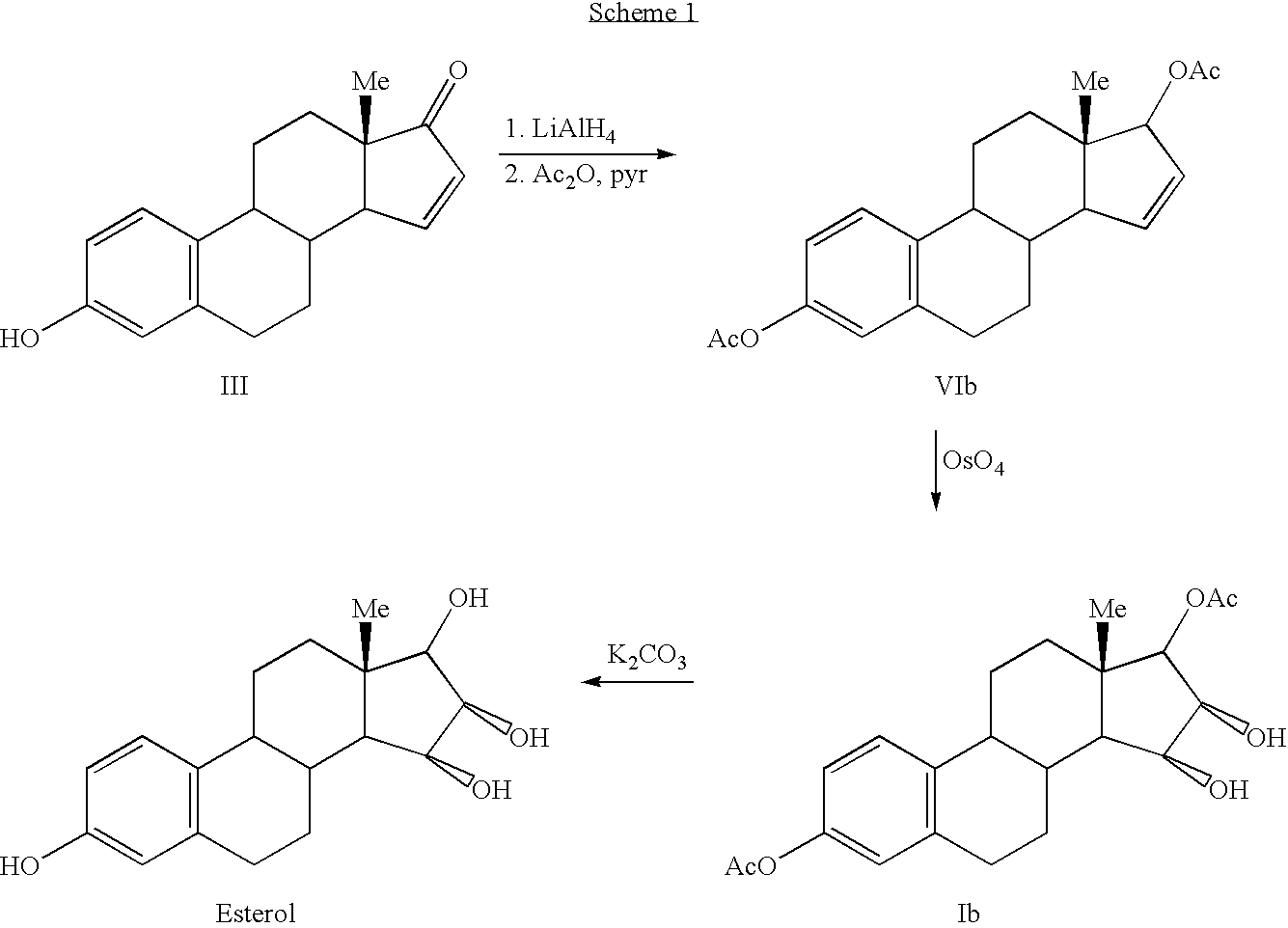
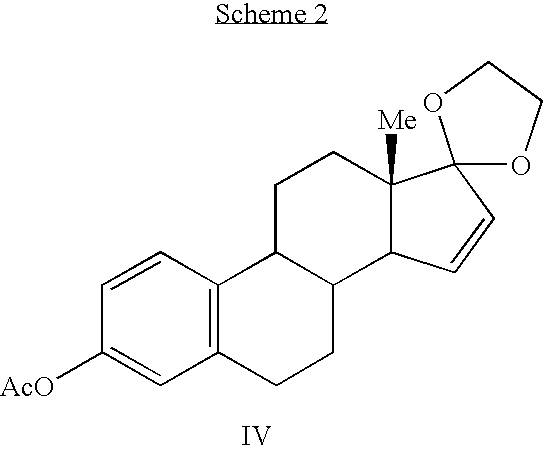
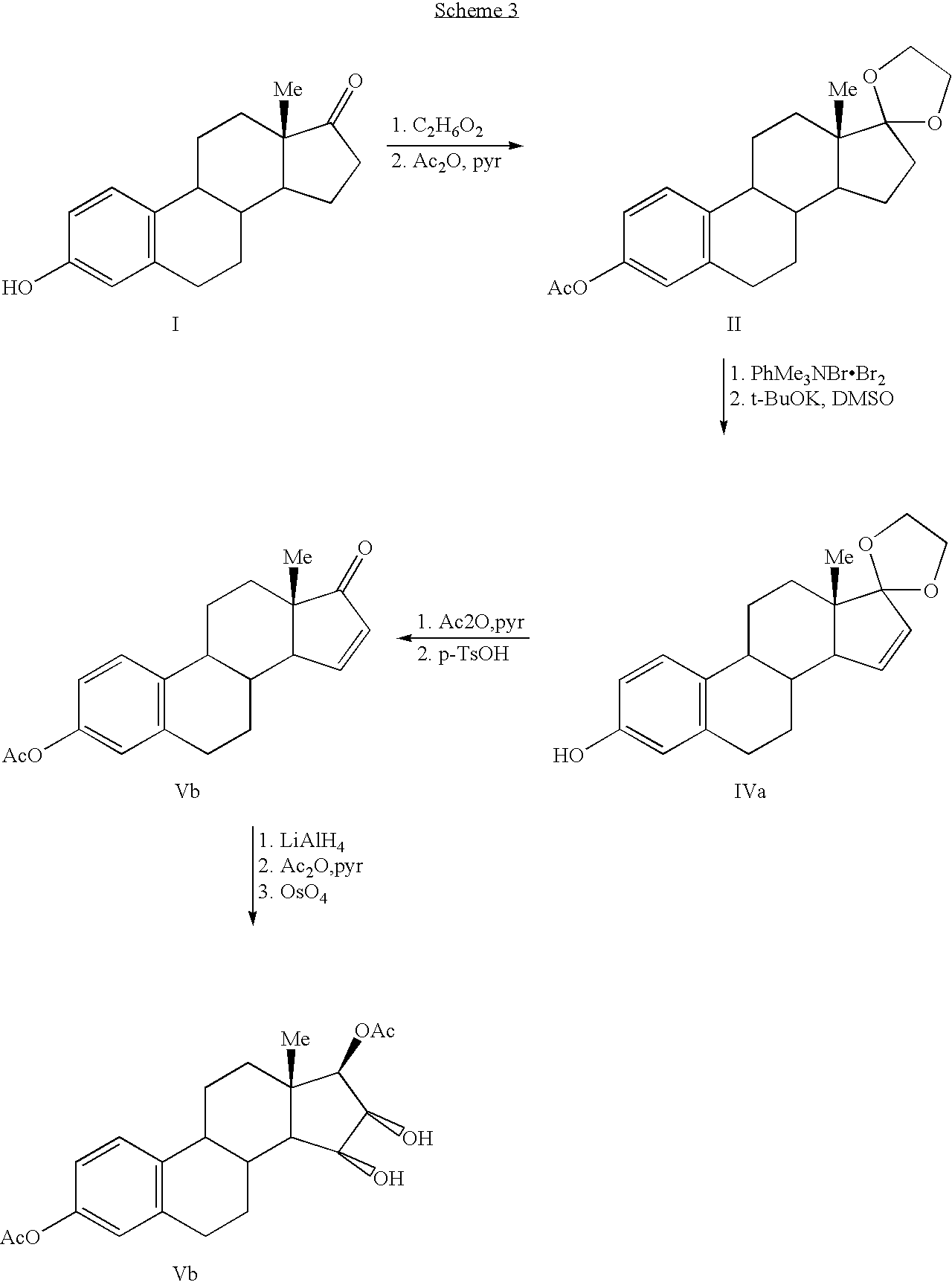
Synthesis of estetrol via estrone derived steroids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-Benzyloxy-estra-1,3,5(10)-trien-17-one (compound 6, A=benzyl)
[0125] To a suspension of estrone (7; 100 g, 0.370 mol) and K2CO3 (160 g, 1.16 mol) in DCM / MeOH (800 mL, 1:1 v / v ratio) at room temperature was added benzyl bromide (132 mL, 1.10 mol) in one portion. The resulting mixture was refluxed for 16 h (50% conversion after 4 h according to TLC). The reaction mixture was cooled to RT and solids were filtered off. The filter-cake was washed with MeOH. The solution was concentrated (to a total volume of ca. 300 mL). The precipitate that had formed was collected by filtration and washed with heptanes to give a white solid. The filtrate was concentrated further (to a total volume of 100 mL) and triturated with heptane. The resulting precipitate was filtered off and combined with the first batch of product. The product (153 g, max 0.370 mol) still contained traces off benzyl bromide but was used without further purification. The product can be purified by recrystallization from DCM / M...
example 2
17,17-Ethylenedioxy-3-benzyloxy estra-1,3,5(10)-trien-17-one (compound 4; A=benzyl, C=ethylene dioxy)
[0126] 3-Benzyl-estrone (compound 6, A=benzyl; 153 g (crude), max. 0.370 mol) was suspended in a mixture of triethyl orthoformate (320 mL) and ethylene glycol (160 mL). p-TsOH monohydrate (5 g, 26.3 mmol) was added and the resulting pinkish suspension was stirred for 3 h at 35° C. (TLC indicated complete conversion after 1.5 h). The mixture was cooled to RT, poured into a mixture of ice-water (2 L) and pyridine (40 mL). The resulting precipitate was collected by filtration and washed with water (150 ml). The remaining white solid was dried azeotropically by stripping with toluene (2×200 mL) to afford the product (153 g, max. 0.370 mmol) as white crystalline material. TLC: Rf=0.3 (heptanes / ethyl acetate=9 / 1); 1H-NMR (200 MHz, CDCl3) δ 7.60-7.24 (m, 5H), 7.29 (d, 1H, J=8.4 Hz), 6.86 (dd, 1H, J1=2.6 Hz, J2=8.4 Hz), 6.80 (d, 1H, J=2.4 Hz), 5.11 (s, 2H), 4.03 (m, 4H), 3.05-2.90 (m, 2H), ...
example 3
17,17-Ethylenedioxy-3-benzyloxy estra-1,3,5(10)-trien-17-one (compound 4; A=benzyl, C=ethylene dioxy)
[0127] A reaction flask equipped with mechanical stirrer, thermometer, nitrogen purge, condenser and dropping funnel was used for the process. The flask was charged with 27 g (100 mmol) of estrone, 50 ml (55 g, 9 equivalents) of glycol and 24 g of triethylorthoformate. The resulting mixture was stirred. 0.5 g of toluenesulfonic acid was added and the reaction temperature was raised to 45° C. At about 35-40° C. an exothermic was observed. The slurry is stirred for 1 hour at 45° C. The conversion is checked with LC. Usually after 1 hour almost complete conversion is observed. To the slurry a solution of sodium methoxide in methanol (30% wt.; 1.1 equivalents) is added from the dropping funnel resulting in a clear yellow solution. The temperature is raised to 65° C. and 15 g of benzyl chloride is added over 5 minutes. Within a few minutes the solution becomes turbid and slowly thickens ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com