Compound, conjugate, kit, and application of kit in detection of estradiol
A technology of compounds and conjugates, which is applied in the field of analysis, can solve problems such as estradiol derivatives to be developed, and achieve the effect of being easy to popularize, apply and obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
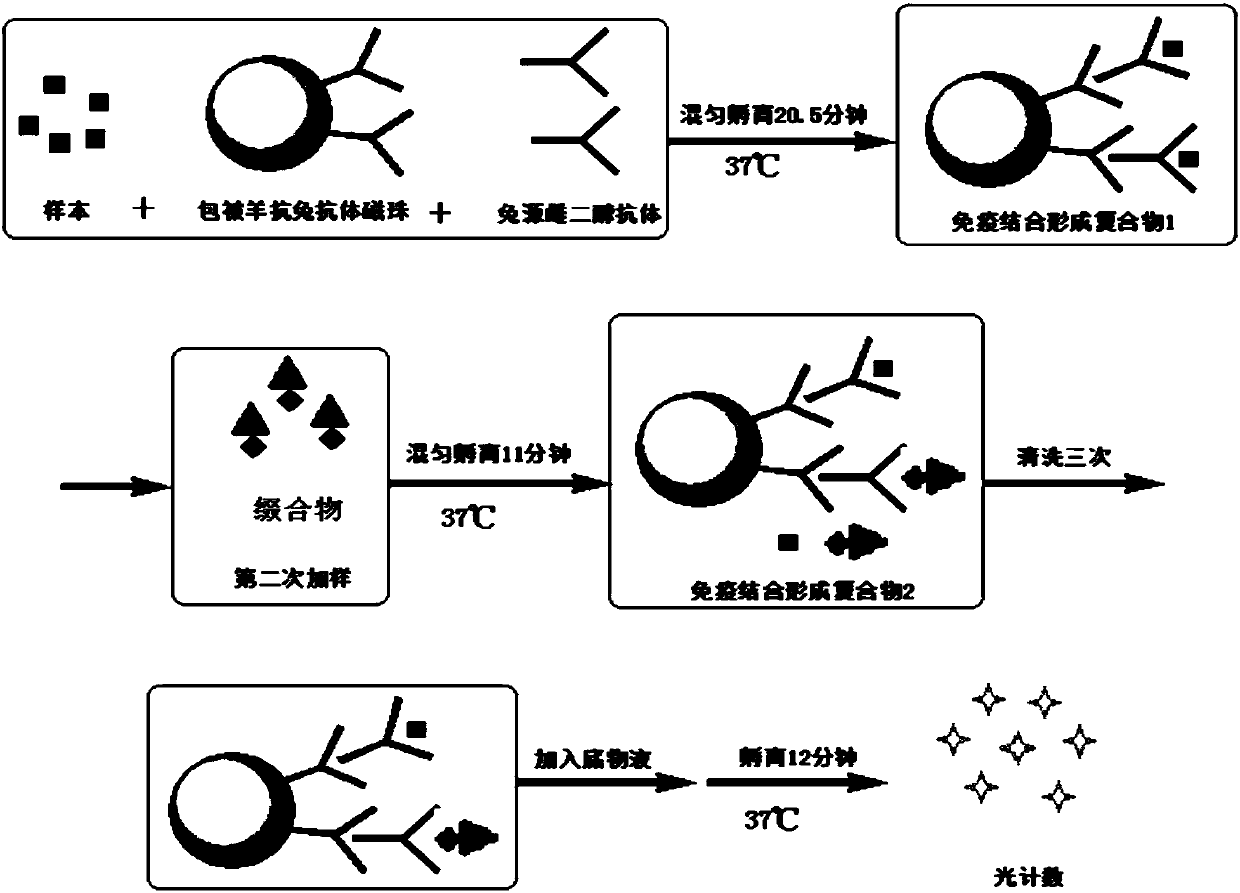
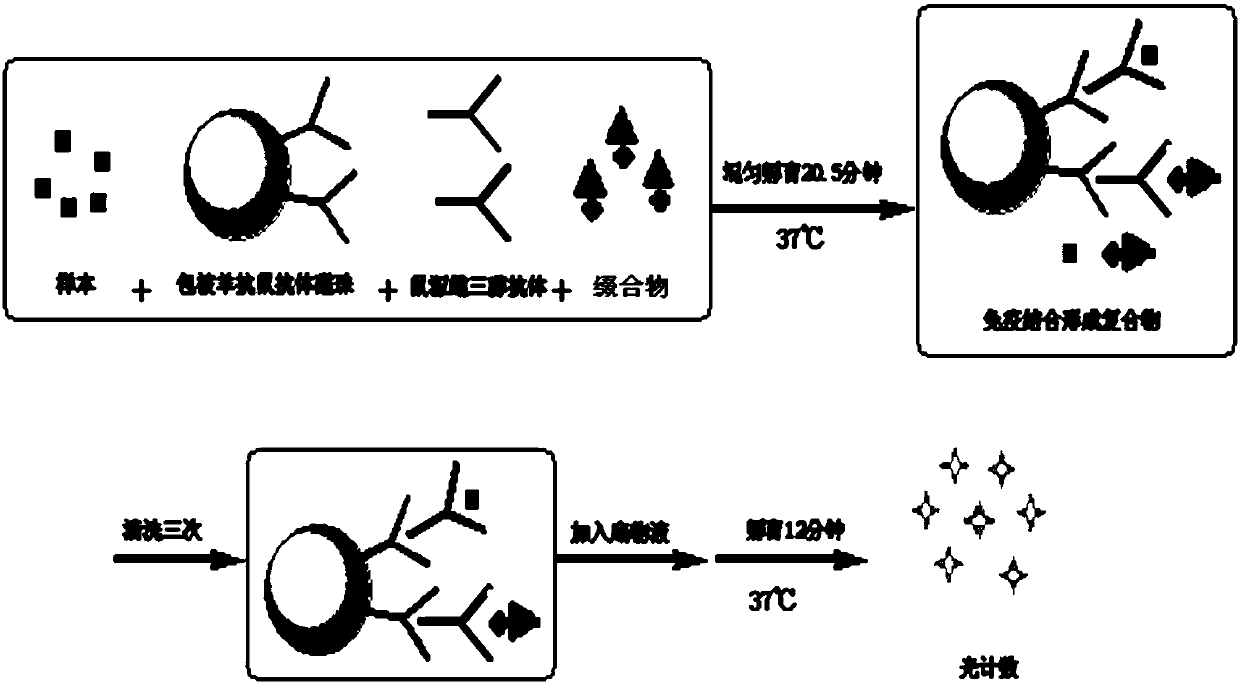
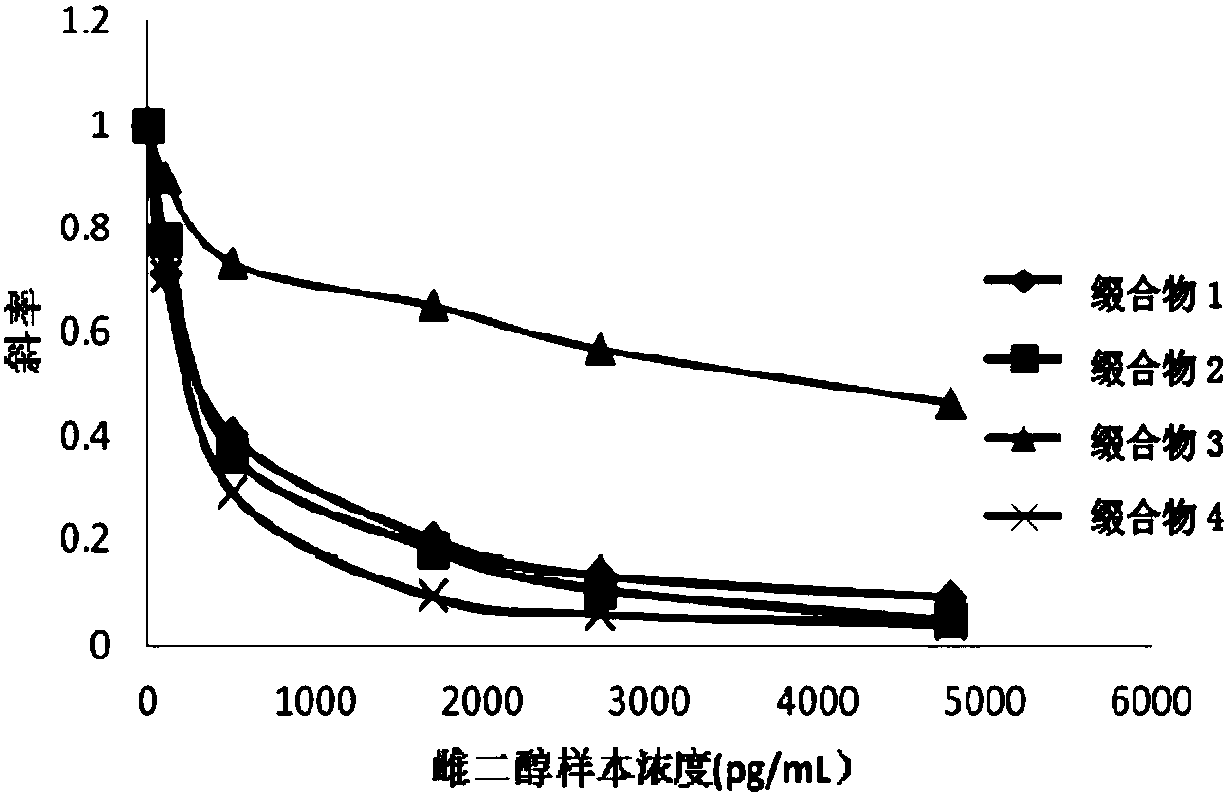
Image
Examples
Embodiment 1
[0058] In this example, the compound represented by formula (2) was synthesized according to the following process:
[0059]
[0060] The synthetic route is as follows:
[0061]
[0062] The specific synthesis steps are as follows:
[0063] 1. Synthesis of compound 2:
[0064] Charge 2.72g (0.01mol) 17β-estradiol (Compound 1) into a 100mL dry and cooled reaction flask, add 40mL dimethylformamide (DMF), stir to dissolve, and then add 3.14g (0.03mol) anhydrous potassium carbonate Under stirring, slowly add 2.17g (0.012mol) methyl 4-bromobutyrate dropwise. After the addition is complete, react for 4 hours. The reaction solution was poured into ice water, extracted three times with ethyl acetate, the organic layers were combined, and then washed three times with saturated brine, then dried over anhydrous sodium sulfate, and concentrated to obtain 4.7 g of crude compound 2.
[0065] MS: positive ion peak: 373.6, 395.6 (+Na peak); negative ion peak: 371.5.
[0066] 2. Synthesis of compound ...
Embodiment 2
[0070] In this example, the compound represented by formula (3) was synthesized according to the following process:
[0071]
[0072] The synthetic route is as follows:
[0073]
[0074] The specific synthesis steps are as follows:
[0075] 1. Synthesis of compound 5:
[0076] Charge 2.70g (0.01mol) estrone (compound 4) in a 100mL dry and cooled reaction flask, add 40mL DMF, stir to dissolve, then add 3.14g (0.03mol) anhydrous potassium carbonate, and slowly add 2.17g( 0.012 mol) Methyl 4-bromobutyrate. After the addition is complete, react for 4 hours. The reaction solution was poured into ice water, extracted three times with ethyl acetate, and the organic layers were combined, washed three times with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 4.6 g of crude compound 5.
[0077] MS: positive ion peak: 371.5, 393.5 (+Na peak); negative ion peak: 369.5.
[0078] 2. Synthesis of compound of formula (3):
[0079] Charge 4.6 g of the crude compound 5 ...
Embodiment 3
[0082] In this example, the compound represented by formula (4) was synthesized according to the following process:
[0083]
[0084] synthetic route
[0085]
[0086] The specific synthesis steps are as follows:
[0087] 1. Synthesis of compound 8:
[0088] Charge 2.88g (0.01mol) estriol (compound 7) in a 100mL dry and cooled reaction flask, add 40mL DMF, stir to dissolve, add 3.14g (0.03mol) anhydrous potassium carbonate, and slowly add 2.17g (0.012 mol) Methyl 4-bromobutyrate. After the addition is complete, react for 4 hours. The reaction solution was poured into ice water, extracted three times with ethyl acetate, and the organic layers were combined, then washed three times with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 4.9 g of crude compound 8.
[0089] MS: positive ion peak: 389.7, 411.7 (+Na peak); negative ion peak: 387.8.
[0090] 2. Synthesis of compound of formula (4):
[0091] Feed 4.9 g of the crude compound 8 obtained in the previ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com