Method for preparing estradiol ultrafine particles with supercritical anti-solvent technology
A supercritical anti-solvent, ultra-fine particle technology, applied in the field of preparation of estradiol ultra-fine particles, can solve the problems of low oral bioavailability and low solubility, and achieves improved bioavailability, small particle size, prominent effect of substantive features
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
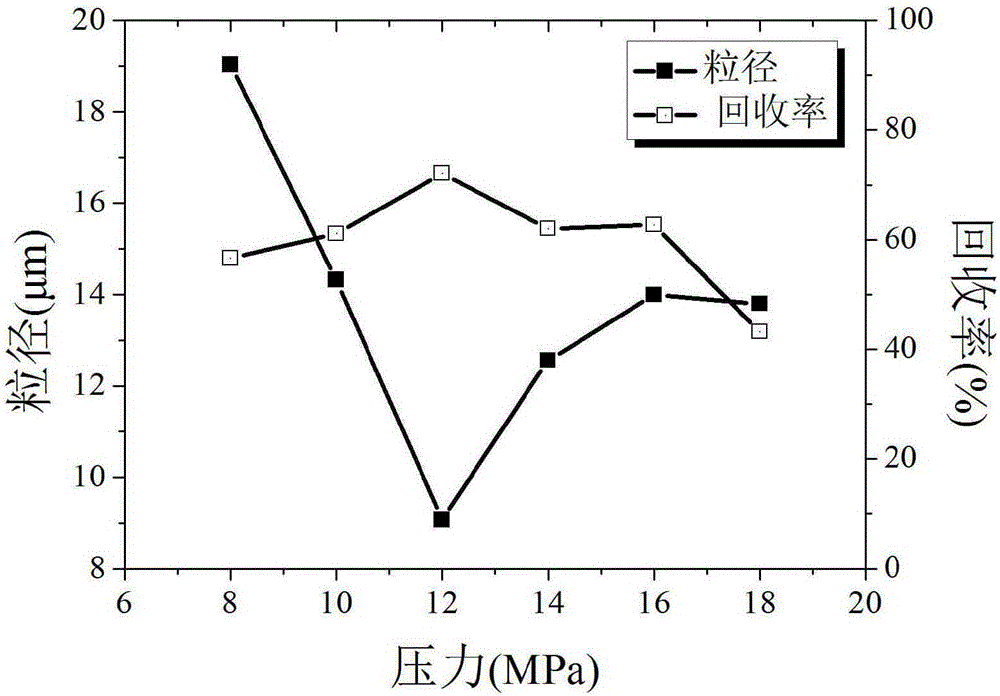
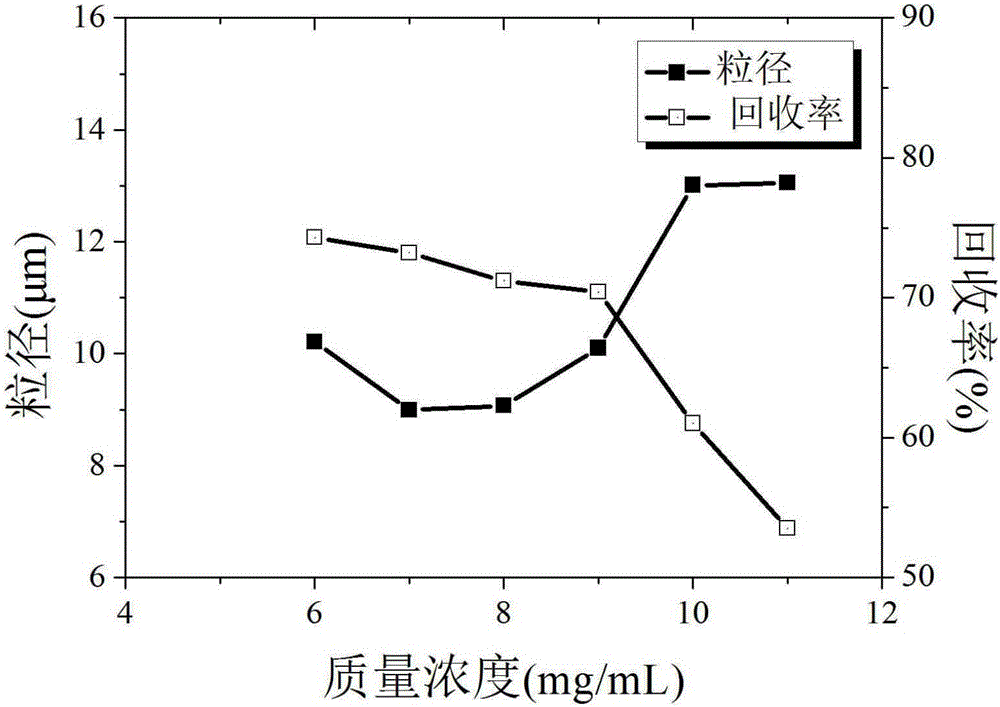
[0032] Embodiment 1: single factor method determines the preferred value range of each key parameter
[0033] Instruments and materials
[0034] See Table 1 for experimental materials and Table 2 for experimental equipment.
[0035] Table 1 Experimental materials
[0036] material name Specification Manufacturer Estradiol ≥99% Wuhan Belka Biomedicine Co., Ltd. CO 2
≥99% Nanjing Tongqi Gas Company ethanol Analytical pure Nanjing Chemical Reagent Co., Ltd. acetone Analytical pure Nanjing Chemical Reagent Co., Ltd. Dichloromethane Analytical pure Nanjing Chemical Reagent Co., Ltd. Methanol Analytical pure Nanjing Chemical Reagent Co., Ltd. Dimethyl sulfoxide Analytical pure Nanjing Chemical Reagent Co., Ltd.
[0037] Table 2 Experimental Instruments
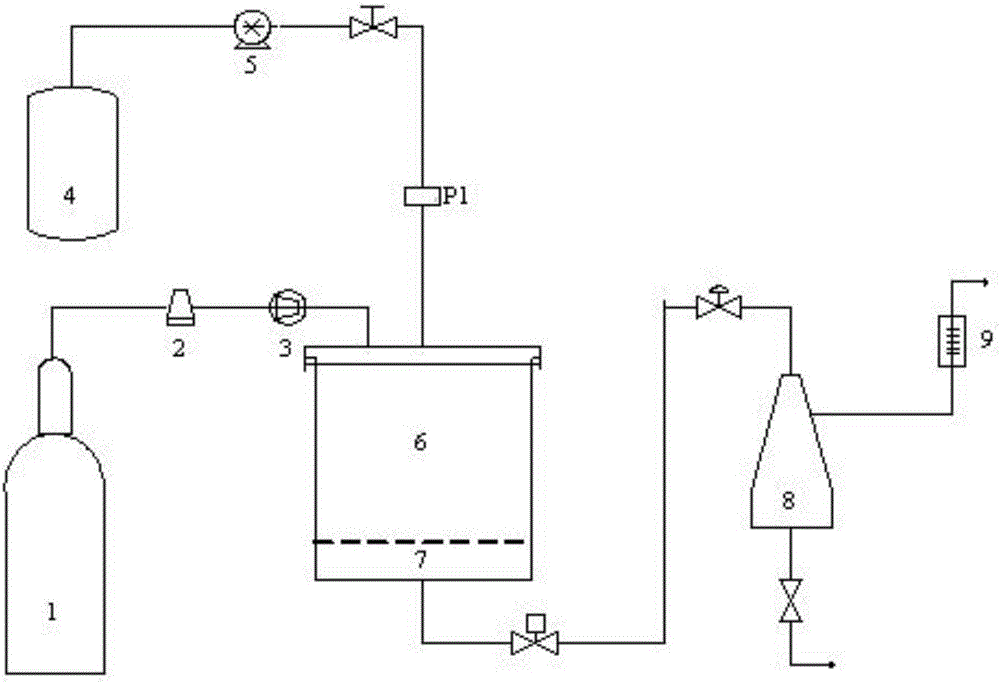
[0038] device name model Manufacturer Supercritical Particle Preparation System Helix Applied Separations, USA Intell...
Embodiment 2
[0071] Embodiment 2: Orthogonal experiment optimizes optimal parameters within the preferred range of each key parameter
[0072] Orthogonal experimental design and results
[0073] Taking the particle size as the main evaluation index, four factors that have a significant impact on the particle size of estradiol particles are selected, namely, the solution volume flow rate (A), the crystallization pressure (B), the crystallization temperature (C) and the mass concentration of estradiol (D ) for investigation, using the L9(34) orthogonal experimental design to carry out experiments, and its factor levels have been determined by the single factor experiment of Example 1, see Table 4. Other process conditions are CO 2 The exhaust volume flow rate is 3L / min, and the volume ratio of acetone and ethanol is 1:1. The experimental design and results are shown in Table 5.
[0074] Table 4 Orthogonal Experimental Factors and Level Table
[0075]
[0076] Table 5 Orthogonal expe...
Embodiment 3
[0093] A method for preparing estradiol ultrafine particles by a supercritical antisolvent method, comprising the steps of:
[0094] Step S1, dissolving estradiol in an organic solvent to obtain an estradiol solution with a mass concentration of 6 mg / mL;
[0095] Step S2, the CO 2 Pass it into the crystallization kettle at a flow rate of 3L / min, adjust the temperature in the crystallization kettle to 55°C, and the pressure to 8MPa;
[0096] Step S3, continue to introduce CO at a flow rate of 3 L / min 2 , maintaining the temperature and pressure in the crystallization tank constant, while passing the estradiol solution prepared in step S1 into the crystallization tank at a volume flow rate of 1.0 mL / min;
[0097] Step S4, after the estradiol solution is passed through, continue to pass through the CO 2, keep the temperature and pressure in the crystallization kettle constant, and release the pressure after 40 minutes; when the pressure in the crystallization kettle drops to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com