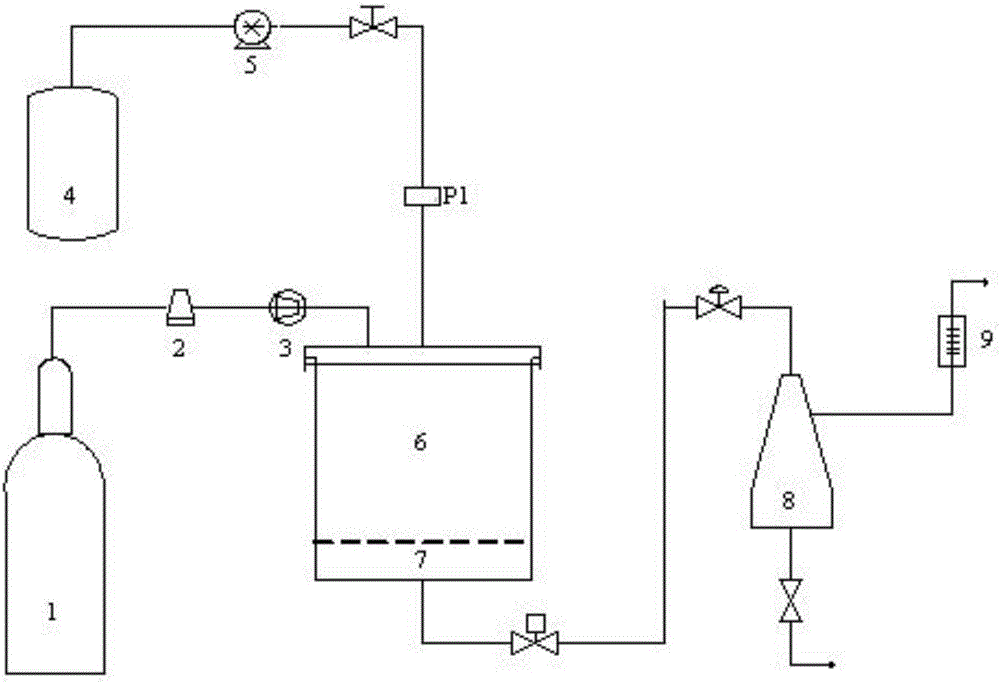
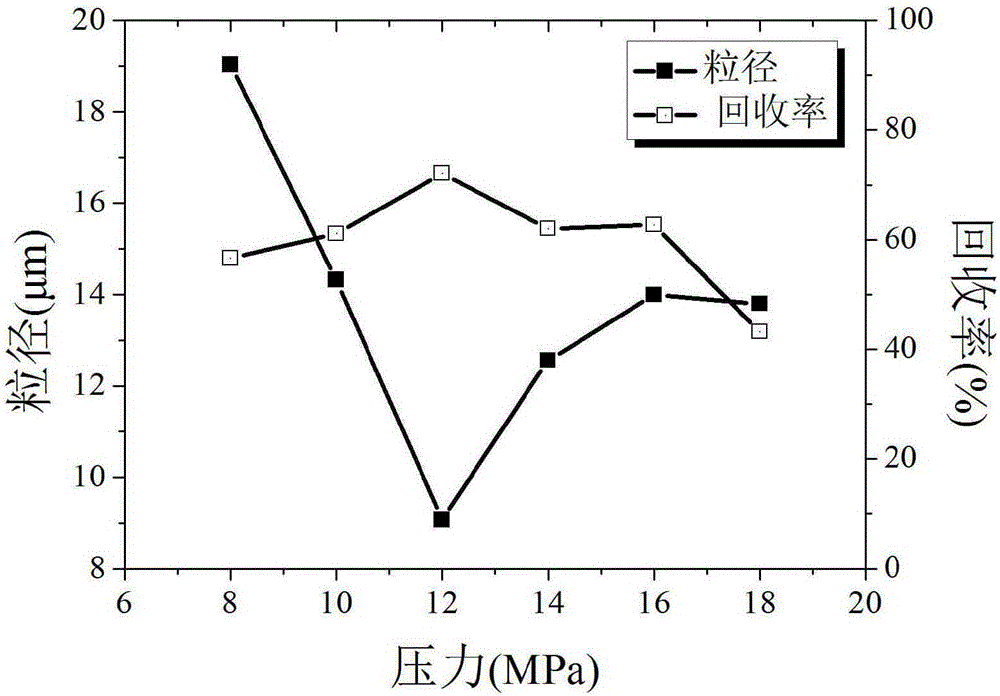
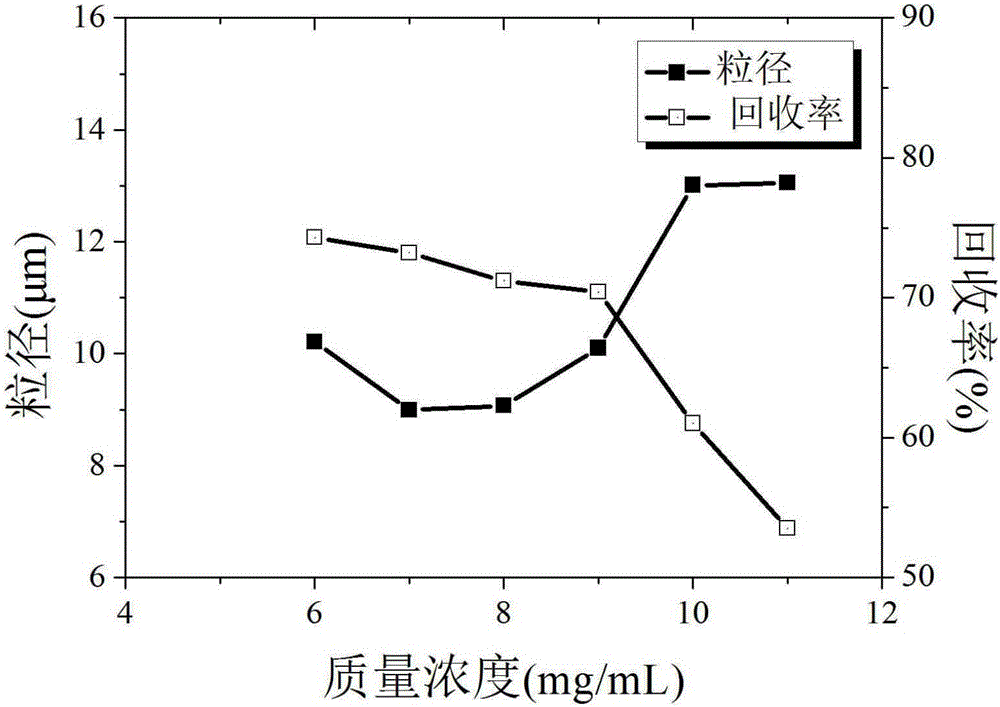
Method for preparing estradiol ultrafine particles with supercritical anti-solvent technology
ActiveCN106432389ASmall particle sizeImprove solubilityEstrane derivativesBulk chemical productionSolubilitySupercritical anti solvent
The invention discloses a method for preparing estradiol ultrafine particles with a supercritical anti-solvent technology. The method comprises the steps as follows: step S1, estradiol is dissolved in an organic solvent, and an estradiol solution is prepared; step S2, CO2 is introduced into a crystallization kettle, and the temperature and pressure in the crystallization kettle are adjusted; step S3, CO2 is introduced continuously at a constant flow rate, and the estradiol solution prepared in step S1 is introduced into the crystallization kettle while the temperature and pressure in the crystallization kettle are kept unchanged; Step S4, CO2 is introduced continuously after the introduction of estradiol solution, the temperature and pressure in the crystallization kettle are kept unchanged, the pressure is relieved after a period of time, and after the pressure in the crystallization kettle is reduced to atmospheric pressure, the crystallization kettle is opened for collecting the estradiol ultrafine particles, wherein the sequence of the step S1 and the step S2 can be changed, and the organic solvent is a mixed solvent of acetone and ethanol. With the adoption of the method, estradiol with fine particle size can be prepared, the solubility of estradiol in water is improved, and accordingly, the bioavailability of estradiol is improved.
Owner:CHINA PHARM UNIV
Estradiol-16alpha-carboxylic acid esters as locally active estrogens
InactiveUS20020143002A1Reduce and prevent likelihoodAffect activityOrganic active ingredientsBiocideCarboxylic acidMedicinal chemistry
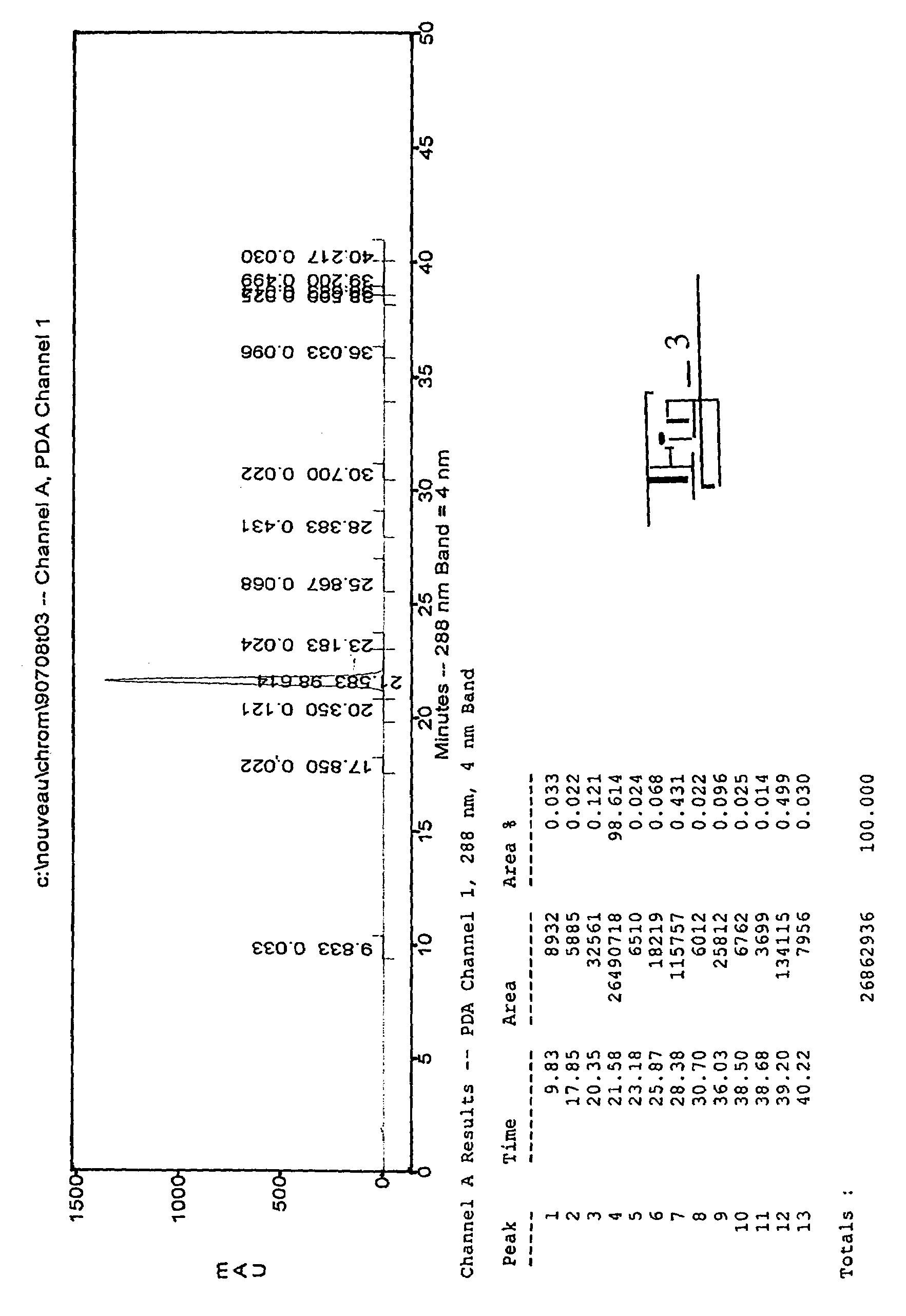
The present invention relates to analogs of estradiol, which, in their most preferred embodiment, act as locally active estrogens without significant systemic action. A series of 16alpha-carboxylic acid substituted steroids and their esters is presented which exhibit excellent biological activity for use in pharmaceutical compositions for the treatment of symptomology associated with menopause. The present invention is therefore directed to compounds according to the structure: Where R is H, a C1 to C5 alkyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3; and m is from 0-2, or a pharmaceutically acceptable salt thereof. Preferably, R is methyl, ethyl, propyl, iso-propyl, butyl, iso-butyl, pentyl, neo-pentyl, vinyl, CF3, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0. More preferably, R is methyl, ethyl, CH2CH2F, CH2CHF2 or CH2CF3 and m is 0.
Owner:YALE UNIV
Method for synthesizing radiopharmaceuticals using a cartridge
ActiveUS9550704B2Guaranteed performancePresent inventionIsotope introduction to sugar derivativesIsotope introduction to heterocyclic compoundsRadioactive drugBiomedical engineering
Owner:FUTURECHEM
Benzopyran-containing compounds and method for their use
InactiveCN1158274CNot easy to convertReduce activationOrganic compound preparationSulfur/selenium/tellurium active ingredientsBenzopyransStereoisomerism
Certain benzopyran antiestrogens are disclosed for treating estrogen sensitive diseases such as breast cancer. Prodrug forms provide ease of manufacturing, good shelf life, and bioavailibility, and preferred stereoisomers are shown to be more effective than racemic mixtures.
Owner:ENDORES & DEV
Method for synthesizing radiopharmaceuticals using a cartridge
ActiveUS20150232392A1Guaranteed performancePresent inventionGlycosidesAmino-hyroxy compound preparationCombinatorial chemistryPolymer
The present invention relates to a method for synthesizing a radiopharmaceutical using a cartridge, which makes it possible to carry out several steps of reaction required for synthesizing a radiopharmaceutical in the cartridge filled with a polymer. A method for synthesizing a radiopharmaceutical according to the present invention enables each step's reaction to be carried out with the solution confined in the cartridge so as not to flow out, thus being simplified compared to the conventional methods for synthesizing radiopharmaceuticals, and expediting the synthesis thereof.
Owner:FUTURECHEM
Compound, conjugate, kit, and application of kit in detection of estradiol
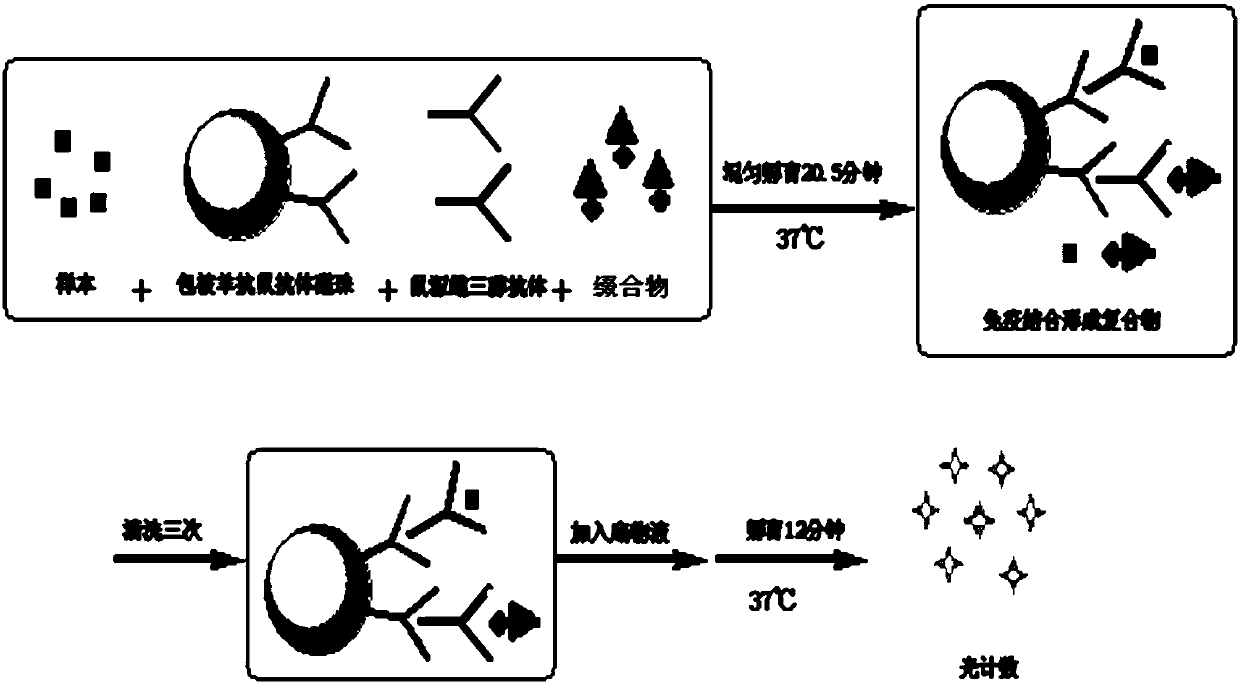
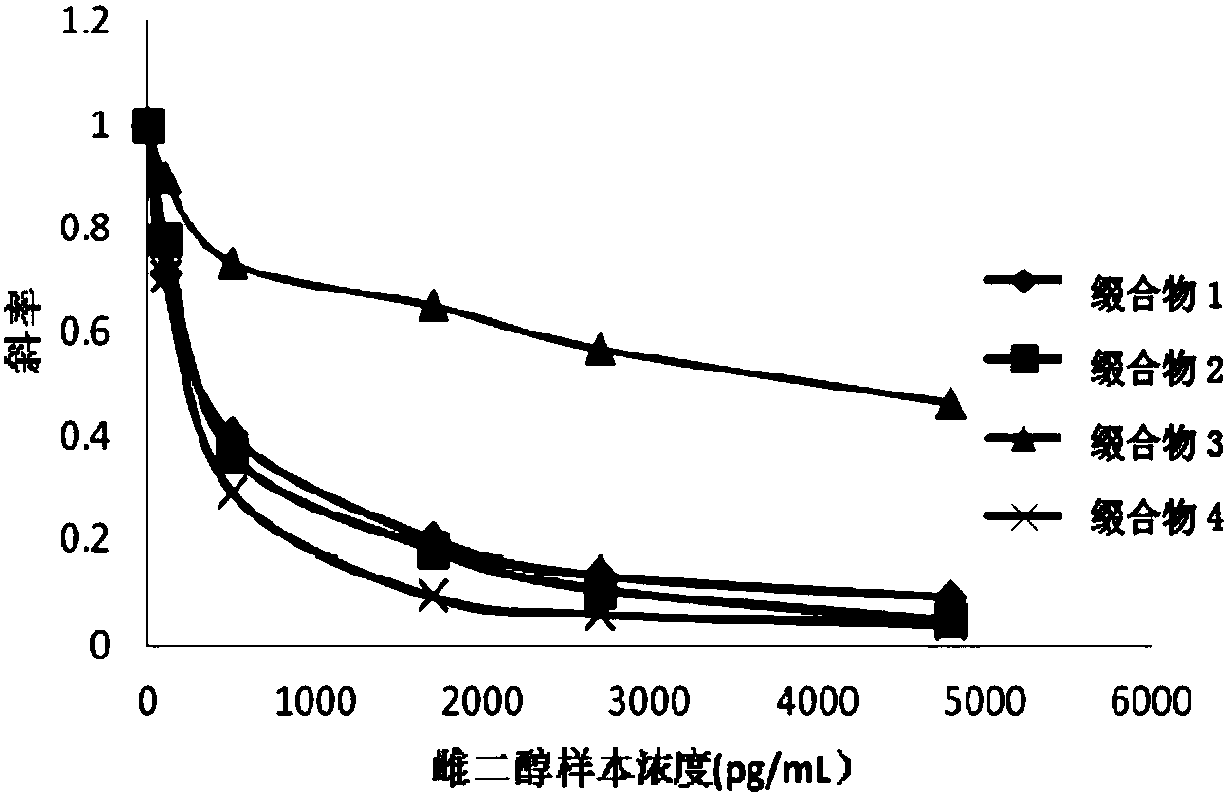
ActiveCN107652343AAccurate detectionEasy to getHydrolasesMicrobiological testing/measurementHydrogenHydroxy compound
The invention discloses a compound, a conjugate, a kit for detecting estradiol, and an application of the kit for detecting the estradiol in detection of the estradiol. The compound has a structure shown as a formula (1), wherein the L represents a connecting arm, the R<1>, R<2>, R<3> and R<4> are respectively independently a hydrogen group, a hydroxyl group, an alkyl of C<1-3>, an alkoxy of C<1-3>, an alkenyl of C<2-3> or an alkynyl of C<2-3>. The compound disclosed by the invention can be used for accurately detecting estradiol.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
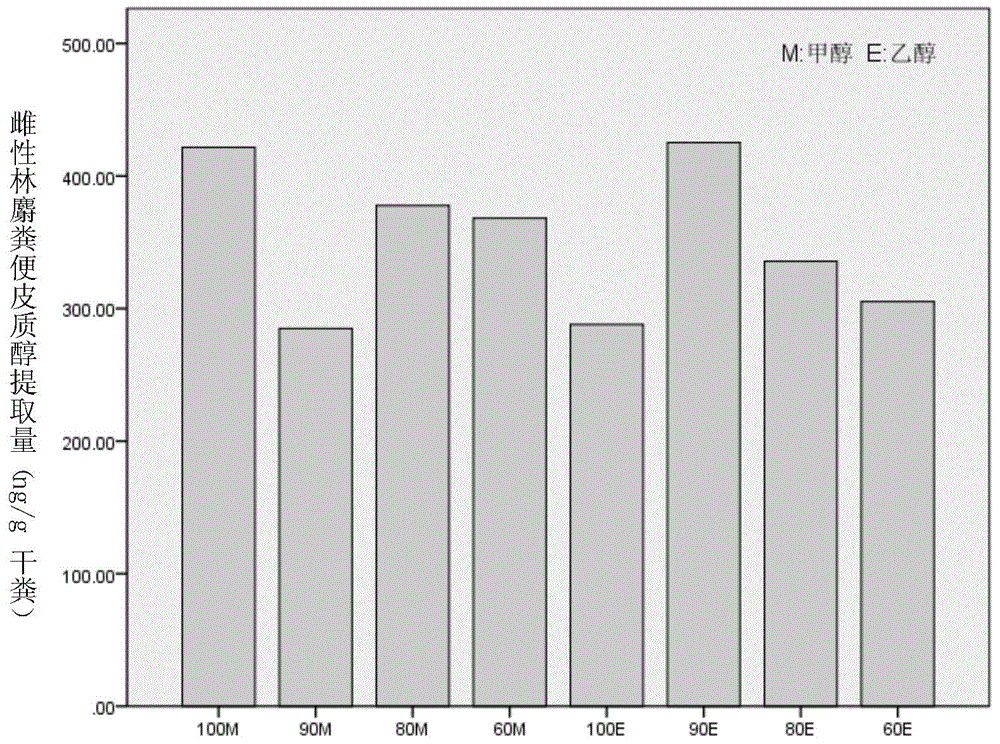
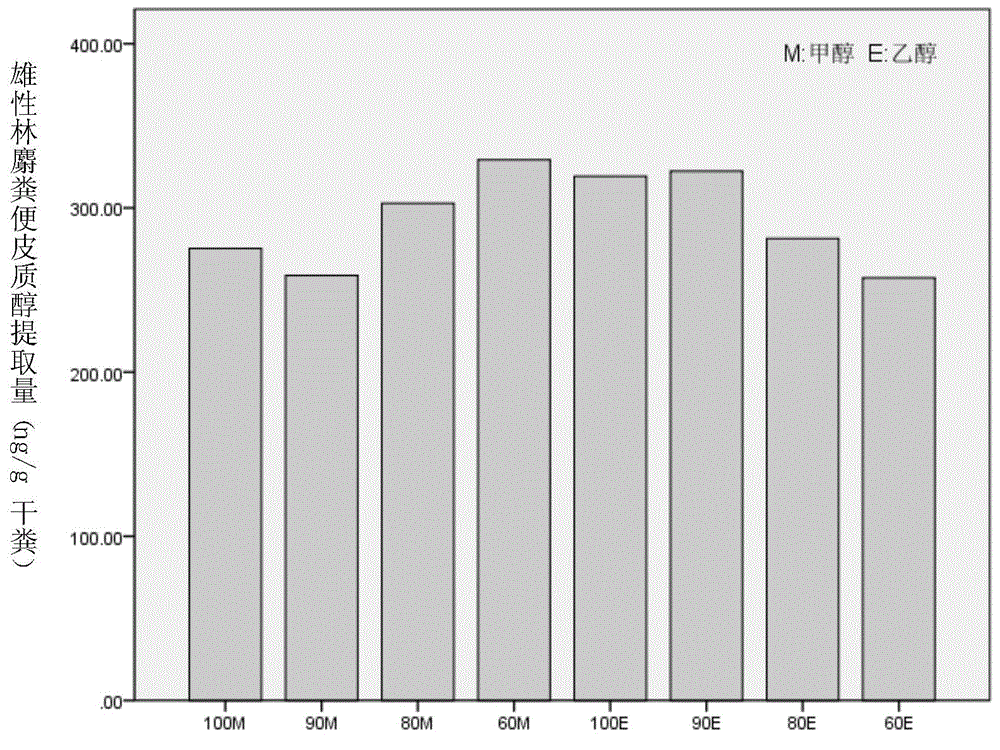
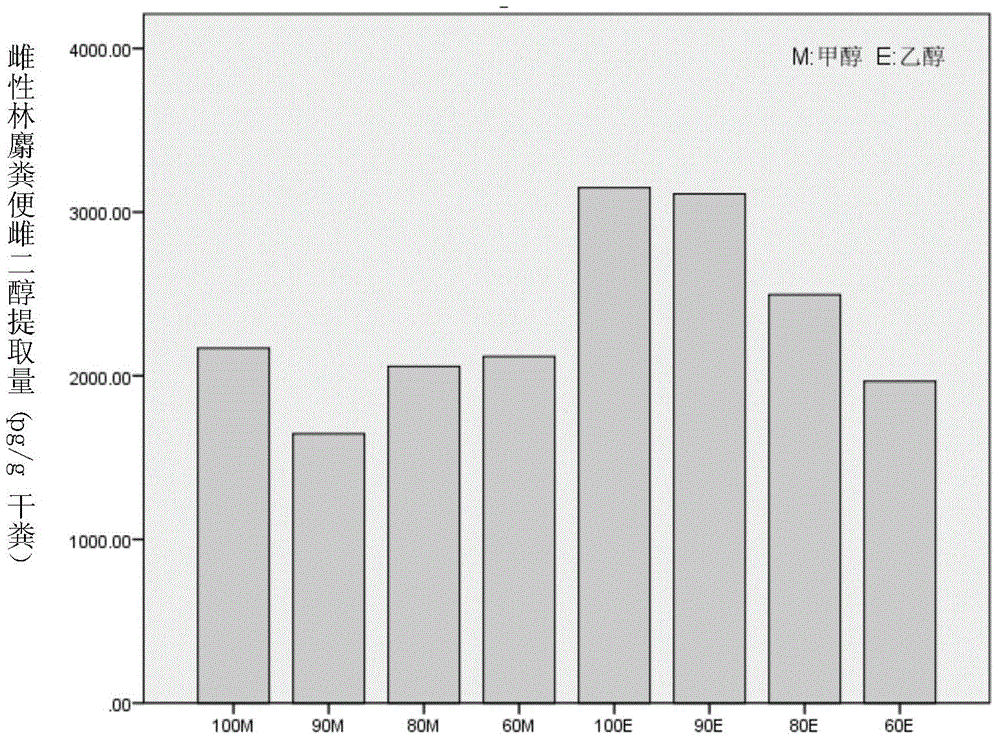
Method for extracting steroid hormone from excrement of forest musk deer
The invention discloses a method for extracting steroid hormone from excrement of forest musk deer. The method comprises the following step of leaching animal excrement by virtue of extraction liquid so as to obtain leaching liquid containing the steroid hormone, wherein the extraction liquid is an ethanol water solution, a methanol water solution, ethanol or methanol; the concentration of volume percent of ethanol in the ethanol water solution is more than or equal to 60% and less than 100%; the concentration of volume percent of methanol in the methanol water solution is more than or equal to 60% and less than 100%; the steroid hormone is one or two or all of cortisol, estradiol and testosterone. An experiment proves that the steroid hormone is extracted from the excrement of the forest musk deer by virtue of the extraction method, the optimal extraction liquid is 90E (10% ethanol water solution).
Owner:BEIJING FORESTRY UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride](https://images-eureka.patsnap.com/patent_img/2a9884c9-3a69-4abf-84a1-b2c6a9383d61/US20170197912A1-20170713-D00001.png)
![Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride](https://images-eureka.patsnap.com/patent_img/2a9884c9-3a69-4abf-84a1-b2c6a9383d61/US20170197912A1-20170713-D00002.png)
![Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride Synthesizing pet tracers using [f-18]sulfonyl fluoride as a source of [f-18]fluoride](https://images-eureka.patsnap.com/patent_img/2a9884c9-3a69-4abf-84a1-b2c6a9383d61/US20170197912A1-20170713-D00003.png)