Patents
Literature
124results about "Ketal steroids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
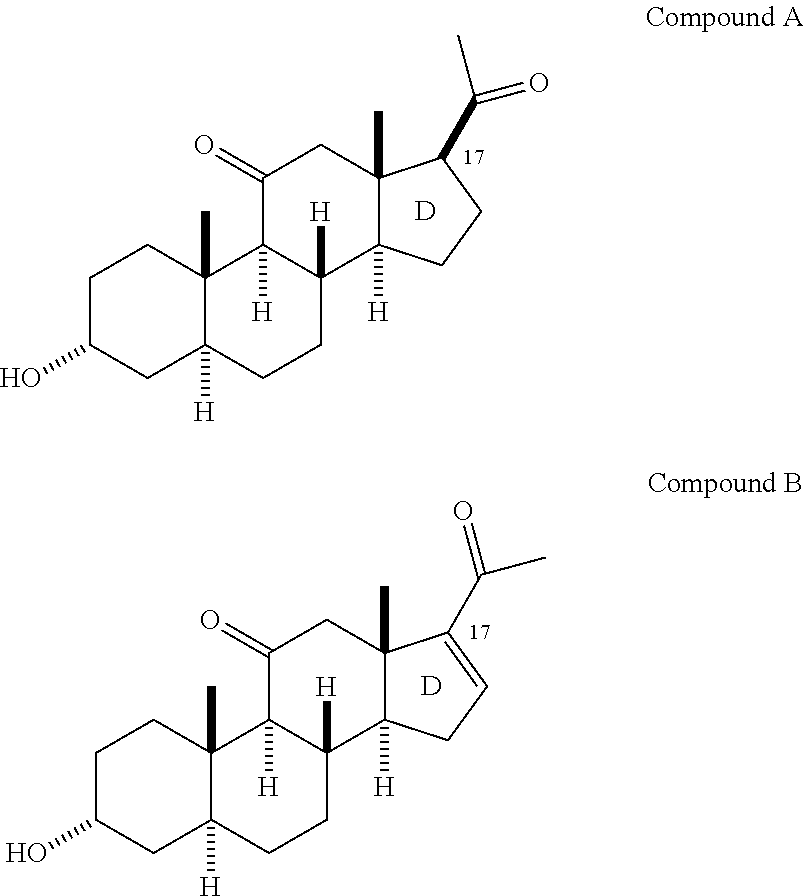
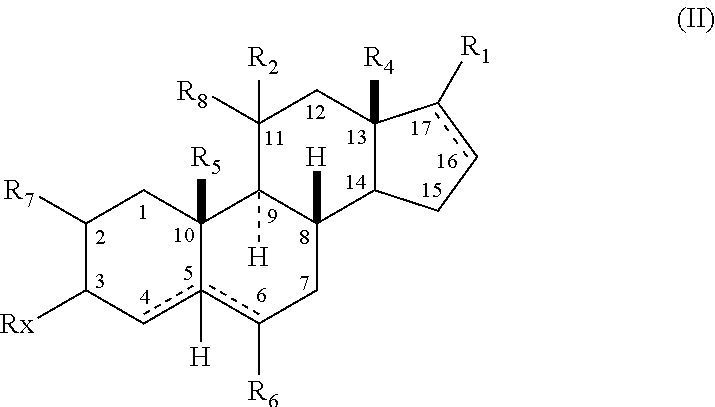
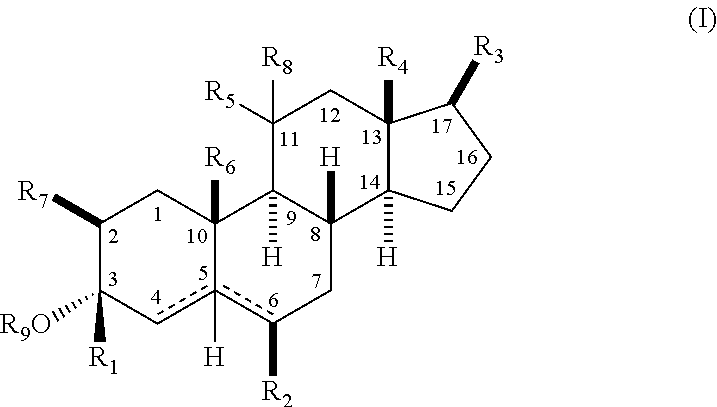
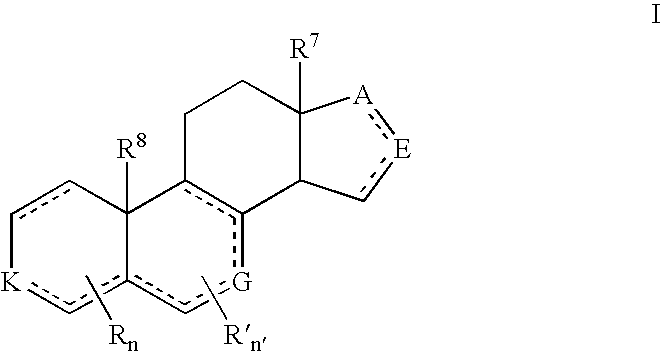
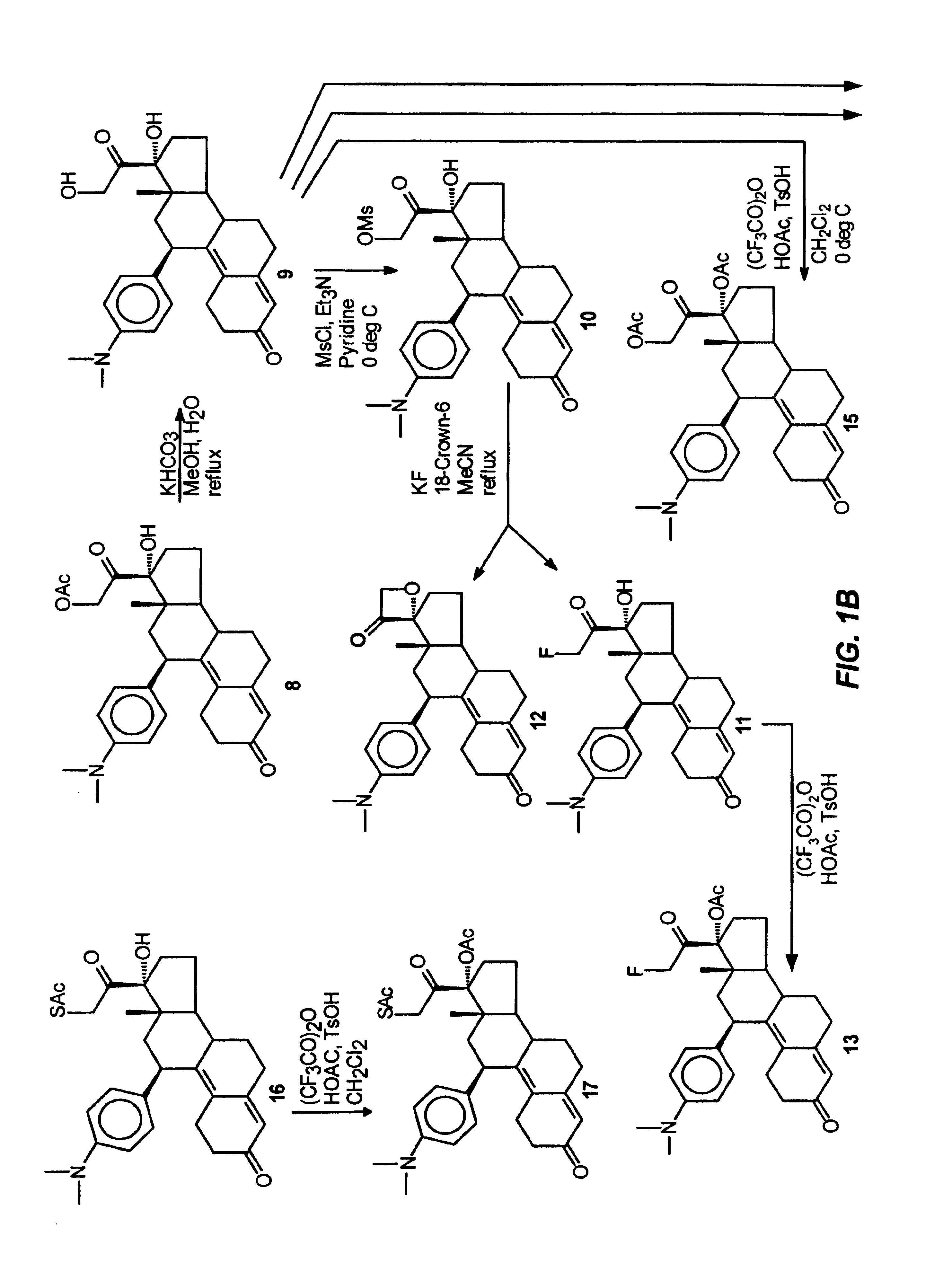
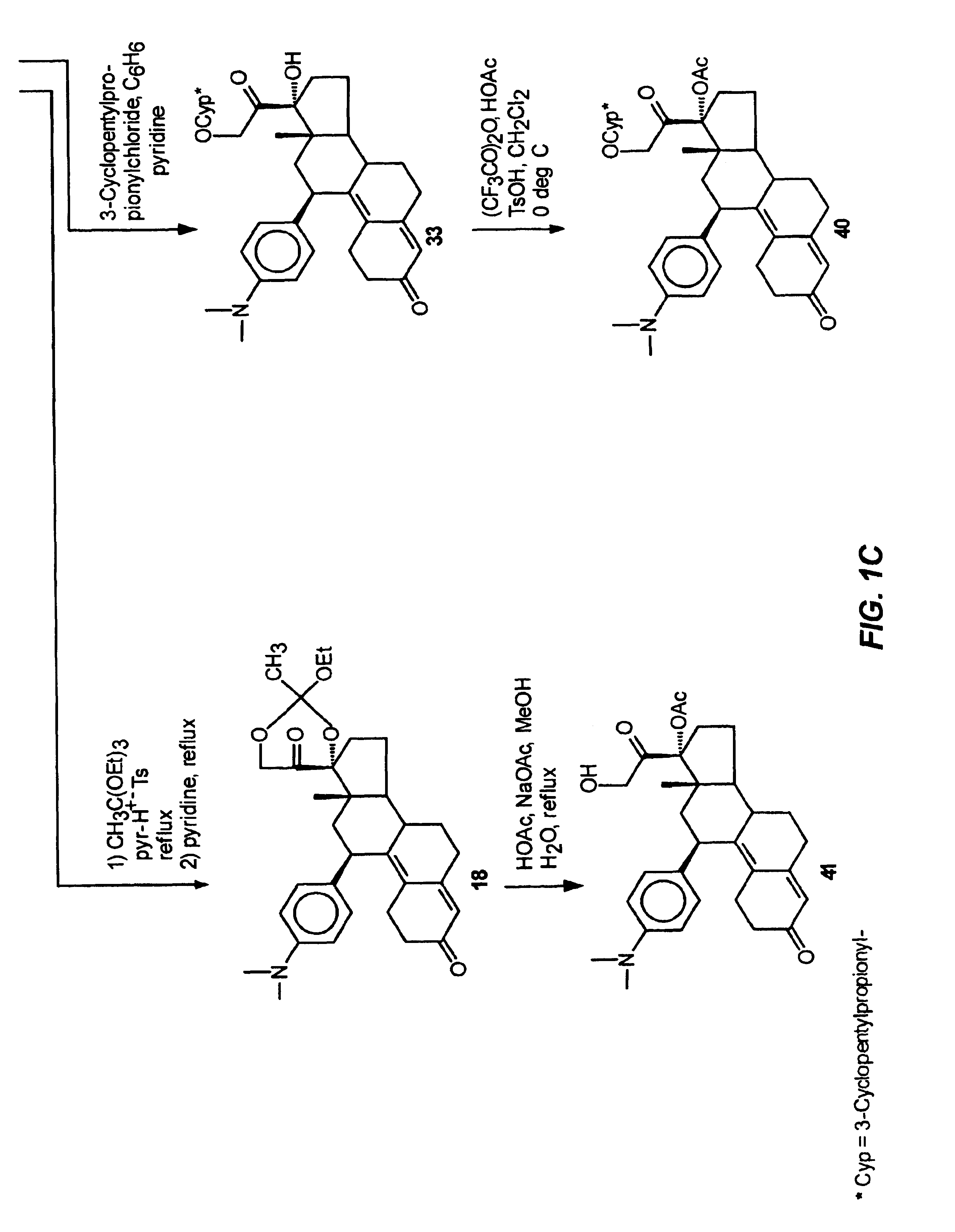
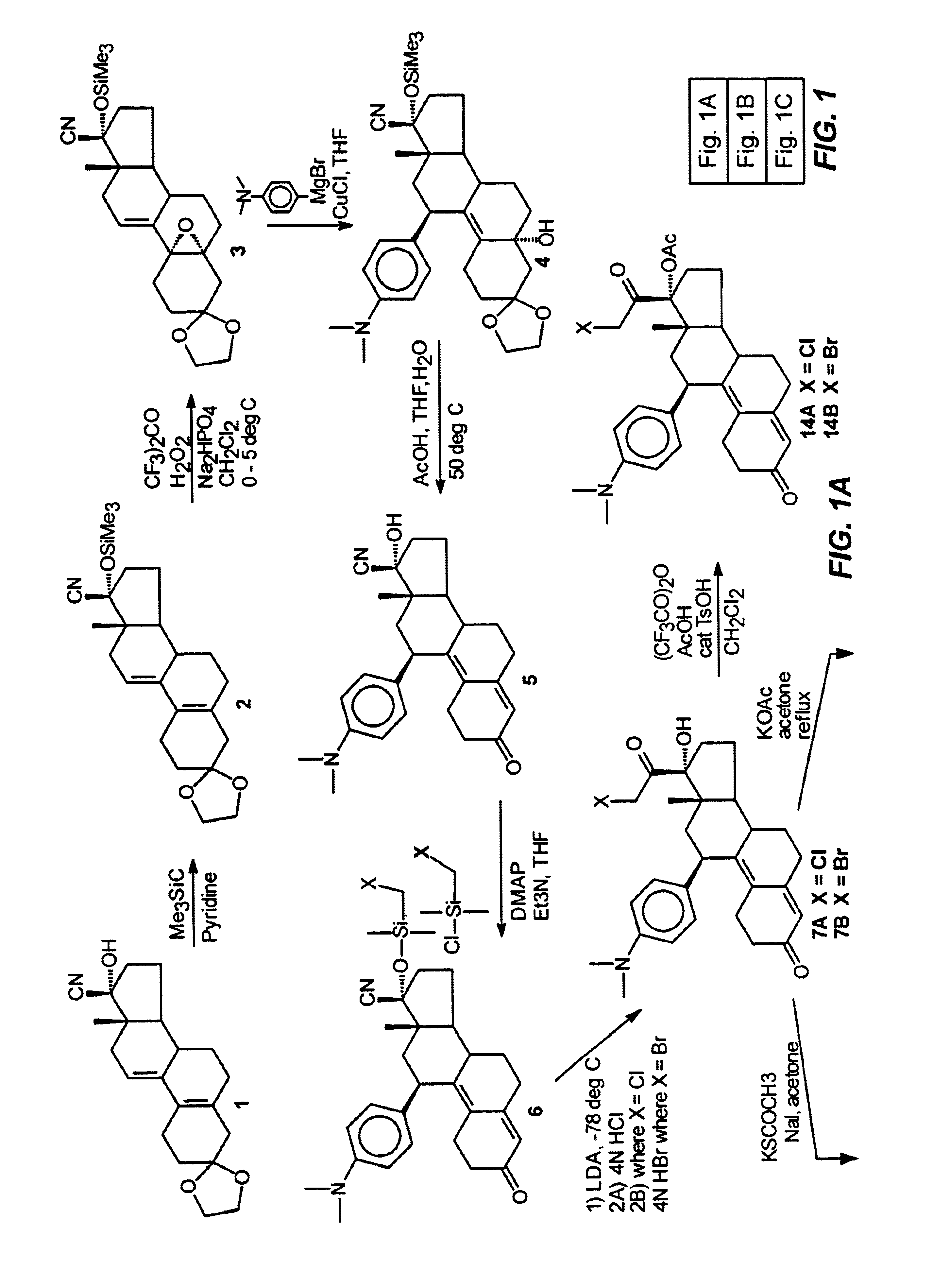
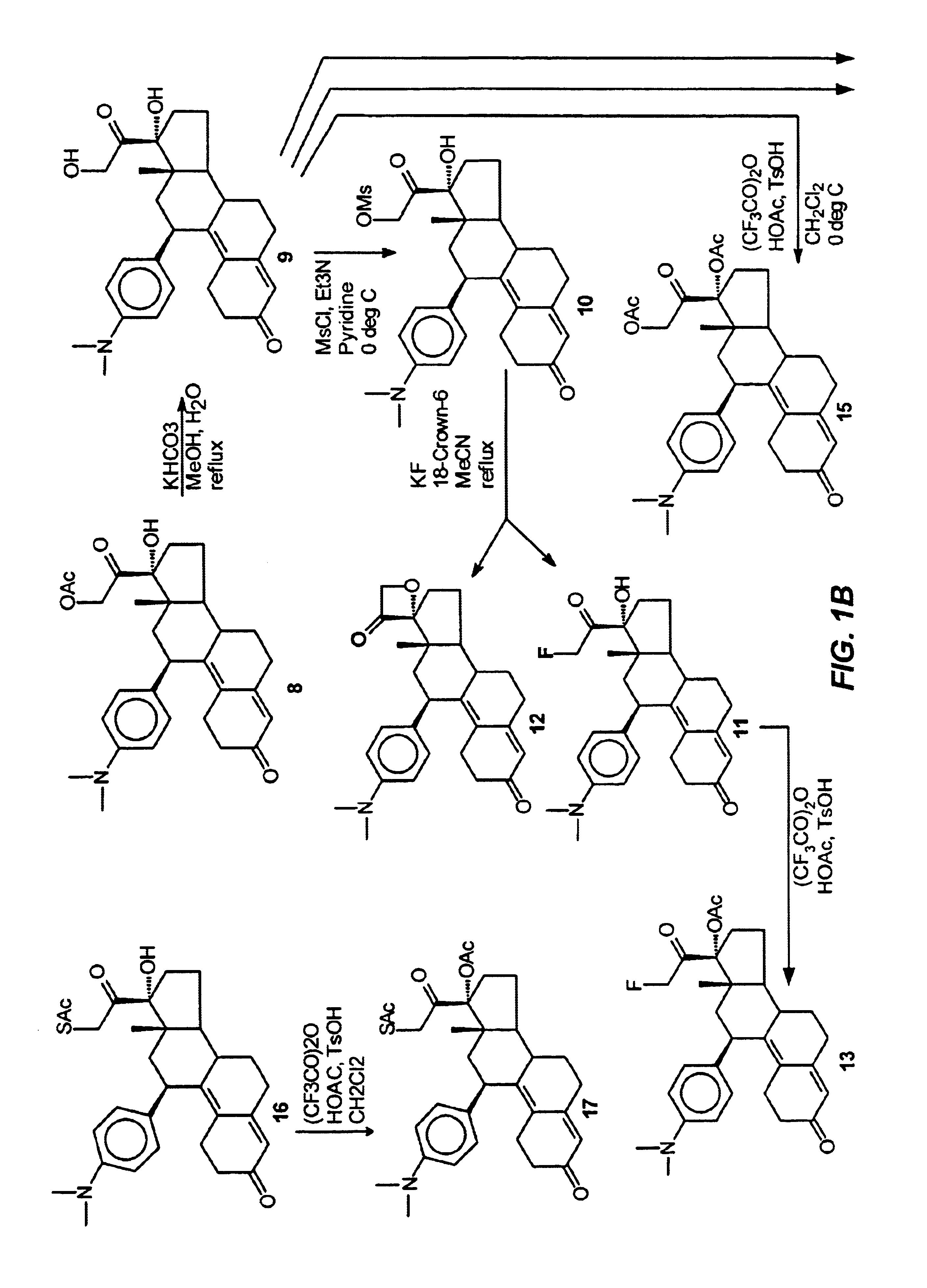
Structural modification of 19-norprogesterone I: 17-α-substituted-11-β-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
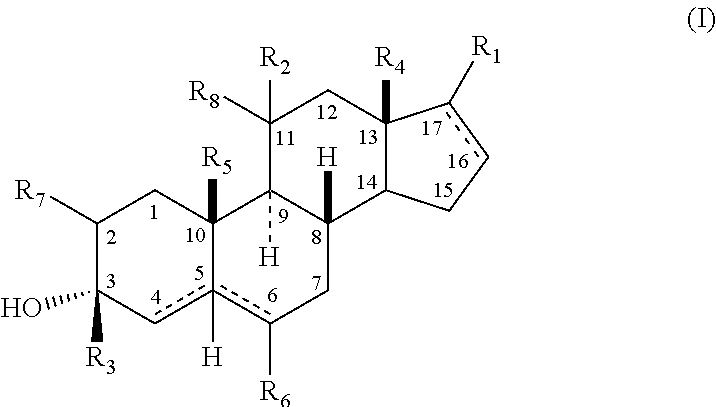
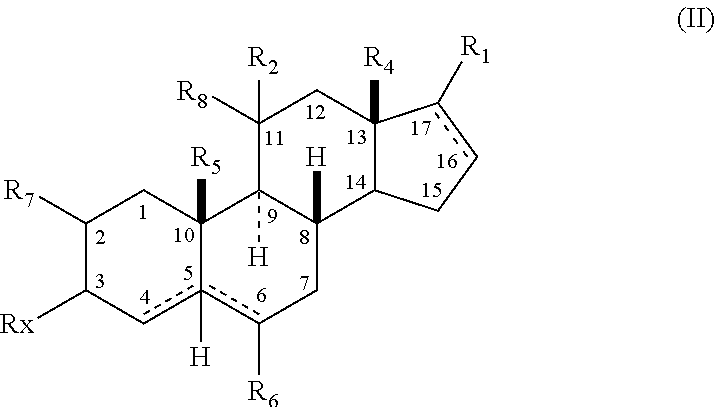
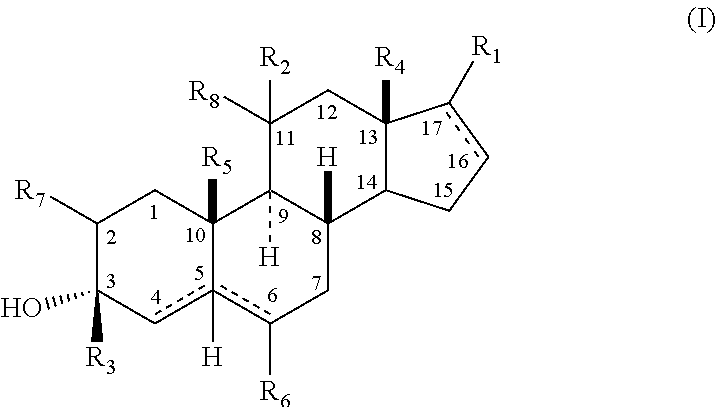
The present invention relates, inter alia, to compounds having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —NC4H8, —NC5H10, —NC4H8O, —CHO, —CH(OH)CH3, —C(O)CH3, —O(CH2)2N(CH3)2, and —O(CH2)2NC5H10; R2 is a member selected from the group consisting of hydrogen, halogen, alkyl, acyl, hydroxy, alkoxy (e.g., methoxy, ethoxy, vinyloxy, ethynyloxy, cyclopropyloxy, etc.), acyloxy (e.g., acetoxy, glycinate, etc.), alkylcarbonate, cypionyloxy, S-alkyl, —SCN, S-acyl and —OC(O)R6, wherein R6 is a functional group including, but not limited to, alkyl (e.g., methyl, ethyl, etc.), alkoxy ester (e.g., —CH2OCH3) and alkoxy (—OCH3); R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat meningiomas; to treat uterine leiomyomas; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce cervical ripening; to induce labor; and for contraception.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
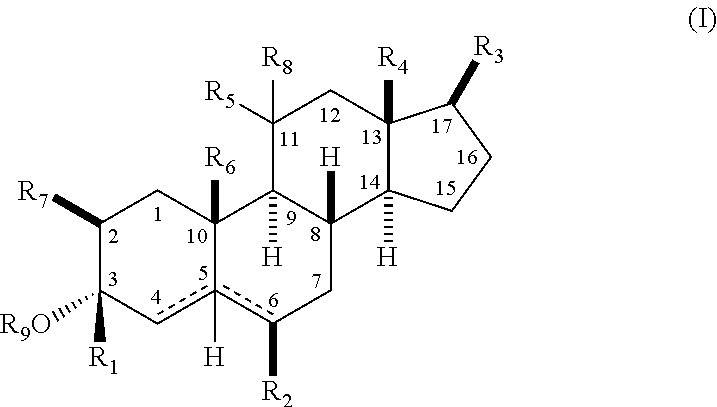
21-substituted progesterone derivatives as new antiprogestational agents
A compound having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —CHO, —COCH3 and —CHOHCH3; R2 is a member selected from the group consisting of halogen, alkyl, acyl, hydroxy, alkoxy, acyloxy, alkyl carbonate, cypionyloxy, S-alkyl and S-acyl; R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce labor; and for contraception.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE
Benzopyran-containing compounds and method for their use
InactiveUS6060503AAvoid conversionEasy to synthesizeBiocideOrganic compound preparationDiseaseBenzopyran
Certain benzopyran antiestrogens are disclosed for treating estrogen sensitive diseases such as breast cancer. Prodrug forms provide ease of manufacturing, good shelf life, and bioavailability, and preferred stereoisomers are shown to be more effective than racemic mixtures.
Owner:ENDORES & DEV
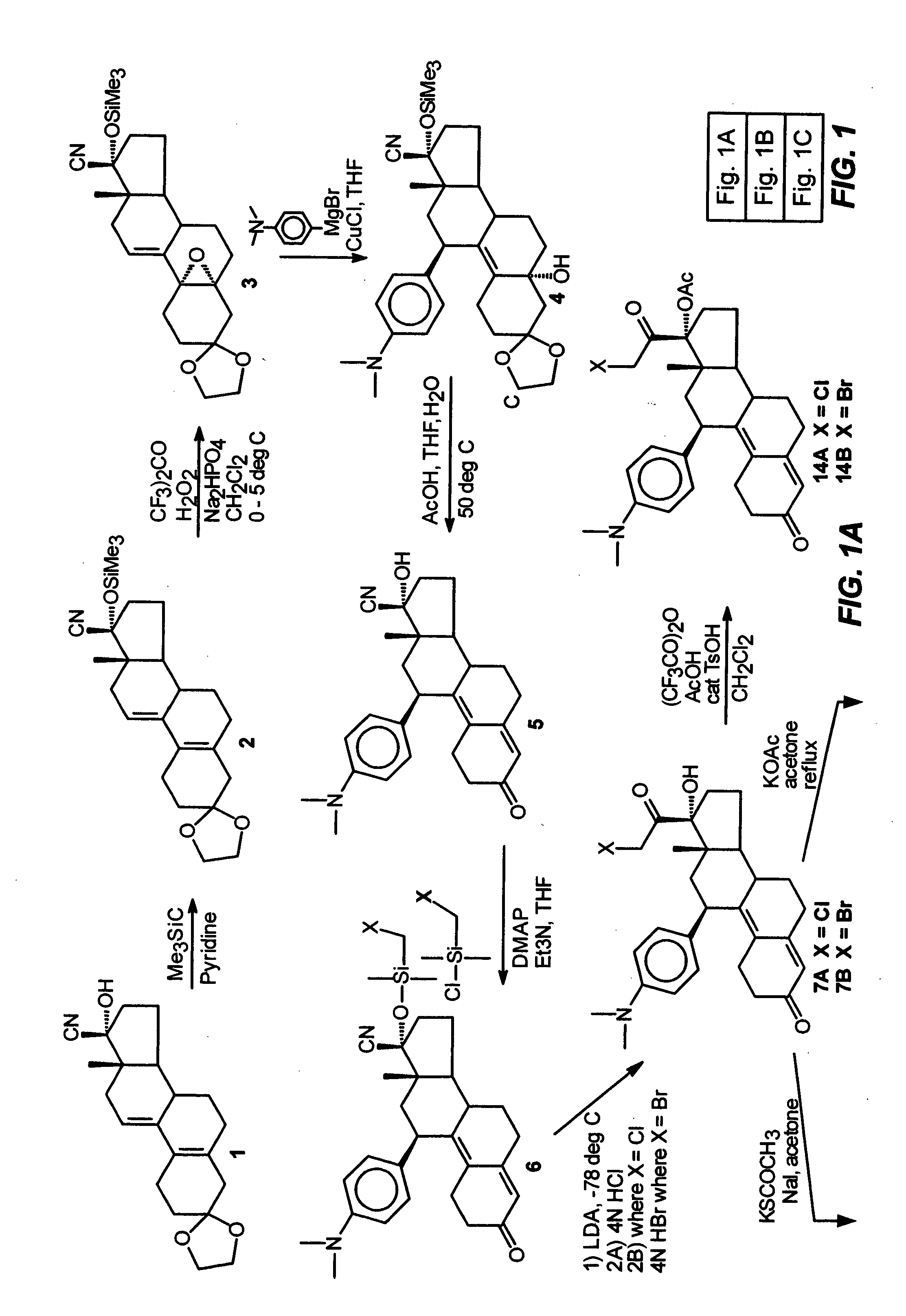
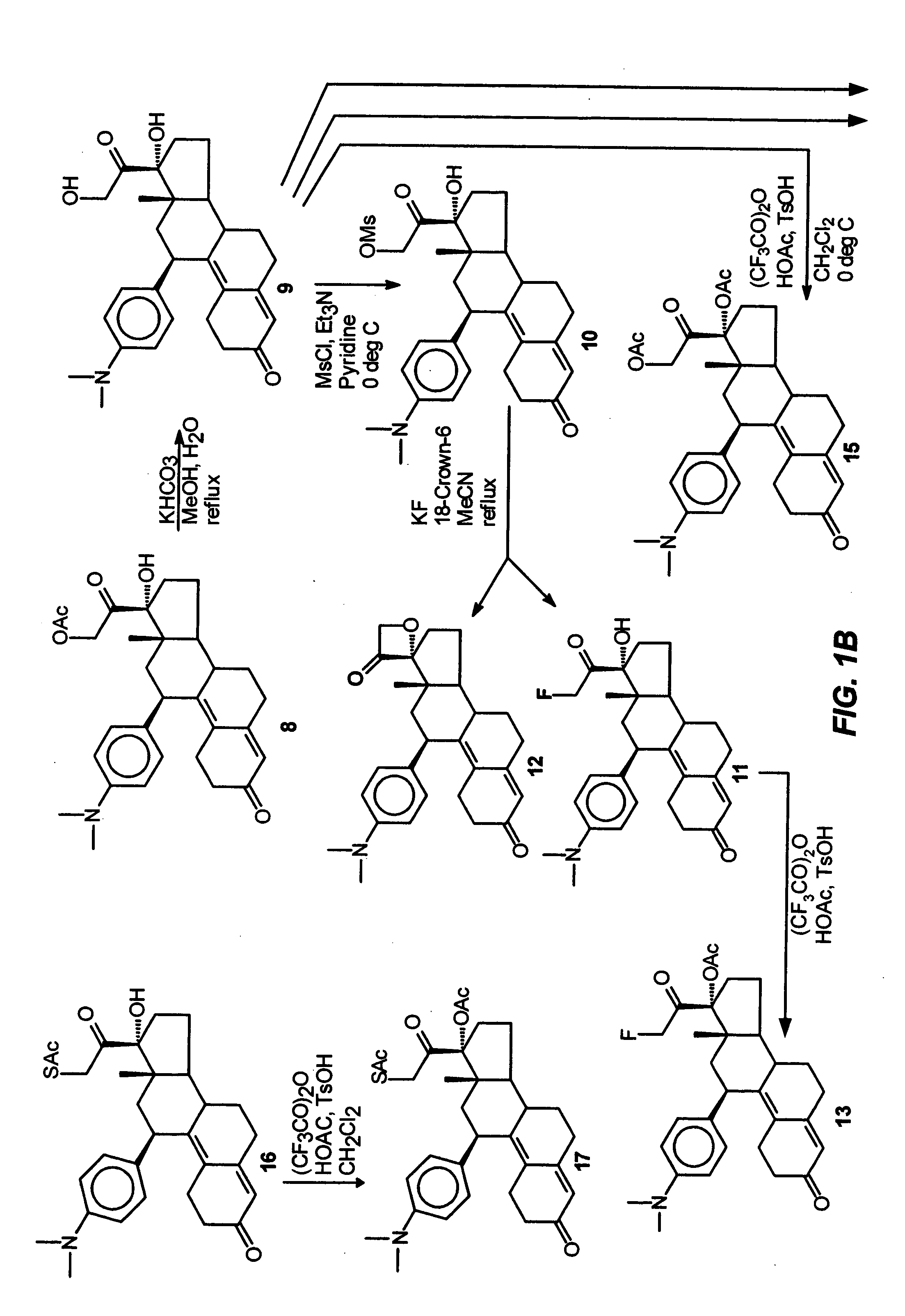
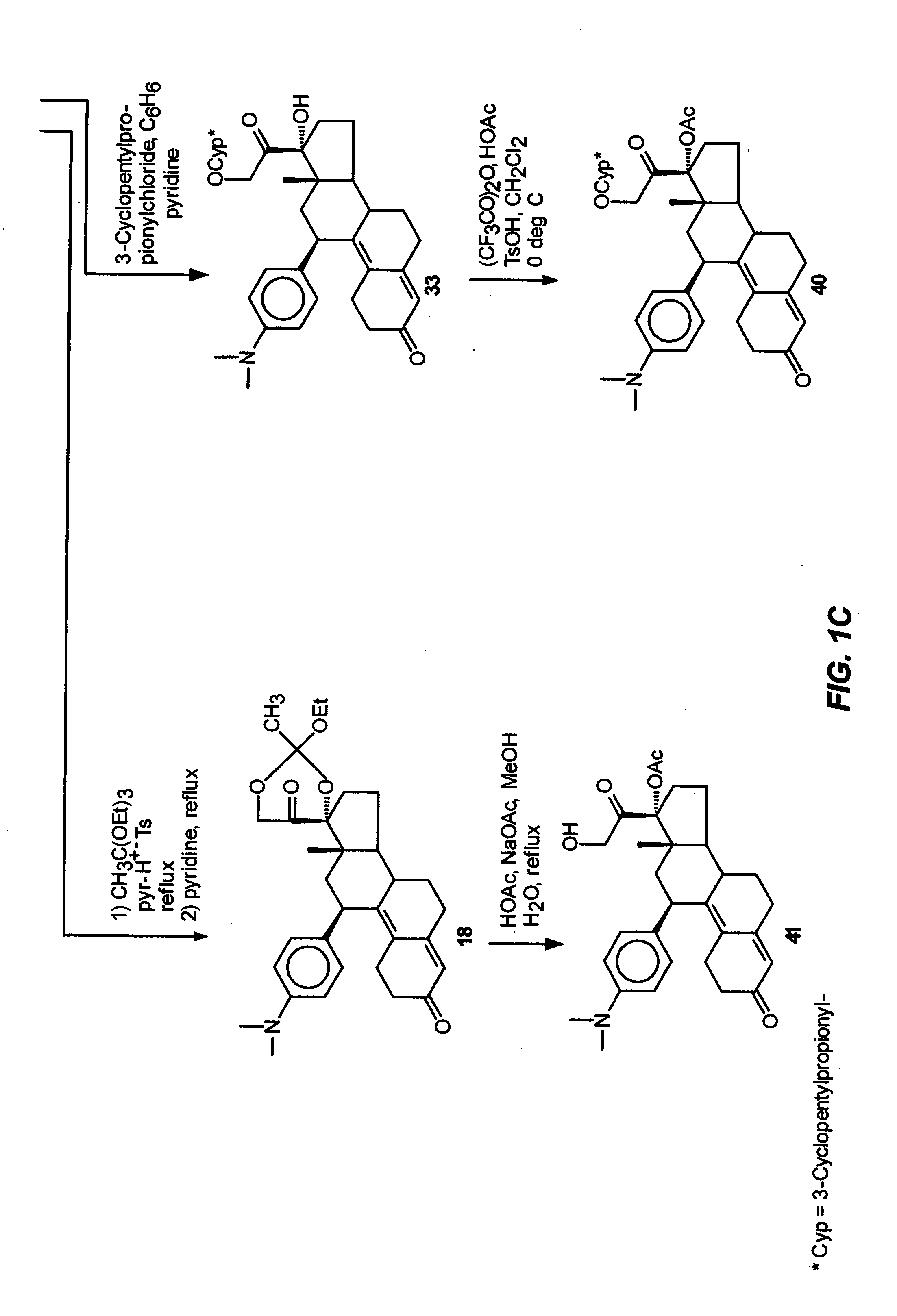
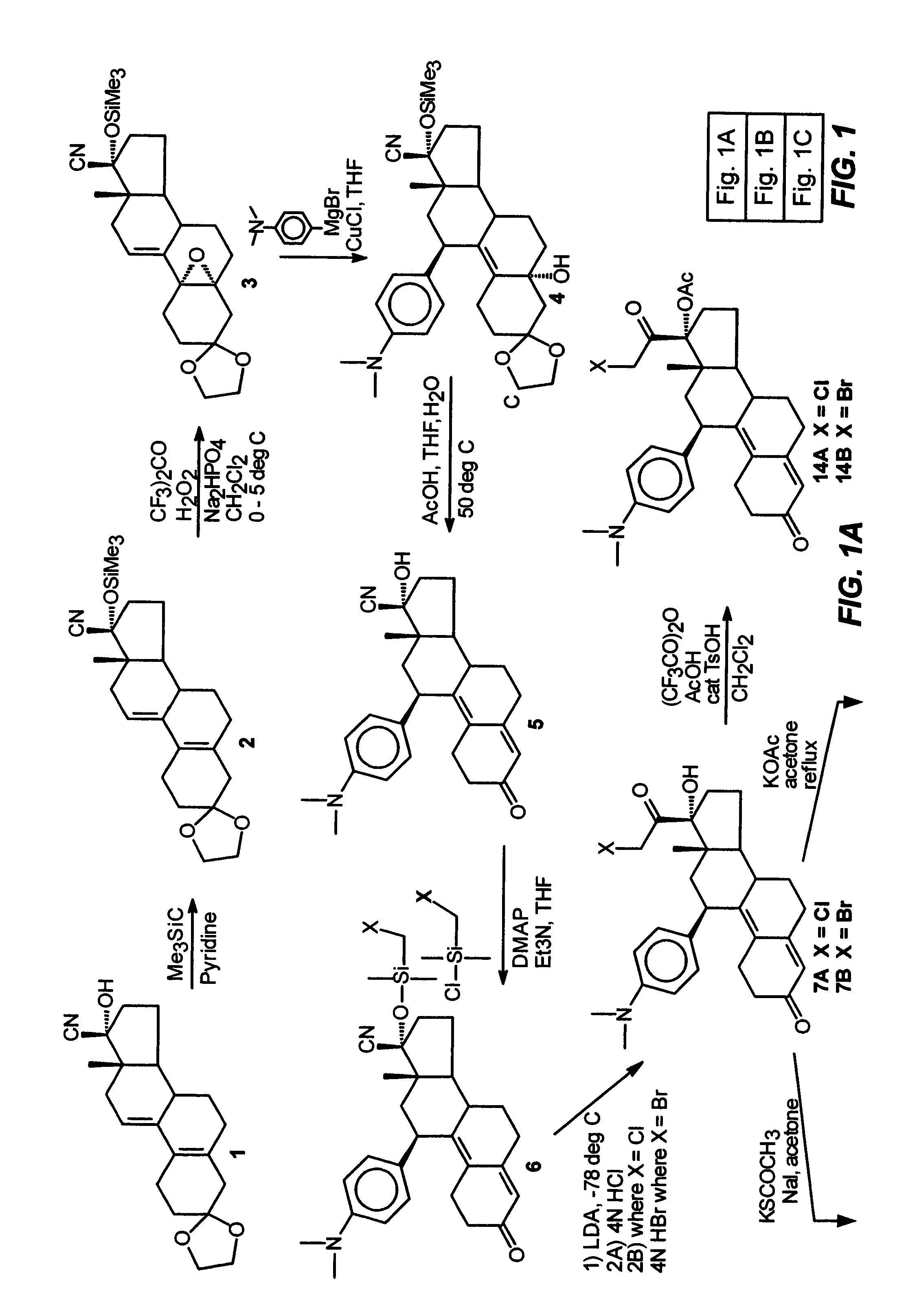
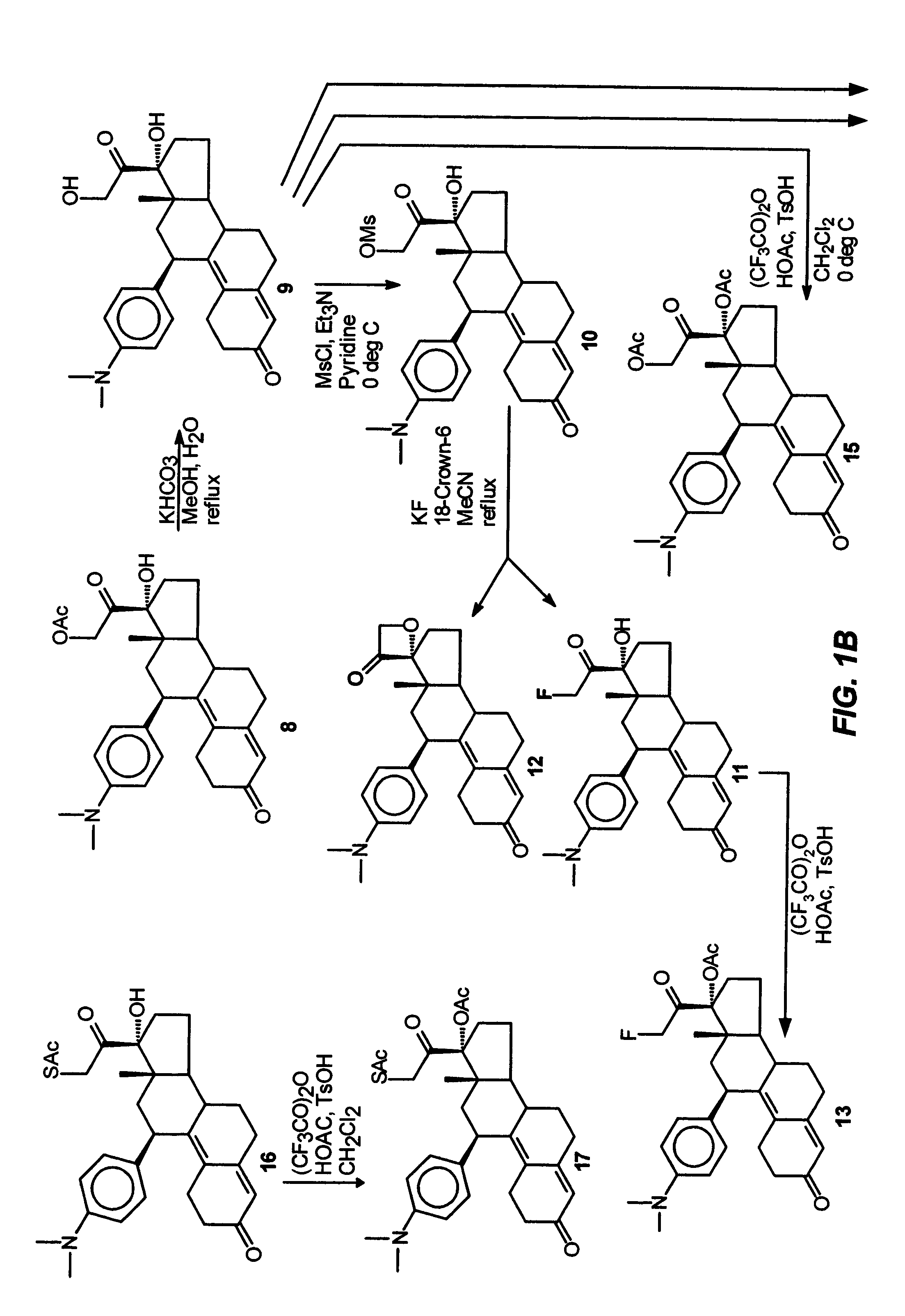
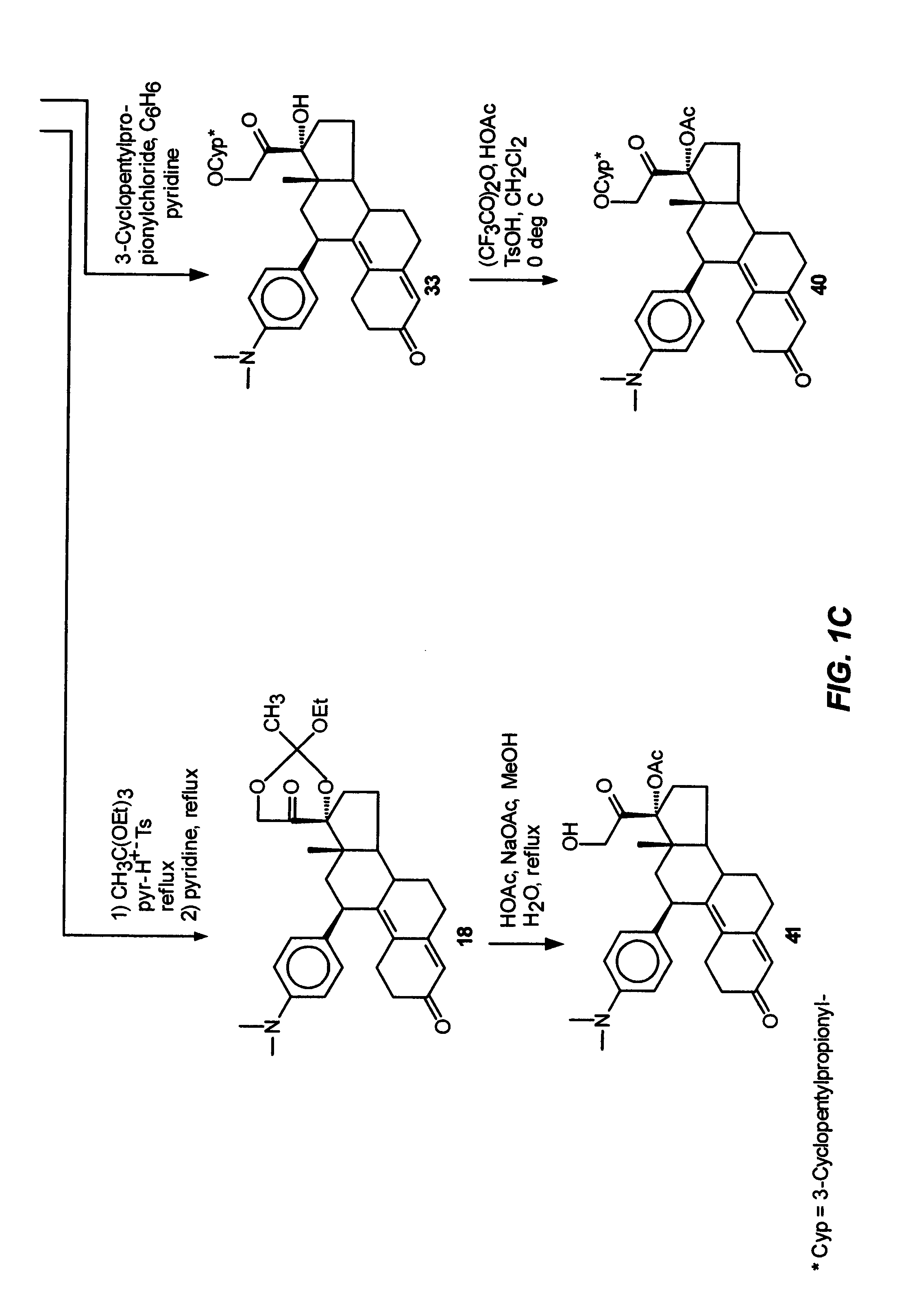
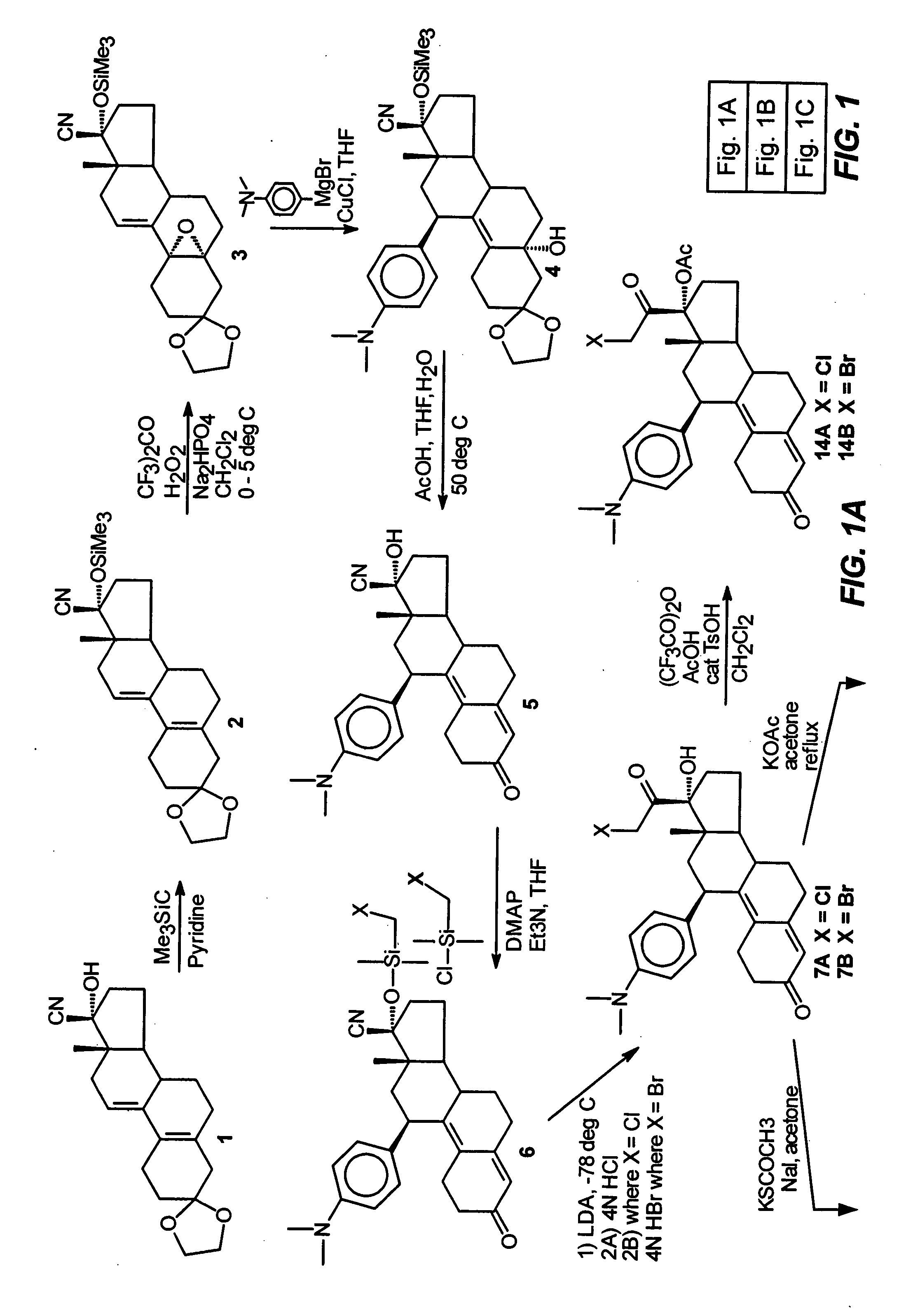
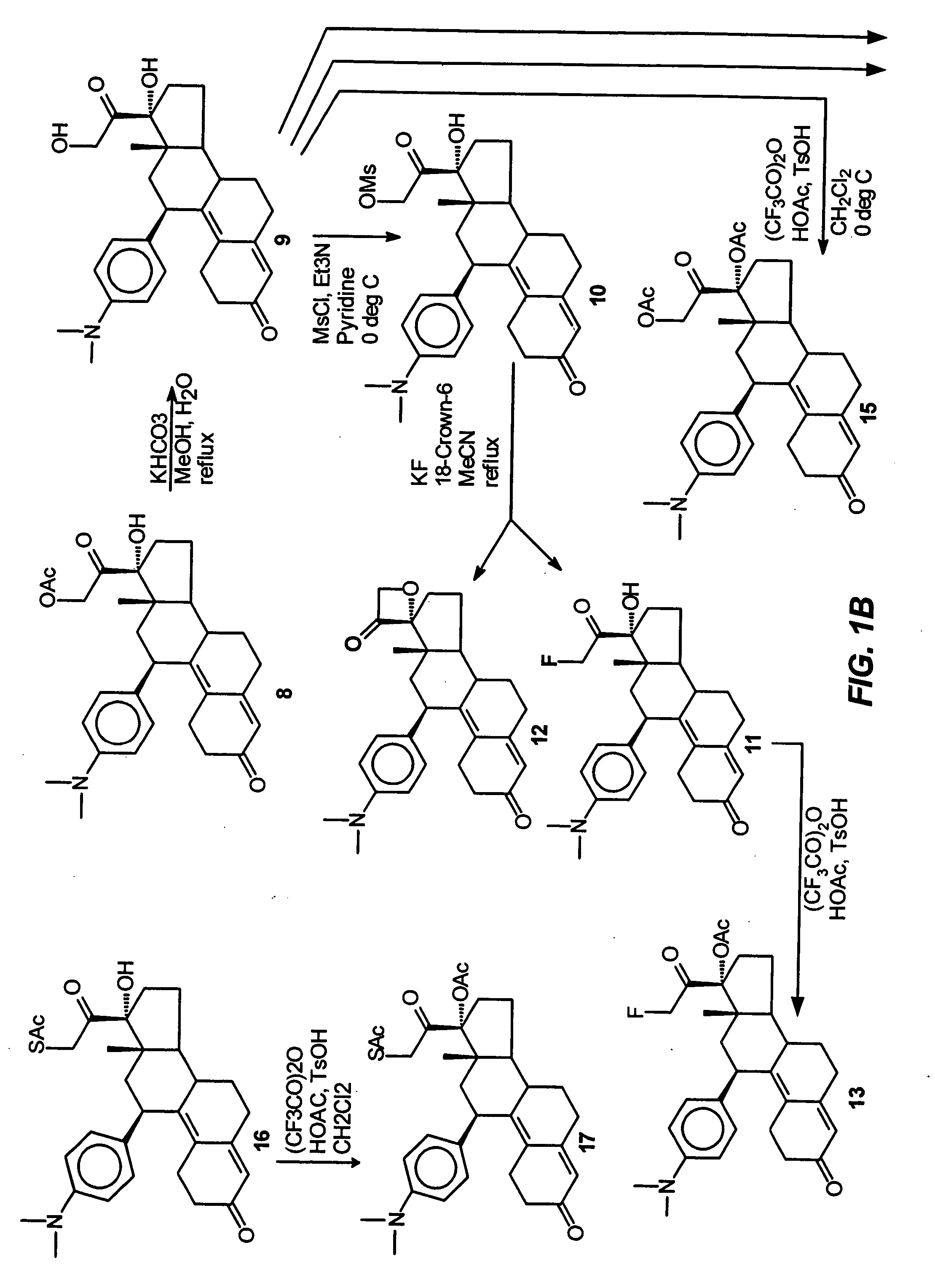
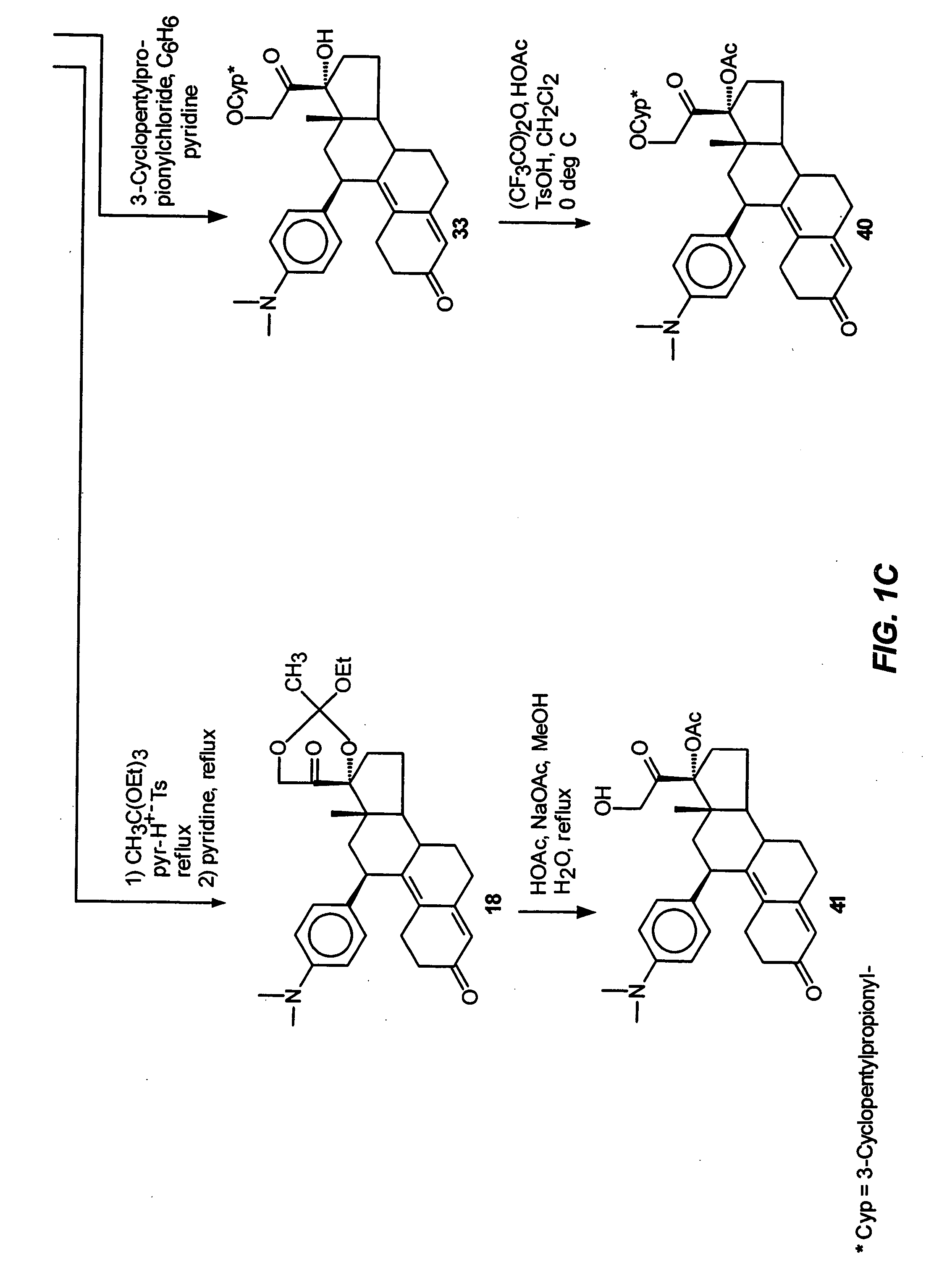
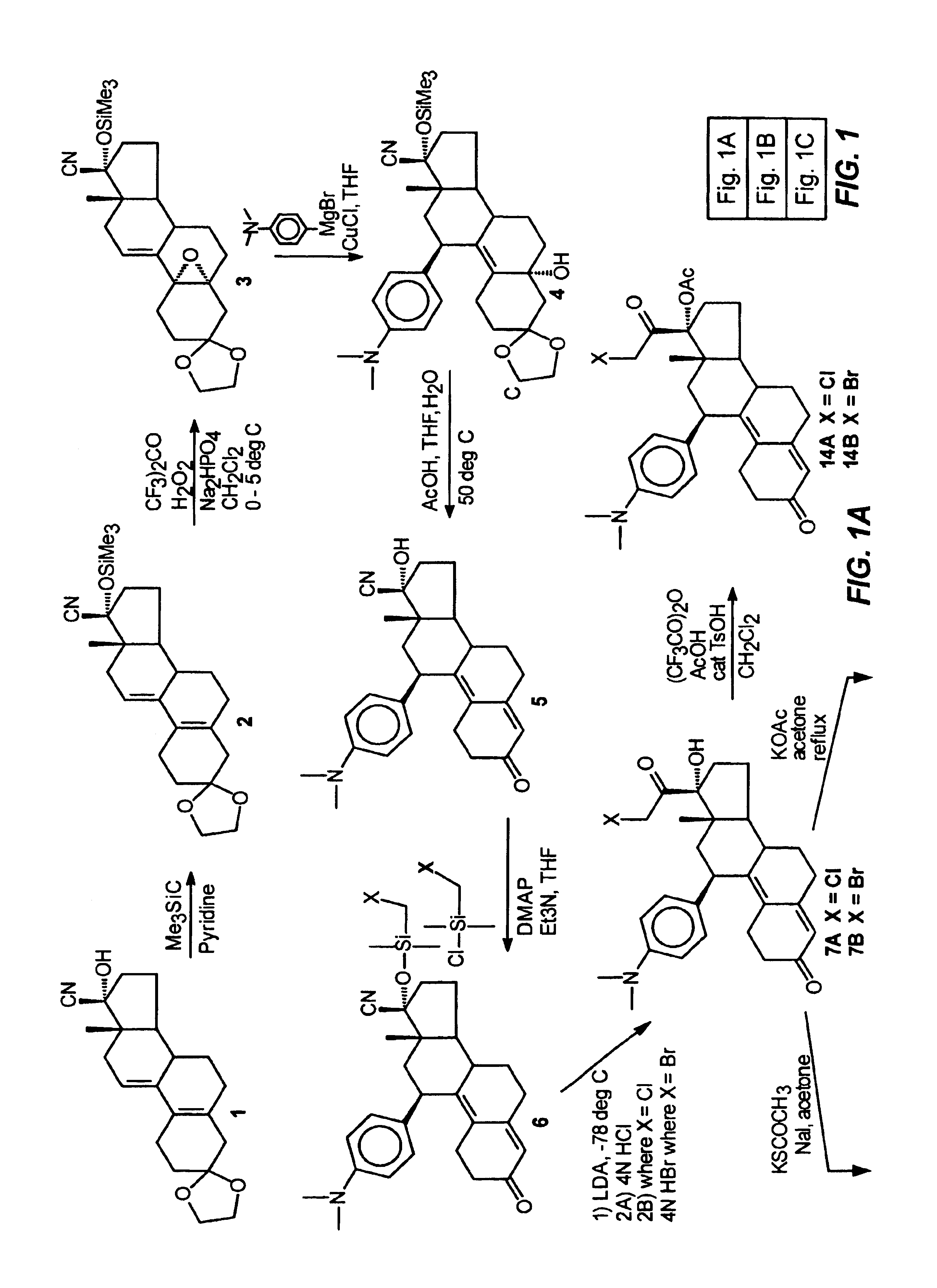
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
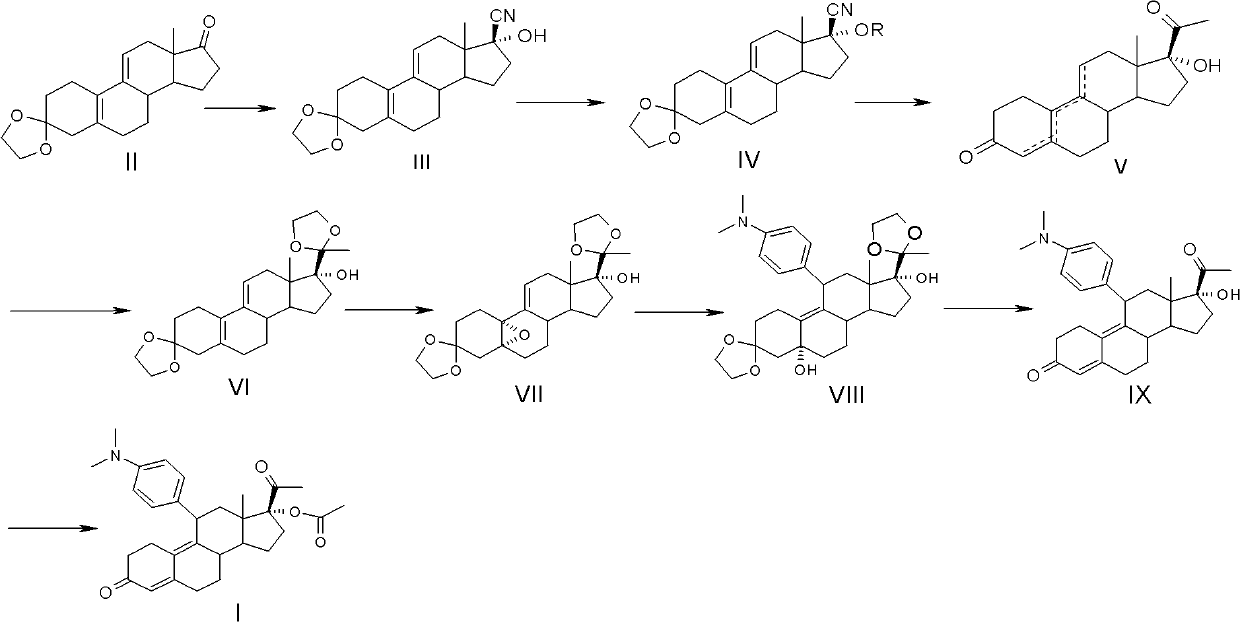
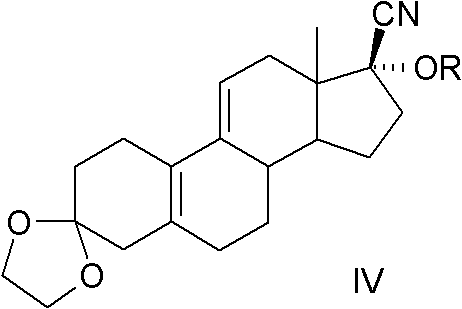
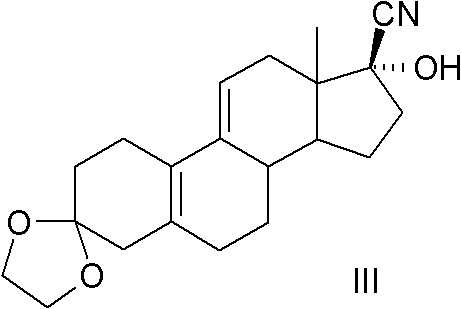
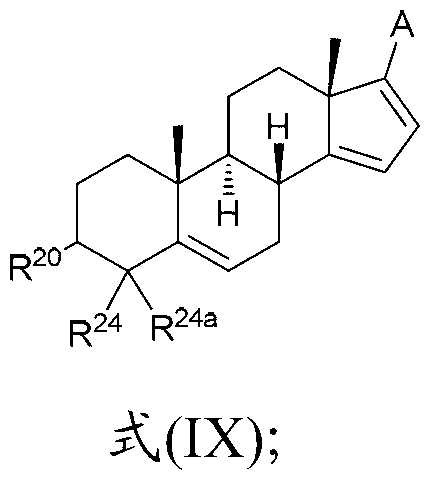
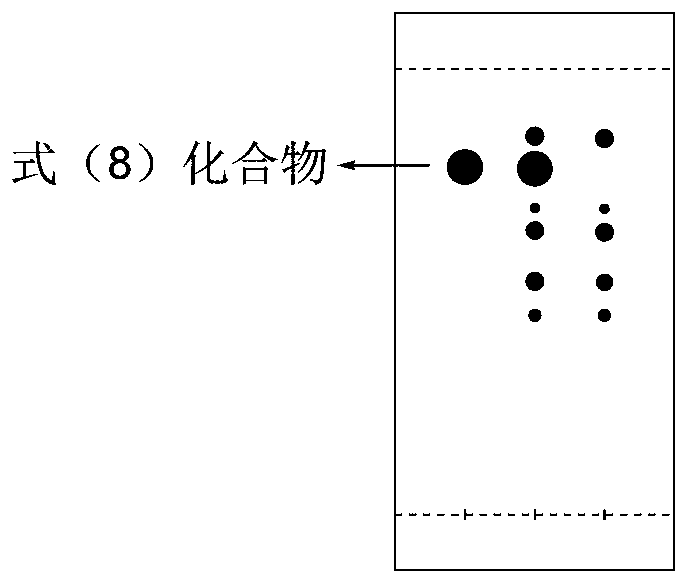
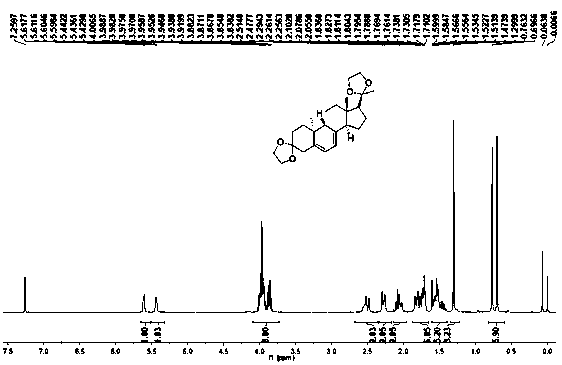
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Neuroactive 19-alkoxy-17-substituted steroids, prodrugs thereof, and methods of treatment using same
The present disclosure is generally directed to neuroactive 19-alkoxy-17-substituted steroids as referenced herein, and pharmaceutically acceptable salts thereof, for use as, for example, an anesthetic, and / or in the treatment of disorders relating to GABA function and activity. The present disclosure is further directed to pharmaceutical compositions comprising such compounds.
Owner:SAGE THERAPEUTICS +1
Methods of making, using and pharmaceutical formulations comprising 7alpha, 11beta-dimethyl-17beta-hydroxyestra-4, 14-dien-3-one and 17 esters thereof
InactiveUS7196074B2High yieldPositively affectingBiocideOrganic active ingredientsParenteral Dosage FormKetone
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Androgen derivatives for use in the inhibition of sex steroid activity
InactiveUS6110906AUseful in treatmentInhibiting sex steroid activityBiocidePhosphorous compound active ingredientsDiseaseAndrogen synthesis
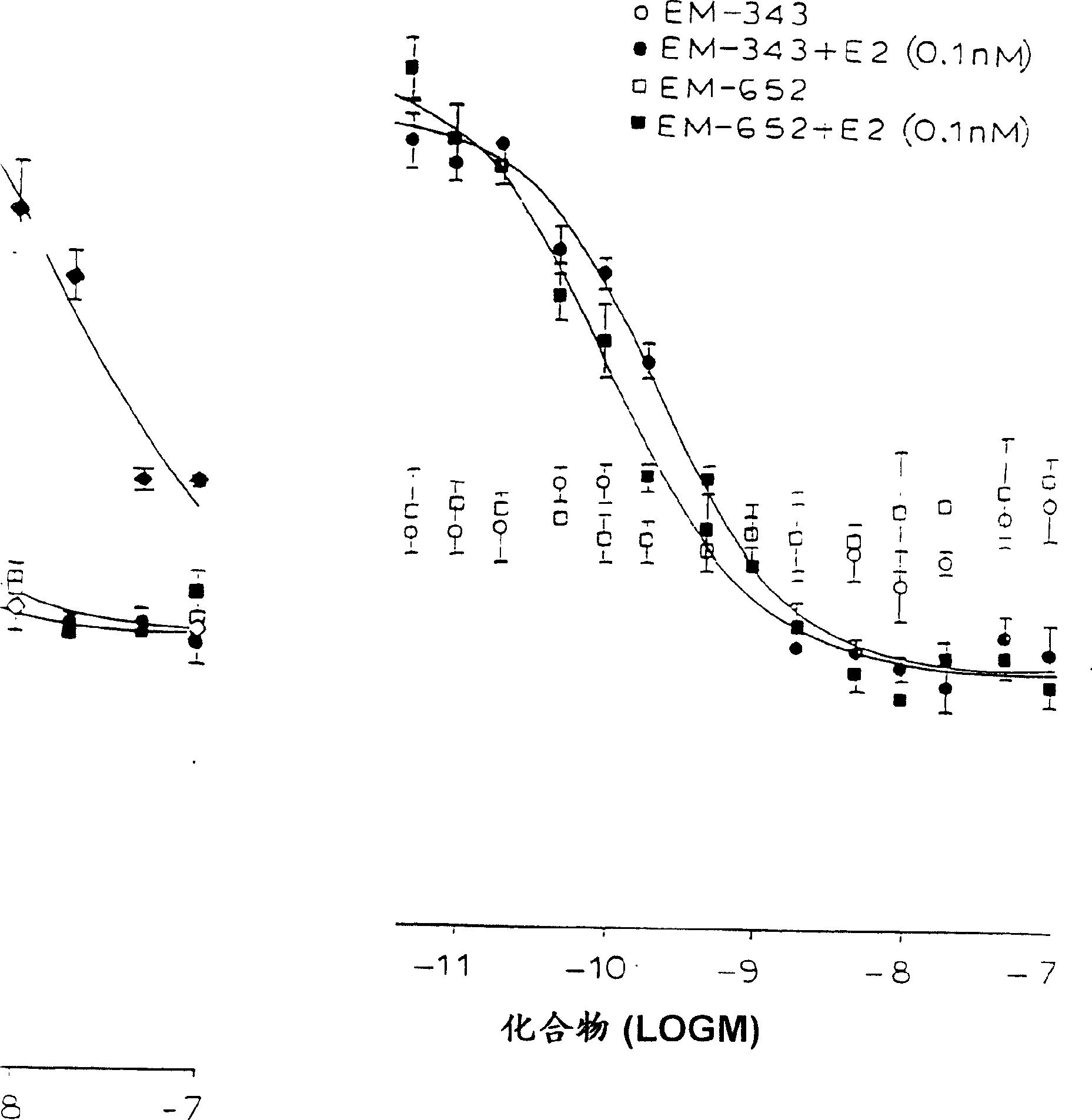
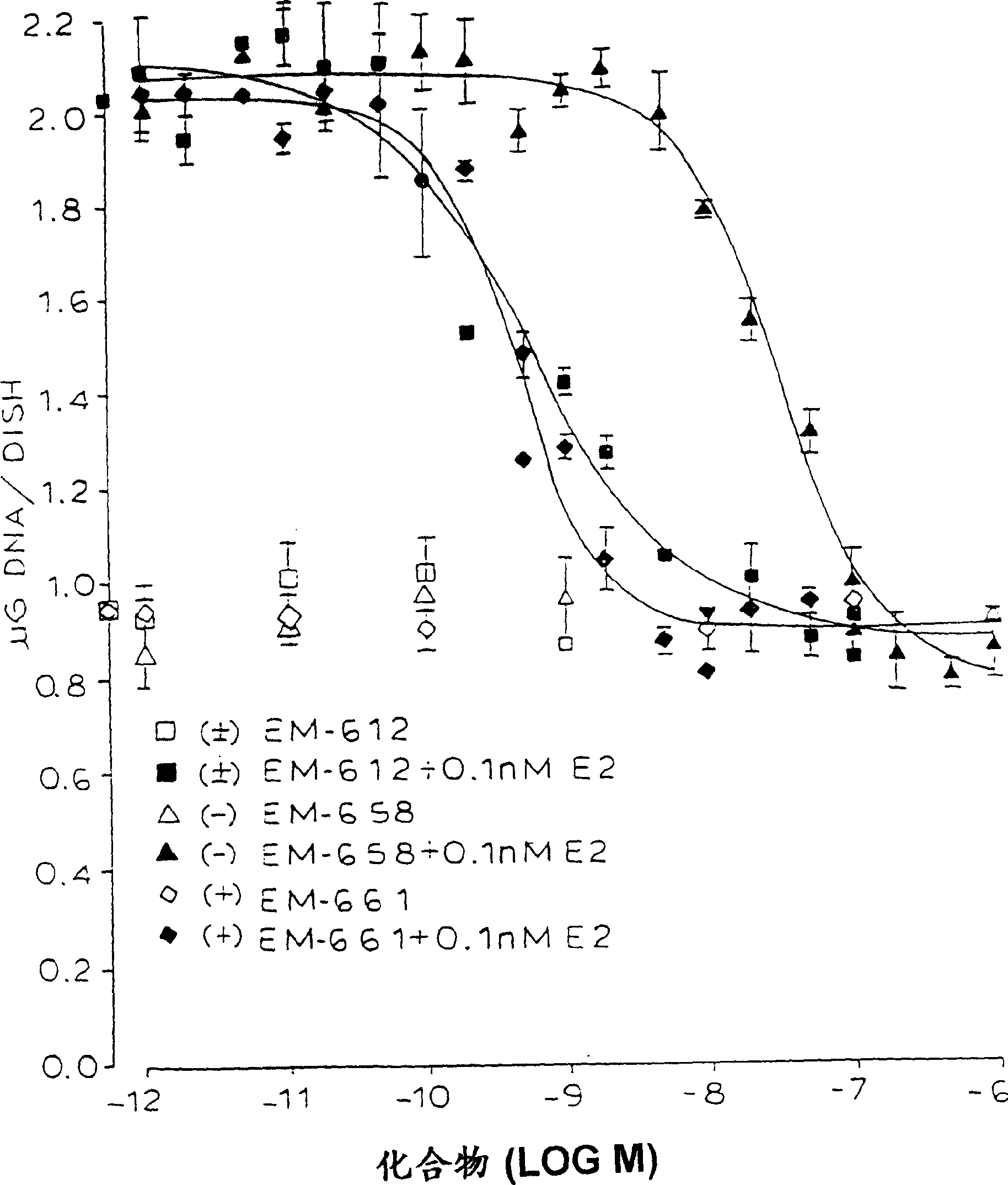
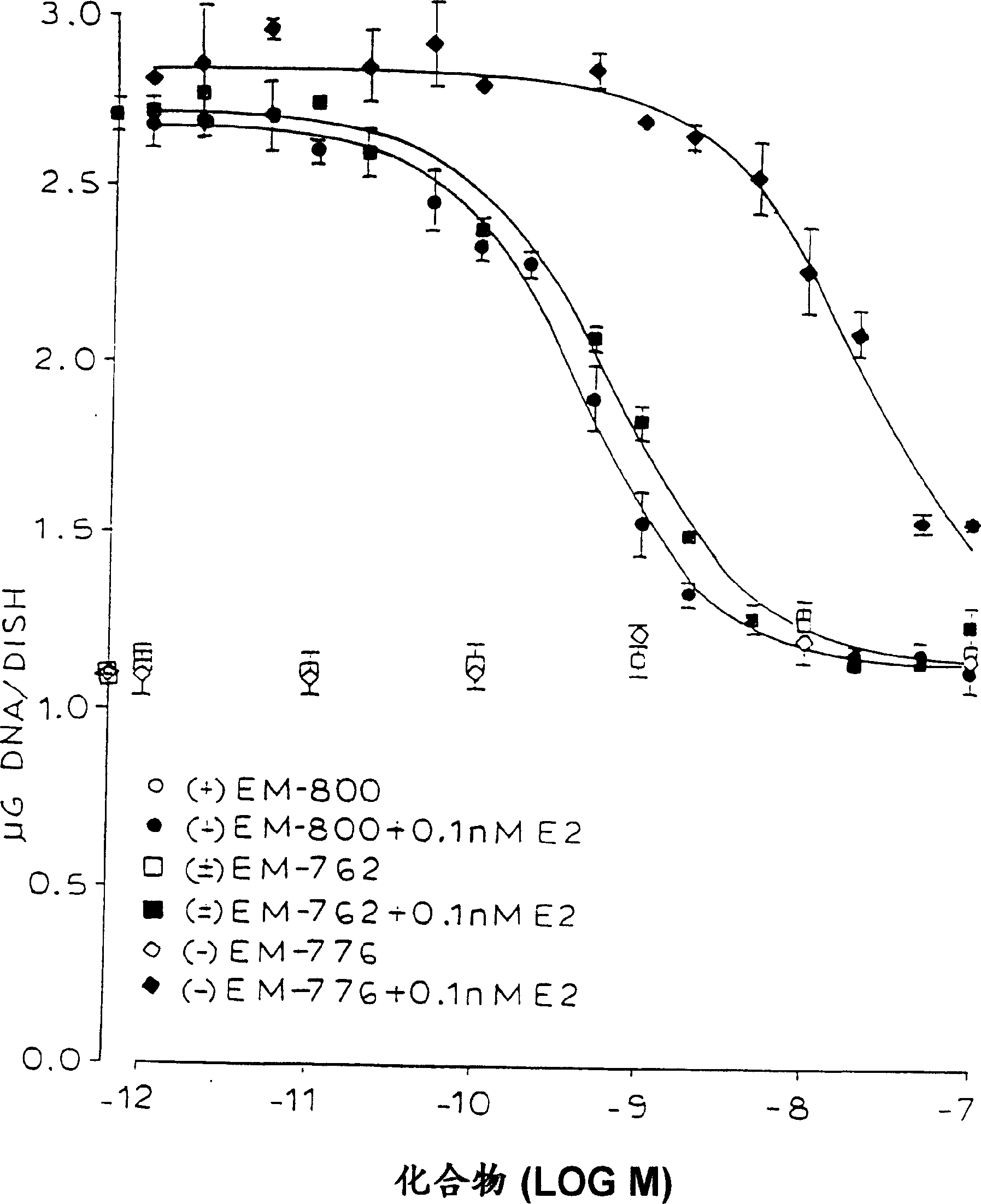
Methods for treating sex steroid-dependent diseases by inhibiting sex steroid activity include administration of novel compounds which include an androgenic nucleus substituted at a ring carbon with at least one substituent specified herein. Such compounds may function by inhibiting sex steroid synthesis (both estrogen and androgen synthesis) and / or by antagonistically blocking androgen receptors.
Owner:ENDORES & DEV
Lif/lifr antagonist in oncology and nonmalignant diseases
InactiveUS20200087340A1Reduce the amount requiredOrganic active ingredientsAndrostane derivativesDiseaseImmunotherapeutic agent
Described herein are methods of using compounds that inhibit leukemia inhibitory factor (LIF) and / or block of the leukemia inhibitory factor receptor for treatment of liver fibrosis, proliferation of spinal tumors, and in combination therapy with an immunotherapeutic agent.
Owner:EVESTRA
Neuroactive steroids, compositions, and uses thereof
InactiveUS20160022701A1Increase and decrease endogenous activityImprove bioavailabilityOrganic active ingredientsNervous disorderMedicineNeuroactive steroid
Owner:SAGE THERAPEUTICS
Preparation method of ulipristal acetate and key intermediate thereof
ActiveCN102516345AHigh puritySimple methodKetal steroidsSteroids preparationEthylenedioxyGrignard reagent
Owner:UTOPHARM SHANGHAI +1
Neuroactive steroids and methods of use thereof
InactiveUS20160031930A1Maintain good propertiesGood metabolic stabilityOrganic active ingredientsSenses disorderNeuroactive steroidPharmacology
Owner:SAGE THERAPEUTICS
Neuroactive 19-alkoxy-17-substituted steroids, prodrugs thereof, and methods of treatment using same
The present disclosure is generally directed to neuroactive 19-alkoxy-17-substituted steroids as referenced herein, and pharmaceutically acceptable salts thereof, for use as, for example, an anesthetic, and / or in the treatment of disorders relating to GABA function and activity. The present disclosure is further directed to pharmaceutical compositions comprising such compounds.
Owner:SAGE THERAPEUTICS +1
Structural modification of 19-norprogesterone I: 17-alpha-substituted-11-beta-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
InactiveUS20050143365A1Organic active ingredientsDrug compositionsAbnormal tissue growthInducing labor
Owner:UNITED STATES OF AMERICA
Neuroactive steroids, compositions, and uses thereof
InactiveUS20160229887A1Useful in therapyReduces avoids symptom causeOrganic active ingredientsSenses disorderSedationNeuroactive steroid
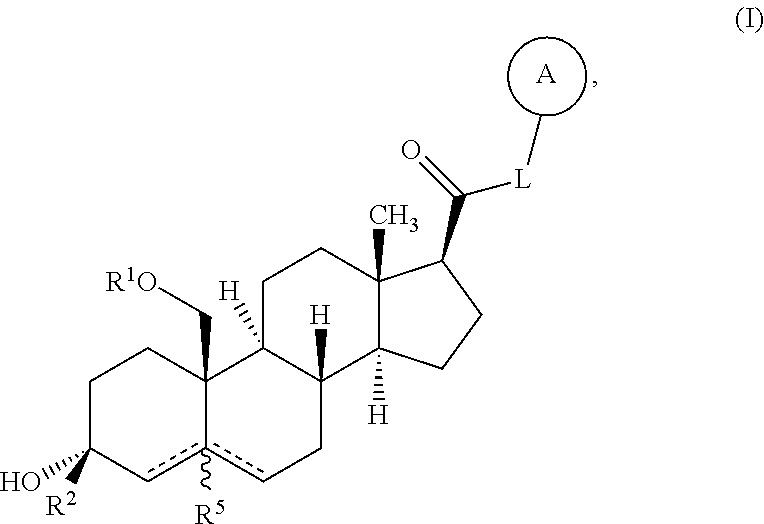
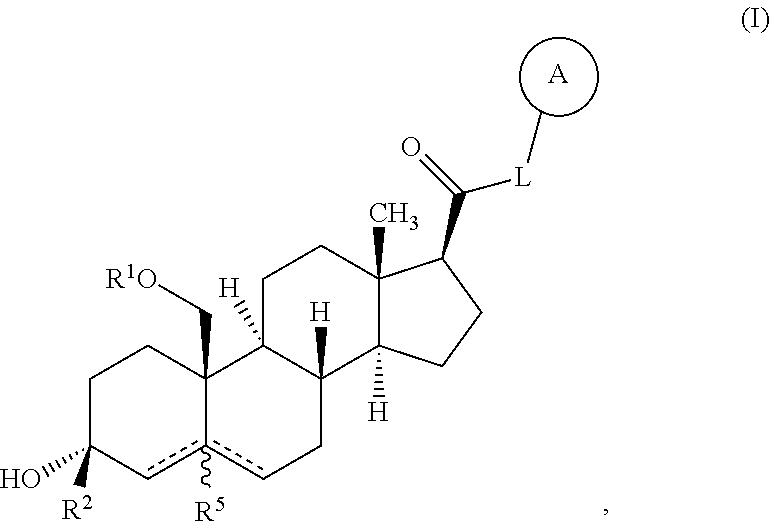
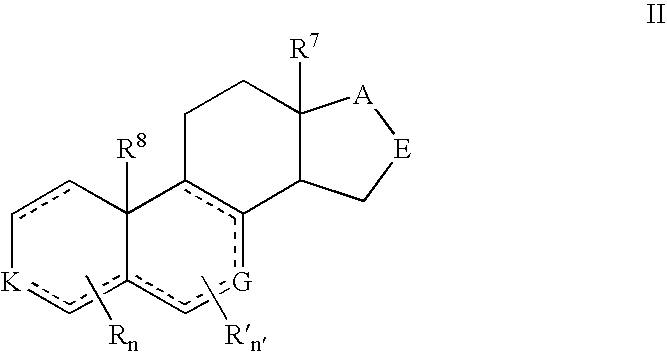
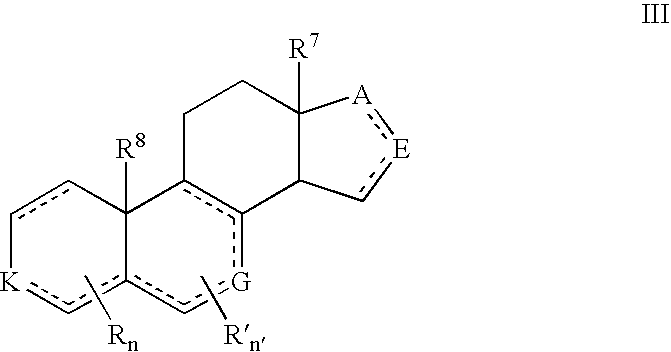
Described herein are neuroactive steroids of the Formula (I): or a pharmaceutically acceptable salt thereof; wherein -------, R1, R2, R5, A and L are as defined herein. Such compounds are envisioned, in certain embodiments, to behave as GABA modulators. The present invention also provides pharmaceutical compositions comprising a compound of the present invention and methods of use and treatment, e.g., such for inducing sedation and / or anesthesia.
Owner:SAGE THERAPEUTICS
Neuroactive 13, 17-substituted steroids as modulators for gaba type-a receptors
The present disclosure is generally directed to neuroactive 13,17-substituted steroids as referenced herein, and pharmaceutically acceptable salts thereof, for use as, for example, an anesthetic, and / or in the treatment of disorders relating to GABA function and activity. The present disclosure is further directed to pharmaceutical compositions comprising such compounds.
Owner:WASHINGTON UNIV IN SAINT LOUIS
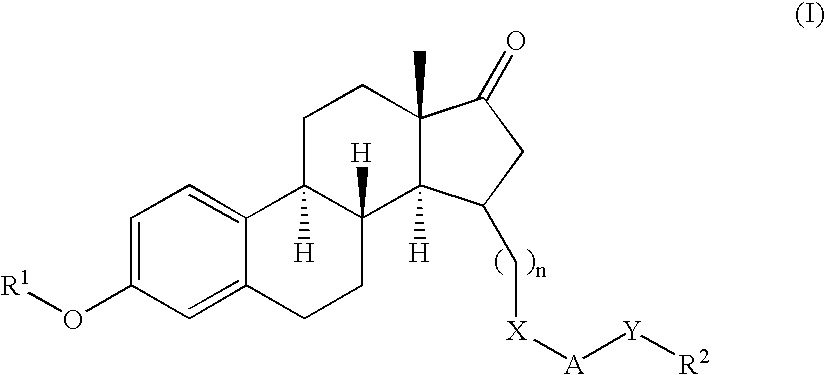
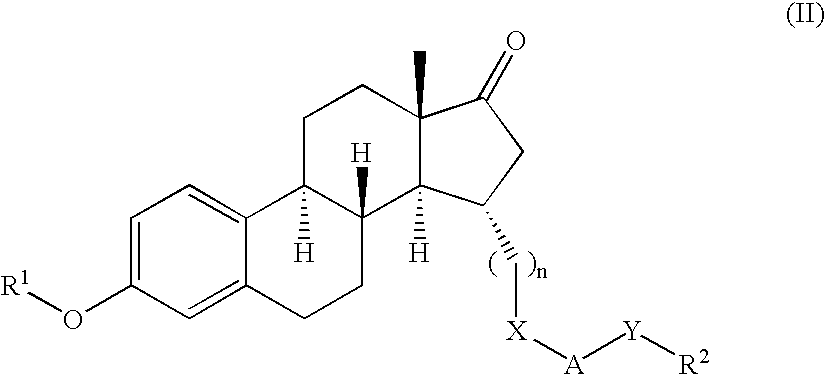
Novel 17beta-hydroxysteroid dehydrogenase type I inhibitors
3,15-substituted estrone compounds which act as inhibitors of 17β-hydroxysteroid dehydrogenase type I (17β-HSD1), salts thereof, pharmaceutical preparations containing such compounds, processes for preparing such compounds, and therapeutic uses of such compounds, particularly in the treatment or inhibition of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration, as well as the general use of selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors which possess in addition no or only pure antagonistic binding affinities to the estrogen receptor for the treatment or inhibition of benign gynecological disorders, particularly endometriosis.
Owner:ABBVIE PHARMA GMBH
Neuroactive steroids, compositions, and uses thereof
ActiveUS10246482B2Reduces avoids symptom causeGood curative effectKetal steroidsSubstance abuserWithdrawal syndrome
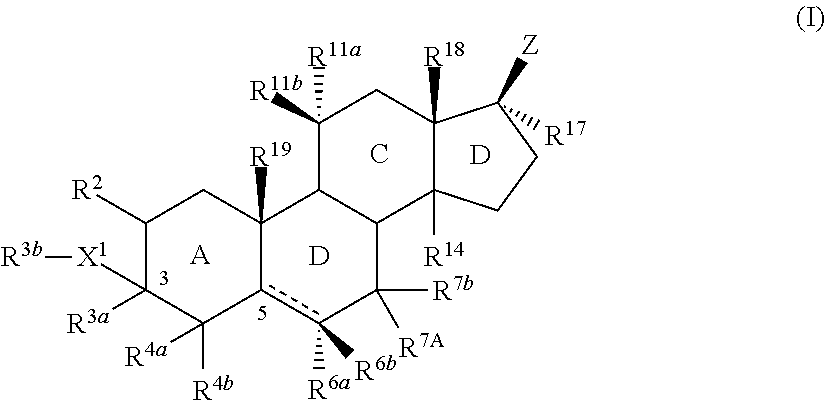
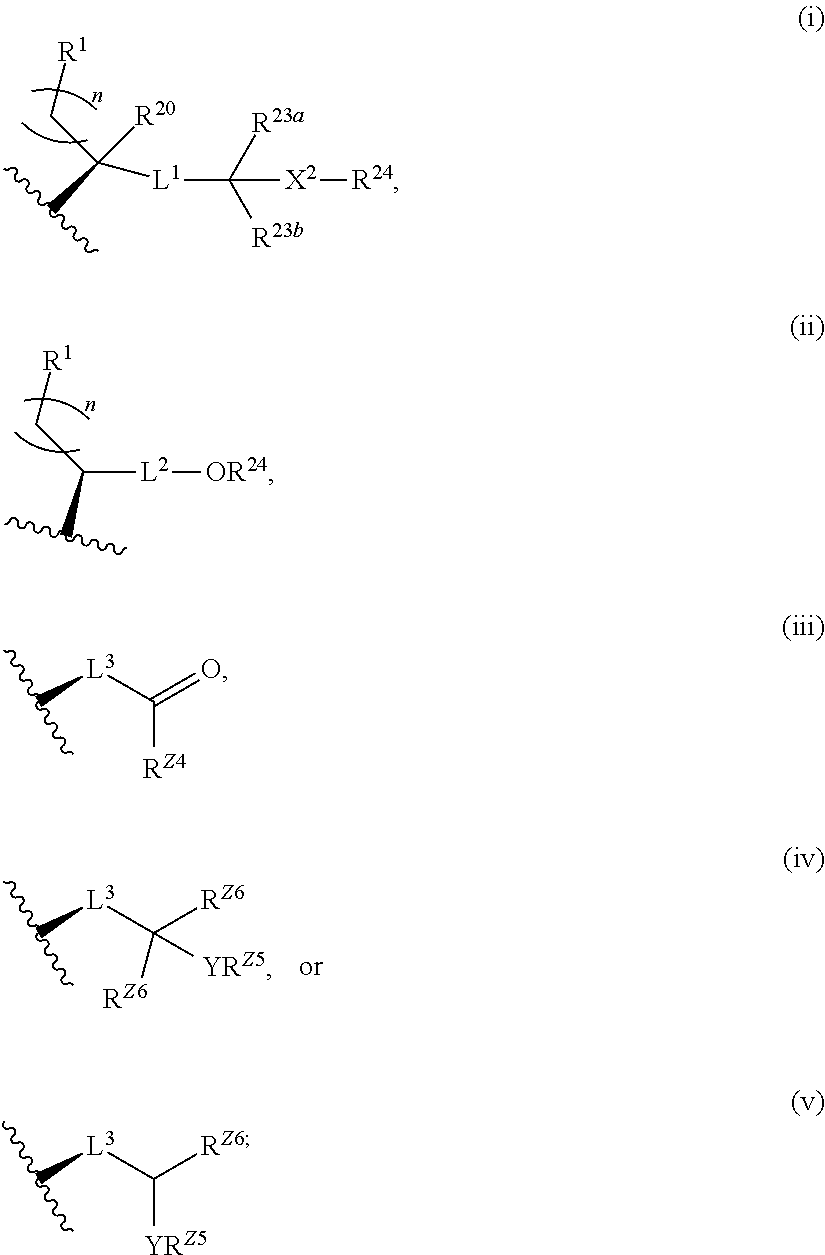
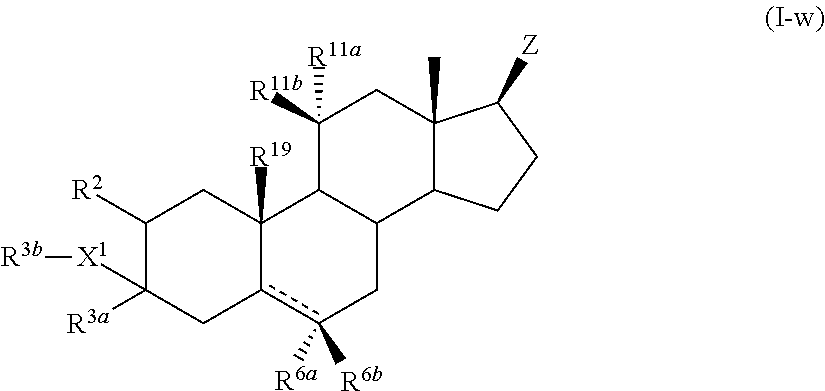
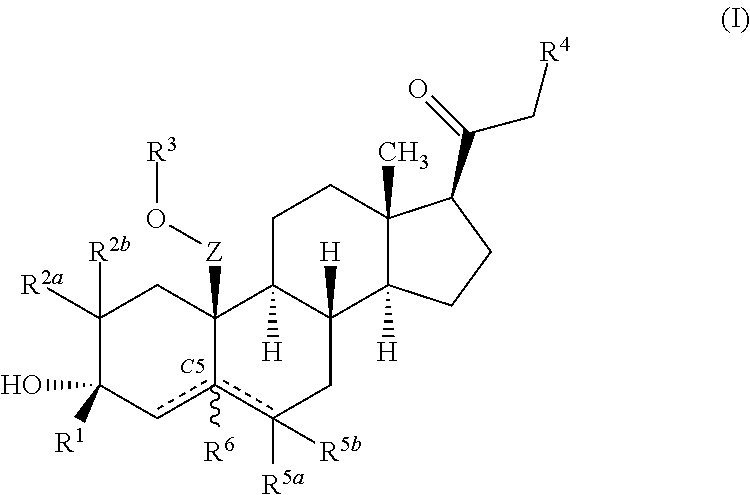
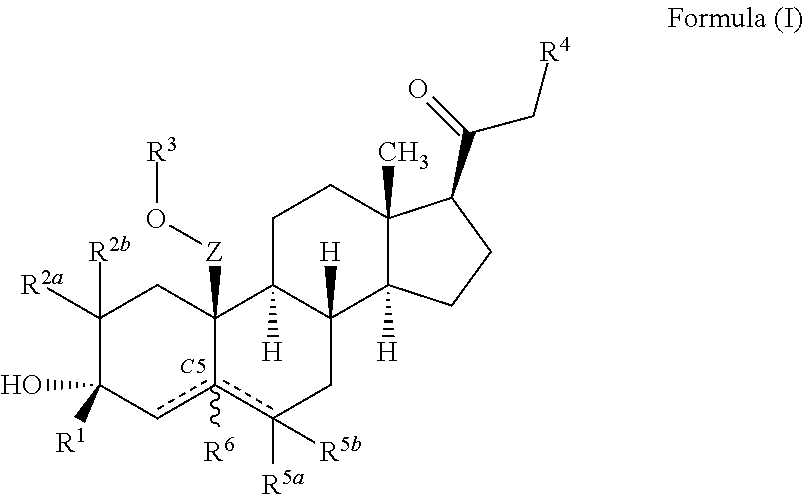
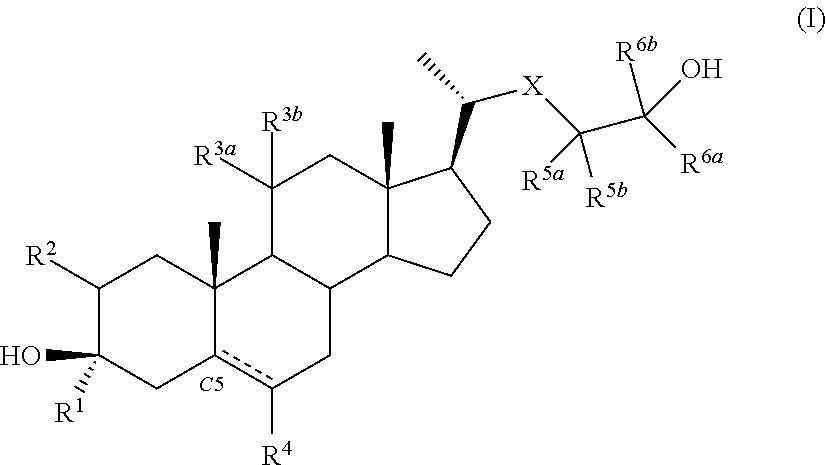
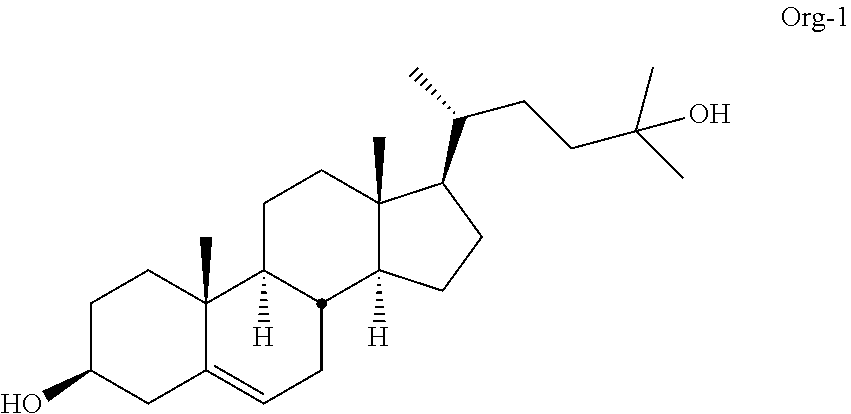
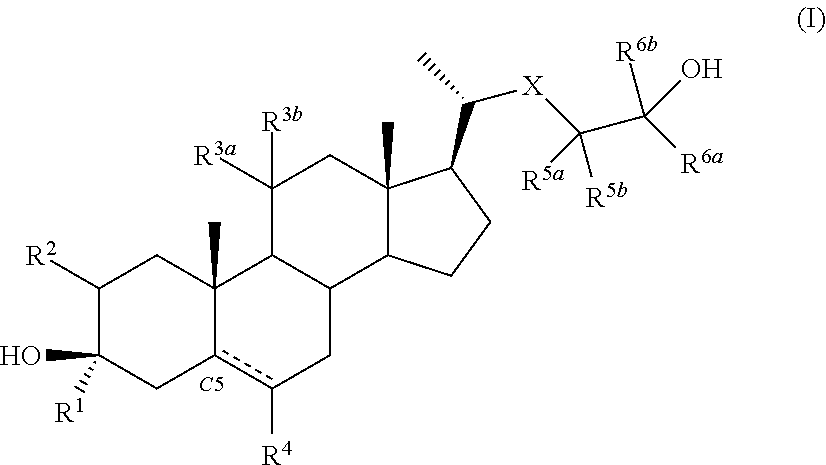
Described herein are steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein R1, R2a, R2b, R3, R4, R5a, R5b, R6, and Z are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Neuroactive steroids and methods of use thereof
InactiveUS20170305960A1Excellent propertyGood metabolic stabilityOrganic active ingredientsSenses disorderNeuroactive steroidStereochemistry
Owner:SAGE THERAPEUTICS
Compounds and Formulations
InactiveUS20080004250A1Organic active ingredientsKetal steroidsWithdrawal syndromeBiological condition
The instant invention provides potent antiandrogen compounds, such as 3β-acetoxyandrost-1,5-diene-17-ethylene ketal and 3β-hydroxyandrost-1,5-diene-17-ethylene ketal, and methods for their use in the prevention and treatment of biological conditions mediated by androgen receptors. Thus, for example, compounds of the invention are useful in the prevention and treatment of prostrate cancer. Furthermore, it has been discovered that compounds of the invention are useful in the prevention and treatment of androgen-independent cancers such as androgen-independent prostrate cancer. Finally, inventive compounds may be used to treat antiandrogen induced withdrawal syndrome.
Owner:NEURMEDIX +2
Cyp11b, cyp17, and/or cyp21 inhibitors
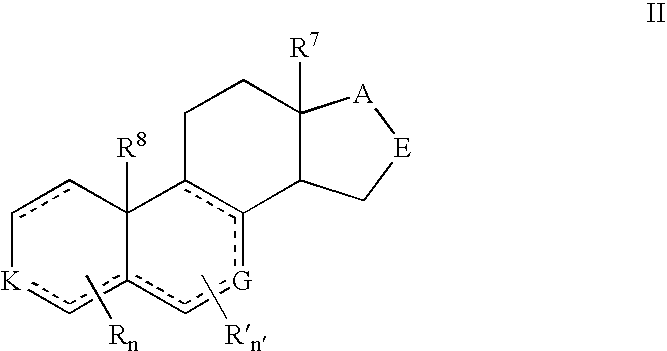
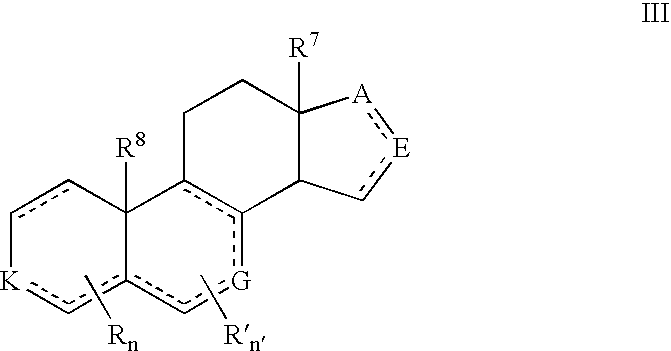
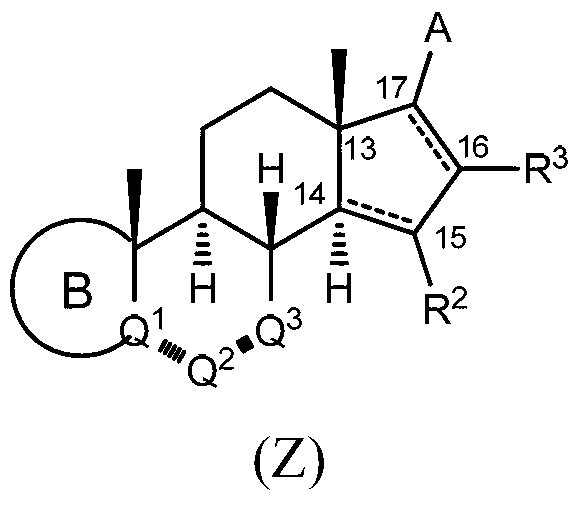
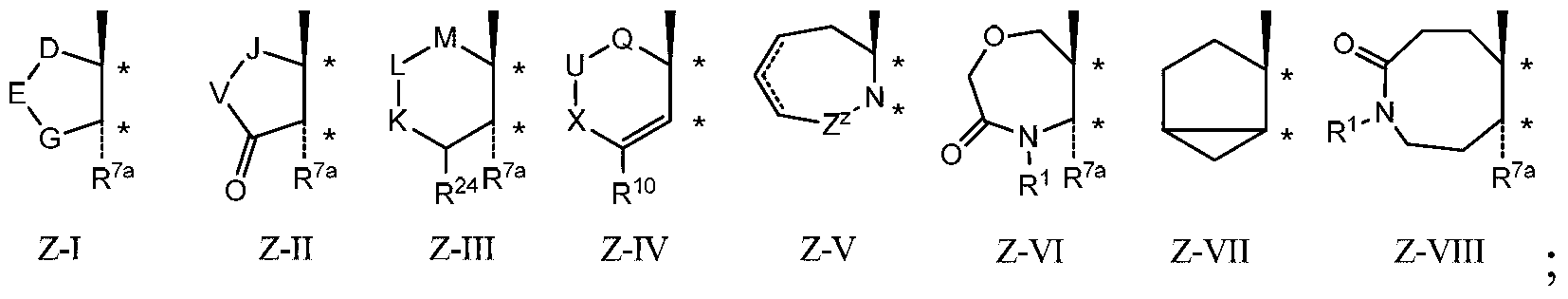
Provided herein are inhibitors of CYP11B, CYP17, and / or CYP21 enzymes of Formula (Z), (IX), (X), (XI), (XII), (XIII), (XIV), (XV), (XVI), or (XVII). Also described herein are pharmaceutical compositions that include at least one compound described herein and the use of a compound or pharmaceutical composition described herein to treat androgen-dependent diseases, disorders and conditions. Formula (Z).
Owner:BIOMARIN PHARMA INC
Preparation method of dydrogesterone intermediate
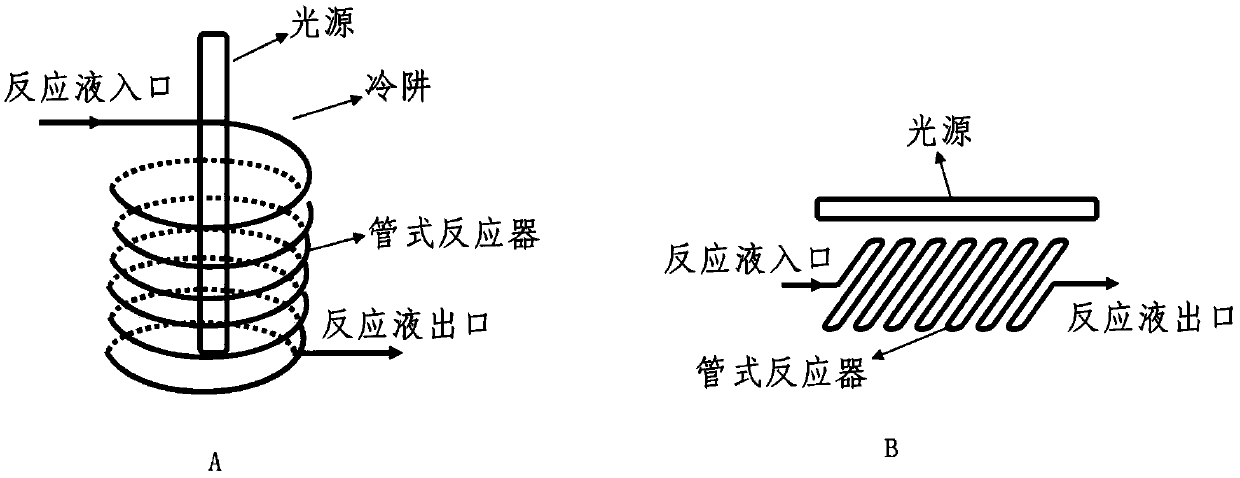
ActiveCN111171101AConcentrated luminous bandsHigh yieldChemical recyclingKetal steroidsPhotocatalytic reactionUltraviolet
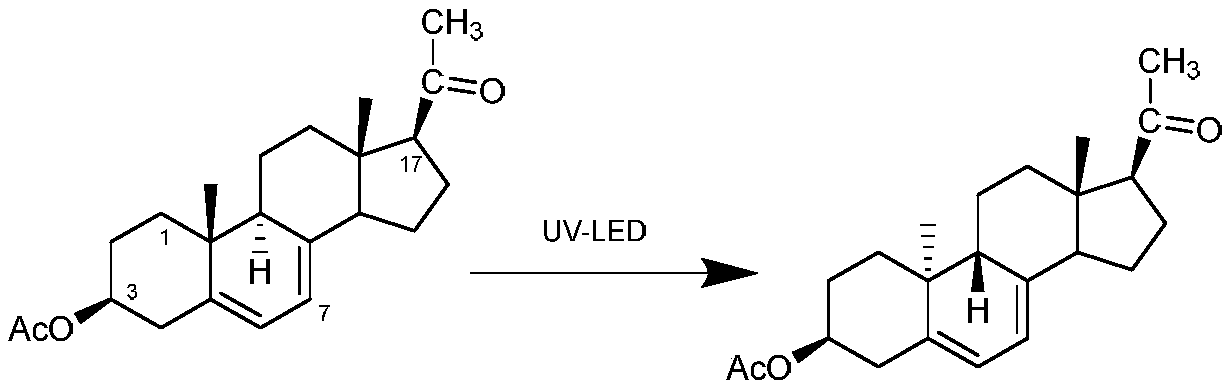
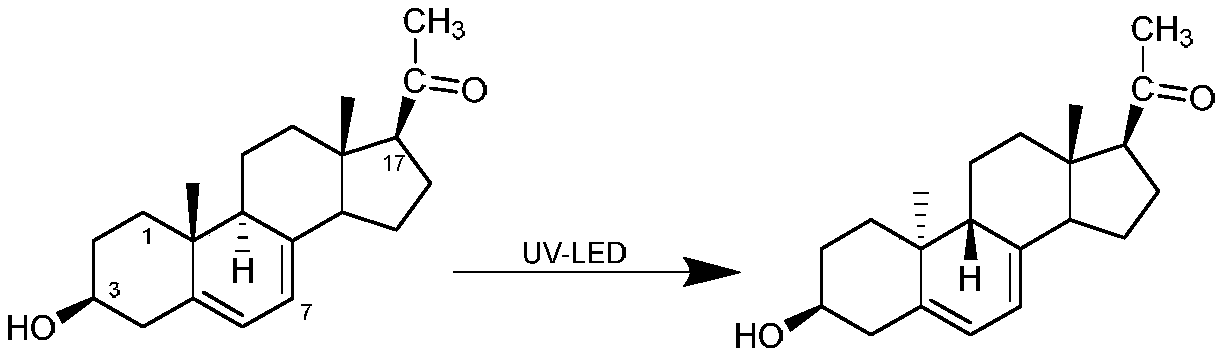
The invention discloses a preparation method of a dydrogesterone intermediate. The preparation method is characterized by comprising the following steps: dissolving 5,7-diene steroid compound in an organic solvent to obtain a solution as a raw material; and carrying out a photocatalytic reaction and separating to obtain the dydrogesterone intermediate, wherein the 5,7-diene steroid compound is 7-dehydropregnenolone acetate, pregna-5,7-diene-3,20-diketodivinyl ketal, 7-dehydropregnenolone, ergosterol or pregna-5,7-diene-3,20-diketo-3-vinyl ketal, and a lamp used in the photocatalytic reaction comprises an LED ultraviolet lamp with the wavelength range of 295-335 nm. The preparation method of the dydrogesterone intermediate is high in yield, low in cost, safer in preparation and friendlier to environment.
Owner:上海璟兆实业有限公司
Preparation method of steroid intermediate
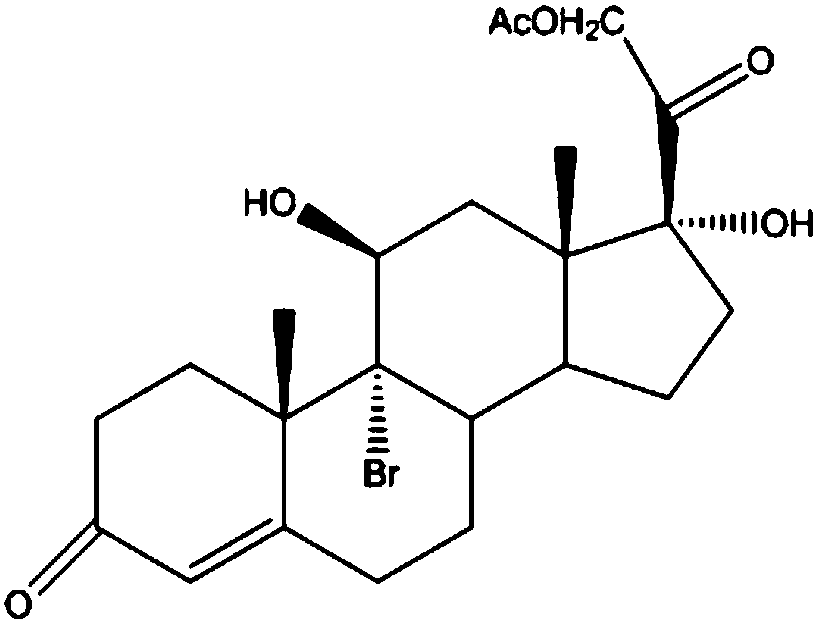
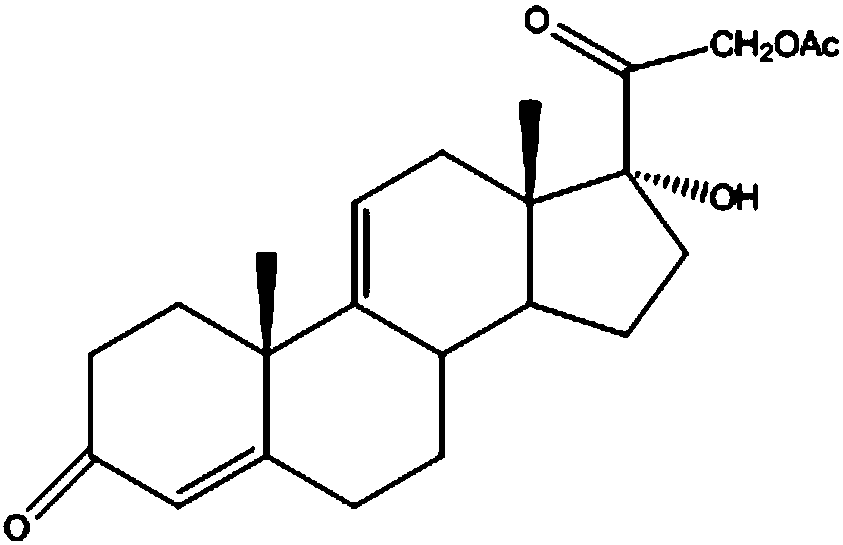
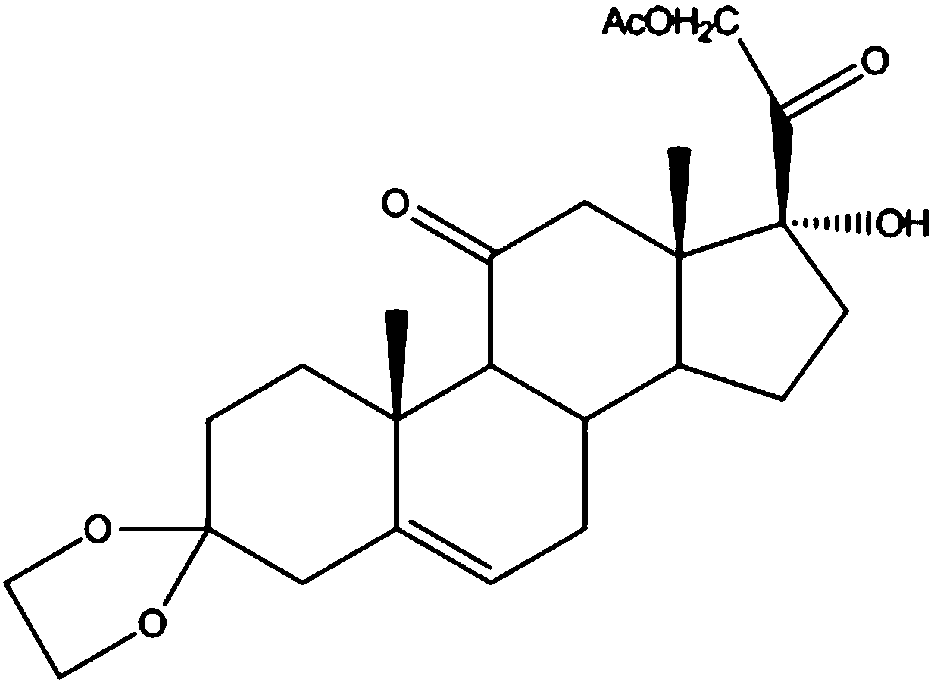
The invention discloses a preparation method of a steroid intermediate. The preparation method comprises the following steps: adding a first solvent, a first acid solution, water and 17-alpha-hydroxypregnenolone steroid-4,9-diene-3,20-diketone acetate into a first reaction tank, and uniformly stirring; adding a bromination agent in four times at 2-4 DEG C, maintaining the temperature for reactingfor 2h, and carrying out thin-layer chromatography analysis until the reaction of the raw materials is finished; and regulating the pH value to be more than 7, regulating the pH value back to 6, carrying out reduced pressure concentration until the first solvent is completely concentrated, adding water, cooling to 5 DEG C, and carrying out centrifugation, cleaning, spin-drying and drying, so as toobtain the steroid intermediate, namely cortisone. According to the preparation method, an oxidant and a catalyst which contain heavy metal ions are not used, the used solvent can be recycled and reused, wastewater does not contain heavy metal ions in the production process, the method belongs to an environment-friendly process and has very good process prospects, and the yield and purity of theproduct are high.
Owner:JIANGSU YUANDA XIANLE PHARMA
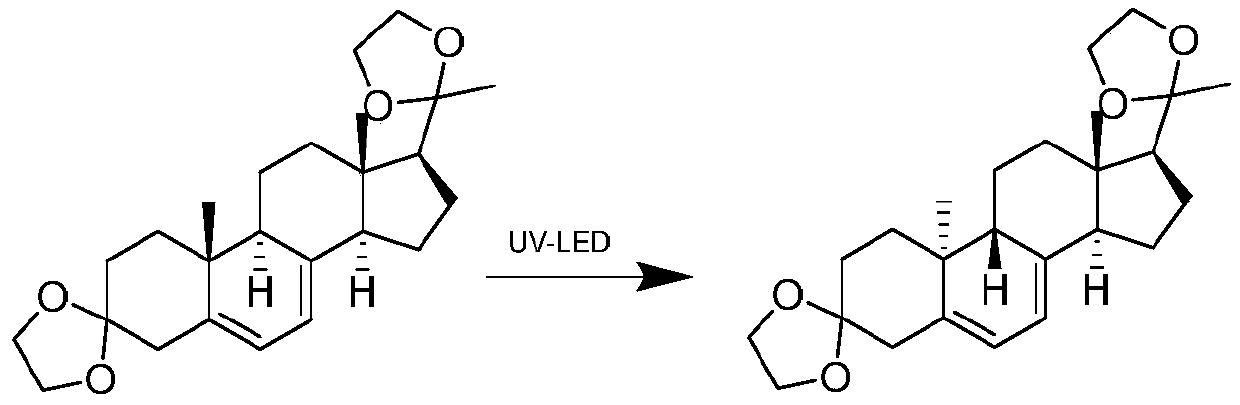
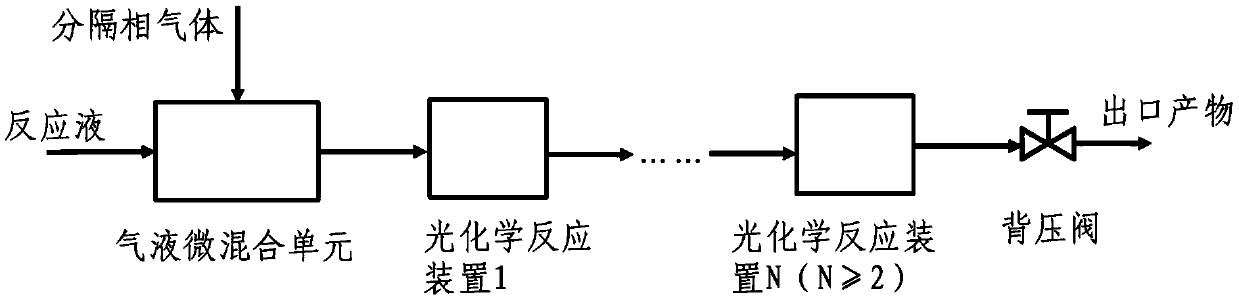
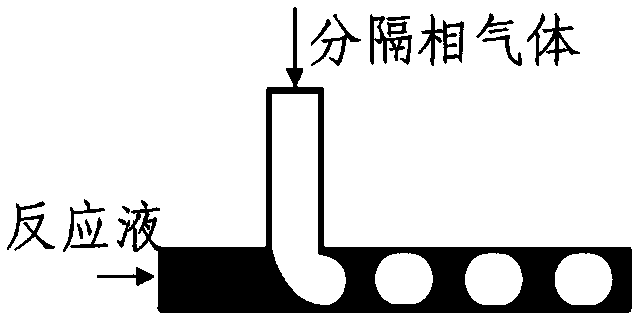
Gas separation flow system and gas separation flow method for photochemical synthesis of 9-beta,10-alpha-dehydroprogesterone ketal
The invention relates to a gas separation flow system and a gas separation flow method for photochemical synthesis of 9-beta,10-alpha-dehydroprogesterone ketal. The method is characterized in that 9-beta,10-alpha-dehydroprogesterone ketal is synthesized in one step or two steps by utilizing ultraviolet light with different wave bands, a raw material 9-alpha,10-beta-dehydroprogesterone ketal reaction solution is separated by gas doing not participate in a reaction, the internal disturbance of the reaction solution is enhanced, and the retention time of the reaction solution in a tubular reactoris ensured to be consistent. The method has the advantages of being continuous, stable, high in light energy utilization rate, low in production cost, easy and convenient to operate and controllablein process, and has huge prospect in the industrial production of 9-beta,10-alpha-dehydroprogesterone ketal through photochemical synthesis.
Owner:SHANDONG TSINGCHUANG CHEMICALSCO LTD +2
Method for synthesizing ursodeoxycholic acid by taking BA as raw material
The invention discloses a synthesis method of ursodeoxycholic acid, and the method comprises the following steps: by using a botanical compound BA as a raw material, carrying out ethylene glycol protection, oxidation, side chain extension reaction, ethylene glycol removal protection, reduction, hydrolysis and the like to synthesize the ursodeoxycholic acid. Raw materials for synthesizing the ursodeoxycholic acid are cheap and easy to obtain, synthesis steps are easy and convenient to operate, the yield is high, environmental friendliness is achieved, and industrial production is facilitated.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Neuroactive steroids, compositions, and uses thereof
ActiveUS10329320B2Reduces avoids symptom causeGood curative effectOrganic active ingredientsNervous disorderSedationNeuroactive steroid
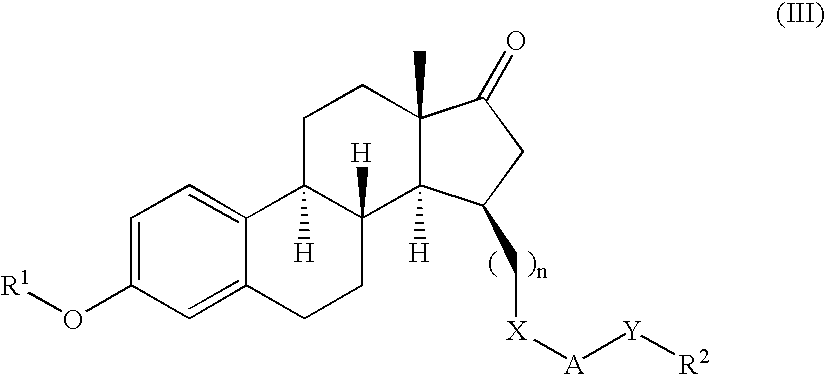
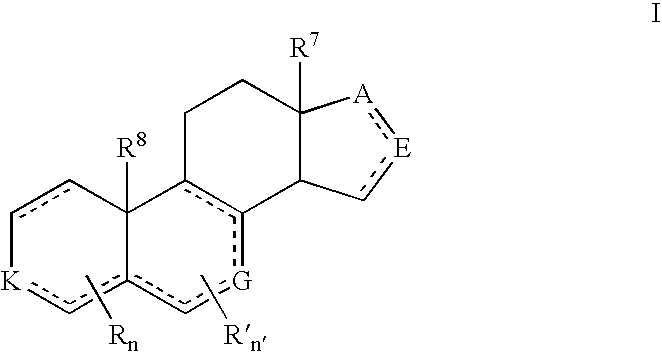
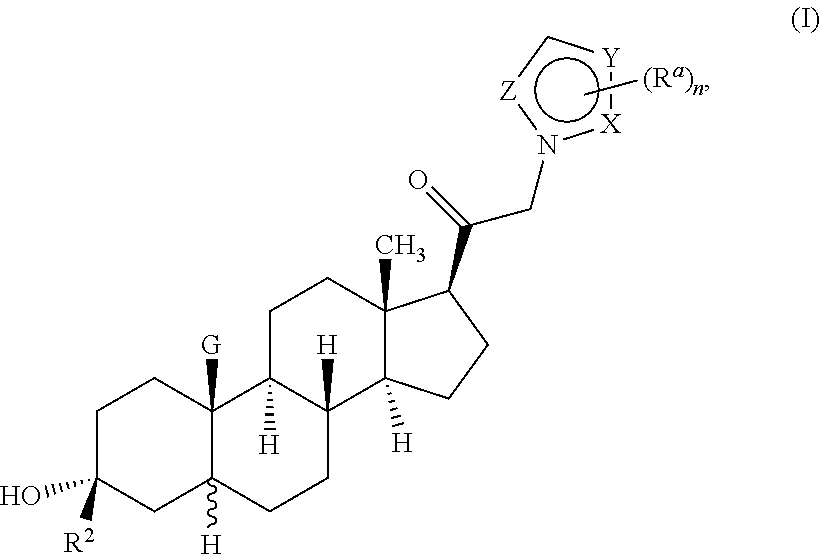
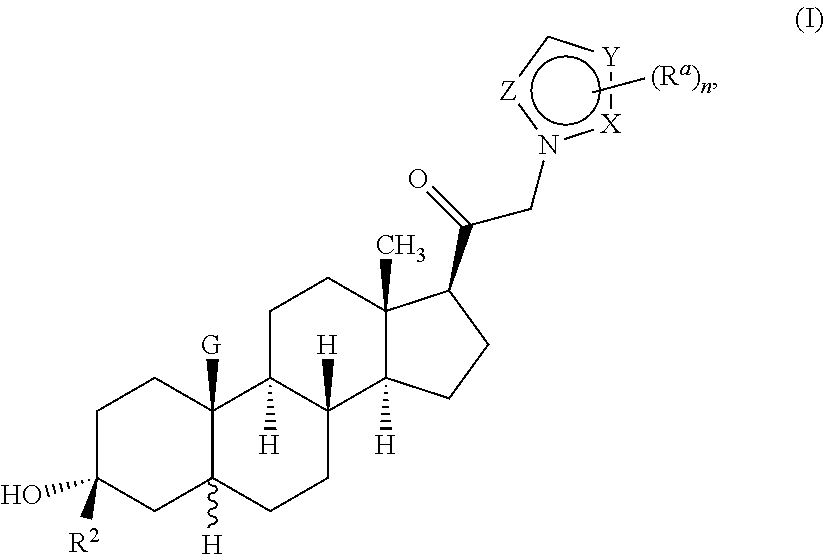
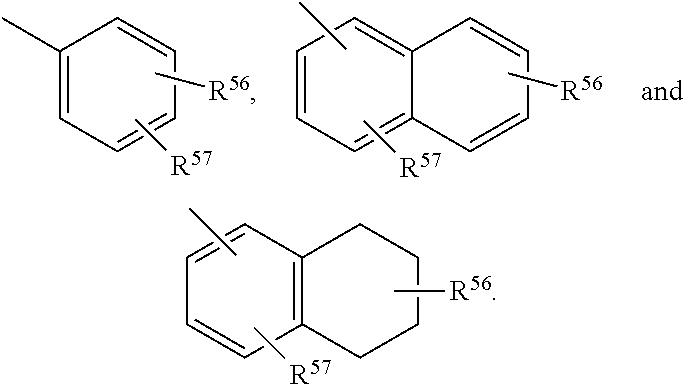
Described herein are neuroactive steroids of the Formula (I):or a pharmaceutically acceptable salt thereof; wherein R1, R2, Ra, G, X, Y, Z, and n are as defined herein. Such compounds are envisioned, in certain embodiments, to behave as GABA modulators. Also provided are pharmaceutical compositions comprising a compound described herein and methods of use and treatment, e.g., such for inducing sedation and / or anesthesia.
Owner:SAGE THERAPEUTICS
Structural modification of 19-norprogesterone I: 17-α-substituted-11-β-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
The present invention relates, inter alia, to compounds having the general formula:in which R1, R2, R3, R4 and X are as defined by the present specification. In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat meningiomas; to treat uterine leiomyomas; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce cervical ripening; to induce labor; and for contraception.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES
Prostate Cancer Treatment
InactiveUS20080009472A1Organic active ingredientsKetal steroidsWithdrawal syndromeBiological condition
The instant invention provides potent antiandrogen compounds, such as 3β-acetoxyandrost-1,5-diene-17-ethylene ketal and 3β-hydroxyandrost-1,5-diene-17-ethylene ketal, and methods for their use in the prevention and treatment of biological conditions mediated by androgen receptors. Thus, for example, compounds of the invention are useful in the prevention and treatment of prostrate cancer. Furthermore, it has been discovered that compounds of the invention are useful in the prevention and treatment of androgen-independent cancers such as androgen-independent prostrate cancer. Finally, inventive compounds may be used to treat antiandrogen induced withdrawal syndrome.
Owner:NEURMEDIX +2
Method for preparing 9 beta, 10 alpha-dehydroprogesterone diethyl diketal by utilizing LED light source
ActiveCN110790807ALow impurity contentWith industrial productionKetal steroidsOrganic chemistryMaterials science
The invention discloses a method for preparing 9 beta, 10 alpha-dehydroprogesterone diethyl diketal by utilizing an LED light source. According to the method, 9 alpha, 10 beta-dehydroprogesterone diethyl diketal is taken as a raw material; the LED light source with a single wavelength is used for carrying out photochemical reaction; so that dydrogesterone key intermediate 9 beta, 10 alpha-dehydroprogesterone diethyl diketal is prepared, the defects in the prior art that when a mercury lamp is used as a light source, photochemical reaction products are complex, the process is not easy to amplify and the like are avoided, and the method which is high in total yield, environmentally friendly, easy and convenient to operate and easy to amplify is provided.
Owner:GUANGXI NORMAL UNIV
Benzopyran-containing compounds and method for their use
InactiveCN1158274CNot easy to convertReduce activationOrganic compound preparationSulfur/selenium/tellurium active ingredientsBenzopyransStereoisomerism
Certain benzopyran antiestrogens are disclosed for treating estrogen sensitive diseases such as breast cancer. Prodrug forms provide ease of manufacturing, good shelf life, and bioavailibility, and preferred stereoisomers are shown to be more effective than racemic mixtures.
Owner:ENDORES & DEV
21-Substituted progesterone derivatives as new antiprogestational agents
A compound having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —CHO, —COCH3 and —CHOHCH3; R2 is a member selected from the group consisting of halogen, alkyl, acyl, hydroxy, alkoxy, acyloxy, alkyl carbonate, cypionyloxy, S-alkyl and S-acyl; R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl. In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce labor; and for contraception.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)