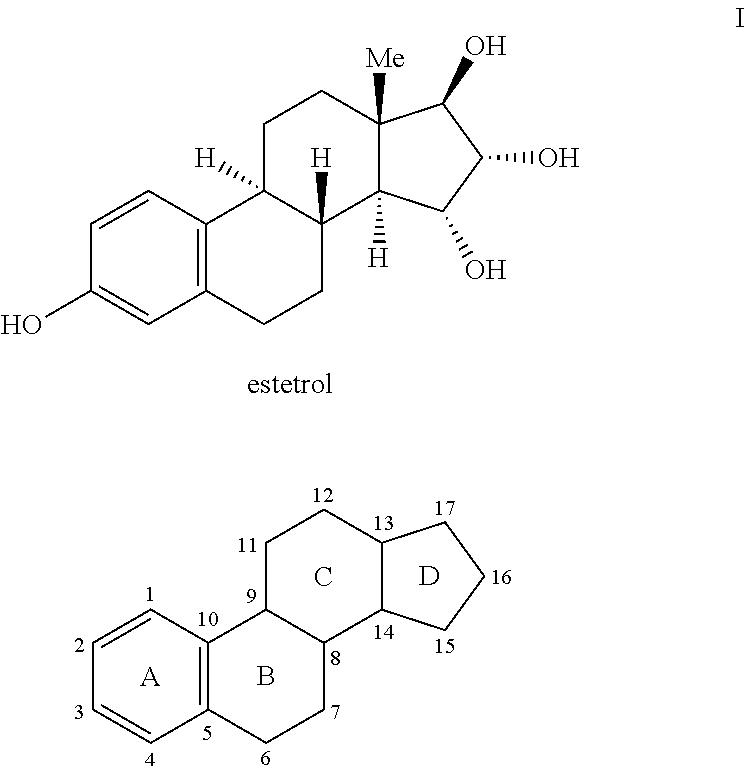
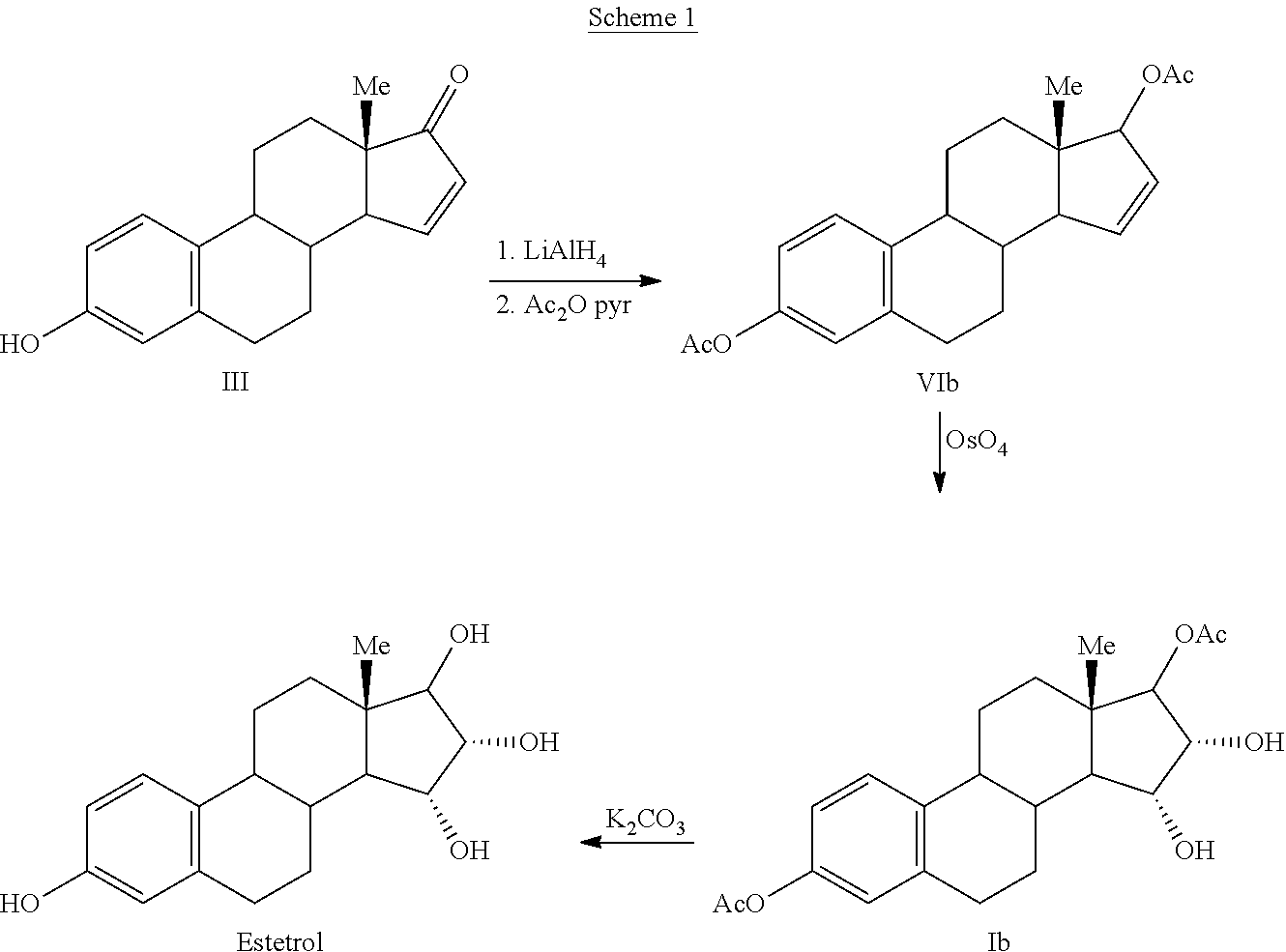
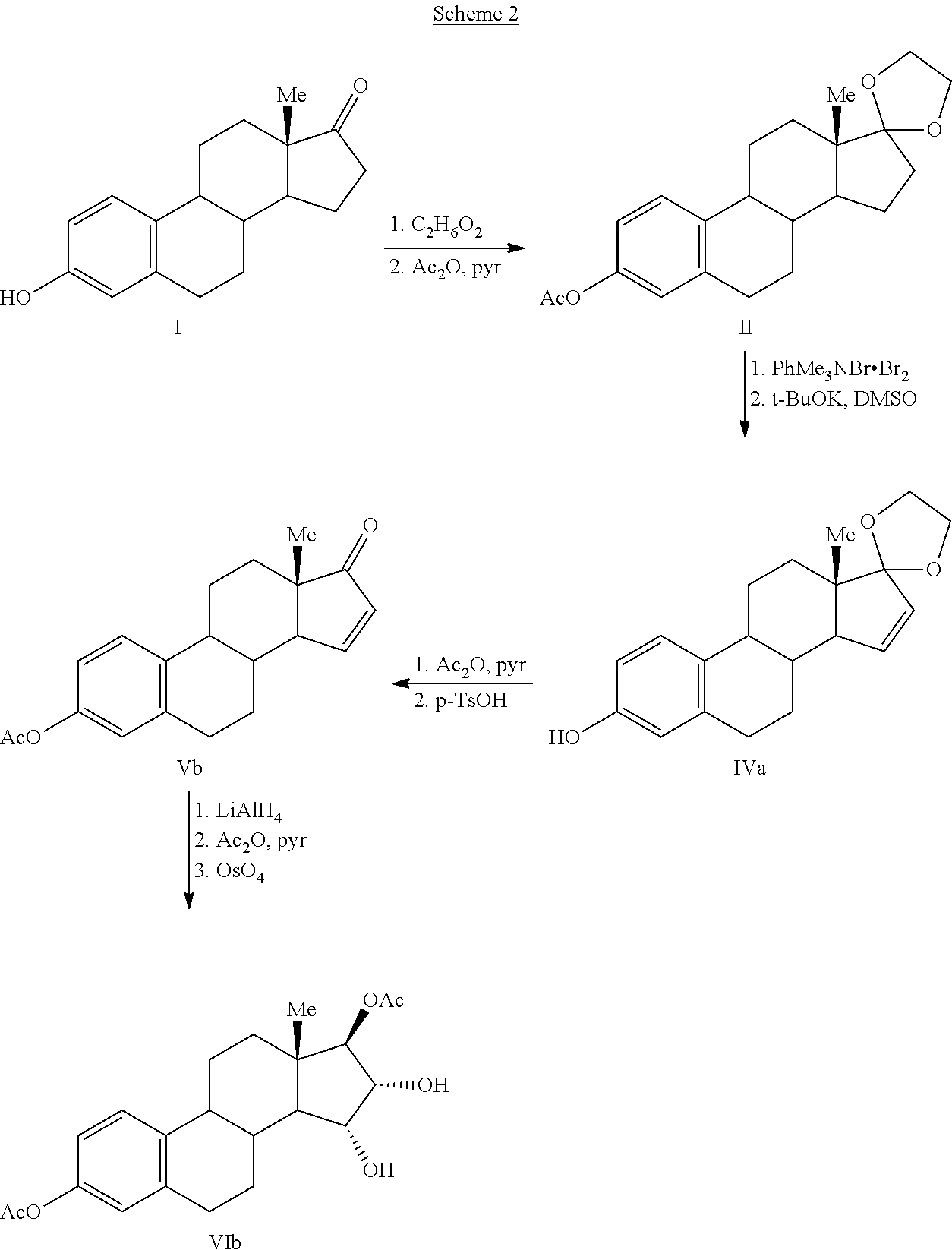
Process for the preparation of estetrol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-Benzyloxy-estra-1,3,5(10)-trien-17-one (3-protected estrone, A is benzyl)
[0139]To a suspension of estrone (II; 100 g, 0.370 mol) and K2CO3 (160 g, 1.16 mol) in DCM / MeOH (800 ml, 1:1 v / v ratio) at room temperature (RT) was added benzyl bromide (132 ml, 1.10 mol) in one portion. The resulting mixture was refluxed for 16 h (50% conversion after 4 h according to TLC). The reaction mixture was cooled to RT and solids were filtered off. The filter-cake was washed with MeOH. The solution was concentrated (to a total volume of ca. 300 ml). The precipitate that had formed was collected by filtration and washed with heptanes to give a white solid. The filtrate was concentrated further (to a total volume of 100 ml) and triturated with heptane. The resulting precipitate was filtered off and combined with the first batch of product. The product (153 g, max 0.370 mol) still contained traces off benzyl bromide but was used without further purification. The product can be purified by recrystalliz...
example 2
3-Benzyloxy-17-trimethylsilyloxy-estra-1,3,5 (10), 16-tetraene (compound III, A is benzyl, B is trimethylsilyl)
[0141]3-Benzyloxy-estra-1,3,5(10)-trien-17-one (3-protected estrone, A is benzyl; 238 mg, 0.660 mmol) was dissolved in DCM (10 ml). Et3N (0.166 ml, 1.188 mmol) and TMS-OTf (0.143 ml, 0.792 mmol) were added and the solution was stirred at ambient temperature for 1 h. According to TLC (alumina, heptane / ethyl acetate 4 / 1 plus Et3N). The entire content of the flask was transferred onto a small column of basic alumina (type II) and eluted with heptane / ethyl acetate 4 / 1 plus Et3N. The product was obtained as a white solid (248 mg, 87%).
example 3
3-Benzyloxy-estra-1,3,5(10),15-tetraen-17-one (compound IV, A is benzyl)
[0142]Unstabilised IBX (1.0 g; 3.6 mmol), a catalytic amount of trimethylamine-N-oxide (40 mg, 10 mol %) and 3 Å molecular sieves (100 mg) were added to 10 ml dry DMSO.
[0143]A fluorobenzene solution containing about 2.8 mmol crude (94% GC) benzylestrone-trimethylsilyl enol ether III (4.5 ml; corresponding to 1.0 g ketone) was added, giving a sudden solidification of the reaction mixture due to precipitated substrate. Mild heating to 40-45° C. was needed for dissolution. After 1 h HPLC showed a clean conversion of the enol ether to the enone with some ketone present due to advantageous hydrolysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com